Respiratory system
Introduction
Respiration is a term used to describe two different but interrelated processes: cellular respiration and mechanical respiration. Cellular respiration is the series of intracellular biochemical processes by which the cell produces energy by metabolism of organic molecules (see Ch. 1). This chapter is concerned with mechanical respiration, which involves the following steps:
• Air is drawn into the body (to the lungs) from the atmosphere by inhalation.
• Before it reaches the furthest parts of the lungs, the air is cleaned by removal of particulate matter, warmed so that its temperature equals that of the body and is also moistened.
• In the lung parenchyma, oxygen is extracted from the air and transferred into the blood vascular system where it bonds tightly with haemoglobin in the red cells for transport in the systemic arterial circulation.
• At the same time that oxygen is passing from air into the blood, carbon dioxide (a by-product of cellular metabolic activity) is transferred from the blood to the air.
• After gaseous exchange, the air is returned to the atmosphere by exhalation.
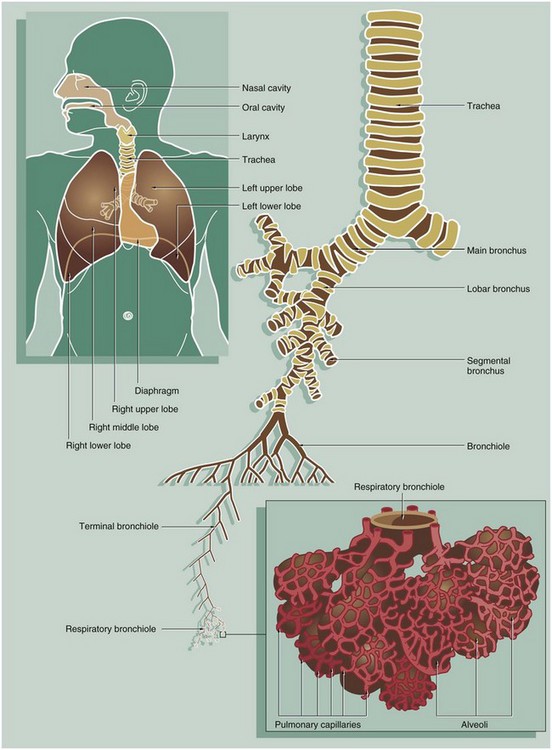
Inhalation and exhalation are achieved by expanding and contracting the thoracic cavity using the intercostal muscles and the diaphragm, drawing air in when the thoracic cavity expands and driving air out when it contracts. The respiratory system has two main functional elements: a conducting/cleaning system and a gaseous interchange mechanism. The conducting system begins as a system of cavities (nasal cavity, paranasal sinuses and nasopharynx) which begin the cleansing, warming and moistening of air drawn in through the anterior nares (nostrils). These cavities are lined by respiratory epithelium with two cell types, one of which secretes mucus which traps particulate matter, whilst the other bears surface cilia which move the thin layer of mucus. Abundant blood vessels beneath the epithelium warm the air and seromucous glands in the submucosa secrete both mucus and a watery fluid which moistens the air. Lymphoid tissue in the nasopharynx provides immunological surveillance against inhaled antigens. Some air is also taken in through the mouth and therefore bypasses these early cavities.
The air then enters a single tube (the trachea) that divides repeatedly to form airways of ever-decreasing diameter (primary or main bronchi, secondary or lobar bronchi and tertiary or segmental bronchi). In the larger airways, the epithelium has a similar structure and function to the upper respiratory tract. The wall of the trachea is held open by hyaline cartilage rings, which become irregular cartilage plates in smaller branches. Smooth muscle is also an important component of the wall, which contracts and relaxes to modify the diameter of the airway and therefore the flow of air, particularly in those air passages with less cartilage. The tertiary bronchi ramify into numerous orders of progressively smaller airways called bronchioles; these have muscle but no cartilage in their walls. The smallest bronchioles are called terminal bronchioles. These are the last of the purely conducting tubes.
The gaseous interchange system is a vast number of blind-ending sacs called alveoli, the walls of the sacs containing an extensive network of thin-walled blood vessels, the pulmonary capillaries. Gaseous exchange occurs between the air in the alveoli and the blood in the capillaries. This arrangement provides a huge surface area where blood and air are separated by a very thin barrier, facilitating gaseous exchange. The continuous process of gaseous diffusion requires appropriate gaseous pressure gradients to be maintained across the alveolar/capillary walls. This is achieved by rapid and continuous perfusion of the pulmonary capillaries by deoxygenated venous blood from the right side of the heart and regular replacement of alveolar gases by breathing. Between the end of the purely conducting part of the system (terminal bronchioles) and the alveoli is a series of transitional airways, the respiratory bronchioles and alveolar ducts, which become increasingly involved in gas exchange. These passages terminate in dilated air spaces called alveolar sacs which open into the alveoli.
The respiratory tract also contains two further elements with separate functions:
• The roof of the nasal cavity contains areas of highly specialised mucosa, the olfactory mucosa, responsible for the detection of smell and the more complex aspects of taste (see Fig. 21.2).
• The larynx is a specialised structure located at the upper end of the trachea. This utilises forcibly expired air from the respiratory tract below it to generate sound by vibrating the true vocal cords.
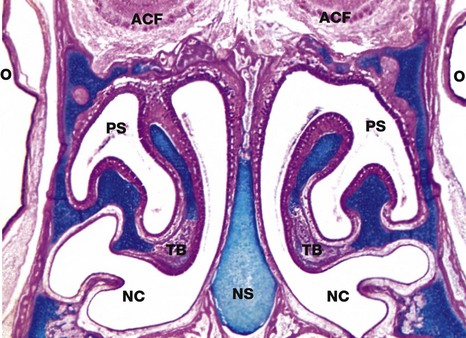
FIG. 12.2 Nasal cavity, kitten
Coronal slice, H&E/Alcian blue (LP)
The nose is subdivided into two nasal cavities NC by the nasal septum NS. The cartilage stains blue using this method.
