Viral Infections
HUMAN HERPES VIRUSES
The term herpes comes from the ancient Greek word meaning to creep or crawl. The human herpesvirus (HHV) family is officially known as Herpetoviridae, and its best-known member is herpes simplex virus (HSV), a DNA virus. Two types of HSV are known to exist: type I (HSV-1 or HHV-1) and type 2 (HSV-2 or HHV-2). Other members of the HHV family include varicella-zoster virus (VZV or HHV-3), Epstein-Barr virus (EBV or HHV-4), cytomegalovirus (CMV or HHV-5), and several more recently discovered members, HHV-6, HHV-7, and HHV-8.
Humans are the only natural reservoir for these viruses, which are endemic worldwide and share many features. All eight types cause a primary infection and remain latent within specific cell types for the life of the individual. On reactivation, these viruses are associated with recurrent infections that may be symptomatic or asymptomatic. The viruses are shed in the saliva or genital secretions, providing an avenue for infection of new hosts. Each type is known to transform cells in tissue culture, with several strongly associated with specific malignancies. Of the various types, the following sections will concentrate on the herpes simplex viruses, varicella-zoster virus, cytomegalovirus, and Epstein-Barr virus. Much less is known about herpesvirus types 6, 7, and 8.
Human herpesviruses 6 and 7 (HHV-6, HHV-7) are closely related, commonly isolated from saliva, usually transmitted by respiratory droplets, and exhibit a prevalence rate of infection close to 90% by age 5 in the United States. Both viruses are associated with a primary infection that usually is asymptomatic but can exhibit an erythematous macular eruption that may demonstrate intermixed slightly elevated papules. The cutaneous manifestation of HHV-6 creates a specific pattern, roseola (exanthema subitum), whereas HHV-7 may cause a similar roseola-like cutaneous eruption. The primary latency resides in CD4 T lymphocytes, and reactivation occurs most frequently in immunocompromised patients. Recurrences can result in widespread multiorgan infection, including encephalitis, pneumonitis, bone-marrow suppression, and hepatitis.
Human herpesvirus 8 (HHV-8) appears to be involved in the pathogenesis of Kaposi’s sarcoma (KS) (see page 557) and has been termed Kaposi’s sarcoma herpesvirus (KSHV). In patients with normal immune systems, primary infection usually is asymptomatic, with sexual contact (especially male homosexual) being the most common pattern of transmission. The virus has been found without difficulty in saliva, suggesting another possible pattern of transmission. Associated symptoms such as transient fever, lymphadenopathy, and arthralgias are rarely reported. Circulating B lymphocytes appear to be the major cell of latency. In addition to Kaposi’s sarcoma, HHV-8 also has been associated with a small variety of lymphomas and Castleman’s disease.
HERPES SIMPLEX VIRUS
The two herpes simplex viruses are similar structurally but different antigenically. In addition, the two exhibit epidemiologic variations.
HSV-1 is spread predominantly through infected saliva or active perioral lesions. HSV-1 is adapted best and performs more efficiently in the oral, facial, and ocular areas. The pharynx, intraoral sites, lips, eyes, and skin above the waist are involved most frequently.
HSV-2 is adapted best to the genital zones, is transmitted predominantly through sexual contact, and typically involves the genitalia and skin below the waist. Exceptions to these rules do occur, and HSV-1 can be seen in a pattern similar to that of HSV-2 and vice versa. The clinical lesions produced by both types are identical, and both produce the same changes in tissue. The viruses are so similar that antibodies directed against one cross-react against the other. Antibodies to one of the types decrease the chance of infection with the other type; if infection does occur, the manifestations often are less severe.
Clinically evident infections with HSV-1 exhibit two patterns. The initial exposure to an individual without antibodies to the virus is called the primary infection. This typically occurs at a young age, often is asymptomatic, and usually does not cause significant morbidity. At this point, the virus is taken up by the sensory nerves and transported to the associated sensory or, less frequently, the autonomic ganglia where the virus remains in a latent state. With HSV-1 infection, the most frequent site of latency is the trigeminal ganglion, but other possible sites include the nodose ganglion of the vagus nerve, dorsal root ganglia, and the brain. The virus uses the axons of the sensory neurons to travel back and forth to the peripheral skin or mucosa.
Secondary, recurrent, or recrudescent HSV-1 infection occurs with reactivation of the virus, although many patients may show only asymptomatic viral shedding in the saliva. Symptomatic recurrences are fairly common and affect the epithelium supplied by the sensory ganglion. Spread to an uninfected host can occur easily during periods of asymptomatic viral shedding or from symptomatic active lesions. When repeatedly tested, approximately one third of individuals with HSV-1 antibodies occasionally shed infectious viral particles, even without active lesions being present. In addition, the virus may spread to other sites in the same host to establish residency at the sensory ganglion of the new location. Numerous conditions such as old age, ultraviolet light, physical or emotional stress, fatigue, heat, cold, pregnancy, allergy, trauma, dental therapy, respiratory illnesses, fever, menstruation, systemic diseases, or malignancy have been associated with reactivation of the virus, but only ultraviolet light exposure has been demonstrated unequivocally to induce lesions experimentally. More than 80% of the primary infections are purported to be asymptomatic, and reactivation with asymptomatic viral shedding greatly exceeds clinically evident recurrences.
HSV does not survive long in the external environment, and almost all primary infections occur from contact with an infected person who is releasing the virus. The usual incubation period is 3 to 9 days. Because HSV-1 usually is acquired from contact with contaminated saliva or active perioral lesions, crowding and poor hygiene promote exposure. Lower socioeconomic status correlates with earlier exposure. In developing countries, more than 50% of the population is exposed by 5 years of age, 95% by 15 years of age, and almost universal exposure by 30 years of age. On the other hand, upper socioeconomic groups in developed nations exhibit less than 20% exposure at 5 years of age and only 50% to 60% in adulthood. Regardless of the socioeconomic group, prevalence tends to increase with age, and many investigators report a frequency of prior infection that approaches 90% of the population by age 60. The low childhood exposure rate in the privileged groups is followed by a second peak during the college years of life. The age of initial infection also affects the clinical presentation of the symptomatic primary infections. In symptomatic cases, individuals exposed to HSV-1 at an early age tend to exhibit gingivostomatitis; those initially exposed later in life often demonstrate pharyngotonsillitis.
As mentioned previously, antibodies to HSV-1 decrease the chance of infection with HSV-2 or lessen the severity of the clinical manifestations. The dramatic increase recently seen in HSV-2 is due partly to lack of prior exposure to HSV-1, increased sexual activity, and lack of barrier contraception. HSV-2 exposure correlates directly with sexual activity. Exposure of those younger than age 14 is close to zero, and most initial infections occur between the ages of 15 and 35. The prevalence varies from near zero in celibate adults to more than 80% in prostitutes. Because many of those infected with HSV-2 refrain from sexual activity when active lesions are present, many investigators believe that at least 70% of primary infections are contracted from individuals during asymptomatic viral shedding.
In addition to clinically evident infections, HSV has been implicated in a number of noninfectious processes. More than 15% of cases of erythema multiforme are preceded by a symptomatic recurrence of HSV 3 to 10 days earlier (see page 776), and some investigators believe that up to 60% of mucosal erythema multiforme may be triggered by HSV. In some instances, the attacks of erythema multiforme are frequent enough to warrant antiviral prophylaxis. An association with cluster headaches and a number of cranial neuropathies has been proposed, but definitive proof is lacking.
On rare occasions, asymptomatic release of HSV will coincide with attacks of aphthous ulcerations. The ulcerations are not infected with the virus. In these rare cases, the virus may be responsible for the initiation of the autoimmune destruction; conversely, the immune dysregulation that produces aphthae may have allowed the release of the virions. In support of the lack of association between HSV and aphthae in the general population of patients with aphthous ulcerations, prophylactic oral acyclovir does not decrease the recurrence rate of the aphthous ulcerations. Although the association between HSV and recurrent aphthous ulcerations is weak, it may be important in small subsets of patients (see page 331).
HSV also has been associated with oral carcinomas, but much of the evidence is circumstantial. The DNA from HSV has been extracted from the tissues of some tumors but not from others. HSV may aid carcinogenesis through the promotion of mutations, but the oncogenic role, if any, is uncertain.
CLINICAL FEATURES: Acute herpetic gingivostomatitis (primary herpes) is the most common pattern of symptomatic primary HSV infection, and more than 90% are the result of HSV-1. In a study of more than 4000 children with antibodies to HSV-1, Jureti found that only 12% of those infected had clinical symptoms and signs severe enough to be remembered by the affected children or their parents. Some health care practitioners suspect that the percentage of primary infections that exhibit clinical symptoms is much higher, whereas others believe the prevalence is lower. Many primary infections may manifest as pharyngitis that mimics the pattern seen in common colds. Further studies are needed to fully answer this question.
found that only 12% of those infected had clinical symptoms and signs severe enough to be remembered by the affected children or their parents. Some health care practitioners suspect that the percentage of primary infections that exhibit clinical symptoms is much higher, whereas others believe the prevalence is lower. Many primary infections may manifest as pharyngitis that mimics the pattern seen in common colds. Further studies are needed to fully answer this question.
Most cases of acute herpetic gingivostomatitis arise between the ages of 6 months and 5 years, with the peak prevalence occurring between 2 and 3 years of age. In spite of these statistics, occasional cases have been reported in patients over 60 years of age. Development before 6 months of age is rare because of protection by maternal anti-HSV antibodies. The onset is abrupt and often accompanied by anterior cervical lymphadenopathy, chills, fever (103° to 105° F), nausea, anorexia, irritability, and sore mouth lesions. The manifestations vary from mild to severely debilitating.
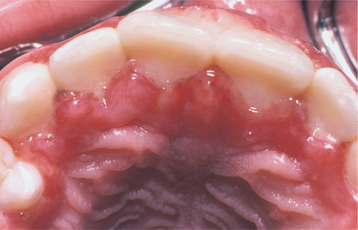
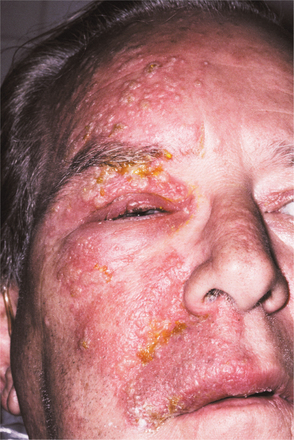
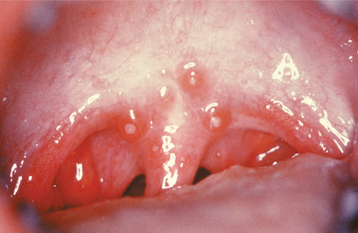
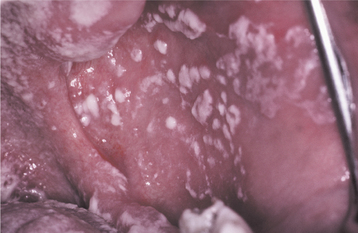
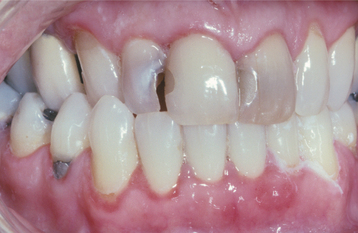
Initially the affected mucosa develops numerous pinhead vesicles, which rapidly collapse to form numerous small, red lesions. These initial lesions enlarge slightly and develop central areas of ulceration, which are covered by yellow fibrin (Fig. 7-1). Adjacent ulcerations may coalesce to form larger, shallow, irregular ulcerations (Fig. 7-2). Both the movable and attached oral mucosa can be affected, and the number of lesions is highly variable. In all cases the gingiva is enlarged, painful, and extremely erythematous (Fig. 7-3). In addition, the affected gingiva often exhibits distinctive punched-out erosions along the midfacial free gingival margins (Fig. 7-4). It is not unusual for the involvement of the labial mucosa to extend past the wet line to include the adjacent vermilion border of the lips. Satellite vesicles of the perioral skin are fairly common. Self-inoculation of the fingers, eyes, and genital areas can occur. Mild cases usually resolve within 5 to 7 days; severe cases may extend to 2 weeks. Rare complications include keratoconjunctivitis, esophagitis, pneumonitis, meningitis, and encephalitis.
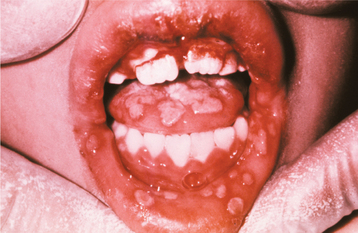
Fig. 7-1 Acute herpetic gingivostomatitis. Widespread yellowish mucosal ulcerations. (Courtesy of Dr. David Johnsen.)
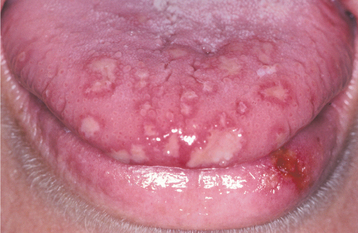
Fig. 7-2 Acute herpetic gingivostomatitis. Numerous coalescing, irregular, and yellowish ulcerations of the dorsal surface of the tongue.
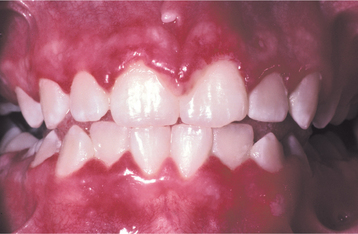
Fig. 7-4 Acute herpetic gingivostomatitis. Painful, enlarged, and erythematous facial gingiva. Note erosions of the free gingival margin.
As mentioned previously, when the primary infection occurs in adults, some symptomatic cases exhibit pharyngotonsillitis. Sore throat, fever, malaise, and headache are the initial symptoms. Numerous small vesicles develop on the tonsils and posterior pharynx. The vesicles rapidly rupture to form numerous shallow ulcerations, which often coalesce with one another. A diffuse, gray-yellow exudate forms over the ulcers in many cases. Involvement of the oral mucosa anterior to Waldeyer’s ring occurs in less than 10% of these cases. HSV appears to be a significant cause of pharyngotonsillitis in young adults who are from the higher socioeconomic groups with previously negative test findings for HSV antibodies. Most of these infections are HSV-1, but increasing proportions are HSV-2. The clinical presentation closely resembles pharyngitis secondary to streptococci or infectious mononucleosis, making the true frequency difficult to determine.
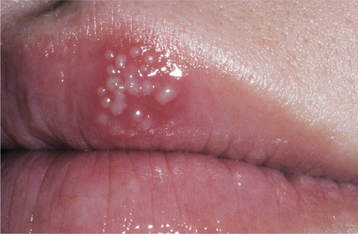
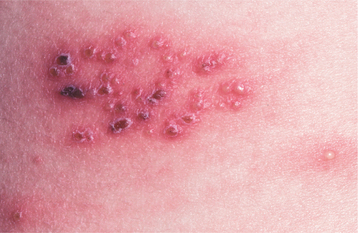
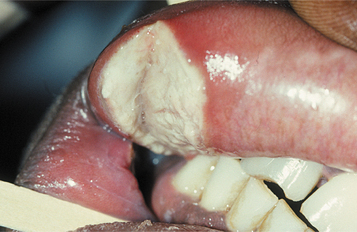
Recurrent herpes simplex infections (secondary herpes, recrudescent herpes) may occur either at the site of primary inoculation or in adjacent areas of surface epithelium supplied by the involved ganglion. The most common site of recurrence for HSV-1 is the vermilion border and adjacent skin of the lips. This is known as herpes labialis (“cold sore” or “fever blister”). Prevalence studies suggest that from 15% to 45% of the United States population have a history of herpes labialis. In some patients, ultraviolet light or trauma can trigger recurrences. Prodromal signs and symptoms (e.g., pain, burning, itching, tingling, localized warmth, erythema of the involved epithelium) arise 6 to 24 hours before the lesions develop. Multiple small, erythematous papules develop and form clusters of fluid-filled vesicles (Fig. 7-5). The vesicles rupture and crust within 2 days. Healing usually occurs within 7 to 10 days. Symptoms are most severe in the first 8 hours, and most active viral replication is complete within 48 hours. Mechanical rupture of intact vesicles and the release of the virus-filled fluid may result in the spreading of the lesions on lips previously cracked from sun exposure (Fig. 7-6). Recurrences are observed less commonly on the skin of the nose, chin, or cheek. The majority of those affected experience approximately 2 recurrences annually, but a small percentage may experience outbreaks that occur monthly or even more frequently.
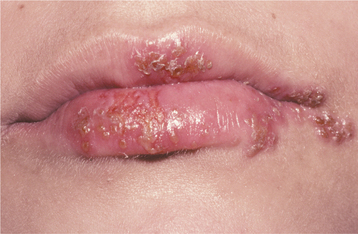
Fig. 7-6 Herpes labialis. Multiple sites of recurrent herpetic infection secondary to spread of viral fluid over cracked lips.
On occasion, some lesions arise almost immediately after a known trigger and appear without any preceding prodromal symptoms. These rapidly developing recurrences tend to respond less favorably to treatment.
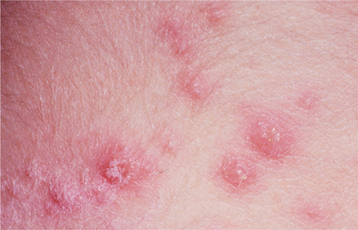
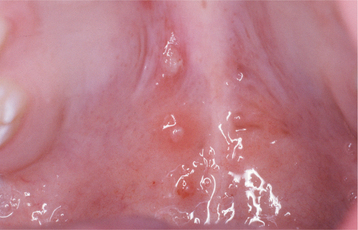
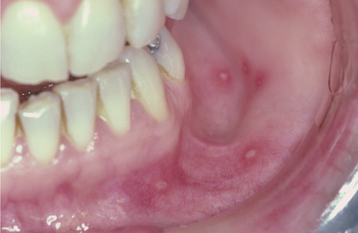
Recurrences also can affect the oral mucosa. In the immunocompetent patient, involvement is limited almost always to keratinized mucosa that is bound to bone (attached gingiva and hard palate). These sites often exhibit subtle changes, and the symptoms are less intense. The lesions begin as 1- to 3-mm vesicles that rapidly collapse to form a cluster of erythematous macules that may coalesce or slightly enlarge (Figs. 7-7 and 7-8). The damaged epithelium is lost, and a central yellowish area of ulceration develops. Healing takes place within 7 to 10 days.
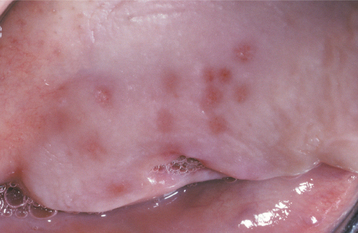
Fig. 7-7 Intraoral recurrent herpetic infection. Early lesions exhibiting as multiple erythematous macules on the hard palate. Lesions appeared a few days after extraction of a tooth.
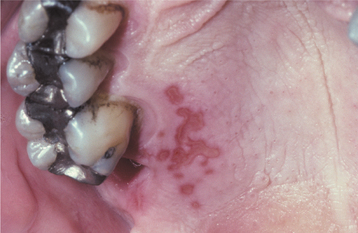
Fig. 7-8 Intraoral recurrent herpetic infection. Multiple coalescing ulcerations on the hard palate.
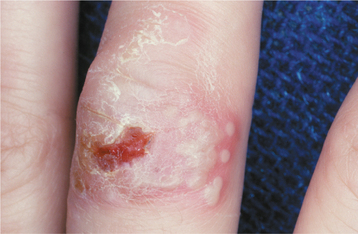
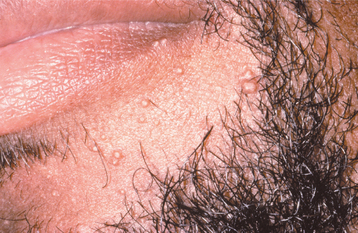
Less common presentations of HSV-1 do occur. Infection of the thumbs or fingers is known as herpetic whitlow (herpetic paronychia), which may occur as a result of self-inoculation in children with orofacial herpes (Fig. 7-9). Before the uniform use of gloves, medical and dental personnel could infect their digits from contact with infected patients, and they were the most likely group affected by this form of HSV-I infection. Recurrences on the digits are not unusual and may result in paresthesia and permanent scarring.
Cutaneous herpetic infections also can arise in areas of previous epithelial damage. Parents kissing areas of dermatologic injury in children represent one vector. Wrestlers and rugby players also may contaminate areas of abrasion, a lesion called herpes gladiatorum or scrumpox. On occasion, herpes simplex has been spread over the bearded region of the face into the minor injuries created by daily shaving, leading to a condition known as herpes barbae (barbae is Latin for “of the beard”). Ocular involvement may occur in children, often resulting from self-inoculation. Patients with diffuse chronic skin diseases, such as eczema, pemphigus, and Darier’s disease, may develop diffuse life-threatening HSV infection, known as eczema herpeticum (Kaposi’s varicelliform eruption). Newborns may become infected after delivery through a birth canal contaminated with HSV, usually HSV-2. Without treatment, there is greater than a 50% mortality rate.
HSV recurrence in immunocompromised hosts can be significant. Without proper immune function, recurrent herpes can persist and spread until the infection is treated with antiviral drugs, until immune status returns, or until the patient dies. On the skin, the lesions continue to enlarge peripherally, with the formation of an increasing zone of superficial cutaneous erosion. Oral mucosa also can be affected and usually is present in conjunction with herpes labialis. Although most oral mucosal involvement begins on the bound mucosa, it often is not confined to these areas. The involved sites begin as areas of necrotic epithelium that are brownish and raised above the surface of the adjacent intact epithelium. Typically, these areas are much larger than the usual pinhead lesions found in immunocompetent patients. With time, the area of involvement spreads laterally. The enlarging lesion is a zone of superficial necrosis or erosion, often with a distinctive circinate, raised, yellow border (Figs. 7-10 and 7-11). This border represents the advancing margin of active viral destruction. Microscopic demonstration of HSV infection in a chronic ulceration on the movable oral mucosa is ominous, and all such patients should be evaluated thoroughly for possible immune dysfunction or underlying occult disease processes.
