52 Antifungal drugs
Overview
Fungal infections (mycoses) are widespread in the population; they are generally associated with the skin (e.g. ‘athlete’s foot’) or mucous membranes (e.g. ‘thrush’).1 In temperate climates such as the UK, and in otherwise healthy people, they are mainly benign, being more of a nuisance than a threat. However, they become a more serious problem when the immune system is compromised or when they gain access to the systemic circulation. When this occurs, fungal infections can be fatal. In this chapter, we will briefly review the main types of fungal infections and discuss the drugs that can be used to treat them.
Fungi and Fungal Infections
Fungi are non-motile eukaryotic cells. Unlike plants, they cannot photosynthesise and many are parasitic in nature. Many thousands of species have been characterised. Many are of economic importance, either because they are edible (e.g. mushrooms), useful in manufacturing other products (e.g. yeast in brewing and in the production of antibiotics) or because of the damage they cause to other animals, crops or to foodstuffs. Approximately 50 are pathogenic in humans. These organisms are present in the environment or may co-exist with humans as commensals without causing any overt risks to health. However, since the 1970s there has been a steady increase in the incidence of serious secondary systemic fungal infections. One of the contributory factors has been the widespread use of broad-spectrum antibiotics, which eradicate the non-pathogenic bacterial populations that normally compete with fungi. Other causes include the spread of AIDS and the use of immunosuppressant or cancer chemotherapy agents. The result has been an increased prevalence of opportunistic infections, i.e. infections that rarely cause disease in healthy individuals. Older people, diabetics, pregnant women and burn wound victims are particularly at risk of fungal infections such as candidiasis. Primary fungal infections, rare in many parts of the temperate world, are also now encountered more often because of the increase in international travel.
Clinically important fungi may be classified into four main types on the basis of their morphological and other characteristics. Of particular taxonomic significance is the presence of hyphae—filamentous projections that may knit together to form a complex mycelium, a mat-like structure giving the characteristic appearance of moulds. Fungi are remarkably specific in their choice of preferred location. The main groups are:
Another organism, Pneumocystis carinii (also kown as P. jirovecii), shares characteristics of both protozoa (see Ch. 53) and fungi; however, it is not susceptible to antifungal drugs and will not be considered here even though it is an important opportunistic pathogen in patients with compromised immune systems (e.g. those suffering from AIDS).
Drugs vary in their efficacy between the different fungal groups. Table 52.1 gives examples of each type of organism and lists some of the diseases caused by these agents and the most common choice of drug classes.
Table 52.1 Some common fungal infections and their sensitivity to various classes of antifungal drugs
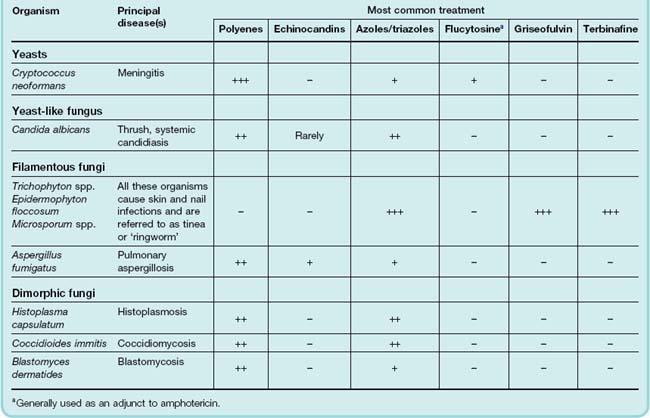
Superficial fungal infections can be classified into the dermatomycoses and candidiasis. Dermatomycoses include infections of the skin, hair and nails (onychomycosis). They are most commonly caused by Trichophyton, Microsporum or Epidermophyton, giving rise to various types of ‘ringworm’ (not to be confused with genuine helminth infections; see Ch. 54) or tinea. Tinea capitis affects the scalp; Tinea cruris, the groin (‘Dhobie itch’); Tinea pedis, the feet (athlete’s foot); and Tinea corporis, the body. In superficial candidiasis, the yeast-like organism may infect the mucous membranes of the mouth or vagina (thrush), or the skin. Secondary bacterial infections may complicate the course and treatment of these conditions.
In the UK, the commonest systemic (or ‘disseminated’) fungal disease is candidiasis. Other more serious conditions are cryptococcal meningitis, endocarditis, pulmonary aspergillosis, and rhinocerebral mucormycosis. Invasive pulmonary aspergillosis is now a leading cause of death in recipients of bone marrow transplants or those with neutropenia. Colonisation by Aspergillus of the lungs of patients with asthma or cystic fibrosis can lead to a similar condition termed allergic bronchopulmonary aspergillosis.
