Chapter 4 Third Week of Human Development
Rapid development of the embryo from the trilaminar embryonic disc during the third week is characterized by:
The third week of development coincides with the week following the first missed menstrual period, that is, 5 weeks after the first day of the last normal menstrual period. Cessation of menstruation is often the first indication that a woman may be pregnant. Approximately 5 weeks after the last normal menstrual period (Fig. 4-1), a normal pregnancy can be detected with ultrasonography.
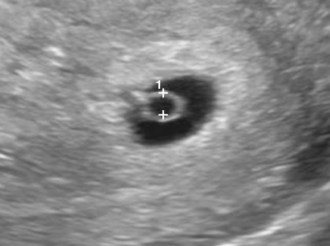
FIGURE 4–1 Ultrasonograph sonogram of a 3.5-week conceptus. Note the surrounding secondary umbilical vesicle (calipers) and the surrounding trophoblast (bright ring of tissue).
(Courtesy of E.A. Lyons, M.D., Professor of Radiology and Obstetrics and Gynecology, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
Pregnancy Symptoms
Frequent symptoms of pregnancy are nausea and vomiting, which may occur by the end of the third week; however, the time of onset of these symptoms varies. Vaginal bleeding at the expected time of menstruation does not rule out pregnancy because sometimes there is a slight loss of blood from the implantation site of the blastocyst. Implantation bleeding results from leakage of blood from a hole in the closing plug in the endometrial epithelium into the uterine cavity from disrupted lacunar networks in the implanted blastocyst (see Fig. 3-5A). When this bleeding is interpreted as menstruation, an error occurs in determining the expected delivery date of the baby.
 Gastrulation: Formation of Germ Layers
Gastrulation: Formation of Germ Layers
Gastrulation is the formative process by which the three germ layers, which are precursors of all embryonic tissues, and axial orientation are established in embryos. During gastrulation, the bilaminar embryonic disc is converted into a trilaminar embryonic disc. Extensive cell shape changes, rearrangement, movement, and alterations in adhesive properties contribute to the process of gastrulation.
Gastrulation is the beginning of morphogenesis (development of body form) and is the significant event occurring during the third week. During this period, the embryo may be referred to as a gastrula. Bone morphogenetic proteins and other signaling molecules such as FGFs, Shh (sonic hedgehog), Tgifs, and Wnts play a crucial role in gastrulation.
Each of the three germ layers (ectoderm, mesoderm, and endoderm) gives rise to specific tissues and organs:
 Primitive Streak
Primitive Streak
The first morphologic sign of gastrulation is formation of the primitive streak on the surface of the epiblast of the embryonic disc (Fig. 4-2B). By the beginning of the third week, a thickened linear band of epiblast—the primitive streak—appears caudally in the median plane of the dorsal aspect of the embryonic disc (Figs. 4-2C and 4-3). The primitive streak results from the proliferation and movement of cells of the epiblast to the median plane of the embryonic disc. As the streak elongates by addition of cells to its caudal end, its cranial end proliferates to form a primitive node (Figs. 4-2F and 4-3).
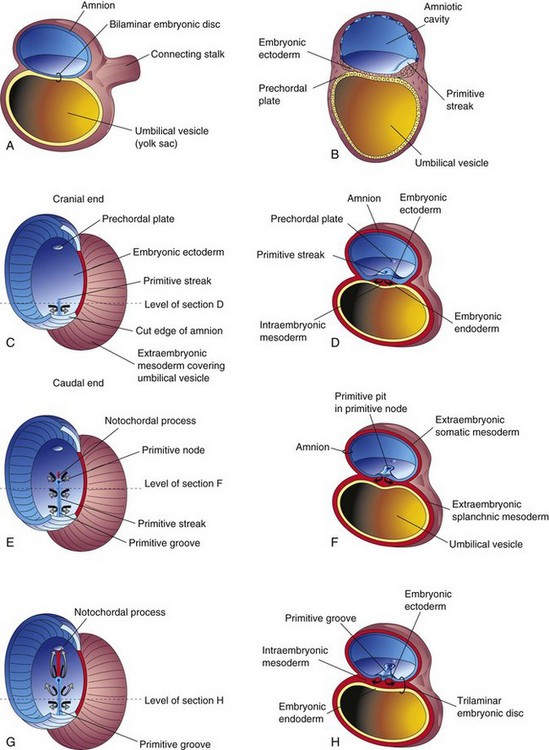
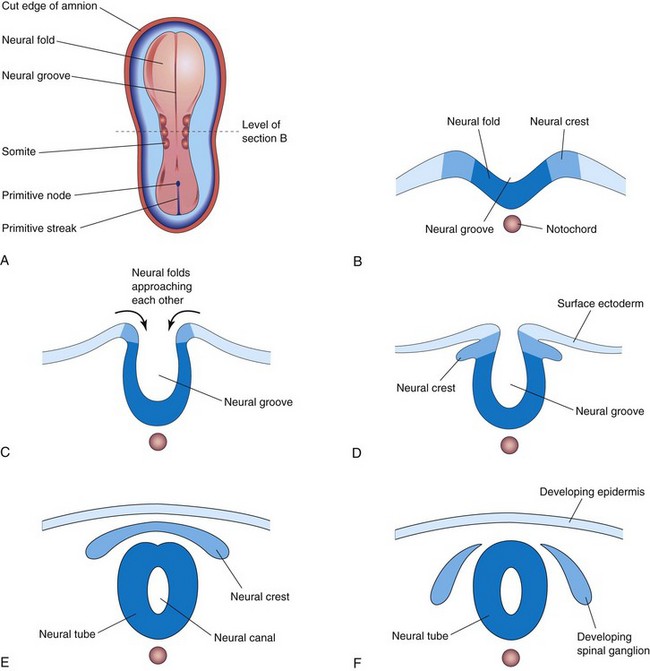
FIGURE 4–2 Illustrations of the formation of the trilaminar embryonic disc (days 15 to 16). The arrows indicate invagination and migration of mesenchymal cells from the primitive streak between the ectoderm and endoderm. C, E, and G, Dorsal views of the trilaminar embryonic disc early in the third week, exposed by removal of the amnion. A, B, D, F, and H, Transverse sections through the embryonic disc. The levels of the sections are indicated in C, E, and G. The prechordal plate, indicating the head region in Fig. 4-2C, is indicated by a light blue oval because this thickening of endoderm cannot be seen from the dorsal surface.