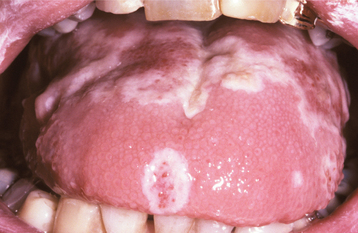
Fig. 7-10 Chronic herpetic infection. Numerous mucosal erosions, each of which is surrounded by a slightly raised, yellow-white border, in a patient with acute myelogenous leukemia.
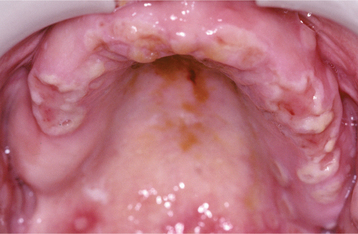
Fig. 7-11 Chronic herpetic infection. Numerous shallow herpetic erosions with raised, yellow and circinate borders on the maxillary alveolar ridge in an immunocompromised patient.
Although a yellow curvilinear border often is present in many chronic herpetic ulcerations noted in immunocompromised patients, this distinctive feature might be missing. Several authors have reported persistent oral ulcerations in patients with acquired immunodeficiency syndrome (AIDS) that lack the distinctive periphery, often are nonspecific clinically, and may mimic aphthous ulcerations, necrotizing stomatitis, or ulcerative periodontal disease. Biopsy of persistent ulcerations in patients with AIDS is mandatory and may reveal any one of a number of infectious or neoplastic processes. These ulcers may reveal histopathologic evidence of herpesvirus, often combined with diagnostic features of CMV (HHV-5) coinfection (see page 255).
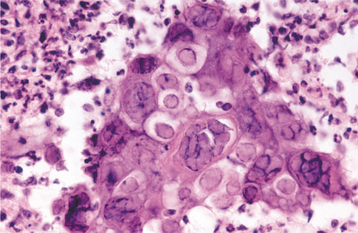
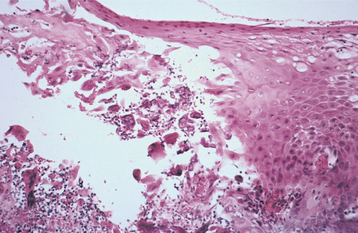
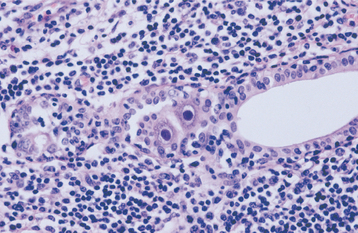
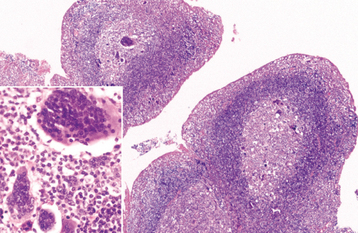
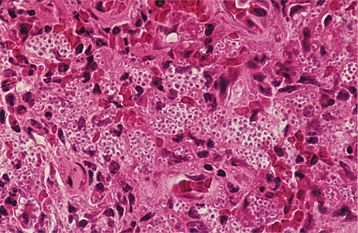
HISTOPATHOLOGIC FEATURES: The virus exerts its main effects on the epithelial cells. Infected epithelial cells exhibit acantholysis, nuclear clearing, and nuclear enlargement, which has been termed ballooning degeneration (Fig. 7-12). The acantholytic epithelial cells are termed Tzanck cells (not specific for herpes; refers to a free-floating epithelial cell in any intraepithelial vesicle). Nucleolar fragmentation occurs with a condensation of chromatin around the periphery of the nucleus. Multinucleated, infected epithelial cells are formed when fusion occurs between adjacent cells (see Fig. 7-12). Intercellular edema develops and leads to the formation of an intraepithelial vesicle (Fig. 7-13). Mucosal vesicles rupture rapidly; those on the skin persist and develop secondary infiltration by inflammatory cells. Once they have ruptured, the mucosal lesions demonstrate a surface fibrinopurulent membrane. Often at the edge of the ulceration or mixed within the fibrinous exudate are the scattered Tzanck or multinucleated epithelial cells.
DIAGNOSIS: With a thorough knowledge of the clinical presentations, the clinician can make a strong presumptive diagnosis of HSV infection. On occasion, HSV infections can be confused with other diseases, and laboratory confirmation is desirable. Viral isolation from tissue culture inoculated with the fluid of intact vesi-cles is the most definitive diagnostic procedure. The problem with this technique in primary infections is that up to 2 weeks can be required for a definitive result. Laboratory tests to detect HSV antigens by direct fluorescent assay or viral DNA by polymerase chain reaction (PCR) of specimens of active lesions also are available. Serologic tests for HSV antibodies are positive 4 to 8 days after the initial exposure. Confirmation of primary infection by serology requires a specimen obtained within 3 days of the presentation and a second sample approximately 4 weeks later. In such cases the initial specimen should be negative, with antibodies discovered only in the convalescent sample. These antibody titers are useful in documenting past exposure and are used primarily in epidemiologic studies.
Intact vesicles are rare intraorally. Therefore, using intraoral viral culture as the sole means of diagnostic confirmation of HSV infection is inappropriate. Research has shown that asymptomatic oral HSV shedding occurs in up to 9% of the general population. During periods of mental or physical stress, asymptomatic viral shedding rises to approximately one third of those previously exposed to the virus. In immunocompromised patients, the prevalence rises to 38%; this percentage is low and most likely would double if the investigation were restricted to those previously exposed to the virus. Therefore, culture of lesions contaminated with saliva that might contain coincidentally released HSV is meaningless unless supplemented by additional diagnostic procedures.
Two of the most commonly used diagnostic procedures are the cytologic smear and tissue biopsy, with cytologic study being the least invasive and most cost-effective. The virus produces distinctive histopathologic alterations within the infected epithelium. Only VZV produces similar changes, but these two infections usually can be differentiated on a clinical basis. Fluorescent monoclonal antibody typing can be performed on the direct smears or on infected cells obtained from tissue culture.
If diagnostic features of herpesvirus are discovered in a biopsy of a persistent ulceration in an immunocompromised patient, immunocytochemical studies for CMV also should be performed to rule out coinfection. The histopathologic features of CMV can be missed easily, resulting in patients not receiving the most appropriate therapy.
TREATMENT AND PROGNOSIS: In the past, primary herpetic gingivostomatitis was treated best symptomatically; however, if the infection is diagnosed early, antiviral medications can have a significant influence. Patients should be instructed to restrict contact with active lesions to prevent the spread to other sites and people. As mentioned previously, autoinoculation of the eyes can result in ocular involvement with the possibility of recurrence. Repeated ocular reinfection can produce permanent damage and blindness. HSV is the leading infectious cause of blindness in the United States.
When acyclovir suspension is initiated during the first 3 symptomatic days in a rinse-and-swallow technique five times daily for 5 days (children: 15 mg/kg up to the adult dose of 200 mg), significant acceleration in clinical resolution is seen. Once therapy is initiated, development of new lesions ceases. In addition, the associated eating and drinking difficulties, pain, healing time, duration of fever, and viral shedding are shortened dramatically. The use of a topical spray with 0.5% or 1.0% dyclonine hydrochloride also dramatically, but temporarily, decreases the mucosal discomfort. Compounding pharmacists also can provide tetracaine lollipops that can be used for rapid and profound numbing of the affected mucosa. Viscous lidocaine and topical benzocaine should be avoided in pediatric patients because of reports of lidocaine-induced seizures in children and an association between topical benzocaine and methemoglobinemia. Nonsteroidal antiinflammatory drugs (NSAIDs), such as ibuprofen, also help alleviate the discomfort. Use of antiviral medications in capsule or tablet form is much less effective because of the increased time these formulations require to exert a significant effect.
Recurrent herpes labialis has been treated with everything from ether to voodoo; nothing has solved the problem for all patients. Of the antiherpetic medications, acyclovir ointment in polyethylene glycol was the initial formulation available for topical therapy. Acyclovir ointment has been of limited benefit for herpes labialis in immunocompetent patients, because its base is thought to prevent significant absorption. Subsequently, penciclovir cream became available in a base that allows increased absorption through the vermilion border. Use of this formulation has resulted in a statistically significant, although clinically minimal, reduction in healing time and pain (duration decreased approximately 1 day). Although the best results are obtained if use of penciclovir cream is initiated during the prodrome, late application has produced a measurable clinical benefit. Other current choices are acyclovir cream and an over-the-counter formulation of 10% n-docosanol cream. Although acyclovir cream does appear more effective than n-docosanol, both of these therapeutic choices are associated with statistically significant, but clinically minimal, reduction in healing time and pain, but at a lesser degree than that associated with penciclovir cream.
Systemic acyclovir and the two newer related medications, valacyclovir and famciclovir, appear to demonstrate similar effectiveness against HSV. However, valacyclovir and famciclovir exhibit improved bioavailability and more convenient oral dosing schedules. Of the three medications, a dosing schedule with valacyclovir, consisting of an initial 2 g taken on recognition of prodromal symptoms followed by another 2 g 12 hours later, has been most successful in minimizing the recurrences. The effects of this treatment are reduced significantly if it is not initiated during the prodrome. Although much less convenient, 400 mg of acyclovir taken five times daily for 5 days appears to produce similar results. For patients whose recurrences appear to be associated with dental procedures, a regimen of 2 g of valacyclovir taken twice on the day of the procedure and 1 g taken twice the next day may suppress or minimize any associated attack. In individuals with a known trigger that extends over a period of time (e.g., skiing, beach vacation), prophylactic short-term use of one of the antivirals (acyclovir, 400 mg twice a day [b.i.d.]; valacyclovir 1 g daily; or famciclovir 250 mg b.i.d.) has been shown to reduce the prevalence and severity of any associated recurrence.
Most cases of recurrent herpes labialis are infrequent; therefore, rarely can regular use of systemic antiviral medications be justified in immunocompetent individuals. Long-term suppression of recurrences with an antiviral medication is reserved by many for patients with more than six recurrences per year, those suffering from HSV-triggered erythema multiforme, and the immunocompromised. In recent years the emergence of acyclovir-resistant HSV has been seen with increasing frequency. Such resistance has arisen almost exclusively in immunocompromised patients receiving intermittent therapy, and the use of prophylactic therapy does not appear to be associated with emergence of resistant strains. In immunocompromised patients, the viral load tends to be high and replication is not suppressed completely by antiviral therapy, creating the environment for generating drug-resistant mutants. Although resistance is seen primarily in immunocompromised patients, cavalier use of antiviral medications for mild cases of recurrent herpes infection probably is inappropriate.
The pain associated with intraoral secondary herpes usually is not intense, and many patients do not require treatment. Some studies have shown chlorhexidine to exert antiviral effects in vivo and in vitro. In addi-tion, acyclovir appears to function synergistically with chlorhexidine. Extensive clinical trials have not been performed, but chlorhexidine alone or in combination with acyclovir suspension may be beneficial in patients who desire or require therapy of intraoral lesions.
Immunocompromised hosts with HSV infections often require intravenous (IV) antiviral medications to control the problem. Furthermore, severely immunosuppressed individuals, such as bone marrow transplant patients and those with AIDS, often need prophylactic doses of oral acyclovir, valacyclovir, or famciclovir. On occasion, viral resistance develops, resulting in the onset of significant herpetic lesions. Any herpes lesions that do not respond to appropriate therapy within 5 to 10 days most likely are the result of resistant strains. At this point the initial antiviral therapy should be repeated at an elevated dose. If this intervention fails, IV trisodium phosphonoformate hexahydrate (foscarnet) is administered. If the infection persists, IV cidofovir is recommended. Another antiviral, adenine arabinoside (vidarabine), is reserved for patients in whom all of the previously described medications have failed. In resistant cases that have been treated successfully, it appears that only the peripheral virus mutates, because future recurrences often are once again sensitive to the first-line antivirals. Ulcerations that reveal coinfection with HSV and CMV respond well to ganciclovir, with foscarnet used in refractory cases.
Although a successful live-virus vaccine has been available for the closely related varicella virus for over 25 years, similar approaches against HSV have produced less satisfactory results. Significant research for a potential vaccine is ongoing and offers hope for the future.
VARICELLA (CHICKENPOX)
The varicella-zoster virus (VZV, HHV-3) is similar to herpes simplex virus (HSV) in many respects. Chick-enpox represents the primary infection with the VZV; latency ensues, and recurrence is possible as herpes zoster, often after many decades. The virus is presumed to be spread through air droplets or direct contact with active lesions. Most cases of chickenpox arise between the ages of 5 and 9, with greater than 90% of the U.S. population being infected by 15 years of age. In contrast to infection with HSV, most cases are symptomatic. The incubation period is 10 to 21 days, with an average of 15 days.
CLINICAL FEATURES: The symptomatic phase of VZV infection usually begins with malaise, pharyngitis, and rhinitis. In older children and adults, additional symptoms (e.g., headache, myalgia, nausea, anorexia, vomiting) occasionally are seen. This is followed by a characteristic, intensely pruritic exanthem. The rash begins on the face and trunk, followed by involvement of the extremities. Each lesion rapidly progresses through stages of erythema, vesicle, pustule, and hardened crust (Figs. 7-14 and 7-15). The early vesicular stage is the classic presentation. The centrally located vesicle is surrounded by a zone of erythema and has been described as “a dewdrop on a rose petal.” In contrast to herpes simplex, the lesions typically continue to erupt for 4 days; in some cases the exanthem’s arrival may extend to 7 or more days. Old crusted lesions intermixed with newly formed and intact vesicles are commonplace. Affected individuals are contagious from 2 days before the exanthem until all the lesions crust. Fever usually is present during the active phase of the exanthem. The severity of the cutaneous involvement is variable and often more severe in adults and in household members secondarily infected by the initial patient.
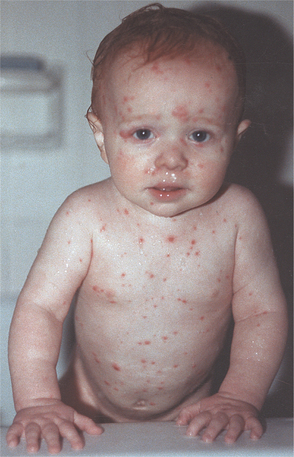
Fig. 7-14 Varicella. Infant with diffuse erythematous and vesicular rash. (Courtesy of Dr. Sherry Parlanti.)
Perioral and oral manifestations are fairly common and may precede the skin lesions. The vermilion border of the lips and the palate are the most common sites of involvement, followed by the buccal mucosa. Occasionally, gingival lesions resemble those noted in primary HSV infections, but distinguishing between the two is not difficult because the lesions of varicella tend to be relatively painless. The lesions begin as 3- to 4-mm, white, opaque vesicles that rupture to form 1- to 3-mm ulcerations (Fig. 7-16). The prevalence and number of the oral lesions correlate with the severity of the extraoral infection. In mild cases, oral lesions are present in about one third of affected individuals. Often only 1 or 2 oral ulcers are evident, and typically these heal within 1 to 3 days. In contrast, patients with severe infections almost always have oral ulcerations, often numbering up to 30 lesions and persisting for 5 to 10 days. In severe cases of chickenpox, old ruptured lesions will often become intermixed with fresh vesicles.
Complications can occur, with the need for hospitalization in children approximating 1 in 600 in the prevaccine era. Possible complications include Reye’s syndrome, secondary skin infections, encephalitis, cerebellar ataxia, pneumonia, gastrointestinal dis-turbances (e.g., vomiting, diarrhea, associated dehydration), and hematologic events (i.e., thrombocytopenia, pancytopenia, hemolytic anemia, sickle cell crisis).
In childhood the most frequent complications are secondary skin infections, followed by encephalitis and pneumonia. With enhanced public education and decreased use of aspirin in children, the prevalence of Reye’s syndrome is decreasing. Although associated bacterial infections had decreased after the introduction of antibiotics, an increased prevalence of significant complications related to secondary infections caused by group A, b-hemolytic streptococci was seen during the 1990s. These organisms have created life-threatening infections and areas of highly destructive necrotizing fasciitis.
The prevalence of complications in adults exceeds that noted in children. The most common and serious complication is varicella pneumonitis, which features dry cough, tachypnea, dyspnea, hemoptysis, chest pain, and cyanosis. Encephalitis and clinically significant pneumonia are diagnosed in 1 in 375 affected adults older than 20 years of age. The central nervous system (CNS) involvement typically produces ataxia but may result in headaches, drowsiness, convulsions, or coma. The risk of death is reported to be 15 times greater in adults compared with children, mostly because of an increased prevalence of encephalitis.
Infection during pregnancy can produce congenital or neonatal chickenpox. Involvement early in the pregnancy can result in spontaneous abortion or congenital defects. Although complications can occur in newborns, the effects of maternal varicella infection appear minimal. A multicenter prospective study of live births associated with maternal varicella infection revealed only a 1.2% prevalence of embryopathy. However, infection of the mother close to delivery can result in a severe fetal infection caused by a lack of maternal antibodies.
Infection in immunocompromised patients also can be most severe. The cutaneous involvement typically is extensive and may be associated with high fever, hepatitis, pneumonitis, pancreatitis, gastrointestinal obstruction, and encephalitis. Before effective antiviral therapy, the mortality rate in immunocompromised individuals was approximately 7%. Secondary bacterial infections often complicate the process.
HISTOPATHOLOGIC FEATURES: The cytologic alterations are virtually identical to those described for HSV. The virus causes acantholysis, with formation of numerous free-floating Tzanck cells, which exhibit nuclear margination of chromatin and occasional multinucleation.
DIAGNOSIS: The diagnosis of chickenpox usually can be made from a history of exposure to VZV within the last 3 weeks and the presence of the typical exanthem. Confirmation can be obtained through a demonstration of viral cytopathologic effects present within the epithelial cells harvested from the vesicular fluid. These cytologic changes are identical to those found in herpes simplex, and further confirmation sometimes is desired. Viral isolation in cell culture or rapid diagnosis from fluorescein-conjugated VZV monoclonal antibodies can be performed. Finally, serum samples can be obtained during the acute stage and 14 to 28 days later. The later sample should demonstrate a significant (fourfold) increase in antibody titers to VZV.
TREATMENT AND PROGNOSIS: Before the current antiviral medications became available, the treatment of varicella primarily was symptomatic. Warm baths with soap or baking soda, application of calamine lotion, and systemic diphenhydramine still are used to relieve pruritus. VZV has a lipid envelope that is destroyed rapidly by soap and other detergents. Lotions with diphenhydramine are not recommended because of reports of toxicity secondary to percutaneous absorption of the medication. Antipyretics other than aspirin should be given to reduce fever.
Use of peroral antiviral medications such as acyclovir, valacyclovir, and famciclovir has been shown to reduce the duration and severity of the infection if it is administered within the first 24 hours of the rash. Routine use of these antiviral medications is not recommended in immunocompetent children with uncomplicated chickenpox. Typically, such therapy is reserved for patients at risk for more severe disease, such as those over 13 years of age and individuals who contract the disease from a family member. Intravenous formulations are used in immunosuppressed patients or those exhibiting a progressive, severe infection. Treatment with one of the available antiviral medications does not alter the antibody response to VZV or reduce immunity later in life.
In patients without evidence of immunity who become exposed to VZV and are at high risk for severe disease or complications, purified varicella-zoster immune globulin can be given to modify the clinical manifestations of the infection. Individuals at risk include immunocompromised patients, pregnant women, premature infants, and neonates whose mothers do not have evidence of immunity. The U.S.-licensed manufacturer of the immune globulin marketed the material under the name VZIG but discontinued production in October 2004. At the time of this writing, the immune globulin is being made by a Canadian company and is known as VariZIG. This product has not completed full U.S. Food and Drug Administration (FDA) approval and is currently classified as an investigational new drug (IND). In an attempt to improve access during this critical period of transition, an expanded access protocol has been approved by a central institutional review board (IRB), with the FDA not requiring additional local IRB approval at the treatment site. VariZIG is most effective if administered within 96 hours of initial exposure. In most instances the material can be delivered from the distributor to the treatment site within 24 hours.
A live attenuated varicella vaccine has been available since 1974 and has been used extensively outside the United States, especially in Japan. In 1995 the vaccine was approved for use in the United States. Before that time, the annual incidence of infection in the United States was approximately 4 million, with an associated 11,000 hospitalizations and 100 deaths. Vaccination is recommended for children between 12 and 18 months of age, as well as for all susceptible individuals over the age of 13. Although the vaccination rates vary by state, the national coverage is approximately 85% and has led to a reduction of reported infection rates that also is around 85%.
During the first year after vaccination, the efficacy appears to be 100% but drops to 95% after 7 years. When breakthrough infections do occur, they usually are very mild. Because of continued exposure to wild virus, previously vaccinated patients have not required boosters to maintain immunity. As the prevalence of the wild virus diminishes, booster vaccines may be required to maintain lifelong immunity. Extensive follow-up of vaccinated groups is ongoing; if antibody levels wane with time, booster immunizations will be recommended. It should be remembered that the vaccine is a live virus that can be spread to individuals in close contact. Vaccine recipients who develop a rash should avoid contact with those at risk, such as immunocompromised or pregnant individuals.
The national health objectives for 2010 included a goal to obtain and maintain ≥95% vaccination coverage among first graders for hepatitis B, diphtheria, tetanus, pertussis, poliovirus, measles, mumps, rubella, and varicella. The measles, mumps, and rubella (MMR) vaccine currently has achieved a 93% vaccination rate, whereas the frequency for the varicella vaccine remains below 90%. Use of a combined measles, mumps, rubella, and varicella (MMRV) vaccine has demonstrated comparable effectiveness and safety. This approach would provide protection via a single injection and have the potential to increase the vaccination rate for varicella more rapidly.
