Pulpal and Periapical Disease
PULPITIS
The initial response of the dental pulp to injury is not significantly different from that seen in other tissues. However, the final result can be different because of the rigid dentinal walls of the pulp chamber. When external stimuli reach a noxious level, degranulation of mast cells, decreased nutrient flow, and cellular damage occur. Numerous inflammatory mediators (e.g., histamine, bradykinin, neurokinins, neuropeptides, prostaglandins) are released. These mediators cause vasodilation, increased blood inflow, and vascular leakage with edema. In normal tissue, increased blood flow promotes healing through removal of inflammatory mediators, and swelling of the injured tissue usually occurs. However, the dental pulp exists in a very confined area.
Pulpal response to noxious stimuli is meant to eliminate any invading organisms, remove cellular debris, and limit tissue damage. Paradoxically, the inflammatory reaction can lead to increased pulpal injury or even death of the pulp. Previous theories have suggested that the associated increased vascular pulpal pressures could compress venous return and lead to “self-strangulation” and pulpal necrosis. Today researchers recognize that the increased fluid pressure usually is localized to the area of inflamed pulp immediately adjacent to the affected dentin. Increased interstitial pressure in areas of inflammation leads to increased flow of fluid back into capillaries of adjacent uninflamed tissue and increased drainage. In this manner, the increased fluid pressure from inflammation is counteracted and typically does not lead to a generalized increase in pulpal fluid pressure, effectively preventing “self-strangulation.” Although many consider the dental pulp very fragile, the defense mechanisms work well the vast majority of time and rarely result in widespread necrosis. Localized pulpal abscesses often are able to heal after formation of reparative dentin and cessation of the noxious stimulus. In spite of numerous defense mechanisms, severe localized pulpal damage can overwhelm the system and spread progressively to the apical portion of the pulp, potentially producing widespread pulpal necrosis. In caries, whenever bacteria reach the tertiary dentin, the defense barriers have been breached, the degree of pulpitis will be severe, and the chance for pulpal recovery is minimal.
Four main types of noxious stimuli are common causes of pulpal inflammation:
1. Mechanical damage. Mechanical sources of injury include traumatic accidents, iatrogenic damage from dental procedures, attrition, abrasion, and barometric changes.
2. Thermal injury. Severe thermal stimuli can be transmitted through large uninsulated metallic restorations or may occur from such dental procedures as cavity preparation, polishing, and exothermic chemical reactions of dental materials.
3. Chemical irritation. Chemical-related damage can arise from erosion or from the inappropriate use of acidic dental materials.
4. Bacterial effects. Bacteria can damage the pulp through toxins or directly after extension from caries or transportation via the vasculature.
Pulpitis can be classified as the following:
The best classification system is one that guides the appropriate treatment. Reversible pulpitis denotes a level of pulpal inflammation in which the tissue is capable of returning to a normal state of health if the noxious stimuli are removed. Irreversible pulpitis implies that a higher level of inflammation has developed in which the dental pulp has been damaged beyond the point of recovery. Often, frank invasion by bacteria is the crossover point from reversible to irreversible pulpitis.
REVERSIBLE PULPITIS: When exposed to temperature extremes, teeth with reversible pulpitis exhibit a sudden mild-to-moderate pain (pulpalgia) of short duration. Although heat may initiate pain, the affected tooth responds most to cold stimuli (e.g., ice, beverages, cold air). Contact with sweet or sour foods and beverages also may cause pain. The pain does not occur without stimulation and subsides within seconds after the stimulus is removed. Typically, the tooth responds to electric pulp testing at lower levels of current than an appropriate control tooth. Mobility and sensitivity to percussion are absent. If the pulpitis is allowed to progress, then the duration of the pain on stimulation can become longer and the pulp may become affected irreversibly.
IRREVERSIBLE PULPITIS: Patients with early irreversible pulpitis generally have sharp, severe pain on thermal stimulation, and the pain continues after the stimulus is removed. Cold is especially uncomfortable, although heat or sweet and acidic foods also can elicit pain. In addition, the pain may be spontaneous or continuous and may be exacerbated when the patient lies down. The tooth responds to electric pulp testing at lower levels of current.
In the early stages of irreversible pulpitis, the pain often can be localized easily to the individual offend-ing tooth; with increasing discomfort, however, the patient is unable to identify the offending tooth within a quadrant.
In the later stages of irreversible pulpitis, the pain increases in intensity and is experienced as a throbbing pressure that can keep patients awake at night. At this point, heat increases the pain; however, cold may produce relief. The tooth responds to electric pulp testing at higher levels of current or demonstrates no response. Mobility and sensitivity to percussion are usually absent because significant inflammation has not spread yet to the apical area. If pulpal drainage occurs (e.g., crown fracture, fistula formation), then the symptoms may resolve—only to return if the drainage ceases.
The dramatic and painful cases of acute pulpitis are the ones that are recalled most easily by both patients and clinicians. In spite of this, the process may take years, the pattern of symptomatology is highly variable, and often the patient may have no symptoms. A number of large retrospective studies of patients presenting for endodontic therapy of teeth with radiographic evidence of periapical inflammatory disease have shown that in approximately half of these cases the associated pulpitis and necrosis were asymptomatic. Severe pulpitis with abscess formation and necrosis may be asymptomatic, whereas mild pulpitis may cause excruciating pain.
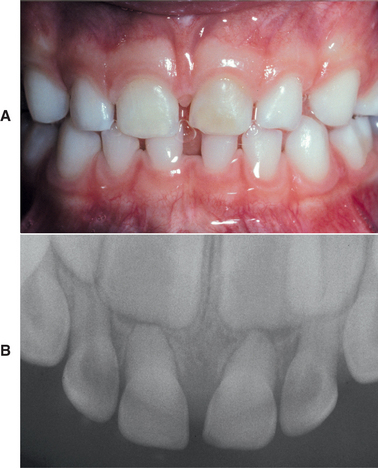
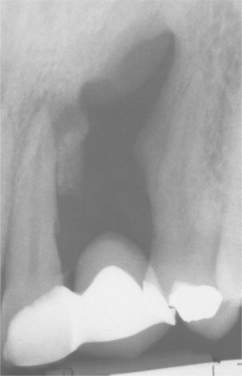
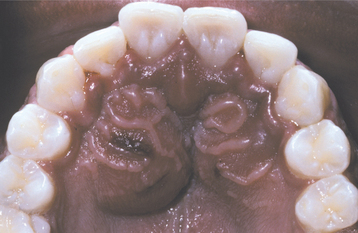
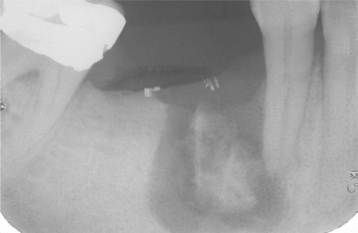
CHRONIC HYPERPLASTIC PULPITIS: One unique pattern of pulpal inflammation is chronic hyperplastic pulpitis (pulp polyp). This condition occurs in children and young adults who have large exposures of the pulp in which the entire dentinal roof often is missing. The most frequently involved teeth are the deciduous or succedaneous molars, which have large pulp chambers in these age groups. Mechanical irritation and bacterial invasion result in a level of chronic inflammation that produces hyperplastic granulation tissue that extrudes from the chamber and often fills the associated dentinal defect (Figs. 3-1 to 3-3). The apex may be open and reduces the chance of pulpal necrosis secondary to venous compression. The tooth is asymptomatic except for a possible feeling of pressure when it is placed into masticatory function.
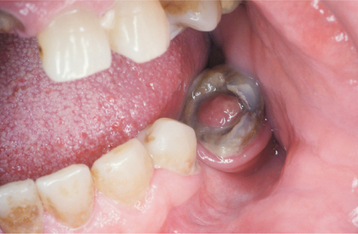
Fig. 3-1 Chronic hyperplastic pulpitis. Erythematous granulation tissue extruding from the pulp chamber of the mandibular first molar.
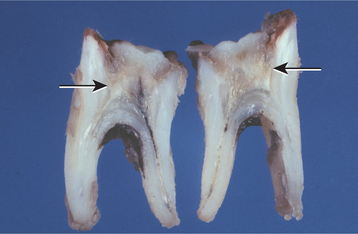
Fig. 3-2 Chronic hyperplastic pulpitis. Gross photograph demonstrating hyperplastic pulp tissue filling a large coronal carious defect. Arrows delineate the previous roof of the pulp chamber.
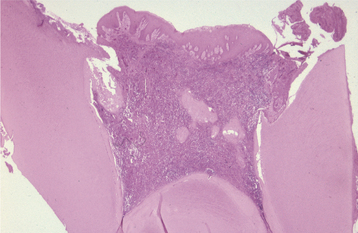
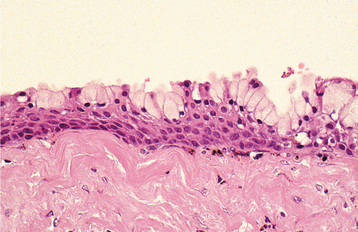
Fig. 3-3 Chronic hyperplastic pulpitis. Same tooth as depicted in Fig. 3-2. Chronically inflamed granulation tissue fills the coronal defect. Note surface stratified squamous epithelium.
Typically, the diagnosis of pulpitis is straightforward and easily correlated with a diseased tooth that can be stimulated to produce the associated symptoms. If such a correlation is not obvious, then it should raise suspicion that the symptoms may not be pulpally related. The tooth that is the source of pulpal pain may be difficult to identify in some instances. Although pulpal pain never crosses the midline, it can be referred from arch to arch, making pulp testing of both arches a necessity in difficult cases. Numerous disorders such as myofascial pain, trigeminal neuralgia, atypical facial neuralgia, migraine headaches, cluster headaches, nasal or sinus pathoses, and angina pectoris have been reported to mimic pulpalgia in some patients. If these conditions are not recognized as causing pain, then sequential extractions or endodontic procedures may be performed inappropriately.
The diagnosis of pulpalgia is made from a combination of the clinical presentation and the response of the teeth to percussion, thermal stimuli, and electric pulp testing. The predictive value of these tests is sometimes less than optimal. When the procedures demonstrate that the pulp is disease free, results are highly reliable. However, when a pulp appears to test positively for irreversible pulpitis, histopathologic examination may demonstrate no obvious evidence of pulpal disease. The practitioner should use all available tests, clinical information, and personal judgment in an attempt to arrive at an appropriate diagnosis. Future improvements in diagnostic methods, such as laser Doppler flowmetry and pulse oximetry devices, may help to increase accuracy.
HISTOPATHOLOGIC FEATURES: Basically, the histopathology is primarily of academic interest and does not usually affect treatment significantly. Numerous investigations have shown a surprising lack of correlation between histopathologic findings and the clinical symptoms in the majority of pulps examined.
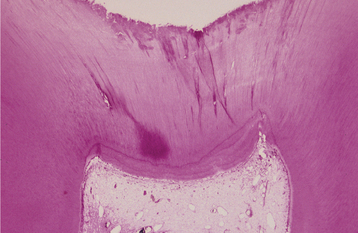
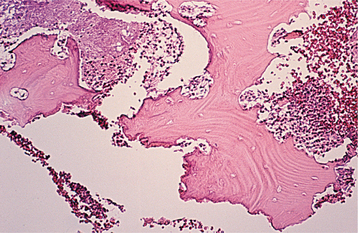
In patients with reversible pulpitis, the pulp usually shows hyperemia, edema, and a few inflammatory cells underlying the area of affected dentinal tubules (Fig. 3-4). Tertiary dentin may be noted in the adjacent dentinal wall, and scattered acute inflammatory cells are found occasionally.
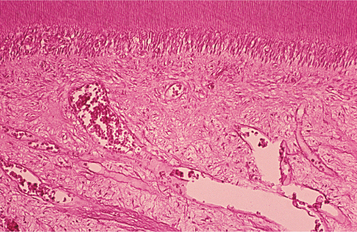
Fig. 3-4 Reversible pulpitis. Dental pulp exhibiting hyperemia and edema. The adjacent dentin was cut recently during placement of a dental restoration.
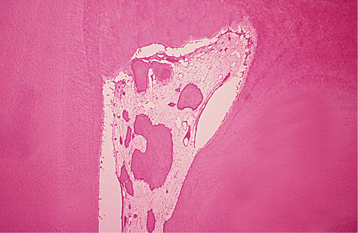
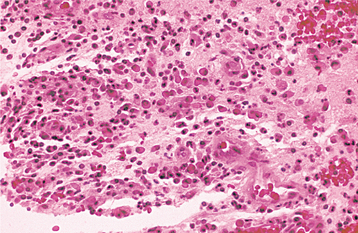
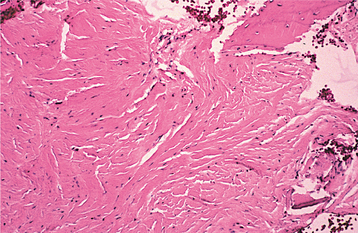
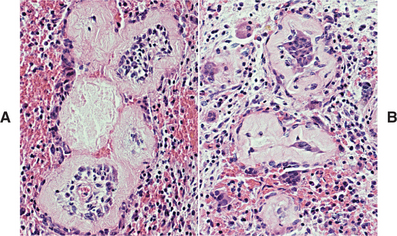
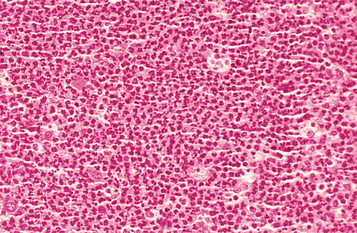
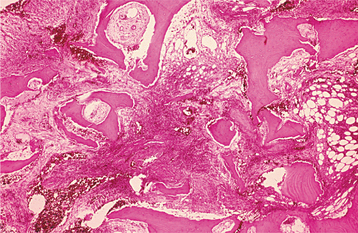
Irreversible pulpitis often demonstrates congestion of the venules that results in focal necrosis. This necrotic zone contains polymorphonuclear leukocytes and histiocytes (Fig. 3-5). The surrounding pulp tissue usually exhibits fibrosis and a mixture of plasma cells, lymphocytes, and histiocytes (Fig. 3-6).
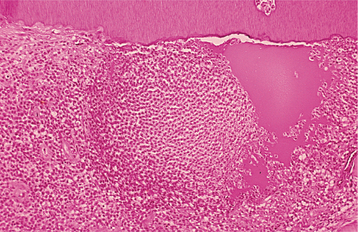
Fig. 3-5 Irreversible pulpitis. Dental pulp exhibiting acute inflammatory infiltrate consisting predominantly of polymorphonuclear leukocytes.
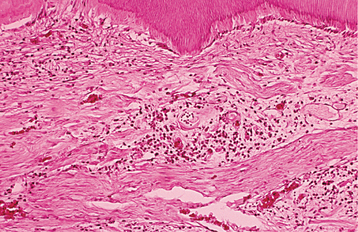
Fig. 3-6 Irreversible pulpitis. Same tooth as depicted in Fig. 3-5. The dental pulp exhibits an area of fibrosis and chronic inflammation peripheral to the zone of abscess formation.
