8 CONCEPTS OF NEUROLOGICAL DYSFUNCTION
INTRODUCTION
There are three main areas, discussed in this chapter, which can affect either the central or the peripheral nervous system. The first is altered cerebral homeostasis, which relates to the function of the brain when exposed to changes in blood flow, blood volume and intracranial pressure. There are many conditions that can cause these changes, such as a bleeding into the brain, and often these are life-threatening. The second involves changes in cognition or an individual’s arousal or consciousness level. Often, changes in cerebral homeostasis can change an individual’s cognitive responses, so the two are strongly related. The third relates to changes in motor function, which can originate from alterations in the central or peripheral nervous systems. It should be noted that changes in any of these areas can be global (widespread) or focal (small area) in relation to the extent of the alteration. For instance, increases in the concentration of neurological irritants, such as urea, cause global metabolic changes to the brain. In contrast, local damage to the brainstem caused by a tumour or bleeding will alter consciousness. Therefore, recognising the extent of the damage is important for determining not only the patient’s prognosis but also the treatment options.
There are many networks that provide an individual with the characteristics that define thought, memory, emotion and intellect. These complex pathways are not discrete but rather involve the interaction of neuronal connections from different regions of the brain. Furthermore, these pathways interact and usually all are required for normal brain functionality. For instance, the neural networks that are basic to cognitive function are: (1) attentional networks, which provide arousal and maintenance of attention over time; (2) memory and language networks, by which information is communicated; and (3) emotional networks, which control feelings and emotions. Collectively, these networks are fundamental to the processes of abstract thinking and reasoning. The combination of abstract thinking and reasoning is organised and made operational through the executive attentional networks — that is, those that regulate our higher responses, such as in stressful situations (e.g. conflict), where we have the ability to understand and respond in several different ways. The normal functioning of these networks is evident through the motor network, in which the behaviour we exhibit is viewed by others as appropriate.
We commence our exploration of these complex processes with changes in cerebral homeostasis. We start with blood flow to the brain, as this is crucial to normal neurological functioning.
ALTERATIONS IN CEREBRAL HOMEOSTASIS
Cerebral haemodynamics
There are several aspects of blood flow to the brain (cerebral blood flow, or CBF) that should be examined before exploring the impact that changes in cerebral haemodynamics have on cerebral homeostasis. The brain receives a constant supply of blood: this is essential for normal brain function. It is required to fuel the brain with oxygen and glucose, as the brain does not store large quantities of ATP and glycogen — the brain needs a regular flow of oxygen and glucose to sustain normal metabolic activity. If cerebral blood flow ceases for only a few minutes, an individual will become unconscious without prompt restoration of this flow.
CBF is the total blood flow to the brain. It is approximately 45–55 mL/100 grams of brain tissue/minute, which is about 15–20% of cardiac output. An important aspect of CBF is that blood flow is greater to grey matter than to white matter. CBF is matched to the local metabolic needs of the brain, thereby maintaining a constant supply of oxygen, glucose and nutrients to meet the requirements of brain activity. CBF is regulated via the diameter of the cerebral blood vessels — that is, the degree of vasoconstriction and vasodilation. Importantly, changes in oxygen and carbon dioxide blood concentrations are the most critical factors in altering blood vessel diameter. However, in most cases, carbon dioxide is the most important, with increases in arterial carbon dioxide concentration (PaCO2) causing increases in CBF and decreases in PaCO2 resulting in decreases in CBF (see Figure 8-1). For instance, it has been calculated that a 1 mmHg decrease in PaCO2 will reduce CBF by approximately 3%.1 In cases of profound hypoxia, that is when arterial oxygen concentration (PaO2) is below 50 mmHg, CBF increases dramatically to counteract the decline in cerebral oxygenation (see Figure 8-2). This protective mechanism is crucial to maintaining neuronal functioning and therefore cerebral homeostasis.
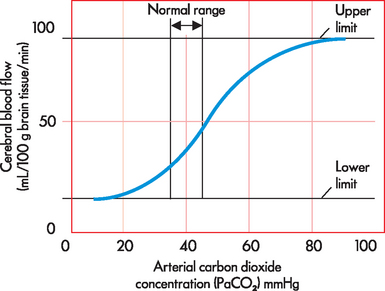
FIGURE 8-1 The relationship between arterial carbon dioxide concentration and cerebral blood flow.
