CHAPTER 25 Disorders of the Pleural Cavity
PYOTHORAX
Etiology
Septic exudate in the pleural cavity is referred to as pyothorax. It is most often idiopathic in origin, particularly in cats. It can result from foreign bodies, puncture wounds through the chest wall, esophageal tears (usually from ingested foreign bodies), and extension of pulmonary infection. Thoracic foreign bodies are usually migrating grass awns. They are rare in cats and most common in sporting breeds of dogs in states where there is a large concentration of foxtail grasses (e.g., California).
Clinical Features
Dogs and cats with pyothorax have clinical signs referable to pleural effusion and abscess formation. Signs may be acute or chronic. Tachypnea, decreased lung sounds, and increased abdominal excursions are typical of pleural effusion. In addition, fever, lethargy, anorexia, and weight loss are common. Animals may be presented in septic shock or demonstrate signs of systemic inflammatory response syndrome.
Diagnosis
The diagnosis of pyothorax is made through thoracic radiography and the cytologic evaluation of pleural fluid. Thoracic radiographs are used to confirm the presence of pleural effusion and to determine whether the disease is localized, unilateral, or bilateral. In most animals fluid is present throughout the pleural space. The finding of a localized accumulation of fluid indicates the possible presence of pleural fibrosis, mass lesions, or lung lobe torsion. Thoracic radiographs are taken again after removal of the fluid to evaluate the pulmonary parenchyma for evidence of underlying disease (e.g., bacterial pneumonia, foreign body) that may have caused the pyothorax. The identification of a septic exudate by pleural fluid analysis establishes the diagnosis of pyothorax.
Septic suppurative inflammation is a consistent finding in pleural fluid examined cytologically, except in animals that are receiving antibiotics (Fig. 25-1; see also Chapter 23). Pleural fluid is best evaluated by Gram staining and aerobic and anaerobic bacterial cultures. Anaerobes are usually present in the fluid, and in many dogs and cats, more than one type of bacteria are present. All of the types of bacteria involved may not grow in the laboratory in spite of cytologic evidence of their presence, possibly because of competition between organisms or an inhibitory effect of the exudative fluid. Organisms such as Actinomyces and Nocardia particularly do not grow well if specimens have been cultured using routine procedures. The absence of growth of bacteria does not rule out a diagnosis of pyothorax.
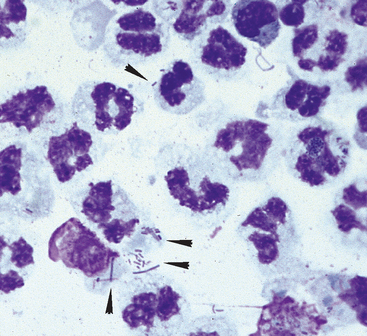
FIG 25-1 Cytologic preparation of a specimen of a pleural effusion from a cat with pyothorax. Degenerative neutrophils predominate, and intracellular and extracellular bacteria are prevalent (arrowheads). Both rods and cocci are seen.
Evaluation of the patient’s systemic status may reveal evidence of active inflammation, systemic inflammatory response syndrome, or sepsis.
Treatment
Medical therapy for pyothorax includes antibiotics, drainage of the pleural cavity, and appropriate supportive care (e.g., fluid therapy). At first, empirically selected antibiotics are administered intravenously. The results of Gram staining and culture and sensitivity testing are helpful in selecting antibiotics. Generally, anaerobes and Pasteurella (a common isolate from cats with pyothorax) are sensitive to amoxicillin-clavulanate. Other gram-negative organisms are often sensitive to amoxicillin-clavulanate, but their antibiotic sensitivities are unpredictable. Unfortunately, this drug is not available for intravenous administration. Ampicillin with sulbactam, a different β-lactamase inhibitor, is an excellent substitute for intravenous use (22 mg/kg of ampicillin q8h). Other drugs that have good activity against anaerobic organisms are chloramphenicol, metronidazole, and clindamycin. If metronidazole or clindamycin is used, additional gram- negative coverage is necessary and is achieved by adding a fluoroquinolone or aminoglycoside antibiotic to the treatment. Addition of one of these antibiotics may also be necessary in patients receiving ampicillin with sulbactam that fail to show improvement in clinical condition, complete blood count (CBC), and fluid cytology within the first few days of treatment.
