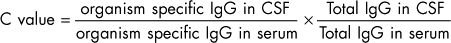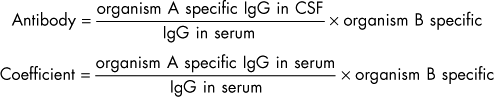CHAPTER 64 Diagnostic Tests for the Neuromuscular System
MINIMUM DATABASE
Patients with disease confined to the central nervous system (CNS) often have no specific abnormalities on a minimum database consisting of a complete blood cell (CBC) count, serum biochemistry profile, and urinalysis. These tests can be useful, however, in the diagnosis of systemic disorders that have neurologic manifestations and in the identification of the clinicopathologic abnormalities associated with some primary neurologic disorders.
Hematologic findings are rarely specific, but leukocytosis suggests inflammatory disease. Severe inflammation and a left shift are expected in patients with bacterial meningitis or encephalitis. Lymphopenia and inclusion bodies within red blood cells (RBCs) and lymphocytes are occasionally seen in dogs with acute canine distemper virus infection. Morulae may be seen within neutrophils in dogs with granulocytic ehrlichiosis. Microcytosis with or without thrombocytopenia is a common finding in dogs with portosystemic shunts. Red cell regeneration with or without accompanying anemia is seen in dogs with recurrent intraperitoneal hemorrhage caused by abdominal hemangiosarcoma. Occasionally, concurrent leukemia is detected in an animal with brain or spinal cord lymphoma.
A serum biochemistry profile is most useful in determining the likelihood of metabolic disorders as the cause of neuropathies, encephalopathies, and seizures. Diabetes mellitus, hypoglycemia, hypocalcemia, hypokalemia, and uremia can be eliminated from the list of differential diagnoses if the biochemistry panel is found to be normal. In dogs with peripheral neuropathy and a greatly increased serum cholesterol concentration, diagnostic tests for hypothyroidism should be considered (see Chapter 51). The finding of high liver enzyme activities (i.e., alanine aminotransaminase, serum alkaline phosphatase) or hypoalbuminemia in a patient with forebrain signs should prompt consideration of liver function tests to rule out hepatic encephalopathy (see Chapter 36). Hepatocellular enzyme elevations are also expected with some multisystemic disorders, such as toxoplasmosis and metastatic neoplasia. Serum creatine kinase is elevated in dogs and cats with muscle inflammation or necrosis. Urine specific gravity differentiates primary renal from prerenal azotemia. Hypernatremia is common when animals stop drinking or develop diabetes insipidus because of intracranial disease. Extremes of hyponatremia and hypernatremia and rapid correction of sodium imbalances cause brain dysfunction (see Chapter 55). Ammonium biurate crystals are occasionally found in the urine of dogs and cats with portosystemic shunts.
OTHER ROUTINE LABORATORY TESTS
Additional biochemical tests are frequently performed during the diagnostic evaluation of patients with neurologic disorders. Preprandial and postprandial bile acids are routinely measured to rule out hepatic encephalopathy in animals with forebrain signs and to monitor liver function in animals being chronically treated with some anticonvulsants. Alternatively, provocative ammonia tolerance testing can be used to assess hepatic function in nonencephalopathic patients, and resting ammonia concentration can be measured in encephalopathic patients. Serum concentrations of anticonvulsants are routinely monitored (see Chapter 67). Whenever CNS hemorrhage is considered as a possible differential diagnosis, coagulation should be assessed by determining either the activated clotting time (ACT) or the prothrombin time (PT) and partial thromboplastin time (PTT). When abnormalities of calcium or glucose regulation are detected on the minimum database, further endocrinologic testing is recommended. Specific endocrine testing is also warranted when thyroid disease, hypoadrenocorticism, or hyperadrenocorticism could be responsible for an animal’s neurologic signs.
IMMUNOLOGY, SEROLOGY, AND MICROBIOLOGY
A number of special diagnostic tests can be performed in patients with neurologic disorders when infectious or immune-mediated diagnoses are being considered. Clinicians should routinely perform bacterial culture of the cerebrospinal fluid (CSF), blood, and urine in patients with inflammatory disease of the brain, spinal cord, or meninges. Concurrent systemic illness, potential for exposure, and vaccination status will determine what additional testing is warranted. When lesions outside of the CNS are identified, such as pneumonia or dermatitis, the most direct route to a diagnosis is usually by sampling those sites. Serum antibody or antigen tests are also available for many of the infectious agents that can affect the CNS. An increased titer of specific antibody in CSF relative to that in serum may be required to make a definitive diagnosis. Alternatively, immunohistochemical staining can be used to identify organisms in tissue (brain, spinal cord, or muscle). In some cases polymerase chain reaction (PCR) analysis is available for diagnosis of active infection by a specific organism.
