Chapter 14 Change and adaptation in pregnancy
The anatomical and physiological adaptations occurring throughout pregnancy affect virtually every body system. The timing and intensity of the changes vary between systems but all are designed to support fetal growth and development and prepare the mother for birth and motherhood. The midwife’s appreciation of the normal adaptations to pregnancy and recognition of abnormal findings are fundamental in the management of normal as well as high risk pregnancies, enabling her to provide appropriate midwifery care to all women including those affected by pre-existing illness. A common feature of these changes is the dynamic and symbiotic partnership between the uteroplacental unit and the mother, which is influenced by physical, mechanical, genetic and hormonal factors. Many aspects of the physiology of pregnancy remain poorly understood and controversies continue to be researched.
Changes in the woman’s emotional state due to hormonal factors are discussed in Chapter 36, section A and changes in the breast are detailed in Chapter 41.
Physiological changes in the reproductive system
The uterus
The uterus plays an essential role in pregnancy by protecting and supporting the fetus, placenta and amniotic fluid (LaMar & Hamernik 2003). For most of the 40 weeks of pregnancy, it expands to accommodate the growing fetus and remains relatively quiescent, yet at the time of labour it is able to contract regularly and forcibly to expel the fetus due to its unique properties of contractility and elasticity. The uterine wall consists of three layers: an external serous epithelial layer or perimetrium, the middle muscle layer or myometrium, and the internal layer of endometrium (decidua) (Blackburn 2007).
The perimetrium
The perimetrium is a thin layer of peritoneum that protects the uterus (see Ch. 8). It provides a relatively inelastic base upon which the myometrium develops tension to increase intrauterine pressure. During pregnancy, the peritoneal sac is greatly distorted as the uterus enlarges and rises out of the pelvis, carrying the adjoining anatomical parts with them (Whitmore et al 2002). The increasing tension exerted on the broad ligaments causes them to become longer and wider and the anterior and posterior folds open out so they are no longer in apposition and can therefore accommodate the greatly enlarged uterine and ovarian arteries and veins (Cunningham et al 1997). The round ligaments undergo considerable hypertrophy and increase in both length and diameter (Cunningham et al 2005, p 24). Frequently, spasm of the round ligaments occurs with movement in pregnancy causing sharp groin pains – usually on the right side due to the dextrorotation of the uterus to the right (Gabbe et al 2004).
Myometrium
The myometrium is the main component in the enlargement of the uterus during pregnancy (Chard & Grudzinskas 1994) and is the distinct muscular layer of the uterine wall which is involved in contraction during labour. The lymphatics, immune cells and myometrial cells all increase in size and number, supported by the accumulating fibrous and elastic tissue which provides an expanding framework as the uterus distends (Rehman et al 2003). Despite historic descriptions of the myometrium having three layers of muscle fibres, it has recently been identified that these layers were observed in other species, such as rodents and lower mammals and that, contrary to traditional understanding, the human myometrium is not composed of well-defined circular and longitudinal layers (Young 2007).
The outer layer of the myometrium lying under the serosal perimetrium is a very thin sheet of smooth muscle, approximately 200μm thick, densely packed with myocytes and devoid of connective tissue (Young & Hession 1999). This structure is so thin in comparison with the remainder of the wall of the uterus that it is unlikely that it contributes significantly to the contractility of the uterus (Young 2007). However, the increase in elastin in this layer with resulting increase in elasticity allows the uterus to grow and stretch to accommodate the growing fetus (Blackburn 2007). Beneath this outer elastic layer lies another thin ‘transitional’ muscle layer approximately 0.5–1mm thick, containing bridging bundles of myocytes spanning from the outer to the inner layer (Young 2007).
The bulk of the uterine wall lies beneath these two thin layers and consists of a thick layer of myocytes organized into cylindrical, sheet-like and ‘fibre’ bundles or fasciculata with communicating bridges which merge and intertwine with each other to form an interlacing network and a contiguous pathway allowing coordinated contraction. The myocytes within each bundle all contract and relax in a longitudinal direction only (as with a spring).These fasciculi are well ordered, running transversely across the fundus of the uterus, obliquely down the anterior and posterior walls of the uterus and transversely across the lower uterine segment (Young & Hession 1999) (Fig. 14.1). Each fasciculata is 1–2mm in diameter and is surrounded and supported by fibrous tissue, collagen and elastin (Martin & Hutchon 2004).
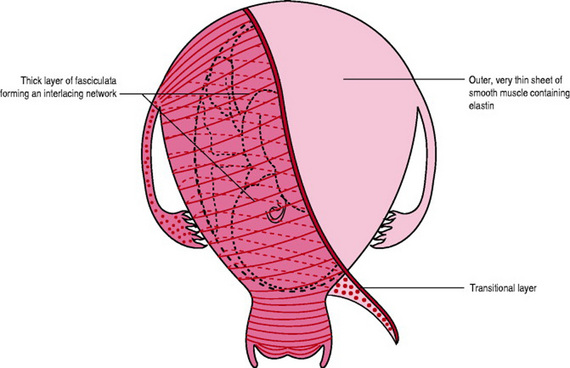
Figure 14.1 Myometrium showing the very thin outer layer, the transitional layer and the inner bulk of myometrium with the arrangement of the fasciculata running transversely across the fundus between the fallopian tubes, obliquely down anterior and posterior walls and transversely around the lower uterine segment.
In the first 12 weeks of pregnancy, uterine growth is due partly to hyperplasia (increased numbers of myocytes due to division) but mainly to hypertrophy (increased size of myocytes) under the influence of high levels of oestradiol and progesterone (Cunningham et al 2005). The myometrial cells stretch in length by up to 15-fold (Baker 2006). Throughout the remainder of the pregnancy, uterine growth is predominantly due to fetal growth causing mechanical tension on the myometrium (Breuiller-Fouche & Germain 2006).
Although the walls of the corpus become considerably thicker during the first few months of pregnancy, as gestation advances they gradually thin so that by term they are only about 1.5cm thick or even less and the uterus is changed into a muscular sac with thin, soft, readily indentable walls through which the fetus can easily be palpated (Cunningham et al 2005; Degani et al 1998). The walls become even thinner during active labour although significant thickening occurs at the implantation site after placental separation (Buhimschi et al 2003).
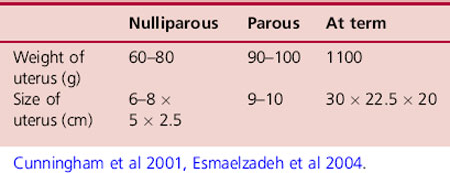
During pregnancy the uterus undergoes a 10-fold increase in weight (Symonds & Symonds 2004) however, the dimensions vary considerably depending on the age and parity of the woman (Cunningham et al 2001). A study by Esmaelzadeh et al (2004) suggested that differences in dimensions may also be due to factors such as race, heredity, environment and diet (Table 14.1).
Although the primary function of the uterus is not to contract for the majority of the pregnancy in order to accommodate the developing fetus (Young 2007), the myometrium is never completely quiescent during pregnancy. By 7 weeks’ gestation contractions are mild, irregular and non-synchronized, although not felt by the woman. As pregnancy progresses they are felt as ‘tightenings’, often appearing unpredictably and sporadically, particularly at night (Blackburn 2007). Late in pregnancy they become more rhythmic and may be as often as every 10–20min, causing some discomfort and accounting for the so-called ‘false labour’ (Cunningham et al 2005) but not causing cervical dilatation. First observed by Braxton Hicks in 1873, they are still known by his name today (Chard & Grudzinskas 1994).
Synchronous contractions of the uterus are dependent on the electrical coupling together of myometrial cells by gap junctions composed of connexin. Connexin 43 forms intercellular channels, which allow the transmission of electrical impulses, and perhaps metabolic communication, among the myometrial cells. Throughout most of pregnancy the number of these cell-to-cell channels is low, which results in poor electrical coupling. This favours myometrial quiescence and the maintenance of pregnancy. Several days prior to the onset of labour, the gap junctions markedly increase, leading to increased electrical conduction and coordination of myometrial cells and tissue necessary for effective contractions. The increase in gap junctions may be controlled by changing oestrogen and progesterone levels in the uterus (Garfield & Maner 2007).
The junctional zone is a separate and distinct functional unit within the uterus and lies between the myometrium and decidua. This area lacks a protective submucosal layer so that the endometrial glands lie in direct contact with the myometrium (Fusi et al 2006). Evidence has accumulated in recent years suggesting that this specialized zone plays a central role in the processes of sperm transport and implantation. Contractions arising in this area can facilitate or compromise the early survival of the embryo (Lesny & Killik 2004).
Decidua
The first signs of the re-modelling of endometrial stromal cells, matrix and blood vessels into the decidua (decidualization) (see Ch. 10) can be seen as early as day 23 of the normal menstrual cycle (Brosens & Gellersen 2006). Decidualization prepares the uterine lining for the invading trophoblasts (Kliman 2000). The decidua in the cervix and the isthmus are less well developed than in the corpus (Honda et al 2005), which prevents implantation in this region. Ultrasonography has identified that implantation usually occurs superiorly in the body of the uterus, and slightly more often on the posterior wall (Moore & Persaud 2003). Driven by rising levels of progesterone, decidualization spreads progressively throughout the endometrium during the first trimester of pregnancy (Jones et al 2006), but is most marked in the early weeks when the endometrial glands are best developed beneath the implantation site (Hempstock et al 2004). Over the first trimester, the decidua basalis gradually reduces from approximately 5mm thick at 6 weeks to 1mm thick at 14 weeks. The glands also gradually regress but still communicate with the intervillous space until at least 10 weeks (Hempstock et al 2004).
The glands within the decidua may provide an important source of nutrients, growth factors and cytokines for the fetoplacental unit (Hempstock et al 2004). Relaxin produced by the decidua plays a part in myometrial quiescence (Carvajal & Weiner 2003). The decidua also produces large amounts of prostaglandins, which either enhances uterine quiescence or initiates labour, depending on the specific receptor to which it is coupled (Blackburn 2007). The full significance of decidual cells is not understood but it has been postulated that they may protect maternal tissue against uncontrolled invasion by the syncytiotrophoblast (Moore & Persaud 2003).
Blood supply
Uterine blood flow in pregnancy supplies the myometrium, endometrium and placenta, with the latter receiving nearly 90% of the total uterine blood flow near term (Ross et al 2002) (see Chs 8 and 11). The diameters of the uterine arteries dilate to 1.5 times those seen in the non-pregnant state. The arcuate arteries that supply the placental bed become 10 times larger (Symonds & Symonds 2004). The highly coiled spiral arteries of the decidua and myometrium undergo marked physiological changes that disrupt their muscular and elastic elements and convert them from narrow spiral arteries into large calibre, uncoiled uteroplacental arteries which reach 30 times their pre-pregnancy diameter and permit expansion of the myometrial smooth muscle as it hypertrophies during pregnancy (Metaxa-Mariatou et al 2002). This re-modelling enhances their capacity to accommodate the increased blood volume needed within the intervillous spaces of the placenta leading to a large pool of blood within the uterus to maintain fetoplacental blood flow and oxygenation. The uterine veins also undergo significant adaptations to accommodate the massively increased uteroplacental blood flow (Blackburn 2007).
For the first 10–12 weeks blood supply into the intervillous spaces is limited due to the temporary occlusion of the tips of the spiral arteries by the invasive trophoblast (Blackburn 2007). Thereafter, the action of the invasive trophoblasts on the maternal spiral arteries leads to a very low resistance uteroplacental circulation which facilitates the marked increase in blood flow seen in these vessels at term (Kliman 2000). As a result of increase in maternal cardiac output and decline in uterine vascular resistance, as well as hormonal and chemical influences, the uterine blood flow progressively increases during gestation, from approximately 50mL/min at 10 weeks’ gestation, increasing to 200mL/min by 28 weeks (Blackburn 2007), reaching as high as 750mL/min at term, representing an almost 17-fold increase in uterine blood flow (Kliman 2000). Thus by term, the uterus is receiving between 10% and 20% of the maternal cardiac output (Blackburn 2007).
The passage of blood through the dilated uterine vessels produces a soft blowing sound synchronous with the maternal pulse known as the uterine souffle. It is heard most distinctly near the lower portion of the uterus. It should not be confused with the placental souffle, a muffled ‘ocean-like’ sound of blood coursing through the placenta which is synchronous with the fetal heart and found in the immediate vicinity of the placenta.
Changes in uterine size
Comparisons between the uterus and fruit has become a fairly reliable mental benchmark for uterine sizing in early pregnancy. At 5 weeks’ gestation, the uterus feels like a small, unripe pear. By 8 weeks it feels like a large orange and by 12 weeks it is about the size of a grapefruit (Margulies & Miller 2001). The traditional method of assessing gestational age is to relate the progressive increase in the height of the fundus at different gestations to abdominal landmarks throughout pregnancy (see Ch 17).
Changes in uterine shape
12th week of pregnancy
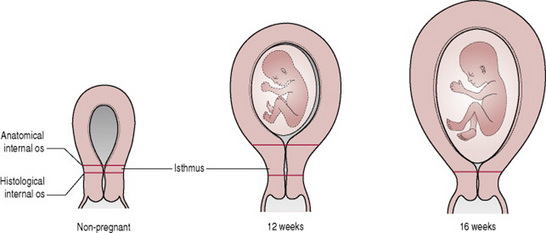
For the first few weeks of pregnancy, the increase in uterine size is limited principally to the anteroposterior diameter and the uterus maintains its original pear shape with the fundus being a flattened convexity between tubal insertions. As pregnancy advances, however, the corpus and fundus assume a more globular form becoming almost spherical by 12 weeks and too large to remain totally within the pelvis (Cunningham et al 2005). Physical movement of the uterus is normal in pregnancy, allowing the uterus to move relatively freely in all planes and thus to rise up out of its state of anteflexion (O’Grady & Pope 2006) (Fig. 14.2).
16th week of pregnancy
Between 12 and 16 weeks’ gestation, the fundus becomes dome-shaped (Blackburn 2007). With the ascent of the uterus from the pelvis it usually undergoes dextrorotation to the right, possibly because of the rectosigmoid on the left side of the pelvis. The uterus now increases more rapidly in length than in width. From about 16 weeks, the internal os gradually relaxes and the lower uterine segment develops from the greatly expanded and thinned out muscular isthmus (Cunningham et al 2005).
20th week of pregnancy
As the uterus rises in the abdomen, it assumes an ovoid shape, the round ligaments appear to insert at the junction of the middle and upper thirds of the organ and the uterine tubes elongate (Cunningham et al 2005). By 20 weeks, the isthmus has fully developed into the lower uterine segment and the cervical canal expands from above downwards in a wedge-shaped fashion
30th week of pregnancy
As the uterus continues to enlarge it contacts the anterior abdominal wall, displacing intestines laterally and superiorly and continues to rise, ultimately reaching almost to the liver. The abdominal wall supports the uterus and unless it is quite relaxed, maintains this relation between the long axis of the uterus and the axis of the pelvic inlet. In the supine position the uterus falls back to rest on the vertebral column and the adjacent great vessels, in particular the inferior vena cava and aorta (Cunningham et al 2005).
36th week of pregnancy
By the end of the 36th week of pregnancy, the enlarged uterus almost fills the abdominal cavity. The fundus is at the tip of the xiphoid cartilage, which is pushed forward. The liver, transverse colon, stomach and spleen are crowded into the vault of the abdominal cavity. The small intestines are crowded above, behind, and to the sides of the uterus. The diaphragm is pressed upward, reducing the vertical diameter of the chest cavity by as much as 4cm (Childbirth Connection 2007). By this stage, about half of the cervical canal is incorporated into the lower uterine segment.
