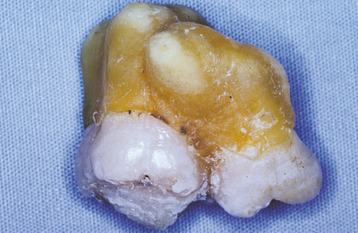
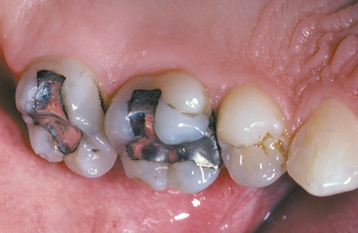
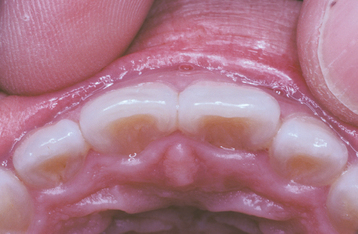
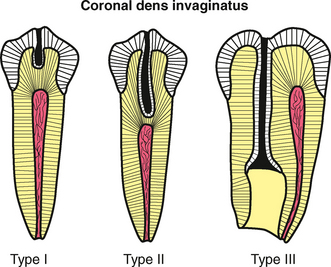
Abnormalities of Teeth
ENVIRONMENTAL ALTERATIONS OF TEETH
Environmental Effects on Tooth Structure Development
Postdevelopmental Loss of Tooth Structure Tooth Wear
Environmental Discoloration of Teeth
Localized Disturbances in Eruption
DEVELOPMENTAL ALTERATIONS OF TEETH
Developmental Alterations in the Number of Teeth
Environmental Alterations of Teeth
The abnormalities of the teeth can be divided into those that are influenced by environmental forces and those that are idiopathic and appear hereditary in nature. Later parts of this chapter delineate the idiopathic and hereditary alterations of teeth. Box 2-1 lists the major categories of tooth alteration that can be affected by environmental influences. In many cases the cause and effect are obvious; in others the primary nature of the problem is less distinct.
ENVIRONMENTAL EFFECTS ON TOOTH STRUCTURE DEVELOPMENT
The ameloblasts in the developing tooth germ are extremely sensitive to external stimuli, and many factors can result in abnormalities in the enamel (Box 2-2). The primary hereditary abnormalities of the enamel that are unrelated to other disorders are termed amelogenesis imperfecta (see page 99).
Dental enamel is unique in that remodeling does not occur after initial formation. Therefore, abnormalities in enamel formation are etched permanently on the tooth surface. The enamel develops in three major stages: (1) matrix formation, (2) mineralization, and (3) maturation. During matrix formation, the enamel proteins are laid down. In the next phase, minerals are deposited and the majority of the original proteins are removed. During the final maturation period, the enamel undergoes final mineralization and the remnants of the original proteins are removed. In the early stage of mineralization, the enamel is dull, white, and relatively soft. During the late stage of maturation, the final hard translucent enamel replaces this diffuse opaque enamel.
The timing of the ameloblastic damage has a great effect on the location and appearance of the defect in the enamel. The cause of the damage does not appear to be of major importance, because many different local and systemic stimuli can result in defects that have similar clinical appearances. The final enamel represents a record of all significant insults received during tooth development. Deciduous enamel contains a neonatal ring, and the rate of enamel apposition is estimated to be 0.023 mm/day. Using this knowledge, the clinician can accurately estimate the timing of an insult to the deciduous teeth to within 1 week. In the permanent dentition, the position of the enamel defects provides a rough estimate of the time of damage; however, available data on the chronology of tooth development are derived from a relatively small sample size, and the ranges of normal values are wide. In addition, gender and racial variations are not established thoroughly.
CLINICAL AND RADIOGRAPHIC FEATURES: Almost all visible environmental enamel defects can be classified into one of three patterns:
Subtle enamel defects can be masked by saliva, plaque, or poor illumination. When attempting to detect areas of altered enamel, the dentition should be cleaned thoroughly; then it should be dried with gauze. Dental operatory lights are an ideal light source (direct sunlight should be avoided). Plaque-disclosing solution can be used to highlight small defects. The altered enamel may be localized or present on numerous teeth, and all or part of the surfaces of each affected tooth may be involved. Enamel hypoplasia occurs in the form of pits, grooves, or larger areas of missing enamel. Diffuse opacities of enamel appear as variations in the translucency of the enamel. The affected enamel is of normal thickness; however, it has an increased white opacity with no clear boundary with the adjacent normal enamel. Demarcated opacities of enamel show areas of decreased translucence, increased opacity, and a sharp boundary with the adjacent enamel. The enamel is of normal thickness, and the affected opacity may be white, cream, yellow, or brown.
The crowns of the deciduous dentition begin to develop at approximately the fourteenth week of gestation and continue until the child is 12 months of age. Development of the crowns of the permanent dentition occurs from approximately 6 months to 15 years of age. The site of coronal damage correlates with the area of ameloblastic activity at the time of the injury; the affected enamel is restricted to the areas in which secretory activity or active maturation of the enamel matrix was occurring.
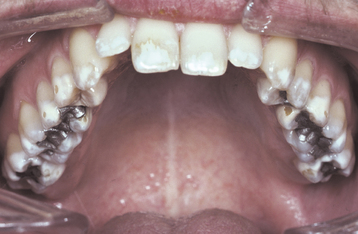
Environmental enamel abnormalities are extremely common. In a review of more than 1500 children from 12 to 15 years of age in an industrialized nation, the prevalence of enamel defects in the permanent dentition was 68.4%. Within this group, 67.2% demonstrated opacities, 14.6% revealed hypoplasia, and both patterns were seen in 13.4% of the children. The average number of affected teeth per individual was 3.6, with greater than 10% of the children having 10 or more teeth involved.
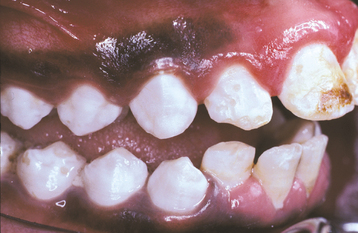
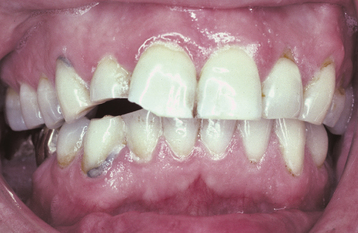
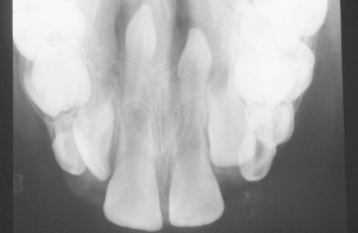
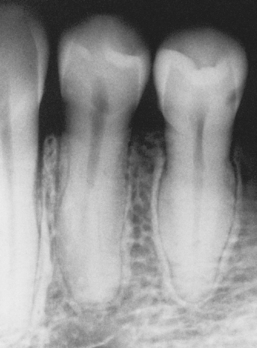
A common pattern is seen as a result of systemic influences, such as exanthematous fevers, that occur during the first 2 years of life. Horizontal rows of pits or diminished enamel are present on the anterior teeth and first molars (Figs. 2-1 and 2-2). The enamel loss is bilaterally symmetric, and the location of the defects correlates well with the developmental stage of the affected teeth. A similar pattern of enamel defects can be seen in the cuspids, bicuspids, and second molars when the inciting event occurs around the age of 4 to 5 years (Fig. 2-3).
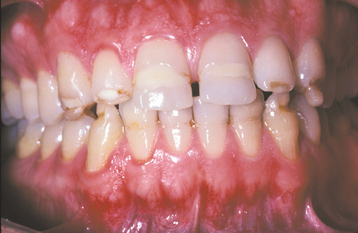
Fig. 2-1 Environmental enamel hypoplasia. Bilaterally symmetrical pattern of horizontal enamel hypoplasia of the anterior dentition. Maxillary central incisors have been restored previously. (From Neville BW, Damm DD, White DK: Color atlas of clinical oral pathology, ed 2, Hamilton, 1999, BC Decker.)
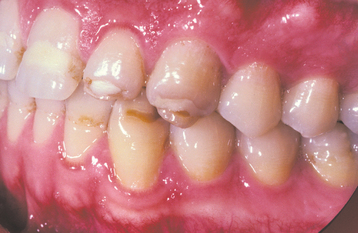
Fig. 2-2 Environmental enamel hypoplasia. Same patient as depicted in Fig. 2-1. Note the lack of enamel damage on bicuspids. (From Neville BW, Damm DD, White DK: Color atlas of clinical oral pathology, ed 2, Hamilton, 1999, BC Decker.)
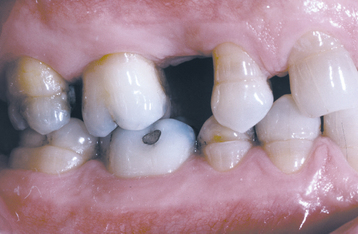
Fig. 2-3 Environmental enamel hypoplasia. Horizontal enamel hypoplasia of the bicuspids and second molars. Note sparing of the first molars. (From Neville BW, Damm DD, White DK: Color atlas of clinical oral pathology, ed 2, Hamilton, 1999, BC Decker.)
TURNER’S HYPOPLASIA: Another frequent pattern of enamel defects seen in permanent teeth is caused by periapical inflammatory disease of the overlying deciduous tooth. The altered tooth is called a Turner’s tooth (after the clinician whose publications allowed this problem to be widely recognized). The appearance of the affected area varies according to the timing and severity of the insult. The enamel defects vary from focal areas of white, yellow, or brown discoloration to extensive hypoplasia, which can involve the entire crown. The process is noted most frequently in the permanent bicuspids because of their relationship to the overlying deciduous molars (Figs. 2-4 and 2-5). Anterior teeth are involved less frequently because crown formation is usually complete before the development of any apical inflammatory disease in the relatively caries-resistant anterior deciduous dentition. Factors that determine the degree of damage to the permanent tooth by the overlying infection include the stage of tooth development, length of time the infection remains untreated, the virulence of the infective organisms, and the host resistance to the infection.
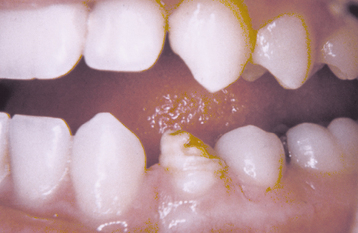
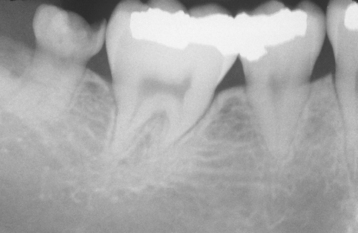
Fig. 2-4 Turner’s hypoplasia. Extensive enamel hypoplasia of mandibular first bicuspid secondary to previous inflammatory process associated with overlying first deciduous molar. (From Halstead CL, Blozis GG, Drinnan AJ et al: Physical evaluation of the dental patient, St Louis, 1982, Mosby.)
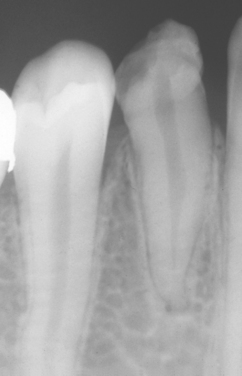
Fig. 2-5 Turner’s hypoplasia. Radiograph of the same tooth depicted in Fig. 2-4. Note the lack of significant enamel and irregularity of the dentin surface. (From Halstead CL, Blozis GG, Drinnan AJ et al: Physical evaluation of the dental patient, St Louis, 1982, Mosby.)
In addition to classic Turner’s teeth, an increased prevalence of demarcated opacities has been reported in the permanent successors of carious primary teeth. In one report, if the primary tooth developed caries, the successor was twice as likely to demonstrate a circumscribed enamel defect. In addition, if the primary tooth was extracted for any reason other than trauma, then the prevalence of a demarcated enamel defect increased fivefold.
Traumatic injury to deciduous teeth also can cause significant alterations of the underlying dentition and the formation of Turner’s teeth. This is not a rare occurrence; up to 45% of all children sustain injuries to their primary teeth. In a prospective study of 114 children with 255 traumatized primary teeth, 23% of the corresponding permanent teeth demonstrated developmental disturbances. The maxillary central incisors are affected in the majority of the cases; the maxillary lateral incisors are altered less frequently (Fig. 2-6). In several large reviews, the prevalence of involvement of the posterior teeth or mandibular incisors was less than 10% of all cases.
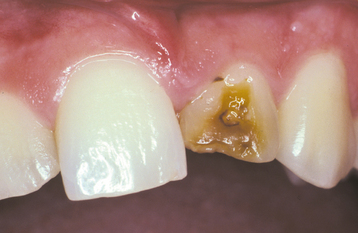
Fig. 2-6 Turner’s hypoplasia. Extensive coronal hypoplasia of permanent maxillary left central incisor secondary to previous trauma to deciduous central incisor.
The frequency of traumatic damage of the anterior maxillary dentition is not surprising, considering the common occurrence of trauma to the deciduous dentition of the prominent anterior maxilla and the close anatomic relationship between the developing tooth bud and the apices of the overlying primary incisors. As would be expected, the clinical appearance of the alteration varies according to the timing and severity of the damage.
Because of the position of the primary apices relative to the tooth bud, the facial surface of the maxillary incisors is the location most frequently affected. Typically, the affected area appears as a zone of white or yellowish-brown discoloration with or without an area of horizontal enamel hypoplasia. The trauma also can cause displacement of the already formed hard-tooth substance in relation to the soft tissue of the remaining developing tooth. This results in a bend of the tooth known as dilaceration and can affect either the crown or the root of a tooth (see page 97). Severe trauma early in the development of the tooth can result in such disorganization of the bud that the resultant product may resemble a complex odontoma (see page 724). Similar levels of damage late in the formative process can lead to partial or total arrest in root formation.
MOLAR INCISOR HYPOMINERALIZATION: Over the last two to three decades, a number of publications have described a unique pattern of defective enamel that has been recognized most frequently in Northern Europe, although the pathosis is not limited to that geographic region. In the past this disorder most likely went undiagnosed because of the high prevalence of caries, but with the dramatic reduction in caries, these tooth changes have become more recognized.
Patients affected with molar incisor hypomineralization have enamel defects of one or more first permanent molars. The altered enamel may be white, yellow, or brown, with a sharp demarcation between the defective and surrounding normal enamel. Often, the involved enamel is soft and porous with a resemblance to discolored chalk or old Dutch cheese (“cheese molars”). Frequently, the incisors also are affected, but the defects generally are much less severe.
The enamel of the affected molars is very fragile and can chip easily. Often, affected molars are sensitive to cold, warm, or mechanical trauma. Toothbrushing is frequently painful, with a tendency for the children to avoid brushing these teeth. As would be expected, the lack of normal enamel and absence of appropriate hygiene lead to rapid development of caries. During attempts at dental therapy, these teeth often are highly sensitive and very difficult to anesthetize.
The cause of molar incisor hypomineralization is unknown, but many investigators believe the condition arises from a systemic influence during the first years of life, coinciding with the period of mineralization of the affected dentition. A number of prevalence studies have been performed with the results ranging from 3.6% to 25%.
HYPOPLASIA CAUSED BY ANTINEOPLASTIC THERAPY: As modern medicine increases the prevalence of successful therapy against childhood cancer, it has become evident that a number of developmental alterations arise secondary to use of therapeutic radiation or chemotherapy. As would be expected, developing teeth are affected most severely, with these therapies producing clinically obvious alterations most commonly in patients younger than 12 years and most extensively in those younger than 5 years. The degree and severity of the developmental alterations are related to the patient’s age at treatment, the form of therapy, and the dose and field of radiation, if used.
Although both chemotherapeutic agents and radiation therapy can be responsible for developmental abnormalities, the most severe alterations are associated with radiation. Doses as low as 0.72 Gy are associated with mild developmental defects in both enamel and dentin. As the dose escalates, so does the effect on the developing dentition and jaws. Frequently noted alterations include hypodontia, microdontia, radicular hypoplasia, and enamel hypoplasia (Fig. 2-7). In addition, mandibular hypoplasia and a reduction of the vertical development of the lower third of the face are not rare. The mandibular hypoplasia may be the direct effect of the radiation, reduced alveolar bone growth secondary to impaired root development, or (possibly) growth failure related to altered pituitary function caused by cranial radiation. Chemotherapy alone results in much less dramatic alterations but can produce an increased number of enamel hypoplasias and discolorations, slightly smaller tooth size, and occasional radicular hypoplasia that is less severe than that secondary to radiation.
DENTAL FLUOROSIS: The ingestion of excess amounts of fluoride also can result in significant enamel defects known as dental fluorosis. In 1901, Dr. Frederick S. McKay suggested the association between this altered enamel and an agent in the Colorado Springs, Colorado, water supply during investigation of the Colorado brown stain seen in the teeth of many of his patients. In 1909, Dr. F.L. Robertson noted a similar association in many of his patients in Bauxite, Arkansas (the home of bauxite mines for aluminum). In 1930, H.V. Churchill, a chemist in Bauxite who was employed by the Aluminum Company of America, discovered high concentrations of fluoride (13.7 ppm) in the water and contacted McKay for samples of the water in affected areas of Colorado. McKay’s samples also demonstrated high levels of fluoride, and the final part of the puzzle was solved.
Although the fluoride produced an unusual and permanent dental stain, a resistance to caries also was noted. In 1931 the National Institutes of Health hired Dr. H. Trendley Dean to investigate the association between fluoride, the presence of dental fluorosis, and the prevalence of caries among children. Ultimately this led to the first water fluoridation clinical trial in Grand Rapids, Michigan. Because of the efforts of these pioneers and the simultaneous work of many others, it was discovered that fluoride in the water at 1.0 ppm reduced caries by 50% to 70%. Since 1962 fluoridation of drinking water is recommended, with the optimum range being 0.7 to 1.2 ppm. The lower concentration is recommended for warmer climates in which water consumption is thought to be higher, but this distinction has been questioned because of an evolving indoor lifestyle and the use of modern air-conditioning. In 1999 the United States Centers for Disease Control and Prevention designated fluoridation of drinking water as one of the ten great public health achievements of the twentieth century in the United States.
Initially, fluoride’s ability to reduce caries was thought to be secondary to its incorporation into developing enamel, resulting in a stronger and more acid-resistant fluorapatite crystal. A number of more recent studies have suggested that the posteruptive effects of fluoride may be of equal or even greater importance. Researchers believe that continued exposure to topical fluoride contained in products such as toothpaste or fluoridated water inhibits demineralization, enhances remineralization, and exhibits antibacterial effects. In addition, they have suggested that preeruptive fluoride is most effective against pit and fissure caries, whereas smooth surface caries is affected most significantly by posteruptive exposure.
Consumption of optimally fluoridated water has been associated with a low prevalence of altered enamel, which usually is mild in degree. However, an increased prevalence of dental fluorosis has been noted in recent years. In addition, the relative caries reduction in fluoridated communities has improved between 8% and 37%. This has been attributed to the diffusion of fluoride to nonfluoridated areas through bottling and processing of foods and beverages with fluoridated water, as well as to the widespread use of fluoride toothpaste. Adult-strength fluoride toothpastes, fluoride supplements, infant foods, soft drinks, fruit juices, and industrial environmental emissions all represent potential sources of fluoride for children in their formative years. Infant formulas also used to contain significant amounts of fluoride; however, in 1979, U.S. manufacturers voluntarily agreed to dramatically limit fluoride in infant formulas. Despite this, some investigators have noted an increased prevalence of fluorosis continuing after 1979 in individuals who consumed powdered, concentrated formula that was reconstituted with optimally fluoridated water. To minimize the chance of fluorosis, the use of ready-to-feed formula or reconstitution with low-fluoride bottled water has been recommended.
Because of this dissemination of fluoride, the need for supplements in nonfluoridated areas is declining. In patients who use fluoride toothpastes, the anticariogenic benefit of supplements is very small or nonexistent and the risk of fluorosis at the community level becomes a certainty. Several investigators have recommended strongly that children younger than 7 years of age apply only a pea-sized amount of fluoride toothpaste on the toothbrush and avoid swallowing. Because young children tend to swallow almost all toothpaste placed on their brush, parents should be warned to avoid fluoridated toothpaste in children younger than 2 years of age and perform oral hygiene with only a toothbrush and water. In addition, fluoride supplements are recommended only in nonfluoridated areas for children who are at high risk for rampant caries. Finally, an effort is under way to alter the 1962 recommendation and lower the optimum level of fluoride in the public water supply to 0.7 ppm.
Fluoride appears to create its significant enamel defects through retention of the amelogenin proteins in the enamel structure, leading to the formation of hypomineralized enamel. These alterations create a permanent hypomaturation of the enamel in which an increased surface and subsurface porosity of the enamel is observed. This enamel structure alters the light reflection and creates the appearance of white, chalky areas. Most of the problems associated with dental fluorosis are aesthetic and concern the appearance of the anterior teeth. Therefore, the critical period for clinically significant dental fluorosis is during the second and third years of life, when these teeth are forming.
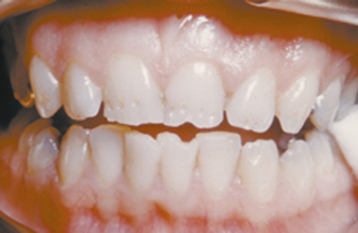
The severity of dental fluorosis is dose dependent, with higher intakes of fluoride during critical periods of tooth development being associated with more severe fluorosis. The affected teeth are caries resistant, and the altered tooth structure appears as areas of lusterless white opaque enamel that may have zones of yellow to dark-brown discoloration (Figs. 2-8 and 2-9). In the past, areas of moderate-to-severe enamel fluorosis were termed mottled enamel. True enamel hypoplasia is uncommon but can occur as deep, irregular, and brownish pits. Because other factors can result in a similar pattern of enamel damage, a definitive diagnosis requires that the defects be present in a bilaterally symmetric distribution, and evidence of prior excessive fluoride intake or elevated levels of fluoride in the enamel or other tissues should be found.
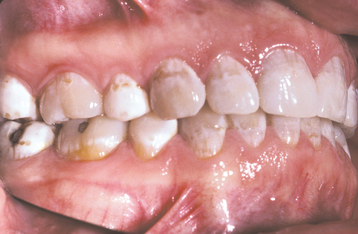
Fig. 2-9 Dental fluorosis. White opaque alteration of the bicuspids and second molars in a patient who also exhibits discoloration of the teeth secondary to tetracycline use. Patient moved to area of endemic fluorosis at 3 years of age.
Recently, an increased prevalence of dental changes similar to dental fluorosis has been linked to amoxicillin use during early infancy. Commonly affected teeth include the permanent first molars and maxillary central incisors. The number of affected teeth appears to correlate with the duration of use. Although the mechanism of this alteration is unclear, the antibiotic may reduce gene expression of selected matrix proteins or reduce the activity of proteinases that hydrolyze matrix proteins. It also should be noted that one of the etiologic theories suggested for molar incisor hypomineralization (see page 58) is prior antibiotic therapy.
SYPHILITIC HYPOPLASIA: Congenital syphilis (see page 190) results in a pattern of enamel hypoplasia that is well known but currently so rare that lengthy discussion is not warranted. Anterior teeth altered by syphilis are termed Hutchinson’s incisors and exhibit crowns that are shaped like straight-edge screwdrivers, with the greatest circumference present in the middle one third of the crown and a constricted incisal edge. The middle portion of the incisal edge often demonstrates a central hypoplastic notch. Altered posterior teeth are termed mulberry molars and demonstrate constricted occlusal tables with a disorganized surface anatomy that resembles the bumpy surface of a mulberry.
