STOOL SPECIMENS
Stool specimens are frequently collected in children to identify parasites and other organisms that cause diarrhea, to assess gastrointestinal function, and to check for occult (hidden) blood. Ideally, stool should be collected without contamination with urine, but in children wearing diapers this is difficult unless a urine bag is applied. Children who are toilet trained should urinate first, flush the toilet, then defecate in the toilet, a bedpan (preferably one that is placed on the toilet to avoid embarrassment), or a commercial potty hat.
Stool specimens should be large enough to obtain an ample sampling, not merely a fecal fragment. Specimens are placed in an appropriate container, which is covered and labeled. If several specimens are needed, the containers are marked with the date and time and kept in a specimen refrigerator. Care is exercised in handling the specimen because of the risk of contamination.
BLOOD SPECIMENS
Whether the specimen is collected by the nurse or others, the nurse is responsible for making certain that specimens, such as serial examinations and fasting specimens, are collected on time and the proper equipment is available. Collecting, transporting, and storing specimens can have a major impact on laboratory results.
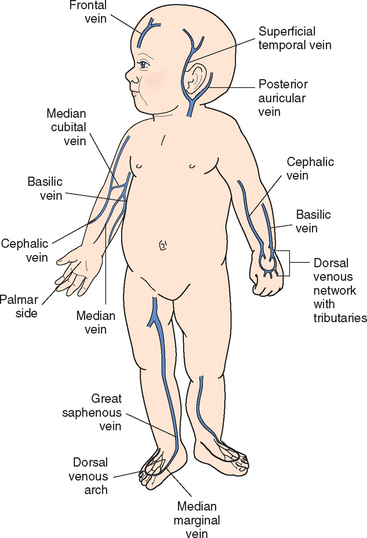
Venous blood samples can be obtained by venipuncture or by aspiration from a peripheral or central access device (see Evidence-Based Practice box). Withdrawing blood specimens through peripheral lock devices in small peripheral veins has met with varying degrees of success. Although it avoids an additional venipuncture for the child, attempting to aspirate blood from the peripheral lock may shorten the life of the device. When using an IV infusion site for specimen collection, consider the type of fluid being infused. For example, a specimen collected for glucose level would be inaccurate if removed from a catheter through which glucose-containing solution was being administered.
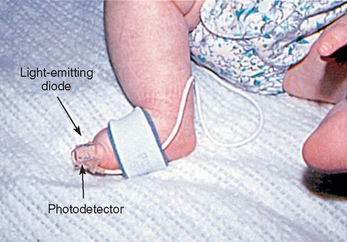
Arterial blood samples are sometimes needed for blood gas measurement, although noninvasive techniques, such as transcutaneous oxygen monitoring and pulse oximetry, are used frequently. Arterial samples may be obtained by arterial puncture using the radial, brachial, or femoral arteries; by deep heel puncture; or from indwelling arterial catheters. Adequate circulation should be assessed before arterial puncture by observing capillary refill or performing the Allen test, a procedure that assesses the circulation of the radial, ulnar, or brachial arteries. Because unclotted blood is required, only heparinized collection tubes are used. In addition, no air bubbles should enter the tube, since they can alter blood gas concentration. Crying, fear, and agitation also affect blood gas values; therefore every effort is made to comfort the child. The nurse should pack the sample in ice to reduce blood cell metabolism and take it to the laboratory for immediate analysis.
Capillary blood samples are taken from children by a finger stick. A common method for taking peripheral blood samples from infants younger than 6 months of age is by a heel stick. Before the blood sample is taken, the heel is warmed for 3 minutes. The area is cleansed with alcohol, the infant’s foot firmly restrained with the free hand, and the heel punctured with an automatic lancet device (Clinical and Laboratory Standards Institute, 2006). An automatic device delivers a more precise puncture depth and is less painful than using a lance (Vertanen, Fellman, Brommels, and others, 2001). A surgical blade of any kind is contraindicated. An example of a safe device is the BD Quickheel Safety Lancet. The Tenderfoot Preemie device was compared with the Monolet lancet and was found to be safer than the lancet and required fewer heel punctures, less collection time, and lower recollection rates (Kellam, Sacks, Wailer, and others, 2001). Shepherd, Glenesk, Niven, and others (2006) reported the Tenderfoot device was more effective and safer than a lancet for newborn screening tests. Although obtaining capillary blood gases is a common practice, these measures may not accurately reflect arterial values.
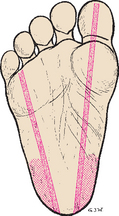
The most serious complications of infant heel puncture are necrotizing osteochondritis from lancet penetration of the underlying calcaneus bone, infection, or abscess (Meehan, 1998). To avoid osteochondritis, the puncture should be no deeper than 2 mm and should be made at the outer aspect of the heel. The boundaries of the calcaneus can be marked by an imaginary line extending posteriorly from a point between the fourth and fifth toes and running parallel to the lateral aspect of the heel and another line extending posteriorly from the middle of the great toe and running parallel to the medial aspect of the heel (Fig. 22-11). Repeated trauma to the walking surface of the heel can cause fibrosis and scarring that may interfere with locomotion.
