Chapter 30 Navicular Disease
Pathophysiology of Navicular Disease
Navicular disease is a chronic forelimb lameness associated with pain arising from the distal sesamoid or navicular bone. It is well recognized that in association with advanced navicular disease, fibrillation of the dorsal aspect of the deep digital flexor tendon (DDFT), with or without adhesion formation between the tendon and the navicular bone, is a common feature. Recent clinical studies using magnetic resonance imaging (MRI)1 and post mortem studies2,3 have demonstrated that there may also be abnormalities of closely related structures, including the collateral sesamoidean ligaments (CSLs), the distal sesamoidean impar ligament (DSIL), and the navicular bursa. These structures and the navicular bone are called the podotrochlear apparatus. For the purposes of this chapter, this complex of degenerative changes will be referred to as navicular disease. Primary injury of the DDFT is considered to be a separate condition,4 which may have a different etiopathogenesis (see Chapter 32).
Although historically considered to be a single disease, given the variety of clinical presentations it is likely that there are a number of different clinical conditions, of different etiologies, that give rise to pain in the podotrochlear apparatus. It is difficult to conceive a single disease that can result in an insidious onset, slowly progressive bilateral forelimb lameness, or an acute onset, relatively severe unilateral forelimb lameness, each with a variety of different radiological manifestations and with some horses never developing radiological changes. It is curious that sometimes clinical signs become apparent in young horses just commencing work, whereas more typically lameness is seen in mature riding horses. It is also seen in horses with vastly different distal limb conformation. It is a common condition in Quarter Horses, which tend to have narrow, upright, boxy feet, small relative to their body size, as well as in European Warmblood horses, many of which have relatively tall narrow feet. It is also common in Thoroughbred horses, which frequently have rather flat feet, with low collapsed heels, often associated with dorsopalmar foot imbalance. Recent evidence suggests there is a heritable tendency toward the development of navicular disease in Dutch and Hanoverian Warmblood horses.5-7
The factors causing pain in the navicular apparatus, and therefore lameness, are poorly understood. Experience with both radiography and MRI suggests that lesions in both the navicular bone and closely related structures are likely to predate the onset of lameness in some horses.8 In some horses, a trigger factor apparently promoting pain and lameness has been a period of enforced rest for an unrelated cause.
There are no good epidemiological studies investigating risk factors for the development of navicular disease. Therefore information is largely anecdotal. The frequency of occurrence of navicular disease appears to vary between breeds. Quarter Horses,9 Warmblood horses,10 and Thoroughbred cross horses11 have a relatively high incidence, whereas the occurrence and/or recognition in some breeds, such as the Finnhorse, Arab,12 and Friesian, is relatively low.
Biomechanical Considerations
From a biomechanical perspective the podotrochlear apparatus comprising the navicular bone, the CSLs, the DSIL, and the navicular bursa, together with the DDFT, and the distal digital annular ligament are integrally related. The navicular bone, which articulates with the middle and distal phalanges, provides a constant angle of insertion and maintains the mechanical advantage of the DDFT, which exerts major compressive forces on the distal third of the bone. Contact studies between the phalanges in isolated limbs have demonstrated that the greatest forces are applied in the propulsion phase of the stride. This occurs during extension of the distal interphalangeal (DIP) joint, with increased pressure of the DDFT on the palmar aspect of the navicular bone, increased contact between the navicular bone and the middle phalanx, and increased tension in the CSLs.13,14 Tension in the DDFT and the distal digital annular ligament promotes stability of the DIP joint. Forces may be altered by foot conformation: in a horse with “weak” heels there is greater extension of the DIP joint compared with a horse with “strong” heels, which results in increased pressure concentrated on the distal aspect of the navicular bone.15
Compressive forces and stress on the navicular bone were compared in clinically sound horses and horses with navicular disease.16 Although the mean peak force and stress were similar, the force and stress in the horses with navicular disease were approximately double early in the stance phase of the stride. This early peak stress resulted in a much higher loading rate of the navicular bone in the navicular disease group. The difference in loading patterns was associated with an increased force in the DDFT in the early and mid-stance phases, probably because of increased contraction of the deep digital flexor (DDF) muscle. Contraction of the DDF muscle may result in toe-first ground contact, seen in some horses with navicular disease. It is suggested that pain associated with the navicular bone may result in a positive feedback by increasing the force in the DDFT to avoid heel-first landing, and hence paradoxically increasing the compressive force on the navicular bone. This hypothesis is supported by reduction in peak forces on the navicular bone throughout the stance phase in horses with navicular disease after perineural analgesia of the palmar digital nerves.17
Although low, collapsed heel conformation has anecdotally been associated with navicular disease, a recent study18 in Irish draught cross-type horses showed no correlation between the peak force exerted on the navicular bone by the DDFT and the conformation of the hoof capsule, contrary to the earlier results.15 However, a 1-degree decrease in angle of the solar border of the distal phalanx resulted in a fourfold increase in peak force on the navicular bone.18 There was no correlation between the angle of the solar border of the distal phalanx and the degree of heel collapse.
The shape of the navicular bone may be determined at birth, and this may influence the biomechanical forces subsequently applied to the bone (Figure 30-1) and hence influence the risk of development of navicular disease.6,19 Finnhorses and Friesian horses tend to have a straight or convex contour of the proximal articular border of the navicular bone and rarely develop navicular disease. There is a much higher incidence of navicular disease in the Dutch Warmblood breed, and horses in which the proximal articular margin is concave or undulating appear to be at highest risk of development of the disease.5,6
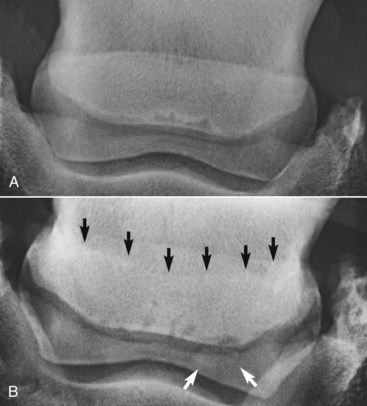
Fig. 30-1 Dorsoproximal-palmarodistal oblique radiographic images of the navicular bone to show differences in shape of the proximal articular margin. A, Straight. There is an axial cluster of radiolucent zones along the distal border of the bone. B, Concave (black arrows). There are variably shaped lucent zones along the distal border of the bone and a large distal border fragment laterally (white arrows).
Histopathological Studies
Navicular disease has not been reproduced experimentally; therefore all proposed etiologies remain speculative. Earlier theories suggesting a vascular etiology with arteriosclerosis20 or thrombosis, resulting in ischemia within the navicular bone,21 have largely been rejected because of failure to identify ischemic bone or thrombosis, failure to reproduce clinical signs or pathological changes by occluding blood supply to the bone, and expanding evidence demonstrating increased bone modeling.22-25 Post mortem studies to date have focused principally on horses with long-term, chronic disease, generally with advanced radiological abnormalities, reflecting the end stage of a disease complex. These studies identified striking similarities between the pathological features of navicular disease and osteoarthritis (Figure 30-2) in both people and horses.24,25
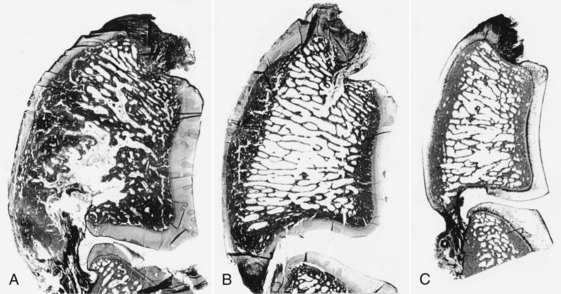
Fig. 30-2 Sagittal sections of the navicular bones of a horse with navicular disease (A), an age-matched control (B), and an immature control horse (C). The subchondral bone has an increased area and porosity in A compared with B and C; the trabecular area is decreased, but the trabeculae are thickened.
(From Wright IM, Kidd L, Thorp BH: Gross, histological and histomorphometric features of the navicular bone and related structures in the horse, Equine Vet J 30:220, 1998.)
Studies of aging changes in the navicular bone of normal immature and mature horses suggested that there is a degenerative aging process similar to that seen in joints.24 However, a more recent study investigating not only the navicular bone but also the DDFT, CSLs, DSIL, and navicular bursa demonstrated no age-related differences between mature horses with no history of foot-related lameness ranging from 4 to 15 years of age.2,3 This suggests that there may be an individual susceptibility to degenerative change. Nonphysiological biomechanical factors may promote this susceptibility to degenerative change.16,24,26
The explanation for pain and lameness in horses with no detectable radiological change has been poorly investigated by post mortem studies. However, recent clinical experience with MRI has indicated that many horses with evidence of increased modeling of the navicular bone based on increased radiopharmaceutical uptake (IRU) detected using nuclear scintigraphy do have pathological abnormalities of the navicular bone detectable using MRI, with or without concurrent changes in the DDFT, CSLs, DSIL, and navicular bursa.27,28
Degenerative changes in the fibrocartilage on the palmar aspect of the navicular bone occur principally in the distal half of the bone, especially centered around the sagittal ridge in both sound and lame horses.2 In horses with navicular disease there is a greater degree of fibrocartilage damage, which may extend into the subchondral bone. Partial-thickness loss of fibrocartilage in this location was one of the most common lesions significantly associated with navicular disease in one study.25 It is likely to represent some of the earliest pathology of one form of this disease but remains difficult to identify in vivo, even with the use of MRI.8 Degenerative change of the spongiosa is generally only seen dorsal to extensive fibrocartilage damage. Physiological forces result in adaptive remodeling of the subchondral bone in immature horses, with cortical thickening.24 Nonphysiological forces may result in focal fibrocartilage and/or flexor cortex damage, with adjacent subchondral sclerosis dorsal to it, associated with thickening of trabeculae and focal areas of lysis. There may also be edema, congestion, and fibrosis of the marrow stroma within the medullary bone, which may result in a cystlike lesion.