TREATMENT AND PROGNOSIS: Most defects in the enamel are cosmetic rather than functional dental problems. Those affected by dental fluorosis often benefit from surface microabrasion, which produces a dramatic and permanent improvement in the surface brown or yellow discoloration. Improvement in the white surface markings usually requires further restorative dentistry. Other types of environmental enamel hypoplasia have been associated with an increased prevalence of caries, with one study reporting more than twice the level in patients with such enamel defects. The decreased caries resistance is thought to be secondary to focal loss of enamel or because of imperfect enamel. The areas most frequently associated with an increased prevalence of caries demonstrate full-thickness enamel defects. Aesthetically or functionally defective teeth can be restored through a variety of cosmetically pleasing techniques, such as the following:
POSTDEVELOPMENTAL LOSS OF TOOTH STRUCTURE
Tooth structure can be lost after its formation by a variety of influences beyond the obvious cases related to caries or traumatic fractures. Destruction can begin on the enamel surface of the crown through abrasion, attrition, erosion, or abfraction. In addition, loss of tooth structure can begin on the dentin or cemen-tal surfaces of the teeth by external or internal resorption.
TOOTH WEAR
Tooth wear, also termed tooth surface loss, is a normal physiologic process that occurs with aging but must be considered pathologic when the degree of destruction creates functional, aesthetic, or dental sensitivity problems. Although the four causes of tooth wear (i.e., attrition, abrasion, erosion, abfraction), often are discussed as independent pathoses, most of these types of tooth loss are the result of a combination of influences. Many cases of attrition are accelerated by the presence of abrasive materials in the mouth. Erosion or abrasion often further damages areas of dentin exposed by attrition or abfraction. Areas softened by erosion are more susceptible to attrition, abrasion, and abfraction. The clinician should appreciate that acquired environmental loss of tooth structure often is multifactorial.
Most researchers agree that the reported prevalence of tooth wear is increasing. This is explained partly by a greater awareness among clinicians and by the adult population retaining more natural teeth as they age. In addition, younger individuals appear to exhibit an increased tooth surface loss that many believe may be caused by a more acidic diet (e.g., acidic soft drinks, diet foods, fresh fruits).
Attrition is the loss of tooth structure caused by tooth-to-tooth contact during occlusion and mastication. The term comes from the Latin verb attritum, which refers to the action of rubbing against another surface. Some degree of attrition is physiologic, and the process becomes more noticeable with age. When the amount of tooth loss is extensive and begins to affect aesthetic appearance and function, the process must be considered pathologic.
The following factors can accelerate tooth destruction:
• Poor-quality or absent enamel (e.g., fluorosis, environmental or hereditary enamel hypoplasia, or dentinogenesis imperfecta)
Abrasion is the pathologic wearing away of tooth structure or restoration secondary to the mechanical action of an external agent. The term arises from the Latin verb abrasum, which literally means to scrape off and implies wear or partial removal through a mechanical process. The most common cause of abrasion is toothbrushing that combines abrasive toothpaste with heavy pressure and a horizontal brushing stroke. Other items frequently associated with dental abrasion include pencils, toothpicks, pipe stems, and bobby pins (hair grips). Chewing tobacco, cracking nuts and seeds, biting fingernails or thread, and using dental floss inappropriately also can cause clinically significant abrasion. When tooth wear is accelerated by chewing an abrasive substance between opposing teeth, the process has been termed demastication and exhibits features of both attrition and abrasion.
Erosion is the loss of tooth structure caused by a nonbacterial chemical process. The term is derived from the Latin verb erosum, which literally means to corrode and implies gradual destruction of a surface by a chemical or electrolytic process. Some investigators have suggested that the term dental corrosion would be a more appropriate designation for this process, but review of modern dictionaries reveals both terms are acceptable, with little need for a disruption in the long-held nomenclature of tooth wear. Typically, the exposure to an acid is to blame, but chelating agents are occasionally the primary cause. Although saliva aids remineralization and contains bicarbonate with a significant buffering ability, this effect can be overwhelmed by xerostomia or excess acid. Causes for salivary gland hypofunction include salivary gland aplasia, dehydration, therapeutic radiation, medications and systemic conditions such as Sjögren syndrome, bulimia nervosa, and diabetes. The acidic source often is foods or drinks, but other causes include some medications (e.g., chewable vitamin C, aspirin tablets), swimming pools with poorly monitored pH, chronic involuntary regurgitation (e.g., hiatal hernia, esophagitis, chronic alcoholism, pregnancy), voluntary regurgitation (e.g., psychologic problems, bulimia, occupations that require low body weight), and industrial environmental exposure. Erosion from dental exposure to gastric secretions is termed perimolysis. Because saliva has the ability to remineralize tooth surfaces exposed to acid, it appears that areas of erosive damage must have some abrasive component that removes the softened enamel before remineralization.
Agreement on the prevalence of dental erosion does not exist. Some investigators believe erosion rarely is responsible solely for loss of tooth structure, although others list erosion as the leading cause of accelerated tooth wear.
Abfraction refers to the loss of tooth structure from occlusal stresses that create repeated tooth flexure with failure of enamel and dentin at a location away from the point of loading. The term is derived from the Latin words ab and fractio, which respectively translate into away and breaking. Dentin is able to withstand greater tensile stress than enamel. When occlusal forces are applied eccentrically to a tooth, the tensile stress is concentrated at the cervical fulcrum, leading to flexure that may produce disruption in the chemical bonds of the enamel crystals in the cervical areas. Once damaged, the cracked enamel can be lost or more easily removed by erosion or abrasion. Some investigators have suggested that the placement of occlusal restorations weakens the tooth’s ability to resist the stresses of occlusion and predisposes to future abfractive lesions.
Like erosion, agreement on the prevalence of abfraction does not exist. Some propose that abfraction causes most cervical tooth loss; others believe that little evidence exists to indicate that this sequence of events actually occurs in the mouth. Some investigators have suggested that the engineering models used to justify abfraction have not taken into consideration the cushioning provided by the surrounding bone and peri odontium, which may dissipate occlusal forces acting on a tooth. The pattern of cervical tooth loss tends to occur at sites with diminished serous salivary flow and could be explained by the initial loss of salivary protection rather than excess occlusal forces. Involvement by abfraction of the facial cervical areas of the anterior maxillary dentition is very puzzling, because the flexure during function would occur on the palatal surface of the tooth, not the facial surface. During function, investigators have found little evidence that strains in lingual enamel and dentin are any different from those that occur in facial sites; however, areas of focal cervical tooth loss occur almost exclusively on the facial surfaces. Finally, review of skulls from ancient Australian aborigines has revealed advanced tooth wear both occlusally and interproximally; and despite evidence of heavy occlusal loads, cervical defects were rare.
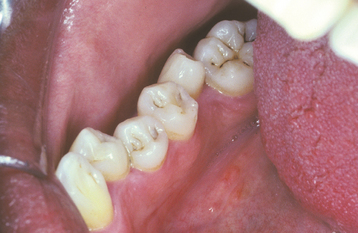
ATTRITION: Attrition can occur in both the deciduous and the permanent dentitions. As would be expected, the surfaces predominantly affected are those that contact the opposing dentition. Most frequently, the incisal and occlusal surfaces are involved, in addition to the lingual of the anterior maxillary teeth and the labial of the anterior mandibular teeth. Large, flat, smooth, and shiny wear facets are found in a relationship that corresponds to the pattern of occlusion. The interproximal contact points also are affected from the vertical movement of the teeth during function. Over time, this interproximal loss can result in a shortening of the arch length. Pulp exposure and dentin sensitivity are rare because of the slow loss of tooth structure and the apposition of reparative secondary dentin within the pulp chamber (Fig. 2-10).
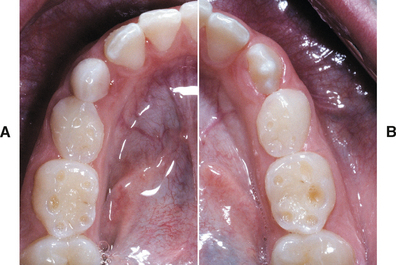
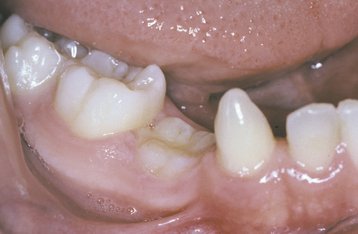
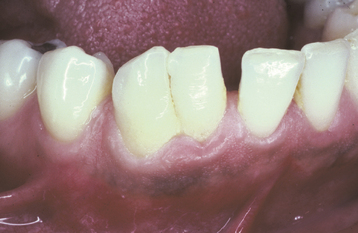
ABRASION: Abrasion has a variety of patterns, depending on the cause. Toothbrush abrasion typically appears as horizontal cervical notches on the buccal surface of exposed radicular cementum and dentin (Fig. 2-11). The defects usually have sharply defined margins and a hard, smooth surface. If acid also is present, then the lesions will be more rounded and shallower. The degree of loss is greatest on prominent teeth (i.e., cuspids, bicuspids, teeth adjacent to edentulous areas) and occasionally is more advanced on the side of the arch opposite the dominant hand. Thread biting or the use of pipes or bobby pins usually produces rounded or V-shaped notches in the incisal edges of anterior teeth (Figs. 2-12 and 2-13). The inappropriate use of dental floss or toothpicks results in the loss of interproximal radicular cementum and dentin.
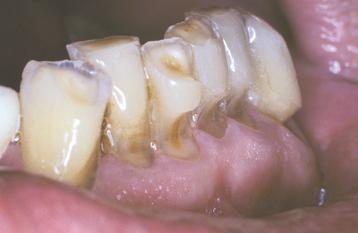
Fig. 2-11 Abrasion. Horizontal cervical notches on the anterior mandibular dentition. Note visible pulp canals that have been filled with tertiary dentin.
EROSION: In patients with erosion, the tooth loss does not correlate with functional wear patterns or with those typically associated with known abrasives. The predominant sites of tooth loss appear to correlate closely with those areas not protected by the serous secretions of the parotid and submandibular glands. The facial and palatal surfaces of the maxillary anterior teeth and the facial and occlusal surfaces of the mandibular posterior teeth are affected most frequently. Involvement of the lingual surfaces of the entire mandibular dentition is uncommon, possibly because of the protective buffering capacity of the submandibular serous saliva.
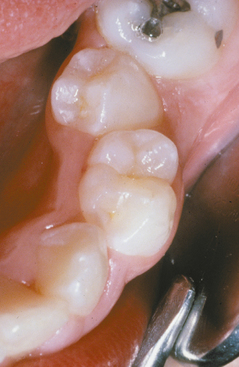
The classic pattern of dental erosion is the cupped lesion in which a central depression of dentin is surrounded by elevated enamel. Cupped areas are seen on the occlusal cusp tips, incisal edges, and marginal ridges (Fig. 2-14). In contrast to abrasion, erosion commonly affects the facial surfaces of the maxillary anteriors and appears as shallow spoon-shaped depressions in the cervical portion of the crown. The posterior teeth frequently exhibit extensive loss of the occlusal surface, and the edges of metallic restorations subsequently may be above the level of the tooth structure (Fig. 2-15). After a portion of the cuspal enamel has been lost, the dentin is destroyed more rapidly than the remaining enamel, often resulting in a concave depression of the dentin surrounded by an elevated rim of enamel (Fig. 2-16). The more rapid dissolution of the dentin can lead to undermined enamel that often is lost easily by chipping. Occasionally, entire buccal cusps are lost and replaced by ski slope–like depressions that extend from the lingual cusp to the buccal cementoenamel junction (Fig. 2-17). When palatal surfaces are affected, the exposed dentin has a concave surface and shows a peripheral white line of enamel (Fig. 2-18). Active erosion typically reveals a clean, unstained surface, whereas inactive sites become stained and discolored.
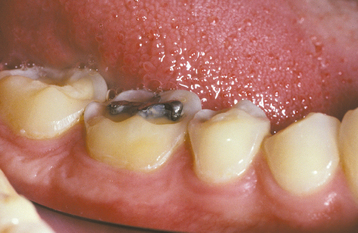
Fig. 2-15 Erosion. Extensive loss of buccal and occlusal tooth structure. Note that the amalgam margins are above the surface of the dentin.
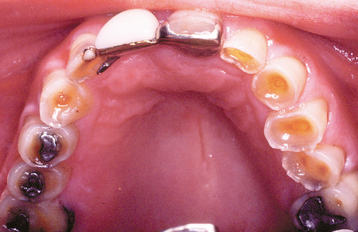
Fig. 2-16 Erosion. Occlusal view of the maxillary dentition exhibiting concave dentin depressions surrounded by elevated rims of enamel.
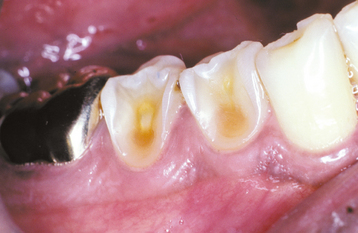
Fig. 2-17 Erosion. Extensive loss of enamel and dentin on the buccal surface of the maxillary bicuspids. The patient had sucked chronically on tamarinds (an acidic fruit).
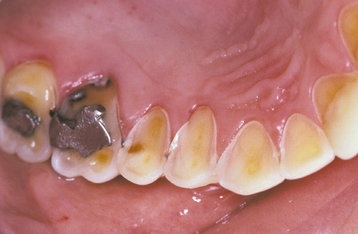
Fig. 2-18 Erosion. Palatal surfaces of the maxillary dentition in which the exposed dentin exhibits a concave surface and a peripheral white line of enamel. The patient had bulimia.
Focal facial tooth wear of the gingival portion has been given the nonspecific term, noncarious cervical lesions, in an attempt to emphasize the multifactorial nature of the process. These cervical defects often are seen in association with loss of occlusal tooth structure, which has features of erosion, attrition, or both.
Erosion limited to the facial surfaces of the maxillary anterior dentition often is associated with dietary sources of acid. When the tooth loss is confined to the incisal portions of the anterior dentition of both arches, an external environmental source is suggested. When erosion is located on the palatal surfaces of the maxillary anterior teeth and the occlusal surfaces of the posterior teeth of both dentitions, regurgitation of gastric secretions is a probable cause. The location of the tooth structure loss may suggest the cause of the damage but is not completely reliable.
ABFRACTION: Abfraction appears as wedge-shaped defects limited to the cervical area of the teeth and may closely resemble cervical abrasion or erosion. Clues to the diagnosis include defects that are deep, narrow, and V-shaped (which do not allow the toothbrush to contact the base of the defect) and often affect a single tooth with adjacent unaffected teeth (Fig. 2-19). In addition, occasional lesions are subgingival, a site typically protected from abrasion and erosion. The lesions predominantly affect bicuspids and molars, are seen almost exclusively on the facial surface, and exhibit a much greater prevalence in those with bruxism.
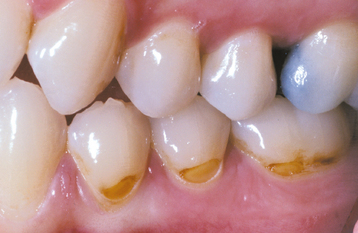
Fig. 2-19 Abfraction. Deep and narrow enamel cervical defects on the facial surface of the mandibular dentition. (From Neville BW, Damm DD, White DK: Color atlas of clinical oral pathology, ed 2, Hamilton, 1999, BC Decker.)
In all forms of tooth wear, the process typically proceeds at a slow rate that allows deposition of tertiary dentin and prevents pulp exposure, even when extensive loss of tooth structure is present (see Fig. 2-11). In some cases, and especially in the deciduous dentition, the tooth loss can proceed at a more accelerated rate that results in a near or frank exposure of the pulp. In a large review of 448 patients with tooth wear, 11.6% revealed near or direct pulp exposure. In addition, hypersensitivity was the presenting symptom in about one third of patients with tooth wear.
TREATMENT AND PROGNOSIS: Normal levels of attrition require no therapy, with intervention reserved for those cases that create a pathologic degree of tooth loss. The presence of advanced tooth wear in the deciduous dentition appears to correlate with subsequent tooth wear in adulthood, suggesting a continuation of the causative influences. Early diagnosis and intervention may assist in preserving the permanent dentition. Before any definitive action, the clinician must remember that tooth wear almost invariably has a multifactorial cause. Failure to recognize the interrelationships of these pathoses can lead to inappropriate therapy and failure of any attempted repair. Intervention should emphasize detailed diagnosis, preventive measures, and long-term monitoring. Immediate therapy should be directed toward resolution of tooth sensitivity and pain, but identifying the causes of tooth structure loss and protecting the remaining dentition also are important goals.
In patients affected by dental erosion, preventive interventions should attempt not only to reduce acid exposure but also to improve the oral cavity’s ability to resist the effects of acid. Upon exposure to an acid, the saliva has the ability to achieve remineralization with time, but teeth are vulnerable to abrasion before completion of this action. Investigators have recommended a minimum 1-hour interval between acid exposure and toothbrushing in an attempt to minimize abrasion of the weakened enamel. Patients with erosion should limit toothbrushing to once a day in the morning because of the increased vulnerability of acid-etched enamel to abrasion and attrition. Low-abrasive toothpaste and professional guidance to prevent inappropriate, overzealous, or too frequent toothbrushing may assist in reducing associated abrasion. Consumption of buffering substances such as milk and cheese also is thought to be beneficial. Proper hydration is extremely important to maintain sufficient salivary flow. A suspected common cause of tooth loss is decreased salivary flow secondary to dehydration, often associated with strenuous work or athletic activities and possibly complicated by use of acidic soft drinks or sports beverages in the place of water. Chewing gum has been suggested as a method for decreasing dental erosion by increasing salivary flow after acid exposure, but others have demonstrated that enamel softened by acid can be damaged by the adjacent soft tissues during the movements of chewing in this time of vulnerability. Patients should be informed of the potential for loss of tooth structure associated with the overuse of acidic foods and drinks (e.g., wine, carbonated beverages, foods pickled in acetic acid, and citrate-containing fruits, fruit juices, and candies), chronic regurgitation, and improper oral hygiene techniques. Mouth guards and occlusal adjustment can be used to slow nocturnal attrition and to protect the teeth from frequent exposure to acid from regurgitation or industrial sources. Dental sensitivity can be reduced through the use of varnishes, mouthwashes, or toothpastes containing strontium chloride, stannous fluoride, or monofluorophosphate. If initially unsuccessful, these agents can be combined with iontophoresis.
Active restorative therapy is premature in the presence of ongoing tooth wear and should be postponed until the patient expresses strong aesthetic concerns, exhibits dental sensitivity that is nonresponsive to conservative interventions, or demonstrates progressive and uncontrollable wear. Once indicated, the minimum treatment necessary to solve the problem should be implemented. In lesions thought to represent abfraction, glass ionomer materials are recommended because of their greater resilience that allows the material to flex with the tooth. In areas of abrasion, a material with optimum resistance to the abrasive process should be chosen. In isolated teeth that continue to lose Class V restorations, continued abfraction is likely, and occlusal trauma should be eliminated. Replacement of lost posterior teeth and avoidance of edge-to-edge occlusion limit the effects of attrition. Lost tooth structure can be restored with composite resins, veneers, onlays, or full crowns. Restorative procedures that do not involve significant removal of remaining tooth structure are preferable in patients demonstrating extensive tooth wear.
The body may adapt to loss of tooth structure by continual eruption of the teeth, appositional alveolar bone deposition, and compensatory skeletal growth. If the process of tooth loss is slow, the vertical dimension often is maintained; in patients with rapid destruction, a loss of facial length occurs. Restoration of extensive loss of tooth structure is complex and should be performed only after a complete evaluation of the dentoalveolar complex.
INTERNAL AND EXTERNAL RESORPTION
In addition to loss of tooth structure that begins on the exposed coronal surfaces, destruction of teeth also can occur through resorption, which is accomplished by cells located in the dental pulp (i.e., internal resorption) or in the periodontal ligament (PDL) (i.e., external resorption). Internal resorption is a relatively rare occurrence, and most cases develop after injury to pulpal tissues, such as physical trauma or caries-related pulpitis. The resorption can continue as long as vital pulp tissue remains and may result in communication of the pulp with the PDL.
By contrast, external resorption is extremely com-mon; with close examination, all patients are most likely to have root resorption on one or more teeth. In one radiographic review of 13,263 teeth, all patients showed evidence of root resorption, and 86.4% of the examined teeth demonstrated external resorption, with an average of 16 affected teeth per patient. Most areas of resorption are mild and of no clinical significance, but 10% of patients exhibit unusual amounts of external resorption.
The potential for resorption is inherent within the periodontal tissue of each patient, and this individual susceptibility to resorption is the most important factor in the degree of resorption that will occur after a stimulus. The factors reported to increase the severity of external resorption are delineated in Box 2-3. Many cases have been termed idiopathic because no factor could be found to explain the accelerated resorption. When pretreatment radiographs of a given patient exhibit a degree of resorption beyond that which is normally seen, the clinician should realize the potential risks involved in initiating procedures (e.g., ortho dontics) that are known to be associated with an increased risk of external resorption.
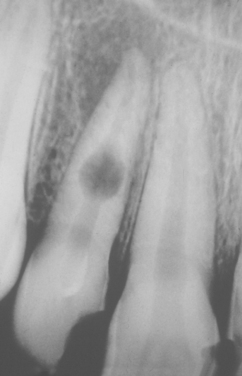
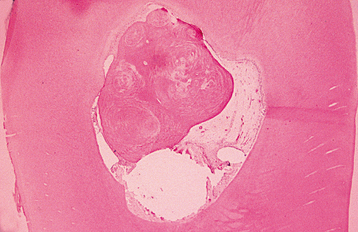
CLINICAL AND RADIOGRAPHIC FEATURES: Resorption of dentin or cementum can occur at any site that contacts vital soft tissue. Internal resorption is usually asymptomatic and discovered through routine radiographs. Pain may be reported if the process is associated with significant pulpal inflammation. Two main patterns are seen: (1) inflammatory resorption and (2) replacement or metaplastic resorption (Fig. 2-20). In inflammatory resorption, the resorbed dentin is replaced by inflamed granulation tissue. Although this pattern may involve any portion of the canal, the cervical zone is affected most frequently (and the pulpal inflammation is usually caused by bacterial invasion). The resorption continues as long as vital pulp remains; typically, the coronal pulp is necrotic, with the apical portion remaining vital. The results of pulp testing are variable. In this pattern the area of destruction usually appears as a uniform, well-circumscribed symmetric radiolucent enlargement of the pulp chamber or canal. When it affects the coronal pulp, the crown can display a pink discoloration (pink tooth of Mummery) as the vascular resorptive process approaches the surface (Fig. 2-21). When it occurs in the root, the original outline of the canal is lost and a balloonlike radiographic dilation of the canal is seen (Fig. 2-22). If the process continues, the destruction eventually can perforate the lateral root surface, which may be difficult to distinguish from external root resorption (Fig. 2-23). Although most cases are progressive, some cases are transient and usually arise in traumatized teeth or those that have recently undergone orthodontic or periodontal therapy.
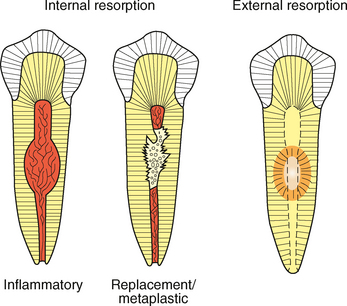
Fig. 2-20 Tooth resorption. Illustration contrasting the common patterns of internal and external tooth resorption. Internal resorption will result in a radiolucent enlargement of the pulp chamber or canal. In external resorption, the radiolucency is superimposed on the pulp canal, which should not be enlarged.
