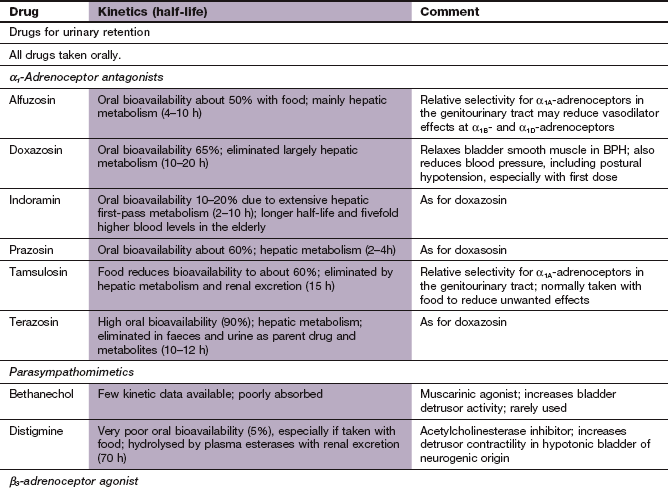
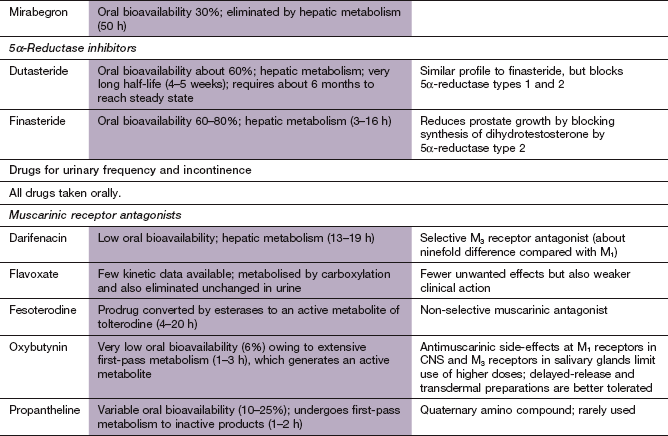
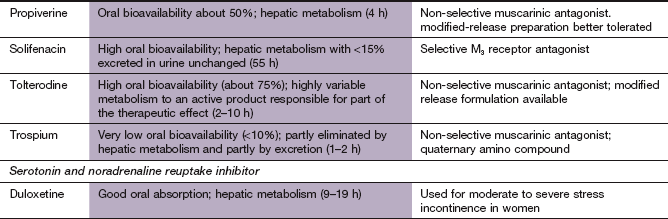
Disorders of micturition
Pathophysiology of micturition
The urinary bladder is a smooth muscle organ, most of which is the detrusor muscle, that relaxes to allow bladder filling up to 500–600 mL. A smaller muscle, the trigone, is found between the ureteric orifices and bladder neck. Internal and external distal sphincter mechanisms are normally constricted to prevent bladder emptying and maintain continence (Fig. 15.1). Coordination of bladder filling, continence and bladder emptying are brought about by the frontal lobe of the cortex and the pontine micturition centre. Conscious sensations of bladder fullness are processed by the cerebral cortex, which then sends signals to the micturition centre.
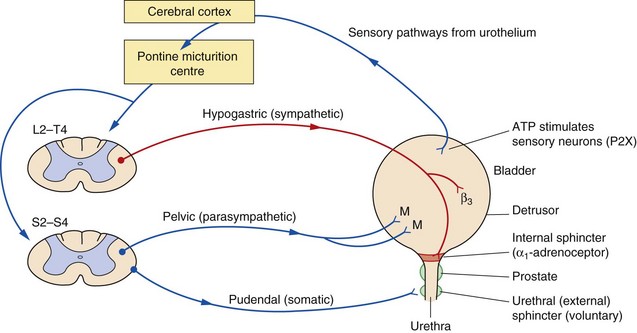
Fig. 15.1 Aspects of the bladder/prostate structures and the innervation involved in the micturition reflex.