38th week of pregnancy
By 38 weeks’ gestation, the insertion of the uterine tubes and broad and round ligaments is located slightly above the middle of the uterus, exerting tension on the ligaments. The lower uterine segment is almost fully developed and the physiological retraction ring develops at the junction between lower and upper segments. The consistency of the lower uterine segment is much less firm. It is distended and much more passive while the upper uterine segment is quite hard, firm and contractile. Because the fasciculata run transversely across this area (Young 2007) and also because of its relative avascularity and quiescence in the puerperium this is the site of choice for the incision for a caesarean delivery.
Descent of the fetal head into the pelvic brim (engagement) leads to slight lowering of the fundus, known as lightening which causes a change in shape of the abdomen. Women describe this as ‘the baby dropped’. When this occurs breathing becomes easier and heartburn occurs less frequently but the increased pressure on the bladder may lead to urinary frequency. As pressure increases in the pelvis constipation may occur and as the pelvic ligaments are stretched more, low backpain may be experienced. All the studies reviewed by Dietze (2001) suggested that engagement in nulliparous women is less common antenatally than is described in textbooks, ranging from 50% to 83%. Ambwani (2004) also commented on the extremely variable incidence, noting that in their study 21% of primigravidae had a floating head at term and calling for an attitude of watchful expectancy to avoid unnecessary intervention.
The cervix
Within 1 month of conception, the cervix becomes softer and cyanosed due to oedema and increased vascularity (Cunningham et al 2005). The collagen fibres become less dense with thinner and more loosely packed fibres. The arrangement of elastin and tightly wound, circumferential, collagen fibres, which are bonded together by a firm ground substance helps to form a rigid, tubular structure, which holds the canal closed and provides strength to retain the fetus in pregnancy (Gee 2004). The elastin:collagen ratio is greatest at the internal os where muscle content is lowest (Blackburn 2007). The glands of the cervix undergo such marked hypertrophy and hyperplasia that by the end of pregnancy they occupy half of the entire cervical mass as opposed to the small fraction in the nonpregnant state. They become everted so that the tissue tends to become red and velvety and bleeds even with minor trauma such as taking Pap smears. The basal cells near the squamocolumnar junction may be more prominent in shape and size due to oestrogen which renders the Pap smear less efficient (Cunningham et al 2005).
The endocervical mucosal cells produce copious amounts of a tenacious mucus resulting in the development of an antibacterial plug in the cervix. The consistency of the mucus changes during pregnancy under the influence of progesterone so that the typical ferning seen in very early pregnancy changes to a beaded pattern (Cunningham et al 2005).
In the last 6 weeks of pregnancy, the cervix undergoes many changes (‘ripening’) in preparation for expelling the fetus (Carbonne et al 2000). Cervical ripening involves inflammatory cells, but is likely dependent upon endogenously produced prostaglandins. Rearrangement and degradation of collagen fibres creates an increase in the space between them, shortens them and increases acidic solubility (Garfield et al 1998) along with reduced capacity to retain water (Chard & Grudzinskas 1994). The ground substance becomes fluid changing the cervix to a soft, distensible structure with reduced resistance to effacement and dilatation (Gee 2004). Cervical thinning, softening and effacement can be readily detected on vaginal examination.
The cervical canal shortens from above downwards from about 2cm long to a mere circular orifice with almost paper-thin edges. The muscular fibres at the level of the internal cervical os are pulled upward or ‘taken up’ into the lower uterine segment while the external os remains unchanged. Effacement can be compared with a funneling process in which the whole length of a narrow cylinder is converted into a very obtuse, flaring funnel with a small circular orifice for an outlet. This process causes the expulsion of the mucus plug as a bloody show at the onset of labour. There are controversies around when cervical shortening occurs (Dijkstra et al 1999). Carvalho et al (2003) demonstrated a spontaneous shortening in the pregnant cervix from the first to the second trimester of pregnancy. Haram et al (2003) observed that the shorter the cervix, the greater the risk of pre-term labour.
Classically cervical effacement and dilatation has been thought to be due to fundal contractions causing a radial pulling of the cervix over the uterine contents. However, Young (2007) suggests effacement and dilatation is primarily the result of increased intrauterine pressure caused by contractions of the uterine wall. In this formulation the weakest point of the sphere – the thinner lower uterine segment and ripened cervix – bulges, thins, and dilates with each episode of increased pressure. In nulliparous women effacement usually takes place prior to the commencement of labour but in parous women effacement may take place simultaneously with cervical dilatation (Gee 2006). By term, assuming a well-fitting presenting part, only about the lower third of the cervical canal plus the external os remain to be dilated in the first stage of labour. Hormonal control of cervical ripening is a complex process that involves a cascade of changes in oestradiol, progesterone and relaxin (Blackburn 2007). Nitric oxide production increases in the cervix at the end of pregnancy (Garfield et al 1998) but its role in cervical ripening remains unclear (Blackburn 2007).
The vagina
During pregnancy, increased vascularity and hyperaemia develop in the skin and muscles of the perineum and vulva with softening of the underlying connective tissue. Increased vascularity affects the vagina and results in the violet colour characteristic of Chadwick’s sign. In preparation for the distension that occurs in labour the vaginal walls undergo striking changes: the mucosa thickens, the connective tissue loosens and the smooth muscle cells hypertrophy. The increased volume of vaginal secretions due to high levels of oestrogen results in a thick, white discharge known as leucorrhoea (Cunningham et al 2005).
In pregnancy, larger amounts of glycogen are deposited in the vaginal epithelium due to high oestrogen availability (Boskey et al 2001). Glycogen is metabolized to lactic acid by the Lactobacillus acidophilus, (‘Döderlein’s bacillus’), a normal commensal of the vagina. This leads to increased vaginal acidity (pH varying from 3.5–6).
Changes in the cardiovascular system
Pregnancy is associated with profound but predominantly reversible changes in maternal haemodynamics and cardiac function. These complex adaptations are necessary to:
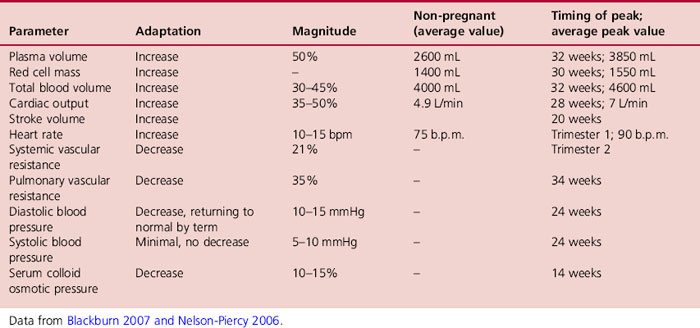
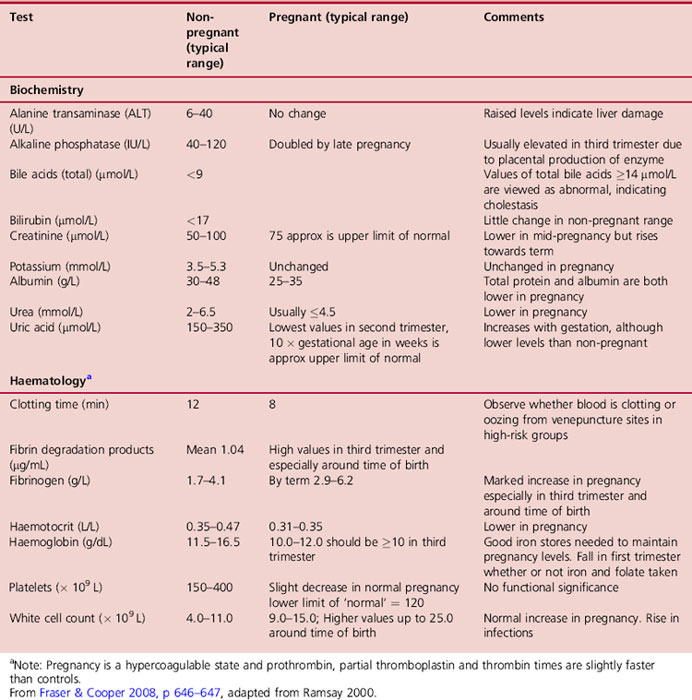
All components of the system undergo a degree of adaptation in pregnancy (Table 14.2). The key physiological changes that occur are; an increase in blood volume, cardiac output, stroke volume and heart rate together with a decrease in systemic vascular resistance, blood pressure, pulmonary vascular resistance and colloid osmotic pressure (Table 14.3). They are accompanied by widespread peripheral vasodilation resulting in the high flow, low resistance haemodynamic state with marked haemodilution, characteristic of a healthy pregnancy.
Table 14.2 A summary of the key components and functions of the cardiovascular system including changes in pregnancy
| Component | Key change in pregnancy |
|---|---|
| The heart | Increases in size |
| Shifted upwards and to left | |
| Arteries | Dramatic systemic and pulmonary vasodilation to increase blood flow |
| Capillaries | Increased permeability |
| Veins | Vasodilation and impeded venous return in lower extremities |
| Blood | Haemodilution |
| Increased capacity for clot formation |
Adapted from Torgersen & Curran 2006.
It is critical to achieve a balance between fetal requirements and maternal tolerance. In most women, these demands are accommodated by physiological adaptations without compromising the mother.
Blood volume
The increase in total blood volume (TBV) is essential to:
The first step in the circulatory changes in pregnancy is extreme vasodilation mediated by rising pregnancy hormone levels (particularly progesterone) and circulating nitric oxide (Van Mook & Peeters 2005).
Vasodilation causes an ‘underfilling’ of the maternal circulation which subsequently initiates fluid and electrolyte retention, expansion of the plasma and extra cellular fluid volumes and a concurrent increase in cardiac output. This occurs prior to full placentation and is accompanied by a parallel increase in renal blood flow and glomerular filtration rate. These changes may be mediated by a systemic and renal vasodilator unique to pregnancy (Varga et al 2000).
The mechanisms for maintaining homeostasis are modified to accommodate and maintain these changes (Weissgerber & Wolfe 2006). Renin and aldosterone activity are increased by oestrogens, progesterone and prostaglandins, leading to increased fluid and electrolyte retention. Oestrogen reduces the transcapillary escape rate of albumin, which promotes intravascular protein retention and shifts extra cellular fluid volume distribution while lowering the osmotic threshold for ADH (anti-diuretic hormone) release. Despite the progressive increase in blood volume as pregnancy progresses the secretion of ANP (atrial natriuretic peptide) is not increased because the ANP-volume relationship is reset during pregnancy (Weissgerber & Wolfe 2006). While ANP levels are slightly reduced, plasma renin activity tends to be increased. This supports the theory that the increase in plasma volume represents ‘underfilling’ due to systemic vasodilatation and the consequent rise in vascular capacity, rather than true blood volume expansion (Varga et al 2000).
Cardiac output
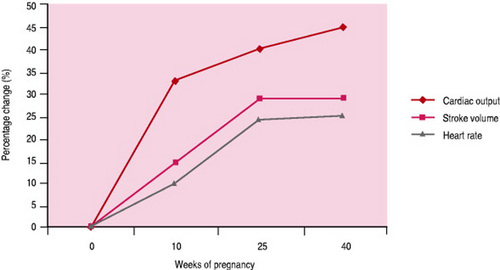
Cardiac output increase allows blood flow to the brain and coronary arteries to remain unchanged, while distribution to other organs is modified as pregnancy advances. The increased cardiac output is due to increases in both stroke volume and heart rate; the relative contributions of these factors to cardiac output vary with gestational age. Most of the increase in heart rate occurs during the first trimester thus contributing to early changes in cardiac output. Increases in stroke volume facilitate second trimester increases in cardiac output augmented by plasma volume expansion. The stroke volume increases by 10% during the first half of pregnancy, and reaches a peak at 20 weeks’ gestation that is maintained until term (Girling 2001) (Fig. 14.3).
Cardiac output in pregnancy is extremely sensitive to changes in body position. This sensitivity increases with lengthening gestation, because the uterus impinges upon the inferior vena cava, thereby decreasing blood return to the heart. Large variations in cardiac output, pulse rate, blood pressure and regional blood flow may follow trivial changes of posture, activity or anxiety.
Blood pressure and vascular resistance
While cardiac output is raised, arterial blood pressure is reduced by 10%, therefore resistance to flow must be decreased (de Sweit 1998a). This can be accounted for by the decrease in systemic vascular resistance, particularly in the peripheral vessels. The decrease begins at 5 weeks’ gestation, reaches a nadir in the second trimester (a 21% reduction) and then gradually rises as term approaches. Numerous modifications occur in the mechanisms controlling vascular activity; agents responsible for peripheral vasodilation include; prostacyclin, nitric oxide and progesterone and vasoactive prostaglandins. The changes are not limited to the uteroplacental circulation but are apparent throughout the body in a healthy pregnancy. Increased heat production in pregnancy further contributes to the reduced resistance by stimulating vasodilation particularly in heat loss areas such as hands and feet (Blackburn 2007).
Early pregnancy is associated with a marked decrease in diastolic blood pressure but minimal reduction in systolic pressure. With reduced peripheral vascular resistance the systolic blood pressure falls an average of 5–10mmHg below baseline levels and the diastolic pressure falls 10–15mmHg by 24 weeks’ gestation. Thereafter, blood pressure gradually rises, returning to the pre-pregnant levels at term. Despite the increased blood volume, systemic venous pressures do not rise significantly in pregnancy; the exception to this is in the lower limbs.
Postural changes that affect cardiac output also have a major effect on blood pressure. The enlarging uterus compresses both the inferior vena cava and the lower aorta when the woman lies supine. This reduces venous return to the heart with a consequential fall in pre-load and cardiac output of 30–40%. Most women are capable of compensating for the resultant decrease in stroke volume by increasing systemic vascular resistance and heart rate. Blood from the lower limbs may also return collateral conduits, however if these are not well developed or adequately perfused, the pregnant woman may suffer from supine hypotensive syndrome. This consists of hypotension, bradycardia, dizziness, light-headedness, nausea and even syncope, if she remains in the supine position too long and occurs in approximately 10% of pregnant women. The fall in blood pressure may be severe enough for the mother to lose consciousness due to reduced cerebral blood flow. By rolling the woman on to her left side, the cardiac output can be instantly restored (Burnett 2001). Compression of the aorta may lead to reduced uteroplacental and renal blood flow and fetal compromise.
The heart
In pregnancy, the heart is enlarged by both chamber dilation and myocardial hypertrophy. Myocardial hypertrophy in early pregnancy leads to a 10–15% increase in ventricular wall muscle. Blood volume expansion in the second and third trimesters results in increased diastolic filling (particularly in the left ventricle), and progressive distension of the heart chambers. Despite cardiac enlargement, efficiency is maintained by lengthening of muscle fibres and reduction in after load, facilitated by marked peripheral vasodilation.
The growing uterus elevates the diaphragm, the great vessels are unfolded and the heart is correspondingly displaced upward and to the left to produce a slight anterior rotation of the heart on its long axis. This can give an exaggerated impression of cardiac enlargement (de Sweit 1998a), and accounts for variations in parameters used for cardiac assessment including ECG and radiographic assessments. Atrial or ventricular extrasystoles are frequent and there is increased susceptibility to supraventricular tachycardia (de Sweit 1998a). While these symptoms are relatively common in normal pregnancies it is imperative that signs of severe disease are not overlooked.
Regional blood flow
As blood volume increases with gestation, a substantial proportion (10–20%), is distributed to the uteroplacental unit.
Renal vasodilation early in pregnancy results in increased renal blood flow and glomerular filtration rate accommodating the increased cardiac output before blood flow significantly increases to the uteroplacental unit (Varga et al 2000). Renal blood flow increases by as much as 70–80% by the 16th week of pregnancy which helps to enhance excretion.
