Physical and Chemical Injuries
Chemical Injuries of the Oral Mucosa
Noninfectious Oral Complications of Antineoplastic Therapy
Bisphosphonate-Associated Osteonecrosis
Orofacial Complications of Methamphetamine Abuse
Oral Trauma from Sexual Practices
Amalgam Tattoo and Other Localized Exogenous Pigmentations
Oral Piercings and Other Body Modifications
Systemic Metallic Intoxication
Drug-Related Discolorations of the Oral Mucosa
Reactive Osseous and Chondromatous Metaplasia
Oral Ulceration with Bone Sequestration
LINEA ALBA
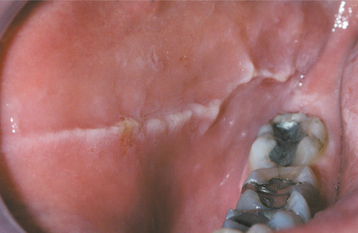
Linea alba (“white line”) is a common alteration of the buccal mucosa that most likely is associated with pressure, frictional irritation, or sucking trauma from the facial surfaces of the teeth. In one study of 256 young men, the alteration was present in 13%; in another study of 993 adolescents, linea alba was the second most common oral mucosal pathosis and affected 5.3%. No other associated problem, such as insufficient horizontal overlap or rough restorations of the teeth, is necessary for the development of linea alba.
CLINICAL FEATURES: As the name implies, the alteration consists of a white line that usually is bilateral. It may be scalloped and is located on the buccal mucosa at the level of the occlusal plane of the adjacent teeth (Fig. 8-1). The line varies in prominence and usually is restricted to dentulous areas. It often is more pronounced adjacent to the posterior teeth.
MORSICATIO BUCCARUM (CHRONIC CHEEK CHEWING)
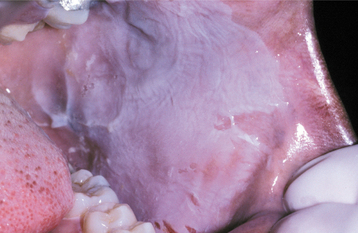
Morsicatio buccarum is a classic example of medical terminology gone astray; it is the scientific term for chronic cheek chewing. Morsicatio comes from the Latin word morsus, or bite. Chronic nibbling produces lesions that are located most frequently on the buccal mucosa; however, the labial mucosa (morsicatio labiorum) and the lateral border of the tongue (morsicatio linguarum) also may be involved. Similar changes have been seen as a result of suction and in glassblowers whose technique produces chronic irritation of the buccal mucosa.
A higher prevalence of classic morsicatio buccarum has been found in people who are under stress or who exhibit psychologic conditions. Most patients are aware of their habit, although many deny the self-inflicted injury or perform the act subconsciously. The occurrence is twice as prevalent in women and three times more prevalent after age 35. At any given time, one in every 800 adults has active lesions.
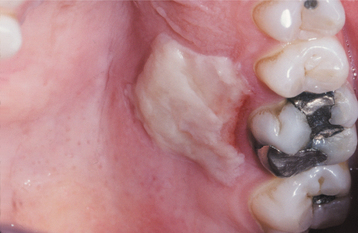
CLINICAL FEATURES: Most frequently, the lesions in patients with morsicatio are found bilaterally on the anterior buccal mucosa. They also may be unilateral, combined with lesions of the lips or the tongue, or isolated to the lips or tongue. Thickened, shredded, white areas may be combined with intervening zones of erythema, erosion, or focal traumatic ulceration (Figs. 8-2 and 8-3). The areas of white mucosa demonstrate an irregular ragged surface, and the patient may describe being able to remove shreds of white material from the involved area.
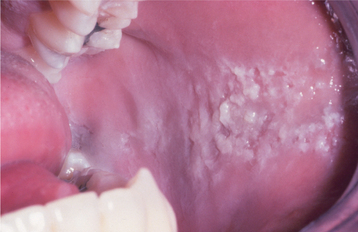
Fig. 8-2 Morsicatio buccarum. Thickened, shredded areas of white hyperkeratosis of the left buccal mucosa.
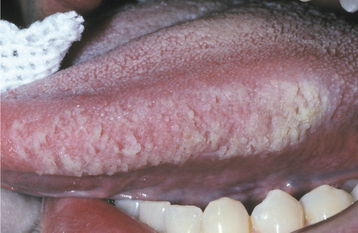
Fig. 8-3 Morsicatio linguarum. Thickened, rough areas of white hyperkeratosis of the lateral border of the tongue on the left side.
The altered mucosa typically is located in the midportion of the anterior buccal mucosa along the occlusal plane. Large lesions may extend some distance above or below the occlusal plane in patients whose habit involves pushing the cheek between the teeth with a finger.
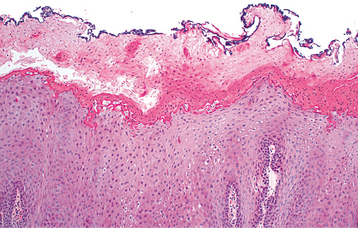
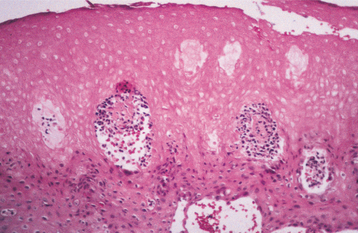
HISTOPATHOLOGIC FEATURES: Biopsy reveals extensive hyperparakeratosis that often results in an extremely ragged surface with numerous projections of keratin. Surface bacterial colonization is typical (Fig. 8-4). On occasion, clusters of vacuolated cells are present in the superficial portion of the prickle cell layer. This histopathologic pattern is not pathog-nomonic of morsicatio and may bear a striking resemblance to oral hairy leukoplakia (OHL), a lesion that most often occurs in people who are infected with the human immunodeficiency virus (HIV) (see page 268), or to uremic stomatitis (see page 851). A similar histopathologic pattern is noted in patients who chronically chew betel quid and has been termed betel chewer’s mucosa (see page 402). Similarities with linea alba and leukoedema also may be seen.
DIAGNOSIS: In most cases the clinical presentation of morsicatio buccarum is sufficient for a strong presumptive diagnosis, and clinicians familiar with these alterations rarely perform biopsy. Some cases of morsicatio may not be diagnostic from the clinical presentation, and biopsy may be necessary. In patients at high risk for HIV infection with isolated involvement of the lateral border of the tongue, further investigation is desirable to rule out HIV-associated OHL.
TREATMENT AND PROGNOSIS: No treatment of the oral lesions is required, and no long-term difficulties arise from the presence of the mucosal changes. For patients who desire treatment, an oral acrylic shield that covers the facial surfaces of the teeth may be constructed to eliminate the lesions by restricting access to the buccal and labial mucosa. For patients desiring either confirmation of the cause or preventive therapy, construction and use of two lateral acrylic shields connected by a labial stainless steel wire can provide quick resolution of the lesions while being acceptable aesthetically and not interfering with speech. Several authors also have suggested psychotherapy as the treatment of choice, but no extensive well-controlled studies have indicated benefits from this approach.
TRAUMATIC ULCERATIONS
Acute and chronic injuries of the oral mucosa are frequently observed. Injury can result from mechanical damage, such as contact with sharp foodstuffs or accidental biting during mastication, overzealous toothbrushing, talking, or even sleeping. Some are self-induced and clinically obvious, whereas others are subtle and difficult to diagnose. Damage also may result from thermal, electrical, or chemical burns. (Oral mucosal manifestations of such burns are discussed later in the chapter.)
Acute or chronic trauma to the oral mucosa may result in surface ulcerations. The ulcerations may remain for extended periods of time, but most usually heal within days. A histopathologically unique type of chronic traumatic ulceration of the oral mucosa is the eosinophilic ulceration (traumatic granuloma, traumatic ulcerative granuloma with stromal eosinophilia [TUGSE], eosinophilic granuloma of the tongue), which exhibits a deep pseudoinva-sive inflammatory reaction and is typically slow to resolve. Interestingly, many of these traumatic granulomas undergo resolution after incisional biopsy. Lesions microscopically similar to eosinophilic ulceration have been reproduced in rat tongues after repeated crushing trauma and in traumatic lesions noted in patients with familial dysautonomia, a disorder characterized by indifference to pain. In addition, similar sublingual ulcerations may occur in infants as a result of chronic mucosal trauma from adjacent anterior primary teeth, often associated with nursing. These distinctive ulcerations of infancy have been termed Riga-Fede disease and should be considered a variation of the traumatic eosinophilic ulceration.
In rare subsets of TUGSE, the lesion does not appear to be associated with trauma, and sheets of large, atypical cells are seen histopathologically. The nature of these atypical cells remains in dispute, although it has been suggested that they may represent reactive myofibroblasts, histiocytes (atypical histiocytic granuloma), or T lymphocytes. Whether these atypical eosinophilic ulcerations represent a single pathosis or a variety of disorders that share stromal eosinophilia is an area for future research. Of these theories, several current investigations have shown the atypical cells to be T lymphocytes with strong immunoperoxidase reactivity for CD30. In these cases, it is thought that this subset of TUGSE may represent the oral counterpart of the primary cutaneous CD30+ lymphoproliferative disorder, which also exhibits sequential ulceration, necrosis, and self-regression.
In most cases of traumatic ulceration, there is an adjacent source of irritation, although this is not present invariably. The clinical presentation often suggests the cause, but many cases resemble early ulcerative squamous cell carcinoma; biopsy is performed to rule out that possibility.
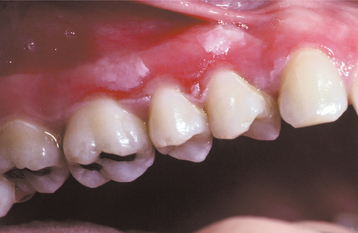
CLINICAL FEATURES: Most injuries are unintentional and arise from a variety of causes. As would be expected, simple chronic traumatic ulcerations occur most often on the tongue, lips, and buccal mucosa—sites that may be injured by the dentition (Fig. 8-5). Lesions of the gingiva, palate, and mucobuccal fold may occur from other sources of irritation. Overzealous toothbrushing can create linear erosions along the free gingival margins. Although these areas may superficially resemble a number of the chronic vesiculoerosive processes, thorough questioning of the patient often leads to the appropriate diagnosis. The individual lesions appear as areas of erythema surrounding a central removable, yellow fibrinopurulent membrane. In many instances, the lesion develops a rolled white border of hyperkeratosis immediately adjacent to the area of ulceration (Fig. 8-6).
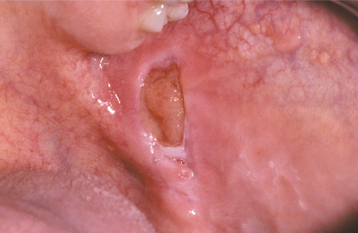
Fig. 8-5 Traumatic ulceration. Well-circumscribed ulceration of the posterior buccal mucosa on the left side.
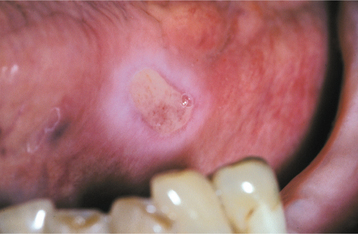
Fig. 8-6 Traumatic ulceration. Mucosal ulceration with a hyperkeratotic collar located on the ventral surface of the tongue.
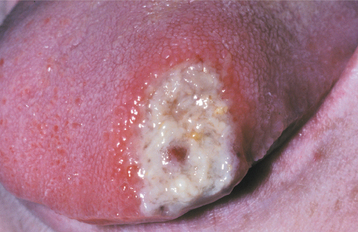
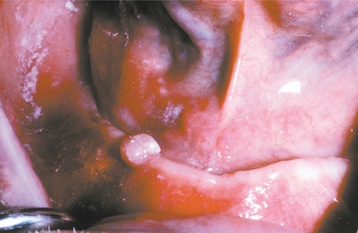
Eosinophilic ulcerations are not uncommon but frequently are not reported. The lesions occur in peopleof all ages, with a significant male predominance. Most have been reported on the tongue, although cases have been seen on the gingiva, buccal mucosa, floor of mouth, palate, and lip. The lesion may last from 1 week to 8 months. The ulcerations appear very similar to the simple traumatic ulcerations; however, on occasion, underlying proliferative granulation tissue can result in a raised lesion similar to a pyogenic granuloma (see page 517) (Fig. 8-7).
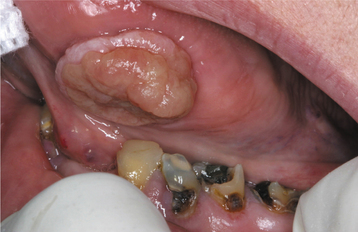
Fig. 8-7 Traumatic granuloma. Exophytic ulcerated mass on the ventrolateral tongue associated with multiple jagged teeth.
Riga-Fede disease typically appears between 1 week and 1 year of age. The condition often develops in association with natal or neonatal teeth (see page 81). The anterior ventral surface of the tongue is the most common site of involvement, although the dorsal surface also may be affected (Fig. 8-8). Ventral lesions contact the adjacent mandibular anterior incisors; lesions on the dorsal surface are associated with the maxillary incisors. A presentation similar to Riga-Fede disease can be the initial finding in a variety of neuro logic conditions related to self-mutilation, such as familial dysautonomia (Riley-Day syndrome), congenital indifference to pain, Lesch-Nyhan syn-drome, Gaucher disease, cerebral palsy, or Tourette syndrome.
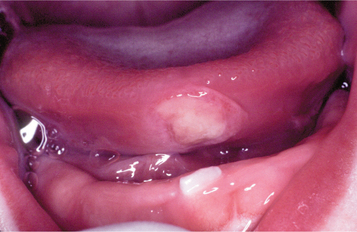
Fig. 8-8 Riga-Fede disease. Newborn with traumatic ulceration of anterior ventral surface of the tongue. Mucosal damage occurred from contact of tongue with adjacent tooth during breastfeeding.
The atypical eosinophilic ulceration occurs in older adults, with most cases developing in patients older than age 40. Surface ulceration is present, and an underlying tumefaction also is seen. The tongue is the most common site, although the gingiva, alveolar mucosa, mucobuccal fold, buccal mucosa, and lip may be affected (Fig. 8-9).
HISTOPATHOLOGIC FEATURES: Simple traumatic ulcerations are covered by a fibrinopurulent membrane that consists of fibrin intermixed with neutrophils. The membrane is of variable thickness, and the adjacent surface epithelium may be normal or may demonstrate slight hyperplasia with or without hyperkeratosis. The ulcer bed consists of granulation tissue that supports a mixed inflammatory infiltrate of lymphocytes, histiocytes, neutrophils, and, occasionally, plasma cells. In patients with eosinophilic ulcerations, the pattern is very similar; however, the inflammatory infiltrate extends into the deeper tissues and exhibits sheets of lymphocytes and histiocytes intermixed with eosinophils. In addition, the vascular connective tissue deep to the ulceration may become hyperplastic and cause surface elevation.
Atypical eosinophilic ulcerations exhibit numerous features of the traumatic eosinophilic ulceration, but the deeper tissues are replaced by a highly cellular proliferation of large lymphoreticular cells. The infiltrate is pleomorphic, and mitotic features are somewhat common. Intermixed with the large atypical cells are mature lymphocytes and numerous eosinophils. Although an associated immunohistochemical profile rarely has been reported, several investigators have shown the large cells to be T lymphocytes, the majority of which react with CD30 (Ki-1). In many instances, molecular studies for T-cell clonality by polymerase chain reaction (PCR) have been performed on the CD30+ cells and demonstrated monoclonal rearrangement. Whether this monoclonal infiltrate represents a true low-grade lymphoma or an unusual reactive lymphoproliferative process has not been determined.
TREATMENT AND PROGNOSIS: For traumatic ulcerations that have an obvious source of injury, the irritating cause should be removed. Dyclonine HCl or hydroxypropyl cellulose films can be applied for temporary pain relief. If the cause is not obvious, or if a patient does not respond to therapy, then biopsy is indicated. Rapid healing after a biopsy is typical even with large eosinophilic ulcerations (Fig. 8-10). Recurrence is not expected.
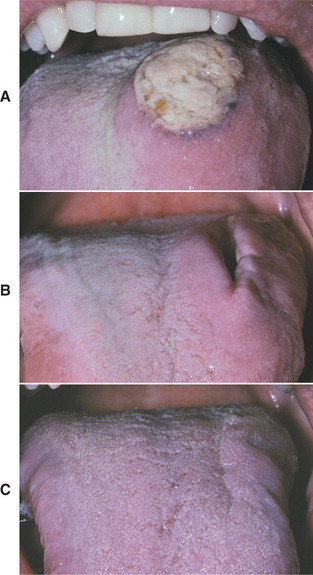
Fig. 8-10 Eosinophilic ulceration. A, Initial presentation of a large ulceration of the dorsal surface of the tongue. B, Significant resolution noted 2 weeks after incisional biopsy. C, Subsequent healing noted 4 weeks after biopsy.
The use of corticosteroids in the management of traumatic ulcerations is controversial. Some clinicians have suggested that use of such medications may delay healing. In spite of this, other investigators have reported success using corticosteroids to treat chronic traumatic ulcerations.
Although extraction of the anterior primary teeth is not recommended, this procedure has resolved the ulcerations in Riga-Fede disease. The teeth should be retained if they are stable. Grinding the incisal mame-lons, coverage of the teeth with a light-cured composite or cellulose film, construction of a protective shield, or discontinuation of nursing have been tried with variable success.
In patients demonstrating histopathologic similarities to the cutaneous CD30+ lymphoproliferative disorder, a thorough evaluation for systemic lymphoma is mandatory, along with lifelong follow-up. Although recurrence frequently is seen, the ulcerations typically heal spontaneously, and the vast majority of patients do not demonstrate dissemination of the process. Further documentation is critical to define more fully this poorly understood process.
ELECTRICAL AND THERMAL BURNS
Electrical burns to the oral cavity are fairly common, constituting approximately 5% of all burn admissions to hospitals. Two types of electrical burns can be seen: (1) contact and (2) arc.
Contact burns require a good ground and involve electrical current passing through the body from the point of contact to the ground site. The electric current can cause cardiopulmonary arrest and may be fatal. Most electrical burns affecting the oral cavity are the arc type, in which the saliva acts as a conducting medium and an electrical arc flows between the electrical source and the mouth. Extreme heat, up to 3000° C, is possible with resultant significant tissue destruction. Most cases result from chewing on the female end of an extension cord or from biting through a live wire.
Most thermal burns of the oral cavity arise from ingestion of hot foods or beverages. Microwave ovens have been associated with an increased frequency of thermal burns because of their ability to cook food that is cool on the exterior but extremely hot in the interior.
CLINICAL FEATURES: Most electrical burns occur in children younger than 4 years of age. The lips are affected most frequently, and the commissure commonly is involved. Initially, the burn appears as a painless, charred, yellow area that exhibits little to no bleeding (Fig. 8-11). Significant edema often develops within a few hours and may persist up to 12 days. Beginning on the fourth day, the affected area becomes necrotic and begins to slough. Bleeding may develop during this period from exposure of the underlying vital vasculature, and the presence of this complication should be monitored closely. The adjacent mucobuccal fold, the tongue, or both also may be involved. On occasion, adjacent teeth may become nonvital, with or without necrosis of the surrounding alveolar bone. Malformation of developing teeth also has been documented. In patients receiving high-voltage electrical injury, resultant facial nerve paralysis is infrequently reported and typically resolves over several weeks to months.
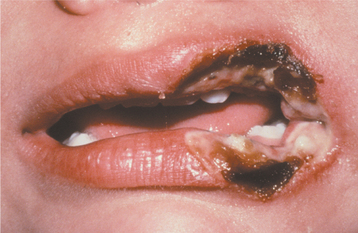
Fig. 8-11 Electrical burn. Yellow charred area of necrosis along the left oral commissure. (Courtesy of Dr. Patricia Hagen.)
The injuries related to thermal food burns usually appear on the palate or posterior buccal mucosa (Fig. 8-12). The lesions appear as zones of erythema and ulceration that often exhibit remnants of necrotic epithelium at the periphery. If hot beverages are swallowed, swelling of the upper airway can occur and lead to dyspnea, which may develop several hours after the injury.
