CHAPTER 33 Dehydration and Replacement Therapy
CHAPTER 33 Dehydration and Replacement Therapy
REPLACEMENT THERAPY
Table 33-1 lists the three sources of normal water loss—the components of maintenance water (see Chapter 32). Insensible losses, composed of evaporative losses from the skin and lungs, represent approximately one third of total maintenance water. Sweating is not insensible and, in contrast to evaporative losses, sweat contains water and electrolytes.
TABLE 33-1 Components of Maintenance Water
| Urine | 60% |
| Insensible losses (skin and lungs) | 35% |
| Stool | 5% |
A variety of clinical situations modify normal maintenance water balance (Table 33-2). Evaporative skin water losses can be significant in neonates, especially premature infants who are under radiant warmers or undergoing phototherapy. Burns can result in massive losses of water and electrolytes (see Chapter 44). Fever leads to a predictable increase in insensible losses, causing a 10% to 15% increase in maintenance water needs for each 1 °C increase in temperature greater than 38 °C. Tachypnea or a tracheostomy increases evaporative losses from the lungs.
TABLE 33-2 Adjustments in Maintenance Water
| Source | Causes of Increased Water Needs | Causes of Decreased Water Needs |
|---|---|---|
| Skin | Radiant warmer Phototherapy Fever Sweat Burns |
Mist tent Incubator (premature infants) |
| Lungs | Tachypnea Tracheostomy |
Humidified ventilator Mist tent |
| Gastrointestinal | Diarrhea Emesis Nasogastric suction |
|
| Renal | Polyuria | Oliguria/anuria |
| Miscellaneous | Surgical drain Third space losses |
Hypothyroidism |
The gastrointestinal tract is potentially a source of considerable water and electrolyte losses. A child who has large amounts of gastrointestinal losses should have these losses measured and replaced with an appropriate replacement solution. It is impossible to predict gastrointestinal losses for the next 24 hours. Hence, losses should be replaced after they occur, using a solution with the same approximate electrolyte concentration as the gastrointestinal fluid. Electrolyte content can be measured directly, or a solution can be selected based on the typical electrolyte composition of diarrhea or gastric losses (Table 33-3). The losses usually are replaced every 1 to 6 hours, depending on the rate of loss, with rapid losses being replaced more frequently. The child should also receive an appropriate maintenance fluid (see Chapter 32).
TABLE 33-3 Adjusting Fluid Therapy for Gastrointestinal Losses
| Average Composition | Approach to Replacement |
|---|---|
| Diarrhea | Replacement of Ongoing Stool Losses |
| Sodium: 55 mEq/L | Solution: 5% dextrose in ¼ normal saline 20 mEq/L sodium bicarbonate 20 mEq/L potassium chloride |
| Potassium: 25 mEq/L | Replace stool mL/mL every 1–6 hr |
| Bicarbonate: 15 mEq/L | |
| Gastric Fluid | Replacement of Ongoing Gastric Losses |
| Sodium: 60 mEq/L | Solution: normal saline 10 mEq/L potassium chloride |
| Potassium: 10 mEq/L | Replace output mL/mL every 1–6 hr |
| Chloride: 90 mEq/L |
Urine output is normally the largest cause of water loss. Diseases such as renal failure and the syndrome of inappropriate antidiuretic hormone (SIADH), can lead to a decrease in urine volume. Continuation of maintenance fluids in a patient with oliguria or anuria produces fluid overload. In contrast, other conditions produce an increase in urine volume; these include polyuric phase of acute tubular necrosis, diabetes mellitus, and diabetes insipidus. The patient must receive more than standard maintenance fluids when the urine output is excessive to prevent dehydration.
The approach to decreased or increased urine output is similar (Table 33-4). Insensible losses are replaced by a solution that is administered at a rate one third of the normal maintenance rate. Placing the anuric child on “insensibles” theoretically maintains an even fluid balance, with the caveat that one third of maintenance fluid is only an estimate of insensible losses. In the individual patient, this rate may need to be adjusted based on monitoring of the patient’s weight and hydration status. An oliguric child needs to receive a urine replacement solution.
TABLE 33-4 Adjusting Fluid Therapy for Altered Renal Output
| Oliguria/Anuria | Polyuria |
|---|---|
| Place the patient on insensible fluids (⅓ maintenance) | Place the patient on insensible fluids (⅓ maintenance) |
| Replace urine output mL/mL with half normal saline | Measure urine electrolytes |
| Replace urine output mL/mL with a solution that is based on the measured urine electrolytes |
Most children with polyuria (except for children with diabetes mellitus [see Chapter 171]) should be placed on insensible fluids plus urine replacement. When urine output is excessive, it is important to measure the sodium and potassium concentration of the urine to determine the electrolyte composition of the urine replacement solution.
