Minerals
A large number of mineral elements are present in nature, of which only a few are essential for the human body. They can be divided into five groups:
1. The first group includes carbon, hydrogen and nitrogen. The body obtains these elements from dietary fats, carbohydrates, proteins, and also from water.
2. The second group includes calcium, phosphorus, magnesium, sodium, potassium, chloride and sulphur. These elements are nutritionally important and are required in relatively larger amount in the diet (100 mg/day). They are called the major elements or macro elements.
3. The elements of the third group, known as the trace elements, are required in diet in much smaller amounts (< 100 mg/day). Chromium, cobalt, copper, iodine, iron, manganese, molybdenum, selenium and zinc are examples of such elements. Fluorine is usually considered to be a trace element, although its role in humans is not clearly understood. Its deficiency is associated with tooth decay.
4. The fourth group contains arsenic, cadmium, nickel, silicon, tin and vanadium. These elements have well-defined role in animals but have no essential (or known) function in humans.
5. The final group consists of certain toxic elements, such as lead and mercury.
In this chapter, the metabolism and functional significance of some biologically important minerals are described. After going through this chapter, the student should be able to understand:
• Absorption, transport and excretion of minerals.
• Mineral deficiency or excess: causes and consequences.
• Sources and daily requirements of minerals.
• Major elements: physiological role of sodium, potassium, magnesium.
• Trace elements: physiological role and deficiency disorders of iron, copper, magnesium, zinc, selenium, and molybdenum.
I Absorption, Transport and Excretion of Minerals
Intestinal absorption of most dietary minerals is difficult, except for sodium and potassium. This is because of the tendency of most minerals to form complexes with fibres or phytates in intestine, which are relatively insoluble. This hinders their absorption, and therefore, most of the ingested minerals are excreted in faeces. Mediation of specific carrier proteins is required for their absorption; for example, zinc absorption requires a mucosal binding factor and iron absorption requires a specific intracellular carrier molecule. Synthesis of these (carrier) proteins, therefore, serves as an important mechanism for control of mineral levels in the body.
Few minerals circulate in free, unbound form, whereas transport of others requires specific binding proteins-β1 e.g. transferrin for iron.
Storage of minerals also requires binding to specific proteins; for example, apoferritin protein is required for storage of iron. Excretion of minerals occurs through the renal or the hepatobiliary route.
II Mineral Deficiency or Excess
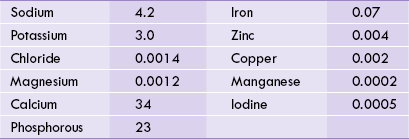
Total body content of some minerals is given in Table 19.1 . Deficiency or excess of these is potentially hazardous. Because circulating levels of a mineral represents net result of its absorption, utilization, storage and excretion, loss of control over these processes (besides inappropriate dietary intake) causes mineral deficiency, or excess. For example, iron deficiency may occur due to any of the following causes:
Since minerals are required for performing specific functions, their deficiency leads to defined clinical syndromes. Iron deficiency, for instance, results in microcytic hypochromic anaemia, described later.
Excess content of almost all minerals causes toxic symptoms. Excess iron causes functional impairment of liver and pancreas (condition called haemochromatosis); copper overload results in hepatolenticular degeneration (condition called Wilsons’s disease), and excessive molybdenum results in several toxic manifestations, collectively referred to as molybdenosis.
III Sources and Daily Requirements of Minerals
The essential minerals are widely distributed in a variety of foodstuffs such as whole-grain cereals, meat, fish, vegetables, fruits and dairy foods. Since concentration of minerals in most foods is very small, it is necessary to consume sufficient quantities of varied foodstuffs to meet the daily requirements of all minerals. Daily requirements and occurrence in enzymes of important minerals are given in Table 19.2 .
Table 19.2
Daily requirement and association with enzymes of certain essential minerals in the human being
| Mineral | Daily requirement | Enzyme(s) in which the mineral is found |
| Ca | 800 mg | Coagulation factors, e.g. prothrombin, second messenger |
| Mg | 300–400 mg | Most ATP-requiring enzymes, e.g. hexokinase, pyruvate kinase |
| Fe | 1–2 mg | Proline hydroxylase, catalase diphosphoribonucleoside dehydrogenase, peroxidases |
| Cu | 2 mg | Cytochrome oxidase, tyrosinase, lysyl oxidase, superoxide dismutases, monoamine oxidase |
| Se | 50–200 μg | Glutathione peroxidase, deiodinase |
| Zn | 16 mg | Over 200 enzymes, e.g. carbonic anhydrase, carboxypeptidases, ALA synthase |
| Mo | 0.15–0.5 mg | Xanthine oxidase, sulphite oxidase, aldehyde oxidase |
| Cr | 50–100 μg | Glucose tolerance factor (GTF) |
| Mn | 2.5–5 mg | Superoxide dismutases |
IV Major Elements
A Sodium
Sodium is the principal cation of the extra-cellular compartment. About 50 mmol/kg body weight of sodium is present in human body, of which about 50% is present in bones, 40% in extracellular fluids and the remaining 10% in soft tissues. The plasma concentration of sodium is 135–145 mmol/L. Other extracellular fluids are also rich in sodium, whereas the intracellular fluid concentration is only about 35 mmol/L (Table 19.3 ).
Table 19.3
Distribution of Na+ and K+ (cations) in body fluids. Concentration of these cations are given in mmol/L (mean values)
| Na+ (chief EC cation) | K+ (chief IC cation) | |
| Extracellular (EC) concentration | 140 mmol/L | 5 mmol/L |
| Intracellular (IC) concentration | 35 mmol/L | 145 mmol/L |
Biochemical Functions
Sodium is involved in maintenance of irritability of excitable tissues, regulation of osmotic pressure and pH of body fluids, and in membrane transport.
1 Neuromuscular excitability
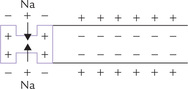
Sodium, together with potassium (the other monovalent cation), increases the neuromuscular excitability; this is counterbalanced by the effect of the divalent cations, calcium and magnesium. The cations are distributed across the cell membrane of nerve fibres in such a way that the membrane exterior is slightly positive compared to the interior. This sets up a potential difference, known as the resting potential.
When a stimulus is applied, the localized area becomes permeable to sodium, resulting in inward movement of these ions. Such an influx of positive ions results in the interior becoming slightly electropositive in relation to the exterior (i.e. action potential).
This generates a nerve impulse, which is further propagated by the same mechanism, i.e. influx of sodium ions along the entire length of the nerve fibre.
2 Fluid balance
Being the major cation of extracellular fluid, sodium plays an important role in maintenance of osmotic pressure, and thus helps to retain water in ECF.