Oral antibiotics are used once significant improvement is noted, usually about the time of chest tube removal. Amoxicillin-clavulanate (20 to 25 mg/kg q8h) is used in patients that have responded to ampicillin with sulbactam. Oral antibiotic therapy is continued for an additional 4 to 6 weeks.
Drainage of the septic exudate is an essential part of the treatment of pyothorax. Although treatment with antibiotics alone often causes dramatic improvement in the animal’s clinical condition initially, the signs generally recur, and complications of the prolonged infection, such as fibrosis or abscesses, are more likely (Fig. 25-2). Indwelling chest tubes provide the best drainage and can be used to keep the exudate from accumulating during the initial days of antibiotic therapy. Dogs and cats in critical condition at presentation are stabilized through the use of needle thoracocentesis and shock therapy before chest tube placement. Intermittent needle thoracocentesis is minimally effective for draining the pleural cavity and is not recommended for treatment unless the owner cannot afford the expense of chest tube management.
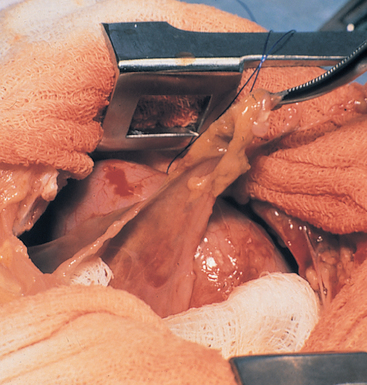
FIG 25-2 Pleural fibrosis manifested by markedly thickened pleura seen during thoracotomy in a cat with chronic pyothorax. Treatment with antibiotics alone was attempted, and several weeks later the cat’s condition deteriorated. Fibrosis was too extensive to allow for routine drainage with chest tubes. Surgical debridement, several lobectomies, drainage through surgically placed tubes, and long-term antibiotic therapy resulted in a cure.
Chest tube placement and assessment of positioning are discussed in Chapter 24. Animals probably respond most rapidly to constant suctioning of the exudate from the chest, although intermittent suction is certainly adequate and often more feasible. Constant suction is applied with a suction pump and collection unit. Disposable pediatric cage-side collection units (e.g., Thora-Seal III, Argyle, Sherwood Medical) are available through hospital supply companies. These units allow monitoring of collected fluid volume and adjustment of suction pressure. An initial suction pressure of 10 to 15 cm H2O is used, but more or less pressure may be necessary depending on the viscosity of the pleural fluid and the collapsibility of the tubes. The collection systems must be carefully monitored for the occurrence of leaks or malfunctions that could cause a fatal pneumothorax.
Intermittent suction by syringe is ideally performed every 2 hours for the first days of treatment, with arrangements made for drainage to continue during the night. Within a few days the volume of fluid produced will decrease, and the interval can then be lengthened. If such intensive care is not possible, an effort should still be made to empty the chest of fluid at least once late in the evening to minimize the accumulation of exudate overnight.
Lavage of the chest cavity is performed twice daily and consists of the removal of any fluid within the chest, followed by the slow infusion of warmed sterile saline solution into the chest. A volume of approximately 10 ml/kg of body weight is infused, but the infusion should be discontinued if any distress is noted. After this the animal is gently rolled from side to side, and the fluid is removed. Sterile technique is used throughout the procedure. The volume recovered should be about 75% of the volume infused. If less fluid is retrieved, this may indicate that the chest tube is no longer providing adequate drainage and should be assessed by radiograph or ultrasonography. There is no obvious benefit from the addition of antibiotics, antiseptics, or enzymes to the lavage solution. The addition of heparin (1500 U/100 ml) to the lavage fluid may decrease fibrin formation.
All adapter ports connected to the chest tube should be covered with sterile caps when not in use. When accessing the ports, the clinician should wear gloves and remember to wipe the ports with hydrogen peroxide before use.
Thoracic radiographs are taken every 24 to 48 hours to ensure that the chest is being completely drained of fluid. Failure to monitor the effectiveness of drainage radiographically can lead to costly prolongation of the intensive care required for maintenance of the chest tube.
