Chapter 20 Carbohydrates
As the name implies, carbohydrates consist of carbon, hydrogen and oxygen with the last two elements usually present in the same proportions as in water. As we have previously noted, carbohydrates are among the first products to arise as a result of photosynthesis. They constitute a large proportion of the plant biomass and are responsible, as cellulose, for the rigid cellular framework and, as starch, for providing an important food reserve. Of special pharmacognostical importance is the fact that sugars unite with a wide variety of other compounds to form glycosides (Chapters 21–25). Mucilages, as found in marshmallow root and psyllium seeds, act as water-retaining vehicles, whereas gums, which are similar in composition and properties, are formed in the plant by injury or stress and usually appear as solidified exudates; both are typically composed of uronic acid and sugar units. The cell walls of the brown seaweeds and the middle lamellae of higher plant tissues contain polysaccharides consisting almost entirely of uronic acid components. All these groups are discussed more fully below, and the drugs and pharmaceutical necessities containing them are listed at the end of the chapter.
SUGARS (SACCHARIDES)
Monosaccharides
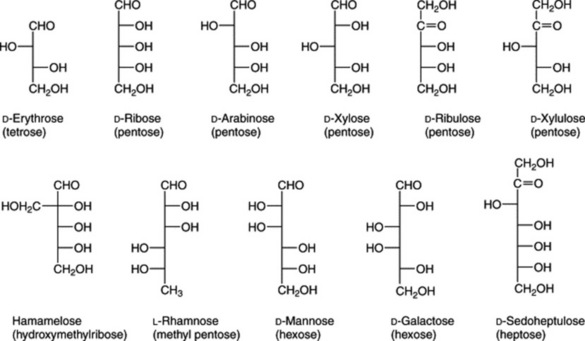
These sugars contain from three to nine carbon atoms, but those with five and six carbon atoms (pentoses, C5H10O5, and hexoses, C6H12O6) are accumulated in plants in greatest quantity.
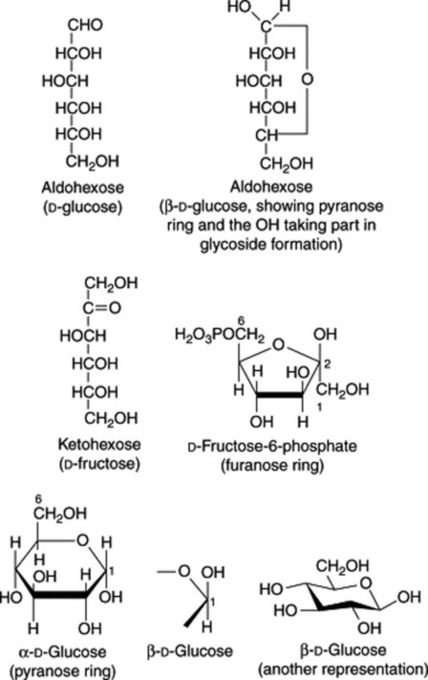
The formulae of sugars and other carbohydrates are written in a number of different ways. The structure of glucose as a straight-chain pentahydroxy aldehyde was established by Kiliani in 1886. Emil Fischer, from 1884 onwards, was the most important of the early workers in this field. Their straight-chain formulae are still useful for illustrating the isomerism and stereochemical relationships and, as shown below, can be written in very abbreviated form. Many of the important biological properties of carbohydrates can, however, best be illustrated by ring formulae which show that the same sugar may exist either as a five-membered ring (furanose) or a six-membered ring (pyranose). Glucose has an aldehyde group and is therefore called an aldose or ‘aldo’ sugar; fructose has a ketone group and is therefore called a ketose. Terms such as ‘aldopentose’ and ‘ketohexose’ are self-explanatory. The formulae (Figs. 20.1, 20.2) illustrate these points.
The furanose structure is comparatively unstable but may be stabilized on glycoside formation. The fructose phosphate of the furanose form illustrated in Fig. 20.1 is an intermediate in glycolysis, the anaerobic degradation of hexoses which provides energy for metabolism (see Fig. 18.5). Fructose in nature is always in the furanose form, but when isolated in crystalline form, it has a pyranose structure.
Uronic acids are produced by oxidation of the terminal groups to –COOH (e.g. glucuronic acid from glucose and galacturonic acid from galactose).
Biosynthesis of monosaccharides
Various monosaccharides arise from the photosynthetic cycle (q.v.). D-Fructose-6-phosphate and D-glucose-6-phosphate are universal in their occurrence. Free sugars may accumulate as a result of hydrolysis of the phosphorylated sugars or the latter may be utilized in respiration, converted to sugar nucleotides (e.g. uridine-diphosphoglucose—UDPG) or, by the action of various epimerases, give rise to other monosaccharides (e.g. galactose).
Di-, tri- and tetrasaccharides
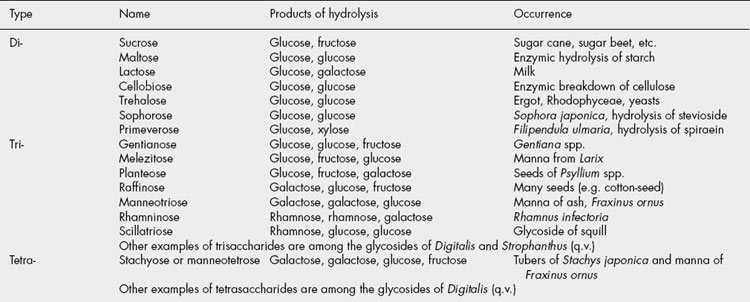
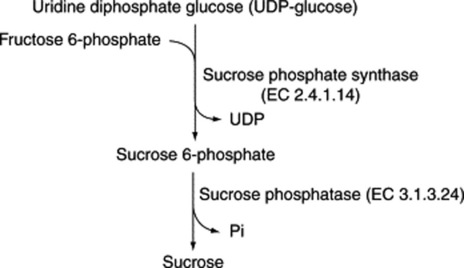
These sugars may also be called bioses, trioses and tetroses. They are theoretically derived from two, three or four monosaccharide molecules, respectively, with the elimination of one, two or three molecules of water (Table 20.1). One of the commonest plant disaccharides is sucrose; it is formed in photosynthesis by the reaction of UDPG with fructose-6-phosphate (Fig. 20.3). Control mechanisms for the build-up of sucrose in leaves, and its breakdown for transport to storage organs, are achieved by metabolite effector control of the appropriate enzymes.
The reverse process, hydrolysis, is brought about by suitable enzymes or by boiling with dilute acid. The same sugars may be linked to one another in various ways. Thus, the disaccharides maltose, cellobiose, sophorose and trehalose are all composed of two molecules of glucose joined by α-1,4-, β-1,4-, β-1,2- and α,α-1,1-(non-reducing) linkages, respectively.
POLYSACCHARIDES
By condensation involving sugar phosphates and sugar nucleotides, polysaccharides are derived from monosaccharides in an exactly similar manner to the formation of di-, tri-and tetrasaccharides. The name ‘oligosaccharide’ (Greek oligo, few) is often applied to saccharides containing from two to 10 units. In polysaccharides the number of sugar units is much larger and the number forming the molecule is often only approximately known. The hydrolysis of polysaccharides, by enzymes or reagents, often results in a succession of cleavages, but the final products are hexoses or pentoses or their derivatives. The term ‘polysaccharide’ may usefully be taken to include polysaccharide complexes which yield in addition to monosaccharides their sulphate esters, uronic acids or amino sugars.
Table 20.2 indicates the character of some of the polysaccharides.
Table 20.2 The character of some polysaccharides.
| Name | Occurrence and nature |
|---|---|
| Containing only monosaccharide units | |
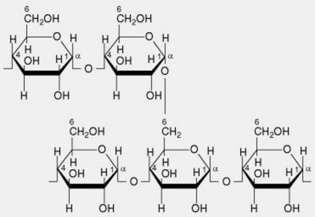
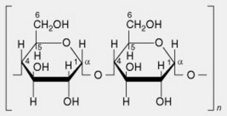
| 1. Amylopectin or α-amylose | The main constituent of most starches (over 80%). The molecule has branched chains each consisting of 20–26 α-1,4-linked glucose residues. Several hundred of these chains are linked by α-1,6 glycosidic bonds to neighbouring chains giving a molecule containing some 50 000 glycosyl units. The branching pattern throughout the molecule is not uniform, resulting in some areas that are apparently amorphous (high degree of branching) and others probably crystalline (linear chains predominate with little branching) |
 |
|
| 2. Amylose or β-amylose | Most starches contain up to 20%, but sometimes absent. Consists essentially of linear chains of α-1,4-linked glucose residues. Several thousand glucose units constitute a chain. It is now recognized that there is a very limited branching (α-1,6-linkages) to the extent of 2–8 branches per molecule |
 |
|
| 3. Glycogen or animal starch | Important reserve carbohydrate of animal tissues. Molecule resembles that of amylopectin |
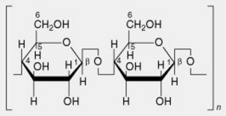
| 4. Cellulose | Chief polysaccharide of plant cell walls. Linear chains of β-1,4-linked glucose residues |
 |
|
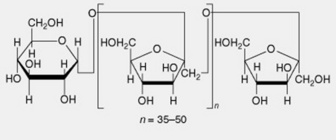
| 5. Inulin | A reserve carbohydrate particularly abundant in the Compositae. Linear chains of up to 50 β-1,2-linked fructofuranose units terminated by a single glucose unit |
 |
|
| 6. Xylans, mannans and galactans | These are often associated with one another and with cellulose. They are difficult to isolate in a pure form. On hydrolysis they yield xylose, mannose and galactose, respectively |
| 7. Hemicelluloses | These polysaccharides occur in the cell wall with cellulose and pectic substances. The nomenclature, dating from 1891, is deceptive because hemicelluloses are not components of cellulose but are formed mainly from hexose and pentose units. Hemicelluloses vary according to source and can be classified as xylans, mannans and galactans according to their principal components |
| 8. Lichenin or lichen starch | A polysaccharide found in lichens. Resembles cellulose but molecule contains about 25% of β-1,3 glucosidic linkages |
| Polysaccharide complexes containing uronic acid or other units | |
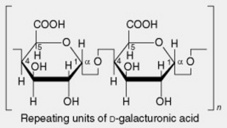
| 1. Pectins | These occur in the middle lamellae of cell walls and are abundant in fruits (e.g. apples, oranges) and roots (beets and gentian). The parent substance protopectin is insoluble but is easily converted by restricted hydrolysis into pectinic acids (pectins). Pectins from different sources vary in their complex constitution, the principal components being blocks of D-galacturonic acid residues linked by α-1,4- glycosidic linkages and interspersed with rhamnose units; some of the carboxyl groups are methylated. These molecules are accompanied by small amounts of neutral arabinans (branched polymers of α-1,5-linked L-arabofuranose units) and galactans (largely linear chains of β-1,4- linked D-galactopyranose units) |
 |
|
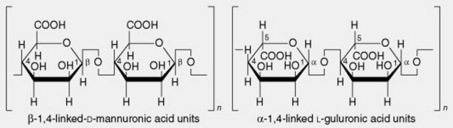
| 2. Algin or alginic acid | Alginic acid is the principal constituent of the cell walls of the brown algae. It was discovered by Stanford in 1880 and is now widely used for the manufacture of alginate salts and fibres (q.v.). The composition varies according to the biological source, thus providing a range of properties which are exploited commercially. It is a heteropolyuronide consisting of chains of β-1,4-linked D-mannuronic acid units interspersed with lengths of α-1,4-linked L-guluronic acid units together with sections in which the two monouronide units are regularly interspersed. In alginic acids from different sources the ratios of the two uronic acids vary from 2:1 to 1:2. The chain length varies with the method of preparation and molecular weight, and viscosity measurements suggest molecules of from 220 to 860 units |
 |
|
| 3. Polysaccharides with sulphuric acid esters | Certain algae, including those yielding agar and carrageen, contain a mixture of polysaccharides. Agar, sulphuric acid esters for example, contains a biose formed from D- and L-galactose but also a more complex agaropectin formed from galactose and uronic acid units partly esterified with sulphuric acid. Carrageen has a similar composition |
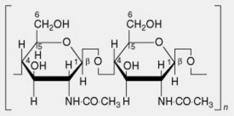
| 4. Chitin | This is found in some of the lower plants, in insects and in crustaceans. The molecule consists of linear chains of β-1,4-linked N-acetyl-D-glycosamine residues. Its inclusion in the microfibrillar component of the fungal cell wall is analogous to that of the cellulose microfibril |
 |
|
| 5. Gums and mucilages | Gums such as acacia and tragacanth and mucilages, such as those found in linseed, psyllium seeds and marshmallow root, are found in many plants, where they are usually formed from the cell wall or deposited on it in layers. They are essentially polyuronides consisting of sugar and uronic acid units. Some gums have methoxyl groups (e.g. tragacanth); in others the acidic complex is united with metals (e.g. acacia) |
In addition to the well-established polysaccharide-containing pharmaceutical materials described later in this chapter there is now considerable interest in a number of polysaccharides with other pharmacological activities. These include immuno-modulating, antitumour, anti-inflammatory, anticoagulant, hypoglycaemic and antiviral properties. Specific examples are the glycyrrhizans of Glycyrrhiza uralensis and G. glabra and the glycans of ginseng and Eleutherococcus (q.v.). In general polysaccharides from fungi exhibit antitumour activity, those from higher plants are immunostimulatory and the algal polysaccharides, which often contain sulphate, are good anticoagulants.
Tests for carbohydrates
The following are some of the more useful tests for sugars and other carbohydrates.
COMMERCIAL PLANT-DERIVED FIBRES AND PRODUCTS
The biological origin and the structure of plant fibres is discussed in Chapter 42; many have important commercial uses and for a review on their botany, chemistry and processing see McDougall et al., J. Sci. Food, Agric., 1993, 62, 1.
