The Biologic Basis of Orthodontic Therapy
PERIODONTAL AND BONE RESPONSE TO NORMAL FUNCTION
Periodontal Ligament Structure and Function
Role of the Periodontal Ligament in Eruption and Stabilization of the Teeth
PERIODONTAL LIGAMENT AND BONE RESPONSE TO SUSTAINED FORCE
Biologic Control of Tooth Movement
Effects of Force Distribution and Types of Tooth Movement
Effects of Force Duration and Force Decay
Drug Effects on the Response to Orthodontic Force
Effects of Local Injury: Corticotomy and Accelerated Tooth Movement
Orthodontic treatment is based on the principle that if prolonged pressure is applied to a tooth, tooth movement will occur as the bone around the tooth remodels. Bone is selectively removed in some areas and added in others. In essence, the tooth moves through the bone carrying its attachment apparatus with it, as the socket of the tooth migrates. Because the bony response is mediated by the periodontal ligament, tooth movement is primarily a periodontal ligament phenomenon.
Forces applied to the teeth can also affect the pattern of bone apposition and resorption at sites distant from the teeth, particularly the sutures of the maxilla and bony surfaces on both sides of the temporomandibular (TM) joint. In addition, it is possible now to apply force to implants in the maxilla or mandible to influence growth at maxillary sutures and at the mandibular condyle. Thus the biologic response to orthodontic therapy includes not only the response of the periodontal ligament but also the response of growing areas distant from the dentition.
In this chapter, the response of periodontal structures to orthodontic force is discussed first, and then the response of skeletal areas distant from the dentition is considered, drawing on the background of normal growth provided in Chapters 2 through 4.
Periodontal and Bone Response to Normal Function
Periodontal Ligament Structure and Function
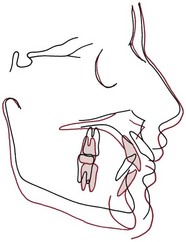
Each tooth is attached to and separated from the adjacent alveolar bone by a heavy collagenous supporting structure, the periodontal ligament (PDL). Under normal circumstances, the PDL occupies a space approximately 0.5 mm in width around all parts of the root. By far the major component of the ligament is a network of parallel collagenous fibers, inserting into cementum of the root surface on one side and into a relatively dense bony plate, the lamina dura, on the other side. These supporting fibers run at an angle, attaching farther apically on the tooth than on the adjacent alveolar bone. This arrangement, of course, resists the displacement of the tooth expected during normal function (Figure 8-1).
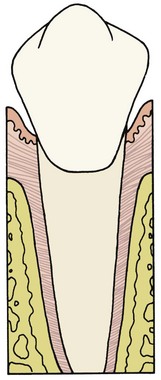
FIGURE 8-1 Diagrammatic representation of periodontal structures (bone in pale red). Note the angulation of the PDL fibers.
Although most of the PDL space is taken up with the collagenous fiber bundles that constitute the ligamentous attachment, two other major components of the ligament must be considered. These are (1) the cellular elements, including mesenchymal cells of various types along with vascular and neural elements, and (2) the tissue fluids. Both play an important role in normal function and in making orthodontic tooth movement possible.
The principal cellular elements in the PDL are undifferentiated mesenchymal cells and their progeny in the form of fibroblasts and osteoblasts. The collagen of the ligament is constantly being remodeled and renewed during normal function. The same cells can serve as both fibroblasts, producing new collagenous matrix materials, and fibroclasts, destroying previously produced collagen.1 Remodeling and recontouring of the bony socket and the cementum of the root is also constantly being carried out, though on a smaller scale, as a response to normal function.
Fibroblasts in the PDL have properties similar to osteoblasts, and new alveolar bone probably is formed by osteoblasts that differentiated from the local cellular population.2 Bone and cementum are removed by specialized osteoclasts and cementoclasts, respectively. These multinucleated giant cells are quite different from the osteoblasts and cementoblasts that produce bone and cementum. Despite years of investigation, their origin remains controversial. Most are of hematogenous origin; some may be derived from stem cells found in the local area.3
Although the PDL is not highly vascular, it does contain blood vessels and cells from the vascular system. Nerve endings are also found within the ligament, both the unmyelinated free endings associated with perception of pain and the more complex receptors associated with pressure and positional information (proprioception).
Finally, it is important to recognize that the PDL space is filled with fluid; this fluid is the same as that found in all other tissues, ultimately derived from the vascular system. A fluid-filled chamber with retentive but porous walls could be a description of a shock absorber, and in normal function, the fluid allows the PDL space to play just this role.
Response to Normal Function
During masticatory function, the teeth and periodontal structures are subjected to intermittent heavy forces. Tooth contacts last for 1 second or less; forces are quite heavy, ranging from 1 or 2 kg while soft substances are being chewed, up to as much as 50 kg against a more resistant object. When a tooth is subjected to heavy loads of this type, quick displacement of the tooth within the PDL space is prevented by the incompressible tissue fluid. Instead, the force is transmitted to the alveolar bone, which bends in response.
The extent of bone bending during normal function of the jaws (and other skeletal elements of the body) is often not appreciated. The body of the mandible bends as the mouth is opened and closed, even without heavy masticatory loads. Upon wide opening, the distance between the mandibular molars decreases by 2 to 3 mm. In heavy function, individual teeth are slightly displaced as the bone of the alveolar process bends to allow this to occur, and bending stresses are transmitted over considerable distances. Bone bending in response to normal function generates piezoelectric currents (Figure 8-2) that appear to be an important stimulus to skeletal regeneration and repair (see further discussion later in this chapter). This is the mechanism by which bony architecture is adapted to functional demands.
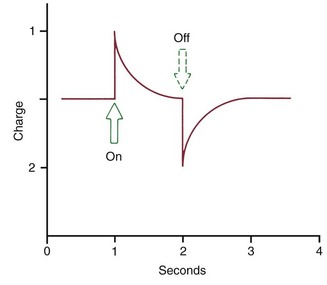
FIGURE 8-2 When a force is applied to a crystalline structure such as bone or collagen, a flow of current is produced that quickly dies away. When the force is released, an opposite current flow is observed. The piezoelectric effect results from migration of electrons within the crystal lattice.
Very little of the fluid within the PDL space is squeezed out during the first second of pressure application. If pressure against a tooth is maintained, however, the fluid is rapidly expressed, and the tooth displaces within the PDL space, compressing the ligament itself against adjacent bone. Not surprisingly, this hurts. Pain is normally felt after 3 to 5 seconds of heavy force application, indicating that the fluids are expressed and crushing pressure is applied against the PDL in this amount of time (Table 8-1). The resistance provided by tissue fluids allows normal mastication, with its force applications of 1 second or less, to occur without pain.
TABLE 8-1
Physiologic Response to Heavy Pressure Against a Tooth
| Time (seconds) | Event |
| <1 | PDL fluid incompressible, alveolar bone bends, piezoelectric signal generated |
| 1-2 | PDL fluid expressed, tooth moves within PDL space |
| 3-5 | PDL fluid squeezed out, tissues compressed; immediate pain if pressure is heavy |
Although the PDL is beautifully adapted to resist forces of short duration, it rapidly loses its adaptive capability as the tissue fluids are squeezed out of its confined area. Prolonged force, even of low magnitude, produces a different physiologic response—remodeling of the adjacent bone. Orthodontic tooth movement is made possible by the application of prolonged forces. In addition, light prolonged forces in the natural environment—forces from the lips, cheeks, or tongue resting against the teeth—have the same potential as orthodontic forces to cause the teeth to move to a different location (see the discussion of equilibrium factors in Chapter 5).
Role of the Periodontal Ligament in Eruption and Stabilization of the Teeth
The phenomenon of tooth eruption makes it plain that forces generated within the PDL itself can produce tooth movement. After a tooth emerges into the mouth, further eruption depends on metabolic events within the PDL, including but perhaps not limited to formation, cross-linkage, and maturational shortening of collagen fibers (see Chapter 3). This process continues, although at a reduced rate, into adult life. A tooth whose antagonist has been extracted will often begin to erupt again after many years of apparent quiescence.
The continuing presence of this mechanism indicates that it may produce not only eruption of the teeth under appropriate circumstances but also active stabilization of the teeth against prolonged forces of light magnitude. It is commonly observed that light prolonged pressures against the teeth are not in perfect balance, as would seem to be required if tooth movement were not to occur (Figure 8-3). The ability of the PDL to generate a force and thereby contribute to the set of forces that determine the equilibrium situation, probably explains this.
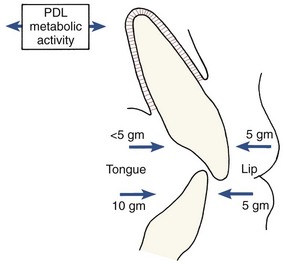
FIGURE 8-3 Resting pressures from the lips or cheeks and tongue are usually not balanced. In some areas, as in the mandibular anterior, tongue pressure is greater than lip pressure. In other areas, as in the maxillary incisor region, lip pressure is greater. Active stabilization produced by metabolic effects in the PDL probably explains why teeth are stable in the presence of imbalanced pressures that would otherwise cause tooth movement.
Active stabilization also implies a threshold for orthodontic force, since forces below the stabilization level would be expected to be ineffective. The threshold, of course, would vary depending on the extent to which existing soft tissue pressures were already being resisted by the stabilization mechanism. In some experiments, the threshold for orthodontic force, if one was found at all, appeared extremely low. In other circumstances, a somewhat higher threshold, but still one of only a few grams, seems to exist. The current concept is that active stabilization can overcome prolonged forces of a few grams at most, perhaps up to the 5 to 10 gm/cm2 often observed as the magnitude of unbalanced soft tissue resting pressures.
Periodontal Ligament and Bone Response to Sustained Force
The response to sustained force against the teeth is a function of force magnitude: heavy forces lead to rapidly developing pain, necrosis of cellular elements within the PDL, and the phenomenon (discussed in more detail later) of “undermining resorption” of alveolar bone near the affected tooth. Lighter forces are compatible with survival of cells within the PDL and a remodeling of the tooth socket by a relatively painless “frontal resorption” of the tooth socket. In orthodontic practice, the objective is to produce tooth movement as much as possible by frontal resorption, recognizing that some areas of PDL necrosis and undermining resorption will probably occur despite efforts to prevent this.
Biologic Control of Tooth Movement
Before discussing in detail the response to orthodontic force, it is necessary to consider the biologic control mechanisms that lead from the stimulus of sustained force application to the response of orthodontic tooth movement. Two possible control elements, biologic electricity and pressure-tension in the PDL that affects blood flow, are contrasted in the two major theories of orthodontic tooth movement. The bioelectric theory relates tooth movement at least in part to changes in bone metabolism controlled by biologic electricity that are produced by light pressure against the teeth. The pressure–tension theory relates tooth movement to cellular changes produced by chemical messengers, traditionally thought to be generated by alterations in blood flow through the PDL. Pressure and tension within the PDL, by reducing (pressure) or increasing (tension) the diameter of blood vessels in the ligament space, could certainly alter blood flow. The two theories are neither incompatible nor mutually exclusive, and it appears that both mechanisms may play a part in the biologic control of tooth movement.4
Biologic Electricity
Electric signals that might initiate tooth movement initially were thought to be piezoelectric. Piezoelectricity is a phenomenon observed in many crystalline materials in which a deformation of the crystal structure produces a flow of electric current as electrons are displaced from one part of the crystal lattice to another. The piezoelectricity of many inorganic crystals like those in bone has been recognized for many years and has been used in everyday technology (e.g., the crystal pickup in inexpensive phonographs). Organic crystals also can be piezoelectric, and collagen in the PDL is an excellent example.
Piezoelectric signals have two unusual characteristics: (1) a quick decay rate (i.e., when a force is applied, a piezoelectric signal is created in response that quickly dies away to zero even though the force is maintained) and (2) the production of an equivalent signal, opposite in direction, when the force is released (see Figure 8-2).
Both of these characteristics are explained by the migration of electrons within the crystal lattice as it is distorted by pressure. When the crystal structure is deformed, electrons migrate from one location to another and an electric current flow is observed. As long as the force is maintained, the crystal structure is stable and no further electric events are observed. When the force is released, however, the crystal returns to its original shape, and a reverse flow of electrons is seen. With this arrangement, rhythmic activity would produce a constant interplay of current flows in one direction and then the other that would be measured as amperes, whereas occasional application and release of force would produce only occasional signal of this type.
Ions in the fluids that bathe living bone interact with the complex electric field generated when the bone bends, causing electric signals in the form of volts as well as temperature changes. As a result, both convection and conduction currents can be detected in the extracellular fluids, and the currents are affected by the nature of the fluids. The small voltages that are observed are called the “streaming potential.” These voltages, though different from piezoelectric current flows, have in common their rapid onset and alteration as changing stresses are placed on the bone.
There is also a reverse piezoelectric effect. Not only will the application of force cause distortion of crystalline structure and with it an electric signal, but also application of an electric field can cause a crystal to deform and produce force in doing so. Reverse piezoelectricity has no place in natural control systems, at least as far as is presently known, but there are intriguing possibilities for using external electric fields to promote bone healing and regeneration after injury.5
There is no longer any doubt that stress-generated signals are important in the general maintenance of the skeleton. Without such signals, bone mineral is lost and general skeletal atrophy ensues—a situation that has proved troublesome for astronauts whose bones no longer flex in a weightless environment as they would under normal gravity. Signals generated by the bending of alveolar bone during normal chewing almost surely are important for maintenance of the bone around the teeth.
On the other hand, sustained force of the type used to induce orthodontic tooth movement does not produce prominent stress-generated signals. As long as the force is sustained, nothing happens. If stress-generated signals were important in producing the bone remodeling associated with orthodontic tooth movement, a vibrating application of pressure would be advantageous. Although earlier experiments indicated little or no advantage in vibrating over sustained force for the movement of teeth,6 this idea has been revived recently and is discussed below in the section of this chapter on possibilities for accelerating tooth movement. It still is the case, however, that the stress-generated signals that are so important for normal skeletal function have little if anything to do with the response to orthodontic tooth movement.
Electromagnetic fields also can affect cell membrane potentials and permeability and thereby trigger changes in cellular activity. In animal experiments, a pulsed electromagnetic field increased the rate of tooth movement, apparently by shortening the initial “lag phase” before tooth movement begins.7 This does not mean that the fields generated by small magnets attached to the teeth to generate tooth-moving forces (see Chapter 9) could change the basic biology of the response to force. Claims that moving teeth with magnetic force reduces pain and mobility are not supported by evidence.
Pressure–Tension in Periodontal Ligament
The pressure–tension theory, the classic theory of tooth movement, relies on chemical rather than electric signals as the stimulus for cellular differentiation and ultimately tooth movement. Chemical messengers are important in the cascade of events that lead to remodeling of alveolar bone and tooth movement, and both mechanical compression of tissues and changes in blood flow can cause their release. Because this does explain the course of events reasonably well,8 it remains the basis of the following discussion.
