Nursing Care of the High Risk Newborn
• Analyze differences in characteristics of preterm, late preterm, term, and postterm neonates.
• Discuss respiratory distress syndrome and the approach to treatment.
• Compare methods of oxygen therapy.
• Analyze the appropriate nursing interventions for nutritional care of the preterm infant.
• Discuss the pathophysiology of retinopathy of prematurity and bronchopulmonary dysplasia (BPD) and the risk factors that predispose preterm infants to these problems.
• Discuss pain assessment and management in the preterm infant.
• Describe the signs and symptoms of perinatal asphyxia.
• Analyze the pathophysiology of meconium aspiration syndrome and its clinical signs.
• Plan developmentally appropriate care for high risk infants.
• Discuss the needs of parents of high risk infants.
• Evaluate a neonatal transport plan.
• Explain appropriate responses and interventions the nurse can use in caring for families of preterm and high risk infants experiencing anticipatory grief or loss and grief in the neonatal period.
• Describe nursing care for late preterm infants admitted to mother-baby units.
• List specific discharge teaching needs for parents of late preterm infants admitted to mother-baby units.
![]() http://evolve.elsevier.com/Lowdermilk/MWHC/
http://evolve.elsevier.com/Lowdermilk/MWHC/
Audio Glossary
Case Study — The Newborn at Risk
Nursing Care Plan
Spanish Guidelines
Video—Nursing Skills
Modern technology and expert nursing care have made important contributions to improving the health and overall survival of high risk infants. However, infants who are born considerably before term and survive are particularly susceptible to the development of problems related to their preterm birth. These problems are not limited to preterm infants. They can also occur in term and late preterm infants, although not so frequently, and include necrotizing enterocolitis, bronchopulmonary dysplasia (BPD), intraventricular and periventricular hemorrhage, and retinopathy of prematurity (ROP).
High risk infants are most often classified according to birth weight, gestational age, and predominant pathophysiologic problems (Box 37-1). Intrauterine growth rates differ among infants; factors such as heredity, placental insufficiency, and maternal disease influence intrauterine growth and birth weight. The classification system in the box encompasses birth weight and gestational age.
For the high risk infant, an accurate assessment of gestational age (see Chapter 24) is critical in helping the nurse identify the potential problems the newborn is likely to have. The response of the preterm, late preterm, or postterm infant to extrauterine life differs from that of the term infant. By understanding the physiologic basis of these differences, the nurse can assess these infants, determine the response of the preterm or postterm infant, and discern which problems are most likely to occur.
Preterm Infants
The vast majority of high risk infants are those born at less than 37 weeks of gestation. This includes preterm and late preterm births. The preterm birth rate in the United States showed a fairly steady increase from the early 1980s to 2006. Then, in 2006, the rate declined to 12.3% from 12.8%. The decrease occurred for all types of births, including cesareans, and induced and noninduced vaginal births (Martin, Osterman, & Sutton, 2010). What caused this decline and whether or not it will continue are subjects for ongoing research.
At times the nurse is able to anticipate problems, such as when a woman is admitted in premature labor. At other times the birth of a high risk infant is unanticipated. In either case the personnel and equipment necessary for immediate care of the infant must be available.
Preterm infants are at risk because their organ systems are immature and they lack adequate reserves of bodily nutrients. The potential problems and care needs of the preterm infant weighing 2000 g differ from those of the term, postterm, or postmature infant of equal weight. If these infants have physiologic disorders and anomalies as well, they affect the infant’s response to treatment. In general, the closer infants are to term from the standpoint of both gestational age and birth weight, the easier their adjustment to the external environment.
Preterm, low birth weight (LBW), and extremely low birth weight infants often require hospitalization beyond the typical 48 hours after birth. Their physiologic immaturity and associated problems can involve extensive use of technologic and pharmacologic interventions. The cost of the care required by preterm and LBW infants is estimated to be in the billions of dollars each year and continues to rise as the use of technology increases.
Varying opinions exist about the practical and ethical dimensions of resuscitation of extremely low birth weight (ELBW) infants (those infants whose birth weight is 1000 g or less). Ethical issues associated with resuscitation that nurses caring for such infants are confronted with include the following:
• Should resuscitation be attempted?
• Is the cost of resuscitation justified?
• Do the benefits of technology outweigh the burdens in relation to the quality of life?
All people involved (health care providers, nurses, parents, ethicists, clergy, attorneys) should participate in discussions addressing these controversial issues. Although there are no clear answers, such discussions help clarify the issues and promote more family-centered approaches to care. That care can involve sustaining life or providing care and support for a peaceful death. Nurses are key to the care of these infants and their families.
Late Preterm Infants
Infants born between 34 and 36 6/7 weeks of pregnancy are called “late preterm”. The term, “late preterm”, was developed by the National Institute of Child Health and Human Development (Raju, Higgins, Stark, & Lereno, 2006), replacing the previous terminology of near-term. By referring to these infants as late preterm, it conveys the concept that they are indeed premature with unique needs and potential problems associated with their early birth.
Late preterm infants are more likely than term infants to experience morbidity and mortality. They are at greater risk for complications such as respiratory distress, are more likely to require intensive and prolonged hospitalization, and to incur higher medical costs. They are more likely to die before 1 year of age and to suffer neurologic injury that results in long-term neurodevelopmental problems (Martin et al., 2010).
Common problems experienced by late preterm infants include thermoregulation, feeding difficulty, hyperbilirubinemia, hypoglycemia, infection, and respiratory problems. Care of the late preterm infant is discussed on pp 922–923.
Physiologic Functions
The preterm infant is likely to have difficulty making the pulmonary transition from intrauterine to extrauterine life. Numerous problems can affect the respiratory systems of preterm infants and can include the following:
• Decreased number of functional alveoli
• Smaller lumen in the respiratory system
• Greater collapsibility or obstruction of respiratory passages
• Insufficient calcification of the bony thorax
• Immature and friable capillaries in the lungs
• Greater distance between functional alveoli and the capillary bed
In combination, these deficits have the potential to severely hinder the preterm infant’s respiratory efforts and can produce respiratory distress or apnea. Nurses must be alert to signs of respiratory distress or apnea and ready to intervene to promote adequate oxygenation.
Respiratory difficulty often follows a progressive pattern. Infants normally breathe between 30 and 60 breaths/min, relying significantly on their abdominal muscles to accomplish this. However, the respiratory rate can increase without a change in rhythm. Early signs of respiratory distress include flaring of the nares and an expiratory grunt. Depending on the cause, retractions can begin as subcostal, suprasternal, or clavicular retractions. If the infant shows increasing respiratory effort (e.g., seesaw breathing patterns, retractions, flaring of the nares, expiratory grunts, and/or apneic spells), this indicates deepening distress. A compromised infant’s color progresses from pink to circumoral cyanosis and then to generalized cyanosis. Acrocyanosis deepens.
Periodic breathing is a respiratory pattern commonly seen in preterm infants. Such infants exhibit 5- to 10-second respiratory pauses followed by 10 to 15 seconds of compensatory rapid respirations. Such periodic breathing should not be confused with apnea, which is a 15- to 20-second cessation of respiration.
Cardiovascular Function
Evaluation of heart rate and rhythm, skin color, blood pressure, perfusion, pulses, oxygen saturation, and acid-base status provides information on the cardiovascular status. The nurse must be prepared to intervene if symptoms of hypovolemia or shock, or both, are found. These symptoms include hypotension, slow capillary refill (longer than 3 seconds), and continued respiratory distress despite the provision of oxygen and ventilation.
An accurate and timely blood pressure (BP) reading can assist in making an early diagnosis of cardiorespiratory disease and in monitoring the effects of fluid therapy. BP is monitored routinely in the sick neonate by internal or external means. Direct recording with arterial catheters is often used but carries the risks inherent in any procedure in which a catheter is introduced into an artery. An umbilical venous catheter can also be used to monitor the neonate’s central venous pressure. Oscillometry (Dinamap) is a noninvasive, effective means for detecting alterations in systemic BP (hypotension or hypertension) and for identifying the need to implement appropriate therapy to maintain cardiovascular function.
Maintaining Body Temperature
Preterm infants are susceptible to temperature instability as a result of numerous factors. Because of their large body surface in relation to their weight, preterm infants are at high risk for heat loss. Other factors that place preterm infants at risk for temperature instability include the following:
• Minimal insulating subcutaneous fat
• Limited stores of brown fat (an internal source for the generation of heat present in normal term infants)
• Decreased or absent reflex control of skin capillaries (shiver response)
• Inadequate muscle mass activity (rendering the preterm infant unable to produce its own heat)
• Poor muscle tone, resulting in more body surface area being exposed to the cooling effects of the environment
The goal of thermoregulation is to create a neutral thermal environment (NTE), which is the environmental temperature at which oxygen consumption is minimal but adequate to maintain the body temperature (Bagwell, 2007). Armed with the knowledge of the four mechanisms of heat transfer (convection, conduction, radiation, and evaporation), the nurse can then create an environment for the preterm infant that prevents temperature instability (see Chapter 24). The infant is kept in a radiant warmer bed or in an incubator with control settings at a temperature to maintain the NTE. Because the preterm infant has few reserves (extra energy calories, minimal or no fat stores), cold sensitivity is a problem. This infant can easily lose heat and develop hypothermia. Physiologically the infant tries to conserve heat and burns more calories, and the metabolic system goes into overdrive, further stressing the already compromised neonate.
A critical nursing role is to prevent or minimize hypothermia and cold stress by recognizing the risk factors and using intervention strategies to prevent and treat such stress. Signs of cold stress are listed in Box 37-2.
The nurse should attempt to prevent hyperthermia. Given that overheating produces an increase in oxygen and calorie consumption, the infant is also jeopardized if he or she becomes hyperthermic. The preterm infant is not able to sweat and thus dissipate heat. Overheating can lead to apnea, tachycardia, and eventually bradycardia, as well as consumption of calories that the preterm infant cannot afford to expend (see Box 37-2).
Central Nervous System Function
The preterm infant’s central nervous system (CNS) is susceptible to injury as a result of the following problems:
• Birth trauma that includes damage to immature structures
• Bleeding from fragile capillaries
• An impaired coagulation process, including prolonged prothrombin time
• Recurrent hypoxic and hyperoxic episodes
• Predisposition to hypoglycemia
• Fluctuating systemic BP with concomitant variation in cerebral blood flow and pressure
In the preterm neonate, neurologic function is dependent on gestational age, associated illness factors, and predisposing factors such as intrauterine asphyxia, which can cause neurologic damage. Clinical signs of neurologic dysfunction can be subtle, nonspecific, or specific. Five categories of clinical manifestations should be thoroughly evaluated in the preterm infant: seizure activity, hyperirritability, CNS depression, elevated intracranial pressure (ICP), and abnormal movements such as decorticate posturing. Primary and tendon reflexes are generally present in preterm infants by 28 weeks of gestation; evaluation of these reflexes should be part of the neurologic examination.
Research evidence indicates that the developing nervous system has the ability to reorganize neural connection after injury, meaning that some injuries that would be permanent in adults are not so in infants. Certain neurologic signs appear to be predictive of later neurologic abnormalities. These signs include hypotonia, a decreased level of activity, weak cry for more than 24 hours, and an inability to coordinate suck and swallow. Ongoing assessment and documentation of these neurologic signs are needed for the purpose of discharge teaching and making follow-up recommendations, as well as for their predictive value.
Maintaining Adequate Nutrition
The goal of neonatal nutrition is to promote normal growth and development. However, the maintenance of adequate nutrition in the preterm infant is complicated by problems with intake and metabolism. The preterm infant has the following disadvantages with regard to intake: weak or absent suck, swallow, and gag reflexes; a small stomach capacity; and weak abdominal muscles. The preterm infant’s metabolic functions are compromised by a limited store of nutrients, a decreased ability to digest proteins or absorb nutrients, and immature enzyme systems.
The nurse must continually assess the infant’s ability to take in and digest nutrients. Some preterm infants require gavage or intravenous (IV) feedings instead of oral feedings. An area of research that holds promise for preterm infants is use of minimal enteral nutrition (MEN) that may br only 1 ml/hr (Anderson, Wood, Keller, & Hay, 2011; Mosqueda, Sapieqiene, Glynn, Wilson-Costello, & Weiss, 2008). These feedings stimulate the gastrointestinal (GI) system with minute amounts of breast milk or formula, usually given via gavage, so that when enteral feedings of greater volume can begin, the GI system is primed for nutrient absorption. They also may help to protect LBW infants from sepsis; however, more evidence is needed to support this relationship (Terrin, Passariello, Canani, Manguso, Paludetto, & Cascioli, 2009).
Maintaining Renal Function
The preterm infant’s immature renal system is unable to (1) adequately excrete metabolites and drugs; (2) concentrate urine; or (3) maintain acid-base, fluid, or electrolyte balance. Therefore, intake and output, as well as specific gravity, must be assessed. Laboratory tests must be done to assess acid-base and electrolyte balance. Medication levels are monitored in preterm infants because certain medications can overwhelm the immature system’s ability to excrete them.
Maintaining Hematologic Status
The preterm infant also is particularly predisposed to hematologic problems because of the following:
• Increased capillary fragility
• Increased tendency to bleed (prolonged prothrombin time and partial thromboplastin time)
• Slowed production of red blood cells resulting from rapid decrease in erythropoiesis after birth
• Loss of blood due to frequent blood sampling for laboratory tests
• Decreased red blood cell survival related to the relatively larger size of the red blood cell and its increased permeability to sodium and potassium
The nurse assesses such infants for any evidence of bleeding from puncture sites and the GI tract. Infants also are examined for signs of anemia (decreased hemoglobin and hematocrit levels, pale skin, increased apnea, lethargy, tachycardia, and poor weight gain). The amount of blood drawn for laboratory testing is closely monitored and recorded.
Resisting Infection
Preterm infants are at increased risk for infection because they have a shortage of stored maternal immunoglobulins, an impaired ability to make antibodies, and a compromised integumentary system (thin skin and fragile capillaries). Preterm infants exhibit various nonspecific signs and symptoms of infection (Box 37-3). Early identification and treatment of sepsis are essential (see Chapter 35). As with all aspects of care, strict attention to hand hygiene is the single most important measure to prevent health care–associated infections.
Growth and Development Potential
Although it is impossible to predict with complete accuracy the growth and development potential of each preterm infant, some findings support an anticipated favorable outcome in the absence of ongoing medical problems that can affect growth, such as BPD, necrotizing enterocolitis, and CNS problems. The lower the birth weight, the greater the likelihood for negative outcomes.
The age of a preterm newborn is corrected by adding the gestational age and the postnatal age. For example, an infant born at 32 weeks of gestation 4 weeks ago would now be considered 36 weeks of age. The infant’s corrected age at 6 months after the birth date is then 4 months, and the infant’s responses are accordingly evaluated against the norm expected for a 4-month-old infant. The growth and development milestones (e.g., motor milestones, vocalization, growth) are corrected for gestational age until the child is approximately 21⁄2 years old.
Certain measurable factors predict normal growth and development. The preterm infant experiences catch-up body growth during the first 2 years of life; this is most likely to occur when the infant has a normal birth length (Kliegman, 2006). The head is the first to experience catch-up growth, followed by a gain in weight and height. At the infant’s discharge from the hospital, which usually occurs between 37 and 40 weeks of postconception age, the infant should exhibit the following characteristics:
• An ability to raise the head when prone and to hold the head parallel with the body when tested for the head-lag response
• An ability to cry with vigor when hungry
• An appropriate amount and pattern of weight gain according to a growth grid
At 39 to 40 weeks of corrected age, the infant should be able to focus on the examiner’s or parent’s face and to follow with his or her eyes.
Very low birth weight (VLBW) (<1500 g) survivors are at high risk for neurologic and/or cognitive disabilities in varying degrees of severity; these include cerebral palsy, borderline intelligence, and learning disabilities (Daily, Carter, & Carter, 2011). Ongoing research is focused on examining other factors including environmental ones that can cause adverse cognitive and neurodevelopmental outcomes for VLBW and ELBW babies by the time they reach infancy or school age.
