Physiologic and Behavioral Adaptations of the Newborn
• Discuss the physiologic adaptations that the neonate must make during the period of transition from the intrauterine to the extrauterine environment.
• Describe the behavioral adaptations that are characteristic of the newborn during the transition period.
• Explain the mechanisms of thermoregulation in the neonate and the potential consequences of hypothermia and hyperthermia.
• Recognize newborn reflexes and differentiate characteristic responses from abnormal responses.
• Discuss the sensory and perceptual functioning of the neonate.
• Identify signs that the neonate is at risk related to problems with each body system.
![]() http://evolve.elsevier.com/Lowdermilk/MWHC/
http://evolve.elsevier.com/Lowdermilk/MWHC/
Anatomy Review
Audio Glossary
Case Study
NCLEX Review Questions
The neonatal period includes the time from birth through day 28 of life. During this time the neonate must make many physiologic and behavioral adaptations to extrauterine life. Physiologic adjustment tasks are those that involve: (1) establishing and maintaining respirations; (2) adjusting to circulatory changes; (3) regulating temperature; (4) ingesting, retaining, and digesting nutrients; (5) eliminating waste; and (6) regulating weight. Behavioral tasks include: (1) establishing a regulated behavioral tempo independent of the mother, which involves self-regulation of arousal, self-monitoring of changes in state, and patterning of sleep; (2) processing, storing, and organizing multiple stimuli; and (3) establishing a relationship with caregivers and the environment. The term infant usually makes these adjustments with little or no difficulty.
Transition to Extrauterine Life
The major adaptations associated with transition from intrauterine to extrauterine life occur during the first 6 to 8 hours after birth. The predictable series of events during transition are mediated by the sympathetic nervous system and result in changes that involve heart rate, respirations, temperature, and gastrointestinal function. This transition period represents a time of vulnerability for the neonate and warrants careful observation by nurses. To detect disorders in adaptation soon after birth, nurses must be aware of normal features of the transition period.
In their classic work on newborn adaptation to extrauterine life, Desmond and associates (1966) proposed three stages of newborn transition. The stages are still considered valid today. (Desmond, Rudolph, & Phitaksphraiwan, 1966).
The first stage of the transition period lasts up to 30 minutes after birth and is called the first period of reactivity. The newborn’s heart rate increases rapidly to 160 to 180 beats/min but gradually falls after 30 minutes or so to a baseline rate of 100 to 120 beats/min. Respirations are irregular, with a rate between 60 and 80 breaths/min. Fine crackles can be present on auscultation. Audible grunting, nasal flaring, and retractions of the chest also can be present, but these should cease within the first hour of birth. The infant is alert and may have spontaneous startles, tremors, crying, and head movement from side to side. Bowel sounds are audible, and meconium may be passed.
After the first period of reactivity the newborn either sleeps or has a marked decrease in motor activity. This period of decreased responsiveness lasts from 60 to 100 minutes. During this time the infant is pink and respirations are rapid and shallow (up to 60 breaths/min), but unlabored. Bowel sounds are audible and peristaltic waves may be noted over the rounded abdomen.
The second period of reactivity occurs roughly between 2 and 8 hours after birth and lasts from 10 minutes to several hours. Brief periods of tachycardia and tachypnea occur, associated with increased muscle tone, changes in skin color, and mucus production. Meconium is commonly passed at this time. Most healthy newborns experience this transition regardless of gestational age or type of birth; extremely and very preterm infants do not because of physiologic immaturity.
Physiologic Adaptations
As the infant emerges from the intrauterine environment and the umbilical cord is severed, profound adaptations are necessary for survival. The most critical of these adaptations is the establishment of effective respirations. Most newborns breathe spontaneously after birth and are able to maintain adequate oxygenation. Preterm infants often encounter respiratory difficulties related to immaturity of the lungs.
Initiation of Breathing
During intrauterine life, oxygenation of the fetus occurs through transplacental gas exchange. However, at birth the lungs must be established as the site of gas exchange. In utero, fetal blood was shunted away from the lungs, but when birth occurs, the pulmonary vasculature must be fully perfused for this purpose. Clamping the umbilical cord causes a rise in blood pressure, which increases circulation and lung perfusion.
It has been recognized that there is no one single trigger for newborn respiratory function. The initiation of respirations in the neonate is the result of a combination of chemical, mechanical, thermal, and sensory factors.
Chemical Factors: The activation of chemoreceptors in the carotid arteries and the aorta results from the relative state of hypoxia associated with labor. With each labor contraction, there is a temporary decrease in uterine blood flow and transplacental gas exchange resulting in transient fetal hypoxia and hypercarbia. Although the fetus is able to recover between contractions, there appears to be a cumulative effect that results in progressive decline in Po2, increased Pco2, and lowered blood pH. Decreased levels of oxygen and increased levels of carbon dioxide seem to have a cumulative effect that is involved in initiating neonatal breathing by stimulating the respiratory center in the medulla. Another chemical factor may also play a role; it is thought that as a result of clamping the cord, there is a drop in levels of a prostaglandin that can inhibit respirations.
Mechanical Factors: Respirations in the newborn can be stimulated by changes in intrathoracic pressure resulting from compression of the chest during vaginal birth. As the infant passes through the birth canal, the chest is compressed. With birth this pressure on the chest is released and the negative intrathoracic pressure helps draw air into the lungs. Crying increases the distribution of air in the lungs and promotes expansion of the alveoli. The positive pressure created by crying helps to keep the alveoli open.
Thermal Factors: With birth the newborn enters the extrauterine environment, in which the temperature is significantly lower. The profound change in environmental temperature stimulates receptors in the skin, resulting in stimulation of the respiratory center in the medulla.
Sensory Factors: Sensory stimulation occurs in a variety of ways with birth. Some of these include handling the infant by the physician or midwife, suctioning the mouth and nose, and drying by the nurses. Pain associated with birth can also be a factor. The lights, sounds, and smells of the new environment can also be involved in stimulation of the respiratory center.
At term the lungs hold approximately 20 ml of fluid per kilogram. Air must be substituted for the fluid that filled the fetal respiratory tract. It has been traditionally thought that the thoracic squeeze occurring during normal vaginal birth resulted in significant clearance of lung fluid. However, it appears that this event plays a minor role. In the days preceding labor, there is reduced production of fetal lung fluid and concomitant decreased alveolar fluid volume. Shortly before the onset of labor, there is a catecholamine surge that seems to promote fluid clearance from the lungs. The movement of lung fluid from the air spaces occurs through active transport into the interstitium, with drainage occurring through the pulmonary circulation and lymphatic system. Retention of lung fluid can interfere with the infant’s ability to maintain adequate oxygenation, especially if other factors (e.g., meconium aspiration, congenital diaphragmatic hernia, esophageal atresia with fistula, choanal atresia, congenital cardiac defect, immature alveoli) that compromise respirations are present. Infants born by cesarean in which labor did not occur prior to birth can experience some lung fluid retention, although it typically clears without deleterious effects on the infant. These infants are also more likely to develop transient tachypnea of the newborn (TTNB) due to the lower levels of catecholamines (Jain & Eaton, 2006).
The alveoli of the term infant’s lungs are lined with surfactant, a protein manufactured in type II cells of the lungs. Lung expansion is largely dependent on chest wall contraction and adequate secretion of surfactant. Surfactant lowers surface tension, therefore reducing the pressure required to keep the alveoli open with inspiration, and prevents total alveolar collapse on exhalation, thereby maintaining alveolar stability. The decreased surface tension results in increased lung compliance, helping to establish the functional residual capacity of the lungs. With absent or decreased surfactant, more pressure must be generated for inspiration, which can soon tire or exhaust preterm or sick term infants.
Breathing movements that began in utero as intermittent become continuous after birth, although the mechanism for this is not well understood. Once respirations are established, breaths are shallow and irregular, ranging from 30 to 60 breaths/min, with periods of breathing that include pauses in respirations lasting less than 20 seconds. These episodes of periodic breathing occur most often during the active (rapid eye movement [REM]) sleep cycle and decrease in frequency and duration with age. Apneic periods longer than 20 seconds are indicative of a pathologic process and should be thoroughly evaluated.
In the majority of newborn infants, auscultation of the chest reveals loud, clear breath sounds that seem very near, because little chest tissue intervenes. Breath sounds should be clear and equal bilaterally. The ribs of the infant articulate with the spine at a horizontal rather than a downward slope; consequently, the rib cage cannot expand with inspiration as readily as that of an adult. Because neonatal respiratory function is largely a matter of diaphragmatic contraction, abdominal breathing is characteristic of newborns. The newborn infant’s chest and abdomen rise simultaneously with inspiration. Characteristics of the respiratory system of the neonate and the effects of these characteristics on respiratory function are listed in Table 23-1.
TABLE 23-1
CHARACTERISTICS OF THE RESPIRATORY SYSTEM OF THE NEONATE
| CHARACTERISTIC | EFFECT ON FUNCTION |
| Decreased lung elastic tissue and recoil | Decreased lung compliance requiring higher pressures and more work to expand; increased risk of atelectasis |
| Reduced diaphragm movement and maximal force potential | Less effective respiratory movement; difficulty generating negative intrathoracic pressures; risk of atelectasis |
| Tendency to nose breathe; altered position of larynx and epiglottis | Enhanced ability to synchronize swallowing and breathing; risk of airway obstruction; possibly more difficult to intubate |
| Small compliant airway passages with higher airway resistance; immature reflexes | Risk of airway obstruction and apnea |
| Increased pulmonary vascular resistance with sensitive pulmonary arterioles | Risk of ductal shunting and hypoxemia with events such as hypoxia, acidosis, hypothermia, hypoglycemia, and hypercarbia |
| Increased oxygen consumption | Increased respiratory rate and work of breathing; risk of hypoxia |
| Increased intrapulmonary right-left shunting | Increased risk of atelectasis with wasted ventilation; lower Pco2 |
| Immaturity of pulmonary surfactant system in immature infants | Increased risk of atelectasis and respiratory distress syndrome; increased work of breathing |
| Immature respiratory control | Irregular respirations with periodic breathing; risk of apnea; inability to rapidly alter depth of respirations |
Pco2, Partial pressure of carbon dioxide.
Source: Blackburn, S. (2007). Maternal, fetal, & neonatal physiology: A clinical perspective (3rd ed.). St. Louis: Saunders.
Signs of Respiratory Distress
Signs of respiratory distress can include nasal flaring, intercostal or subcostal retractions (in-drawing of tissue between the ribs or below the rib cage), or grunting with respirations. Suprasternal or subclavicular retractions with stridor or gasping most often represent an upper airway obstruction. Seesaw or paradoxical respirations (exaggerated rise in abdomen, with respiration, as the chest falls) instead of abdominal respirations are abnormal and should be reported. A respiratory rate of less than 30 or greater than 60 breaths/min with the infant at rest must be thoroughly evaluated. The respiratory rate of the infant can be slowed, depressed, or absent due to the effects of analgesics or anesthetics administered to the mother during labor and birth. Apneic episodes can be related to several events (rapid increase in body temperature, hypothermia, hypoglycemia, or sepsis) that require thorough evaluation. Tachypnea can result from inadequate clearance of lung fluid, or it can be an indication of newborn respiratory distress syndrome.
Changes in the infant’s color can indicate respiratory distress. Acrocyanosis, the bluish discoloration of hands and feet is a normal finding in the first 24 hours after birth. Transient periods of duskiness while crying are not uncommon immediately after birth; however, central cyanosis is abnormal and signifies hypoxemia. With central cyanosis, the lips and mucous membranes are bluish. Central cyanosis can be the result of inadequate delivery of oxygen to the alveoli, poor perfusion of the lungs that inhibits gas exchange, or cardiac dysfunction. Because central cyanosis is a late sign of distress, newborns usually have significant hypoxemia when cyanosis appears (Askin, 2009).
Infants who experience mild TTNB often have signs of respiratory distress during the first 1 to 2 hours after birth as they transition to extrauterine life. Tachypnea with rates up to 100 breaths/min can be present along with intermittent grunting, nasal flaring, and mild retractions. Supplemental oxygen may be needed.
In neonates with more serious respiratory problems, symptoms of distress are more pronounced and tend to last beyond the first 2 hours after birth. Respiratory rates can exceed 120 breaths/min. Moderate to severe retractions, grunting, pallor, and central cyanosis can occur. The respiratory symptoms can be accompanied by hypotension, temperature instability, hypoglycemia, acidosis, and signs of cardiac problems. Common respiratory complications affecting neonates include respiratory distress syndrome (RDS), meconium aspiration, pneumonia, and persistent pulmonary hypertension of the newborn (PPHN) (Askin, 2009) (see Chapter 35).
