Anaemia and haematopoietic colony-stimulating factors
Anaemia
The definition of anaemia is rather arbitrary and the absolute normal ranges for haemoglobin concentration in blood vary among laboratories. In adults, anaemia equates to a blood concentration of haemoglobin in males below about 130 g⋅L−1 (normal is about 130–170 g⋅L−1) or in non-pregnant females below about 120 g⋅L−1 (normal is about 120–150 g⋅L−1). Many individuals, however, have concentrations below these ranges without apparent detriment. Lower concentrations can be normal in children and during pregnancy. Anaemia can cause many symptoms, including shortness of breath and fatigue. There are many causes of anaemia (Box 47.1), the forms of which are classified by red cell size and haemoglobin content (Box 47.2).
There are three key dietary factors that are required for normal red cell synthesis, referred to as haematinics:
Iron
Dietary iron is absorbed from the duodenum and upper jejunum. In an omnivorous diet most iron is absorbed from meat, in which it is present as haem. Haem is the ferrous form of iron (Fe2+) complexed with a porphyrin ring. Haem is readily absorbed from the gut, but non-haem iron in a vegetarian diet, which is mainly in the ferric state (Fe3+), is inefficiently absorbed. Absorption of ferric iron is facilitated by several factors:
 gastric acid, which increases its solubility,
gastric acid, which increases its solubility,
 conversion to ferrous iron by ferric reductase on the brush border of enterocytes, which is enhanced by dietary reducing agents such as ascorbic acid, fructose and some amino acids,
conversion to ferrous iron by ferric reductase on the brush border of enterocytes, which is enhanced by dietary reducing agents such as ascorbic acid, fructose and some amino acids,
 intestinal absorption mediated by the divalent metal transporter (DMT-1), mainly in the duodenum. Expression of DMT-1 is increased in iron deficiency and in hereditary haemochromatosis.
intestinal absorption mediated by the divalent metal transporter (DMT-1), mainly in the duodenum. Expression of DMT-1 is increased in iron deficiency and in hereditary haemochromatosis.
Within enterocytes, iron is oxidized to the ferric state and transported to the circulation by the protein ferroportin. In the blood, ferric iron is bound to the globulin transferrin and transported to the bone marrow and iron stores. Cellular iron uptake occurs via transferrin receptors, and in most cells iron is stored as ferritin (a complex of iron with the apoferritin protein). In some tissues iron is also found as relatively insoluble aggregates of degraded forms of ferritin, known as haemosiderin. Two-thirds of the iron in the body is present in circulating red cells, and about half of the remainder is found in macrophages, reticuloendothelial cells and hepatocytes. The rest is present in myoglobin in muscle cells or associated with various intracellular enzymes.
When ageing red cells are broken down by the reticuloendothelial system, most of the released iron is recycled via macrophages for further erythropoiesis. Iron loss from the body is normally low, and occurs through shedding of mucosal cells containing ferritin; there is negligible renal loss of iron.
Iron deficiency
The main cause of iron deficiency in the UK is abnormal loss of blood, particularly from the gut or from exaggerated menstrual loss. Iron malabsorption can result from disease of the upper small intestine, for example coeliac disease, or following partial gastrectomy. Dietary deficiency is rarely a major cause in Western societies, although worldwide a vegetarian diet low in absorbable forms of iron is the commonest contributory cause of iron deficiency.
Therapeutic iron preparations
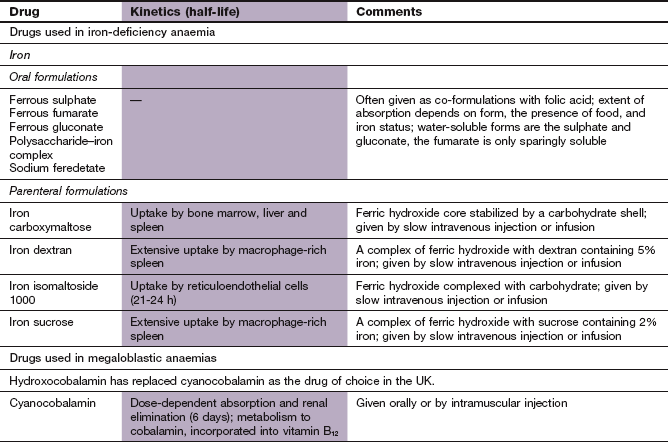
Oral iron: Oral iron supplements are preferred and are given as ferrous salts; for example, ferrous sulphate, fumarate or gluconate. Tablets are normally used, but some people find that a syrup is more palatable. In the presence of iron deficiency, a daily oral dose equivalent to 100–200 mg of elemental iron produces the maximum rate of rise of haemoglobin (200 mg ferrous sulphate contains 65 mg iron). About one-third of this dose will be absorbed. Some oral iron preparations contain vitamin C, but the therapeutic advantage is minimal compared to the ferrous salt alone.