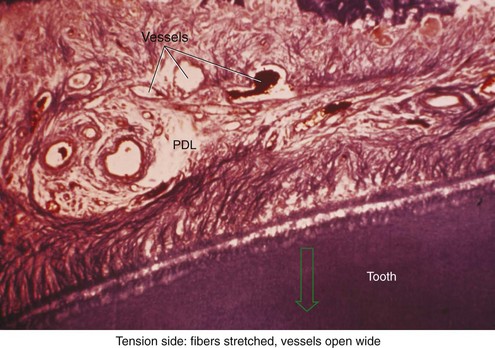
There is no doubt that sustained pressure against a tooth causes the tooth to shift position within the PDL space, compressing the ligament in some areas while stretching it in others. The mechanical effects on cells within the ligament cause the release of cytokines, prostaglandins, and other chemical messengers. In addition, blood flow is decreased where the PDL is compressed (Figure 8-4), while it is maintained or increased where the PDL is under tension (Figure 8-5). These alterations in blood flow also quickly create changes in the chemical environment. For instance, oxygen levels certainly would fall in the compressed area and carbon dioxide (CO2) levels would increase, while the reverse might occur on the tension side. These chemical changes, acting either directly or by stimulating the release of other biologically active agents, then would stimulate cellular differentiation and activity. In essence, this view of tooth movement shows three stages: (1) initial compression of tissues and alterations in blood flow associated with pressure within the PDL, (2) the formation and/or release of chemical messengers, and (3) activation of cells.
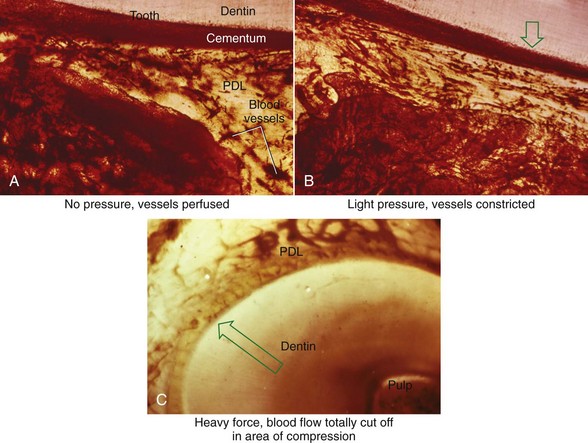
FIGURE 8-4 In experimental animals, changes in blood flow in the PDL can be observed by perfusing India ink into the vascular system while the animal is being sacrificed. The vessels are filled with India ink, so that their size can be seen easily. A, Normal perfusion of the PDL—note the dark areas indicating blood flow. B, 50 gm force compressing the PDL. Note the decreased amount of perfusion, but there still is blood flow through the compressed area. C, Heavy force with almost complete obliteration of blood flow in the compressed area. This specimen is seen in horizontal section, with the tooth root on the left and the pulp chamber just visible in the upper left. The PDL is below and to the right. Cells disappear in the compressed areas, and the area is sometimes said to be hyalinized because of its resemblance to hyaline cartilage. (Courtesy Dr. F. E. Khouw.)
Effects of Force Magnitude
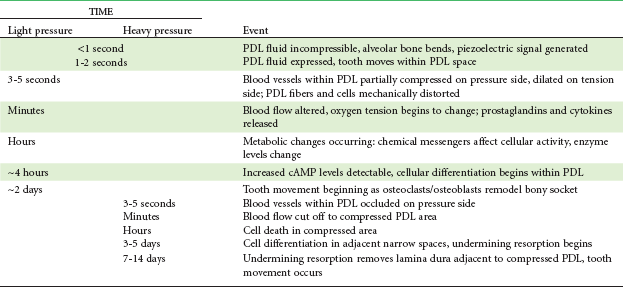
The heavier the sustained pressure, the greater should be the reduction in blood flow through compressed areas of the PDL, up to the point that the vessels are totally collapsed and no further blood flows (Figure 8-6). That this theoretical sequence actually occurs has been demonstrated in animal experiments, in which increasing the force against a tooth causes decreasing perfusion of the PDL on the compression side (see Figures 8-4 and 8-5).9 Let us consider the time course of events after application of orthodontic force, contrasting what happens with heavy versus light force (Table 8-2).
TABLE 8-2
Physiologic Response to Sustained Pressure Against a Tooth
PDL, Periodontal ligament; cAMP, cyclic adenosine monophosphate.
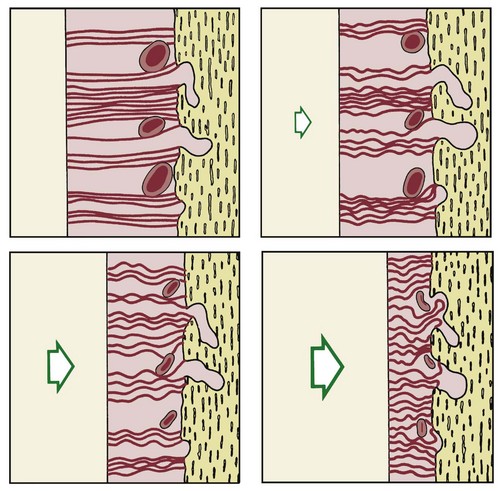
FIGURE 8-6 Diagrammatic representation of the increasing compression of blood vessels as pressure increases in the PDL. At a certain magnitude of continuous pressure, blood vessels are totally occluded and a sterile necrosis of PDL tissue ensues.
When light but prolonged force is applied to a tooth, blood flow through the partially compressed PDL decreases as soon as fluids are expressed from the PDL space and the tooth moves in its socket (i.e., in a few seconds). Within a few hours at most, the resulting change in the chemical environment produces a different pattern of cellular activity. Animal experiments have shown that increased levels of cyclic adenosine monophosphate (cAMP), the “second messenger” for many important cellular functions, including differentiation, appear after about 4 hours of sustained pressure. This amount of time to produce a response correlates rather well with the human response to removable appliances. If a removable appliance is worn less than 4 to 6 hours per day, it will produce no orthodontic effects. Above this duration threshold, tooth movement does occur.
What happens in the first hours after sustained force is placed against a tooth, between the onset of pressure and tension in the PDL and the appearance of second messengers a few hours later? Experiments have shown that prostaglandin and interleukin-1 beta levels increase within the PDL within a short time after the application of pressure, and it is clear now that prostaglandin E (PgE) is an important mediator of the cellular response. Since prostaglandins are released when cells are mechanically deformed, it appears that prostaglandin release is a primary rather than a secondary response to pressure. At the molecular level, we are beginning to understand how these effects are created. Focal adhesion kinase (FAK) appears to be the mechanoreceptor in PDL cells, and its compression is at least part of the reason that PgE2 is released.10 Experiments have shown that concentrations of the receptor activator of nuclear factor-kappa ligand (RANKL) and osteoprotegerin (OPG) in gingival crevicular fluid increase during orthodontic tooth movement, which suggests that PDL cells under stress may induce the formation of osteoclasts through upregulation of RANKL.11 Other chemical messengers, particularly members of the cytokine family but also nitric oxide (NO) and other regulators of cellular activity, also are involved.12 Since drugs of various types can affect both prostaglandin levels and other potential chemical messengers, it is clear that pharmacologic modification of the response to orthodontic force is more than just a theoretical possibility (see further discussion below).
For a tooth to move, osteoclasts must be formed so that they can remove bone from the area adjacent to the compressed part of the PDL. Osteoblasts also are needed to form new bone on the tension side and remodel resorbed areas on the pressure side. Prostaglandins have the interesting property of stimulating both osteoclastic and osteoblastic activity, making it particularly suitable as a mediator of tooth movement. If parathyroid hormone is injected, osteoclasts can be induced in only a few hours, but the response is much slower when mechanical deformation of the PDL is the stimulus, and it can be up to 48 hours before the first osteoclasts appear within and adjacent to the compressed PDL. Studies of cellular kinetics indicate that they arrive in two waves, implying that some (the first wave) may be derived from a local cell population, while others (the larger second wave) are brought in from distant areas via blood flow. These cells attack the adjacent lamina dura, removing bone in the process of “frontal resorption,” and tooth movement begins soon thereafter. At the same time, but lagging somewhat behind so that the PDL space becomes enlarged, osteoblasts (recruited locally from progenitor cells in the PDL) form bone on the tension side and begin remodeling activity on the pressure side.13
The course of events is different if the sustained force against the tooth is great enough to totally occlude blood vessels and cut off the blood supply to an area within the PDL. When this happens, rather than cells within the compressed area of the PDL being stimulated to develop into osteoclasts, a sterile necrosis ensues within the compressed area. In clinical orthodontics it is difficult to avoid pressure that produces at least some avascular areas in the PDL, and it has been suggested that releasing pressure against a tooth at intervals, while maintaining the pressure for enough hours to produce the biologic response, could help in maintaining tissue vitality. This seems to be the mechanism by which chewing on a plastic wafer or chewing gum after orthodontic force is applied reduces pain—chewing force briefly displaces the tooth and allows a spurt of blood into compressed areas, thereby reducing the size of necrotic areas in the PDL.
Because of its histologic appearance as the cells disappear, an avascular area in the PDL traditionally has been referred to as hyalinized (see Figure 8-4). Despite the name, the process has nothing to do with the formation of hyaline connective tissue. It represents the inevitable loss of all cells when the blood supply is totally cut off. When this happens, remodeling of bone bordering the necrotic area of the PDL must be accomplished by cells derived from adjacent undamaged areas.
After a delay of several days, cellular elements begin to invade the necrotic (hyalinized) area. More importantly, osteoclasts appear within the adjacent bone marrow spaces and begin an attack on the underside of the bone immediately adjacent to the necrotic PDL area (Figure 8-7). This process is appropriately described as undermining resorption, since the attack is from the underside of the lamina dura. When hyalinization and undermining resorption occur, an inevitable delay in tooth movement results. This is caused first by a delay in stimulating differentiation of cells within the marrow spaces, and second because a considerable thickness of bone must be removed from the underside before any tooth movement can take place. The different time course of tooth movement when frontal resorption is compared with undermining resorption is shown graphically in Figure 8-8.
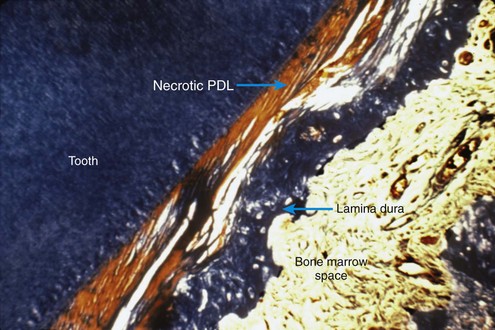
FIGURE 8-7 Histologic specimen of compressed PDL area after several days. When the PDL is compressed to the point that blood flow is totally cut off, differentiation of osteoclasts within the PDL space is not possible. After a delay of several days, osteoclasts within adjacent marrow spaces attack the underside of the lamina dura in the process called undermining resorption. (Courtesy Dr. F. E. Khouw.)
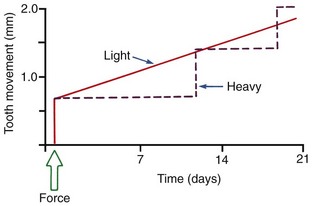
FIGURE 8-8 Diagrammatic representation of the time course of tooth movement with frontal resorption vs. undermining resorption. With frontal resorption, a steady attack on the outer surface of the lamina dura results in smooth continuous tooth movement. With undermining resorption, there is a delay until the bone adjacent to the tooth can be removed. At that point, the tooth “jumps” to a new position, and if heavy force is maintained, there will again be a delay until a second round of undermining resorption can occur.
Not only is tooth movement more efficient when areas of PDL necrosis are avoided, but pain is also lessened. However, even with light forces, small avascular areas are likely to develop in the PDL, and tooth movement will be delayed until these can be removed by undermining resorption. The smooth progression of tooth movement with light force shown in Figure 8-8 may be an unattainable ideal when continuous force is used. In clinical practice, tooth movement usually proceeds in a more stepwise fashion because of the inevitable areas of undermining resorption.
Effects of Force Distribution and Types of Tooth Movement
From the previous discussion, it is apparent that the optimum force levels for orthodontic tooth movement should be just high enough to stimulate cellular activity without completely occluding blood vessels in the PDL. Both the amount of force delivered to a tooth and the area of the PDL over which that force is distributed are important in determining the biologic effect. The PDL response is determined not by force alone, but by force per unit area, or pressure. Since the distribution of force within the PDL, and therefore the pressure, differs with different types of tooth movement, it is necessary to specify the type of tooth movement, as well as the amount of force in discussing optimum force levels for orthodontic purposes.
The simplest form of orthodontic movement is tipping. Tipping movements are produced when a single force (e.g., a spring extending from a removable appliance) is applied against the crown of a tooth. When this is done, the tooth rotates around its “center of resistance,” a point located about halfway down the root. (A further discussion of the center of resistance and its control follows in Chapter 9.) When the tooth rotates in this fashion, the PDL is compressed near the root apex on the same side as the spring and at the crest of the alveolar bone on the opposite side from the spring (Figure 8-9). Maximum pressure in the PDL is created at the alveolar crest and at the root apex. Progressively less pressure is created as the center of resistance is approached, and there is minimum pressure at that point.
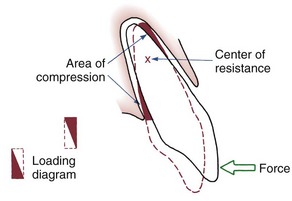
FIGURE 8-9 Application of a single force to the crown of a tooth creates rotation around a point approximately halfway down the root. Heavy pressure is felt at the root apex and at the crest of the alveolar bone, but pressure decreases to zero at the center of resistance. The loading diagram therefore consists of two triangles as shown.
In tipping, only one-half of the PDL area that could be loaded actually is. As shown in Figure 8-9, the “loading diagram” consists of two triangles, covering half of the total PDL area. On the other hand, pressure in the two areas where it is concentrated is high in relation to the force applied to the crown. For this reason, forces used to tip teeth must be kept quite low. Both experiments with animals and clinical experience with humans suggest that tipping forces for a single-rooted tooth should not exceed approximately 50 gm, and lighter forces are better for smaller teeth (which have a smaller PDL).
If two forces are applied simultaneously to the crown of a tooth, the tooth can be moved bodily (translated), that is, the root apex and crown move in the same direction the same amount. In this case, the total PDL area is loaded uniformly (Figure 8-10). It is apparent that to produce the same pressure in the PDL and therefore the same biologic response, twice as much force would be required for bodily movement as for tipping. To move a tooth so that it is partially tipped and partially translated would require forces intermediate between those needed for pure tipping and bodily movement (Table 8-3).
TABLE 8-3
Optimum Forces for Orthodontic Tooth Movement
| Type of movement | Force* (gm) |
| Tipping | 35-60 |
| Bodily movement (translation) | 70-120 |
| Root uprighting | 50-100 |
| Rotation | 35-60 |
| Extrusion | 35-60 |
| Intrusion | 10-20 |
*Values depend in part on the size of the tooth; smaller values appropriate for incisors, higher values for multirooted posterior teeth.
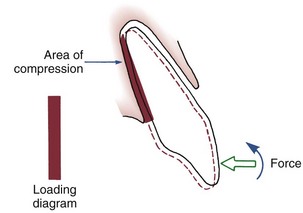
FIGURE 8-10 Translation or bodily movement of a tooth requires that the PDL space be loaded uniformly from alveolar crest to apex, creating a rectangular loading diagram. Twice as much force applied to the crown of the tooth would be required to produce the same pressure within the PDL for bodily movement as compared with tipping.