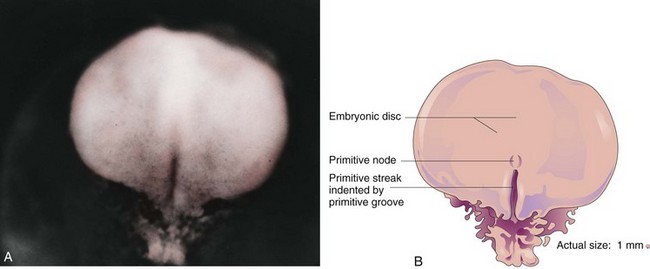
FIGURE 4–3 A, Dorsal view of an embryo approximately 16 days old. B, Drawing of structures shown in A.
(A, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
Concurrently, a narrow groove—primitive groove—develops in the primitive streak that is continuous with a small depression in the primitive node—the primitive pit. As soon as the primitive streak appears, it is possible to identify the embryo’s craniocaudal axis, cranial and caudal ends, dorsal and ventral surfaces, and right and left sides. The primitive groove and pit result from the invagination (inward movement) of epiblastic cells, which is indicated by arrows in Figure 4-2E.
Shortly after the primitive streak appears, cells leave its deep surface and form mesenchyme, an embryonic connective tissue consisting of small, spindle-shaped cells loosely arranged in an extracellular matrix of sparse collagen (reticular) fibers (Fig. 4-4B). Mesenchyme forms the supporting tissues of the embryo, such as most of the connective tissues of the body and the connective tissue framework of glands. Some mesenchyme forms mesoblast (undifferentiated mesoderm), which forms the intraembryonic, or embryonic, mesoderm (Fig. 4-2D).
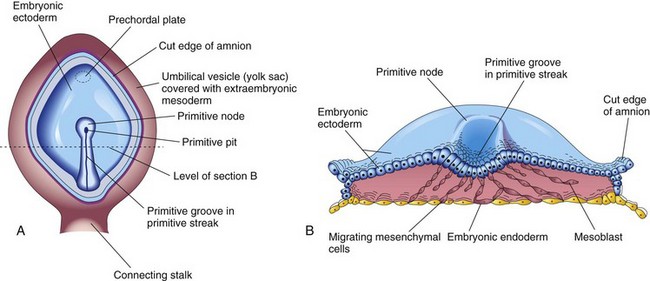
FIGURE 4–4 A, Drawing of a dorsal view of a 16-day embryo. The amnion has been removed to expose the primitive node, primitive pit and primitive streak. B, Drawing of the cranial half of the embryonic disc. The trilaminar embryonic disc has been cut transversely to show the migration of mesenchymal cells from the primitive streak to form mesoblast that soon organizes to form the intraembryonic mesoderm. This illustration also shows that most of the embryonic endoderm also arises from the epiblast. Most of the hypoblastic cells are displaced to extraembryonic regions such as the wall of the umbilical vesicle.
Cells from the epiblast, as well as from the primitive node and other parts of the primitive streak, displace the hypoblast, forming the embryonic endoderm in the roof of the umbilical vesicle (Fig. 4-2H). The cells remaining in the epiblast form the embryonic ectoderm.
Research data suggest that signaling molecules (nodal factors) of the transforming growth factor β superfamily induce formation of mesoderm. The concerted action of other signaling molecules (e.g., Wnt3a, Wnt5a, FGFs) also participates in specifying germ cell layer fates. Moreover, transforming growth factor β (nodal), a T-box transcription factor (veg T), and the Wnt signaling pathway appear to be involved in specification of the endoderm.
Mesenchymal cells derived from the primitive streak migrate widely. These pluripotential cells differentiate into diverse types of cells, such as fibroblasts, chondroblasts, and osteoblasts (see Chapter 5). In summary, cells of the epiblast, through the process of gastrulation, give rise to all three germ layers in the embryo, the primordia of all its tissues and organs.
Fate of Primitive Streak
The primitive streak actively forms mesoderm by the ingression of cells until the early part of the fourth week; thereafter, production of mesoderm slows down. The primitive streak diminishes in relative size and becomes an insignificant structure in the sacrococcygeal region of the embryo (Fig. 4-5D). Normally the primitive streak undergoes degenerative changes and disappears by the end of the fourth week.
