1. Lees P., Landoni M.F., Giraudel J. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J Vet Pharmacol Ther. 2004;27(6):479-490.
2. Vane J., Botting R. Inflammation and the mechanism of action of antiinflammatory drugs. FASEB J. 1987;1:89-96.
3. Botting R. Inhibitors of cyclooxygenase: Mechanisms, selectivity and uses. J Phys Pharm. 2006;57(5):113-124.
4. D’Acquisto F., May M.J., Ghosh S. Inhibition of nuclear factor kappa B (NFκB): an emerging theme in anti-inflammatory therapies. Mol Interv. 2002;2:22-35.
5. Mohammed F.F., Smookler D.S., Khokha R. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis. 2003;62:43-47.
6. Boynton C.S., Dick C.F., Mayor G.H. NSAIDs: an overview. J Clin Pharmacol. 1988;28:512-517.
7. Hochberg M.C. NSAIDs: mechanisms and pathways of action. Hosp Pract. 1989;15:185-198.
8. Robinson D.R. Eicosanoids, inflammation, and antiinflammatory drugs. Clin Exp Rheumatol. 1989;7:155-161.
9. Warner T.D. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18(7):790-804.
10. Weissmann G. The actions of NSAIDs. Hosp Pract. 1991;15:60-76.
11. FitzGerald G.A., Burke A., Smyth E. Recent developments with traditional NSAIDs and COX-2 inhibitors” (Update). In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill, 2006.
12. Griswold D.E., Adams J.L. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): rationale for selective inhibition and progress to date. Med Res Rev. 1996;16(2):181-206.
13. Williams C.S., DuBois R.N. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol. 1996;270(3 Pt 1):G393-G400.
14. Crawford L.J. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol. 1997;49(Suppl 24):15-19.
15. Cashman J.N. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52(Suppl 5):13-23.
16. Donnelly M.T., Hawkey C.J. Review article: COX-II inhibitors: a new generation of safer NSAIDs? Alimentary Pharmacol Ther. 1997;11(2):227-236.
17. Kiefer W., Dannhardt G. Novel insights and therapeutical applications in the field of inhibitors of COX-2. Curr Med Chem. 2004;11:3147-3161.
18. Hinz B., Brune K. Pain and osteoarthritis: new drugs and mechanisms. Curr Opin Rheumatol. 2004;16(5):628-633.
19. Krause D.L., Müller N. Neuroinflammation, microglia and implications for anti- inflammatory treatment in Alzheimer’s disease. Int J Alzheimers Dis. 2010. Jun 14;2010. pii: 732806. PubMed PMID: 20798769; PubMed Central PMCID: PMC2925207
19a. Izarraga I., Chambers J.P., Johnson C.B. Synergistic depression of NMDA receptor-mediated transmission by ketamine, ketoprofen and L-NAME combinations in neonatal rat spinal cords in vitro. Br J Pharmacol. 2008;153(5):1030-1042.
20. Warner T.D., Giulano F., Vojnovic I., et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96(13):7563-7568.
20a. Jones P., Lamdin R. Oral cyclo-oxygenase 2 inhibitors versus other oral analgesics for acute soft tissue injury: systematic review and meta-analysis. Clin Drug Investig. 2010;30(7):419-437.
20b. Vuolteenaho K., Moilanen T., Moilanen E. Non-steroidal anti-inflammatory drugs, cyclooxygenase-2 and the bone healing process. Basic Clin Pharmacol Toxicol. 2008;102(1):10-14.
20c. Souyri C., Olivier P., Grolleau S., et al. Severe necrotizing soft-tissue infections and nonsteroidal anti-inflammatory drugs. Clin Exp Dermatol. 2008;33(3):249-255.
20d. Roumie C.L., Choma N.N., Kaltenbach L., et al. Non-aspirin NSAIDs, cyclooxygenase-2 inhibitors and risk for cardiovascular events-stroke, acute myocardial infarction, and death from coronary heart disease. Pharmacoepidemiol Drug Saf. 2009;18(11):1053-1063.
21. Goodman L., Torres B., Punke J., et al. Effects of firocoxib and tepoxalin on healing in a canine gastric mucosal injury model. J Vet Intern Med. 2009;23(1):56-62.
22. Bertolini A., Ottan A., Sandrini M. Dual-acting antinflammatory drugs: a reappraisal. Pharm Res. 2001;44(6):437-450.
23. Knapp D.W. Expression of cyclooxygenase-1 and -2 in naturally occurring canine cancer. Prostaglandins Leukot Essent Fatty Acids. 2004;70(5):479-483.
24. Hennig R., Grippo P., Ding X.Z., et al. 5-Lipoxygenase, a marker for early pancreatic intraepithelial neoplastic lesions. Cancer Res. 2005;65(14):6011-6016.
25. Higgins A.J. The biology, pathophysiology and control of eicosanoids in inflammation. J Vet Pharmacol Ther. 1985;8:1-18.
26. Lees P., Taylor J.B.O., Higgins A.J., et al. Phenylbutazone and oxyphenbutazone distribution into tissue fluids in the horse. J Vet Pharmacol Ther. 1986;9:204-212.
27. Laudanno O.M., Cesolari J.A., Esnarriaga J., et al. In vivo selectivity of nonsteroidal anti-inflammatory drugs on COX-1–COX-2 and gastrointestinal ulcers, in rats [in Spanish]. Acta Gastroenterol Latinoam. 1998;28(3):249-255.
28. Little D., Jones S.L., Blikslager A.T. Cyclooxygenase (COX) inhibitors and the intestine. J Vet Intern Med. 2007;21:367-377.
29. Streppa H.K., Jones C.H., Busberg S.C. Cyclooxygenase selectivity of nonsteroidal anti-inflammatory drugs in canine blood. Am J Vet Res. 2002;63:91-94.
30. Kawai S., Nishida S., Kato M., et al. Comparison of cyclooxygenase-1 and -2 inhibitory activities of various nonsteroidal anti-inflammatory drugs using human platelets and synovial cells. Eur J Pharmacol. 1998;347(1):87-94.
31. Wilson J.E., Chandrasekharan V., Westover K.D., et al. Determination of expression of cyclooxygeans 1 and 2 isoenzyme in canine tissues and their differential sensitivity to nonsteroidal anti-inflammatory drugs. Am J Vet Res. 2004;65:910-918.
32. Ricketts A.P., Lundy K.M., Seibel S.B. Evaluation of selective inhibition of canine cyclooxygenase 1 and 2 by carprofen and other nonsteroidal anti-inflammatory drugs. Am J Vet Res. 1998;59(11):1441-1446.
33. Brideau C., Van Staden C., Chan C.C. In vitro effects of cyclooxygenase inhibitors in whole blood of horses, dogs, and cats. Am J Vet Res. 2001;62(11):1755-1760.
34. Giraudel J.M., Toutain P.L., Lees P. Development of in vitro assays for the evaluation of cyclooxygenase inhibitors and predicting selectivity of nonsteroidal anti-inflammatory drugs in cats. Am J Vet Res. 2005;66(4):700-709.
35. Bryant C.E., Farnfield B.A., Janicke H.J. Evaluation of the ability of carprofen and flunixin meglumine to inhibit activation of nuclear factor kappa B. Am J Vet Res. 2005;64(2):211-215.
36. Braten D.C. Clinical pharmacology of NSAIDs. J Clin Pharmacol. 1988;28:518-523.
37. Kenyon C.J., Hooper G., Tierney D., et al. The effect of food on the gastrointestinal transit and systemic absorption of naproxen from a novel sustained release formulation,. J Contro Release. 1995;34(1):31-36.
38. Munsiff I.J., Koritz G.D., McKiernan B.C., et al. Plasma protein binding of theophylline in dogs. J Vet Pharmacol Ther. 1988;11:112-114.
39. Galbraith E.A., McKellar Q.A. Protein binding and in vitro serum thromboxane B2 inhibition by flunixin meglumine and meclofenamic acid in dog, goat and horse blood. Res Vet Sci. 1996;61(1):78-81.
40. Toutain P.L., Bousquet-Mélou A. Free drug fraction vs. free drug concentration: a matter of frequent confusion. J Vet Pharmacol Ther. 2002;25:460-463.
41. Paulson S.K., Engel L., Reitz B., et al. Evidence for polymorphisn in the canine metabolism of the cyclooxygenase 2 inhibitor, celecoxib. Drug Metab Dispos. 1999;27:1133-1142.
42. Aitken M.M., Sanford J. Plasma levels following administration of sodium meclofenamate by various routes. Res Vet Sci. 1975;19:241-244.
43. Lepist E.-I., Jusko W.J. Modeling and allometric scaling of s(+)-ketoprofen pharmacokinetics and pharmacodynamics: a retrospective analysis. J Vet Pharmacol Ther. 2004;27:211-218.
43a. Morris T., Stables M., Hobbs A., et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183(3):2089-2096.
44. Lees P., Higgins A.J. Clinical pharmacology and therapeutic uses of non-steroidal anti-inflammatory drugs in the horse. Equine Vet J. 1985;17:83-96.
44a. Lees P., Giraudel J., Landoni M.F., et al. PK-PD integration and PK-PD modelling of nonsteroidal anti-inflammatory drugs: principles and applications in veterinary pharmacology. J Vet Pharmacol Ther. 2004;27(6):491-502.
45. Giraudel J.M., King J.N., Jeunesse E.C., et al. Use of a pharmacokinetic/pharmacodynamic approach in the cat to determine a dosage regimen for the COX-2 selective drug rebenacoxib. J Vet Pharmacol Ther. 2008;32:18-30.
46. Laird J.M., Herrero J.F., Garcia de la Rubia P., et al. Analgesic activity of the novel COX-2 preferring NSAID, meloxicam in mono-arthritic rats: central and peripheral components. Inflamm Res. 1997;46(6):203-210.
47. Greisman L.A., Mackowia P.A. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241-245.
48. Brandt K.D. The mechanism of action of nonsteroidal antiinflammatory drugs. J Rheumatol. 1991;18:120-121.
48a. Martel-Pelletier J., Lajeunesse D., Reboul P., et al. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs,. Ann Rheum Dis. 2003;62(6):501-509.
