Chapter 29 Physiology and management of the third stage of labour
Postpartum haemorrhage (PPH) is still ranked among the top three major causes of maternal death globally (WHO 2007). Although the majority (99%) of deaths reported occur in developing countries, the risk of PPH should not be underestimated for any birth, nor should the potential for the third stage of labour to be the most dangerous stage of labour be underestimated (McDonald et al 2004, WHO 2007). Maternal mortality rates in high resource countries are relatively low when compared to low resource countries, however, maternal morbidity is similar in significance. During this stage, the mother’s focus and sense of emotional and physical relief often spontaneously shift from the concentrated exertions of the actual birth to that of exploration and familiarization with her newborn baby. To facilitate a safe and healthy outcome for the mother and her baby, antenatal health as well as intrapartum preparation and postnatal skill, diligence and expertise of the midwife are crucial factors. Research evidence is clearer for some aspects of third stage management than others.
Physiological processes
The third stage is defined as the period from the birth of the baby to complete expulsion of the placenta and membranes, involving the separation, descent and expulsion of the placenta and membranes and control of haemorrhage from the placenta site (Johnson & Taylor 2000). It is an understanding of what has already occurred during pregnancy and labour and the changes that take place that guides the midwife’s practice. During the third stage, separation and expulsion of the placenta and membranes occur as the result of mechanical and haemostatic factors. The time at which the placenta actually separates from the uterine wall varies. It may shear off during the final expulsive contractions accompanying the birth of the baby or remain adherent for some considerable time. The third stage usually lasts between 5 and 15 min, but any period up to 1 hr may be considered to be within normal limits.
Separation and descent of the placenta
Mechanical factors
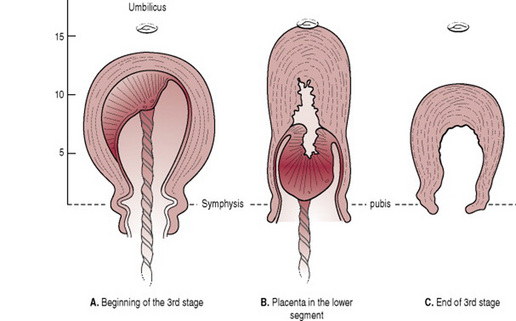
The unique characteristic of uterine muscle lies in its power of retraction. During the second stage of labour, the uterine cavity progressively empties, enabling the retraction process to accelerate. Thus, by the beginning of the third stage, the placental site has already diminished in area by about 75% (Baldock & Dixon 2006). As this occurs, the placenta becomes compressed and the blood in the intervillous spaces is forced back into the spongy layer of the decidua basalis. Retraction of the oblique uterine muscle fibres exerts pressure on the blood vessels so that blood does not drain back into the maternal system. The vessels during this process become tense and congested. With the next contraction the distended veins burst and a small amount of blood seeps in between the thin septa of the spongy layer and the placental surface, stripping it from its attachment (Fig. 29.1). As the surface area for placental attachment reduces, the relatively non-elastic placenta begins to detach from the uterine wall.
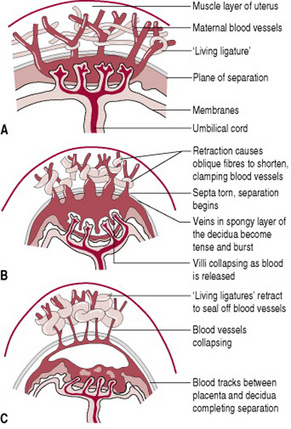
Figure 29.1 The placental site during separation. (A) Uterus and placenta before separation. (B) Separation begins. (C) Separation is almost complete.
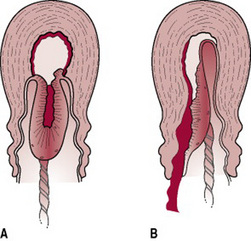
Separation usually begins centrally so that a retroplacental clot is formed (Fig. 29.2). This further aids separation by exerting pressure at the midpoint of placental attachment so that the increased weight helps to strip the adherent lateral borders and peel the membranes off the uterine wall so that the clot thus formed becomes enclosed in a membranous bag as the placenta descends, fetal surface first. This process of separation (first described by Schultze) is associated with more complete shearing of both placenta and membranes and less fluid blood loss (Fig. 29.3A). Alternatively, the placenta may begin to detach unevenly at one of its lateral borders. The blood escapes so that separation is unaided by the formation of a retroplacental clot. The placenta descends, slipping sideways, maternal surface first. This process (first described by Matthews Duncan in the nineteenth century) takes longer and is associated with ragged, incomplete expulsion of the membranes and a higher fluid blood loss (Fig. 29.3B).
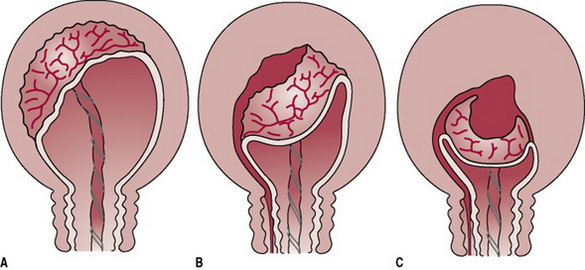
Figure 29.2 The mechanism of placental separation. (A) Uterine wall is partially retracted, but not sufficiently to cause placental separation. (B) Further contraction and retraction thicken the uterine wall, reduce the placental site and aid placental separation. (C) Complete separation and formation of the retroplacental clot. Note: The thin lower segment has collapsed like a concertina following the birth of the baby.
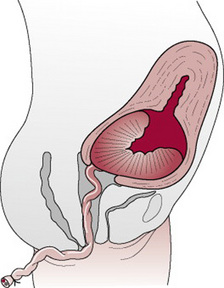
Once separation has occurred, the uterus contracts strongly, forcing placenta and membranes to fall into the lower uterine segment (Fig. 29.4) and finally into the vagina.
Haemostasis
The normal volume of blood flow through the placental site is 500–800 mL/min. At placental separation, this has to be arrested within seconds, as otherwise serious haemorrhage will occur. The interplay of three factors within the normal physiological processes that control bleeding are critical in minimizing blood loss and the serious sequelae of maternal morbidity or mortality, or both, which may result. They are:
Management of the third stage
The midwife’s care of the mother should be based on an understanding of the normal physiological processes at work, including having access to as much information as possible about the woman’s pregnancy and labour history. Progress of the first and second stages of labour are likely to impact on management of the third stage of labour and should not be reviewed in isolation. The midwife’s actions can help to reduce the very real risks of haemorrhage, infection, retained placenta and shock, any of which may increase maternal morbidity and even result in death. A mother’s ability to withstand these complications depends, to a large degree, upon her general health and the avoidance of debilitating, predisposing problems, such as anaemia, ketosis, exhaustion and prolonged hypotonic uterine action. Factors that may influence the risk of haemorrhage are discussed in more detail later.
Uterotonics or uterotonic agents
These are drugs (e.g. Syntometrine, Syntocinon, ergometrine and prostaglandins) that stimulate the smooth muscle of the uterus to contract. They may be administered with crowning of the baby’s head, at the time of birth of the anterior shoulder of the baby, after the birth of the baby but prior to placental delivery or following the delivery of the placenta.
Whether women should routinely receive uterotonic drugs, have the umbilical cord clamped or be given assistance with placental delivery has been the subject of a great deal of debate and many research trials. What is of primary importance is that the health professional (whether a midwife, GP or obstetrician) providing clinical care and advice should ensure that, in order to facilitate decision-making by the woman, adequate time for deliberation and questions should be made available, where possible, during the course of her routine antenatal consultations. Information related to the best available research information on the use of uterotonic drugs during the third stage of labour should be offered in an objective manner, which could perhaps be supported with pamphlets that cover topics such as possible management options for the woman in the setting in which she intends to birth, types of uterotonics, explanation of their different applications, risks and benefits, route of administration and timing and method of placental delivery.