Immune-mediated CNS disorders such as steroidresponsive meningitis-arteritis (SRMA) and granulomatous meningoencephalomyelitis (GME) are relatively common in dogs. Diagnosis requires finding typical clinical and clinicopathologic abnormalities and eliminating the possibility of infectious disorders, as previously. Dogs with SRMA commonly have elevated serum and CSF IgA levels, and some have concurrent immune-mediated polyarthritis, which contributes to the diagnosis. In dogs with polyneuropathies, polymyositis, or apparent multisystemic immune-mediated disease, it may be useful to measure antinuclear antibody (ANA) titers to support a diagnosis of systemic lupus erythematosus (SLE). Most dogs with acquired myasthenia gravis have detectable circulating antibodies against acetylcholine receptors, and some dogs with masticatory muscle myositis have serum antibodies directed against type 2M myofibers (See Chapter 72).
RADIOGRAPHY
RADIOGRAPHS
Radiographs of the thorax and abdomen can be useful as screening tests for infectious and neoplastic diseases and as a means of evaluating liver size. These are noninvasive tests that should be performed routinely.
Spinal radiographs are necessary and useful in the diagnosis of congenital malformations, fractures and luxations, disk disease, diskospondylitis, and primary or metastatic vertebral neoplasia. In most cases general anesthesia is required to obtain lateral and ventrodorsal radiographs of sufficient quality to permit the detection of subtle abnormalities. Radiographs should be centered on the region of clinical interest as established by the neurologic examination. Neoplasia of the soft tissues of the brain or spinal cord generally does not cause radiographic abnormalities. Although skull radiographs are a low-yield procedure, they are often performed in animals with disease above the foramen magnum, because finding an area of lysis, a region of tumor calcification, or an intranasal mass aids in diagnosis.
CEREBROSPINAL FLUID COLLECTION AND ANALYSIS
INDICATIONS
Analysis of CSF can be useful in the diagnostic evaluation of patients with CNS disease. Specific neurologic disorders often cause typical alterations in CSF cytology or protein concentration, aiding diagnosis. In addition, special techniques such as bacterial culture, organism identification, antibody determination, and PCR can be performed on CSF, leading to a definitive diagnosis in some patients with infectious CNS disease. CSF examination is indicated in most animals with certain or suspected neurologic disease in which a diagnosis is not readily apparent, once traumatic, metabolic, and congenital abnormalities have been excluded. Analysis of CSF is most likely to be diagnostic in dogs and cats with intracranial lesions causing progressive forebrain signs and in animals with fever and axial pain. In animals with evidence of spinal cord disease, CSF analysis should always be performed before myelography to rule out inflammatory disease.
CONTRAINDICATIONS
If the proper technique is followed, the procedure for obtaining CSF is safe and simple. The animal is first placed under general anesthesia, and the puncture site is prepared in a sterile fashion, thereby minimizing the risk of damage resulting from animal movement and the risk of iatrogenic infections. Spinal puncture should not be performed in an animal that is an obvious anesthetic risk or that has a severe coagulopathy. General anesthesia and collection of CSF should not be performed in any patient with suspected increased intracranial pressure (Box 64-1) without first taking steps to lower the intracranial pressure in order to decrease the risk of brain herniation (Box 64-2).
TECHNIQUE
In dogs and cats the most reliable source of CSF for analysis is the cerebellomedullary cistern. The L5-L6 site may also be used, but it is more difficult to obtain a large volume of uncontaminated fluid from this site. Although it has been stated that CSF obtained from the cerebellomedullary cistern best reflects the nature of intracranial disease, whereas fluid from a lumbar tap is more useful in characterizing spinal cord disease, diagnostically the two are not significantly different. Lumbar CSF from normal dogs may have a slightly higher protein content and lower nucleated cell count than CSF obtained from the cerebellomedullary cistern.
Cisternal Puncture
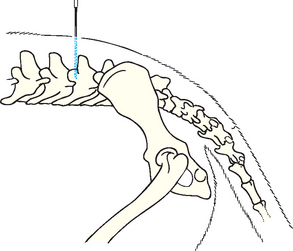
With the animal under general anesthesia, the clinician should prepare the back of its neck between the ears from the occipital protuberance to C2 for surgery. If the clinician is right-handed, the animal should be placed in right lateral recumbency with its neck flexed so that the median axis of the head is perpendicular to the spine. The nose should be elevated slightly so that its midline is parallel to the surface of the table. With the thumb and third finger of the left hand, the clinician should palpate the cranial edges of the wings of the atlas and draw an imaginary line at their most cranial aspect.
The examiner can then use the left index finger to palpate the external occipital protuberance and draw a second imaginary line caudally from that site along the dorsal midline. The needle should be inserted where the two imaginary lines intersect (Fig. 64-1).
