Chapter 39The Antebrachium
Anatomy
The antebrachium lies between the elbow and carpus and is composed principally of the radius and small vestigial portion of the ulna and the flexor and extensor muscles. The tendons of the superficial and deep digital flexor muscles, the accessory ligament of the superficial digital flexor tendon, and the carpal sheath are discussed elsewhere (see Chapters 69, 70, and 75). The medial aspect of the antebrachium is relatively devoid of soft tissue coverage, and this is important when considering fractures of the radius. Major neurovascular structures include the median artery (continuation of the brachial artery in the proximal antebrachium), vein, and nerve; the radial, ulnar, and cutaneous antebrachial nerves; and the accessory cephalic and cephalic veins.
Clinical Diagnosis and Imaging Considerations
Lameness associated with the antebrachial region is relatively unusual. Clinical signs are obvious in horses with unstable radial fractures or in those with marked soft tissue swelling. In others diagnosis can be challenging, and ruling out other causes of forelimb lameness and then using diagnostic imaging to reach a definite diagnosis may be necessary. Perineural analgesia of the median and ulnar nerves (see Chapter 10) is performed to rule out more distal sources of pain.
Definitive diagnosis of most lameness problems of the antebrachium can be made using conventional radiography and ultrasonography, but nuclear scintigraphy is useful for diagnosing incomplete and stress fractures of the radius, enostosis-like lesions, and enthesopathy at the origin of the accessory ligament of the superficial digital flexor tendon.
Osteochondroma of the Distal Aspect of the Radius
See Chapter 75 for a discussion of osteochondroma of the distal aspect of the radius.
Physeal Dysplasia of the Distal Aspect of the Radius (Physitis)
See Chapter 57 for a discussion of physitis.
Traumatic Physitis and Closure of the Distal Radial Physis
A syndrome of vague forelimb lameness believed to be associated with inflammation or pain originating from the distal radial physis has been recognized in young racehorses in early training. Anecdotally the condition appears to be more prevalent in 2-year-old colts. Distal radial physeal closure determined radiologically occurred earlier in fillies (701 days) than in colts (748 days).1 Presumably the condition results from repetitive trauma to an open physis. The term open knees is commonly used to describe the state of skeletal immaturity. This condition is distinct from physeal dysplasia (physitis), because the condition is not a developmental abnormality, is not associated with clinically apparent enlargement of the metaphyseal region, and occurs in 2-year-old horses in active training.
Mild to moderate forelimb lameness is vague, without indications of a problem elsewhere in the limb. The condition is usually bilateral, but horses can show unilateral lameness, because one limb is more painful than the other. Horses often have a choppy stride, with the legs carried wide. Focal heat may be present, but swelling is usually absent or minimal, and pain may be difficult to detect. Presumptive diagnosis is made on the basis of history, clinical signs, and ruling out other causes of lameness. A positive response to median and ulnar nerve blocks can be used to confirm that the distal aspect of the antebrachium is the source of pain, but this can also abolish subchondral bone pain in the carpus. Definitive radiological abnormalities are rarely present, but a radiologically open physis supports the diagnosis. Nuclear scintigraphy is generally not helpful, because all horses of this age have moderate-to-intense increased radiopharmaceutical uptake (IRU) at the physis. However, scintigraphy is useful for identifying or ruling out other potential causes of lameness, and asymmetrical radiopharmaceutical uptake (greater in the affected physis of the more severely affected limb) may support the diagnosis.
Treatment consists primarily of rest or a reduction in exercise intensity and systemic nonsteroidal antiinflammatory drugs (NSAIDs). Duration of rest varies with the skeletal maturity of the horse and severity of the condition. Local injection of corticosteroids or other drugs, such as homeopathic remedies, and systemic treatment with anabolic steroids have been used but are of dubious value. For some horses, slow jogging for 4 to 6 weeks may be all that is required; for others, stall confinement with hand-walking exercise progressing to paddock turnout for several months may be necessary. Follow-up radiographs can be used to monitor physeal closure, which is often used to determine the appropriate time to resume harder training.