Concurrently there may be fibrillation of the dorsal surface of the DDFT, which may predispose to adhesion formation between the DDFT and regions of partially or fully eroded fibrocartilage on the palmar aspect of the navicular bone. Whether lesions in the DDFT are primary or secondary to preexisting damage of the fibrocartilage currently remains open to debate. However, recent post mortem evidence suggests that there may be non–age-related degenerative vascular and matrix changes in the dorsal aspect of the DDFT in both lame and clinically normal horses.3 Although it has been suggested that vascular occlusion and matrix changes in the DDFT may be age-related,25 the results of a recent study showed that the severity of these changes was greater in horses with palmar foot pain than in age-matched control horses.3 Minor fibrillation of the dorsal aspect of the DDFT was seen in both lame and control horses, whereas deep sagittal splits were only seen in lame horses. Complete occlusion of blood vessels, replacement of normal tendon architecture by focal fibroplasia, and areas of fibrocartilaginous metaplasia were common in the lame horses. As these changes are predominantly seen in the intratendonous septa, there is a strong possibility that they predispose to the development of sagittal splits in the dorsal surface of the tendon along these septal planes. Sharp edges of splits in the DDFT extending from the dorsal surface may cause ulceration of the fibrocartilage of the navicular bone and thus predispose to lesions extending into the medulla.
There is an association between changes of the flexor aspect and distal and proximal borders of the navicular bone.2 Similar types of change occur at the proximal and distal aspects but tend to be more extensive distally. Enlarged synovial invaginations are the result of recruitment and activation of osteoclasts following the course of the nutrient vessels into the spongiosa. This may be associated with local medullary osteonecrosis and the presence of foci of fibrocartilaginous metaplasia and/or entheseous new bone close to the interface between the DSIL and the navicular bone.
Aging changes were described in the articular cartilage of the navicular bone and the opposing face of the distal phalanx.29 There was loss of proteoglycan and tidemark advancement, which was thought to reflect excessive shear stress in the zone between the calcified and noncalcified articular cartilage. A greater number of tidemarks were seen in horses with clinical signs of navicular disease than normal horses of similar age. However, a more recent study failed to identify significant age-related changes, and low-grade degenerative changes in the articular cartilage were common in both control horses and those with navicular disease.2
Observations from Nuclear Scintigraphy and MRI
Previous studies using tetracycline labeling of bone22 and scintigraphic studies27,30 have indicated that there is evidence of increased bone turnover in association with some forms of navicular disease, even in the absence of radiological abnormalities of the bone. IRU predominantly reflects increased osteoblastic activity31 but is not synonymous with either pain or lameness.32 IRU may reflect a functional adaptation to foot conformation and the biomechanical forces on the navicular bone. Comparison between scintigraphy and MRI has demonstrated that many horses with focal moderate or intense IRU have abnormalities of the navicular bone detectable using MRI.27 However, scintigraphy can also produce false-negative results, indicating that pathological abnormalities of the navicular bone are not always associated with increased osteoblastic activity.
A comparison of MRI findings in control horses with no history of foot-related pain and horses with chronic palmar foot pain showed significant alterations of the podotrochlear apparatus in the lame horses.33 A comparative MRI and post mortem study showed good correlation between the lesions identified using MRI and histopathological findings.34 Clinical experience with MRI in horses with foot pain provides support for the progression of lesions as outlined above and has demonstrated some earlier lesions than those investigated post mortem.8
However, a group of horses has also been identified with no detectable abnormalities of the flexor fibrocartilage or cortex but with diffuse abnormalities of the medulla characterized by increased signal intensity in fat-suppressed magnetic resonance (MR) images. Post mortem examination of several such horses revealed evidence of early fat necrosis with a moth-eaten appearance of the trabeculae, with necrosis of bone edges. This may have a different etiopathogenesis.
Hyperintense signal in the medulla of the navicular bone has been ascribed to the presence of edema in the marrow spaces,35 but this was not validated post mortem. Further research is required to determine the true causes of this phenomenon. In our studies, mild or moderate focal or generalized increased signal intensity in fat-suppressed MR images was associated with trabecular thinning and widened intertrabecular spaces.34 High-intensity increased signal associated with irregular decreased signal intensity in T1- and T2-weighted images was associated with generalized osteonecrosis and fibrosis, with irregular trabeculae, adjacent adipose tissue edema, and prominent capillary infiltration. A recent post mortem study of feet with advanced radiological abnormalities of the navicular bone demonstrated that increased signal intensity in fat-suppressed images correlated with areas of degenerate adipose tissues, hemorrhage or replacement by fibrocollagenous material, or fluid-filled cystic spaces.36
In some other horses, fluid-filled osseous cystlike lesions were seen in the distal aspect of the bone, apparently separate from synovial invaginations, and not associated with any detectable abnormality of the flexor aspect of the bone. Such lesions have not yet been characterized histologically, and their etiology remains speculative, although they may be associated with lesions of the DSIL.
Occasionally horses have been identified with new bone on the palmar aspect of the navicular bone, centered on the sagittal ridge. The cause of this is currently unknown.
Entheseous Changes
The presence of entheseous new bone on the proximal border of the navicular bone, reflecting previous insertional desmopathy of the CSL, is well documented radiologically37,38 and at post mortem examination25,37 in both clinically normal horses and horses with navicular disease. Its clinical significance remains uncertain, although more extensive new bone in this location tends to be associated with other signs of navicular disease.37,38 Recent experience with MRI has confirmed these findings.1 Rarely, an avulsion fracture is identified at the insertion of the CSL into the navicular bone.8,39 Mineralized and osseous fragments (Figure 30-3) in the DSIL have also been recognized in both normal horses and in horses with navicular disease, and their clinical significance remains difficult to determine. Fragments were unusual in sound horses undergoing prepurchase radiographic examination,40 although their true incidence may be underestimated by radiographic examination compared with MRI or computed tomography (CT). In two post mortem studies, fragments associated with a defect in the distal margin of the navicular bone were more common in horses with navicular disease than in age-matched controls.2,25 This has also been my clinical experience.
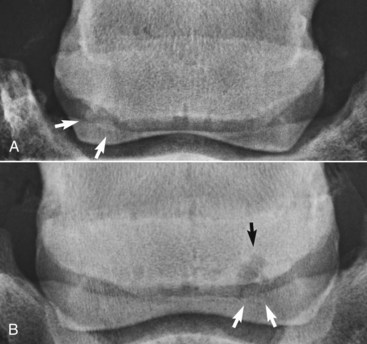
Fig. 30-3 Dorsoproximal-palmarodistal oblique radiographic images of two navicular bones showing a large discrete mineralized fragment on the distal medial sloping border of the bone (arrows) (A) and a distal border fragment (white arrows) located distal to the lateral angle of the navicular bone (B); there is a large radiolucent zone in the adjacent navicular bone (black arrow).
Fibrocartilaginous metaplasia in the body of the DSIL was more extensive in horses with navicular disease compared with age-matched control horses.2 However, no significant differences between groups were seen in the CSLs.
Aging changes have been seen in the region of insertion of the DSIL and DDFT, with a change in fibroblast shape and an increase in proteoglycans.14 The functional significance of this is not yet known. Evidence of inflammation was recently recognized histologically at the intersection of the DSIL and DDFT in horses with clinical signs of navicular syndrome.29,41 Changes reflecting “abnormal stress” at the insertion of the DSIL and DDFT have been demonstrated in horses with poor foot conformation.29 This region is rich in sensory nerve endings, with many arteriovenous complexes that are damaged in horses with navicular disease.42
Clinical experience with MRI has demonstrated that structural abnormalities of the DSIL are often seen in association with abnormal modeling of the distal palmar aspect of the navicular bone, with focal increased signal in fat-suppressed MR images.1,28 It has been suggested that this focally increased signal in the distal aspect of the navicular bone at the origin of the DSIL on fat-suppressed images may represent an important early event in the etiopathogenesis of navicular disease.33 Increased signal intensity may also be seen in the navicular bone close to the insertion of the CSLs in fat-suppressed images.1,28 In some horses there is a linear band of increased signal intensity in fat-suppressed images, extending through the middle third of the navicular bone, from the insertion of the CSL to the origin of the DSIL. Abnormalities of the CSL have also been associated with concurrent abnormalities of the navicular bone. Based on clinical experience, it seems that these lesions may be the result of abnormal stresses at the attachments of the CSL and DSIL on the navicular bone, and this may reflect a different mechanism of navicular disease development.8
Although endosteal irregularity at the insertion of the DSIL on the distal phalanx may be seen in both horses with and without foot pain,33,34 in some lame horses there is evidence of insertional desmopathy, characterized by enthesophyte formation, axial cortical disruption, osseous cystlike leions, and/or increased signal intensity in the bone at this site in fat-suppressed images, reflecting bone edema or necrosis.8
The Navicular Bursa
The incidence and etiology of primary bursitis of the navicular bursa are not known, nor is the relationship to the development of navicular disease. Villous hypertrophy, hyperplasia of synovial lining cells, and venous congestion have been described in association with navicular disease, whereas the synovial membrane appeared uniform in six normal horses of undetermined age.43 However, in another study comparing immature horses, horses with navicular disease, and age-matched controls, 3 of 25 age-matched controls had evidence of asymptomatic chronic synovitis. In both the navicular disease group and the age-matched controls, mild hyperplasia and hypertrophy were seen compared with immature horses up to 3 years of age.25 In a more recent study, there was no evidence of acute inflammation within the navicular bursa in horses with palmar foot pain or age-matched control horses2; however, lame horses had marked chronic synovial proliferation compared with control horses.2,33 There was a positive association between abnormalities of the bursa and lesions of either the dorsal aspect of the DDFT or the flexor aspect of the navicular bone. Clinical experience with MRI has indicated that abnormal distention of the bursa is a frequent finding in lame horses but is rarely seen in isolation.1
Associations between Injuries
It is clear from our recent post mortem study, as well as from clinical experience using MRI, that frequently several structures are affected concurrently. It is common to see various combinations of abnormalities of the navicular bone, DDFT, DSIL, CSL, and the collateral ligaments (CLs) of the DIP joint. Clinical MR examination of 263 horses with forelimb foot pain revealed 6 with abnormalities of the navicular bone alone; 29 with concurrent DDFT and navicular bone abnormalities; 60 with various combinations of abnormalities of the navicular bone, DSIL, DDFT, or CSL; 46 horses with CL injury of the DIP joint combined with lesions of the DDFT, CSL, DSIL, or navicular bone; and 25 horses with abnormalities of five or more structures.28 The sequence of injury occurrence remains speculative. It is possible that degenerative changes in several structures may predispose to concurrent injury. The navicular bone, CSL, and DSIL act as a unit and so presumably undergo similar biomechanical stresses. Alternatively, injury to one structure may cause low-grade instability, predisposing to injury of closely related structures.
What Causes Pain?