In other parts of the world, the commonest systemic fungal infections include blastomycosis, histoplasmosis, coccidiomycosis and paracoccidiomycosis; these are often primary infections, i.e. they are not secondary to reduced immunological function or altered commensal microorganisms.
Drugs Used To Treat Fungal Infections
The current therapeutic agents can be broadly classified into two groups: first, the naturally occurring antifungal antibiotics such as the polyenes and echinocandins, and second, synthetic drugs including azoles and fluorinated pyrimidines. Because many infections are superficial, there are many topical preparations. Many antifungal agents are quite toxic, and when systemic therapy is required these agents must often be used under strict medical supervision.
Figure 52.1 shows sites of action of common antifungal drugs.
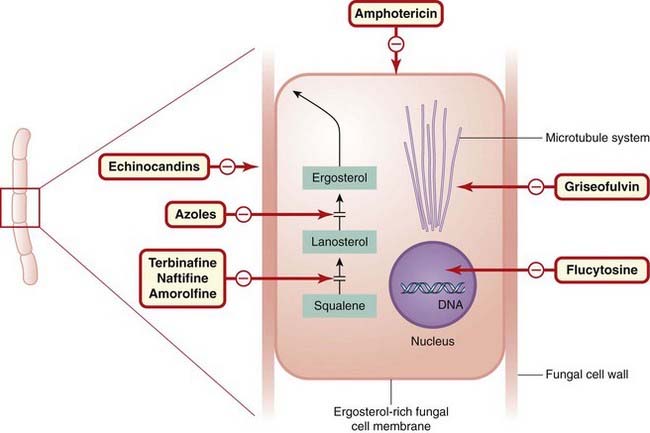
Fig. 52.1 Sites of action of common antifungal drugs.
Fungi are morphologically very diverse organisms, and this diagram of a ‘typical’ fungus is not intended to be technically accurate. The principal sites of action of the main antifungal agents mentioned in this chapter (in red-bordered boxes) are indicated as shown.
Antifungal Antibiotics
Amphotericin
Amphotericin (also called amphotericin B) is a mixture of antifungal substances derived from cultures of Streptomyces. Structurally, these are very large (‘macrolide’) molecules belonging to the polyene group of antifungal agents.
Like other polyene antibiotics (see Ch. 50), the site of amphotericin action is the fungal cell membranes, where it interferes with permeability and with transport functions. Its most important property is probably its ability to form large pores in the membrane. The hydrophilic core of the doughnut-shaped molecule creates a transmembrane ion channel, causing gross disturbances in ion balance including the loss of intracellular K+. Amphotericin has a selective action, binding avidly to the membranes of fungi and some protozoa, less avidly to mammalian cells and not at all to bacteria. The basis of this relative specificity is the drug’s greater avidity for ergosterol, a fungal membrane sterol that is not found in animal cells (where cholesterol is the principal sterol). Amphotericin is active against most fungi and yeasts, and is the gold standard for treating disseminated infections caused by several organisms including Aspergillus and Candida. Amphotericin also enhances the antifungal effect of flucytosine (see below), providing a useful synergistic combination.
Pharmacokinetic aspects
Amphotericin is very poorly absorbed when given orally, and this route is used only for treating fungal infections of the upper gastrointestinal tract. It can be used topically, but for systemic infections it is generally administered by slow intravenous injection complexed with liposomes or other lipid-containing preparations. This improves the pharmacokinetics and reduces the considerable burden of side effects. Long-circulating or so-called ‘stealth’ liposomes containing amphotericin have been used to good effect.
Amphotericin is very highly protein bound. It penetrates tissues and membranes (such as the blood–brain barrier) poorly, although it is found in fairly high concentrations in inflammatory exudates and may cross the blood–brain barrier more readily when the meninges are inflamed, and intravenous amphotericin is used with flucytosine to treat cryptococcal meningitis. It is excreted very slowly via the kidney, traces being found in the urine for 2 months or more after administration has ceased.
Unwanted effects
The commonest and most serious unwanted effect of amphotericin is renal toxicity. Some degree of reduction of renal function occurs in more than 80% of patients receiving the drug; although this generally recovers after treatment is stopped, some impairment of glomerular filtration may remain. Hypokalaemia occurs in 25% of patients, requiring potassium chloride supplementation. Hypomagnesaemia also occurs, and anaemia can be a further problem. Other unwanted effects include impaired hepatic function, thrombocytopenia and anaphylactic reactions. Injection frequently results initially in chills, fever, tinnitus and headache, and about one in five patients vomits. The drug is irritant to the endothelium of the veins, and local thrombophlebitis is sometimes seen after intravenous injection. Intrathecal injections can cause neurotoxicity, and topical applications cause a skin rash. The (considerably more expensive) liposome-encapsulated and lipid-complexed preparations have no greater efficacy than the native drug but cause fewer adverse reactions.