The issue of distal radial physeal closure, the role of radiographs in making this determination, and how to determine the point when training should commence are controversial. Many trainers and veterinarians customarily obtain radiographs of the distal aspect of the radius of 2-year-olds, and those with open knees (radiological evidence that bony union at the physis is incomplete) are withheld from hard training until the physes have closed. Radiological closure of the distal radial physis generally occurs by 20 to 24 months of age2 or slightly later.1 Scintigraphic activity of the distal radial physis persists well after radiological evidence of closure is observed.3 However, endochondral ossification ceases (biological closure) before fusion is evident radiologically, and in our experience horses with a thin or faintly visible physeal remnant visible radiologically are at low risk for traumatic physitis.
Because the diagnosis is difficult to substantiate and pain may originate from an undetermined source, giving an accurate prognosis is difficult. If other more common conditions have been ruled out and the diagnosis of traumatic physitis is accurate, the prognosis is excellent. Many horses remaining in training develop signs of carpal lameness, and traumatic physitis may simply represent a prodromal phase of early osteoarthritis and bone pain. Finally, no correlation between age or month of closure of the distal radial physes and money won, races won, fastest mile, or fastest win mile during the 2-year-old year was found in Standardbreds.4
Radial Fractures
Radial fractures almost always result from external trauma, often a kick from another horse in adults, or from being stepped on or kicked by a mare in foals. Stress fractures of the radius also occur,5,6 but in our experience true stress fractures of the radius are rare, and the description of those reported by others is similar to what we have termed enostosis-like lesions (see the following discussion). One of us (MWR) recently evaluated images of a 4-year-old Thoroughbred colt in race training, with a history of sudden left forelimb lameness, with a genuine radial stress fracture involving the medial, middiaphyseal cortex (Figure 39-1). Cortical location of stress fractures as determined scintigraphically and radiologically differentiates this injury from enostosis-like lesions.
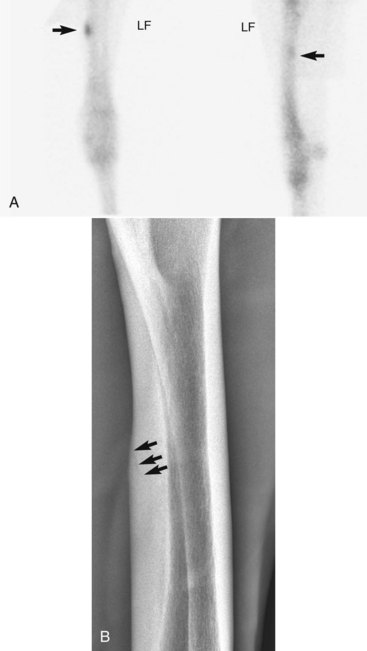
Fig. 39-1 A, Delayed-phase cranial (left image; medial is to the left and proximal is uppermost) and lateral (cranial is left) scintigraphic images of a 4-year-old Thoroughbred colt with acute left forelimb lameness. A focal area (arrows) of increased radiopharmaceutical uptake (IRU) involves the caudomedial cortex of the radius. In the cranial and lateral images, IRU can clearly be seen to involve the cortex rather than the medullary cavity of the radius. Cortical location is diagnostic for a stress fracture rather than an enostosis-like lesion. B, Cranial 45° medial-caudolateral oblique digital radiographic image of the left radius showing an oblique stress fracture (arrows) of the caudomedial cortex.
(Courtesy Dean Richardson, Kennett Square, Pennsylvania, United States.)
Clinical signs depend on the severity and location of the fracture. Horses with complete fractures (which are nearly always displaced) are severely lame (non–weight bearing, grade 5) and have marked soft tissue swelling associated with the fracture itself or the site of the original wound. The limb may have an unusual angle, and crepitus is usually audible and palpable. Often an associated wound results from the initial injury or is caused by fragment penetration, especially on the medial aspect of the antebrachium. Horses with incomplete or nondisplaced fractures have moderate to severe lameness (grade 3 to 5) shortly after the injury, but within 12 to 72 hours they are often fully weight bearing and walking with minimal lameness. However, resumption of exercise or turnout often results in the fracture becoming displaced within 1 to 2 days (see Figure 3-4). Horses with true stress fractures have moderate lameness (grade 1 to 3) at a trot.
