Health Problems of Newborns
COMMON PROBLEMS IN THE NEWBORN
NURSING CARE OF THE HIGH-RISK NEWBORN AND FAMILY
HIGH RISK RELATED TO DYSMATURITY
HIGH RISK RELATED TO PHYSIOLOGIC FACTORS
Hemolytic Disease of the Newborn
Nursing Care Plan: The High-Risk Infant with Respiratory Distress Syndrome
HIGH RISK RELATED TO INFECTIOUS PROCESSES
HIGH RISK RELATED TO MATERNAL CONDITIONS
On completion of this chapter the reader will be able to:
• Recognize common deviations from normal characteristics in the newborn.
 Perform a systematic assessment of a high-risk newborn.
Perform a systematic assessment of a high-risk newborn.
 Outline a general care plan for a high-risk infant.
Outline a general care plan for a high-risk infant.
 Recognize physiologic factors that compromise the preterm infant’s health status.
Recognize physiologic factors that compromise the preterm infant’s health status.
 Discuss the role of the nurse in facilitating positive parent-infant relationships.
Discuss the role of the nurse in facilitating positive parent-infant relationships.
 Contrast physical characteristics of preterm and full-term infants.
Contrast physical characteristics of preterm and full-term infants.
 Discuss the basis for screening newborns for health problems.
Discuss the basis for screening newborns for health problems.
 Discuss the rationale for performing newborn screening and genetic counseling when a newborn has a hereditary condition.
Discuss the rationale for performing newborn screening and genetic counseling when a newborn has a hereditary condition.
 Modify a general care plan to meet the needs of an infant with specific high-risk health needs.
Modify a general care plan to meet the needs of an infant with specific high-risk health needs.
BIRTH INJURIES
Several factors predispose an infant to birth injuries (Mangurten, 2006; Paige and Moe, 2006). Maternal factors include uterine dysfunction that leads to prolonged or precipitous labor, preterm or postterm labor, and cephalopelvic disproportion. Injury may result from dystocia caused by fetal macrosomia, multifetal gestation, abnormal or difficult presentation (not caused by maternal uterine or pelvic conditions), and congenital anomalies. Intrapartum events that can result in scalp injury include the use of intrapartum monitoring of fetal heart rate and collection of fetal scalp blood for acid-base assessment. Obstetric birth techniques can cause injury. Forceps birth, vacuum extraction, version and extraction, and cesarean birth are potential contributory factors. Often more than one factor is present, and multiple predisposing factors may be related to a single maternal condition.
SOFT-TISSUE INJURY
Various types of soft-tissue injury may be sustained during the process of birth, primarily in the form of bruises or abrasions secondary to dystocia. Soft-tissue injury usually occurs when there is some degree of disproportion between the presenting part and the maternal pelvis (cephalopelvic disproportion). The use of forceps to facilitate a difficult vertex delivery may produce bruising or abrasion on the sides of the neonate’s face. Petechiae or ecchymoses may be observed on the presenting part after a breech or brow delivery. After a difficult or precipitous delivery, the sudden release of pressure on the head can produce scleral hemorrhages or generalized petechiae over the face and head. Petechiae and ecchymoses may also appear on the head, neck, and face of an infant born with a nuchal cord, giving the infant’s face a cyanotic appearance. A well-defined circle of petechiae and ecchymoses or abrasions may also be seen on the occipital region of the newborn’s head when a vacuum suction cup is applied during delivery. Rarely, lacerations occur during cesarean section.
These traumatic lesions generally fade spontaneously within a few days, without treatment. However, petechiae may be a manifestation of an underlying bleeding disorder or a systemic illness such as an infection and should be further evaluated as to their origin. Nursing care is primarily directed toward assessing the injury and providing an explanation and reassurance to the parents.
HEAD TRAUMA
Trauma to the head and scalp that occurs during the birth process is usually benign but occasionally results in more serious injury. The injuries that produce serious trauma, such as intracranial hemorrhage and subdural hematoma, are discussed in relation to neurologic disorders in the newborn (see Table 9-9). Skull fractures are discussed in association with other fractures sustained during the birth process. The three most common types of extracranial hemorrhagic injury are caput succedaneum, subgaleal hemorrhage, and cephalhematoma.
Caput Succedaneum
The most commonly observed scalp lesion is caput succedaneum, a vaguely outlined area of edematous tissue situated over the portion of the scalp that presents in a vertex delivery (Fig. 9-1, A). The swelling consists of serum or blood, or both, accumulated in the tissues above the bone, and it often extends beyond the bone margins. The swelling may be associated with overlying petechiae or ecchymoses. No specific treatment is needed, and the swelling subsides within a few days.
Cephalhematoma
Infrequently, a cephalhematoma is formed when blood vessels rupture during labor or delivery to produce bleeding into the area between the bone and its periosteum. The injury occurs most often with primiparous delivery and is often associated with forceps delivery and vacuum extraction. Unlike caput succedaneum, the boundaries of the cephalhematoma are sharply demarcated and do not extend beyond the limits of the bone (suture lines) (see Fig. 9-1, B). The cephalhematoma may involve one or both parietal bones. The occipital bones are less commonly affected, and the frontal bones are rarely affected. The swelling is usually minimal or absent at birth and increases in size on the second or third day. Blood loss is usually not significant.
No treatment is indicated for uncomplicated cephalhematoma. Most lesions are absorbed within 2 weeks to 3 months. Lesions that result in severe blood loss to the area or that involve an underlying fracture require further evaluation. Hyperbilirubinemia may result during resolution of the hematoma. A local infection can develop and is suspected when a sudden increase in swelling occurs. Parents should be counseled that, in some cases, a small area of calcification may develop and persist.
Subgaleal Hemorrhage
Subgaleal hemorrhage is bleeding into the subgaleal compartment (see Fig. 9-1, C). The subgaleal compartment is a potential space that contains loosely arranged connective tissue; it is located beneath the galea aponeurosis, the tendinous sheath that connects the frontal and occipital muscles and forms the inner surface of the scalp. The injury occurs as a result of forces that compress and then drag the head through the pelvic outlet (Paige and Moe, 2006). There have been reports of concern regarding the increased use of the vacuum extractor at birth and an association with cases of subgaleal hemorrhage, neonatal morbidity, and deaths (Boo, Foong, Mahdy, and others, 2005; Uchil and Arulkumaran, 2003); however, the rates of such outcomes have reportedly declined (Putta and Spencer, 2000). The bleeding extends beyond bone, often posteriorly into the neck, and continues after birth, with the potential for serious complications such as anemia or hypovolemic shock.
Early detection of the hemorrhage is vital; serial head circumference measurements and inspection of the back of the neck for increasing edema and a firm mass are essential. A boggy fluctuant mass over the scalp that crosses the suture line and moves as the baby is repositioned is an early sign of subgaleal hemorrhage (Doumouchtsis and Arulkumaran, 2006). Other signs include pallor, tachycardia, and increasing head circumference (Putta and Spencer, 2000). An early sign of subgaleal hemorrhage is a forward and lateral positioning of the newborn’s ears because the hematoma extends posteriorly. Computed tomography or magnetic resonance imaging is useful in confirming the diagnosis. Replacement of lost blood and clotting factors is required in acute cases of hemorrhage. Monitoring the infant for changes in level of consciousness and a decrease in the hematocrit are also key to early recognition and management. An increase in serum bilirubin levels may be seen as a result of the degradation of red blood cells within the hematoma.
Nursing Care Management
Nursing care is directed toward assessment and observation of the common scalp injuries and vigilance in observing for possible associated complications such as infection or, rarely, acute blood loss and hypovolemia. Because these visible injuries resolve spontaneously, parents need reassurance of their usual benign nature.
FRACTURES
The clavicle, or collarbone, is the bone most frequently fractured during the birth process. It is often associated with shoulder dystocia or a difficult vertex or breech delivery of infants who are large for gestational age. Crepitus (the coarse crackling sensation produced by the rubbing together of fractured bone fragments) may be felt or heard on examination. A palpable, spongy mass, representing localized edema and hematoma, may also be a sign of a fractured clavicle. The infant may be reluctant to move the arm on the affected side, and the Moro reflex may be asymmetric. Radiographs usually reveal a complete fracture with overriding of the fragments.
Fractures of long bones, such as the femur or the humerus, are sometimes difficult to detect by radiographic examination. Although osteogenesis imperfecta is a rare finding, a newborn infant with a fracture should be assessed for other evidence of this congenital disorder.
Fractures of the neonatal skull are uncommon. The bones, which are less mineralized and more compressible than bones in older infants and children, are separated by membranous seams that allow sufficient alteration in the head contour so that it adjusts to the birth canal during delivery. Skull fractures usually follow a prolonged, difficult delivery or forceps extraction. Most fractures are linear, but some may be visible as depressed indentations that compress or decompress like a Ping-Pong ball. Management of depressed skull fractures is controversial; many resolve without intervention. Nonsurgical elevation of the indentation using a hand breast pump or vacuum extractor has been reported (Mangurten, 2006). Surgery may be required in the presence of bone fragments or signs of increased intracranial pressure (Uhing, 2004). A similar finding in neonates is craniotabes, which is usually benign or may be associated with prematurity or hydrocephalus (Johnson, 2003). In this condition the cranial bone(s) move freely on palpation and may easily compress.
Nursing Care Management
Often, no intervention is needed other than maintaining proper body alignment, careful dressing and undressing of the infant, and handling and carrying that support the affected bone. For example, if the infant has a fractured clavicle, it is important to support the upper and lower back rather than pull the infant up from under the arms. Placing the infant in a side-lying position with the affected side down is also avoided. Linear skull fractures usually require no treatment. A Ping-Pong ball–type skull fracture may require decompression by surgical intervention. The infant is carefully observed for signs of neurologic complications. The parents of infants with a fracture of any bone should be involved in caring for the infant during hospitalization as part of discharge planning for care at home.
PARALYSIS
Pressure on the facial nerve (cranial nerve VII) during delivery may result in injury to that nerve. The primary clinical manifestations are loss of movement on the affected side, such as an inability to completely close the eye, drooping of the corner of the mouth, and absence of wrinkling of the forehead and nasolabial fold (Fig. 9-2). The paralysis is most noticeable when the infant cries. The mouth is drawn to the unaffected side, the wrinkles are deeper on the normal side, and the eye on the involved side remains open.
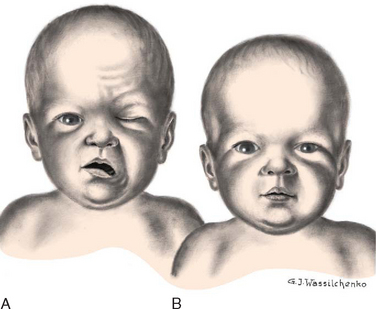
FIG. 9-2 A, Paralysis of right side of face 15 minutes after forceps delivery. Absence of movement on affected side is especially noticeable when infant cries. B, The same infant 24 hours later.
No medical intervention is necessary. The paralysis usually disappears spontaneously in a few days but may take as long as several months.
Brachial Palsy
Plexus injury results from forces that alter the normal position and relationship of the arm, shoulder, and neck. Erb palsy (Erb-Duchenne paralysis) is caused by damage to the upper plexus and usually results from stretching or pulling away of the shoulder from the head, as might occur with shoulder dystocia or with a difficult vertex or breech delivery. Other identified risk factors include an infant with birth weight of over 4000 g (8.8 pounds), a second stage of labor of less than 15 minutes, maternal body mass index greater than 29, and a vacuum-assisted extraction (Hudic, Fatusic, Sinanovic, and others, 2006). The less common lower plexus palsy, or Klumpke palsy, results from severe stretching of the upper extremity while the trunk is relatively less mobile.
The clinical manifestations of Erb palsy are related to the paralysis of the affected extremity and muscles. The arm hangs limp alongside the body while the shoulder and arm are adducted and internally rotated. The elbow is extended, and the forearm is pronated, with the wrist and fingers flexed; a grasp reflex may be present because finger and wrist movement remain normal (Tappero, 2003) (Fig. 9-3). In lower plexus palsy the muscles of the hand are paralyzed, with consequent wrist drop and relaxed fingers. In a third and more severe form of brachial palsy, the entire arm is paralyzed and hangs limp and motionless at the side. The Moro reflex is absent on the affected side for all forms of brachial palsy.
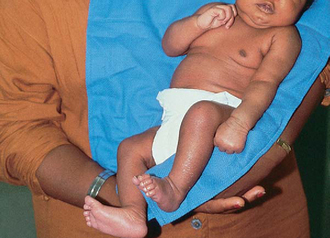
FIG. 9-3 Left-sided brachial plexus (Erb) palsy. Note extended, internally rotated arm and pronated wrist on affected side.
Treatment of the affected arm is aimed at preventing contractures of the paralyzed muscles and maintaining correct placement of the humeral head within the glenoid fossa of the scapula. Complete recovery from stretched nerves usually takes 3 to 6 months. Full recovery is expected in 88% to 92% of infants (Paige and Moe, 2006). However, avulsion of the nerves (complete disconnection of the ganglia from the spinal cord that involves both anterior and posterior roots) results in permanent damage. For those injuries that do not improve spontaneously by 3 months, surgical intervention may be needed to relieve pressure on the nerves or to repair the nerves with grafting (Joyner, Soto, and Adam, 2006). In some cases injection of botulinum toxin A into the pectoralis major muscle may be effective in reducing muscle contractures after birth-related brachial plexus injuries (Price, Ditaranto, Yaylali, and others, 2007).
Phrenic Nerve Paralysis
Phrenic nerve paralysis results in diaphragmatic paralysis as demonstrated by ultrasonography, which shows paradoxic chest movement and an elevated diaphragm. Initially, radiography may not demonstrate an elevated diaphragm if the neonate is receiving positive pressure ventilation (Volpe, 2001). The injury sometimes occurs in conjunction with brachial palsy. Respiratory distress is the most common and important sign of injury. Because injury to the phrenic nerve is usually unilateral, the lung on the affected side does not expand, and respiratory efforts are ineffectual. Breathing is primarily thoracic, and cyanosis, tachypnea, or complete respiratory failure may be seen. Pneumonia and atelectasis on the affected side may also occur.
Nursing Care Management
Nursing care of the infant with facial nerve paralysis involves aiding the infant in sucking and helping the mother with feeding techniques. Because part of the mouth cannot close tightly around the nipple, the use of a soft rubber nipple with a large hole may be helpful. The infant may require gavage feeding to prevent aspiration. Breastfeeding is not contraindicated, but the mother will need additional assistance in helping the infant grasp and compress the areolar area.
If the lid of the eye on the affected side does not close completely, artificial tears can be instilled daily to prevent drying of the conjunctiva, sclera, and cornea. The lid is often taped shut to prevent accidental injury. If eye care is needed at home, the parents are taught the procedure for administering eye drops before the infant is discharged from the nursery (see Chapter 22).
Nursing care of the newborn with brachial palsy is concerned primarily with proper positioning of the affected arm. The affected arm should be gently immobilized on the upper abdomen; passive range-of-motion exercises of the shoulder, wrist, elbow, and fingers are initiated at 7 to 10 days of age (Joyner, Soto, and Adam, 2006). Wrist flexion contractures may be prevented with the use of supportive splints. In dressing the infant, preference is given to the affected arm. Undressing begins with the unaffected arm, and redressing begins with the affected arm to prevent unnecessary manipulation and stress on the paralyzed muscles. Parents are taught to use the “football” position when holding the infant and to avoid picking the child up from under the axillae or by pulling on the arms.
The infant with phrenic nerve paralysis requires the same nursing care as any infant with respiratory distress.
The family’s emotional needs are also an important part of nursing care; the family will need reassurance regarding the neonate’s progress toward an optimal outcome.
Follow-up is also essential because of the extended length of recovery. Parents may wish to contact the Brachial Plexus Palsy Foundation* and visit the website for further information.
COMMON PROBLEMS IN THE NEWBORN
Erythema toxicum neonatorum, also known as flea-bite dermatitis or newborn rash, is a benign, self-limiting eruption of unknown cause that usually appears within the first 2 days of life. The lesions are firm, 1- to 3-mm, pale yellow or white papules or pustules on an erythematous base; they resemble flea bites. The rash appears most commonly on the face, proximal extremities, trunk, and buttocks, but it may be located anywhere on the body except the palms and soles. The rash is more obvious during crying episodes. There are no systemic manifestations, and successive crops of lesions heal without pigmentation changes. The rash usually lasts about 5 to 7 days. The etiology is unknown. However, a smear of the pustule will show numerous eosinophils and a relative absence of neutrophils. When the diagnosis is questionable, bacterial, fungal, or viral cultures should be obtained. Although no treatment is necessary, parents are usually concerned about the rash and need to be reassured of its benign and transient nature.
