CHAPTER 87 Disorders of Hemostasis
GENERAL CONSIDERATIONS
Spontaneous or excessive bleeding is relatively common in small animals, particularly in dogs. As a general rule, a systemic hemostatic abnormality is the underlying cause of excessive bleeding in dogs and cats that have sustained trauma or are undergoing a surgical procedure and in dogs evaluated because of spontaneous bleeding tendencies (spontaneous bleeding is rare in cats with hemostatic abnormalities). Approaching these patients’ bleeding in a logical and systematic fashion allows the clinician to confirm the presumptive diagnosis in most cases.
In addition to bleeding, abnormal hemostatic mechanisms can also cause thrombosis and thromboembolism, potentially leading to organ failure. Spontaneous bleeding disorders are extremely common in dogs evaluated at our clinic but are rare in cats. Thromboembolic disorders are rare in both dogs and cats without underlying cardiovascular disorders (e.g., cats with hypertrophic cardiomyopathy and aortic thromboembolism; see Chapter 12). The most common disorder leading spontaneous bleeding in dogs seen at our clinic is thrombocytopenia, mainly of immune-mediated pathogenesis. Other common hemostatic disorders leading to spontaneous bleeding in dogs evaluated at our hospital include disseminated intravascular coagulation (DIC) and rodenticide poisoning. Congenital clotting factor deficiencies resulting in spontaneous bleeding are rare. Although von Willebrand disease (vWD) is common in certain breeds (see p. 1251), it is not a common cause of spontaneous bleeding. Abnormalities in hemostasis screens are frequently noted in cats with liver disease, feline infectious peritonitis (FIP), or neoplasia; however, spontaneous bleeding tendencies are extremely rare in these patients. Decreased production of platelets (thrombocytopenia) or virus-induced thrombocytopathia resulting in spontaneous bleeding is occasionally seen in cats with retrovirus-induced bone marrow disorders.
PHYSIOLOGY OF HEMOSTASIS
Under normal conditions, injury to a blood vessel leads to immediate vascular changes (e.g., vasoconstriction) and rapid activation of the hemostatic system. Changes in axial blood flow lead to exposure of circulating blood to subendothelial collagen, resulting in rapid adhesion of platelets to the affected area. The adhesion of platelets to the subendothelium is mediated by adhesive proteins, such as von Willebrand factor (vWF) and fibrinogen. After adhering to the area of endothelial damage, platelets aggregate and form the primary hemostatic plug, which is short lived (seconds) and unstable. The primary hemostatic plug serves as a framework in which secondary hemostasis occurs because most of the clotting factors “assemble” the thrombus or clot on the platelet plug.
Although the intrinsic, extrinsic, and common coagulation pathways have been well characterized and are still used to teach physiology of hemostasis, coagulation in vivo does not necessarily follow these distinct pathways. For example, factors XII and XI do not appear to be necessary for the initiation of coagulation (e.g., dogs and cats with factor XII deficiency do not have spontaneous bleeding tendencies). In the past 2 decades the traditional coagulation cascade has been thought of as a common pathway from early in the process; the traditional intrinsic, extrinsic, and common pathways are now known to be interrelated (Schenone et al., 2004).
In the traditional scheme, activation of the contact phase of the coagulation cascade occurs almost simultaneously with platelet adhesion and aggregation (Fig. 87-1) and leads to the formation of fibrin through the intrinsic coagulation cascade. A good mnemonic is to refer to the intrinsic system as the “dime store” coagulation cascade: “it is not $12, but $11.98” (for factors XII, XI, IX, and VIII). Factor XII is activated by contact with the subendothelial collagen and by the platelet plug; once it has been activated, fibrin, or the secondary hemostatic plug, forms. Prekallikrein (Fletcher factor) and high-molecular-weight kininogen are important co-factors for factor XII activation. The role of the contact phase of coagulation in vivo is questionable. The secondary hemostatic plug is stable and long lasting. In addition, whenever tissue trauma occurs, the release of tissue procoagulants (collectively referred to as tissue factor) results in activation of the extrinsic coagulation cascade, also leading to the formation of fibrin (see Fig. 87-1). Tissue factor is ubiquitous and is present on the membrane of most cells, with the exception of normal endothelium.
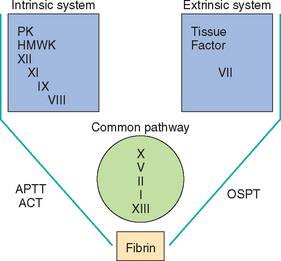
FIG 87-1 The traditional intrinsic, extrinsic, and common coagulation pathways. PK, Prekallikrein; HMWK, high-molecular-weight kininogen; APTT, activated partial thromboplastin time; ACT, activated coagulation time; OSPT, one-stage prothrombin time.
The stimuli that activate the contact phase of coagulation also activate the fibrinolytic and kinin pathways. Fibrinolysis is extremely important as a safeguard mechanism because it prevents excessive clot or thrombus formation. When plasmin lyses fibrinogen and fibrin, it generates fibrin degradation products (FDPs), which impair additional platelet adhesion and aggregation in the site of injury. Once fibrin has been stabilized by complexing factor XIII, plasmin biodegradation generates d-dimers instead. The activation of plasminogen into plasmin results in the destruction (lysis) of an existing clot (or thrombus) and interferes with the normal clotting mechanisms (inhibition of platelet aggregation and clotting factor activation in the affected area). Therefore excessive fibrinolysis usually leads to spontaneous bleeding. Two molecules stimulate plasminogen activation into plasmin: tissue plasminogen activator (tPA) and urokinase-type plasminogen activator. Three plasminogen activator inhibitors (PAI) termed PAI-1, -2, and -3 inhibit fibrinolysis, thus leading to thrombosis.
Other systems that oppose blood coagulation also become operational once intravascular clotting has occurred. The best-characterized ones include antithrombin (AT), a protein synthesized by hepatocytes that acts as a co-factor for heparin and inhibits the activation of factors IX, X, and thrombin. AT also inhibits tPA. Proteins C and S are two vitamin K–dependent anticoagulants also produced by hepatocytes. These three factors are some of the natural anticoagulants that prevent excessive clot formation.
CLINICAL MANIFESTATIONS OF SPONTANEOUS BLEEDING DISORDERS
In the evaluation of a cat or dog with spontaneous or excessive bleeding, the clinician should ask the owners the following questions, which may provide additional clues to the pathogenesis of the coagulopathy:
The clinical manifestations of primary hemostatic abnormalities are quite different from those of secondary hemostatic abnormalities (Box 87-1). Indeed, the clinician should be able to classify the type of coagulopathy on the basis of the physical examination findings before submitting any samples for laboratory evaluation. This is rather easy to conceptualize by thinking about the normal coagulation mechanisms. For example, a primary hemostatic plug cannot form in a cat or dog with severe thrombocytopenia or platelet dysfunction. Because this plug is short lived and eventually covered with fibrin (generated through the secondary hemostatic mechanisms), multiple, short-lived bleeds occur that are arrested as soon as fibrin is formed, resulting in multiple small and superficial hemorrhages. This is analogous to turning on and off a faucet connected to a garden hose with multiple perforations (i.e., an irrigator); multiple spurts of water (i.e., blood) form adjacent to the hose (i.e., the vessel). On the other hand, a short-lived primary hemostatic plug can form in a cat or dog with severe clotting factor deficiencies (e.g., hemophilia, rodenticide poisoning); enough functional platelets are present, but fibrin cannot be generated. The result of this is a delayed, continuous, long-lasting bleed, leading to hematoma formation or bleeding into a body cavity. This is analogous to turning on a faucet connected to a regular garden hose with a single large opening; in this situation, water (i.e., blood) continues to flow and collect in large amounts next to the opening in the hose (i.e., vessel).
 BOX 87-1 Clinical Manifestations of Primary and Secondary Hemostatic Defects
BOX 87-1 Clinical Manifestations of Primary and Secondary Hemostatic Defects
| Primary Hemostatic Defect | Secondary Hemostatic Defect |
|---|---|
| Petechiae common | Petechiae rare |
| Hematomas rare | Hematomas common |
| Bleeding in skin and mucous membranes | Bleeding into muscles, joints, and body cavities |
| Bleeding immediately after venipuncture | Delayed bleeding after venipuncture |
Spontaneous bleeding infrequently occurs in cats and dogs with excessive fibrinolysis. I have evaluated four dogs with protein-losing nephropathy and nephrotic syndrome in which spontaneous bleeding (i.e., petechiae and ecchymoses) appeared to result from enhanced fibrinolysis. We have recently documented delayed postoperative bleeding in retired racing Greyhounds that may be associated with hyperfibrinolysis (Lara et al., 2007).
Cats and dogs with primary hemostatic defects (i.e., platelet disorders) therefore have typical manifestations of superficial bleeding, consisting of petechiae, ecchymoses, bleeding from mucosal surfaces (e.g., melena, hematochezia, epistaxis, hematuria), and prolonged bleeding immediately after venipuncture. In clinical practice, the majority of primary hemostatic disorders are caused by decreased numbers of circulating platelets (thrombocytopenia). Primary hemostatic defects occasionally result from platelet dysfunction (e.g., uremia, von Willebrand disease [vWD], monoclonal gammopathies, vector-borne diseases). Primary hemostatic defects caused by vascular disorders are extremely rare in cats and dogs and are not discussed here.
Clinical signs in cats and dogs with secondary hemostatic defects (i.e., clotting factor deficiencies) consist of deep bleeding, including bleeding into body cavities and joints, and deep hematomas, most of which are discovered as a lump. Certain congenital coagulopathies, including factor XII, prekallikrein, and high-molecular-weight kininogen deficiencies, result in a marked prolongation of the activated coagulation time (ACT) or activated partial thromboplastin time (APTT) without spontaneous or prolonged bleeding (see below).
Most secondary bleeding disorders seen in clinical practice are caused by rodenticide poisoning or liver disease; selective congenital clotting factor deficiencies occasionally can lead to spontaneous secondary bleeding disorders. A combination of primary and secondary bleeding disorders (mixed disorders) is seen almost exclusively in dogs and cats with DIC.
