Chapter 15 Muscular System
The muscular system develops from mesoderm, except for the muscles of the iris, which develop from neuroectoderm, and the muscles of the esophagus, which are believed to develop by transdifferentiation from smooth muscle. Myoblasts (embryonic muscle cells) are derived from mesenchyme (embryonic connective tissue). Three types of muscle—skeletal, cardiac, and smooth—are formed during the embryonic period.
MyoD, a member of the family of myogenic regulatory factors, activates transcription of muscle-specific genes and is considered an important regulatory gene for the induction of myogenic differentiation. The induction of myogenesis in mesenchymal cells by MyoD is dependent on their degree of mesenchymal cell differentiation.
Much of the mesenchyme in the head is derived from the neural crest (see Chapters 4 and 5), particularly the tissues derived from the pharyngeal arches (see Chapter 9); however, the original mesenchyme in these arches gives rise to the musculature of the face and neck (see Table 9-1).
Development of Skeletal Muscle
Limb and axial muscles develop by epitheliomesenchymal transformation from myogenic precursor cells. Studies show that myogenic precursor cells originate from the somatic mesoderm and from the ventral dermomyotome of somites in response to molecular signals from nearby tissues (Figs. 15-1 and 15-2).
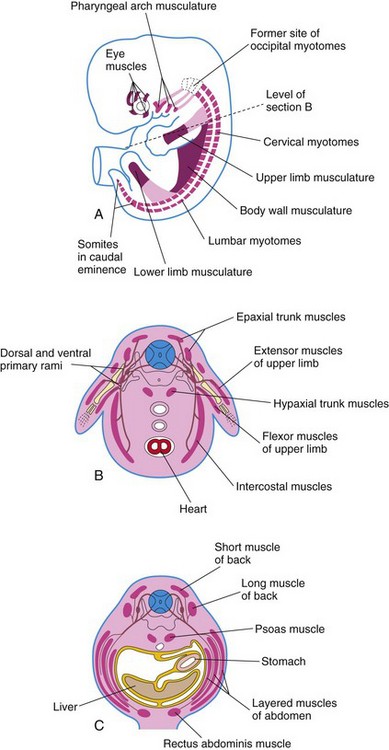
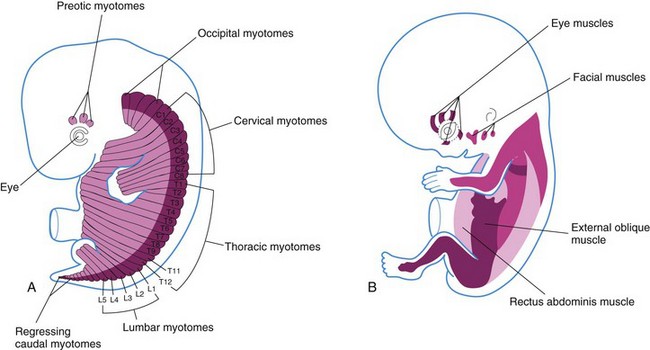
FIGURE 15–1 A, Sketch of an embryo (approximately 41 days) showing the myotomes and developing muscular system. B, Transverse section of the embryo illustrating the epaxial and hypaxial derivatives of a myotome. C, Similar section of a 7-week embryo showing the muscle layers formed from the myotomes.
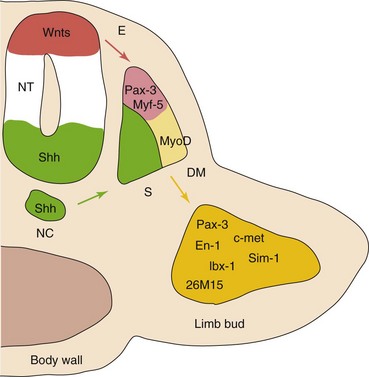
FIGURE 15–2 A model for molecular interactions during myogenesis. Shh and Wnts, produced by the neural tube (NT) and notochord (NC), induce Pax-3 and Myf-5 in the somites. Either of them can activate the initiation of MyoD transcription and myogenesis. Surface ectoderm (E) is also capable of inducing Myf-5 and MyoD. In addition, Pax-3 regulates the expression of c-met, necessary for the migratory ability of myogenic precursor cells, that also express En-1, Sim-1, lbx-1, and 26M15. DM, dermamyotome; S, sclerotome.
(From Kablar B, Rudnicki MA: Skeletal muscle development in the mouse embryo. Histol Histopathol 15:649, 2000.)
