The Etiology of Orthodontic Problems
Malocclusion is a developmental condition. In most instances, malocclusion and dentofacial deformity are caused, not by some pathologic process, but by moderate (occasionally severe) distortions of normal development. Occasionally, a single specific cause is apparent, for example, in mandibular deficiency secondary to a childhood fracture of the jaw or the characteristic malocclusion that accompanies some genetic syndromes. More often, these problems result from a complex interaction among multiple factors that influence growth and development, and it is impossible to describe a specific etiologic factor (Figure 5-1).
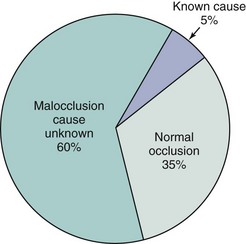
FIGURE 5-1 From a broad perspective, only about one-third of the U.S. population has normal occlusion, while two-thirds have some degree of malocclusion. In the malocclusion group, only a small minority (not more than 5%) have problems attributable to a specific known cause; the remainder are the result of a complex and poorly understood combination of inherited and environmental influences.
Although it is difficult to know the precise cause of most malocclusions, we do know in general what the possibilities are, and these must be considered when treatment is considered. In this chapter, we examine etiologic factors for malocclusion under three major headings: specific causes, hereditary influences, and environmental influences. The chapter concludes with a perspective on the interaction of hereditary and environmental influences in the development of the major types of malocclusion.
Specific Causes of Malocclusion
Disturbances in Embryologic Development
Defects in embryologic development usually result in death of the embryo. As many as 20% of early pregnancies terminate because of lethal embryologic defects, often so early that the mother is not even aware of conception. Although most defects in embryos are of genetic origin, effects from the environment also are important. Chemical and other agents capable of producing embryologic defects if given at the critical time are called teratogens. Most drugs do not interfere with normal development or, at high doses, kill the embryo without producing defects, and therefore are not teratogenic. Teratogens typically cause specific defects if present at low levels but if given in higher doses, do have lethal effects. Teratogens known to produce orthodontic problems are listed in Table 5-1.
TABLE 5-1
Teratogens Affecting Dentofacial Development
| Teratogens | Effect |
| Aminopterin | Anencephaly |
| Aspirin | Cleft lip and palate |
| Cigarette smoke (hypoxia) | Cleft lip and palate |
| Cytomegalovirus | Microcephaly, hydrocephaly, microphthalmia |
| Dilantin | Cleft lip and palate |
| Ethyl alcohol | Central midface deficiency |
| 6-Mercaptopurine | Cleft palate |
| 13-cis Retinoic acid (Accutane) | Similar to craniofacial microsomia and Treacher Collins syndrome |
| Rubella virus | Microphthalmia, cataracts, deafness |
| Thalidomide | Malformations similar to craniofacial microsomia, Treacher Collins syndrome |
| Toxoplasma | Microcephaly, hydrocephaly, microphthalmia |
| X-radiation | Microcephaly |
| Valium | Similar to craniofacial microsomia and Treacher Collins syndrome |
| Vitamin D excess | Premature suture closure |
There are five principal stages in craniofacial development (Table 5-2), and effects on the developing face and jaws can arise during each stage:
TABLE 5-2
Stages of Embryonic Craniofacial Development
| Stage | Time in humans (postfertilization) | Related syndromes |
| Germ layer formation and initial organization of structures | Day 17 | Fetal alcohol syndrome (FAS) |
| Neural tube formation | Days 18-23 | Anencephaly |
| Origin, migration, and interaction of cell populations | Days 19-28 | Craniofacial microsomia Mandibulofacial dysostosis (Treacher Collins syndrome) Limb abnormalities |
| Formation of organ systems Primary palate Secondary palate |
Days 28-38 Days 42-55 |
Cleft lip and/or palate, other facial clefts Cleft palate |
| Final differentiation of tissues | Day 50-birth | Achondroplasia Synostosis syndromes (e.g., Crouzon’s, Apert’s) |
1. Germ layer formation and initial organization of craniofacial structures
2. Neural tube formation and initial formation of the oropharynx
3. Origins, migrations, and interactions of cell populations, especially neural crest cells
4. Formation of organ systems, especially the pharyngeal arches and the primary and secondary palates
5. Final differentiation of tissues (skeletal, muscular, and nervous elements)
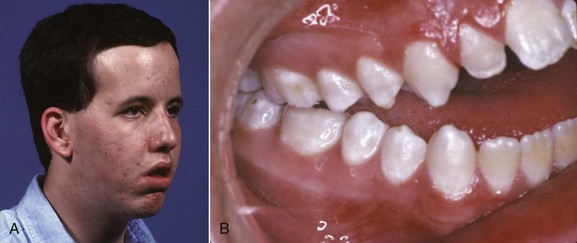
The best example of a problem that can be traced to the very early first and second stages is the characteristic facies of fetal alcohol syndrome (FAS; Figure 5-2). This is due to deficiencies of midline tissue of the neural plate very early in embryonic development caused by exposure to very high levels of ethanol. Although such blood levels can be reached only in extreme intoxication in chronic alcoholics, the resulting facial deformity and developmental delay occur frequently enough to be implicated in many cases of midface deficiency.1 In these unfortunate children, the delay in dental development matches the skeletal delay.2
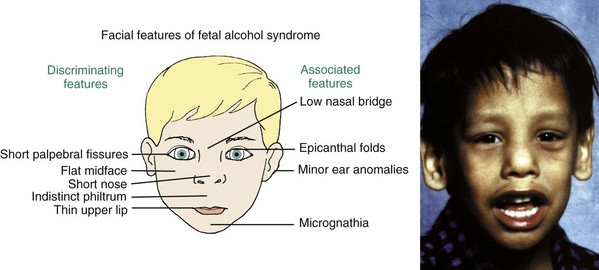
FIGURE 5-2 The characteristic facial appearance of fetal alcohol syndrome (FAS), caused by exposure to very high blood alcohol levels during the first trimester of pregnancy.
Many of the problems that result in craniofacial anomalies arise in the third stage of development and are related to neural crest cell origin and migration. Since most structures of the face are ultimately derived from migrating neural crest cells (Figure 5-3), it is not surprising that interferences with this migration produce facial deformities. At the completion of the migration of the neural crest cells in the fourth week of human embryonic life, they form practically all of the loose mesenchymal tissue in the facial region that lies between the surface ectoderm and the underlying forebrain and eye and most of the mesenchyme in the mandibular arch. Most of the neural crest cells in the facial area later differentiate into skeletal and connective tissues, including the bones of the jaw and the teeth.
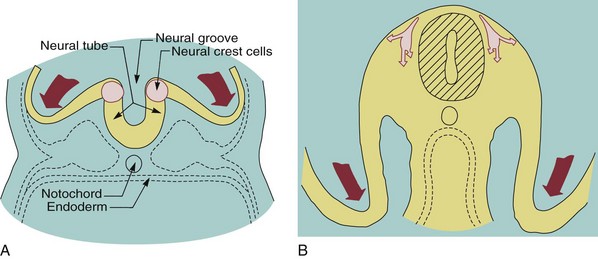
FIGURE 5-3 Diagrammatic lateral sections of embryos at 20 and 24 days, showing formation of the neural folds, neural groove, and neural crest. A, At 20 days, neural crest cells (pink) can be identified at the lips of the deepening neural groove, forerunner of the central nervous system. B, At 24 days, the neural crest cells have separated from the neural tube and are beginning their extensive migration beneath the surface ectoderm. The migration is so extensive and the role of these neural crest cells is so important in formation of structures of the head and face that they can almost be considered a fourth primary germ layer.
The importance of neural crest migration and the possibility of drug-induced impairment of the migration have been demonstrated clearly by unfortunate experience. In the 1960s and 1970s, exposure to thalidomide caused major congenital defects, including facial anomalies in thousands of children. In the 1980s, severe facial malformations related to the anti-acne drug isotretinoin (Accutane) were reported. The similarities in the defects make it likely that both these drugs affect neural crest cells. Retinoic acid plays a crucial role in the ontogenesis of the midface, and recent work suggests that loss of retinoic acid receptor genes affects postmigratory activity of crest cells, clarifying the timing of Accutane effects.3 The danger with isotretinoin is that it affects a developing embryo before the mother knows she is pregnant.
Altered development of cells derived from the neural crest also has been implicated in Treacher Collins syndrome (Figure 5-4), which is characterized by a generalized lack of mesenchymal tissue and now known to be due (at least in some instances) to mutations in a specific gene (TCOF1) that lead to loss of a specific exon.4
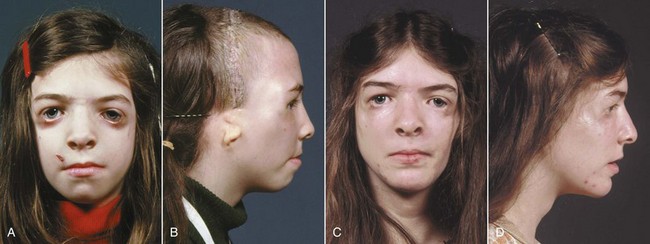
FIGURE 5-4 In the Treacher Collins syndrome (also called mandibulofacial dysostosis), a generalized lack of mesenchymal tissue in the lateral part of the face is the major cause of the characteristic facial appearance. Note the underdevelopment of the lateral orbital and zygomatic areas. The ears also may be affected. Patient at age 12 before (A) and immediately after (B) surgical treatment to advance the midface. Note the ear deformity that usually is concealed by hair. C and D, Age 16. Note the change in the lateral orbital margins.
Craniofacial microsomia (formerly called hemifacial microsomia) is characterized by a lack of development in lateral facial areas. Typically, the external ear is deformed and both the ramus of the mandible and associated soft tissues (muscle, fascia) are deficient or missing (Figure 5-5). Although facial asymmetry is always seen (thus the former name), cranial as well as facial structures are affected. The cause is loss of neural crest cells (for an unknown reason) during migration. Neural crest cells with the longest migration path, those taking a circuitous route to the lateral and lower areas of the face, are most affected, whereas those going to the central face tend to complete their migratory movement, so midline facial defects, including clefts, rarely are part of the syndrome.5

FIGURE 5-5 In craniofacial microsomia, both the external ear and the mandibular ramus are deficient or absent on the affected side. (From Proffit WR, White RP, Sarver DM. Contemporary Treatment of Dentofacial Deformity. St. Louis: Mosby; 2003.)
Neural crest cells migrating to lower regions are important in the formation of the great vessels (aorta, pulmonary artery, aortic arch), and they also are likely to be affected by problems in crest cell migration. For this reason, defects in the great vessels (as in the tetralogy of Fallot) are common in children with craniofacial malformations.
The most common congenital defect involving the face and jaws, second only to clubfoot in the entire spectrum of congenital deformities, is clefting of the lip, palate, or (less commonly) other facial structures. Clefts arise during the fourth developmental stage. Exactly where they appear is determined by the locations at which fusion of the various facial processes failed to occur (Figures 5-6 and 5-7), and this in turn is influenced by the time in embryologic life when some interference with development occurred.
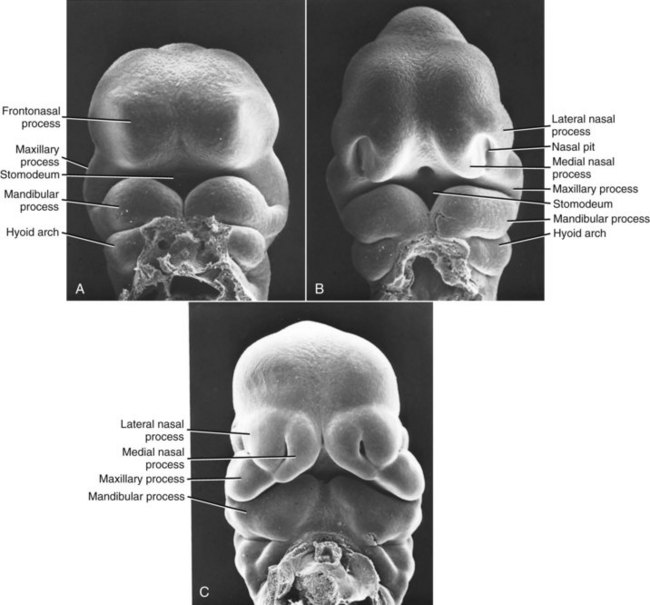
FIGURE 5-6 Scanning electron micrographs of mouse embryos (which are very similar to human embryos early in embryogenesis), showing the stages in facial development. A, Early formation of the face, about 24 days after conception in the human. B, At a stage equivalent to about 31 days in the human, the medial and lateral nasal processes can be recognized alongside the nasal pit. C, Fusion of the median nasal, lateral nasal, and maxillary processes forms the upper lip, and fusion between the maxillary and mandibular processes establishes the width of the mouth opening. This stage is reached at about 36 days in humans. (Courtesy Dr. K. Sulik.)