3 Acid-base balance
In form of sodium bicarbonate, sodium is a component of the bicarbonate buffer. The latter is the chief buffer system in the extracellular fluid (Chapter 1). The sodium-potassium exchange in renal tubules helps to acidify urine.
4 Membrane transport
Active absorption of a number of substances across the membranous barrier requires sodium cotransport (Fig. 7.16). This is termed the secondary active transport.
Absorption and Elimination
Sodium absorption occurs throughout the small and the large intestine. Because sodium concentration in the intestinal fluid (145 mmol/L) is several folds higher than that in the intestinal mucosal cells (10 mmol/L), sodium passively diffuses into the cell. The intracellular sodium is then actively moved into the plasma by sodium pump through expenditure of ATP energy.
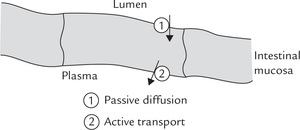
Sodium is eliminated from the body via urine. It is a meticulously regulated process, in which mineralocorticoids (e.g. aldosterone) play a major role: they act at distal convoluted tubules to cause retention of sodium and loss of potassium from the body. Glucocorticoids and sex hormones also have the same effect, but they are far less potent than aldosterone.
Requirement and Dietary Sources
Normal Indian diet contains about 5–10 g of sodium, mainly in form of table salt (sodium chloride), though meat, fish eggs, milk, cheese, cauliflower, spinach, legumes and nuts are also good sources. The daily requirement in a tropical country like India is 4–6 g. Excessive intake must be guarded against since it has been shown to lead to hypertension.
Disturbances of Serum Sodium
Hyponatraemia
In this condition the serum sodium level falls below normal. It can be caused by (a) excess sodium loss or it may be (b) secondary to excessive water retention (dilutional hyponatraemia).
It is a condition in which serum sodium level is elevated. It may occur as a result of excessive loss of water relative to the sodium loss in the body. Some more common conditions are Cushing’s disease, hyperaldosteronism, prolonged cortisone therapy, dehydration and nephrogenic diabetes insipidus.
Raised blood pressure and blood volume are important manifestations of hypernatraemia.
B Potassium
Potassium is the chief intracellular cation. Body content of potassium is about 40 mEq/kg body weight, nearly 98% of which is located intracellularly. The extracellular concentration is much smaller (about 5 mmol/L) than the intracellular concentration of 145 mmol/L (Table 19.3).
Biochemical Functions
Functions of potassium in the body include regulation of neuromuscular excitability, contraction of the heart, intracellular fluid volume and hydrogen ion concentration.
Neuromuscular excitability
Together with sodium, potassium helps maintenance of normal excitability of nerves and ensures smooth conduction of nerve impulses.
Contraction of heart
The potassium concentration in the ECF has a major influence on the contraction of cardiac muscles. A high concentration leads to slowing of heart rate, electrocardiographic abnormalities and possibly cardiac arrhythmia. These may be due to lowering of the membrane potential, which decreases the cell’s action-potential intensity.
A low concentration of K+ increases the membrane potential, decreases irritability, and produces other ECG abnormalities and muscle paralysis. The heart may cease to contract in extreme cases.
Intracellular fluid volume
Potassium in the cell maintains intracellular osmotic pressure and hence, intracellular fluid volume. Nearly half the ICF osmolarity is due to potassium.
Hydrogen ion concentration
The potassium concentration has a significant influence on hydrogen ion concentration in the blood. Movement of K+ into a blood-cell is normally counter-balanced by movement of H+ out of the cell. In hypokalaemia (low serum potassium), these counter-balanced movements are decreased, and less H+ moves out of cell. The hydrogen ion concentration is, therefore, decreased in serum, resulting in alkalosis.
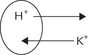
Secondary active transport
Like sodium, potassium is also involved in secondary active transport of many substances. The sodium pump that is involved in active transport of glucose, galactose amino acids, etc. is actually a sodium-potassium pump: it causes active efflux of sodium and influx of potassium.
Dietary Sources, Absorption and Elimination
Potassium is abundant in foodstuffs of plant and animal origin since it is the principal intracellular cation. Therefore, its daily requirement of about 4 g per day can be easily met and so dietary deficiency is rarely seen. Some important food sources are meat, fish, cereals, vegetables, oranges and peaches. Potassium absorption occurs by passive diffusion along a concentration gradient in both small and large intestine. Excretion mainly occurs through renal route.
Disturbances of Serum Potassium
Hypokalaemia
Fall in serum potassium levels below 3 mmol/L is called hypokalaemia. It may occur when excess potassium is lost from the body, when there is a reduced dietary intake, or when potassium is redistributed within the body.
(a) Loss of potassium may occur via gastrointestinal route, in renal tubular acidosis, K+-losing nephritis, hyperaldosteronism, etc.
(b) Decreased dietary intake occurs with chronic starvation or anorexia nervosa.
(c) Hypokalaemia due to redistribution may be caused by insulin: insulin induces potassium to move into the cells. Magnesium deficiency may also lead to potassium deficiency.
Manifestations of hypokalaemia: Hypokalaemia manifests as muscular weakness, confusion, irregular heart beat, tachycardia, and altered ECG pattern (flattening of ECG waves).
Hyperkalaemia
Although less common, hyperkalaemia (serum potassium > 5.5 mmol/L) is dangerous because of its effect on cardiac muscles. It may be caused by:
(a) Failure of the kidneys to excrete potassium, as in Addison’s disease, in which low aldosterone production prevents potassium excretion. Renal failure can cause hyperkalaemia when daily urine output drops below 400–500 L per day.
(b) Redistribution of potassium, which occurs in acidosis and crush injuries. These conditions lead to hyperkalaemia even though total body potassium is not increased. In acidosis, potassium moves out of the cells as hydrogen ions move in, and in crush injuries, the damaged tissues release their potassium content into blood circulation.
(c) Others: Dehydration, massive blood transfusion and indiscriminate potassium therapy are other important causes of hypokalaemia.
Clinical manifestations: The symptoms of hyperkalaemia are similar to those of hypokalaemia in that myocardial irritability, irregular heartbeat, ECG changes, and muscle weakness may occur in either condition. The greatest danger, however, is the possibility of cardiac arrest at levels greater than 7.0 mmol/L.
C Chloride
It is the major anion in the body. Normal serum chloride levels (with sodium levels), in general, undergo parallel alterations. The chloride in the CSF is about 125 mmol/L, which is higher than in any other body fluid. Total body content of an average adult is about 80 g.