TREATMENT AND PROGNOSIS: For patients with electrical burns of the oral cavity, tetanus immunization, if not current, is required. Most clinicians prescribe a prophylactic antibiotic, usually penicillin, to prevent secondary infection in severe cases. The primary problem with oral burns is contracture of the mouth opening during healing. Without intervention, significant microstomia can develop and may produce such restricted access to the mouth that hygiene and eating become impossible in severe cases. Extensive scarring and disfigurement are typical in untreated patients.
To prevent the disfigurement, a variety of microstomia prevention appliances can be used to eliminate or reduce the need for subsequent surgical reconstruction. Compliance is the most important consideration when choosing the most appropriate device. Tissue-supported appliances appear most effective for infants and young children; older, more cooperative patients usually benefit from tooth-supported devices. In most cases, splinting is maintained for 6 to 8 months to ensure proper scar maturation. Evaluation for possible surgical reconstruction is usually performed after a 1-year follow-up.
Most thermal burns are of little clinical consequence and resolve without treatment. When the upper airway is involved and associated with breathing difficulties, antibiotics and corticosteroids often are administered. In rare cases, swelling of the airway mandates intubation or tracheotomy to resolve the associated dyspnea. In these severe cases, oral intake of food often is discontinued temporarily with nutrition provided by a nasogastric tube.
CHEMICAL INJURIES OF THE ORAL MUCOSA
A large number of chemicals and drugs come into contact with the oral tissues. A percentage of these agents are caustic and can cause clinically significant damage.
Patients often can be their own worst enemies. The array of chemicals that have been placed within the mouth in an attempt to resolve oral problems is amazing. Aspirin, sodium perborate, hydrogen peroxide, gasoline, turpentine, rubbing alcohol, and battery acid are just a few of the more interesting examples.
Certain patients, typically children or those under psychiatric care, may hold medications within their mouths rather than swallow them. A surprising number of medications are potentially caustic when held in the mouth long enough. Aspirin, bisphosphonates, and two psychoactive drugs, chlorpromazine and promazine, are well-documented examples.
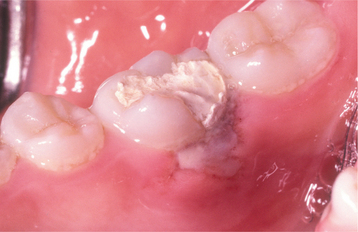
Topical medications for mouth pain can compound the problem. Mucosal damage has been documented from many of the topical medicaments sold as treatments for toothache or mouth sores. Products containing isopropyl alcohol, phenol, hydrogen peroxide, or eugenol have produced adverse reactions in patients. Over-the-counter tooth-whitening products also contain hydrogen peroxide or one of its precursors, carbamide peroxidase, which has been shown to create mucosal necrosis (Fig. 8-13).
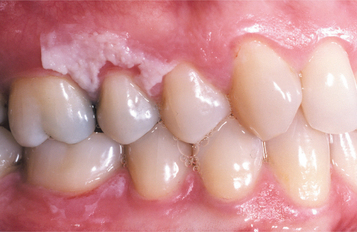
Fig. 8-13 Mucosal burn from tooth-whitening strips. Sharply demarcated zone of epithelial necrosis on the maxillary facial gingiva, which developed from the use of tooth-whitening strips. Less severe involvement also is present on the mandibular gingiva.
Health care practitioners are responsible for the use of many caustic materials. Silver nitrate, formocresol, sodium hypochlorite, paraformaldehyde, chromic acid, trichloroacetic acid, dental cavity varnishes, and acid-etch materials all can cause patient injury. Education and use of the rubber dam have reduced the frequency of such injuries.
The improper use of aspirin, hydrogen peroxide, silver nitrate, phenol, and certain endodontic materials deserves further discussion because of their frequency of misuse, the severity of related damage, and the lack of adequate documentation of these materials as harmful agents.
ASPIRIN: Mucosal necrosis from aspirin being held in the mouth is not rare (Fig. 8-14). Aspirin is available not only in the well-known tablets but also as powder.
HYDROGEN PEROXIDE: Hydrogen peroxide became a popular intraoral medication for prevention of periodontitis in the late 1970s. Since that time, mucosal damage has been seen more frequently as a result of this application. Concentrations at 3% or greater are associated most often with adverse reactions. Epithelial necrosis has been noted with dilutions as low as 1%, and many over-the-counter oral medications exceed this concentration (Fig. 8-15).
SILVER NITRATE: Silver nitrate remains a popular treatment for aphthous ulcerations, because the chemical cautery brings about rapid pain relief by destroying nerve endings. In spite of this, its use should be strongly discouraged. In all cases the extent of mucosal damage is increased by its use. In some patients an abnormal reaction is seen, with resultant significant damage and enhanced pain. In one report, an application of a silver nitrate stick to a small aphthous ulceration led to a necrotic defect that exceeded 2 ×- 2 cm and had to be débrided surgically. In addition, rare reports have documented irreversible systemic argyria secondary to habitual intraoral use of topical silver nitrate after recommendation by a dentist (see page 315).
PHENOL: Phenol has occasionally been used in dentistry as a cavity-sterilizing agent and cauterizing material. It is extremely caustic, and judicious use is required. Over-the-counter agents advertised as “canker sore” treatments may contain low concentrations of phenol, often combined with high levels of alcohol. Extensive mucosal necrosis and (rarely) underlying bone loss have been seen in patients who placed this material (phenol concentration 0.5%) in attempts to resolve minor mucosal sore spots (Fig. 8-16).
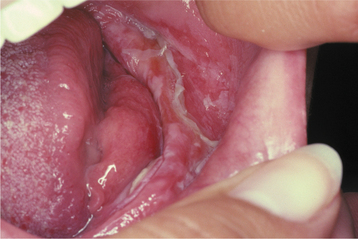
Fig. 8-16 Phenol burn. Extensive epithelial necrosis of the mandibular alveolar mucosa on the left side. Damage resulted from placement of an over-the-counter, phenol-containing, antiseptic and anesthetic gel under a denture. (Courtesy of Dr. Dean K. White.)
A prescription therapy containing 50% sulfuric acid, 4% sulfonated phenol, and 24% sulfonated phenolic agents is being marketed heavily to dentists for treatment of aphthous ulcerations. Because extensive necrosis has been seen from use of medicaments containing 0.5% phenol, this product must be closely monitored and used with great care.
ENDODONTIC MATERIALS: Some endodontic materials are dangerous because of the possibility of soft tissue damage (Fig. 8-17) or their injection into hard tissue with resultant deep spread and necrosis. Because of the past difficulty of obtaining profound anesthesia in some patients undergoing root canal therapy, some clinicians have used arsenical paste or paraformaldehyde formulations to devitalize the inflamed pulp. Gingival and bone necrosis have been documented as a consequence of leakage of this material from the pulp chamber into the surrounding tissues. In addition, extrusion of filling material containing paraformaldehyde into the periapical tissues has led to significant difficulties, and its use should be discouraged. Sodium hypochlorite produces similar results when it leaks into the surrounding supporting tissues or is injected past the apex, leading some to suggest chlorhexidine as a safer irrigant. The following can reduce the chances of tissue damage:
CLINICAL FEATURES: The previously discussed caustic agents produce similar damage. With short exposure, the affected mucosa exhibits a superficial white, wrinkled appearance. As the duration of exposure increases, the necrosis proceeds and the affected epithelium becomes separated from the underlying tissue and can be desquamated easily. Removal of the necrotic epithelium reveals red, bleeding connective tissue that subsequently will be covered by a yellowish, fibrinopurulent membrane. Mucosa bound to bone is keratinized and more resistant to damage, whereas the nonkeratinized movable mucosa is destroyed more quickly. In addition to mucosal necrosis, significant tooth erosion has been seen in patients who chronically chew aspirin or hold the medication in their teeth as it dissolves.
The use of the rubber dam can dramatically reduce iatrogenic mucosal burns. When cotton rolls are used for moisture control during dental procedures, two problems may occur. On occasion, caustic materials can leak into the cotton roll and be held in place against the mucosa for an extended period, with mucosal injury resulting from the chemical absorbed by the cotton. In addition, oral mucosa can adhere to dry cotton rolls, and rapid removal of the rolls from the mouth often can cause stripping of the epithelium in the area. The latter pattern of mucosal injury has been termed cotton roll burn (cotton roll stomatitis) (Fig. 8-18).
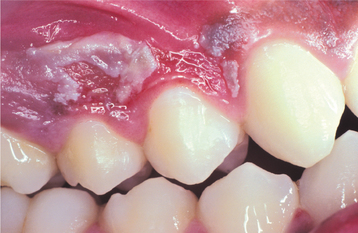
Fig. 8-18 Cotton roll burn. Zone of white epithelial necrosis and erythema of the maxillary alveolar mucosa.
Caustic materials injected into bone during endodontic procedures can result in significant bone necrosis, pain, and perforation into soft tissue. Necrotic surface ulceration and edema with underlying areas of soft tissue necrosis may occur adjacent to the site of perforation.
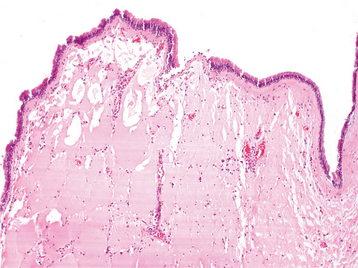
HISTOPATHOLOGIC FEATURES: Microscopic examination of the white slough removed from areas of mucosal chemical burns reveals coagulative necrosis of the epithelium, with only the outline of the individual epithelial cells and nuclei remaining (Fig. 8-19). The necrosis begins on the surface and moves basally. The amount of epithelium affected depends on the duration of contact and the concentration of the offending agent. The underlying connective tissue contains a mixture of acute and chronic inflammatory cells.
TREATMENT AND PROGNOSIS: The best treatment of chemical injuries is prevention of exposure of the oral mucosa to caustic materials. When using potentially caustic drugs (e.g., aspirin, chlorpromazine), the clinician must instruct the patient to swallow the medication and not allow it to remain in the oral cavity for any significant length of time. Children should not use chewable aspirin immediately before bedtime, and they should rinse after use.
Superficial areas of necrosis typically resolve completely without scarring within 10 to 14 days after discontinuation of the offending agent. For temporary protection, some clinicians have recommended coverage with a protective emollient paste or a hydroxypropyl cellulose film. Topical dyclonine HCl provides excellent but temporary pain relief. When large areas of necrosis are present, such as that related to the use of silver nitrate or accidental intrabony injection of offending materials, surgical débridement and antibiotic coverage often are required to promote healing and prevent spread of the necrosis.
NONINFECTIOUS ORAL COMPLICATIONS OF ANTINEOPLASTIC THERAPY
No systemic anticancer therapy currently available is able to destroy tumor cells without causing the death of at least some normal cells, and tissues with rapid turnover (e.g., oral epithelium) are especially susceptible. The mouth is a common site (and one of the most visible areas) for complications related to cancer therapy. Both radiation therapy and systemic chemotherapy may cause significant oral problems. The more potent the treatment, the greater the risk of complications. Each year almost 400,000 patients in the United States suffer acute or chronic oral side effects from anticancer treatments. With the advancement of medical practice, these complications are becoming more common as more patients have longer survival times and as intense therapies, such as bone marrow transplantation, become more commonplace. Oral complications are noted almost uniformly in patients receiving head and neck radiation, and close to 75% of bone marrow transplant patients are affected. The prevalence of chemotherapy-associated oral complications varies from 10% to 75%, depending on the type of associated cancer and the form of chemotherapy used.
CLINICAL FEATURES: A variety of noninfectious oral complications are seen regularly as a result of both radiation and chemotherapy. Two acute changes, mucositis and hemorrhage, are the predominant problems associated with chemotherapy, especially in cancers, such as leukemia, that involve high treatment doses.
Painful acute mucositis and dermatitis are the most frequently encountered side effects of radiation, but several chronic alterations continue to plague patients long after their courses of therapy are completed. Depending on the fields of radiation, the radiation dose, and the age of the patient, the following outcomes are possible:
HEMORRHAGE: Intraoral hemorrhage is typically secondary to thrombocytopenia, which develops from bone marrow suppression. Intestinal or hepatic damage, however, may cause lower vitamin K–dependent clotting factors, with resultant increased coagulation times. Conversely, tissue damage related to therapy may cause release of tissue thromboplastin at levels capable of producing potentially devastating disseminated intravascular coagulation (DIC). Oral petechiae and ecchymosis secondary to minor trauma are the most common presentations. Any mucosal site may be affected, but the labial mucosa, tongue, and gingiva are involved most frequently.
MUCOSITIS: Recent research suggests that mucosal damage secondary to cancer therapy is much more complex than previously thought and appears to arise from an extended series of molecular and cellular events that take place not only in the epithelium but also in the underlying stroma. Genetic differences in the rate of tissue apop tosis, microvascular injury from endothelial apoptosis, and increased peripheral blood levels of tumor necrosis factor a and interleukin-6 appear to be involved. Beyond the direct effects of the antineoplastic agent, additional risk factors include young age, female sex, poor oral hygiene, oral foci of infection, poor nutrition, impaired salivary function, tobacco use, and alcohol consumption.
Cases of oral mucositis related to radiation or chemotherapy are similar in their clinical presentations. The manifestations of chemotherapy develop after a few days of treatment; radiation mucositis may begin to appear during the second week of therapy. Both chemotherapy and radiation-induced mucositis will resolve slowly 2 to 3 weeks after cessation of treatment. Oral mucositis associated with chemotherapy typically involves the nonkeratinized surfaces (i.e., buccal mucosa, ventrolateral tongue, soft palate, floor of the mouth), whereas radiation therapy primarily affects the mucosal surfaces within the direct portals of radiation.
The earliest manifestation is development of a whitish discoloration from a lack of sufficient desquamation of keratin. This is soon followed by loss of this layer with replacement by atrophic mucosa, which is edematous, erythematous, and friable. Subsequently, areas of ulceration develop with formation of a removable yellowish, fibrinopurulent surface membrane (Figs. 8-20 to 8-22). Pain, burning, and discomfort are significant and can be worsened by eating and oral hygiene procedures.
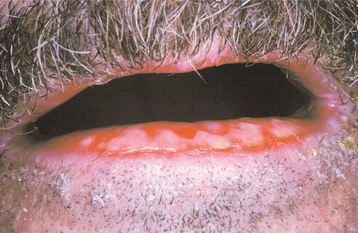
Fig. 8-20 Chemotherapy-related epithelial necrosis. Vermilion border of the lower lip exhibiting epithelial necrosis and ulceration in a patient receiving systemic chemotherapy.
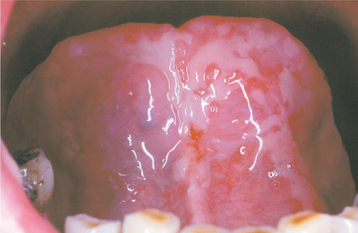
Fig. 8-21 Chemotherapy-related epithelial necrosis. Large, irregular area of epithelial necrosis and ulceration of the anterior ventral surface of the tongue in a patient receiving systemic chemotherapy.
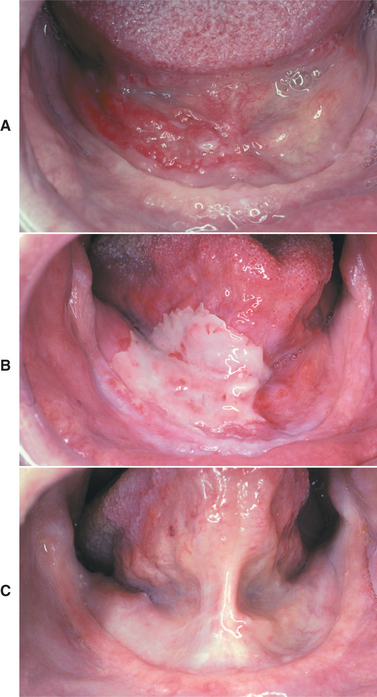
Fig. 8-22 Radiation mucositis. A, Squamous cell carcinoma before radiation therapy. Granular erythroplakia of the floor of the mouth on the patient’s right side. B, Same lesion after initiation of radiation therapy. Note the large, irregular area of epithelial necrosis and ulceration of the anterior floor of the mouth on the patient’s right side. C, Normal oral mucosa after radiation therapy. Note resolution of the tumor and the radiation mucositis.
DERMATITIS: Acute dermatitis of the skin in the fields of radiation is common and varies according to the intensity of the therapy. Patients with mild radiation dermatitis experience erythema, edema, burning, and pruritus. This condition resolves in 2 to 3 weeks after therapy and is replaced by hyperpigmentation and variable hair loss. Moderate radiation causes erythema and edema in combination with erosions and ulcerations. Within 3 months these alterations resolve, and permanent hair loss, hyperpigmentation, and scarring may ensue. Necrosis and deep ulcerations can occur in severe acute reactions. Radiation dermatitis also may become chronic and be characterized by dry, smooth, shiny, atrophic, necrotic, telangiectatic, depilated, or ulcerated areas.
XEROSTOMIA: Salivary glands are very sensitive to radiation, and xerostomia is a common complication. When a portion of the salivary glands is included in the fields of radiation, the remaining glands undergo compensatory hyperplasia in an attempt to maintain function. The changes begin within 1 week of initiation of radiation therapy, with a dramatic decrease in salivary flow noted during the first 6 weeks of treatment. Even further decreases may be noted for up to 3 years.
Serous glands exhibit an increased radiosensitivity when compared with the mucous glands. On significant exposure, the parotid glands are affected dramatically and irreversibly. In contrast, the mucous glands partially recover and, over several months, may achieve flow that approaches 50% of preradiation levels. Symptomatic dry mouth appears most strongly associated with a decrease in palatal mucous secretions, with the loss of parotid serous secretion exerting a less noticeable effect. In addition to the discomfort of a mouth that lacks proper lubrication, diminished flow of saliva leads to a significant decrease of the bactericidal action and self-cleansing properties of saliva.
Without intervention, patients often develop symptomatic dry mouth that affects their ability to eat comfortably, wear dentures, speak, and sleep. In addition, there often is an increase in the caries index (xerostomia-related caries), regardless of the patient’s past caries history (Fig. 8-23). The decay is predominantly cervical in location and secondary to xerostomia (not a direct effect of the radiation).
LOSS OF TASTE: In patients who receive significant radiation to the oral cavity, a substantial loss of all four tastes (hypogeusia) often develops within several weeks. Although these tastes return within 4 months for most patients, some patients are left with permanent hypogeusia; others may have persistent dysgeusia (altered sense of taste) (see page 875).
OSTEORADIONECROSIS: Osteoradionecrosis is one of the most serious complications of radiation to the head and neck; however, it is seen less frequently today because of better treatment modalities and prevention. The current prevalence rate is approximately 5%, whereas the frequency approached 15% less than 20 years ago. Although the risk is low, it increases dramatically if a local surgical procedure is performed within 21 days of therapy initiation or within 12 months after therapy.
In the past, many researchers believed that radiation induced an osseous endarteritis that led to tissue hypoxia, hypocellularity, and hypovascularity and created a predisposition to necrosis if a minor injury occurred. This theory led to widespread use of hyperbaric oxygen in the prevention and treatment of this pathosis. However, many now believe the process is more complex and may involve radiation damage to osseous cells; when these cells lose normal function, bone turnover is suppressed in a manner similar to the effect on osteoclasts associated with use of bisphosphonates (see page 299). Researchers believe that damage to these cells not only disrupts their primary function but also affects bone vascularity through a complex interaction of cytokines and growth factors.
Previously, investigators suggested that radiation-associated reduction in healing capacity might be permanent, with the risk for osteonecrosis remaining high for many years. Currently, others have proposed that repair of damaged osseous cells occurs as they are replaced by future cellular generations. Investigations have suggested that significant recovery does occur, and the prevalence of osteonecrosis is reduced significantly after a 1-year recovery period, with or without use of hyperbaric oxygen. More recent clinical trials of irradiated patients have shown a low prevalence of osteonecrosis if a period of postradiation healing (1 year or longer) is combined with atraumatic surgical technique.
Although most instances of osteoradionecrosis arise secondary to local trauma, a minority appear sponta-neous. The mandible is involved most frequently, although a few cases have involved the maxilla (Fig. 8-24). Affected areas of bone reveal ill-defined areas of radiolucency that may develop zones of relative radiopacity as the dead bone separates from the residual vital areas (Fig. 8-25). Intractable pain, cortical perforation, fistula formation, surface ulceration, and pathologic fracture may be present (Fig. 8-26).