Bladder filling provides neuronal signals to the micturition centre via sensory input from purinoceptors on neurons in the urothelium. To accommodate filling and continence, sympathetic stimulation both relaxes the smooth muscle of the bladder via β2- and β3-adrenoceptors and stimulates sphincter mechanisms through α1-adrenoceptor subtypes. Somatic control of the external sphincter also aids continence. Voluntary urination involves parasympathetic stimulation of bladder smooth muscle through M3 and M2 muscarinic receptor subtypes (M) and inhibition of the sympathetic and somatic outflow. Aspects of bladder control may involve other less understood transmitter substances. For example, γ-aminobutyric acid (GABA) interneurons inhibit bladder contraction. P2X, purinergic receptors.
During bladder filling, sympathetic nervous system stimulation via the hypogastric nerve relaxes the bladder smooth muscle (via β2- and β3-adrenoceptors in the detrusor which generate intracellular cAMP). At the same time sympathetic stimulation of α1-adrenoceptors (α1A- and to a lesser extent α1B-adrenoceptor subtypes), via the vesical nerve, contracts the smooth muscle of the internal urethral sphincter. Stimulation of the striated muscle of the external urethral sphincter, which is under voluntary control via the pudendal nerve, and aided by pelvic muscle control in women, contributes to maintenance of internal urethral sphincter tone and continence. The sensation of urge to micturate occurs in adults at a bladder volume of 200–300 mL.
Bladder emptying is initiated by myogenic stretch receptor activity produced by distention of the trigone, and by sensory signals from the urothelium (the epithelial cell lining of the bladder). Release of ATP from the urothelium stimulates P2X purinoceptors which along with other local receptor modulators initiate sensory impulses in the afferent nerves (see Ch. 1). The afferent sensory nerves project to the pontine micturition centre, which then initiates activity in efferent motor pathways. Stimulation of the pelvic nerve, acting through parasympathetic muscarinic M3 receptors in the detrusor muscle, leads to bladder contraction and voiding. Acetylcholine, acting through these receptors, leads to generation of intracellular inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). At the same time, stimulation of muscarinic M2 receptors on presynaptic nerve terminals inhibits intracellular cAMP production, and therefore opposes the effects of sympathetic activity. Non-cholinergic-mediated efferent impulses (ATP neurotransmission acting via P2X purinoreceptors) also contribute to bladder contraction, and this component becomes more prominent in unstable bladders. Contraction of the detrusor is coordinated with inhibition of the tonic control of distal sphincter mechanisms and the bladder neck, thus relaxing the bladder outflow tract. Bladder emptying may be augmented by contraction of the diaphragm and abdominal muscles. M3 and M2 receptors are present in detrusor muscle but the M3 subtype appears to be more important for detrusor contraction.
Disorders of micturition
Disorders of micturition can arise from a disturbance of bladder function or from abnormalities affecting bladder outflow. Although there are distinct clinical syndromes, many people with incontinence have mixed incontinence; for example, both stress and urge incontinence (see below). Management of disorders of micturition should also consider possible contributory factors, such as diuretics, α1-adrenoceptor antagonists used for treatment of hypertension, and stool impaction, which inhibits sacral parasympathetic neurotransmission.
Overactive bladder syndrome
Detrusor instability produces uncontrolled bladder contractions during normal filling. Symptoms from this include urinary urgency, nocturia and frequency (overactive bladder syndrome), often accompanied by urge incontinence (a sudden compelling desire to urinate). Most cases in women are idiopathic but probably have a neurogenic component, while in men bladder outflow obstruction is the commonest cause. Upper motor neuron lesions, such as those produced by stroke, spinal cord injuries or multiple sclerosis, can also produce an overactive bladder. First-line approaches to management include reduction in excessive fluid intake, weight loss, smoking cessation and behavioural training that includes pelvic floor muscle rehabilitation and suppression of urge.
