Local Complications
A number of potential complications are associated with the administration of local anesthetics. For purposes of convenience, these complications may be separated into those that occur locally in the region of the injection and those that are systemic. Systemic complications associated with the administration of local anesthesia are discussed in Chapter 18, and include overdosage (toxic reaction), allergy, and psychogenic reactions. The following localized complications are described in this chapter:
It must be emphasized that with any complication associated with the administration of a local anesthetic, a written note should be entered onto the patient’s dental chart. For complications that become chronic, a note should appear whenever the patient is re-evaluated.
Needle Breakage
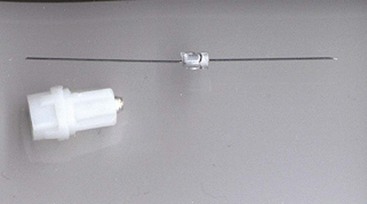
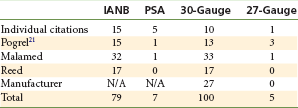
Since the introduction of non-reusable, stainless steel dental local anesthetic needles, needle breakage has become an extremely rare complication of dental local anesthetic injections (Fig. 17-1). Pogrel has (roughly) estimated the risk of needle breakage among Northern California dentists at 1 in 14 million inferior alveolar nerve blocks.1 In the United States, 1.43 million boxes of dental needles (100 needles per box; 143,000,000 needles) were sold by one needle manufacturer in 2004, 1.56 million boxes in 2005, and 1.43 million boxes in 2006.2 Reports of broken dental needles in the published literature appear only infrequently, but they do appear. A MedLine search for broken dental needles from 1951 through February 2010 uncovered 26 published reports of broken dental needles, including their cause and management.1,3-27 Review of 20 of these reports, for which information regarding needle gauge and length and technique of anesthesia employed is available, reveals that 15 were inferior alveolar nerve block (IANB) and 5 were posterior superior alveolar (PSA) nerve block. All 5 PSA reports described adult patients, whereas 9 of the 15 broken needle reports following IANB occurred in children. Needle gauge and/or length was presented in 11 papers. Ten of the 11 needles were 30-gauge short; only one case reported long needle breakage (27-gauge) with the needle remaining in the tissues.12
Pogrel reported on 16 patients whom he evaluated over a 25-year period (1983-2008) following needle breakage.1 Fifteen patients had received IANB, one a PSA. Thirteen of the 16 needles were 30-gauge short, 3 were 27-gauge short.
Independent of the cited literature, this author is aware of 51 cases that progressed to litigation in which broken dental needle fragments remained within the soft tissues of the patient receiving the injection.28 Fifty of these events involved 30-gauge short needles; a 27-gauge short was involved in the other case. All but 1 involved administration of an IANB. A posterior superior alveolar nerve block was used in the other case.
A manufacturer of dental local anesthetic needles reported that over a 6-year period (1997-2002), 27 doctors contacted the company reporting instances of broken dental needles. All incidents involved 30-gauge short needles.29
Long dental needles most likely have broken during injection. However, because the long needle is unlikely to have been inserted to its full length (approximately 32 mm) into soft tissue, some portion of the needle would remain visible in the patient’s mouth. Retrieval of the fragment with a hemostat is easily accomplished. Litigation does not occur in such incidents.
Table 17-1 summarizes the accumulated findings presented to this point. Although some reports may have been duplicated, the factual information clearly identifies commonalities in most cases: use of 30-gauge short or ultra-short needles in injection techniques in which the needle is inserted to its hub (“hubbing the needle”). All reported cases involved the inferior alveolar or posterior superior alveolar nerve block. In all situations in which it is mentioned, needle fracture occurred at the hub—never along the shaft of the needle (Fig. 17-2). Additional factors include (1) intentional bending of the needle by the doctor before injection (Fig. 17-3, A) (2) sudden unexpected movement by the patient while the needle is still embedded in tissue; and (3) forceful contact with bone.
TABLE 17-1
Summary of Reports of Broken Dental Needles
From Malamed SF, Reed K, Poorsattar S: Needle breakage: incidence and prevention, Dent Clin N Am 54:745–756, 2010.
Figure 17-2 Hub of broken needle from Figure 17-3, B.
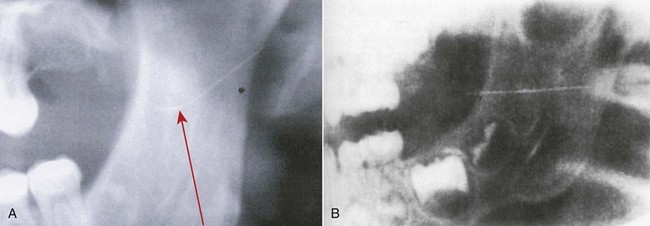
Figure 17-3 A, Radiograph of a broken dental needle (note bend in needle: arrow). B, Radiograph of a broken dental needle in the pterygomandibular space. (B, From Marks RB, Carlton DM, McDonald S: Management of a broken needle in the pterygomandibular space: report of a case, J Am Dent Assoc 109:263–264, 1984. Reprinted by permission.)
The exact cause of needle breakage is rarely discernible. In cases in which the needle has been surgically retrieved and/or forensic metallurgists have examined the hub of the needle, no evidence has revealed manufacturing defects in the needle (Fig. 17-4).
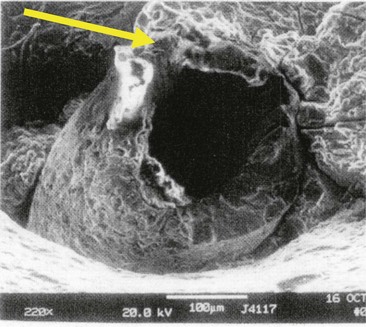
Figure 17-4 Scanning electron microscopy of a broken dental needle. Arrow at 11 o’clock position indicates area where needle was bent superiorly before injection, per court testimony of forensic metallurgist.
Problem
Needle breakage per se is not a significant problem if the needle can be removed without surgical intervention. Ready access to a hemostat enables the doctor or the assistant to grasp the visible proximal end of the needle fragment and remove it from the soft tissue.
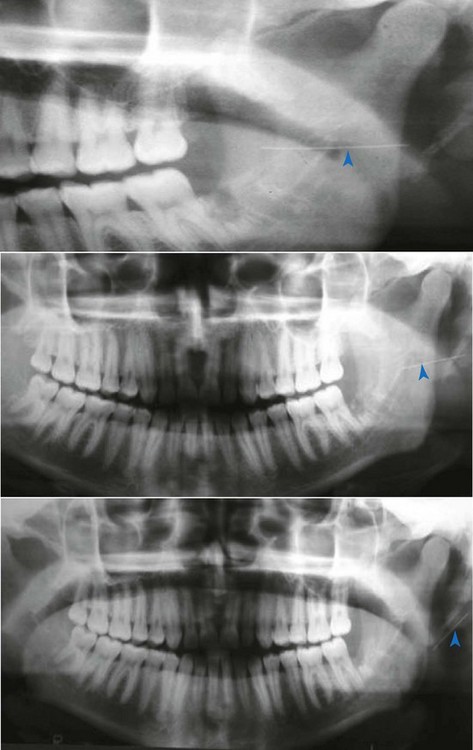
Where the needle has been inserted to its hub and the soft tissue has dimpled under pressure from the syringe, the broken fragment will not be visible when the syringe is withdrawn from the patient’s mouth.The needle fragment remaining in the tissue poses a risk of serious damage being inflicted on the soft tissues for as long as the fragment remains. Although it does not often occur, needle fragments can migrate, as is illustrated by the series of panoramic films taken at 3-month intervals (Fig. 17-5).
Management
Management of the broken dental needle involves immediate referral of the patient to an appropriate specialist (e.g., an oral and maxillofacial surgeon) for evaluation and possible attempted retrieval. Conventional management involves locating the retained fragment through panoramic and computed tomographic (CT) scanning.25
More recently, three-dimensional CT scanning has been recommended to identify the location of the retained needle fragment.1,30 A surgeon in the operating theater then removes the retained needle fragment while the patient is under general anesthesia (Fig. 17-6).
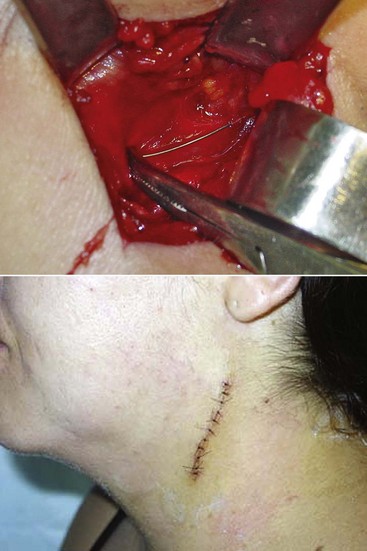
Figure 17-6 Surgical excision of needle fragment (see patient from Fig. 17-5). Courtesy Dr. Carlos Elias De Freitas CVM. From Malamed SF, Reed KR, Poorsattar S: Needle breakage: incidence and prevention, Dent Clin N Amer 54:745–756, 2010.)