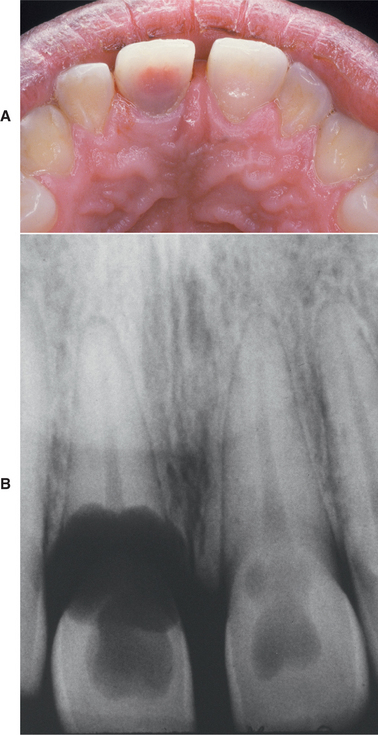
Fig. 2-21 Internal resorption (pink tooth of Mummery). A, Pink discoloration of the maxillary central incisor. B, Radiograph of same patient showing extensive resorption of both maxillary central incisors.
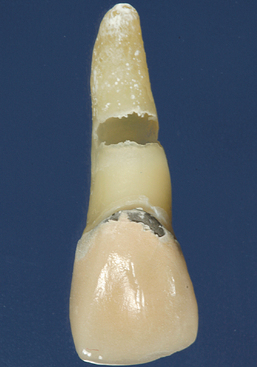
Fig. 2-23 Internal resorption. The destruction has resulted in perforation of the lateral root surface.
The remaining pattern of internal resorption is termed replacement or metaplastic resorption. In this form, portions of the pulpal dentinal walls are resorbed and replaced with bone or cementum-like bone (see Fig. 2-20). Radiographically, replacement resorption appears as an enlargement of the canal, which is filled with a material that is less radiodense than the surrounding dentin. Because a central zone of the pulp is replaced with bone, the radiographic appearance often demonstrates partial obliteration of the canal. The outline of destruction is less defined than that seen in inflammatory resorption.
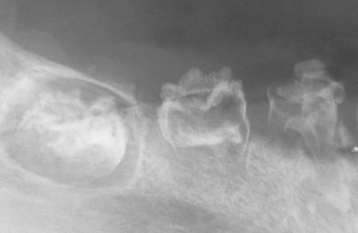
By contrast, external resorption typically appears as a “moth-eaten” loss of tooth structure in which the radiolucency is less well defined and demonstrates variations in density (Figs. 2-24 to 2-27). If the lesion overlies the pulp canal, then close examination demonstrates the retention of the unaltered canal through the area of the defect. Most cases involve the apical or midportions of the root. External resorption can create significant defects in the crowns of teeth before eruption (see Fig. 2-26). This pattern frequently is misdiagnosed as preeruptive caries and is thought by some investigators to be caused by defects in the enamel epithelium that allow connective tissue to come into direct contact with the enamel.
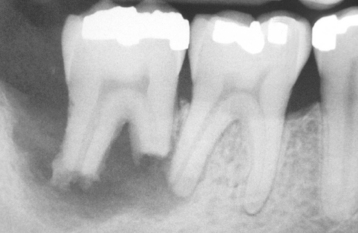
Fig. 2-24 External resorption. Extensive irregular destruction of both roots of the mandibular second molar associated with chronic periodontitis. (Courtesy of Dr. Tommy Shimer.)
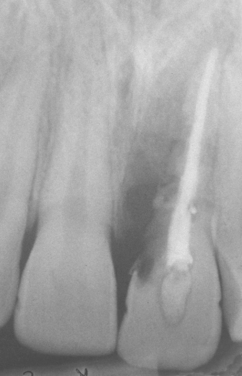
Fig. 2-25 External resorption. “Moth-eaten” radiolucent alteration of the maxillary left central incisor. The tooth had been reimplanted after traumatic avulsion. (Courtesy of Dr. Harry Meyers.)
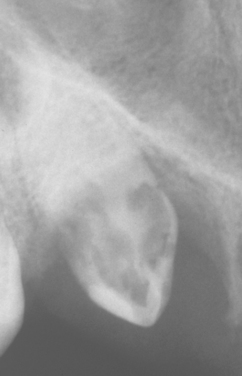
Fig. 2-26 External resorption. Extensive external resorption of the crown of the impacted right maxillary cuspid. Histopathologic examination revealed resorption without bacterial contamination or caries.
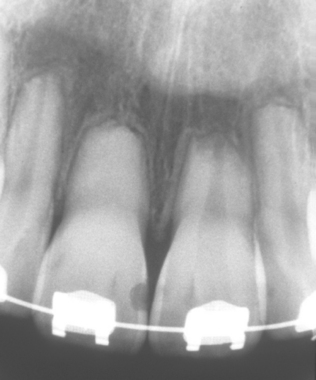
Fig. 2-27 External resorption. Diffuse external resorption of radicular dentin of maxillary dentition. This process arose after initiation of orthodontics.
In reimplanted avulsed teeth, extensive external resorption of the root is extremely common without rapid and appropriate intervention (see Fig. 2-25). If the tooth remains outside of the socket without being placed in a proper storage medium, then the PDL cells will undergo necrosis. Without vital PDL cells, the surrounding bone will view the tooth as a foreign object and initiate resorption and replacement by bone.
External resorption occurring during orthodontics does not appear to be affected significantly by the patient’s sex or age, the severity of malocclusion, or the type of mechanics used during therapy. Although the patient’s individual susceptibility has the strongest influence, the single most important skeletodental predictor is the distance a tooth is moved during therapy. The maxillary anterior teeth typically are the most severely affected, particularly in patients who have been treated with premolar extractions. Movement of teeth with an abnormal root shape such as dilaceration also has been associated with an increased severity of external resorption.
Occasionally, external resorption may begin in the cervical area and extend from a small opening to involve a large area of the dentin between the cementum and the pulp. The resorption can extend apically into the pulp or coronally under the enamel and simulate the pink tooth seen in internal resorption. The cervical pattern of external resorption often is rapid and has been termed invasive cervical resorption. In some instances, several teeth may be involved, and an underlying cause for the accelerated destruction may not be obvious (multiple idiopathic root resorption) (Fig. 2-28). The exact cause of this pattern of resorption has been elusive, and it may result from a variety of inflammatory, traumatic, or bacterial stimuli affecting the clastic cells within the PDL. The process has been noted after orthodontic therapy, orthognathic surgery, other dentoalveolar surgery, root scaling or planing, internal bleaching of endodontically treated teeth, local trauma, bruxism, and tooth fracture. Other investigators believe this pattern of resorption can be triggered by periodontal pathogens and have seen good response to local mechanical débridement combined with systemic antibiotics.
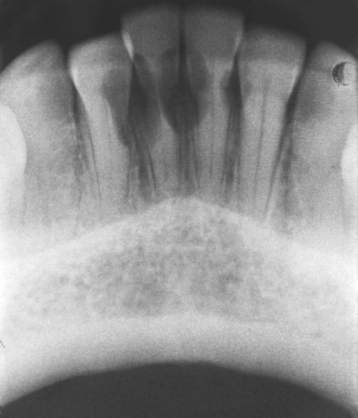
Fig. 2-28 Multiple idiopathic root resorption. Extensive invasive cervical resorption of several anterior mandibular teeth. (Courtesy of Dr. Keith Lemmerman.)
In addition to invasive cervical resorption, generalized and progressive external resorption also can affect the apical portion of the roots. Although this pattern can occur secondary to an endocrine disturbance or one of a small number of systemic conditions, many of these cases are idiopathic and difficult to arrest.
If difficulty arises in distinguishing external from internal resorption, then the mesial-buccal-distal rule can be used through two radiographic exposures: one perpendicular and one mesial (objects closer to the source of radiation will shift distally). With this technique, the sites of external resorption appear to shift away from the pulp canal when the radiographs are compared. In addition, the radiographs can reveal which side of the root is affected in cases of external resorption.
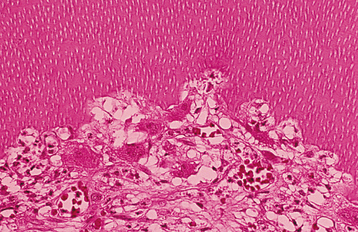
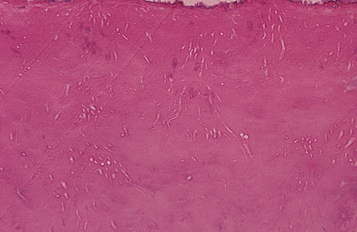
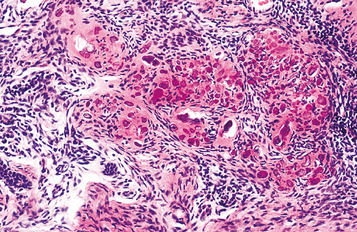
HISTOPATHOLOGIC FEATURES: In patients with internal inflammatory resorption, the pulp tissue in the area of destruction is vascular and exhibits increased cellularity and collagenization. Immediately adjacent to the dentinal wall are numerous multinucleated dentinoclasts, which are histologically and functionally identical to osteoclasts (Fig. 2-29). An inflammatory infiltrate characterized by lymphocytes, histiocytes, and polymorphonuclear leukocytes is not uncommon. In replacement resorption, the normal pulp tissue is replaced by woven bone that fuses with the adjacent dentin. External resorption is similar in appearance, with numerous multinucleated dentinoclasts located in the areas of structure loss. Areas of resorption often are repaired through deposition of osteodentin. In large defects, external inflammatory resorption results in deposition of inflamed granulation tissue, and areas of replacement with woven bone may also be seen. Extensive bony re-placement in areas of external resorption can lead to ankylosis.
TREATMENT AND PROGNOSIS: The treatment of internal and external resorption centers on the removal of all soft tissue from the sites of dental destruction. Internal resorption can be stopped consistently if endodontic therapy successfully removes all vital pulp tissue before the process perforates into the PDL. Once perforation occurs, therapy becomes more difficult and the prognosis is poor. In such cases, initial placement of calcium hydroxide paste occasionally may result in remineralization of the site of perforation and stop the resorptive process. If remineralization of cervical sites of perforation is not successful, then surgical exposure and restoration of the defect may halt the process. Extraction often is necessary for radicular perforations that do not respond to therapy.
The first step in treating external resorption is the identification and elimination of any accelerating factor. Apically located sites cannot be approached without significant damage created by attempts at access. Those cases located in the cervical areas can be treated by surgical exposure, removal of all soft tissue from the defects, and restoration of the lost structure of the tooth. Because the cells responsible for the resorption are located within the PDL, endodontic therapy is not effective in stopping the process. In one report of generalized cervical resorption, therapy directed against local periodontal pathogens (débridement combined with systemic metronidazole and amoxicillin) stopped the resorption and was associated with an increased density of the adjacent crestal bone.
For avulsed teeth, the best way to prevent resorption is to maintain PDL vitality by immediate reimplantation or short-term use of a physiologic storage solution. Teeth reimplanted with an open apex should be monitored monthly; for teeth with a closed apex, endodontic therapy is necessary. Avulsed teeth with an open apex and nonvital PDL cells should not be implanted.
ENVIRONMENTAL DISCOLORATION OF TEETH
The color of normal teeth varies and depends on the shade, translucency, and thickness of the enamel. Abnormal colorations may be extrinsic or intrinsic. Extrinsic stains occur from surface accumulation of an exogenous pigment and typically can be removed with a surface treatment, whereas intrinsic discolorations arise from an endogenous material that is incorporated into the enamel or dentin and cannot be removed by prophylaxis with toothpaste or pumice. Box 2-4 lists the most frequently documented causes of tooth discolorations.
Dental fluorosis is discussed in the section on environmental effects on the structural development of the teeth (see page 58). The alterations associated with amelogenesis imperfecta (see page 99) and dentinogenesis imperfecta (see page 106) are presented later in this chapter in the text devoted to primary developmental alterations of the teeth.
EXTRINSIC STAINS: Bacterial stains are a common cause of surface staining of exposed enamel, dentin, and cementum. Chromogenic bacteria can produce colorations that vary from green or black-brown to orange. The discoloration occurs most frequently in children and is usually seen initially on the labial surface of the maxillary anterior teeth in the gingival one third. In contrast to most plaque-related discolorations, the black-brown stains most likely are not primarily of bacterial origin but are secondary to the formation of ferric sulfide from an interaction between bacterial hydrogen sulfide and iron in the saliva or gingival crevicular fluid.
Extensive use of tobacco products, tea, or coffee often results in significant brown discoloration of the surface enamel (Fig. 2-30). The tar within the tobacco dissolves in the saliva and easily penetrates the pits and fissures of the enamel. Smokers (of tobacco or marijuana) most frequently exhibit involvement of the lingual surface of the mandibular incisors; users of smokeless tobacco often demonstrate involvement of the enamel in the area of tobacco placement. Stains from beverages also often involve the lingual surface of the anterior teeth, but the stains are usually more widespread and less intense. In addition, foods that contain abundant chlorophyll can produce a green discoloration of the enamel surface.
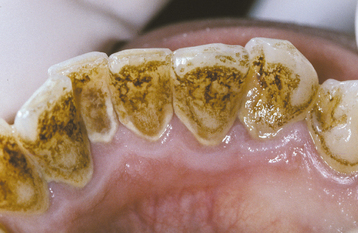
Fig. 2-30 Tobacco discoloration. Extrinsic brown stains of the enamel on the lingual surfaces of the anterior mandibular dentition secondary to long-term tobacco abuse.
The green discoloration associated with chromogenic bacteria or the frequent consumption of chlorophyll-containing foods can resemble the pattern of green staining seen secondary to gingival hemorrhage. As would be expected, this pattern of discoloration occurs most frequently in patients with poor oral hygiene and erythematous, hemorrhagic, and enlarged gingiva. The color results from the breakdown of hemoglobin into green biliverdin.
A large number of medications may result in surface staining of the teeth. In the past, use of products containing high amounts of iron or iodine was associated with significant black pigmentation of the teeth. Exposure to sulfides, silver nitrate, or manganese can cause stains that vary from gray to yellow to brown to black. Copper or nickel may produce a green stain; cadmium, essential oils, and co-amoxiclav may be associated with a yellow to brown discoloration. Multiple recent reports have documented a yellow-brown staining of teeth associated with doxycycline, which can be removed by professional abrasive cleaning; the cause of this discoloration is unclear.
More recently, the most frequently reported culprits include stannous fluoride and chlorhexidine. Fluoride staining may be associated with the use of 8% stannous fluoride and is thought to be secondary to the combination of the stannous (tin) ion with bacterial sulfides. This black stain occurs predominantly in people with poor oral hygiene in areas of a tooth previously affected by early carious involvement. The labial surfaces of anterior teeth and the occlusal surfaces of posterior teeth are the most frequently affected. Chlorhexidine is associated with a yellow-brown stain that predominantly involves the interproximal surfaces near the gingival margins. The degree of staining varies with the concentration of the medication and the patient’s susceptibility. Although an increased frequency has been associated with the use of tannin-containing beverages, such as tea and wine, effective brushing and flossing or frequent gum chewing can minimize staining. Chlorhexidine is not alone in its association with tooth staining; many oral antiseptics, such as Listerine and sanguinarine, also may produce similar changes.
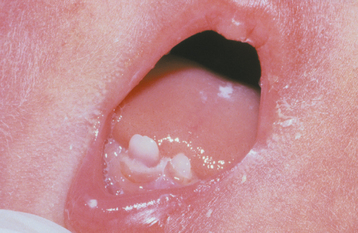
INTRINSIC STAINS: Congenital erythropoietic porphyria (Gönther disease) is an autosomal recessive disorder of porphyrin metabolism that results in the increased synthesis and excretion of porphyrins and their related precursors. Significant diffuse discoloration of the dentition is noted as a result of the deposition of porphyrin in the teeth (Fig. 2-31). Affected teeth demonstrate a marked red-brown coloration that exhibits a red fluorescence when exposed to a Wood’s ultraviolet (UV) light. The deciduous teeth demonstrate a more intense coloration because porphyrin is present in the enamel and the dentin; in the permanent teeth, only the dentin is affected. Excess porphyrins also are present in the urine, which may reveal a similar fluorescence when exposed to a Wood’s light.
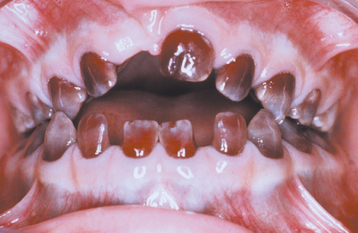
Fig. 2-31 Erythropoietic porphyria–related discoloration. Red-brown discoloration of the maxillary dentition.
Another autosomal recessive metabolic disorder, alkaptonuria, is associated with a blue-black discoloration termed ochronosis that occurs in connective tissue, tendons, and cartilage. On rare occasions, a blue discoloration of the dentition may be seen in patients who also are affected with Parkinson’s disease.
Bilirubin is a breakdown product of red blood cells, and excess levels can be released into the blood in a number of conditions. The increased amount of bilirubin can accumulate in the interstitial fluid, mucosa, serosa, and skin, resulting in a yellow-green discoloration known as jaundice (see page 821). During periods of hyperbilirubinemia, developing teeth also may accumulate the pigment and become stained intrinsically. In most cases the deciduous teeth are affected as a result of hyperbilirubinemia during the neonatal period. The two most common causes are erythroblastosis fetalis and biliary atresia. Other diseases that less frequently display intrinsic staining of this type include the following:
• Neonatal respiratory distress
• Significant internal hemorrhage
• Metabolic diseases (tyrosinemia, α1-antitrypsin deficiency)
Erythroblastosis fetalis is a hemolytic anemia of newborns secondary to a blood incompatibility (usually Rh factor) between the mother and the fetus. Currently, this disorder is relatively uncommon because of the use of antiantigen gamma globulin at delivery in mothers with Rh-negative blood.
Biliary atresia is a sclerosing process of the biliary tree and is the leading cause of death from hepatic failure in children in North America. However, many affected children live after successful liver transplantation.
The extent of the dental changes correlates with the period of hyperbilirubinemia, and most patients exhibit involvement limited to the primary dentition. Occasionally, the cusps of the permanent first molars may be affected. In addition to enamel hypoplasia, the affected teeth frequently demonstrate a green discoloration (chlorodontia). The color is the result of the deposition of biliverdin (the breakdown product of bilirubin that causes jaundice) and may vary from yellow to deep shades of green (Fig. 2-32). The color of tooth structure formed after the resolution of the hyperbilirubinemia appears normal. The teeth often demonstrate a sharp dividing line, separating green portions (formed during hyperbilirubinemia) from normal-colored portions (formed after normal levels of bilirubin were restored).
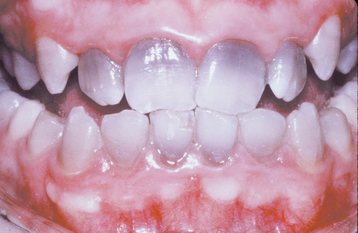
Fig. 2-32 Hyperbilirubinemia-related discoloration. Diffuse grayish-blue discoloration of the dentition. Cervical portions are stained most intensely. (Courtesy of Dr. John Giunta.)
Coronal discoloration is a frequent finding after trauma, especially in the deciduous dentition. Posttraumatic injuries may create pink, yellow, or dark-gray discoloration. Temporary pink discoloration that arises 1 to 3 weeks after trauma may represent localized vascular damage and often returns to normal in 1 to 3 weeks. In these instances, periapical radiographs are warranted to rule out internal resorption that may produce a similar clinical presentation. A yellow discoloration is indicative of pulpal obliteration, termed calcific metamorphosis, and is discussed more fully in Chapter 3 (see page 123). The dark-gray discoloration is long-term and occurs in teeth with significant pulpal pathosis in which blood degradation products have diffused into the dentinal tubules. Endodontic therapy initiated before or shortly after the total death of the pulp often prevents the discoloration. The pulpal necrosis may be aseptic and not associated with significant tenderness to percussion, mobility, or associated periapical inflammatory disease. A related process secondary to localized red blood cell destruction also can result in discoloration of the teeth. Occasionally, during a postmortem examination, a pink discoloration of teeth is found. The crowns and necks of the teeth are affected most frequently, and the process is thought to arise from hemoglobin breakdown within the necrotic pulp tissue in patients in whom blood has accumulated in the head.
A similar pink or red discoloration of the maxillary incisors has been reported in living patients with le-promatous leprosy (see page 198). Although controversial, some investigators believe these teeth are involved selectively because of the decreased temperature preferred by the causative organism. This process is thought to be secondary to infection-related necrosis and the rupture of numerous small blood vessels within the pulp, with a secondary release of hemoglobin into the adjacent dentinal tubules.
Dental restorative materials, especially amalgam, can result in black-gray discolorations of teeth. This most frequently arises in younger patients who presumably have more open dentinal tubules. Large Class II proximal restorations of posterior teeth can pro-duce discoloration of the overlying facial surface. In addition, deep lingual metallic restorations on anterior incisors can significantly stain underlying dentin and produce visible grayish discoloration on the labial surface. To help reduce the possibility of discoloration, the clinician should not restore endodontically treated anterior teeth with amalgam (Fig. 2-33).
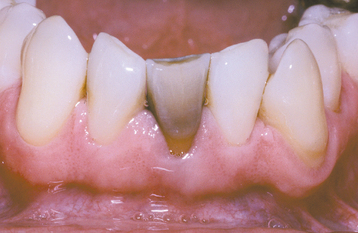
Fig. 2-33 Amalgam discoloration. Green-gray discoloration of mandibular central incisor, which had endodontic access preparation restored with amalgam.
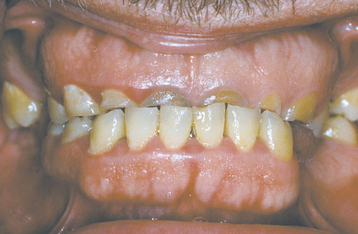
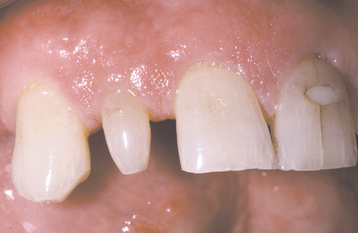
Several different medications can become incorporated into the developing tooth and result in clinically evident discoloration. The severity of the alterations is dependent on the time of administration, the dose, and the duration of the drug’s use. The most infamous is tetracycline, with the affected teeth varying from bright yellow to dark brown and, in UV light, showing a bright-yellow fluorescence (Fig. 2-34). After chronic exposure to ambient light, the fluorescent yellow discoloration fades over months to years into a nonfluorescent brown discoloration. Often the facial surfaces of the anterior teeth will darken while the posterior dentition and lingual surfaces remain a fluorescent yellow. The drug and its homologues can cross the placental barrier; therefore, administration should, if possible, be avoided during pregnancy and in children up to 8 years of age. All homologues of tetracycline are associated with discoloration and include chlortetracycline (gray-brown discoloration) and demethylchlortetracycline and oxytetracycline (yellow).
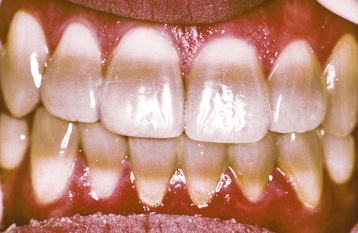
Fig. 2-34 Tetracycline-related discoloration. Diffuse brownish discoloration of the permanent dentition. (Courtesy of Dr. John Fantasia.)
One semisynthetic derivative of tetracycline, minocycline hydrochloride, has been shown to produce significant discoloration of the dentition and also may affect teeth that are fully developed. Minocycline is a widely used medication for the treatment of acne and also is occasionally prescribed to treat rheumatoid arthritis. Its prevalence of use is increasing (and, presumably, so will the number of patients affected with discolored teeth and bone).
Although the mechanism is unknown, minocycline appears to bind preferentially to certain types of collagenous tissues (e.g., dental pulp, dentin, bone, dermis). Once in these tissues, oxidation occurs and may produce the distinctive discoloration. Some investigators believe supplementation with ascorbic acid (an antioxidant) can block formation of the discoloration. No matter the cause, once the pulp tissues are stained, the coloration can be seen through the overlying translucent dentin and enamel. The staining is not universal; only 3% to 6% of long-term users become affected. In those affected, the period of time before discoloration becomes evident can range from just 1 month to several years.