Active management
This is a policy whereby prophylactic administration of a uterotonic, as a precautionary measure aimed at reduction in the risk for postpartum haemorrhage, is applied regardless of the assessed obstetric risk status of the woman. An active management policy usually includes the routine administration of a uterotonic agent, either intravenously, intramuscularly or even orally. This is undertaken in conjunction with clamping of the umbilical cord shortly after birth of the baby and delivery of the placenta by the use of controlled cord traction. In situations where women may also be assessed as being at higher risk for PPH (e.g. multiple birth, grande multiparity), a prophylactic infusion of larger doses of uterotonics diluted in intravenous solutions may be administered over several hours following the birth. This would also be considered to be part of an active management policy. Active management in the third stage is the policy of third stage labour management most widely practised throughout the developed world. Like all interventions performed, skill in assisting the delivery of the placenta and membranes is extremely important.
Expectant or physiological management
In expectant management, routine administration of a uterotonic drug is withheld, the umbilical cord is left unclamped until cord pulsation has ceased or the mother requests it to be clamped, or both, and the placenta is expelled by use of gravity and maternal effort. With this approach, therapeutic uterotonic administration would be administered either to stop bleeding once it has occurred or to maintain the uterus in a contracted state when there are indications that excessive bleeding is likely to occur. Emergency use usually indicates an event of uncontrolled haemorrhage. In this situation, it is important for the midwife to be aware of whether, what and how much of a uterotonic agent has already been administered.
No matter what an individual midwife’s personal practice experience may be or what the best available research evidence recommends, it is still ultimately the woman’s decision as to how she would ideally wish her pregnancy and birth plan to be followed. There may be philosophical, religious or cultural beliefs that influence her decision.
However, it is also fair to suggest that the midwife also has rights and responsibilities. Detailed, accurate, written (contemporaneous wherever possible) documentation is extremely important in all aspects of care, particularly in areas where evidence-based information is relied upon to assess whether due care has been delivered. In the case of third stage management, an example might be: in the circumstance where a woman specifically requests that uterotonic drugs be withheld from routine use in her third stage care, the midwife should clarify the circumstances in which this decision may be reversed. If a uterotonic drug is not to be used, the woman’s preference for care must be recorded in her notes antenatally. A record of the discussion may be signed by the woman. It would be prudent for the midwife to notify her clinical manager or the attending medical practitioner of such a request if it is contrary to local guidelines.
In practice, one of the following uterotonic drugs is usually used.
Intravenous ergometrine 0.25 mg
This drug acts within 45 s; therefore it is particularly useful in securing a rapid contraction where hypotonic uterine action results in haemorrhage. If a doctor is not present in such an emergency, a midwife may give the injection. In an overview of the choice of uterotonics for use in the third stage, Prendiville et al (1988a) found that there was no supportive evidence for the continued routine use of intravenous ergometrine, which is associated with an increased risk of retained placenta and this drug is more often used to treat a PPH rather than as a prophylactic drug. If an intravenous cannula is not already in situ, any difficulty encountered in locating a vein or sudden movement by the woman may result in failed venepuncture or at least a delay in administration.
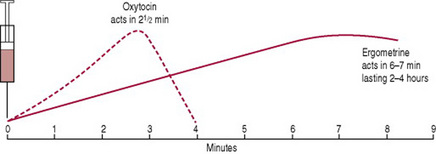
Combined ergometrine and oxytocin (a commonly used brand is Syntometrine)
A 1 mL ampoule contains 5 IU of oxytocin and 0.5 mg ergometrine and is administered by i.m. injection. The oxytocin acts within 2½ min, and the ergometrine within 6–7 min (Fig. 29.5). Their combined action results in a rapid uterine contraction enhanced by a stronger, more sustained contraction lasting several hours. It is usually administered as the anterior shoulder of the baby is born, thus stimulating good uterine action at the beginning of the third stage. The use of combined ergometrine/oxytocin or any ergometrine-based drug is associated with side-effects such as elevation of the blood pressure and vomiting (McDonald et al 2004).
Oxytocin (a commonly used brand is Syntocinon)
Oxytocin is a synthetic form of the natural oxytocin produced in the posterior pituitary, and is safe to use in a wider context than combined ergometrine/oxytocin agents. It can be administered as an intravenous and or intramuscular injection. However, an intravenous bolus of oxytocin can cause profound, fatal hypotension, especially in the presence of cardiovascular compromise. The recommendation of the CEMD (Lewis & Drife 2001, p 21) is that ‘when given as an intravenous bolus the drug should be given slowly in a dose of not more than 5 IU’.
Research evidence to date suggests that this is an effective uterotonic choice where routine prophylactic management of the third stage of labour is practised (Choy et al 2002, Khan et al 1995, McDonald et al 1993), more specifically in women who experience a blood loss exceeding 1000 mL. There still, however, does not appear to be an absolute recommendation for practice with regard to choice of uterotonic. This is perhaps partly due to the varied circumstances in which women seek care and clinicians work. It is also perhaps indicative of the need to encompass all the factors that surround the management of labour itself, including induction, use of uterotonics in labour, use of epidurals and tolerance of longer second stages.
Carbetocin originally developed for veterinary use and not widely used for prophylactic use in management of the third stage, is a long-acting synthetic oxytocin analogue which can be administered as a single dose 100 μg injection. This drug has been compared favourably with other injectable oxytocics, such as Syntometrine (oxytocin-ergometrine) and Syntocinon (oxytocin alone) for prevention of PPH (Chaparro et al. 2006, Prendiville & Elbourne 1989, WHO 1990). However, carbetocin still requires refrigeration for stability, so while it provides an alternative uterotonic, there is insufficient evidence as yet regarding its benefit over the drugs that are currently available.
Prostaglandins
The use of prostaglandins for third stage management has up until now been more often associated with the treatment of postpartum haemorrhage than with prophylaxis. This may be partly due to prostaglandin agents being more expensive and associated with side-effects, such as diarrhoea (Chua et al 1995) and cardiovascular complications of increased stroke volume and heart rate (van Selm et al 1995). Prostaglandin administration is most effective when used intramurally (injected directly into the uterine wall) or by intrauterine irrigation (Peyser & Kupfermine 1990). The procedures are time consuming and invasive and the expertise required for undertaking the procedures is unlikely to always be readily available in routine labour management.
In more recent years, a great deal of research time and investment has been invested in seeking alternate ways of implementing strategies to reduce the risk of PPH. Misoprostol (a prostaglandin E1 analogue) was first used to treat gastric ulcers, but when its potential as a uterotonic agent was discovered, optimism regarding its suitability in low resource settings was high. It is cheap, not prone to loss of potency, does not need to be sterile or refrigerated and can be administered vaginally, orally or rectally negating the need for syringes. The Cochrane Library contains several reviews evaluating route and dose of misoprostol compared with either placebo or another uterotonic agent. The largest and most comprehensive trial was conducted by Gülmezoglu et al 2004. The primary endpoint of the study was the number of women reported as having had a PPH >1000 mL. Considering many of the intended settings, which included low resource communities, this seemed unusual as many women in these settings would already be chronically anaemic and would very likely suffer considerable morbidity or even death at a much lower threshold. The difference in the incidence of PPH between the women given 600 mg of misoprostol orally and the women who received other uterotonic agents was 3.6% versus 2.7%. This translated to a >20% difference, which was the tolerance level chosen beyond which it was deemed misoprostol was not as effective. Misoprostol was also found to have unpleasant side-effects, such as severe shivering and higher temperature, both of which were transient but unacceptable to some women. Its use appeared to be no more likely to necessitate manual removal of the placenta. Even though the recommendation was that misoprostol should not replace other uterotonics in settings where they are available, the authors suggest that it may be useful in circumstances where nothing else is available. The transient side-effects associated with misoprostol may not be any more debilitating than the nausea, vomiting (Ng et al 2007) and hypertensive episodes experienced by some women receiving Syntometrine, which remains the most commonly prescribed and administered uterotonic globally.
Clamping of the umbilical cord
This may have been carried out during birth of the baby if the cord was tightly around the neck. However, opinions vary as to the most beneficial time for clamping the cord during the third stage of labour.
Early clamping is normally applied in the first 1–3 min immediately after birth, regardless of whether the cord pulsation has ceased. It has been suggested that this practice may have the following effects:
Proponents of late clamping suggest that no action be taken until cord pulsation ceases or the placenta has been completely delivered, thus allowing the physiological processes to take place without intervention. Postulated advantages of late clamping include:
There is very little evidence concerning how much, if any, of a uterotonic agent the baby receives following birth. In five documented cases of accidental administration of an adult dose of Syntometrine to a newborn infant, no long-term adverse effects were reported (Whitfield & Salfield 1980).