The nasal cavities and paranasal sinuses PS are lined by respiratory mucosa, the major function of which is to adjust the temperature and humidity of inspired air. Particulate matter entering the nares is usually trapped by the hairs at that site, but some smaller particles are caught on the respiratory mucosa. These functions are enhanced by a large surface area provided by the turbinate bones TB which project into the nasal cavities.
Part of the nasal mucosa, the olfactory mucosa, contains receptors for the sense of smell (see Fig. 21.2). Olfactory mucosa is extensive in lower mammals, but in man it is confined to a small area in the roof of the nasal cavity. Note the close proximity of the nasal cavities to the orbital cavities O and the anterior cranial fossa ACF.
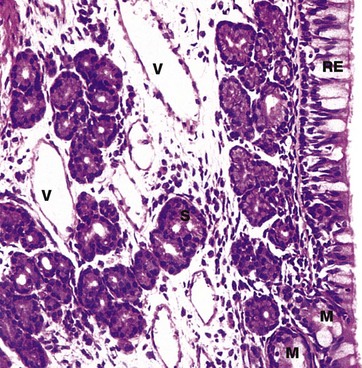
FIG. 12.3 Nasal mucosa
H&E (HP)
The mucosa of the nasal cavities (and paranasal sinuses) consists of a pseudostratified ciliated columnar epithelium RE containing numerous mucin-secreting goblet cells. This is called respiratory epithelium and is found elsewhere in the conducting part of the respiratory tract. The respiratory epithelium has an unusually thick basement membrane (not seen at this magnification).
It is supported by a lamina propria rich in blood vessels V and serous S and mucous M glands. The secretions of these glands and epithelial goblet cells trap small particles in the inspired air in a thin layer of surface mucous. This mucous layer is propelled towards the pharynx by the coordinated movement of the cilia. This is sometimes described as the muco-ciliary escalator. From the pharynx, most of the mucus is swallowed and gastric acid destroys any trapped bacteria.
The temperature of the inspired air is adjusted close to that of the body as a result of warming by the rich plexus of blood vessels (mainly thin-walled vessels). The air is also humidified by contact with the gland secretions, particularly those of the serous glands.
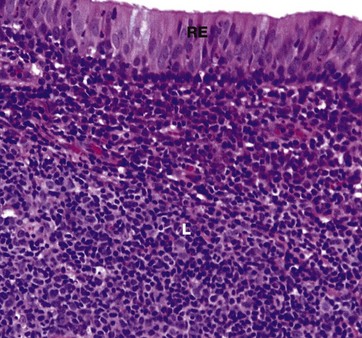
FIG. 12.4 Nasopharynx
H&E (HP)
The nasopharynx is lined by pseudostratified ciliated columnar (respiratory) epithelium RE similar to that seen in Fig. 12.3, but patches of squamous epithelium occur with increasing age, particularly near the lower end and most extensively in smokers.
The lamina propria contains some serous and mucous glands, but the dominant feature of the mucosa at this site is the presence of large masses of lymphoid tissue L which forms a component of Waldeyer ring of lymphoid tissue, protecting the entry portals of the respiratory and gastrointestinal systems.
This lymphoid tissue is particularly prominent in children and young adults and usually bulges outwards into the lumen of the nasopharynx, producing an appearance similar to that seen in the lingual tonsil (see Fig. 13.13) with epithelial crypts. This is called the nasopharyngeal tonsil or adenoid.
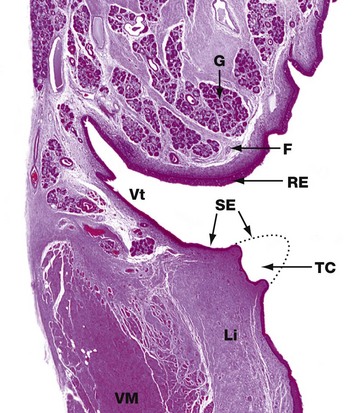
FIG. 12.5 Larynx
H&E (LP)
This low-power photomicrograph shows the constituents of one half of the larynx. It comprises two folds which protrude into the airway. The upper fold is the false vocal cord F which is covered by columnar ciliated respiratory-type epithelium RE and contains seromucous glands G.
The lower fold is the true vocal cord TC. In this surgically removed human larynx, the sharp tip of the true cord has been removed by diathermy in the distant past and a dotted line shows its normal outline. The true cord contains the vocalis muscle VM and vocalis ligament Li which are responsible for moving the true cord so that it moves towards or away from the true cord on the other side, thus controlling the pitch of the sound made. The true cords are covered by stratified squamous epithelium SE which is more resistant to the effects of physical trauma caused by the free margins of the true cords contacting each other during speech.
Between the true and false cords, there is a narrow cleft, the ventricle Vt, which terminates in a blind-ending saccule (not shown). The ventricle and saccule are lined by respiratory-type columnar epithelium and also contain seromucous glands.
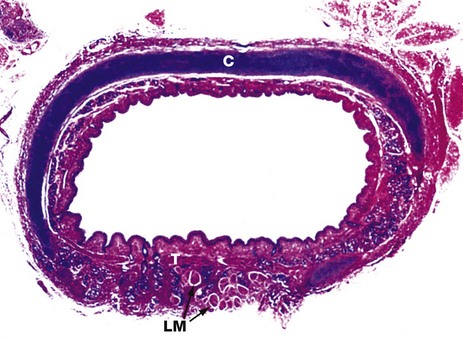
FIG. 12.6 Trachea
H&E/Alcian blue (LP)
This specimen from a newborn child shows the general structure of the trachea. This is a flexible tube of fibroelastic tissue and cartilage which permits expansion in diameter and extension in length during inspiration, and passive recoil during expiration. A series of C-shaped rings of hyaline cartilage C (stained blue) support the tracheal mucosa and prevent its collapse during inspiration.