Prevention
Although rare, dental needle breakage can, and does, occur. Review of the literature and personal experience of the authors bring into focus several commonalities, which, when avoided, can minimize the risk of needle breakage with the fragment being retained. These include the following:
• Do not use short needles for inferior alveolar nerve block in adults or larger children.
• Do not use 30-gauge needles for inferior alveolar nerve block in adults or children.
• Do not bend needles when inserting them into soft tissue.
• Do not insert a needle into soft tissue to its hub, unless it is absolutely essential for the success of the injection.
• Observe extra caution when inserting needles in younger children or in extremely phobic adult or child patients.
Prolonged Anesthesia or Paresthesia
On occasion, a patient reports feeling numb (“frozen”) many hours or days after a local anesthetic injection. Normal distribution of patient response to drugs allows for the rare individual (e.g., hyperreactor) who may experience prolonged soft tissue anesthesia after local anesthetic administration that persists for many hours longer than expected. This is not a problem.
When anesthesia persists for days, weeks, or months, the potential for the development of problems is increased. Paresthesia or persistent anesthesia is a disturbing yet often-times unpreventable complication of local anesthetic administration. Paresthesia is one of the most frequent causes of dental malpractice litigation.
A patient’s clinical response to this can be profuse and varied, including sensations of numbness, swelling, tingling, and itching. Associated oral dysfunction, including tongue biting, drooling, loss of taste, and speech impediment, may be noted.31-34
Paresthesia is defined as persistent anesthesia (anesthesia well beyond the expected duration), or altered sensation well beyond the expected duration of anesthesia. In addition, the definition of paresthesia should include hyperesthesia and dysesthesia, in which the patient experiences both pain and numbness.35
Causes
Trauma to any nerve may lead to paresthesia. Paresthesia is a not uncommon complication of oral surgical procedures and mandibular dental implants.32,36-38 In an audit of 741 mandibular third molar extractions, Bataineh found postoperative lingual nerve anesthesia in 2.6%; inferior alveolar nerve paresthesia was 3.9%, developing in 9.8% of patients younger than 20 years of age. Also, a significant correlation was noted between the incidence of paresthesia and the experience of the operator.38
Injection of a local anesthetic solution contaminated by alcohol or sterilizing solution near a nerve produces irritation, resulting in edema and increased pressure in the region of the nerve, leading to paresthesia. These contaminants, especially alcohol, are neurolytic and can produce long-term trauma to the nerve (paresthesia lasting for months to years).
Trauma to the nerve sheath can be produced by the needle during injection. Many patients report the sensation of an “electric shock” throughout the distribution of the involved nerve. Although it is exceedingly difficult (and is highly unlikely) to actually sever a nerve trunk or even its fibers with the small needles used in dentistry, trauma to a nerve produced by contact with the needle is all that may be needed to produce paresthesia.32,33 Insertion of a needle into a foramen, as in the second division (maxillary) nerve block via the greater palatine foramen, also increases the likelihood of nerve injury.
Hemorrhage into or around the neural sheath is another cause. Bleeding increases pressure on the nerve, leading to paresthesia.31-33,35
The local anesthetic solution itself may contribute to the development of paresthesia after local anesthetic injection.39 Haas and Lennon took a retrospective look at paresthesia after injection of local anesthetic in dentistry in the province of Ontario, Canada, over a 20-year period (1973-1993).31 This report included voluntary submissions by dentists to their insurance carriers for claims of paresthesia. Only cases where no surgery was performed were considered. One hundred forty-three cases of paresthesia unrelated to surgery were reported in this period. All reported cases involved the inferior alveolar or the lingual nerve or both, with anesthesia of the tongue reported most often, followed by anesthesia of the lip. Pain (hyperesthesia) was reported by 22% of patients. Paresthesia was reported more often after administration of a 4% local anesthetic—prilocaine HCl and articaine HCl. Observed frequencies of paresthesia after administration of articaine HCl and prilocaine HCl were greater than expected, based on the distribution of local anesthetic use in Ontario in 1993.31 According to Haas, the incidence of paresthesia resulting from all local anesthetics is approximately 1 : 785,000; for 0.5%, 2%, and 3% local anesthetics, it is approximately 1 : 1,250,000, and for 4% local anesthetics, it is approximately 1 : 485,000.31
In 2006 Hillerup and Jensen in Denmark, reviewing insurance claims, suggested that articaine should not be used by inferior alveolar nerve block because it had, in their opinion, a greater propensity for paresthesia.40 Yet, of the 54 case reports of paresthesia reviewed, 42 (77%) involved not the inferior alveolar nerve but the lingual nerve (see later discussion) (Table 17-2).
TABLE 17-2
Distribution of Analgesic Solution and Nerve Affected, Including 54 Nerve Injuries in 52 Patients
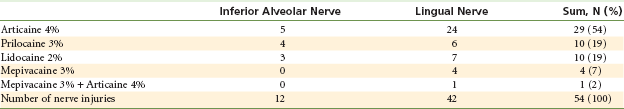
From Hillerup S, Jensen R: Nerve injury caused by mandibular block analgesia, Int J Oral Maxillofac Surg 35:437–443, 2006.
In response to Hillerup and Jensen’s article, the Pharmacovigilance Working Committee of the European Union reviewed reports of paresthesia associated with articaine and other local anesthetics in 57 countries, estimating that the number of patients treated with articaine is approximately 100 million annually.41 Their published report (October 30, 2006) states the following: “This investigation is a follow-up to an inquiry initiated in 2005. This enquiry resulted from suspicions that were raised in Denmark, that a local anesthetic, articaine, was responsible for an increased risk of nerve injuries compared with the risk associated with other local anesthetics (mepivacaine, prilocaine, lidocaine).” The report concluded: “Regarding articaine, the conclusion is that [the] safety profile of the drug has not significantly evolved since its initial launch (1999). Thus, no medical evidence exists to prohibit the use of articaine according to the current guidelines listed in the summary of product characteristics.41 All local anesthetics may cause nerve injury (they are neurotoxic in nature). The occurrence of sensory impairment is apparently slightly more frequent following use of articaine and prilocaine. However, considering the number of patients treated, sensory impairments rarely occur. For example, the incidence of sensory impairment following the use of articaine is estimated to be 1 case in 4.6 million treated patients.” Further they report, “Nerve injuries may result from several incidents: mechanical injury due to needle insertion; direct toxicity from the drug; and neural ischaemia.”
In 2007 Pogrel reported the first, and still the only, clinical evaluation of cases of paresthesia.42 Evaluation of 57 cases of paresthesia following local anesthetic administration (over a 3-year period) revealed that lidocaine was responsible for 35% of cases, articaine 29.8%, and prilocaine 29.8%. He presented the following as the reason for his research and writing of the paper: “We were aware of the discussion in dental circles as to the use of articaine for inferior alveolar nerve blocks, and are aware of recommendations suggesting that it not be used for IANBs. This was the predominant reason for submitting this paper at this time.”
Table 17-3 presents the incidence of paresthesia as reported by Pogrel along with the market share of each drug at that time.42 If all local anesthetics were equally neurotoxic, then the percentage of reported cases of paresthesia for any given drug would be equal to its percentage of market share. For example, if a drug had a 30% market share, it should then account for 30% of the reported cases of paresthesia—a 1 : 1 ratio (reported as 1.0).
TABLE 17-3
Number of cases of nerve damage with percentage of U.S. National Sales Figures
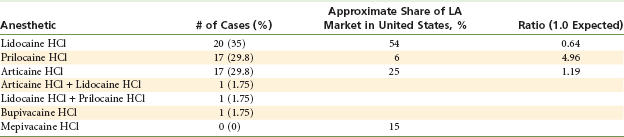
From Pogrel MA: Permanent nerve damage from inferior alveolar nerve blocks—an update to include articaine, J Calif Dent Assoc 35:271–273, 2007 (modified to include ratio).
From Pogrel’s statistics, lidocaine, with 54% of the market share and 35% of the reported cases of paresthesia, had a 0.64% ratio—better than expected. Prilocaine, on the other hand, with a 6% market share, had 29.8% of the reported cases—a 4.96% ratio. Articaine, with 25% market share at the time, had 29.8% of the reported cases—a 1.19% ratio.
Pogrel concluded, “using our previous assumption that approximately half of local anesthetic used is for inferior alveolar nerve blocks, then on the figures we have generated from our clinic, we do not see disproportionate nerve involvement for articaine.”
