18 Cannabinoids
Overview
Modern pharmacological interest in cannabinoids dates from the discovery that Δ9-tetrahydrocannabinol (THC) is the active principle of cannabis, and took off with the discovery of specific cannabinoid receptors—termed CB receptors—and endogenous ligands (endocannabinoids), together with mechanisms for their synthesis and elimination. Drugs that act on this endocannabinoid system have considerable therapeutic potential. Here we consider plant-derived cannabinoids, cannabinoid receptors, endocannabinoids, physiological functions, pathological mechanisms, synthetic ligands and potential clinical applications. More detailed information is given by Kano et al. (2009). The pharmacology of cannabinoids in the central nervous system (CNS) is discussed in Chapters 36, 47 and 48.
Plant-Derived Cannabinoids and Their Pharmacological Effects
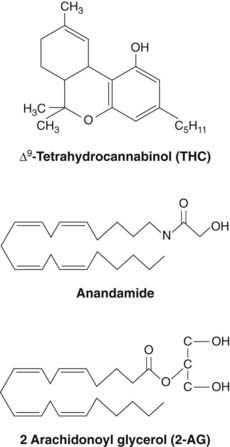
Cannabis sativa, the hemp plant, has been used for its psychoactive properties for thousands of years (Ch. 47). Its medicinal use was advocated in antiquity, but serious interest resurfaced only in 1964 with the identification of Δ9-tetrahydrocannabinol (THC, see Fig. 18.1), as the main psychoactive component. Cannabis extracts contain numerous related compounds, called cannabinoids, most of which are insoluble in water. The most abundant cannabinoids are THC, its precursor cannabidiol, and cannabinol, a breakdown product formed spontaneously from THC. Cannabidiol and cannabinol lack the psychoactive properties of THC, but can exhibit anticonvulsant activity and induce hepatic drug metabolism (see Ch. 9).
Pharmacological Effects
THC acts mainly on the central nervous system (CNS), producing a mixture of psychotomimetic and depressant effects, together with various centrally mediated peripheral autonomic effects. The main subjective effects in humans consist of the following:
These effects are similar to, but usually less pronounced than, those produced by psychotomimetic drugs such as lysergic acid diethylamide (LSD; see Ch. 47). Subjects report that time passes extremely slowly. The alarming sensations and paranoid delusions that often occur with LSD are seldom experienced after cannabis. Some studies support a connection between chronic use and subsequent schizophrenia and mood disorder (Henquet et al., 2005; Leweke & Koethe, 2008).
Central effects that can be directly measured in human and animal studies include:
The main peripheral effects of cannabis are:
Cannabis ![]()
Pharmacokinetic and Analytical Aspects
The effect of cannabis, taken by smoking, takes about 1 h to develop fully and lasts for 2–3 h. A small fraction of THC is converted to 11-hydroxy-THC, which is more active than THC itself and probably contributes to the pharmacological effect of smoking cannabis, but most is converted to inactive metabolites that are subject to conjugation and enterohepatic recirculation. Being highly lipophilic, THC and its metabolites are sequestered in body fat, and excretion continues for several days after a single dose. Radioimmunoassay of THC is bedevilled by cross-reactivity, and accurate identification and quantification of THC in biological fluids, important for medicolegal reasons, depends on mass spectrometry.
Adverse Effects
In overdose, THC is relatively safe, producing drowsiness and confusion but not life-threatening respiratory or cardiovascular depression. In this respect, it is safer than most abused substances, particularly opiates and ethanol. Even in low doses, THC and synthetic derivatives such as nabilone (see below) produce euphoria and drowsiness, sometimes accompanied by sensory distortion and hallucinations. These effects, together with legal restrictions on the use of cannabis, have precluded the widespread therapeutic use of cannabinoids.
In rodents, THC produces teratogenic and mutagenic effects, and an increased incidence of chromosome breaks in circulating white cells has been reported in humans. Such breaks are, however, by no means unique to cannabis, and epidemiological studies have not shown an increased risk of fetal malformation or cancer among cannabis users.