In susceptible individuals, minocycline creates discoloration in the skin, oral mucosa (see page 318), nails, sclera, conjunctiva, thyroid, bone, and teeth. Coloration of the bone occasionally results in a distinctive blue-gray appearance of the palate, mandibular tori, or anterior alveolar mucosa, which represents the black bone showing through the thin, translucent oral mucosa (see page 317). Several patterns of staining are noted in the dentition. Fully erupted teeth typically reveal a blue-gray discoloration of the incisal three fourths, with the middle one third being maximally involved. The exposed roots of erupted teeth demonstrate a dark-green discoloration, although the roots of developing teeth are stained dark black.
Another antibiotic, ciprofloxacin, is given intravenously to infants for Klebsiella spp. infections. Although less notable than tetracycline, this medication also has been associated with intrinsic tooth staining, usually a greenish discoloration.
TREATMENT AND PROGNOSIS: Careful polishing with fine pumice can remove most extrinsic stains on the teeth; typically, normal prophylaxis paste is insufficient. Stubborn stains often are resolved by mixing 3% hydrogen peroxide with the pumice or by using bicarbonated spray solutions. The use of jet prophylactic devices with a mild abrasive is the most effective. Recurrence of the stains is not uncommon unless the cause is reduced or eliminated. Improving the level of oral hygiene often minimizes the chance of recurrence.
Intrinsic discoloration is much more difficult to resolve because of the frequent extensive involvement of the dentin. Suggested aesthetic remedies include external bleaching of vital teeth, internal bleaching of nonvital teeth, bonded restorations, composite buildups, laminate veneer crowns, and full crowns. The treatment must be individualized to fulfill the unique needs of each patient and his or her specific pattern of discoloration.
LOCALIZED DISTURBANCES IN ERUPTION
Eruption is the continuous process of movement of a tooth from its developmental location to its functional location. Teeth that cease to erupt before emergence are impacted. Some authors subdivide these nonerupted teeth into those that are obstructed by a physical barrier (impacted) and those that appear to exhibit a lack of eruptive force (embedded). In many cases a tooth may appear to be embedded; however, on removal a previously undetected overlying odontogenic hamartoma or neoplasm is discovered. There-fore, it appears appropriate to classify all these teeth as impacted.
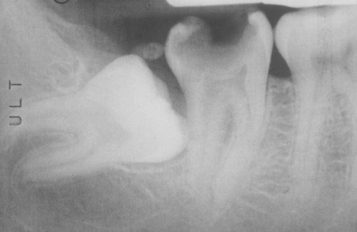
CLINICAL AND RADIOGRAPHIC FEATURES: Impaction of deciduous teeth is extremely rare; when seen, it most commonly involves second molars (Fig. 2-35). Analysis of cases suggests that ankylosis plays a major role in the pathogenesis. In the permanent dentition, the most frequently impacted teeth are the mandibular third molar, followed by maxillary third molars and maxillary cuspids. In decreasing order of frequency, impaction also may occur with mandibular premolars, mandibular canines, maxillary premolars, maxillary central incisors, maxillary lateral incisors, and mandibular second molars. First molars and maxillary second molars are rarely affected.
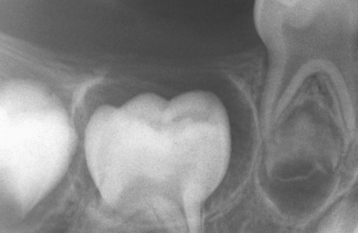
Fig. 2-35 Impaction of deciduous tooth. The right secondary primary molar demonstrates delayed eruption and enlarged pericoronal radiolucency. (Courtesy of Dr. G. Thomas Kluemper.)
Lack of eruption most frequently is caused by crowding and insufficient maxillofacial development. Procedures that create more space, such as removal of bicuspids for orthodontic purposes, are associated with a decreased prevalence of third molar impaction. Impacted teeth are frequently diverted or angulated and eventually lose their potential to erupt (on completion of root development). Other factors known to be associated with impaction include the following:
Impacted teeth may be partially erupted or completely encased within the bone (i.e., full bony impaction). In addition, the impaction may be classified according to the angulation of the tooth in relationship to the remaining dentition: mesioangular, distoangular, vertical, horizontal, or inverted. On occasion, a small spicule of nonvital bone may be seen radiographically or clinically overlying the crown of partially erupted permanent posterior tooth (Fig. 2-36). The process is termed an eruption sequestrum and occurs when the osseous fragment becomes separated from the contiguous bone during eruption of the associated tooth. On occasion, mild sensitivity is noted in the area, especially during eating.
TREATMENT AND PROGNOSIS: The choices of treatment for impacted teeth include the following:
The presence of infection, nonrestorable carious lesions, cysts, tumors, or destruction of adjacent tooth and bone mandates extraction. Surgical removal of impacted teeth is the procedure performed most frequently by oral and maxillofacial surgeons. The choice of therapy in asymptomatic cases is an area of hot debate, and no immediate resolution is obvious. The risks associated with nonintervention include the following:
• Resorption, caries, and worsening of the periodontal status of adjacent teeth (Fig. 2-37)
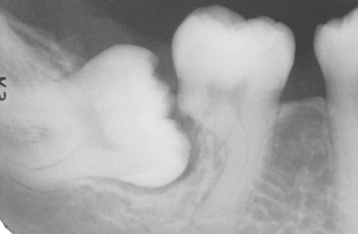
Fig. 2-37 Impaction-related tooth resorption. Mesioangular impaction of the right mandibular third molar associated with significant resorption of the distal root of the second molar. (Courtesy of Dr. Richard Brock.)
• Development of pathologic conditions, such as infections, cysts, and tumors
The risks of intervention include the following:
Dental referral patterns provide a variety of perspectives of different dental practitioners. Many specialists (e.g., oral and maxillofacial surgeons, oral and maxillofacial pathologists) see a large percentage of significant pathologic conditions associated with impacted teeth compared with the experience of other clinicians. Although pathology rarely is associated with impacted teeth in children and young adults, numerous reports have documented an increased prevalence of problems in the later decades; therefore, any meaningful prospective studies must be lifelong rather than confined to just a few years. One review of 2646 pericoronal lesions submitted to an active oral pathology service revealed that 32.9% of cases had pathol-ogically significant lesions, with strong relationship between increasing age and the prevalence of pericoronal pathosis. In this 6-year review were six primary squamous cell carcinomas arising from dentigerous cysts in addition to numerous odontogenic keratocysts and odontogenic tumors. Because of the frequent occurrence of significant pericoronal pathology, specialists often recommend extraction over close observation of impacted teeth.
The eruption sequestrum requires no therapy and usually undergoes spontaneous resorption or exfoliation.
ANKYLOSIS
Eruption continues after the emergence of the teeth to compensate for masticatory wear and the growth of the jaws. The cessation of eruption after emergence is termed ankylosis and occurs from an anatomic fusion of tooth cementum or dentin with the alveolar bone. Although the areas of union may be too subtle to be detected clinically and radiographically, histopathologic examination will demonstrate fusion between the affected tooth and the adjacent bone in almost all cases. Other terms for this process within the literature include infraocclusion, secondary retention, submergence, reimpaction, and reinclusion. Secondary retention is an acceptable term but may be confused with retained primary teeth, which maintain their emergence. Submergence, reimpaction, and reinclusion connote an active depression, and this is not the case.
The pathogenesis of ankylosis is unknown and may be secondary to one of many factors. Disturbances from changes in local metabolism, trauma, injury, chemical or thermal irritation, local failure of bone growth, and abnormal pressure from the tongue have been suggested. The periodontal ligament (PDL) might act as a barrier that prevents osteoblasts from applying bone directly onto cementum. Ankylosis could arise from a variety of factors that result in a deficiency of this natural barrier. Such loss could arise from trauma or a genetically decreased PDL gap. Other theories point to a disturbance between normal root resorption and hard tissue repair. Several investigators believe genetic predisposition has a significant influence and point to monozygotic twins who demonstrate strikingly similar patterns of ankylosis to support this hypothesis.
CLINICAL AND RADIOGRAPHIC FEATURES: Ankylosis may occur at any age; however, clinically the condition is most obvious if the fusion develops during the first two decades of life. Most patients reported in the literature with obvious alterations in occlusion are between the ages of 7 and 18 years, with a peak prevalence occurring in 8- to 9-year-old children. The reported prevalence of clinically detectable ankylosis in children varies from 1.3% to 8.9% and has been reported to be as high as 44% in siblings of those affected.
Although any tooth may be affected, the most commonly involved teeth in order of frequency are the mandibular primary first molar, the mandibular primary second molar, the maxillary primary first molar, and the maxillary primary second molar. Ankylosis of permanent teeth is uncommon. In the deciduous dentition, mandibular teeth are affected 10 times as often as the maxillary dentition. The occlusal plane of the involved tooth is below that of the adjacent dentition (infraocclusion) in a patient with a history of previous full occlusion (Fig. 2-38). A sharp, solid sound may be noted on percussion of the involved tooth but can be detected only when more than 20% of the root is fused to the bone. Radiographically, absence of the PDL space may be noted; however, the area of fusion is often in the bifurcation and interradicular root surface, making radiographic detection most difficult (Fig. 2-39).
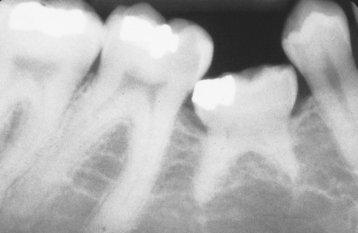
Fig. 2-39 Ankylosis. Radiograph of an ankylosed deciduous molar. Note the lack of periodontal ligament (PDL) space.
Ankylosed teeth that are allowed to remain in position can lead to a number of dental problems. The adjacent teeth often incline toward the affected tooth, frequently with the development of subsequent occlusal and periodontal problems. In addition, the opposing teeth often exhibit overeruption. Occasionally, the ankylosed tooth leads to a localized deficiency of the alveolar ridge or impaction of the underlying permanent tooth. An increased frequency of lateral open bite and crossbite is seen.
TREATMENT AND PROGNOSIS: Because they are fused to the adjacent bone, ankylosed teeth fail to respond to normal orthodontic forces, with attempts to move the ankylosed tooth occasionally resulting in intrusion of the anchor teeth. Recommended therapy for ankylosis of primary molars is variable and often is determined by the severity and timing of the process. When an underlying permanent successor is present, extraction of the ankylosed primary molar should not be performed until it becomes obvious that exfoliation is not proceeding normally or adverse occlusal changes are developing. After extraction of an ankylosed molar, the permanent tooth will erupt spontaneously in the majority of cases. In permanent teeth or primary teeth without underlying successors, prosthetic buildup can be placed to augment the occlusal height. Severe cases in primary teeth are treated best with extraction and space maintenance. Luxation of affected permanent teeth may be attempted with extraction forceps in an effort to break the ankylosis. It is hoped that the subsequent inflammatory reaction results in the formation of a new fibrous ligament in the area of previous fusion. In these cases, reevaluation in 6 months is mandatory. Finally, several reports have documented successful repositioning of an ankylosed permanent tooth with a combination of orthodontics, segmental osteotomy, and distraction osteogenesis.
Developmental Alterations of Teeth
Numerous developmental alterations of teeth can occur. Box 2-5 delineates the major reported alterations, and the following text pertains to these entities. These alterations may be primary or arise secondary to environmental influences (e.g., concrescence, hypercementosis, dilaceration). For the sake of convenience, both the primary and the environmental forms will be discussed together.
DEVELOPMENTAL ALTERATIONS IN THE NUMBER OF TEETH
Variations in the number of teeth that develop are common. Several terms are useful in the discussion of the numeric variations of teeth. Anodontia refers to a total lack of tooth development. Hypodontia denotes the lack of development of one or more teeth; oligodontia (a subdivision of hypodontia) indicates the lack of development of six or more teeth. Hyperdontia is the development of an increased number of teeth, and the additional teeth are termed supernumerary. Terms such as partial anodontia are oxymorons and should be avoided. In addition, these terms pertain to teeth that failed to develop and should not be applied to teeth that developed but are impacted or have been removed.
Genetic control appears to exert a strong influence on the development of teeth. Hypodontia and hyperdontia have been noted in patients with a variety of syndromes (Boxes 2-6 and 2-7). In all of these syndromes, an increased prevalence of hypodontia or hyperdontia exists, but the strength of the association varies. Furthermore, the actual genetic contribution to the increased or decreased number of teeth may be unclear in some of these conditions. In addition to these syndromes, an increased prevalence of hypodontia is noted in patients with nonsyndromic cleft lip (CL) or cleft palate (CP).
Genetic influences still may affect nonsyndromic numeric alterations of teeth, because more than 200 genes are known to play a role in odontogenesis. Because of the complexity of the system, variations in tooth number arise in a wide variety of patterns. A large percentage of primary hypodontia cases appear to be inherited in an autosomal dominant fashion, with incomplete penetrance and variable expressivity, whereas a minority of examples present an autosomal recessive or sex-linked pattern. The environment is not without its influence, with occasional examples suggesting multifactorial inheritance. Several investigators have reported variable expression of hypodontia in monozygotic twins (confirmed by DNA fingerprinting). This discordance confirms the occasional multifactorial nature of the process. Overall, hypodontia most likely represents a variety of disorders caused by variable genetic and epigenetic factors.
Research has identified a gene mutation in only a small percentage of nonsyndromic hypodontia cases. Although this list will continue to lengthen over time, the currently implicated genes include the PAX9 gene, the MSX1 gene, the AXIN2 gene, and He-Zhao deficiency, which is associated with an unknown gene that maps to chromosome 10q11.2. Although variable expressivity is common, most of these examples represent oligodontia and exhibit numerous missing teeth. Interestingly, the affected gene tends to correlate to the pattern of missing teeth. It must be stressed that these genes are involved in only a very small number of affected patients with hypodontia, and the genetic basis for the vast majority of hypodontia cases remains elusive.
Less information is available on the genetics of hyperdontia; however, like hypodontia, almost every possible pattern of inheritance has been suggested. In all likelihood, many cases are multifactorial and arise from a combination of genetics and environmental influences. In spite of this, studies on certain kindreds have suggested an autosomal dominant pattern of inheritance with incomplete penetrance, autosomal recessive inheritance with lesser penetrance in females, and X-linked inheritance.
Some investigators have implied that hypodontia is a normal variant, suggesting that humans are in an intermediate stage of dentitional evolution. A proposed future dentition would contain one incisor, one canine, one premolar, and two molars per quadrant. Conversely, others have suggested that hyperdontia represents atavism—the reappearance of an ancestral condition. The latter hypothesis is difficult to accept because some patients have had as many as four premolars in one quadrant, a situation that has never been reported in other mammals. The most widely accepted theory is that hyperdontia is the result of a localized and independent hyperactivity of dental lamina.
In contrast, hypodontia correlates with the absence of appropriate dental lamina. As discussed, the loss of the developing tooth buds in most instances appears to be genetically controlled. In spite of this, the environment most likely influences the final result or, in some cases, may be responsible completely for the lack of tooth formation. The dental lamina is extremely sensitive to external stimuli, and damage before tooth formation can result in hypodontia. Trauma, infection, radiation, chemotherapeutic medications, endocrine disturbances, and severe intrauterine disturbances have been associated with missing teeth.
HYPODONTIA: Failure of teeth to form is one of the most common dental developmental abnormalities, with a reported prevalence of 1.6% to 9.6% in permanent teeth when absence of third molars is excluded. The prevalence increases to 20% if third molars are considered. A female predominance of approximately 1.5:1 is reported. Anodontia is rare, and most cases occur in the presence of hereditary hypohidrotic ectodermal dysplasia (see page 741). Indeed, when the number of missing teeth is high or involves the most stable teeth (i.e., maxillary central incisors, first molars), the patient should be evaluated for ectodermal dysplasia. Hypodontia is uncommon in the deciduous dentition, with a prevalence that ranges from 0.5% to 0.9% and, when present, most frequently involves the lateral incisors. Absence of a deciduous tooth is associated strongly with an increased prevalence of a missing successor. Missing teeth in the permanent dentition are not rare, with third molars being the most commonly affected. After the molars, the second premolars and lateral incisors are absent most frequently (Fig. 2-40). Hypodontia is associated positively with microdontia (see page 83), reduced alveolar development, increased freeway space, and retained primary teeth (Fig. 2-41). In whites with missing teeth, approximately 80% will demonstrate loss of only one or two teeth.
Fig. 2-40 Hypodontia. Developmentally missing maxillary lateral incisors. Radiographs revealed no underlying teeth, and there was no history of trauma or extraction.
Fig. 2-41 Hypodontia. A, Multiple developmentally missing permanent teeth and several retained deciduous teeth in a female adult. B, The panoramic radiograph shows no unerupted teeth in either jaw.
Mutation of the PAX9 gene creates an autosomal dominant pattern of oligodontia that can involve various teeth but most commonly affects most of the permanent molars. In severe cases, loss of the primary molars, second premolars, and permanent mandibular central incisors also may be seen. Mutation of the MSX1 gene also is inherited as an autosomal dominant trait. Those affected with this mutation tend to demonstrate loss of the distal tooth of each type, with more severely affected individuals also revealing anterior progression of the agenesis. In these patients the most commonly missing teeth are the second premolars and third molars. In more severe cases, often the maxillary first premolars and maxillary lateral incisors also are missing. With the MSX1 mutation, the degree of oligodontia is severe, with an average of approximately 12 missing teeth per patient. The He-Zhao deficiency arose in a large kindred from northwest China and includes a highly variable pattern of missing teeth that occurs only in the permanent dentition. The missing teeth may affect the entire dentition, but the condition most commonly involves the third molars, second premolars, and maxillary lateral incisors.
For dentists and their patients, the most critical discovery related to hypodontia revolves around the mutation of the AXIN2 gene. This pattern of oligodontia is inherited as an autosomal dominant disorder, with the most commonly missing teeth being the permanent second and third molars, second premolars, lower incisors, and maxillary lateral incisors. The maxillary central incisors always are present and usually accompanied by the canines, first premolars, and first molars. However, the number and type of missing teeth are highly variable, a typical finding of inheritable oligodontia. Although the missing teeth can produce a significant oral problem, the presence of the AXIN2 mutation in these kindreds also has been associated with development of adenomatous polyps of the colon and colorectal carcinoma. This suggests that patients with similar examples of oligodontia should be questioned closely for a family history of colon cancer, with further medical evaluation recommended for those possibly at risk.
Even in kindreds with an obviously inherited pattern of hypodontia or oligodontia, it must be stressed that, in the majority of the cases, the genes are yet to be discovered. The most common form of inherited hypodontia is an autosomal dominant pattern in which the average number of missing teeth is slightly more than two. Excluding the third molars, the most commonly missing teeth in these cases are the lower second premolars, upper second premolars, maxillary lateral incisors, and lower central incisors.
HYPERDONTIA: The prevalence of supernumerary permanent teeth in whites is between 0.1% and 3.8%, with a slightly higher rate seen in Asian populations. The frequency in the deciduous dentition is much lower and varies from 0.3% to 0.8%. Approximately 76% to 86% of cases represent single-tooth hyperdontia, with two supernumerary teeth noted in 12% to 23%, and three or more extra teeth noted in less than 1% of cases. Single-tooth hyperdontia occurs more frequently in the permanent dentition, and approximately 95% present in the maxilla, with a strong predilection for the anterior region. The most common site is the maxillary incisor region, followed by maxillary fourth molars and mandibular fourth molars, premolars, canines, and lateral incisors (Fig. 2-42). Supernumerary mandibular incisors are very rare. Although supernumerary teeth may be bilateral, most occur unilaterally (Figs. 2-43 and 2-44). In contrast to single-tooth hyperdontia, nonsyndromic multiple supernumerary teeth occur most frequently in the mandible. These multiple supernumerary teeth occur most often in the premolar region, followed by the molar and anterior regions, respectively (Fig. 2-45).
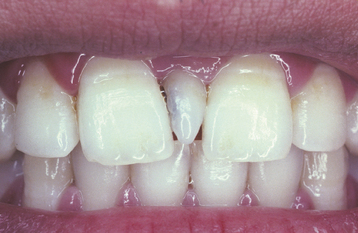
Fig. 2-42 Hyperdontia (mesiodens). Erupted supernumerary, rudimentary tooth of the anterior maxilla.
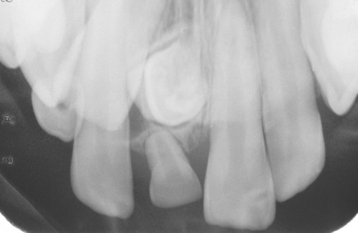
Fig. 2-43 Hyperdontia (mesiodens). Unilateral supernumerary tooth of the anterior maxilla, which has altered the eruption path of the maxillary right permanent central incisor.
Although most supernumerary teeth occur in the jaws, examples have been reported in the gingiva, maxillary tuberosity, soft palate, maxillary sinus, sphenomaxillary fissure, nasal cavity, and between the orbit and the brain. The eruption of accessory teeth is variable and dependent on the degree of space available; 75% of supernumerary teeth in the anterior maxilla fail to erupt. Unlike hypodontia, hyperdontia is positively correlated with macrodontia (see page 83) and exhibits a 2:1 male predominance. Although examples may be identified in older adults, most supernumerary teeth develop during the first two decades of life.
Several terms have been used to describe supernumerary teeth, depending on their location. A supernumerary tooth in the maxillary anterior incisor region is termed a mesiodens (see Fig. 2-42); an accessory fourth molar is often called a distomolar or distodens. A posterior supernumerary tooth situated lingually or buccally to a molar tooth is termed a paramolar (Fig. 2-46).
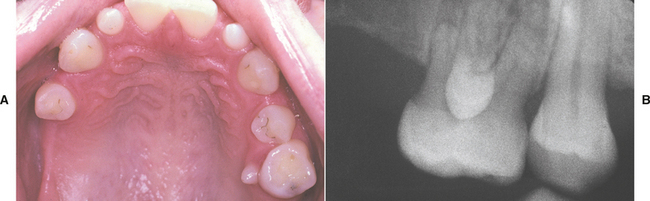
Fig. 2-46 Paramolar. A, Rudimentary tooth situated palatal to a maxillary molar in a patient who also exhibits hypodontia. B, Radiograph of the same patient showing a fully formed tooth overlying the crown of the adjacent molar.
Supernumerary teeth are divided into supplemental (normal size and shape) or rudimentary (abnormal shape and smaller size) types. Rudimentary supernumerary teeth are classified further into conical (small, peg-shaped), tuberculate (barrel-shaped anterior with more than one cusp), and molariform (small premolar-like or molarlike). Although odontomas are considered hamartomas and could be placed within this classification, these lesions traditionally are in-cluded in the list of odontogenic neoplasms and are discussed in Chapter 15 (page 724). The conical mesiodens represents one of the more common supernumerary teeth and can erupt spontaneously, whereas tuberculate examples are less frequent and rarely erupt.
Occasionally, normal teeth may erupt into an inappropriate position (e.g., a canine present between two premolars). This pattern of abnormal eruption is called dental transposition. Such misplaced teeth have been confused with supernumerary teeth; but in reality, patients exhibiting dental transposition have been reported to exhibit an increased prevalence of hypodontia, not hyperdontia. The teeth involved most frequently in transposition are the maxillary canines and first premolars. Crowding or malocclusion of these normal teeth may dictate reshaping, orthodontics, or extraction.