Pain associated with navicular disease may be caused by venous congestion of the navicular bone. Dilated venules and sinusoids entrapped in fibrous marrow have only been identified in horses with navicular disease.24 Increased intraosseous pressure has been measured in horses with navicular disease.44,45 Distention of the navicular bursa may cause pain. The contribution of other causes or sources of pain remains open to speculation, although many sensory nerve endings have also been identified in the CSLs and the DSIL,46,47 and given the high frequency of occurrence of concurrent abnormalities in these structures, it is likely that these nerve endings may be important in pain mediation.
The Future
It is clear that degrees of adaptive and reactive change occur in the podotrochlear apparatus and DDFT of all horses. We need to understand better both the factors that stimulate their progression and what causes pain. Identification of genetic and biomechanical risk factors would be useful. Study of horses with early navicular disease should help to establish better the interrelationship between abnormalities of the DDFT, navicular bone, CSL, and DSIL. We need to determine what factors lead to vascular and matrix changes in the DDFT. Further research into the sensory nerve supply to the podotrochlear apparatus and DDFT may help in understanding what causes pain—and therefore lameness—and how it may be treated.
Diagnostic Considerations
History
Most horses are presented with a history of an insidious onset of loss of performance, shortening of stride, or intermittent shifting bilateral forelimb lameness that usually is worst on firm ground. The complaint from the owner may be loss of action, stiffness, unwillingness to jump, especially drop fences, and inability to lengthen stride. Less commonly a horse may have acute-onset, moderate to severe, usually unilateral but sometimes bilateral, forelimb lameness. The condition is rare in ponies compared with horses, and although hindlimb lameness associated with navicular disease is unusual, it does occasionally occur in both ponies and horses.48,49
Clinical signs often are first apparent when the horse is approximately 7 to 9 years of age, although the disease can occur in young horses of 3 to 4 years of age, which may have fairly advanced radiological abnormalities. Lameness may first become apparent after a period of enforced rest because of some other unrelated problem or after a change in management. Development of lameness soon after change of ownership, associated with a change in trimming and shoeing, different work patterns, and altered periods of turnout, is not uncommon.
Clinical Signs
Pain when the horse is standing at rest may be a feature of navicular disease; however, this is a variable finding, and some clinically normal horses habitually point one or both front feet. A horse that has resting pain associated with navicular disease may stand pointing one front foot, sometimes alternating between feet. This is also a feature of horses with primary lesions of the DDFT. Alternatively, an affected horse may pack bedding under the heel or have a tendency to sit on a manger to relieve pressure on the palmar aspect of the foot. Navicular disease is recognized in horses with a wide variety of foot shapes: narrow, boxy upright feet of the Quarter Horse and low, collapsed heels typical of many Thoroughbreds. Navicular disease often is seen in association with poor mediolateral or dorsopalmar foot balance. If lameness is consistently worse on one forelimb, the feet may become asymmetrical in shape, with the lamer foot narrower with a taller heel.
The digital vessels sometimes are palpably enlarged, but this is an inconsistent, nonspecific finding. The response to hoof testers applied to the frog region is often negative,22,49,50 although other authors51 have described a positive response as a fairly consistent feature of navicular disease. Pain may be elicited at the toe if the horse has been repeatedly overloading the toe, resulting in subsolar bruising. Differences in clinical signs observed by different clinicians may reflect genuine differences in horse populations in different geographical locations. Distention of the DIP joint capsule is sometimes seen in association with navicular disease but not invariably so. Increased intraarticular pressure (>40 mm Hg) is said to be associated with navicular disease,52 although in my experience there is considerable variability in the degree of DIP joint distention and pressure in both normal and lame horses.
Lameness is sometimes apparent when the horse is moving on a hard surface in straight lines. It may fluctuate in degree within an examination period or between examinations performed on different days. The horse may show a tendency to stumble associated with an altered foot placement. Overt unilateral lameness may be evident, but in some horses there is only marginal shortening of stride and reduced lift to the stride, which may be difficult to detect if the horse was not previously known to the observer. The horse may move better on a soft surface, even in circles. No clinical signs are detectable in some horses when examined moving in hand on a hard surface. Lameness generally is accentuated if the horse moves in circles on a hard surface, especially with the lame limb on the inside of the circle. In some horses, lameness is apparent only under these circumstances. Less commonly lameness is accentuated when the lame limb is on the outside of a circle. Sometimes lameness cannot be detected unless the horse is ridden, when it may move in a slightly stiff, “flat,” and restricted fashion. Recognition of this requires previous knowledge of the horse, knowledge of the expected quality of movement of a horse of that type, or respecting the opinion of the rider that the horse has “lost some action.” A vast difference may be detected by desensitizing both front feet. This may be easier to appreciate when riding the horse than watching it.
The response to distal limb flexion is extremely variable. Many horses with navicular disease show a transient, mild increase in lameness.22,49,50 Resistance to flexion or marked accentuation of lameness after flexion is unlikely to reflect navicular bone pain. Distal limb flexion of one forelimb may result in increased lameness in the contralateral forelimb because of increased loading of the podotrochlear apparatus, but the same response can also be seen in horses with primary injuries of the DDFT. Elevation of the toe of the foot on a wedge or wooden board, with the contralateral limb picked up, resulting in extension of the DIP joint, may increase lameness, but this response is neither consistent nor pathognomonic for navicular disease.
If the horse’s foot conformation is poor and the feet are not trimmed and shod optimally, it is worthwhile improving the trimming and shoeing and reassessing the lameness after several weeks. If the lameness has markedly improved, it is unlikely to reflect navicular disease.
Response to Local Analgesic Techniques
Perineural analgesia of the palmar digital nerves, using 1.5 to 2 mL of mepivacaine hydrochloride (2%) per site, performed immediately axial to the cartilages of the foot usually results in improvement in lameness. However, lameness often is not alleviated fully.50,53 Occasionally there is no response. Lameness is generally alleviated completely after perineural analgesia of the palmar nerves, performed at the base of the proximal sesamoid bones, unless there is another concurrent source of pain. Horses should always be reevaluated in both straight lines and circles to determine whether lameness becomes apparent or is accentuated on the contralateral limb.
Intraarticular analgesia of the DIP joint using 4 to 6 mL of mepivacaine can alleviate or improve pain associated with the navicular bone within 5 minutes of injection.53,54 A negative response does not preclude navicular pain because approximately 20% of horses have a negative response to intraarticular analgesia of the DIP joint and a positive response to intrathecal analgesia of the navicular bursa53; this has been correlated with both radiological and post mortem abnormalities of the navicular bone. A positive response to intraarticular analgesia of the DIP joint is a nonspecific result (Figures 30-4 and 30-5) because this technique can alleviate solar pain55 and pain associated with the palmar processes of the distal phalanx, the DSIL, the DDFT, and the joint itself.46,53,54 Misleading positive results are more likely to occur with larger volumes of local anesthetic solution and evaluation of the response more than 10 minutes after injection.56
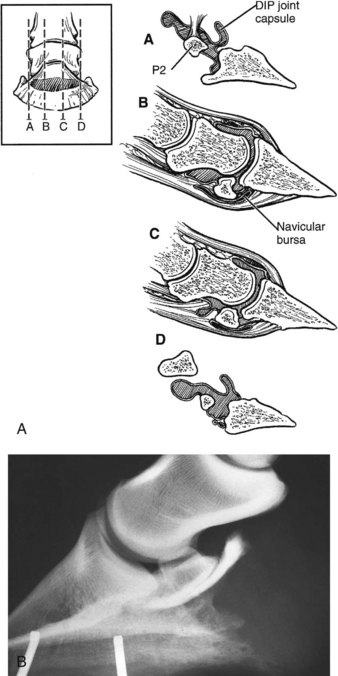
Fig. 30-4 A, Diagram of the relationship between the distal interphalangeal (DIP) joint capsule, the collateral ligaments of the navicular bone, and the distal sesamoidean impar ligament. The four sagittal sections through the foot are denoted with lines A, B, C, and D. B, Lateromedial radiographic image of a foot. Three milliliters of radiodense contrast medium has been injected into the navicular bursa to show its proximodistal extent.
Fig. 30-5 Histological sample stained to show substance P, a small peptide, within thinly myelinated sensory nerves in the collateral ligaments of the navicular bone, appearing to innervate microvessels and pass toward the navicular bone.
(Courtesy Dr. R. Bowker.)
A positive response to analgesia of the navicular bursa (3 to 4 mL of mepivacaine) usually reflects primary navicular bone pain, primary bursal pain, or a primary lesion of the DDFT. For accurate injection into the navicular bursa, the needle should be sited in the middle of the flexor surface of the navicular bone. Placement of a needle into the navicular bursa is best performed under radiographic control or should be followed by injection of a radiographic contrast agent to check the accuracy of injection. If the needle is positioned too far proximally, there is danger of injection into the proximal palmar outpouching of the DIP joint or the digital flexor tendon sheath. If the needle is too far distal, it may enter the distal palmar outpouching of the DIP joint. If synovial fluid appears spontaneously in the needle hub, it is unlikely that the needle is positioned correctly. Improvement in lameness is usually seen within 5 minutes of injection of local anesthetic solution. A negative response to analgesia of both the DIP joint and the navicular bursa makes it unlikely that the horse has navicular disease.
Radiographic Examination
Radiographic examination of the navicular bone should be performed after removal of the shoes and appropriate preparation of the feet, and should include lateromedial (LM), dorsoproximal-palmarodistal oblique (DPr-PaDiO), and palmaroproximal-palmarodistal oblique (PaPr-PaDiO) images (Figure 30-6). Flexed oblique images of the DIP joint also are useful for evaluation of its articular margins. Appropriate positioning of the feet is critical for high-quality images. Correct angulation of the x-ray beam for PaPr-PaDiO images is crucial for diagnostic images. Artifacts can be created unless the x-ray beam is tangential to the palmar surface of the navicular bone.8,57 The correct angle can vary between 35 and 50 degrees, depending on the shape of the foot.