In theory, forces to produce rotation of a tooth around its long axis could be much larger than those to produce other tooth movements, since the force could be distributed over the entire PDL rather than over a narrow vertical strip. In fact, however, it is essentially impossible to apply a rotational force so that the tooth does not also tip in its socket, and when this happens, an area of compression is created just as in any other tipping movement. For this reason, appropriate forces for rotation are similar to those for tipping.
Extrusion and intrusion are also special cases. Extrusive movements ideally would produce no areas of compression within the PDL, only tension. Like rotation, this is more a theoretical than a practical possibility, since if the tooth tipped at all while being extruded, areas of compression would be created. Even if compressed areas could be avoided, heavy forces in pure tension would be undesirable unless the goal was to extract the tooth rather than to bring alveolar bone along with the tooth. Extrusive forces, like rotation, should be about the same magnitude as those for tipping.
For many years, it was considered essentially impossible to produce orthodontic intrusion of teeth. Now it is clear that clinically successful intrusion can be accomplished, but only if very light forces are applied to the teeth. Light force is required for intrusion because the force will be concentrated in a small area at the tooth apex (Figure 8-11). As with extrusion, the tooth probably will tip somewhat as it is intruded, but the force still will be concentrated at the apex. Only if the force is kept very light can intrusion be expected.
Effects of Force Duration and Force Decay
The key to producing orthodontic tooth movement is the application of sustained force, which does not mean that the force must be absolutely continuous. It does mean that the force must be present for a considerable percentage of the time, certainly hours rather than minutes per day. As we have noted previously, animal experiments suggest that only after force is maintained for approximately 4 hours do cyclic nucleotide levels in the PDL increase, indicating that this duration of pressure is required to produce the “second messengers” needed to stimulate cellular differentiation.
Clinical experience suggests that there is a threshold for force duration in humans in the 4 to 8 hour range and that increasingly effective tooth movement is produced if force is maintained for longer durations. Although no firm experimental data are available, a plot of efficiency of tooth movement as a function of force duration would probably look like Figure 8-12. Continuous forces, produced by fixed appliances that are not affected by what the patient does, produce more tooth movement than removable appliances unless the removable appliance is present almost all the time. Removable appliances worn for decreasing fractions of time produce decreasing amounts of tooth movement.
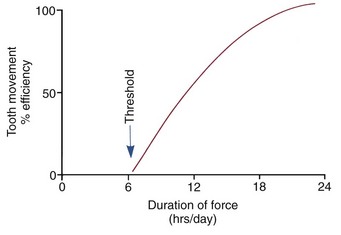
FIGURE 8-12 Theoretical plot of tooth movement efficiency versus duration of force in hours per day. Continuous force, 24 hours per day, produces the most efficient tooth movement, but successful tooth movement can be produced by shorter durations, with a threshold at about 6 hours.
Duration of force has another aspect, related to how force magnitude changes as the tooth responds by moving. Only in theory is it possible to make a perfect spring, one that would deliver the same force day after day, no matter how much or how little the tooth moved in response to that force. In reality, some decline in force magnitude (i.e., force decay) is noted with even the springiest device after the tooth has moved a short distance (though with the superelastic nickel–titanium materials discussed in Chapter 10, the decrease is amazingly small). With many orthodontic devices, the force may drop all the way to zero. From this perspective, orthodontic force duration is classified (Figure 8-13) by the rate of decay as:
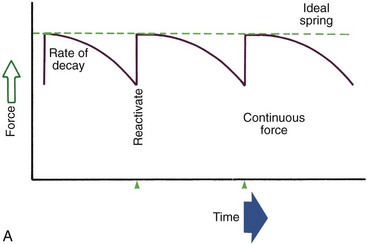
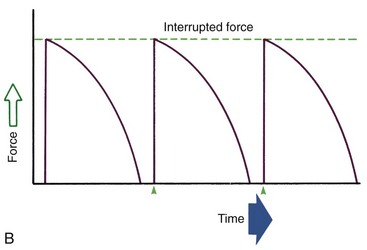
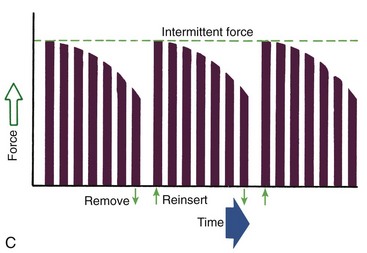
FIGURE 8-13 Diagrammatic representation of force decay. A, An ideal spring would maintain the same amount of force regardless of distance a tooth had moved, but with real springs the force decays at least somewhat as tooth movement occurs. Forces that are maintained between activations of an orthodontic appliance, even though the force declines, are defined as continuous. B, In contrast, interrupted forces drop to zero between activations. C, Intermittent forces fall to zero when a removable appliance is taken out, only to resume when the appliance is reinserted into the mouth. These forces also decay as tooth movement occurs.
• Continuous—force maintained at some appreciable fraction of the original from one patient visit to the next
• Interrupted—force levels decline to zero between activations
Both continuous and interrupted forces can be produced by fixed appliances that are constantly present.
• Intermittent—force levels decline abruptly to zero intermittently, when an orthodontic appliance or elastic attached to a fixed appliance is removed by the patient, and then return to the original level some time later. When tooth movement occurs, force levels will decrease as they would with a fixed appliance (i.e., the intermittent force can also become interrupted between adjustments of the appliance).
Intermittent forces are produced by all patient-activated appliances such as removable plates, headgear, and elastics. Forces generated during normal function (e.g., chewing, swallowing, speaking) can be viewed as a special case of intermittently applied forces, most of which are not maintained for enough hours per day to have significant effects on the position of the teeth.
There is an important interaction between force magnitude and how rapidly the force declines as the tooth responds. Consider first the effect of a nearly continuous force. If this force is quite light, a relatively smooth progression of tooth movement will result from frontal resorption. If the continuous force is heavy, however, tooth movement will be delayed until undermining resorption can remove the bone necessary to allow the tooth movement. At that time, the tooth will change its position rapidly, and the constant force will again compress the tissues, preventing repair of the PDL and creating the need for further undermining resorption and so on. Such a heavy continuous force can be quite destructive to both the periodontal structures and the tooth itself.
Consider now the effect of forces that decay fairly rapidly, so that the force declines to zero after the tooth moves only a short distance. If the initial force level is relatively light, the tooth will move a small amount by frontal resorption and then will remain in that position until the appliance is activated again. If the force level is heavy enough to produce undermining resorption, the tooth will move when the undermining resorption is complete. Then, since the force has dropped to zero at that point, it will remain in that position until the next activation. Although the original force is heavy, after the tooth moves there is a period for regeneration and repair of the PDL before force is applied again.
Theoretically, there is no doubt that light continuous forces produce the most efficient tooth movement. Despite the clinician’s best efforts to keep forces light enough to produce only frontal resorption, some areas of undermining resorption are probably produced in every clinical patient. The heavier forces that produce this response are physiologically acceptable only if the force level quickly drops to zero so that there is a period of repair and regeneration before the next activation or if the force decreases at least to the point that no second and third rounds of undermining resorption occur.
The bottom line: heavy continuous forces are to be avoided; heavy intermittent forces, though less efficient, can be clinically acceptable. To say it another way: the more perfect the spring in the sense of its ability to provide continuous force, the more careful the clinician must be that only light force is applied. Some of the cruder springs used in orthodontic treatment have the paradoxical virtue of producing forces that rapidly decline to zero and are thus incapable of inflicting the biologic damage that can occur from heavy continuous forces. Several clinical studies have indicated that heavy force applications may produce more tooth movement than lighter ones, which can be understood only from consideration of force decay characteristics.
Experience has shown that orthodontic appliances should not be reactivated more frequently than at 3-week intervals. A 4- to 6-week appointment cycle is more typical in clinical practice. Undermining resorption requires 7 to 14 days (longer on the initial application of force, shorter thereafter). When this is the mode of tooth movement and when force levels decline rapidly, tooth movement is essentially complete in this length of time. The wisdom of the interval between adjustments now becomes clear. If the appliance is springy and light forces produce continuous frontal resorption, there is no need for further activation. If the appliance is stiffer and undermining resorption occurs, but then the force drops to zero, the tooth movement occurs in the first 10 days or so, and there is an equal or longer period for PDL regeneration and repair before force is applied again. This repair phase is highly desirable and needed with many appliances. Activating an appliance too frequently, short circuiting the repair process, can produce damage to the teeth or bone that a longer appointment cycle would have prevented or at least minimized.
Drug Effects on the Response to Orthodontic Force
At present, drugs that stimulate tooth movement are unlikely to be encountered, although efforts to produce them continue. A major problem is how they would be applied to the local area where an effect on tooth movement is desired. Direct injection of prostaglandin into the PDL has been shown to increase the rate of tooth movement, but this is quite painful (a bee sting is essentially an injection of prostaglandin) and not very practical. Relaxin, a “pregnancy hormone” discovered in the 1980s, facilitates birth by causing a softening and lengthening of the cervix and pubic symphysis. It works by simultaneously reducing collagen synthesis and increasing collagen breakdown. The effects on collagen seem to make it more than just a pregnancy hormone, especially since its peak levels in pregnancy occur well before birth. Preliminary data in rats showed faster tooth movement with relaxin treatment, but a well-done, double-blinded clinical trial at the University of Florida, in which relaxin or just physiologic saline was injected adjacent to a tooth to be moved, did not show a consistent positive effect,14 and further clinical trials have been delayed. It seems likely that at some point in the future, drugs to facilitate tooth movement will become clinically useful—but there is no way to know how long it will take to develop them.
Drugs that inhibit tooth movement as a side effect of their use for other problems, however, already are encountered frequently, though not yet prescribed for their tooth-stabilizing effect. Two types of drugs are known to depress the response to orthodontic force and may influence current treatment: prostaglandin inhibitors for pain control (especially the more potent members of this group that are used in treatment of arthritis, like indomethacin),15 and the bisphosphonates used in treatment of osteoporosis (e.g., Fosamax, Actonel, Boniva, Reclast, Atelvia).
Prostaglandin Inhibitors
If PgE plays an important role in the cascade of signals that leads to tooth movement, one would expect inhibitors of its activity to affect tooth movement. Drugs that affect prostaglandin activity fall into two categories: (1) corticosteroids and nonsteroidal antiinflammatory drugs (NSAIDs) that interfere with prostaglandin synthesis and (2) other agents that have mixed agonistic and antagonistic effects on various prostaglandins. In the body, prostaglandins are formed from arachidonic acid, which in turn is derived from phospholipids. Corticosteroids reduce prostaglandin synthesis by inhibiting the formation of arachidonic acid; NSAIDs inhibit the conversion of arachidonic acid to prostaglandins.
Most over-the-counter analgesics are NSAIDs and therefore are prostaglandin inhibitors (aspirin, ibuprofen, Naprosyn [Aleve], and many others). The major exception is acetaminophen (Tylenol), which acts centrally rather than peripherally. This raises the interesting possibility that the medication used by many patients to control pain after orthodontic appointments could interfere with tooth movement. Fortunately, with the low doses and short durations of analgesic therapy in orthodontic patients, this does not occur, but it can become a problem in adults or children being treated for arthritis. Control of pain related to orthodontic treatment is discussed later in more detail.
Several other classes of drugs can affect prostaglandin levels and therefore could affect the response to orthodontic force. Tricyclic antidepressants (doxepin, amitriptyline, imipramine), antiarrhythmic agents (procaine), antimalarial drugs (quinine, quinidine, chloroquine), and methylxanthines fall into this category. In addition, the anticonvulsant drug phenytoin has been reported to decrease tooth movement in rats, and some tetracyclines (e.g., doxycycline) inhibit osteoclast recruitment, an effect similar to bisphosphonates.16 It is possible that unusual responses to orthodontic force could be encountered in patients taking any of these medications.
Bisphosphonates
Osteoporosis is a problem particularly in postmenopausal females but is associated with aging in both sexes and now also is being used in children who require long-term steroids. Estrogen therapy, which was used frequently in the past to prevent loss of bone in older women, now has been shown to carry significant risks with it and is not widely used. Estrogens have little or no effect on orthodontic treatment, but pharmacologic agents that inhibit bone resorption are a potential problem. At present, bisphosphonates, synthetic analogues of pyrophosphate that bind to hydroxyapatite in bone, are the major class of drugs of this type. They act as specific inhibitors of osteoclast-mediated bone resorption, so it is not surprising that the bone remodeling necessary for tooth movement is slower in patients on this medication.
Bisphosphonates are a particular problem for two reasons:
1. Their use has been associated with an unusual necrosis of mandibular bone. This typically occurs after extraction of a tooth or other injury to the bone, which fails to heal and becomes the center of an expanding necrotic area. Fortunately, this is rare and occurs most often in patients with metastatic bone cancer who receive high doses of potent bisphosphonates, but elective extractions for orthodontic purposes should be avoided for a patient who has been taking any of these drugs.
2. They are incorporated into the structure of bone, then slowly eliminated over a period of years—so stopping the drug does not eliminate all of its effects. It appears that there are two elimination rates: a fast elimination from the surface of the bones within some weeks and slower elimination from bone structure. Fortunately, most of the drug is only on the surface, which makes orthodontic treatment possible after about 3 months with no further bisphosphonate therapy.17 Obviously, treatment would be possible only if the physician were willing for the patient to have a drug holiday or if the patient could be switched to Evista (the estrogen analogue with the most effect on bone), at least temporarily.
Effects of Local Injury: Corticotomy and Accelerated Tooth Movement
Because remodeling of alveolar bone is the key component of orthodontic tooth movement and bone remodeling is accelerated during wound healing,18 the idea that teeth could be moved faster after local injury to the alveolar process first appeared early in the history of orthodontics. The pioneer American oral surgeon Hullihan is said to have experimented with moving teeth after making cuts in alveolar bone in the late nineteenth century, and sporadic experiments with this continued into the early twentieth century. The approach was not widely adopted, however, for several reasons that included concerns about infections and bone loss in this pre-antibiotic era. In mid-century, the German surgeon Köle revived the idea that cuts between teeth could produce faster tooth movement.19 This method was advocated in the United States at that time by Merrill at the University of Oregon and was again suggested in 1978 by Gunderson et al,20 but it was viewed as unnecessarily invasive and was not widely accepted.
The idea that local injury to alveolar bone, in the form of cuts through the cortex of the bone between the teeth, could speed tooth movement was presented again in the late 1990s and has achieved some acceptance as its mechanism has become better understood. At this point, there still are remarkably few papers in the peer-reviewed literature to document the results. The next section is an effort to put injury to alveolar bone, now usually called corticotomy, in a current perspective.
Surgical Techniques
Köle’s approach of 50 years ago to using local injury to accelerate tooth movement called for flap surgery to reflect the gingiva, then vertical cuts facially and lingually between and under the teeth that did not penetrate all the way through to the other side. An orthodontic appliance (placed before the surgery) was activated as soon as possible, using relatively stiff archwires, and the teeth were pulled into alignment. The surgery was conceptualized as creating blocks of bone around the teeth that could be repositioned without depending on remodeling created by the PDL responses described previously. The method therefore would be considered a variation of distraction osteogenesis from a current perspective. As with distraction for other purposes, enough initial force would be needed to fracture any small areas that were not cut at surgery (see the discussion of distraction osteogenesis and the principles on which it is based at the end of this chapter).