49. Knapp D.W., Richardson R.C., Bottoms G.D., et al. Phase I. Trial of piroxicam in 62 dogs bearing naturally occurring tumors. Cancer Chemother Pharmacol. 1992;29:214-218.
50. Knapp D.W., Richardson R.C., Chan T.C., et al. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 1994;8:273-278.
51. Knapp D.W., Chan T.C., Kuczek T., et al. Evaluation of in vitro cytotoxicity of nonsteroidal anti-inflammatory drugs against canine tumor cells. Am J Vet Res. 1995;56(6):801-805.
51a. Martinez J.M., Sali T., Okazaki R., et al. Drug-induced expression of nonsteroidal anti-inflammatory drug-activated gene/macrophage inhibitory cytokine-1/prostate-derived factor, a putative tumor suppressor, inhibits tumor growth. J Pharmacol Exp Ther. 2006;318(2):899-906.
52. Braun D.P., Bonomi P.D., Taylor S.G., et al. Modification of the effects of cytotoxic chemotherapy on the immune responses of cancer patients with a nonsteroidal, antiinflammatory drug, piroxicam. A pilot study of the Eastern Cooperative Oncology Group. J Biol Response Mod. 1987;6:331-345.
53. Trepanier L.A. Potential interactions between non-steroidal anti-inflammatory drugs and other drugs. J Vet Emerg Crit Care. 2005;15(4):248-253.
54. Hulisz D., Lagzdins M. Drug-induced hypertension. US Pharamcist. 2008;33(9):HS11-HS20.
55. Fillastre J., Leroy A., Borsa-Lebas F., et al. Effects of ketoprofen (NSAID) on the pharmacokinetics of pefloxacin and ofloxacin in healthy volunteers. Drugs Exp Clin Res. 1992;18:487-492.
56. Jayaraman L., Sood J. An unusual cause of convulsions following general anesthesia. J Anaesth Clin Pharmcol. 2005;21(3):333-334.
57. Loke Y.K., Trivedi A.N., Singh S. Meta-analysis; gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27(1):31-40.
58. Woodward K.N. Veterinary pharmacovigilance. Part 6. Predictability of adverse reactions in animals from laboratory toxicology studies. J Vet Pharmacol Ther. 2005;28:213-231.
59. Jonnes R.D., Baynes R.E., Nimitz C.T. Nonsteroidal anti-inflammatory drug toxicosis in dogs and cats: 240 cases. J Am Vet Med Assoc. 1992;201:475-477.
59a.http://www.fda.gov/AnimalVeterinary/SafetyHealth/ProductSafetyInformation/ucm055394.htm (accessed November 22, 2010).
60. Horii Y., Ikenaga M., Shimoda M., et al. Pharmacokinetics of flunixin in the cat: enterohepatic circulation and active transport mechanism in the liver. J Vet Pharmacol Ther. 2004;27(2):65-69.
61. Ellison G.W. NSAIDs: How ulcerogenic are they? Proc Am Coll Vet Int Med. 1995:445-446.
62. Shaw N., Burrows C.F., King R.R. Massive gastric hemorrhage induced by buffered aspirin in a greyhound. J Am Anim Hosp Assoc. 1997;33(3):215-219.
63. Dye T.L. Naproxen toxicosis in a puppy. Vet Hum Toxicol. 1997;39(3):157-159.
64. Giannoukas A.D., Baltoyiannis G., Milonakis M., et al. Protection of the gastroduodenal mucosa from the effects of diclofenac sodium: role of highly selective vagotomy and misoprostol. World J Surg. 1996;20(4):501-506.
65. Vonderhaar M.A., Salisbury S.K. Gastroduodenal ulceration associated with flunixin meglumine administration in three dogs. J Am Vet Med Assoc. 1993;203:92-95. Published erratum appears in J Am Vet Med Assoc 203:869, 1993
66. Mathews K.A., Paley D.M., Foster R.A., et al. A comparison of ketorolac with flunixin, butorphanol, and oxymorphone in controlling postoperative pain in dogs,. Can Vet J. 1996;37(9):557-567.
67. McCormack K., Brune K. Classical absorption theory and the development of gastric mucosal damage associated with the non-steroidal anti-inflammatory drugs. Arch Toxicol. 1987;60:261-269.
68. Peura D.A., Goldkind L. Balancing the gastrointestinal benefits and risks of nonselective NSAIDs. Arthritis Res Ther. 2005;7(Suppl 4):S7-S13.
69. Wallace J.L. The 21st Gaddum Memorial Lecture, Building a better aspirin: gaseous solutions to a century-old problem. Br J Pharmacol. 2007;152:421-428.
70. Chastain C.B. Aspirin: new indications for an old drug. Compend Small Anim. 1987;9:165-170.
71. Silverstein F.D. Improving the gastrointestinal safety of NSAIDs. The development of misoprostol—from hypothesis to clinical practice. Dig Dis Sci. 1998;43:447-458.
72. Mazué G., Richez P., Berthe J. Pharmacology and comparative toxicology of non-steroidal anti-inflammatory agents. In: Ruckebusch Y., Toutain P.L., Koritz G.D., editors. Veterinary pharmacology and toxicology. Boston: MTP Press Limited; 1983:321-331.
73. Dickman A., Ellershaw J. NSAIDs: gastroprotection or selective COX-2 inhibitor? Palliative Medicine. 2004;18(4):275-286.
74. Boston S.E., Moens N.M., Kruth S.A., et al. Endoscopic evaluation of the gastroduodenal mucosa to determine the safety of short-term concurrent administration of meloxicam and dexamethasone in healthy dogs. Am J Vet Res. 2003;64(11):1369-1375.
75. Meddings J.B., Kirk D., Olson M.E. Noninvasive detection of nonsteroidal anti-inflammatory drug-induced gastropathy in dogs. Am J Vet Res. 1995;56(8):977-981.
76. Neiger R. NSAID-induced gastrointestinal adverse effects in dogs—can we avoid them? J Vet Intern Med. 2003;17(3):259-261.
77. Palmer R.H., DeLapp R. Gastrointestinal toxicity in elderly osteoarthritis patients treated with NSAIDs. Inflammopharmacology. 2000;8(1):19-30.
78. Dow S.W., Rosychuk R.A., McChesney A.E., et al. Effects of flunixin and flunixine plus prednisone on the gastrointestinal tract of dogs. Am J Vet Res. 1990;51:1131-1137.
79. Kietzmann M., Meyer-Lindenberg A., Engelke A., et al. Pharmacokinetics and tolerance of an orally administered combination preparation containing phenylbutazone and prednisolone in the dog. Deutsche Tierarztl Wochenschr. 1996;103(1):14-16.
80. Lascelles B.D., Blikslager A.T., Fox S.M., et al. Gastrointestinal tract perforation in dogs treated with a selective cyclooxygenase-2 inhibitor: 29 cases (2002-2003). J Am Vet Med Assoc. 2005;227:1112-1117.
81. Brune K. Safetyof anti-inflammatory treatment—new ways of thinking. Rheumatology. 2004;43(Suppl 1):i16-i20.
82. Peterson K.D., Keefe T.J. Effects of meloxciam on severity of lameness and other clinical signs of osteoarthritis. J Am Vet Med Assoc. 2004;225:1056-1060.
83. Reimer M.E., Johnston S.A., Leib M.S., et al. The gastroduodenal effects of buffered aspirin, carprofen, and etodolac in healthy dogs,. J Vet Intern Med. 1999;13(5):472-477.
84. Mason L., Moore R.A., Edwards J.E., et al. Topical NSAIDs for acute pain: a meta-analysis. BMC Family Practice. 2004;5:10.
85. Collins L.G., Tyler D.E. Experimentally induced phenylbutazone toxicosis in ponies: description of the syndrome and its prevention with synthetic prostaglandin E2. Am J Vet Res. 1985;46:1605-1615.
86. Boulay J.P., Lipowitz A.J., Klausner J.S. Effect of cimetidine on aspirin-induced gastric hemorrhage in dogs. Am J Vet Res. 1986;47(8):1744-1746.
86a. Watkins .P. Omeprazole induction of cytochrome P45O1A2: the importance of selecting an appropriate human model. Hepatology. 1993;17:748-750.
86b. Li X.Q., Andersson T.B., Ahlström M. Comparison of inhibitory effects of the proton pump inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole and rabeprazole on human cytochrome P45O activities. Drug Met Rev. 2004;32:821-837.
87. Bowersox T.S., Lipowitz A.J., Hardy R.M., et al. The use of a synthetic prostaglandin E1 analog as a gastric protectant against aspirin-induced hemorrhage in the dog,. J Am Anim Hosp Assoc. 1996;32(5):401-407.
88. Murtaugh R.J., Matz M.E., Labato M.A., et al. Use of synthetic prostaglandin E1 (misoprostol) for prevention of aspirin-induced gastroduodenal ulceration in arthritic dogs. J Am Vet Med Assoc. 1993;202:251-256.
89. Johnston S.A., Leib M.S., Forrester S.D., et al. The effect of misoprostol on aspirin-induced gastroduodenal lesions in dogs. J Vet Int Med. 1995;19:32-38.
90. Shield M.J. Diclofenac/misoprostol: novel findings and their clinical potential. J Rheumatol. 1998;25(Suppl) 51):31-41.
90a. Cook D., Guyatt G., Marshall J., et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation,. N Engl J Med. 338, 1998. 791–791
91. Cho C.-H., Liu E.S., Shin V.Y. Polysaccharides: A new role in gastrointestinal protection. Cho C.-H., Wang J.-Y., editors. Gastrointestinal mucosal repair and experimental therapeutics, Front Gastrointest Res. 25:180-189. 2002.
92. Bjarnason I., Hayliar J., Smethurst P., et al. Metronidazole reduces intestinal inflammation and blood loss in non-steroidal anti-inflammatory drug induced enteropathy. Gut. 1992;33:1204-1208.
93. Canadian Agency for Drugs and Technologies in Health (CADTH): Preventing NSAID induced GI complications: an economic evaluation of alternative strategies in Canada, 2007. Accessed November 9, 2009, at cadth.ca/media/compus/pdf/compus_economic_evaluation_pud_model_e.pdf.