CANDIDIASIS
Candidiasis, also known as moniliasis, is not uncommon in the newborn. Candida albicans, the usual organism responsible, may cause disease in any organ system. It is a yeastlike fungus (it produces yeast cells and spores) that can be acquired from a maternal vaginal infection during delivery; from person-to-person transmission (especially from poor hand-washing technique); or from contaminated hands, bottles, nipples, or other articles. Mucocutaneous, cutaneous, and disseminated candidal infections are all observed in this age-group. Candidiasis is usually a benign disorder in the neonate, often confined to the oral and diaper regions. Diaper dermatitis caused by Candida organisms manifests as a moist, erythematous eruption with small white or yellow pebbly pustules. Small areas of skin erosion may also be seen.
Oral Candidiasis
Oral candidiasis (thrush) is characterized by white, adherent patches on the tongue, palate, and inner aspects of the cheeks (Fig. 9-4). It is often difficult to distinguish from coagulated milk. The infant may refuse to suck because of pain in the mouth.
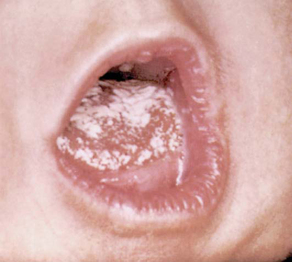
FIG. 9-4 Oral candidiasis (thrush). (From Variations and minor departures in infants, Evansville, Ind, 1978, Mead Johnson & Co.) Mead Johnson & Co.
This condition tends to be acute in the newborn and chronic in infants and young children. Thrush appears when the oral flora is altered as a result of antibiotic therapy or poor hand washing by the infant’s caregiver. Although the disorder is usually self-limiting, spontaneous resolution may take as long as 2 months, during which time lesions may spread to the larynx, trachea, bronchi, and lungs and along the gastrointestinal tract. The disease is treated with good hygiene, application of a fungicide, and correction of any underlying disturbance. The source of infection should be treated to prevent reinfection.
Topical application of 1 ml nystatin (Mycostatin) over the surfaces of the oral cavity four times a day, or every 6 hours, is usually sufficient to prevent spread of the disease or prolongation of its course. Several other drugs may be used, including amphotericin B (Fungizone), clotrimazole (Lotrimin, Mycelex), fluconazole (Diflucan), or miconazole (Monistat, Micatin) given intravenously, orally, or topically. To prevent relapse, therapy should be continued for at least 2 days after the lesions disappear (Lawrence and Lawrence, 2005). Gentian violet solution may be used in addition to one of the antifungal drugs in chronic cases of oral thrush; however, the former does not treat gastrointestinal Candida infection and may be irritating to the oral mucosa.
Nursing Care Management
Nursing care is directed toward preventing spread of the infection and correctly applying the prescribed topical medication. For candidiasis in the diaper area, the caregiver is taught to keep the diaper area clean and to apply the medication to affected areas as prescribed (see also Diaper Dermatitis, Chapter 30). Infants with candidal diaper dermatitis can introduce the yeast into the mouth from contaminated hands. Placing clothes over the diaper can prevent this cycle of self-infection.
In cases of oral thrush, nystatin is administered after feedings. The medication is distributed over the surface of the oral mucosa and tongue with an applicator or syringe; the remainder of the dose is deposited in the mouth to be swallowed by the infant to treat any gastrointestinal lesions.
In addition to good hygienic care, other measures to control thrush include rinsing the infant’s mouth with plain water after each feeding before applying the medication and boiling reusable nipples and bottles for at least 20 minutes after a thorough washing (spores are heat resistant). If used, pacifiers should be boiled for at least 20 minutes once daily. If the mother is breastfeeding it is recommended that simultaneous treatment of the infant and mother occur if either is infected (Lawrence and Lawrence, 2005).
HERPES
Neonatal herpes is one of the most serious viral infections in the newborn, with a mortality rate of up to 60% in infants with disseminated disease. Approximately 86% to 90% of herpes simplex transmission occurs during passage through the birth canal (Parker and Montrowl, 2004). The risk of infection during vaginal birth in the presence of genital herpes is estimated to be as high as 57% with active primary infection at term (Brown, Wald, Morrow, and others, 2003). However, in up to 80% of cases of neonatal herpes simplex virus (HSV) infection, the mother has no history or symptoms of infection at the time of birth, but serologic testing reveals evidence of the herpesvirus (Kimberlin, 2005).
Neonatal herpes manifests in one of three ways: (1) with skin, eye, and mouth involvement; (2) as localized central nervous system (CNS) disease; or (3) as disseminated disease involving multiple organs. In skin and eye disease a rash appearsas vesicles or pustules on an erythematous base. Clusters of lesions are common. The lesions ulcerate and crust over rapidly. Most infants with neonatal herpes eventually develop this characteristic rash, but up to 20% of neonates with disseminated disease do not develop a skin rash (Kimberlin, 2007). Ophthalmologic clinical findings include chorioretinitis and microphthalmia; neurologic involvement such as microcephaly and encephalomalacia may also develop (Kimberlin, 2007). Disseminated infections may involve virtually every organ system, but the liver, adrenal glands, and lungs are most commonly affected. In HSV meningitis infants develop multiple lesions of cortical hemorrhagic necrosis. It can occur alone or with oral, eye, or skin lesions. The presenting symptoms, which may occur in the second to fourth week of life, include lethargy, poor feeding, irritability, and local or generalized seizures.
Nursing Care Management
Neonates with herpesvirus or suspected infection (as a result of exposure) should be carefully evaluated for clinical manifestations. The absence of skin lesions in the neonate exposed to maternal herpesvirus does not indicate absence of disease. Contact precautions (in addition to standard precautions) should be instituted according to American Academy of Pediatrics and American College of Obstetricians and Gynecologists guidelines or hospital protocol. It is recommended that swabs of the mouth, nasopharynx, conjunctivae, rectum, and any skin vesicles be obtained from the exposed neonate; in addition, urine, stool, blood, and CSF specimens should be obtained for culture. Antiviral therapy with acyclovir is initiated if the cultures are positive or if there is strong suspicion of herpesvirus infection (American Academy of Pediatrics, 2006a).
BIRTHMARKS
Discolorations of the skin are common findings in the newborn infant (see discussion on skin assessment of the newborn, Chapter 8). Most, such as mongolian spots or telangiectatic nevi, involve no therapy other than reassurance to parents of the benign nature of these discolorations. However, some can be a manifestation of a disease that suggests further examination of the child and other family members (e.g., the multiple light brown café-au-lait spots that often characterize the autosomal dominant hereditary disorder neurofibromatosis and are common findings in Albright syndrome).
Darker or more extensive lesions demand further scrutiny, and excision of the lesion is recommended when feasible. Such lesions include the reddish brown solitary nodule that appears on the face or upper arm and usually represents a spindle and epithelioid cell nevus (juvenile melanoma); a giant pigmented nevus (or bathing trunk nevus), a dark brown to black, irregular plaque that is at risk of transformation to malignant melanoma; and the dark brown or black macules that become more numerous with age (junctional or compound nevi).
Vascular birthmarks may be divided into the following categories: vascular malformations, capillary hemangiomas, and mixed hemangiomas. Vascular stains (malformations) are permanent lesions that are present at birth and are initially flat and erythematous. Any vascular structure, capillary, vein, artery, or lymphatic may be involved. The two most common vascular stains are the transient macular stain (stork bite, salmon patch, or angel kiss) and the port-wine stain, or nevus flammeus. The port-wine lesions are pink, red, or, rarely, purple stains of the skin that thicken, darken, and proportionately enlarge as the child grows (Fig. 9-5, A). The macular stain is most often located on the eyelids, glabella, or nape of the neck and usually fades by 1 year of age (Conlon and Drolet, 2004).
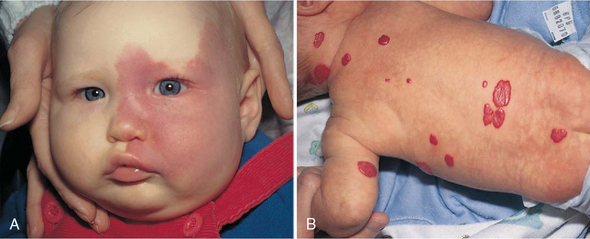
FIG. 9-5 A, Port-wine stain. B, Strawberry hemangioma. (From Zitelli BJ, Davis HW: Atlas of pediatric physical diagnosis, ed 5, St Louis, 2007, Mosby.)
Port-wine stains may also be associated with structural malformations, such as glaucoma or leptomeningeal angiomatosis (tumor of blood or lymph vessels in the pia-arachnoid) (Sturge-Weber syndrome) or bony or muscular overgrowth (Klippel-Trenaunay-Weber syndrome). Children with port-wine stains on the eyelids, forehead, or cheeks should be monitored for these syndromes with periodic ophthalmologic examination, neurologic imaging, and measurement of extremities.
The treatment of choice for port-wine stains is the use of the flashlamp-pumped pulsed dye laser. A series of treatments is usually needed. The treatments can significantly lighten or completely clear the lesions with almost no scarring or pigment change.
Capillary hemangiomas, sometimes referred to as strawberry hemangiomas, are benign cutaneous tumors that involve only capillaries. These hemangiomas are bright red, rubbery nodules with a rough surface and a well-defined margin (see Fig. 9-5, B). Strawberry hemangiomas may not be apparent at birth but may appear within a few weeks and enlarge considerably during the first year of life and then begin to involute spontaneously. It may take 5 to 12 years for complete resolution. As many as 50% of patients may be left with residual findings such as telangiectasia, redundant fatty tissue, or skin atrophy (Alster and Railan, 2006). Cavernous venous hemangiomas involve deeper vessels in the dermis and have a bluish red color and poorly defined margins. These latter forms may be associated with the trapping of platelets (Kasabach-Merritt syndrome) and subsequent thrombocytopenia (Witt, 2003; Conlon and Drolet, 2004).
Although most hemangiomas require no treatment because of their high rate of spontaneous involution, some vision and airway obstruction may necessitate therapy. The pulsed dye laser can effectively reduce some hemangiomas; systemic prednisone administered for 2 to 3 weeks or longer may also deter further growth. Subcutaneous injections of interferon alfa-2a or interferon alfa-2b may be required if prednisone therapy and the pulsed dye laser fail to control a problematic hemangioma; however, associated side effects may outweigh the benefits of therapy in some cases (Dohil, Baugh, and Eichenfield, 2000).
Nursing Care Management
Birthmarks, especially those on the face, are upsetting to parents. Families need an explanation of the type of lesion, its significance, and possible treatment.* They can benefit from seeing photographs of other infants before and after treatment for port-wine stains or after the passage of time for hemangiomas. Pictures taken to follow the involution process may further help parents gain confidence that progress is taking place.
If laser therapy is performed, the lesion will have a purplish black appearance for 7 to 10 days, after which the blackness fades and gives way to redness with an eventual lightening of the treated area. During the treatment phase parents are cautioned to avoid any trauma to the lesion or picking at the scab. The child’s fingernails are trimmed as an added precaution. Washing the area gently with water and dabbing it dry is adequate, although in some cases a topical antibiotic ointment may be used. No salicylates should be taken during the treatment phase because they decrease the effects of the therapy. The child should be kept out of the sun for several weeks and then protected with a sunscreen of at least SPF 25. Complications associated with laser treatment include redness and bruising and, less commonly, hyperpigmentation, hypopigmentation, and atrophic scarring (Alster and Railan, 2006).
NURSING CARE OF THE HIGH-RISK NEWBORN AND FAMILY
IDENTIFICATION OF HIGH-RISK NEWBORNS
The high-risk neonate can be defined as a newborn, regardless of gestational age or birth weight, who has a greater-than-average chance of morbidity or mortality because of conditions or circumstances associated with birth and the adjustment to extrauterine existence. The high-risk period encompasses human growth and development from the time of viability (the gestational age at which survival outside the uterus is believed to be possible, or as early as 23 weeks of gestation) up to 28 days following birth; thus it includes threats to life and health that occur during the prenatal, perinatal, and postnatal periods.
Classification of High-Risk Newborns
High-risk infants are most often classified according to birth weight, gestational age, and predominant pathophysiologic problems. The more common problems related to physiologic status are closely associated with the state of maturity of the infant and usually involve chemical disturbances (e.g., hypoglycemia, hypocalcemia) or consequences of immature organs and systems (e.g., hyperbilirubinemia, respiratory distress, hypothermia). Because high-risk factors are common to several specialty areas—particularly obstetrics, pediatrics, and neonatology—specific terminology is needed to describe the developmental status of the newborn (Box 9-1).
Formerly, weight at birth was considered to reflect a reasonably accurate estimation of gestational age; that is, if an infant’s birth weight exceeded 2500 g (5.5 pounds), the infant was considered to be mature. However, accumulated data have shown that intrauterine growth rates are not the same for all infants and that other factors (e.g., heredity, placental insufficiency, maternal disease) influence intrauterine growth and birth weight. From these data a more definitive and meaningful classification system that encompasses birth weight, gestational age, and neonatal outcome has been developed. (See Fig. 8-2 for size comparison of newborn infants.)
CARE OF HIGH-RISK NEWBORNS
A thorough systematic physical assessment is an essential component in the care of the high-risk infant (see Nursing Care Guidelines box). Subtle changes in feeding behavior, activity, color, oxygen saturation (Sao2), or vital signs often indicate an underlying problem. The low-birth-weight (LBW) preterm infant, especially the very low–birth-weight (VLBW) or extremely low–birth-weight (ELBW) infant, is ill equipped to withstand prolonged physiologic stress and may die within minutes of exhibiting abnormal symptoms if the underlying pathologic process is not corrected. The alert nurse is aware of subtle changes and reacts promptly to implement interventions that promote optimum functioning in the high-risk neonate. Changes in the infant’s status are noted through ongoing observations of the infant’s adaptation to the extrauterine environment.
Observational assessments of the high-risk infant are made according to the infant’s acuity; the critically ill infant requires close observation and assessment of respiratory function, including continuous pulse oximetry, electrolytes, and evaluation of blood gases. Accurate documentation of the infant’s status is an integral component of nursing care. With the aid of continuous, sophisticated cardiopulmonary monitoring, nursing assessments and daily care may be coordinated to allow for minimal handling of the infant (especially the VLBW or ELBW infant) to decrease the effects of environmental stress.
Monitoring Physiologic Data
Most neonates under intensive observation are placed in a controlled thermal environment and monitored for heart rate, respiratory activity, and temperature. The monitoring devices are equipped with an alarm system that indicates when the vital signs are above or below preset limits. However, it is essential to check the apical heart rate and compare it with the monitor reading.
Blood pressure (BP) is monitored routinely in the sick neonate by either internal or external means. Direct recording with arterial catheters is often used but carries the risks inherent in any procedure in which a catheter is introduced into an artery. Normal BP ranges for healthy preterm infants are listed in Table 9-1. Infants who have birth asphyxia, have low Apgar scores, or are mechanically ventilated may have lower mean BPs. In the neonatal intensive care unit (NICU), frequent laboratory examinations and their interpretation are integral parts of the ongoing assessment of infants’ progress. Accurate intake and output records are kept on all acutely ill infants. An accurate output can be obtained by collecting urine in a plastic urine collection bag specifically made for preterm infants (see Urine Specimens, Chapter 22) or by weighing the diapers, which is the simplest and least traumatic means of measuring urinary output. The preweighed wet diaper is weighed on a gram scale, and the gram weight of the urine is converted directly to milliliters (e.g., 25 g = 25 ml).
TABLE 9-1
Blood Pressure Ranges in Different Weight Groups of Healthy Preterm Infants*
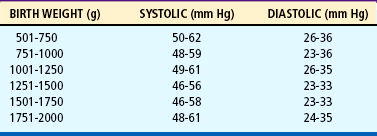
*Defined as infants without a history of maternal hypertension, Apgar scores of <3 at 1 minute and <6 at 5 minutes, pneumothorax, hematocrit 32%, serum pH 7.1, use of dopamine, infusion of erythrocytes or colloid, mechanical ventilation, or cardiopulmonary resuscitation.
Modified from Hegyi T, Carbone MT, Anwar M, and others: Blood pressure ranges in premature infants, part I, The first hours of life, J Pediatr 124(4):630, 1994.