Bands of smooth muscle called the trachealis muscle T join the free ends of the rings posteriorly. Contraction of the trachealis reduces tracheal diameter and thereby assists in raising intrathoracic pressure during coughing. A few strands of longitudinal muscle LM can be seen disposed behind the trachealis muscle.
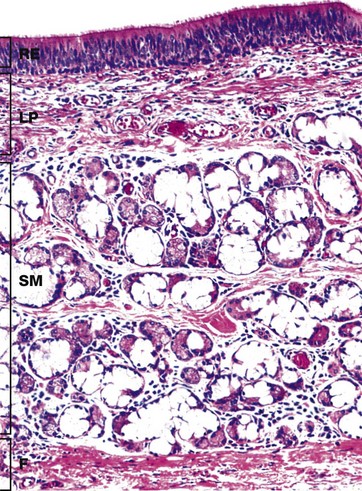
FIG. 12.7 Trachea
H&E (MP)
The inner layers of the tracheal wall are shown in this specimen from a young adult. The respiratory epithelium RE of the trachea is similar to the rest of the bronchial tree and nasal epithelium. A variety of cell types is found in the epithelium, including:
• Tall pseudostratified columnar cells with cilia
• Serous cells identical to the cells of the submucosal serous glands
• Basal cells which are part of the diffuse neuroendocrine system
• Basal stem cells which are able to divide and differentiate to replace other cell types
The various cell types are present in different proportions in different parts of the trachea. Ciliated columnar cells are more plentiful in the lower trachea whilst goblet and basal cells are more common in the upper trachea. Beneath the basement membrane, the lamina propria LP consists of loose, highly vascular supporting tissue which becomes more condensed at its deeper aspect to form a band of fibroelastic tissue.
Underlying the lamina propria is the loose submucosa SM containing numerous mixed seromucinous glands which decrease in number in the lower trachea. The serous cells stain strongly and the mucous cells poorly with H&E. The submucosa merges with the perichondrium of the underlying hyaline cartilage rings (not seen here) or, as here, with the dense fibroelastic tissue F between the cartilage rings.
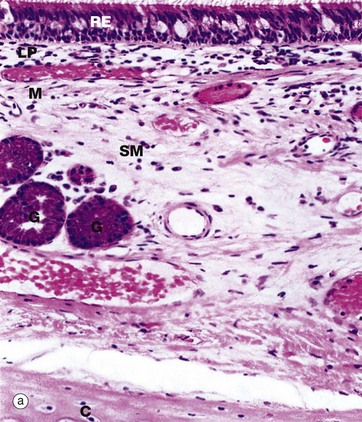
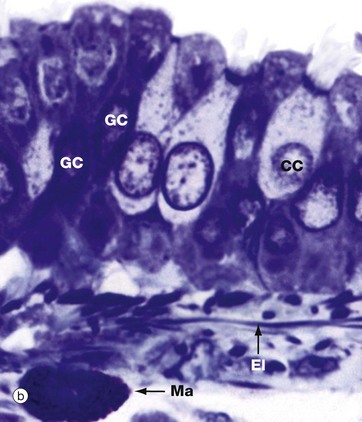
FIG. 12.8 Primary bronchus
(a) H&E (MP) (b) Thin resin section, toluidine blue (HP)
The basic structure of the wall of a main bronchus (a) is similar to that of the trachea but differs in several details:
• The respiratory epithelium RE is less tall and contains fewer goblet cells.
• The upper lamina propria LP contains more elastin.
• The lamina propria is separated from the submucosa SM by a layer of smooth muscle M which becomes more prominent in more distal bronchi.
• The submucosa contains fewer seromucinous glands G.
• The cartilage support C is in flattened interconnected plates rather than distinct rings.
Micrograph (b) shows the epithelial layer at very high magnification. The cells are pseudostratified, the bases of all the cells contacting the basement membrane but not all the cells reaching the luminal surface. The ciliated CC and goblet GC cells can be easily distinguished. The underlying lamina propria contains elastic fibres El and occasional mast cells Ma.
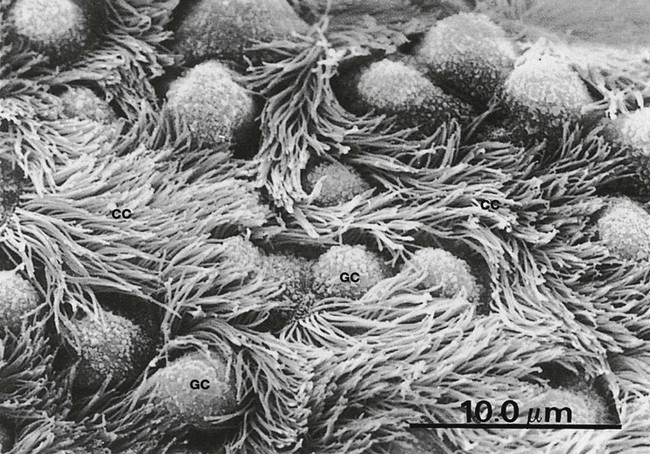
FIG. 12.9 Primary bronchus
SEM ×2000
This scanning electron micrograph illustrates the surface of a primary bronchus. The film of surface mucus has been removed.
The ciliated epithelial cells CC have numerous surface cilia, each several microns long, that move in a coordinated fashion in order to sweep mucus up the bronchus.