Is the timing of uterotonic administration and cord clamping clinically important in influencing the incidence of PPH?
Background
At the time of birth, the baby is still attached to the mother via the umbilical cord, which is part of the placenta. When the third stage or placental delivery stage is managed actively, an injection of an oxytocic drug is given to the mother at about the same time as the baby’s shoulders are born and the umbilical cord is clamped twice. One clamp is placed closer to the baby’s navel end. Care should be taken to apply the clamp to the cord end nearer the baby, 3–4 cm clear of the abdominal wall, to avoid pinching the skin or clamping a portion of gut, which, in rare instances, may be in the cord. A greater length of cord is left when umbilical vessels are needed for transfusion, for example in pre-term babies and cases of Rhesus haemolytic disease, and the second clamp is placed closer to the placental end of the cord. The cord between the two clamps is then cut. At this time, the baby may be placed on the mother’s abdomen, put to the breast or be more closely examined on a warmed cot if resuscitation is required. Once the placenta is felt to have separated from the wall of the uterus, downward traction may be applied to the remaining length of umbilical cord to assist delivery of the placenta.
Timing of cord clamping is also supposedly routine, but, in practice it varies greatly. Early cord clamping, which is usually part of active management, is in general regarded as clamping of the umbilical cord within 30s of the birth of the baby. Late cord clamping, a physiological approach, involves clamping of the umbilical cord when cord pulsation has ceased. However, definitions of what constitutes early and late cord clamping vary (Prendiville & Elbourne 1989) and again, in practice, unavoidable factors (e.g. if the cord is around the neck, the number of clinicians in the room, the need for active resuscitation of the infant) can make it difficult to adhere to a particular policy (McDonald 1996). There is no published evidence that this delay is of consequence in term infants (Chaparro et al 2006, McDonald 1996, Mercer 2006, Rabe et al 2004).
Investigations have been undertaken into the advantages and disadvantages of maternal–fetal transfusion and the effect of early or late cord clamping in relation to respiratory distress in the preterm infant (Dunn 1966, Inch 1985, Linderkamp 1982). There is a considerable amount of literature published on timing of cord clamping and associated placental transfusion. Debate continues over the effect of the extra 90–100 mL of blood received by the baby when late cord clamping is practised (Mercer 2006). Recent evidence suggests that the effects of early versus late cord clamping may be different for pre-term and term infants (Rabe et al 2004). Timing of cord clamping appears to be less of an issue in term infants, probably because the normal physiological process of transfer is completed within the first 1–2 min of birth for the majority of these infants (McDonald 1996).
Although active management leads to reduced risk of PPH, it is important to establish which of the components of this package lead to this reduced risk. Given the difficulties of adhering to an active management policy and the preferences of some women for physiological management, it is important to explore practice behaviours to clarify whether it is necessary to continue to promote the policy as it currently stands.
In light of the above information, it may be that whereas there is an obvious advantage to the prophylactic administration of a uterotonic drug, future third stage management policies may be less prescriptive about the necessity to clamp and cut the cord immediately following the birth.
Delivery of the placenta and membranes
Controlled cord traction (CCT). This manoeuvre is believed to reduce blood loss, shorten the third stage of labour and therefore minimize the time during which the mother is at risk from haemorrhage. It is designed to enhance the normal physiological process. Successful results depend upon understanding the principles of placental separation described at the beginning of this chapter.
If CCT is to be used, there are several checks to be made before proceeding:
It is important not to manipulate the uterus in any way as this may precipitate incoordinate action. No further step should be taken until a strong contraction is palpable. If tension is applied to the umbilical cord without this contraction, uterine inversion may occur. This is an acute obstetric emergency with life-threatening implications for the mother (see Ch. 33 Lewis 2007).
When CCT is the preferred method of management, the following sequence of actions is usually undertaken.
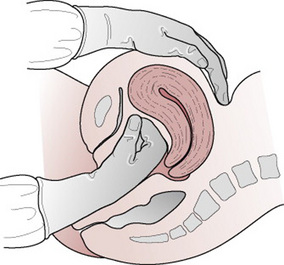
Once the uterus is found on palpation to be contracted, one hand is placed above the level of the symphysis pubis with the palm facing towards the umbilicus exerting pressure in an upwards direction. This is counter-traction. The other hand, firmly grasping the cord, applies traction in a downward and backward direction following the line of the birth canal (Fig. 29.7). Some resistance may be felt but it is important to apply steady tension by pulling the cord firmly and maintaining the pressure. Jerky movements and force should be avoided. The aim is to complete the action as one continuous, smooth, controlled movement. However, it is only possible to exert this tension for a short time as it may be an uncomfortable procedure for the mother and the midwife’s hand will tire.
Downward traction on the cord must be released before uterine counter-traction is relaxed as sudden withdrawal of counter-traction while tension is still being applied to the cord may also facilitate uterine inversion. If the manoeuvre is not immediately successful there should be a pause before the uterine contraction is again checked and a further attempt is made. Should the uterus relax, tension is temporarily released until a good contraction is again palpable. Once the placenta is visible it may be cupped in the hands to ease pressure on the friable membranes. A gentle upward and downward movement or twisting action will help to coax out the membranes and increase the chances of delivering them intact. Artery forceps may be applied to gradually ease the membranes out of the vagina. This process should not be hurried; great care should be taken to avoid tearing the membranes.
Expectant management
This management policy allows the physiological changes within the uterus that occur at the time of birth to take their natural course with minimal intervention. The processes of placental separation and expulsion are quite distinct from one another and the signs of separation and descent must be evident before maternal effort can be used to expedite expulsion. If the mother is sitting or squatting at this stage, gravity will aid expulsion.
If good uterine contractions are sustained, maternal effort will usually bring about expulsion. The mother simply pushes as during the second stage of labour. Encouragement is important, as by now she may be exhausted and the contractions will feel weaker and less expulsive than those during the second stage of labour. Providing that fresh blood loss is not excessive, the mother’s condition remains stable and her pulse rate normal, there need be no anxiety. This spontaneous process can take from 20 min to 1 hr to complete. It is important that the midwife monitors uterine action by placing a hand lightly on the fundus. She can thus palpate the contraction while checking that relaxation does not result in the uterus filling with blood. Vigilance is crucial as it should be remembered that the longer the placenta remains undelivered, the greater is the risk of bleeding because the uterus cannot contract down fully while the bulk of the placenta is in situ. Dombrowski et al (1995) found that the frequency of haemorrhage increased between 10 min and 40 min after the birth of the baby. Patience and confidence are required on the part of the midwife to secure a successful conclusion. A uterotonic agent is usually not administered unless uterine tone is poor.
Early attachment of the baby to the breast may enhance these physiological changes by stimulating the reflex release of oxytocin from the posterior lobe of the pituitary gland, which helps to secure good uterine action.
Evidence for active versus expectant management
There is an increasing amount of appropriate, rigorously conducted research evidence available that strongly suggests that the prophylactic administration of a uterotonic significantly reduces the risk of PPH, results in a lower mean blood loss, fewer blood transfusions are required and there is a reduced need for therapeutic uterotonics (Begley 1990, Khan et al 1997, Prendiville et al 1988a, Rogers et al 1998, Thilaganathan et al 1993). It has also been highlighted by the wide range of ‘risk status’ of women included in several studies that it is in fact very difficult to define a group of women who are not at risk for PPH. Taking all the best available evidence into consideration, a systematic review of the literature by Prendiville et al (2002) recommended that all women who birth in circumstances where this option is available should be encouraged to do so. Although the evidence is strongly in favour of prophylactic administration of a uterotonic, there are other aspects of active versus expectant management that may be worth exploring, e.g. prophylactic uterotonic (that is the uterotonic is given as soon as is practicable following the birth of the baby, 2–3 min) administration alone versus active management.
The FIGO/ICM Joint Statement released in November 2006, supports the use of active management by all skilled birth attendants regardless of the setting in which they practise and supplies clear guidelines related to alternative uterotonics and management strategies to be used in the absence of uterotonic drugs.
