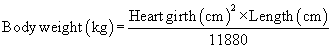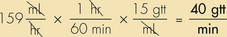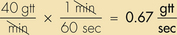Patient Preparation
After you have completed this chapter, you will be able to:
• Explain the importance of effective communication and the role of the veterinary technician in communication.
• List the reasons for preoperative patient evaluation.
• List the parts of a minimum patient database.
• Take a complete history, and identify findings that affect anesthetic event planning.
• Identify ways in which patient signalment influences the anesthetic procedure and patient management.
• Discuss the rationale for obtaining the owner’s consent for anesthesia.
• Perform a preanesthetic physical assessment.
• Relate the patient signalment, body weight, and patient condition to the selection and use of anesthetic agents and adjuncts.
• Assign a patient to one of the five physical status classifications as specified by the American Society of Anesthesiologists.
• Describe the components of preanesthetic preparation, including diagnostic testing, choice of protocol, withholding of food, and correction of preexisting problems.
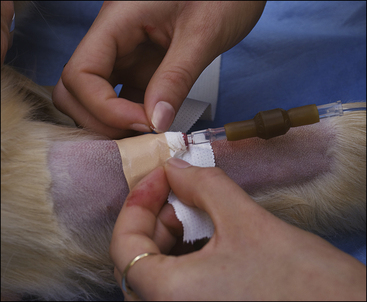
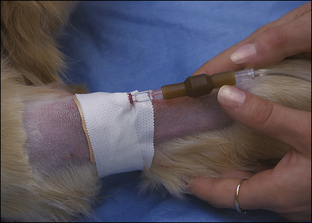
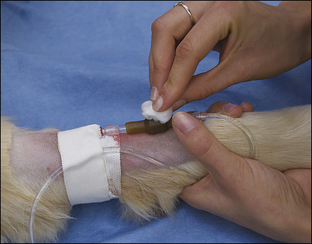
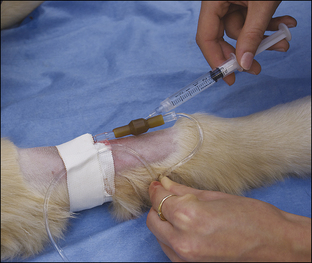
• List the reasons why an intravenous (IV) catheter is advisable for anesthetized patients.
• Describe the types of IV fluids that are used during anesthesia and why each might be chosen.
All successful anesthetic procedures begin with careful patient preparation. Completion of this mundane but important aspect of anesthesia requires attention to several diverse tasks. First, the anesthetist must assist the veterinarian-in-charge (VIC) in developing a minimum patient database—a compilation of pertinent information gleaned from the patient history, physical examination, and diagnostic tests—which the VIC will use to determine the patient’s physical status and anesthetic risk and to select an appropriate anesthetic protocol. Next, the anesthetist must ensure that the patient has been appropriately fasted. She must also provide preinduction care of the patient, including medication administration, intravenous (IV) catheterization, fluid administration, stabilization, and any other care ordered by the VIC. She must confirm that the necessary equipment and supplies are available and in good working order (further described in Chapter 4). Finally, she must administer preanesthetic medications ordered by the veterinarian and monitor the patient until the time of induction. Box 2-1 lists “to-do” items during the preanesthetic period. Like the pieces of a puzzle, each step is necessary to create a finished whole, and if any step is missed, the likelihood of a positive outcome decreases.
COMMUNICATION—A KEY TO SUCCESS
A key element of successful preparation is effective communication. Veterinarians depend on accurate information at all stages of the procedure to make effective patient care decisions. Clients need clear instructions and answers to questions before the procedure, as well as progress reports and home care instructions after the procedure. In addition, the technician often acts as a liaison between the doctor and client, conveying necessary information between these two parties.
The bond between most clients and pets is very strong. Surveys of pet owners over the past several decades consistently show that a vast majority of dog and cat owners consider their pets to be members of the family. Consequently, most clients are very protective of, and concerned about, the well-being of their animals and are very attuned to real or perceived risks to their safety. The risks inherent in the practice of anesthesia often produce or heighten feelings of anxiety.
These feelings can be minimized, however, when the relationship between health care providers and the client is strong. A strong relationship is based not only on confidence in the competency of the veterinarian and staff, but also on assurance that they truly care about the welfare of the patient. The saying, “Clients don’t care how much you know until they know how much you care,” has become an oft-repeated adage in the veterinary community. Although not originally used in reference to the veterinary profession, anyone with even a little practice experience can tell you that it accurately reflects the way most clients feel.
Exceptional communication is probably the best way to convey the caring that clients desire and expect. They feel more confident and less anxious about the procedure when informed. When unanticipated complications occur, clients are thus more able to come to terms with the outcome. A few extra minutes spent answering questions and explaining the risks and benefits before a procedure is well worth the time in terms of increased client satisfaction and lessening of the sadness, anger, and disappointment that occur when events do not go as hoped and that, if not adequately addressed, can result in ill will, the loss of a client, or even legal action.
In fact, there is no better predictor of success than the technician’s communication skills, and for this important reason communication will remain a centerpiece of this text. (See Case Presentation 2-1 for an example of the importance of good communication.)
THE MINIMUM PATIENT DATABASE
Technicians soon become accustomed to wide variety in size, age, temperament, conformation, and condition among the patients they see. Veterinary patients can range in size from 20 g to over 1000 kg and may be neonatal, pediatric, mature, or geriatric. They may be docile, spirited, fearful, agitated, or aggressive. They may be emaciated, lean, obese, brachiocephalic, deep-chested, or pregnant. Some are healthy, and others have varying degrees of illness ranging from mild to critical. Some are brought in for minor procedures; others will undergo complicated and lengthy operations. Given this diversity, it is unrealistic to assume that the same anesthetic techniques will work for all patients. Furthermore, it is dangerous to expect all patients to react to a given anesthetic agent in the same way.
Therefore the veterinarian and technician must gather as much information as possible to uncover any factors that might lead to anesthetic complications. The information, collectively referred to as a minimum patient database, is used to make patient care decisions. If the information obtained about a given animal reveals a potential problem, the veterinarian may choose to alter, postpone, or even cancel the anesthetic procedure.
The minimum patient database consists of the following:
Many veterinary clinics routinely recommend that animals scheduled for elective surgery be brought into the clinic for an appointment before the day of surgery so that this information can be gathered. During the visit, the veterinarian may also administer necessary vaccinations, give information about preanesthetic fasting of the animal, obtain signed consent forms, and present a fee estimate. If such an appointment is scheduled several days before the planned procedure, unforeseen problems can be discovered and addressed well in advance of surgery.
Before embarking on a study of anesthesia, the student should be familiar with the process required to obtain a patient history as well as the procedures required to perform a complete physical examination and diagnostic workup. Consequently the sections that follow will focus specifically on how the anesthetist can best obtain information needed to make effective and safe anesthetic management decisions. Emphasis will be placed on specific historical questions to ask, physical findings to focus on, and diagnostic test results that are most pertinent to anesthetic procedures, as well as the ways in which common abnormal findings from each of these sources of information affect outcomes.
Patient History
A patient history is information about the patient’s health acquired by asking the client questions. A thorough patient history is an essential part of a minimum patient database and, in fact, is often considerably more important for making effective patient care decisions than results of diagnostic testing.
Obtaining a thorough patient history requires skill and care, and, like all arts, it must be continually practiced and refined. The technician must know the most important questions to ask as well as the most effective way to frame those questions. Otherwise the patient history will be incomplete or misleading.
A common error is to ask a question that can be answered “yes” or “no.” For instance, the question “Does your dog drink a normal amount of water?” will yield a “yes” or “no” answer that reflects that owner’s assumption regarding what is normal, as opposed to facts that can be accurately evaluated. In contrast, the question “About how much water does your dog drink per day?” prompts the owner to quantify the amount of water consumed but not to judge whether he or she believes the amount to be normal. If the client replies, “About 1 quart (or liter),” the VIC will then be able to determine whether or not this volume is excessive, despite the owner’s opinion regarding its significance.
Another common error is to ask leading questions. In the previous example, the question “Your dog doesn’t drink much water, does she?” might bias the owner toward a particular response such as “No, I guess not,” resulting in misleading information.
In a busy practice it is often difficult to set aside adequate time to obtain a good history. Under these circumstances, the technician may be tempted to take shortcuts or omit questions altogether—an inadvisable practice that will lead to unpleasant surprises. For instance, an owner may not realize that coughing and exercise intolerance may be associated with a heart problem. If an adequate history is not taken, the preexisting heart disease may not be detected, and the technician may become aware of the problem only when the anesthetized animal is in danger.
When inquiring about signs of illness, the technician not only should note information the client freely offers but should always strive to get as much detail as possible. For instance, if a client indicates that the patient is vomiting, the doctor will want to know other details in order to interpret its significance. No matter what the sign (for example vomiting, coughing, diarrhea, polyuria, seizures, or weakness), it is always helpful to know the following four points,∗ which give the doctor specific information necessary to arrive at a diagnosis:
1. The duration (how long has it been going on?)
Confirmation of the Scheduled Procedure
Because miscommunications occur and clerical errors may be made on medical charts, in appointment books, and on surgery schedules, the technician must verbally confirm the procedure to be performed. Periodically stories appear in the news about devastating consequences of a miscommunication in the human medical field such as amputation of the wrong limb or administration of chemotherapy to the wrong patient. Similar errors may occur in veterinary patients and frequently result in ill will, patient harm, or legal proceedings.
Attention to this simple but important element of effective communication will prevent these and other embarrassing, dangerous, or potentially devastating errors such as a missed diagnosis from failing to submit a biopsy sample, anesthetizing the wrong patient, neglecting to perform a necessary procedure, or performing a procedure the patient does not need. When confirming the procedure, check the following specifics:
• When performing surgery on a limb, confirm the affected limb (left or right; fore or hind).
• When a tumor is being removed, be sure that the exact location of the tumor is known. Skin tumors can be obscured by the hair coat, and if small can be extremely difficult to locate unless pointed out by the client. Once the tumor has been located, mark the location by clipping the hair or using a surgical marker.
• If removing tumors or other mass lesions, confirm the owner’s wishes regarding histopathology (biopsy) or cytology.
• Determine if the client wishes the doctor to use his or her judgment regarding decisions that must be made during the procedure (such as the number of teeth that need to be extracted during a dental cleaning) or if the client would like a telephone call before proceeding.
After confirming this information with the client, be sure it is accurately transferred to the medical record and communicated to the VIC.
Obtaining a Patient History
When a complete patient history is being obtained, the following specific questions should be asked.
What Are the Species, Breed, Age, Sex, and Reproductive Status of the Patient?: The species, breed, age, sex, and reproductive status are collectively known as the signalment. The signalment is important in planning the anesthetic procedure, as each data point influences the anesthetic plan, drug doses, and many other actions related to patient management as noted in the following sections.
Species: Each species has unique responses to anesthetic agents and adjuncts as well as unique needs associated with anesthesia. Following are examples of notable species differences that must be considered:
• Horses and cats are more sensitive to opioids than dogs and ruminants. Therefore some of these agents must be used with caution, at lower doses or not at all in these species.
• Each species has unique dosing requirements (e.g., cats require a lower dose of lidocaine but are more resistant to the effects of phenothiazine tranquilizers than dogs).
• Horses tend to have rougher recoveries from inhalant anesthetics than other species.
• The use of anticholinergics should be avoided in ruminants, as it can make their saliva thick and ropy, which can lead to airway occlusion. Ruminants may also regurgitate at any point during anesthesia, and the anesthetist should take steps to avoid aspiration.
• Ruminants are more sensitive to xylazine, requiring about one tenth the dose of horses.
• Cats can tolerate administration of dissociative agents alone, whereas dogs may experience seizure-like activity unless the dissociative is combined with another agent.
• Large animals are prone to respiratory depression and dependent atelectasis and thus often require ventilatory support.
• Large animals experience pressure necrosis of tissues lying over pressure points such as the shoulder and hip and thus require measures to pad dependent areas when in lateral recumbency.
• Horses may fracture limbs during anesthetic recovery and thus require special attention during the recovery period.
• Cats, small dogs, and small animal pediatric patients are prone to hypoxemia and hypercarbia caused by increased mechanical dead space.
• Cats and ruminants are prone to airway blockage because of development of excess airway secretions.
• Ruminants are prone to bloat.
• Exotic animals such as birds and reptiles must be managed very differently than common domestic species. The technician should consult appropriate references and the veterinarian before administering anesthetics to these animals.
Breed: Differences in anatomy and physiology among the various breeds also may affect the animal’s response to anesthetic agents and procedures.
• Sighthounds such as greyhounds and salukis are sensitive to barbiturates (e.g., thiopental sodium) because of their relative lack of body fat and slow metabolism of these agents compared with other breeds. Consequently these drugs must be used cautiously or not at all in these patients.
• Boxers and giant breeds are more sensitive to acepromazine than other breeds, whereas terriers are resistant.
• Brachycephalic animals are difficult to intubate and must be watched closely to ensure a patent airway before, during, and after any anesthetic procedure. Also, members of these breeds often require use of smaller endotracheal tubes than most other breeds.
• Many exotic or rare breed animals are perceived to be sensitive to anesthetics by their owners, although many of these idiosyncrasies are not proven.
• Draft horses are typically sensitive to sedatives in the same way that giant breed dogs are. They are also more likely to experience complications during recovery because of their large body mass compared with average-sized horses.
Age: The age of the patient can be an important consideration when deciding what drugs to use. Neonates (up to 2 weeks of age) or pediatric patients (2 to 8 weeks of age) are much less capable of metabolizing injectable drugs than are adult animals because the necessary liver metabolic pathways are not fully developed. Geriatric patients may be unable to tolerate normal doses of some drugs because of poor hepatic or renal function. The result in either case may be a slow recovery from anesthesia, particularly if doses of injectable drugs are not adjusted accordingly. In addition, young patients are more difficult to intubate and to catheterize, are more subject to dosing errors, are more prone to hypothermia and hypoxia, and have a weaker respiratory drive. As patients age, they become less able to tolerate the demands that anesthesia places on their major organ systems. These factors put neonatal, pediatric, and geriatric patients at higher risk for complications.
Sex and Reproductive Status: Always confirm the patient’s sex by physical examination. Owners often are not aware of their pet’s sex or may misidentify it. If not corrected, misidentification of the patient’s sex can lead to an undesirable outcome. For instance, attempting to spay a male cat (because the patient’s sex was misidentified by the client and not confirmed by the technician) is unnecessary for the patient and very embarrassing and difficult to explain to the client.