Scattered goblet cells GC are recognisable by their bulbous surface outline, lack of cilia and the presence of small surface projections associated with mucus secretion. The fragile cilia are particularly vulnerable to damage and destruction by inhaled toxic chemicals (cigarette smoke, car exhaust fumes) and by bacterial and viral infections.
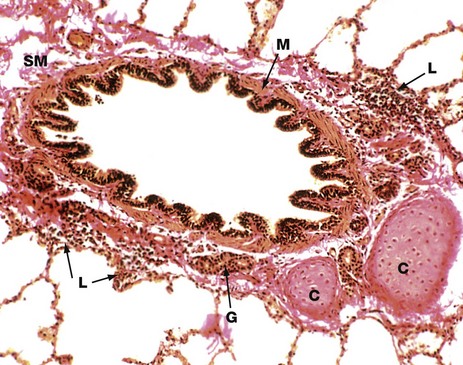
FIG. 12.10 Tertiary (segmental) bronchus
Elastic van Gieson (MP)
As bronchi diminish in diameter, the structure progressively changes to resemble more closely that of large bronchioles. The epithelium, just visible in this image, is tall and columnar with little pseudostratification. Goblet cell numbers are greatly diminished.
The lamina propria is thin, elastic and completely encircled by smooth muscle M which is disposed in a spiral manner. This arrangement permits contraction of the bronchi in both length and diameter during expiration. Seromucinous glands G are sparse in the submucosa. These glands are rarely found within smaller airways. The cartilage framework C is reduced to a few irregular plates. Cartilage does not usually extend beyond tertiary bronchi.
Note that the submucosa SM merges with the surrounding adventitia and then with the lung parenchyma. Small aggregates of lymphocytes L, part of the mucosa-associated lymphoid tissue (MALT), are seen in the adventitia.
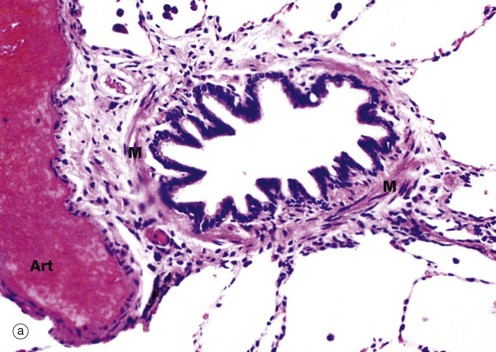
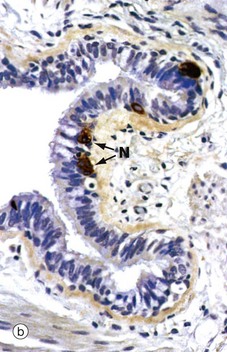
FIG. 12.11 Bronchiole
(a) H&E (MP) (b) Immunohistochemical staining for chromogranin (HP)
A bronchiole (a) is an airway of less than 1 mm diameter which has neither cartilage or submucosal glands in its wall. The epithelium is composed of ciliated columnar cells and few goblet cells. In the terminal and respiratory bronchioles, goblet cells are replaced by Clara cells (see Fig. 12.12), tall columnar cells with apical secretory granules. The wall is also composed of smooth muscle M, the tone of which controls the bore of the tube and therefore resistance to airflow within the lungs. A distended thin-walled pulmonary artery branch Art lies next to the bronchiole.
Micrograph (b) demonstrates the presence of neuroendocrine cells N using an immunohistochemical method. These cells form part of the diffuse neuroendocrine system (see Ch. 17), secreting a number of peptide hormones including 5-HT (serotonin) and bombesin which regulate muscle tone in bronchial and vessel walls.
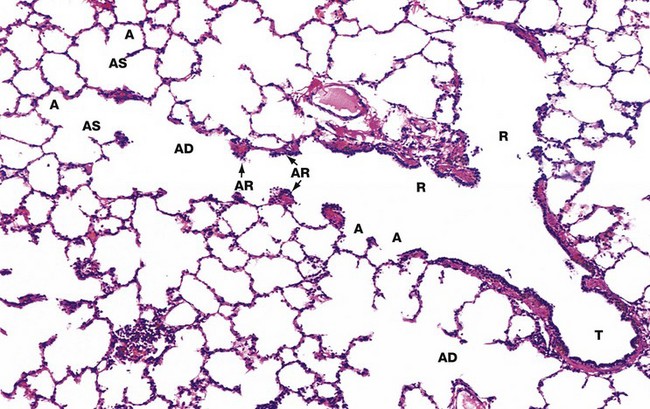
FIG. 12.12 Terminal portion of the respiratory tree (illustration opposite)
H&E (LP)
Terminal bronchioles T are the smallest diameter passages of the purely conducting portion of the respiratory tree. Beyond this, branches become increasingly involved in gaseous exchange.
Each terminal bronchiole divides to form short, thinner walled branches called respiratory bronchioles R which contain a small number of single alveoli A in their walls. The epithelium of the respiratory bronchioles is devoid of goblet cells and largely consists of ciliated cuboidal cells and smaller numbers of non-ciliated cells called Clara cells. In the most distal part of the respiratory bronchioles. Clara cells become the predominant cell type. Clara cells have three functions:
• They produce one of the components of surfactant.
• They act as stem cells, i.e. they are able to divide, differentiate and replace other damaged cell types.
• They contain enzyme systems which can detoxify noxious substances.
Each respiratory bronchiole divides further into several alveolar ducts AD which have numerous alveoli A opening along their length. The alveolar ducts end in an alveolar sac AS, which in turn opens into several alveoli.