Chronic hyperplastic pulpitis demonstrates a cap of subacutely inflamed granulation tissue that fills the entire space of the original pulp chamber and histopathologically resembles a pyogenic granuloma (see page 518). The surface of the polyp may or may not be covered with stratified squamous epithelium, which migrates from the adjacent gingiva or arises from sloughed epithelium within the oral fluids (see Fig. 3-3). The deeper pulp tissue within the canals typically demonstrates fibrosis and a chronic inflammatory infiltrate. Pulpal calcifications are common in both the radicular and coronal portions. Often the apical portion of the pulp tissue is normal, with minimal inflammation or fibrosis.
TREATMENT AND PROGNOSIS: Reversible pulpitis is treated by removal of the local irritant. On occasion, analgesic medications sometimes are desirable. The prognosis of reversible pulpitis is good if action is taken early enough. The tooth should be tested for vitality after the symptoms have subsided to ensure that irreversible damage has not occurred.
Irreversible and chronic hyperplastic pulpitis are treated by extraction of the tooth or by root canal therapy.
SECONDARY AND TERTIARY DENTIN
Formation of dentin proceeds throughout life. The dentin formed before completion of the crown is called primary dentin. This process is followed by the formation of secondary dentin. The same odontoblasts that formed the primary dentin remain functional and produce secondary dentin. With advancing age, deposition of secondary dentin leads to smaller pulp chambers and canal systems. The deposition of dentin is slow and gradual but does increase after the age of 35 to 40 years. Forensic scientists have shown that the formation of secondary dentin occurs so consistently that the width ratio of the dentin taken at three different root levels correlates very closely with age. Early widespread formation of secondary dentin has been seen in association with progeria, a condition associated with accelerated aging. On occasion, significant traumatic injury can lead to early obliteration of the pulp chamber and canal (calcific metamorphosis) in the affected tooth.
In functioning teeth, deposition begins in the coronal portions of the tooth and proceeds to the apical areas. Many investigators believe that this type of dentin, termed physiologic secondary dentin, occurs as a result of aging. A significantly decreased amount of secondary dentin has been described in impacted teeth, suggesting that functional forces of occlusion promote the deposition. Interestingly, the deposition in impacted teeth appears to begin in the apical areas and spreads coronally.
Although production of physiologic secondary dentin and a resultant decrease in pulpal size are related most strongly to aging, the process is more advanced in males and has been associated positively with calcification-related diseases (e.g., arthritis, gout, kidney stones, gall stones, atherosclerosis, hypertension). Deposition within the pulp chamber often is not totally uniform. In posterior teeth, the greatest deposition is seen on the pulpal floor, to a lesser extent on the roof, and least on the sidewalls. Therefore, with age, pulp chambers decrease significantly in height but not extensively in width.
Localized new dentin also is laid down in areas of focal injury. This dentin is more haphazardly organized and is termed tertiary (reactionary, reparative, irregular, or irritation) dentin. This localized dentin formation may occur in response to the following:
Injury of the peripheral odontoblastic processes is all that is required to initiate tertiary dentin formation. If the stimulus is mild to moderate, then the tertiary dentin typically is produced by surviving odontoblasts and is termed reactionary dentin. This type of tertiary dentin is more regular in appearance and continuous with the tubules of the primary and secondary dentin. If the stimulus is more severe and leads to the death of the primary odontoblasts, then a new generation of odontoblasts may arise from undifferentiated cells within the pulp and continue to form tertiary dentin that is termed reparative dentin. Researchers believe that these new odontoblasts arise from subodontoblastic cells or pericytes. During primary dentin formation, the odontoblasts incorporate a number of growth factors, such as transforming growth factor-b (TGF-b), into the intertubular matrix. Investigators have suggested that these growth factors may be released secondary to dentinal injury or cavity restoration and may be involved in the signaling for differentiation of the secondary generation of odontoblasts. Demineralization of dentin during caries also releases significant amounts of calcium and phosphates. These minerals often diffuse toward the pulp and assist in sclerosis of the tubules as calcium phosphate. In many ways, dentin not only acts as the backbone of the tooth but also as a storehouse of bioactive materials awaiting release during critical times of injury.
The initial layer of reparative dentin is atubular and known as interface dentin (fibrodentin). This thin band may be acellular or exhibit scattered nuclear inclusions. After deposition of the interface dentin, the remainder of the reparative dentin is tubular but not continuous with the primary, secondary, or reactionary dentin. This lack of communication further assists in protecting the pulp from the external stimulus. When the primary odontoblasts die, their dentinal tubules are filled with degenerated odontoblastic processes and are termed dead tracts. These tubules usually are sealed off from the pulp by the reparative dentin.
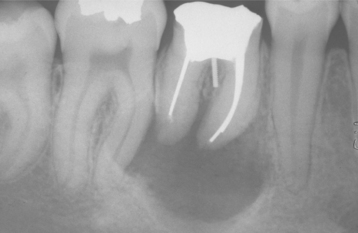
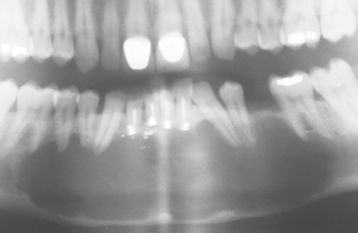
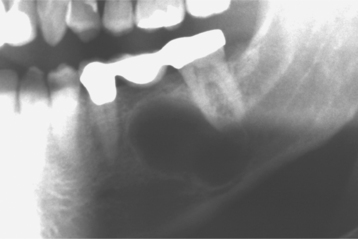
CLINICAL AND RADIOGRAPHIC FEATURES: As noted on periapical radiographs, the deposition of secondary dentin results in diminishing size of pulp chambers and canals. In addition to being used as an estimate of age, secondary dentin appears to reduce sensitivity of the affected teeth, susceptibility to dentinal caries, and the trauma of dental procedures. Although production of secondary dentin makes pulp exposure during operative procedures less likely, it also increases the difficulty of locating the pulp chamber and canals during endodontic therapy. On occasion, large inflammatory lesions may involve more than one apex; the size of the canals can be used to help determine the original focus of infection because the canal may be larger in the tooth that became nonvital earlier (Fig. 3-7). Teeth affected by calcific metamorphosis often are discovered clinically by a yellow discoloration of the crown; radiographically, the affected teeth exhibit an accelerated closure of the pulp chamber and canal when compared with adjacent or contralateral teeth (Fig. 3-8). In such cases the pulpal space may appear to be obliterated completely or reduced dramatically. This alteration usually follows trauma to the tooth and may be seen as early as 3 months after the traumatic episode; however, usually the condition is not detected for about 1 year.
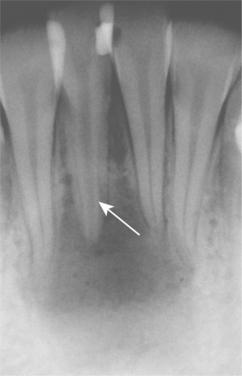
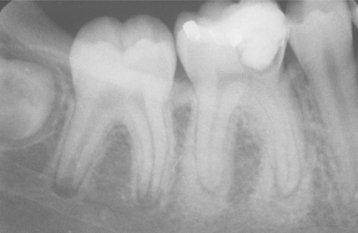
Fig. 3-7 Physiologic secondary dentin. Periapical abscess with all four teeth nonresponsive to electric pulp testing. Decreased deposition of physiologic secondary dentin on the right central incisor (arrow) delineated the origin of the infection; endodontic treatment of this tooth resolved the lesion.
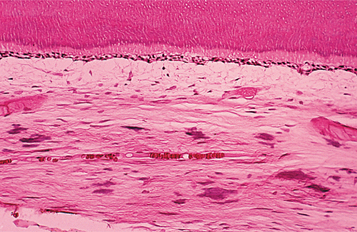
HISTOPATHOLOGIC FEATURES: Physiologic secondary dentin consists of regular tubular dentin that is applied onto the primary dentin. These two layers of dentin can be separated by a line of demarcation, often indicated by a bending of the tubules (Fig. 3-9). With advancing age, as the odontoblasts undergo degenerative changes, the physiologic secondary dentin becomes more irregular with fewer tubules.

Fig. 3-9 Physiologic secondary dentin. A distinct line of demarcation (arrow) separates the primary dentin and physiologic secondary dentin.
The quality and appearance of tertiary dentin depend on the severity of the noxious stimulus that promoted its formation. Tertiary dentin is localized to the pulpal end of the odontoblastic processes that were affected (Fig. 3-10). With a mild stimulus, such as abrasion or attrition, reactionary dentin exhibits slow deposition characterized by tubules that are continuous with the secondary dentin and only slightly irregular. With more severe damage (e.g., a rapidly progressing carious lesion), reparative dentin is formed, a process that occurs more rapidly and consists of a thin layer of interface dentin on which is deposited irregular dentin with widely scattered, disorganized tubules.
TREATMENT AND PROGNOSIS: In studies of teeth exhibiting calcific metamorphosis, the vast majority of affected teeth never develop clinical or radiographic features suggestive of periapical inflammatory disease; therefore, endodontic therapy should be performed only if periapical pathosis or negative vitality testing is present. Even if a canal space cannot be identified radiographically, conventional root canal therapy usually can locate and negotiate the pulp canal. Because of the dramatically reduced canal space, location of the pulp canal can be difficult, and care must be exercised during access preparation to prevent perforation. If endodontic therapy is unsuccessful, then periapical surgery can be performed in those cases with evidence of periapical inflammatory disease. If vitality testing is positive, then periodic reevaluation appears prudent. To improve dental aesthetics, full coverage is recommended for discolored anterior teeth with large restorations. Otherwise, bleaching often effectively resolves the discoloration.
PULPAL CALCIFICATIONS
Calcifications within the dental pulp are not rare, but the frequency is difficult to determine. Reported rates vary from 8% to 90%, but several investigators have documented a prevalence of approximately 20% in individual teeth reviewed radiographically. Because radiographically detectable pulp stones typically exceed 200 mm in diameter, the prevalence in a histopathologic review would be expected to be much higher. There appears to be a strong association between long-standing chronic pulpitis and the presence of pulpal calcification; in addition, the prevalence of pulpal calcifications increases with age. However, some examples appear to be developmental with a familial tendency.
The three types of pulpal calcifications are:
All pulpal calcifications start out as free bodies within the pulp tissue, but many may become attached or embedded in the dentinal walls of the pulp.
Denticles are believed to form as a result of an epitheliomesenchymal interaction within the developing pulp. Epithelial strands originating from the root sheath, or cervical extensions into the pulp chamber adjacent to furcations, induce odontoblastic differentiation of the surrounding mesenchyme of the dental papilla, forming the core of the denticle. Odontoblasts deposit tubular dentin as they move away from the central epithelium and produce thimble-shaped structures surrounding the epithelium. Denticles form during the period of root development and occur in the root canal and the pulp chamber adjacent to the furcation areas of multirooted teeth. Because denticle development typically precedes completion of the primary dentin, most denticles become attached to or embedded in the dentin.
Pulp stones are believed to develop around a central nidus of pulp tissue (e.g., collagen fibril, ground substance, necrotic cell remnants). Initial calcification begins around the central nidus and extends outward in a concentric or radial pattern of regular calcified material. Pulp stones are formed within the coronal portions of the pulp and may arise as a part of age-related or local pathologic changes. Most pulp stones develop after tooth formation is completed and are usually free or attached. In rare instances, stones may become embedded.
Diffuse linear calcifications do not demonstrate the lamellar organization of pulp stones; they exhibit areas of fine, fibrillar, irregular calcification that often parallel the vasculature. These calcifications may be present in the pulp chamber or canals, and the frequency increases with age.
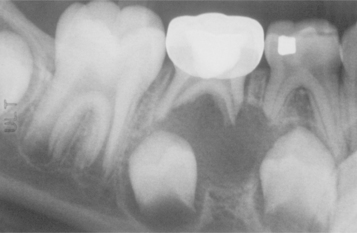
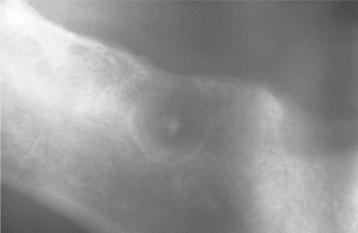
CLINICAL AND RADIOGRAPHIC FEATURES: Denticles and pulp stones can reach sufficient size to be detected on intraoral radiographs as radiopaque enlargements within the pulp chamber or canal (Fig. 3-11). Diffuse calcifications are not detectable radiographically.
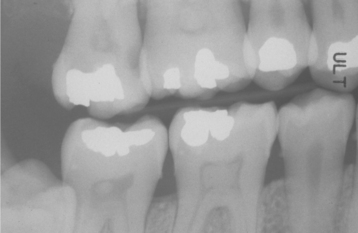
Fig. 3-11 Pulp stones. Multiple teeth demonstrating radiographically obvious calcifications within the pulp chambers.
Other than rare difficulties during endodontic procedures, pulpal calcifications are typically of little clinical significance. Some investigators associate the calcifications with dental neuralgias, but the high frequency of these lesions in the absence of clinical symptoms argues against this relationship. On occasion, the pulpal calcifications may become very large and may interfere with root formation, possibly leading to early periodontal destruction and tooth loss. Prominent pulpal calcifications have been noted in association with certain disease processes, such as the following:
• Dentin dysplasia type Id (see page 108)
• Dentin dysplasia type II (see page 108)
• Pulpal dysplasia (see page 110)
• Ehlers-Danlos syndrome (see page 755)
If all of the patient’s teeth have enlarged pulp chambers containing calcifications, the possibility of dentin dysplasia type II should be investigated. If the stones are located in the coronal portion of root canals that exhibit enlargement and bulging, then dentin dysplasia type 1d should be considered. Both of these rare conditions are associated with abnormally shaped pulp chambers or canals; therefore, such a diagnosis should not be considered when pulp stones are noted in the absence of pulpal changes.
HISTOPATHOLOGIC FEATURES: Denticles consist of tubular dentin surrounding a central nest of epithelium. With time, the central epithelium degenerates and the tubules undergo sclerosis, making their detection difficult. Most denticles are attached or embedded. Those that remain free in the pulp occasionally develop outer layers of irregular fibrillar calcification or lamellated layers of calcification similar to those seen in pulp stones.
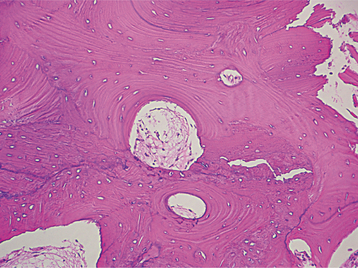
Pulp stones demonstrate a central amorphous mass of irregular calcification surrounded by concentric lamellar rings of regular calcified material (Fig. 3-12). Occasionally, a peripheral layer of tubular dentin may be applied by odontoblasts, which arise from the surrounding pulp tissue in response to the presence of the pulp stone. In addition, fibrillar irregular calcified material also may be evident on the periphery of pulp stones.
Diffuse linear calcifications consist entirely of fine, fibrillar, and irregular calcifications that develop in the pulp chambers and canals (Fig. 3-13). This material often is deposited in a linear fashion along the course of a blood vessel or nerve.
