Chapter 16 The Electroencephalogram and Sensory-Evoked Potentials
1. All areas of the cerebral cortex share common histological features.
2. The electroencephalogram is a common clinical tool.
3. The collective behavior of cortical neurons can be studied noninvasively through the use of macroelectrodes on the scalp.
4. Stimulation of sensory pathways can be recorded as evoked potentials.
When many excitable cells are present in a living tissue, their electrical behavior can be detected by macroelectrodes placed on the body at a distance from these cells. Several clinically important electrophysiological diagnostic procedures are based on this concept.
Underlying these procedures is a theory called volume conduction. This theory describes the spread of ionic currents within the extracellular fluid from a group of neurons or muscle cells to more distant points in the body, such as the skin. These ionic currents can be measured from the skin. Their waveforms are characteristic of the tissues from which they arise. The best-known electrophysiological recording is the electrocardiogram from heart muscle (Chapter 20). The electromyogram from skeletal muscle (Chapter 6) and electroretinogram (Chapter 14) are other examples.
This chapter introduces two additional clinical electrophysiological tools: the electroencephalogram and sensory-evoked potentials, particularly brainstem auditory-evoked responses. These tools represent two general types of clinical electrophysiological recordings. The first is a record of the spontaneous activity of tissue. The second is a record of potentials that are artificially evoked by electrical or magnetic stimulation of tissue or by activation of sensory receptor organs. Before discussing the electroencephalogram and sensory-evoked potentials, it is necessary to understand more about the histology and electrophysiology of the cerebral cortex.
All Areas of the Cerebral Cortex Share Common Histological Features
Different regions of the cerebral cortex have different functions. For example, the motor cortices (Chapter 10) project to the brainstem and spinal cord to initiate skilled, learned, conscious movement. The occipital cortex processes visual information received from the retina of the eye (Chapter 14). The temporal cortex processes similar information from the ear (Chapter 17). However, even though different cortical regions have different functions, they have an underlying histological similarity. Therefore, cortical synaptic processing of information shares common features across regions, but differences in the origin of input signals and the destination of output signals contribute significantly to functional differences among regions. Cerebral cortical cells can work collectively over vast regions of the brain in such normal states as sleep and wakefulness and in such disease states as coma and seizures.
The cerebral cortex contains several different cell types, but most belong to two major classes: pyramidal cells and stellate cells (Figure 16-1). These cells are arranged in six layers (I-VI). The pyramidal cells, so named because their cell bodies are pyramid shaped, have dendrites projecting up toward the pial surface of the cortex, usually reaching and branching within layer I. These cells also have basal dendrites that extend horizontally from the cell body. Pyramidal cells are projection neurons, with their axons leaving their cortical region of origin and projecting to other parts of the central nervous system (CNS) or to a different region of cerebral cortex. Pyramidal cells are generally excitatory at their axon’s synapse. Stellate cells, so named because most have a starlike appearance, are local-circuit interneurons within the cortex and can be either excitatory or inhibitory. The majority of subcortical information arrives at the cortex through a massive input from the thalamic nuclei, most of which is targeted to layer IV. Input from some portions of the thalamus, as well as from other regions of cerebral cortex, have a more diffuse termination across cortical layers. Information arriving from cortical afferents is processed by local cortical circuitry, and pyramidal cells then carry the processed information to other CNS regions.
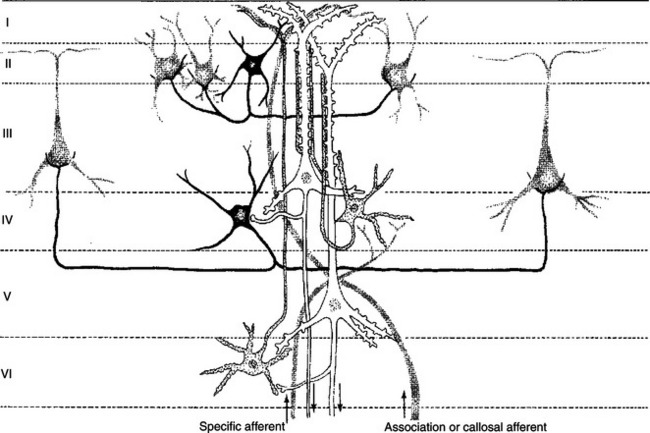
FIGURE 16-1 The principal neuron types and their interconnections have a basic similarity in the various regions of the cerebral cortex. Note that the two large pyramidal cells (white) in layers III and V receive multiple synaptic contacts from the star-shaped interneuron (stellate cell, stippled) in layer IV. Basket cell (black) inhibition is directed to the somata of cortical neurons. Major input to the cortex derives from specific thalamic nuclei (specific afferents) and is directed mostly to layer IV; association and callosal inputs (association and callosal afferents) have a more widespread termination pattern among the cortical layers.