There is an upper and a lower limit of CBF where changes in PaCO2 do not alter CBF. Also, note that small changes in PaCO2 result in larger changes in CBF.
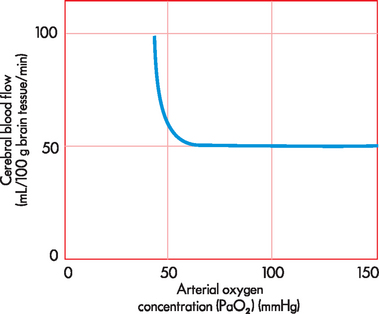
FIGURE 8-2 The relationship between arterial oxygen concentration and cerebral blood flow.
Note that PaO2 over 50 mmHg causes an increase in CBF to increase the supply of oxygen to brain tissue.
Autoregulation is another important factor in understanding CBF. The brain requires a constant blood supply to deliver oxygen, glucose and nutrients but also to remove waste. To achieve this, CBF is kept relatively constant, even when there are changes in blood pressure. The exact mechanism is not yet fully understood, but it appears that complex physiological mechanisms in the local smooth muscle of the cerebral arteries and metabolic changes (such as arterial carbon dioxide concentration) are responsible for maintaining a near constant blood flow. In addition, the vascular wall (the endothelium) may play a role, although this has not been fully elucidated.2 Nonetheless, the main aspect of cerebral autoregulation is that CBF is near-constant across a blood pressure range of 50–150 mmHg (see Figure 8-3). This is very important to understand, because alterations in neurological function, such as brain tumours and stroke, can greatly affect the ability of the brain to maintain cerebral autoregulation.2,3
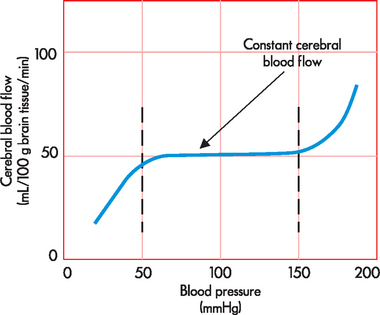
FIGURE 8-3 Cerebral autoregulation.
CBF is maintained at near-constant levels over a wide range of blood pressure (50–150 mmHg).
Cerebral blood volume is the amount of blood in the cranial vault at any given time: it is approximately 10% of total intracranial volume and therefore is crucial to CBF. However, during times of neurological injury, such as haemorrhage in the brain, the blood volume will increase and exceed 10% of total volume. This can lead to significant problems because the brain is enclosed in a fixed space and therefore another component in the brain will need to decrease in volume by an equivalent amount. We now explore how changes in these volumes can lead to increases in intracranial pressure.
Intracranial pressure
Three main components occupy the cranial vault: blood, cerebrospinal fluid (CSF) and brain tissue. As we have already noted, blood makes up approximately 10% of the cranial volume. The CSF that surrounds the brain and spinal cord makes up about 10%; and brain tissue accounts for the other 80%. The skull does not allow for expansion and thus, because these components are in a fixed space, any increase in the volume of one must be compensated by a decrease in the volume of the others. This phenomenon is called the Monro-Kellie hypothesis, and it describes the relationship between the three volumes (see Figure 8-4). When small increases in one occur, the other two must reduce sufficiently so as to maintain a stable pressure. However, larger changes in one cannot be sufficiently compensated for by the decrease in the other two. This leads to increases in intracranial pressure (more commonly referred to as ICP).
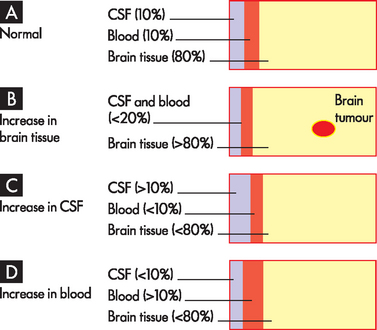
A Normal state, approximately 80% is brain tissue, with CSF and blood accounting for 10% each. B An increase in brain tissue with displacement of CSF and blood. This is the most common change. C An increase in CSF causes displacement of brain tissue and blood. D An increase in blood volume causes displacement of brain tissue and CSF. Note the changes are representative only.