PERIAPICAL GRANULOMA (CHRONIC APICAL PERIODONTITIS)
The term periapical granuloma refers to a mass of chronically or subacutely inflamed granulation tissue at the apex of a nonvital tooth. This commonly used name is not totally accurate because the lesion does not show true granulomatous inflammation microscopically. Although the term apical periodontitis may be more appropriate, it may prove confusing to the clinician. Formation of apical inflammatory lesions represents a defensive reaction secondary to the presence of microbial infection in the root canal with spread of related toxic products into the apical zone. Initially, the defense reaction eliminates noxious substances that exit the canals. With time, however, the host reaction becomes less effective with microbial invasion or spread of toxins into the apical area. Although the infection typically is bacterial in origin, the presence of yeasts occasionally is demonstrated. Although controversial, an increased prevalence of human cytomegalovirus and, to a lesser extent, Epstein-Barr virus has been documented in symptomatic periapical inflammatory disease; some clinicians believe this to be more than a secondary infestation.
In the early stages of infection, neutrophils predominate and radiographic alterations are not present; this phase of periapical inflammatory disease is termed acute apical periodontitis. The involved inflammatory cells are primarily neutrophils and release prostaglandins, which activate osteoclasts to resorb the sur-rounding bone, leading to a detectable periapical radiolucency. Researchers believe that this bone destruction is an attempt to prevent the spread of the infection and provide space for the arrival of defense cells specialized against the infectious process. With time, chronic inflammatory cells begin to dominate the host response. Mediators released by lymphocytes reduce further osteoclastic activity while also stimulating fibroblasts and the microvasculature. Because of these actions, chronic lesions often are asymptomatic and demonstrate little additional change radiographically.
Periapical granulomas may arise after quiescence of a periapical abscess or may develop as the initial periapical pathosis. These lesions are not necessarily static. In addition to possible periapical cyst formation, a worsening of the pulpal infection can lead to a reappearance of inflammation, redevelopment of symptoms, and possible enlargement of the associated radiolucency. Secondary acute inflammatory changes within a periapical granuloma have been termed a phoenix abscess, after the mythical bird that would die, only to arise again from its own ashes. In progressive periapical granulomas, the enlargement often is not continuous but occurs in spurts associated with periodic acute exacerbations.
CLINICAL AND RADIOGRAPHIC FEATURES: The initial phase of periapical inflammatory disease—acute periapical periodontitis—creates a constant dull, throbbing pain. The associated tooth responds negatively to vitality testing or reveals a delayed positive result. Typically, pain on biting or percussion is present, and no obvious radiographic alterations are noted. If the acute inflammatory process evolves into a chronic pattern, then the associated symptoms diminish. In many instances, chronic periapical inflammatory disease is detected without any previous recollection of a prior acute phase.
Most periapical granulomas are asymptomatic, but pain and sensitivity can develop if acute exacerbation occurs. Typically, the involved tooth does not demonstrate mobility or significant sensitivity to percussion. The soft tissue overlying the apex may or may not be tender. The tooth does not respond to thermal or electric pulp tests unless the pulpal necrosis is limited to a single canal in a multirooted tooth. Periapical granulomas represent approximately 75% of apical inflammatory lesions and 50% of those that have failed to respond to conservative endodontic measures.
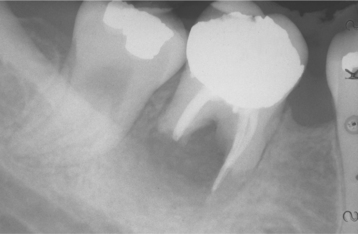
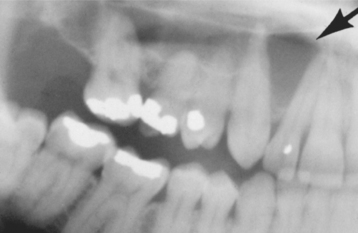
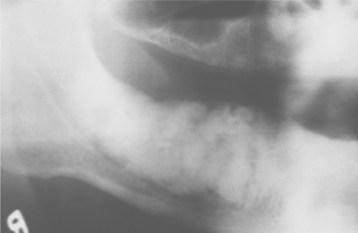
Most lesions are discovered on routine radiographic examination. The associated radiolucencies are variable, ranging from small, barely perceptible lesions to lucencies exceeding 2 cm in diameter (Figs. 3-14 to 3-16). Affected teeth typically reveal loss of the apical lamina dura. The lesion may be circumscribed or ill-defined and may or may not demonstrate a surrounding radiopaque rim. Root resorption is not uncommon (Fig. 3-17). Although lesions greater than 200 mm2 often represent periapical cysts, numerous investigators have been unable to distinguish periapical granulomas from periapical cysts simply on the basis of size and radiographic appearance. Because periapical inflammatory disease is not static and granulomas can transform into cysts or abscesses (and vice versa) without significant radiographic change, it is not surprising that the radiographic features are not diagnostic.
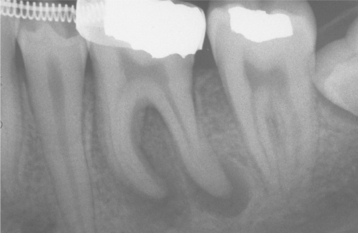
Fig. 3-14 Periapical granulomas. Discrete periapical radiolucencies associated with the apices of the mandibular first molar. (Courtesy of Dr. Garth Bobrowski.)
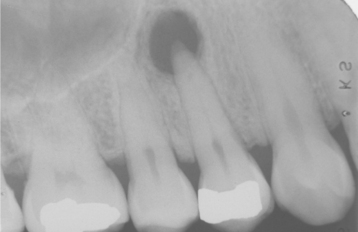
Fig. 3-15 Periapical granuloma. Well-defined radiolucency associated with the apex of the maxillary first bicuspid. (Courtesy of Dr. Frank Beylotte.)
HISTOPATHOLOGIC FEATURES: Periapical granulomas consist of inflamed granulation tissue surrounded by a fibrous connective tissue wall. The granulation tissue demonstrates a variably dense lymphocytic infiltrate that is intermixed frequently with neutrophils, plasma cells, histiocytes, and, less frequently, mast cells and eosinophils (Fig. 3-18). When numerous plasma cells are present, scattered eosinophilic globules of gamma globulin (Russell bodies) may be seen. In addition, clusters of lightly basophilic particles (pyronine bodies) also may be present in association with the plasmacytic infiltrate. Both of these plasma cell products are not specific for the periapical granuloma and may be found within any accumulation of plasma cells. Epithelial rests of Malassez may be identified within the granulation tissue. Collections of cholesterol clefts, with associated multinucleated giant cells and areas of red blood cell extravasation with hemosiderin pigmentation, may be present. Although the source of the cholesterol is unclear, this material often is noted in areas of long-term inflammation and may accumulate from dying inflammatory cells, disintegrating red blood cells, or degenerating cystic epithelium. The cholesterol attracts macro-phages and foreign body giant cells, which are unable to degrade the material but release inflammatory and bone resorptive mediators. Significant cholesterol accumulation can continue the inflammatory process in the absence of active microbial infection. Small foci of acute inflammation with focal abscess formation may be seen but do not warrant the diagnosis of periapical abscess.
TREATMENT AND PROGNOSIS: Apical inflammatory lesions result from the presence of microorganisms or their toxic products in the root canal, the apical tissues, or both. Successful treatment depends on the reduction and control of the offending organisms. Because of the anatomic complexity of the root canal systems, some investigators believe absolute eradication of all microorganisms is unlikely; the goal of endodontics is to reduce the microbial load to a level that is insufficient to maintain periapical inflammation. If the tooth can be maintained, then root canal therapy can be performed. Nonrestorable teeth must be ex-tracted, followed by curettage of all apical soft tissue. In symptomatic cases, nonsteroidal antiinflammatory drugs (NSAIDs) are beneficial; use of systemic antibiotic medications is not recommended unless associated swelling or systemic changes are present.
Teeth treated endodontically should be evaluated at 1- and 2-year intervals (at a minimum) to rule out possible lesional enlargement and to ensure appropriate healing. In addition, many clinicians believe that evaluations at 1, 3, and 6 months are appropriate. Strong emphasis should be placed on the importance of the recall appointments.
Lesions may fail to heal for several reasons:
• Persistent pulpal infection (e.g., poor access design, missed canals, perforated canals, vertical root fractures, inadequate aseptic technique or instrumentation, leaking fillings)
• Extraradicular infection (usually localized periapical actinomycotic colonization)
• Accumulation of endogenous debris (e.g., cholesterol crystals)
• Associated periodontal disease
If initial conventional therapy is unsuccessful, endodontic retreatment represents the best approach for minimizing the bacterial contamination and should be considered before periapical surgery. Periapical surgery remains an important tool for resolution of periapical inflammatory disease, but often it is reserved for lesions larger than 2 cm or those associated with teeth that are not appropriate for conventional endodontic therapy. Periapical surgery should include thorough curettage of all periradicular soft tissue, amputation of the apical portion of the root, and sealing the foramen of the canal.
All soft tissue removed during periapical surgical procedures should be submitted for histopathologic examination. These surgical sites represent areas that have failed to respond to appropriate therapy; as such, histopathologic examination and diagnostic confirmation are mandatory. The primary motivation for this examination is not to discover whether the lesion represents a periapical granuloma or cyst; the examination is conducted to eliminate the possibility of a more serious process unrelated to periapical inflammatory disease. In an active oral and maxillofacial pathology service, discovery of unexpected neoplasms within specimens removed during periapical surgery is not rare.
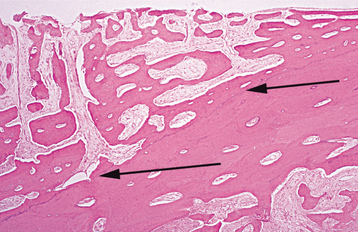
On occasion, the defect created by periapical inflammatory lesions may fill with dense collagenous tissue rather than normal bone (Fig. 3-19). These fibrous (periapical) scars occur most frequently when both the facial and lingual cortical plates have been lost (Fig. 3-20); however, they occasionally arise in areas with intact cortical plates. If during surgery both plates are discovered to be missing, then the patient should be informed of the possibility of scar formation. The development of a periapical scar is not an indication for future surgery.
PERIAPICAL CYST (RADICULAR CYST; APICAL PERIODONTAL CYST)
Epithelium at the apex of a nonvital tooth presumably can be stimulated by inflammation to form a true epithelium-lined cyst, or periapical cyst. The inflammatory response appears to increase the production of keratinocyte growth factor by periodontal stroma cells, leading to increased proliferation of normally quiescent epithelium in the area. The source of the epithelium is usually a rest of Malassez but also may be traced to crevicular epithelium, sinus lining, or epithelial lining of fistulous tracts. Cyst development is common; the reported frequency varies from 7% to 54% of periapical radiolucencies.
The wide disparity of prevalence most likely is related to the stringency of the diagnostic criteria used in a particular study. Several investigators believe the diagnosis of a periapical cyst can be made only after a lesion has been examined entirely with serial or step sectioning of the specimen. Review of random sections of a fragmented and epithelialized periapical granuloma could appear to be an epithelium-lined cavity that did not exist in reality. When strict criteria are used, the prevalence of periapical cysts appears to be approximately 15%. When the cyst and root are removed totally, two variations of periapical cyst have been described. Periapical pocket cysts are characterized by an incomplete epithelial lining because of extension of the apical portion of the tooth into the cyst lumen. Periapical true cysts form a complete epithelium-lined baglike structure that is adjacent to, but separated from, the tooth apex. Because distinguishing between an epithelialized periapical granuloma, a “pocket” cyst, or a “true” cyst has little postsurgical implications, laborious histopathologic examination and subclassification are impractical.
Periapical cysts represent a fibrous connective tissue wall lined by epithelium with a lumen containing fluid and cellular debris. Theoretically, as the epithelium desquamates into the lumen, the protein content is increased. Fluid enters the lumen in an attempt to equalize the osmotic pressure, and slow enlargement occurs. Most periapical cysts grow slowly and do not attain a large size.
On occasion, a similar cyst, best termed a lateral radicular cyst, may appear along the lateral aspect of the root. Like the periapical cyst, this lesion also usually arises from rests of Malassez, and the source of inflammation may be periodontal disease or pulpal necrosis with spread through a lateral foramen. Radiographically, these cysts mimic developmental lateral periodontal cysts (see page 692). Histopathologically, however, they are consistent with cysts of inflammatory origin.
Periapical inflammatory tissue that is not curetted at the time of tooth removal may give rise to an inflammatory cyst called a residual periapical cyst. With time, many of these cysts exhibit an overall reduction in size, and spontaneous resolution can occur from a lack of continued inflammatory stimulus.
CLINICAL AND RADIOGRAPHIC FEATURES:
PERIAPICAL CYST: Typically, patients with periapical cysts have no symptoms unless there is an acute inflammatory exacerbation. In addition, if the cyst reaches a large size, then swelling and mild sensitivity may be noted. Movement and mobility of adjacent teeth are possible as the cyst enlarges. The tooth from which the cyst originated does not respond to thermal and electric pulp testing.
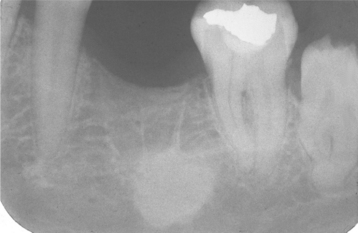
The radiographic pattern is identical to that of a periapical granuloma. Cysts may develop even in small periapical radiolucencies, and the radiographic size cannot be used for the definitive diagnosis. A loss of the lamina dura is seen along the adjacent root, and a rounded radiolucency encircles the affected tooth apex (Fig. 3-21). Root resorption is common (Fig. 3-22). With enlargement, the radiolucency often flattens out as it approaches adjacent teeth. Significant growth is possible, and lesions occupying an entire quadrant have been noted (Fig. 3-23). Although periapical cysts more frequently achieve greater size than periapical granulomas, neither the size nor the shape of the lesion can be considered a definitive diagnostic criterion. Periapical cysts also are known to involve deciduous teeth. These are most frequently associated with molar teeth and appear as a radiolucent zone that surrounds the roots and fills the interradicular space at the bifurcation (Fig. 3-24).
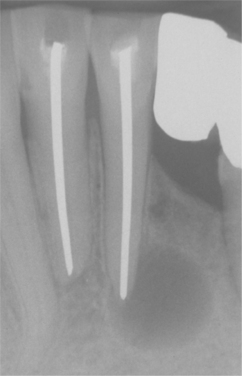
Fig. 3-21 Periapical cyst. Well-circumscribed radiolucency intimately associated with the apex of the mandibular central incisor. Note the loss of lamina dura in the area of the lesion.
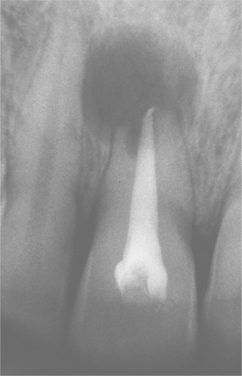
Fig. 3-22 Periapical cyst. Radiolucency associated with the maxillary central incisor, which exhibits significant root resorption.
LATERAL RADICULAR CYST: Lateral radicular cysts appear as discrete radiolucencies along the lateral aspect of the root (Fig. 3-25). Loss of lamina dura and an obvious source of inflammation may not be detected without a high index of suspicion. Before surgical exploration of laterally positioned radiolucencies, a thorough evaluation of the periodontal status and vitality of adjacent teeth should be performed. Many examples of the so-called globulomaxillary cyst (see page 28) prove to be of inflam-matory origin and represent lateral radicular cysts (Fig. 3-26).