CLINICOPATHOLOGIC EVALUATION OF THE BLEEDING PATIENT
Clinicopathologic evaluation of the hemostatic system is indicated primarily in two subsets of patients: in those with spontaneous or prolonged bleeding and before surgery in patients with disorders commonly associated with bleeding tendencies (e.g., splenic hemangiosarcoma [HSA] and DIC in dogs; liver disease and clotting factor deficiency) or a suspected congenital coagulopathy (e.g., before ovariohysterectomy in a Doberman Pinscher suspected of having subclinical vWD).
When evaluating a cat or dog with a spontaneous bleeding disorder, the clinician should keep in mind that the preliminary clinical diagnosis can usually be confirmed by performing a handful of simple cage-side tests. If these tests do not yield a definitive answer or if a more specific diagnosis is desirable (e.g., the identification of specific clotting factor deficiencies), a plasma sample can be submitted to a referral veterinary diagnostic laboratory or a specialized coagulation laboratory (e.g., New York State Diagnostic Laboratory, Cornell University, Ithaca).
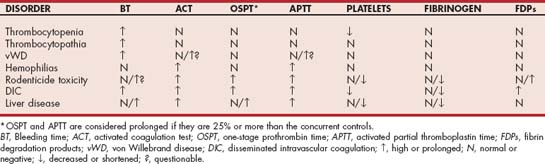
Some simple cage-side tests include evaluation of a blood smear; determination of the ACT, one-stage prothrombin time (OSPT), and APTT; quantification of FDP concentration or d-dimer assays; and the buccal mucosa bleeding time (BMBT) (Table 87-1). Examination of a good-quality, well-stained blood smear (e.g., Diff-Quik, Medion GmbH, Düdingen, Switzerland) provides important clues regarding platelet numbers and morphology. The first aspect of this examination should be to scan the smear at low power to identify platelet clumps; platelet clumping commonly results in pseudothrombocytopenia. Next, the oil immersion lens should be used to examine several representative monolayer fields (i.e., where approximately 50% of the red blood cells [RBCs] touch each other), and the number of platelets in five fields should be averaged. In dogs, 12 to 15 platelets should be present in each oil immersion field; in normal cats, 10 to 12 platelets per field should be seen. As a general rule, each platelet in an oil immersion field represents 12,000 to 15,000 platelets/μL (i.e., number of platelets/oil immersion field × 15,000 = platelets/μL). Cats and dogs with platelet counts of more than 30,000/μL and normal platelet function do not bleed spontaneously. Therefore the cause of bleeding is usually not thrombocytopenia if more than two or three platelets are visualized in each oil immersion field. The evaluation of platelet numbers should also include evaluation of the morphology of individual platelets because abnormal platelet morphology may reflect impaired platelet function.
 TABLE 87-1 Simple Cage-Side Tests for the Rapid Classification of Hemostatic Disorders
TABLE 87-1 Simple Cage-Side Tests for the Rapid Classification of Hemostatic Disorders
| TEST | RESULTS | MOST LIKELY DISORDER(S) IF PROLONGED (OR POSITIVE) |
|---|---|---|
| Platelet estimation in blood smear | Low | Thrombocytopenia |
| ACT | Prolonged | Intrinsic/common system defect |
| FDP/D-dimer | Positive | Enhanced fibrinolysis; DIC |
| BMBT | Prolonged | Thrombocytopenia, thrombocytopathia |
ACT, Activated clotting time; FDP, fibrin degradation products; DIC, disseminated intravascular coagulation; BMBT, buccal mucosal bleeding time.
The second set of cage-side tests of hemostatic ability are the ACT, OSPT, and APTT. For the APTT, 2mL of whole fresh blood is added to a tube containing diatomaceous earth; this activates the contact phase of coagulation, thus assessing the integrity of the intrinsic and common pathways (factors XII, XI, IX, VIII, X, V, II, and I) (see Fig. 87-1). If the activity of individual clotting factors involved in these pathways has decreased by more than 70% to 75%, the ACT is prolonged (normal, 60 to 90 seconds). Common coagulopathies associated with prolongation of the ACT are listed in Table 87-2. A cage-side instrument has recently been validated in dogs and cats (SCA 2000, Synbiotics Corp., San Diego, Calif.); a new easy-to-use instrument is now commercially available (CoagDx Analyzer, IDEXX, Westbrook, Maine). These units perform evaluation of the APTT or OSPT with only a small volume of blood for each test. The reference ranges for the APTT with this instrument are different than for the APTT obtained in referral diagnostic laboratories.
The third cage-side test that can be easily performed in practice is the determination of the FDP concentration (or titer) with the commercially available Thrombo Wellcotest (Thermo Fisher Scientific, Lenexa, Kan.). This latex agglutination test can detect circulating FDPs, which are generated during the cleavage of fibrin and fibrinogen (i.e., fibrinolysis). This test is commonly positive in dogs and in some cats with DIC. The FDP test is also positive in more than half of dogs with bleeding caused by rodenticide poisoning (e.g., warfarin). The mechanism of the latter is unknown; however, these results cannot be reproduced by the intracavitary or intramuscular injection of anticoagulated blood in normal dogs. Vitamin K antagonists are believed to activate fibrinolysis by inhibiting the production of PAI-1. Recently a point-of-care d-dimer assay has been validated in the dog (Stokol, 2003).
A fourth cage-side test that can be performed primarily in dogs is the BMBT (Box 87-2), in which a template (SimPlate, IDEXX) is used to make an incision in the buccal mucosa and the time until bleeding completely ceases is determined. The BMBT is abnormal in cats and dogs with thrombocytopenia or with platelet dysfunction. In an animal with clinical signs of a primary bleeding disorder (e.g., petechiae, ecchymoses, mucosal bleeding) and a normal platelet count, a prolonged bleeding time indicates an underlying platelet dysfunction (e.g., resulting from NSAID therapy or vWD) or, less likely, a vasculopathy. Unfortunately, the BMBT has high interoperator and intraoperator variability (as high as 80%), and the results are not reproducible, even by the same operator. The PFA-100 (see below) has replaced the BMBT in most veterinary teaching hospitals.
 BOX 87-2 Procedure for Determining the BMBT in Dogs
BOX 87-2 Procedure for Determining the BMBT in Dogs
BMBT, Buccal mucosal bleeding time.
By performing these simple tests after evaluating the clinical features of the bleeding disorder, the clinician should be able to narrow down the number of differential diagnoses. For example, the blood smear evaluation reveals whether the patient is thrombocytopenic. If the patient is not thrombocytopenic but petechiae and ecchymoses are present, a prolonged bleeding time supports the existence of a platelet function defect. A prolonged ACT or APTT indicates that an abnormality in the intrinsic or common pathways; a prolonged OSPT documents a defect in the extrinsic pathway (i.e., factor VII); and a positive test result for FDPs supports the presence of primary or secondary fibrinolysis.
If further confirmation of a presumptive diagnosis is required, plasma can be submitted to a referral laboratory or a specialized coagulation laboratory (see p. 1244). Most commercial veterinary diagnostic laboratories routinely evaluate hemostatic profiles. Samples should be submitted in a purple-topped tube (sodium ethylene diamine tetraacetic acid) for platelet count, a blue-topped tube (sodium citrate) for coagulation studies (OSPT, APTT, fibrinogen concentration), and a special blue-topped tube (Thrombo Wellcotest) for FDP determination (the last tube is usually supplied by the diagnostic laboratory). The blue-topped tubes are now available in two different sodium citrate concentrations: 3.2% and 3.8%. The results of routine hemostasis assays are not affected by the concentration of citrate used (Morales et al., 2007). It is important to submit the right samples in the appropriate anticoagulant. The guidelines for sample submission to commercial laboratories are summarized in Table 87-3.
 TABLE 87-3 Specimens Required for Laboratory Evaluation of Hemostasis
TABLE 87-3 Specimens Required for Laboratory Evaluation of Hemostasis
| SAMPLE | TUBE TOP COLOR | TEST(S) |
|---|---|---|
| EDTA blood | Purple | Platelet count |
| Citrated blood | Blue | OSPT, APTT, fibrinogen, AT, vWF, clotting factor assays, d-dimer, TEG, PFA-100 |
| Thrombin | Blue | FDPs |
EDTA, Ethylenediamine tetraacetic acid; OSPT, one-stage prothrombin time; APTT, activated partial thromboplastin time; AT, antithrombin, vWF, von Willebrand factor assay; TEG, Thromboelastograph; PFA-100, platelet function analyzer; FDP, fibrin degradation product.
A routine coagulation screen (or hemostatic profile) usually contains the OSPT, APTT, platelet count, fibrinogen concentration, and FDP concentration (or titer). In some laboratories a d-dimer test and AT activity may also be included. The OSPT primarily evaluates the extrinsic pathway, whereas the APTT primarily evaluates the intrinsic pathway. Because the end product in these assays is always fibrin formation, both tests also evaluate the common pathway (see Fig. 87-1). The d-dimer assay evaluates for systemic fibrinolysis, as does the FDP test; however, the d-dimer is formed after fibrin as been stabilized by factor XIII. Thus it is more indicative of intravascular thrombus formation. The interpretation of routine hemostasis profiles is summarized in Table 87-2.
New instruments now allow evaluation of other aspects of hemostasis. For example, the platelet function analyzer PFA-100 (Siemens Healthcare Diagnostics, Deerfield, Ill.) is a simple, cage-side instrument to evaluate platelet adhesion and aggregation (Couto et al., 2006). This instrument is available in several specialized clinical hemostasis laboratories and has been extensively evaluated in dogs. The PFA-100 is quite sensitive in the diagnosis of vWD. The Thromboelastograph (TEG; Haemoscope, Niles, Ill.), also available in some specialized hemostasis laboratories, uses native or anticoagulated blood that is activated with a variety of agonists. This instruments evaluates global hemostasis, including platelet adhesion and aggregation, fibrin formation, fibrinolysis, and clot retraction. The TEG is ideal to monitor response to blood component therapy in patients with coagulopathies. I have found it to provide a wealth of information in patients with hypercoagulability and those with spontaneous bleeding and normal results of hemostasis profiles.