Care Management
The goal of care for the preterm infant is to provide an extrauterine environment that approximates the healthy intrauterine environment to promote normal growth and development. Physicians, nurses, nurse practitioners, infant developmental specialists, and respiratory therapists work together as a team to provide the intensive care needed.
The admission of a preterm newborn to the intensive care nursery usually represents an emergency situation. Immediately after admission, a rapid initial evaluation is done to determine the infant’s need for lifesaving treatment. Resuscitation is started in the birthing unit, and the newborn’s needs for warmth and oxygen are provided for during transfer to the nursery.
Nursing care is focused on the continuous assessment and analysis of the infant’s physiologic status. Nurses fulfill many roles in providing the intensive and extended care that these infants require. In addition, they are the support persons and teachers during the first phase of the parents’ adjustment to the birth of their preterm infant.
The nurse uses many technologic support systems to monitor the body responses and maintain the body functions of the infant. Technical skill must be combined with a gentle touch and concern about the traumatic effects of harsh lighting and the volume of machinery noise. Provision of individualized behavioral and environmental care has been shown to reduce infant stress, conserve energy, and promote better neurobehavioral outcomes (Gardner & Goldson, 2011). (See Nursing Process box on p. 899 and Nursing Care Plan on pp. 900-901.)
Physical Care
The environmental support measures for the preterm infant typically consist of the following equipment and procedures:
• An incubator or radiant warmer placed over the infant to control body temperature (NTE)
• Oxygen administration, depending on the infant’s cardiopulmonary and circulatory status
• Electronic monitors as needed for the observation of respiratory and cardiac functions
• Assistive devices for positioning the infant in neutral flexion and with boundaries
• Clustering of care and minimization of stimulation according to infant cues
Various metabolic support measures that can be instituted consist of the following:
• Parenteral fluids to help support nutrition and maintain normal arterial blood gas (ABG) levels and acid-base balance
• IV access to facilitate the administration of antibiotic therapy if sepsis is a concern
• Blood work to monitor ABG levels, pH, blood glucose levels, electrolytes, and the status of blood cultures
Maintaining Body Temperature
The high risk infant is susceptible to heat loss and its complications. In addition, LBW infants can be unable to increase their metabolic rate because of impaired gas exchange, caloric intake restrictions, or poor thermoregulation. Transepidermal water loss is greater because of skin immaturity in very preterm infants (those at less than 28 weeks of gestation) and can contribute to temperature instability.
The preterm infant should be transferred from the birth room in a prewarmed incubator; ELBW infants can be placed in a polyethylene bag to decrease heat and water loss (Fig. 37-1). Skin-to-skin contact (kangaroo care) between the stable preterm infant and parent is a viable option for interaction because of the maintenance of appropriate body temperature by the infant (see pp. 910-911 for further discussion of kangaroo care).
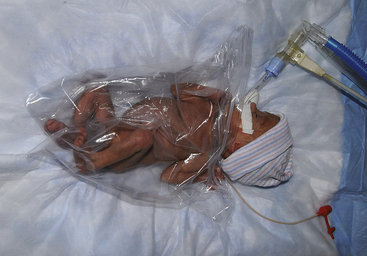
FIG. 37-1 Preterm infant in polyethylene bag to protect against heat loss. (Courtesy Cheryl Briggs, RNC, Annapolis, MD.)
High risk infants are cared for in the thermoneutral environment created by use of an external heat source. A probe to an external heat source supplied by a radiant warmer or a servo-controlled incubator is attached to the infant. The infant acts as a thermostat to regulate the amount of heat supplied by the external source. This idealized environment maintains an infant’s normal body temperature between 36.5° and 37.2° C. Maintaining a thermoneutral condition in the youngest, most immature infants decreases the need for them to generate additional heat. The rationale is that this should increase physiologic stability and decrease oxygen consumption (Bosque & Haverman, 2009; Soll, 2008).
Care of the Hypothermic Infant: The hypothermic infant can appear pale and mottled; the skin is cool to touch, especially the extremities. Acrocyanosis and respiratory distress can occur as oxygen consumption increases in an effort to generate heat. As hypothermia worsens, the infant can have apnea, bradycardia, and central cyanosis.
When an infant becomes hypothermic, rewarming should begin immediately by providing external heat. However, rapid changes in body temperature can cause apnea and acidosis. For the infant with mild hypothermia, slow rewarming is recommended. External heat sources should be slightly warmer than skin temperature and increased gradually until the infant’s temperature is within the range of NTE. For the severely hypothermic infant (body temperature less than 35°C), more rapid rewarming is needed. Use of radiant heaters or heated water mattresses helps to prevent prolonged metabolic acidosis and hypoglycemia and reduces mortality (Brown & Landers, 2011).
Oxygen Therapy
Clinical criteria for identifying the need for oxygen administration include increased respiratory effort, respiratory distress with apnea, tachycardia, bradycardia, and central cyanosis with or without hypotonia. The need for oxygen should be substantiated by biochemical data (arterial oxygen pressure [Pao2] of less than 60 mm Hg or an oxygen saturation of less than 92%). High risk infants often require saturations of more than 95% to maintain respiratory stability because their hemoglobin levels are frequently low. As the Pao2 decreases, less oxygen is released from the hemoglobin, which increases the risk for cellular hypoxia.
Oxygen administered to an infant is warmed and humidified to prevent cold stress and drying of the respiratory mucosa. During the administration of oxygen, the concentration, volume, temperature, and humidity of the gas are carefully controlled. Delivery of oxygen for more than a few minutes requires the use of special equipment (hood, nasal cannula, positive-pressure mask, or endotracheal tube) because the concentration of free-flow oxygen cannot be monitored accurately. Free-flow oxygen into an incubator should not be used because the concentration fluctuates dramatically each time the doors or portholes are opened. The indiscriminate use of oxygen can be hazardous. Possible complications of oxygen therapy include ROP and BPD.
Infants who need oxygen should have their respiratory status assessed accurately at least every hour. This includes a continuous pulse oximetry reading and at least one ABG measurement. There should also be hourly documentation of pulse oximetry readings as well as the amount of oxygen being administered and the mode of delivery (Gardner, Enzman-Hines, & Dickey, 2011). The interventions implemented are then determined on the basis of the findings yielded by the clinical assessment, including telemetry (pulse oximetry or tcPo2 [skin oxygen tension] monitoring) and laboratory tests (Cifuentes & Carlo, 2007). The interventions ordered are those that can directly manage the underlying disease process and range from hood oxygen administration to ventilator therapy.
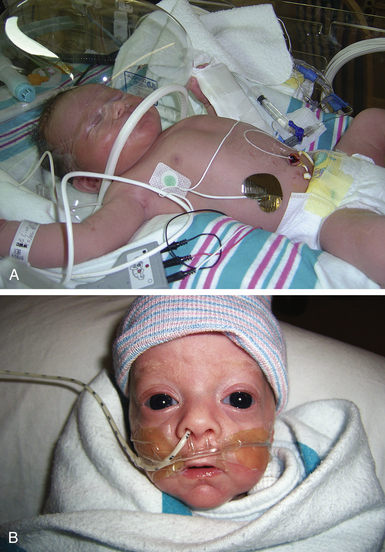
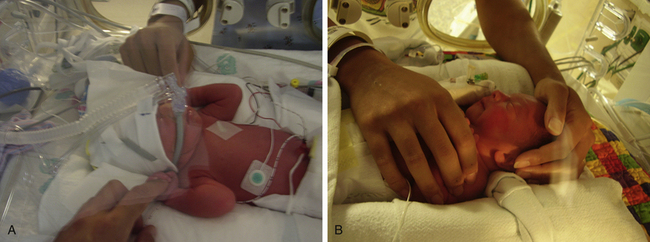
Hood Therapy: A hood can be used to administer oxygen to infants who do not require mechanical pressure support. The hood is a clear plastic cover that is sized to fit over the head and neck of the infant (Fig. 37-2, A). Inside the hood the infant receives the correct amount of oxygen. The nurse checks the oxygen level at least every hour because the concentration must be adjusted in response to the infant’s condition. If the hood is removed for holding, feeding, or suctioning, an alternative source of oxygen must be provided (Gardner et al., 2011).
Nasal Cannula: Infants requiring low-flow amounts of oxygen can benefit from the use of a nasal cannula (see Fig. 37-2, B). These are of particular value for older infants who are recuperating but still require supplemental oxygen. They are the preferred method for home oxygen administration. Nasal cannulas permit the infant to receive an adequate, continuous flow of oxygen while allowing optimal vision, positioning, and parental holding. Infants also can breastfeed or bottle-feed while receiving oxygen by this method. Nasal cannulas come in different sizes; proper fit is important. The nasal prongs must be inspected and cleaned frequently to make sure they are not partially obstructed by milk or secretions. Nasal cannulas allow easier feedings and psychosocial interactions.
Continuous Positive Airway Pressure Therapy: Infants who are unable to maintain an adequate Pao2 despite the administration of oxygen by hood or nasal cannula may require the delivery of oxygen by using continuous positive airway pressure (CPAP). CPAP infuses oxygen or air under a preset pressure by means of nasal prongs, a face mask, or an endotracheal tube (Fig. 37-3). It is often achieved by sending the oxygen bubbling through water to the infant; this is referred to as bubble CPAP. Researchers are investigating whether the work of neonatal breathing is improved with bubble CPAP versus variable-flow devices (Polin, 2009). In either case, an orogastric tube should be used for decompression of the stomach during use of nasal prongs. CPAP increases the functional residual capacity, improves the diffusion time of pulmonary gases, including oxygen, and can decrease pulmonary shunting. If implemented early enough, CPAP may preclude the need for mechanical ventilation (Cifuentes & Carlo, 2007). CPAP can cause vascular shunting in the pulmonary beds, which can lead to persistent pulmonary hypertension and severe respiratory distress.
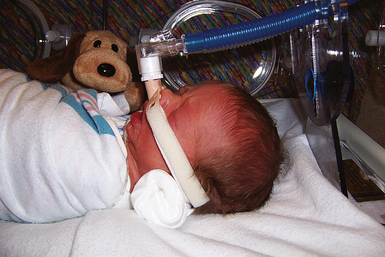
Mechanical Ventilation: Mechanical ventilation must be implemented if other methods of therapy cannot correct abnormalities in oxygenation (Fig. 37-4). Its use is indicated whenever blood gas values reveal the existence of severe hypoxemia or severe hypercapnia. The condition of the infant who has apnea with bradycardia, ineffective respiratory effort, shock, asphyxia, infection, meconium aspiration syndrome, respiratory distress syndrome (RDS), or congenital defects that affect ventilation also can deteriorate and require intubation to reverse the process (Cifuentes & Carlo, 2007). Dexamethasone may be administered to prevent chronic lung disease in ventilator-dependent infants who are unlikely to survive without corticosteroids. It is not recommended for LBW infants (AAP & Canadian Paediatric Society, 2006).
The ventilator settings are determined by the infant’s particular needs. The ventilator is set to provide a predetermined amount of oxygen to the infant during spontaneous respirations and mechanical ventilation in the absence of spontaneous respirations. Newer technologies in ventilation allow oxygen to be delivered at lower pressures and in assist modes, thereby preventing the overriding of the infant’s spontaneous breathing and providing distending pressures within a physiologic range. Barotrauma and associated complications such as pneumothorax (accumulation of air in the pleural space) and pulmonary interstitial emphysema (PIE) (free air that accumulates in interstitial tissue) are decreased. See Table 37-1 for a description of the types of mechanical ventilation used in newborns.
Neonatal Resuscitation: In 2005 the American Heart Association published neonatal resuscitation guidelines (American Heart Association [AHA], 2005). A rapid assessment of infants can identify those who do not require resuscitation: those born at term gestation, with no evidence of meconium or infection in the amniotic fluid; those who are breathing or crying; and those with good muscle tone. If any of these characteristics is absent, the infant should receive the following actions in sequence: (1) initial steps in stabilization: provide warmth by placing the baby under a radiant warmer, position the head in a position to open the airway, clear the airway with a bulb syringe or suction catheter, dry the baby, stimulate breathing, and reposition the baby; (2) ventilation; (3) chest compressions; and (4) administration of epinephrine or volume expansion or both. The decision to move from one category of action to the next is based on the assessment of respirations, heart rate, and color. Rapid decision making is imperative; 30 seconds are allotted for each step. The condition of the infant is reevaluated and the decision made whether to progress to the next step (Fig. 37-5).
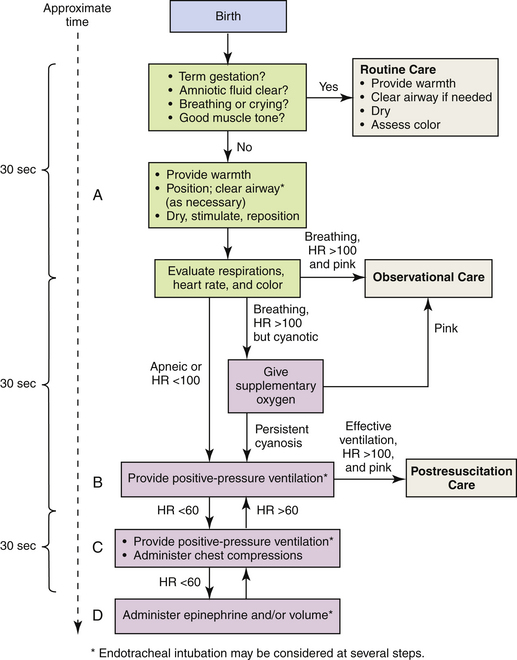
FIG. 37-5 Neonatal resuscitation flow algorithm. (From American Heart Association. [2005]. Neonatal resuscitation guidelines. Circulation, 112[24 Suppl], IV-188–IV-195.)
Resuscitation of asphyxiated newborns with 21% oxygen rather than 100% oxygen shows promise. Proponents for room air resuscitation suggest that fewer complications are associated with oxidative stress and hyperoxemia when room air is administered. The 2005 American Heart Association resuscitation standards for neonatal resuscitation stress that resuscitation may begin with no supplemental oxygen (i.e., 21% or room air) but that if the infant’s condition does not improve within 90 seconds, supplemental oxygen should be available for use. The stated goal is to minimize oxygen free radicals by preventing hyperoxia using supplemental oxygen at levels less than 100% (AHA, 2005). A review of several studies indicates that neonatal mortality is reduced by 30% to 40% when room air instead of 100% oxygen is used for neonatal resuscitation (Saugstad, 2007). Fluctuations in oxygen saturation are also deemed harmful. Experts recommend that oxygen saturations for ELBW infants be maintained between 85% and 93% but definitely not exceeding 95% (Saugstad).
Surfactant Administration: Surfactant is a surface-active phospholipid secreted by the alveolar epithelium. Acting much the same as a detergent, this substance reduces the surface tension of fluids that line the alveoli and respiratory passages, resulting in uniform expansion and maintenance of lung expansion at low intraalveolar pressure. Before 34 weeks of gestation, most infants do not produce enough surfactant to survive extrauterine life. As a result, lung compliance is decreased, and not enough gas exchange occurs as the lungs become atelectatic and require greater pressures to expand.
Surfactant can be administered as an adjunct to oxygen and ventilation therapy. With administration of artificial surfactant, respiratory compliance is improved until the infant can generate enough surfactant on his or her own. Exogenous surfactant is either artificial or natural and is given in several doses through an endotracheal tube. The American Academy of Pediatrics (AAP) (Engle & AAP Committee on Fetus and Newborn, 2008) recommends the use of surfactant in infants with RDS as soon as possible after birth, especially ELBW infants and those not exposed to maternal antenatal steroids. The administration of antenatal steroids to the mother and surfactant replacement has decreased the incidence of RDS and concomitant morbidities. Use of artificial surfactant has been associated with a significantly reduced length of time on ventilators and oxygen therapy, and an increased survival rate in preterm infants. As with any drug therapy, the infant must be monitored for the occurrence of potential side effects such as a patent ductus arteriosus (PDA) and pulmonary hemorrhage (see the Medication Guide).