Nystatin (also called fungicidin) is a polyene macrolide antibiotic similar in structure to amphotericin and with the same mechanism of action. It is not absorbed through mucous membranes or skin, and its use is mainly limited to Candida infections of the skin, mucous membranes and the gastrointestinal tract. Unwanted effects may include nausea, vomiting and diarrhoea.
Griseofulvin
Griseofulvin is a narrow-spectrum antifungal agent isolated from cultures of Penicillium griseofulvum. It interferes with mitosis by binding to fungal microtubules. It can be used to treat dermatophyte infections of skin or nails when local treatment is ineffective, but treatment needs to be very prolonged. It has largely been superseded by other drugs.
Pharmacokinetic aspects
Griseofulvin is given orally. It is poorly soluble in water, and absorption varies with the type of preparation, in particular with particle size. It is taken up selectively by newly formed skin and concentrated in the keratin. The plasma half-life is 24 h, but it is retained in the skin for much longer. It potently induces cytochrome P450 enzymes and causes several clinically important drug interactions.
Echinocandins
Echinocandins comprise a ring of six amino acids linked to a lipophilic side-chain. All drugs in this group are based on the structure of echinocandin B, which is found naturally in A. nidulans. The echinocandins inhibit the synthesis of 1,3-β-glucan, a glucose polymer that is necessary for maintaining the structure of fungal cell walls. In the absence of this polymer, fungal cells lose integrity and lyse.
Caspofungin is active in vitro against a wide variety of fungi, and it has proved effective in the treatment of candidiasis and forms of invasive aspergillosis that are refractory to amphotericin. Oral absorption is poor, and it is given intravenously, once daily. Anidulafungin is used mainly for invasive candidiasis; again it is given intravenously. The principal side effects of both drugs include nausea, vomiting and diarrhoea, and skin rash.
Synthetic Antifungal Drugs
Azoles
The azoles are a group of synthetic fungistatic agents with a broad spectrum of activity based on the imidazole (clotrimazole, econazole, fenticonazole, ketoconazole, miconazole, tioconazole and sulconazole) or triazole nucleus (itraconazole, voriconazole and fluconazole).
The azoles inhibit the fungal cytochrome P450 3A enzyme, lanosine 14α-demethylase, which is responsible for converting lanosterol to ergosterol, the main sterol in the fungal cell membrane. The resulting depletion of ergosterol alters the fluidity of the membrane, and this interferes with the action of membrane-associated enzymes. The net effect is an inhibition of replication. Azoles also inhibit the transformation of candidal yeast cells into hyphae—the invasive and pathogenic form of the parasite. Depletion of membrane ergosterol reduces the binding of amphotericin.
Ketoconazole
Ketoconazole was the first azole that could be given orally to treat systemic fungal infections. It is effective against several different types of organism (see Table 52.1). It is, however, toxic (see below), and relapse is common after apparently successful treatment. It is well absorbed from the gastrointestinal tract. It is distributed widely throughout the tissues and tissue fluids but does not reach therapeutic concentrations in the central nervous system unless high doses are given. It is inactivated in the liver and excreted in bile and in urine. Its half-life in the plasma is 8 h.
Unwanted effects
The main hazard of ketoconazole is liver toxicity, which is rare but can prove fatal. Liver function is monitored before and during treatment. Other side effects that occur are gastrointestinal disturbances and pruritus. Inhibition of adrenocortical steroid and testosterone synthesis has been recorded with high doses, the latter resulting in gynaecomastia in some male patients. There may be adverse interactions with other drugs. Ciclosporin and astemizole all interfere with cytochrome P450 drug-metabolising enzymes, causing increased plasma concentrations of ketoconazole or the interacting drug or both. Rifampicin, histamine H2 receptor antagonists and antacids decrease the absorption of ketoconazole.
Fluconazole
Fluconazole is well absorbed and can be given orally or intravenously. It reaches high concentrations in the cerebrospinal fluid and ocular fluids, and is used to treat most types of fungal meningitis. Fungicidal concentrations are also achieved in vaginal tissue, saliva, skin and nails. It has a half-life of ~25 h, and is mainly excreted unchanged in the urine.
