22 The Pelvis and Reproductive Organs of the Horse
This chapter is concerned with the pelvic cavity and its contents and with the extrapelvic parts of the reproductive organs of both sexes. It also includes a brief account of the udder. The general conformation of the region and the surface landmarks created by the pelvic skeleton are dealt with in Chapter 24.
GENERAL ANATOMY OF THE PELVIS AND PERINEUM
The pelvic cavity is roofed by the sacrum and first two or three caudal vertebrae; it is impossible to be more specific because an arbitrary element exists in the definition of the outlet. The roof narrows from front to back and is slightly concave in its length. The ischial tuber and spine are both less prominent than in cattle, and the contribution of the substantial sacrosciatic ligament to the lateral wall is therefore relatively greater (Figure 22–1/7). The floor is solid because the symphysis is firmly fused in mature animals. It is more or less horizontal and flat in its length, though somewhat hollowed from side to side. The pubic region presents a median swelling or ridge in young animals, and it retains this conformation in the stallion; however, the bone thins and the upper surface becomes markedly excavated in mares, especially those that have carried several foals.

Figure 22–1 Lateral view of the bony pelvis and sacrosciatic ligament.
1, Coxal tuber; 2, sacral tuber; 3, lateral border of sacrum; 4, Cd1; 5, ischial tuber; 6, caudal part of greater trochanter; 7, sacrosciatic ligament; 8, dorsal sacroiliac ligament; 9, greater sciatic foramen; 10, lesser sciatic foramen; 11, gluteus profundus; 12, cranial gluteal nerve; 13, sciatic nerve; 13′, common peroneal nerve; 13″, tibial nerve; 14, caudal gluteal nerve; 15, caudal cutaneous femoral nerve; 16, pudendal nerve; 17, trochanteric bursa.
The entrance to the pelvic cavity faces cranioventrally; its slope places the pubic brim below the third, or even fourth, sacral vertebra in the mare but only the second in the stallion. Viewed from the front, the inlet to the female pelvis is wide and rounded while that of the male is more angular and cramped, particularly ventrally (Figure 22–2, B). In both sexes, the outlet from the cavity is much smaller than the inlet; it is bounded by a caudal vertebra, the free edges of the sacrosciatic ligaments, and the ischial tubers and arch.

Figure 22–2 Cranial view of the pelvis of the mare (A), stallion (B), and cow (C). The terminal line is emphasized in the smaller pictures; observe the differences in the shape of the pelvic inlet and position of the ischial spines.
1, Coxal tuber; 2, sacral tuber; 3, wing of ilium; 4, promontory; 5, shaft of ilium; 6, acetabulum; 7, brim of pubis; 8, ischial spines; 9, ischial tuber.
The cavity has the approximate form of a truncated cone, and the longitudinal axis is almost straight between the entrance and the exit (Figure 22–3). The pelvis of the mare is thus more favorably formed for ease of parturition than that of the cow: the entrance is wide, the exit less confined, the cavity generally more capacious, the axis without marked deflection, and a greater part of the lateral walls composed of soft tissue.

Figure 22–3 Schematic median section of the mare’s pelvis illustrating certain obstetrical terms.
1, Promontory; 2, cranial end of the pelvic symphysis; 3, conjugata; 4, vertical diameter; 5, diagonal conjugata. The arrow indicates the axis of the pelvic canal.
The reader is referred to page 43 for a general account of the structure of the pelvis and to Figures 22–8 and 22–19 for an indication of the topography and peritoneal relationships of the viscera.
The most distinctive feature of the perineum is its confinement between the semimembranosus muscles, which extend ventrally from their vertebral heads of origin. These muscles cover the ischial tubers and also the ischiorectal fossae, which therefore do not contribute to the surface contour. Since the muscles bury the caudal borders of the sacrosciatic ligaments, they hamper recognition of the softening that is so useful an indication of the approach of parturition in cattle.
The thin, sparsely haired, and deeply pigmented perineal skin obtains a surface sheen from the secretion of sebaceous glands. It is raised over the caudal part of the anal canal, forming a projection whose shape and salience vary with the functional state. The unusual outline of the vulva and its variable position are the subject of a later comment (p. 570). In the male the urethra may be palpated where it bends around the ischial arch.
The deeper structures of the perineum closely resemble their bovine counterparts, to which reference may be made (p. 700); differences in detail, though numerous, are not of practical significance.
INNERVATION, VASCULARIZATION, AND LYMPH DRAINAGE OF THE PELVIC WALLS
The branches of the lumbosacral plexus that traverse the pelvis are considered at length on page 323, and only a few features are mentioned here. The obturator nerve follows the usual course over the medial aspect of the shaft of the ilium to reach the obturator foramen, and this exposes it to risk of injury in fractures of the bone or by compression when the mare is giving birth (Figure 22–4/15). The nervous web from which the cranial gluteal, sciatic, and caudal gluteal nerves arise is exposed to similar risk where it lies against the ventral aspect of the sacrum, en route to the greater sciatic foramen (Figure 22–4/13).
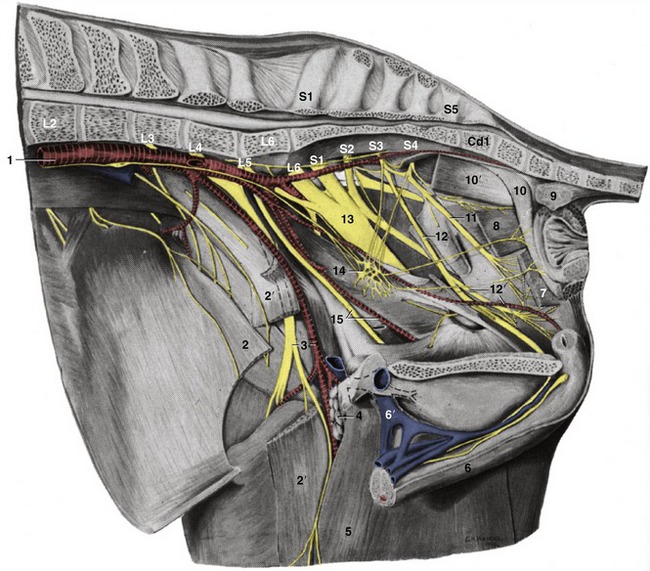
Figure 22–4 Dissection of the pelvic wall; medial view.
1, Aorta; 2, internal abdominal oblique; 2′, sartorius, resected; 3, femoral a. and n.; 4, deep inguinal lymph nodes; 5, gracilis; 6, penis; 6′, (accessory) external pudendal v.; 7, levator ani, resected; 8, coccygeus; 9, rectococcygeus; 10, retractor penis; 10′, ventral tail muscle; 11, caudal rectal n.; 12, pudendal n.; 12′, deep perineal n. and internal pudendal a.; 13, sciatic n.; 14, pelvic plexus; 15, obturator n. and vessels.
The pudendal nerve (Figure 22–4/12) arises from the middle sacral nerves (S[2]3–4) and heads in the direction of the ischial tuber. The nerve first runs internal to the sacrosciatic ligament but later becomes embedded within its substance. As the nerve passes the lesser sciatic foramen, it exchanges fibers with the caudal cutaneous nerve of the thigh through the opening. It later splits into several branches of which the most important is the deep perineal nerve (Figure 22–4/12′). The main trunk continues to the clitoris or penis. The deep perineal is concerned with the innervation of the striated musculature of the perineum. The superficial branch is sensory to the anus, vulva, and perineal skin as far ventrally as the udder (or scrotum and prepuce).
The caudal rectal nerve (Figure 22–4/11), which arises from the same sacral nerves (S[2]3–4), is motor to the striated muscles of the dorsal part of the perineum and sensory to the rectum, the wall of the anal canal, and adjacent skin.
The pelvic nerves (Figure 22–4/14) are deployed in the usual fashion and are composed of parasympathetic fibers from the second, third, and fourth sacral nerves.
The blood supply to the pelvic contents and walls is attended to by the internal iliac arteries, terminal branches of the abdominal aorta (see Figure 22–4). The very short internal iliac artery passes below the wing of the ilium and soon divides into internal pudendal and caudal gluteal arteries. The internal pudendal artery has a mainly visceral distribution. It runs caudoventrally on the deep face of the sacrosciatic ligament, close to the pudendal nerve, before swinging medially to divide about the level of the ischial spine. Its branches include the umbilical artery, which conveys a little blood to the vertex of the bladder (and the adjacent part of the deferent duct in the male), and a much more important branch that supplies the bulk of the intrapelvic reproductive organs. This is known as the vaginal artery in the female, in which it supplies the greater part of the bladder, the urethra, the caudal part of the uterus, the vagina, and, by way of the middle rectal artery, a substantial part of the rectum. The homologous prostatic artery supplies the bladder, the urethra, the accessory genital glands, and the corresponding part of the rectum. End branches of the internal pudendal (Figure 22–4/12′) include the caudal rectal artery to the rectum and anus, a (ventral) perineal artery for the tissues between the anus and vulva, and branches to the vestibule and the vestibular bulb; the male counterpart of the last-named is the artery of the penis, which anastomoses with divisions of the obturator.
The caudal gluteal artery passes caudally in the dorsolateral wall of the pelvis; it branches off the obturator and cranial gluteal arteries. The trunk pierces the sacrosciatic ligament before supplying the hamstring muscles and the tail. The obturator artery leaves the pelvis through the obturator foramen, and the cranial gluteal artery exits through the greater sciatic foramen.
The veins largely mirror the patterns of the arteries.
The lymph nodes associated with the pelvic walls display the usual species characteristics, comprising numerous, closely packed, and individually small nodes that aggregate to form sizable masses. The major groupings are related to the termination and parietal branches of the aorta. Sacral nodes lie between the divergent internal iliac arteries, medial iliac nodes lie at the origin (from the external iliac) of the deep circumflex iliac arteries, and lateral iliac nodes lie at the terminal division of the latter.
Other (anorectal) nodes lie over the caudal part of the rectum. In the horse the deep inguinal nodes (Figure 22–4/4) lie outside the pelvic cavity, within the femoral triangle and at no great distance from the superficial inguinal nodes. The latter are interposed between the prepuce and scrotum (or udder) and the trunk. They drain lymph from the external reproductive organs (and udder) and from the skin and deeper structures over a considerable part of the ventral trunk. This lymph is then channeled to the deep inguinal nodes, which also receive most lymph from the hindlimb, of which a part has already been filtered through the nodes in the popliteal fossa. The outflow goes to the medial iliac nodes, which constitute the collecting center for lymph emanating from the caudal abdominal and pelvic walls and from the pelvic viscera. Much of this lymph has already passed through anorectal, sacral, or lateral iliac nodes. The outflow is either to the aortic lumbar nodes of the abdominal roof or directly to an erratically formed lumbar trunk.
THE RECTUM AND ANAL CANAL
The principal features of visceral topography and peritoneal disposition are shown in Figures 22–5, 22–6, and 22–7.