Distraction of an alveolar segment containing a tooth has been considered in at least two circumstances. The first is bringing an ankylosed tooth into position, and of course, the only way to move an ankylosed tooth would be to move the segment of bone to which it has become attached (Figure 8-14). The technique is to make bone cuts that either totally free the segment or leave only a small area of bone attached. After a latency period of approximately 5 days to allow bone healing to reach the callus stage, either a jackscrew attached to the segment and to adjacent alveolar bone or an archwire is used to move the segment.
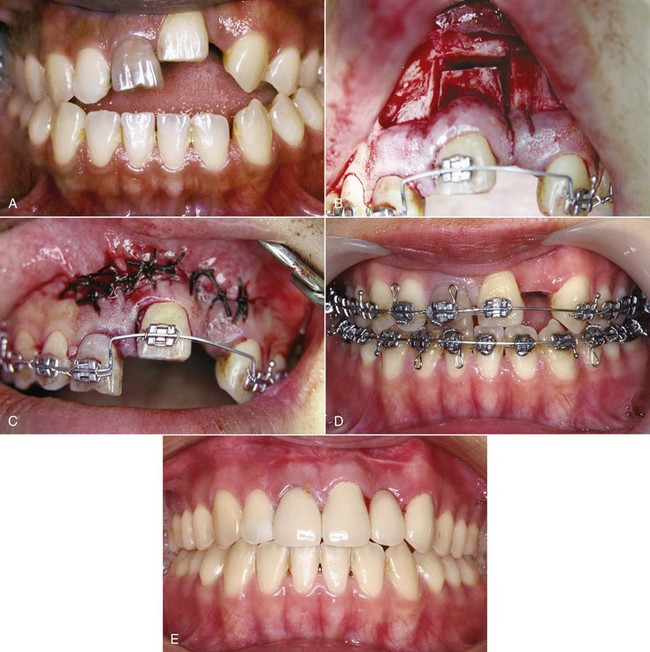
FIGURE 8-14 An ankylosed tooth can be moved only by moving the bone to which it is attached. Distraction osteogenesis allows that to be done. A, Age 21, maxillary central incisor that ankylosed after an accident at age 8 (the lateral incisor was lost at that time). B, Creation of the bony segment to be moved. C, Closure of the wound. A period of initial healing, usually 5 to 7 days, is allowed before the archwire is activated to begin movement of the segment. D, The tooth nearly in final position, 3 weeks later. E, Treatment completed, with prosthetic replacement of the missing lateral incisor. (Courtesy Dr. H. Chen.)
For repositioning an ankylosed maxillary incisor, the most frequent indication for alveolar distraction, even the smallest jackscrew is bulky and obvious. A relatively stiff archwire does not give as precise control over the rate of movement but is much more tolerable to the patient and can be quite effective (see Figure 8-14).21 In theory, a similar approach could be used to move teeth affected by primary failure of eruption (PFE; see Chapter 4), but this is feasible only if PFE developed after a tooth had erupted at least partially and is difficult to impossible when more than one posterior tooth in a quadrant is involved.
The other circumstance is retraction of a tooth, usually a maxillary canine, across an extraction site created adjacent to it. Interestingly, this does not involve moving a segment of bone that contains the tooth. Instead, cuts are made in the walls of the socket where the premolar was extracted, and then the canine is moved with a spring providing heavy force so that the PDL is extremely stretched. Although this is called PDL distraction, it is not distraction osteogenesis in the usual sense. Only selected case reports have appeared in the literature,22 and the amount of reduction in total treatment time has not been established.
More recently, rapid tooth movement after corticotomy has come to be viewed as a demineralization/remineralization phenomenon that produces a regional acceleration of bone remodeling that allows faster tooth movement, rather than movement of blocks of bone that contain a tooth. Now, lighter force to move teeth more physiologically while taking advantage of more widespread remodeling of alveolar bone is recommended, and the surgical approach has been broadened into “accelerated osteogenic orthodontics” (AOO) by adding areas of decortication over the facial surfaces of alveolar bone that are then covered with particulate bone grafting material (demineralized freeze-dried bone or a mixture of this with bovine bone or allograft bone; Figure 8-15).23 This adds modeling (changing the external shape of bone) to remodeling after local injury. One of the risks of expansion of the dental arches, of course, is fenestration of the alveolar bone, and the AOO approach is said to generate new bone that allows facial movement of teeth without this risk.
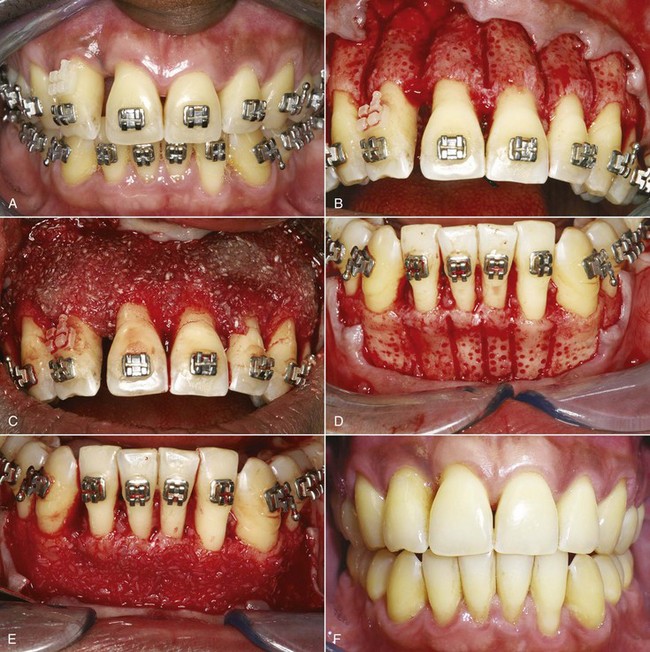
FIGURE 8-15 A, For this adult with an upper incisor that was brought down into position and crowding in the lower incisor region, corticotomy and bone grafting on the facial surface (AOO) was planned. B, After reflection of a flap, corticotomy cuts between the teeth were made and small circular depressions were placed in the facial surface of the bone over the maxillary anterior teeth. C, Bone graft material in the form of a slurry of demineralized freeze-dried bone was placed over the facial surface. D, Corticotomy and preparation of the bone over the lower incisor was done at the same time, and E, bone graft material was placed to reduce the chance of bone loss as the lower incisors were advanced. F, 11 months later, after completion of treatment that required 6 months, with good healing of the alveolar bone. (Courtesy Dr. S. Dibart.)
Treatment Outcomes in Corticotomy-Assisted Tooth Movement
In order to evaluate the outcomes of corticotomy-assisted orthodontics, as with any other type of treatment, an analysis of benefit versus cost and risk is needed. The primary benefit claimed for corticotomy is a reduction in treatment time, with facilitation of arch expansion via AOO as a secondary benefit.
Reduction of treatment time has been presented primarily as case reports that show a reduction in time to alignment for selected patients. After a fracture, bone healing takes about 6 weeks; after distraction osteogenesis, which would be a more pertinent comparison, 2 months of stabilization is recommended and mature bone in the bone regenerate area is seen after 4 months—so one would expect that bone remodeling after corticotomy could be accelerated for 2 to 4 months. Experiments in dogs and rats that show faster tooth movement after corticotomy have not provided data as to the duration of the accelerated bone response.24,25
Alignment is the first phase of comprehensive orthodontic treatment. Its duration obviously depends on the extent of crowding, but even severe crowding rarely requires more than 5 months with superelastic archwires. If corticotomy reduced that to 1 month, the 4-month reduction in total treatment time would represent about 20% of the typical treatment time of 18 to 21 months. Can such a reduction be expected routinely? Is a greater reduction possible? If so, what would be the mechanism?
Does corticotomy reduce treatment time for tooth movement other than alignment? A special indication might be intrusion, which requires remodeling of the denser bone that lies beneath the tooth roots and often takes several months. Skeletal anchorage now makes it possible to intrude posterior teeth, which allows correction of anterior open bite primarily by posterior intrusion (see Chapter 18). The rate of intrusion typically is 1 mm/month. One recent paper, again just a case report, reported that after an osteotomy beneath the incisors and application of the AOO approach in conjunction with skeletal anchorage, it still took several months to obtain the desired intrusion.26 No published evidence in a peer-reviewed journal supports the claim of more rapid intrusion.
In the cost and risk side of the evaluation, cost includes all aspects of the “burden of treatment.” In addition to the economic cost of the surgery (which can require several hours), morbidity and inconvenience of all types need to be evaluated. This information simply is not available at present. On the risk side, what is the chance of complications of the surgery and what problems are most likely to arise? Wilcko et al commented that in adolescents there appeared to be no loss in alveolar bone height, but some (insignificant?) decrease in bone height would be expected in adults. No data were provided, and this report did not note any other complications or problems.27
Modified Corticotomy
A frequent concern with corticotomy/AOO is that the surgery is quite extensive. This has led to modifications of the corticotomy technique that typically involve incisions in the interproximal gingiva so that reflecting flaps is not necessary and less extensive cuts in the bone (Figure 8-16).28 It still is possible to tunnel beneath the gingiva over the root to add graft material if this is desired, and case reports suggest that the modified approach produces similar results to full-fledged AOO.
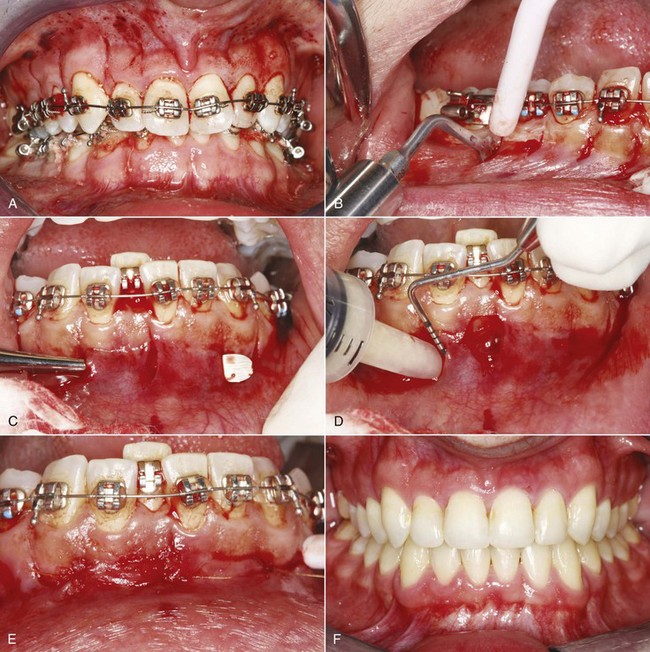
FIGURE 8-16 A, Modified corticotomy avoids reflecting a flap, using thin micro-incisions through the facial tissue. B, A piezoelectric knife is used to penetrate the cortical bone and extend into the medullary bone between the teeth. C, If a bone graft is desired, a tunnel under the soft tissue is established, and D, the graft slurry is placed into the area with a syringe. E, Appearance at the end of the procedure, with the graft material in place. F, 10 months later. (Courtesy Dr. S. Dibart.)
An approach that is even less extensive in the amount of local injury has been proposed recently and now is in the clinical trial stage of development (PROPEL, Alveologic LLC, Briarcliff Manor, NY). This is based on “microperforation,” in which screws like those used for skeletal anchorage (discussed in some detail later) are placed through the gingiva into interproximal alveolar bone and then removed. It is said that three such perforations in each interproximal area are enough to generate a regional acceleration of bone remodeling, and thereby produce faster tooth movement.
Perhaps a fair conclusion is that for some patients, the cost-risk/benefit ratio is favorable and corticotomy and/or AOO, especially with the modified approaches that reduce the amount of surgical intervention, would be a valuable adjunct to orthodontic treatment. Until evidence is available to document this when specific indications are present, caution in recommending it seems prudent.
Other Proposed Approaches to Faster Tooth Movement
Three other methods intended to accelerate tooth movement have been proposed quite recently: vibration of the teeth, application of light to the alveolar process, and application of therapeutic ultrasound to the teeth and adjacent bone. For all three, commercialization is proceeding, presumably with scientific evidence of effectiveness to follow.
The AcceleDent vibratory system (OrthoAccel Technologies, Inc., Houston, TX), unlike the efforts 40 years ago to induce piezoelectric currents that we have already discussed, is based on delivery of high-frequency vibration (30 Hz) to the teeth for approximately 20 minutes per day (Figure 8-17). The rationale is that this stimulates cell differentiation and maturation, so that the bone remodeling that is necessary for tooth movement occurs more quickly. From that perspective, the effect appears to be analogous to local injury (i.e., creation of microfractures in the alveolar bone) just with a different and less invasive way to produce the injury effect than corticotomy or bone perforation. Bone remodeling in general can be conceptualized as the ongoing cleanup of microfractures produced by function.
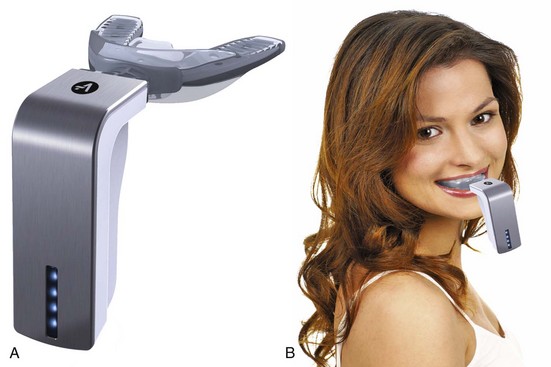
FIGURE 8-17 A, The AcceleDent vibration device consists of a mouthpiece that is energized by a battery-powered device into which it is inserted. B, The patient bites down onto the mouthpiece, which vibrates at 30 Hz for 20 minutes daily. (Courtesy OrthoAccel Technologies, Inc., Houston, TX.)
A patent application for the use of phototherapy to speed tooth movement was filed in late 2010, and clinical trials now are being conducted. Phototherapy (Biolux) uses light with an 800 to 850 nanometer wave length (just above the visible spectrum) that penetrates soft tissue and “infuses light energy directly into the bone tissue” (Figure 8-18). Experiments have shown that about 97% of the light energy is lost before it penetrates through the cheeks and alveolar bone to the interior of a recent premolar extraction site, but the remaining 3% is said to have enough energy to excite intracellular enzymes and increase cellular activity in the PDL and bone. This presumably would increase the rate of bone remodeling and tooth movement. Phototherapy in other applications has been shown to increase blood flow, and this also might affect the rate of tooth movement. An interesting aspect of the Biolux device is that it can be adjusted to apply light to only the anterior teeth, the whole arch, or only the posterior teeth, which certainly would potentially improve anchorage control if the light application does speed up tooth movement in the illuminated area.
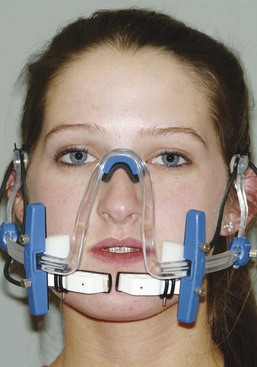
FIGURE 8-18 A prototype Biolux device, which delivers light at a frequency above the human visible spectrum that penetrates the cheeks and the soft tissue over the alveolar bone. It also is to be worn 20 minutes per day.
Ultrasound to the alveolar process during orthodontic tooth movement also is intended to alter the biology, with the expectation that it will reduce root resorption and facilitate tooth movement. It is known that therapeutic ultrasound (which is different from diagnostic ultrasound) increases blood flow in the treated area, and the theory is that increased blood flow in the PDL would decrease or perhaps eliminate the formation of hyalinized areas and thereby reduce root resorption. Perhaps this also would increase the rate of bone remodeling and tooth movement.