Serum electrolyte concentrations are also monitored. Many dogs and cats with pyothorax are dehydrated and anorectic at presentation and require intravenous fluid therapy. Supplementation of the intravenous fluid with potassium may be necessary.
The decision to discontinue drainage and remove the chest tube is based on the fluid volume and cytologic characteristics. The volume of fluid recovered should have decreased to less than 2 ml/kg/day. Slides of the fluid are prepared daily and evaluated cytologically. Bacteria should no longer be visible intracellularly or extracellularly. Neutrophils will persist but should no longer appear degenerative (Fig. 25-3). When these criteria have been met and no pockets of fluid are seen on thoracic radiographs, the chest tube is removed and the animal is monitored clinically for at least 24 hours for the development of pneumothorax or the recurrence of effusion. Thoracic radiographs can be taken to more sensitively evaluate the animal for these potential problems.
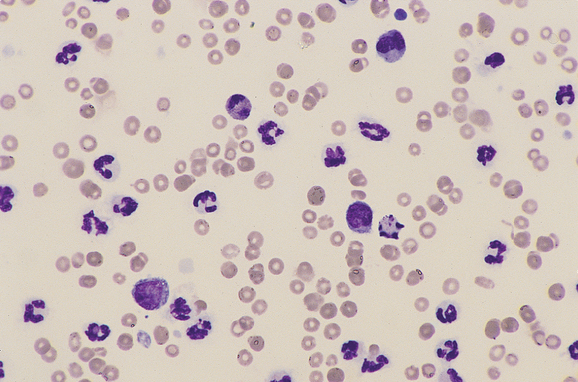
FIG 25-3 Cytologic preparation of a specimen of a pleural effusion from a cat being treated successfully for pyothorax with chest tube drainage and antibiotics. Compared with the fluid shown in Fig. 25-1, the nucleated cell count is low, the neutrophils are nondegenerative, organisms are not present, and mononuclear cells are appearing (Cytocentrifuge prep).
Thoracic radiographs are evaluated 1 week after removal of the chest tube and 1 week and 1 month after discontinuation of the antibiotic therapy. These radiographs are obtained so that a localized nidus of disease such as a foreign body or an abscess can be identified and also so that recurrence of a pyothorax can be detected before large volumes of pleural fluid accumulate. Such niduses are often invisible when large volumes of pleural fluid are present or while aggressive therapy is in progress.
Exploratory thoracotomy is indicated for the removal of a suspected nidus of infection and in those animals that do not respond to medical therapy. In the latter instance surgery may be necessary to remove fibrotic and diseased tissue or a foreign body. Failure to respond is suggested by the continued need for a chest tube for longer than 1 week after the start of appropriate antibiotic treatment and drainage, although reported cases that have undergone complete recovery after medical management have required drainage by chest tubes for longer periods. Furthermore, persistence of large pockets of fluid in spite of appropriate chest tube placement may necessitate the decision to perform a thora cotomy earlier. Computed tomography of the chest may be a more sensitive method for detecting persistent pulmonary lesions than thoracic radiography. Rooney et al. (2002) recommended consideration for thoracotomy particularly in dogs that have radiographic evidence of mediastinal or pulmonary lesions or if Actinomyces spp. are identified in the pleural fluid.
Prognosis
Most cases of pyothorax are idiopathic. The prognosis for animals with pyothorax is fair to good if it is recognized early and treated aggressively. Waddell et al. (2002) reported a survival rate for cats of 66%, excluding those that were euthanatized before treatment. In their report, 5 of 80 cats required thoracotomy. Treatment success in dogs has been reported to be as high as 100% with medical therapy alone (Piek et al., 2000). However, in a study by Rooney et al. (2002) of 26 dogs, only 25% of dogs were successfully treated medically whereas 78% responded favorably to thoracotomy. One possible explanation for the poor success of medical management in the latter study is the geographic location in a region of the country where grass awn migration is common.
Exploratory surgery is necessary to ensure complete resolution of the problem in dogs or cats with foreign bodies in the thoracic cavity. Radiolucent foreign bodies can be difficult to find, however, and the prognosis for pyothorax secondary to them is more guarded. Long-term complications of pyothorax such as pleural fibrosis and restrictive lung disease are uncommon.