ICP is normally about 5–15 mmHg. However, it is actually difficult to quantify because it represents the sum of the pressure exerted by the three components. Therefore, in most cases ICP is equivalent to CSF pressure.4 In clinical practice, ICP is usually measured either by placing a catheter into the lateral ventricle, thereby measuring CSF pressure, or by placing a catheter directly into brain tissue.
Increased intracranial pressure may result from an increase in intracranial content (as occurs with tumour growth), oedema (swelling of the brain), excess CSF or haemorrhage. It necessitates an equal reduction in volume of the other cranial contents. The most readily displaced content is CSF. This is because, if the brain swells, the volume of CSF can be reduced. However, if ICP remains high after CSF is displaced out of the cranial vault, the cerebral blood volume is then altered. If compensation is inadequate, ICP will continue to rise and life-threatening conditions precipitate (see explanation of the various stages below). Since the brain is enclosed in a rigid skull, there is relatively little scope for it to move or compress, so when ICP becomes excessive, the brain will be forced through the foramen magnum (the main hole at the base of the skull) or blood flow will be severely restricted — either way leading to death. Figure 8-5 demonstrates the relationship between intracranial pressure and volume.
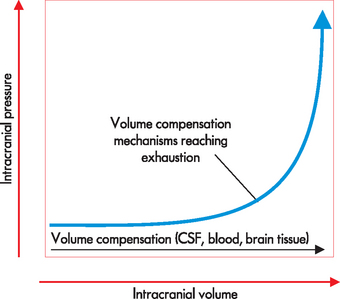
FIGURE 8-5 The relationship between intracranial volume and intracranial pressure.
The horizontal portion of the curve indicates that if one component is expanding, the other two have the ability to compensate. (Note: this compensation mainly comes about by displacement of CSF and blood.) If the volume expands even more, the compensation of the other components is not enough to maintain intracranial pressure — small-volume increments result in large increases in intracranial pressure.
Source: Based on Miller RD et al. Miller’s anesthesia. 7th edn. Philadelphia: Churchill Livingstone; 2010.
The first stage of compensation occurs when there is an increase in ICP due to expansion of one intracranial component, with vasoconstriction and external compression of the venous system occurring in an attempt to decrease the intracranial pressure. Thus, ICP may not change because of effective compensatory mechanisms and there may be few symptoms (see Figure 8-6). However, small increases in volume cause an increase in pressure and the pressure may take longer to return to baseline. This can be detected with ICP monitoring.
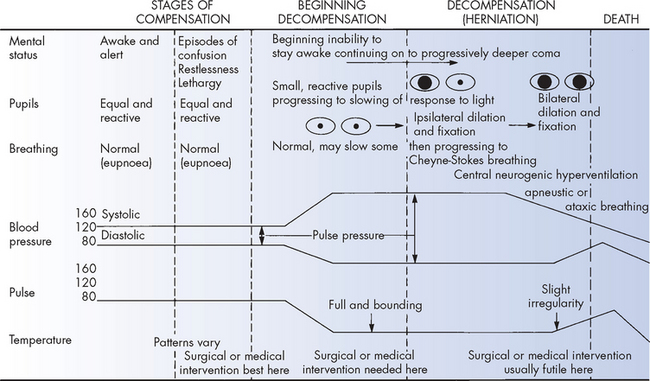
FIGURE 8-6 Clinical manifestations of compensated and uncompensated raised intracranial pressure.
Source: Beare PG, Myers JL. Principles and practice of adult health nursing. 3rd edn. St Louis: Mosby; 1998.
With continued expansion of the intracranial content, the resulting increase in ICP may exceed the brain’s compensatory capacity to adjust. The pressure begins to compromise neuronal oxygenation, and systemic arterial vasoconstriction occurs in an attempt to elevate the systemic blood pressure sufficiently to overcome the raised ICP. Clinical manifestations at this stage usually are subtle and transient, such as episodes of confusion, restlessness, drowsiness and slight pupillary and breathing changes (see Figure 8-6).
As ICP begins to approach cranial arterial pressure, the brain tissues begin to experience hypoxia (low oxygen level) and hypercapnia (high carbon dioxide level) and the individual’s condition deteriorates rapidly. Clinical manifestations include decreasing levels of arousal or central neurogenic hyperventilation (abnormal deep and rapid breathing), widened pulse pressure (the difference between systolic and diastolic blood pressure), bradycardia and pupils that become small and sluggish (see Figure 8-6).