High-Frequency Ventilation.
High frequency ventilation (HFV) is accomplished through the use of jet ventilators, oscillators, or high-frequency flow interrupters (Gardner et al., 2011). These methods provide smaller volumes of oxygen at a significantly more rapid rate (more than 300 breaths/min) than do traditional mechanical ventilators. As a result, the intrathoracic pressure is decreased, and along with this, the risk of barotrauma.
Additional Therapies
Inhaled nitric oxide (INO), delivered as a gas, causes potent and sustained pulmonary vasodilation in the pulmonary circulation. NO binds with hemoglobin in red blood cells and is inactivated after metabolism. INO is used in term and late preterm infants with conditions such as persistent pulmonary hypertension, meconium aspiration syndrome, pneumonia, sepsis, and congenital diaphragmatic hernia to decrease or reverse pulmonary hypertension, pulmonary vasoconstriction, acidosis, and hypoxemia. NO is a colorless, highly diffusible gas that can be administered through the ventilator circuit blended with oxygen. INO therapy can be used in conjunction with surfactant replacement therapy, high-frequency ventilation, or extracorporeal membrane oxygenation (ECMO). In the few studies conducted with human infants, positive results were seen: oxygen saturation improved, and no toxic effects from methemoglobin or increased levels of nitrogen oxide were documented. NO shows much promise in reducing adverse respiratory sequelae of being born prematurely. Its use has reduced the need for invasive technologies such as ECMO (Carlo, 2007; Gardner et al., 2011).
Extracorporeal Membrane Oxygenation (ECMO).
ECMO is a very complex and costly treatment that is sometimes used to support life and allow treatment of intractable hypoxemia due to severe cardiac or respiratory failure. This therapy involves a modified heart-lung machine, although in ECMO the heart is not stopped, and blood does not entirely bypass the lungs. Blood is shunted from a catheter in the right atrium or right internal jugular vein by gravity to a servo-regulated roller pump, pumped through a membrane lung where it is oxygenated, and through a small heat exchanger where it is warmed, and then returned to the systemic circulation via a major artery such as the carotid artery to the aortic arch. ECMO provides oxygen to the circulation, allowing the lungs to “rest,” and decreases pulmonary hypertension and hypoxemia in such conditions as persistent pulmonary hypertension of the newborn, congenital diaphragmatic hernia, sepsis, meconium aspiration,
and severe pneumonia. ECMO is contraindicated for preterm infants younger than 34 weeks of gestation because of the anticoagulant therapy required in the pump and circuits, which can increase the potential for intraventricular hemorrhage in such infants (Carlo, 2007; Lund, 2010).
Partial Liquid Ventilation (PLV).
For infants with severe RDS, the use of partial liquid ventilation (PLV) can improve outcomes. Perfluorocarbon liquid is instilled into the lungs during gaseous (mechanical) ventilation. PLV is beneficial to the surfactant deficient or immature lung because it reduces or eliminates surface tension, improves oxygenation through the re-creation of a fetal lung environment, and helps re-expand atelectatic areas. The safety and efficacy of PLV are being evaluated in the United States (Gardner et al., 2011).
Weaning from Respiratory Assistance.
Respiratory assistance is weaned slowly as the infant’s status improves. The infant is ready to be weaned from respiratory assistance once the ABG and oxygen saturation levels are maintained within normal limits. A spontaneous, adequate respiratory effort must be present, and the infant must show improved muscle tone during increased activity. Weaning is done in a stepwise and gradual manner. This can consist of the infant being extubated, placed on CPAP, and then weaned to oxygen by means of a hood or nasal cannula. Throughout the weaning process, the infant’s oxygen levels are monitored by pulse oximetry, TcPo2 monitoring, and blood gas levels.
The goal of weaning is the withdrawal of all oxygen support. However, some infants do not achieve this before discharge from the hospital and can require home oxygen therapy for several months. Throughout the weaning period the infant is assessed for signs and symptoms indicating poor tolerance of the process. These include an increased pulse, respiratory distress, or cyanosis, or a combination of these. If these occur, the amount of oxygen being delivered is increased, and weaning proceeds more slowly while further assessments are done. Underlying causes of intolerance of weaning may be BPD, a PDA, or CNS damage.
Nutritional Care
It is not always possible to provide enteral (by the GI route) nourishment to a high risk infant. Such infants are often too ill or weak to breastfeed or bottle feed because of respiratory distress or sepsis. Early enteral feeding of the asphyxiated neonate with a low Apgar score also is avoided to prevent bowel necrosis. In such cases, nutrition is provided parenterally. Those infants who require parenteral nutrition may have one or more of the following problems:
• Lack of a coordinated suck-and-swallow reflex
• Inability to suck because of a congenital anomaly
• Respiratory distress requiring aggressive ventilator support
• Asphyxiation with a potential for necrotizing enterocolitis
Type of Nourishment: The type, mode, and volume of feedings and the feeding schedule of the infant are determined on the basis of the findings yielded by assessment of the following variables:
• Initially, the birth weight, and then the current weight of the preterm infant
• Pattern of weight gain or loss (infants weighing less than 1500 g require more energy for growth and thermoregulation and may gain weight poorly with either breast- or bottle feedings)
• Presence or absence of suck-and-swallow reflex in all infants at less than 35 weeks of gestation
• Behavioral readiness to take oral feedings
• Physical condition, including presence or absence of bowel sounds, abdominal distention, or bloody stools, as well as presence and degree of respiratory distress or apneic episodes
• Residual from previous feeding, if being gavage fed
• Malformations (especially GI defects such as gastroschisis, omphalocele or esophageal atresia), including the need for a gastrostomy feeding tube
• Renal function, including urinary output and laboratory values (nitrogen balance, electrolyte balance, glucose level); preterm infants are especially susceptible to altered renal function
Human milk is the best source of nutrition for term and preterm infants. Even small preterm infants (28 to 36 weeks) are able to breastfeed if they have adequate sucking and swallowing reflexes and no other contraindications, such as respiratory complications or concurrent illness, are present. Preterm infants who are breastfed rather than bottle fed demonstrate fewer oxygen desaturations, absence of bradycardia, warmer-than-normal skin temperature, and improved coordination of breathing, sucking, and swallowing (Gardner & Lawrence, 2011). Mothers who wish to breastfeed their preterm infants are encouraged to pump their breasts until their infants are sufficiently stable to tolerate feeding at the breast. Appropriate guidelines for the storage of expressed mother’s milk should be used to decrease the risk of milk contamination and destruction of its beneficial properties (Jones & Tully, 2006).
Commercially available preterm formulas are cow’s milk–based and whey predominant, and have a higher concentration of protein, calcium, and phosphorus than term formulas to meet the unique needs of the preterm infant (AAP Committee on Nutrition, 2009). Most preterm formulas are either 22 or 24 cal/oz. Human milk with fortifier (protein, phosphorus, and calcium) is recommended for LBW preterm infants because it increases weight gain and improves bone mineralization better than nonfortified human milk (Gardner & Lawrence, 2011). Supplementation with iron, vitamin D, and multivitamins may be considered in exclusively breastfed LBW infants.
Weight and Fluid Loss or Gain: The caloric, nutrient, and fluid requirements of high risk infants are greater than those of the term, normal newborn. Premature or dysmature (malnourished) newborns often have limited stores of nutrients and fluids. In addition, symptomatic or asymptomatic hypoglycemia, electrolyte imbalances, or other metabolic disturbances can develop in an infant whose nutritional intake is poor. Such hypoglycemia can cause serious damage to carbohydrate-dependent brain cells.
The infant’s weight is measured and recorded daily, and the rate of weight loss or gain is calculated. Further depletion of weight and metabolic stores can occur as a result of one or a combination of the following factors:
• Increased respirations or respiratory effort
• Insensible fluid loss caused by evaporation (with radiant heat or phototherapy)
• Vomiting, diarrhea, and dysfunctional absorption from the GI tract
• Growth demands (a preterm infant’s growth rate approximates that of fetal growth during the last trimester and is at least two times faster than a term infant’s growth rate after birth)
• Inability of the renal system to concentrate urine and maintain an adequate rate of urea excretion, as well as infant’s inadequate response to antidiuretic hormone
The high risk newborn is predisposed to have weight and fluid losses because of the greater amount of fluid needed to meet the demands of the increased cellular metabolic processes (resulting from stress, repair, or growth). The body weight of preterm infants has a higher water content than that of their full-term counterparts (Hulzebos & Sauer, 2007). Most of this water is in the extracellular fluid compartment. Even with the early institution of fluid and nutrition intake, the preterm infant’s weight and fluid losses seem exaggerated. Inadequate fluid intake, resulting from either delayed administration or insufficient volume, can further cause weight and fluid losses in the preterm infant.
Insensible water loss (IWL) is an evaporative loss that occurs largely through the skin (70%) and through the respiratory tract (30%). The basal IWL in a term infant is approximately 20 ml/kg/24 hr. It is significantly increased in preterm infants, and especially in ELBW infants with thin, gelatinous skin (Bell & Oh, 2005; Blackburn, 2007). The effects of radiant warmers, incubators, phototherapy, and other factors can increase the IWL. Humidifying the respiratory gases administered can prevent some of this loss.
During the first week of extrauterine life, the preterm infant can lose up to 15% of his or her birth weight. In contrast, a weight loss of up to only 7% to 10% is acceptable in a term, appropriate for gestational age (AGA) infant. After the initial week, a preterm infant’s loss or gain during each 24-hour period should not exceed 2% of the previous day’s weight. (To calculate a weight loss or gain, see Box 37-4.)
Increased stooling or voiding, increased evaporative losses, inadequate volume or incorrect fluid administration, and problems with malabsorption can cause weight loss. Implementation of interventions and frequent reassessment of the infant and the environment are necessary to correct the problems. Such interventions include adjusting the incubator temperature; “swamping” or providing high levels of humidity under a cover over the radiant warmer; monitoring and adjusting the volume and type of fluids being administered; assessing the urinary output, including the specific gravity; and assessing the blood glucose levels. Hyperglycemia results in urinary loss of glucose that can cause osmotic diuresis, which increases the risk of dehydration (Armentrout, 2010).
If the infant is gaining more than the expected amount of weight, this may be due to overfeeding or fluid retention. The nurse reports and records the findings and continues to assess the infant’s fluid status, urinary output, and blood glucose levels. The interventions implemented are determined by the infant’s specific disorder and nutritional needs.
Elimination Patterns: The infant’s elimination patterns are assessed. This includes the frequency of urination, as well as the amount, color, pH, and specific gravity of the urine. The assessment of the infant’s bowel movements includes the frequency of stooling and the character of the stool, as well as whether there is constipation, diarrhea, or loss of fats (steatorrhea). All of these findings are documented. The nurse may request guaiac tests to assess for blood in the stool, tests to detect stool-reducing substances, and a pH determination to assess for malabsorption. Infants with unexplained abdominal distention are assessed carefully to rule out the presence of hypomotility obstructions of the GI tract, or necrotizing enterocolitis (NEC).
Oral Feeding: Nourishment by the oral route is preferred for the infant who has adequate strength and GI function. The best milk for an infant is from the mother. Breast milk can be fed by breast, bottle, cup, or spoon. Throughout the feeding the nurse assesses the newborn’s tolerance of the procedure. Preterm infants can be put to breast for practice feeds and nonnutritive suckling as soon as medically stable. The nurse assists the mother by providing support and help as necessary when the infant breastfeeds. Referral to a lactation consultant is important.
The needs of the high risk infant must be considered when determining the type and frequency of the feedings. Many high risk infants cannot suck well enough to breastfeed or bottle feed until they have recovered from their initial illness or matured physically (corrected age more than 32 weeks of gestation). Mothers of high risk infants are encouraged to continue pumping breast milk, especially if theirs is a very premature infant who will not breastfeed for many weeks. Because of the significant breastfeeding attrition rates among these mothers, they need ongoing support and encouragement to continue pumping while their infant is not yet able to nurse. If no breast milk is available (from the mother or a milk bank), commercial formula is used. The calories, protein, and mineral content of commercial formulas vary. The type of nipple selected (“preemie,” regular, orthodontic) depends on the infant’s ability to suck from the specific type of nipple. The nurse also considers the energy the infant needs to expend in the process. However, the practice of delaying breastfeeding until the baby is able to effectively bottle-feed is not evidence based because studies continue to confirm that breastfeeding is less stressful than bottle feeding (Gardner & Lawrence, 2011).
Overfeeding of the preterm infant should be avoided because this can lead to abdominal distention, with apnea, vomiting, and possibly aspiration of the feeding. The nurse monitors the infant’s abdominal girth when distention is obvious.
Gavage Feeding: Gavage feeding is a method of providing nourishment to the infant who is compromised by respiratory distress, the infant who is too immature to have a coordinated suck-and-swallow reflex, or the infant who is easily fatigued by sucking. In gavage feeding, breast milk or formula is given to the infant through a nasogastric or orogastric tube (Fig. 37-6). This spares the infant the work of sucking.
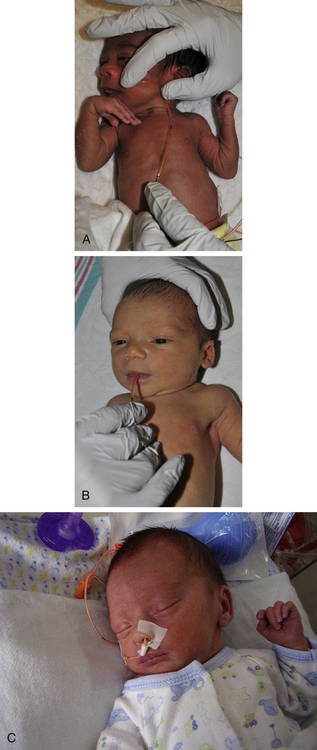
FIG. 37-6 Gavage feeding. A, Measurement of gavage feeding tube from tip of nose to earlobe and to midpoint between end of xiphoid process and umbilicus. Tape may be used to mark correct length on tube. For accurate measure, the infant should be facing up. B, Insertion of gavage tube using orogastric route. C, Indwelling gavage tube, nasogastric route. After feeding by orogastric or nasogastric tube, infant is propped on right side or placed prone (preterm infant) for 1 hour to facilitate emptying of stomach into small intestine. (A and B, Courtesy Cheryl Briggs, RNC, Annapolis, MD; C, courtesy Randi and Jacob Wills, Clayton, NC.)
Gavage feeding can be done either with an intermittently placed tube providing a bolus feeding or continuously through an indwelling catheter. Infants who cannot tolerate large bolus feedings (those on ventilators for more than a week) are given continuous feedings. Minimal enteral nutrition (MEN) can be used to stimulate or prime the GI tract to achieve better absorption of nutrients when bolus or regular intermittent gavage feedings can be given (Blackburn, 2007).
Breast milk or formula can be supplied intermittently by using a syringe with gravity-controlled flow, or it can be given continuously by using an infusion pump. The type of fluid instilled is recorded with every syringe change. The volume of the continuous feedings is recorded hourly, and the residual gastric aspirate is measured every 2 to 4 hours. Aspirates of less than a one hour volume can be refed to the infant. For intermittent feedings, residuals of less than 50% of the previous feeding can be re-fed to the infant to prevent the loss of gastric electrolytes. Feeding is usually stopped if the residual is greater than 50% of the feeding or if residuals are increasing and is not resumed until the infant can be assessed for a possible feeding intolerance (Anderson, Wood, Keller, & Hay, 2011).
The orogastric route for gavage feedings is preferred because most infants are preferential nose breathers. Also when indwelling nasogastric tubes are used, there is a tendency toward nares necrosis; however, some infants do not tolerate oral tube placement. A small nasogastric feeding tube can be placed in older infants who would otherwise gag or vomit or in ones who are learning to suck. To insert the tube and give the feeding, the nurse should follow the sequence given in the Procedure box.