Cardiovascular System
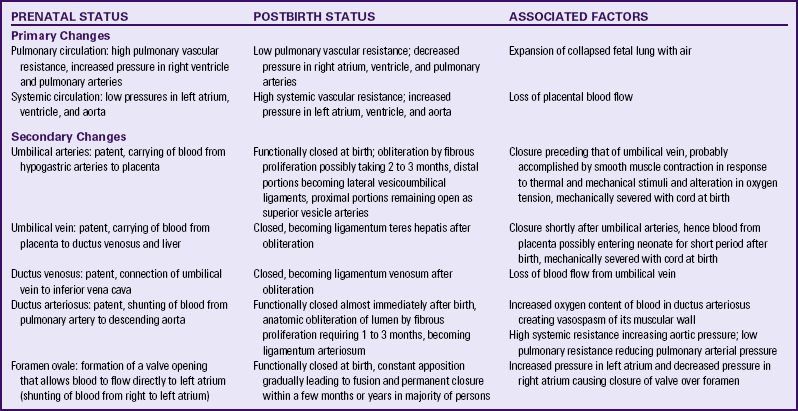
The cardiovascular system changes significantly after birth. The infant’s first breaths, combined with increased alveolar capillary distention, inflate the lungs and reduce pulmonary vascular resistance to the pulmonary blood flow from the pulmonary arteries. Pulmonary artery pressure drops, and pressure in the right atrium declines. Increased pulmonary blood flow from the left side of the heart increases pressure in the left atrium, which causes a functional closure of the foramen ovale. During the first few days of life, crying can reverse the flow through the foramen ovale temporarily and lead to mild cyanosis.
In utero, fetal Po2 is 27 mm Hg. After birth, when the Po2 level in the arterial blood approximates 50 mm Hg, the ductus arteriosus constricts in response to increased oxygenation. Circulating levels of the hormone prostaglandin E2 (PGE2) also have an important role in closure of the ductus arteriosus. In term infants it functionally closes within the first hours after birth; permanent closure usually occurs within 3 to 4 weeks and the ductus arteriosus becomes a ligament. The ductus arteriosus can open in response to low oxygen levels in association with hypoxia, asphyxia, or prematurity. With auscultation of the chest a patent ductus arteriosus can be detected as a heart murmur.
There is rapid constriction of the umbilical vein and arteries within the first 2 minutes after birth. It is thought that this is related to exposure of the cord to the cooler extrauterine environment and to increased oxygenation as the infant begins to breathe. With the clamping and severing of the cord, the umbilical arteries, the umbilical vein, and the ductus venosus are functionally closed; they are converted into ligaments within 2 to 3 months. The hypogastric arteries also occlude and become ligaments. Table 23-2 summarizes the cardiovascular changes at birth.
Heart Rate and Sounds
The term newborn has a resting heart rate between 100 and 160 beats/min, with brief fluctuations above and below these values, usually noted during sleeping and waking states. Shortly after the first cry the infant’s heart rate can accelerate as high as 180 beats/min. The range of the heart rate in the term infant is about 85 to 100 beats/min during deep sleep and 120 to 160 beats/min while the infant is awake. A heart rate of 180 beats/min is not unusual when the infant cries. A heart rate that is either high (more than 160 beats/min) or low (fewer than 100 beats/min) should be reevaluated within 30 minutes to 1 hour or when the activity of the infant changes. Immediately after birth the heart rate can be palpated by grasping the base of the umbilical cord.
By term the infant’s heart lies midway between the crown of the head and the buttocks, and the axis is more transverse than that in an adult. The apical impulse (point of maximal impulse [PMI]) in the newborn is at the fourth intercostal space and to the left of the midclavicular line. The PMI is often visible because of the thin chest wall.
Apical pulse rates should be determined for all infants. Auscultation should be for a full minute, preferably when the infant is asleep. An irregular heart rate is not uncommon in the first few hours of life. After this time an irregular heart rate not attributed to changes in activity or respiratory pattern should be further evaluated.
Heart sounds during the neonatal period are of higher pitch, shorter duration, and greater intensity than those during adult life. The first sound (S1) is typically louder and duller than the second sound (S2), which is sharp. The third and fourth heart sounds are not auscultated in newborns. Most heart murmurs heard during the first few days of life have no pathologic significance, and more than one half of the murmurs disappear by 6 months. However, the presence of a murmur and accompanying signs such as poor feeding, apnea, cyanosis, or pallor are considered abnormal and should be further investigated. There can be significant cardiac defects without symptoms in the early newborn period. This reinforces the importance of ongoing assessment (Askin, 2009).
Blood Pressure
Blood pressure varies according to weight and gestational age. The newborn infant’s average systolic blood pressure (BP) is 60 to 80 mm Hg, and the average diastolic pressure is 40 to 50 mm Hg. The BP increases by the second day of life, with minor variations noted during the first month of life. A drop in systolic BP (approximately 15 mm Hg) in the first hour of life is common. Crying and movement usually cause increased systolic pressure. The measurement of BP is best accomplished with an oscillometric device while the infant is at rest. A correctly sized cuff must be used for accurate measurement of an infant’s BP.
Unless a specific indication exists, BP is not usually measured in the newborn on a routine basis except as a baseline. In some institutions nurses obtain four extremity blood pressures in the presence of any cardiovascular symptoms such as tachycardia, persistent murmur, abnormal pulses, poor perfusion, or abnormal precordial activity. The value of four extremity BPs in the early newborn period to detect coarctation of the aorta (COA) has been questioned (Razmus & Lewis, 2006). This is based on evidence that COA defects do not occur in the immediate postbirth period but more typically at approximately 12 to 14 days of age, a time when the ductus arteriosus closes.
Blood Volume
The blood volume of the newborn is approximately 80 to 85 ml/kg of body weight. Immediately after birth the total blood volume averages 300 ml, but this can increase by as much as 100 ml, depending on the length of time before the cord is clamped and cut. The preterm infant has a relatively greater blood volume than the term newborn because the preterm infant has a proportionately greater plasma volume, not a greater red blood cell (RBC) mass.
Early or late clamping of the umbilical cord changes the circulatory dynamics of the newborn. Late clamping expands the blood volume from the so-called placental transfusion of blood to the newborn. Delayed cord clamping (≥2 minutes after birth) has been reported to be beneficial in improving hematocrit and iron status and in decreasing anemia; such benefits can last up to 6 months. Polycythemia that occurs with delayed clamping is usually not harmful, although there can be an increased risk of jaundice that requires phototherapy (Arca, Botet, Palacio, & Carbonell-Estrany, 2010; Hutton & Hassan, 2007; McDonald & Middleton, 2008).
Signs of Risk for Cardiovascular Problems
Close monitoring of the infant’s vital signs is important for early detection of impending problems. Persistent tachycardia (more than 160 beats/min) can be associated with anemia, hypovolemia, hyperthermia, or sepsis. Persistent bradycardia (less than 100 beats/min) can be a sign of a congenital heart block or hypoxemia.
The newborn’s skin color can be reflective of cardiovascular problems. Pallor in the immediate postpartum period is often symptomatic of underlying problems such as anemia or marked peripheral vasoconstriction as a result of intrapartum asphyxia or sepsis. Any prolonged cyanosis other than in the hands or feet can indicate respiratory and/or cardiac problems. The presence of jaundice can indicate ABO or Rh factor incompatibility problems (see Chapter 36).
Congenital heart defects are the most common type of congenital malformations (see Chapter 36). Although the more serious defects such as tetralogy of Fallot are likely to have clinical manifestations such as cyanosis, dyspnea, and hypoxia, others such as small ventricular septal defects can be asymptomatic. The prenatal history can provide information regarding risk factors for congenital heart defects so that the nurse knows to be more alert for symptoms. Maternal illness such as rubella, metabolic disease such as diabetes, and drug ingestion are associated with an increased risk of cardiac defects.
Hematopoietic System
The hematopoietic system of the newborn exhibits certain variations from that of the adult. Levels of RBCs and leukocytes differ, but platelet levels are relatively the same.
Red Blood Cells
Because fetal circulation is less efficient at oxygen exchange than the lungs, the fetus needs additional RBCs for transport of oxygen in utero. Therefore, at birth the average levels of RBCs, hemoglobin, and hematocrit are higher than those in the adult; these levels fall slowly over the first month. At birth the RBC count ranges from 4.8 to 7.1 × 106/mcl. The term newborn may have a hemoglobin concentration of 14 to 24 g/dl, decreasing gradually to 12 to 20 g/dl during the first 2 weeks. On the first day, the hematocrit ranges from 44% to 64% and by 8 weeks, it is between 39% and 59% (Pagana & Pagana, 2009). Polycythemia (central venous hematocrit greater than 65%) can occur in term and preterm infants as a result of delayed cord clamping, maternal hypertension or diabetes, or intrauterine growth restriction.
The source of the sample is a significant factor in levels of RBCs, hemoglobin, and hematocrit because capillary blood yields higher values than venous blood. The timing of blood sampling is also significant; the slight rise in RBCs after birth is followed by a substantial drop. At birth the infant’s blood contains an average of 70% fetal hemoglobin, but because of the shorter life span of the cells containing fetal hemoglobin, the percentage falls to 55% by 5 weeks and to 5% by 20 weeks. Iron stores generally are sufficient to sustain normal RBC production for 4 to 5 months in the term infant, at which time a transient physiologic anemia can occur.
Leukocytes
Leukocytosis, with a white blood cell (WBC) count of approximately 18,000 cells/mm3 (range, 9000 to 30,000 cells/mm3), is normal at birth (Pagana & Pagana, 2009). The number of WBCs increases to 23,000 to 24,000 cells/mm3 during the first day after birth. This initial high WBC count of the newborn decreases rapidly, and a resting level of 12,000 cells/mm3 is normally maintained during the neonatal period. Serious infection is not well tolerated by the newborn; leukocytes are slow to recognize foreign protein and to localize and fight infection early in life. Sepsis can be accompanied by a concomitant rise in WBCs (neutrophilia); however, some infants exhibit clinical signs of sepsis without a significant elevation in WBCs. In addition, events other than infection can cause neutrophilia in the newborn. These events include prolonged crying, maternal hypertension, asymptomatic hypoglycemia, hemolytic disease, meconium aspiration syndrome, labor induction with oxytocin, surgery, difficult labor, high altitude, and maternal fever.
Platelets
The platelet count ranges between 150,000 and 300,000 cells/mm3 and is essentially the same in newborns as in adults (Pagana & Pagana, 2009). The levels of factors II, VII, IX, and X, found in the liver, are decreased during the first few days of life because the newborn cannot synthesize vitamin K. However, bleeding tendencies in the newborn are uncommon, and unless the vitamin K deficiency is great, clotting is sufficient to prevent hemorrhage.
Blood groups
The infant’s blood group is genetically determined and established early in fetal life. However, during the neonatal period the strength of the agglutinogens present in the RBC membrane gradually increases. Cord blood samples can be used to identify the infant’s blood type and Rh status.
Thermogenic System
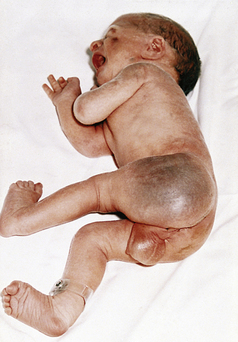
Next to establishing respirations and adequate circulation, heat regulation is most critical to the newborn’s survival. During the first 12 hours after birth the neonate attempts to achieve thermal balance in adjusting to the extrauterine environmental temperature. Thermoregulation is the maintenance of balance between heat loss and heat production. Newborns attempt to stabilize their core body temperatures within a narrow range. Hypothermia from excessive heat loss is a common and dangerous problem.
Anatomic and physiologic characteristics of neonates place them at risk for heat loss. Newborns have a thin layer of subcutaneous fat. The blood vessels are closer to the surface of the skin. Changes in environmental temperature alter the temperature of the blood, thereby influencing temperature regulation centers in the hypothalamus. Newborns have larger body surface-to-body weight (mass) ratios than do children and adults (Sedin, 2006).
Heat Loss
The body temperature of newborn infants is dependent on the heat transfer between the infant and the external environment. Factors that influence heat loss to the environment include the temperature and humidity of the air, the flow and velocity of the air, and the temperature of surfaces in contact with and around the infant. The goal of care is to maintain a neutral thermal environment for the neonate in which heat balance is maintained. The neutral thermal environment is the ideal environmental temperature that allows the neonate to maintain a normal body temperature to minimize oxygen and glucose consumption.