Dramatic sustained rises in ICP are not seen until all compensatory mechanisms have been exhausted. Then dramatic rises in ICP occur over a very short period. Autoregulation fails to maintain a constant blood flow and is lost with progressively increased ICP. Accumulating carbon dioxide may still cause vasodilation locally, but without autoregulation this vasodilation causes the blood pressure in the vessels to drop and blood volume to increase. The brain volume is thus further enhanced and ICP continues to rise. Small increases in volume cause dramatic increases in ICP (see Figure 8-5) and the pressure takes much longer to return to baseline. As ICP begins to approach systemic blood pressure, the blood pressure in the brain (the cerebral perfusion pressure, or CPP) falls and cerebral perfusion slows dramatically. The brain tissues experience severe hypoxia and acidosis (an increase in hydrogen ion concentration; see Chapter 29).
ICP in one compartment of the cranial vault is not evenly distributed throughout the other vault compartments. As a result, the brain tissue shifts from the compartment of greater pressure to a compartment of lesser pressure (this is termed herniation) (see Figures 8-6 and 8-7). The herniating brain tissue’s blood supply then becomes compromised, causing further ischaemia and hypoxia in the herniating tissues. The volume of content within the lower pressure compartment increases, exerting pressure on the brain tissue that normally occupies that compartment and impairing its blood supply. Small haemorrhages often develop in the involved brain tissue. The herniation process markedly and rapidly increases intracranial pressure. Mean systolic arterial pressure soon equals ICP and cerebral blood flow ceases at this point. The types of herniation syndromes are outlined in Box 8-1.
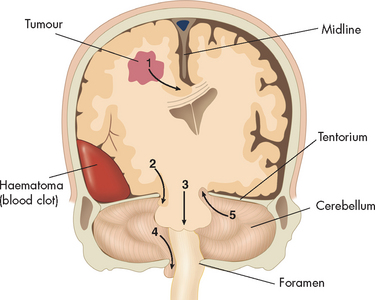
FIGURE 8-7 Herniation of the brain.
Schematic diagram showing the various types of brain herniation. 1 Cingulate herniation, in this case caused by an adjacent tumour. Note that the brain moves sideways, which is termed midline shift. 2 Transtentorial uncal herniation with midbrain compression, in this case caused by an acute haematoma. 3 Central herniation with vertical descent of the brainstem. 4 Cerebellar tonsillar herniation through the foramen magnum with compression of the medulla. 5 Upward cerebellar herniation with upper brainstem compression.
Source: Based on Schapira AHV. Neurology and clinical neuroscience. St Louis: Mosby; 2008.
Supratentorial herniation
Uncal herniation. Occurs when the uncus shifts from the middle fossa through the tentorial notch into the posterior fossa, compressing the third cranial nerve and the mesencephalon. Uncal herniation is generally caused by an expanding mass in the lateral region of the middle fossa. The classical manifestations of uncal herniation are a decreasing level of consciousness, pupils that become sluggish before fixing and dilating, Cheyne-Stokes breathing, and the appearance of decorticate and then decerebrate posturing.
Central herniation. Involves the straight downward shift of the diencephalon through the tentorial notch. It may be caused by injuries or masses located around the outer perimeter of the frontal, parietal or occipital lobes, extracerebral injuries around the central apex (top) of the cranium, and bilaterally positioned injuries or masses. The individual rapidly becomes unconscious; moves from Cheyne-Stokes breathing to apnoea (no breathing); develops small, reactive pupils and then dilated, fixed pupils; and passes from decortication to decerebration.
Infratentorial herniation
In the most common syndrome the cerebellar tonsil shifts through the foramen magnum because of increased pressure within the posterior fossa. The clinical manifestations are an arched stiff neck, paraesthesias in the shoulder area, decreased consciousness, respiratory abnormalities and pulse rate variations. Occasionally the force produces an upward transtentorial herniation of a cerebellar tonsil or the lower brainstem. No specific set of clinical manifestations is associated with infratentorial herniation.
