Conception and Fetal Development
• Summarize the process of fertilization.
• Describe the development, structure, and functions of the placenta.
• Describe the composition and functions of amniotic fluid.
• Identify three organs or tissues arising from each of the three primary germ layers.
• Summarize the growth and development of the embryo and fetus.
• Identify the potential effects of teratogens during vulnerable periods of embryonic and fetal development.
• Relate selected congenital defects to stage of fetal development.
This chapter presents an overview of the process of fertilization and the development of the normal embryo and fetus.
Conception
Conception, defined as the union of a single egg and sperm, marks the beginning of a pregnancy. Conception occurs not as an isolated event but as part of a sequential process. This sequential process includes gamete (egg and sperm) formation, ovulation (release of the egg), fertilization (union of the gametes), and implantation in the uterus.
Cell Division
Cells are reproduced by two different methods: mitosis and meiosis. In mitosis, body cells replicate to yield two cells with the same genetic makeup as the parent cell. First the cell makes a copy of its deoxyribonucleic acid (DNA), and then it divides; each daughter cell receives one copy of the genetic material. Mitotic division facilitates growth and development or cell replacement.
Meiosis, the process by which germ cells divide and decrease their chromosomal number by half, produces gametes (eggs and sperm). Each homologous pair of chromosomes contains one chromosome received from the mother and one from the father; thus meiosis results in cells that contain one of each of the 23 pairs of chromosomes. Because these germ cells contain 23 single chromosomes, half of the genetic material of a normal somatic cell, they are called haploid. This halving of the genetic material is accomplished by replicating the DNA once and then dividing twice. When the female gamete (egg or ovum) and the male gamete (spermatozoon) unite to form the zygote, the diploid number of human chromosomes (46, or 23 pairs) is restored.
The process of DNA replication and cell division in meiosis allows different alleles (genes on corresponding loci that code for variations of the same trait) for genes to be distributed at random by each parent and then rearranged on the paired chromosomes. The chromosomes then separate and proceed to different gametes. Many combinations of genes are possible on each chromosome because parents have genotypes derived from four different grandparents. This random mixing of alleles accounts for the variation of traits seen in the offspring of the same two parents.
Gametogenesis
Oogenesis, the process of egg (ovum) formation, begins during fetal life in the female. All the cells that may undergo meiosis in a woman’s lifetime are contained in her ovaries at birth. The majority of the estimated 2 million primary oocytes (the cells that undergo the first meiotic division) degenerate spontaneously. Only 400 to 500 ova will mature during the approximately 35 years of a woman’s reproductive life. The primary oocytes begin the first meiotic division (i.e., they replicate their DNA) during fetal life but remain suspended at this stage until puberty (Fig. 12-1, A). Then usually monthly, one primary oocyte matures and completes the first meiotic division, yielding two unequal cells: the secondary oocyte and a small polar body. Both contain 22 autosomes and one X sex chromosome.
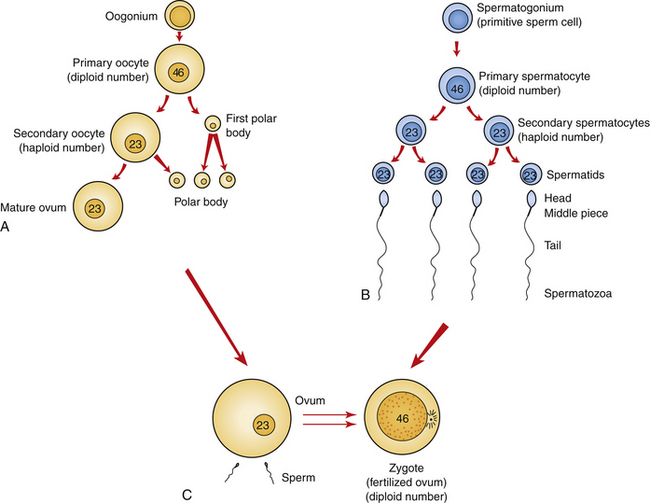
FIG. 12-1 Gametogenesis. A, Oogenesis. Gametogenesis in the female produces one mature ovum and three polar bodies. Note the relative difference in overall size between the ovum and sperm. B, Spermatogenesis. Gametogenesis of the male produces four mature gametes, the sperm. C, Fertilization results in the single-cell zygote and the restoration of the diploid number of chromosomes.
At ovulation the second meiotic division begins; however, the ovum does not complete the second meiotic division unless fertilization occurs. At fertilization, when the sperm is united with the mature ovum, a second polar body and the zygote (the united egg and sperm) are produced (see Fig. 12-1, C). The three polar bodies degenerate.
When a male reaches puberty, his testes begin the process of spermatogenesis. The cells that undergo meiosis in the male are called spermatocytes. The primary spermatocyte, which undergoes the first meiotic division, contains the diploid number of chromosomes. The cell has already copied its DNA before division, so four alleles for each gene are present. The cell is still considered diploid because the copies are bound together (i.e., one allele plus its copy on each chromosome).
During the first meiotic division, two haploid secondary spermatocytes are formed. Each secondary spermatocyte contains 22 autosomes and one sex chromosome; one contains the X chromosome (plus its copy), and the other, the Y chromosome (plus its copy). During the second meiotic division, the male produces two gametes with an X chromosome and two gametes with a Y chromosome, all of which will develop into viable sperm (Fig. 12-1, B).
When homologous chromosomes fail to separate during gametogenesis (nondisjunction), some gametes have 24 chromosomes and others have 22 (Fig. 12-2). If a gamete with 24 chromosomes unites with a normal gamete with 23 chromosomes, the resulting zygote has 47 chromosomes. This produces a trisomy as occurs in Down syndrome. When a gamete with 22 chromosomes unites with a normal gamete with 23 chromosomes, a zygote with 45 chromosomes results, producing a monosomy. Abnormal gametogenesis can occur in both sex chromosomes and in autosomes (Moore & Persaud, 2008).
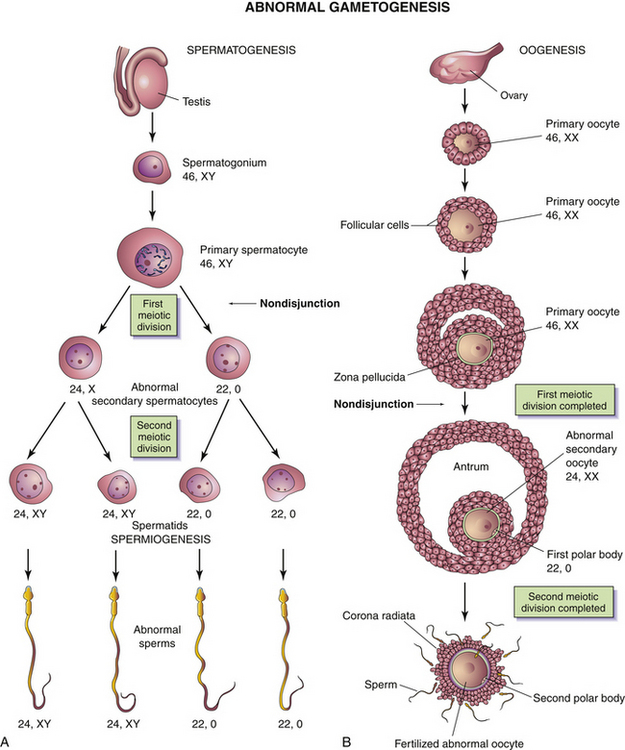
FIG. 12-2 Abnormal gametogenesis: nondisjunction. A, When nondisjunction occurs during the first meiotic division of spermatogenesis, one secondary spermatocyte contains 22 autosomes plus an X and a Y chromosome, whereas the other one contains 22 autosomes and no sex chromosomes. B, Nondisjunction during oogenesis may give rise to an oocyte with 22 autosomes and two X chromosomes (as shown) or one with 22 autosomes and no sex chromosome. (From Moore, K., & Persaud, T. [2008]. Before we are born: Essentials of embryology and birth defects [7th ed.]. Philadelphia: Saunders.)
Ovum
Meiosis occurs in the female in the ovarian follicles and produces an egg, or ovum. Each month, one ovum matures with a host of surrounding supportive cells. At ovulation the ovum is released from the ruptured ovarian follicle. High estrogen levels increase the motility of the uterine tubes so their cilia are able to capture the ovum and propel it through the tube toward the uterine cavity. An ovum cannot move by itself.
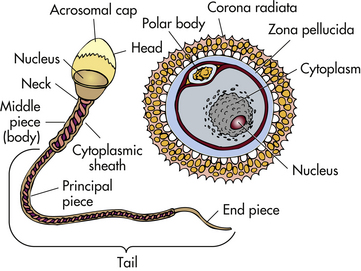
Two protective layers surround the ovum (Fig. 12-3). The inner layer is a thick, acellular layer, the zona pellucida. The outer layer, the corona radiata, is composed of elongated cells.