In histological sections, all that can be seen of the walls of the alveolar ducts are small aggregations of smooth muscle cells, collagen and elastin fibres which form alveolar rings AR surrounding the alveolar ducts and the openings of the alveolar sacs and alveoli. The smooth muscle of the respiratory bronchioles and alveolar ducts regulates alveolar air movements.
Each alveolus consists of a pocket, open at one side, lined by flattened epithelial cells (pneumocytes). The alveolar septa contain occasional small openings about 8 µm diameter, the alveolar pores (of Kohn), which allow some movement of air between adjacent alveoli. The collagen and elastic fibres of the septum condense around the openings of the alveoli and form a supporting meshwork for the lung parenchyma.
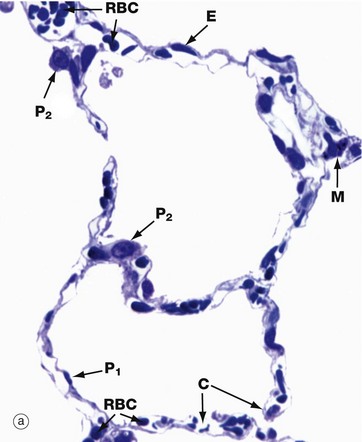
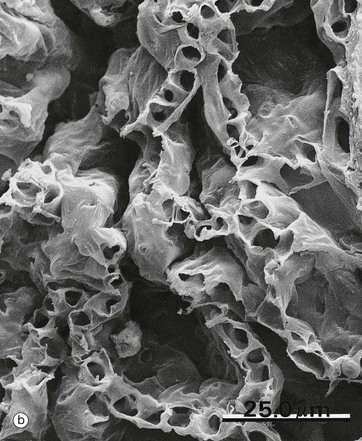
FIG. 12.13 Alveoli
(a) Thin resin section, toluidine blue (HP) (b) SEM ×500
The alveolar wall consists of three tissue components: surface epithelium, supporting tissue and blood vessels.
The epithelium provides a continuous lining to each alveolus and consists of cells of two types. Most of the alveolar surface area is covered by large squamous cells called type I pneumocytes (alveolar lining cells). Because the cytoplasm of these cells covers such an extensive area, the characteristic densely stained nuclei of type I pneumocytes P1 are relatively infrequently seen in histological section. The type II pneumocyte P2 represents some 60% of cells in the lining epithelium, but is rounded in shape and thus occupies a much smaller proportion (about 5%) of the alveolar surface area.
Type II pneumocytes secrete a surface-active material called surfactant which reduces alveolar surface tension, preventing alveolar collapse during expiration. Clara cells of the respiratory bronchioles probably synthesise other components of surfactant. Type II pneumocytes retain the capacity for cell division and can differentiate into type I pneumocytes if required.
Supporting tissue forms an attenuated layer surrounding the blood vessels of the alveolar wall. This layer consists of reticular, collagenous and elastic fibres and occasional fibroblasts. Blood vessels, mainly capillaries C, form an extensive plexus around each alveolus. In most of the alveolar wall, the basement membrane of the capillary endothelium is directly applied to the basement membrane of the surface epithelium. In such sites, the two basement membranes are fused and supporting tissue is absent. This arrangement provides an interface of minimal thickness between alveolar air and blood.
In micrograph (a), red blood cells within the capillary lamina are seen as densely stained round or elliptical structures RBC. Nuclei of the endothelial cells E lining the capillaries are elongated, flat and usually sparse. Micrograph (b) is a low-magnification scanning electron micrograph of a group of alveoli, showing their three-dimensional architecture. The capillaries in the alveolar walls have been distended by injection. No cellular detail is visible.
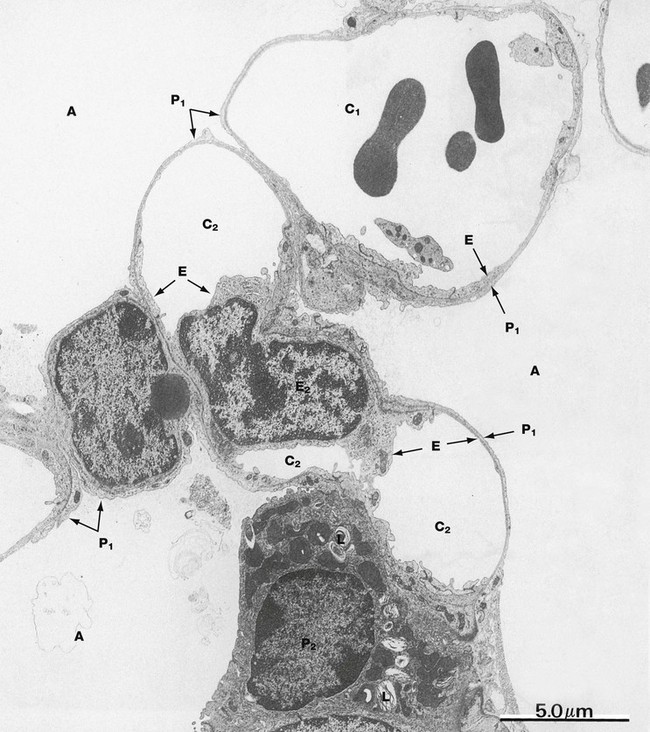
FIG. 12.14 Alveolar wall
EM ×6000
This electron micrograph shows the alveolar wall between three alveoli A at low magnification. Capillaries make up the bulk of the alveolar wall, branching and anastomosing to create a basket-like arrangement around each alveolus. This field shows parts of several capillaries, the uppermost C1 containing erythrocytes and a platelet. The plane of section has cut the lumen of a second capillary C2 in three places and includes the nucleus of one of its lining endothelial cells E. The cytoplasm of type I pneumocytes P1, which cover most of the alveolar surface, and capillary endothelial cells E are both extremely attenuated, and distinction between them is best made by tracing their basement membranes. Alveolar lining cells lie on the convex side of the basement membrane, whilst endothelial cells are on the concave side and adjacent to any erythrocytes within the capillary.