(From Kandel ER, Schwartz JH: Principles of neural science, ed 2, New York, 1985, Elsevier Science Publishing.)
As with other regions of the brain, the cerebral cortex contains about 10 to 50 times more glial cells than neurons. Three types of glial cells are present in the cortex: astrocytes, oligodendrocytes, and microglia. They do not develop action potentials, but as noted in Chapter 3, they may indirectly monitor neuronal electrical activity and modulate the effectiveness of neural communication. Glia also take up excess potassium ions, neurotransmitter, and toxins from the extracellular space and play a role in immune function. In addition, they help guide the course of developing neurons and stabilize the position of neurons, thus the origin of the term glia (“glue”).
The Electroencephalogram Is a Common Clinical Tool
It has been known since the 1930s that a fluctuating electrical voltage reflecting brain activity could be recorded from macroelectrodes on the scalp (Figure 16-2). Such a recording is known as an electroencephalogram (EEG). The frequency of the waveform recorded varies inversely with its amplitude. Both frequency and amplitude change with changes in levels of arousal (Figure 16-3). An alert animal has a fairly high-frequency, fairly low-amplitude EEG, whereas a more relaxed animal has a slower-frequency, higher-amplitude EEG. A sleeping animal usually begins sleep by exhibiting a slow-wave, high-amplitude EEG. Paradoxically, there are periods of high-frequency, low-amplitude EEG during the sleep cycle. Four frequency ranges have been given names: alpha (8-13 Hz), beta (13-30 Hz), delta (0.5-4 Hz), and theta (4-7 Hz).
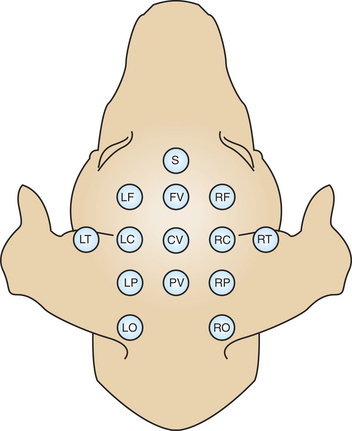
FIGURE 16-2 Commonly used points of scalp electrode attachment (electrode montage) for recording the electroencephalogram (EEG). Recordings are typically made from multiple paired combinations of electrodes.
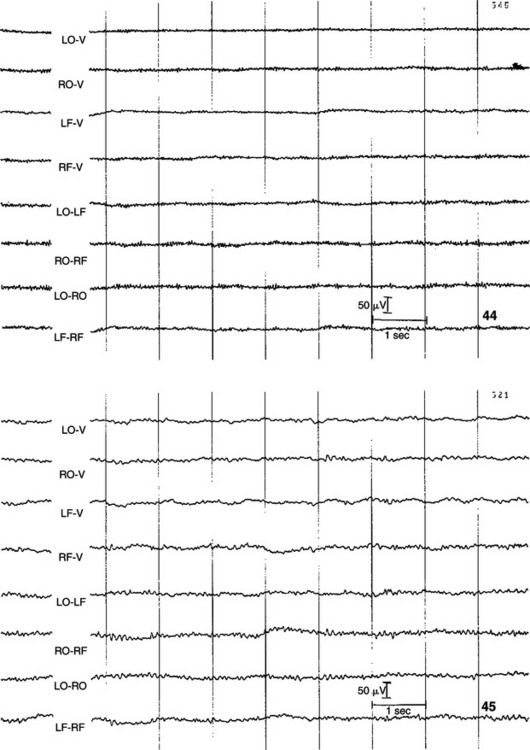
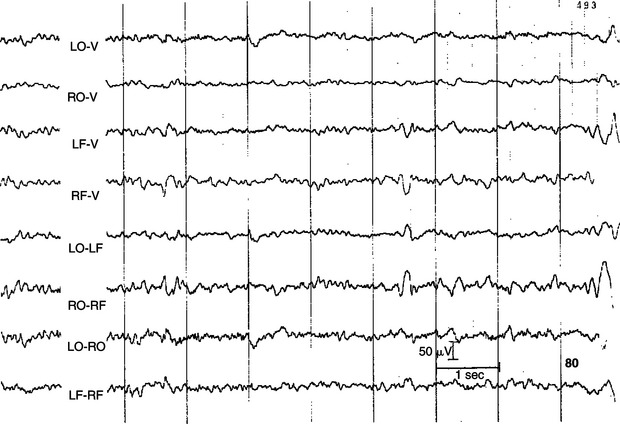
FIGURE 16-3 EEG recorded from various combinations of lead points using an older, simpler electrode configuration (the Redding configuration). It shows the difference in frequency and amplitude between an alert animal (44), a relaxed animal (45), and an animal in light sleep (80). Note the decrease in frequency and increase in amplitude in the progression from alert to relaxed to light sleep.