Blood flow to the brain, coronary arteries and liver is not significantly changed in pregnancy. Pulmonary blood flow increases secondary to the increase in cardiac output and is facilitated by reduced pulmonary vascular resistance. Blood flow in the lower limbs is slowed in late pregnancy by compression of the iliac veins and inferior vena cava by the enlarging uterus and the hydrodynamic effects of increased venous return from the uterus (Broughton-Pipkin 2001). Reduced venous return and increased venous pressure in the legs contributes to the increased distensibility and pressure in the veins of the legs, vulva, rectum and pelvis, leading to dependent oedema, varicose veins of legs and vulva and haemorrhoids. These changes are more pronounced in the left leg due to compression of the left iliac vein by the overlying right iliac artery and the ovarian artery. This anatomical variation accounts for the fact that 85% of venous thrombosis in pregnancy occur in the left leg (Nelson-Piercy 2006) (Box 14.1).
Varicosities develop in approximately 40% of women, and are usually seen in the veins of the legs, but may also occur in the vulva and as haemorrhoids in the anal area. The effects of progesterone and relaxin on the smooth muscles of the vein walls, and the increased weight of the growing uterus all contribute to the increased risk of valvular incompetence. A family tendency is also a factor (Blackburn 2007). Some suggestions for alleviating them include: spraying the legs with hot and cold water, resting with the legs elevated and wearing supportive stockings.
Blood flow is increased to the capillaries of the mucous membranes and skin, particularly in hands and feet. This helps to eliminate the excess heat produced by the increased metabolism of the maternal-fetal mass and cardiorespiratory work of pregnancy. The associated peripheral vasodilation explains why pregnant women ‘feel the heat’, sweat profusely at times, have clammy hands and often suffer from nasal congestion (Broughton-Pipkin 2001).
Haematological changes
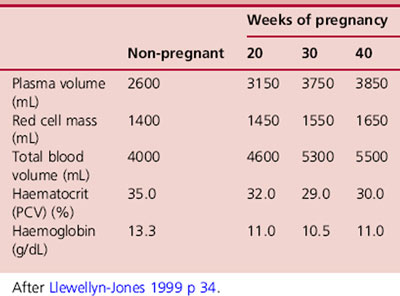
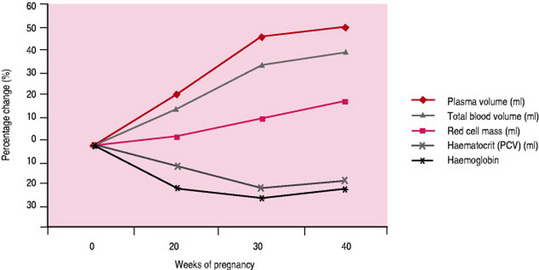
In parallel with the 30–45% increase in maternal blood volume, plasma volume increases by 50% (1250–1600mL) over the course of the pregnancy (Burnett 2001), followed by a relatively smaller increase in red blood cell volume (Table 14.4). These changes are responsible for the hypervolaemia of pregnancy leading to numerous modifications to parameters commonly assessed in blood tests (Table 14.5). The changes begin at 6–8 weeks of pregnancy. Plasma volume, placental mass and birth weight positively correlate in a healthy pregnancy (Duffy 2004). Excessive increases in plasma volume have been associated with multiple pregnancy, prolonged pregnancy, maternal obesity, and infants large for gestational age while inadequate increases have been associated with pre-eclampsia.
Red cell mass, which represents the total volume of red cells in circulation, increases during pregnancy by approx 18% in response to increased levels of erythropoietin. This is stimulated by maternal hormones (prolactin, progesterone, human placental lactogen and oestrogen) and the extra oxygen requirements of maternal and placental tissue (Cunningham et al 1997). This homeostatic mechanism is discrete from that which controls fluid balance and increased plasma volumes. Therefore in spite of the increased production of red blood cells, the marked increase in plasma volume causes dilution of many circulating factors. As a result the red cell count, haematocrit and haemoglobin concentration all decrease (Letsky 1998), resulting in ‘apparent anaemia’, characteristic of a healthy pregnancy (Fig. 14.4). The disproportionate increase in plasma volume is clearly advantageous. By reducing blood viscosity, resistance to blood flow is reduced leading to improved placental perfusion and reduced maternal cardiac effort (Cunningham et al 1997).
Red blood cells become more spherical with increased diameter due to the drop in plasma colloid pressure encouraging more water to cross the erythrocyte cell membrane. Mean cell volume also increases due to the higher proportion of young larger red blood cells (reticulocytes). There is still disagreement as to the exact increase in red cell mass and measurements have been influenced by iron medication.
While total haemoglobin increases from 85–150g, the mean haemoglobin concentration falls. In healthy women with adequate iron stores this drops by about 2g/dL from an average of 13.3g/dL in the non-pregnant state to 11.0g/dL in early pregnancy. It is at its lowest at around 32 weeks’ gestation when plasma volume expansion is maximal, and after this time rises by approximately 0.5g/dL, returning to 11g/dL around the 36th week of pregnancy. A haemoglobin level below 10.5g/100mL at 28 weeks should be investigated (NICE 2008aCh. 21).
Iron metabolism
A fall in haemoglobin is a physiological adjustment to pregnancy, whereas a high haemoglobin level can be a sign of pathology (McFadyen 1995). The total iron requirements of pregnancy average about 1000mg. About 500mg are required to increase the red blood cell mass, and about 300mg are transported to the fetus, mainly in the last 12 weeks of pregnancy. The remaining 200mg are needed to compensate for insensible loss in skin, stool and urine. Practically all of the increased iron requirements occur in the last half of the pregnancy, averaging 6–7mg/day. Since this amount is not available from body stores in most women, the red cell volume and haemoglobin level fall with the rising plasma volume. In spite of this, even if the mother has severe iron deficiency anaemia, the placenta is still able to provide the needed iron from maternal serum for the fetal production of haemoglobin. However, if the woman enters pregnancy with depleted iron stores, in spite of the moderate increase in iron absorption from the gut the amount of iron absorbed from the diet plus that mobilized from stores may be insufficient to meet the demands imposed by pregnancy. The purpose of iron supplementation, therefore, is to maintain iron stores in order to prevent the development of true anaemia, rather than to raise the haemoglobin level (see Ch. 21) (Letsky 1998). Iron supplementation, however, should not be offered routinely to all pregnant women as it is of no benefit to mother or baby and has unpleasant maternal side-effects (NICE 2008a).
Plasma protein
Haemodilution leads to a fall in total serum protein content within the first trimester which remains reduced throughout pregnancy. Despite oestrogen reducing the transcapillary escape rate of albumin, albumin concentration falls abruptly in early pregnancy and then more slowly until late pregnancy (Table 14.5). Albumin plays an important role, not only as a carrier protein for some hormones, drugs, free fatty acids and unconjugated bilirubin, but also because of its influence in decreasing colloid osmotic pressure. A 10–15% fall in colloid osmotic pressure (Nelson-Piercy 2006) allows water to move from the plasma into the cells or out of vessels, and plays a part in the increased fragility of red blood cells and oedema of the lower limbs. It is now accepted that peripheral oedema in the lower limbs in late pregnancy is a feature of normal, uncomplicated pregnancy (Girling 2001).
Clotting factors
Changes in the coagulation system lead to the characteristic hypercoagulable state of normal pregnancy. The increased tendency to clot is caused by increases in clotting factors and fibrinogen accompanied by reduced plasma fibrinolytic activity and an increase in circulating fibrin degradation products in the plasma.
From 12 weeks’ gestation there is a 50% increase in synthesis of plasma fibrinogen concentration (factor I). This may be necessary for the body to deal with the frequent disruptions in the integrity of the vascular tree in the placental bed (Coustan 1995). It is also critical in the prevention of haemorrhage at the time of placental separation. The development of a fibrin mesh to cover the placental site to control the bleeding requires 5–10% of all the circulating fibrinogen. When this process is impaired, as for example in inadequate uterine action or incomplete placental separation, there is rapid depletion of fibrinogen reserves, which can lead to exsanguination and death (Campbell & Lees 2000).
Coagulation factors VII, VIII and X increase in pregnancy (Burnett 2001), while factors II (prothrombin) and V remain constant or show a slight fall. Both the prothrombin time (normal 10–14s) and the partial thromboplastin time (normal 35–45s) are shortened slightly as pregnancy advances. The clotting times of whole blood, however, are not significantly different in normal pregnancy. The platelet count declines slightly as pregnancy advances, which is explained partially by haemodilution. However, there is a substantial increase in platelet volume, which may be due to the hyper-destruction of platelets in pregnancy, and as young platelets are larger than old ones the balance is pushed towards an overall increase in size (Steinfeld & Wax 2001).
A decrease in some endogenous anticoagulants (antithrombin, protein S and activated protein C resistance) occur in pregnancy and are intended to reduce the risk of haemorrhage at the time of birth; however, along with the physiological vasodilation of pregnancy, this contributes to a six-fold increase in the risk of thromboembolism in pregnancy (Girling 2001).
White blood cells (leucocytes) and immune function
Pregnancy presents a paradox for the mother’s immune system as the mechanisms which are essential to protect her from infection have the potential to destroy the genetically disparate conceptus.
The total white cell count rises from 8 weeks’ gestation and reaches a peak at 30 weeks. This is mainly because of the increase in numbers of neutrophil polymorphonuclear leucocytes, monocytes and granulocytes, the latter two producing a far more active and efficient phagocytosis function, which enhances the blood’s phagocytic and bactericidal properties. Numbers of eosinophils, basophils, monocytes, lymphocytes and circulating T cells and B cells remain relatively constant. Lymphocyte function is depressed, and natural killer cytokine activity is down regulated by progesterone particularly in latter stages of pregnancy. Chemotaxis is suppressed resulting in a delayed response to some infections. There is decreased resistance to viral infections such as herpes, influenza, rubella, hepatitis, poliomyelitis and malaria. The metabolic activity of granulocytes increases during pregnancy, possibly resulting from the stimulation of oestrogen (Steinfeld & Wax 2001).
It is clear that the immunological relationship between the mother and the fetus involves a two-way communication involving fetal antigen presentation and maternal recognition of and reaction to these antigens by the immune system. There is evidence that immunological recognition of pregnancy is important for the maintenance of gestation and inadequate recognition of fetal antigens might result in failed pregnancy (Szekeres-Bartho 2001).
Maternal immune response is biased toward an enhancement of innate (humoral) immunity and away from cell-mediated response that could be harmful to the fetus. The stimulus for these changes is predominantly hormonal involving progesterone, HPL, prostaglandins, corticosteroids, human chorionic gonadotrophin (hCG), prolactin and serum proteins.
Despite the placental barrier, small trophoblastic fragments have been shown to enter the maternal circulation stimulating the maternal inflammatory response. This is modified in specific areas such as uteroplacental interface by pregnancy zone protein. This has been shown to increase in pregnancy by 100–200% (Blackburn 2007).
Changes in the respiratory system
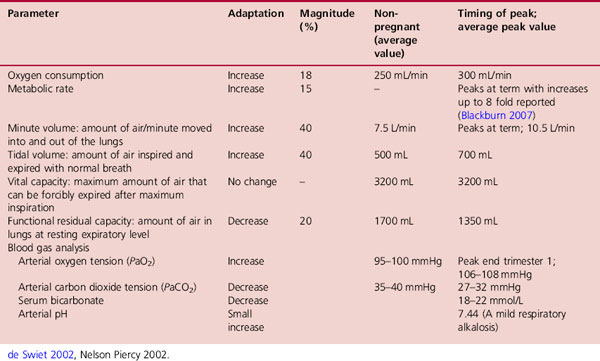
Pregnancy is associated with marked changes in respiratory physiology mediated by biochemical and mechanical factors. These accommodate the progressive increase in oxygen consumption and the physical impact of the enlarging uterus. Normal oxygen consumption is 250mL/min at rest and increases by 20% in pregnancy in order to meet the 15% increase in the maternal metabolic rate (Nelson-Piercy 2006). Changes in pregnancy result in an overcompensation to this respiratory demand. The resulting hyperventilation causes the arterial oxygen tension to increase and arterial carbon dioxide tension to fall, accompanied by a compensatory fall in serum bicarbonate. A mild respiratory alkalosis is therefore normal in pregnancy (Table 14.6).
The driving force for change is the stimulatory effect of progesterone on the respiratory centre, which lowers the threshold and increases sensitivity to carbon dioxide (Jensen et al 2005). Up to 75% of pregnant women with no underlying pre-existing respiratory disease experience some dyspnoea possibly due to an increased awareness of the physiological hyperventilation (Nelson-Piercy 2006). Hyperventilation can be extremely uncomfortable and may lead to dyspnoea and dizziness. Although it is not usually associated with pathological processes, care must be taken not to dismiss it lightly and miss a warning sign of cardiac or pulmonary disease (Steinfeld & Wax 2001) (Box 14.2).
Breathlessness during pregnancy occurs in approximately 60% of women with exertion and under 20% at rest. This physiological dyspnoea often occurs early in pregnancy and does not interfere with daily activities and usually diminishes as term approaches. Although mechanical impediment by the uterus is often blamed, hyperventilation is due to altered sensitivity to CO2. Distinguishing this physiological dyspnoea from breathlessness caused by disorders complicating pregnancy or diseases that might coexist with pregnancy is essential. It can be alleviated by maintaining an upright posture and holding hands above the head while taking deep breaths. Avoiding excessive exertion is advisable.
From early pregnancy onwards, the overall shape of the chest alters; the lower ribs flare outwards prior to any mechanical pressure from the growing uterus. This progressively increases the subcostal angle, from 68 ° in early pregnancy to 103 ° at term (Fig 14.5). The shape of the chest changes as the anteroposterior and transverse diameters increase, by about 2cm, resulting in a 5–7cm expansion of the chest circumference. The flaring of the lower ribs, causes the diaphragm to rise by up to 4cm, its contribution to the respiratory effort increasing with no evidence of being impeded by the uterus. These changes are thought to be mediated by the effect of progesterone, which together with relaxin, increases ribcage elasticity by relaxing ligaments. Progesterone also mediates bronchial and tracheal smooth muscle relaxation thereby reducing airway resistance. This improves air flow along the bronchial tree, and explains why women with respiratory problems in pregnancy rarely deteriorate as much as women suffering from other chronic disorders (Campbell & Lees 2000).
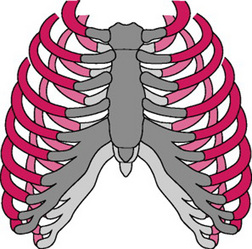
Figure 14.5 Displacement of the ribcage in pregnancy (dark) and the non-pregnancy state (light) showing elevated diaphragm, the increased transverse diameter and circumference, flaring out of ribs and the increased subcostal angle
(de Sweit, 1998b, p 115, with permission from Wiley-Blackwell Publishing Ltd).
Expansion of the rib cage causes tidal volume to increase by 30–40%. It rises early in pregnancy continuing to rise until term. Although the respiratory rate is little changed in pregnancy from the normal 14 or 15 breaths/min, breathing is deeper even at rest. The minute volume that facilitates gas exchange is increased by 30–40%, from 7.5–10.5L/min, and minute oxygen uptake increases appreciably as pregnancy advances (Cunningham et al 1997). The enhanced tidal volume contributes to an increase in inspiratory capacity while vital capacity is unchanged. As a result the functional residual capacity is decreased by 20%. This reduces the amount of used gas mixing with each new inspiration thereby enhancing alveolar gas exchange by 50–70%.