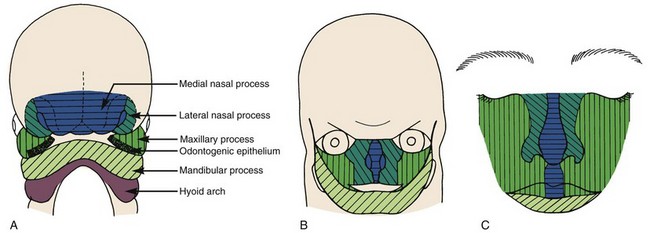
FIGURE 5-7 Schematic representations of fusion of the facial processes. A, Diagrammatic representation of structures at 31 days, when fusion is just beginning. B, Relationships at 35 days, when the fusion process is well advanced. C, Schematic representation of the contribution of the embryonic facial processes to the structures of the adult face. The medial nasal process contributes the central part of the nose and the philtrum of the lip. The lateral nasal process forms the outer parts of the nose, and the maxillary process forms the bulk of the upper lip and the cheeks. (B redrawn from Ten Cate AR. Oral Histology. 3rd ed. St Louis: Mosby; 1989; C redrawn from Sulik KK, Johnston MC. Scan Elect Microsc 1:309-322, 1982.)
Clefting of the lip occurs because of a failure of fusion between the median and lateral nasal processes and the maxillary prominence, which normally occurs in humans during the sixth week of development. At least theoretically, a midline cleft of the upper lip could develop because of a split within the median nasal process, but this almost never occurs. Instead, clefts of the lip occur lateral to the midline on either or both sides (Figure 5-8). Since the fusion of these processes during primary palate formation creates not only the lip but the area of the alveolar ridge containing the central and lateral incisors, it is likely that a notch in the alveolar process will accompany a cleft lip even if there is no cleft of the secondary palate.
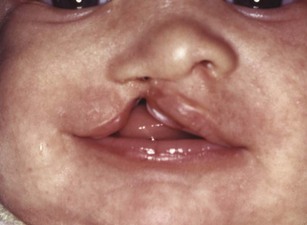
FIGURE 5-8 Unilateral cleft lip in an infant. Note that the cleft is not in the midline but lateral to the midline.
Closure of the secondary palate by elevation of the palatal shelves (Figures 5-9 and 5-10) follows that of the primary palate by nearly 2 weeks, which means that an interference with lip closure that still is present can also affect the palate. About 60% of individuals with a cleft lip also have a palatal cleft (Figure 5-11). An isolated cleft of the secondary palate is the result of a problem that arose after lip closure was completed. Incomplete fusion of the secondary palate produces a notch in its posterior extent (sometimes only a bifid uvula). This indicates a very late-appearing interference with fusion.
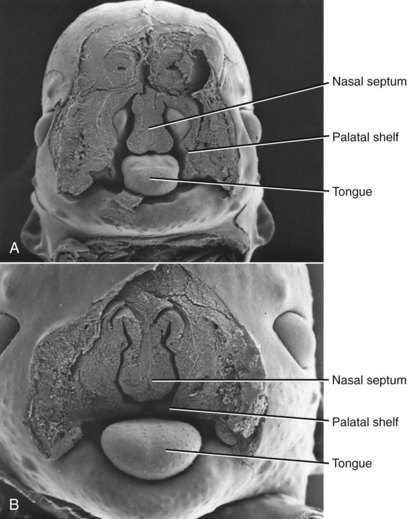
FIGURE 5-9 Scanning electron micrographs of mouse embryos sectioned in the frontal plane. A, Before elevation of the palatal shelves. B, Immediately after depression of the tongue and elevation of the shelves. (Courtesy Dr. K. Sulik.)
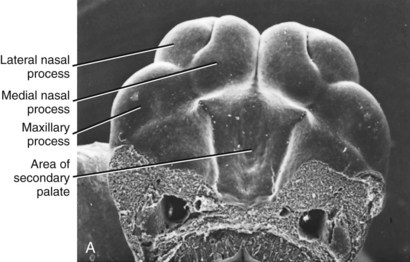
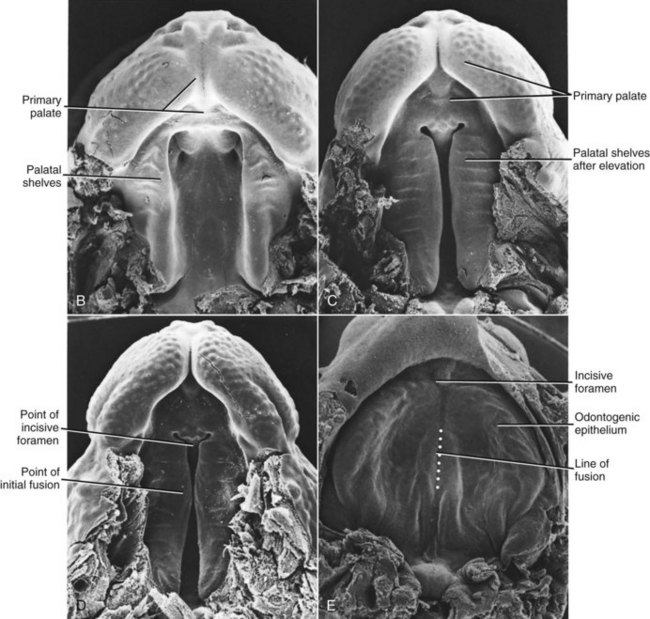
FIGURE 5-10 Scanning electron micrographs of the stages in palate closure (mouse embryos sectioned so that the lower jaw has been removed), analogous to the same stages in human embryos. A, At the completion of primary palate formation. B, Before elevation of the palatal shelves, equivalent to Figure 3-8, A. C, Shelves during elevation. D, Initial fusion of the shelves at a point about one third of the way back along their length. E, Secondary palate immediately after fusion. (Courtesy Dr. K. Sulik.)
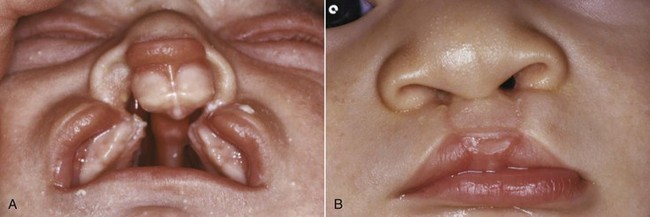
FIGURE 5-11 A, Bilateral cleft lip and palate in an infant. The separation of the premaxilla from the remainder of the maxilla is shown clearly. B, Same child after lip repair.
The width of the mouth is determined by fusion of the maxillary and mandibular processes at their lateral extent, so a failure of fusion in this area could produce an exceptionally wide mouth, or macrostomia. Failure of fusion between the maxillary and lateral processes could produce an obliquely directed cleft of the face. Other patterns of facial clefts are possible, based on the details of fusion, and were classified by Tessier.6 Fortunately, these conditions are rare.
Morphogenetic movements of the tissues are a prominent part of the fourth stage of facial development. As these have become better understood, the way in which clefts of the lip and palate develop has been clarified. For example, it is known now that cigarette smoking by the mother is an etiologic factor in the development of cleft lip and palate,7 and even passive smoke increases the risk of cleft palate.8 An important initial step in development of the primary palate is a forward movement of the lateral nasal process, which positions it so that contact with the median nasal process is possible. The hypoxia associated with smoking probably interferes with this movement.
Another major group of craniofacial malformations arise considerably later than the ones discussed so far, during the final stage of facial development and in the early fetal rather than the embryologic period of prenatal life. These are the craniosynostosis syndromes, which result from early closure of the sutures between the cranial and facial bones. In fetal life, normal cranial and facial development depends on growth adjustments at the sutures in response to growth of the brain and facial soft tissues. Early closure of a suture, called synostosis, leads to characteristic distortions, depending on the location of the early fusion.9
Crouzon’s syndrome is the most frequently occurring member of this group. It is characterized by underdevelopment of the midface and eyes that seem to bulge from their sockets (Figure 5-12). This syndrome arises because of prenatal fusion of the superior and posterior sutures of the maxilla along the wall of the orbit. The premature fusion frequently extends posteriorly into the cranium, producing distortions of the cranial vault as well. If fusion in the orbital area prevents the maxilla from translating downward and forward, the result is severe underdevelopment of the middle third of the face. The characteristic protrusion of the eyes is largely an illusion—the eyes appear to bulge outward because the area beneath them is underdeveloped. There may be a component of true extrusion of the eyes, however, because when cranial sutures become synostosed, intracranial pressure increases.
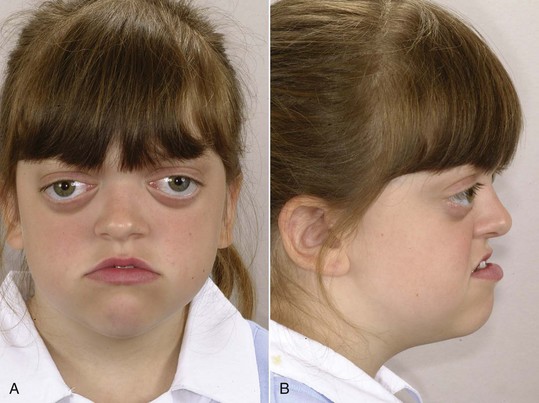
FIGURE 5-12 A and B, Facial appearance in Crouzon’s syndrome of moderate severity, at age 8 years 8 months. Note the wide separation of the eyes (hypertelorism) and deficiency of the midfacial structures, both of which are characteristic of this syndrome. Because of premature suture fusion, forward development of the midface is retarded, which produces the apparent protrusion of the eyes.
Although the characteristic deformity is recognized at birth, the situation worsens as growth disturbances caused by the fused sutures continue postnatally. Surgery to release the sutures is necessary at an early age.
Growth Disturbances in the Fetal and Perinatal Period
Fetal Molding and Birth Injuries
Injuries apparent at birth fall into two major categories: (1) intrauterine molding and (2) trauma to the mandible during the birth process, particularly from the use of forceps in delivery.
Intrauterine Molding: Pressure against the developing face prenatally can lead to distortion of rapidly growing areas. Strictly speaking, this is not a birth injury, but because the effects are noted at birth, it is considered in that category. On rare occasions, an arm is pressed across the face in utero, resulting in severe maxillary deficiency at birth (Figure 5-13). Occasionally, a fetus’ head is flexed tightly against the chest in utero, preventing the mandible from growing forward normally. This is related to a decreased volume of amniotic fluid, which can occur for any of several reasons. The result is an extremely small mandible at birth, usually accompanied by a cleft palate because the restriction on displacement of the mandible forces the tongue upward and prevents normal closure of the palatal shelves.
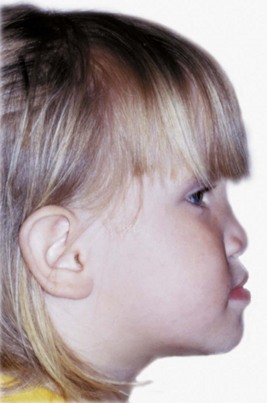
FIGURE 5-13 Midface deficiency in a 3-year-old still apparent though much improved from the severe deficiency that was present at birth because of intrauterine molding. Prior to birth, one arm was pressed across the face. (From Proffit WR, White RP, Sarver DM. Contemporary Treatment of Dentofacial Deformity. St. Louis: Mosby; 2003.)
This extreme mandibular deficiency at birth is termed the Pierre Robin anomalad or sequence. It is not a syndrome that has a defined cause; instead, multiple causes can lead to the same sequence of events that produce the deformity. The reduced volume of the oral cavity can lead to respiratory difficulty at birth, and it may be necessary to perform a tracheostomy so the infant can breathe. Early mandibular advancement via distraction osteogenesis has been used recently in these severely affected infants to provide more space for an airway so that the tracheostomy can be closed.
Because the pressure against the face that caused the growth problem would not be present after birth, there is the possibility of normal growth thereafter and perhaps eventually a complete recovery. Some children with Pierre Robin sequence at birth do have favorable mandibular growth in childhood, but a smaller than normal mandible typically persists (Figure 5-14), and a recent study found no catch-up growth during adolescence.10 It has been estimated that about one-third of the Pierre Robin patients have a defect in cartilage formation and can be said to have Stickler syndrome. Not surprisingly, this group has limited growth potential. Catch-up growth is most likely when the original problem was mechanical growth restriction that no longer existed after birth.
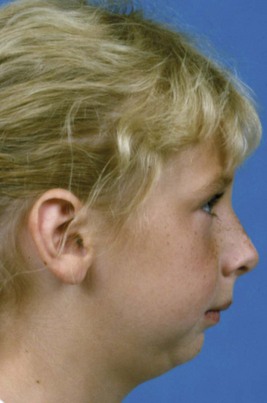
FIGURE 5-14 This girl was diagnosed at birth as having the Pierre Robin sequence, which results in a very small mandible, airway obstruction, and cleft palate. Some children with this condition have enough postnatal mandibular growth to largely correct the jaw deficiency, but the majority do not. At age 9, her mandibular deficiency persists. (From Proffit WR, White RP, Sarver DM. Contemporary Treatment of Dentofacial Deformity. St. Louis: Mosby; 2003.)
Birth Trauma to the Mandible: Many facial deformity patterns now known to result from other causes once were blamed on injuries during birth. Many parents, despite explanations from their doctors, will refer to their child’s facial deformity as being caused by a birth injury even if a congenital syndrome is evident. No matter what the parents say later, a recognizable syndrome obviously did not arise because of birth trauma.
In some difficult births, however, the use of forceps to the head to assist in delivery might damage either or both of the temporomandibular (TM) joints. At least in theory, heavy pressure in the area of the TM joints could cause internal hemorrhage, loss of tissue, and a subsequent underdevelopment of the mandible. At one time, this was a common explanation for mandibular deficiency. If the cartilage of the mandibular condyle were an important growth center, of course, the risk from damage to a presumably critical area would seem much greater. In light of the contemporary understanding that the condylar cartilage is not critical for proper growth of the mandible, it is not as easy to blame underdevelopment of the mandible on birth injuries. Children with deformities involving the mandible are much more likely to have a congenital syndrome.