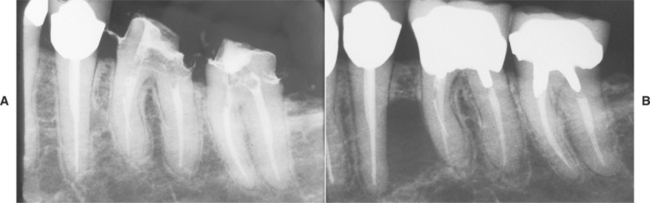
Fig. 3-25 Lateral radicular cyst. A, Periapical radiograph of the left side of the posterior mandible taken at time of completion of endodontic therapy of the bicuspid and molars. B, Subsequent radiograph taken 27 months later. Note radiolucency between bicuspid and first molar extending laterally from the mesial root of the first molar. (Courtesy of Dr. Carroll Gallagher.)
RESIDUAL PERIAPICAL CYST: The residual periapical cyst appears as a round-to-oval radiolucency of variable size within the alveolar ridge at the site of a previous tooth extraction (Figs. 3-27 and 3-28). As the cyst ages, degeneration of the cellular contents within the lumen occasionally leads to dystrophic calcification and central luminal radiopacity (Fig. 3-29).
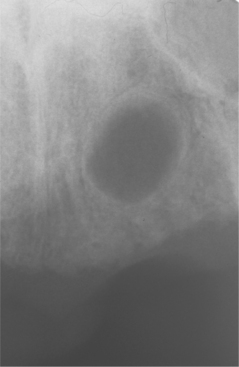
Fig. 3-27 Residual periapical cyst. Persistent radiolucency of the mandibular body at the site of previous tooth extraction.
HISTOPATHOLOGIC FEATURES: The histopathologic features of all three types of inflammatory cysts are similar. The cyst is lined by stratified squamous epithelium, which may demonstrate exocytosis, spongiosis, or hyperplasia (Fig. 3-30). As seen in dentigerous cysts, scattered mucous cells or areas of ciliated pseudostratified columnar epithelium may be noted in periapical cysts (Fig. 3-31). Although some maxillary periapical cysts lined by pseudostratified columnar epithelium may have originated from the adjacent sinus lining, the presence of mucous cells or respiratory-like epithelium also can be observed in mandibular cysts. The ability of odontogenic epithelium to demonstrate such specialized differentiation represents an example of prosoplasia (forward metaplasia) and highlights the diverse potential of odontogenic epithelium. The cyst lumen may be filled with fluid and cellular debris. On occasion, the lining epithelium may demonstrate linear or arch-shaped calcifications known as Rushton bodies (Fig. 3-32). Dystrophic calcification, cholesterol clefts with multinucleated giant cells, red blood cells, and areas of hemosiderin pigmentation may be present in the lumen, wall, or both. The wall of the cyst consists of dense fibrous connective tissue, often with an inflammatory infiltrate containing lymphocytes variably intermixed with neutrophils, plasma cells, histiocytes, and (rarely) mast cells and eosinophils.
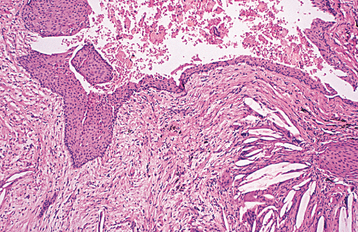
Fig. 3-30 Periapical cyst. Cyst lined by stratified squamous epithelium. Note connective tissue wall, which contains a chronic inflammatory infiltrate and numerous cholesterol clefts.
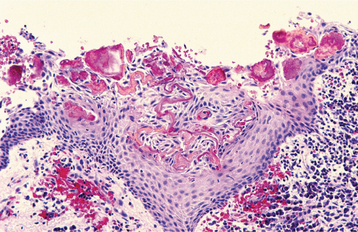
Fig. 3-32 Periapical cyst. Squamous epithelial cyst lining exhibiting numerous irregular and curvilinear Rushton bodies.
Occasionally, the walls of inflammatory cysts will contain scattered hyaline bodies (pulse granuloma, giant-cell hyaline angiopathy). These bodies appear as small circumscribed pools of eosinophilic material that exhibits a corrugated periphery of condensed collagen often surrounded by lymphocytes and multinucleated giant cells (Fig. 3-33). The eosinophilic material may be uniform or contain a variable mixture of lymphocytes, plasma cells, multinucleated giant cells, neutrophils, necrotic debris, and dystrophic calcification. Initially, these foci were thought to be a vascular degenerative process or a foreign body reaction to machinery oil or vegetable matter. Subsequently, these bodies have been shown to represent pools of inflammatory exudate (i.e., extravasated serum) that ultimately undergoes fibrosis and occasionally dystrophic calcification. The multinucleated giant cells are drawn to the site for removal of insoluble hemosiderin granules. Hyaline bodies may be found in any area of chronic intraosseous inflammation, especially periapical inflammatory disease.
TREATMENT AND PROGNOSIS: A periapical cyst is treated in the same manner as a periapical granuloma. When clinical and radiographic features indicate a periapical inflammatory lesion, extraction or conservative nonsurgical endodontic therapy is performed. Although some authors believe that large cystic lesions cannot be resolved with conventional endodontics, experienced clinicians have successfully used nonsurgical root canal therapy for large areas of periapical inflammatory disease that approach 2 cm in diameter. Larger lesions associated with restorable teeth have been treated successfully with conservative endodontic therapy when combined with biopsy and marsupialization, decompression, or fenestration. As with any periapical inflammatory lesion, minimal follow-up at 1 and 2 years is advised strongly.
If the radiolucency fails to resolve, then the lesion often can be managed successfully by nonsurgical endodontic retreatment. As previously mentioned, periapical surgery typically is performed for lesions exceeding 2 cm and those associated with teeth that are not suitable for conventional endodontics. Biopsy is indicated to rule out other possible pathologic processes.
Because any number of odontogenic and nonodontogenic cysts and tumors can mimic the appearance of a residual periapical cyst, all of these cysts should be excised surgically. All inflammatory foci in the area of a lateral radicular cyst should be eliminated and the patient observed in a manner similar to that described for the periapical cyst. In some instances, lateral radicular cysts are removed before tooth vitality testing or periodontal evaluation for an adjacent focus of infection. If this diagnosis is made, then a thorough evaluation for an inflammatory source is mandatory.
Cysts of inflammatory origin do not recur after appropriate management. Fibrous scars are possible, especially when both cortical plates have been lost; once diagnosed, no further therapy for fibrous scars is indicated. In rare instances, development of squamous cell carcinoma has been reported within periapical cysts; therefore, even in the absence of symptoms, treatment is required for all persistent intrabony pathoses that have not been diagnosed definitively by histopathologic examination.
PERIAPICAL ABSCESS
The accumulation of acute inflammatory cells at the apex of a nonvital tooth is termed a periapical abscess. Acute inflammatory lesions with abscess formation may arise as the initial periapical pathosis or from an acute exacerbation of a chronic periapical inflammatory lesion (see discussion of phoenix abscess, page 128). Frequently, the source of the infection is obvious. On occasion, however, pulpal death may be trauma related, and the tooth may contain neither a cavity nor a restoration.
In the earliest stage of all forms of periapical inflammatory disease, the periapical periodontal ligament (PDL) fibers may exhibit acute inflammation but no frank abscess formation. This localized alteration, best termed acute apical periodontitis, may or may not proceed to abscess formation. Although this process often occurs in association with a nonvital tooth, acute apical periodontitis may be found in vital teeth secondary to trauma, high occlusal contacts, or wedging by a foreign object. The clinical presentation often closely resembles that of a periapical abscess and must be considered in the differential diagnosis.
CLINICAL AND RADIOGRAPHIC FEATURES: Many investigators subdivide periapical abscesses into acute and chronic types. However, these are misnomers because both types represent acute inflammatory reactions. Periapical abscesses should be designated as symptomatic or asymptomatic on the basis of their clinical presentations.
Periapical abscesses become symptomatic as the purulent material accumulates within the alveolus. The initial stages produce tenderness of the affected tooth that often is relieved by direct application of pressure. With progression, the pain becomes more intense, often with extreme sensitivity to percussion, extrusion of the tooth, and swelling of the tissues. The offending tooth does not respond to cold or electric pulp testing. Headache, malaise, fever, and chills may be present.
Radiographically, abscesses may demonstrate a thickening of the apical periodontal ligament, an ill-defined radiolucency, or both; however, often no appreciable alterations can be detected because insufficient time has occurred for significant bone destruction. Phoenix abscesses demonstrate the outline of the original chronic lesion, with or without an associated ill-defined bone loss.
With progression, the abscess spreads along the path of least resistance. The purulence may extend through the medullary spaces away from the apical area, resulting in osteomyelitis, or it may perforate the cortex and spread diffusely through the overlying soft tissue (as cellulitis). Each of these occurrences is described later in the chapter.
Once an abscess is in soft tissue, it can cause cellulitis or may channelize through the overlying soft tissue. The cortical plate may be perforated in a location that permits entrance into the oral cavity. The purulent material can accumulate in the connective tissue overlying the bone and can create a sessile swelling or perforate through the surface epithelium and drain through an intraoral sinus (Figs. 3-34 and 3-35). At the intraoral opening of a sinus tract, a mass of subacutely inflamed granulation tissue often is found, known as a parulis (gum boil) (Figs. 3-36 and 3-37). Occasionally, the nonvital tooth associated with the parulis may be difficult to determine, and insertion of a gutta-percha point into the tract can aid in detection of the offending tooth during radiographic examination (Fig. 3-38). Dental abscesses also may channelize through the overlying skin and drain via a cutaneous sinus (Fig. 3-39).
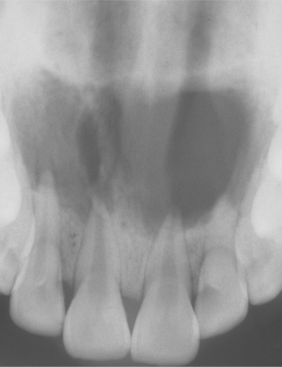
Fig. 3-35 Periapical abscess. Same patient as depicted in Fig. 3-34. Multiple, overlapping radiolucencies of the anterior maxilla are present. All four maxillary incisors exhibit pulpal necrosis.
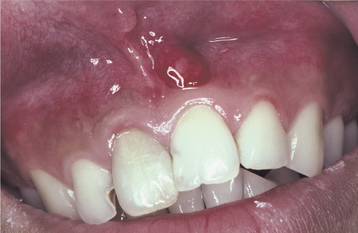
Fig. 3-36 Parulis. Erythematous mass of granulation tissue overlying the left maxillary central incisor. Note discoloration of the maxillary right central incisor.
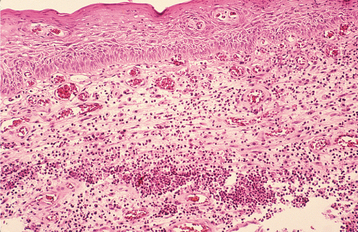
Fig. 3-37 Parulis. Normal connective tissue has been replaced by acutely inflamed granulation tissue, which exhibits focal areas of neutrophilic abscess formation. Note the central sinus tract, which courses from the base of the specimen toward the surface epithelium.
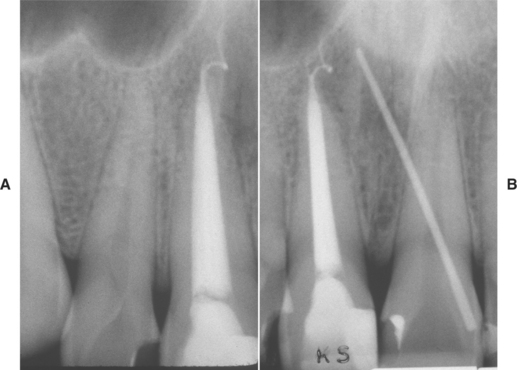
Fig. 3-38 Periapical abscess. A, Same patient as depicted in Fig. 3-36. None of the incisors demonstrates an obvious periapical radiolucency. (The large radiolucency at the top is the anterior portion of the maxillary sinus.) B, Gutta-percha point revealed that the right maxillary incisor was the source of the infection.
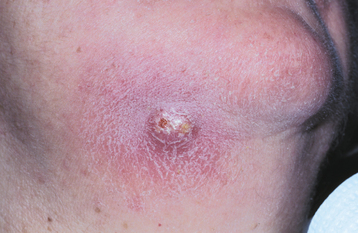
Fig. 3-39 Cutaneous sinus. Erythematous, firm, and sensitive enlargement of the skin inferior to the right body of the mandible.
Most dental-related abscesses perforate buccally because the bone is thinner on the buccal surface. However, infections associated with maxillary lateral incisors, the palatal roots of maxillary molars, and mandibular second and third molars typically drain through the lingual cortical plate.
If a chronic path of drainage is achieved, a periapical abscess typically becomes asymptomatic because of a lack of accumulation of purulent material within the alveolus. Occasionally, such infections are discovered during a routine oral examination after detection of a parulis or drainage through a large carious defect (Figs. 3-40 and 3-41). If the drainage site becomes blocked, then signs and symptoms of the abscess frequently become evident in a short time. On occasion, periapical infections can spread through the bloodstream and result in systemic symptoms such as fever, lymphadenopathy, and malaise. The risk of dissemination appears to be less for periapical abscesses that drain freely.
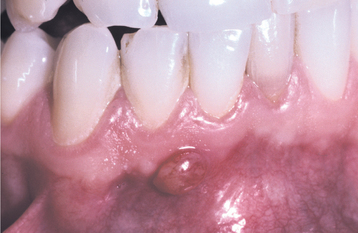
Fig. 3-40 Parulis. Asymptomatic yellow-red nodule of the anterior mandibular alveolar mucosa. The adjacent teeth were asymptomatic and appeared clinically normal.
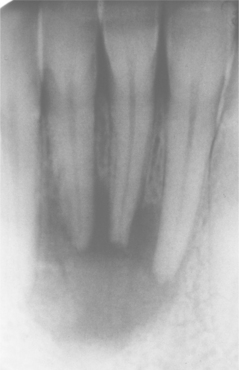
Fig. 3-41 Periapical abscess. Same patient as depicted in Fig. 3-40. Periapical radiolucency associated with the nonvital mandibular lateral incisor.
HISTOPATHOLOGIC FEATURES: Biopsy specimens from pure abscesses are uncommon because the material is in liquid form. Abscesses consist of a sea of polymorphonuclear leukocytes often intermixed with inflammatory exudate, cellular debris, necrotic material, bacterial colonies, or histiocytes (Fig. 3-42). Phoenix abscesses can maintain a soft tissue component; they present as subacutely inflamed periapical granulomas or cysts intermixed with areas of significant abscess formation. In these cases the pathologist typically diagnoses the primary lesion but comments about the abscess formation.
TREATMENT AND PROGNOSIS: Treatment of the patient with a periapical abscess consists of drainage and elimination of the focus of infection. Those abscesses associated with a patent fistulous tract may be asymptomatic but, nevertheless, should be treated. With localized periapical abscesses, the signs and symptoms typically diminish significantly within 48 hours of initiation of appropriate drainage. When the abscess causes clinical expansion of the bone or soft tissue adjacent to the apex of the affected tooth, incisional drainage of the swelling should be considered because this technique appears to be associated with more rapid resolution of the inflammatory process when compared with drainage through the root canal. If the affected tooth is extruded, then reduction of the occlusion is recommended because chronic occlusal trauma has been shown to delay resolution of the inflammatory process. Unless contraindicated, treatment with NSAIDs usually is appropriate preoperatively, immediately postoperatively, and for subsequent pain control. Typically, use of antibiotic medications for a well-localized and easily drained periapical abscess in a healthy patient is unnecessary. Antibiotic coverage should be reserved for the medically compromised and patients with significant cellulitis or clinical evidence of dissemination (i.e., fever, lymphadenopathy, malaise). Medical conditions that favor more widespread infection include diabetes mellitus, neutropenia, malignancy, immunosuppression, or use of therapeutic corticosteroid medications or cytotoxic drugs. Patients with significant cellulitis must be treated aggressively and monitored closely. Complications, such as cavernous sinus thrombosis, mediastinitis, cervical necrotizing fasciitis, and cerebral abscess, can be life threatening. Once the infection has been resolved by extraction or appropriate endodontic therapy, the affected bone typically heals.