Output from surgical drains and chest tubes, when significant, should be measured and replaced. Third space losses manifest with edema and ascites and are due to a shift of fluid from the intravascular space into the interstitial space. Third space losses cannot be quantitated easily. Nonetheless, these losses can be large and lead to intravascular volume depletion, despite weight gain. Replacement of third space fluid is empirical but should be anticipated in patients who are at risk, such as children who have burns or abdominal surgery. Third space losses and chest tube output are isotonic and usually require replacement with an isotonic fluid, such as normal saline or Ringer’s lactate. Adjustments in the amount of replacement fluid for third space losses are based on continuing assessment of the patient’s intravascular volume status.
DEHYDRATION
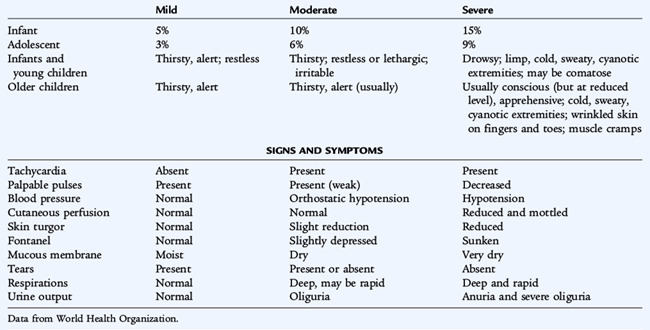
Dehydration, most often due to gastroenteritis, is common in children. The first step in caring for a child with dehydration is to assess the degree of dehydration. The degree of dehydration dictates the urgency of the situation and the volume of fluid needed for rehydration. Table 33-5 summarizes the clinical features that are present with varying degrees of dehydration.
An infant with mild dehydration (3% to 5% of body weight dehydrated) has few clinical signs or symptoms. The infant may be thirsty; the alert parent may notice a decline in urine output. The history describes decreased intake and increased fluid losses. An infant with moderate dehydration has demonstrable physical signs and symptoms. Intravascular space depletion is evident by an increased heart rate and reduced urine output. The patient is 10% dehydrated and needs fairly prompt intervention. An infant with severe dehydration is gravely ill. The decrease in blood pressure indicates that vital organs may be receiving inadequate perfusion (shock) (see Chapter 40). The infant is at least 15% dehydrated and should receive immediate and aggressive intravenous (IV) therapy. Mild, moderate, and severe dehydration represent 3%, 6%, and 9% of body weight lost in older children and adults. This difference is because water is a higher percentage of body weight in infants (see Chapter 32). Clinical assessment of dehydration is only an estimate; the patient must be continually re-evaluated during therapy. The degree of dehydration is underestimated in hypernatremic dehydration because the osmotically driven shift of water from the intracellular space to the extracellular space helps to preserve the intravascular volume.
Laboratory Evaluation
Serum blood urea nitrogen (BUN) and creatinine concentrations are useful in assessing a child with dehydration. Volume depletion without renal insufficiency may cause a disproportionate increase in the BUN, with little or no change in the creatinine concentration. This situation is secondary to increased passive reabsorption of urea in the proximal tubule caused by appropriate renal conservation of sodium and water. This increase in the BUN may be absent or blunted in a child with poor protein intake because urea production depends on protein degradation. Conversely, the BUN may be disproportionately increased in a child with increased urea production, as occurs in a child with a gastrointestinal bleed or a child who is receiving glucocorticoids. A significant elevation of the creatinine concentration suggests renal injury.
The urine specific gravity is usually elevated (≥1.025) in cases of significant dehydration but decreases after rehydration. With dehydration, a urinalysis may show hyaline and granular casts, a few white blood cells and red blood cells, and 30 to 100 mg/dL of proteinuria. These findings usually are not associated with significant renal pathology, and they remit with therapy. Hemoconcentration from dehydration causes an increase in the hematocrit and hemoglobin.
Calculation of Fluid Deficit
A child with dehydration has lost water; there is usually a concurrent loss of sodium and potassium. The fluid deficit is the percentage of dehydration multiplied by the patient’s weight (for a 10-kg child, 10% of 10 kg >1 L deficit).
Approach to Dehydration
The child with dehydration requires acute intervention to ensure that there is adequate tissue perfusion (see Chapter 40). This resuscitation phase requires rapid restoration of the circulating intravascular volume which should be done with an isotonic solution, such as normal saline (NS) or Ringer’s lactate. Blood is an appropriate fluid choice for a child with acute blood loss. The child is given a fluid bolus, usually 20 mL/kg of the isotonic solution, over about 20 minutes. A child with severe dehydration may require multiple fluid boluses and may need to receive fluid at a faster rate. The initial resuscitation and rehydration is complete when the child has an adequate intravascular volume. Typically the child has some general clinical improvement, including a lower heart rate, normalization of the blood pressure, improved perfusion, and a more alert affect.