Ova are considered fertile for about 24 hours after ovulation. If not fertilized by a sperm, the ovum degenerates and is resorbed.
Sperm
Ejaculation during sexual intercourse normally propels about a teaspoon of semen, containing as many as 200 to 500 million sperm, into the vagina. The sperm swim propelled by the flagellar movement of their tails. Some sperm can reach the site of fertilization within 5 minutes, but average transit time is 4 to 6 hours. Sperm remain viable within the woman’s reproductive system for an average of 2 to 3 days. Most sperm are lost in the vagina, within the cervical mucus, or in the endometrium, or they enter the uterine tube that contains no ovum.
As the sperm travel through the female reproductive tract, enzymes are produced to aid in their capacitation. Capacitation is a physiologic change that removes the protective coating from the heads of the sperm. Small perforations then form in the acrosome (a cap on the sperm) and allow enzymes (e.g., hyaluronidase) to escape (see Fig. 12-3). These enzymes are necessary for the sperm to penetrate the protective layers of the ovum before fertilization.
Fertilization
Fertilization takes place in the ampulla (outer third) of the uterine tube. When a sperm successfully penetrates the membrane surrounding the ovum, both sperm and ovum are enclosed within the membrane, and the membrane becomes impenetrable to other sperm; this process is termed the zona reaction. The second meiotic division of the secondary oocyte is then completed, and the nucleus of the ovum becomes the female pronucleus. The head of the sperm enlarges to become the male pronucleus, and the tail degenerates. The nuclei fuse, and the chromosomes combine, restoring the diploid number (46) (Fig. 12-4). Conception, the formation of the zygote (the first cell of the new individual), has been achieved.
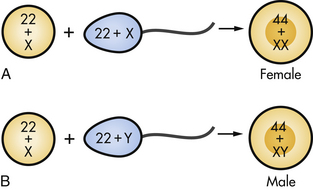
FIG. 12-4 Fertilization. A, Ovum fertilized by X-bearing sperm to form female zygote. B, Ovum fertilized by Y-bearing sperm to form male zygote.
Mitotic cellular replication, called cleavage, begins as the zygote travels the length of the uterine tube into the uterus. This transit takes 3 to 4 days. Because the fertilized egg divides rapidly with no increase in size, successively smaller cells, blastomeres, are formed with each division. A 16-cell morula, a solid ball of cells, is produced within 3 days and is still surrounded by the protective zona pellucida (Fig. 12-5, A). Further development occurs as the morula floats freely within the uterus. Fluid passes through the zona pellucida into the intercellular spaces between the blastomeres, separating them into two parts, the trophoblast (which gives rise to the placenta) and the embryoblast (which gives rise to the embryo). A cavity forms within the cell mass as the spaces come together, forming a structure called the blastocyst cavity. When the cavity becomes recognizable, the whole structure of the developing embryo is known as the blastocyst. Stem cells are derived from the inner cell mass of the blastocyst. The outer layer of cells surrounding the blastocyst cavity is the trophoblast. The trophoblast differentiates into villous and extravillous trophoblast (Fig. 12-6).
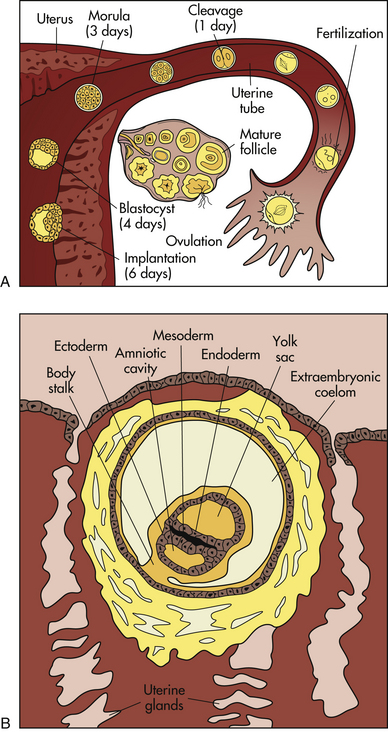
FIG. 12-5 First weeks of human development. A, Follicular development in the ovary, ovulation, fertilization, and transport of the early embryo down the uterine tube and into the uterus, where implantation occurs. B, Blastocyst embedded in endometrium. Germ layers forming. (A from Carlson, B. [2004]. Human embryology and developmental biology (3rd ed.). Philadelphia: Mosby; B adapted from Langley, L. [1980]. Dynamic anatomy and physiology [5th ed.]. New York: McGraw-Hill.)
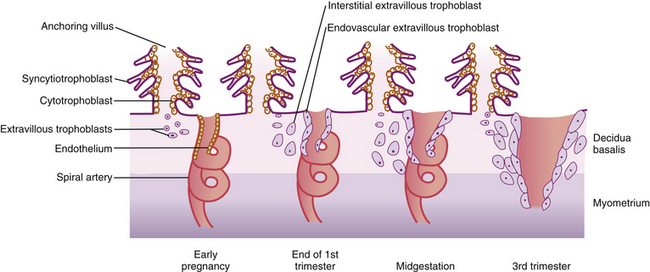
FIG. 12-6 Extravillous trophoblasts are found outside the villus and can be subdivided into endovascular and interstitial categories. Endovascular trophoblasts invade and transform spiral arteries during pregnancy to create low-resistance blood flow that is characteristic of the placenta. Interstitial trophoblasts invade the decidua and surround spiral arteries. (From Cunningham, F., Leveno, K., Bloom, S., Hauth, J., Rouse, D., & Spong, C. (2010). Williams obstetrics [23rd ed.]. New York: McGraw-Hill.)
Implantation
The zona pellucida degenerates, the trophoblast cells displace endometrial cells at the implantation site, and the blastocyst embeds in the endometrium, usually in the anterior or posterior fundal region. Between 6 and 10 days after conception, the trophoblast secretes enzymes that enable it to burrow into the endometrium until the entire blastocyst is covered. This is termed implantation. Endometrial blood vessels erode, and some women have slight implantation bleeding (slight spotting or bleeding at the time of the first missed menstrual period). Chorionic villi, finger-like projections, develop out of the trophoblast and extend into the blood-filled spaces of the endometrium. These villi are vascular processes that obtain oxygen and nutrients from the maternal bloodstream and dispose of carbon dioxide and waste products into the maternal blood.
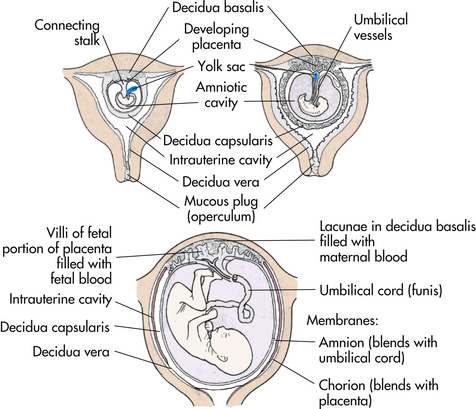
After implantation the endometrium is termed the decidua. The portion directly under the blastocyst, where the chorionic villi tap into the maternal blood vessels, is the decidua basalis. The portion covering the blastocyst is the decidua capsularis, and the portion lining the rest of the uterus is the decidua vera (Fig. 12-7).
Embryo and Fetus
Pregnancy lasts approximately 10 lunar months, 9 calendar months, 40 weeks, or 280 days. Length of pregnancy is computed from the first day of the last menstrual period (LMP) until the day of birth. However, conception occurs approximately 2 weeks after the first day of the LMP; thus the postconception age of the fetus is 2 weeks less, for a total of 266 days or 38 weeks. Postconception age is used in the discussion of fetal development.
Intrauterine development is divided into three stages: ovum or preembryonic, embryo, and fetus (Fig. 12-8). The stage of the ovum lasts from conception until day 14. This period covers cellular replication, blastocyst formation, initial development of the embryonic membranes, and establishment of the primary germ layers.