Progressive Deformities in Childhood
A progressive deformity is one that steadily becomes worse, which, of course, indicates early treatment. These problems, fortunately, arise much less frequently than the severe but stable deformities that comprise most of the jaw problems encountered in children.
Childhood Fractures of the Jaw
In the frequent falls and impacts of childhood, the condylar neck of the mandible is particularly vulnerable, and fractures of this area in childhood are relatively common. Fortunately, the condylar process tends to regenerate well after early fractures. The best human data suggest that about 75% of children with early fractures of the mandibular condylar process have normal mandibular growth afterward and therefore do not develop malocclusions that they would not have had in the absence of such trauma. Unilateral condylar fracture is much more frequent than bilateral fractures. It seems to be relatively common for a child to crash the bicycle, chip a tooth and fracture a condyle, cry a bit, and then continue to develop normally, complete with total regeneration of the condyle. Often, the diagnosis of condylar fracture was never made.
When a problem does arise following condylar fracture, it usually is asymmetric growth deficiency, with the injured side (or, in bilateral fractures, the more severely injured side) lagging behind (Figure 5-15). After an injury, if there is enough scarring around the TM joint to restrict translation of the condyle, so that the mandible cannot be pulled forward as much as the rest of the growing face, subsequent growth will be restricted.
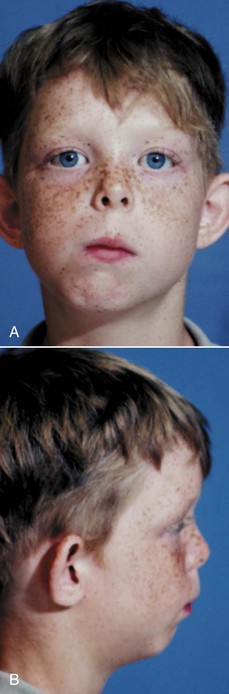
FIGURE 5-15 Mandibular asymmetry in an 8-year-old boy caused by deficient growth on the affected side after fracture of the left condylar process, probably at age 2. For this patient, growth was normal despite the complete loss of the mandibular condyle until age 6, when an attachment of the condylar process to the underside of the zygomatic arch on the injured side began to restrict growth; then facial asymmetry developed rapidly. (From Proffit WR, White RP, Sarver DM. Contemporary Treatment of Dentofacial Deformity. St. Louis: Mosby; 2003.)
This concept is highly relevant to the management of condylar fractures in children. It suggests, and clinical experience confirms, that there would be little if any advantage from surgical open reduction of a condylar fracture in a child. The additional scarring produced by surgery could make things worse. The best therapy therefore is conservative management at the time of injury and early mobilization of the jaw to minimize any restriction on movement. If deficient growth is observed, however, early treatment is needed (see Chapter 12).
Although an old condylar fracture is the most likely cause of asymmetric mandibular deficiency in a child, other destructive processes that involve the TM joint, such as rheumatoid arthritis (Figure 5-16), or a congenital absence of tissue as in craniofacial microsomia also can produce this problem.
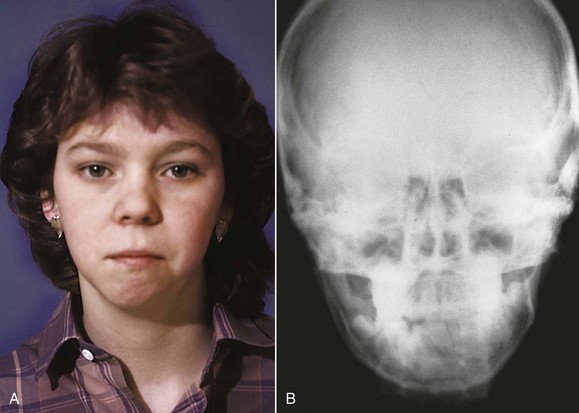
FIGURE 5-16 Rheumatoid arthritis is an uncommon cause of facial asymmetry, but in the polyarticular form of the disease (multiple joints affected), the temporomandibular (TM) joints often are involved, and asymmetry may develop as one side is affected more than the other. A, Facial appearance age 12, 2 years after the diagnosis of polyarticular rheumatoid arthritis. B, Posteroanterior (P-A) cephalometric radiograph, age 12. Note the jaw asymmetry.
Muscle Dysfunction
The facial muscles can affect jaw growth in two ways. First, the formation of bone at the point of muscle attachments depends on the activity of the muscle; second, the musculature is an important part of the total soft tissue matrix whose growth normally carries the jaws downward and forward. Loss of part of the musculature is most likely to result from damage to the motor nerve (muscle atrophies when its motor nerve supply is lost). The result would be underdevelopment of that part of the face, with a deficiency of both soft and hard tissues (Figure 5-17).
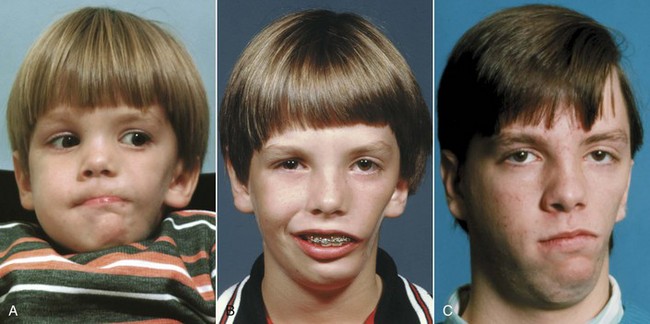
FIGURE 5-17 Facial asymmetry in an 11-year-old boy whose masseter muscle was largely missing on the left side. The muscle is an important part of the total soft tissue matrix; in its absence growth of the mandible in the affected area also is deficient. A, Age 4. B, Age 11. C, Age 17 after surgery to advance the mandible more on the left than right side. The soft tissue deficiency from the missing musculature on the left side still is evident.
Excessive muscle contraction can restrict growth in much the same way as scarring after an injury. This effect is seen most clearly in torticollis, a twisting of the head caused by excessive tonic contraction of the neck muscles on one side (primarily the sternocleidomastoid) (Figure 5-18). The result is a facial asymmetry because of growth restriction on the affected side, which can be quite severe unless the contracted neck muscles are surgically detached at an early age.11 Conversely, a major decrease in tonic muscle activity (as in muscular dystrophy, some forms of cerebral palsy, and various muscle weakness syndromes) allows the mandible to drop downward away from the rest of the facial skeleton. The result is increased anterior face height, distortion of facial proportions and mandibular form, excessive eruption of the posterior teeth, narrowing of the maxillary arch, and anterior open bite (Figure 5-19).12
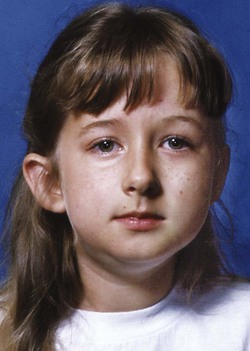
FIGURE 5-18 Facial asymmetry in a 6-year-old girl with torticollis. Excessive muscle contraction can restrict growth in a way analogous to scarring after an injury. Despite surgical release of the contracted neck muscles at age 1, moderate facial asymmetry developed in this case, and a second surgical release of the left sternocleidomastoid muscle was performed at age 7. Note that the asymmetry reflects deficient growth of the entire left side of the face, not just the mandible.
Disturbances Arising in Adolescence or Early Adult Life
Occasionally, unilateral excessive growth of the mandible occurs in individuals who seem metabolically normal. Why this occurs is entirely unknown. It is most likely in girls between the ages of 15 and 20 but may occur as early as age 10 or as late as the early 30s in either sex. The condition formerly was called condylar hyperplasia, and proliferation of the condylar cartilage is a prominent aspect; however, because the body of the mandible also is affected (Figure 5-20), hemimandibular hypertrophy now is considered a more accurate descriptive term.13 The excessive growth may stop spontaneously, but in severe cases removal of the affected condyle and reconstruction of the area are necessary.
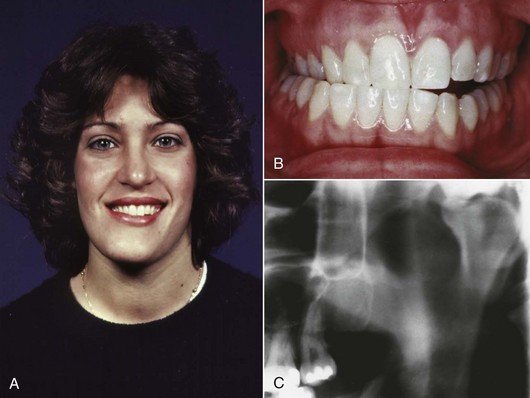
FIGURE 5-20 A, Facial asymmetry in this 21-year-old woman developed gradually in her late teens, after orthodontic treatment for dental crowding during which there was no sign of jaw asymmetry, due to excessive growth of the mandible on the right side. B, The dental occlusion shows an open bite on the affected right side, reflecting the vertical component of the excessive growth. C, Note the grossly enlarged mandibular condyle on the right side. Why this type of excessive but histologically normal growth occurs and why it is seen predominantly in females is unknown.
In acromegaly, which is caused by an anterior pituitary tumor that secretes excessive amounts of growth hormone, excessive growth of the mandible may occur, creating a skeletal Class III malocclusion in adult life (Figure 5-21). Often (but not always—sometimes the mandible is unaffected while hands and/or feet grow), mandibular growth accelerates again to the levels seen in the adolescent growth spurt, years after adolescent growth was completed.14 The condylar cartilage proliferates, but it is difficult to be sure whether this is the cause of the mandibular growth or merely accompanies it. Although the excessive growth stops when the tumor is removed or irradiated, the skeletal deformity persists and orthognathic surgery to reposition the mandible is likely to be necessary (see Chapter 19).
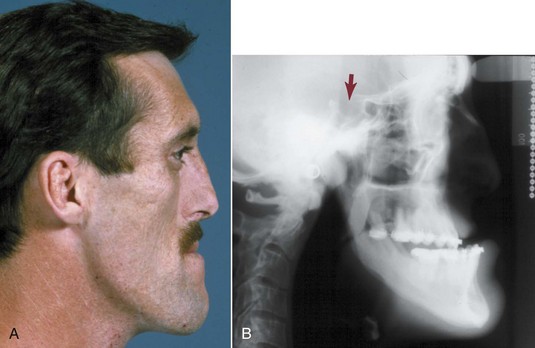
FIGURE 5-21 Profile view (A) and cephalometric radiograph (B) of a 32-year-old man with acromegaly, which was diagnosed 3 years previously when he went to a dentist because his lower jaw was moving forward. After irradiation of the anterior pituitary area, growth hormone levels dropped and mandibular growth ceased. Note the enlargement of sella turcica and loss of bony definition of its bony outline, reflecting the secretory tumor in that location. (From Proffit WR, White RP, Sarver DM. Contemporary Treatment of Dentofacial Deformity. St. Louis: Mosby; 2003.)
Disturbances of Dental Development
Most disturbances of dental development are contributors to isolated Class I malocclusion, and these conditions (such as drift of permanent teeth after early loss of primary teeth) are discussed in Chapter 11. Dental problems that are related to larger congenital or health problems include:
Congenitally Missing Teeth
Congenital absence of teeth results from disturbances during the initial stages of formation of a tooth—initiation and proliferation. Anodontia, the total absence of teeth, is the extreme form. The term oligodontia refers to congenital absence of many but not all teeth, whereas the rarely used term hypodontia implies the absence of only a few teeth. Since the primary tooth buds give rise to the permanent tooth buds, there will be no permanent tooth if its primary predecessor was missing. It is possible, however, for the primary teeth to be present and for some or all the permanent teeth to be absent.
Anodontia and oligodontia are usually associated with a systemic abnormality, ectodermal dysplasia. Individuals with ectodermal dysplasia have thin, sparse hair and an absence of sweat glands in addition to their characteristically missing teeth (Figure 5-22). Occasionally, oligodontia occurs in a patient with no apparent systemic problem or congenital syndrome. In these children, it appears as if there is a random pattern to the missing teeth.
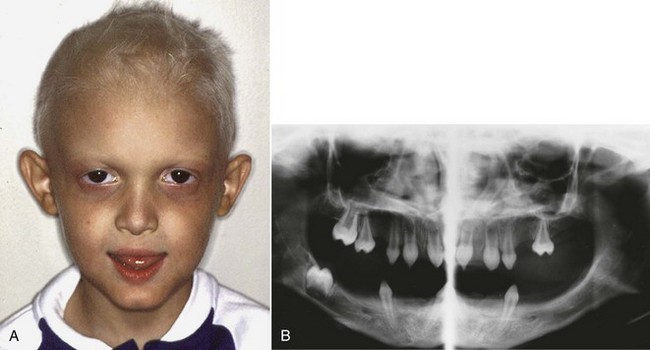
FIGURE 5-22 A, A child with ectodermal dysplasia, in addition to the characteristic thin and light-colored hair, is likely to have an overclosed appearance because of lack of development of the alveolar processes. B, Panoramic radiograph of the same boy, showing the multiple missing teeth. When this many teeth are congenitally missing, ectodermal dysplasia is the most likely cause.