CHYLOTHORAX
Etiology
Chylothorax is the accumulation of chyle within the thoracic cavity. The chyle originates from the thoracic duct, which carries triglyceride-rich fluid from the intestinal lymphatics and empties into the venous system in the anterior thorax. The fluid also contains lymphocytes, protein, and fat-soluble vitamins. Thoracic duct rupture after thoracic trauma can result in transient chylothorax. However, most cases are not the result of a ruptured duct. Possible causes of nontraumatic chylothorax include generalized lymphangiectasia, inflammation, and obstruction of lymphatic flow. Flow can be obstructed for physical reasons, such as neoplasia, or as a result of increased venous pressures.
Chylothorax can be categorized as congenital, traumatic, or nontraumatic. A congenital predisposition may exist in animals in which chylothorax develops later in life. Traumatic events that induce chylothorax can be surgical (e.g., thoracotomy) or nonsurgical (e.g., being hit by a car). Nontraumatic causes of chylothorax include neoplasia, particularly mediastinal lymphoma in cats; cardiomyopathy, dirofilariasis, pericardial disease, and other causes of right-sided heart failure; lung lobe torsion; diaphragmatic hernia; and systemic lymphangiectasia. No underlying disease can be identified in most animals, in which case idiopathic chylothorax is diagnosed.
Fibrosing pleuritis and pericarditis can be associated with chylothorax. Cats, in particular, may develop fibrosing pleuritis, which can interfere with normal expansion of the lungs even after thoracocentesis. Inflammation and thickening of the pericardium could contribute to the further formation of chylous effusion.
Clinical Features
Chylothorax can occur in dogs or cats of any age. Afghan Hounds and Shiba Inus appear to be predisposed to the disorder. The primary clinical sign is respiratory distress typical of pleural effusion. Although the distress is often acute in onset, more subtle signs have generally been present for more than a month. Lethargy, anorexia, weight loss, and exercise intolerance are common. In some cases cough is the only presenting sign.
Diagnosis
Chylothorax is diagnosed by thoracic radiographs and the identification of chyle through cytologic and biochemical evaluation of pleural fluid obtained by thoracocentesis (see Chapter 23). Lymphopenia and panhypoproteinemia may be present in peripheral blood.
Once chylothorax has been diagnosed, further diagnostic tests are performed to identify potential underlying disease (Box 25-1). These tests include thoracic ultrasonography; echocardiography; microfilarial examination and adult antigen testing for heartworm disease; and, in cats, the measurement of thyroid hormone concentrations. Lymphangiography can be used to identify lymphangiectasia, sites of obstruction, and, rarely, sites of leakage from the thoracic duct. Lymphangiography is performed before the surgical ligation of lymphatics is attempted.
Treatment
Thoracocentesis and appropriate fluid therapy are used to stabilize dogs and cats with chylothorax, as needed, at presentation. Electrolyte abnormalities may be present. A concerted effort is made to identify any underlying cause of the chylothorax so that it can be directly treated. Elimination of the underlying problem may result in resolution of the chylothorax, although medical management (as described later for idiopathic chylothorax) is generally required for several weeks or even months. The exception is chylothorax of traumatic origin, which generally resolves within 1 to 2 weeks.
A routinely successful treatment for idiopathic chylothorax has not been established. Medical management is initially attempted because spontaneous remission occurs in some cases. In the absence of resolution with medical therapy, thoracic duct ligation and pericardectomy are recommended.
Medical management consists primarily of intermittent thoracocentesis and a low-fat diet. Thoracocentesis is performed as needed on the basis of the owner’s observation of increased respiratory rate or effort or decreased activity or appetite. Initially, thoracocentesis may need to be performed every 1 to 2 weeks. The interval between thoracocenteses will gradually lengthen if the chylothorax is responsive to medical management. Ultrasound guidance of the needle during thoracocentesis is especially helpful in removing pockets of chyle from the pleural cavity, and by increasing the effectiveness of drainage, it can prolong the interval between thoracocenteses.
A low-fat, nutritionally complete diet is fed (see Chapter 54). In humans medium-chain triglyceride oil is absorbed directly into the bloodstream, bypassing the lymphatics, and can be used as a fat supplement. Unfortunately, in dogs these triglycerides have been shown to enter the thoracic duct. Nevertheless, they can be added to the diet if additional calories are desired.