A number of vegetable fibres have importance in pharmacy, particularly as components of surgical dressings and for the manufacture of artificial fibres and haemostatic dressings. The subject of surgical dressings was, and in many Schools still is, regarded as pharmacognosy-related. However with the more recent advances in the management and concept of wound-healing, many materials of non-vegetable origin are now used which, for an in-depth coverage, bring the topic outside the scope of this book. Described below are the more important primary carbohydrate materials involved.
COTTON, RAW COTTON
Cotton consists of the epidermal trichomes of the seeds of Gossypium herbaceum and other cultivated species of Gossypium (Malvaceae). The plants are shrubs or small trees which produce three- to five-celled capsules containing numerous seeds. The USA produces about half of the world’s cotton, other important sources being Egypt, India and South America. The chief American cottons are derived from G. barbadense (Sea Island cotton) and G. herbaceum (Upland, Texas or New Orleans cotton).
The hairs of the different species vary in length or ‘staple’. The staples of important commercial varieties of cotton are as follows: (1) Sea Island, up to 54.5 mm; (2) Egyptian, 31–38 mm; (3) Brazilian and Peruvian, 29–30 mm; (4) American Upland, about 25.9 mm; (5) Indian, 21.4–29.2 mm.
Preparation
When ripe the bolls are collected, dried and subjected to a ginning process to separate the hairs from the seed. The gin, which may be of a roller or a pneumatic type, is designed to pull the hairs through a narrow space which is too small to allow the seed to pass. In ordinary American or Upland cotton the gin leaves the seeds with a coating of short hairs which have to be removed by a second type of gin known as a ‘linter’. These short hairs are used for making the lower grades of cotton wool and rayons. The seeds are used for the preparation of cottonseed oil (q.v.) and cattle cake. Raw cotton contains various impurities, such as immature and broken seeds, fragments of leaf, etc., most of which are removed during the manufacture of yarn.
For spinning very fine yarns Sea Island cotton is used, but for coarser yarns it is possible to use shorter staple cottons. Different machines are used for these two types of yarn, which are known as combed and carded, respectively. The cotton-combing machine separates all the shorter fibres and a thread is spun consisting of long, well-paralleled, uniform fibres. The short fibres of comber waste are used for making the best grades of cotton wool. The carding machine uses fibres which are shorter and less uniform in length, and the absence of combing is shown in the yarn by the irregular arrangement of the fibres, the ends of which often project from the surface.
Microscopy of unbleached cotton
Cotton consists of unicellular hairs the appearance of which has been likened to that of empty, twisted fire-hoses. Their length is up to about 5 cm, diameter 9–24 μm, and the number of twists varies from about 75 cm−1 in the Indian to 150 cm−1 in the Sea Island. Pieces of ‘shell’ or seed coat, which can often be picked from samples of raw cotton, show hair bases fitting between the thick-walled epidermal cells. The apex is rounded and solid. The cotton hair is cylindrical when young but becomes flattened and twisted as it matures, the large lumen, which contains the remains of protoplasm being much elongated in transverse section. The cellulose wall of the hair is covered with a waxy cuticle which renders it non-absorbent. The cuticle may be stained with ruthenium red. Bleached cotton yarn and absorbent cotton wool (see below) are readily wetted by water.
Tests
The following tests are applicable to cotton.
ABSORBENT COTTON WOOL, ABSORBENT WOOL
Cotton wool is mainly prepared from linters, card strips, card fly and comber waste. Bales of these short-fibred cotton wastes pass from the yarn manufacturers to the makers of cotton wool. For best-quality cotton wool the comber waste of American and Egyptian cottons is preferred. In this the fibres are reasonably long and twisted and thus suitable for producing a cotton wool having an average staple that will offer appreciable resistance when pulled and not shed a significant quality of dust when shaken gently.
The preparation may be outlined as follows. The comber waste (which arrives in bales) is loosened by machinery and then heated with dilute caustic soda and soda ash solution at a pressure of 1–3 atmospheres for 10–15 h. This removes much of the fatty cuticle and renders the trichome wall absorbent. It is then well washed with water, bleached with dilute sodium hypochlorite solution and treated with very dilute hydrochloric acid. After washing and drying it is in a matted condition and is therefore opened up by machines and then ‘scutched’; that is, it is converted into a continuous sheet of fairly even thickness with the fibres loosened ready for the carding machine. The carding machine effects a combing operation and forms a thin continuous film of cotton wool. Several such films are superimposed on one another, interleaved with paper and packaged in rolls.
Structure of fibre
Like the cell wall in general, that of cotton consists of a primary and secondary wall. The formation of the former is modified to embrace the enormous longitudinal development of the cell, some thousands of times greater than the width. The microfibrils themselves are unexpandable and are initially laid down in hoops around the fibre, restricting its lateral growth. However, because of their different orientation at the fibre end, longitudinal extension can take place. Owing to the build-up of pressure from successive layers of cellulose the original orientation becomes lost as the fibre matures. Matrix polysaccharides and proteins derived from the Golgi apparatus are also present in the primary wall. The secondary wall, some 5–10 μm thick and consisting almost entirely of pure cellulose, constitutes the main bulk of the mature cotton fibre.
Chemical nature
Raw cotton consists of cellulose approximately 90% and moisture 7%, the remainder being wax, fat, remains of protoplasm and ash.
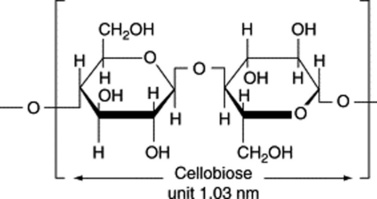
Absorbent cotton is a very pure form of cellulose and its chemical and physical properties have been extensively studied. The cellulose molecule is built up of glucose residues united by 1,4-β-glucosidic links (contrast starch). The wall of the cotton fibre, like that of plant cells in general, shows anisotropic properties. When swollen in water, the swelling is in a direction at right angles to the long axis. In the direction of the long axis it shows considerable tensile strength. Examined in polarized light, it shows birefringence, the value of the double refraction depending on the liquid in which the fibre is immersed. This phenomenon, characteristic of mixed bodies with rod-like structural elements, has been termed rodlet-double refraction. Stained with chlor-zinc-iodine and examined microscopically in polarized light (analyser removed), the fibre shows greater absorption when orientated with its long axis parallel to the plane of polarization than when orientated with the long axis at right angles (dichroism). These physical properties suggest that the fibre wall is built up of elongated structural units orientated in some definite manner. The study of the cotton fibre by X-ray analysis has confirmed this and has shown that its cell wall is composed of elongated chain-like molecules (built up of repeating units 1.03 nm long) and orientated in a spiral manner, the spiral making an angle of 30° with the long axis of the fibre. The length of the repeating unit of structure corresponds to that of two glucose residues fully extended. This unit is the ‘cellobiose unit’, many of which are united in the polysaccharide molecule of cellulose (Table 20.2).
The biosynthesis of cellulose in the cotton trichome would appear to involve UDP-glucose originating from sucrose.
Jute
Jute consists of the strands of phloem fibres from the stem bark of Corchorus capsularis, C. olitorius and other species of Corchorus (Tiliaceae). These are annual plants about 3–4 m high which are cultivated in Bengal, in the delta region of the Ganges and Brahmaputra rivers, and in Assam, Bihar and Orissa.
The fibres are separated from the other plant material by retting and then spun into yarn which can be made up into hessian and sacking. Short fibres left over from the preparation of the yarns and ropes constitute tow and in pharmacy the term ‘tow’ refers to jute, although it can also be applied to hemp and flax. The commercial strands are 1–3 m long and about 30–140 μm in diameter. Each consists of a bundle of phloem fibres composed of lignocellulose. The heavily lignified middle lamella is destroyed by oxidizing agents; a mixture of nitric acid and potassium chlorate may therefore be used to disintegrate the bundles, the individual fibres being then teased out and sketched. Prepared transverse sections should also be examined and compared with those of hemp and flax.
Flax
Flax is prepared from the pericyclic fibres of the stem of Linum usitatissimum (Linaceae).
The commercial fibres show fine transverse injuries received during the preparation. Good-quality flax fibre is non-lignified except for the middle lamella. Lignification of the secondary wall, however, takes place as the stem matures, starting at the base, and if the stems are too old before retting, the fibre is coarse and harsh in texture.
Hemp
Hemp is prepared from the pericyclic fibres of the stem of Cannabis sativa (Cannabinaceae). The fibre is composed chiefly of cellulose, but some lignification has usually taken place and the percentage of cellulose is lower than in flax.
The fibre ends, in contrast to those of flax, are bluntly rounded. Some of the fibre ends are forked, this bifurcation arising from injuries to the stem. The lumen of the hemp fibre is flattened or oval, in contrast to the small round lumen of flax. Transverse striations seen in commercial fibres arise from beating, the fibres being prepared by partial retting.
Jute, hemp and flax fibres are compared in Table 20.3.
REGENERATED CARBOHYDRATE MATERIAL AND CHEMICALLY MODIFIED FIBRES
Regenerated fibres are those produced from naturally occurring, long-chain molecules which have been isolated, controlled and, if necessary, modified to give a suitable fibre form. The term ‘rayon’, as in viscose, acetate and cuprammonium rayon, is applied to those derived from the polysaccharide cellulose. The term ‘artificial silk’ is now out of date. Also in this class is alginate fibre, derived from alginic acid (q.v.).
Viscose (regenerated cellulose, rayon)
This has been developed from a process introduced by three British chemists (Beadle, Bevan and Cross) in 1892, and accounts for the bulk of the world rayon output today. It is also the principal type used in surgical dressings.
The starting material is a cellulose prepared either from coniferous wood, particularly spruce, or scoured and bleached cotton linters. The wood is usually delignified at source (Canada, Scandinavia, etc.) by a process similar to that used for cellulose wadding. It reaches the rayon manufacturers as boards of white pulp, containing 80–90% of cellulose and some hemicellulose (mainly pentosans). The latter, being alkali-soluble, are removed in the first stage of the process, which consists of steeping in sodium hydroxide solution. After most of the excess alkaline liquor has been pressed out, alkali-cellulose (sodium cellulosate) remains. This is dissolved by treatment with carbon disulphide and sodium hydroxide solution to give a viscous (whence the name ‘viscose’) solution of sodium cellulose xanthate. After ‘ripening’ and filtering, the solution is forced through a spinneret, a jet with fine nozzles, immersed in a bath which includes dilute sulphuric acid and sodium sulphate, when the cellulose is regenerated as continuous filaments. These are drawn together as a yarn, which is twisted for strength, desulphurized by removing free sulphur with sodium sulphide, bleached, washed, dried and conditioned to a moisture content of 10%.
The viscose yarn may be left as such (i.e. continuous filament rayon) for use in such things as blouse fabrics, or it may be cut up to give staple rayon (‘Fibro’) of fixed length from 1 to 8 in. That used in surgical dressings and many other fabrics is made to resemble cotton in dimensions. Suitable spinnerets are used to give a diameter of 15–20 μm and the fibre is cut into lengths usually of 4.8 cm. This staple can be processed on types of spinning and weaving machines used for cotton dressings or it may be left in a loose fibre form as viscose rayon absorbent wool.
Viscose rayon is a very pure form of cellulose. It yields a trace of ash which contains sulphur. The cellulose molecules of the original natural material, whether wood or cotton, become more separated from one another in the viscose solution than in the vegetable material and in the regenerated fibre are still less closely packed. Radiography has shown that the side-to-side aggregation of the long-chain molecules is different from that in natural celluloses. The size of the molecules is also reduced, wood cellulose having molecules of the order of 9000 glucose residue units, while those of viscose rayon have only about 450.
Viscose rayon gauze and other rayon dressings have the advantage over cotton dressings in that they show no loss of absorbency on storage.
Macroscopical characters
As normally produced, this rayon is a white, highly lustrous fibre (natural or glossy viscose). Its tensile strength varies from two-thirds to one-and-a-half times that of cotton. When wetted it loses about 60% of its tensile strength, a proportionately greater loss than is found with cotton. Where more than a certain amount of rayon is used in a dressing, the fabric may be required to be rendered water-repellent (e.g. cotton crêpe bandage).
Microscopical characters
The fibres are solid and transparent and 15–20 μm in diameter. They have a slight twist, and show grooves along their length which are principally caused by the spinnerets being immersed in the regenerating solution (compare nylon). The grooves give a characteristic appearance to the transverse section. The ends of the fibres are abrupt and characteristic. The fibres are clearly seen in chloral hydrate solution or in lactophenol, but are almost invisible in cresol (having the same refractive index of 1.53). They appear bright in polarized light with crossed Nicols.
Chemical tests
Delustring and dyeing of fibres
Rayon and other artificial fibres with a natural lustrous appearance may be delustred by addition of the white pigment titanium oxide to the solution (e.g. viscose) or to the melt (e.g. nylon) before extrusion of the filaments. In this way the pigment is evenly distributed inside each filament and delustring is permanent. These fibres may be similarly ‘spun-dyed’ by addition of an appropriate dye instead of the titanium oxide. The method results in an exceptional degree of colour fastness.
Matt Viscose (delustrated viscose rayon) is the form normally used in the manufacture of surgical dressings; hence, in general appearance these are very similar to those manufactured from cotton. The individual filaments have the appearance already described, except for the matt white colour and on microscopical examination the pigment particles, which appear black by transmitted light and are scattered throughout the filament. The amount of pigment is controlled by the ash value. Titanium is detected in the ash by dissolving in sulphuric acid, diluting and adding hydrogen peroxide, 3%, when a yellow colour is produced.
Cellulose ethers
These are prepared from purified alkali cellulose derived from cotton linters or delignified wood pulp by the action of caustic soda, as in the initial stages of the production of viscose rayon.