Biochemical functions
Chloride is the major extracellular anion, and so, along with sodium, it is involved in regulation of osmotic pressure of extracellular fluids. It helps maintenance of pH of blood (see chloride shift, Chapter 1), and is also involved in the formation of hydrochloric acid in stomach.
Absorption
Chloride in food is almost completely absorbed, mostly in the proximal ileum by passive diffusion. In distal ileum and colon, chloride absorption occurs in exchange for bicarbonate ions.
Requirement and dietary sources
The daily requirement of chloride is 5–10 g. Most important source is sodium chloride, the table salt. Foods that provide sodium also provide chloride, e.g. meat, fish, cheese, cereals, eggs, etc. Thus, intake of sodium and chloride runs parallel.
Alterations of serum chloride levels
With a possible exception of metabolic alkalosis, serum chloride level undergoes parallel changes with sodium concentration.
Hyperchloraemia (increased chloride concentration) occurs in conditions of (a) dehydration, (b) adrenocorti-cal hyperactivity: adrenal steroids cause increased reabsorption from renal tubules, (c) severe diarrhoea, leading to loss of bicarbonate and compensatory retention of chloride, and (d) respiratory alkalosis, metabolic acido-sis and renal tubular acidosis.
Hypochloraemia is seen in (a) severe vomiting: loss of HCl decreases chloride concentration, and so compensatory rise in bicarbonate occurs, (b) Addison’s disease: mineralocorticoid depletion causes impaired reabsorption of chloride from renal tubules, (c) prolonged gastric suction, and (d) respiratory acidosis and metabolic alkalosis.
E Magnesium
Magnesium, an essential activator of over 300 enzymes in humans, is the fourth most abundant cation in the body after sodium, potassium and calcium (Table 19.1). In intracellular fluid, it is the second most abundant cation after potassium. It is also an important constituent of chlorophyll, just as iron is an essential component of haemoglobin. An average adult contains about 25 g of magnesium, about 70% of which is present in skeleton. The remaining 30% occurs in soft tissues (mainly liver and muscles) and body fluids. Only about 1% of the body magnesium is in the blood and ECF. The skeletal and ECF magnesium pools exchange freely with each other but not with the intracellular pool.
Serum concentration of magnesium is 1.6–2.3 mg/dL (0.7–1.0 mmol/L), of which, about 20–25% is protein bound. In a manner similar to calcium, the unbound (ionized) portion of serum magnesium is the biologically active fraction. The intracellular magnesium concentration is much higher (about 10 mmol/L) than that in ECF, most of which is bound to organelles. Inside RBCs, the concentration is about 20 mmol/L.
Biochemical Functions
Enzyme activator
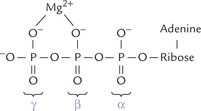
Magnesium activates a number of enzymes, particularly those in which ATP4–Mg2+ (a complex of ATP and magnesium) is a substrate. The magnesium ion is chelated between the β- and the γ-phosphates of ATP, thus diminishing the anionic character of the latter (Fig. 19.1 ). This permits smooth interaction of the ATP with specific protein sites. The enzymes needing magnesium are involved in intermediary metabolism, transcellular ion transport, muscle contraction, and oxidative phosphorylation. Some examples are hexokinase, phosphofructokinase, pyruvate kinase, thiokinase, squalene synthase, glutamine synthetase, adenylate cyclase, etc.
Neuromuscular excitability
It is decreased by magnesium. A low magnesium level results in increased nerve excitability.
Glucose tolerance
Magnesium improves glucose tolerance, probably by enhancing insulin dependent uptake of glucose.
Others
Magnesium binds to other nucleoside phosphates and nucleic acids and is required for DNA replication, transcription and translation. The DNA helix is stabilized by binding of histones and magnesium to the exposed phosphate groups. In green plants, chlorophyll—a magnesium porphyrin complex similar to haem—is vital for photosynthesis.
Absorptions and Elimination
Magnesium is mostly absorbed from the small intestine but a small amount is also absorbed from colon. About 50% of the ingested magnesium is absorbed on an average diet, and the extent of absorption decreases as the dietary content of this element increases. Parathormones appear to increase the absorption.
Kidneys are the main organs responsible for maintaining plasma magnesium concentration within normal limits. Most of the magnesium (about 98%) filtered at glomeruli is reabsorbed. Urinary excretion varies with plasma magnesium concentration. PTH enhances the tubular reabsorption of magnesium, whereas aldosterone decreases it.
Requirement and Dietary Sources
Daily requirement is about 350 mg in adult men, 300 mg in adult women and 150–50 mg in children. In pregnancy and during lactation about 450 mg/day is needed to maintain positive magnesium balance.
Major food sources are nuts, cereals, beans and fish; almonds are a particularly rich source. Leafy green vegetables are a good source because of the high chlorophyll content.
Disturbances of Serum Magnesium
Serum magnesium level may fall (hypomagnesaemia) in a number of clinical settings and contributes to a poorer prognosis for the patient, especially when below 1.7 mg/ dL. Some important causes are diabetes, chronic alcoholism, liver cirrhosis, hyperparathyroidism, aldosteronism, protein calorie malnutrition and drug therapy. Hypo-magnesaemia leads to neuromuscular hyperirritability, tremors, increased vascular resistance, coronary vaso-spasm and hypertension.
Increase in serum magnesium level (hypermagnesaemia) is commonly seen in renal failure.
V Trace Elements
Almost a dozen trace minerals are known to be essential in diet. Most of these are toxic at higher doses. Therefore, they are complexed with specific intracellular binding proteins, which not only store the mineral but also prevent toxicity.
Iron, copper, selenium, zinc, molybdenum, chromium, manganese, iodine and fluoride are some important trace elements, discussed below.
A Iron
Iron is a very important element in medical practice. Though it is one of the most abundant elements on earth, yet only trace amounts are present in the living cells. A normal adult of 70 kg body weight contains about 3–4 g of iron. Daily turnover is high (30–40 mg) since iron is continually circulated in various metabolic pathways. About 65–70% of the total iron in human body is found in haemoglobin and approximately 10% in myoglobin and other iron-containing enzymes and proteins. The remaining 20–25% consists of storage pool of iron.
By comparison, the average adult woman has only 2–3 g of iron in her body. This difference is attributable to (a) lower haemoglobin concentration, (b) smaller vascular volume, and (c) lower iron reserves in women.
Iron distribution is summarized in Table 19.4 .