Reproductive status refers to whether or not the patient has been spayed or castrated and, if the patient is intact, whether or not the patient is being used for breeding. For intact female patients, it also includes the current estrous cycle status and pregnancy status. These points are of particular importance in terms of the anesthetic plan as well as patient management as indicated in the following examples:
• When an intact female animal is in heat, the uterus is enlarged and has a more extensive blood supply. These patients also may bleed excessively because of the effects of estrogen on the clotting cascade. For an ovariohysterectomy, these factors increase the length, difficulty, risk, and often the cost of the surgery.
• During pregnancy, the gravid uterus and its blood supply gradually enlarge, reaching a size many times larger than a nongravid uterus. As is the case with estrus, these changes substantially increase the length, difficulty, and risk of an ovariohysterectomy. The prospect of spaying a pregnant animal also creates an ethical dilemma that must be discussed with the client before proceeding.
• Acepromazine is considered to be contraindicated by many clinicians in stallions because it may cause penile prolapse, which can lead to permanent injury.
• Xylazine has been shown to cause uterine contractions in the third trimester of pregnancy in sheep and cattle.
Is the Patient Receiving or Has It Recently Received Treatment with Any Drugs, Neutraceuticals, or Pesticides?: Many medications, including anticonvulsants, behavior-modifying drugs, drugs that affect the autonomic nervous system, and antibiotics may influence the effect of anesthetics.
• Sympathomimetics such as epinephrine increase the incidence of cardiac arrhythmias when given with cyclohexamines, xylazine, barbiturates, and halothane.
• Tricyclic antidepressants such as amitriptyline and clomipramine may predispose patients to cardiac arrhythmias and excessive responses to anticholinergics and central nervous system (CNS) depressants.
• The antibiotic chloramphenicol may decrease biotransformation of barbiturate anesthetics, leading to significantly prolonged recovery, and may prolong the action of propofol and ketamine.
• When given within 14 days of one another, some monoamine oxidase inhibitors such as amitraz and selegiline may increase the effects of morphine and other opioids.
• Some antihistamines can increase CNS and respiratory depression when given with opioids and other anesthetic agents that depress these body systems.
Is There Any History of Allergies or Drug Reactions?: Past adverse reactions to drugs must be documented in the medical record and conveyed to the VIC. Severe allergic reactions such as anaphylaxis preclude the use of the offending drug in the future. A past history of prolonged recovery, personality changes, organ dysfunction, or other postanesthetic adverse reactions may influence a decision to use these agents and must be brought to the veterinarian’s attention. For example, some cats will have prolonged recoveries from ketamine and other dissociatives that can last several days. Some dogs can experience behavioral changes after sedation with acepromazine that can result in bites. It may be best to choose another agent in the future for a patient experiencing these and other adverse reactions.
Is the Patient up to Date on Routine Preventive Care?: Check the date and type of vaccines administered and the results of fecal analysis and routine testing for contagious diseases such as heartworm disease in dogs and cats and feline leukemia and feline immunodeficiency virus in cats. The last date of tetanus antitoxoid administration to an equine patient should be known. Many veterinary clinics require current vaccinations before hospitalization to prevent the spread of contagious diseases.
Has the Patient Ever Had or Does It Currently Have Any Illness or Medical Problems, and, If So, What Was the Medical or Surgical Treatment?: Information about current and past medical problems is obtained from the patient’s medical record or verbally from the client. Animals with preexisting disease may be at increased risk for anesthetic complications. Sick animals may also introduce pathogens into the hospital, posing a risk to other patients unless they are placed in an isolation ward.
Asking if the patient has any of the signs listed in the following paragraphs helps the VIC make a diagnosis, determine risk, and guide patient management decisions. These common signs of disease often indicate a variety of problems, which must be explored and acted on through physical examination, diagnostic testing, patient treatment and stabilization, or even postponement of the procedure. Remember to determine the details regarding duration, volume or severity, frequency, and appearance or character.
Anorexia, Vomiting, Diarrhea, Coughing, Sneezing, Polyuria, Polydipsia, Tenesmus, or Dysuria: Anorexia, vomiting, diarrhea, coughing, sneezing, polyuria, polydipsia, tenesmus, or dysuria may indicate one of many disorders, which may need to be stabilized before anesthesia. Parvovirus in dogs, upper respiratory infections in cats, inflammatory bowel disease, urinary blockage, endocrine disease, and organ dysfunction are examples of problems associated with these signs.
A Change in Behavior: Changes in behavior may be a sign of CNS disease, pain, systemic illness, and many other conditions.
Exercise Intolerance: Exercise intolerance may indicate a variety of problems including heart disease, anemia, and musculoskeletal pain.
Weakness: Weakness is a nonspecific sign caused by many disorders and always warrants investigation to determine a cause.
Fainting or Seizures: Both fainting episodes and seizures vary widely in appearance and sometimes may be difficult to tell apart. In many cases these disorders can be differentiated by careful observation, but other cases require careful analysis of information derived from the patient history, physical examination, and diagnostic tests. Despite any similarities in appearance, these disorders have completely different causes. Fainting (also called syncope) often indicates hypoxemia, low blood pressure, or cardiac disease, whereas seizures are often associated with CNS disease, toxin ingestion, or metabolic disorders such as hypoglycemia.
Other Considerations: Before the procedure, it is advisable to give the owner a written estimate of the expected charges. It is also customary to obtain a signed consent form authorizing anesthesia and surgery. It is illegal in most jurisdictions to undertake surgery or anesthesia on an animal without the owner’s written or oral consent, and in any case is unadvisable. Such consent must be informed, meaning that the owner is warned beforehand of risks associated with the procedure. Standard consent forms (available from practice management consultants and from state and provincial veterinary associations) often state that anesthesia and surgery are never without risk (Figure 2-1).
Many consent forms also include a statement giving the veterinarian permission to perform cardiopulmonary-cerebrovascular resuscitation (CPCR) if the patient’s condition requires it. Owners should be asked to provide a telephone number at which they may be reached during the day, in case an emergency or unforeseen complication should arise. Consent forms may also state that some anesthetic drugs may be used that have not received approval from the Food and Drug Administration for this purpose. This is termed extra-label drug use and is common in veterinary anesthesia.
It is ironic that although cell phones have become ubiquitous, clients are often difficult to reach because they rely on voice mail and don’t always feel compelled to answer their phones promptly. An inability to contact a client presents a problem, particularly with procedures such as exploratory surgery, in which the diagnosis is not known. In these situations decisions that require the client’s input (such as whether to biopsy or remove a tumor or whether to proceed with a difficult, high-risk, or expensive procedure) must sometimes be made quickly. Therefore it is imperative that the client knows to be present and available by phone at the time of surgery, so that he or she can be contacted immediately should the need arise.
Obviously a great deal of information must be exchanged before anesthesia is initiated. Although it may be appropriate for a trained receptionist to assist in gathering this information, particularly when a young, healthy animal is scheduled for an elective operation such as castration or ovariohysterectomy, the veterinarian or technician should handle more complex cases. The hospital employee who admits a patient and speaks to the owner must not just obtain this information but must also relay that information to the anesthetist by means of a written record or oral report.
In some cases it may be difficult to obtain the history in person. Some clinics prefer to have the owner fill out a prepared history form, particularly if the clinic has a high volume of patients. In any practice, difficulties may arise when an animal’s owner is in a hurry and reluctant to stop and answer questions. However, it is usually possible to obtain a telephone number and call for more information at a prearranged time. Occasionally the person bringing the animal into the clinic is not the owner and is unfamiliar with the pet. In this case, every effort should be made to contact the owner by telephone to obtain the required information.
Physical Examination and Physical Assessment
A physical examination is a complete evaluation of a patient’s physical condition using the hands, eyes, ears, and nose. Although the basic technique used to perform a physical examination is the same whether the examination is performed by a veterinarian or veterinary technician, there is a qualitative difference in the way the results of the examination are used by these two parties. A veterinarian uses the physical findings to arrive at a diagnosis and plan treatment. In contrast, a veterinary technician uses physical findings to provide effective patient care, respond to patient needs, and alert the veterinarian to changes in patient condition that influence patient management. Coordinated application of these two very different but complementary approaches enables the veterinary team to provide high-quality care at a level that would not otherwise be possible.
In order to differentiate this contrast in purpose, in this text the term physical examination (PE) will be used to indicate evaluation by a veterinarian for the purpose of diagnosis and treatment planning, and the term physical assessment (PA) will be used to indicate evaluation by a technician for the purpose of maximizing quality of care and influencing patient management through communication with the VIC. No value judgment is implied in either term, as both physical examinations and assessments as defined herein are interdependent techniques—equally necessary and of equal importance in delivery of high-quality patient care. With this in mind, discussion of this vital part of the minimum patient database follows.
A complete physical examination should be conducted on every animal scheduled for anesthesia. Although a veterinarian usually does this before scheduling a procedure, the technician should, in addition, always perform a brief physical assessment immediately before the procedure. Following are common examples of findings revealed during physical examination or physical assessment that will influence patient anesthetic management.
• Dehydration increases the risk for anesthetic complications including hypotension, poor tissue perfusion, and kidney damage.
• Anemia decreases the oxygen-carrying capacity of the blood and predisposes the patient to hypoxemia.
• Bruising lesions on the skin or mucous membranes, in the absence of trauma, often indicate a clotting disorder, which will increase the risk of potentially life-threatening intraoperative and postoperative bleeding.
• Respiratory or cardiovascular disease increases the risk of anesthetic complications and death.
• Abnormalities of abdominal organs such as an enlarged liver or abnormally small kidneys may be associated with abnormal organ function and a reduced ability to metabolize or excrete anesthetic agents.
• General conditions that require veterinary attention such as ear mite or flea infestations, otitis externa, dental disease, overgrown nails, and anal sac impaction. These conditions are often most easily treated during the anesthetic procedure. Owners are often not aware these conditions are present and, once informed, will frequently authorize treatment.
• Physical abnormalities that may influence the procedure. One example of this is an abdominally retained testicle in a patient presented for castration. This condition increases the complexity and cost of surgery, so the owner must be informed before the veterinarian proceeds.
In most jurisdictions, veterinary technicians must perform a physical assessment under the supervision of a licensed veterinarian. The veterinary medical licensing board practice act should be consulted to determine the laws regulating technician duties in your state or province.
There are many different ways to approach a physical examination or physical assessment. Any technique is valid as long as the patient is examined entirely and in a systematic manner (e.g., from head to tail or by organ system). Include each element listed in the following sections, with emphasis on the nervous, cardiovascular, and pulmonary systems, as these systems are generally most affected by anesthetic agents and most directly influence outcome. When possible, it is helpful to have the owner present during the physical examination or physical assessment to give pertinent history about any physical abnormalities that are found. Any unusual findings must be brought to the attention of the veterinarian, because ultimately it is the veterinarian’s responsibility to make a diagnosis, formulate an appropriate treatment plan, and advise the owner and hospital staff accordingly.
Patient Identification
In practice, inattention to seemingly simple considerations, such as proper patient identification, may result in undesirable outcomes, because they are easily overlooked. Examination of the news will occasionally reveal stories of accidents at human hospitals related to patient identification such as a surgery performed on the wrong patient. Needless to say, serious errors such as these can and do occur in veterinary medicine.
In a busy animal hospital, patients may easily be confused if not positively identified with cage tags and patient identification collars, as well as documentation of external characteristics such as species, breed, size, hair coat length, color, and other visual identifying characteristics. This is because most individuals within a particular breed look very similar. Patients may be placed in the wrong cage, or one may simply be mistaken for another. Therefore, careful attention must always be given to patient identification.
Tags on the front of each cage should identify the patient so that those passing the cage can quickly identify the patient before opening the cage. Identification collars confirm patient identification in case a patient is inadvertently placed in the wrong cage.
Before performing any procedure, make positive patient identification by matching the cage tag, the ID collar, and the patient’s external characteristics with the information contained in the medical record. In other words, do whatever needs to be done to make sure the animal in your hands is the correct one!