The first indication of myogenesis (muscle formation) is the elongation of the nuclei and cell bodies of mesenchymal cells as they differentiate into myoblasts. Soon these primordial muscle cells fuse to form elongated, multinucleated, cylindrical structures—myotubes.
At the molecular level, these events are preceded by gene activation and expression of the MyoD family of muscle-specific, basic helix-loop-helix transcription factors (MyoD, myogenin, Myf-5, and myogenic regulatory factor 4) in the precursor myogenic cells. Retinoic acid enhances skeletal myogenesis by upregulating the expression of mesodermal markers and myogenic regulatory factors. It has been suggested that signaling molecules (Shh, from the ventral neural tube and notochord), and others from the dorsal neural tube (Wnts, bone morphogenetic protein [BMP]-4) and overlying ectoderm (Wnts, BMP-4) regulate the beginning of myogenesis and the induction of the myotome (Fig. 15-3). Further muscle growth in the fetus results from the ongoing fusion of myoblasts and myotubes.
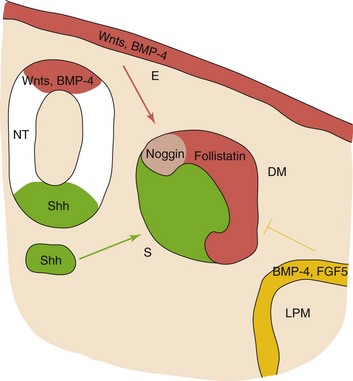
FIGURE 15–3 Embryonic structures and myogenesis. The current view suggests that the dorsal neural tube (NT) and the overlying non-neural ectoderm (E) are sources of signaling molecules belonging to the family of Wnt-secreted proteins and bone morphogenetic protein (BMP)-4, whereas the notochord (NC) and the ventral neural tube (green) are sources of the Shh. They positively regulate the onset of myogenesis and the induction of the myotome. By contrast, the lateral plate mesoderm (LPM) produces BMP-4 and FGF5 (fibroblast growth factor 5), negatively regulating muscle terminal differentiation in the lateral part of the myotome lineage. Response to the BMP-4 signal may be mediated by its binding proteins noggin and follistatin. DM, dermamyotome; S, sclerotome.
(From Kablar B, Rudnicki MA: Skeletal muscle development in the mouse embryo. Histol Histopathol 15:649, 2000.)
During or after fusion of the myoblasts, myofilaments develop in the cytoplasm of the myotubes. Other organelles characteristic of striated muscle cells, such as myofibrils, also form. As the myotubes develop, they become invested with external laminae, which segregate them from the surrounding connective tissue. Fibroblasts produce the perimysium and epimysium layers of the fibrous sheath of the muscle; the endomysium is formed by the external lamina and reticular fibers.
Most skeletal muscles develop before birth and almost all remaining ones are formed by the end of the first year. The increase in the size of a muscle after the first year results from an increase in the diameter of the fibers because of the formation of more myofilaments. Muscles increase in length and width to grow with the skeleton. Their ultimate size depends on the amount of exercise that is performed. Not all embryonic muscle fibers persist; many of them fail to establish themselves as necessary units of the muscle and soon degenerate.
Myotomes
Each typical myotome part of a somite divides into a dorsal epaxial division and a ventral hypaxial division (Fig. 15-1B). Every developing spinal nerve also divides and sends a branch to each myotome division; the dorsal primary ramus supplies the epaxial division and the ventral primary ramus, the hypaxial division. The myoblasts that form the skeletal muscles of the trunk are derived from mesenchyme in the myotome regions of the somites (Fig. 15-1). Some muscles, such as the intercostal muscles, remain segmentally arranged like the somites, but most myoblasts migrate away from the myotome and form nonsegmented muscles.
Gene targeting studies in the mouse embryo show that myogenic regulatory factors (MyoD, MrfH, Myf-5, and myogenin) are essential for the development of the hypaxial and epaxial muscles, as well as the abdominal and intercostal muscles.
Myoblasts from epaxial divisions of the myotomes form the extensor muscles of the neck and vertebral column (Fig. 15-4). The embryonic extensor muscles derived from the sacral and coccygeal myotomes degenerate; their adult derivatives are the dorsal sacrococcygeal ligaments. Myoblasts from the hypaxial divisions of the cervical myotomes form the scalene, prevertebral, geniohyoid, and infrahyoid muscles (Fig. 15-4). The thoracic myotomes form the lateral and ventral flexor muscles of the vertebral column, and the lumbar myotomes form the quadratus lumborum muscle. The sacrococcygeal myotomes form the muscles of the pelvic diaphragm and probably the striated muscles of the anus and sex organs.