Usually, a sinus tract resolves spontaneously after the offending tooth is extracted or endodontically treated. Sinus tracts that persist are thought to contain sufficient infectious material along the fistulous tract to maintain the surface granulation tissue, and surgical removal with curettage of the tract is required for resolution.
CELLULITIS
If an abscess is not able to establish drainage through the surface of the skin or into the oral cavity, it may spread diffusely through fascial planes of the soft tissue. This acute and edematous spread of an acute inflammatory process is termed cellulitis. Although numerous patterns of cellulitis can be seen from the spread of dental infections, two especially dangerous forms warrant further discussion: (1) Ludwig’s angina and (2) cavernous sinus thrombosis.
Ludwig’s angina, named after the German physician who described the seriousness of the disorder in 1836, refers to cellulitis of the submandibular region. Angina comes from the Latin word angere, which means to strangle (an apt term, considering the clinical features described in the following section). In approximately 70% of cases, Ludwig’s angina develops from spread of an acute infection from the lower molar teeth. Other situations associated with this clinical presentation are peritonsillar or parapharyngeal abscesses, oral lacerations, fractures of the mandible, or submandibular sialadenitis. Although the process may occur in otherwise healthy individuals, there is an increased prevalence in patients who are immunocompromised secondary to disorders such as diabetes mellitus, organ transplantation, acquired immunodeficiency syndrome (AIDS), and aplastic anemia.
The cavernous sinus is a major dural sinus that is encased between the meningeal and periosteal layers of the dura. The meningeal layer contains the trochlear and oculomotor nerves and the maxillary and ophthalmic branches of the trigeminal nerve. In addition, the internal carotid artery and abducens nerve travel within the sinus. The sinus receives venous drainage from the orbit via the superior and inferior ophthalmic veins. Infection of the sinus can produce a variety of clinical symptoms related to the numerous anatomic structures that course through this site.
Cavernous sinus thrombosis can occur via an anterior or posterior pathway. Infection from the maxillary anterior teeth can perforate the facial maxillary bone and spread to the canine space. A septic thrombus develops in the valveless facial veins coursing through this space, propagating in a retrograde fashion from the angular vein to the inferior ophthalmic vein through the inferior orbital fissure into the cavernous sinus. The posterior pathway is followed by infections originating from maxillary premolar or molar teeth, which demonstrate buccal or infratemporal space involvement that may spread via the emissary veins from the pterygoid venous plexus to the inferior petrosal sinus and into the cavernous sinus. Overall, cavernous sinus thrombosis is relatively uncommon, and orodental infections are responsible in approximately 10% of the cases.
LUDWIG’S ANGINA: Ludwig’s angina is an aggressive and rapidly spreading cellulitis that involves the sublingual, submandibular, and submental spaces. Once the infection enters the submandibular space, it may extend to the lateral pharyngeal space and then to the retropharyngeal space. This extension may result in spread to the mediastinum, with several serious consequences.
Ludwig’s angina creates massive swelling of the neck that often extends close to the clavicles (Fig. 3-43). Involvement of the sublingual space results in elevation, posterior enlargement, and protrusion of the tongue (woody tongue), which can compromise the airway. Submandibular space spread causes enlargement and tenderness of the neck above the level of the hyoid bone (bull neck). Although initially unilateral, spread to the contralateral neck typically occurs. Pain in the neck and floor of mouth may be seen in addition to restricted neck movement, dysphagia, dysphonia, dysarthria, drooling, and sore throat. Involvement of the lateral pharyngeal space can cause respiratory obstruction secondary to laryngeal edema. Tachypnea, dyspnea, tachycardia, stridor, restlessness, and the patient’s need to maintain an erect position suggest airway obstruction. Fever, chills, leukocytosis, and an elevated sedimentation rate may be seen. Classically, obvious collections of pus are not present.
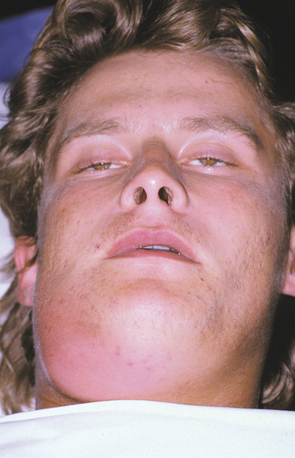
CAVERNOUS SINUS THROMBOSIS: Cavernous sinus thrombosis appears as an edematous periorbital enlargement with involvement of the eyelids and conjunctiva. In cases involving the canine space, swelling is also typically present along the lateral border of the nose and may extend up to the medial aspect of the eye and periorbital area (Fig. 3-44). Protrusion and fixation of the eyeball often are evident, in addition to induration and swelling of the adjacent forehead and nose. Pupil dilation, lacrimation, photophobia, and loss of vision may occur. Pain over the eye and along the distribution of the ophthalmic and maxillary branches of the trigeminal nerve often are present. Proptosis, chemosis, and ptosis are noted in greater than 90% of affected patients. The cavernous sinuses freely communicate via the intercavernous sinus. Although many cases are initially unilateral, without appropriate therapy, the infection may spread to the contralateral side.
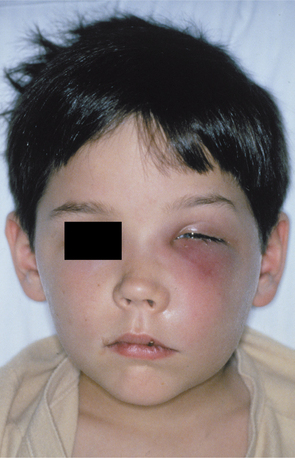
Fig. 3-44 Cellulitis involving canine space. Erythematous and edematous enlargement of the left side of the face with involvement of the eyelids and conjunctiva. Patients with odontogenic infections involving the canine space are at risk for cavernous sinus thrombosis. (Courtesy of Dr. Richard Ziegler.)
Fever, chills, headache, sweating, tachycardia, nausea, and vomiting can occur. With progression, signs of central nervous system (CNS) involvement develop. Meningitis, tachycardia, tachypnea, irregular breathing, stiffening of the neck, and deepening stupor with or without delirium indicate advanced toxemia and meningeal involvement. Occasionally, brain abscesses may result.
LUDWIG’S ANGINA: Treatment of Ludwig’s angina centers around four activities:
Of primary importance is management of an intact airway. On initial observation, many clinicians administer systemic corticosteroid medications, such as intravenous (IV) dexamethasone, in an attempt to reduce the cellulitis. This procedure often protects the airway and allows more rapid penetration of antibiotic medications in the infected fascial spaces. Such therapy significantly reduces the need for an artificial airway; in the majority of the cases, tracheotomy or intubation is not required.
If signs or symptoms of impending airway obstruction develop, fiber-optic nasotracheal intubation or tracheostomy should be performed. Orotracheal intubation often is very difficult because of the presence of trismus and swollen soft tissues. Because intubation is difficult in patients with such massive neck enlargement and may cause laryngospasm or discharge of pus into the bronchial tree, tracheostomy is preferred if there is any chance of significant intubation complications. On occasion, cricothyroidotomy is performed instead of a tracheostomy because of a perceived lower risk of spreading the infection to the mediastinum.
High-dose penicillin is the antibiotic of choice. Aminoglycoside therapy is given for resistant organisms, and clindamycin or chloramphenicol is used in penicillin-sensitive patients. The antibiotic medication is adjusted according to the patient’s response and culture results from aspirates of fluid from the enlargements.
Although large accumulations of purulent material are rare, decompression of the sublingual, submental, and submandibular spaces should be performed when fluctuance is present. If the infection remains diffuse, indurated, and brawny, then surgical intervention is at the discretion of the clinician and often is governed by the patient’s response to noninvasive therapy. Computed tomography (CT) of the neck and chest is recommended for patients with extensive cervical infection to rule out spread into the mediastinum.
Before the use of modern antibiotic medications, the mortality from Ludwig’s angina often exceeded 50%. Although this rate has been reduced to less than 10%, deaths still occur as the result of complications such as pericarditis, pneumonia, mediastinitis, sepsis, empyema, and respiratory obstruction.
CAVERNOUS SINUS THROMBOSIS: The therapeutic cornerstones for cavernous sinus thrombosis secondary to dental infections are surgical drainage combined with high-dose antibiotic medications similar to those administered for patients with Ludwig’s angina. The offending tooth should be extracted, and drainage is required if fluctuance is present. Administration of systemic corticosteroid drugs is indicated only in patients who have developed pituitary insufficiency in advanced cases of cavernous sinus thrombosis. Some investigators also prescribe anticoagulant medications to prevent thrombosis and septic emboli; conversely, others believe that thrombosis limits the infection and that the use of anticoagulant drugs may promote hemorrhagic lesions in the orbit and brain.
In older series the mortality rate approached 75%. Even with current medical advances and modern antibiotic medications, the mortality rate remains at approximately 30%, with fewer than 40% of patients achieving full recovery.
OSTEOMYELITIS
Osteomyelitis is an acute or chronic inflammatory process in the medullary spaces or cortical surfaces of bone that extends away from the initial site of involvement. The term osteomyelitis has been used to encompass a wide variety of pathoses. This section describes the classic pattern of osteomyelitis. The vast majority of osteomyelitis cases are caused by bacterial infections and result in an expanding lytic destruction of the involved bone, with suppuration and sequestra formation. Many believe that this condition is more appropriately termed suppurative osteomyelitis, bacterial osteomyelitis, or secondary osteomyelitis. This pattern of osseous pathosis is in contrast to an ill-defined group of idiopathic inflammatory disorders of bone that do not respond consistently to antibacterial medications and typically demonstrate ultimate sclerosis of bone without suppuration or sequestra formation. This second pattern of inflammatory bone disease is most appropriately termed primary chronic osteomyelitis but often is included under the term diffuse sclerosing osteomyelitis. This disorder and several other patterns of inflammatory bone disease (e.g., focal sclerosing osteomyelitis, proliferative periostitis, alveolar osteitis) are unique and are covered separately later in the chapter. Osteoradionecrosis is excluded from this discussion because this is primarily a problem of hypoxia, hypocellularity, and hypovascularity in which the presence of bacteria represents a secondary colonization of nonhealing bone rather than a primary bacterial infection (see page 296). In addition, bisphosphonate-associated osteonecrosis represents another unique pattern that is discussed in a later chapter and appears more strongly related to altered bone metabolism (see page 299).
Suppurative osteomyelitis of the jaws is uncommon in developed countries, but it continues to be a source of significant difficulty in developing nations. In Europe and North America, most cases arise after odontogenic infections or traumatic fracture of the jaws. In addition, many cases reported in Africa occur in the presence of acute necrotizing ulcerative gingivitis (ANUG) or noma.
Chronic systemic diseases, immunocompromised status, and disorders associated with decreased vascularity of bone appear to predispose people to osteomyelitis. Tobacco use, alcohol abuse, IV drug abuse, diabetes mellitus, exanthematous fevers, malaria, sickle cell anemia, malnutrition, malignancy, collagen vascular diseases, and AIDS have been associated with an increased frequency of osteomyelitis. In addition to radiation, several diseases (e.g., osteopetrosis, dysosteosclerosis, late Paget’s disease, end-stage cemento-osseous dysplasia) may result in hypovascularized bone that is predisposed to necrosis and inflammation.
Acute suppurative osteomyelitis exists when an acute inflammatory process spreads through the medullary spaces of the bone and insufficient time has passed for the body to react to the presence of the inflammatory infiltrate. Chronic suppurative osteomyelitis exists when the defensive response leads to the production of granulation tissue, which subsequently forms dense scar tissue in an attempt to wall off the infected area. The encircled dead space acts as a reservoir for bacteria, and antibiotic medications have great difficulty reaching the site. This pattern begins to evolve about 1 month after the spread of the initial acute infection and results in a smoldering process that is difficult to manage unless the problem is approached aggressively.
CLINICAL AND RADIOGRAPHIC FEATURES: Patients of all ages can be affected by osteomyelitis. There is a strong male predominance, approaching 75% in some reviews. Most cases involve the mandible. Maxillary disease becomes important primarily in pediatric patients and in cases that arise from ANUG or noma (in African populations).
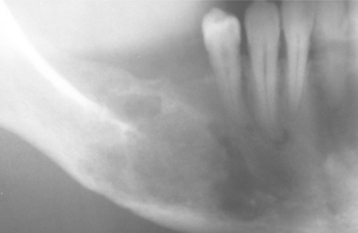
ACUTE SUPPURATIVE OSTEOMYELITIS: Patients with acute osteomyelitis have signs and symptoms of an acute inflammatory process that has typically been less than 1 month in duration. Fever, leukocytosis, lymphadenopathy, significant sensitivity, and soft tissue swelling of the affected area may be present. The radiographs may be unremarkable or may demonstrate an ill-defined radiolucency (Fig. 3-45). Periosteal new bone formation also may be seen in response to subperiosteal spread of the infection. This proliferation is more common in young patients and presents as a single-layered linear radiopaque line separated from the normal cortex by an intervening radiolucent band. On occasion, paresthesia of the lower lip, drainage, or exfoliation of fragments of necrotic bone may be discovered. A fragment of necrotic bone that has separated from the adjacent vital bone is termed a sequestrum. Sequestra often exhibit spontaneous exfoliation (Fig. 3-46). On occasion, fragments of necrotic bone may become surrounded by new vital bone, known as an involucrum.
CHRONIC SUPPURATIVE OSTEOMYELITIS: If acute osteomyelitis is not resolved expeditiously, the entrenchment of chronic osteomyelitis occurs, or the process may arise primarily without a previous acute episode. Swelling, pain, sinus formation, purulent discharge, sequestrum formation, tooth loss, or pathologic fracture may occur. Patients may experience acute exacerbations or periods of decreased pain associated with chronic smoldering progression (Fig. 3-47).
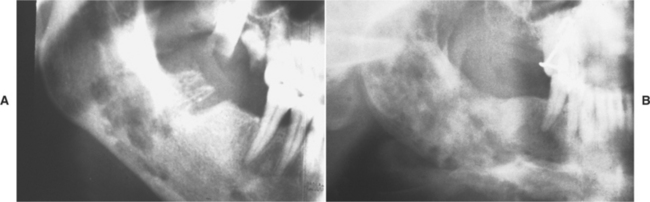
Fig. 3-47 Chronic osteomyelitis. A, Ill-defined area of radiolucency of the right body of the mandible adjacent to a recent extraction site. B, After the initial intervention, the patient failed to return for follow-up because of lack of significant pain. An enlarged, ill-defined radiolucency of the right body of the mandible was discovered 2 years after the initial surgery. (Courtesy of Dr. Charles Waldron.)