Gastrostomy Feedings: Gastrostomy feedings are used for infants with neurologic problems or certain congenital malformations that require long-term gavage feedings (Ditzenberger, 2010). This involves the surgical placement of a tube through the skin of the abdomen into the stomach. The tube is then taped in an upright position to prevent trauma to the incision site. After the site heals, the nurse initiates small bolus feedings per the physician’s orders. Feedings by gravity are done slowly over 20- to 30-minute periods. Special care must be taken to prevent rapid bolusing of the fluid because this can
lead to abdominal distention, GI reflux into the esophagus, or respiratory compromise. Meticulous skin care at the tube insertion site is necessary to prevent skin breakdown or infections. In addition, intake and output are monitored scrupulously because these infants are prone to diarrhea until regular feedings are established.
Parenteral Nutrition: Supplemental parenteral fluids are indicated for infants who are unable to obtain sufficient fluids or calories by enteral feeding. Some of these infants are dependent on total parenteral nutrition (TPN) for extensive periods. The nurse assesses and documents the following in infants receiving parenteral fluids or TPN:
• Type and infusion rate of the solution
• Functional status of the infusion equipment, including the tubing and infusion pump
• Infusion site for possible complications (phlebitis, infiltration, dislodgment)
The physician or nurse practitioner orders TPN per the hospital protocol. These orders must specify the electrolytes and nutrients desired, as well as the volume and rate of infusion. The composition of calories, protein, and fats is calculated on an individual basis.
While caring for the infant receiving parenteral fluids or TPN, the nurse secures and protects the insertion site. Scrupulous hand hygiene is used before handling the TPN tubing or IV sites. Strict sterile technique is implemented for dressing changes (Ditzenberger, 2010). The nurse must observe the principles of neonatal skin care. The nurse also should inspect the infusion site for signs of infiltration and reposition the infant frequently to maintain body alignment and protect the site. Parents of infants need to be given explanations about TPN and the way in which the IV equipment and solutions affect their infant.
Advancing Infant Feedings: Feedings are advanced as assessment data and the infant’s ability to tolerate the feedings warrant. Documentation of a preterm infant’s sucking patterns also can be used to determine readiness to nipple feed. Feedings are advanced from passive (parenteral and gavage) to active (nipple and breastfeeding). At each step the nurse must carefully assess the infant’s response to prevent overstressing the infant.
The infant receiving nutrition parenterally is gradually weaned off this type of nutrition. To do this the nourishment given by continuous or intermittent gavage feedings is increased while the parenteral fluids are decreased. Even the smallest infant is sometimes given MEN to stimulate the GI system to mature and to enhance caloric intake (Blackburn, 2007).
Feedings are advanced slowly and cautiously because if advanced too rapidly, the infant can develop vomiting (with an attendant risk of aspiration), diarrhea, abdominal distention, and apneic episodes. Rapid advancement also can cause fluid retention with cardiac compromise or a pronounced diuresis with hyponatremia.
If the infant needs additional calories, a commercial human milk fortifier can be added to the gavaged breast milk, or the number of calories per 30 ml of commercial formula can be increased. Soy and elemental formulas are used only for infants with very special dietary needs, such as allergies to cow’s milk or chronic malabsorption. Calories in breast milk can be lost if the cream separates and adheres to the tubing during continuous infusion. This problem is decreased if microbore tubing is used for both continuous and intermittent gavage feedings.
The infant receiving gavage feedings progresses to bottle feeding or breast milk feedings. To do this the gavage feedings are decreased as the infant’s ability to suckle breast milk or formula improves. Often during this transition, the infant is fed by both bottle or breast and gavage feeding to ensure the intake of both the prescribed volume of food and nutrients. However, when there is an indwelling tube, during breast or bottle feedings, some infants experience an increased respiratory effort, so nurses must watch for this. The parents need support during this transition because many families measure their parenting competence by how well they can feed their infant. For breastfed infants, it is important to weigh the infant before and after breastfeeding to determine the infant’s intake (Hurst, 2007).
As the time of discharge nears, the appropriate method of feeding, as well as the assessments pertaining to the method (e.g., tolerance of feedings, status of gavage tube placement), are reviewed with the parents. The parents should be encouraged to interact with the infant by talking and making eye contact with the infant during the feeding. This is encouraged to stimulate the psychosocial development of the infant and to facilitate bonding and attachment.
Nonnutritive Sucking: If the gavage or the parenteral route nourishes the infant, nonnutritive sucking is encouraged for several reasons (Fig. 37-7). Allowing the infant to suck on a pacifier during gavage or between oral feedings can improve oxygenation. In addition, such nonnutritive sucking can lead to a decreased energy expenditure with less restlessness. It also promotes positive weight gain and better sucking skills (Harding, Law, & Pring, 2006).
Mothers of preterm infants should be encouraged to allow their infants to start sucking at the breast during kangaroo care (skin-to-skin). In some infants, the suck-and-swallow reflexes are coordinated as early as 32 weeks of gestation. If the neonate is unable to suck, the mother can place the infant near the nipple to encourage nuzzling or licking.
Infants with intrauterine growth restriction (IUGR) can have an age-appropriate sucking reflex but require thermoregulatory support, making it difficult to breastfeed. These infants also may benefit from nonnutritive sucking at the breast for short periods.
Skin Care
The skin of preterm infants is characteristically immature relative to that of full-term infants. Because of its increased sensitivity and fragility, the use of alkaline-based soap that might destroy the acid mantle of the skin is avoided. Vernix caseosa has benefits for the preterm infant’s skin. Vernix acts as an epidermal barrier, decreases bacterial contamination of the skin through its antimicrobial peptides and proteins, and decreases transepidermal water loss (Lund, Kuller, Raines, Ecklund, Archambault, & O’Flaherty, 2007). Experts recommend that a validated skin assessment tool such as the Braden Q Scale or the Neonatal Skin Condition Score (NSCS) be used once daily to evaluate the high risk infant’s skin condition so as to implement interventions aimed at minimizing skin breakdown (Curley, Razmus, Roberts, & Wypij, 2003; Lund & Osborne, 2004).
Environmental Concerns
Infants in NICUs also are exposed to high levels of auditory input from the various machine alarms, and this can have adverse effects (Fig. 37-8). In addition, continuous noise levels of 45 to 85 decibels (db) are common in NICUs. An incubator alone produces a constant noise level of 60 to 80 db, and each new piece of life-support equipment used adds another 20 db to the background noise. The infant’s hearing may be damaged if it is exposed to a constant decibel level of 90 db or frequent decibel swings higher than 110 db. Cochlear damage has been recognized as a side effect of the NICU environment. Thus both conductive and sensorineural hearing losses have been identified in NICU graduates; these losses lead to long-term speech and language deficits (Haubrich, 2007; Krueger, Wall, Parker, & Nealis, 2005). Over time more emphasis has been placed on noise in the NICU and the adverse or long-term effects on neonates (White, 2007).
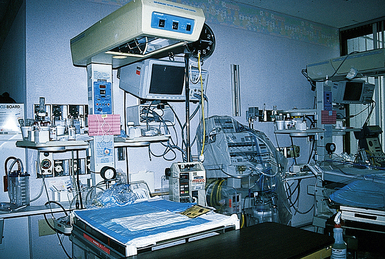
FIG. 37-8 Although necessary, neonatal intensive care unit equipment can contribute to significant environmental stimulation. Note bed, wall oxygen attachments, monitor, ventilator, incubator, and pumps, all of which have alarm systems. (Courtesy Marjorie Pyle, RNC, Lifecircle, Costa Mesa, CA.)
Respiratory equipment or a phototherapy mask can alter the infant’s vision, making it difficult for the infant to interact with caregivers and family members. The infant also may be unable to establish diurnal and nocturnal rhythms because of the continuous exposure to overhead lighting. In addition, sedation or pain medications affect the way in which the infant perceives the environment.
An additional concern in the care of infants is that some drugs used for infant therapy can potentiate environmental hazards. Diuretics (especially furosemide [Lasix]), antibiotics (gentamicin), and antimalarial agents can potentiate noise-induced hearing loss (Haubrich, 2007).
Research is ongoing to determine effects of light and noise on the preterm infant. Long-term problems are the focus of much research (Symington & Pinelli, 2006). Cycling of light and covering of incubators to reduce direct light hitting the retina are two areas of research. The retina of the immature infant has little protection from the nearly translucent eyelid, thus allowing light to almost continuously penetrate the retina unless it is artificially protected by dimming the lights or using incubator covers. Cycled lighting has been shown to have a positive effect on growth (White, 2007). Light and sound are adverse stimuli that add to an already stressed preterm infant. The result is stress cues, increased metabolic rate, increased oxygen and caloric use, and depression of the immune system. The nurse must monitor the macroenvironment and the microenvironment (unit and immediate environments) for sources of overstimulation. Providing a developmentally supportive environment can lead to decreased complications and length of stay. There are national recommendations for sound and light levels in the NICU.
Nurses can modify the environment to provide a developmentally supportive milieu. In that way the infant’s neurobehavioral and physiologic needs can be better met, the infant’s developing organization can be supported, and growth and development can be fostered (Symington & Pinelli, 2006).
Developmental Care
The goal of developmental care is to support each infant’s efforts to become as well organized, competent, and stable as possible. Developmental care includes all care procedures and the physical and social aspects of care in the NICU (Als, Duffy, McAnulty, Rivkin, Vajapeyam, Mulkern, et al., 2004). The caregiver uses the infant’s own behavior and physiologic functioning as the basis for planning care and providing interventions. Through caregiver observation, the infant’s strengths, thresholds for disorganization, and vulnerable areas can be identified. The family is included in developmental care as the primary coregulators (Als et al.). Working together, the family and other caregivers provide opportunities to enhance the strengths of the family and the infant and to reduce the stress that is associated with the birth and care of high risk infants.
Reducing light and noise levels by instituting “quiet hours” at regularly scheduled times and positioning are just two of the ways in which nurses can support infants in their development. Sleep interruptions are minimized, and positioning and bundling the infant help promote self-regulation and prevent disorganization (Symington & Pinelli, 2006).
Positioning: The motor development of preterm infants permits less flexion than in term infants. Caregivers can provide a variety of positions for infants; side-lying and prone are preferred to supine (but only in the nursery) (Fig. 37-9). Body containment with use of blanket rolls, swaddling, holding the infant’s arms in a crossed position, and secure holding provide boundaries. Use of facilitated tucking promotes self-regulation during feeding, procedures, and other stressful interventions The prone position encourages flexion of the extremities; a sling or hip roll assists in maintaining flexion. Keeping the extremities close to the body helps calm the infant and decreases stimulation. Proper body alignment is necessary to prevent developmental problems that can affect the ability to walk as the child matures (Carrier, 2010).
Reducing Inappropriate Stimuli: Staff can reduce unnecessary noise by closing doors or portholes on incubators quietly, placing only necessary objects gently on top of incubators, keeping radios at low volume, speaking quietly, and handling equipment noiselessly. Another source is internal noise created by mechanical sources such as CPAP. These noise sources must be considered when thinking about long-term effects on hearing. Earmuffs can be used during scans and transports (Mathur, Neil, McKinstry, & Inder, 2008).
Infants can be protected from light by dimming the lights during the night, placing a blanket over the incubator, or covering the infant’s eyes with a mask. Sleep-wake cycles can be induced with such measures. Infants need periods in which there are no disruptions and sleep can occur. Clustering of care can promote longer uninterrupted periods of sleep (Carrier, 2010).
Infant Communication: Infants communicate their needs and ability to tolerate sensory stimulation through physiologic responses. The nurses and parents of high risk infants must therefore be alert to such cues. Although term infants may thrive on stimulation, this same stimulation in high risk infants can provoke physical symptoms of stress and anxiety (Symington & Pinelli, 2006).
Problems with noxious stimuli and barriers to normal contact can cause anxiety and tension. Clues to overstimulation include averting the gaze, hiccuping, gagging, or regurgitating food. Term infants exhibit a startle reflex, and preterm infants move all of their limbs in an uncoordinated fashion in response to noxious stimuli. An irregular respiratory rate or an increased heart rate can develop in severely distressed infants, and they may be unable to regain a calm state.
A relaxed infant state is indicated by stabilization of vital signs, closed eyes, and a relaxed posture. Nonintubated infants may make soothing verbal sounds when they are relaxed. Infants requiring artificial ventilation cannot cry audibly and often show their distress through posturing; they relax once their needs are met. As high risk infants heal and mature, they increasingly respond to stimuli in a self-regulated manner rather than with a dissociated response. Infants who do not show increased self-regulation should be evaluated for a neurologic problem.
Infant Stimulation: The Newborn Individualized Developmental Care and Assessment Program (NIDCAP, 2009) routinely integrates aspects of neurodevelopmental theory with caregivers’ observations, environmental interventions, and parental support. Routine reassessment is built into the program’s design. Developmental stimuli may consist of such simple measures as placing a waterbed mattress on the top of the infant’s mattress, or kangaroo holding. The simplest calming technique is for the caregiver to use both hands to contain the infant’s extremities close to the body. The care of the infant is organized to allow extended periods of undisturbed rest and sleep. Pain medications or sedatives should be administered consistently per the unit’s protocol.
Infants acquire a sense of trust as they learn the feel, sound, and smell of their parents. High risk infants also must learn to trust their caregivers to obtain comfort. However, caregivers in the nursery also can inflict pain as part of the care they must give. For this reason it is important for parents and caregivers to use comforting interventions such as removing painful stimuli, stopping hunger, and changing wet or soiled clothing to foster trust. They can offer nonnutritive sucking or use oral sucrose for pain relief and topical creams before procedures to avoid pain. All of these techniques are part of developmental, supportive care (Carrier, 2010).
When the infant is ready for stimulation, the nurse has many options. Most infants can tolerate being held, even if only for short periods. Additional ways for the nurse or parents to stimulate infants include cuddling, rocking, singing, use of music therapy, and talking to the infant. These activities are beneficial and promote growth and weight gain as well as shorten the length of hospital stay. Stroking the infant’s skin during medical therapy can provide tactile stimulation. The caregiver responds to the infant’s cues by offering reassurance, providing nonnutritive sucking, stroking the infant’s back, and talking to the infant. Infant massage is gaining evidence as a way to promote weight gain (Gardner & Goldson, 2011).
Mobiles and decals that can be changed frequently may be placed within the infant’s visual range to stimulate the infant visually. Wind-up musical toys provide rhythmic distractions as long as they are not too loud. If the infant is receiving phototherapy, the protective eyepatches are removed periodically (e.g., during feeding) so that the infant can see the caregiver’s face for short, comforting sessions.
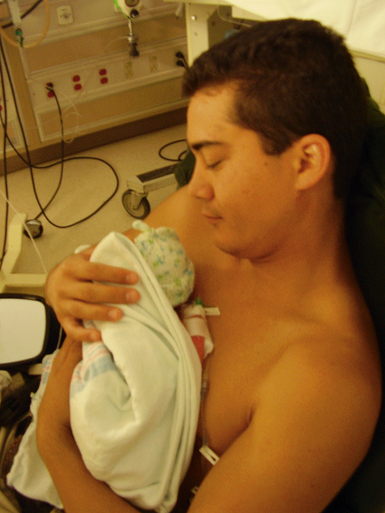
Kangaroo Care: Although it must be individually adjusted, kangaroo care and short periods of gentle massage can help reduce stress in preterm infants (Fig. 37-10). The parent is bare-chested or may wear a loose-fitting, open-front top that has a modified marsupial-like pocket carrier for the infant. The undressed (except for diaper) infant is placed in a vertical position on the parent’s bare chest, which permits direct eye contact, skin-to-skin sensations, and close proximity. Skin-to-skin contact can have a positive healing effect for the mother who had a high risk pregnancy. Additional benefits include early contact with mechanically ventilated infants, maintenance of neonatal thermal stability and oxygen saturation, increased feeding vigor and enhanced breastfeeding, maintenance of organized state, decreased pain perception during painful heelsticks, and minimal untoward effects of being held. The National Association of Neonatal Nurses developed a clinical practice guideline for kangaroo care for the stable healthy preterm infant ages 30 weeks or more of gestation (Ludington-Hoe, Morgan, & Abouelfettoh, 2008).