Drugs for treatment of overactive bladder syndrome
Increased understanding of the neural pathways involved in initiating micturition is opening up new avenues for drug therapy to augment the relatively ineffective treatments currently available. Those used at present to treat overactive bladder act at peripheral muscarinic receptors to decrease bladder activity.
Muscarinic receptor antagonists
These drugs act with various degrees of selectivity at muscarinic receptor subtypes. Antimuscarinic unwanted effects (Ch. 4) are most troublesome with oxybutynin, particularly central nervous system (CNS) effects such as sedation, insomnia, confusion and cognitive problems (from M1 receptor blockade) and dry mouth (from M3 receptor blockade in salivary glands). The need for continued use of these drugs should be reviewed after 6 months.
 Oxybutynin is a selective antagonist of M1 and M3 receptors and has additional weak muscle relaxant properties through calcium channel blocking actions and local anaesthetic activity. Oxybutynin is rapidly absorbed from the gut and metabolised in the liver to an active metabolite. Modified-release and transdermal formulations are available because oxybutynin has a short half-life (1–3 h) and use of standard formulations can result in large fluctuations in plasma drug concentrations and increase the severity of unwanted effects.
Oxybutynin is a selective antagonist of M1 and M3 receptors and has additional weak muscle relaxant properties through calcium channel blocking actions and local anaesthetic activity. Oxybutynin is rapidly absorbed from the gut and metabolised in the liver to an active metabolite. Modified-release and transdermal formulations are available because oxybutynin has a short half-life (1–3 h) and use of standard formulations can result in large fluctuations in plasma drug concentrations and increase the severity of unwanted effects.
 Tolterodine, fesoterodine and trospium are non-selective muscarinic receptor antagonists with no additional properties and less lipophilicity than oxybutynin. They cross the blood–brain barrier less readily and have fewer cognitive unwanted effects. Both tolterodine and trospium have short half-lives. Tolterodine is better tolerated in a modified-release formulation.
Tolterodine, fesoterodine and trospium are non-selective muscarinic receptor antagonists with no additional properties and less lipophilicity than oxybutynin. They cross the blood–brain barrier less readily and have fewer cognitive unwanted effects. Both tolterodine and trospium have short half-lives. Tolterodine is better tolerated in a modified-release formulation.
 Darifenacin and solifenacin are more selective antagonists of M3 receptors and may have fewer CNS actions.
Darifenacin and solifenacin are more selective antagonists of M3 receptors and may have fewer CNS actions.
Beta3-adrenoceptor agonist
Example: mirabegron: Stimulation of β3-adrenoceptors in the bladder trigone flattens and lengthens the bladder base, which facilitates urine storage. Mirabegron reduces symptoms of urinary frequency and urgency with similar efficacy when compared to the muscarinic receptor antagonists. The main adverse effects are an increase in blood pressure and heart rate. It may be an option for those who fail to respond to muscarinic receptor antagonists, or who cannot tolerate them.
Other drugs
 Topical vaginal oestrogen-replacement therapy (Ch. 45) reverses atrophic changes in the lower genital tract in postmenopausal women and may be helpful for overactive bladder syndrome.
Topical vaginal oestrogen-replacement therapy (Ch. 45) reverses atrophic changes in the lower genital tract in postmenopausal women and may be helpful for overactive bladder syndrome.
 Desmopressin, a synthetic antidiuretic hormone (ADH) analogue (Ch. 43), is sometimes helpful to reduce nocturia in unstable bladder syndrome. It is taken orally; the nasal spray is no longer licensed for this indication because of the risk of water intoxication in children.
Desmopressin, a synthetic antidiuretic hormone (ADH) analogue (Ch. 43), is sometimes helpful to reduce nocturia in unstable bladder syndrome. It is taken orally; the nasal spray is no longer licensed for this indication because of the risk of water intoxication in children.