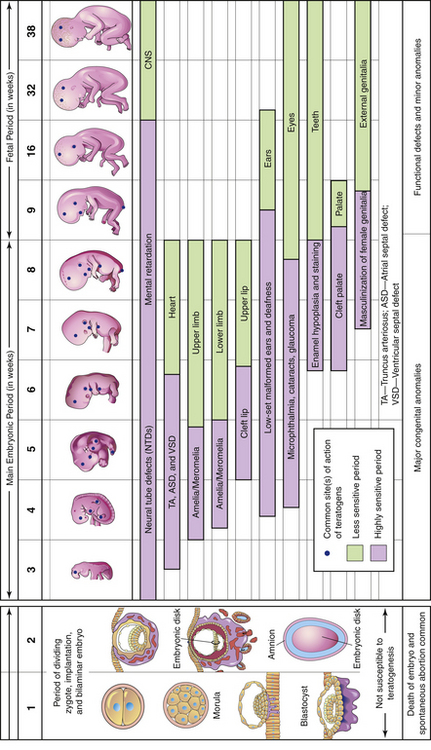
FIG. 12-8 Sensitive or critical periods in human development. During the first 2 weeks of development the embryo usually is not susceptible to teratogens. At that time a teratogen damages all or most of the cells, resulting in death of the embryo, or damages only a few cells, allowing the conceptus to recover and the embryo to develop without birth defects. The dark color denotes highly sensitive periods; the light color indicates stages that are less sensitive to teratogens. (From Moore, K., & Persaud, T. [2008]. Before we are born: Essentials of embryology and birth defects [7th ed.]. Philadelphia: Saunders.)
Primary Germ Layers
During the third week after conception, the embryonic disk differentiates into three primary germ layers: the ectoderm, the mesoderm, and the endoderm (or entoderm) (see Fig. 12-5, B). All tissues and organs of the embryo develop from these three layers.
The upper layer of the embryonic disk, the ectoderm, gives rise to the epidermis, the glands (anterior pituitary, cutaneous, and mammary), the nails and hair, the central and peripheral nervous systems, the lens of the eye, the tooth enamel, and the floor of the amniotic cavity.
The middle layer, the mesoderm, develops into the bones and teeth, the muscles (skeletal, smooth, and cardiac), the dermis and connective tissue, the cardiovascular system and spleen, and the urogenital system.
The lower layer, the endoderm, gives rise to the epithelium lining the respiratory and digestive tracts, and the glandular cells of associated organs, including the oropharynx, the liver and pancreas, the urethra, the bladder, and the vagina. The endoderm forms the roof of the yolk sac.
Development of the Embryo
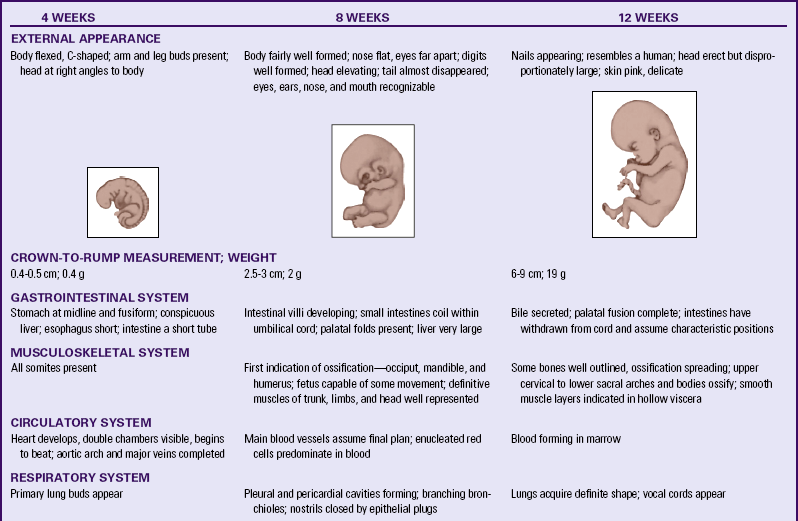
The stage of the embryo lasts from day 15 until approximately 8 weeks after conception, when the embryo measures 3 cm from crown to rump. This embryonic stage is the most critical time in the development of the organ systems and the main external features. Developing areas with rapid cell division are the most vulnerable to malformation caused by environmental teratogens (substances or exposure that causes abnormal development). At the end of the eighth week, all organ systems and external structures are present, and the embryo is unmistakably human (see Fig. 12-8 and Visible Embryo, www.visembryo.com/baby, for a pictorial view of normal and abnormal development).
Membranes
At the time of implantation, two fetal membranes that will surround the developing embryo begin to form. The chorion develops from the trophoblast and contains the chorionic villi on its surface. The villi burrow into the decidua basalis and increase in size and complexity as the vascular processes develop into the placenta. The chorion becomes the covering of the fetal side of the placenta. It contains the major umbilical blood vessels as they branch out over the surface of the placenta. As the embryo grows the decidua capsularis stretches. The chorionic villi on this side atrophy and degenerate, leaving a smooth chorionic membrane.
The inner cell membrane, the amnion, develops from the interior cells of the blastocyst. The cavity that develops between this inner cell mass and the outer layer of cells (trophoblast) is the amniotic cavity (see Fig. 12-5, B). As it grows larger, the amnion forms on the side opposite the developing blastocyst (see Figs. 12-5 and 12-7). The developing embryo draws the amnion around itself, forming a fluid-filled sac. The amnion becomes the covering of the umbilical cord and covers the chorion on the fetal surface of the placenta. As the embryo grows larger, the amnion enlarges to accommodate the embryo/fetus and the surrounding amniotic fluid. The amnion eventually comes into contact with the chorion surrounding the fetus.
Amniotic Fluid
The amniotic cavity initially derives its fluid by diffusion from the maternal blood. Fluid secreted by the respiratory and gastrointestinal tracts of the fetus also enters the amniotic cavity (Moore & Persaud, 2008). The amount of fluid increases weekly, and 700 to 1000 ml of transparent liquid is normally present at term. The amniotic fluid volume changes constantly. The fetus swallows fluid, and fluid flows into and out of the fetal lungs. Beginning in week 11, the fetus urinates into the fluid, increasing its volume.
Amniotic fluid serves many functions. It helps maintain a constant body temperature. It serves as a source of oral fluid and a repository for waste and assists in maintenance of fluid and electrolyte homeostasis. It allows freedom of movement for musculoskeletal development. It cushions the fetus from trauma by blunting and dispersing outside forces. It acts as a barrier to infection and allows fetal lung development (Moore & Persaud, 2008). The fluid keeps the embryo from tangling with the membranes, facilitating symmetric growth. If the embryo does become tangled with the membranes, amputations of extremities or other deformities can occur from constricting amniotic bands.
The volume of amniotic fluid is an important factor in assessing fetal well-being. Having less than 300 ml of amniotic fluid (oligohydramnios) is associated with fetal renal abnormalities. Having more than 2 L of amniotic fluid (hydramnios) is associated with gastrointestinal and other malformations.
Amniotic fluid contains albumin, urea, uric acid, creatinine, lecithin, sphingomyelin, bilirubin, fructose, fat, leukocytes, proteins, epithelial cells, enzymes, and lanugo hair. Study of fetal cells in amniotic fluid through amniocentesis yields much information about the fetus. Genetic studies (karyotyping) provide knowledge about the sex and the number and structure of chromosomes (see Chapter 3). Other studies, such as lecithin/sphingomyelin ratio, determine the health or maturity of the fetus (see Chapter 26).
Yolk Sac
When the amniotic cavity and amnion are forming, another blastocyst cavity forms on the other side of the developing embryonic disk (see Fig. 12-5, B). This cavity becomes surrounded by a membrane, forming the yolk sac. The yolk sac aids in transferring maternal nutrients and oxygen, which have diffused through the chorion, to the embryo. Blood vessels form to aid transport. Blood cells and plasma are manufactured in the yolk sac during the second and third weeks while uteroplacental circulation is being established and is forming primitive blood cells until hematopoietic activity begins. At the end of the third week the primitive heart begins to beat and circulate the blood through the embryo, the connecting stalk, the chorion, and the yolk sac.
The folding in of the embryo during the fourth week results in incorporation of part of the yolk sac into the embryo’s body as the primitive digestive system. Primordial germ cells arise in the yolk sac and move into the embryo. The shrinking remains of the yolk sac degenerate (see Fig. 12-7). By the fifth or sixth week the remnant has separated from the embryo.
Umbilical Cord
By day 14 after conception, the embryonic disk, the amniotic sac, and the yolk sac are attached to the chorionic villi by the connecting stalk. During the third week the blood vessels develop to supply the embryo with maternal nutrients and oxygen. During the fifth week the embryo has curved inward on itself from both ends, bringing the connecting stalk to the ventral side of the embryo. The connecting stalk becomes compressed from both sides by the amnion and forms the narrower umbilical cord (see Fig. 12-7). Two arteries carry blood from the embryo to the chorionic villi, and one vein returns blood to the embryo. Approximately 1% of umbilical cords contain only two vessels: one artery and one vein. This occurrence is sometimes associated with congenital malformations.
The cord rapidly increases in length. At term, the cord is 2 cm in diameter and ranges from 30 to 90 cm long (with an average of 55 cm). It twists spirally on itself and loops around the embryo/fetus. A true knot is rare, but false knots occur as folds or kinks in the cord and may jeopardize circulation to the fetus. Connective tissue called Wharton’s jelly prevents compression of the blood vessels and ensures continued nourishment of the embryo/fetus. Compression can occur if the cord lies between the fetal head and the maternal pelvis or is twisted around the fetal body. When the cord is wrapped around the fetal neck, it is called a nuchal cord.