Not all research has a clearly defined outcome (Sandall & McCandlish 2006). Discussion and practice of management of the third stage of labour also need to take into account that the ‘package’ of care, whether active or expectant is reliant on the other components of the package being carried out as prescribed. For example, if management is expectant, then the introduction of an oxytocic, cord clamping or pulling on the cord will disrupt the intended sequence of the care process leading to what is often described as a fragmented approach. Once the sequence of the processes is altered, the clinician should commit to completing the process. That is, if the protocol for expectant management is interrupted the clinician should proceed to completing the process with an active management approach. This practice has been shown to significantly reduce the incidence of PPH in a birth centre setting (Patterson 2005).
Position of the woman
The effect of the position adopted by the woman at the time of placental delivery is still largely unclear. It may vary according to the mother’s personal preference, the normality of progress and the experience and confidence of the attendant midwife, and may be influenced by the need for the midwife to monitor closely such factors as uterine contraction and blood loss.
Adoption of a dorsal position allows easy palpation of the uterine fundus. However, blood is more likely to pool in the uterus and vagina, thus disguising the true blood loss. Upright, kneeling and all-fours positions may enhance the effect of gravity and increase intra-abdominal pressure, which may in turn hasten the placental delivery process. Palpation of the uterus and/ or use of traction on the umbilical cord are contraindicated in this situation, as there is evidence that these manoeuvres elevate the risk for increased bleeding and retention of the placenta (Sinclair 2004). Blood loss can be more easily observed as fluids will drain out of the vagina. The squatting position has been reported to increase visible blood loss (Gupta & Nikodem 2002). Whichever position is adopted, the use of aids such as wedges, pillows and physical support from her partner will help to ensure the woman’s comfort while completion of the third stage is being accomplished. Some women feel cold and shivery at this time, especially if labour has progressed rapidly or has been long and exhausting. This is usually transient and not abnormal.
Asepsis
The need for asepsis is even greater now than in the preceding stages of labour. Laceration and bruising of the cervix, vagina, perineum and vulva provide a route for the entry of micro-organisms. At the placental site, a raw surface provides an ideal medium for infection. Strict attention to the prevention of infection is therefore vital.
Cord blood sampling
This may be required for a variety of conditions:
The sample should be taken as soon as possible from the fetal surface of the placenta where the blood vessels are congested and easily visible. If the cord has not been clamped prior to placental delivery the fetal vessels will not be congested, but a sample of sufficient volume may still be easily obtained. The appropriate containers should be used for any investigations requested. These may include the baby’s blood group, Rhesus type, haemoglobin estimation, serum bilirubin level, Coombs’ test or electrophoresis. Maternal blood for Kleihauer testing can be taken upon completion of the third stage.
Completion of the third stage
Once the placenta is delivered, the midwife must first check that the uterus is well contracted and fresh blood loss is minimal. Careful inspection of the perineum and lower vagina is important. A strong light is directed onto the perineum in order to assess trauma accurately prior to instigating repair. This should be carried out as gently as possible as the tissues are often bruised and oedematous. If perineal suturing (see Ch. 28) is required it should be carried out as expediently as possible to prevent unnecessary blood loss, increased risk of oedema at the site of trauma and perhaps unnecessary re-infiltration of additional local anaesthetics.
Blood loss estimation
Blood loss is difficult to measure and is frequently underestimated (Duthie 1990, Prastertcharoensuk et al 2000, WHO 2004). This is an important factor to be considered when assessing blood loss in the immediate postnatal period. The site of the blood loss does not necessarily alter the impact in terms of potential debility for affected women.
Other blood loss studies have been more specifically related to caesarean section. In his paper on blood loss at caesarean section, Brandt (1966) makes a valid point that haemodynamically women can withstand perhaps a 1000–1500 mL blood loss. However, any further blood loss may not be tolerated so readily. Women who undergo elective caesarean section will for the most part have been adequately prepared. Women who undergo emergency caesarean section or vaginal birth who are dehydrated or anaemic may not withstand sudden large volumes of blood loss.
In his study of the importance and difficulties of precise estimation of PPH, Brandt (1967) calculated that 20% of women lose >500 mL of blood after a vaginal birth. It was estimated that 3940 mL of circulating blood volume were required to maintain the central venous pressure at 10 cm of water. Most measurement techniques are not sufficiently sensitive to detect a rapid volume change in the immediate setting when decisions need to be made.
Note. It should also be remembered that any amount of blood loss that causes a physical deterioration such as feeling faint, sudden onset of tachycardia, drop in blood pressure should be immediately investigated.
Examination of placenta and membranes
This should be performed as soon after birth as practicable so that, if there is doubt about their completeness, further action may be taken before the woman leaves the birth room or the midwife prepares to leave the home. A thorough inspection must be carried out in order to make sure that no part of the placenta or membranes has been retained. The membranes are the most difficult to examine as they become torn during delivery and may be ragged. Every attempt should be made to piece them together to give an overall picture of completeness. This is easier to see if the placenta is held by the cord, allowing the membranes to hang. The hole through which the baby was born can then usually be identified and a hand can be spread out inside the membranes to aid inspection (Fig. 29.8). The placenta should then be laid on a flat surface and both placental surfaces minutely examined in a good light. The amnion should be peeled from the chorion right up to the umbilical cord, which allows the chorion to be fully viewed.
Any clots on the maternal surface need to be removed and kept for measuring. Broken fragments of cotyledon must be carefully replaced before an accurate assessment is possible.
The lobes of a complete placenta fit neatly together without any gaps, the edges forming a uniform circle. Blood vessels should not radiate beyond the placental edge. If they do, this denotes a succenturiate lobe, which has developed separately from the main placenta (see Ch. 11). When such a lobe is visible there is no cause for concern, but if the tissue has been retained the vessels will end abruptly at a hole in the membrane. If there is any suspicion that the placenta or membranes are incomplete, they must be kept for inspection and a doctor informed immediately in case a PPH occurs or there is the possibility that a surgical intervention may be required. Account must be taken of blood that has soaked into linen and swabs as well as measurable fluid loss and clot formation.
Upon completion of the examination, the midwife should return her attention to the mother. The empty uterus should be firmly contracted. If the fundus has risen in the abdomen a blood clot may be present. This should be expelled while the uterus is in a state of contraction by pressing the fundus gently in a downward and backward direction – with due regard to the risk of inversion and acute discomfort to the woman. Force should never be used.
Immediate care
It is advisable for mother and baby to remain in the midwife’s care for at least 1 hr after birth, regardless of the birth setting. Much of this time will be spent in clearing up and completion of records but careful observation of mother and infant is very important. If an epidural cannula is in situ it is usually removed and checked at this time.
Early physiological observations including ensuring a well contracted uterus, assessment of vaginal blood loss and a gentle inspection of the genital tract to inspect for trauma should be undertaken (NICE 2006).
The woman should be encouraged to pass urine because a full bladder may impede uterine contraction. She may not actually feel an urge to do so, especially if she has passed urine immediately prior to giving birth or an effective epidural has been in progress, but she should be asked to try. Uterine contraction and blood loss should be checked on several occasions during this first hour. Once basic procedures to ensure the woman’s and baby’s safety and comfort have been completed, there is no evidence to suggest that restriction of food or fluids is necessary.
Most women intending to breastfeed will wish to put their babies to the breast during these early moments of contact. This is especially advantageous, as babies are usually very alert at this time and their sucking reflex is particularly strong. There is also evidence to suggest that women who breastfeed soon after birth successfully breastfeed for a longer period of time (Salariya et al 1979). An additional benefit lies in the reflex release of oxytocin from the posterior lobe of the pituitary gland, which stimulates the uterus to contract. This may result in the mother experiencing a sudden fresh blood loss as the uterus empties and she should be pre-warned and reassured that it is a normal response. The desire to feed a newborn baby is a warm, loving and instinctive response. While breastfeeding should be actively encouraged, a formula feed should be available for those who do not wish to breastfeed.
Records
A complete and accurate account of the labour, including the documentation of all drugs, physical examination and observations, is the midwife’s responsibility. This should also include details of examination of the placenta, membranes and cord with attention drawn to any abnormalities. The volume of blood loss is particularly important. This record not only provides information that may be critical in the future care of both mother and infant but is a legal document that may be used as evidence of the care given. Signatures are therefore essential, with co-signatories where necessary. Many mothers now carry their own notes related to pregnancy and details of the birth. The completed records are a vital communication link between the midwife responsible for the birth and other caregivers, particularly those who take over care and provide ongoing community support services once the woman returns home.