Tolerance and Dependence
Tolerance to cannabis, and physical dependence, occur only to a minor degree and mainly in heavy users. The abstinence symptoms are similar to those of ethanol or opiate withdrawal, namely nausea, agitation, irritability, confusion, tachycardia and sweating, but are relatively mild and do not result in a compulsive urge to take the drug. Psychological dependence does occur with cannabis, but it is less compelling than with the major drugs of addiction (Ch. 48), and it has been argued whether cannabis should be classified as addictive (see Fattore et al., 2008 and reviews by Maldonado & Rodríguez de Fonseca, 2002; Taber & Hurley 2009).
Cannabinoid Receptors
Cannabinoids, being highly lipid soluble, were originally thought to act in a similar way to general anaesthetics. However, in 1988, saturable high-affinity binding of a tritiated cannabinoid was demonstrated in membranes prepared from homogenised rat brain. This led to the identification of specific cannabinoid receptors in brain. These are now termed CB1 receptors to distinguish them from the CB2 receptors subsequently identified in peripheral tissues. Cannabinoid receptors are typical members of the family of G-protein-coupled receptors (Ch. 3). CB1 receptors are linked via Gi/o to inhibition of adenylyl cyclase and of voltage-operated calcium channels, and to activation of G-protein-sensitive inward-rectifying potassium (GIRK) channels, causing hyperpolarisation (Fig. 18.2). These effects are similar to those mediated by opioid receptors (Ch. 41). CB1 receptors are located in the plasma membrane of nerve endings and inhibit transmitter release from presynaptic terminals, which is caused by depolarisation and Ca2+ entry (Ch. 4). CB receptors also influence gene expression, both directly by activating mitogen-activated protein kinase, and indirectly by reducing the activity of protein kinase A as a result of reduced adenylyl cyclase activity (see Ch. 3).
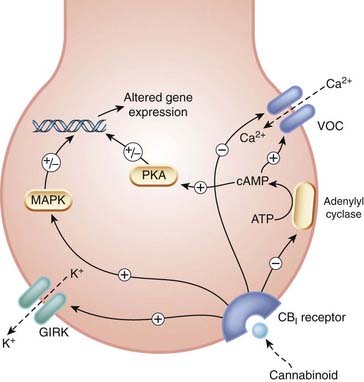
Fig. 18.2 Cellular actions of cannabinoids.
CB1 receptor activation inhibits neurotransmitter release via inhibition of Ca2+ entry and hyperpolarisation due to activation of potassium channels. It also alters gene expression. GIRK: G-protein-sensitive inward-rectifying potassium channel; MAPK: mitogen-activated protein kinase; PKA: protein kinase A; VOC, voltage-operated calcium channel.
(Redrawn from Devane et al. 1992.)
CB1 receptors are among the most abundant receptors in the brain, being comparable in this regard with receptors for glutamate and GABA—the main central excitatory and inhibitory neurotransmitters (Ch. 37). They are not homogeneously distributed, being concentrated in the hippocampus (relevant to effects of cannabinoids on memory), cerebellum (relevant to loss of coordination), hypothalamus (important in control of appetite and body temperature; see Ch. 29 and below), substantia nigra, mesolimbic dopamine pathways that have been implicated in psychological ‘reward’ (Ch. 48), and in association areas of the cerebral cortex. There is a relative paucity of CB1 receptors in the brain stem, perhaps explaining the lack of serious respiratory or cardiovascular toxicity of the cannabinoids. At a cellular level, CB1 receptors are localised presynaptically, and inhibit transmitter release as explained above. Like opioids, they can, however, increase the activity of some neuronal pathways by inhibiting inhibitory connections, including GABA-ergic interneurons in the hippocampus and amygdala.
In addition to their well-recognised location in the CNS, CB1 receptors are also expressed in peripheral tissues, for example on endothelial cells, adipocytes and peripheral nerves. Cannabinoids promote lipogenesis through activation of CB1 receptors, an action that could contribute to their effect on body weight (Cota et al., 2003).