HERPES ZOSTER (SHINGLES)
After the initial infection with VZV (chickenpox), the virus is transported up the sensory nerves and presumably establishes latency in the dorsal spinal ganglia. Clinically evident herpes zoster occurs after reactivation of the virus, with the involvement of the distribution of the affected sensory nerve. Zoster occurs during the lifetime of 10% to 20% of individuals, and the prevalence of attacks increases with age. With the increasing average age of the population, an increased prevalence of herpes zoster is expected. Unlike herpes simplex virus (HSV), single rather than multiple recurrences are the rule. Immunosuppression, HIV-infection, treatment with cytotoxic or immunosuppressive drugs, radiation, presence of malignancies, old age, alcohol abuse, stress (emotional or physical), and dental manipulation are predisposing factors for reactivation.
CLINICAL FEATURES: The clinical features of herpes zoster can be grouped into three phases: (1) prodrome, (2) acute, and (3) chronic. During initial viral replication, active ganglionitis develops with resultant neuronal necrosis and severe neuralgia. This inflammatory reaction is responsible for the prodromal symptoms of intense pain that precedes the rash in more than 90% of the cases. As the virus travels down the nerve, the pain intensifies and has been described as burning, tingling, itching, boring, prickly, or knifelike. The pain develops in the area of epithelium innervated by the affected sensory nerve (dermatome). Typically, one dermatome is affected, but involvement of two or more can occur. The tho racic dermatomes are affected in about two thirds of cases. This prodromal pain, which may be accompanied by fever, malaise, and headache, normally is present 1 to 4 days before the development of the cutaneous or mucosal lesions. During this period (before the exanthem) the pain may masquerade as sensitive teeth, otitis media, migraine headache, myocardial infarction, or appendicitis, depending on which dermatome is affected.
Approximately 10% of affected individuals will exhibit no prodromal pain. Conversely, on occasion there may be recurrence in the absence of vesiculation of the skin or mucosa. This pattern is called zoster sine herpete (zoster without rash), and affected patients have severe pain of abrupt onset and hyperesthesia over a specific dermatome. Fever, headache, myalgia, and lymphadenopathy may or may not accompany the recurrence.
The acute phase begins as the involved skin develops clusters of vesicles set on an erythematous base (Fig. 7-17). Within 3 to 4 days, the vesicles become pustular and ulcerate, with crusts developing after 7 to 10 days. The lesions tend to follow the path of the affected nerve and terminate at the midline (Fig. 7-18). The exanthem typically resolves within 2 to 3 weeks in otherwise healthy individuals. On healing, scarring with hypopigmentation or hyperpigmentation is not unusual.
Oral lesions occur with trigeminal nerve involvement and may be present on the movable or bound mucosa. The lesions often extend to the midline and frequently are present in conjunction with involvement of the skin overlying the affected quadrant. Like varicella, the individual lesions manifest as 1- to 4-mm, white, opaque vesicles that rupture to form shallow ulcerations (Fig. 7-19). Involvement of the maxilla may be associated with devitalization of the teeth in the affected area.
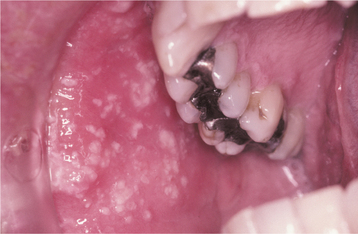
Fig. 7-19 Herpes zoster. Numerous white opaque vesicles on the right buccal mucosa of the same patient depicted in Fig. 7-18.
Several reports have documented significant bone necrosis with loss of teeth in areas involved with herpes zoster. Because of the close anatomic relationship between nerves and blood vessels within neurovascular bundles, inflammatory processes within nerves have the potential to extend to adjacent vessels. It is postulated that the gnathic osteonecrosis may be secondary to damage of the blood vessels supplying the alveolar ridges and teeth, leading to focal ischemic necrosis. Of the reported cases, there is almost an equal distribution between the maxilla and mandible, with both sexes affected similarly. Although the average patient age is approximately 55, a wide range has been seen from the second to late eighth decade. The average interval between the appearance of the exanthem and the osteonecrosis is 21 days, but it has been reported as late as 42 days.
Ocular involvement is not unusual and can be the source of significant morbidity, including permanent blindness. The ocular manifestations are highly variable and may arise from direct viral-mediated epithelial damage, neuropathy, immune-mediated damage, or secondary vasculopathy. If the tip of the nose is involved, this is a sign that the nasociliary branch of the fifth cranial nerve is involved, suggesting the potential for ocular infection. In these cases, referral to an ophthalmologist is mandatory.
Facial paralysis has been seen in association with herpes zoster of the face or external auditory canal. Ramsay Hunt syndrome is the combination of cutaneous lesions of the external auditory canal and involvement of the ipsilateral facial and auditory nerves. The syndrome causes facial paralysis, hearing deficits, vertigo, and a number of other auditory and vestibular symptoms. In multiple studies of patients thought to have Bell’s palsy (see page 859), evidenced of active VZV infection was detected in approximately 30% of patients by polymerase chain reaction (PCR) or via demonstration of appropriate antibody titers, suggesting an underlying viral cause for many cases of “idiopathic” facial paralysis. Similar associations also have been demonstrated with HSV and EBV.
Approximately 15% of affected patients progress to the chronic phase of herpes zoster, which is characterized by pain (postherpetic neuralgia) that persists longer than 3 months after the initial presentation of the acute rash. Postherpetic neuralgia is uncommon in individuals under the age of 50 but affects at least 50% of patients older than 60 years of age. The pain is described as burning, throbbing, aching, itching, or stabbing, often with flares caused by light stroking of the area or from contact with adjacent clothing. Most of these neuralgias resolve within 1 year, with half of the patients experiencing resolution after 2 months. Rare cases may last up to 20 years, and patients have been known to commit suicide as a result of the severe, lancinating quality of the pain. Although the cause is unknown, some investigators believe chronic VZV ganglionitis is responsible. Clearance of the pain has been reported within days after initiation of long-term famciclovir, with recurrence of the pain if the medication is stopped. Additional double-blind, placebo-controlled studies will need to be performed to confirm this observation.
HISTOPATHOLOGIC FEATURES: The active vesicles of herpes zoster are identical microscopically to those seen in the primary infection, varicella. For more information, refer to the previous portions of the chapter on the histopathologic presentation of varicella and herpes simplex.
DIAGNOSIS: The diagnosis of herpes zoster often can be made from the clinical presentation, but other procedures may be necessary in atypical cases. Viral culture can confirm the clinical impression but takes at least 24 hours. Cytologic smears demonstrate viral cytopathologic effects, as seen in varicella and HSV. In most cases the clinical presentation allows the clinician to differentiate zoster from HSV, but cases of zosteriform recurrent HSV infection, although uncommon, do exist. A rapid diagnosis can be obtained through the use of direct staining of cytologic smears with fluorescent monoclonal antibodies for VZV. This technique gives positive results in almost 80% of the cases. Molecular techniques such as dot-blot hybridization and PCR also can be used to detect VZV.
TREATMENT AND PROGNOSIS: Before the development of the current antiviral medications, therapy for herpes zoster was directed toward supportive and symptomatic measures. Fever should be treated with antipyretics that do not contain aspirin. Antipruritics, such as diphenhydramine, can be administered to decrease itching. Skin lesions should be kept dry and clean to prevent secondary infection; antibiotics may be administered to treat such secondary infections.
Early therapy with appropriate antiviral medications such as acyclovir, valacyclovir, and famciclovir has been found to accelerate healing of the cutaneous and mucosal lesions, reduce the duration of the acute pain, and decrease the duration of postherpetic neuralgia. These medications are most effective if initiated within 72 hours after development of the first vesicle. The newer generation of antiviral drugs, famciclovir and valacyclovir, may be more successful than acyclovir in reducing the prevalence of postherpetic neuralgia.
Once the skin lesions have healed, the neuralgia may become the worst aspect of the disease and often is the most difficult to resolve successfully. This intense pain has been treated with variable results by a variety of methods, including analgesics, narcotics, tricyclic antidepressants, anticonvulsants, gabapentin, percutaneous electric nerve stimulation, biofeedback, nerve blocks, and topical anesthetics. As mentioned previously, postherpetic neuralgia may be related to chronic VZV ganglionitis and respond to long-term famciclovir. In those who do not respond to famciclovir, IV acyclovir often leads to clinical improvement.
One topical treatment, capsaicin, has had significant success, with almost 80% of patients experiencing some pain relief; however, the medication’s effect often does not occur until 2 weeks or more of therapy. Capsaicin is derived from red peppers and is not recommended for placement on mucosa or open cutaneous lesions. Capsaicin has been associated with significant burning, stinging, and redness in 40% to 70% of patients, with up to 30% discontinuing therapy because of this side effect. After use, patients must be warned to wash their hands and avoid contact with mucosal surfaces.
Corticosteroid therapy has been used in the hope it might decrease the neural inflammation and associated chronic pain. Although conflicting research has been published, studies have shown no long-term benefit when corticosteroids are added to an acyclovir regimen. In addition, an increased prevalence of side effects was noted in groups treated with corticosteroids.
A live attenuated VZV vaccine has been approved for use in adults 60 years of age or older. The vaccine, Zostavax, is 14 times more potent than Varivax, the vaccine for chickenpox. In a study of more than 38,000 adults, Zostavax markedly decreased the prevalence of herpes zoster, as well as the morbidity and frequency of postherpetic neuralgia in those who did develop the infection. In an October 2006 press release, the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommended that the vaccine be given to all people 60 years of age and older. This recommendation is being reviewed and becomes official only when published in the CDC’s Morbidity and Mortality Weekly Report.
INFECTIOUS MONONUCLEOSIS (MONO; GLANDULAR FEVER; “KISSING DISEASE”)
Infectious mononucleosis is a symptomatic disease resulting from exposure to Epstein-Barr virus (EBV, HHV-4). The infection usually occurs by intimate contact. Intrafamilial spread is common, and once a person is exposed, EBV remains in the host for life. Children usually become infected through contaminated saliva on fingers, toys, or other objects. Adults usually contract the virus through direct salivary transfer, such as shared straws or kissing, hence, the nickname “kissing disease.” Exposure during childhood usually is asymptomatic, and most symptomatic infections arise in young adults. In developing nations, exposure usually occurs by age 3 and is universal by adolescence. In the United States, introduction to the virus often is delayed, with close to 50% of college students lacking previous exposure. These unexposed adults become infected at a rate of 10% to 15% per year while in college. Infection in adulthood is associated with a higher risk (i.e., 30% to 50%) for symptomatic disease.
Besides infectious mononucleosis, EBV has been demonstrated in the lesions of oral hairy leukoplakia (OHL) (see page 268) and has been associated with a number of lymphoproliferative disorders, a variety of lymphomas (most notably African Burkitt’s lymphoma) (see page 600), nasopharyngeal carcinoma (see page 428), some gastric carcinomas, possibly breast and hepatocellular carcinomas, salivary lymphoepithelial carcinomas, and occasional smooth muscle tumors. However, direct proof of a cause-and-effect relationship is lacking.
CLINICAL FEATURES: Most EBV infections in children are asymptomatic. In children younger than 4 years of age with symptoms, most have fever, lymphadenopathy, pharyngitis, hepatosplenomegaly, and rhinitis or cough. Children older than 4 years of age are affected similarly but exhibit a much lower prevalence of hepatosplenomegaly, rhinitis, and cough.
Most young adults experience fever, lymphadenopathy, pharyngitis, and tonsillitis. Hepatosplenomegaly and rash are seen less frequently. In adults older than 40 years of age, fever and pharyngitis are the predominant findings, with less than 30% demonstrating lymphadenopathy. Less frequent signs and symptoms in this group include hepatosplenomegaly, rash, and rhinitis or cough. Possible significant complications include splenic rupture, thrombocytopenia, autoimmune hemolytic anemia, aplastic anemia, and neurologic problems with seizures. These complications are uncommon at any age but more frequently develop in children.
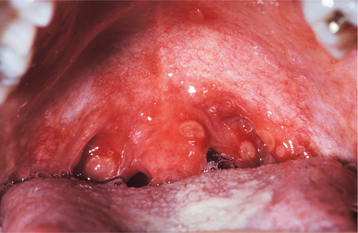
In classic infectious mononucleosis in a young adult, prodromal fatigue, malaise, and anorexia occur up to 2 weeks before the development of pyrexia. The body temperature may reach 104° F and lasts from 2 to 14 days. Prominent lymphadenopathy is noted in more than 90% of the cases and typically appears as enlarged, symmetrical, and tender nodes, frequently with involvement of the posterior and anterior cervical chains. Enlargement of parotid lymphoid tissue rarely has been reported and can be associated with facial nerve palsy. More than 80% of affected young adults have oropharyngeal tonsillar enlargement, sometimes with diffuse surface exudates and secondary tonsillar abscesses (Fig. 7-20). The lingual tonsils, which are located on the base of the tongue and extend from the circumvallate papilla to the epiglottis, can become hyperplastic and compromise the airway. Rare fatalities have been reported from respiratory difficulties secondary to the combined effects of hyperplasia of the lingual and palatine tonsils, arytenoid hypertrophy, pharyngeal edema, uvular edema, and epiglottal swelling.
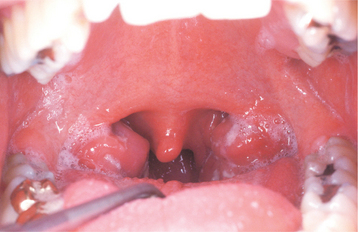
Fig. 7-20 Infectious mononucleosis. Hyperplastic pharyngeal tonsils with yellowish crypt exudates. (Courtesy of Dr. George Blozis.)
Oral lesions other than lymphoid enlargement also may be seen. Petechiae on the hard or soft palate are present in about 25% of patients (Fig. 7-21). The petechiae are transient and usually disappear within 24 to 48 hours. Necrotizing ulcerative gingivitis (NUG) (see page 157) also is fairly common. NUG-like pericoronitis (see page 171) and necrotizing ulcerative mucositis (see page 158) occur less frequently. Cases of NUG that are refractory to normal therapy should be evaluated to rule out the possibility of EBV.
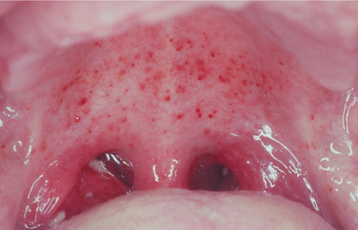
Fig. 7-21 Infectious mononucleosis. Numerous petechiae of the soft palate. (Courtesy of Dr. George Blozis.)
A controversial symptom complex called chronic fatigue syndrome has been described, and several investigators have tried to associate EBV with this problem. Patients complain of rather nonspecific symptoms of chronic fatigue, fever, pharyngitis, myalgias, headaches, arthralgias, paresthesias, depression, and cognitive defects. These patients often demonstrate elevations in EBV antibody titers, but this finding alone is insufficient to prove a definite cause-and-effect relationship. Several studies have cast serious doubt on a relationship between EBV and the chronic fatigue syndrome.
DIAGNOSIS: The diagnosis of infectious mononucleosis is suggested by the clinical presentation and should be confirmed through laboratory procedures. The white blood cell (WBC) count is increased, with the differential count showing relative lymphocytosis that can become as high as 70% to 90% during the second week. Atypical lymphocytes usually are present in the peripheral blood. The classic serologic finding in mononucleosis is the presence of Paul-Bunnell heterophil antibodies (immunoglobulins that agglutinate sheep erythrocytes). A rapid test for these antibodies is available and in-expensive. More than 90% of infected young adults have positive findings for the heterophil antibody, but infected children younger than age 4 frequently have negative results. Indirect immunofluorescent testing to detect EBV-specific antibodies should be used in those suspected of having an EBV infection but whose findings were negative on the Paul-Bunnell test. Enzyme-linked immunosorbent assays (ELISA) and recombinant DNA-derived antigens also may be used in place of the indirect immunofluorescent test.
TREATMENT AND PROGNOSIS: In most cases, infectious mononucleosis resolves within 4 to 6 weeks. Non–aspirin-containing antipyretics and NSAIDs can be used to minimize the most common symptoms. Infrequent complications include splenic rupture, EBV-related hepatitis, and Bell’s palsy. Patients with significant enlargement of the spleen should avoid contact sports to prevent the rare possibility of splenic rupture. On occasion, the fatigue may become chronic. In immunocompromised patients, a polyclonal B-lymphocyte proliferation may occur and possibly lead to death.
The tonsillar involvement may, on occasion, resemble streptococcal pharyngitis or tonsillitis (see page 183). However, treatment with ampicillin and penicillin should be avoided because the use of these antibiotics in infectious mononucleosis has been associated with a higher than normal prevalence of allergic morbilliform skin rashes.
Corticosteroid use is the recommended therapy in many textbooks. Such drugs, however, should not be used indiscriminately because the person’s immune response appears to be the most important factor in fighting the infection and preventing a potentially fatal polyclonal B-lymphocyte proliferation. In addition, an increased prevalence of encephalitis and myocarditis has been noted in patients who have infectious mononucleosis and are treated with steroids. Corticosteroid use produces a shortened duration of fever and shrinkage of enlarged lymphoid tissues, but its use should be restricted to life-threatening cases (e.g., those with upper-airway obstruction because of massive lymphadenopathy, tonsillar hyperplasia, and oropharyngeal edema). If corticosteroid therapy fails to resolve the airway obstruction, acute tonsillectomy and tracheostomy may be necessary.
Although antiviral medications such as acyclovir, valacyclovir, and famciclovir have been used successfully for temporary resolution of oral hairy leukoplakia, these medications do not demonstrate clinically obvious benefit for patients with infectious mononucleosis. Although the medications most likely have an effect on viral replication, the main clinical manifestations appear to be secondary to the immune response to EBV-infected activated B lymphocytes and are not altered by the medical intervention.
CYTOMEGALOVIRUS
Cytomegalovirus (CMV, HHV-5) is similar to the other human herpes viruses (i.e., after the initial infection, latency is established and reactivation is possible under conditions favorable to the virus). CMV can reside latently in salivary gland cells, endothelium, macrophages, and lymphocytes. Most clinically evident disease is found in neonates or in immunosuppressed adults. In infants, the virus is contracted through the placenta, during delivery, or during breast-feeding. The next peak of transmission occurs during adolescence, predominantly from the exchange of bodily fluids as this group begins sexual activity. Transmission also has been documented from blood transfusion and organ transplantation. The prevalence of neonatal CMV infection varies from 0.5% to 2.5%. By the age of 30, almost 40% of the population is infected; by age 60, 80% to 100% are infected. Screening of healthy middle-aged adult blood donors reveals that approximately 50% have been exposed to CMV.
CLINICAL FEATURES: At any age, almost 90% of CMV infections are asymptomatic. In clinically evident neonatal infection, the infant appears ill within a few days. Typical features include hepatosplenomegaly, extramedullary cutaneous erythropoiesis, and thrombocytopenia (often with associated petechial hemorrhages). Significant encephalitis frequently leads to severe mental and motor retardation.
Although the majority of acute CMV infections are asymptomatic, less than 10% may include a nonspecific pattern of symptoms that ranges from an influenza-like presentation to lethal multiorgan involvement. In a review of 115 hospitalized immunocompetent adults with CMV infection, the most common symptoms (in order) include fever, joint and muscle pain, shivering, abdominal pain, nonproductive cough, cutane-ous eruption (maculopapular rash), and diarrhea. Associated signs include hepatomegaly, splenomegaly, adenopathy, pharyngitis, jaundice, and evidence of meningeal irritation. The authors stress that symptomatic CMV infection should not be dismissed in immunocompetent patients and should be considered in any patient with unexplained persistent fever.
In contrast to patients with infectious mononucleosis, only about one third of patients with CMV infection demonstrate pharyngitis and lymphadenopathy. Rarely, immunocompetent patients may show signs of an acute sialadenitis that diffusely involves all of the major and minor salivary glands. In such cases, xerostomia often is noted and the affected glands are painful. Involvement of the major glands usually results in clinically obvious enlargements of the parotid and submandibular glands. Unusual complications of primary CMV infection include myocarditis, pneumonitis, and septic meningitis.
Evident CMV involvement is not unusual in immunocompromised transplant patients. In some cases a temporary mild fever is the only evidence; in others, the infection becomes aggressive and is characterized by significant hepatitis, leukopenia, pneumonitis, gastroenteritis, and, more rarely, a progressive wasting syndrome.
CMV disease is common in patients with AIDS (see page 264). CMV chorioretinitis affects almost one third of patients with AIDS and tends to progress rapidly, often resulting in blindness. Bloody diarrhea from CMV colitis is fairly common but may respond to appropriate antiviral medications.
Although oral lesions from CMV infection have been documented in a number of immunosuppressive conditions, reports of oral involvement by CMV have been increasing since the advent of the AIDS epidemic. Most affected patients have chronic mucosal ulcerations, and CMV changes are found on biopsy. Occasionally, chronic oral ulcerations in immunocompromised patients will demonstrate coinfection (usually CMV combined with HSV).
Neonatal CMV infection also can produce developmental tooth defects. Examination of 118 people with a history of neonatal CMV infection revealed tooth defects in 40% of those with symptomatic infections and slightly more than 5% of those with asymptomatic infections. The teeth exhibited diffuse enamel hypoplasia, significant attrition, areas of enamel hypomaturation, and yellow coloration from the underlying dentin.
HISTOPATHOLOGIC FEATURES: Biopsy specimens of intraoral CMV lesions usually demonstrate changes within the vascular endothelial cells. Scattered infected cells are extremely swollen, showing both intracytoplasmic and intranuclear inclusions and prominent nucleoli. This enlarged cell has been called an “owl eye” cell. Gomori’s methenamine silver and periodic acid-Schiff (PAS) stains demonstrate the cytoplasmic inclusions but not the intranuclear changes. Salivary ductal epithelium also may be affected and form “owl eye” cells (Fig. 7-22).
DIAGNOSIS: The diagnosis of CMV infection is made by considering a combination of the clinical features and by conducting other examinations. Biopsy material can demonstrate cellular changes that suggest infection. Because effective therapies exist for CMV infections in immunocompromised patients, biopsies are recommended for chronic ulcerations that are not responsive to conservative therapy. More specific verification can be made by electron microscopy, detection of viral antigens by immunohistochemistry, in situ hybridization, polymerase chain reaction (PCR), demonstration of rising viral antibody titers, or viral culture. Enzyme-linked immunosorbent assay (ELISA) serologic testing for CMV is inexpensive, demonstrates good specificity, and should be considered in any patient with an unexplained fever or signs of CMV infection.