Unwanted effects
Unwanted effects, which are generally mild, include nausea, headache and abdominal pain. However, exfoliative skin lesions (including, on occasion, Stevens–Johnson syndrome2) have been seen in some individuals—primarily in AIDS patients who are being treated with multiple drugs. Hepatitis has been reported, although this is rare, and fluconazole, in the doses usually used, does not produce the inhibition of hepatic drug metabolism and of steroidogenesis that occurs with ketoconazole.
Itraconazole
Itraconazole is active against a range of dermatophytes. It may be given orally but, after absorption (which is variable), undergoes extensive hepatic metabolism. It is highly lipid soluble (and water insoluble), and a formulation in which the drug is retained within pockets of β-cyclodextrin is available. In this form, itraconazole can be administered intravenously, thereby overcoming the problem of variable absorption from the gastrointestinal tract. Administered orally, its half-life is about 36 h, and it is excreted in the urine. It does not penetrate the cerebrospinal fluid.
Unwanted effects
Though rare, the most serious are hepatoxicity and Stevens–Johnson syndrome (see above). Gastrointestinal disturbances, headache and allergic skin reactions can occur. Inhibition of steroidogenesis has not been reported. Drug interactions as a result of inhibition of cytochrome P450 enzymes occur (similar to those described above for ketoconazole).
Miconazole
Miconazole is given topically for oral and other infections of the gastrointestinal tract. It has a short plasma half-life and needs to be given every 8 h. It reaches therapeutic concentrations in bone, joints and lung tissue but not in the central nervous system, and it is inactivated in the liver.
Unwanted effects
Unwanted effects are relatively infrequent, those most commonly seen being gastrointestinal disturbances, but pruritus, blood dyscrasias and hyponatraemia are also reported. There are isolated reports of liver damage, and it should not be given to patients with impaired hepatic function.
Other azoles
Clotrimazole, econazole, tioconazole and sulconazole are used only for topical application. Clotrimazole interferes with amino acid transport into the fungus by an action on the cell membrane. It is active against a wide range of fungi, including candidal organisms. These drugs are sometimes combined with anti-inflammatory glucocorticoids (see Ch. 26). Poscanazole and voriconazole are used mainly for the treatment of invasive life-threatening infections such as aspergillosis.
Other Antifungal Drugs
Flucytosine is a synthetic, orally active antifungal agent that is effective against a limited range (mainly yeasts) of systemic fungal infections. If given alone, drug resistance commonly arises during treatment, so it is usually combined with amphotericin for severe systemic infections such as candidiasis and cryptococcal meningitis.
Flucytosine is converted to the antimetabolite 5-fluorouracil in fungal but not human cells. 5-Fluorouracil inhibits thymidylate synthetase and thus DNA synthesis (see Chs 5 and 55). Resistant mutants may emerge rapidly, so this drug should not be used alone.
Flucytosine is usually given by intravenous infusion but can also be given orally. It is widely distributed throughout the body fluids, including the cerebrospinal fluid. About 90% is excreted unchanged via the kidneys, and the plasma half-life is 3–5 h. The dosage should be reduced if renal function is impaired.
Unwanted effects are infrequent. Gastrointestinal disturbances, anaemia, neutropenia, thrombocytopenia and alopecia have occurred (possibly due to formation of fluorouracil by gut bacteria), but these are usually mild (but may be more significant in AIDS patients) and are easily reversed when therapy ceases. Uracil is reported to decrease the toxic effects on the bone marrow without impairing the antimycotic action. Hepatitis has been reported but is rare.
Terbinafine is a highly lipophilic, keratinophilic fungicidal compound active against a wide range of skin pathogens. It is particularly useful against nail infections. It acts by selectively inhibiting the enzyme squalene epoxidase, which is involved in the synthesis of ergosterol from squalene in the fungal cell wall. The accumulation of squalene within the cell is toxic to the organism.
When used to treat ringworm or fungal infections of the nails, it is given orally. The drug is rapidly absorbed and is taken up by skin, nails and adipose tissue. Given topically, it penetrates skin and mucous membranes. It is metabolised in the liver by the cytochrome P450 system, and the metabolites are excreted in the urine.
Unwanted effects occur in about 10% of individuals and are usually mild and self-limiting. They include gastrointestinal disturbances, rashes, pruritus, headache and dizziness. Joint and muscle pains have been reported and, more rarely, hepatitis.
Naftifine is similar in action to terbinafine. Among other developments, a morpholine derivative, amorolfine, which interferes with fungal sterol synthesis, is available as a nail lacquer, being effective against onchomycoses.