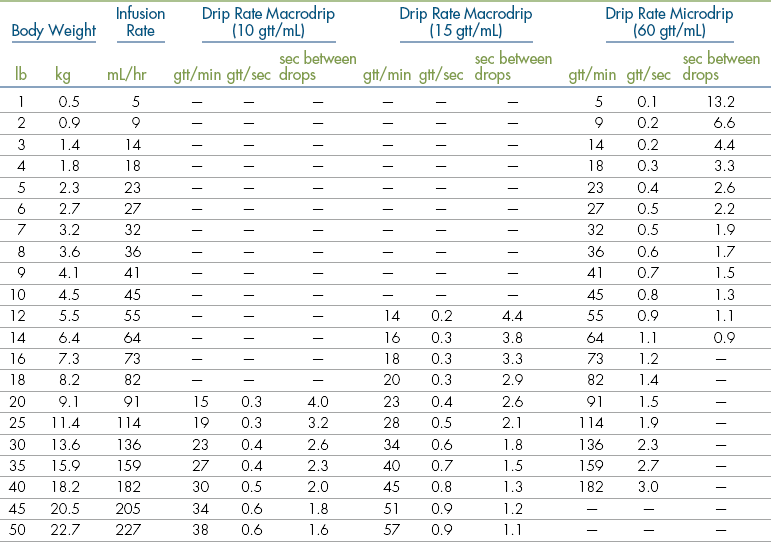
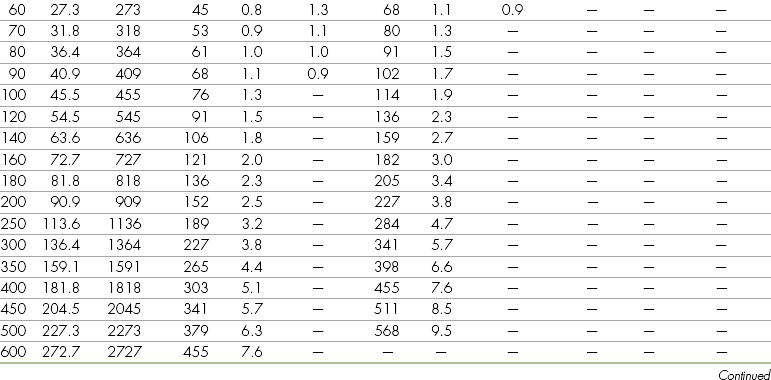
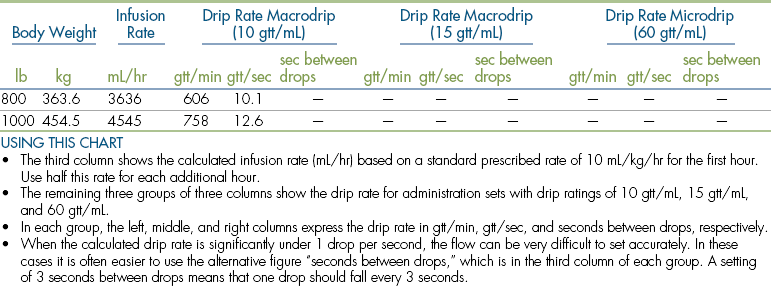
Body Weight
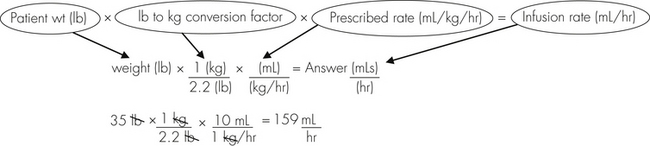
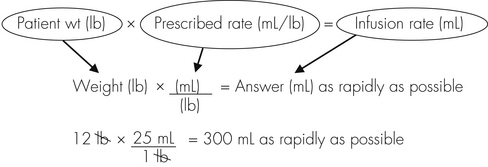
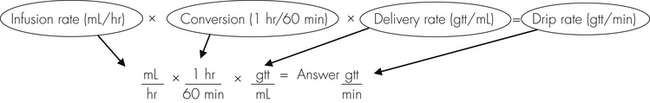
Anesthetic agents as a rule have very narrow therapeutic indexes. Therefore accurate dosing is critical to a successful outcome. With the exception of inhalant anesthetics, most drug dosages and IV fluid administration rates are calculated according to body weight. All animals should therefore be accurately weighed immediately before any anesthetic procedure. Animals under 5 kg should be weighed on a pediatric scale, and those under 1 kg should be weighed on a gram scale. It is not appropriate to estimate a body weight for purposes of anesthetic administration unless working with a large animal that cannot be weighed because of temperament or lack of a large animal scale. In this case, techniques will be used to estimate weight based on experience, or measurement of girth and length in horses, but must also take into account the patient’s body condition score. The basic formula for determining the body weight in horses is as follows:
where heart girth equals the circumference of the chest behind the point of the elbow, and length equals the distance from the point of the shoulder to the point of the pelvis.
The current weight should be compared with previous weights (found in the patient’s medical record) to determine whether weight gain or loss has occurred. Changes in weight may reflect changes in the patient’s food intake, hydration, activity level, or overall state of health.
Body Condition Score
A body condition score is a numeric assessment of the patient’s weight compared with the ideal body weight. Some professionals use a five-level system, and others use a nine-level system. In the nine-level system, the number 5 in cats and 4 or 5 in dogs represents ideal body weight. Lower numbers and higher numbers represent a body weight less than or greater than the ideal weight respectively, with a score of 1 representing extreme cachexia and 9 representing overt obesity (Figure 2-2). Although it has fewer gradations, the five-level system is similar to the nine-level system, except that a score of 3 represents normal weight and 5 represents overt obesity.


FIGURE 2-2 Nine-level body condition scoring for dogs and cats. A, Body condition scoring system for dogs. B, Body condition scoring system for cats. (Used with permission from Nestle-Purina.)
The body condition score is important because changes in body weight influence patient management. Excessive thinness may indicate the presence of an underlying disorder such as hyperthyroidism or chronic parasitism, which may increase patient risk. Animals with little body fat are more sensitive to the effects of some anesthetics such as the ultra–short-acting barbiturates and also are more prone to hypothermia than patients of normal body weight.
Obesity also poses difficulties for the anesthetist. Obese animals may have compromised cardiovascular function and decreased functional lung volume. In addition, venipuncture and auscultation are more difficult in obese patients. When patients are significantly overweight, anesthetics should be dosed according to lean body weight (excluding body fat) instead of the total body weight. This is because body fat increases the total body weight, but not the volume or weight of the nervous tissue on which anesthetics exert their effect. Administration of a dose calculated using total body weight results in an anesthetic overdose. Therefore the anesthetist must use the body condition score along with the actual patient weight to estimate lean body weight.
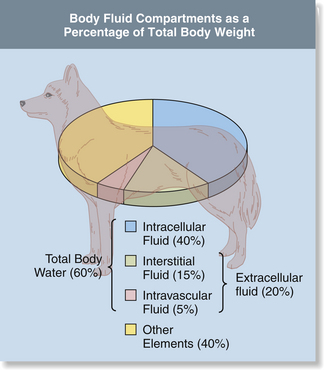
Assessment of Hydration
Hydration status is assessed using physical parameters that enable the anesthetist to estimate percent dehydration. Table 2-1 outlines these parameters including skin turgor, position of the eye in the orbit, mucous membrane color, refill and moisture level, heart rate, and pulse strength. As these parameters are affected by body fat content, age, and other factors, assessment of hydration is a somewhat subjective procedure at best and is naturally subject to many inaccuracies. For instance, young and obese patients appear more hydrated than they really are, whereas old and cachectic patients appear less hydrated. Panting may dry mucous membranes, causing the patient to appear less hydrated. Therefore these physical parameters can be expected to give the anesthetist only a general idea of hydration and must be used with other clinical data such as careful serial monitoring of body weight. This is an excellent indicator of hydration, with a sudden loss of 1 kg corresponding to 1 L of fluid loss.
TABLE 2-1
Physical Signs Associated with Dehydration
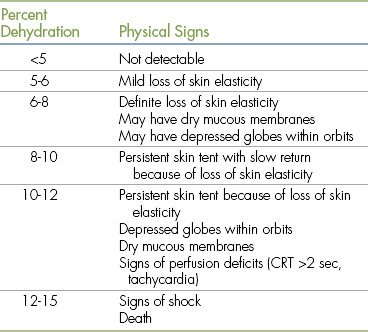
From DiBartola S: Fluid, electrolyte, and acid-base disorders, ed 3, St Louis, 2006, Elsevier.
Some clinical chemistry and hematology tests such as the plasma protein and hematocrit also reflect hydration status and will be discussed in the following section on diagnostic testing.
In any case, if the animal appears significantly dehydrated, this should be corrected before anesthesia, because dehydration will impair tissue perfusion and predispose the patient to hypotension. Fluid therapy is further discussed on p. 28.
Level of Consciousness
Level of consciousness (LOC) refers to the patient’s responsiveness to stimuli or how easily it can be aroused, and is used to assess brain function. A decreased LOC indicates abnormal brain function and is caused by a variety of factors including hypoxia, drugs, dehydration, and neurologic disease.
Consciousness of healthy patients is often described as alert and responsive or alert and oriented. Patients can further be classified as “bright” if noticeably engaged and interested in the environment, or “quiet” or “subdued” if this is not the case. Therefore “B/A/R” is an abbreviation that may be used to describe patients that are bright, alert, and responsive, and “Q/A/R” may be used for patients that are quiet, alert, and responsive.
Patients with a mildly decreased LOC that can be aroused with minimal difficulty are said to be lethargic. The word lethargy is a noun describing this state. Patients that are more depressed and that cannot be fully aroused are referred to as obtunded (noun form obtundity). A stuporous patient (noun form stupor) is in a sleeplike state and can be aroused only with a painful stimulus. A comatose patient (noun form coma) cannot be aroused and is unresponsive to all stimuli including pain.
A system commonly used in human patients is referred to as the AVPU scale. Each letter represents one of four levels of consciousness: A for alert (which includes the classifications B/A/R, Q/A/R, and lethargic); V for a patient that responds to a verbal stimulus (equivalent to obtunded); P for a patient that responds to only a painful stimulus (equivalent to stuporous); and U for an unresponsive patient (equivalent to comatose). Although this system is not currently in common use in veterinary medicine, it can easily be applied to veterinary patients. Table 2-2 summarizes the methods used to assess LOC.
TABLE 2-2
Assessment of Level of Consciousness (LOC)
| Signs | LOC (Traditional) | AVPU Scale |
| Fully conscious, alert, engaged and interested in the environment. | Bright, alert, responsive (B/A/R) | A (Alert) |
| Fully conscious and alert but not engaged, owing to fear, pain, illness, or any other cause. Subdued or quiet. | Quiet, alert, responsive (Q/A/R) | A (Alert) |
| Mildly depressed. Is aware of surroundings. Can be aroused with minimal difficulty (verbal or tactile stimulus). | Lethargic | A (Alert) |
| Very depressed. Uninterested in surroundings. Responds to but cannot be fully aroused by a verbal or tactile stimulus. | Obtunded | V (Responds to a verbal stimulus) |
| A sleeplike state. Nonresponsive to a verbal stimulus. Can be aroused only by a painful stimulus. | Stuporous | P (Responds only to a painful stimulus) |
| Sleeplike state. Cannot be aroused by any means. | Comatose | U (Unresponsive) |
Pain Score
Assessment of the patient’s level of pain should be included as a routine part of the patient assessment. The pain score will help guide selection of preanesthetic and anesthetic agents. Refer to Chapter 7 for a discussion of methods used to determine the pain score.
Body Temperature
Body temperature is best determined using a rectal thermometer. Table 2-3 shows normal body temperatures in nonanesthetized animals. A high body temperature most commonly indicates an inflammatory condition, which must be identified and may require pretreatment with antibiotics, antiinflammatories, or other medication. A significantly low body temperature may be associated with one of a number of serious systemic disorders.
TABLE 2-3
Normal Vital Signs in Nonanesthetized Patients
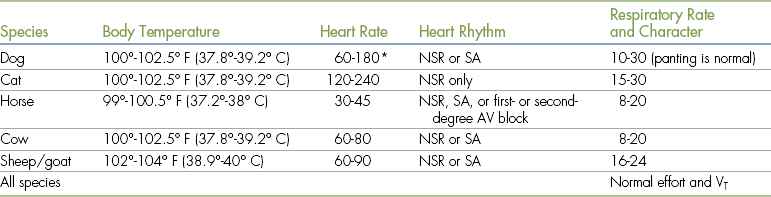
AV, Atrioventricular; NSR, normal sinus rhythm; SA, sinus arrhythmia; VT, tidal volume.
∗Owing to the extreme variability of size, large dogs tend to have lower rates, whereas small dogs and puppies have higher rates.
General Condition
The patient’s general condition refers to findings that are revealed by visually examining the patient at a distance including gait, temperament, and activity level.
Gait: Gait refers to the manner in which the patient moves. A patient with a normal gait places approximately equal weight on all limbs as it walks. A lame patient places unequal weight on the limbs, limps or carries a limb, and may have sensitive or painful areas. Any of these signs may indicate a musculoskeletal disorder that may require treatment during the procedure or, in some cases, may indicate a nervous or systemic disorder that would increase anesthetic risk.
Temperament and Activity Level: The animal’s temperament and activity level will affect the selection of anesthetic agents. For instance, an animal that is anxious or excited may override the effects of a phenothiazine tranquilizer. In this case, combining a phenothiazine tranquilizer with an opioid or giving a more potent agent such as an alpha2-agonist may be preferable. An ill patient may be excessively sedated with a standard dose of acepromazine, and the veterinarian may choose to reduce the dose of acepromazine, give a milder agent, or omit sedation entirely. Special handling techniques such as anesthetic chamber inductions, oral administration of ketamine, or the use of intramuscular (IM) alpha2-agonists or Telazol (tiletamine-zolazepam) may be necessary to restrain feral or extremely aggressive patients without endangering hospital staff.
Exercise intolerance or weakness can indicate a variety of disorders that may affect anesthetic outcome including anemia, heart disease, or electrolyte disturbances. When observed from a distance, a weak patient will be reluctant to rise, may not stand for long, or may appear unsteady on its feet. An exercise-intolerant patient will tire quickly. These patients may require alternative anesthetic protocols or treatment before the anesthetic procedure.
Examination of Exterior Surfaces: Examination of exterior surfaces consists of examination of the hair coat, the skin, lymph nodes, mammary glands, and body openings.
Coat Condition: The hair coat should be shiny, full, and smooth. A rough hair coat, alopecia, and external parasites should be noted and reported. Although external infestations with ear mites, lice, fleas, ticks, or other parasites may not directly affect the anesthetic management, affected animals may require treatment during or after the anesthetic procedure.
Skin: Part the hair and examine the skin over the entire body including ventral surfaces. Run your hands over the entire body surface, as many masses may be hidden in the fur or on a part of the body difficult to see such as the axilla or groin. Redness, inflammation, masses, or wounds should be noted and reported. Ecchymoses, purpura, or petechiae on the skin or mucous membranes, in the absence of trauma, indicate a clotting disorder, which will increase the risk of potentially life-threatening intraoperative and postoperative bleeding.
Lymph Nodes and Mammary Glands: Examine the superficial lymph nodes and mammary glands for masses or swellings. This is best done with a combination of visual examination and palpation. Lymph nodes, if normal, should be very small or nonpalpable in small animal patients. Enlarged lymph nodes in any patient may indicate the presence of infection, inflammation, or neoplasia.
Body Openings: Examine all body openings for odors and discharges. In intact females, examine the patient for signs of estrus or milk production. Common causes of putrid (foul smelling) odors include dental disease, external ear infections, abscesses, urogenital infections, diarrhea, and skin infections. Patients with severe kidney failure may have azotemic (urine-like) breath, and patients with ketonemia from complicated diabetes mellitus may have a characteristic odor to the breath. Some of the conditions that cause odors or discharges, such as kidney failure and diabetes, may necessitate treatment before anesthesia. Some, such as pyometra, may necessitate a change in the anesthetic protocol, and many others including dental disease, abscesses, and ear infections may require treatment while the patient is anesthetized.
Examination of the Eyes, Ears, Nose, and Oral Cavity: The eyes, ears, nose, and oral cavity are often referred to as the “eyes, ears, nose, and throat” or EENT. Normal patients should not have discharges, inflammation, or swelling involving the oral cavity, ears, or nose. The eyes should be central, corneas should be clear, and eye discharge or redness should not be present.
