Clinical Action of Specific Agents
Selection Of A Local Anesthetic
Although many drugs are classified as local anesthetics and find use within the health professions, only a handful are currently used in dentistry. In 1980, when the first edition of this text was published, five local anesthetics were available in dental cartridge form in the United States: lidocaine, mepivacaine, prilocaine, and the combination of procaine and propoxycaine.1 In the years since that first edition, increased demand for longer-acting local anesthetics led to the introduction, in dental cartridges, of bupivacaine (1982 Canada, 1983 United States) and etidocaine (1985). In 1975 articaine became available in Germany, and later throughout Europe. Articaine came to North America in 1983 (Canada) and to the United States in 2000. Articaine is classified as an intermediate-duration local anesthetic.
The combination of procaine and propoxycaine was withdrawn from the U.S. market in January 1996.
As this sixth edition of Handbook of Local Anesthesia goes to press, the local anesthetic armamentarium in North American dentistry includes articaine, bupivacaine, lidocaine, mepivacaine, and prilocaine.
With the availability of these local anesthetics, in various combinations with and without vasoconstrictors, it is possible for a doctor to select a local anesthetic solution that possesses the specific pain controlling properties necessary for the patient for any given dental procedure. Table 4-1 lists local anesthetics and the various combinations in which they are currently available in the United States and Canada. Box 4-1 lists these combinations by their expected duration of clinical action (durations of pulpal and soft tissue anesthesia).
TABLE 4-1
Local Anesthetics Available in North America (August 2011)
| Local Anesthetic (+ Vasoconstrictor) | Duration of Action* |
| Articaine HCl | |
| 4% + epinephrine 1 : 100,000 | Intermediate |
| 4% + epinephrine 1 : 200,000 | Intermediate |
| Bupivacaine HCl | |
| 0.5% + epinephrine 1 : 200,000 | Long |
| Lidocaine HCl | |
| 2% + epinephrine 1 : 50,000 | Intermediate |
| 2% + epinephrine 1 : 100,000 | Intermediate |
| Mepivacaine HCl | |
| 3% | Short |
| 2% + levonordefrin 1 : 20,000 | Intermediate |
| Prilocaine HCl | |
| 4% | Short (infiltration); intermediate (nerve block) |
| 4% + epinephrine 1 : 200,000 | Intermediate |
*The classification of duration of action is approximate, for extreme variations may be noted among patients. Short-duration drugs provide pulpal or deep anesthesia for less than 30 minutes; intermediate-duration drugs for about 60 minutes; and long-duration drugs for longer than 90 minutes.
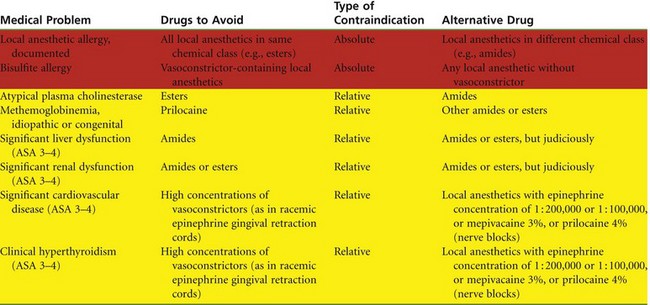
In this chapter, each of the available local anesthetics in its various combinations is described. In addition, the rationale for selection of an appropriate local anesthetic for a given patient at a given appointment is presented. It is strongly suggested that the reader—the potential administrator of these drugs—become familiar with this material, including contraindications to the administration of certain local anesthetic combinations (Table 4-2).
In the following discussion of the clinical properties of specific local anesthetic combinations, several concepts are presented that require some explanation. These include the duration of action of the drug and determination of the maximum recommended dose.
Duration
The duration of pulpal (hard tissue) and soft tissue (total) anesthesia cited for each drug is an approximation. Many factors affect both the depth and the duration of a drug’s anesthetic action, prolonging or (much more commonly) decreasing it. These factors include but are not limited to the following:
1. Individual response to the drug (the “bell-shaped” curve)
2. Accuracy in deposition of the local anesthetic
3. Status of tissues at the site of drug deposition (vascularity, pH)
5. Type of injection administered (supraperiosteal [“infiltration”] or nerve block)
In the subsequent discussion of individual local anesthetics, the durations of anesthesia (pulpal and soft tissue) are presented as a range (e.g., 40 to 60 minutes). This approach attempts to take into account the factors mentioned that can influence drug action:
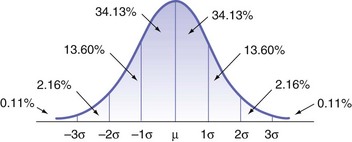
1. Normal distribution curve (bell-shaped curve): Variation in individual response to a drug is common and expected and is depicted in the so-called bell or normal distribution curve (Fig. 4-1). Most patients will respond in a predictable manner to a drug’s actions (e.g., 40 to 60 minutes). However, some patients (with none of the other factors that influence drug action obviously present) will have a shorter or longer duration of anesthesia. This is to be expected and is entirely normal.
For example, if 100 persons are administered an appropriate dose of 2% lidocaine HCl with epinephrine 1 : 100,000 via supraperiosteal injection over a maxillary lateral incisor, and a pulp tester is used to assess the duration of anesthesia, approximately 70% (68.26%) will have pulpal anesthesia for approximately 60 minutes. These represent the normo-responders. Approximately 15% will have pulpal anesthesia that lasts beyond the expected 60 minutes—perhaps 70 or 80 minutes, and even longer for some. These persons are termed hyperresponders. No dentist complains about these patients because their dental treatment proceeds and is completed with no pain or need for repeated injection of local anesthetic. However, it is the final 15%, the hyporesponders, who are well remembered by the dentist. These patients, given lidocaine with epinephrine, are anesthetized for 45 minutes, 30 minutes, 15 minutes, or even less. These are the patients about whom the doctor states (incorrectly): “They metabolize the drug rapidly.” As was mentioned in Chapter 2, metabolism (biotransformation, detoxification) has nothing to do with the clinical effects of a local anesthetic dissipating. The duration of anesthesia is based simply on the way some persons respond to this drug (or group of drugs).
2. Accuracy in administration of the local anesthetic is another factor that influences drug action. Although not as significant in certain techniques (e.g., supraperiosteal) or with certain drugs (e.g., articaine), accuracy in deposition is a major factor in many nerve blocks in which a considerable thickness of soft tissue must be penetrated to access the nerve being blocked. Inferior alveolar nerve block (IANB) is the prime example of a technique in which the depth and duration of anesthesia are greatly influenced by the accuracy of injection. Deposition of local anesthetic close to the nerve provides greater depth and duration of anesthesia compared with an anesthetic deposited at a greater distance from the nerve to be blocked.
3. The status of the tissues into which a local anesthetic is deposited influences the observed duration of anesthetic action. The presence of normal healthy tissue at the site of drug deposition is assumed. Inflammation, infection, or pain (acute or chronic) usually decreases depth and anticipated duration of anesthesia. Increased vascularity at the site of drug deposition results in more rapid absorption of the local anesthetic and a decreased duration of anesthesia. This is most notable in areas of inflammation and infection but is also a consideration in “normal” anatomy. The recent introduction (February 2011) of buffered local anesthetics promises to help overcome this negative effect of inflammation and infection.2 The neck of the mandibular condyle, the target for local anesthetic deposition in the Gow-Gates mandibular nerve block, is considerably less vascular than the target area for the IANB. The expected duration of anesthesia for any local anesthetic will be greater in a less vascular region.
4. Anatomic variation also influences clinical anesthesia. The normal anatomy of the maxilla and the mandible is described in Chapter 12. The most notable aspect of “normal” anatomy is the presence of extreme variation (e.g., in size and shape of the head or in thickness of bone) from person to person. The techniques presented in subsequent chapters are based on the middle of the bell curve, the so-called normal responders. Anatomic variations away from this “norm” may influence the duration of clinical drug action. Although most obvious in the mandible (height of the mandibular foramen, width of the ramus, thickness of the cortical plate of bone), such variation also may be noted in the maxilla. Supraperiosteal infiltration, usually effective in providing pulpal anesthesia for all maxillary teeth, provides a shorter duration than expected or an inadequate depth of anesthesia when alveolar bone is more dense than usual. Where the zygomatic arch is lower (primarily in children, but occasionally in adults), infiltration anesthesia of the maxillary first and second molars may provide a shorter duration or may even fail to provide adequate depth of pulpal anesthesia. In other cases, the palatal root of maxillary molars may not be adequately anesthetized, even in the presence of normal thickness of the buccal alveolar bone, when that root flares greatly toward the midline of the palate. In the mandible, it is stated that supraperiosteal infiltration is not effective in adults because their cortical plate of bone is too thick; however, according to the bell-shaped curve, 15% of adult patients should have cortical bone that is thinner, perhaps allowing mandibular infiltration to be effective. The use of articaine HCl by mandibular infiltration in adults has been demonstrated to be highly effective (and is discussed in detail in Chapters 15 and 20).3
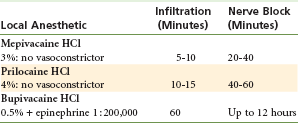
5. Finally, the duration of clinical anesthesia is influenced by the type of injection administered. For all drugs presented, administration of a nerve block provides a longer duration of pulpal and soft tissue anesthesia than is provided by supraperiosteal injection (e.g., infiltration). This assumes that the recommended minimum volume of anesthetic is injected. Less than recommended volumes decrease the duration of action. Larger than recommended doses do not provide increased duration. For example, a duration of pulpal anesthesia of 10 to 15 minutes may be expected to follow supraperiosteal injection with prilocaine 4% (no vasoconstrictor), whereas a 40- to 60-minute duration is normal following nerve block (Table 4-3).
Maximum Doses of Local Anesthetics
Doses of local anesthetic drugs are presented in terms of milligrams of drug per unit of body weight—as milligrams per kilogram (mg/kg) or as milligrams per pound (mg/lb). These numbers, similar to the ones presented for duration, reflect estimated values because there is a wide range (the bell-shaped curve also is seen here) in patient response to blood levels of local anesthetics (or of any drug).