 Gastrointestinal intolerance is common, especially nausea and dyspepsia. The prevalence of these effects depends both on the dose of elemental iron and on psychological factors, rather than on the iron salt used. They can be minimised by taking iron supplements with food or by reducing the dose. Modified-release iron formulations have been developed to improve tolerability, but much of the iron is released beyond the duodenum, the site where it is best absorbed. These formulations should only be used when other methods for improving iron tolerance are ineffective. Diarrhoea or constipation also occur, but are not dose-related.
Gastrointestinal intolerance is common, especially nausea and dyspepsia. The prevalence of these effects depends both on the dose of elemental iron and on psychological factors, rather than on the iron salt used. They can be minimised by taking iron supplements with food or by reducing the dose. Modified-release iron formulations have been developed to improve tolerability, but much of the iron is released beyond the duodenum, the site where it is best absorbed. These formulations should only be used when other methods for improving iron tolerance are ineffective. Diarrhoea or constipation also occur, but are not dose-related.
Parenteral iron: Iron can be given by slow intravenous injection or infusion, or less commonly by deep intramuscular injection. Formulations involve complexing ferric hydroxide to a carrier to form iron sucrose, iron dextran (the only formulation for intramuscular use), ferric carboxymaltose or iron isomaltoside 1000. The iron in these formulations is not bound to transferrin in plasma but accumulates in reticuloendothelial cells. When calculating the amount of iron to give, the approximate total body iron deficit (haemoglobin and body stores) is estimated from the person's size and haemoglobin concentration.
Therapeutic use of iron
The cause of iron deficiency should always be sought when starting symptomatic treatment with iron. If this is not done, then serious disorders such as gastrointestinal malignancy can be overlooked. Oral iron supplements are adequate for most mild or moderate iron-deficiency anaemias. After an initial delay of a few days while new red cells are formed, oral iron supplements should raise the blood haemoglobin concentration by about 20 g⋅L−1 over the first 3–4 weeks, and about 10 g⋅L−1 per week thereafter. Oral iron supplements should be continued for 3 months after the haemoglobin concentration has been restored, in order to replenish tissue iron stores.
Failure to respond to oral iron can be caused by several factors:
 incorrect diagnosis, for example anaemia of chronic disorder, thalassaemia,
incorrect diagnosis, for example anaemia of chronic disorder, thalassaemia,
 poor adherence to oral iron therapy,
poor adherence to oral iron therapy,
 inadequate iron dosage, for example in some modified-release formulations,
inadequate iron dosage, for example in some modified-release formulations,
 continuing excessive blood loss,
continuing excessive blood loss,
 concurrent deficiency of other substances necessary for haemoglobin synthesis.
concurrent deficiency of other substances necessary for haemoglobin synthesis.
Parenteral iron preparations are used if there are intractable unwanted effects from oral preparations, if there is severe uncorrectable malabsorption or continuing heavy blood loss and when adherence to oral treatment is poor. Parenteral iron does not raise the haemoglobin concentration any faster than oral iron, except during haemodialysis for severe renal failure.
Oral iron supplements are occasionally given for prophylaxis against iron deficiency at times of high demand for iron, for example pregnancy, menorrhagia or if there is a poor dietary intake. The reduced iron absorption after subtotal or total gastrectomy can also be overcome by long-term iron supplements.
