CHAPTER 24 Diagnostic Tests for the Pleural Cavity and Mediastinum
RADIOGRAPHY
PLEURAL CAVITY
The pleura surrounds each lung lobe and lines the thoracic cavity. It is not normally visible radiographically, and individual lung lobes cannot be distinguished. Abnormalities of the pleura and pleural cavity include pleural thickening, pleural effusion, and pneumothorax. The mediastinum in the dog and cat is not an effective barrier between the left and right side of the thorax, and effusion or pneumothorax is therefore usually bilateral.
Pleural Thickening
Pleural thickening results in a thin, fluid-dense line between lung lobes, where the pleura is perpendicular to the X-ray beam. These lines arc from the periphery toward the hilar region and are known as pleural fissure lines. The lines can occur as a result of prior pleural disease and subsequent fibrosis, mild active pleuritis, or low-volume pleural effusion. They can be an incidental finding in older dogs. Infiltration of the pleura with neoplastic cells generally results in effusion rather than thickening.
Pleural Effusion
Pleural effusion is visible radiographically after about 50 to 100 ml has accumulated in the pleural cavity, depending on the size of the animal. An early effusion assumes the appearance of pleural fissure lines and can be confused with pleural thickening. As fluid accumulates, the lung lobes retract and the lung lobe borders become rounded. Rounding of the caudodorsal angles of the caudal lung lobes is especially noticeable. The fluid silhouettes with the heart and diaphragm, obscuring their borders. The lungs float on top of the fluid, displacing the trachea dorsally and causing the illusion of a mediastinal mass or cardiomegaly (Fig. 24-1, A). As more fluid accumulates, the lung parenchyma appears abnormally dense as a result of incomplete expansion. Collapsed lobes should be examined carefully for evidence of torsion (see Chapter 20). Pockets of fluid accumulation or unilateral effusion indicates the possibility of concurrent pleural adhesions (Fig. 24-1, B).
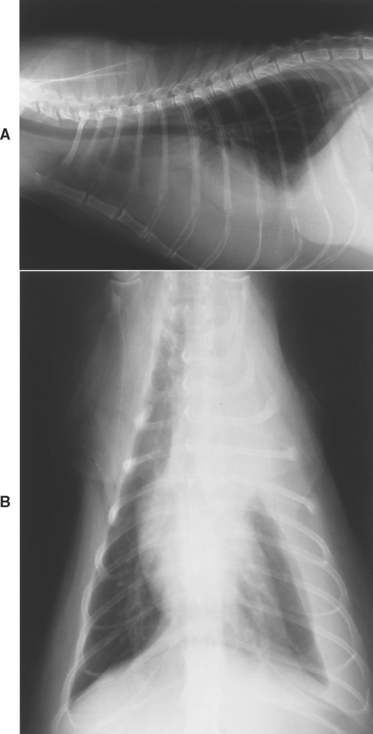
FIG 24-1 A, Lateral thoracic view of a cat with pleural effusion. See text. B, Ventrodorsal view showing that the effusion is unilateral.
Critical radiographic evaluation of intrathoracic structures, including the lungs, heart, diaphragm, and mediastinum, cannot be performed in animals with pleural effusion until the fluid has been removed. The interpretation of radiographs obtained in the presence of fluid is prone to error. An exception to this rule is the finding of gas-filled intestinal loops in the thorax, which is diagnostic for diaphragmatic hernia. Both left and right lateral views should be evaluated, in addition to a ventrodorsal view, to improve the sensitivity of detecting masses.
Pneumothorax
Pneumothorax is the presence of air in the pleural space. Air opacity without vessels or airways can be seen between the lung lobes and chest wall on radiographs. It may be necessary to carefully scrutinize the films using high intensity lighting to detect mild pneumothorax. As a greater volume of air accumulates in the pleural space, the lung parenchyma becomes more dense because of incomplete expansion, facilitating the radiographic diagnosis. The heart is generally elevated above the sternum, with air opacity apparent between these two structures (Fig. 24-2). Radiographs should be examined carefully for evidence of possible causes of the pneumothorax, such as cavitary lesions or rib fractures (indicating trauma). To accurately evaluate the pulmonary parenchyma, the air must be removed and the lungs allowed to expand. Cavitary lesions are not always apparent radiographically. Further evaluation for cavitary lesions in patients with spontaneous pneumothorax includes computed tomography.