A type II pneumocyte P2 is also seen, typically located at a branching point of the alveolar septum. The cytoplasm is filled with vesicles containing phospholipid in the form of lamellar bodies L. These bodies are discharged into the alveolar air space where they contribute to a surfactant layer at the epithelium/air interface.
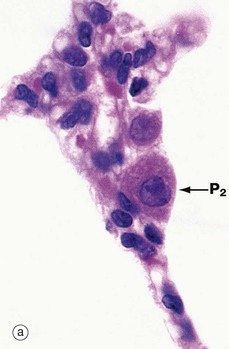
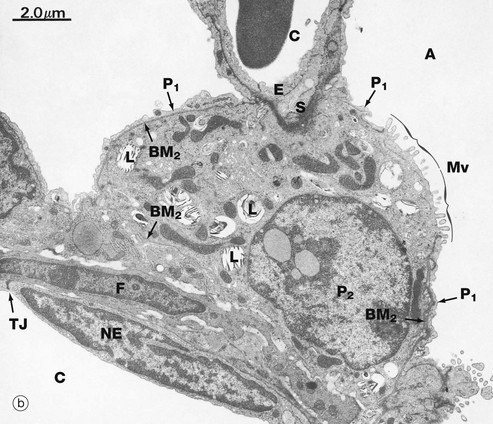
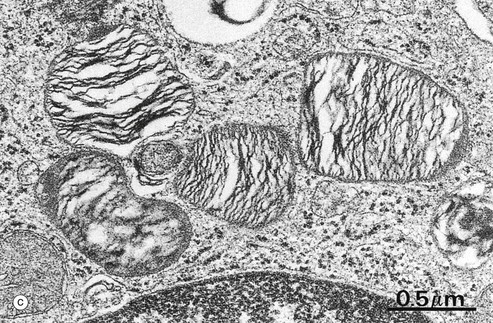
FIG. 12.15 Type II pneumocytes
(a) H&E (HP) (b) EM ×9000 (c) EM ×35 000
Micrograph (a) shows the light microscopic appearance of type II pneumocytes P2 or surfactant cells, which are responsible for surfactant production. Their nuclei are large and plump with dispersed chromatin and prominent nucleoli. The plentiful eosinophilic cytoplasm is filled with fine unstained vacuoles representing lamellar bodies, the phospholipid of which is dissolved out during tissue preparation. In comparison, the nuclei of type I pneumocytes (alveolar lining cells) and capillary endothelial cells are small, dense and flattened.
Micrograph (b) shows a branch point in an alveolar wall, typically containing a type II pneumocyte P2, recognisable by its lamellar bodies L. Most of the type II pneumocyte is surrounded by basement membrane BM2, and only a small proportion of its surface is exposed directly to the alveolar space A, where it exhibits numerous small microvilli Mv associated with surfactant secretion. Elsewhere, the alveolar aspect of the type II pneumocyte is invested by a thin layer of cytoplasm of type I pneumocytes P1 but separated by a common basement membrane. At the top of the field, the type II pneumocyte abuts a capillary C, its cytoplasm being separated from that of the capillary endothelium E by the basement membranes of each cell and a little intervening supporting tissue S. At the lower left of the field, the type II cell rests upon a thin layer of septal supporting tissue containing a fibroblast F. Beyond this lies the flattened nucleus of a capillary endothelial cell NE, its attenuated cytoplasm spreading out to line the capillary lumen C. A tight junction TJ is seen where this endothelial cell abuts an adjacent cell. Basement membranes can be traced on both aspects of this alveolar supporting tissue. The surfactant cell contains rough endoplasmic reticulum, free ribosomes and moderate numbers of elongated mitochondria.
Micrograph (c) shows lamellar bodies at high magnification. These are membrane bound and the lamellae within them are composed mainly of phospholipids, particularly palmitoyl phosphatidylcholine. Phospholipid is released by exocytosis, spreading out over the alveolar surface where it combines with other carbohydrate- and protein-containing secretory products (some of which are derived from bronchiolar Clara cells) to form a tubular lattice of lipoprotein described as tubular myelin. In the event of two alveolar surfaces coming together, this overcomes the effects of surface tension which would otherwise cause them to adhere. This allows for normal inflation of the alveoli at birth (see textbox) and for the reinflation of alveoli which collapse after airway obstruction.
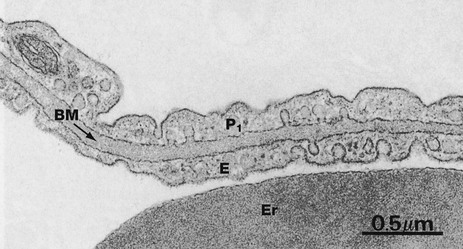
FIG. 12.16 Air-blood barrier
EM ×34 000
This micrograph illustrates, at very high magnification, the components of the narrow diffusion barrier which lies between the blood in the pulmonary capillaries and the alveolar air. This consists of the attenuated cytoplasm of a type I pneumocyte P1, the fused basement membrane BM and the thin cytoplasm of a capillary endothelial cell E. Note part of an erythrocyte Er within the capillary lumen.