Because the placenta develops from the chorionic villi, the umbilical cord is usually located centrally. A peripheral location is less common and is termed battledore placenta. The blood vessels are arrayed out from the center to all parts of the placenta.
Placenta
The placenta begins to form at implantation. During the third week after conception, the trophoblast cells of the chorionic villi continue to invade the decidua basalis. As the uterine capillaries are tapped, the endometrial spiral arteries fill with maternal blood. The chorionic villi grow into the spaces with two layers of cells: the outer syncytium and the inner cytotrophoblast. A third layer develops into anchoring septa, dividing the projecting decidua into separate areas called cotyledons. In each of the 15 to 20 cotyledons, the chorionic villi branch out, and a complex system of fetal blood vessels forms. Each cotyledon is a functional unit. The whole structure is the placenta (Fig. 12-9).
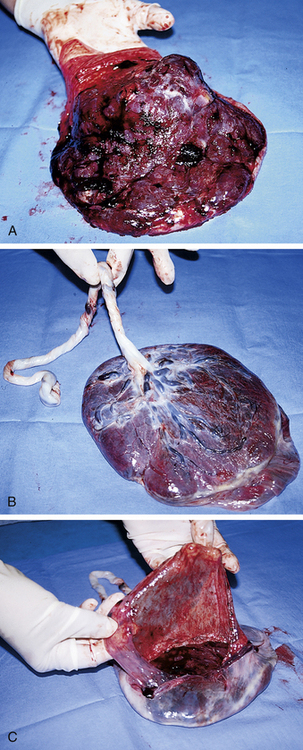
FIG. 12-9 Term placenta. A, Maternal (or uterine) surface, showing cotyledons and grooves. B, Fetal (or amniotic) surface, showing blood vessels running under amnion and converging to form umbilical vessels at attachment of umbilical cord. C, Amnion and smooth chorion are arranged to show that they are (1) fused and (2) continuous with margins of placenta. (Courtesy Marjorie Pyle, RNC, Lifecircle, Costa Mesa, CA.)
The maternal-placental-embryonic circulation is in place by day 17, when the embryonic heart starts beating. By the end of the third week, embryonic blood is circulating between the embryo and the chorionic villi. In the intervillous spaces, maternal blood supplies oxygen and nutrients to the embryonic capillaries in the villi (Fig. 12-10). Waste products and carbon dioxide diffuse into the maternal blood.
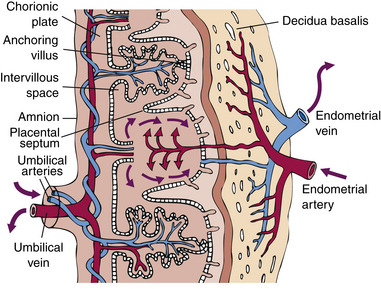
FIG. 12-10 Schematic drawing of the placenta illustrating how it supplies oxygen and nutrition to the embryo and removes its waste products. Deoxygenated blood leaves the fetus through the umbilical arteries and enters the placenta, where it is oxygenated. Oxygenated blood leaves the placenta through the umbilical vein, which enters the fetus via the umbilical cord.
The placenta functions as a means of metabolic exchange. Exchange is minimal at this time because the two cell layers of the villous membrane are too thick. Permeability increases as the cytotrophoblast thins and disappears; by the fifth month, only the single layer of syncytium is left between the maternal blood and the fetal capillaries. The syncytium is the functional layer of the placenta. By the eighth week, genetic testing may be done on a sample of chorionic villi by aspiration biopsy; however, limb defects have been associated with chorionic villus sampling done before 10 weeks. The structure of the placenta is complete by the twelfth week. The placenta continues to grow wider until 20 weeks, when it covers approximately half of the uterine surface. It then continues to grow thicker. The branching villi continue to develop within the body of the placenta, increasing the functional surface area.
Functions
One of the early functions of the placenta is as an endocrine gland that produces hormones necessary to maintain the pregnancy and support the embryo and fetus. The hormones are produced in the syncytium.
The protein hormone human chorionic gonadotropin (hCG) can be detected in the maternal serum by 8 to 10 days after conception, shortly after implantation. This hormone is the basis for pregnancy tests. The hCG preserves the function of the ovarian corpus luteum, ensuring the continued supply of estrogen and progesterone needed to maintain the pregnancy. Miscarriage occurs if the corpus luteum stops functioning before the placenta is producing sufficient estrogen and progesterone. The amount of hCG reaches its maximal level at 50 to 70 days and then begins to decrease.
The other protein hormone produced by the placenta is human chorionic somatomammotropin (hCS) formerly known as human placental lactogen (hPL). This substance is similar to a growth hormone and stimulates the maternal metabolism to supply nutrients needed for fetal growth. This hormone increases the resistance to insulin, facilitates glucose transport across the placental membrane, and stimulates breast development to prepare for lactation (Fig. 12-11).
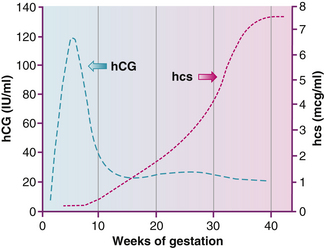
FIG. 12-11 Distinct profile for the concentrations of human chorionic gonadotropin (hCG) and human chorionic somatomammotropin (hCS) in serum of women through normal pregnancy. IU, International units. (Adapted from Cunningham, F., Leveno, K., Bloom, S., Hauth, J., Rouse, D., & Spong, C. [2010]. Williams obstetrics [23rd ed.]. New York: McGraw-Hill.)
The placenta eventually produces more of the steroid hormone progesterone than the corpus luteum does during the first few months of pregnancy. Progesterone maintains the endometrium, decreases the contractility of the uterus, and stimulates maternal metabolism and development of breast alveoli.
By 7 weeks after fertilization the placenta is producing most of the maternal estrogens, which are steroid hormones. The major estrogen secreted by the placenta is estriol, whereas the ovaries produce mostly estradiol. Estriol levels may be measured to determine placental functioning. Estrogen stimulates uterine growth and uteroplacental blood flow. It causes a proliferation of the breast glandular tissue and stimulates myometrial contractility. Placental estrogen production increases greatly toward the end of pregnancy. One theory for the cause of the onset of labor is the decline in the ratio of circulating levels of progesterone to the increased levels of estrogen (Fig. 12-12).
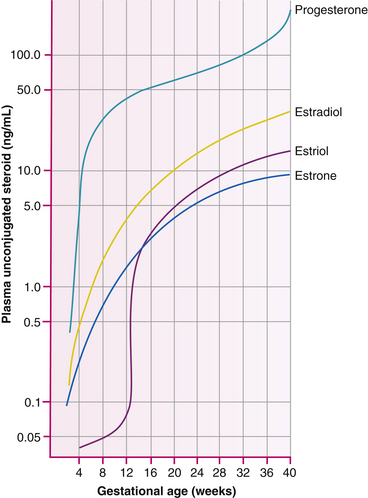
FIG. 12.12 Plasma level of progesterone, estradiol, estrone, and estriol in women during the course of gestation. (From Cunningham, F., Leveno, K., Bloom, S., Hauth, J., Rouse, D., & Spong, C. [2010]. Williams obstetrics [23rd ed.]. New York: McGraw-Hill.)
The metabolic functions of the placenta are respiration, nutrition, excretion, and storage. Oxygen diffuses from the maternal blood across the placental membrane into the fetal blood, and carbon dioxide diffuses in the opposite direction. In this way the placenta functions as lungs for the fetus.
Carbohydrates, proteins, calcium, and iron are stored in the placenta for ready access to meet fetal needs. Water, inorganic salts, carbohydrates, proteins, fats, and vitamins pass from the maternal blood supply across the placental membrane into the fetal blood, supplying nutrition. Water and most electrolytes with a molecular weight below 500 readily diffuse through the membrane. Hydrostatic and osmotic pressures aid the flow of water and some solutions. Facilitated and active transport assist in the transfer of glucose, amino acids, calcium, iron, and substances with higher molecular weights. Amino acids and calcium are transported against the concentration gradient between the maternal blood and fetal blood.
The fetal concentration of glucose is lower than the glucose level in the maternal blood because of its rapid metabolism by the fetus. This fetal requirement demands larger concentrations of glucose than simple diffusion can provide. Therefore maternal glucose moves into the fetal circulation by active transport.
Pinocytosis is a mechanism used for transferring large molecules, such as albumin and gamma globulins, across the placental membrane. This mechanism conveys the maternal immunoglobulins that provide early passive immunity to the fetus.