Hypotonic bladder
Hypotonic bladder is often a result of lower motor neuron lesions, or can arise from bladder distension following chronic urinary retention. Drugs with antimuscarinic properties (such as tricyclic antidepressants; Ch.22) and the specific antimuscarinic drugs above can make the symptoms worse. Hypotonic bladder leads to incomplete bladder emptying, with urinary retention and overflow incontinence. Treatment depends on the cause.
 Chronic urinary retention is often caused by bladder outlet obstruction. If renal function is impaired, it should be managed by bladder catheterisation and correction of the underlying cause.
Chronic urinary retention is often caused by bladder outlet obstruction. If renal function is impaired, it should be managed by bladder catheterisation and correction of the underlying cause.
 Neurogenic problems are sometimes treated with anticholinesterases (such as distigmine) which may increase the force of detrusor contraction (Ch. 4), although they are probably ineffective. Cholinergic drugs should not be used in the presence of urinary outflow obstruction.
Neurogenic problems are sometimes treated with anticholinesterases (such as distigmine) which may increase the force of detrusor contraction (Ch. 4), although they are probably ineffective. Cholinergic drugs should not be used in the presence of urinary outflow obstruction.
Urethral sphincter incompetence
Urethral sphincter incompetence produces stress incontinence in women (urine leakage with effort, exertion, sneezing or coughing) or sphincter weakness incontinence in men. The most common cause in women is loss of collagenous support in the pelvic floor or perineum; it also arises from trauma to the membranous urethra (sphincter mechanism), such as may occur from pelvic trauma or following prostatectomy in males. Drugs such as α1-adrenoceptor antagonists (see below) can make the symptoms worse. Pelvic floor muscle training may be helpful, while minimal access surgical sling procedures or colposuspension to provide urethral support are among the surgical options. Drug therapy is limited, and only recommended if surgical treatment is not suitable.
Duloxetine is a serotonin and noradrenaline reuptake inhibitor (SNRI; see Ch. 22). It is believed to augment sympathetic activity, which relaxes the detrusor, and to enhance external urethral sphincter activity by increasing efferent impulses in the motor neurons of the pudendal nerve when the bladder is placed under stress. It reduces the frequency of incontinence episodes significantly in about half of those treated.
Benign prostatic hypertrophy
Benign prostatic hypertrophy (BPH) produces symptoms in more than 25% of men above the age of 60 years, and up to 70% of men over the age of 70 years. The spectrum of symptoms is often called prostatism (Box 15.1). Left untreated, spontaneous improvement occurs or symptoms remain stable in up to half of all those with prostatism. Acute urinary retention occurs at a rate of 1–2% per year. Scoring systems can reliably quantify the extent to which symptoms affect the quality of life and therefore as a guide to treatment.
Drugs for prostatism
Alpha1-adrenoceptor antagonists
Selective α1-adrenoceptor antagonists inhibit contraction in prostatic and bladder neck smooth muscle, without affecting the detrusor. Relaxation of these muscles improves urine flow rate and symptoms of BPH. Tamsulosin is claimed to be a more selective antagonist in the smooth muscle of the urinary tract and may produce fewer peripheral vasodilatory unwanted effects compared with many other α1-adrenoceptor antagonists. This was assumed to be due to a selective action at the α1A-adrenoceptor subtype found in the prostate, but is more likely to be due to selective access to the target tissue. More information about α1-adrenoceptor antagonists is found in Chapter 6.
5α-Reductase inhibitors
Inhibition of 5α-reductase reduces the enzymatic conversion of testosterone to dihydrotestosterone (DHT) in prostatic cells, but does not affect circulating testosterone levels. DHT is involved in prostate growth, and inhibition of its production can reduce prostate volume by up to 30%. There are two isoenzymes of 5α-reductase, both found in the prostate. Finasteride only inhibits the type 2 isoenzyme, while dutasteride inhibits both the type 1 and 2 isoenzymes, but it is not yet known whether this confers any clinical advantage.