Figure 22–5 Schematic median section of the pelvis of the mare.
1, 1′, Peritoneal and retroperitoneal parts of the rectum; 2, anal canal; 3, uterus; 4, cervix; 5, vagina; 6, vestibule; 7, bladder; 8, urethra; 9, caudal extent of peritoneum.

Figure 22–6 The disposition of the peritoneum in the pelvis of the mare (transverse section).
1, Rectum; 2, vagina; 3, bladder; 4, parietal peritoneum; 5, broad ligament; 6, lateral ligament of bladder; 7, median ligament of bladder; 8, rectogenital pouch; 8′, pararectal fossa; 9, vesicogenital pouch; 10, pubovesical pouch; 11, ureter.

Figure 22–7 The disposition of the peritoneum in the pelvis of the stallion (transverse section).
1, Rectum; 2, deferent duct; 3, ureter; 4, vesicular gland; 5, bladder; 6, genital fold; 7, lateral ligament of bladder; 8, median ligament of bladder; 9, rectogenital pouch; 9′, pararectal fossa; 10, vesicogenital pouch; 11, pubovesical pouch.
The rectum continues the descending colon beyond the pelvic inlet. Initially it resembles the colon in structure and in relationship to the peritoneum, but as it proceeds caudally the mesentery shortens and the peritoneal covering is gradually lost (commencing with the dorsal aspect); finally the rectum is wholly retroperitoneal and embedded in a fat-rich connective tissue. The proportion of the rectum that is retroperitoneal appears to vary between individuals and is relevant to the perforations of the wall of the rectum that are the unfortunate and highly embarrassing mishaps that occasionally complicate rectal exploration. The terminal part of the rectum loses the sacculated character and forms a wide flasklike expansion (ampulla) just before it joins the anal canal. The ampulla stores feces before evacuation. A change of lesser importance is the regrouping of the dorsal and lateral longitudinal muscles into bundles that break free, pass above the anus, and anchor the rectum to the fourth or fifth caudal vertebra; these bundles constitute the smooth rectococcygeus (Figure 22–4/9).
The relations of the rectum depend on its fullness and on the sex. In the mare, the rectum lies on the uterus and vagina unless, as often happens, these are displaced to one side and the rectum is enabled to make contact with the bladder. In male animals the ventral surface lies on the bladder, the urethra, and the accessory reproductive glands; the extents of the individual contacts depend on the state of the bladder and the development of the glands, which are naturally smaller in the gelding.
The anal canal continues the rectum but, unlike this, is generally empty of feces. It is closed by the apposition and interdigitation of longitudinal mucosal folds and by the contraction of the internal and external anal sphincters. The extent of the canal is sharply defined by anorectal and anocutaneous lines marking the limits of epithelial specialization. The canal is embraced by the pelvic diaphragm (Figure 22–4/7,8); the part caudal to the pelvic diaphragm projects as a cylindrical eminence within the perineal region.
THE BLADDER AND FEMALE URETHRA
The neck region of the bladder lies directly on the pelvic floor, and when the organ is fully contracted it forms a firm, globular swelling about the size of a clenched fist; it is so far withdrawn into the pelvic cavity that it is almost wholly retroperitoneal. As the bladder fills, it gradually assumes a more ovoid form and extends cranially over the abdominal wall.
The relations of the bladder depend on the degree of filling and on the sex. When empty, its vertex is generally in contact with the pelvic flexure of the colon, but as the bladder enlarges, the vertex and adjacent parts obtain a more extensive and more varied relationship to the intestine. In the mare the dorsal surface is in contact with the cranial part of the vagina, the cervix, a variable part of the body of the uterus, and sometimes the rectum (Figure 22–8). The corresponding relations in the male are the genital fold, the deferent ducts, the vesicular glands, the prostate, and the rectum.