Medical management may be facilitated by the administration of rutin, a benzopyrone drug. Rutin has been used in humans for the treatment of lymphedema. It is thought to decrease the protein content of the effusion by affecting macrophage function. The resorption of effusion may thereby be enhanced and fibrosis of the pleura minimized. The drug is available over the counter at health food stores. A dosage of 50 to 100 mg/kg given orally every 8 hours is recommended.
Surgical management is considered if clinical signs have not improved within 2 to 3 months of medical therapy or if signs are intolerable. The recommended surgical management of chylothorax includes thoracic duct ligation and pericardectomy. Thoracic duct ligation is technically difficult and is ideally performed by an experienced surgeon. Multiple ligations of the thoracic duct and its collaterals are performed. The ducts are identified by lymphangiography before surgery, and lymphangiography is repeated after ligation to assess the success of ligation. Pericardectomy is recommended at the time of thoracic duct ligation and is associated with an improved outcome (Fossum et al., 2004).
Placement of pleuroperitoneal or pleurovenous shunts or mesh within the diaphragm to allow fluid to drain away from the pleural space has also been recommended for the management of chylothorax and should be considered if medical and surgical treatment are unsuccessful. These drainage procedures provide a route for the leaking chyle to reenter the circulation without producing the respiratory compromise associated with pleural effusion. Unfortunately, drains often become nonfunctional within months of placement.
Prognosis
The prognosis for chylothorax has generally been regarded as guarded unless the chylothorax was traumatically induced or the result of a reversible condition. However, a study by Fossum et al. (2004) indicated an overall success rate for thoracic duct ligation and pericardectomy of 100% in dogs and 90% in cats. It is not possible to predict the contribution of fibrosing pleuritis to clinical signs in cats with this complication. In cats with continued respiratory difficulties following resolution of effusion, decortication of the lung is considered.
SPONTANEOUS PNEUMOTHORAX
Spontaneous pneumothorax occurs when preexisting pulmonary cavitary lesions rupture. It is much less common than traumatic pneumothorax and occurs more often in dogs than cats. Rapid, profound respiratory distress occurs in the subset of animals in which a tension pneumothorax develops. Cavitary lesions can be congenital or idiopathic or result from prior trauma, chronic airway disease (e.g., idiopathic feline bronchitis), or Paragonimus infection. Necrotic centers can develop in neoplasms, thromboembolized regions (e.g., from dirofilariasis), abscesses, and granulomas involving the airways, and these can rupture, allowing air to escape into the pleural space. (See Chapter 20 for further discussion of cavitary lesions.)
Thoracocentesis is useful for initial stabilization of the animal’s condition. If frequent thoracocentesis is needed to control the pneumothorax, a chest tube is placed (see Chapter 24). Dogs and cats are evaluated for underlying disease with thoracic radiographs (repeated after full lung expansion), computed tomography of the thorax, multiple fecal examinations for Paragonimus ova (see Chapter 20), heartworm tests, and possibly tracheal wash fluid analysis or bronchoscopy. Computed tomography is much more sensitive for the identification of bullae or blebs and should be performed before thoracotomy. In a study by Au et al. (2006), thoracic radiography identified bullae or blebs in only 2 of 12 dogs with spontaneous pneumothorax whereas computed tomography was successful in identifying lesions in 9 of these dogs.
Patients with Paragonimus infections generally respond to medical treatment (See Chapter 22). Otherwise, surgical therapy is indicated for most animals. In a review of 21 cases, Holtsinger et al. (1993) found that most dogs with spontaneous pneumothorax managed medically with chest tubes and suction ultimately required surgery during the initial hospitalization or upon subsequent recurrence of pneumothorax to resolve the problem. Because unobserved recurrence of spontaneous pneumothorax can be fatal, conservative treatment is believed to carry more risk than surgery. Furthermore, a report of 64 cases by Puerto et al. (2002) showed that recurrence and mortality rates for dogs with spontaneous pneumothorax were lower in dogs that had surgery compared with dogs that were treated conservatively. A median sternotomy is generally recommended to allow exposure of all lung lobes because it is often not possible to localize all cavitary lesions preoperatively (Fig. 25-4). Abnormal tissue is evaluated histologically and microbiologically for a definitive diagnosis.