Parental Adaptation to Preterm Infant
Parents of premature infants often have difficulty in bonding and relating to their babies. The need to be empowered to recognize their competence and achieve competence is the basis of the Creating Opportunities for Parent Empowerment (COPE) program. This early educational-behavioral intervention model promotes more positive parent-infant interactions and enhanced ability to read and respond to infant cues (Melnyk, Feinstein, Alpert-Gillis, Fairbanks, Crean, Sinkin, et al., 2006; Siegel, Gardner, & Dickey, 2011).
Parental Tasks: Parents of preterm infants must accomplish numerous psychologic tasks before effective relationships and parenting patterns can evolve. These tasks include the following:
• Experiencing anticipatory grief over the potential loss of an infant. The parent grieves in preparation for the infant’s possible death, although the parent clings to the hope that the infant will survive. This process begins during labor and lasts until the infant dies or shows evidence of surviving. Anticipatory grief occurs when families have knowledge of an impending loss, such as when a baby is admitted to an NICU with problems or when a diagnosis of an anencephalic fetus is made with ultrasonography. The baby is still alive, but the prognosis is poor. Being able to anticipate the loss gives families an opportunity to plan, feel more in control of their situation, and say good-bye in a special way. However, some individuals or family members distance or detach themselves from the experience or from their loved ones as a way of protecting themselves from the pain of loss and grief. How a parent responds to this situation depends on religious, spiritual, and cultural beliefs. These must be considered when planning care. The nurse’s role is to advocate for the family so that other health professionals realize that the family is grieving. Being fully present for these families and practicing active listening is important (see Chapter 38).
• The mother’s acceptance of her failure to give birth to a healthy full-term infant. Grief and depression typify this phase, which persists until the infant is out of danger and is expected to survive.
• Resuming the process of relating to the infant. As the baby’s condition begins to improve and the baby gains weight, is able to breastfeed or bottle-feed, and is weaned from the incubator or radiant warmer, the parent can begin the process of developing an attachment to the infant that was interrupted by the infant’s critical condition at birth.
• Learning about the ways in which this baby differs in terms of his or her special needs and growth patterns, caregiving needs, and growth and development expectations.
• Adjusting the home environment to accommodate the needs of the new infant. Parents are encouraged to limit the number of visitors to minimize exposure of the infant to pathogens. The environmental temperature may have to be altered to optimize conditions for the infant.
Parental Responses: Physical contact with the infant is important to establish early bonding. If it is not possible for parents to hold the infant, they can touch and stroke the baby as they speak softly (Fig. 37-11). As the infant’s condition improves, parents can hold the neonate and provide kangaroo care (see Fig. 37-10). They gradually begin to participate in infant activities, such as feeding, bathing, and changing. Parents go through numerous phases of adjustment as they learn to parent their infant. Nurses facilitate the transition to parenthood through their teaching and support of parental efforts.
Parental Support: The nurse as the support person and teacher is responsible for shaping the environment and making the caregiving responsive to the needs of the parents and infant. Nurses are instrumental in helping parents learn who their infant is and to recognize behavioral cues in his or her development and to use these cues in the care they provide (Aagaard & Hall, 2008; Carrier, 2010).
When a high risk birth is anticipated, the family can be given a tour of the NICU or shown a video to prepare them for the sights and activities of the unit. After an unanticipated preterm birth, the parents can be given a booklet, view a video, or have someone describe what they will see when they go to the unit to see their infant. As soon as possible, the parents should see and touch their infant so that they can begin to acknowledge the reality of the birth and the infant’s true appearance and condition. They will need encouragement as they begin to accomplish the psychologic tasks imposed by the high risk birth. For the following reasons, a nurse or physician should be present during the parents’ first visit to see the infant:
• To help them “see” the infant rather than focus on the equipment. The importance and purpose of the equipment that surrounds their infant should be explained to them.
• To explain the characteristics normal for an infant of their baby’s gestational age; in this way parents do not compare their child with a term, healthy infant.
• To encourage the parents to express their feelings about the pregnancy, labor, and birth and the experience of having a high risk infant
• To assess the parents’ perceptions of the infant to determine the appropriate time for them to become actively involved in care
As soon as possible after the birth, the parents are given the opportunity to meet the infant in the en face position, to touch the infant, and to see his or her favorable characteristics. The premature or sick baby’s appearance can be stressful to the parents. As soon as possible, depending primarily on her physical condition, the mother is encouraged to visit the nursery as desired and help with the infant’s care. When the family cannot be present physically, staff members devise appropriate methods to keep them in almost constant touch with the newborn, such as with daily phone calls, notes written as if by the infant, or photographs of the infant.
The birth of a preterm or high risk infant affects the entire family. Nurses need to consider responses and reactions of grandparents and siblings as they provide individualized family-centered care for the infant and family. Grandparents often experience grief and sadness as they watch their own children experiencing the difficulties and challenges of having a preterm or high risk infant. They worry and are concerned about the well-being of their grandchild. Siblings also react to the birth of the preterm or high risk infant. If they are old enough to realize that the mother was supposed to be bringing home a new baby, they can be very confused when the baby must remain in the hospital. When possible it can be helpful to allow siblings to visit the new baby in the NICU environment so that they can see the infant (Fig. 37-12). Once the infant is brought home, some children are bewildered and angry at the seemingly disproportionate amount of parental time spent on the newborn. Nurses can facilitate visits by grandparents and siblings while the infant is hospitalized and can help parents anticipate possible reactions of siblings once the baby is discharged.
FIG. 37-12 Sibling visits newborn in the neonatal intensive care unit. A, Father prepares older child for visit. B, Sibling observes neonate at a safe distance. C, He reaches out to touch the infant. (Courtesy Lauren and Brian LiVecchi, Raleigh, NC.)
Some hospitals have support groups for the parents of infants in NICUs. These groups help parents experiencing anxiety and grief by encouraging them to share their feelings. Hospitals also often arrange to have an experienced NICU parent make contact with a new group member to provide additional support. The volunteer parents provide support by making hospital visits, phone calls, and home visits. Mothers in particular are prone to posttraumatic stress that can hinder their ability to interact with or care for their infant (Holditch-Davis, Bartlett, Blickman, & Miles, 2003). They need help in expressing their feelings and, when appropriate, referral for psychologic or family counseling.
Many NICUs use volunteers in varying capacities. After they have gone through the orientation program, volunteers can perform tasks such as holding the infants, stocking bedside cabinets, assembling parent packets, and, in some nurseries, feeding the infants.
Parental Maladaptation: The incidence of physical and emotional abuse is increased in infants who, because of preterm birth or high risk condition, are separated from their parents for a time after birth. Physical abuse includes varying degrees of poor nutrition, poor hygiene, and bodily harm. Emotional abuse ranges from subtle disinterest to outright dislike of the infant. Appropriate resources should be made available to assess the parent’s feelings regarding the preterm infant’s birth. In addition, proper guidance and counseling are made available, including posthospital discharge, to help families adjust to and care for the preterm infant. The ultimate goal is for the family to accept the infant and incorporate this new member into the existing family structure.
Parent Education
Some high risk infants can be discharged earlier than the expected time. The criteria showing an infant’s readiness for early discharge are that the infant’s physiologic condition is stable, the infant is receiving adequate nutrition, and the infant’s body temperature is stable. The parents, or other caregivers, also need to exhibit physical, emotional, and educational readiness to assume responsibility for the care of the infant. Ideally, the home environment is adequate for meeting the needs of the infant. The parents also need to show that they know how to take the infant’s temperature, know the signs and symptoms to report, and understand the dietary needs of the infant. Resources for parents and health care providers include http://premature-infant.com, www.vort.com/age/premature.html, www.neonatology.org, and www.familyvillage.wisc.edu.
Complications in High Risk Infants
Respiratory Distress Syndrome: Respiratory distress syndrome (RDS) refers to a lung disorder usually affecting preterm infants. Maternal and fetal conditions associated with a decreased incidence and severity of RDS include female gender, African-American race, maternal gestational hypertension, maternal drug abuse, maternal steroid therapy (betamethasone), chronic retroplacental abruption, prolonged rupture of membranes, and IUGR. The incidence and severity of RDS increase with a decrease in the gestational age. Perinatal asphyxia, hypovolemia, male gender, Caucasian race, maternal diabetes (types 1 and 2), second-born twin, familial predisposition, maternal hypotension, cesarean birth without labor, hydrops fetalis, and third-trimester bleeding are all factors that place an infant at increased risk for RDS. The incidence of RDS in infants weighing less than 1500 g is between 40% and 60% (Carlo, 2007; Cifuentes & Carlo, 2007).
RDS is caused by a lack of pulmonary surfactant, which leads to progressive atelectasis, loss of functional residual capacity, and a ventilation-perfusion imbalance with an uneven distribution of ventilation. This surfactant deficiency can be caused by insufficient surfactant production, abnormal composition and function, disruption of surfactant production, or a combination of these factors. The weak respiratory muscles and an overly compliant chest wall, common among preterm infants, further compromise the sequence of events that occurs. Lung capacity is further compromised by the presence of proteinaceous material and epithelial debris in the airways. The resulting decreased oxygenation, cyanosis, and metabolic or respiratory acidosis can cause the pulmonary vascular resistance (PVR) to be increased. This increased PVR can lead to right-to-left shunting and a reopening of the ductus arteriosus and foramen ovale (Fig. 37-13).
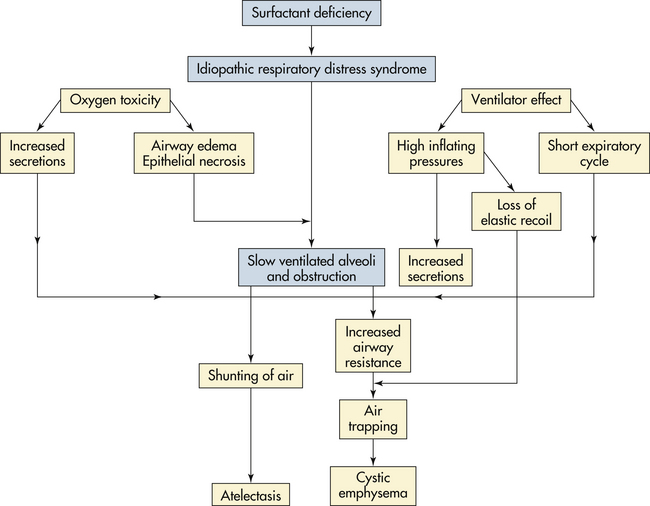
FIG. 37-13 Pathogenesis of respiratory distress syndrome (RDS). (Source: Gardner, S., Enzman-Hines, M., & Dickey, L. [2011]. Respiratory diseases. In S. Gardner, B. Carter, M. Enzman-Hines, & J. Hernandez [Eds.], Merenstein and Gardner's handbook of neonatal intensive care [7th ed.]. St. Louis: Mosby.)
Clinical symptoms of RDS usually appear immediately after or within 6 hours of birth. Physical examination reveals crackles, poor air exchange, pallor, the use of accessory muscles (retractions) and, occasionally, apnea. Radiographic findings include a uniform reticulogranular appearance and air bronchograms (Rodriguez, Martin, & Fanaroff, 2006). The infant’s clinical course typically is variable, usually with an increased oxygen requirement and increased respiratory effort as atelectasis, a loss of functional residual capacity, and ventilation-perfusion imbalance worsen.
RDS is a self-limiting disease that abates after 72 hours. This disappearance of respiratory signs coincides with the production of surfactant in the type 2 cells of the alveoli.
The treatment for RDS is supportive. Adequate ventilation and oxygenation must be established and maintained in an attempt to prevent ventilation-perfusion mismatch and atelectasis. Exogenous surfactant may be administered at birth or shortly after; this has the effect of altering the typical course of RDS. Positive-pressure ventilation, bubble CPAP, and oxygen therapy can be necessary during the respiratory illness. The prevention of complications associated with mechanical ventilation is critical. These complications include pulmonary interstitial emphysema, pneumothorax, pneumomediastinum, and pneumopericardium. The mortality and morbidity rates associated with RDS are attributed to the immature organ systems of the infant and the complications associated with the treatment of the disease (Rodriguez et al., 2006).
Acid-base balance is evaluated by monitoring the ABG values (Table 37-2). Frequent blood sampling requires arterial access either by umbilical artery catheterization or by a peripheral arterial line. Pulse oximetry and transcutaneous carbon dioxide and oxygen monitors document trends in ventilation and oxygenation. Capillary blood gas values indicate the pH and Pco2 status in infants who are in more stable condition.
The maintenance of an NTE is critical to the care of infants with RDS. Infants with hypoxemia are unable to increase their metabolic rate when they experience cold stress (Rodriguez et al., 2006).
The clinical and radiographic presentation (radiodense lung fields and air bronchograms) of neonatal pneumonia can be similar to that of RDS. Fluid in the minor tissue also can be noted in infants with neonatal pneumonia. Therefore, sepsis evaluation, including blood culture and complete blood count (CBC) with differential, is done in infants with RDS to rule out neonatal pneumonia. Occasionally a lumbar puncture is done as part of the sepsis evaluation. Broad-spectrum antibiotics are begun while the results of cultures are awaited.
Fluid and nutrition must be maintained in the critically ill infant with RDS. Parenteral nutrition can be implemented to provide protein and fats to promote a positive nitrogen balance. Daily monitoring of the electrolyte values, urinary output, specific gravity, and weight help evaluate the infant’s hydration status.
Frequent blood sampling can make blood transfusions necessary. The critically ill infant usually needs to have a venous hematocrit level of more than 40% to maintain adequate oxygen-carrying capacity.
Reassuring the family that stringent testing of all blood products is done can help alleviate some of their anxiety about the transmission of blood-borne pathogens such as human immunodeficiency virus (HIV) and hepatitis B. Because some religions prohibit the use of blood transfusions, it is critical to obtain a complete history from the family, including their religious preference. Alternative strategies for maintaining the infant’s hematocrit may be used in these instances.
Complications Associated with Oxygen Therapy:
Retinopathy of Prematurity: Retinopathy of prematurity (ROP) is a complex multicausal disorder that affects the developing retinal vessels of preterm infants. The normal retinal vessels begin to form in utero at approximately 16 weeks in response to an unknown stimulus. These vessels continue to develop until they reach maturity at approximately 42 to 43 weeks after conception. Once the retina is completely vascularized, the retinal vessels are not susceptible to ROP. The mechanism of injury in ROP is unclear. Oxygen tensions that are too high for the level of retinal maturity initially result in vasoconstriction. After oxygen therapy is discontinued, neovascularization occurs in the retina and vitreous, with capillary hemorrhages, fibrotic resolution, and possible retinal detachment. Scar tissue formation and consequent visual impairment can be mild or severe. The entire disease process in severe cases can take as long as several months to evolve. Examination by an ophthalmologist before discharge and a schedule for repeated examinations thereafter are recommended for the parents’ guidance (Askin & Diehl-Jones, 2009b; Strodtbeck, 2007).
The key to the management of ROP is prevention of preterm birth and early detection. Blood oxygen levels in the preterm infant should be closely monitored and significant fluctuations avoided. Oxygen and ventilator settings should be adjusted to keep oxygen saturations within acceptable levels. Cryotherapy and laser photocoagulation are common treatments for ROP (Askin & Diehl-Jones, 2009b). Researchers are examining the effect of the NICU environment on the development of ROP in relation to light that shines directly through the very thin eyelid of very immature infants. Ambient lighting in the NICU is known to have an effect on the developing eye; although it may not directly cause ROP, it does contribute to other visual problems for the preterm infant. Another potential contributor to ROP is hyperglycemia, which apparently fosters vasoproliferation (Ertl, Gyarmati, Gaal, & Szabo, 2006). This factor needs much more study.