Anodontia and oligodontia are rare, but hypodontia is a relatively common finding. It appears that a polygenic multifactorial model of etiology is the best explanation of etiology. As a general rule, if only one or a few teeth are missing, the absent tooth will be the most distal tooth of any given type. If a molar tooth is congenitally missing, it is almost always the third molar; if an incisor is missing, it is nearly always the lateral; if a premolar is missing, it almost always is the second rather than the first. Rarely is a canine the only missing tooth.
Malformed and Supernumerary Teeth
Abnormalities in tooth size and shape result from disturbances during the morphodifferentiation stage of development, perhaps with some carryover from the histodifferentiation stage. The most common abnormality is a variation in size, particularly of maxillary lateral incisors (Figure 5-23) and second premolars. About 5% of the total population have a significant “tooth size discrepancy” because of disproportionate sizes of the upper and lower teeth. Unless the teeth are matched for size, normal occlusion is impossible. As might be expected, the most variable teeth, the maxillary lateral incisors, are the major culprits.
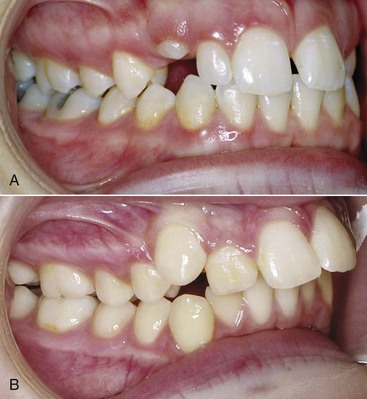
FIGURE 5-23 Disproportionately small (A) or large (B) maxillary lateral incisors are relatively common. This creates a tooth-size discrepancy that makes normal alignment and occlusion almost impossible. It is easier to build up small laterals than reduce the size of large ones, because dentin is likely to be exposed interproximally after more than 1 to 2 mm in width reduction.
Supernumerary or extra teeth also result from disturbances during the initiation and proliferation stages of dental development. The most common supernumerary tooth appears in the maxillary midline and is called a mesiodens. Supernumerary lateral incisors also occur; extra premolars occasionally appear; a few patients have fourth, as well as third, molars. The presence of an extra tooth obviously has great potential to disrupt normal occlusal development (Figure 5-24), and early intervention to remove it is usually required to obtain reasonable alignment and occlusal relationships. Multiple supernumerary teeth are most often seen in the congenital syndrome of cleidocranial dysplasia (see Figure 3-15), which is characterized by missing clavicles (collar bones), many supernumerary and unerupted teeth, and failure of the succedaneous teeth to erupt.
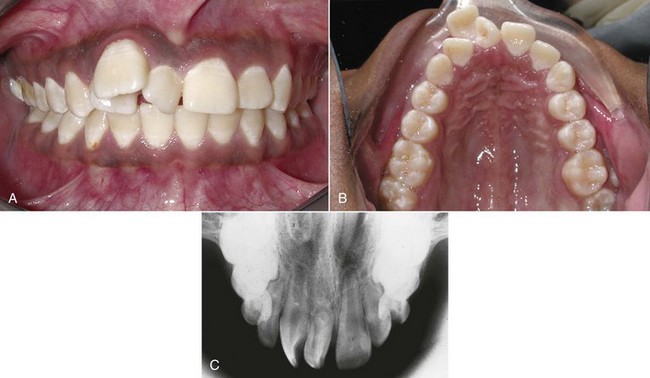
FIGURE 5-24 A to C, The maxillary midline is the most common location for a supernumerary tooth, often called a mesiodens because of its location. It can be of almost any shape. The supernumerary may block the eruption of one or both the central incisors or, as in this girl, may separate them widely and also displace the lateral incisors.
Traumatic Displacement of Teeth
Almost all children fall and hit their teeth during their formative years. When trauma to a primary tooth displaces the permanent tooth bud underlying it, there are two possible results. First, if the trauma occurs while the crown of the permanent tooth is forming, enamel formation will be disturbed and there will be a defect in the crown of the permanent tooth.
Second, if the trauma occurs after the crown is complete, the crown may be displaced relative to the root. Root formation may stop, leaving a permanently shortened root. More frequently, root formation continues, but the remaining portion of the root then forms at an angle to the traumatically displaced crown (Figure 5-25). This distortion of root form is called dilaceration, which is defined as a distorted root form.
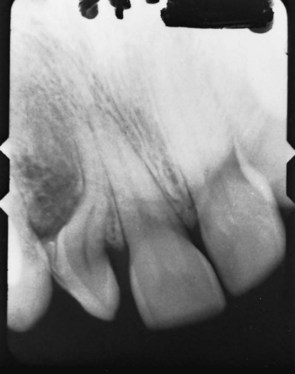
FIGURE 5-25 Distortion of the root (termed dilaceration) of this lateral incisor resulted from trauma at an earlier age that displaced the crown relative to the forming root. This is a more severe dilaceration than what is usually observed (see Figure 3-17), but even in this child, the tooth erupted—dilaceration does not prevent eruption.
If distortion of root position is severe enough, it is almost impossible for the crown to assume its proper position—that might require the root to extend out through the alveolar bone. For this reason, it may be necessary to extract a severely dilacerated tooth. Traumatically displaced permanent teeth in children should be repositioned as early as possible (see Chapter 12). Immediately after the accident, an intact tooth usually can be moved back to its original position rapidly and easily. After healing (which takes 2 to 3 weeks), it is difficult to reposition the tooth, and ankylosis may develop that makes it impossible.
Genetic Influences
A strong influence of heredity on facial features is obvious at a glance—it is easy to recognize familial tendencies in the tilt of the nose, the shape of the jaw, and the look of the smile. Certain types of malocclusion run in families. The Hapsburg jaw, the prognathic mandible of this European royal family, is the best known example (Figure 5-26), but dentists routinely see repeated instances of similar malocclusions in parents and their offspring. The pertinent question for the etiology of malocclusion is not whether there are inherited influences on the jaws and teeth, because obviously there are, but whether different types of malocclusion can be directly caused by inherited characteristics.

FIGURE 5-26 Mandibular prognathism in the Hapsburg family became known as the Hapsburg jaw as it recurred over multiple generations in European royalty and was recorded in many portraits. A, Phillip II and Prince Ferdinand, 1575 (Titian). B, Phillip IV, 1638 (Velasquez). C, Charles IV and family, 1800 (Goya). C, Note the strong lower jaw in baby, father, and grandmother but not in mother.
For much of the twentieth century, thoughts about how malocclusion could be produced by inherited characteristics focused on two major possibilities. The first would be an inherited disproportion between the size of the teeth and the size of the jaws, which would produce crowding or spacing. The second would be an inherited disproportion between the size or shape of the upper and lower jaws, which would cause improper occlusal relationships. The more independently these characteristics are determined, the more likely that disproportions could be inherited. Could a child inherit relatively large teeth but a jaw too small to accommodate them, for instance, or a large upper jaw and a small lower one? That would be quite possible if jaw and tooth sizes were inherited independently, but if dentofacial characteristics tended to be linked, an inherited mismatch of this type would be unlikely.
Primitive human populations in which malocclusion is less frequent than in modern groups are characterized by genetic isolation and uniformity. If everyone in a group carried the same genetic information for tooth size and jaw size, there would be no possibility of a child inheriting discordant characteristics. In the absence of processed food, one would expect strong selection pressure for traits that produced good masticatory function. Genes that introduced disturbances into the masticatory system would tend to be eliminated from the population (unless they conferred some other advantage). The result should be exactly what is seen in primitive populations: individuals in whom tooth size–jaw size discrepancies are infrequent, and groups in which everyone tends to have the same jaw relationship (not necessarily one that produces ideal dental occlusion). Different human groups have developed impressive variations in facial proportions and jaw relationships. What happens, then, when there is outbreeding between originally distinct human population groups?
One of the characteristics of civilization is the collection of large groups of people into urban centers, where the opportunities for mating outside one’s own small population group are greatly magnified. If inherited disproportions of the functional components of the face and jaws were frequent, one would predict that modern urban populations would have a high prevalence of malocclusion and a great variety of orthodontic problems. The United States, reflecting its role as a “genetic melting pot,” should have one of the world’s highest rates of malocclusion, which it does. In the 1930s and 1940s, as knowledge of the new science of genetics developed, it was tempting to conclude that the great increase in outbreeding that occurred as human populations grew and became more mobile was the major explanation for the increase in malocclusion in recent centuries.
This view of malocclusion as primarily a genetic problem was greatly strengthened by breeding experiments with animals carried out in the 1930s. By far the most influential individual in this regard was Professor Stockard, who methodically crossbred dogs and recorded the interesting effects on body structure (Figure 5-27).15 Present-day dogs, of course, come in a tremendous variety of breeds and sizes. What would happen if one crossed a Boston terrier with a collie? Might the offspring have the collie’s long, pointed lower jaw and the terrier’s diminutive upper jaw? Could unusual crowding or spacing result because the teeth of one breed were combined in the offspring with the jaw of the other? Stockard’s experiments indicated that dramatic malocclusions did occur in his crossbred dogs, more from jaw discrepancies than from tooth size–jaw size imbalances. These experiments seemed to confirm that independent inheritance of facial characteristics could be a major cause of malocclusion and that the rapid increase in malocclusion accompanying urbanization was probably the result of increased outbreeding.
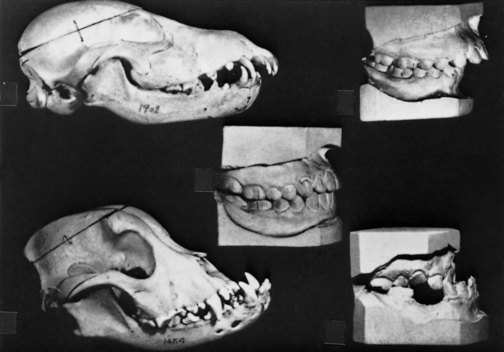
FIGURE 5-27 In breeding experiments with dogs in the 1930s, Professor Stockard demonstrated that severe malocclusions could be developed by crossing morphologically different breeds. His analogy to human malocclusion was a powerful influence in the rejection of the prevailing belief of the 1920s that improper jaw function caused malocclusion. (From Stockard CR, Johnson AL. Genetic and Endocrinic Basis for Differences in Form and Behavior. Philadelphia: The Wistar Institute of Anatomy and Biology; 1941.)
These dog experiments turned out to be misleading, however, because many breeds of small dogs carry the gene for achondroplasia. Animals or humans affected by this condition have deficient growth of cartilage. The result is extremely short extremities and an underdeveloped midface. The dachshund is the classic achondroplastic dog, but most terriers and bulldogs also carry this gene. Achondroplasia is an autosomal dominant trait. Like many dominant genes, the gene for achondroplasia shows variable expressivity, meaning simply that the trait will be expressed more dramatically in some individuals than in others. Most of the unusual malocclusions produced in Stockard’s breeding experiments can be explained not on the basis of inherited jaw size but by the extent to which achondroplasia was expressed in that animal.
Achondroplasia is rare in humans, but it does occur and produces the expected changes (Figure 5-28). In addition to short limbs, the cranial base does not lengthen normally because of the deficient growth at the synchondroses, the maxilla is not translated forward to the normal extent, and a relative midface deficiency occurs. In a number of relatively rare genetic syndromes like achondroplasia, influences on the form of the face, jaws, and teeth can be discerned, but those cause only a fraction of 1% of orthodontic problems.

FIGURE 5-28 In this 14-year-old girl with moderately severe achondroplasia, note the deficient midface, particularly at the bridge of the nose. This results from decreased growth of cartilage in the cranial base, with a resulting lack of forward translation of the maxilla. (From Proffit WR, White RP, Sarver DM. Contemporary Treatment of Dentofacial Deformity. St. Louis: Mosby; 2003.)
A careful examination of the results of outbreeding in human populations also casts doubt on the hypothesis that independently inherited tooth and jaw characteristics are a major cause of malocclusion. The best data are from investigations carried out in Hawaii by Chung et al.16 Before its discovery by the European explorers of the eighteenth century, Hawaii had a homogeneous Polynesian population. Large scale migration to the islands from Europe, China, and Japan, as well as the arrival of smaller numbers of other racial and ethnic groups, resulted in an exceptionally heterogeneous modern population. Tooth size, jaw size, and jaw proportions were all rather different for the Polynesian, Asian, and European contributors to the Hawaiian melting pot. Therefore, if tooth and jaw characteristics were inherited independently, a high prevalence of severe malocclusion would be expected in this population.
The prevalence and types of malocclusion in the current Hawaiian population, though greater than the prevalence of malocclusion in the original population, do not support this concept. The effects of interracial crosses appear to be more additive than multiplicative. For example, about 10% of the Chinese who migrated to Hawaii had Class III malocclusion, whereas about 10% of the Polynesians had crowded teeth. The offspring of this cross seem to have about a 10% prevalence of each characteristic, but there is no evidence of dramatic facial deformities like those seen in the crossbred dogs. In other words, if malocclusion or a tendency to malocclusion is inherited, the mechanism is not the independent inheritance of discrete morphologic characteristics like tooth and jaw sizes.
The classic way to determine to what extent a characteristic is determined by inheritance is to compare monozygotic (identical) with dizygotic (fraternal) twins. Monozygotic twins occur because of the early division of a fertilized egg, so each individual has the same chromosomal DNA and the two are genetically identical. Any differences between them should be solely the result of environmental influences. Twins also occur when two eggs are released at the same time and fertilized by different spermatozoa. These dizygotic twins are not more similar than ordinary siblings except that they have shared the same intrauterine and family environment. By comparing identical twins, fraternal twins, and ordinary siblings, the proportion of the variability in that characteristic due to heredity can be estimated.