In immunocompromised patients with chronic ulcerations, the typical “owl eye” cells may be few and difficult to discover on routine light microscopy. When biopsy is performed on a chronic oral ulceration in these patients, in situ hybridization or immunohistochemical evaluation for CMV should be performed, even in the absence of “owl eye” cells. In addition, close examination to rule out coinfection by HSV also should be performed.
TREATMENT AND PROGNOSIS: Although most CMV infections resolve spontaneously, therapy often is required in the immunosuppressed patient. Ganciclovir has resolved clinical symptoms in more than 75% of treated immunocompromised patients. However, the medication must be continued to prevent a relapse if the immune dysfunction persists. In patients with oral ulcerations coinfected with CMV and HSV, intravenous (IV) ganciclovir will produce resolution in most instances. The development of resistance to ganciclovir has been reported; other effective medications include foscarnet, cidofovir, and valganciclovir. In spite of these antiviral medications, the best therapy in immunocompromised patients remains improvement of their immune status, such as that achieved with highly active anti-retroviral therapy (HAART) therapy in many patients with AIDS (see page 280).
Immunocompetent patients with clinically evident CMV infection usually are treated symptomatically with antipyretic medications and NSAIDs. Corticosteroids or IV gammaglobulins have been used in patients with hemolytic anemia or severe thrombocytopenia. There is no consensus related to the use of antiviral agents in immunocompetent patients.
ENTEROVIRUSES
Human enterovirus infections traditionally have been classified into echoviruses, coxsackieviruses A and B, and polioviruses. Beginning in the 1960s, newly discovered enteroviruses have been assigned a numeric designation (e.g., enterovirus 71) rather than being placed into one of the traditional groups. The clinical presentations associated with these viruses are diverse and vary from a minor febrile illness to a severe and potentially fatal infection. In addition, some of these viruses have been associated with an increased prevalence of type 1 diabetes mellitus and dilated cardiomyopathy. The estimated annual incidence of symptomatic infections in the United States is 10 to 15 million.
Of the enteroviruses, more than 30 exist that can result in symptomatic infections associated with rashes. Few are distinctive enough clinically to allow differentiation from one another. Most are asymptomatic or subclinical. These infections may arise at any age, but most occur in infants or young children. Neonatal cases also have been reported. Only herpangina, hand-foot-and-mouth disease, and acute lymphonodular pharyngitis deserve discussion. These three clinical patterns are closely related and should not be considered as entirely separate infections. In reports of epidemics in which a large number of patients acquire the same strain of the virus, the clinical presentations often are variable and include both herpangina and hand-foot-and-mouth disease.
Herpangina usually is produced by coxsackievirus A1 to A6, A8, A10, or A22. However, it also may represent infection by coxsackievirus A7, A9, or A16; coxsackievirus B2 to 6; echovirus 9, 16, or 17; or enterovirus 71. Hand-foot-and-mouth disease usually is caused by coxsackievirus A16, but may also arise from coxsackievirus A5, A9, or A10; echovirus 11; or enterovirus 71. Acute lymphonodular pharyngitis is less recognized, and coxsackievirus A10 has been found in the few reported cases. The incubation period for these viruses is 4 to 7 days.
Most cases arise in the summer or early fall in nontropical areas, with crowding and poor hygiene aiding their spread. The fecal-oral route is considered the major path of transmission, and frequent hand washing is emphasized in an attempt to diminish spread during epidemics. During the acute phase, the virus also can be transmitted through saliva or respiratory droplets. Infection confers immunity against reinfection to that one strain. In spite of the developed immunity, people may become infected numerous times with different enterovirus types over several years while still remaining susceptible to other different strains.
CLINICAL FEATURES: In many countries, epidemics occur every 2 to 3 years and primarily affect children aged 1 to 4 years. The timing of the epidemics appears to be correlated to the accumulation of a new population of susceptible young children. In all three clinical patterns, the severity and significant complications are variable and appear associated with the particular strain that is responsible. In general, most strains produce a self-limiting disease that requires no therapy, but occasional strains can produce epidemics with an increased number of significant complications and occasional mortalities. Systemic complications include pneumonia, pulmonary edema and hemorrhage, acute flaccid paralysis, encephalitis, meningitis, and carditis. Infection with coxsackie B virus during pregnancy occasionally has been associated with fetal and neonatal death, whereas cardiac anomalies have been noted in infants who survive the initial infection.
In 1998 a massive epidemic spread over Taiwan (population 21,178,000), and it is estimated that approximately 1.5 million people developed clinical evidence of the infection. A group of sentinel physicians (8.7% of primary physicians) documented 129,106 infected patients. Of these patients, the vast majority were infected with enterovirus 71; a much lesser number were infected with one of a number of coxsackieviruses (predominantly A16). When patients infected with the same strain were examined, clinical patterns diagnostic of both herpangina and hand-foot-and-mouth disease were detected. In this epidemic, more than 75% had symptoms of hand-foot-and-mouth disease, but it is clear these two clinical patterns represent variations of the same disorder.
In the surveillance document by the Centers for Disease Control and Prevention (CDC) of the reported enterovirus infections between 1970 and 2005, 44.2% occurred in infants younger than 1 year of age, 15% in children aged 1 to 4 years, 11.6% in children aged 5 to 9 years, 11.9% in those aged 10 to 19 years, and 17.3% in patients older than 20 years. During this period, 131 deaths were reported secondary to complications such as aseptic meningitis, encephalitis, paralysis, myocarditis, and neonatal enteroviral sepsis. The most common viruses to produce herpangina or hand-foot-and-mouth disease were, in order, coxsackieviruses B5, A9, B3, B1, and A16, followed by enterovirus 71. In patients younger than the age of 20, there was a male predominance. In those older than 20, females were infected more frequently, most likely because of exposure as the primary caregivers to infected young children.
HERPANGINA: Herpangina begins with an acute onset of significant sore throat, dysphagia, and fever, occasion ally accompanied by cough, rhinorrhea, anorexia, vomiting, diarrhea, myalgia, and headache. Most cases, however, are mild or subclinical. A small number of oral lesions, usually two to six, develop in the posterior areas of the mouth, usually the soft palate or tonsillar pillars (Fig. 7-23). The affected areas begin as red macules, which form fragile vesicles that rapidly ulcerate. The ulcerations average 2 to 4 μm in diameter. The systemic symptoms resolve within a few days; as would be expected, the ulcerations usually take 7 to 10 days to heal.
HAND-FOOT-AND-MOUTH DISEASE: Hand-foot-and-mouth disease is the most well-known enterovirus infection. Like herpangina, the skin rash and oral lesions typically are associated with flulike symptoms (e.g., sore throat, dysphagia, fever), occasionally accompanied by cough, rhinorrhea, anorexia, vomiting, diarrhea, myalgia, and headache.
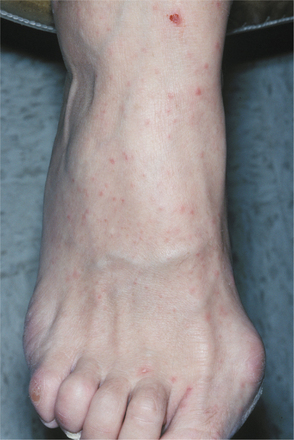
The name fairly well describes the location of the lesions. Oral lesions and those on the hands almost always are present; involvement of other cutaneous sites is more variable. The oral lesions arise without prodromal symptoms and precede the development of the cutaneous lesions. Sore throat and mild fever are present. The cutaneous lesions range from a few to dozens and primarily affect the borders of the palms and soles and the ventral surfaces and sides of the fingers and toes (Fig. 7-24). Rarely other sites, especially the buttocks, external genitals, and legs, may be involved. The individual cutaneous lesions begin as erythematous macules that develop central vesicles and heal without crusting (Fig. 7-25).
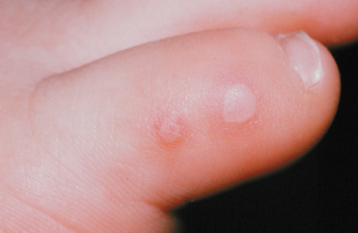
Fig. 7-24 Hand-foot-and-mouth disease. Multiple vesicles of the skin of the toe. (Courtesy of Dr. Samuel J. Jasper.)
The oral lesions resemble those of herpangina but may be more numerous and are not confined to the posterior areas of the mouth. The number of lesions ranges from 1 to 30. The buccal mucosa, labial mucosa, and tongue are the most common sites to be affected, but any area of the oral mucosa may be involved (Fig. 7-26). The individual vesicular lesions rapidly ulcerate and are typically 2 to 7 μm in diameter but may be larger than 1 cm. Most of these ulcerations resolve within 1 week.
ACUTE LYMPHONODULAR PHARYNGITIS: Acute lymphonodular pharyngitis is characterized by sore throat, fever, and mild headache, which may last from 4 to 14 days. Low numbers (one to five) of yellow to dark-pink nodules develop on the soft palate or ton sillar pillars (Fig. 7-27). The nodules represent hyperplastic lymphoid aggregates and resolve within 10 days without vesiculation or ulceration. Few cases have been described, and whether this represents a distinct clinical entity is as yet unresolved. The possibility that the sore throat and palatal lymphoid hyperplasia represent features of herpangina or some other infection cannot be excluded without further documentation of additional cases.
HISTOPATHOLOGIC FEATURES: In patients with herpangina and hand-foot-and-mouth disease, the areas of affected epithelium exhibit intracellular and intercellular edema, which leads to extensive spongiosis and the formation of an intraepithelial vesicle. The vesicle enlarges and ruptures through the epithelial basal cell layer, with the resultant formation of a subepithelial vesicle. Epithelial necrosis and ulceration soon follow. Inclusion bodies and multinucleated epithelial cells are absent.
DIAGNOSIS: The diagnoses of herpangina, hand-foot-and-mouth disease, and acute lymphonodular pharyngitis usually are made from the distinctive clinical manifestations. In patients with atypical presentations, laboratory confirmation appears prudent. Viral isolation from culture can be performed, and analysis of stool specimens is the best technique in patients with only mucosal lesions. Throat culture findings tend to be positive predominantly during the early acute stage. The culture of cutaneous lesions is best for the diagnosis of hand-foot-and-mouth disease. A serologic demonstration of rising enteroviral antibody titers between the acute and convalescent stages can be used to confirm the diagnosis in questionable cases. Polymerase chain reaction (PCR) assay is increasingly available and replacing viral culture in many diagnostic laboratories.
TREATMENT AND PROGNOSIS: In most instances, the infection is self-limiting and without significant complications. Therapy for patients with an enterovirus infection is directed toward symptomatic relief. Nonaspirin antipyretics and topical anesthetics, such as dyclonine hydrochloride, often are beneficial.
Occasionally, certain strains produce infections with a more aggressive clinical course. During the 1998 epidemic in Taiwan, a large group of physicians reported 405 patients with severe disease and 78 deaths. Patients with more significant complications demonstrated higher body temperature (>102° F), fever for longer than 3 days, more serious vomiting, and greater lethargy. When these findings are present, the physician must monitor the patient more closely for the development of more serious complications.
RUBEOLA (MEASLES)
Rubeola is an infection produced by a virus in the family Paramyxovirus, genus Morbillivirus, and exhibits a variable prevalence that is correlated to the degree of vaccine use. Measles vaccine has been in wide use in the United States since 1963 and is 95% effective, resulting in a 98% reduction in the prevalence of this infection. Before 1963, virtually all children acquired measles, but the vaccine produced a continued and significant decline until the late 1980s. From 1989 to 1991, a major resurgence occurred with an increas-ing proportion of cases among unvaccinated pre-school-aged children, particularly minority residents of densely populated urban areas. In addition, a smaller number of cases appeared to be associated with vaccine failure.
CLINICAL FEATURES: Most cases of measles arise in the winter and are spread through respiratory droplets. The incubation period is from 10 to 12 days, and affected individuals are infectious from 2 days before becoming symptomatic until 4 days after appearance of the associated rash. The virus is associated with significant lymphoid hyperplasia that often involves sites such as the lymph nodes, tonsils, adenoids, and Peyer’s patches. Giant cell infiltration is noted in various tissues along with a vasculitis that is responsible for the characteristic skin rash.
There are three stages of the infection, with each stage lasting 3 days and justifying the designation nine-day measles. The first 3 days are dominated by the three Cs: Coryza (runny nose), Cough (typically brassy and uncomfortable), and Conjunctivitis (red, watery, and photophobic eyes). Fever typically accompanies these symptoms. During this initial stage, the most distinctive oral manifestation, Koplik’s spots, is seen. Multiple areas of mucosal erythema are visible on the buccal and labial mucosa, and less often on the soft palate; within these areas are numerous small, blue-white macules (Fig. 7-28). In addition, similar spots rarely are noted on the inner conjunctival folds of the eye or the vaginal mucosa. These pathognomonic spots represent foci of epithelial necrosis and have been described as “grains of salt” on a red background.
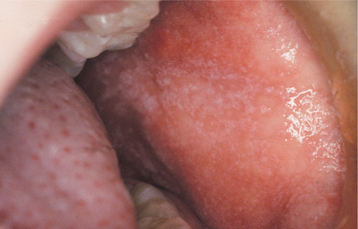
Fig. 7-28 Rubeola. Numerous blue-white Koplik’s spots of buccal mucosa. (Courtesy of Dr. Robert J. Achterberg.)
As the second stage begins, the fever continues, the Koplik’s spots fade, and a maculopapular and erythematous (morbilliform) rash begins. The face is involved first, with eventual downward spread to the trunk and extremities. Ultimately, a diffuse erythematous maculopapular eruption is formed, which tends to blanch on pressure (Fig. 7-29). Abdominal pain secondary to lymphatic involvement is not rare.
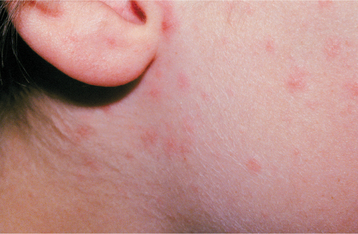
Fig. 7-29 Rubeola. Erythematous maculopapular rash of the face. (Courtesy of Dr. Robert J. Achterberg.)
In the third stage, the fever ends. The rash begins to fade and demonstrates a similar downward progression with replacement by a brown pigmentary staining. Ultimately, desquamation of the skin is noted in the areas previously affected by the rash.
Common complications in young children are otitis media, pneumonia, persistent bronchitis, and diarrhea. Acute appendicitis occasionally is seen secondary to vascular obstruction created by the swelling of Peyer’s patches. Encephalitis develops in approximately 1 in 1000 cases, often resulting in death or permanent brain damage and mental retardation. In about 1 in 100,000 cases, a delayed complication termed subacute sclerosing panencephalitis (SSPE) arises as late as 11 years after the initial infection. This degenerative disorder of the CNS leads to personality changes, seizures, coma, and death. Widespread vaccine use has virtually eliminated SSPE in developed nations. In the United States, one to two deaths occur for every 1000 reported cases of measles. In developing countries, the infection often is more severe, and the case-to-fatality rate can be as high as 25%. The most common causes of death are pneumonia and acute encephalitis.
Measles in immunocompromised patients can be serious, with a high risk of complications and death. Most of these patients exhibit either an atypical rash or no exanthem. Pneumonitis is the primary complication. The fatality rate of measles in patients with a malignancy is greater than 50%; AIDS-associated measles results in the death of more than one third of affected patients.
Koplik’s spots are not the only oral manifestation that may be associated with measles. Candidiasis, necrotizing ulcerative gingivitis (NUG), and necrotizing stomatitis may occur if significant malnutrition also is present. Severe measles in early childhood can affect odontogenesis and result in pitted enamel hypoplasia of the developing permanent teeth. Enlargement of accessory lymphoid tissues such as the lingual and pharyngeal tonsils also may be noted.
HISTOPATHOLOGIC FEATURES: Because of the reduced prevalence of measles and the transient nature of Koplik’s spots, few oral and maxillofacial pathologists have had the opportunity to view these lesions microscopically. Initially, Koplik’s spots represent areas of focal hyperparakeratosis in which the underlying epithelium exhibits spongiosis, intercellular edema, dyskeratosis, and epithelial syncytial giant cells. The number of nuclei within these giant cells ranges from three to more than 25. Close examination of the epithelial cells often reveals pink-staining inclusions in the nuclei or, less commonly, in the cytoplasm. On electron microscopy, the inclusions have been shown to represent microtubular aggregates characteristic of the causative paramyxovirus. As the spot ages, the epithelium exhibits heavy exocytosis by neutrophils leading to microabscess formation, epithelial necrosis, and, ultimately, ulceration. Frequently, examination of the epithelium adjacent to the ulceration will reveal the suggestive syncytial giant cells.
Examination of hyperplastic lymphoid tissue during the prodromal stage of measles often reveals a similar alteration. In 1931, Warthin and Finkeldey, in two separate publications, reported an unusual finding in patients who had their tonsils removed within 1 to 5 days of the clinical appearance of measles. Within the hyperplastic lymphoid tissue, there were numerous multinucleated giant lymphocytes (Fig. 7-30). These multinucleated cells subsequently have been termed Warthin-Finkeldey giant cells and were thought for a time to be specific for measles. Since that time, however, similar-appearing cells have been noted in a variety of lymphoproliferative conditions such as lymphoma, Kimura’s disease, AIDS-related lymphoproliferative disease, and lupus erythematosus.
DIAGNOSIS: The diagnosis of typical measles in an epidemic setting usually is straightforward and based on the clinical features and history. Laboratory confirmation can be of value in isolated or atypical cases. Viral isolation or rapid detection of viral antigens is possible, but confirmation usually is established through a demonstration of rising serologic antibody titers. The antibodies appear within 1 to 3 days after the beginning of the exanthem and peak in about 3 to 4 weeks.
TREATMENT AND PROGNOSIS: With a complication rate of 21%, the best treatment for measles is a good vaccination program; rubeola is part of the widely used MMR vaccine. In an attempt to stop the resurgence of measles that began in 1989, the vaccination schedule was altered and the pockets of young, unvaccinated children were targeted. This action brought the transmission of indigenous measles to record lows. Although the number is variable from year to year, since 1993 the annual incidence of reported cases in the United States typically is well below 500. Total eradication of the infection is technically feasible with existing vaccines but will require universal cooperation and enthusiasm from across the globe. Renewed emphasis in the noncompliant sections of society must be stressed. In addition, a new two-dose vaccination schedule has been adopted in an attempt to decrease the vaccine failures. Currently, routine vaccination is recommended for all children between the ages of 12 and 15 months, with a second dose administered between the ages of 4 and 6 years.
In otherwise healthy patients with measles, fluids and nonaspirin antipyretics are recommended for symptomatic relief. Immunocompromised patients also may be treated with one of a number of medications that have shown promise but definitively have not been proven to be efficacious. The most promising is ribavirin; however, immunoglobulin, interferon, and vitamin A also are being used.
RUBELLA (GERMAN MEASLES)
Rubella is a mild viral illness that is produced by a virus in the family Togavirus, genus Rubivirus. The greatest importance of this infection lies not in its effects on those who contract the acute illness, but in its capacity to induce birth defects in the developing fetus. The infection occurs primarily in the winter and spring, is contracted through respiratory droplets, and it is transmitted to nearly 100% of individuals in close living conditions. The incubation time is from 14 to 21 days, and infected patients are contagious from 1 week before the exanthem to about 5 days after the development of the rash. Infants with a congenital infection may release virus for up to 1 year.
In the past, this infection occurred in cycles, with localized epidemics every 6 to 9 years and pandemics every 10 to 30 years. The last pandemic occurred from 1962 to 1964. In 1964 and 1965, the United States alone had more than 12.5 million cases, which resulted in more than 10,000 fetal deaths (direct effects or secondary to therapeutic abortions) and 20,000 infants born with congenital rubella syndrome (CRS).
An effective vaccine, first released in 1969, is used widely and has dramatically affected the epidemiology of the infection and broken the cycle of occurrences. The vaccine is contraindicated in the following groups:
• Patients with acute febrile illnesses
• Patients with a known allergy to components of the vaccine
Researchers postulated that the protection of children also would eliminate the risk of exposure to women in the childbearing years. A 99% decrease in the infection was seen between 1969 and 1988, but young adults remain susceptible. Like rubeola, 1989 and 1990 demonstrated a slight resurgence of rubella, which was the result of a lack of vaccination diligence. More than 70% of the current cases occur in patients older than 15 years of age, and 10% to 25% of young adults remain susceptible. Of course, this should change when the previously vaccinated children grow into adults. For the present, the vaccination of postpubertal females must be stressed. Persons are presumed to be immune if they have received at least one dose of the MMR or were born before 1957. For pregnant females who have not received the vaccine, their immunity should be confirmed by demonstration of serum rubella IgG.
Initially, a single dose of the vaccine was thought to be sufficient to confer permanent immunity. Continued immunity appears to be permanent only if exposure to the virus occurs periodically, essentially serving as “booster doses.” With almost total vaccination coverage throughout the population of the United States, reexposures are not occurring and immunity is waning. To combat this loss of immunity in a highly vaccinated population, additional boosters may become necessary. Already, two doses of MMR vaccine are recommended, the first at 12 to 15 months of age and the second at the age of 4 to 6 years. Some believe a third dose may become necessary in the future.
CLINICAL FEATURES: A large percentage of infections are asymptomatic; the frequency of symptoms is greater in adolescents and adults. Prodromal symptoms may be seen 1 to 5 days before the exanthem and include fever, headache, malaise, anorexia, myalgia, mild conjunctivitis, coryza, pharyngitis, cough, and lymphadenopathy. The lymphadenopathy may persist for weeks and is noted primarily in the suboccipital, postauricular, and cervical chains. The most common complication is arthritis, which increases in frequency with age and usually arises subsequent to the rash. Rare complications include encephalitis and thrombocytopenia.