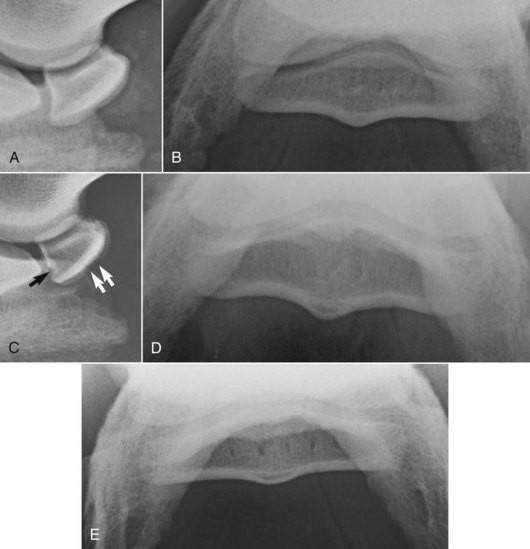
Fig. 30-6 A, Lateromedial radiographic image of a normal navicular bone. B, Palmar 45° proximal-palmarodistal oblique radiographic image of a normal navicular bone. C, Lateromedial radiographic image of a normal navicular bone; note the smoothly outlined concave depression in the sagittal ridge (white arrows) and the concavity in the distal border, a normal fossa (black arrow). D, Palmar 45° proximal-palmarodistal oblique radiographic image of a normal navicular bone with a well-defined, crescent-shaped radiolucent zone in the central eminence of the flexor cortex. E, Palmar 50° proximal-palmarodistal oblique image of a normal navicular bone in a horse with upright foot conformation. The flexor cortex is flatter and thinner compared with C.
The proximal border of the navicular bone has two margins: the flexor surface, which is always convex in a DPr-PaDiO image, and the articular margin, which is variable in shape. It can be categorized as concave, undulating, straight, or convex. In the Dutch Warmblood an enhanced risk of the development of navicular disease has been suggested if the articular margin is concave or undulating rather than convex.6
The following features should be evaluated in all images57 (Figures 30-6 through 30-9): the presence, number, shape, size, and location of radiolucent zones along the distal borders of the navicular bone; the presence of radiolucent zones along the proximal border of the navicular bone; radiolucent zones within the medulla of the bone; trabecular pattern within the medulla; enthesophyte formation on the proximal or distal aspects of the bone; presence of mineralization within a CSL; presence of articular osteophytes; thickness of the flexor cortex; regularity of outline of the flexor cortex; radiolucent areas in the flexor cortex; new bone formation on the flexor surface; corticomedullary definition; and presence of mineralized fragments distal to the navicular bone.
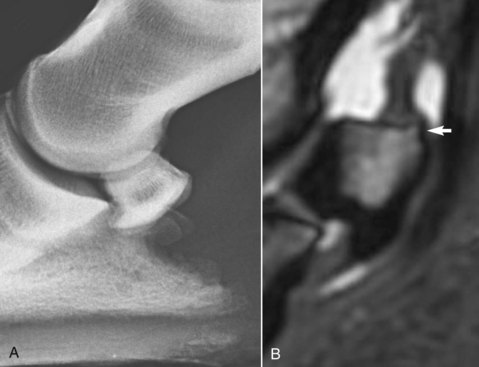
Fig. 30-7 A, Lateromedial radiographic image of the left front foot of a 6-year-old Warmblood dressage horse with bilateral foot pain. The flexor cortex is abnormally thick. B, Sagittal T2*-weighted spoiled gradient-echo MR image of the same foot as in A. The dorsal, distal, and palmar cortices are thickened, and there is a small enthesophyte proximally (arrow) at the insertion of the collateral sesamoidean ligament.

Fig. 30-8 A, Dorsoproximal-palmarodistal oblique radiographic image of a navicular bone of a horse with clinical signs of navicular disease. Note the variably shaped and sized radiolucent zones along the distal aspect of the bone. There is proximodistal elongation of the bone. B, Dorsoproximal-palmarodistal oblique radiographic image of the navicular bone of a horse with clinical signs of navicular disease. Lateral is to the right. There are multiple variably shaped and sized radiolucent zones along the distal border. The modeling of the proximal medial and lateral margins of the bone is the result of enthesophyte formation.
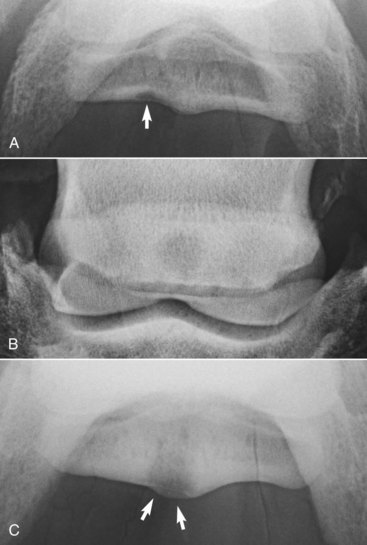
Fig. 30-9 A, Dorsoproximal-palmarodistal oblique radiographic image of a navicular bone with a radiolucent defect in the flexor cortex of the navicular bone medially (arrow). The bone appeared normal in all other radiographic projections. The horse had a bilateral forelimb lameness, which was improved by analgesia of the distal interphalangeal joint or navicular bursa. B, Dorsoproximal-palmarodistal oblique radiographic image of a navicular bone with a large cystlike lesion within the spongiosa of the bone, which penetrated the flexor cortex of the bone (see C).The horse had a bilateral forelimb lameness that was worse on soft ground with the lamer limb on the outside of a circle. Lameness was improved by palmar (abaxial sesamoid) nerve blocks. C, Palmaroproximal-palmarodistal oblique radiographic image of the same navicular bone as in B, with a large lucent area penetrating the flexor cortex of the bone. There were extensive adhesions between this defect and the deep digital flexor tendon.
A grading system for evaluation of the navicular bone in LM and DPr-PaDiO views was devised by Dik.58 An adapted version is presented in Box 30-1. The degree of lameness and the degree of radiological abnormality often are poorly correlated. Some horses with navicular bone pain have no detectable radiological change. Some horses, especially young horses, have relatively advanced radiological changes when lameness is first recognized. In many horses with suspected navicular disease the radiological abnormalities are equivocal. The ability to document the progression of radiological change over time (years) is relatively unusual, although the development of a cystlike lesion penetrating the flexor cortex has been noted within 21 days of the onset of acute lameness.59
BOX 30-1 Radiological Findings of the Navicular Bone in Normal and Diseased Horses
| Grade | Condition | Radiological Findings |
|---|---|---|
| 0 | Excellent | Good corticomedullary demarcation; fine trabecular pattern. Flexor cortex of uniform thickness and opacity. No radiolucent zones along the distal border of the bone, or several (<6) narrow conical radiolucent zones along the horizontal distal border. Right and left navicular bones symmetrical in shape. |
| 1 | Good | As above, but radiolucent zones on the distal border of the navicular bone more variable in shape. |
| 2 | Fair | Slightly poor definition between the palmar cortex and the medulla as a result of subcortical increased radiopacity. Crescent-shaped radiolucent zone in the central eminence of the flexor cortex of the bone. Several (<8) radiolucent zones of variable shape along the distal horizontal border of the navicular bone. Mild enthesophyte formation on the proximal border of the navicular bone. Navicular bones asymmetrical in shape. Proximal or distal extension of the flexor border of the navicular bone. |
| 3 | Poor | Poor corticomedullary definition resulting from increased medullary radiopacity. Thickening of the dorsal and flexor cortices. Poorly defined radiolucent areas in the flexor cortex of the bone. Many (>7) radiolucent zones along the distal horizontal or sloping borders of the navicular bone. Radiolucent zones along the proximal border of the bone. Large enthesophyte formation on the proximal border of the bone. Discrete mineralization within a collateral sesamoidean ligament. Radiopaque fragment on the distal border of the navicular bone. |
| 4 | Bad | Large cystlike lesion within the medulla of the navicular bone. Radiolucent region in the flexor cortex of the navicular bone. New bone on the flexor cortex of the navicular bone. |
The interpretation of distal border radiolucent zones, which represent synovial invaginations from the DIP joint,60,61 has long been subject to controversy. It is generally accepted that the larger the number of radiolucent zones (>7), of variable size and shape, the more likely they are to be of clinical significance. Such radiolucent zones may be seen in association with localized increased radiopacity. Radiolucent zones positioned on the medial and lateral sloping borders of the bone are considered more likely to be of clinical significance. Clusters of axially positioned radiolucent zones, combined with distal elongation of the flexor border of the navicular bone, may reflect abnormal stress at the origin of the DSIL. Comparison between DPr-PaDiO and PaPr-PaDiO views helps to determine the dorsopalmar extent of the radiolucent zones. Proximal border radiolucent zones are occasionally seen and are not normal. They have been seen in association with lesions of the CSL.
Large radiolucent zones close to, but apparently discrete from, the distal border of the navicular bone may occur alone or in association with distal border synovial invaginations. Such radiolucent zones usually extend from the palmar to dorsal cortices when assessed in a PaPr-PaDiO view and are not normally seen in clinically sound horses.
Osseous fragments on the distal border of the navicular bone usually occur at the distal medial and lateral angles of the navicular bone, although less commonly involve the medial or lateral sloping border. Several studies have shown that such fragments occur more commonly in lame horses with other abnormalities of the navicular bone than in clinically normal horses.2,25,38,62 Prevalence was low in a group of clinically normal horses undergoing a prepurchase examination.40 Such fragments may reflect ectopic mineralization in the DSIL, a fracture of the distal border of the navicular bone, or a fracture of an enthesophyte at the origin of the DSIL. The presence of a radiolucent area at the medial or lateral angle of the distal border of the navicular bone suggests the presence of a fragment, which can be present uniaxially or biaxially. Fragments can be seen as an incidental abnormality, especially if the opacity of the adjacent navicular bone is normal, but if seen in association with a radiolucent area, they are more likely to be associated with lameness. Such fragments have been associated with marked reactions in the adjacent navicular bone seen in MR images, presumably the result of chronic movement, or associated with adhesions to the DDFT and/or associated lesions of the DSIL. Fragments are most easily detected in a DPr-PaDiO image, but the ease with which they can be seen is somewhat position dependent. In some horses the presence of a fragment can be verified in a PaPr-PaDiO image, in which an opacity representing the fragment is usually seen superimposed over the spongiosa of the navicular bone. Fragments displaced from the proximal border of the navicular bone are rare and in my experience have been associated with lameness and presumably reflect an avulsion at the insertion of the CSL.39
A crescent-shaped lucent zone in the flexor cortex of the navicular bone in the sagittal ridge (see Figure 30-6, D) seen in a PaPr-PaDiO image is considered to be a normal variant, representing a reinforcement line.63 The crescent-shaped lucent zone in the sagittal ridge of the navicular bone is rarely seen in very young horses. It represents early navicular bone modeling in response to stress and is of unknown clinical significance. A relatively sclerotic reinforcement line develops in the subchondral bone parallel with the flexor cortex in the region of the sagittal ridge. The intervening bone is relatively radiolucent and is projected in the PaPr-PaDiO image as the crescent-shaped lucent zone in the sagittal ridge. If the bone between the reinforcement line and the flexor cortex becomes compacted, then the radiolucent zone becomes less clear and may be obliterated. An ill-defined loss of opacity at the most palmar aspect of the sagittal ridge has been seen as an incidental finding, with no other pathological change, but may be a precursor to the development of clinically significant degeneration.