Will any of these approaches really prove to be effective in humans? The fact that Food and Drug Administration (FDA) approval is being sought for vibration and phototherapy (AcceleDent already has FDA clearance), and is likely to be required for therapeutic ultrasound, means that some data for clinical outcomes will have to be supplied before these devices are cleared for clinical use in the United States. Most innovations in orthodontic therapy (including corticotomy and AOO) have been developed outside any type of regulatory review and have been marketed well in advance of scientific data—so FDA clearance for any new orthodontic devices represents a step forward toward evidence-based therapy.
Anchorage and its Control
Anchorage: Resistance to Unwanted Tooth Movement
The term anchorage, in its orthodontic application, is defined in an unusual way: the definition as “resistance to unwanted tooth movement” includes a statement of what the dentist desires. The usage, though unusual, is clearest when presented this way. The dentist or orthodontist always constructs an appliance to produce certain desired tooth movements. For every (desired) action, there is an equal and opposite reaction. Inevitably, reaction forces can move other teeth as well if the appliance contacts them. Anchorage, then, is the resistance to reaction forces that is provided usually by other teeth, occasionally by the palate, sometimes by the head or neck (via extraoral force), and more and more often by anchors screwed to the jaws.
At this point, let us focus first on controlling unwanted tooth movement when some teeth are to serve as anchors. In planning orthodontic therapy, it is simply not possible to consider only the teeth whose movement is desired. Reciprocal effects throughout the dental arches must be carefully analyzed, evaluated, and controlled. An important aspect of treatment is maximizing the tooth movement that is desired, while minimizing undesirable side effects.
Relationship of Tooth Movement to Force
An obvious strategy for anchorage control would be to concentrate the force needed to produce tooth movement where it is desired, and then to dissipate the reaction force over as many other teeth as possible, keeping the pressure in the PDL of anchor teeth as low as possible. A threshold, below which pressure would produce no reaction, could provide perfect anchorage control, since it would only be necessary to be certain that the threshold for tooth movement was not reached for teeth in the anchorage unit. A differential response to pressure, so that heavier pressure produced more tooth movement than lighter pressure, would make it possible to move some teeth more than others, even though some undesired tooth movement occurred.
In fact, the threshold for tooth movement appears to be quite low, but there is a differential response to pressure, and so this strategy of “divide and conquer” is reasonably effective. As Figure 8-19 indicates, teeth behave as if orthodontic movement is proportional to the magnitude of the pressure, up to a point. When that point is reached, the amount of tooth movement becomes more or less independent of the magnitude of the pressure, so that a broad plateau of orthodontically effective pressure is created.29 The optimum force level for orthodontic movement is the lightest force and resulting pressure that produces a near-maximum response (i.e., at the edge of the plateau). Forces greater than that, though equally effective in producing tooth movement, would be unnecessarily traumatic and, as we will see, unnecessarily stressful to anchorage.
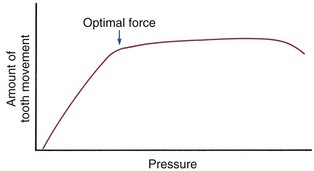
FIGURE 8-19 Theoretical representation of the relationship of pressure within the PDL to the amount of tooth movement. Pressure in the PDL is determined by the force applied to a tooth divided by the area of the PDL over which that force is distributed. The threshold for tooth movement is very small. Tooth movement increases as pressure increases up to a point, remains at about the same level over a broad range, and then may actually decline with extremely heavy pressure. The best definition of the optimum force for orthodontic purposes is the lightest force that produces a maximum or near-maximum response (i.e., that brings pressure in the PDL to the edge of the nearly-constant portion of the response curve). The magnitude of the optimum force will vary, depending on the way it is distributed in the PDL (i.e., is different for different types of tooth movement [tipping, bodily movement, intrusion, and so on]).
Anchorage Situations
From this background, we can now define several anchorage situations.
Reciprocal Tooth Movement: In a reciprocal situation, the forces applied to teeth and to arch segments are equal, and so is the force distribution in the PDL. A simple example is what would occur if two maxillary central incisors separated by a diastema were connected by an active spring (Figure 8-20). The essentially identical teeth would feel the same force distributed in the same way through the PDL and would move toward each other by the same amount.
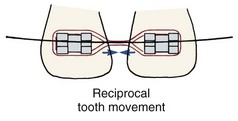
FIGURE 8-20 Reciprocal tooth movement is produced when two teeth or resistance units of equal size pull against each other, as in this example of the reciprocal closure of a maxillary midline diastema.
A somewhat similar situation would arise if a spring were placed across a first premolar extraction site, pitting the central incisor, lateral incisor, and canine in the anterior arch segment against the second premolar and first molar posteriorly. Whether this would really produce reciprocal tooth movement requires some thought. Certainly the same force would be felt by the three anterior teeth and the two posterior teeth, since the action of the spring on one segment has an equal and opposite reaction on the other. Reciprocal movement would require the same total PDL area over which the force was distributed.
Conceptually, the “anchorage value” of a tooth, that is, its resistance to movement, can be thought of as a function of its root surface area, which is the same as its PDL area. The larger the root, the greater the area over which a force can be distributed, and vice versa. As Figure 8-21 shows, the PDL area for the two posterior teeth in this example is slightly larger than the total anterior PDL area. Therefore, with a simple spring connecting the segments, the anterior teeth would move slightly more than the posterior teeth. The movement would not be truly reciprocal but would be close to it.
FIGURE 8-21 The “anchorage value” of any tooth is roughly equivalent to its root surface area. As this diagram shows, the first molar and second premolar in each arch are approximately equal in surface area to the canine and two incisors. (Modified from Freeman DC. Root Surface Area Related to Anchorage in the Begg Technique. Memphis: University of Tennessee Department of Orthodontics, M.S. Thesis; 1965.)
Reinforced Anchorage: Continuing with the extraction site example: if it was desired to differentially retract the anterior teeth, the anchorage of the posterior teeth could be reinforced by adding the second molar to the posterior unit (see Figure 8-21). This would change the ratio of the root surface areas so that there would be relatively more pressure in the PDL of the anterior teeth and therefore relatively more retraction of the anterior segment than forward movement of the posterior segment.
Note that reinforcing anchorage by adding more resistance units is effective because with more teeth (or extraoral structures) in the anchorage, the reaction force is distributed over a larger PDL area. This reduces the pressure on the anchor units, moving them down the slope of the pressure–response curve. Now the shape of the pressure–response curve becomes important. Keeping the force light has two virtues. Not only does it minimize trauma and pain but also it makes it possible to create anchorage by taking advantage of different PDL areas in the anchor segments. As Figure 8-22 illustrates, too much force destroys the effectiveness of reinforced anchorage by pulling the anchor teeth up onto the flatter portion of the pressure–response curve. Then the clinician is said to have slipped, burned, or blown the anchorage by moving the anchor teeth too much.
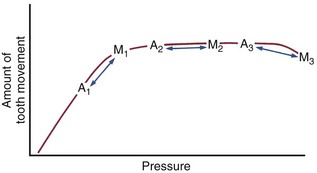
FIGURE 8-22 Consider the response of anchor teeth (A on the chart) and teeth to be moved (M) in three circumstances. In each case, the pressure in the PDL of the anchor teeth is less than the pressure in the PDL of the teeth to be moved because there are more teeth in the anchor unit. In the first case (A1-M1), the pressure for the teeth to be moved is optimal, whereas the pressure in the anchor unit is suboptimal, and the anchor teeth move less (anchorage is preserved). In the second case (A2-M2), although the pressure for the anchor teeth is less than for the teeth to be moved, both are on the plateau of the pressure-response curve, and the anchor teeth can be expected to move as much as the teeth that are desired to move (anchorage is lost). With extremely high force (A3-M3), the anchor teeth might move more than the teeth it was desired to move. Although the third possibility is theoretical and may not be encountered clinically, both the first and second situations are seen in clinical orthodontics. This principle explains the efficacy of light forces in controlling anchorage, and why heavy force destroys anchorage.
Stationary Anchorage: The term stationary anchorage, traditionally used though inherently less descriptive than the term reinforced anchorage, refers to the advantage that can be obtained by pitting bodily movement of one group of teeth against tipping of another (Figure 8-23). Using our same example of a premolar extraction site, if the appliance were arranged so that the anterior teeth could tip lingually while the posterior teeth could only move bodily, the optimum pressure for the anterior segment would be produced by about half as much force as if the anterior teeth were to be retracted bodily. This would mean that the reaction force distributed over the posterior teeth would be reduced by half, and as a consequence, these teeth would move half as much.
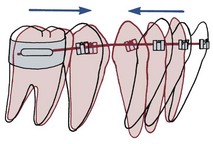
FIGURE 8-23 Displacement of anchor teeth can be minimized by arranging the force system so that the anchor teeth must move bodily if they move at all, while movement teeth are allowed to tip, as in this example of retracting incisors by tipping them posteriorly. The approach is called stationary anchorage. In this example, treatment is not complete because the roots of the lingually tipped incisors will have to be uprighted at a later stage, but two-stage treatment with tipping followed by uprighting can be used as a means of controlling anchorage. Distributing the force over a larger PDL area of the anchor teeth reduces pressure there.
If PDL areas were equal, tipping the anterior segment while holding the posterior segment for bodily movement would have the effect of doubling the amount of anterior retraction compared with posterior forward movement. It is important to note again, however, that successful implementation of this strategy requires light force. If the force were large enough to bring the posterior teeth into their optimum movement range, it would no longer matter whether the anterior segment tipped or was moved bodily. Using too much force would disastrously undermine this method of anchorage control.
Differential Effect of Very Large Forces: If tooth movement were actually impeded by very high levels of pressure, it might be possible to structure an anchorage situation so that there was more movement of the arch segment with the larger PDL area. This result could happen, of course, if such high force were used that the smaller segment was placed beyond the greatest tooth movement range, while the larger segment was still in it (see Figure 8-22). Because the effect would be highly traumatic, it would be an undesirable way to deliberately manage anchorage.
In fact, it is not certain that the amount of tooth movement in response to applied force really decreases with very high force levels in any circumstance, and so this type of differential movement may not really exist. By using too much force, however, it is certainly possible to produce more movement of the anchor segment than was expected, even if the mechanism is merely a differential movement of the anchor segment up the slope of the pressure–response curve rather than a decline in the response of the movement segment. Differential force is understood best in terms of the plateau portion of the curve in Figures 8-19 and 8-22, not the questionable decline at the far right.
Cortical Anchorage: Another consideration in anchorage control is the different response of cortical compared with medullary bone. Cortical bone is more resistant to resorption, and tooth movement is slowed when a root contacts it. Some authors have advocated torquing the roots of posterior teeth outward against the cortical plate as a way to inhibit their mesial movement when extraction spaces are to be closed. Since the mesial movement would be along rather than against the cortical plate, it is doubtful that this technique greatly augments anchorage (although it has the potential to create root resorption). However, a layer of dense cortical bone that has formed within the alveolar process can certainly affect tooth movement. This situation may be encountered at an old extraction site, for example, in an adult in whom a molar or premolar was lost many years previously (Figure 8-24). It can be very difficult to close such an extraction site because tooth movement is slowed to a minimum as the roots encounter cortical bone along the resorbed alveolar ridge.
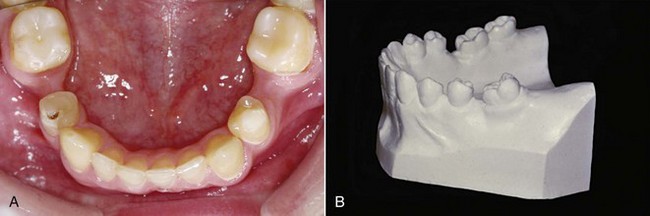
FIGURE 8-24 Loss of alveolar bone at an old extraction site can create an area of cortical bone between adjacent teeth, as the alveolar process resorbs and narrows. A, This child lost second primary molars early and was congenitally missing the second premolars. The greater ridge resorption on the right than the left side indicates that the right second primary molar was lost first. This is one situation in which “cortical anchorage” definitely can be a factor. Closing such an extraction site is extremely difficult because of the resistance of cortical bone to remodeling. B, In adults who “lost” permanent first molars in adolescence, the second molar tips mesially, but resorption of alveolar bone at the extraction site narrows the ridge. Closing these spaces also is difficult and slow because remodeling of cortical bone is required.
As a general rule, torquing movements are limited by the facial and lingual cortical plates. If a root is persistently forced against either of these cortical plates, tooth movement is greatly slowed and root resorption is likely, but penetration of the cortical bone may occur. Although it is possible to torque the root of a tooth labially or lingually out of the bone (Figure 8-25), fortunately, it is difficult to do so.
Skeletal Anchorage: It has long been realized that if structures other than the teeth could be made to serve as anchorage, it would be possible to produce tooth movement or growth modification without unwanted side effects. Until the turn of the twenty-first century, extraoral force (headgear) and to a lesser extent the anterior palate were the only ways to obtain anchorage that was not from the teeth. Although headgear can be used to augment anchorage, there are two problems: (1) it is impossible for a patient to wear headgear all the time, and most wear it half the time at best, and (2) when headgear is worn, the force against the teeth is larger than optimal. The result is a force system that is far from ideal. Heavy intermittent force from headgear is simply not a good way to counterbalance the effect of light continuous force from the orthodontic appliance. It is not surprising that headgear to the anchor segment of a dental arch usually does not control its movement very well. In theory, additional anchorage can be obtained from the rugae area of the palate; in fact, this is not very effective (see Chapter 15).
With the development of successful bone implant techniques to replace missing teeth, it was quickly realized that implants also could be used for orthodontic anchorage. A successful implant is like an ankylosed tooth: it does not move unless pathologic degeneration of the bone around it develops. Recently, it has become apparent that the osseointegration needed for long-term implant success is not necessary, and perhaps not desirable, for temporary attachments to bone to provide orthodontic anchorage. A number of options for skeletal anchorage exist at present, the principal ones being titanium screws that penetrate through the gingiva into alveolar bone (Figure 8-26, A) and bone anchors placed beneath the soft tissue, usually in the zygomatic buttress area of the maxilla (Figure 8-26, B).
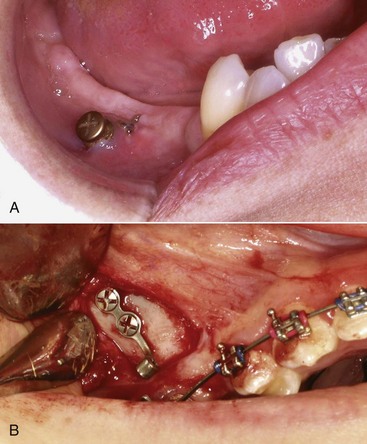
FIGURE 8-26 Skeletal (absolute) anchorage can be provided in two major ways: A, Screws placed through the gingiva into the alveolar bone, as in this patient in whom the screw will be used for anchorage so that the lower incisors can be aligned before prosthodontic replacement of the missing teeth; or B, bone anchors placed beneath the soft tissue, usually at the base of the zygomatic arch, so that the posterior teeth can be intruded or the anterior teeth retracted. After soft tissues are sutured back over the plate and screws, only the tube for attachment of springs will extend into the oral cavity.