Heat loss in the newborn occurs by four modes:
• Convection is the flow of heat from the body surface to cooler ambient air. Because of heat loss by convection the ambient temperature in the nursery or mother’s room is kept at approximately 24° C, and newborns in open bassinets are wrapped to protect them from the cold.
• Radiation is the loss of heat from the body surface to a cooler solid surface not in direct contact but in relative proximity. To prevent this type of loss, newborn cribs and examining tables are placed away from outside windows, and care is taken to avoid direct air drafts.
• Evaporation is the loss of heat that occurs when a liquid is converted to a vapor. In the newborn, heat loss by evaporation occurs as a result of vaporization of moisture from the skin. This heat loss can be intensified by failure to dry the newborn directly after birth or by drying the infant too slowly after a bath. The less mature the newborn is, the more severe the evaporative heat loss will be. Evaporative heat loss, as a component of insensible water loss, is the most significant cause of heat loss in the first few days of life.
• Conduction is the loss of heat from the body surface to cooler surfaces in direct contact. Soon after birth, if or when the infant is placed in a warmer or crib, it should be pre-warmed to prevent heat loss. The scales used for weighing the newborn should have a protective cover to minimize conductive heat loss as well.
Loss of heat must be controlled to protect the infant. Control of such modes of heat loss is the basis of caregiving policies and techniques. One method for promoting maternal-newborn interaction is to place the naked healthy newborn on the mother’s bare chest or abdomen and cover both with a blanket. This skin-to-skin contact promotes stabilization of newborn temperature while enhancing mother-infant attachment (Fig. 23-1).
Thermogenesis
In response to cold the neonate attempts to generate heat (thermogenesis) by increasing muscle activity. Cold infants may cry and appear restless. Because of vasoconstriction the skin can feel cool to touch, and acrocyanosis can be present. There is an increase in cellular metabolic activity, primarily in the brain, heart, and liver; this also increases oxygen and glucose consumption.
In an effort to conserve heat, term newborns assume a position of flexion that helps guard against heat loss because it diminishes the amount of body surface exposed to the environment. Infants also can reduce the loss of internal heat through the body surface by constricting peripheral blood vessels.
Whereas adults are able to produce heat through shivering, the shivering mechanism of heat production is rarely operable in the newborn. Nonshivering thermogenesis is accomplished primarily by metabolism of brown fat, which is unique to the newborn, and secondarily by increased metabolic activity in the brain, heart, and liver. Brown fat is located in superficial deposits in the interscapular region and axillae, as well as in deep deposits at the thoracic inlet, along the vertebral column, and around the kidneys. Brown fat has a richer vascular and nerve supply than ordinary fat. Heat produced by intense lipid metabolic activity in brown fat can warm the newborn by increasing heat production as much as 100%. Reserves of brown fat, usually present for several weeks after birth, are rapidly depleted with cold stress. The amount of brown fat reserve increases with the weeks of gestation. A full-term newborn has greater stores than a preterm infant.
Cold Stress
Cold stress imposes metabolic and physiologic demands on all infants, regardless of gestational age and condition. The respiratory rate increases in response to the increased need for oxygen. In the cold-stressed infant, oxygen consumption and energy are diverted from maintaining normal brain and cardiac function and growth to thermogenesis for survival. If the infant cannot maintain an adequate oxygen tension, vasoconstriction follows and jeopardizes pulmonary perfusion. As a consequence, the Po2 is decreased, and the blood pH drops. These changes can prompt a transient respiratory distress or aggravate existing respiratory distress syndrome. Moreover, decreased pulmonary perfusion and oxygen tension can maintain or reopen the right-to-left shunt across the ductus arteriosus.
The basal metabolic rate increases with cold stress. If cold stress is protracted, anaerobic glycolysis occurs, resulting in increased production of acids. Metabolic acidosis develops, and if a defect in respiratory function is present, respiratory acidosis also develops (Fig. 23-2). Excessive fatty acids can displace the bilirubin from the albumin-binding sites and exacerbate hyperbilirubinemia.
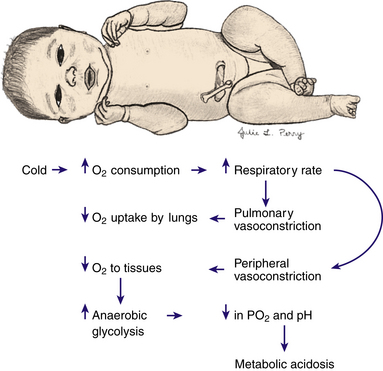
FIG. 23-2 Effects of cold stress. When an infant is stressed by cold, oxygen consumption increases, and pulmonary and peripheral vasoconstriction occurs, thereby decreasing oxygen uptake by the lungs and oxygen to the tissues; anaerobic glycolysis increases; and the Po2 and pH decrease, leading to metabolic acidosis.
Hypoglycemia is another metabolic consequence of cold stress. The process of anaerobic glycolysis uses approximately three to four times the amount of blood glucose, thereby depleting existing stores. If the infant is sufficiently stressed and low glucose stores are not replaced, hypoglycemia, which can be asymptomatic in the newborn, can develop.
Hyperthermia
Although occurring less frequently than hypothermia, hyperthermia can occur and must be corrected. A body temperature greater than 37.5° C is considered to be abnormally high and is typically caused by excess heat production related to sepsis or to a decrease in heat loss. The clinical appearance of the infant who is hyperthermic often indicates the causative mechanism. Infants who are overheated due to environmental factors such as being swaddled in too many blankets, exhibit signs of heat-losing mechanisms: skin vessels dilate, skin appears flushed, hands and feet are warm to touch, and the infant assumes a posture of extension. The newborn who is hyperthermic because of sepsis will appear stressed: vessels in the skin are constricted, color is pale, and hands and feet are cool. Hyperthermia develops more rapidly in a newborn than in an adult because of the relatively larger surface area of an infant. Sweat glands do not function well. Serious overheating of the newborn can cause cerebral damage from dehydration or even heat stroke and death (Sedin, 2006).
Renal System
At term gestation the kidneys occupy a large portion of the posterior abdominal wall. The bladder lies close to the anterior abdominal wall and is an abdominal organ and a pelvic organ. In the newborn almost all palpable abdominal masses are renal.
A small quantity (approximately 40 ml) of urine is usually present in the bladder of a full-term infant at birth. Voiding at the time of birth often occurs. During the first 30 hours after birth, 98% of newborns will void. If a newborn has not voided within 48 hours, it can be a sign of renal impairment (Vogt, Dell, & Davis, 2006).
The frequency of voiding varies from 2 to 6 times per day during the first and second days of life and from 5 to 25 times per day thereafter. Approximately 6 to 8 voidings per day of pale straw-colored urine are indicative of adequate fluid intake after the first 3 to 4 days. Generally, term infants void 15 to 60 ml of urine/kg/day.
Full-term infants have limited capacity to concentrate urine; therefore the specific gravity of the urine can range from 1.001 to 1.020. The ability to concentrate urine fully is attained by approximately 3 months of age. After the first voiding the infant’s urine can appear cloudy (because of mucus content) and have a much higher specific gravity. This level decreases as fluid intake increases. Normal urine during early infancy is usually straw colored and almost odorless. During the first days after birth, urine contains an abundance of uric acid crystals that can appear as pink or orange stains (“brick dust”) on the diaper. If this occurs after the first week, it can be an indication of insufficient intake and dehydration.
Fluid and Electrolyte Balance
In the term neonate 75% of body weight consists of water. At birth approximately 40% of the body weight is held within the extracellular fluid compartment. A reduction in extracellular fluid occurs with diuresis during the first few days after birth.
The daily fluid requirement for full-term neonates during the first 2 days of life is 60 to 80 ml/kg. From 3 to 7 days the requirement is 100 to 150 ml/kg/day; and from 8 to 30 days it is 120 to 180 ml/kg/day (Dell & Davis, 2006).
At birth, the glomerular filtration rate (GFR) of a newborn is approximately 30% to 50% that of the adult. This results in a decreased ability to remove nitrogenous and other waste products from the blood. The GFR rapidly increases during the first month of life as a result of postnatal physiologic changes including decreased renal vascular resistance, increased renal blood flow, and increased filtration pressure.
Sodium reabsorption is decreased as a result of a lowered sodium- or potassium-activated adenosine triphosphatase activity. The decreased ability to excrete excessive sodium results in hypotonic urine compared with plasma, leading to a higher concentration of sodium, phosphates, chloride, and organic acids and a lower concentration of bicarbonate ions. The infant has a higher renal threshold for glucose than adults.
Bicarbonate concentration and buffering capacity are decreased. This can lead to acidosis and electrolyte imbalance.
Signs of Risk for Renal System Problems
The renal system has a wide range of functions. Dysfunction resulting from physiologic abnormalities can range from the lack of a steady stream of urine to gross anomalies such as hypospadias and exstrophy of the bladder, which can be identified easily at birth. Enlarged or cystic kidneys can be identified as masses during abdominal palpation. Some kidney anomalies also can be detected by ultrasound examination during pregnancy (see Chapter 36).
Gastrointestinal System
The term newborn is capable of swallowing, digesting, metabolizing, and absorbing proteins and simple carbohydrates and emulsifying fats. With the exception of pancreatic amylase the characteristic enzymes and digestive juices are present even in low-birth-weight neonates.
In the adequately hydrated infant the mucous membrane of the mouth is moist and pink. The hard and soft palates are intact. The presence of moderate to large amounts of mucus is common in the first few hours after birth. Small whitish areas (Epstein pearls) may be found on the gum margins and at the juncture of the hard and soft palate. The cheeks are full because of well-developed sucking pads. These pads, like the labial tubercles (sucking calluses) on the upper lip, disappear around the age of 12 months, when the sucking period is over.
Even though fetal sucking motions have been recorded by ultrasound, these motions are not coordinated with swallowing in any infant born before 32 to 33 weeks of gestation. Sucking behavior is influenced by factors such as neuromuscular maturity, physiologic status, sleep-wake state, and the type of feeding (e.g., breast, bottle, or orogastric).
A special mechanism in healthy term newborns coordinates the breathing, sucking, and swallowing reflexes necessary for oral feeding. Sucking takes place in small bursts of 3 or 4 and up to 8 to 10 sucks at a time, with a brief pause in between bursts. The infant is unable to move food from the lips to the pharynx; therefore, placing the nipple (breast or bottle) well inside the baby’s mouth is necessary. Peristaltic activity in the esophagus is uncoordinated in the first few days of life but quickly becomes coordinated in healthy full-term infants, and they swallow easily.
Teeth begin developing in utero, with enamel formation continuing until approximately age 10 years. Tooth development is influenced by neonatal or infant illnesses, medications, and maternal illnesses or medications taken by the mother during pregnancy. The fluoride level in the water supply also influences tooth development. Occasionally an infant is born with one or more teeth. These natal teeth have poorly formed roots and as they loosen place the infant at risk of aspiration. Therefore, they are usually extracted.
Bacteria are not present in the infant’s gastrointestinal tract at birth. Soon after birth, oral and anal orifices permit entry of bacteria and air. Generally the highest bacterial concentration is found in the lower portion of the intestine, particularly in the large intestine. Normal colonic bacteria are established within the first week after birth, and normal intestinal flora help synthesize vitamin K, folate, and biotin. Bowel sounds can usually be heard shortly after birth.
The capacity of the newborn stomach varies widely depending on the size of the infant from less than 30 ml on day one to more than 90 ml on day 3. After birth the newborn stomach becomes increasingly more compliant and relaxed to accommodate larger volumes (Spangler, Randenberg, Brenner, & Howett, 2008). Emptying time for the stomach is highly variable and can be affected by several factors such as time and volume of feedings or type and temperature of food. The cardiac sphincter and nervous control of the stomach are immature, thus some regurgitation can occur. Regurgitation during the first day or two of life can be decreased by avoiding overfeeding, by burping the infant, and by positioning the infant with the head slightly elevated.
Digestion
The infant’s ability to digest carbohydrates, fats, and proteins is regulated by the presence of certain enzymes. Most of these enzymes are functional at birth except for amylase and lipase. Amylase is produced by the salivary glands after approximately 3 months and by the pancreas at approximately 6 months of age. This enzyme is necessary to convert starch into maltose and occurs in high amounts in colostrum. The other exception is lipase, also secreted by the pancreas; it is necessary for the digestion of fat. Therefore, the normal newborn is capable of digesting simple carbohydrates and proteins but has a limited ability to digest fats.