Air should move freely and quietly through the nares during breathing. Stridor may indicate an upper airway infection or obstruction. Any disorder that could impede endotracheal intubation, such as the presence of redundant tissue in the oropharynx, tumors, or jaw fractures, should be noted, because it may be necessary to intubate a patient so affected through a tracheotomy incision. The gingivae and mucous membranes should be pink and should not be inflamed, bleed, or show bruising.
Note the amount of dental tartar. Because dental cleanings must be performed using chemical restraint, it is commonplace to clean the teeth during the same anesthetic event used to perform another surgery or procedure as long as there is no anticipated detrimental effect of performing both procedures together. Combining two procedures minimizes the number of anesthetic events, decreases the number of visits to the clinic, and in most cases also costs less. Therefore identification of dental disease before anesthesia increases safety, value, convenience, and client satisfaction.
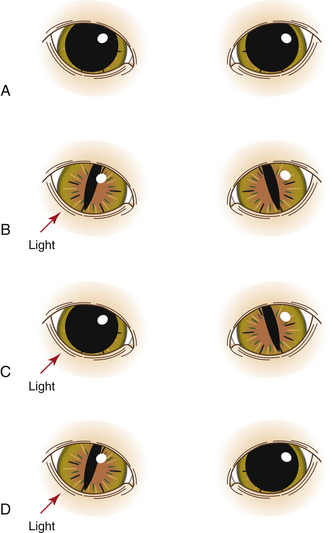
Assessment of the Pupillary Light Reflex: To check the pupillary light reflex (PLR), first observe and compare the size of both pupils, which should be of equal diameter. The size depends on the amount of ambient light entering the eye, the patient’s level of excitement, patient health, and the effect of medications. Next direct a bright light (usually from a penlight or other portable light source) into the right eye with the beam directed toward the medial aspect of the retina, as this region is more sensitive to light. Note the size of the right pupil (direct reflex). Pupil constriction (miosis) is normal, whereas failure to constrict in the presence of light is abnormal. While still directing the light in the right eye, observe the pupil size in the left eye (consensual reflex); in a normal patient the left pupil should constrict the same amount as the right pupil (Figure 2-3). Finally repeat the process on the left eye, looking for both a direct and consensual response. Note that the PLR may be diminished in excited animals or after the administration of some anesthetic agents and adjuncts including anticholinergics and opioids.
Cardiovascular System Examination
Determination of the Heart Rate and Rhythm: The heart rate and rhythm are generally most easily evaluated by auscultation of the heart over the left chest wall at the point of maximal intensity. With experience, the heart rate in beats per minute (bpm) can be accurately determined in small animals by counting the number of beats in 10 seconds and multiplying the number by 6. Large animal patients have slower heart rates; therefore counting for a period of 30 seconds and multiplying by 2 is more accurate. The heart may be difficult to assess, however, in obese patients, panting dogs, and purring cats, so the anesthetist must be familiar with techniques to deal with these situations. In obese patients (especially cats) the audible intensity of the heartbeat is often decreased. Sometimes palpation for the apical pulse is helpful to locate the optimal area for placement of the diaphragm of the stethoscope. When the apical pulse is not palpable, the anesthetist must carefully search in the region of the left axilla for a spot where the heart is most audible. This requires systematic, small measured changes in the position of the stethoscope head until the optimal area is located. A decreased heart sound intensity in a nonobese patient may indicate pericardial or pleural effusion. Suspicion of either of these conditions should be communicated to the VIC.
When panting prevents determination of a dog’s heart rate, the muzzle can be gently held closed for a brief time to stop the pant, although this will cause some patients to struggle. In these patients, blow gently in the nose or distract the patient. One of these actions may stop the panting long enough to determine the rate. Do not attempt these techniques in aggressive patients if you are concerned for your safety.
Purring in cats can sometimes be stopped by distracting the patient or by turning on the tap in a sink and allowing the patient to see a gentle stream of running water from a distance. If this does not work, gradually bring the patient closer to the stream or increase the flow until the purring stops. Use caution as some cats are more fearful of water than others and may scratch or bite if brought too close to the water.
Normal heart rates (see Table 2-3) are generally inversely proportional to body size (faster in smaller species and breeds and slower in larger species and breeds). In all species, pediatric patients tend to have higher rates than adults. Exercise or the stress of handling may cause the heart rate to increase. The heart rate should be measured when the patient is calm, not after stressful events such as inserting a rectal thermometer or collecting a blood sample.
Normal heart rhythms vary according to the species (see Table 2-3). Normal dogs may have either a normal sinus rhythm (NSR) or a sinus arrhythmia (SA). Cats and most other exotic mammals such as rabbits, ferrets, and rodents should always have an NSR, as an SA is not normal in these species. Horses and ruminants usually have an NSR or SA. Horses may also exhibit first- or second-degree block when at rest but will have an NSR after exercise. The sound of these rhythms is summarized below in the following paragraphs.
Normal Sinus Rhythm: Normal sinus rhythm is a completely regular rhythm with no irregularities or pauses between beats, although the rate may change in response to excitement level.
Sinus Arrhythmia: Sinus arrhythmia is a rhythm in which the heart rate cyclically increases during inspiration and decreases during expiration. This rhythm may be pronounced in young, healthy dogs and can sound to the inexperienced anesthetist as if there are skipped or premature beats. Abnormal rhythms can be differentiated from sinus arrhythmia by observing the respirations while listening to the heart. Abnormal rhythms are not associated with the breathing.
First-Degree Atrioventricular Heart Block: First-degree atrioventricular (AV) heart block is caused by a conduction delay through the AV node and is recognized by a prolonged PR interval on an electrocardiographic tracing. This rhythm causes no noticeable change in the heart sounds and therefore can be detected only by electrocardiography.
Second-Degree Atrioventricular Heart Block: Second-degree AV heart block is caused by a periodic block of electrical conduction through the AV node and is recognized by missing QRS complexes. On auscultation, periodic pauses representing skipped beats are audible. Note that it is not normal for more than one beat to be skipped in a row.
Any abnormal rhythms must be reported to the VIC, as they may be exacerbated or become life-threatening during anesthesia. (More detail regarding these rhythms may be found in Chapter 5.)
Examination for Murmurs: During auscultation, also check for heart murmurs. Listen over each valve and place the stethoscope diaphragm into the cranial-most aspect of the left axilla, as some murmurs including those associated with patent ductus arteriosus are often audible only in this location. Murmurs are caused by turbulence associated with abnormal flow of blood and often indicate a variety of conditions such as a leaking valve, a stenotic valve or vessel, or an abnormal communication between heart chambers, any of which may increase anesthetic risk.
Palpation of the Pulse and Comparison with the Heart Rate: In the conscious dog and cat the pulse is most easily palpated at the femoral artery, on the medial side of the rear leg. The patient should be standing quietly, and the femoral artery should be located by cupping the hand around the medial aspect of the thigh with the pad of the first or second finger in the groin just over the femur (see Figure 5-17, B). Other sites that may be palpable on medium to large dogs include the metatarsal and metacarpal arteries. In large animals the pulse may be palpated at the facial artery, digital artery, ventral tail, and auricular artery.
A strong, regular pulse should closely follow each audible heartbeat. If there are more heartbeats than pulses, a pulse deficit exists, which may indicate the presence of cardiovascular disease.
Palpation of the pulse also may give a crude estimate of blood pressure. A weak or nonpalpable pulse suggests hypotension, whereas an exaggerated pulse suggests hypertension. Realize that this is not always reliable because many other factors including body conformation, drugs, patient temperament, and excitement may affect either the strength of the pulse or the ability of the anesthetist to feel it.
Assessment of Mucous Membrane Color and Capillary Refill Time: The standard site for assessment is the gingiva at the base of a tooth. Normal mucous membrane color is sometimes described as “bubblegum pink.” Capillary refill time is assessed by applying digital pressure on the gingiva until blanching occurs, releasing pressure, and then observing the amount of time it takes for the return of normal color (see Figure 5-16). Normal refill time is less than 2 seconds. Pale mucous membranes or prolonged capillary refill time is indicative of decreased perfusion from shock, vasoconstriction, hypotension, or a variety of other conditions. Pale mucous membranes can also be associated with anemia. Cyanotic mucous membranes indicate reduced oxygen saturation, which is a medical emergency. Any of these conditions will increase anesthetic risk and must be treated before the procedure. If the gingivae are pigmented, mucous membrane color and capillary refill time may be observed at other sites, such as the conjunctiva of the lower eyelid, the entrance to the vulva, or the tip of the prepuce.
Respiratory System Examination
Determination of Respiratory Rate and Character: The respiratory rate in breaths per minute and respiratory character are best evaluated by visually observing chest excursions. In most cases the respiratory rate can be accurately determined in small and large animals by counting the breaths in 30 seconds and multiplying the result by 2. Auscultation is not a particularly useful tool for assessing respirations. As with the heart rate, the respiratory rate is generally inversely proportional to body size and age and will be affected by exercise or stress. Normal dogs may pant during examination. In this situation, as long as respiratory effort is normal, the rate may be entered in the medical record as “pant” instead of attempting to count the rate. (See Table 2-3 for normal respiratory rates in nonanesthetized animals.)
Respiratory character refers to other aspects of respirations including effort, relative length of inhalation and exhalation, and regularity. Patients with a normal respiratory cycle should inhale, immediately exhale, and then briefly rest before the next inhalation. Exhalation should be about twice as long as inhalation. Normal respirations should be even, smooth, and minimally visible when the patient is at rest.
Dyspneic patients may exhibit mouth breathing, flared nostrils, excessive panting, exaggerated chest or abdominal movements on inspiration, wheezing, and reluctance to lie down. In extreme cases a dyspneic animal may exhibit cyanosis. Dyspnea and cyanosis are both medical emergencies and should be brought to the veterinarian’s attention immediately. Avoid stressing dyspneic or cyanotic patients, as they are very intolerant of handling and can die during examination.
Auscultation of the Lungs: Assess the lungs by auscultating each of the four quadrants of the thorax (the right and left anteroventral lung fields and the right and left dorsal lung fields). In normal, calm patients the lung sounds should be very quiet. The presence of discontinuous lung sounds (crackles, rales, or rhonchi) or continuous sounds (wheezes) may indicate either pulmonary conditions (including pneumonia, bronchial disease, or asthma) or heart failure.
Abdominal Palpation and Auscultation
The normal abdomen should be soft to the touch and not painful. In small animals, most normal organs are difficult to feel except for a full urinary bladder, full colon, and, in cats, kidneys. Any firm or painful structure or any organ that feels larger than normal should be reported. Abdominal distention may indicate ascites, pregnancy, organ enlargement, or the presence of tumors. In large animals abdominal auscultation is used to detect normal borborygmus (gut sounds). Borborygmus should be present in all four abdominal quadrants in horses. In ruminants, normal contraction of the rumen is audible in the left paralumbar fossa.
Preanesthetic Diagnostic Workup
After the history and physical examination are completed, the veterinarian will decide which diagnostic tests (if any) are recommended for a given patient. The technician will then obtain appropriate samples (blood, urine, feces, or other samples) and will either perform the tests or forward the samples to a diagnostic laboratory for testing.
There are no universal guidelines for preanesthetic diagnostic tests, but each facility has standard policies. Some tests may be routinely recommended for every animal scheduled for anesthesia; different test groupings may be recommended for different patient groups such as geriatric patients, patients undergoing elective surgeries, and sick patients; or recommended tests may be based on the physical status class. The veterinarian may customize tests on the basis of the patient’s age, the history, and the results of the physical examination. Financial and other considerations may affect the number and type of tests the client chooses to approve. If the client declines one or more tests, a waiver should be signed indicating that the client understands the risk of proceeding without the information that would be provided by the declined tests. Table 2-4 lists sample preanesthetic diagnostic testing recommendations.
TABLE 2-4
Preanesthetic Diagnostic Test Recommendations
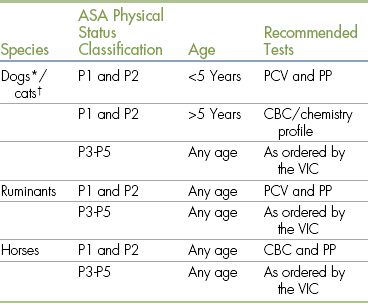
ASA, American Society of Anesthesiologists; CBC, complete blood count; PCV, packed cell volume; PP, plasma protein.
∗Heartworm testing is recommended for all dogs. Some clinics may extend this requirement to feline patients as well.
†All cats should be screened for feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) before anesthesia.
Common preanesthetic diagnostic tests and procedures include the complete blood count (CBC), blood chemistries, parasite screens, complete urinalysis, serologic tests, coagulation screen, electrocardiogram (ECG), and thoracic radiographs. Other tests may be ordered based on other conditions or illnesses that may be present. All test results must be recorded in the medical record and reviewed by the VIC before commencement of the procedure.
Complete Blood Cell Count
The CBC is a comprehensive evaluation of blood cell numbers and morphology that includes packed cell volume (PCV), plasma protein (PP), hemoglobin, and total red blood cell (RBC), white blood cell (WBC), platelet, and absolute leukocyte counts. Normal values for these parameters are given in Table 2-5.
TABLE 2-5
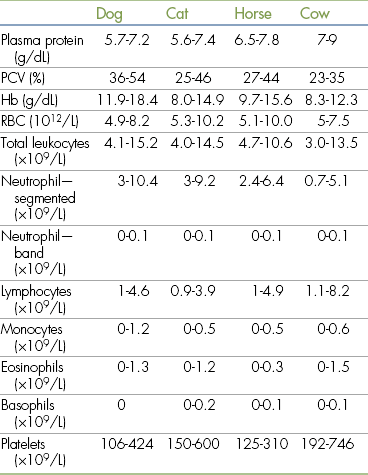
Hb, Hemoglobin; PCV, packed cell volume; RBC, red blood cells.
Modified from Muir WW, Hubbell JA, Bednarski RM: Handbook of veterinary anesthesia, ed 4, St Louis, 2006, Mosby.
The PCV is a measurement of the percent of the total blood volume that is made up of whole RBCs. The total RBC count (erythrocyte count) is a direct measurement of the total number of RBCs in a fixed volume of blood. Both tests are indicators of the oxygen-carrying capacity of the blood.
An elevated PCV or RBC count is most often caused by dehydration and is of concern to the anesthetist because of the associated decrease in blood volume, which may adversely affect cardiac output, blood pressure, and tissue perfusion.