Central or acentric radiolucent zones, cystlike lesions, in the spongiosa of the navicular bone seen in DPr-PaDiO images are almost invariably of clinical significance (see Figure 30-9, B and C). They usually involve the middle third of the bone from proximal to distal, but not invariably so. In some horses such lesions can be seen to involve the flexor cortex of the bone in PaPr-PaDiO images. However, not all such lesions involve the flexor cortex. Moreover, some lesions penetrate the flexor cortex, but this cannot be verified radiologically. This depends on which part of the flexor cortex is tangential to the x-ray beam, the location of the lesion, and its size. A large radiolucent area in the flexor cortex of the navicular bone is invariably of clinical significance, and in some horses is the only detectable radiological abnormality. Such lesions usually occur at the sagittal ridge or abaxial to it. The lesion usually reflects erosion of the overlying fibrocartilage and adhesions of the DDFT. There may be associated increased radiopacity of the trabecular bone dorsal to the lesion.
New bone formation on the flexor border of the navicular bone is usually only detectable in a PaPr-PaDiO image and is usually centered around the sagittal ridge. It has only been seen in association with lameness and has not necessarily been seen in conjunction with other abnormalities of the navicular bone.
The thickness of the cortices of the navicular bone varies within and between horses. The thickness of the flexor cortex is easiest to evaluate in a well-positioned LM image (see Figure 30-6). Like any bone, shape is influenced by Wolff’s law. In a sound horse with one foot that is much more upright than the other, the flexor cortex of the more upright foot is often thinner than the contralateral foot. In one form of navicular disease there is progressive thickening of the cortices of the bone (see Figure 30-7) and the trabeculae of the spongiosa.25 Abnormal thickness of the flexor cortex of the navicular bone probably reflects pathological change.
A normal navicular bone has a regular trabecular architecture in the spongiosa that is clearly defined from the dorsal and palmar cortices in both LM and PaPr-PaDiO images. In one form of navicular disease there is generalized increased radiopacity of the spongiosa, involving particularly the middle third of the bone from laterally to medially. This can also develop as a sequel to acute trauma to the bone. However, a thick overlying frog can create increased radiopacity in this region, and this should be differentiated from genuine increased radiopacity. Alternatively, increased radiopacity may be localized toward the palmar aspect of the bone, affecting the endosteal aspect of the flexor cortex. This can be seen in association with abnormal thickness of the cortices of the bone and may be associated with a defect in the flexor cortex not detectable radiologically.
Demarcation between the spongiosa and the flexor cortex of the navicular bone must be assessed from both LM and PaPr-PaDiO images. However, it must be borne in mind that positioning of the foot during image acquisition may influence the appearance of the navicular bone in the skyline projection, and give a false impression of increased radiopacity of the spongiosa. The two views should be compared carefully.
The shape of the navicular bone should be assessed carefully in a LM image.37 Distal extension of the flexor border of the navicular bone may reflect chronic stress at the origin of the DSIL. Proximal extension of the flexor border of the navicular bone may reflect chronic stress at the insertion of the CSLs.
Enthesophytes at the proximal medial and lateral aspects of the navicular bone are best identified in DPr-PaDiO or dorsopalmar images. Small lateral enthesophytes are a common incidental abnormality and presumably reflect asymmetrical stress at the insertion of the lateral and medial CSLs. However, the presence of a large lateral enthesophyte, or a medial enthesophyte, is more likely to reflect abnormal stress on the podotrochlear apparatus and potentially to be associated with lameness.
The position of the navicular bone in a LM radiograph relative to the middle and distal phalanges is partly influenced by the position of the limb during image acquisition. However, occasionally the bone is abnormally close to the distal phalanx. This has been seen in young horses with lameness recognized soon after the introduction of work and has generally been associated with advanced pathological changes of the navicular bone and the DIP joint. This may reflect a congenital abnormality.
Generally the larger number of radiological changes that are present in all radiographic projections, the more likely it is that the horse has clinical navicular disease. The most clinically significant radiological changes likely to reflect navicular disease include cystlike lesions within the medulla (see Figure 30-9, A) that are discrete from distal border lucencies, generalized increased radiopacity of the medulla, reduced corticomedullary demarcation, new bone on the flexor surface, and erosions of the flexor cortex of the bone (see Figure 30-9, B and C).
In some horses, concurrent degenerative changes of the DIP joint are also seen, with modeling* of the proximal dorsal articular margin of the navicular bone, the dorsal and palmar articular margins of the middle phalanx, and the extensor process of the distal phalanx.
In some horses the development of advanced radiological changes precedes the recognition of clinical signs. However, the absence of radiological abnormalities of the navicular bone does not preclude the presence of pain associated with the navicular bone and scintigraphic evidence of increased bone modeling.
*There is confusion between the histological and radiological usage of the terms remodeling and modeling. Histologically, remodeling refers to resorption and formation of bone that is coupled and occurs in basic multicellular units. This regulates the microstructure of bone without altering its shape and is a continuous process, replacing damaged bone with new bone. Thus it cannot be appreciated on radiographs. The term has been used with regard to radiographs to describe the reshaping of bone to match form and function (e.g., after fracture repair), but strictly speaking, the term modeling should be used.
Histologically, modeling refers to resorption and formation of bone that is not coupled and occurs at anatomically different sites (bone drift). It is a continuous process that regulates the macroscopic appearance of bone according to Wolff’s law. In terms of radiography, modeling has been used to describe the formation of bone relative to the cartilage model that is being replaced (i.e., the normal formation of bone). Thus the two definitions do not agree; to avoid confusion, strictly speaking the term modeling should be applied to describe the change in shape of a bone as it adapts to the stresses applied to it.
Contrast Radiography of the Navicular Bursa
Contrast radiography of the navicular bursa may reveal a number of abnormalities not detectable on plain radiographs, including thinning or erosion of the flexor fibrocartilage of the navicular bone, loss of the radiopaque contrast layer thought to be caused by adhesion formation between the DDFT and the navicular fibrocartilage, and filling defects on the palmar aspect of the bursa perhaps associated with surface fibrillation of the DDFT.49,64 The clinical significance of these findings is currently questionable because it has been well recognized that thinning of the flexor fibrocartilage is a normal aging change, and the clinical significance of surface fibrillation of the DDFT is still open to debate.
Nuclear Scintigraphy
Tetracycline labeling of bone has revealed increased bone turnover in the navicular bone in navicular disease.22,23 Nuclear scintigraphy offers a sensitive method of detecting increased bone turnover; however, this is not necessarily synonymous with either pathological change or pain.
Lateral pool (soft tissue) and lateral and palmar (solar) bone phase images of the front feet are required for accurate diagnosis of navicular disease (Figures 30-10 and 30-11).27,28,30,32,65 In solar images of normal horses, uptake of the radiopharmaceutical in the navicular bone and the distal phalanx is approximately similar. However, uneven uptake in various regions of the distal phalanx can complicate interpretation. Care must be taken when interpreting lateral images not to confuse uptake in the cartilages of the foot with uptake in the navicular bone. In solar images, radiopharmaceutical uptake associated with the proximal interphalangeal joint can potentially be superimposed over the navicular bone, unless the foot is well extended during image acquisition and the pastern region is masked with a lead shield. Interpretation can also be difficult because some horses with unilateral lameness have IRU in both the left and right front navicular bones while apparently experiencing pain in only one limb.
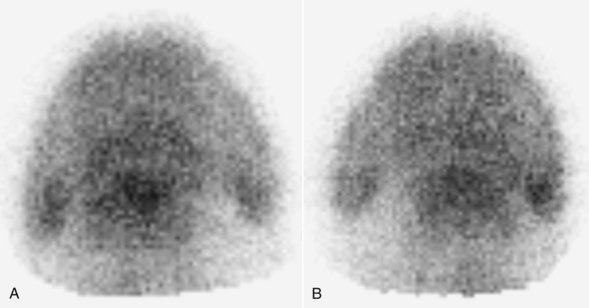
Fig. 30-10 Solar scintigraphic bone phase images of the left (A) and right (B) front feet of a 7-year-old Warmblood with left forelimb lameness. There is focal moderate increased radiopharmaceutical uptake (IRU) in the left front navicular bone, with mild diffuse IRU in the region of insertion of the deep digital flexor tendon on the distal phalanx and mild focal IRU in the medial and lateral palmar processes of the distal phalanx. In the right forelimb there is mild diffuse IRU in the navicular bone, mild focal IRU in the lateral palmar process, and moderate IRU in the medial palmar process of the distal phalanx.
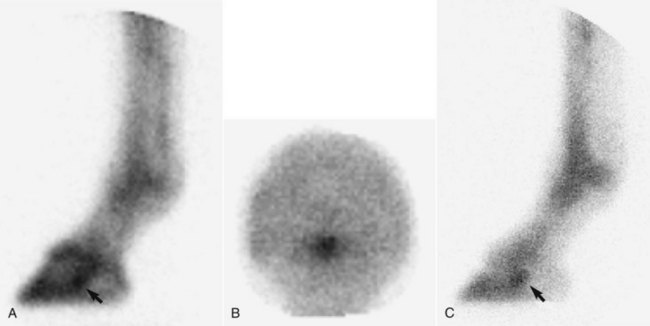
Fig. 30-11 Scintigraphic images of the left front foot of a 5-year-old Warmblood dressage horse with acute-onset left forelimb lameness abolished by palmar digital analgesia and intraarticular analgesia of the distal interphalangeal joint. A, Lateral pool phase image. B, Solar bone phase image. C, Lateral bone phase image. There is focal intense increased radiopharmaceutical uptake in the navicular bone (arrow) in all images.