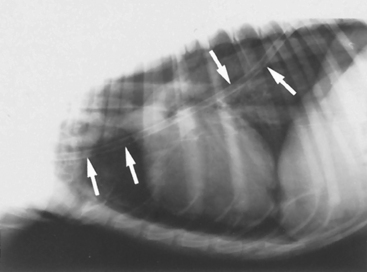
FIG 24-2 Lateral view of a dog with pneumothorax and pneumomediastinum. The pneumothorax is mild and is demonstrated by elevation of the heart above the sternum. When high-intensity lighting was placed behind the original radiographs, retraction of lung borders could also be seen. It is possible to visualize the outer wall of the trachea and major blood vessels in the anterior mediastinum because of the pneumomediastinum. A chest tube placed to stabilize the dog’s condition is also visible (arrows).
MEDIASTINUM
The cranial and caudal mediastinum contains the heart and great vessels, esophagus, lymph nodes, and associated support structures. Radiographic abnormalities involving the mediastinum include pneumomediastinum, and alterations in size (e.g., mass lesions), displacement, and abnormalities involving the structures within the mediastinum (e.g., megaesophagus).
Pneumomediastinum is the accumulation of air in the mediastinum. If a pneumomediastinum is present, the outer wall of the trachea and the other cranial mediastinal structures, such as the esophagus, major branches of the aortic arch, and cranial vena cava, are contrasted against the air (see Fig. 24-2). These structures are not normally visible.
Abnormal soft tissue opacities can occur in the cranial mediastinum, although concurrent pleural effusion often obscures mass lesions. Localized lesions can represent neoplasia, abscesses, granulomas, or cysts (see Fig. 23-2). Less discrete disease can cause a general widening of the mediastinum that is seen to exceed the width of the vertebra on ventrodorsal views. Exudates, edema, hemorrhage, tumor infiltration, and fat can cause a widened mediastinum. Megaesophagus can often be observed in the cranial mediastinum, especially on lateral views.
The caudal vena cava and aorta are normally visible in the caudal mediastinum. The most common caudal mediastinal abnormalities are megaesophagus and diaphragmatic hernia. Megaesophagus is an important consideration in animals with respiratory signs because it is a common cause of aspiration pneumonia.
The mediastinum is normally located in the center of the thoracic cavity. An abnormal shift of the mediastinum is identified by a lateral change in the position of the heart on ventrodorsal or dorsoventral views. Atelectasis (i.e., lung lobe collapse), lobectomy, and adhesions of the mediastinum to the chest wall can all cause the mediastinum to shift toward the abnormality. Space-occupying lesions can cause the mediastinum to shift in the opposite direction.
The lymph nodes and heart are mediastinal structures but are considered separately to ensure a careful evaluation. The sternal nodes are located immediately dorsal to the sternum near the thoracic inlet at the level of the first to third sternebrae (Fig. 24-3). Enlargement is seen on lateral views and has the appearance of a discrete mass lesion. The hilar nodes are located at the heartbase around the carina. Enlargement is seen as a generalized increased soft tissue opacity in the perihilar region and is most easily seen on the lateral view. Common differential diagnoses for hilar lymphadenopathy are lymphoma and fungal infections (especially histoplasmosis). Other differential diagnoses include metastatic neoplasia, eosinophilic pulmonary granulomatosis, and mycobacterial infections. Any inflammatory disease can potentially cause lymphadenopathy. Other considerations in animals with an increased perihilar opacity on radiographs include atrial enlargement and heartbase tumors.
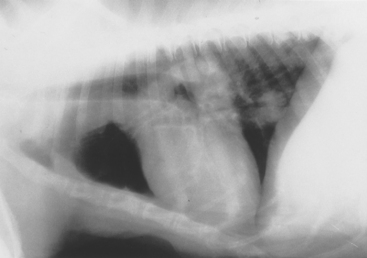
FIG 24-3 Lateral thoracic radiograph obtained in a dog with pulmonary neoplasia and sternal and hilar lymphadenopathy. The sternal node is the soft tissue opacity resting on the caudal half of the second sternebra. The hilar nodes are identified by the increased soft tissue opacity around the carina. Several discrete pulmonary nodules are also present.
Evaluation of the heart is described in Chapters 1 and 2. Right-sided heart failure and pericardial effusion can cause pleural fluid accumulation.