Accessory teeth may be present at or shortly after birth. Historically, teeth present in newborns have been called natal teeth; those arising within the first 30 days of life are designated neonatal teeth. This is an artificial distinction, and it appears appropriate to call all of these teeth natal teeth (Fig. 2-47). Although some authors have suggested that these teeth may represent predeciduous supernumerary teeth, most are prematurely erupted deciduous teeth (not supernumerary teeth). Approximately 85% of natal teeth are mandibular incisors, 11% are maxillary incisors, and 4% are posterior teeth.
TREATMENT AND PROGNOSIS: Sequelae associated with hypodontia include abnormal spacing of teeth, delayed tooth formation, delayed deciduous tooth exfoliation, late permanent tooth eruption, and altered dimension of the associated gnathic regions. The management of the patient with hypodontia depends on the severity of the case. No treatment may be required for a single missing tooth; prosthetic replacement often is needed when multiple teeth are absent. Therapeutic options include removable partial dentures, traditional fixed prosthodontics, resin-bonded bridges, or osseointegrated implants with associated prosthetic crowns. Use of fixed prosthodontics typically is not recommended for children because of the risk of pulp exposure during abutment preparation and because further growth can lead to infraocclusion and ankylosis of teeth held together by the prosthesis. Likewise, because implants act more like ankylosed teeth than erupting teeth, their use is not recommended before completion of skeletal growth except for patients with anodontia. For these reasons, a removable appliance or resin-bonded bridge often is appropriate in children and young adults while waiting for full dental and skeletal maturation.
In some cases of hypodontia, orthodontic therapy may improve the restorative treatment or even negate its need in selected patients. Patients with oligodontia exhibit an increased prevalence of orthodontics-associated external root resorption. This may be due to the altered root anatomy or to the extensive tooth movement that is required in some patients. Follow-up radiographs are recommended after 6 to 9 months of therapy to evaluate the root morphology for evidence of excessive resorption.
The presence of supernumerary teeth should be suspected if a significant delay is observed in the eruption of a localized portion of the dentition. Because of the decreased clarity in the anterior portion of a panoramic radiograph, this image should be combined with occlusal and periapical radiographs to fully visualize the area. Supernumerary teeth may develop long after eruption of the permanent dentition. Several publications have documented supernumerary bicuspids arising up to 11 years after completion of normal teeth development. In patients previously diagnosed with supernumerary teeth, or in those genetically predisposed, long-term monitoring for additional tooth development is warranted.
Early diagnosis and treatment often are crucial in minimizing the aesthetic and functional problems of the adjacent teeth. Because only 7% to 20% of supernumerary teeth exist without clinical complications, the standard of care is removal of the accessory tooth during the time of the early mixed dentition. Complications created by anterior supernumerary teeth tend to be more significant than those associated with extra teeth in the posterior regions. Reports have documented spontaneous eruption of the adjacent dentition in 75% of the cases if the supernumerary tooth is removed early. After removal of the supernumerary tooth, full eruption typically occurs within 18 months to 3 years. Impacted permanent teeth having closed apices or those associated with a tuberculate mesiodens may show a reduced tendency for spontaneous eruption. Permanent teeth that fail to erupt are treated best by surgical exposure with orthodontic eruption. Removal of unerupted deciduous teeth is not recommended, because most will erupt spontaneously.
A consequence of late therapy may include the delayed eruption, resorption of the adjacent teeth, displacement of the teeth with associated crowding, dilaceration, malocclusion, diastema formation, or eruption into the nasal cavity. Supernumerary teeth also predispose the area to subacute pericoronitis, gingivitis, periodontitis, abscess formation, and the development of any one of a large number of odontogenic cysts and tumors. In selected cases, clinical judgment may not dictate surgical removal, or patient resistance to therapy may be present. In these instances, regular monitoring is appropriate.
Natal teeth must be approached individually, with sound clinical judgment guiding appropriate therapy. As stated, the erupted teeth in most cases represent the deciduous dentition, and removal should not be performed hastily. If the teeth are mobile and at risk for aspiration, then removal is indicated. If mobility is not a problem and the teeth are stable, then they should be retained. Traumatic ulcerations of the adjacent soft tissue (Riga-Fede disease) (see page 287) may occur during breast-feeding but often can be resolved with appropriate measures.
DEVELOPMENTAL ALTERATIONS IN THE SIZE OF TEETH
Tooth size is variable among different races and between the sexes. The presence of unusually small teeth is termed microdontia; the presence of teeth larger than average is termed macrodontia. Although heredity is the major factor, both genetic and environmental influences affect the size of developing teeth. The deciduous dentition appears to be affected more by maternal intrauterine influences; the permanent teeth seem to be more affected by environment.
CLINICAL FEATURES: Although the size of teeth is variable, the two sides of the jaws are usually symmetrical. Despite this, when significant size variation is present, the entire dentition rarely is affected. Typically, only a few teeth are altered significantly in size. Differences in tooth sizes cannot be considered in isolation. Microdontia is associated strongly with hypodontia (see page 79); macrodontia often is seen in association with hyperdontia (see page 80). Females demonstrate a higher frequency of microdontia and hypodontia; males have a greater prevalence of macrodontia and hyperdontia.
MICRODONTIA: The term microdontia should be applied only when the teeth are physically smaller than usual. Normal-sized teeth may appear small when widely spaced within jaws that are larger than normal. This appearance has been historically termed relative microdontia, but it represents macrognathia (not microdontia). Diffuse true microdontia is uncommon but may occur as an isolated finding in Down syndrome, in pituitary dwarfism, and in association with a small number of rare hereditary disorders that exhibit multiple abnormalities of the dentition (Fig. 2-48).
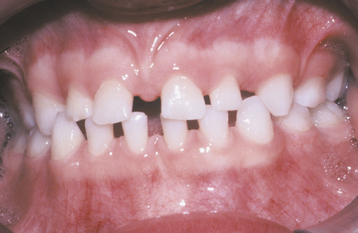
Fig. 2-48 Diffuse microdontia. Dentition in which the teeth are smaller than normal and widely spaced within the arch.
Isolated microdontia within an otherwise normal dentition is not uncommon. The maxillary lateral incisor is affected most frequently and typically appears as a peg-shaped crown overlying a root that often is of normal length (Fig. 2-49). The mesiodistal diameter is reduced, and the proximal surfaces converge toward the incisal edge. The reported prevalence varies from 0.8% to 8.4% of the population, and the alteration appears to be autosomal dominant with incomplete penetrance. In addition, isolated microdontia often affects third molars. Interestingly, the maxillary lateral incisors and the third molars are among the most frequent teeth to be congenitally missing. When a peg-shaped tooth is present, the remaining perma-nent teeth often exhibit a slightly smaller mesiodistal size.
MACRODONTIA: Analogous to microdontia, the term macrodontia (megalodontia, megadontia) should be applied only when teeth are physically larger than usual and should not include normal-sized teeth crowded within a small jaw (previously termed relative macrodontia). In addition, the term macrodontia should not be used to describe teeth that have been altered by fusion or gemination. Diffuse involvement is rare, and typically only a few teeth are abnormally large. Diffuse macrodontia has been noted in association with pituitary gigantism (see page 831), otodental syndrome, XYY males, and pineal hyperplasia with hyperinsulinism. Macrodontia with unilateral premature eruption is not rare in hemifacial hyperplasia (see page 38). Authors have postulated that the unilateral bone growth resulting from this condition may also affect developing teeth on the altered side. Isolated macrodontia is reported to occur most frequently in incisors or canines but also has been seen in second premolars and third molars. In such situations, the alteration often occurs bilaterally.
DEVELOPMENTAL ALTERATIONS IN THE SHAPE OF TEETH
GEMINATION, FUSION, AND CONCRESCENCE
Double teeth (connated teeth, conjoined teeth) are two separate teeth exhibiting union by dentin and (perhaps) their pulps. The union may be the result of fusion of two adjacent tooth buds or the partial splitting of one into two. The development of isolated large or joined (i.e., double) teeth is not rare, but the literature is confusing when the appropriate terminology is presented. Historically, gemination was defined as an attempt of a single tooth bud to divide, with the resultant formation of a tooth with a bifid crown and, usually, a common root and root canal. Conversely, fusion was considered the union of two normally separated tooth buds with the resultant formation of a joined tooth with confluence of dentin. Finally, concrescence was the union of two teeth by cementum without confluence of the dentin.
Many investigators have found these definitions confusing and open to debate. A double tooth found in the place of a maxillary permanent central incisor is a good example of the controversy. If the joined tooth is counted as one and the tooth number is correct, then the anomaly could result from the division of a single tooth bud or the fusion of the permanent tooth bud with the bud of an adjacent mesiodens. Some have suggested that the terms gemination, fusion, and concrescence should be discontinued, and all of these anomalies should be termed twinning. This also is confusing because other investigators use twinning to refer to the development of two separate teeth that arose from the complete separation of one tooth bud (this also is arguable).
Because of this confusion in terminology, the use of the term twinning cannot be recommended. Extra teeth are termed supernumerary, and another name is not necessary. Even though the exact pathogenesis may be questionable in some cases (whether caused by fusion of adjacent buds or partial split of one bud), the terms gemination, fusion, and concrescence serve a useful purpose because they are the most descriptive of the clinical presentation. Gemination is defined as a single enlarged tooth or joined (i.e., double) tooth in which the tooth count is normal when the anomalous tooth is counted as one. Fusion is defined as a single enlarged tooth or joined (i.e., double) tooth in which the tooth count reveals a missing tooth when the anomalous tooth is counted as one. Concrescence is union of two adjacent teeth by cementum alone, without confluence of the underlying dentin. Unlike fusion and gemination, concrescence may be developmental or postinflammatory. When two teeth develop in close proximity, developmental union by cementum is possible. In addition, areas of inflammatory damage to the roots of teeth are repaired by cementum once the inciting process resolves. Concrescence of adjacent teeth may arise in initially separated teeth in which cementum deposition extends between two closely approximated roots in a previous area of damage.
GEMINATION AND FUSION: Double teeth (gemination and fusion) occur in both the primary and the permanent dentitions, with a higher frequency in the anterior and maxillary regions (Figs. 2-50 to 2-54). In the permanent dentition, the prevalence of double teeth in whites is approximately 0.3% to 0.5%, whereas the frequency in deciduous teeth is greater, with a reported prevalence from 0.5% to 2.5%. Asian populations tend to demonstrate a higher occurrence that exceeds 5% in some studies. In both dentitions, incisors and canines are the most commonly affected teeth. Involvement of posterior primary teeth, premolars, and permanent molars also can occur. Gemination is more common in the maxilla, whereas fusion tends to occur more frequently in the mandible. Bilateral cases are uncommon (Fig. 2-55).
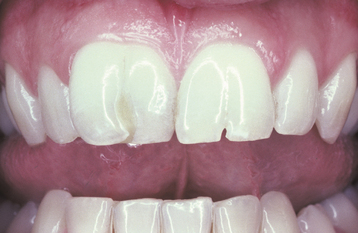
Fig. 2-50 Bilateral gemination. Two double teeth. The tooth count was normal when each anomalous tooth was counted as one.
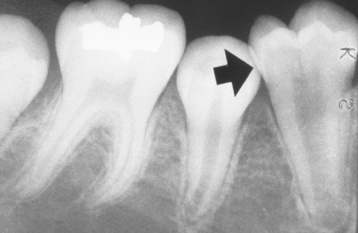
Fig. 2-52 Gemination. Same patient as depicted in Fig. 2-51. Note the bifid crown and shared root canal.
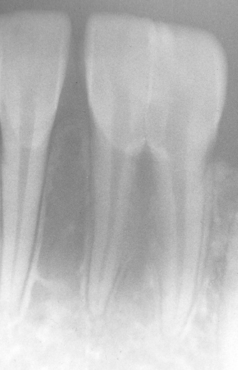
Fig. 2-54 Fusion. Radiographic view of double tooth in the place of the mandibular central and lateral incisors. Note separate root canals.
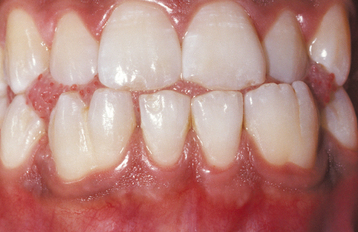
Fig. 2-55 Fusion. Bilateral double teeth in the place of the mandibular lateral incisors and cuspids.
Gemination and fusion appear similar and may be differentiated by assessing the number of teeth in the dentition. Some authors have suggested that gemination demonstrates a single root canal. Separate canals are present in fusion, but this does not hold true in all cases (Fig. 2-56). A variety of appearances are noted with both fusion and gemination. The processes may result in an otherwise anatomically correct tooth that is greatly enlarged. A bifid crown may be seen overlying two completely separated roots, or the joined crowns may blend into one enlarged root with a single canal.
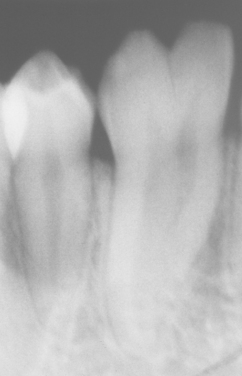
Fig. 2-56 Fusion. Radiograph of the same patient depicted in Fig. 2-55. Note the bifid crown overlying the single root canal; the contralateral radiograph revealed a similar pattern.
CONCRESCENCE: Concrescence is two fully formed teeth, joined along the root surfaces by cementum. The process is noted more frequently in the posterior and maxillary regions. The developmental pattern often involves a second molar tooth in which its roots closely approximate the adjacent impacted third molar (Fig. 2-57). The postinflammatory pattern frequently involves carious molars in which the apices overlie the roots of horizontally or distally angulated third molars. This latter pattern most frequently arises in a carious tooth that exhibits large coronal tooth loss. The resultant large pulpal exposure often permits pulpal drainage, leading to a resolution of a portion of the intrabony pathosis. Cemental repair then occurs (Figs. 2-58 and 2-59).
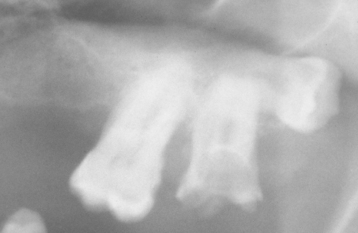
Fig. 2-58 Concrescence. Union by cementum of maxillary second and third molars. Note the large carious defect of the second molar.
Fig. 2-59 Concrescence. Gross photograph of the same teeth depicted in Fig. 2-58. Histopathologic examination revealed that union occurred in the area of cemental repair previously damaged by a periapical inflammatory lesion.
TREATMENT AND PROGNOSIS: The presence of double teeth (i.e., gemination or fusion) in the deciduous dentition can result in crowding, abnormal spacing, and delayed or ectopic eruption of the underlying permanent teeth. When detected, the progression of eruption of the permanent teeth should be monitored closely by careful clinical and radiographic observation. When appropriate, extraction may be necessary to prevent an abnormality in eruption. Occasionally, fusion in the primary dentition is associated with absence of the underlying permanent successor.
Several approaches are available for the treatment of joined teeth in the permanent dentition, and the treatment of choice is determined by the patient’s particular needs. Rare reports of successful surgical division have been documented. In most cases of surgical division, endodontic therapy was performed. Selected shaping with or without placement of full crowns has been used in many cases. Other patients exhibit pulpal or coronal anatomic features that are resistant to reshaping and require surgical removal with prosthetic replacement. Double teeth often will demonstrate a pronounced labial or lingual groove that may be prone to develop caries. In such cases, placement of a fissure sealant or composite restoration is appropriate if the tooth is to be retained.
Patients with concrescence often require no therapy unless the union interferes with eruption; then surgical removal may be warranted. Postinflammatory concrescence must be kept in mind whenever extraction is planned for nonvital teeth with apices that overlie the roots of an adjacent tooth. Significant extraction difficulties can be experienced on attempted removal of a tooth that is unexpectedly joined to its neighbor. Surgical separation often is required to complete the procedure without loss of a significant portion of the surrounding bone.
ACCESSORY CUSPS
The cuspal morphology of teeth exhibits minor variations among different populations; of these, three distinctive patterns deserve further discussion: (1) cusp of Carabelli, (2) talon cusp, and (3) dens evaginatus. When an accessory cusp is present, the other permanent teeth often exhibit a slightly increased tooth size.
CLINICAL AND RADIOGRAPHIC FEATURES:
CUSP OF CARABELLI: The cusp of Carabelli is an accessory cusp located on the palatal surface of the mesiolingual cusp of a maxillary molar (Fig. 2-60). The cusp may be seen in the permanent or deciduous dentitions and varies from a definite cusp to a small indented pit or fissure. When present, the cusp is most pronounced on the first molar and is increasingly less obvious on the second and third molars. When a cusp of Carabelli is present, the remaining permanent teeth often are larger than normal mesiodistally, but a similar association in deciduous tooth size is typically not noted. A significant variation exists among different populations, with the prevalence reported to be as high as 90% in whites and rare in Asians. An analogous accessory cusp is seen occasionally on the mesiobuccal cusp of a mandibular permanent or deciduous molar and is termed a protostylid.
TALON CUSP: A talon cusp is a well-delineated additional cusp that is located on the surface of an anterior tooth and extends at least half the distance from the cementoenamel junction to the incisal edge. A talon cusp is thought to represent the end of a continuum that extends from a normal cingulum, to an enlarged cingulum, to a small accessory cusp, and, finally, to a full-formed talon cusp. Investigators have muddied the literature associated with this spectrum by categorizing all enlarged cingula as talon cusps and developing a classification system for the degree of enlargement. These classification systems make prevalence data difficult to evaluate and should be discouraged.
Three fourths of all reported talon cusps are located in the permanent dentition. The cusps predominantly occur on permanent maxillary lateral (55%) or central (33%) incisors but have been seen less frequently on mandibular incisors (6%) and maxillary canines (4%) (Fig. 2-61). Their occurrence in the deciduous dentition is very rare, with the vast majority noted on maxillary central incisors. In almost all cases the accessory cusp projects from the lingual surface of the affected tooth and forms a three-pronged pattern that resembles an eagle’s talon. On rare occasions, the cusp may project from the facial surface or from both surfaces of a single tooth. A deep developmental groove may be present where the cusp fuses with the underlying surface of the affected tooth. Most, but not all, talon cusps contain a pulpal extension. Radiographically, the cusp is seen overlying the central portion of the crown and includes enamel and dentin (Fig. 2-62). Only a few cases demonstrate visible pulpal extensions on dental radiographs.
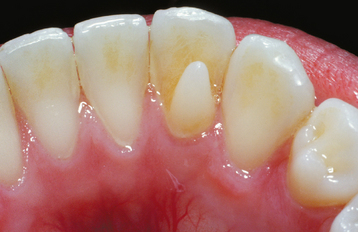
Fig. 2-61 Talon cusp. Accessory cusp present on the lingual surface of a mandibular lateral incisor.
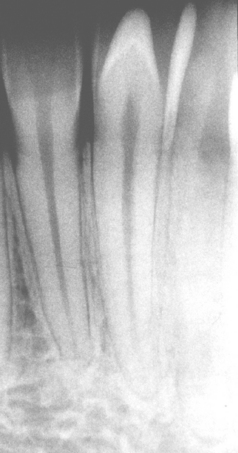
Fig. 2-62 Talon cusp. Radiograph of same patient shown in Fig. 2-61. Note the enamel and dentin layers within the accessory cusp.
Extensive prevalence studies have not been performed, but estimates suggest the frequency of talon cusp in the population ranges from less than 1% to 8%. Variations among different population groups and inconsistent definitions of a talon cusp make a definitive calculation difficult. The process does appear to occur more frequently in Asians, Native Americans, the Inuit, and those of Arab descent. Both sexes may be affected, and the occurrence may be unilateral or bilateral. The accessory cusp has been seen in association with other dental anomalies (e.g., supernumerary teeth, odontomas, impacted teeth, peg-shaped lateral incisors, dens invaginatus, posterior dens evaginatus).
In isolated cases, genetic influences appear to have an effect, because identical talon cusps occasionally have been documented in twins. Talon cusps also have been seen in patients with Rubinstein-Taybi syndrome, Mohr syndrome, Ellis-van Creveld syndrome, incontinentia pigmenti achromians, and Sturge-Weber angiomatosis. Although the strength of association between the presence of talon cusps and these syndromes generally is not clear, Rubinstein-Taybi syndrome is strongly correlated as demonstrated by a study of 45 affected patients in which 92% demonstrated talon cusps. Other characteristic features of this syndrome include growth and mental retardation, broad thumbs and great toes, and a number of other orodental features (thin upper lip, retrognathia, micrognathia, narrow high-arched palate, submucous cleft palate, and cleft palate [rarely]).
DENS EVAGINATUS: Dens evaginatus (central tubercle, tuberculated cusp, accessory tubercle, occlusal pearl, evaginated odontome, Leong premolar, tuberculated premolar) is a cusplike elevation of enamel located in the central groove or lingual ridge of the buccal cusp of premolar or molar teeth (Fig. 2-63). Although this pattern of accessory cusps has been reported on molars, dens evaginatus typically occurs on premolar teeth, is usually bilateral, and demonstrates a marked mandibular predominance. Deciduous molars are affected infrequently. The accessory cusp normally consists of enamel and dentin, with pulp present in about half of the cases. Although the prevalence is variable, most reviews suggest a frequency between 1% and 4%. The anomaly is encountered most frequently in Asians, the Inuit, and Native Americans but is rare in whites. Researchers expect an increased prevalence of this anomaly in the United States secondary to immigration by Asians and by Hispanics of mestizo heritage (i.e., those of mixed European and Native American ancestry). Radiographically, the occlusal surface exhibits a tuberculated appearance, and often a pulpal extension is seen in the cusp (Fig. 2-64). The accessory cusp frequently creates occlusal interferences that are associated with significant clinical problems. In one large study, more than 80% of the tubercles were worn or fractured, with pulpal pathosis noted in more than 25% of patients. Pulpal necrosis is common and may occur through a direct exposure or invasion of patent, immature dentinal tubules. In addition to abnormal wear and pulpal pathosis, the accessory cusp also may result in dilaceration, displacement, tilting, or rotation of the tooth.
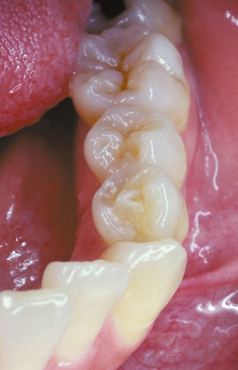
Fig. 2-63 Dens evaginatus. Cusplike elevation located in the central groove of mandibular first bicuspid.
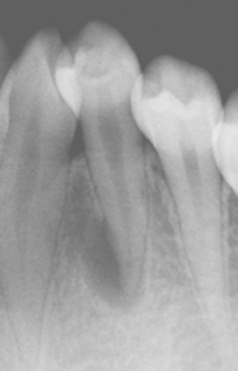
Fig. 2-64 Dens evaginatus. Radiograph of teeth depicted in Fig. 2-63. Note the tuberculated occlusal anatomy. Attrition on the accessory cusp led to pulpal necrosis and periapical inflammatory disease.
Frequently, dens evaginatus is seen in association with another variation of coronal anatomy, shovel-shaped incisors. This alteration also occurs predominantly in Asians, with a prevalence of approximately 15% in whites but close to 100% in Native Americans and the Inuit. Affected incisors demonstrate prominent lateral margins, creating a hollowed lingual surface that resembles the scoop of a shovel (Fig. 2-65). Typically, the thickened marginal ridges converge at the cingulum; not uncommonly, a deep pit, fissure, or dens invaginatus is found at this junction. Maxillary lateral and central incisors most frequently are affected, with mandibular incisors and canines less commonly reported.
TREATMENT AND PROGNOSIS: Patients with cusps of Carabelli require no therapy unless a deep groove is present between the accessory cusp and the surface of the mesiolingual cusp of the molar. These deep grooves should be sealed to prevent carious involvement.