Methylcellulose BP/EP is a whitish, fibrous powder prepared by the action of methyl chloride under pressure on an alkali cellulose, when hydroxyl groups become methylated. A useful grade is that in which two of the three hydroxyl groups of the glucose residue units of the cellulose chain are methylated, and this has the optimum solubility in water. In pharmacy a grade giving a low viscosity is used both to increase the viscosity and to stabilize lotions, suspensions, pastes and some ointments and ophthalmic preparations; one giving a high viscosity is used as a tablet disintegrant. In medicine it is used as a hydrophilic colloid laxative in chronic constipation and can be used in obese persons to curb the appetite, because it gives a feeling of fullness. Ethylcellulose is similarly prepared and has like applications.
Carmellose Sodium EP/BP (sodium carboxymethylcellulose) is an odourless and tasteless white hygroscopic powder or granules prepared by the action of monochloroacetic acid on alkali cellulose and removal of the byproduct salts. Substitution of hydroxyl groups by carboxymethyl groups occurs over a range depending on the conditions and the cellulose used; there are prescribed limits for the sodium content. It is water-soluble, and a grade giving a medium viscosity contains 0.7 carboxymethyl groups per glucose residue unit. It is insoluble in organic solvents. Its pharmaceutical and medical uses are similar to those of methylcellulose, but as well as being used as a laxative it is a useful antacid.
Pyroxylin BP (Cellulose nitrate)
Pyroxylin is prepared by the action of nitric and sulphuric acids on wood pulp or cotton linters that have been freed from fatty materials. When dry it is explosive and must be carefully stored, dampened with not less than 25% its weight of isopropyl alcohol or industrial methylated spirits. It is used for making Flexible Collodion BP.
Absorbable haemostatic dressings
The control of bleeding is of vital concern in surgery, and the great disadvantage of the old-type dressing such as a cotton gauze plug is that it has to be removed after bleeding has been checked with a consequent danger of a recurrence of the haemorrhage. Gelatin sponge, oxidized cellulose and alginate dressings overcome this, in that there is no need to remove them after the bleeding has been checked, since they are absorbed by the tissues.
Oxidized cellulose
Oxidized cellulose originated in the USA as a result of the work published by Yackel and Kenyon in 1942. Cotton wool or gauze is treated with nitrogen dioxide until the number of carboxyl groups formed bythe oxidation of the primary alcohol groups of the glucose residue units of the cellulose molecules reaches 16–22%. The original cellulose now has glucuronic acid residue units (compare alginic acid) as well as some glucose residue units.
Appearance
Gauze, lint or knitted material, very similar to normal cotton but with an off-white colour, a harsher texture, charred odour and an acid taste. It does not go pasty on chewing. The wool tends to disintegrate on handling. In microscopical appearance the fibres are very similar to those of absorbent cotton.
Tests
Alginate fibres
These originated about 1938 in Britain and were further developed during World War II.
The fibres are prepared by a process similar to that for viscose rayon. An aqueous solution of sodium alginate (see this chapter) is pumped through a spinneret immersed in a bath of calcium chloride solution (acidified with hydrochloric acid), when water-insoluble calcium alignate is precipitated as continuous filaments. These are collected, washed and dried. For use in surgical dressings and bacteriological swabs they are reduced to a staple form which may then be processed to a calcium alginate wool or a fabric (e.g. gauze) in the same manner as used for viscose staple or cotton.
As indicated in Table 20.2, alginic acid is composed of polymers of both mannuronic and guluronic acids. The properties of the two are variable and alginates of different origin have different compositions and properties. This is illustrated by the two commercial haemostatic dressings—Kalostat (BritCair Limited) and Sorbsan (Steriseal—Pharmaplast Limited). The former is derived from the seaweed Laminaria hyperborea collected off the Norwegian coast and yields an alginate with a guluronic:mannuronic ratio of 2:1; the latter is prepared from Laminaria and Ascophyllum species collected off the west coast of Scotland and gives an alginate with a guluronic:mannuronic acid ratio of about 1:2. On a wound surface the α-linkages of the guluronic acid polymer are not easily broken so that fibre strength is retained and a strong gel is formed on contact with the wound exudate. A high ratio of mannuronic acid polymer (β-linkages) yields a product giving a weaker gel and less retention of fibre strength. In practice this means that the Kalostat dressing can be removed from the wound with forceps and Sorbsan is removed by irrigation with, for example, sodium citrate solution.
Calcium alginate fibres of commerce contain substantial traces of substances used to inhibit mould and bacterial growth in the sodium alginate spinning solution. Spinning lubricants such as lauryl or cetyl pyridinium bromide (antibacterial) are also applied to the filaments. These substances must not be used or must be removed in the case of calcium alginate staple for use in, for example, bacteriological swabs.
Before use as an absorbable haemostatic dressing some calcium alginate dressings must be immersed in sodium chloride to give a fibre of the calcium alginate covered by sodium alginate. The degree of conversion is conditioned to give the desired rate of absorption when in use; the greater the proportion of sodium alginate the faster the absorption rate.
Alginate filaments are composed of salts of the long-chain molecules of alginic acid (see Table 20.2) and there is little cross-linking between the chains in the fibre.
Appearance
Fairly lustrous, pale cream-coloured fibres which in microscopical appearance are very similar to those of viscose rayon, being solid grooved rods. The haemostatic dressing (‘Calgitex’) is almost tasteless and odourless and rather harsh to touch. The gauze is usually a knitted fabric and has little sheen. That with a fast rate of absorption when chewed readily assumes a pasty form somewhat like that of mashed potato. That with a slow rate of absorption remains smoothly coarser in the same time. They do not disintegrate easily on handling. These points and the tests below will serve to distinguish alginate haemostatic dressings from those of oxidized cellulose. First-aid dressings frequently embody an alginate gauze impregnated with a local anaesthetic.
Tests
These refer to calcium alginate fibre or the mixed sodium and calcium salt fibre. They give the general tests for vegetable and regenerated carbohydrate fibres. For distinctions from rayons and oxidized cellulose see earlier.
Uses
The alginate absorbable haemostatic dressings are non-toxic and non-irritant. They have advantages over oxidized cellulose, which include selective rate of absorption, sterilization (and resterilization) by autoclaving or dry heat and compatibility with antibiotics such as penicillin. They may be used internally in neurosurgery, endaural and dental surgery to be subsequently absorbed. Externally, they may be used (e.g. for burns or sites from which skin grafts have been taken) to arrest bleeding and form a protective dressing which may be left or later removed in a manner appropriate to the type of dressing employed (see above). Protective films of calcium alginate may also be used by painting the injured surface with sodium alginate solution and then spraying it with calcium chloride solution.
Calcium alginate wool as a swab for pathological work or bacterial examination of such things as food processing equipment and tableware has the great advantage over cotton wool in that it permits release of all the organisms by disintegration and solution of the swab in, for example, Ringer’s solution containing sodium hexametaphosphate.
In fabrics the calcium alginate fibres would disintegrate in alkaline solutions (laundering), but this advantage is turned to a commercial virtue by the use of the yarn as a scaffolding thread to support yarns normally too fine to survive the weaving process. The scaffold is removed by an alkaline bath to leave a lightweight fabric.
Cellulose wadding
Cellulose wadding was official in the BP 1989. It is prepared from high-grade bleached sulphite wood pulp which is received by the manufacturer in the form of boards about 0.75 m square and 1 mm thick. These are packed in bales containing about 180 kg pulp. The pulp is put in a ‘beater’, where it is mixed with about 20 times its weight of water and the mixture circulates between a power-driven roll and the bed-plate of the ‘beater’. The effect of this is to break up the pulp into separate fibres. When this process is complete, the contents of the beater are mixed with a further quantity of water and then allowed to run in a steady flow on to the ‘wire’ of the paper machines. This ‘wire’ is a very fine wire gauze through which water runs, leaving a fine web of fibres on top of the ‘wire’. This web is then dried and crêped to give a thin, soft, absorbent sheet. About 30 of these thin sheets are laid together to form cellulose wadding.
When examined microscopically, chemical wood pulps or cellulose wadding show characteristic woody elements, which, however, give no lignin reaction (distinction from mechanical wood pulp). Tracheids with bordered pits and characteristic medullary ray cells are usually observed. The cellulose nature of the walls is shown by the blue colour obtained with iodine followed by 80% sulphuric acid and by their solubility in an ammoniacal solution of copper oxide.
STARCHES
Starch constitutes the principal form of carbohydrate reserve in the green plant and is to be found especially in seeds and underground organs. The green parts of plants exposed to sunlight contain small granules of transitional starch which arise from photosynthesis. During the hours of darkness these are removed to the storage organs. Starch occurs in the form of granules (starch grains) the shape and size of which are characteristic of the species as is also the ratio of the content of the principal constituents, amylose and amylopectin.
A number of starches are recognized for pharmaceutical use. They include maize (Zea mays L.), rice (Oryza sativa L.), wheat (Triticum aestivum L.) and potato (Solanum tuberosum L.). Tapioca or cassava starch (Manihot utilissima) may be used in place of the above in tropical and subtropical countries.
The more important commercial starches are listed in Table 20.4.
Table 20.4 Commercial starches.
| Family | Plant | Economic product |
|---|---|---|
| Cycadaceae | Zamia floridana | Florida arrowroot |
| Gramineae | Zea mays | Maize or corn |
| Oryza sativa | Rice | |
| Triticum aestivum | Wheat | |
| Avena sativa | Oats | |
| Hordeum sp. | Barley | |
| Secale cereale | Rye | |
| Palmae | Metroxylon rumphii | Sago |
| Musaceae | Musa spp. | Bananas and plantains |
| Zingiberaceae | Zingiber officinale | Ginger |
| Curcuma spp. | East Indian arrowroot and turmeric | |
| Cannaceae | Canna edulis | Queensland arrowroot or tous les mois |
| Polygonaceae | Polygonum fagopyrum | Buckwheat |
| Euphorbiaceae | Manihot utilissima | Manihot or cassava starch and tapioca |
| Leguminosae | Phaseolus vulgaris | Bean flour |
| Ervum lens | Lentil flour | |
| Pisum sativum | Pea flour | |
| Convolvulaceae | Ipomoea batatas | Sweet potato |
| Solanaceae | Solanum tuberosum | Potato |
Preparation of starches
Commercial starches, the preparation of which is described below, are not chemically pure and contain small amounts of nitrogenous and inorganic matter.
Many patented processes are in use for particular starches, and the procedure adopted depends on the degree of purity desired and the nature of the compounds from which the starch has to be freed. Cereal starches, for example, have to be freed from cell debris, oil, soluble protein matter and the abundant insoluble proteins (glutelins and prolamins) known as ‘gluten’. Potato starch, on the other hand, is associated with vegetable tissue, mineral salts and soluble proteins.
Wheat and similar starches were at one time prepared by kneading the ground material in a stream of water, the gluten remaining as a sticky mass, while the starch separated on standing from the milky washings. The following methods are now employed.
Preparation of maize starch
The grain is first softened by soaking at 50°C for about 2 days in a 0.2% solution of sulphurous acid. This assists disintegration, enabling the embryo or germ to be easily liberated intact and permitting the starch to be readily freed from fibre. During this time lactic acid bacteria are active and metabolize soluble sugars extracted from the maize. The grain, in water, is then distintegrated by attrition mills; these do not break the liberated oil-containing embryos, which, in the older process, were skimmed off. Nowadays the germs are continuously separated from the suspension by liquid cyclones (hydroclones) which operate in batteries of about 12. The germs are used for the preparation of germ oils, which are an important source of vitamins. The remainder of the grain is ground wet and the starch and gluten separated from fibrous material in rotating, slightly inclined stainless steel reels covered with perforated metal sheets. The retained fibre is washed and the total mixture of starch and protein (mill starch) is fractionated into gluten and starch by the use of special starch purification centrifuges; separation depends on the fact that gluten is lighter than starch. In older processes this separation was accomplished by repeated ‘tabling’, in which the suspension was allowed to flow very slowly through troughs about 40 m long and 0.7 m wide, when the heavier starch was deposited first. The starch suspension from the centrifuge is further purified in other centrifuges and hydroclones, which reduces the protein level. The subsequent drying process may involve flash dryers or a moving-belt dryer; considerable flexibility in drying time is required to accommodate the various modified starches which are now produced.
Preparation of rice starch
Rice is soaked in successive quantities of 0.4% caustic soda until the material can be easily disintegrated. The softened grain is ground (the compound grains separating into their components), made into a dilute suspension which is repeatedly screened, and the starch separated by standing or by means of a centrifuge. The damp starch is next cut into blocks and dried at 50–60°C for 2 days. The brown outer layer which forms is then scraped from the blocks and drying is continued at a lower temperature for about 14 days, during which time the blocks gradually crack into irregular masses. For pharmaceutical use this ‘crystal’ starch is powdered.
Preparation of potato starch
The potatoes are washed and reduced to a fine pulp in a rasping machine or in a disintegrator of the hammer-mill type. Much of the cell debris is removed from the pulp by rotary sieves and the milky liquid which passes through the sieve contains starch, soluble proteins and salts, and some cell debris. On standing, the starch separates more rapidly than the other, insoluble, matter and in older processes was purified by techniques resembling the ‘tabling’ described above; again high-speed centrifugal separators, for use with potato starch (and cassava starch), are now employed for separation and washing. At two or three points during the isolation, sulphur dioxide is added to prevent discoloration of the product by the action of oxidative enzymes. The washed starch is collected, dried to contain about 18% moisture and packaged.
Macroscopical characters
Starch occurs in irregular, angular masses or as a white powder. It is insoluble in cold water but forms a colloidal solution on boiling with about 15 times its weight of water, the solution forming a translucent jelly on cooling. A starch mucilage is coloured deep blue with solution of iodine, the colour disappearing on heating to 93°C but reappearing on cooling. When starches are heated with water, the granules first swell and then undergo gelatinization. The temperatures at which these changes commence and are complete vary with different starches. Starch granules also undergo gelatinization when treated with caustic potash, concentrated solutions of calcium or zinc chlorides, or concentrated solution of chloral hydrate.
Maize starch is neutral, but other commercial starches frequently show an acid (wheat and potato) or alkaline (rice) reaction. The USP gives microbial limit tests for Salmonella spp. and for Escherichia coli.