For patients whose responses to anesthetic blood levels lie within the middle of the normal distribution curve, administration of a maximum dose based on body weight produces a local anesthetic blood level below the usual threshold for an overdose (toxic) reaction. The response observed if an overdose reaction occurs at that dose is mild (e.g., tremor of the arms and legs, drowsiness). Patients who are hypo-responders to elevated local anesthetic blood levels may not experience any adverse reaction until their local anesthetic blood level is considerably above this “normal” overdose threshold. These patients represent little increased risk when local anesthetics are administered in “usual” dental doses. However, hyperresponders may demonstrate clinical signs and symptoms of local anesthetic overdose at blood levels that are considerably lower than those normally necessary to produce such reactions. To increase safety for all patients during administration of local anesthetics, but especially in this latter group, one should always minimize drug doses and use the smallest clinically effective dose. Recommended volumes of local anesthetics are presented for each injection technique in Chapters 13, 14, and 15.
The maximum recommended dose (MRD) of local anesthetics has been modified in this 6th edition. In previous editions, both the manufacturer’s recommended dose (MRD-m) and the author’s recommended dose (MRD-a) were listed. In some instances, these doses differed. Where doses differed, those recommended by this author were more conservative than those recommended by the drug’s manufacturer. In this 6th edition of Local Anesthesia, only MRDs that have been approved by the U.S. Food and Drug Administration (FDA) are listed (Table 4-4).
TABLE 4-4
Maximum Recommended Dosages (MRDs) of Local Anesthetics Available in North America
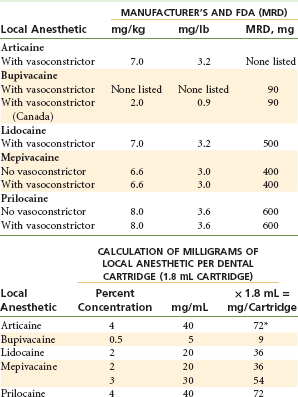
MRD, Maximum recommended dose.
*Cartridges of some drugs in the United States read, “1.7 mL. each.” The actual volume of all local anesthetic cartridges is approximately 1.76 mL.
Maximum doses are unlikely to be reached in most dental patients, especially adults of normal body weight, for most dental procedures. Two groups of patients, however, represent potentially increased risk from overly high local anesthetic blood levels: the smaller, lighter-weight (and well-behaved) child, and the debilitated elderly individual. Considerable attention must be given to drug administration in these two groups. The maximum recommended dose calculated should always be decreased in medically compromised, debilitated, or elderly persons.
Changes in liver function, plasma protein binding, blood volume, and other important physiologic functions influence the manner in which local anesthetics are distributed and biotransformed in the body.4 The net result of these changes is increased plasma blood levels of the drug, associated with increased relative risk of overdose reaction. The half-lives of the amide local anesthetics are significantly increased in the presence of decreased liver function or perfusion.5 Peak plasma local anesthetic blood levels tend to be higher and to remain so longer in these situations. The calculated drug dose (based on body weight) should be decreased in all “at risk” individuals. Unfortunately, there is no magical formula that can aid in determining the degree of dose reduction for a given patient. It is suggested that the doctor evaluate each patient’s dental care needs and then devise a treatment plan that takes into account that person’s requirement for smaller doses of local anesthetic at every treatment appointment.
A point that has come up in several medicolegal situations related to overdosage (OD) of local anesthetics involves the maximum number of milligrams administered and the effect on the patient. Assume, for example, that the MRD for a local anesthetic in a given patient is 270 mg, and the patient is administered 271 mg. The thinking among laypersons (and, unfortunately, some health care professionals too) is that an overdose will definitely occur. However, this may not be the case. As mentioned, many factors interact to determine how a patient will respond to a given drug. When the MRD is exceeded, there is no guarantee that an OD will occur, only that there is a greater likelihood of its occurrence. Indeed, in certain individuals, an OD may be seen with dosages below the calculated MRD (hyperresponders to the drug). Another factor in determining whether an OD will occur is the time over which the local anesthetic dose was administered. If all 271 mg is administered within a brief time frame, the resulting local anesthetic blood level will be greater than in a situation in which the same dose is administered a little at a time over several hours. These points are discussed in greater detail in Chapter 18.
Box 4-2 provides examples of how to calculate maximum dosages and numbers of local anesthetic cartridges to be administered to various patients.
A minor point, but one that has led to some confusion primarily among dental and dental hygiene students, and also doctors and hygienists in practice, is that labeling changes on some cartridges of local anesthetics indicate that the volume of solution contained in the cartridge is 1.7 mL, not the “traditional” 1.8 mL. In actual fact, dental cartridges did not always contain 1.8 mL of solution. In the late 1990s, when articaine was undergoing the FDA approval process, the question was asked of its manufacturer: “Can you guarantee that each and every cartridge contains at least 1.8 mL of solution?” The answer was “No.” Cartridges are filled mechanically, and very slight variation in volume is noted from one cartridge to the next. When asked if the manufacturer could guarantee that each and every cartridge contains at least 1.7 mL of solution, the answer was “Yes.” In actual fact, the average volume of local anesthetic solution in a dental cartridge in the United States is 1.76 mL.6 When the MRD of a local anesthetic for a given patient is calculated, it is advised that a volume of 1.8 mL should be employed.
A commonly asked question is this: “How do I determine the dose of each local anesthetic administered in clinical situations in which more than one drug is necessary?” The answer is again that no guaranteed formula exists for determining this number. One method is simply to ensure that the total dose of both local anesthetics does not exceed the lower of the two maximum doses for the individual agents.
For example, a 45-kg (100-lb) patient receiving 4% prilocaine with epinephrine may be given 8.0 mg/kg (3.6 mg/lb) (or 360 mg) during a 90-minute procedure (the approximate elimination half-life of prilocaine). She receives two cartridges (144 mg), but anesthesia is inadequate for the treatment to proceed. As is commonly the case, the doctor blames the lack of anesthesia on the anesthetic drug (“I’ve got a bad batch of local”), not on technique error or unusual patient anatomy, as is more likely. The doctor elects to switch to lidocaine 2% with epinephrine 1 : 100,000 to provide anesthesia. How does one determine the maximum dose of lidocaine that may be used?
If lidocaine were being administered alone to this patient, its MRD would be 7.0 mg/kg (3.2 mg/lb) or 315 mg. However, she has already received 144 mg of prilocaine within the past few minutes. The amount of lidocaine suggested is the smaller total maximum dose (which in this case is 315 mg [lidocaine] vs. 360 mg [prilocaine]) minus the dose of prilocaine already administered (144 mg), which permits a dose of 171 mg of lidocaine, or about 4.5 cartridges, to be administered to this patient (Box 4-3).
It is extremely unlikely that a “bad batch” of local anesthetic has been distributed to the doctor. The most common causes for failure to achieve adequate pain control are anatomic variation and faulty technique. (However, blaming failure to obtain adequate pain control on the local anesthetic drug serves to soothe the doctor’s ego.)
The concept of maximum recommended dose is discussed more fully in Chapter 18.
Clinically available local anesthetics (the amides: articaine, bupivacaine, lidocaine, mepivacaine, and prilocaine) are discussed in detail here. Esters (procaine and propoxycaine) are mentioned in passing, more as a matter of historical interest than of necessity. Agents available for topical application (topical anesthetics) also are discussed.
Ester-Type Local Anesthetics
Pertinent Information
Excretion: More than 2% unchanged in the urine (90% as para-aminobenzoic acid [PABA], 8% as diethylaminoethanol).
Comments
Procaine HCl, the first synthetic injectable local anesthetic, is no longer available in North America in dental cartridges. However, its proprietary name, Novocain, is synonymous throughout the world with dental local anesthesia. Until 1996, procaine was found in dental cartridges in combination with a second ester anesthetic, propoxycaine.
Used as the sole local anesthetic agent for pain control in dentistry, as it was from its introduction in 1904 until the introduction of the amide local anesthetic lidocaine in the mid-1940s, 2% procaine (plain) provides essentially no pulpal anesthesia and from 15 to 30 minutes of soft tissue anesthesia. This is a result of its profound vasodilating properties. Procaine produces the greatest vasodilation of all clinically used local anesthetics. Thus a clean (e.g., bloodless) surgical field is more difficult to maintain with procaine because of increased bleeding.
Procaine is of importance in the immediate management of inadvertent intra-arterial (IA) injection of a drug; its vasodilating properties are used to aid in breaking arteriospasm.7
Although not extremely common, the incidence of allergy to both procaine and other ester local anesthetics is significantly greater than to amide local anesthetics.8
Metabolized in the blood by plasma cholinesterase, procaine does not exhibit increased toxicity in patients with hepatic dysfunction.
The maximum recommended dose of procaine, used for peripheral nerve blocks, is 1000 mg.9
With a dissociation constant (pKa) of 9.1, procaine has a slow clinical onset of anesthesia (6 to 10 minutes)—a reason for inclusion of propoxycaine in the anesthetic cartridge.
Propoxycaine HCl
Comments
Propoxycaine was combined with procaine in solution to provide more rapid onset and a more profound and longer-lasting anesthesia than could be obtained with procaine alone. Propoxycaine was not available alone because its higher toxicity (seven to eight times that of procaine) limited its usefulness as a sole agent.
Procaine HCl + Propoxycaine HCl
Although it is no longer manufactured or available in the United States, the combination of two ester anesthetics, propoxycaine + procaine, was worthy of consideration for inclusion in a dentist’s armamentarium of local anesthetics. It was useful when the amides were absolutely contraindicated (e.g., because of documented allergy [although this is an extremely unlikely occurrence]), or when several amide local anesthetics failed to provide clinically adequate anesthesia. Until its removal from the U.S. market in January 1996, the combination of procaine and propoxycaine was the only ester local anesthetic available in dental cartridge form.
A dose of 0.4% propoxycaine/2% procaine with 1 : 20,000 levonordefrin (United States) or with 1 : 30,000 norepinephrine (Canada) provided approximately 40 minutes of pulpal anesthesia and 2 to 3 hours of soft tissue anesthesia. The use of norepinephrine in local anesthetic solutions is no longer recommended, especially in areas where prolonged ischemia can lead to tissue necrosis. In the oral cavity, this is most likely to be seen in the palate.
Maximum Recommended Dose
The manufacturer’s maximum recommended dose was 3.0 mg/lb or 6.6 mg/kg of body weight for the adult patient.10 For children, a dose of 3.0 mg/lb was recommended up to a maximum of five cartridges.
Amide-Type Local Anesthetics
Pertinent Information
Potency: 2 (compared with procaine) (procaine = 1; lidocaine remains the standard of comparison [lidocaine = 1] for all local anesthetics).
Metabolism: In the liver, by the microsomal fixed-function oxidases, to monoethylglyceine and xylidide; xylidide is a local anesthetic that is potentially toxic11 (see Fig. 2-3).
Maximum Recommended Dose
The maximum recommended dose by the FDA of lidocaine with or without epinephrine is 3.2 mg/lb or 7.0 mg/kg of body weight for the adult and pediatric patient, not to exceed a an absolute maximum dose of 500 mg12 (Table 4-5).