A meta-analysis of the efficacy and safety of articaine versus lidocaine, published in 2010, concluded, “This systematic review supports the argument that articaine as compared with lignocaine provides a higher rate of anaesthetic success, with comparable safety to lignocaine when used as infiltration or blocks for routine dental treatments.”43
In July of 2010, Garisto and associates reported on the occurrence of paresthesia after dental local anesthetic administration in the United States.44 Data were gathered from the U.S. Food and Drug Administration Adverse Event Reporting System (AERS). Over a 10-year reporting period (November 1997–August 2008), 248 cases of paresthesia following dental local anesthesia were reported, of which 94.5% involved inferior alveolar nerve block. Of the reported cases, 89.0% involved only the lingual nerve. Table 17-4 reports the incidence of paresthesia calculated for each dental local anesthetic. As a comparison, the reported risk of being struck by lightning in a given year in the United States is 1 : 750,000.45
TABLE 17-4
Reported incidences of paresthesia to AERS from 1997 to 2008
| Mepivacaine | 1 : 623,112,900 |
| Lidocaine | 1 : 181,076,673 |
| Bupivacaine | 1 : 124,286,050 |
| Overall | 1 : 13,800,970 |
| Articaine | 1 : 4,159,848 |
| Prilocaine | 1 : 2,070,678 |
| Being struck by lightning (annual risk) | 1 : 750,000 |
AERS, Adverse event reporting system.
Data derived and modified from Garisto GA, Gaffen AS, Lawrence HP, et al: Occurrence of paresthesia after dental local anesthetic administration in the United States, J Am Dent Assoc 141:836–844, 2010.
Of the 248 cases, 108 reported on resolution of the paresthesia, which ranged from 1 day to 736 days. Confirmed resolution occurred in 34 of the 108 cases (31.4%). Of these, 25 resolved within 2 months, and the remaining 9 resolved within 240 days.44
However, the report by Garisto and colleagues relied on data from the AERS. The FDA Website for AERS displays the following warning: “AERS data do have limitations. First, there is no certainty that the reported event was actually due to the product. FDA does not require that a causal relationship between a product and event be proven, and reports do not always contain enough detail to properly evaluate an event. Further, FDA does not receive all adverse event reports that occur with a product. Many factors can influence whether or not an event will be reported, such as the time a product has been marketed and publicity about an event. Therefore, AERS cannot be used to calculate the incidence of an adverse event in the U.S. population.”46
So, as of January 2012, “the jury is still out,” as the saying goes. Proponents of one side of the argument adamantly believe that 4% local anesthetics do carry a greater risk of paresthesia, be it transient or permanent, but the others believe that other factors are usually involved, primarily mechanical trauma, especially when the paresthesia involves only the lingual nerve, as is the case in 89% of the cases cited by Garisto and colleages.44
So what should the doctor do? As with all procedures under consideration for use by a doctor, as well as with any drugs being considered for administration, the doctor must weigh the benefit to be gained from use of the drug or therapeutic procedure against the risks involved in its use. Only when, in the mind of the treating doctor, the benefit to be gained clearly outweighs the risk should the drug or the procedure be used.
Problem
Persistent anesthesia, rarely total, in most cases partial, can lead to self-inflicted soft tissue injury. Biting or thermal or chemical insult can occur without a patient’s awareness until the process has progressed to a serious degree. When the lingual nerve is involved, the sense of taste (via the chorda tympani nerve) also may be impaired.
In some instances, loss of sensation (paresthesia) is not the clinical manifestation of nerve injury. Hyperesthesia (an increased sensitivity to noxious stimuli) and dysesthesia (a painful sensation occurring to usually nonnoxious stimuli) also may be noted. Haas and Lennon reported that pain was present in 22% of the 143 cases of paresthesia that they reviewed.31
Prevention
Strict adherence to injection protocol and proper care and handling of dental cartridges help minimize the risk of paresthesia. Nevertheless, cases of paresthesia will still occur in spite of care taken during the injection. Whenever a needle is inserted into soft tissues, anywhere in the body, in an attempt to deposit a drug (e.g., local anesthetic) as close to a nerve as possible without actually contacting it, it is simply a matter of time before such contact does occur. As Pogrel opined: “It is reasonable to suggest that during a career, each dentist may encounter at least one patient with an inferior alveolar nerve block resulting in permanent nerve involvement. The mechanisms are unknown and there is no known prevention or treatment.”32
Management
Nichol reported that most paresthesias resolve within approximately 8 weeks without treatment.47 Only when damage to the nerve is severe will the paresthesia be permanent, and this occurs only rarely.
In most situations, paresthesia is minimal, with the patient retaining most of the sensory function to the affected area. Therefore, the risk of self-inflicted tissue injury is minimal.
Garisto and coworkers, in reviewing 248 reports of paresthesia, had data on resolution in 108 cases. The period of resolution ranged from as little as 1 day to as many as 736 days. Confirmed resolution of paresthesia was reported in 34 of the 108 cases (31.4%). Of the 34 cases that did resolve, 25 did so within 2 months; the remaining 9 cases resolved within 240 days.44
McCarthy48 and Orr49 have recommended the following sequence in managing the patient with a persistent sensory deficit after local anesthesia:
1. Be reassuring. The patient usually telephones the office the day after the dental procedure complaining of still being a little numb.
a. Speak with the patient personally. Do not relegate the duty to an auxiliary. Remember that if patients cannot get through to speak to their doctor, they can always get the doctor’s attention through litigation.
b. Explain that paresthesia is not uncommon after local anesthetic administration. Sisk and associates have reported that paresthesia may develop in up to 22% of patients in very select circumstances.50
2. Examine the patient in person.
a. Determine the degree and extent of paresthesia.
b. Explain to the patient that paresthesia normally persists for at least 2 months before resolution begins, and that it may last up to a year or longer.
c. “Tincture of time” (e.g., observation) is the recommended treatment, although surgery might be considered as an option.
d. Record all findings on the patient’s chart using the patient’s own descriptors, such as “hot,” “cold,” “painful,” “tingling,” “increasing,” “decreasing,” and “staying the same.”
e. Suggest that simple observation for 1 to 2 months is recommended, but on that same day, offer to send the patient for a second opinion to an oral and maxillofacial surgeon (OMS), who will be able to map out the affected area and would be able to perform the surgical repair, if that is deemed necessary.
f. If surgical repair is suggested by this first consultant, a second opinion should be sought from another OMS. It generally is deemed appropriate to observe the situation for minimally 1 to 2 months before considering the surgical option.
3. Reschedule the patient for examination every 2 months for as long as the sensory deficit persists.
4. Dental treatment may continue, but avoid readministering local anesthetic into the region of the previously traumatized nerve. Use alternate local anesthetic techniques if possible.
5. It would be prudent to contact your liability insurance carrier should the paresthesia persist without evident improvement beyond 1 to 2 months.
Facial Nerve Paralysis
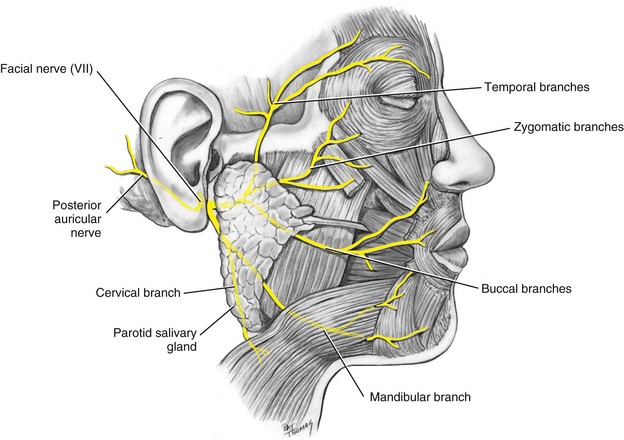
The seventh cranial nerve carries motor impulses to the muscles of facial expression, of the scalp and external ear, and of other structures. Paralysis of some of its terminal branches occurs whenever an infraorbital nerve block is administered, or when maxillary canines are infiltrated. Muscle droop is also observed when, occasionally, motor fibers are anesthetized by inadvertent deposition of local anesthetic into their vicinity. This may occur when anesthetic is introduced into the deep lobe of the parotid gland, through which terminal portions of the facial nerve extend (Fig. 17-7).
The facial nerve branches and the muscles they innervate are listed below:
3. Buccal branches: supplying the region inferior to the eye and around the mouth
4. Mandibular branch: supplying muscles of the lower lip and chin
Cause
Transient facial nerve paralysis is commonly caused by the introduction of local anesthetic into the capsule of the parotid gland, which is located at the posterior border of the mandibular ramus, clothed by the medial pterygoid and masseter muscles.35,50-53 Directing the needle posteriorly or inadvertently deflecting it in a posterior direction during an IANB, or overinserting during a Vazirani-Akinosi nerve block, may place the tip of the needle within the body of the parotid gland. If local anesthetic is deposited, transient paralysis can result. The duration of the paralysis is equal to that of the soft tissue anesthesia usually noted for that drug.
Problem
Loss of motor function to the muscles of facial expression produced by local anesthetic deposition is normally transitory. It lasts no longer than several hours, depending on the local anesthetic formulation used, the volume injected, and proximity to the facial nerve. Usually, minimal or no sensory loss occurs.
During this time, the patient has unilateral paralysis and is unable to use these muscles (see Fig. 17-8). The primary problem associated with transient facial nerve paralysis is cosmetic: the person’s face appears lopsided. No treatment is known, other than waiting until the action of the drug resolves.