94. Lewis J.H. Hepatic toxicity of nonsteroidal anti-inflammatory drugs. Clin Pharmacol. 1984;3:128-138.
95. Martin K., Andersson L., Stridsberg M., et al. Plasma concentration, mammary excretion and side-effects of phenylbutazone after repeated oral administration in healthy cows. J Vet Pharmacol Ther. 1984;7:131-138.
96. Carlisle C.H., Penny R.H.C., Prescott C.W., et al. Toxic effects of phenylbutazone on the cat. Br Vet J. 1968;124:560-566.
97. Watson A.D.J., Wilson J.T., Turner D.M., et al. Phenylbutazone-induced blood dyscrasias suspected in three dogs. Vet Rec. 1980;107:239-241.
98. Markel M.D. What is your diagnosis? J Am Vet Med Assoc. 1986;188:307-308.
99. Wallau J.L. Distribution and expression of cyclooxygenase (COX) isoenzymes, their physiologic role, and the categorization of nonsteroidal antiinflammatory drugs (NSAIDs). Am J Med. 1999;107:11S-16S.
100. Dunn J.M., Simonson J., Davidson E.W., et al. Nonsteroidal anti-inflammatory drugs and renal function. J Clin Pharmacol. 1988;28:524-529.
101. Angio R.G. Nonsteroidal antiinflammatory drug-induced renal dysfunction related to inhibition of renal prostaglandins. Drug Intell Clin Pharmacol. 1987;21:954-960.
102. Efrati S., Berman S., Siman-Tov Y., et al. N-acetylcysteine attenuates NSAID-induced rat renal failure by restoring intrarenal prostaglandin synthesis. Nephrol Dial Transplant. 2007;22:1873-1881.
103. Selig C.B., Maloley P.A., Campbell J.R. Nephrotoxicity associated with concomitant ACE inhibitor and NSAID therapy. South Med J. 1990;83:1144-1148.
104. Swan S.K., Rudy D.W., Lasseter K.C., et al. Effect of cyclooxygenase-2 inhibtion on renal function in persons receiving a low salt diet. Ann Intern Med. 2000;133:1-9.
105. Crandell D.E., Mathews K.A., Dyson D.H. Effect of meloxicam and carprofen on renal function when administered to healthy dogs prior to anesthesia and painful stimulation. Am J Vet Res. 2004;65(10):1384-1390.
106. Lobetti R.G., Joubert K.E. Effect of administration of nonsteroidal anti-inflammatory drugs before surgery on renal function in clinically normal dogs. Am J Vet Res. 2000;61(12):1501-1506.
107. Steagall P.V.M., Moutinho F.Q., Mantovani F.B., et al. Evaluation of the adverse effects of subcutaneous carprofen over six days in healthy cats. Res Vet Sci. 2009;86:115-120.
108. Gunew M.N., Menrath V.H., Marshall R.D. Long-term safety, efficacy and palatability of oral meloxicam at 0.01-0.03 mg/kg for treatment of osteoarthritic pain in cats. J Fel Med Surg. 2008;10:235-241.
109. Fullerton T., Sica D.A., Blum R.A. Evaluation of the renal protective effect of misoprostol in elderly, osteoarthritic patients at risk for nonsteroidal anti-inflammatory drug-induced renal dysfunction. J Clin Pharmacol. 1993;33:1225-1232.
110. Pouteil-Noble C., Chapuis F., Berra N., et al. Misoprostol in renal transplant recipients: a prospective, randomized, controlled study on the prevention of acute rejection episodes and cyclosporin Anephrotoxicity. Dial Transplant. 1994;9(5):552-555.
111. Boers M., Bensen W.G., Ludwin D., et al. Cyclosporine nephrotoxicity in rheumatoid arthritis: no effect of short term misoprostol treatment. J Rheumatol. 1992;19(4):534-537.
112. Aitio M- L. N-acetylcysteine—passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006;61(1):1-15.
113. Levin A., Pate G.E., Shalansky S., et al. N-acetylcysteine reduces urinary albumin excretion following contrast administration: evidence of biological effect. Nephrol Dial Transplant. 2007.
114. Jackson M.L. Platelet physiology and platelet function: inhibition by aspirin. Compend Contin Educ Pract Vet. 1987;9:627-638.
115. Konstantinopoulos P.A., Lehmann D.F. The cardiovascular toxicity of selective and nonselective cyclooxygenase inhibitors: comparisons, contrasts, and aspirin confounding. J Clin Pharmacol. 2005;45(7):742-750.
116. Hennan J.K., Huang J., Barrett T.D., et al. Effects of selective cyclooxygenase-2 inhibition on vascular responses and thrombosis in canine coronary arteries. Circulation. 2001;104(7):820-825.
116a. Rimon G., Sidhu R.S., Lauver D.A., et al. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1. Proc Natl Acad Sci USA 5. 2010;107(1):28-33.
117. Daminet S., Croubels S., Duchateau L., et al. Influence of acetylsalicylic acid and ketoprofen on canine thyroid function tests. Vet J. 2003;166(3):224-232.
118. Panciera D.L., Johnston S.A. Results of thyroid function tests and concentrations of plasma proteins in dogs administered etodolac. Am J Vet Res. 2002;63(11):1492-1495.
119. Sauve F., Paradis M., Refsal K.R., et al. Effects of oral administration of meloxicam, carprofen, and a nutraceutical on thyroid function in dogs with osteoarthritis. Can Vet J. 2003;44:474-479.
120. Clemmons R.M., Meyers K.M. Acquisition and aggregation of canine blood platelets: basic mechanisms of function and differences because of breed origin. Am J Vet Res. 1984;45:137-144.
121. Berliner S., Weinberger A., Shoenfeld Y., et al. Ibuprofen may induce meningitis in (NZB X NZW) mice. Arthritis Rheum. 1985;28:104-107.
122. Syvlia L.M., Forlenza S.W., Brocavich J.M. Aseptic meningitis associated with naproxen. DICP Ann Pharmacother. 1988;22:399-401.
122a. Botting R. Vane’s discovery of the mechanism of aspirin changed our understanding of its clinical pharmacology. Pharmacol Rep. 2010;62:518-525.
123. Romano M. Lipid mediators: lipoxin and aspirin-riggered 15-epi-lipoxins. Inflamm Allergy Drug Targets. 2006;5:81-90.
123a. Morris T., Stables M., Hobbs A., et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183(3):2089-2096.
124. Conlon P.D. Nonsteroidal drugs used in the treatment of inflammation. Vet Clin North Am Small Anim Pract. 1988;18:1115-1131.
125. Davis L.E., Westfall B.A. Species differences in biotransformation and excretion of salicylate. Am J Vet Res. 1972;33:1253-1262.
126. Short C.R., Hsieh L.C., Malbrough M.S., et al. Elimination of salicylic acid in goats and cattle. Am J Vet Res. 1990;51:1267-1270.
127. USP. Veterinary pharmaceutical information monographs, anti-inflammatories anonymous. J Vet Pharmacol Therap. 27(Suppl 1), 2004.
128. Parton K., Balmer T.V., Boyle J. The pharmacokinetics and effects of intravenously administered carprofen and salicylate on gastrointestinal mucosa and selected biochemical measurements in healthy cats,. J Vet Pharmacol Ther. 2000;23(2):73-79.
129. Robinson M.G. New oral salicylates in therapy of chronic idiopathic inflammatory bowel disease. Gastroenterol Clin North Am. 1989;18:43-50.
130. Takaya T., Sawada K., Suzuki H., et al. Application of a colon delivery capsule to 5-aminosalicylic acid and evaluation of the pharmacokinetic profile after oral administration to beagle dogs. J Drug Targeting. 1997;4(5):271-276.
131. Beasley V.R., Buck W.B. Acute ethylene glycol toxicosis: a review. Vet Hum Toxicol. 1980;22:255.
132. Larson E.J. Toxicity of low doses of aspirin in the cat. J Am Vet Med Assoc. 1963;143:837-840.
133. Palmoski M.J., Colyer R.A., Brandt K.D. Marked suppression by salicylate of the augmented proteoglycan synthesis in osteoarthritic cartilage. Arthritis Rheum. 1980;23(1):83-91.
134. Morton D.L., Knottenbelt D.C. Pharmacokinetics of aspirin and its application in canine veterinary medicine. J S Afr Vet Assoc. 1989;60:191-194.
135. Lipowitz A.J., Boulay J.P., Klausner J.S. Serum salicylate concentrations and endoscopic evaluation of the gastric mucosa in dogs after oral administration of aspirin-containing products. Am J Vet Res. 1986;47:1586-1589.
136. Konturek S.J. Physiology and pharmacology of prostaglandin. Dig Dis Sci. 1986;31:6S-19S.
137. Jezyk P.F. Metabolic diseases: an emerging area of veterinary pediatrics. Compend Contin Educ Pract Vet. 1983;5:1026-1031.
138. Yeary R.A., Swanson W. Aspirin dosages for the cat. J Am Vet Med Assoc. 1973;163:1117-1178.
139. Davis L.E., Westfall B.A., Short C.R. Biotransformation and pharmacokinetics of salicylate in newborn animals. Am J Vet Res. 1973;34:1105-1108.
140. Oehme F.W. Aspirin and acetaminophen. In: Kirk R., editor. Current veterinary therapy (small animal practice) X. Philadelphia: Saunders; 1986:188-190.
141. Hardie E.M., Kolata R.J., Rawlings C.A. Canine septic peritonitis: treatment with flunixin meglumine. Circ Shock. 1983;11:159-173.
142. Moore J.N., Hardee M.M., Hardee G.E. Modulation of arachidonic acid metabolism in endotoxic horses: comparison of flunixin meglumine, phenylbutazone, and a selective thromboxane synthetase inhibitor. Am J Vet Res. 1986;47:110-113.
143. Templeton C.B., Bottoms G.D., Fessler J.F., et al. Endotoxin-induced hemodynamic and prostaglandin changes in ponies: effects of flunixin meglumine, dexamethasone, and prednisolone. Circ Shock. 1987;23:231-240.