Folic acid
Folate is required for a number of cellular biochemical processes, including DNA synthesis, and is essential for cell replication, including the formation of red cells. Folic acid (pteroylglutamic acid) is ingested as conjugated folate polyglutamates, found mainly in fresh leaf vegetables (in which it is heat-labile) and in liver (where it is more heat-stable). Before absorption, the polyglutamates are deconjugated to the monoglutamate. Folate monoglutamate is absorbed principally in the duodenum and jejunum, and is methylated and reduced to 5-methyltetrahydrofolate by dihydrofolate reductase during absorption. Methyltetrahydrofolate enters cells, where it is demethylated and converted back to folate polyglutamates. These are coenzymes in the synthesis of pyrimidines and purines and hence of DNA (see also Ch. 52).
Folate deficiency
The most obvious consequence of folate deficiency is a macrocytic anaemia with the presence of megaloblasts in the marrow, a feature it shares with vitamin B12 deficiency. Folate deficiency can arise for a number of reasons (Box 47.3). Unlike iron, folate cannot be recycled from old red cells that are removed from the circulation.
Therapeutic use of folic acid
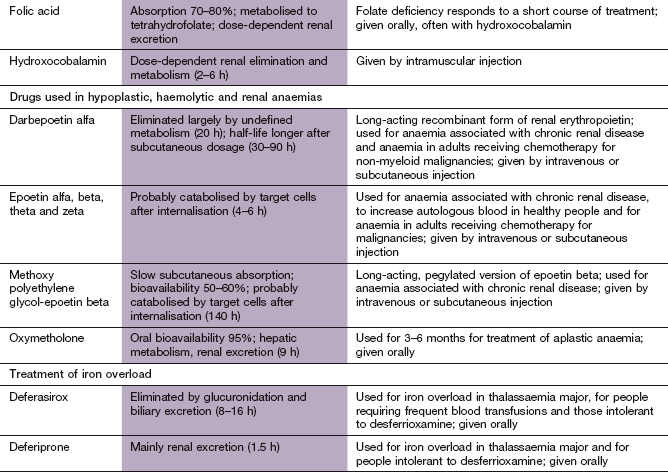
Folate deficiency almost always responds to oral folic acid supplements. Folic acid is a poor substrate for dihydrofolate reductase, and is largely absorbed unchanged and then converted to tetrahydrofolic acid in the plasma and liver. Most causes of folate deficiency are self-limiting, and folic acid treatment is usually given for 4 months to correct the anaemia and replace folate stores.
Folic acid is given prophylactically in pregnancy. It is given in higher doses if there is an increased risk of conceiving a child with a neural tube defect. Those at higher risk include a partner with a neural tube defect, history of neural tube defect in a previous pregnancy, or if the woman has coeliac disease, diabetes mellitus, sickle-cell anaemia or is taking antiepileptic drugs (Ch. 23). Folic acid is also given prophylactically to premature infants, during renal dialysis, and for chronic haemolytic anaemia.
Treatment of deficiencies of both vitamin B12 and folate using only folic acid may correct the anaemia, but irreversible neurological damage can be precipitated (see below). Therefore, vitamin B12 deficiency must be excluded before folic acid is used, or both vitamin B12 and folic acid should be given if there is a possibility of vitamin B12 deficiency.
For folate deficiency produced by drugs that inhibit dihydrofolate reductase (e.g. methotrexate; Ch. 52 and Fig. 51.4) it is necessary to ‘bypass’ this enzyme blockade by giving the synthetic tetrahydrofolic acid, folinic acid (5-formyl tetrahydrofolic acid). This is the basis of ‘folinic acid rescue’ to reduce the toxic effects on healthy tissues of high-dose methotrexate used for treatment of malignancy (Ch. 52). Folinic acid is formulated as a salt and given orally, usually as calcium folinate. When low-dose methotrexate is used in a once-a-week regimen for immunosuppression folic acid can be given on a separate day to reduce toxicity.
Vitamin B12
Vitamin B12 has many roles in the body, including participation in DNA synthesis and fatty acid synthesis. The term vitamin B12 refers to a group of cobalt-containing compounds, also known as cobalamins. Bacteria are the only organisms that can synthesise cobalamins de novo. Humans obtain vitamin B12 from meat (particularly liver), from animal products (milk, cheese, eggs, etc.) or from vegetables contaminated by bacteria. Absorption is by an unusual mechanism: dietary vitamin B12 binds in the stomach to a glycoprotein called intrinsic factor that is produced by gastric parietal cells. This complex is absorbed principally from the terminal ileum after binding to receptors on the luminal membranes of ileal cells.