Blood volume expansion and vasodilation of pregnancy result in hyperaemia and oedema of the upper respiratory mucosa, which predispose the pregnant woman to nasal congestion, epistaxis and even changes in voice. The changes to the upper respiratory tract may lead to upper airway obstruction and bleeding making both mask anaesthesia and tracheal intubation more difficult. These can be further exacerbated by fluid overload or oedema associated with pregnancy-induced hypertension or pre-eclampsia.
Blood gases
Despite overcompensation of the respiratory system to maternal oxygen requirements, alveolar oxygen partial pressure and the arterial oxygen partial pressure (PaO2) are only slightly increased from non-pregnant values (98–100mmHg) to pregnant values of (101–104mmHg). This is accounted for by the increased oxygen consumption and oxygen carrying capacity of the blood (Broughton-Pipkin 2001). The ‘hyperventilation of pregnancy’ causes a 15–20% decrease in maternal arterial carbon dioxide partial pressure (PaCO2) from an average of 35–40mmHg in the non-pregnant woman to 30mmHg or lower in late pregnancy (Girling 2004). Because fetal PaCO2 is 44mmHg, the carbon dioxide gradient from fetus to mother is increased, which facilitates the transfer of CO2 from the fetus to the mother and causes carbon dioxide to be washed out of the lungs (Campbell & Lees 2000). Clinical implications of these changes are that maternal blood gases should never be performed in the supine position which may give a false impression of hypoxia.
The body has a considerable capacity for storing carbon dioxide in blood, largely as bicarbonate. To compensate, renal excretion of bicarbonate is significantly increased which may limit the buffering capacity in pregnancy. The fall in PaCO2 is therefore matched by an equivalent fall in plasma bicarbonate concentration from the non-pregnant values of 24–30mEq/L to the pregnant values of 18–21mEq/L. Although maternal arterial pH changes very little, the resulting mild alkalaemia (arterial pH 7.40–7.45) further facilitates oxygen release to the fetus (Steinfeld & Wax 2001).
Central nervous system
Research suggests that the dramatic hormonal fluctuations occurring throughout childbearing may re-model the female brain, increasing the size of neurons in some regions and producing structural changes in others (Russell et al 2001). The pituitary gland increases in size by 30–50% in pregnancy (Carlson 2002). Prolactin and β endorphin production increase progressively as pregnancy advances accounting for much of the increased pituitary activity. The opioid active form of β endorphin produced by the pituitary is thought to play a major role in raising the maternal threshold for pain and discomfort in the latter stages of pregnancy.
The adaptations in neural circuitry in the mother’s brain are initiated by pregnancy hormones. Oestrogen and progesterone readily enter the brain acting on a multitude of nerve cells changing the balance between inhibition and stimulation. Other pregnancy hormones, such as relaxin and lactogen also act on the brain. The stage of pregnancy is signalled to the brain by the pattern of secretion of these hormones.
Oxytocin is imperative for labour stimulating expulsive uterine contractions and plays a significant role in the bonding process (Ginesi & Niescierowicz 1998). While oxytocin production is increased in pregnancy, release is inhibited and levels build up in the posterior pituitary. Oxytocin neurons are inhibited from releasing the stored oxytocin prematurely through several hormonal mechanisms involving progesterone, oestrogen and opioid peptides. At term, progesterone secretion falls and the inhibitory mechanism modified to allow gradual release of oxytocin in labour followed by a surge at the time of birth.
Pregnant women’s sleep patterns are affected by both mechanical and hormonal influences. These include nocturia, dyspnoea, nasal congestion, stress and anxiety as well as muscular aches and pains, leg cramps and fetal activity (Box 14.3).
Sleep disturbances are a common complaint of pregnancy. Various hormonal and mechanical influences promote insomnia leading to disturbed sleep during pregnancy in most women (Santiago et al 2001). This worsens toward the end of pregnancy and continues to some extent for 3 months postpartum (Hedman et al 2002).
Interventions include establishing sleep – wake habits, avoiding caffeine, relaxation techniques, massage, heat and support for lower back pain, modifying sleep environment, limiting fluids in the evening and avoiding passive smoking. Sleep medications should be avoided. Some studies have shown that sleep loss in the last few weeks of pregnancy are associated with increased labour length and LSCS rates (Lee & Gay 2004).
Changes in the urinary system
Kidneys and ureters
The changes to renal physiology in healthy pregnancy can both hide and mimic renal disease. Due to the increase in total blood volume renal blood flow increases. This causes a swelling of the kidneys so that they appear larger on ultrasonography. The renal pelvis, calyces, and ureters dilate and can appear obstructed (Williams 2004). The overall dimensions of the kidney increase by approximately 1cm and renal volume increases by as much as 30%. Dilatation of the collecting system occurs in 80% of women by mid-pregnancy leading to a physiological hydronephrosis and hydroureter which can persist for several weeks postpartum (Jeyabalan & Lain 2007). The dilated collecting system can hold up to 300mL of urine, serving as an excellent reservoir for bacteria. After the uterus rises out of the pelvis it rests on the ureters laterally displacing and compressing them at the pelvic brim. During the last half of pregnancy, the upper portion of the ureters above the pelvic brim elongate and become more tortuous, being thrown into single or double curves of varying sizes (Cunningham et al 2005). Dilatation of the ureters, which is rarely present below the pelvic brim, is possibly due to mechanical compression by the enlarging uterus and ovarian plexus. However, the early onset of ureteral dilatation suggests that smooth muscle relaxation caused by progesterone possibly plays an additional role (Gabbe et al 2004). The dilatation is more marked on the right side than on the left, because of the cushioning effect of the sigmoid colon on the left and also the uterine tendency to dextrorotation (Girling 2004).
Significant anatomical changes occur in the bladder from 12 weeks. Due to the increased size of the uterus, the hyperaemia affecting all pelvic organs, and the hyperplasia of the muscle and connective tissues, the bladder trigone is elevated and its posterior margin is thickened. As pregnancy continues, the trigone becomes increasingly deep and wide. The bladder mucosa becomes more oedematous and the blood vessels increase in size and become more tortuous (Cunningham et al 2005). Decreased bladder tone leading to incompetence of the vesicoureteral valve and reflux of urine is seen in up to 3.5% of pregnant women especially in the third trimester (Blackburn 2007). Although decreased bladder tone may lead to increased capacity, the enlarging uterus displaces the bladder superiorly and anteriorly and flattens it. The normal convex surface is converted into a concavity. On cystoscopy an indentation of the bladder dome by the enlarged uterus is visible and the ureteric orifices are visualized in a higher position than in the non-pregnant state. This doubles the intravesical pressure and may therefore decrease capacity. To compensate for reduced bladder capacity urethral length increases, and to preserve continence intraurethral pressure increases. In spite of this, most women experience a degree of urinary incontinence during pregnancy (Box 14.4). Pressure of the presenting part impairs drainage of blood and lymph from the base of the bladder which may cause the area to become oedematous, easily traumatized and more susceptible to infection (Cunningham et al 2005). Progesterone may be involved in the relaxation of bladder smooth muscle, and in extreme cases, detrusor inactivity and retention of urine (Fitzgerald & Graziano 2007). All of the above factors can lead to urinary stasis and an increased risk of urinary tract infection in pregnancy (Fig. 14.6) (see Ch. 21). Glycosuria provides substrates for bacterial growth and is therefore another cause of asymptomatic bacteriuria (Blackburn 2007) (Box 14.5).
Urinary incontinence can begin early in pregnancy and the incidence increases as pregnancy progresses. Stress incontinence appears to be more common than urge incontinence although mixed symptoms are frequent. Women’s descriptions of their incontinence range from mild to ‘terrible’. There is some evidence that pelvic floor strengthening can prevent incontinence during pregnancy and in the postpartum period. Normal function usually returns for most women soon after the birth of the baby (Fitzgerald & Graziano 2007).
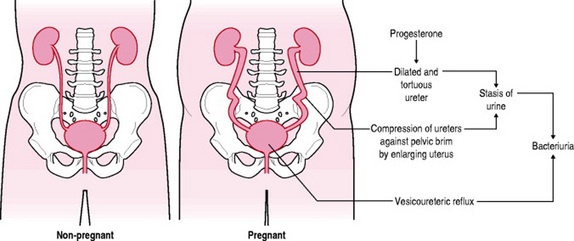
Figure 14.6 Changes in urinary tract in pregnancy and the factors predisposing women to urinary tract infection in pregnancy.
Box 14.5 Asymptomatic bacteriuria
Asymptomatic bacteriuria, the presence of a positive urine culture in an asymptomatic person, occurs in 2–7% of pregnancies, often developing in the first month (Hooton & Stamm 2007). This diagnosis has traditionally been identified with greater than 100 000 colony-forming units (CFU)/mL on two consecutive first-void clean-catch urine specimens. More recently, isolation of >20 000 CFU/mL of a single bacterium has been considered to represent infection and has been associated with subsequent development of pyelonephritis (Wing 2002).
Alterations in renal haemodynamics are among the earliest and most dramatic maternal adaptations to pregnancy (Jeyabalan & Conrad 2007). Due to reduced resistance in the afferent and efferent arterioles of the kidney, and mediated by relaxin, renal plasma flow (RPF) increases most dramatically in the first trimester, from about 1.2L/min in the non-pregnant state to at least 1.5L/min. A 60–70% increase in RPF is reached by the beginning of the third trimester, with a subsequent decline towards term (Jeyabalan & Lain 2007), but is very dependent on posture. The GFR rises 25% by week 4 and 50% by the beginning of the second trimester (Williams 2004), remaining elevated until term even although renal plasma flow decreases during late pregnancy (Cunningham et al 2005). Because they are freely filtered at the level of the glomerulus there is a significant decrease in serum urea, creatinine, blood urea nitrogen (BUN) and uric acid levels during normal pregnancy (Blackburn 2007).
Serum urea levels may be only 63% of non-pregnant values by the third trimester (Blackburn 2007), falling from around 4.3–3.1mmol/L. Serum creatinine also falls. By the end of the first and second trimesters the upper limit for serum creatinine is 80μmol/L and 65μmol/L, respectively (Nelson-Piercy 2004; de Swiet et al 2002), thus levels which are normal in the non-pregnant woman could represent quite severe renal disease in the pregnant woman (de Swiet et al 2002). It is important that appropriate pregnancy-specific normal ranges are used when managing both normal pregnancies and those of women with renal diseases (Baker 2006).
When measured by 24h creatinine clearance test the GFR increases from 100 to around 150mL/min by the beginning of the second trimester (Williams 2004). While the dilated collecting system can interfere with the accuracy of 24-h urine collections (Blackburn 2007), creatinine clearance is a useful test to estimate renal function in pregnancy.
As a consequence of increased GFR and/or reduced proximal tubular reabsorption, serum uric acid concentrations fall by 25% from non-pregnant values by 8 weeks’ gestation, and by 35% throughout most of normal pregnancy. The rise toward non-pregnant levels near term may be due to a progressively increasing renal tubular reabsorption (Jeyabalan & Conrad 2007). The increased GFR increases concentration of solutes and volume of fluid within the tubules by 50–100%. Tubular reabsorption increases in order to prevent rapid depletion of sodium, chloride, glucose, potassium and water from the body. However, tubular reabsorption is not always able to cope with the increased filtered load, resulting in an increase in the excretion of substances such as glucose, amino acids, protein, electrolytes and vitamins (Blackburn 2007).
Glycosuria is common in pregnancy and can vary from day-to-day and within any 24-h period. Urinary glucose values may be 10–100-fold greater than the non-pregnant values, particularly in the third trimester (Blackburn 2007). Gestational glycosuria usually reflects reduced tubular glucose reabsorption rather than abnormal carbohydrate metabolism (Williams 2004). Glucose reabsorption occurs secondarily to the absorption of sodium and therefore other factors contributing to volume homeostasis and sodium retention may be involved in the physiological glycosuria of pregnancy (Baker 2006). Because of these changes in renal handling of glucose and the normal appearance of glucose in the urine, the use of glycosuria as a screen for pregnancy-related glucose intolerance is not particularly helpful (Jeyabalan & Lain 2007) and is not recommended (NICE 2008b).
Excretion of amino acids, urea and protein is markedly increased in pregnancy. Protein excretion rises from <100mg/24h up to 300mg/24h, with marked day-to-day variations. Proteinuria occurs more frequently during pregnancy due to the capacity of tubular reabsorption being exceeded. Values of 1+ protein on dipsticks are common and do not necessarily indicate glomerular pathology or pre-eclampsia. Potassium excretion is decreased with retention of an additional 300–350mEq due to increased proximal tubular reabsorption. Serum potassium levels do not rise as the extra potassium is used for maternal tissues and by the fetus. Urinary calcium excretion is increased, possibly due to the increased GFR, and serum calcium and phosphorus levels decrease. This is balanced by increased intestinal absorption of calcium from the diet (Blackburn 2007).
The amount of water filtered by the kidneys increases by 50% due to the increased GFR (Blackburn 2007), but in pregnancy the woman must retain additional fluid and electrolytes to meet her own needs and the needs of the fetus. On average she gains 6–8kg of fluid: 1.2L is intravascular. Plasma volume expansion is positively correlated with fetal size (Williams 2004). Interstitial fluid volume increases gradually with the greatest accumulation in the second half of pregnancy. Accumulation of more than 1.5L of interstitial fluid is associated with oedema (Blackburn 2007). During the day, pregnant women tend to accumulate water in the form of dependent oedema and at night while recumbent, they mobilize this fluid and excrete it via the kidneys (Cunningham et al 2005).
Urinary frequency (>7 daytime voidings), urgency, incontinence (Box 14.4) and nocturia may be experienced. It is primarily due to the effects of hormonal changes, hypervolaemia, increased renal blood flow and glomerular filtration rate (Blackburn 2007) although the increased fluid intake during pregnancy may also play a part (Fitzgerald & Graziano 2007). Later in pregnancy it is likely to be caused by the enlarged uterus, or descent of the presenting part.
Due to the increased GFR, the filtered load of sodium increases by up to 50%, however adaptation of the renal tubule results in 99% being reabsorbed in the tubules. Sodium retention occurs gradually with an increase in late pregnancy. It is used by the fetus and placenta and the rest is distributed in maternal blood and extracellular fluid. In spite of these alterations the woman responds normally to changes in water and sodium balance in pregnancy. Water excretion is enhanced by the lateral recumbent position although this position interferes with the ability of the woman to concentrate urine. Sodium excretion may be decreased in the supine and sitting positions.
Sodium retention is associated with weight gain and ankle oedema that comes and goes rapidly according to the woman’s activities. During the day, water and sodium are trapped in the lower extremities because of venous stasis and pressure of the uterus on the iliac vein and inferior vena cava. This pressure is reduced at night when the woman is lying down resulting in increased venous return, cardiac output, renal blood flow and glomerular filtration rate with subsequent increase in urinary output. Nocturia may also be due to the large amounts of sodium (and therefore water), which are excreted at night as opposed to daytime (Blackburn 2007).
The kidney also acts as an endocrine organ that produces erythropoietin, vitamin D and renin. The production of these hormones increases during healthy pregnancy, but their effects are masked by other changes, e.g. lowered blood pressure, physiological anaemia and halving of parathyroid hormone levels (Williams 2004).