144. Jarlov N., Andersen P.H., Haubro P., et al. Pathophysiology of experimental bovine endotoxicosis: endotoxin induced synthesis of prostaglandins and thromboxane and the modulatory effect of some non-steroidal anti-inflammatory drugs. Acta Vet Scand. 1992;33:1-8.
145. Davidson J.R., Lantz G.C., Salisbury S.K., et al. Effects of flunixin meglumine on dogs with experimental gastric dilatation-volvulus. Vet Surg. 1992;21:113-120.
146. Hardie E.M., Hardee G.E., Rawlings C.A. Pharmacokinetics of flunixin meglumine in dogs. Am J Vet Res. 1985;46:235-237.
147. McKellar Q.A., Galbraith E.A., Bogan J.A., et al. Flunixin pharmacokinetics and serum thromboxane inhibition in the dog. Vet Rec. 1989;24:651-654.
148. Scherkl R., Frey H.H. Pharmacokinetics of ibuprofen in the dog. J Vet Pharmacol Ther. 1987;10:261-265.
149. Dunayer E. Ibuprofen toxicosis in dogs, cats, and ferrets. Vet Med. 2004;7:580-586.
150. Williams R.L., Upton R.A. The clinical pharmacology of ketoprofen,. J Clin Pharmacol. 1988;28:S13-S22.
151. Schmitt M., Guentert T.W. Biopharmaceutical evaluation of ketoprofen following intravenous, oral, and rectal administration in dogs. J Pharm Sci. 1990;79:614-616.
152. Castro E., Soraci A., Fogel F., et al. Chiral inversion of R(-) fenoprofen and ketoprofen enantiomers in cats. J Vet Pharmacol Therap. 2000;23:265-271.
153. Cailleteau J.G. Ketoprofen in dentistry: a pharmacologic review. Oral Surg Oral Med Oral Pathol. 1988;66:620-624.
154. Beaver W.T. Ketoprofen: a new nonsteroidal anti-inflammatory analgesic. J Clin Pharmacol. 1988;28:S1.
155. Stambough J., Drew J. A double-blind parallel evaluation of the efficacy and safety of a single dose of ketoprofen in cancer pain. J Clin Pharmacol. 1988;28:S34-S39.
156. Avouac B., Teule M. Ketoprofen: the European experience. J Clin Pharmacol. 1988;28:S2-S7.
157. Turek M.D., Baird W.M. Double-blind parallel comparison of ketoprofen, acetaminophen plus codeine, and placebo in postoperative pain. J Clin Pharmacol. 1988;28:S23-S28.
158. Glew A., Aviad A.D., Keister D.M., et al. Use of ketoprofen as an antipyretic in cats. Can Vet J. 1996;37:222-225.
159. Slingsby L.S., Waterman-Pearson A.E. Comparison of pethidine, buprenorphine and ketoprofen for postoperative analgesia after ovariohysterectomy in the cat. Vet Rec. 1998;143:185-189.
160. Insel P.A. Analgesic, antipyretic and anti-inflammatory agents and drugs employed in treatment of gout. In: Hardman J.G., Limbird L.D., editors. Goodman and Gilman’s the pharmacological basis of therapeutics. ed 9. New York: McGraw-Hill; 1996:617-659.
161. Paddleford R.R. Analgesia and pain management. In Paddleford R.R., editor: Manual of small animal anesthesia, ed 2, Philadelphia: Saunders, pp 227–246, 1999.
162. Pasloske K., Renaud R., Burger J., et al. Pharmacokinetics of ketorolac after intravenous and oral single dose administration in dogs. J Vet Pharmacol Ther. 1999;22:314-319.
163. Frey H.H., Rieh B. Pharmacokinetics of naproxen in the dog. Am J Vet Res. 1981;42:1615-1617.
164. Zech R., Scherkl R., Hashem A., et al. Plasma and tissue kinetics of phenylbutazone and naproxen in dogs. Arch Int Pharmacodyn Ther. 1993;325:113-128.
165. Gfeller R.W., Sandors A.D. Naproxen-associated duodenal ulcer complicated by perforation and bacteria- and barium sulfate-induced peritonitis in a dog. J Am Vet Med Assoc. 1991;198:644-646.
166. Roudebush P., Morse G.E. Naproxen toxicosis in a dog. J Am Vet Med Assoc. 1981;179:805-806.
167. Ratliffe A., Rosenwasser M.P., Mahmud F., et al. The in vivo effects of naproxen on canine experimental osteoarthritic articular cartilage: composition, metalloproteinase activity and metabolism. Agents Actions. 1993;39(Suppl):207-211.
168. De Backer P., Braeckman R., Belpaire F., et al. Bioavailability and pharmacokinetics of phenylbutazone in the cow. J Vet Pharmacol Ther. 1980;3:29-33.
169. Mills P.C., Ng J.C., Hrdlicka J., et al. Disposition and urinary excretion of phenylbutazone in normal and febrile greyhounds. Res Vet Sci. 1995;59(3):261-266.
170. Mills P.C., Ng J.C., Skelton K.V., et al. Phenylbutazone in racing greyhounds: plasma and urinary residues 24 and 48 hours after a single intravenous administration. Aust Vet J. 1995;72(8):304-308.
171. Murray M.J. Phenylbutazone toxicity in a horse. Compend Contin Educ Pract Vet. 1985;7:S389-S394.
172. Tandy J., Thorpe E. A fatal syndrome in the dog following administration of phenylbutazone,. Vet Rec. 1967;81:398-399.
173. Kalbhen D.A. The influence of NSAIDs on morphology of articular cartilage. Scand J Rheumatol Suppl. 1988;77:13-22.
174. Jolly W.T., Whittem T., Jolly A.C., et al. The dose-related effects of phenylbutazone and a methylprednisolone acetate formulation (Depo-Medrol) on cultured explants of equine carpal articular cartilage. J Vet Pharmacol Ther. 1995;18(6):429-437.
175. Thomas A.D., Bowater I.C., Vine J.H., et al. Uptake of drugs from topically applied anti-inflammatory preparations applied to racing animals. Aust Vet J. 1997;75:897-901.
176. Weiss D.J., Klausner J.S. Drug associated aplastic anemia in dogs: eight cases (1984-1988). J Am Vet Med Assoc. 1990;196:472.
177. Galbraith E.A., McKellar Q.A. Pharmacokinetics and pharmacodynamics of piroxicam in dogs. Vet Rec. 1991;128:561.
178. Heeb H.L., Chun R., Koch D.E., et al. Single dose pharmacokinetics of piroxicam in cats. J Vet Pharmacol Ther. 2003;26(4):259-263.
179. Bergh M.S., Budsberg S.C. The coxib NSAIDs: potential clinical and pharmacologic importance in veterinary medicine. J Vet Intern Med. 2005;19(5):633-643.
180. Fox S.M., Johnston S.A. Use of carprofen for the treatment of pain and inflammation in dogs. J Am Vet Med Assoc. 1997;210(10):1493-1498.
181. McKellar Q.A., Delatour P., Lees P. Stereospecific pharmacodynamics and pharmacokinetics of carprofen in the dog. J Vet Pharmacol Ther. 1994;17(6):447-454.
182. Clark T.P., Chieffo C., Huhn J.C., et al. The steady-state pharmacokinetics and bioequivalence of carprofen administered orally and subcutaneously in dogs. J Vet Pharmacol Therap. 2003;26:187-192.
183. Priymenko N., Garnier F., Ferre J.P., et al. Enantioselectivity of the enterohepatic recycling of carprofen in the dog. Drug Metab Dispos. 1998;26(2):170-176.
184. Vasseur P.B., Johnson A.L., Budsberg S.C., et al. Randomized, controlled trial of the efficacy of carprofen, a nonsteroidal antiinflammatory drug, in the treatment of osteoarthritis in dogs. J Am Vet Assoc. 1995;206:807-811.
185. Forsyth S.F., Guilford W.G., Haslett S.J., et al. Endoscopy of the gastroduodenal mucosa after carprofen, meloxicam and ketoprofen administration in dogs. J Small Anim Pract. 1998;39(9):421-424.
186. Craven M., Chandler M.L., Steiner J.M., et al. Acute effects of carprofen and meloxicam on canine gastrointestinal permeability and mucosal absorptive capacity. J Vet Intern Med. 2007;21:917-923.
187. MacPhail C.M., Lappin M.R., Meyer D.J., et al. Hepatocellular toxicosis associated with administration of carprofen in 21 dogs. J Am Vet Med Assoc. 1998;212(12):1895-1901.
188. Pfizer Carprofen Technical Report: 1998; Pfizer Animal Health.
189. Forsyth S.F., Guilford W.G., Pfeiffer D.U. Effect of NSAID administration on creatinine clearance in healthy dogs undergoing anaesthesia and surgery. J Small Anim Pract. 2000;41(12):547-550.
190. Bostrom I.M., Nyman G.C., Lord P.E., et al. Effects of carprofen on renal function and results of serum biochemical and hematologic analyses in anesthetized dogs that had low blood pressure during anesthesia. Am J Vet Res. 2002;63(5):712-721.
191. Hickford F.H., Barr S.C., Erb H. Effect of carprofen on hemostatic variables. Am J Vet Res. 2001;62:1642-1646.
192. Benton H.P., Vasseur P.G., Broderick-Villa A., et al. Effect of carprofen on sulfated glycosaminoglycan metabolism, protein synthesis and prostaglandin release by cultured osteoarthritic canine chondrocytes. Am J Vet Res. 1997;58:286-291.
193. Lascelles B.D., Butterworth S.J., Waterman A.E. Postoperative analgesic and sedative effects of carprofen and pethidine in dogs. Vet Rec. 1994;134(8):187-191.
194. Lascelles B.D., Cripps P.J., Jones A., et al. Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet Surg. 1998;27(6):568-582.
195. Welsh E.M., Nolan A.M., Reid J. Beneficial effects of administering carprofen before surgery in dogs. Vet Rec. 1997;141:251-253.
