CHAPTER 86 Combined Cytopenias and Leukoerythroblastosis
DEFINITIONS AND CLASSIFICATION
Combined cytopenias commonly result from decreased bone marrow production or, less frequently, from increased destruction or sequestration of circulating cells. Following are the definitions of several terms used throughout this chapter. Bicytopenia is a decrease in the numbers of two circulating blood cell lines (anemia and neutropenia, anemia and thrombocytopenia, or neutropenia and thrombocytopenia). If all three cell lines are affected (anemia, neutropenia, thrombocytopenia), this is called pancytopenia (from the Greek word pan, meaning “all”). In most cases, if anemia is present it is nonregenerative. If regenerative anemia occurs in association with other cytopenias, the cause usually is peripheral destruction of cells. Leukoerythroblastic reaction (LER) (or leukoerythroblastosis) refers to the presence of immature white blood cells (WBCs) and nucleated red blood cells (nRBCs) in the circulation (i.e., nRBCs and a left shift). In these cases the WBC count is usually high, but it can be normal or low.
As mentioned, cytopenias can develop as a result of decreased production or increased peripheral destruction of the affected cell line(s). In general, bicytopenias and pancytopenias result from primary bone marrow disorders (i.e., there is a problem in the “cell factory”) (Box 86-1), although they may also result from peripheral blood cell destruction, such as what occurs in sepsis, disseminated intravascular coagulation (DIC), and some immune-mediated blood disorders.
 BOX 86-1 Causes of Bicytopenia and Pancytopenia in Dogs and Cats
BOX 86-1 Causes of Bicytopenia and Pancytopenia in Dogs and Cats
Common; relatively common; uncommon. DIC, Disseminated intravascular coagulation.FELV,Feline leukemia virus.
Decreased cell production
Bone Marrow Hypoplasia-Aplasia
Chemicals (e.g., benzene derivatives)
Estrogen (endogenous or exogenous)
Drugs (chemotherapeutic agents, antibiotics, anticonvulsants, colchicine, nonsteroidal antiinflammatories)
Infectious (parvovirus, FeLV, feline immunodeficiency virus, Ehrlichia canis, and plasmosis)
Bone Marrow Necrosis
Infectious disorders sepsis, parvovirus)
Neoplasms (acute and chronic leukemias, metastatic neoplasia)
LERs result from a variety of mechanisms (Box 86-2), but in general the presence of immature blood cells in the circulation is secondary to their premature release from the bone marrow or from other hematopoietic organs (spleen, liver). This premature release can result from (1) an increased demand for blood cells (e.g., hemolytic anemia, blood loss, peritonitis), resulting in a shorter transit time through the bone marrow compartments or extramedullary hematopoietic sites; or (2) the crowding out of normal bone marrow precursors (e.g., leukemia, bone marrow lymphoma). They may also be prematurely released from a site of extramedullary hematopoiesis (EMH) (i.e., spleen, liver) as a result of the absence of normal feedback mechanisms.
 BOX 86-2 Causes of Leukoerythroblastosis in Dogs and Cats
BOX 86-2 Causes of Leukoerythroblastosis in Dogs and Cats
Common; relatively common; uncommon.
H may play a role in the pathogenesis of the LER in several of the disorders mentioned in the text.
EMH, Extramedullary hematopoiesis; DIC, disseminated intravascular coagulation; LER, leukoerythroblastic reaction.
Clinicopathologic Features
The clinical signs and physical examination findings in dogs and cats with combined cytopenias or LERs are usually related to the underlying disorder rather than the hematologic abnormalities per se, with the exception of pallor and spontaneous bleeding (petechiae, ecchymoses) secondary to anemia and thrombocytopenia, respectively. Pyrexia may be present if the patient is markedly neutropenic and is septic or bacteremic.
An important aspect of the clinical evaluation of these patients is the history. A detailed history should be obtained, with particular inquiries about the therapeutic use of drugs (e.g., estrogen or phenylbutazone in dogs, griseofulvin or chloramphenicol in cats), exposure to benzene derivatives (rare), travel history, vaccination status, and exposure to other animals, among others. Most drugs that cause anemia or neutropenia can also cause combined cytopenias (see Boxes 83-2 and 85-1).