At this point, application of bone screws or plates for skeletal anchorage has become a routine aspect of clinical orthodontics. These devices are reviewed in the fixed appliance section of Chapter 10, and clinical applications of temporary skeletal anchorage are described in Chapter 18.
Deleterious Effects of Orthodontic Force
Mobility and Pain Related to Orthodontic Treatment
Orthodontic tooth movement requires not only a remodeling of bone adjacent to the teeth but also a reorganization of the PDL itself. Fibers become detached from the bone and cementum, then reattach at a later time. Radiographically, it can be observed that the PDL space widens during orthodontic tooth movement. The combination of a wider ligament space and a somewhat disorganized ligament means that some increase in mobility will be observed in every patient.
A moderate increase in mobility is an expected response to orthodontic treatment. The heavier the force, however, the greater the amount of undermining resorption expected, and the greater the mobility that will develop. Excessive mobility is an indication that excessive forces are being encountered. This may occur because the patient is clenching or grinding against a tooth that has moved into a position of traumatic occlusion. If a tooth becomes extremely mobile during orthodontic treatment, it should be taken out of occlusion and all force should be discontinued until the mobility decreases to moderate levels. Unlike root resorption, excessive mobility will usually correct itself without permanent damage.
If heavy pressure is applied to a tooth, pain develops almost immediately as the PDL is literally crushed. There is no excuse for using force levels for orthodontic tooth movement that produce immediate pain of this type. If appropriate orthodontic force is applied, the patient feels little or nothing immediately. Several hours later, however, pain usually appears. The patient feels a mild aching sensation, and the teeth are quite sensitive to pressure, so that biting a hard object hurts. The pain typically lasts for 2 to 4 days, then disappears until the orthodontic appliance is reactivated. At that point, a similar cycle may recur, but for almost all patients, the pain associated with the initial activation of the appliance is the most severe. It is commonly noted that there is a great deal of individual variation in any pain experience, and this is certainly true of orthodontic pain. Some patients report little or no pain even with relatively heavy forces, whereas others experience considerable discomfort with quite light forces.
The pain associated with orthodontic treatment is related to the development of ischemic (hyalinized) areas in the PDL that will undergo sterile necrosis. The increased tenderness to pressure suggests inflammation at the apex, and the mild pulpitis that usually appears soon after orthodontic force is applied probably also contributes to the pain. There does seem to be a relationship between the amount of force used and the amount of pain: all other factors being equal, the greater the force, the greater the pain. This is consistent with the concept that ischemic areas in the PDL are the major pain source, since greater force would produce larger areas of ischemia.
If the source of pain is the development of ischemic areas, strategies to temporarily relieve pressure and allow blood flow through compressed areas should help. In fact, if light forces are used, the amount of pain experienced by patients can be decreased by having them engage in repetitive chewing (of sugarless gum, a plastic wafer placed between the teeth, or whatever) during the first 8 hours after the orthodontic appliance is activated. Presumably this works by temporarily displacing the teeth enough to allow some blood flow through compressed areas, thereby preventing buildup of metabolic products that stimulate pain receptors. Light forces, however, are the key to minimizing pain as a concomitant of orthodontic treatment.
As we have noted previously, many drugs used to control pain have the potential to affect tooth movement because of their effects on prostaglandins. It has been suggested that acetaminophen (Tylenol) should be a better analgesic for orthodontic patients than aspirin, ibuprofen, naproxen, and similar prostaglandin inhibitors because it acts centrally rather than as a prostaglandin inhibitor. The counterargument against acetaminophen is that inflammation in the PDL contributes to the pain. Acetaminophen does not reduce inflammation, but the peripherally acting agents such as ibuprofen do, so they may offer more effective pain control. Based on a number of clinical studies, it now is widely accepted that acetaminophen and the over-the-counter NSAIDs are equally acceptable for controlling pain over the 3 to 4 days after an orthodontic appliance has been activated. It also is of interest that there is a strong placebo effect: reassuring patients and calling them at home the evening after appliances were placed was as effective in reducing pain as either type of drug in a recent and well-done study.30
It is rare but not impossible for orthodontic patients to develop pain and inflammation of soft tissues, not because of the orthodontic force, but because of an allergic reaction. There are two major culprits when this occurs: a reaction to the latex in gloves or elastics and a reaction to the nickel in stainless steel bands, brackets, and wires. Latex allergies can become so severe as to be life threatening. Extreme care should be taken to avoid using latex products in patients reporting a latex allergy. Nickel is allergenic, and nearly 20% of the U.S. population show some skin reaction to nickel-containing materials (such as cheap jewelry and earrings). Fortunately, most children with a skin allergy to nickel have no mucosal response to stainless steel orthodontic appliances (which are about 8% nickel) and tolerate treatment perfectly well, but some do not.31 The typical symptoms of nickel allergy in an orthodontic patient are widespread erythema and swelling of oral tissues, developing 1 to 2 days after a stainless steel appliance is placed. For such patients, titanium brackets and tubes can be substituted for stainless steel (see Chapter 10), and beta-titanium archwires can be used instead of nickel-titanium (NiTi) or steel wires. If there is doubt about how a patient with a known nickel allergy will react to an orthodontic appliance, it is wise to bond one or two steel brackets and wait for a week or two to see if there will be an allergic response before placing a complete appliance.
Effects on the Pulp
Although pulpal reactions to orthodontic treatment are minimal, there is probably a modest and transient inflammatory response within the pulp, at least at the beginning of treatment. As we have noted previously, this may contribute to the discomfort that patients often experience for a few days after appliances are placed, but the mild pulpitis has no long-term significance.
There are occasional reports of loss of tooth vitality during orthodontic treatment. Usually, there is a history of previous trauma to the tooth, but poor control of orthodontic force also can be the culprit. If a tooth is subjected to heavy continuous force, a sequence of abrupt movements occurs, as undermining resorption allows increasingly large increments of change. A large enough abrupt movement of the root apex could sever the blood vessels as they enter. Loss of vitality has also been observed when incisor teeth were tipped distally to such an extent that the root apex, moving in the opposite direction, was actually moved outside the alveolar process (see Figure 8-25). Again, such movements probably would sever the blood vessels entering the pulp canal.
Since the response of the PDL, not the pulp, is the key element in orthodontic tooth movement, moving endodontically treated teeth is perfectly feasible. It may be necessary to treat some teeth endodontically, especially in adults receiving adjunctive orthodontic treatment (see Chapter 18), and then reposition them orthodontically. There is no contraindication to doing this. Severe root resorption should not be expected as a consequence of moving a nonvital tooth that has had proper endodontic therapy. One special circumstance is a tooth that experienced severe intrusive trauma and required pulp therapy for that reason.32 If such a tooth must be repositioned orthodontically, resorption seems less likely if a calcium hydroxide fill is maintained until the tooth movement is completed, and then the definitive root canal filling is placed.33
Effects on Root Structure
Orthodontic treatment requires remodeling of bone adjacent to the tooth roots. For many years, it was thought that the roots were not remodeled in the same way as bone. More recent research has made it plain that when orthodontic forces are applied, there usually is some remodeling of the cementum on the root surface as well as the adjacent bone.
Rygh and coworkers have shown that cementum adjacent to hyalinized (necrotic) areas of the PDL is “marked” by this contact and that clast cells attack this marked cementum when the PDL area is repaired.34 This observation helps explain why heavy continuous orthodontic force can lead to severe root resorption. Even with the most careful control of orthodontic force, however, it is difficult to avoid creating some hyalinized areas in the PDL. It is not surprising therefore that careful examination of the root surfaces of teeth that have been moved reveals repaired areas of resorption of both cementum and dentin of the root (Figure 8-27). It appears that cementum (and dentin, if resorption penetrates through the cementum) is removed from the root surface, then cementum is restored in the same way that alveolar bone is removed and then replaced. Root remodeling, in other words, is a constant feature of orthodontic tooth movement, but permanent loss of root structure would occur only if repair did not replace the initially resorbed cementum.
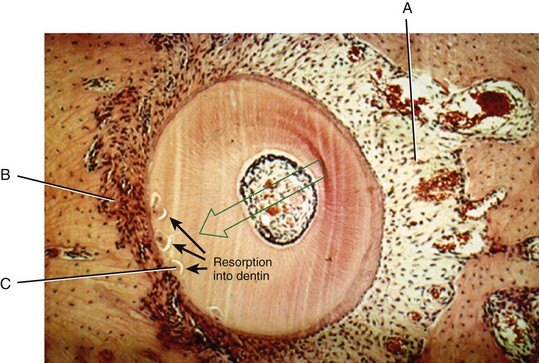
FIGURE 8-27 Coronal section through the root of a premolar being moved to the left (arrow). Note the zone of PDL compression to the left and tension to the right. Dilation of blood vessels and osteoblastic activity (A) can be seen on the right. Osteoclasts removing bone are present on the left (B). Areas of beginning root resorption that will be repaired by later deposition of cementum also can be seen on the left (C). If resorption penetrates through the cementum and into the dentin, the result will be cementum repair that fills in craters in the dentin. (Courtesy Professor B. Melsen.)
Repair of the damaged root restores its original contours, unless the attack on the root surface produces large defects at the apex that eventually become separated from the root surface (Figure 8-28). Once an island of cementum or dentin has been cut totally free from the root surface, it will be resorbed and will not be replaced. On the other hand, even deep defects in the form of craters into the root surface will be filled in again with cementum once orthodontic movement stops. Therefore permanent loss of root structure related to orthodontic treatment occurs primarily at the apex. Sometimes there is a reduction in the lateral aspect of the root in the apical region.
FIGURE 8-28 During tooth movement, clast cells attack cementum, as well as bone, creating defects in surface of the roots. During the repair phase, these defects fill back in with cementum. Shortening of the root occurs when cavities coalesce at the apex, so that peninsulas of root structure are cut off as islands. These islands resorb, and although the repair process places new cementum over the residual root surface, a net loss of root length occurs. This is why, although both the sides and the apex of the root experience resorption, roots become shorter but not thinner as a result of orthodontic tooth movement.
Shortening of tooth roots during orthodontic treatment occurs in three distinct forms that must be distinguished when the etiology of resorption is considered.
Moderate Generalized Resorption
Despite the potential for repair, careful radiographic examination of individuals who have undergone comprehensive orthodontic treatment shows that most of the teeth show some loss of root length, and this is greater in patients whose treatment duration was longer (Table 8-4). The average shortening of root length of maxillary incisors is somewhat greater than for other teeth, but all teeth included in the typical fixed orthodontic appliance show slight average shortening. In the Seattle study from which the data of Table 8-4 were derived, all teeth except upper second molars were banded. Note that these were the only unaffected teeth. Although 90% of maxillary incisors and over half of all teeth show some loss of root length during treatment, for the great majority of the patients, this modest shortening is almost imperceptible and is clinically insignificant.
TABLE 8-4
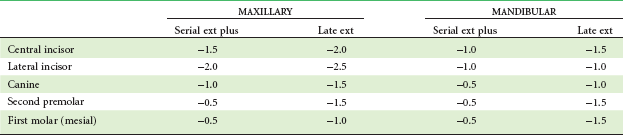
Data from Kennedy DB, Joondeph DR, Osterburg SK, et al. Am J Orthod 84:183, 1983.
Occasionally, however, loss of one-third or one-half or more of the root structure is observed in patients who received what seemed to be only routine orthodontic therapy (Figure 8-29). Again, it is important to distinguish between two forms of severe resorption.
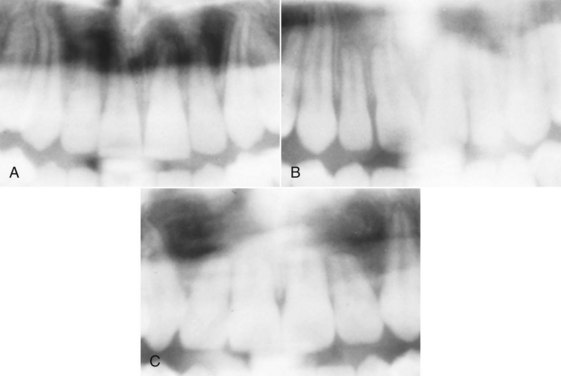
FIGURE 8-29 Root resorption accompanying orthodontic treatment can be placed into three categories as illustrated here for maxillary central and lateral incisors: A, Category 1, slight blunting; B, category 2, moderate resorption, up to  of root length; C, category 3, severe resorption, greater than
of root length; C, category 3, severe resorption, greater than 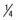 of root length. See Table 8-5 for data for prevalence of these levels of resorption. (From Kaley JD, Phillips C. Angle Orthod 61:125-131, 1991.)
of root length. See Table 8-5 for data for prevalence of these levels of resorption. (From Kaley JD, Phillips C. Angle Orthod 61:125-131, 1991.)
Severe Generalized Resorption
Severe root resorption of all the teeth, fortunately, is rare. Some individuals are prone to root resorption, even without orthodontic treatment—severe generalized resorption has been observed many times in individuals who never were orthodontic patients. If there is evidence of root resorption before orthodontic treatment, the patient is at considerable risk of further resorption during orthodontic treatment, much more so than a patient with no pretreatment resorption. Although hormonal imbalances and other metabolic derangements have been suspected in these susceptible patients, little evidence supports these theories. It was reported in the 1940s that a deficiency of thyroid hormone could lead to generalized root resorption, and occasionally thyroid supplements for orthodontic patients are suggested as a way to prevent this, but almost all patients with generalized resorption have no endocrine problems.
At this point the etiology of severe generalized resorption must be considered entirely unknown. Orthodontic treatment is not the major etiologic factor. Various reports have suggested that above-average resorption can be anticipated if the teeth have conical roots with pointed apices, distorted tooth form (dilaceration), or a history of trauma (whether endodontic treatment was or was not required). These characteristics, however, are best considered indicators of somewhat more extensive moderate resorption than as risk factors for severe resorption.
Severe Localized Resorption
In contrast to severe generalized resorption, severe localized resorption (i.e., severe resorption of a few teeth) is caused by orthodontic treatment in many instances. It has been known for many years that excessive force during orthodontic treatment increases the risk of root resorption, particularly if heavy continuous forces are used. Prolonged duration of orthodontic treatment also increases the amount of resorption.
It is increasingly apparent that some individuals are more susceptible to root resorption. It seems reasonable to presume that the large individual differences relate to genetic factors, although there is not yet any way to use genetic testing to evaluate resorption risk.35 Perhaps the best way to detect those who are likely to experience unusually large amounts of resorption is to take a panoramic radiograph 6 to 9 months into treatment and evaluate the amount of resorption during this time. Patients who show significant resorption in the initial stage of treatment are likely to have greater resorption at the end of treatment.36
The risk of severe localized resorption is much greater for maxillary incisors (3% affected versus <1% for all other teeth; Table 8-5). Kaley and Phillips reported a twentyfold increase in the risk of severe resorption for maxillary incisors if their roots were forced against the lingual cortical plate during treatment (Table 8-6).37 This is likely to occur during camouflage treatment for skeletal problems, when the maxillary incisors are torqued (as in Class II patients) or tipped (as in Class III treatment) so that the root apices are thrust against the lingual cortical plate. Contact with the cortical plates also can explain other patterns of localized root resorption, such as resorption of lower molar roots when buccal root torque is used in an effort to augment anchorage for Class II elastics.