Bronchopulmonary Dysplasia: Bronchopulmonary dysplasia (BPD) is a chronic pulmonary iatrogenic condition caused by barotrauma from pressure ventilation and oxygen toxicity. The etiology of BPD is multifactorial and includes pulmonary immaturity, surfactant deficiency, lung injury and stretch, barotrauma, inflammation caused by oxygen exposure, fluid overload, ligation of a PDA, and a familial predisposition. With the advent of prenatal use of maternal steroids when preterm birth is expected coupled with use of exogenous surfactant in the neonate, most BPD or chronic lung disease (CLD) has been eliminated (Askin & Diehl-Jones, 2009a).
Clinical signs of BPD include tachypnea, retractions, nasal flaring, increased work of breathing, exercise intolerance (to handling and feeding), and tachycardia. Infants with BPD can have an increase in ventilatory requirements or are unable to be weaned from the ventilator. Auscultation of the lung fields in affected infants typically reveals crackles, decreased air movement, and occasionally expiratory wheezing. Hypoxia, hypercapnia, and respiratory acidosis are common (Askin, 2010).
The treatment for BPD includes oxygen therapy, nutrition, fluid restriction, and medications (diuretics, corticosteroids, bronchodilators). However, the key to the management of BPD is prevention of prematurity and RDS. Other therapies that can aid in prevention of BPD include antenatal steroids, prophylactic surfactant, avoidance of mechanical ventilation when possible, use of CPAP, gentle ventilation in the delivery room, and administration of vitamin A. Oxygen therapy may be continued in the home setting (Askin & Diehl-Jones, 2009a).
The prognosis for infants with BPD depends on the degree of pulmonary dysfunction and on the infant’s overall health status. There is usually progressive normalization of pulmonary function, although abnormalities of small airways can persist. Mortality rates after hospital discharge are less than 10%; deaths are often due to complications such as respiratory infection (Askin, 2010).
Patent Ductus Arteriosus: The ductus arteriosus is a normal muscular contractile structure in the fetus connecting the left pulmonary artery and the dorsal aorta, diverting blood to the placenta for gas exchange. The duct constricts after birth as oxygenation, the levels of circulating prostaglandins, and the muscle mass increase. Other factors that promote ductal closure include catecholamines, low pH, bradykinin, and acetylcholine. When the fetal ductus arteriosus fails to close after birth, patent ductus arteriosus (PDA) occurs. During the first few days of life when a preterm or sick infant is under stress, the ductus arteriosus can reopen, leading to mottling and cyanosis. It can last only a few minutes until the stress is past, or it can remain open if the infant is quite unstable. The incidence of PDA in preterm infants is 20%, with an increasing incidence in VLBW infants and those with pulmonary disease (Carlo, Martin, & Fanaroff, 2006).
Although a small PDA can be asymptomatic, the clinical presentation in an infant with a significant PDA includes systolic murmur, active precordium, bounding peripheral pulses, tachycardia, tachypnea, crackles, and hepatomegaly. The systolic murmur is heard best at the second or third intercostal space at the upper left sternal border. An increased left ventricular stroke volume causes an active precordium.
Radiographic studies in infants with PDA typically show cardiac enlargement and pulmonary edema. ABG findings reveal hypercapnia and metabolic acidosis. Definitive diagnosis is through echocardiography, which can visualize a PDA and measure the amount of blood shunting across the PDA (Sadowski, 2010).
Medical management consists of ventilatory support, fluid restriction, and the administration of diuretics and indomethacin or ibuprofen (Ohlsson, Walia, & Shah, 2008; Sadowski, 2010). Ibuprofen and indomethacin inhibit prostaglandin synthesis and cause the PDA to constrict. There is some concern that indomethacin reduces blood flow to the brain, kidneys, and GI tract (Ohlsson et al.).
Ventilatory support is adjusted based on the ABG values. Fluid restriction and diuretic therapy are implemented to decrease cardiovascular volume overload. Surgical ligation is done when a PDA is clinically significant and medical management is ineffective.
Nursing management of the infant with PDA focuses on supportive care. The infant needs an NTE, adequate oxygenation, meticulous fluid balance, and parental support.
Germinal Matrix Hemorrhage–Intraventricular Hemorrhage: Germinal matrix hemorrhage–intraventricular hemorrhage (GMH-IVH) is one of the most common types of brain injury in neonates and is among the most severe from the standpoint of both short- and long-term outcomes. It is the most common type of intracranial hemorrhage, occurring almost exclusively in preterm infants. The risk of GMH-IVH increases with decreasing gestational age. The incidence of GMH-IVH is estimated to be 5% to 11%; it has shown a decline in recent years.(de Vries, 2006). The decline in incidence is attributed to the prenatal use of corticosteroids and postnatal use of surfactant.
The germinal matrix is a loose network of cells abundantly supplied with tiny, fragile, thin-walled vessels. It lies beneath the lining of the lateral ventricles. This area is present and active until 34 to 35 weeks of gestation as a site for production of neurons and glial cells that gradually migrate to the cerebral cortex. The germinal matrix is especially vulnerable to alterations in cerebral blood flow related to blood pressure changes. Hemorrhage occurs when the tiny blood vessels rupture; the hemorrhage can extend into the lateral ventricles, then to the third and fourth ventricles, the subarachnoid space, and even into the white matter of the brain. Large clots can develop and create outflow obstruction from the ventricles (Volpe, 2008).
Infants who experience GMH-IVH are usually less than 34 weeks of gestation with a history of hypoxia, birth asphyxia, RDS, or other events causing impaired venous return or increased venous pressure. GMH-IVH is diagnosed in 50% of preterm infants within the first 24 hours and in 90% within the first 4 days. The hemorrhage progresses during the first few days of life in 20% to 40% of infants. Infants with GMH-IVH can be asymptomatic, develop symptoms gradually, or have an acute catastrophic presentation. Clinical signs suggestive of hemorrhage include decreasing hematocrit, full anterior fontanel, changes in activity level, and decreased muscle tone. With a catastrophic incident, the infant can develop stupor, coma, respiratory distress that progresses to apnea, decerebrate posturing, and seizures; there is a high mortality rate in these cases (Volpe, 2008).
Morbidity and mortality related to GMH-IVH are based on the severity of the hemorrhage and the associated problems. Small hemorrhages are usually associated with good outcomes and high survival rates. Infants with more severe hemorrhages, posthemorrhagic ventricular dilation, or periventricular leukomalacia have higher mortality rates and often long-term morbidity. Neurodevelopmental outcomes of GMH-IVH include hydrocephalus, cerebral palsy, developmental retardation, learning disorders, and sensory and attention problems (Volpe, 2008).
Care management begins with prevention of preterm birth, birth trauma, and hypoxic-ischemic injury. Antenatal steroids help to reduce the risk of GMH-IVH. Prompt and skilled resuscitation at birth minimizes hypoxia and ischemia. Nursing care focuses on recognition of factors that increase the risk of GMH-IVH, interventions to decrease the risk of bleeding, and supportive care to infants who have bleeding episodes. Ongoing assessment of vulnerable infants involves monitoring oxygenation and perfusion and avoiding or minimizing activities that increase cerebral blood flow. If GMH-IVH occurs, care is focused on maintaining oxygenation and perfusion, an NTE, and normoglycemia. The infant is positioned with the head in midline and the head of the bed elevated slightly to prevent or minimize fluctuations in intracranial blood pressure. Rapid infusions of fluids should be avoided. Blood pressure is monitored closely for fluctuations. The use of developmental interventions such as swaddling or containment during painful procedures can help promote greater physiologic stability (Blackburn & Ditzenberger, 2007).
Nursing support for parents includes assessment of their understanding and concerns related to the infant’s condition. They need opportunities to discuss their feelings and ask questions about the infant’s condition and care as well as the long-term prognosis. Nurses can demonstrate to parents how they can interact with the infant in a developmentally appropriate manner. Nurses can provide anticipatory guidance regarding ways the infant’s needs and care will change as he or she matures. For many of these infants, a multidisciplinary approach to care is needed to address the neurodevelopmental sequelae of GMH-IVH and to plan for care after hospital discharge (Blackburn & Ditzenberger, 2007).
Necrotizing Enterocolitis: Necrotizing enterocolitis (NEC) is an acute inflammatory disease of the GI mucosa, commonly complicated by bowel necrosis and perforation. NEC occurs in up to 10% of all NICU admissions; 90% of cases are preterm infants (Caplan, 2006; Lambert, Christensen, Henry, Besner, Baer, Wiedmeier, et al., 2007). Reported mortality rates due to NEC range from 9% to 50% (Caplan). A national study showed rates of hospitalized neonates with NEC at about 16% and increasing to 20% among VLBW infants (Holman, Stoll, Curns, Yorita, Steiner, & Schonberger, 2006).
The exact etiology and pathophysiology of NEC are unclear, although many factors seem to contribute to its development (risk factors are listed in Box 37-5). Three primary conditions appear to be involved in the etiology of NEC. The first is intestinal ischemia that occurs as a result of asphyxia/hypoxia or events that cause a redistribution of blood flow away from the GI tract (e.g., hypotension, hypovolemia, severe stress). A second condition involved in the development of NEC seems to be bacterial colonization of the initially sterile GI tract with harmful organisms prior to the establishment of normal intestinal flora. Klebsiella, Escherichia coli, and Clostridium are common organisms involved in NEC. A third condition associated with the development of NEC is enteral feeding. The majority of infants with NEC had received some type of enteral feeding. It is thought that the feedings can provide a substrate for bacterial proliferation or that feedings can increase intestinal oxygen demand during absorption and results in tissue hypoxia. Breast milk seems to have a protective effect against the development of NEC—it is rare among infants who are exclusively fed breast milk. The use of probiotics shows promise in reducing the risk of NEC. Use of natural prophylactic probiotics such as Bifidobacterium infantis and, Streptococcus thermophilus to enhance bowel flora appears to decrease the incidence of NEC (AlFaleh & Bassler, 2008; Barclay, Stenson, Simpson, Weaver, & Wilson, 2007). In addition, minimal enteral nutrition may help reduce the risk of NEC.
The onset of NEC in the term infant usually occurs between 1 and 3 days after birth, but can occur as late as 1 month. In the preterm infant, NEC usually occurs within the first 7 days, but can be delayed for up to 30 days. The signs of developing NEC are nonspecific, which is characteristic of many neonatal diseases. Some generalized signs include decreased activity, hypotonia, pallor, recurrent apnea and bradycardia, decreased oxygen saturation values, respiratory distress, metabolic acidosis, oliguria, hypotension, decreased perfusion, temperature instability, and cyanosis. GI symptoms include abdominal distention, increasing or bile-stained residual gastric aspirates, vomiting (bile or blood), grossly bloody stools, abdominal tenderness, and erythema of the abdominal wall (Bradshaw, 2010).
A diagnosis is confirmed by a radiographic examination that reveals bowel loop distention, pneumatosis intestinalis (air in the wall of the bowel), pneumoperitoneum, portal air, or a combination of these findings. The abnormal radiographic findings are caused by the bacterial colonization of the GI tract associated with NEC, resulting in ileus. Pneumatosis intestinalis, pneumoperitoneum, and portal air are caused by gas produced by the bacteria that invade the wall of the intestines and escape into the peritoneum and portal system when perforation occurs. The laboratory evaluation in such infants consists of a CBC with differential, coagulation studies, ABG analysis, measurement of serum electrolyte levels, and blood culture. The white blood cell count on the CBC can be either increased or decreased. The platelet count and coagulation study findings can be abnormal, showing thrombocytopenia and disseminated intravascular coagulation (DIC). Electrolyte levels can be abnormal, with leaking capillary beds and fluid shifts with the infection (Bradshaw, 2010; Caplan, 2006).
Management strategies are based on the degree of bowel involvement and the severity of the disease. The goal of treatment is to prevent progression of the NEC, intestinal perforation, and shock.
For the infant with suspected or confirmed NEC, oral or tube feedings are discontinued to rest the GI tract. An orogastric tube is placed and attached to low wall suction to provide gastric decompression. Parenteral therapy (often TPN) is begun. Because NEC is an infectious disease, control of the infection is imperative, with an emphasis on careful hand hygiene before and after infant contact. Antibiotic therapy may be instituted, and surgical resection is performed if perforation or clinical deterioration occurs. Therapy is usually prolonged, and recovery can be delayed by the formation of adhesions, the development of the complications associated with bowel resection, the occurrence of short-bowel syndrome (especially if the ileocecal valve is removed), or the development of intolerance to oral feedings. Some of these infants are candidates for intestinal transplants if they truly have short-bowel syndrome (Bradshaw, 2010; Caplan, 2006).
Families need education and support when faced with the crisis of having an infant with NEC. As part of the health care team, nurses can help parents understand the severity of the disease, treatment options, and care needed by the infant. When the disease is severe and the prognosis is poor, nurses are instrumental in supporting families with decision making and anticipatory grieving (Hughes, Baez, & McGrath, 2009).
Infant Pain Responses
The physiology of pain and pain assessment in the newborn are discussed in Chapter 24. This discussion focuses on pain assessment and management in the preterm infant.
Pain Assessment: Assessment of pain in the neonate is difficult because evaluation must be based on physiologic changes and behavioral observations. Pain is now considered the fifth vital sign, and its assessment is a requirement of The Joint Commission (TJC). A scale that examines multiple dimensions facilitates accurate assessment of neonatal pain (Spence, Gillies, Harrison, Johnston, & Nagy, 2005). Although behaviors such as vocalizations, facial expressions, body movements, and general state are common to all infants, they vary with different situations. Crying associated with pain is more intense and sustained. Facial expression is the most consistent and specific characteristic; scales are available for systematic evaluation of facial features, such as eye squeeze, brow bulge, and open mouth and taut tongue (Walden, 2007) (see Fig. 24-22). Most infants respond with increased body movements, but may be experiencing pain even when lying quietly with eyes closed. The preterm infant’s response to pain may be behaviorally blunted or absent. An infant who receives a muscle-paralyzing agent such as vecuronium will be incapable of mounting a behavioral or visible pain response (Box 37-6), yet still feel pain.
Several tools have been developed for the assessment of pain in the neonate. The CRIES assessment tool is discussed in Chapter 24 (see Table 24-4). Other instruments are the Pain Assessment Tool (PAT) (Hodgkinson, Bear, Thorn, & Van Blaricum, 1994); Scale for Use in Newborns (SUN) (Blauer & Gerstmann, 1998); Behavioral Pain Score (BPS) (Pokela, 1994); Distress Scale for Ventilated Newborn Infants (DSVNI) (Sparshott, 1995); Neonatal Infant Pain Scale (NIPS) (Lawrence, Alcock, McGrath, Kay, MacMurray, & Dulberg, 1993); and the Premature Infant Pain Profile (PIPP) (Stevens, Johnston, Petryshen, & Taddio, 1996). The PIPP is one of the most widely used scales for preterm infants because it considers behavioral, physiologic, and contextual indicators (Walden, 2007).
Memory of Pain: Preterm infants are subjected to a variety of repeated noxious stimuli, including multiple heelsticks, venipuncture, endotracheal intubation and suctioning, arterial sticks, chest tube placement, and lumbar puncture. The effects of pain caused by such procedures are not fully known, but researchers have begun to investigate potential consequences. From preliminary reports, it appears that a rewiring of the pain responses occurs in preterm infants who have been subjected to multiple painful treatments early in their lives. The nervous system networks of the preterm infant appear more dense and have more branches than those in the average infant, leading to the conclusion that the pain threshold and sensitivity in once preterm infants is heightened for life. There are also changes when the infant has undergone anesthesia (Anand, Johnston, Oberlander, Taddio, Lehr, & Walco, 2005; Aranda, Carlo, Hummel, Thomas, Lehr, & Anand, 2005).