ULTRASONOGRAPHY
Ultrasonography is indicated in the diagnostic evaluation of dogs and cats with pleural effusion to search for masses, diaphragmatic hernia, lung lobe torsion, and cardiac disease. Mediastinal masses, masses involving the pulmonary parenchyma adjacent to the body wall, and masses extending into the thorax from the body wall may be identified and their echogenicity evaluated. Ultrasonography can also be used to guide aspiration needles or biopsy instruments to the lesion, although biopsies can be done safely only on solid masses. Ultrasonography is also useful for directing needle placement during thoracocentesis in animals with localized accumulations of pleural fluid. Air interferes with the sound waves, so structures surrounded by aerated lung cannot be examined.
COMPUTED TOMOGRAPHY
As discussed in Chapter 20, computed tomography is more sensitive than radiographs for evaluating the thorax. It is useful to determine the extent of mass lesions prior to thoracotomy and to increase the likelihood of localizing cavitary lesions in patients with spontaneous pneumothorax.
THORACOCENTESIS
Thoracocentesis is indicated for the collection of diagnostic specimens in dogs and cats with pleural effusion, for removal of pleural fluid or air to stabilize the condition of dogs and cats with impaired ventilation, and before radiographic evaluation of intrathoracic structures in dogs and cats with pleural fluid or air. Possible complications of thoracocentesis are pneumothorax caused by lung laceration, hemothorax, and iatrogenic pyothorax. Complications are extremely rare if careful technique is used.
Thoracocentesis is performed with the animal in lateral or sternal recumbency, depending on which position is less stressful. Fluid or air is usually present bilaterally throughout the pleural space and can be retrieved from the seventh intercostal space (ICS) by placing the needle approximately two thirds of the distance from the costochondral junction toward the spine. If initial attempts are unsuccessful, other sites are tried or the animal’s position is changed. Fluid may be more successfully retrieved from gravity-dependent sites (i.e., closer to the costochondral junctions) and air from nondependent sites. Thoracic radiographs are useful in choosing sides for thoracocentesis in the event of unilateral effusions. Ultrasonography is useful for guiding needle placement in patients in which fluid collection proves difficult.
A local anesthetic can be administered at the site of thoracocentesis. Sedation is rarely required but may be useful for decreasing patient stress. The site is shaved and surgically prepared, and the procedure is performed using sterile technique. Most often, a butterfly catheter, three-way stopcock, and syringe are used. The removal of fluid or air by syringe is associated with movement of the syringe, and the tubing of the butterfly catheter prevents this movement from affecting the position of the needle within the thoracic cavity. Air and most fluids can be retrieved through a 21-gauge butterfly catheter. A larger needle may be required to collect extremely viscous fluids, such as fluid from feline infectious peritonitis or pyothorax. The three-way stopcock is attached to the catheter to keep air from entering the thorax during emptying or changing of the syringe.
With the syringe snugly attached and the stopcock open between the catheter and syringe (closed to room air), the needle is advanced through the skin only. The needle and skin are then moved about two rib spaces to the actual collection site. This technique prevents air from entering the chest through the needle tract after the procedure (an unlikely scenario). The needle is then advanced into the thorax immediately in front of the rib to avoid the intercostal vessels and nerves. The needle is held with a hand resting on the chest wall so that it will not move relative to the respirations or movement of the animal. Slight negative pressure is applied to the catheter by the syringe so that entry into the pleural space is immediately identified by the recovery of fluid or air. Once the needle has entered the pleural space, the tip is aimed away from the lung by lowering the wings of the catheter toward the body wall. Ideally, the bevel of the needle should face toward the lungs.
An alternative to a butterfly catheter is an intravenous over-the-needle catheter. In large dogs a 31/4- or 51/4-inch (8- or 13-cm) 14- to 16-gauge catheter can be used. These catheters are soft and produce less trauma than butterfly catheters while in the pleural space and permit the animal to be repositioned or rolled to improve fluid or air removal. The longer length, compared with a butterfly needle, may be needed to reach the pleural space in large-breed or obese dogs. A few side holes can be added to the distal end of the catheter using a surgical blade and sterile technique to increase the sites where fluid can enter. The holes should be spaced far apart, should not take up more than one fifth of the circumference of the catheter, and should have no rough edges because the catheter might then break off in the animal during removal. Extension tubing and a three-way stopcock are attached to the catheter immediately after placement. A small skin incision, just slightly larger than the catheter, will facilitate placement. As with the butterfly catheter, slight negative pressure is maintained by syringe so that entry into the pleural space is immediately identified. The catheter tip is then directed cranially to allow positioning of the catheter between the lungs and chest wall, preventing trauma to the lung tissue.