As previously discussed, if an unusual coagulopathy or a specific clotting factor deficiency is suspected, blood should be submitted to a specialized veterinary coagulation laboratory (see p. 1244). Congenital and acquired clotting factor deficiencies that occur in cats and dogs are listed in Box 87-3.
 BOX 87-3 Congenital and Acquired Clotting Factor Defects
BOX 87-3 Congenital and Acquired Clotting Factor Defects
DIC, Disseminated intravascular coagulation.
Congenital Clotting Factor Defects
Factor I, or hypofibrinogenemia and dysfibrinogenemia (St. Bernards and Borzois)
Factor II, or hypoprothrombinemia (Boxers, Otterhounds, English Cocker Spaniels)
Factor VII, or hypoproconvertinemia (Beagles, Malamutes, Boxers, Bulldogs, Miniature Schnauzers)
Factor VIII, or hemophilia A (many breeds but mainly German Shepherd dogs)
Factor IX, or hemophilia B (many breeds of dogs; domestic short-haired and British Shorthair cats)
Factor X, or Stuart-Prower trait (Cocker Spaniels, Jack Russell Terriers)
Factor XI, or hemophilia C (English Springer Spaniels, Great Pyrenees, Kerry Blue Terriers)
Factor XII, or Hageman factor (Miniature and Standard Poodles, Shar-Peis, German Shorthair Pointers; cats)
Prekallikrein (Fletcher factor) deficiency (various dog breeds)
Thrombocytopenia can be from either decreased production or increased destruction, consumption, or sequestration of platelets; therefore a bone marrow aspiration for cytologic evaluation is indicated in cats and dogs with thrombocytopenia of unknown cause. Other tests can also be performed in thrombocytopenic cats and dogs, including determinations of titers or polymerase chain reaction (PCR) for tick-borne diseases, evaluation for retrovirus infection, radioactive platelet scanning, and antiplatelet antibody tests (see p. 1250).
Finally, clinicians occasionally encounter a patient with abnormal results of hemostasis profiles but without spontaneous bleeding. The most common “abnormality” in the hemostasis profile of a dog or cat without a tendency to bleed is a prolongation of the APTT. Quite frequently the prolongation is marked (more than 50% above the control or upper limit of the reference range for the laboratory). If this “abnormality” is found during a presurgical evaluation, the surgery may be delayed needlessly if the clinician is not familiar with some of the following clinical conditions. As previously discussed, dogs and cats with factor XII deficiency do not bleed, yet they have a prolonged APTT; determination of factor XII activity will resolve this issue. Prekallikrein and high-molecular-weight kininogen (HMWK) are co-factors for contact activation of factor XII. Dogs with prekallikrein or HMWK deficiencies have prolonged APTT but do not bleed; incubation of the plasma samples for a few hours overrides the factor deficiency and corrects the APTT. Finally, the presence of circulating anticoagulants, also referred to as lupus anticoagulants, results in prolongation of the APTT without bleeding. A simple test to determine if the patient with a prolonged APTT has a clotting factor deficiency (e.g., factor XII) or circulating anticoagulants is to perform an APTT after diluting the patient’s sample 50 : 50 with normal or pooled dog plasma (dilution assay). As previously discussed, the APTT becomes prolonged when the patient has less than 30% activity of an individual factor. If the patient has factor XII deficiency, for example, and 0% factor XII activity, mixing the sample 50 : 50 with normal dog plasma (with a factor XII activity of 100%), will result in a final factor XII activity of 50% and thus the APTT will be normal. Circulating anticoagulants also inhibit the clotting factors in the normal dog plasma, so when the samples are mixed 50 : 50 the APTT remains prolonged.
MANAGEMENT OF THE BLEEDING PATIENT
Several basic principles apply to the management of cats and dogs with spontaneous bleeding disorders. Specific principles are discussed in the following paragraphs. In general, a cat or dog with a spontaneous bleeding disorder should be managed aggressively because these disorders are potentially life threatening, but iatrogenic bleeding should be minimized. As a general rule, trauma should be minimized and the patient must be kept quiet, preferably confined to a cage and leash walked, if necessary. Exercise should be avoided or markedly restricted.
Venipunctures should be done with the smallest gauge needle possible, and pressure should be applied to the puncture site for a minimum of 5 minutes. A compressive bandage should also be applied to the area once pressure has been released. If repeated samples for packed cell volumes (PCVs) and plasma protein determinations are necessary, they should be obtained from a peripheral vein with a 25-gauge needle to fill one or two microhematocrit tubes by capillarity. A bandage should be applied after each venipuncture.
Invasive procedures should be minimized. For example, urine samples should never be collected by cystocentesis because of the risk of intraabdominal, intravesical, or intramural bladder bleeding. Certain invasive procedures, however, can be performed quite safely. These include bone marrow aspiration from the iliac crest or wing of the ilium, fine-needle aspiration of lymph nodes or superficial masses, fine-needle aspiration of the spleen (the thick fibromuscular capsule of the carnivore spleen seals the needle hole as soon as the needle is removed), and intravenous catheter placement (although seepage from the catheter is common in thrombocytopenic patients).
Certain types of surgery can also be safely performed in some cats and dogs with coagulopathies. For example, pedicle surgery (e.g., splenectomy) can be performed with minimal bleeding (i.e., seepage from the abdominal wound) in dogs with marked thrombocytopenia (i.e., less than 25,000 platelets/μL).
A transfusion of blood or blood components is indicated in some dogs and cats with spontaneous bleeding disorders. Whole fresh blood (or a combination of packed RBCs and fresh frozen plasma) should be used if the animal is anemic and lacking one or more clotting factors; plasma transfusions are of no benefit in thrombocytopenic animals. Fresh frozen plasma can be used to replenish clotting factors in a cat or dog with a normal or mildly decreased packed cell volume (i.e., the animal is not symptomatic). Stored blood or frozen plasma is deficient in factors V and VIII. In general, whole fresh blood, platelet-rich plasma, and platelet transfusions rarely provide sufficient platelets to halt spontaneous bleeding in a cat or dog with thrombocytopenia, particularly if the bleeding is the result of platelet consumption. (Some guidelines for transfusion therapy are discussed in Chapter 83.)
PRIMARY HEMOSTATIC DEFECTS
Primary hemostatic defects are characterized by the presence of superficial and mucosal bleeding (e.g., petechiae, ecchymoses, hematuria, epistaxis) and are usually associated with thrombocytopenia. Platelet dysfunction is a rare cause of spontaneous bleeding in dogs and cats. Primary hemostatic defects caused by vascular problems are extremely rare and thus are not discussed here. Primary hemostatic defects are the most common cause of spontaneous bleeding in dogs seen at our hospital.
THROMBOCYTOPENIA
Thrombocytopenia represents the most common cause of spontaneous bleeding in dogs seen at our clinic. Decreased numbers of circulating platelets can be the result of one or more of the following abnormalities (Box 87-4):
 BOX 87-4 Causes of Thrombocytopenia in Dogs and Cats
BOX 87-4 Causes of Thrombocytopenia in Dogs and Cats
Common; relatively common; rare. IMT, Immune-mediated thrombocytopenia; DIC, disseminated intravascular coagulation.
Decreased Platelet Production
Immune-mediated megakaryocytic hypoplasia Idiopathic bone marrow aplasia
Drug-induced megakaryocytic hypoplasia (estrogens, phenylbutazone, melphalan, lomustine βlactams)
Increased platelet destruction represents the most common cause of thrombocytopenia in dogs in our clinic, but it is rare in cats. Most commonly the peripheral destruction of platelets results from immune-mediated, drug-related (including vaccination with modified-live viruses), and sepsis-related (see Box 87-4) mechanisms. Increased platelet consumption occurs most commonly in dogs and cats with DIC (see below), and sequestration is usually caused by splenomegaly or, rarely, hepatomegaly (see Box 87-4).
Approach to the Patient with Thrombocytopenia
Before assessing a patient with primary hemostatic bleeding, the clinician must remember than in some breeds platelet counts below the reference range for dogs are common. Platelet counts in Greyhounds typically range between 80,000 and 120,000/μL, whereas in Cavalier King Charles Spaniels with macrothrombocytopenia platelet counts <50,000/μL are common. In the latter the global platelet function is normal. Once thrombocytopenia has been confirmed by a platelet count or evaluation of a blood smear, its pathogenesis should be identified. The absolute platelet count may offer clues to its cause; for example, platelet counts of less than 25,000/μL are common in dogs with immune-mediated thrombocytopenia (IMT), whereas platelet counts of 50,000 to 75,000//μL are more common in dogs with ehrlichiosis, lymphoma affecting the spleen, or rodenticide toxicity.
The patient’s drug history should be obtained from the owner. If the animal is receiving any medication, the thrombocytopenia should be considered drug related until proven otherwise. The drug should be discontinued if possible and the platelet count reevaluated within 2 to 6 days. If the count returns to normal, a retrospective diagnosis of drug-associated thrombocytopenia is made. Drugs that have been associated with thrombocytopenia in cats and dogs can also cause anemia and neutropenia (see Boxes 83-2 and 85-1).
Because retroviral disorders commonly affect the bone marrow and may result in thrombocytopenia in cats, bone marrow aspiration is indicated in a thrombocytopenic cat with no history of previous medication. The risk of bleeding during or after bone marrow aspiration in a thrombocytopenic animal is minimal. Feline leukemia virus (FeLV) and feline immunodeficiency virus tests should also be performed. If determined by the laboratory, a mean platelet volume is high in most cats with FeLV infection (i.e., macrothrombocytosis); however, macrothrombocytes are also seen in cats and dogs with peripheral platelet destruction, consumption, or sequestration, in which they may be analogous to reticulocytes (young, immature, large platelets).