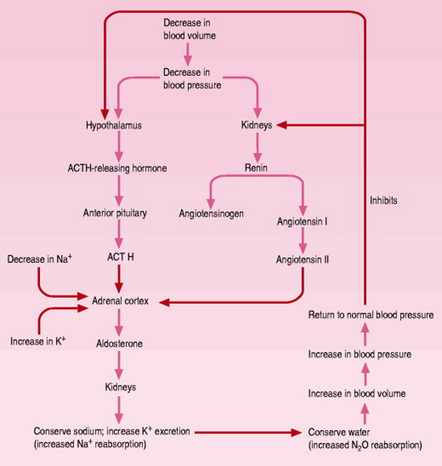
Renin leads to a cascade of events. The renin-angiotensin-aldosterone system is important in fluid and electrolyte homeostasis and maintaining arterial blood pressure (Fig. 14.7). This system must be altered in pregnancy to react appropriately to the new equilibrium. Renin peaks at levels two to three times higher than normal during the first trimester and remains elevated, then reaches a plateau at about 32 weeks’ gestation. Renin release is stimulated by oestrogens, lower blood pressure, increased levels of plasma and urinary prostaglandin and progesterone. There is a 60% decrease in sensitivity to the hypertensive effect of angiotensin II so that rather than the blood pressure rising, it decreases along with the peripheral vascular resistance. Angiotensinogen levels also peak at 30–32 weeks. Aldosterone reaches levels 8–10 times higher than those in nonpregnant women by 36 weeks. This opposes the sodium-losing effects of progesterone and allows a progressive accumulation of sodium in maternal and fetal tissues. It may also be necessary to maintain the expanded extracellular volume (Blackburn 2007). Although various hormones have been implicated in the gestational renal haemodynamic alterations, their effects are unclear and researchers offer conflicting opinions (Jeyabalan & Conrad 2007).
Changes in the gastrointestinal system
Anatomical and physiological changes take place in each organ of the gastrointestinal system. Influenced by oestrogen the gums become highly vascularized and oedematous. Associated with this is dental plaque, calculus and debris deposits which increase during pregnancy. Advanced gingivitis can lead to a specific angiogranuloma known as epulis. This is a purplish red mass, 2cm in diameter, often between the upper anterior maxillary teeth. It is very friable, bleeds easily and often interferes with chewing. It is usually painless but may ulcerate and become painful. It normally regresses spontaneously after birth but may recur in the same location in subsequent pregnancies. Occasionally these growths require to be excised (Blackburn 2007).
Pregnancy does not cause tooth decay. Fetal calcium requirements are drawn from maternal body stores, not from the teeth. Due to gingival alterations, however, the pregnant woman may become more aware of pre-existing or newly developed dental caries which may deteriorate as a result of the more acidic saliva (Box 14.6) and the nausea and vomiting of pregnancy. There may also be a transient increase in tooth mobility.
Ptyalism (excessive salivation) is rare in pregnancy. It begins early in pregnancy and ceases with the birth of the baby. It may be associated with gastro-oesophageal reflux (Blackburn 2007) or due to stimulation of the salivary glands by the ingestion of starch. Much more common is the perception of an increased saliva production rather than an actual increase in saliva volume, due to difficulty in swallowing saliva during the period of nausea and vomiting in early pregnancy.
Nausea and vomiting is experienced by more than half of all pregnant women (Box 14.7). In spite of this, an increase in appetite is common in pregnancy and may be due to the effects of progesterone, which acts as an appetite stimulant (Blackburn 2007) or due to the movement of glucose and other nutrients to the fetus (Williamson 2006) or to the alterations in taste threshold. Food consumption has been reported to increase up to 20%, beginning early in pregnancy, peaking at mid-gestation and decreasing near term (Chamberlain & Steer 2001).
Nausea and vomiting (popularly known as ‘morning sickness’) is experienced by more than half of all pregnant women and may occur at any time of day or night. Symptoms usually begin between 4 and 7 weeks and usually resolve by 16–20 weeks. Women need to know that it is not usually associated with a poor pregnancy outcome and should be advised that ginger, P6 (wrist) acupressure and antihistamines appear to be effective in reducing symptoms (NICE 2008a). Most women do not require treatment but it can have profound effects on the life of the woman and her family. If the condition persists and is severe it can progress to hyperemesis gravidarum (see Ch. 19) (Festin 2006). The exact cause and function is unknown. Many theories have been proposed but none are sufficiently supported by evidence (Blackburn 2007). Nausea is frequently triggered by hypoglycaemia, hence its occurrence on wakening in the morning. If severe it may be accompanied by food cravings and aversions.
Reduced physical activity as well as changes in metabolism lead to more efficient utilization and absorption of nutrients during pregnancy, which means that for many nutrients an increase in dietary intake over and above that which is normally required is not necessary (Williamson 2006).
Taste often changes early in pregnancy. Even before the first missed period, there may be a loss of taste for something usually enjoyed (Chamberlain & Steer 2001). The development of cravings or aversions to food is also often reported. The most common cravings are for dairy products and sweet foods. Common aversions include tea and coffee, alcohol, fried foods and eggs and later in pregnancy, sweet foods (Williamson 2006). Pica, the persistent craving and compulsive consumption of non-food substances is poorly understood (Box 14.8). The increase in thirst in pregnancy noticed by many women may be due to a resetting of the central osmostat controlling thirst and vasopressin secretion as a result of the actions of relaxin on the brain (McKinley & Johnson 2004).
Pica is the persistent craving and compulsive consumption of substances such as ice, clay, soap, coal or starch. Several theories have been proposed to explain the condition, such as nutritional deficiencies of zinc or iron, or the sensory enjoyment of the taste, texture or smell of the substance. Some pica practices have cultural roots and some believe it is a behavioural response to stress, a habit or disorder or a manifestation of an oral fixation (Mills 2007). Pica can cause a number of medical problems, such as nutritional deficiencies, constipation, electrolyte imbalance, gastrointestinal and metabolic disturbances, lead poisoning, dental complications and weight gain (Mills 2007). Some cravings are potentially dangerous, such as eating mothballs, which can cause fetal haemolytic anaemia (Cunningham et al 2005). Patel et al (2004) noted that the prevalence of anaemia was 15% in women with pica compared with 6% in those without it, and the rate of pre-term birth at <35 weeks was noted to be twice as high in women with pica. The incidence of pica within the UK appears to have declined in recent years (Williamson 2006), however it may be commoner than generally believed, since it is known to be underreported by women due to embarrassment and guilt. When diagnosed it is important that a non-judgemental understanding and culturally sensitive approach to care is taken. Treatment will depend on the cause but may include iron supplementation, homeopathic remedies and counselling (Mills 2007).
Gastrointestinal tone and the resting pressure of the lower gastro-oesophageal sphincter is decreased in pregnancy due to increased circulating levels of progesterone, oestrogen and motilin resulting in prolonged intestinal transit time (Suresh & Radfar 2004). The abdominal distension, which ensues can cause the woman to feel ‘bloated’ (Box 14.9). Progesterone is also responsible for the common problems of constipation and haemorrhoids (Box 14.10). By 20 weeks’ gestation the gravid uterus exerts pressure on the stomach causing its upward displacement and rotation which alters the angle of the gastro-oesphageal junction and increases intragastric pressure (Rudra 2005). The altered position of the stomach, increased intragastric pressure due to the enlarging fetus, and decreased pressure of the lower gastro-oesophageal sphincter contribute to the common problem of heartburn (Cunningham et al 2005) (Box 14.11). While some confusion remains about how pregnancy affects gastric contents, recent evidence suggests that gastric emptying of fluids is not altered during pregnancy (O’Sullivan & Scrutton 2003) and gastric emptying of solids does not appear to be delayed during at least the first half of pregnancy (Rayner & Micell 2005). Following an extensive review of research studies for the development of fasting guidelines, the American Society of Anesthesiologists support the view that gastric emptying is normal in all three trimesters of pregnancy (Maltby 2000).
Abdominal distension and a ‘bloated’ feeling occur when nutrients and fluids remain in the intestinal tract for longer, particularly in the third trimester due to the prolonged transit time. Increased flatulence may also occur due to decreased motility and pressure of the uterus on the bowel (Blackburn 2007).
Box 14.10 Constipation and haemorrhoids
Constipation occurs because progesterone enhances absorption of sodium and water in the colon resulting in smaller stools with lower water content. Iron supplements may also aggravate constipation. Pregnant women are advised to consider changing the type of iron supplement (if used), to increase their intake of bran or wheat fibre and fluids and to take gentle exercise to alleviate this problem. Dietary bulking agents may also be helpful (Williamson 2006).
Haemorrhoids are also fairly common in pregnancy due to both constipation and pressure in veins below the level of the enlarging uterus (Cunningham et al 2005). Poor support for haemorrhoidal veins in the anorectal area and lack of valves in these vessels can lead to reversal in the direction of blood flow and stasis of blood (Blackburn 2007). Women should be offered dietary advice and if symptoms remain troublesome should consider standard haemorrhoid creams (NICE 2008a).
Heartburn or acid reflux into the lower oesophagus during pregnancy occurs in 30–80% of women, particularly during the third trimester (Rayner & Micell 2005). Frequent or more severe heartburn can interfere with sleep and deter the woman from eating adequately. Lifestyle modifications may be necessary, for example elevating the head of the bed 6 inches, stopping smoking, sleeping on the left side, avoiding reclining for 2–3 hrs after a meal. Dietary modifications which may be helpful include eating less fat and more protein, avoiding chocolate and certain drinks such as coffee, citrus juices, tomato products, and alcoholic or fizzy drinks (Charan & Katz 2001). Antacids such as Gaviscon or ranitidine may provide relief, however, it should be remembered that long-term therapy affecting gastric acidity can impair iron absorption (Rayner & Micell 2005).
Although gastric acidity is reduced due to the influence of oestrogen during the first and second trimesters it is increased in the third trimester (Blackburn 2007). The comparative study by Hong et al (2005) confirms that women fasting prior to elective caesarean delivery at term had much greater and more acidic gastric contents than the non-pregnant patients preoperatively, however, it is recognized that this may be influenced by preoperative anxiety (Blackburn 2007).
The enlarging uterus causes the intestines to extend upwards and laterally (Cunningham et al. 2005) leading to a gradual displacement and lateral rotation of the caecum and appendix. In the first trimester the appendix remains within the right iliac fossa, moving to the pelvic brim during the second trimester and to lower right upper quadrant in the third trimester (Chawla et al 2003). Recent MRI studies have suggested that in spite of these anatomical changes the most common presenting symptom of appendicitis in pregnancy is pain in the right lower quadrant of the abdomen regardless of gestational age rather than the upper quadrant as previously believed (Oto et al 2006).
Gall bladder volume is increased and emptying rate is decreased, especially during the second and third trimesters due to its reduced muscle tone and motility under the influence of progesterone. The residual gall bladder volume after fasting is nearly twice that of the non- pregnant woman. As a result bile is more dilute with a decreased ability to make cholesterol soluble. The sequestered cholesterol precipitates formation of crystals and stones which increases the tendency to gall stones. Reduced gall bladder tone leads also to the tendency to retain bile salts which can lead to pruritus (Blackburn 2007).
Although the size of the liver remains the same during pregnancy it is displaced superiorly, posteriorly and anteriorly by the enlarging uterus. Hepatic blood flow is reduced by 35% in spite of the increased cardiac output, due to diversion to the uteroplacental circulation. Liver production of plasma proteins, bilirubin, serum enzymes and serum lipids is altered due to effects of oestrogen and haemodilution (see Table 14.5).
Changes in metabolism
The major changes in the utilization of carbohydrate, fat and protein during pregnancy are closely linked with the functions of the various endocrine glands. The placenta is already secreting hormones that affect metabolism within a few weeks of conception (see Ch. 11). Metabolic changes are essential for the continuous supply of glucose and amino acids for fetal growth as well as for meeting the increased physiological demands of the woman during pregnancy, labour and lactation. Food intake and appetite are increased, activity is decreased, approximately 3.5kg of fat is deposited, energy reserves of approximately 30000kcal are established and 900g of new protein is synthesized by the mother, fetus and placenta.
The products of conception, uterus and maternal blood are relatively rich in protein. In the first half of pregnancy, maternal storage of protein increases. Most of it is transported to the fetus but some is retained in maternal tissues. During the second half of pregnancy more protein is conserved with decreased urinary nitrogen excretion (Blackburn 2007). This increasing nitrogen balance suggests more efficient use of dietary protein as pregnancy progresses (Cunningham et al 2005).
Changes in lipid metabolism promote the accumulation of maternal fat stores in early and mid-pregnancy and enhance fat mobilization in late pregnancy (Butte 2000). Later in pregnancy, as fetal nutritional demands increase, maternal fat storage decreases. Leptin, a peptide hormone, is secreted by both placenta and adipose tissue and plays a key role in the regulation of body fat and energy expenditure. Serum leptin levels progressively increase, peaking during the second trimester and rising to a plateau at term in concentrations three to four times higher than the non-pregnant woman. Fats are used by the woman as an alternative energy substrate allowing her to conserve glucose for the fetus and her central nervous system during the second half of pregnancy. After a meal maternal fat stores are replenished by increased glucose uptake, incorporation of glucose to glycerol and taking up of fatty acids by adipocytes (Blackburn 2007).
Carbohydrate metabolism, however, demonstrates the most dramatic changes. During the first two trimesters in response to fetal and maternal needs, glucose secretion increases by 15–30% with increased peripheral glucose use without increases in insulin resistance. Maternal glucose levels are generally 10–20% lower than in non-pregnant women. From 20 weeks’ gestation until term, insulin secretion increases three-fold and resistance increases with decreased glucose uptake by muscle and adipose tissue. Increasing insulin resistance promotes the flow of nutrients from the mother to the fetus and promotion of adipose tissue accumulation. Insulin resistance is mediated by the increasing levels of oestrogen, progesterone, hPL, cortisol and prolactin. In late pregnancy, although basal insulin levels are elevated, maternal blood glucose levels are similar to non-pregnant levels and do not drop as rapidly as usual even with higher circulating levels of insulin. This diabetogenic state protects the fetus even if the mother is fasting by keeping glucose in the blood and thus available for placental transfer (Blackburn 2007).
After a meal however, the pregnant woman’s levels of glucose and insulin are higher than those of non-pregnant woman and glycogen is suppressed, resulting in hyperinsulinaemia, hyperglycaemia and insulin resistance. These changes increase glucose availability for transport to the fetus. Maternal blood glucose levels may rise transiently to 7.2–7.8mmol/L and insulin resistance is increased by 60–70%. The hyperinsulinaemic response is most marked during the third trimester because of hypertrophy and hyperplasia of islet β cells, which become more responsive to alterations in blood glucose and amino acid levels. The increased insulin levels after eating overcome the insulin resistance to allow glucose uptake by muscles for storage as glycogen. Even with increased production of insulin however, overall glucose levels are maintained although at a relatively lower level than in the non-pregnant woman because of the counterbalancing effects of oestrogen, progesterone and hPL (Blackburn 2007).
The hyperglycaemic state after meals changes rapidly to a fasting state characterized by decreased levels of plasma glucose and amino acids. During fasting plasma concentrations of fatty acids, triglycerides and cholesterol are higher. This pregnancy-induced switch in fuels from glucose to lipids is known as accelerated starvation and ketonaemia rapidly appears (Cunningham et al 2005). During the overnight fast, plasma glucose levels decline more dramatically in the pregnant woman due to the continuous transfer of glucose and amino acids to the fetus (the fetal siphon). The woman compensates by using fat stores to meet her energy needs with breakdown of glycerol to glucose. As early as 15 weeks’ gestation, maternal glucose levels after a 12–14h fast are 0.8–1.1mmol/L lower than levels in non-pregnant women. This becomes more noticeable during the second and third trimesters when the switch to fat oxidation takes place after 2–3h in comparison with 14–18h in the non-pregnant woman. Hypoglycaemia and lower insulin levels between meals and overnight lead to more rapid development of ketosis. This may contribute to the increased maternal appetite and sometimes a feeling of faintness during pregnancy. Optimal blood glucose levels in the pregnant woman range between 4.4 and 5.5mmol/L.