Microscopical characters
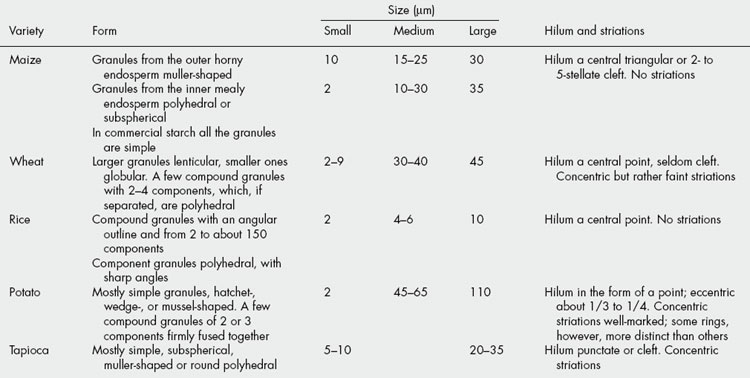
Starches can be identified by microscopical examination. They should be mounted in water or Smith’s starch reagent (equal parts of water, glycerin and 50% acetic acid). The size, shape and structure of the starch granules from any particular plant only vary within definite limits, so that it is possible to distinguish between the starches derived from different species. Starch granules may be simple or compound, and the description of a starch granule as 2-, 3-, 4- or 5-compound refers to the number of component granules present in the compound granule. In some cases the compound granule is formed by the aggregation of a large number of simple granules (e.g. rice and cardamoms).
The starting point of formation of the granule in the amyloplast is marked by the hilum, which may be central or eccentric. Granules with an eccentric hilum are usually longer than broad. On drying, fissures often appear in the granule and are seen to originate from the hilum. On microscopical examination, the hilum takes the form of a rounded dot or of a simple, curved or multiple cleft.
The starch granule is built up by the deposition of successive layers around the hilum, and concentric rings or striations are often clearly visible in larger granules, e.g. potato. The striations probably arise from the diurnal deposition of the starch giving variations in refractive index, density and crystallinity. The position and form of the hilum and the presence or absence of well-defined striations are of importance in the characterization of starches.
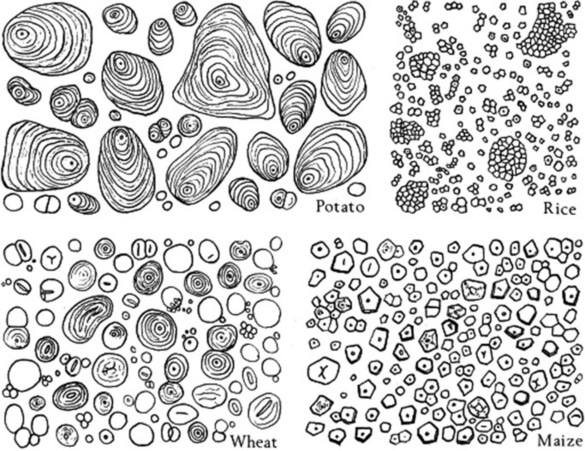
Some of the more important microscopic characters of the principal starches are set out in Table 20.5 and Figure 20.4.
Starch granules show double refraction when examined between crossed Nicols, the granules appearing in the dark field as illuminated objects marked by a dark cross, the bars of which intersect at the hilum.
Starch is required to comply with specified limits for viable microbial contamination including, specifically, Escherichia coli.
Chemical composition of starch
Starch granules usually contain two carbohydrates, amylopectin (α-amylose) and amylose (β-amylose); the former constitutes over 80% of most starches. Fractionation of the two components can be achieved by selective precipitation involving the formation of an insoluble complex of amylose with such polar organic substances as butanol or thymol. As indicated in Table 20.2, β-amylose consists essentially of linear chains; these have a helical arrangement with each turn comprising six glucosyl units and giving a diameter of 1.3 nm. Conversely, amylopectin has a branched structure; these differences give the two substances different properties and it is their variation in proportion that contributes towards the distinctive characteristics of a starch from a particular biological source.
Amylose, although water-soluble, gives an unstable solution, which irreversibly precipitates. It is mainly responsible for the deep blue coloration (λmax c.660 nm) given by starch and iodine in which the latter as I5 becomes trapped as an inclusion complex in the amylose helix. The strong affinity of amylose for iodine means that it will take up to 19% of its weight of iodine and this figure can be used in the determination of amylose in starch. Dilute solutions in water or alkali have an appreciable vicosity and the molecule is extensively degraded by β-amylase to maltose. The course of the hydrolytic reaction may be followed: (1) by treating with iodine and observing the colour changes (starch giving a blue; dextrins purple to reddish-brown; maltose, and glucose, if acid hydrolysis, no colour); (2) by testing portions at intervals with Fehling’s solution (the amount of reduction increases with the amounts of sugar formed); or (3) by successive measurements of viscosity (viscosity decreases as hydrolysis proceeds). On the other hand, solutions of amylopectin are relatively stable, the colour given with iodine is purple (λmax c.540 nm), and the iodine-binding is low. β-Amylase can only attack the outer linear chains, not being able to bypass the 1–6 interchain links; as a consequence, amylopectin is hydrolysed to the extent of 50–60% only by the enzyme; complete hydrolysis is achieved by mineral acids and other enzymes.
A very small amount of covalently bound phosphate appears to be a normal component of starch; its exact location within the molecule is uncertain but may represent the phosphorylation of some 1 in 300 glucose molecules. Cereal starches also contain about 1% of lipid which occupies the same helix space as does added iodine.
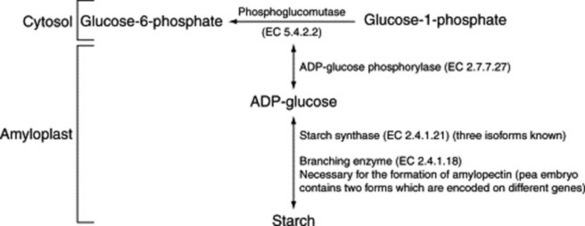
Biosynthesis of starch
The final stages of the synthesis of starch are associated with amyloplasts—double membrane organelles which develop, like chloroplasts, from protoplasts. Sucrose appears to be the primary substrate which by the mediation of the reversible sucrose synthase and other enzymes is converted to fructose, glucose-1-phosphate and glucose-6-phosphate in the cytosol. The precise pathway involved and the specific substrate which passes into the amyloplast for the final stages of synthesis are a current area of study. One problem is the difficulty of isolating intact amyloplasts for biochemical study. α-Amylase activity in barley has been extensively studied; the endosperm of germinating maize seeds contains four isozymes of α-amylases (α-amylase-1 to -4) and one isozyme of β-amylase (K. B. Subbarao et al., Phytochemistry, 1998, 49, 657). The probable final reactions are indicated in Fig. 20.5. (For a review with 81 refs. see A. M. Smith and K. Denyer, New Phytologist, 1992, 122, 21.)
Mutant varieties
A number of mutant varieties of maize and other crops produce abnormal starch grains some of which have commercial use and possibilities. Thus ‘waxy’ maize starch contains principally amylopectin producing a tapioca-like starch. It derives its name from the shiny appearance of the broken endosperm. Another mutant, ‘amylose extender’ is deficient in one of the enzymes responsible for producing the branching of the amylopectin molecule. At least six specific enzyme deficiencies have been identified as associated with abnormal maize starch mutants.
Uses
Starch finds extensive use in dusting powders, in which its absorbent properties are important. In mucilage form it is used as askin emollient, as a basis for some enemas and as an antidote in the treatment of iodine poisoning. Starch is also used as a tablet disintegrant. For the US food and drinks industry large quantities of maize starch are converted to high-fructose corn syrup by a process involving hydrolysis (glucose producing) and subsequent isomerization. Starch has also provided the plastics industry with a number of new products, including biodegradable polyvinylchloride and polyethylene plastics.
Sterilizable Maize Starch BP is used as a lubricant for surgeons’ gloves; it is maize starch subjected to physical and chemical treatments so that it does not gelatinize on exposure to moisture of steam sterilization. Unlike talc, it is completely absorbed by body tissues.
Brazilian arrowroot
This is the starch obtained from the tubers of the sweet potato, Ipomoea batatas (Convolvulaceae). The granules are rounded, polyhedral or muller-shaped, the larger ones being 25–55 μm in diameter.
Portland arrowroot
The English hedgerow plant, Arum maculatum (Araceae) is known by a number of names, including Lords and Ladies, Cuckoo Pint and Wake Robin. The tuberous rootstock is rich in starch and was formerly extracted to give Portland arrowroot. Used for starching Elizabethan ruffs, it was a cause of dermatitis among laundrymaids.
Modified starches
As with cellulose, the starch molecule can be considerably modified by chemical treatments and some of the products have a use in the paper, food, textile, adhesive and other industries. Treatments include acetylation, hydroxyethylation, phosphorylation, inorganic esterification and cross-linking. For pharmaceutical purposes maranta starch (St Vincent Arrowroot), which is no longer commercially available, has been replaced by an ester starch of cassava which has similar properties.
Pregelatinized starch
This is prepared from maize, rice or potato starch by suitable mechanical rupturing of the grains in the presence of water either with or without heat followed by drying. There are no added materials but the product may be further manipulated to improve flow rate and compressibility properties. It is widely used as a tablet excipient.
Soluble starch
Soluble starch is prepared by treating commercial potato starch with hydrochloric acid until, after washing, it forms a limpid, almost clear solution in hot water. A soluble starch solution should show little reduction with Fehling’s solution and gives a deep blue colour with iodine. On heating with 5% potassium hydroxide solution, it gives a canary-yellow colour; no colour is afforded by ordinary starch and the dextrins give a brown colour when similarly treated.
Commercial dextrins
High-grade dextrins are prepared by heating starch which has been moistened with a small quantity of dilute nitric acid and dried, at 110–115°C. The product is known as white dextrin. Inferior dextrins, which have a yellow or brown colour, are prepared by roasting starch at 150–250°C without the addition of acid.
White dextrins may contain up to 15% of soluble starch, the remainder consisting largely of erythrodextrin. Yellow dextrins are more completely hydrolysed and, unlike the white variety, contain appreciable quantities of maltose, which may be detected and estimated by means of Fehling’s solution.
FRUCTANS
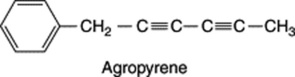
Fructans are D-fructose polymers each chain being terminated by a single D-glucosyl residue. They are found in nature as oligosaccharides with up to 10 units and as polysaccharides with up to 50 units. The best-known fructan, and the most important pharmaceutically, is inulin, a reserve carbohydrate found in many roots of members of the Compositae and Campanulaceae. The tubers of the Jerusalem artichoke (Helianthus tuberosus) and roots of chicory (Cichorium intybus) are particularly rich sources. Other fructans are the phleins found in grasses e.g. in Phleum pratense, agropyrene in couch grass (q.v.), and sinistrin a component of Urginea maritima (q.v.).
Unlike the biosynthesis of starch and cellulose, that of fructan does not originate by the conjugation of identical monosaccharide units but in all cases starts with a molecule of sucrose (glucose + fructose) to which is successively added further molecules of furanofructose. A further distinction from starch biosynthesis is that no monosaccharide nucleotide (cf. glucose adenine diphosphate) is involved in the addition of the fructose units. Various trisaccharides composed of one glucose and two fructose molecules occur in nature and the mode of the linkage of the second fructosyl unit to the sucrose and the extent of the addition of more fructosyl units to the trisaccharide determine the properties of the final polymer. Thus, inulin biosynthesis proceeds via the enzymatic transfer of a fructosyl group from sucrose to another molecule of sucrose giving the trisaccharide 1-ketose and free glucose. A second enzyme (a fructan fructosyl transferase) then mediates the addition of further fructosyl units from other oligomeric fructans. Thus, the final molecule is terminated at one end by a glucosyl unit. However, for the formation of some other fructans different fructosylsucrose trisaccharides are involved and elongation of the polymer chain may occur at either end of the trisaccharide so that the final fructan molecule has a glucosyl residue situated towards the middle of the chain.
INULIN
Inulin BP/EP is obtained from the tubers of Dahlia variabilis, Helianthus tuberosus and other genera of the Compositae; it derives its name from the dahlia, Inula helenium, from which it was first isolated in the 19th century. It occurs either in solution in the cell sap (cf. starchgranules which are formed in plastids) or in alcohol-preserved material as sphaerocrystalline masses (Fig. 42.1F). It is sparingly soluble in cold water but readily dissolves at around 70°C without gelatinizing. It is neither stained by iodine solution nor hydrolysed by mammalian enzymes.
Chemically inulin consists of a chain of 35–50 1,2-linked fructofuranose units terminated by one glucose unit. The furanose ring systems render the molecules much less rigid than either those of cellulose or starch. In any sample of inulin there is a mixture of molecular species the smaller molecules being probably intermediates in the polymerizing chain.
BP/EP tests include a thin-layer chromatography examination; clarity, colour and specific optical rotation (−36.5° to −40.5°, 2% solution) of solutions and limits for acidity, sulphated ash (0.1%), heavy metals, oxalate etc.
Inulin is not metabolized by the body and is excreted unchanged. As Inulin Injection it is used for the measurement of glomerular filtration rate.
Dandelion root
The root of the dandelion (Taraxacum officinale) is an important drug of herbal medicine. Among other constituents it contains up to 40% of carbohydrates, particularly inulin, in the autumn and about 2% inulin in the spring. The fructose content reaches about 18% in the spring. The drug is described in Chapter 29.
ALGAL GELLING AGENTS
The two most important pharmaceutical products in this class are the alginates and agar.
ALGINIC ACID
Large quantities of brown seaweeds are collected from many of the colder waters of the world. Principal producers, approximately in order of quantity, are the USA (California), Norway, Chile, China, Canada (Nova Scotia), Irish Republic, Australia (Tasmania), Iceland, UK (Scotland), South Africa. Some years ago it was reported that the Chinese had developed strains of brown seaweed which would flourish in the warmer East and South China Seas.