Nurses’ anecdotal reports suggest that infants show memory by exhibiting defensive behaviors when painful procedures are repeated. Nurses often describe infants who stiffen and withdraw when touched because human touch has repeatedly been associated with pain. Such infants often become hypervigilant and gaze intently at the hands rather than at the eyes of people who approach them.
These reports not only indicate that infants remember painful events but also show that continual exposure to pain affects development, especially in response to human contact.
Consequences of Untreated Pain in Infants: Despite research on the neonate’s experience of pain, infant pain remains inadequately managed. This mismanagement is partially due to misconceptions regarding the effects of pain on the neonate, as well as a lack of knowledge of immediate and long-term consequences of untreated pain. Infants respond to noxious stimuli through physiologic indicators (increased heart rate and blood pressure, variability in heart rate and intracranial pressure, and decreases in arterial oxygen saturations and skin blood flow) and behavioral indicators (muscle rigidity, facial expression, crying, withdrawal, and sleeplessness). The physiologic and behavioral indicators, as well as a variety of neurophysiologic responses to noxious stimulation, are responsible for short- and long-term consequences of pain.
Pain Management: The International Evidence-Based Group for Neonatal Pain developed a Consensus Statement for the Prevention and Management of Pain in the Newborn (Anand & The International Evidence-Based Group for Neonatal Pain, 2001), which states that pain must be anticipated and prevented to avoid long-term consequences. Nonpharmacologic measures to alleviate pain include repositioning, swaddling, containment, cuddling,
rocking, playing music, reducing environmental stimulation, providing tactile comfort measures and nonnutritive sucking, and using oral sucrose. However, nonpharmacologic measures may not be sufficient to decrease physiologic distress, even if behavioral responses such as crying are lessened. In preterm infants, additional stimulation such as stroking or environmental light or noise can increase physiologic distress (Walden, 2007). The effect of the NICU environment must be considered along with other forms of stimuli that can produce stress and pain.
Morphine is the most widely used opioid analgesic for pharmacologic management of neonatal pain, with fentanyl as an effective alternative. Continuous or bolus epidural or IV infusion of opioids provides effective and safe pain control. Other methods are epidural/intrathecal infusion, local and regional nerve blocks, and topical anesthetics, as well as general anesthesia for surgery (Walden, 2007).
Parents are universally concerned that their infants are feeling pain during procedures. Nurses need to address these concerns and encourage the parents to speak with the health care professionals involved. Parents have the right to withhold consent for invasive procedures and are entitled to honest answers from those responsible for the infant’s care. When appropriate, they also can help provide comfort measures for the infant. Kangaroo care is one parental intervention that comforts and calms the infant.
Parents want to know that nurses recognize pain in their infants and that the infants will be comfortable when they, the parents, are not present. They want to know that the nurse will advocate for comfort care for their baby. Although pain is considered a fifth vital sign, it cannot be assessed only at the time of vital signs. It must receive an ongoing evaluation of the pain level and the effectiveness of comfort measures used. This assessment is not lengthy but can be as simple as walking to the bedside and really looking at the infant’s color, posture, movements, and breathing. Pain is a real phenomenon that is preventable in many instances. Pain management is a standard of care, and it is considered unethical not to prevent and effectively treat pain. Another growing area of neonatal nursing is end-of-life and palliative care. Most of this care centers on pain management. Palliative care is really comfort care that supports the needs of the preterm, sick neonate.
Late Preterm Infants
Late preterm infants are those born between 34 0/7 and 36 6/7 weeks of gestation (Raju et al., 2006). Because birth weights of late preterm infants often range from 2000 to 2500 g and they appear relatively mature in comparison to the smaller less mature infant, they are often cared for as if they are normal term infants. Risk factors for late preterm infants can easily be overlooked. Compared with term infants, late preterm infants are at increased risk for problems with thermoregulation, hypoglycemia, hyperbilirubinemia, feeding, sepsis, and respiratory function (Bakewell-Sachs, 2007; Darcy, 2009). Many of these healthy-appearing infants are admitted directly to postpartum units with their mothers or stay in the NICU only briefly (i.e., less than 24 hours). They are commonly discharged home at 2 to 3 days of age with their mothers. Discharge before 48 hours after birth is not recommended (Ramachandrappa & Jain, 2009).
Recognition of late preterm infants is essential to providing effective care. Initial physical assessment and assessment of gestational age are crucial to identifying these infants. Although the mother’s estimated date of birth (EDB) can indicate a longer gestation, infant appearance, behaviors, and/or weight can indicate otherwise. The obstetric estimate of gestational age is usually reliable if it is based on a first-trimester ultrasound. However, if there is a discrepancy between the gestational age based on the obstetric estimate and newborn examination, it is better to rely on the estimate based on the newborn examination (Ramachandrappa & Jain, 2009).
The Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) published the Late Preterm Infant Assessment Guide (Santa-Donato, Medoff-Cooper, Bakewell-Sachs, Askin, & Rosenberg, 2007) for the education of perinatal nurses regarding the late preterm infant’s risk factors and appropriate care and follow-up (Table 37-3).
Components of nursing care for late preterm infants and their parents are discussed in the following text. Frequent assessment is important. On mother-baby units, late preterm infants should be assessed more often than term infants—at least every 4 hours throughout their hospital stay. This includes vital signs and observation of a feeding.
Respiratory Distress: Late preterm infants are at increased risk for respiratory problems, including apnea. Close monitoring of respiratory status is essential and any changes are reported promptly to the primary health care provider. Car seat testing is indicated. Infants may go home on an apnea monitor. If possible, parents should attend an infant cardiopulmonary resuscitation (CPR) class or view a video about infant CPR. To help prevent respiratory infections the nurse should instruct the parents to limit the infant’s contact with others outside the home. The nurse should review signs and symptoms of respiratory problems with the parents.
Thermoregulation: Late preterm infants have more difficulty with thermoregulation than term infants, and cold stress is a greater concern. They have less body fat than term infants, a higher ratio of surface area to body weight, decreased glycogen stores, and less mature mechanisms for increasing metabolism for heat. Stores of brown fat are smaller and quickly depleted. Cold stress can quickly lead to hypoglycemia. Nurses must closely monitor body temperature and teach parents to take the infant’s temperature. Parents are encouraged to keep a record of this at home and to report high or low temperatures. It is important to remember that temperature instability is often an early sign of neonatal sepsis (Darcy, 2009).
Nutrition: Feedings should occur at least every 3 to 4 hours and according to infant feeding cues. Late preterm infants eat less than term neonates and have less energy reserves to do so. They can have difficulty coordinating sucking, swallowing, and breathing. Breastfeeding can be more problematic if the infant is sleepy and difficult to arouse for feedings. Late preterm infants are prone to early fatigue during feedings and fall asleep before consuming adequate volumes of milk. Nurses should observe at least one feeding every 8 hours. Early and extended skin-to-skin contact promotes breastfeeding. If supplementation is needed, expressed breast milk is the best option (Walker, 2008). To maximize the milk supply and milk transfer to the infant, mothers should use the electric breast pump after feedings while in the hospital and continue this process at home until the infant is able to successfully remove milk from the breasts and until the milk supply is well established. The mother-baby nurse or lactation consultant can provide instruction and assist breastfeeding mothers with using a breast pump. Mothers also need information regarding safe milk handling and storage guidelines (Jones & Tully, 2006). Parents should keep an intake and output record until their health care provider tells them otherwise and should share this record with the health care provider at each visit (Cleaveland, 2010; Walker, 2008).
Hypoglycemia: Late preterm infants are at risk for hypoglycemia. Hospital protocols may require routine monitoring of blood glucose until levels are stabilized and the infant is feeding adequately. Although the cut-off value for treatment remains uncertain (Garg & Devaskar, 2006), blood glucose values of less than 45 mg/dl, or symptoms of hypoglycemia, should be treated. Bedside monitoring of blood glucose levels at frequent intervals throughout the late preterm infant’s hospital stay is recommended.
Hyperbilirubinemia: Neonatal hyperbilirubinemia is more common in late preterm infants because of immaturity of the liver, decreased gastric motility, and increased breakdown of red blood cells (RBCs) (Pappas & Walker, 2010). They are less able to conjugate and excrete bilirubin. Serum bilirubin levels tend to peak at 5 to 7 days and persist longer than in term infants. Hyperbilirubinemia is the most frequent reason for hospital readmission during the first week of life. Bilirubin levels are closely monitored before discharge and parents are instructed regarding signs of jaundice and when to notify the health care provider. Follow-up visits soon after hospital discharge are important for monitoring rising bilirubin levels (Darcy, 2010).
Infection: The immune systems of late preterm infants are immature; thus they are more likely to experience infections. Nurses should assess the infant for signs and symptoms of infection including temperature instability, lethargy, irritability, poor feeding, or vomiting. Before discharge, nurses provide education for the parents about the common signs and symptoms of infection.
Postmature Infants
A pregnancy that is prolonged beyond 42 weeks is a postterm pregnancy, and the infant who is born is called postterm or postmature. Postmaturity can be associated with placental
insufficiency, resulting in a fetus that has a wasted appearance (dysmaturity) at birth because of loss of subcutaneous fat and muscle mass. However, not all postmature infants will show signs of dysmaturity; some will continue to grow in utero and will be large at birth. Most postmature infants are oversized but otherwise normal, with advanced development and bone age. A postmature infant will have some, but not necessarily all, of the following physical characteristics:
• Generally a normal skull, but the reduced dimensions of the rest of the body in the presence of dysmaturity make the skull look inordinately large
• Dry, cracked (desquamating), parchment-like skin at birth
• Hard nails extending beyond the fingertips
• Depleted subcutaneous fat layers, leaving the skin loose and giving the infant an “old person” appearance
• Often meconium staining (golden yellow to green) of skin, nails, and cord, indicative of a hypoxic episode in utero or a perinatal infection such as listeriosis
• Can have an alert, wide-eyed appearance symptomatic of chronic intrauterine hypoxia
The perinatal mortality rate is significantly higher in the postmature fetus and neonate. One reason for this is that during labor and birth the increased oxygen demands of the postmature fetus may not be met. Insufficient gas exchange in the postmature placenta also increases the likelihood of intrauterine hypoxia, which can result in the passage of meconium in utero, thereby increasing the risk for meconium aspiration syndrome (MAS).
Parents may be concerned about the appearance of the postmature infant. Nurses can help them understand reasons for the dry, peeling, skin and other characteristics of postmaturity. Initial bathing should be done with a mild soap. It can be helpful to moisturize the skin with a petrolatum-based ointment. Nurses need to be alert to common problems associated with postmaturity such as hypoglycemia and meconium aspiration (Lund & Kuller, 2007).
Meconium Aspiration Syndrome: Meconium staining of the amniotic fluid can be indicative of fetal distress, especially in a vertex presentation. It appears in 10% to 15% of all births (Dudell & Stoll, 2007). Many infants with meconium staining exhibit no signs of depression at birth; however, the presence of meconium in the amniotic fluid necessitates careful supervision of labor and close monitoring of fetal well-being. The presence of a team skilled in neonatal resuscitation is required at the birth of any infant with meconium-stained amniotic fluid (Fig. 37-14). The mouth and nares of the infant are not routinely suctioned on the perineum before the infant’s first breath. However, for infants with meconium staining who are not vigorous, endotracheal suctioning should be performed immediately. Vigorous infants need no special handling (AHA, 2005; Velaphi & Vidyasagar, 2008).
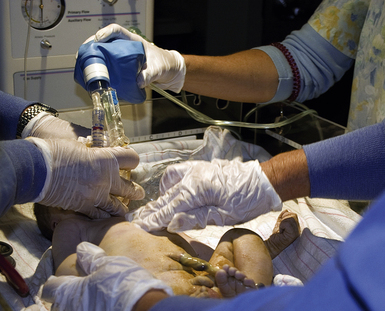
FIG. 37-14 Infant being resuscitated at birth. Meconium was present on the abdomen, and umbilical cord. Infant was not breathing, and heart rate was 65 beats/min at birth. Respirations and heart rate were normal at 2 minutes. (Courtesy Shannon Perry, Phoenix, AZ.)
If the infant is very depressed and the meconium is not removed from the airway at birth, it can migrate down to the terminal airways, causing mechanical obstruction leading to meconium aspiration syndrome. It also is possible that the fetus aspirated meconium in utero. Such meconium aspiration can cause a chemical pneumonitis. These infants can develop persistent pulmonary hypertension of the newborn, further complicating their management. Infants with MAS who receive surfactant can experience improved oxygenation, decreased severity of respiratory failure, and reduced need for ECMO (Engle & AAP Committee on Fetus and Newborn, 2008).
Persistent Pulmonary Hypertension of the Newborn: The term persistent pulmonary hypertension of the newborn (PPHN) is applied to the combined findings of pulmonary hypertension, right-to-left shunting, and a structurally normal heart. PPHN can occur either as a single entity or as the main component of MAS, congenital diaphragmatic hernia, RDS, hyperviscosity syndrome, or neonatal pneumonia or sepsis. PPHN also is called persistent fetal circulation (PFC) because the syndrome includes a reversion to fetal pathways for blood flow.
A brief review of the characteristics of fetal blood flow can help in visualizing the problems with PPHN (see Fig. 12-13). In utero, oxygen-rich blood leaves the placenta via the umbilical vein, goes through the ductus venosus, and enters the inferior vena cava. From there it empties into the right atrium and is mostly shunted across the foramen ovale to the left atrium, effectively bypassing the lungs. This blood enters the left ventricle, leaves via the aorta, and preferentially perfuses the carotid and coronary arteries. Thus the heart and brain receive the most oxygenated blood. Blood drains from the brain into the superior vena cava, reenters the right atrium, proceeds to the right ventricle, and exits via the main pulmonary artery. The lungs are a high-pressure circuit, needing only enough perfusion for growth and nutrition. The ductus arteriosus (connecting the main pulmonary artery and the aorta) is the path of least resistance for the blood leaving the right side of the fetal heart, shunting most of the cardiac output away from the lungs and toward the systemic system. This right-to-left shunting is the key to fetal circulation.
After birth, both the foramen ovale and the ductus arteriosus close in response to various biochemical processes, pressure changes within the heart, and dilation of the pulmonary vessels. This dilation allows virtually all of the cardiac output to enter the lungs, become oxygenated, and provide oxygen-rich blood to the tissues for normal metabolism. PPHN characteristically proceeds into a downward spiral of exacerbating hypoxia and pulmonary vasoconstriction. Prompt recognition and aggressive intervention are required to reverse this process (Cifuentes & Carlo, 2007).
The infant with PPHN is typically born at term or after term and has tachycardia and cyanosis. Management depends on the underlying cause of the persistent pulmonary hypertension. The use of ECMO has improved the chances of survival in these infants (see earlier discussion); however, it is considered a very invasive procedure. Use of NO as a pharmacologic intervention has increased, with great success. It acts as a vasodilator to decrease the pulmonary hypertension while increasing oxygenation (Konduri & Kim, 2009). This therapy is proving to work well either alone or with high-frequency ventilation. Another pharmacologic treatment is use of exogenous surfactant because some of these infants appear to be surfactant deficient. Use of environmental strategies such as decreasing adverse stimuli (excessive light and noise) to reduce stress is an area of ongoing research. This intervention is used in conjunction with other therapies.
Another mode of treatment for PPHN and other respiratory disorders of the newborn is high-frequency ventilation, a group of assisted ventilation methods that deliver small volumes of gas at high frequencies and limit the development of high airway pressure, thus reducing barotrauma. High-frequency ventilation decreases carbon dioxide while increasing oxygenation. It can be effectively used in conjunction with NO.
It is important to understand that PPHN is considered a cardiovascular and a respiratory problem. The lungs of these infants are healthy, but the hypertension of the cardiovascular system leads to their oxygenation problems.