Future Developments
Increasing numbers of fungal strains are becoming resistant to the current antifungal drugs (fortunately, drug resistance is not transferable in fungi), and toxicity and low efficacy also contribute to the need for better antifungal drugs. An additional problem is that new strains of commensal-turned-pathogenic fungi have emerged. Fungal infections are also on the rise because of the prevalence of cancer chemotherapy and transplant-associated immunosuppression. Encouragingly, new compounds are in development, some with novel mechanisms of action and the prospect of using combination therapies has been explored in more depth (see Lupetti et al., 2003).
At the time of writing, a new echinocandin, micafungin, has just been introduced into the UK for treating invasive candidiasis. Unwanted effects are mild, and their incidence less than that seen with amphotericin. Several ‘new-generation’ triazoles are also in prospect (see Boucher et al., 2004).
Because fungal infections are often secondary to compromised host defence, attempts have been made to boost this by administration of the cytokine granulocyte macrophage colony stimulating factor (GMCSF, see Ch. 17) and other factors that increase host leukocyte numbers or function (see also Lupetti et al., 2003). Finally, the possibility of developing an antifungal vaccine, first mooted in the 1960s, has recently met with limited success in animals (see Torosantucci et al., 2005 for an account of a Candida vaccine). It is hoped that such advances will soon find their way into clinical practice.
References and Further Reading
Boucher H.W., Groll A.H., Chiou C.C., Walsh T.J. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs. 2004;64:1997-2020. (A useful review of the newer echinocandins and triazoles)
Como J.A., Dismukes W.E. Oral azole drugs as systemic antifungal therapy. N. Engl. J. Med.. 1994;330:263-272. (A bit dated now but still worth reading for the review of ketoconazole, fluconazole and itraconazole)
Dan J.M., Levitz S.M. Prospects for development of vaccines against fungal diseases. Drug Resist. Updat.. 2006;9:105-110. (A short review of some recent attempts to develop antifungal vaccines)
Deepe G.S.Jr. Preventative and therapeutic vaccines for fungal infections: from concept to implementation. Expert. Rev. Vaccines. 2004;3:701-709. (An interesting, and optimistic, overview of the quest for antifungal vaccines)
Denning D.W. Echinocandin antifungal drugs. Lancet. 2003;362:1142-1151. (General review on the echinocandins, focusing on their clinical use)
Dodds E.S., Drew R.H., Perfect J.R. Antifungal pharmacodynamics: review of the literature and clinical applications. Pharmacotherapy. 2000;20:1335-1355. (Good review of antifungals used to treat systemic infections; somewhat clinical in tone)
Gupta A.K., Tomas E. New antifungal agents. Dermatol. Clin.. 2003;21:565-576. (Quite a comprehensive review that deals mainly with the newer antifungals, their mechanisms of action and resistance)
Hodgson M.J., Morey P., Leung W.Y., et al. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. J. Occup. Environ. Med.. 1998;40:241-249. (Strictly for those of you who want to delve into this fascinating branch of fungal biology)
Hoeprich P.D. Antifungal chemotherapy. Prog. Drug Res.. 1995;44:88-127. (A bit dated now but contains very detailed coverage of the main classes of drug: chemical formulae, mode of action, pharmacokinetics, adverse effects)
Kauffman C.A. Fungal infections in older adults. Clin. Infect. Dis.. 2001;33:550-555. (Interesting account of fungal infections and their treatment)
Lupetti A., Nibbering P.H., Campa M., et al. Molecular targeted treatments for fungal infections: the role of drug combinations. Trends Mol. Med.. 2003;9:269-276. (Interesting and accessible article that deals with the use of combination antifungal therapy. Some good diagrams)
Torosantucci A., Bromuro C., Chiani P., et al A novel glyco-conjugate vaccine against fungal pathogens J. Exp. Med. 202:2005:597-606 (An experimental paper demonstrating the development of a novel type of vaccine effective against Candida infections in mice)
Van Spriel A.B. Novel immunotherapeutic strategies for invasive fungal disease. Curr. Drug Targets Cardiovasc. Haematol. Disord.. 2003;3:209-217. (A paper that discusses the role of the host immune response in fungal infection and examines novel strategies for antifungal therapy drawing on these data)
Vermes A., Guchelaar H.J., Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother.. 2000;46:171-179. (The title is self-explanatory!)
http://www.doctorfungus.org (This is an excellent site sponsored by a consortium of pharmaceutical companies. It covers all aspects of fungal infections and drug therapy, and has many compelling images and some video clips. Highly recommended—and fun!)