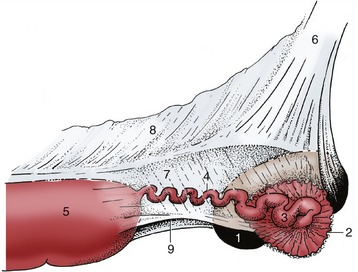
Figure 22–8 Caudal abdominal and pelvic organs of the mare in situ; the organs have been sectioned in a paramedian plane with the pelvis. Because of the absence of the intestines, the ovaries hang much lower than they would in the intact animal.
1, Sacrum; 2, Cd2; 3, floor of pelvis; 4, rectum; 5, anal canal; 6, cervix; 7, vaginal part of cervix; 8, vagina; 9, vestibule; 10, bladder; 11, urethra; 12, clitoris; 13, vulva; 14, left uterine horn; 15, uterine tube; 16, ovary; 17, broad ligament (largely cut away); 18, descending mesocolon; 19, left kidney.
The relatively large neonatal bladder is entirely intraabdominal. It slowly adjusts to the adult proportions and position with the postnatal enlargement of the pelvis and the development of the intestines. Leakage at the navel from a still-patent urachus is not uncommon in the first period after birth and provides a potential portal for infection.
The female urethra is very short (only 6 cm or thereabouts) and opens into the vestibule, immediately caudal to the transverse fold of the hymen. It is rather wide; it admits one finger without difficulty and by gentle manipulation may be persuaded, with low epidural anesthesia, to accept a small hand, which is convenient when returning a bladder prolapse or removing a kidney or bladder stone from the bladder. The shortness, wide caliber, and dilatable nature of the urethra permit occasional prolapse of the bladder into the vestibule.
THE FEMALE REPRODUCTIVE ORGANS
The anatomy of the female reproductive organs is strongly influenced by age, present status, and previous reproductive history. The initial description refers to the mature, parous but nongravid mare (Figure 22–9).

Figure 22–9 The female reproductive organs in relation to the pelvis, dorsal view.
1, Coxal tuber; 2, sacral tuber; 3, ischial tuber; 4, obturator foramen; 5, ovary; 6, uterine horn; 7, body of uterus; 8, cervix; 9, vagina; 10, vulva.
THE OVARIES
The ovaries scarcely descend from the sites where they develop initially, and they commonly lie in the dorsal part of the abdomen, cranioventral to the iliac wings, approximately in the plane of the fifth lumbar vertebra. Each is suspended by a thick mesovarium that allows the ovary considerable latitude in position (Figure 22–8/16). The length of the mesovarium is such that the ovary may generally be brought into, but not through, a flank incision.
In comparison with those of other species, the ovaries of the mare are conspicuously large; indeed, in a large draft mare they may measure as much as 8 to 10 cm along the major axis. They are also remarkable for their shape because the free border is deeply indented to form an “ovulation fossa,” the site of rupture of the mature follicles (Figure 22–10). The internal structure also shows a departure from the usual arrangement. The follicles and corpora lutea are scattered within the central part of the organ and toward the ovulation fossa. They are enclosed within a dense, richly vascularized connective tissue casing that corresponds to the medulla of the ovary of other species. Because of this, even large follicles and corpora lutea do not form prominent surface elevations, and their identification on rectal exploration is more difficult than in the cow. A change in hue marks the boundary between the covering of the fossa and the common peritoneum that clothes the remainder of the organ. The position, the form, the consistency, and the general absence of marked surface projections characterize the ovaries sufficiently to allow them to be easily recognized on rectal examination.

Figure 22–10 Sections of ovaries in various functional states. A, Ovary with corpora lutea and small follicles. B, Ovary with developing corpus luteum. C, Ovary with fully developed corpus luteum. D, Ovary with mature follicle. E, Ovary with follicles of various sizes and a rather large corpus luteum. The corpus luteum of the mare does not protrude from the ovary as in other species.
1, Corpora lutea; 2, follicles; 3, blood vessels; 4, ovulation fossa.
THE UTERINE TUBES
The uterine tube measures about 20 cm when extended but in nature follows a tortuous course that brings its beginning and end close together. The infundibulum is margined by ragged fimbriae that spread over the surface of the ovary where some make permanent attachment (Figure 22–11/2). A small opening in the depth of the infundibulum leads to the ampulla (Figure 22–11/3), which is approximately 10 cm long and about 6 mm wide; its caliber at all stages of the cycle is greater than that of the isthmus, which is only half as wide. The isthmus (Figure 22–11/4), also about 10 cm long, opens into the apex of the uterine horn through a small orifice on the summit of an eccentrically placed papilla. Strangely, this uterotubal junction is able in some way to distinguish between fertilized and infertile ova; the former are admitted to the uterus after the appropriate delay, but the latter are denied entry. The tubal mucosa is plicated, especially within the ampulla, where the elaborate major folds carry secondary and even tertiary ridges. The mesosalpinx, which supports the tube, branches from the lateral surface of the mesovarium and, with this, encloses a large but shallow ovarian bursa (Figure 22–11/9 and see Figure 5–60, B/5).
THE UTERUS
The uterus has a large body and two divergent horns. The horns, which are about 25 cm long, lie wholly within the abdomen and diverge sharply from each other. They are suspended from the abdominal roof by the broad ligaments, whose width varies such that the extremities of each horn are more tightly tethered than the intermediate part (Figure 22–8/14). However, in life, the horns are usually raised toward the abdominal roof on the mass of intestines. The body of the uterus is a little shorter (≈20 cm) than the horns and lies partly within the abdomen and partly within the pelvis. Although its relations vary, they always include the terminal part of the descending colon and rectum dorsally and the bladder and various parts of the gut ventrally. The body is often displaced to one side by a distended bladder or by pressure from the gut. When the uterus is empty, both horns and body are flattened and the lumen almost obliterated.
The cervix (Figure 22–8/6) is rather short (≈6 cm). Although its position and extent are not readily distinguishable on visual inspection, they are at once revealed on palpation as the cervix has a somewhat firmer consistency. The difference is less pronounced at estrus. The caudal part of the cervix projects into the lumen of the vagina, where it is surrounded by an annular space (fornix) of more or less uniform depth. This intravaginal part (Figure 22–8/7) has a lobed appearance created by the extension through the external ostium of the mucosal folds lining the cervical canal. These folds continue onto the vaginal wall, where they gradually subside. Except at estrus and parturition the cervical canal is closed; however, it will still admit a finger on gentle probing (Figure 22–12).
THE VAGINA
The vagina is about as long as the body of the uterus. It lies ventral to the rectum, dorsal to the bladder and urethra, and in lateral contact with the pelvic wall (Figures 22–8 and 22–13/8). Although it is largely retroperitoneal, the extent of the covering depends on the degrees of filling of the bladder and rectum (see Figure 22–5). A small cranial part of the ventral aspect and a somewhat larger part of the dorsal aspect are always clad in peritoneum. This arrangement is useful because the dorsal part of the vaginal fornix provides a convenient approach to the peritoneal cavity for various procedures, including the recovery of ova.