Patients with talon cusps on mandibular teeth often require no therapy; talon cusps on maxillary teeth frequently interfere with occlusion and should be removed. Other complications include compromised aesthetics, displacement of teeth, caries, periodontal problems, and irritation of the adjacent soft tissue (e.g., tongue or labial mucosa). Because many of these cusps contain pulp, rapid removal often results in pulpal exposure. Removal without the loss of vitality may be accomplished through periodic grinding of the cusp, with time allowed for tertiary dentin deposition and pulpal recession. At the end of each grinding session, the exposed dentin should be coated with a desensitizing agent such as fluoride varnish, which also may speed the rate of pulpal recession. Even with slow reduction and no direct pulp exposure, loss of vitality is possible when large numbers of immature dentin tubules are exposed. After successful removal of the cusp, the exposed dentin can be covered with calcium hydroxide, the peripheral enamel etched, and a composite resin placed.
On eruption, the affected tooth should be inspected for the presence of a deep fissure at the junction between the talon cusp and the surface of the tooth. If a fissure is present, it should be restored to avoid early carious extension into the nearby dental pulp. Reports also have documented the continuation of this fissure down the surface of the root, with subsequent development of lateral radicular inflammatory lesions secondary to the access provided to oral flora by the deep groove. In these latter cases, further surgery is required to expose the groove for appropriate cleansing.
Dens evaginatus typically results in occlusal problems and often leads to pulpal death. In affected teeth, removal of the cusp often is indicated, but attempts to maintain vitality have met with only partial success. Slow, periodic grinding of the cusp exposes immature patent dentinal tubules and may lead to irreversible pulpitis without direct exposure. To reduce the chance of pulpal pathosis, elimination of opposing occlusal interferences combined with removal of minimal dentin and treatment of the area with stannous fluoride has been recommended. More rapid cuspal removal with indirect or direct pulp capping also has proven beneficial in some patients. Other investigators support removal of occlusal interferences, protection of the cusp from fracture by the placement of surrounding resin reinforcement, and delaying cuspal removal until evidence of significant dentinal maturation, pulpal recession, and apical root closure are present.
Shovel-shaped incisors should be inspected for surface defects at the point where the marginal ridges converge. Any deep fissures or invaginations should be restored shortly after eruption to prevent carious exposure of the adjacent pulp.
DENS INVAGINATUS (DENS IN DENTE)
Dens invaginatus is a deep surface invagination of the crown or root that is lined by enamel. Oehlers described this condition thoroughly in three classic articles published from 1957 to 1958. Two forms, coronal and radicular, are recognized.
CLINICAL AND RADIOGRAPHIC FEATURES: By a great margin, coronal dens invaginatus is seen more frequently; the reported prevalence varies from 0.04% to 10% of all patients. In order of decreasing frequency, the teeth affected most often include the permanent lateral incisors, central incisors, premolars, canines, and molars. Involvement of deciduous teeth has been reported but is uncommon. A strong maxillary predominance is seen.
The depth of the invagination varies from a slight enlargement of the cingulum pit to a deep infolding that extends to the apex. As would be expected, before eruption the lumen of the invagination is filled with soft tissue similar to the dental follicle (i.e., reduced enamel epithelium with a fibrous connective tissue wall). On eruption, this soft tissue loses its vascular supply and becomes necrotic.
Historically, coronal dens invaginatus has been classified into three major types (Fig. 2-66). Type I exhibits an invagination that is confined to the crown. The invagination in type II extends below the cemento enamel junction and ends in a blind sac that may or may not communicate with the adjacent dental pulp (Figs. 2-67 and 2-68). Large invaginations may become dilated and contain dystrophic enamel in the base of the dilatation (Fig. 2-69). Type III extends through the root and perforates in the apical or lateral radicular area without any immediate communication with the pulp. In this latter type, the enamel that lines the invagination is often replaced by cementum close to the radicular perforation. This perforation provides direct communication from the oral cavity to the intraosseous periradicular tissues and often produces inflammatory lesions in the presence of a vital pulp (Figs. 2-70 and 2-71).
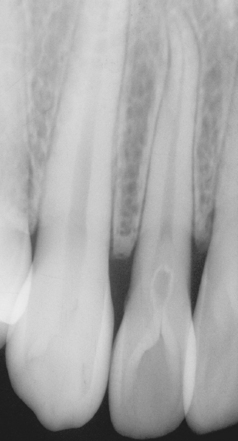
Fig. 2-67 Coronal dens invaginatus type II. Maxillary lateral incisor exhibiting invagination of the surface enamel that extends below the cementoenamel junction.
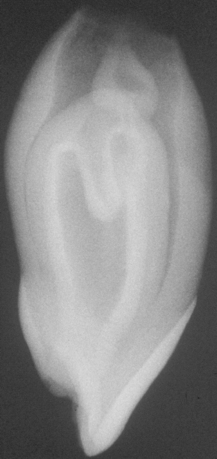
Fig. 2-68 Coronal dens invaginatus type II. Bulbous maxillary cuspid exhibiting a dilated invagination lined by enamel.
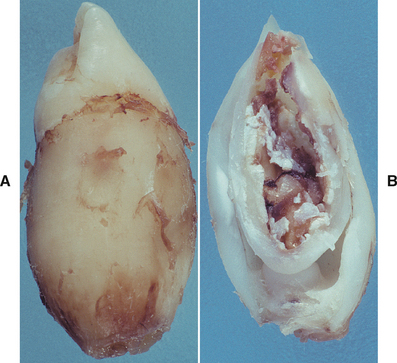
Fig. 2-69 Coronal dens invaginatus type II. Gross photograph of a sectioned tooth. Note the dilated invagination with apical accumulation of dystrophic enamel.
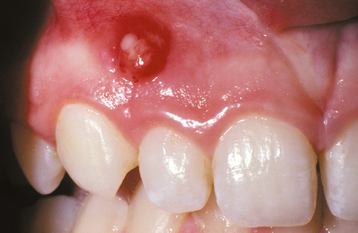
Fig. 2-70 Coronal dens invaginatus type III. Parulis overlying vital maxillary cuspid and lateral incisor. The cuspid contained a dens invaginatus that perforated the mesial surface of its root.
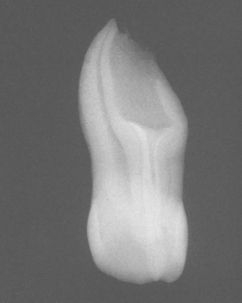
Fig. 2-71 Coronal dens invaginatus type III. Maxillary cuspid exhibiting an enamel invagination that parallels the pulp canal and perforates the lateral root surface. (Courtesy of Dr. Brian Blocher.)
Occasionally, the invagination may be rather large and resemble a tooth within a tooth, hence the term dens in dente. In other cases the invagination may be dilated and disturb the formation of the tooth, resulting in anomalous tooth development termed dilated odontome. Involvement may be singular, multiple, or bilateral.
Radicular dens invaginatus is rare and thought to arise secondary to a proliferation of Hertwig’s root sheath, with the formation of a strip of enamel that extends along the surface of the root. This pattern of enamel deposition is similar to that frequently seen in association with radicular enamel pearls (see Ectopic Enamel). Rather than protrude from the surface (as seen in an enamel pearl), the altered enamel forms a surface invagination into the dental papilla (Fig. 2-72). Cementum-lined invaginations of the root have been reported, but these represent a simple variation of root morphology and should not be included under the term radicular dens invaginatus.
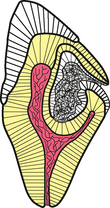
Fig. 2-72 Radicular dens invaginatus. Illustration depicting the radicular form of dens invaginatus.
Radiographically, the affected tooth demonstrates an enlargement of the root. Close examination often reveals a dilated invagination lined by enamel, with the opening of the invagination situated along the lateral aspect of the root.
TREATMENT AND PROGNOSIS: On eruption, the invagination of the affected tooth communicates with the oral cavity, and the soft tissue within the lumen undergoes necrosis (providing an excellent environment for growth of bacteria). In small type I invaginations, the opening of the invagination should be restored after eruption in an attempt to prevent carious involvement and subsequent pulpal inflammation. If the invagination is not detected quickly, then pulpal necrosis frequently results. With larger invaginations the contents of the lumen and any carious dentin must be removed; then a calcium hydroxide base may be placed to help treat any possible microcommunications with the adjacent pulp. In cases with obvious pulpal communication or signs of pulpal pathosis, both the invagination and the adjacent pulp canal require endodontic therapy. In teeth with open apices, apexification with calcium hydroxide or mineral trioxide aggregate often is successful, followed by final obturation.
Type III invaginations associated with periradicular inflammatory lesions require endodontic-like therapy of the perforating invagination. Once again, before final obturation with gutta-percha, temporary placement of calcium hydroxide helps to build dentinal bridges and maintain vitality of the adjacent pulp. If vitality is lost, endodontic therapy of the parallel root canal also becomes necessary. Some cases do not respond to conservative endodontic therapy and require periapical surgery and retrofill. Large and extremely dilated invaginations often have abnormal crowns and need to be extracted.
If the invagination does not significantly disrupt the morphologic appearance of the tooth, then complications of radicular dens invaginatus are rare unless the radicular opening is exposed to the oral cavity. After exposure occurs, carious involvement often leads to pulpal necrosis. Openings close to the anatomic neck of the tooth should be exposed and restored to minimize damage to the tooth and surrounding structures.
ECTOPIC ENAMEL
Ectopic enamel refers to the presence of enamel in unusual locations, mainly the tooth root. The most widely known are enamel pearls. These are hemispheric structures that may consist entirely of enamel or contain underlying dentin and pulp tissue. Most enamel pearls project from the surface of the root and are thought to arise from a localized bulging of the odontoblastic layer. This bulge may provide prolonged contact between Hertwig’s root sheath and the developing dentin, triggering induction of enamel formation. Similar internal projections of enamel into the underlying dentin rarely have been reported in the crowns of teeth.
In addition to enamel pearls, cervical enamel extensions also occur along the surface of dental roots. These extensions represent a dipping of the enamel from the cementoenamel junction toward the bifurcation of molar teeth. This pattern of ectopic enamel forms a triangular extension of the coronal enamel that develops on the buccal surface of molar teeth directly overlying the bifurcation. The base of the triangle is continuous with the inferior portion of the coronal enamel; the leading point of the triangle extends directly toward the bifurcation of the tooth. These areas of ectopic enamel have been called cervical enamel projections, but this terminology is confusing because no significant exophytic projections are seen.
CLINICAL AND RADIOGRAPHIC FEATURES:
ENAMEL PEARLS: Enamel pearls are found most frequently on the roots of maxillary molars (mandibular molars are the second most frequent site). It is uncommon for maxillary premolars and incisors to be affected. Involvement of deciduous molars is not rare. The prevalence of enamel pearls varies (1.1% to 9.7% of all patients) according to the population studied and is highest in Asians. In most cases, one pearl is found, but as many as four pearls have been documented on a single tooth. The majority occur on the roots at the furcation area or near the cementoenamel junction (Fig. 2-73). Radiographically, pearls appear as well-defined, radiopaque nodules along the root’s surface (Fig. 2-74). Mature internal enamel pearls appear as well-defined circular areas of radiodensity extending from the dentinoenamel junction into the underlying coronal dentin.
Fig. 2-73 Enamel pearl. Mass of ectopic enamel located in the furcation area of a molar tooth. (Courtesy of Dr. Joseph Beard.)
Fig. 2-74 Enamel pearl. Radiopaque nodule on the mesial surface of the root of the maxillary third molar. Another less distinct enamel pearl is present on the distal root of the second molar.
The enamel surface of pearls precludes normal periodontal attachment with connective tissue, and a hemidesmosomal junction probably exists. This junction is less resistant to breakdown; once separation occurs, rapid loss of attachment is likely. In addition, the exophytic nature of the pearl is conducive to plaque retention and inadequate cleansing.
CERVICAL ENAMEL EXTENSIONS: As mentioned previously, cervical enamel extensions are located on the buccal surface of the root overlying the bifurcation (Fig. 2-75). Mandibular molars are affected slightly more frequently than maxillary molars. In reviews of extracted teeth in the lower 48 United States, the prevalence is surprisingly high, with approximately 20% of molars being affected. Similar studies demonstrate an even greater prevalence in other locations such as Japan, China, and Alaska, with cervical enamel extensions discovered in 50% to 78% of extracted molars. Cervical enamel extensions may occur on any molar, but they are seen less frequently on third molars. Because connective tissue cannot attach to enamel, these extensions have been correlated positively with localized loss of periodontal attachment with furcation involvement. On review of a large number of dentitions with periodontal furcation involvement, a significantly higher frequency of cervical enamel extensions was found compared with dentitions without furcation involvement. In addition, the greater the degree of cervical extension, the higher the frequency of furcation involvement.
Fig. 2-75 Cervical enamel extension. Illustration of a normal molar adjacent to a molar exhibiting V-shaped elongation of enamel extending toward the bifurcation.
In addition to periodontal furcation involvement, cervical enamel extensions (in some cases) have been associated with the development of inflammatory cysts that are histopathologically identical to inflammatory periapical cysts. The cysts develop along the buccal surface over the bifurcation and most appropriately are called buccal bifurcation cysts (see page 698). The association between cervical enamel extensions and this unique inflammatory cyst is controversial.
TREATMENT AND PROGNOSIS: When enamel pearls are detected radiographically, the area should be viewed as a weak point of peri-odontal attachment. Meticulous oral hygiene should be maintained in an effort to prevent localized loss of periodontal support. If removal of the lesion is contemplated, then the clinician must remember that enamel pearls occasionally contain vital pulp tissue.
For teeth with cervical enamel extensions and associated periodontal furcation involvement, therapy is directed at achieving a more durable attachment and providing access to the area for appropriate cleaning. Reports have suggested that flattening or removing the enamel in combination with an excisional new attachment procedure and furcation plasty may accomplish this.
TAURODONTISM
Taurodontism is an enlargement of the body and pulp chamber of a multirooted tooth, with apical displacement of the pulpal floor and bifurcation of the roots. This pattern of molar formation has been found in ancient Neanderthals, and the overall shape of the taurodont resembles that of the molar teeth of cud-chewing animals (tauro = bull; dont = tooth).
CLINICAL AND RADIOGRAPHIC FEATURES: Affected teeth tend to be rectangular and exhibit pulp chambers with a dramatically increased apicoocclusal height and a bifurcation close to the apex (Fig. 2-76). The diagnosis usually is made subjectively from the radiographic appearance. The degree of taurodontism has been classified into mild (hypotaurodontism), moderate (mesotaurodontism), and severe (hypertaurodontism), according to the degree of apical displacement of the pulpal floor (Fig. 2-77). Witkop and colleagues and Shifman and Chanannel presented useful biometric criteria for the determination of taurodontism. These reports contain information that is useful in epidemiologic studies of the process.
Fig. 2-76 Taurodontism. Mandibular molar teeth exhibiting increased pulpal apicoocclusal height with apically positioned pulpal floor and bifurcation. (Courtesy of Dr. Michael Kahn.)
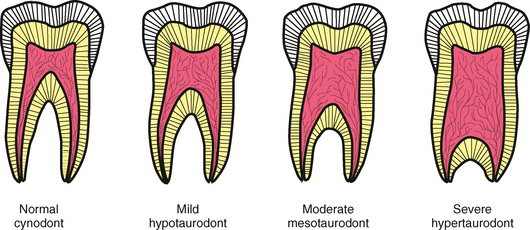
Fig. 2-77 Taurodontism. Illustration exhibiting the classification of taurodontism according to the degree of apical displacement of the pulpal floor.
Some investigators include examples of tau-rodontism in premolar teeth; others argue that tau-rodontism is not shown by premolars. This argument is academic because the presence of taurodontism in premolars cannot be documented in situ. Investiga-tions of taurodontism in premolar teeth require the examination of extracted teeth, because the necess-ary radiographs depict the tooth in a mesiodistal orientation.
Taurodontism may be unilateral or bilateral and affects permanent teeth more frequently than deciduous teeth. There is no sex predilection. The reported prevalence is highly variable (0.5% to 46%) and most likely is related to different diagnostic criteria and racial variations. In the United States most reports indicate a prevalence of 2.5% to 3.2% of the population. Some investigators believe the alteration is more of a variation of normal rather than a definitive pathologic anomaly. The process often demonstrates a field effect, with the involvement of all molars. When this occurs, the first molar is usually affected least, with increasing severity noted in the second and third molars, respectively.
Taurodontism may occur as an isolated trait or as a component of a specific syndrome (Box 2-8). An increased frequency of taurodontism has been reported in patients with hypodontia, cleft lip, and cleft palate. Investigations have shown that taurodontism may develop in the presence of any one of a large number of different genetic alterations. These findings suggest that chromosomal abnormalities may disrupt the development of the tooth’s form and that taurodontism is not the result of a specific genetic abnormality.
TREATMENT AND PROGNOSIS: Patients with taurodontism require no specific therapy. Coronal extension of the pulp is not seen; therefore, the process does not interfere with routine restorative procedures. Some investigators have suggested the taurodontic shape may exhibit decreased stability and strength as an abutment tooth in prosthetic procedures, but this hypothesis has not been verified. If endodontic therapy is required, then the shape of the pulp chamber frequently increases the difficulty of locating, instrumenting, and obturating the pulp canals. One bit of good news is that patients have to demonstrate significant periodontal destruction before bifurcation involvement occurs.
HYPERCEMENTOSIS
Hypercementosis (cemental hyperplasia) is a nonneoplastic deposition of excessive cementum that is continuous with the normal radicular cementum.
CLINICAL AND RADIOGRAPHIC FEATURES: Radiographically, affected teeth demonstrate a thickening or blunting of the root, but the exact amount of increased cementum often is difficult to ascertain because cementum and dentin demonstrate similar radiodensities (Fig. 2-78). The enlarged root is surrounded by the radiolucent PDL space and the adjacent intact lamina dura. On occasion, the enlargement may be significant enough to suggest the possibility of a cementoblastoma (see page 655). However, the cementoblastoma usually can be distinguished on the basis of associated pain, cortical expansion, and continued enlargement.
Hypercementosis may be isolated, involve multiple teeth, or appear as a generalized process. In a study of more than 22,000 affected teeth, the mandibular molars were affected most frequently, followed by the mandibular and maxillary second premolars and mandibular first premolars. In this study, a 2.5:1 mandibular predominance was noted.
Hypercementosis occurs predominantly in adulthood, and the frequency increases with age, most likely secondary to cumulative exposure to causative influences. Its occurrence has been reported in younger patients, and many of these cases demonstrate a familial clustering, suggesting hereditary influence.
Box 2-9 lists several local and systemic factors that have been associated with an increased frequency of the cemental deposition. All of the listed systemic factors exhibit a weak association with hypercementosis except for Paget’s disease of bone (see page 623). Numerous authors have reported significant hypercementosis in patients with Paget’s disease, and this disorder should be considered whenever generalized hypercementosis is discovered in a patient of the appropriate age. In spite of the association with a number of disorders, most localized cases of hypercementosis are not related to any systemic disturbance.
HISTOPATHOLOGIC FEATURES: The periphery of the root exhibits deposition of an excessive amount of cementum over the original layer of primary cementum. The excessive cementum may be hypocellular or exhibit areas of cellular cementum that resemble bone (osteocementum). Often the material is arranged in concentric layers and may be applied over the entire root or be limited to the apical portion. On routine light microscopy, distinguishing between dentin and cementum often is difficult, but viewing the section with polarized light helps to discriminate between the two different layers (Fig. 2-79).
DILACERATION
Dilaceration is an abnormal angulation or bend in the root or, less frequently, the crown of a tooth (Figs. 2-80 and 2-81). Although most examples are idiopathic, a number of teeth with dilaceration appear to arise after an injury that displaces the calcified portion of the tooth germ, and the remainder of the tooth is formed at an abnormal angle. The damage frequently follows avulsion or intrusion of the overlying primary predecessor, an event that usually occurs before 4 years of age. Injury-related dilaceration more frequently affects the anterior dentition and often creates both a functional and a cosmetic dental problem. Less frequently the bend develops secondary to the presence of an adjacent cyst, tumor, or odontogenic hamartoma (e.g., odontoma, supernumerary tooth) (Fig. 2-82).
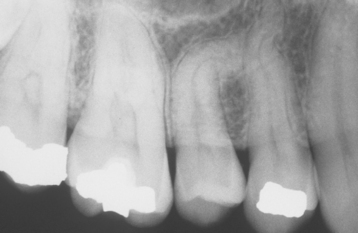
Fig. 2-81 Dilaceration. Maxillary second bicuspid exhibiting mesial inclination of the root. The patient reported no history of injury to this area. (Courtesy of Dr. Lawrence Bean.)
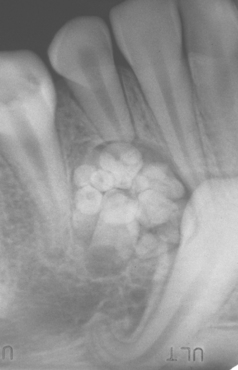
Fig. 2-82 Dilaceration. Root angulation of a mandibular cuspid. Development has been altered by the presence of an adjacent compound odontoma. (Courtesy of Dr. Brent Bernard.)
CLINICAL AND RADIOGRAPHIC FEATURES: In one review of 1166 randomly selected patients, 176 dilacerated teeth were identified. Of these teeth, the most commonly affected were the mandibular third molars, followed by the maxillary second premolars and mandibular second molars. The maxillary and mandibular incisors were the least frequently affected, representing approximately 1% of the series. This contrasts with other authors who have reported a high frequency of dilaceration involving anterior teeth. In reality the molars most likely demonstrate the highest prevalence of dilaceration but are not highlighted because of a lack of associated clinical problems in most instances. Occasionally, involvement of the deciduous teeth is reported, and some have been associated with prior trauma secondary to neonatal laryngoscopy and endotracheal intubation. The age of the patient and the direction and degree of force appear to determine the extent of the tooth’s malformation. The abnormal angulation may be present anywhere along the length of the tooth.
Altered maxillary anterior teeth frequently demonstrate the bend in the crown or the coronal half of the root; failure of eruption is often seen. Affected mandibular incisors also exhibit involvement of the crown or the superficial portion of the root, but more frequently they erupt into full occlusion. Those that achieve eruption often follow an altered path and present in a labial or lingual position. Many of the affected teeth, especially anterior mandibular teeth, are nonvital and associated with periapical inflammatory lesions. Typically, altered posterior teeth demonstrate involvement of the apical half of the root and frequently do not exhibit delayed eruption.
TREATMENT AND PROGNOSIS: The treatment and prognosis vary according to the severity of the deformity. Altered deciduous teeth often demonstrate inappropriate resorption and result in delayed eruption of the permanent teeth. Extraction is indicated when necessary for the normal eruption of the succedaneous teeth. Patients with minor dilaceration of permanent teeth frequently require no therapy. Those teeth that exhibit delayed or abnormal eruption may be exposed and orthodontically moved into position. In some cases with extensive deformation of the affected tooth, perforation of the buccal alveolar ridge by the malpositioned root may occur on repositioning. In such cases, amputation of the root apex with subsequent endodontic therapy may be necessary. Grossly deformed teeth require surgical removal. The extraction of affected teeth may be difficult and result in root fracture on removal. When attempting to perform endodontic procedures, the clinician must use great care to avoid root perforation of teeth with significant dilaceration.
Root dilaceration concentrates stress if the affected tooth is used as an abutment for a dental prosthetic appliance. This increased stress may affect the stability and longevity of the abutment tooth. Splinting of the dilacerated tooth to an adjacent tooth results in a multirooted abutment and overcomes the stress-related problems.
SUPERNUMERARY ROOTS
The term supernumerary roots refers to the development of an increased number of roots on a tooth compared with that classically described in dental anatomy.