TABLE 8-5
Percentage of Patients with Root Resorption by Degree of Resorption (200 Consecutive Full-Treatment Patients)
*Values are for the right tooth in each instance (no significant right-left differences): 0 = no apical root resorption; 1 = slight blunting of the root apex; 2 = moderate resorption, up to  of root length; 3 = severe resorption, greater than
of root length; 3 = severe resorption, greater than 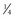 of root length. (See Figure 8-29.)
of root length. (See Figure 8-29.)
Data from Kaley JD, Phillips C. Angle Orthod 61:125-131, 1991.
Effects of Treatment on the Height of Alveolar Bone
At the root apex, if the balance between apposition and resorption of the root surface moves too far toward resorption, irreversible shortening of the root may occur. It seems logical to suspect that this might also happen at the alveolar bone crest and that another effect of orthodontic treatment might be loss of alveolar bone height. Since the presence of orthodontic appliances increases the amount of gingival inflammation, even with good hygiene, this potential side effect of treatment might seem even more likely.
Fortunately, excessive loss of crestal bone height is almost never seen as a complication of orthodontic treatment. Loss of alveolar crest height in one large series of patients averaged less than 0.5 mm and almost never exceeded 1 mm, with the greatest changes at extraction sites.38 Minimal effects on crestal alveolar bone levels also are observed on long-term follow-up of orthodontic patients. The reason is that the position of the teeth determines the position of the alveolar bone. When teeth erupt or are moved, they bring alveolar bone with them. The only exception is tooth movement in the presence of active periodontal disease, and even adults who have had bone loss from periodontal disease can have orthodontic treatment with good bone responses, if the periodontal disease is well controlled.
The relationship between the position of a tooth and alveolar bone height can be seen clearly when teeth erupt too much or too little. In the absence of pathologic factors, a tooth that erupts too much simply carries alveolar bone with it, often for considerable distances. It does not erupt out of the bone. But unless a tooth erupts into an area of the dental arch, alveolar bone will not form there. If a tooth is congenitally absent or extracted at an early age, a permanent defect in the alveolar bone will occur unless another tooth is moved into the area relatively rapidly. This is an argument against very early extraction, as, for instance, the enucleation of an unerupted premolar. Early removal of teeth poses a risk of creating an alveolar bone defect that cannot be overcome by later orthodontic treatment.
Because an erupting tooth brings alveolar bone with it, orthodontic tooth movement can be used to create the alveolar bone needed to support an implant to replace a congenitally missing tooth. For instance, if a maxillary lateral incisor is missing and a prosthetic replacement is planned, it is advantageous to have the permanent canine erupt mesially, into the area of the missing lateral incisor, and then to move it back into its proper position toward the end of the growth period. This stimulates the formation of alveolar bone in the lateral incisor region that otherwise would have not formed.39
The same effects on alveolar bone height are seen with orthodontic extrusion as with eruption: as long as the orthodontic treatment is carried out with reasonable force levels and reasonable speed of tooth movement, a tooth brought into the dental arch by extrusive orthodontic forces will bring alveolar bone with it. The height of the bone attachment along the root will be about the same at the conclusion of movement as at the beginning. In some circumstances, it is possible to induce bone formation where an implant will be required, by extruding the root of an otherwise hopelessly damaged tooth, so that new hard and soft tissue forms in the area. If a tooth is intruded, bone height tends to be lost at the alveolar crest, so that about the same percentage of the root remains embedded in bone as before, even if the intrusion was over a considerable distance.
In most circumstances, this tendency for alveolar bone height to stay at the same level along the root is a therapeutic plus. Occasionally, it would be desirable to change the amount of tooth embedded in bone. For instance, the bone support around periodontally involved teeth could be improved by intruding the teeth and forcing the roots deeper into the bone, if the alveolar bone did not follow the intruding tooth. There are reports of therapeutic benefit from intruding periodontally involved teeth,40 but the reduced pocketing relates to the formation of a long junctional epithelium, not to reattachment of the PDL or more extensive bony support. On occasion, it is desirable to elongate the root of a fractured tooth to enable its use as a prosthetic abutment without crown-lengthening surgery. If heavy forces are used to extrude a tooth quickly, a relative loss of attachment may occur, but this deliberately nonphysiologic extrusion is at best traumatic and at worst can lead to ankylosis and/or resorption. Physiologic extrusion or intrusion that brings the alveolar bone along with the tooth, followed by surgical recontouring of gingiva and bone, is preferable.
Skeletal Effects of Orthodontic Force: Growth Modification
Principles in Growth Modification
Orthodontic force applied to the teeth has the potential to radiate outward and affect distant skeletal locations, and it now is possible to apply force to implants or screws in the jaws to affect their growth. Orthodontic tooth movement can correct dental malocclusions; to the extent that the distant effects change the pattern of jaw growth, there also is the possibility of correcting skeletal malocclusions.
Our current knowledge of how and why the jaws grow is covered in some detail in Chapters 2 through 4. In brief summary, the maxilla grows by apposition of new bone at its posterior and superior sutures in response to being pushed forward by the lengthening cranial base and pulled downward and forward by the growth of the adjacent soft tissues. Tension at the sutures as the maxilla is displaced from its supporting structures appears to be the stimulus for new bone formation. Somewhat similarly, the mandible is pulled downward and forward by the soft tissues in which it is embedded. In response, the condylar process grows upward and backward to maintain the temporomandibular articulation. If this is so, it seems entirely reasonable that pressures resisting the downward and forward movement of either jaw should decrease the amount of growth, while adding to the forces that pull them downward and forward should increase their growth.
The possibility of modifying the growth of the jaws and face in this way has been accepted, rejected, and then accepted again during the past century. Although the extent to which treatment can produce skeletal change remains controversial, the clinical effectiveness of procedures aimed at modifying growth has been demonstrated in recent years. The possibilities for growth modification treatment and the characteristics of patients who would be good candidates for it are described in Chapter 12. Here, the focus is on how the effects on growth are produced.
Effects of Orthodontic Force on the Maxilla and Midface
Manipulation and control of tooth eruption is properly considered an aspect of orthodontic tooth movement and therefore has been reviewed in some detail in the previous section. The focus of the discussion below is on changes in the jaws, not on dentoalveolar structures, but it is important to keep in mind that in treatment of patients, the dentoalveolar and skeletal effects cannot be divorced so readily.
Restraint of Maxillary Growth
Besides the dentoalveolar process, the important sites of growth of the maxilla, where it might be possible to alter the expression of growth, are the sutures that separate the middle of the palate and attach the maxilla to the zygoma, pterygoid plates, and frontonasal area. These sutures are similar in some respects to the PDL but are neither as complex in their structure nor nearly as densely collagenous (Figure 8-30). For modification of excessive maxillary growth, the concept of treatment would be to add a force to oppose the natural force that separates the sutures, preventing the amount of separation that would have occurred (Figure 8-31). For deficient growth, the concept would be to add additional force to the natural force, separating the sutures more than otherwise would have occurred.
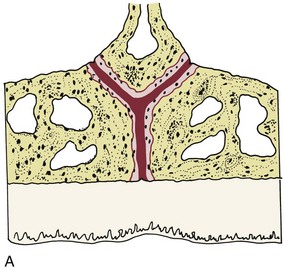
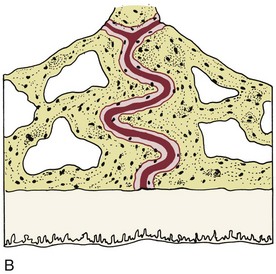
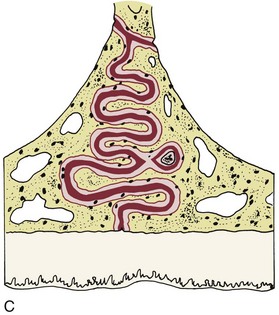
FIGURE 8-30 Like the other sutures of the facial skeleton, the midpalatal suture becomes increasingly tortuous and interdigitated with increasing age. These diagrams show the typical histologic appearance of the midpalatal suture in (A) infancy, when the suture is almost a straight line; B, childhood (early mixed dentition); and (C) early adolescence. In childhood, sutural expansion can be accomplished with almost any type of expansion device (e.g., a lingual arch). By early adolescence interdigitation of spicules in the suture has reached the point that a jackscrew with considerable force is required to create microfractures before the suture can open. By the late teens, interdigitation and areas of bony bridging across the suture develop to the point that skeletal maxillary expansion becomes impossible. (Redrawn from Melsen B. Am J Orthod 668:42-54, 1975.)
FIGURE 8-31 Extraoral force applied to the maxillary teeth radiates to the sutures of the maxilla, where it can affect the pattern of skeletal maxillary growth.
It is difficult to measure compression or tension within sutures, and there is no way to know theoretically what is required to alter growth. Clinical experience suggests that moderate amounts of force against the maxillary teeth can impede forward growth of the maxilla, but heavier force is needed for separation of sutures and growth stimulation. When force is applied to the teeth, only a small fraction of the pressure in the PDL is experienced at the sutures because the area of the sutures is so much larger. For this reason, even the moderate forces recommended for restraint of forward maxillary growth tend to be heavier than those recommended for tooth movement alone. For instance, a force of 250 gm per side (500 gm total) probably is about the minimum for impeding forward movement of the maxilla, and often this force or more is applied only to the first molar teeth via a facebow.
The effect of this much force on the dentition is a justifiable matter for concern. During growth modification treatment, tooth movement is undesirable—the objective is to correct the jaw discrepancy, not move teeth to camouflage it. As we have noted in the first part of this chapter, heavy continuous force can damage the roots of the teeth and the periodontium. Heavy intermittent force is less likely to produce damage, and intermittent force is a less effective way to induce tooth movement, probably because the stimulus for undermining resorption is diluted during the times that the heavy force is removed. It follows logically that to minimize damage to the teeth, full-time application of heavy force to the maxillary dentition is unwise.
Because tooth movement is an undesirable side effect, it would be convenient if part-time application of heavy force produced relatively more skeletal than dental effect. At one time, it was thought that the skeletal effect of headgear was about the same with 12 to 16 or 24 hours of wear per day, while much more tooth movement occurred with solid 24-hour wear. This would be another argument for part-time rather than full-time headgear wear. However, very little data exist to support this hypothesis, and intermittent headgear wear cannot be relied on to produce a differential between tooth movement and skeletal change.
For tooth movement, there is a definite threshold for the duration of force: unless force is applied to a tooth for at least 6 hours per day, no bone remodeling occurs. Whether a similar duration threshold applies to sutures is unknown, but clinical experience suggests that it may. See Roberts et al23 for a recent review of influences on bone growth and remodeling.
Until recently the time of day when force was applied to the jaws was not considered important. It is clear now that in both experimental animals and humans, short-term growth is characterized by fluctuations in growth rates, even within a single day. It has been known for some time that in growing children, growth hormone is released primarily during the evening, so it is not surprising that addition of new bone at the epiphyseal plates of the long bones occurs mostly—perhaps entirely—at night.41 We do not know whether facial growth follows this pattern, but it seems likely that it does. Growth hormone release begins in the early evening, however, so it probably is important to stress that a patient should begin wearing headgear or a functional appliance immediately after dinner rather than waiting until bedtime.
Based on these considerations, the following “force prescription” for headgear to restrain maxillary growth in patients with Class II problems now is considered optimal:
• Force of 500 to 1000 gm total (half of that on each side)
• Force direction slightly above the occlusal plane (through the center of resistance of the molar teeth, if the force application is to the molars by a facebow)
• Force duration at least 12 hours per day, every day, with emphasis on wearing it from early evening (right after dinner) until the next morning
• Typical treatment duration approximately 12 months, depending on rapidity of growth and patient cooperation (Figure 8-32)
Augmentation of Maxillary Growth
Although modest changes can be produced by a face mask (reverse headgear), increasing the amount of forward growth of the maxilla by producing tension in the sutures has not been as successful clinically as restraining growth. This difficulty probably reflects our inability to produce enough force at the posterior and superior sutures to separate them in older children, but that is not the whole story. Part of the problem also is the extent of interdigitation of bony spicules across the sutural lines (see Figure 8-30).42 As the sutures become more and more highly interdigitated with increasing age, it becomes more and more difficult to separate them. In an adolescent, enough force can be applied across the palate with a jackscrew to open a moderately interdigitated midpalatal suture, but extraoral force from a facemask cannot produce that much force in the extensive suture system above and behind the maxilla, once even a moderate level of interdigitation has been reached.
Tooth movement is undesirable when any type of growth modification is being attempted, but it is a particular problem in efforts to displace the maxilla forward. One way to overcome this is to apply the force to bone anchors or bone screws in the maxilla. Another possibility is to use Class III elastics to bone plates in the maxilla and mandible (an approach that is reviewed in more detail in Chapter 13).43 Skeletal anchorage totally eliminates unwanted tooth movement, but this should not be taken to mean that then there would be no constraints on the amount of possible skeletal change. Forward growth, after all, seems to be largely controlled by the soft tissue matrix in which the maxilla is embedded. Clinical experience to date suggests that without surgical intervention, more than 4 to 5 mm forward displacement of the maxilla is unlikely.
Effects of Orthodontic Force on the Mandible
If the mandible, like the maxilla, grows largely in response to growth of the surrounding soft tissues, it should be possible to alter its growth in somewhat the same way maxillary growth can be altered, by pushing back against it or pulling it forward. To some extent, that is true, but the attachment of the mandible to the rest of the facial skeleton via the TM joint is very different from the sutural attachment of the maxilla. Not surprisingly, the response of the mandible to force transmitted to the temporomandibular joint also is quite different.
Restraint of Mandibular Growth
As we have discussed in Chapter 7, efforts to restrain mandibular growth by applying a compressive force to the mandibular condyle have never been very successful. Experiments with monkeys, in which quite heavy and prolonged forces can be used, suggest that restraining forces can stop mandibular growth and cause remodeling within the temporal fossa.44 Tooth movement is not a major problem, because the force is applied to the chin rather than the mandibular teeth. The major difficulty in getting this to work with human children is their unwillingness to cooperate with the necessary duration and magnitude of force (which, after all, is both inconvenient and likely to be painful).
The duration of the chin cup force (hours/day) is an important difference between children and experimental animals. In the animal experiments in which a force against the chin has been shown to impede mandibular growth, the force was present essentially all the time. The effect of functional ankylosis in children (see Chapter 5) demonstrates that when there is a constant interference with translation of the condyles out of the glenoid fossa, growth is inhibited. An experimental monkey has no choice but to wear a restraining device full-time (and tolerate heavy force levels). Children will wear a growth-modifying appliance for some hours per day but are quite unlikely to wear it all the time even if they promise to do so. Headgear against the maxilla works well with 12 to 14 hours per day, or even less, but the mandible is different. It appears that restraint of mandibular growth may require prevention of translation on a full-time or nearly full-time basis. For the first time in humans, remodeling of the TM joint so that the mandible moves backward has been observed in children wearing Class III elastics to bone anchors essentially full-time.43 This suggests that force duration is more important than force magnitude—as it is for tooth movement and other orthodontic treatment effects.
Another apparent difficulty with a chin cup to restrain mandibular growth is that it is difficult to load the entire top of the condyle, and the line of force is likely to be below the theoretical ideal (Figure 8-33). For this reason, a chin cup is likely to rotate the mandible downward and to produce any restraint of forward growth of the chin primarily by this mechanism. Class III functional appliances produce exactly the same type of downward and backward rotation. The problem, of course, is that a patient who had excessive face height and mandibular prognathism would not be a good candidate for this type of treatment—and two-thirds of the prognathic patients of European descent have a long face as well.