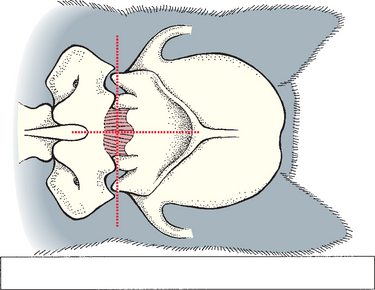
FIG 64-1 Landmarks for cerebrospinal fluid (CSF) collection at the cerebellomedullary cistern. The site of needle entry is at the intersection of the dorsal midline and the most cranial aspect of the wings of the atlas.
A 1½- or 3-inch (3.75 to 7.5 cm) long styletted spinal needle is then directed straight in through the skin, perpendicular to the spine, and into the underlying tissues. The needle is advanced 1 to 2 mm at a time, and the stylette is removed so that the clinician can look for CSF fluid. While the right hand is used to remove the stylette, the thumb and first finger of the left hand, which is rested against the spine for support, should grasp and stabilize the hub of the needle. A sudden “pop” may be felt as the dorsal atlantooccipital membrane and the dura mater and arachnoid mater are penetrated simultaneously (Fig. 64-2). This is not a reliable sign, however, and the level at which the subarachnoid space is reached varies greatly with the breed and individual animal. It is often very close to the skin surface in toy breeds and some cats.
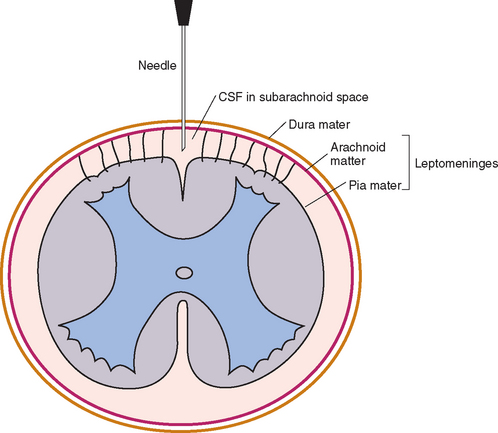
FIG 64-2 Transverse section showing the relationship among the meninges, the cerebrospinal fluid (CSF), and the spinal cord. The tip of the needle is in the subarachnoid space, as it would be for CSF collection or myelography.
If the needle strikes bone, it should be withdrawn, the patient position and landmarks reassessed, and the procedure repeated. If whole blood appears in the spinal needle, the needle should be withdrawn and the procedure repeated with another sterile needle. When CSF is observed, the fluid should be allowed to drip directly from the needle into a test tube. The clinician should check with the laboratory to determine the type of tube preferred for collection of CSF. The amount of CSF collected ranges from 0.5 to 3 ml depending on the size of the animal. Simultaneous jugular vein compression may hasten flow but will transiently increase intracranial pressure. Blood in the CSF may be the result of the disease or of the tap. If it is caused by the procedure, the amount of blood should decrease as the CSF drips from the needle. If this occurs, some of the less contaminated fluid should be collected in a second tube for cytologic evaluation. Mild CSF contamination with hemorrhage (<500 RBCs/μl) does not alter the CSF protein and leukocyte determinations. Grossly hemorrhagic CSF should always be collected into a tube containing ethylenediaminetetraacetic acid (EDTA) to prevent clotting.
Lumbar Puncture
The animal is placed in lateral recumbency with its trunk flexed. Foam cushions are placed between its limbs and beneath the lumbar region to achieve true lateral positioning. A 3½-inch (8.75-cm) spinal needle is inserted on midline at the cranial edge of the dorsal spinal process of the L5 or L6 vertebra and directed ventrally into the ligamentum flavum (Fig. 64-3). The needle is passed in a smooth motion through or alongside the caudal spinal cord and cauda equina into the ventral subarachnoid space. The animal’s tail and pelvic limbs may twitch when the cord is penetrated. Because CSF flow is slower from this site and more likely to be contaminated by blood, cerebellomedullary collection is usually preferred for diagnostic purposes.
ANALYSIS
Normal CSF is clear and colorless. A cell count should be performed and a cytologic preparation made for examination as soon as possible because white blood cells (WBCs) in the CSF deteriorate rapidly. If the sample must be stored for longer than 1 hour before analysis, the specimen should be refrigerated to slow cellular degeneration. The addition of autologous serum (10% by volume of the sample) will preserve CSF so that cytologic analysis 24 to 48 hours after collection will yield reliable results, but a separate sample must be saved for protein analysis. Alternatively, one drop of buffered 10% formalin can be added for each 0.25 ml of CSF to preserve cytologic features without affecting the protein measurement.