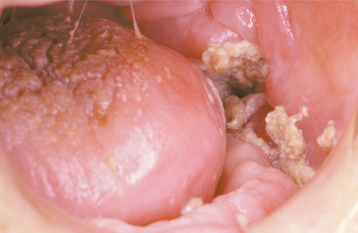
Fig. 8-24 Osteoradionecrosis. Ulceration overlying left body of the mandible with exposure and sequestration of superficial alveolar bone.
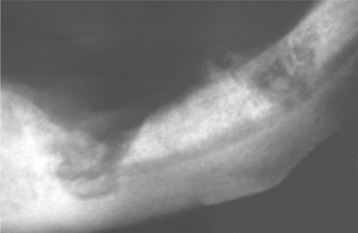
Fig. 8-25 Osteoradionecrosis. Multiple ill-defined areas of radiolucency and radiopacity of the mandibular body.
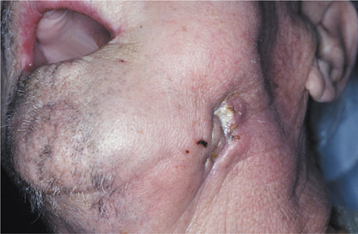
Fig. 8-26 Osteoradionecrosis. Same patient as depicted in Fig. 8-24. Note fistula formation of the left submandibular area resulting from osteoradionecrosis of the mandibular body.
The radiation dose is the main factor associated with bone necrosis, although the proximity of the tumor to bone, the presence of remaining dentition, and the type of treatment also exert an effect. Additional factors associated with an increased prevalence include older age, male sex, poor health or nutritional status, and continued use of tobacco or alcohol.
Prevention of bone necrosis is the best course of action. Before therapy, all questionable teeth should be extracted or restored, and oral foci of infection should be eliminated; excellent oral hygiene should be initiated and maintained. A healing time of at least 3 weeks between extensive dental procedures and the initiation of radiotherapy significantly decreases the chance of bone necrosis. Extraction of teeth or any bone trauma is strongly contraindicated during radiation therapy.
TRISMUS: Trismus may develop and can produce extensive difficulties concerning access for hygiene and dental treatment. Tonic muscle spasms with or without fibrosis of the muscles of mastication and the temporomandibular joint (TMJ) capsule can cause difficulties in jaw opening. When these structures are radiated heavily, jaw-opening exercises may help to decrease or prevent problems.
DEVELOPMENTAL ABNORMALITIES: Antineoplastic therapy during childhood can affect growth and development. The changes vary according to the age at treatment and the type and severity of therapy. Radiation can alter the facial bones and result in micrognathia, retrognathia, or malocclusion. Developing teeth are very sensitive and can exhibit a number of changes, such as root dwarfism, blunting of roots, dilaceration of roots, incomplete calcification, premature closure of pulp canals in deciduous teeth, enlarged canals in permanent teeth, microdontia, and hypodontia (see page 58).
TREATMENT AND PROGNOSIS: Optimal treatment planning involves the oral health practitioner before initiation of antineoplastic therapy. Elimination of all current or potential oral foci of infection is paramount, along with patient education about maintaining ultimate oral hygiene. Proper nutrition, cessation of tobacco use, and alcohol abstinence will minimize oral complications. Once cancer therapy is initiated, efforts must be directed toward relieving pain, preventing dehydration, maintaining adequate nutrition, eliminating foci of infection, and ensuring continued appropriate oral hygiene.
MUCOSITIS: In an attempt to decrease the severity, duration, and symptoms associated with oral mucositis, a large number of treatments such as anesthetic, analgesic, antimicrobial, and coating agents have been tried with mixed reviews. Few have been proven to be consistently beneficial in well-designed placebo-controlled trials. A low-cost salt and soda rinse often has demonstrated similar effectiveness and a lower adverse reaction profile when compared with many other interventions. When all treatments fail, the degree of mucositis and associated pain may mandate systemic morphine therapy.
Cryotherapy (placement of ice chips in the mouth 5 minutes before chemotherapy and continued for 30 minutes) has been shown to reduce significantly the prevalence and severity of oral mucositis secondary to bolus injection of chemotherapeutic drugs with a short half-life, such as 5-fluorouracil or edatrexate. Although limited by the hardware expense and necessity for specialized training, low-level helium-neon laser therapy also appears to reduce the frequency and severity of chemotherapy-associated mucositis.
A new avenue of research is concentrating on growth factors that may affect the prevalence of oral mucositis. Recently, intravenous (IV) recombinant human keratinocyte growth factor (palifermin) became the first compound approved by the U.S. Food and Drug Administration (FDA) for reduction of oral mucositis related to myelotoxic chemotherapy. In clinical studies, this expensive biologic response modifier provided a modest, but statistically significant, reduction in the severity of mucositis, severity of oral pain, use of narcotic analgesia, and need for total parenteral nutrition.
One of the more effective mechanisms to reduce radiation-associated mucositis has been placement of midline radiation blocks or use of three-dimensional radiation treatment to limit the volume of irradiated mucosa. Although not approved in the United States but available in Canada and Europe, topical benzydamine reduces the frequency, severity, and pain of radiation-associated oral mucositis. This unique nonsteroidal antiinflammatory drug (NSAID) also exhibits analgesic, anesthetic, and antimicrobial properties.
XEROSTOMIA: Xerostomic patients should be counseled to avoid all agents that may decrease salivation, especially the use of tobacco products and alcohol. To combat xerostomia-related caries, a regimen of daily topical fluoride application should be instituted.
The problem of chronic xerostomia has been approached through the use of salivary substitutes and sialagogues. Because the mucous glands often demonstrate significant recovery after radiation, the sialagogues show promise because they stimulate the residual functional glands. Moisturizing gels, sugarless candies, and chewing gum are used, but the most efficacious product in controlled clinical studies has been systemic use of one of the cholinergic drugs, pilocarpine or cevimeline. Although these drugs may be beneficial for many patients, they are contraindicated in patients with asthma, gastrointestinal ulcerations, labile hypertension, glaucoma, chronic obstructive pulmonary disease, and significant cardiovascular disease. Adverse reactions are uncommon but include excess sweating, rhinitis, headache, nausea, uropoiesis, flatulence, and circulatory disorders.
Other systemic salivary stimulants that are associated with a less dramatic influence on flow include bethanechol and anetholetrithione. Anetholetrithione appears to act by increasing the number of salivary gland receptors. Although somewhat effective when used alone, this medication has been combined with one of the cholinergic medications and achieved improvement in patients who failed to respond to the use of a single agent.
LOSS OF TASTE: Although the taste buds often regenerate within 4 months after radiation therapy, the degree of long-term impairment is highly variable. In those with continu-ing symptoms, zinc sulfate supplements greater than the usual recommended daily doses appear to be beneficial.
OSTEORADIONECROSIS: Although prevention must be stressed, cases of osteoradionecrosis do occur. Use of hyperbaric oxygen has numerous contraindications and possible adverse reactions. Because of the newer theories of pathogenesis for osteoradionecrosis, many clinicians are less inclined to use hyperbaric oxygen except in selected cases. Therapy consists of antibiotics, débridement, irrigation, and removal of diseased bone. The amount of bone removed is determined by clinical judgment, with the surgery extended until brightly bleeding edges are seen.
BISPHOSPHONATE-ASSOCIATED OSTEONECROSIS
The initial association between use of bisphosphonates and subsequent development of gnathic osteonecrosis was documented in 2003. Bisphosphonates comprise a unique class of medications that has been shown to inhibit osteoclasts and possibly interfere with angiogenesis through actions such as inhibition of vascular endothelial growth factor. These medications can be delivered by mouth (PO) or intravenously (IV) and are used primarily to slow osseous involvement of a number of cancers (multiple myeloma and metastatic breast or prostate carcinoma), to treat Paget’s disease (see page 623), and to reverse osteoporosis.
The first-generation bisphosphonates have a relatively low potency and are readily metabolized by osteoclasts. Addition of a nitrogen side chain was added subsequently, creating a more potent second generation of these drugs, designated aminobisphosphonates (Box 8-1). In contrast to the first generation, these formulations are incorporated into the skeleton and demonstrate an extended half-life (e.g., estimated to be 12 years for alendronate). Currently, strong association with gnathic osteonecrosis has been limited to the aminobisphosphonates.
Although bone may seem to be a stable tissue, it constantly is undergoing a process of resorption and reapposition throughout life. The typical lifespan of an osteoblast/osteocyte is approximately 150 days. Osteoclasts also play a critical role in the maintenance of normal bone by repairing microfractures and resorbing areas of bone that contain foci of older nonvital osteocytes. On resorption of bone, cytokines and growth factors such as bone morphogenetic protein are released and induce formation of active bone-forming osteoblasts. If osteoclastic function declines, then microfractures accumulate and the lifespan of the entrapped osteocytes is exceeded, creating areas of vulnerable bone. When osteoclasts ingest bisphos-phonate-treated bone, the medication is cytotoxic and induces osteoclastic apoptosis, a reduction in recruitment of additional osteoclasts, and a stimulation of osteoblasts to release an osteoclast-inhibiting factor. The incorporation of the medication is highest in areas of active remodeling, such as the jaws. Although the vast majority of osteonecrosis cases have occurred in the jaws, extragnathic involvement has been documented (e.g., osteonecrosis of ear after removal of exostosis of the external ear canal).
CLINICAL AND RADIOGRAPHIC FEATURES: In an excellent systematic review of 368 reported cases of bisphosphonate-associated osteonecrosis (BON), 94% were discovered in patients who were treated with the IV formulations (primarily pamidronate and zoledronic acid) for cancer, with 85% being reported in patients with multiple myeloma. Current estimates indicate that the prevalence of osteonecrosis in patients taking aminobisphosphonates for cancer is 6% to 10%. Prospective trials will be necessary to confirm these figures.
Osteonecrosis related to use of oral aminobisphosphonates is most uncommon (conservative estimate by drug industry: annual incidence is 0.7 per 100,000); however, prospective trials for the true incidence of this complication have yet to be performed. Risk factors for BON associated with the PO formulations include advanced patient age (older than 65 years), corticosteroid use, use of chemotherapy drugs, diabetes, smoking or alcohol use, poor oral hygiene, and duration of drug use exceeding 3 years. A predictive test for those at risk for bisphosphonate osteonecrosis has not been confirmed. Some investigators recently have suggested use of a serum marker for bone turnover, serum C-telopeptide (CTX), but additional prospective studies are needed to confirm the utility of this test.
Although a mandibular predominance has been noted, involvement of the maxilla or both jaws is not uncommon (Fig. 8-27). In 60% of these patients, the necrosis has followed an invasive dental procedure, with the remainder occurring spontaneously (Fig. 8-28). On occasion, the necrosis can occur after minor trauma to bony prominences, such as tori or other exostoses (Fig. 8-29). Affected patients have areas of exposed, necrotic bone that is asymptomatic in approximately one third.
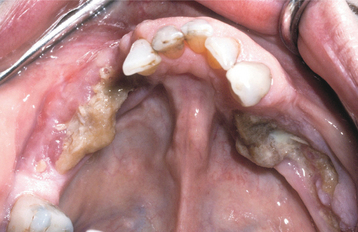
Fig. 8-27 Bisphosphonate-associated osteonecrosis. Bilateral necrotic exposed bone of the mandible in a patient receiving zoledronic acid for metastatic breast cancer. (Courtesy of Dr. Brent Mortenson.)
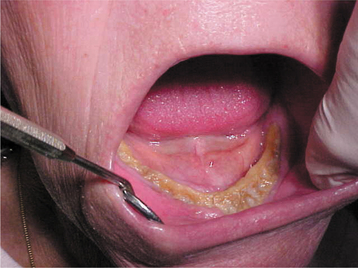
Fig. 8-28 Bisphosphonate-associated osteonecrosis. Extensive necrosis of the mandible, which followed extraction of multiple teeth. The patient was receiving zoledronic acid for metastatic breast cancer. (Courtesy of Dr. Benny Bell.)
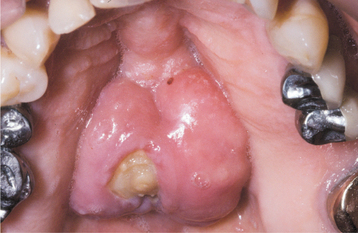
Fig. 8-29 Bisphosphonate-associated osteonecrosis. Lobulated palatal torus with an area of exposed necrotic bone in a patient taking alendronate for osteoporosis.
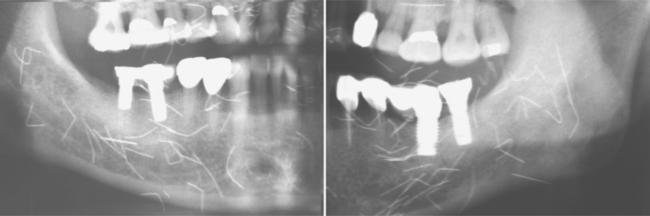
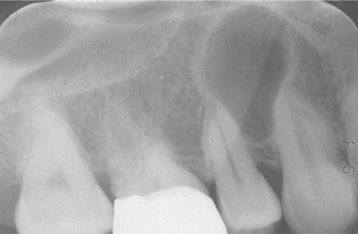
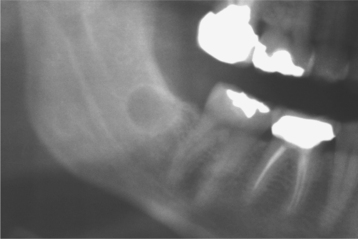
Investigators have suggested that bone at imminent risk for osteonecrosis often will demonstrate increased radiopacity before clinical evidence of frank necrosis. These changes typically occur predominantly in areas of high bone remodeling, such as the alveolar ridges. Panoramic radiographs often will reveal a marked radiodensity of the crestal portions of each jaw, with a more normal appearance of the bone away from tooth-bearing portions. Periosteal hyperplasia also is not rare. In more severe cases, the osteonecrosis creates a moth-eaten and ill-defined radiolucency with or without central radiopaque sequestra (Fig. 8-30). In some cases the necrosis can lead to development of a cutaneous sinus or pathologic fracture (Fig. 8-31).
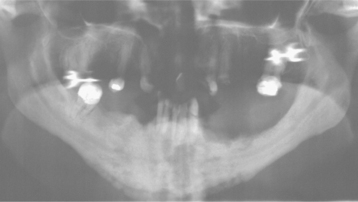
Fig. 8-30 Bisphosphonate-associated osteonecrosis. Panoramic radiograph of patient depicted in Fig. 8-27. Note sclerosis of tooth-bearing areas along with multiple radiolucencies and periosteal hyperplasia of the lower border of the mandible. (Courtesy of Dr. Brent Mortenson.)
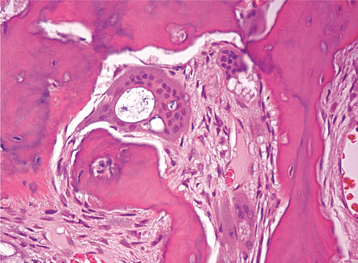
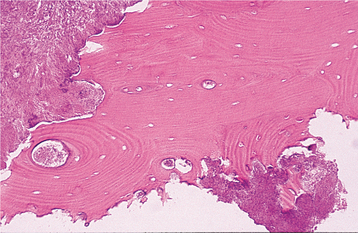
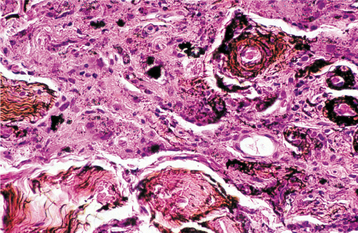
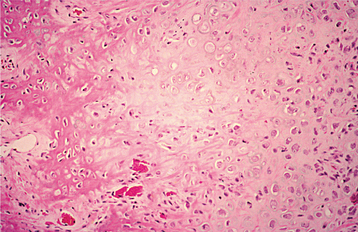
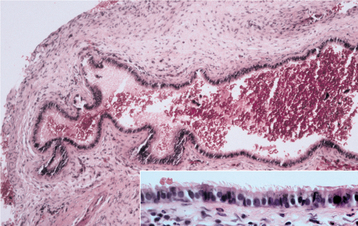
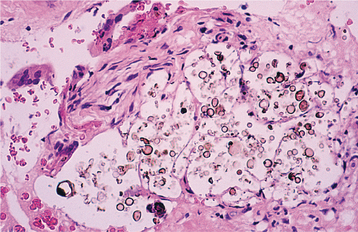
HISTOPATHOLOGIC FEATURES: Biopsy of vital bone altered by aminobisphosphonates is not common. In such cases the specimen often reveals irregular trabeculae of pagetoid bone, with adjacent enlarged and irregular osteoclasts that often demonstrate numerous intracytoplasmic vacuoles (Fig. 8-32). Specimens of active areas of BON reveal trabeculae of sclerotic lamellar bone, which demonstrate loss of the osteocytes from their lacunae and frequent peripheral resorption with bacterial colonization (Fig. 8-33). Although the peripheral bacterial colonies often resemble actinomycetes, the infestation is not consistent with cervicofacial actinomycosis.
TREATMENT AND PROGNOSIS: The clinical approach to patients treated with aminobisphosphonates varies according to the formulation of the drug, the disease being treated, and the duration of drug use. All patients who take these medications should be warned of the risks and instructed to obtain and maintain ultimate oral hygiene. The oral medications are extremely caustic; patients should be warned to minimize oral mucosal contact and ensure the medication is swallowed completely.
Routine dental therapy, in most cases, should not be modified solely on the basis of oral aminobisphosphonate use. All restorative, prosthodontic, conventional endodontic, and routine periodontic procedures can be implemented as needed. Although orthodontic treatment is not contraindicated, progress should be evaluated after 2 to 3 months of active therapy. At that point, therapy can proceed if the tooth movement is occurring predictably with normal forces. Invasive orthodontic techniques such as orthognathic surgery, four-tooth extraction cases, and miniscrew anchorage should be avoided, if possible. Because the medica-tion is drawn to sites of active bone remodeling, a drug holiday (or switching to a nonaminobisphosph-onate agent) during active orthodontics may be advantageous.
When manipulation of bone is considered (e.g., surgical endodontics, periodontal bone recontouring, oral surgery, implant placement), the patient should be advised of the potential complications of aminobisphosphonate use and the risk of BON. Written informed consent and documentation of a discussion of the benefits, risks, and alternative therapies are highly advised. For elective surgical procedures in patients with a duration of drug use exceeding 3 years, discontinuation of the medication 3 months before and 3 months after surgery has been suggested. Because of the reduced angiogenesis, osseous grafts should be used judiciously. If multiple sites require surgery, then one sextant should be treated with a 2-month disease-free follow-up before completion of the remaining sextants. During this interval, use of chlorhexidine twice daily is recommended. The sextant-by-sextant approach is not necessary for periapical inflammatory disease or abscesses, both of which can lead directly to osteonecrosis and demand immediate resolution.
Dental therapy in patients taking an IV formulation for cancer is much more problematic. Prevention is paramount. In patients evaluated before initiation of an IV aminobisphosphonate, the goal is to eliminate all dental infections and improve dental health to prevent future invasive therapy; this includes removal of large tori or partially impacted teeth. If only noninvasive therapy is necessary, then the initiation of the medication need not be delayed. If surgical procedures are performed, then a month-long delay in initiation of the medication is recommended, along with prophylactic antibiotic therapy (i.e., penicillin; quinolone and metronidazole, or erythromycin and metronidazole for those allergic to penicillin).
For patients presenting in the midst of active IV therapy, a number of clinicians have suggested that manipulation of bone should be avoided. Conventional endodontics is a better option than extraction. If a nonvital tooth is not restorable, then endodontics should be performed and followed by crown amputation. Teeth with 1+ or 2+ mobility should be splinted; those with 3+ mobility can be extracted.