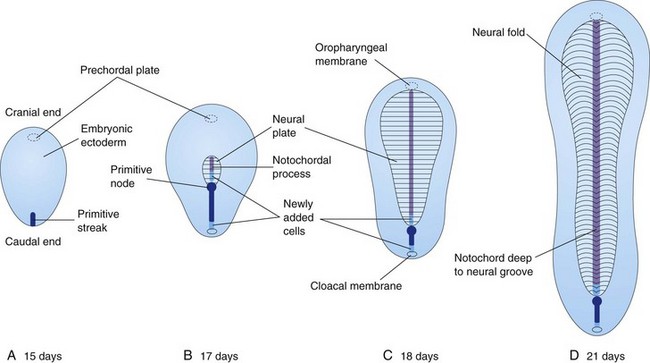
FIGURE 4–5 Diagrammatic sketches of dorsal views of the embryonic disc showing how it lengthens and changes shape during the third week. The primitive streak lengthens by addition of cells at its caudal end, and the notochordal process lengthens by migration of cells from the primitive node. The notochordal process and adjacent mesoderm induce the overlying embryonic ectoderm to form the neural plate, the primordium of the CNS. Observe that as the notochordal process elongates, the primitive streak shortens. At the end of the third week, the notochordal process is transformed into the notochord.
Sacrococcygeal Teratoma
Remnants of the primitive streak may persist and give rise to a sacrococcygeal teratoma (Fig. 4-6). A teratoma is a type of germ cell tumor. Because they are derived from pluripotent primitive streak cells, these tumors contain tissues derived from all three germ layers in incomplete stages of differentiation. Sacrococcygeal teratomas are the most common tumor in newborns and have an incidence of approximately one in 35,000; most affected infants (80%) are female. Sacrococcygeal teratomas are usually diagnosed on routine antenatal ultrasonography; most tumors are benign. These teratomas are usually surgically excised promptly, and the prognosis is good. A presacral teratoma may cause bowel or urinary obstruction in the newborn.

FIGURE 4–6 Female infant with a large sacrococcygeal teratoma that developed from remnants of the primitive streak. The tumor, a neoplasm made up of several different types of tissue, was surgically removed.
(Courtesy of A.E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
 Notochordal Process and Notochord
Notochordal Process and Notochord
Some mesenchymal cells migrate through the primitive streak and, as a consequence, acquire mesodermal cell fates. These cells then migrate cranially from the primitive node and pit, forming a median cellular cord, the notochordal process (Fig. 4-7C). This process soon acquires a lumen, the notochordal canal.
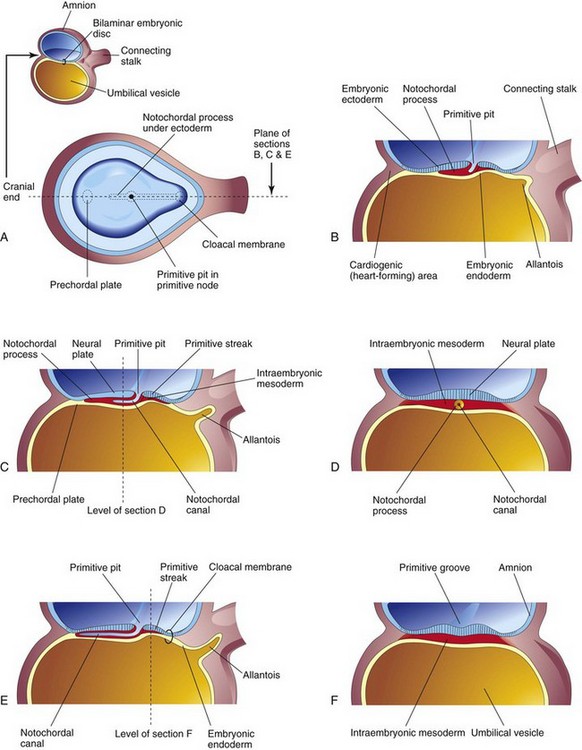
FIGURE 4–7 Illustrations of developing notochordal process. The small sketch at the upper left is for orientation. A, Dorsal view of the embryonic disc (approximately 16 days) exposed by removal of the amnion. The notochordal process is shown as if it were visible through the embryonic ectoderm. B, C, and E, Median sections at the plane shown in A, illustrating successive stages in the development of the notochordal process and canal. The stages shown in C and E occur at approximately 18 days. D and F, Transverse sections through the embryonic disc at the levels shown in C and E.
The notochordal process grows cranially between the ectoderm and endoderm until it reaches the prechordal plate, a small circular area of columnar endodermal cells where the ectoderm and endoderm are fused. Prechordal mesoderm is a mesenchymal population of neural crest origin, rostral to the notochord. The prechordal plate gives rise to the endoderm of the oropharyngeal membrane, located at the future site of the oral cavity (Fig. 4-8C). The prechordal plate serves as a signaling center (Shh and PAX6) for controlling development of cranial structures, including the forebrain and eyes.