Further digestion and absorption of nutrients occur in the small intestine in the presence of pancreatic secretions, secretions from the liver through the common bile duct, and secretions from the duodenal portion of the small intestine.
Stools
Meconium fills the lower intestine at birth. It is formed during fetal life from the amniotic fluid and its constituents, intestinal secretions (including bilirubin), and cells (shed from the mucosa). Meconium is greenish black and viscous and contains occult blood. The first meconium passed is usually sterile, but within hours all meconium passed contains bacteria. The majority of healthy term infants pass meconium within the first 12 to 24 hours of life, and almost all do so by 48 hours (Blackburn, 2007). The number of stools passed varies during the first week, being most numerous between the third and sixth days. Newborns fed early pass stools sooner. Progressive changes in the stooling pattern indicate a properly functioning gastrointestinal tract (Box 23-1).
Feeding Behaviors
Variations occur among infants regarding interest in food, signs of hunger, and amount ingested at one time. The amount of food that the infant takes in at any feeding depends on the size, hunger level, and alertness of the infant. When put to breast some infants feed immediately, whereas others require a longer learning period. Random hand-to-mouth movement and sucking of fingers are well developed at birth and intensify when the infant is hungry. Caregivers should be alert and responsive to these hunger cues (Lawrence & Lawrence, 2005).
Signs of Risk for Gastrointestinal Problems
The time, color, and character of the infant’s first stool should be noted. Failure to pass meconium can indicate bowel obstruction related to conditions such as an inborn error of metabolism (e.g., cystic fibrosis) or a congenital disorder (e.g., Hirschsprung disease or an imperforate anus). An active rectal “wink” reflex (contraction of the anal sphincter muscle in response to touch) is a sign of good sphincter tone.
Fullness of the abdomen above the umbilicus can be due to problems such as hepatomegaly, duodenal atresia, or distention. Abdominal distention at birth usually indicates a serious disorder such as a ruptured viscus (from abdominal wall defects) or tumors. Distention that occurs later can be the result of overfeeding or can signal gastrointestinal disorders. A scaphoid (sunken) abdomen, with bowel sounds heard in the chest and signs of respiratory distress indicate a diaphragmatic hernia. Fullness below the umbilicus can indicate a distended bladder.
Some infants are intolerant of certain commercial infant formulas. If an infant is allergic to or unable to digest a formula, the stools can become very soft with a high water content that is signaled by a distinct water ring around the stool on the diaper. Forceful ejection of stool and a water ring around the stool are signs of diarrhea. Care must be taken to avoid misinterpreting transitional stools for diarrhea. The loss of fluid in diarrhea can rapidly lead to fluid and electrolyte imbalance. Passage of meconium from the vagina or urinary meatus is a sign of a possible fistulous tract from the rectum.
The amount and frequency of regurgitation (“spitting up”) after feedings should be documented. Color change, gagging, and projectile (very forceful) vomiting occur in association with esophageal and tracheoesophageal anomalies (see Chapter 36).
Hepatic System
The liver and gallbladder are formed by the fourth week of gestation. In the newborn the liver can be palpated approximately 1 cm below the right costal margin because it is enlarged and occupies approximately 40% of the abdominal cavity. The infant’s liver plays an important role in iron storage, carbohydrate metabolism, conjugation of bilirubin, and coagulation.
Iron Storage
The fetal liver, which serves as the site for production of hemoglobin after birth, begins storing iron in utero. The infant’s iron store is proportional to total body hemoglobin content and length of gestation. At birth the term infant has an iron store sufficient to last 4 to 6 months. Iron stores of preterm and small-for-gestational age infants are often lower and are depleted sooner than in healthy term infants. Although both breast milk and cow’s milk contain iron, the bioavailability of iron in breast milk is far superior. Full-term infants who are breastfed do not need supplemental iron for 6 months, whereas formula-fed infants should receive a formula that contains supplemental iron (Luchtman-Jones, Schwartz, & Wilson, 2006).
Carbohydrate Metabolism
At birth the newborn is cut off from the maternal glucose supply and, as a result, has an initial decrease in serum glucose levels. The newborn’s increased energy needs, decreased hepatic release of glucose from glycogen stores, increased RBC volume, and increased brain size can initially contribute to the rapid depletion of stored glycogen within the first 24 hours after birth. In most healthy term newborns blood glucose levels stabilize at 50 to 60 mg/dl during the first several hours after birth; by the third day of life the blood glucose levels should be approximately 60 to 70 mg/dl. The initiation of feedings assists in the stabilization of the newborn’s blood glucose levels. In general, blood glucose levels less than 40 mg/dl are considered abnormal and warrant intervention. The hypoglycemic infant can display the classic symptoms of jitteriness, lethargy, apnea, feeding problems, or seizures, or the infant can be asymptomatic. Hypoglycemia in the initial newborn period is most often transient and easily corrected through feeding. Persistent or recurrent hypoglycemia necessitates intravenous glucose therapy and possible pharmacologic intervention.
Conjugation of Bilirubin and Newborn Jaundice
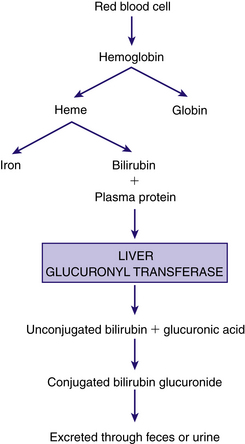
Jaundice, the visible yellowish color of the skin and sclera, is due to elevated serum levels of bilirubin (hyperbilirubinemia). The liver is responsible for the conjugation of bilirubin, which results from the breakdown of red blood cells. When RBCs reach the end of their life span, their membranes rupture and hemoglobin is released. The hemoglobin is phagocytosed by macrophages; it then splits into heme and globin. The heme is broken down by the reticuloendothelial cells, converted to bilirubin, and released in an unconjugated form. The unconjugated (indirect) bilirubin is relatively insoluble and almost entirely bound to circulating albumin, a plasma protein. Bilirubin that is not bound to albumin, or free bilirubin, can easily cross the blood-brain barrier and cause neurotoxicity (Bagwell, 2007; Wong, DeSandre, Sibley, & Stevenson, 2006).
The unconjugated bilirubin must be conjugated so that it becomes soluble and excretable. In the liver the unbound bilirubin is conjugated with glucuronic acid in the presence of the enzyme glucuronyl transferase. The conjugated form of bilirubin (direct bilirubin) is soluble and is excreted from liver cells as a constituent of bile. Along with other components of bile, direct bilirubin is excreted into the biliary tract system that carries the bile into the duodenum. Bilirubin is converted to urobilinogen and stercobilinogen within the duodenum through the action of the bacterial flora. Urobilinogen is excreted in urine and feces; stercobilinogen is excreted in the feces (Fig. 23-3). The effectiveness of bilirubin excretion through the feces depends on the stooling pattern of the newborn and on the substances in the intestine that break down conjugated bilirubin. In the newborn intestine the enzyme β-glucuronidase is able to convert conjugated bilirubin into the unconjugated form, which is subsequently reabsorbed by the intestinal mucosa and transported to the liver; this is called enterohepatic circulation. Feeding is important in reducing serum bilirubin levels because it stimulates peristalsis and produces more rapid passage of meconium, thus diminishing the amount of reabsorption of unconjugated bilirubin. Feeding also introduces bacteria to aid in the reduction of bilirubin to urobilinogen. Colostrum, a natural laxative, facilitates meconium evacuation.
When levels of unconjugated bilirubin exceed the liver’s ability for conjugation, plasma levels of bilirubin increase and jaundice appears. Jaundice is generally noticeable first in the head, especially in the sclera and mucous membranes, then progresses gradually to the thorax, abdomen, and extremities. The degree of jaundice is determined by serum total bilirubin measurements. Jaundice is likely to appear when bilirubin levels exceed 2.5 mg/dl (Bagwell, 2007).
The newborn is at risk for hyperbilirubinemia because of distinctive aspects of normal neonatal physiology. The higher red blood cell mass at birth and shorter life span of neonatal red blood cells mean that there is the need for greater bilirubin synthesis. The liver’s ability to conjugate bilirubin is reduced during the first few days after birth; it can metabolize and excrete only about two thirds of the circulating bilirubin. In addition, there are fewer bilirubin binding sites because newborns have lower serum albumin levels. In the intestines, conjugated bilirubin becomes unconjugated and recirculated through the enterohepatic circulation, which increases serum bilirubin levels.
Traditionally newborn jaundice has been categorized as either physiologic or pathologic (nonphysiologic) depending primarily on the time it appears and on the serum bilirubin levels. Controversy surrounds the definitions of normal or physiologic ranges of total serum bilirubin. Total serum bilirubin levels in newborns are affected by variables such as length of gestation, age, weight, race, nutritional status, and mode of feeding (Wong et al., 2006).
Physiologic Jaundice: Physiologic jaundice or nonpathologic unconjugated hyperbilirubinemia occurs in as many as 60% of newborn infants and in 80% of preterm newborns. Physiologic jaundice usually resolves without treatment.
Two phases of physiologic jaundice have been identified in full-term infants. In the first phase, bilirubin levels of formula-fed Caucasian and African-American infants gradually increase to approximately 5 to 6 mg/dl by 60 to 72 hours of life, then decrease to a plateau of 2 to 3 mg/dl by the fifth day (Blackburn, 2007). In Asian and Asian-American infants, levels reach a peak of 10 to 14 mg/dl around the third to fifth days of life; the levels gradually fall to 2 to 3 mg/dl by the seventh to tenth days. Bilirubin levels maintain a steady plateau state in the second phase without increasing or decreasing until approximately 12 to 14 days, at which time levels fall to the normal value of 1 mg/dl (Blackburn). This pattern varies according to racial group, method of feeding (breast versus bottle), and gestational age. In preterm formula-fed infants, serum bilirubin levels can peak as high as 10 to 12 mg/dl at 5 to 6 days of life and decrease slowly over a period of 2 to 4 weeks.
Some characteristics of physiologic jaundice include the following:
• The infant is otherwise well relative to cardiorespiratory status, neurologic status, carbohydrate metabolism, feeding pattern, and elimination.
• In term infants, jaundice first appears after 24 hours and disappears by the end of the seventh day.
• In preterm infants, jaundice is first evident after 48 hours and disappears by the ninth or tenth day.
• The infant’s predischarge total serum bilirubin falls below the high risk category (less than the 95th percentile) on the hour-specific nomogram (see Fig. 24-8).
• The serum concentration of unconjugated bilirubin usually does not exceed 12 mg/dl in term infants and 15 mg/dl in preterm infants.
• Direct bilirubin does not exceed 1 to 1.5 mg/dl.
• Indirect or unconjugated bilirubin concentration does not increase by more than 5 mg/dl per day.
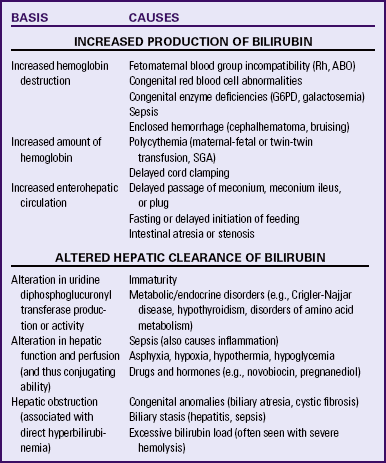
Table 23-3 lists the varying causes of neonatal indirect hyperbilirubinemia.
Pathologic Jaundice: Although physiologic jaundice is usually considered benign, unconjugated bilirubin (indirect) can accumulate to hazardous levels and lead to a pathologic condition. Pathologic or nonphysiologic jaundice is unconjugated hyperbilirubinemia that is either pathologic in origin or severe enough to warrant further evaluation and treatment (see Chapter 36). Jaundice is usually considered pathologic or nonphysiologic if it appears within 24 hours of birth, if total serum bilirubin levels increase by more than 5 mg/dl in 24 hours, and if the serum bilirubin level exceeds 15 mg/dl at any time. High levels of unconjugated bilirubin are usually due to excessive production of bilirubin through hemolysis, Hemolytic disease of the newborn, caused by maternal/newborn blood group incompatibility is the most common cause of hyperbilirubinemia. Other causes are listed in Table 23-3.
If increased levels of unconjugated bilirubin are left untreated, neurotoxicity can result as bilirubin is transferred into the brain cells. Acute bilirubin encephalopathy refers to the acute manifestations of bilirubin toxicity that occur during the first weeks after birth. This can include a range of symptoms such as lethargy, hypotonia, irritability, seizures, coma, and death. Kernicterus refers to the irreversible, long-term consequences of bilirubin toxicity such as hypotonia, delayed motor skills, hearing loss, cerebral palsy, and gaze abnormalities (American Academy of Pediatrics [AAP], 2004; Bradshaw, 2010).