The CB2 receptor has only approximately 45% amino acid homology with CB1 and is located mainly in lymphoid tissue (spleen, tonsils and thymus as well as circulating lymphocytes, monocytes and tissue mast cells). CB2 receptors are also present on microglia—immune cells in the CNS (Ch. 36). The localisation of CB2 receptors on cells of the immune system was unexpected, but may account for inhibitory effects of cannabis on immune function. CB2 receptors differ from CB1 receptors in their responsiveness to cannabinoid ligands (see Table 18.1). They are linked via Gi/o to adenylyl cyclase, GIRK channels and mitogen-activated protein kinase similarly to CB1, but not to voltage-operated calcium channels (which are not expressed in immune cells). So far, rather little is known about their function. They are present in atherosclerotic lesions (see Ch. 22), and CB2 agonists have antiatherosclerotic effects (Mach & Steffens, 2008).
Table 18.1 Definite and possible endocannabinoids
| Endocannabinoid | Selectivity |
|---|---|
| Definite endocannabinoids | |
| Anandamide | CB1 > CB2 |
| 2-Arachidonoyl glycerol | CB1 = CB2 |
| Less well-established endocannabinoid candidates | |
| Virhodamine | CB2 > CB1 |
| Noladin | CB1 >> CB2 |
| N-Arachidonoyl dopamine | CB1 >> CB2 |
Some endocannabinoids turned out, surprisingly,1 to activate vanilloid receptors, ionotropic receptors that stimulate nociceptive nerve endings (see Ch. 41). Other as-yet-unidentified G-protein-coupled receptors are also implicated, because cannabinoids exhibit analgesic actions and activate G-proteins in the brain of CB1 knockout mice despite the absence of CB1 receptors.
Endocannabinoids
The discovery of specific cannabinoid receptors led to a search for endogenous mediators. The first success was chalked up by a team that screened fractions of extracted pig brain for ability to compete with a radiolabelled cannabinoid receptor ligand (Devane et al., 1992). This led to the purification of N-arachidonylethanolamide, an eicosanoid mediator (see Ch. 17), the structure of which is shown in Figure 18.1. This was christened anandamide.2 Anandamide not only displaced labelled cannabinoid from synaptosomal membranes in the binding assay, but also inhibited electrically evoked twitches of mouse vas deferens, a bioassay for psychotropic cannabinoids (Fig. 18.3). A few years later, a second endocannabinoid, 2-arachidonoyl glycerol (2-AG, Fig 18.1), was identified, and more recently three further endocannabinoid candidates with distinct CB1/CB2 (see below) receptor selectivities have been added to the list (Table 18.1). Endocannabinoids are made ‘on demand’ like eicosanoids (see Ch. 17), rather than being presynthesised and stored for release when needed.
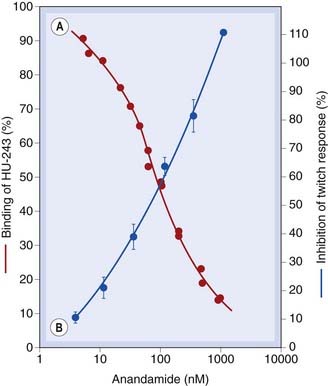
Fig. 18.3 Anandamide as an endocannabinoid.
Anandamide is an endogenous cannabinoid. [A] Competitive inhibition of tritiated HU-243 (a cannabinoid receptor ligand) binding to synaptosomal membranes from rat brain by natural anandamide (red circles, left hand ordinate axis). [B] Inhibition of vas deferens twitch response (a bioassay for cannabinoids) by natural anandamide (blue symbols, right hand ordinate). Note the similarity between the binding and bioactivity.
(Redrawn from Devane et al. 1992.)
Biosynthesis of Endocannabinoids
Biosynthesis of anandamide and of 2-AG is summarised in Figure 18.4. A fuller account of biosynthesis and degradation is given by Di Marzo (2008).