Pharmacokinetics: Both finasteride and dutasteride are well absorbed after oral administration and eliminated by hepatic metabolism. Finasteride has a half-life of about 6 h, whereas the half-life of dutasteride is extremely long at about 4 weeks.
Plant extracts
These products are available direct to consumers, but their composition varies among suppliers. Very limited trial evidence suggests that they produce modest short-term improvements in symptoms of prostatism, but a more rigorous study found no benefit for a preparation of saw palmetto extract compared to placebo. It is uncertain how they might work, but these extracts may reduce the synthesis of DHT or inhibit expression of prostatic growth factors. Plant extracts are well tolerated, with unwanted effects mainly confined to gastrointestinal upset.
Treatment of prostatism
Many symptomatic individuals do not require or want treatment, and a policy of ‘watchful waiting’ will be appropriate. The aim of drug treatment is either to reduce prostatic size or to relax the smooth muscle that restricts urine outflow. There is no evidence that medical treatment avoids the need for surgery in the long term.
Selective α1-adrenoceptor antagonists are the first-choice drugs for improving symptoms and urinary flow rates. Symptomatic improvement usually occurs within 1 month, and is seen in about two-thirds of those treated. 5α-Reductase inhibitors usually take 3–6 months to improve symptoms of prostatism, but the improvements are maintained. These drugs may be more effective with larger-volume prostates. Additional symptomatic benefit can be obtained from combining finasteride or dutasteride with an α1-adrenoceptor antagonist.
Surgical treatment is usually required for severe symptoms or complications of BPH (Box 15.2). Transurethral resection of the prostate improves symptoms in 70–90% of those with prostatism. Long-term sequelae include impotence (5–10%), retrograde ejaculation (80–90%) and incontinence (<5%). Several less invasive procedures are now available, but they may be less successful for relieving symptoms and do not reduce the risk of long-term consequences, although they produce fewer immediate postoperative complications.
True/false questions
1. Urinary bladder function is controlled by both voluntary and involuntary nervous pathways.
2. The antimuscarinic drug darifenacin causes urinary frequency and urge incontinence.
3. The antidepressant drug duloxetine can be used to treat stress incontinence.
4. The anticholinesterase distigmine can be given safely if there is urinary outflow obstruction.
5. Blockade of α1-adrenoceptors in the bladder neck smooth muscle improves urine flow rates.
6. Finasteride reduces prostate volume by blocking testosterone receptors.
Extended-matching-item questions
Choose the most appropriate pharmacological option A–F for each case scenarios 1–3 below.
1. A 50-year-old man with a 2-year history of difficulty in urinating and hesitancy was diagnosed with benign prostatic hypertrophy (BPH) and an enlarged prostate. He was given a 1-month trial of an α1-adrenoceptor antagonist, which did not improve his symptoms. He did not at this stage want to undergo surgery. What pharmacological treatment might be of benefit?
2. A 30-year-old woman with normal bladder function complained of difficulty in urination after being prescribed new medication for her depression. She was found to have urinary retention. What class of antidepressant could cause this effect?
3. A 60-year-old woman had severe urge incontinence. She urinated 16–20 times a day and had leakage two to three times a day and at night. What treatment could she be given?
Case-based question
A 65-year-old man developed progressive urinary problems over a 5-year period. He had difficulty passing urine and was getting up three times in the night to pass urine. A rectal examination by his GP showed an enlarged prostate. Ultrasound, flow tests and prostate-specific antigen measurements suggested BPH.
1. True. Bladder function is controlled by involuntary parasympathetic and sympathetic innervation of the detrusor and sphincter muscles and by voluntary control via the somatic nervous system.