Pharyngeal Arch Muscles
Myoblasts from the pharyngeal arches, which originate from the unsegmented paraxial mesoderm and prechordal plate form the muscles of mastication, facial expression, pharynx, and larynx as described in Chapter 9. These muscles are innervated by pharyngeal arch nerves.
Ocular Muscles
The origin of the extrinsic eye muscles is unclear, but they may be derived from mesenchymal cells near the prechordal plate (Figs. 15-1 and 15-4). The mesenchyme in this area is thought to give rise to three preotic myotomes. Myoblasts differentiate from mesenchymal cells derived from these myotomes. Groups of myoblasts, each supplied by its own nerve (cranial nerve [CN] III, CN IV, or CN VI), form the extrinsic muscles of the eye.
Tongue Muscles
Initially there are four occipital (postotic) myotomes; the first pair disappears. Myoblasts from the remaining myotomes form the tongue muscles, which are innervated by the hypoglossal nerve (CN XII).
Limb Muscles
The musculature of the limbs develops from myoblasts surrounding the developing bones (Fig. 15-1). The myoblasts form a mass of tissue on the dorsal (extensor) and ventral (flexor) aspects of the limbs. Grafting and gene targeting studies in birds and mammals have demonstrated that the precursor myogenic cells in the limb buds originate from the somites. These cells are first located in the ventral part of the dermomyotome and are epithelial in nature (Fig. 14-1D). After epitheliomesenchymal transformation, the cells then migrate into the primordium of the limb.
Molecular signals from the neural tube and notochord induce Pax-3, MyoD, and Myf-5 in the somites. Pax-3 regulates the expression of c-met in the limb bud (a migratory peptide growth factor), which regulates migration of the precursor myogenic cells.
Development of Smooth Muscle
Smooth muscle fibers differentiate from splanchnic mesenchyme surrounding the endoderm of the primordial gut and its derivatives (Fig. 15-1). The somatic mesoderm provides smooth muscle in the walls of many blood and lymphatic vessels. The muscles of the iris (sphincter and dilator pupillae) and the myoepithelial cells in mammary and sweat glands are thought to be derived from mesenchymal cells that originate from ectoderm.
The first sign of differentiation of smooth muscle is the development of elongated nuclei in spindle-shaped myoblasts. During early development, additional myoblasts continue to differentiate from mesenchymal cells but do not fuse as in skeletal muscle; they remain mononucleated.
During later development, division of existing myoblasts gradually replaces the differentiation of new myoblasts in the production of new smooth muscle tissue. As smooth muscle cells differentiate, filamentous but nonsarcomeric contractile elements develop in their cytoplasm, and the external surface of each cell acquires a surrounding external lamina. As smooth muscle fibers develop into sheets or bundles, they receive autonomic innervation. Muscle cells and fibroblasts synthesize and lay down collagenous, elastic, and reticular fibers.
Development of Cardiac Muscle
Cardiac muscle develops from the lateral splanchnic mesoderm, which gives rise to the mesenchyme surrounding the developing heart tube (Chapter 13). Cardiac myoblasts differentiate from the primordial myocardium. Heart muscle is recognizable in the fourth week and likely develops through expression of cardiac-specific genes. Recent studies suggest that Pbx proteins interacting with the transcription factors Hand2, promote cardiac muscle differentiation. Immunohistochemical studies have revealed a spatial distribution of tissue-specific antigens (myosin heavy chain isoforms) in the embryonic heart between the fourth and eighth weeks.
Cardiac muscle fibers arise by differentiation and growth of single cells, unlike striated skeletal muscle fibers, which develop by fusion of cells. Growth of cardiac muscle fibers results from the formation of new myofilaments. The myoblasts adhere to each other as in developing skeletal muscle, but the intervening cell membranes do not disintegrate; these areas of adhesion give rise to intercalated discs. Late in the embryonic period, special bundles of muscle cells develop with relatively few myofibrils and relatively larger diameters than typical cardiac muscle fibers. These atypical cardiac muscle cells—Purkinje fibers—form the conducting system of the heart (Chapter 13).
Anomalies of Muscles
Absence of one or more skeletal muscles is more common than is generally recognized; common examples are the sternocostal head of the pectoralis major (Fig. 15-5), the palmaris longus, trapezius, serratus anterior, and quadratus femoris. Usually only a single muscle is absent on one side of the body, or only part of the muscle fails to develop. Occasionally the same muscle or muscles may be absent on both sides of the body.