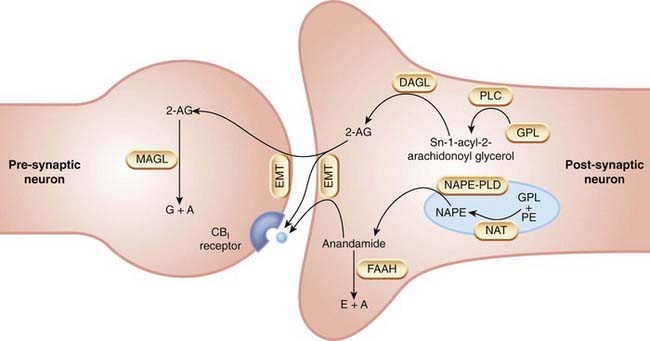
Fig. 18.4 Biosynthesis and inactivation of endocannabinoids.
2-AG, 2-arachidonoyl glycerol; A, arachidonic acid; DAGL, diacylglycerol lipase; E, ethanolamine; EMT, endocannabinoid membrane transporter; FAAH, fatty acid amide hydrolase; GPL, glycerophospholipid; MAGL, monoacyl glycerol lipase; NAPE, N-acyl-phosphatidylethanolamine; NAPE-PLD, N-acyl phosphatidylethanolamine-specific phopholipase D; NAT, N-acyl-transferase; PE, phosphatidylethanolamine; PLC, phospholipase C.
 Anandamide is formed by a distinct phospholipase D (PLD) selective for N-acyl-phosphatidylethanolamine (NAPE) but with low affinity for other membrane phospholipids, and known as NAPE-PLD. NAPE-PLD is a zinc metallohydrolase that is stimulated by Ca2+ and also by polyamines. Selective inhibitors for NAPE-PLD are being sought. The precursors are produced by an as-yet-uncharacterised but Ca2+-sensitive transacylase that transfers an acyl group from the sn-1 position of phospholipids to the nitrogen atom of phosphatidylethanolamine.
Anandamide is formed by a distinct phospholipase D (PLD) selective for N-acyl-phosphatidylethanolamine (NAPE) but with low affinity for other membrane phospholipids, and known as NAPE-PLD. NAPE-PLD is a zinc metallohydrolase that is stimulated by Ca2+ and also by polyamines. Selective inhibitors for NAPE-PLD are being sought. The precursors are produced by an as-yet-uncharacterised but Ca2+-sensitive transacylase that transfers an acyl group from the sn-1 position of phospholipids to the nitrogen atom of phosphatidylethanolamine.
2-AG is also produced by hydrolysis of precursors derived from phospholipid metabolism. The key enzymes are two recently cloned sn-1-selective diacylglycerol lipases (DAGL-α and DAGL-β), which belong to the family of serine lipases. Both these enzymes, like NAPE-PLD, are Ca2+ sensitive, consistent with intracellular Ca2+ acting as the physiological stimulus to endocannabinoid synthesis. The DAGLs are located in axons and presynaptic axon terminals during development, but postsynaptically in dendrites and cell bodies of adult neurons, consistent with a role for 2-AG in neurite growth, and with a role as a retrograde mediator (see below) in adult brain.
Little is known as yet about the biosynthesis of the more recent endocannabinoid candidates noladin, virhodamine and N-arachidonoyl dopamine. pH-dependent non-enzymatic interconversion of vihrodamine and anandamide is one possibility, and could result in a switch between CB2- and CB1-mediated responses (see Table 18.1).
Termination of the Endocannabinoid Signal
Endocannabinoids are rapidly taken up from the extracellular space. Being lipid soluble, they diffuse through plasma membranes down a concentration gradient. There is also evidence for a saturable, temperature-dependent, facilitated transport mechanism for anandamide and 2-AG, dubbed the ‘endocannabinoid membrane transporter’, for which selective uptake inhibitors (e.g. UCM-707) have been developed. Pathways of endocannabinoid metabolism are summarised in Figure 18.4. The key enzyme for anandamide is a microsomal serine hydrolase known as fatty acid amide hydrolase (FAAH). FAAH converts anandamide to arachidonic acid plus ethanolamine and also hydrolyses 2-AG, yielding arachidonic acid and glycerol.