The exanthematous rash is often the first sign of the infection and begins on the face and neck, with spread to the entire body within 1 to 3 days. The rash forms discrete pink macules, then papules, and finally fades with flaky desquamation. The rash fades as it spreads and often exhibits facial clearing before the completion of its spread into the lower body areas. Generally, the rash is resolved completely by day 3, giving rise to the designation three-day measles.
Oral lesions, known as Forchheimer’s sign, have been reported to be present in about 20% of the cases. These consist of small, discrete, dark-red papules that develop on the soft palate and may extend onto the hard palate. This enanthem arises simultaneously with the rash, becoming evident in about 6 hours after the first symptoms and not lasting longer than 12 to 14 hours. Palatal petechiae also may occur.
The risk of CRS correlates with the time of infection. The frequency of transmission from an infected mother is greater than 80% during the first 12 weeks of pregnancy, with the risk of fetal damage decreasing dramatically at 8 weeks and becoming rare after 20 weeks of gestation. The classic triad of CRS consists of deafness, heart disease, and cataracts. Deafness is the most common manifestation, affecting more than 80% of patients. This hearing loss may not become evident until 2 years of age and usually is bilateral. Less common, late emerging complications include encephalopathy, mental retardation, diabetes mellitus, and thyroid disorders.
DIAGNOSIS: The diagnosis of rubella is contingent on laboratory tests because the clinical presentation of the acquired infection is typically subclinical, mild, or nonspecific. Although viral culture is possible, serologic analysis is the mainstay of diagnosis.
TREATMENT AND PROGNOSIS: Rubella is mild, and therapy usually is not required. Nonaspirin antipyretics and antipruritics may be useful in patients with significant fever or symptomatic cutaneous involvement. Passive immunity may be provided by the administration of human rubella immunoglobulin. If immunoglobulin is given within a few days of exposure, it decreases the severity of the infection. This therapy typically is reserved for pregnant patients who decline abortion.
During the 1962 to 1965 worldwide rubella epidemic, the estimated number of infections in the United States was 12.5 million. These infections were associated with 20,000 infants born with CRS, 11,250 fetal deaths, and 2100 neonatal deaths. Because of the two-dose vaccination schedule with the MMR and at least 95% vaccination coverage among school-aged children, in 2004, rubella was declared no longer to be endemic in the United States. The annual reported incidence of rubella was 23 in 2001, 18 in 2002, 7 in 2003, and 9 in 2004. Approximately half of these infections occurred in individuals born outside the United States. Likewise, from 2001 to 2004, only four cases of CRS were reported, and three of the mothers of these infants were not born in the United States.
MUMPS (EPIDEMIC PAROTITIS)
Mumps is an infection caused by a virus in the family Paramyxovirus, genus Rubulavirus, which causes a diffuse disease of exocrine glands. Although the salivary glands are the best known sites of involvement, the pancreas, choroid plexus, and mature ovaries and testes also frequently are involved. The involved glands exhibit edema and lymphocytic infiltration. As with measles and rubella, the epidemiology has been affected dramatically by the MMR vaccine. Before the advent of widespread vaccination, epidemics were seen every 2 to 5 years; nearly everyone was exposed with 90% of the infections occurring before age 15. The vaccine directed against mumps was released in 1967, but its use was not accepted nationally until 1977. At that time, vaccination became the norm for children 12 to 15 months of age. The vaccine has a success rate of 75% to 95%. Most individuals born before 1957 are thought to have immunity from exposure to naturally occurring mumps virus. Although most authorities assume that natural infection is associated with lifelong immunity, rare cases of recurrent mumps have been well documented in patients with a confirmed history of prior natural infection.
The annual incidence of mumps decreased by 98% and reached an all-time low in 1985. In 1986 a resurgence developed. In the past, most cases occurred in children aged 5 to 9 years; during the resurgence, the disease was more prevalent in 10- to 19-year-old patients. Outbreaks have been reported in high schools, on college campuses, and in the workplace. This increased incidence has been attributed to lack of vaccination, not vaccine failure. Subsequently, in the early 1990s, isolated outbreaks were reported in highly vaccinated populations and thought to be the result of large-scale vaccination failure. Not long after these reports, a second immunization as part of the MMR vaccine was recommended at 4 to 6 years of age. When compared with the prevaccine era, the two-dose MMR vaccination schedule has reduced the prevalence of mumps by 99%. In addition, in an attempt to decrease the prevalence in the older age groups, it is recommended that individuals lacking a history of mumps or MMR vaccination be immunized. This primarily affects those born between 1967 and 1977 and, to a lesser extent, those born between 1957 and 1967. For health care workers born before 1957, a single dose of MMR is recommended unless there is a physician’s diagn-osis of mumps or laboratory evidence of mumps immunity.
In 2006 an outbreak of mumps virus occurred in the United States, with an epicenter in Iowa and surrounding states. During this outbreak, only 7% of infected individuals were proven to be unvaccinated, and 49% had received at least the recommended two doses of the MMR. After two doses of MMR, the vaccine appears to be 98% effective against measles but only 90% effective against mumps. In spite of the vaccine failures in the recent outbreak, the high vaccination coverage most likely prevented thousands of additional cases of mumps. Some investigators wonder if such outbreaks may lead to a recommendation for a third dose of the MMR vaccine in the future.
The mumps virus can be transmitted through urine, saliva, or respiratory droplets. The incubation period usually is 16 to 18 days, with a range of about 2 to 4 weeks. Patients are contagious from 1 day before the clinical appearance of infection to 14 days after its clinical resolution.
CLINICAL FEATURES: Approximately 30% of mumps infections are subclinical. In symptomatic cases, prodromal symptoms of low-grade fever, headache, malaise, anorexia, and myalgia arrive first. Most frequently, these nonspecific findings are followed within 1 day by significant salivary gland changes. The parotid gland is involved most frequently, but the sublingual and submandibular glands also can be affected. Discomfort and swelling develop in the tissues surrounding the lower half of the external ear and extending down along the posterior inferior border of the adjacent mandible (Fig. 7-31). The enlargement typically peaks within 2 to 3 days, and the pain is most intense during this period of maximal enlargement. Chewing movements of the jaw or eating saliva-stimulating foods tends to increase the pain. Enlargement of the glands usually begins on one side and is followed by contralateral glandular changes within a few days. Unilateral involvement is seen in about 25% of patients.
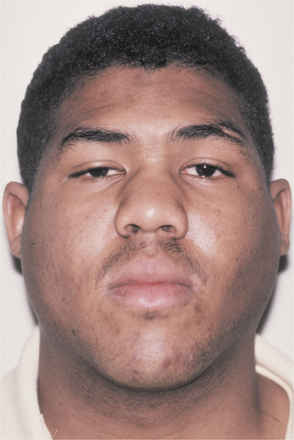
Fig. 7-31 Mumps. Bilateral parotid enlargement. (From Neville BW, Damm DD, White DK: Color atlas of clinical oral pathology, ed 2, Hamilton, 1999, BC Decker.)
The second most common finding is epididymo-orchitis, which occurs in about 25% of postpubertal males. In those affected the testicle exhibits rapid swelling, with significant pain and tenderness. The enlargement can range from a minimal swelling to a fourfold increase in size. Unilateral involvement is most common. On resolution of the swelling, atrophy occurs in the affected testicle. Permanent sterility from testicular changes is rare. Less commonly, oophoritis and mastitis can be seen in postpubertal females. In addition, spontaneous abortion occurs in approximately 25% of women who contract mumps during the first trimester of pregnancy.
Less commonly, meningoencephalitis (from involvement of the choroid plexus), cerebellar ataxia, hearing loss, pancreatitis, arthritis, carditis, and decreased renal function may occur. The most common symptom associated with CNS involvement is headache, whereas involvement of the pancreas can lead to nausea and vomiting. Isolated changes, such as orchitis or meningitis, may occur in the absence of salivary gland involvement, thereby making diagnosis difficult in nonepidemic settings. Mumps-related mortality is exceedingly rare and most frequently associated with mumps encephalitis.
The most frequently reported oral manifestation is redness and enlargement of Wharton’s and Stensen’s salivary gland duct openings. In addition, involvement of the sublingual gland may produce bilateral enlargements of the floor of the mouth.
DIAGNOSIS: The diagnosis of mumps can be made easily from the clinical presentation when the infection is occurring in an epidemic fashion; however, isolated cases must be differentiated from other causes. The most frequently used confirmatory measures are demonstration of mumps-specific IgM or a fourfold rise of mumps-specific IgG titers when measured during the acute phase and about 2 weeks later. In addition, a swab of secretions obtained from parotid or other affected salivary gland ducts can be used for viral isolation or reverse-transcriptase–polymerase chain reaction testing.
TREATMENT AND PROGNOSIS: The treatment of mumps is palliative in nature. Frequently, nonaspirin analgesics and antipyretics are administered. In an attempt to minimize orchitis, bed rest is recommended for males until the fever breaks. Avoidance of sour foods and drinks helps to decrease the salivary gland discomfort. As with measles and rubella, the best results come from prior vaccination, thereby preventing the infection.
HUMAN IMMUNODEFICIENCY VIRUS AND ACQUIRED IMMUNODEFICIENCY SYNDROME
During the last 2 decades, more articles have been written on human immunodeficiency virus (HIV) and its related disease states than any other infectious process. A complete bibliography alone easily would be thicker than this chapter. Entire texts dedicated to HIV infection and acquired immunodeficiency syndrome (AIDS) are available and should be consulted for more detailed information.
The first cases of AIDS reported in the United States were documented by the Centers for Disease Central and Prevention (CDC) in the Morbidity and Mortality Weekly Report on June 5, 1981. This publication detailed Pneumocystis carinii pneumoniae in five previously healthy men from Los Angeles, California. More than 25 years have passed. During this time, 65 million individuals worldwide have become infected with HIV and more than 25 million individuals have died of AIDS. Through 2004, a total of 529,113 deaths as a result of AIDS in the United States have been reported to the CDC, and more than 1 million individuals are living with HIV. Worldwide in 2005 alone, 4.1 million new infections occurred, 38.6 million were living with HIV, and an estimated 2.8 million individuals died of AIDS.
At the time of publication of the first edition of this text, the infection was thought to be nearly 100% fatal. Through treatment advances, the annual incidence of AIDS and related deaths have been altered dramatically in the United States. Cases of AIDS in the United States expanded rapidly during the 1980s, peaked in 1992 (estimated 78,000), and decreased each year from that time until 1998 when the annual incidence stabilized at about 40,000. Highly active anti-retroviral therapy (HAART) (see Treatment and Prognosis section) is changing the face of HIV infection, with affected individuals demonstrating extended survival (resulting in an increased percentage of the population living with the virus). The percentage of individuals surviving 2 years after the diagnosis of AIDS has increased from 44% in 1981 to 1992, to 64% in 1993 to 1995, to 85% in 1996 to 2000.
In infected individuals, the virus can be found in most bodily fluids. HIV has been recovered from serum, blood, saliva, semen, tears, urine, breast milk, ear secretions, and vaginal secretions. The most frequent routes of transmission are sexual contact, parenteral exposure to blood, or transmission from mother to fetus during the perinatal period. Infection also has been documented to be caused by artificial insemination, breast-feeding from infected mothers, and organ transplantation. Although heterosexual transmission is increasing, most of the adults infected in the United States have been homosexual or bisexual men, intravenous (IV) drug abusers, hemophiliac patients receiving factor VIII before 1985, recipients of blood products, or heterosexual contacts with one of the other high-risk groups.
Researchers have debated the infectiousness of oral fluids. HIV has been found to be present in oral fluids, but saliva appears to reduce the ability of HIV to infect its target cells, lymphocytes. Reports of transmission by oral fluids are rare, and it appears this is not a significant source for the transmission of AIDS. In spite of this, anecdotal reports have documented the transmission of AIDS during breast-feeding from the oral fluids of postpartum infected infants to their previously noninfected mothers. In addition, rare examples have been documented reporting the transmission of HIV infection by contamination of the oral fluids during cunnilingus or repeated passionate kissing. These rare anecdotal reports point out that oral fluids can be infectious and are not completely protective against oral introduction of HIV. Although saliva is known to contain a number of anti-HIV inhibitory factors, the presence of aphthae, erosions, ulcerations, and hemorrhagic inflammatory pathoses (e.g., gingivitis, periodontitis) may predispose an individual to oral transmission. In summary, the best precaution against infection is avoidance of all body fluids of infected patients.
Initially in the United States, AIDS was thought to be a disease that primarily affected whites and male homosexuals. Although men who have sex with men (MSM) remains the largest single risk factor, the nature of the epidemic is shifting because of numerous public health interventions directed against particularly vulnerable populations. These changing patterns are demonstrated well by comparing the data related to the early years of the infection with those noted in the initial portion of the twenty-first century (Table 7-1).
Since the initial years of the epidemic, blood-screening methods have improved dramatically and reduced the risk for HIV infection to as low as 1 in 2 million blood donations. The risk of transmission from infected mothers to newborns has been reduced from 25%-30% to 2% (95% reduction) because of widespread prenatal HIV testing, prophylactic use of antivirals, elective cesarean section performed before onset of labor, and avoidance of breast-feeding.
In the early years, non-Hispanic whites were the predominant ethnic group diagnosed with AIDS; however, with evolution of the epidemic, non-Hispanic blacks have become the predominant racial group. In addition, an increasing prevalence of HIV infection is being seen in females and heterosexuals. Of 35 areas reported to the CDC in 2004, 51% of all HIV infections and cases of AIDS were documented in blacks, even though this group accounted for only 13% of the population in the United States. Of these, 11% of the black men and 54% of the black women were infected through heterosexual contact. During this time, HIV infection was the leading cause of death for black women aged 25 to 34 years; the rates of infection per 100,000 in blacks, Hispanics, and whites were 76.3, 29.5, and 9.0, respectively. In comparing the racial rates of infection in the sexes in 2004, black males were affected seven times more frequently than whites, whereas black females demonstrated prevalence 21 times higher than white females.
The primary target cell of HIV is the CD4+ helper T lymphocyte. The DNA of HIV is incorporated into the DNA of the lymphocyte and is present for the life of the cell. In most viral infections, host antibodies that are protective against the organism usually are formed. In people with HIV infection, antibodies are developed but are not protective. The virus may remain silent, cause cell death, or produce syncytial fusion of the cells, which disrupts their normal function. A subsequent decrease in T-helper cell numbers occurs, with a resultant loss in immune function. The normal response to viruses, fungi, and encapsulated bacteria is diminished.
On introduction of HIV, an indefinite percentage of those infected will have an acute self-limited viral syndrome. This is followed by an asymptomatic stage, which averages 8 to 10 years. The length of the asymptomatic period is variable and may be affected by the nature of the virus, the host immune reaction, or external factors that may delay or accelerate the process. Almost inevitably, the final symptomatic stage develops.
CLINICAL FEATURES: HIV infection initially may be asymptomatic, or an acute response may be seen. The acute viral syndrome that occurs typically develops within 1 to 6 weeks after exposure in 50% to 70% of infected patients. The symptoms bear some resemblance to those of infectious mononucleosis (e.g., generalized lymphadenopathy, sore throat, fever, maculopapular rash, headache, myalgia, arthralgia, diarrhea, photophobia, peripheral neuropathies). Oral changes may include mucosal erythema and focal ulcerations.
The acute viral syndrome clears within a few weeks; during this period, HIV infection usually is not considered or investigated. A variable asymptomatic period follows. Some patients have persistent generalized lymphadenopathy (PGL), which may later resolve. In some patients (before development of overt AIDS), there is a period of chronic fever, weight loss, diarrhea, oral candidiasis, herpes zoster, and/or oral hairy leukoplakia (OHL). This has been termed AIDS-related complex (ARC).
The presentation of symptomatic, overt AIDS is highly variable and often is affected by a person’s prior exposure to a number of chronic infections. The signs and symptoms described under ARC are often present, along with an increasing number of opportunistic infections or neoplastic processes. In 50% of the cases, pneumonia caused by the protozoan Pneumocystis carinii is the presenting feature leading to the diagnosis. Other infections of diagnostic significance include disseminated cytomegalovirus (CMV) infection, severe herpes simplex virus (HSV) infection, atypical mycobacterial infection, cryptococcal meningitis, and cen-tral nervous system (CNS) toxoplasmosis. Persistent diarrhea is commonplace and may be bacterial or protozoal in origin. Clinically significant neurologic dysfunction is present in 30% to 50% of patients, and the most common manifestation is a progressive encephalopathy known as AIDS-dementia complex.
The most widely accepted classification of the oral manifestations of AIDS was compiled by the EC-Clearinghouse on Problems Related to HIV Infection and the WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus. This classification divided the manifestations into three groups: (1) strongly associated, (2) less commonly associated, and (3) seen in patients HIV infection (Box 7-1). The discussion here concentrates primarily on the clinical presentations. (For detailed information on the histopathology, diagnosis, and treatment of each condition, see the text covering the individual disease.) When the infections are treated differently in HIV-infected patients, these variations are presented here. The most common manifestations are presented first, followed by a selection of the less frequently encountered disorders.
The prevalence and mixture of oral manifestations noted in HIV-infected patients has been altered dramatically by the current antiretroviral therapies. Numerous investigations of patients receiving HAART (see Treatment and Prognosis section) have demonstrated an increase in CD4+ count and a reduction in viral load that appear to be correlated with a reduced prevalence of many oral manifestations. Other factors that appear to affect the frequency of oral manifestations include xerostomia, poor oral hygiene, and smoking. Subsequent to HAART, the overall prevalence of oral manifestations decreased, including significant reductions in the frequency of oral candidiasis, OHL, HIV-associated periodontal disease, and Kaposi’s sarcoma. Although the prevalence of certain lymphomas has decreased because of HAART, the frequency of all HIV-related lymphomas has not demonstrated significant change. In contrast, many researchers have reported an increased prevalence of benign human papillomavirus (HPV)-induced pathoses. A similar increased frequency of HIV-related salivary gland disease has been noted by some, but disputed by others.
The detection of oral manifestations can be critical, because it may suggest possible HIV infection in an unaware individual. The discovery in a patient with known HIV infection who is not yet on active therapy may signal progression of HIV disease. Finally, if new oral manifestations surface in a patient receiving antiretroviral therapy, it may lead to reevaluation and possible adjustment of the therapeutic regimen
ORAL AND MAXILLOFACIAL LESIONS STRONGLY ASSOCIATED WITH HIV INFECTION:
CANDIDIASIS: Oral candidiasis is the most common intraoral manifestation of HIV infection and often is the presenting sign that leads to the initial diagnosis (Fig. 7-32). Although a number of Candida species have been encountered intraorally, the most common organism identified in association with oral candidiasis is Candida albicans. The presence of oral candidiasis in a patient infected with HIV is not diagnostic of AIDS but appears to be predictive for the subsequent development of full-blown AIDS in untreated patients within 2 years. Prevalence studies vary widely, but approximately one third of HIV-infected individuals and more than 90% of patients with AIDS develop oral candidiasis at some time during their disease course. The following four clinical patterns are seen:
The first two variants constitute most of the cases (see page 213). Although infrequently seen in immunocompetent patients, chronic multifocal oral involvement is common in patients who are infected with HIV. Erythematous candidiasis typically begins to appear when the CD4 lymphocyte count drops below 400 cells/mm3, with the pseudomembranous pattern being noted when the counts drop below 200 cells/mm3. When comparing immunocompromised patients of different causations, those secondary to HIV infection have a greater prevalence of oral candidiasis, suggesting that HIV may play a role in initiation of the infection. Some studies have shown that development of candidiasis may be associated more closely with viral load than CD4 cell count. Oral candidiasis can be painful and associated with a reduction in taste and smell, which may lead to decreased intake of food and further wasting.
The diagnosis of candidiasis often is obvious from the clinical presentation but can be confirmed by cytologic smear or biopsy. Biopsy specimens of involved mucosa demonstrate the candidal organisms embedded in the superficial keratin, but the typical inflammatory reaction often is deficient (Fig. 7-33). Because C. albicans is a portion of the normal flora in 60% of healthy adults, positive microbiological cultures do not necessarily imply an active infection.
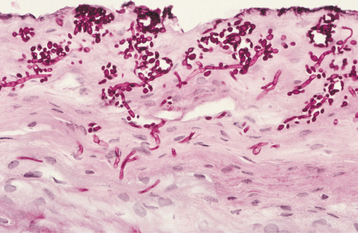
Fig. 7-33 HIV-associated candidiasis. Periodic acid-Schiff (PAS) stain of histopathologic section exhibiting numerous fungal organisms embedded in superficial keratin.
Treatment is much more difficult in patients with AIDS. Nystatin often is ineffective. Topical clotrimazole is associated with an improved response and typically produces a clinical cure rate that equals that of the systemic azoles. In spite of this success, topical therapy is associated with a high recurrence rate. The systemic azoles (i.e., fluconazole, ketoconazole, itraconazole) produce longer disease-free intervals but are associated with another set of problems. Itraconazole and ketoconazole require gastric acidity for adequate absorption, and all three agents are associated with a number of drug interactions. In addition, widespread use of systemic azoles has led to an increased prevalence of drug-resistant candidiasis in this patient population. In an attempt to reduce recurrences, patients have been encouraged to improve oral hygiene and assist mechanical cleansing of the mouth by rinsing with normal saline or home-made salt water several times daily.
In patients who are receiving effective antiretroviral therapy, have a CD4+ count exceeding 50 cells/mm3, and have no signs of esophageal involvement, topical clotrimazole is the treatment of choice. Systemic therapy is recommended for patients not receiving effective antiretroviral therapy or for those with either esophageal involvement, a CD4+ count below 50 cells/mm3, or a high viral load. Fluconazole is considered by many to be the drug of choice and has been shown to be the most effective prophylactic medication. In spite of this, non-albicans species such as Candida glabrata and Candida krusei have been isolated in HIV-infected patients, and these organisms are less susceptible to fluconazole. In many patients, itraconazole in an oral solution has been shown to be particularly effective in a swish-and-swallow method. Although gentian violet is not widely used, its effectiveness is better than nystatin and approaches the azoles, making this therapy a low-cost alternative in areas with inadequate funding or insufficient access to many of the newer medications. In several studies, antiretroviral protease inhibitors have been shown to exhibit a synergistic effect with the antifungal azoles and are thought possibly to interfere directly with candidal secretory aspartyl protease, an important virulence factor. Patients failing systemic azole therapy are candidates for intravenous amphotericin B if the patient’s health supports its use. Amphotericin B oral suspension is available but reserved by many for azole-resistant candidiasis. Prophylactic antifungal therapy is not recommended unless frequent and severe recurrences are present.