Comments
Lidocaine HCl was synthesized in 1943 and in 1948 was the first amide local anesthetic to be marketed. Its entry into clinical practice transformed dentistry; it replaced procaine (Novocain) as the drug of choice for pain control. Compared with procaine, lidocaine possesses a significantly more rapid onset of action (3 to 5 minutes vs. 6 to 10 minutes), produces more profound anesthesia, has a longer duration of action, and has greater potency.
Allergy to amide local anesthetics is virtually nonexistent; true, documented, and reproducible allergic reactions are extremely rare, although possible.13-18 This is a major clinical advantage of lidocaine (and all amides) over ester-type local anesthetics.8
Within only a few years of its introduction, lidocaine had replaced procaine as the most widely used local anesthetic in both medicine and dentistry—a position it maintains today in most countries. It represents the “gold standard,” the drug against which all new local anesthetics are compared.
Lidocaine HCl is available in North America in two formulations: 2% with epinephrine 1 : 50,000, and 2% with epinephrine 1 : 100,000 (Fig. 4-2). A 2% lidocaine with epinephrine 1 : 300,000 formulation is available in some countries (although not in North America as of August 2011). 2% lidocaine without epinephrine (2% “plain”) is no longer available in dental cartridges in North America.
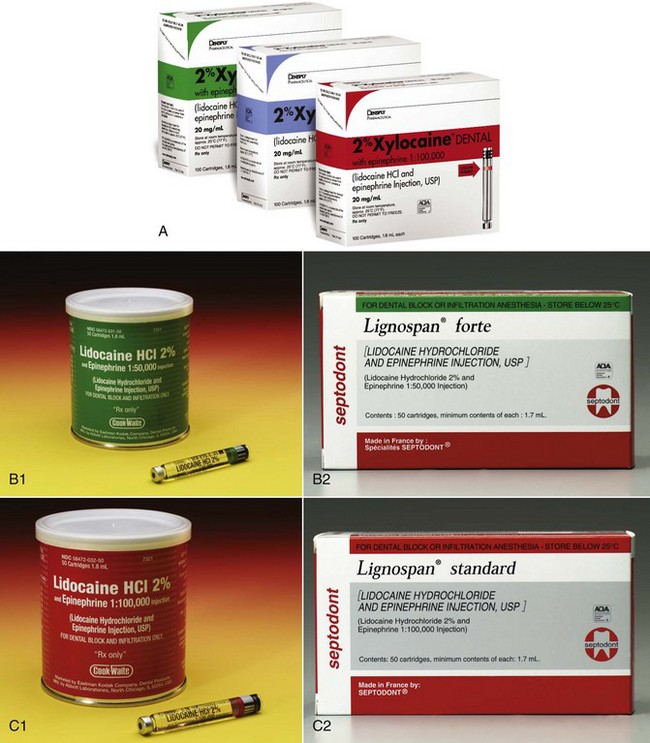
Figure 4-2 A, Lidocaine 2%. B, Lidocaine 2% with epinephrine 1 : 50,000. C, Lidocaine with epinephrine 1 : 100,000. (A, Courtesy Dentsply, York, Pa. B and C, Courtesy Eastman Kodak, New York, NY, and Septodont, New Castle, Del.)
Two Percent Lidocaine HCl Without a Vasoconstrictor (Lidocaine Plain): Its vasodilating properties severely limit the duration and the depth of pulpal anesthesia (5 to 10 minutes). This vasodilatory effect leads to (1) higher blood levels of lidocaine, with an attendant increase in the risk of adverse reactions, along with (2) increased perfusion in the area of drug deposition. Few clinical indications are known for the use of 2% lidocaine without a vasoconstrictor in the typical dental practice. As of August 2011, 2% lidocaine without epinephrine (2% “plain”) was no longer available in dental cartridges in North America.
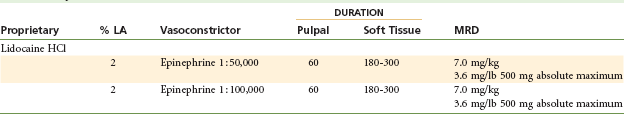
Two Percent Lidocaine With Epinephrine 1 : 50,000: (Table 4-6)
TABLE 4-6
Lidocaine 2% With Epinephrine 1 : 50,000*†
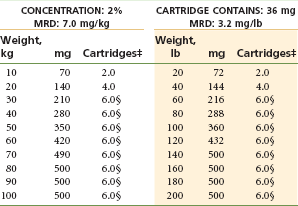
MRD, Maximum recommended dose.
*As with all local anesthetics, the dose varies, depending on the area to be anesthetized, the vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dose needed to provide effective anesthesia should be administered.
†Doses indicated are the maximum suggested for normal healthy individuals (ASA 1); they should be decreased for debilitated or elderly patients.
‡Rounded down to the nearest half-cartridge.
§200 µg of epinephrine is the dose-limiting factor (1 : 50,000 contains 36 µg/cartridge).
Inclusion of epinephrine produces a decrease in blood flow (perfusion), leading to decreased bleeding in the area of drug administration caused by the α-stimulating actions of epinephrine. Because of this decrease in perfusion, the local anesthetic is absorbed into the cardiovascular system more slowly (thereby remaining at the site of administration longer, it is hoped near the nerve), leading to an increase in both depth and duration of anesthesia: approximately 60 minutes of pulpal anesthesia and 3 to 5 hours of soft tissue anesthesia. The resultant blood level of local anesthetic is also decreased. The 1 : 50,000 epinephrine concentration is equal to 20 µg/mL, or 36 µg per cartridge. For patients weighing more than 45 kg (100 lb), the limiting factor in determining the MRD of this local anesthetic combination is the maximum epinephrine dose of 200 µg for the healthy patient. The MRD for epinephrine-sensitive individuals (e.g., certain cardiovascularly compromised patients [American Society of Anesthesiologists Physical Status classification system {ASA} 3] and clinically hyperthyroid patients [ASA 3]) is 40 µg per appointment. This is equivalent to about one cartridge of 1 : 50,000 epinephrine (see Chapter 20).
The only recommended use of 2% lidocaine with a 1 : 50,000 epinephrine concentration is for hemostasis (a situation wherein only small volumes are infiltrated directly into the surgical site).
Two Percent Lidocaine With Epinephrine 1 : 100,000: (Table 4-7)
TABLE 4-7
Lidocaine 2% With Epinephrine 1 : 100,000*†‡
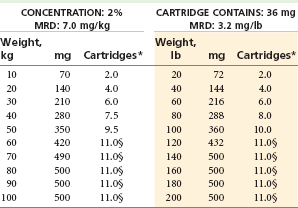
MRD, Maximum recommended dose.
*As with all local anesthetics, the dose varies, depending on the area to be anesthetized, the vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dose needed to provide effective anesthesia should be administered.
†Doses indicated are the maximum suggested for normal healthy individuals (ASA 1); they should be decreased for debilitated or elderly patients.
‡Rounded to the nearest half-cartridge.
§200 µg of epinephrine is the dose-limiting factor (1 : 100,000 contains 18 µg/cartridge).
Administration of 2% lidocaine with epinephrine 1 : 100,000 decreases blood flow into the area of injection. Duration of action is increased: approximately 60 minutes of pulpal anesthesia and 3 to 5 hours of soft tissue anesthesia. In addition to the lower blood level of lidocaine, less bleeding occurs in the area of drug administration. The epinephrine dilution is 10 µg/mL, or 18 µg per cartridge. Epinephrine-sensitive patients (see earlier discussion for lidocaine with 1 : 50,000 epineprhine) should be limited to two cartridges of 1 : 100,000 epinephrine per appointment.
The duration and depth of pulpal anesthesia attained with both lidocaine-epinephrine solutions (1 : 50,000 and 1 : 100,000) are equivalent. Each may provide approximately 60 minutes of pulpal anesthesia in ideal circumstances and soft tissue anesthesia of 3 to 5 hours’ duration. Indeed, 2% lidocaine with 1 : 200,000 or 1 : 300,000 epinephrine provides the same duration of pulpal and soft tissue anesthesia, although not the same level of hemostasis.19
In terms of duration and depth of anesthesia for most procedures in a typical dental patient, 2% lidocaine with 1 : 100,000 epinephrine is preferred to 2% lidocaine with 1 : 50,000 epinephrine. Both formulations provide equal duration and depth, but the 1 : 100,000 solution contains only half as much epinephrine as the 1 : 50,000 solution. Although the dose of epinephrine in the 1 : 50,000 solution is not dangerous to most patients, ASA-3 and -4 risks with histories of cardiovascular disorders might prove overly sensitive to these concentrations. Also, an elderly patient is likely to be more hyper-responsive to vasoconstrictors. In these individuals, the more dilute formulation (1 : 100,000 or 1 : 200,000) should be used.
For hemostasis in procedures in which bleeding is definitely or potentially a problem, 2% lidocaine with 1 : 50,000 epinephrine is recommended because it decreases bleeding (during periodontal surgery) by 50% compared with a 1 : 100,000 epinephrine dilution.20 Vasoconstrictors act directly at the site of administration to decrease tissue perfusion, and the 1 : 50,000 solution provides excellent hemostatic action. The 1 : 100,000 dilution also may be used for hemostasis, but it is not as effective. Rebound vasodilation occurs with both 1 : 50,000 and 1 : 100,000 epinephrine concentrations as the tissue concentration of epinephrine decreases. Minimum volumes of solution should be administered to provide excellent hemostasis.
Signs and symptoms of lidocaine toxicity (overdose) may be the same (central nervous system [CNS] stimulation followed by CNS depression) as those described in Chapter 2. However, the stimulatory phase may be brief or may not develop at all.21 Although muscle tremor and seizures commonly occur with overly high lidocaine blood levels, the first signs and symptoms of lidocaine overdose may include drowsiness, leading to loss of consciousness and respiratory arrest.
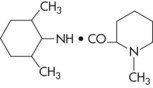
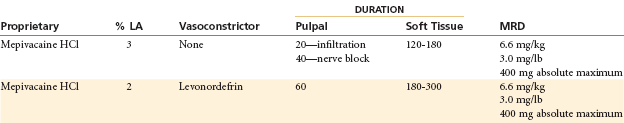
Mepivacaine HCl
Prepared by: A.F. Ekenstam, 1957; introduced into dentistry in 1960 as a 2% solution containing the synthetic vasopressor levonordefrin, and in 1961 as a 3% solution without a vasoconstrictor.