Most vitamin B12 in plasma is bound to a glycoprotein, transcobalamin I, from which it is rapidly taken up by the tissues, especially the liver, which stores about 50% of the body content of vitamin B12. A second protein, transcobalamin II, is mainly responsible for rapid transport of vitamin B12 to tissues, and for enhancing its uptake by the bone marrow via specific receptors. Vitamin B12 is essential as a coenzyme in nucleic acid synthesis, and in other metabolic pathways in conjunction with folate. Many functions of vitamin B12 can be performed by folic acid, but there are two enzyme families that only vitamin B12 can facilitate. These are responsible for isomerisation of methylmalonyl coenzyme A to succinyl coenzyme A, isomerisation of α-leucine to β-leucine, and methylation of homocysteine to methionine (a reaction that results in demethylation of methyltetrahydrofolate).
Vitamin B12 deficiency
Impairment of vitamin B12-dependent enzyme reactions affects DNA synthesis. The major organs affected by vitamin B12 deficiency are those with a rapid cell turnover, particularly the bone marrow and the gastrointestinal tract.
Vitamin B12 deficiency presents with a macrocytic anaemia and a megaloblastic bone marrow. The tongue becomes smooth, and changes to the lining of the small bowel can lead to malabsorption. Damage to the posterior and lateral neuronal tracts in the spinal cord can also occur, leading to a condition known as subacute combined degeneration of the cord. The biochemical basis for the neurological damage is poorly understood, and it may not be fully reversible after correction of vitamin B12 deficiency.
Causes of vitamin B12 deficiency include:
 diet: strict vegetarians (vegans) only,
diet: strict vegetarians (vegans) only,
 intestinal malabsorption due to damage to the terminal ileum; for example, Crohn's disease, lymphoma,
intestinal malabsorption due to damage to the terminal ileum; for example, Crohn's disease, lymphoma,
 deficiency of intrinsic factor: pernicious anaemia (destruction of gastric parietal cells with achlorhydria and failure of intrinsic factor production), total and subtotal gastrectomy.
deficiency of intrinsic factor: pernicious anaemia (destruction of gastric parietal cells with achlorhydria and failure of intrinsic factor production), total and subtotal gastrectomy.
Therapeutic use of vitamin B12
Most people with vitamin B12 deficiency have problems absorbing it from the gut, and treatment is usually by intramuscular injection of vitamin B12 in aqueous solution. Hydroxocobalamin, the form of vitamin B12 produced by bacteria, is used for treatment of deficiency. Following initial injections on alternate days for 2 weeks to replenish stores, maintenance injections every 3 months for life are adequate. In the rare dietary causes of vitamin B12 deficiency, oral cyanocobalamin supplements can be given, but otherwise oral treatment is never indicated.
Erythropoietin
Erythropoietin is a glycosylated protein hormone produced mainly by the kidney. It regulates red cell production by reducing apoptosis and stimulating differentiation and proliferation of erythroid progenitor cells. Erythropoetin binds to its receptor, which is found in high concentration on erythroid precursors, and enables the receptor to activate several intracellular signalling pathways. Deficiency of erythropoietin in end-stage renal disease contributes to the anaemia that characterises this disorder. Interestingly, the hormone has also been found to have a protective effect on ischaemic neurons in the brain. Human erythropoietin has been synthesised using recombinant DNA technology (epoetin): it is produced in four forms – alfa, beta, theta and zeta – which have similar clinical effects. Erythropoietin is also available as two longer-acting derivatives: a hyperglycosylated derivative, darbepoetin alfa, and methoxy polyethylene glycol-epoetin beta.
Pharmacokinetics
Epoetin can be given intravenously or, more conveniently, subcutaneously, when a 25–50% lower dose can be used. The red cell response is more rapid after intravenous use, but ultimately greater after subcutaneous injection. Epoetin has a half-life of about 4–6 h, and is normally given two or three times a week. Darbepoetin has a longer half-life and is given once a week, and methoxy polyethylene glycol-epoetin beta is given every 2–4 weeks. The route of elimination of epoetin is uncertain, but may be largely by receptor-mediated uptake in the bone marrow and subsequent intracellular degradation.