Table 19.4
Distribution of iron in the body
| Compound | Per cent of total | Function |
| Haemoglobin | 65 | Oxygen transport, blood |
| Myoglobin | 10 | Oxygen storage, muscle |
| Ferritin* | 10 | Iron storage |
| Haemosiderin* | 9 | Iron storage |
| Transferrin* | 0.2 | Iron transport |
| Enzymes (catalase/peroxidase) | < 1.0 | H2O2 decomposition, oxidation |
| Iron-sulphur* | Electron transfer | |
| Cytochromes | < 0.5 | Electron transport |
| Other | < 5 | Oxidation-reduction reactions |
Biochemical Functions
Iron is essential for several biological processes, and therefore, biologically indispensable.
1. Tissue respiration: Iron can change readily between the ferrous and the ferric states, and can therefore function in electron transfer reactions. Both the haem iron of the cytochromes and non-haem iron in iron-sulphur proteins (e.g. NADH dehydrogenase and succinate dehydrogenase) are used in this way.
2. Transport of gases: Iron is able to bind a variety of ligands, including molecular oxygen and carbon dioxide. This property is exploited by oxygen-binding proteins, such as haemoglobin and myoglobin for its transport and storage.
3. Oxidative reactions: Iron is a component of various oxido-reductase enzymes (Table 6.1), and so plays a vital role in a number of oxidative reactions in the body.
4. Others: Iron is required for an effective immune response due to a variety of reasons. For instance, it is required for activity of the lysosomal peroxidase, which helps in phagocytic and bactericidal activities of neutrophils.
Requirements and Dietary Sources
| Adult men | 10 mg/day |
| Pre-menopausal women | 18 mg/day |
| Post-menopausal women | 10 mg/day |
| Pregnant women | 40 mg/day |
| Children | 10 mg/day |
About 1 mg iron is eliminated each day from the human body, mainly by shedding of skin epithelial cells and cells lining the urinary tracts, and a to a smaller extent in urine and sweat. This much amount must be replaced by dietary intake. An average Indian diet provides about 10–20 mg of iron, of which only about 10% is absorbed. This balances the average daily iron loss in men and post-menopausal women.
However, requirements are higher in the women since blood is lost in each menstrual cycle. Each cycle drains 20–40 mg of iron, and therefore, an additional 1 mg of iron per day is required. The diversion of iron to the fetus during pregnancy, blood loss during delivery, and subsequent breast feeding of the infant consumes 900 mg or iron on average. This increases daily iron demand to 3 mg or 4 mg in pregnant and lactating women.
Dietary sources include meat, fish, eggs, cereals and green leafy vegetables. Milk is a poor source. Iron obtained from animal sources is more readily available than that obtained from vegetarian food. This is because the latter is complexed with certain inorganic molecules, such as phytates, which impede its absorption.
Absorption
Intestinal absorption is especially important in case of iron. It is a regulated process and the efficiency of absorption increases or decreases depending on the body requirement. The iron stores in the body are regulated by intestinal absorption. Exact mechanism of absorption is still unknown, a working hypothesis is as below:
The dietary iron, which exists mostly in the ferric form, is converted to the more soluble ferrous form, which is readily absorbed. The ferric to ferrous conversion is brought about by action of HCl and reducing agents such as ascorbic acid, cyseine and –SH groups of proteins.

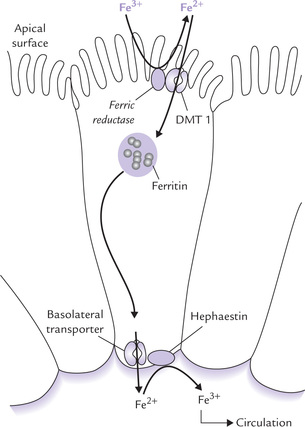
Entry of Fe2+ into the mucosal cell may be aided by an enzyme on the brush-border of the enterocyte (the enzyme possesses ferric reductase activity also). The ferrous iron is then transported in the cell by a divalent metal transporter (DMT-1), as shown in Figure 19.2 .
Iron in Intestinal Cells
Within the intestinal cell, the iron may be either stored or transported out.
(a) Stored by incorporation into ferritin in those individuals who have adequate plasma iron concentration. A ferroxidase converts the absorbed ferrous iron to the ferric form, which then combines with apoferritin to form ferritin.

(b) Transported to a transport protein at basolateral cell membrane and released into the circulation. However, the basolateral-transport-protein has not yet been identified. It is believed to work in combination with hephaestin, a copper-containing protein, which oxidizes Fe2+ back to Fe3+.
The intestinal cells internalize more iron than the amount that eventually will enter the circulation. The surplus, incorporated into ferritin for storage, is subsequently mobilized, if necessary. The ferritin stores are gradually built-up, but most are lost when the mucosal cells are shed. New cells take their place, and the cycle of iron build-up starts again.
Factors Affecting Iron Absorption
1. Gastric acid and dietary components that form soluble-ferrous-chelates (such as ascorbic acid, sugars and amino acids) keep iron in solution and increase its absorption.
2. Substances that form insoluble complexes with iron, such as oxalates and phytates (in vegetables), phosphates (in milk and eggs), and tannates (in tea), decrease iron absorption.
3. When body needs for iron increase, such as in iron deficiency, pregnancy and accelerated erythropoiesis, iron absorption is stimulated three-folds or more. On the other hand, the absorption is reduced after the consumption of iron or iron poisoning.
4. Marginal decrease in iron absorption occurs by tea and eggs.
The iron balance is controlled by changes in absorption, which speeds up or slows down depending on the body needs. No other nutrient is regulated in this manner. The overall effect is to prevent the absorption of excess iron (excess iron is toxic because it can bind to and disrupt structure of many proteins) while maintaining an adequate supply for current needs.
Absorption of haem iron: The above description relates to non-haem iron. Absorption of the haem iron, which is present in non-vegetarian foods, is a simpler and more rapid process. After haem is released from the polypeptide chain, it is absorbed intact by the intestinal cells, where porphyrin ring is split and iron is liberated. This process is not only more efficient, but also is not affected by dietary factors.
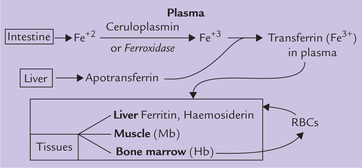
Transport in Plasma and Cell-uptake
The iron absorbed from intestine (or that liberated from the ferritin of mucosal cells) is converted to ferric form by a copper-containing enzyme ceruloplasmin, which possesses ferroxidase activity. Ferric iron then binds with transferrin, a liver derived ß-globulin, which binds essentially all the iron in plasma (Fig. 19.3 ). Need for a specific carrier protein arises because free, unbound iron is quite toxic due to two reasons:
• It can disrupt native conformation of several biologically active proteins, and
• Initiates oxidative damage by forming reactive hydroxyl and peroxide radicals in the presence of molecular oxygen.