One aspect of raised ICP needs a special mention. The blood pressure that supplies the neurons of the brain is termed the cerebral perfusion pressure (CPP). This pressure determines whether neurons receive blood or not and is often altered during raised ICP. In clinical practice CPP is quantified from the blood pressure and ICP using the following equation:
The normal range of CPP is approximately 70 to 100 mmHg. Three injury states are possibly related to cerebral blood flow: too little cerebral perfusion; normal cerebral perfusion but an elevated intracranial pressure exists; and too much cerebral blood volume. Treatments for these injury states are directed at improving or maintaining CPP, as well as controlling intracranial pressure. An injured brain requires a CPP of greater than 70 mmHg.
Also, if ICP is sufficiently elevated, CPP will decrease. In fact, a vicious cycle can occur, with increasing ICP leading to a decrease in CPP. This decreases blood flow to the neurons and, if this is sustained, hypoxic tissue will eventually die (infarction). The dead tissue causes swelling (oedema), which further increases ICP. This is summarised in Figure 8-8.
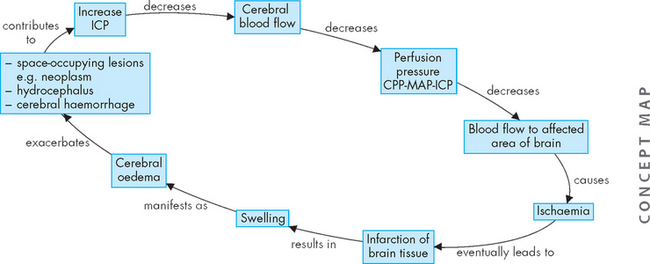
FIGURE 8-8 Cycle of increased intracranial pressure leading to reductions in cerebral perfusion pressure.
This cycle is continued as the tissue dies and swells, leading to further increases in intracranial pressure. CPP = cerebral perfusion pressure; ICP = intracranial pressure; MAP = mean arterial pressure.
Cerebral oedema
Cerebral oedema is an increase in the fluid content of brain tissue (see Figure 8-9). The result is increased extracellular or intracellular tissue volume. It occurs after brain insult from trauma, infection, haemorrhage, tumour, ischaemia, infarct or hypoxia. The harmful effects of cerebral oedema are caused by distortion of the blood vessels, displacement of brain tissues and eventual herniation of brain tissue from one brain compartment to another (see Figure 8-7).
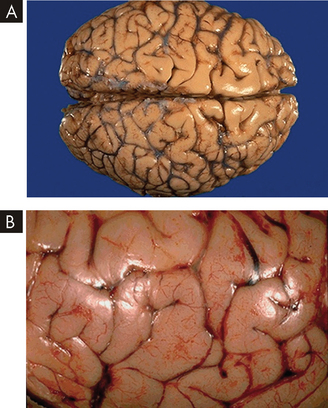
A Normal brain. Note the pattern of gyri and sulci. B Gross cerebral oedema. Note the widened, flattened gyri and narrowed sulci from the swelling of the brain tissue.
Source: Klatt E. Robbins & Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
PATHOPHYSIOLOGY
There are four different types of cerebral oedema — left untreated, all can be devastating.
 Vasogenic oedema is clinically the most important type and is caused by the increased permeability of the capillary endothelium (the lining cells of the blood vessels) of the brain after injury to the vascular structure. The blood–brain barrier is disrupted and plasma proteins leak into the extracellular spaces, drawing water to them and increasing the water content of the brain parenchyma (neuronal, glial and endothelial cells). Vasogenic oedema starts in the area of injury and spreads, with fluid accumulating in the white matter of the ipsilateral side (on the same side) because the parallel myelinated fibres separate more easily. Oedema promotes more oedema because of ischaemia from the increasing pressure. Vasogenic oedema can arise due to tumours, haemorrhage, infections and brain trauma.
Vasogenic oedema is clinically the most important type and is caused by the increased permeability of the capillary endothelium (the lining cells of the blood vessels) of the brain after injury to the vascular structure. The blood–brain barrier is disrupted and plasma proteins leak into the extracellular spaces, drawing water to them and increasing the water content of the brain parenchyma (neuronal, glial and endothelial cells). Vasogenic oedema starts in the area of injury and spreads, with fluid accumulating in the white matter of the ipsilateral side (on the same side) because the parallel myelinated fibres separate more easily. Oedema promotes more oedema because of ischaemia from the increasing pressure. Vasogenic oedema can arise due to tumours, haemorrhage, infections and brain trauma.