For patients with BON, the goal of therapy is to stop pain. A number of clinicians believe that removal of bone typically results in further bone necrosis, and hyperbaric oxygen has not been beneficial. Asymptomatic patients should rinse daily with chlorhexidine and be monitored closely. Any rough edges of exposed bone should be smoothed. If the exposed bone irritates adjacent tissues, then coverage with a soft splint may prove beneficial. In symptomatic patients, systemic antibiotic therapy (i.e., penicillin with or without metronidazole, ciprofloxacin or erythromycin and metronidazole for those allergic to penicillin) and chlorhexidine usually lead to pain relief. If the antibiotics fail to stop the pain, then hospitalization with IV therapy is indicated. In recalcitrant cases the bulk of the dead bone is reduced surgically, followed by administration of systemic antibiotics. Because of the long half-life of bisphosphonates, discontinuation of the drug offers no short-term benefit. In isolated anecdotal reports of recalcitrant cases, discontinuation of the medication for 6 to 12 months occasionally has been associated with spontaneous sequestration and resolution.
As mentioned in the discussion of the radiographic features, the alveolar ridges of patients affected with osteonecrosis exhibit significant sclerosis because of increased drug deposition in areas of high remodeling. Some investigators have successfully treated patients with BON by resection of all of the osteosclerotic areas with extension to the underlying more lucent and normally bleeding bone. As adjunctive therapy to enhance healing, the osseous defect can be covered with a resorbable collagen membrane impregnated with platelet-rich plasma.
Overall, the benefits of aminobisphosphonate therapy for osteoporosis and metastatic cancer appear to greatly outweigh the risk of developing BON. However, the oral medications should be prescribed only for those individuals with inadequate bone density and should be discontinued or switched to a nonaminobisphosphonate once the bone density returns to an acceptable level.
Increased bone density does not correlate necessarily to good bone quality. The negative effects of oversuppression of bone metabolism must be considered in all patients prescribed oral aminobisphosphonates. Several reports have documented spontaneous nontraumatic stress fractures with associated delayed healing in patients on long-term aminobisphosphonate therapy. Many physicians now believe that aminobisphosphonate therapy should be stopped after 5 years and not reinitiated until bone density studies confirm redevelopment of significant osteoporosis.
Dentists should be proactive and strongly encour-age patients to speak to their attending physicians for consideration of these factors during planning and execution of long-term care. For individuals scheduled to receive IV aminobisphosphonate therapy as part of their cancer management, the involved oncologist should recommend pretreatment dental evaluation and preventive care, with long-term, close follow-up.
OROFACIAL COMPLICATIONS OF METHAMPHETAMINE ABUSE
Methamphetamine (“meth”) is a drug with stimulant effects on the central nervous system (CNS). In 1937 the drug was approved in the United States for the treatment of narcolepsy and attention deficit hyperactivity disorder. Within a few years, many began to use the drug to increase alertness, control weight, and combat depression. Because methamphetamine users perceive increased physical ability, greater energy, and euphoria, illegal use and manufacture of the drug began to develop. Because of greater control over the main ingredient, pseudoephedrine, production of homemade methamphetamine is decreasing but often being replaced by illegal importation of the finished product. The drug is a powdered crystal that dissolves easily in liquid and can be smoked, snorted, injected, or taken orally. The drug is known by nicknames that include chalk, crank, crystal, fire, glass, ice, meth, and speed.
CLINICAL FEATURES: Although methamphetamine abuse may occur throughout society, most users are men between the ages of 19 and 40 years. The effects of the medication last up to 12 hours, and the typical abuser reports use that exceeds 20 days per month, creating an almost continuous effect of the drug. The short-term effects of methamphetamine include insomnia, aggressiveness, agitation, hyperactivity, decreased appetite, tachycardia, tachypnea, hypertension, hyperthermia, vomiting, tremors, and xerostomia. Long-term effects additionally include strong psychologic addiction, violent behavior, anxiety, confusion, depression, paranoia, auditory hallucinations, delusions, mood changes, skin lesions, and a number of cardiovascular, CNS, hepatic, gastrointestinal, renal, and pulmonary disorders.
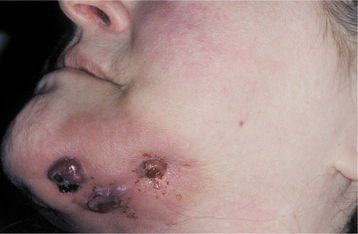
Many addicts develop delusions of parasitosis (formication, from the Latin word formica, which translates to ant), a neurosis that produces the sensation of snakes or insects crawling on or under the skin. This sensation causes the patient to attempt to remove the perceived parasites, usually by picking at the skin with fingernails, resulting in widespread traumatic injury. The factitial damage can alter dramatically the facial appearance in a short period of time, and these lesions have been nicknamed speed bumps, meth sores, or crank bugs.
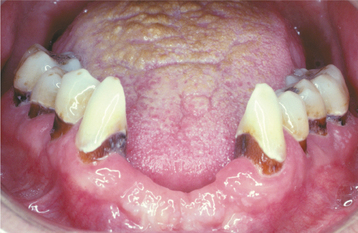
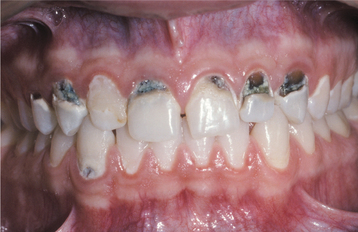
Rampant dental caries is another common manifestation and exhibits numerous similarities with milk-bottle caries. The carious destruction initially affects the facial smooth and interproximal surfaces, but without intervention, the coronal structure of the entire dentition can be destroyed (Fig. 8-34). The carious destruction appears to be caused by poor oral hygiene combined with extreme drug-related xerostomia, which leads to heavy consumption of acidic and sugar-filled soft drinks or other refined carbohydrates.
TREATMENT AND PROGNOSIS: The oral health practitioner should be alerted when an emaciated, agitated, and nervous young adult presents with tachycardia, tachypnea, hypertension, hyperthermia, and rampant smooth surface and interproximal caries. Failure to recognize these signs can be serious. For up to 6 hours after ingestion, methamphetamine potentiates the effects of sympathomimetic amines. Use of local anesthetics with epinephrine or levonordefrin can lead to a hypertensive crisis, cerebral vascular accident, or myocardial infarction. Caution also should be exercised when administering sedatives, general anesthesia, nitrous oxide, or prescriptions for narcotics. Although cessation of the illicit drug use is paramount, patients should be encouraged during periods of xerostomia to discontinue use of highly acidic and sugar-filled soft drinks and to avoid diuretics such as caffeine, tobacco, and alcohol. In addition, the importance of personal and oral hygiene should be stressed. Preventive measures such as topical fluorides may assist in protecting the remaining dentition. A medical consultation with referral to a substance abuse center should be encouraged.
ANESTHETIC NECROSIS
Administration of a local anesthetic agent can, on rare occasions, be followed by ulceration and necrosis at the site of injection. Researchers believe that this necrosis results from localized ischemia, although the exact cause is unknown and may vary from case to case. Faulty technique, such as subperiosteal injection or administration of excess solution in tissue firmly bound to bone, has been blamed. The epinephrine contained in many local anesthetics also has received attention as a possible cause of ischemia and secondary necrosis.
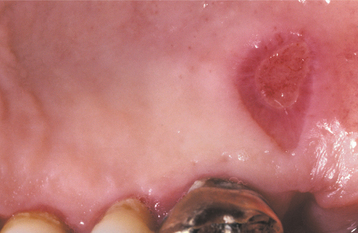
CLINICAL FEATURES: Anesthetic necrosis usually develops several days after the procedure and most commonly appears on the hard palate (Fig. 8-35). A well-circumscribed area of ulceration develops at the site of injection. The ulceration often is deep, and, on occasion, healing may be delayed. One report has documented sequestration of bone at the site of tissue necrosis.
TREATMENT AND PROGNOSIS: Treatment of anesthetic necrosis usually is not required unless the ulceration fails to heal. Minor trauma, such as that caused by performing a cytologic smear, has been reported to induce resolution in these chronic cases. Recurrence is unusual but has been reported in some patients in association with use of epinephrine-containing anesthetics. In these cases the use of a local anesthetic without epinephrine is recommended.
EXFOLIATIVE CHEILITIS
Exfoliative cheilitis is a persistent scaling and flaking of the vermilion border, usually involving both lips. The process arises from excessive production and subsequent desquamation of superficial keratin. A significant percentage of cases appears related to chronic injury secondary to habits such as lip licking, biting, picking, or sucking. Those cases proven to arise from chronic injury are termed factitious cheilitis.
Many patients deny chronic self-irritation of the area. The patient may be experiencing associated personality disturbances, psychologic difficulties, or stress. In a review of 48 patients with exfoliative cheilitis, 87% exhibited psychiatric conditions and 47% also demonstrated abnormal thyroid function. Evidence suggests that there may be a link between thyroid dysfunction and some psychiatric disturbances.
In other cases, no evidence of chronic injury is evident. In these patients other causes should be ruled out (e.g., atopy, chronic candidal infection, actinic cheilitis, cheilitis glandularis, hypervitaminosis A, photosensitivity). In a review of 165 patients with acquired immunodeficiency syndrome (AIDS), more than one quarter had alterations that resembled exfoliative cheilitis. In this group the lip alterations appeared secondary to chronic candidal infestation. The most common presentation of bacterial or fungal infections of the lips is angular cheilitis (see page 216). Diffuse primary infection of the entire lip is very unusual; most diffuse cases represent a secondary candidal infection in areas of low-grade trauma of the vermilion border of the lip (cheilocandidiasis).
In one review of 75 patients with chronic cheilitis, a thorough evaluation revealed that more than one third represented irritant contact dermatitis (often secondary to chronic lip licking). In 25% of the patients, the cheilitis was discovered to be an allergic contact mucositis (see page 350). Atopic eczema was thought to be the cause in 19% of cases; the remaining portion was related to a wide variety of pathoses.
In spite of a thorough investigation, there often remain a number of patients with classic exfoliative cheilitis for which no underlying cause can be found. These idiopathic cases are most troublesome and often resistant to a wide variety of interventions.
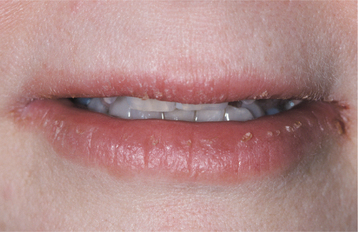
CLINICAL FEATURES: A marked female predominance is seen in cases of factitious origin, with most cases affecting those younger than 30 years of age. Mild cases feature chronic dryness, scaling, or cracking of the vermilion border of the lip (Fig. 8-36). With progression, the vermilion can become covered with a thickened, yellowish hyperkeratotic crust that can be hemorrhagic or that may exhibit extensive fissuring. The perioral skin may become involved and exhibit areas of crusted erythema (Fig. 8-37). Although this pattern may be confused with perioral dermatitis (see page 352), the most appropri ate name for this process is circumoral dermatitis. Both lips or just the lower lip may be involved.
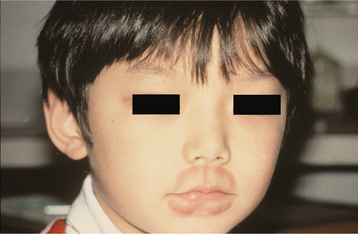
Fig. 8-37 Circumoral dermatitis. Crusting and erythema of the skin surface adjacent to the vermilion border in a child who chronically sucked on both lips.
In patients with chronic cheilitis, development of fissures on the vermilion border is not rare. In a prevalence study of more than 20,000 patients, these fissures involved either lip and were slightly more common in the upper lip. In contrast to typical exfoliative cheilitis, these fissures demonstrate a significant male predilection and a prevalence rate of approximately 0.6%. The majority arise in young adults, with rare occurrence noted in children and older adults.
Although the cause is unknown, proposed contributing factors include overexposure to sun, wind, and cold weather; mouth breathing; bacterial or fungal infections; and smoking. An increased prevalence of lip fissures has been noted in patients with Down syndrome and may be the result of the high frequency of mouth breathing or the tendency to develop orofacial candidiasis. Application of lipstick or lip balm appears to be protective. Fissure occurrence also may be related to a physiologic weakness of the tissues. Those affecting the lower lip typically occur in the midline, whereas fissures on the upper vermilion most frequently involve a lateral position. These are the sites of prenatal merging of the mandibular and maxillary processes.
TREATMENT AND PROGNOSIS: In those cases associated with an obvious cause, elimination of the trigger typically results in resolution of the changes. In those cases with no underlying physical, infectious, or allergic cause, psychotherapy (often combined with mild tranquilization or stress reduction) may achieve resolution. In cases for which no cause can be found, therapeutic interventions often are not successful.
Cases that result from candidal infections often do not resolve until the chronic trauma also is eliminated. Initial topical antifungal agents, antibiotics, or both can be administered to patients in whom chronic trauma is not obvious or is denied. If the condition does not resolve, then further investigation is warranted in an attempt to discover the true source of the lip alterations.
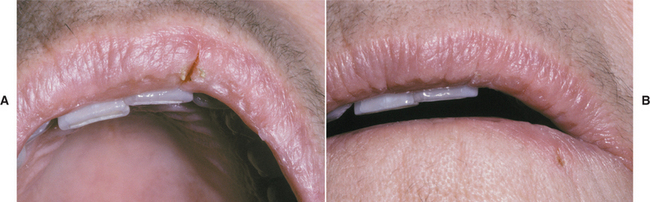
Hydrocortisone and iodoquinol (antibacterial and antimycotic) cream has been used to resolve chronic lip fissures in some patients (Fig. 8-38). Other reported therapies include various corticosteroid preparations, topical tacrolimus, sunscreens, and moisturizing preparations. In many cases, resistance to topical therapy or frequent recurrence is noted. In these cases, cryotherapy or excision with or without Z-plasty has been used successfully.
SUBMUCOSAL HEMORRHAGE
Everyone has experienced a bruise from minor trauma. This occurs when a traumatic event results in hemorrhage and entrapment of blood within tissues. Different terms are used, depending on the size of the hemorrhage:
• Minute hemorrhages into skin, mucosa, or serosa are termed petechiae.
• If a slightly larger area is affected, the hemorrhage is termed a purpura.
• Any accumulation greater than 2 cm is termed an ecchymosis.
• If the accumulation of blood within tissue produces a mass, this is termed a hematoma.
Blunt trauma to the oral mucosa often results in hematoma formation. Less well known are petechiae and purpura, which can arise from repeated or prolonged increased intrathoracic pressure (Valsalva maneuver) associated with such activities as repeated coughing, vomiting, convulsions, or giving birth (Fig. 8-39). When considering a diagnosis of traumatic hemorrhage, the clinician should keep in mind that hemorrhages can result from nontraumatic causes, such as anticoagulant therapy, thrombocytopenia, disseminated intravascular coagulation (DIC), and a number of viral infections, especially infectious mononucleosis and measles.
With the increased frequency of dental implant placement, a number of reports have surfaced related to potentially life-threatening floor of mouth hemorrhage secondary to a tear in the lingual periosteum or perforation of the cortical plate during implant site preparation. Similar spontaneous sublingual hemorrhage has been documented in patients with severe hypertension or a systemic coagulopathy. Another unusual hemorrhagic event involves the development of an organized hematoma within the maxillary sinus. The blood originates from a variety sources, the most common of which is intranasal hemorrhage that drains through the ostia and collects in the antrum.
CLINICAL FEATURES: Submucosal hemorrhage appears as a nonblanching flat or elevated zone with a color that varies from red or purple to blue or blue-black (Fig. 8-40). As would be expected, traumatic lesions are located most frequently on the labial or buccal mucosa. Blunt facial trauma often is responsible, but injuries such as minor as cheek biting may produce a hematoma or areas of purpura (Fig. 8-41). Mild pain may be present.
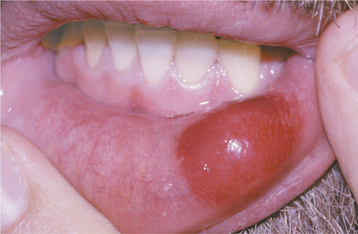
Fig. 8-40 Purpura. Submucosal hemorrhage of the lower labial mucosa on the left side secondary to blunt trauma.
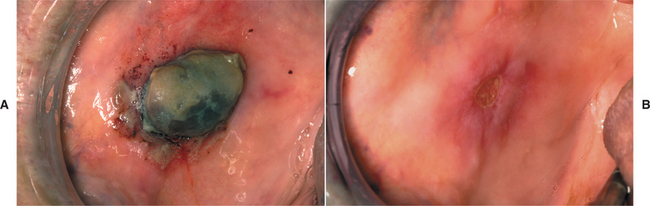
Fig. 8-41 Hematoma. A, Dark-purple nodular mass of the buccal mucosa in a patient on coumadin therapy. B, Near resolution of the lesion 8 days later after discontinuation of the medication. (Courtesy of Dr. Charles Ferguson.)
The hemorrhage associated with increased intrathoracic pressure usually is located on the skin of the face and neck and appears as widespread petechiae that clear within 24 to 72 hours. Although it has not been as well documented as the cutaneous lesions, mucosal hemorrhage can be seen in the same setting and most often appears as soft palatal petechiae or purpura.
Hematoma formation associated with surgical implant preparation usually is associated with damage of the soft tissues adjacent to the lingual surface of the mandible and produces swelling and elevation of the floor of the mouth. Although the hemorrhage is noticeable immediately in most patients, the problem may not become evident clinically for 4 to 6 hours. Distension, elevation, and protrusion of the tongue may occur and be associated with inability to swallow or significant dyspnea.
Patients with antral hematomas typically complain of frequent nasal bleeding but also may experience unilateral nasal obstruction, hyposmia, headache, and malar swelling. Facial paresthesia and vision problems occur less frequently. Computed tomography (CT) typically demonstrates a soft tissue mass filling and expanding the antrum, often associated with proptosis and partial destruction or displacement of the sinus walls. In many instances, the radiographic appearance is worrisome for a malignant neoplasm.
TREATMENT AND PROGNOSIS: Often, no treatment is required if the hemorrhage is not associated with significant morbidity or related to systemic disease. The areas should resolve spontaneously. Large hematomas may require several weeks to resolve. If the hemorrhage occurs secondary to an underlying disorder, then treatment is directed toward control of the associated disease.
In cases of emergent hemorrhage associated with surgical implant placement, the first priority is to secure the airway. Although the hemorrhage can be controlled with conservative measures in some cases, the majority require surgical exploration with isolation and repair of the damaged vessel. Antral hematomas usually are removed via a Caldwell-Luc procedure for both therapeutic and diagnostic purposes. After successful removal, a search for the bleeding source or any underlying coagulopathy is recommended to prevent recurrence.
ORAL TRAUMA FROM SEXUAL PRACTICES
Although orogenital sexual practices are illegal in many jurisdictions, they are extremely common. Among homosexual men and women, orogenital sexual activity almost is universal. For married heterosexual couples younger than age 25, the frequency has been reported to be as high as 90%. Considering the prevalence of these practices, the frequency of associated traumatic oral lesions is surprisingly low.
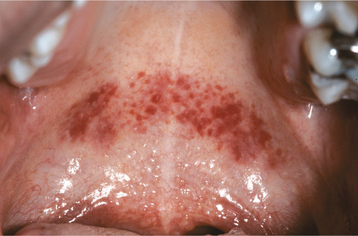
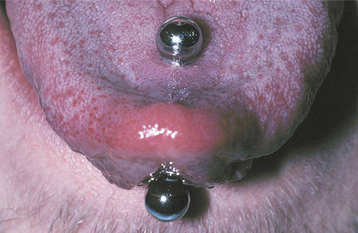
CLINICAL FEATURES: The most commonly reported lesion related to orogenital sex is submucosal palatal hemorrhage secondary to fellatio. The lesions appear as erythema, petechiae, purpura, or ecchymosis of the soft palate. The areas often are asymptomatic and resolve without treatment in 7 to 10 days (Fig. 8-42). Recurrences are possible with repetition of the inciting (exciting?) event. The erythrocytic extravasation is thought to result from the musculature of the soft palate elevating and tensing against an environment of negative pressure. Similar lesions have been induced from coughing, vomiting, or forceful sucking on drinking straws and glasses. Forceful thrusting against the vascular soft palate has been suggested as another possible cause.
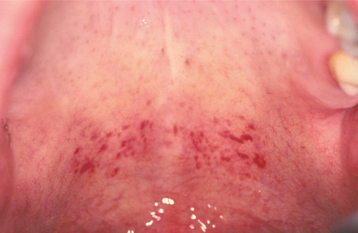
Fig. 8-42 Palatal petechiae from fellatio. Submucosal hemorrhage of the soft palate resulting from the effects of negative pressure.