Transferrin is a single chain glycoprotein with a molecular weight of 78 kDa and a plasma concentration of approximately 300 mg/dL. Each transferrin molecule has two high affinity binding sites for ferric (but not ferrous) iron. Only approximately one-third of the binding sites are occupied. This is expressed as an iron saturation of 33%. In the laboratory, the transferrin concentration is measured as the total iron-binding capacity (TIBC).
The transferrin delivers iron to the cells with specific surface receptors for this protein. These receptors bind with the iron loaded transferrin, and the transferrin-receptor complex is then taken into the cell by endocytosis. The endocytotic vesicle becomes acidified to a pH of approximately 5.0. At this pH, the iron dissociates from transferrin and transported into the cytoplasm. The receptor-transferrin complex is then returned to the cell surface, where it undergoes additional rounds of iron transport and uptake.
Iron Storage
After the iron is delivered to the peripheral cells, it can either be used for various biological activities, or stored. The storage occurs in two proteins: ferritin and haemosiderin, which abound in liver, spleen, bone marrow, intestinal mucosa, pancreas, myocardium and other tissues. Storage is important because it serves to package and isolate iron atoms from the intracellular environment, thus preventing any toxic action on cell constituents.
Ferritin: It consists of a multi-subunit protein shell, known as apoferritin (MW 500,000). This shell with an external diameter of 13 nm and an internal cavity of 6 nm across surrounds a core of up to 4500 ferric ions. Ferritin is a readily mobilized form of storage iron.
Haemosiderin: It is derived from ferritin that loses some of its surface protein and undergoes aggregation to form micellar complexes. These complexes, now called haemosiderin, have higher iron content than ferritin, but release iron more slowly.
Substantial quantities of iron can be stored (up to several grams) in some older males. About one-third of the storage iron is present in liver, one-third in bone marrow and the remainder in spleen and other tissues. On the other extreme are many children and menstruating women, in whom the storage iron is nearly absent.
The principal pathways of iron metabolism are outlined in Figure 19.3. Significance of RBCs is highlighted in Box 19.1.
Pathological Conditions
Iron overload
In iron overload, the iron molecules combine with the available apoferritin resulting in excessive production of ferritin. As mentioned earlier, surplus iron may cause excessive formation and accumulation of haemosiderin; the condition is termed haemosiderosis. Various parameters in iron overload (and deficiency) are given in Table 19.5 .
Table 19.5
Iron parameters in iron overload and deficiency
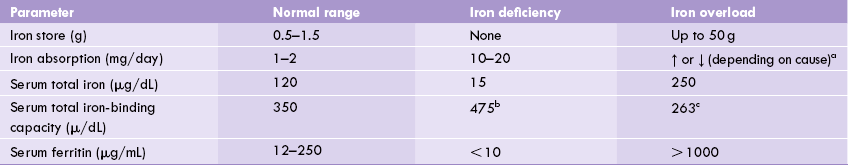
a↑ in idiopathic haemochromatosis, ↓ in most other conditions of iron overload.
breflects increased serum transferrin levels.
calmost equal to total serum iron.
Iron overload is symptom-less initially. Gradually, however, massive deposits of haemosiderin may develop, which may cause functional impairment of the involved organs. The condition is then called haemochromatosis. It may be genetic (e.g. primary haemochromatosis) or acquired, called secondary haemochromatosis (Box 19.2).
Signs and symptoms of haemochromatosis
These depend on the organ system involved. Accumulation in liver can result in cirrhosis. In the pancreas, the excess iron can damage the β-cells to cause diabetes mellitus (seen in two-thirds of patients). The latter condition is termed bronze diabetes.
Cardiomyopathy, hyperpigmentation of the skin, and arthralgia (joint pain) are the other frequent signs.
Treatment
Haemochromatosis is treated by repeated phlebotomy. This is indeed the only disease in which the traditional use of leech (Hirudo sp.) is the treatment of choice. Iron-chelating agents, e.g. desferrioxamine, are used to remove excess iron. They form soluble iron complex that is rapidly excreted in urine.
Iron deficiency
Iron deficiency is a serious health problem. With possible exception of protein calorie malnutrition, iron deficiency is the second most prevalent nutritional deficiency worldwide, with a total of at least half a billion people affected. The deficiency may be caused by inadequate dietary intake of iron, impaired absorption, or by chronic bleeding due to piles, peptic ulcer, hookworm infestation or menstrual irregularities. Growing children, adolescent girls, pregnant and lactating women are the worst sufferers. Iron deficiency results in a microcytic hypochromic anaemia (microcytic = small RBCs; hypochromic = low haemoglobin content per cell). Biochemical indices of the deficiency are summarized in Table 19.5 and other relevant details are presented in (Case 19.1).
B Copper
Copper primarily functions as a component of metalloenzymes or proteins that participate in redox reactions. Adult human body contains 60–100 mg of copper which is widely distributed in a number of tissues, but largest amounts are present in muscles (30–50 mg), bones (10–20 mg), and liver (10–15 mg).
Biochemical Functions
1. Copper is an essential component of several oxidases.As a rule, these enzymes use either molecular oxygen or an oxygen derivative as one of their substrate. Examples include:
• Cytochrome oxidase, which transfers electrons to oxygen, the ultimate acceptor of electron transport chain.
• Lysyl oxidase, which catalyzes cross linking in collagen and elastin by converting certain lysine residues to allysine.
• Tyrosinase, which is necessary for the synthesis of melanin; ALA synthase, which is required for haem synthesis; and several others, e.g. superoxide dismutase, ascorbic acid oxidase, uricase, dopamine hydroxylase and monoamine oxidase.
2. Ceruloplasmin, a copper-containing enzyme in plasma possesses ferroxidase activity; it converts Fe2+ to Fe3+, the form in which the transferrin iron is transported in plasma.
3. Certain non-enzyme proteins also require copper, although their functions are not clearly known. These include hepatocuperin (in liver), cerebrocuperin (in brain) and haemocuperin (in red cells).
Requirements and Dietary Sources
Only about 2 mg/day copper is required by adults (Table 19.2) and about half this amount by children. The average cereal-based Indian diet provides about 4 mg copper each day, which can meet the body’s requirements. Cereals, legumes, raisins, nuts, green leafy vegetables, liver, kidney, meat, and egg yolk are common dietary sources. Milk is a poor source.
Absorption
Intestinal absorption of copper occurs mainly from duodenum. Less than one-third of the dietary copper is absorbed from an average Indian diet, because dietary constituents (e.g. phytates, fibres, Zn, Mo) reduce its absorbability (from western diet 50% copper content is absorbed). Moreover, the intestinal absorption is a sluggish process because of highly insoluble nature of copper. Mechanisms, therefore exist to enhance its solubility. For example, in the intestinal lumen, copper complexes with a low molecular weight substance secreted by saliva and gastric juice, which keeps it soluble at the intestinal pH. Precise nature of this substance is still unknown.