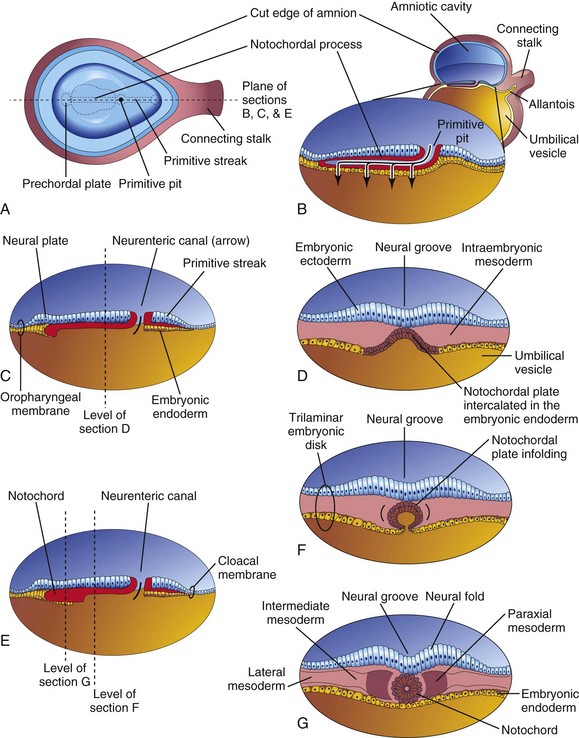
FIGURE 4–8 Illustrations of notochord development by transformation of the notochordal process. A, Dorsal view of the bilaminar embryonic disc at 18 days, exposed by removing the amnion. B, Three-dimensional median section of the embryo. C and E, Similar sections of slightly older embryos. D, F, and G, Transverse sections of the trilaminar embryonic disc at the levels shown in C and E.
Mesenchymal cells from the primitive streak and notochordal process migrate laterally and cranially, among other mesodermal cells, between the ectoderm and endoderm until they reach the margins of the embryonic disc. These cells are continuous with the extraembryonic mesoderm covering the amnion and umbilical vesicle (Fig. 4-2C and D). Some mesenchymal cells from the primitive streak that have mesodermal fates migrate cranially on each side of the notochordal process and around the prechordal plate. Here they meet cranially to form cardiogenic mesoderm in the cardiogenic area where the heart primordium begins to develop at the end of the third week (see Fig. 4-11B).
Caudal to the primitive streak there is a circular area—the cloacal membrane, which indicates the future site of the anus (Fig. 4-7E). The embryonic disc remains bilaminar here and at the oropharyngeal membrane because the embryonic ectoderm and endoderm are fused at these sites, thereby preventing migration of mesenchymal cells between them (Fig. 4-8C). By the middle of the third week, intraembryonic mesoderm separates the ectoderm and endoderm everywhere except
Instructive signals from the primitive streak region induce notochordal precursor cells to form the notochord, a cellular rod-like structure. The molecular mechanism that induces these cells involves (at least) Shh signaling from the floor plate of the neural tube. The notochord
The notochord develops as follows:
The notochord extends from the oropharyngeal membrane to the primitive node. The notochord degenerates as the bodies of the vertebrae form, but small portions of it persist as the nucleus pulposus of each intervertebral disc.
The notochord functions as the primary inductor (signaling center) in the early embryo. The developing notochord induces the overlying embryonic ectoderm to thicken and form the neural plate (Fig. 4-8C), the primordium of the CNS.
 Allantois
Allantois
The allantois appears on approximately day 16 as a small diverticulum (outpouching) from the caudal wall of the umbilical vesicle that extends into the connecting stalk (Figs. 4-7B, C, and E and 4-8B).
In reptiles, birds, and most mammals, this endodermal sac has a respiratory function and/or acts as a reservoir for urine during embryonic life. In humans, the allantoic sac remains very small, but allantoic mesoderm expands beneath the chorion and forms blood vessels that will serve the placenta. The proximal part of the original allantoic diverticulum persists throughout much of development as a stalk called the urachus, which extends from the bladder to the umbilical region. The urachus is represented in adults by the median umbilical ligament. The blood vessels of the allantoic stalk become the umbilical arteries (see Fig. 4-12). The intraembryonic part of the umbilical veins has a separate origin.
Allantoic Cysts
Allantoic cysts, remnants of the extraembryonic portion of the allantois, are usually found between the fetal umbilical vessels and can be detected by ultrasonography. They are most commonly detected in the proximal part of the umbilical cord, near its attachment to the anterior abdominal wall. The cysts are generally asymptomatic until childhood or adolescence, when they can become infected and inflamed.
 Neurulation: Formation of Neural Tube
Neurulation: Formation of Neural Tube
The processes involved in the formation of the neural plate and neural folds and closure of the folds to form the neural tube constitute neurulation. Neurulation is completed by the end of the fourth week, when closure of the caudal neuropore occurs (Chapter 5).
Neural Plate and Neural Tube
As the notochord develops, it induces the overlying embryonic ectoderm, located at or adjacent to the midline, to thicken and form an elongated neural plate of thickened epithelial cells. The neuroectoderm of the neural plate gives rise to the CNS—the brain and spinal cord. Neuroectoderm also gives rise to various other structures, for example, the retina. At first, the neural plate corresponds in length to the underlying notochord. It appears rostral (head end) to the primitive node and dorsal (posterior) to the notochord and the mesoderm adjacent to it (Fig. 4-5B). As the notochord elongates, the neural plate broadens and eventually extends cranially as far as the oropharyngeal membrane (Figs. 4-5C and 4-8C). Eventually the neural plate extends beyond the notochord.
On approximately the 18th day, the neural plate invaginates along its central axis to form a longitudinal median neural groove, which has neural folds on each side (Fig. 4-8G). The neural folds become particularly prominent at the cranial end of the embryo and are the first signs of brain development. By the end of the third week, the neural folds have begun to move together and fuse, converting the neural plate into the neural tube, the primordium of the brain vesicles and the spinal cord (Figs. 4-9 and 4-10). The neural tube soon separates from the surface ectoderm as the neural folds meet. Neural crest cells undergo an epithelial to mesenchymal transition and migrate away as the neural folds meet and the free edges of the surface ectoderm (non-neural ectoderm) fuse so that this layer becomes continuous over the neural tube and the back of the embryo (Fig. 4-10E and F). Subsequently, the surface ectoderm differentiates into the epidermis. Neurulation is completed during the fourth week. Neural tube formation is a complex cellular and multifactorial process involving a cascade of molecular mechanisms and extrinsic factors (see Chapter 17).