IRU in the navicular bone, with or without IRU at the region of insertion of the DDFT, has correlated well with a positive response to intrathecal analgesia of the navicular bursa32 and with abnormalities of the navicular bone detected using MRI.27,28 False-positive results can be found in clinically normal horses, although a relatively high percentage have subsequently developed lameness associated with palmar foot pain.32,49
There is a relatively high incidence of horses with clinical signs compatible with navicular disease and a positive response to intraarticular analgesia of the DIP joint or intrathecal analgesia of the navicular bursa that have no detectable radiological abnormalities of the navicular bone but have IRU in the navicular bone.27,28,32 Thus nuclear scintigraphy offers a sensitive method of diagnosis of navicular disease, although if bone necrosis is the principal abnormality or the disease is end stage, radiopharmaceutical uptake may be normal.28
Computed Tomography and Magnetic Resonance Imaging
CT66,67 (see Chapter 20) and MRI* (see Chapter 21) are potentially more sensitive than radiography in determining structural lesions of the navicular bone (Figures 30-12 through 30-15) and identifying degenerative changes in the articular cartilage of the DIP joint and primary lesions in the DDFT, the DSIL, and the CSLs. A number of different pathological changes have been identified in the navicular bone that were not detectable with high-quality computed radiography and that may have a different etiopathogenesis. These include:
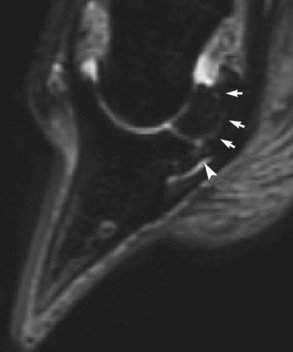
Fig. 30-12 Sagittal short tau inversion recovery (STIR) MR image of an 8-year-old dressage horse with loss of forelimb action. There is linear increased signal intensity extending through the navicular bone between the insertion of the collateral sesamoidean ligament and the origin of the distal sesamoidean impar ligament (DSIL) (white arrows). There is also increased signal intensity in the DSIL (arrowhead).
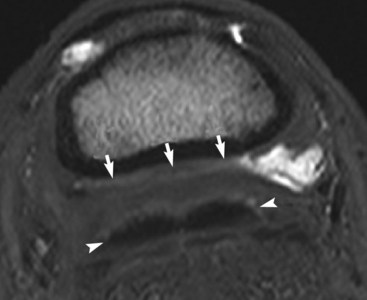
Fig. 30-13 Transverse T2* gradient-echo magnetic resonance (MR) image of the left front foot at the level of the middle phalanx of a 7-year-old pleasure riding horse with some structural abnormalities of the navicular bone seen on MR images. The collateral sesamoidean ligament (arrows) is substantially thickened and is in close apposition to the deep digital flexor tendon (arrowheads), which has a rather irregular dorsal border. Fluid can only be seen in the navicular bursa to the right of the image.
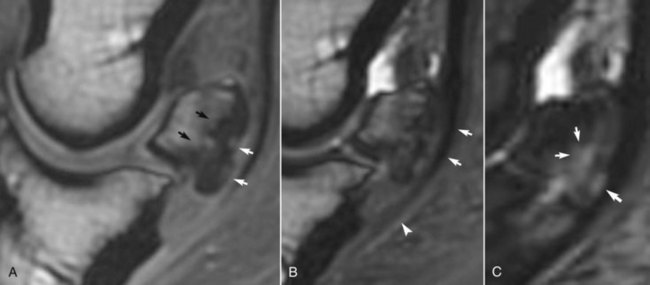
Fig. 30-14 A, Sagittal T1-weighted spoiled gradient-echo magnetic resonance (MR) image; B, three-dimensional T2* gradient-echo MR image; and C, short tau inversion recovery (STIR) MR image of a Grand Prix show jumper with lameness improved by analgesia of the navicular bursa. There were no detectable radiological abnormalities, but there was focal intense increased radiopharmaceutical uptake in the navicular bone. A, There are two focal areas of increased signal intensity in the flexor cortex of the navicular bone; the flexor cortex is thickened, and there is marked endosteal irregularity (black arrows). B, There is loss of separation between the deep digital flexor tendon (arrows) and the palmar aspect of the navicular bone and close apposition of the tendon and the distal sesamoidean impar ligament (arrowhead). C, There is increased signal intensity in the flexor cortex of the navicular bone and in the spongiosa (arrows).
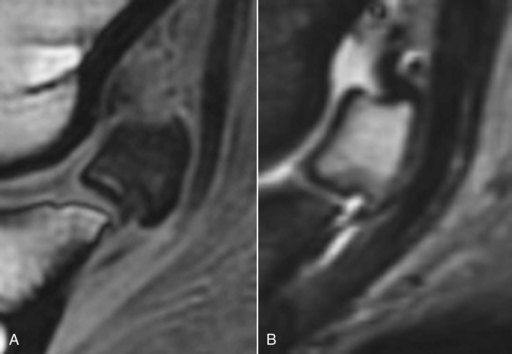
Fig. 30-15 A, Sagittal spoiled gradient-echo magnetic resonance (MR) image; and B, sagittal short tau inversion recovery (STIR) MR image of the left front navicular bone of a 8-year-old Warmblood show jumper with recurrent lameness alleviated by palmar digital analgesia. There is diffuse decreased signal intensity throughout the spongiosa of the navicular bone in the T1-weighted image and diffuse hyperintense signal in the STIR image. Radiopharmaceutical uptake was normal. Post mortem examination revealed trabecular and adipose tissue necrosis of the spongiosa of the navicular bone.
It must also be borne in mind that in a substantial proportion of horses there is a combination of injuries involving not only the navicular bone but also the CSL, DSIL, navicular bursa, and DDFT. In a U.K. study of 347 horses with foot pain that underwent MRI, 12 horses (3.5%) had lesions of the navicular bone alone, 40 horses had lesions of the navicular bone and DDFT (11.5%), and a further 184 (53.0%) had combinations of lesions of the podotrochlear apparatus, navicular bursa, and DDFT.70 In a U.S. study of 151 horses in which Quarter Horses predominated, there was a much higher incidence of primary navicular bone lesions.71 However, horses with more chronic lameness (>6 months’ duration) were more likely to have concurrent soft tissue lesions, especially in the CSL.
Endoscopic Evaluation of the Navicular Bursa
Endoscopic examination of the navicular bursa (see Chapter 24) permits evaluation of the fibrocartilage on the flexor surface of the navicular bone, the navicular bursa itself, the overlying dorsal surface of the DDFT, and a limited view of the distal sesamoidean impar ligament.49,72 Thus it is possible to definitively identify adhesions between the DDFT and the palmar aspect of the navicular bone, thinning or full-thickness erosion of the flexor fibrocartilage of the bone, fibrillation of the dorsal aspect of the DDFT, and synovitis of the bursa. Tears in the DSIL have also been identified,49 but these are thought to be of traumatic origin rather than part of the navicular disease complex. It is important to differentiate between age-related changes and pathological abnormalities.
Other Soft Tissue Causes of Palmar Foot Pain
Our understanding of soft tissue pain associated with the palmar aspect of the foot has been limited until recently to identification of pain seen in association with poor foot balance, poor shoeing, or both factors that responded to corrective trimming and shoeing (see Chapter 27). Horses that respond satisfactorily to trimming and shoeing alone are unlikely to have chronic navicular disease, and although navicular bone pain may be present, the precise source of pain cannot be determined. A hemodynamic function for the cartilages of the foot and associated vascular system may contribute to force dissipation (see Chapter 29).73 Peptidergic sensory nerve endings also have been identified in the vascular channels of the cartilages of the foot, close to pacinian-like corpuscles, indicating that this part of the foot may respond to sensory and proprioceptive stimuli. In horses with long toes and underrun heels, the cartilages of the foot and associated vasculature are less well developed than in a better conformed foot. This may result in less effective force distribution, predisposing to the development of palmar foot pain. In addition, morphological differences have been identified in the digital cushions between “healthy” and “weak” feet, so the digital cushion also may be involved in force dissipation.
With the advent of MRI other soft tissue causes of palmar foot pain were confirmed. These horses usually have no detectable radiological abnormalities. Primary lesions of the DDFT are discussed in detail in Chapter 32. Other injuries that should be considered include the following:
Definitive diagnosis of most of these conditions requires MRI, although ultrasonography performed via the bulbs of the heel or the frog may give some information. Inflammation of the navicular bursa or the DIP joint is rarely seen as single disease entities but accompanies many other causes of foot pain. Injury of the CSL is usually seen in conjunction with other soft tissue injuries or lesions of the navicular bone and rarely alone. Lesions of the DSIL are principally degenerative in nature, although a focal tear in the DSIL has been identified by endoscopic examination of the navicular bursa in an event horse with acute-onset severe lameness that improved after perineural analgesia of the palmar digital nerves or intraarticular analgesia of the DIP joint (Figure 30-16).74
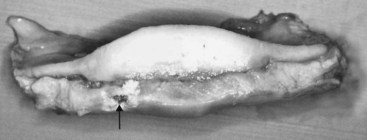
Fig. 30-16 Gross post mortem specimen of the distal aspect of the navicular bone and the distal sesamoidean impar ligament (DSIL). There is a focal tear in the DSIL (arrow), which had been identified by endoscopic evaluation of the navicular bursa. Surface fibrillation of the deep digital flexor tendon had also been seen.
Treatment of Primary Navicular Disease*
Navicular disease is managed because there currently is no cure for this complex condition (Figure 30-17). Rest may not be a useful strategy in the long-term management of many horses with pain in the navicular region because although lameness improves somewhat in most horses after a period of rest, it often returns shortly after the horse resumes exercise. A period of rest for some other cause often precedes the onset of clinical signs of navicular disease. When reviewing literature related to the management of navicular disease it should be borne in mind that much of it relates to palmar foot pain with no definitive diagnosis. The work of Wilson et al16 suggests that relief of pain is vitally important to alleviate a potentially vicious circle. The horse should be encouraged to land normally, rather than toe first, to avoid increased forces on the navicular bone from the DDFT. However, it must be acknowledged that in some horses with acute lameness in which the principal pathological abnormality is increased signal intensity in fat-suppressed MR images in the spongiosa of the navicular bone, which may reflect bone trauma, the most appropriate treatment may be rest.