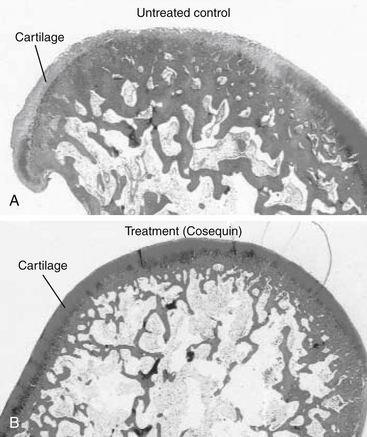
196. Borer L.R., Peel J.E., Seewald W., et al. Effect of carprofen, etodolac, meloxicam, or butorphanol in dogs with induced acute synovitis. Am J Vet Res. 2003;64(11):1429-1437.
197. Moreau M., Dupuis J., Bonneau N.H., et al. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152(11):323-329.
198. Aragon C.L., Hofmeister E.H., Budsberg S.C. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc. 2007;230(4):514-521.
199. Taylor P.M., Delatour P., Landoni F.M., et al. Pharmacodynamics and enantioselective pharmacokinetics of carprofen in the cat. Res Vet Sci. 1996;60(2):144-151.
200. Lascelles B.D., Cripps P., Mirchandarin S., et al. Carprofen as an analgesic for postoperative pain in cats: dose titration and assessment of efficacy in comparison to pethidine hydrochloride. Small Anim Prac. 1995;36:535-541.
201. Balmer T.V., Irvine D., Jones R.S., et al. Comparison of carprofen and pethidine as postoperative analgesics in the cat. J Small Anim Pract. 1998;39(4):158-164.
202. Runk A., Kyles A.E., Downs M.O. Duodenal perforation in a cat following the administration of nonsteroidal antiinflammatory medication. J Am Anim Hosp Assoc. 1999;35:52-55.
203. Slingsby L.S., Waterman-Pearson A.E. Postoperative analgesia in the cat after ovariohysterectomy by use of carprofen, ketoprofen, meloxicam or tolfenamic acid. J Small Anim Pract. 2000;41(10):447-450.
204. Taylor P.M., Steagall P.V.M., Dixon M.J., et al. Carprofen and buprenorphine prevent hyperalgesia in a model of inflammatory pain in cats. Res Vet Sci. 2007;83:369-375.
205. Simmons K: Personal communication, Novartis Animal Health, October, 2004.
206. Gierse J.K., Staten N.R., Casperson G.F., et al. Cloning, expression, and selective inhibition of canine cyclooxygenase-1 and cyclooxygenase-2. Vet Ther. 2002;3(3):270-280.
207. Millis D.L., Weigel J.P., Moyers T., et al. The effect of deracoxib, a new COX-2 inhibitor, on the prevention of lameness induced by chemical synovitis in dogs. Vet Ther. 2002;3(4):7-18.
208. Sennello K.A., Leib M.S. Effects of deracoxib or buffered aspirin on the gastric mucosa of healthy dogs. J Vet Intern Med. 2006;20:1291-1296.
209. Glaser K.B. Cyclooxygenase selectivity and NSAIDS: Cyclooxygenase-2 selectivity of etodolac (Lodine). Inflammopharmacology. 1995;3:335-345.
210. Cayan M.N., Kraml M., Gerdinandi E.S., et al. The metabolic disposition of etodolac in rats, dogs, and man. Drug Metab Rev. 1981;12:339.
211. Lippiello L., Han M.S., Henderson T. Protective effect of the chondroprotective agent CosequinDS on bovine articular cartilage exposed in vitro to nonsteroidal antiinflammatory agents. Vet Ther. 2002;2(3):128-135.
211a. Klauss G., Giuliano E.A., Moore C.P., et al. Keratoconjunctivitis sicca associated with administration of etodolac in dogs: 211 cases (1992-2002). J Am Vet Med Assoc. 2007;230(4):541-547.
212. Budsberg S., Johnston S., Schwarz P., et al. Evaluation of etodolac for the treatment of osteoarthritis of the hip in dogs: a prospective multicenter study (abstract). Vet Surg. 1996;25:420.
213. McCann M.E., Andersen D.R., Zhang D., et al. In vitro effects and in vivo efficacy of a novel cyclooxygenase-2 inhibitor in dogs with experimentally induced synovitis. Am J Vet Res. 2004;65(4):503-512.
214. Pollmeier M., Toulemonde C., Fleishman C., et al. Clinical evaluation of firocoxib and carprofen for the treatment of dogs with osteoarthritis. Vet Rec. 2006;159:547-551.
215. Engelhardt G., Bogel R., Schnitzer C., et al. Meloxicam: influence on arachidonic acid metabolism, Part 1. In vitro findings. Biochem Pharmacol. 1996;51:21.
216. Engelhardt G., Bogel R., Schnitzer C., et al. Meloxicam: influence on arachidonic acid metabolism, Part II. In vivo findings. Biochem Pharmacol. 1996;51:29.
217. Engelhardt G., Homma D., Schlegel K., et al. General pharmacology of meloxicam, Part II. Effects on blood pressure, blood flow, heart rate, ECG, respiratory minute volume and interactions with paracetamol, pirenzepine, chlorthalidone, phenprocoumon and tolbutamide. Gen Pharmacol. 1996;27(4):679-688.
218. Hare J.E., Darling H., Doig P.A. Metacam oral suspension: current safety data, Boehringer Ingelheim Vetmedica. Toronto, Ontario: c/o Jonathan Hare, Janssen Animal Health, 19 Green Belt Drive; 1998.
219. Kay-Mugford P.A., Grimm K.A., Weingarten A.J., et al. Effect of preoperative administration of tepoxalin on hemostasis and hepatic and renal function dogs. Vet Ther. 2004;5:120-127.
220. Busch U., Schmid J., Heinzel G., et al. Pharmacokinetics of meloxicam in animals and the relevance to humans. Drug Metab Dispos. 1998;26(6):576-584.
221. Staerkel P., Horsmans Y. Meloxicam-induced liver toxicity. Acta Gastroenterol Belg. 1999;62(2):255-256.
222. Enberg T.B., Braun L.D., Kuzman A.B. Gastrointestinal perofration in five dogs associated with the administration of meloxicam. J Vet Emerg Crit Care. 2006;16(1):34-43.
223. Niza N.M., Felix N., Vilela C.L. Cutaneous and ocular adverse reactions in a dog following meloxicam administration. Vet Dermatol. 2007;18:45-49.
224. Doig P.A., Purbrick K.A., Hare J.E., et al. Clinical efficacy oand tolerance of meloxicam in dogs with chronic osteoarthritis. Can Vet J. 2000;41:296-300.
225. Van Bree H., Justus C., Quirke J.F. Preliminary observations on the effects of meloxicam in a new model for acute intra-articular inflammation in dogs. Vet Res Commun. 1994;18(3):217-224.
226. Jones C.J., Streppa H.K., Harmon B.G., et al. In vivo effects of meloxicam and aspirin on blood, gastric mucosal, and synovial fluid prostanoid synthesis in dogs. Am J Vet Res. 2002;63(11):1527-1531.
227. Lascelles B.D., Henderson A.J., Hackett I.J. Evaluation of the clinical efficacy of meloxicam in cats with painful locomotor disorders. J Small Anim Pract. 2001;42(12):587-593.
228. Slingsby L.S., Waterman-Pearson A.E. Comparison between meloxicam and carprofen for postoperative analgesia after feline ovariohysterectomy. J Small Anim Pract. 2002;43(7):286-289.
229. Mathews K.A., Pettifer G., Foster R., et al. Safety and efficacy of preoperative administration of meloxicam, compared with that of ketoprofen and butorphanol in dogs undergoing abdominal surgery. Am J Vet Res. 2001;62(6):882-888.
230. Budsberg S.C., Cross A.R., Quandt J.E., et al. Evaluation of intravenous administration of meloxicam for perioperative pain management following stifle joint surger in dogs. Am J Vet Res. 2002;63:1557-1563.
231. Rainsford K.D., Skerry T.M., Chindemi P., et al. Effects of the NSAIDs meloxicam and indomethacin on cartilage proteoglycan synthesis and joint responses to calcium pyrophosphate crystals in dogs. Vet Res Commun. 1999;23(2):101-113.
232. Justus C., Quirke J.F. Dose-response relationship for the antipyretic effect of meloxicam in an endotoxin model in cats. Vet Res Commun. 1995;19(4):321-330.
233. Metacam Symposium on Arthritic Disease in Cats, Seville, Spain, June 1-3, 2007.
234. Giraudel J.M., Toutain P.L., King J.N., et al. Differential inhibition of cyclooxygenase isoenzymes in the cat by the NSAID robenacoxib. J Vet Pharmacol Ther. 2009;32(1):31-40.
235. Jung M., Lees P., Seewald W., et al. Analytical determination and pharmacokinetics of robenacoxib in the dog. J Vet Pharmacol Therap. 2008;32:41-48.
236. Hoeijmakers M., Coert A., van Helden H., et al. The pharmacokinetics of vedaprofen and its enantiomers in dogs after single and multiple dosing,. J Vet Pharmacol Ther. 2005;28:305-312.
237. Nell T., Bergman J., Hoeijmakers M., et al. Comparison of vedaprofen and meloxicam in dogs with musculoskeletal pain and inflammation. J Small Anim Pract. 2002;43(5):208-212.
238. Paulson S.K., Zhang J.Y., Jessen S., et al. Comparison of celecoxib metabolism and excretion in mouse, rabbit, dog, cynomolgus monkey and rhesus monkey. Xenobiotica. 2000;30(7):731-744.
239. Hunter R.P., Radlinsky M., Koch D.E., et al. Single and multiple dose pharmacokinetics and synovial fluid concentrations of celecoxib in greyhound dogs. Proc ACVIM. #286, 2003.
240. Moreau M., Daminet S., Martel-Pelletier J., et al. Superiority of the gastroduodenal safety profile of licofelone over rofecoxib, a COX-2 selective inhibitor, in dogs. J Vet Pharmacol Therap. 2005;28:81-86.
241. Halpin R.A., Geer L.A., Zhang K.E., et al. The absorption, distribution, metabolism and excretion of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in rats and dogs. Drug Metab Dispos. 2000;28(10):1244-1254.
242. Toutain P.L., Cester C.C., Haak T., et al. Pharmacokinetic profile and in vitro selective cyclooxygenase-2 inhibition by nimesulide in the dog. J Vet Pharmacol Ther. 2001;24(1):35-42.