It is usually the midwife who completes the birth notification form. Timely notification and referral may prevent delay in a woman receiving appropriate assistance should she need it.
Transfer from the birth room
The midwife is responsible for seeing that all observations are made and recorded prior to transfer of mother and baby to the postnatal ward or before the midwife leaves the home following the birth.
The postnatal ward midwife should verify these details prior to transfer of mother and baby. Following a domiciliary birth, the midwife should leave details of a telephone number where she may be contacted should the parents feel any cause for concern.
Complications of the third stage of labour
Postpartum haemorrhage
Primary postpartum haemorrhage is defined as excessive bleeding from the genital tract at any time following the baby’s birth up to 24 hrs following the birth (WHO 2000).
PPH is one of the most alarming and serious emergencies a midwife may face and is especially terrifying if it occurs immediately following a straightforward birth. It is always a stressful experience for the woman and any support persons present and may undermine her confidence, influence her attitude to future childbearing and delay her recovery. Although the maternal mortality rate (MMR) in developed countries such as the UK, Australasia, Canada, Japan and USA is quoted as approximately 20/100 000 live births (WHO 2004) the reported MMR for lower resource countries, such as Africa is 830/100 000 live births and Asia 330/100 000 live births with region-specific areas experiencing much higher figures (for example, sub-Saharan Africa best estimates report 920/100 000 live births (WHO 2004). A significant number of the deaths recorded were due to PPH. The midwife is often the first and may be the only professional person present when a haemorrhage occurs, so her prompt, competent action will be crucial in controlling blood loss and reducing the risk of maternal morbidity or even death.
Primary postpartum haemorrhage
Fluid loss is extremely difficult to measure with any degree of accuracy, especially when a mixture of blood and fluid has soaked into the bed linen and spilled onto the floor. It should also be remembered that measurable solidified clots represent only about half the total fluid loss. With these factors in mind, the best yardstick is that any blood loss, however small, that adversely affects the mother’s condition constitutes a PPH. Much will therefore depend upon the woman’s general well-being. In addition, if the measured loss reaches 500 mL, it must be treated as a PPH, irrespective of maternal condition.
There are several reasons why a PPH may occur, including atonic uterus, retained placenta, trauma and blood coagulation disorder.
Atonic uterus
This is a failure of the myometrium at the placental site to contract and retract and to compress torn blood vessels and control blood loss by a living ligature action. When the placenta is attached, the volume of blood flow at the placental site is approximately 500–800 mL/min. Upon separation, the efficient contraction and retraction of uterine muscle staunch the flow and prevent a haemorrhage, which would otherwise ensue with horrifying speed (Box 29.1).
Incomplete placental separation
If the placenta remains fully adherent to the uterine wall, it is unlikely to cause bleeding. However, once separation has begun, maternal vessels are torn. If placental tissue remains partially embedded in the spongy decidua, efficient contraction and retraction are interrupted.
Precipitate labour
When the uterus has contracted vigorously and frequently resulting in a duration of labour that is less than 1 hr, then the muscle may have insufficient opportunity to retract.
Prolonged labour
In a labour where the active phase lasts >12 hrs uterine inertia (sluggishness) may result from muscle exhaustion.
Polyhydramnios or multiple pregnancy
The myometrium becomes excessively stretched and therefore less efficient.
Placenta praevia
The placental site is partly or wholly in the lower segment where the thinner muscle layer contains few oblique fibres: this results in poor control of bleeding.
Placental abruption
Blood may have seeped between the muscle fibres, interfering with effective action. At its most severe this results in a Couvelaire uterus (see Ch. 20).
Induction or augmentation of labour with oxytocin
In some circumstances, the use of oxytocin during labour may result in hyperstimulation of the uterus and cause a precipitate, expulsive birth of the baby. In this instance the uterus may still be responding in a stimulated, but ineffective manner in terms of contracting the empty uterus. In the case of induction or augmentation of a labour, that continues over a prolonged period without establishing efficient uterine contractions, physical and emotional fatigue of the mother, and uterine fatigue or inertia may occur. This inertia inhibits the uterine muscle from providing strong, sustained contraction and retraction of the empty uterus that aids in the prevention of a postpartum haemorrhage occurring.
General anaesthesia
Anaesthetic agents may cause uterine relaxation, in particular the volatile inhalational agents, for example halothane.
Mismanagement of the third stage of labour
‘Fundus fiddling’ or manipulation of the uterus may precipitate arrhythmic contractions so that the placenta only partially separates and retraction is lost.
A full bladder
If the bladder is full, its proximity to the uterus in the abdomen on completion of the second stage may interfere with uterine action. This also constitutes mismanagement.
Aetiology unknown
A precipitating cause may never be discovered.
There are in addition a number of factors that do not directly cause a PPH, but do increase the likelihood of excessive bleeding (Box 29.2).
Previous history of PPH or retained placenta
There is a risk of recurrence in subsequent pregnancies. A detailed obstetric history taken at the first antenatal visit will ensure that arrangements are made for such a mother to give birth in a consultant unit.
High parity
With each successive pregnancy, fibrous tissue replaces muscle fibres in the uterus; this reduces its contractility and the blood vessels become more difficult to compress. Women who have had five or more births are at increased risk.
Fibroids (fibromyomata)
These are normally benign tumours consisting of muscle and fibrous tissue, which may impede efficient uterine action.
Anaemia
Women who enter labour with reduced haemoglobin concentration (below 10 g/dL) may succumb more quickly to any subsequent blood loss, however small. Anaemia is associated with debility, which is a more direct cause of uterine atony.
HIV/AIDS
Women who have HIV/AIDS are often in a state of severe immunosuppression, which lowers the platelet count to such a degree that even a relatively minor blood loss may cause severe morbidity or death.
Ketosis
The influence of ketosis upon uterine action is still unclear. Foulkes & Dumoulin (1983) demonstrated that, in a series of 3500 women, 40% had ketonuria at some time during labour. They reported that if labour progressed well, this did not appear to jeopardize either the fetal or maternal condition. However, there was a significant relationship between ketosis and the need for oxytocin augmentation, instrumental delivery and PPH when labour lasted >12 hrs. Correction of ketosis is therefore advisable and can be facilitated by ensuring women have an adequate intake of fluids and light solid nourishment as tolerated throughout labour. There is no evidence to suggest restriction of food or fluids is necessary during the normal course of labour.
Prophylaxis
By using the above list, it is possible for the midwife to apply some preventive screening in an attempt to identify women who may be at greater risk and to recognize causative factors. During the antenatal period a thorough and accurate history of previous obstetric experiences will identify risk factors such as previous PPH or precipitate labour. Arrangements can then, after careful explanation and in full consultation with the woman, be made for birth to take place in a unit where facilities for dealing with emergencies are available. The early detection and treatment of anaemia will help ensure that women enter labour with a haemoglobin level, ideally, in excess of 10 g/dL. The midwife should check that blood tests, if needed, are taken regularly and the results recorded and explained to the woman. If necessary, action can be taken to restore the haemoglobin level before birth. Women more prone to anaemia should be closely monitored, e.g. those with multiple pregnancies.
During labour, good management practices during the first and second stages are important to prevent prolonged labour and ketoacidosis. A mother should not enter the second or third stage with a full bladder. Prophylactic administration of a uterotonic agent is recommended for the third stage, by either intramuscular injection or intravenous infusion. Two units of cross-matched blood should be kept available for any woman known to have a placenta praevia or is known to have pre-disposing risk factors for PPH.
Treatment of PPH
Whatever the stage of labour or crisis that may occur, the midwife should adhere to the underlying principle of always reassuring the woman and her support persons by continually relaying appropriate information and involving them in decision-making.
Three basic principles of care should be applied immediately upon observation of excessive bleeding:
Call for medical aid
This is an important initial step so that help is on the way whatever transpires. If the bleeding is brought under control before the doctor arrives, then no action by the doctor will be needed. However, the woman’s condition can deteriorate very rapidly, in which case medical assistance will be required urgently. If the mother is at home or in a midwife-led unit, the emergency department of the closest obstetric unit should be contacted and, depending on the policy of the region, an obstetric emergency team summoned or ambulance transfer arranged.