2. False. Darifenacin blocks muscarinic receptors with some selectivity for the M3 subtype, inhibiting the parasympathetic effects on the detrusor muscle. It is used for treatment of overactive bladder.
3. True. Duloxetine inhibits the reuptake of serotonin and noradrenaline and increases the contractility of the urethral sphincters.
4. False. The anticholinesterase distigmine increases detrusor muscle contractility, which is undesirable in the presence of urinary outflow obstruction.
5. True. Selective antagonism of the α1A-adrenoceptor subtype in bladder smooth muscle may reduce unwanted vasodilator effects.
6. False. Finasteride and dutasteride block the conversion of testosterone to dihydrotestosterone by inhibiting 5α-reductase in the prostate.
Extended-matching-item answers
1. Answer B. Finasteride inhibits the conversion of testosterone to dihydrotestosterone, which is a promoter of prostatic cell growth. A reduction of up to 30% in prostate size can be obtained. Symptomatic benefit may be increased if finasteride and an α1-adrenoceptor antagonist are given together
2. Answer D. The tricyclic antidepressant amitriptyline is also an antagonist at muscarinic receptors and may inhibit the micturition reflex.
3. Answer C. Duloxetine could be tried if the condition were caused by sphincter incompetence. Duloxetine increases the levels of noradrenaline and serotonin in the synapse and the activity of the motor neurons in the pudendal nerve. Pelvic floor exercises should also be suggested as drug therapy is of limited benefit.
Case-based answers
A Drugs may be used in mild disease and while awaiting a transurethral resection of the prostate. Selective α1-adrenoceptor antagonists increase urine flow to a limited extent but also decrease urgency, frequency and hesitancy. The 5α-reductase inhibitor finasteride slowly reduces prostate size.
B Alpha1-adrenoceptor antagonists can cause postural hypotension, especially with the first dose. They cause dizziness and can interact with other drugs to lower blood pressure. Finasteride can reduce libido and cause impotence.
C The outcome is variable; symptoms may not worsen appreciably for many years, but moderate symptoms can lead to a poor quality of life. Complications include urinary retention, incontinence and renal insufficiency owing to hydronephrosis.
Barendrecht, MM, Oelke, M, Laguna, MP, et al. Is the use of parasympathetics for treating an underactive urinary bladder evidence-based? BJU Int. 2007;99:749–752.
Connolly, SS, Fitzpatrick, JM. Medical treatment of benign prostatic hyperplasia. Postgrad Med J. 2007;83:73–78.
Foley, CL, Kirby, RS. 5-Alpha-reductase inhibitors: what's new? Curr Opin Urol. 2003;13:31–37.
Gerber, GS. Phytotherapy for benign prostatic hyperplasia. Curr Urol Rep. 2002;3:285–291.
Hashim, H, Abrams, P. Pharmacological management of women with mixed incontinence. Drugs. 2006;66:591–606.
Marinkovic, SP, Rovner, ES, Moldwin, RM, et al. The management of overactive bladder syndrome. BMJ. 2012;344:e2365.
Norton, P, Brubaker, L. Urinary incontinence in women. Lancet. 2006;367:57–67.
Nygaard, I. Idiopathic urgency urinary incontinence. N Engl J Med. 2010;363:1156–1162.
Robinson, D, Cardozo, L. Antimuscarinic drugs to treat overactive bladder. BMJ. 2012;344:e2130.
Rogers, RG. Urinary stress incontinence in women. N Engl J Med. 2008;358:1029–1036.
Shamliyan, TA, Kane, RL, Wyman, J, et al. Randomised, controlled trials of non-surgical treatments for urinary incontinence in women. Ann Intern Med. 2008;148:459–473.
Thorpe, A, Neal, D. Benign prostatic hyperplasia. Lancet. 2003;361:1359–1367.
Wilt, TJ, Dow, JN. Benign prostatic hyperplasia. Part 2 – Management. BMJ. 2008;336:206–210.