Radiographs reveal a patchy, ragged, and ill-defined radiolucency that often contains central radiopaque sequestra. On CT scan, the osteolytic change is contin uous and may exhibit spread to the periosteum by direct extension. This is in contrast to primary chronic osteomyelitis, in which multifocal and separate areas of osteolysis are present within zones of sclerosis. Occasionally, the surrounding bone may exhibit an increased radiodensity, and the cortical surface can demonstrate significant osteogenic periosteal hyperplasia. In spite of the potential for peripheral sclerosis, the main radiographic feature of suppurative osteomyelitis is one of an expanding radiolucent osteolytic change.
Because of an anatomic peculiarity, large portions of each jawbone receive their blood supply through multiple arterial loops originating from a single vessel. Involvement of this single feeder vessel can lead to necrosis of a large portion of the affected bone. Sequestration that has involved an entire quadrant of the jaw has been reported in long-standing cases of chronic osteomyelitis.
ACUTE SUPPURATIVE OSTEOMYELITIS: Generation of biopsy material from patients with acute osteomyelitis is not common because of the predominantly liquid content and lack of a soft tissue component. When submitted, the material consists predominantly of necrotic bone. The bone shows a loss of the osteocytes from their lacunae, peripheral resorption, and bacterial colonization (Fig. 3-48). The periphery of the bone and the haversian canals contain necrotic debris and an acute inflammatory infiltrate consisting of polymorphonuclear leukocytes. The submitted material will be diagnosed as a sequestrum unless a good clinicopathologic correlation points to the appropriate diagnosis of acute osteomyelitis.
CHRONIC SUPPURATIVE OSTEOMYELITIS: Biopsy material from patients with chronic osteomyelitis demonstrates a significant soft tissue component that consists of chronically or subacutely inflamed fibrous connective tissue filling the intertrabecular areas of the bone (Fig. 3-49). Scattered sequestra and pockets of abscess formation are common.
ACUTE SUPPURATIVE OSTEOMYELITIS: If obvious abscess formation is noted, the treatment of acute osteomyelitis consists of antibiotic medications and drainage. Microbiologic study of the infectious material typically reveals a polymicrobial infection of organisms normally present in the oral cavity. The antibiotic medications most frequently selected include penicillin, clindamycin, cephalexin, cefotaxime, tobramycin, and gentamicin.
In most patients a sufficient and appropriate antibiotic regimen aborts the infection and averts the need for surgical intervention. In patients receiving appropriate antibiotic medications, investigators have suggested that nonvital bone fragments could be allowed to remain in place as scaffolding for the future development of new bone.
CHRONIC SUPPURATIVE OSTEOMYELITIS: Chronic osteomyelitis is difficult to manage medically, presumably because pockets of dead bone and organisms are protected from antibiotic drugs by the surrounding wall of fibrous connective tissue. Surgical intervention is mandatory. The antibiotic medications are similar to those used in the acute form but must be given intravenously in high doses.
The extent of the surgical intervention depends on the spread of the process; removal of all infected material down to good bleeding bone is mandatory in all cases. For small lesions, curettage, removal of necrotic bone, and saucerization are sufficient. In patients with more extensive osteomyelitis, decortication or saucerization often is combined with transplantation of cancellous bone chips. In cases of persisting osteomyelitis, resection of the diseased bone followed by immediate reconstruction with an autologous graft is required. Weakened jawbones must be immobilized.
The goal of surgery is removal of all infected tissue. Persistence of chronic osteomyelitis is typically the result of incomplete removal of diseased tissue. On successful elimination of all infected material, resolution is expected. Adjunctive procedures (e.g., hyperbaric oxygen) are rarely necessary if thorough surgical curettage and sequestrectomy have been accomplished. In an attempt to remove all areas of necrotic bone thoroughly, tetracycline has been administered 48 hours in advance of surgery and used as a fluorescent marker of vital bone. At the time of surgery, necrotic bone will not fluoresce under the ultraviolet (UV) light of a Wood’s lamp, indicating that it should be removed.
Management of persistent cases of chronic osteomyelitis often requires use of more sophisticated techniques. Scintigraphic techniques with technetium 99m (99mTc-labeled phosphorus compounds) can be used to evaluate the therapeutic response and progress of treatment. Hyperbaric oxygen is recommended primarily for the rare patient who does not respond to standard therapy or for disease arising in hypovascularized bone (e.g., osteoradionecrosis, osteopetrosis, Paget’s disease, cemento-osseous dysplasia).
DIFFUSE SCLEROSING OSTEOMYELITIS
Diffuse sclerosing osteomyelitis is an ill-defined, highly controversial, evolving area of dental medicine. This diagnosis encompasses a group of presentations that are characterized by pain, inflammation, and varying degrees of gnathic periosteal hyperplasia, sclerosis, and lucency. On occasion, diffuse sclerosing osteomyelitis can be confused with secondarily inflamed intraosseous pathoses (florid cemento-osseous dysplasia) (see page 641) or Paget’s disease of bone (see page 623). In spite of the clinical and radiographic similarities, these processes can be separated from diffuse sclerosing osteomyelitis because of various clinical, radiographic, and histopathologic differences.
The remaining pathoses can be grouped under three categories:
Although the concepts regarding the nature of these conditions continue to be clarified, many believe chronic tendoperiostitis may represent a subset of primary chronic osteomyelitis, whereas two other disorders, synovitis-acne-pustulosis hyperostosis-osteomyelitis (SAPHO) syndrome and chronic recurrent multifocal osteomyelitis (CRMO), constitute primary chronic osteomyelitis with additional extragnathic manifestations. Whether these represent variations of a single disorder or different pathologic processes is highly debated with no clear answer at this time. It appears prudent for clinicians to consider all possibilities in an effort to ensure the most appropriate care for patients affected by these conditions.
In the purist’s view, diffuse sclerosing osteomyelitis is different from primary chronic osteomyelitis and all its variants. This term should be used only when an obvious infectious process directly is responsible for sclerosis of bone. In these cases, chronic intraosseous bacterial infection creates a smoldering mass of chronically inflamed granulation tissue that incites sclerosis of the surrounding bone.
Primary chronic osteomyelitis often is confused with, but must be distinguished from, chronic suppurative osteomyelitis (secondary chronic osteomyelitis). In contrast to suppurative osteomyelitis, an association with a bacterial infection is not obvious, and suppura-tion and sequestration characteristically are absent. A number of causes have been proposed, such as an altered immune response to an organism of low virulence, but no single theory has received widespread acceptance. In contrast to suppurative osteomyelitis, a primary infectious cause cannot be proven, because many studies have been unable to culture organisms and the condition does not respond to long-term antibiotic therapy.
Although initially thought to be an obscure infectious process, the clinical presentation of chronic tendoperiostitis is similar to that of primary chronic osteomyelitis; today many clinicians believe it represents a reactive alteration of bone that is initiated and exacerbated by chronic overuse of the masticatory muscles, predominantly the masseter and digastric. In a large series of patients, parafunctional muscle habits (e.g., bruxism, clenching, nail biting, co-contraction, inability to relax jaw musculature) were known or became evident during follow-up. In neurophysiologic studies, masseter inhibitory reflexes were abnormal in the vast majority of patients studied. The cause of chronic tendoperiostitis is controversial, and some investigators believe this disorder may represent a variation of primary chronic osteomyelitis, in which parafunctional muscle habits exacerbate the process but are not the initial cause.
On occasion, gnathic lesions presenting as primary chronic osteomyelitis occur in patients with other significant systemic manifestations. As the name implies, CRMO includes involvement of multiple bones that is thought by many to represent a widespread variant of primary chronic osteomyelitis. As previously discussed, SAPHO syndrome is an acronym for a complex clinical presentation that includes synovitis, acne, pustulosis, hyperostosis, and osteitis in which the osseous lesions mirror those of primary chronic osteomyelitis and CRMO. The cause of SAPHO is unknown, but it is thought to arise in genetically predisposed individuals who develop an autoimmune disturbance secondary to exposure to dermatologic bacteria. Although not found consistently, an increased prevalence of histocompatibility antigen 27 (HLA 27) in patients with SAPHO has been noted by several in-vestigators. Researchers theorize that an abnormal immune response to the microorganism cross-reacts with normal bone or joint structures, leading to the variety of clinical manifestations. Other immunologic factors have been suggested to cause chronic osteomyelitis and CRMO, but again this theory currently is unproven.
CLINICAL AND RADIOGRAPHIC FEATURES:
DIFFUSE SCLEROSING OSTEOMYELITIS: Diffuse sclerosing osteomyelitis is similar to the localized variant (condensing osteitis; see page 147); how-ever, the disorder is also very different. It arises almost exclusively in adulthood, does not exhibit a sex predominance, and primarily occurs in the mandible. An increased radiodensity develops around sites of chronic infection (e.g., periodontitis, pericoronitis, apical inflammatory disease) in a manner very similar to the increased radiodensity that may be seen surrounding areas of chronic suppurative osteomyelitis. Typically, the altered area is restricted to a single site but may be multifocal or extend to fill an entire quadrant.
The sclerosis centers on the crestal portions of the tooth-bearing alveolar ridge and does not appear to originate in the areas of attachment of the masseter or digastric muscle (Fig. 3-50). The radiodensities do not develop from previously radiolucent fibro-osseous lesions and do not exhibit the predilection for black females, as is found in those patients with florid cemento-osseous dysplasia. Pain and swelling are not typical.
PRIMARY CHRONIC OSTEOMYELITIS: Primary chronic osteomyelitis is most commonly discovered as an isolated process that typically is localized to the mandible. Extragnathic evidence of SAPHO syndrome or CRMO is seen much less frequently. The onset of symptoms tends to demonstrate two peaks, one in adolescence and the other in adults after the fifth decade of life. Affected patients have recurrent episodes of pain, swelling, local induration, and limited mouth opening that is not associated with any obvious dental infection. During periods of disease activity, regional lymphadenopathy and reduced sensation in the distribution of the inferior alveolar nerve may be present. Absence of fever, purulence, sequestration, and sinus formation are characteristic. The lack of an obvious association with an odontogenic infection and the nonsuppurative presentation clearly separate this condition from chronic suppurative osteomyelitis.
In the early stages of primary chronic osteomyelitis, radiographs tend to demonstrate a mixed pattern, with areas of radiolucent osteolysis intermingled with zones of sclerosis. In contrast to the pattern noted in CT images of suppurative osteomyelitis, the osteolytic areas are not continuous and alternate with zones of sclerosis. The affected area of the bone typically is thickened and demonstrates a periosteal proliferation that is more solid than the typical laminated proliferative periostitis of inflammatory origin. Facial asymmetry is not uncommon and often takes years to resolve secondary to slow remodeling. Over time, the affected area becomes predominantly sclerotic, but during subsequent periods of disease activity, new foci of osteolysis and cortical bone destruction appear. These newly affected areas subsequently undergo sclerosis, awaiting the next cycle of disease activity. With disease progression, the clinical symptoms typically diminish and the affected bone demonstrates progressive sclerosis and a reduction in the volume. Radiolucent osteolytic areas may remain, but they tend to be relatively small and widely scattered. Overall, the predominant radiographic alteration of primary chronic osteomyelitis is medullary sclerosis, a pattern that is noted invariably in affected patients. Skeletal scintigraphy demonstrates significant uptake in the affected areas and should be performed in all patients in an effort to rule out extragnathic involvement.
CHRONIC TENDOPERIOSTITIS: Although the mean age of occurrence is 40 years, chronic tendoperiostitis may occur in people of all ages. There is no sex predilection. Recurrent pain, swelling of the cheek, and trismus are classic symptoms. Suppuration and an associated infectious cause are not found. Microbiologic cultures are typically negative, with the lesions failing to respond to appropriate antibiotic medications. Uncommon spontaneous resolution with development of radiographic normalcy has been noted.
In most instances, the sclerosis is limited to a single quadrant and centers on the anterior region of the mandibular angle and posterior portion of the mandibular body (i.e., attachment of the masseter muscle). Occasionally, the cuspid and premolar region and the anterior mandible (i.e., attachment of the digastric muscle) may be involved. Relatively radiolucent zones are apparent within the areas of radiodensity, but histopathologic examination reveals only dense bone, formation of reactive bone, and relatively few signs of inflammation. The inferior border of the mandibular body is typically affected, and significant erosion of the inferior border appears just anterior to the angle of the mandible.
SAPHO SYNDROME: Patients with SAPHO syndrome are usually younger than 60 years old and have chronic multifocal osteomyelitis that is typically associated with negative microbiologic cultures and is nonresponsive to antibiotic therapy. In contrast to bacterial osteomyelitis, the osteolytic areas are scattered randomly within areas of sclerotic bone. Periosteal new bone formation is common but not related to cortical bone perforation. Investigation of the entire skeleton by bone scintigraphy classically reveals involvement of multiple sites. The most frequently involved location is the anterior chest wall, with the sternum, clavicles, and ribs being affected individually or together. Other bones occasionally involved include the spine, pelvis, and long bones.
In early gnathic lesions, diffuse osteolytic zones are more prominent than sclerosis; the affected bone is enlarged because of significant production of periosteal new bone. With time, the bone becomes more sclerotic and decreases in size because of diminished periosteal apposition, while the osteolytic zones be-come smaller and fewer. External bone resorption and deformity of the mandible are characteristic in older lesions.
In some instances, multiple bone lesions are present without associated skin involvement. In these cases the osseous abnormalities have been termed CRMO. Scintigraphy and radiographs will demonstrate synchronous or metachronous involvement of multiple bones such as the clavicle, humerus, radius, femur, or tibia. Mandibular involvement in CRMO occurs in less than 10% of reported cases. Dermatologic involvement may be absent, appear after some delay, or be so subtle as to escape detection. The interval between initial recognition of bone lesions and ultimate development of skin alterations has been as long as 20 years. Commonly associated skin lesions include palmoplantar pustulosis, pustular or plain psoriasis, acne conglobata or ulcerans, and hidradenitis suppurativa.
DIFFUSE SCLEROSING OSTEOMYELITIS: Diffuse sclerosing osteomyelitis demonstrates sclerosis and remodeling of bone. The haversian canals are scattered widely and little marrow tissue can be found. Although the sclerosis occurs adjacent to areas of inflammation, the bone is not typically intermixed with a significant inflammatory soft tissue component. If the adjacent inflammatory process extends into the sclerotic bone, then necrosis often occurs. The necrotic bone separates from the adjacent vital tissue and becomes surrounded by subacutely inflamed granulation tissue. Secondary bacterial colonization often is visible.
PRIMARY CHRONIC OSTEOMYELITIS: Similar histopathologic features are seen in primary chronic osteomyelitis, SAPHO syndrome, and CRMO. In the areas of sclerosis, numerous irregular trabeculae of pagetoid bone are present and demonstrate extensive evidence of remodeling with prominent reversal lines, osteoblastic rimming, and focal areas of osteoclastic activity (Fig. 3-51). Intertrabecular fibrosis is present, with scattered lymphocytes and plasma cells. Present in many, but not all examples, are foci of microabscess formation, hyalinization around small blood vessels, and subperiosteal bone formation. The microabscesses have been correlated with the osteolytic foci noted during active phases of the disease. In obvious contrast to chronic suppurative osteomyelitis, bone necrosis, bacterial colonization, and frank purulence are absent.