Metabolic waste products of the fetus cross the placental membrane from the fetal blood into the maternal blood. The maternal kidneys then excrete them. Many viruses can cross the placental membrane and infect the fetus. Some bacteria and protozoa first infect the placenta and then infect the fetus. Drugs also can cross the placental membrane and may harm the fetus. Caffeine, alcohol, nicotine, carbon monoxide, and other toxic substances in cigarette smoke, as well as prescription and recreational drugs (such as cocaine and marijuana) readily cross the placenta (Box 12-1).
Although no direct link exists between the fetal blood in the vessels of the chorionic villi and the maternal blood in the intervillous spaces, only one cell layer separates them. Breaks occasionally occur in the placental membrane. Fetal erythrocytes then leak into the maternal circulation, and the mother may develop antibodies to the fetal red blood cells. This is often the way an Rh-negative mother becomes sensitized to the erythrocytes of her Rh-positive fetus. (See discussions of isoimmunization in Chapters 23 and 34.)
Although the placenta and the fetus are analogous to living tissue transplants, they are not destroyed by the host mother (Mor & Abrahams, 2009). Either the placental hormones suppress the immunologic response, or the tissue evokes no response.
Placental function depends on the maternal blood pressure supplying circulation. Maternal arterial blood, under pressure in the small uterine spiral arteries, spurts into the intervillous spaces (see Fig. 12-10). As long as rich arterial blood continues to be supplied, pressure is exerted on the blood already in the intervillous spaces, pushing it toward drainage by the low-pressure uterine veins. At term gestation 10% of the maternal cardiac output goes to the uterus.
If there is interference with the circulation to the placenta, the placenta cannot supply the embryo or fetus. Vasoconstriction, such as that caused by hypertension and cocaine use, diminishes uterine blood flow. Decreased maternal blood pressure or cardiac output also diminishes uterine blood flow.
When a woman lies on her back with the pressure of the uterus compressing the vena cava, blood return to the right atrium is diminished (see discussion of supine hypotension in Chapter 19 and Fig. 19-5). Excessive maternal exercise that diverts blood to the muscles away from the uterus compromises placental circulation. Optimal circulation is achieved when the woman is lying at rest on her side. Decreased uterine circulation may lead to intrauterine growth restriction of the fetus and to infants who are small for gestational age.
Braxton Hicks contractions appear to enhance the movement of blood through the intervillous spaces, aiding placental circulation. Prolonged contractions or too-short intervals between contractions during labor, however, reduce blood flow to the placenta.
Fetal Maturation
The stage of the fetus lasts from 9 weeks (when the fetus becomes recognizable as a human being) until the pregnancy ends. Changes during the fetal period are not so dramatic, because refinement of structure and function are taking place. The fetus is less vulnerable to teratogens, except for those affecting central nervous system functioning.
Viability refers to the capability of the fetus to survive outside the uterus. With modern technology and advancements in maternal and neonatal care, infants who are 22 to 25 weeks of gestation are now considered to be on the threshold of viability (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010). The limitations on survival outside the uterus when an infant is born at this early stage are based on central nervous system function and the oxygenation capability of the lungs.
Fetal Circulatory System
The cardiovascular system is the first organ system to function in the developing human. Blood vessel and blood cell formation begins in the third week and supplies the embryo with oxygen and nutrients from the mother. By the end of the third week the tubular heart begins to beat, and the primitive cardiovascular system links the embryo, connecting stalk, chorion, and yolk sac. During the fourth and fifth weeks the heart develops into a four-chambered organ. By the end of the embryonic stage the heart is developmentally complete.
The fetal lungs do not function for respiratory gas exchange, so a special circulatory pathway, the ductus arteriosus, bypasses the lungs. Oxygen-rich blood from the placenta flows rapidly through the umbilical vein into the fetal abdomen (Fig. 12-13). When the umbilical vein reaches the liver, it divides into two branches; one branch circulates some oxygenated blood through the liver. Most of the blood passes through the ductus venosus into the inferior vena cava. There it mixes with the deoxygenated blood from the fetal legs and abdomen on its way to the right atrium. Most of this blood passes straight through the right atrium and through the foramen ovale, an opening into the left atrium. There it mixes with the small amount of deoxygenated blood returning from the fetal lungs through the pulmonary veins.
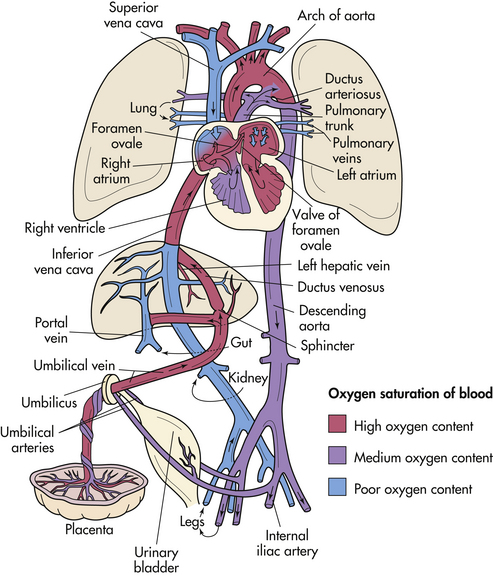
FIG. 12-13 Schematic illustration of the fetal circulation. The colors indicate the oxygen saturation of the blood, and the arrows show the course of the blood from the placenta to the heart. The organs are not drawn to scale. Observe that three shunts permit most of the blood to bypass the liver and lungs: (1) ductus venosus, (2) foramen ovale, and (3) ductus arteriosus. A small amount of highly oxygenated blood from the inferior vena cava remains in the right atrium and mixes with poorly oxygenated blood from the superior vena cava. This medium oxygenated blood then passes into the right ventricle. The poorly oxygenated blood returns to the placenta for oxygen and nutrients through the umbilical arteries. (Modified with permission from Moore, K., & Persaud, T. [2008]. Before we are born: Essentials of embryology and birth defects [7th ed.]. Philadelphia: Saunders).
The blood flows into the left ventricle and is squeezed out into the aorta, where the arteries supplying the heart, head, neck, and arms receive most of the oxygen-rich blood. This pattern of supplying the highest levels of oxygen and nutrients to the head, neck, and arms enhances the cephalocaudal (head-to-rump) development of the embryo/fetus. Deoxygenated blood returning from the head and arms enters the right atrium through the superior vena cava. This blood is directed downward into the right ventricle, where it is squeezed into the pulmonary artery. A small amount of blood circulates through the resistant lung tissue, but the majority follows the path with less resistance through the ductus arteriosus into the aorta, distal to the point of exit of the arteries supplying the head and arms with oxygenated blood. The oxygen-poor blood flows through the abdominal aorta into the internal iliac arteries, where the umbilical arteries direct most of it back through the umbilical cord to the placenta. There the blood gives up its wastes and carbon dioxide in exchange for nutrients and oxygen. The blood remaining in the iliac arteries flows through the fetal abdomen and legs, ultimately returning through the inferior vena cava to the heart.
The following three special characteristics enable the fetus to obtain sufficient oxygen from the maternal blood:
Hematopoietic System
Hematopoiesis, the formation of blood, occurs in the yolk sac (see Fig. 12-5, B) beginning in the third week. Hematopoietic stem cells seed the fetal liver during the fifth week, and hematopoiesis begins there during the sixth week. This accounts for the relatively large size of the liver between the seventh and ninth weeks. Stem cells seed the fetal bone marrow, spleen, thymus, and lymph nodes between weeks 8 and 11 (for more information about stem cells see http://stemcells.nih.gov).
The antigenic factors that determine blood type are present in the erythrocytes soon after the sixth week. For this reason the Rh-negative woman is at risk for isoimmunization in any pregnancy that lasts longer than 6 weeks after fertilization.
Gastrointestinal System
During the fourth week the shape of the embryo changes from being almost straight to a C shape, as both ends fold in toward the ventral surface. A portion of the yolk sac is incorporated into the body from head to tail as the primitive gut (digestive system).
The foregut produces the pharynx, part of the lower respiratory tract, the esophagus, the stomach, the first half of the duodenum, the liver, the pancreas, and the gallbladder. These structures evolve during the fifth and sixth weeks. The malformations that can occur in these areas are esophageal atresia, hypertrophic pyloric stenosis, duodenal stenosis or atresia, and biliary atresia (see Chapter 36).
The midgut becomes the distal half of the duodenum, the jejunum and the ileum, the cecum and the appendix, and the proximal half of the colon. The midgut loop projects into the umbilical cord between weeks 5 and 10. A malformation (omphalocele) results if the midgut fails to return to the abdominal cavity, causing the intestines to protrude from the umbilicus (see Fig. 36-9, A). Meckel’s diverticulum, the most common malformation of the midgut, occurs when a remnant of the yolk stalk that has failed to degenerate attaches to the ileum, leaving a blind sac.