Unwanted effects
 Influenza-like symptoms early in treatment.
Influenza-like symptoms early in treatment.
 Hypertension, which is dose-dependent and can be severe, leading to encephalopathy with seizures.
Hypertension, which is dose-dependent and can be severe, leading to encephalopathy with seizures.
 Thrombosis of arteriovenous shunts.
Thrombosis of arteriovenous shunts.
 Pure red cell aplasia (not affecting white cells or platelets) occurs rarely during subcutaneous administration in renal failure; this is usually associated with formation of antibodies to epoetin, and treatment must be discontinued if this occurs.
Pure red cell aplasia (not affecting white cells or platelets) occurs rarely during subcutaneous administration in renal failure; this is usually associated with formation of antibodies to epoetin, and treatment must be discontinued if this occurs.
Therapeutic uses of epoetin
 Anaemia of end-stage renal disease. Other causes of anaemia should be excluded. Adequate iron stores are essential, since erythropoiesis demands large amounts of iron, and iron supplements (often intravenously) may be needed to maximise the response. Anaemia can be corrected in more than 90% of those treated, and treatment improves quality of life. Epoetin also modulates lipid metabolism, creating a less atherogenic plasma lipid profile, which may reduce the high cardiovascular mortality in renal failure. However, recent evidence suggests that cardiovascular mortality and morbidity may be increased if the haemoglobin concentration is raised above 120 g⋅L−1.
Anaemia of end-stage renal disease. Other causes of anaemia should be excluded. Adequate iron stores are essential, since erythropoiesis demands large amounts of iron, and iron supplements (often intravenously) may be needed to maximise the response. Anaemia can be corrected in more than 90% of those treated, and treatment improves quality of life. Epoetin also modulates lipid metabolism, creating a less atherogenic plasma lipid profile, which may reduce the high cardiovascular mortality in renal failure. However, recent evidence suggests that cardiovascular mortality and morbidity may be increased if the haemoglobin concentration is raised above 120 g⋅L−1.
 To increase red cell production prior to surgery. Autologous blood transfusion is becoming more popular to reduce the use of banked blood. Epoetin given twice weekly for 3 weeks before surgery can increase the number of units of blood that can be obtained.
To increase red cell production prior to surgery. Autologous blood transfusion is becoming more popular to reduce the use of banked blood. Epoetin given twice weekly for 3 weeks before surgery can increase the number of units of blood that can be obtained.
 Anaemia associated with human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS).
Anaemia associated with human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS).
 Anaemia associated with cytotoxic chemotherapy of non-myeloid malignant disease (Ch. 52).
Anaemia associated with cytotoxic chemotherapy of non-myeloid malignant disease (Ch. 52).
 Epoetin is sometimes abused by athletes to increase haematocrit and improve performance. This abuse is associated with an increased risk of arterial and venous thromboses.
Epoetin is sometimes abused by athletes to increase haematocrit and improve performance. This abuse is associated with an increased risk of arterial and venous thromboses.
Drug treatment in other anaemias
Certain other anaemias require specific drug therapy.
Aplastic anaemia
Failure of haematopoietic stem cell production has many causes, including certain drugs (Box 47.4). Drugs do not have a major role in treatment of aplastic anaemia. The anabolic steroid oxymetholone (Ch. 46; available in the UK only on a named-patient basis) is sometimes used, but its effectiveness is unpredictable. Antilymphocyte globulin is helpful in some acquired aplastic anaemias, and is sometimes used in combination with ciclosporin (Ch. 38).
Sideroblastic anaemia
This can also be caused by drugs (Box 47.5). It is characterised by accumulation of iron in the mitochondria of erythroblasts, which lie in a ring around the nucleus. Staining for iron reveals the characteristic ring sideroblasts. Pyridoxine supplements can increase the haemoglobin concentration in idiopathic acquired and hereditary forms of the disorder. They can also be useful for reversible sideroblastic anaemia associated with pregnancy, haemolysis, alcohol dependence or during treatment with the antituberculous drug isoniazid (Ch. 51).