One study has shown that urine obtained from cloth diapers or disposable diapers containing absorbent gelling material yields inaccurate results for urine specific gravity, pH, and protein. Urine samples obtained from cotton balls of 100% cotton that were strategically placed in the diaper proved to be the most accurate (Kirkpatrick, Alexander, and Cain, 1997).
Blood examinations are a necessary part of the ongoing assessment and monitoring of the high-risk newborn’s progress. The tests most often performed are blood glucose, bilirubin, calcium, hematocrit, serum electrolytes, and blood gases. Samples may be obtained from the heel; by venipuncture; by arterial puncture; or by an indwelling catheter in an umbilical vein, an umbilical artery, or a peripheral artery (see Atraumatic Care box, p. 224, and Collection of Specimens, Chapter 22).
When numerous blood samples must be drawn, it is important to maintain an accurate record of the amount of blood being removed, especially in ELBW and VLBW infants, who can ill afford to have their blood supply depleted during the acute phase of their illness. There is an increased emphasis on drawing as little blood as possible from high-risk neonates to minimize the depletion of blood volume and avoid blood transfusions and associated complications. To obtain frequent samples for monitoring arterial blood gas levels without repeated arterial punctures, pulse oximetry, which measures the saturation or percentage of oxygen in the hemoglobin, is typically used. Although used less frequently than pulse oximetry, transcutaneous carbon dioxide (tcPco2) is monitored in some situations. The nurse notes changes in oxygenation (or other aspects being monitored) associated with handling and adjusts the infant’s care accordingly. The frequency of vital signs is determined by the infant’s acuity level (seriousness of condition) and response to handling.
The nursing process in the care of the high-risk newborn and family is described in the Nursing Process box.
Respiratory Support
The primary objective in the care of high-risk infants is to establish and maintain respiration. Many infants require supplemental oxygen and assisted ventilation. All infants require appropriate positioning to maximize oxygenation and ventilation. Oxygen therapy is provided on the basis of the infant’s requirements and illness (see Respiratory Distress Syndrome, p. 284).
Thermoregulation
After or concurrent with the establishment of respiration, the most crucial need of the LBW infant is application of external warmth. Prevention of heat loss in the distressed infant is absolutely essential for survival, and maintaining a neutral thermal environment is a challenging aspect of neonatal intensive nursing care. Heat production is a complicated process that involves the cardiovascular, neurologic, and metabolic systems, and the immature neonate has all of the problems related to heat production that are faced by the full-term infant(see Thermoregulation, Chapter 8). However, LBW infants are placed at further disadvantage by a number of additional problems. They have an even smaller muscle mass and fewer deposits of brown fat for producing heat, lack insulating subcutaneous fat, and have poor reflex control of skin capillaries.
To delay or prevent the effects of cold stress, at-risk newborns are placed in a heated environment immediately after birth, where they remain until they are able to maintain thermal stability—the capacity to balance heat production and conservation with heat dissipation. Because overheating produces an increase in oxygen and calorie consumption, the infant is also jeopardized in a hyperthermic environment. A neutral thermal environment is one that permits the infant to maintain a normal core temperature with minimum oxygen consumption and calorie expenditure. Studies indicate that optimum thermoneutrality cannot be predicted for every high-risk infant’s needs. In healthy term infants it is recommended that axillary temperatures be maintained at 36.5° to 37.5° C (97.7° to 99.5° F), whereas in preterm infants axillary temperatures of 36.3° and 36.9° C (97.3° and 98.4° F) are considered appropriate (Blake and Murray, 2006). Other guidelines for VLBW infants suggest that a skin temperature of 36° to 36.3° C (96.8° to 97.3° F) may be appropriate for some infants providing the infant is medically stable and growing appropriately (Kenner, 2003). The lower temperature range in preterm infants reflects the increased risk of apnea of prematurity and thermal stress in these infants.
VLBW and ELBW infants, with thin skin and almost no subcutaneous fat, can control body heat loss or gain only within a limited range of environmental temperatures. In these infants heat loss from radiation, evaporation, and transepidermal water loss is three to five times greater than in largerinfants, and a decrease in body temperature is associated with an increase in mortality. Further research is needed to define a neutral thermal environment for the ELBW infant.
The consequences of cold stress that produce additional hazards to the neonate are (1) hypoxia, (2) metabolic acidosis, and (3) hypoglycemia. Increased metabolism in response to chilling creates a compensatory increase in oxygen and calorie consumption. If available oxygen is not increased to accommodate this need, arterial oxygen tension is decreased. This is further complicated by a smaller lung volume in relation to the metabolic rate, which creates diminished oxygen in the blood and concurrent pulmonary disorders. A small advantage is gained by the presence of fetal hemoglobin because its increased capacity to carry oxygen allows the infant to exist for longer periods in conditions of lowered oxygen tension.
The three primary methods for maintaining a neutral thermal environment are the use of an incubator, a radiant warming panel (Fig. 9-6), and an open bassinet with cotton blankets. The dressed infant under blankets can maintain a certain temperature within a wider range of environmental temperatures; however, the close observations required with a high-risk infant are best accomplished if the infant remains partially unclothed. The incubator should always be prewarmed before placing an infant in it. The use of double-walled incubators significantly improves the infant’s ability to maintain a desirable temperature and reduce energy expenditure related to heat regulation. Inside or outside the incubator, head coverings are effective in preventing heat loss. A fabric-insulated or wool cap is more effective than one fashioned from stockinette. The use of a heated gel mattress with radiant heat has been shown to significantly decrease the incidence of radiation heat loss and preserve an adequate neutral thermal environment for the VLBW neonate (L’Herault, Petroff, and Jeffrey, 2001).

FIG. 9-6 Nurse caring for infant in a radiant warmer. (Photo courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
An effective means for maintaining the desired range of temperature in the infant is the use of a manually adjusted or automatically controlled (servo-controlled) incubator. The latter mechanism, when set at the upper and lower limits of the desired circulating air temperature range, adjusts automatically in response to signals from a thermal sensor attached to the abdominal skin. If the infant’s temperature drops, the warming device is triggered to increase heat output. The servo control is usually set to a desired skin temperature between 36° and 36.5° C (96.8° and 97.7° F) (Blake and Murray, 2006).
A high-humidity atmosphere contributes to body temperature maintenance by reducing evaporative heat loss. A number of “microenvironments” may be used with the VLBW and ELBW infant to minimize evaporative and insensible water losses. These include items such as food-grade plastic bags or plastic wrap, humidified reservoirs for incubators, and humidified plastic heat shields covered with plastic wrap (Fig. 9-7). When such environments are used, special care must be taken to avoid bacterial contamination of the warm and humid environment by organisms such as Pseudomonas and Serratia, which have an affinity for moist environments; postnatally acquired pneumonia from such organisms may be fatal, particularly in VLBW infants. A systematic review of practices to decrease hypothermia at birth in LBW infants found that plastic wraps or bags kept preterm infants warmer, leading to higher temperatures on admission to neonatal units and less hypothermia (McCall, Alderdice, Halliday, and others, 2005). This practice is now recommended in the Neonatal Resuscitation Program guidelines published by the American Heart Association (2005).
FIG. 9-7 Infant under plastic wrap, which produces a draft-free environment. (Photo courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Skin-to-skin (kangaroo) contact between the stable preterm infant and parent is also a viable option for interaction because of the maintenance of appropriate body temperature by the infant. Other benefits of skin-to-skin contact are discussed later in this chapter.
Protection from Infection
Protection from infection is an integral part of all newborn care, but preterm and sick neonates are particularly susceptible. The protective environment of a regularly cleaned and changed incubator provides effective isolation from airborne infective agents. However, thorough, meticulous, and frequent hand washing is the foundation of a preventive program. Thisincludes all persons who come in contact with infants and their equipment. After handling another infant or equipment, no one ever touches an infant without first washing hands.
Personnel with infectious disorders are either barred from the unit until they are no longer infectious or are required to wear suitable shields, such as masks or gloves, to reduce the likelihood of contamination. An annual influenza vaccination is recommended for NICU personnel. Standard precautions as a method of infection control are instituted in all nursery areas to protect the infants and staff (see Chapter 22). The benefit of “gowning” by visitors and hospital staff to control infection is not supported by research. Sibling visitation in the NICU has not been shown to increase nosocomial infections (Polak, Ringler, and Daugherty, 2004).
The sources of infection rise in direct relationship to the number of persons and pieces of equipment coming in contact with the infants. Equipment used in the care of infants is cleaned on a regular basis in accordance with the manufacturer’s recommendations or institutional protocol; this includes cleaning of cribs, mattresses, incubators, radiant warmers, cardiorespiratory monitors, pulse oximeters, and vital sign–monitoring equipment after usage with one infant and before usage with another. Because organisms thrive best in water, plumbing fixtures and humidifying equipment are particularly hazardous. Disposable equipment used for water-related therapies, such as nebulizers and plastic tubing, is changed regularly.
Hydration
High-risk infants often receive supplemental parenteral fluids to supply additional calories, electrolytes, or water. Adequate hydration is particularly important in preterm infants because their extracellular water content is higher (70% in full-term infants and up to 90% in preterm infants), their body surface is larger, and the capacity for osmotic diuresis is limited in preterm infants’ underdeveloped kidneys. Therefore these infants are highly vulnerable to fluid depletion.
Parenteral fluids may be given to the high-risk neonate via several routes depending on the nature of the illness, the duration and type of fluid therapy, and unit preference. Common routes of fluid infusion include peripheral, peripherally inserted central venous (or percutaneous central venous), surgically inserted central venous, and umbilical venous catheters. The preferred sites for peripheral intravenous (IV) infusions in neonates are the peripheral veins on the dorsal surfaces of the hands or feet. Alternative sites are scalp veins and antecubital veins. Special precautions and frequent observations must accompany the use of peripheral lines (Beauman and Swanson, 2006). In many neonatal centers the percutaneous central venous catheter is used for parenteral therapy and medication administration because of less expense and decreased neonatal trauma.
In most facilities NICU nurses insert peripheral IV catheters and maintain the infusions. IVs must always be delivered by continuous infusion pumps that deliver minute volumes at a preset flow rate. The catheter is secured to the skin with a minimum amount of tape (see Skin Care, p. 259), with care taken not to cause undue pressure from the catheter hub and tubing. Because all infants, especially those who are ELBW and VLBW, are highly vulnerable to any fluid shifts, infusion rates are carefully regulated and checked hourly to prevent tissue damage from extravasation, fluid overload, or dehydration. Pulmonary edema, congestive heart failure, patent ductus arteriosus, and intraventricular hemorrhage may occur with fluid overload. Dehydration may cause electrolyte disturbances with potentially serious CNS effects.
Infants who are ELBW, tachypneic, receiving phototherapy, or in a radiant warmer have increased insensible water losses that require appropriate fluid adjustments. Nurses must monitor fluid status by daily (or more frequent) weights and accurate intake and output of all fluids, including medications and blood products. Urine-specific gravity and dipstick measurements are monitored per unit protocol, and serum electrolytes are obtained as warranted by the infant’s condition. ELBW infants often require more frequent monitoring of these parameters because of their inordinate transepidermal fluid loss, immature renal function, and propensity to dehydration or overhydration. Intolerance of even dextrose 5% is not uncommon in the ELBW infant, with subsequent glycosuria and osmotic diuresis. Alterations in behavior, alertness, or activity level in these infants receiving IV fluids may signal an electrolyte imbalance, hypoglycemia, or hyperglycemia. The nurse is also observant for tremors or seizures in the VLBW or ELBW infant, since these may be a sign of hyponatremia or hypernatremia.
A common problem observed in infants who have an umbilical artery catheter in place is vasoconstriction of peripheral vessels, which can seriously impair circulation. The response is triggered by arterial vasospasm caused by the presence of the catheter, the infusion of fluids, or injection of medication. Blanching of the buttocks, genitalia, or legs or feet is an indication of vasospasm. The problem is recognized promptly and reported to the practitioner. The nurse must also observe for signs of thrombi in infants with umbilical venous or arterial lines. The precipitation of microthrombi in the vascular bed with the use of such catheters is commonly manifested by a sudden bluish discoloration seen in the toes, called catheter toes. The problem is promptly reported to the practitioner because failure to alleviate the existing pathologic condition may result in the loss of toes or even a foot or leg.
Nutrition
Optimum nutrition is critical in the management of LBW and preterm infants, but there are difficulties in providing for their nutritional needs. The various mechanisms for ingestion and digestion of foods are not fully developed; the more immaturethe infant, the greater the problem. In addition, the nutritional requirements for this group of infants are not known with certainty. It is known that all preterm infants are at risk because of poor nutritional stores and several physical and developmental characteristics.
An infant’s nutritional needs for rapid growth and daily maintenance must be met in the presence of several anatomic and physiologic disabilities. Although some sucking and swallowing activities are demonstrated before birth and in preterm infants, coordination of these mechanisms does not occur until approximately 32 to 34 weeks of gestation, and they are not fully synchronized until 36 to 37 weeks. Initial sucking is not accompanied by swallowing, and esophageal contractions are uncoordinated. Consequently, infants are highly prone to aspiration and its attendant dangers. As infants mature, the suck-swallow pattern develops but is slow and ineffectual, and these reflexes may also become easily exhausted.
The amount and method of feeding are determined by the infant’s size and condition. Nutrition can be provided by either the parenteral or enteral route or by a combination of the two. Infants who are ELBW, VLBW, or critically ill often obtain the majority of their nutrients by the parenteral route because of their inability to digest and absorb enteral nutrition. Illness factors resulting in hypoxia and major organ immaturity further preclude the use of enteral feeding until the infant’s condition has stabilized; necrotizing enterocolitis (NEC) has previously been associated with enteral feedings in acutely ill or distressed infants (see Necrotizing Enterocolitis, p. 297). Total parenteral nutritional support of acutely ill infants may be accomplished successfully with commercially available IV solutions specifically designed to meet the infant’s nutritional needs, including protein, amino acids, trace minerals, vitamins, carbohydrates (dextrose), and fat (lipid emulsion).
Studies have shown that there are benefits to the early introduction of small amounts of enteral feedings in metabolically stable preterm infants. These minimal enteral (trophic gastrointestinal priming) feedings have been shown to stimulate the infant’s gastrointestinal tract, preventing mucosal atrophy and subsequent enteral feeding difficulties. Minimal enteral feedings with as little as 0.1 to 4 ml/kg breast milk or preterm formula may be given by gavage as early as the second or third postnatal day. These minimal enteral feedings have been shown to simulate the infant’s gastrointestinal tract, preventing mucosal atrophy and subsequent enteral feeding difficulties. Parenteral hydration and nutrition are continued until the infant is able to tolerate an amount of enteral feeding sufficient to sustain growth. An increased incidence of NEC in those VLBW infants receiving minimal enteral nutrition has not been substantiated (Reynolds and Thureen, 2007). In fact, minimal enteral feedings have been proved to increase mineral absorption, increase serum calcium and alkaline phosphatase activity, and substantially decrease the incidence of bilious gastric residuals and feeding intolerance in preterm infants (Schanler, Shulman, Lau, and others, 1999). Minimal enteral feedings have been recommended as the standard of care for feeding VLBW infants (Kliegman, 2003).
Although the timing of the first feeding has been a matter of controversy, most authorities now believe that early feeding (provided that the infant is medically stable) reduces the incidence of complicating factors, such as hypoglycemia and dehydration, and the degree of hyperbilirubinemia. The feeding regimen used varies in different units. The initial enteral feeding is usually not attempted until infants have adapted to extrauterine existence as evidenced by adequate oxygenation; gastrointestinal motility, including passage of meconium; and stable cardiopulmonary status.
Breastfeeding.: There is now sufficient evidence indicating that human milk is the best source of nutrition for term and preterm infants. Studies indicate that preterm infants as young as 32 weeks are able to breastfeed if they have adequate sucking and swallowing reflexes and there are no other contraindications, such as respiratory complications or concurrent illness (McCain, 2003). Mothers who wish to breastfeed their preterm infants are encouraged to pump their breasts until their infants are sufficiently stable to tolerate breastfeeding. Appropriate guidelines for the storage of expressed mother’s milk should be followed to decrease the risk of milk contamination and destruction of its beneficial properties.