The absorptive process consists of three phases:
1. Mucosal uptake: The ingested copper enters the intestinal epithelial cells by moving across luminal aspect of the cell membrane.
2. Intracellular transport: Inside the cell, copper is transported by a low molecular weight, binding protein, called metallothionein.
3. Extrusion: The intracellular copper is then moved across the serosal aspect of the mucosal cell membrane by an enzyme copper-binding P-type ATPase. The enzyme, present in intestinal mucosa (and some other cell types), effectively transfers copper into portal circulation from where it reaches the liver.
Within hepatocytes, an intracellular protein facilitates incorporation of copper into apoceruloplasmin, an endogenously synthesized α2-globulin. This results in formation of ceruloplasmin, which contains six to eight atoms of copper. Half of these are in cuprous state and the half in the cupric state. Failure to synthesize this protein is implicated in pathogenesis of Wilson’s disease, discussed later.
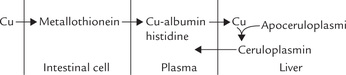
Transport and Excretion
Plasma concentration of copper is 100–200 mg/dL, about 95% of which is bound to ceruloplasmin. Ceruloplasmin is an enzyme (ferroxidase) and an antioxidant, but not a transport protein since the copper is so tightly bound to it that it is not readily exchangeable with other molecules. The major carrier of copper is albumin, which loosely binds with copper. A small fraction of plasma copper is complexed with amino acids, mainly histidine.
Excretion of copper occurs mainly in the bile, which eliminates 1.5–2.0 mg/day. The urinary excretion is normally < 400 μg/day. A transport protien, the copper binding ATPase moves copper from lines cells into bile.
Disorders of Copper Metabolism
1. Copper deficiency: It is most likely to occur from reduced intake or excessive loss, e.g. in renal dialysis. Deficiency manifests as microcytic (small erythrocytes) hypochromic (pale erythrocytes) anaemia that is resistant to iron therapy. There is decreased number of leukocytes in the blood (neutropenia), degeneration of vascular tissue with bleeding (due to defects in elastin and collagen production), and skin de-pigmentation (because melanin synthesis requires tyrosinase, a copper-containing enzyme). Demyelination of neural tissue, myocardial fibrosis, and grey hair are the other manifestations.
2. Menkes’ disease: It is an X-linked genetic defect of deficient production of the copper-binding P-type ATPase in intestinal mucosa (and most other tissues). Copper accumulates in intestinal mucosa but cannot be released into circulation. Decreased copper in plasma and urine results, and in untreated cases more severe manifestations, such as mental retardation, abnormal collagen formation, greying of hair, and temperature instability results.
3. Wilson’s disease (hepatolenticular degeneration): It is a rare, autosomal recessive disorder in which excessive accumulation of copper occurs in tissues. The possible causes are:
(a) The gene encoding the copper-binding ATPase in hepatocytes, which is required for excretion of copper from liver cells, may be defective. In its absence copper is not eliminated (in bile) and accumulates.
(b) Some workers suggest failure to synthesize ceruloplasmin or an impairment in the binding capacity of copper to this protein as the possible cause. Ceruloplasmin level less than 20 mg/dL is considered pathognomonic of Wilson’s hepatocellular degeneration.
Excess copper accumulates in liver and brain, leading to liver damage or neurological degeneration, or both. Copper deposition in kidney may cause renal damage, with consequent excretion of amino acids, glucose, peptides and haemoglobin in urine. Copper deposits in bone marrow cause haemolytic anaemia and those in cornea form green or golden pigmented ring, the KayserFleischer ring. These and some other manifestations of the disease are given in Case 19.2. D-Pencillamine, a copper chelator, is used in treatment. It forms a soluble copper complex, and permits its urinary excretion.
4. Copper toxicity: Copper is generally non-toxic, but in large doses it accumulates in tissues and can interfere with other metal ions, specifically iron and zinc. Acute toxicity manifests as blue-green stools and saliva, acute haemolysis, and abnormalities of kidney functions.
5. Indian childhood cirrhosis: Chronic excessive intake of copper and consequent deposition in liver occurs in this disorder. Feeding of milk boiled in brass utensils to infants is probably the cause of the excess.
C Other Trace Elements
A number of other trace elements serve as prosthetic groups or cofactors in various enzymatic reactions and are, therefore, required in our diet, though the daily requirement is very low. Examples include selenium, chromium, cobalt, iodine, manganese, and molybdenum, zinc.
The daily requirements and biological roles of these elements are outlined in Table 19.2.
Selenium
Total selenium content in adult human is only about 6 mg. Skin, liver, pancreas, and renal cortex contain relatively large amounts of this mineral. It is known that in plants selenium is present as selenomethionine (in animals as selenocysteine).
Biochemical Functions
1. Antioxidant role: In the form of selenocysteine, this element occurs in glutathione peroxidase (GP), an enzyme with strong antioxidant properties. It prevents peroxidation of lipids and other compounds by hydrogen peroxide.

The above reaction may be considered as a detoxification reaction in which selenium acts as a nonspecific antioxidant (being a component of glutathione peroxidase). This protects the tissues against potentially toxic effect of hydrogen peroxide and permits the life forms to survive in an oxygen atmosphere.
2. Synergism with vitamin E: Selenium prevents lipid peroxidation and offers protection against the free radicals. Vitamin E also has the same effect (antioxidant), though by a different mechanism (Chapter 18). Thus, selenium and vitamin E supplement each other. Availability of vitamin E reduces the selenium requirement, and conversely, in selenium-deficient tissue, vitamin E content is depleted.
3. Others: The other possible roles of selenium are that it is part of coenzyme Q, cofactor for the enzyme deiodinase, and plays a role in immune reactions. It binds with certain toxic, heavy metals (Cd and Hg) and protects the body against their harmful effects.
Requirements and Dietary Sources
Daily requirement of selenium is 50–200 mg for an adult (Table 19.2). It is present in sea-foods and organ meats such as kidney and liver.
Deficiency and Toxicity
Both selenium deficiency and toxicity are well known. Keshan disease, an endemic cardiomyopathy (occurs in Keshan Province of China), is caused by low selenium content of locally grown foodstuff. In fact, deficiency of this mineral has been reported when soil is deficient in it. For instance, certain mountainous regions are deficient in selenium, which is reflected in the low selenium content of the food plants grown in these soils (Case 19.3).