Once the fluid is collected, a total cell count is performed and the concentration of RBCs and WBCs is determined. The normal range of values varies with each laboratory, but in general there should be fewer than five WBCs per microliter. An increased number of CSF WBCs is referred to as a pleocytosis. A pleocytosis should be further characterized by microscopic examination and differential cell count to determine the predominant leucocyte present. Cytologic analysis of CSF is necessary even if the WBC count is normal because there may be abnormal cell types or organisms present.
A concentration procedure is usually required to obtain sufficient cells for cytologic assessment if the CSF WBC count is less than 500 cells/μl. Cytocentrifuge concentration of CSF is available in most institutions and commercial laboratories, and results are best if samples are processed within 30 minutes of collection or if samples are preserved as described earlier. Alternatively, an in-clinic sedimentation technique can be used, in which 0.5 ml of CSF is allowed to sediment over a region of a slide within a sedimentation chamber that is attached to the slide with paraffin or petroleum jelly (Fig. 64-4). The CSF supernate is then gently aspirated with a needle and syringe and can be used for protein or antibody titer determination. Any remaining fluid is then removed by applying blotting paper to the fluid edge, and the slide is quickly dried by vigorously waving it in the air. Once the slide is dry, the remaining paraffin or petroleum jelly should be scraped off. Slides should be evaluated by a veterinary cytopathologist. If the slide cannot reach a commercial laboratory within a few hours, it should be fixed and stained with Diff-Quik Differential Stain Set (American Scientific Products) or Wright’s or Giemsa stain.
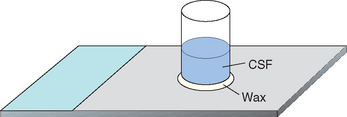
FIG 64-4 A sedimentation chamber can be made from a cut section of a glass vacutainer or plastic specimen tube that is attached to a glass microscope slide with paraffin or petroleum jelly. Cerebrospinal fluid (CSF; 0.5 ml) is placed in the chamber. After 30 minutes the supernate is gently aspirated with a needle and syringe and the slide is dried rapidly. The slide is then fixed, and the paraffin or petroleum jelly is removed with a scalpel.
Most of the cells in the CSF of normal dogs and cats are small, well-differentiated lymphocytes (60% to 70%). Minimally vacuolated, large mononuclear phagocytes normally compose up to 40% of the cells. Occasional neutrophils and eosinophils are present, but these cells should not normally make up more than 2% of the cell population. The typical CSF findings in some specific disorders in dogs and cats are summarized in Box 64-3. It is important to realize, however, that CSF cytologic findings must always be interpreted in relation to the signalment, history, and clinical findings.
 BOX 64-3 Interpreting Cerebrospinal Fluid Cytology
BOX 64-3 Interpreting Cerebrospinal Fluid Cytology
Italics signify unusual presentation.
Mixed Cell Pleocytosis (>50 White Blood Cells/μl; Lymphocytes, Mononuclear Phagocytes, Neutrophils, Plasma Cells)
If blood contamination is severe, it can influence the cytologic findings, but even grossly apparent iatrogenic contamination with peripheral blood will have only a minor impact on WBC count and protein analysis. To approximate the maximum effect blood contamination will have on the WBC count in CSF, one WBC per microliter can be expected for every 500 RBCs per microliter.
The protein concentration in samples collected from the lumbar site (<40 mg protein/dl) is normally higher than the protein content of CSF collected from the cerebellomedullary cistern (<25 mg protein/dl). The protein content of the collected CSF should be determined and, whenever possible, protein electrophoresis performed. An increase in the CSF protein content can occur in diseases that disrupt the blood-brain barrier, cause local necrosis, interrupt normal CSF flow and absorption, or result in intrathecal globulin production. Information from CSF protein electrophoresis can be used to determine whether the high protein content in CSF is a result of blood-brain barrier disruption, the intrathecal production of immunoglobulin, or both (Box 64-4). CSF protein electrophoresis patterns typical of inflammation, degeneration, and neoplasia of the CNS have been established and can be used with some degree of accuracy to predict the mechanism of disease involved. The immunoglobulins in CSF can also be quantified, helping to differentiate inflammatory from noninflammatory disorders.
 BOX 64-4 Diagnostic Interpretation of Protein and Antibody Concentrations in Cerebrospinal Fluid
BOX 64-4 Diagnostic Interpretation of Protein and Antibody Concentrations in Cerebrospinal Fluid
CSF, Cerebrospinal fluid.