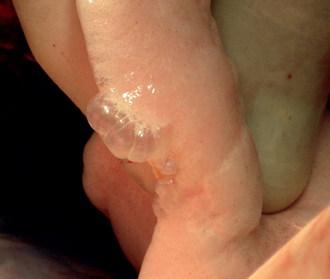
FIG 25-4 Blebs can be seen in this intra-operative image of the lung of a dog that presented with spontaneous pneumothorax. The size of these blebs precluded their identification by either thoracic radiography or computed tomography.
(Courtesy Dr. Guillaume Pierre Chanoit.)
Conservative therapy consists of cage rest and chest tube placement with continuous suction (see the section on pyothorax). In large dogs a one-way Heimlich valve can be used rather than suction.
Regardless of the treatment used, recurrence is a possibility. Accurate diagnosis of the underlying lung disease and determination of the extent of involvement through a thoracotomy assist in determining the prognosis.
NEOPLASTIC EFFUSION
Neoplastic effusions resulting from mediastinal lymphoma are treated with radiation or chemotherapy (see Chapter 80). Effusions caused by mesothelioma or carcinoma of the pleural surfaces may respond to palliative therapy with intracavitary infusions of cisplatin or carboplatin (see Moore, 1992). Placement of pleuroperitoneal shunts or intermittent thoracocentesis to alleviate the degree of respiratory compromise can also be considered to prolong the life of patients that have no clinical signs beyond those resulting from the accumulation of pleural effusion.
Au JJ, et al. Use of computed tomography for evaluation of lung lesions associated with spontaneous pneumothorax in dogs: 12 cases (1999-2002). J Am Vet Med Assoc. 2006;228:733.
Fossum TW, et al. Chylothorax in cats: 37 cases (1969–1989). J Am Vet Med Assoc. 1991;198:672.
Fossum TW, et al. Chylothorax associated with right-sided heart failure in 5 cats. J Am Vet Med Assoc. 1994;204:84.
Fossum TW. Small animal surgery, ed 3. St Louis: Mosby, 2007.
Holtsinger RH, et al. Spontaneous pneumothorax in the dog: a retrospective analysis of 21 cases. J Am Anim Hosp Assoc. 1993;29:195.
Lipscomb VJ, et al. Spontaneous pneumothorax caused by pulmonary blebs and bullae in 12 dogs. J Am Anim Hosp Assoc. 2003;39:435.
Moore AS. Chemotherapy for intrathoracic cancer in dogs and cats. Problems in Vet Med. 1992;4:351.
Piek CJ, et al. Pyothorax in 9 dogs. Vet Q. 2000;22:107.
Puerto DA, et al. Surgical and nonsurgical management of and selected risk factors for spontaneous pneumothorax in dogs: 64 cases (1986–1999). J Am Vet Med Assoc. 2002;220:1670.
Rooney MB, et al. Medical and surgical treatment of pyothorax in dogs: 26 cases (1991-2001). J Am Vet Med Assoc. 2002;221:86.
Scott JA, et al. Canine pyothorax: clinical presentation, diagnosis, and treatment. Compend Contin Educ Pract Vet. 2003;25:180.
Smeak DD, et al. Treatment of chronic pleural effusion with pleuroperitoneal shunts in dogs: 14 cases (1985–1999). J Am Vet Med Assoc. 2001;219:1590.
Thompson MS, et al. Use of rutin for the medical management of idiopathic chylothorax in four cats. J Am Vet Med Assoc. 1999;215:245.
Waddell LS, et al. Risk factors, prognostic indicators, and outcome of pyothorax in cats: 80 cases (1986–1999). J Am Vet Med Assoc. 2002;221:819.
Walker AL, et al. Bacteria associated with pyothorax of dogs and cats: 98 cases (1989–1998). J Am Vet Med Assoc. 2000;216:359.
White HL, et al. Spontaneous pneumothorax in two cats with small airway disease. J Am Vet Med Assoc. 2003;222:1573.
 BOX 25-1
BOX 25-1