Stop the bleeding
The initial action is always the same, regardless of whether bleeding occurs with the placenta in situ or later.
Rub up a contraction
The fundus is first felt gently with the fingertips to assess its consistency. If it is soft and relaxed, the fundus is massaged with a smooth, circular motion, applying no undue pressure. When a contraction occurs, the hand is held still.
Give a uterotonic to sustain the contraction
In many instances, oxytocin 5 units or 10 units, or combined ergometrine/oxytocin 1 mL, has already been administered and this may be repeated. Alternatively, ergometrine 0.25–0.5 mg may be injected intravenously, which will be effective within 45 s. No more than two doses of ergometrine should be given (including any dose of combined ergometrine/oxytocin), as it may cause pulmonary hypertension. Several reports have described the dramatic haemostatic effects of prostaglandins used in cases of uterine atony. Misoprostol or carboprost (Hemabate) are the most common prostaglandin drugs used to increase uterine contractility for the treatment of PPH. However, the side-effects (nausea, vomiting, pyrexia, hypertension, diarrhoea) associated with these drugs can make their use limited (Anderson & Etches 2007).
The baby may be put to the breast to enhance the physiological secretion of oxytocin from the posterior lobe of the pituitary gland, thus stimulating a contraction.
Empty the uterus
Once the midwife is satisfied that it is well contracted, she should ensure that the uterus is emptied. If the placenta is still in the uterus, it should be delivered; if it has been expelled, any clots should be expressed by firm but gentle pressure on the fundus.
Resuscitate the mother
An intravenous infusion should be commenced while peripheral veins are easily negotiated. This will provide a route for an oxytocin infusion or fluid replacement. As an emergency measure, the mother’s legs may be lifted up in order to allow blood to drain from them into the central circulation. However, the foot of the bed should not be raised as this encourages pooling of blood in the uterus, which prevents the uterus contracting.
It is usually expedient to catheterize the bladder to ensure a full bladder is not impeding uterine contraction and thus precipitating further bleeding and to minimize trauma should an operative procedure be necessary.
On no account must a woman in a collapsed condition be moved prior to resuscitation and stabilization.
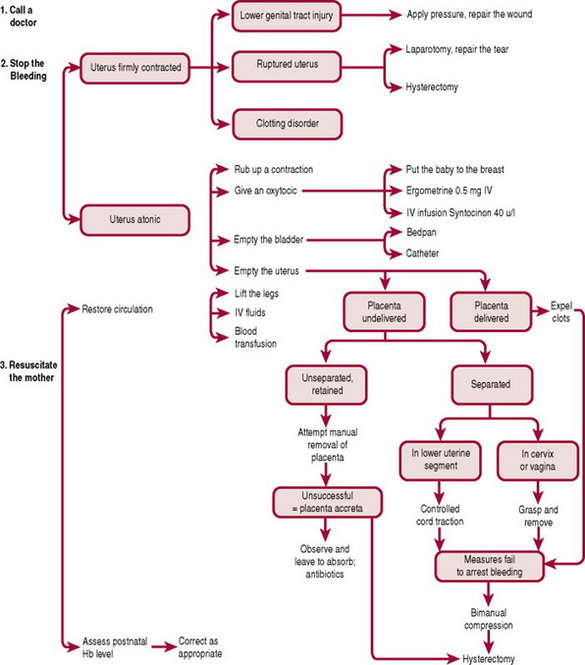
The flow chart (Fig. 29.9) briefly sets out the possible courses of action that may be taken depending upon whether or not bleeding persists. If the above measures are successful in controlling any further loss, administration of oxytocin, 40 units in 1 L of intravenous solution (e.g. Hartmann’s or saline) infused slowly over 8–12 hrs, will ensure continued uterine contraction. This will help to minimize the risk of recurrence. Before the infusion is connected, 10 mL of blood should be withdrawn for haemoglobin estimation and for cross-matching compatible blood. If bleeding continues uncontrolled, the choice of further action will depend largely upon whether the placenta remains undelivered.
Placenta delivered
If the uterus is atonic following delivery of the placenta, light fundal pressure may be used to expel residual clots while a contraction is stimulated. If an effective contraction is not maintained, 40 units of Syntocinon in 1 L of intravenous fluid should be started. The placenta and membranes must be re-examined for completeness because retained fragments are often responsible for uterine atony.
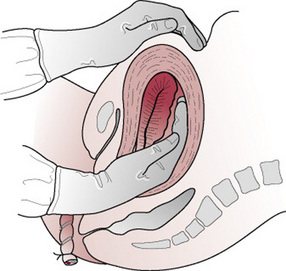
Bimanual compression
If bleeding continues, bimanual compression of the uterus may be necessary in order to apply pressure to the placental site. It is desirable for an intravenous infusion to be in progress. The fingers of one hand are inserted into the vagina like a cone; the hand is formed into a fist and placed into the anterior vaginal fornix, the elbow resting on the bed. The other hand is placed behind the uterus abdominally, the fingers pointing towards the cervix. The uterus is brought forwards and compressed between the palm of the hand positioned abdominally and the fist in the vagina (Fig. 29.10). If bleeding persists, a clotting disorder must be excluded before exploration of the vagina and uterus is performed under a general anaesthetic (see also Ch. 55 for aortic compression).
Retained placenta
This diagnosis is reached when the placenta remains undelivered after a specified period of time (up to 1 hr following the baby’s birth). The conventional treatment is to separate the placenta from the uterine wall digitally, effecting a manual removal.
Breaking of the cord
This is not an unusual occurrence during completion of the third stage of labour. Before further action, it is crucial to check that the uterus remains firmly contracted. If the placenta remains adherent, no further action should be taken before a doctor is notified. It is possible that manual removal may be indicated. If the placenta is palpable in the vagina, it is probable that separation has occurred and when the uterus is well contracted then maternal effort may be encouraged (see Expectant management, above). If there is any doubt, the midwife applies fresh sterile gloves before performing a vaginal examination to ascertain whether this is so. As a last resort, if the woman is unable to push effectively then fundal pressure may be used. A uterotonic drug must be given prior to this. Great care is exercised to ensure that placental separation has already occurred and the uterus is well contracted. The woman should be relaxed as the midwife exerts downward and backward pressure on the firmly contracted fundus. This method can cause considerable pain and distress to the woman and result in the stretching and bruising of supportive uterine ligaments. If it is performed without good uterine contraction, acute inversion may ensue. This is an extremely dangerous procedure in unskilled hands and is not advocated in everyday practice when alternative, safer methods may be employed.
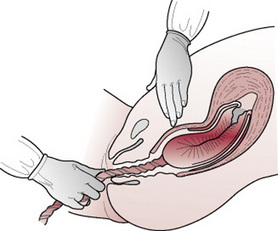
Manual removal of the placenta
This should be carried out by a doctor. An intravenous infusion must first be sited and an effective anaesthetic in progress. The choice of anaesthesia will depend upon the woman’s general condition. If an effective epidural anaesthetic is already in progress, a top-up may be given in order to avoid the hazards of general anaesthesia. A spinal anaesthetic offers an alternative but where time is an urgent factor a general anaesthetic will be initiated.
Management
Manual removal is performed with full aseptic precautions and, unless in a dire emergency situation, should not be undertaken prior to adequate analgesia being ensured for the woman. With the left hand, the umbilical cord is held taut while the right hand is coned and inserted into the vagina and uterus following the direction of the cord. Once the placenta is located the cord is released so that the left hand may be used to support the fundus abdominally, to prevent rupture of the lower uterine segment (Fig. 29.11). The operator will feel for a separated edge of the placenta. The fingers of the right hand are extended and the border of the hand is gently eased between the placenta and the uterine wall, with the palm facing the placenta. The placenta is carefully detached with a sideways slicing movement. When it is completely separated, the left hand rubs up a contraction and expels the right hand with the placenta in its grasp. The placenta should be checked immediately for completeness, so that any further exploration of the uterus may be carried out without delay. A uterotonic drug is given upon completion.