Figure 17-8 Facial nerve paralysis. Inability to close eyelid (A) and drooping of lip on affected side (patient’s left) (B).
A secondary problem is that the patient is unable to voluntarily close one eye. The protective lid reflex of the eye is abolished. Winking and blinking become impossible. The cornea, however, does retain its innervation; thus if it is irritated, the corneal reflex is intact, and tears lubricate the eye.
Prevention
Transient facial nerve paralysis is almost always preventable by adhering to protocol with the inferior alveolar and Vazirani-Akinosi nerve blocks (as described in Chapter 14), although in some situations, branches of the facial nerve may lie close to the site of local anesthetic deposition in the IANB and Vazirani-Akinosi nerve blocks.
A needle tip that comes in contact with bone (medial aspect of the ramus) before depositing local anesthetic solution essentially precludes the possibility that anesthetic will be deposited into the parotid gland during an IANB. If the needle deflects posteriorly during this block and bone is not contacted, the needle should be withdrawn almost entirely from the soft tissues, the barrel of the syringe brought posteriorly (thereby directing the needle tip more anteriorly), and the needle readvanced until it contacts bone.
Because no contact is made with bone during the Vazirani-Akinosi nerve block, overinsertion of the needle, either absolute (>25 mm) or relative (25 mm in a smaller patient), should be avoided, if possible.
Management
Within seconds to minutes after deposition of local anesthetic into the parotid gland, the patient senses weakening of the muscles on the affected side of the face. Sensory anesthesia is not present in this situation. Management includes the following:
1. Reassure the patient. Explain that the situation is transient, will last for a few hours, and will resolve without residual effect. Mention that it is produced by the normal action of local anesthetic drugs on the facial nerve, which is a motor nerve to the muscles of facial expression.
2. Contact lenses should be removed until muscular movement returns.
3. An eye patch should be applied to the affected eye until muscle tone returns. If resistance is offered by the patient, advise the patient to manually close the affected eyelid periodically to keep the cornea lubricated.
4. Record the incident on the patient’s chart.
5. Although no contraindication is known to reanesthetizing the patient to achieve mandibular anesthesia, it may be prudent to forego further dental care at this appointment.
Trismus
Trismus, from the Greek trismos, is defined as a prolonged, tetanic spasm of the jaw muscles by which the normal opening of the mouth is restricted (locked jaw). This designation was originally used only in tetanus, but because an inability to open the mouth may be seen in a variety of other conditions, the term is currently used in restricted jaw movement, regardless of the cause.54 Although postinjection pain is the most common local complication of local anesthesia, trismus can become one of the more chronic and complicated problems to manage.55-57
Causes
Trauma to muscles or blood vessels in the infratemporal fossa is the most common causative factor in trismus associated with dental injection of local anesthetics.
Local anesthetic solutions into which alcohol or cold sterilizing solutions have diffused produce irritation of tissues (e.g., muscle), leading potentially to trismus. Local anesthetics have been demonstrated to be slightly myotoxic to skeletal muscles. The injection of local anesthetic solution intramuscularly or supramuscularly leads to a rapidly progressive necrosis of exposed muscle fibers.58-60
Hemorrhage is another cause of trismus. Large volumes of extravascular blood can produce tissue irritation, leading to muscle dysfunction as the blood is slowly resorbed (over approximately 2 weeks). Low-grade infection after injection can also cause trismus.61
Every needle insertion produces some damage to the tissue through which it passes. It stands to reason, then, that multiple needle penetrations correlate with a greater incidence of postinjection trismus. In addition, Stacy and Hajjar found that of 100 needles used for the administration of IANB, 60% were barbed on removal from the tissues. The barb occurred when the needle came into contact with the medial aspect of the mandibular ramus. Withdrawal of the needle from tissue increased the likelihood of involvement of the lingual or inferior alveolar nerve (e.g. paresthesia) and the development of trismus.62
Excessive volumes of local anesthetic solution deposited into a restricted area produce distention of tissues, which may lead to postinjection trismus. This is more common after multiple missed IANBs.
Problem
Although the limitation of movement associated with postinjection trismus is usually minor, it is possible for much more severe limitation to develop. The average interincisal opening in cases of trismus is 13.7 mm (range, 5 to 23 mm).59 Average interincisal opening for males is 44.8 (±9.4) mm and for females is 39.2 (±10.8) mm.83 Stone and Kaban reported four cases of severe trismus after multiple inferior alveolar (IA) or PSA nerve blocks, three of which required surgical intervention.63 Before surgery, patients had limited mandibular openings of approximately 2 mm, despite usual treatment regimens.
In the acute phase of trismus, pain produced by hemorrhage leads to muscle spasm and limitation of movement.64,65 The second, or chronic, phase usually develops if treatment is not begun. Chronic hypomobility occurs secondary to organization of the hematoma, with subsequent fibrosis and scar contracture.66 Infection may produce hypomobility through increased pain, increased tissue reaction (irritation), and scarring.61
Prevention
1. Use a sharp, sterile, disposable needle.
2. Properly care for and handle dental local anesthetic cartridges.
3. Use aseptic technique. Contaminated needles should be changed immediately.
4. Practice atraumatic insertion and injection technique.
5. Avoid repeat injections and multiple insertions into the same area by gaining knowledge of anatomy and proper technique. Use regional nerve blocks instead of local infiltration (supraperiosteal injection) wherever possible and rational.
6. Use minimum effective volumes of local anesthetic. Refer to specific protocols for recommendations.
Management
In most instances of trismus, the patient reports pain and some difficulty opening his or her mouth on the day after dental treatment in which a posterior superior alveolar or, more commonly, an inferior alveolar nerve block was administered. Hinton and associates reported that the onset of trismus occurred 1 to 6 days post treatment (average, 2.9 days).59 The degree of discomfort and dysfunction varies but is usually mild.
With mild pain and dysfunction, the patient reports minimum difficulty opening his or her mouth. Arrange an appointment for examination. In the interim, prescribe heat therapy, warm saline rinses, analgesics, and, if necessary, muscle relaxants to manage the initial phase of muscle spasm.67,68 Heat therapy consists of applying hot, moist towels to the affected area for approximately 20 minutes every hour. For a warm saline rinse, a teaspoon of salt is added to a 12-ounce glass of warm water; the rinse is held in the mouth on the involved side (and spit out) to help relieve the discomfort of trismus. Aspirin (325 mg) is usually adequate as an analgesic in managing pain associated with trismus. Its anti-inflammatory properties are also beneficial. Diazepam (approximately 10 mg bid) or another benzodiazepine is used for muscle relaxation if deemed necessary.
The patient should be advised to initiate physiotherapy consisting of opening and closing the mouth, as well as lateral excursions of the mandible, for 5 minutes every 3 to 4 hours. Chewing gum (sugarless, of course!) is yet another means of providing lateral movement of the temporomandibular joint.
Record the incident, findings, and treatment on the patient’s dental chart. Avoid further dental treatment in the involved region until symptoms resolve and the patient is more comfortable.
If continued dental care in the area is urgent, as with an infected painful tooth, it may prove difficult to achieve effective pain control when trismus is present. The Vazirani-Akinosi mandibular nerve block usually provides relief of the motor dysfunction, permitting the patient to open his or her mouth, allowing administration of the appropriate injection for clinical pain control, if needed.
In virtually all cases of trismus related to intraoral injections that are managed as described, patients report improvement within 48 to 72 hours. Therapy should be continued until the patient is free of symptoms. If pain and dysfunction continue unabated beyond 48 hours, consider the possibility of infection. Antibiotics should be added to the treatment regimen described and continued for 7 full days. Complete recovery from injection-related trismus takes about 6 weeks (range, 4 to 20 weeks).59
For severe pain or dysfunction, if no improvement is noted within 2 or 3 days without antibiotics or within 5 to 7 days with antibiotics, or if the ability to open the mouth has become limited, the patient should be referred to an oral and maxillofacial surgeon for evaluation. Other therapies, including the use of ultrasound or appliances, are available for use in these situations.69,70
Temporomandibular joint involvement is rare in the first 4 to 6 weeks after injection. Surgical intervention to correct chronic dysfunction may be indicated in some instances.59,63
Soft Tissue Injury
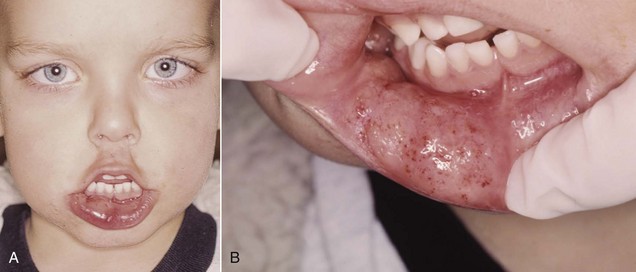
Self-inflicted trauma to the lips and tongue is frequently caused by the patient inadvertently biting or chewing these tissues while still anesthetized (see Fig. 17-9).