(From Oliver JE, Hoerlein BF, Mayhew IG, editors: Veterinary neurology, Philadelphia, 1987, Saunders.)
This technique has been applied clinically since the early 1960s. Abnormal EEG activity has been associated empirically with several brain diseases. In human neurology, EEGs have been used to classify the epilepsies, to localize lesions, and to help define “brain death.” EEGs have not been as widely used in veterinary medicine but still have clinical utility in veterinary neurology.
We now discuss where these scalp recordings originate and how they relate to brain function.
The Collective Behavior of Cortical Neurons Can Be Studied Noninvasively Through the Use of Macroelectrodes on the Scalp
The scalp EEG records a fluctuating voltage resulting from changes in postsynaptic potentials in thousands of neurons below the electrode. Each change in voltage has a polarity.
By convention, changes in voltage measured by extracellular electrodes, such as those on the scalp, have a standard direction of recorder deflection. When the voltage change is in a positive direction, the recorded deflection is “down”; when in a negative direction, the deflection is “up” (Figure 16-4). The polarity of the voltage change at the scalp depends on the nature and location of the postsynaptic potential change. If an excitatory postsynaptic potential (EPSP) occurs in a deep cortical layer, positive ions (e.g., Na+) enter the cell there, leaving the extracellular fluid at that location relatively negative. Through principles of volume conduction, this leaves the extracellular fluid near the cortical surface positive with respect to the deeper, negatively charged region of extracellular fluid (see Figure 16-4; for simplicity, only one cell is indicated). This results in a positive voltage change being recorded at the scalp macroelectrode near the cortical surface. Based on the same principles, if the EPSP occurs near the cortical surface (see Figure 16-4), the voltage recorded from the scalp is negative. The polarity of these changes would be reversed for inhibitory postsynaptic potentials (IPSPs).
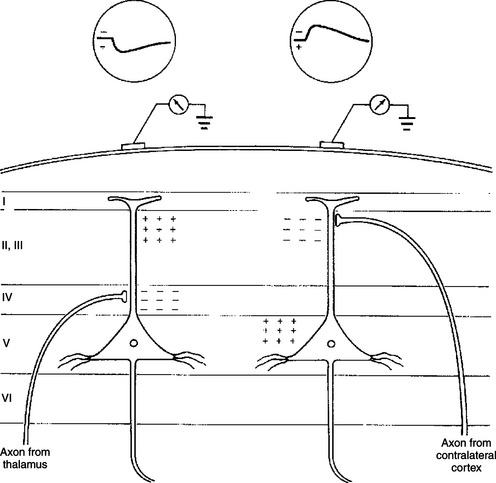
FIGURE 16-4 Scalp recordings and underlying synaptic mechanisms. Left, Potential recorded from a scalp electrode after activation of thalamic inputs. The terminals of thalamocortical neurons make excitatory connections on cortical neurons predominantly in layer IV. Thus the site of inward current flow (sink) in layer IV leaves the extracellular fluid at that location relatively negative and the extracellular fluid near the cortical surface relatively positive. Because the recording electrode is located on the scalp, near the cortical surface, it records a positive potential. By convention, a positive extracellularly recorded potential is, unlike intracellular recordings, a downward deflection. Right, Potential recorded from an excitatory callosal afferent originating in the contralateral cortex. The axon of this callosal neuron terminates in a superficial cortical layer. A negative potential (upward deflection) is recorded because the electrode is closer to the site of inward current flow, which leaves the extracellular fluid near the cortical surface relatively negative.