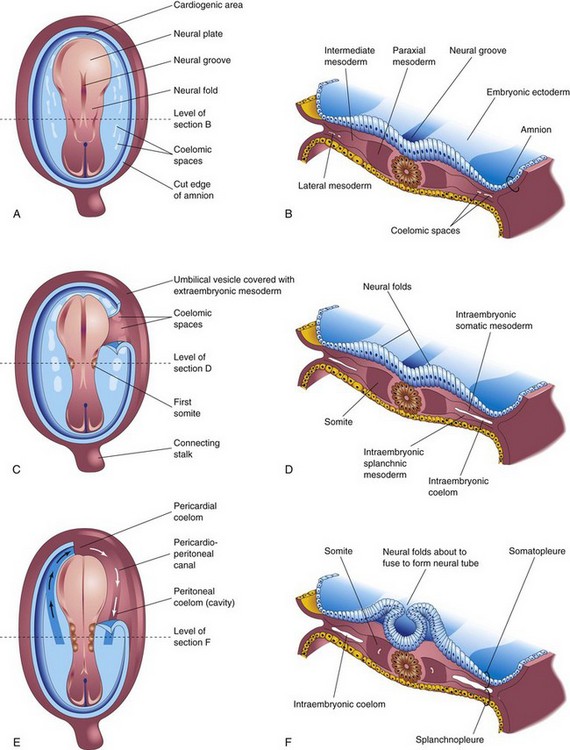
FIGURE 4–9 Drawings of embryos at 19 to 21 days illustrating development of the somites and intraembryonic coelom. A, C, and E, Dorsal views of the embryo, exposed by removal of the amnion. B, D, and F, Transverse sections through the trilaminar embryonic disc at the levels shown. A, Presomite embryo of approximately 18 days. C, An embryo of approximately 20 days showing the first pair of somites. Part of the somatopleure on the right has been removed to show the coelomic spaces in the lateral mesoderm. E, A three-somite embryo (approximately 21 days) showing the horseshoe-shaped intraembryonic coelom, exposed on the right by removal of part of the somatopleure.
Neural Crest Formation
As the neural folds fuse to form the neural tube, some neuroectodermal cells lying along the inner margin of each neural fold lose their epithelial affinities and attachments to neighboring cells (Fig. 4-10). As the neural tube separates from the surface ectoderm, neural crest cells form a flattened irregular mass, the neural crest, between the neural tube and the overlying surface ectoderm (Fig. 4-10E). Wnt/β-catenin signaling activates the Gbx2 homeobox gene and is essential for the development of the neural crest. The neural crest soon separates into right and left parts that shift to the dorsolateral aspects of the neural tube; here they give rise to the sensory ganglia of the spinal and cranial nerves. Neural crest cells subsequently move both into and over the surface of somites. Although these cells are difficult to identify, special tracer techniques have revealed that neural crest cells disseminate widely but usually along predefined pathways. Differentiation and migration of neural crest cells are regulated by molecular interactions of specific genes (e.g., FoxD3, Snail2, Sox9, and Sox10), signaling molecules, and transcription factors.
Neural crest cells give rise to the spinal ganglia (dorsal root ganglia) and the ganglia of the autonomic nervous system. The ganglia of cranial nerves V, VII, IX, and X are also partly derived from neural crest cells. In addition to forming ganglion cells, neural crest cells form the neurolemma sheaths of peripheral nerves and contribute to the formation of the leptomeninges arachnoid mater and pia mater (Chapter 17). Neural crest cells also contribute to the formation of pigment cells, the suprarenal (adrenal) medulla, and many connective tissue components in the head (see Chapter 9).
Laboratory studies indicate that cell interactions both within the surface epithelium and between it and underlying mesoderm are required to establish the boundaries of the neural plate and specify the sites where epithelial-mesenchymal transformation will occur. These are mediated by bone morphogenetic proteins, Wnt, Notch, and FGF signaling systems. Also, molecules such as ephrins are important in guiding specific streams of migrating neural crest cells. Many human diseases result from defective migration and/or differentiation of neural crest cells.
Birth Defects Resulting from Abnormal Neurulation
Because the neural plate, the primordium of the CNS, appears during the third week and gives rise to the neural folds and the beginning of the neural tube, disturbance of neurulation may result in severe birth defects of the brain and spinal cord (see Chapter 17). Neural tube defects are among the most common congenital anomalies. Meroencephaly (partial absence of the brain) is the most severe neural tube defect and is also the most common anomaly affecting the CNS. Although the term anencephaly (Greek an, without + enkephalos, brain) is commonly used, it is a misnomer because a remnant of the brain is present. Available evidence suggests that the primary disturbance (e.g., a teratogenic drug; see Chapter 20) affects cell fates, cell adhesion, and the mechanism of neural tube closure. This results in failure of the neural folds to fuse and form the neural tube. Neural tube defects may also be secondary to or linked to lesions affecting the degree of flexion imposed on the neural plate during folding of the embryo.