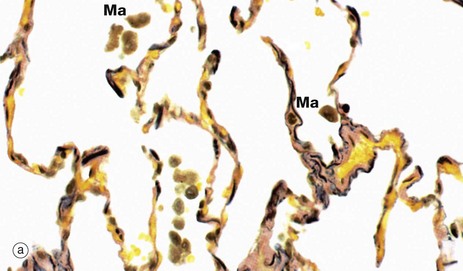
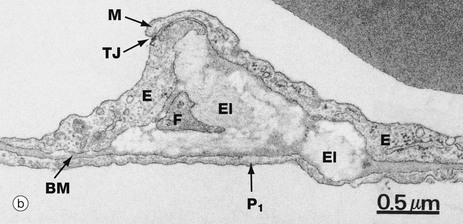
FIG. 12.17 Pulmonary elastic tissue
(a) Elastic van Gieson (HP) (b) EM ×26 000
The staining method used in micrograph (a) demonstrates the large amount of elastin (stained black) in the alveolar walls. At the margins of the openings into the alveoli, the elastin is condensed to form a supporting ring. The elastin and septal collagen of the alveolar wall are continuous with those of adjacent alveoli, forming a fibroelastic supporting framework for the lung parenchyma as a whole. Occasional alveolar macrophages Ma can be seen within the lumen of the alveoli.
Micrograph (b) shows part of an alveolar septum containing elements of the elastin meshwork. The septum consists of the thin cytoplasmic layers of a type I pneumocyte P1 and two capillary endothelial cells E separated by a common basement membrane BM. Note the marginal fold M of one endothelial cell overlapping the other, creating a seal and reinforced by a tight junction TJ.
The elastin El is an amorphous, moderately electron-dense mass which is insinuated between the two cell layers. This space also contains a fine cytoplasmic extension from a fibroblast F.
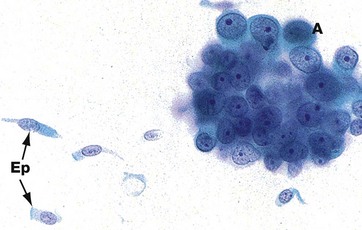
FIG. 12.18 Lung cancer cytology
FNA sample Giemsa (HP)
This micrograph demonstrates the difference between normal epithelial cells Ep and adenocarcinoma cells A. The malignant cells are much larger, with prominent nuclei, large nucleoli and scanty cytoplasm. In contrast, the normal epithelial cells exhibit the expected columnar shape. Cilia can just be discerned on the luminal surface of the normal cells in this image.
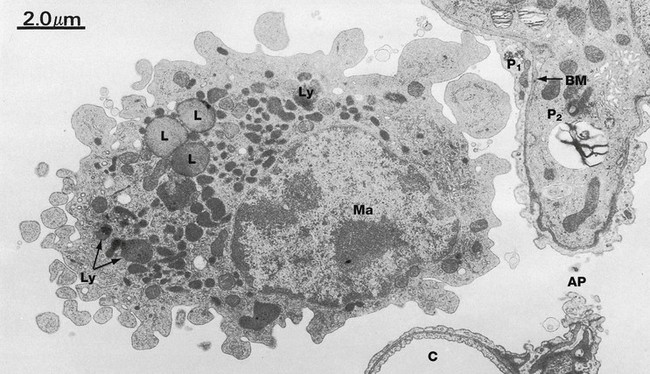
FIG. 12.19 Alveolar macrophage
EM ×9000
The lung contains macrophages, both free within the alveolar spaces and in the alveolar septa. They are derived from circulating blood monocytes, although some may arise by mitotic division of macrophages already present in the lung. Their function is the phagocytosis and removal of unwanted material which gains access to the air spaces, such as inhaled particulate matter and bacteria. The most common particles are carbon. In city dwellers, these are derived from car exhaust fumes and industrial smoke. Numerous carbon particles are typically seen in cigarette smokers. After phagocytosing the particles, most macrophages pass into the airways to become trapped in mucus and coughed up as sputum. Others stay in the septa whilst some gain access to the lymphatic system and pass with their phagocytosed material to the hilar lymph nodes.
The macrophage Ma in this micrograph lies within an alveolus adjacent to a septal capillary C and a type II pneumocyte P2. Between these, there is an alveolar pore AP. The aspect of the type II peumocyte seen here is typically invested by the thin cytoplasm of a type I pneumocyte P1, the two being separated by a common basement membrane BM (see Fig. 12.16). The alveolar macrophage exhibits the typical features of macrophages elsewhere in the body (see Fig. 4.19), but in particular contains numerous secondary lysosomes Ly and lipid droplets L.
Pulmonary Vasculature
The lungs have a double blood supply:
The major supply is the pulmonary vascular system. Deoxygenated blood is carried by the systemic veins to the right atrium and into the right ventricle. The right ventricle pumps the blood through the pulmonary valve into the main pulmonary arterial trunk and then into the right and left pulmonary arteries. The main right and left pulmonary arteries enter the lungs at the lung hila alongside the main bronchi and follow the course of the bronchi into the lungs, dividing into progressively smaller branches as the bronchi divide. With each division, the arteries become smaller and the structure of their wall changes. The proximal pulmonary arteries, the main pulmonary trunk and large pulmonary arteries, are elastic arteries similar to the aorta (see Fig. 8.9) but thinner walled, with elastic fibres an important component of the tunica media. Beyond the point where the bronchi lose their cartilage plates to become bronchioles, the pulmonary arteries become muscular arteries with distinct elastic laminae and a tunica media that is almost completely composed of smooth muscle. The transition from elastic to muscular arteries is gradual. The distal pulmonary arteries continue to follow the distribution of bronchioles and become progressively smaller as the tunica media becomes thinner, eventually becoming discontinuous in the pulmonary arterioles. The small pulmonary arterioles transfer blood into the pulmonary capillaries (see Figs 12.13 and 12.14) where it becomes oxygenated and it is then passed through pulmonary venules (indistinguishable from arterioles) into a series of gradually enlarging venules and veins. Some of these run in the fibrocollagenous septa of the lung before becoming medium-sized veins with a distinct tunica media and ill-formed elastic laminae. The largest pulmonary veins, that leave the lungs at the hilar regions and pass to the left atrium, show elastic fibres scattered in the media, rather than in distinct elastic laminae.