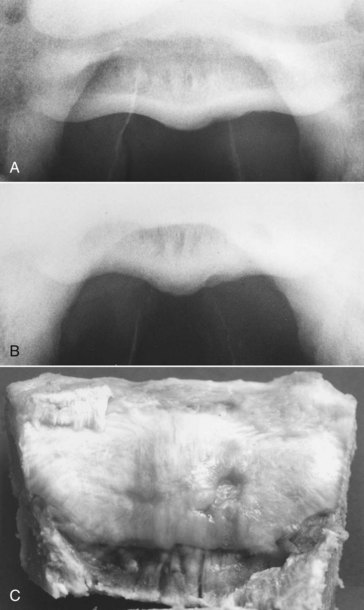
Fig. 30-17 A, Palmar 45° proximal-palmarodistal oblique image of a navicular bone with an irregular contour of the lateral aspect of the flexor cortex. Lateral is to the right. The horse had bilateral forelimb lameness, which was improved by intraarticular analgesia of the distal interphalangeal joint or analgesia of the navicular bursa. B, Palmar 35° proximal-palmarodistal oblique image of the same navicular bone as in A. The irregularity of the flexor cortex is more obvious. Apparent reduction in corticomedullary demarcation is an artifact, the result of the angle of projection. Lateral is to the right. C, The same horse as in A. The gross pathological specimen has a defect in the flexor surface, lateral to the sagittal ridge. Lateral is to the right.
In horses with early navicular disease without major radiological abnormalities, medical management using nonsteroidal antiinflammatory drugs (NSAIDs), isoxsuprine, tiludronate, and careful trimming and shoeing may be effective. The aim of medical management is to attempt to return the horse to regular work as soon as possible, starting initially with work predominantly in straight lines. Horses should be exercised as much as possible daily, combining ridden exercise with either turnout or walking on a horse walker. In some horses, use of corrective trimming and shoeing combined with isoxsuprine is sufficient, whereas in others additional analgesia is required using an NSAID. In horses with marked increased radiopacity of the medulla, lesions involving the flexor surface of the navicular bone, or central osseous cystlike lesions, the response to medical management often is unsatisfactory, and surgical treatment may be indicated.
Management Strategies
Trimming and Shoeing
Successful management depends on a good relationship with an accomplished, reliable farrier. There is an art and science to farriery, and finding the right combination of the method of trimming and the selection of an appropriate shoe for a given horse often requires some trial and error. Each foot and each horse must be examined individually with regard to the distal limb and foot conformation, limb flight, foot placement, and the intended use of the horse. Both the farrier and the veterinarian must agree, and both must also agree to alter the initial approach if it does not work. Some degree of compromise is always involved. However, some principles apply for all horses with navicular disease. The degree to which lameness can be improved by trimming and shoeing alone depends on the horse’s hoof-pastern angle and the previous suitability of trimming and shoeing. If the horse already had a well-conformed foot, little will be achieved.
Care must be taken either to correct or preserve dorsopalmar and lateromedial foot balance whenever possible. This depends to some extent on the natural shape of the horse’s foot and its distal limb conformation. Ideally, the hoof-pastern axis should be straight, but whether this can be achieved depends on the horse’s conformation. Radical changes in foot trimming may temporarily result in increased lameness; therefore it may be necessary to achieve correct foot balance in stages. The foot should be trimmed to maintain heel mass and shorten the toe to facilitate breakover. Sufficient shortening of the toes usually requires trimming the horn from the solar and dorsal aspects of the foot. The use of the so-called four-point or natural balance trim has recently been favored in some quarters, but the same principles of breakover can probably be achieved with more traditional trimming, provided that the toe is shortened sufficiently (see Chapter 27). Although the four-point trim results in clinical improvement in some horses, in others lameness has been increased, possibly caused by excessive trimming, resulting in altered hoof wall strain,2 or by excessive sole pressure.
Elevation of the heel may relieve pressure from the DDFT on the palmar aspect of the navicular bone, with subsequent pain relief. In a short-term study of 12 horses with navicular disease, elevation of the heel using a 3-degree wedge-heel aluminum shoe resulted in significant gait improvement as assessed by force plate analysis.75 In a study of normal Dutch Warmblood horses, elevation of the heel using a 6-degree wedge reduced the maximal force on the navicular bone by 24% compared with flat shoes.3 However, not all horses with palmar foot pain need to have the heel elevated, and in many the response is only temporary. A hoof angle between 50 and 55 degrees is considered ideal. Achieving a straight foot-pastern axis is also a goal, but poor foot conformation may prohibit this. Many horses with a low, collapsed heel have a natural angle of no more than 35 degrees, and it is impractical to achieve an angle of 50 to 55 degrees. Lateromedial radiographic images may be helpful to demonstrate to a less-skilled farrier whether a horse needs more or less heel. If the horse’s lameness is worsened after elevating the toe using the wedge test, some degree of heel elevation may be beneficial. Elevation of the heel can be achieved by using a wedge heel shoe or a rim or complete wedge pad.
A variety of shoes have been used successfully in the management of horses with navicular disease, including egg bar, egg bar–heart bar, straight bar shoes, and the so-called Tennessee shoe, which moves the point of breakover to just in front of the apex of the frog by placing rails on the palmar aspect of the branches of the shoe. Success has also been achieved in some horses using the natural balance shoe.76
The strategy of using shoes that move the weight-bearing axis in a palmar direction in horses with a low, collapsed, and underrun heel is reasonably successful. However, a recent study has shown that using egg bar shoes in clinically normal Dutch Warmblood horses with well-conformed feet did not reduce force on the navicular bone compared with flat shoes.77 The forelimb gait was also described as less animated with egg bar shoes compared with flat shoes. However, these findings cannot necessarily be translated to lame horses with less than ideal foot conformation. Many top-level show jumpers and dressage horses have been shod with egg bar shoes with no apparent detriment to the quality of the gaits. Egg bar shoes provide a greater surface area through which forces are transmitted and clinically seem to help reduce pain associated with either navicular bone pain or the DIP joint. Of 55 horses with clinically diagnosed navicular disease, 53% had permanent relief of lameness after application of egg bar shoes in a follow-up period of 12 to 40 months.78 In some horses, clinical improvement took many weeks. Resolution of lameness was seen despite the persistence of a low, collapsed heel. In another study, treated horses showed histomorphometric evidence of altered navicular bone modeling compared with untreated controls.79 The shoes should be made-to-measure and of sufficient size and length to provide adequate support to the heel region (Figure 30-18).
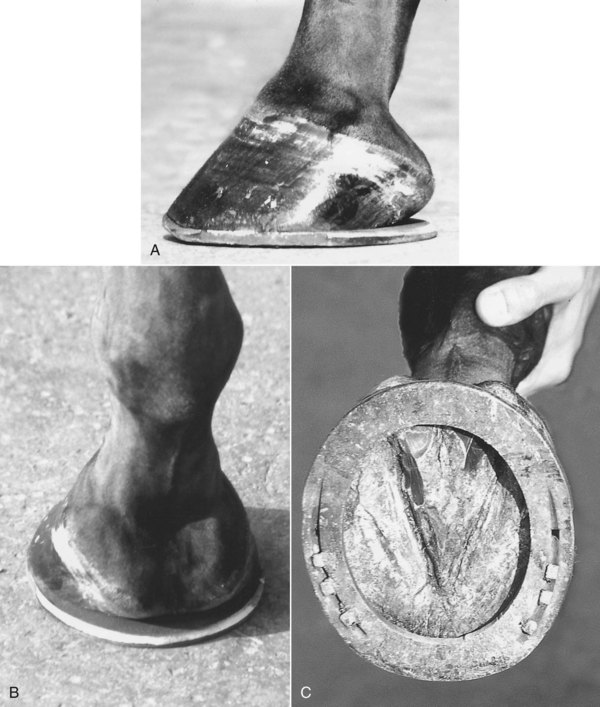
Fig. 30-18 Lateral (A), palmar (B), and solar (C) photographs of a correctly fitted egg bar shoe. The shoe projects beyond the ground-bearing surface of the foot to cover the bulbs of the heel.
Fitting an egg bar shoe is not necessarily easy and depends on the shape of the horse’s foot. If the quarters are very wide it can be difficult to “pick up” the heel bulbs. In such horses a traditional open shoe fitted long and wide at the heel or a straight bar shoe may be more appropriate. A correctly fitted egg bar shoe should project beyond the ground-bearing surface of the foot. It is more likely to be pulled off than a standard shoe, and the use of overreach boots should always be considered, at least when the horse is worked or turned out. These shoes are not suitable for a horse that is going to work in deep mud because they are readily sucked off. In summary, the following principles may aid in the selection of a shoe: correction and then maintenance of dorsopalmar and lateromedial balance, ease of breakover achieved by rolling the toe of the shoe, maintenance of foot (and especially heel) mass, and protection of the palmar aspect of the foot from concussion.
Screw-in studs are used in many competition horses to enhance traction. Some riders just use studs in the lateral branch of the shoe to avoid the risks of tread injuries created by a medial stud in the contralateral limb. This inevitably creates mediolateral imbalance, and therefore care should be taken either to use studs in both branches or to avoid the use of studs. The studs should be positioned as far palmarly as possible to avoid shortening the effective length of the shoe. When the ground is hard, small, pointed studs are preferable to large, square-shaped studs.
Nonsteroidal Antiinflammatory Medication
A wide variety of NSAIDs are used in horses. In general, these drugs should be administered at the lowest dose necessary to achieve the desired effect. There are anecdotal reports that certain NSAIDs are better for horses with foot pain (e.g., meclofenamic acid [Arquel, Pfizer, New York, New York, United States]), but there are no controlled studies to support these claims. Phenylbutazone is widely used, and many, but not all, horses with navicular pain respond to this drug. To determine the lowest effective dose of phenylbutazone, the horse is given a dose of 4 mg/kg twice daily for 3 days, and the dose is then gradually decreased until the lameness returns. Depending on lameness severity, NSAID treatment may only be necessary when the horse is being used. It should be borne in mind that it has been demonstrated that phenylbutazone may influence bone turnover.80 After bone biopsy of the tibia, mineral apposition rate was significantly decreased in horses treated with phenylbutazone (4.4 mg/kg twice daily) compared with untreated, control horses. The clinical significance of this finding in the management of navicular disease is unknown. For horses that are intolerant of phenylbutazone, carprofen ([Rimadyl; Pfizer] 1.0 to 1.5 mg/kg once daily) may be a useful alternative. A short-term study using etodolac, a COX-2 inhibitor, demonstrated significant gait improvement based on force plate data in horses with chronic navicular disease (23 mg/kg orally, once daily)81; however, the horses were not in work, and its effectiveness in a clinical setting is unknown.