243. Toutain P.L., Cester C.C., Haak T., et al. A pharmacokinetic/pharmacodynamic approach vs. a dose titration for the determination of a dosage regimen: the case of nimesulide, a Cox-2 selective nonsteroidal anti-inflammatory drug in the dog,. J Vet Pharmacol Ther. 2001;24(1):43-55.
244. Ewing G.O. Indomethacin-associated gastrointestinal hemorrhage in a dog. J Am Vet Med Assoc. 1972;161:1665-1668.
245. Mburu D.N., Mbugua S.W., Skoglund L.A., et al. Effects of paracetamol and acetylsalicylic acid on the post-operative course after experimental orthopaedic surgery in dogs. J Vet Pharmacol Ther. 1988;11:163-171.
246. St. Omer V.V., McKnight E.D. Acetylcysteine for treatment of acetaminophen toxicosis in the cat. J Am Vet Med Assoc. 1980;176:911-913.
247. Cullison R.F. Acetaminophen toxicosis in small animals: clinical signs, mode of action, and treatment. Compend Contin Educ. 1984;6:315-321.
248. Savides M.C., Oehme F.W., Leipold H.W. Effects of various antidotal treatment on acetaminophen toxicosis and biotransformation in cats. Am J Vet Res. 1985;46:1485-1489.
249. Jackson J.E. Cimetidine protects against acetaminophen toxicity. Life Sci. 1982;31:31-35.
250. Ruffalo R.L., Thompson J.F. Cimetidine and acetylcysteine as antidote for acetaminophen overdose. South Med J. 1982;75:954-958.
251. Wallace K.P., Center S.A., Hickford F.H., et al. S-Adenosyl-L-Methionine (SAMe) for the trestment of acetaminophen toxicity in a dog. J Am Anim Hosp Assoc. 2002;38:246-254.
252. Aronson L.R., Drobatz K. Acetaminophen toxicosis in 17 cats. J Vet Emerg Crit Care. 1996;6(2):65-69.
253. Cartier L.-J., Leclerc P., Pouliot M., et al. Toxic levels of acetaminophen produce a major positive interference on glucometer elite and accu-check advantage glucose meters. Clin Chem. 1998;44(4):893-894.
253a. Flood A.R. The role of acetaminophen in the treatment of osteoarthritis,. Am J Manag Care. 2010;16:S48-S54.
254. Johnston S.A., Budsberg S.C. Nonsteroidal anti-inflammatory drugs and corticosteroids for the management of canine osteoarthritis. Vet Clin North Am Small Anim Pract. 1997;27:841-862.
255. Hjelle J.J., Grauer G.F. Acetaminophen induced toxicosis in dogs and cats. J Am Vet Med Assoc. 1986;188:742-746.
256. Savides M.C., Oehme F.W. Acetaminophen and its toxicity. J Appl Toxicol. 1983;3:96-111.
257. Francavilla A., Makowka L., Polimeno L., et al. A dog model for acetaminophen-induced fulminant hepatic failure. Gastroenterology. 1989;96:470-478.
258. Goodman L., Coles T.B., Budsberg S. Leukotriene inhibition in small animal medicine. J Vet Pharmacol Ther. 2008;31:387-398.
259. Newcombe D.S. Leukotrienes: regulation of biosynthesis, metabolism, and bioactivity. J Clin Pharmacol. 1988;28:530-549.
260. Parkinson J.F. Lipoxin and synthetic Lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006;5:91-106.
261. Schmelzer R., Kubala L., Newman J.W., et al. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Nat Acad Sci. 2005;102(28):9772-9777.
262. Ding X.Z., Hennig R., Adrian T.E. Lipoxygenase and cyclooxygenase metabolism: new insights in treatment and chemoprevention of pancreatic cancer. Mol Cancer. 2003;2:10-22.
263. Daffonchio L., Rossoni G., Clavenna G., et al. Protective activity of ketoprofen lysine salt against the pulmonary effects induced by bradykinin in guinea-pigs. Inflammation Res. 1996;45(5):259-264.
264. Martel-Pelletier J., Lajeunesse D., Reboul P., et al. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501-509.
265. Jovanovic D.V., Fernandes J.C., Martel-Pelletier J., et al. In vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis: suppression of collagenase 1 and interleukin-1 b synthesis. Arthritis Rheum. 2000;44:2320-2330.
266. Agnello K.A., Reynolds L.R., Budsberg S.C. In vivo effects of tepoxalin, an inhibitor of cyclooxygenase and lipoxygenase, on prostanoid and leukotriene production in dogs with chronic osteoarthritis. Am J Vet Res. 2005;66(6):966-972.
267. Punke J.P., Speas A.L., Reynolds L.R., et al. Effects of firocoxib, meloxicam, and tepoxalin on prostanoid and leukotriene production by duodenal mucosa and other tissues of osteoarthritic dogs. Am J Vet Res. 2008;69(9):1203-1209.
268. Hall G. Personal communication, Technical Service Veterinarian. Schering-Plough. April, 2009.
269. Bosmans T., Gasthuys F., Duchateau L., et al. A comparison of tepoxalin-buprenorphine combination and buprenorphine for postoperative analgesia in dogs: a clinical study,. J Vet Med A Physiol Pathol Clin Med. 2007;54(7):364-369.
269a. Gupat A., Kumar A., Kulkarni S. Licofelone attenuates MPTP-induced neuronal toxicity: behavioral, biochemical and cellular evidence. Inflammopharmacology. 2010;18:223-232.
270. Undem B.J. Pharmacotherapy of asthma. In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill, pp 717–736, 2006.
271. Serafin W.E. Drugs used in the treatment of asthma. In: Hardman J.G., Limbird L.D., editors. Goodman and Gilman’s the pharmacological basis of therapeutics. ed 9. New York: McGraw-Hill; 1996:659-682.
272. Ambrus J.L., Anain J.M., Anain S.M., et al. Dose-response effects of pentoxifylline on erythrocyte filterability: clinical and animal model studies. Clin Pharmacol Ther. 1990;48:50-56.
273. Ambrus J.L., Stadler S., Kulaylat M. Hemorrheologic effects of metabolites of pentoxifylline (Trental). J Med. 1995;26:65-75.
274. Zargari O. Pentoxifylline: a drug with wide spectrum applications in dermatology. Dermatology Online Journal. 14. 11. 2008. 2. http://dermatology.cdlib.org/1411/reviews/pentoxy/zargari.html. Accessed February 12, 2009, at(1 of 10)
275. Rees C., Boothe D.M., Boeckh A., et al. Dosing regimen and hemotologic effects of pentoxifylline and its active metabolites in normal dogs. Vet Ther. 2003;4(2):188-196.
276. Quezado Z.M.N., Hoffman W.D., Banks S.M. Increasing doses of pentoxifylline as a continuous infusion in canine septic shock. J Pharmacol Experiment Therapeut. 1999;288:107-113.
277. Apaydin B.B., Paksoy M., Arb T., et al. Influence of pentoxifylline and interferon-alpha on prevention of stricture due to corrosive esphagitis. Eur Surg Res. 2001;33:225-231.
278. Skidgel R.A., Erdös E.G. Histamine, bradykinin, and their antagonists. In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill, pp 629–651, 2006.
278a. Moy A.B., Winter M., Kamath A., et al. Histamine alters endothelial barrier function at cell-cell and cell-matrix sites. Am J Physiol Lung Cell Mol Physiol. 2000;278(5):L888-L898.
279. Obradovic T., Dobson G.G., Shingaki T., et al. Assessment of the first and second generation antihistamines brain penetration and role of P-glycoprotein. Pharm Res. 2007;24(2):318-327.
280. Hansson H., Bergvall K., Bondesson U., et al. Clinical pharmacology of clemastine in healthy dogs. Vet Dermatol. 2004;15(3):152-158.
281. Bizikova P., Papich M.G., Olivry T. Hydroxyzine and cetirizine pharmacokinetics and pharmacodynamics after oral and intravenous administration of hydroxyzine to healthy dogs. Vet Dermatol. 2008;19(6):348-357.
282. Papich M.G., Schooley E.K., Reinero C.R. Pharmacokinetics of cetirizine in healthy cats. Am J Vet Res. 2008;69(5):670-674.
283. Devillier P. Comparing the new antihistamines: the role of pharmacological parameters. Clin Exp Allergy. 2006;36:5-7.
284. Cook C.P., Scott D.W., Miller W.H.Jr., et al. Treatment of canine atopic dermatitis with cetirizine, a second generation antihistamine: a single-blinded, placebo-controlled study. Can Vet J. 2004;45(5):414-417.
285. DeBoer D.J., Griffin C.E. The ACVD task force on canine atopic dermatitis XXI: antihistamine pharmacotherapy,. Vet Immunol Immunopathol. 2001;81:323-329.
286. Scott D.W., Miller W.H.Jr. Antihistamines in the management of allergic pruritus in dogs and cats. J Small Anim Pract. 1999;40:359-364.
287. Fajardo M., Di Cesare P.E. Disease-modifying therapies for osteoarthritis: current status. Drugs Aging. 2005;22(2):141-161.
288. Gardner D.L. The nature and causes of osteoarthrosis,. Br Med J. 1983;286:418-424.
289. Jones A.C., Doherty M. The treatment of osteoarthritis. Br J Clin Pharmacol. 1992;33:357-363.
290. Vaughan-Scott T., Taylor J.H. The pathophysiology and medical management of canine osteoarthritis. J S Afr Vet Assoc. 1997;68(1):21-25.
291. Kelly G.S. The role of glucosamine sulfate and chondroitin sulfates in the treatment of degenerative joint disease,. Altern Med Rev. 1998;3(1):27-39.
292. Pelletier J.P., DiBattista J.A., Roughley P., et al. Cytokines and inflammation in cartilage degradation. Rheum Dis Clin North Am. 1993;19:545-568.
293. Pelletier J.P., Mineau F., Ranger P., et al. The increased synthesis of nitric oxide induced by IL-1 in human chondrocytes markedly reduced the synthesis of IL-1 receptor antagonists: a possible role in osteoarthritic cartilage degradation,. Osteoarthritis Cartilage. 1996;4(1):77-84.