Other Problems Related to Gestation
Small for Gestational Age and Intrauterine Growth Restriction: Infants who are small for gestational age (SGA; e.g., weight is below the 10th percentile expected at term) and infants who have IUGR (rate of growth does not meet expected growth pattern) are considered high risk. Among these infants perinatal mortality rates are 5 to 20 times greater than for normal term infants (Kliegman, 2006).
Various conditions can affect and impede growth in the developing fetus. Conditions occurring in the first trimester that affect all aspects of fetal growth (e.g., infections, teratogens, chromosomal abnormalities) or extrinsic conditions early in pregnancy result in symmetric IUGR (i.e., head circumference, length, and weight, are all less than the 10th percentile). Conditions causing symmetric growth restriction result in an SGA infant, usually with a head circumference that is smaller than that of a term infant and reduced brain capacity. Growth restriction in later stages of pregnancy, as a result of maternal or placental factors, results in asymmetric growth restriction (with respect to gestational age, weight will be less than the 10th percentile, whereas length and head circumference will be greater than the 10th percentile). Infants with asymmetric IUGR have the potential for normal growth and development. There is relative sparing of head and brain growth while weight and somatic organ growth are more seriously altered (Furdon & Benjamin, 2010).
Several physical findings are characteristic of the SGA neonate (Furdon & Benjamin, 2010):
• Generally a normal skull, but the reduced dimensions of the rest of the body make the skull look inordinately large
• Reduced subcutaneous fat stores
• Diminished muscle mass, especially over buttocks and cheeks
• Sunken abdomen (scaphoid) as opposed to the well-rounded abdomen seen in normal infants
• Thin, yellowish, dry, and dull umbilical cord (normal cord is gray, glistening, round, and moist)
Care of the SGA infant is based on the clinical problems present and is the same for preterm infants with similar problems. Gas exchange is supported by maintaining a clear airway and preventing cold stress. Hypoglycemia is treated with oral feedings (e.g., breast, formula) or IV dextrose as the infant’s condition warrants. An external heat source (radiant warmer or incubator) is used until the infant is able to maintain an adequate body temperature. Nursing support of parents is the same as that given to parents of preterm infants.
Common problems that affect SGA (IUGR) infants are perinatal asphyxia, meconium aspiration, immunodeficiency, hypoglycemia, polycythemia, and temperature instability.
Perinatal Asphyxia.: Commonly, IUGR infants have been exposed to chronic hypoxia for varying periods before labor and birth. Labor is a stressor to the normal fetus, but it is an even greater stressor for the growth-restricted fetus. The chronically hypoxic infant is severely compromised even by a normal labor and has difficulty compensating after birth. Appropriate management and resuscitation are essential for these depressed infants.
The birth of SGA babies with perinatal asphyxia can be associated with a maternal history of heavy cigarette smoking; preeclampsia; low socioeconomic status; multifetal gestation; gestational infections such as rubella, cytomegalovirus, and toxoplasmosis; advanced diabetes mellitus; and cardiac problems. The nursing staff must be alert to and prepared for possible perinatal asphyxia during the birth of an infant to a woman with such a history. Sequelae to perinatal asphyxia include MAS (see p. 924) and hypoglycemia.
Hypoglycemia: All high risk infants have an increased likelihood of developing hypoglycemia. Infants who experience physiologic stress can experience hypoglycemia as a result of a decreased glycogen supply, inadequate gluconeogenesis, or overutilization of glycogen stored during fetal and postnatal life. Preterm infants can also become hypoglycemic because of inadequate intake and increased metabolic demands as a result of illness factors. Evidence to support the concept that the preterm or high risk infant can tolerate lower levels of serum glucose any better than healthy term infants is insufficient (Blackburn, 2007) (see Chapter 23, p. 537, for discussion of hypoglycemia). The SGA infant, not unlike the preterm infant, is at increased risk for hypoglycemia as a result of decreased fetal stores and decreased rate of gluconeogenesis (McGowan, Rozance, Price-Douglas, & Hay, 2011).
Symptoms of hypoglycemia include poor feeding, hypothermia, and diaphoresis. CNS symptoms can include tremors and jitteriness, weak cry, lethargy, floppy posture, convulsions, or coma. Diagnosis is confirmed by blood glucose determinations performed by the laboratory, when suspected, or by unit visual methods with reagent strips such as Chemstrip-BG or Dextrostix (Kliegman, 2006). Blood glucose screening should be done on all high risk infants soon after birth and frequently during the first few hours until glucose levels stabilize.
Hyperglycemia: Hyperglycemia is defined as a blood glucose level greater than 125 mg/dl (whole blood) or a plasma glucose level of 145 to 150 mg/dl (Blackburn, 2007). This condition is seen primarily in ELBW and VLBW infants receiving parenteral nutrition with dextrose concentrations of 5% or higher. Hyperglycemia can be just as harmful to the preterm infant as hypoglycemia. Increased circulating levels of glucose can lead to osmotic changes, increased urine output, and fluid shifts in the already compromised CNS of the preterm infant. The net result of hyperglycemia can be cellular dehydration and intraventricular hemorrhage. Preterm infants undergoing stress such as surgical intervention can also become hyperglycemic with increased catecholamine release, which inhibits insulin release and glucose utilization (Blackburn). Therefore ELBW and VLBW infants should be monitored closely for both hypoglycemia and hyperglycemia during the acute phase of illness, while receiving parenteral nutrition, and perioperatively.
Polycythemia: Polycythemia or hyperviscosity of the blood is another common problem of the SGA infant. With polycythemia, there is an excess in circulating RBC mass. This condition is a result of fetal hypoxia and intrauterine stress that forces the body to produce more RBCs in an attempt to provide oxygen to the developing fetus. Polycythemia is associated with maternal preeclampsia, maternal smoking, maternal diabetes, and delayed cord clamping (Diehl-Jones & Askin, 2010). With hematocrit greater than 65% or venous hemoglobin greater than 22 g/dl, blood viscosity is increased. This can lead to compromised blood flow and reduced oxygenation of the body organs. Many infants with polycythemia are asymptomatic. Others present with plethora, cyanosis, CNS abnormalities (lethargy, jitteriness, seizures), respiratory distress, tachycardia, congestive heart failure, or hypoglycemia. Infants with polycythemia are at increased risk for hyperbilirubinemia. In some cases, a partial exchange transfusion to reduce the viscosity of the blood is necessary.
Heat Loss: SGA infants are particularly susceptible to temperature instability as a result of decreased brown fat deposits, decreased adipose tissue, large body surface exposure, and, in many instances, poor flexion, as well as decreased glycogen storage in major organs such as the liver and heart. Therefore, close attention must be given to maintain an NTE. Nursing considerations focus on maintenance of thermoneutrality to promote recovery from perinatal asphyxia because cold stress jeopardizes such recovery.
Large for Gestational Age Infants: The LGA infant is defined as an infant weighing 4000 g or more at birth. An infant is considered LGA despite gestation when the weight is more than the 90th percentile on growth charts or two standard deviations above the mean weight for gestational age. The LGA infant is at greater risk for morbidity than the SGA or preterm infant; such infants have an increased incidence of birth injuries, asphyxia, and congenital anomalies such as heart defects (Stoll & Adams-Chapman, 2007).
All pregnancies of longer than 42 weeks of gestation must be thoroughly evaluated. All large fetuses are monitored during a trial of labor, and preparation is made for a cesarean birth if abnormal fetal heart pattern or poor progress of labor occurs. LGA newborns can be preterm, term, or postterm; they may be infants of mothers with diabetes; or they can be postmature. Each of these problems carries special concerns. Regardless of coexisting potential problems, the LGA infant is at risk by virtue of size alone.
The nurse assesses the LGA infant for hypoglycemia and trauma resulting from vaginal or cesarean birth. Any specific birth injuries are identified and treated appropriately (see Chapter 35).
Discharge Planning
Discharge planning for the high risk newborn begins early in the hospitalization. Throughout the infant’s hospitalization the nurse gathers information from the health care team members and the family. This information is used to determine the infant’s and family’s readiness for discharge. Discharge teaching for the high risk newborn family is extensive, requires time and planning, and cannot be adequately accomplished on the day of discharge. Information is provided about infant care, especially as it pertains to the particular infant’s home care needs (e.g., supplemental oxygen, gastrostomy feedings, follow-up medical visits). Parents should be allowed to spend a night or two in a predischarge room providing care for the infant away from the NICU to become better acquainted with the necessary care and to have a time of transition in which questions can be answered regarding home care. Additional parent teaching should include bathing and skin care; requirements for meeting nutritional needs following discharge; safety in the home, including supine sleep position and prevention of infection (e.g., RSV); and medication administration.
Durable medical equipment and supplies required for the care of the infant in the home should be delivered to the home before the infant is discharged; parents and care providers should have ample opportunity and education in the use of the equipment. Parents of infants being discharged with special needs such as gavage or gastrostomy feedings, nasal cannula oxygen, tracheostomy, or colostomy should receive several days of thorough education in the procedure before discharge. Preterm infants have a high rate of emergency department visits and readmission to acute care centers; the family absolutely must have a health professional they can contact for questions regarding infant care and behavior once they are home. Parents should obtain an age-appropriate car seat before the discharge of their infant and demonstrate its use. Car seat safety is an essential aspect of discharge planning, and infants who were born at less than 37 weeks of gestation should have a period of observation in an appropriate car seat to monitor for possible apnea, bradycardia, and decreased Sao2.
Before discharge all high risk or preterm infants should receive the appropriate immunizations, metabolic screening, hematologic assessment (bilirubin risk as appropriate), and evaluation of hearing. Successful discharge of high risk infants to their homes requires a multidisciplinary approach. Medical, nursing, social services, and other professionals (physical therapy, occupational therapy, developmental follow-up specialist) are crucial to the smooth transition of these infants and their families to the community and home. If the infant is transported back to the community hospital that referred either the mother before birth or the infant after birth, interfacility communication is essential to continuity of care.
Instruction in CPR is essential for parents of all infants but especially for those of infants at risk for life-threatening events. Infants considered at risk include those who are preterm, have apnea or bradycardia, or have a tendency to choke. Before taking their infant home, parents must be able to administer CPR. All parents should be encouraged to obtain instruction in CPR at their local Red Cross or other community agency, if it is not provided by the NICU.
Transport to and from a Regional Center
If a hospital is not equipped to care for a high risk mother and fetus or a high risk infant, transfer to a specialized perinatal or tertiary care center is arranged. Maternal transport ideally occurs with the fetus in utero because this has two distinct advantages: (1) the associated neonatal morbidity and mortality are decreased; and (2) infant-parent attachment is supported, thereby avoiding separation of the parents and infant. For a variety of reasons, however, it is not always possible to transport the mother before the birth. These reasons include imminent birth and unanticipated problems; therefore, physicians and nurses in level 1 and 2 facilities must have the skills and equipment necessary for making an accurate diagnosis and implementing emergency interventions to stabilize the infant’s condition until transport can occur (Rojas, Shirley, & Rush, 2011). The goal of these interventions is to maintain the infant’s condition within the normal physiologic range. Specific attention is given to the following areas:
Transport teams can include physicians, nurse practitioners, nurses with expertise in neonatal intensive care, and respiratory therapists. The team must have expertise in resuscitation, stabilization, and provision of critical care during the transport, which can occur on the ground or in the air. In a neonatal transport, the team should provide information for the parents about the tertiary center. Transport teams can integrate an individual developmental plan of care into their caregiving efforts, thereby initiating multidisciplinary interventions early in the infant’s life.
Health care professionals who are responsible for the early stabilization of newborns need specialized training to provide timely, efficient, and effective care. The S.T.A.B.L.E. training program (Box 37-7) is an evidence-based continuing education program that focuses on the postresuscitation and pretransport stabilization of sick neonates (Taylor & Price-Douglas, 2008). It has been endorsed by the March of Dimes and the American Academy of Pediatrics. Training includes an interactive didactic presentation and a posttest (www.stableprogram.org).
The birth of any high risk infant can cause profound parental stress. Parents can grieve the loss of the ideal infant. They are fearful of the possible eventual outcomes for the infant. They also must deal with the technologic world surrounding their infant, and amid all the equipment, it is sometimes difficult for them to perceive the infant and respond to his or her needs. Parents of high risk infants who have been transported to regional centers therefore need special support. As one way to deal with this problem, many intensive care units provide the family with a handbook or pictures of the tertiary care unit to help them understand what is going on around them. Parents should have the name and telephone number of a contact person at the regional center.
Infants are sometimes transferred back (back transport) to the referring facility; however, in most cases the infant is discharged home from the tertiary-care center. Preterm infants who require thermoregulation and gavage feedings may be cared for in community hospitals closer to home, which allows parents to visit their infant more easily and to work with their personal health care provider on the long-range outcomes for the infant. Specialized incubators make these trips possible (Fig. 37-15). However, parents may express mixed feelings about such return transports and may be reluctant to adapt to a different facility and group of caregivers. To minimize some of these concerns, giving the parents clear information about return transports during the initial discharge planning is important.
Anticipatory Grief
Families experience anticipatory grief when they are told of the impending death of their infant. Anticipatory grief prepares and protects parents who are facing a loss. Parents who have an infant with a debilitating disease (with or without a congenital deformity), but one that may not necessarily threaten the life of the child, also may experience anticipatory grief. An alteration in relationships, a change in lifestyle, and a very real threat to their hopes and dreams for the future may affect the day-to-day interaction of the family with their infant and the staff. Nurses can help facilitate the family’s grieving process. If the nurse observes that a family member’s daily interactions with the infant change, the nurse should assess the situation and request psychosocial support or intervention by a chaplain or social worker, if necessary.
Loss of an Infant: Parents who know their infant is going to die have a very difficult time. The parents need to direct their attention, energy, and caregiving activities toward the dying infant. However, some parents find it difficult to visit their infant even for short periods once a terminal diagnosis has been made. Grandparents also grieve but often are unsure how to comfort their own child (the infant’s parent) during the period of impending death. Health care professionals can help by involving the family in the infant’s care, providing privacy, answering questions, and preparing them for the inevitability of the death (see Chapter 38). There is a growing emphasis on hospice and palliative care for infants and their families.
The nursing staff also experiences grief. Many primary staff nurses find themselves grieving as if the infant were their own because they often have worked closely with the infant and family for weeks, or even months. Managers and other staff members must acknowledge this grief. Talking about the infant or attending the funeral can help the affected staff members resolve their feelings about the infant’s death.
KEY POINTS
• Preterm infants are at risk for problems stemming from the immaturity of their organ systems.
• Nurses who work with preterm, late preterm, and other high risk infants observe them for respiratory distress and other early symptoms of physiologic disorders.
• The adaptation of parents to preterm, late preterm, or high risk infants differs from that of parents to normal term infants.
• Nurses can facilitate the development of a positive parent-child relationship.
• Nurses’ skills in interpreting data, making decisions, and initiating therapy in newborn intensive care units are crucial to ensuring infants’ survival.
• Pain management requires vigilant ongoing assessment, anticipation of painful events, and early interventions to prevent and diminish such a response.
• Nurses need to assess the macroenvironments and microenvironments of the infant and family to create a developmentally positive atmosphere.
• Developmental care is a philosophy that embraces family-centered care and awareness of the effect of environmental stimuli on the physical and psychologic well-being of the infant and family.
• Parents need special instruction (e.g., CPR, oxygen therapy, suctioning, developmental care) before they take home a high risk infant.
• SGA infants are considered at risk because of fetal growth restriction.
• The high incidence of fetal distress among postmature infants is related to the progressive placental insufficiency that can occur in a postterm pregnancy.
• Multidisciplinary health care teams including specially trained nurses transport high risk infants to and from special care units.
• Parents need assistance as they cope with anticipatory grief or loss and grief.
![]() Audio Chapter Summaries Access an audio summary of the Key Points on
Audio Chapter Summaries Access an audio summary of the Key Points on ![]()