The phenotype of FAAH ‘knockout’ mice gives some clues to endocannabinoid physiology; such mice have an increased brain content of anandamide and an increased pain threshold. Selective inhibitors of FAAH have analgesic and anxiolytic properties in mice (see Ch. 43 for an explanation of how drugs are tested for anxiolytic properties in rodents). In contrast to anandamide, brain content of 2-AG is not increased in FAAH knockout animals, indicating that another route of metabolism of 2-AG is likely to be important. Other possible routes of metabolism include esterification, acylation and oxidation by cyclo-oxygenase-2 to prostaglandin ethanolamides (‘prostamides’), or by 12- or 15-lipoxygenase (see Ch. 17).
Physiological Mechanisms
Stimuli that release endocannabinoids, leading to activation of CB1 receptors and the linkage to downstream events including behavioural or psychological effects, are very incompletely defined. Increased intracellular Ca2+ concentration is probably an important cellular trigger because, as mentioned above, Ca2+ activates NAPE-PLD and other enzymes involved in endocannabinoid biosynthesis.
Activation of CB receptors is implicated in a phenomenon known as depolarisation-induced suppression of inhibition (DSI). DSI occurs in hippocampal pyramidal cells; when these are depolarised by an excitatory input, this suppresses the GABA-mediated inhibitory input to the pyramidal cells, implying a retrograde flow of information from the depolarised pyramidal cell to inhibitory axons terminating on it. Such a reverse flow of information from post- to presynaptic cell is a feature of other instances of neuronal plasticity, such as ‘wind-up’ in nociceptive pathways (Fig. 41.3) and long-term potentiation in the hippocampus (Fig. 37.7). DSI is blocked by the CB1 antagonist rimonabant. The presynaptic location of CB1 receptors and cellular distributions of the DAGL and MAGL enzymes (Fig. 18.4) fit nicely with the idea that the endocannabinoid 2-AG could be a ‘retrograde’ messenger in DSI (see Fig. 38.8).
Neuromodulatory actions of endocannabinoids could influence a wide range of physiological activities, including nociception, cardiovascular, respiratory and gastrointestinal function. Hypothalamic hormone interactions could influence food intake and reproductive function. Mouse models lacking CB receptors support important and balanced roles of endocannabinoid signalling in male and female fertility and are implicated in spermatogenesis, fertilisation, preimplantation development of the early embryo, implantation and postimplantation growth of the embryo (reviewed by Wang et al., 2006). Effects of endocannabinoids on food intake are of particular interest, because of the importance of obesity (Ch. 29).
The endocannabinoid system ![]()
Pathological Involvement
There is evidence, both from experimental animals and from human tissue, that endocannabinoid signalling is abnormal in various neurodegenerative diseases (see Ch. 39). Other diseases where abnormalities of cannabinoid signalling have been reported in human tissue as well as experimental models include hypotensive shock (both haemorrhagic and septic; see Ch. 22), advanced cirrhosis of the liver (where there is evidence that vasodilatation is mediated by endocannabinoids acting on vascular CB1 receptors—see Bátkai et al., 2001), miscarriage (see Wang et al., 2006) and malignant disease. It seems likely that in some disorders endocannabinoid activity is a compensatory mechanism limiting the progression of disease or occurrence of symptoms, whereas in others it may be ‘too much of a good thing’ and actually contribute to disease progression. Consequently, there may be a place in therapeutics for drugs that potentiate or inhibit the cannabinoid system; see Di Marzo & Petrosino (2007) for a fuller discussion.
Synthetic Cannabinoids
Cannabinoid receptor agonists were developed in the 1970s in the hope that they would prove useful non-opioid/non-NSAID analgesics (cf. Chs 41 and 26, respectively, for limitations of opioids and NSAIDs), but adverse effects, particularly sedation and memory impairment, were problematic. Nevertheless, one such drug, nabilone, is sometimes used clinically for nausea and vomiting caused by cytotoxic chemotherapy if this is unresponsive to conventional antiemetics (Ch. 29). The cloning of CB2 receptors, and their absence from healthy brain, led to the synthesis of CB2-selective agonists in the hope that these would lack the CNS-related adverse effects of plant cannabinoids. Several such drugs are being investigated for possible use in inflammatory and neuropathic pain.