Metabolism: In the liver, by microsomal fixed-function oxidases. Hydroxylation and N-demethylation play important roles in the metabolism of mepivacaine.
Vasodilating Properties: Mepivacaine produces only slight vasodilation. The duration of pulpal anesthesia with mepivacaine without a vasoconstrictor is 20 to 40 minutes (lidocaine without a vasoconstrictor is but 5 to 10 minutes; procaine without a vasoconstrictor may produce effects up to 2 minutes).
Maximum Recommended Dose
MRD is 6.6 mg/kg or 3.0 mg/kg of body weight, not to exceed 400 mg22 (Table 4-8).
Comments
The milder vasodilating property of mepivacaine leads to a longer duration of pulpal anesthesia than is observed with most other local anesthetics when administered without a vasoconstrictor. Mepivacaine 3% plain provides 20 to 40 minutes of pulpal anesthesia (20 minutes via infiltration; 40 minutes via nerve block) and approximately 2 to 3 hours of soft tissue anesthesia.
Three Percent Mepivacaine Without a Vasoconstrictor: (Fig. 4-3 and Table 4-9)
TABLE 4-9
Mepivacaine 3% Without Vasoconstrictor*†
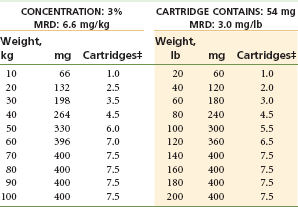
MRD, Maximum recommended dose.
*As with all local anesthetics, the dose varies, depending on the area to be anesthetized, the vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dose needed to provide effective anesthesia should be administered.
†Doses indicated are the maximum suggested for normal healthy individuals (ASA 1); they should be decreased for debilitated or elderly patients.
‡Rounded down to the nearest half-cartridge.
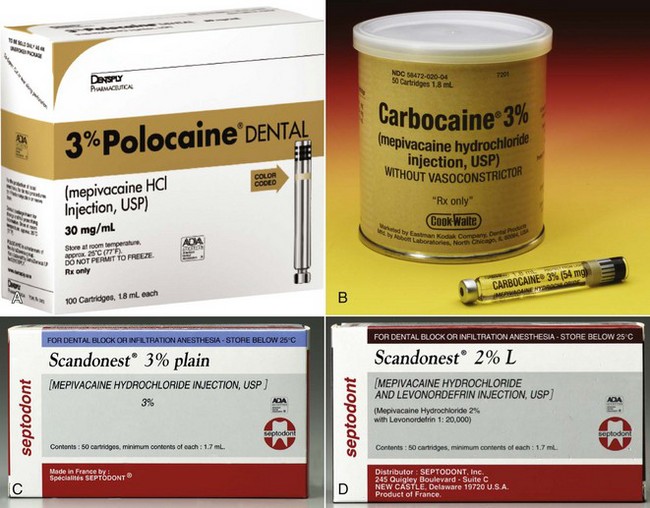
Figure 4-3 A through C, Mepivacaine 3%. D, Mepivacaine 2% with levonordefrin 1 : 20,000. (A, Courtesy Dentsply, York, Pa. B, Courtesy Eastman Kodak, New York, NY. D, Courtesy Septodont, New Castle, Del.)
This is recommended for patients in whom a vasoconstrictor is not indicated and for dental procedures requiring neither lengthy nor profound pulpal anesthesia. Mepivacaine plain is the most used local anesthetic in pediatric patients when the treating doctor is not a pediatric dentist (e.g., is a general practitioner) and often is quite appropriate in the management of geriatric patients.
Two Percent Mepivacaine With a Vasoconstrictor, Levonordefrin 1 : 20,000: (Table 4-10)
TABLE 4-10
Mepivacaine 2% With Vasoconstrictor*†
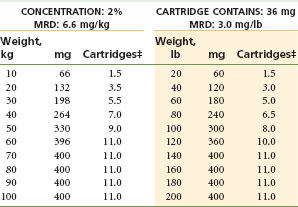
MRD, Maximum recommended dose.
*As with all local anesthetics, the dose varies, depending on the area to be anesthetized, the vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dose needed to provide effective anesthesia should be administered.
†Doses indicated are the maximum suggested for normal healthy individuals (ASA 1); they should be decreased for debilitated or elderly patients.
‡Rounded down to the nearest half-cartridge.
This solution provides depth and duration of pulpal (hard tissue) and total (soft tissue) anesthesia similar to those observed with lidocaine-epinephrine solutions. Pulpal anesthesia of approximately 60 minutes’ duration and soft tissue anesthesia of 3 to 5 hours are to be expected. Mepivacaine is available in combination with levonordefrin (1 : 20,000). Where hemostasis is desired, epinephrine is preferred to levonordefrin.
The incidence of true, documented, and reproducible allergy to mepivacaine, an amide local anesthetic, is virtually nonexistent.
Signs and symptoms of mepivacaine overdose usually follow the more typical pattern of CNS stimulation followed by depression. Although possible, absence of stimulation with immediate CNS depression (e.g., drowsiness and unconsciousness, as is seen more commonly with lidocaine) is rare with mepivacaine.
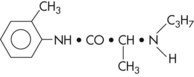
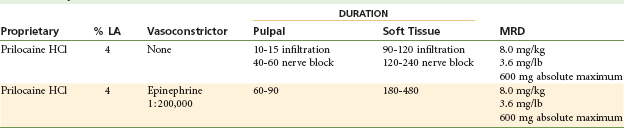
Prilocaine HCl
Metabolism: The metabolism of prilocaine differs significantly from that of lidocaine and mepivacaine. Because it is a secondary amine, prilocaine is hydrolyzed straightforwardly by hepatic amidases into orthotoluidine and N-propylalanine. Carbon dioxide is a major end product of prilocaine biotransformation. The efficiency of the body’s degradation of prilocaine is demonstrated by the extremely small fraction of intact prilocaine recoverable in the urine.23 Orthotoluidine can induce the formation of methemoglobin, producing methemoglobinemia if large doses are administered. Minor degrees of methemoglobinemia have been observed following both benzocaine and lidocaine administration,24,25 but prilocaine consistently reduces the blood’s oxygen-carrying capacity, at times sufficiently to cause observable cyanosis.26,27 Limiting the total prilocaine dose to 600 mg (FDA recommendation) avoids symptomatic cyanosis. Methemoglobin blood levels less than 20% usually do not produce clinical signs or symptoms (which include grayish or slate blue cyanosis of the lips, mucous membranes, and nail beds and [infrequently] respiratory and circulatory distress). Methemoglobinemia may be reversed within 15 minutes with administration of 1 to 2 mg/kg body weight of 1% methylene blue solution intravenously over a 5-minute period.25 The mechanism of methemoglobin production is discussed in Chapter 10. Prilocaine undergoes biotransformation more rapidly and completely than lidocaine; this takes place not only in the liver but also to a lesser degree in the kidneys and lungs.27 Plasma levels of prilocaine decrease more rapidly than those of lidocaine.28 Prilocaine thus is considered to be less toxic systemically than comparably potent local anesthetic amides.29 Signs of CNS toxicity after prilocaine administration in humans are briefer and less severe than after the same intravenous (IV) dose of lidocaine.30
Excretion: Prilocaine and its metabolites are excreted primarily via the kidneys. Renal clearance of prilocaine is faster than for other amides, resulting in its faster removal from the circulation.31
Vasodilating Properties: Prilocaine is a vasodilator. It produces greater vasodilation than is produced by mepivacaine but less than lidocaine and significantly less than procaine.
Topical Anesthetic Action: Not in clinically acceptable concentrations.
Prilocaine, in its uncharged base form, is an integral part of EMLA cream (eutectic mixture of the local anesthetics lidocaine and prilocaine), a formulation that permits anesthetics to penetrate the imposing anatomic barrier of intact skin. EMLA cream is used to provide topical anesthesia of skin before venipuncture and other painful cosmetic procedures.33,34
Maximum Recommended Dose
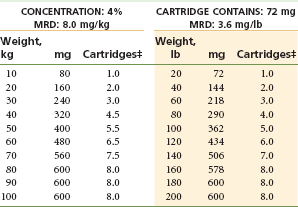
The MRD for prilocaine is 8.0 mg/kg or 3.6 mg/lb of body weight for the adult patient, to a maximum recommended dose of 600 mg (Table 4-11).31
Comments
Clinical actions of prilocaine plain (Fig. 4-4) vary significantly with the type of injection technique used. Although true for all anesthetics, the variation between supraperiosteal infiltration and nerve block is more pronounced with prilocaine plain (and mepivacaine plain). Infiltration provides short durations of pulpal (10 to 15 minutes) and soft tissue ( to 2 hours) anesthesia, whereas regional nerve block (e.g., inferior alveolar nerve block) provides pulpal anesthesia for up to 60 minutes (commonly 40 to 60 minutes) and soft tissue anesthesia for 2 to 4 hours.32 Thus prilocaine plain frequently is able to provide anesthesia that is equal in duration to that attained with lidocaine or mepivacaine with a vasoconstrictor.
to 2 hours) anesthesia, whereas regional nerve block (e.g., inferior alveolar nerve block) provides pulpal anesthesia for up to 60 minutes (commonly 40 to 60 minutes) and soft tissue anesthesia for 2 to 4 hours.32 Thus prilocaine plain frequently is able to provide anesthesia that is equal in duration to that attained with lidocaine or mepivacaine with a vasoconstrictor.
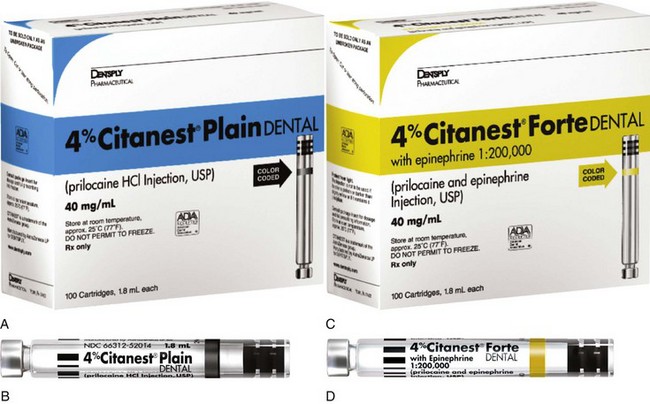
Figure 4-4 A and B, Prilocaine 4%. C and D, Prilocaine 4% with epinephrine 1 : 200,000. (Courtesy Dentsply, York, Pa.)