Beta-thalassaemia major
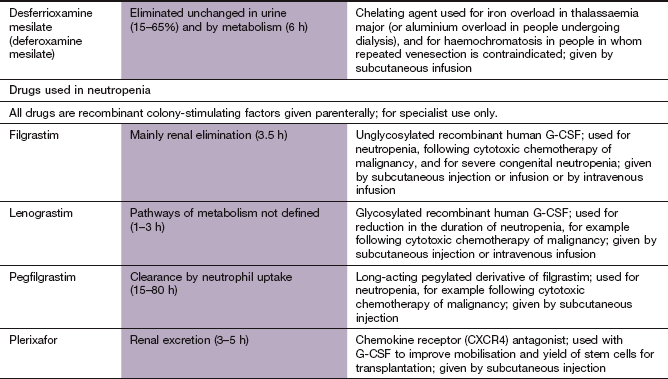
This is a genetic disorder of haemoglobin synthesis with a hyperplastic bone marrow and refractory anaemia. Blood transfusions or excessive iron supplements lead to iron overload, with damage to the liver, heart and pancreas. Iron overload can be prevented with infusions of desferrioxamine mesilate (Ch. 53) together with vitamin C, which enhances iron excretion. The oral iron chelators, deferiprone or deferasirox, are used when desferrioxamine is poorly tolerated or contraindicated.
Sickle cell anaemia
This inherited disorder occurs when more than 80% of the haemoglobin is HbS; fetal haemoglobin (HbF) forms the remainder. Hydroxycarbamide (see Ch. 52) reduces the frequency and severity of sickle cell crises. It raises the HbF concentration and also reduces the number of young red cells, which are those most likely to adhere to endothelium and occlude blood vessels.
Drugs as a cause of anaemia
 Iron deficiency: especially drugs causing bleeding from the upper gut, for example non-steroidal anti-inflammatory drugs (NSAIDs).
Iron deficiency: especially drugs causing bleeding from the upper gut, for example non-steroidal anti-inflammatory drugs (NSAIDs).
 Aplastic anaemia: see Box 47.4.
Aplastic anaemia: see Box 47.4.
 Sideroblastic anaemia: see Box 47.5.
Sideroblastic anaemia: see Box 47.5.
 Haemolysis in glucose-6-phosphate dehydrogenase (G6PD) deficiency: see Box 47.6. G6PD is involved in generating reduced glutathione, which protects red cells against oxidative stresses. Oxidant drugs produce haemolysis in GP6D-deficient individuals, who are usually male (Ch. 53).
Haemolysis in glucose-6-phosphate dehydrogenase (G6PD) deficiency: see Box 47.6. G6PD is involved in generating reduced glutathione, which protects red cells against oxidative stresses. Oxidant drugs produce haemolysis in GP6D-deficient individuals, who are usually male (Ch. 53).
Neutropenia
Leucocytes are part of the first line of defence against pathogens. They include phagocytic cells (neutrophils, monocytes and eosinophils) and non-phagocytic cells (lymphocytes and basophils). In addition to their role in acute inflammation, all these cells participate in regulation of cellular and humoral immunity through the production of cytokines (Ch. 38). A reduction in the number of circulating neutrophils (neutropenia) in particular increases the risk of serious infection. There are several causes of neutropenia (Box 47.7). Neutropenia does not give rise to symptoms, but predisposes to infection, especially if the neutrophil count falls below 0.5×109 L−1.
Drugs for neutropenia
Granulocyte colony-stimulating factors
Granulocyte colony-stimulating factors are produced by many cells, such as endothelial cells, monocytes and fibroblasts, and stimulate the maturation of pluripotent stem cells in the bone marrow. Granulocyte colony-stimulating factor (G-CSF) is produced by recombinant DNA technology. Therapeutic agents include:
G-CSF is glycosylated in its natural state, but this does not seem to be a prerequisite for effectiveness. A transient fall in circulating neutrophils occurs within minutes of the injection, followed a few hours later by a substantial rise.