The hindgut develops into the distal half of the colon, the rectum and parts of the anal canal, the urinary bladder, and the urethra. Anorectal malformations are the most common abnormalities of the digestive system.
The fetus swallows amniotic fluid beginning in the fifth month. Gastric emptying and intestinal peristalsis occur. Fetal nutrition and elimination needs are taken care of by the placenta. As the fetus nears term, fetal waste products accumulate in the intestines as dark green to black, tarry meconium. Normally this substance is passed through the rectum within 24 hours of birth. Sometimes with a breech presentation or fetal hypoxia, meconium is passed in utero into the amniotic fluid. The failure to pass meconium after birth may indicate atresia somewhere in the digestive tract, an imperforate anus (see Fig. 36-10), or meconium ileus, in which a firm meconium plug blocks passage (seen in infants with cystic fibrosis).
The metabolic rate of the fetus is relatively low, but the fetus has great growth and development needs. Beginning in week 9 the fetus synthesizes glycogen for storage in the liver. Between 26 and 30 weeks the fetus begins to lay down stores of brown fat in preparation for extrauterine cold stress. Thermoregulation in the neonate requires increased metabolism and adequate oxygenation.
The gastrointestinal system is mature by 36 weeks. Digestive enzymes (except pancreatic amylase and lipase) are present in sufficient quantity to facilitate digestion. The neonate cannot digest starches or fats efficiently. Little saliva is produced.
Hepatic System
The liver and biliary tract develop from the foregut during the fourth week of gestation. Hematopoiesis begins during the sixth week and requires that the liver be large. The embryonic liver is prominent, occupying most of the abdominal cavity. Bile, a constituent of meconium, begins to form in the twelfth week.
Glycogen is stored in the fetal liver beginning at week 9 or 10. At term, glycogen stores are twice those of the adult. Glycogen is the major source of energy for the fetus and neonate stressed by in utero hypoxia, extrauterine loss of the maternal glucose supply, the work of breathing, or cold.
Iron also is stored in the fetal liver. If the maternal intake is sufficient, the fetus can store enough iron to last for 5 months after birth.
During fetal life the liver does not have to conjugate bilirubin for excretion because the unconjugated bilirubin is cleared by the placenta. Therefore the glucuronyl transferase enzyme needed for conjugation is present in the fetal liver in amounts less than those required after birth. This predisposes the neonate, especially the preterm infant, to hyperbilirubinemia.
Coagulation factors II, VII, IX, and X cannot be synthesized in the fetal liver because of the lack of vitamin K synthesis in the sterile fetal gut. This coagulation deficiency persists after birth for several days and is the rationale for the prophylactic administration of vitamin K to the newborn.
Respiratory System
The respiratory system begins development during embryonic life and continues through fetal life and into childhood. The development of the respiratory tract begins in week 4 and continues through week 17 with formation of the larynx, the trachea, the bronchi, and the lung buds. Between 16 and 24 weeks the bronchi and terminal bronchioles enlarge, and vascular structures and primitive alveoli are formed. Between 24 weeks and term more alveoli form. Specialized alveolar cells, type I and type II cells, secrete pulmonary surfactants to line the interior of the alveoli. After 32 weeks sufficient surfactant is present in developed alveoli to provide infants with a good chance of survival.
Pulmonary Surfactants: The detection of the presence of pulmonary surfactants, surface-active phospholipids, in amniotic fluid has been used to determine the degree of fetal lung maturity, or the ability of the lungs to function after birth. Lecithin (L) is the most critical alveolar surfactant required for postnatal lung expansion. It is detectable at approximately 21 weeks and increases in amount after week 24. Another pulmonary phospholipid, sphingomyelin (S), remains constant in amount. Thus the measure of lecithin in relation to sphingomyelin, or the L/S ratio, is used to determine fetal lung maturity. When the L/S ratio reaches 2:1, the infant’s lungs are considered to be mature. This occurs at approximately 35 weeks of gestation (Mercer, 2009).
Certain maternal conditions that cause decreased maternal blood flow, such as maternal hypertension, placental dysfunction, infection, or corticosteroid use, can accelerate fetal lung maturity. This apparently is caused by the resulting fetal hypoxia, which stresses the fetus and increases the blood levels of corticosteroids that accelerate alveolar and surfactant development.
Conditions such as gestational diabetes and chronic glomerulonephritis can retard fetal lung maturity. The use of intrabronchial synthetic surfactant in the treatment of respiratory distress syndrome in the newborn has greatly improved the chances of survival for preterm infants.
Fetal respiratory movements have been seen on ultrasound examination as early as week 11. These fetal respiratory movements may aid in development of the chest wall muscles and regulate lung fluid volume. The fetal lungs produce fluid that expands the air spaces in the lungs. The fluid drains into the amniotic fluid or is swallowed by the fetus.
Before birth, secretion of lung fluid decreases. The normal birth process squeezes out approximately one third of the fluid. Infants born by cesarean do not benefit from this squeezing process, thus they may have more respiratory difficulty at birth. The fluid remaining in the lungs at birth is usually reabsorbed into the infant’s bloodstream within 2 hours of birth.
Renal System
The kidneys form during the fifth week and begin to function approximately 4 weeks later. Urine is excreted into the amniotic fluid and forms a major part of the amniotic fluid volume. Oligohydramnios is indicative of renal dysfunction. Because the placenta acts as the organ of excretion and maintains fetal water and electrolyte balance, the fetus does not need functioning kidneys while in utero. At birth, however, the kidneys are required immediately for excretory and acid-base regulatory functions.
A fetal renal malformation can be diagnosed in utero. Corrective or palliative fetal surgery may treat the malformation successfully, or plans can be made for treatment immediately after birth.
At term the fetus has fully developed kidneys. However, the glomerular filtration rate (GFR) is low, and the kidneys lack the ability to concentrate urine. This makes the newborn more susceptible to both overhydration and dehydration.
Most newborns void within 24 hours of birth. With the loss of the swallowed amniotic fluid and the metabolism of nutrients provided by the placenta, the amount voided for the first days of life is scanty until fluid intake increases.
Neurologic System
The nervous system originates from the ectoderm during the third week after fertilization. The open neural tube forms during the fourth week. It initially closes at what will be the junction of the brain and spinal cord, leaving both ends open. The embryo folds in on itself lengthwise at this time, forming a head fold in the neural tube at this junction. The cranial end of the neural tube closes, and then the caudal end closes. During week 5, different growth rates cause more flexures in the neural tube, delineating three brain areas: the forebrain, the midbrain, and the hindbrain.
The forebrain develops into the eyes (cranial nerve II) and the cerebral hemispheres. The development of all areas of the cerebral cortex continues throughout fetal life and into childhood. The olfactory system (cranial nerve I) and the thalamus also develop from the forebrain. Cranial nerves III and IV (oculomotor and trochlear) form from the midbrain. The hindbrain forms the medulla, the pons, the cerebellum, and the remainder of the cranial nerves. Brain waves can be recorded on an electroencephalogram by week 8.
The spinal cord develops from the long end of the neural tube. Another ectodermal structure, the neural crest, develops into the peripheral nervous system. By the eighth week, nerve fibers traverse throughout the body. By week 11 or 12 the fetus makes respiratory movements, moves all extremities, and changes position in utero. The fetus can suck his or her thumb and swim in the amniotic fluid pool, turn somersaults, and occasionally tie a knot in the umbilical cord. Sometime between 16 and 20 weeks, when the movements are strong enough to be perceived by the mother as “the baby moving,” quickening has occurred. The perception of movement occurs earlier in the multipara than in the primipara. The mother also becomes aware of the sleep and wake cycles of the fetus.
Sensory Awareness: Purposeful movements of the fetus have been demonstrated in response to a firm touch transmitted through the mother’s abdomen. Because it can feel, the fetus requires anesthesia when invasive procedures are done.
Fetuses respond to sound by 24 weeks. Different types of music evoke different movements. The fetus can be soothed by the sound of the mother’s voice. Acoustic stimulation can be used to evoke a fetal heart rate response. The fetus becomes accustomed (i.e., habituates) to noises heard repeatedly. Hearing is fully developed at birth.
The fetus is able to distinguish taste. By the fifth month, when the fetus is swallowing amniotic fluid, a sweetener added to the fluid causes the fetus to swallow faster. The fetus also reacts to temperature changes. A cold solution placed into the amniotic fluid can cause fetal hiccups.
The fetus can see. Eyes have both rods and cones in the retina by the seventh month. A bright light shone on the mother’s abdomen in late pregnancy causes abrupt fetal movements. During sleep time, rapid eye movements have been observed similar to those occurring in children and adults while dreaming.