Bone marrow evaluation may also be indicated in dogs with thrombocytopenia. Given the high prevalence of IMT, at our clinic we usually elect to treat a dog with a presumed diagnosis of IMT. If the patient does not respond to immunosuppressive drugs within 2 to 3 days, a bone marrow aspiration may be performed.
Hyperplasia of megakaryocytes occurs in response to peripheral destruction, consumption, or sequestration of platelets. Occasionally dogs and cats with IMT have decreased numbers of megakaryocytes and abundant free megakaryocyte nuclei in the bone marrow. This is thought to be mediated by antibodies directed against platelets that also destroy the megakaryocytes. Infiltrative or dysplastic bone marrow disorders causing thrombocytopenia are easy to identify on a bone marrow smear.
Because IMT is a diagnosis of exclusion, tick-borne diseases (e.g., canine ehrlichiosis, Rocky Mountain spotted fever, cyclic thrombocytopenia, babesiosis, bartonellosis) should theoretically be ruled out by evaluating the appropriate serology or PCR and a blood smear. However, if the animal does not have clinical signs unrelated to the bleeding, the thrombocytopenia is not likely caused by sepsis or tick-borne diseases, although occasionally asymptomatic thrombocytopenic dogs have subclinical rickettsial diseases. If sepsis is suspected on the basis of clinical signs and clinicopathologic findings (e.g., fever, tachycardia, poor perfusion, degenerative left shift in the leukogram, hypoglycemia, hyperbilirubinemia), urine and blood should be obtained for bacterial cultures.
The presence of spherocytic hemolytic anemia or autoagglutination in a dog with thrombocytopenia is highly suggestive of Evans syndrome (combination of IMT and immune hemolytic anemia [IHA]). A direct Coombs test is usually positive in these cases. On rare occasions a direct Coombs test is positive in a dog with IMT and borderline anemia, further supporting a diagnosis of Evans syndrome.
A hemostasis screen should always be performed to rule out DIC in a thrombocytopenic animal found to have RBC fragments in a blood smear or evidence of secondary bleeding (e.g., hematomas, bleeding into body cavities). The remainder of the hemostasis screen is usually normal in dogs and cats with selective thrombocytopenia.
Several tests are available to evaluate antiplatelet antibodies, including direct immunofluorescence of bone marrow megakaryocytes and enzyme-linked immunosorbent assays for circulating or platelet-bound antibodies (see Chapter 92). However, most of these are not clinically reliable, and a diagnosis of IMT can be made only after other causes of thrombocytopenia have been excluded (i.e., regardless of the results of the antiplatelet antibody tests).
Abdominal radiographs and ultrasonograms may reveal an enlarged spleen not evident during physical examination. Diffuse splenomegaly (i.e., splenic sequestration of platelets) may be the cause of the thrombocytopenia, or it may reflect “work hypertrophy” (i.e., mononuclear phagocytic system hyperplasia) and extramedullary hematopoiesis in a dog with IMT. Splenic nodules are usually an incidental finding in dogs with thrombocytopenia, and they may represent extramedullary hematopoiesis or hyperplasia; fine-needle aspiration of the nodules should establish a cytologic diagnosis. Despite the low platelet counts, clinically relevant bleeding rarely occurs.
Often a specific diagnosis of IMT is obtained only after a therapeutic trial with corticosteroids (see later discussion) results in resolution of the thrombocytopenia. If the clinician is in doubt regarding whether the thrombocytopenia is caused by a rickettsial disease or IMT (in dogs), immunosuppressive doses of corticosteroids can be administered in conjunction with doxycycline (5 to 10mg/kg PO q12-24h) until serologic or PCR test results become available. This combination of agents has no deleterious effects on dogs with rickettsial diseases.
Blood or blood products should be transfused as needed (see Chapter 83). However, the transfusion of whole fresh blood, platelet-rich plasma, or platelets rarely, if ever, results in normalization of the platelet count or even in increases in the platelet count to “safe” levels.
Immune-Mediated Thrombocytopenia
IMT is the most common cause of spontaneous bleeding in dogs but is rare in cats. It affects primarily middle-aged, female dogs, and Cocker Spaniels and Old English Sheepdogs are overrepresented. The clinical signs are those of a primary hemostatic defect and include petechiae, ecchymoses, and mucosal bleeding. Acute collapse may occur if bleeding is pronounced; if the anemia is mild, most dogs are fairly asymptomatic. IMT is acute or peracute in onset in most dogs. During physical examination, signs of primary hemostatic bleeding (e.g., petechiae, ecchymoses, mucosal bleeding) with or without splenomegaly may be found.
The complete blood count in dogs with IMT is characterized by thrombocytopenia with or without anemia (depending on the degree of spontaneous bleeding and the presence or absence of concurrent IHA); leukocytosis with a left shift may also be present. As a general rule, hematologic changes are limited to the thrombocytopenia. If IHA is associated with IMT (i.e., Evans syndrome), a Coombs-positive, regenerative anemia with spherocytosis or autoagglutination is usually present. Bone marrow cytologic studies typically reveal megakaryocytic hyperplasia, although megakaryocytic hypoplasia with free megakaryocyte nuclei is occasionally present. In addition to the thrombocytopenia, the bleeding time is the only other abnormal test result (ACT, APTT, OSPT, FDP, and fibrinogen concentration are normal). An inverse linear correlation is usually present between the platelet count and the BMBT (i.e., a longer BMBT with lower platelet counts). Ideally, tick-borne diseases and drug-induced thrombocytopenia should be ruled out before establishing a definitive diagnosis of IMT.
If the index of suspicion for IMT is high (i.e., a fairly asymptomatic dog with spontaneous primary hemostatic bleeding and thrombocytopenia as the sole hematologic abnormality), a therapeutic trial with immunosuppressive doses of corticosteroids (equivalent to 2 to 8mg/kg/day of prednisone) should be instituted. Responses are usually seen within 24 to 96 hours. No clinical evidence exists that dexamethasone is more effective than prednisone in controlling IMT. Indeed, in my experience acute gastrointestinal tract ulceration is considerably more prevalent in dogs receiving dexamethasone than in those receiving prednisone. Because an acute upper gastrointestinal tract bleed is usually catastrophic in a dog with thrombocytopenia, prednisone is my drug of choice. H2-antihistamines, such as famotidine (0.5mg/kg PO q24h), should be used in combination with the corticosteroids.
Fresh whole blood, stored blood, packed RBCs, or hemoglobin solutions should be administered as needed to maintain adequate oxygen-carrying capacity (see Transfusion Therapy in Chapter 83). In addition to immunosuppressive doses of corticosteroids, cyclophosphamide, given intravenously or orally in a single dose of 200 to 300mg/m2, is effective for inducing remission. However, it should not be used as a maintenance agent because it usually causes sterile hemorrhagic cystitis when used on a long-term basis. Vincristine, at a dose of 0.5mg/m2 given intravenously, traditionally has been recommended for dogs with IMT. This drug stimulates megakaryocyte endomitosis, resulting in early platelet release from the bone marrow. However, because vinca alkaloids bind to tubulin, the platelets released prematurely are not fully functional (tubulin is responsible for platelet aggregation), and the patients may have further bleeding before the platelet count increases. As discussed in Chapters 85 and 93, human intravenous immunoglobulin (0.5 to 1g/kg, single dose) can also be used successfully in dogs with refractory or life-threatening IMT.
Failure to induce remission (i.e., to normalize the platelet count) is usually the result of insufficient drug (low doses or the need for a second agent), insufficient duration of therapy (the drugs have not yet had time to become effective), or an incorrect diagnosis. In the event of one of these, the treatment protocol can easily be amended, with the thrombocytopenia usually resolving as a result. Azathioprine (50mg/m2 PO q24-48h) is effective in maintaining remission but is not a good agent for inducing remission. In some dogs azathioprine is better tolerated than long-term corticosteroid therapy, although close hematologic monitoring is recommended given its myelosuppressive properties and potential for hepatotoxicity. The androgenic steroid danazol or cyclosporine A may also be beneficial in dogs with IMT. (See Chapter 93 for additional information and drug dosages.)
The prognosis is good in most dogs with IMT, although they may require lifelong treatment. Dogs with refractory IMT can be successfully treated with vinca-loaded platelets, pulse-dose cyclophosphamide, human immunoglobulin, or splenectomy.
IMT has become more prevalent in cats over the past few years. The typical clinical presentation is different from dogs in that most cats have chronic thrombocytopenia that does not lead to spontaneous bleeding. A platelet count of 10,000 to 30,000/μL is relatively common in an otherwise healthy cat without spontaneous bleeding. I have followed up several of these cats for months to years, and their platelet counts do not increase markedly with treatment. Interestingly, a high proportion of these cats also have regenerative or nonregenerative anemia, neutropenia, lymphocytosis, or combinations thereof. The cytopenias may resolve for no apparent reason, only to have a decrease in another cell line months later. Because most of these cats do not bleed, the clinician should be aware that increasing drug dosages or adding drugs may cause more problems than monitoring the platelet count. My treatment of choice for cats with IMT or immune-mediated cytopenias is a combination of dexamethasone (4mg q1-2wk) and chlorambucil (20-30mg/m2 PO q2wk). I have also successfully used human intravenous immunoglobulin G in a limited number of cats with immune-mediated cytopenias.
PLATELET DYSFUNCTION
The presence of primary hemostatic bleeding in a patient with a normal platelet count is highly suggestive of a platelet dysfunction syndrome, although vasculopathies and enhanced fibrinolysis should also be considered. Platelet dysfunction syndromes can be congenital or acquired (Box 87-5); however, they rarely result in spontaneous bleeding. More often a prolonged BMBT is noted preoperatively in an otherwise healthy animal, or the animal has a history of pronounced bleeding during a previous surgery. Congenital platelet dysfunction syndromes are rare, with the notable exception of vWD. Some authors classify vWD among the congenital clotting factor deficiencies; however, because its clinical manifestations are those of a primary hemostatic defect, I include it in this section. Acquired platelet function disorders are more common; clinically they are mainly secondary to uremia, monoclonal gammopathies, ehrlichiosis, retroviral infections, or drug therapy.