The physical examination of dogs and cats with combined cytopenias may reveal the presence of spontaneous hemorrhages compatible with a primary hemostatic disorder (e.g., thrombocytopenia) or pallor secondary to the attendant anemia. Several physical examination findings may help the clinician establish a more presumptive or definitive diagnosis in patients with cytopenias or LER. Of particular interest is the finding of male-feminizing signs in a male dog (usually a cryptorchid) with pancytopenia, which may indicate the presence of a Sertoli cell tumor or, less frequently, an interstitial cell tumor or a seminoma with secondary hyperestrogenism. The finding of generalized lymphadenopathy, hepatomegaly or splenomegaly, or intraabdominal or intrathoracic masses may direct the clinician toward a specific group of presumptive diagnoses. For example, the finding of a cranial or mid-abdominal mass in a dog with anemia, thrombocytopenia, and LER is highly suggestive of splenic hemangiosarcoma.
The presence of diffuse splenomegaly indicates that the spleen may be sequestering or destroying circulating blood cells or that EMH is occurring in response to a primary bone marrow disorder. Cytologic evaluation of spleen specimens obtained by percutaneous fine-needle aspiration is always indicated in dogs and cats with cytopenias and diffuse splenomegaly to determine whether the enlarged spleen is the cause or consequence of the cytopenia (see Chapter 88).
Serologic studies or polymerase chain reaction (PCR) for infectious diseases is usually indicated in dogs and cats with bicytopenias or pancytopenias. Infectious diseases associated with bicytopenias and pancytopenias commonly diagnosed on serologic PCR findings include monocytic ehrlichiosis in dogs, Babesia gibsoni infection in dogs (combined anemia and thrombocytopenia), and feline leukemia virus (FeLV) and feline immunodeficiency virus infections in cats. If the clinical and hematologic features of the case point toward an immune-mediated disease (e.g., presence of polyarthritis or proteinuria, spherocytosis) a direct Coombs’ test and an antinuclear antibody test should be done (see Chapter 92). It is also helpful to submit fluid obtained from one or more joints for cytologic evaluation because the presence of suppurative nonseptic arthritis suggests an immune pathogenesis or a rickettsial disease.
Because establishing whether the cytopenia is the result of peripheral cell destruction or a bone marrow disorder is important, evaluation of the “cell factory” is logical if no evidence of RBC regeneration in the blood smear or CBC exists (see Chapter 85). Therefore bone marrow aspiration and, ideally, bone marrow core biopsy to obtain specimens for histopathologic studies should be performed in all dogs and cats with combined cytopenias, except for dogs with highly likely or confirmed Evans syndrome and dogs and cats with DIC (i.e., the anemia is regenerative; thus it is assumed that the factory is working properly). Algorithms for the evaluation of bone marrow findings in dogs and cats with bicytopenia and pancytopenia are shown in Figs. 86-1 and 86-2. In private practice obtaining a bone marrow aspirate is usually easier; bone marrow core biopsies are usually performed at referral practices.
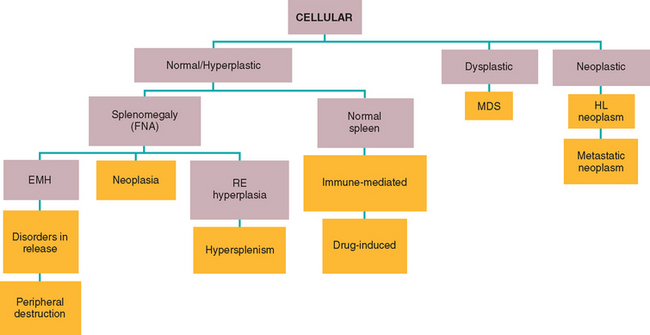
FIG 86-1 Algorithm for the diagnosis of a pancytopenic animal with hypercellular bone marrow. FNA, Fine-needle aspiration; MDS, myelodysplastic syndrome; HL, hemolymphatic; EMH, extramedullary hematopoiesis; RE, reticuloendothelium. Orange boxes indicate final diagnoses.w
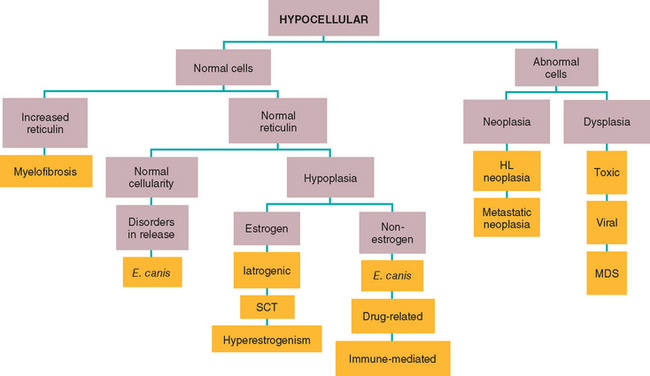
FIG 86-2 Algorithm for the diagnosis of a pancytopenic animal with hypocellular bone marrow. HL, Hemolymphatic; MDS, myelodysplastic syndrome; SCT, Sertoli cell tumor. Orange boxes indicate final diagnoses.