After fluid specimens are saved for cytologic and microbiologic analysis, as much fluid or air as possible is removed, except in patients with acute hemothorax (see Chapter 26).
CHEST TUBES: INDICATIONS AND PLACEMENT
Chest tube placement is indicated for the treatment of dogs and cats with pyothorax (see Chapter 25). Chest tubes are also indicated for the management of pneumothorax if air continues to accumulate despite multiple thoracocenteses. Chest tubes provide a means to prevent fluid and air from accumulating in the pleural space until the underlying cause of the pleural disorder resolves. If possible, needle thoracocentesis and therapy for shock are performed to stabilize dogs and cats in critical condition before chest tubes are placed.
The major complication of chest tubes is pneumothorax caused by a leak in the apparatus. Animals with chest tubes must be carefully monitored at all times to make sure that they do not disrupt the tubing connections, pull the tube part of the way out of the chest so that there are fenestrations outside the body wall, or bite through the tubing. Any leaks in the system can result in a life-threatening pneumothorax within minutes. If an animal with a chest tube must be left unattended, the tube should be clamped off close to the body wall and should be well protected by bandage material. Hemothorax, iatrogenic pyothorax, and pneumothorax caused by lung laceration can also occur, but these problems are generally prevented through the use of careful aseptic technique.
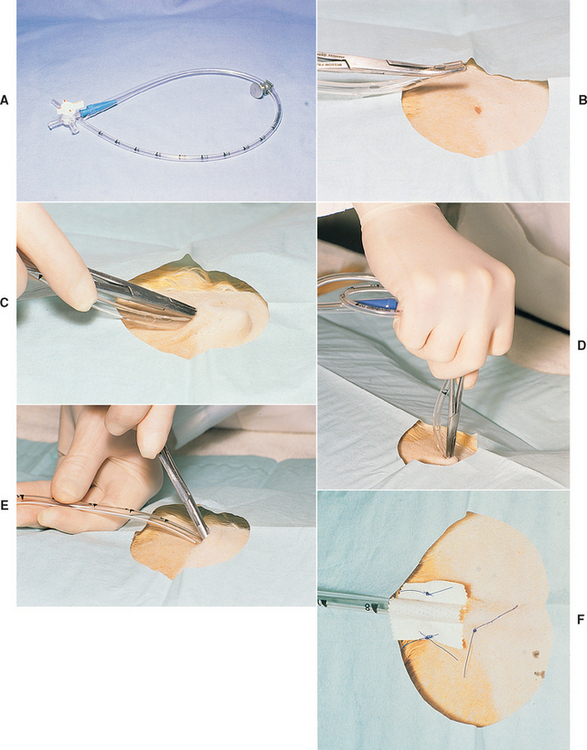
Pediatric chest tubes can be obtained from hospital supply companies. These tubes have multiple fenestrations, are calibrated along their length, and are radiopaque. For treating pyothorax, the tube should be as large as will fit between the ribs. The size of the tube is less critical for control of pneumothorax. Before placement the end of the tube is occluded with a syringe adapter, a three-way valve, and a hose clamp (Fig. 24-4, A).
Sterile technique is used during placement of the chest tube. In an animal with unilateral disease, the tube is placed in the involved side of the thorax. Either side can be used in an animal with bilateral disease. The lateral side of the animal over the caudal rib cage is shaved and surgically prepared. The animal is anesthetized or heavily sedated. If the animal is sedated, a local anesthetic is placed subcutaneously at the tenth ICS and within the subcutaneous tissues, intercostal muscles, and pleura at the seventh ICS. The dorsoventral orientation is one half to two thirds the distance from the costochondral junction to the thoracolumbar musculature. This distance should correspond to the level where the ribs are maximally bowed.
The length of tube to be advanced into the chest must be determined from thoracic radiographs or by external landmarks on the animal. The tube should extend from the tenth ICS to the first rib. The fenestrations in the tube must not extend outside the point of exit from the pleural cavity.