 Development of Somites
Development of Somites
In addition to the notochord, cells derived from the primitive node form paraxial mesoderm. Close to the primitive node, this cell population appears as a thick, longitudinal column of cells (Figs. 4-8G and 4-9B). Each column is continuous laterally with the intermediate mesoderm, which gradually thins into a layer of lateral mesoderm. The lateral mesoderm is continuous with the extraembryonic mesoderm covering the umbilical vesicle and amnion. Toward the end of the third week, the paraxial mesoderm differentiates, condenses, and begins to divide into paired cuboidal bodies, the somites (Greek soma, body), which form in a craniocaudal sequence. These blocks of mesoderm are located on each side of the developing neural tube (Fig. 4-9C to F). About 38 pairs of somites form during the somite period of human development (days 20 to 30). By the end of the fifth week, 42 to 44 pairs of somites are present. The somites form distinct surface elevations on the embryo and are somewhat triangular in transverse sections (Fig. 4-9C to F). Because the somites are so prominent during the fourth and fifth weeks, they are used as one of several criteria for determining an embryo’s age (see Chapter 5, Table 5-1).
Somites first appear in the future occipital region of the embryo. They soon develop craniocaudally and give rise to most of the axial skeleton and associated musculature as well as to the adjacent dermis of the skin. The first pair of somites appears a short distance caudal to the site at which the otic placode forms (Fig. 4-9C). Motor axons from the spinal cord innervate muscle cells in the somites, a process that requires the correct guidance of axons from the spinal cord to the appropriate target cells.
Formation of somites from the paraxial mesoderm involves the expression of Notch pathway genes (Notch signaling pathway), Hox genes, and other signaling factors. Moreover, somite formation from paraxial mesoderm is preceded by expression of the forkhead transcription factors FoxC1 and FoxC2 and the craniocaudal segmental pattern of the somites is regulated by the Delta-Notch signaling. A molecular oscillator or clock has been proposed as the mechanism responsible for the orderly sequencing of somites.
 Development of Intraembryonic Coelom
Development of Intraembryonic Coelom
The primordium of the intraembryonic coelom (embryonic body cavity) appears as isolated coelomic spaces in the lateral mesoderm and cardiogenic (heart-forming) mesoderm (Fig. 4-9A). These spaces soon coalesce to form a single horseshoe-shaped cavity, the intraembryonic coelom (Fig. 4-9E), which divides the lateral mesoderm into two layers (Fig. 4-9D):
The somatic mesoderm and overlying embryonic ectoderm form the embryonic body wall or somatopleure (Fig. 4-9F), whereas the splanchnic mesoderm and underlying embryonic endoderm form the embryonic gut or splanchnopleure. During the second month, the intraembryonic coelom is divided into three body cavities: pericardial cavity, pleural cavities, and peritoneal cavity. For a description of these divisions of the intraembryonic coelom, see Chapter 8.
Early Development of Cardiovascular System
At the end of the second week, embryonic nutrition is obtained from the maternal blood by diffusion through the extraembryonic coelom and umbilical vesicle. At the beginning of the third week, vasculogenesis and angiogenesis, or blood vessel formation, begins in the extraembryonic mesoderm of the umbilical vesicle, connecting stalk, and chorion (Fig. 4-11). Embryonic blood vessels begin to develop approximately 2 days later. The early formation of the cardiovascular system is correlated with the urgent need for blood vessels to bring oxygen and nourishment to the embryo from the maternal circulation through the placenta. During the third week, a primordial uteroplacental circulation develops (Fig. 4-12).

FIGURE 4–11 Successive stages in the development of blood and blood vessels. A, Lateral view of the umbilical vesicle and part of the chorionic sac (approximately 18 days). B, Dorsal view of the embryo exposed by removing the amnion (approximately 20 days). C to F, Sections of blood islands showing progressive stages in the development of blood and blood vessels.
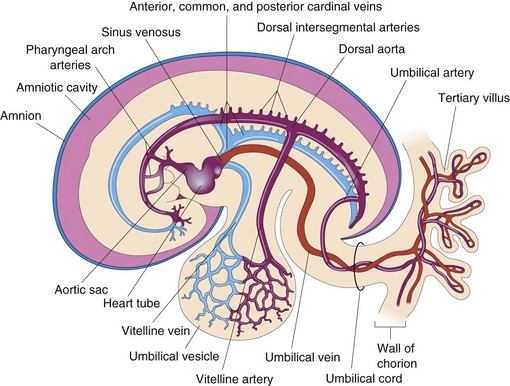
FIGURE 4–12 Diagram of the primordial cardiovascular system in an embryo of approximately 21 days, viewed from the left side. Observe the transitory stage of the paired symmetrical vessels. Each heart tube continues dorsally into a dorsal aorta that passes caudally. Branches of the aortae are (1) umbilical arteries establishing connections with vessels in the chorion, (2) vitelline arteries to the umbilical vesicle, and (3) dorsal intersegmental arteries to the body of the embryo. Vessels on the umbilical vesicle form a vascular plexus that is connected to the heart tubes by vitelline veins. The cardinal veins return blood from the body of the embryo. The umbilical vein carries oxygenated blood and nutrients to f the chorion that provides nourishment to the embryo. The arteries carry poorly oxygenated blood and waste products to the chorionic villi for transfer to the mother’s blood.