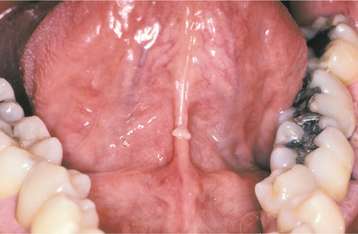
Oral lesions also can occur from performing cunnilingus, resulting in horizontal ulcerations of the lingual frenum. As the tongue is thrust forward, the taut frenum rubs or rakes across the incisal edges of the mandibular central incisors. The ulceration created coincides with sharp tooth edges when the tongue is in its most forward position. The lesions resolve in 7 to 10 days but may recur with repeated performances. Linear fibrous hyperplasia has been discovered in the same pattern in individuals who chronically perform the act (Fig. 8-43).
HISTOPATHOLOGIC FEATURES: With an appropriate index of suspicion, biopsy usually is not required; however, a biopsy has been performed in some cases of palatal lesions secondary to fellatio. These suction-related lesions reveal subepithelial accumulations of red blood cells that may be extensive enough to separate the surface epithelium from underlying connective tissue. Patchy degeneration of the epithelial basal cell layer can occur. The epithelium classically demonstrates migration of erythrocytes and leukocytes from the underlying lamina propria.
TREATMENT AND PROGNOSIS: No treatment is required, and the prognosis is good. In patients who request assistance, palatal petechiae can be prevented through the use of less negative pressure and avoidance of forceful thrusting. Smoothing and polishing the rough incisal edges of the adjacent mandibular teeth can minimize the chance of lingual frenum ulceration.
AMALGAM TATTOO AND OTHER LOCALIZED EXOGENOUS PIGMENTATIONS
A number of pigmented materials can be implanted within the oral mucosa, resulting in clinically evident pigmentations. Implantation of dental amalgam (amalgam tattoo) occurs most often, with a frequency that far outdistances that for all other materials. Localized argyrosis has been used as another name for amalgam tattoo, but this nomenclature is inappropriate because amalgam contains not only silver but also mercury, tin, copper, zinc, and other metals.
Amalgam can be incorporated into the oral mucosa in several ways. Previous areas of mucosal abrasion can be contaminated by amalgam dust within the oral fluids. Broken amalgam pieces can fall into extraction sites. If dental floss becomes contaminated with amalgam particles of a recently placed restoration, then linear areas of pigmentation can be created in the gingival tissues as a result of hygiene procedures (Fig. 8-44). Amalgam from endodontic retrofill procedures can be left within the soft tissue at the surgical site (Fig. 8-45). Finally, fine metallic particles can be driven through the oral mucosa from the pressure of high-speed air turbine drills.
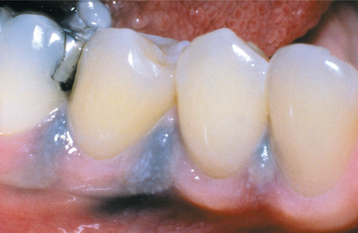
Fig. 8-44 Floss-related amalgam implantation. Linear strips of mucosal pigmentation that align with the interdental papillae. The patient used dental floss on the mandibular first molar immediately after the placement of the amalgam restoration. Because the area was still anesthetized, the patient impaled the floss on the gingiva, then continued forward using the amalgam-impregnated floss in the bicuspid area to create additional amalgam tattoos.
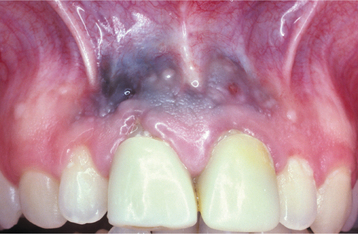
Fig. 8-45 Endodontic-related amalgam implantation. Multifocal areas of mucosal discoloration overlying the maxillary anterior incisors, which have been treated with apical retrofill procedures.
Theoretically, the use of the rubber dam should decrease the risk; however, immediately after removal of the dam, the occlusion often is adjusted with the potential for amalgam contamination of any areas of mucosal damage. Submucosal implantation of pencil graphite, coal and metal dust, fragments of broken carborundum disks, dental burs, and, in the past, charcoal dentifrices, have resulted in similar-appearing areas of discoloration.
Intentional tattooing, which can be found in approximately 25% of the world’s population, also may be performed in the oral cavity. Although some cases are culturally related, health professionals also are responsible for a number of intentional oral and facial tattoos for the purpose of demonstrating landmarks, judging tumor response to antineoplastic therapies, repigmenting areas of vitiligo, cosmetically disguising disfigured areas, and applying permanent cosmetic makeup. Injudicious intraoral use of these marking agents can cause diffusion of the pigment with discoloration of the adjacent skin surface.
CLINICAL AND RADIOGRAPHIC FEATURES: Amalgam tattoos appear as macules or, rarely, as slightly raised lesions. They may be black, blue, or gray. The borders can be well defined, irregular, or diffuse (Fig. 8-46). Lateral spread may occur for several months after the implantation. In most cases, only one site is affected, although multiple tattoos in a single patient may be present. Any mucosal surface can be involved, but the most common sites are the gingiva, alveolar mucosa, and buccal mucosa (Fig. 8-47).
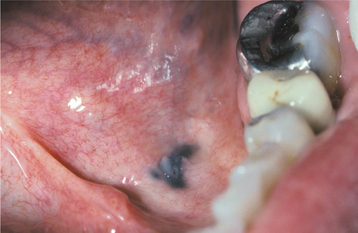
Fig. 8-46 Amalgam tattoo. Area of mucosal discoloration of the floor of the mouth on the patient’s left side.
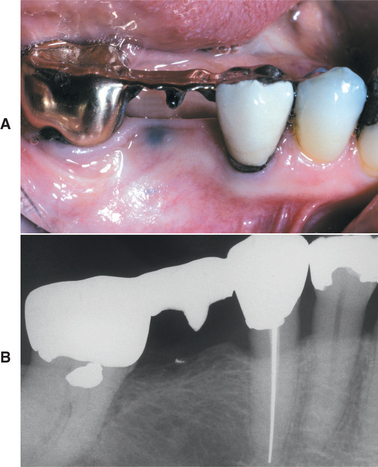
Fig. 8-47 Amalgam tattoo. A, Area of mucosal discoloration of the mandibular alveolar ridge immediately below the bridge pontic. B, Radiograph of the same patient showing radiopaque metallic fragment present at the site of mucosal discoloration.
Periapical radiographs, when taken, are negative in many cases. When metallic fragments are visible radiographically, the clinical area of discoloration typically extends beyond the size of the fragment. The fragments are densely radiopaque, varying from several millimeters to pinpoint in size (see Fig. 8-47). On occasion, the pattern of the amalgam dispersal has been sufficiently unique to be used as a distinctive characteristic in the identification of unknown deceased individuals.
The pattern of accidental localized foreign body tattoo other than amalgam is diverse and depends on the associated trauma that impacted the material. Mucosal graphite implantation is rarely documented, but it most likely occurs with a higher frequency than indicated by the number of cases reported. Examples in the literature have been presented as grayish areas of mucosal discoloration of the hard palate, the most likely site for pencil-related trauma.
Cosmetic tattooing is gaining in popularity and may include injection of permanent cosmetic inks into the eyelids and vermilion border of the upper and lower lips. On occasion, patients may react to the material and experience swelling, burning, and itching at the site, followed by enlargement and induration. In such cases, biopsy often reveals a granulomatous reaction to the foreign material.
The intentional intraoral tattoos that are not placed by health professionals occur most frequently on the anterior maxillary facial gingiva of individuals from a number of African countries and have been documented at institutions in the United States (Fig. 8-48). In these cases the anterior maxillary facial gingiva is given a heavy blue-black discoloration. Occasionally, tattoos (usually blue or black) are placed on the labial mucosa of adults in the United States to convey a personal, often vulgar, message (Fig. 8-49).
HISTOPATHOLOGIC FEATURES: Microscopic examination of amalgam tattoos reveals pigmented fragments of the metal within the connective tissue. Scattered, large, dark, solid fragments or numerous fine, black, or dark-brown granules may be seen (Fig. 8-50). The silver salts of the dental amalgam preferentially stain the reticulin fibers, especially those encircling nerves and vascular channels (Fig. 8-51).
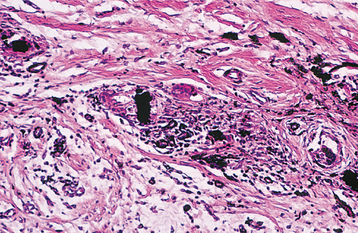
Fig. 8-50 Amalgam tattoo. Numerous dark, solid fragments of amalgam are surrounded by a lymphohistiocytic inflammatory infiltrate.
The biologic response to amalgam appears related to particle size and the elemental composition of the amalgam. Large fragments often become surrounded by dense fibrous connective tissue with mild inflammation. Smaller particles typically are associated with a more significant inflammatory response that may be granulomatous or a mixture of lymphocytes and plasma cells. Graphite implantation appears similar microscopically to amalgam but can be differentiated by its pattern of birefringence after treatment with ammonium sulfide and by the lack of staining of the reticulin fibers. In addition, energy dispersive x-ray microanalysis can be used to identify the type of material present within areas of foreign body tattoos.
TREATMENT AND PROGNOSIS: To confirm the diagnosis of amalgam tattoo, the clinician can obtain radiographs of the areas of mucosal discoloration in an attempt to demonstrate the metallic fragments. The films should be capable of high detail, because many of the fragments are no larger than the point of a pin.
No treatment is required if the fragments can be detected radiographically. If no metallic fragments are found and the lesion cannot be diagnosed clinically, then biopsy may be needed to rule out the possibility of melanocytic neoplasia. On occasion, the amalgam implantation may create pigmentation in a cosmetically objectionable location such as the anterior maxillary facial gingiva. In such cases, conservative surgical excision can be performed; alternatively amalgam tattoos have been removed successfully with Q-switched ruby or alexandrite lasers. With respect to cosmetic tattoos, a variety of treatments such as corticosteroids and lasers have been used with variable results.
ORAL PIERCINGS AND OTHER BODY MODIFICATIONS
Historical evidence from almost every continent shows that body piercing is an ancient practice with a strong association with religious, cultural, or superstitious beliefs. In the Western world, body piercing beyond the earlobes has become increasingly popular as a method of self-expression during the past decade. In a 2001 survey of 481 college students in the United States, 51% admitted body piercing, and the prevalence still appears to be rising. Usually, the piercing is performed to place jewelry in sites such as the eyebrows, helix of the ears, nose, navel, nipples, genitals, and a number of intraoral sites.
Forked tongue (split tongue, bifid tongue) is a rather recent addition to the art of body modification, with few associated publications. In this practice the anterior one third of the tongue is split down the middle. This has been performed slowly by pulling fishing line through a pierced hole and tightening the loop over a period of 3 weeks or using a surgical instrument or laser to quickly separate the two halves. Some form of cautery is necessary to prevent the halves from reuniting. Forked tongue also has been reported as a complication of tongue piercing.
Another practice with unique orofacial manifestations is implantation of a form of talisman (magical charm) called susuk (charm needles, charm pins). This practice is common in southeast Asia, especially Malaysia, Thailand, Singapore, Indonesia, and Brunei. The susuk is placed by a native magician or medicine man termed bomoh and is thought to enhance or preserve beauty, relieve pain, bring success in business, or provide protection against harm. The majority of the individuals with susuk are Muslims, although Islam strictly prohibits black magic. Therefore, many affected individuals will deny placement of susuk, even when confronted directly with hard evidence.
CLINICAL AND RADIOGRAPHIC FEATURES: Intraoral piercings are noted most frequently in adolescents and young adults, with a female predominance. The most commonly affected sites are the tongue, lips, buccal mucosa, and, rarely, the uvula. The selected site typically is pierced with a 14- to 16-gauge sheeted needle, then the jewelry of choice is threaded through the wound. The jewelry most often is gold, silver, or stainless steel; in the tongue, the most frequent adornment is a barbell (dumbbell might be a better name!) consisting of a metal rod with a ball that screws onto each end (Fig. 8-52). The lip jewelry is termed a labret and most often consists of a ring or a rod with a flat end attached to the mucosal side and a round ball for the cutaneous sur-face (Fig. 8-53).
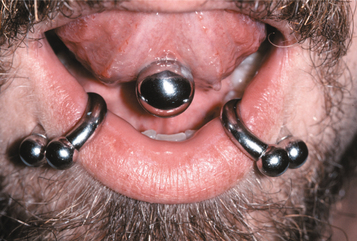
Fig. 8-53 Labial piercing. Lower lip pierced bilaterally with labrets consisting of a circular rod with terminating balls. The patient also has a lingual barbell.
If no complications occur, healing of the piercing site takes place within 4 to 6 weeks. Potential acute complications include pain, prolonged or profuse bleeding, swelling (to the point of airway obstruction in rare cases), infection including Ludwig’s angina and cerebellar brain abscess, lingual nerve damage, speech impediment, and allergy to the jewelry. Chronic complications include mucosal or gingival trauma, chipped or fractured teeth, hypersalivation, aspiration or swallowing of jewelry, tissue hyperplasia around the posts, and embedded jewelry. Gingival recession (labrets, barbells) and tooth fracture (barbells) are extremely common, with the prevalence closely related to the duration of use. Molars and premolars are fractured most frequently, with the damage associated more often with a short-stem barbell, whereas greater severity of gingival recession is associated with long-stem barbells and labrets.
In individuals with forked tongues, the anterior half of the tongue is split down the middle (Fig. 8-54). Risks of the procedure include inflammation, infection, profuse or prolonged hemorrhage, and permanent neurovascular damage. After healing, some individuals have developed the ability to control each half independently.
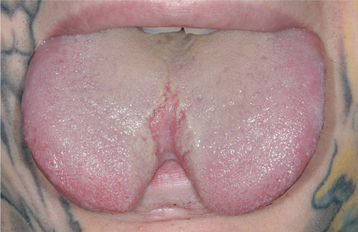
Fig. 8-54 Forked tongue. Anterior portion of the tongue divided into two separate lobes, each of which can be controlled independently. (Courtesy Dr. Fleming Chisholm.)
Susuk usually is shaped like a needle that is pointed on one end and blunt on the other. Most are made from silver or gold, measure 0.5 mm in diameter, and vary from 0.5 to 1.0 cm in length. Rarely, they are made from diamonds. The pins vary in number from one to many and are inserted subcutaneously, often in a symmetrical fashion. The orofacial region is the most common location, but some choose the chest, arms, breasts, and pubic region. In most instances, the individuals are middle-aged adults. Normally, no clinical evidence exists, either visually or by palpation, and the pins are discovered during routine radiography for unrelated medical or dental problems (Fig. 8-55).
TREATMENT AND PROGNOSIS: As mentioned, intraoral barbells and labrets are associated with an increasing prevalence of oral complications that relate closely with duration of use. The patient should be strongly encouraged to remove the jewelry. On removal, if the site demonstrates significant inflammation, local débridement, antibiotic therapy, and chlorhexidine mouthwash may be appropriate.
Except for slight sibilant distortions and shortening of the protrusive length of the tongue, few long-term adverse events have been noted in patients with forked tongues.
Susuk have not been associated with harmful effects, and no treatment is required. If the needles have a ferrous content, then magnetic resonance imaging (MRI) would be contraindicated. On occasion, affected individuals request removal of the susuk before they die because they believe their death will be excruciatingly painful.
SYSTEMIC METALLIC INTOXICATION
Ingestion or exposure to any one of several heavy metals can cause significant systemic and oral abnormalities. Exposure to heavy metals may be massive, resulting in acute reactions, or it may be minimal over a longer period, producing chronic changes. Oral alterations from ingestion of lead, mercury, silver, bismuth, arsenic, and gold are rare but may occur and warrant discussion. Oral complications from excessive zinc, iron, tin, and manganese are extremely rare.
LEAD: Little is known about the prevalence of lead poisoning (plumbism), but lead is one of the most widespread environmental toxins affecting children in the United States. Lead solder for plumbing was not banned until 1986. Homes built before then have the potential for significant water contamination, and one of the primary causes of lead intoxication in infants is formula preparation using tap water tainted by the metal.
Another significant source of lead poisoning in children is lead-based paint; children may ingest chips of the paint in older homes or be exposed to the fumes or dust during sanding and renovation. Paint with a high lead content was not restricted until 1977 and still remains in many homes. Removal of lead from gasoline began in 1972 but was not completed until 1995. These sources of lead combined with previous industrial emissions have resulted in sites with highly contaminated soil, especially in urban areas. Despite the widespread publicity and significant efforts to restrict exposure to lead during childhood, significant risk remains.
Adult exposure also occurs and often is related to industry. The potential for exposure exists during handling of lead oxide batteries, in lead-processing industries, and from the welding of lead-covered surfaces. Some food and drink containers or vegetables grown in lead-contaminated soil also may contain inappropriate levels of the metal. Lead contamination in illicit alcohol has made the distinction between symptoms of lead intoxification and chronic alcohol abuse very difficult in certain sections of the American Deep South. Lead also can be found in brass fixtures, ceramics, crystal, electrical cable, radiation shielding, folk remedies, and cosmetics. Rarely, plumbism arises from retained lead bullet fragments in gunshot victims.
MERCURY: The danger of mercury exposure is well known. Elemental mercury is poorly absorbed, and its ingestion is relatively harmless. In contrast, inhalation of mercury vapor is very hazardous, with a high rate of absorption and systemic retention. Ingestion of mercury salts (e.g., mercurous chloride) also is associated with significant adverse reactions. Exposure has occurred in association with the use of mercury in teething powders, cathartic agents, and anthelmintic preparations.
A great deal of attention has been directed toward the mercury released from dental amalgams, but no well-documented adverse health effects have been identified (except for relatively rare contact hypersensitivity to mercury, see page 354). The level of mercury that is released from amalgams does not appear sufficiently high to cause disease and has been shown not to exceed the range expected from background exposure to environmental mercury. In an attempt to shed light on this controversy, the National Institutes of Health (NIH) funded two large randomized clinical trials that compared the neurologic and renal effects of dental amalgam in a 7-year study of a large cohort of children. In these pivotal investigations, no adverse effects from dental amalgam were seen. Interestingly, the control group that received only composite restorations demonstrated a 50% higher need for additional restorative treatment because of the failure of their restorations during the long-term study.
SILVER: Silver has known antibacterial properties and has been associated with a number of additional health benefits. In the past, silver compounds were used topically in nose drops and systemically for a variety of disorders including mental illness, epilepsy, nicotine addition, common colds, sinusitis, gastrointestinal ulcerations, syphilis, and gonorrhea. Because of the numerous complications, including silver intoxification, the FDA concluded that the risk of systemic silver products exceeded their benefits. In 1999 the use of colloidal silver or silver salts was banned in over-the-counter products. Several silver nitrate and silver sulfadiazine formulations remain available by prescription. These products should be used only under strict supervision. Well-documented examples of generalized argyria have been seen secondary to long-term treatment of aphthous ulcerations, denture sores, and minor gingival hemorrhage with topical silver nitrate.
Devices for production of homemade colloidal silver suspension and a number of colloidal silver proteins continue to be marketed over the Internet and in health food stores as essential mineral supplements for diseases such as arthritis, cancer, diabetes, AIDS, and herpes. These unregulated silver products have no known physiologic function, and their continued use cannot be supported.
BISMUTH AND ARSENIC: In the United States, exposure to bismuth and arsenic currently is rare. The medical use of these metals has diminished dramatically; most current cases arise from occupational exposure. Bismuth was used in the past for treatment of venereal diseases and various dermatoses, whereas arsenic compounds were prescribed for asthma and skin disorders such as psoriasis. Bismuth iodoform paraffin paste continues to be used by otolaryngologists and oral surgeons as a surgical pack, with rare reports of associated toxicity. In addition, bismuth subsalicylate tablets (Pepto-Bismol) have been reported to produce localized mucosal discoloration. Chronic exposure to arsenic continues in some lesser developed areas of the world from drinking contaminated water.
GOLD: Gold has been used in medical treatment in the past and continues to be used today in selected cases of active rheumatoid arthritis and other immunologically mediated diseases. In these cases the side effects are well known, and physicians observe the patients closely. In reviews of large-scale skin testing, gold has been found to be among the top 10 most frequent allergens, with positive reactions seen in about 10% of the population, including increased prevalence in patients who have gold dental restorations.