Figure 22–13 A-B, Dorsal view of the female reproductive organs. The dorsal wall of the caudal part of the tract has been opened in B. C, An enlargement of the vulva, shows the glans of the clitoris within the ventral commissure.
1, Right ovary; 1′, proper ligament of ovary; 2, uterine tube; 3, horn of uterus; 4, body of uterus; 5, cervix; 6, vaginal part of cervix; 7, fornix; 8, vagina; 9, vestibule; 9′, wall of vestibule; 10, vulva; 11, right labium; 12, glans of clitoris.
The vagina is thin walled, and although its lumen is normally closed by the dorsal and ventral walls falling together, the organ is remarkably distensible in length and circumference. The vaginal mucosa is ridged lengthwise, although the ridges are readily effaced on distention. The mucosa is normally pale pink but darkens when suffused with blood, as tends to happen on prolonged exposure to air during vaginoscopy. A transverse fold cranial to the opening of the urethra represents the remains of the hymen; although variable, it is generally more prominent than in other domestic species.
THE VESTIBULE AND VULVA
The dorsal wall of the vestibule only gradually departs from the line of the rectum and anal canal; the longer ventral wall slopes more steeply downward beyond the ischial arch (Figure 22–8/9). Noteworthy features are the urethral opening at the cranial limit and the clitoris within the ventral commissure of the vulva. The clitoris varies much in development and is largely covered by a transverse preputial fold that attaches to the dorsal surface of its glans (Figure 22–13, C/12). The fold and ventral commissure together constitute the prepuce. The clitoris is very prominent in mares in heat when exposed by “winking” movements of the labia. Laterally and ventrally it is separated from the labia by a clitoral fossa. Several sinuses of varying depth invade the glans. These may harbor the organism responsible for contagious equine metritis. Further mucosal recesses are present in the ventral parts of the clitoral fossa and labia. Although no major vestibular glands exist, numerous minor glands discharge within small depressions, ranked in ventral and dorsolateral rows. The mucosa overlying the vestibular bulb, situated in the lateral wall toward the vulva, is more darkly colored.
The vulva is unusual in having rounded ventral and pointed dorsal commissures, which is a reversal of the usual arrangement (Figure 22–14/3). The relationship of the vulva to the pelvic skeleton varies considerably. Usually it is largely ventral to the pelvic floor with the cleft closed. Sometimes, and quite commonly in Thoroughbreds, the opening is more dorsal and closure is less effective; in this circumstance, air may be drawn into or expelled from the tract with each change in intraabdominal pressure. Bacteria may be introduced, and the contamination may spread to the endometrium, which may result in sterility. The same fault (wind-sucking) may be due to laceration of the vulva at a previous parturition.
VASCULARIZATION AND INNERVATION
The reproductive organs are principally supplied by the ovarian, uterine, and vaginal arteries. The ovarian artery, a direct branch from the aorta, divides into uterine and ovarian branches. The ovarian branch pursues a tortuous course within the mesovarium before dividing into several branches that spread over the surface of the ovary; this contrasts with the arrangement in other species, in which the vessels penetrate the ovary immediately on arrival. The other branch passes to the cranial part of the horn. The corresponding vein is disproportionately large and drains much of the uterus in addition to the ovary. Little transfer of prostaglandins from venous to arterial blood occurs in the mare, which is a fact that may be correlated with the less intimate relationship of the ovarian artery and vein than exists in many other species.
The uterine artery, a branch of the external iliac, is the foremost supply to the uterus. It divides into several branches within the broad ligament, and these approach the mesometrial border of the horn and body separately. The antimesometrial aspect is reached only by small vessels, thus lending itself to relatively bloodless incision. Anastomoses with branches of the ovarian and vaginal arteries are present.
The vaginal artery takes origin from the internal pudendal in common with the middle rectal artery. It passes through the retroperitoneal tissue lateral to the vagina before bending forward to divide and supply the larger part of the vagina, the cervix, the caudal part of the body of the uterus, the bladder, and the urethra. The remaining part of the vagina and the vestibule are supplied from the vestibular branch of the internal pudendal artery.
The veins draining the genital organs are satellite to the arteries. The innervation displays no noteworthy special features.
GROWTH AND CYCLICAL CHANGES IN THE REPRODUCTIVE ORGANS
At midgestation the fetal ovaries are much larger than those of the dam but they later regress; by birth they are reduced to one tenth of their greatest fetal size. They then grow slowly until puberty when a sudden spurt occurs. The first estrus is generally at the beginning of a breeding season, and the age at which it occurs therefore varies with the date of the individual’s birth as well as with breed and nutrition. It usually occurs sometime between the 18th and 27th months. The neonatal ovary is ellipsoidal; the peculiar indented adult form develops during the first 2 or 3 years (Figure 22–15). In the mature ovary the larger follicles are concentrated near the ovulation fossa to which they migrate as they enlarge (Figure 22–10/2). Two or three (perhaps spread between the two ovaries) reach full size in each cycle, but usually only one ruptures; its diameter is then about 5 cm. After rupture, the cavity contains some blood, and for a time the soft clot may be appreciated on rectal examination. It then gradually fills with luteal cells, but even when mature the corpus luteum hardly projects above the surrounding surface. The corpus luteum is initially brick-red but becomes ocherous as it matures. Its regression begins about the 10th day and is more or less complete when its successor forms. The cycle averages 22 days. The left ovary is generally the more active; despite this the right uterine horn is slightly more favored by conceptuses. Transuterine migration by a conceptus must be common.
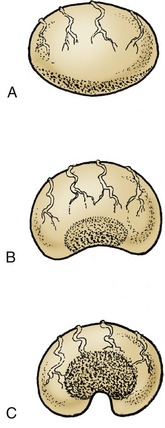
Figure 22–15 The postnatal development of the ovary. The more rapid growth at the poles confines the germinal epithelium (stippled) to a small central area.
A, At birth; the germinal epithelium is widespread over the surface. B, At 6 months of age. C, Adult; the germinal epithelium surrounds an indentation known as the ovulation fossa.
Ultrasonic examination may be used to follow the stages of follicular development, to determine the occurrence of ovulation, and to trace the fate of the resulting follicular cavity. It is generally successful in determining the course of events a little before this is possible by palpation per rectum. It may allow the prediction of ovulation by about a day because it can reveal the change in form, from spherical to pyriform, of the ripening follicle. A further advantage lies in its success in recognizing the parallel maturation of multiple follicles that may result in twin pregnancy.
The juvenile reproductive tract is small, symmetrical, and thin walled. The endometrium is pale, and the layers of the uterine wall are difficult to differentiate with the naked eye. The broad ligaments are thin and transparent, and the blood vessels are narrow and relatively inconspicuous. Growth is initially isometric—it keeps pace with growth of the body as a whole—until a prepubertal acceleration occurs. Cyclical changes in the uterus, including increased retention of water, a greater blood flow, and activation of the glands thickening the wall in preparation for the reception of the blastocyst, broadly resemble those in other species. If pregnancy does not result, these changes recede with the regression of the corpus luteum. Cyclical changes in muscular tonus are the subject of some controversy, but most authorities hold that tonus is greatest about a week after ovulation.
The cervix softens during estrus when the intravaginal part droops so that its orifice is lost to view on vaginoscopic examination (Figure 22–12, B). When stimulated by handling, it becomes firmer, returns to the horizontal, and may exhibit rhythmic contractions. It is also moist, swollen, and pink at this time. It is paler in appearance and firmer during metestrus and diestrus when the lumen is closed by a plug of thick mucus (see Figure 22–12). Although the vaginal wall is pink and moist during estrus, its liability to change color on prolonged exposure to air denies diagnostic significance to its appearance. Cytological changes in the vaginal epithelium are slight and also of little diagnostic value.
THE REPRODUCTIVE TRACT DURING PREGNANCY
The ovaries continue to show cyclical activity during the first months of pregnancy. Although the first corpus luteum does not persist beyond the usual term, it is replaced by a succession of other corpora over the next 5 months; some are formed after rupture of follicles, others apparently by direct luteinization. The accessory corpora lutea survive longer than the original one and are a rich source of progesterone. The growth, ripening, and luteinization of the new follicles are controlled by gonadotrophic hormones derived from the endometrial cups that are so distinctive a feature of the species. After 5 months the accessory corpora lutea also regress and pregnancy is then maintained by progesterone of placental origin. The enormous enlargement of the fetal gonads, peculiar to the horse among domestic species, reaches a peak between 6 and 8 months. Despite assertions that fetal hypophysial luteinizing hormone (LH) is responsible for the enlargement, unpublished information indicates that the enlargement continues in the decapitated fetus; this points to the endometrial gonadotrophins as a contributing if not sole source (see Figure 22–18, B and see p. 576). The temporary enlargement of the fetal testes influences the timing and the success of their descent, which is normally completed about full term.
The proliferative changes of the endometrium that occur with each cycle continue and intensify if pregnancy has occurred. The early diagnosis of pregnancy and, because of the prevalence of early embryonic death, the confirmation of its continuation through the critical early stages have particular importance in equine practice. An additional significance is provided by the desirability of recognizing twin pregnancies at an early stage. Twin pregnancies are rarely completed successfully, and the clinician and client may choose to destruct one of the twins by manual crushing to lessen the risk of losing a breeding season. The crushing has to be carried out before implantation. Although various laboratory methods of pregnancy diagnosis exist, the principal reliance remains on careful internal examination per rectum, supplemented by ultrasonography (Figure 22–16). The experienced clinician may recognize a loss of uterine tone at the location of a conceptus, compared with the tone of neighboring parts, as early as the 20th day—possibly even a day or two before this. The location of the conceptus at this time is within the part of a uterine horn adjacent to the junction with the body of the organ; at this stage the conceptus has a diameter of approximately 30 to 40 mm, and a slight bulge of the ventral aspect of the gravid horn should be detectable. Ultrasonic examination may bring forward the time of recognition of the presence of a conceptus to as early as the 11th or 12th day, occasionally even the 9th day. Because the conceptus has a diameter of only a few millimeters at this stage, it is clear that very systematic examination is required to detect, or confidently exclude, its presence. The identification of the body of the embryo becomes possible a week or so later (about day 19), and this removes any lingering suspicion that a cavity identified at an earlier examination might be attributable to an endometrial cyst. The differentiation of pregnancy from pathology will probably receive additional confirmation from a shift in location of the conceptus, which is still mobile, unlike a lesion.