Clinical actions of prilocaine with 1 : 200,000 epinephrine are not as dependent on anesthetic technique. Prilocaine with epinephrine provides lengthy anesthesia while offering a less concentrated epinephrine dilution: 1 : 200,000. Pulpal anesthesia of 60 to 90 minutes’ duration and soft tissue anesthesia of 3 to 8 hours are common. The cartridge contains 9 µg of epinephrine; therefore epinephrine-sensitive individuals, such as the ASA 3 cardiovascular disease patient, may receive up to four cartridges (36 µg) of prilocaine with epinephrine.
In epinephrine-sensitive patients requiring prolonged pulpal anesthesia (≥60 minutes), prilocaine plain or with 1 : 200,000 epinephrine is strongly recommended. It is rapidly biotransformed and, for this reason, is considered to be a safe local anesthetic (e.g., lower toxicity).28
Prilocaine is relatively contraindicated in patients with idiopathic or congenital methemoglobinemia, hemoglobinopathies (sickle cell anemia), anemia, or cardiac or respiratory failure evidenced by hypoxia, because methemoglobin levels are increased, decreasing oxygen-carrying capacity. Prilocaine administration is also relatively contraindicated in patients receiving acetaminophen or phenacetin, both of which produce elevations in methemoglobin levels (Table 4-12).
TABLE 4-12
Prilocaine 4% With and Without Vasoconstrictor*†
MRD, Maximum recommended dose.
*As with all local anesthetics, the dose varies, depending on the area to be anesthetized, the vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dose needed to provide effective anesthesia should be administered.
†Doses indicated are the maximum suggested for normal healthy individuals (ASA 1); they should be decreased for debilitated or elderly patients.
‡Rounded to the nearest half-cartridge.
It has been claimed that prilocaine HCl 4% solution (with or without vasopressor) is associated with a higher risk of paresthesia, primarily of the lingual nerve, than other anesthetic formulations following inferior alveolar nerve block.34,35 Although the “evidence” remains anecdotal, it appears that this drug, as formulated in North America [as a 4% solution], might be more neurotoxic than other commonly used local anesthetic formulations.36 This question will be discussed in greater detail in Chapter 17.
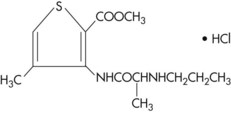
Articaine HCl
Classification: Hybrid molecule. Classified as an amide; however, it possesses both amide and ester characteristics.
Metabolism: Articaine is the only amide-type local anesthetic that contains a thiophene group. Because articaine HCl is the only widely used amide-type local anesthetic that contains an ester group, biotransformation of articaine HCl occurs in both plasma (hydrolysis by plasma esterase) and liver (hepatic microsomal enzymes). Degradation of articaine HCl is initiated by hydrolysis of the carboxylic acid ester groups to obtain free carboxylic acid.37 Its primary metabolite, articainic acid, is pharmacologically inactive, undergoing additional biotransformation to form articainic acid glucuronide.37 Additional metabolites have been detected in animal studies.38 From this point, the reaction can follow several pathways: cleavage of carboxylic acid, formation of an acid amino group by internal cyclization, and oxidation.
Excretion: Via the kidneys; approximately 5% to 10% unchanged, approximately 90% metabolites (M1 at 87%, M2 at 2%).
Vasodilating Properties: Articaine has a vasodilating effect equal to that of lidocaine. Procaine is slightly more vasoactive.
pH of Plain Solution: Not available in North America (4% articaine HCl “plain” is available in Germany).
Onset of Action: Articaine 1 : 200,000, infiltration 1 to 2 minutes, mandibular block 2 to 3 minutes; articaine 1 : 100,000, infiltration 1 to 2 minutes, mandibular block 2 to 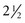 minutes.
minutes.
Effective Dental Concentration: 4% with 1 : 100,000 or 1 : 200,000 epinephrine. Articaine HCl is available with epinephrine 1 : 400,000 in Germany.
Anesthetic Half-Life: 0.5 hours [27 minutes].39
Maximum Recommended Dose
The FDA maximum recommended dose is 7.0 mg/kg or 3.2 mg/lb of body weight for the adult patient (Tables 4-13 and 4-14).22,40
TABLE 4-14
Articaine 4% With Epinephrine 1 : 100,000 or 1 : 200,000*†
MRD, Maximum recommended dose.
*As with all local anesthetics, the dose varies, depending on the area to be anesthetized, the vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dose needed to provide effective anesthesia should be administered.
†Doses indicated are the maximum suggested for normal healthy individuals (ASA 1); they should be decreased for debilitated or elderly patients.
‡Rounded to the nearest half-cartridge.
Comments
Originally known as carticaine, the generic nomenclature of this local anesthetic was changed in 1984 to articaine. Literature appearing before 1984 should be reviewed under the original name.
Articaine is the only anesthetic of the amide type to possess a thiophene ring as its lipophilic moiety. It has many of the physicochemical properties of other local anesthetics, with the exception of the aromatic moiety and the degree of protein binding.
Articaine has been available in Europe since 1976 and in Canada since 1984 in two formulations: 4% with 1 : 100,000 epinephrine and 4% with 1 : 200,000 epinephrine (Fig. 4-5). In 2000 the FDA approved articaine HCl with epinephrine 1 : 100,000 for marketing in the United States.41-43 The formulation with 1 : 100 : 000 epinephrine provides between 60 and 75 minutes of pulpal anesthesia; the 1 : 200,000 formulation, approximately 45 to 60 minutes.44,45
Figure 4-5 Articaine 4% with epinephrine 1 : 100,000 and 1 : 200,000. (Courtesy Septodont, New Castle, Del.)
Since its introduction into the U.S. dental market in June 2000, articaine has steadily become increasingly popular. As of May 2011 articaine was the second most used dental local anesthetic in the United States (≈40% market share).46 Articaine, as the newest local anesthetic drug marketed, has been the subject of intense discussion and of many (anecdotal) claims made by dentists, some good (faster onset, increased success rates; “don’t miss as often”), some bad (increased risk of paresthesia). It has been claimed that articaine is able to diffuse through soft and hard tissues more reliably than other local anesthetics.47,48 Clinically, it is claimed that following maxillary buccal infiltration, articaine on occasion may provide palatal soft tissue anesthesia, obviating the need for palatal injection, which, in many hands, is traumatic.48
Some initial claims about articaine have been shown to be true, specifically, the significant success of articaine administered by buccal infiltration in the mandible of adult patients. 49-55 This is discussed more fully in Chapter 20.
The success of articaine in the United States mirrors its success elsewhere. In Germany, the first country to have articaine (1976), by 1989 it was used by 71.7% of German dentists,56 and by 2010 it commanded 95% of the dental local anesthetic market.57 Articaine has become the leading local anesthetic in Canada, which acquired it in 1983; in the United States, where articaine has been available since June of 2000, it presently (May 2011) commands 40% of the local anesthetic market.46
Reports of paresthesia following local anesthetic administration became more frequent after the introduction of articaine into the United States. An overwhelming majority of reported cases occurred following inferior alveolar nerve block and primarily involved the lingual nerve. The question of paresthesia related to local anesthetic drug administration is addressed in depth in Chapter 17.
In previous editions of this textbook, methemoglobinemia was listed as a potential side effect of administration of large doses of articaine.58 Such reactions had been noted after the IV administration of articaine for regional anesthetic purposes; however, no cases have ever been reported when articaine was administered in the usual manner and volume for dental procedures.
Articaine HCl with epinephrine is contraindicated in persons with known sensitivity to amide-type local anesthetics (few to none) and in persons with sulfite sensitivity (such as some asthmatic patients with allergic-type asthma, as epinephrine-containing LA formulations contain the antioxidant Na metabisulfite). Articaine HCl should be used with caution in persons with hepatic disease and significant impairment in cardiovascular function because amide-type local anesthetics undergo biotransformation in the liver and possess myocardial depressant properties. Articaine is listed by the U.S. FDA as a Class C drug during pregnancy. Use with caution in females who are breast-feeding because it is not known whether articaine is excreted in milk.59 Administration to children younger than 4 years is not recommended because insufficient data are available to support such usage.
Cartridges of articaine marketed in the United States are listed as containing “1.7 mL” (Fig. 4-6), in contrast to other local anesthetic cartridges, which are labeled as “1.8 mL.” Some might interpret this to mean that the cartridge consists of 68 mg. This is incorrect. Articaine HCl cartridges are identical in all ways to other dental cartridges. However, as discussed earlier in this chapter, a change was made to labeling, not to content.
Bupivacaine HCl
Chemical Formula: 1-Butyl-2′,6′-pipecoloxylidide hydrochloride; structurally related to mepivacaine except for a butyl group replacing a methyl group.
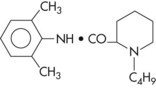
Vasodilating Properties: Relatively significant: greater than those of lidocaine, prilocaine, and mepivacaine, yet considerably less than those of procaine.
Maximum Recommended Dose
The FDA maximum recommended dose of bupivacaine is 90 mg. There is no recommended dosage for bupivacaine based on body weight in the United States (Table 4-15).60 In Canada, the maximum dose is based on 2.0 mg/kg (0.9 mg/lb).
Comments
Bupivacaine has been available in cartridge form since February 1982 in Canada and July 1983 in the United States. Bupivacaine is available as a 0.5% solution with 1 : 200,000 epinephrine (Fig. 4-7); there are two primary indications for its utilization in dentistry:
1. Lengthy dental procedures for which pulpal (deep) anesthesia in excess of 90 minutes is necessary (e.g., full mouth reconstruction, implant surgery, extensive periodontal procedures)
2. Management of postoperative pain (e.g., endodontic, periodontal, postimplant, surgical)
The patient’s requirement for postoperative opioid analgesics is considerably lessened when bupivacaine is administered for pain control.61 For postoperative pain control after a short surgical procedure (<30 minutes), bupivacaine may be administered at the start of the procedure; however, for postoperative pain control after lengthy surgical procedures, it might be reasonable to administer bupivacaine at the conclusion of the procedure, shortly before the patient’s discharge from the office.
A regimen for management of postsurgical pain has been developed that is clinically quite effective.62-64 It suggests the pretreatment administration of one oral dose of a nonsteroidal anti-inflammatory drug (NSAID), followed by administration of any suitable (e.g., intermediate-duration) local anesthetic to manage periprocedural pain. A long-duration local anesthetic (bupivacaine) is administered immediately before patient discharge (if deemed necessary), with the patient continuing to take the oral dose of NSAID every “x” hours as indicated (not “prn pain”) for “y” number of days. The requirement for opioid agonist analgesics is significantly diminished with this protocol. Hargreaves demonstrated that the preoperative oral dose of NSAID is not necessary as long as the initial oral dose can be ingested within 1 hour of the start of the surgical procedure.65
Onset of anesthesia with bupivacaine is commonly delayed for from 6 to 10 minutes, a fact that is understandable in view of its pKa of 8.1. If this occurs, it may be advisable, at subsequent appointments, to initiate procedural pain control with a more rapid-acting amide (e.g., articaine, mepivacaine, lidocaine, prilocaine), which provides clinically acceptable pain control within a few moments, allowing the procedure to commence more promptly. Follow this with an injection of bupivacaine for long-duration anesthesia.