Studies of this type are limited in several ways. Not only is it difficult to obtain the twin pairs for study, but also it can be difficult to establish zygosity and confirm that the environments were in fact the same for both members of a twin pair. Nevertheless, well-done twin studies are the best way to evaluate heritability.17 Using twins with siblings as controls, Hughes et al reported that the hereditary component for variations in spacing and tooth position within the dental arches was 69% to 89%. It was 53% for overbite, but only 28% for overjet (which therefore appears to have a greater environmental component than crowding/spacing or overbite).18 Corruccini and coworkers19 have argued that with appropriate corrections for unsuspected environmental differences within twin pairs, the heritability for some dental characteristics such as overjet is almost zero.
The other classic method of estimating the influence of heredity is to study family members, observing similarities and differences between mother–child, father–child, and sibling pairs. From an examination of longitudinal cephalometric radiographs and dental casts of siblings who participated in the Bolton-Brush growth study, Harris and Johnson20 concluded that the heritability of craniofacial (skeletal) characteristics was relatively high but that of dental (occlusal) characteristics was low. For skeletal characteristics, the heritability estimates increased with increasing age; for dental characteristics, the heritability estimates decreased, indicating an increasing environmental contribution to the dental variation. These findings were confirmed and extended in a more recent study of heritability in Icelandic families.21 To the extent that the facial skeleton determines the characteristics of a malocclusion, therefore, a hereditary component is likely to be present. When parent–child correlations are used to assist in predicting facial growth, errors are reduced, which in itself strongly indicates the hereditary influence on these dimensions.22 Purely dental variation, however, seems to be much more environmentally determined.
As was noted in European royal families (see Figure 5-26), the influence of inherited tendencies is particularly strong for mandibular prognathism. In a recent study of 55 families in Brazil with over 2000 individuals, the heritability of mandibular prognathism was estimated to be 0.316. The majority of the pedigrees suggested autosomal dominant inheritance with incomplete penetration, and the investigators concluded that there is a major gene that influences the expression of mandibular prognathism.23 It is apparent that what we call Class III malocclusion really is a group of different phenotypes, and determining the heritability of these phenotypes is a necessary step toward unraveling the genetics of Class III problems.24
The long-face pattern of facial deformity seems to be the second most likely type of deformity to run in families. In general, similar malocclusions are likely to be seen in siblings, especially if the malocclusion is severe, perhaps because their genetically influenced facial types and growth patterns lead to similar responses to environmental factors. Knowing the type of growth associated with different genetic patterns could help greatly with both the type and timing of orthodontic and surgical treatment.
The extent to which other types of malocclusion are related to genetic influences is less clear. If dental variations that contribute to malocclusion are not tightly linked to gene expression, a condition like open bite could be largely due to external influences, for example, sucking habits or tongue posture. Let us now examine the role of the environment in the etiology of malocclusion.
Environmental Influences
Environmental influences during the growth and development of the face, jaws, and teeth consist largely of pressures and forces related to physiologic activity. A relationship between anatomic form and physiologic function is apparent in all animals. Over evolutionary time, adaptations in the jaws and dental apparatus are prominent in the fossil record. Form–function relationships at this level are controlled genetically and, though important for a general understanding of the human condition, have little to do with any individual’s deviation from the current norm.
On the other hand, there is every reason to suspect that form–function relationships during the lifetime of an individual may be significant in the development of malocclusion. Although the changes in body form are minimal, an individual who does heavy physical work has both heavier and stronger muscles and a sturdier skeletal system than one who is sedentary. If function could affect the growth of the jaws, altered function would be a major cause of malocclusion, and it would be logical for chewing exercises and other forms of physical therapy to be an important part of orthodontic treatment. But if function makes little or no difference in the individual’s pattern of development, altering his or her jaw function would have little if any impact, etiologically or therapeutically. Because of its importance in contemporary orthodontics, particular emphasis is placed here on evaluating potential functional contributions to the etiology of malocclusion and to possible relapse after treatment.
Equilibrium Considerations
The laws of physics state that an object subjected to unequal forces will be accelerated and thereby will move to a different position in space. It follows that if any object is subjected to a set of forces but remains in the same position, any forces must be in balance or equilibrium. From this perspective, the dentition is obviously in equilibrium, since the teeth are subjected to a variety of forces but do not move to a new location under usual circumstances.
The effectiveness of orthodontic treatment is itself a demonstration that forces on the dentition are normally in equilibrium. Teeth normally experience forces from masticatory effort, swallowing, and speaking but do not move. If a tooth is subjected to a continuous force from an orthodontic appliance, it does move, so the force applied by the orthodontist has altered the previous equilibrium. The nature of the forces necessary for tooth movement is discussed in detail in Chapter 8, but at this point, we must briefly preview what is known about force magnitude and force duration in producing changes in tooth position.
A key consideration is that the supporting structures of the dentition (periodontal ligament [PDL] and alveolar bone) are constructed to withstand heavy forces of short duration such as those from mastication. During mastication, the fluid in the PDL space acts as a shock absorber, so that the soft tissues in the PDL are not compressed although bending of alveolar bone occurs. Only if pressure is maintained long enough to squeeze out the fluid (a few seconds) is there an impact on the soft tissues. Then, since that hurts, the pressure is released and the fluid returns before the next chewing stroke. The result is that only light force of long duration (6 hours or so per day) is important in determining whether there is enough of an imbalance of forces to lead to tooth movement, which means if the balance between tongue versus lip/cheek pressure changes, tooth movement would be expected.
It is easy to demonstrate that this is indeed the case. For example, if an injury to the soft tissue of the lip results in scarring and contracture, the incisors in this vicinity will be moved lingually as the lip tightens against them (Figure 5-29). On the other hand, if restraining pressure by the lip or cheek is removed, the teeth move outward in response to unopposed pressure from the tongue (Figure 5-30, A). Pressure from the tongue, whether from an enlargement of the tongue from a tumor or other source or because its posture has changed, will result in labial displacement of the teeth even though the lips and cheeks are intact because the equilibrium is altered (Figure 5-30, B).
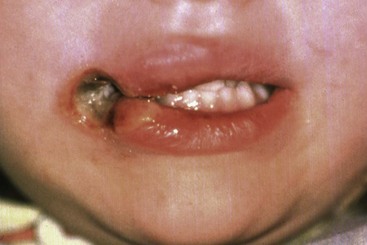
FIGURE 5-29 Scarring of the corner of the mouth in this child will occur as the burn from biting an electrical cord heals. From equilibrium theory, one would expect a distortion in the form of the dental arch in the region of the contracting scar, and exactly this occurs after an injury of this type.
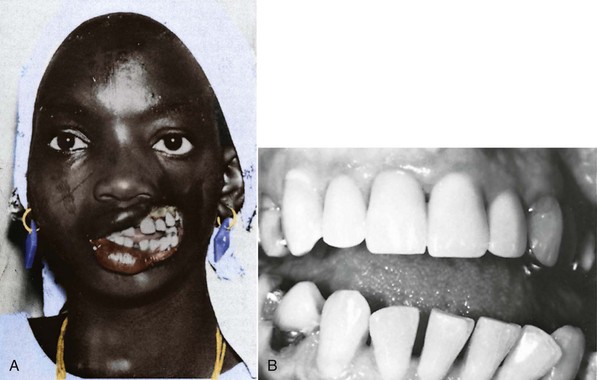
FIGURE 5-30 A, In this individual, a large part of the cheek was lost because of a tropical infection. Note the outward splaying of the teeth on the affected side after the restraining force of the cheek was lost. B, After a paralytic stroke, this patient’s tongue rested against the mandibular posterior teeth. Before the stroke, the occlusion was normal. In this patient, an outward splaying of the teeth occurred on the affected side because of the increase in resting tongue pressure. (A courtesy Professor J.P. Moss; B courtesy Dr. T. Wallen.)
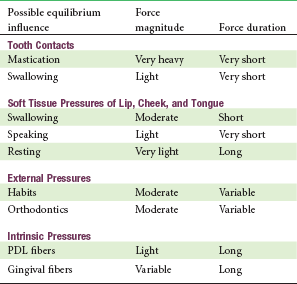
These observations make it plain that, in contrast to forces from mastication, light sustained pressures from lips, cheeks, and tongue at rest are important determinants of tooth position. It seems unlikely, however, that the intermittent short-duration pressures created when the tongue and lips contact the teeth during swallowing or speaking would have any significant impact on tooth position. As with masticatory forces, the pressure magnitudes would be great enough to move a tooth, but the duration is inadequate (Table 5-3).
TABLE 5-3
Possible Equilibrium Influences: Magnitude and Duration of Force Against the Teeth During Function
Equilibrium considerations also apply to the skeleton, including the facial skeleton. Skeletal alterations occur all the time in response to functional demands and are magnified under unusual experimental situations. As discussed in Chapter 2, the bony processes to which muscles attach are especially influenced by the muscles and the location of the attachments. The form of the mandible, because it is largely dictated by the shape of its functional processes, is particularly prone to alteration. The density of the facial bones, like the skeleton as a whole, increases when heavy work is done and decreases in its absence.
Let us now consider the role of function in the etiology of malocclusion and dentofacial deformity from this perspective.
Masticatory Function
The pressures generated by chewing activity potentially could affect dentofacial development in two ways: (1) greater use of the jaws, with higher and/or more prolonged biting force, could increase the dimensions of the jaws and dental arches or (2) less use of the jaws might lead to underdeveloped dental arches and crowded and irregular teeth and the resulting decreased biting force could affect how much the teeth erupt, thereby affecting lower face height and overbite/open bite relationships.
Function and Dental Arch Size
The size and shape of the muscular processes of the jaws should reflect muscle size and activity. Enlargement of the mandibular gonial angles can be seen in humans with hypertrophy of the mandibular elevator muscles (Figure 5-31), and changes in the form of the coronoid processes occur in children when temporalis muscle function is altered after injuries, so there is no doubt that the muscular processes of human jaws are affected by muscle function. The heavy intermittent forces produced during mastication should have little direct effect on tooth positions, so the size of the dental arches would be affected by function only if their bony bases were widened. Does the extent of masticatory activity affect the width of the base of the dental arches?
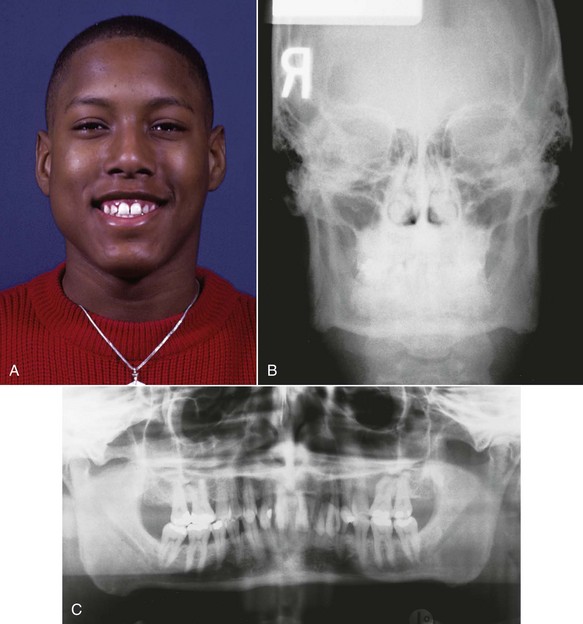
FIGURE 5-31 Hypertrophy of the masseter muscles leads to excessive bone formation at the angles of the mandible, as would be expected in a bony area that responds to muscle attachment. Note the bony enlargement at the gonial angles, especially on the right side of the face.
It seems likely that differences between human racial groups, to some extent, reflect dietary differences and the accompanying masticatory effort. The characteristic craniofacial morphology of Eskimos, which includes broad dental arches, is best explained as an adaptation to the extreme stress they traditionally have placed on jaws and teeth, and changes in craniofacial dimensions from early to modern human civilizations have been related to the accompanying dietary changes.25 A number of studies by physical anthropologists indicate that changes in dental occlusion and an increase in malocclusion occur along with transitions from a primitive to modern diet and lifestyle, to the point that Corruccini has labeled malocclusion a “disease of civilization.”26 In the context of adaptations to changes in diet over even a few generations, it appears that dietary changes probably have played a role in the modern increase in malocclusion. During the development of a single individual, vertical jaw relationships clearly are affected by muscular activity (the effect on tooth eruption is discussed later). Whether masticatory effort influences the size of the dental arches and the amount of space for the teeth is not so clear.27
Animal experiments with soft versus hard diets show that morphologic changes can occur within a single generation when diet consistency is altered. When a pig, for instance, is raised on a soft rather than a normal diet, there are changes in jaw morphology, in the orientation of the jaws to the rest of the facial skeleton, and in dental arch dimensions.28 In humans, if dietary consistency affects dental arch size and the amount of space for the teeth as an individual develops, it must do so early in life because dental arch dimensions are established early. Is it possible that a preadolescent child’s masticatory effort plays a major role in determining dental arch dimensions? That seems unlikely, but the precise relationship remains unknown.
Biting Force and Eruption
Patients who have excessive overbite or anterior open bite usually have posterior teeth that are infra- or supra-erupted, respectively. It seems reasonable that how much the teeth erupt should be a function of how much force is placed against them during function. Is it possible that differences in muscle strength and therefore in biting force are involved in the etiology of short- and long-face problems?