In contrast, a decreased PCV or RBC count indicates anemia caused by decreased production, loss, or destruction of RBCs. Anemia results in a decreased capacity to supply oxygen to the tissues. Because the effects of anesthesia often increase the risk of tissue hypoxia, anemia must not be ignored. When a significant anemia is present, the veterinarian may recommend that anesthesia be postponed until it is corrected. A PCV less than 25% in a dog or less than 20% in a cat, horse, or cow should be reported immediately.
The PP is a measurement of blood protein including albumin, globulins, and fibrinogen. As with an elevated PCV, hyperproteinemia may be associated with dehydration and the same potential adverse consequences mentioned previously.
Hypoproteinemia usually results from decreased protein production by the liver or increased loss from the gastrointestinal tract or kidneys or from blood loss. Many anesthetics circulate in the blood partially bound to plasma proteins and partially free. Only the portion of the drug that is free and unbound can exert an effect. In patients with hypoproteinemia, the unbound portion proportionately increases, increasing drug potency. Hypoproteinemic patients also have difficulty maintaining adequate blood volume and may develop tissue edema because of the decreased oncotic pressure in the vascular system. For these reasons, a PP less than 4.0 in a patient of any species should be reported immediately.
The total WBC and absolute leukocyte counts (calculated from the total WBC and the differential WBC counts) measure the total number of leukocytes and the numbers of each type of leukocyte (neutrophils, lymphocytes, monocytes, eosinophils, and basophils). Changes in these counts may be associated with infection, parasitism, leukemias, and many other conditions that may be exacerbated by anesthesia and surgery or may increase anesthetic risk. The blood smear evaluation reveals changes in blood cell morphology, inclusions, parasites, and other conditions that may influence preanesthetic patient management. Interpretation of these tests is complex and beyond the scope of this chapter.
The platelet count is necessary to evaluate the mechanical component of blood coagulation. Patients with thrombocytopenia are at higher risk for abnormal intraoperative and postoperative bleeding, and their condition must be stabilized before surgery. Any decrease in platelet numbers should be reported to the VIC immediately.
Urinalysis
The complete urinalysis provides information about both the urinary and nonurinary systems.
The kidney function is of particular interest to the anesthetist, as it plays an important role in regulating electrolyte and water balance, blood pressure, and elimination of many anesthetics. If there is protein in the urine or if the urine specific gravity is less than 1.030 in a canine sample, 1.035 in a feline sample, or 1.025 in a large animal sample, the veterinarian should be notified because further tests may be needed to accurately assess kidney function.
Abnormalities in the macroscopic examination findings (color, clarity, and odor), biochemical analysis, and microscopic examination findings also may reveal evidence of diabetes mellitus, liver or kidney disease, or other systemic disorders that may affect patient anesthetic management. Abnormalities in any of these parts of the urinalysis should be reported and investigated.
Blood Chemistry Tests
A wide variety of blood chemistry tests are available that assess circulating enzymes, electrolytes, proteins, and metabolites. These tests give information about organ health and function, help the veterinarian formulate the anesthetic plan, screen for conditions that increase patient risk, influence patient perianesthetic management, and allow preparation for potential complications. Chemistry tests are often grouped in preanesthetic profiles that may assess one body organ such as the kidney, the liver, or the pancreas or may provide a comprehensive evaluation of all organ systems. The interpretation of these tests is complex, so any abnormalities should be reported to the veterinarian.
Blood Coagulation Screens
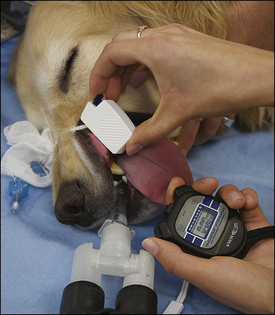
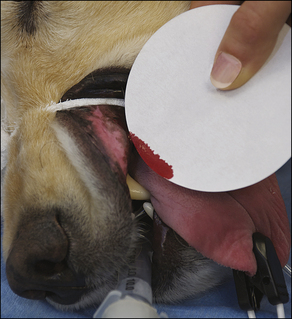
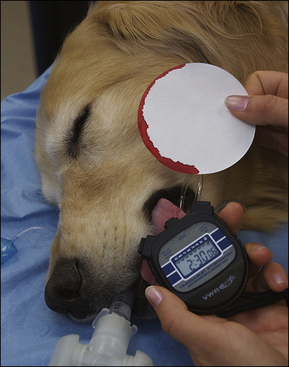
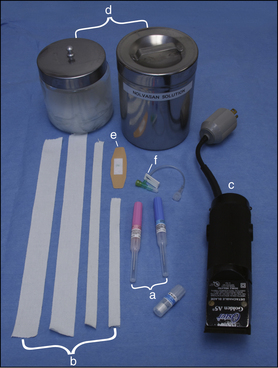
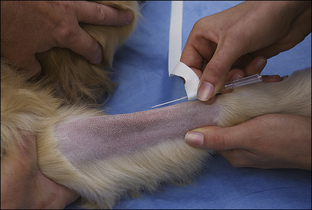
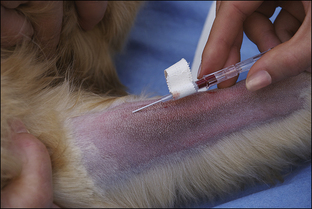
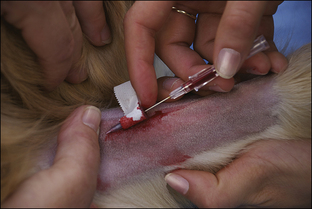
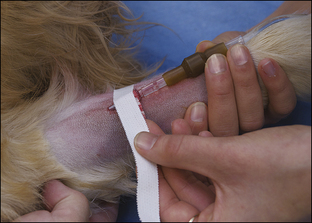
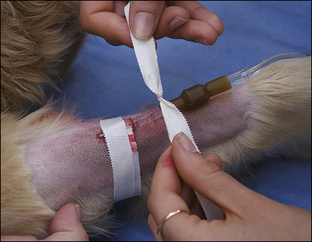
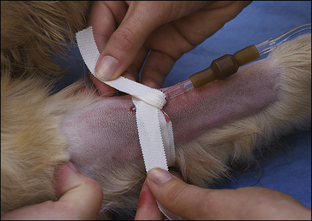
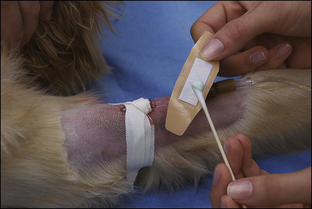
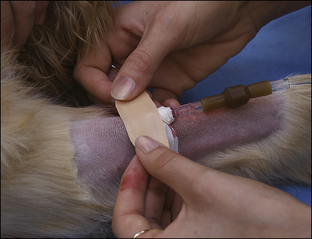
Blood coagulation screens evaluate the chemical, and sometimes mechanical components of blood coagulation. This is particularly important before nonelective surgeries because various disorders may adversely affect blood coagulation and put surgery patients at high risk for intraoperative and postoperative hemorrhage. A coagulation panel, including a prothrombin time (PT) and activated partial thromboplastin time (APTT), should be performed on any patient that may have a preexisting coagulation disorder (such as those with end-stage liver disease) or animals of breeds known to be commonly affected by hereditary coagulation disorders, such as Doberman pinschers, Rottweilers, and Scottish terriers. Coagulation panels may be performed at reference laboratories or in house. Any abnormal coagulation test result should be reported to the VIC immediately. The buccal mucosal bleeding time is an in-house screening test that can be used in patients, including those with normal platelet counts and coagulation panels, to determine the likelihood of perioperative bleeding (Procedure 2-1, p. 42).
Electrocardiogram
The ECG records the electrical activity of the heart, allowing the veterinarian to assess heart rhythm. Although not routine, an ECG is recommended for those patients with known or suspected heart disease, chest trauma, gastric dilatation-volvulus, splenic disease, or electrolyte disturbances or that are on medications that affect heart rhythm. The ECG also can be used to screen for cardiac disease in geriatric or other high-risk patients. Because most anesthetic agents alter heart rate, cardiac output, and oxygen consumption to some degree, patients with heart disease are at much greater risk for anesthetic complications. (ECGs are further discussed in Chapter 5.)
Radiography
Although not routine, thoracic radiography is warranted in animals that show signs of cardiac or pulmonary disease. Thoracic and abdominal radiographs are indicated for animals with major trauma (such as having been hit by a car) to rule out conditions that could increase patient risk or require modification of the protocol such as diaphragmatic hernia, pneumothorax, pleural effusion, pulmonary contusions, bladder rupture, or intraabdominal bleeding.
Miscellaneous Tests
Depending on patient need, existing illness, the geographic location of the practice, and other factors, other diagnostic tests may be routinely performed before anesthesia. For example, most veterinary practices in areas where heartworm disease is endemic require a heartworm test for all dogs and in some cases cats before any anesthetic procedure.
DETERMINATION OF THE PHYSICAL STATUS CLASSIFICATION
Before selecting the anesthetic protocol, the VIC should evaluate the minimum patient database and assign a physical status classification. The most widely accepted classification system is the one adopted by the American Society of Anesthesiologists (ASA), which is summarized in Table 2-6. This system rates patient risk from minimal (class P1) to extreme (class P5) based on the patient’s health.
TABLE 2-6
American Society of Anesthesiologists Physical Status Classifications
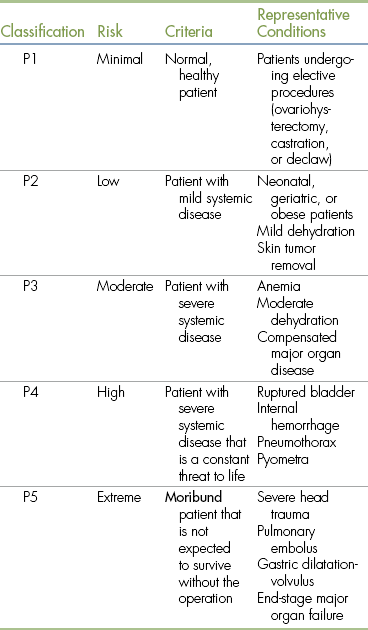
From Bassert JM, McCurnin DM: McCurnin’s clinical textbook for veterinary technicians, ed 7, St Louis, 2010, Elsevier.
In general, class P1 and class P2 patients can be safely anesthetized with standard anesthetic protocols. Class P3 to P5 patients often require special protocols, and their condition should be stabilized before surgery if possible. Patients undergoing emergency anesthesia may also be assigned an additional letter E regardless of class (e.g., P2E or P5E).
Classification of risk is somewhat subjective and may change over time. Two anesthetists might disagree, for example, on whether a patient with a moderate anemia should be assigned to class P2 (low risk) or class P3 (moderate risk). A patient in shock might be downgraded to a lower risk classification after receiving appropriate IV fluid therapy. Nevertheless, this system gives the anesthetist some basis for making patient management decisions that will minimize risk. The physical status class should be recorded in the animal’s medical record and in the anesthetic logbook.
SELECTION OF THE ANESTHETIC PROTOCOL
As mentioned in the previous sections, the technician acting as anesthetist should not hesitate to discuss abnormal findings from the minimum database with the veterinarian because such information may lead to changes in the planned anesthetic protocol. The presence of severe disease does not necessarily require that the procedure be postponed or canceled, although this may be the wisest course in some situations. It does often require selection of agents with which the anesthetist may be less familiar but that are less likely to produce adverse effects. For example the VIC may choose to use etomidate instead of propofol in a geriatric dog with heart failure because etomidate is much less likely to adversely affect cardiovascular function.
If the patient is very ill, the veterinarian may decide that the animal’s condition must be stabilized before an anesthetic is administered. Patients that are severely dehydrated, are profoundly anemic, or have a serious systemic disease or electrolyte imbalance are poor anesthetic risks. Every attempt should be made to correct these conditions before anesthesia, if time allows. If the planned procedure is not immediately necessary to save the patient’s life, it is possible that anesthesia may safely be postponed.
Factors That Influence Selection
In all jurisdictions in the United States and Canada, the veterinarian is the only health care provider legally allowed to choose (that is, prescribe) anesthetic drugs for animals. In most hospitals however, the veterinarian establishes one or two standard anesthetic protocols for physical status class P1 and P2 patients, with which the technician will quickly become familiar. However, each patient must be evaluated individually based on the minimum patient database, and changes must be made to the standard protocol when necessary. Although a technician with a strong knowledge base can help the veterinarian make an appropriate choice by communicating observations and suggestions regarding the patient, ultimately the VIC bears responsibility for the patient and must make the final decision regarding the anesthetic protocol.
When choosing the anesthetic protocol, the following factors must be considered.
Facilities and Equipment
Some anesthetic protocols require the use of specialized equipment. For example, isoflurane and sevoflurane require the use of a precision vaporizer and an oxygen supply. In some circumstances such as equine field anesthesia, use of a machine is impractical or impossible.
Familiarity with the Agent
Although for most patients any one of several anesthetic protocols is likely to result in a successful outcome, practitioners frequently choose the protocol with which they are most familiar. It is seldom advisable to anesthetize a high-risk patient with a new combination of drugs that the anesthetist may have heard or read about but has never tried before.
Nature of the Procedure
Procedures vary in their duration and complexity. They also require different degrees of analgesia, immobilization, muscle relaxation, and CNS depression. For example, local anesthesia may be suitable for short procedures such as a skin biopsy in which the patient requires only anesthesia of the biopsy site and physical restraint but no CNS depression. On the other hand, general anesthesia is required for thoracic or abdominal surgery, which necessitates unconsciousness, immobility, generalized visceral and somatic analgesia, and muscle relaxation.
Circumstances Specific to the Procedure
Anesthetics that may be appropriate for animals undergoing a routine operation (such as ovariohysterectomy or orchiectomy) may not be appropriate for a nonelective surgical procedure. For example, a cesarean section will require a protocol that minimizes respiratory depression in the neonates. In contrast, excellent muscle relaxation may be important for a fracture repair.
Cost
In a situation in which two agents are comparable in terms of patient safety, it is reasonable to choose the less expensive option. In other situations such as the presence of cardiac disease, it may be important to choose a cardiac-sparing drug such as etomidate even though it is considerably more expensive than thiobarbiturates or propofol.