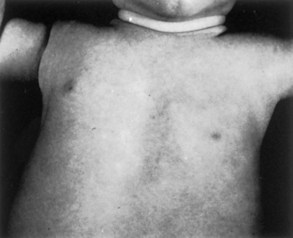
FIGURE 15–5 The thorax of an infant with congenital absence of the left pectoralis major muscle. Note the absence of the anterior axillary fold on the left and the low location of the left nipple.
(From Behrman RE, Kliegman RM, Arvin AM [eds]: Nelson Textbook of Pediatrics, 15th ed. Philadelphia, WB Saunders, 1996.)
Absence of the pectoralis major, often its sternal part, is usually associated with syndactyly (fusion of digits). These birth defects are part of the Poland syndrome (absence of the pectoralis major and minor muscles, ipsilateral breast hypoplasia, and absence of two to four ribs). Absence of the pectoralis major is occasionally associated with absence of the mammary gland in the breast and/or hypoplasia of the nipple.
Some muscular birth defects, such as congenital absence of the diaphragm, cause difficulty in breathing, which is usually associated with incomplete expansion of the lungs or part of a lung (pulmonary atelectasis) and pneumonitis (pneumonia). Absence of muscles of the anterior abdominal wall may be associated with severe gastrointestinal and genitourinary defects, for example, exstrophy of the bladder (see Chapter 12). Both muscle development and muscle repair have distinctive dependence on expression of muscle regulatory genes.
Arthrogryposis
The term arthrogryposis (arthrogryposis multiplex congenita) is a clinical term used to describe multiple congenital joint contractions that affect different parts of the body (Fig. 15-6). The incidence of arthrogryposis is 1 : 3000 live births. It includes more than 300 heterogeneous disorders. The causes of arthrogryposis are unclear. In about 30% of cases, genetic factors are involved. Neuropathic disorders and muscle and connective tissue abnormalities restrict intrauterine movement and may lead to fetal akinesia and joint contractures.
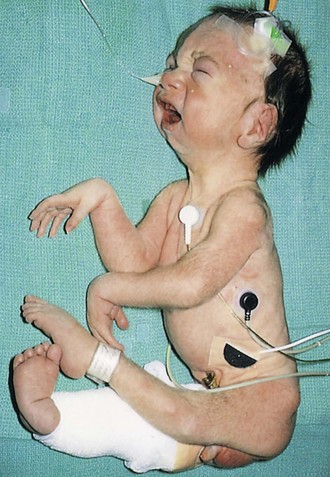
FIGURE 15–6 Neonate with multiple joint contractures: arthrogryposis. Infants with this syndrome have stiffness of the joints associated with hypoplasia of the associated muscles.
(Courtesy of Dr. A.E. Chudley, Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital and University of Manitoba, Winnipeg, Manitoba, Canada.)
Variations in Muscles
All muscles are subject to a certain amount of variation, but some are affected more often than others. Certain muscles are functionally vestigial (rudimentary), such as those of the external ear and scalp. Some muscles present in other primates appear in only some humans (e.g., the sternalis muscle, a band sometimes found parallel to the sternum). Variations in the form, position, and attachments of muscles are common and are usually functionally insignificant.
Congenital Torticollis
Some cases of torticollis (wryneck) may result from tearing of fibers of the sternocleidomastoid (SCM) muscle during childbirth. Bleeding into the muscle occurs in a localized area, forming a hematoma (a small swelling due to a collection of blood). Later a solid mass develops because of necrosis (death) of muscle fibers and fibrosis (formation of fibrous tissue). Shortening of the muscle usually follows, which causes lateral bending of the head to the affected side and a slight turning away of the head from the side of the short muscle (Fig. 15-7).
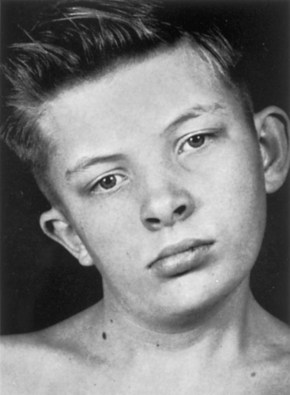
FIGURE 15–7 The head and neck of a 12-year-old boy with congenital torticollis (wryneck). Shortening of the right sternocleidomastoid muscle has caused tilting of the head to the right and turning of the chin to the left. There is also asymmetrical development of the face and cranium.