There is a two-fold increase in intestinal absorption of calcium during pregnancy to meet the calcium needs for fetal bone mineralization and breastmilk production. In spite of this maternal bone loss may occur in the last months of pregnancy when the fetal skeleton is rapidly mineralizing. The average daily transfer of calcium in the first trimester is 2–3mg/day, whereas the rate at 35–36 weeks’ gestation is estimated to be 250mg/day. This places the woman at an increased risk of osteoporosis later in life. Maternal diet has little effect on the amount of mineral transferred (Kalwarf & Specker 2002). Serum albumin is required for binding calcium, however since albumin levels are decreased due to the greatly expanded intravascular fluid volume, maternal plasma calcium concentration falls (Cunningham et al 2005).
Low calcium levels are no longer believed to be associated with leg cramp and the most effective intervention for cramp remains unclear (Blackburn 2007). Calcium supplements are not generally thought to be necessary, however, women at risk of vitamin D deficiency are advised to take 10μg of vitamin D per day (NICE 2008a).
Maternal weight
Most of the weight gain during pregnancy is attributable to the uterus and its contents, the breasts, increases in blood volume and extracellular fluid (see Table 14.7). A smaller fraction of the increased weight is the result of metabolic alterations known as maternal reserves (Cunningham et al 2005). Approximately 62% of weight gain consists of water, which is retained in all systems of the body.
Table 14.7 Distribution of average increase in weight
| Weight gain (kg) | Percentage of total weight | |
|---|---|---|
| Maternal | ||
| Uterus | 0.9 |
 |
| Breasts | 0.4 | |
| Fat | 4.0 | |
| Blood | 1.2 | |
| Extracellular fluid | 1.2 | |
| Total | 7.7 | |
| Fetal | ||
| Fetus | 3.3 | 25 |
| Placenta | 0.7 |
 |
| Amniotic fluid | 0.8 | |
| Total | 4.8 | |
| Grand total | 12.5 | |
While initial maternal weight and the weight gained during pregnancy are highly associated with birth weight, it remains unclear what role maternal fat or water have in fetal growth. Studies in well-nourished women at term suggest that maternal body water rather than fat contributes more significantly to infant birth weight (Cunningham et al 2005). The recommended pattern for normal weight gain is approximately 3kg in the first trimester followed by about 0.4kg/week for the remainder of the pregnancy. This pattern results in approximately 3kg of fat stores accumulating in the first half of pregnancy, while weight gained in the second half goes toward the growth of the fetus and maternal supportive tissues. Fetal growth is slow in the first 2 months during organogenesis but then accelerates rapidly. Maximum growth rate is achieved between the 4th and 8th month when the fetus grows at the rate of 5–9% per week. Until 15–16 weeks the placenta is larger than the fetus but by term the fetus is five to six times heavier than the placenta. Amniotic fluid increases from 7mL at 8 weeks, 30mL by 10 weeks, 190mL by 16 weeks, to a mean of about 800mL by 35 weeks after which it decreases to about 400mL by 42 weeks. The increase in the weight of the uterus is more rapid in the first 20 weeks (Blackburn 2007). The weight of breasts, blood volume and total body water increases steadily throughout pregnancy. The pitting oedema of ankles and legs, which is seen in most pregnant women, especially at the end of the day may amount to over 1L (Cunningham et al 2005).
There are currently no official recommendations for weight gain during pregnancy in the UK. Weight gain averages between 11 and 16kg but variations are large. A birth weight of 3.1–3.6kg has been associated with optimal maternal and fetal outcomes. Maternal nutritional status at the time of conception is very important for fetal growth and development. It is important to attain a healthy body weight prior to conception (see Ch. 13)
Skeletal changes
Mechanical and hormonal influences result in progressive modifications of posture, gait and joint mobility throughout pregnancy. The evolutionary derived curvature and reinforcement of the lumbar vertebrae enable women to compensate for the bipedal obstetric load (Whitcome et al 2007). Posture alters to compensate for the enlarging uterus, and a progressive lordosis shifts the woman’s centre of gravity back over her legs. There is increased mobility of the sacroiliac and sacrococcygeal joints, which may contribute to the alteration in maternal posture leading to the characteristic ‘waddling’ gait of pregnancy and low back pain (Box 14.12). The muscles of the abdominal wall may stretch and lose some tone further aggravating back pain (Lowdermilk & Perry 2004).
Back pain occurs in approximately 70% of pregnant women. The weight of the pregnant uterus and altered posture (compensatory lordosis) increase susceptibility which is exacerbated by progesterone and relaxin causing softening and relaxation of the ligaments of the pelvis. Simple measures to reduce pain include limiting physical activity, alteration of posture, wearing low-heeled shoes and adequate rest. Supportive pillows beneath the knees and abdomen and local application of heat may relieve pain. A physiotherapist may teach the woman back and abdominal muscle exercises to strengthen these muscles, or apply a sacroiliac or trochanteric support (see Ch. 16). Research suggests that exercises and acupuncture can reduce back pain (Pennick & Young 2007). While not substantiated by research, reports suggest a range of alternatives may be beneficial including massage, yoga, relaxation therapy, water exercises and osteopathy (Wang et al 2005).
Relaxation of the pelvic joints is predominantly due to hormonal influences. Oestrogen modifies the connective tissue making it more pliable. This causes the joint capsules to relax, making the pelvic joints mobile. Progesterone has the effect of relaxing or weakening the pelvic ligaments. Relaxin plays a major role in the changes, remodelling collagen fibres and softening pelvic joints and ligaments in preparation for birth (Marnach et al 2003). This allows some expansion of the pelvic cavity during descent of the fetal head in labour but further predisposes the woman to pelvic girdle instability.
Skin changes
Changes in the skin, hair, nails, sebaceous and sweat glands are predominantly modulated by hormonal, immunologic, and metabolic factors (Box 14.13).Certain changes have been shown to have a genetic predisposition, particularly striae gravidarum and pigmentation changes (Muallem & Rubeiz 2006).
Hair growth has been shown to follow a common pattern in pregnancy. Women commonly report a thickening and increased volume of scalp hair. Stimulated by oestrogen, the growing period for hairs is increased in pregnancy so the woman reaches the end of pregnancy with many overaged hairs. This ratio is reversed after birth so that sometimes alarming amounts of hair are shed during brushing or washing. Normal hair growth is usually restored by 6–12 months. Mild hirsutism is common during pregnancy, particularly on the face (Muallem & Rubeiz 2006). Actions that may help include reducing damage to the hair by not combing when it is wet, and avoiding hairstyles that pull and stress hair, using shampoos and conditioners that contain biotin and silica. Diet that is high in fruits and vegetables containing flavonoids and antioxidants may provide protection for the hair follicles and encourage growth.
Almost all women note some degree of skin darkening as one of the earliest signs of pregnancy. While the exact pathogenesis remains unclear, it is generally attributed to an increase in melanocyte stimulating hormone, progesterone and oestrogen serum levels. Hyperpigmentation is more marked in dark-skinned women and is more pronounced in areas that are normally pigmented, e.g. areola, genitalia and umbilicus, in areas prone to friction, such as the axillae and inner thighs (Muallem & Rubeiz 2006) and in recent scars.
The linea alba is a line that lies over the midline of the rectus muscles from the umbilicus to the symphysis pubis. Hyperpigmentation causes it to darken resulting in the linea nigra. Pigmentation of the face affects up to 75% of pregnant women (Muallem & Rubeiz 2006). Known as chloasma or melasma, or ‘mask of pregnancy’ it is caused by melanin deposition into epidermal or dermal macrophages, further exacerbated by sun exposure. The chloasma usually regresses postpartum but may persist in approx 10% of women. Oral contraceptives may aggravate melasma and should be avoided in susceptible women (Cunningham et al 1997). If chloasma persists postpartum it can be treated with a variety of topical agents, including hydroquinone, tretinoin, kojic acid and vitamin C (Katsambas & Stratigos 2001).
As maternal size increases, stretching occurs in the collagen layer of the skin, particularly over the breasts, abdomen and thighs. In some women, this results in striae gravidarum (stretch marks) caused by thin tears occurring in the dermal collagen. These appear as red stripes changing to glistening, silvery white lines approximately 6 months after birth. The aetiology of striae has yet to be defined but may be compounded by hormones adrenocorticoids, oestrogens and relaxin which modify collagen and possibly elastic tissue.
Pruritus in pregnancy (not due to liver disease) can be distressing. In the absence of a rash, aspirin is recommended. If there is a rash chlorphenindione (chlorpheniramine) may be more effective (Young & Jewell 2002).
A rise in temperature by 0.2–0.4 °C occurs as a result of the effects of progesterone and the increased basal metabolic rate (BMR). As a result, pregnant women ‘feel the heat’ and often sweat profusely, particularly in hot, humid climates. Peripheral vasodilation and acceleration of sweat gland activity help to dissipate the excess heat produced by maternal, placental and fetal metabolism (Lowdermilk & Perry 2004).
Angiomas or vascular spiders (minute red elevations on the skin of the face, neck, arms and chest) and palmar erythema (reddening of the palms) frequently occur, possibly as a result of high oestrogen levels. They are of no clinical significance, and disappear after pregnancy (Cunningham et al 1997). Most conditions have a tendency to resolve spontaneously within a few months postpartum. Nevertheless, changes may mask more serious conditions such as malignant neoplasms as well as herpes gestationis, and intrahepatic cholestasis of pregnancy. It is therefore imperative to assess for specific dermatoses of pregnancy which may be associated with maternal systemic symptoms and fetal mortality and morbidity if severe and untreated.
Changes in the breasts are summarized in Table 14.8.
Table 14.8 Breast changes in chronological order
| Time | Changes |
|---|---|
| 3–4 weeks | Prickling, tingling sensation due to increased blood supply particularly around nipple |
| 6–8 weeks | Increase in size, painful, tense and nodular due to hypertrophy of the alveoli. Delicate, bluish surface veins become visible just beneath the skin |
| 8–12 weeks | Montgomery’s tubercles become more prominent on the areola. These hypertrophic sebaceous glands secrete sebum, which keeps the nipple soft and supple. The pigmented area around the nipple (the primary areola) darkens and may enlarge and become more erectile |
| 16 weeks | Colostrum can be expressed. The secondary areola develops with further extension of the pigmented area that is often mottled in appearance |
| Late pregnancy | Colostrum may leak from the breasts; progesterone causes the nipple to become more prominent and mobile |
Changes in the endocrine system
Successful physiological adaptation to pregnancy is due to the alterations in steroid and protein hormone production by the maternal endocrine system and the trophoblast (Cunningham et al 2005). Early effects of placental hormones are described in Chapter 10. Later physiological effects caused by hormones are now summarized.
Placental hormones
hCG levels increase rapidly in early pregnancy, doubling every 2 days, with maximal levels being attained at about 8–10 weeks’ gestation. Thereafter levels begin to decline and a nadir is reached by around 20 weeks, after which this lower level is maintained for the remainder of pregnancy (Fig. 14.8). The detection of hCG in blood or urine is almost always indicative of pregnancy. Since hCG is also synthesized in the fetal kidneys it is also found in fetal blood and amniotic fluid (Cunningham et al 2005). Besides stimulating the maternal thyroid gland, the main function of hCG is to maintain the corpus luteum in order to ensure secretion of progesterone (and to a lesser extent relaxin) until placental production is adequate after 10–11 weeks, after which concentrations of hCG gradually decrease until it has completely disappeared 2 weeks after birth (Blackburn 2007).
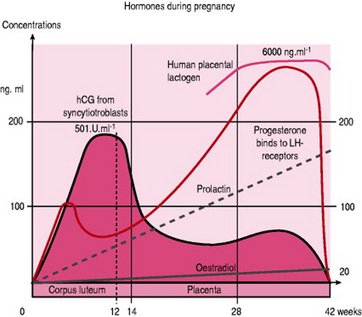
Figure 14.8 Variations in plasma hormone concentrations during a normal pregnancy.
(From Paulev P 1999, with permission of Copenhagen Medical Publishers. Online. Available: http://www.mfi.ku.dk/ppaulev/chapter29/kap29.htm)
The steady rise in maternal hPL plasma level, until it peaks around 34–36 weeks’ gestation, is linked mainly to placental mass (Cunningham et al 2005). The primary role of hPL is to regulate glucose availability for the fetus. hPL is an insulin antagonist that increases maternal metabolism and use of fat for energy and reduces glucose uptake and use by maternal cells. As a result more glucose is available for transport to the fetus. When there is less glucose in the blood there is increased hPL secretion. By thus altering maternal protein, carbohydrate and fat metabolism, hPL promotes fetal growth (Blackburn 2007). While prolonged maternal starvation in the first half of pregnancy leads to an increase in the plasma concentration of hPL, short-term changes in plasma glucose or insulin have little effect on plasma levels of hPL (Cunningham et al 2005).
The placenta secretes over 20 different oestrogens into the maternal circulation but the major ones are oestradiol, oestrone, oestriol, the latter being the largest fraction. Oestradiol and oestrone are directly synthesized by the syncytiotrophoblast. Urinary and plasma oestriol levels increase progressively throughout pregnancy until 38 weeks’ gestation (Symonds & Symonds 2004). The production of oestrogens is dependent on interaction of the maternal-fetal-placental unit. Approximately 90% of the precursors for oestriol are derived from the fetal adrenal glands and the liver (Cunningham et al 2005). Oestrogen levels are low in the first trimester, then increase markedly in the second trimester and remain steadily elevated until labour (Ticconi et al 2006). During the last few weeks of pregnancy, huge amounts of oestrogens, in particular oestradiol, are produced each day by the syncytiotrophoblast, the majority of which are released into maternal circulation and eventually excreted in maternal urine (Cunningham et al 2005).
High oestrogen levels of pregnancy stimulate production of thyroxine-binding proteins and induce an increase in cortisol binding globulin (CBG) which in turn increases corticotrophin releasing hormone (CRH), adrenocorticotrophin hormone (ACTH), and total cortisol levels to maintain an adequate free cortisol concentration (Utz 2005). Oestrogens act to increase the number of glandular ducts of the breast, enhance myometrial activity, promote myometrial vasodilation, increase sensitivity of the maternal respiratory centre to carbon dioxide, soften fibres in the cervical collagen tissue, increase pituitary secretion of prolactin, increase serum binding proteins and fibrinogen, decrease plasma proteins and increase the sensitivity of the uterus to progesterone in late pregnancy (Blackburn 2007).
Progesterone is the principal pro-pregnancy factor. Placental production of progesterone increases steadily throughout pregnancy until it reaches maximal levels around 38 weeks. It is produced initially by the corpus luteum under the influence of hCG during the first 6–8 weeks after conception. Thereafter it is synthesized primarily by the syncytiotrophoblast. Some 90% is secreted into maternal circulation and the remaining 10% is used by the fetal adrenals to produce glucocorticoids and mineralocorticoids. During pregnancy progesterone acts to maintain myometrial quiescence, constrict myometrial vessels, inhibit prolactin secretion, help suppress maternal immunological responses to fetal antigens and therefore prevent rejection of the fetus, relax smooth muscle in the gastrointestinal and urinary systems, increase basal body temperature and increase sodium and chloride excretion (Blackburn 2007). It also stimulates the appetite, fat storage and the respiratory centres (de Sweit et al 2002).
Pituitary gland and its hormones
The anterior pituitary gland develops a more convex, dome-shaped surface and increases in size and weight during pregnancy by 30–50% from an average of 660mg in the non-pregnant woman to 760mg during pregnancy. The weight increase is due largely to an oestrogen induced hypertrophy and hyperplasia of the prolactin-secreting cells (Blackburn 2007). Pituitary FSH secretion is inhibited by the negative feedback of progesterone and oestrogen and also by inhibin (a glycoprotein hormone produced in the corpus luteum and placenta) and thus ovulation is prevented during pregnancy (Cunningham et al 2005) (see Ch. 10). Levels of FSH and LH are low by 6–7 weeks and are undetectable by mid-pregnancy (Blackburn 2007).