Bupivacaine is not recommended in younger patients or in those for whom the risk of postoperative soft tissue injury produced by self-mutilation is increased, such as physically and mentally disabled persons. Bupivacaine is rarely indicated in children because pediatric dental procedures are usually of short duration.
Etidocaine HCl
Chemical Formula: 2-(N-Ethylpropylamino) butyro-2,6-xylidide hydrochloride; structurally similar to lidocaine.
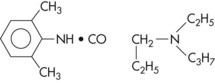
Dental cartridges of etidocaine HCl with epinephrine 1 : 200,000 were withdrawn from the North American market in 2002. For a detailed description of etidocaine HCl, the reader is referred to the 5th edition of this textbook.66
Anesthetics For Topical Application
Use of topically applied local anesthetics is an important component of the atraumatic administration of intraoral local anesthesia (see Chapter 11). Conventional topical anesthetics are unable to penetrate intact skin but do diffuse through abraded skin (e.g., sunburn) and any mucous membranes.
The concentration of a local anesthetic applied topically is typically greater than that of the same local anesthetic administered by injection. The higher concentration facilitates diffusion of the drug through the mucous membrane. Higher concentration also increases the risk of toxicity, both locally to tissues and systemically if the drug is efficiently absorbed.67 Because topical anesthetic formulations do not contain vasoconstrictors and local anesthetics have vasodilatory properties, vascular absorption of some topical formulations is rapid, and blood levels may quickly reach those achieved by direct IV administration.67
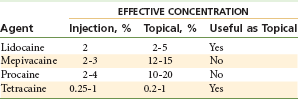
Many local anesthetics used effectively via injection prove ineffective when applied topically (e.g., articaine HCl, mepivacaine HCl, prilocaine HCl, procaine HCl) because the concentrations necessary to produce anesthesia via topical application are high, with significantly increased overdose and local tissue toxicity potential (Table 4-16).
As a general rule, topical anesthetics are effective only on surface tissues (2 to 3 mm). Tissues deep to the area of application are poorly anesthetized, if at all. However, surface anesthesia does allow for atraumatic needle penetration of the mucous membrane.68,69
The topical anesthetics benzocaine and lidocaine base (not the HCl form used by injection) are insoluble in water. However, they are soluble in alcohol, propylene glycol, polyethylene glycol, and other vehicles suitable for surface application. The base forms of benzocaine and lidocaine are slowly absorbed into the cardiovascular system and therefore are less likely to produce an overdose reaction following typical dental application.
Some topical anesthetics are marketed in pressurized spray containers. Although they are no more effective than other forms, it is difficult to control the amount of anesthetic expelled and to confine it to the desired site of application. Spray devices that do not deliver measured doses should not be used intraorally.
Benzocaine
Benzocaine (ethyl p-aminobenzoate) is an ester local anesthetic:
2. Poor absorption into the cardiovascular system
3. Systemic toxic (overdose) reactions virtually unknown
4. Remains at the site of application longer, providing a prolonged duration of action
6. Localized allergic reactions may occur after prolonged or repeated use. Although allergic reaction to ester anesthetics is rare, ester local anesthetics are more allergenic than amide local anesthetics.70
7. Inhibits the antibacterial action of sulfonamides71
8. Availability (Fig. 4-8): Benzocaine is available in the following formulations in numerous dosages: aerosol, gel, gel patch, ointment, and solution.
Benzocaine, Butamben, and Tetracaine HCl
Aerosol, gel, ointment, and solution: benzocaine 140 mg/mL; butamben 20 mg/mL; tetracaine HCl, 20 mg/mL.
Cocaine Hydrochloride
Cocaine hydrochloride (benzoylmethylecgonine hydrochloride) occurs naturally as a white crystalline solid that is highly soluble in water:
1. Used exclusively via topical application. Injection of cocaine is contraindicated because of the ready availability of more effective and much less toxic local anesthetics. Cocaine is an ester local anesthetic.
2. Onset of topical anesthetic action is quite rapid, usually occurring within 1 minute.
3. Duration of anesthetic action may be as long as 2 hours.
4. It is absorbed rapidly but eliminated slowly (elimination half-life = 42 minutes).
5. It undergoes metabolism in the liver and plasma.
6. Unmetabolized cocaine may be found in the urine.
7. Cocaine is the only local anesthetic consistently demonstrated to produce vasoconstriction, which develops as a result of its ability to potentiate the actions of endogenous epinephrine and norepinephrine.27 Addition of vasoconstrictors to cocaine therefore is unnecessary and is potentially dangerous, increasing the likelihood of dysrhythmias, including ventricular fibrillation.
8. Classified as a Schedule II drug under the Controlled Substances Act. Repeated use results in psychological dependence and tolerance.
9. Overdose of cocaine is not uncommon following illicit use, primarily because the drug is readily absorbed from the nasal mucosa and its dosage is not carefully monitored.
10. Clinical manifestations of mild overdose include euphoria, excitement, restlessness, tremor, hypertension, tachycardia, and tachypnea.
11. Clinical manifestations of acute cocaine overdose include excitement, restlessness, confusion, tremor, hypertension, tachycardia, tachypnea, nausea and vomiting, abdominal pain, exophthalmos, and mydriasis; these are followed by depression (CNS, cardiovascular, respiratory) and death from respiratory arrest.
12. It is available in concentrations ranging from 2% to 10%.
13. It is recommended that the concentration of cocaine not exceed 4% for topical application to oral mucous membranes.
14. Solutions of cocaine are unstable and deteriorate on standing.
15. Because of the extreme abuse potential of cocaine, its use as a topical anesthetic in dentistry is not recommended.
16. Topically applied cocaine is used occasionally before nasal-endotracheal intubation is performed in the operating theater to minimize bleeding from this highly vascular region and pain as the endotracheal tube is passed.
Dyclonine Hydrochloride
Dyclonine hydrochloride (4′-butoxy-3-piperidinopropiophenone hydrochloride) is a ketone derivative without an ester or amide linkage that may be used in patients who are allergic to the common anesthetics. Dyclonine offers advantages over other topical anesthetic agents. Extensive experience with the topical preparation has shown it to be effective and safe.72 Dyclonine is marketed in 0.5% and 1% solutions:
1. Cross-sensitization with other local anesthetics does not occur; therefore dyclonine may be used in patients with known sensitivities to local anesthetics of other chemical groups.
3. Potency equal to that of cocaine
4. Onset of anesthesia slow, requiring up to 10 minutes
5. Duration of anesthesia may be as long as 1 hour.
6. Systemic toxicity is extremely low, primarily because of the agent’s poor water solubility.
7. Not indicated for use by injection; irritating to tissues at the site of application
8. A 0.5% solution is used in dentistry. Maximum recommended dose is 200 mg (40 mL of a 0.5% solution).
9. Dyclonine was available as Dyclone in a 0.5% solution: each 100 mL of solution contained 500 mg of dyclonine HCl, 300 mg of chlorobutanol as a preservative, sodium chloride for isotonicity, and hydrochloric acid, as needed, to adjust pH. The Dyclone brand was withdrawn from the North American market in 2001.
EMLA (Eutectic Mixture of Local Anesthetics)
EMLA cream (composed of lidocaine 2.5% and prilocaine 2.5%) is an emulsion in which the oil phase is a eutectic mixture of lidocaine and prilocaine in a ratio of 1 : 1 by weight. It was designed as a topical anesthetic able to provide surface anesthesia for intact skin (other topical anesthetics do not produce anesthesia on intact skin, only abraded skin), and as such is used primarily before painful procedures such as venipuncture and other needle insertions. Originally marketed for use in pediatrics, EMLA has gained popularity among needle-phobic adults and persons having other superficial, but painful, procedures performed (e.g., hair removal).
EMLA use has become almost routine during circumcision,73 during leg ulcer débridement,74 and in gynecologic procedures.75 Because intact skin is a barrier to drug diffusion, EMLA must be applied 1 hour before the procedure. Satisfactory numbing of the skin occurs 1 hour after application, reaches a maximum at 2 to 3 hours, and lasts for 1 to 2 hours after removal.
EMLA is supplied in a 5-g or 30-g tube or as an EMLA anesthetic disc. The EMLA disc is a white, round, cellulose disc preloaded with EMLA, packaged in protective laminate foil surrounded by adhesive tape.
EMLA is contraindicated for use in patients with congenital or idiopathic methemoglobinemia, infants younger than 12 months who are receiving treatment with methemoglobin-inducing agents, and patients with known sensitivity to amide-type local anesthetics or any other component of the product.76
Because EMLA is effective in penetrating intact skin, its ability to produce effective topical anesthesia in the oral cavity seems obvious. Although the drug package insert76 stated originally that “EMLA is not recommended for use on mucous membranes,” subsequent clinical trials have demonstrated satisfactory results.77-80
Bernardi and associates demonstrated statistically significant analgesia in 52 dental patients requiring removal of metal maxillary or mandibular splints used to contain fractures.77 The authors concluded, “the analgesic effect[s] of EMLA cream on oral mucosa allow the application of contact anesthesia to be broadened to oral surgery and dentistry, limiting it to those procedures that do not involve deep tissues and only require short-term anesthesia.”77
Munshi and associates reported on the use of EMLA cream in 30 pediatric patients undergoing a variety of clinical procedures, including extraction of mobile primary teeth and root stumps and pulpal therapy procedures in the primary teeth, in which EMLA is used as the sole anesthetic agent.78 Results show that use of EMLA could eliminate to some extent use of the needle in procedures performed in pediatric dentistry.79,80
Lidocaine
Lidocaine is available in two forms for topical application: lidocaine base, which is poorly soluble in water, is used as a 5% concentration, and is indicated for use on ulcerated, abraded, or lacerated tissue; and lidocaine hydrochloride, its water-soluble preparation, which is used as a 2% concentration. The water-soluble form of lidocaine penetrates tissue more efficiently than the base form. However, systemic absorption is also greater, providing greater risk of toxicity than the base form:
1. Lidocaine is an amide local anesthetic with an exceptionally low incidence of allergic reactions.
2. Maximum recommended dose following topical application is 200 mg.
3. Availability: Lidocaine base is available as an aerosol spray, ointment, patch, and solution in various dosage forms (Fig. 4-9).