At term the fetal brain is approximately one fourth the size of an adult brain. Neurologic development continues. Stressors on the fetus and neonate (e.g., chronic poor nutrition or hypoxia, drugs, environmental toxins, trauma, disease) cause damage to the central nervous system long after the vulnerable embryonic time for malformations in other organ systems. Neurologic insult can result in cerebral palsy, neuromuscular impairment, mental retardation, and learning disabilities.
Endocrine System
The thyroid gland develops along with structures in the head and neck during the third and fourth weeks. The secretion of thyroxine begins during the eighth week. Maternal thyroxine does not readily cross the placenta; therefore, the fetus who does not produce thyroid hormones will be born with congenital hypothyroidism. If untreated, hypothyroidism can result in severe mental retardation. Screening for hypothyroidism is typically included in the testing when screening for phenylketonuria (PKU) after birth.
The adrenal cortex is formed during the sixth week and produces hormones by the eighth or ninth week. As term approaches, the fetus produces more cortisol. This is believed to aid in initiation of labor by decreasing the maternal progesterone and stimulating production of prostaglandins.
The pancreas forms from the foregut during the fifth through eighth weeks. The islets of Langerhans develop during the twelfth week. Insulin is produced by week 20. In infants of mothers with uncontrolled diabetes, maternal hyperglycemia produces fetal hyperglycemia, stimulating hyperinsulinemia and islet cell hyperplasia. This results in a macrosomic (large) fetus. The hyperinsulinemia also blocks lung maturation, placing the neonate at risk for respiratory distress and hypoglycemia when the maternal glucose source is lost at birth. Control of the maternal glucose level before and during pregnancy minimizes problems for the fetus and infant.
Reproductive System
Sex differentiation begins in the embryo during the seventh week. Female and male external genitalia are indistinguishable until after the ninth week. Distinguishing characteristics appear around the ninth week and are fully differentiated by the twelfth week. When a Y chromosome is present, testes are formed. By the end of the embryonic period, testosterone is being secreted and causes formation of the male genitalia. By week 28 the testes begin descending into the scrotum. After birth low levels of testosterone continue to be secreted until the pubertal surge.
The female, with two X chromosomes, forms ovaries and female external genitalia. By the sixteenth week oogenesis has been established. At birth the ovaries contain the female’s lifetime supply of ova. Most female hormone production is delayed until puberty. However, the fetal endometrium responds to maternal hormones, and withdrawal bleeding or vaginal discharge (pseudomenstruation) may occur at birth when these hormones are lost. The high level of maternal estrogen also stimulates mammary engorgement and secretion of fluid (“witch’s milk”) in newborn infants of both sexes.
Musculoskeletal System
Bones and muscles develop from the mesoderm by the fourth week of embryonic development. At that time the cardiac muscle is already beating. The mesoderm next to the neural tube forms the vertebral column and ribs. The parts of the vertebral column grow toward each other to enclose the developing spinal cord. Ossification, or bone formation, begins. If there is a defect in the bony fusion, various forms of spina bifida may occur. A large defect affecting several vertebrae may allow the membranes and spinal cord to pouch out from the back, producing neurologic deficits and skeletal deformity.
The flat bones of the skull develop during the embryonic period, and ossification continues throughout childhood. At
birth connective tissue sutures exist where the bones of the skull meet. The areas where more than two bones meet (called fontanels) are especially prominent. The sutures and fontanels allow the bones of the skull to mold, or move during birth, enabling the head to pass through the birth canal.
The bones of the shoulders, arms, hips, and legs appear in the sixth week as a continuous skeleton with no joints. Differentiation occurs, producing separate bones and joints. Ossification will continue through childhood to allow growth. Beginning during the seventh week, muscles contract spontaneously. Arm and leg movements are visible on ultrasound examination, although the mother does not perceive them until sometime between 16 and 20 weeks.
Integumentary System
The epidermis begins as a single layer of cells derived from the ectoderm at 4 weeks. By the seventh week there are two layers of cells. The cells of the superficial layer are sloughed and become mixed with the sebaceous gland secretions to form the white, cheesy vernix caseosa, the material that protects the skin of the fetus. The vernix is thick at 24 weeks but becomes scant by term.
The basal layer of the epidermis is the germinal layer, which replaces lost cells. Until 17 weeks the skin is thin and wrinkled, with blood vessels visible underneath. The skin thickens and all layers are present at term. After 32 weeks as subcutaneous fat is deposited under the dermis, the skin becomes less wrinkled and red in appearance.
By 16 weeks the epidermal ridges are present on the palms of the hands, the fingers, the bottom of the feet, and the toes. These handprints and footprints are unique to that infant.
Hairs form from hair bulbs in the epidermis that project into the dermis. Cells in the hair bulb keratinize to form the hair shaft. As the cells at the base of the hair shaft proliferate, the hair grows to the surface of the epithelium. Very fine hairs, called lanugo, appear first at 12 weeks on the eyebrows and upper lip. By 20 weeks they cover the entire body. At this time the eyelashes, eyebrows, and scalp hair are beginning to grow. By 28 weeks the scalp hair is longer than the lanugo, which thins and may disappear by term gestation.
Fingernails and toenails develop from thickened epidermis at the tips of the digits beginning during the tenth week. They grow slowly. Fingernails usually reach the fingertips by 32 weeks, and toenails reach toe tips by 36 weeks.
Immunologic System
During the third trimester albumin and globulin are present in the fetus. The only immunoglobulin that crosses the placenta, immunoglobulin G (IgG), provides passive acquired immunity to specific bacterial toxins. The fetus produces IgM by the end of the first trimester. These are produced in response to blood group antigens, gram-negative enteric organisms, and some viruses. IgA is not produced by the fetus; however, colostrum, the precursor to breast milk, contains large amounts of IgA and can provide passive immunity to the neonate who is breastfed.
The normal term neonate can fight infection but not so effectively as an older child. The preterm infant is at much greater risk for infection.
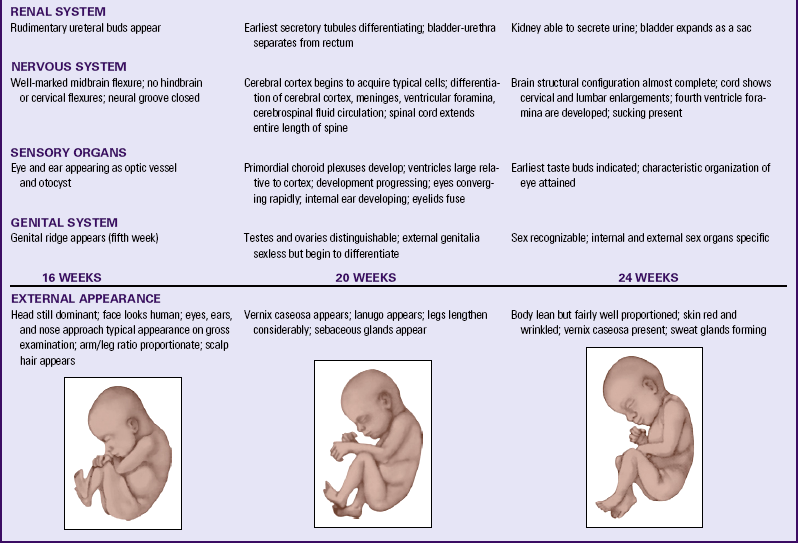
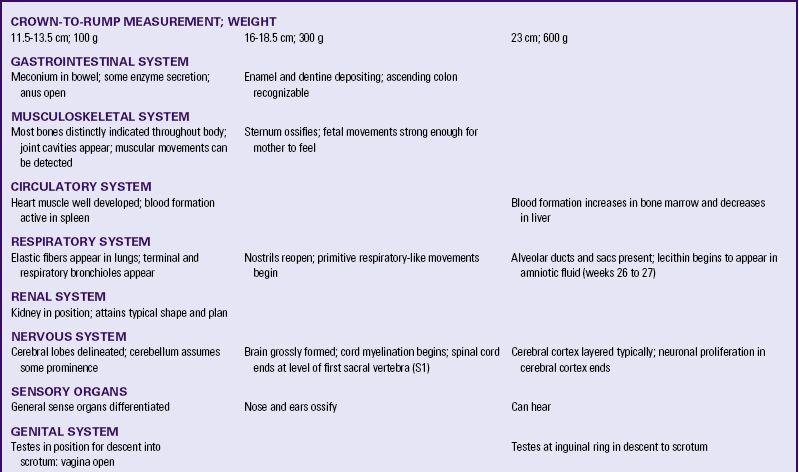
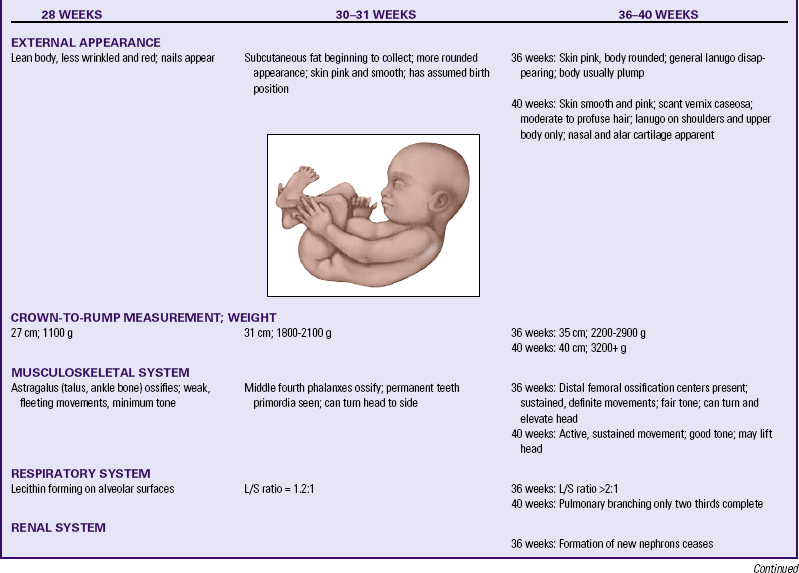
Table 12-1 summarizes embryonic and fetal development.