(From Behrman RE, Vaughan VC III: Nelson Textbook of Pediatrics, 13th ed. Philadelphia, WB Saunders, 1987.)
Although birth trauma may be a cause of congenital torticollis, the fact that the condition has been observed in infants delivered by cesarean section suggests that there are other causes as well, including intrauterine crowding and primary SCM myopathy.
Abdominal Muscle Deficiency
Congenital partial or complete absence of abdominal muscles (also called prune belly syndrome) results from abdominal laxity secondary to abdominal distension from ascites (accumulation of serous fluid in the peritoneal cavity). The accumulation of fluid distends the abdomen, resulting in atrophy of the abdominal muscles. Male neonates with this syndrome have associated cryptorchidism (failure of one or both testes to descend) and megaureters (dilation of ureters). Usually the abdominal wall is so thin that the viscera (e.g., intestines) are visible and easily palpated.
Accessory Muscles
Accessory muscles occasionally develop; for example, an accessory soleus muscle is present in approximately 3% of people. It has been suggested that the primordium of the soleus muscle undergoes early splitting to form an accessory soleus. In some cases, accessory muscles cause clinically significant symptoms.
Summary of Muscular System
Clinically Oriented Problems
Case 15–1
An infant presented with absence of the left anterior axillary fold. In addition, the left nipple was much lower than usual.
Case 15–2
A medical student was concerned when she learned that she had only one palmaris longus muscle.
Case 15–3
The parents of a 4-year-old girl observed that she always held her head slightly tilted to the right side and that one of her neck muscles was more prominent than the others. The clinical history revealed that her delivery had been a breech birth, one in which the buttocks presented.
Case 15–4
A neonate presented with an abdominal wall defect. Failure of striated muscle to develop in the median plane of the anterior abdominal wall is associated with the formation of a severe congenital birth defect of the urinary system.
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Bamshad M, Van Heest AE, Pleasure D. Arthrogryposis: A review and update. J Bone Joint Surg Am. 2009;91(Suppl 4):40.
Bonnet A, Dai F, Brand-Saberi B, et al. Vestigial-like 2 acts downstream of MyoD activation and is associated with skeletal muscle differentiation in chick myogenesis. Mech Dev. 2010;127:120.
Bothe I, Tenin G, Oseni A, et al. Dynamic control of head mesoderm patterning. Development. 2011;138:2807.
Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16:525.
Goncalves LF, Kusanovic JP, Gotsch F, et al. The fetal musculoskeletal system. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: Elsevier, 2008.
Cooperman DR, Thompson GH. Musculoskeletal disorders. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Cheng JCY, Tang SP, Chen MWN, et al. The clinical presentation and outcome of treatment of congenital muscular torticollis in infants—a study of 1,086 cases. J Pediatr Surg. 2000;35:1091.
Gasser RF. The development of the facial muscle in man. Am J Anat. 1967;120:357.
Giacinti C, Giodano A. Cell cycle regulation in myogenesis. In: Giordano A, Galderisi U, editors. Cell Cycle Regulation and Differentiation in Cardiovascular and Neural Systems. New York: Springer, 2010.
Gibb S, Maroto M, Dale JK. The segmentation clock mechanism moves up a notch. Trends Cell Biol. 2010;20:593.
Kablar B, Krastel K, Ying C, et al. Myogenic determination occurs independently in somites and limb buds. Dev Biol. 1999;206:219.
Kablar B, Tajbakhsh S, Rudnick MA. Transdifferentiation of esophageal smooth muscle is myogenic bHLH factor-dependent. Development. 2000;127:1627.
Kalcheim C, Ben-Yair R. Cell rearrangements during development of the somite and its derivatives. Curr Opin Genet Dev. 2005;15:371.
Martin J, Afouda BA, Hoppler S. Wnt/beta-catenin signaling regulates cardiomyogenesis via GATA transcription factors. J Anat. 2010;216:92.
Mathew SJ, Hansen JM, Merrell AJ, et al. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371.
Maves L, Tyler A, Moens CB, et al. Pbx acts with Hand 2 in early myocardial differentiation. Dev Biol. 2009;333:409.
Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Noden DM. Vertebrate craniofacial development—the relation between ontogenetic process and morphological outcome. Brain Behav Evol. 1991;38:190.
Messina G, Biressi S, Monteverde S, et al. NFix regulates fetal-specific transcription in developing skeletal muscle. Cell. 2010;140:554.