ORAL HAIRY LEUKOPLAKIA: Although EBV is thought to be associated with several forms of lymphoma in HIV-infected patients, the most common EBV-related lesion in patients with AIDS is oral hairy leukoplakia (OHL). This lesion clinically presents as a white mucosal plaque that does not rub off and is characterized histopathologically by a some what distinctive (but not diagnostic) pattern of hyperkeratosis and epithelial hyperplasia.
Most cases of OHL occur on the lateral border of the tongue and range in appearance from faint white vertical streaks to thickened and furrowed areas of leukoplakia, exhibiting a shaggy keratotic surface (Fig. 7-34). The lesions infrequently may become extensive and cover the entire dorsal and lateral surfaces of the tongue. Rarely, the buccal mucosa, soft palate, pharynx, or esophagus may be involved.
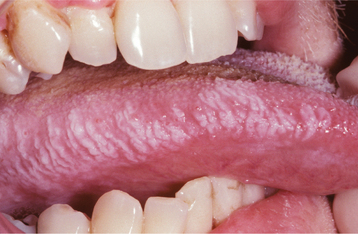
Fig. 7-34 HIV-associated oral hairy leukoplakia (OHL). Vertical streaks of keratin along the lateral border of the tongue.
Histopathologically, OHL exhibits thickened parakeratin that demonstrates surface corrugations or thin projections (Fig. 7-35). The epithelium is acanthotic and exhibits a bandlike zone of lightly stained cells with abundant cytoplasm (“balloon cells”) in the upper spinous layer (Fig. 7-36). Close examination of the superficial epithelium reveals scattered cells with nuclear clearing and a characteristic pattern of peripheral margination of chromatin termed nuclear beading (see Fig. 7-36, inset), caused by extensive EBV replication that displaces the chromatin to the nuclear margin. Dysplasia is not noted. Heavy candidal infestation of the parakeratin layer is typical, although the normal inflammatory reaction to the fungus usually is absent.
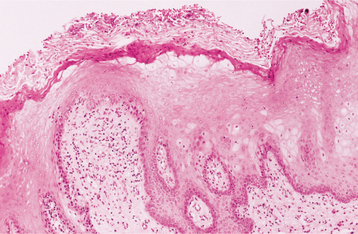
Fig. 7-35 HIV-associated oral hairy leukoplakia (OHL). Oral mucosa exhibiting hyperparakeratosis with surface corrugations.
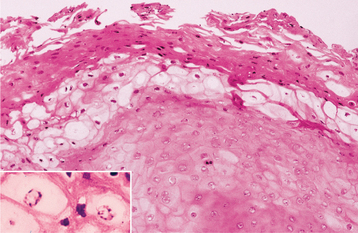
Fig. 7-36 HIV-associated oral hairy leukoplakia (OHL). Oral epithelium exhibiting hyperparakeratosis and layer of “balloon cells” in the upper spinous layer. Inset reveals high-power magnification of epithelial cells that demonstrate nuclear beading.
In the routine management of patients with HIV infection, the clinical features of OHL typically are sufficient for a presumptive diagnosis. When definitive diagnosis is necessary, demonstration of EBV within the lesion is required and can be achieved by in situ hybridization, PCR, immunohistochemistry, Southern blotting, or electron microscopy (Fig. 7-37).
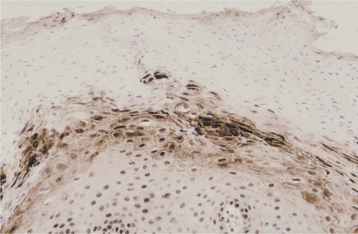
Fig. 7-37 HIV-associated oral hairy leukoplakia (OHL). Immunoperoxidase evaluation for Epstein-Barr virus (EBV) revealing positive reaction within numerous epithelial cells.
Treatment of OHL usually is not needed, although slight discomfort or aesthetic concerns may necessitate therapy. Systemic antiherpesviral drugs produce rapid resolution, but recurrence is expected with discontinuation of therapy. Topical treatment with retinoids or podophyllum resin has resulted in temporary remissions. Surgical excision or cryotherapy has been used by some. A significantly reduced prevalence of OHL has been noted in patients on HAART who have demonstrated decreased viral load and an improved CD4 count. In underdeveloped countries with patients located great distances from urban health centers, the presence or absence of OHL can be used along with oral candidiasis as a clinical guide to assist in judging the effectiveness of antiviral therapy.
Although rare instances of OHL have been reported in immunocompetent individuals, most cases arise in immunocompromised persons. OHL also has been reported in heart, kidney, liver, and bone marrow transplant recipients, but its presence in the absence of a known cause of immunosuppression strongly suggests HIV infection. Discovery of OHL in “normal” patients mandates a thorough physical evaluation to rule out immunocompromised status. The presence of OHL in HIV-infected patients is a signal of severe immune suppression and more advanced disease.
KAPOSI’S SARCOMA: Kaposi’s sarcoma (KS) is a multifocal neoplasm of vascular endothelial cell origin that was described initially in patients over the age of 60 (see page 557). However, since the beginning of the AIDS epidemic, most cases in the United States have been seen in association with HIV infection, with about 15% to 20% of patients with AIDS demonstrating KS. Human herpesvirus type 8 (HHV-8, Kaposi’s sarcoma–associated herpesvirus [KSHV]) is noted within the tumor and believed to be responsible for the neoplasm’s development. In Western countries, AIDS-associated KS has been reported primarily in homosexuals and is thought to be related to sexual transmission of HHV-8. Further evidence from Africa has demonstrated acquisition of HHV-8 infection before sexual activity and suggests alternate transmission pathways. HHV-8 has been found in saliva, serum, plasma, throat swabs, and bronchoalveolar lavage fluids. In Africa, HIV-related KS does not demonstrate a strong association with homosexual activity, exhibits an equal prevalence in men and women, and is not rare in children. In a recent North American study of healthy adults, HHV-8 was detected in oral epithelial cells and in the oropharynx, suggesting that the oral cavity may represent the predominant reservoir of infectious virus. These studies suggest that saliva represents an important route for transmission of HHV-8 in both heterosexual and homosexual populations.
KS typically manifests as multiple lesions of the skin or oral mucosa, although occasionally a solitary lesion is identified first. The trunk, arms, head, and neck are the most commonly involved anatomic sites (Fig. 7-38). Approximately 70% of individuals with HIV-related KS of skin or viscera demonstrate oral lesions; in 22% the oral cavity is the initial site of involvement. Although any mucosal site may be involved, the hard palate, gingiva, and tongue are affected most frequently (Figs. 7-39 and 7-40). When present on the palate or gingiva, the neoplasm can invade bone and create tooth mobility. The lesions begin as brown or reddish purple macular lesions that do not blanch with pressure. With time, the macules typically develop into plaques or nodules (Fig. 7-41). Pain, bleeding, and necrosis may become a problem and necessitate therapy (Fig. 7-42).
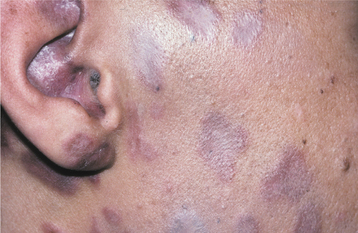
Fig. 7-38 HIV-associated Kaposi’s sarcoma (KS). Multiple purple macules on the right side of the face.
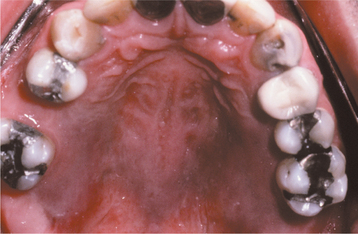
Fig. 7-39 HIV-associated Kaposi’s sarcoma (KS). Large zones of KS exhibiting as a flat, brownish, and M-shaped discoloration of the hard palate.
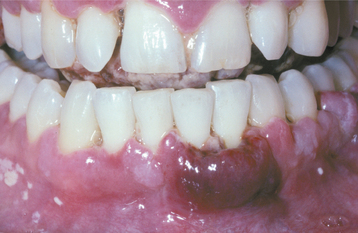
Fig. 7-40 HIV-associated Kaposi’s sarcoma (KS). Raised, dark-red enlargement of the mandibular anterior facial gingiva on the left side.
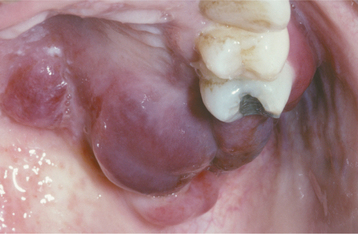
Fig. 7-41 HIV-associated Kaposi’s sarcoma (KS). Diffuse, red-blue nodular enlargement of the left hard palate.
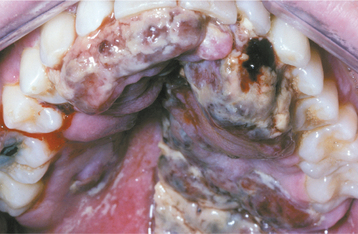
Fig. 7-42 HIV-associated Kaposi’s sarcoma (KS). Diffuse, red-blue gingival enlargement that demonstrates widespread necrosis.
A biopsy is required to make the definitive diagnosis, although a presumptive diagnosis is sometimes made from the clinical presentation and history. Lesions that have a similar clinical appearance can occur in HIV-infected patients who exhibit bacillary angiomatosis, the multifocal vascular proliferation associated with the cat-scratch bacillus (see page 206). HIV-related lymphomas also may resemble KS at times.
Before HAART, KS was considered a progressive malignancy that often disseminated widely to lymph nodes and various organ systems. HAART with protease inhibitors has been associated with a 30% to 50% reduction in the prevalence of KS and has produced significant regression of lesions in patients already affected. Because KS typically arises in immunocompromised patients and frequently demonstrates regression on return of immunocompetence, many researchers question whether KS is a true sarcoma. In cases not responding to HAART, single-agent chemotherapy such as alkaloids, bleomycin, doxorubicin, epirubicin, etoposide, and paclitaxel have shown effectiveness. Multiagent combinations have an increased effect but are associated with more severe adverse reactions and poor results in patients with widespread disease.
Oral lesions are frequently a cause of major morbidity, as a result of pain, bleeding, and functional interferences. Problematic lesions may be removed surgically or with cryotherapy. Intralesional injection of oral lesions with vinblastine (a chemotherapeutic agent) is effective. Intralesional injection of sodium tetradecyl sulfate, a sclerosing agent, has been effective for problematic intraoral lesions less than 2.5 cm in diameter. Laser ablation or electrosurgery have been used to treat KS, although theoretical concerns have been raised with respect to aerosolization of viral particles, which may place the surgical team at risk.
PERSISTENT GENERALIZED LYMPHADENOPATHY: After seroconversion, HIV disease often remains silent except for persistent generalized lymphadenopathy (PGL). The prevalence of this early clinical sign varies; however, in several studies it approaches 70%. PGL consists of lymphadenopathy that has been present for longer than 3 months and involves two or more extrainguinal sites. The most frequently involved sites are the posterior and anterior cervical, submandibular, occipital, and axillary nodes. Nodal enlargement fluctuates, usually is larger than 1 cm, and varies from 0.5 to 5.0 cm (Fig. 7-43).
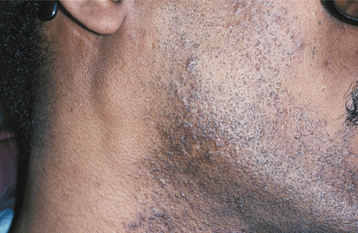
Fig. 7-43 HIV-associated lymphadenopathy. Enlarged cervical lymph nodes in a patient with persistent generalized lymphadenopathy (PGL).
Because lymphoma is known to occur in this population, a lymph node biopsy may be indicated for localized or bulky adenopathy, when cytopenia or an elevated erythrocyte sedimentation rate is present, or when requested for patient reassurance. Histopathologic examination reveals florid follicular hyperplasia. Although not as predictive as oral candidiasis or hairy leukoplakia, PGL does warn of progression to AIDS; almost one third of affected and untreated patients will have diagnostic features of AIDS within 5 years.
NON-HODGKIN’S LYMPHOMA: Non-Hodgkin’s lymphoma (NHL) is the second most common malignancy in HIV-infected individuals. This neoplasm occurs in approximately 3% to 5% of those with the virus, a prevalence 60 times greater than the normal population. Typically, NHL in patients with AIDS presents as a high-grade and aggressive disease that frequently is associated with widespread involvement and short survival times. In AIDS the relative risk for developing a low-grade NHL is 15 times greater, compared with a 400 times greater risk for a high-grade NHL. The majority of the NHLs are B-cell lymphomas and include AIDS Burkitt’s lymphoma, anaplastic large cell lymphoma, diffuse large cell lymphoma, immunoplasmacytoid lymphoma, primary effusion lymphoma, and plasmablastic lymphoma. Although a large number of these neoplasms demonstrate a relationship with EBV, studies have suggested that plasmablastic lymphoma and primary effusion lymphoma may be associated with both EBV and HHV-8.
Lymphoma in patients with AIDS usually occurs in extranodal locations, with the CNS being the most common site. Oral lesions are seen in approximately 4% of patients with AIDS-related NHL and most frequently involve the gingiva, palate, tongue, tonsil, or maxillary sinus (Fig. 7-44). Intraosseous involvement also has been documented and may resemble diffuse progressive periodontitis with loss of periodontal attachment and loosening of teeth. In these cases, widening of the periodontal ligament and loss of lamina dura frequently are noted and represent clues to the diagnosis.
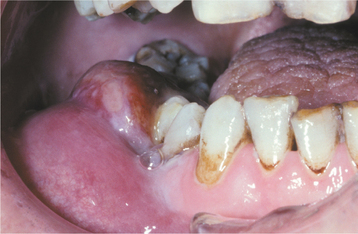
Fig. 7-44 HIV-associated lymphoma. Erythematous and ulcerated soft tissue enlargement of the posterior mandibular gingiva and mucobuccal fold on the right side.
The treatment usually is combination chemotherapy, and radiation is reserved for local control of the disease. These malignancies are aggressive, and survival usually is measured in months from the date of discovery. Although HAART has dramatically reduced the prevalence of many opportunistic infections and KS in HIV-infected patients, the effect on NHL appears to vary with the type of lymphoma and has been inconsistent. Although many forms of NHL continue to contribute to the morbidity and mortality of HIV-infected patients, others categories of NHL, such as plasmablastic lymphoma, have declined significantly because of HAART.
HIV-ASSOCIATED PERIODONTAL DISEASE: Three atypical patterns of periodontal disease are associated strongly with HIV infection:
Linear gingival erythema initially was termed HIV-related gingivitis, but ultimately was noted in association with other disease processes. This unusual pattern of gingivitis appears with a distinctive linear band of erythema that involves the free gingival margin and extends 2 to 3 μm apically (Fig. 7-45). In addition, the alveolar mucosa and gingiva may demonstrate punctate or diffuse erythema in a significant percentage of the cases. This diagnosis should be reserved for gingivitis that does not respond to improved plaque control and exhibits a greater degree of erythema than would be expected for the amount of plaque in the area. The literature related to linear gingival erythema is difficult to evaluate, because it appears that conventional marginal gingivitis often is misinterpreted as linear gingival erythema. Although some investigators believe linear gingival erythema results from an abnormal host immune response to subgingival bacteria, data suggest that this pattern of gingivitis may represent an unusual pattern of candidiasis. Most instances respond to systemic antifungal medications such as fluconazole or ketoconazole.
Necrotizing ulcerative gingivitis (NUG) (see page 157) refers to ulceration and necrosis of one or more interdental papillae with no loss of periodontal attachment. Patients with NUG have interproximal gingival necrosis, bleeding, pain, and halitosis (Fig. 7-46).
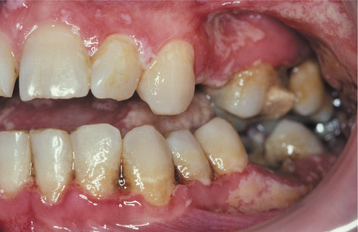
Fig. 7-46 HIV-associated necrotizing ulcerative gingivitis (NUG). Multiple punched-out interdental papillae of the mandibular gingiva. Note diffuse pseudomembranous candidiasis of the surrounding mucosa.
Necrotizing ulcerative periodontitis (NUP) previously was termed HIV-associated periodontitis; however, it has not been deemed to be specific for HIV infection. NUP is characterized by gingival ulceration and necrosis associated with rapidly progressing loss of periodontal attachment. Although severe cases can affect all teeth, multiple isolated defects often are seen and contrast with the diffuse pattern associated with typical chronic periodontitis. Edema, severe pain, and spontaneous hemorrhage are common and often lead affected patients to seek care. Deep pocketing usually is not seen because extensive gingival necrosis typically coincides with loss of the adjacent alveolar bone (Fig. 7-47). Loss of more than 6 μm of attachment within a 6-month period is not unusual. HIV-associated periodontitis does not respond to conventional periodontal therapy.
Fig. 7-47 HIV-associated periodontitis. Extensive loss of periodontal support without deep pocketing.
The treatment of NUG and NUP revolves around débridement, antimicrobial therapy, immediate follow-up care, and long-term maintenance. The initial re-moval of necrotic tissue is necessary, combined with povidone-iodine irrigation. The use of systemic antibiotics usually is not necessary, but metronidazole (narrow spectrum to suppress periodontal pathogens without strongly promoting candidal overgrowth) has been administered to patients with extensive involvement that is associated with severe acute pain. All patients should use chlorhexidine mouth rinses initially and for long-term maintenance. After initial débridement, follow-up removal of additional diseased tissue should be performed within 24 hours and again every 7 to 10 days for two to three appointments, depending on the patient’s response. At this point, monthly recalls are necessary until the process stabilizes; evaluations then are performed every 3 months.
In patients with gingival necrosis, the process occasionally extends away from the alveolar ridges and creates massive areas of tissue destruction termed necrotizing stomatitis (Fig. 7-48). The process clinically resembles noma (see page 201) and may involve predominantly soft tissue or extend into the underlying bone, resulting in extensive sequestration (Fig. 7-49). Although this process initially was thought to be an extension of NUP, necrotizing stomatitis has arisen on the oral mucosa separate from the gingiva (not overlying bone).
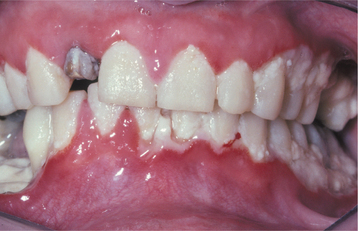
Fig. 7-48 HIV-associated periodontitis with necrotizing stomatitis. Diffuse gingival necrosis with extension onto alveolar mucosa.
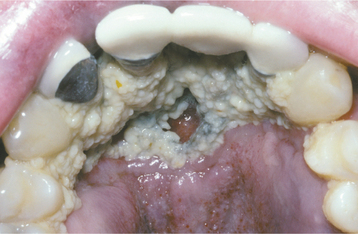
Fig. 7-49 HIV-associated necrotizing stomatitis. Massive necrosis of soft tissue and bone of the anterior maxilla.
In the absence of gingival involvement, the clinical features of necrotizing stomatitis are nonspecific and mandate biopsy. In many instances, the areas of soft tissue ulceration and necrosis demonstrate infection with one of more agents, such as HSV, CMV, and EBV.
In addition to these three atypical forms of HIV-related periodontal disease, patients also may demonstrate conventional gingivitis, chronic periodontitis, and progressive nonnecrotizing periodontitis. Studies have shown that periodontal attachment loss can be combated successfully with regular professional removal of supra- and subgingival plaque in patients who optimize their personal oral hygiene techniques. Because smoking has been associated strongly with all forms of periodontal disease, patients should be encouraged to discontinue their tobacco habit.
LESS COMMON ORAL AND MAXILLOFACIAL MANIFESTATIONS OF HIV INFECTION:
MYCOBACTERIAL INFECTION: The best known mycobacterial infection is tuberculosis (TB), which typically is caused by Mycobacterium tuberculosis (see page 195). Infections with other mycobacteria include M. avium and M. intracellulare (M. avium-intracellulare complex), M. bovis, M. scrofulaceum, M. africanum, and M. haemophilum, although these usually are found only in the immunocompromised patient. Worldwide, one in three individuals contracts TB sometime during their life, with more than 2 million associated deaths each year. Those coinfected with HIV are at greater risk of death and account for 15% of AIDS-related deaths worldwide. It is estimated that more than 4.4 million individuals are coinfected with TB and HIV, with more than half a million individuals exhibiting active TB.
Oral lesions are uncommon and occur in less than 5% of individuals with active TB. When present, the tongue is affected most frequently, but lesions also can develop on the buccal mucosa, gingiva, floor of mouth, lips, and palate. The affected areas present as chronic ulcerations, granular leukoplakias, or exophytic proliferative masses. Jaw involvement also has been reported. Confirming the diagnosis of TB often can be difficult in AIDS patients, because up to 80% do not react to tuberculin skin tests. In such cases, identifying the organism by examining AFB-stained sections of biopsy material and confirming its presence on culture of infected tissue are important.
Management is difficult because of increasing drug resistance and difficulty in ensuring patient compliance with the extended treatment protocols. Agents frequently used in the triple-drug regimens include rifampicin, isoniazid, and pyrazinamide, with ethambutol added when isoniazid resistance is likely.
HYPERPIGMENTATION: Hyperpigmentation of the skin, nails, and mucosa has been reported in HIV-infected patients. The changes are similar microscopically to focal melanosis, with increased melanin pigmentation observed in the basal cell layer of the affected epithelium. Several medications taken by AIDS patients (e.g., ketoconazole, clofazimine, pyrimethamine, zidovudine) may cause the increased melanin pigmentation. Adrenocortical destruction has been reported from several of the infections associated with AIDS, resulting in an addisonian pattern of pigmentation. Finally, pigmentation with no apparent cause has arisen in HIV-infected patients, and some investigators have theorized that this may be a direct result of the HIV infection.