Cause
Trauma occurs most frequently in younger children, in mentally or physically disabled children or adults, and in older-old patients; however, it can and does occur in patients of all ages. The primary reason is the fact that soft tissue anesthesia lasts significantly longer than does pulpal anesthesia. Dental patients receiving local anesthetic during their treatment usually are dismissed from the dental office with residual soft tissue numbness. (See the discussion in Chapter 20 of the local anesthesia reversal agent, phentolamine mesylate.)
Problem
Trauma to anesthetized tissues can lead to swelling and significant pain when the anesthetic effects resolve. A young child or a handicapped individual may have difficulty coping with the situation, and this may lead to behavioral problems. The possibility that infection will develop is remote in most instances.
Prevention
A local anesthetic of appropriate duration should be selected if dental appointments are brief. (Refer to the discussion of lip chewing and duration of anesthesia for specific drugs, p. 281.)
A cotton roll can be placed between the lips and the teeth if they are still anesthetized at the time of discharge. The cotton roll is secured with dental floss wrapped around the teeth (to prevent inadvertent aspiration of the roll) (Fig. 17-10).

Figure 17-10 Cotton roll placed between lips and teeth, secured with dental floss, minimizes risk of accidental mechanical trauma to anesthetized tissues.
Warn the patient and the guardian against eating, drinking hot fluids, and biting on the lips or tongue to test for anesthesia. A self-adherent warning sticker may be used on children (Fig. 17-11).
Hematoma
The effusion of blood into extravascular spaces can be caused by inadvertent nicking of a blood vessel (artery or vein) during administration of a local anesthetic. A hematoma that develops subsequent to the nicking of an artery usually increases rapidly in size until treatment is instituted because of the significantly greater pressure of blood within an artery. Nicking of a vein may or may not result in the formation of a hematoma. Tissue density surrounding the injured vessel is a determining factor. The denser the surrounding tissues (e.g., palate), the less likely a hematoma is to develop, but in looser tissue (e.g., infratemporal fossa), large volumes of blood may amass before a swelling is ever noted and therapy instituted.
Cause
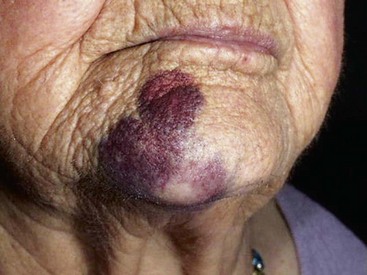
Because of the density of tissue in the hard palate and its firm adherence to bone, hematoma rarely develops after a palatal injection. A rather large hematoma may result from arterial or venous puncture after a posterior superior alveolar or inferior alveolar nerve block. The tissues surrounding these vessels more readily accommodate significant volumes of blood. The blood effuses from vessels until extravascular exceeds intravascular pressure, or until clotting occurs. Hematomas that occur after the inferior alveolar nerve block usually are only visible intraorally, whereas PSA hematomas are visible extraorally.
Problem
A hematoma rarely produces significant problems, aside from the resulting “bruise,” which may or may not be visible extraorally. Possible complications of hematoma include trismus and pain. Swelling and discoloration of the region usually subside gradually over 7 to 14 days.
A hematoma constitutes an inconvenience to the patient and an embarrassment to the person administering the drug (Fig. 17-12).
Prevention
1. Knowledge of the normal anatomy involved in the proposed injection is important. Certain techniques are associated with a greater risk of visible hematoma. The PSA nerve block is the most common, followed by the IANB (a distant second) and the mental/incisive nerve block (a close third when the foramen is entered, a distant third if the technique described in Chapter 14 is adhered to).
2. Modify the injection technique as dictated by the patient’s anatomy. For example, the depth of penetration for a PSA nerve block may be decreased in a patient with smaller facial characteristics.71,72
3. Use a short needle for the PSA nerve block to decrease the risk of hematoma.
Hematoma is not always preventable. Whenever a needle is inserted into tissue, the risk of inadvertent puncturing of a blood vessel is present.
Management
When swelling becomes evident during or immediately after a local anesthetic injection, direct pressure should be applied to the site of bleeding. For most injections, the blood vessel is located between the surface of the mucous membrane and the bone; localized pressure should be applied for not less than 2 minutes. This effectively stops the bleeding.
Inferior Alveolar Nerve Block: Pressure is applied to the medial aspect of the mandibular ramus. Clinical manifestations of the hematoma, which are visible intraorally, include possible tissue discoloration and probable tissue swelling on the medial (lingual) aspect of the mandibular ramus.
Anterior Superior Alveolar (Infraorbital) Nerve Block: Pressure is applied to the skin directly over the infraorbital foramen. The clinical manifestation is discoloration of the skin below the lower eyelid. Hematoma is unlikely to occur with anterior superior alveolar (ASA) nerve block because the technique described requires application of pressure to the injection site throughout drug administration and for a period of at least 1 to 2 minutes thereafter.
Incisive (Mental) Nerve Block: Pressure is placed directly over the mental foramen, externally on the skin or intraorally on the mucous membrane. Clinical manifestations include discoloration of the skin of the chin in the area of the mental foramen and/or swelling in the mucobuccal fold in the region of the mental foramen (see Fig. 17-12). As with the ASA nerve block, pressure applied during administration of the drug effectively minimizes the risk of hematoma formation during incisive (but not mental) nerve block.
Buccal Nerve Block or Any Palatal Injection: Place pressure at the site of bleeding. In these injections, the clinical manifestations of hematoma usually are visible only within the mouth.
Posterior Superior Alveolar Nerve Block: The PSA nerve block usually produces the largest and most esthetically unappealing hematoma. The infratemporal fossa, into which bleeding occurs, can accommodate a large volume of blood. The hematoma usually is not recognized until a colorless swelling appears on the side of the face around the temporomandibular joint area (usually a few minutes after the injection is completed). It progresses over a period of days, extending inferiorly and anteriorly toward the lower anterior region of the cheek. It is difficult to apply pressure to the site of bleeding in this situation because of the location of the involved blood vessels. It is also relatively difficult to apply pressure directly to the posterior superior alveolar artery (the primary source of bleeding), the facial artery, and the pterygoid plexus of veins. They are located posterior, superior, and medial to the maxillary tuberosity. Bleeding normally ceases when external pressure on the vessels exceeds internal pressure, or when clotting occurs. Digital pressure can be applied to the soft tissues in the mucobuccal fold as far distally as can be tolerated by the patient (without eliciting a gag reflex). Apply pressure in a medial and superior direction. If available, ice should be applied (extraorally) to increase pressure on the site and help constrict the punctured vessel.
Subsequent
The patient may be discharged once bleeding stops. Note the hematoma on the patient’s dental chart. Advise the patient about possible soreness and limitation of movement (trismus). If either of these develops, begin treatment as described for trismus. Discoloration will likely occur as a result of extravascular blood elements; it is gradually resorbed over 7 to 14 days.
If soreness develops, advise the patient to take an analgesic such as aspirin or NSAID. Do not apply heat to the area for at least 4 to 6 hours after the incident. Heat produces vasodilation, which may further increase the size of the hematoma if applied too soon. Heat may be applied to the region beginning the next day. It serves as an analgesic, and its vasodilating properties may increase the rate at which blood elements are resorbed, although the latter benefit is debatable. The patient should apply warm moist heat to the affected area for 20 minutes every hour.
Ice may be applied to the region immediately on recognition of a developing hematoma. It acts as both an analgesic and a vasoconstrictor, and it may aid in minimizing the size of the hematoma. Time (tincture of time) is the most important element in managing a hematoma. With or without treatment, a hematoma will be present for 7 to 14 days. Avoid additional dental therapy in the region until symptoms and signs resolve.
Pain on Injection
Pain on injection of a local anesthetic can best be prevented through careful adherence to the basic protocol of atraumatic injection. (See Chapter 11.)
Causes
1. Careless injection technique and a callous attitude (“Palatal injections always hurt” or “This will hurt a little”) all too often become self-fulfilling prophecies.
2. A needle can become dull from multiple injections.
3. Rapid deposition of the local anesthetic solution may cause tissue damage.
4. Needles with barbs (from impaling bone) may produce pain as they are withdrawn from tissue.62
Problem
Pain on injection increases patient anxiety and may lead to sudden unexpected movement, increasing the risk of needle breakage, traumatic soft tissue injury to the patient, or needle-stick injury to the administrator.
Prevention
1. Adhere to proper techniques of injection, both anatomic and psychological.
3. Use topical anesthetic properly before injection.
4. Use sterile local anesthetic solutions.
5. Inject local anesthetics slowly.
6. Make certain that the temperature of the solution is correct. A solution that is too hot or too cold may be more uncomfortable than one at room temperature.
7. Buffered local anesthetics, at a pH of approximately 7.4, have been demonstrated to be more comfortable on administration.73,74 Buffered local anesthetics are discussed further in Chapter 20.
Burning on Injection
A burning sensation that occurs during injection of a local anesthetic is not uncommon. Several potential causes are known.