The specimens are quickly collected, and pressure is applied to the puncture site with dry gauze until bleeding stops. The arm is kept extended, not flexed, while pressure is applied for a few minutes after venipuncture in the antecubital fossa to reduce bruising. The site is covered with an adhesive bandage. In young children, adhesive bandages pose an aspiration hazard; they should be avoided or removed as soon as the bleeding stops. Applying warm compresses to ecchymotic areas increases circulation, helps remove extravasated blood, and decreases pain.
No matter how or by whom the specimen is collected, children, even some older ones, fear the loss of their blood. This is particularly true for children whose condition requires frequent blood specimens. They mistakenly believe that blood removed from their bodies is a threat to their lives. Explaining to them that their body is continuously producing blood provides them with a measure of reassurance. When the blood is drawn, a comment such as “Just look how red it is. You–re really making a lot of nice red blood,” confirms this information and affords them an opportunity to express their concern. An adhesive bandage gives them added reassurance that the vital fluids will not leak out.
Children also dislike the discomfort associated with venous, arterial, or capillary punctures. Children have identified these procedures as the ones most frequently causing pain during hospitalization and arterial punctures as being one of the most painful of all procedures experienced (Van Cleve, Johnson, and Pothier, 1996). The ones most distressed by venipunctures are toddlers, followed by school-age children and then adolescents. Consequently nurses need to institute pain reduction techniques to lessen the discomfort of these procedures (see Atraumatic Care box).
RESPIRATORY SECRETION SPECIMENS
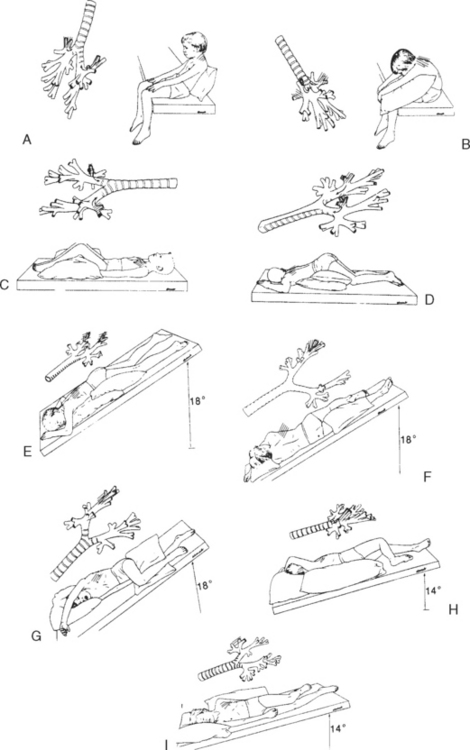
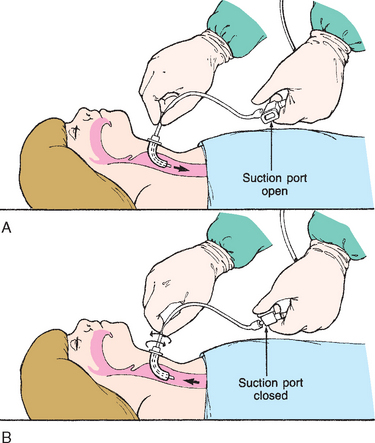
Collection of sputum or nasal discharge is sometimes required for diagnosis of respiratory infections, especially tuberculosis and respiratory syncytial virus (RSV). Older children and adolescents are able to cough and supply sputum specimens when given proper directions. It must be made clear to them that a coughed specimen, not mucus that is cleared from the throat, is needed. It is helpful to demonstrate a deep cough. Infants and small children are unable to follow directions to cough and will swallow any sputum produced; therefore gastric washings (lavage) may be used to collect a sputum specimen. Sometimes a satisfactory specimen can be obtained using a suction device such as a mucus trap if the catheter is inserted into the trachea and the cough reflex is elicited. A catheter inserted into the back of the throat is not sufficient. For children with a tracheostomy, a specimen is easily aspirated from the trachea or major bronchi by attaching a collecting device to the suction apparatus.
Nasal washings are usually obtained to diagnose an infection of RSV. The child is placed supine, and 1 to 3 ml sterile normal saline is instilled with a sterile syringe (without needle) into one nostril. The contents are aspirated using a small, sterile bulb syringe and are placed in a sterile container. Another method uses a syringe with 5 cm (2 inches) of 18- to 20-gauge tubing. The saline is quickly instilled and then aspirated to recover the nasal specimen. To prevent additional discomfort, all of the equipment should be ready before beginning the procedure.
Other respiratory secretion collection methods include nasopharyngeal swabs to diagnose Bordetella pertussis and throat cultures. The nurse swabs both the tonsils and the posterior pharynx when obtaining a throat culture. The swab stick is inserted into the culture tube. Some culture kits require squeezing an ampule to release the culture medium.

 ATRAUMATIC CARE
ATRAUMATIC CARE