Figure 22–16 Ultrasonographic view of 31-day equine twin embryos. The scale is in centimeters.
1, Twin embryos; 2, junction of the two conceptuses; 3, developing allantoic membrane; 4, uterine wall.
The early conceptus enjoys considerable mobility before adopting a fixed location within the uterus. There is evidence to suggest that, though most equine conceptuses are located within the body of the uterus about the 10th day, they will have settled within a horn a week or so later.
Ultrasonography may be employed at a somewhat later stage of pregnancy to determine the sex of the fetus, which is revealed by the location of the genital tubercle; it is found close to the umbilical cord in the male, nearer the tail in the female. Such examinations are performed after 55 days.
The whole gravid horn (which is more commonly the right one) then gradually enlarges, followed by the body and, although to a lesser degree, the nonpregnant horn. As the uterus enlarges it sinks into the abdomen, dragging the body and the cervix out of the pelvis (Figure 22–17). The broad ligaments exert constraint on the mesometrial margins, and the horns therefore enlarge asymmetrically and become more flexed on themselves; the ovaries are drawn ventrocranially. The uterine arteries, which are pulled in the same direction, develop a characteristic vibration (fremitus or thrill) in the pregnant mare. This feature may be appreciated on rectal examination, and its diagnostic value is greatest at that stage of pregnancy (between the 3rd and 5th month) when the uterus has sunk out of reach. The position of the foal adapts to the form of the uterus; by midpregnancy it has come to lie with its back against the greater curvature of the horn (and thus ventrally) and with its head generally (99% of the time) raised toward the cervix. In the circumstances that most favor easy parturition, the bulky body of the foal is preceded into the cervix by the extended forelimbs, on which rest the relatively small head and slender neck. The foal is delivered with its back uppermost. Because of the general enlargement and considerable size of the body of the uterus, it is possible for the occasional fetus to lie transversely, extending from one horn into the other; clearly this bodes ill for parturition. Enlargement of the uterus displaces the other abdominal contents forward and upward; in later pregnancy the uterus dominates the entire abdominal topography, extending forward on the abdominal floor and under the rib cage; however, it generally remains to the left of the cecum.
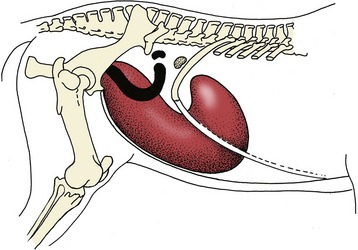
Figure 22–17 Changes in the topography of the uterus and ovary between the beginning (black) and the end (red) of pregnancy.
A prominent feature of the uterus in early months of pregnancy is the presence of a ring or horseshoe formation of scablike structures, disfiguring the endometrium of the caudal part of the horn, the location where the young conceptus comes to rest. These so-called endometrial cups (Figure 22–18, B) are unique to Equidae and are the source of both equine chorionic gonadotropin (formerly known as pregnant mare’s serum gonadotropin [PMSG]), the hormone responsible for the unusual activity of the ovary of the pregnant mare (p. 574) and the even more remarkable, though temporary, enlargement of the gonads of equine fetuses of both sexes. The cups have their origin in cells that invade the endometrium from a limited region of the chorion: the (allanto-) chorionic girdle that marks the boundary between the allantochorionic and omphalochorionic (yolk sac) portions of the embryonic vesicle and provides the area of initial adhesion of the conceptus to the uterus (Figure 22–18, A). The migration of chorionic cells begins about the 35th day, and the cups soon become visible as low endometrial elevations. They continue to grow, forming irregular centrally depressed saliences that reach their zenith about the 60th day, only to enter a process of degeneration and necrosis shortly thereafter. The process culminates in their separation and sloughing from the endometrium, which are events largely concluded by the 120th day (or thereabout), although a few may persist much longer. The fetal (chorionic) cells penetrate some way into the endometrial stroma, and although they provide the essential endocrine components of the cups, they become admixed with connective tissue cells, blood vessels, and glandular debris and secretion contributed by the endometrium. Some detached cups come to lie between the endometrium and chorion; other detachments of this material push into the allantoic cavity, enclosed within pedunculated sacs of allantochorion, and these protrusions may be the origin of some of the hippomanes mentioned shortly.