The primary cause of a mild burning sensation is the pH of the solution being deposited into the soft tissues. The pH of “plain” local anesthetics (i.e., no vasopressor included) is approximately 6.5, whereas solutions that contain a vasopressor are considerably more acidic (around 3.5). Wahl and associates compared the pain on injection of prilocaine plain versus lidocaine with epinephrine (1 : 100,000) and found no statistical difference in patient perception75; however, when bupivacaine with epinephrine (1 : 200,000) was compared with prilocaine plain, significantly more pain was reported by patients receiving bupivacaine.76
Rapid injection of local anesthetic, especially in the denser, more adherent tissues of the palate, produces a burning sensation.
Contamination of local anesthetic cartridges can result when they are stored in alcohol or other sterilizing solutions, leading to diffusion of these solutions into the cartridge. Solutions warmed to normal body temperature usually are considered “too hot” by the patient.
Problem
Although usually transient, the sensation of burning on injection of a local anesthetic indicates that tissue irritation is occurring. If this is caused by the pH of the solution, it rapidly disappears as the anesthetic action develops. Usually no residual sensitivity is noted when the anesthetic action terminates.
When a burning sensation occurs as a result of rapid injection, a contaminated solution, or an overly warm solution, the likelihood that tissue may be damaged is greater, and subsequent complications such as postanesthetic trismus, edema, or possible paresthesia are reported.
Prevention
By buffering the local anesthetic solution to a pH of approximately 7.4 immediately before injection, it is possible to eliminate the burning sensation that some patients experience during injection of a local anesthetic solution containing a vasopressor.73,74
Slowing the speed of injection also helps. The ideal rate of injectable drug administration is 1 mL/min. Do not exceed the recommended rate of 1.8 mL/min.
The cartridge of anesthetic should be stored at room temperature in the container (blister-pack or tin) in which it was shipped, or in a suitable container without alcohol or other sterilizing agents. (See Chapter 7 for proper care and handling of dental cartridges.)
Management
Because most instances of burning on injection are transient and do not lead to prolonged tissue involvement, formal treatment usually is not indicated. In those few situations in which postinjection discomfort, edema, or paresthesia becomes evident, management of the specific problem is indicated.
Infection
Infection subsequent to local anesthetic administration in dentistry is an extremely rare occurrence since sterile disposable needles and glass cartridges have been introduced.
Causes
The major cause of postinjection infection is contamination of the needle before administration of the anesthetic. Contamination of a needle always occurs when the needle touches mucous membrane in the oral cavity. This cannot be prevented, nor is it a significant problem because the normal flora of the oral cavity does not lead to tissue infection.
Improper technique in the handling of local anesthetic equipment and improper tissue preparation for injection are other possible causes of infection.
Injecting Local Anesthetic Solution Into an Area of Infection
As discussed in the section on local anesthetic requirements in endodontics (see Chapter 16), local anesthetics are less effective when injected into infected tissues. However, if deposited under pressure, as in the periodontal ligament injection, the force of their administration might transport bacteria into adjacent, healthy tissues, thereby spreading infection.
Problem
Contamination of needles or solutions may cause a low-grade infection when the needle or solution is placed in deeper tissue. This may lead to trismus if it is not recognized and if proper treatment is not initiated.61
Prevention
1. Use sterile disposable needles.
2. Properly care for and handle needles. Take precautions to avoid contamination of the needle through contact with nonsterile surfaces; avoid multiple injections with the same needle, if possible.
3. Properly care for and handle dental cartridges of local anesthetic.
a. Use a cartridge only once (one patient).
b. Store cartridges aseptically in their original container, covered at all times.
c. Cleanse the diaphragm with a sterile disposable alcohol wipe immediately before use.
4. Properly prepare the tissues before penetration. Dry them and apply topical antiseptic (optional).
Management
Low-grade infection, which is rare, is seldom recognized immediately. The patient usually reports postinjection pain and dysfunction 1 or more days after dental care. Overt signs and symptoms of infection occur rarely. Immediate treatment consists of those procedures used to manage trismus: heat and analgesic if needed, muscle relaxant if needed, and physiotherapy. Trismus produced by factors other than infection normally responds with resolution or improvement within several days. If signs and symptoms of trismus do not begin to respond to conservative therapy within 3 days, the possibility of a low-grade infection should be entertained and the patient started on a 7- to 10-day course of antibiotics. Prescribe 29 (or 41, if 10 days) tablets of penicillin V (250-mg tablets). The patient takes 500 mg immediately and then 250 mg four times a day until all tablets have been taken. Erythromycin may be substituted if the patient is allergic to penicillin.
Record the progress and management of the patient on the dental chart.
Edema
Swelling of tissues is not a syndrome, but it is a clinical sign of the presence of some disorder.
Causes
3. Allergy: Angioedema is a possible response to ester-type topical anesthetics in an allergic patient (localized tissue swelling occurs as a result of vasodilation secondary to histamine release).
4. Hemorrhage (effusion of blood into soft tissues produces swelling)
5. Injection of irritating solutions (alcohol- or cold sterilizing solution–containing cartridges)
6. Hereditary angioedema is a condition characterized by the sudden onset of brawny nonpitting edema affecting the face, extremities, and mucosal surfaces of the intestine and respiratory tract, often without obvious precipitating factors. Manipulation within the oral cavity, including local anesthetic administration, may precipitate an attack. Lips, eyelids, and the tongue are often involved.77 Karlis and associates noted that 15% to 33% of untreated angioedema patients died from acute airway obstruction as a result of laryngeal edema.78
Problem
Edema related to local anesthetic administration is seldom intense enough to produce significant problems such as airway obstruction. Most instances of local anesthetic–related edema result in pain and dysfunction of the region and embarrassment for the patient.
Angioneurotic edema produced by topical anesthetic in an allergic individual, although exceedingly rare, can compromise the airway. Edema of the tongue, pharynx, or larynx may develop and represents a potentially life-threatening situation that requires vigorous management.79
Management
The management of edema is predicated on reduction of the swelling as quickly as possible and on the cause of the edema. When produced by traumatic injection or by introduction of irritating solutions, edema is usually of minimal degree and resolves in several days without formal therapy. In this and all situations in which edema is present, it may be necessary to prescribe analgesics for pain.
After hemorrhage, edema resolves more slowly (over 7 to 14 days) as extravasated blood elements are resorbed into the vascular system. If signs of hemorrhage (e.g., bluish discoloration progressing to green, yellow, and other colors) are evident, management follows that discussed for hematoma.
Edema produced by infection does not resolve spontaneously but may, in fact, become progressively more intense if untreated. If signs and symptoms of infection (pain, mandibular dysfunction, edema, warmth) do not appear to resolve within 3 days, antibiotic therapy should be instituted as outlined previously.
Allergy-induced edema is potentially life threatening. Its degree and location are highly significant. If swelling develops in buccal soft tissues and there is absolutely no airway involvement, treatment consists of intramuscular and oral histamine blocker administration and consultation with an allergist to determine the precise cause of the edema.
If edema occurs in any area where it compromises breathing, treatment consists of the following:
1. P (position): if unconscious, the patient is placed supine.
2. A-B-C (airway, breathing, circulation): basic life support is administered, as needed.
3. D (definitive treatment): emergency medical services (e.g., 9-1-1) is summoned.
4. Epinephrine is administered: 0.3 mg (0.3 mL of a 1 : 1000 epinephrine solution) (adult), 0.15 mg (0.15 mL of a 1 : 1000 epinephrine solution) (child [15 to 30 kg]), intramuscularly (IM) or 3 mL of a 1 : 10,000 epinephrine solution intravenously (IV-adult), every 5 minutes until respiratory distress resolves.
5. Histamine blocker is administered IM or IV.
6. Corticosteroid is administered IM or IV.
7. Preparation is made for cricothyrotomy if total airway obstruction appears to be developing. This is extremely rare but is the reason for summoning emergency medical services early.
8. The patient’s condition is thoroughly evaluated before his or her next appointment to determine the cause of the reaction.
Sloughing of Tissues
Prolonged irritation or ischemia of gingival soft tissues may lead to a number of unpleasant complications, including epithelial desquamation and sterile abscess.
Problem
Pain, at times severe, may be a consequence of epithelial desquamation or a sterile abscess. It is remotely possible that infection may develop in these areas.
Prevention
Use topical anesthetics as recommended. Allow the solution to contact the mucous membranes for 1 to 2 minutes to maximize its effectiveness and minimize toxicity.
When using vasoconstrictors for hemostasis, do not use overly concentrated solutions. Norepinephrine (Levophed) 1 : 30,000 is the agent most likely to produce ischemia of sufficient duration to cause tissue damage and a sterile abscess (Fig. 17-13). Norepinephrine is not available in any dental local anesthetic solution in North America. Epinephrine (1 : 50,000) also may produce this problem, if reinjection of the solution occurs whenever ischemia resolves, over a long period of time (e.g., several hours). The palatal tissues are likely the only place in the oral cavity where this phenomenon may arise.