Jaundice Related to Breastfeeding: Two forms of breastfeeding-related jaundice are recognized: breastfeeding-associated jaundice and breast milk jaundice. These typically occur in otherwise healthy infants. Both types can occur in the same infant and are not easily differentiated (Blackburn, 2007).
Breastfeeding-associated jaundice (early-onset jaundice) begins at 2 to 4 days of age and occurs in approximately 10% to 25% of breastfed newborns. Breastfeeding does not cause the jaundice; instead, it is a lack of effective breastfeeding that contributes to the hyperbilirubinemia. If the infant is not feeding effectively, there is less caloric and fluid intake and possible dehydration. Hepatic clearance of bilirubin is reduced. With less intake, there are fewer stools. As a result, bilirubin is reabsorbed from the intestine back into the bloodstream and must be conjugated again so that it can be excreted (Blackburn, 2007; Page-Goertz, 2008).
Breast-milk jaundice (late-onset jaundice) can initially begin as the early-onset variety or can begin at age 4 to 6 days and occurs in 2% to 3% of breastfed infants. Infants are usually feeding well and gaining weight appropriately. Rising levels of bilirubin peak during the second week and gradually diminish. Despite high levels of bilirubin that may persist for 3 to 12 weeks, these infants have no signs of hemolysis or liver dysfunction. The etiology of breast milk jaundice is uncertain. However, it seems to be related to factors in the breast milk (e.g., pregnanediol, fatty acids, and β-glucuronidase) that either inhibit the conjugation or decrease the excretion of bilirubin (Blackburn, 2007). (See Chapter 25 for a discussion of these conditions in relation to newborn nutrition.)
Coagulation
The liver plays an important role in blood coagulation. Coagulation factors, which are synthesized in the liver, are activated by vitamin K. The lack of intestinal bacteria needed to synthesize vitamin K results in transient blood coagulation deficiency between the second and fifth days of life. The levels of coagulation factors slowly increase to reach adult levels by age 9 months. The administration of intramuscular vitamin K shortly after birth helps prevent clotting problems. Any bleeding problems noted in the newborn should be reported immediately, and tests for clotting ordered (Luchtman-Jones et al., 2006).
Signs of Risk for Hepatic System Problems
Preterm infants are at greater risk for hepatic system problems such as hyperbilirubinemia and hypoglycemia because of their immaturity. The hematologic status of all newborns should be assessed for anemia. Because infants can develop a coagulation deficiency, a male neonate who has been circumcised must be observed closely for signs of hemorrhage. Hemorrhage also can be caused by a clotting defect, indicating a serious problem such as hemophilia (see Chapter 36).
Immune System
Beginning early in gestation, the immune system of the fetus is developing the capacity to respond to foreign antigens. The development of the immune system is necessary to equip the neonate to meet the numerous environmental challenges (microorganisms) associated with life in the extrauterine world.
At birth most of the circulating antibodies in the newborn are immunoglobulin G (IgG) antibodies that were transported across the placenta from the maternal circulation. This transfer of antibodies from the mother begins as early as 14 weeks of gestation and is greatest during the third trimester. By term, the IgG levels in the cord blood of the infant are higher than those in maternal blood. The passive immunity afforded the infant through the placental transfer of IgG usually provides sufficient antimicrobial protection during the first 3 months of life. Production of adult concentrations of IgG is reached by 4 to 6 years of age (Kapur, Yoder, & Polin, 2006).
The fetus is capable of producing IgM by the eighth week of gestation, and low levels are present at term (less than 10% of adult levels). By the age of 2 years, IgM reaches adult levels. The production of IgA, IgD and IgE is much more gradual, and maximal levels are not attained until early childhood (Kapur et al., 2006).
Natural barrier mechanisms such as the acidity of the stomach and the production of pepsin and trypsin, which maintain sterility of the small intestine, are not fully developed until age 3 to 4 weeks.
The membrane-protective IgA is missing from the respiratory and urinary tracts, and unless the newborn is breastfed, it also is absent from the gastrointestinal tract. Breast milk provides the newborn with important immunity. The secretory IgA in human milk acts locally in the intestines to neutralize bacterial and viral pathogens. It may also lessen the risk of allergy and food intolerance through modulation of exposure to foreign milk protein antigens.
The newborn is capable of producing a protective immune response to vaccines, given as early as a few hours after birth. For example, when hepatitis B vaccine is administered at birth to the infant born to a mother with hepatitis B, there is an excellent immune response. This holds true even if the infant does not receive additional hepatitis B immunoglobulins.
The white blood cells of the newborn display a delayed response to invading bacteria. The influx of phagocytic cells to areas of inflammation is somewhat slowed, although the ability of these cells to attack and destroy bacteria is equivalent to that of adults (Kapur et al., 2006).
Risk for Infection
All newborns, and preterm newborns especially, are at high risk for infection during the first several months of life. During this period infection is one of the leading causes of morbidity and mortality. The newborn cannot limit the invading pathogen to the portal of entry because of the generalized hypofunctioning of the inflammatory and immune mechanisms.
Early signs of infection must be recognized so that prompt diagnosis and treatment can occur. Temperature instability or hypothermia can be symptomatic of serious infection; newborns do not typically exhibit fever, although hyperthermia can occur (temperature greater than 38° C). Lethargy, irritability, poor feeding, vomiting or diarrhea, decreased reflexes, and pale or mottled skin color are some of the clinical signs that are suggestive of infection. Respiratory symptoms such as apnea, tachypnea, grunting, or retracting can be associated with infection such as pneumonia (Lott, 2010).
Any unusual discharge from the infant’s eyes, nose, mouth, or other orifice must be investigated. If a rash appears it must be evaluated closely; many normal rashes in the newborn are not associated with any infection. Infants must be protected from infections by the use of good hand hygiene techniques.
The greatest risk factor for neonatal infection is prematurity because of immaturity of the immune system. Other risk factors include premature rupture of membranes, chorioamnionitis, maternal fever, antenatal or intrapartal asphyxia, invasive procedures, stress, and congenital anomalies (Lott, 2010).
Integumentary System
All skin structures are present at birth. The epidermis and dermis are loosely bound and extremely thin. After 35 weeks of gestation, the skin is covered by vernix caseosa (a cheeselike, whitish substance) that is fused with the epidermis and serves as a protective covering. Vernix caseosa is a product of the sebaceous glands. The amount of vernix decreases with age and is shed into the amniotic fluid. In the term newborn, vernix is present in the creases of the neck, the axilla, and the groin. Postterm infants have little vernix.
The term infant has erythematous (red) skin for a few hours after birth, after which it fades to its normal color. The skin often appears blotchy or mottled, especially over the extremities. The hands and feet appear slightly cyanotic (acrocyanosis); this is caused by vasomotor instability, capillary stasis, and a high hemoglobin level. Acrocyanosis is normal and can appear intermittently over the first 7 to 10 days, especially with exposure to cold.
The healthy term newborn usually appears plump. Subcutaneous fat accumulated during the last trimester acts as insulation. Fine lanugo hair may be noted over the face, shoulders, and back. Edema of the face and ecchymosis (bruising) or petechiae can be present as a result of face presentation, forceps-assisted birth, or vacuum extraction (see Fig. 24-6). Petechiae appearing on areas of the skin other than the face or neck can be associated with serious infection or hematologic problems (Lissauer, 2006).
Creases are located on the palms of the hands and on the soles of the feet. The simian line, a single palmar crease, is often seen in Asian infants or in infants with Down syndrome. The soles of the feet should be inspected for the number of creases during the first few hours after birth; as the skin dries, more creases appear. Increasing numbers of creases correlate with a greater maturity rating. Premature newborns have few if any creases.
Sweat Glands
Sweat glands are present at birth but do not respond to increases in ambient or body temperature. Some fetal sebaceous gland hyperplasia and secretion of sebum result from the hormonal influences of pregnancy. Distended, small, white sebaceous glands (milia) may be noticeable on the newborn’s face.
Desquamation
Desquamation (peeling) of the skin of the term infant does not usually occur until a few days after birth. Large generalized areas of skin desquamation present at birth may be an indication of postmaturity.
Mongolian Spots
Mongolian spots, bluish black areas of pigmentation, can appear over any part of the exterior surface of the body, including the extremities. They are more commonly noted on the back and buttocks (Fig. 23-4). These pigmented areas are most frequently noted in newborns whose ethnic origins are in the Mediterranean area, Latin America, Asia, or Africa. They are more common in dark-skinned individuals but occur in 5% to 13% of Caucasians as well (Blackburn, 2007). They fade gradually over months or years.
Nevi
Telangiectatic nevi, known as “stork bites,” are pink and easily blanched (Fig. 23-5, A). They appear on the upper eyelids, nose, upper lip, lower occipital area, and nape of the neck. They have no clinical significance and fade by the second year of life.
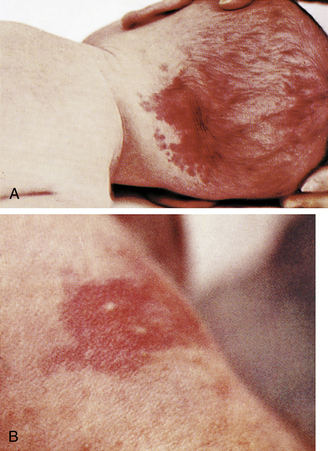
FIG. 23-5 A, Telangiectatic nevi (stork bite). B, Erythema toxicum (flea bite dermatitis). (Courtesy Mead Johnson & Co., Evansville, IN.)
The nevus vasculosus is a common type of capillary hemangioma. It consists of dilated newly formed capillaries occupying the entire dermal and subdermal layers, with associated connective tissue hypertrophy. The typical lesion is a raised, sharply demarcated, bright red or dark red, rough-surfaced swelling. As the infant grows the hemangioma may proliferate and become more vascular, thus often called a strawberry hemangioma. Lesions are usually single but may be multiple, with 75% occurring on the head. These lesions can remain until the child is of school age or sometimes even longer but can be removed successfully with pulsed dye laser therapy and prednisone administration. In some cases, subcutaneous injections of interferon alfa-2a or interferon alfa-2b may be required if prednisone therapy and the pulsed dye laser fail to control a problematic hemangioma.
A port-wine stain, or nevus flammeus, when present, is usually observed at birth and is composed of a plexus of newly formed capillaries in the papillary layer of the corium. It is red to purple; varies in size, shape, and location; and is not elevated. True port-wine stains do not blanch with pressure or disappear. They are most commonly found on the face and neck.
Erythema Toxicum
A transient rash, erythema toxicum, is also called erythema neonatorum, newborn rash, or flea bite dermatitis. It is found in term neonates during the first 3 weeks of life. Erythema toxicum produces lesions in different stages: erythematous macules, papules, and small vesicles (see Fig. 23-5, B). The lesions may appear suddenly anywhere on the body. The rash is thought to be an inflammatory response. Eosinophils, which help decrease inflammation, are found in the vesicles. Although the appearance is alarming, the rash has no clinical significance and requires no treatment.
Signs of Risk for Integumentary Problems
Close observation of the newborn’s skin color can lead to early detection of potential problems. Any pallor, plethora (deep purplish color from increased circulating RBCs), petechiae, central cyanosis, or jaundice should be noted and described. The skin should be examined for signs of birth injuries, such as forceps marks and lesions related to fetal monitoring. Bruises or petechiae may be present on the head, neck, and face of an infant born with a nuchal cord (cord around the neck) or in an infant who had a face presentation at birth. Bruising can increase the risk of hyperbilirubinemia. Petechiae can be present if increased pressure was applied to an area. Petechiae scattered over the infant’s body should be reported to the pediatrician because their presence can indicate underlying problems such as low platelet count or infection. Unilateral or bilateral periauricular papillomas (skin tags) occur fairly frequently. Their occurrence is usually a family trait and of no consequence.
Reproductive System
At birth the ovaries contain thousands of primitive germ cells. These cells represent the full complement of potential ova; no oogonia form after birth in term infants. The ovarian cortex, which is made up primarily of primordial follicles, occupies a larger portion of the ovary in the newborn female than in the adult female. From birth to sexual maturity the number of ova decreases by approximately 90%.
An increase of estrogen during pregnancy followed by a decrease after birth results in a mucoid vaginal discharge and even some slight bloody spotting (pseudomenstruation). External genitals (i.e., labia majora and minora) are usually edematous with increased pigmentation. In term infants the labia majora and minora cover the vestibule (Fig. 23-6, A). In preterm infants, the clitoris is prominent, and the labia majora are small and widely separated. Vaginal or hymenal tags are common findings and have no clinical significance. Vernix caseosa can be present between the labia and should not be forcibly removed during bathing.