Degree of Urgency
Critically injured animals may require rapid induction of anesthesia to initiate emergency therapy. For example, a patient that is in danger of shock because of ongoing uncontrolled hemorrhage cannot wait 15 to 20 minutes for a premedication given subcutaneously or intramuscularly to take effect. Selection of agents that allow for preservation of adequate blood pressure and rapid induction is desirable in this case.
PREINDUCTION PATIENT CARE
During the preinduction period the technician should ensure that the patient is fasted and receives appropriate nursing care, fluid therapy, medication administration, and any other care ordered by the veterinarian. All treatments should be recorded in the medical record. A checklist of procedures may be helpful, particularly if more than one person is responsible for preanesthetic care.
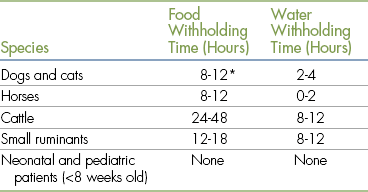
Withholding Food before Anesthesia
Animals that are anesthetized without prior fasting are prone to a variety of mild to serious complications that result from reflux of stomach contents into the distal esophagus, aspiration of stomach contents into the pulmonary tree, and bloating in ruminants (see Chapter 10).
Esophageal reflux commonly occurs during the anesthetic period as a result of decreased lower esophageal sphincter tone and flow of stomach acid into the esophagus that occurs when a patient is in a prone position. Esophageal reflux may cause irritation, inflammation, or, in extreme cases, severe tissue damage of the distal esophagus, resulting in stricture, and is recognized as a common cause of postoperative nausea, dysphagia, vomiting, and anorexia.
Vomiting is an active expulsion of stomach contents, preceded by retching, that occurs only in conscious patients. In contrast, regurgitation is a passive process that may occur in an unconscious or conscious patient, is not preceded by retching, and results in flow of stomach contents into the esophagus and mouth. Pulmonary aspiration occurs if the patient vomits or regurgitates during a time when the swallowing reflex is decreased or absent. Pulmonary aspiration is always serious and may lead to pneumonia that is difficult to treat, permanent disability, and in some cases even immediate respiratory arrest and death.
To prevent vomiting and minimize reflux during the anesthetic period, food should be withheld from most patients except for neonatal, pediatric, and some exotic patients. Table 2-7 lists fasting recommendations. The technician must ensure that fluids are administered as prescribed, because fasted patients are at higher risk for dehydration, especially if ill.
If a patient is known to have eaten within 12 hours of the proposed surgery and the surgery cannot be postponed, the veterinarian may choose to administer a preanesthetic with antiemetic properties (such as acepromazine), administer an agent that is likely to induce vomiting (such as xylazine in small animals) in order to empty the stomach, or proceed with heightened monitoring for regurgitation or vomiting.
Even if fasting recommendations are observed, vomiting may occur during the anesthetic or recovery period. Fasted patients sometimes vomit foam, bile, or mucus. At other times food may be vomited if a disorder exists that prevents the stomach from emptying, such as a foreign body, ileus, or a stricture. If vomiting occurs in the anesthetized patient, there is some protection against aspiration if a cuffed endotracheal tube is in place. This is the reason that the endotracheal tube must be left in place during recovery until the animal regains the swallowing reflex. (Methods for preventing and managing vomiting during anesthesia are discussed further in Chapter 12.)
Animals undergoing gastrointestinal procedures such as enterotomy, colonoscopy, or intestinal biopsy may require longer withholding times, enemas, or cathartics to minimize the amount of ingesta within the gastrointestinal tract at the time of surgery.
The anesthetist should be aware that although preanesthetic fasting is recommended, prolonged fasting is detrimental. Many seriously ill animals are anorexic and may refuse to eat. For example, a dog with severe trauma that requires prolonged hospitalization may be too uncomfortable, frightened, or weak to eat, and by the time it is released it may have gone without eating for several days. This lack of adequate intake impedes the healing process and prolongs recovery. For these reasons, efforts should be made to reestablish caloric intake by hand-feeding palatable foods, feeding by syringe bolus, or using feeding tubes or total parenteral nutrition.
Patient Stabilization
Seriously ill patients may require significant nursing care before surgery for stabilization. For example, a patient with a pneumothorax and femoral fracture will need to have the pneumothorax stabilized before the fracture repair is performed. This care can be labor- and time-intensive but is a very important part of minimizing patient risk. Veterinary technicians are usually intimately involved in this process of stabilization. At times (for example, with a patient with a gastric torsion or uncontrolled internal bleeding) the veterinarian may decide that the risk of delaying surgery outweighs the increased anesthetic risk and will elect to proceed. These patients often pose the greatest test of the technician’s skills and knowledge.
INTRAVENOUS CATHETERIZATION
Reasons for Intravenous Catheterization
Not all animals are catheterized before anesthesia; however, the presence of an IV catheter is of benefit to both the patient and the anesthetist for the following reasons:
1. Fluid administration helps to maintain blood volume and support blood pressure. Although not mandatory for routine operations in healthy patients, fluid administration is highly recommended under the following circumstances:
 Patients undergoing any procedure that may result in significant blood loss (such as a cesarean section or removal of a splenic tumor).
Patients undergoing any procedure that may result in significant blood loss (such as a cesarean section or removal of a splenic tumor).
 Debilitated or dehydrated patients.
Debilitated or dehydrated patients.
 Patients with organ dysfunction or failure.
Patients with organ dysfunction or failure.
 Patients with electrolyte abnormalities (for example, hyperkalemia).
Patients with electrolyte abnormalities (for example, hyperkalemia).
 Prolonged anesthesia (more than 1 hour).
Prolonged anesthesia (more than 1 hour).
 Patients at risk for hypotension or shock. Even mild hypotension is a potential problem in anesthetized animals because it leads to decreased blood flow to the kidneys and other vital organs.
Patients at risk for hypotension or shock. Even mild hypotension is a potential problem in anesthetized animals because it leads to decreased blood flow to the kidneys and other vital organs.
2. IV access allows rapid administration of emergency drugs such as epinephrine.
3. An IV catheter can be used for constant rate infusion (CRI) of anesthetics, analgesics, electrolytes, or other drugs such as insulin. CRI is slow, continuous administration of a drug at a rate sufficient to achieve the desired effect. For example, propofol is administered by CRI to maintain general anesthesia or to control seizures in patients with status epilepticus. Drugs given by CRI are either administered through an IV catheter with a syringe pump or added to an IV fluid bag and administered via an administration set and IV catheter.
The use of an IV catheter is necessary for safe CRI of drugs because it is difficult to keep a needle seated in a vein for more than a few minutes, especially if the patient is moved from one location to another. Therefore attempting to administer CRIs via a needle and syringe is likely to result in perivascular drug injection.
4. Vesicants (anesthetic agents that damage tissues if injected perivascularly, such as thiopental) can be administered safely. Some vesicants are so irritating to tissues that injection of even an extremely small amount can cause tissue irritation and sloughing. Perivascular injection is much more likely when a syringe and needle are used.
5. Incompatible drugs can be administered more easily via an IV catheter. For example diazepam and hydromorphone will precipitate when mixed because hydromorphone is water-soluble and diazepam is not. When administering this drug combination intravenously, each drug must be injected separately with saline flush in between to prevent mixing. Sequential injections are very cumbersome to administer by venipuncture with a needle and syringe without the risk of inadvertent perivascular injection or without breaking aseptic technique.
Choosing and Placing an Intravenous Catheter
Two main types of IV catheters are in common use for fluid and drug administration in veterinary patients: through-the-needle catheters and over-the-needle catheters. Although commonly used in critical care patients, through-the-needle catheters are not frequently used for anesthesia because they are more complex, expensive, and time-consuming to place, especially if the technician is not experienced with their use.
Over-the-needle catheters are more commonly used in general practice for patients receiving anesthesia and in most cases serve this purpose well because they are inexpensive, readily available, and relatively easy to place. Procedure 2-2, p. 44, shows the sequence of events used to place a catheter in a small animal patient. Typically, 16- to 24-gauge, ¾- to 2-inch catheters are used in small animal anesthesia, and 12- to 16-gauge, 5¼-inch catheters are used in cattle and horses.
When used for anesthetic management, the basic principles of catheter placement and maintenance are no different than when used for any other purpose. However, there are several special considerations that apply specifically to catheter maintenance and fluid administration during anesthesia:
• It is important to choose a catheter of sufficient length and to secure it carefully to prevent it from being dislodged during patient transfer (such as from surgical preparation to the operating room to recovery).
• When possible, choose a large-diameter catheter in case rapid fluid administration is necessary, as would be the case with excessive blood loss or hypotension.
• The catheter must be placed in a location that will not interfere with the procedure. For instance, if an orthopedic procedure is to be performed on the left front limb, the catheter must be placed in the right front limb, a rear limb, or some other site.
• Be sure to use an administration set with an injection port, so that IV medications can be administered when necessary. Procedure 2-3, p. 46, shows the sequence of events used to administer medications through an IV administration set port.
• Because surgery patients are placed in various positions most conducive to exposure of the surgery site, limb ties or the limb position itself may impede flow of fluids. This must be kept in mind when positioning and securing the patient to ensure that fluids are flowing freely.
• Movement of the catheter and patient during patient transfer may result in the introduction of air into the vein (known as an air embolism) through the administration line. This must be avoided, because a large air embolus is life-threatening.
• Drugs administered via a catheter must be given slowly. Most drugs should be given over a period of 15 to 60 seconds, although some, such as sodium bicarbonate and potassium chloride, must be given much more slowly.
• When administering drugs IV, be sure to flush the entire dose of drug into the vein after each bolus. This will prevent dosage errors.
FLUID ADMINISTRATION
All animals have a fundamental physiologic need for a constant source of oxygen delivered to all body tissues in quantities necessary to perform basic metabolic functions. An absence of adequate oxygen will rapidly damage any tissues so deprived and will result in cell death in high-demand tissues such as the brain and heart muscle within minutes.
One of the primary functions of the cardiovascular and respiratory systems is to supply this need by extracting oxygen from the air and conveying it directly to every cell in the body. For this reason, agents that adversely affect cardiopulmonary function have the potential for decreasing oxygen delivery. As nearly all anesthetic agents adversely affect these systems, constant attention to cardiopulmonary support is one of the cornerstones of the successful practice of anesthesia.
There are several specific ways in which anesthetic agents affect cardiopulmonary function. Almost all anesthetic agents decrease the force of heart muscle contraction (referred to as inotropy) and cause bradycardia. These factors in turn decrease the flow of blood from the heart (cardiac output). Almost all agents also relax the muscle tone of blood vessels, which in turn causes an increase in the intravascular volume (vasodilatation). Together the decreased cardiac output and vasodilatation cause hypotension and decrease the perfusion of tissues with blood.
Administration of intravenous fluids is one of the primary tools available to the anesthetist to support oxygen delivery. Fluids increase circulating blood volume and cardiac output—two physiologic changes that support blood pressure and tissue perfusion.
Composition of Body Fluids
Water is the most prevalent substance in the body. In adult animals, about 60% of the body weight is water. Because of variation associated with age and body fat content, young and lean patients have a somewhat higher percentage, whereas old and obese patients have a somewhat lower percentage. Body water is separated by cell membranes into intracellular (within the cells) and extracellular (outside of the cells) fluid compartments. The extracellular fluid compartment is subdivided by the vascular endothelium into the interstitial fluid compartment (fluid in the tissues outside of the cells) and the intravascular compartment (fluid within the vascular system).
Of the 60% of the body weight that consists of water, about two thirds (or 40% of the body weight) is intracellular fluid (ICF). Although estimates of extracellular fluid (ECF) vary widely (about 15% to 30% of body weight for small animals), most clinicians use the figure 20% for the purpose of calculating fluid needs. About three fourths of the ECF (15% of the body weight) is interstitial fluid (fluid between the cells), and one fourth (5% of the total body weight) is intravascular fluid (plasma) (Figure 2-4). When blood cells are added, blood volume is considered to be 8% to 9% of body weight in dogs and large animals and 6% to 7% of body weight in cats. For this reason the figures 90 mL/kg for dogs and large animals and 60 mL/kg for cats are commonly used for the purpose of calculating blood volume.
Body fluids consist of water and solutes. Body fluid solutes are either atoms or molecules dissolved in body water. The solutes most important in fluid therapy are small–molecular-weight electrically charged particles called ions, large–molecular-weight plasma proteins called colloids, and small nonionic particles such as glucose and small proteins.
Electrolytes are substances that when dissolved separate into positively charged ions, called cations (so called because they migrate toward the cathode during electrolysis), and negatively charged ions, called anions (so called because they migrate toward the anode). Sodium chloride (NaCl or table salt) is an example of an electrolyte; when dissolved, it separates into the cation sodium and the anion chloride. Important cations in body fluids include sodium (Na+), potassium (K+), magnesium (Mg2+) and calcium (Ca2+). Important anions include chloride (Cl−), bicarbonate (HCO3−), phosphates (HPO42−, H2PO4−), and proteins.
Solutes serve diverse purposes. Electrolytes provide osmotic pressure and are essential for many fundamental physiologic processes such as blood clotting, heart function, and neuromuscular function. Proteins participate in a wide variety of processes including drug transport, regulation of blood pressure by providing oncotic pressure, and blood clotting. Glucose provides energy to the cells.
Fluid Homeostasis
Homeostasis refers to a constant state within the body created and maintained by normal physiologic processes. Maintenance of fluid homeostasis is a highly delicate and dynamic process. In health, water and solutes constantly move through cell membranes between fluid compartments as needed to maintain solute concentrations within a narrow and specific range. Many solutes move freely between body compartments by passive diffusion along gradients from areas of high concentration to those of low concentration. Others are confined to or concentrated in a particular space. For instance, owing to its extremely large size, albumin does not travel freely through the normal vascular endothelium but tends to concentrate within the intravascular space. Another example is potassium, which, because of active transport through the cell membranes, is concentrated within the cells (98% of K+ is located within the cells). In contrast, sodium is largely excluded from the cells and thus is found in high concentrations in the extracellular space.
Consequently, the concentration of each of the major solutes often differs widely among the fluid compartments. For instance, ICF is very rich in potassium, magnesium, protein, and phosphate when compared with ECF. In contrast, ECF has much higher concentrations of sodium, chloride, and bicarbonate (Figure 2-5).
FIGURE 2-5 Comparison of the average electrolyte concentrations in the extracellular and intracellular fluid compartments. (Modified from DiBartola S: Fluid, electrolyte, and acid-base disorders, ed 3, St Louis, 2006, Elsevier.)