LEAD: Lead poisoning results in nonspecific systemic signs and symptoms, thereby making the ultimate diagnosis very difficult. The presentation is extremely variable and determined by the type of lead (organic or inorganic) and the age of the patient. Patients with acute cases most often have abdominal colic, which may occur along with anemia, fatigue, irritability, and weakness. Encephalopathy and renal dysfunction also may occur. Chronic exposure causes dysfunction of the nervous system, kidneys, marrow, bone, and joints. Symptoms generally include fatigue, musculoskeletal pain, and headache. Bones and teeth represent a major reservoir in patients with chronic plumbism, with 90% of the body’s deposition being within bone. In radiographs of the long bones in infants, a radiopaque lead line often is noted along the epiphyseal plates. In addition, abdominal radiographs often will reveal small radiopaque paint chips in the gastrointestinal tract.
Oral manifestations include ulcerative stomatitis and a gingival lead line (Burton’s line). The lead line appears as a bluish line along the marginal gingiva resulting from the action of bacterial hydrogen sulfide on lead in the gingival sulcus to produce a precipitate of lead sulfide. Gray areas also may be noted on the buccal mucosa and tongue. Additional manifestations include the following:
MERCURY: Mercury poisoning also may be acute or chronic. With acute cases, abdominal pain, vomiting, diarrhea, thirst, pharyngitis, and gingivitis typically are present. With chronic cases, gastrointestinal upset and numerous neurologic symptoms occur. Oral changes include a metallic taste and ulcerative stomatitis combined with inflammation and enlargement of the salivary glands, gingiva, and tongue. The gingiva may become blue-gray to black. Mercuric sulfide can be generated by the bacterial action on the metal and can cause significant destruction of the alveolar bone with resultant exfoliation of teeth.
Chronic mercury exposure in infants and children is termed acrodynia (pink disease, Swift disease). The children have cold, clammy skin, especially on the hands, feet, nose, ears, and cheeks. An erythematous and pruritic rash is present. Severe sweating, increased lacrimation, irritability, insomnia, photophobia, hypertension, weakness, tachycardia, and gastrointestinal upset also may be present. On occasion, these highly irritable children have torn out patches of their hair. Oral signs include excessive salivation, ulcerative gingivitis, bruxism, and premature loss of teeth. Because mercury salts were formerly used in the processing of felt, hat makers in past centuries were exposed to the metal and experienced similar symptoms, giving rise to the phrase “mad as a hatter.”
SILVER: Acute silver intoxication can produce coma, pleural edema, hemolysis, and bone marrow failure. Chronic systemic silver intoxication is known as argyria. Silver is disseminated throughout the body with substantial amounts accumulating as subepithelial deposits in the skin. These deposits result in a diffuse grayish discoloration that develops primarily in the sun-exposed areas (Fig. 8-56). The sclerae and nails also may be pigmented. One of the first signs of argyria occurs in the oral cavity and appears as a slate-blue silver line along the gingival margins. This discoloration is secondary to deposition of metallic silver and silver sulfide pigments. In addition, the oral mucosa often exhibits a diffuse blue-black discoloration.
Fig. 8-56 Argyria. Grayish discoloration of the face compared with a more normal facial complexion in an individual who used a silver-containing nutritional supplement. Before development of silver intoxication, this blue-eyed, red-haired individual had a very light complexion. (Courtesy of Bradford R. Williams.)
BISMUTH: Chronic bismuth exposure can result in a diffuse blue-gray discoloration of the skin. The conjunctiva and oral cavity also may be involved. A blue-gray line along the gingival margin similar to that seen from lead intoxication is the most common intraoral presentation. Bismuth combines with bacterial hydrogen sulfide to form bismuth sulfide, which is irritating locally but not as destructive as mercuric sulfide. Associated ptyalism, burning, stomatitis, and ulceration may be seen. Intoxication from bismuth-containing surgical packs has been associated with CNS symptoms such as delirium. Chronic use of bismuth subsalicylate tablets can create a removable black discoloration of the otherwise normal filiform papillae. Although the lingual alteration may resemble black hairy tongue, the papillae are not elongated.
ARSENIC: In addition to widespread effects on numerous organ systems, significant dermatologic alterations frequently occur. Prolonged ingestion of arsenic often results in a diffuse macular hyperpigmentation. The discoloration is due to both the presence of the metal and an increased melanin production. In addition, palmar and plantar hyperkeratosis often is noted, as well as numerous premalignant skin lesions called arsenical keratoses. Development of basal cell carcinoma and cutaneous squamous cell carcinoma has been seen after years of exposure. Oral manifestations are rare and typically appear as excessive salivation and painful areas of necrotizing ulcerative stomatitis. In the past, extensive dorsal hyperkeratosis of the tongue was seen in patients with syphilis and may be related to arsenic therapy used before the advent of antibiotic therapy.
GOLD: The most common complication of gold therapy is dermatitis, which often is preceded by a warning signal: pruritus. Although a generalized exfoliative dermatitis with resultant alopecia and loss of nails can be seen, dermatitis about the face, eyelids, and at direct sites of skin contact is the most common presentation. Because of the high frequency of allergy to gold, skin testing often is performed before administration of gold drug therapy.
The second most common adverse reaction to gold is severe oral mucositis, which most frequently involves the buccal mucosa, lateral border of the tongue, palate, and pharynx. These mucosal changes represent a systemic allergic reaction and are different from intraoral contact gold hypersensitivity (see page 347). A metallic taste often precedes development of the oral lesions and should be considered another warning signal. Therapy with gold rarely can bring about a slate-blue discoloration of sun-exposed skin (chrysiasis).
TREATMENT AND PROGNOSIS: The management of heavy metal intoxication involves removal from further exposure to the agent, supportive care, decontamination, and use of chelating agents. In some cases a medication may be responsible and can be discontinued; however, sometimes the source of the metal may be difficult to determine. In infants with radiographic evidence of gastrointestinal lead-containing paint chips, bowel irrigation with a polyethylene glycol electrolyte lavage solution may be warranted. In the past, two chelators, ethylenediaminetetraacetic acid (EDTA) (calcium disodium ethylenediaminetetraacetate) and BAL (2,3-dimercaptopropanol), were first-line therapy in the treatment of lead poisoning, whereas arsenic and mercury intoxication were treated with BAL. These medications may have significant side effects, and less toxic alternatives such as DMSA (2,3-dimercaptosuccinic acid) and DMPS (2,3-dimercaptopropane-1-sulfonate) now are available. No antidote exists for silver intoxication, and treatment is limited to supportive measures. Attempts to remove the bluish discoloration of facial argyria with dermabrasion have been unsuccessful. Encephalopathy associated with use of bismuth-containing surgical packs clears on removal of the material.
SMOKER’S MELANOSIS
Oral pigmentations are increased significantly in heavy smokers. In one investigation of more than 31,000 whites, 21.5% of tobacco smokers exhibited areas of melanin pigmentation compared with 3% among those not using tobacco. In another study of an ethnically pigmented population, smokers had more oral surfaces exhibiting melanin pigmentation.
Melanin pigmentation in the skin exerts a well-known protective effect against ultraviolet (UV) damage. Investigations of melanocytes located away from sun-exposed areas have shown the ability of melanin to bind to noxious substances. Exposure to polycyclic amines (e.g., nicotine and benzpyrene) has been shown to stimulate melanin production by melanocytes that also are known to bind strongly to nicotine. Research has suggested that melanin production in the oral mucosa of smokers serves as a protective response against some of the harmful substances in tobacco smoke. This concept is supported by the findings in “reverse” smokers, who smoke with the lit end of the cigarette inside the mouth and demonstrate heavy melanin pigmentation of the palate. In some reverse smokers, areas of melanocytes are lost and zones of depigmented red mucosa can develop. Cancer is found in 12% of patients with these red zones, further delineating the probable protective effects of melanocytes against toxic substances.
CLINICAL FEATURES: Although any mucosal surface may be affected, smoker’s melanosis most commonly affects the anterior facial gingiva (Fig. 8-57). Most people affected by this condition are cigarette users. In contrast, pipe smokers frequently exhibit pigmentations located on the commissural and buccal mucosae. Reverse smokers show alterations of the hard palate.
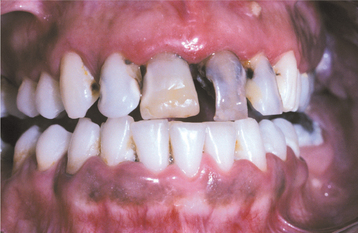
Fig. 8-57 Smoker’s melanosis. Light, diffuse melanin pigmentation in a white female who is a heavy smoker. Pigmentary changes are limited to the anterior facial gingiva.
The areas of pigmentation significantly increase during the first year of smoking and appear correlated to the number of cigarettes smoked each day. A higher frequency is seen in females, and researchers have suggested that female sex hormones exert a synergistic effect when combined with smoking. Reports from Sweden, Germany, and Japan have shown tobacco smoking to be the most common cause for mucosal pigmentation in light-skinned adult populations.
HISTOPATHOLOGIC FEATURES: Biopsy specimens of affected areas in people with smoker’s melanosis reveal increased melanin pigmentation of the basal cell layer of the surface epithelium, similar to a melanotic macule (see page 379). In addition, collections of incontinent melanin pigmentation are seen free within the superficial connective tissue and in scattered melanophages.
DIAGNOSIS: The clinician can make the diagnosis by correlating the smoking history with the clinical presentation and medical history. Other causes of melanin pigmentation, such as trauma, neurofibromatosis, Peutz-Jeghers syndrome, drug-related pigmentation, endocrine disturbances, hemochromatosis, chronic pulmonary disease, and racial pigmentation should be excluded.
TREATMENT AND PROGNOSIS: Cessation of smoking results in gradual disappearance of the areas of related pigmentation over a 3-year period. Biopsy should be considered when the pigmentation is in unexpected locations, such as the hard palate, or when there are unusual clinical changes, such as increased melanin density or surface elevation.
DRUG-RELATED DISCOLORATIONS OF THE ORAL MUCOSA
An expanding number of medications have been implicated as a cause of oral mucosal discolorations. Although many medications stimulate melanin production by melanocytes, deposition of drug metabolites is responsible for the color change in others. These pigmentary alterations have been associated with use of phenolphthalein, minocycline, tranquilizers, antimalarial medications, estrogen, chemotherapeutic agents, and some medications used in the treatment of patients with AIDS.
The antimalarial agents that are most frequently implicated are chloroquine, hydrochloroquine, quinidine, and quinacrine; chlorpromazine represents the most frequently implicated tranquilizer. Besides treating malaria, antimalarial agents are used for many other disorders, including lupus erythematosus and rheumatoid arthritis.
Oral mucosal pigmentation associated with chemotherapeutic medications is most commonly reported with use of doxorubicin, busulfan, cyclophosphamide, or 5-fluorouracil. Although idiopathic hyperpigmentation also may occur, AIDS patients receiving zidovudine (AZT), clofazimine, or ketoconazole have demonstrated increased melanin pigmentation.
CLINICAL FEATURES: The clinical presentations of pigmentations related to drug use vary. Most agents produce a diffuse melanosis of the skin and mucosal surfaces, but others may cause a unique pattern. As in many cases of increased melanin pigmentation, females are more sensitive, most likely as a result of an interaction with sex hormones.
Use of phenolphthalein as a laxative has been associated with numerous small, well-circumscribed areas of hyperpigmentation on the skin. Similar areas of oral mucosal melanosis also can occur.
Long-term use of minocycline, a semisynthetic derivative of tetracycline, results in discoloration of the bone and developing teeth. The affected bone is dark green but creates a blue-gray discoloration as seen through the translucent oral mucosa. The most com-mon presentations include a linear band above the facial attached gingiva near the mucogingival junction and a broad zone of discoloration on the hard palate (Fig. 8-58).
Fig. 8-58 Minocycline-related discoloration. Blue-gray discoloration of the facial surface of the anterior mandibular alveolus because stained alveolar bone is visible through the thin mucosa.
Rare soft tissue pigmentation of the lips, tongue, eyes, and skin also has been reported with use of minocycline. The cutaneous pigmentation appears to be dose-dependent and is seen in up to 15% of patients being treated for acne and as many as 70% of those treated for rheumatoid arthritis. The pigmentation presents in three patterns, two of which are thought to be caused by deposition of drug metabolites that become chelated with iron. In the third pattern, the medication triggers a diffuse muddy-brown or gray cutaneous pigmentation resulting from increased melanosis of the epithelial basal cell layer and superficial connective tissue. This latter pattern is accentuated in sun-exposed areas, and the diagnosis can be made only when there is a direct association between initiation and cessation of the medication and the appearance and fading of the pigmentation. Minocycline-related pigmentation of the oral mucosa unrelated to discoloration of the underlying bone also has been reported, with patients exhibiting either widespread increased melanosis or focal accumulations of iron-containing particles (Figs. 8-59 and 8-60). Although the cutaneous and oral mucosal staining fade after discontinuation of the medication, the dental discoloration remains.
Fig. 8-59 Minocycline-associated pigmentation. Sharply demarcated brown pigmentation on the vermilion border of the lips, which arose in association with long-term minocycline use and is the result of increased melanin deposition.
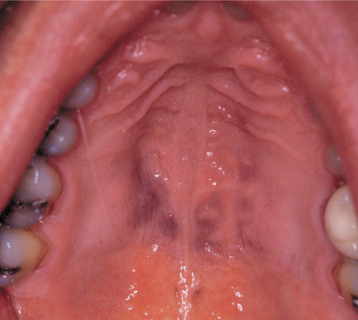
Fig. 8-60 Minocycline-associated pigmentation. Multifocal areas of palatal pigmentation secondary to deposition of drug metabolites chelated to iron in association with long-term minocycline use. (From Treister NS, Magalnick D, Woo S-B: Oral mucosal pigmentation secondary to minocycline therapy: report of two cases and a review of the literature, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:718-725, 2004.)
The classic presentation of intraoral pigmentation from use of antimalarial medications or tranquilizers is a blue-black discoloration limited to the hard palate (Fig. 8-61). In addition, the intake of antimalarial medications may occasionally lead to a more diffuse brown melanosis of the oral mucosa and skin.
Estrogen, chemotherapeutic agents, and medications used in the treatment of AIDS patients may result in a diffuse brown melanosis of the skin and mucosal surfaces. Any mucosal surface may be involved, but the attached gingiva and buccal mucosa are most frequently affected. The pattern and appearance of the oral mucosal involvement are similar to those seen in racial pigmentation.
REACTIVE OSSEOUS AND CHONDROMATOUS METAPLASIA (CUTRIGHT LESION)
On occasion, cartilage or bone may be discovered within soft tissue specimens removed from the oral cavity. Cartilaginous rests are known to exist in the area of the nasopalatine duct. In the past, several investigators have reported the presence of cartilage within flabby soft tissue removed from maxillary edentulous alveolar ridges of long-term denture wearers. This finding was thought to represent cartilaginous metaplasia secondary to chronic denture trauma. In retrospect, the islands of cartilage within these cases most likely represent embryologic remnants, not traumatic metaplasia. These rests are also occasionally discovered during histopathologic examination of naso-palatine duct cysts and maxillary gingivectomy specimens.
Despite the suggestion that the anterior maxillary lesions are embryologic and not traumatic, development of osseous and chondromatous metaplasia from mechanical denture irritation does occur. Although such metaplasia is probably uncommon in the anterior maxilla, its development is not rare along the crest of the posterior mandibular alveolar ridge in long-term denture wearers with atrophic ridges.
CLINICAL AND RADIOGRAPHIC FEATURES: In patients with reactive osseous and chondromatous metaplasia, an extremely tender and localized area of the alveolar ridge is typically noted that may be associated with local enlargement (Fig. 8-62). These changes almost always arise in patients with extensive atrophy of the mandibular alveolar ridge leading to a knife edge–like crest. Although most examples involve the posterior mandible, similar areas may rarely be seen overlying the maxillary alveolar ridge or associated with anterior portions of the mandible. Because of significant associated symptoms and occasional enlargement, biopsy is frequently performed.
HISTOPATHOLOGIC FEATURES: Histopathologic examination of reactive osseous and chondromatous metaplasia typically demonstrates a mass of hypercellular periosteum that blends into areas of osseous and chondromatous tissue. The bone and cartilage frequently exhibit atypical features, such as hypercellularity, pleomorphism, nuclear hyperchromatism, and occasional binucleated or multinucleated cells (Fig. 8-63). These alterations are worrisome for sarcoma, but the appropriate diagnosis can be made when an appropriate clinicopathologic correlation is made. In contrast, the cartilaginous rest discovered incidentally in maxillary specimens is usually very bland without any atypical features that would suggest malignancy.
TREATMENT AND PROGNOSIS: The thin mandibular ridges may be recontoured or supplemented with graft material to improve shape and to alleviate the symptoms associated with the localized periosteal hyperplasia. Implants also may reduce the traumatic injury to the ridge and lessen the chance of recurrence. If the ridge modification is not made, then the continued injury to the site occasionally results in recurrence of the lesion.
ORAL ULCERATION WITH BONE SEQUESTRATION (SPONTANEOUS SEQUESTRATION; TRAUMATIC SEQUESTRATION)
Focal superficial sequestration of a fragment of cortical bone not related to systemic disease, infection, or a major traumatic event is uncommon. In such instances the cause of the focal osteonecrosis is not known, although many believe the primary event is a traumatic or aphthous ulceration that leads to osteitis and necrosis of a small focus of adjacent cortical bone. Others have suggested the blood supply of the peripheral cortical plate may be delivered by the periosteal microvasculature, and loss of this supply leads to focal bone necrosis and sequestration. Such lesions tend to occur in anatomically unique sites in which a bony prominence is covered by a thin mucosal surface.
CLINICAL AND RADIOGRAPHIC FEATURES: In most instances, the sequestration arises without the patient’s awareness of any preceding trauma. The most frequent site of sequestrum development is the lingual surface of the posterior mandible along the mylohyoid ridge (Fig. 8-64). Focal involvement of exostoses also may occur. Although any exostosis may be involved, mandibular tori are affected most frequently.
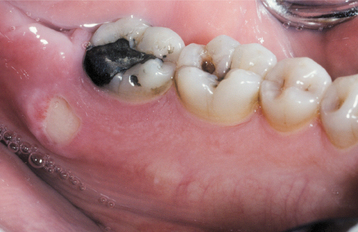
Fig. 8-64 Spontaneous sequestration. Mucosal ulceration with exposed bone of the posterior lingual surface of the mandible.
The overlying mucosa typically demonstrates a focal area of ulceration that has been present for a period of time that varies from a few days to several months. The presence and intensity of associated pain are variable. Although most cases are unilateral, bilateral involvement may occur. On occasion an occlusal radiograph will reveal a faint radiopaque mass superimposed and partially lingual to the intact cortical plate.
The mylohyoid ridge often is prominent but typically protected from trauma by the lingual inclination of the adjacent molars. Absence of the adjacent molars or restorations that do not replace the normal inclination could predispose the area to repeated trauma; such alterations have been noted in the majority of affected patients.
HISTOPATHOLOGIC FEATURES: The sequestra consist of well-organized lamellar bone that exhibits loss of the osteocytes from their lacunae, along with peripheral resorption and bacterial colonization.
TREATMENT AND PROGNOSIS: Spontaneous loss of the dead bone or surgical removal of the sequestrum results in rapid healing. Recurrence is uncommon. In some instances, the dead bone is freely movable and easily removed. In other cases, the fragment is adherent to the underlying vital bone and must be surgically excised. In an attempt to avoid surgery, some clinicians use a tetracycline rinse in addition to topical corticosteroids. Alternatively, these patients can be recalled weekly for 2 to 4 weeks, during which time the dead bone may exfoliate spontaneously without the need for invasive therapy.
PSEUDOCYSTS AND CYSTS OF THE MAXILLARY SINUS
Antral pseudocysts are common findings on panoramic radiographs. They appear as dome-shaped, faintly radiopaque lesions arising from the floor of the maxillary sinus. In the past these sinus changes were incorrectly termed sinus mucoceles, because previous investigators thought the lesions resulted from mucus extravasation similar to that seen in salivary glands of soft tissue. In fact, it appears that no comparable mucus extravasation occurs in the maxillary sinus.