For horses with complete, displaced (unstable) fractures of the radius the diagnosis is straightforward. Radiology is needed only to define fracture configuration and to determine if repair is possible. Radiographs are essential in the initial evaluation of any horse with a wound in the antebrachium or over the proximal aspect of the carpus that has a history of acute, moderate-to-severe lameness associated with the injury. Lameness associated with incomplete or hairline fractures of the radius may be transient, but radiographs often reveal obvious or suspicious fracture lines (Figure 39-2, A). Any radiological evidence of bone injury, often a localized cortical fragmentation or compression fracture, warrants high suspicion of an incomplete fracture, and a full series of radiographs should be obtained. Any horse that has persistent lameness after antebrachial trauma in which original radiological findings were negative should be reevaluated within 7 to 10 days, when a fracture may be evident. The horse should be confined to box rest in the interim. Diagnosis in horses with incomplete fractures or stress fractures can sometimes be difficult. Scintigraphic examination is important to differentiate fracture from other problems of the radius, such as enostosis-like lesions.
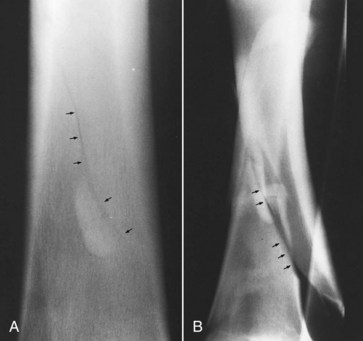
Fig. 39-2 A, Craniocaudal radiographic image of the distal radius of a horse revealing a nondisplaced fracture (arrows). The horse had been found acutely severely lame with a small wound over the distal cranial aspect of the antebrachium earlier that day. B, Craniocaudal radiographic image 1 day later. Catastrophic fracture occurred even though the horse was confined to a box stall. The original fracture line (arrows) and the edge of a plastic fence post used as a splint are visible.
Emergency management of horses with long-bone fractures has been well described7 (see Chapter 86). Horses with unstable fractures should be sedated, wounds should be treated, and a full-limb Robert Jones dressing with splints should be applied. A caudal splint extending from the ground to the point of the elbow (olecranon process) and a lateral splint extending to the withers are attached with tape. The bandage should have a flat surface to allow the lateral splint to be in contact with the skin of the upper limb and torso to prevent distal limb abduction. An oversized bandage reduces the effectiveness of the splints. Properly applying external coaptation is time-consuming and difficult, but appropriate stabilization is essential to reduce the risks of further fracture displacement and skin penetration. NSAIDs and broad-spectrum antimicrobial therapy should be administered. Horses should travel facing backward.
Confinement to a stall is mandatory for any horse with any radiological abnormality, including focal cortical defects, or with known trauma but with no detectable lesions. Catastrophic failure of the radius often results when small cortical defects or incomplete fractures become displaced (see Figure 39-2, B). We recommend conservative management in adult horses with incomplete or nondisplaced radial fractures. If possible, the horse should be transported to a surgical facility even if conservative management is chosen, because the fracture could become displaced and immediate surgical repair may be necessary. However, transport also involves risks that must be weighed against economic considerations and other factors affecting prognosis. Horses with incomplete fractures should be strictly confined to a stall for 8 weeks. External coaptation is applied as described previously for 4 to 8 weeks but can be difficult to maintain. Cross-tying is generally unnecessary, because horses tend not to lie down with bulky external coaptation in place. If radiographs reveal acceptable progression of healing after 8 weeks, the horse is restricted to box stall rest with hand-walking exercise for another 2 months. The majority of fractures are clinically healed in 4 months, although complete radiological healing may take up to 6 months or more in adult horses. Paddock turnout is generally allowed after 4 months, and horses return to work 5 to 6 months after injury.
NSAIDs are administered as needed to provide comfort and minimize the potential for contralateral limb laminitis. NSAID administration is usually necessary for only 7 to 10 days, and if pain is not adequately controlled, the horse should be reassessed carefully for fracture displacement or progression or infection associated with any wounds. Antibiotic therapy should be given to horses with wounds or deep pressure sores associated with the bandage and splints.