(From Kandel ER, Schwartz JH: Principles of neural science, ed 2, New York, 1985, Elsevier Science Publishing.)
Voltage changes recorded from the scalp are the result of the summated extracellular voltage changes caused by the postsynaptic potentials of a large number of active cortical neurons, primarily pyramidal cells, because the voltage change from any one neuron is too small to record. Action potentials contribute little to the EEG with scalp electrodes.
The amplitude (height) of voltage fluctuations in the scalp-recorded EEG is a function of how many cortical cells are changing their postsynaptic potentials in the same direction at the same time. Because a high-amplitude voltage change would result from a large number of neurons firing synchronously, a high-amplitude, slow-frequency EEG is said to be a synchronized EEG. When neurons are firing more or less at random, a low-amplitude, high-frequency EEG results, said to be a desynchronized EEG.
The frequency with which EEG voltage changes occur is largely determined by the reticular activating system. As noted in Chapter 10, ascending projections of the reticular formation play an important role in modulating consciousness, arousal, and attention. Many of these projections synapse primarily in the thalamus, hypothalamus, or directly in the cerebral cortex in a diffuse fashion. Diffuse cortical projections from portions of the thalamus (intralaminar nuclei) and hypothalamus (lateral hypothalamus), along with diffuse, direct cortical projections from the reticular formation, likely regulate consciousness and arousal. Neurons that project to cortex from specific sensory relay nuclei of the thalamus and receive reticular formation input probably influence attention. The term reticular activating system collectively refers to these ascending reticular formation neurons and the neurons that relay their activity to the cortex, both of which affect consciousness, arousal, and attention.
Stimulation of Sensory Pathways Can Be Recorded as Evoked Potentials
Large areas of the brain and the spinal cord are not reflected in the EEG. Other clinical electrophysiological recordings can help examine the function of these areas.
Synaptic activity in a sensory pathway can be recorded from the scalp by a computerized technique that averages out the more random background EEG activity and averages in the electrical response to multiple stimulations of a sensory system. Such signals are called sensory-evoked potentials.
Because scalp macroelectrodes can more easily record the EEG electrical signals generated from the closer cerebral cortical cells, these higher-voltage signals must be eliminated; otherwise, they would mask the sensory-evoked potentials. Because the background EEG signals are relatively random, a computer can average them together and functionally erase them from the recording while at the same time averaging the nonrandom sensory-evoked potential signals recorded from multiple stimulations of a sensory pathway. In this way, scalp macroelectrodes can be used to record electrical events generated in brain locations distant from the recording electrode. For this reason, these sensory-evoked potentials are often called far-field potentials.
One such sensory-evoked potential is the brainstem auditory-evoked response (BSAER). This clinical electrophysiological procedure, in which the electrode placement is configured to include activity from the brainstem, records the electrical events for 10 msec after a click stimulus to the ear (Figure 16-5). Usually, seven waves are recorded, thought to be generated by neural activity in components of the auditory pathway from the auditory nerve through the auditory radiations leaving the medial geniculate nucleus of the thalamus. Recordings longer than 10 msec are sometimes taken. These later waves reflect cortical response to auditory stimulation. BSAER is used in animals and humans to assess brainstem function in general and auditory function in particular. Other sensory-evoked potentials can be recorded from the visual system, the somatosensory system, and other sensory modalities.
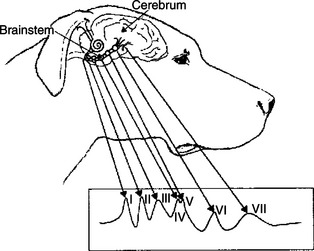
FIGURE 16-5 Brainstem auditory-evoked response (BSAER): idealized diagram of waveforms recorded by signal averaging. Neural elements believed to generate sequentially the auditory waves are grouped as follows: wave I reflects the cochlea, spiral ganglia, and cranial nerve VIII; wave II reflects the cochlear nuclei; wave III reflects the nucleus of the trapezoid body; waves IV and V reflect the lateral lemniscus and lemniscal nuclei and caudal colliculus, respectively (these two waves are frequently combined to form one wave); wave VI reflects the medial geniculate body; and wave VII reflects auditory radiations. Positive is upward.