ANTRAL PSEUDOCYST: Antral pseudocyst is the best term for the dome-shaped lesion of the sinus floor. The process usually consists of an inflammatory exudate (serum, not mucin) that has accumulated under the maxillary sinus mucosa and caused a sessile elevation (Fig. 8-65). The exudate is surrounded by connective tissue, and the epithelial lining of the sinus is superior to the fluid. Reviews of large numbers of radiographs have determined the prevalence, which varies from 1.5% to 14% of the population. The cause of the inflammatory infiltrate has not been definitively determined, but in a radiographic review, most cases showed a possible source from an adjacent odontogenic infection. Primary irritation of the sinus lining, such as that seen from a sinus infection or allergies, also can theoretically result in the subperiosteal inflammatory infiltrate.
Fig. 8-65 Antral pseudocyst and true sinus mucocele. An antral pseudocyst is an accumulation of serum beneath the sinus lining. A sinus mucocele is an epithelium-lined cystic structure separate from the sinus.
An increased prevalence of pseudocysts has been noted during the cold winter months, leading some investigators to associate these lesions with an increased frequency of upper respiratory tract infections or irritation from dry, forced-air heating. Although allergies have been proposed as a cause, no increased prevalence has been noted during the time of peak pollen exposure.
SINUS MUCOCELES: True sinus mucoceles are accumulations of mucin that are completely encased by epithelium. They occur in two situations. One type of sinus mucocele occurs after trauma or surgery to the sinus; this type is best known as a surgical ciliated cyst, traumatic ciliated cyst, or postoperative maxillary cyst. A portion of the sinus lining becomes separated from the main body of the sinus and forms an epithelium-lined cavity into which mucin is secreted (see Fig. 8-65). The cyst most frequently originates after a Caldwell-Luc operation but may arise from difficult extraction of a maxillary tooth in which the floor of the maxillary sinus is damaged. Authors have suggested that sinus or nasal epithelium rarely can be transplanted accidentally to the mandible during genioplasty or chin augmentation procedures and lead to formation of ciliated cysts in ectopic locations.
The second type of sinus mucocele arises from an obstruction of the sinus ostium, thereby blocking normal drainage. This blocked sinus then acts like a separate cystlike structure lined by epithelium and filled with mucin. Sinus mucoceles enlarge in size as the intraluminal pressure increases and can distend the walls of the sinus and erode through bone, often clinically mimicking malignancy of antral origin.
Postoperative maxillary cysts appear to be uncommon in the United States and Europe but are reported more frequently in Japan. Mucoceles arising from ostial obstruction are much more numerous and most frequently involve the frontal sinus, with the ethmoid and sphenoid sinuses being affected less often. Maxillary sinus mucoceles are relatively rare and account for less than 10% of paranasal sinus mucoceles.
RETENTION CYSTS: Retention cysts of the maxillary sinus arise from the partial blockage of a duct of the seromucous glands or from an invagination of the respiratory epithelium. The mucin is surrounded by epithelium, and no extravasation occurs. Most retention cysts are located around the ostium or within antral polyps. The majority of cysts are small, not evident clinically, and discovered during histopathologic examination of antral polyps.
CLINICAL AND RADIOGRAPHIC FEATURES: Many symptoms have been attributed to sinus mucoceles; however, because of the confusion between pseudocysts and true mucoceles, it is unclear which symptoms are associated with pseudocysts and which are related to true sinus mucoceles. Most pseudocysts are asymptomatic; although it is rare, affected patients may exhibit facial fullness or report paresthesia, pain, or soreness on palpation. As true sinus mucoceles enlarge and expand bone, symptoms may develop and vary according to the location and the degree of expansion and destruction.
Radiographically, the pseudocyst classically appears as a dome-shaped and slightly radiopaque lesion overlying the intact floor of the maxillary sinus (Figs. 8-66 and 8-67). Maxillary cysts and neoplasms can simulate the dome-shaped pattern of an antral pseudocyst, but close examination of these pathoses typically reveals the floor of the sinus covering the superior aspect of the lesion. When the maxillary sinus is involved by a true sinus mucocele, the entire sinus will be cloudy. As the lesion enlarges, the walls of the sinus may become thinned and eventually eroded. Surgical ciliated cysts are spherical lesions that are separate from the sinus and lack the dome-shaped appearance of pseudocysts (Fig. 8-68). As these postoperative cysts enlarge, they too can lead to perforation of the sinus walls. Retention cysts rarely reach a size that would produce detectable radiographic changes.
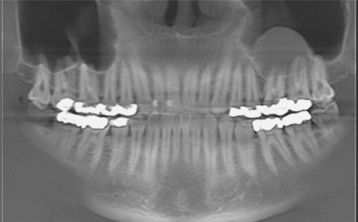
Fig. 8-66 Antral pseudocyst. Three-dimensional cone-beam radiograph showing dome-shaped radiopacity within the maxillary sinus. (Courtesy of Dr. Scott Jenkins and Dr. Nick Morrow.)
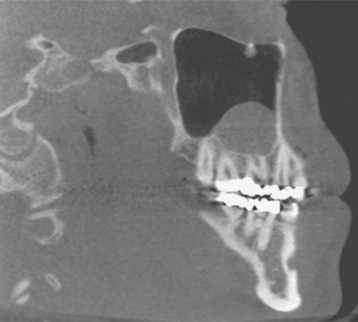
Fig. 8-67 Antral pseudocyst. Three-dimensional cone-beam sagittal section of same patient depicted in Fig. 8-66. Note that the floor of the sinus remains intact below the lesion. (Courtesy of Dr. Scott Jenkins and Dr. Nick Morrow.)
HISTOPATHOLOGIC FEATURES: Antral pseudocysts are covered by sinus epithelium and demonstrate a subepithelial inflammatory exudate that consists of serum occasionally intermixed with inflammatory cells (Fig. 8-69). Collections of cholesterol clefts and scattered small dystrophic calcifications may be seen. True sinus mucoceles and surgical ciliated cysts are true cystic structures lined by ciliated pseudostratified columnar epithelium, squamous epithelium with mucous cells, or metaplastic squamous epithelium (Fig. 8-70). A sinus retention cyst shows focal dilatation of a duct associated with the seromucous glands of the sinus lining. The lumen of the dilated duct is filled with thick mucus, often intermixed with chronic inflammatory cells.
TREATMENT AND PROGNOSIS: Typically, pseudocysts of the maxillary sinus are harmless, and no treatment is necessary. The adjacent teeth should be evaluated thoroughly, and any foci of infection should be eliminated. A few clinicians prefer to confirm their radiographic impression and rule out a tumor through drainage of the inflammatory exudate. Removal by means of a Caldwell-Luc operation should be performed on any radiographically diagnosed lesion that produces significant expansion or is associated definitively with symptoms such as headache.
Because sinus mucoceles and surgical ciliated cysts are expansile and destructive lesions, the traditional therapy for these pathoses is assured surgical removal. Numerous investigators also have shown that sinus mucoceles arising from ostial obstruction often do not require surgical excision and respond well to endoscopic middle meatal antrostomy and marsupialization of the mucocele.
CERVICOFACIAL EMPHYSEMA
Cervicofacial emphysema arises from the introduction of air into subcutaneous or fascial spaces of the face and neck. The forced air may spread through the spaces to the retropharyngeal and mediastinal areas. The first case was reported almost 100 years ago and occurred as a result of blowing into a bugle a short time after tooth extraction.
Cervicofacial emphysema of dental origin may arise in several ways:
• After the use of compressed air by the clinician
• After difficult or prolonged extractions
• As a result of increased intraoral pressure (e.g., sneezing, blowing) after an oral surgical procedure
Introduction of air within tissue has been seen after a large number of dental procedures, but most instances involve either surgical extraction of teeth, osteotomies, significant trauma, or the use of air or water syringes. In addition, the prevalence has increased as a result of the use of air-driven handpieces during oral surgery. On occasion, cervicofacial emphysema has resulted from compressed air being accidentally forced into small intraoral lacerations located away from the field of operation. Conservative surgical flap design without extension into fascial planes and limited use of air-driven handpieces during surgical procedures may minimize the chance of occurrence. Rare reports of cervicofacial emphysema after self-induced oral injury have been reported in prisoners attempting to escape by simulation of a medical emergency and a drug abuser with Munchausen syndrome who was trying to access unnecessary medical intervention.
An analogous problem termed pneumoparotid can arise when air enters the parotid duct, leading to enlargement of the parotid gland caused by air insufflation. This can be accidental, self-induced, or occupational (e.g., glassblowers, wind instrument players). Stensen’s duct has numerous redundant mucosal folds that seal as intraoral pressure is increased; in addition, contraction of the buccinator muscle further prevents entrance of air by compressing the duct. In spite of this protection, dramatic increases in intraoral pressures can result in air filling the parotid ductal system.
CLINICAL AND RADIOGRAPHIC FEATURES: More than 90% of cases of cervicofacial emphysema develop during surgery or within the first postoperative hour. Cases with delayed onset are associated with increased postoperative pressure created by the patient. The initial change is one of soft tissue enlargement from the presence of the air in deeper tissues (Fig. 8-71). Pain is usually minimal, and crepitus is detected easily with gentle palpation. Subsequently, the enlargement increases and spreads because of secondary inflammation and edema. Variable pain, facial erythema, dysphagia, dysphonia, vision difficulties, and mild fever may occur. The facial enlargement often is confused with an angioedema, but the diagnosis can be made by identifying crepitus within the swelling.
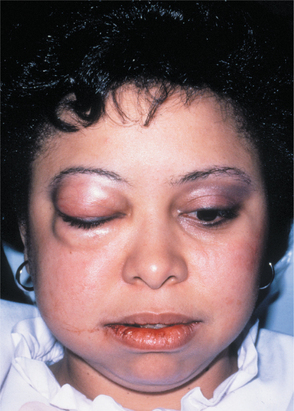
Fig. 8-71 Cervicofacial emphysema. Periorbital and facial enlargement caused by use of an air-driven handpiece during third molar removal.
Significant spread into the mediastinum can result in dysphonia, dysphagia, or dyspnea. Cardiac auscultation often reveals crepitus synchronous with the heart beat (Hamman’s crunch) in cases with mediastinal involvement. Pneumomediastinum can be confirmed on chest radiographs by observing displacement of the mediastinal pleura.
Pneumoparotid typically appears as a unilateral enlargement of the parotid that demonstrates crepitus on gentle palpation. Milking the parotid duct produces a frothy, air-filled saliva, rather than the typical clear, waterlike secretion.
TREATMENT AND PROGNOSIS: Broad-spectrum antibiotic coverage is recommended in all dental-related cases of cervicofacial emphysema. The body gradually removes the entrapped air during a 2- to 5-day period. Most cases spontaneously resolve without significant difficulty. Rare cases of respiratory distress have been noted, and assisted ventilation was required.
The first goal of therapy for pneumoparotid is discovery of the inciting event. In occupation-related cases, such as those seen in trumpet players, the individual should be coached to compress the cheeks during playing. This procedure contracts the buccinator muscle and compresses the parotid duct. Acute symptoms are treated with antibiotics, massage, hydration, sialogogues, and warm compresses.
MYOSPHERULOSIS
Placement of topical antibiotic in a petrolatum base into a surgical site may occasionally result in a unique foreign body reaction, known as myospherulosis. The resultant histopathologic pattern is most unusual and was thought initially to represent a previously undescribed endosporulating fungus.
CLINICAL AND RADIOGRAPHIC FEATURES: Myospherulosis may occur at any site within soft tissue or bone where the antibiotic has been placed. The initial report described involvement of the arms, legs, and gluteal and scapular regions. Most cases in the dental literature have occurred within bone at previous extraction sites where an antibiotic had been placed in an attempt to prevent alveolar osteitis. Although maxillary and oral soft tissue examples have been documented, most cases have occurred within mandibular surgical sites. In addition, myospherulosis is reported occasionally in a paranasal sinus after a surgical procedure in which a gauze packing coated with an antibiotic ointment was used.
The involved area may exhibit swelling or be discovered as an asymptomatic and circumscribed radiolucency in a previous extraction site (Fig. 8-72). In some cases pain and purulent drainage have resulted. On exploration of the lesion, a black, greasy, tarlike material is found.
HISTOPATHOLOGIC FEATURES: The histopathologic pattern is unique; it is the result of a tissue interaction with both the petroleum base and the antibiotic, typically tetracycline. Dense collagenous tissue is intermixed with a granulomatous inflammatory response showing macrophages and multinucleated giant cells. Within the connective tissue are multiple cystlike spaces that contain numerous brown- to black-staining spherules (Fig. 8-73). The collections of spherules sometimes are surrounded by an outer membrane known as a parent body, forming structures that resemble a “bag of marbles.” The spherules represent red blood cells that have been altered by the medication. The unusual dark coloration is due to the degradation of hemoglobin. To complicate matters, myospherulosis arising in a paranasal sinus occasionally is contaminated with respiratory fungal organisms, such as the zygomycetes or aspergillus.
TREATMENT AND PROGNOSIS: Myospherulosis is treated by surgical removal of the foreign material and associated tissue. Histopathologic examination of the altered tissue provides the definitive diagnosis. Recurrence is not expected. Those arising in a paranasal sinus and exhibiting fungal infestation respond well to local measures and do not require systemic antimicrobial therapy. Some investigators have recommended discontinuation of nasal packing with antibiotic ointment, because patients with antral myospherulosis have been found to be almost three times more likely than controls to develop adhesions and require revision of their sinus surgery.
A similar clinical and radiographic pattern has been seen in association with the use of powdered tetracycline in a polymer dressing. Although somewhat different histopathologically, this formulation also leads to a granulomatous foreign body reaction. Because of complications associated with both formulations, the practice of applying topical antibiotics to oral wounds should be approached with caution, and other methods of delivery should be considered. If topical antibiotics are used, then they should be accompanied by close follow-up to ensure appropriate clinical and radiographic evidence of healing of the surgical site.
BIBLIOGRAPHY
Kashani, HG, Mackenzie, IC, Kerber, PE. Cytology of linea alba using a filter imprint technique. Clin Prev Dent. 1980;2:21–24.
Parlak, AH, Koybasi, S, Yavuz, et al. Prevalence of oral lesions in 13- to 16-year old students in Duzce, Turkey. Oral Dis. 2006;12:553–558.
Wood, NK, Goaz, PW. Differential diagnosis of oral and maxillofacial lesions, ed 5, St Louis: Mosby; 1997:98–99.
Bouquot, JE. Common oral lesions found during a mass screening examination. J Am Dent Assoc. 1986;112:50–57.
Hjørting-Hansen, E, Holst, E. Morsicatio mucosae oris and suctio mucosae oris: an analysis of oral mucosal changes due to biting and sucking habits. Scand J Dent Res. 1970;78:492–499.
Kocsard, E, Schwarz, L, Stephen, BS, et al. Morsicatio buccarum. Br J Dermatol. 1962;74:454–457.
Reichart, PA, Philipsen, HP. Betel chewer’s mucosa—a review. J Oral Pathol Med. 1998;27:239–242.
Romero, M, Vicente, A, Bravo, LA. Prevention of habitual cheek biting: a case report. Spec Care Dentist. 2005;25:214–216.
Schiödt, M, Larsen, V, Bessermann, M. Oral findings in glassblowers. Community Dent Oral Epidemiol. 1980;8:195–200.
Sewerin, I. A clinical and epidemiologic study of morsicatio buccarum/labiorum. Scand J Dent Res. 1971;79:73–80.
Van Wyk, CW, Staz, J, Farman, AG. The chewing lesion of the cheeks and lips: its features and the prevalence among a selected group of adolescents. J Dent. 1977;5:193–199.
Aloebeid, B, Pan, L-X, Milligan, L, et al. Eosinophil-rich CD30+ lymphoproliferative disorder of the oral mucosa. Am J Clin Pathol. 2004;121:43–50.
Baghdadi, ZD. Riga-Fede disease: report of a case and review. J Clin Pediatr Dent. 2001;25:209–213.
Bhaskar, SN, Lilly, GE. Traumatic granuloma of the tongue (human and experimental). Oral Surg Oral Med Oral Pathol. 1964;18:206–218.
El-Mofty, SK, Swanson, PE, Wick, MR, et al. Eosinophilic ulcer of the oral mucosa: report of 38 new cases with immunohistochemical observations. Oral Surg Oral Med Oral Pathol. 1993;75:716–722.
Eversole, LR, Leider, AS, Jacobsen, PL, et al. Atypical histiocytic granuloma: light microscopic, ultrastructural, and histochemical findings in an unusual pseudomalignant reactive lesion of the oral cavity. Cancer. 1985;55:1722–1729.
Ficarra, G, Prignano, F, Romagnoli, P. Traumatic eosinophilic granuloma of the oral mucosa: a CD30+ (Ki-1) lymphoproliferative disorder. Oral Oncol. 1997;33:375–379.
Hirshberg, A, Amariglio, N, Akrish, S, et al. Traumatic ulcerative granuloma with stromal eosinophilia. Am J Clin Pathol. 2006;126:522–529.
Kabani, S, Cataldo, E, Folkerth, R, et al. Atypical lymphohistiocytic infiltrate (pseudolymphoma) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1988;66:587–592.
Regezi, JA, Zarbo, RJ, Daniels, TE, et al. Oral traumatic granuloma: characterization of the cellular infiltrate. Oral Surg Oral Med Oral Pathol. 1993;75:723–727.
Throndson, RR, Wright, JM, Watkins, D. Atypical histiocytic granuloma of the oral mucosa: an unusual clinicopathologic entity simulating malignancy. J Oral Maxillofac Surg. 2001;59:822–826.
Zaenglein, AL, Chang, MU, Meehan, SA, et al. Extensive Riga-Fede disease of the lip and tongue. J Am Acad Dermatol. 2002;47:445–447.
Czerepak, CS. Oral splint therapy to manage electrical burns of the mouths in children. Clin Plast Surg. 1984;11:685–692.
Goto, R, Miyabe, K, Mori, N. Thermal burn of the pharynx and larynx after swallowing hot milk. Auris Nasus Larynx. 2002;29:301–303.
Gormley, MB, Marshall, J, Jarrett, W, et al. Thermal trauma: a review of 22 electrical burns of the lip. J Oral Surg. 1972;30:531–533.
Leake, JE, Curtin, JW. Electrical burns of the mouth in children. Clin Plast Surg. 1986;11:669–683.
Taylor, LB, Walker, J. A review of selected microstomia prevention appliances. Pediatr Dent. 1997;19:413–418.
Oral Adverse Reactions to Chemicals
Baruchin, AM, Lustig, JP, Nahlieli, O, et al. Burns of the oral mucosa: report of 6 cases. J Craniomaxillofac Surg. 1991;19:94–96.
Buck, IF, Zeff, S, Kalnins, L, et al. The treatment of intraoral chemical burns. J Oral Ther. 1965;2:101–106.
Frost, DE, Barkmeier, WW, Abrams, H. Aphthous ulcer—a treatment complication. Oral Surg Oral Med Oral Pathol. 1978;45:863–869.
Gernhardt, CR, Eppendorf, K, Kozlowski, A, et al. Toxicity of concentrated sodium hypochlorite used as an endodontic ir-rigant. Int Endod J. 2004;37:272–280.
Grace, EG, Sarlani, E, Kaplan, S. Tooth erosion caused by chewing aspirin. J Am Dent Assoc. 2004;135:911–914.
Kawakami, J, Muto, T, Shigeo, K, et al. Tooth exfoliation and necrosis of the crestal bone caused by use of formocresol. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:736–738.
Özgöz, M, Yaggiz, H, Çiçek, Y, et al. Gingival necrosis following the use of a paraformaldehyde-containing paste: a case report. Int Endod J. 2004;37:157–161.
Rawal, SY, Claman, LJ, Kalmar, JR, et al. Traumatic lesions of the gingiva: a case series. J Periodontol. 2004;75:762–769.
Rees, TD, Orth, CF. Oral ulcerations with use of hydrogen peroxide. J Periodontol. 1986;57:689–692.
Noninfectious Complications of Antineoplastic Therapy
Annane, D, Depondt, J, Aubert, P, et al. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo-controlled, double-blind trial from the ORN96 study group. J Clin Oncol. 2004;22:4893–4900.
Dreizen, S. Description and incidence of oral complications. Consensus Development Conference on Oral Complications of Cancer Therapies: Diagnosis, Prevention, and Treatment. NCI Monogr. 1990;9:11–15.