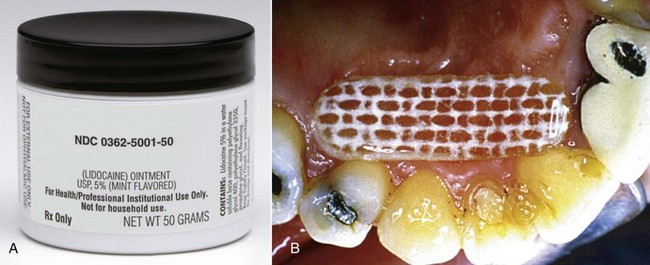
Figure 4-9 Topical anesthetics—Lidocaine. A, Octocaine ointment. B, Dentipatch. (A, Courtesy Septodent, New Castle, DE. B, Courtesy Noven Pharmaceuticals Inc., Miami, FL, www.noven.com.)
4. Availability: Lidocaine HCl is available as an oral topical solution in 20 mg/mL (viscous) and 40 mg/mL (solution).
Tetracaine Hydrochloride
Tetracaine hydrochloride (2-dimethylaminoethyl-4-butylaminobenzoate hydrochloride) is a long-duration ester local anesthetic that can be injected or applied topically.
2. Applied topically, five to eight times more potent than cocaine
3. Onset of action after topical application is slow.
4. Duration of action is approximately 45 minutes after topical application.
5. Metabolized in plasma and the liver by plasma pseudocholinesterase at a slower rate than procaine
6. When used by injection, available as a 0.15% concentration
7. 2% concentration is used for topical application.
8. Rapidly absorbed through mucous membranes. Use should be limited to small areas to avoid rapid absorption. Other, more slowly or poorly absorbed agents should be used in lieu of tetracaine when larger areas of topical anesthesia are necessary.
9. Maximum recommended dose of 20 mg when used for topical application. This represents 1 mL of a 2% solution.
10. Caution is urged because of great potential for systemic toxicity.
12. Tetracaine in a 3% concentration, with the vasoconstrictor oxymetazocine, has been shown to provide pulpal anesthesia of maxillary teeth when administered by aerosol spray into a patient’s nares.81 Use of intranasal local anesthesia for dental pain control in discussed in Chapter 20.
Selection Of A Local Anesthetic
Because of the many local anesthetic combinations available for injection, it sometimes is difficult to select an ideal drug for a given patient. Many dentists simply deal with this by using one local anesthetic for all procedures, regardless of their duration. For example, the dentist may elect to use 2% lidocaine with 1 : 100,000 epinephrine for procedures lasting 5 to 10 minutes and for procedures involving 90 minutes of treatment time. Although the duration of pulpal anesthesia achievable with this drug, in ideal circumstances, may permit pain-free treatment in both these instances, the patient who requires only 10 minutes of pulpal anesthesia will remain anesthetized unnecessarily for an additional 3 to 5 hours (soft tissues), whereas the patient requiring 90 minutes of pulpal anesthesia will likely experience pain toward the end of the procedure.
A rational approach to selection of an appropriate local anesthetic for a patient includes consideration of several factors: (1) the length of time for which pain control is necessary; (2) the need for posttreatment pain control; (3) the need for hemostasis; and (4) whether any contraindications exist to administration of the selected local anesthetic.1,22 Table 4-17 lists currently available local anesthetic formulations according to their expected duration of pulpal and soft tissue anesthesia. Again, it must be noted that these numbers are approximations, and the actual duration of clinical anesthesia may be somewhat longer or shorter than indicated.
A second consideration in the selection of a local anesthetic must be the requirement for pain control after treatment. A long-duration local anesthetic can be administered when postoperative pain is thought to be a factor. Local anesthetics providing a shorter duration of soft tissue anesthesia can be used for nontraumatic procedures. When postoperative pain is considered likely, 0.5% bupivacaine (for 8 to 12 hours of soft tissue anesthesia [via nerve block]) is suggested.
For patients in whom postoperative anesthesia represents a potential risk, a shorter-duration anesthetic should be considered. These patients include younger children, the older-old patient, and the physically or mentally disabled, who might accidentally bite or chew their lips or tongue, and persons unable to miss a meal (e.g., those with type 1 diabetes) because of residual soft tissue anesthesia. For these patients, 3% mepivacaine is recommended for use in short procedures. However in situations in which these patients require a more profound and/or longer duration of pulpal anesthesia, use of a local anesthetic containing a vasoconstrictor is necessary. The introduction of the local anesthesia reversal agent, phentolamine mesylate (Oraverse), has made it possible to significantly shorten the duration of residual soft tissue anesthesia, thereby minimizing the risk of accidental self-inflicted soft tissue injury (e.g., biting of the lip or tongue).82-84 Reversal of soft tissue anesthesia in discussed in Chapter 20.
A third factor in choosing a local anesthetic is the requirement for hemostasis during the procedure. Anesthetic solutions containing epinephrine in a 1 : 50,000 or 1 : 100,000 concentration are recommended, via local infiltration into the surgical site, when hemostasis is considered necessary. More dilute epinephrine formulations (e.g., 1 : 200,000, 1 : 400,000) are not effective for hemostasis, nor is levonordefrin or felypressin.
A fourth factor in the selection of a local anesthetic involves the presence of any contraindications to use of the selected local anesthetic (see Table 4-2).
Absolute contraindications require that the offending drug(s) is not administered to the patient under any condition. The risk that a life-threatening situation will arise is unacceptably elevated. Most absolute contraindications to local anesthetic administration are, in fact, medical contraindications to the delivery of elective dental care (e.g., the patient is too ill to tolerate dentistry). However, one absolute contraindication to local anesthetic administration does exist: true, documented reproducible allergy. Although the incidence of “alleged” local anesthetic allergy is high, true, documented and reproducible allergy is an extremely rare occurrence with amide local anesthetics. Management of alleged and documented allergy to local anesthetics is discussed in Chapter 18.
In cases of a relative contraindication, it is preferable to avoid administration of the drug in question because the risk that an adverse reaction will occur is increased. An alternative drug that is not contraindicated is recommended. However, if an acceptable alternative is not available, the drug in question may be used, but judiciously, with the minimum dose that will provide adequate pain control administered. One example of a relative contraindication is the presence of atypical plasma (pseudo)cholinesterase, which decreases the rate of biotransformation of ester local anesthetics. Amides may be used without increased risk in these patients. Contraindications (both absolute and relative) are reviewed in Chapter 10.
Box 4-4 summarizes the criteria used in selection of a local anesthetic for administration to a given patient at a given dental appointment.
Table 4-18 lists the proprietary names of injectable local anesthetics available in the United States (as of November 2011).
TABLE 4-18
Proprietary Names of Injectable Local Anesthetics (United States)
| Articaine HCl | Articadent, Orabloc, Septocaine, Zorcaine |
| Bupivacaine HCl | Marcaine, Vivacaine |
| Lidocaine HCl | Lignospan, Octocaine, Xylocaine |
| Mepivacaine HCl | Carbocaine, Isocaine, Polocaine, Scandanest |
| Prilocaine HCl | Citanest |
The local anesthetic armamentarium for a dentist or dental hygienist therefore should include drugs of varying durations of action, such as the selection that follows. A minimum of two drugs is recommended for most offices. Amides are preferred to esters whenever possible:
References
1. Malamed, SF. Handbook of local anesthesia. St Louis: Mosby; 1980.
2. Malamed, SF. Buffering local anesthetics in dentistry. ADSA Pulse. 2011;44:8–9.
3. Meechan, JG. Infiltration anesthesia in the mandible. Dent Clin North Am. 2010;54:621–629.
4. Iwatsubo, T, Hirota, N, Ooie, T, et al. Prediction of in vivo drug metabolism in the human liver from in vitro metabolism data. Pharmacol Ther. 1997;73:147–171.
5. Thompson, P, Melmon, K, Richardson, J, et al. Lidocaine pharmacokinetics in advanced heart failure, liver disease, and renal failure in humans. Ann Intern Med. 1973;78:499.
6. Haase, A, Reader, A, Nusstein, J, Beck, M, Drum, M. Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block. J Amer Dent Assoc. 2008;139:1228–1235.
7. Malamed, SF. Sedation: a guide to patient management, ed 4. St Louis: Mosby; 2003.
8. Wilson, AW, Deacock, S, Downie, IP, et al. Allergy to local anesthetic: the importance of thorough investigation. Br Dent J. 2000;188:320–322.
9. Covino, BG. Clinical pharmacology of local anesthetic agents. In Cousins MJ, Bridenbaugh PO, eds.: Neural blockade in clinical anesthesia and management of pain, ed 2, Philadelphia: JB Lippincott, 1988.
10. Prescribing information: Ravocaine and Novocain with Levophed. New York: Cook-Waite, Sterling Winthrop, 1993.
11. Moore, PA, Hersh, EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010;54:587–599.
12. Prescribing information: 2% Xylocaine Dental. York, Pa: Dentsply Pharmaceutical, March 2009.
13. Brown, RS, Paluvoi, S, Choksi, S, et al. Evaluating a dental patient for local anesthesia allergy. Comp Contin Educ Dent. 2002;23:225–228. [131–132, 134, 140].
14. Jackson, D, Chen, AH, Bennett, CR. Identifying true lidocaine allergy. J Am Dent Assoc. 1994;125:1362–1366.
15. Sindel, LJ, deShazo, RD. Accidents resulting from local anesthetics: true or false allergy? Clin Rev Allergy. 1991;9:379–395.
16. Ball, IA. Allergic reactions to lignocaine. Br Dent J. 1999;186:524–526.
17. Baluga, JC. Allergy to local anaesthetics in dentistry: myth or reality? Rev Alerg Mex. 2003;50:176–181.
18. Thyssen, JP, Menne, T, Elberling, J, et al. Hypersensitivity to local anaesthetics—update and proposal of evaluation algorithm. Contact Dermatitis. 2008;59:69–78.
19. Young, ER, Mason, DR, Saso, MA, et al. Some clinical properties of Octocaine 200 (2 percent lidocaine with epinephrine 1:200,000). J Can Dent Assoc. 1989;55:987–991.
20. Buckley, JA, Ciancio, SG, McMullen, JA. Efficacy of epinephrine concentration in local anesthesia during periodontal surgery. J Periodontol. 1984;55:653–657.