 BOX 87-5 Platelet Function Defects in Dogs and Cats
BOX 87-5 Platelet Function Defects in Dogs and Cats
vWD, von Willebrand disease.
von Willebrand Disease
vWD is the most common inherited bleeding disorder in human beings and dogs but is rare in cats. The term von Willebrand syndrome is reserved for an acquired vWF deficiency. vWD can be classified into three types (Table 87-4). Dogs with the disease typically have a decreased concentration or activity (type 1 vWD), absence of circulating vWF (type 3 vWD), or low to normal concentrations of an abnormal vWF (type 2 vWD), which result in mild (if any) spontaneous bleeding or, more likely, prolonged surgical bleeding. In dogs vWD can be inherited as an autosomal dominant trait with incomplete penetrance or, more rarely, an autosomal recessive trait (see later discussion). This disorder has been reported to occur in more than 50 breeds of dogs but is more common in Doberman Pinschers, German Shepherd dogs, Poodles, Golden Retrievers, and Shetland Sheepdogs. In these breeds the defect is inherited as an autosomal dominant trait with incomplete penetrance. In Scottish Terriers and Shetland Sheepdogs, it can be inherited as an autosomal recessive trait; homozygous dogs have no detectable vWF concentrations and are usually severely affected. Type 1 vWD may purportedly occur in association with clinical hypothyroidism in dogs; however, most scientifically controlled studies have failed to prove an association between vWD and hypothyroidism. Type 2 vWD was recently reported in dogs with aortic valvular disease; in those dogs, the high shear associated with turbulent flow across the valve resulted in selective depletion of high-molecular-weight VWF multimers (Tarnow et al., 2005).
 TABLE 87-4 Classification of vWD in Dogs
TABLE 87-4 Classification of vWD in Dogs
| TYPE | DEFECT | BREEDS |
|---|---|---|
| 1 | Low concentration of normal vWF | Airedale Terrier, Akita, Corgi, Dachshund, Doberman Pinscher, German Shepherd dog, Golden Retriever, Greyhound, Irish Wolfhound, Manchester Terrier, Poodle, Schnauzer, Shetland Sheepdog |
| 2 | Low concentration of abnormal vWF | German Shorthaired Pointer, German Wirehaired Pointer |
| 3 | Absence of vWF | Familial: Chesapeake Bay Retriever, Scottish Terrier, Shetland Sheepdog Sporadic: Border Collie, Bull Terrier, Cocker Spaniel, Labrador Retriever, Pomeranian |
vWD, von Willebrand disease; vWF, von Willebrand factor.
Modified from Brooks M: von Willebrand disease. In Feldman BF et al, editors: Schalm’s veterinary hematology, ed 5, Philadelphia, 2000, Lippincott Williams & Wilkins, p 509.
In human beings vWF is produced by megakaryocytes and endothelial cells, circulates in plasma complexed to factor VIII coagulant (factor VIII : C), and is one of the major adhesive proteins in the body. In the dog, platelets do not contribute as much vWF to plasma as in human beings. vWF is mainly responsible for causing platelets to adhere to the subendothelial structures (e.g., collagen) in areas of high shear once endothelial cell damage has occurred, thus initiating the formation of the primary hemostatic plug (Fig. 87-2). The vWF molecule circulates coiled; it uncoils at the site of endothelial damage, binds to the subendothelium and then to the platelet receptors, and the platelets are “reeled” in to the site of injury. As a consequence, vWD is usually characterized by primary hemostatic defects (e.g., petechiae, ecchymoses, mucosal bleeding). However, most dogs with vWD do not bleed spontaneously but rather bleed excessively during or after surgery; excessive bleeding during teething or estrus can also occur, but petechiae and ecchymoses are rarer. Most dogs with vWD and spontaneous bleeding seen at our clinic are brought in for evaluation of diffuse oropharyngeal or vaginal bleeding. People with vWD can also have low circulating concentrations of factor VIII leading to spontaneous secondary hemostatic bleeding (i.e., the clinical findings of hemophilia A); however, this is extremely rare in dogs. Perinatal death or abortions/stillbirths are common in litters with vWD.
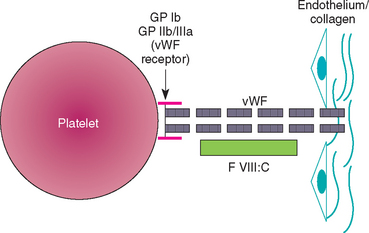
FIG 87-2 The interaction between vWF, platelet, and subendothelial surfaces. GP, Glycoprotein; vWF, von Willebrand factor; FVIII : C, factor VIII coagulant.
The hemostasis screen results are normal in most dogs with vWD. The platelet counts in dogs with vWD are also normal. However, the results of a PFA-100 or BMBT usually inversely correlate with the degree of vWF deficiency (i.e., the PFA-100 closure time or the BMBT is prolonged if the vWF concentration or activity is low). Indeed, the BMBT may be the most cost-effective method for screening dogs for vWD, although its results are not foolproof. It can be done before surgery in breeds at risk or if the owner or breeder is interested in determining whether the dog is likely to have this disorder. However, a normal bleeding time does not necessarily rule out vWD. At our clinic we routinely use the PFA-100 before surgery in dogs at high risk for vWD so that appropriate therapy can be instituted before or during surgery. A diagnosis of vWD can be confirmed by quantifying vWF in specialized veterinary coagulation laboratories. Genetic testing for vWD in specific breeds is available through commercial diagnostic laboratories.
Most dogs with type 1 vWD can be successfully treated before surgery or during a bleeding episode with desmopressin acetate (DDAVP), which causes a massive release of vWF from the endothelial cells and results in shortening of the BMBT and the PFA-100 closure times within 30 minutes of administration. A single 1μg/kg dose of DDAVP (intranasal preparation) given subcutaneously consistently lessens bleeding in dogs with type 1 vWD despite modest increases in vWF concentration. DDAVP is not effective in dogs with types 2 or 3 vWD because these dogs either lack or have an abnormal (i.e., nonfunctional) vWF. Cryoprecipitate is the blood component of choice for dogs with vWD; a unit of cryoprecipitate is defined as the volume obtained from a unit of fresh frozen plasma. We dose it at 1U cryoprecipitate per 10kg of body weight; therefore, a Doberman Pinscher typically receives 3U. If cryoprecipitate is not available, fresh frozen plasma or whole fresh blood can be used. DDAVP can also be administered to the blood donor dog 1 hour before blood is collected to maximize the yield of vWF. The use of topical hemostatic agents such as fibrin, collagen, or methacrylate is also indicated to control the local bleeding. As is the case in dogs with other inheritable disorders, dogs with congenital vWD should not be bred.
Other Congenital Platelet Function Defects
Platelet function defects leading to spontaneous primary hemostatic bleeding have been reported in at least three breeds of dogs (Otterhounds, Foxhounds, and Basset Hounds). The clinical signs and clinicopathologic abnormalities are similar to those seen in dogs with vWD, but the vWF concentrations are normal or high. A syndrome of spontaneous and postoperative bleeding resembling Scott syndrome in human beings from a lack of platelet procoagulant activity was described in German Shepherd dogs (Brooks et al., 2002).
SECONDARY HEMOSTATIC DEFECTS
Dogs with secondary hemostatic defects are usually evaluated because of collapse, exercise intolerance, dyspnea, abdominal distention, lameness, or masses. The collapse and exercise intolerance are usually caused by anemia resulting from intracavitary bleeding, as is the dyspnea and abdominal distention. The lameness is usually caused by hemarthrosis, and the masses or lumps usually represent hematomas. Cats and dogs with secondary hemostatic disorders do not have petechiae or ecchymoses, and mucosal bleeding (e.g., melena, epistaxis) is rarely seen. In general the severity of the bleeding is directly related to the severity of the deficiency of the clotting factor(s). Liver disease and rodenticide poisoning leading to vitamin K deficiency are the two most common causes of secondary hemostatic defects seen at our clinic. As previously noted, these disorders are more common in dogs than in cats.
CONGENITAL CLOTTING FACTOR DEFICIENCIES
Congenital clotting factor deficiencies, as well as the breeds affected, are listed in Box 87-3. They are relatively common in dogs but are rare in cats. Most genetic mutations leading to these defects have been well characterized, and some laboratories now offer genetic testing for congenital coagulopathies. Hemophilia A and B are sex-linked traits; the modes of inheritance of other coagulopathies vary. In affected animals the severity of the bleeding is usually inversely proportional to the concentration of the individual clotting factor affected (e.g., bleeding is more severe in association with a very low factor activity). Clinical signs usually include spontaneous hematoma formation, which the owners may describe as “lumps,” and bleeding into body cavities as well as signs compatible with “fading puppy syndrome” and protracted umbilical cord bleeding after birth. Abortions or stillbirths in the litter are common. Petechiae and ecchy moses are not present in dogs with congenital clotting factor deficiencies. Cats with congenital clotting factor deficiency usually do not bleed spontaneously, but rather have intraoperative or delayed postoperative bleeding.
Carriers of the defect may be asymptomatic but usually have prolonged clotting times in vitro. Certain factor deficiencies (so-called “contact factors”), including factors XII and XI, Fletcher factor (prekallikrein), and HMWK, are also found in otherwise asymptomatic animals (i.e., no excessive bleeding) with markedly prolonged APTTs. However, massive and often life-threatening postoperative bleeding starting 24 to 36 hours after surgery is common in dogs with factor XI deficiency.
Most dogs and cats with congenital coagulopathies are treated with supportive and transfusion therapies; no other treatments appear to be beneficial. As with animals with other congenital defects, dogs and cats with coagulopathies should not be bred.
VITAMIN K DEFICIENCY
Vitamin K deficiency in small animals usually results from the ingestion of vitamin K antagonists (warfarin, diphacinone, or their derivatives, brodifacoum and bromadiolone), although it can also occur as a consequence of malabsorption in dogs and cats with obstructive cholestasis, infiltrative bowel disease, or liver disease. Four clotting factors are vitamin K dependent: factors II, VII, IX, and X. Proteins C and S, two natural anticoagulants, are also vitamin K dependent. Because of its clinical relevance, the following discussion focuses only on rodenticide poisoning, which is more common in dogs and extremely rare in cats.