Horses with complete or displaced radial fractures require open reduction and internal fixation, but the prognosis in adult horses is poor to grave. The current method of choice is the application of two bone plates, one on the cranial surface and one on the lateral or medial surface, depending on fracture configuration.8,9 In adult horses, use of the dynamic condylar screw plate can be considered and use of locking compression plates should be encouraged. Repair of fractures located at the most proximal or distal aspect of the radius or those with severe comminution is considerably more difficult, and these horses have a grave prognosis. In foals surgical repair is more successful, and a midshaft transverse or short oblique fracture with minor comminution is most common. Although infection is still a concern, implant or bone failure, seen commonly in adult horses, is less frequent. Physeal fractures result in premature closure of the physis, even if repaired surgically. Plate removal is necessary for foals intended to be used for racing.
Management of horses with rare radial stress fractures consists of rest for 4 months. Horses are given 2 months of box stall rest and 2 months of individual turnout in a small paddock before being returned to training. Follow-up radiographic and scintigraphic examinations are recommended.
Prognosis for horses with incomplete or nondisplaced fractures of the radius is good, but clients should be warned of the possible complications of fracture propagation and contralateral laminitis, if initial lameness is severe. Those that do not develop complications, and in which fractures heal uneventfully, have an excellent prognosis for return to full athletic function. Prognosis for surgical repair of radial fractures in adult horses is poor but depends greatly on fracture configuration and whether the fracture is open. Horses with open fractures have a worse prognosis, because infection is an important and frequent complication. Adult horses with long spiral or oblique fractures are the best surgical candidates, whereas those with comminution or fractures involving the proximal and distal aspects of the radius, in particular those involving the antebrachiocarpal or elbow joints, have a poor-to-grave prognosis. Overall the success rate of surgical management of an adult horse with a complete radial fracture is no better than 10% and is expensive. If severe comminution exists, the fracture is open, or it involves a joint, euthanasia should be recommended. In foals, prognosis is considerably better, with a good prognosis for survival and a fair-to-good prognosis for future athletic use.8,9 Foals with radial fractures involving the distal physis will likely develop angular limb deformity, and prognosis for athletic use is worse than in those with middiaphyseal fractures. The prognosis for horses with rare radial stress fractures is excellent.
Enostosis-like Lesions of the Radius
Enostosis-like lesions are an unusual condition affecting many long bones, including the radius (see Chapters 12 and 19). Enostosis is defined as “bone within a bone,” and because the cause has yet to be determined, the term enostosis-like lesion is used. Enostosis-like lesions are focal or multifocal intramedullary mild-to-dense radiopacities. Scintigraphically, enostosis-like lesions are associated with mild-to-intense IRU on delayed (bone phase) images (Figure 39-3, A). Lesions may occur in one or more bones simultaneously but are not necessarily associated with lameness.10,11 Previously, enostosis-like lesions were described as bone infarcts or bone islands. Histological examination of specimens harvested from an enostosis-like lesion in a humerus revealed changes compatible with ischemia of cancellous bone and bone marrow.12 Histological changes were similar to those in specimens examined from horses with medullary infarcts or those in bone adjacent to diaphyseal fractures and cortical stress fractures. However, the findings were not pathognomonic or exclusively characteristic of either of these entities.12 We believe enostosis-like lesions may be caused by primary disruption of medullary vasculature and secondary development of bone sclerosis. Enostosis-like lesions are possibly an atypical form of bone infarct. Enostosis-like lesions are frequently found close to a nutrient foramen.10 Because enostosis-like lesions occur in adult horses of all ages and performance categories, they are not likely to be stress fractures.
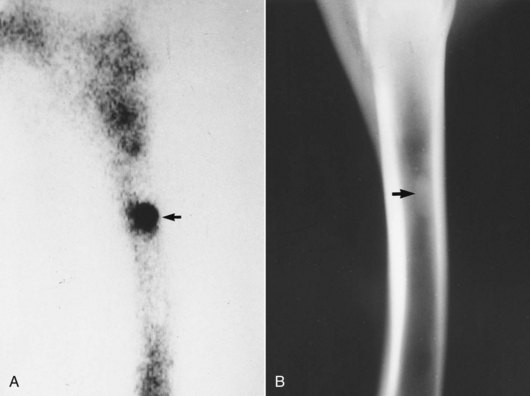
Fig. 39-3 A, Lateral delayed (bone) phase scintigraphic image showing focal, intense increased radiopharmaceutical uptake (arrow) in the medullary cavity consistent with an enostosis-like lesion. Two scintigraphic images were compared, and increased radiopharmaceutical uptake was determined to involve the medullary cavity rather than the cortex. B, Lateromedial radiographic image reveals intramedullary increased radiopacity (arrow) on the endosteal surface and adjacent to the nutrient foramen, radiological signs typical in horses with enostosis-like lesions.