HIV-ASSOCIATED SALIVARY GLAND DISEASE: HIV-associated salivary gland disease also can arise anytime during infection. Clinically obvious salivary gland disease is noted in approximately 5% to 10% of HIV-infected patients, with a greater prevalence noted in children. The main clinical sign is salivary gland enlargement, particularly affecting the parotid. Bilateral involvement is seen in about 60% of patients with glandular changes and often is associated with cervical lymphadenopathy.
As a result of a genetically influenced alteration of the immune response to HIV infection, some patients develop diffuse infiltrative lymphocytosis syndrome (DILS), which is associated with a more favorable prognosis for their HIV infection but also associated with a forty-fourfold greater chance of lymphoma. Affected individuals reveal CD8 lymphocytosis and lymphadenopathy, along with salivary gland enlargement. Although the pathosis may involve any of the major or minor salivary glands, the parotid is affected most commonly. Other sites of involvement include the lacrimal glands, kidneys, muscles, nerves, and lungs. The glandular involvement arises from CD8-lymphocytic infiltration and often is followed by lymphoepithelial cyst formation in the parotid.
The most widely accepted therapy for DILS is oral prednisone or antiretroviral therapy, although some patients have been treated with parotidectomy or radiation therapy. The effect of HAART is unclear. Some investigators have reported an increased prevalence (possibly because of partial immune reconstitution), whereas others have noted regression after initiation of antiviral therapy. Because of the increased risk for B-cell lymphoma, observation with histopathologic monitoring by fine-needle aspiration is recommended by some. In patients with large lymphoepithelial cysts, aspiration or sclerotherapy with tetracycline or doxycycline has been associated with temporary improvement. Associated xerostomia is variable and treated in a manner similar to that of cases associated with non-HIV disease (i.e., maintenance of good oral health and the use of sialogogues and saliva substitutes).
THROMBOCYTOPENIA: Thrombocytopenia (see page 584) has been reported in up to 40% of patients with HIV infection, may occur at any time during the course of the disease, and frequently is the first clinical manifestation of HIV infection. The causes are diverse and include direct infection by HIV, immune dysfunction, alteration of platelet production, loss because of associated infectious diseases, and drug reactions. Cutaneous lesions are present in most cases, but oral lesions do occur with petechiae, ecchymosis, or spontaneous gingival hemorrhage.
Platelets have been shown to engulf HIV and play an important role in the immune response to the virus. HIV-infected patients with extended thrombocytopenia have demonstrated decreased survival. HAART has reduced the prevalence of thrombocytopenia and is the first line of defense. Additional therapies used in nonresponsive patients include splenectomy, intravenous immunoglobulin (IVIG), IV anti-Rho immunoglobulin (anti-D), and transfusion of platelets. Corticosteroids, danazol, and vinca alkaloids are additional approaches proven to be effective in the general population but associated with increased risks in HIV-infected patients.
HERPES SIMPLEX VIRUS: Recurrent HSV infections occur in about the same percentage of HIV-infected patients as they do in the immunocompetent population (10% to 15%); however, the lesions are more widespread, occur in an atypical pattern, and may persist for months (Fig. 7-50). The prevalence of HSV lesions increases significantly once the CD4+ count drops below 50 cells/mm3. Herpes labialis may extend to the facial skin and exhibit extensive lateral spread. Persistence of active sites of HSV infection for more than 1 month in a patient infected with HIV is one accepted definition of AIDS. The clinical presentations of recurrences in immunocom-promised patients and appropriate therapy and maintenance have been discussed in the text on herpesvirus (see page 244).
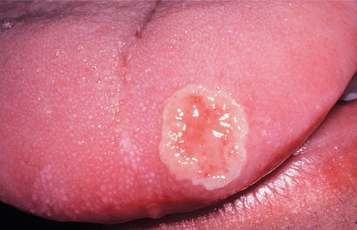
Fig. 7-50 HIV-associated recurrent herpetic infection. Mucosal erosion of the anterior dorsal surface of the tongue on the left side. Note the yellowish circinate border.
As mentioned in the discussion of necrotizing stomatitis, evaluation for HSV should be performed in all persistent oral ulcerations in HIV-infected individuals. In these ulcerations, investigators have discovered HSV in 10% to 19% (with an additional 10% to 28% exhibiting coinfection by HSV and CMV).
VARICELLA-ZOSTER VIRUS: Recurrent varicella-zoster virus (VZV) infection (herpes zoster) is fairly common in HIV-infected patients, but the course is more severe, with increased morbidity and mortality rates. Many of these patients are younger than age 40, in contrast to cases in immunocompetent patients that usually arise later in life. In the early stages of HIV-related immunosuppression, herpes zoster usually is confined to a dermatome but persists longer than usual. In full-blown AIDS, herpes zoster usually begins in a classic dermatomal distribution; however, subsequent cutaneous dissemination is not unusual. When present intraorally, the involvement often is severe and occasionally leads to bone sequestration and loss of teeth. In many instances, the associated osteonecrosis and tooth exfoliation may be delayed a month or more after the initial onset of the herpes zoster. Associated pain typically is intense. Although peroral antiviral medications are beneficial in immunocompetent patients, intravenous acyclovir is recommended for severe herpes zoster in the absence of an intact immune system.
HUMAN PAPILLOMAVIRUS: Human papillomavirus (HPV) is responsible for several facial and oral lesions in immunocompetent patients, the most frequent of which are the verruca vulgaris (common wart) (see page 364) and oral squamous papilloma (see page 362). An increased prevalence of HPV-related lesions is noted in HIV-infected patients, and most are located in the anogenital areas. Oral involvement also may be seen. Although usual types of HPV may be present in intraoral lesions, HIV-infected patients often demonstrate more unusual variants such as HPV-7 (associated with butcher’s warts) or HPV-32 (often noted in multifocal epithelial hyperplasia) (see page 367).
An increased prevalence of HPV-related lesions has been reported from several centers in patients responding to HAART. Although the exact cause is not clear, some wonder if the virus remains latent in many patients until partial immune reconstitution leads to a local inflammatory response, viral reactivation, and the initiation of clinically evident lesions. In several cohorts, the risk of oral HPV lesions increased with the effectiveness of the antiretroviral therapy.
The oral lesions usually are multiple and may be located on any mucosal surface. The labial mucosa, tongue, buccal mucosa, and gingiva are frequent sites. The lesions may exhibit a cluster of white, spikelike projections, pink cauliflower-like growths, or slightly elevated sessile papules (Fig. 7-51).
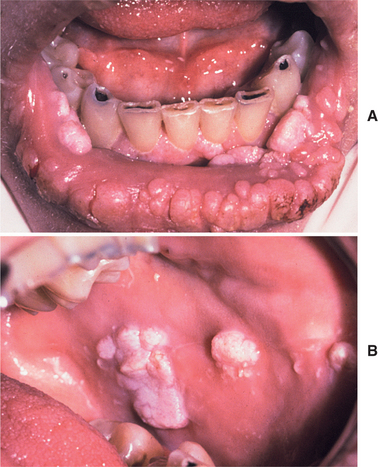
Fig. 7-51 HIV-associated human papillomavirus (HPV) infection. Multiple exophytic and somewhat papillary nodules of the lip, buccal mucosa, and gingiva.
Histopathologically, the lesions may be sessile or papillary and covered by acanthotic or even hyperplastic stratified squamous epithelium (Fig. 7-52). The affected epithelium often demonstrates vacuolization of numerous epithelial cells (i.e., koilocytosis) and occasionally may exhibit mild variation in nuclear size (Fig. 7-53). Immunohistochemistry or DNA in situ hybridization often is used to confirm the presence and type of HPV within histopathologic specimens (Fig. 7-54).
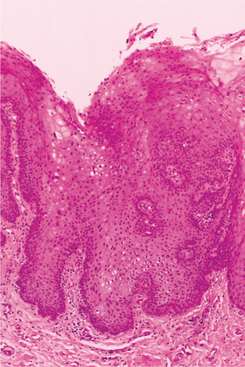
Fig. 7-52 HIV-associated human papillomavirus (HPV) infection. Oral mucosa exhibiting acanthosis and mild nuclear pleomorphism.
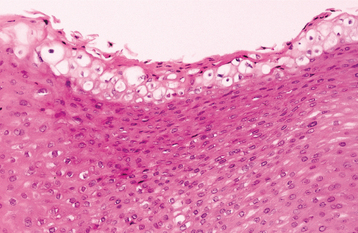
Fig. 7-53 HIV-associated human papillomavirus (HPV) infection. Oral mucosa exhibiting extensive koilocytosis in the superficial spinous cell layer.
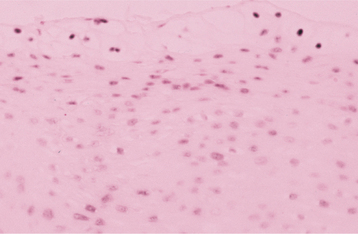
Fig. 7-54 HIV-associated human papillomavirus (HPV) infection. DNA in situ hybridization of oral mucosal biopsy that reveals diffuse cellular positivity for HPV.
Dysplasia has been noted within HPV-related lesions in patients with AIDS and mandates close observation of affected patients for development of squamous cell carcinoma. The treatment of choice is surgical removal; however, recurrences are common, especially in patients with significant immune deficiency. Other therapeutic modalities that have been used include topical podophyllin, imiquimod, interferon, cryosurgery, laser ablation, and electrocoagulation. If one of the latter two choices is used, the surgical team must be wary of the resultant plume that may contain infectious HPV.
OTHER ORAL AND MAXILLOFACIAL LESIONS SEEN IN HIV INFECTION:
HISTOPLASMOSIS: Histoplasmosis, the most common endemic respiratory fungal infection in the United States, is produced by Histoplasma capsulatum (see page 224). In healthy patients the infection typically is subclinical and self-limiting, but clinically evident infections do occur in immunocompromised individuals. Although a number of deep fungal infections are possible in patients with AIDS, histoplasmosis is the most common, with disseminated disease noted in approximately 5% of AIDS patients residing in areas where the fungus is endemic. In patients with AIDS, diagnosis of histoplasmosis also has been documented in nonendemic areas, possibly from reactivation of a previous subclinical infection.
The signs and symptoms associated with dissemination are nonspecific and include fever, weight loss, splenomegaly, and pulmonary infiltrates. Oral lesions are not uncommon and usually are caused by bloodborne organisms or spread from pulmonary involvement. On occasion, the initial diagnosis is made from the oral changes, with some patients demonstrating involvement isolated to the oral cavity. Although intrabony infection in the jaws has been reported, the most common oral presentation of histoplasmosis is a chronic, indurated mucosal ulceration with a raised border (Fig. 7-55). The oral lesions may be single or multiple, and any area of the oral mucosa may be involved.
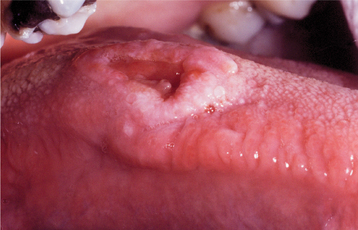
Fig. 7-55 HIV-associated histoplasmosis. Indurated ulceration with a rolled border on the dorsal surface of the tongue on the right side.
Microscopically, the small fungal organisms are visible within the cytoplasm of histiocytes and multinucleated giant cells. These phagocytic cells may be present in sheets or in organized granulomas (Fig. 7-56). The therapy of choice for disseminated histoplasmosis has been intravenous amphotericin B, but itraconazole has been shown to be effective with fewer adverse reactions and better patient compliance. Ketoconazole is another alternative, but its hepatotoxicity makes this approach a less desirable form of therapy.
APHTHOUS ULCERATIONS: Lesions that are clinically similar to aphthous ulcerations occur with increased frequency in patients infected with HIV. All three forms (minor, major, and herpetiform) are seen; surprisingly, however, almost two thirds of the patients have the usually uncom-mon herpetiform and major variants (Fig. 7-57). As immunosuppression becomes more profound, major aphthous ulcerations demonstrate an increased prevalence.
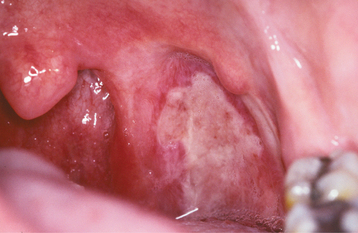
Fig. 7-57 HIV-associated aphthous ulceration. Large superficial ulceration of the posterior soft palate.
Treatment with potent topical or intralesional corticosteroids has been successful in a number of patients. Not all lesions respond, and recurrences are common. Secondary candidiasis may be a complication of therapy. Systemic corticosteroid drugs also may prove beneficial but typically are avoided in an attempt to prevent further immune depression. For lesions nonresponsive to topical corticosteroids, thalidomide has been found to be advantageous in many patients. Thalidomide must be used cautiously for only a short term because of its association with an irreversible peripheral neuropathy and its ability to enhance the production of HIV. In a limited number of patients, granulocyte colony-stimulating factor (G-CSF) has produced rapid and sustained resolution of aphthous ulcerations that were resistant to therapy with topical corticosteroids, cyclosporine, and thalidomide.
Biopsy of any chronic mucosal ulceration clinically diagnosed as an aphthous ulceration should be considered if the lesion is atypical clinically or does not respond to therapy (Fig. 7-58). In such cases, biopsy often reveals another cause, such as HSV, CMV, deep fungal infection, or neoplasia. (For further information on aphthous ulcerations and the pathogenesis of these lesions in patients infected with HIV, see page 331.)
MOLLUSCUM CONTAGIOSUM: Molluscum contagiosum is an infection of the skin caused by a poxvirus (see page 371). The lesions are small, waxy, dome-shaped papules that often demonstrate a central depressed crater. In immunocompetent individuals, the lesions are self-limiting and typically involve the genital region or trunk. In patients with AIDS, hundreds of lesions may be present, with many exhibiting little tendency to undergo spontaneous resolution, and some occasionally obtaining large size. Approximately 5% to 10% of HIV-infected patients are affected, and the facial skin commonly is involved (Fig. 7-59). Rare intraoral examples of molluscum contagiosum have been reported, appearing as erythematous papules. These lesions may involve either the keratinized or nonkeratinized mucosa.
Histopathologically, the surface epithelium forms several hyperplastic downgrowths. This involuting epithelium contains numerous large, intracytoplasmic inclusions known as molluscum bodies (Fig. 7-60). In the center of the lesion, the keratin layer often disintegrates and releases the adjacent molluscum bodies, hence the central crater.
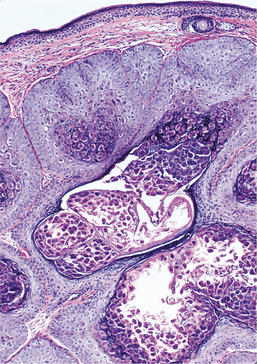
Fig. 7-60 HIV-associated molluscum contagiosum. Downgrowth of surface epithelium exhibiting numerous “molluscum bodies.”
Numerous reports have documented resolution of widespread and recalcitrant lesions after successful initiation of HAART. It is not known if these responses are secondary to immune reconstitution or the antiviral effects of the therapy. Because viral particles have been identified in perilesional skin of HIV-infected patients, local therapy (e.g., curettage, cryosurgery, cautery, photodynamic therapy, podophyllotoxin) often is associated with recurrences. Anecdotal reports of positive responses to immunomodulatory (imiquimod) or antiviral therapy (cidofovir) have been documented in severely immunocompromised patients with previously recalcitrant disease.
ORAL SQUAMOUS CELL CARCINOMA: Squamous cell carcinoma of the oral cavity, pharynx, and larynx has been reported in HIV-infected patients. These neoplasms are associated with the same cancer risk factors as the general population but tend to occur at a younger age. Similar clinical presentations and anatomic distribution of these carcinomas are noted (Fig. 7-61). It appears that HIV infection may accelerate the development of squamous cell carcinoma, possibly because of impaired immune surveillance.
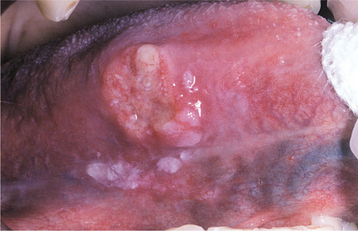
Fig. 7-61 HIV-associated squamous cell carcinoma. Ulceration with raised, indurated borders on the lateral tongue.
Treatment of squamous cell carcinoma is not significantly different for HIV-infected patients and consists of surgical resection, radiation therapy, or combined radiation and chemotherapy. Clinical staging can be problematic because of HIV-related cervical lymphadenopathy. In these cases, cross-sectional computed tomography (CT) or magnetic resonance imaging (MRI) could be performed in an attempt to distinguish lymph nodes enlarged by lymphoproliferative disease from those containing metastatic carcinoma. The majority of HIV-infected patients with a diagnosis of squamous cell carcinoma have advanced disease and exhibit a less favorable prognosis.
DIAGNOSIS: Confirmation of HIV infection can be made by viral culture or by detection of HIV antibodies or antigens. The standard screening tool is the enzyme immunoassay (EIA) for antibodies to HIV. This test can have false-positive results or cross-reactions; therefore, it should be repeated and followed by the more accurate Western blot antibody assay. Other alternatives include radioimmunoprecipitation (RIPA), rapid latex agglutination assay, and dot-blot immunobinding assay. All of these evaluations are used to detect antibodies to HIV.
In an attempt to improve the safety of the blood supply, a few assays have been approved by the FDA to detect viral antigens before development of anti-HIV antibodies. These tests are not used widely and include the p24 antigen capture assay and polymerase chain reaction (PCR) for detection of HIV DNA that may be integrated into the host DNA. This latter method may be used to identify someone who was infected recently or HIV carriers who otherwise have negative antigen or antibody findings.
The diagnosis of AIDS is indicated if the patient has laboratory evidence of HIV infection combined with documentation of less than 200 CD4+ T lymphocytes per microliter or a CD4+ T-lymphocyte percentage of total lymphocytes that is less than 14. In addition, the diagnosis of AIDS can be made in an HIV-infected person if one of the indicator diseases listed in Box 7-2 has been documented.
TREATMENT AND PROGNOSIS: As mentioned previously, HIV infection initially was considered fatal; however, the introduction of HAART has altered the course of the epidemic. A wide variety of antiretroviral agents is available and continues to expand (Box 7-3), increasing the effectiveness of medical therapy. Although numerous drug combinations are possible, HAART often consists of two nucleoside analogue reverse-transcriptase inhibitors, at least one protease inhibitor, and/or one nonnucleoside analogue reverse-transcriptase inhibitor. Alternatively, one nucleoside analogue reverse-transcriptase inhibitor can be combined with one nonnucleoside ana-logue reverse-transcriptase inhibitor and one protease inhibitor.
The current therapeutic approaches have driven HIV to undetectable levels in many patients, with a resultant clinically significant reconstitution of the immune system. With the current antiretroviral medications, total HIV eradication would take at least a decade and presently is not a realistic goal. Although no cure exists, survival times are increasing as a result of earlier diagnosis and improved therapy.
Although antiretroviral therapy is effective for many patients, it is expensive. In addition, this treatment often is associated with significant adverse reactions, may not be effective in all patients, or may fail after a period of initial success. Work is proceeding toward the development of a safe and effective vaccine against HIV infection, but complex issues slow the progress. Advances in therapy and prevention of HIV infection occur daily; however, the best defense against the disease is prevention of the initial infection.
Despite the continuing advances in medical therapy and the ongoing work toward a successful vaccine, many of the positive gains against the epidemic have been achieved through epidemiologically directed public health interventions. The ever-changing data continue to be important and highlight increasingly important areas (e.g., increasing prevalence in heterosexuals and blacks) for present and future interventions. Routine HIV testing is critical, because it represents the best avenue toward life-saving early therapy and prevention of HIV transmission to others. It is estimated that more than 300,000 individuals in the United States are unaware that they are infected with HIV. To appropriately emphasize early diagnosis and prevention, the CDC recommends HIV testing as a part of routine clinical care in all health care settings.
Some health professionals have been concerned about the risk of occupational transmission of HIV. The average risk of seroconversion after a percutaneous exposure to HIV-infected blood is estimated to be approximately 0.3%. Mucous membrane exposure to HIV-infected blood results in seroconversion in only 0.09% of cases, and the rate is even lower after a nonintact skin exposure. The risk of transmission after exposure to fluids or tissues other than blood has not been quantified but most likely is considerably lower. On exposure to HIV-contaminated material, the risk of seroconversion can be reduced by greater than 75% through postexposure prophylaxis (PEP) with antiretroviral medications if initiated within hours of the event. Four weeks of therapy is recommended; however, this often is difficult to complete because of adverse reactions; therefore, the regimen probably should not be used for exposures that pose a negligible risk for transmission. The basic PEP consists of a two-drug regimen that may be expanded to a three-drug combination for more severe exposures. Because of the complexity of choosing and administering the regimen, involvement of an infectious disease specialist or a physician experienced in antiretroviral therapy is strongly recommended. Regardless, promptness in the initiation of the therapy is paramount.
BIBLIOGRAPHY
Amir, J. Clinical aspects and antiviral therapy in primary herpetic gingivostomatitis. Paediatr Drugs. 2001;3:593–597.
Arduino, PG, Porter, SR. Oral and perioral herpes simplex virus type I (HSV-I) infection: review of its management. Oral Dis. 2006;12:254–270.
Chilukuri, S, Rosen, T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311–320.
Cohen, PR, Kazi, S, Grossman, ME. Herpetic geometric glossitis: a distinctive pattern of lingual herpes simplex virus infection. South Med J. 1995;88:1231–1235.
Cohen, SG, Greenberg, MS. Chronic oral herpes simplex virus infection in immunocompromised patients. Oral Surg Oral Med Oral Pathol. 1985;59:465–471.
Flaitz, CM, Nichols, CM, Hicks, MJ. Herpesviridae-associated persistent mucocutaneous ulcers in acquired immunodeficiency syndrome: a clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:433–441.
Hess, GP, Walson, PD. Seizures secondary to oral viscous lidocaine. Ann Emerg Med. 1988;17:725–727.