It was noted some years ago that short-face individuals have higher and long-face persons lower maximum biting forces than those with normal vertical dimensions. The difference between long- and normal-face patients is highly significant statistically for occlusal tooth contacts during swallow, simulated chewing, and maximum biting (Figure 5-32).29 Such an association between facial morphology and occlusal force does not prove a cause-and-effect relationship. In the rare muscle weakness syndromes discussed earlier, there is a downward and backward rotation of the mandible associated with excessive eruption of the posterior teeth, but this is almost a caricature of the more usual long-face condition, not just an extension of it. If there were evidence of decreased occlusal forces in children who were showing the long-face pattern of growth, a possible causative relationship would be strengthened.
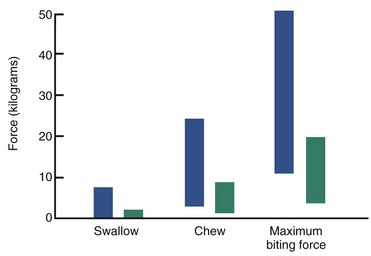
FIGURE 5-32 Comparison of occlusal force for swallowing, simulated chewing, and maximum effort at 2.5 mm molar separation in normal face (blue) and long face (green) adults. Note that the normal subjects have much greater occlusal force during swallowing and chewing as well as at maximum effort. The differences are highly significant statistically. (From Proffit WR, Fields HW, Nixon WL. J Dent Res 62:566-571, 1983.)
It is possible to identify a long-face pattern of growth in prepubescent children. Measurement of occlusal forces in this group produces a surprising result: there are no differences between children with long faces and normal faces, nor between either group of children and long-face adults.30 All three groups have forces far below those of normal adults (Figure 5-33). Therefore it appears that the differences in occlusal force arise at puberty, when the normal group gains masticatory muscle strength and the long-face group does not. Because the long-face growth pattern can be identified before the differences in occlusal force appear, it seems more likely that the different biting force is an effect rather than a cause of the malocclusion.
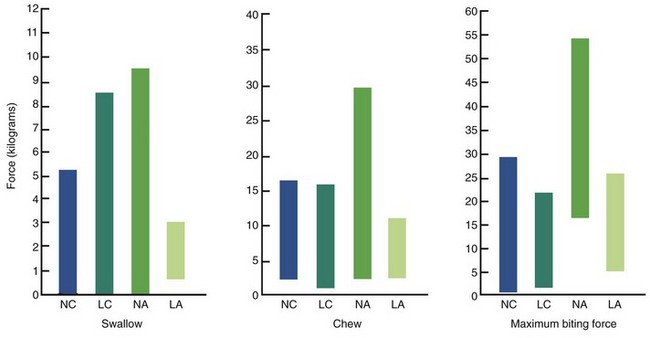
FIGURE 5-33 Comparison of occlusal forces in normal-face children (NC, blue), long-face children (LC, aqua), normal-face adults (NA, green), and long-face adults (LA, light green). Values for both groups of children and the long-face adults are similar; values for normal adults are significantly higher than any of the other three groups. The implication is that the differences in occlusal force in adults result from failure of the long-face group to gain strength during adolescence, not to the long condition itself. (From Proffit WR, Fields HW, Nixon WL. J Dent Res 62:566-571, 1983.)
These findings suggest that the force exerted by the masticatory muscles is not a major environmental factor in controlling tooth eruption and not an etiologic factor for most patients with deep bite or open bite. The effect of muscular dystrophy and related syndromes shows that there can be definite effects on growth if the musculature is abnormal, but in the absence of syndromes of this type, there is no reason to believe that how a patient bites is a major determinant of either dental arch size or vertical dimensions.
Sucking and Other Habits
Almost all normal children engage in non-nutritive sucking of a thumb or pacifier, and as a general rule, sucking habits during the primary dentition years have little if any long-term effect. If these habits persist beyond the time that the permanent teeth begin to erupt, however, malocclusion characterized by flared and spaced maxillary incisors, lingually positioned lower incisors, anterior open bite, and a narrow upper arch is the likely result (Figure 5-34). The characteristic malocclusion associated with sucking arises from a combination of direct pressure on the teeth and an alteration in the pattern of resting cheek and lip pressures.
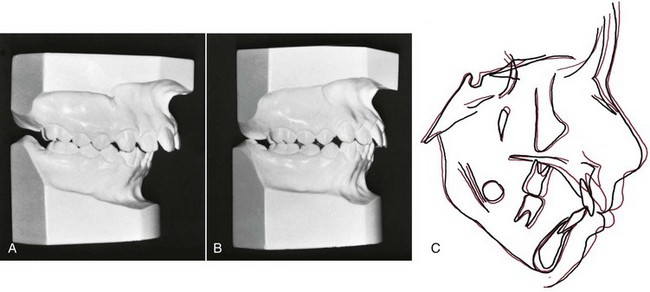
FIGURE 5-34 In this pair of identical twins, one sucked her thumb up to the time of orthodontic records at age 11 and the other did not. A, Occlusal relationships in the thumbsucking girl and (B) her non-thumbsucking twin. Note the increased overjet and forward displacement of the dentition of the thumbsucker. C, Cephalometric tracings of the two girls superimposed on the cranial base of the two girls. As one would expect with identical twins, the cranial base morphology is nearly identical. Note the forward displacement of not only the maxillary dentition but also the maxilla itself. (Courtesy Dr. T. Wallen.)
When a child places a thumb or finger between the teeth, it is usually positioned at an angle so that it presses lingually against the lower incisors and labially against the upper incisors (Figure 5-35). There can be considerable variation in which teeth are affected and how much. From equilibrium theory, one would expect that how much the teeth are displaced would correlate better with the number of hours per day of sucking than with the magnitude of the pressure. Children who suck vigorously but intermittently may not displace the incisors much if at all, whereas others, particularly those who sleep with a thumb or finger between the teeth all night, can cause a significant malocclusion.

FIGURE 5-35 A child sucking their thumb usually places it against the roof of the mouth, causing pressure that pushes the lower incisors lingually and the upper incisors labially. In addition, the jaw is positioned downward, providing additional opportunity for posterior teeth to erupt, and cheek pressure is increased while the tongue is lowered vertically away from the maxillary posterior teeth, altering the equilibrium that controls width dimensions. If the thumb is placed on one side instead of in the midline, the symmetry of the arch may be affected.
The anterior open bite associated with thumbsucking arises by a combination of interference with normal eruption of incisors and excessive eruption of posterior teeth. When a thumb or finger is placed between the anterior teeth, the mandible must be positioned downward to accommodate it. The interposed thumb directly impedes incisor eruption. At the same time, the separation of the jaws alters the vertical equilibrium on the posterior teeth, and as a result, there is more eruption of posterior teeth than might otherwise have occurred. Because of the geometry of the jaws, 1 mm of elongation posteriorly opens the bite about 2 mm anteriorly, so this can be a powerful contributor to the development of anterior open bite (Figure 5-36).
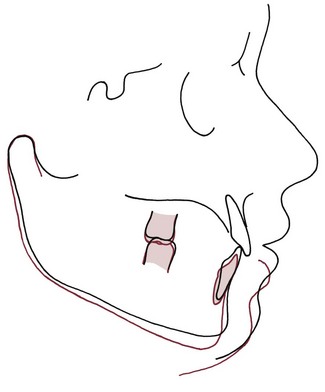
FIGURE 5-36 Cephalometric tracing showing the effects of posterior eruption on the extent of anterior opening. The only difference between the red and black tracings is that the first molars have been elongated 2 mm in the red tracing. Note that the result is 4 mm of separation of the incisors because of the geometry of the jaw.
Although negative pressure is created within the mouth during sucking, there is no reason to believe that this is responsible for the constriction of the maxillary arch that usually accompanies sucking habits. Instead, arch form is affected by an alteration in the balance between cheek and tongue pressures. If the thumb is placed between the teeth, the tongue must be lowered, which decreases pressure by the tongue against the lingual of upper posterior teeth. At the same time, cheek pressure against these teeth is increased as the buccinator muscle contracts during sucking (Figure 5-37). Cheek pressures are greatest at the corners of the mouth, and this probably explains why the maxillary arch tends to become V-shaped, with more constriction across the canines than the molars. A child who sucks vigorously is more likely to have a narrow upper arch than one who just places the thumb between the teeth.
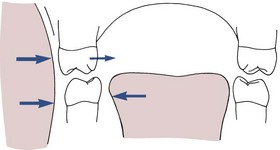
FIGURE 5-37 Diagrammatic representation of soft tissue pressures in the molar region in a child with a sucking habit. As the tongue is lowered and the cheeks contract during sucking, the pressure balance against the upper teeth is altered, and the upper but not the lower molars are displaced lingually.
Mild displacement of the primary incisor teeth is often noted in a 3- or 4-year-old thumbsucker, but if sucking stops at this stage, normal lip and cheek pressures soon restore the teeth to their usual positions. If the habit persists after the permanent incisors begin to erupt, orthodontic treatment may be necessary to overcome the resulting tooth displacements. The constricted maxillary arch is the aspect of the malocclusion least likely to correct spontaneously. In many children with a history of thumbsucking, if the maxillary arch is expanded transversely, both the incisor protrusion and anterior open bite will improve spontaneously (see Chapter 12). There is no point in beginning orthodontic therapy, of course, until the habit has stopped.
Whether a habit can serve in the same way as an orthodontic appliance to change the position of the teeth has been the subject of controversy since at least the first century AD, when Celsus recommended that a child with a crooked tooth be instructed to apply finger pressure against it to move it to its proper position. From our present understanding of equilibrium, we would expect that this might work, but only if the child kept finger pressure against the tooth for 6 hours or more per day.
This concept also makes it easier to understand how playing a musical instrument might relate to the development of a malocclusion. In the past, many clinicians have suspected that playing a wind instrument might affect the position of the anterior teeth, and some have prescribed musical instruments as part of orthodontic therapy. Playing a clarinet, for instance, might lead to increased overjet because of the way the reeds are placed between the incisors, and this instrument could be considered both a potential cause of a Class II malocclusion and a therapeutic device for treatment of Class III. String instruments like the violin and viola require a specific head and jaw posture that affects tongue versus lip/cheek pressures and could produce asymmetries in arch form. Although the expected types of displacement of teeth are seen in professional musicians,31 even in this group the effects are not dramatic, and little or no effect is observed in most children.32 It seems quite likely that the duration of tongue and lip pressures associated with playing the instrument is too short to make any difference, except in the most devoted musician.
Can habits affect development of the jaws? In Edward Angle’s era, a “sleeping habit” in which the weight of the head rested on the chin once was thought to be a major cause of Class II malocclusion. Facial asymmetries have been attributed to always sleeping on one side of the face or even to “leaning habits,” as when an inattentive child leans the side of his face against one hand to doze without falling out of the classroom chair. It is not nearly as easy to distort the facial skeleton as these views implied. Sucking habits often exceed the time threshold necessary to produce an effect on the teeth, but even prolonged sucking has little impact on the underlying form of the jaws. On close analysis, most other habits have such a short duration that dental effects, much less skeletal effects, are unlikely.
Tongue Thrusting
Much attention has been paid at various times to the tongue and tongue habits as possible etiologic factors in malocclusion. The possible deleterious effects of “tongue thrust swallowing” (Figure 5-38), defined as placement of the tongue tip forward between the incisors during swallowing, received particular emphasis in the 1950s and 1960s.
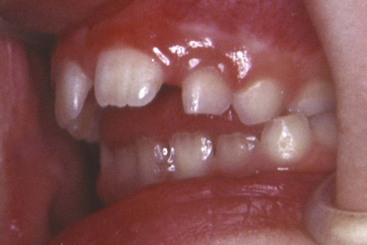
FIGURE 5-38 The typical appearance of a “tongue thrust swallow” with the lip pulled back. Note the tongue tip between the incisors protruding forward toward contact with the elevated lower lip.
Laboratory studies indicate that individuals who place the tongue tip forward when they swallow usually do not have more tongue force against the teeth than those who keep the tongue tip back; in fact, tongue pressure may be lower.33 The term tongue thrust is therefore something of a misnomer because it implies that the tongue is forcefully thrust forward. Swallowing is not a learned behavior but is integrated and controlled physiologically at subconscious levels, so whatever the pattern of swallow, it cannot be considered a habit in the usual sense. It is true, however, that individuals with an anterior open bite malocclusion place the tongue between the anterior teeth when they swallow, while those who have a normal incisor relationship usually do not, and it is tempting to blame the open bite on this pattern of tongue activity.
As discussed in detail in Chapter 2, the mature or adult swallow pattern appears in some normal children as early as age 3 but is not present in the majority until about age 6 and is never achieved in 10% to 15% of a typical population. Tongue thrust swallowing in older patients superficially resembles the infantile swallow (described in Chapter 3), and sometimes children or adults who place the tongue between the anterior teeth are spoken of as having a retained infantile swallow. This is clearly incorrect. Only brain-damaged children retain a truly infantile swallow in which the posterior part of the tongue has little or no role.