Figure 22–18 A, Young conceptus (horse).
1, Yolk sac; 2, chorionic girdle; 3, allantochorion.
B, Endometrial cups (mare) during early pregnancy. C, The placenta of the horse fetus is not very complex. The villi do not penetrate deep into the endometrium.
The cervix of the pregnant mare is firm and closed by a plug of mucus (see Figure 22–12). The pale vaginal wall is also coated with mucus that becomes stickier and more inspissated as pregnancy progresses. The connective tissues of the cervix, vagina, and vulva and the sacrotuberal ligaments soften shortly before birth, which is generally speedily executed, facilitated by the generous dimensions of the pelvic cavity. It is necessary that it should be so, as rupture of the membranes with loss of fetal fluids allows separation of the loose attachment between the chorion and the endometrium, jeopardizing fetal respiration.
Puerperal changes follow the same pattern as in other species but run a rapid course. Involution of the uterus is completed sooner than in the cow, and because there is no endometrial damage to repair, mares covered at the “foal heat”—about the 8th to 10th day after giving birth—often conceive.
PLACENTATION AND PRENATAL DEVELOPMENT
In the horse, unlike other domestic species, a choriovitelline (or omphalo-) placenta provides the principal organ of exchange for the first third or so of intrauterine life. Thereafter, with the establishment of the chorioallantoic placenta, the yolk sac wanes. The definitive chorioallantoic placenta is of the epitheliochorial type and is commonly described as diffuse. The outer surface of the chorion carries innumerable branched villi that penetrate into crypts of the endometrial surface to form a loose attachment that is reinforced by the radial pressure exerted by the fetal fluids. Although the villi are widely spread, their distribution is not uniform, and they are clumped together in groups sometimes known as microcotyledons (because they resemble the cotyledonary arrangement in ruminants on a smaller scale). Small spaces between the microcotyledons face the openings of the uterine glands and fill with their secretions.
The capillaries of both fetal and maternal parts of the placenta reach directly below the corresponding epithelia, and only a thin tissue layer separates the two bloodstreams. Even so, the passage of large molecules, including antibodies, is impossible, and the passive transfer of immunity from mother to offspring is dependent on the foal ingesting colostrum.
A peculiar feature is the presence of so-called hippomanes in the allantoic (and, to a lesser extent, amniotic) fluid. These are soft brownish bodies; most are formed by the deposition of organic material on nuclei provided by solid particles within the fluids, but some have their origin in material flaked from endometrial cups when these have completed their role. The latter are sometimes found anchored to the chorioallantoic membrane by attenuated stalks. Hippomanes have no clinical (or residual physiological) importance, but laypeople sometimes credit them with the most fantastic origins and various properties—often rather lurid and wholly mythical.
Although detailed information must be sought elsewhere, it may be useful to have this bare guide to the estimation of fetal age (Table 22–1). Crown–rump measurements are of limited value in this species because of its wide range of body size.
Table 22–1 Guide to the Aging of Horse Fetuses
From Evans HE, Sack WO: Prenatal development of domestic and laboratory animals. Growth curves, external features and selected references, Anat Histol Embryol 2:11–45, 1973.
| Month | Crown–Rump Length | External Features |
|---|---|---|
| 1 | — | The embryo is about 1–1.5 cm long. |
| 2 | ≈7 cm | The species is recognizable and the sex determinable from the external genitalia. |
| 3 | ≈14 cm | The parts of the hoof are distinct. |
| 4 | ≈25 cm | Some hair is present around the mouth. |
| 5 | ≈36 cm | Hairs are present above the eyes. |
| 6 | ≈50 cm | Eyelashes are present. |
| 7 | ≈65 cm | Hair is present at the tail tip. |
| 8 | ≈80 cm | Hair has appeared along the back and on the limbs. |
| 9 | ≈95 cm | Fine hair covers most of the body (the belly excepted). |
| 10 | ≈110 cm | The body is completely haired. |
| 11 | Full term (generally in the range of 330–345 days) |
THE MALE REPRODUCTIVE ORGANS
THE SCROTUM AND TESTES
The scrotum lies below the pubic brim, where it is concealed from lateral inspection by the thigh. It is broadly globular, commonly asymmetrical, and divided by an external raphe that extends cranially onto the prepuce and caudally onto the perineum. The scrotal skin is thin, supple, and sparsely haired and is usually deeply pigmented; it glistens from sebaceous secretion. The deeper layers of the scrotal wall are constructed in the usual fashion.


Figure 22–19 A, The reproductive organs of the stallion in situ.
1, Rectum; 2, external anal sphincter; 3, ureter; 4, bladder; 5, urethra; 6, floor of pelvis; 7, floor of abdomen; 8, cremaster; 9, left deferent duct; 10, vaginal ring; 11, right testicular artery and vein; 12, ampulla of deferent duct; 13, vesicular gland; 14, prostate; 15, bulbourethral gland; 16, penis; 17, left crus (in section); 18, glans penis; 19, ischiocavernosus; 20, bulbospongiosus.
B, Testis and spermatic cord within exposed vaginal process.
The testes are imperfectly ellipsoidal, being slightly compressed from side to side (Figure 22–19 and Figure 5–41). They generally lie with their long axes horizontal but become almost vertical on strong contraction of the cremaster muscles that attach to the vaginal tunic near the cranial poles. The tunica albuginea is less thick than in ruminants, and the testes yield on gentle compression; even so, the grayish pink parenchyma is contained under some pressure and bulges through any incision of the tunic. The septa that extend inward from the capsule do not join to form a visibly distinct mediastinum. The epididymis lies along the dorsal border and projects a little beyond the poles of the testis, where it is most firmly attached. It leaves a distinct testicular bursa that opens laterally. The ligament of the tail of the epididymis is quite thick and must be severed in castration by the “open” method. Wartlike growths (appendices testis) on the testis near the head of the epididymis are very common; they are remnants of the paramesonephric duct.
The spermatic cord is broad and thin where it attaches to the testis but rounds when followed toward the superficial inguinal ring. The cranial vascular part (Figure 5–41/5) is clearly distinguished from the caudal part that carries the deferent duct. The constituents diverge in the usual manner on entering the abdomen (see Figure 22–19 and Figure 22–24, B). The course of the deferent duct then takes it across the dorsal face of the bladder, beside the medial border of the vesicular gland, before it penetrates the prostate to reach the urethra. The subterminal part (≈20 cm) of the duct is widened to form an ampulla, which is an inappropriate term because it is the wall and not the lumen that is enlarged. The ampulla is less distinct in geldings, particularly those castrated early.
The wide inguinal canal makes inguinal hernias a relatively common occurrence.
Although the process of testicular descent may be presumed to be governed by the same factors (p. 173) as in other species, it is marked by one circumstance unique among domestic mammals. The testes of the fetal colt exhibit an inordinate though temporary increase in size between the 100th and 250th days of gestation, attaining a peak on about the 215th day. (A comparable enlargement affects the ovaries of the fetal filly.) In consequence, although each testis arrives in the vicinity of the vaginal ring on about the 120th day, it is delayed here and does not resume its migration until it has shrunk to a fraction of its maximal size. It does not arrive in the scrotum until close to the time of birth and may even arrive after this event (probably within 2 weeks either way).
Not infrequently a testis fails to reach the scrotum even then but remains hidden within the abdomen or delayed within the inguinal canal. Retention may be temporary or permanent, confined to one side or bilateral, and if bilateral, the sites of lodgement may be asymmetrical. The condition, known as cryptorchidism, may resolve spontaneously, and the testis may make a delayed appearance in the scrotum at some time within the first year of postnatal life or possibly even later. In such cases it may be assumed that the testis was held up within the inguinal canal because the vaginal ring normally contracts shortly after birth, preventing a late entry to the canal from the abdomen. Testes that fail to make an appearance within a reasonable time require surgical removal, for which a variety of techniques is available depending on the location of the arrest. The diagnosis of cryptorchidism is sometimes less obvious than might be supposed. Cryptorchid animals that have changed hands may be presented in good faith as geldings, and suspicion may only arise when stallion characters of conformation and behavior develop. Moreover, in young horses of nervous disposition, successfully descended testes may initially escape detection by being withdrawn into the groins, against the superficial inguinal rings, when the scrotum and inguinal regions are palpated.
THE PELVIC REPRODUCTIVE ORGANS
The short (≈12 cm) pelvic urethra lies directly over the pelvic symphysis. Although generally remarkably wide (≈6 cm), its lumen is narrowed in two places: one level with the body of the prostate, and the other where the urethra crosses the ischial arch (Figure 22–20). The deferent ducts (Figure 22–20/2) penetrate the urethral wall close to the origin of the urethra from the bladder. Each combines with the duct of the neighboring vesicular gland to form a common passage, the ejaculatory duct. This is only a few millimeters long and opens into the urethra to the side of the dorsal thickening, the seminal colliculus.
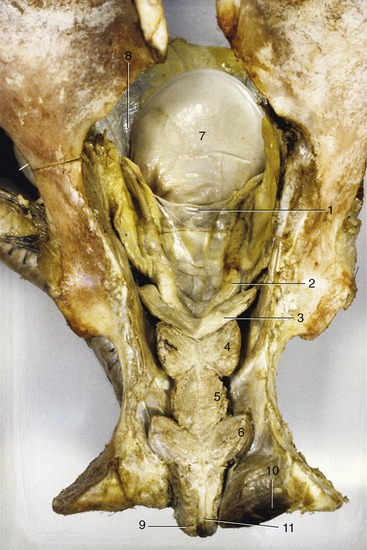
Figure 22–20 Dorsal view of the pelvic urethra and accessory reproductive glands (in situ).
1, Genital fold; 2, ampulla of deferent duct; 3, vesicular gland; 4, prostate; 5, urethralis; 6, bulbourethral gland; 7, bladder; 8, lateral ligament of bladder; 9, bulbospongiosus; 10, ischiocavernosus; 11, retractor penis.
The vesicular glands (Figure 22–20/3) of the horse merit the alternative name seminal vesicles because they take the form of smooth-surfaced, pear-shaped bladders, approximately 12 cm long, with large central lumina. Each is contained within the genital fold.
The prostate (Figure 22–20/4) is largely retroperitoneal and entirely compact. It consists of two lateral lobes joined by a narrow isthmus that crosses the dorsal aspect of the urethra close to the bladder neck. Each lateral lobe is pressed against the border of the urethra and extends cranially along the caudolateral edge of the adjacent vesicular gland. Because the prostate is firm and lobulated, the two glands are easily distinguished on rectal examination. Numerous ductules drain from the prostate to discharge into the urethra through tiny slits beside the colliculus (see Figure 5–50/7).
The paired bulbourethral glands lie dorsolateral to the urethra at the pelvic outlet. They are thinly covered by striated muscle (bulboglandularis), about 4 cm long, and so oriented that their pointed caudal ends converge (Figure 22–20/6). These glands discharge through numerous small pores that open into the urethra where it leaves the pelvis.
All accessory reproductive glands are of course much reduced in geldings.
THE PENIS AND PREPUCE
The penis of the horse is composed of the usual triad of structures and is of the musculocavernous variety. The two dorsal elements, the crura penis, arise from the ischial arch, bend forward between the thighs, and soon unite in a single corpus cavernosum, which is divided in its proximal part by a median septum that reflects the compound origin (Figure 22–22, A/3). The septum fades and finally disappears when followed toward the apex. The corpus cavernosum is somewhat compressed laterally and carries ventrally a groove into which the third erectile body, the corpus spongiosum, fits.
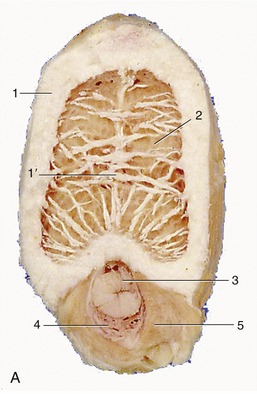