Vasculogenesis and Angiogenesis
The formation of the embryonic vascular system involves two processes: vasculogenesis and angiogenesis. Vasculogenesis is the formation of new vascular channels by assembly of individual cell precursors called angioblasts. Angiogenesis is the formation of new vessels by budding and branching from preexisting vessels. Blood vessel formation (vasculogenesis) in the embryo and extraembryonic membranes during the third week may be summarized as follows (Fig. 4-11):
Blood cells develop from the endothelial cells of vessels as they grow on the umbilical vesicle and allantois at the end of the third week (Fig. 4-11E and F), and later in specialized sites along the dorsal aorta. Blood formation (hematogenesis) does not begin in the embryo until the fifth week. It occurs first along the aorta and then in various parts of the embryonic mesenchyme, mainly the liver, and later in the spleen, bone marrow, and lymph nodes. Fetal and adult erythrocytes are derived from different hematopoietic progenitor cells (hemangioblasts).
Primordial Cardiovascular System
The heart and great vessels form from mesenchymal cells in the cardiogenic area (Fig. 4-11B). Paired, longitudinal endothelial-lined channels—the endocardial heart tubes—develop during the third week and fuse to form a primordial heart tube. The tubular heart joins with blood vessels in the embryo, connecting stalk, chorion, and umbilical vesicle to form a primordial cardiovascular system (Fig. 4-12). By the end of the third week, the blood is circulating and the heart begins to beat on the 21st or 22nd day.
The cardiovascular system is the first organ system to reach a functional state. The embryonic heartbeat can be detected using Doppler ultrasonography during the fifth week, approximately 7 weeks after the last normal menstrual period (Fig. 4-13).
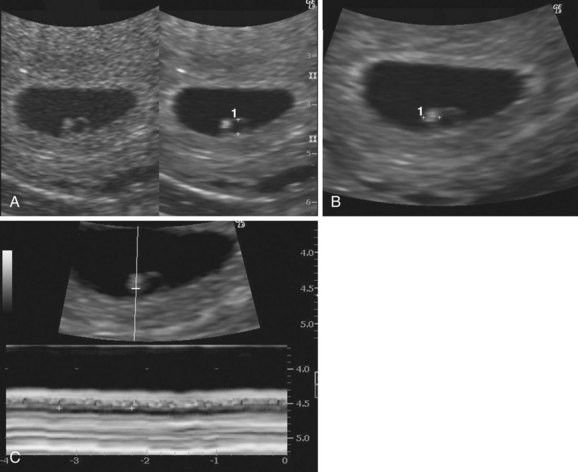
FIGURE 4–13 Endovaginal ultrasonogram of a 4-week embryo A, Secondary umbilical vesicle (calipers, 2 mm). B, Bright (echogenic) 4-week embryo (calipers, 2.4 mm) C, Cardiac activity of 116 beats per minute demonstrated with motion mode. The calipers are used to encompass two beats.
(Courtesy of E.A. Lyons, M.D., Professor of Radiology and Obstetrics and Gynecology, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
Abnormal Growth of Trophoblast
Sometimes the embryo dies and the chorionic villi (Fig. 4-14A) do not complete their development; that is, they do not become vascularized to form tertiary villi. These degenerating villi form cystic swellings—hydatidiform moles—which resemble a bunch of grapes. The moles exhibit variable degrees of trophoblastic proliferation and produce excessive amounts of human chorionic gonadotropin. Some moles develop after spontaneous abortions, and others occur after normal deliveries. Three percent to 5% of moles develop into malignant trophoblastic lesions—choriocarcinomas.
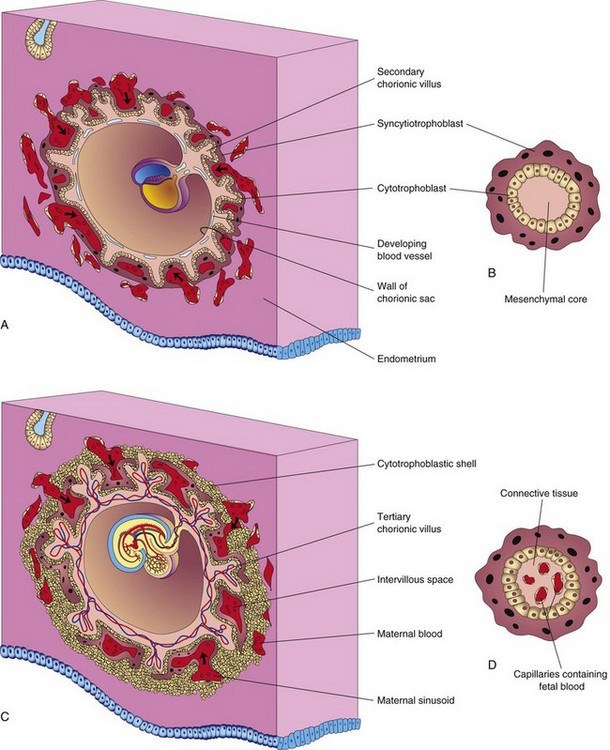
FIGURE 4–14 Diagrams illustrating development of secondary chorionic villi into tertiary chorionic villi. Early formation of the placenta is also shown. A, Sagittal section of an embryo (approximately 16 days). B, Section of a secondary chorionic villus. C, Section of an implanted embryo (approximately 21 days). D, Section of a tertiary chorionic villus. The fetal blood in the capillaries is separated from the maternal blood surrounding the villus by the endothelium of the capillary, embryonic connective tissue, cytotrophoblast, and syncytiotrophoblast.
Choriocarcinomas invariably metastasize (spread) through the bloodstream to various sites, such as the lungs, vagina, liver, bone, intestine, and brain.
The main mechanisms for development of complete hydatidiform moles follow:
A partial (dispermic) hydatidiform mole usually results from fertilization of an oocyte by two sperms (dispermy). Most complete hydatidiform moles are monospermic. For both types, the genetic origin of the nuclear DNA is paternal.