Pharmacokinetics: Granulocyte colony-stimulating factors are given by prolonged intravenous infusion or subcutaneous infusion or injection. Daily injections of filgrastim or lenograstim are given until there is an adequate neutrophil response. Pegfilgrastim has a longer duration of action than filgrastim, and is only given once after chemotherapy. Filgrastim and lenograstim are eliminated both by the kidney and by neutrophil uptake. Pegfilgrastim is not eliminated by the kidney, and has a prolonged effect in neutropenia, since few neutrophils are available to contribute to its elimination.
Therapeutic use of colony-stimulating factors
The use of these drugs remains controversial in many indications.
Congenital neutropenia
Survival is prolonged by G-CSF which reduces life-threatening infection, but 10% of people develop acute myeloid leukaemia as a result of treatment.
Chemotherapy-induced neutropenia
The duration of neutropenia may be reduced, with a limitation of associated sepsis. However, with many chemotherapy regimens there is no evidence that long-term survival is improved by G-CSF, and with some regimens the risk of acute myeloid leukaemia may be increased. G-CSF treatment is therefore reserved for those regimens that have greater than 20% historical risk of febrile neutropenia. It is also used when chemotherapy has previously been associated with a febrile neutropenic episode and the drug dosage cannot be reduced for subsequent courses.
Mobilisation of progenitor cells into peripheral blood for harvesting prior to bone marrow transplantation
The white blood cell count rises 7–12 days after treatment and is accompanied by an increase in haematopoietic stem cells, which are collected via a cell-separation machine. G-CSF use can be followed by the chemokine receptor antagonist plerixafor, which mobilises haematopoietic stem cells into peripheral blood.
True/false questions
1. Dietary iron is transported in the blood mostly bound to ferritin.
2. Pernicious anaemia is caused by reduced vitamin B12 absorption.
3. In vitamin B12 deficiency treatment is rarely required for more than 3 months.
4. The blood film in pernicious anaemia shows microcytosis.
5. Both vitamin B12 and folate are essential for DNA synthesis.
6. Folic acid cannot be given orally.
7. Phenytoin can cause folate deficiency.
8. Erythropoietin reduces apoptosis of red blood cell progenitors.
One-best-answer (OBA) questions
1. Identify the correct statement below concerning the properties of erythropoietin.
A Erythropoietin is mainly synthesised by the adrenal glands.
B Erythropoietin can correct anaemia in end-stage renal disease.
C Erythropoietin is an effective anaemia treatment even if iron levels are low.
D Erythropoietin impairs athletic performance by increasing hamatocrit.
2. Identify the incorrect statement below concerning the usage and properties of folic acid and its metabolites.
A Tetrahydrofolate is involved in the synthesis of the nucleotide bases in DNA.
B Folic acid is often given with hydroxocobalamin.
C Folate is absorbed in the stomach.
D Tetrahydrofolic acid is given rather than folic acid to correct the folate deficiency caused by methotrexate.
E Folic acid in pregnancy reduces the risk of neural tube defects.
Case-based questions
A 40-year-old woman complained to her GP of fatigue and heavy menstrual periods lasting 7 days and occurring every 28 days. Her GP noted that she was pallid; her haemoglobin level was 67 g⋅L−1 and mean cell volume (MCV) was 61 fL (normal 76–96). Other blood measurements of platelets and white cell counts were unremarkable.
A How would you interpret these data and what were the possible reasons?
B What biochemical tests could have helped the diagnosis?
C The tests confirmed iron-deficiency anaemia.
What pharmaceutical preparation should have been given?
D Several iron formulations were tried, as the woman felt unwell taking ferrous sulphate.
What unwanted effects might she have experienced?
E Where was the iron absorbed?
F After 2 months of oral iron therapy, the haemoglobin value was 80 g⋅L−1.
Was this a sufficient response?
G The woman was intolerant of oral iron.
With the new treatment regimen her haemoglobin rose to 115 g⋅L−1 over 2–3 weeks.
1. False. Iron is transported in the blood bound to transferrin and stored in tissues as ferritin and haemosiderin.
2. True. Autoimmune loss of gastric parietal cells reduces production of intrinsic factor, which is needed for vitamin B12 absorption in the distal ileum.
3. False. In pernicious anaemia vitamin B12 is given (as hydroxocobalamin) by intramuscular injection every 2–3 months for life.