Most dogs with toxicity are evaluated because of acute collapse and a possible history of rodenticide ingestion. Coughing, thoracic pain, and dyspnea are also common. These dogs usually have clinical signs compatible with secondary bleeding, such as hematomas and bleeding into body cavities. The most common site of bleeding in dogs evaluated at our clinic is the thorax; some dogs have superficial skin bruising in areas of friction, such as the axilla or the groin. Other abnormalities include pale mucous membranes, anemia (usually regenerative if sufficient time has elapsed since the acute bleeding episode), and hypoproteinemia. Sudden death may occur as a result of central nervous system or pericardial hemorrhage.
If the rodenticide has been ingested minutes to hours before presentation, induced vomiting and the administration of activated charcoal may eliminate or neutralize most of it. If the ingestion is questionable and no clinical signs of coagulopathy are present (e.g., hemothorax, hemoabdomen, bruising), determination of the OSPT is recommended. Because factor VII is the shortest lived vitamin K–dependent protein (circulating half-life of 4 to 6 hours), the OSPT is usually prolonged before spontaneous bleeding becomes evident. Newer tests for proteins induced by vitamin K absence may also aid in the early diagnosis of rodenticide toxicity, but they are not used in our clinic because they seem to lack clinical relevance.
The typical hemostasis screen in a dog with symptomatic vitamin K deficiency reveals marked prolongation of the OSPT and APTT; this is one of the few clinical situations where the OSPT is typically longer than the APTT. The FDP test is positive in more than half of affected dogs and mild thrombocytopenia is present (70,000 to 125,000/μL), which is likely caused by an excessive consumption of platelets from protracted bleeding.
These animals usually require immediate transfusions of whole fresh blood or fresh frozen plasma (or cryo-poor plasma) to replenish the coagulation factors (and packed RBCs if the animal is anemic). From 8 to 12 hours may elapse before vitamin K therapy appreciably shortens the OSPT and subsequently decreases bleeding.
Vitamin K is available in several forms, but vitamin K1 is the most effective. It is available for oral or parenteral use. Intravenous administration of vitamin K is not recommended because of the risk of anaphylactic reactions or Heinz body formation; intramuscular injections in a dog with a coagulopathy usually result in hematoma formation. Subcutaneous administration of vitamin K1 with a 25-gauge needle (loading dose of 5mg/kg, followed in 8 hours by 2.5mg/kg SQ divided q8h) is preferred if the patient is properly hydrated. Administration of oral loading doses of vitamin K1 has been advocated for the treatment of dogs with rodenticide poisoning (5mg/kg with a fatty meal, then 2.5mg/kg divided q8-12h); this is the treatment used in our clinic. Because vitamin K is lipid soluble, its absorption is enhanced if it is given with fatty meals. Animals with cholestatic or malabsorptive syndromes may require continued subcutaneous injections of vitamin K. In critical cases the OSPT should be monitored every 8 hours until it normalizes.
If the anticoagulant is known to be warfarin or another first-generation hydroxycoumarin, 1 week of oral vitamin K1 is usually sufficient to reverse the coagulopathy. However, if it is indanedione or any of the second- or third-generation anticoagulants, oral vitamin K1 therapy must be maintained for at least 3 weeks and possibly as long as 6 weeks. Most currently available rodenticides contain second- and third-generation anticoagulants. If the rodenticide ingested is unknown, the animal should be treated for 1 week, at which time vitamin K treatment is discontinued. An OSPT is then determined within 24 to 48 hours of the last dose. If the OSPT is prolonged, therapy should be reinstituted and maintained for 2 more weeks and the OSPT reevaluated at the end of this period.
MIXED (COMBINED) HEMOSTATIC DEFECTS
DISSEMINATED INTRAVASCULAR COAGULATION
DIC, previously called consumptive coagulopathy or defibrination syndrome, is a complex syndrome in which excessive intravascular coagulation leads to multiple-organ microthrombosis (multiple organ failure [MOF]) and paradoxic bleeding caused by the inactivation or excessive consumption of platelets and clotting factors as a result of enhanced fibrinolysis. DIC is not a specific disorder but rather a common pathway in a variety of disorders. Moreover, DIC constitutes a dynamic phenomenon in which the patient’s status and the results of coagulation tests change markedly, rapidly, and repeatedly during treatment. This syndrome is relatively common in dogs and cats.
Pathogenesis
Several general mechanisms can lead to activation of intravascular coagulation and therefore to the development of DIC, including the following:
Endothelial damage commonly results from electrocution or heat stroke, although it may also play a role in sepsis-associated DIC. Platelets can be activated by a variety of stimuli, but mainly they are activated by viral infections (e.g., FIP in cats) or sepsis. Tissue procoagulants (likely tissue factor) are released in several common clinical conditions, including trauma, hemolysis, pancreatitis, bacterial infections, acute hepatitis, and possibly some neoplasms (e.g., HSA).
The best way to understand the pathophysiologic process of DIC is to think of the entire vascular system as a single, giant blood vessel and the pathogenesis of the disorder as an exaggeration of the normal hemostatic mechanisms. Once the coagulation cascade has been activated in this “giant vessel” (i.e., it is widespread within the microvasculature in the body), several events take place. Although they are described sequentially, most of them actually occur simultaneously, and the intensity of each varies with time, thus making for an extremely dynamic process.
First, the primary and secondary hemostatic plugs are formed (see p. 1242). Because this is happening in thousands or tens of thousands of small vessels simultaneously, multiple thrombi form in the microcirculation. If this process is left unchecked, ischemia (resulting in MOF) eventually develops. During this excessive intravascular coagulation, platelets are consumed and destroyed in large quantities, leading to thrombocytopenia. Second, the fibrinolytic system is activated systemically, resulting in clot lysis and the inactivation (or lysis) of clotting factors and impaired platelet function. Third, AT and possibly proteins C and S are consumed in an attempt to halt intravascular coagulation, leading to “exhaustion” of the natural anticoagulants. Fourth, the formation of fibrin within the microcirculation leads to the development of hemolytic anemia and further compounds the thrombocytopenia as the RBCs are sheared by these fibrin strands (i.e., fragmented RBCs or schistocytes).
When all these events are considered, it is easy to understand (1) why an animal with multiple organ thrombosis (caused by excessive intravascular coagulation and the depletion of natural anticoagulants) is bleeding spontaneously (as a result of thrombocytopenia, impaired platelet function, and inactivation of clotting factors) and (2) why one of the therapeutic approaches that appears to be beneficial in halting the bleeding in dogs and cats with DIC is to paradoxically administer heparin or other anticoagulants (i.e., if sufficient AT is available, heparin halts intravascular coagulation, which in turn decreases activation of the fibrinolytic system, thus releasing its inhibitory effect on the clotting factors and platelet function).
In addition to the events just described, impaired tissue perfusion results in the development of secondary “enhancers” of DIC, including hypoxia; acidosis; and hepatic, renal, and pulmonary dysfunction; and the release of myocardial depressant factor. The function of the mononuclearphagocytic system also is impaired so that FDPs and other byproducts, as well as bacteria absorbed from the intestine, cannot be cleared from the circulation. These factors also must be dealt with therapeutically (see p. 1256).
The prevalence of primary disorders associated with DIC in 50 dogs and 21 cats recently evaluated at The Ohio State University Veterinary Teaching Hospital (OSU-VTH) is depicted in Table 87-5. Neoplasia (primarily HSA), liver disease, and immune-mediated blood diseases were the most common disorders associated with DIC in dogs; liver disease (primarily hepatic lipidosis), neoplasia (mainly lymphoma), and FIP were the disorders most frequently associated with DIC in cats.
 TABLE 87-5 Primary Disorders Associated with DIC in 50 Dogs and 21 Cats Evaluated at The OSU-VTH
TABLE 87-5 Primary Disorders Associated with DIC in 50 Dogs and 21 Cats Evaluated at The OSU-VTH
| DISEASE | DOGS (%) | CATS (%) |
|---|---|---|
| Neoplasia | 18 | 29 |
| HSA | 8 | 5 |
| Carcinoma | 4 | 10 |
| LSA | 4 | 14 |
| HA | 2 | 0 |
| Liver disease | 14 | 33 |
| Cholangiohepatitis | 4 | 0 |
| Lipidosis | 0 | 24 |
| PSS | 4 | 0 |
| Cirrhosis | 2 | 0 |
| Unspecified | 4 | 10 |
| Pancreatitis | 4 | 0 |
| Immune-mediated diseases | 10 | 0 |
| IHA | 4 | 0 |
| IMT | 2 | 0 |
| Evans syndrome | 2 | 0 |
| IMN | 2 | 0 |
| Infectious diseases | 10 | 19 |
| FIP | 0 | 19 |
| Sepsis | 8 | 0 |
| Babesiosis | 2 | 2 |
| Rodenticide* | 8 | 0 |
| GDV | 6 | 0 |
| HBC | 4 | 0 |
| Miscellaneous | 18 | 19 |
Reprinted from Couto CG: Disseminated intravascular coagulation in dogs and cats, Vet Med 94:547, 1999. This table originally appeared in the June 1999 issue of Veterinary Medicine. It is reprinted here by permission of Thomson Veterinary Healthcare Communications, 8033 Flint, Lenexa, KS 66214; (913) 492-4300; fax: (913) 492-4157; www.vetmedpub.com. All rights reserved.
DIC, Disseminated intravascular coagulation; OSU-VTH, Ohio State University Veterinary Teaching Hospital; HSA, hemangiosarcoma; LSA, lymphoma; HA, hemangioma; PSS, portosystemic shunt; IHA, immune-mediated hemolytic anemia; IMT, immune-mediated thrombocytopenia; IMN, immune-mediated neutropenia; FIP, feline infectious peritonitis; GDV, gastric dilation-volvulus; HBC, hit by car.
* The results of hemostasis profiles in dogs with rodenticide toxicity mimic those seen in DIC.