Figure 22–21 Transections of the penis, directly distal to the root (A), midshaft (B), and in its free part (C).
1, Tunica albuginea; 1′, incomplete septum penis; 2, corpus cavernosum; 3, urethra; 4, corpus spongiosum; 5, bulbospongiosus; 6, retractor penis; 7, dorsal process of glans.
The corpus spongiosum expands over the apex of the organ to form the distinctively shaped glans (Figure 22–22, A/1). This has a resemblance to a mushroom; the widest part, the corona, is some distance proximal to the apex, where the terminal part of the urethra protrudes into a central fossa (Figure 22–22/3). The glans is constricted to form a neck behind the corona and is then prolonged in a tapering process over the dorsal aspect of the body; this feature is not visible externally (Figure 22–21/7).




Figure 22–22 Extremity of penis exposed (A), within prepuce in median section (B), and the entire organ after dismount (C) enlarged glans penis (D).
1, Glans; 1′, corona glandis; 1″, collum glandis; 2, urethra; 2′, corpus spongiosum; 3, urethral process within fossa glandis; 3′, urethral sinus; 4, corpus cavernosum; 5, preputial fold; 5′, preputial ring; 6, prepuce, forming preputial orifice with the body wall.
A considerable portion of the quiescent penis projects into the preputial cavity. The equine prepuce (sheath) is peculiar in being thrown into an additional fold that allows for the considerable lengthening of the penis on erection (Figure 22–22, C). The entrance (preputial ring; Figure 22–22, B/5′) to this inner sleeve lies just within the preputial orifice. Sometimes as a congenital defect, the ring is unduly tight and prevents protrusion of the penis (phimosis). The condition may be corrected by section of the responsible encircling band of muscle that is included within the ring. The preputial lining contains many glands and is commonly fouled by their secretion, the smegma. An inspissated mass of this dark material—the “bean” of the penis is the stable term—commonly fills a small (urethral) sinus above the urethral process (Figure 22–22).
The penis of the horse obtains blood from the obturator and external pudendal arteries in addition to the usual internal pudendal source.
Unusually, the bulbospongiosus continues along the ventral aspect of the penis well beyond the point of incorporation of the urethra (Figure 22–21/5). The muscle, which is the direct continuation of the urethralis, bridges the ventral groove of the corpus cavernosum and on contraction compresses the corpus spongiosum (and urethra), assisting in the expulsion of urine and semen. The ischiocavernosus muscles are powerful but in no way remarkable. The smooth retractor penis muscles loop round the rectum before passing onto the ventral surface of the penis (Figure 22–21/6). They continue forward, gradually weaving through the transverse fibers of the bulbospongiosus, to find attachment on the glans.
Erection
Since the penis is of the musculocavernous type, it becomes considerably engorged with blood when erect. When erection is complete, a process requiring some time and achieved by the relaxation of the helicine arteries* and the pumping action of the ischiocavernosi, the organ is much enlarged in both length and girth (Figure 22–22, C). A very considerable pressure, perhaps as much as 3700 mm Hg, is attained within the blood spaces of the corpus cavernosum, and as in other species, this occasionally results in rupture of the fibrous capsule. The ejaculate is relatively large (≈65 mL on average) and is mainly the product of the vesicular glands.
Dismounting after service is often followed by a remarkable “flaring” or enlargement of the glans, in which the corona may briefly attain a diameter of 12 cm or so before it subsides. The return of the flaccid penis to the sheath is effected by the retractor muscles assisted by the smooth muscle component of the walls of the cavernosus spaces. Indeed, the resting posture of the penis is dependent on the tonus of this muscle. If this is reduced or lost—a relatively common occurrence in horses that are fatigued or in poor condition—the penis limply droops from the prepuce. It is vulnerable to injury when exposed in this way. The resistance of the muscle may also be overcome by sustained traction when it is necessary to expose the organ for clinical examination or for washing as part of routine stable hygiene.
THE ANATOMY OF RECTAL EXPLORATION
Exploration per rectum is an important diagnostic technique in the horse. A hand can very easily be introduced into the rectum and descending colon and then be passed in various directions to examine the pelvic and caudal abdominal wall, the pelvic contents, and a variable amount of the abdominal contents (Figure 22–23/7). Rectal examinations are not free from risk of injury to the mucosa or even, in extreme cases, of perforation of the intestinal wall—a mishap most likely to occur when invasion of the rectum induces straining. The novice should not attempt the procedure without appropriate supervision. Some organs can always be identified with certainty and others less consistently, for the results of the investigation depend not only on the relative sizes of the investigator and patient but also on the condition of the organs. It is one thing to palpate an organ through the gut wall and quite another to recognize enough of its nature to be confident of identification. The greater part of the pelvic skeleton can be identified with absolute certainty, although the part of the floor about the symphysis may be made inaccessible by overlying organs. The caudal part of the abdominal wall is also within reach, although it rarely reveals much of interest other than the caudal margin of the internal oblique muscle bordering the deep inguinal ring and the vaginal ring (Figure 22–24/1) within that opening. The vaginal ring can be recognized most easily in the stallion, in which the deferent duct may be picked up where it lies on the bladder and traced to its disappearance.