(From Oliver JE, Hoerlein BF, Mayhew IG, editors: Veterinary neurology, Philadelphia, 1987, Saunders.)
BSAER is also often used with simultaneous EEG recording in the confirmation of brain death. A flat EEG, a very crude indicator of brain death, combined with a viable BSAER can suggest that the functional deficit may not be irreversible.
CLINICAL CORRELATIONS
Brain Tumor
History.
You examine a 13-year-old Boston terrier. The owner states that during the past 3 weeks the dog has had seizures of increasing frequency, characterized by turning the head to the right, rigidity of the right front and right hind legs, collapsing to the ground, and urination. More recently, the dog has seemed weak, drowsy, and confused. He tends to walk in circles and seems weak on the right front leg.
Clinical Examination.
Important physical examination deficits are referable to the nervous system. The dog seems weak, drowsy, confused, and unsteady in gait. He tends to walk in counterclockwise circles. Cranial and spinal segmental reflexes are within normal limits. Proprioceptive placing reaction is abnormal in the right front leg and normal in the other three legs (see Chapters 6 and 10). An EEG reveals that the dominant frequency is slower and the amplitude higher over the left parietal cortex than over the rest of the brain. Occasional bursts of electrical spiking activity can also be seen from the area of the left parietal cortex. A computed tomography (CT) or magnetic resonance imaging (MRI) scan is warranted to determine the presence and nature of a tumor suspected from the EEG patterns. MRI provides the best imaging of intracranial lesions to determine whether this is a primary tumor (originating from the brain tissue) or a secondary tumor (originating from other tissue; e.g., osteosarcoma, lymphosarcoma).
Comment.
This is an old dog, with a recent history of progressive, asymmetric brain disease. The history suggests a focal intracranial lesion, perhaps a brain tumor. A focal lesion is further confirmed by the EEG and brain imaging. Brain tumors within the cerebral hemispheres often cause focal slowing of the EEG frequency with increased amplitude. This is called a slow-wave focus. The tumor itself is electrically silent, but its effects on the surrounding cerebral cortex are slowing, and the intermittent bursts of electrical spikes represent seizure activity within the cortex. Between clinical seizures, these spikes can still be seen with the EEG, but they do not spread widely enough within the cortex to cause a clinical seizure. During a clinical seizure, this abnormal electrical activity spreads more widely to incorporate normal brain, causing the various motor and other events of the seizure. Why such spikes only occasionally spread to incorporate more distant parts of the brain to cause seizures, and why seizures stop, is still unknown.
Treatment.
Many forms of seizure disorders can be managed successfully by removing the underlying cause, or the frequency of the seizures can be reduced with antiepileptic medication. In this dog the cause is likely a brain tumor. Depending on the nature of the tumor, surgery and radiation therapy may be possible and may extend the dog’s life. However, the prognosis is likely poor. Antiepileptic and steroid medication may improve the quality of the dog’s remaining life.
Bagley RS. Fundamentals of veterinary clinical neurology. Ames, Iowa: Blackwell Publishing, 2005.
Bear MF, Connors BW, Paradiso MA. Neuroscience: exploring the brain, ed 3. Philadelphia: Lippincott Williams & Wilkins, 2007.
Bergman RL: Intracranial neoplasia in dogs. ACVIM 23rd Annual Proceedings, Baltimore, 2005.
Ducote JM, Dewey CW. Neurodiagnostics. In: Dewey CW, ed. A practical guide to canine and feline neurology. Ames: Iowa State University Press, 2003.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Haines DE. Fundamental neuroscience for basic and clinical applications. Philadelphia: Churchill Livingstone, 2006.
Kandel ER, Schwartz JH, Jessell TM. Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
Lorenz MD, Kornegay J. Oliver and Lorenz’s handbook of veterinary neurology, ed 4. Philadelphia: Saunders, 2004.
Strain GM. Consciousness and higher cortical function. Reece WO, ed. Duke’s physiology of domestic animals, ed 12, Ithaca, NY: Comstock Cornell University Press, 2004.
PRACTICE QUESTIONS
1. Which of the following regarding EEG is false?
2. A lesion in which of the following brain structures would be least likely to have a significant effect on the EEG?
3. Which of the following statements is true?
4. The BSAER requires averaging out the random background EEG activity before it can be observed.
5. A brain tumor may cause focal slowing of the EEG from the brain tissue immediately surrounding the tumor.