Huber, MA. Herpes simplex type-1 virus infection. Quintessence Int. 2003;34:453–457.
Jureti , M. Natural history of herpetic infection. Helv Paediatr Acta. 1966;21(4):356–368.
, M. Natural history of herpetic infection. Helv Paediatr Acta. 1966;21(4):356–368.
Kameyama, T, Futami, M, Nakayoshi, N, et al. Shedding of herpes simplex virus type I into saliva in patients with orofacial fracture. J Med Virol. 1989;28:78–80.
Kameyama, T, Sujaku, C, Yamamoto, S, et al. Shedding of herpes simplex virus type 1 into saliva. J Oral Pathol. 1988;17:478–481.
Kolokotronis, A, Doumas, S. Herpes simplex virus infection, with particular reference to the progression and complications of primary herpetic gingivostomatitis. Clin Microbiol Infect. 2006;12:202–211.
Krause, PR, Straus, SE. Herpesvirus vaccines. Development, controversies, and applications. Infec Dis Clin North Am. 1999;13:61–81.
Jensen, LA, Hoehns, JD, Squires, CL. Oral antivirals for the acute treatment of recurrent herpes labialis. Ann Pharmacother. 2004;38:705–709.
Nahmias, AJ. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl. 1990;69:19–36.
Nesbit, SP, Gobetti, JP. Multiple recurrence of oral erythema multiforme after secondary herpes simplex: report of case and review of literature. J Am Dent Assoc. 1986;112:348–352.
Overall, JC. Oral herpes simplex: pathogenesis, clinical and virologic course, approach to treatment. In: Hooks JJ, Jordan G, eds. Viral infections in oral medicine. New York: Elsevier; 1982:53–78.
Park, JB, Park, N. Effect of chlorhexidine on the in vitro and in vivo herpes simplex virus infection. Oral Surg Oral Med Oral Pathol. 1989;67:149–153.
Parlette, EC, Polo, JM. Inoculation herpes barbae. Skinmed. 2005;4:186–187.
Raborn, GW, Grace, MGA. Recurrent herpes simplex labialis: selected therapeutic options. J Can Dent Assoc. 2003;69:498–503.
Raborn, GW, Martel, AY, Lassonde, M, et al. Effective treatment of herpes simplex labialis with penciclovir cream. Combined results of two trials. J Am Dent Assoc. 2002;133:303–309.
Scott, DA, Coulter, WA, Lamey, P-J. Oral shedding of herpes simplex virus type 1: a review. J Oral Pathol Med. 1997;26:441–447.
Spruance, SL, Freeman, DJ, Stewart, JC, et al. The natural history of ultraviolet radiation–induced herpes simplex labialis and response to therapy with perioral and topical formulations of acyclovir. J Infect Dis. 1991;163:728–734.
Spruance, SL, Nett, R, Marbury, T, et al. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46:2238–2243.
Starr, JR, Daling, JR, Fitzgibbons, ED, et al. Serologic evidence of herpes simplex virus 1 infection and oropharyngeal cancer risk. Cancer Res. 2001;61:8459–8464.
Stanberry, LR. Herpes: vaccines for HSV. Dermatol Clin. 1998;16:811–816.
Stoopler, ET, Greenberg, MS. Update on herpesvirus infections. Dent Clin North Am. 2003;47:517–532.
Weathers, DR, Griffin, JW. Intraoral ulcerations of recurrent herpes simplex and recurrent aphthae: two distinct clinical entities. J Am Dent Assoc. 1970;81:81–88.
Woo, S-K, Challacombe, SJ. Management of recurrent oral herpes simplex infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(suppl 1):S12.e1–S12.e18.
Wormser, GP, Mack, L, Lenox, T, et al. Lack of effect of oral acyclovir on prevention of aphthous stomatitis. Otolaryngol Head Neck Surg. 1988;98:14–17.
Alper, BS, Lewis, PR. Does treatment of acute herpes zoster prevent or shorten postherpetic neuralgia? J Fam Pract. 2000;49:255–264.
Badger, GR. Oral signs of chickenpox (varicella): report of two cases. J Dent Child. 1980;47:349–351.
Balfour, HH, Jr., Bean, B, Laskin, OL, et al. Acyclovir halts progression of herpes zoster in immunocompromised patients. N Engl J Med. 1983;308:1448–1453.
Barrett, AP, Katelaris, CH, Morris, JGL, et al. Zoster sine herpete of the trigeminal nerve. Oral Surg Oral Med Oral Pathol. 1993;75:173–175.
Centers for Disease Control and Prevention. Decline in annual incidence of varicella-selected states. MMWR Morb Mortal Wkly Rep. 2003;52:884–885.
Centers for Disease Control and Prevention. A new product (VariZIG) for postexposure prophylaxis of varicella available under an investigational new drug application expanded access protocol. MMWR Morb Mortal Wkly Rep. 2006;55:209–210.
Centers for Disease Control and Prevention. CDC’s advisory committee recommends “shingles” vaccination. Available at http://www.cdc.gov/od/media/pressrel/r061026.htm. [Accessed December 21, 2007.].
Dunkle, LM, Arvin, AM, Whitley, RJ, et al. A controlled trial of acyclovir for chickenpox in normal children. N Engl J Med. 1991;325:1539–1544.
Furuta, Y, Ohtani, F, Aizawa, H, et al. Varicella-zoster virus reactivation is an important cause of acute peripheral facial paralysis in children. Pedatric Infect Dis. 2005;24:97–101.
Gilden, DH, Cohrs, RJ, Hayward, AR, et al. Chronic varicella-zoster virus ganglionitis—possible cause of postherpetic neuralgia. J Neurovirol. 2003;9:404–407.
Kolokotronis, A, Louloudiadis, K, Fotiou, G, et al. Oral manifestations of infections due to varicella zoster virus in otherwise healthy children. J Clin Pediatr Dent. 2001;25:107–112.
Lieberman, JM, Williams, WR, Miller, JM, et al. The safety and immunogenicity of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. A study of manufacturing consistency and persistence of antibody. Pediatr Infect Dis J. 2006;25:615–622.
Meer, S, Coleman, H, Altini, M, et al. Mandibular osteomyelitis and tooth exfoliation following zoster-CMV co-infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:70–75.
Mendieta, C, Miranda, J, Brunet, LI, et al. Alveolar bone necrosis and tooth exfoliation following herpes zoster infection: a review of the literature and case report. J Periodontol. 2005;76:148–153.
Ogita, S, Terada, K, Niizuma, T, et al. Characteristics of facial nerve palsy during childhood in Japan: frequency of varicella-zoster virus association. Pediatr Int. 2006;48:245–249.
Oxman, MN, Levin, MJ, Johnson, GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284.
Stankus, SJ, Dlugopolski, M, Packer, D. Management of herpes zoster (shingles) and postherpetic neuralgia. Am Fam Physician. 2000;61:2437–2448.
Straus, SE, Ostrove, JM, Inchauspe, G, et al. NIH conference. Varicella-zoster virus infections. Biology, natural history, treatment and prevention. Ann Intern Med. 1988;108:221–237.
Wutzler, P. Antiviral therapy of herpes simplex and varicella-zoster virus infections. Intervirology. 1997;40:343–356.
Courant, P, Sobkov, T. Oral manifestations of infectious mononucleosis. J Periodontol. 1979;40:279–283.
Fraser-Moodie, W. Oral lesions in infectious mononucleosis. Oral Surg Oral Med Oral Pathol. 1959;12:685–691.
Har-EI, G, Josephsen, JS. Infectious mononucleosis complicated by lingual tonsillitis. J Laryngol Otol. 1990;104:651–653.
Niedobitek, G, Meru, N, Delecluse, H-J. Epstein-Barr virus infection and human malignancies. Int J Exp Pathol. 2001;82:149–170.
Roberge, RJ, Simon, M, Russell, M, et al. Lingual tonsillitis: an unusual presentation of mononucleosis. Am J Emerg Med. 2001;19:173–175.
Ryan, C, Dutta, C, Simo, R. Role of screening for infectious mononucleosis in patients admitted with isolated, unilateral peritonsillar abscess. J Laryngol Otol. 2004;118:362–365.
Slote, J, Saygun, I, Sabeti, M, et al. Epstein-Barr virus in oral diseases. J Periodontol Res. 2006;41:235–244.
Torre, D, Tambini, R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scand J Infect Dis. 1999;31:543–547.
van der Horst, C, Joncas, J, Ahronheim, G, et al. Lack of effect of peroral acyclovir for the treatment of acute infectious mononucleosis. J Infect Dis. 1991;164:788–791.
Bonnet, F, Neau, D, Viallard, J-F, et al. Clinical and laboratory findings of cytomegalovirus infection in 115 hospitalized non-immunocompromised adults. Ann Med Interne (Paris). 2001;152:227–235.
Epstein, JB, Scully, C. Cytomegalovirus: a virus of increasing relevance to oral medicine and pathology. J Oral Pathol Med. 1993;22:348–353.
Epstein, JB, Sherlock, CH, Wolber, RA. Oral manifestations of cytomegalovirus infection. Oral Surg Oral Med Oral Pathol. 1993;75:443–451.
Flaitz, CM, Nichols, CM, Hicks, MJ. Herpesviridae-associated persistent mucocutaneous ulcers in acquired immunodeficiency syndrome: a clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:433–441.
Guntinas-Lichius, O, Wagner, M, Krueger, GRF, et al. Severe acute cytomegalovirus sialadenitis in an immunocompetent adult: case report. Clin Infect Dis. 1996;22:1117–1118.
Jones, AC, Freedman, PD, Phelan, JA, et al. Cytomegalovirus infections of the oral cavity: a report of six cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1993;75:76–85.
Regezi, JA, Eversole, LR, Barker, BF, et al. Herpes simplex and cytomegalovirus coninfected oral ulcers in HIV-infected patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:55–62.
Schubert, MM, Epstein, JB, Lloid, ME. Oral infections due to cytomegalovirus in immunocompromised patients. J Oral Pathol Med. 1993;22:268–273.
Slots, J. Update on human cytomegalovirus in destructive periodontal disease. Oral Microbiol Immunol. 2004;19:217–223.
Stagno, S, Pass, RF, Thomas, JP, et al. Defects of tooth structure in congenital cytomegalovirus infection. Pediatrics. 1982;69:646–648.
Torres, R, Cottrell, D, Reebye, UN. Ulcerative tongue lesion secondary to cytomegalovirus. J Mass Dent Soc. 2004;53:36–37.
Buchner, A. Hand, foot, and mouth disease. Oral Surg Oral Med Oral Pathol. 1976;41:333–337.
Khetsuriani, N, Lamonte-Fowlkes, A, Oberst, S, et al. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ. 2006;55:1–20.
Chang, L-Y, Lin, T-Y, Huang, Y-C, et al. Comparison of enterovirus 71 and coxsackievirus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr Infect Dis J. 1999;18:1092–1096.
Ho, M, Hsu, K-H, Twu, S-J, et al. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999;341:929–935.
Ornoy, A, Tenenbaum, A. Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reprod Toxicol. 2006;21:446–457.
Steigman, AJ, Lipton, MM, Braspennickx, H. Acute lymphonodular pharyngitis: a newly described condition due to coxsackie virus. J Pediatr. 1962;61:331–336.
Kaplan, LJ, Daum, RS, Smaron, M, et al. Severe measles in immunocompromised patients. JAMA. 1992;267:1237–1241.
Koplik, H. The diagnosis of the invasion of measles from a study of the exanthema as it appears on the buccal mucosa membrane. Arch Pediatr. 1896;13:918–922.
Nozawa, Y, Ono, N, Abe, M, et al. An immunohistochemical study of Warthin-Finkeldey cells in measles. Pathol Int. 1994;44:442–447.
Pomerance, HH. The usual childhood diseases: forgotten but not gone. Fetal Pediatr Pathol. 2005;24:169–189.
Roberts, GBS, Bain, AD. The pathology of measles. J Pathol Bacteriol. 1958;76:111–118.
Suringa, DWR, Bank, LJ, Ackerman, AB. Role of measles virus in skin lesions and Koplik’s spots. N Engl J Med. 1970;283:1139–1142.
Warthin, AS. Occurrence of numerous large giant cells in the tonsils and pharyngeal mucosa in the prodromal stage of measles. Arch Pathol. 1931;11:864–874.
Centers for Disease Control and Prevention. Elimination of rubella and congenital rubella syndrome—United States, 1969-2004. MMWR Morb Mortal Wkly Rep. 2005;54:279–282.
Forchheimer, F. German measles (Rubella). In: Stedman TL, ed. Twentieth century practice, an international encyclopedia of modern medical science by leading authorities of Europe and America. New York: W Wood; 1898:175–188.
Pomerance, HH. The usual childhood diseases: forgotten but not gone. Fetal Pediatr Pathol. 2005;24:169–189.
Watson, JC, Hadler, SC, Dykewicz, CA, et al. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep. 1998;47(RR-8):1–57.
Anonymous. Global status of mumps immunization and surveillance. Wkly Epidemiol Rec. 2005;80:418–424.
Campos-Outcalt, D. Mumps epidemic in 2006: are you prepared to detect and prevent it? J Fam Pract. 2006;55:500–502.
Centers for Disease Control and Prevention. Update: mumps activity—United States, January 1-October 7, 2006. MMWR Morb Mortal Wkly Rep. 2006;55:1152–1153.
Centers for Disease Control and Prevention. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) for the control and elimination of mumps. MMWR Morb Mortal Wkly Rep. 2006;55:629–630.
Linder, TE, Brestel, R, Schlegel, C. Mumps virus infection: case report of an unusual head and neck manifestation. Am J Otolaryngol. 1996;17:420–423.
van Loon, FP, Holmes, SJ, Sirotkin, BI, et al. Mumps surveillance—United States 1988-1993. MMWR CDC Surveill Summ. 1995;44:1–14.
Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome
Baccaglini, L, Atkinson, JC, Patton, LL, et al. Management of oral lesions in HIV-positive patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2007;103(suppl 1):S50.e1–S50.e23.
Blignaut, E, Patton, LL, Nittayananta, W, et al. HIV phenotypes, oral lesions, and management of HIV-related disease. Adv Dent Res. 2006;19:122–129.
Campo, J, Perea, MA, del Romero, J, et al. Oral transmission of HIV, reality or fiction? An update. Oral Dis. 2006;12:219–228.
Centers for Disease Control and Prevention. Twenty-five years of HIV/AIDS—United States, 1981-2006. MMWR Morb Mortal Wkly Rep. 2006;55:585–589.
Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women. MMWR Recomm Rep. 2006;55:1–7.
Centers for Disease Control and Prevention. Updated U.S. public health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1–17.
Cherry-Peppers, G, Daniels, CO, Meeks, V, et al. Oral manifestations in the era of HAART. J Nat Med Assoc. 2003;95:21S–32S.
Cioc, AM, Allen, C, Kalmar, JR, et al. Oral plasmablastic lymphoma in AIDS patients are associated with human herpesvirus 8. Am J Surg Pathol. 2004;28:41046.
Cohen, PT, Sande, MA, Volberding, PA. The AIDS knowledge base: a textbook on HIV disease from the University of California, San Francisco, and the San Francisco General Hospital, ed 3. Waltham, Mass: Medical Publishing Group, 1999.
Coogan, MM, Greenspan, J, Challacombe, SJ. Oral lesions in infection with human immunodeficiency virus. Bull World Health Organ. 2005;83:700–706.
EC-Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus. Classification and diagnostic criteria for oral lesions in HIV infection. J Oral Pathol Med. 1993;22:289–291.
Epstein, JB, Cabay, RJ, Glick, M. Oral malignancies in HIV disease: changes in disease presentation, increasing understanding of molecular pathogenesis, and current management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:571–578.
Fornatora, ML, Reich, RF, Gray, RG, et al. Intraoral molluscum contagiosum: a report of a case and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:318–320.
Frezzini, C, Leao, JC, Porter, S. Current trends in HIV disease of the mouth. J Oral Pathol Med. 2005;34:513–531.
Gaitán-Cepeda, LA, Martínez-González, M, Ceballos-Salobreña, A. Oral candidosis as a clinical marker of immune failure in patients with HIV/AIDS on HAART. AIDS Patient Care STDS. 2005;19:70–77.
Greenspan, D, Greenspan, JS. Significance of oral hairy leukoplakia. Oral Surg Oral Med Oral Pathol. 1992;73:151–154.
Hernández, SL, López de Blanc, SA, Sambuelli, RH, et al. Oral histoplasmosis associated with HIV infection: a comparative study. J Oral Pathol Med. 2004;33:445–450.
Hofer, D, Hämmerle, Grassi, M, et al. Long-term results of supportive periodontal therapy (SPT) in HIV-seropositive and HIV-seronegative patients. J Clin Periodontol. 2002;29:630–637.
Holmstrup, P, Westergaard, J. HIV infection and periodontal diseases. Periodontol 2000. 1998;18:37–46.
Jones, AC, Gulley, ML, Freedman, PD. Necrotizing ulcerative stomatitis in human immunodeficiency virus-seropositive individuals: a review of the histopathologic, immunohistochemical, and virologic characteristics of 18 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:323–332.
Kerr, AR, Ship, JA. Management strategies for HIV-associated aphthous stomatitis. Am J Clin Dermatol. 2003;4:669–680.
King, MD, Reznik, DA, O’Daniels, CM, et al. Human papillomavirus-associated oral warts among human immunodeficiency virus-positive patients in the era of highly active antiretroviral therapy: an emerging pattern. Clin Inf Dis. 2002;34:641–648.
Lager, I, Altini, M, Coleman, H. Oral Kaposi’s sarcoma: a clinicopathologic study from South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:701–710.
Lester, R, Li, CH, Phillips, P, et al. Improved outcome of human immunodeficiency virus-associated plasmablastic lymphoma of the oral cavity in the era of high active antiretroviral therapy: a report of two cases. Leuk Lymphoma. 2004;45:1881–1885.
Mandel, L, Kim, D, Uy, C. Parotid gland swellings in HIV diffuse infiltrative CD8 lymphocytosis syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:565–568.
Mandel, L, Surattanont, F. Regression of HIV parotid swellings after antiviral therapy: case reports with computed tomographic scan evidence. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:454–459.
Manders, SM, Kostman, JR, Mendez, L, et al. Thalidomide-resistant HIV-associated aphthae successfully treated with granulocyte colony-stimulating factor. J Am Acad Dermatol. 1995;33:380–382.
Mercante, DE, Leigh, JE, Lilly, EA, et al. Assessment of the association between HIV viral load and CD4 cell count on the occurrence of oropharyngeal candidiasis in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;42:483–578.
Migliorati, CA, Birman, EG, Cury, AE, et al. Oropharyngeal candidiasis in HIV-infected patients under treatment with protease inhibitors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:301–310.
Miguez-Burbano, MJ, Jackson, J, Jr., Hadrigan, S. Thrombocytopenia in HIV disease: clinical relevance, physiopathology, and management. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:365–376.
Miziara, ID, Weber, R. Oral candidosis and oral hairy leukoplakia as predictors of HAART failure in Brazilian HIV-infected patients. Oral Dis. 2006;12:402–407.
Pienaar, ED, Young, T, Holmes, H. Interventions for the prevention and management of oropharyngeal candidiasis associated with HIV infection in adults and children. Cochrane Database Syst Rev. 2006;3:CD003940.
Piluso, S, Ficarra, G, Lucatorto, FM, et al. Cause of oral ulcers in HIV-infected patients: a study of 19 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:166–172.
Ramírez-Amador, V, Esquivel-Pedraza, L, Sierra-Madero, J, et al. The changing clinical spectrum of human immunodeficiency virus (HIV)-related oral lesions in 1,000 consecutive patients: a 12-year study in a referral center in Mexico. Medicine. 2003;82:39–50.
Reznik, DA. Oral manifestations of HIV disease. Top HIV Med. 2006;13:143–148.
Rivera, H, Nikitakis, NG, Castillo, S, et al. Histopathological analysis and demonstration of EBV and HIV p-24 antigen but not CMV expression in labial minor salivary glands of HIV patients affected by diffuse infiltrative lymphocytosis syndrome. J Oral Pathol Med. 2003;32:431–437.
Samaranayake, LP. Re-emergence of tuberculosis and its variants: implications for dentistry. Int Dent J. 2002;52:330–336.
Ship, JA, Vissink, A, Challacombe, SJ. Use of prophylactic antifungals in the immunocompromised host. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(suppl 1):S6.e1–S6.e14.
Siwamogstham, P, Kuansuwan, C, Reichart, P. Herpes zoster in HIV infection with osteonecrosis of the jaw and tooth exfoliation. Oral Dis. 2006;12:500–505.
Strauss, RM, Doyle, EL, Mohsen, AH, et al. Successful treatment of molluscum contagiosum with topical imiquimod in a severely immunocompromised HIV-positive patient. Int J STD AIDS. 2001;12:264–266.
Syrjänen, S, Leimola-Virtanen, R, Schmidt-Westhausen, A, et al. Oral ulcers in AIDS patients frequently associated with cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections. J Oral Pathol Med. 1999;28:204–209.
Triantos, D, Porter, SR, Scully, C, et al. Oral hairy leukoplakia: clinicopathologic features, pathogenesis, diagnosis, and clinical significance. Clin Infect Dis. 1997;25:1392–1396.
Umadevi, M, Adeyemi, O, Patel, M. Periodontal diseases and other bacterial infections. Adv Dent Res. 2006;19:139–145.
Völter, C, He, Y, Delius, H, et al. Novel HPV types in oral papillomatous lesions from patients with HIV infection. Int J Cancer. 1996;66:453–456.
Walling, DM, Flaitz, CM, Nichols, CM. Epstein-Barr virus replication in oral hairy leukoplakia: response, persistence, and resistance to treatment with valacyclovir. J Inf Dis. 2003;188:883–890.
Webster-Cyriaque, J, Duus, K, Cooper, C, et al. Oral EBV and KSHV infection in HIV. Adv Dent Res. 2006;19:91–95.
Younai, FS. Oral HIV transmission. J Can Dent Assoc. 2001;29:142–148.