294. Mitchell P.G., Yocum S.A., Lopresti L.L., et al. Collagenase 1 and collagenase 3 expression in IL 1 treated cartilage explants: regulation of net activity by endogenous produced inhibitors. Trans ORS. 459, 1997.
295. Singer I.I., Scott S., Kawka D.W., et al. Aggrecanase and metalloproteinase specific aggrecan neo-epitopes are induced in the articular cartilage of mice with collagen 11 arthritis. Osteoarthritis Cartilage. 1997;5(6):407-418.
296. Pinals R.S. Pharmacologic treatment of osteoarthritis. Clin Ther. 1992;14:336-346.
297. Altman R.D., Kapila P., Dean D.D., et al. Future therapeutic trends in osteoarthritis. Scand J Rheumatol Suppl. 1989;77:37-42.
298. Kelly G.S. The role of glucosamine sulfate and chondroitin sulfates in the treatment of degenerative joint disease,. Altern Med Rev. 1998;3(1):27-39.
299. White G.W. Adequan: a review for the practicing veterinarian. Vet Rev. 1988;8:463-467.
300. Francis D.J., Forrest M.J., Brooks P.M., et al. Retardation of articular cartilage degradation by glycosaminoglycan polysulfate, pentosan polysulfate, and DH-40J in the rat air pouch model. Arthritis Rheum. 1989;32:608-616.
301. Hannan N., Ghosh P., Bellenger C., et al. Systemic administration of glycosaminoglycan polysulphate (arteparon) provides partial protection of articular cartilage from damage produced by meniscectomy in the canine. J Orthop Res. 1987;5:47-59.
302. Nethery A., Giles I., Jenkins K., et al. The chondroprotective drugs, arteparon and sodium pentosan polysulphate, increase collagenase activity and inhibit stromelysin activity in vitro,. Biochem Pharmacol. 1992;44:1549-1553.
303. Halverson P.B., Cheung H.S., Struve J., et al. Suppression of active collagenase from calcified lapine synovium by arteparon. J Rheumatol. 1987;14:1013-1017.
304. Rao N.V., Kennedy T.P., Rao G., et al. Sulfated polysaccharides prevent human leukocyte elastase-induced acute lung injury and emphysema in hamsters. Am Rev Respir Dis. 1990;142:407-412.
305. Montefiori D.C., Robinson W.E., Modliszewski A., et al. Differential inhibition of HIV-1 cell binding and HIV-1-induced syncytium formation by low molecular weight sulphated polysaccharides. J Antimicrob Chemother. 1990;25:313-318.
306. Biffoni M., Paroli E. Complement in vitro inhibition by a low sulfate chondroitin sulfate (matrix). Drugs Exp Clin Res. 1991;27:35-39.
307. Arsenis C., McDonnell J. Effects of antirheumatic drugs on the interleukin-1a induced synthesis and activation of proteinases in articular cartilage explants in culture. Agents Actions. 1989;27:261-264.
308. Greinacher A., Michels I., Schäfer M., et al. Heparin associated thrombocytopenia in a patient treated with polysulphated chondroitin sulphate: evidence for immunological crossreactivity between heparin andpolysulphated glycosaminoglycan. Br J Haematol. 1992;81:252-254.
309. Jepsen J.V., Sall M., Rhodes P.R., et al. Long-term experience with pentosan polysulfate in interstitial cystitis. Urology. 1998;51(3):381-387.
310. Barrington J.W., Stephenson T.P. Pentosan polysulphate for interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(5):293-295.
311. Ghosh P., Hutadilok N. Interactions of pentosan polysulfate with cartilage matrix proteins and synovial fibroblasts derived from patients with osteoarthritis. Osteoarthritis Cartilage. 1996;4(1):43-53.
312. Read R.A., Cullis-Hill D., Jones M.P. Systemic use of pentosan polysulphate in the treatment of osteoarthritis. J Small Anim Pract. 1996;37(3):108-114.
313. Budsberg S.C., Bergh M.S., Reynolds L.R., et al. Evaluation of pentosan polysulfate sodium in the postoperative recovery from cranial cruciate injury in dogs: a randomized, placebo-controlled clinical trial. Vet Surg. 2007;36(3):234-244.
314. Hannon R.L., Smith J.G., Cullis-Hill D., et al. Safety of Cartrophen Vet in the dog: review of adverse reaction reports in the UK2003. J Small Anim Pract. 2003:202-208.
315. Elliot S.J., Striker L.J., Stetler W.G., et al. Pentosan polysulfate decreases proliferation and net extracellular matrix production in mouse mesangial cells. J Am Soc Nephrol. 1999;10:62-68.
316. Striker G.E., Lupia E., Elliot S., et al. Glomerulosclerosis, arteriosclerosis, and vascular graft stenosis: treatment with oral heparinoids. Kidney Int Suppl. 1997;63:S120-S123.
317. Senthil D., Malini M.M., Varalakshmi P. Sodium pentosan polysulphate—a novel inhibitor of urinary risk factors and enzymes in experimental urolithiatic rats. Ren Fail. 1998;20(4):573-580.
318. Schwartsmann G., Sprinz E., Kalakun L., et al. Phase II study of pentosan polysulfate (PPS) in patients with AIDS-related Kaposi’s sarcoma. Tumori. 1996;82(4):360-363.
319. Suzuki Y., Yamaguchi T. Effects of hyaluronic acid on macrophage phagocytosis and active oxygen release. Agents Actions. 1993;38(1-2):32-37.
320. Asheim A., Lindblad G. Intra-articular treatment of arthritis in race-horses with sodium hyaluronate. Acta Vet Scand. 1976;17:379-394.
321. Campos J.F.A. Efficacy of sodium hyaluronate in the treatment of hip dysplasia in dogs. Ahora Vet. 1998;17:59-61.
322. Fajardo M., Di Cesare P.E. Disease-modifying therapies for osteoarthritis; current status. Drugs Aging. 2006;22(2):141-161.
323. Anderson M. Oral chondroprotective agents, part I: common drugs used today. Compend Contin Educ Small Anim Pract. 1999;21(7):601-609.
324. Anderson M. Oral chondroprotective agents, Part II: evaluation of products and future compounds. Compend Contin Educ Small Anim Pract. 1999;21(9):861-865.
325. Boothe D.M. Nutraceuticals in veterinary medicine: Part I. Definitions and regulatory considerations. Compend Contin Educ Pract Vet. 1997;19:1248-1255.
326. Boothe D.M. Nutracueticals in veterinary medicine: Part II. Evaluating safety and efficacy. Compend Contin Educ Pract Vet. 1997;20:15-21.
327. Deal C.L., Moskowitz R.W. Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate, and collagen hydrolysate. Rheum Dis Clin North Am. 1999;25(2):379-395.
328. American Association of Feed Control Officials (AAFCO). Accessed November 9, 2009, at www.aafco.org.
329. National Animal Supplement Council (NASC). Accessed November 9, 2009, at www.nasc.cc.
329a. Adebowale A.O., Cox D.S., Liang Z., et al. Analysis of glucosamine and chondroitin sulfate content in marketed products and the Caco-2 permeability of chondroitin sulfate raw materials. J Am Nutr Assoc. 2000;3:37-44.
329b. Russell A.S., Aghazadeh-Habashi A., Jamali F. Active ingredient consistency of commercially available glucosamine sulfate products. J Rheumatol. 2002;29(11):2407-2409.
330. ConsumerLab. Accessed November 9, 2009, at www.Consumerlabs.com.
331. Adebowale A., Du J., Liang Z., et al. The bioavailability and pharmacokinetics of glucosamine hydrochloride and low molecular weight chondroitin sulfate after single and multiple doses to beagle dogs. Biopharm Drug Dispos. 2002;23(6):217-225.
332. Hanson R.R., Smalley L.R., Huff G.K., et al. Treatment with an oral glucosamine-chondroitin sulfate compound for degenerative joint disease in horses: 25 cases. Proc Vet Orthoped Soc. 1996;24:5.
333. Hanson R.R. Oral glycosaminoglycans in treatment of degenerative joint diseases in horses. Equine Pract. 1997;18:18-22.
334. White G.W., Sanders T., Jones E.W., et al. Efficacy of an orally administered sulfated glycosaminoglycan supplement in an induced equine carpitis model. Proc Am Assoc Equine Practitioners. 1996;42:139-141.
335. Anderson M.A., Slater M.R., Hammad T.A. Results of a survey of small-animal practitioners on the perceived clinical efficacy and safety of an oral nutraceutical. Prev Vet Med. 1999;38:65-73.
336. Bucci L.R. Chondroprotective agents: glucosamine salts and chondroitin sulfates. Townsend Letters for Doctors. 1994:52-55. January
337. McNamara P.S., Barr S.C., Idouaraine A., et al. Effects of an oral chondroprotective agent (Cosequin) on cartilage metabolism and canine serum. Proc Vet Orthoped Soc. 1997;24:35.
338. Phillipi A.F. Glucosamine, chondroitin and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Mil Med. 1999;164:85-91.
339. McCarty M.F. Enhanced synovial production of hyaluronic acid may explain rapid clinical response to high-dose glucosamine in osteoarthritis. Med Hypoth. 1998;50(6):507-510.
340. Barclay T.S., Tsourounis C., McCart G.M. Glucosamine. Ann Pharmacother. 1998;32(5):574-579.
341. McNamara P.S., Barr S.C., Hollis N.E. Hematologic, hemostatic and biochemical effects in dogs receiving an oral chondroprotective agent for thirty days. Am J Vet Res. 1996;57:1390-1394.
342. Neil K.M., Caron J.P., Orth M.W. The role of glucosamine and chondroitin sulfate in treatment for prevention of osteoarthritis in animals. J Am Vet Med Asoc. 2005;226:1079-1089.
342a. Reginster J.Y., Bruyere 0, Neuprez A. Current role of glucosamine in the treatment of osteoarthritis. Rheumatology. 2007;46(5):730-735.
343. Anderson J.W., Nicolosi R.J., Borzelleca J.F. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol. 2005;43(2):187-201.
344. Setnikar I., Rovati L.C. Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review. Arzneimittelforschung. 2001;51(9):699-725.