Selenium toxicity, called selenosis, occurs by accidental ingestion of metal polishes or antirust compounds. It manifests as garlicky odor in breath, hair and weight loss, diarrhoea and falling of nails.
Zinc
Zinc is the intracellular cation present in all body tissues and fluids and, next to iron, is the second most abundant of the trace metals in humans. Prostate, liver, kidneys, muscles, heart, skin, bones, teeth, etc. are particularly rich in zinc. Total zinc content of the adult body is about 2 g, out of which 60% is in muscles, 28% in bones and 0.5% in blood. Erythrocytes contain 75-88% of the blood zinc. In the plasma, about 18% of the zinc (normal range 700-1200 μg/L) is tightly bound to an a2-mac-roglobulin, 80% is loosely bound to albumin, 2% is bound to transferrin, ceruloplasmin, or the amino acids, and a small fraction is present as free zinc.
Biochemical Functions
1. Zinc in the form of zinc fingers, is an important constituent of the regulatory proteins that control transcription.
2. More than 300 metalloenzymes require zinc for their catalytic activity, including the enzymes critical for nucleic acid and protein synthesis, e.g. DNA and RNA polymerases, and reverse transcriptase. Thus, zinc is a necessary component for gene expression and cell replication. Other important zinc containing enzymes are carbonic anhydrase, extramitochondrial superoxide dismutase, carboxypeptidases A and B, ethanol dehydrogenase, lactate dehydrogenase, malate dehydrogenase, alkaline phosphatase and glutamate dehydrogenase.
3. Insulin when stored in the β-cells of pancreas contains zinc that is probably required for stabilization of the hormone molecule and its release.
4. Along with other trace metals (Cu and Mn), zinc is associated with a group of enzymes called superoxide dismutases, which scavenge the superoxide anion. The latter are by-products of various redox reactions or the electron transport system and may give rise to the very destructive hydroxy (OH) radicals (Chapter 27).
5. Zinc is also required for immunological functions.
6. Zinc induces synthesis of metallothionein, a small protein of 61 amino acids (MW 7000). Both Zn and Cu are stored in tissues bound to this protein. The protein binds copper more firmly than zinc and forms an unabsorbable complex in the gastrointestinal tract, hence reducing copper absorption. Therefore, dietary zinc supplementation is known to significantly lower the absorption of Cu and may elicit Cu deficiency symptoms.
(Zinc fingers, defined as domains of zinc-binding proteins that also bind to DNA, are involved in the gene expression of metallothionein.)
Requirement and Dietary Sources
| Infants | 5 mg/day |
| Children and adolescents | 10 mg/day |
| Adults (males) | 15 mg/day |
| Adults (females) | 12 mg/day |
In pregnant and lactating females, the daily requirement increases to 15 mg/day and 19 mg/day, respectively.
Seafood, red meat, fish, eggs and milk are good sources of zinc. Although vegetables contain appreciable amounts of zinc, the fiber and phytate in them bind zinc and hence diminishes its bioavailability. Hence, vegetarians are at greater risk for zinc deficiency.
Metabolism
Zinc is absorbed from the duodenum. Its absorption is incomplete because of the presence of certain interfering substances (e.g. fibres and phytates) in the food, which form insoluble complexes with zinc to impair absorption. Copper, iron, cadmium and calcium also interfere with zinc absorption.
In the plasma, there is no specific binding protein, and zinc is transported mainly in association with albumin. It is stored in many tissues in the form of metallothionein, which also binds copper and some other heavy metals. Metallothionein protects the cells from the toxic effects of the free, unbound metal ions. Zinc is present in pancreatic secretions, and the stools are the major route for its excretion. Urine, sweat and seminal fluid are the excretory routes of minor importance.
Deficiency
Acute zinc deficiency in humans, especially in growing children, manifests as skin lesions, testicular atrophy, poor growth, delayed sexual development and increased susceptibility to infections. Neuropsychiatric impairments and decreased taste acuity are the other prominent features. As noted above, red meat being prime source of bioavailable zinc, vegetarians are at much greater risk for zinc deficiency.
Zinc deficiency may occur in acrodermatitis enteropathica, a rare, recessively inherited disease characterized by dermatitis, diarrhoea, and alopecia (hair loss). It is caused by an impairment of intestinal zinc absorption. High doses of orally administered zinc are curative.
Molybdenum
Biochemical Functions
Very small amounts of this element are present in the human body, mainly in liver and kidneys. It is known to function at active sites of at least three enzymes: xanthine oxidase, aldehyde oxidase and sulphite oxidase. The last one is a haem-protein similar to cytochrome C. The plant enzyme nitrite reductase, required for nitrogen fixation, also contains molybdenum.
Requirement, Dietary Sources and Absorption
The dietary requirements for molybdenum are not known. It is present in a variety of foodstuffs and is adequately absorbed (60–70%) in the small intestine. Therefore, deficiency of Mo is rarely seen in adults in natural conditions. However, among infants with pertinent genetic lesions, the deficiency may occur, resulting in neurological pathologies.
Toxicity
Excess consumption of molybdenum may cause toxic manifestations, such as impairment in growth, diarrhoea and anaemia. The condition is called molybdenosis. Copper deficiency may also develop in this condition because molybdenum is known to decrease mobilization and utilization of copper in the body.
Cobalt
The only known role of cobalt is that it is a component of vitamin B12. Inorganic cobalt is not absorbed from the alimentary tract, so it must be provided in the diet as vitamin B12.
In high doses for a prolonged period it may produce polycythaemia (increased RBC count), probably through stimulating synthesis of erythropoietin.
Chromium
An average adult male contains about 6 mg of chromium. Concentration in the blood is 20 μg/dl. It plays important role in metabolism, but excess chromium can be toxic.
Biochemical Functions
1. Chromium is a component of “glucose tolerance factor”, a poorly characterized complex of trivalent chromium, nicotinic acid and glutathione (MW 1500), which potentiates action of insulin on its target tissues. Brewer’s yeast is a good source of the glucose tolerance factor.
2. Chromium is essential for the normal metabolism of carbohydrates and lipids. It lowers the total serum cholesterol and the LDL levels, and elevates the HDL level, thereby providing health benefits.
3. It is postulated that chromium binds to DNA, RNA and nuclear proteins, and is thereby involved in maintenance of the structural integrity of the nuclear strands and in the regulation of gene expression.
4. Amino acid transport into the cells (liver and heart) may require participation of chromium.
Absorption
An average adult consumes about 10–100 μg of chromium each day. But the intestinal absorption is sluggish: less than 1% of the ingested element is absorbed. Stainless steel utensils contain chromium which can be readily absorbed.