Although enostosis-like lesions can be an incidental radiological or scintigraphic finding of the radius, or most long bones, lameness apparently attributable to the condition occurs in approximately 50% of affected horses. Lameness from enostosis-like lesions is most common in horses with lesions involving the humerus and femur, whereas in other bones such as the radius and tibia, signs of lameness may be subtle or nonexistent.13 An unusual clustering of Thoroughbred racehorses has been documented, with acute, forelimb or hindlimb lameness referable to enostosis-like lesions of the radii and tibiae, respectively, mimicking lameness seen in racehorses with stress fractures of these bones.13 In horses with clinically relevant enostosis-like lesions, lameness is usually mild but may be severe. Although enostosis-like lesions can affect many bones simultaneously, lameness is usually restricted to a single limb.
Because completely abolishing pain originating from the radius may be difficult using diagnostic analgesia, in most horses with enostosis-like lesions diagnosis is made by ruling out other potential causes of lameness. Enostosis-like lesions must be differentiated from true stress fractures. It is imperative that two perpendicular scintigraphic images be obtained, to allow differentiation of cortical and medullary IRU (see Figure 39-1). Although treatment of horses with enostosis-like lesions and of those with stress fractures is similar, cause and recurrence are different. Enostosis-like lesions are associated with mild-to-intense IRU in the medulla. In our experience enostosis-like lesions causing lameness are usually moderately to intensely active. Radiographs reveal corresponding single or multiple, focal or multifocal areas of increased radiopacity within the medullary cavity (see Figure 39-3, B). The lesions are frequently in contact with the endosteal surface and are most often in close proximity to the nutrient foramen. In the radius, follow-up scintigraphic and radiological evaluation 4 to 9 months after initial diagnosis often reveals resolution of the enostosis-like lesions, although resolution of radiological changes lags behind those visible scintigraphically.
Treatment of horses with enostosis-like lesions thought to be causing lameness is nearly identical to that for those with stress fractures. NSAIDs (phenylbutazone, 2.2 mg/kg, bid) are administered for 5 to 10 days or longer depending on degree of lameness. Horses are restricted to stall rest with hand-walking exercise for 2 months, followed by a minimum of 2 months in an individual small paddock. Follow-up clinical, scintigraphic, and radiographic evaluation is recommended. Prognosis for most horses with enostosis-like lesions is excellent, and recurrent lameness is rare, although occasionally longer convalescence (6 to 9 months) is required.
Osseous Cystlike Lesions of the Distal Radius
Osseous cystlike lesions occur in immature14 and adult horses15 and may result from osteochondrosis or trauma. Occasionally, osseous cystlike lesions occur secondary to severe osteoarthritis of the antebrachiocarpal joint. Lameness ranges from mild to severe (grade 1 to 4) and may be sudden or insidious in onset.14,15 Often no localizing clinical signs are present, unless osseous cystlike lesions are secondary to osteoarthritis of the antebrachiocarpal joint, when effusion may be present.
Lameness is localized to the antebrachiocarpal joint and distal aspect of the radius by intraarticular analgesia or by using median and ulnar nerve blocks. Response to intraarticular analgesia of the antebrachiocarpal joint is inconsistent; most horses show partial improvement. Scintigraphy is useful in horses in which diagnostic analgesia fails to localize lameness. Radiographs reveal a well-defined radiolucent defect in the subchondral bone, often with a sclerotic margin (Figure 39-4), with or without communication with the antebrachiocarpal joint.