In very exceptional circumstances, when no doctor is available to be called, a midwife would be expected to carry out a manual removal of the placenta. Once she has diagnosed a retained placenta as the cause of PPH, the midwife must act swiftly to reduce the risk of onset of shock and exsanguination. It must be remembered that the risk of inducing shock by performing a manual removal of the placenta is greater when no anaesthetic is given. In a developed country, the midwife is unlikely to find herself dealing with this situation.
At home
If the placenta is retained following a home birth, emergency obstetric help must be summoned. Under no circumstances should a woman be transferred to hospital until an intravenous infusion is in progress and her condition stabilized.
It is best if the placenta can be delivered without moving the mother but if this is not possible, or if further treatment is needed, she should be transferred to a consultant unit. The baby should accompany her.
Morbid adherence of placenta
Very rarely, the placenta remains morbidly adherent; this is known as placenta accreta. If it is totally adherent, then bleeding is unlikely to occur and it may be left in situ to absorb during the puerperium. If, however, only part of the placenta remains embedded then the risks of fatal haemorrhage are high and an emergency hysterectomy may be unavoidable.
Trauma as a cause of haemorrhage
If bleeding occurs despite a well-contracted uterus, it is almost certainly the consequence of trauma to the uterus, vagina, perineum or labia, or a combination of these. Poeschmann et al (1991) cautioned that episiotomy may contribute up to 30% of total blood loss; in their study the severity of blood loss was linked to the length of time that elapsed between incision of the perineum and the commencement of repair. Predictably, the longer the wait the greater is the blood loss.
In order to identify the source of bleeding, the mother is placed in the lithotomy position under a good directional light. An episiotomy wound or tears to the anterior labia, clitoris and perineum often bleed freely. These external injuries are easily identified and torn vessels may be clamped with artery forceps prior to ligation. Internal trauma to the vagina, cervix or uterus more commonly occurs following instrumental or manipulative delivery. A speculum is inserted to enable the cervix and vagina to be clearly visualized and examined. Tissue or artery forceps may be used to apply pressure prior to suturing under general anaesthesia.
If bleeding persists when the uterus is well contracted and no evidence of trauma can be found, uterine rupture must be suspected. Following a laparotomy this is repaired, but if bleeding remains uncontrolled a hysterectomy may become inevitable.
Blood coagulation disorders
As well as the causes already listed above, PPH may be the result of coagulation failure (see Ch. 20). The failure of the blood to clot is such an obvious sign that it can be overlooked in the midst of the frantic activity that accompanies torrential bleeding. It can occur following severe pre-eclampsia, APH, massive PPH, amniotic fluid embolus, intrauterine death or sepsis. Evaluation should include coagulation status and replacing appropriate blood components (Anderson & Etches 2007). Fresh blood is usually the best treatment, as this will contain platelets and the coagulation factors V and VIII. The expert advice of a haematologist will be needed in assessing specific replacement products such as fresh frozen plasma and fibrinogen.
Maternal observation following PPH
Once bleeding is controlled, the total volume lost must be estimated as accurately as possible. Large amounts appear less than they are in reality. Maternal pulse and blood pressure are recorded every 15 min and the temperature taken every 4 hrs. The uterus should be palpated frequently to ensure that it remains well contracted and lochia lost must be observed. Intravenous fluid replacement should be carefully calculated to avoid circulatory overload. Monitoring the central venous pressure (see Ch. 33) will provide an accurate assessment of the volume required, especially if blood loss has been severe. Fluid intake and urinary output are recorded as indicators of renal function. The output should be accurately measured on an hourly basis by the use of a self-retaining urinary catheter.
The woman will, if possible, be transferred to a high dependency care unit if closer monitoring is required, until her condition is stable. All records should be meticulously completed and signed contemporaneously. Continued vigilance will be important for 24–48 hrs. As this woman will need a period of recovery, she will not be suitable for early transfer home.
Secondary postpartum haemorrhage
Secondary postpartum haemorrhage is any abnormal or excessive bleeding from the genital tract occurring between 24 hrs and 12 weeks postnatally. In developed countries, 2% of postnatal women are admitted to hospital with this condition, half of them undergoing uterine surgical evacuation (Alexander et al 2007). It is most likely to occur between 10 and 14 days after birth. Bleeding is usually due to retention of a fragment of the placenta or membranes, or the presence of a large uterine blood clot. Typically occurring during the second week, the lochia is heavier than normal and will have changed from a serous pink or brownish loss to a bright red blood loss. The lochia may also be offensive if infection is a contributory factor. Subinvolution, pyrexia and tachycardia are usually present. As this is an event that is most likely to occur at home, women should be alerted to the possible signs of secondary PPH prior to discharge from midwifery care.
Management
The following steps should be taken:
If the bleeding occurs at home and the woman has telephoned the hospital, midwife or her GP, she should be told to lie down flat until professional assistance arrives (the front door should be left unlocked if the woman is alone). On arrival, the doctor, midwife or paramedic will assess the amount of blood loss and the woman’s condition and attempt to arrest the haemorrhage. If the loss is severe or uncontrolled, the nearest emergency obstetric unit will be called and the mother and baby prepared for transfer to hospital. The doctor, midwife or paramedic who attends will start an intravenous infusion and ensure that the mother’s condition is stable first.
Careful assessment is usually undertaken prior to the uterus being explored under general anaesthetic. The use of ultrasound as a diagnostic tool is invaluable in minimizing the number of mothers who have operative intervention. If retained products of conception cannot be seen on a scan, the mother may be treated conservatively with antibiotic therapy and oral ergometrine. The haemoglobin should be estimated prior to discharge. If it is below 9 g/dL, options for iron replacement should be discussed with the woman. The severity of the anaemia will assist in determining the most appropriate care, which may be dependent on whether the woman is symptomatic (e.g. feeling faint, dizzy, short of breath). Management may vary from increased intake of iron-rich foods, iron supplements or, in extreme cases, blood transfusion. It is also important to discuss the common symptoms that may be experienced as a result of anaemia following PPH, including extreme tiredness and general malaise. Encourage the woman to seek assistance and stress the importance of making an appointment to see her GP to have her general health and haemoglobin levels checked.
Haematoma formation
PPH may also be concealed as the result of progressive haematoma formation. This may be obvious at such sites as the perineum or lower vagina, but it is more difficult to diagnose if it occurs into the broad ligament or vault of the vagina. A large volume of blood may collect insidiously (up to 1 L). Involution and lochia are usually normal, the main symptom being increasingly severe maternal pain. This is often so acute that the haematoma has to be drained in theatre under a general anaesthetic. Secondary infection is a strong possibility.
Care after a postpartum haemorrhage
Whatever the cause of the haemorrhage, the woman will need the continued support of her midwife until she regains her confidence. Her partner may also be fearful of a recurrence and need much reassurance. If the mother is breast-feeding, lactation may be impaired but this will only be temporary and she should be encouraged to persevere. The midwife is often the first and may be the only professional person present when a haemorrhage occurs, so her prompt, competent action will be crucial in controlling blood loss and reducing the risk of maternal morbidity or even mortality.
Key issues in the management of the third stage of labour are listed in Box 29.3.
Box 29.3 Key issues in the management of the third stage of labour
Alexander J, Thomas P, Sanghera J. Treatments for secondary postpartum haemorrhage. The Cochrane Database of Systematic Reviews. (Issue 2):2007. CD002867
Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. American Family Physician. 2007;75:875-882.
Baldock S, Dixon L. Physiological changes in labour and the postnatal period. In: Pairman S, Pincombe J, Thorogood C, et al, editors. Midwifery: preparation for practice. Australia: Elsevier, Marrickville, 2006.
Begley CM. A comparison of ‘active’ and ‘physiological’ management of the third stage of labour. Midwifery. 1990;6:3-17.
Brandt HA. Blood loss at caesarean section. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1966;73:456-459.
Brandt HA. Precise estimation of postpartum haemorrhage: difficulties and importance. British Medical Journal. 1967;1:398-400.
Chaparro CM, Neufeld LM, Alavez GT, et al. Effect of timing of umbilical cord clamping on iron status in Mexican infants: A randomised controlled trial. Lancet. 2006;367(9527):1997-2004.