Manganese
The total manganese content in adult humans is 12-20 mg, of which 25% is in skeleton. Liver, pancreas and kidneys also contain relatively higher amounts of this element. It is found mainly in the nuclei, where it is believed to impart stability to the nucleic acid structure.
Biochemical Functions
1. Manganese is required for glycoprotein and proteoglycan synthesis, and so plays important role in formation of the matrix of bones and cartilages.
2. Manganese stimulates the activity of several enzymes, such as glucosyl transferases, arginase, RNA polymerase, pyruvate carboxylase and superoxide dismutase (mitochondrial). However, in most cases it can be replaced by magnesium.
3. Normal functioning of the central nervous system, physical growth and development of reproductive functions requires manganese.
Requirements, Dietary Sources and Absorption
A daily intake of 2.5–5.0 mg is recommended for an adult, though exact requirement is not known. Cereals, leafy vegetables, fruits and tea are good sources of manganese. Absorption occurs in the small intestine (3–4% absorbed), and is inhibited by iron and the other divalent cations.
Iodine
Iodine, atomic weight 127, is a group VII halogens in the periodic table. Although it is widely distributed throughout the earth’s surface, the sea is the major source of this element. The iodide concentration of sea water is 50 μg/L, which is approximately similar to that of human serum. In human body, iodide is present in small amounts (4550 mg), of which 10–15 mg is present in thyroid gland, 25 mg in muscles, 5 mg in skin, 3 mg in skeleton and 2 mg in liver.
Biochemical Functions
Iodine is a constituent of the hormones secreted by thyroid gland, thyroxine (T4) and triiodothyronine (T3). These hormones are synthesized by iodination of tyro-sine and are essential for healthy growth, differentiation, and development (Chapter 30).
Requirement and Dietary Sources
Daily iodine requirement varies with age as here.
| Infants | 40–50 μg |
| Children | 70–90 μg |
| Adults | 100–150 μg |
| Pregnancy | 175 μg |
| Lactation | 200 mg |
Uptake of iodide by foodstuffs is directly proportional to the soil and water content of this element. Marine fish, lobsters and seaweed, growing in iodine rich sea-water, are therefore the best sources. Drinking water is also an important source. Prolonged consumption of crops growing in areas where soil is depleted of iodide leads to iodide deficiency.
Absorption Transport and Excretion
Iodine is absorbed mainly from the small intestine. Normally, about 30% of the dietary iodine is absorbed. Skin may also absorb iodine, so toxic manifestations may result from prolonged use of iodine containing skin ointments. The absorbed iodine is released into blood circulation where it is mostly (90%) present in the form of thyroid hormones bound to protein (protein bound iodine). Only about 10% is present in the form of inorganic iodide. Excretion of iodine occurs mainly through urine, and to a lesser extent through bile and saliva. Urine inorganic iodide correlates with plasma level.
Deficiency
Iodide deficiency is an important cause of hypothyroid-ism in certain areas of the world, where the soil and the plants grown in it, are deficient in this mineral. Such areas constitute the “goiter belt” and the sub-Himalayan regions are a part of this belt.
The follicular cells of thyroid gland undergo hypertrophy and hyperplasia in iodine deficiency because decreased thyroid hormone synthesis leads to over-stimulation of the gland by TSH (TSH release from the anterior pituitary is disinhibited whenever the levels of circulating thyroid hormones are abnormally low). This phenomenon is known as simple goiter (or diffuse goiter).
Use of iodized salt (potassium iodate mixed in common salt in the ratio of 1:10,000 to 20,000) in the goiter belt has considerably reduced the incidence of simple goiter.
Fluoride
Like iodine, fluorine (atomic weight 19) also belongs to group VII halogens of the periodic table of elements. Only about 2.5 mg of this element is present in an average adult body, of which, more than 95% is present in bones and teeth.
Biochemical Functions
1. Fluorine makes the teeth resistant to cavities (dental caries) and bones to development of osteoporosis in later life by improving quality of hydroxyapatite crystals in teeth and bones. The fluoride anion may substitute for the hydroxyl ion in the hydroxyapatite crystal structure to produce a “harder” crystal. This is believed to account for the protective effect against dental caries (cavities), caused by certain bacteria, which are normal inhabitants of the oral cavity, and which act on dietary carbohydrates and convert them into lactic acid. The latter corrodes the enamel of the teeth and produces cavities.
2. Hardening of hydroxyapatite by fluoride also explains its therapeutic use, either alone or in combination with vitamin D, in therapy of osteoporosis.
3. Fluoride is a potent inhibitor of activities of certain enzymes. Fluoroacetate inhibits aconitase (of TCA cycle) and sodium fluoride inhibits enolase (of glycolysis).
Requirements and Dietary Sources
The daily requirement of fluoride is 1–2 mg per day. In warm Indian climate, where the water intake is usually high, the drinking water, having a fluoride content of 0.5–0.8 ppm easily meets this meager daily requirement (ppm = parts per million; 1ppm = 1 gram of fluoride in million gram of water, which equals 1 mg/1000 ml).
Traces of fluoride are present in most foods; seafood and cheese are particularly rich. Drinking water is also a major source; fluoride can be present in drinking water, either naturally or because of artificial supplementation. In fact, a directly inverse association between the incidence of dental caries and fluoride concentration of < 1 mg/dL in drinking water has long been recognized.
Deficiency and Toxicity
Decreased fluoride intake is associated with dental caries and excessive intake with fluorosis. When drinking water contains less than 0.5 ppm of fluoride, dental caries results.
A prolonged high intake of fluoride is potentially hazardous. Fluorine levels more than 5 ppm cause dental fluorosis characterized by discoloured and mottled teeth. In more severe cases (fluorine levels > 20 ppm) bones are also affected, leading to alternate areas of osteoporosis and osteosclerosis and calcification of tendons and ligaments. The condition is called skeletal fluorosis. Fluorosis is mostly seen in geographical areas where water content of this mineral is high.
Exercises
Essay type questions
1. Describe metabolism of copper and zinc. Discuss metabolic importance of ceruloplasmin.
2. Discuss biochemical significance and disease states associated with trace elements.
3. Describe intestinal absorption of iron and various factors involved in its regulation.
4. What is the nutritional role of selenium? How do vitamin E and selenium supplement each other?
5. What are the biochemical roles of trace elements in humans. Illustrate your answer with two examples.
 Decreased intake, impaired absorption, and excessive loss are examples of conditions that could result in deficiency of a trace element, which in turn is associated with impairment of functions. Excess concentration may be toxic.
Decreased intake, impaired absorption, and excessive loss are examples of conditions that could result in deficiency of a trace element, which in turn is associated with impairment of functions. Excess concentration may be toxic.