Epstein, JB, Klasser, GD. Emerging approaches for prophylaxis and management of oropharyngeal mucositis in cancer therapy. Expert Opin Emerging Drugs. 2006;11:353–373.
Keller, EE. Placement of dental implants in the irradiated mandible: a protocol without adjunctive hyperbaric oxygen. J Oral Maxillofac Surg. 1997;55:972–980.
Marx, RE, Johnson, RP, Kline, SN. Prevention of osteoradionecrosis: a randomized prospective clinical trial of hyperbaric oxygen versus penicillin. J Am Dent Assoc. 1985;111:49–54.
Rubenstein, EB, Peterson, DE, Schubert, M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100(suppl 9):2026–2046.
Scully, C, Sonis, S, Diz, PD. Oral mucositis. Oral Dis. 2006;12:229–241.
Sulaiman, F, Huryn, JM, Zlotolow, IM. Dental extraction in the irradiated head and neck patient: a retrospective analysis of Memorial Sloan-Kettering Cancer Center protocols, criteria, and end results. J Oral Maxillofac Surg. 2003;61:1123–1131.
Teng, MS, Futran, ND. Osteonecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg. 2005;13:217–221.
Bisphosphonate-Related Osteonecrosis
Adornato, MC, Morcos, I, Rozanski, J. The treatment of bisphosphonate-associated osteonecrosis of the jaws with bone resection and autologous platelet-derived growth factors. J Am Dent Assoc. 2007;138:971–977.
American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy. Expert panel recommendations. J Am Dent Assoc. 2006;137:1144–1150.
Cohen D, Bhattacharyya N, Islam J et al: Case based evidence for expanding the criteria for the diagnosis of bisphosphonate induced osteochemonecrosis, Abstract 31, American Academy of Oral and Maxillofacial Pathology, Kansas City, Mo, May 8, 2007.
Odvina, CV, Zerwekh, JE, Rao, DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301.
Ott, SM. Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005;90:1897–1899.
Marx, RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic (letter to the editor). J Oral Maxillofac Surg. 2003;61:1115–1117.
Marx, RE, Cillo, JE, Jr., Ulloa, JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65:2397–2410.
Marx, RE, Sawatari, Y, Fortin, M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575.
Ruggiero, SL, Fantasia, J, Carlson, E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:433–441.
Ruggiero, SL, Mehrotra, B, Rosenberg, TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534.
Schneider, JP. Should bisphosphonates be continued indefinitely? An unusual fracture in a healthy woman on long-term alendronate. Geriatrics. 2006;61:31–33.
Woo, S-B, Hellstein, JW, Kalmar, JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761.
Orofacial Complications of Methamphetamine Abuse
Curtis, EK. Meth mouth: a review of methamphetamine abuse and its oral manifestations. Gen Dent. 2006;54:125–129.
Goodchild, JH, Donaldson, M, Mangini, DJ. Methamphetamine abuse and the impact on dental health. Dent Today. 2007;26:124–131.
Rhodus, NL, Little, JW. Methamphetamine abuse and “meth mouth,”. Northwest Dent. 2005;84:29. [31, 33-37].
Richards, JR, Brofeldt, BT. Patterns of tooth wear associated with methamphetamine use. J Periodontol. 2000;71:1371–1374.
Shaner, JW, Kimmes, N, Saini, T, et al. “Meth mouth”: rampant caries in methamphetamine abusers. AIDS Patient Care STDs. 2006;20:146–150.
Carroll, MJ. Tissue necrosis following a buccal infiltration. Br Dent J. 1980;149:209–210.
Giunta, J, Tsamsouris, A, Cataldo, E, et al. Postanesthetic necrotic defect. Oral Surg Oral Med Oral Pathol. 1975;40:590–593.
Schaffer, J, Calman, HI, Levy, B. Changes in the palate color and form (case 9). Dent Radiogr Photogr. 1966;39:3–6. [19-22].
Axéll, T, Skoglund, A. Chronic lip fissures. Prevalence, pathology and treatment. Int J Oral Surg. 1981;10:354–358.
Connolly, M, Kennedy, C. Exfoliative cheilitis successfully treated with topical tacrolimus. Br J Dermatol. 2004;151:241–242.
Daley, TD, Gupta, AK. Exfoliative cheilitis. J Oral Pathol Med. 1995;24:177–179.
Freeman, S, Stephens, R. Cheilitis: analysis of 75 cases referred to a contact dermatitis clinic. Am J Contact Dermat. 1996;7:146–151.
Leyland, L, Field, EA. Case report: exfoliative cheilitis managed with antidepressant medication. Dent Update. 2004;31:524–526.
Reade, PC, Sim, R. Exfoliative cheilitis—a factitious disorder? Int J Oral Maxillofac Surg. 1986;15:313–317.
Rosenquist, B. Median lip fissure: etiology and suggested treatment. Oral Surg Oral Med Oral Pathol. 1991;72:10–14.
Scully, C, van-Bruggen, W, Diz-Dios, P, et al. Down syndrome: lip lesions (angular stomatitis and fissures) and Candida albicans. Br J Dermatol. 2002;147:37–40.
Taniguchi, S, Kono, T. Exfoliative cheilitis: a case report and review of the literature. Dermatology. 1998;196:253–255.
Kalpidis, CDR, Setayesh, RM. Hemorrhaging associated with endosseous implant placement in the anterior mandible: a review of the literature. J Periodontol. 2004;75:631–645.
Kravitz, P. The clinical picture of “cough purpura,” benign and nonthrombocytopenic eruption. Va Med. 1979;106:373–374.
Lee, B-J, Park, H-J, Heo, S-C. Organized hematoma of the maxillary sinus. Acta Otolaryngol. 2003;123:869–872.
Tabaee, A, Kacker, A. Hematoma of the maxillary sinus presenting as a mass—a case report and review of the literature. Int J Pediatr Otorhinolaryngol. 2002;65:153–157.
Woo, BM, Al-Bustani, S, Ueeck, BA. Floor of mouth haemorrhage and life-threatening airway obstruction during immediate implant placement in the anterior mandible. Int J Oral Maxillofac Surg. 2006;35:961–964.
Oral Trauma from Sexual Practices
Damm, DD, White, DK, Brinker, CM. Variations of palatal erythema secondary to fellatio. Oral Surg Oral Med Oral Pathol. 1981;52:417–421.
Elam, AL. Sexually related trauma: a review. Ann Emerg Med. 1986;15:576–584.
Farman, AG, Van Wyk, CW. The features of non-infectious oral lesions caused by fellatio. J Dent Assoc S Afr. 1977;32:53–55.
Leider, AS. Intraoral ulcers of questionable origin. J Am Dent Assoc. 1976;92:1177–1178.
Mader, CL. Lingual frenum ulcer resulting from orogenital sex. J Am Dent Assoc. 1981;103:888–890.
Terezhalmy, GT. Oral manifestations of sexually related diseases. Ear Nose Throat J. 1983;62:287–296.
Van Wyk, CW. Oral lesions caused by habits. Forensic Sci. 1976;7:41–49.
Localized Exogenous Pigmentations
Buchner, A, Hansen, LS. Amalgam pigmentation (amalgam tattoo) of the oral mucosa: a clinicopathologic study of 268 cases. Oral Surg Oral Med Oral Pathol. 1980;49:139–147.
Daley, TD, Gibson, D. Practical applications of energy dispersive x-ray microanalysis in diagnostic oral pathology. Oral Surg Oral Med Oral Pathol. 1990;69:339–344.
Kaufman, T, Bloch, C, Schmidt, W, et al. Chronic inflammation and pain inside the mandibular jaw and a 10-year forgotten amalgam filling in an alveolar cavity of an extracted molar tooth. Ultrastruct Pathol. 2005;29:405–413.
Klontz, KC, Lambert, LA, Jewell, RE, et al. Adverse effects of cosmetic tattooing: an illustrative case of granulomatous dermatitis following the application of permanent makeup. Arch Dermatol. 2005;141:918–919.
Mani, NJ. Gingival tattoo: a hitherto undescribed mucosal pigmentation. Quintessence Int. 1985;16:157–159.
Muller, H, van der Velden, Samderubun, EM. Tattooing in maxillofacial surgery. J Craniomaxillofac Surg. 1988;16:382–384.
Peters, E, Gardner, DG. A method of distinguishing between amalgam and graphite in tissue. Oral Surg Oral Med Oral Pathol. 1986;62:73–76.
Shah, G, Alster, TS. Treatment of an amalgam tattoo with a Q-switched alexandrite (755 nm) laser. Dermatol Surg. 2002;28:1180–1181.
Slabbert, H, Ackermann, GL, Altini, M. Amalgam tattoo as a means for person identification. J Forensic Odontostomatol. 1991;9:17–23.
Weaver, T, Auclair, PL, Taybos, GM. An amalgam tattoo causing local and systemic disease? Oral Surg Oral Med Oral Pathol. 1987;63:137–140.
Oral Piercings and Forked Tongue
Benecke, M. First report of nonpsychotic self-cannibalism (autophagy), tongue splitting, and scar patterns (scarification) as an extreme form of cultural body modification in a western civilization. Am J Forensic Med. 1999;20:281–285.
Brennan, M, O’Connell, B, O’Sullivan, M. Multiple dental fractures following tongue barbell placement: a case report. Dent Traumatol. 2006;22:41–43.
Bressmann, T. Self-inflicted cosmetic tongue split: a case report. J Can Dent Assoc. 2004;70:156–157.
Campbell, A, Moore, A, Williams, E, et al. Tongue piercing: impact of time and barbell stem length on lingual gingival recession and tooth chipping. J Periodontol. 2002;73:289–297.
Fleming, PS, Flood, TR. Bifid tongue—a complication of tongue piercing. Br Dent J. 2005;198:265–266.
Kapferer, I, Benesch, T, Gregoric, N, et al. Lip piercing: prevalence of associated gingival recession and contributing factors. A cross-sectional study. J Periodontol Res. 2007;42:177–183.
Loh, FC, Yeo, JF. Talisman in the orofacial region. Oral Surg Oral Med Oral Pathol. 1989;68:252–255.
Nor, MM, Yushar, A, Razali, M, et al. Incidental radiologic findings of susuk in the orofacial region. Dentomaxillofac Radiol. 2006;35:473–474.
Ahnlide, I, Ahlgren, C, Björkner, B, et al. Gold concentration in blood in relation to the number of gold restorations and contact allergy to gold. Acta Odontol Scand. 2002;60:301–305.
Bellinger, DC, Trachtenberg, F, Barregard, L, et al. Neuropsychological and renal effects of dental amalgam in children. A randomized trial. J Am Med Assoc. 2006;295:1775–1783.
DeRouen, TA, Martin, MD, Leroux, BG, et al. Neurobehavioral effects of dental amalgam in children. A randomized trial. J Am Med Assoc. 2006;295:1784–1792.
Dodes, JE. The amalgam controversy. An evidence-based analysis. J Am Dent Assoc. 2001;132:348–356.
Drake, PL, Hazelwood, KJ. Exposure-repeated health effects of silver and silver compounds: a review. Ann Occup Hyg. 2005;49:575–585.
Dummet, CO. Systemic significance of oral pigmentation and discoloration. Postgrad Med J. 1971;49(1):78–82.
Eisler, R. Mammalian sensitivity to elemental gold (Au degrees). Biol Trace Elem Res. 2004;100:1–18.
Fowler, J, Jr., Taylor, J, Storrs, F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3–5.
Gordon, NC, Brown, S, Khosla, VM, et al. Lead poisoning: a comprehensive review and report of a case. Oral Surg Oral Med Oral Pathol. 1979;47:500–512.
Harris, RA, Poole, A. Beware of bismuth: post maxillectomy delirium. ANZ J Surg. 2002;72:846–847.
Ioffreda, MD, Gordon, CA, Adams, DR, et al. Black tongue. Arch Dermatol. 2001;137:968–969.
Jacobs, R. Argyria: my life story. Clin Dermatol. 2006;24:66–69.
Langworth, S, Björkman, L, Elinder, C-G, et al. Multidisciplinary examination of patients with illness attributed to dental fillings. J Oral Rehabil. 2002;29:705–713.
Lee, SM, Lee, SH. Generalized argyria after habitual use of AgNO3. J Dermatol. 1994;21:50–53.
Marshall, JP, Schneider, RP. Systemic argyria secondary to topical silver nitrate. Arch Dermatol. 1977;113:1077–1179.
Martin, MD, Williams, BJ, Charleston, JD, et al. Spontaneous exfoliation of teeth following severe elemental mercury poisoning: case report and histological investigation for mechanism. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:495–501.
Mirsattari, SM, Hammond, RR, Sharpe, MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408–1410.
Su, M, Barrueto, F, Jr., Hoffman, RS. Childhood lead poisoning from paint chips: a continuing problem. J Urban Health. 2002;79:491–501.
Wadhera, A, Fung, M. Systemic argyria associated with ingestion of colloidal silver. Dermatol Online J. 2005;11(1):12.
Axéll, T, Hedin, CA. Epidemiologic study of excessive oral melanin pigmentation with special reference to the influence of tobacco habits. Scand J Dent Res. 1982;90:434–442.
Hedin, CA. Smokers’ melanosis. Arch Dermatol. 1977;113:1533–1538.
Hedin, CA, Axéll, T. Oral melanin pigmentation in 467 Thai and Malaysian people with special emphasis on smoker’s melanosis. J Oral Pathol Med. 1991;20:8–12.
Hedin, CA, Larsson, Å. The ultrastructure of the gingival epithelium in smokers’ melanosis. J Periodontal Res. 1984;19:177–190.
Hedin, CA, Pindborg, JJ, Daftary, DK, et al. Melanin depigmentation of the palatal mucosa in reverse smokers: a preliminary study. J Oral Pathol Med. 1992;21:440–444.
Ramer, M, Burakoff, RP. Smoker’s melanosis. N Y State Dent J. 1997;63:20–21.
Drug-Related Discolorations of the Oral Mucosa
Birek, C, Main, JHP. Two cases of oral pigmentation associated with quinidine therapy. Oral Surg Oral Med Oral Pathol. 1988;66:59–61.
Cale, AE, Freedman, PD, Lumerman, H. Pigmentation of the jawbones and teeth secondary to minocycline hydrochloride therapy. J Periodontol. 1988;59:112–114.
Cockings, JM, Savage, NW. Minocycline and oral pigmentation. Aust Dent J. 1998;43:14–16.
Dummet, CO. Oral mucosal discolorations related to pharmacotherapeutics. J Oral Ther Pharmacol. 1964;1:106–110.
Granstein, RD, Sober, AJ. Drug- and heavy metal-induced hyperpigmentation. J Am Acad Dermatol. 1981;5:1–18.
Hood, AF. Cutaneous side effects of cancer chemotherapy. Med Clin North Am. 1986;70:187–209.
Langford, A, Pohle, H-D, Gelderblom, H, et al. Oral hyperpigmentation in HIV-infected patients. Oral Surg Oral Med Oral Pathol. 1989;67:301–307.
LaPorta, VN, Nikitakis, NG, Sindler, AJ, et al. Minocycline-associated intra-oral soft-tissue pigmentation: clinicopathologic correlations and review. J Clin Periodontol. 2005;32:119–122.
Pérusse, R, Morency, R. Oral pigmentation induced by Premarin. Cutis. 1991;48:61–64.
Treister, NS, Magalnick, D, Woo, S-B. Oral mucosal pigmentation secondary to minocycline therapy: report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:718–725.
Traumatic Osseous and Chondromatous Metaplasia
Cutright, DE. Osseous and chondromatous metaplasia caused by dentures. Oral Surg Oral Med Oral Pathol. 1972;34:625–633.
Daley, TD, Damm, DD, Wysocki, GP, et al. Atypical cartilage in reactive osteocartilagenous metaplasia of the traumatized edentulous mandibular ridge. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:26–29.
Lello, GE, Makek, M. Submucosal nodular chondrometaplasia in denture wearers. J Prosthet Dent. 1985;54:237–240.
Magnusson, BC, Engström, H, Kahnberg, K-E. Metaplastic formation of bone and chondroid in flabby ridges. Br J Oral Maxillofac Surg. 1986;24:300–305.
Oral Ulceration with Bone Sequestration
Farah, CS, Savage, NW. Oral ulceration with bone sequestration. Aust Dent J. 2003;48:61–64.
Kessler, HP. Lingual mandibular sequestration with ulceration. Tex Dent J. 2005;122:198–199. [206-207].
Peters, E, Lovas, GL, Wysocki, GP. Lingual mandibular sequestration and ulceration. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1993;75:739–743.
Scully, C. Oral ulceration: a new and unusual complication. Br Dent J. 2002;192:139–140.
Sonnier, KE, Horning, GM. Spontaneous bony exposure: a report of 4 cases of idiopathic exposure and sequestration of alveolar bone. J Periodontol. 1997;68:758–762.
Pseudocysts and Cysts of the Maxillary Sinus
Allard, RHB, van der Kwast, WAM, van der Waal, I. Mucosal antral cysts: review of the literature and report of a radiographic survey. Oral Surg Oral Med Oral Pathol. 1981;51:2–9.
Bockmühl, U, Kratzsch, B, Benda, K, et al. Surgery for paranasal sinus mucoceles: efficacy of endonasal micro-endoscopic management and long-term results of 185 patients. Rhinology. 2006;44:62–67.
Bourgeois, SL, Nelson, BL. Surgical ciliated cyst of the mandible secondary to simultaneous LeFort I osteotomy and genioplasty: report of case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:36–39.
Carter, LC, Calamel, A, Haller, A, et al. Seasonal variation in maxillary antral pseudocyst in a general clinical population. Dentomaxillofac Radiol. 1998;27:22–24.
Gardner, DG. Pseudocysts and retention cysts of the maxillary sinus. Oral Surg Oral Med Oral Pathol. 1984;58:561–567.
Gardner, DG, Gullane, PJ. Mucoceles of the maxillary sinus. Oral Surg Oral Med Oral Pathol. 1986;62:538–543.
Kaneshiro, S, Nakajima, T, Yoshikawa, Y, et al. The postoperative maxillary cyst: report of 71 cases. J Oral Surg. 1981;39:191–198.
Horowitz, I, Hirshberg, A, Freedman, A. Pneumomediastinum and subcutaneous emphysema following surgical extraction of mandibular third molars: three case reports. Oral Surg Oral Med Oral Pathol. 1987;63:25–28.
Karras, SC, Sexton, JJ. Cervicofacial and mediastinal emphysema as the result of a dental procedure. J Emerg Med. 1996;14:9–13.
López-Peláez, MF, Roldán, J, Mateo, S. Cervical emphysema, pneumomediastinum, and pneumothorax following self-induced oral injury: report of four cases and review of the literature. Chest. 2001;120:306–309.
Martìn-Granizo, R, Herrera, M, Garcìa-Gonzàlez, D, et al. Pneumoparotid in childhood: report of two cases. J Oral Maxillofac Surg. 1999;57:1468–1471.
Stanton, DC, Balasanian, E, Yepes, JF. Subcutaneous cervicofacial emphysema and pneumo-mediastinum: a rare complication after a crown preparation. Gen Dent. 2005;53:122–124.
Tosun, F, Ozer, C, Akcam, T, et al. A patient with severe cervicofacial subcutaneous emphysema associated with Munchausen’s syndrome. J Craniofac Surg. 2005;16:661–664.
Dunlap, CL, Barker, BF. Myospherulosis of the jaws. Oral Surg Oral Med Oral Pathol. 1980;50:238–243.
Lynch, DP, Newland, JR, McClendon, JL. Myospherulosis of the oral hard and soft tissues. J Oral Maxillofac Surg. 1984;42:349–355.
Moore, JW, Brekke, JH. Foreign body giant cell reaction related to placement of tetracycline-treated polylactic acid: report of 18 cases. J Oral Maxillofac Surg. 1990;48:808–812.
Sarkar, S, Gangane, N, Sharma, S. Myospherulosis of maxillary sinus—a case report with review of literature. Indian J Pathol Microbiol. 1998;41:491–493.
Sindwani, R, Cohen, JT, Pilch, BZ, et al. Myospherulosis following sinus surgery: pathological curiosity or important clinical entity. Laryngoscope. 2003;113:1123–1127.
Wallace, ML, Neville, BW. Myospherulosis: report of a case. J Periodontol. 1990;61:55–57.