Choy CMY, Lau WC, Tam WH, et al. A randomised controlled trial of intramuscular Syntometrine and intravenous oxytocin in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology. 2002;109:173-177.
Chua S, Shaw SI, Yeoh CL, et al. A randomised controlled study of prostaglandin 15 methyl F2 alpha compared with Syntometrine for prophylactic use in the third stage of labour. Journal of Australian and New Zealand Obstetrics and Gynaecology. 1995;35(4):413.
Dombrowski MP, Bottoms SF, Saleb AAA, et al. Third stage of labour: analysis of duration and clinical practice. American Journal of Obstetrics and Gynecology. 1995;172:1279-1284.
Dunn PM. Management of childbirth in normal women: the third stage and fetal adaptation. In: Perinatal medicine. Proceedings of the IX European Congress on Perinatal Medicine, Dublin, September 1984. Lancaster: MTP Press; 1985:47-54.
Dunn PM. The placental venous pressure during and after the third stage of labour following early cord ligation. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1966;73:747-756.
Duthie SJ, Ven D, Yung GLK, et al. Discrepancy between laboratory determination and visual estimation of blood loss during normal delivery. European Journal of Obstetrics, Gynaecology and Reproductive Biology. 1990;38:119-124.
FIGO/ICM (International Federation of Gynaecology and Obstetrics/International Confederation of Midwives) Joint Statement. Prevention and treatment of post-partum haemorrhage: New Advances for Low Resource Settings, November, 2006.
Foulkes J, Dumoulin JG. Ketosis in labour. British Journal of Hospital Medicine. 1983;29(6):562-564.
Gülmezoglu AM, Forna F, Villar J, et al. Prostaglandins for prevention of postpartum hemorrhage. Cochrane Review. 2004. Issue 1:CD000494
Gupta JK, Nikodem VC. Position for women during second stage of labour. Cochrane Review, Issue 2. 2002. CD002006
Inch S. Management of the third stage of labour – another cascade of intervention. Midwifery. 1985;1:114-122.
Johnson R, Taylor W. Third stage issues. In: Johnson R, editor. Skills for midwifery practice. London: Harcourt, 2000.
Khan QK, John IS, Chan T, et al. Abu Dhabi third stage trial: oxytocin versus Syntometrine in the active management of the third stage of labour. European Journal of Obstetrics, Gynaecology and Reproductive Biology. 1995;58:147-151.
Khan GQ, John IS, Wani S, et al. Abstract 56: ‘Controlled cord traction’ versus ‘minimal intervention’ techniques in the delivery of the placenta: a randomised controlled trial. American Journal of Obstetrics and Gynecology. 1997;177(4):770-774.
Lewis G, Drife J, editors. Why mothers die 1997–1999. The Confidential Enquiries into Maternal Deaths in the United Kingdom, RCOG Press, London, 2001;21.
Lewis G, editor. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving mothers’ lives: reviewing maternal deaths to make motherhood safer 2003–2005. The seventh report on Confidential Enquiries into Maternal Deaths in the United Kingdom. CEMACH, London, 2007.
Linderkamp O. Placental transfusion: determinants and effects. Clinical Perinatology. 1982;9:589-593.
McDonald S, Abbott JM, Higgins SP. Prophylactic ergometrine-oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews. (Issue 1):2004. CD000201
McDonald SJ, Middleton P. Effect of timing of umbilical cords clamping of term infants on maternal and neonatal outcomes. Cochrane Database of Systematic Reviews 2008 Issue 2 Art no CD 004074. 2008.
McDonald SJ. Timing of interventions in the third stage of labour. In: McDonald SJ, editor. Management in the third stage of labour (Doctoral thesis). University of Western Australia: Faculty of Medicine, Department of Obstetrics and Gynaecology; 1996:60-81.
McDonald SJ, Prendiville WJ, Blair E. Randomised controlled trial of oxytocin alone versus oxytocin and ergometrine in active management of the third stage of labour. British Medical Journal. 1993;307:1167-1171.
Mercer JS. Current best evidence: a review of the literature on umbilical cord clamping. Wickham S, editor. Midwifery, Vol. 4. London: Elsevier, 2006. Best practice
Newton M, Mosey LM, Egli GE, et al. Blood loss during and immediately after delivery. Obstetrics and Gynaecology. 1961;17:9-18.
Ng PS, Lai CY, Sahota DS, et al. A double-blind randomized controlled trial of oral misoprostol and intramuscular Syntometrine in the management of the third stage of labor. Gynecologic and Obstetric Investigations. 2007;63:55-60.
NICE (National Institute for Health and Clinical Excellence). Postnatal care: Routine postnatal care of women and their babies, 2006, http://www.nice.org.uk, Online. Available
Patterson D. The views and experiences of childbirth educators providing a breastfeeding intervention during pregnancy. Proceedings of 27th Congress of the International Confederation of Midwives on ‘Midwifery: Pathways to Healthy Nations’. Brisbane, Australia, 2005.
Peyser RM, Kupfermine MJ. Management of postpartum haemorrhage by uterine irrigation with prostaglandin. American Journal of Obstetrics and Gynecology. 1990;162(3):694-696.
Poeschmann RP, Docsburg WH, Eskis TKABA. Randomised comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology. 1991;98:528-530.
Prastertcharoensuk W, Swadpanich U, Lumbiganon P. Accuracy of the blood loss estimation in the third stage of labor. International Journal Gynecological Obstetrics. 2000;71:9-70.
Prendiville W, Elbourne D. Care during the third stage of labour. In: Chalmers I, Enkin M, Keirse MJNC, editors. Effective care in pregnancy and childbirth. Oxford: Oxford University Press; 1989:1145-1169.
Prendiville W, Elbourne D, Chalmers I. The effects of routine uterotonic administration in the management of the third stage of labour: an overview of the evidence from controlled trials. British Journal of Obstetrics and Gynaecology. 1988;95:3-16.
Prendiville WJ, Elbourne DR, Chalmers I. The Bristol third stage trial: active versus physiological management of the third stage of labour. British Medical Journal. 1988;297:1295-1300.
Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management of the third stage of labour. Cochrane Review. (Issue 4):2002. CD001808
Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database of Systematic Reviews. (Issue 4):2004. CD003248
Rogers J, Wood J, McCandlish R, et al. Active versus expectant management of third stage of labour: the Hinching- brooke randomised controlled trial. Lancet. 1998;351:693-699.
Salariya E, Easton P, Cater J. Early and often for best results. Nursing Mirror. 1979;148:15-17.
Sandall J, McCandlish R. Why Do Research? In: Page L, McCandlish R, editors. The new midwifery: science and sensitivity in practice. 2nd edn. Edinburgh: Churchill Livingstone; 2006:251.
Sinclair CA. The midwife’s handbook. Missouri: W B Saunders, 2004.
Thilaganathan B, Cutner A, Latimer J, et al. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics and Gynecology and Reproductive Biology. 1993;48:19-22.
van Rheenen P, Brabin BJ. Late umbilical cord clamping as an intervention for reducing iron deficiency anaemia in term infants in developing and industrialized countries: a systematic review. Annals of Tropical Paediatrics. 2004;24:3-16.
van Selm M, Kanhai HHH, Keiser MINC. Preventing the recurrence of atonic postpartum haemorrhage: a double-blind trial. Acta Obstetrica et Gynecologica Scandinavica. 1995;74:270-274.
Whitfield MF, Salfield SAW. Accidental administration of Syntometrine in adult dosage to the newborn. Archives of Disease in Childhood. 1980;55:68-70.
WHO (World Health Organization). The prevention and management of postpartum hemorrhage. WHO report of technical working group, No. WHO/MCH/90.7. Geneva: WHO, 1990.
WHO (World Health Organization). Managing complications in pregnancy and childbirth: a guide for midwives and doctors, WHO/RHR/00.7. Geneva: WHO, 2000.
WHO (World Health Organization). Maternal mortality in 2000: estimates developed by WHO, UNICEF and UNFPA. Geneva: WHO, 2004.
WHO (World Health Organization). Maternal mortality in 2005. Estimates developed by WHO, UNICEF, UNFPA, and The World Bank. Geneva: WHO, 2007.
Yao AC, Lind J. Placental transfusion. American Journal of Diseases of Children. 1974;127:128-141.