Figure 22–23 Drawings of the abdominal and pelvic cavities in left lateral (A) and dorsal (B) outline, indicating the scope of rectal exploration. The dorsal outline encloses a ring of the relatively fixed organs (9, 9′, 10, 11, 12) with the pancreas (13) in the center.
1, Thoracic cavity; 1′, thoracic inlet; 1″, costal arch; 2, diaphragm; 3, coxal tuber; 3′, shaft of ilium; 4, terminal line; 5, pelvic cavity; 5′, inguinal canal; 6, thigh and stifle; 7, approximate range in rectal palpation in the median plane (A) and directly ventral to the kidneys (B); 8, deep inguinal ring; 9, 9′, left and right kidneys; 10, spleen; 11, stomach; 12, liver; 13, pancreas.


Figure 22–24 A, Dissection showing the vaginal ring. B, Endoscopic view of the ring.
1, Vaginal ring 2, testicular artery and vein 3, deferent duct; 4, descending colon.
Of the viscera, the small colon is the most easily recognized because its identity is betrayed by the chain of sacculations that are usually filled with firmish feces; even when empty, this part of the gut can be distinguished by single tenia following the free border (the tenia along the opposite mesenteric border is not normally palpable). Although the small colon has a mobile disposition, a mass of coils is generally found just in front of the pelvic inlet and mainly to the left. A considerable part of the ascending colon is also within reach. The pelvic flexure, the part most easily identified, is usually found immediately before or even within the pelvic cavity. Most often it lies just to the left of the median plane but it may cross to the right. The adjoining parts of the left ventral and dorsal parts of the ascending colon can be followed for some distance. They are most easily recognized when gas-filled, as this emphasizes the contrast between the sacculations of the wide ventral part and the smooth surface of the narrower dorsal part. Although the names of these parts are indicative, it must not be assumed that they necessarily lie directly one above the other. The dorsal diaphragmatic flexure and right parts of the colon are out of reach of even the longest arm, although sometimes it is just possible for the fingertips to touch and trace the junction of the ascending and transverse parts of the colon. The base and the dorsal part of the body of the cecum are consistently within reach; however, unless they are inflated, little beyond position exists to identify them. The cranial mesenteric artery, adherent to the left face of the cecal base, may sometimes be identified when thickened by reaction to nematode larval invasion. Even in the most favorable circumstances it is barely within reach.
Although much of the small intestine is accessible, it is usually impossible to identify it with certainty; the exception is the firmer terminal part of the ileum, which may be picked up as it approaches the medial aspect of the cecal base. Identification is easiest when it is impacted. When distended with gas the caudal flexure of the duodenum may be identified as it crosses the root of the mesentery.
A small horse and a long arm are the prerequisites if any of the contents of the cranial part of the abdomen are to be reached. The caudal pole of the left kidney may usually be felt, and it is theoretically possible to trace both ureters over the abdominal roof; in practice, healthy ureters cannot be identified. The caudal margin of the spleen is also accessible, although it may not always be appreciated; a greater part of this organ may be brought within reach when the stomach is distended.
An emergency means of euthanasia, of little relevance today, is available in transection of the abdominal aorta per rectum.
The bladder is invariably identifiable, regardless of its degree of filling and despite the fact that it is partly overlain by reproductive organs. In the mare, the vagina is distinguishable as a rather lax organ interposed between the rectum and the bladder; if followed forward, it leads to the somewhat firmer cervix. Beyond the cervix, the body of the uterus may be traced to its bifurcation, and the horns may then be followed laterally toward the ovaries. The dimensions and the texture of the uterus vary greatly with its state, and the experienced equine clinician can date an early pregnancy with quite remarkable precision by palpating the uterus. The ovaries are among the easiest organs to identify because they have a very characteristic shape and consistency. They are rather movable and are not always found exactly where expected. Only the largest follicles may be appreciated individually.
The pelvic urethra of the stallion is easily identified as a wide slack tube, although its outline is partly concealed by the associated glands (see Figure 22–20). The bulbourethral glands at the pelvic exit, the smooth pear-shaped vesicular glands, the more knobby prostate, and the fusiform enlargements of the ampullae of the deferent ducts are almost always individually distinctive. Manipulation may stimulate the urethral muscle, which may firm the urethra and cause it to exhibit rhythmic contractions.
THE UDDER
The mammary glands are consolidated in a rather small udder situated below the caudal part of the abdominal floor and cranial part of the pelvis and concealed from casual inspection by the thigh (Figure 22–25). The form and size of the udder vary with the present state and previous history of the mare; the udder is very small in young virgin animals. A prominent external groove indicates its formation from right and left halves; each half has the form of a laterally compressed cone and, though carrying a single teat, is composed of two (occasionally three) separate duct systems.

Figure 22–25 The udder is consolidated from right and left halves. The apices of the teats are perforated by the papillary ducts.
The skin over the udder is thin, strongly pigmented, and sparsely haired; it is supplied with many sweat and sebaceous glands and usually glistens. The teat is small and cylindrical, except in the lactating mare, in which it is both larger and more conical. Two (or three) openings perforate the apex; each leads through a short papillary duct to a small lactiferous sinus spread between the teat and gland mass and associated with an independent set of lactiferous ducts (Figure 22–26, A-C). The tissues of the individual glands of each side interdigitate, and it is impossible to demonstrate their independence on dissection. Although much less developed, the suspensory apparatus resembles that of the cow’s udder and combines medial elastic and lateral fibrous ligaments, which together encapsulate the udder and supply the lamellae that support the parenchyma. The medial ligaments provide a cleavage plane between the apposed surfaces of the udder halves.



Figure 22–26 A, A sagittal section of the udder demonstrating the construction of the teat and the location of the lactiferous sinus. B, C, Transected teats showing internal division.
The blood supply comes from the external pudendal artery, and the principal venous return is by the corresponding vein, which does not follow the usual course through the inguinal canal (p. 550). As in the cow, a subcutaneous venous connection with a superficial vein of the thoracic wall develops as an alternative drainage route during the first pregnancy. Lymph drains to the mammary (superficial inguinal) nodes. The cutaneous innervation is divided between the nerves of the flank and a descending (mammary) branch of the pudendal nerve; the contributing spinal nerves are thus those of cord segments L2–4 and S2–4 (Figure 22–27). The substance of the gland is supplied by the genitofemoral nerve (L3–4). The glands develop rapidly during the second half of the first pregnancy and commence secretion before birth. Sebaceous secretion, epithelial debris, and possibly colostrum that escape through the teat openings during the last days of pregnancy dry to give the apex a waxy covering, which is a useful indication that parturition impends.
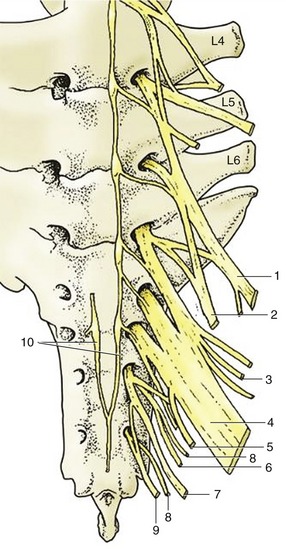
Figure 22–27 Ventral view of sacrum and caudal lumbar vertebrae with emerging ventral rami forming the lumbosacral plexus.
1, Femoral n., 2, obturator n., 3, cranial gluteal n. 4, sciatic n. 5, caudal cutaneous femoral n. 6, caudal gluteal n. 7, pudendal n. 8, pelvic n. 9, caudal rectal n. 10, continuation of sympathic cord.