The first selective CB1 receptor antagonist, rimonabant, also has inverse agonist properties in some systems. It was licensed in Europe for treating obesity, but was withdrawn because of psychiatric problems including depression. Synthetic inhibitors of endocannabinoid uptake and/or metabolism have shown potentially useful effects in animal models of pain, epilepsy, multiple sclerosis, Parkinson’s disease, anxiety and diarrhoea.
Clinical Applications
Clinical uses of drugs that act on the cannabinoid system remain controversial, but in both the UK and the USA cannabinoids have been used as antiemetics and to encourage weight gain in patients with chronic disease such as HIV-AIDS and malignancy. A substantial randomised controlled trial of THC in patients with multiple sclerosis found no objective evidence of benefit on spasticity but improved mobility (see also Ch. 39). Adverse events were generally mild at the doses used—see UK MS Research Group (2003). Endocannabinoids have been implicated in shock and hypotension in liver disease (Malinowska et al., 2008), and modulation of this system is a potential therapeutic target. Other potential clinical uses (see Pacher et al., 2006 for a review of emerging therapeutic targets) are given in the clinical box.
Potential and actual clinical uses of cannabinoid agonists and antagonists ![]()
Cannabinoid agonists and antagonists are undergoing evaluation for a wide range of possible indications, including the following.
The CB1 receptor antagonist rimonabant, combined with a reduced calorie diet, caused a dose-related weight loss (of approximately 6 kg at the higher dose) after 12 months’ treatment in one placebo-controlled trial (see Fig. 18.5, and see also Ch. 29). Adverse effects included nausea and diarrhoea, and importantly depression and other psychological disturbances which led to its withdrawal from clinical use as an anorectic agent, but interest remains in the therapeutic potential of blocking the endocannabinoid system in order to reduce weight and improve cardiometabolic risk factors (reviewed by Samaha & Chou, 2009).
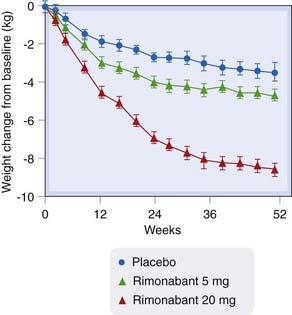
Fig. 18.5 Change from baseline in body weight in a double-blind, placebo-controlled trial of rimonabant versus placebo in 1507 overweight patients.
(Redrawn from Van Gaal et al., 2005.)
References and Further Reading
Freund T.F., Katona I., Piomelli D. Role of endogenous cannabinoids in synaptic signaling Physiol. Rev. 83:2003:1017-1066 (The fine-grain anatomical distribution of the neuronal cannabinoid receptor CB1 is described, and possible functions of endocannabinoids as retrograde synaptic signal molecules discussed in relation to synaptic plasticity and network activity patterns)
Kano M., Ohno-Shosaku T., Hashimotodani Y., et al. Physiol. Rev.. 2009;89:309-380. (Integrates current pharmacological with anatomical, electrophysiological and behavioural knowledge)
Maldonado R., Rodríguez de Fonseca F. Cannabinoid addiction: behavioral models and neural correlates. J. Neurosci.. 2002;22:3326-3331. (Deals mainly with animal models and argues that cannabis meets criteria for classification as addictive)
Samaha F.F., Chou C.M. Blockade of the endocannabinoid system for the reduction of cardiometabolic risk factors. Obesity. 2009;17:220-225. (Reviews rimonabant action in animals and clinical trials in context of cardiovascular risk factors, including future prospects)
Wilson R.I., Nicoll R.A. Endocannabinoid signaling in the brain. Science. 2002;296:678-682.