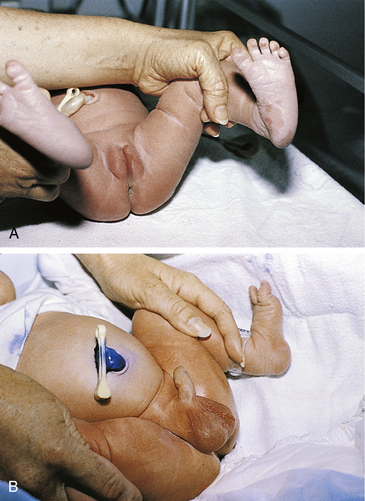
FIG. 23-6 External genitalia. A, Genitals in female term infant. Note mucoid vaginal discharge. B, Genitals in male infant. Uncircumcised penis. Rugae cover scrotum, indicating term gestation. Cord has been swabbed with ethylene blue to prevent infection. (Courtesy Marjorie Pyle, RNC, Lifecircle, Costa Mesa, CA.)
If the infant was born in the breech position, the labia can be edematous and bruised. The edema and bruising resolve in a few days.
Male
The foreskin completely covers the glans. The urethral opening may be completely covered by the prepuce, which is not retractable for 3 to 4 years. The position of the urethra should be at the tip of the penis. With hypospadias, the urethral opening is located in an abnormal position, on or adjacent to the glans, although it can be placed on the penile shaft or perineum. Small, white, firm lesions called epithelial pearls may be seen at the tip of the prepuce. By 28 to 36 weeks of gestation, the testes can be palpated in the inguinal canal, and a few rugae appear on the scrotum. At 36 to 40 weeks of gestation, the testes are palpable in the upper scrotum, and rugae appear on the anterior portion. After 40 weeks the testes can be palpated in the scrotum, and rugae cover the scrotal sac. The postterm neonate has deep rugae and a pendulous scrotum. Hydroceles, caused by an accumulation of fluid around the testes, may be present. They can be easily transilluminated with a light and usually resolve without treatment.
The scrotum is usually more deeply pigmented than the rest of the skin (see Fig. 23-6, B), particularly in darker-skinned infants. A bluish discoloration of the scrotum suggests testicular torsion, which needs immediate attention. If the male infant is born in a breech presentation, the scrotum can be very edematous and bruised (Fig. 23-7). The swelling and discoloration subside within a few days.
Swelling of Breast Tissue
Swelling of the breast tissue in term infants of both sexes is caused by the hyperestrogenism of pregnancy. In a few infants a thin discharge (witch’s milk) can be seen. This finding has no clinical significance, requires no treatment, and subsides within a few days as the maternal hormones are eliminated from the infant’s body.
The nipples should be symmetric on the chest. Breast tissue and areola size increase with gestation. The areola appears slightly elevated at 34 weeks of gestation. By 36 weeks a breast bud of 1 to 2 mm is palpable and increases to 12 mm by 42 weeks.
Signs of Risk for Reproductive System Problems
The infant must be closely inspected for ambiguous genitalia and other abnormalities. Normally in a female infant the urethral opening is located behind the clitoris. Any deviation from this can incorrectly suggest that the clitoris is a small penis, which can occur in conditions such as adrenal hyperplasia. Nearly all female infants are born with hymenal tags; absence of such tags can indicate vaginal agenesis. Fecal discharge from the vagina indicates a rectovaginal fistula. Any of these findings must be reported to the physician or neonatal nurse practitioner for further evaluation.
Hypospadias, undescended or maldescended testes, and other abnormalities of the male genitalia must be reported. Circumcision is contraindicated in the presence of hypospadias since the foreskin is used in repair of this anomaly.
Inguinal hernias can be present and become more obvious when the infant cries. They are common, especially in African-American neonates, and usually require no treatment because they resolve with time (Lissauer, 2006).
Skeletal System
The infant’s skeletal system undergoes rapid development during the first year of life. At birth more cartilage is present than ossified bone. Because of cephalocaudal (head-to-rump) development the newborn appears somewhat out of proportion.
At term the head is one fourth of the total body length. The arms are slightly longer than the legs. In the newborn the legs are one third of the total body length but only 15% of the total body weight. As growth proceeds the midpoint in head-to-toe measurements gradually descends from the level of the umbilicus at birth to the level of the symphysis pubis at maturity.
The face appears small in relation to the skull, which appears large and heavy. Cranial size and shape can be distorted by molding (the shaping of the fetal head by the overlapping of cranial bones to facilitate movement through the birth canal during labor) (Fig. 23-8). It is not uncommon to see edematous areas on the newborn’s head after birth, especially following a prolonged second stage of labor. Caput succedaneum and cephalhematoma are seen most often; these are usually self-limiting and resolve without treatment. A more serious injury is subgaleal hemorrhage (Fig. 23-9, C), which is discussed in Chapter 35.
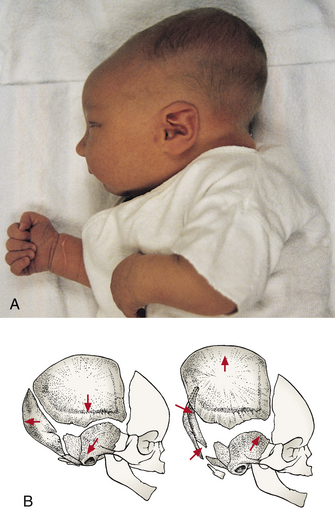
FIG. 23-8 Molding. A, Significant molding, soon after birth. B, Schematic of bones of skull when molding is present. (A, Courtesy Kim Molloy, Knoxville, IA.)
Caput succedaneum is a generalized, easily identifiable edematous area of the scalp, most commonly found on the occiput (Fig. 23-9, A). With vertex presentation the sustained pressure of the presenting vertex against the cervix results in compression of local vessels, slowing venous return. The slower venous return causes an increase in tissue fluids within the skin of the scalp, and an edematous swelling develops. This edematous swelling, present at birth, extends across suture lines of the skull and disappears spontaneously within 3 to 4 days. Infants who are born with the assistance of vacuum extraction usually have a caput in the area where the cup was applied. Bruising of the scalp is often seen in the presence of caput succedaneum.
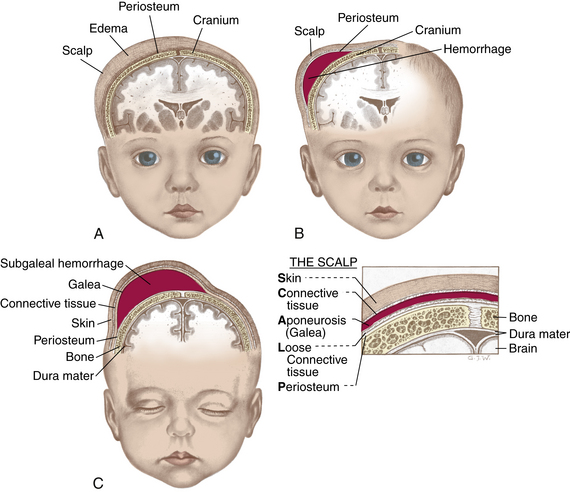
FIG. 23-9 Differences between caput succedaneum, cephalhematoma, and subgaleal hemorrhage. A, Caput succedaneum: edema of scalp noted at birth; crosses suture lines. B, Cephalhematoma: bleeding between periosteum and skull bone appearing within first 2 days; does not cross suture lines. C, Subgaleal hemorrhage: bleeding into the subgaleal compartment; bleeding extends beyond bone, often posteriorly into the neck and continuous after birth. (From Seidel, H., Ball, J., Dains, J., & Benedict, G. [2006]. Mosby’s guide to physical examination [6th ed.]. St. Louis: Mosby.)
Cephalhematoma is a collection of blood between a skull bone and its periosteum; therefore, a cephalhematoma does not cross a cranial suture line (see Fig. 23-9, B). Caput succedaneum and cephalhematoma often occur simultaneously.
Bleeding can occur with spontaneous birth from pressure against the maternal bony pelvis. Low forceps birth and difficult forceps rotation and extraction can also cause bleeding. This soft, fluctuating, irreducible fullness does not pulsate or bulge when the infant cries. It appears several hours or the day after birth and may not become apparent until a caput succedaneum is absorbed. A cephalhematoma is usually largest on the second or third day, by which time the bleeding stops. The fullness of a cephalhematoma spontaneously resolves in 3 to 6 weeks. It is not aspirated because infection can develop if the skin is punctured. As the hematoma resolves, hemolysis of RBCs occurs, and jaundice can result. Hyperbilirubinemia and jaundice can occur after the newborn is discharged home.
The bones in the vertebral column of the newborn form two primary curvatures—one in the thoracic region and one in the sacral region. Both are forward, concave curvatures. As the infant gains head control at approximately age 3 months, a secondary curvature appears in the cervical region. The newborn’s spine appears straight and can be easily flexed. The newborn can lift the head and turn it from side to side when prone. The vertebrae should appear straight and flat. If a pilonidal dimple is noted, further inspection is required to determine whether a sinus is present. A pilonidal dimple, especially with a sinus and nevus pilosis (hairy nevus), is significant because it can be associated with spina bifida.
The infant’s extremities should be symmetric and of equal length. Fingers and toes should be equal in number (five fingers on each hand and five toes on each foot) and should have nails present. Digits may be missing (oligodactyly). Extra digits (polydactyly) are sometimes found on hands or feet. Fingers or toes may be fused (syndactyly).
The infant is examined for developmental dysplasia of the hips (DDH). At birth the hip is rarely dislocated, but instead is dislocatable. Postnatal factors determine whether the hip dislocates, subluxates, or remains stable. DDH occurs more often in female infants, in breech presentations (Fig. 23-10), and in infants with family history of DDH (Lissauer, 2006). If the hip is dislocated, the femoral head is above and lateral to the acetabulum. With subluxation or laxity of ligaments, the femoral head is within the acetabulum, but it can be manipulated to the edge of the acetabulum. The majority of unstable hips will become clinically normal by one month of age.
FIG. 23-10 Position of infant’s legs after breech birth. (Courtesy Cheryl Briggs, RNC, Annapolis, MD.)
The hips are inspected for symmetry. Gluteal skinfolds and thigh skinfolds should be equal and symmetric, and legs should be of equal length (Fig. 23-11, A). The level of the knees in flexion should be equal (see Fig. 23-11, C). Hip integrity is assessed by using the Barlow test and the Ortolani maneuver. For the Barlow test the examiner places the middle finger over the greater trochanter and the thumb along the midthigh. The hip is flexed to 90 degrees and adducted followed by gentle downward pushing of the femoral head. If the hip can be dislocated with this maneuver, the femoral head moves out of the acetabulum and the examiner feels a “clunk.” The hip is then checked to determine if the femoral head can be returned into the acetabulum using the Ortolani test. As the hip is abducted and upward leverage is applied, a dislocated hip will return to the acetabulum with a clunk (see Fig. 23-11, B and D).
FIG. 23-11 Signs of developmental dysplasia of the hip. A, Asymmetry of gluteal and thigh folds with shortening of the thigh (Galeazzi sign). B, Limited hip abduction, as seen in flexion (Ortolani maneuver). C, Apparent shortening of the femur, as indicated by the level of the knees in flexion (Allis sign). D, Ortolani maneuver with femoral head moving in and out of acetabulum (in infants 1 to 2 months of age). (From Hockenberry, M., & Wilson, D. [2009]. Wong’s essentials of pediatric nursing [8th ed.]. St. Louis: Mosby.)
Signs of Risk for Skeletal Problems
Abnormalities of the skeletal system can be congenital, developmental, drug induced, or the result of intrapartum or postnatal factors. Signs of DDH, additional digits or webbing of digits, and any other abnormality should be documented and reported to the primary health care provider.
Fractured clavicle often occurs in macrosomic infants and in those whose who had a difficult birth (e.g., shoulder dystocia). Unequal movement of the upper extremities or a crepitant feeling over the clavicular area can indicate fracture.
The feet of the newborn can appear to be abnormally positioned. This can be indicative of congenital deformity or can be related to fetal positioning in utero. For example, clubfoot (talipes equinovarus), a deformity in which the foot turns inward and is fixed in a plantar-flexion position, is a congenital condition that warrants attention. If the foot is turned inward in the plantar-flexion position but can be moved into the normal position, it is likely due to fetal positioning and should gradually resolve.