The North Atlantic rockweeds (littoral types) (e.g. Ascophyllum nodosum) are cut either by hand with sickles or by means of various designs of floating ‘combine harvesters’. The remaining world total is mainly storm-cast. After collection, the raw, wet seaweed may either go immediately for processing or be fuel- or sun-dried to 12–17% moisture content, in which form it has an indefinite storage life.
Alginic acid, a hard, horny polysaccharide, was first isolated by the English chemist Stanford (1883) and in Britain was first marketed in 1910; the 1976 estimated world production of alginate was 19 507 tonnes. New methods of extraction are continually being patented but the pattern of Stanford’s process is still much followed. The dried milled seaweed is macerated with dilute sodium carbonate solution and the resulting pasty mass diluted with sufficient soft water to make practicable the separation of the insoluble matter by modern super-decanters or continuous-settling devices. Soft water is essential to avoid the precipitation of insoluble alginates. The resulting clear liquor, which contains most of the alginate originally present in the algae, may now be treated in one of two ways: (1) it is poured into dilute sulphuric acid or dilute calcium chloride solution, when the insoluble alginic acid or its salt, calcium alginate, is precipitated as a bulky, heavily hydrated gel, from which liquor is removed by roller- or expeller-presses. The product obtained looks and handles like wood pulp. By moving the calcium alginate with constant agitation against a stream of hydrochloric acid, the calcium is removed and the highly swollen pulp of alginic acid is roller-pressed and then neutralized with sodium carbonate to give sodium alginate. (2) The clear liquor can be made to precipitate sodium alginate of high purity by the addition of ethyl alcohol directly or after partial evaporation.
Alginic acid BP/EP (formula, Table 20.2) is composed of residues of D-mannuronic and L-guluronic acids; the chain length is long and varies (mol. wt. from 35 000 to 1.5 × 106) with the method of isolation and the source of the algae. The degree of polymerization can be varied to meet the properties required. A small proportion of the carbonyls may be neutralized, the pharmacopoeial material having not less than 19.0% and not more than 25.0% carbonyl groups calculated with reference to the dried material. The assay involves back-titration with standardized acid. There are also tests for chlorides, heavy metals, microbial contamination, loss on drying and sulphated ash.
Alginic acid is insoluble in cold water (but swells and absorbs many times its own weight) and slightly soluble in hot water. It is insoluble in most organic solvents. It liberates carbon dioxide from carbonates. With compounds containing ions of alkali metals, or ammonium or magnesium, it reacts to give salts (alginates) which are water-soluble and form viscous solutions typical of hydrophilic colloids. The salts of most other metals are water-insoluble.
The alginates, particularly the sodium salt, have, because of their greater chemical reactivity, certain advantages over agar, starch, pectin, vegetable gums and gelatin. Alginates find applications as stabilizing, thickening, emulsifying, deflocculating, gelling and film- and filament-forming agents in the rubber, paint, textile, dental, food (including ice-cream), cosmetic and pharmaceutical industries. The formulation of creams, ointments, pastes, jellies and tablets are examples in the last-named industry. Alginic acid is also used in tablet and liquid preparations for the control of gastro-oesophageal reflux. Alginate textile fibres and their uses, for example, as absorbable haemostatic dressings have been discussed earlier.
AGAR
Agar (Japanese Isinglass) is the dried colloidal concentrate from a decoction of various red algae, particularly species of Gelidium, Pterocladia (both Gelidaceae, order Gelidiales), and Gracilaria (Gracilariaceae, order Gigartinales). Agar is obtained from Japan (Gelidium amansii), Korea, South Africa, both Atlantic and Pacific Coasts of the USA, Chile, Spain and Portugal. Some 6500 tonnes are produced annually, of which about one-third originates from Japan. The genus Gelidium provides about 35% of the total source material.
Collection and preparation
On the Japanese coast the algae are largely cultivated in special areas, poles being planted in the sea to form supports on which they develop. From time to time the poles are withdrawn and the algae stripped off. Some are also collected from small boats by means of rakes or shovels, or even by diving. The algae are taken ashore and dried; beaten and shaken to remove sand and shells; bleached by watering and exposure to sunlight, the washing also serving to remove salt. They are then boiled with acidulated water for several hours (about 1 part of dry algae to 55 or 60 parts of water), and the mucilaginous decoction filtered, while hot, through linen. On cooling, a jelly is produced which is cut into bars, these being afterwards forced through wire netting to form strips. The manufacture of agar takes place only in winter (November to February), and moistureis removed by successively freezing, thawing and drying at about 35°C. In Japan the algae are collected from May to October.
Characters
Agar occurs in two forms: (1) bundles of somewhat agglutinated, translucent, yellowish-white strips, these being about the thickness of leaf gelatin, 4 mm wide and about 60 cm long; (2) coarse powder or flakes. Agar swells in cold water but only a small fraction dissolves. A 1% solution may be made by boiling and a stiff jelly separates from this on cooling. When Japanese agar is not used, jellies of similar stiffness may be obtained by using 0.7% New Zealand, 1% South African or 2% Australian agar.
A nearly boiling 0.2% solution gives no precipitate with an aqueous solution of tannic acid (distinction from gelatin). Agar also differs from gelatin in that it contains no nitrogen and therefore gives no ammonia when heated with soda lime. When hydrolysed by boiling with dilute acid, galactose and sulphate ions are produced, the former reducing Fehling’s solution and the latter precipitating with barium chloride. If agar is ashed and the residue, after treatment with dilute hydrochloric acid, examined microscopically, the silica skeletons of diatoms and sponge spicules will be found. More perfect diatoms can often be isolated by centrifuging a 0.5% solution. The large discoid diatom Arachnoidiscus, which is about 0.1–0.3 mm in diameter, species of Grammatophora and Cocconeis, and sponge spicules are readily discernible in the ash of Japanese agar (see Fig. 20.6). Powdered agar is distinguished from powdered acacia and tragacanth by giving a deep crimson to brown colour with 0.05 M iodine and by staining pink when mounted in a solution of ruthenium red.
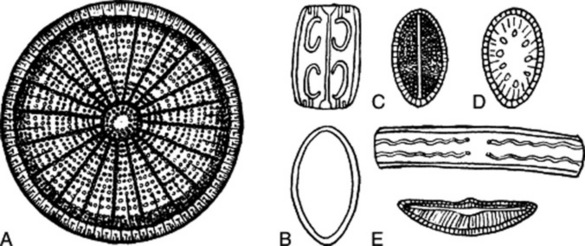
Fig. 20.6 Diatoms associated with Japanese agar. A, Arachnoidiscus (diameter 130 μm); B, species of Grammatophora; C, Cocconeis; D, Campyloneis; E, other diatoms.
Agar BP/EP is required to comply with tests for the absence of Escherichia coli and Salmonella, and general microbial contamination should not exceed a level of 103 microorganisms per g−1 as determined by a plate count. It has a swelling index (q.v.) of not less than 10 and the determined value must be quoted on the product label.
Constituents
Agar has long been known to yield on hydrolysis D- and L-galactose and sulphate ions. It is now known to be a heterogeneous polysaccharide the two principal constituents of which are agarose and agaropectin. Agarose is a neutral galactose polymer (free from sulphate) which is principally responsible for the gel strength of agar. It consists of alternate residues of 3,6-anhydro-L-galactose and -D-galactose (the disaccharide known as agarobiose). The structure of agaropectin, responsible for the viscosity of agar solutions, is less well established, but it appears to be a sulphonated polysaccharide in which galactose and uronic acid units are partly esterified with sulphuric acid. Pure agarose is commercially available and its gels are recommended for the electrophoresis of, for example, proteins.
Irish Moss
Chondrus (Carrageen) is obtained from the variable red alga Chondrus crispus and to some extent from Gigartina stellata (Gigartinaceae). Commercial supplies are derived from the north and north-west coast of Ireland, from Brittany and from the Massachusetts coast south of Boston.
Collection and preparation
The algae grow on rocks just below low-water mark, being covered by about 5 or 7 m of water at high tide. In Ireland collection takes place during the autumn; in America, during the summer. The collectors put out in small boats at about half-tide and, after detaching a load of algae from the rocks by means of long rakes, return with them at half-flood. Carrageen is bleached by spreading it on the shore and submitting it for some weeks to the action of sun and dew with about four or five soakings in seawater at suitable intervals. Bleaching by sulphur dioxide has not proved particularly satisfactory. After drying in sheds, the drug is packed in bales weighing up to 300 kg. World production of Irish moss is estimated to be around 20 000 tonnes.
Carrageenan USP/NF (1995) is obtained from red seaweeds by extraction with water or aqueous alkali and recovered by alcoholic precipitation, drum drying or by freezing.
Characters
Chondrus when fresh varies in colour from purplish-red to purplish-brown, but the bleached drug is yellowish-white, translucent and horny. It consists of complete, dichotomously branched thalli about 5–15 cm long and of very variable form, some thalli having broad fan-like segments, others having ribbon-like ones. Many samples of Chondrus contain large quantities of the related alga Gigartina mamillosa, the mixture being officially sanctioned in many pharmacopoeias. In some districts (e.g. south of Boston) almost pure Chondrus crispus may be collected, while in others (e.g. north of Boston) it is almost invariably closely associated with G. mamillosa. These algae may be distinguished from one another by the form of their large compound cystocarps, which contain carpospores. Chondrus has oval cystocarps about 2 mm long which are sunk in the thallus, while G. mamillosa has peg-like ones about 2–5 mm long, as also has G. pistillata. The latter species is rare round the coast of Britain, and its presence would indicate a drug of French origin.
Chondrus is sometimes covered with calcareous matter which effervesces with hydrochloric acid. The drug has a slight odour, and a mucilaginous and saline taste.
Chondrus swells in cold water, about 47% slowly dissolving, while on boiling about 75% passes into solution. A 5% decoction forms a jelly on cooling. A cooled 0.3% solution gives no precipitate with solution of tannic acid (distinction from gelatin), and gives no blue colour with iodine (distinction from Iceland moss and absence of starch).
Constituents
The constituents of chondrus resemble those of agar. At least five galactans, known as carrageenans, are present. Three important ones are κ-, ι- and λ-carrageenans, which differ in the amount of 3,6-anhydro-D-galactose they contain and in the number and position of the ester sulphate groups. Like other members of the Gigartinaceae C. crispus produces different carrageenans in the two phases of its life cycle; κ-carrageenan predominates in the gametophyte generation and λ-carrageenan in the diploid tetrasporophyte generation (for research on the heterogeneity of both types of plants see B. Matsuhiro and C. C. Urzua, Phytochemistry, 1992, 31, 531). The drug is rich in halogen salts, and, according to Schulzen (1964) the extract differs from that of agar in that it has a higher sulphate and ash content.
GUMS AND MUCILAGES
Gums and mucilages have similar constitutions and on hydrolysis yield a mixture of sugars and uronic acids. Gums are considered to be pathological products formed upon injury of the plant or owing to unfavourable conditions, such as drought, by a breakdown of cell walls (extracellular formation; gummosis). Conversely, mucilages are generally normal products of metabolism formed within the cell (intracellular formation) and may represent storage material, a water-storage reservoir or a protection for germinating seeds. They are often found in quantity in the epidermal cells of leaves, e.g. senna, in seed coats (linseed, psyllium etc.), roots (marshmallow) and barks (slippery elm).
TRAGACANTH
The BP/EP defines Tragacanth as ‘the air-hardened gummy exudate, flowing naturally or obtained by incision, from the trunk and branches of Astragalus gummifer Labillardière and certain other species of Astragalus from Western Asia’. The genus (Leguminosae) contains some 2000 species and those that yield gum are chiefly thorny shrubs found in the mountainous districts of Anatolia, Syria, Iraq, Iran and the former USSR. So-called Persian tragacanth has been traditionally employed in the UK, with Anatolian tragacanth finding a considerable market on the continent of Europe. The term Persian tragacanth is used by pharmacists to denote the better grades of tragacanth produced in Iran and Turkish Kurdistan.
Formation
The mode of formation of tragacanth is entirely different from that of acacia, the gum exuding immediately after injury and therefore being preformed in the plant, whereas acacia is slowly produced after injury. A section of a tragacanth stem shows that the cell walls of the pith and medullary rays are gradually transformed into gum, the change being termed ‘gummosis’. The gum absorbs water and a considerable pressure is set up within the stem. Hanbury, having cut off branches of living plants, stated, ‘there immediately exudes from the centre a stream of soft, solid tragacanth, pushing itself out like a worm, to the length of three-quarters of an inch, sometimes in the course of half an hour’.
Botanical sources
The requirement for precise botanical specifications and satisfactory analytical procedures for tragacanth, necessitated by the legal aspects covering its use as a food additive, has rendered the above BP/EP definition somewhat inadequate. A survey of the Turkish gum-producing species has indicated that A. microcephalus is the principal species employed with smaller amounts of A. gummifer and A. kurdicus being collected. Also, in an investigation of tragacanth production in Iran in 1957, Gentry (Econ. Bot., 1957, 11, 40) reported A. echidnaeformis, A. gossypinus and A. microcephalus to be important species. The approximate distribution of a number of gum-producing species found in the areas where tragacanth is collected is shown in Table 20.6.
Table 20.6 Distribution of gum-yielding Astragalus species.
| Species | Geographical distribution |
|---|---|
| A. gummifer | Anatolia and Syria |
| A. kurdicus | Northern Iraq, Turkey and Syria |
| A. brachycalyx | Western and S.W. Iran |
| A. eriostylus | S.W. Iran |
| A. verus | Western Iran |
| A. leioclados | Western and central Iran |
| A. echidnaeformis | Isfahan region of Iran |
| A. gossypinus | Isfahan region of Iran |
| A. microcephalus (syn. A. pycnocladus) | Shiraz and Kerman regions of Iran, Turkey |
| A. adscendens | South western and southern Iran |
| A. strobiliferus | Eastern Iran |
| A. heratensis | Khorasan to Afghanistan |
Collection
Most of the plants from which tragacanth is collected grow at an altitude of 1000–3000 m. The shrubs are very thorny; each of their compound leaves has a stout, sharply pointed rachis which persists after the fall of the leaflets. The mode of collection varies somewhat in different districts, but the following details of collection in the province of Fars are typical.