Preterm infants may be able to successfully breastfeed earlier than previously believed (32 to 36 weeks); in addition, preterm infants who are breast-fed rather than bottle-fed demonstrate fewer incidences of oxygen desaturation; absence of bradycardia; warmer skin temperature; and better coordination of breathing, sucking, and swallowing (Gardner, Snell, and Lawrence, 2006). The preterm infant should be carefully evaluated for readiness to breastfeed, including assessment of behavioral state, ability to maintain body temperature outside an artificial heat source, respiratory status, and readiness to suckle at the mother’s breast. The latter may be accomplished with nonnutritive suckling at the breast during skin-to-skin (kangaroo) contact so that the mother and newborn may become accustomed to each other (Gardner, Snell, and Lawrence, 2006). Nasal cannula oxygen may also be provided during preterm breastfeeding on the basis of the infant’s assessed requirements.
Time, patience, and dedication on the part of the mother and the nursing staff are needed to help infants with breastfeeding. The process is begun slowly—beginning with one feeding daily and gradually increasing the feedings as the infant tolerates them. Supplementary bottle feeding is inefficient because the infant expends energy and calories to feed twice. Supplementing by gavage feeding or using a training nipple is more energy and calorie efficient. Breastfeeding the preterm infant often requires additional guidance by a lactation consultant; continued support and encouragement by the nursing staff and family members are essential. In addition, postdischarge breastfeeding often requires further guidance, counseling, and support by nursing staff (McCain, 2003).
For those infants who cannot be breast-fed but who also cannot survive except on human milk, banked donor milk is important. Because of the antiinfective and growth-promoting properties of human milk, as well as its superior nutrition, donor milk is used in many NICUs for preterm or sick infants when the mother’s own milk is not available (American Academy of Pediatrics, 2005a). Donor milk is also used therapeutically for medical purposes, such as in transplant recipients who are immunocompromised. Unprocessed human milk from unscreened donors is not recommended because of the risk of transmission of infectious agents (American Academy of Pediatrics, 2005a).
The Human Milk Banking Association of North America* has established guidelines for the operation of donor humanmilk banks. Donor milk banks collect, screen, process (pasteurize), and distribute milk donated by breastfeeding mothers who are feeding their own infants and pumping a few extra ounces each day for the milk bank.
Nipple Feeding.: Vigorous infants can be fed from a nipple with little difficulty, whereas compromised preterm infants will require alternative methods. The amount to be fed is determined largely by the infant’s weight gain and tolerance of previous feeding and is increased by small increments until a satisfactory caloric intake is ensured.
The rate of increase that is well tolerated varies from one infant to another, and determining this rate is often a nursing responsibility. Preterm infants require more time and patience to feed compared with full-term infants, and the oropharyngeal mechanism may be stressed by an attempt to feed too rapidly. It is important not to tire the infants or overtax their capacity to retain the feedings. When infants require a prolonged time (arbitrarily, more than 30 minutes) to complete a feeding, gavage feeding may be considered for the next time.
A developmental approach to feeding considers the individual infant’s readiness rather than initiating feedings based on weight and age or a predetermined time schedule. Feeding readiness is determined by each infant’s medical status, energy level, ability to sustain a brief quiet alert state, gag reflex (demonstrated with a gavage tube insertion), spontaneous rooting and sucking behaviors, and functional sucking reflex (McCain, 2003). The preterm infant may experience difficulty coordinating sucking, swallowing, and breathing, with resultant apnea, bradycardia, and decreased oxygen saturation. The infant’s ability to suck on a pacifier does not indicate complete readiness for nipple feeding or ability to coordinate the above-mentioned activities without some degree of stress; a gradual introduction of nippling in preterm infants is based on careful evaluation of their ability to maintain adequate cardiopulmonary functions while feeding. When infants are unable to tolerate bottle feedings, intermittent feedings by gavage are instituted until they gain enough strength and coordination to use the nipple.
The nipple used should be relatively firm and stable. Although a high-flow, pliable nipple requires less energy to use, it may provide a flow rate that is too rapid for some preterm infants to manage without risk of aspiration. A firmer nipple facilitates a more “cupped” tongue configuration and allows for a more controlled, manageable flow rate.
The infant is positioned in the feeder’s arms or placed semiupright in the lap (Fig. 9-8) and is held with the back curved slightly to simulate the position assumed naturally by most full-term newborns. The use of gentle cheek and jaw support for preterm infants has been shown to facilitate feedings. Stroking the infant’s lips, cheeks, and tongue before feeding helps promote oral sensitivity. Inward and upward support to the infant’s cheeks and a slightly upward lift to the chin are provided by the fingers to assist nipple compression during feeding.
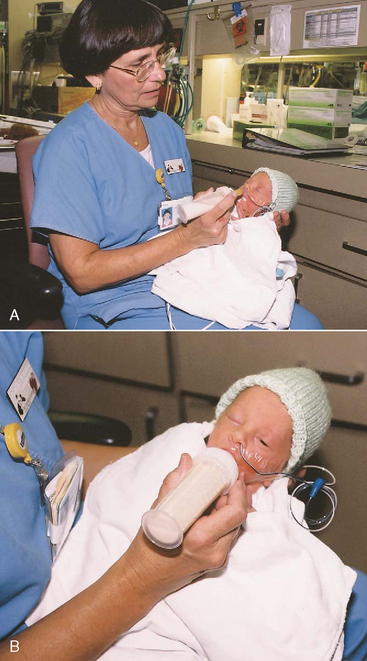
FIG. 9-8 Nipple feeding the preterm infant. A, Infant is first brought to a quiet alert state in preparation for feeding. B, After readiness is demonstrated, infant is nipple fed. (Courtesy Jeff Barnes, Education and Eastern Oklahoma Perinatal Center, St. Francis Hospital, Tulsa.)
Bottle feedings are continued if infants are able to tolerate the feedings and take the required amount. Some preterm infants respond more slowly than full-term infants; therefore the feeding interval and the amount of the feeding are individualized. Preterm infants are often slow feeders and require patience, frequent rest periods, and burping (or bubbling).
Gavage Feeding.: Gavage feeding is a safe means of meeting the nutritional requirements of infants who are unable to feed orally. These infants are usually too weak to suck effectively, are unable to coordinate swallowing, and lack a gag reflex. Gavage feedings may be provided by continuous drip regulated via infusion pump or by intermittent bolus feedings. Studies have demonstrated an overall decrease in total milk fat concentration delivery when continuous gavage infusions are administered, which suggests that intermittent or bolus gavage of expressed mother’s milk be administered when possible (Premji, Paes, Jacobson, and others, 2002). Intermittentgavage feeding is used as an energy-conserving technique for infants learning to nipple feed who become excessively tired, listless, or cyanotic.
A size 3.5, 5, 6, or 8 French feeding tube is used to instill the feeding, and the usual methods for determining correct placement are used (see Chapter 22 for technique). Although the more relaxed lower esophageal sphincter makes passage of the tube easier, there may be changes in heart rate and BP in response to vagal stimulation. For intermittent feeding it is preferable to insert the tube through the mouth rather than the nares. Nasal insertion obstructs nose breathing and may irritate the delicate nasal mucosa. Passage through the mouth also provides an opportunity to observe the sucking response. However, because of less stimulation of the gag reflex, nasal tube gavage may be used in certain situations, such as when continuous or frequent feedings (every 2 hours) are required or in older preterm infants who need supplementation after nipple feeding but who fight, gag, and vomit with oral tube management. When an indwelling tube is required, consideration should be given to using a product made of Silastic rather than polyvinyl chloride (PVC), since PVC becomes stiff when exposed to body fluids.
The stomach is aspirated, the contents measured, and the aspirate returned as part of the feeding. However, this practice may vary depending on circumstances and individual unit protocol. The amount of aspirate depends on the time since the previous feeding or concurrent illness. Some advocate deducting the amount aspirated to avoid overdistending the stomach.
The milk or formula is allowed to flow by gravity, and the length of time varies. This procedure is not used as a timesaving method for the nurse. Complications of indwelling tubes include aspiration, obstructed nares, mucous plugs, purulent rhinitis, epistaxis, infection, and possible stomach perforation.
The nurse must observe preterm infants closely for behaviors that indicate readiness for oral feedings. These include:
When these behaviors are noted, infants can be challenged with nipple feedings that are introduced slowly.
The infant may be held during gavage feedings by the caregiver or parent. Oxygen may be supplied via nasal cannula to facilitate handling. It is not recommended that the infant be removed from a primary source of oxygen for feedings, since doing so decreases oxygen availability. Nonnutritive sucking (NNS) on a pacifier may help bring the infant to a quiet alert state in preparation for feeding. Proposed benefits of NNS include improved weight gain, improved milk intake, more stable heart rate and oxygen saturation, earlier age at full oral feeds, and improved behavioral state. A systematic review of NNS found that infants receiving NNS were discharged significantly earlier than non-NNS infants and also experienced a more rapid transition from tube to bottle feedings and better bottle-feeding performance. Support for the other benefits of NNS were less consistent (Pinelli and Symington, 2005).
Feeding Resistance
Any feeding technique that bypasses the mouth precludes the opportunity for the child to practice sucking and swallowing, or to experience normal hunger and satiation cycles. Infants may demonstrate aversion to oral feedings by such behaviors as averting the head to the presentation of the nipple, extruding the nipple by tongue thrust, gagging, or even vomiting.
Other observations include disinterest in or active resistance to oral play, diminished spontaneity and motivation, and shallow interpersonal relationships, probably related to the absence of some early incorporative patterns of normal oral experiences. The longer the period of nonoral feeding, the more severe the feeding problems, especially if this period occurs during a time when the infant progresses from reflexive to learned and voluntary feeding actions. Infancy is the period during which the mouth is the primary instrument for reception of stimulation and pleasure.
Infants identified as being at risk for feeding resistance should be provided with regular oral stimulation such as stroking the oral area from cheeks to lips, touching the tongue, placing some of the feeding on the lips and tongue, and associating feeding with pleasurable activities (holding, talking, making eye contact) based on the child’s developmental level. Those who exhibit feeding aversion should begin a stimulation program to overcome resistance and acquire the ability to take nourishment by the oral route. Because management requires long-term commitment, successful implementation of a plan for oral stimulation depends on maximum parental involvement and promotion of primary nursing (see Family Focus box).
Energy Conservation
One of the major goals of care for the high-risk infant is conservation of energy. Much of the care described in this section is directed toward this end (e.g., disturbing the infant as little as possible, maintaining a neutral thermal environment, gavage feeding as appropriate, promoting oxygenation, judiciously implementing any caregiving activities that increase oxygen intake and caloric consumption). The infant who is not required to expend excess energy to breathe, eat, or alter body temperature can use this energy for growth and development. Diminishing environmental noise levels and shading the infant from bright lights also promote rest (see Developmental Outcome, p. 261).
Early in hospitalization, the prone position is best for most preterm infants and results in improved oxygenation, better-tolerated feedings, and more organized sleep-rest patterns. Infants exhibit less physical activity and energy expenditure when placed in the prone position (Fig. 9-9). Prolonged supine positioning for preterm infants is not desirable, since they appear to lose their sense of equilibrium when supine and use vital energy in attempts to recover balance by postural changes. In addition, prolonged supine positioning is associated with long-term problems such as decreased flexion of the limbs, pelvis, and trunk; widely abducted hips (frog-leg position); retracted and abducted shoulders; ankle and foot eversion; increased neck extension; and increased trunk extension with neck and back arching (Holditch-Davis, Blackburn, and VandenBerg, 2003). The American Academy of Pediatrics (2005b) continues to affirm its position that healthy infants be placed to sleep in a supine position.* When medically stable, preterm infants should also be placed in a supine position to sleep unless conditions such as gastroesophageal reflux or upper airway anomalies make this impractical (see also Sudden Infant Death Syndrome, Chapter 11). Prone positioning for play should be provided in the nursery and encouraged after discharge.
Skin Care
The skin of preterm infants is characteristically immature relative to that of full-term infants. In most preterm infants the skin barrier properties resemble those of the term infant by 2 to 4 weeks’ postnatal age, regardless of gestational age at birth. Because of its increased sensitivity and fragility, no alkaline-based soap that might destroy the skin’s acid mantle is used. The increased permeability of the skin facilitates absorption of ingredients. All skin products (e.g., alcohol or povidone-iodine) should be used with caution; the skin is rinsed with water afterward because these substances may cause severe irritation and chemical burns in VLBW and ELBW infants.
The skin is easily excoriated and denuded; therefore care must be taken to avoid damage to the delicate structure. The total skin is thinner than that of full-term infants and lacks rete pegs, appendages that anchor the epidermis to the dermis. Therefore there is less cohesion between the thinner skin layers. The use of adhesive tape or bandages may excoriate the skin or adhere to the skin surface so well that the epidermis can be separated from the dermis and pulled away with the tape. The use of pectin barriers and hydrocolloid adhesives may be useful, since these products mold well to skin contours and adhere in moist conditions. Recommendations for protecting the integrity of the skin of preterm infants include using minimal adhesive tape, backing the tape with cotton, and delaying adhesive and pectin barrier removal until adherence is reduced (Lund and Kuller, 2003). Emollients such as Eucerin or Aquaphor have been used to promote skin integrity and prevent dry, cracking, and peeling skin in infants at risk for skin breakdown; however, the use of such agents should be weighed against the increased risk for coagulase-negative infections in preterm infants (Edwards, Conner, Soll, and others, 2004).
It is unsafe to use scissors to remove dressings or tape from the extremities of very small and immature infants, since it is easy to snip off tiny extremities or nick loosely attached skin. Solvents used to remove tape are avoided because they tend to dry and burn the delicate skin. Guidelines for skin care are listed in the Nursing Care Guidelines box.
During skin assessment of preterm infants, nurses are alert to the subtle signs that indicate zinc deficiency, a problem sometimes seen in infants who have inadequate intake or abnormal losses of zinc. Breakdown usually occurs in the areas around the mouth, buttocks, fingers, and toes. In preterm and VLBW infants it may also occur in the creases of the neck, wrists, and ankles and around wounds. Zinc deficiency is most likely to appear in preterm infants with inadequate zinc intake, an ileostomy, short-bowel syndrome, or chronic diarrhea. Suspicious lesions are reported to the practitioner so that zinc supplements can be prescribed.
Skin injuries have been reported during the use of phototherapy blankets. Caution is warranted in using these products in ELBW infants or infants who are at risk for skin breakdown.
Administration of Medications
Administration of therapeutic agents such as drugs, ointments, IV infusions, and oxygen requires judicious handling and meticulous attention to detail. The computation, preparation, and administration of drugs in minute amounts often require collaboration between nurses, physicians, and pharmacists to reduce the chance for error. In addition, the immaturity of an infant’s detoxification mechanisms and inability to demonstrate symptoms of toxicity (e.g., signs of auditory nerve involvement from ototoxic drugs such as gentamicin) complicate drug therapy and require that nurses be particularly alert for signs of adverse reaction (see Administration of Medication, Chapter 22).
Nurses should be aware of the hazards of administering bacteriostatic and hyperosmolar solutions to infants. Benzyl alcohol, a common preservative in bacteriostatic water and saline, has been shown to be toxic to newborns, and products containing this preservative should not be used to flush IV catheters, to dilute or reconstitute medications, or as an anesthetic to start IVs. It is recommended that medications with preservative such as benzyl alcohol be avoided whenever possible. Nurses must read labels carefully to detect the presence of preservatives in any medication to be administered to an infant.
Hyperosmolar solutions present a potential danger to preterm infants. Hyperosmolar solutions given orally to infants can produce clinical, physiologic, and morphologic alterations, the most serious of which is NEC. Oral and parenteral medications should be sufficiently diluted to prevent complications related to hyperosmolality.
There has been heightened awareness of the impact of medication errors and subsequent poor outcomes for high-risk neonates. Nurses, physicians, and pharmacists must work in cooperation to implement strategies in the NICU environment to eradicate medication errors. Technology alone has not proved to be the solution; therefore nurses must be extremely vigilant when administering medications to preterm and high-risk infants.
Developmental Outcome
Much attention has been focused on the effects of early developmental intervention on both normal and preterm infants. Infants respond to a great variety of stimuli, and the atmosphere and activities of the NICU are overstimulating. Consequently, infants in the NICU are subjected to inappropriate stimulation that can be harmful. For example, the noise levelthat results from monitoring equipment, alarms, and general unit activity has been correlated with the incidence of intracranial hemorrhage, especially in the ELBW or VLBW infant. Personnel should reduce noise-generating activities, such as closing doors (including incubator portholes), listening to loud radios, talking loudly, and handling equipment (e.g., trash containers). Byers, Waugh, and Lowman (2006) suggest monitoring sound levels in the nursery to address problem areas. Nursing care activities, such as taking vital signs, changing the infant’s position, weighing, and changing diapers, are associated with frequent periods of hypoxia, oxygen desaturation, and elevated intracranial pressure. The more immature the infant, the less able he or she is to habituate to a single procedure, such as taking an oscillometric BP, without becoming overstimulated.