CLINICAL AND RADIOGRAPHIC FEATURES: Any tooth may develop accessory roots, and involvement has been reported in both the deciduous and the permanent dentitions. Data on the frequency of supernumerary roots are sparse, but the prevalence appears to vary significantly among different races. The most frequently affected teeth are the permanent molars (especially third molars) from either arch and mandibular cuspids and premolars (Fig. 2-83). In some instances the supernumerary root is divergent and seen easily on radiographs; in other cases the additional root is small, superimposed over other roots, and difficult to ascertain.
TREATMENT AND PROGNOSIS: No treatment is required for supernumerary roots, but the detection of the accessory root is of critical importance when endodontic therapy or exodontia is undertaken. Extracted teeth always should be examined closely to ensure that all roots have been removed successfully, because accessory roots may not be obvious on the presurgical radiographs. Just as important is the search for accessory canals during endodontic access procedures, because failure to discover these additional openings often results in a lack of resolution of the associated inflammatory process.
DEVELOPMENTAL ALTERATIONS IN THE STRUCTURE OF TEETH
Amelogenesis imperfecta encompasses a complicated group of conditions that demonstrate developmental alterations in the structure of the enamel in the absence of a systemic disorder. Box 2-2 (see page 55) lists several systemic diseases associated with enamel disorders that are not considered isolated amelogenesis imperfecta.
At least 14 different hereditary subtypes of amelogenesis imperfecta exist, with numerous patterns of inheritance and a wide variety of clinical manifestations. As proof of the complicated nature of the process, several different classification systems exist. The most widely accepted is that developed by Witkop (Table 2-1), and this part of the text adheres to this classification. The dissertation by Witkop and Sauk (and Witkop’s 1988 review) are works of art, and they should be used if the clinician desires more information.
Table 2-1
Classification of Amelogenesis Imperfecta
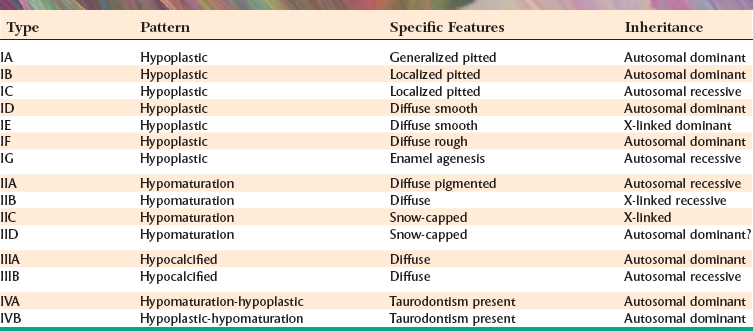
Modified from Witkop CJ Jr: Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification, J Oral Pathol 17:547-553, 1988.
An ideal classification system for amelogenesis imperfecta has not been established yet. Witkop’s classification relies on the phenotype and pedigree (i.e., clinical appearance and apparent pattern of inheritance). Classification by clinical appearance is problematic, because different phenotypes have been noted within a single affected family. In addition, similar phenotypes may be seen in individuals with very different molecular patterns of disease. One example of the potential confusion occurs in kindreds affected with certain variants of autosomal dominant amelogenesis imperfecta in which homozygotes exhibit generalized thin hypoplasia, whereas heterozygotes exhibit localized enamel pitting. Using the current nomenclature, different individuals within this kindred would be placed into multiple categories (e.g., types IB, ID, IF).
Although the molecular basis underlying the majority of amelogenesis imperfecta remains poorly defined, the genetics associated with several variations of amelo genesis imperfecta has been clarified. This has led investigators to suggest a future classification system based primarily on the mode of inheritance with secondary discriminators that include the phenotype and molecular basis (site of chromosomal mutation, when known). Although the push for a new classification system is not new, the progress in defining the molecular genetics of amelogenesis imperfecta has been slow and currently prevents complete agreement on a transition to a genetics-directed system of classification. As the molecular basis of the disease becomes better clarified, the move to a new pattern of classification seems inevitable.
Investigations into the genetics are ongoing and producing results that are not only interesting but also directly applicable to patient care. To date, mutations in five genes have been associated with amelogenesis imperfecta. Each gene can be mutated in a variety of ways, often creating diverse and distinct phenotypic patterns.
The AMELX gene is associated with the enamel protein amelogenin, which constitutes up to 90% of enamel matrix. AMELX-associated variants of amelogenesis imperfecta are X-linked with 14 different mutations currently known. Because of the effect of lyonization, the male and female phenotypes are variable but often associated with the genotype. The male phenotypes include both the diffuse smooth hypoplastic and the hypomaturation variants.
The ENAM gene is associated with another enamel protein, enamelin, which represents approximately 1% to 5% of enamel matrix. Mutations of the ENAM gene have been correlated with some autosomal dominant and recessive patterns of hypoplastic amelogenesis imperfecta, ranging from minor pitting to diffuse generalized thin enamel.
The MMP-20 gene codes for a proteinase named enamelysin; mutation of this gene has been associated with the autosomal recessive, pigmented hypomaturation variant of amelogenesis imperfecta.
The protease, kallikrein-4, is associated with the KLK4 gene, the mutation of which has been shown to be involved with some forms of hypomaturation amelogenesis imperfecta. Both enamelysin and kallikrein-4 are thought necessary for the removal of enamel matrix proteins during the maturation stage of enamel development.
The DLX3 gene is in a group of genes that code for a number of proteins that are critical for craniofacial, tooth, hair, brain, and neural development; mutation of this gene has been associated with the hypoplastic-hypomaturation variants of amelogenesis imperfecta with taurodontism.
Another strong candidate is the AMBN gene that codes for the protein ameloblastin, which constitutes about 5% of enamel matrix. Although not proven to be associated with amelogenesis imperfecta, this gene locus is a strong candidate for some of the autosomal dominant patterns.
Although no one has compiled a complete list of amelogenesis imperfecta types using the proposed new classification, Table 2-2 provides a rough idea of how this might be organized. Despite these exciting molecular genetic discoveries, it must be stressed how little is known and how much remains to be investigated. When studying large numbers of kindreds affected by amelogenesis imperfecta, only rare families will demonstrate mutation of one of the currently known genes.
Table 2-2
Modified Classification of Amelogenesis Imperfecta
| Inheritance | Phenotype | Related Genes |
| Autosomal dominant | Generalized pitted | |
| Autosomal dominant | Localized hypoplastic | ENAM |
| Autosomal dominant | Generalized thin | ENAM |
| Autosomal dominant | Hypocalcification | |
| Autosomal dominant | With taurodontism | DLX3 |
| Autosomal recessive | Localized hypoplastic | |
| Autosomal recessive | Generalized thin | |
| Autosomal recessive | Pigmented hypomaturation | MMP20, KLK4 |
| Autosomal recessive | Hypocalcification | |
| X-linked | Generalized thin | AMELX |
| X-linked | Diffuse hypomaturation | AMELX |
| X-linked | Snow-capped hypomaturation |
The formation of enamel is a multistep process, and problems may arise in any one of the steps. In general, the development of enamel can be divided into three major stages:
The hereditary defects of the formation of enamel also are divided along these lines: hypoplastic, hypocalcified, and hypomaturation.
CLINICAL AND RADIOGRAPHIC FEATURES: Amelogenesis imperfecta may be inherited as an autosomal dominant, autosomal recessive, or X-linked disorder, with an estimated frequency between 1:718 and 1:14,000 of the population. As in any hereditary condition, clustering of affected patients in certain geographic areas may occur (resulting in an increased prevalence of the disorder in those areas). Additionally, the stringency of the diagnostic criteria may influence the reported prevalence in any given study. In general, both the deciduous and the permanent dentitions are diffusely involved.
HYPOPLASTIC AMELOGENESIS IMPERFECTA: In patients with hypoplastic amelogenesis imperfecta, the basic alteration centers on inadequate deposition of enamel matrix. Any matrix present is mineralized appropriately and radiographically contrasts well with the underlying dentin. In the generalized pattern, pinpoint-to-pinhead–sized pits are scattered across the surface of the teeth and do not correlate with a pattern of environmental damage (Fig. 2-84). The buccal surfaces of the teeth are affected more severely, and the pits may be arranged in rows or columns. Staining of the pits may occur. Variable expressivity is seen within groups of affected patients. The enamel between the pits is of normal thickness, hardness, and coloration.
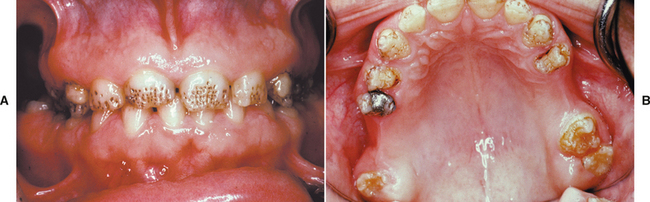
Fig. 2-84 Hypoplastic amelogenesis imperfecta, generalized pitted pattern. A, Note the numerous pinpoint pits scattered across the surface of the teeth. The enamel between the pits is of normal thickness, hardness, and coloration. B, Occlusal view of same patient showing diffuse involvement of all maxillary teeth, which would be inconsistent with environmental damage. (A from Stewart RE, Prescott GH: Oral facial genetics, St Louis, 1976, Mosby; B courtesy of Dr. Joseph S. Giansanti.)
In the localized pattern, the affected teeth demonstrate horizontal rows of pits, a linear depression, or one large area of hypoplastic enamel surrounded by a zone of hypocalcification. Typically, the altered area is located in the middle third of the buccal surfaces of the teeth. The incisal edge or occlusal surface usually is not affected. Both dentitions (or only the primary teeth) may be affected. All the teeth may be altered, or only scattered teeth may be affected. When the involvement is not diffuse, the pattern of affected teeth does not correlate with a specific time in development. The autosomal recessive type (type IC) is more severe and typically demonstrates involvement of all teeth in both dentitions.
In the autosomal dominant smooth pattern, the enamel of all teeth exhibits a smooth surface and is thin, hard, and glossy (Fig. 2-85). The absence of appropriate enamel thickness results in teeth that are shaped like crown preparations and demonstrate open contact points. The color of the teeth varies from opaque white to translucent brown. Anterior open bite is not rare. Radiographically, the teeth exhibit a thin peripheral outline of radiopaque enamel. Often, unerupted teeth exhibiting resorption are seen.
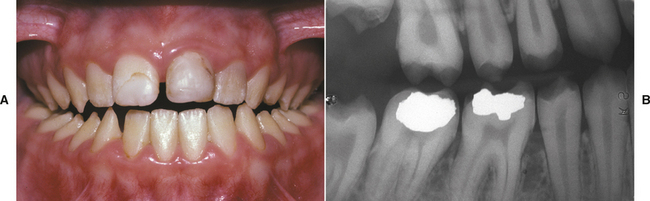
Fig. 2-85 Hypoplastic amelogenesis imperfecta, autosomal dominant smooth pattern (generalized thin pattern). A, Small, yellowish teeth exhibiting hard, glossy enamel with numerous open contact points and anterior open bite. B, Radiograph of the same patient demonstrating thin peripheral outline of radiopaque enamel. (B courtesy of Dr. John G. Stephenson.)
The X-linked smooth pattern has been stated to arise from an X-linked dominant mutation and is a lesson in the lyonization effect. On approximately the sixteenth day of embryonic life in all individuals with two X chromosomes, one member of the pair is inactivated in each cell. As a result of this event, females are mosaics, with a mixture of cells, some with active maternal X chromosomes and others with active paternal X chromosomes. Usually the mix is of approximately equal proportions. If one X were to direct the formation of defective enamel and the other X were to form normal enamel, then the teeth would exhibit alternating zones of normal and abnormal enamel. Aldred and Crawford have argued against subdivision of X-linked amelogenesis imperfecta into dominant and recessive variants, because both heterozygous females and hemizygous males are affected regardless of the mutation being dominant or recessive. Although the intelligence of this suggestion is less evident in an X-linked dominant pattern of amelogenesis imperfecta, the argument is quite convincing when associated with the X-linked “recessive” hypomaturation pattern of amelogenesis imperfecta (see the discussion of the X-linked pattern of hypomaturation amelogenesis imperfecta on page 103).
Males with the X-linked smooth pattern exhibit diffuse thin, smooth, and shiny enamel in both dentitions. The teeth often have the shape of crown preparations, and the contact points are open. The color varies from brown to yellow-brown. Radiographs demonstrate a peripheral outline of radiopaque enamel. Unerupted teeth may undergo resorption. On the other hand, heterozygous females exhibit vertical furrows of thin hypoplastic enamel, alternating between bands of normal thickness. The banding often is detectable with dental radiographs. An open bite is seen in almost all males and in a minority of females.
In the rough pattern, the enamel is thin, hard, and rough-surfaced. As in the smooth forms, the teeth taper toward the incisal-occlusal surface and demonstrate open contact points (Fig. 2-86). The color varies from white to yellow-white. The enamel is denser than that seen in the smooth patterns, and the teeth are less vulnerable to attrition. Radiographs exhibit a thin peripheral outline of radiodense enamel. Unerupted teeth, often undergoing resorption, may be seen. An anterior open bite is common.
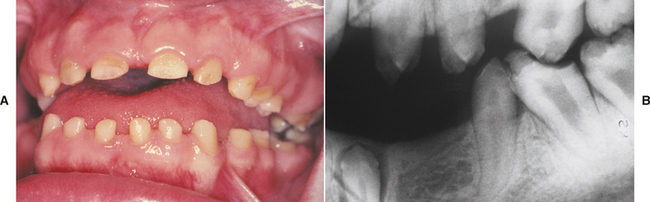
Fig. 2-86 Hypoplastic amelogenesis imperfecta, rough pattern (generalized thin pattern). A, Small, yellow teeth with rough enamel surface, open contact points, significant attrition, and anterior open bite. B, Radiograph of the same patient. Note the impacted tooth and the thin peripheral outline of radiodense enamel.
As the name implies, enamel agenesis demonstrates a total lack of enamel formation. The teeth are the shape and color of the dentin, with a yellow-brown hue, open contact points, and crowns that taper toward the incisal-occlusal surface. The surface of the dentin is rough, and an anterior open bite is seen frequently. Radiographs demonstrate no peripheral enamel overlying the dentin. A lack of eruption of many teeth with significant resorption frequently occurs.
Many investigators have difficulty subdividing the diffuse hypoplastic forms of amelogenesis imperfecta into categories such as smooth, rough, and enamel agenesis. In the autosomal recessive pattern of amelogenesis imperfecta previously termed enamel agenesis, the presence of a thin band of enamel has been confirmed in many affected patients. In addition, the separation between smooth and rough forms can be highly subjective and problematic. Therefore, some investigators have suggested discontinuation of these subdivisions and combining these phenotypic patterns into one category termed generalized thin hypoplastic amelogenesis imperfecta.
HYPOMATURATION AMELOGENESIS IMPERFECTA: In a person with hypomaturation amelogenesis im-perfecta, the enamel matrix is laid down appropriately and begins to mineralize; however, there is a defect in the maturation of the enamel’s crystal structure. Affected teeth are normal in shape but exhibit a mottled, opaque white-brown-yellow discoloration. The enamel is softer than normal and tends to chip from the underlying dentin. Radiographically, the affected enamel exhibits a radiodensity that is similar to dentin.
In the pigmented pattern the surface enamel is mottled and agar-brown. The enamel often fractures from the underlying dentin and is soft enough to be punctured by a dental explorer. Anterior open bite and unerupted teeth exhibiting resorption are uncommon. Occasionally, the surface enamel may be affected severely and be similar in softness to that of hypocalcified patterns. These cases often demonstrate extensive calculus deposition.
The X-linked pattern is another lesson in lyonization; however, the lyonization is not as obvious as that seen in the X-linked hypoplastic pattern. Although the mutation has been said to be recessive, hemizygous males and heterozygous females will demonstrate clinical changes. Affected males exhibit different patterns in the deciduous and permanent dentitions. The deciduous teeth are opaque white with a translucent mottling; the permanent teeth are opaque yellow-white and may darken with age (Fig. 2-87, A). The enamel tends to chip and often can be pierced with a dental explorer point. The degree of enamel loss is more rapid than that in normal teeth but does not approach that seen in the hypocalcified forms. Focal areas of brown discoloration may develop within the white opaque enamel. Radiographically, the contrast between enamel and dentin is reduced.
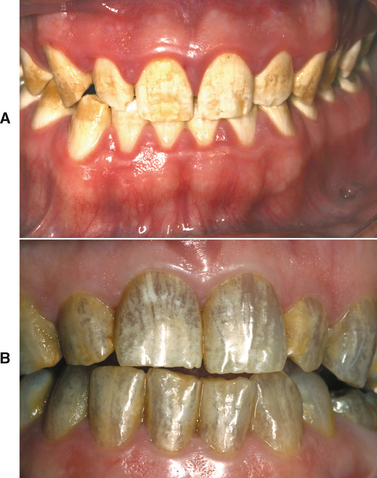
Fig. 2-87 Hypomaturation amelogenesis imperfecta, X-linked. A, Male patient exhibiting diffuse yellow-white dentition. B, The patient’s mother exhibits vertical bands of white, opaque enamel and translucent enamel. (Courtesy of Dr. Carlos Salinas.)
Heterozygous females exhibit a similar pattern in both dentitions. The teeth demonstrate vertical bands of white opaque enamel and translucent enamel; the bands are random and asymmetric (Fig. 2-87, B). The banding can be seen under regular lighting but is more obvious with transillumination. Radiographically, the bands are not perceptible, and the contrast between enamel and dentin is within normal limits. The obvious clinical evidence of the mutation in heterozygous females strongly argues for the pathosis to be designated as simply X-linked rather than X-linked recessive.
The snow-capped patterns exhibit a zone of white opaque enamel on the incisal or occlusal one quarter to one third of the crown (Fig. 2-88). The altered areas do not exhibit a distribution that would support an environmental origin, and the surface lacks the iridescent sheen seen with mild fluorosis. The affected teeth often demonstrate an anterior-to-posterior distribution and have been compared with a denture dipped in white paint (only affected anteriors, the anteriors back to the bicuspids, or the anteriors back to the molars). Both the deciduous and the permanent dentitions are affected. Most cases demonstrate an X-linked pattern of inheritance, but an autosomal dominant form is possible.
HYPOCALCIFIED AMELOGENESIS IMPERFECTA: In this type the enamel matrix is laid down appropriately but no significant mineralization occurs. In both patterns of hypocalcified amelogenesis imperfecta, the teeth are appropriately shaped on eruption, but the enamel is very soft and easily lost. On eruption the enamel is yellow-brown or orange, but it often becomes stained brown to black and exhibits rapid calculus apposition (Fig. 2-89). With years of function much of the coronal enamel is removed, except for the cervical portion that is occasionally calcified better. Unerupted teeth and anterior open bite are not rare. Both patterns are similar, but the autosomal recessive examples are generally more severe than the autosomal dominant cases. Radiographically, the density of the enamel and dentin are similar. Before eruption the teeth are normal in shape; however, after a period of function much of the cuspal enamel is lost, with the occlusal surface becoming the most irregular.
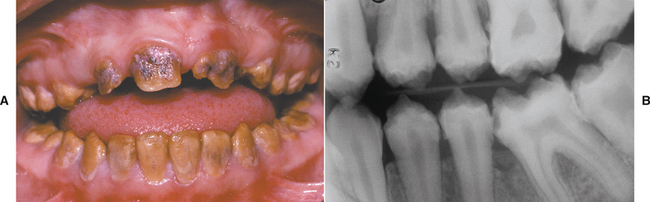
Fig. 2-89 Hypocalcified amelogenesis imperfecta. A, Dentition exhibiting diffuse yellow-brown discoloration. Note numerous teeth with loss of coronal enamel except for the cervical portion. B, Radiograph of the same patient. Note the extensive loss of coronal enamel and the similar density of enamel and dentin.
AMELOGENESIS IMPERFECTA WITH TAURODONTISM (HYPOMATURATION/HYPOPLASTIC AMELOGENESIS IMPERFECTA): This type of amelogenesis imperfecta exhibits enamel hypoplasia in combination with hypomaturation. The deciduous and the permanent dentitions are diffusely involved. Historically, two patterns have been recognized that are similar but differentiated by the thickness of the enamel and the overall tooth size. When studying a single kindred, phenotypic variation is seen that would place members of the same family in both divisions; therefore, many believe these divisions should be joined into one phenotype termed merely amelogenesis imperfecta with taurodontism.
In the presentation known as the hypomaturation-hypoplastic pattern, the predominant defect is one of enamel hypomaturation in which the enamel appears as mottled yellow-white to yellow-brown. Pits are seen frequently on the buccal surfaces of the teeth. Radiographically, the enamel appears similar to dentin in density, and large pulp chambers may be seen in single-rooted teeth in addition to varying degrees of taurodontism.
In the hypoplastic-hypomaturation pattern, the predominant defect is one of enamel hypoplasia in which the enamel is thin but also hypomature. Except for the decrease in the thickness of the enamel, this pattern is radiographically similar to the hypomaturation-hypoplastic variant.
A pattern of teeth alteration similar to amelogenesis imperfecta with taurodontism is seen in the systemic disorder, tricho-dento-osseous syndrome. This autosomal dominant disorder is mentioned here because the diagnosis may not be readily apparent without a high index of suspicion (Fig. 2-90). In addition to the dental findings, the predominant systemic changes are present variably and include kinky hair, osteosclerosis, and brittle nails. The kinky hair is present at birth but may straighten with age. The osteosclerosis primarily affects the base of the skull and the mastoid process. The mandible often exhibits a shortened ramus and an obtuse angle.
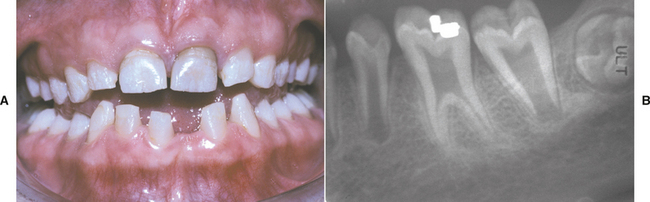
Fig. 2-90 Tricho-dento-osseous syndrome. A, Dentition exhibiting diffuse enamel hypoplasia and hypomaturation. At birth, the patient exhibited a kinky “steel wool” texture to her hair; with time, the hair straightened. A high index of suspicion was required to arrive at the diagnosis. B, Radiograph of the same patient showing significant taurodontism of the first molar and thin enamel, which is similar in density to the dentin.
Some authors have suggested that amelogenesis imperfecta with taurodontism may represent partial expression of the tricho-dento-osseous syndrome. Recent studies have identified distinctly different gene mutations that are responsible for tricho-dento-osseous syndrome and amelogenesis imperfecta with taurodontism. However, other investigators dispute this fact, showing evidence that some examples of this pattern of amelogenesis imperfecta appear allelic (different mutation on the same gene) to the syndrome. If only dental changes are seen in the absence of hair or bone changes, either in the individual or within the family, then the diagnosis of amelogenesis imperfecta appears appropriate.
HISTOPATHOLOGIC FEATURES: The histopathologic alterations present in amelogenesis imperfecta are not evident in routine preparations. Because decalcification of the teeth is necessary before processing to allow sectioning of paraffin-embedded specimens, all the enamel is lost. To examine the enamel structure of altered teeth, ground sections of nondecalcified specimens are prepared. The alterations discovered are highly diverse and vary with each clinical type of amelogenesis imperfecta. Detailed descriptions of such alterations were provided by Witkop and Sauk.
TREATMENT AND PROGNOSIS: The clinical implications of amelogenesis imperfecta vary according to the subtype and its severity, but the main problems are aesthetics, dental sensitivity, and loss of vertical dimension. In addition, in some types of amelogenesis imperfecta there is an increased prevalence of caries, anterior open bite, delayed eruption, tooth impaction, or associated gingival inflammation.
Patients with generalized thin enamel hypoplasia demonstrate minimal normal enamel associated with rapid attrition. These variants require full coverage as soon as is practical; if the treatment is delayed, a loss of usable crown length occurs. In those patients without sufficient crown lengths, full dentures (overdentures in some cases) often become the only satisfactory approach.