The bronchial vascular system is minor and provides lung structures such as bronchi with oxygenated blood at systemic pressure. The bronchial arteries are lateral branches of the thoracic aorta and run with the bronchial tree as far as respiratory bronchiole level where they anastomose with the pulmonary vascular system. The small bronchial veins and venules also anastomose freely with pulmonary veins and venules. The main bronchial veins drain into the azygos and hemiazygos veins.
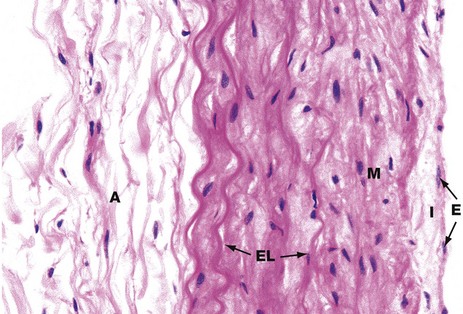
FIG. 12.20 Large pulmonary artery
H&E (HP)
The pulmonary trunk, main right and left pulmonary arteries and their major lobar branches have a structure similar to that of the aorta (see Fig. 8.9), i.e. an elastic artery with prominent elastic lamellae as an important component of the tunica media. However, because the intravascular pressures are so much less in the pulmonary vascular system, the layers are thinner and less substantial than their equivalents in the aorta. The tunica intima I has surface endothelial cells E, and the tunica media M is composed of smooth muscle cells, collagen and prominent elastic lamellae EL. The adventitia A comprises loose fibrocollagenous tissue.
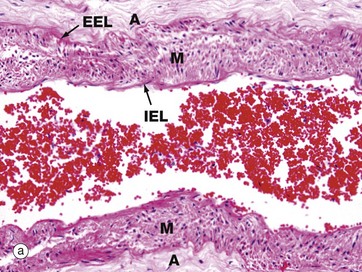
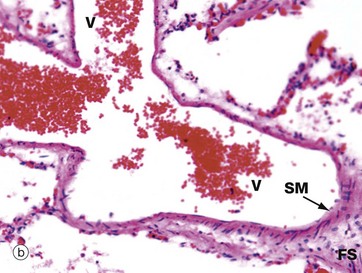
FIG. 12.21 Smaller pulmonary vessels
(a) Muscular pulmonary artery, H&E (MP) (b) Small pulmonary vein, H&E (MP)
More distally, the elastic pulmonary artery progressively loses most of the elastic fibres in the media. A muscular pulmonary artery is illustrated in micrograph (a). Most of the remaining elastic is in the form of internal IEL and external EEL elastic laminae so that the media M is largely composed of smooth muscle and collagen.
The intima is thin and indistinct, and the fibrocollagenous tunica adventitia A merges with the fibrocollagenous support tissue of the lung septa and peribronchial areas.
Pulmonary capillaries and venules empty oxygenated blood into thin-walled pulmonary veins V, as illustrated in image (b). The amount of smooth muscle media SM in the vein wall increases progressively along the venous network, and the largest pulmonary veins have a distinct muscular tunica media containing elastin fibres. Small pulmonary veins of the size shown here run in the fibrous septa FS of the lungs.
The Pleura
The two cavities in the thorax which house the right and left lungs, the pleural cavities, are lined internally by a thin smooth layer, the pleura, which is also reflected over the external surfaces of the lungs. The part of the pleura which forms the internal lining of the chest cavities is called the parietal pleura and that which externally coats the lungs, the visceral pleura. The parietal and visceral pleurae are normally in contact but separated by a potential space containing a small amount of serous fluid that lubricates the movement of visceral upon parietal pleura during breathing.
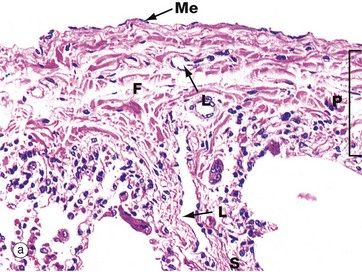
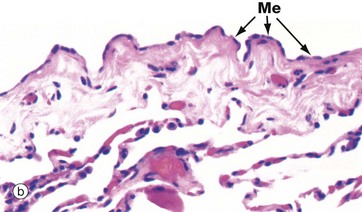
FIG. 12.22 Visceral pleura
(a) H&E (MP) (b) H&E (HP)
Micrograph (a) illustrates visceral pleura P. The outer surface is lined by a layer of flattened mesothelium Me, supported by a thin basement membrane. The underlying fibrous supporting tissue F consists primarily of collagen and elastin fibres. The fibrous layer of visceral pleura extends into the lung as fibrous septa FS which are continuous with the fibroelastic framework of the lung parenchyma.
The visceral pleura contains a superficial plexus of lymph vessels which drain via the septa into a deep plexus surrounding the pulmonary blood vessels and airways. Lymph from the deep plexuses drains into the thoracic duct via lymph nodes in the hilar region. Lymphatic capillaries are not found in alveolar walls, but they are present in the walls of respiratory bronchioles and in all larger airways. Several lymphatic vessels L can be seen in the pleura in this micrograph. The visceral pleura also contains numerous small blood vessels and capillaries.
Micrograph (b) is a higher magnification view of the pleura showing the flattened cuboidal mesothelial cells Me. These cells stretch to accommodate the movement of the lungs so that the height of the cells varies from flattened to columnar. Ultrastructurally, mesothelial cells have plentiful long surface microvilli which serve to trap hyaluronic acid, thus enhancing the lubrication of the two pleural surfaces. Mesothelial cells contain keratin intermediate filaments.