A bone marrow evaluation should also be part of the clinical workup in animals with LERs to determine whether the immature WBCs and RBCs in the circulation are secondary to a primary bone marrow disorder or a disorder such as EMH. Because abdominal neoplasms, particularly hemangiosarcoma, are commonly associated with LERs in dogs, abdominal ultrasonography should be done. If diffuse splenomegaly is detected, percutaneous fine-needle aspiration of the spleen should be performed. If splenic or hepatic masses or both are present, the patient should be evaluated as described in Chapter 90.
Weiss (2006) recently reviewed bone marrow aspirates, core biopsies, and medical records of 717 dogs evaluated for presumptive bone marrow disorders. Approximately 2% of the specimens evaluated were nondiagnostic, 22% were normal, 26% had changes secondary to another primary disease, 24% had nondysplastic and nonneoplastic conditions, 9% had dysplasia, and 18% had neoplasia. Less than 5% of the specimens evaluated had bone marrow hypoplasia and approximately 20% were hyperplastic; acute leukemias were more common than chronic leukemias.
Bone Marrow Aplasia-Hypoplasia
Bone marrow aplasia-hypoplasia is a disorder characterized by peripheral blood cytopenias and a paucity or absence of hematopoietic precursors in the bone marrow. As previously discussed, bone marrow aplasia-hypoplasia is commonly associated with the administration of certain drugs, such as griseofulvin or chloramphenicol in cats and phenylbutazone or estrogen in dogs. It is also commonly associated with infectious diseases, such as canine monocytic ehrlichiosis and FeLV infection. A corticosteroid-responsive syndrome of combined cytopenias or pancytopenia has been recognized in dogs and cats in the author’s clinic. Some patients with pancytopenia have hypercellular bone marrow (see below), suggesting that the cells are destroyed peripherally or at the late stages of bone marrow production.
Bone marrow aspirates from dogs and cats with bone marrow aplasia or hypoplasia typically show hypocellularity or acellularity, and a bone marrow biopsy is frequently necessary to obtain specimens for histopathologic analysis so that a definitive diagnosis can be made. Once infectious diseases (e.g., Ehrlichia canis titer, SNAP test [IDEXX, Westbrook, Maine], or PCR; FeLV p27 determination) and drug exposure have been ruled out, a therapeutic trial of immunosuppressive doses of corticosteroids (with or without other immunosuppressive drugs; see Chapter 93) may be warranted. Anabolic steroids and erythropoietin do not appear to be beneficial in these patients.
Myelophthisis
Infiltration of the bone marrow with neoplastic or inflammatory cells can lead to the crowding out of normal hematopoietic precursors and therefore the development of peripheral blood cytopenias. Disorders resulting in myelophthisis are listed in Box 86-1. Often these animals are evaluated because of anemia, although fever and bleeding caused by neutropenia and thrombocytopenia, respectively, can also be presenting complaints. The presence of hepatomegaly, splenomegaly, or lymphadenopathy in a dog or cat with anemia or combined cytopenias is highly suggestive of some of the neoplastic or infectious disorders listed in Box 86-1.
A definitive diagnosis in dogs and cats with myelophthisis is obtained by evaluating the cytologic or histopathologic characteristics of a bone marrow specimen. Given the fact that certain neoplastic or granulomatous disorders can show a patchy or multifocal distribution, the findings yielded by a bone marrow core biopsy specimen are usually more reliable than those yielded by an aspirate. Once a cytologic or histopathologic diagnosis is obtained, treatment is aimed at the primary neoplasm (i.e., with chemotherapy) or infectious agent (see specific sections for detailed discussion).