Twenty-four-hour surveillance of sick infants implies maximum visibility and often bright lights. Units should establish a night-day sleep pattern by either darkening the room, covering cribs with blankets, or placing eye patches over the infant’s eyes at night. Infants need scheduled rest periods during which the lights are dimmed, the incubators are covered with blankets, and the infants are not disturbed for handling of any kind (Holditch-Davis, Blackburn, and VandenBerg, 2003). Sleep periods should be undisturbed for at least 50 minutes to allow complete sleep cycles.
Infants’ eyes should be shielded from bright procedure lights to prevent potential harm. Many experts suggest that the human face, especially the parent’s, is the best visual stimulus and that visual stimuli be kept to a minimum early in development. Developmental care, accentuating the infant’s unique ability to achieve behavioral state organization, is tailored to the developmental level and tolerance of each infant based on a comprehensive behavioral assessment. During the early stages of development (especially before 33 weeks of gestation), external stimulation produces uncoordinated, random activity, such as jerky limb extension, hyperflexion, and irregular vital signs. At this stage infants need to have minimum environmental stimulation. Using the developmental model of supportive care, the nurse closely monitors physiologic and behavioral signs to promote organization and well-being of the high-risk infant during handling. Softly calling the infant by name and then gently placing a hand on the body signal care is beginning and alleviate the abrupt interruption that precedes caregiving. Infants are handled with slow, controlled movements (some infants are unstable if moved abruptly), and their random movements are controlled with limbs held flexed close to their bodies during turning or other position changes. This containment or facilitated tucking may also be used before invasive procedures such as heel stick to alleviate distress. Blanket swaddling and nesting or containment have been shown to decrease physiologic and behavioral stress during routine care procedures such as bathing, weighing, and heel stick. A nest constructed by placing blanket rolls underneath the bed sheet helps infants maintain an attitude of flexion when prone or side lying.
Although it must be individually adjusted, skin-to-skin contact (kangaroo care) and short periods of gentle massage can help reduce stress in preterm infants. Regular passive skin-to-skin contact between parents (mother or father) and LBW infants has been shown to alleviate stress. The parent wears a loose-fitting, open-front top that has a modified marsupial-like pocket carrier for the infant. The undressed (except for diaper) infant is placed in a vertical position on the parent’s bare chest, which permits direct eye contact, skin-to-skin sensations, and close proximity (Fig. 9-10). Skin-to-skin contact between parent and infant, in addition to being a safe and effective method for VLBW infant-parent acquaintance, can have a positive healing effect for the mother with a high-risk pregnancy. Mothers may experience psychologic healing related to preterm delivery and regain the mothering role through early skin-to-skin contact with their VLBW infants. Additional benefits of skin-to-skin care include earlier contact with mechanically ventilated infants, maintenance of neonatal thermal stability and oxygen saturation, increased feeding vigor, maintenance of organized state, and minimal untoward effects of being held (Conde-Agudelo, Diaz-Rossello, and Belizan, 2000; Anderson, 1999; Dodd, 2005). In full-term newborns, skin-to-skin contact has a strong analgesic effect during procedures such as heel lance (Ludington-Hoe, Hosseini, and Torowicz, 2005). LBW infants receiving skin-to-skin contact with breastfeeding mothers maintained higher oxygen saturation and were less likely to have desaturations below 90%, and their mothers were more likely to continue breastfeeding both in the hospital and for 1 month after discharge. Kangaroo care of preterm infants fosters appropriate neurobehavioral development by promoting stability of heart and respiratory function, minimizes purposeless movements, offers maternal proximity for attention, improves the infant’s behavioral state, and permits self-regulating behaviors (McCain, Ludington-Hoe, Swinth, and others, 2005).
Cobedding of twins (or multiples) is another developmental intervention that has been implemented in neonatal intensive care and newborn nurseries to provide a better environment for neonatal growth and development (Altimier and Lutes, 2001; DellaPorta, Aforismo, and Butler-O’Hara, 1998). Cobedding involves placing twins or other multiples together in the same crib or incubator. Preliminary data from a multicenter study indicate that twins who are cobedding have improved thermoregulation, have significantly fewer apnea and bradycardia episodes, gain weight more quickly than their single counterparts, and have decreased length of stay. Parental satisfaction is also significantly greater with cobedded newborns. One major concern with cobedding is cross-transmission of infection between the neonates, but increased infection rates have not occurred with cobedding (LaMar and Dowling, 2006).
Additional research studies have confirmed the beneficial effects of developmental care with preterm infants. In addition to requiring fewer days of mechanical ventilation, preterm infants who received individualized developmental care had shorter hospital stays; a significant decrease in complications such as intraventricular hemorrhage and bronchopulmonary dysplasia; less need for sedation when critically ill; improved neurodevelopmental scores at 9, 18, and 36 months of life; and a decrease in feeding intolerance (Symington and Pinelli, 2003; Westrup, Sizun, and Lagercrantz, 2007).
The arena of developmental care for preterm infants has expanded to include a wide variety of interventions such as infant massage, soothing soft music, recordings of parents reading stories, positioning to enhance self-regulatory abilities, enhancement of hand-to-mouth activities, uninterrupted sleep periods, decreased environmental light and noise, and even the use of stuffed animals to facilitate infant positioning. As a result of such interventions, parents may perceive the NICU environment as less threatening. Active participation in providing such an environment for their special infant also involves the parents in the provision of daily care when the newborn is critically ill and cannot be fed or held.*
When infants have reached sufficient developmental organization and stability, interventions are designed and implemented to support their growing abilities. Nurses and parents become adept at learning to read infants’ behavioral cues and supplying appropriate interventions (Table 9-2). Clues include both approach and avoidance behaviors. Approach behaviors that are supported and enhanced include tongue extension, hand clasp, hand-to-mouth movements, sucking, looking, and cooing. Signs of stress or fatigue that signal the infant’s need for “time-out” are described in Table 9-2.
TABLE 9-2
Signs of Stress or Fatigue in Neonates
Data from Als H: Toward a synactive theory of development: promise for the assessment and support of infant individuality, Infant Mental Health J 3(4):229-243, 1982; Als H: A synactive model of neonatal behavior organization: framework for the assessment of neurobehavioral development in the premature infant and for support of infants and parents in the neonatal intensive care environment, Phys Occup Ther Pediatr 6:3-55, 1986; Hunter JG: The neonatal intensive care unit. In Case-Smith J, Allen AS, Pratt PN, editors: Occupational therapy for children, ed 4, St Louis, 2001, Mosby.
When infants are recovering and are free of support systems, medically stable, and on room air or smaller amounts of oxygen, they are assessed to document behavioral state organization and ability to self-regulate. When the infant is stable and mature enough to begin developmental intervention, activities are individualized according to each infant’s cues, temperament, state, behavioral organization, and particular needs. Intervention periods are short (e.g., 2 to 3 minutes of voices, 5 minutes of quiet music). Hearing and vestibular interventions are initiated earlier than visual stimulation. One type of intervention at a time is applied to document the infant’s tolerance and response (see Nursing Care Guidelines box). An intervention program for convalescing infants includes parents and siblings early in the infant’s hospitalization; teaching parents to be responsive to the infant’s individual cues is an important function of the NICU nurse. Parents, siblings, and health care providers are encouraged to adhere to the established developmental care plan to avoid disruption in sleep-wake cycles and minimize inappropriate stimuli.
Developmental care of the preterm neonate is an ongoing process in the NICU and is incorporated into the daily care given to each infant. The nurse is cognizant of the preterm infant’s developmental needs, temperament, and newborn state, as well as environmental conditions that adversely affect the infant; nursing care is planned accordingly to enhance optimum physical, psychosocial, and neurologic development. This task is often difficult to accomplish when invasive treatments or interventions are required to stabilize the critically ill neonate.
Family Support and Involvement
Professional health workers often are so absorbed in the lifesaving physical aspects of care that they ignore the emotional needs of infants and their families. The significance of early parent-child interaction and infant stimulation has been documented by reliable research. Nurses, aware of these infant and family needs, must incorporate activities that facilitate family interaction into the nursing care plan.
The birth of a preterm infant is an unexpected and stressful event for which families are emotionally unprepared. They find themselves simultaneously coping with their own needs, the needs of their infant, and the needs of their family (especially when there are other children). To compound the situation, their infant’s precarious condition engenders an atmosphere of apprehension and uncertainty. They are faced with multiple crises and overwhelming feelings of responsibility, helplessness, and frustration.
All parents have some anxieties about the outcome of a pregnancy, but after a preterm birth the concern is heightened regarding both the viability and the normalcy of their infant. Mothers may see their infant only briefly before the newborn is removed to the intensive care unit or even to another hospital, leaving them with just the recollection of the infant’s very small size and unusual appearance. They often feel alone or lost on the mother-baby unit, belonging neither with mothers who have lost their infants nor with those who have delivered healthy, full-term infants. The staff and physicians are often guarded in discussing the infant’s condition; mothers are continually expecting to hear that their infant has died, and they are sensitive to the anxieties of other mothers and staff members. Going home without their infant only compounds their feelings of disappointment, failure, and deprivation.
When an infant is to be transported from the hospital, the parents need a description of the facility where the infant is going. They need to know the location, reputation, and nature of the facility and the care that the infant is expected to receive. The name of the infant’s physician and the telephone number of the nursery should be given to them, and unfamiliar terms such as neonatologist, ventilator, infusion, and incubator should be explained. Explanations are kept simple, and parents are given the opportunity to ask questions. If booklets are available that describe the facility, they are given to the family.
Perhaps most important, the parents should have some contact with the infant before the transport. Being able to see, touch, and (if possible) hold their infant may help decrease the parents’ anxiety. Often a photograph, or even a videotape, of their infant can serve as tangible evidence of the newborn’s existence until the parents are able to travel to the regional facility. When possible, it is often advisable to transfer the mother to the same institution as her infant.
Parents need to be informed of their infant’s progress and reassured that the infant is receiving proper care. They need to understand the smallest aspects of the infant’s condition and treatment. Parents need a realistic, honest, and direct assessment of the situation. Using nonmedical terminology, moving at a pace that is comfortable for parents to assimilate the information, and avoiding lengthy technical explanations facilitate communication with family members. Psychologic tasks that must be accomplished by parents during their infant’s care are presented in Box 9-2.
Facilitating Parent-Infant Relationships
Because of their physiologic instability, infants are separated from their mothers immediately and surrounded by a complex, impenetrable barrier of glass windows, mechanical equipment, and special caregivers. There is some evidence indicating that the emotional separation that accompanies the physical separation of mothers and infants may interfere with the normal maternal-infant attachment process discussed in Chapter 8. Maternal attachment is a cumulative process that begins before conception, strengthens by significant events during pregnancy, and matures through maternal-infant contact during the neonatal period and infancy.
When an infant is sick, the necessary physical separation appears to be accompanied by an emotional estrangement by the parents, which may seriously damage the capacity for parenting their infant. This detachment is further hampered by the tenuous nature of the infant’s condition. When survival is in doubt, parents may be reluctant to establish a relationship with their infant. They prepare themselves for the infant’s death while continuing to hope for recovery. This anticipatory grief (see Chapter 18) and hesitancy to embark on a relationship are evidenced by behaviors such as delay in giving the infant a name, reluctance in visiting the nursery (or when they do visit, focusing on equipment and treatments rather than on their infant), and hesitancy to touch or handle the infant when given the opportunity.
Family-centered care of high-risk newborns includes encouraging and facilitating parental involvement rather than isolating parents from their infant and associated care. This is particularly important in relation to mothers; to reduce the effects of physical separation, mothers are united with their newborn at the earliest opportunity.
Preparing the parents to see their infant for the first time is a nursing responsibility. The nurse prepares parents for their infant’s appearance, the equipment attached to the child, and the general atmosphere of the unit. The initial encounter with the intensive care unit is a stressful experience, and the frightening array of people, equipment, and activity is likely to be overwhelming. A book of photographs or pamphlets describing the NICU environment (infants in incubators or under radiant warmers, monitors, mechanical ventilators, and IV equipment) provides a useful and nonthreatening introduction to the NICU.
Parents are encouraged to visit their infant as soon as possible. Even if they saw the infant at the time of transport or shortly after birth, the infant may have changed considerably, especially if a number of medical and equipment requirements are associated with the infant’s hospitalization. At the bedside the nurse should explain the function of each piece of equipment and the role it plays in facilitating recovery. Explanations may often need to be patiently repeated because parents’ anxiety over the infant’s condition and the surroundings may prevent them from really “hearing” what is being said. When possible, some items related to therapy can be removed; for example, phototherapy can be temporarily discontinued and eye patches removed to permit eye-to-eye contact.
Parents appreciate the support of a nurse during the initial visit with their infant, but they may also appreciate some time alone with the infant for a short while. It is important during the early visits to emphasize the positive aspects oftheir infant’s behavior and development so that parents can focus on their infant as an individual rather than on the equipment that surrounds the child. For example, the nurse may describe the infant’s spontaneous behaviors during care, such as the grasp reflex and spontaneous movement, or make comments about the infant’s biologic functions. Most institutions have open visiting policies so that parents and siblings may visit their infant as often as they wish.
Parents vary greatly in the degree to which they are able to interact with their infant. Some may wish to touch or hold their infant during the first visit, whereas others may not feel comfortable enough to even enter the nursery. These reactions depend on a variety of prenatal and postnatal factors, such as the parity of the mother and her preparation before birth; the infant’s size, condition, and physical appearance; and the type of treatment the infant is receiving. It is essential to recognize that the individualized pacing and quality of the interactions are more important than an early onset of these interactions. Parents may not be receptive to early and extended infant contact because they need time to adjust to the impact of an infant with birth problems and must be helped to grieve before they can accept their infant.
The parents’ inability to focus on their infant is a clue for the nurse to assist the parents in expressing feelings of guilt, anxiety, helplessness, inadequacy, anger, and ambivalence. Nurses can help parents deal with these distressing feelings and recognize that they are normal responses shared by other parents. It is important to point out and reinforce the positive aspects of parents’ behavior and interactions with their infant.
Most parents feel shaky and insecure about initiating interaction with their infant. Nurses can sense parents’ level of readiness and offer encouragement in these initial efforts. Parents of preterm infants follow the same acquaintance process as do parents of term infants. They may quickly proceed through the process or may require several days, or even weeks, to complete the process. Parents begin by touching their infant’s extremities with their fingertips and poking the infant tenderly, and then proceed to caresses and fondling (figs. 9-11 and 9-12). Touching is the first act of communication between parents and child. Parents need to be prepared for their infant’s exaggerated and generalized startle responses to touch so that they will not interpret these as negative reactions to their overtures. It may be necessary to limit tactile stimuli when the infant is critically ill and labile, but the nurse can offer other options such as speaking softly or sitting at the bedside.

FIG. 9-12 Mother and father interacting with their preterm infant. (Photo courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Parents of acutely ill preterm infants may express feelings of helplessness and lack of control. Involving the parent in some type of caregiving activity, no matter how minor it may seem to the nurse, enables the parent to “take on” a more active role. Examples of such caregiving for the acutely ill infant who cannot be held and is seemingly not responding positively include moistening the infant’s lips with a small amount of sterile water on a cotton-tipped swab or slipping the diaper from under the infant when it is wet or soiled.
Eventually, parents begin to endow their infant with an identity—as part of the family. When an infant no longer appears as a foreign object and begins to take on aspects of family members, such as the father’s chin or the sister’s nose, nurses can facilitate this incorporation. Parents are encouraged to bring in clothes, a toy, a stuffed animal, or a family snapshot for their infant, and the nurse can help parents set goals for themselves and for the infant. Parents may become involved by reading a children’s storybook or nursery rhymes in a soft, soothing voice. Some families tape record the parents’ voices telling or reading stories and play the tapes when the infant is able to cope with such stimuli. Feeding schedules are discussed, and parents are encouraged to visit at times when they can become involved in the care of their infant (Fig. 9-13).

FIG. 9-13 Father feeding preterm infant. (Photo courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Throughout the parent-infant acquaintance process, the nurse listens carefully to what the parents say to assess their concerns and their progress toward incorporating their infant into their lives. The manner in which parents refer to their infant and the questions they ask reveal their worries and feelings and can serve as valuable clues to future relationships with the infant. The alert nurse is attuned to these subtle indications of parents’ needs, which provide guidelines for nursing intervention. Often all that the parents need is reassurance that they will have the support of the nurse during caregiving activities and that the behaviors about which they are concerned are normal reactions and will disappear as the infant matures.