Management
Usually, no formal management is necessary for epithelial desquamation or sterile abscess. Be certain to reassure the patient of this fact.
Management may be symptomatic. For pain, analgesics such as aspirin or other NSAIDs and a topically applied ointment (Orabase) are recommended to minimize irritation to the area. Epithelial desquamation resolves within a few days; the course of a sterile abscess may run 7 to 10 days. Record data on the patient’s chart.
Postanesthetic Intraoral Lesions
Patients occasionally report that approximately 2 days after an intraoral injection of local anesthetic, ulcerations developed in their mouth, primarily around the site(s) of the injection(s). The primary initial symptom is pain, usually of a relatively intense nature.
Cause
Recurrent aphthous stomatitis or herpes simplex can occur intraorally after a local anesthetic injection or after any trauma to the intraoral tissues.
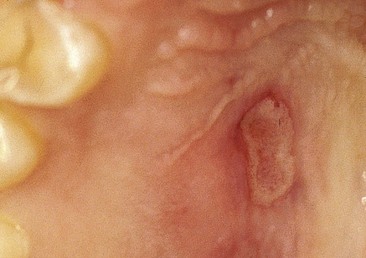
Recurrent aphthous stomatitis (recurrent aphthous ulceration) is the most common oral mucosal disease known to human beings.80 Recurrent aphthous stomatitis is more frequently observed than herpes simplex, typically developing on gingival tissues that are not attached to underlying bone (e.g., movable tissue), such as the buccal vestibule (Fig. 17-14). In spite of much continuing research, the causes remain poorly understood, the ulcers are not preventable, and treatment remains symptomatic.
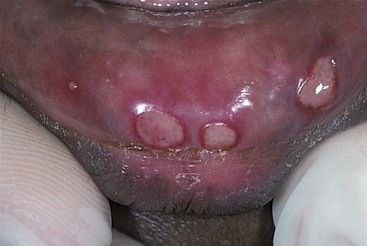
Figure 17-14 Aphthous stomatitis. (From Eisen D, Lynch D: The mouth: diagnosis and treatment, St Louis, 1998, Mosby.)
Herpes simplex can develop intraorally, although more commonly it is observed extraorally. It is viral and becomes manifest as small bumps on tissues that are attached to underlying bone (e.g., fixed) such as the soft tissue of the hard palate (Fig. 17-15).
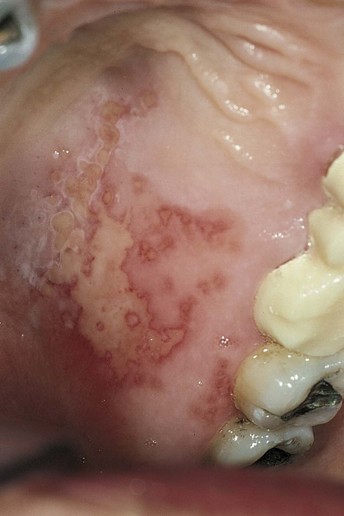
Figure 17-15 Intraoral lesion (herpes simplex) on the palate. (From Eisen D, Lynch D: The mouth: diagnosis and treatment, St Louis, 1998, Mosby.)
Trauma to tissues by a needle, a local anesthetic solution, a cotton swab, or any other instrument (e.g., rubber dam clamp, handpiece) may activate the latent form of the disease process that was present in the tissues before injection.
Problem
The patient describes acute sensitivity in the ulcerated area. Many consider that the tissue has become infected as a result of the local anesthetic injection they received; however, the risk of a secondary infection developing in this situation is minimal.
Prevention
Unfortunately, there is no means of preventing these intraoral lesions from developing in susceptible patients. Extraoral herpes simplex, on occasion, may be prevented or its clinical manifestations minimized if treated in its prodromal phase. The prodrome consists of a mild burning or itching sensation at the site where the virus is present (e.g., lip). Antiviral agents, such as acyclovir, applied qid to the affected area effectively minimize the acute phase of this process.
Management
Primary management is symptomatic. Pain is the major initial symptom, developing approximately 2 days after injection. Reassure the patient that the situation is not caused by a bacterial infection secondary to the local anesthetic injection, but in fact is an exacerbation of a process that was present, in latent form, in the tissues before injection. Indeed, most of these patients have experienced this response before and are resigned to it happening again.
No management is necessary if the pain is not severe. However, if pain causes the patient to complain, treatment can be instituted, usually with varying degrees of success. The objective is to keep the ulcerated areas covered or anesthetized.
Topical anesthetic solutions (e.g., viscous lidocaine) may be applied as needed to the painful areas. A mixture of equal amounts of diphenhydramine (Benadryl) and milk of magnesia rinsed in the mouth effectively coats the ulcerations and provides relief from pain. Orabase, a protective paste, without Kenalog can provide a degree of pain relief. Kenalog, a corticosteroid, is not recommended because its anti-inflammatory actions increase the risk of viral or bacterial involvement. A tannic acid preparation (Zilactin) can be applied topically to the lesions extraorally or intraorally (dry the tissues first). Studies from the University of Alabama have demonstrated that most patients achieve substantial pain relief for up to 6 hours.81,82
The ulcerations usually last 7 to 10 days with or without treatment. Maintain records on the patient’s chart.
References
1. Pogrel, MA. Broken local anesthetic needles: a case series of 16 patients, with recommendations. J Am Dent Assoc. 2009;140:1517–1522.
2. 2006 Septodont reported wholesale sales. Newark, Del: Septodont NA, 2006.
3. Amies, AB. Broken needles. Aust Dent J. 1951;55:403–406.
4. Muller, EE, Lernoud, R. Surgical extraction of needles broken during local anesthesia of the mandibular nerve. Acta Odontol Venez. 1967;5:229–237.
5. Dudani, IC. Broken needles following mandibular injections. J Indian Dent Assoc. 1971;43:14–17.
6. Kennett, S, Curran, JB, Jenkins, GR. Management of a broken hypodermic needle: report of a case. J Can Dent Assoc. 1972;38:414–416.
7. Kennett, S, Curran, JB, Jenkins, GR. Management of a broken hypodermic needle: report of a case. Anesth Prog. 1973;20:48–50.
8. Bump, RL, Roche, WC. A broken needle in the pterygomandibular space: report of a case. Oral Surg Oral Med Oral Pathol. 1973;36:750–752.
9. Hai, HK. Retrieval of a broken hypodermic needle: a new technique of localising. Singapore Dent J. 1983;8:27–29.
10. Orr, DL, 2nd. The broken needle: report of case. J Am Dent Assoc. 1983;107:603–604.
11. Marks, RB, Carlton, DM, McDonald, S. Management of a broken needle in the pterygomandibular space: report of case. J Am Dent Assoc. 1984;109:263–264.
12. Burke, RH. Management of a broken anesthetic needle. J Am Dent Assoc. 1986;112:209–210.
13. Fox, IJ, Belfiglio, EJ. Report of a broken needle. Gen Dent. 1986;34:102–106.
14. Pietruszka, JF, Hoffman, D, McGivern, BE, Jr. A broken dental needle and its surgical removal: a case report. N Y State Dent J. 1986;52:28–31.
15. Chaikin, L. Broken needles. N Y State Dent J. 1987;53:8.
16. Burgess, JO. The broken dental needle—a hazard. Spec Care Dentist. 1986;8:71–73.
17. Ho, KH. A simple technique for localizing a broken dental needle in the pterygomandibular region. Aust Dent J. 1988;33:308–309.
18. Mima, T, Shirasuna, K, Morioka, S, et al. A broken needle in the pterygomandibular space. Osaka Daigaku Shigaku Zasshi. 1989;34:418–422.
19. McDonogh, T. An unusual case of trismus and dysphagia. Br Dent J. 1996;180:465–466.
20. Bhatia, S, Bounds, G. A broken needle in the pterygomandibular space: report of a case and review of the literature. Dent Update. 1998;25:35–37.
21. Bedrock, RD, Skigen, A, Dolwick, MF. Retrieval of a broken needle in the pterygomandibular space. J Am Dent Assoc. 1999;130:685–687.
22. Faura-Sole, M, Sanchez-Garces, MA, Berini-Aytes, L, et al. Broken anesthetic injection needles: report of 5 cases. Quintessence Int. 1999;30:461–465.
23. Dhanrayani, PJ, Jonaidel, O. A forgotten entity: “broken needle while inferior dental block. Dent Update. 2000;27:101.
24. Murray, M. A forgotten entity: “broken needle while administering inferior dental block. Dent Update. 2000;27:306.
25. Zeltser, R, Cohen, C, Casap, N. The implications of a broken needle in the pterygomandibular space: clinical guidelines for prevention and retrieval. Pediatr Dent. 2002;24:153–156.
26. Thompson, M, Wright, S, Cheng, LH, et al. Locating broken dental needles. Int J Oral Maxillofac Surg. 2003;32:642–644.