Thyroid-stimulating hormone (TSH) falls during the first trimester as a result of the mild thyrotrophic effect of hCG (British Thyroid Association 2006). It then rises rapidly by 20 weeks and plateaus until term. TSH increases both the synthesis and release of thyroxine (T4) and triiodothyronine (T3) (Shennan 2004). ACTH secretion and plasma levels increase progressively in the second and third trimesters to a peak during labour, thus stimulating release of cortisol by the adrenal gland (Blackburn 2007).
Prolactin concentrations in pregnancy reach levels that would be considered pathological in the non-pregnant woman (Baker 2006), increasing 10-fold to peak at birth. Although the anterior pituitary is responsible for most of the increase, prolactin is also produced by the breasts, the decidua and is found in high concentrations in amniotic fluid. Prolactin has many effects, the most important of which is to promote mammary development and stimulate lactation (see Ch. 41).
The posterior pituitary gland produces two hormones: vasopressin and oxytocin. Vasopressin levels are within normal ranges in pregnancy, however, the threshold at which it is secreted is reset with a decline in plasma osmolality. It also modulates blood pressure, electrolyte balance and ACTH release (Blackburn 2007). Oxytocin is produced not only by the posterior maternal and fetal pituitary glands but also by the myometrium, decidua, placenta and fetal membranes. Under the influence of oestrogen, the uterus becomes increasingly sensitive to oxytocin throughout pregnancy as oxytocin receptors in the myometrium increase 100-fold by 32 weeks and up to 300-fold by term. In spite of this, oxytocin concentration in the maternal circulation does not begin to rise until the expulsive stage of labour begins (Heffner & Schust 2006), when it stimulates contractions in the myometrium, and after the baby is born, causes contraction of myoepithelial cells of the breast, resulting in milk ejection (see Ch. 41).
Thyroid function
There is moderate enlargement of the thyroid gland in pregnancy due to a relative iodide deficiency as a result of the siphoning of maternal iodide by the fetus and also as a result of the increased glomerular filtration rate which increases renal clearance and excretion (Symonds & Symonds 2004). Enlargement can also be attributed to increased thyroid volume and blood flow in the intra-thyroid vessels (Fister et al 2006). However, enlargement should not be pathological and any goitre requires to be investigated (Cunningham et al 2005). While many studies have reported an increase of up to 50% in thyroid size in iodine-deficient areas, enlargement up to 15% has also been reported in areas with adequate iodine intake.
Thyroid function per se does not change during pregnancy (Blackburn 2007). In response to high oestrogen levels and the elevated hCG, there is a marked increase in levels of thyroid-binding globulin (TBG) by the liver which reach a peak around 20 weeks and then stabilize for the remainder of the pregnancy (Fig. 14.9). This leads to an increase in the bound forms of serum thyroxine (T4) and triiodothyronine (T3), which rise to a peak at 10–15 weeks’ gestation and then plateau at levels 40–100% higher than non-pregnant values (Cunningham et al 2005). This suppresses thyroid stimulating hormone (TSH) secretion which decreases transiently from 8–14 weeks’ gestation at the time of the hCG peak, returning to non-pregnant levels by the second trimester (Blackburn 2007). The circulating concentrations of unbound/free (and therefore active) forms of T3 and T4 decrease in the second and third trimesters and may fall below the reference range derived from non-pregnant women. The changes in maternal thyroid hormones are essential for the normal development of the fetal brain (British Thyroid Association 2006) and for supporting the altered carbohydrate, protein and lipid metabolism of pregnancy and changes in basal metabolic rate. However, as these changes mimic hyperthyroidism by causing palpitations, sweating and heat intolerance they can cause diagnostic confusion (Becks & Burrow 2000).
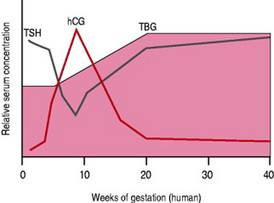
Figure 14.9 Relative levels of thyroid-stimulating hormone (TSH), thyroid-binding globulin (TBG) and human chorionic gonadotrophin (hCG) in pregnancy
(From Bowen 1999, with permission.)
Adrenal glands
The adrenal gland increases only slightly in size during pregnancy due to hypertrophy and widening in the glucocorticoid area which suggests increased secretion of these hormones. Regulated by corticotrophin releasing hormone (CRH) and adrenocortocotrophin (ACTH), glucocorticoids control metabolism and blood glucose and suppress immune functions (Blackburn 2007). Because CRH is also produced by the trophoblast cells, maternal CRH levels increase dramatically and have a role in regulating the activity of the fetal adrenal glands and the myometrium and possibly the maternal adrenal gland (Baker 2006).
During pregnancy, the adrenal gland is more responsive to ACTH (Blackburn 2007). Although levels of ACTH are reduced strikingly in early pregnancy (Symonds & Symonds 2004), they increase approximately two-fold after the first trimester which stimulates a parallel increase in cortisol. Most of the cortisol circulates bound either to globulin or albumin (total cortisol), and only 10% is free (measured by 24h urinary free cortisol) with the result that the free cortisol fraction remains within the normal range (Garner 2002). Consequently, although total plasma cortisol increases from 3 months and peaks at term at levels 3–8 times higher, the pregnant woman does not show signs of hypercortisolism. Urinary cortisol increases up to three-fold by term and the diurnal secretion is blunted but maintained (Blackburn 2007).
Aldosterone, an anti-natriuretic hormone is the other major circulating mineralocorticoid produced by the adrenal gland. It regulates fluid and electrolyte imbalance by controlling sodium reabsorption and potassium and bicarbonate excretion in the distal renal tubule. Aldosterone levels in pregnancy increase four-fold by 8 weeks and continue to increase reaching a 10-fold increase by term (Garner 2002). Rising levels of progesterone with its natriuretic properties possibly influence the production of aldosterone (Baker 2006). The function of the adrenal medulla remains unchanged so that levels of catecholamine remain as in the non-pregnant state. However, during labour there are often massive increases in both adrenaline and noradrenaline as a result of stress and muscle activity (Symonds & Symonds 2004).
Diagnosis of pregnancy
The signs and symptoms of pregnancy are often enough to cause a woman to suspect pregnancy (Table 14.9) (Baker 2006). Diagnosis of pregnancy usually begins when a woman presents with such symptoms and possibly a positive home pregnancy test (Cunningham et al 2005).
| Sign | Time of occurrence | Differential diagnosis |
|---|---|---|
| Possible (presumptive) signs | ||
| Early breast changes (unreliable in multigravida) | 3–4 weeks + | Contraceptive pill |
| Amenorrhoea | 4 weeks + | Hormonal imbalance |
| Emotional stress | ||
| Illness | ||
| Morning sickness | 4–14 weeks | Gastrointestinal disorders |
| Pyrexial illness | ||
| Cerebral irritation, etc. | ||
| Bladder irritability | 6–12 weeks | Urinary tract infection |
| Pelvic tumour | ||
| Quickening | 16–20 weeks + | Intestinal movement, ‘wind’ |
| Probable signs | ||
| Presence of human chorionic gonadotrophin (hCG) in: | ||
| Blood | 9–10 days | Hydatidiform mole |
| Urine | 14 days | Choriocarcinoma |
| Softened isthmus (Hegar’s sign) | 6–12 weeks | |
| Blueing of vagina (Chadwick’s sign) | 8 weeks + | |
| Pulsation of fornices (Osiander’s sign) | 8 weeks + | Pelvic congestion |
| Tumours | ||
| Changes in skin pigmentation | 8 weeks + | |
| Uterine souffle | 12–16 weeks | Increased blood flow to uterus as in large uterine myomas or ovarian tumours |
| Braxton Hicks contractions | 16 weeks | |
| Ballottement of fetus | 16–28 weeks | |
| Positive signs | ||
| Visualization of gestational sac by | ||
| Transvaginal ultrasound | 4.5 weeks | |
| Transabdominal ultrasound | 5.5 weeks | |
| Visualization of heart pulsation by | ||
| Transvaginal ultrasound | 5 weeks | |
| Transabdominal ultrasound | 6 weeks | |
| Fetal heart sounds by | ||
| Doppler | 11–12 weeks | |
| Fetal stethoscope | 20 weeks + | No alternative diagnosis |
| Fetal movements | ||
| Palpable | 22 weeks + | |
| Visible | Late pregnancy | |
| Fetal parts palpated | 24 weeks + | |
| Visualization of fetus by X-ray | 16 weeks + | |
Traditionally diagnosis has been based on history and physical examination. Important aspects of the menstrual history must be obtained. Items that may confuse the diagnosis of early pregnancy are an atypical last menstrual period, contraceptive use and a history of irregular periods. As many as 25% of women bleed during their first trimester, which may further complicate the assessment (Likes & Rittenhouse 2004). The signs of pregnancy described below are mainly of historic significance and although it is possible that they may still be of value in some parts of the world, they have generally been rendered obsolete in the developed world by more modern and sophisticated methods.
Hegar’s (or Goodell’s) sign
On bimanual examination, a firm cervix is felt in contrast with the softer body and compressible, softer isthmus at about 6–8 weeks menstrual age (Cunningham et al 2005).
Chadwick’s sign
This is the dark purplish red discoloration and congestion of the vulva and vaginal mucous membranes (Cunningham et al 2005). First detected between the 4th and 8th weeks of pregnancy it reaches its maximum intensity at the 16th week and then persists throughout pregnancy (Mudaliar et al 1990).
Osiander’s sign
This is the stronger and harder vaginal pulsations caused by the greatly increased blood supply and the enlarged uterine artery. This may also occur in the non-pregnant woman due to fibroids and pelvic inflammation.
Quickening
The first fluttering movements of the fetus are felt around 20 weeks in a first pregnancy and 18 weeks in subsequent pregnancies, however many women experience fetal movements earlier than these times. The woman’s report should be recorded. Fetal movements can begin to be detected by the examiner around 20 weeks (see Table 14.9).
Most women use a home pregnancy test (HPT), which is private and easy to use. Measurement of hCG produced by the trophoblast from the day of implantation forms the basis for the standard modern pregnancy test using maternal urine although progesterone and early pregnancy factor (EPF) can also be measured (Likes & Rittenhouse 2004). hCG can be detected in maternal serum and urine 7–8 days after ovulation or around the time of implantation and can give a positive indication of pregnancy by 3 weeks after conception. Concentrations of hCG in maternal serum double every 2–3 days until peak values are reached 60–90 days after conception (Blackburn 2007). Women should be made aware that accuracy is variable and is dependent on how, when and by whom the test is used and on the brand of the test. By waiting 2 weeks after the missed period, pregnancy will be confirmed accurately in 97% of women (Symonds & Symonds 2004). Early pregnancy detection allows for the commencement of prenatal care, potential medication changes and lifestyle changes to promote a healthy pregnancy (Likes & Rittenhouse 2004).
Common disorders arising from adaptations to pregnancy
Throughout this chapter, reference has been made to the multitude of symptoms the physiological changes may produce within a woman’s body. While deemed physiological, women may experience these as unpleasant, and even distressing or debilitating. Due to their common, natural and non-pathological nature and the fact that they generally resolve spontaneously, caregivers are often guilty of a dismissive or trivializing approach towards them. Since these changes are expressions of the normal physiology of pregnancy it is both difficult and possibly ill-advised to take measures to prevent them.
The two key issues for midwives to build into their practice are:
Ambwani B. Primigravidas with floating head at term or onset of labour. Internet Journal of Gynecology and Obstetrics, 3 1, 2004. Online. Available: http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijgo/vol3n1/float.xml.
Baker P, editor. Obstetrics by ten teachers, 18th edn., London: Edward Arnold, 2006.
Becks G, Burrow G. Thyroid disorders and pregnancy. Thyroid foundation of Canada. 2000. Online. Available: http://www.thyroid.ca/Articles/ENGe11a.HTML, January 2008
Blackburn S. Maternal, fetal and neonatal physiology, 2nd edn. Philadelphia: W B Saunders, 2007.
British Thyroid Association, Association of Clinical Biochemistry, British Thyroid Foundation. UK guidelines for the use of thyroid function tests. 2006. Online. Available: http://acb.org.uk/docs/tftguidelinefinal.pdf, April 2008
Broughton-Pipkin F. Clinical physiology in obstetrics. Oxford: Blackwell Science, 2001;114-115.
Boskey E, Cone R, Whaley K, et al. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Human Reproduction. 2001;16(9):1809-1813.
Bowen R. Thyroid and parathyroid glands. Pathophysiology of the endocrine system, 1999. Online. Available: http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/thyroid/thyroid_preg.html, January 2008
Breuiller-Fouche M, Germain G. Gene and protein expression in the myometrium in pregnancy and labor. Reproduction. 2006;131:837-850.
Brosens J, Gellersen B. Death or survival – progesterone-dependent cell fate decisions in the human endometrial stroma. Journal of Molecular Endocrinology. 2006;36:389-398.
Buhimschi C, Buhimshci I, Malinow A, et al. Myometrial thickness during human labour and immediately post partum. American Journal of Obstetrics and Gynecology. 2003;188(2):553-559.
Burnett A. Clinical obstetrics and gynaecology. Oxford: Blackwell Science, 2001.
Butte N. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. American Journal of Clinical Nutrition. 2000;71(5):1256s-1261s.
Campbell S, Lees C, editors. Obstetrics by ten teachers. New York: Oxford University Press, 2000.
Carbonne B, Dallot E, Haddad B, et al. Effects of progesterone on prostaglandin E2-induced changes in glycosaminoglycan synthesis by human cervical fibroblasts in culture. Molecular Human Reproduction. 2000;6(7):661-664.
Carlson H. The pituitary gland in pregnancy and the puerperium. In: Melmed S, editor. The pituitary. Oxford: Blackwell Science, 2002.
Carvajal J, Weiner C. Mechanisms underlying myometrial quiescence during pregnancy. Fetal and Maternal Medicine Review. 2003;14(3):209-237.
Carvalho M, Bittar R, Brizot M, et al. Cervical length at 11–14 weeks’ and 22–24 weeks’ gestation evaluated by transvaginal sonography, and gestational age at delivery. Ultrasound in Obstetrics and Gynaecology. 2003;21(2):135-139.
Chamberlain G, Steer P. Turnbull’s obstetrics, 3rd edn. Edinburgh: Churchill Livingstone, 2001.
Charan M, Katz P. Gastrooesphageal reflux disease in pregnancy. Current Treatment Options in Gastroenterology. 2001;4:73-81.
Chard T, Grudzinskas J. The uterus. Cambridge: Cambridge University Press, 1994.
Chawla S, Vardhan S, Jog S. Appendicitis during pregnancy. Medical Journal of the Armed Forces India. 2003;59(3):212-215.
Childbirth Connection. Online. Available: http://www.childbirthconnection.org/article.asp?ck=10242, 2007. January 2008
Coustan D. Maternal physiology. In: Coustan D, Haning R, Singer D, editors. Human reproduction – growth and development. London: Little, Brown, 1995.
Cunningham F, MacDonald P, Gant N, et al. William’s obstetrics, 20th edn. London: Prentice Hall, 1997.
Cunningham F, Gant N, Leveno K, et al. William’s obstetrics, 21st edn. New York: McGraw-Hill, 2001.
Cunningham F, Leveno K, Bloom S, et al. William’s obstetrics, 22nd edn. New York: McGraw-Hill, 2005.