Parents need guidance in their relationships with their infant and assistance in their efforts to meet their infant’s physical and developmental needs. The nursing staff must help parents understand that their preterm infant offers few behavioral rewards and show them how to accept small rewards from their infant. The infant’s reactions and behaviors are explained to parents, who take their infant’s jerky, rejective behavior personally. They need reassurance that these behaviors are not a reflection on their parenting skills. Parents are taught to recognize their infant’s cues regarding stimulation, handling, and other interaction, especially aversive behaviors that indicate a need for rest. Nurses need to include parents in planning their infant’s care and sensory stimulation materials, such as a music box or recording.
Above all, nurses must encourage and reinforce parents during their caregiving activities and interactions with their infant to promote healthy parent-child relationships. It is also helpful for the parents to have contact and communication with a consistent group of nurses. This decreases the different information given to parents and often instills confidence that, although the parents cannot be at their infant’s bedside 24 hours a day, there are competent and caring nurses whom they may call to inquire about the infant’s status. Periodic parent conferences involving the staff caring for the child serve to clarify misunderstandings or problems related to the infant’s condition.
Siblings.: In the past, concerns about sibling visitation in the NICU focused on fears of infection and disruption of nursing routines. These fears have not been substantiated, and sibling visits should be a part of the normal operation of NICUs (Fig. 9-14). Clearly defined policies and procedures should be developed to facilitate sibling visitation (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2007; Moore, Coker, DuBuisson, and others, 2003).

FIG. 9-14 Siblings visiting in the NICU. (Photo courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
The birth of a preterm infant is a difficult time for siblings, who rely on the support of understanding parents. When the happy anticipation is changed to sadness, worry, and altered routines, siblings are bewildered and deprived of their parents’ attention. They know something is wrong, but they have only a dim understanding of what it is. Concern about the negative effects on visiting siblings of seeing the ill newborn has not been confirmed. Children have not hesitated to approach or touch the infant, and children younger than 5 years of age have been less reluctant than older children; in addition, there have been no measurable differences between previsit and postvisit behaviors.
The potential benefits of sibling visits must be weighed against exposure of the child to the environment of the NICU. Children must be prepared for the unfamiliar NICU atmosphere, but contact with the infant appears to have a positive effect on siblings by helping them deal with the reality rather than the bizarre fantasies that are characteristic of young children. Such visits also help to bond the family as a unit.
Support Groups.: Parents need to feel that they are not alone. Parent support groups have been of immeasurable value to families of infants in the NICU. Some groups consist of parents who have infants in the hospital and share the same anxieties and concerns. Other groups include parents who have had infants in the NICU and who have dealt with the crisis effectively. The groups are usually under the leadership of a staff person and involve physicians, nurses, and social workers, but the parents can offer other parents something that no one else can provide.
An excellent resource for parents of preterm infants is the book by J. Zaichkin, Newborn Intensive Care: What Every Parent Needs to Know (2002). This resource has technical and anecdotal information regarding different problems facing preterm infants, common treatments and therapies, preparation for home discharge, and home care for the preterm infant. Another resource for parents wanting more information about kangaroo care is by S.M. Ludington-Hoe, Kangaroo Care: The Best You Can Do to Help Your Preterm Infant (1993).
Discharge Planning and Home Care
Parents become apprehensive and excited as the time for discharge approaches. They have many concerns and insecurities regarding the care of their infant. They fear that the child may still be in danger, that they will be unable to recognize signs of distress or illness in their infant, and that the infant may not yet be ready for discharge. Nurses need to begin early to assist parents in acquiring or increasing their skills in the care of their infant. Appropriate instruction must be provided and sufficient time allowed for the family to assimilate the information and learn the continuing special care requirements. Where rooming-in or other live-in arrangements are available, parents can stay for a few days and nights and assume the care of their infant under the supervision and support of the nursery staff.
There should be appropriate medical and nursing follow-up and referrals to services that can benefit the family, including developmental follow-up. Parents of preterm infants should also be given adequate information about immunizations with other discharge planning information. Most home health agencies provide nursing supervision, counseling, and referrals for nursing visits. With the trend toward early discharge, many hospital-based home health care agencies become involved in the follow-up and care of the NICU “graduate” in the home. For the parents of an infant being discharged with equipment such as an oxygen tank, apnea monitor, or even a ventilator, discharge planning requires multidisciplinary collaborative practice to ensure that the family has not only the appropriate resources, but also the available assistance for dealing with the infant’s needs. Many communities have organized support groups, including those discussed previously, those designed for parents of infants who require special care because of specific defects or disabilities, and those for parents of multiple births.
Car seat safety is an essential aspect of discharge planning, and infants younger than 37 weeks of gestation should have a period of observation in an appropriate car seat to monitor for possible apnea, bradycardia, and decreased Sao2 (American Academy of Pediatrics, 1999) (see Community Focus box). Several models can be adapted for small infants with the placement of blanket rolls on each side of the infant to support the head and trunk. For adequate support without slumping, the seat back–to-crotch strap distance must be 14 cm (5.5 inches) or less; a small rolled blanket may be placed between the crotch strap and the infant to reduce slouching. The distance from the lower harness strap to the seat bottom should be 25.5 cm (10 inches) or less to decrease the potential for the harness straps to cross the infant’s ears (Howard-Salsman, 2006). The rear-facing position provides support for the head, neck, and back, thereby reducing the stress to the neck and spinal cord in a vehicle crash. An infant who cannot tolerate a semireclining position in a standard car seat may be placed in a car bed restraint. Car seat manufacturers must specify recommended minimum and maximum weights for the occupant; therefore it is important to check the manufacturer’s recommendations before purchasing a car seat for a smaller infant. Additional guidelines are available from the American Academy of Pediatrics (2002), including a videotape for the safe transportation of preterm and LBW infants. (See Chapter 10 for a discussion of infant car restraints and the American Academy of Pediatrics website * for a complete list of appropriate car seats for infants.)
An important part of discharge planning and care of the preterm infant is nutrition for continued growth; thus choice of feeding must be carefully addressed. Human milk should be fortified according to the infant’s corrected age and physiologic needs. An enriched postdischarge formula (usually 22 kcal/oz) is required for preterm infants born at less than 36 weeks to meet appropriate growth standards (Lucas, Fewtrell, Morley, and others, 2001; Carver, Wu, Hall, and others, 2001). Full-term infant formulas are not considered adequate for proper growth in preterm infants.
Knowing that staff members are available for telephone or personal contact when the parents take the infant home provides a measure of security to anxious parents. Many NICU facilities maintain a policy of open communication between staff and parents both during the infant’s hospitalization and after discharge. It is the responsibility of the NICU staff to make certain that parents are prepared to care for their infant, both emotionally and physically. At the same time, it is important that parents establish a trusting relationship with the infant’s primary care provider in the community before discharge from the acute care facility.
Neonatal Loss
The precarious nature of many high-risk infants makes death a real and ever-present possibility (see Ethical Case Study). Although infant mortality has been reduced sharply with improved technology, the mortality rate is still greatest during the neonatal period. Nurses in the NICU are the persons who must prepare the parents for an inevitable death, provide end-of-life care for the infant and family, and facilitate a family’s grieving process after an expected or unexpected death.
The loss of an infant has special meaning for the grieving parents. It represents a loss of a part of themselves (especially for mothers), a loss of the potential for immortality that offspring represent, and the loss of the dream child that has been fantasized throughout the pregnancy. There is often a sense of emptiness and failure. In addition, when an infant has lived for such a short time, there may be few, if any, pleasant memories to serve as a basis for the identification and idealization that are part of the resolution of a loss.
To help parents understand that the death is a reality, it is important that they be encouraged to hold their infant before death and, if possible, be present at the time of death so that their infant can die in their arms if they choose. Many who deny the need to hold the infant later regret the decision.
Parents are given the opportunity to actually “parent” the infant in any manner they wish or are able to do before and after the death. This may include seeing, touching, holding, caressing, and talking to their infant privately; the parents may also wish to bathe and dress the infant. If parents are hesitant about seeing their dead infant, it is advisable to keep the body in the unit for a few hours, since many parents change their minds after the initial shock of the death.
Parents may need to see and hold the infant more than once—the first time to say “hello” and the last time to say “good-bye.” If parents wish to see the infant after the body has been taken to the morgue, the infant should be retrieved, wrapped in a blanket, rewarmed in a radiant warmer, and taken to the mother’s room or other private place. The nurse should stay with the parents and provide them an opportunity for private time alone with their dead infant. Individual grief responses of the mother and father should be recognized and handled appropriately; gender differences and cultural and religious beliefs will affect the parents’ grief responses.
Some neonatal units have a hospice approach for families with infants for whom the decision has been made to not prolong life and who are receiving only palliative care. A special “family” room is set aside and contains all supportive equipment needed for the care of the infant. It also provides a homelike atmosphere for the family. All hospice services are available to the family, and the infant remains under the care and supervision of a primary nurse on the NICU staff. (See Chapter 18 for further discussion of hospice care.)
A photograph of the infant taken before or after death is highly desirable. Parents may wish to have a special family portrait taken with the infant and other family members; this often helps personalize and make the experience more tangible. The parents may not wish to see the photograph at the time of death, but the chance to refer to it later will help make their infant seem more real, which is a part of the normal grief process. A photograph of their infant being held by the hand or touched by an adult offers a more positive image than a morgue type of photograph. A bereavement or memory packet can be given to the grieving parents and family; it may include the infant’s handprints and footprints, a lock of hair, the bedside name card, name tags, armbands, and, as appropriate to the family’s religious beliefs, a certificate of baptism.
Naming the deceased infant is an important step in the grieving process. Some parents may hesitate to give the newborn a name that had been chosen during the pregnancy for their special “baby.” However, having a tangible person for whom to grieve is an important component of the grieving process.
A primary nurse who is familiar to the family should be present during the discussion about the dead or dying infant. The nurse should talk with parents openly and honestly about funeral arrangements, since few parents have had experience with this aspect of death. Many funeral homes now offer inexpensive arrangements for these special cases. Someone from the NICU should take the responsibility for acquiring this type of information. It is often helpful to parents for the NICU to have a list of local funeral homes, services offered, and prices. Families need to be informed of the options available, but a funeral is preferable because the ritual provides an opportunity for parents to feel the support of friends and relatives. A member of the clergy of the appropriate faith may be notified if the parents wish. Issues regarding an autopsy or organ donation (when appropriate) are approached in a multidisciplinary fashion (primary practitioner and primary nurse) with respect, sensitivity to cultural and religious beliefs, tact, and consideration of the family’s wishes. (For additional suggestions for helping families who experience neonatal loss, see Grief and Perinatal Loss in Merenstein and Gardner, 2006, and Jansen, 2003.)
Before the parents leave the hospital, they are given the telephone number of the unit (if they do not have it) and invited to call any time they have any further questions. Many intensive care units make it a point to contact the parents several weeks after a neonatal death to assess the parents’ coping mechanisms, evaluate the grieving process, and provide support as needed. Several organizations are available to offer support and understanding to families who have lost a newborn; these organization include the Compassionate Friends,* Aiding Mothers and Fathers Experiencing Neonatal Death (AMEND),† and Share Pregnancy and Infant Loss Support, Inc.‡ (See also Chapter 18 for further discussion of the family and the grief process.)
Nurses who care for critically ill infants also experience grief; NICU nurses may feel helpless and sorrowful. It is important that such grief be allowed and that nurses attend the funeral or memorial service as a part of working through the grief process. Nurses may fear that showing emotion is unprofessional and that the expression of grief indicates “loss of control.” These fears are unfounded. Studies have demonstrated that to continue to be effective managers and providers of care, nurses must be allowed to grieve and support each other through the process (Gardner, Hauser, and Merenstein, 2006).
Baptism.: Because many Christian parents wish to have their child baptized if death is anticipated or is a decided possibility, this becomes a nursing responsibility. Whenever possible, it is most desirable that a representative of the parents’ faith (e.g., a Roman Catholic priest or a Protestant minister) perform such a ritual. When death is imminent, a nurse or a physician can perform the baptism by simply pouring water on the infant’s forehead (a medicine dropper is a convenient means) while repeating the words, “I baptize you in the name of the Father and of the Son and of the Holy Spirit.” This includes a birth of any gestational age, particularly when the parents are Roman Catholic.
When the parents’ faith is uncertain, a conditional baptism can be carried out by saying, “If you are capable of receiving baptism, I baptize you in the name of the Father and of the Son and of the Holy Spirit.” The baptism is recorded in the infant’s chart, and a notice is placed on the crib or incubator. Parents are informed at the first opportunity.
HIGH RISK RELATED TO DYSMATURITY
Prematurity accounts for the largest number of admissions to an NICU. Immaturity of most organ systems places infants at risk for a variety of neonatal complications (e.g., hyperbilirubinemia, respiratory distress syndrome). The actual cause of prematurity is not known in most instances (Box 9-3). The incidence of prematurity is lowest in the middle to high socioeconomic classes, in which pregnant women are generally in good health, are well nourished, and receive prompt and comprehensive prenatal care. The incidence is highest in the lower socioeconomic class, in which a combination of deleterious circumstances is present. Other factors, such as multiple pregnancies, pregnancy-induced hypertension, and placental problems that interrupt the normal course of gestation before completion of fetal development, are responsible for a large number of preterm births.
The outlook for preterm infants is largely, but not entirely, related to the state of physiologic and anatomic immaturity of the various organs and systems at the time of birth. Infants at term have advanced to a state of maturity sufficient to allow a successful transition to the extrauterine environment. Preterm infants must make the same adjustments but with functional immaturity proportional to the stage of development reached at the time of birth. The degree to which infants are prepared for extrauterine life can be predicted to some extent by birth weight and estimated gestational age (see Clinical Assessment of Gestational Age, Chapter 8).
Within the last decade infants previously designated as being preterm yet who were born between 34 and 36 6/7 weeks’ gestation have been labeled as being near-term or late-preterm infants. Such infants have some of the same risk factors as those born before 34 weeks’ gestation, but physical characteristics and adaptation to extrauterine life are variable. Late-preterm infants have metabolic and physical immaturity that places them at risk for greater mortality and morbidity than term infants (Engle, Tomashek, Wallman, and others, 2007). In the following sections the discussion of the preterm infant continues to apply to all infants who are born before a completed gestational age of 37 weeks. Because prematurity now encompasses a wider age, weight, and physiologic maturity range, physical characteristics described may also vary; such descriptions are generalized for description purposes.
Diagnostic Evaluation
Preterm infants have a number of distinct characteristics at various stages of development. Identification of these characteristics provides valuable clues to the gestational age and hence to the infant’s physiologic capabilities. The general, outward physical appearance changes as the fetus progresses to maturity. Characteristics of skin, general attitude (or posture) when supine, appearance of hair, and amount of subcutaneous fat provide cues to a newborn’s physical development. Observation of spontaneous, active movements and response to stimulation and passive movement contributes to the assessment of neurologic status. The appraisal is made as soon as possible after admission to the nursery, since much of the observation and management of infants depends on this information.
On inspection, preterm infants are very small and appear scrawny because they have only minimal subcutaneous fat deposits (or none in some cases) and have a proportionately large head in relation to the body, which reflects the cephalocaudal direction of growth. The skin is bright pink (often translucent, depending on the degree of immaturity), smooth, and shiny, with small blood vessels clearly visible underneath the thin epidermis. The fine lanugo hair is abundant over the body (depending on gestational age) but is sparse, fine, and fuzzy on the head. The ear cartilage is soft and pliable, and the soles and palms have minimal creases, resulting in a smooth appearance. The bones of the skull and the ribs feel soft, and the eyes may be closed. Male infants have few scrotal rugae, and the testes are undescended; in females the labia and clitoris are prominent. Fig. 9-15 compares the features of full-term and preterm infants.