Gum can be obtained from the plants in their first year but is then said to be of poor quality and unfit for commercial use. The plants are therefore tapped in the second year. The earth is taken away from the base to a depth of 5 cm and the exposed part is incised with a sharp knife having a thin cutting edge. A wedge-shaped piece of wood is used by the collector to force open the incision so that the gum will exude more freely. The wedge is generally left in the cut for some 12–24 h before being withdrawn. The gum exudes and is collected 2 days after the incision. Some of the plants are burned at the top after having had the incision made. The plant then sickens and gives off a greater quantity of gum. However, this practice is not universal, as many plants cannot recover their strength and are killed by the burning. The gum obtained after burning is of lower quality than that obtained by incision only, and is reddish and dirty looking. The crop becomes available in August–September.
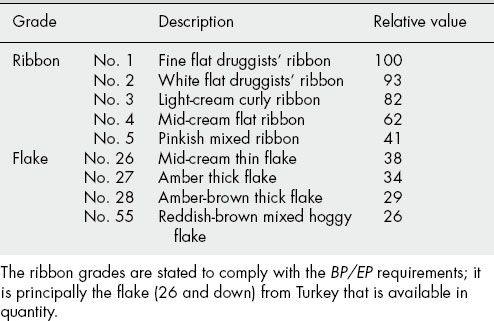
Grades
Tragacanth is graded into several qualities by the exporter or middle man. The best grades form the official drug, while the lower grades are used in the food, textile and other industries; their approximate relative values are listed in Table 20.7.
Tragacanth is an expensive commodity; not only has the supply situation increased the price, but also the extra treatment and tests required to meet the BP/EP microbial requirements have added to the cost.
Characters
The official Persian tragacanth occurs in flattened ribbons up to 25 mm long and 12 mm wide. The surface shows a number of ridges which indicates the successive, temporary stoppages of flow from the incision. Fine furrows parallel to the margin of the flake are produced by the uneven edges of the incision. The gum is white or very pale yellowish-white in colour, translucent and horny. It breaks with a short fracture, is odourless and has little taste.
Tragacanth swells into a gelatinous mass when placed in water, but only a small portion dissolves. On the addition of a dilute solution of iodine to a fragment previously soaked in water, relatively few blue points are visible (distinction from Smyrna tragacanth, which contains more starch). With stronger iodine solution the gum acquires a greenish colour (cf. ‘Agar’).
Constituents
Tragacanth consists of a water-soluble fraction known as tragacanthin and a water-insoluble fraction known as bassorin; they have molecular weights of the order of 840 000. Both are insoluble in alcohol. Tragacanthin and bassorin may be separated by ordinary filtration of an extremely dilute mucilage and the tragacanthin may be estimated by the evaporation of an aliquot portion of the filtrate. Tests show that the best grades of gum contain the least tragacanthin. If the tragacanthin content and moisture content are known, the amount of bassorin may be calculated.
Like other gums, tragacanth is composed of sugar and uronic acid units. Among the products of hydrolysis galacturonic acid, D-galactopyranose, L-arabinofuranose and D-xylopyranose have been identified. C. A. Tischer et al., have reported on the structure of the arabinogalactan fraction (Carbohydr. Res., 2002, 337, 1647). The BP/EP thin-layer chromatography test for identification is based on the products of acid hydrolysis of the gum. Von Fellenberg (1918) pointed out that in the tragacanthin fraction there are no methoxyl groups, but that the bassorin fraction contained about 5.38% methoxyl. Rowson (1937) showed that gums with high methoxyl contents and high bassorin contents gave the most viscous mucilages. Heating or fine powdering produces demethylation with loss of viscosity. Anderson and Bridgeman (Phytochemistry, 1985, 24, 2301) showed that the gum-exudates of the three principal species of Turkish gum-producers are proteinaceous polysaccharides and represent a protein content of about 3–4%, involving 18 amino acids. The relative amino-acid proportions differed in the three gums.
The BP/EP includes a test for minimum apparent viscosity of the powdered gum, a flow time for gum to be used in the preparation of emulsions, and a microbial limit test, together with compliance tests for Escherichia coli and Salmonella. Peroxidase enzymes are usually considered to be absent, but their presence has been detected in commercial samples of genuine drug. The presence of peroxidase enzymes appears to be related to a high starch content; both disappear as gummosis proceeds.
Allied drugs
Non-pharmaceutical grades of tragacanth. Large quantities of tragacanth of the lower grades are imported and used in the textile industry and pickle manufacture. The pieces vary in shape and are from a yellow ivory colour to almost black. The lower grades are much contaminated with earth, and their ashes give a strong reaction for iron. The viscosity of mucilages prepared from these grades of tragacanth falls rapidly from the No. 1 to the No. 55, the marked difference in price being fully justified. The lower grades of tragacanth are known as hog gum or hog tragacanth.
Acacia gum admixed with tragacanth can be detected by TLC of the hydrolysed sample—the presence of rhamnose indicates adulteration.
Chitral gum of Indian commerce is said to be obtained from Astragalus strobiliferus.
Sterculia gum (Karaya gum) is itself an important article of commerce and is described below. Its presence in tragacanth gum is detected by the gel formation in alcoholic solution and by an acidity test.
Insoluble Shiraz gum is a gum of doubtful botanical origin imported from Iran. When of good quality, it resembles a mixture of bleached and natural Kordofan acacia. It may be distinguished from tragacanth by the fact that it contains no starch and that it gives a reaction for oxidase enzyme.
STERCULIA GUM
Sterculia (Karaya Gum, Indian Tragacanth, Bassora Tragacanth) is the dried gummy exudate obtained from the tree Sterculia urens (Sterculiaceae). It is produced in India, Pakistan and, to a small extent, in Africa. The gum is of relatively recent introduction, being generally regarded during the early part of the last century as an adulterant and inferior substitute for tragacanth. Now, however, having been shown to be superior to other gums in certain respects, it constitutes an integral part of the gum industry and is official in the BP.
Collection and preparation
In central and northern India (Andhra Pradesh, Madhya Pradesh, Rajasthan and Uttar Pradesh) two collections are made each year, before and after the monsoon season: in April–June and in September, respectively. The first collection gives a gum affording the highest viscosity. Blazes, up to about one square foot in area, are made in the larger trees (smaller trees are tapped) and the gum immediately starts to exude; the flow is greatest during the first 24 h and continues for several days. The dried, irregular masses, weighing up to several pounds, are picked off and transported to village centres for purchase by Mumbai merchants. The Indian merchants remove excess bark and roughly sort the gum into two grades; it is further graded in Europe and the USA according to colour and presence of foreign organic matter (mainly bark). It is finally sold as a granulated (crystal), or finely powdered, product.
Unfortunately, in some areas over-production and destructive tapping methods have led to a serious decline in the natural tree population and have necessitated the introduction of 10-year bans on collection. This has stimulated research on in vitro propagation using seedling explants, nodal explants and somatic embryogenesis (S. D. Purohit and A. Dave, Plant Cell Reports, 1996, 15, 704; V. G. Sunnichan et al., ibid., 1998, 17, 951).
Characters
Good-quality gum occurs in irregular almost colourless, translucent, striated masses weighing up to 25 g or more. Medium grades have a marked pinkish tinge, while the lower grades are very dark and contain a considerable amount of bark. Karaya gum has a marked odour of acetic acid, and when hydrolysed with 5% phosphoric acid, has a volatile acidity (BP) of not less than 14% (tragacanth, about 2–3%). According to Janot and Gounard (1938), sterculia has a methoxyl value of 0 (tragacanth 30–40). When boiled with solution of potash, it becomes slightly brownish (tragacanth, canary yellow). Karaya also differs from tragacanth, in that it contains no starch and stains pink with solution of ruthenium red.
In water, sterculia gum has low solubility but swells to many times its original volume. This means that the processing of the gum influences the final product—the coarser granulated grades give a discontinuous grainy dispersion, whereas the fine powder affords an apparently homogeneous dispersion.
Constituents
Partial acid hydrolysis of sterculia yields D-galactose, L-rhamnose, D-galacturonic acid, aldobiouronic acids, an acid trisaccharide and acetic acid; the galacturonic acid and rhamnose units are the branching points within the molecule. Uronic acid residues represent about 37% of the gum.
Uses
The granular grades are used as a bulk laxative, being second only to psyllium seed in use in this respect. The powdered gum is used in lozenges, pastes and denture fixative powders, and it has proved particularly useful as an adhesive for stoma appliances. As a bulk laxative and stimulant it is available, with frangula, as granules.
ACACIA GUM
Acacia (Gum Arabic) is a dried gum obtained from the stem and branches of Acacia senegal Wild, other species of Acacia of African origin and Acacia seyal Del. (Leguminosae). A. senegal is a tree about 6 m high, which is abundant in the Sudan, particularly in the province of Kordofan, in central Africa and in West Africa. The tree is known in Kordofan as Hashab and in Senegambia as Verek. The best gum is that produced in Kordofan from tapped trees, but some Senegal and Nigerian gum is of good quality. Apart from ‘acacia gardens’, wild, self-sown plants are the main source of the gum.
History
Gum was brought from the Gulf of Aden to Egypt in the seventeenth century BC, and in the works of Theophrastus it is spoken of as a product of Upper Egypt. The West African product was imported by the Portuguese in the fifteenth century. Previously, commerce in the Sudan was in the hands of a number of local merchants, but it is now entirely controlled by the ‘Gum Arabic Company Ltd’, a concessional company set up by the Sudanese Government.
Collection and preparation
Some gum exudes from trees as a result of the cracking of the bark, but the most esteemed, Kordofan variety, is obtained from trees, about 6 years old, tapped in February and March, or earlier, in September after the rains, when the leaves fall. The tapper, with a blow from a small axe, makes a transverse incision in a branch and so twists the axe that the bark is loosened, strips of it being then pulled off above and below the cut. The portion of branch so bared to the cambium measures about 0.5–1.0 m long and 5–7.5 cm wide. This cambium produces new phloem and in about 20–30 days the tears of gum which have formed on the surface may be picked off. The gum is collected in leather bags and is conveyed in sacks to El Obeid and other centres, mostly located along the railway. Here the gum is garbled to free it from sand and vegetable debris, and is sorted. Other acacia gums such as talka gum, the product obtained from A. seyal (the talka of the Arabs), are also separated. At one time some of the gum was ‘ripened’ by exposure to the sun, when it became bleached and dried, developing numerous cracks. During this process, which took 3–4 months, the gum lost about 30% of its weight. Subsequently, this bleached gum became unobtainable.
From El Obeid the drug is sent by rail to Port Sudan, whence large quantities are shipped to London, the USA and other countries. In the London Market Reports three grades are usually quoted, namely hand-picked selected (h.p.s.) Kordofan, cleaned Kordofan and talka. Gum arabic is sold in one currency only ($US), so that currency changes also affect the price. The Senegal acacia gum is largely used for pharmaceutical purposes on the Continent and is shipped to Marseilles and Bordeaux. This also occurs in three grades, namely ‘gomme du bas du fleuve’, ‘gomme du haut du fleuve’ and ‘gomme friable’.
For comments on the impact of the recent Sudan war on the gum arabic industry, see K. Purcell, HerbalGram, 2005, 65, 25; K. Purcell and N. Dennis, HerbalGram, 2006, 71, 24.
Spray-dried acacia, produced by the importers, is becoming of increasing importance and is included as a monograph in the BP. In addition to its general usefulness, it has the further advantage of a low viable bacterial count (see below).
Characters
Bleached Kordofan acacia, when available, occurs in rounded or ovoid tears up to about 3 cm diameter, or in angular fragments. The outer surface bears numerous fine cracks which form during the ‘ripening’ and make the tears opaque. The gum is white or very pale yellow in colour. The tears break rapidly with a somewhat glassy fracture, and much of the drug consists of small pieces. It is odourless and has a bland and mucilaginous taste.
Cleaned and h.p.s. Kordofan gum differs from the above in having fewer cracks which causes it to be more transparent, and in being more yellowish or pinkish in colour. The tears are usually of less uniform size, some being quite small, while others have a diameter of 4 cm or more. The better qualities of Senegal gum closely resemble the Kordofan, but some of the tears are vermiform in shape and, speaking generally, the gum is rather more yellowish in colour.
Tests
Acacia is almost completely soluble in an equal weight of water, solution taking place rather slowly. The solution is slightly acid and becomes more so on keeping, especially if hot water is used to make the solution. It is viscid, but not glairy, and when diluted does not deposit on standing.
A 10% aqueous solution is laevorotatory, gives no precipitate with dilute solution of lead acetate (distinction from tragacanth and agar), gives no colour with solution of iodine (absence of starch and dextrin), and, if of pharmacopoeial quality, gives no reaction for tannin with ferric chloride. A very weak solution precipitates with lead subacetate solution. The mucilage gives a blue colour when treated with solution of benzidine and a few drops of hydrogen peroxide, which indicates the presence of a peroxidase (possible distinction from tragacanth). Because benzidine has carcinogenetic properties, this test is no longer advocated; however, as some pharmaceutical grades of tragacanth have now been shown to contain a peroxidase enzyme system (q.v.), the test has probably less significance than was previously considered. A tincture of guaiacum can also be used to test for the enzyme.
The BP/EP thin-layer chromatography test involves fractionation of an acid hydrolysate of the gum and visualization of the separated sugars with anisaldehyde reagent followed by heating.
There are official tests for compliance with limits for microbial contamination (total viable aerobic count, Escherichia coli and in the USP/NF, Salmonella spp.).
Constituents
Acacia consists mainly of arabin, the calcium (with traces of magnesium and potassium) salt of arabic acid. Arabic acid may be prepared by acidifying a mucilage with hydrochloric acid and dialysing.