Since coordinated movements of the posterior tongue and elevation of the mandible tend to develop before protrusion of the tongue tip between the incisor teeth disappears, what is called “tongue thrusting” in young children is often a normal transitional stage in swallowing. During the transition from an infantile to a mature swallow, a child can be expected to pass through a stage in which the swallow is characterized by muscular activity to bring the lips together, separation of the posterior teeth, and forward protrusion of the tongue between the teeth. This is also a description of the classic tongue thrust swallow. A delay in the normal swallow transition can be expected when a child has a sucking habit.
When there is an anterior open bite and/or upper incisor protrusion, as often occurs from sucking habits, it is more difficult to seal off the front of the mouth during swallowing to prevent food or liquids from escaping. Bringing the lips together and placing the tongue between the separated anterior teeth is a successful maneuver to close off the front of the mouth and form an anterior seal. In other words, a tongue thrust swallow is a useful physiologic adaptation if you have an open bite, which is why an individual with an open bite also has a tongue thrust swallow. The reverse is not true—protruding the tongue between the anterior teeth during swallowing is often present in children with good anterior occlusion. After a sucking habit stops, the anterior open bite tends to close spontaneously, but the position of the tongue between the anterior teeth persists for a while as the open bite closes. Until the open bite disappears, an anterior seal by the tongue tip remains necessary.
The modern viewpoint is, in short, that tongue thrust swallowing is seen primarily in two circumstances: in younger children with reasonably normal occlusion, in whom it represents only a transitional stage in normal physiologic maturation; and in individuals of any age with displaced incisors, in whom it is an adaptation to the space between the teeth. The presence of a large overjet (often) and anterior open bite (nearly always) conditions a child or adult to place the tongue between the anterior teeth. A tongue thrust swallow therefore is more likely to be the result of displaced incisors, not the cause. It follows, of course, that correcting the tooth position should cause a change in swallow pattern, and this usually happens. It is neither necessary nor desirable to try to teach the patient to swallow differently before beginning orthodontic treatment.
This is not to say that the tongue has no etiologic role in the development of open bite malocclusion. From equilibrium theory, light but sustained pressure by the tongue against the teeth would be expected to have significant effects. Tongue thrust swallowing simply has too short a duration to have an impact on tooth position. Pressure by the tongue against the teeth during a typical swallow lasts for approximately 1 second. A typical individual swallows about 800 times per day while awake but has only a few swallows per hour while asleep. The total per day therefore is usually under 1000. One thousand seconds of pressure, of course, totals only a few minutes, not nearly enough to affect the equilibrium.
On the other hand, if a patient has a forward resting posture of the tongue, the duration of this light pressure could affect tooth position, vertically or horizontally. Tongue tip protrusion during swallowing is sometimes associated with a forward tongue posture. If the position from which tongue movements start is different from normal, so that the pattern of resting pressures is different, there is likely to be an effect on the teeth, whereas if the postural position is normal, the tongue thrust swallow has no clinical significance.
Perhaps this point can best be put in perspective by comparing the number of children who have an anterior open bite malocclusion with the number of children of the same age reported to have a tongue thrust swallow. As Figure 5-39 shows, at every age above 6, the number of children reported to have a tongue thrust swallow is about 10 times greater than the number reported to have an anterior open bite. Thus there is no reason to believe that a tongue thrust swallow always implies an altered rest position and will lead to malocclusion. In a child who has an open bite, tongue posture may be a factor, but the swallow itself is not.
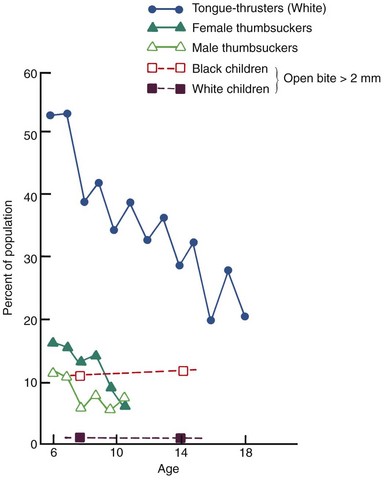
FIGURE 5-39 Prevalence of anterior open bite, thumbsucking, and tongue thrust swallowing as a function of age. Open bite occurs much more frequently in blacks than in whites. Note that the prevalence of anterior open bite at any age is only a small fraction of the prevalence of tongue thrust swallowing and is also less than the prevalence of thumbsucking. (Data from Fletcher SG, Casteel RL, Bradley DP. J Speech Hear Disord 26:201-208, 1961; Kelly JE, et al. DHEW Pub No [HRA] 77-144, 1977.)
Respiratory Pattern
Respiratory needs are the primary determinant of the posture of the jaws and tongue (and of the head itself, to a lesser extent). Therefore it seems entirely reasonable that an altered respiratory pattern, such as breathing through the mouth rather than the nose, could change the posture of the head, jaw, and tongue. This in turn could alter the equilibrium of pressures on the jaws and teeth and affect both jaw growth and tooth position. In order to breathe through the mouth, it is necessary to lower the mandible and tongue, and extend (tip back) the head. If these postural changes were maintained, face height would increase, and posterior teeth would super-erupt; unless there was unusual vertical growth of the ramus, the mandible would rotate down and back, opening the bite anteriorly and increasing overjet; and increased pressure from the stretched cheeks might cause a narrower maxillary dental arch.
Exactly this type of malocclusion often is associated with mouth breathing (note its similarity to the pattern also blamed on sucking habits and tongue thrust swallow). The association has been noted for many years: the descriptive term adenoid facies has appeared in the English literature for at least a century, probably longer (Figure 5-40). Unfortunately, the relationship between mouth breathing, altered posture, and the development of malocclusion is not so clear-cut as the theoretical outcome of shifting to oral respiration might appear at first glance.34 Recent experimental studies have only partially clarified the situation.
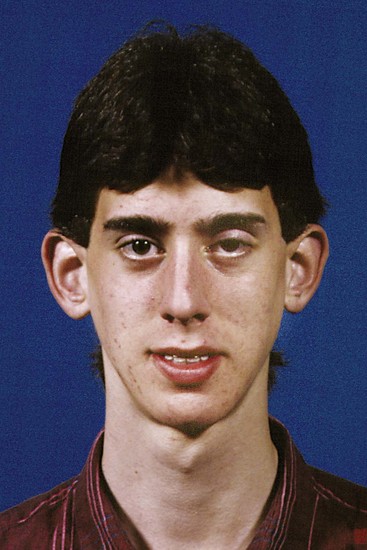
FIGURE 5-40 The classic “adenoid facies,” characterized by narrow width dimensions, protruding teeth, and lips separated at rest, has often been attributed to mouth breathing. Since it is perfectly possible to breathe through the nose with the lips separated, simply by creating an oral seal posteriorly with the soft palate, the facial appearance is not diagnostic of the respiratory mode. On careful study, many patients with this facial type are found not to be obligatory mouth breathers.
In analyzing this, it is important to understand first that although humans are primarily nasal breathers, everyone breathes partially through the mouth under certain physiologic conditions, the most prominent being an increased need for air during exercise. For the average individual, there is a transition to partial oral breathing when ventilatory exchange rates above 40 to 45 L/min are reached. At maximum effort, 80 or more L/min of air are needed, about half of which is obtained through the mouth. At rest, minimum airflow is 20 to 25 L/min, but heavy mental concentration or even normal conversation lead to increased airflow and a transition to partial mouth breathing.
During resting conditions, greater effort is required to breathe through the nose than through the mouth—the tortuous nasal passages introduce an element of resistance to airflow as they perform their function of warming and humidifying the inspired air. The increased work for nasal respiration is physiologically acceptable up to a point, and indeed respiration is most efficient with modest resistance present in the system. If the nose is partially obstructed, the work associated with nasal breathing increases, and at a certain level of resistance to nasal airflow, the individual switches to partial mouth breathing. This crossover point varies among individuals but is usually reached at resistance levels of about 3.5 to 4 cm H2O/L/min.35 The swelling of the nasal mucosa accompanying a common cold occasionally converts all of us to mouth breathing at rest by this mechanism.
Chronic respiratory obstruction can be produced by prolonged inflammation of the nasal mucosa associated with allergies or chronic infection. It can also be produced by mechanical obstruction anywhere within the nasorespiratory system, from the nares to the posterior nasal choanae. Under normal conditions, the size of the nostril is the limiting factor in nasal airflow. The pharyngeal tonsils or adenoids normally are large in children, and partial obstruction from this source may contribute to mouth breathing in children. Individuals who have had chronic nasal obstruction may continue to breathe partially through the mouth even after the obstruction has been relieved. In this sense, mouth breathing can sometimes be considered a habit.
If respiration had an effect on the jaws and teeth, it should do so by causing a change in posture that secondarily altered long-duration pressures from the soft tissues. Experiments with human subjects have shown that a change in posture does accompany nasal obstruction. For instance, when the nose is completely blocked, usually there is an immediate change of about 5 degrees in the craniovertebral angle (Figure 5-41). The jaws move apart, as much by elevation of the maxilla because the head tips back, as by depression of the mandible. When the nasal obstruction is removed, the original posture immediately returns. This physiologic response occurs to the same extent, however, in individuals who already have some nasal obstruction, which indicates that it may not totally result from respiratory demands.
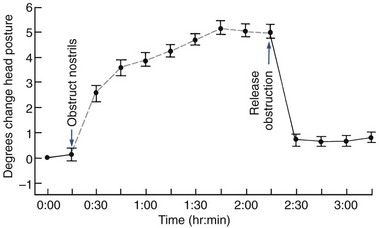
FIGURE 5-41 Data from an experiment with dental students, showing the immediate change in head posture when the nostrils are totally blocked: the head tips back about 5 degrees, increasing the separation of the jaws. When the obstruction is relieved, head posture returns to its original position. (From Vig PS, Showfety KJ, Phillips C. Am J Orthod 77:258-268, 1980.)
Harvold’s classic experiments with growing monkeys showed that totally obstructing the nostrils for a prolonged period in this species leads to the development of malocclusion, but not the type commonly associated with mouth breathing in humans.36 Instead, the monkeys tend to develop some degree of mandibular prognathism, although their response shows considerable variety. In evaluating these experiments, it must be kept in mind that mouth breathing of any extent is completely unnatural for monkeys, who will die if the nasal passages are obstructed abruptly. To carry out the experiments, it was necessary to gradually obstruct their noses, giving the animals a chance to learn how to survive as mouth breathers. The variety of responses in the monkeys suggests that the type of malocclusion is determined by the individual animal’s pattern of adaptation.
Total nasal obstruction is extremely rare in humans. There are only a few well-documented cases of facial growth in children with long-term total nasal obstruction, but it appears that under these circumstances the growth pattern is altered in the way one would predict (Figure 5-42). Because total nasal obstruction in humans is so rare, the important clinical question is whether partial nasal obstruction, of the type that occurs occasionally for a short time in everyone and chronically in some children, can lead to malocclusion; or more precisely, how close to total obstruction does partial obstruction have to come before it is clinically significant?
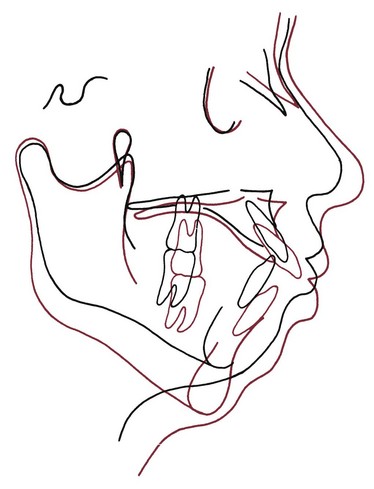
FIGURE 5-42 Cephalometric superimposition showing the effect of total nasal obstruction produced by a pharyngeal flap operation (for cleft palate speech) that sealed off the nose posteriorly. From age 12 (black) to 16 (red), the mandible rotated downward and backward as the patient experienced considerable growth. (Redrawn from McNamara JA. Angle Orthod 51:269-300, 1981.)
The question is difficult to answer, primarily because it is difficult to know what the pattern of respiration really is at any given time in humans. Observers tend to equate lip separation at rest with mouth breathing (see Figure 5-40), but this is simply not correct. It is perfectly possible for an individual to breathe through the nose while the lips are apart. To do this, it is only necessary to seal off the mouth by placing the tongue against the palate. Since some lip separation at rest (lip incompetence) is normal in children, many children who appear to be mouth breathers may not be.
Simple clinical tests for mouth breathing can also be misleading. The highly vascular nasal mucosa undergoes cycles of engorgement with blood and shrinkage. The cycles alternate between the two nostrils: when one is clear, the other is usually somewhat obstructed. For this reason, clinical tests to determine whether the patient can breathe freely through both nostrils nearly always show that one is at least partially blocked. One partially obstructed nostril should not be interpreted as a problem with normal nasal breathing.
The only reliable way to quantify the extent of mouth breathing is to establish how much of the total airflow goes through the nose and how much through the mouth, which requires special instrumentation to simultaneously measure nasal and oral airflow. This allows the percentage of nasal or oral respiration (nasal/oral ratio) to be calculated for the length of time the subject can tolerate being continuously monitored. It seems obvious that a certain percentage of oral respiration maintained for a certain percentage of the time should be the definition of significant mouth breathing, but despite years of effort such a definition has not been produced.
The best experimental data for the relationship between malocclusion and mouth breathing are derived from studies of the nasal/oral ratio in normal versus long-face children.37 The relationship is not nearly as clear-cut as theory might predict. It is useful to represent the data as in Figure 5-43, which shows that both normal and long-face children are likely to be predominantly nasal breathers under laboratory conditions. A minority of the long-face children had less than 40% nasal breathing, while none of the normal children had such low nasal percentages. When adult long-face patients are examined, the findings are similar: the number with evidence of nasal obstruction is increased in comparison to a normal population, but the majority are not mouth breathers in the sense of predominantly oral respiration.