21. DeToledo, JC. Lidocaine and seizures. Ther Drug Monit. 2000;22:320–322.
22. Malamed, SF, Yagiela, J. Local anesthetics. In: ADA Guide to Dental Therapeutics. Chicago, Ill: American Dental Association; 1998.
23. Geddes, IC. Metabolism of local anesthetic agents. Int Anesthesiol Clin. 1967;5:525–549.
24. Severinghaus, JW, Xu, F-D, Spellman, MJ. Benzocaine and methemoglobin: recommended actions. Anesthesiology. 1991;74:385–386.
25. Schroeder, TH, Dieterich, HJ, Muhlbauer, B. Methemoglobinemia after maxillary block with bupivacaine and additional injection of lidocaine in the operative field. Acta Anaesthesiol Scand. 1999;43:480–482.
26. Wilburn-Goo, D, Lloyd, LM. When patients become cyanotic: acquired methemoglobinemia. J Am Dent Assoc. 1999;130:626–631.
27. de Jong, RH. Local anesthetics. St Louis: Mosby; 1994.
28. Akerman, B, Astrom, A, Ross, S, et al. Studies on the absorption, distribution, and metabolism of labeled prilocaine and lidocaine in some animal species. Acta Pharmacol Toxicol. 1966;24:389–403.
29. Foldes, FF, Molloy, R, McNall, PG, et al. Comparison of toxicity of intravenously given local anesthetic agents in man. JAMA. 1960;172:1493–1498.
30. Englesson, S, Eriksson, E, Ortengren, B. Differences in tolerance to intravenous Xylocaine and Citanest. Acta Anaesthesiol Scand Suppl. 1965;16:141–145.
31. Deriksson, E, Granberg, PO. Studies on the renal excretion of Citanest and Xylocaine. Acta Anaesthesiol Scand Suppl. 1985;16:79–85.
32. Smith, DW, Peterson, MR, DeBerard, SC. Local anesthesia: topical application, local infiltration, and field block. Postgrad Med. 1999;106:27–60. [64–66].
33. Akinturk, S, Eroglu, A. A clinical comparison of topical piroxicam and EMLA cream for pain relief and inflammation in laser hair removal. Lasers Med Sci. 2009;24:535–538.
34. Haas, DA, Lennon, D. A 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc. 1995;61:319–320. [323–326, 329–330].
35. Garisto, GA, Gaffen, AS, Lawrence, HP, et al. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010;141:836–844.
36. Pogrel, MA. Permanent nerve damage from inferior alveolar nerve blocks—an update to include articaine. J Calif Dent Assoc. 2007;35:271–273.
37. van Oss, GE, Vree, TB, Baars, AM, et al. Pharmacokinetics, metabolism, and renal excretion of articaine and its metabolite articainic acid in patients after epidural administration. Eur J Anaesthesiol. 1989;6:19–56.
38. van Oss, GE, Vree, TB, Baars, AM, et al. Clinical effects and pharmacokinetics of articainic acid in one volunteer after intravenous administration. Pharm Weekbl (Sc). 1988;10:284–286.
39. Vree, TB, Baars, AM, van Oss, GE, et al. High performance liquid chromatography and preliminary pharmacokinetics of articaine and its 2-carboxy metabolite in human serum and urine. J Chromatogr. 1988;424:240–444.
40. Prescribing information: Septocaine. Louisville, Colo: Septodont Inc, 2011.
41. Malamed, SF, Gagnon, S, Leblanc, D. Safety of articaine: a new amide local anesthetic. J Am Dent Assoc. 2001;132:177–185.
42. Malamed, SF, Gagnon, S, Leblanc, D. Articaine hydrochloride in pediatric dentistry: safety and efficacy of a new amide-type local anesthetic. Pediatr Dent. 2000;22:307–311.
43. Malamed, SF, Gagnon, S, Leblanc, D. Efficacy of articaine: a new amide local anesthetic. J Am Dent Assoc. 2000;131:535–642.
44. Donaldson, D, James-Perdok, L, Craig, BJ, et al. A comparison of Ultracaine DS (articaine HCl) and Citanest Forte (prilocaine HCl) in maxillary infiltration and mandibular nerve block. J Can Dent Assoc. 1987;53:38–42.
45. Knoll-Kohler, E, Rupprecht, S. Articaine for local anaesthesia in dentistry: a lidocaine controlled double blind cross-over study. Eur J Pain. 1992;13:59–63.
46. Septodont Inc., NA: Personal communications, May 2011.
47. Schulze-Husmann, M, Experimental evaluation of the new local anesthetic Ultracaine in dental practice [doctoral dissertation]. University of Bonn: Bonn, 1974.
48. Clinicians guide to dental products and techniques, Septocaine. CRA Newsletter. June 2001.
49. Kanaa, MD, Whitworth, JM, Corbett, IP, et al. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J. 2009;42:238–246.
50. Meechan, JG. Infiltration anesthesia in the mandible. Dent Clin North Am. 2010;54:621–629.
51. Yonchak, T, Reader, A, Beck, M, et al. Anesthetic efficacy of infiltrations in mandibular anterior teeth. Anesth Prog. 2001;48:55–60.
52. Meechan, JG, Ledvinka, JI. Pulpal anaesthesia for mandibular central incisor teeth: a comparison of infiltration and intraligamentary injections. Int Endod J. 2002;35:629–634.
53. Kanaa, MD, Whitworth, JM, Corbett, IP, et al. Articaine and lidocaine mandibular buccal infiltration anesthesia: a prospective randomized double-blind cross-over study. J Endod. 2006;32:296–298.
54. Robertson, D, Nusstein, J, Reader, A, et al. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth. J Am Dent Assoc. 2007;138:1104–1112.
55. Haase, A, Reader, A, Nusstein, J, et al. Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block. J Am Dent Assoc. 2008;139:1228–1235.
56. Jakobs, W. Status of dental anesthesia in Germany. Anesth Prog. 1989;36:10–212.
57. Deutscher Dentalmarkt Jahresbericht (DDM) 2010 (German Dental Market Annual Report 2010) GfK HealthCare, Nuremberg, Germany.
58. Malamed, SF. Handbook of local anesthesia, ed 4. St Louis: Mosby; 1997.
59. Articaine, epinephrine: contraindications/precautions, MD Consult. Available at http://www.mdconsult.com/php/250679983–2/homepage. [Revised April 21, 2010. Accessed May 24, 2011].
60. Jakobs W: Actual aspects of dental anesthesia in Germany. Presented at the 10th International Dental Congress on Modern Pain Control, IFDAS, Edinburgh, Scotland, UK, June 2003.
61. Prescribing information: Bupivacaine HCl. Lake Forest Ill: Hospira, Inc, November 2009.
62. Moore, PA. Bupivacaine: a long-lasting local anesthetic for dentistry. Oral Surg. 1984;58:369.
63. Acute Pain Management Guideline Panel, Acute pain management: operative or medical procedures and trauma. Clinical practice guideline [AHCPR Publication Number 92-0032]. Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services: Rockville, Md, 1992.
64. Oxford League Table of Analgesics in Acute Pain. Bandolier Website. Available at http://www.medicine.ox.ac.uk/bandolier/booth/painpag/acutrev/analgesics/lftab.html. [Accessed May 24, 2011].
65. Hargreaves, KM, Keiser, K. Development of new pain management strategies. J Dent Educ. 2002;66:113–121.
66. Malamed, SF. Handbook of local anesthesia, ed 5. St Louis: CV Mosby; 2003. [pp 74–75].
67. Adriani, J, Campbell, D. Fatalities following topical application of local anesthetics to mucous membranes. JAMA. 1956;162:1527.
68. Jeske, AH, Blanton, PL. Misconceptions involving dental local anesthesia. Part 2. Pharmacology. Texas Dent J. 2002;119:310–314.
69. Rosivack, RG, Koenigsberg, SR, Maxwell, KC. An analysis of the effectiveness of two topical anesthetics. Anesth Prog. 1990;37:290–292.
70. Patterson, RP, Anderson, J. Allergic reactions to drugs and biologic agents. JAMA. 1982;248:2637–2645.
71. Alston, TA. Antagonism of sulfonamides by benzocaine and chloroprocaine. Anesthesiology. 1992;76:375–476.
72. Adriani, J, Zepernick, R. Clinical effectiveness of drugs used for topical anesthesia. JAMA. 1964;188:93.
73. Taddio, A. Pain management for neonatal circumcision. Paediatr Drugs. 2001;3:101–111.
74. Vanscheidt, W, Sadjadi, Z, Lillieborg, S. EMLA anaesthetic cream for sharp leg ulcer debridement: a review of the clinical evidence for analgesic efficacy and tolerability. Eur J Dermatol. 2001;11:20–96.
75. Wright, VC. Vulvar biopsy: techniques for reducing patient discomfort. Adv Nurse Pract. 2001;9:17–60.
76. EMLA, MD Consult. Available at http://www.mdconsult.com/php/250679983–2/homepage. [Revised May 12, 2010. Accessed May 24, 2011].
77. Bernardi, M, Secco, F, Benech, A. Anesthetic efficacy of a eutectic mixture of lidocaine and prilocaine (EMLA) on the oral mucosa: prospective double-blind study with a placebo. Minerva Stomatol. 1999;48:9–43.
78. Munshi, AK, Hegde, AM, Latha, R. Use of EMLA: is it an injection free alternative? J Clin Pediatr Dent. 2001;25:215–219.
79. Franz-Montan, M, Ranali, J, Ramacciato, JC, et al. Ulceration of gingival mucosa after topical application of EMLA: report of four cases. Br Dent J. 2008;204:133–134.
80. Nayak, R, Sudha, P. Evaluation of three topical anaesthetic agents against pain: a clinical study. Indian J Dent Res. 2006;17:155–160.
81. University of Buffalo, The State University of New York. Nasap spray may end dental needle injections for upper teeth repair. Available at www.buffalo.edu/news/9911. [Accessed February 17, 2009].
82. Moore, PA, Hersh, EV, Papas, AS, et al. Pharmacokinetics of lidocaine with epinephrine following local anesthesia reversal with phentolamine mesylate. Anesth Prog. 2008;55:40–48.
83. Hersh, EV, Moore, PA, Papas, AS, et al. Soft Tissue Anesthesia Recovery Group: Reversal of soft-tissue local anesthesia with phentolamine mesylate in adolescents and adults. J Am Dent Assoc. 2008;139:1080–1093.
84. Tavares, M, Goodson, JM, Studen-Pavlovich, D, et al. Soft Tissue Anesthesia Reversal Group: Reversal of soft-tissue local anesthesia with phentolamine mesylate in pediatric patients. J Am Dent Assoc. 2008;139:1095–1104.