The balance of water and solutes in body fluids is governed by a number of principles, which, if known, help the anesthetist administer fluids in a way that is most beneficial to the patient. Some of these principles are as follows:
• At any given time, the number of negatively and positively charged particles in any given fluid compartment must be equal. Thus if the number of positively charged particles in a compartment increases, the number of negatively charged particles must increase by the same amount. This state of electrical balance in any fluid compartment is called electroneutrality.
• In health, a solute concentration or osmolarity of approximately 300 mOsm/L is maintained in all body fluids. Conditions including dehydration, exercise, heat stroke, and some cases of vomiting and diarrhea that involve primarily water loss will increase the osmolarity, and others such as chronic congestive heart failure, in which large quantities of solutes are lost, will decrease it.
• The solutes in each fluid compartment provide osmotic pressure, which draws water into that compartment in proportion to the number of particles present. For example, if the osmolarity in the vascular space increases or decreases, water will follow, increasing or decreasing the blood volume respectively.
• Small particle solutes such as ions diffuse freely through vascular endothelium, taking water with them. Thus they equilibrate relatively quickly between the intravascular and interstitial fluid spaces. The interstitial fluid compartment is at least twice the size of the intravascular compartment. Therefore at most only about one third of fluid administered intravenously remains in the vascular space after equilibration.
• Colloids including albumin do not diffuse freely through vascular endothelium. Their presence in the vascular space provides colloid osmotic pressure (also called oncotic pressure) that tends to draw water into the blood vessels and is thus important for maintenance of blood volume and pressure.
• The plasma concentration of certain solutes such as potassium and calcium must be kept in a very narrow range in order to maintain normal muscle and heart function. Relatively small deviations in levels of these cations can cause significant clinical signs and can endanger the patient.
Fluid Needs during Anesthesia
During anesthesia, many factors including disease conditions, surgery, and effects of drugs often disrupt fluid homeostasis and require the anesthetist to intervene. Appropriate fluid therapy requires knowledge of the nature of fluid losses associated with disease conditions. Following are some common associations with general anesthesia:
• Fluid losses that occur from dehydration and many general disease conditions initially deplete the ECF space. For this reason, when replacing recent fluid losses in patients with disease, dehydration, or anorexia, fluids should be chosen with solute profiles similar to ECF.
• Perioperative hemorrhage involves fluid loss from the intravascular space, part of the ECF space. Thus, as in the previous example, fluids should be chosen with solute profiles similar to ECF, unless hemorrhage is profound.
• Profound perioperative hemorrhage involves significant loss of albumin, blood cells, and other constituents of blood in addition to electrolytes and water. To support patients experiencing severe perioperative hemorrhage, administration of blood products may be necessary to provide RBCs or hemoglobin to support oxygen-carrying capacity, and in some cases clotting factors and platelets to support normal coagulation.
• Patients with perioperative hemorrhage may also benefit from hypertonic saline or colloid solutions, both of which draw water into the vascular space and raise blood pressure.
• Patients with low albumin may require colloids or blood plasma (fluids containing large solutes), which provide oncotically active particles that remain in the vascular space for longer periods and help maintain blood volume and pressure.
Classification of Intravenous Fluids
All IV fluids are solutions consisting of one or more solutes dissolved in water. Most IV fluids contain one or more electrolytes. Dextrose, a naturally occurring form of glucose, is another ingredient present in some fluids. Some fluids also contain the buffers lactate, gluconate, or acetate, which are converted to HCO3− by the liver and help regulate pH. Others contain colloids (large–molecular-weight solutes).
There are many intravenous solutions, each with different solute profiles. These fluids are most commonly classified as either crystalloids or colloids based in the molecular weight of the primary solutes they contain.
Either crystalloids or colloids may also be classified according to the mix and quantity of solutes as replacement or maintenance; balanced or unbalanced; and isotonic, hypotonic, or hypertonic. Replacement fluids have high concentrations of Na+ and Cl− (as ECF does) and are designed to replace fluid losses. Maintenance fluids have lower concentrations of Na+ and Cl− but somewhat more K+ (as total body water does) and are designed to maintain fluid balance over a longer period. Balanced fluids contain a solute profile similar to that of ECF, whereas unbalanced fluids do not. Isotonic fluids have an osmolarity near that of blood plasma (300 mOsm/L). Hypotonic and hypertonic fluids have an osmolarity significantly lower or higher than plasma, respectively.
Crystalloid Solutions
Crystalloid solutions (also referred to as crystalloids) contain water and small–molecular-weight solutes such as electrolytes that pass freely through vascular endothelium. In addition to electrolytes, some crystalloids contain dextrose and alkalinizing agents (buffers). Table 2-8 shows the composition of selected commercially available crystalloid fluids. Other constituents are sometimes added to commercially available crystalloids, including 50% dextrose, potassium, and B-complex vitamins. Crystalloids are routinely used in most anesthetized patients, except those that have low blood protein, low RBC mass, or low platelet count. These solutions are generally appropriate, provided the PCV is 20% or greater and the plasma protein is 3.5 g/dL or greater. There are five basic types of crystalloid solutions.
TABLE 2-8
Solute Composition of Selected Commercially Available Crystalloid Fluids
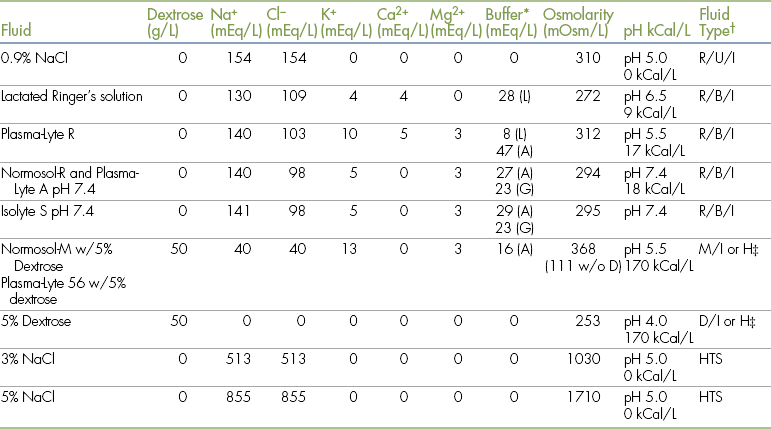
∗Buffers used: A = acetate; G = gluconate; L = lactate.
†Fluid types: B = balanced; D = dextrose solution; H = hypotonic; HTS = hypertonic saline; I = isotonic; M = maintenance; R = replacement; U = unbalanced.
‡Note that because of the rapid metabolism of dextrose, these solutions may be classified as hypotonic.
Modified from Plumb D: Plumb’s veterinary drug handbook, ed 6, Ames, 2008, Blackwell.
Isotonic, Polyionic Replacement Solutions: As the name implies, isotonic, polyionic replacement solutions are balanced solutions that contain several ions (most often sodium, potassium, and chloride and in some cases magnesium and/or calcium) in concentrations that reflect the solute composition of ECF. Lactated Ringer’s solution (LR), Normosol-R (NR), Plasma-Lyte A and R (PA and PR), and Isolyte S (IS) are examples of some commonly used solutions in this class. They all have somewhat similar solute profiles with the following exceptions. Each contains one or two buffers (lactate, gluconate, and/or acetate), but the concentration of buffer differs among these five solutions. PA, PR, NR, and IS contain magnesium, whereas LR does not. LR and PR contain calcium, whereas NR, PA, and IS do not. Because calcium may cause transfused blood to clot, LR and PR may not be administered with blood products.
Isotonic, Polyionic Maintenance Solutions: These crystalloid solutions are designed for maintenance fluid therapy over a longer period of time. Normosol-M in 5% dextrose (NM5) and Plasma-Lyte 56 in 5% dextrose (PL5) are examples in this class. They contain less sodium and chloride and more potassium than the corresponding replacement solutions and consequently more closely reflect the solute composition of total body water. These solutions also contain lower concentrations of buffer than their replacement counterparts and contain dextrose.
Normal Saline Solution: Also called physiologic saline, 0.9% saline, or sodium chloride 0.9%, normal saline solution (NS) contains only sodium and chloride ions in water and is therefore an unbalanced replacement solution. Normal saline is sometimes used instead of an isotonic, polyionic replacement crystalloid and is recommended as the preferred fluid in some specific circumstances (e.g., patients with Addison’s disease and for administering blood transfusions). NS is also used to bathe exposed tissues during surgery, to flush IV catheters (with or without heparin added), and to flush body cavities. Normal saline is somewhat more acidic than polyionic solutions and does not contain any potassium and therefore can cause hypokalemia if not supplemented.
Hypertonic Saline Solutions: Concentrated saline solution (3%, 5%, 7%, and 23.4%) is given with isotonic crystalloids in acute care settings to treat patients with hypovolemic, traumatic, or endotoxic shock. Hypertonic saline rapidly but temporarily draws water into the intravascular space and supports blood pressure, but, like other crystalloids, rapidly diffuses into the interstitial space and so must be followed with colloids if the patient needs long-term blood volume expansion.
These solutions are seldom used during the perioperative period but may be used in special circumstances such as profound hemorrhage when blood products are unavailable, or for patients with increased intracranial pressure or low sodium.
Dextrose Solutions: Five percent dextrose in water is an isotonic solution that contains dextrose as the only solute. Replacement polyionic solutions, LR, NR, PA, PR, half-strength (0.45%) or full-strength (0.9%) saline, or maintenance polyionic solutions containing 2.5% or 5% dextrose are available or may be mixed in house. Once infused, the dextrose in these solutions is rapidly metabolized to CO2 and water, and then most of the remaining plain water diffuses into the interstitial compartment. For this reason, some clinicians classify 5% dextrose in water as hypotonic.
Although dextrose solutions are not used for replacement fluid therapy, they are used for specific purposes. Dextrose solutions with or without electrolytes are used to support blood glucose in neonatal, hypoglycemic, or debilitated patients and in patients with diabetes mellitus that are receiving insulin. They are also used as a part of therapy for hyperkalemia.
Plain 5% dextrose is used to replace the pure water deficit that accompanies simple dehydration or heat stroke. It is not suitable for patients in shock as a result of blood loss, however, because it does not expand the blood volume, and it should not be used as a sole maintenance fluid because it will dilute the electrolytes in body water.
Colloid Solutions
Colloids solutions (also referred to as colloids) contain large–molecular-weight solutes that do not freely diffuse across vascular endothelium and therefore stay in the intravascular space. Colloids are used to support expansion of blood volume and blood pressure. During the perioperative period, colloids are used for patients with blood protein less than 3.5 g/dL. There are two basic types of colloids.
Synthetic Colloid Solutions: Synthetic colloid solutions contain the large–molecular-weight solutes dextran, pentastarch, hetastarch, or gelatin products. Like natural colloids, these solutes tend to remain in the intravascular space, expanding the blood volume. Depending on the agent, 30% to 60% of a synthetic colloid remains in the plasma after 24 hours, and a smaller percentage remains in the plasma for as long as days to weeks after administration. Hetastarch is the most commonly used synthetic colloid in veterinary patients.
Blood Products: Blood products such as plasma and whole blood contain albumin and other natural colloids. During the perioperative period, blood products are used for a variety of indications including anemia, hypoproteinemia, coagulation disorders, and thrombocytopenia. Whole blood or packed RBCs are used to support oxygen-carrying capacity of blood for patients that have profound blood loss. Plasma is primarily used to support expansion of blood volume or treat hypoproteinemia.
Hemoglobin-Based Oxygen Carriers: Hemoglobin-based oxygen-carrying solutions, made by extracting human or bovine hemoglobin from RBCs, are intended as blood substitutes to treat patients with anemia or hemorrhage. They were originally developed for human use on the battlefield. As compared with blood, oxygen carriers have a long shelf life, less intensive storage requirements, and no need for cross-matching or blood typing. The first preparations had significant adverse effects, but over time, techniques were developed to cross-link or polymerize the hemoglobin (bind molecules together or into chains), which increased safety.
Oxyglobin is a proprietary veterinary oxygen carrier containing polymerized bovine hemoglobin in a modified Lactated Ringer’s solution labeled for use in dogs. At the time of the writing of this text, however, Oxyglobin was no longer in production.
Intravenous Fluid Selection and Administration Rates
Fluid therapy is an inexact science. Although there are generally accepted fluid choices and administration rates for animals that are in shock, ill, experiencing blood loss, or undergoing surgery, each patient must be managed in a unique manner appropriate to its condition. So even though these standard rates are used at a starting point, ultimately the doctor will use his or her professional judgment to determine the final rate for each patient.
Isotonic Crystalloids
As mentioned in the previous section, isotonic, polyionic replacement crystalloids including LR, NR, and PR are the first choice for fluid therapy for healthy patients undergoing routine surgery as well as many sick patients as long as the PCV is over 20% and the plasma protein is over 3.5 gm/dL. Consequently these fluids are used in the vast majority of anesthetized patients.
For both small and large animals, a rate of 10 mL/kg/hr during the first hour followed by 5 mL/kg/hr for the remainder of the procedure is an accepted IV administration rate for crystalloids during routine anesthesia and surgery. These rates are safe for most patients. These rates are significantly higher than the volume needed to maintain hydration because they are intended to compensate for the vasodilation, decreased cardiac output, and increase in insensible fluid loss that can occur during anesthesia. (Table 2-9 is a quick reference chart for fluid administration rates during surgery.)
Patients with excessive bleeding or hypotension will need significantly higher rates of administration. Healthy young dogs tolerate an IV infusion rate of 40 mL/kg/hr for a maximum of 1 hour, with half of this given over the first 15 minutes. Cats are more susceptible to overhydration than dogs, and for this reason the infusion rate should not exceed 20 mL/kg/hr. Large animal infusion rates are similar to those used in dogs. Overhydration in these species is unlikely however, because the infusion rate is somewhat limited by the size of the catheter. When blood loss occurs, about 3 mL of fluids must be given for every 1 mL of blood lost. This is because the interstitial fluid compartment is about twice the volume of the intravascular compartment, and, like all crystalloids, these fluids equilibrate throughout the entire ECF compartment. A saturated 3 × 3 or 4 × 4 gauze sponge holds an average of about 3 to 6 or 5 to 10 mL of blood, respectively. Although not a completely reliable measure, these guidelines can be used to estimate approximate blood loss during surgery. In contrast, if a blood transfusion is given, the amount of blood given should approximately equal the amount of blood lost. After this initial infusion, additional volumes of blood must be based on results of laboratory testing.