Multifetal Pregnancy
The incidence of twinning is 1 in 30 pregnancies. There has been a steady rise in multiple births since 1973 (Benirschke, 2009). This is partly attributed to the availability of assisted reproductive technologies and the increasing age at which women give birth (Malone & D’Alton, 2009).
Dizygotic Twins: When two mature ova are produced in one ovarian cycle, both have the potential to be fertilized by separate sperm. This results in two zygotes, or dizygotic twins (Fig. 12-14). There are always two amnions, two chorions, and two placentas, which may be fused (Fig. 12-15). These dizygotic, or fraternal, twins can be the same sex or different sexes and are genetically no more alike than siblings born at different times. Dizygotic twinning occurs in families, more often among African-American women than among Caucasian women, and least often among Asian women. Dizygotic twinning increases in frequency with maternal age up to 35 years, with parity, and with the use of fertility drugs.
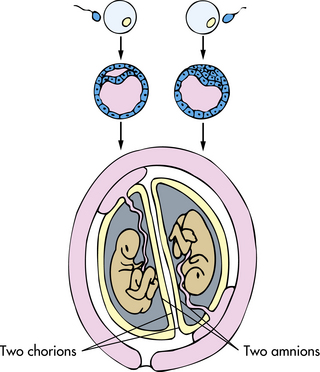
FIG. 12-14 Formation of dizygotic twins, with fertilization of two ova, two implantations, two placentas, two chorions, and two amnions.
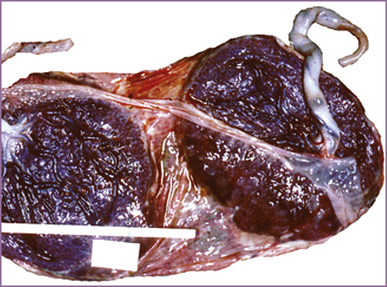
FIG. 12-15 Diamniotic dichorionic (separate) twin placenta. (From Benirschke, K. [2009]. Multiple gestation. The biology of twinning. In R. Creasy, R. Resnik, J. Iams, C. Lockwood, & T. Moore (Eds.), Creasy and Resnik’s maternal-fetal medicine: Principles and practice (6th ed.). Philadelphia: Saunders.
Monozygotic Twins: Identical or monozygotic twins develop from one fertilized ovum, which then divides (Fig. 12-16). They are the same sex and have the same genotype. If division occurs soon after fertilization, two embryos, two amnions, two chorions, and two placentas that can be fused will develop. Most often, division occurs between 4 and 8 days after fertilization, and there are two embryos, two amnions, one chorion, and one placenta. Rarely, division occurs after the eighth day after fertilization. In this case, there are two embryos within a common amnion and a common chorion with one placenta. This often causes circulatory problems because the umbilical cords may tangle together, and one or both fetuses may die. If division occurs very late, cleavage may not be complete, and conjoined twins could result. Monozygotic twinning occurs approximately 3.5 to 4 per 1000 births (Benirschke, 2009). There is no association with race, heredity, maternal age, or parity. Fertility drugs increase the incidence of monozygotic twinning.
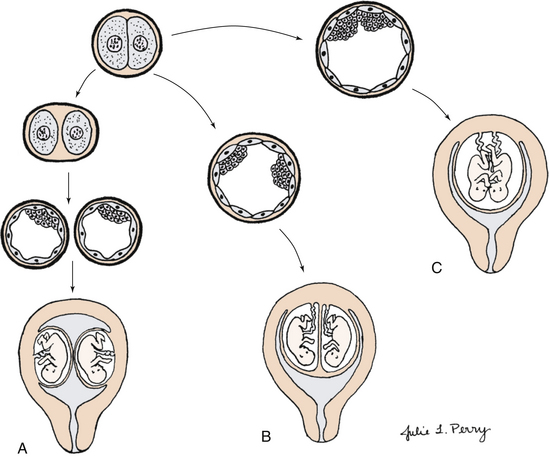
FIG. 12-16 Formation of monozygotic twins. A, One fertilization: blastomeres separate, resulting in two implantations, two placentas, and two sets of membranes. B, One blastomere with two inner cell masses, one fused placenta, one chorion, and separate amnions. C, One blastomere with incomplete separation of cell mass, resulting in conjoined twins.
Conjoined Twins: Conjoined twins are a type of monozygotic twins in which there is incomplete embryonic division at 13 to 15 days postconception (see Fig. 12-16). The estimated frequency is 1 in 50,000 births (Malone & D’Alton, 2009). Prenatal diagnosis is possible with three-dimensional ultrasonography. Cesarean birth minimizes trauma to mother and fetuses.
Other Multifetal Pregnancies
The occurrence of multifetal pregnancies with three or more fetuses has increased with the use of fertility drugs and in vitro fertilization. Triplets occur in approximately 1 of 1341 pregnancies (Benirschke, 2009). They can occur from the division of one zygote into two, with one of the two dividing again, producing identical triplets. Triplets also can be produced from two zygotes, one dividing into a set of identical twins, and the second zygote developing as a single fraternal sibling, or from three zygotes. Quadruplets, quintuplets, sextuplets, and so on, likewise have similar possible derivations.
Nongenetic Factors Influencing Development
Congenital disorders may be inherited, may be caused by environmental factors, or by inadequate maternal nutrition. Congenital means that the condition was present at birth. Some congenital malformations may be the result of teratogens, that is, environmental substances or exposures that result in functional or structural disability. In contrast to other forms of developmental disabilities, disabilities caused by teratogens are theoretically totally preventable. Known human teratogens are certain drugs and chemicals, infections, exposure to radiation, and certain maternal conditions such as diabetes and PKU (see Box 12-1). A teratogen has the greatest effect on the organs and parts of an embryo during its periods of rapid growth and differentiation. This occurs during the embryonic period, specifically from days 15 to 60. During the first 2 weeks of development, teratogens either have no effect or have effects so severe that they cause miscarriage. Brain growth and development continue during the fetal period, and teratogens can severely affect mental development throughout gestation (see Fig. 12-8).
In addition to the genetic makeup and the influence of teratogens, the adequacy of maternal nutrition influences development. The embryo and fetus must obtain the nutrients they need from the mother’s diet; they cannot tap the maternal reserves. Malnutrition during pregnancy produces low-birth-weight newborns who are susceptible to infection. Malnutrition also affects brain development during the latter half of gestation and can result in learning disabilities in the child. Inadequate folic acid is associated with neural tube defects.
KEY POINTS
• Mitosis is the process by which body cells replicate for growth and development and cell replacement of the organism.
• Meiosis is the process by which gametes are formed for reproduction of the organism.
• Human gestation lasts approximately 280 days after the last menstrual period or 266 days after conception.
• Fertilization occurs in the uterine tube within 24 hours of ovulation. The zygote undergoes mitotic divisions, creating a 16-cell morula.
• Implantation begins 6 days after fertilization.
• The organ systems and external features develop during the embryonic period, that is, the third to the eighth week after fertilization.
• Refinement of structure and function occurs during the fetal period, and the fetus becomes capable of extrauterine survival.
• During critical periods in human development, the embryo and fetus are vulnerable to environmental teratogens.
![]() Audio Chapter Summaries Access an audio summary of these Key Points on
Audio Chapter Summaries Access an audio summary of these Key Points on ![]()