A stab incision is made through the skin at the tenth ICS. A purse-string suture is then placed around the opening but is not tied. Some chest tubes made for humans contain a stylet. Smaller chest tubes are inserted with the aid of curved hemostats. The tip of the tube is grasped with the tip of the hemostats with the tube parallel to the body of the clamps (see Fig. 24-4, B).
The tube, with the stylet or hemostats, is then tunneled subcutaneously from the tenth to the seventh ICS. If hemostats are used, the tips are directed away from the animal’s body (see Fig. 24-4, C). Once the tip reaches the seventh ICS, the stylet or hemostats are raised perpendicular to the chest wall. The palm of the hand is placed over the end of the stylet or the hemostat handles, and the tube is thrust through the body wall with one rapid motion (see Fig. 24-4, D). Once the tube has entered the pleural space, it is quickly advanced forward until the predetermined length has entered the chest while the stylet or hemostats are withdrawn (see Fig. 24-4, E).
An alternative technique can be used to minimize trauma to the lungs caused when thrusting the tube through the body wall. In this technique, after the skin incision has been made and a purse-string suture placed, an assistant standing at the head of the animal draws the skin of the thorax cranially to pull the skin opening forward from the tenth to the seventh ICS (Fig. 24-5). With the skin held in this position, hemostats are used to bluntly dissect through the thoracic and intercostal musculature to the pleura. At this point the chest tube with the stylet or hemostats is easily popped through the pleura into the chest with minimal force. The tube is then advanced and the skin released.
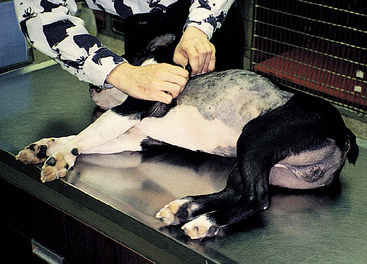
FIG 24-5 After an assistant pulls the skin forward, an incision can be made through the skin at the seventh intercostal space and blunt dissection is used to reach the pleura. A chest tube can be popped into the pleural space with minimal trauma to the underlying lung. When the skin is released, the tube will course through a subcutaneous tunnel to prevent air leaks around the tube.
Air will be sucked into the pleural cavity during tube placement regardless of the method used. This air must be immediately removed through the tube using a 35-ml syringe. The purse-string suture is then tied around the tube. Immediately external to the skin entrance, the tube is attached to the body wall by suturing the tape that is formed as a butterfly around the tube to the skin on either side of it (see Fig. 24-4, F) or by using a Chinese finger trap suture around the tube and attached to the skin. This prevents the chest tube from being withdrawn if tension is accidentally applied to the tubing. The opening in the skin is covered with a sterile sponge with antiseptic ointment. A light wrap is placed around the tube to hold it against the chest wall. The wrap must not be too tight. A wrap that is too tight can greatly decrease chest wall compliance and increase the work of breathing in these compromised animals. The hose clamp is placed on the tube between the animal and the three-way valve to further protect against pneumothorax whenever suction is not being applied to the tube. An Elizabethan collar is always placed on the animal because a single bite through the tube can be fatal.
Thoracic radiographs are taken to evaluate the tube position and the effectiveness of drainage. Two views must be evaluated. Ideally, the tube should extend along the ventral aspect of the pleural space to the thoracic inlet. The most important sign of adequate tube placement is the absence of areas of persistent fluid or air accumulation. If areas of fluid or air persist, it may be necessary to replace the tube or place a second tube in the opposite side.
Once a chest tube is in place and is determined to be in a satisfactory position, its effectiveness must be monitored regularly by thoracic radiography, generally every 24 to 48 hours. The animal must also be monitored for the development of secondary complications. These include infection and the leakage of air. The bandage should be removed at least daily. The site where the tube enters the skin should be evaluated for signs of inflammation or subcutaneous emphysema. The tube and skin sutures should be examined for signs of motion. The skin around the tube is kept clean, and a sterile sponge is replaced over the entry site of the tube before rebandaging. Stopcock ports should be protected with sterile caps when not in use. Gloves should be worn and the stopcock ports wiped with hydrogen peroxide before use.
THORACOSCOPY AND THORACOTOMY
A definitive diagnosis for the cause of pleural effusion is sometimes elusive. In such cases, thoracoscopy or thoracotomy may be necessary to allow visual assessment of the thoracic cavity and the collection of specimens for histologic and bacteriologic analysis. Mesotheliomas and pleural carcinomatosis are often diagnosed through these methods.