When hydrolysed with dilute sulphuric acid, it yields L-rham- nopyranose, D-galactopyranose, L-arabinofuranose and the aldobionic acid 6-β-D-glucuronosido-D-galactose. This major polysaccharide fraction consists of branched β-(1,3)-linked galactose units with side-chains of arabinose, rhamnose and uronic acids linked through the 1,6-positions. A second component of the gum is a hydroxyproline-rich glycoprotein of high molecular weight; it has a high amino acid composition with a repetitive polypeptide backbone. For further studies on this fraction, see L. J. Goodrum et al., Phytochemistry, 2000, 54, 99.
The glycan composition of the gum is variable, depending on source. Thus that derived from A. senegal contains a high proportion of D-galactose relative to L-arabinose, whereas the reverse holds for the gum obtained from A. seyal. Also, the latter contains significantly more 4-0-methyl-D-glucuronic acid but less L-rhamnose and unsubstituted D-glucuronic acid than does the gum from A. senegal.
Acacia also contains an oxidase enzyme and about 14% of water. It yields about 2.7–4% of ash.
The gum is formed in the cambial region of the plant with gum-containing cysts developing in the inner bark (J.-P. Joseleau and G. Ullmann, Phytochemistry, 1990, 29, 3401).
Hairy root cultures
These produce a mucilage with a different polysaccharide composition to that of the parent plant.
Uses
As a general stabilizer in emulsions and as a pharmaceutical necessity in lozenges, etc. Its demulcent properties are employed in various cough, diarrhoea and throat preparations but it is incompatible with readily oxidized materials such as phenols, and the vitamin A of cod-liver oil. It has widespread use in the food, drinks and other industries.
Allied drugs
Talka gum is usually much broken and of very variable composition, some of the tears being almost colourless and others brown.
Ghatti or Indian gum is derived from Anogeissus latifolia (Combretaceae). It is produced in much the same localities as sterculia gum, and is harvested and prepared in a similar manner. It resembles talka in possessing tears of various colours. Some of the tears are vermiform in shape and their surface shows fewer cracks than even the natural acacia. Aqueous dispersions of the gum have a viscosity intermediate between those of acacia and sterculia gums.
West African Gum Combretum, obtained from Combretum nigricans, is not permitted as a food additive but is exploited as an adulterant of gum arabic. Unlike the latter in which the rhamnose and uronic acid units are chain terminal, in gum combretum these moieties are located within the polysaccharides chain. The leaves of this plant contain cytotoxic dammar-3-one pentacyclic triterpene derivatives (G. Simon et al., Fitoterapia, 2003, 74, 339).
Many other gums of the acacia type are occasionally met with in commerce, and many gum exudates of the large genus Acacia have been given chemotaxonomic consideration.
GUAR GUM
Guar BP/EP is obtained from the ground endosperms of the leguminous plant Cyamopsis tetragonolobus (L.) Taub., a species cultivated in India as a fodder crop. The gum is a white or off-white powder which readily forms a mucilage with water. Examined under the microscope the powder, in a glycerol mountant, shows the thick-walled endosperm cells with granular contents.
The principal constituent of the gum is a galactomannan which on hydrolysis gives galactose and mannose; these sugars of the hydrolysate constitute the basis of the pharmacopoeial thin-layer chromatography test for the drug. Other tests refer to the absence of other gums, viscosity, loss on drying, ash and microbial contamination.
Fatty acids, both free and combined as esters, have been reported by GLC-MS analysis.
Guar is available as an oral hypoglycaemic drug; it produces changes in gastric emptying and in the gastrointestinal transition time, which can delay absorption of sugars and oligosaccharides from the gut. Guar also lowers cholesterol levels, possibly by binding bile salts in the gut. However its efficacy in the treatment of diabetes is not considered by all to be fully proven. The gum, with 5–6 times the thickening power of starch, is also used in the food industry.
XANTHAN GUM
This gum is produced artificially by the pure culture fermentation of the bacterium Xanthomonas campestris on glucose. It, like cellulose, consists of 1,4-β-glycosidically linked chains of glucose with, additionally, trisaccharide side-chains on alternating anhydroglucose units. These side-chains are composed of two mannose units which encompass a glucuronic acid unit. Pyruvate groups are attached to most of the terminal units with acetyl groups at the C-6 of a number of the mannose moieties next to the glucose chain. The BP/EP assay is based on the amount of the pyruvic acid produced by the acid hydrolysis of the gum and should correspond to a content of  1.5%.
1.5%.
Other tests include a limit for 2-propanol (750 ppm as determined by gas chromatography), foreign polysaccharides, a limit of 103 bacteria and 102 fungi per gram, and a total-ash range of 6.5–16.0% consistent with xanthan gum occurring as the sodium, potassium or calcium salt.
Xanthan gum is used as a pharmaceutical aid, and also finds use in the food and cosmetics industries.
PSYLLIUM
(Flea Seed)
The dried, ripe seeds of Plantago afra (P. psyllium), P. indica (P. arenaria) and P. ovata (Plantaginaceae) are used in medicine. The US National Formulary includes all three species under the name ‘Plantago Seed’. The BP/EP describes the seeds of the first two species under the title ‘Psyllium’ and the husks of seeds of P. ovata are included under ‘Ispaghula Husk’. The latter consists of the epidermis and collapsed adjacent layers removed from the ripe seeds.
The seeds of P. afra and P. indica are known in commerce as Spanish or French psyllium, while those of P. ovata are known as blond psyllium, ispaghula, spogel seeds or Indian plantage seeds.
Constituents
All the seeds contain mucilage in the epidermis of the testa. The seeds may be evaluated by measuring the volume of mucilage produced after shaking the seeds with water and allowing to stand (see swelling index, Chapter 16).
Two fractions have been separated from the mucilage; one is soluble in cold water, and the other in hot water giving a highly viscous solution which gels on cooling. On hydrolysis fractions yield D-xylose, L-arabinose and aldobiuronic acid. The seeds also contain fixed oil, aucubin glycoside, various bases, sugars, sterols and protein. The aucubin content differs appreciably in different seed samples and species.
Allied drugs
Wild seeds of P. ovata and related species are reported to contain less mucilage than the cultivated variety. P. asiatica, a species used in Chinese medicine, contains mucilage the backbone chain of which is composed of β-1,4-linked D-xylopyranose residues having three kinds of branches.
Seeds of P. major are reportedly used as a medicinal substitute for P. ovata; the seed oil contains an unusual hydroxyolefinic acid. For a review of the traditional uses, chemical constitutents and biological activities of the P. major plant see A. B. Samuelsen, J. Ethnopharm., 2000, 71, 1.
MARSHMALLOW LEAF
Marshmallow leaf BP/EP is the whole, cut or dried leaf of Althaea officinalis L. The alternate petiolate leaves, about 7–10 cm long, arise from tall, erect, velvety, stems; the lower leaves are roundish, 3–8 cm across, and the upper ones are narrower and ovate; both are slightly 3–5 lobed, the upper more deeply so, and folded, toothed and covered with a soft velvety down. Microscopically, the powdered drug exhibits numerous long, unicellular trichomes, a few glandular trichomes, anomocytic or paracytic stomata, cluster crystals of calcium oxalate and mucilage-containing parenchymatous cells staining red with ruthenium red solution.
As with marshmallow root, mucilage is the effective constituent, giving the leaves emollient, slightly laxative and antitussive properties. Traditionally, the leaves have been used as a poultice for abcesses. Medicinally, non-specific constituents include flavonoids, e.g. quercetin and kaempferol (see Table 21.5), coumarin and polyphenolic acids.
The leaf is characterized by the pharmacopoeial TLC test, which, with the genuine drug, gives a number of fluorescent zones when viewed in UV light at 365 nm. The swelling index, minimum 12, is higher than that for the root (10).
MARSHMALLOW ROOT
Marshmallow root is derived from Althaea officinalis (Malvaceae), a perennial herb which is found wild in moist situations in southern England and Europe. In general appearance it closely resembles the common hollyhock, Althaea rosea. The plant has a woody rootstock from which arise numerous roots up to 30 cm in length. The drug is chiefly collected on the Continent from cultivated plants at least 2 years old. The roots are dug up in the autumn, scraped free from cork and dried, either entire or after slicing. The EP/BP drug may be peeled or unpeeled, whole or sliced.
The drug occurs in whitish, fibrous pieces about 15–20 cm long and 1–2 cm in diameter, or in small transverse slices. Odour, slight; taste, sweetish and mucilaginous. A transverse section shows a bark about 1–2 mm thick which is separated by a greyish, sinuate cambium from the white, radiate wood. The section shows numerous mucilage cells, the contents of which are coloured a deep yellow by a solution of sodium hydroxide. Structures to be seen in the powdered drug include: variously thickened vessels; fibres; calcium oxalate as cluster crystals 20–25–30–35 μm in diameter; starch granules, mainly single, 3–25 μm in size; thin-walled cork cells.
Marshmallow root contains about 10% of mucilage, the amount being season-dependent; it contains a polysaccharide giving on hydrolysis galactose, rhamnose, galacturonic acid and glucuronic acid. Other components of the mucilage are glucans and an arabinan. The mucilage content of the drug is indicated by the swelling index which, for BP purposes should be not less than 10. The upper limit for the total ash of the peeled root is 6.0% and that for the unpeeled 8.0%. Starch, pectin and sugars, and about 2% of asparagine are also present. The latter, which is the amide of aspartic (amino-succinic) acid, is also found in asparagus, potatoes, liquorice, etc.
Marshmallow root, and also the leaves, are used as demulcents, particularly for irritable coughs and throat and gastric inflammation.
MULLEIN FLOWER
Mullein flower BP/EP is the dried flower, reduced to the corolla and androecium, of Verbascum thapsus L., V. densiflorum Bertol. (V. thapsiforma Schrad.) and/or V. phlomoides L. Family Scrophulariceae.
V. thapsus (great mullein, Aaron’s rod, blanket leaf and a number of other synonyms) is common throughout Europe and has long been naturalized in the US from the Atlantic coast west to South Dakota and the south-western states. In general, it is found in waste places, roadsides and on sunny banks. Reaching a height of 2 m, it is a biennial terminating in a dense cylindrical spike of yellow flowers. Oval to lanceolate shortly petiolate leaves alternate on the simple stem. The whole plant is blanketed with woolly hairs. V. densiflorum (large-flowered mullein), found throughout continental Europe but a rare casual in Britain, differs from the above species in having larger quite flat corollas and decurrent leaves. V. phlomoides (orange mullein) is native to central and southern Europe and West Asia. It is an occasional casual in Britain but is widely cultivated as an ornamental. The flowers are yellow to yellow–orange.
A microscopical examination of the yellow to brown or orange powder shows many branched clothing trichomes (see Fig. 42.3), yellow fragments of petals and numerous ovoid pollen grains with a finely pitted exine and three pores.
Constituents
The three species contain the same groups of chemical constituents but with varying proportions and nature of individual compounds. V. densiflorum and V. phlomoides have been the most intensively studied as these species are the most commonly used on continental Europe. Flavonoids include, among others, apigenin, luteolin, their 7-glucosides and verbascoside. Iridoids include aucubin, catapol and their 6-xylosides (Fig. 20.7). Polysaccharides constitute the important mucilage components of the flowers; a water-soluble acidic arabinogalactan, MW 70 000, has been isolated from commercial material. As a standard for the mucilage content of the drug the BP specifies a minimum swelling index of 9 determined on the powdered flowers. A number of saponins have been recorded, including verbascosaponin, the structure of which was finally elucidated in 1992. Other constituents include phenolic acids not specific to mullein.
The Pharmacopoeia includes a TLC comparison of an extract of the flowers against reference substances and a test for the presence of iridoids.
COUCH GRASS RHIZOME
Couch Grass Rhizome BP/EP, BHP consists of the washed and dried rhizomes of Agropyron repens (L.) Beauv. (Elymus repens (L.) Gould), family Gramineae, sliced or whole with most of the adventitious roots and leaves removed.
The plant, although an invasive and troublesome weed (twitch), has a long history of medicinal use; it was known to Dioscorides and has subsequently been included in many herbals and pharmacopoeias. In Britain it was last included in the BP 1914 but was, in 1999, returned as a result of its EP status. S.E. Europe (Hungary) supplies most of the commercial material.
Characters
The dried, rigid rhizome is pale yellow, shiny, 2–3 mm thick and usually cut into pieces up to 6 mm in length. It is strongly furrowed longitudinally and, except at the nodes, hollow. The drug is odourless with a slightly sweet taste. Features of the powder include: epidermal cells of two types—one, narrow elongated with wavy and pitted, lignified walls—the second, alternating with the first type, somewhat rounded and unlignified; endodermal cells with U-shaped thickenings; fibres; pitted vessels with annular or spiral thickenings, pitted parenchyma. Calcium oxalate and starch are absent.
Constituents
The rhizomes contain about 10% mucilage and up to 8% of the fructosan polysaccharide triticin. Unlike the inulins (β, 2-1 linkages) the fructosans of the Gramineae, termed levans, are fructofuranose units linked by β,2-6 glycosidic linkages and, as with the inulins, terminated with a sucrose unit. Other constituents are 2–3% of sugar alcohols (mannitol, inositol), 0.01–0.05% volatile oil containing the polyacetylene agropyrene and other volatiles, vanillin and its monoglucoside, phenolic carboxylic acids and silicates.
Tests
The BP/EP specifies a water-soluble extractive of  25% and foreign matter consisting of greyish-black pieces of rhizome
25% and foreign matter consisting of greyish-black pieces of rhizome  15percnt;. Tests, depending on the presence of starch and thickening of the endodermic cells are given to detect Cynodon dactylon (Bermuda grass), found on sandy soils in warmer temperate regions of the world including the shores of S.W. England, and Imperata cylindrica, a troublesome weed of subtropical regions.