Degani S, Leibovitz Z, Shapiro I, et al. Myometrial thickness in pregnancy: longitudinal sonographic study. Journal of Ultrasound in Medicine. 1998;17:661-665.
de Sweit M. The cardiovascular system. In Chamberlain G, Broughton Pipkin F, editors: Clinical physiology in obstetrics, 3rd edn., Oxford: Blackwell Science, 1998.
de Sweit M. The respiratory system. In Chamberlain G, Broughton Pipkin F, editors: Clinical physiology in obstetrics, 3rd edn., Oxford: Blackwell Science, 1998.
de Sweit M. Medical disorders in obstetric practice, 4th edn. Oxford: Blackwell, 2002.
de Swiet M, Chamberlain G, Bennett P, editors. Basic science in obstetrics and gynaecology, 3rd edn., Edinburgh: Churchill Livingstone, 2002.
Dietze M. A Re-evaluation of the mechanism of labour for contemporary midwifery practice. Midwifery Matters, 2001;88:3-8. Online. Available: http://www.radmid.demon.co.uk/physiology.htm, July 2007
Dijkstra K, Janssen H, Kuczynski E, Lockwood C. Cervical length in uncomplicated pregnancy: A study of sociodemographic predictors of cervical changes across gestation. American Journal of Obstetrics and Gynecology. 1999;180(3):639-644.
Duffy T. Haematologic aspects of pregnancy. In Burrows GN, Duffey TP, Copel JA, editors: Medical complications in pregnancy, 6th edn., Philadelphia: Saunders, 2004.
Esmaelzadeh S, Rezael N, HajiAhmadi M. Normal uterine size in women of reproductive age in northern Islamic Republic of Iran. Eastern Mediterranean Health Journal. 2004;10(3):437-441.
Festin M. Nausea and vomiting in early pregnancy. British Medical Journal Clinical Evidence. 2006. Online. Available: www.clinicalevidence.com.
Fister P, Gaberscek S, Zaletel K, et al. Thyroid volume and intrathyroidal blood flow increase during pregnancy. Clinical Endocrinology. 2006;65:828-829.
Fitzgerald M, Graziano S. Anatomic and functional changes of the lower urinary tract during pregnancy. Urologic Clinics of North America. 2007;34:7-12.
Fraser DM, Cooper MA. Survival Guide to Midwifery. Edinburgh: Churchill Livingstone Elsevier, 2008.
Fusi L, Cloke B, Brosens J. The uterine junctional zone. Best Practice and Research Clinical Obstetrics and Gynaecology. 2006;20(4):479-491.
Gabbe S, Niebyl J, Simpson J, et al, editors. Obstetrics: normal and problem pregnancies, 4th edn., Edinburgh: Churchill Livingstone, 2004.
Garfield R, Saade G, Buhimschi C, et al. Control and assessment of the uterus and cervix during pregnancy and labour. Human Reproduction Update. 1998;4(5):673-695.
Garfield R, Maner W. Physiology and electrical activity of uterine contractions. Seminars in Cell and Developmental Biology. 2007;18:289-295.
Garner P. Adrenal disorders of pregnancy. 2002. Ch. 2B. Online. Available: http://www.endotext.com/pregnancy/pregnancy2/pregnancy2b.htm, January 2008
Gee H. Routine intrapartum care: an overview. In: Luesley D, Baker P, editors. Obstetrics and gynaecology – an evidence-based text for MRCOG. London: Arnold, 2004.
Gee H. Poor progress in labour. In: James D, Steer P, Weiner C, et al, editors. High risk pregnancy. Philadelphia: Elsevier, 2006.
Ginesi L, Niescierowicz R. Neuroendocrinology and birth 1: stress. British Journal of Midwifery. 1998;6(10):659-663.
Girling J. Physiology of pregnancy. Obstetrics, anaesthesia and intensive care medicine, Medicine Publishing Company, Abingdon, 2001;167-170. Online. Available: www.medicinepublishing.com/girl_1-4.
Girling J. Physiology of pregnancy. Anaesthesia and Intensive Care Medicine. 2004;5(7):215-218.
Hanretty K. Obstetrics illustrated, 6th edn. Edinburgh: Churchill Livingstone, 2003.
Haram K, Mortensen J, Wollen A. Preterm delivery: an overview. Acta Obstetricia et Gynecologica Scandinavica. 2003;82:687-704.
Hedman C, Pohjasvaara T, Tolonen U, et al. Effects of pregnancy on mothers’ sleep. Sleep Medicine. 2002;1:37-42.
Heffner L, Schust D. The reproductive system at a glance, 2nd edn. Oxford: Blackwell, 2006.
Hempstock J, Cindrova-Davies T, Jauniaux E, et al. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: A morphological and immunohistochemical study. Reproductive Biology and Endocrinology. 2004;2:58.
Honda T, Hasegawa M, Nakahori T, et al. Perinatal management of cervicoisthmic pregnancy. Journal of Obstetrics and Gynaecology Research. 2005;31(4):332-336.
Hong J, Park J, Jong I. Comparison of preoperative gastric contents and serum gastrin concentrations in pregnant and nonpregnant women. Journal of Clinical Anesthesia. 2005;17(6):451-455.
Hooton T, Stamm W. Urinary tract infections and asymptomatic bacteriuria. 2007. UpToDate. Online. Available: http://patients.uptodate.com/print.asp?print=true&file=uti_infe/7516, January 2008
Jensen D, Wolfe L, Slatkovska L, et al. Effects of human pregnancy on the ventilatory chemoreflex response to carbon dioxide. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2005;288:R1369-R1375.
Jeyabalan A, Conrad K. Renal function during normal pregnancy and preeclampsia. Frontiers in Bioscience. 2007;12:2425-2437.
Jeyabalan A, Lain K. Anatomic and functional changes of the upper urinary tract during pregnancy. Urologic Clinics of North America. 2007;34:1-6.
Jones R, Findlay J, Salamonsen L. The role of activins during decidualization of human endometrium. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2006;46:245-249.
Kalwarf H, Specker B. Bone mineral changes during pregnancy and lactation. Endocrine. 2002;17(1):49-53.
Katsambas AD, Stratigos AJ. Depigmenting and bleaching agents: coping with hyperpigmentation. Clinical Dermatology. 2001;19:483-488.
Kliman H. Uteroplacental blood flow. The story of decidualization, menstruation and trophoblast invasion. American Journal of Pathology. 2000;157(6):1759-1768.
LaMar K, Hamernik C. Life inside the womb: implications for newborn and infant nurses. Newborn & Infant Nursing Reviews. 2003;3(4):136-142.
Lee KA, Gay G. Sleep in late pregnancy predicts length of labor and type of delivery. American Journal of Obstetrics and Gynecology. 2004;191(6):2041-2046.
Lesny P, Killik S. The junctional zone of the uterus and its contractions. British Journal of Obsetrics and Gynaecology. 2004;111:1182-1189.
Letsky E. The haematological system. In: Chamberlain G, Broughton Pipkin F, editors. Clinical physiology in obstetrics. Oxford: Blackwell Science, 1998.
Likes R, Rittenhouse E. Pregnancy diagnosis. 2004. Online. Available: http://www.emedicine.com/med/topic3277.htm, January 2008
Llewellyn-Jones D. Fundamentals of obstetrics and gynaecology, 7th edn. London: Mosby, 1999.
Lowdermilk D, Perry S. Maternity and Women’s Health Care, 8th edn. Missouri: Mosby, 2004.
Maltby J. Pre-operative fasting guidelines, 2000, 2. Issue 12, article 2, Online. Available: www.nda.ox.ac.uk/wfsa/html/u12/u1202_02.htm#dela, January 2008
Margulies R, Miller L. Fruit size as a model for teaching first trimester uterine sizing in bimanual examination. American Journal of Obstetricians and Gynecologists. 2001;98(2):341-344.
Marnach ML, Ramin KD, Ramsey PS, et al. Characterization of the relationship between joint laxity and maternal hormones in pregnancy. Obstetrics and Gynecology. 2003;101(2):331-335.
Martin W, Hutchon S. Mechanism and management of normal labour. Current Obstetrics and Gynaecology. 2004;14:301-308.
Metaxa-Mariatou V, McGavigan C, Robertson K, et al. Elastin distribution in the myometrial and vascular and smooth muscle of the human uterus. Molecular Human Reproduction. 2002;8(6):559-565.
McFadyen I. Maternal physiology in pregnancy. In Chamberlain G, editor: Turnbull’s obstetrics, 2nd edn., Edinburgh: Churchill Livingstone, 1995. Ch. 7
McKinley M, Johnson A. The physiological regulation of thirst and fluid intake. News in Physiological Sciences. 2004;19:1-6.
Mills M. Craving more than food – the implications of pica in pregnancy. Nursing for Women’s Health. 2007;11(3):267-273.
Moore K, Persaud T. The developing human, 7th edn. Saunders, Philadelphia, 2003:47. 120
Muallem M, Rubeiz N. Physiological and biological skin changes in pregnancy. Clinics in Dermatology. 2006;24(2):80-83.
Mudaliar A, Menon M, Palaniappan B. Mudaliar and Menon’s clinical obstetrics, 9th edn. Orient Longman, Anna Salai, Madras, India, 1990;49. Online. Available: http://books.google.com/books?id=Iipd0GIpw4cC&pg=PA49&lpg=PA49&dq=osiander’s+sign&source=web&ots=KDgr7YVxpw&sig=W6wSqWMecKTtltudd1TADSnocwA, January 2008
Nelson-Piercy C. Renal disease. In: Luesley D, Baker P, editors. Obstetrics and gynaecology – An evidence-based text for MRCOG. London: Arnold, 2004.
Nelson-Piercy C. Handbook of obstetric medicine. Oxford: Taylor and Francis, 2006.
NICE. Antenatal care: routine care for the healthy pregnant woman. London: RCOG Press, 2008.
NICE. Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. Clinical guideline 63. RCOG Press, London, 2008.
O’Grady J, Pope C. Malposition of the uterus. 2006. Online. Available: http://emedicine.com/med/topic3473.htm, January 2008
O’Sullivan G, Scrutton M. NPO during labour. Is there any scientific validation? Anesthesiology clinics of North America. 2003;21(1):87-98.
Oto A, Srinivasan P, Ernst R, et al. Revisiting MRI for appendix location during pregnancy. AJR Women’s Imaging. 2006;186:883-887.
Patel M, Nuthalapaty F, Ramsey P, et al. A neglected risk factor for preterm birth. Obstetrics and Gynecology. 2004;103:68S.
Paulev P. Essentials and clinical problems, Section vii: Sexual Satisfaction, Reproduction and Disorders. Endocrine glands in humans. Paulev P, editor. Textbook in medical physiology and pathophysiology, Ch. 29, Copenhagen Medical Publishers, Copenhagen, 1999. Online. Available: http://www.mfi.ku.dk/ppaulev/chapter29/kap29.htm, January 2008
Pennick VE, Young G. Interventions for preventing and treating pelvic and back pain in pregnancy (Cochrane Review). The Cochrane Database of Systematic Reviews. (Issue 2):2007.
Rayner C, Micell G. Dealing with gastro-oesophageal reflux disease during pregnancy. European gastroenterology review 2005. 2005. Online. Available: http://www.touchgastroenterology.com/dealing-with-gastro-oesophageal-a1351-1.html, January 2008
Rehman K, Yin S, Mayhew B, et al. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Molecular Human Reproduction. 2003;9(11):681-700.
Ross M, Ervin M, Novak D. Placental and fetal physiology. In Gabbe S, Neibyl J, Simpson J, editors: Obstetric – normal and problem pregnancies, 4th edn., Edinburgh: Churchill Livingstone, 2002.
Rudra A. Airway management in obstetrics. Indian Journal of Anaesthesia. 2005;49(4):328-335.
Russell J, Douglas A, Windle R. The maternal brain: neurobiological and neuroendocrine adaptation and disorders in pregnancy & post partum. Edinburgh: Elsevier Science, 2001.
Santiago J, Nolledo M, Kinzler W, et al. Sleep and sleep disorders in pregnancy. Annals of Internal Medicine. 2001;134(5):396.
Shennan A. Thyroid disease. In: Luesley D, Baker P, editors. Obstetrics and Gynaecology – An evidence-based text for MRCOG. London: Arnold; 2004:59.
Steinfeld J, Wax J. Maternal physiologic adaptations to pregnancy. In: Seifer D, Samuels P, Kniss D, editors. The physiologic basis of gynecology and obstetrics. London: Lippincott, Williams and Wilkins, 2001.
Suresh L, Radfar L. Medical management update – pregnancy and lactation. Oral Surgery, Oral Medicine, Oral Pathology. 2004;97(6):672. 668
Symonds E, Symonds I. Essential obstetrics and gynaecology, 4th edn. Edinburgh: Churchill Livingstone, 2004.
Szekeres-Bartho J. Progesterone dependent immunomodulation. Chemical Immunology and Allergy. 2001;89:118.
Torgersen KL, Curran CA. A systematic approach to the physiologic adaptations of pregnancy. Critical Care Nursing Quarterly. 2006;29:2-19.
Ticconi C, Belmonte A, Piccione E, et al. Feto-placental communication system with the myometrium in pregnancy and parturition: The role of hormones, neurohormones, inflammatory mediators, and locally active factors. Journal of Maternal-Fetal and Neonatal Medicine. 2006;19(3):125-133.
Utz A. Physiologic cortisol dynamics and Cushing’s syndrome in pregnancy. The Neuroendocrine Clinical Center Bulletin. 11(2), 2005.
Van Mook W, Peeters L. Severe cardiac disease in pregnancy, Part II: impact of congenital and acquired cardiac diseases during pregnancy. Current Opinion in Critical Care. 2005;11(5):435-448.
Varga I, Rigó J, Somos P, et al. Analysis of maternal circulation and renal function in physiologic pregnancies: Parallel examinations of the changes in the cardiac output and the glomerular filtration rate. Journal of Maternal Fetal Medicine. 2000;9(2):97-104.
Wallace W. Endocrine Function. In Montague S, Watson R, Hubert R, editors: Physiology for Nursing Practice, 3rd edn., Elsevier, 2005.
Wang SM, De Zinno P, Fermo L. Complementary and alternative medicine for low-back pain in pregnancy: a cross-sectional survey. Journal of Alternative and Complementary Medicine. 2005;11(3):459-464.
Weissgerber T, Wolfe L. Physiological adaptation in early human pregnancy: adaptation to balance maternal-fetal demands Applied Physiology. Nutrition and Metabolism. 2006;31:1-11.
Whitcome K, Shapiro L, Lieberman D. Fetal load and the evolution of lumbar lordosis in bipedal hominis. Nature. 2007;450:1075-1078.
Whitmore I, Willan P, Gosling J, et al. Human anatomy: colour atlas and text, 4th edn. Edinburgh: Mosby, 2002.
Williams D. Renal disease in pregnancy. Current Obstetrics and Gynaecology. 2004;14:166-174.
Williamson C. Nutrition in pregnancy. Briefing Paper. British Nutrition Foundation Nutrition Bulletin. 2006;31:28-59.
Wing D. Urinary tract infection in pregnancy: from asymptomatic bacteriuria to pyelonephritis. In Mishell D, Goodwin T, Brenner P, editors: Management of common problems in obstetrics and gynecology, 4th edn., Oxford: Blackwell, 2002.
Young R, Hession R. Three-dimensional structure of the smooth muscle in the term-pregnant human uterus. Obstetrics and Gynecology. 1999;93(1):94-99.
Young G, Jewell D. Antihistamines versus aspirin for itching in late pregnancy (Cochrane review). The Cochrane Library. Update Software Oxford, 2002. Issue 1. Online. Available: www.update-software.com, accessed 23 October 2007
Young R. Myocytes, myometrium, and uterine contractions. Annals of New York Academy of Sciences. 2007;1101:72-84.