With adequate intravascular volume, it is now appropriate to plan the fluid therapy for the next 24 hours (Table 33-6). In order to assure that the intravascular volume is restored, the patient receives an additional 20 mL/kg bolus of isotonic fluid over 2 hours. The child’s total fluid needs are added together (maintenance + deficit). The volume of isotonic fluids the patient has received as acute resuscitation is subtracted from this total. The remaining fluid volume is then administered over 24 hours. Potassium usually is not included in the IV fluids until the patient voids, unless significant hypokalemia is present. Children with significant ongoing losses need to receive an appropriate replacement solution.
Monitoring and Adjusting Therapy
All calculations in fluid therapy are only approximations. Thus, the patient needs to be monitored during treatment with therapy modifications based on the clinical situation (Table 33-7).
Hyponatremic dehydration occurs in children who have diarrhea and consume a hypotonic fluid (water or diluted formula). Volume depletion stimulates secretion of antidiuretic hormone, preventing the water excretion that should correct the hyponatremia. Some patients develop symptoms, predominantly neurologic, from the hyponatremia (see Chapter 35). Most patients with hyponatremic dehydration do well with the same general approach outlined in Table 33-6. Overly rapid correction of hyponatremia (>12 mEq/L/24 hr) should be avoided because of the remote risk of central pontine myelinolysis.
Hypernatremic dehydration is usually a consequence of an inability to take in fluid, because of a lack of access, a poor thirst mechanism (neurologic impairment), intractable emesis, or anorexia. The movement of water from the intracellular space to the extracellular space during hypernatremic dehydration partially protects the intravascular volume. Urine output may be preserved longer, and there may be less tachycardia. Children with hypernatremic dehydration are often lethargic and irritable. Hypernatremia may cause fever, hypertonicity, and hyperreflexia. More severe neurologic symptoms may develop if cerebral bleeding or thrombosis occurs.
Overly rapid treatment of hypernatremic dehydration may cause significant morbidity and mortality. Idiogenic osmoles are generated within the brain during the development of hypernatremia. These idiogenic osmoles increase the osmolality within the cells of the brain, providing protection against brain cell shrinkage secondary to movement of water out of cells into the hypertonic extracellular fluid. These idiogenic osmoles dissipate slowly during correction of hypernatremia. With rapid lowering of the extracellular osmolality during correction of hypernatremia, there may be a new gradient created that causes water movement from the extracellular space into the cells of the brain, producing cerebral edema. Possible manifestations of the resultant cerebral edema include seizures, brain herniation, and death.
To minimize the risk of cerebral edema during correction of hypernatremic dehydration, the serum sodium concentration should not decrease more than 12 mEq/L every 24 hours. The deficits in severe hypernatremic dehydration may need to be corrected over 2 to 4 days (Table 33-8). The choice and rate of fluid are not nearly as important as vigilant monitoring of the serum sodium concentration and adjustment of the therapy based on the result. Nonetheless, the initial resuscitation-rehydration phase of therapy remains the same as for other types of dehydration.
IV, intravenous.
Oral Rehydration
Mild to moderate dehydration from diarrhea of any cause can be treated effectively using a simple, oral rehydration solution (ORS) containing glucose and electrolytes (see Chapter 112). The ORS relies on the coupled transport of sodium and glucose in the intestine. Oral rehydration therapy has significantly reduced the morbidity and mortality from acute diarrhea but is underused in developed countries. It should be attempted for most patients with mild to moderate diarrheal dehydration. Oral rehydration therapy is less expensive than IV therapy and has a lower complication rate. IV therapy may still be required for patients with severe dehydration; patients with uncontrollable vomiting; patients unable to drink because of extreme fatigue, stupor, or coma; or patients with gastric or intestinal distention.
As a guideline for oral rehydration, 50 mL/kg of the ORS should be given within 4 hours to patients with mild dehydration, and 100 mL/kg should be given over 4 hours to patients with moderate dehydration. Supplementary ORS is given to replace ongoing losses from diarrhea or emesis. An additional 10 mL/kg of ORS is given for each stool. Fluid intake should be decreased if the patient appears fully hydrated earlier than expected or develops periorbital edema. After rehydration, patients should resume their usual diet (breast milk, formula),
When rehydration is complete, maintenance therapy should be started, using 100 mL of ORS/kg in 24 hours until the diarrhea stops. Breastfeeding or formula-feeding should be maintained and not delayed for more than 24 hours. Patients with more severe diarrhea require continued supervision. The volume of ORS ingested should equal the volume of stool losses. If stool volume cannot be measured, an intake of 10 to 15 mL of ORS/kg/hr is appropriate.