Whenever the CSF is cellular, it should be submitted for Gram’s staining and anaerobic and aerobic bacterial culture. If infectious disorders are considered likely (see the discussion of meningitis, Chapter 69), specific culture techniques can be applied or, when available, PCR can be used to identify infectious agents in CSF. Antibody titers to a variety of infectious organisms can be measured in CSF, but leakage of antibodies from the serum to the CSF can be problematic. An immunogolobin G (IgG) index greater than 1 indicates that there is significant intrathecal production of immunoglobulin (see Box 64-4). Comparison of CSF and serum titers against a specific organisms can be performed, with a C-value greater than 1 indicating active CNS infection with an organism (see Box 64-4).
ADVANCED DIAGNOSTIC IMAGING
MYELOGRAPHY
In animals with clinical evidence of spinal cord disease or compression, myelography may be used to confirm, localize, and characterize lesions. This procedure is particularly valuable for identifying compression of the spinal cord by herniated disks or tumors. Myelography is rapid and is more readily available and less expensive than other advanced imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), but it is also associated with a higher rate of complications.
To perform myelography, the clinician anesthesizes the animal and injects a nonionic contrast material into the subarachnoid space at the atlanto-occipital or lumbar (L5/6 or L4/5) space. The contrast material most commonly used for this purpose is iohexol (Omnipaque; Nycomed). Injection of iohexal (0.25 to 0.50 ml/kg of 240 or 300 mgI/ml contrast media) is associated with a relatively low (<10%) prevalence of postmyelographic adverse effects, such as seizure, hyperesthesia, and vomiting. Lumbar injections are technically more difficult but associated with decreased risk of iatrogenic spinal cord trauma and improved delineation of thoracic and lumbar compressive spinal cord lesions because the contrast material can be injected under increased pressure and forced around a site of severe compression.
Myelography should be performed only after it has been confirmed that the CSF is not inflammatory because contrast injection will worsen the inflammation and clinical symptoms in an animal with meningitis. Additionally, injection of contrast will cause mild inflammation, making diagnostic evaluation of CSF cytology very difficult for at least 1 week after myelography. CSF collection and analysis should always precede myelography.
In cisternal myelography the needle is inserted using the same technique and landmarks as for cisternal puncture for CSF collection (see Fig 64-1), and the bevel of the needle is directed caudally. The contrast material is injected slowly and allowed to flow caudally the length of the spinal subarachnoid space. Needle removal and elevation of the animal’s head, neck, and thorax promote caudal flow, resulting in opacification of the caudal limit of the subarachnoid space within 10 minutes. During myelography the flow of the contrast agent is visualized fluoroscopically (when available), and lateral, ventrodorsal, and sometimes obliquely positioned radiographs are taken directly over each region of interest. In some instances dynamic views (traction, extension, and flexion) may be obtained. If contrast medium filling is inadequate in some regions, the animal is tilted and manipulated to allow gravity-assisted pooling of contrast medium at the site of interest.
Lumbar myelography is performed with the needle at the L5/L6 (large dogs) or L6/L7 (small dogs and cats) site. Needle insertion is as described for lumbar CSF collection, with the bevel of the needle oriented cranially. Once the needle is in place, a small test volume (0.2 ml) of contrast medium should be injected and lateral radiography or fluoroscopy performed to make sure the injection is not directed into the spinal cord parenchyma. If the needle is positioned correctly, the injection is completed during fluorosocopic visualization and then lateral radiographic views are taken with the needle in place. Once the spinal needle is removed, ventrodorsal, oblique, and dynamic radiographs should be taken rapidly because epidural leakage may occur through the puncture site.
Seizures occasionally occur in animals recovering from anesthesia after myelography. Seizures are most common in dogs larger than 29 kg, when cisternal myelography is per formed, and when more than two injections of contrast agent are administered. These seizures can usually be controlled with diazepam (5 to 20 mg, administered intravenously).
Neurologic deterioration occurs in some animals after myelography. Large-breed dogs with cervical spondylomyelopathy (Wobbler syndrome), dogs and cats with inflammatory CNS disease or extradural tumors, and dogs with degenerative myelopathy are most often affected. Fortunately, this deterioration is usually transient.
A normal myelogram will show contrast material filling the subarachnoid space. This appears as a column of contrast agent on each side of the cord on ventrodorsal views and in the ventral and dorsal columns on lateral views (Fig. 64-5). In normal myelograms a slight elevation and thinning of the ventral column of the contrast agent can be seen as it passes over each intervertebral disk space; however, a wide dorsal column remains, indicating that spinal cord compression is not present. Based on the features of the myelogram, a spinal cord lesion can be characterized as extradural compression, intradural extramedullary compression, or intramedullary swelling (Figs. 64-6 and 64-7).