Bátkai S., Járai Z., Wagner JA., et al Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis Nat. Med. 7:2001:827-832 (Rats with cirrhosis have low blood pressure, which is elevated by a CB1 receptor antagonist. Compared with non-cirrhotic controls, in cirrhotic human livers there was a three-fold increase in CB1 receptors on isolated vascular endothelial cells)
Cota D., Marsicano G., Tschöp M., et al The endogenous cannabinoid system affects energy balance via central orixogenic drive and peripheral lipogenesis J. Clin. Invest. 112:2003:423-431 (Investigation of CB1 knockout mice, implicating this receptor in regulation of energy homeostasis via both central effect on food intake and on peripheral lipogenesis; see also related commentary by Horvath, T.L. pp. 323–326 of the same issue, on endocannabinoids and regulation of body fat)
Devane W.A., Hanu L., Breurer A., et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946-1949. (Identification of arachidonylethanolamide, extracted from pig brain, both chemically and via a bioassay, as a natural ligand for the cannabinoid receptor; the authors named it anandamide after a Sanskrit word meaning ‘bliss’ + amide)
Di Marzo V. Endocannabinoids: synthesis and degradation. Rev. Physiol. Biochem. Pharmacol.. 2008;160:1-24. (Reviews current knowledge)
Di Marzo V., Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr. Opin. Lipidol.. 2007;18:129-140. (Gastrointestinal disorders, inflammation, neurodegeneration)
Fattore L., Fadda P., Spano M.S., et al. Neurobiological mechanisms of cannabinoid addiction. Mol. Cell. Endocrinol.. 2008;286:S97-S107. (Addiction mechanisms)
Henquet C., Krabbendam L., Spauwen J., et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. Br. Med. J.. 2005;330:11-14. (Cannabis use moderately increased the risk of psychotic symptoms but had a much stronger effect in young people with evidence of predisposition for psychosis)
Karst M., Salim K., Burstein S., et al. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain. A randomized controlled trial. JAMA. 2003;290:1757-1762. (CT-3, a potent cannabinoid, produces marked antiallodynic and analgesic effects in animals. In a preliminary randomised cross-over study in 21 patients with chronic neuropathic pain, CT-3 was effective in reducing chronic neuropathic pain compared with placebo)
Leweke F., Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addict. Biol.. 2008;13:264-275. (Association of chronic use with subsequent psychiatric disorder)
Mach F., Steffens S. The role of the endocannabinoid system in atherosclerosis. J. Neuroendocrinol.. 2008;20:53-57. (Review)
Malinowska B., Lupinski S., Godlewski G., et al. Role of endocannabinoids in cardiovascular shock. J. Physiol. Pharmacol.. 2008;59:91-107.
Pacher P., Batkai S., Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev.. 2006;58:390-462.
Steffens S. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice Nature 434:2005:782-786 (Oral administration of THC (1 mg/kg per day) resulted in significant inhibition of disease progression in apoE knockout mice. CB2 receptors were expressed in both human and mouse atherosclerotic plaques. Lymphoid cells isolated from THC-treated mice showed diminished proliferation capacity and decreased interferon secretion. Macrophage chemotaxis, crucial for the development of atherosclerosis, was also inhibited in vitro by THC. All these effects were completely blocked by a specific CB2 receptor antagonist. Concludes that cannabinoids with activity at the CB2 receptor may be valuable targets for treating atherosclerosis. See also News and Views, p. 708 of the same issue, for comment by Roth, M.D.)
Taber K.H., Hurley R.A. Endocannabinoids: stress, anxiety and fear. J. Neuropsychiat. Clin. Neurosci.. 2009;21:108-113. (Succinctly reviews the involvement of the endocannabinoid system in brain function, and potential therapeutic applications in treating mood/anxiety, degenerative disease and brain injury)
UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet. 2003;362:1517-1526. (Randomised, placebo-controlled trial in 667 patients with stable multiple sclerosis and muscle spasticity. Trial duration was 15 weeks. There was no treatment effect of THC or cannabis extract on the primary outcome of spasticity assessed with a standard rating scale, but there was an improvement in patient-reported spasticity and pain, which might be clinically useful)
Van Gaal L.F., Rissanen A.M., Scheen A.J., et alfor the RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389-1397. (A total of 1507 overweight patients treated with rimonabant 5 or 20 mg or with placebo daily for 1 year in addition to dietary advice: significant dose-related decrease in weight and improvement in cardiovascular risk factors in actively treated patients; adverse effects were mild)
Wang H., Dey S.K., Maccarrone M. Jekyll and Hyde: two faces of cannabinoid signaling in male and female fertility. Endocr. Rev.. 2006;27:427-448. (Reviews lipid signalling in reproductive endocrinology, and potential for clinical interventions to treat infertility)