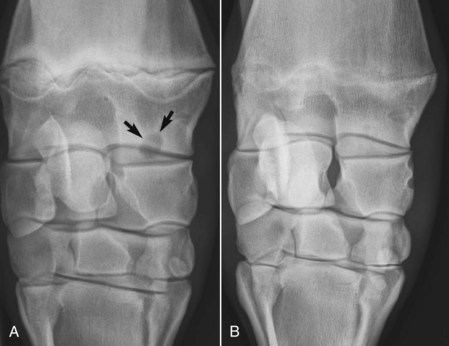
Fig. 39-4 A, Craniocaudal digital radiographic image of the distal aspect of the right radius of a Standardbred weanling with an osseous cystlike lesion (arrows) of the distal, medial aspect of the radius, close to the antebrachiocarpal joint. This weanling was given stall rest for 4 months, and three intraarticular injections with hyaluronan and methylprednisolone acetate were performed. B, Seven-month follow-up craniocaudal image showing near complete resolution of the bone cyst.
Management remains controversial, and a universally accepted method is not currently available. Successful surgical management using an extraarticular approach, debridement, and cancellous bone grafting has been described.15 Osseous cystlike lesions that enter the antebrachiocarpal joint can be debrided using an intraarticular approach, whereas those without communication are best managed using an extraarticular approach. Nonsurgical management has been successful and consisted of restricted exercise, with or without intraarticular administration of hyaluronan.14 We treat horses conservatively. Duration of restricted exercise and number and type of intraarticular injections are determined on a case-by-case basis. Horses are given a minimum of 4 to 6 months of rest, or longer if gradual improvement in clinical signs is seen. Conservative management is the first choice in immature horses (<2 years of age). Extraarticular corticosteroid injection using a small drill bit has been successful in other sites and may be applicable for lesions involving the distal aspect of the radius. If little improvement is seen after 6 months, then surgical debridement (with or without grafting) should be considered. Limited data are available on which to base prognosis, which may be better in immature horses (<2 years of age).
Desmitis of the Accessory Ligament of the Superficial Digital Flexor Tendon
See Chapter 75 for a discussion of desmitis of the accessory ligament of the superficial digital flexor tendon.
Acute Caudal Antebrachial Myositis
Myositis and traumatic injury of the muscles in the caudal aspect of the antebrachium are relatively rare. Horses that compete over jumps at speed such as Three Day Event, timber, and steeplechase horses appear to be at risk to traumatize these muscles, presumably as a result of hyperextension of the metacarpophalangeal and carpal joints during landing. Occasionally, horses develop inexplicable myositis or injury of these muscle unassociated with a known traumatic event. Infectious myositis can result from puncture or kick wounds (see the following discussion).
Presumptive diagnosis can usually be made on the basis of history and clinical signs. The antebrachium should be carefully evaluated for even small puncture wounds. A recent but seemingly unrelated area of injury or small wound in the elbow or axillary region may be an important part of the history or physical examination. The clinician should measure rectal temperature. Pyrexia indicates infection. Radiography should be performed to rule out bony injury, such as an incomplete fracture of the radius. Ultrasonographic examination reveals heterogeneous echogenicity within the bellies and between the fascia of the affected muscles, compatible with fiber disruption and hematoma formation.
Treatment consists of systemic NSAID administration (7 to 10 days) and hydrotherapy. Cold water hosing is administered for 15 to 20 minutes twice a day for 5 days, and then warm water hosing is initiated for 5 days. Broad-spectrum antimicrobial therapy is given if infection is suspected (see the following discussion). Horses are confined to a stall for 6 weeks, and hand-walking exercise is initiated, beginning at 5 minutes twice a day and increasing by 5 minutes each week. Passive flexion and extension of the carpus (30 to 50 repetitions daily) helps to improve range of motion and possibly fiber alignment. Clinical and ultrasonographic evaluation is recommended after 6 weeks. Work should not commence until carpal flexion is normal and the horse is sound. The prognosis for return to full athletic function is good.