Myelodysplastic Syndromes
Myelodysplastic syndromes include a host of hematologic and cytomorphologic changes that may precede the development of acute leukemias by months or years. In addition to the morphologic abnormalities in blood and bone marrow, functional abnormalities of granulocytes and platelets have been well documented in human beings with MDS. Therefore recurrent infections, spontaneous bleeding tendencies, or both are common in such patients, even when the neutrophil and platelet counts are within normal limits. These abnormalities have also been observed in cats with MDS.
MDS has been recognized in both dogs and cats but appears to be more common in retrovirus-infected cats. All dogs are lethargic, depressed, and anorectic. Physical examination findings include hepatosplenomegaly, pallor, and pyrexia; hematologic changes include pancytopenia or bicytopenia, macrocytosis, metarubricytosis, and reticulocytopenia. acute myelogenous leukemia (AML) subsequently developed 3 months after the initial diagnosis of MDS in one of the author’s patients (Couto et al., 1984). The cytologic bone marrow abnormalities were similar to those described in cats and are discussed below. Some authors have proposed classifying dogs with primary myelodysplastic syndromes into those with refractory anemia and those with true myelo dysplasia, following similar classification schemes used in human beings (Weiss et al., 2000). However, because almost no clinical information was provided for the dogs evaluated, that classification scheme is of questionable clinical relevance.
Several reports of MDS in cats have appeared in the literature. More than 80% of cats in whom the FeLV status was investigated were found to be viremic. Most cats were evaluated because of nonspecific clinical signs such as lethargy, weight loss, and anorexia. Other signs, such as dyspnea, recurrent infections, and spontaneous bleeding, were observed in a few cats. Physical examination revealed hepatosplenomegaly in more than half of the cats; generalized lymphadenopathy and pyrexia were detected in approximately one third.
Hematologic abnormalities in cats with MDS are similar to those seen in dogs; they include isolated or combined cytopenias, macrocytosis, reticulocytopenia, metarubricytosis, and macrothrombocytosis. Morphologic changes in the bone marrow include a normal to increased cellularity, less than 30% blasts, an increased myeloid/erythroid ratio, dyserythropoiesis, dysmyelopoiesis, and dysthrombopoiesis. Megaloblastic RBC precursors are common, with occasional binucleated, trinucleated, or tetranucleated rubricytes or metarubricytes. The morphologic abnormalities in the myeloid cell line include giant metamyelocytes and asynchronous nuclear-cytoplasmic maturation.
Acute leukemia subsequently developed within weeks to months of the diagnosis in approximately one third of cats with MDS described in the literature. MDS commonly progresses to AML in human beings, with only isolated reports of progression to acute lymphocytic leukemia (ALL). However, according to Maggio and colleagues (1978), in one series of 12 cats with MDS, ALL subsequently developed in nine. This may reflect the fact that cytochemical staining was not done to classify the leukemic cells, and cells were thus morphologically classified as lymphoid when they were myeloid. However, because all the cats that showed progression to ALL were also viremic with FeLV, the hematologic changes preceding the development of leukemia did not reflect a “spontaneous” hematologic disorder (as seen in human beings and dogs) but were rather a manifestation of the morphologic and functional changes induced by FeLV.
The management of dogs and cats with MDS is still controversial. A variety of treatments have been used in human beings with MDS; however, none has proved effective. Chemotherapy, supportive therapy, anabolic steroids, inductors of differentiation, hematopoietic growth factors, and androgenic steroids, among others, have been reported to be of benefit in some human beings with MDS. Currently the preferred approach in human beings is treatment with supportive therapy and inductors of differentiation or hematopoietic growth factors. Because most patients are older, chemotherapy does not constitute the first treatment option, given its toxicity. The author recommends supportive therapy (e.g., fluids, blood components, antibiotics) and low-dose cytosine arabinoside as an inductor of differentiation (see Box 81-3). Aclarubicin (5 mg/m2 IV q24h for 5 days), a drug not currently available in the United States, was reported to be of benefit in a Shih Tzu with myelodysplasia (Miyamoto et al., 1999). Novel therapeutic approaches in human beings with MDS have been discussed by Warlick and Smith (2007).