CHRONIC TENDOPERIOSTITIS: Chronic tendoperiostitis demonstrates sclerosis and remodeling of the cortical and subcortical bone with a resultant increase in bone volume. If chronic inflam matory cells are present, then they are located in cortical resorption defects and the subcortical bone adjacent to sites of muscle insertion.
DIFFUSE SCLEROSING OSTEOMYELITIS: Diffuse sclerosing osteomyelitis is treated best through resolution of the adjacent foci of chronic infection. After resolution of the infection, the sclerosis remodels in some patients but remains in others. The persistent sclerotic bone is hypovascular, does not exhibit typical remodeling, and is very sensitive to inflammation. The patient and the clinician should work together to avoid future problems with periodontitis or apical inflammatory disease. With long-term alveolar resorption after denture placement, the altered bone does not exhibit typical resorption and exposure with secondary osteomyelitis can develop. These secondary lesions can be treated in the same way as a primary acute or chronic osteomyelitis (see page 143).
PRIMARY CHRONIC OSTEOMYELITIS: Even with significant surgical and medical intervention, the disease course is characterized by flares separated by partial remissions. Most treatments directed toward elimination of infection have been proven ineffective. Long-term antibiotic treatment with or without hyperbaric oxygen therapy has not produced consistent long-term success. Surgical decortication has decreased the intensity and frequency of symptoms but has failed to resolve the process totally. Because of inconsistent results from surgical intervention, extensive surgery is contraindicated, especially in young, growing patients. Corticosteroid medications, NSAIDs, and calcitonin have been reported to relieve symptoms but usually are associated with incomplete resolution. In a limited number of publications, IV administration of bisphosphonates has shown significant therapeutic benefits. Of the bisphosphonates that have been evaluated (alendronate, disodium clodronate, and pamidronate), a single infusion of alendronate produced the most remarkable response, resulting in complete disappearance of pain within 24 hours, dramatic sup-pression of bone turnover as confirmed by skeletal scintigraphy, and long-term remission.
CHRONIC TENDOPERIOSTITIS: Treatment of chronic tendoperiostitis as a form of osteomyelitis has been most unsatisfactory. Large series of patients have been treated with antibiotic medications, explorations, intraoral decortication, implantation of gentamicin beads, hyperbaric oxygen, and corticosteroid drugs with no significant effect. Treatment directed toward resolution of muscle overuse has resulted in significantly decreased symptoms in most patients and total resolution in a minority. Therapeutic approaches include the following:
CONDENSING OSTEITIS (FOCAL SCLEROSING OSTEOMYELITIS)
Localized areas of bone sclerosis associated with the apices of teeth with pulpitis (from large carious lesions or deep coronal restorations) or pulpal necrosis are termed condensing osteitis. The association with an area of inflammation is critical, because these lesions can resemble several other intrabony processes that produce a somewhat similar pattern.
CLINICAL AND RADIOGRAPHIC FEATURES: This secondary sclerosis of bone is seen most frequently in children and young adults but also can occur in older adults. The classic alteration consists of a localized, usually uniform zone of increased radiodensity adjacent to the apex of a tooth that exhibits a thickened periodontal ligament space or an apical inflammatory lesion (Fig. 3-52). Clinical expansion should not be present. Most cases occur in the premolar and molar areas of the mandible, and the dental pulp of the involved tooth demonstrates pulpitis or necrosis. The lesion does not exhibit a radiolucent border, as is seen in cases of focal cemento-osseous dysplasia (see page 640), although an adjacent radiolucent inflammatory lesion may be present. In addition, the radiopacity is not separated from the apex as would be seen in idiopathic osteosclerosis (see page 620).
TREATMENT AND PROGNOSIS: Treatment of the patient with condensing osteitis consists of resolution of the odontogenic focus of infection. After extraction or appropriate endodontic therapy of the involved tooth, approximately 85% of cases of condensing osteitis will regress, either partially or totally. Typically, resolution of the lesion is associated with normalization of the associated periodontal membrane. If the lesion persists and the periodontal membrane remains wide, then reevaluation of the endodontic therapy should be considered. A residual area of condensing osteitis that remains after resolution of the inflammatory focus is termed a bone scar (Fig. 3-53).
OSTEOMYELITIS WITH PROLIFERATIVE PERIOSTITIS (PERIOSTITIS OSSIFICANS)
Bone formation within a periosteal reaction is a common finding that occurs in a wide variety of intraosseous pathoses and in all age groups. Causes of periosteal new bone formation include osteomyelitis, trauma, cysts, infantile cortical hyperostosis, fluorosis, avitaminosis C, hypertrophic osteoarthropathy, congenital syphilis, and neoplasms such as Ewing sarcoma, Langerhans cell histiocytosis, and osteogenic sarcoma. Of these, osteomyelitis and malignant neoplasms are associated most frequently with formation of bone within a periosteal reaction.
In 1893 a Swiss physician, Carl Garré, reported in the German literature on patterns of acute osteomyelitis. Since that time, numerous articles have been written that associate Garré’s report with a form of inflammatory periosteal hyperplasia demonstrating an onionskin-like reduplication of the cortical plate. (In these subsequent articles, Garré’s name was misspelled consistently as Garré, with an improper accent.) However, Garré did not have any pathologic specimens for microscopic examination, and Roentgen did not discover x-rays until 2 years after Garré’s publication. Nowhere in the original publication is there any mention of periostitis, periosteal duplication, or “onionskinning.” Although the term Garré’s osteomyelitis often is used synonymously for this condition, it is an improper designation that should be disassociated with the entity described in the text that follows.
CLINICAL AND RADIOGRAPHIC FEATURES: Proliferative periostitis represents a periosteal reaction to the presence of inflammation. The affected periosteum forms several rows of reactive vital bone that parallel each other and expand the surface of the altered bone. Affected patients tend to be primarily children and young adults, with a mean age of 13 years. No sex predominance is noted.
As expected, the most frequent cause is dental caries with associated periapical inflammatory disease, although lesions have been reported secondary to periodontal infections, fractures, buccal bifurcation cysts, and nonodontogenic infections. Most cases arise in the premolar and molar area of the mandible. The hyperplasia is located most frequently along the lower border of the mandible, but buccal cortical involvement also is common. Isolated lingual cortical enlargement is infrequent. Most cases are unifocal, although multiple quadrants may be affected.
Appropriate radiographs demonstrate radiopaque laminations of bone that roughly parallel each other and the underlying cortical surface (Fig. 3-54). The laminations vary from 1 to 12 in number, and radiolucent separations often are present between the new bone and the original cortex. Less frequently, the new bone formation exhibits consolidation and contains numerous fine bony projections that radiate perpendicular from the underlying and intact periosteum. Within the new bone, areas of small sequestra or osteolytic radiolucencies may be found.
Fig. 3-54 Proliferative periostitis. A, Firm swelling of the lateral and inferior border of the right mandible that arose after traumatic injury. B, Computed tomography (CT) image demonstrating new periosteal bone growth with onionskin laminations. C, Panoramic radiograph exhibiting new periosteal bone formation along the right inferior border of the mandible. (Courtesy of Drs. Sherif Mekhail and Benjamin Lin.)
Because of difficulty in proper angulation and problems related to superimposition of the underlying bone, CT scanning has proved to be consistently superior to conventional radiography in demonstrating proliferative periostitis. On plain films, the alterations are typically seen best on a panoramic or lateral oblique radiograph. The latter type often is favored because of finer detail of the final image. If lateral oblique radiographs fail to demonstrate the lesion, then occlusal views and, less frequently, posteroanterior radiographs may be successful.
HISTOPATHOLOGIC FEATURES: Usually, biopsy is not required unless the clinical diagnosis is in question. Specimens often reveal parallel rows of highly cellular and reactive woven bone in which the individual trabeculae are frequently oriented perpendicular to the surface. The trabeculae sometimes form an interconnecting meshwork of bone or are scattered more widely, resembling the pattern seen in immature fibrous dysplasia (Fig. 3-55). Between the cellular trabeculae, relatively uninflamed fibrous connective tissue is evident. Sequestra, if included, demonstrate the typical features of bone necrosis (see Osteomyelitis, page 141).
TREATMENT AND PROGNOSIS: Most cases of proliferative periostitis of the jaws are associated with periapical inflammatory lesions, and treatment in these cases (either extraction of the offending tooth or appropriate endodontic therapy) is directed toward eliminating the source of the infection. After the focus of infection has been eliminated and inflammation has resolved, the layers of bone will consolidate in 6 to 12 months as the overlying muscle action helps to remodel the bone to its original state.
If a unifocal periosteal reaction similar to proliferative periostitis appears in the absence of an obvious source of inflammation, biopsy is recommended because several neoplastic conditions can result in a similar pattern. On occasion, periosteal hyperplasia has been found in patients without any detectable cause except for the close proximity of an unerupted tooth surrounded by its dental follicle. The cause of the periosteal hyperplasia in these instances is unclear.
ALVEOLAR OSTEITIS (DRY SOCKET; FIBRINOLYTIC ALVEOLITIS)
After extraction of a tooth, a blood clot is formed at the site, with eventual organization of the clot by granulation tissue, gradual replacement by coarse fibrillar bone, and, finally, replacement by mature bone. Destruction of the initial clot prevents appropriate healing and causes the clinical condition known as alveolar osteitis.
Extensive investigations have shown that the clot is lost secondary to transformation of plasminogen to plasmin, with subsequent lysis of fibrin and formation of kinins (fibrinolytic alveolitis); these are potent pain mediators. Local trauma, estrogens, and bacterial pyrogens are known to stimulate fibrinolysins. This knowledge correlates well with the increased frequency of alveolar osteitis in association with deeply impacted mandibular third molars, poor oral hygiene, inexperienced surgeons, traumatic extractions, oral contraceptive use, and presurgical infections. In addition, inadequate irrigation at surgery and the use of tobacco products have been related to the development of the problem. In fact, studies have shown that the prevalence of alveolar osteitis is 20% in patients who smoke more than one pack of cigarettes per day and increases to 40% for those who smoke on the day of surgery or within 24 hours after surgery. Heavy sucking or spitting by the patient after surgery also has been implicated in dislodgement and loss of the alveolar clot.
CLINICAL FEATURES: The frequency of alveolar osteitis is higher in the mandible and the posterior areas. After oral contraceptive use is taken into account, there does not appear to be a significant sex predilection. The prevalence is between 1% and 3% of all extractions, but it increases to 25% to 30% for impacted mandibular third molars. The frequency appears to be decreased when impacted teeth are removed prophylactically rather than for therapeutic reasons after development of chronic inflammation of pericoronal tissues. The overall prevalence is highest between 20 and 40 years of age (when the majority of teeth are extracted), although the likelihood of developing alveolar osteitis appears greatest for extractions in the 40- to 45-year-old age group.
The affected extraction site is filled initially with a dirty gray clot that is lost and leaves a bare bony socket (dry socket). The detection of the bare socket may be hindered by partial retention of the clot or by overlying inflamed tissue that covers the site. The diagnosis is confirmed by probing of the socket, which reveals exposed and extremely sensitive bone. Typically, severe pain, foul odor, and (less frequently) swelling and lymphadenopathy develop 3 to 4 days after extraction of the tooth. On occasion, the pain radiates from the socket to the ipsilateral ear, temporal region, or eye. Rarely, trismus also may be noted. The signs and symptoms may last from 10 to 40 days.
TREATMENT AND PROGNOSIS: On evaluation of the patient complaining of postextraction pain, a radiograph should be taken of the affected area to rule out the possibility of a retained root tip or a foreign body. All sutures should be removed. The socket is irrigated with warm saline, followed by thorough clinical inspection of the socket for any unexpected pathosis. Curettage of the socket is not recommended, because this typically increases the associated pain. Potent oral analgesics should be prescribed, and the patient should be given a plastic syringe with instructions to keep the socket clean via home irrigation with a chlorhexidine or saline solution. This irrigation should continue until debris no longer collects within the healing socket (usually 3 to 4 weeks).
Use of an obtundent and antiseptic dressing such as iodoform gauze containing eugenol is controversial. Although the dressing may reduce the symptoms and help keep out food debris, many believe the dressing acts as foreign material and delays healing of the extraction socket. If a dressing is used, then it should be changed every 24 hours for the first 3 days, then every 2 to 3 days until granulation tissue covers the exposed bone. The dressing should be discontinued as soon as the patient is pain free. After that time, the patient should be given a plastic syringe with instructions for home irrigation.
Many investigators have studied preventive measures for alveolar osteitis. For female patients using oral contraceptives, the extractions could be scheduled on days without estrogen supplementation (typically days 23 to 28 of the menstrual cycle). Before the formation of the initial blood clot, thorough irrigation of the surgical site with sterile saline to remove any small fragments of debris, tooth, and bone appears beneficial. One popular method for prevention of alveolar osteitis that is associated with a low chance of an adverse reaction is gentle rinsing with chlorhexidine on the day of the surgery and for several days after the surgical procedure. Placement of a prophylactic dressing into the extraction socket is thought by many to increase the chance of possible side effects and add unnecessary additional cost to the procedure. In spite of this, some clinicians use systemic or topical antifibrinolytics, topical antibiotic medications, or systemic antibiotic drugs. Of the used intraalveolar antibiotic medications, tetracycline is chosen most frequently, but lincomycin, clindamycin, and metronidazole also show favorable results. The antibiotic should not be in an ointment form, because such use has resulted in chronic foreign body reactions (e.g., myospherulosis) (see page 324). As mentioned, many surgeons are hesitant to place medicaments into an extraction socket; those who do often restrict their use to certain “high-risk” patients, such as those who:
BIBLIOGRAPHY
Cohen, S, Hargreaves, KM. Pathways of the pulp, ed 9. St Louis: Mosby, 2005.
Ingle, JI, Bakland, LK. Endodontics, ed 5. Ontario, Hamilton: BC Decker, 2002.
Banchs, F, Trope, M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196–200.
Barrieshi-Nusair, KM, Qudeimat, MA. A prospective clinical study of mineral trioxide aggregate for partial pulpotomy in cariously exposed permanent teeth. J Endod. 2006;32:731–735.
Çal kan, MK, Öztop, F, Çal
kan, MK, Öztop, F, Çal kan, G. Histological evaluation of teeth with hyperplastic pulpitis caused by trauma or caries: case reports. Int Endod J. 2002;36:64–70.
kan, G. Histological evaluation of teeth with hyperplastic pulpitis caused by trauma or caries: case reports. Int Endod J. 2002;36:64–70.
Heyeraas, KJ, Sveen, OB, Mjör, IA. Pulp-dentin biology in restorative dentistry. Part 3: pulpal inflammation and its sequelae. Quintessence Int. 2001;32:611–625.
Holland, GR. Pulpalgia, the Pimpernel of pain. Tex Dent J. 2003;120:239–247.
Hyman, JL, Cohn, ME. The predictive value of endodontic diagnostic tests. Oral Surg Oral Med Oral Pathol. 1984;58:343–346.
Michaelson, PL, Holland, GR. Is pulpitis painful? Int J Endod. 2002;35:829–832.
Modaresi, J, Dianat, O, Mozayeni, MA. The efficacy comparison of ibuprofen, acetaminophen-codeine, and placebo premedication therapy on the depth of anesthesia during treatment of inflamed teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:339–403.
Rafter, M. Apexification: a review. Dent Traumatol. 2005;21:1–8.