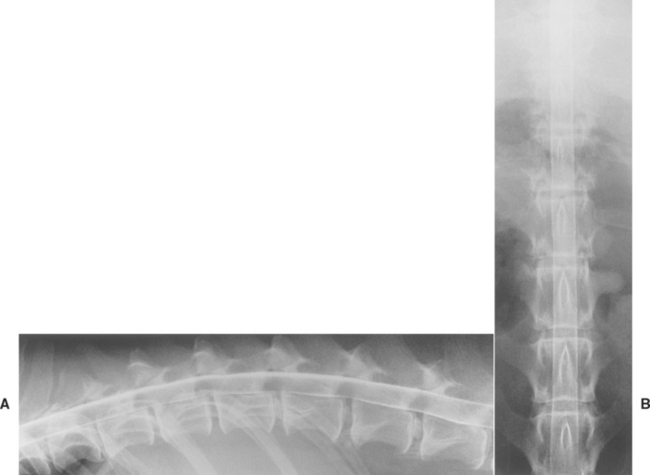
FIG 64-5 Lateral (A) and ventrodorsal (B) views of a normal myelogram of the thoracolumbar region in a dog. Multiple calcified intervertebral disks can be seen, but no spinal cord compression is evident.
(Courtesy Dr. John Pharr, University of Saskatchewan.)
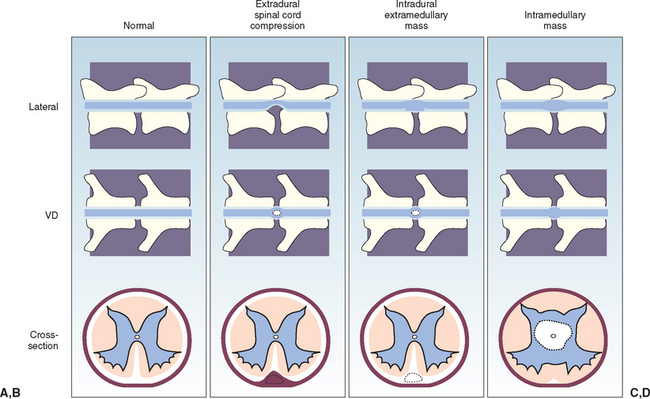
FIG 64-6 The myelographic appearance of extradural, intradural-extramedullary, and intramedullary spinal cord masses. A, Normal myelogram. B, Ventral extradural spinal cord compression. The leading edge of the contrast material tapers toward the spinal cord, away from the bone on the lateral view. The dorsal column is thinned in this region. On the ventrodorsal view the spinal cord appears widened or flattened, resulting in narrow columns of contrast material. C, Ventral intradural, extramedullary spinal cord compression. The leading edge of the contrast material expands and outlines the lesion, tapering toward the spinal cord and toward the bony margin of the osseous canal, resulting in a filling defect at the site of the lesion and the appearance of a “golf tee sign.” On the ventrodorsal view the spinal cord appears widened or flattened, resulting in narrow columns of contrast material. D, Intramedullary mass or swelling. The leading edges of the contrast material taper toward the bony margin of the osseous canal on both views, with diverging columns of contrast material indicating spinal cord enlargement.
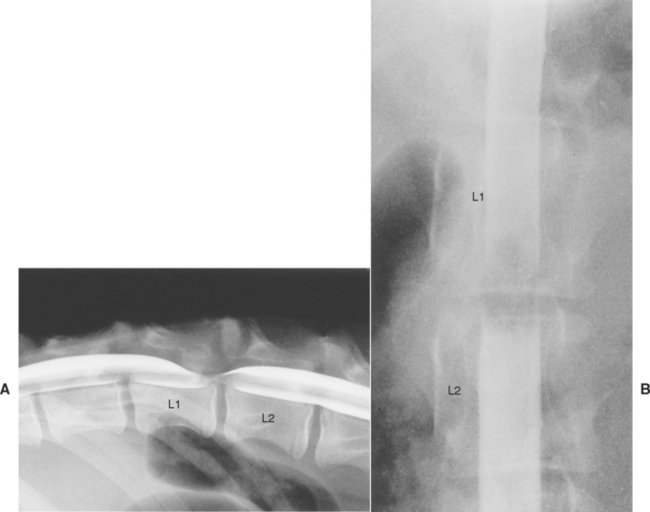
FIG 64-7 Lateral (A) and ventrodorsal (B) views of a myelogram in a 5-month-old German Shepherd Dog with a 3-week history of progressive ataxia. A dorsally located extradural compression of the spinal cord within the caudal portion of the L1 vertebra can be seen. At necropsy the dog was found to have a single focal cartilaginous exostosis of the roof of the L1 vertebra.