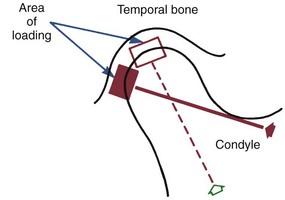
FIGURE 8-33 Extraoral force aimed at the condyle of the mandible tends to load only a small portion of the rounded surface, which is one explanation for the relative ineffectiveness of this type of growth modification.
It is fair to say that controlling excessive mandibular growth is an important unsolved problem in contemporary orthodontics. At this point, we simply cannot restrain mandibular growth with anything like the effectiveness of similar treatment for the maxilla.
Augmentation of Mandibular Growth
On the other hand, the condyle translates forward away from the temporal bone during normal function, and the mandible can be pulled into a protruded position and held there for long durations with moderate and entirely tolerable force. If the current theory is correct, that should stimulate growth. Arguments have raged for many years over whether it really does. If growth stimulation is defined as an acceleration of growth, so that the mandible grows faster while it is being protruded, growth stimulation can be shown to occur for many (but not all) patients (see Figure 7-13). If stimulation is defined as producing a larger mandible at the end of the total growth period than would have existed without treatment, it is much harder to demonstrate a positive effect. Many reports have found that the ultimate size of mandibles in treated and untreated patients is remarkably similar.
It is possible that exactly how the mandible is held forward out of the fossa is important in determining the response. There are two mechanisms for protrusion. One is passive, that is, the mandible is held forward by the orthodontic appliance. The other is active, that is, the patient responds to the appliance by using his or her muscles, especially the lateral pterygoid, to hold the mandible forward. Stimulating (activating) the muscles was thought to be important from the beginning of functional appliance therapy, hence both the generic functional name and the specific term activator.
Up to a certain point, posturing the mandible forward does activate the mandibular musculature—both the elevators and the less powerful muscles involved in protrusion. Some clinicians argue that it is important in taking the construction bite for a functional appliance to advance the mandible only a few millimeters because this gives maximum activation of the muscles. If the mandible is brought forward a considerable distance, 1 cm or more, the muscles tend to be electrically silenced rather than activated. But appliances made with such extreme construction bites can be quite effective clinically and may be just as potent in modifying mandibular (and maxillary) growth as appliances made with smaller advancements. Muscle activation, in short, is not necessary to obtain growth modification. The argument is about whether muscle activation makes these appliances work better, not whether it is necessary to get them to work at all.
When the mandible is protruded (or restrained), changes can occur on the temporal as well as the mandibular side of the TM joint. Sometimes, growth of the mandible has much less than the expected effect on a skeletal Class II malocclusion because the articular fossa remodels posteriorly at the same time the mandible is growing longer (see Figure 4-9), and occasionally, forward displacement of the joint contributes noticeably to Class II correction. There are no data to suggest, however, that forward relocation of the temporomandibular joint area is a major factor in the usual clinical response to functional appliances.
Holding the mandible forward passively requires a force of a few hundred grams. If the musculature relaxes, the reaction force is distributed to the maxilla and, to the extent that the appliance contacts them, to the maxillary and mandibular teeth. The restraint of forward maxillary growth that often accompanies functional appliance treatment is another indication that extremely heavy force is not required to affect the maxilla. On the other hand, headgear usually produces a greater effect on the maxilla than a functional appliance. This implies that the reactive forces from posturing the mandible forward are below the optimum level for altering maxillary growth. When a functional appliance contacts the teeth, as most do, a force system identical to Class II elastics is created, which would move the upper teeth backward and the lower teeth forward. To maximize skeletal effects and minimize dental effects, it is clear that the reactive forces should be kept away from the teeth, in so far as possible.
From this perspective, whether the patient actively uses his musculature to posture the mandible forward or passively rests against the appliance may or may not affect the amount of mandibular growth, but it definitely affects how much tooth movement occurs and may determine the effect on the maxilla. The difference between active and passive protrusion shows up most clearly when the Herbst appliance, a fixed functional appliance, is used (see Figure 10-7). With the Herbst appliance, the condyle is displaced anteriorly at all times, but the amount of force against the teeth is very much under the patient’s control. The patient can use his or her own muscles to hold the mandible forward, with the Herbst appliance serving only as a stimulus to do so; or the appliance can passively hold the jaw forward, with no contribution from the musculature. If the muscles hold the jaw forward, there is little or no reactive force against the teeth and minimal tooth movement; if the jaw repositioning is entirely passive, force against the teeth can displace them quite significantly.
All of these possible outcomes can be seen in cephalometric tracings of patients treated with functional appliances. The Herbst appliance is potentially the most effective of the functional appliances in altering jaw growth because of its full-time action, but it is also rather unpredictable in terms of the amount of skeletal versus dental change likely to be produced (Figure 8-34). Cooperation in terms of active versus passive posturing of the jaw probably explains much of the variability in the results. The Frankel appliance (see Figure 10-8), which is supported mostly by the soft tissues rather than the teeth, should be and probably is the functional appliance least likely to displace the teeth, but a Class II elastics effect can be seen even with it.
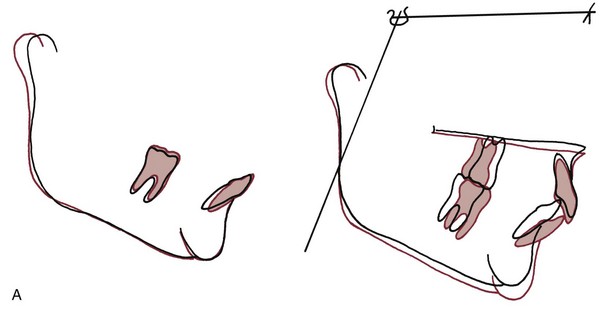
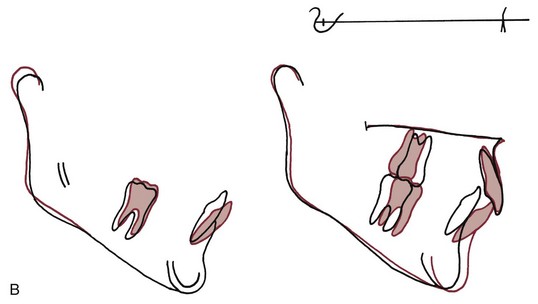
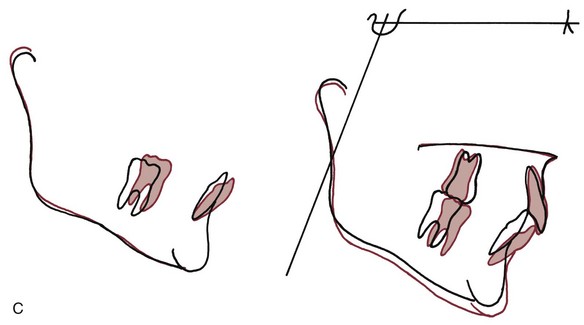
FIGURE 8-34 Functional appliance treatment can result in any combination of differential mandibular growth relative to the maxilla and cranial base (skeletal effect) and displacement of the mandibular and maxillary teeth (dental effect). Note in these tracings of the response to Herbst appliance treatment the almost total skeletal response in A, the combination of skeletal and dental changes in B, and the almost totally dental response in C. Although the changes in B are typical, it is important to keep in mind that responses like A and C can occur. (Redrawn from Pancherz H. Am J Orthod 82:104-113, 1982.)
The various types of functional appliances and their use in clinical treatment are reviewed in detail, along with other growth-modifying appliances, in Chapter 13.
References
1. Bumann, A, Carvalho, RS, Schwarzer, CL, et al. Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur J Orthod. 1997;19:29–37.
2. Basdra, EK, Komposch, G. Osteoblast-like properties of human periodontal ligament cells: An in vitro analysis. Eur J Orthod. 1997;19:615–621.
3. Yokoya, K, Sasaki, T, Shibasaki, Y. Distributional changes of osteoclasts and pre-osteoclastic cells in periodontal tissues during experimental tooth movement. J Dent Res. 1997;76:580–587.
4. Thilander, B. Tissue reactions in orthodontics. In Graber LW, Vanarsdall R, Vig KWL, eds.: Orthodontics: Current Principles and Techniques, 5th ed, St. Louis: Elsevier, 2011.
5. Pilla, AA. Low-intensity electromagnetic and mechanical modulation of bone growth and repair: are they equivalent? J Orthop Sci. 2002;7:420–428.
6. Shapiro, E. Orthodontic movement using pulsating force-induced piezoelectricity. Am J Orthod. 1979;73:59–66.
7. Darendeliler, MA, Zea, A, Shen, G, et al. Effects of pulsed electromagnetic field vibration on tooth movement induced by magnetic and mechanical forces: a preliminary study. Australian Dent J. 2007;52:282–287.
8. Krishnan, V, Davidovitch, Z. On a path to unfolding the biologic mechanisms of orthodontic tooth movement. J Dent Res. 2009;88:597–608.
9. Khouw, FE, Goldhaber, P. Changes in vasculature of the periodontium associated with tooth movement in the rhesus monkey and dog. Arch Oral Biol. 1970;15:1125–1132.
10. Kang, YG, Nam, JH, Kim, KH, et al. FAK pathway regulates PGE2 production in compressed periodontal ligament cells. J Dent Res. 2010;89:1444–1449.
11. Yamaguchi, M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res. 2009;12:113–119.
12. Nifforoushan, D, Manolson, MF. Expression of nitric oxide synthases in orthodontic tooth movement. Angle Orthod. 2009;79:502–508.
13. Roberts, WE. Bone physiology, metabolism, and biomechanics in orthodontic practice. In Graber LW, Vanarsdall RL, Vig KWL, eds.: Orthodontics: Current Principles and Techniques, 5th ed, St Louis: Elsevier, 2011.
14. McGorray SP, Dolce C, Kramer S, et al. A randomized, placebo-controlled clinical trial on the effects of recombinant human relaxin on tooth movement and retention. Am J Orthod Dentofac Orthop, in press.
15. Zhou, D, Hughes, B, King, GJ. Histomorphometric and biochemical study of osteoclasts at orthodontic compression sites in the rat during indomethacin inhibition. Arch Oral Biol. 1997;42:717–726.
16. Bartzela, T, Turp, JC, Motschall, E, et al. Medication effects on the rate of orthodontic tooth movement: a systematic literature review. Am J Orthod Dentofac Orthop. 2009;135:16–26.
17. Zahrowski, JJ. Optimizing orthodontic treatment in patients taking bisphosphonates for osteoporosis. Am J Orthod Dentofac Orthop. 2009;135:361–374.
18. Frost, HM. Wolff’s Law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64:175–188.
19. Köle, H. Surgical operations of the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12:515–529.
20. Gunderson, RM, Porter, JM, Zell, A, et al. Combined surgical and orthodontic management of anterior open bite using corticotomy. J Oral Surg. 1978;34:216–219.
21. Chang, HY, Chang, YL, Chen, HL. Treatment of a severely ankylosed central incisor and a missing lateral incisor by distraction osteogenesis and orthodontic treatment. Am J Orthod Dentofac Orthop. 2010;138:829–838.
22. Lv, T, Kang, N, Wang, C, et al. Biologic response of rapid tooth movement with periodontal ligament distraction. Am J Orthod Dentofac Orthop. 2009;136:401–411.
23. Roberts, WE, Epker, BN, Burr, DB, et al. Remodeling of mineralized tissues, part II: control and physiology. Sem Orthod. 2006;12:238–253.
24. Mostafa, YA, Fayed, MMS, Mehanni, S, et al. Comparison of corticotomy-facilitated vs standard tooth-movement techniques in dogs with miniscrews as anchor units. Am J Orthod Dentofac Orthop. 2009;136:570–577.
25. Liu, SS, Lee, W, Lei, D, et al. Tissue responses in corticotomy- and osteotomy-assisted tooth movements in rats: histology and immunostaining. Am J Orthod Dentofac Orthop. 2009;136:770e1–770e11. [eds summary and author interview, 136:770-771].
26. Kim, S, Yoon, A, Jeong, D, et al. Clinical application of accelerated osteogenic orthodontics and partially osseointegrated mini-implants for minor tooth movement. Am J Orthod Dentofac Orthop. 2009;136:431–439.
27. Wilcko, MT, Wilcko, WM, Pulver, JJ, et al. Accelerated osteogenic orthodontics technique: a 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J Oral Maxillofacial Surg. 2009;67:2149–2159.
28. Dibart, S, Sebaoun, JD, Surmenian, J. Piezocision: a minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Compend Contin Educ Dent. 2009;30:342–344. [346, 348-350].
29. Quinn, RS, Yoshikawa, DK. A reassessment of force magnitude in orthodontics. Am J Orthod. 1985;88:252–260.
30. Murdock, S, Phillips, C, Khondker, Z, et al. Treatment of pain after initial arch wire placement: a noninferiority randomized clinical trial comparing over-the-counter analgesics and bite-wafer use. Am J Orthod Dentofac Orthop. 2010;137:316–323.
31. Kusy, RP. Clinical response to allergies in patients. Am J Orthod Dentofac Orthop. 2004;125:544–547.
32. Llamas-Carreras, JM, Amarilla, A, Solano, E, et al. Study of external root resorption during orthodontic treatment in root filled teeth compared with their contralateral teeth with vital pulps. Int Endod J. 2010;43:654–662.
33. Chaushu, S, Shapira, J, Heling, I, et al. Emergency orthodontic treatment after the traumatic intrusive luxation of maxillary incisors. Am J Orthod Dentofac Orthop. 2004;126:162–172.
34. Brudvik, P, Rygh, P. Transition and determinants of orthodontic root resorption-repair sequence. Eur J Orthod. 1995;17:177–188.
35. Hartsfield, JK, Jr. Pathways in external apical root resorption associated with orthodontia. Orthod Craniofac Res. 2009;12:236–242.
36. Artun, J, Van t’Hullenaar, R, Doppel, D, et al. Identification of orthodontic patients at risk of severe apical root resorption. Am J Orthod Dentofac Orthop. 2009;135:448–455.
37. Kaley, JD, Phillips, C. Factors related to root resorption in edgewise practice. Angle Orthod. 1991;61:125–131.
38. Kennedy, DB, Joondeph, DR, Osterburg, SK, et al. The effect of extraction and orthodontic treatment on dentoalveolar support. Am J Orthod. 1983;84:183–190.
39. Kokich, VO, Kinzer, GA. Managing congenitally missing lateral incisors, Part III: implant replacement. J Esthetic Restorative Dent. 2005;17:202–210.
40. Melsen, B, Agerbaek, N, Markenstam, G. Intrusion of incisors in adult patients with marginal bone loss. Am J Orthod Dentofac Orthop. 1989;96:232–241.
41. Beier, F. Cell-cycle control and the cartilage growth plate. J Cell Physiol. 2005;202:1–8.
42. Melsen, B. Palatal growth studied on human autopsy material. Am J Orthod. 1975;68:42–54.
43. DeClerck, H, Cevidanes, L, Baccetti, T. Dentofacial effects of bone-anchored maxillary protraction: a controlled study of consecutively-treated Class III patients. Am J Orthod Dentofac Orthop. 2010;138:577–581.
44. Janzen, EK, Bluher, JA. The cephalometric, anatomic and histologic changes in Macaca mulatta after application of a continuous-acting retraction force on the mandible. Am J Orthod. 1965;51:832–855.