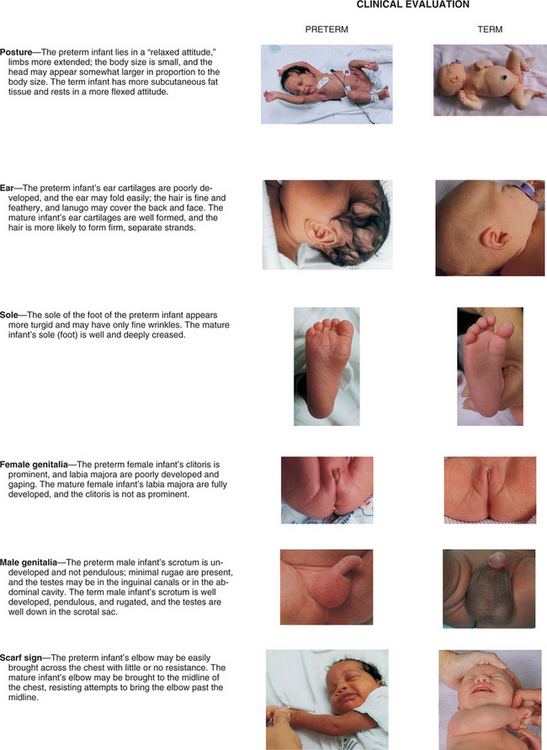
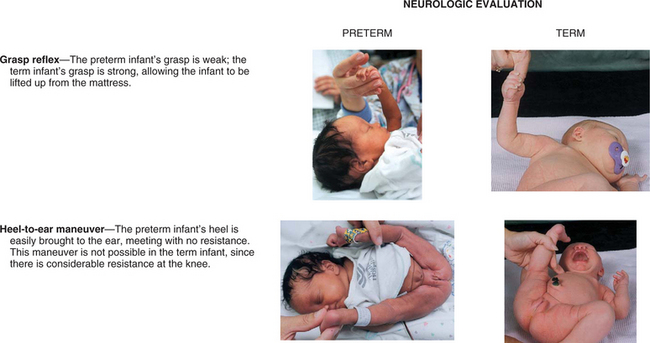
FIG. 9-15 Clinical and neurologic examinations comparing preterm and full-term infants. (Data from Pierog SH, Ferrara A: Medical care of the sick newborn, ed 2, St Louis, 1976, Mosby.)
In contrast to full-term infants’ overall attitude of flexion and continuous activity, preterm infants may be inactive and listless. The extremities maintain an attitude of extension and remain in any position in which they are placed. Reflex activity is only partially developed—sucking is absent, weak, or ineffectual; swallow, gag, and cough reflexes are absent or weak; and other neurologic signs are absent or diminished. Physiologically immature, preterm infants are unable to maintain body temperature, have limited ability to excrete solutes in the urine, and have increased susceptibility to infection. A pliable thorax, immature lung tissue, and an immature regulatory center lead to periodic breathing, hypoventilation, and frequent periods of apnea. They are more susceptible to biochemical alterations such as hyperbilirubinemia and hypoglycemia, and they have a higher extracellular water content that renders them more vulnerable to fluid and electrolyte derangements. Preterm infants exchange fully half of their extracellular fluid volume every 24 hours as compared with one seventh of the volume in adults.
The soft cranium is subject to characteristic unintentional deformation caused by positioning from one side to the other on a mattress. The head looks disproportionately longer from front to back, is flattened on both sides, and lacks the usual convexity seen at the temporal and parietal areas. This positional molding is often a concern to parents and may influence the parents’ perception of the infant’s attractiveness and their responsiveness to the infant. Positioning the infant on a waterbed or gel mattress can reduce or minimize cranial molding.
Neurologic impairment (such as intraventricular hemorrhage) and serious sequelae correlate with the size and gestational age of infants at birth and with the severity of neonatal complications. The greater the degree of immaturity, the greater the degree of potential disability. A greater incidence of cerebral palsy, attention deficit hyperactivity disorder (ADHD), visual-motor deficits, and altered intellectual functioning is observed in preterm than in full-term infants. However, behavioral development can be enhanced when families are provided with support and infants are referred to appropriate services for neurologic and developmental interventions. Parental interest and involvement are important variables in the developmental progress of infants.
Therapeutic Management
When delivery of a preterm infant is anticipated, the intensive care nursery is alerted and a team approach implemented. Ideally, a neonatologist, an advanced practice nurse, a staff nurse, and a respiratory therapist are present for the delivery. Infants who do not require resuscitation are immediately transferred in a heated incubator to the NICU, where they are weighed and where IV lines, oxygen therapy, and other therapeutic interventions are initiated as needed. Resuscitation is conducted in the delivery area until infants can be safely transported to the NICU.
Subsequent care is determined by the infant’s status. The general care of the preterm infant differs from that of the full-term infant primarily in the areas of respiratory support, temperature regulation, nutrition, susceptibility to infection, activity intolerance, and other consequences of physical immaturity.
POSTTERM INFANTS
Infants born of a gestation that extends beyond 42 weeks as calculated from the mother’s last menstrual period (or by gestational age assessment) are considered to be postterm, or postmature, regardless of birth weight. This constitutes 3.5% to 15% of all pregnancies. The cause of delayed birth is unknown. Some infants are appropriate for gestational age but show the characteristics of progressive placental dysfunction. These infants display characteristics such as absence of lanugo, little if any vernix caseosa, abundant scalp hair, and long fingernails. The skin is often cracked, parchment-like, and desquamating. A common finding in postterm infants is a wasted physical appearance that reflects intrauterine deprivation. Depletion of subcutaneous fat gives them a thin, elongatedappearance. The little vernix caseosa that remains in the skinfolds may be stained a deep yellow or green, which is usually an indication of meconium in the amniotic fluid.
There is a significant increase in fetal and neonatal mortality in postterm infants as compared with those born at term. They are especially prone to fetal distress associated with the decreasing efficiency of the placenta, macrosomia, and meconium aspiration syndrome. The greatest risk occurs during the stresses of labor and delivery, particularly in infants of primigravidas, or women delivering their first child. Close surveillance with fetal assessment and induction of labor is usually recommended when infants are significantly overdue.
HIGH RISK RELATED TO PHYSIOLOGIC FACTORS
The term hyperbilirubinemia refers to an excessive level of accumulated bilirubin in the blood and is characterized by jaundice, or icterus, a yellowish discoloration of the skin, sclerae, and nails. Hyperbilirubinemia is a common finding in the newborn and in most instances is relatively benign. However, in extreme cases, it can indicate a pathologic state.
Hyperbilirubinemia may result from increased unconjugated or conjugated bilirubin. The unconjugated form or indirect hyperbilirubinemia (Table 9-3) is the type most commonly seen in newborns. The following discussion ofhyperbilirubinemia is limited to unconjugated hyperbilirubinemia.
TABLE 9-3
Comparison of Major Types of Unconjugated Hyperbilirubinemia*
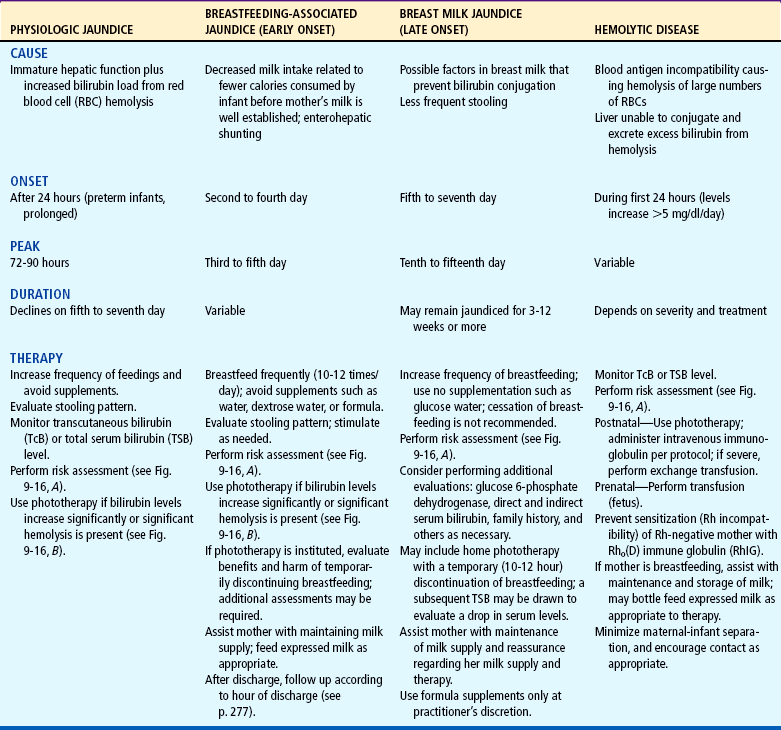
*Table depicts patterns of jaundice in term infants; patterns in preterm infants will vary according to factors such as gestational age, birth weight, and illness.
Pathophysiology
Bilirubin is one of the breakdown products of the hemoglobin that results from red blood cell (RBC) destruction. When RBCs are destroyed, the breakdown products are released into the circulation, where the hemoglobin splits into two fractions: heme and globin. The globin (protein) portion is used by the body, and the heme portion is converted to unconjugated bilirubin, an insoluble substance bound to albumin.
In the liver the bilirubin is detached from the albumin molecule and, in the presence of the enzyme glucuronyl transferase, is conjugated with glucuronic acid to produce a highly soluble substance, conjugated bilirubin, which is then excreted into the bile. In the intestine, bacterial action reduces the conjugated bilirubin to urobilinogen, the pigment that gives stool its characteristic color. Most of the reduced bilirubin is excreted through the feces; a small amount is eliminated in the urine.
Normally the body is able to maintain a balance between the destruction of RBCs and the use or excretion of by-products. However, when developmental limitations or a pathologic process interferes with this balance, bilirubin accumulates in the tissues to produce jaundice. Possible causes of hyperbilirubinemia in the newborn are:
 Physiologic (developmental) factors (prematurity)
Physiologic (developmental) factors (prematurity)
 An association with breastfeeding or breast milk
An association with breastfeeding or breast milk
 Excess production of bilirubin (e.g., hemolytic disease, biochemical defects, bruises)
Excess production of bilirubin (e.g., hemolytic disease, biochemical defects, bruises)
 Disturbed capacity of the liver to secrete conjugated bilirubin (e.g., enzyme deficiency, bile duct obstruction)
Disturbed capacity of the liver to secrete conjugated bilirubin (e.g., enzyme deficiency, bile duct obstruction)
 Combined overproduction and undersecretion (e.g., sepsis)
Combined overproduction and undersecretion (e.g., sepsis)
 Some disease states (e.g., hypothyroidism, galactosemia, infant of a diabetic mother)
Some disease states (e.g., hypothyroidism, galactosemia, infant of a diabetic mother)
 Genetic predisposition to increased production (Native Americans, Asians)
Genetic predisposition to increased production (Native Americans, Asians)
The most common cause of hyperbilirubinemia is the relatively mild and self-limited physiologic jaundice, or icterus neonatorum. Unlike hemolytic disease of the newborn (HDN) (see p. 281), physiologic jaundice is not associated with any pathologic process. Although almost all newborns experience elevated bilirubin levels, only about half demonstrate observable signs of jaundice.
Two phases of physiologic jaundice have been identified in full-term infants. In the first phase, bilirubin levels of formula-fed Caucasian and African-American infants gradually increase to a peak by day of life 3 to 4, with a subsequent rapid decrease in serum levels for 2 to 3 days; in the second phase, serum bilirubin levels gradually decrease to less than 1 mg/dl over 1 to 2 weeks (Blackburn, 2007). This pattern varies according to racial group, method of feeding (breast vs bottle), and gestational age. In term breastfed infants, serum bilirubin levels peak later than those in formula-fed infants and at higher levels (7 to 14 mg/dl); in term breastfed infants, the gradual decline in serum bilirubin levels may occur over 2 to 4 weeks, and in some cases may last as long as 6 weeks (Blackburn, 2007).
As noted above, infants of Asian descent (as well as Native Americans) have mean bilirubin levels almost twice those seen in Caucasians or African Americans. An increased incidence of hyperbilirubinemia is seen in newborns from certain geographic areas, particularly areas around Greece. These populations may have glucose-6-phosphate dehydrogenase (G6PD) deficiency, which can cause hemolytic anemia.
On average, newborns produce twice as much bilirubin as do adults because of higher concentrations of circulating erythrocytes and a shorter life span of RBCs (only 70 to 90 days, in contrast to 120 days in older children and adults). In addition, the liver’s ability to conjugate bilirubin is reduced because of limited production of glucuronyl transferase. Newborns also have a lower plasma-binding capacity for bilirubin because of reduced albumin concentrations as compared with older children. Normal changes in hepatic circulation after birth may contribute to excess demands on liver function.
Normally, conjugated bilirubin is reduced to urobilinogen by the intestinal flora and excreted in feces. However, the sterile and less motile newborn bowel is initially less effective in excreting urobilinogen. In the newborn intestine the enzyme β-glucuronidase is able to convert conjugated bilirubin into the unconjugated form, which is subsequently reabsorbed by the intestinal mucosa and transported to the liver. This process, known as enterohepatic circulation, or shunting, is accentuated in the newborn and is thought to be a primary mechanism in physiologic jaundice (Blackburn, 2007). Feeding (1) stimulates peristalsis and produces more rapid passage of meconium, thus diminishing the amount of reabsorption of unconjugated bilirubin; and (2) introduces bacteria to aid in the reduction of bilirubin to urobilinogen. Colostrum, a natural cathartic, facilitates meconium evacuation.
Breastfeeding is associated with an increased incidence of jaundice as a result of two distinct processes. Breastfeeding-associated jaundice (early-onset jaundice) begins at 2 to 4 days of age and occurs in approximately 12% to 35% of breast-fed newborns (Blackburn, 2007). The jaundice is related to the process of breastfeeding and probably results from decreased caloric and fluid intake by breast-fed infants before the milk supply is well established, since fasting is associated with decreased hepatic clearance of bilirubin (Porter and Dennis, 2002). Reduced fluid intake results in dehydration, which also concentrates the bilirubin in the blood.
Breast milk jaundice (late-onset jaundice) begins at age 5 to 7 days and occurs in 2% to 4% of breast-fed infants (Blackburn, 2007). Rising levels of bilirubin peak during the second week and gradually diminish. Despite high levels of bilirubin that may persist for 3 to 12 weeks, these infants are well. The jaundice may be caused by factors in the breast milk (pregnanediol, fatty acids, and β-glucuronidase) that either inhibit the conjugation or decrease the excretion of bilirubin. Less frequent stooling by breast-fed infants may allow for extended time for reabsorption of bilirubin from stools.
Diagnostic Evaluation
The degree of jaundice is determined by serum bilirubin measurements. Normal values of unconjugated bilirubin are 0.2 to 1.4 mg/dl. In the newborn, levels must exceed 5 mg/dl before jaundice (icterus) is observable. It is important to note, however, that the evaluation of jaundice is not based solely on serum bilirubin levels, but also on the timing of the appearance of clinical jaundice; gestational age at birth; age in days since birth; family history, including maternal Rh factor; evidence of hemolysis; feeding method; infant’s physiologic status; and the progression of serial serum bilirubin levels. The following criteria are indicators of pathologic jaundice that, when present,warrant further investigation as to the cause of the jaundice. It is not an all-inclusive list; other factors are also evaluated:
 Appearance of jaundice within 24 hours of birth
Appearance of jaundice within 24 hours of birth
 Persistent jaundice over 2 weeks in a full-term formula-fed infant
Persistent jaundice over 2 weeks in a full-term formula-fed infant
 Total serum bilirubin levels over 12.9 mg/dl (term infant) or over 15 mg/dl (preterm infant); upper limit for breast-fed infant: 15 mg/dl
Total serum bilirubin levels over 12.9 mg/dl (term infant) or over 15 mg/dl (preterm infant); upper limit for breast-fed infant: 15 mg/dl
 Increase in serum bilirubin by 5 mg/dl/day
Increase in serum bilirubin by 5 mg/dl/day
 Direct bilirubin exceeding 1.5 to 2 mg/dl
Direct bilirubin exceeding 1.5 to 2 mg/dl
 Total serum bilirubin level over 95th percentile for age (in hours) on hour-specific nomogram (Fig. 9-16)
Total serum bilirubin level over 95th percentile for age (in hours) on hour-specific nomogram (Fig. 9-16)
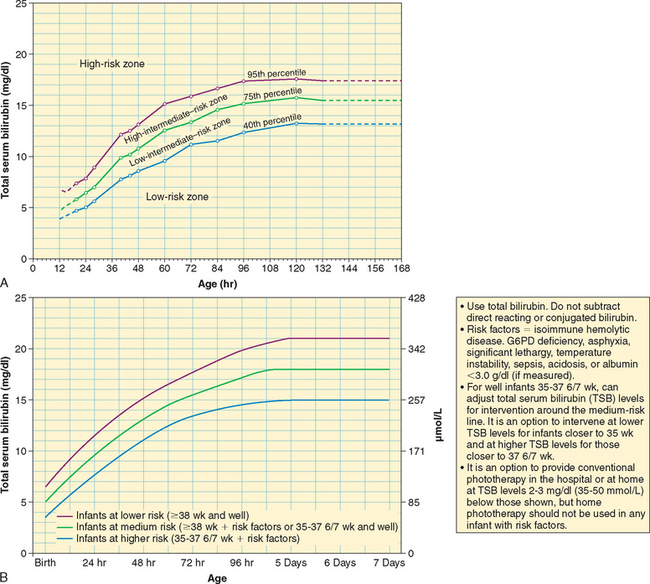
FIG. 9-16 A, Nomogram for designation of risk in 2840 well newborns at 36 or more weeks of gestational age with birth weight of 2000 g (4.4 pounds) or more, or 35 or more weeks of gestational age and birth weight of 2500 g (5.5 pounds) or more, based on the hour-specific serum bilirubin values. (This nomogram should not be used to represent the natural history of neonatal hyperbilirubinemia.) B, Guidelines for phototherapy in hospitalized infants of 35 or more weeks’ gestation. (A, From Bhutani VK, Johnson L, Sivieri EM: Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns, Pediatrics 103(1):6-14, 1999. B, From American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation, (Pediatrics 114(1):297-316, 2004.)