At our clinic, symptomatic DIC in dogs (i.e., that associated with bleeding) is most commonly associated with HSA, followed by sepsis, pancreatitis, hemolytic anemia, gastric dilation-volvulus, and liver disease. Symptomatic DIC is extremely rare in cats but hemostatic evidence of DIC is common, accounting for approximately two thirds of the abnormal hemostatic profiles in this species in our clinic. As previously discussed, DIC is common in cats with liver disease, malignant neoplasms, or FIP. We have also observed symptomatic DIC in two cats receiving methimazole. The pathogenesis of DIC in dogs with HSA appears to be complex and multifactorial; the major mechanism triggering intravascular coagulation in dogs with this neoplasm was believed to be the abnormal irregular endothelium in the neoplasm (i.e., exposure to subendothelial collagen and the activation of coagulation). However, some canine HSAs appear to synthesize a cancer procoagulant because dogs with small HSAs can have severe DIC, whereas some dogs with widely disseminated HSA have normal hemostasis.
Clinical Features
Dogs with DIC can have several clinical presentations; the two common forms are chronic silent (subclinical) and acute (fulminant) DIC. In the chronic silent form, the patient does not have evidence of spontaneous bleeding, but clinicopathologic evaluation of the hemostatic system reveals abnormalities compatible with this syndrome (see the next page). This form of DIC appears to be common in dogs with malignancy and other chronic disorders. The acute form may represent a true acute phenomenon (e.g., after heatstroke, electrocution, or acute pancreatitis) or, more commonly, it represents acute decompensation of a chronic, silent process (e.g., HSA). Acute DIC is extremely rare in cats. Regardless of the pathogenesis, dogs with acute DIC often are brought in because of profuse spontaneous bleeding and constitutional signs attributable to anemia or parenchymal organ thrombosis (i.e., MOF). The clinical signs of bleeding indicate both primary bleeding (e.g., petechiae, ecchymoses, mucosal bleeding) and secondary bleeding (blood in body cavities). Clinical and clinicopathologic evidence of organ dysfunction is also present. Most cats with DIC seen at our clinic do not have evidence of spontaneous bleeding; clinical signs in these cats are those associated with the primary disease.
In a recent retrospective study of 50 dogs with DIC conducted in our clinic, only 26% had evidence of spontaneous bleeding, whereas only one of 21 cats with DIC had evidence of spontaneous bleeding. Most patients were presented for evaluation of their primary problem and were not bleeding spontaneously; DIC was diagnosed as part of the routine clinical evaluation.
Diagnosis
Because clinical DIC is uncommon in cats, the discussion on diagnosis and treatment focuses on dogs. Several hematologic findings help support a presumptive clinical diagnosis of DIC and include a regenerative hemolytic anemia (although occasionally, because the animal has a chronic disorder such as cancer, the anemia is nonregenerative), hemoglobinemia (caused by intravascular hemolysis), RBC fragments or schistocytes, thrombocytopenia, neutrophilia with a left shift, and rarely neutropenia. Most of these features are evident with evaluation of a spun hematocrit and a blood smear.
Serum biochemical abnormalities in dogs with DIC include hyperbilirubinemia from hemolysis or hepatic thrombosis, azotemia and hyperphosphatemia if severe renal microembolization has occurred, an increase in liver enzyme activities caused by hypoxia or hepatic microembolization, a decreased total carbon dioxide content caused by metabolic acidosis, and panhypoproteinemia if the bleeding is severe enough. Another manifestation of MOF is the development of multifocal ventricular premature contractions detected in an electrocardiogram.
Urinalysis usually reveals hemoglobinuria and bilirubinuria and occasionally proteinuria and cylindruria. Urine samples in dogs with acute DIC should not be obtained by cystocentesis because severe intravesical or intramural bleeding may result.
Hemostatic abnormalities in dogs with DIC include thrombocytopenia, a prolongation of the OSPT or APTT (more than 25% of the concurrent control), normal or low fibrinogen concentration, a positive FDP or d-dimer test, and a decreased AT concentration. Using a TEG, fibrinolysis can be enhanced in these animals. At our clinic, DIC is diagnosed if the patient has four or more of the hemostatic abnormalities just described, particularly if schistocytes are present.
The hemostatic abnormalities in 50 dogs and 21 cats with DIC evaluated in our clinic are listed in Table 87-6. In dogs thrombocytopenia, prolongation of the APTT, anemia, and schistocytosis were common; in contrast with previous descriptions of the syndrome in dogs, regenerative anemia, prolongation of the OSPT, and hypofibrinogenemia were not. In cats prolongation of the APTT and/or OSPT, schistocytosis, and thrombocytopenia were common, whereas the presence of FDPs and hypofibrinogenemia were rare.
 TABLE 87-6 Hemostatic Abnormalities in 50 Dogs and 21 Cats with DIC Evaluated at The OSU-VTH
TABLE 87-6 Hemostatic Abnormalities in 50 Dogs and 21 Cats with DIC Evaluated at The OSU-VTH
| ABNORMALITY | DOGS (%) | CATS (%) |
|---|---|---|
| Thrombocytopenia | 90 | 57 |
| Prolonged APTT | 88 | 100 |
| Schistocytosis | 76 | 67 |
| Positive FDPs | 64 | 24 |
| Prolonged OSPT | 42 | 71 |
| Hypofibrinogenemia | 14 | 5 |
DIC, Disseminated intravascular coagulation; OSU-VTH, Ohio State University Veterinary Teaching Hospital; APTT, activated partial thromboplastin time; FDPs, fibrin degradation products; OSPT, one-stage prothrombin time.
From Couto CG: Disseminated intravascular coagulation in dogs and cats, Vet Med 94:547, 1999.
Treatment
Once a diagnosis of DIC has been established (or even if the degree of suspicion is high that DIC is present), treatment should be instituted without delay. Unfortunately, no controlled clinical trials have been performed in veterinary medicine evaluating the effects of different treatments in dogs with DIC. Therefore the following discussion reflects my beliefs in the management of dogs with this disorder (Box 87-6).
 BOX 87-6 Treatment of Dogs and Cats with DIC
BOX 87-6 Treatment of Dogs and Cats with DIC
DIC, Disseminated intravascular coagulation; AT, antithrombin.
Unquestionably, removing or eliminating the precipitating cause constitutes the main therapeutic goal in patients with DIC. However, this is not always possible. Conditions in which the precipitating causes can be eliminated include a primary HSA (surgical excision), disseminated or metastatic HSA (chemotherapy), sepsis (appropriate antimicrobial treatment), and IHA (immunosuppressive treatment). In most other situations (e.g., electrocution, heatstroke, pancreatitis) the cause can rarely be eliminated within a short time. Therefore the treatment of dogs with DIC is aimed at the following:
Of note, if blood and blood products were available in an unlimited supply (such as is the case in most human hospitals), dogs with DIC would not die of hypovolemic shock. Most dogs with DIC die of pulmonary or renal dysfunction. At our clinic, “DIC lungs” (i.e., intrapulmonary hemorrhages with alveolar septal microthrombi) appear to be a common cause of death in these patients.
Halting intravascular coagulation
I use a dual approach to halt intravascular coagulation: the administration of heparin and blood or blood products. As previously mentioned, heparin is a cofactor for AT and therefore is not effective in preventing the activation of coagulation unless AT activity in the plasma is sufficient. Because AT activity in animals with DIC is usually low as a result of consumption and possibly inactivation, the patient should be provided with sufficient quantities of this anticoagulant. The most cost-efficient way of achieving this is to administer fresh frozen plasma. The old adage that administering blood or blood products to a dog with DIC is analogous to “adding logs to a fire” has not been true in my experience. Therefore blood or blood products should never be withheld based solely on this belief.
Heparin has been used historically to treat DIC in human beings and dogs. However, controversy still exists regarding whether it is beneficial. At our clinic the survival rate in dogs with DIC has increased markedly since we routinely started using heparin and blood products. Although this can also be attributed to improvement in patient care, I believe that heparin is beneficial in such patients and indeed may be responsible for the increased survival rate.
Sodium heparin is given in a wide range of doses. Following are the four traditional dose ranges:
I routinely use low-dose heparin in combination with the transfusion of blood or blood components. The rationale for this is that this dose of heparin does not prolong the ACT or APTT in normal dogs (a minimum of 150 to 250IU/kg q8h is required to prolong the APTT in normal dogs), and it appears to be biologically active in these animals given that some of the clinical signs and hemostatic abnormalities are reversed in animals receiving this dosage. The fact that it does not prolong the APTT or ACT is extremely helpful in dogs with DIC. For example, if a dog with DIC is receiving intermediate-dose heparin, it is then impossible to predict, on the basis of hemostatic parameters, whether a prolongation of the APTT is caused by excessive heparin administration or progression of this syndrome. As laboratory heparin determinations become widely available, this may become a moot point. Until then, my clinical impression is that if an animal with DIC receiving mini- or low-dose heparin shows a prolonged ACT or APTT, the intravascular coagulation is deteriorating and a treatment change is necessary. The use of low-molecular-weight heparin in dogs with DIC is currently being investigated. In an experimental model of DIC in Beagles, high doses of low-molecular-weight heparin resulted in resolution of the clinicopathologic abnormalities associated with DIC (Mischke et al., 2005).
Lepirudin, a novel leech recombinant AT, recently proved beneficial in preventing MOF in an experimental model of sepsis with enteric organisms in Greyhounds. However, this treatment is currently cost prohibitive.
If evidence of severe microthrombosis is present (e.g., marked azotemia, increase in liver enzyme activity, ventricular premature contractions), dyspnea, or hypoxemia, intermediate- or high-dose heparin can be used, with the goal of prolonging the ACT to 2 to 2.5 times the baseline value, or normal if the baseline time was already prolonged. If overheparinization occurs, protamine sulfate can be administered by slow intravenous infusion (1mg for each 100IU of the last dose of heparin; 50% of the calculated dose is given 1 hour after the heparin and 25% 2 hours after the heparin). The remainder of the dose can be administered if clinically indicated. Protamine sulfate should be administered with caution because it can be associated with acute anaphylaxis in dogs. Once improvement in the clinical and clinicopathologic parameters has been achieved, the heparin dose should be tapered gradually (over 1 to 3 days) to prevent rebound hypercoagulability, a phenomenon commonly observed in human beings.