IV infusion rates for animals in shock are even higher (e.g., up to 90 mL/kg as rapidly as possible for dogs and for large animals, and 55 mL/kg as rapidly as possible for cats). In a clinical setting, for patients with blood loss, hypotension, or shock, a 10- to 20-mL/kg bolus is commonly administered, and the patient is reevaluated. Further boluses are given as necessary. Because of the complexity involved in determining administration rates, the technician should consult with the veterinarian about the optimum rate and should carefully monitor the total volume of fluids being given to the patient during surgery.
Hypertonic Saline
Sometimes 3%, 5%, and 7% hypertonic saline solutions are administered in small volumes in patients with shock and blood loss when blood volume expansion is necessary. The standard rate of administration for 7% hypertonic saline in both large and small animals is 3 to 4 mL/kg slowly over a 5-minute period, followed by administration of isotonic crystalloids. If hypertonic solutions are given too quickly, serious side effects can occur, including hypotension, bradycardia, rapid, shallow breathing, and bronchoconstriction. Therefore the rate of administration must be monitored carefully.
Colloids
Synthetic colloids are administered IV in moderate volumes (10 to 20 mL/kg/day for dogs and for large animals and 5 to 10 mL/kg/day for cats). Because colloids expand blood volume, the infusion rate and total volume of colloids administered must be watched closely to prevent volume overload. These agents can also infrequently cause coagulation disorders and rarely allergic reactions. If given too rapidly, hetastarch can induce nausea and vomiting. Therefore colloids are usually administered to dogs and large animals as a slow bolus over 15 to 60 minutes and to cats as a slow bolus over 30 to 60 minutes. These solutions may also be administered as a CRI of 1 to 2 mL/kg/hr.
Adverse Effects of Fluid Administration
If fluid administration is too rapid, the volume may overwhelm the circulation and cause problems such as pulmonary or cerebral edema. This condition is known as volume overload. Animals weighing less than 5 kg and those with cardiac or renal disease are at greatest risk. For these patients, a slower infusion rate of 3 to 5 mL/kg/hr of crystalloid fluids during surgery may be more appropriate. When monitoring any anesthetized patient that is receiving IV fluids, the anesthetist should be alert for signs of overhydration. These include ocular and nasal discharge, chemosis (edema and swelling of the conjunctiva), subcutaneous edema, increased lung sounds, increased respiratory rate, and dyspnea. When the patient is awake, coughing and restlessness may be seen. Although not commonly done, measurement of central venous pressure (see Chapter 5) allows early detection of overhydration and is recommended for patients receiving more than this amount.
Another potential adverse effect of excessive fluid administration is dilution of the RBCs and plasma proteins, a condition known as hemodilution. Anemic patients, hypoproteinemic patients, and patients that lose a lot of blood from tissue oozing during surgery are particularly at risk. In these patients, fluid therapy should therefore be based on careful monitoring of the packed cell volume (PCV), plasma protein (PP), and physical parameters.
To avoid overhydration, fluids should either be administered using a fluid pump or monitored carefully. In either case, the fluid bag should be labeled with a scale indicating the starting fluid level and anticipated fluid levels by the hour so that the volume of fluid administered can be closely monitored. The use of a burette is advisable in small patients because it allows accurate measurement and administration of small volumes of fluids rather than direct administration from a bag or bottle. See Figure 2-6 for an illustration of an infusion pump, a scale, and a burette.
FIGURE 2-6 Infusion pump, tape scale, and burette. A, Infusion pump. Place the administration set line in the pump as indicated on the owner’s manual. Most pumps require an infusion rate in milliliters per hour, and a total volume to be infused (VTBI) in milliliters. Note that this pump is programmed to deliver 240 mL/hr, and the VTBI is 467 mL. Most pumps will stop and sound an alarm if an occlusion or air bubble is detected in the line. Consequently, infusion pumps must be monitored frequently to ensure proper operation. B, Tape scale used to monitor the fluid administration rate. The tape should be labeled with the infusion rate (250 mL/hr in this case) and the drip rate (1 gtt/sec in this case) and should have lines drawn indicating the expected fluid level each hour. This way, any staff member can determine if the patient has received the proper volume of fluids. C, Burette. Used to administer small volumes of fluids to small, pediatric, or exotic patients. The burette is filled with the total volume of fluids to be infused by opening the clamp between the burette and the fluid bag. When it is filled to the desired level (in this case 100 mL), the upper clamp is closed. The lower clamp is then opened to allow the fluids to flow into the patient at the desired rate.
Calculating Fluid Administration Rates
When fluids are administered, the prescribed rates (in volume/body weight/unit time) listed in the previous section must be converted into a form that will allow the anesthetist to program an infusion pump or set the roller clamp on an infusion set. Conversion requires use of mathematic formulas. Any discussion of fluid rate calculations can be confusing, however, because the terminology of fluid administration is not standard throughout the literature. So to avoid confusion, the discussion that follows will begin with a list of terms and definitions related to fluid administration rate calculations (Box 2-2).
The infusion rate (mL/hr) is the value used to program an IV infusion pump, and the drip rate (gtt/min or gtt/sec) is the value used to adjust the administration set. In order to perform the necessary calculations, the anesthetist must be familiar with the formulas used to determine these rates as well as the delivery rates of available administration sets.
There are two general types of administration sets. Macrodrip sets deliver fluids at a rate of 10 or 15 drops per milliliter and are used to deliver fluids at infusion rates equal to or greater than 100 mL/hr. Microdrip sets deliver fluids at a rate of 60 drops per milliliter and are used for infusion rates less than 100 mL/hr (Figure 2-7).
FIGURE 2-7 Fluid administration set chambers, comparing the size of the drops delivered. A, Macrodrip set chamber (15 gtt/mL). Used for infusion rates ≥100 mL/hr. Compare the size of this drop with B, Microdrip set chamber (60 gtt/mL). Used for infusion rates of <100 mL/hr. C, Macrodrip (15 gtt/mL) and microdrip (60 gtt/mL) fluid administration sets on the left and right, respectively. Note that the delivery rate in gtt/mL is listed on the package.
As an alternative, the appropriate set can be chosen based on patient body weight. Macrodrip sets are appropriate for patients that weigh 10 kg or more, and microdrip sets are appropriate for patients that weigh less than 10 kg. This guideline works well unless delivering fluids at very high rates as for shock therapy, in which case the guideline based on the infusion rate will work better. If the appropriate set is not used, the anesthetist will find it difficult to adjust the drip rate accurately.
Calculation of fluid administration rates may be divided into two basic steps. (1) Use the patient body weight and the prescribed rate to calculate the infusion rate in milliliters per hour. (2) Using the infusion rate, the delivery rate, and conversion factors, calculate the drip rate in drops per minute or some other unit. Box 2-3 and Figure 2-8 summarize the steps required to calculate infusion rates and drip rates during the perioperative period for routine administration during surgery and for treatment of blood loss, hypotension, and shock.
OTHER PREANESTHETIC CARE
In addition to the care summarized on the previous pages, the veterinarian may direct the technician or another staff member to provide additional preoperative nursing care including administration of medications.
Antibiotics are often ordered for animals that have infections or that are scheduled for procedures involving a contaminated area (such as gastrointestinal or dental procedures). Administration of pain medication before painful procedures, a practice known as preemptive analgesia (discussed further in Chapter 7), is proven to be considerably more effective than waiting until the pain is manifest and significantly improves patient recovery.
Some patients may require a variety of other medications such as insulin, anticonvulsants, or antiemetic or antiinflammatory drugs. The technician should actively seek specific instructions from the veterinarian regarding care required, as the well-being of the patient and the outcome of the procedure are significantly influenced by the attention that is given to this final but important facet of patient preparation.
KEY POINTS
1. Effective communication is key to the ability of the veterinary health care team to provide high-quality patient care. The veterinary technician often acts as a liaison between the veterinarian-in-charge (VIC), the client, and other members of the health care team.
2. During the preanesthetic period, the technician has many duties. He or she must help the VIC develop a minimum patient database, ensure that fasting instructions were followed, place an intravenous (IV) catheter, administer fluids, stabilize the patient, prepare equipment, and administer medications.
3. An accurate and complete patient history is at least as important, if not more important, than results of diagnostic tests in shaping patient management. Acquisition of a patient history requires skill in choosing and framing questions.
4. There are many ways in which patient signalment influences response to anesthesia. The anesthetist must consider these factors when managing patients.
5. Dehydration, anemia, abnormal bleeding, respiratory or cardiovascular system disease, kidney or liver dysfunction, and conditions that require treatment while the patient is under anesthesia are physical findings that may influence anesthetic management.
6. Immediately before any procedure, definitively identify and weigh the patient; assess body condition, hydration, level of consciousness, vital signs, and general condition; and determine a pain score.
7. Before any procedure, always present a consent form and fee estimate and acquire signatures.
8. No single anesthetic protocol is ideal for all patients. Rather, the anesthetic techniques and agents used are tailored to the needs of the individual patient. Factors such as previous or concurrent illness, nature of the procedure, urgency, and preference of the veterinarian are all considered in selecting a protocol.
9. Diagnostic tests such as the complete blood cell count (CBC), chemistries, urinalysis, radiography, and electrocardiography may provide valuable information regarding the patient’s ability to tolerate anesthesia.
10. The risk of anesthesia to the patient should be assessed before initiating any procedure. The patient should be assigned to a class on the basis of physical condition, as outlined by the American Society of Anesthesiologists.
11. The patient should be in stable condition, when possible, before being anesthetized. Preexisting problems such as dehydration or shock should be corrected.
12. IV catheter placement in surgery patients gives the anesthetist the ability to safely administer IV anesthetics, provide fluid support, maintain anesthesia via constant rate infusion (CRI), and administer emergency drugs if needed.
13. Knowledge of fluid homeostasis and composition of body fluids enables the anesthetist to administer fluids safely and effectively.
14. Crystalloids may be classified according to the mix and quantity of solutes as replacement or maintenance; balanced or unbalanced; and isotonic, hypotonic, or hypertonic.
15. Crystalloids are routinely used in most anesthetized patients, except those with a plasma protein level less than 3.5 g/dL, a packed cell volume (PCV) less than 20%, or a low platelet count.
16. Colloids and/or blood products are used to support expansion of blood volume, blood pressure, oxygen-carrying capacity, and/or blood coagulation in patients with low plasma protein, PCV, and/or platelet count.
17. Although IV catheterization and fluid administration increase patient safety, the procedure is associated with the risk of accidental overhydration. A fluid infusion rate of 10 mL/kg/hr for the first hour followed by 5 mL/kg/hr for each additional hour is considered safe for most patients.
REVIEW QUESTIONS
1. In gathering a patient history, which of the following would be the best way to frame a question about a patient’s exercise level?
a. “Your dog does not exercise much, does he, Mrs. Jones?”
b. “Does your dog exercise, Mrs. Jones?”
c. “How many times a week does your pet go for a walk or exercise, Mrs. Jones?”
d. “You don’t give your dog as much exercise as you should, do you, Mrs. Jones?”
2. Which of the following examples of species associations is not correct?
a. Horses and cats are more sensitive to opioids than dogs and ruminants.
b. The use of anticholinergics is recommended in ruminants to avoid airway occlusion.
c. Horses may fracture limbs during anesthetic recovery and thus require special attention during the recovery period.
d. Large animals are prone to respiratory depression and dependent atelectasis and thus often require ventilatory support.
3. Which of the following statements regarding physical examination findings is incorrect?
a. Dehydration increases the risk for hypotension.
b. Anemia predisposes the patient to hypoxemia.
c. Patients with bruising may be at higher risk for potentially life-threatening intraoperative and postoperative bleeding.
d. A dog with a body condition score of 8/9 will require more anesthetic per unit body weight than a dog of the same breed with a body condition score of 5/9.
4. You are evaluating a patient’s LOC and find the patient in a sleeplike state, nonresponsive to a verbal stimulus but arousable by a painful stimulus. This patient is:
5. You are evaluating a patient’s hydration. The skin elasticity is somewhat slowed, the patient’s mucous membranes are tacky, the CRT is 1.5 seconds, but the eyes are in a normal position in the orbit. Your patient is:
a. Approximately 5% to 6% dehydrated
b. Approximately 6% to 8% dehydrated
6. Which of the following fasting times is least advisable?
7. Which of the following is not a crystalloid solution?
8. Using the ASA Physical Status Classification system, a patient that is moderately anemic or moderately dehydrated would be classified as:
9. Which of the following signs of disease in a calm canine patient would be most significant in terms of the potential to increase the risk of anesthesia?
10. Which of the following species or breeds must be watched especially closely during any anesthetic procedure to ensure a patent airway?
11. Using the standard fluid infusion rate of 10 mL/kg/hr for the first hour, and a macrodrip administration set with a delivery rate of 15 gtt/mL, a 53-lb patient would require which of the following infusion and drip rates?
12. Which of the following statements regarding IV catheter placement and use in surgery patients is incorrect?
a. Choose an administration set with an injection port.
b. Give all IV drugs slowly unless told otherwise.
c. Always follow IV injections through a catheter with sterile saline flush.
d. Choose a catheter that is small in diameter to minimize the risk of bleeding.
13. Which of the following general guidelines about body fluids in a normal adult animal is incorrect?
a. About 40% of the body weight is water.
b. About two thirds of the total body water is inside the cells.
14. The fluid type most appropriate to replace moderate losses from dehydration would be:
15. Which of the following figures represents a fluid “infusion rate” as defined in this chapter?
16. The delivery rate of a microdrip administration set is:
17. Which of the following statements regarding electrolyte composition of fluids is incorrect?
a. Extracellular fluid contains more sodium than intracellular fluid.
b. Intracellular fluid contains more potassium than intravascular fluid.
c. The osmolarity of intracellular fluid is similar to that of extracellular fluid.
d. Intravascular fluid has more negatively charged particles than positively charged particles.
18. Regarding fluid infusion rates:
a. Standard shock doses of fluids are about the same as doses used during routine surgery.
b. Surgery patients with blood loss may require colloids instead of crystalloids.
c. Crystalloids are generally given at lower administration rates than colloids.
d. Hypertonic saline is administered in large volumes to patients in shock.
19. Which of the following is not a sign of fluid overload?
20. Which of the following heart rhythms is not normal in a resting horse?