Neuromuscular System
The neuromuscular system is almost completely developed at birth. The term newborn is a responsive and reactive being with remarkable capacity for social interaction and self-organization.
Growth of the brain after birth follows a predictable pattern of rapid growth during infancy and early childhood, and growth becomes more gradual during the remainder of the first decade and minimal during adolescence. By the end of the first year the cerebellum ends its growth spurt, which began at approximately 30 gestational weeks.
The brain requires glucose as a source of energy and a relatively large supply of oxygen for adequate metabolism. Such requirements signal a need for careful assessment of the infant’s respiratory status. The necessity for glucose requires attentiveness to those neonates who are at risk for hypoglycemia (e.g., infants of mothers who have diabetes, infants who are macrosomic or small for gestational age, and newborns experiencing prolonged birth, hypoxia, or preterm birth).
Spontaneous motor activity can be seen as transient tremors of the mouth and chin, especially during crying episodes, and of the extremities, notably the arms and hands. Transient tremors are normal and can be observed in nearly every newborn. These tremors should not be present when the infant is quiet and should not persist beyond 1 month of age. Persistent tremors or tremors involving the total body can indicate pathologic conditions. Normal tremors, tremors of hypoglycemia, and central nervous system (CNS) disorders must be differentiated so corrective care can be instituted as necessary.
The posture of the term newborn demonstrates flexion of the arms at the elbows and the legs at the knees. Hips are abducted and partially flexed. Intermittent fisting of the hands is common.
Muscle tone and strength are directly related. The infant with normal tone and strength exhibits some resistance to passive movement, such as when being pulled to sit, or when the arm or leg is extended by the examiner. The hypotonic neonate shows little resistance and can feel like a “rag doll.” Hypertonia is evidenced by increased resistance to passive movement.
Neuromuscular control, although very limited, can be noted. If newborns are placed face down on a firm surface, they will turn their heads to the side. They attempt to hold their heads in line with their bodies if they are raised by their arms. Various reflexes serve to promote safety and adequate food intake.
Newborn Reflexes
The newborn has many primitive reflexes. The times at which these reflexes appear and disappear reflect the maturity and intactness of the developing nervous system. The most common reflexes found in the normal newborn are described in Table 23-4.
TABLE 23-4
ASSESSMENT OF NEWBORN’S REFLEXES∗
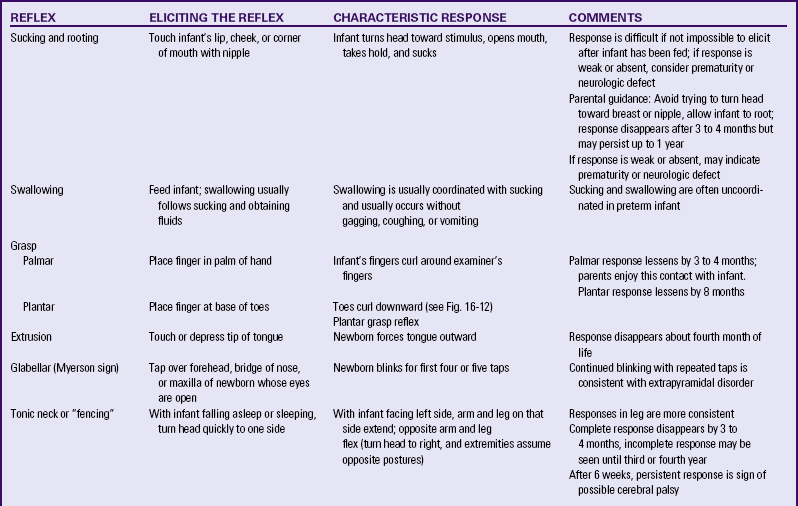
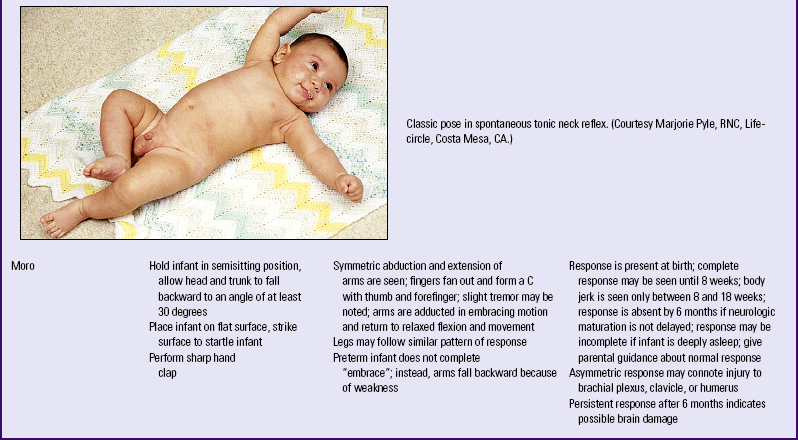
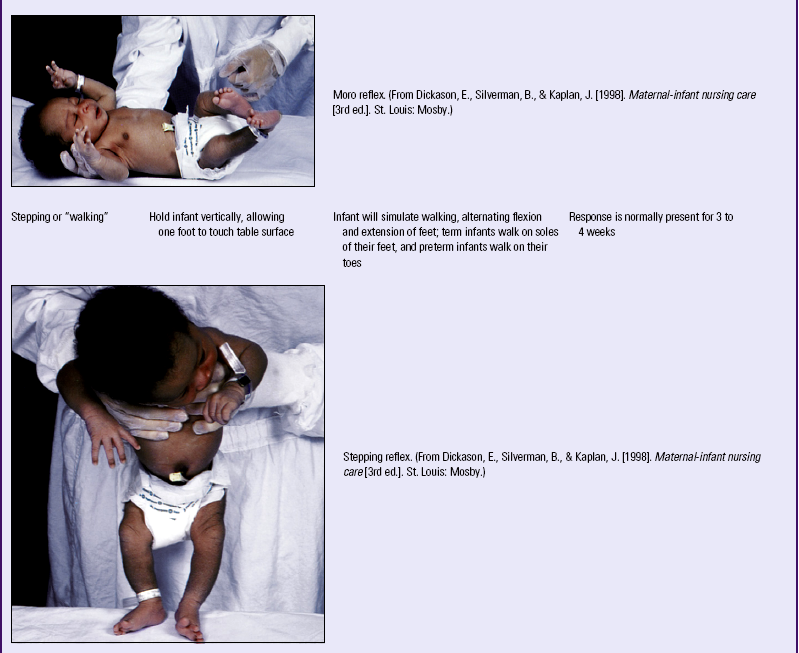
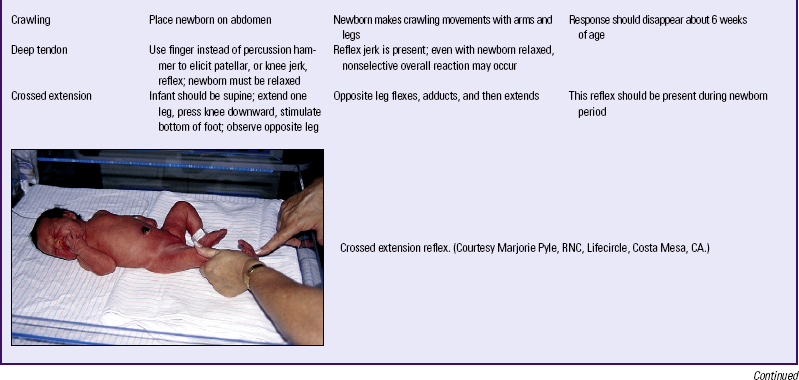
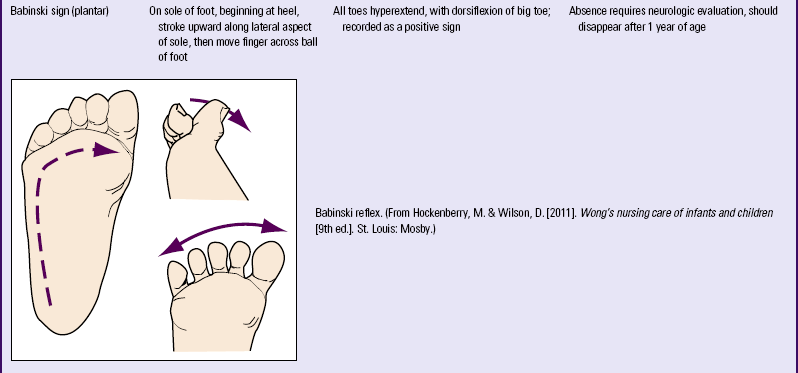
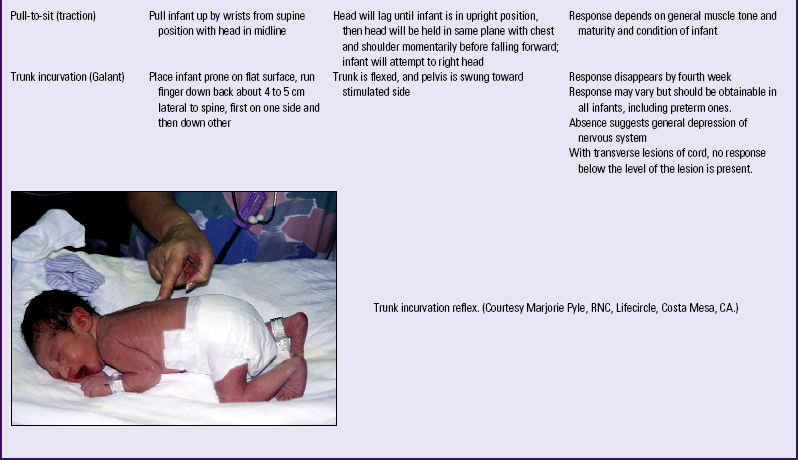
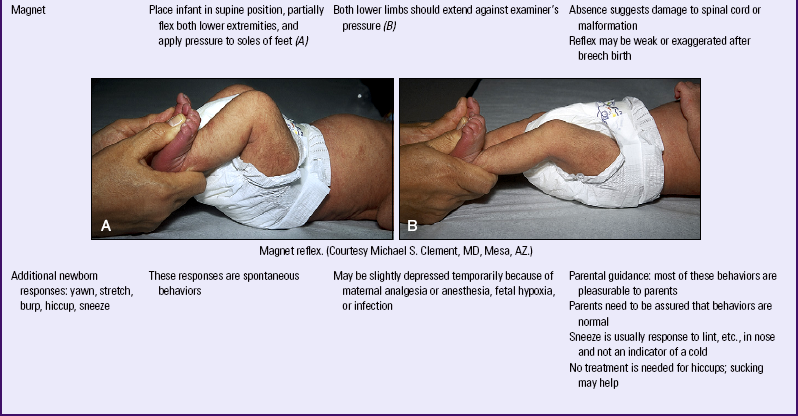
∗All durations for persistence of reflexes are based on time elapsed after 40 weeks of gestation; that is, if this newborn was born at 36 weeks of gestation, add 1 month to all time limits given.
Signs of Risk for Neuromuscular Problems
Any absence of a newborn reflex can indicate major neurologic problems. Birth trauma can cause nerve damage that results in facial asymmetry and paralysis. CNS depression resulting from maternal medications received during labor and birth also will influence neuromuscular functioning.
The cry of the healthy term newborn is strong and “lusty.” A weak cry is often associated with prematurity, neuromuscular disease, or sepsis. A high-pitched or shrill cry can suggest CNS abnormality. A low-pitched, husky cry can be related to hypothyroidism. Stridor with crying can indicate airway obstruction.
Observation of the neonate for any abnormalities must be documented. A thorough physical examination of the newborn assists in detecting any potential complications (see Table 24-2).
Behavioral Characteristics
The healthy infant must accomplish behavioral and biologic tasks to develop normally. Behavioral characteristics form the basis of the social capabilities of the infant. Healthy newborns differ in their activity levels, feeding patterns, sleeping patterns, and responsiveness. Parents’ reactions to their newborns are often determined by these differences. Showing parents the unique characteristics of their infant assists parents to develop a more positive perception of the infant with increased interaction between infant and parent.
Behavioral responses, as well as physical characteristics, change during the period of transition. The Brazelton Neonatal Behavioral Assessment Scale (BNBAS) can be used to assess the infant’s behavior systematically (Brazelton, 1999; Brazelton & Nugent, 1996). The BNBAS is an interactive examination that assesses the infant’s response to 28 areas organized according to the clusters in Box 23-2. It is generally used as a research or diagnostic tool and requires special training.