Clinical manifestations of vasogenic oedema include focal neurological deficits (such as cranial nerve disturbances or hemiparesis (one-sided weakness), which provide clues as to the location of damage in the brain), disturbances of consciousness and severe increases in ICP. Vasogenic oedema resolves by slow diffusion.
 In cytotoxic oedema, toxic factors directly affect the cellular elements of the brain parenchyma, causing failure of the active transport systems. The cells lose their potassium and gain larger amounts of sodium. Water follows sodium and moves by osmosis into the cells, so that the cells swell. Cytotoxic oedema occurs principally in the grey matter and may increase vasogenic oedema. Cytotoxic oedema can significantly alter global neurological function and result in coma.
In cytotoxic oedema, toxic factors directly affect the cellular elements of the brain parenchyma, causing failure of the active transport systems. The cells lose their potassium and gain larger amounts of sodium. Water follows sodium and moves by osmosis into the cells, so that the cells swell. Cytotoxic oedema occurs principally in the grey matter and may increase vasogenic oedema. Cytotoxic oedema can significantly alter global neurological function and result in coma. Ischaemic oedema follows cerebral infarction (death of neurons, often following a stroke). The ischaemia has components of both vasogenic and cytotoxic oedema. The initial oedema is confined to the intracellular compartment, but over several days, brain cells begin to undergo necrosis and die, releasing lysosomes (see Chapter 3). In this autodigestive process, the blood–brain barrier’s permeability increases, thereby contributing to the oedema.
Ischaemic oedema follows cerebral infarction (death of neurons, often following a stroke). The ischaemia has components of both vasogenic and cytotoxic oedema. The initial oedema is confined to the intracellular compartment, but over several days, brain cells begin to undergo necrosis and die, releasing lysosomes (see Chapter 3). In this autodigestive process, the blood–brain barrier’s permeability increases, thereby contributing to the oedema. Interstitial oedema is seen most often with non-communicating hydrocephalus (see next section). The oedema is caused by movement of CSF from the ventricles into the extracellular spaces of the brain tissues. The brain fluid volume increases predominantly around the ventricles, with increased hydrostatic pressure within the white matter. The size of the white matter reduces because of the rapid disappearance of myelin lipids.
Interstitial oedema is seen most often with non-communicating hydrocephalus (see next section). The oedema is caused by movement of CSF from the ventricles into the extracellular spaces of the brain tissues. The brain fluid volume increases predominantly around the ventricles, with increased hydrostatic pressure within the white matter. The size of the white matter reduces because of the rapid disappearance of myelin lipids.CLINICAL MANIFESTATIONS
The signs and symptoms of cerebral oedema can vary. If cerebral oedema is compensated by a decrease in CSF and blood flow, then neurological function may not be impaired. However, when cerebral oedema is significant or ongoing, increase in ICP may occur. The clinical manifestations of raised ICP include — but are not limited to — headache, nausea and vomiting, and a decrease in consciousness. If left untreated, these clinical manifestations will progress and a decrease in global cerebral function will result. Patients with severe cerebral oedema may present with coma and changes in pupillary light reactions.
EVALUATION AND TREATMENT
The treatment of cerebral oedema is dependent on the type of oedema and the extent of the increased ICP. For instance, if a brain tumour is causing localised oedema, patients will often be treated with corticosteroids, such as dexamethasone. Moreover, the tumour can be surgically removed. In the acute phase of cerebral oedema, osmotic diuretics (mannitol) can reduce the water volume and lower the effects of oedema.
There are other options for clinicians to manage cerebral oedema if there is an accompanying raised ICP. They include hyperventilation to lower (‘blow off’) carbon dioxide, which will cause cerebral vasoconstriction; or alternatively, a catheter can be inserted into the lateral ventricles and excess CSF can be drained to lower the ICP (see Figure 8-10). These two methods employ other mechanisms to reduce the pressure and do not actively treat the cause of the cerebral oedema. It should be noted that changing the CSF or blood flow involves using the principles of the Monro-Kellie hypothesis — that is, when one volume increases, the other volumes must decrease by a similar amount to maintain homeostasis between the brain tissue, blood and CSF.
Hydrocephalus
The term hydrocephalus refers to various conditions characterised by excess fluid in the cranial vault or subarachnoid space, or both. Hydrocephalus occurs because of interference with CSF flow caused by increased fluid production, obstruction within the ventricular system or defective reabsorption of the fluid. The types of hydrocephalus are reviewed in Table 8-1.