Myelofibrosis, Osteosclerosis, and Osteopetrosis
Fibroblasts or osteoblasts within the bone marrow can proliferate in response to retroviral infections, chronic noxious stimuli, or unknown causes, leading to fibrous or osseous replacement of the bone marrow cavity, thereby displacing the hematopoietic precursors. These syndromes are termed myelofibrosis and osteosclerosis-osteopetrosis, respectively. Although both syndromes are rare, they have been observed in FeLV-infected cats and in dogs with chronic hemolytic disorders, such as the pyruvate kinase deficiency anemia that occurs in Basenjis and Beagles. Peripheral blood elliptocytosis and dacryocytosis appear to be a common feature in dogs with myelofibrosis. A limited number of dogs and cats with idiopathic myelofibrosis have been reported; in some of these cases, previous exposure to drugs (e.g., phenobarbital, phenytoin, phenylbutazone, colchicine) was documented. In the author’s experience, the clinical and hematologic features associated with myelofibrosis in dogs frequently resolve after immunosuppressive treatment with a combination of corticosteroids and azathioprine (see Chapter 93); the author has limited experience with myelofibrosis in FeLV-negative cats.
A presumptive diagnosis of osteosclerosis/osteopetrosis is made on the basis of the presence of combined cytopenias together with increased osseous radiographic density and can be confirmed by a core biopsy of the bone marrow. Unfortunately, no effective treatment is available.
Brazzell JL, Weiss DJ. A retrospective study of aplastic pancyto-penia in the dog: 9 cases (1996-2003). Vet Clin Pathol. 2006;35:413.
Couto CG, et al. Preleukemic syndrome in a dog. J Am Vet Med Assoc. 1984;184:1389.
Feldman BF, et al. Schalm’s veterinary hematology, ed 5. Philadelphia: Lippincott Williams & Wilkins, 2000.
Gilmour M, et al. Investigating primary acquired pure red cell aplasia in dogs. Vet Med. 1991;86:1199.
Harvey JW. Canine bone marrow: normal hematopoiesis, biopsy techniques, and cell identification and evaluation. Compend Cont Educ. 1984;6:909.
Kunkle GA, et al. Toxicity of high doses of griseofulvin in cats. J Am Vet Med Assoc. 1987;191:322.
Maggio L, et al. Feline preleukemia: an animal model of human disease. Yale J Biol Med. 1978;51:469.
Miura N, et al. Bone marrow hypoplasia induced by administration of estradiol benzoate in male Beagle dogs. Jpn J Vet Sci. 1985;47:731.
Miyamoto T, et al. Long-term case study of a myelodysplastic syndrome in a dog. J Am Anim Hosp Assoc. 1999;35:475.
Peterson ME, et al. Propylthiouracil-associated hemolytic anemia, thrombocytopenia, and antinuclear antibodies in cats with hyperthyroidism. J Am Vet Med Assoc. 1984;184:806.
Scott-Moncrieff JCR, et al. Treatment of nonregenerative anemia with human gamma-globulin in dogs. J Am Vet Med Assoc. 1995;206:1895.
Smith M, et al. Radiophosphorus (32P) treatment of bone marrow disorders in dogs: 11 cases (1970–1987). J Am Vet Med Assoc. 1989;194:98.
Warlick ED, Smith BD. Myelodysplastic syndromes: review of pathophysiology and current novel treatment approaches. Curr Cancer Drug Targets. 2007;7:541.
Watson ADJ, et al. Phenylbutazone-induced blood dyscrasias suspected in three dogs. Vet Rec. 1980;107:239.
Weiss DJ. Antibody-mediated suppression of erythropoiesis in dogs with red blood cell aplasia. Am J Vet Res. 1986;47:2646.
Weiss DJ, et al. A retrospective study of canine pancytopenia. Vet Clin Pathol. 1999;28:83.
Weiss DJ, Smith SA. Primary myelodysplastic syndromes of dogs: a report of 12 cases. J Vet Intern Med. 2000;14:491.
Weiss DJ, Smith SA. A retrospective study of 19 cases of canine myelofibrosis. J Vet Intern Med. 2002;16:174.
Weiss DJ. Flow cytometric evaluation of canine bone marrow based on intracytoplasmic complexity and CD45 expression. Vet Clin Pathol. 2004;33:96.
Weiss DJ. Bone marrow necrosis in dogs: 34 cases (1996-2004). J Am Vet Med Assoc. 2005;227:263.
Weiss DJ. Recognition and classification of dysmyelopoiesis in the dog: a review. J Vet Intern Med. 2005;19:147.
Weiss DJ. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996-2004). J Vet Intern Med. 2006;20:955.
Weiss DJ. Hemophagocytic syndrome in dogs: 24 cases (1996-2005). J Am Vet Med Assoc. 2007;230:697.