345. Setnikar I., Giacchetti C., Zanolo G. Pharmacokinetics of glucosamine in the dog and man. Pharmatherapeutica. 1984;3:538-549.
346. McNamara P.S., Barr S.C., Erb H.N., et al. Hematological, hemostatic, and biochemical effects in cats receiving an oral chondroprotective agent for thirty days. Vet Ther. 2000;1(2):108-117.
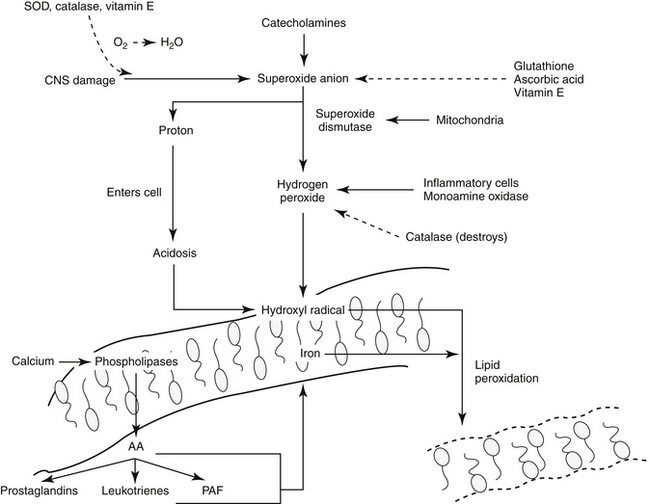
347. Volpi N. Oral bioavailability of chondroitin sulfate (Condrosulf) and its constituents in healthy male volunteers. Osteoarthritis Cartilage. 2002;10(10):768.
348. Echard B.W., Talpur N.A., Funk K.A., et al. Effects of oral glucosamine and chondroitin sulfate alone and in combination on the metabolism of SHR and SD rats. Mol Cell Biochem. 2001;225(1-):85-91.
348a. Scholtissen S., Bruyère O., Neuprez A., et al. Glucosamine sulphate in the treatment of knee osteoarthritis: cost-effectiveness comparison with paracetamol. Int J Clin Pract. 2010;64(6):756-762.
349. Scroggie D.A., Albright A., Harris M.D. The effect of glucosamine-chondroitin supplementation on glycosylated hemoglobin levels in patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomized clinical trial,. Arch Intern Med. 2003;163(13):1587-1590.
350. Hoffer L.J., Kaplan L.N., Hamadeh M.J., et al. Sulfate could mediate the therapeutic effect of glucosamine sulfate. Metabolism. 2001;50(7):767-770.
351. Reginster J.Y., Deroisy R., Rovati L.C., et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357(9252):251-256.
352. Bruyere O., Pavelka K., Rovati L.C., et al. Glucosamine sulfate reduces osteoarthritis progression in postmenopausal women with knee osteoarthritis: evidence from two 3-year studies. Menopause. 2004;11(2):138-143.
353. Ruane R., Griffiths P. Glucosamine therapy compared to ibuprofen for joint pain. Br J Community Nurs. 2002;7(3):148-152.
354. Conte A., Volpi N., Palmieri L. Biochemical and pharmacokinetic aspects of oral treatment with chondroitin sulfate. Arzneimittelforschung. 1995;45(8):918-925.
355. Gregory S., Kelly N.D. The role of glucosamine sulfate and chondroitin sulfates in the treatment of degenerative joint disease. Altern Med Rev. 1998;3:27-39.
356. Leffler C.T., Philippi A.F., Leffler S.G., et al. Glucosamine, chondroitin and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Mil Med. 1999;164(2):85-91.
357. Das A.K., Hammad T.A. Efficacy of a combination of FCHG49 glucosamine hydrochloride, TRHI22 low molecular weight sodium chondroitin sulfate and manganese ascorbate in the management of knee osteoarthritis. Osteoarthritis Cartilage. 2000;8(5):343-350.
358. Leffler C.T., Philippi A.F., Leffler S.G., et al. Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Mil Med. 1999;164(2):85-91.
359. McAlindon T., LaValley M., Gulin J., et al. Glucosamine and chondroitin for treatment of osteoarthritis - a systematic quality assessment and meta-analysis. J Am Med Assoc. 2000;283(11):1469-1475.
360. Fajardo M., Di Cesare P.E. Disease-modifying therapies for osteoarthritis: current status. Drugs Aging. 2005;22(2):141-161.
361. Clegg D.O., Reda D.J., Harris C.L., et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795-808.
362. Lippiello L., Idouraine A., McNamara P.S., et al. Cartilage stimulatory and antiproteolytic activity is present in sera of dogs treated with a chondroprotective agent. Canine Pract. 1999;24(1):18-19.
363. Canapp S.O., McLaughlin R.M., Hoskinson J.J., et al. Scintigraphic evaluation of glucosamine HCl and chondroitin sulfate as treatment for acute synovitis in dogs. Am J Vet Res. 1999;60(12):1552-1557.
364. Ameye L.G., Chee W.S.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidence. Arthritis Res Ther. 2006;8:R127.
365. Lippiello L., Nardo J.V., Harlan R., et al. Metabolic effects of avocado/soy unsaponifiables on articular chondrocytes. Evid Based Complement Alternat Med. 2008;5(2):191-197.
366. Soeken K.L., Lee W.-L., Bausell R.B., et al. Safety and efficacy of S-adenosylmethionine (SAMe) for osteoarthritis. J Family Pract. 2002;51:425-430.
367. Romay Ch, Gonzalez R., Ledon N., et al. C-Phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 2003;4:207-216.
368. Reddy C.M., Bhat V.B., Kiranmai G., et al. Selective inhibition of cyclooxygenase-2 by C-Phycocyanin, a biliprotein from Spirulina platensis. Biochem Biophys Res Commun. 2000;277:599-603.
369. Salin M., McCord J.M. Free radicals and inflammation: protection of phagocytosing leukocytes by superoxide dismutase. J Clin Invest. 1975;56:1319-1323.
370. Tobin T. Pharmacology review: the nonsteroidal anti-inflammatory drugs. II. Equiproxen, meclofenamic acid, flunixin and others. J Equine Med Surg. 1979;6:298-302.
371. Breshears D.E., Brown C.D., Riffel D.M., et al. Evaluation of orgotein in treatment of locomotor dysfunction in dogs. Mod Vet Pract. 1974;55:85-93.
372. Ahlengard S., Tufvesson G., Pettersson H., et al. Treatment of traumatic arthritis in the horse with intra-articular orgotein (Palosein). Equine Vet J. 1978;10:122-124.
373. Brayton C.F. Dimethyl sulfoxide (DMSO). Cornell Vet. 1986;76:61-90.
374. Alsup E.M. Dimethyl sulfoxide. J Am Vet Med Assoc. 1984;185:1011-1014.
375. Wong L.K., Reinertson E.L. Clinical considerations of dimethyl sulfoxide. Iowa State Univ Vet. 1984;46:89-95.
376. Spitzer W.O. Drugs as determinants of health and disease in the population. J Clin Epidemiol. 1991;44:823-830.
377. Olson N.C., Hellyer P.W., Dodam J.R. Mediators and vascular effects in response to endotoxin. Br Vet J. 1995;151:489-522.
378. Whittle B.J.R. Nitric oxide in physiology and pathology. Histochem J. 1995;27:727-737.
379. Howe L.B. Treatment of endotoxic shock: glucocorticoids, lazaroids, nonsteroidals and others. Vet Clin North Am. 1998;28:249-267.
380. Vincent J.L. Evidence-based medicine in the ICU: important advances and limitations. Chest. 2004;126:592-600.
381. Minneci P., Deans K., Natanson C., et al. Increasing the efficacy of anti-inflammatory agents used in the treatment of sepsis. Eur J Clin Microbiol Infect Dis. 2003;22:1-9.
382. Kettelhut I.C., Fiers W., Goldgerg A.L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors,. Proc Natl Acad Sci USA. 1987;84:4273.
383. Shuster R., Traub-Dargatz J., Baxter G. Survey of diplomates of the American College of Veterinary Internal Medicine and the American College of Veterinary Surgeons regarding clinical aspects and treatment of endotoxemia in horses. J Am Vet Med Assoc. 1997;210:87-92.
384. Sigurdsson G.H., Youssef H. Amelioration of respiratory and circulatory changes in established endotoxic shock by ketoprofen. Acta Anesthesiol Scand. 1994;38:33-39.
385. Boumpas D.T., Chrousos G.P., Wilder R.L., et al. Glucocorticoid therapy for immune-mediated diseases. Basic and clinical correlates. Ann Intern Med. 1993;119:1198-1208.
386. Bone R.C., Fisher C.J., Clemmer T.P., et al. A controlled clinical trial of high dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653-658.
387. Aoki N., Lefer A.M. Protective effects of a novel non-glucocorticoid 21 aminosteroid (U74006F) during traumatic shock in rats. J Cardiovasc Pharmacol. 1990;15:205-210.
388. Hall E.D., Yonkers P.A., McCall J.M. Attenuation of hemorrhagic shock by the non-glucocorticoid 21-aminosteroid U74006F. Eur J Pharmacol. 1988;147:299-303.
389. Semrad S.D., Rose M., Putnam M.L. Efficacy and toxicity of lazaroid (U74006F) in neonatal endotoxemia. Circ Shock. 1989;27:358-359.
390. Semrad S.D., Rose M.L., Adams J.L. Effects of tirilazad mesylate (U74006F) on eicosanoid and tumor necrosis factor generation in healthy and endotoxic neonatal calves. Circ Shock. 1993;40:235-242.
391. Zhang H., Spapen H., Minikis P., et al. Tirilazad mesylate (U-74006F) inhibits effects of endotoxin in dogs. Am J Physiol. 1995;286:H1847-H1855.
392. Morris P.E., Light R.B., Garber G.E. Identifying patients with severe sepsis who should not be treated with drotrecogin alfa (activated). Am J Surg. 2002;184:19S-24S.
393. Kavanagh R.J., Kam P.C. Lazaroids: efficacy and mechanism of action of the 21-aminosteroids in neuroprotection. Br J Anaesth. 2001;86(1):110-119.