ULTRASONOGRAPHY
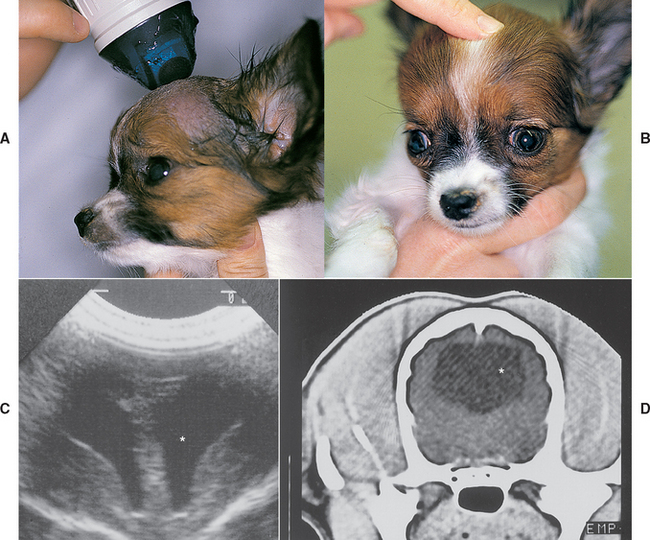
Ultrasound is not useful in routine imaging of the brain and spinal cord because these structures are normally encased in bone. This modality can, however, provide valuable information in the diagnostic evaluation of patients with neurologic disease. Abdominal ultrasound is recommended to search for a primary tumor whenever metastatic neoplasia is considered as a possible cause of neurologic signs. Ultrasound can also be used to identify portosystemic shunts in dogs and cats. Brain ultrasound through open fontanelles can be performed to identify hydrocephalus in dogs and cats (Fig. 64-8). Ultrasound of the axilla in a sedated animal can be useful in the identification and biopsy of soft tissue masses within the brachial plexus.
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
CT and MRI are available for the diagnosis of neurologic disease at most major veterinary referral centers. These techniques are noninvasive and valuable in the localization, identification, and characterization of many brain and spinal cord lesions (Figs. 64-9 and 64-10). CT is most useful for identification and characterization of bony abnormalities of the vertebral bodies and skull. Contrast-enhanced CT will help identify soft tissue lesions that disrupt vascular endothelium. MRI can be used to determine very small density differences in soft tissues; therefore it is the imaging modality of choice for the brain, spinal cord, and peripheral nerves. These techniques allow precise topographic mapping of lesions, making them valuable tools in the evaluation of compressive lesions of the brain, spinal cord, or cauda equina when surgery is being considered.
ELECTRODIAGNOSTIC TESTING
Electrophysiologic studies can be used to record electrical activity from muscle or neural tissue and aid in lesion localization and characterization. These tests are minimally invasive but usually require sedation or general anesthesia. The cost of the equipment and the experience needed to conduct the studies limit their use to academic and referral clinics.
ELECTROMYOGRAPHY
Normal muscle is electrically silent. As a needle is inserted into normal muscle, a short burst of electrical activity is elicited, which stops when the needle insertion is stopped. Severance, destruction, or demyelination of the peripheral nerve supplying the muscle results in the development of spontaneous fibrillations and positive sharp waves (i.e., denervation potentials) and prolonged insertional activity in affected muscles 5 to 7 days after denervation. These changes may also be seen in some primary muscle disorders. Electromyography (EMG) is most useful to confirm a suspected diagnosis of a muscle or peripheral nerve disorder and to identify abnormal muscles for subsequent biopsy.
NERVE CONDUCTION VELOCITIES
The conduction velocity of motor nerves can be determined by stimulating a nerve at two separate sites and recording the time it takes for an evoked muscle potential to occur. The motor nerve conduction velocity in that segment of nerve can be determined by measuring the distance between the two sites and the difference in the time it takes for the evoked potentials to appear. The conduction velocity of sensory nerves can be measured using a similar technique. Slow conduction times are seen in demyelinating disorders, allowing the diagnosis of peripheral neuropathies. Nerves that have been injured or avulsed and that have degenerated (onset typically 4 to 5 days after injury) do not conduct an impulse; thus nerve conduction velocity testing can also be used to diagnose and localize peripheral nerve injuries.
ELECTRORETINOGRAPHY
An electroretinogram (ERG) is a recording of the electrical response of the retina to a flashing light stimulus. It is an objective way to evaluate retinal function, assessing both rod and cone receptors. The ERG is most useful for the evaluation of blind animals in which the retina appears normal on ophthalmic examination (e.g., diagnosing sudden acquired retinal degeneration) or in which the retina cannot be visualized (e.g., determining whether animals with cataracts have concurrent retinal degeneration). The ERG is abnormal with degenerative disorders of the retina, but it is normal if the lesion causing visual dysfunction is located caudal to the retina (in the optic nerves, optic chiasm, optic tract, or cerebral cortex). The ERG can be performed under general anesthesia or under sedation if the patient is uncooperative.