Development of Chorionic Villi
Shortly after primary chorionic villi appear at the end of the second week, they begin to branch. Early in the third week, mesenchyme grows into these primary villi, forming a core of mesenchymal tissue. The villi at this stage—secondary chorionic villi—cover the entire surface of the chorionic sac (Fig. 4-14A and B). Some mesenchymal cells in the villi soon differentiate into capillaries and blood cells (Fig. 4-14C and D). Villi are called tertiary chorionic villi when blood vessels are visible in them.
The capillaries in the chorionic villi fuse to form arteriocapillary networks, which soon become connected with the embryonic heart through vessels that differentiate in the mesenchyme of the chorion and connecting stalk (Fig. 4-12). By the end of the third week, embryonic blood begins to flow slowly through the capillaries in the chorionic villi. Oxygen and nutrients in the maternal blood in the intervillous space diffuse through the walls of the villi and enter the embryo’s blood (Fig. 4-14C and D). Carbon dioxide and waste products diffuse from blood in the fetal capillaries through the wall of the chorionic villi into the maternal blood. Concurrently, cytotrophoblastic cells of the chorionic villi proliferate and extend through the syncytiotrophoblast to form an extravillous cytotrophoblastic shell (Fig. 4-14C), which gradually surrounds the chorionic sac and attaches it to the endometrium.
Villi that attach to the maternal tissues through the cytotrophoblastic shell are stem chorionic villi (anchoring villi). The villi that grow from the sides of the stem villi are branch chorionic villi. It is through the walls of the branch villi that the main exchange of material between the blood of the mother and the embryo takes place. The branch villi are bathed in continually changing maternal blood in the intervillous space (Fig. 4-14C).
Summary of Third Week
Clinically Oriented Problems
Case 4–1
A 30-year-old woman became pregnant 2 months after discontinuing use of oral contraceptives. Approximately 3 weeks later, she had an early spontaneous abortion.
Case 4–2
A 25-year-old woman with a history of regular menstrual cycles was 5 days overdue on menses. Owing to her mental distress related to the abnormal bleeding and the undesirability of a possible pregnancy, the doctor decided to do a menstrual extraction or uterine evacuation. The tissue removed was examined for evidence of a pregnancy.
Case 4–3
A woman who had just missed her menstrual period was concerned that a glass of wine she had consumed the week before may have harmed her embryo.
Case 4–4
A female infant was born with a large tumor situated between her anus and sacrum. A diagnosis of sacrococcygeal teratoma was made and the mass was surgically removed.
References and Suggested Reading
Barembaum M, Bronner-Fraser M. Early steps in neural crest specification. Semin Cell Dev Biol. 2005;16:642.
De Val S. Key transcriptional regulators of early vascular development. Arterioscler Thromb Vasc Biol. 2011;31:1469.
Downs KM. The enigmatic primitive streak: prevailing notions and challenges concerning the body axis of mammals. Bioessays. 2009;31:892.
Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69:73.
Dubrulle J, Pourquie O. Coupling segmentation to axis formation. Development. 2004;131:5783.
Flake AW. The fetus with sacrococcygeal teratoma. In Harrison MR, Evans MI, Adzick NS, Holzgrev W, editors: The Unborn Patient: The Art and Science of Fetal Therapy, ed 3, Philadelphia: WB Saunders, 2001.
Gasser RF. Evidence that some events of mammalian embryogenesis can result from differential growth, making migration unnecessary. Anat Rec B New Anat. 2006;289B:53.
Gibb S, Maroto M, Dale JK. The segmentation clock mechanism moves up a notch. Trends Cell Biol. 2010;20:593.
Hall BK. Bones and Cartilage: Developmental Skeletal Biology. Philadelphia: Elsevier; 2005.
Hardin J, Walston T. Models of morphogenesis: the mechanisms and mechanics of cell rearrangement. Curr Opin Genet Dev. 2004;14:399.
Harvey NL, Oliver G. Choose your fate: artery, vein or lymphatic vessel? Curr Opin Genet Dev. 2004;14:499.
Hollway G, Currie P. Vertebrate myotome development. Birth Defects Res C Embryo Today. 2005;75:172.
Hur E-M, Zhou F-Q. GSK3 signalling in neural development. Nature Rev Neurosci. 2010;11:539.
Lewis J, Hanisch A, Holder M. Notch signaling, the segmentation clock, and the patterning of vertebrate somites. J Biol. 2009;8:44.
Liu W, Komiya Y, Mezzacappa C, et al. MIM regulates vertebrate neural tube closure. Development. 2011;138:2035.
Monsoro-Burq AH. Sclerotome development and morphogenesis: when experimental embryology meets genetics. Int J Dev Biol. 2005;49:301.
Ohls RK, Christensen RD. Development of the hematopoietic system. In Behrman RE, Kliegman Jenson HB, editors: Nelson Textbook of Pediatrics, ed 17, Philadelphia: Elsevier/Saunders, 2004.
Robb L, Tam PP. Gastrula organiser and embryonic patterning in the mouse. Semin Cell Dev Biol. 2004;15:543.
Slack JMW. Essential Developmental Biology, ed 2. Oxford: Blackwell Publishing; 2006.
Tovar JA. The neural crest in pediatric surgery. J Ped Surg. 2007;42:915.
Wang Y, Steinbeisser H. Molecular basis of morphogenesis during vertebrate gastrulation. Cell Mol Life Sci. 2009;66:2263.
Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221.