Isoxsuprine
Isoxsuprine is a β-agonist that causes peripheral vasodilation in people. Its mode of action in the treatment of navicular disease is unknown. It has been suggested that there are no measurable cardiovascular effects of isoxsuprine given orally in the horse.78,79 However, orally administered slow-release isoxsuprine resin was absorbed and resulted in thermographic evidence of increased skin temperature of the distal aspect of the forelimbs for about 8 hours after administration.80 Isoxsuprine also binds strongly to α-adrenoreceptors81 and therefore may be active despite insignificant measurable levels in plasma.80 The drug also may have some antiinflammatory or hemorheological properties that could explain why some horses with navicular disease appear to respond to isoxsuprine therapy. A double-blind clinical trial evaluating the response to isoxsuprine in the treatment of navicular disease demonstrated a decrease in lameness in the treated horses.82
If the horse responds to a trial dose of 0.6 mg/kg twice a day for 30 days, then continued treatment at the same dose given once or twice a day indefinitely may be indicated. Some horses have been treated for 12 weeks and responded satisfactorily and have then shown remission of clinical signs for up to 9 months without treatment. If no response is seen within 30 days, it is unlikely that further treatment will be beneficial. However, some horses that had no response to a dose of 0.6 mg/kg twice daily responded satisfactorily to 0.9 mg/kg twice daily. If lameness is moderate to severe, then treatment should be combined with an NSAID for the first 3 to 4 weeks of treatment. The response to treatment in horses with major radiological abnormalities is generally very poor.
Intraarticular and Intrathecal Medication
It has been demonstrated that potentially therapeutic concentrations of methylprednisolone acetate or triamcinolone acetonide can be achieved in the navicular bursa after injection of the DIP joint.83 However, although lameness associated with navicular disease may be improved by intraarticular analgesia of the DIP joint, the intraarticular administration of hyaluronan with or without corticosteroids (e.g., triamcinolone acetonide, 10 mg) does not usually result in a similar degree of improvement, unless there is concurrent synovitis of the DIP joint. In a study of 128 horses in which lameness was improved by intraarticular analgesia of the DIP joint, the presence of radiological abnormalities of the navicular bone (mild to moderate in degree) resulted in an 18 times lesser likelihood of a successful response to intraarticular medication of the DIP joint with either polysulfated glycosaminoglycan (PSGAG) or methylprednisolone acetate compared with horses without radiological abnormalities.88 Intrathecal injection of corticosteroids into the navicular bursa is often more effective clinically and may provide transient relief of clinical signs for up to 2 to 5 months.89-92 In my experience, this treatment is effective only in horses with low-grade radiological abnormalities but can result in very substantial, although temporary, improvement. Repeated treatment may result in the development of dystrophic mineralization. However, the response in horses with radiological91 or MRI evidence91,92 of ulcers (erosions) on the palmar aspect of the navicular bone has been poor.
Other Drugs
Warfarin previously was used to treat horses with “navicular disease,” and in one series an 80% success rate was claimed.93 However, these were predominantly pleasure horses and low-level competition horses. Experience in higher-level competition horses produced disappointing results.49 Warfarin therapy requires careful adjustment of the dose guided by monitoring of prothrombin time at least at monthly intervals. This, combined with the drug’s limited efficacy, has largely resulted in the abandonment of this treatment.
Pentoxyfylline and propentofylline are hemorheological drugs that alter the deformability of the red blood cell membrane and inhibit platelet aggregation.83,94 These drugs are advocated by some clinicians for the treatment of navicular disease, although clinical efficacy to date is largely unknown. No measurable effect on digital and laminar blood flow was seen in healthy horses after oral administration of pentoxifylline.83 A small clinical study using propentofylline resulted in improvement but not alleviation of lameness associated with navicular disease during a 6-week treatment period.94 A long-term clinical trial using metrenperone found similar efficacy to isoxsuprine; only 27% of horses had long-term resolution of lameness.96
The pathological similarities between navicular disease and osteoarthritis have prompted the use of drugs used for the management of osteoarthritis. Systemic administration of a PSGAG (Adequan, Novartis Animal Health, Basel, Switzerland; 500 mg intramuscularly every 4 days for 7 treatments) resulted in clinical improvement in lameness in treated horses compared with controls during the treatment period.96 In my experience, PSGAG treatment has not had any long-lasting benefit on lameness associated with navicular disease.
Tiludronate
Tiludronate is a bisphosphonate that inhibits osteoclastic activity. In a placebo-controlled clinical trial, horses with navicular disease treated by intravenous infusion of tiludronate showed significantly improved lameness scores, but in no horse was lameness completely abolished.97 I have treated approximately 20 horses in which increased signal intensity in the spongiosa of the navicular bone in fat-suppressed MR images was the principal abnormality, but the results were disappointing.91 There is anecdotal suggestion that regional perfusion with tiludronate may be more effective.
Shockwave Therapy
There is anecdotal information that shockwave therapy may temporarily alleviate pain associated with palmar foot pain. In an uncontrolled clinical trial in 42 horses with chronic (>3 months) foot-related lameness, the effect of transcuneal shockwave therapy or therapy applied between the bulbs of the heel was compared. The latter was more efficacious, although improvement was observed with both treatments.98
Chemical “Neurectomy”: Cryoneurectomy
Temporary resolution of palmar foot pain can be achieved by chemical ablation of sensory fibers in the palmar digital nerves. Several products are capable of causing a temporary loss of sensation when injected over the nerves. These include Sarapin (a plant alkaloid that is thought to alter transmission in type C fibers; High Chemical Company, Levittown, Pennsylvania, United States), P block, and cobra venom. The product usually is injected over, or directly into, the nerve with a corticosteroid. Injection of absolute alcohol into the perineurium may also be used. Any of these products may result in localized perineural fibrosis, which makes subsequent surgical neurectomy more complicated. Alternatively, the digital nerves may be frozen percutaneously using liquid nitrogen. Both methods result in 2 to 3 months of clinical improvement when successfully applied. In the Editors’ experience, these methods work well on occasion but are unreliable. Return of sensation in most horses is accompanied by a return of the lameness.
Desmotomy of the Collateral Sesamoidean Ligaments
Desmotomy of the CSLs has been described as a treatment for navicular disease.99,100 In an initial report from the United Kingdom, 13 of 16 horses treated with this technique were able to work without lameness,99 and in a subsequent report, 76% of 118 horses were sound at 6 months, but only 43% remained sound 3 years later.101 Diehl100 from Germany reported clinical improvement in 50% of 57 horses. In a study from New Zealand, 12 of 17 horses were sound at least 6 months postoperatively.102 In my experience there is often only short-term (6 to 12 months) improvement in lameness. It was originally suggested that this surgical procedure resulted in alteration of the biomechanical forces on the navicular bone, but clinical improvement may also be the result of sectioning the sensory nerve fibers that run in the CSLs. The original surgical technique described transecting the ligaments near the origin through oblique skin incisions, but the proximal interphalangeal joint capsule can be penetrated by this approach. Better results have been achieved via vertical skin incisions further distally, centered over the CSLs.
Desmotomy of the Accessory Ligament of the Deep Digital Flexor Tendon
Desmotomy of the accessory ligament of the DDFT was described as a treatment for navicular disease in horses with markedly upright foot conformation.103 Long-term follow-up results are not available.
Periarterial “Sympathectomy”
Litzke et al104 described a technique for cutting the sympathetic nerve supply to the lateral and medial digital arteries, either alone or with palmar digital neurectomy. This technique is still practiced in Germany, but the results have not been well documented.
Palmar Digital Neurectomy
If lameness associated with navicular disease is completely eliminated by perineural analgesia of the palmar digital nerves, then palmar digital neurectomy may be considered as a treatment of last resort.106-110 The technique has been associated with a clinically significant number of complications, including failure to alleviate lameness, recurrence of lameness, complete or partial rupture of the DDFT, subluxation or luxation of the DIP joint, neuroma formation, and failure to recognize either subsolar infection or penetrating injuries of the palmar aspect of the foot. Case selection is very important. Horses with preexisting pathology of the DDFT are probably at highest risk of subsequent rupture of the DDFT. If there is radiological evidence of a substantial defect in the flexor surface of the navicular bone, DDFT lesions are likely, and these horses should not be treated by neurectomy. A variety of techniques of palmar digital neurectomy have been described. Recent work suggests that the simple guillotine technique results in the longest period of desensitization and less neuroma formation compared with epineural capping and carbon dioxide laser division.107 Surgery should be delayed for at least 7 days after perineural analgesia. For neurectomy, I use two long (3- to 4-cm) incisions just dorsal to the medial and lateral edges of the superficial digital flexor tendon starting just below the base of the proximal sesamoid bone. The nerve is gently stretched and transected using a scalpel blade as far distally and proximally as possible. Approximately 50% of horses have one or more (up to five) accessory branches that must be divided to achieve complete desensitization of the heel region. In most instances the accessory branches are located abaxial to the palmar digital nerve and run in a palmar direction and parallel to the ligament of the ergot. If an accessory branch is located at one surgical site, there are invariably accessory branches associated with the remaining palmar digital nerves. The incision is closed in a single layer, and the horse is confined to a box stall with handwalking only for 30 days. Phenylbutazone (2.2 mg/kg twice daily) is administered for 10 days. This limited exercise, combined with minimal handling of the nerve(s) intraoperatively, seems to reduce the risk of neuroma formation.
The horse is shod in the manner that was most successful before surgery. This procedure usually provides relief of clinical symptoms in approximately 65% to 70% of horses for approximately 12 to 18 months postoperatively.108,109 Individual horses may have recurrence of lameness sooner.
With careful handling of the nerve intraoperatively, followed by conservative postoperative management, neuroma formation is uncommon. A painful neuroma, which may occur several months after surgery, may be successfully managed with perineural injections of Sarapin and/or triamcinolone acetonide. Occasionally a second neurectomy done proximal to the neuroma is required.
If lameness occurs because of reinnervation, surgery may be repeated, assuming that perineural analgesia of the palmar digital nerves still results in relief of pain. However, reinnervation may be caused by axons sprouting from the proximal nerve stump and anastomosing with other local nerves. Therefore repeated surgery may be less successful.
Decompression of the Navicular Bone by Surgical Drilling
A technique for drilling cystlike lesions in the navicular bone through an endoscopic approach was recently described, but long-term follow-up results are not available.111,112 The rationale is to provide decompression to reduce intraosseous pressure.