4. False. Macrocytes (enlarged red cells) are found in the blood in pernicious anaemia.
5. True. Folate is necessary for synthesis of purines and pyrimidines, and vitamin B12 is a cofactor in their synthesis.
6. False. Folic acid is given orally each day for up to 4 months to replenish stores.
7. True. Phenytoin and a number of other drugs interfere with folate metabolism.
8. True. Erythropoietin increases survival of erythroid progenitor cells in the bone marrow.
9. False. Filgrastim is a recombinant form of granulocyte colony-stimulating factor (G-CSF), which promotes formation of neutrophils and other granulocytes in the bone marrow.
10. True. Plerixafor is an antagonist of the CXCR4 chemokine receptor and is used with a recombinant G-CSF to mobilise stem cells for harvesting.
OBA answers
A Incorrect. The kidneys are the main site of erythropoietin production.
B Correct. Anaemia due to renal disease is commonly treated with erythropoietin.
C Incorrect. Adequate iron stores are necessary for erythropoietin to be successful.
D Incorrect. Erythropoietin may enhance performance by increasing haematocrit, but with an increased risk of thrombosis.
E Incorrect. It is a glycoprotein given by intravenous or subcutaneous routes.
2. Answer C is the incorrect statement.
A Correct. Tetrahydrofolate is a folic acid metabolite utilised in the synthesis of the purine and pyrimidine bases in DNA.
B Correct. Neurological damage can be caused if folic acid is given alone when both folate and vitamin B12 are deficient.
C Incorrect. Folate is absorbed in the proximal jejunum, and absorption is deficient in coeliac disease.
D Correct. Methotrexate inhibits the synthesis of tetrahydrofolate by dihydrofolate reductase. Synthetic tetrahydrofolate (folinic acid) bypasses this block.
E Correct. Folic acid is given prophylactically in pregnancy, and in higher amounts if there is a history of a neural tube defect in a previous pregnancy.
Case-based answers
A The haemoglobin concentration (67 g⋅L−1) is below normal for a non-pregnant woman (115 g⋅L−1), indicating anaemia. The MCV (61 fL) is also low. A common cause of low MCV is iron-deficiency anaemia, which is common in menstruating women. Another cause is gastrointestinal bleeding, including haemorrhoids.
B Serum ferritin would be low and total iron-binding capacity elevated.
C Oral ferrous salts (e.g. ferrous sulphate, the form most easily absorbed).
D Gastrointestinal distension and loose bowel movements are common.
E Iron is absorbed from the duodenum and upper jejunum.
F The rise in haemoglobin was insufficient: it should be about 10 g⋅L−1 each week.
G Poor response could be due to poor adherence to treatment, continued bleeding or malabsorption.
H Alternative treatment could have been slow intravenous injection or infusion of iron sucrose or iron dextran.
Cappellini, MD, Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. 2008;371:64–74.
Crawford, J, Dale, DC, Lyman, GH. Chemotherapy-induced neutropenia: risks, consequences and new directions for its management. Cancer. 2004;100:228–237.
Frewin, R, Henson, A, Provan, D. Iron deficiency anaemia. BMJ. 1997;314:360–363.
Henry, DH, Bowers, P, Romano, MT, et al. Epoetin alpha. Clinical evolution of a pleiotropic cytokine. Arch Intern Med. 2004;164:262–276.
Hoffbrand, V, Provan, D. Macrocytic anaemias. BMJ. 1997;314:430–433.
Hubell, K, Engert, A. Clinical applications of granulocyte colony-stimulating factor: an update and summary. Ann Haematol. 2003;82:207–213.
Kaushansky, K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–2046.
Lyman, GH, Shayne, M. Granulocyte colony-stimulating factors: finding the right indication. Curr Opin Oncol. 2007;19:299–307.
Macdougall, IC, Eckardt, K-U. Novel strategies for stimulating erythropoiesis and potential new treatments for anaemia. Lancet. 2006;368:947–953.
Provan, D, Weatherall, D. Red cells II: acquired anaemias and polycythaemias. Lancet. 2000;355:1260–1268.
Umbreit, J. Iron deficiency: a concise review. Am J Haematol. 2005;78:225–231.
Weatherall, D, Provan, D. Red cells I: inherited anaemias. Lancet. 2000;355:1169–1175.