Witherspoon, DE, Small, JC, Harris, GZ. Mineral trioxide aggregate pulpotomies. A case series outcome assessment. J Am Dent Assoc. 2006;137:610–618.
Amir, FA, Gutmann, JL, Witherspoon, DE. Calcific metamorphosis: a challenge in endodontic diagnosis and treatment. Quintessence Int. 2001;32:447–455.
Mjör, IA, Ferrari, M. Pulp-dentin biology in restorative dentistry. Part 6: reactions to restorative materials, tooth-restoration interfaces, and adhesive techniques. Quintessence Int. 2002;33:35–63.
Mjör, IA, Sveen, OB, Heyeraas, KJ, et al. Pulp-dentin biology in restorative dentistry. Part 1: normal structure and physiology. Quintessence Int. 2001;32:46–427.
Morse, DR. Age-related changes of the dental pulp complex and their relationship to systemic aging. Oral Surg Oral Med Oral Pathol. 1991;72:721–745.
Paewinsky, E, Pfeiffer, H, Brinkmann, B. Quantification of secondary dentine formation from orthopantomograms—a contribution to forensic age estimation methods in adults. Int J Legal Med. 2005;119:27–30.
Pashley, DH, Pashley, EL, Carvalho, RM, et al. The effects of dentin permeability on restorative dentistry. Dent Clin North Am. 2002;46:211–245.
Smith, AJ, Murray, PE, Lumley, PJ. Preserving the vital pulp in operative dentistry: I. A biological approach. Dent Update. 2002;29:64–69.
Solheim, T. Amount of secondary dentin as an indicator of age. Scand J Dent Res. 1992;100:193–199.
West, JD. The aesthetics and endodontic dilemmas of calcific metamorphosis. Pract Periodontics Aesthet Dent. 1997;9:286–293.
Woods, MA, Robinson, QC, Harris, EF. Age-progressive changes in pulp widths and root lengths during adulthood: a study of American blacks and whites. Gerodontology. 1990;9:41–50.
Al-Hadi Hamasha, A, Darwazeh, A. Prevalence of pulp stones in Jordanian adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:730–732.
Comer, TL, Ground, TG. Hereditary pattern for dentinal dysplasia type Id: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:51–53.
Morse, DR. Age-related changes of the dental pulp complex and their relationship to systemic aging. Oral Surg Oral Med Oral Pathol. 1991;72:721–745.
Moss-Salentijn, L, Hendricks-Klyvert, M. Calcified structures in human dental pulps. J Endod. 1988;14:184–189.
Parekh, S, Kyriazidou, A, Bloch-Zupan, A, et al. Multiple pulp stones and shortened roots of unknown etiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e139–e142.
Rao, SR, Witkop, CJ, Yamane, GM. Pulpal dysplasia. Oral Surg Oral Med Oral Pathol. 1970;30:682–689.
Selden, HS. Radiographic pulpal calcifications: normal or abnormal—a paradox. J Endod. 1991;17:34–37.
Trowbridge, HO. Pulp biology: progress during the past 25 years. Aust Endod J. 2003;29:5–12.
Periapical Inflammatory Disease (Granuloma; Cyst; Abscess)
Bhaskar, SN. Periapical lesions—types, incidence, and clinical features. Oral Surg Oral Med Oral Pathol. 1966;21:657–671.
Çal kan, MK. Prognosis of large cyst-like periapical lesions following nonsurgical root canal treatment: a clinical review. Int Endod J. 2004;37:408–416.
kan, MK. Prognosis of large cyst-like periapical lesions following nonsurgical root canal treatment: a clinical review. Int Endod J. 2004;37:408–416.
Chen, S-Y, Fantasia, JE, Miller, AS. Hyaline bodies in the connective tissue wall of odontogenic cysts. J Oral Pathol. 1981;10:147–157.
Dahlén, G. Microbiology and treatment of dental abscesses and periodontal-endodontic lesions. Periodontol 2000. 2002;28:206–239.
Gao, Z, Flaitz, CM, Mackenzie, IC. Expression of keratinocyte growth factor in periapical lesions. J Dent Res. 1996;75:1658–1663.
Harn, WM, Chen, MC, Chen, YHM. Effect of occlusal trauma on healing of periapical pathoses: report of two cases. Int Endod J. 2001;34:554–561.
High, AS, Hirschmann, PN. Age changes in residual radicular cysts. J Oral Pathol. 1986;15:524–528.
Kuriyama, T, Absi, EG, Williams, DW, et al. An outcome audit of the treatment of acute dentoalveolar infection: impact of penicillin. Br Dent J. 2005;198:759–763.
Matthews, DC, Sutherland, S, Basrani, B. Emergency management of acute apical abscesses in the permanent dentition: a systematic review of the literature. J Can Dent Assoc. 2003;69:601–660. [661].
Nair, PNR. New perspectives on radicular cysts: do they heal? Int Endod J. 1998;31:155–160.
Nair, PNR. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006;39:249–281.
Nair, PNR, Pajarola, G, Schroeder, HE. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1993;81:93–102.
Ricucci, D, Mannocci, F, Pitt, TR. A study of periapical lesions correlating the presence of a radiopaque lamina with histological findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:389–394.
Slots, J, Nowzari, H, Sabeti, M. Cytomegalovirus infection in symptomatic periapical pathosis. Int Endod J. 2004;37:519–524.
Stockdale, CR, Chandler, NP. The nature of the periapical lesion—a review of 1108 cases. J Dent. 1988;16:123–129.
Sutherland, S, Matthews, DC. Emergency management of acute apical periodontitis in the permanent dentition: a systematic review of the literature. J Can Dent Assoc. 2003;69:160–161. [160i].
Tronstad, L. Recent development in endodontic research. Scand J Dent Res. 1992;100:52–59.
Trowbridge, HO, Stevens, BH. Microbiologic and pathologic aspects of pulpal and periapical disease. Curr Opin Dent. 1992;2:85–92.
Baqain, ZH, Newman, L, Hyde, N. How serious are oral infections? J Laryngol Otol. 2004;118:561–565.
Busch, RF, Shah, D. Ludwig’s angina: improved treatment. Otolaryngol Head Neck Surg. 1997;117:S172–S175.
Dolan, RW, Chowdhury, K. Diagnosis and treatment of intracranial complications of paranasal sinus infections. J Oral Maxillofac Surg. 1995;53:1080–1087.
Hutchinson, IL, James, DR. New treatment for Ludwig’s angina. Br J Oral Maxillofac Surg. 1989;27:83–84.
Iwu, CO. Ludwig’s angina: report of seven cases and review of the current concepts in management. Br J Oral Maxillofac Surg. 1990;28:189–193.
Lazow, SK. Orofacial infection in the 21st century. N Y State Dent J. 2005;71:36–41.
Moreland, LW, Corey, J, McKenzie, R. Ludwig’s angina: report of a case and review of the literature. Arch Intern Med. 1988;148:461–466.
Ogundiya, DA, Keith, DA, Mirowski, J. Cavernous sinus thrombosis and blindness as complication of an odontogenic infection. J Oral Maxillofac Surg. 1989;47:1317–1321.
Pynn, BR, Sands, T, Pharoah, MJ. Odontogenic infections: part 1. Anatomy and radiology. Oral Health. 1995;85:7–21.
Sands, T, Pynn, BRT, Katsikeris, N. Odontogenic infections: part 2. Microbiology, antibiotics and management. Oral Health. 1995;85:11–28.
Adekeye, EO, Cornah, J. Osteomyelitis of the jaws: a review of 141 cases. Br J Oral Maxillofac Surg. 1985;23:24–35.
Daramola, JO, Ajagbe, HA. Chronic osteomyelitis of the mandible in adults: a clinical study of 34 cases. Br J Oral Surg. 1982;20:58–62.
Harvey, BR, Ephros, H, DeFalco, RJ. Tetracycline bone labeling in surgical management of chronic osteomyelitis: a case report. J Oral Maxillofac Surg. 2004;62:752–754.
Koorbusch, GF, Fotos, P, Terhark, K. Retrospective assessment of osteomyelitis: etiology, demographics, risk factors, and management in 35 cases. Oral Surg Oral Med Oral Pathol. 1992;74:149–154.
Marx, RE. Chronic osteomyelitis of the jaws. Oral Maxillofac Clin North Am. 1991;3:367–381.
Rohlin, M. Diagnostic value of bone scintigraphy in osteomyelitis of the mandible. Oral Surg Oral Med Oral Pathol. 1993;75:650–655.
Schuknecht, B, Valavanis, A. Osteomyelitis of the mandible. Neuroimaging Clin North Am. 2003;13:605–618.
van Merkesteyn, JPR, Bakker, DJ, van der Waal, I, et al. Hyperbaric oxygen treatment of chronic osteomyelitis of the jaws. Int J Oral Surg. 1984;13:386–395.
van Merkesteyn, JPR, Groot, RH, van der Akker, HP, et al. Treatment of chronic suppurative osteomyelitis of the mandible. Int J Oral Maxillofac Surg. 1997;26:450–454.
Diffuse Sclerosing Osteomyelitis
Groot, RH, van Merkesteyn, JP, Bras, J. Diffuse sclerosing osteomyelitis and florid osseous dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:333–342.
Baltensperger, M, Grätz, K, Bruder, E, et al. Is primary chronic osteomyelitis a uniform disease? Proposal of a classification based on a retrospective analysis of patients treated in the past 30 years. J Craniomaxillofac Surg. 2004;32:43–50.
Eyrich, GKH, Baltensperger, MM, Bruder, E, et al. Primary chronic osteomyelitis in childhood and adolescence: a retrospective analysis of 11 cases and review of the literature. J Oral Maxillofac Surg. 2003;61:561–573.
Groot, RH, Ongerboer de Visser, BW, van Merkesteyn, JP, et al. Changes in masseter inhibitory reflex responses in patients with diffuse sclerosing osteomyelitis of the mandible. Oral Surg Oral Med Oral Pathol. 1992;74:727–732.
Groot, RH, van Merkesteyn, JP, van Soest, JJ, et al. Diffuse sclerosing osteomyelitis (chronic tendoperiostitis) of the mandible: an 11-year follow-up report. Oral Surg Oral Med Oral Pathol. 1992;74:557–560.
Heggie, AA, Shand, JM, Aldred, MJ, et al. Juvenile mandibular chronic osteomyelitis: a distinct clinical entity. Int J Oral Maxillofac Surg. 2003;32:459–468.
Hino, S, Murase, R, Terakado, N, et al. Response of diffuse sclerosing osteomyelitis of the mandible to alendronate: follow-up study by 99mTc scintigraphy. Int J Oral Maxillofac Surg. 2005;34:576–578.
Montonen, M, Kalso, E, Pylkkären, L, et al. Disodium clodronate in the treatment of diffuse sclerosing osteomyelitis (DSO) of the mandible. Int J Oral Maxillofac Surg. 2001;30:313–317.
Soubrier, M, Dubost, JJ, Ristori, JM, et al. Pamidronate in the treatment of diffuse sclerosing osteomyelitis of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:637–640.
Suei, Y, Taguchi, A, Tanimoto, K. Diagnostic point and possible origin of osteomyelitis in synovitis, acne, pustulosis, hyperostosis and osteitis (SAPHO) syndrome: a radiographic study of 77 mandibular osteomyelitis cases. J Rheumatol. 2003;42:1398–1403.
Suei, Y, Taguchi, A, Tanimoto, K. Diagnosis and classification of mandibular osteomyelitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:207–214.
van Merkesteyn, JPR, Groot, RH, Bras, J, et al. Diffuse sclerosing osteomyelitis of the mandible: a new concept of its etiology. Oral Surg Oral Med Oral Pathol. 1990;70:414–419.
Boyne, PJ. Incidence of osteosclerotic areas in the mandible and maxilla. J Oral Surg Anesth Hosp Dent Serv. 1960;18:486–491.
Eliasson, S, Halvarsson, C, Ljungheimer, C. Periapical condensing osteitis and endodontic treatment. Oral Surg Oral Med Oral Pathol. 1984;57:195–199.
Eversole, LR, Stone, CE, Strub, D. Focal sclerosing osteomyelitis/focal periapical osteopetrosis: radiographic patterns. Oral Surg Oral Med Oral Pathol. 1984;58:456–460.
Farman, AG, de V Joubert, JJ, Nortjé, C. Focal osteosclerosis and apical periodontal pathoses in “European” and Cape Coloured dental outpatients. Int J Oral Surg. 1978;7:549–557.
Hedin, M, Polhagen, L. Follow-up study of periradicular bone condensation. Scand J Dent Res. 1979;79:436–440.
Osteomyelitis with Proliferative Periostitis
Eversole, LR, Leider, AS, Corwin, JO, et al. Proliferative periostitis of Garré: its differentiation from other neoperiostoses. J Oral Surg. 1979;37:725–731.
Jacobson, HLJ, Baumgartner, JC, Marshall, JG, et al. Proliferative periostitis of Garré: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:111–114.
Kannan, SK, Sandhya, G, Selvarani, R. Periostitis ossificans (Garrè’s osteomyelitis) radiographic study of two cases. Int J Paediatr Dent. 2006;16:59–64.
Kawai, T, Hiranuma, H, Kishino, M, et al. Gross periostitis ossificans in mandibular osteomyelitis. Review of the English language literature and radiographic variation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:376–381.
Kawai, T, Murakami, S, Sakuda, M, et al. Radiographic investigation of mandibular periostitis ossificans in 55 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:704–712.
Nortjé, CJ, Wood, RE, Grotepass, F. Periostitis ossificans versus Garrè’s osteomyelitis: part II. Radiologic analysis of 93 cases in the jaws. Oral Surg Oral Med Oral Pathol. 1988;66:249–260.
Wood, RE, Nortjé, CJ, Grotepass, F, et al. Periostitis ossificans versus Garrè’s osteomyelitis: part I. What did Garrè really say? Oral Surg Oral Med Oral Pathol. 1988;65:773–777.
Awang, MN. The aetiology of dry socket: a review. Int Dent J. 1989;39:236–240.
Birn, H. Etiology and pathogenesis of fibrinolytic alveolitis (“dry socket”). Int J Oral Surg. 1973;2:215–263.
Blum, IR. Contemporary views on dry socket (alveolar osteitis): a clinical appraisal of standardization, aetiopathogenesis and management: a critical review. Int J Oral Maxillofac Surg. 2003;31:309–317.
Caso, A, Hung, L-K, Beirne, OR. Prevention of alveolar osteitis with chlorhexidine: a meta-analytic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:155–159.
Catellani, JE. Review of factors contributing to dry socket through enhanced fibrinolysis. J Oral Surg. 1979;37:42–46.
Fazakerley, M, Field, EA. Dry socket: a painful post-extraction complication (a review). Dent Update. 1991;18(1):31–34.
Garcia, AG, Grana, PM, Sampedro, FG, et al. Does oral contraceptive use affect the incidence of complications after extraction of a mandibular third molar? Br Dent J. 2003;194:453–455.
Houston, JP, McCollum, J, Pietz, D, et al. Alveolar osteitis: a review of its etiology, prevention, and treatment modalities. Gen Dent. 2002;50:457–463.
Larsen, PE. Alveolar osteitis after surgical removal of impacted mandibular third molars: identification of the patient at risk. Oral Surg Oral Med Oral Pathol. 1992;73:393–397.
Swanson, AE. Prevention of dry socket: an overview. Oral Surg Oral Med Oral Pathol. 1990;70:131–136.