15percnt;. Tests, depending on the presence of starch and thickening of the endodermic cells are given to detect Cynodon dactylon (Bermuda grass), found on sandy soils in warmer temperate regions of the world including the shores of S.W. England, and Imperata cylindrica, a troublesome weed of subtropical regions.
‘Aloe vera’ products
Aloe vera products are derived from the mucilage located in the parenchymatous cells of the Aloe vera leaf and should not be confused with aloes, described in Chapter 21, which originate from the aloetic juice of the pericyclic region. The mucilaginous gel has been used from early times for the treatment of numerous conditions but in recent years its use in the herbal and cosmetic industries has become very big business in the USA, Europe and elsewhere. Raw materials are obtained from plantations in the southern states of the USA, South America and elsewhere. Exaggerated claims have inevitably brought scepticism about the true usefulness of the products which feature as suntan lotions, tonics and food additives.
Research over the last 10 years has, however, largely upheld a number of the therapeutic properties ascribed to the gel. These include the anti-inflammatory properties (wound healing, burn healing, frostbite), gastrointestinal activity (peptic ulcer), antidiabetic activity, anticancer activity (principally animal tests), antifungal activity, antibacterial activity and radiobiological protection. Not all these properties have been unequivocally accepted.
The complex chemistry of the gel makes the attribution of the various activities to specific compounds difficult. Indeed beneficial clinical results for a particular condition may arise from more than one component. Thus wound-healing benefits may derive from the anti-inflammatory, fibroblast-stimulating, antibacterial and hydrophilic properties of the gel.
Some of the conflicting results of tests may be due, in some measure, to the different methods of collecting and subsequent processing of the gel. Thus anthraquinones have been reported as constituents of the gel but their presence may, or may not be, due to some admixture with the aloetic juice from the pericyclic region of the leaf. Also, variations in carbohydrate composition arise due to varietal differences within the species and to seasonal, climatic and soil factors.
Glucomannans constitute a principal component of the gel; some consist of glucose and mannose only or glucose, mannose and glucuronic acid, others are acetylated. Other polysaccharides are galactogalacturans (galactose + galacturonic acid) and galactoglucoarabinomannans. Molecular weights range from 200 000–450 000. An acetylated mannan is available commercially and has a range of reported biological activities. Glycoprotein fractions of the gel which may also influence the immune system have been shown to have proliferation-promoting activity on human and hamster cells in vitro (A. Yagi et al., Planta Medica, 1997, 63, 18; 2000, 66, 180). In a series of papers N. Okamura et al. (Phytochemistry, 1996, 43, 495; 1997, 45, 1511; 1998, 49, 219) have reported on the isolation of some eleven new chromones. Pectic substances, the triterpenoid lupeol, plant sterols (cholesterol, campestrol, β-sitosterol), possible prostanoids and other organic and inorganic compounds have also been identified in the gel. Five phytosterols which, in animals, show long-term blood-glucose-level control, could be beneficial in the treatment of type 2 diabetes. These were identified as cycloartenol (Fig. 23.1), its 24-methylene derivate, lophenol and its 24-methyl and ethyl derivatives (M. Tanaka et al., Biol. Pharm. Bull., 2006, 29, 1418).
The intense interest in aloe vera products both from the commercial and scientific viewpoints has made this a topic for very active research. For an extensive review update on the subject (over 300 refs) see T. Reynolds and A. C. Dweck, J. Ethnopharmacology, 1999, 68, 3.
Gels from other species of Aloe, e.g. A. arborescens, A. ferox, have also been examined for their polysaccharide composition.
Quince seeds
Quince seeds are obtained from Cydonia oblonga Mill. (Rosaceae), a tree cultivated in South Africa, Central Europe and the Middle East. Iran supplies about 75% of the total world production.
The seeds are separated from the apple- or pear-shaped fruits and dried. They resemble apple pips and frequently adhere together in masses, owing to their surface coating of dried mucilage. The latter is derived from the outer epidermis of the testa. Quince seeds contain about 20% of mucilage, composed of units of arabinose, xylose and uronic acid derivatives, 15% of fixed oil and a small quantity of cyanogenetic glycoside and an enzyme which effects its hydrolysis. The seeds are used as a demulcent, as an emulsifying agent and in the preparation of hair-fixing lotions.
Slippery elm bark
Slippery elm bark is obtained from Ulmus rubra Muhlenberg (Ulmus fulva Michaux) (Ulmaceae), a tree 15–20 m in height which is widely distributed in the USA and in Canada. In the spring fairly old bark is stripped from the trees. The outer part of the bark is then removed, only the inner part, which forms the commercial drug, being dried. After this has been sawn into convenient lengths, it is bound into bundles with wire.
The drug occurs in broad, flat strips about 50–100 cm in length and from 1 to 4 mm in thickness. A few reddish-brown patches of the imperfectly removed rhytidome may be seen but the remainder consists only of secondary phloem. The outer surface is brownish-yellow and striated, the inner surface yellowish-white and finely ridged. The bark is easily identified by the characteristic, fenugreek-like odour, the strongly fibrous fracture and the fact that it yields mucilage when moistened.
The chief constituent of the bark is mucilage, which is a mixture of two or more polyuronides. The mucilage has demulcent, emollient and nutritive properties. A poultice of the powdered bark is sometimes used.
Other mucilage-containing medicinal plants
Coltsfoot BHP 1983 consists of the dried leaves of Tussilago farfara, Compositae. It is used (and also the flowers) as a herbal expectorant and contains up to 10% mucilage which on hydrolysis yields a number of sugars and uronic acids. The leaves also contain tannin and a small percentage of pyrrolizidine alkaloids, e.g. senkirkine, which can prove hepatotoxic in sufficient doses. Probably for this reason the herb is no longer included in recent editions of the BHP; the German Commission E sets limits for the daily dose of these compounds, as occurring in coltsfoot. Further information on the drug will be found in a review by M. Berry (Pharm. J., 1996, 256, 234).
For other drugs containing appreciable mucilage see fenugreek seeds and linseed.
MISCELLANEOUS CARBOHYDRATE-CONTAINING DRUGS
HONEY
Honey is a saccharine substance deposited by the hive bee, Apis mellifera (order Hymenoptera, Apidae), and other species of Apis, in the cells of the honeycomb. Honey is produced in England, but the chief sources of supply are the West Indies, California, Chile, various parts of Africa, Australia and New Zealand.
Collection and preparation
The worker bees, by means of a long, hollow tube formed from the maxillae and labium, take nectar from the flowers they visit and pass it through the oesophagus into the honey-sac or crop. The nectar, which consists largely of sucrose, is mixed with salivary secretion containing the enzyme invertase and while in the honey-sac is hydrolysed into invert sugar. On arrival at the hive the bee brings back the contents of the honey-sac and deposits them in a previously prepared cell of the honeycomb.
The best honey is that derived from flowers such as clover and heather, obtained from hives that have never swarmed, and separated from the cut comb either by draining or by means of a centrifuge. Honey obtained by expression is liable to be contaminated with the wax. The nectar of certain flowers (e.g. of species of Eucalyptus or Banksia) may give the honey a somewhat unpleasant odour and taste; nectar from Datura stramonium, ragworts and Rhododendron spp. are known to give poisonous honey.
Appropriate cultivation measures should be in force to prevent the build-up of undue levels of pesticide and herbicide residues in the honey.
Preparation of the honey may involve melting at a moderate temperature, skimming off any impurities that collect on standing and, if necessary, adjusting the water content using refractive index measurement.
Characters
Honey, when freshly prepared, is a clear, syrupy liquid of a pale yellow or reddish-brown colour. On keeping, it tends to crystallize and become opaque and granular. The odour and taste depend very largely on the flowers used in its preparation.
Constituents and tests
Honey consists mainly of invert sugar and water. It contains small quantities of sucrose, dextrin, formic acid, volatile oil, wax and pollen grains. Microscopical examination of the latter afford valuable evidence of the source. The most likely adulterants are artificial invert sugar, sucrose and commercial liquid glucose. The tests for purity of the BP/EP purified honey should be noted. The limit tests for chloride and sulphate are important, as starch and sucrose may be hydrolysed with acids to give commercial liquid glucose and artificial invert sugar, respectively. Artificial invert sugar contains furfural, which gives a red colour with resorcinol in hydrochloric acid, but this may be formed in genuine honey by prolonged heating or lengthy storage.
The pharmacopoeia limits 5-hydroxymethylfurfural to a maximum of 80 ppm, calculated on dry solids, determined by absorbance measurements at 284 μm; the TLC test identifies glucose and fructose and eliminates excess sucrose. Water content is limited to 20% determined by refractive index measurements (minimum value 1.487). Conductivity (maximum 800 μS.cm−1) and optical rotation (maximum +0.6°) are further standards.
FIGS
Fig BP is the sun-dried succulent fruits of Ficus carica L. (Moraceae). It is widely produced in the Mediterranean countries, particularly Turkey (Smyrna figs), Greece and Spain.
The fruit is produced by the union of the cymose inflorescence to form a hollow, fleshy axis bearing the flowers on its inner surface. The young fruit is rich in latex, but when mature, no latex is found and the fleshy axis contains much sugar. Figs contain about 50% of sugars (chiefly glucose), appreciable quantities of vitamins A and C, smaller amounts of vitamins B and D, and enzymes (protease, lipase and diastase). There is an official requirement for a water-soluble extractive of not less than 60.0%. Figs are used by the BP and BPC in laxative preparations (e.g. Compound Fig Elixir).
For a review of Ficus spp. covering the ethnobotany and potential as anticancer and anti-inflammatory agents see E. P. Lansky et al., J. Ethnobotany, 2008, 119, 195.
FUCUS
Fucus, or bladderwrack, consists of the dried thallus of Fucus vesiculosus L., F. serratus L. or Ascophyllum nodosum Le Jolis., family Fucaceae. The BP/EP, under the title Kelp, admits all three species, whereas the BHP (1990) monograph is restricted to F. vesiculosus. Although the name ‘kelp’ is often used in connection with Fucus spp. it more strictly applies to the larger brown seaweeds of the genera Laminaria and Macrocystis family, Laminariaceae.
The dried grey to black thallus, sometimes having a whitish coating, is hard and brittle but readily softens in water to become mucilaginous. Depending on the species, air vesicles may, or may not be present.
Bladderwrack contains mucilaginous polysaccharides such as alginic acid, for the extraction of which it may be used (q.v.). It is also rich in trace elements and iodine. The latter is in the form of inorganic salts, bound to protein, and as a constituent of iodo-amino acids such as di-iodotyrosine. Other constituents are polyphenols consisting of phloroglucinol units, sterols and complex lipids.
Note the official limit tests for heavy metals and arsenic, the high total ash value ( 24.0%) (mainly soluble in dilute hydrochloric acid) and loss on drying (
24.0%) (mainly soluble in dilute hydrochloric acid) and loss on drying ( 15.0%). Other standards are the swelling index (
15.0%). Other standards are the swelling index ( 6.0) and total iodine content (0.03–0.2%) (sodium thiosulphate titration).
6.0) and total iodine content (0.03–0.2%) (sodium thiosulphate titration).
Bladderwrack has thyroactive properties and has, in the past, been employed in iodine therapy; however other preparations with a more consistent iodine content are now preferred. Again arising from its iodine content, bladderwrack has been promoted in teas as a slimming agent. In suitable doses it serves as a dietary supplement for trace elements and as a bulk laxative.
CETRARIA
Cetraria or Iceland moss, Cetraria islandica (L.) Acharius s.l. (Parmeliaceae), is a foliaceous lichen growing amidst moss and grass in central Europe, Siberia and North America, and on the lower mountain slopes of central Europe and Spain. For medicinal purposes it is usually collected in Scandinavia and central Europe.
The BP/EP drug consists of irregularly lobed, leafy thalli, about 5–10 cm long and about 0.5 mm thick. The upper surface is greenish-brown and sometimes covered with reddish points, while the lower surface is pale brown or greyish-green and marked with white, irregular spots. At frequent intervals along the margin of the thallus are minute projections. The dried drug is brittle but becomes cartilaginous on moistening with water. Odour, slight; taste, mucilaginous and bitter. A 5% decoction forms a jelly on cooling, which is stained blue by iodine (distinction from carrageen). It has an official swelling value of  4.5 determined on the powdered drug. Sections of the drug reveal the dual nature of the plant, the small rounded cells of the unicellular alga Cystococcus humicola being enclosed by the more or less closely woven hyphae of the fungus.
4.5 determined on the powdered drug. Sections of the drug reveal the dual nature of the plant, the small rounded cells of the unicellular alga Cystococcus humicola being enclosed by the more or less closely woven hyphae of the fungus.
The drug contains carbohydrates, known as lichenin and isolichenin. Lichenin is only soluble in hot water and is not coloured blue by iodine, while isolichenin is soluble in cold water and gives a blue colour with iodine. Both on hydrolysis give glucose. Cetraria also contains a very bitter depsidone, cetraric acid, and other acids such as lichestearic and usnic acids. The latter compound is an antibiotic which may not be unimportant in contributing to the efficacy of Iceland moss as a demulcent for the treatment of cough involving throat irritation. Iceland moss has been used as a bitter tonic and for disguising the taste of nauseous medicines.
Prunes
Prunes (Rosaceae) are dried plums derived from Prunus domestica L. Of the many cultivated forms, var. Juliana de Candolle, which is largely grown in France, produces those commonly used in European medicine. Prunes of excellent quality are also produced in California.
The appearance of prunes requires no description. The pulp contains about 44% of sugars (mainly glucose), malic acid and water. The kernel contains 45% of fixed oil, and small quantities of amygdalin and benzoic acid. Prunes are used in Confection of Senna.
Weymouth-Wilson AC. The role of carbohydrates in biologically active natural products. Natural Product Reports. 1997;14(2):99-110.
Whistler RL, BeMiller JN, editors. Industrial gums; polysaccharides and their derivatives, 3rd edn., London, UK: Academic Press, 1993.
Whistler RL, BeMiller JN, editors. Carbohydrate chemistry for food scientists. St Paul, Minnesota: Eagan Press, 1997.