Infectious Myositis and Cellulitis
Small puncture wounds in the antebrachium, elbow, and pectoral region can result in infections that cause tremendous antebrachial swelling associated with subcutaneous or deeper tissues. Small wounds may seal over quickly but result in inoculation of bacteria into deeper tissues, causing diffuse cellulitis or infectious myositis. Abrasions from equipment such as hobbles in a Standardbred racehorse can lead to deep infections of the proximal aspect of the antebrachium. Usually swelling is also present in the distal aspect of the limb, and the carpal sheath may show sympathetic effusion. Infectious myositis may lead to deep abscess formation. Lameness is usually moderate to severe. The horse may be depressed and is usually pyrexic. White blood cell count and fibrinogen concentration are raised. Skin sloughing may develop with aggressive infections caused by Streptococcus or Staphylococcus species. Radiographs should be obtained, because a radial or ulnar fracture may occur concurrent with infection, but radiological findings are usually negative unless osteitis of the radius or ulna exists (see the following discussion).
Treatment involves administration of broad-spectrum antimicrobial drugs and NSAIDs and the application of topical warm water hydrotherapy. Full-limb bandaging is recommended to reduce swelling in the antebrachium and distal aspect of the limb. Horses with diffuse cellulitis usually respond quickly (within 3 to 5 days), whereas those with deeper infections require prolonged therapy. Horses with an abscess need surgical drainage.
Osteitis and Osteomyelitis of the Radius and Ulna
Osteitis and osteomyelitis of the radius and/or ulna can result from penetrating trauma, most commonly a kick. Clinical signs are similar to those seen with extensive soft tissue infection (see the preceding discussion), and some component of infectious myositis or cellulitis is present in most horses with osteomyelitis and in many with osteitis. Radiographs are diagnostic (Figure 39-5). Focal cortical trauma or fractures may also be present (see preceding section on radial fractures). Radiographs are indicated in all horses with infectious antebrachial myositis or cellulitis that exhibit a poor response to appropriate antimicrobial treatment and other ancillary therapy. Osteitis and osteomyelitis should be suspected in all horses with radial or ulnar fractures or focal cortical trauma associated with an open wound that subsequently develop signs of soft tissue infection. Infectious arthritis of the elbow or carpus may be present, either from direct involvement with a wound, or extension of the bone infection into the joint.16
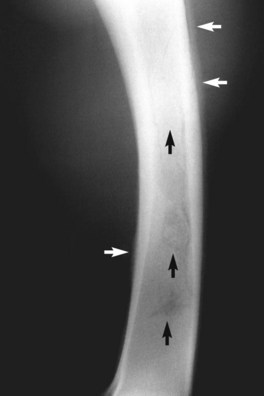
Fig. 39-5 Lateromedial radiographic image of the right radius of a horse with extensive osteomyelitis resulting from a penetrating wound. Note the characteristic periosteal proliferative reaction (white arrows) and the osteolytic and osteoproliferative changes within the medullary cavity (black arrows).
Treatment is similar to that outlined for infectious myositis or cellulitis. Additional therapeutic options include surgical debridement and local antimicrobial delivery, such as intraosseous antimicrobial perfusion and use of antimicrobial-impregnated bone cement. The reader is referred to Chapter 65 for details on the diagnosis and management of infectious arthritis. Prognosis for horses with localized osteitis is generally favorable. Prognosis for those with osteomyelitis is more guarded and is dependent on the extent and severity of the infection, as well as the nature of any concurrent problems or injuries.
Swelling of the Antebrachium Associated with Other Conditions
Horses with infectious arthritis of the elbow joint, fractures of the proximal aspect of the radius or ulna, or distal humeral fractures often have swelling of the antebrachium. These conditions should be kept in mind if diagnosis of a primary problem in the antebrachium cannot be made.
Hypertrophic Osteopathy
Hypertrophic osteopathy is an unusual disorder involving bilaterally symmetrical proliferation of fibrous tissue and periosteal bone in the appendicular and, less frequently, the axial skeleton, involving the metaphyseal and diaphyseal regions of several bones of all limbs.17,18 Hypertrophic osteopathy may be associated with disease in the thorax or abdomen or with vascular abnormalities. Cause is unknown but may be neurogenic, mediated by the vagus nerve, or hormonal, leading to changes in regional blood flow. Clinical signs include soft tissue swelling and stiffness or lameness, often associated with elevated fibrinogen levels. Radiology of affected bones reveals palisading periosteal new bone. Identification of the primary lesion is important, because bony lesions may resolve with successful treatment of the underlying disease.17-20 NSAIDs may ameliorate clinical signs.