In addition to use as initial and ongoing tools to assess neurologic and behavioral responses, the scales can assess initial parent-infant relationships and help parents focus on their infant’s individuality and develop a deeper attachment to their child. See Chapter 22 for further discussion of attachment.
Sleep-Wake States
Variations in the state of consciousness of infants are called sleep-wake states. The six states form a continuum from deep sleep to extreme irritability (Fig. 23-12): two sleep states (deep sleep and light sleep) and four wake states (drowsy, quiet alert, active alert, and crying) (Blackburn, 2007). Each state has specific characteristics and state-related behaviors. The optimal state of arousal is the quiet alert state. During this state infants smile, vocalize, move in synchrony with speech, watch their parents’ faces, and respond to people talking to them. Infants respond to internal and external environmental factors by controlling sensory input and regulating the sleep-wake states; the ability to make smooth transitions between states is called state modulation. The ability to regulate sleep-wake states is essential in the infant’s neurobehavioral development. As infants approach term gestation, they are better able to cope with external or internal factors that affect the sleep-wake patterns.
FIG. 23-12 Summary of newborn sleep-wake states. States of consciousness: A, Deep sleep. B, Light sleep. C, Drowsy. D, Quiet alert. E, Active alert. F, Crying. (Courtesy Marjorie Pyle, RNC, Lifecircle, Costa Mesa, CA.)
Infants use purposeful behavior to maintain the optimal arousal state as follows: (1) actively withdrawing by increasing physical distance, (2) rejecting by pushing away with hands and feet, (3) decreasing sensitivity by falling asleep or breaking eye contact by turning the head, or (4) using signaling behaviors, such as fussing and crying. These behaviors permit infants to quiet themselves and reinstate readiness to interact.
The first 6 weeks of life involve a steady decrease in the proportion of active REM sleep to total sleep. A steady increase in the proportion of quiet sleep to total sleep also occurs. Periods of wakefulness increase. For the first few weeks the wakeful periods seem dictated by hunger but soon a need for socializing appears as well. The newborn sleeps on average approximately 17 hours a day, with periods of wakefulness gradually increasing. By the fourth week of life, some infants stay awake from one feeding to the next.
Other Factors Influencing Newborn Behavior
The gestational age of the infant and level of CNS maturity affect the observed behavior. In an infant with an immature CNS (preterm) the entire body responds to a pinprick of the foot although the response may not be observed by an untrained observer. The more mature infant withdraws only the foot. CNS immaturity is reflected in reflex development, sleep-wake states, and ability (or lack thereof) to regulate or modulate a smooth transition between different states. Preterm infants have brief periods of alertness but have difficulty maintaining alertness without becoming overstimulated, which leads to autonomic instability unless intervention is implemented. Premature or sick infants show signs of fatigue or physiologic stress sooner than full-term healthy infants.
Time
The time elapsed since birth affects the behavior of infants as they attempt to become organized initially. Time elapsed since the previous feeding and time of day also can influence infants’ responses.
Stimuli
Environmental events and stimuli affect the behavioral responses of infants. The newborn responds to animate and inanimate stimuli. Nurses in intensive care nurseries observe that infants respond to loud noises, bright lights, monitor alarms, and tension in the unit. If a mother is tense, nervous, or uncomfortable while feeding her infant, the infant may sense her tension and demonstrate difficulty feeding.
Medication
There is no conclusive evidence regarding the effects of maternal analgesia or anesthesia during labor on neonatal behavior. Some researchers note that infants of mothers given certain analgesic medications can have disturbances in newborn behavior such as more crying, an increase in temperature, and some difficulty in breastfeeding (Ransjö-Arvidson, Matthiesen, Lilja, Nissen, Widström, & Uvnäs-Moberg, 2001). Other researchers maintain that the effect on infant behavior is negligible. Researchers who have studied the effects of epidural medications on breastfeeding behaviors have been unable to show a cause-and-effect relationship (Chang & Heaman, 2006; Devroe, De Coster, & Van de Velde, 2009; Torvaldsen, Roberts, Simpson, Thompson, & Ellwood, 2006).
Sensory Behaviors
From birth, infants possess sensory capabilities that indicate a state of readiness for social interaction. Infants effectively use behavioral responses in establishing their first dialogues. These responses, coupled with the newborns’ “baby appearance” (e.g., facial proportions of forehead, eyes larger than the lower portion of the face) and their small size and helplessness, rouse feelings of wanting to hold, protect, and interact with them.
Vision
At birth the eye is structurally incomplete and the muscles are immature. The process of accommodation is not present but improves over the first 3 months of life. The pupils react to light, the blink reflex is easily stimulated, and the corneal reflex is activated by light touch. Term newborns can see objects as far away as 50 cm (2.5 feet). The clearest visual distance is 17 to 20 cm (8 to 12 inches), which is approximately the distance between the mother’s face and the infant’s face during breastfeeding or cuddling. Infants are sensitive to light; they will frown if a bright light is flashed in their eyes, and will turn toward a soft, red light. If the room is darkened, they will open their eyes wide and look about. By 2 months of age they can detect color; but at 5 days of age and younger, they seem more attracted by black-and-white patterns.
Response to movement is noticeable. If a bright light is shown to newborns (even at 15 minutes of age), they will follow it visually; some will even turn their heads to do so. Because human eyes are bright shiny objects, newborns will track their parents’ eyes. Parents often comment on their excitement in observing this behavior. The development of eye-to-eye contact is very important for parent-infant attachment. Children of blind parents, and parents who have blind children, must circumvent this obstacle for the formation of a relationship.
Visual acuity is surprising. Even at 2 weeks of age infants can distinguish patterns with stripes 3 mm apart. By 6 months their vision is as acute as that of an adult. They prefer to look at patterns rather than plain surfaces, even if the latter are brightly colored. Infants prefer more complex patterns to simple ones. They prefer novelty (changes in pattern) by 2 months of age. The infant of a few weeks of age is therefore capable of responding actively to an enriched environment.
Hearing
As soon as the amniotic fluid drains from the ears the infant’s hearing is similar to that of an adult. Loud sounds of approximately 90 decibels cause the infant to respond with a Mono reflex. The newborn responds to low-frequency sounds such as a heartbeat or lullaby by decreasing motor activity or stopping crying. High-frequency sound elicits an alerting reaction.
The infant responds readily to the mother’s voice. Studies indicate a selective listening to maternal voice sounds and rhythms during intrauterine life that prepares newborns for recognition and interaction with their primary caregivers—their mothers. Newborns are accustomed in the uterus to hearing the regular rhythm of the mother’s heartbeat. As a result, they respond by relaxing and ceasing to fuss and cry if a regular heartbeat simulator is placed in their cribs.
The internal and middle portions of the ear are larger at birth, but the external canal is small. The mastoid process and bony parts of the external canal have not developed; therefore, the tympanic membrane and facial nerve are very close to the surface and can be easily damaged. Hearing loss is common at birth; 1 in 1000 newborn infants is afflicted with a sensorineural hearing loss significant enough to affect speech and language development. Approximately half of those are due to hereditary loss and the other half are due to an acquired loss. The most common cause of acquired hearing loss is infection that is transmitted from the mother to the fetus transplacentally (Arnold & Sprecher, 2006). To identify affected infants the hearing of all infants is screened before discharge from the birth institution (Fig. 24-9).
Smell
Newborns have a highly developed sense of smell and can detect and discriminate distinct odors. It has been shown that preterm infants as early as 28 weeks are capable of reacting to odors. Newborns react to strong odors such as alcohol or vinegar by turning their heads away, but are attracted to sweet smells. By the fifth day of life, newborn infants can recognize their mother’s smell (Brazelton, 1999). Breastfed infants are able to smell breast milk and can differentiate their mothers from other lactating women (Lawrence & Lawrence, 2005).
Taste
The newborn can distinguish among tastes, and various types of solutions elicit differing facial expressions. A tasteless solution produces no response; a sweet solution elicits eager sucking. A sour solution causes a puckering of the lips, and a bitter liquid produces a grimace.
Young infants are particularly oriented toward the use of their mouths, both for meeting their nutritional needs for rapid growth and for releasing tension through sucking. The early development of circumoral sensation, muscle activity, and taste would seem to be preparation for survival in the extrauterine environment.
Touch
The infant is responsive to touch on all parts of the body. The face (especially the mouth), the hands, and the soles of the feet seem to be the most sensitive. Reflexes can be elicited by stroking the infant. The newborn’s responses to touch suggest that this sensory system is well prepared to receive and process tactile messages. Touch and motion are essential to normal growth and development. However, each infant is unique, and variations can be seen in newborns’ responses to touch. Birth trauma or stress and depressant drugs taken by the mother decrease the infant’s sensitivity to touch or painful stimuli.
Response to Environmental Stimuli
Classic studies (Thomas, Birch, Chess, & Robbins, 1961; Thomas, Chess, & Birch, 1970) identified individual variations in the primary reaction pattern of newborns and described them as temperament. Their style of behavioral response to stimuli is guided by the temperament affecting the newborn’s sensory threshold, ability to habituate, and response to maternal behaviors. The newborn possesses individual characteristics that affect selective responses to various stimuli present in the internal and external environment.
Habituation
Habituation is a protective mechanism that allows the infant to become accustomed to environmental stimuli. Habituation is a psychologic and physiologic phenomenon in which the response to a constant or repetitive stimulus is decreased. In the term newborn, habituation can be demonstrated in several ways. Shining a bright light into a newborn’s eyes will cause a startle or squinting the first two or three times. The third or fourth flash will elicit a diminished response, and by the fifth or sixth flash the infant ceases to respond (Brazelton, 1999; Brazelton & Nugent, 1996). The same response pattern holds true for the sounds of a rattle or a pinprick to the heel.
The ability to habituate also allows the newborn to select stimuli that promote continued learning about the social world, thus preventing overload. The intrauterine experience seems to have programmed the newborn to be especially responsive to human voices, soft lights, soft sounds, and sweet tastes.
The newborn quickly learns the sounds in the home environment and is able to sleep in their midst. The selective responses of the newborn indicate cerebral organization capable of memory and making choices. The ability to habituate depends on state of consciousness, hunger, fatigue, and temperament. These factors also affect consolability, cuddliness, irritability, and crying.
Consolability
Newborns vary in their ability to console themselves or to be consoled. In the crying state, most newborns initiate one of several methods for reducing their distress. Hand-to-mouth movements are common, with or without sucking, as well as alerting to voices, noises, or visual stimuli.
Cuddliness
Cuddliness is especially important to parents because they often gauge their ability to care for the child by the child’s responses to their actions. Variability is noted in the degree to which newborns will mold into the contours of the persons holding them. Babies are soothed and become alert with the vestibular stimulation of being picked up and moved.
Irritability
Some newborns cry longer and harder than others. For some infants the sensory threshold seems low. They are readily upset by unusual noises, hunger, wetness, or new experiences and respond intensely to these stimuli. Others with a high sensory threshold require a great deal more stimulation and variation to reach the active, alert state.
Crying
Crying in an infant may signal hunger, pain, desire for attention, or fussiness. Most mothers learn to distinguish among the cries. The duration of crying is highly variable in each infant; newborns may cry for as little as 5 minutes or as much as 2 hours or more per day. The amount of crying peaks in the second month and then decreases. A diurnal rhythm of crying can be noted, with more crying in the evening hours. Crying does not seem to differ with different caregivers.
KEY POINTS
• By full term the infant’s various anatomic and physiologic systems have reached a level of development and functioning that permits a physical existence apart from the mother.
• The healthy term infant has sensory capabilities that indicate a state of readiness for social interaction.
• Several significant differences exist among the respiratory, renal, and thermogenic systems of the newborn and those of an adult.
• Physiologic jaundice occurs in approximately 60% of term newborns and 80% of preterm newborns.
• Jaundice is considered pathologic or nonphysiologic if it appears within the first 24 hours of life, if serum bilirubin levels increase by more than 5 mg/dl in 24 hours, or if the serum bilirubin level exceeds 15 mg/dl at any time.
• Heat loss in the healthy term newborn can exceed the capacity to produce heat, causing cold stress which can lead to metabolic and respiratory complications that threaten the newborn’s well-being.
• Some reflex behaviors are important for the newborn’s survival.
• Individual personalities and behavioral characteristics of infants play major roles in their ultimate relationships with their parents.
• Sleep-wake states and other factors influence the newborn’s behavior.
• Each full-term newborn has a predisposed capacity to handle the multitude of stimuli in the external world.
![]() Audio Chapter Summaries Access an audio summary of these Key Points on
Audio Chapter Summaries Access an audio summary of these Key Points on ![]()