27. Baart, JA, van Amerongen, WE, de Jong, KJ, et al. Needle breakage during mandibular block anaesthesia: prevention and retrieval. Ned Tijdschr Tandheelkd. 2006;113:520–523.
28. Malamed, SF, Reed, K, Poorsattar, S. Needle breakage: incidence and prevention. Dent Clin N Am. 2010;54:745–756.
29. Personal communication, Dentsply-MPL Technologies. Franklin Park, Ill, 2003.
30. Ethunandan, M, Tran, AL, Anand, R, et al. Needle-breakage following inferior alveolar nerve block: implications and management. Br Dent J. 2007;202:395–397.
31. Haas, DA, Lennon, D. A 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc. 1995;61:319–320. [323–326, 329–330].
32. Pogrel, MA, Thamby, S. Permanent nerve involvement resulting from inferior alveolar nerve blocks. J Am Dent Assoc. 2000;131:901–907.
33. Pogrel, MA, Thamby, S. The etiology of altered sensation in the inferior alveolar, lingual, and mental nerves as a result of dental treatment. J Calif Dent Assoc. 1999;27:531–538.
34. Dower, JS, Jr. A review of paresthesia in association with administration of local anesthesia. Dent Today. 2003;22:64–69.
35. Haas, DA. Localized complications from local anesthesia. J Calif Dent Assoc. 1998;26:677–682.
36. Malden, NJ, Maidment, YG. Lingual nerve injury subsequent to wisdom teeth removal: a 5-year retrospective audit from a High Street dental practice. Br Dent J. 2002;193:203–205.
37. Heller, AA, Shankland, WE, II. Alternative to the inferior alveolar nerve block anesthesia when placing mandibular dental implants posterior to the mental foramen. J Oral Implantol. 2001;27:127–133.
38. Bataineh, AB. Sensory nerve impairment following mandibular third molar surgery. J Oral Maxillofac Surg. 2001;59:1012–1017.
39. Kasaba, T, Onizuka, S, Takasaki, M. Procaine and mepivacaine have less toxicity in vitro than other clinically used local anesthetics. Anesth Analg. 2003;97:85–90.
40. Hillerup, S, Jensen, R. Nerve injury caused by mandibular block analgesia. Int J Oral Maxillofac Surg. 2006;35:437–443.
41. Stenver DI: Pharmacovigilance Working Party of the European Union—Laegemiddelstyrelsen Danish Medicines Agency. Adverse effects from anaesthetics used in relation with dental care with a special focus on anesthetics containing articaine, October 20, 2006.
42. Pogrel, MA. Permanent nerve damage from inferior alveolar nerve blocks—an update to include articaine. J Calif Dent Assoc. 2007;35:271–273.
43. Katyal, V. The efficacy and safety of articaine versus lignocaine in dental treatments: a meta-analysis. J Dent. 2010;38:307–317.
44. Garisto, GA, Gaffen, AS, Lawrence, HP, et al. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010;141:836–844.
45. National Weather Service, Lightning Safety. Odds of becoming a lightning victim. Available at www.lightningsafety.noaa.gov/medical, 2011. [Accessed February 15].
46. U.S. Food and Drug Administration Center for Drug Evaluation and Research Office of Post-Marketing Drug Risk Assessment. Available at www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects, 2011. [Accessed October 14].
47. Nickel, AA, Jr. A retrospective study of paresthesia of the dental alveolar nerves. Anesth Prog. 1990;37:42–45.
48. Personal communication. F. M. McCarthy. 1979.
49. Personal communication. D. Orr. 2011.
50. Sisk, AL, Hammer, WB, Shelton, DW, et al. Complications following removal of impacted third molars. J Oral Maxillofac Surg. 1986;44:855–859.
51. Cooley, RL, Coon, DE. Transient Bell’s palsy following mandibular block: a case report. Quintessence Int. 1978;9:9.
52. Crean, SJ, Powis, A. Neurological complications of local anaesthetics in dentistry. Dent Update. 1999;26:344–349.
53. Malamed, SF. The possible secondary effects in cases of local anesthesia. Rev Belge Medec Dent. 2000;55:19–28.
54. Tveter-as, K, Kristensen, S. The aetiology and pathogenesis of trismus. Clin Otolaryngol. 1986;11:383–387.
55. Dhanrajani, PJ, Jonaidel, O. Trismus: etiology, differential diagnosis and treatment. Dent Update. 2002;29:88–92. [94].
56. Leonard, M. Trismus: what is it, what causes it, and how to treat it. Dent Today. 1999;18:74–77.
57. Marien, M, Jr. Trismus: causes, differential diagnosis, and treatment. Gen Dent. 1997;45:350–355.
58. Benoit, PW, Yagiela, JA, Fort, NF. Pharmacologic correlation between local anesthetic-induced myotoxicity and disturbances of intracellular calcium distribution. Toxic Appl Pharmacol. 1980;52:187–198.
59. Hinton, RJ, Dechow, PC, Carlson, DS. Recovery of jaw muscle function following injection of a myotonic agent (lidocaine-epinephrine). Oral Surg Oral Med Oral Pathol. 1986;59:247–251.
60. Jastak, JT, Yagiela, JA, Donaldson, D. Complications and side effects. In: Jastak JT, Yagiela JA, Donaldson D, eds. Local anesthesia of the oral cavity. Philadelphia: WB Saunders, 1995.
61. Kitay, D, Ferraro, N, Sonis, ST. Lateral pharyngeal space abscess as a consequence of regional anesthesia. J Am Dent Assoc. 1991;122:56–59.
62. Stacy, GC, Hajjar, G. Barbed needle and inexplicable paresthesias and trismus after dental regional anesthesia. Oral Surg Oral Med Oral Pathol. 1994;77:585–588.
63. Stone, J, Kaban, LB. Trismus after injection of local anesthetic. Oral Surg. 1979;48:29–32.
64. Eanes, WC. A review of the considerations in the diagnosis of limited mandibular opening. Cranio. 1991;9:137–144.
65. Luyk, NH, Steinberg, B. Aetiology and diagnosis of clinically evident jaw trismus. Aust Dent J. 1990;35:523–529.
66. Brooke, RI. Postinjection trismus due to formation of fibrous band. Oral Surg Oral Med Oral Pathol. 1979;47:424–426.
67. Himel, VT, Mohamed, S, Luebke, RG. Case report: relief of limited jaw opening due to muscle spasm. LDA J. 1988;47:6–7.
68. Kouyoumdjian, JH, Chalian, VA, Nimmo, A. Limited mandibular movement: causes and treatment. J Prosthet Dent. 1988;59:330–333.
69. Carter, EF. Therapeutic ultrasound for the relief of restricted mandibular movement. Dent Update. 1986;13:503. [504, 506, 508–509].
70. Lund, TW, Cohen, JI. Trismus appliances and indications for use. Quintessence Int. 1993;24:275–279.
71. Harn, SD, Durham, TM, Callahan, BP, Kent, DK. The triangle of safety: a modified posterior superior alveolar injection technique based on the anatomy of the PSA artery. Gen Dent. 2002;50:554–557.
72. Harn, SD, Durham, TM, Callahan, BP, et al. The posterior superior alveolar injection technique: a report on technique variations and complications. Gen Dent. 2002;50:544–550.
73. Malamed, SF, Hersh, E, Poorsattar, S, Falkel, M. Reduction of local anesthetic injection pain using an automated dental anesthetic cartridge buffering system: A randomized, double-blind, crossover study. J Amer Dent Assoc October. 2011.
74. Malamed, SF. Buffering local anesthetics in dentistry. ADSA Pulse. 2011;44(1):3. [8–9].
75. Wahl, MJ, Overton, D, Howell, J, et al. Pain on injection of prilocaine plain vs. lidocaine with epinephrine: a prospective double-blind study. J Am Dent Assoc. 2001;132:1398–1401.
76. Wahl, MJ, Schmitt, MM, Overton, DA, et al. Injection pain of bupivacaine with epinephrine vs. prilocaine plain. J Am Dent Assoc. 2002;133:1652–1656.
77. Nzeako, U, Frigas, E, Tremaine, W. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001;161:2417–2429.
78. Karlis, V, Glickman, RS, Stern, R, et al. Hereditary angioedema: case report and review of management. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont. 1997;83:462–464.
79. Hayes, SM. Allergic reaction to local anesthetic: report of a case. Gen Dent. 1980;28:30–31.
80. Ship, JA. Recurrent aphthous stomatitis: an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont. 1996;82:118.
81. Raborn, GW, McGaw, WT, Grace, M, et al. Herpes labialis treatment with acyclovir 5% modified aqueous cream: a double-blind randomized trial. Oral Surg Oral Med Oral Pathol. 1989;67:676–679.
82. Raborn, GW, McGaw, WT, Grace, M, et al. Treatment of herpes labialis with acyclovir: review of three clinical trials. Am J Med. 1988;85:39–42.
83. Mezitis, M, Rallis, G, Zachariades, N. The normal range of mouth opening. J Oral Maxillofac Surg. 2009;47:1028–1029.