Aspirin and other antiplatelet agents can also be given to prevent platelet activation and thus halt intravascular coagulation. Doses of 0.5 to 10mg/kg of aspirin given orally every 12 hours in dogs and every third day in cats have been recommended, although in my experience aspirin is rarely of clinical benefit. If it is used, the patient should be closely watched for severe gastrointestinal tract bleeding, because this NSAID can cause gastroduodenal ulceration, which could be catastrophic in a dog with a severe coagulopathy such as DIC.
Maintaining good parenchymal organ perfusion
Good parenchymal organ perfusion is best achieved with aggressive fluid therapy consisting of crystalloids or plasma expanders such as dextran (see Table 87-6). The purpose of this therapy is to dilute out the clotting and fibrinolytic factors in the circulation, flush out microthrombi from the microcirculation, and maintain the precapillary arterioles patent so that blood is shunted to areas in which oxygen exchange is efficient. However, care should be taken not to overhydrate an animal with compromised renal or pulmonary function.
Preventing secondary complications
As previously discussed, numerous complications occur in dogs with DIC. Attention should be directed toward maintaining oxygenation (by oxygen mask, cage, or nasopharyngeal catheter), correcting acidosis, eliminating cardiac arrhythmias, and preventing secondary bacterial infections (i.e., the ischemic gastrointestinal mucosa no longer functions as an effective barrier to microorganisms, bacteria are absorbed and cannot be cleared by the hepatic mononuclear-phagocytic system, and sepsis occurs).
Prognosis
The prognosis for dogs with DIC is still grave. Despite the numerous acronyms for DIC coined over the past few decades (e.g., “death is coming,” “dead in cage,” “dog in cooler”), if the inciting cause can be controlled most patients recover with appropriate treatment (see Fig. 87-2). In the retrospective study of DIC in dogs conducted at OSU-VTH the mortality rate was 54%; however, the mortality rate in dogs with minor changes in the hemostasis screen (i.e., fewer than three abnormalities) was 37%, whereas that in the dogs with severe hemostatic abnormalities (i.e., more than three hemostatic abnormalities) was 74%. In addition, marked prolongation of the APTT and marked thrombocytopenia were negative prognostic factors. The median APTT in dogs that survived was 46% over the controls, whereas it was 93% over the controls in dogs that did not survive. Likewise, the median platelet count in dogs that survived was 110,000/μL, and in dogs that did not survive it was 52,000/μL.
THROMBOSIS
Thrombotic and thromboembolic disorders appear to be considerably less common in cats and dogs than in human beings. Several situations can result in thrombosis or thromboembolism (TE), including stasis of blood, activation of intravascular coagulation in an area of abnormal or damaged endothelium, decreased activity of natural anticoagulants, and decreased or impaired fibrinolysis. Thrombosis has been recognized clinically as associated with cardiomyopathy, hyperadrenocorticism, protein-losing enteropathy and nephropathy, and IHA.
Diagnosing TE is not an easy task. Clinical signs are variable and include signs associated with parenchymal organ ischemia (e.g., dyspnea from pulmonary TE, high liver enzyme activities in patients with hepatic TE, intermittent rear limb claudication in dogs with aortic thrombosis). A positive d-dimer test has been reported to be associated with TE disease in dogs (Nelson & Andreasen, 2003). In our experience TEG is a rapid and sensitive test to diagnose TE disease in dogs (Fig. 87-3).
FIG 87-3 A, TEG (Haemoscope, Niles, Ill.) tracing in a normal dog; the MA (maximum amplitude) provides information on the strength of the clot and is within the reference range (53.9 mm). B, TEG tracing in a dog with hypercoagulability; notice that the MA is 80.3 mm.
Stasis of blood and possibly an irregular endothelial surface appear to be the major causes in cats with aortic (iliac) TE secondary to hypertrophic cardiomyopathy. Decreased activity of the natural anticoagulant AT plays a major role in the thrombosis seen in dogs with protein-losing nephropathy or protein-losing enteropathy; in addition, human beings with hypertension frequently have high concentration of PAI-1, which in turns inhibits fibrinolysis, thus resulting in a net procoagulant effect. This mechanism may also be important in dogs with protein-losing nephropathy and hypertension. The decreased AT activity stems from the fact that this is a relatively small molecule (approximately 60kD) that is easily lost in the urine or gut contents in dogs with either of these two disorders. The thrombosis commonly seen in dogs with hyperadrenocorticism is likely related to the induction of PAI-1 synthesis by corticosteroids (corticosteroids inhibit fibrinolysis). An increased risk for TE has been recognized in dogs with IHA. Although the pathogenesis of these disorders is obscure, the release of procoagulants from the lysed RBCs has been postulated as a cause; sludging of autoagglutinated RBCs in the microcirculation is also likely to contribute to this procoagulant state.
Dogs and cats at high risk for thrombosis or TE should receive anticoagulants. The two drugs commonly used in cats and dogs at risk for this condition are aspirin and heparin. Coumarin derivatives are commonly used in human beings, but in dogs and cats they can result in excessive bleeding. In recent reports of human beings with AT deficiency, anabolic steroids such as stanozolol have been suggested to also decrease the risk of thrombotic disorders as a result of their stimulatory effect on the fibrinolytic system. The recognition and management of pulmonary TE are discussed in Chapter 22.
Bateman SW, et al. Diagnosis of disseminated intravascular coagulation in dogs admitted to an intensive care unit. J Am Vet Med Assoc. 1999;215:805.
Bateman SW, et al. Evaluation of point-of-care tests for diagnosis of disseminated intravascular coagulation in dogs admitted to an intensive care unit. J Am Vet Med Assoc. 1999;215:798.
Bick RL. Disseminated intravascular coagulation: current concepts of etiology, pathophysiology, diagnosis, and treatment. Hematol Oncol Clin North Am. 2003;17:149.
Brooks M. von Willebrand disease. In: Feldman BF, et al, editors. Schalm’s veterinary hematology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2000:509.
Brooks M, et al. Epidemiologic features of von Willebrand’s disease in Doberman Pinschers, Scottish Terriers, and Shetland Sheepdogs. J Am Vet Med Assoc. 1992;200:1123.
Brooks MB, et al. A hereditary bleeding disorder of dogs caused by a lack of platelet procoagulant activity. Blood. 2002;99:2434.
Callan MB, Giger U. Assessment of a point-of-care instrument for identification of primary hemostatic disorders in dogs. Am J Vet Res. 2001;62:652.
Callan MB, Giger U. Effect of desmopressin acetate administration on primary hemostasis in Doberman Pinschers with type-1 von Willebrand disease as assessed by a point-of-care instrument. Am J Vet Res. 2002;63:1700.
Couto CG. Clinical approach to the bleeding patient. Vet Med. 1999;94:450.
Couto CG. Disseminated intravascular coagulation in dogs and cats. Vet Med. 1999;94:547.
Couto CG. Managing thrombocytopenia in the dog and cat. Vet Med. 1999;94:460.
Couto CG, et al. Disorders of hemostasis. In Sherding RG, editor: The cat: diseases and clinical management, ed 2, New York: Churchill Livingstone, 1994.
Couto CG, et al. Evaluation of platelet aggregation using a point-of-care instrument in retired racing Greyhounds. J Vet Intern Med. 2006;20:365.
Dodds WJ. Hereditary and acquired hemorrhagic disorders in animals. Prog Hemost Thromb. 1974;2:215.
Dodds WJ. Other hereditary coagulopathies. In: Feldman BF, et al, editors. Schalm’s veterinary hematology. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2000:1030.
Feldman BF, et al. Disseminated intravascular coagulation: antithrombin, plasminogen, and coagulation abnormalities in 41 dogs. J Am Vet Med Assoc. 1981;179:151.
Grindem CB, et al. Epidemiologic survey of thrombocytopenia in dogs: a report on 987 cases. Vet Clin Pathol. 1991;20:38.
Kraus KH, et al. Effect of desmopressin acetate on bleeding times and plasma von Willebrand factor in Doberman Pinscher dogs with von Willebrand’s disease. Vet Surg. 1989;18:103.
Lara García A, et al. Postoperative bleeding in retired racing Greyhounds. J Vet Intern Med. 2008;22:525.
Mischke R, et al. Efficacy of low molecular weight heparin in a canine model of thromboplastin-induced acute disseminated intravascular coagulation. Res Vet Sci. 2005;79:69.
Morales F, et al. Effects of 2 concentrations of sodium citrate on coagulation test results, von Willebrand factor concentration, and platelet function in dogs. J Vet Intern Med. 2007;21:472.
Nelson OL, Andreasen C. The utility of plasma d-dimer to identify thromboembolic disease in dogs. J Vet Intern Med. 2003;17:830.
Peterson JL, et al. Hemostatic disorders in cats: a retrospective study and review of the literature. J Vet Intern Med. 1995;9:298.
Ramsey CC, et al. Use of streptokinase in four dogs with thrombosis. J Am Vet Med Assoc. 1996;209:780.
Schenone M, et al. The blood coagulation cascade. Curr Opin Hematol. 2004;11:272.
Sheafor S, et al. Clinical approach to the dog with anticoagulant rodenticide poisoning. Vet Med. 1999;94:466.
Stokol T. Plasma d-dimer for the diagnosis of thromboembolic disorders in dogs. Vet Clin North Am Small Anim Pract. 2003;33:1419.
Tarnow I, et al. Dogs with heart diseases causing turbulent high-velocity blood flow have changes in platelet function and von Willebrand factor multimer distribution. J Vet Intern Med. 2005;19:515.
Wiinberg B, et al. Validation of human recombinant tissue factor-activated thromboelastography on citrated whole blood from clinically healthy dogs. Vet Clin Pathol. 2005;34:389.