Noninvasive monitoring of bilirubin via cutaneous reflectance measurements (transcutaneous bilirubinometry [TcB]) allows for repetitive estimations of bilirubin and, when used correctly, may decrease the need for invasive monitoring (Thayyil and Marriott, 2005). The new TcB monitors provide accurate measurements within 2 to 3 mg/dl in most neonatal populations at serum levels below 15 mg/dl (American Academy of Pediatrics, 2004). TcB monitors must be used according to published guidelines as a screening tool, not as a predictor of need for therapy; multiple readings over time at a consistent site (e.g., sternum or forehead) are of more value than a single reading. After phototherapy has been initiated, TcB is no longer useful as a screening tool.
The use of hour-specific serum bilirubin levels to predict newborns at risk for rapidly rising levels has now become an official recommendation by the American Academy of Pediatrics (2004) for the monitoring of healthy neonates of 35 weeks of gestation or older before discharge from the hospital. The use of a nomogram with three levels (high, intermediate, or low risk) of rising total serum bilirubin values assists in the determination of which newborns might need further evaluation after discharge (Bhutani, Johnson, and Sivieri, 1999; Bhutani, Johnson, and Keren, 2004) (see Fig. 9-16, A). The hour-specific bilirubin risk nomogram is used to determine the infant’s risk for developing hyperbilirubinemia requiring medical treatment or closer screening. Risk factors recognized to place infants in the high-risk category include gestational age of less than 38 weeks, breastfeeding, a sibling who had significant jaundice, and jaundice appearing before discharge (American Academy of Pediatrics, 2004).
It is also recommended that healthy near-term and term infants (older than 34⅞ weeks) receive follow-up care and assessment of bilirubin within 3 days of discharge if discharged at less than 24 hours and a risk assessment with TcB or the hour-specific nomogram. Newborns discharged at 24 to 47.9 hours should receive follow-up evaluation within 4 days (96 hours), and those discharged between 48 and 72 hours should receive follow-up within 5 days (American Academy of Pediatrics, 2004). The serum bilirubin may be obtained at the time of the metabolic screening, thus precluding the need for additional blood sampling. The newest guidelines for monitoring and treating neonatal hyperbilirubinemia are published extensively elsewhere, and the reader is referred to the American Academy of Pediatrics (2004) reference for an in-depth overview of management guidelines.
End-tidal carbon monoxide (ETCO) levels (measured in exhaled breath) may be of value in determining the presence of hemolysis and the rate of heme degradation and bilirubin production in some infants; these determinations may be useful in determining the need for surveillance during the first week of life (American Academy of Pediatrics, 2004). ETCO measurements are reported to have limited value in neonates whose mothers smoked during pregnancy and in neonates with G6PD deficiency (Steffensrud, 2004).
Complications.: Unconjugated bilirubin is highly toxic to neurons; therefore an infant with severe jaundice is at risk of developing bilirubin encephalopathy, a syndrome of severe brain damage resulting from the deposition of unconjugated bilirubin in brain cells. Kernicterus describes the yellow staining of the brain cells that may result in bilirubin encephalopathy (Sgro, Shah, and Campbell, 2005). The damage occurs when the serum concentration reaches toxic levels, regardless of cause. There is evidence that a fraction of unconjugated bilirubin crosses the blood-brain barrier in neonates with physiologic hyperbilirubinemia. When certain pathologic conditions exist in addition to elevated bilirubin levels, there is an increase in the permeability of the blood-brain barrier to unconjugated bilirubin and, thus, potential irreversible damage. The exact level of serum bilirubin required to cause damage is not yet known.
Multiple factors contribute to bilirubin neurotoxicity; therefore serum bilirubin levels alone do not predict the risk of brain injury. Factors that enhance the development of bilirubin encephalopathy include metabolic acidosis, lowered serum albumin levels, intracranial infections such as meningitis, and abrupt fluctuations in BP. In addition, any condition that increases the metabolic demands for oxygen or glucose (e.g., fetal distress, hypoxia, hypothermia, hypoglycemia) also increases the risk of brain damage at lower serum levels of bilirubin.
The signs of bilirubin encephalopathy are those of CNS depression or excitation. Prodromal symptoms consist of decreased activity, lethargy, irritability, hypotonia, and seizures. Later these subtle findings are followed by development of athetoid cerebral palsy, mental retardation, and deafness (Sgro, Shah, and Campbell, 2005). Those who survive may eventually show evidence of neurologic damage, such as mental retardation, ADHD, delayed or abnormal motor movement (especially ataxia or athetosis), behavior disorders, perceptual problems, or sensorineural hearing loss.
Therapeutic Management
The primary goals in the treatment of hyperbilirubinemia are to prevent bilirubin encephalopathy and, as in any blood group incompatibility, to reverse the hemolytic process (p. 281). The main form of treatment involves the use of phototherapy. Exchange transfusion is generally used for reducing dangerously high bilirubin levels that occur with hemolytic disease.
The pharmacologic management of hyperbilirubinemia with phenobarbital has centered primarily on the infant with hemolytic disease, and phenobarbital is most effective when given to the mother several days before delivery. Phenobarbital promotes (1) hepatic glucuronyl transferase synthesis, which increases bilirubin conjugation and hepatic clearance of the pigment in bile; and (2) protein synthesis, which may increase albumin for more bilirubin binding sites. However, the use of phenobarbital in either the antenatal or the postnatal period has not proved to be as effective as other treatments in reducing bilirubin. Bilirubin production in the newborn can be decreased by inhibiting heme oxygenase—an enzyme needed for heme breakdown (to biliverdin)—with metalloporphyrins, especially tin protoporphyrin and tin mesoporphyrin. The use of heme-oxygenase inhibitors provides a preventive approach to hyperbilirubinemia (Bhutani, Gourley, Adler, and others, 2000; Drummond and Kappas, 2004).
Healthy near-term and full-term infants with jaundice may also benefit from early initiation of feedings and frequent breastfeeding. These preventive measures are aimed at promoting increased intestinal motility, decreasing enterohepatic shunting, and establishing normal bacterial flora in the bowel to effectively enhance the excretion of unconjugated bilirubin.
Phototherapy consists of the application of fluorescent light to the infant’s exposed skin (Fig. 9-17). Light promotes bilirubin excretion by photoisomerization, which alters the structure of bilirubin to a soluble form (lumirubin) for easier excretion.
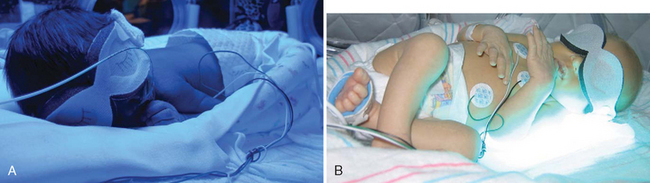
FIG. 9-17 A, Infant receiving phototherapy; note nested boundaries for comfort and eye protection.B, Newborn laying on BiliBlanket, which may be used with overhead lights to provide intensive phototherapy. (Courtesy E. Jacobs, Texas Children’s Hospital, Houston.)
Studies indicate that blue fluorescent light is more effective than white fluorescent in reducing bilirubin. However, because blue light alters the infant’s coloration, the normal light of fluorescent bulbs in the spectrum of 420 to 460 nm is often preferred so that the infant’s skin can be better observed for color (jaundice, pallor, cyanosis) or other conditions. For phototherapy to be effective, the infant’s skin must be fully exposed to an adequate amount of the light source. When serum bilirubin levels are rapidly increasing or approximating critical levels, double or intensive phototherapy is recommended. Intensive phototherapy with a higher irradiance is considered to be more effective than standard phototherapy for rapid reduction of serum bilirubin levels (Beachy, 2007). The color of the infant’s skin does not influence the efficacy of phototherapy. Best results occur within the first 24 to 48 hours of treatment. Phototherapy alone is not effective in the management of hyperbilirubinemia when levels are at a critical level or are rising rapidly; it is designed primarily for the treatment of moderate hyperbilirubinemia.
An alternative to traditional phototherapy “bililights” is the fiberoptic blanket or panel (Wallaby, BiliBlanket), which consists of a light-generating illuminator; a bundle of plastic fibers affixed to a panel that distributes the energy; and a soft, disposable, light-permeable cover to protect the infant. The blanket delivers therapeutic light consistently and continuously to the infant and achieves the same photoisomerization as conventional phototherapy. The fiberoptic blanket is especially suited for home phototherapy. The portable blanket permits more infant-parent interaction and better temperature control because the infant can be covered. It also eliminates the need for using eye patches (if eyes are not exposed to a direct light source).
A Cochrane review of 24 studies indicated that conventional phototherapy was more effective at lowering serum bilirubin values than fiberoptic lights alone; when two fiberoptic devices were used simultaneously in preterm infants, the therapy was as effective as conventional therapy at reducing serum bilirubin. Combination phototherapy (fiberoptic and conventional) was found to be more effective than conventional therapy alone. The authors further concluded that fiberoptic phototherapy is a safe and effective alternative to conventional therapy in preterm infants. The authors also pointed out that no trials were available to show that fiberoptic therapy is more effective than conventional phototherapy (Mills and Tudehope, 2005).
The American Academy of Pediatrics (2004) practice parameter guidelines provide suggestions for initiating phototherapy (see Fig. 9-16, B) and for implementing exchange transfusion in healthy, term infants.
Some clinicians believe that preterm infants have a higher risk of developing pathologic jaundice at lower serum bilirubin levels than do healthy, term infants because of associated illness factors that may increase the entry of bilirubin into the brain; however, research has failed to confirm this belief (Cashore, 2000). Until further research is completed, the recommendations for starting phototherapy in infants of 36 weeks of gestation is at levels of 14.6 mg/dl; for those at 32 weeks, levels of 8.8 mg/dl; for those at 28 weeks, levels of 5.8 mg/dl; and for 24 week infants, 4.7 mg/dl (Maisels and Watchko, 2003). However, each infant should be carefully evaluated with other illness and risk factors in mind, rather than depending on absolute values for all infants in a specific group.
Phototherapy has not been found to cause long-term adverse effects. The effectiveness of treatment is determined by a decrease in total serum bilirubin levels. Concurrently, the infant’s total physical status is assessed continually because the suppression of jaundice by phototherapy may mask signs of sepsis, hemolytic disease, or hepatitis.
Recommendations for prevention and management of early-onset jaundice in breast-fed infants include encouraging frequent breastfeeding, preferably every 2 hours; avoiding glucose water, formula, or water supplementation; and monitoring for early stooling. The infant’s weight, voiding, and stooling should be evaluated along with the breastfeeding pattern (Lawrence and Lawrence, 2005). Parents are taught to evaluate the number of voids and evidence of adequate breastfeeding once the infant is home and are encouraged to bring the newborn to the hospital, clinic, or primary care practitioner if there are indications of hyperbilirubinemia (Wheeler, 2000).
Bilirubin levels are monitored in late-onset jaundice, and treatment options vary. If the serum bilirubin levels remain above 16 mg/dl for more than 24 hours, obtain a bilirubin reading 2 hours after breastfeeding, which may then be interrupted for 10 to 12 hours (provide fluid and calories during this time with supplementation) and repeat levels drawn; with a serum bilirubin level decrease of 2 mg/dl or more, the infant may resume breastfeeding if levels are below 15 mg/dl.In the event that levels do not drop significantly, further evaluation is necessary (Lawrence and Lawrence, 2005). Parents should be offered the option of continuing breastfeeding, since no harmful effects have been found in healthy, term infants with bilirubin levels less than 20 mg/dl who continue to consume human milk. Based on these findings, Maisels (2005) suggests that, when feasible, phototherapy be implemented without the interruption of breastfeeding. Home phototherapy and continued breastfeeding are another option. Phototherapy as a treatment for hyperbilirubinemia is further discussed on pp. 279–280.
Prognosis.: Early recognition and treatment of hyperbilirubinemia prevent unnecessary medical therapies, parent-infant separation, breastfeeding disruption and possibly failure, and severe brain damage (bilirubin encephalopathy). The characteristic impaired neurologic functions associated with bilirubin encephalopathy include athetosis (involuntary writhing movements), sensorineural hearing loss, paralytic gaze palsy, and other gaze abnormalities. Motor skills are delayed, and dental enamel hypoplasia may also occur.
 IN TEXT
IN TEXT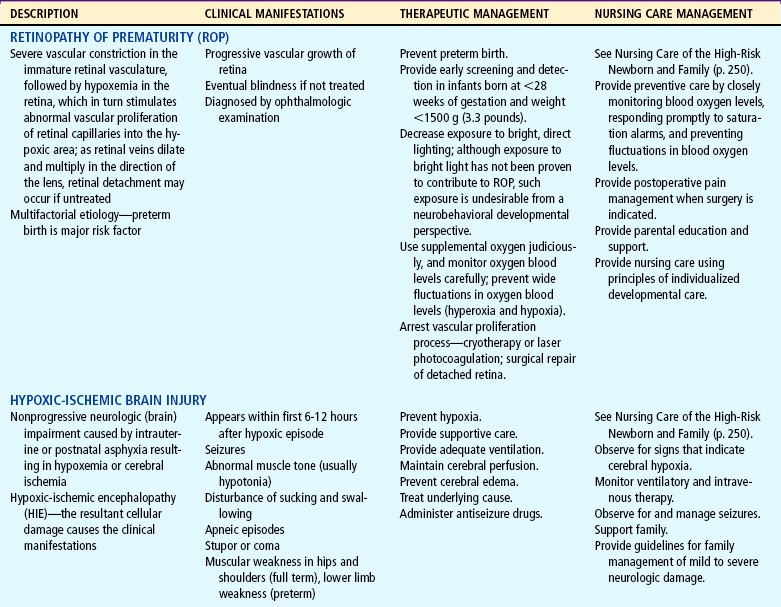

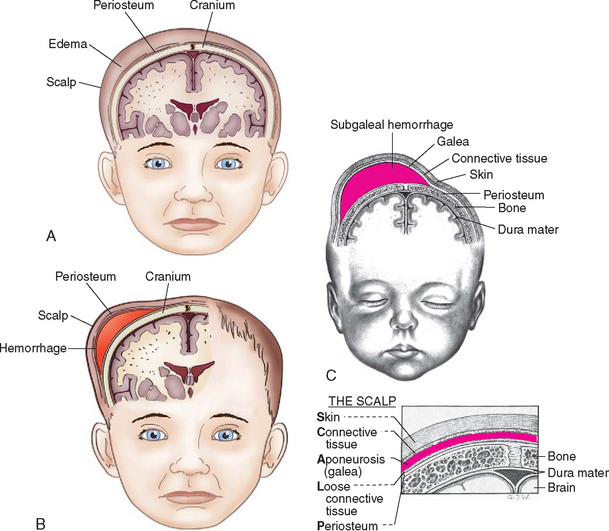
 FAMILY FOCUS
FAMILY FOCUS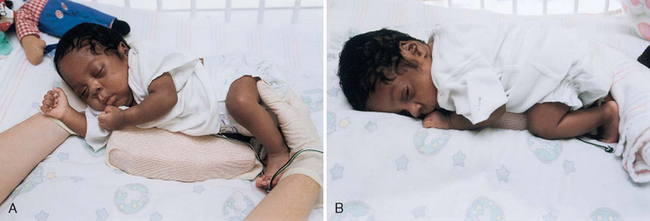


 ETHICAL DECISION MAKING MODEL
ETHICAL DECISION MAKING MODEL