BRAINSTEM AUDITORY EVOKED RESPONSE
The brainstem auditory evoked response (BAER) depicts the response of nervous tissues to an auditory stimulus (a click). The response is a series of waveforms representing activity beginning in the cochlea and being relayed up the auditory pathway in the brainstem. Lesions of the outer, middle, or inner ear; the peripheral vestibulocochlear nerve; and the brainstem caudal to the midbrain cause characteristic changes in the response, aiding in lesion localization. This test has been most widely used for detecting unilateral and bilateral congenital deafness in dogs.
ELECTROENCEPHALOGRAPHY
Electroencephalography provides a graphic record of the spontaneous electrical activity of the cerebral cortex. Results may help determine whether a cerebral disorder is focal or diffuse. Some dogs with epilepsy will have abnormal electroencephalograms (EEGs) between seizures.
BIOPSY OF MUSCLE AND NERVE
MUSCLE BIOPSY
Muscle biopsy specimens should be evaluated when there is clinical and electrophysiologic evidence of muscular disease. A biopsy may provide a definitive diagnosis or indicate the nature of the disease process. For best results, muscle that is affected should be biopsied and in generalized disorders two different muscles should be sampled. For investigation of myopathic disorders, proximal limb muscles such as the vastus lateralis or triceps should be biopsied, whereas neuropathies are more evident in distal limb muscles such as the cranial tibial or extensor carpi radialis. Because complete histopathologic examination of muscle requires fresh-frozen tissue, most laboratories request that fresh muscle samples be wrapped in a saline-moistened gauze and shippped overnight under refrigeration. Whenever formalin-fixed samples are submitted, the sample should be attached to a splint, such as a tongue depressor, to prevent contraction during fixation. Routine histologic studies may reveal inflammatory or neoplastic changes and the etiologic agent if the disease is infectious.
When fresh-frozen tissue is evaluated using a full range of enzymatic and immunohistochemical techniques, many characteristics of the muscle can be determined. Based on enzymatic staining characteristics, muscle fibers can be classified according to type and the proportion and distribution of myofiber types described. Some myopathies result in a selective loss of one fiber type. Denervation with reinnervation, as occurs in many neuropathies, results in “type grouping,” wherein the normal checkerboard pattern disappears and large clusters of fibers of the same type appear. Muscle fiber shape and size, the presence of degeneration or necrosis, the location of nuclei, the presence of vacuoles or inclusions, and the presence of cellular infiltrates are all evaluated. Immunostains are also available to identify some parasites (Neospora) and evaluate muscles for normal structural components. Muscle samples should be sent to a laboratory with a special interest in muscle disorders to ensure that optimal results are obtained and accurately interpreted. Clinicians should consult the laboratory that will process the biopsy to learn the proper technique of obtaining and preparing specimens and the other procedures to be followed.
NERVE BIOPSY
It may be useful to obtain nerve biopsy specimens in an effort to evaluate peripheral nerve disorders. Nerves are biopsied by transecting approximately one third of the width of the nerve and removing fascicles about 1 cm in length, leaving most of the nerve trunk intact. It is important to biopsy nerves that are affected. The common peroneal nerve and the ulnar nerve are the mixed (i.e., motor and sensory) nerves most commonly biopsied. As with muscle biopsy specimens, nerve biopsy specimens require special handling to ensure that maximal information is obtained. Samples should be laid out on a piece of wooden tongue depressor and pinned at each end to keep them oriented longitudinally, but they should not be stretched. They should then be fixed in 2.5% glutaraldehyde or buffered 10% formalin for light microscopy. Fresh nerve samples can be frozen in liquid nitrogen and stored for biochemical analysis.
Bienzle D, McDonnell JJ, Stanton JB. Analysis of cerebrospinal fluid from dogs and cats after 24 and 48 hours of storage. J Am Vet Med Assoc. 2000;216:1761.
Bohn A, Wills T, West C, et al. Cerebrospinal fluid analysis and magnetic resonance imaging in the diagnosis of neurologic diseases in dogs: a retrospective study. Vet Clin Pathol. 2006;35:315-320.
Chrisman CL. Cerebrospinal fluid analysis. Vet Clin N Am Small Anim Pract. 1992;22:781.
Dickinson PJ, LeCouter RA. Muscle and nerve biopsy. Vet Clin N Am Small Anim Pract. 2002;32:63.
Hurtt AE, Smith MO. Effects of iatrogenic blood conatmination on results of cerebrospinal fluid analysis in clinically normal dogs and dogs with neurologic disease. J Am Vet Med Assoc. 1997;211:866.
Olby NJ, Thrall DE. Neuroradiology. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
Wamsley H, Alleman AR. Clinical pathology. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
 BOX 64-2
BOX 64-2