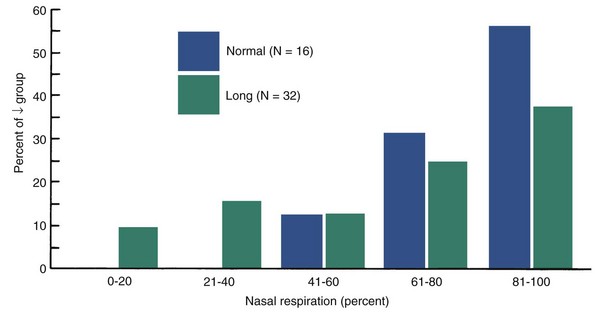
FIGURE 5-43 Comparison of the percentage of nasal respiration in long-face versus normal-face adolescents. About one-third of the long-face group have less than 50% nasal respiration, whereas none of the normal-face group have such a low nasal percentage, but most of the long-face group are predominantly nasal breathers. The data suggest that impaired nasal respiration may contribute to the development of the long-face condition but is not the sole or even the major cause. (Data redrawn from Fields HW, Warren DW, Black K, et al. Am J Orthod Dentofac Orthop 99:147-154, 1991.)
It seems reasonable to presume that children who require adenoidectomy and/or tonsillectomy for medical purposes, or those diagnosed as having chronic nasal allergies, would have some degree of nasal obstruction. Studies of Swedish children who underwent adenoidectomy showed that on the average, children in the adenoidectomy group had a significantly longer anterior face height than control children (Figure 5-44). They also had a tendency toward maxillary constriction and more upright incisors.38 Furthermore, when children in the adenoidectomy group were followed after their treatment, they tended to return toward the mean of the control group, though the differences persisted (Figure 5-45). Similar differences from normal control groups were seen in other groups requiring adenoidectomy and/or tonsillectomy.39
FIGURE 5-44 Composite (mean) cephalometric tracings for a group of Swedish children requiring adenoidectomy for medical purposes, compared with a group of normal controls. The adenoidectomy group had statistically significantly greater anterior face height and steeper mandibular plane angles than the controls, but the differences were quantitatively not large. (From Linder-Aronson S. Acta Otolaryngol Scand [suppl]:265, 1970.)
FIGURE 5-45 Comparison of mandibular plane angles in a group of postadenoidectomy children compared with normal controls. Note that the differences existing at the time of adenoidectomy decreased in size but did not totally disappear. (From Linder-Aronson S. In: Cook JT, ed. Transactions of the Third International Orthodontic Congress. St. Louis: Mosby; 1975.)
Although the differences between normal children and those in the allergy or adenoidectomy groups were statistically significant and undoubtedly real, they were not large. Face height on the average was about 3 mm greater in the adenoidectomy group. It appears therefore that research to this point on respiration has established two opposing principles, leaving a large gray area between them: (1) total nasal obstruction is highly likely to alter the pattern of growth and lead to malocclusion in experimental animals and humans, and individuals with a high percentage of oral respiration are overrepresented in the long-face population; but (2) the majority of individuals with the long-face pattern of deformity have no evidence of nasal obstruction and must therefore have some other etiologic factor as the principal cause. Perhaps the alterations in posture associated with partial nasal obstruction and moderate increases in the percentage of oral respiration are not great enough by themselves to create a severe malocclusion. Mouth breathing, in short, may contribute to the development of orthodontic problems but is difficult to indict as a frequent etiologic agent.
It is interesting to consider the other side of this relationship: can malocclusion sometimes cause respiratory obstruction? Sleep apnea has been recognized recently as a more frequent problem than had been appreciated, and it is apparent that mandibular deficiency can contribute to its development (see Chapter 18). Its etiology, however, is by no means determined just by orofacial morphology—obesity, age/gender, and cephalometric characteristics seem to be important, in that order.40
Etiology in Contemporary Perspective
Part of the philosophy of the early orthodontists was their belief in the perfectibility of man. Edward Angle and his contemporaries, influenced by the romanticized view of primitive peoples commonly held 100 years ago, took it for granted that malocclusion was a disease of civilization and blamed it on improper function of the jaws under the “degenerate” modern conditions. Changing jaw function in order to produce proper growth and improve facial proportions was an important goal of treatment, which unfortunately proved difficult to achieve.
Classical (Mendelian) genetics developed rapidly in the first part of the twentieth century, and a different view of malocclusion gradually replaced the earlier one. This new view was that malocclusion is primarily the result of inherited dentofacial proportions, which may be altered somewhat by developmental variations, trauma, or altered function, but which are basically established at conception. If that were true, the possibilities for orthodontic treatment also would be rather limited. The orthodontist’s role would be to adapt the dentition to the existing facial structures, with little hope of producing underlying changes.
In the 1980s, there was a strong swing back toward the earlier view, as the failure of heredity to explain most variation in occlusion and jaw proportions was appreciated and as the new theories of growth control indicated how environmental influences could operate by altering posture. The earlier concept that jaw function is related to the development of malocclusion was revived and strengthened, both by the evidence against simple inheritance and by a more optimistic view of the extent to which the human skeleton can be altered. Clinical applications, some already recognized as unfortunate, reflected extreme optimism about arch expansion and growth modification.
As the twenty-first century moves ahead, a more balanced view seems to be emerging. Contemporary research has refuted the simplistic picture of malocclusion as resulting from independent inheritance of dental and facial characteristics, but the research findings consistently have shown also that there are no simple explanations for malocclusion in terms of oral function. Mouth breathing, tongue thrusting, soft diet, sleeping posture—none of these can be regarded as the sole or even the major reason for most malocclusions. Along the same lines, it is fair to say that the research has not yet clarified the precise role of heredity as an etiologic agent for malocclusion. The relatively high heritability of craniofacial dimensions and the relatively low heritability of dental arch variations now have been established, but exactly how this relates to the etiologic process of malocclusions that have both skeletal and dental components remains unknown. Conclusions about the etiology of most orthodontic problems are difficult because several interacting factors probably played a role. At least, at this point we are more aware of how much we really do not yet know about the etiology of orthodontic problems.
References
1. Moore, ES, Ward, RE, Jamison, PL, et al. New perspectives on the face in fetal alcohol syndrome: what anthropometry tells us. Am J Med Genet. 2002;109:249–260.
2. Naidoo, S, Norval, G, Swanevelder, S, et al. Foetal alcohol syndrome: a dental and skeletal age analysis of patients and controls. Eur J Orthod. 2006;28:247–253.
3. Dupé, V, Pellerin, I. Retinoic acid receptors exhibit cell-autonomous functions in cranial neural crest cells. Dev Dyn. 2009;238:2701–2711.
4. Macaya, D, Katsanis, SH, Hefferon, TW, et al. A synonymous mutation in TCOF1 causes Treacher Collins syndrome due to mis-splicing of a constitutive exon. Am J Med Genet A. 2009;149:1624–1627.
5. Johnston, MC, Bronsky, PT. Abnormal craniofacial development: an overview. Crit Rev Oral Biol Med. 1995;6:368–422.
6. Tessier, P. Anatomical classification of facial, craniofacial and latero-facial clefts. J Maxillofac Surg. 1976;4:69–92.
7. Shaw, GM, Carmichael, SL, Vollset, SE. Mid-pregnancy cotinine and risks of orofacial clefts and neural tube defects. J Pediatr. 2009;154:17–19.
8. Li, Z, Liu, J, Ye, R, et al. Maternal passive smoking and risk of cleft lip with or without cleft palate. Epidemiology. 2010;21:240–242.
9. Turvey, TA, Vig, KWL, Fonseca, RJ. Facial Clefts and Craniosynostosis: Principles and Management. Philadelphia: WB Saunders; 1996.
10. Suri, S, Ross, RB, Tompson, BD. Craniofacial morphology and adolescent facial growth in Pierre Robin sequence. Am J Orthod Dentofac Orthop. 2010;137:763–774.
11. Yu, CC, Wong, FH, Lo, LJ, et al. Craniofacial deformity in patients with uncorrected congenital muscular torticollis: an assessment from three-dimensional computed tomography imaging. Plast Reconstr Surg. 2004;113:24–33.
12. Kiliaridis, S, Katsaros, C. The effects of myotonic dystrophy and Duchenne muscular dystrophy on the orofacial muscles and dentofacial morphology. Acta Odontol Scand. 1998;56:369–374.
13. Obwegeser, HL. Mandibular Growth Anomalies. Berlin: Springer-Verlag; 2000.
14. Fleseriu, M, Delashaw, JB, Jr., Cook, DM. Acromegaly: a review of current medical therapy and new drugs on the horizon. Neurosurg Focus. 2010;29:E15.
15. Stockard, CR, Johnson, AL. Genetic and Endocrinic Basis for Differences in Form and Behavior. Philadelphia: The Wistar Institute of Anatomy and Biology; 1941.
16. Chung, CS, Niswander, JD, Runck, DW, et al. Genetic and epidemiologic studies of oral characteristics in Hawaii’s schoolchildren. II. Malocclusion. Am J Human Genet. 1971;23:471–495.
17. Townsend, G, Hughes, T, Bockmann, M, et al. How studies of twins can inform our understanding of dental morphology. Front Oral Biol. 2009;13:136–141.
18. Hughes, T, Thomas, C, Richards, L, et al. A study of occlusal variation in the primary dentition of Australian twins and singletons. Arch Oral Biol. 2001;46:857–864.
19. Corruccini, RS, Sharma, K, Potter, RHY. Comparative genetic variance and heritability of dental occlusal variables in U.S. and northwest Indian twins. Am J Phys Anthropol. 1986;70:293–299.
20. Harris, EF, Johnson, MG. Heritability of craniometric and occlusal variables: a longitudinal sib analysis. Am J Orthod Dentofac Orthop. 1991;99:258–268.
21. Johannsdottir, B, Thorarinsson, F, Thordarson, A, et al. Heritability of craniofacial characteristics between parents and offspring estimated from lateral cephalograms. Am J Orthod Dentofac Orthop. 2005;127:200–207.
22. Suzuki, A, Takahama, Y. Parental data used to predict growth of craniofacial form. Am J Orthod Dentofac Orthop. 1991;99:107–121.
23. Cruz, RM, Krieger, H, Ferreira, R, et al. Major gene and multifactorial inheritance of mandibular prognathism. Am J Med Genet A. 2008;146A:71–77.
24. Bui, C, King, T, Proffit, W, et al. Phenotypic characterization of Class III patients. Angle Orthod. 2006;76:564–569.
25. Larsen, CS. Bioarchaeology: Interpreting Behavior from the Human Skeleton. Cambridge: Cambridge University Press; 1997.
26. Corruccini, RS. Anthropological aspects of orofacial and occlusal variations and anomalies. In: Kelly MA, Larsen CS, eds. Advances in Dental Anthropology. New York: Wiley-Liss, 1991.
27. Kiliaridis, S. Masticatory muscle influence on craniofacial growth. Acta Odontol Scand. 1995;53:196–202.
28. Ciochon, RL, Nisbett, RA, Corruccini, RS. Dietary consistency and craniofacial development related to masticatory function in minipigs. J Craniofac Genet Dev Biol. 1997;17:96–102.
29. Proffit, WR, Fields, HW, Nixon, WL. Occlusal forces in normal and long face adults. J Dent Res. 1983;62:566–571.
30. Proffit, WR, Fields, HW. Occlusal forces in normal and long face children. J Dent Res. 1983;62:571–574.
31. Kovero, O, Kononen, M, Pirinen, S. The effect of professional violin and viola playing on the bony facial structures. Eur J Orthod. 1997;19:39–45.
32. Kindisbacher, I, Hirschi, U, Ingervall, B, et al. Little influence on tooth position from playing a wind instrument. Angle Orthod. 1990;60:223–228.
33. Proffit, WR. Lingual pressure patterns in the transition from tongue thrust to adult swallowing. Arch Oral Biol. 1972;17:555–563.
34. Vig, KWL. Nasal obstruction and facial growth: the strength of evidence for clinical assumptions. Am J Orthod Dentofac Orthop. 1998;113:603–611.
35. Warren, DW, Mayo, R, Zajac, DJ, et al. Dyspnea following experimentally induced increased nasal airway resistance. Cleft Palate-Craniofac J. 1996;33:231–235.
36. Harvold, EP, Tomer, BS, Vargervik, K, et al. Primate experiments on oral respiration. Am J Orthod. 1981;79:359–372.
37. Fields, HW, Warren, DW, Black, K, Phillips, C. Relationship between vertical dentofacial morphology and respiration in adolescents. Am J Orthod Dentofac Orthop. 1991;99:147–154.
38. Linder-Aronson, S. Adenoids: their effect on mode of breathing and nasal airflow and their relationship to characteristics of the facial skeleton and dentition. Acta Otolaryngol Scand. 1970;265:1–132.
39. Woodside, DG, Linder-Aronson, S, Lundstrom, A, et al. Mandibular and maxillary growth after changed mode of breathing. Am J Orthod Dentofac Orthop. 1991;100:1–18.
40. Friedman F, ed. Sleep Apnea and Snoring: Surgical and Non-surgical Therapy. Edinburgh: Saunders-Elsevier, 2009.