The other types of amelogenesis imperfecta demonstrate less rapid tooth loss, and the aesthetic appearance often is the prime consideration. Many less severe cases can be improved by the placement of full crowns or facial veneers on clinically objectionable teeth. In some cases a lack of good enamel bonding of veneers occurs and does not result in a durable restoration. The use of glass ionomer cements with dentinal adhesives often overcomes this weakness.
DENTINOGENESIS IMPERFECTA (HEREDITARY OPALESCENT DENTIN; CAPDEPONT’S TEETH)
Dentinogenesis imperfecta is a hereditary developmental disturbance of the dentin in the absence of any systemic disorder. Similar dental changes may be seen in conjunction with the systemic hereditary disorder of bone, osteogenesis imperfecta (see page 613). Dentin defects associated with this bone disease are termed osteogenesis imperfecta with opalescent teeth. Extensive pedigrees of individuals with dentinogenesis imperfecta have been studied, and none have exhibited other changes suggestive of osteogenesis imperfecta. Genetic research has confirmed that osteogenesis imperfecta with opalescent teeth clearly is a separate disease from dentinogenesis imperfecta.
The various types of osteogenesis imperfecta have been associated with mutation of the COL1A1 or COL1A2 gene that encodes production of type I collagen; in contrast, dentinogenesis imperfecta is associated with mutation of the DSPP (dentin sialophosphoprotein) gene. Currently, eight mutations of the DSPP gene are known; seven are associated with dentinogenesis imperfecta, with the eighth known to create dentin dysplasia type II.
Like amelogenesis imperfecta, the classification of the disorders of dentin is gradually evolving as the result of these recent molecular genetic findings. Two systems, one by Witkop and the other by Shields, historically were well accepted but not totally satisfactory (Table 2-3). Dentinogenesis imperfecta formerly was divided into hereditary opalescent dentin (Shields type II) and the Brandywine isolate (Shields type III).
Table 2-3
| Shields | Clinical Presentation | Witkop |
| Dentinogenesis imperfecta I | Osteogenesis imperfecta with opalescent teeth | Dentinogenesis imperfecta |
| Dentinogenesis imperfecta II | Isolated opalescent teeth | Hereditary opalescent teeth |
| Dentinogenesis imperfecta III | Isolated opalescent teeth | Brandywine isolate |
Data from Shields ED: A new classification of heritable human enamel defects and a discussion of dentin defects. In Jorgenson RJ, Paul NW: Dentition: genetic effects (birth defects original article series), vol 19, no. 1, pp 107-127, New York, 1983, Alan R Liss; Witkop CJ Jr: Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification, J Oral Pathol 17:547-553, 1988.
The defining phenotypic feature of the Brandywine isolate was the presence of unusual pulpal enlargement known as shell teeth. Current evidence strongly suggests that the Brandywine isolate represents nothing more than variable expressivity of the gene for dentinogenesis imperfecta. The original review of the isolate revealed only 8% of the kindred with shell teeth. Investigators have documented enlarged pulps in affected individuals whose parents and children have classic dentinogenesis imperfecta. Finally, identical patterns of expression have been seen in other large kindreds with no connection to the Brandywine isolate. Subsequently, a single mutation of the DSPP gene has been shown to cause both phenotypic patterns, strongly supporting the assumption that the phenotypes previously termed dentinogenesis imperfecta type II and dentinogenesis imperfecta type III represent a single disease with variable expressions. A modified classification of dentin disorders therefore seems warranted (Table 2-4), and continued use of the Shields or Witkop nomenclature does not appear justified.
Table 2-4
Modified Classification of Hereditary Disorders Affecting Dentin
| Disorder | Inheritance | Involved Gene or Genes |
| Osteogenesis imperfecta with opalescent teeth | Autosomal dominant or recessive | COL1A1, COL1A2 |
| Dentinogenesis imperfecta | Autosomal dominant | DSPP |
| Dentin dysplasia type I | Autosomal dominant | |
| Dentin dysplasia type II | Autosomal dominant | DSPP |
CLINICAL AND RADIOGRAPHIC FEATURES: The prevalence of dentinogenesis imperfecta is not randomly distributed throughout the United States and Europe. Most cases can be traced to whites (people of English or French ancestry) from communities close to the English Channel. The disorder is autosomal dominant and occurs in about 1:8000 whites in the United States.
The dental alterations in dentinogenesis imperfecta and osteogenesis imperfecta with opalescent teeth are similar clinically, radiographically, and histopathologically. All teeth in both dentitions are affected. The severity of the dental alterations varies with the age at which the tooth developed. Deciduous teeth are affected most severely, followed by the permanent incisors and first molars, with the second and third molars being least altered.
The dentitions have a blue-to-brown discoloration, often with a distinctive translucence (Fig. 2-91). The enamel frequently separates easily from the underlying defective dentin. Once exposed, the dentin often demonstrates significantly accelerated attrition (Fig. 2-92). Radiographically, the teeth have bulbous crowns, cervical constriction, thin roots, and early obliteration of the root canals and pulp chambers (Fig. 2-93).
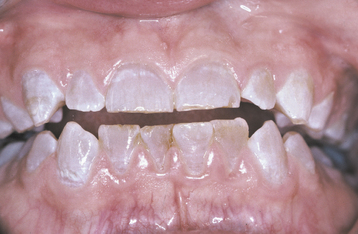
Fig. 2-91 Dentinogenesis imperfecta. Dentition exhibiting diffuse brownish discoloration and slight translucence.
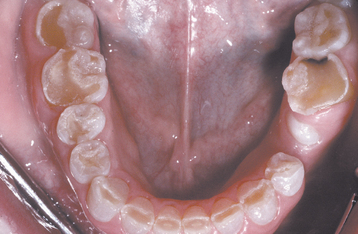
Fig. 2-92 Dentinogenesis imperfecta. Dentition exhibiting grayish discoloration with significant enamel loss and attrition.
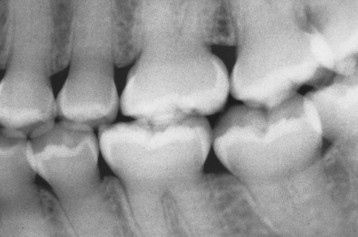
Fig. 2-93 Dentinogenesis imperfecta. Radiograph of dentition exhibiting bulbous crowns, cervical constriction, and obliterated pulp canals and chambers.
The trait exhibits close to 100% penetrance but variable expressivity. Significant, clinically obvious enamel hypoplasia is noted in some patients (Fig. 2-94). Researchers believe that the enamel abnormality is a secondary defect and not a direct expression of the dentinogenesis imperfecta gene. Although the pulps are usually obliterated by excess dentin production, some teeth may show normal-sized pulps or pulpal enlargement (shell teeth).
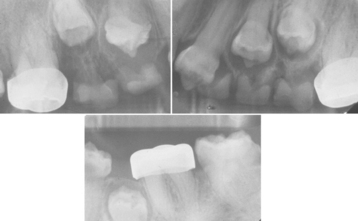
Fig. 2-94 Dentinogenesis imperfecta. Radiograph of dentition exhibiting bulbous crowns, early obliteration of the pulp, and enamel hypoplasia. (From Levin LS, Leaf SH, Jelmine RJ et al: Dentinogenesis imperfecta in the Brandywine isolate (DI type III): clinical, radiologic, and scanning electron microscopic studies of the dentition, Oral Surg Oral Med Oral Pathol 56:267-274, 1983.)
Shell teeth demonstrate normal-thickness enamel in association with extremely thin dentin and dramatically enlarged pulps (Fig. 2-95). The thin dentin may involve the entire tooth or be isolated to the root. This rare abnormality has been seen most frequently in deciduous teeth in the presence of dentinogenesis imperfecta. The alteration may be unassociated with dentinogenesis imperfecta as an isolated finding in both dentitions and demonstrate normal tooth shape and coloration, a negative family history, and diffuse involvement. In the isolated variant, slow but progressive root resorption occurs.
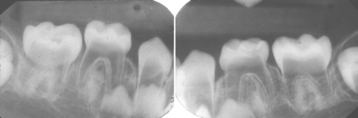
Fig. 2-95 Shell teeth. Dentition exhibiting normal thickness enamel, extremely thin dentin, and dramatically enlarged pulps.
Several kindreds affected with dentinogenesis imperfecta also have been shown to demonstrate progressive, sensorineural, high-frequency hearing loss. Jaw position has been shown to affect the anatomy of the inner ear, and premature tooth loss has been associated with hearing deficits. At this time, it is unclear if the hearing loss is correlated with the DSPP mutation or an alteration secondary to the primary gnathic changes. Investigators wonder if dental restoration may prevent the hearing loss or if the DSPP gene may directly affect bone formation and the structure of the inner ear.
HISTOPATHOLOGIC FEATURES: As expected, affected teeth demonstrate altered dentin. The dentin adjacent to the enamel junction appears similar to normal dentin, but the remainder is distinctly abnormal. Short misshapen tubules course through an atypical granular dentin matrix, which often demonstrates interglobular calcification (Fig. 2-96). Scanty atypical odontoblasts line the pulp surface, and cells can be seen entrapped within the defective dentin. In ground sections the enamel is normal in most patients; however, about one third of the patients have hypoplastic or hypocalcified defects.
TREATMENT AND PROGNOSIS: The entire dentition is at risk because of numerous problems. The root canals become threadlike and may develop microexposures, resulting in periapical inflammatory lesions. In spite of the risk of enamel loss and significant attrition, the teeth are not good candidates for full crowns because of cervical fracture. The success of full coverage is best in teeth with crowns and roots that exhibit close to a normal shape and size. Overlay dentures placed on teeth that are covered with fluoride-releasing glass ionomer cement have been used with success in some cases.
Additional therapeutic approaches have been used, but long-term follow-up is incomplete. In patients with extensive attrition, the vertical dimension has been rebuilt by placing nonprecious metal castings with adhesive luting agents on teeth that have received no preparation and are not subject to significant occlusal stress. The newer composites combined with a dentin-bonding agent have been used in areas subject to occlusal wear. When large kindreds have been followed over a long term, most of those affected are candidates for full dentures or implants by 30 years of age in spite of the numerous interventions. Newer materials and interventions may alter this outlook.
DENTIN DYSPLASIA
Dentin dysplasia was initially categorized in 1939. Two major patterns exist: type I and type II. By definition, dentin dysplasia should have no correlation with sys temic disease or dentinogenesis imperfecta. An unusual combination of type I and type II dentin dysplasia has been reported, but these cases represent variable pulpal anatomy that has been documented well in dentin dysplasia type I. Systemic diseases reported to be associated with similar dentin changes are listed in Box 2-10.
As evidenced by the clinical and radiographic descriptions that follow, dentin dysplasia type II is closely related to dentinogenesis imperfecta. In addition, genetic evaluation has shown that dentin dysplasia type II arises from mutation of the DSPP gene (see Dentinogenesis Imperfecta) and is allelic (different mutation of the same gene) to dentinogenesis imperfecta. The phenotypic and genotypic findings are so close that some might choose to classify dentin dysplasia type II as a variation of dentinogenesis imperfecta rather than grouping it with dentin dysplasia type I. Indeed, before the current era of molecular genetics, Witkop remarked on the close similarities of these two diseases and mentioned the possibility of reclassification.
CLINICAL AND RADIOGRAPHIC FEATURES:
DENTIN DYSPLASIA TYPE I: Dentin dysplasia type I (radicular dentin dysplasia), has been referred to as rootless teeth, because the loss of organization of the root dentin often leads to a shortened root length. The process exhibits an autosomal dominant pattern of inheritance and an approximate prevalence of 1:100,000. The enamel and coronal dentin are normal clinically and well formed (Fig. 2-97), but the radicular dentin loses all organization and subsequently is shortened dramatically (Fig. 2-98). Wide variation in root formation is produced because dentinal disorganization may occur during different stages of tooth development. If the dentin organization is lost early in tooth development, markedly deficient roots are formed; later disorganization results in minimal root malformation. The variability is most pronounced in permanent teeth and may vary not only from patient to patient but also from tooth to tooth in a single patient. Because of the shortened roots, the initial clinical signs are extreme tooth mobility and premature exfoliation, spontaneously or secondary to minor trauma. Less frequently, delayed eruption is the presenting symptom. The strength of the radicular dentin is reduced, with the teeth being predisposed to fracture during extractions.
Fig. 2-97 Dentin dysplasia type I. Dentition exhibiting attrition but otherwise normal coronal coloration and morphology.
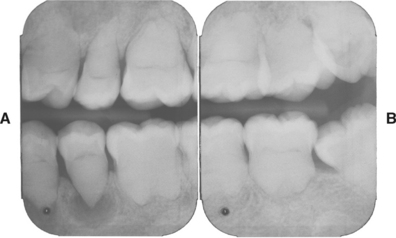
Fig. 2-98 Dentin dysplasia type I. Posterior dentition exhibiting dramatically shortened roots, absence of pulp canals, and small, crescent-shaped pulp chambers. Note radiolucency at apex of mandibular bicuspid. (Courtesy of Dr. Michael Quinn.)
Radiographically, the deciduous teeth often are affected severely, with little or no detectable pulp, and roots that are markedly short or absent. The permanent teeth vary according to the proportion of organized versus disorganized dentin (Fig. 2-99). Several years ago, a subclassification of dentin dysplasia type I was proposed and has become widely accepted (Box 2-11).
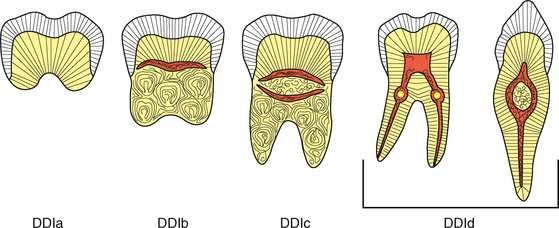
Fig. 2-99 Dentin dysplasia type I. Illustration demonstrating the variability of the radiographic appearance according to the degree of dentin disorganization within the root.
In general, the teeth without root canals are those that frequently develop periapical radiolucencies without obvious cause (see Fig. 2-98). The radiolucencies represent periapical inflammatory disease and appear secondary to caries or spontaneous coronal exposure of microscopic threads of pulpal remnants present within the defective dentin. The subclassification system has proven to be beneficial, because it assists in highlighting the mild DDId variant that often is not quickly recognized as dentin dysplasia and can be confused with dentin dysplasia type II. On occasion, reports have demonstrated patients with classic DDId-like involvement of the anteriors and bicuspids but without affected molars.
A similar but unrelated disorder is fibrous dysplasia of dentin. This autosomal dominant disorder exhibits teeth that are normal clinically. Radiographically the teeth are normal in shape but demonstrate a radiodense product filling the pulp chambers and canals. In contrast to dentinogenesis imperfecta, small foci of radiolucency can be seen in the pulp. In contrast to dentin dysplasia type I, no crescent pulp chambers and no decrease in root length are seen. The radiodense intrapulpal material consists of fibrotic dentin.
DENTIN DYSPLASIA TYPE II: Dentin dysplasia type II (coronal dentin dysplasia) is inherited as an autosomal dominant hereditary disorder that exhibits numerous features of dentinogenesis imperfecta. In contrast to dentin dysplasia type I, the root length is normal in both dentitions. The deciduous teeth closely resemble those of dentinogenesis imperfecta. Clinically, the teeth demonstrate a blue-to-amber-to-brown translucence. Radiographically, the dental changes include bulbous crowns, cervical constriction, thin roots, and early obliteration of the pulp. The permanent teeth demonstrate normal clinical coloration; however, radiographically, the pulp chambers exhibit significant enlargement and apical extension. This altered pulpal anatomy has been described as thistle tube–shaped or flame-shaped (Figs. 2-100 and 2-101). Pulp stones develop in the enlarged pulp chambers.
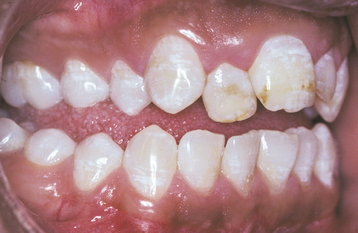
Fig. 2-100 Dentin dysplasia type II. Permanent dentition that does not exhibit translucence, as noted in the deciduous teeth. The patient also exhibits mild fluorosis of the enamel.
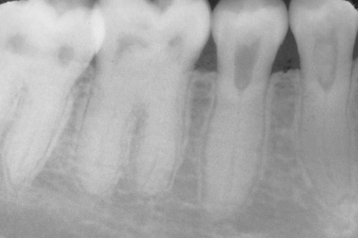
Fig. 2-101 Dentin dysplasia type II. Radiographic appearance of the dentition depicted in Fig. 2-100. Note thistle tube–shaped enlargements of the pulp chambers and numerous pulp stones.
A similar but unrelated disorder is pulpal dysplasia. This process develops in teeth that are normal clinically. Radiographically, both dentitions exhibit thistle tube–shaped pulp chambers and multiple pulp stones.
HISTOPATHOLOGIC FEATURES: In patients with dentin dysplasia type I, the coronal enamel and dentin are normal. Apical to the point of disorganization, the central portion of the root forms whorls of tubular dentin and atypical osteodentin. These whorls exhibit a peripheral layer of normal dentin, giving the root the appearance of a “stream flowing around boulders” (Fig. 2-102).
Fig. 2-102 Dentin dysplasia type I. Polarized light view of affected tooth demonstrating a classic “stream flowing around boulders” appearance.
In patients with dentin dysplasia type II, the deciduous teeth demonstrate the pattern described in dentinogenesis imperfecta. The permanent teeth exhibit normal enamel and coronal dentin. Adjacent to the pulp, numerous areas of interglobular dentin are seen. The radicular dentin is atubular, amorphous, and hypertrophic. Pulp stones develop in any portion of the chamber (Fig. 2-103).
TREATMENT AND PROGNOSIS: In patients with dentin dysplasia type I, preventive care is of foremost importance. Perhaps as a result of shortened roots, early loss from periodontitis is frequent. In addition, pulp vascular channels extend close to the dentinoenamel junction; therefore, even shallow occlusal restorations can result in pulpal necrosis. Meticulous oral hygiene must be established and maintained.
If periapical inflammatory lesions develop, the root length guides the therapeutic choice. Conventional endodontic therapy requires mechanical creation of canal paths and has been successful in teeth without extremely short roots. Teeth with short roots demonstrate pulpal ramifications that eliminate conventional endodontic treatment as an appropriate therapeutic option. Periapical curettage and retrograde amalgam seals have demonstrated short-term success.
Dentin dysplasia type II demonstrates similar problems, and meticulous oral hygiene must be established. The deciduous teeth can be approached in a manner similar to that used for dentinogenesis imperfecta. In the permanent teeth, an increased risk of periapical inflammatory lesions is also seen. Because the pulp canals are not usually obliterated completely, endodontic therapy is accomplished more readily.
REGIONAL ODONTODYSPLASIA (GHOST TEETH)
Regional odontodysplasia is a localized, nonhereditary developmental abnormality of teeth with extensive adverse effects on the formation of enamel, dentin, and pulp. Most cases are idiopathic, but a number have been related to various syndromes, growth abnormalities, neural disorders, and vascular malformations (Box 2-12). A number of causes have been proposed (Box 2-13), but the most popular theory revolves around an alteration in the vascular supply. Several cases have occurred in patients with vascular nevi of the head and neck; in addition, similar changes have been induced in animals by restricting the vascular flow to an area of the jaws.
CLINICAL AND RADIOGRAPHIC FEATURES: Regional odontodysplasia is an uncommon finding that occurs in both dentitions and exhibits no racial predilection and a slight female predominance. A review of the age at the time of diagnosis reveals a bimodal peak that correlates with the normal time of eruption of the deciduous (2 to 4 years) and permanent (7 to 11 years) dentitions. Typically, the process affects a focal area of the dentition, with involvement of several contiguous teeth. A maxillary predominance exists with a predilection for the anterior teeth. Occasionally, an unaffected tooth may be intermixed within a row of altered teeth. Ipsilateral involvement of both arches and bilateral changes in the same jaw have been reported. Although rare generalized involvement has been documented, the presence of regional odontodysplasia in more than two quadrants is rare. Involvement of the deciduous dentition is typically followed by similarly affected permanent teeth. In the area of altered teeth, the surrounding bone often exhibits a lower density; in addition, hyperplasia of the soft tissue may be noted overlying affected teeth that are impacted.
Many of the affected teeth fail to erupt. Erupted teeth demonstrate small irregular crowns that are yellow to brown, often with a very rough surface. Caries and associated periapical inflammatory lesions are fairly common. Because of dentinal clefts and very long pulp horns, pulpal necrosis is common (often in the absence of an obvious cause). Radiographically, the altered teeth demonstrate extremely thin enamel and dentin surrounding an enlarged radiolucent pulp, resulting in a pale wispy image of a tooth; hence the term ghost teeth (Fig. 2-104). A lack of contrast is seen between the dentin and the enamel, with an indistinct or “fuzzy” appearance of the coronal silhouette. Short roots and open apices may be seen. The enlarged pulps frequently demonstrate one or more prominent pulp stones. The most common presenting signs and symptoms include delayed or failure of eruption, early exfoliation, abscess formation, malformed teeth, and noninflammatory gingival enlargement.
HISTOPATHOLOGIC FEATURES: In ground sections the thickness of the enamel varies, resulting in an irregular surface. The prism structure of the enamel is irregular or lacking with a laminated appearance. The dentin contains clefts scattered through a mixture of interglobular dentin and amorphous material. Globular areas of poorly organized tubular dentin and scattered cellular inclusions often are seen. The pulp tissue contains free or attached stones that may exhibit tubules or consist of laminated calcification. The follicular tissue surrounding the crown may be enlarged and typically exhibits focal collections of basophilic enamel-like calcifications called enameloid conglomerates (Fig. 2-105). This pattern of calcification is not specific for regional odontodysplasia and has been seen in other processes with disturbed enamel formation, such as amelogenesis imperfecta. Scattered islands of odontogenic epithelium and other patterns of intramural calcification also are seen.
TREATMENT AND PROGNOSIS: The basic approach to therapy of regional odontodysplasia is directed toward retention of the altered teeth, whenever possible, to allow for appropriate development and preservation of the surrounding alveolar ridge. Endodontic therapy on nonvital teeth that have sufficient hard tissue to allow restoration has been performed successfully. Unerupted teeth should remain untouched, restoring function with a removable partial prosthesis until the skeletal growth period has passed. Erupted teeth can be covered with etched-retained restorations or stainless steel crowns until final restorations can be placed after the completion of growth. Because of the fragile nature of the coronal hard tissue and the ease of pulp exposure, tooth preparation is contraindicated. Severely affected and infected teeth often are not salvageable and need to be removed. Although ankylosis has been seen with autotransplanted teeth, normal bicuspids have been autotransplanted into the extraction sites of the abnormal dentition. This approach can successfully restore masticatory function, allowing appropriate facial development, and preventing ridge atrophy and supereruption of the opposing dentition. Osseointegrated implants have been placed in growing children with hypodontia and could be used in the setting of regional odontodysplasia. Because implants essentially become ankylosed, they have the potential to become embedded upon continued gnathic growth in a child. For this reason, implants generally should be reserved for patients who have completed pubertal growth.
Although vitality of the abnormal dentition often is difficult to maintain, such efforts may bring significant rewards. Several investigators have shown continued dentinal development of teeth affected by regional odontodysplasia. In cases monitored for many years, the teeth lost their ghostly appearance and revealed a resultant decrease in pulp size, a significant increase in dentin thickness, and ultimate relative normalization of the radicular anatomy. In contrast, the enamel remained hypoplastic. The surrounding bone became well developed and lost its diminished density. Only a few reports of this phenomenon exist, however, most likely because the prior treatment of choice has been extraction.