Table 8-1 TYPES OF HYDROCEPHALUS
| TYPE | MECHANISM | CAUSE |
|---|---|---|
| Non-communicating | Obstruction of CSF flow between ventricles | |
| Communicating | Impaired absorption of CSF within subarachnoid space |
Hydrocephalus may develop from infancy through to adulthood. Congenital hydrocephalus (i.e. ventricular enlargement before birth) results in an increased volume of CSF (see below). Non-communicating hydrocephalus — obstruction within the ventricular system — is seen more often in children; and communicating hydrocephalus — defective reabsorption of CSF from the cerebral subarachnoid space — is found more often in adults.
Most cases of hydrocephalus develop gradually and insidiously over time. Acute hydrocephalus, however, may develop in a couple of hours in people who have sustained head injuries. Acute hydrocephalus contributes significantly to raised ICP.
PATHOPHYSIOLOGY
The obstruction of CSF flow associated with hydrocephalus produces dilation of the ventricles proximal to the obstruction. This is evident on computed tomography (CT) and magnetic resonance imaging (MRI) scans (see Figure 8-11). Obstructed CSF is under pressure, causing atrophy of the cerebral cortex and degeneration of the white matter tracts. There is selective preservation of grey matter. When excess CSF fills a defect caused by atrophy, a degenerative disorder or a surgical excision, this fluid is not under pressure; therefore, atrophy and degenerative changes are not induced.
CLINICAL MANIFESTATIONS
Acute hydrocephalus presents with signs of rapidly developing increased ICP. The person quickly deteriorates into a deep coma if not treated promptly. Normal-pressure hydrocephalus (dilation of the ventricles without the increased pressure) develops slowly, with the individual or family having a decline in memory and cognitive function. An unsteady, broad-based gait (walking style) with a history of falling is common. Additional clinical manifestations are apathy, inattentiveness and indifference to self, family and the environment. Urinary incontinence is often present.
EVALUATION AND TREATMENT
The diagnosis is based on physical examination and CT and MRI scans. Hydrocephalus can be treated by surgery to resect cysts, neoplasms or haematomas. A ventricular bypass into the normal intracranial channel or into an extracranial compartment using a shunting procedure is one of the most common neurosurgical procedures for the treatment of hydrocephalus. In normal-pressure hydrocephalus, reduction in CSF through a diuresis regimen is often used.
Congenital hydrocephalus
Congenital hydrocephalus is characterised by an increased volume of CSF. It may be caused by blockage within the ventricular system where the CSF flows, an imbalance in the production of CSF or a reduced reabsorption of CSF. The pressure within the ventricular system pushes and compresses the brain tissue against the skull cavity. When hydrocephalus develops before fusion of the cranial sutures, the skull can accommodate this additional space-occupying volume and preserve neuronal function. The overall incidence of hydrocephalus is approximately 3 per 1000 live births.5 The incidence of hydrocephalus, excluding hydrocephalus associated with myelomeningocele — a common neural tube defect at birth, where the vertebrae (bones of the spinal column) do not completely form, causing the spinal cord and meninges to protrude through the back — is approximately 0.5 to 1 per 1000 live births.6
ALTERATIONS IN COGNITIVE FUNCTION
We have looked at the changes in brain function that arise from blood supply alterations or from lesions that increase ICP. Now we turn to explore the changes to cognitive function in the brain and how these changes may manifest in patients.
Alterations in arousal
Full consciousness is a state of awareness of oneself and the environment and a set of responses to that environment. The fully conscious individual responds to external stimuli, such as noise, light, pain and temperature changes, with a wide array of responses. Any decrease in this state of awareness and varied responses is considered a decrease in consciousness.
Consciousness involves arousal and content of thought. Arousal is an individual’s state of awareness — that is, is the person aware of their surroundings? This complex state is mediated by the reticular activating system, which includes the brainstem and fibres that project to and from the cerebral cortex (see Chapter 6). When a person temporarily loses cerebral function, such as following a blow to the head, the reticular activating system and brainstem can maintain the vital functions of the body, with arousal usually returning in a short period of time. However, if there are widespread permanent alterations in cerebral function, then vital autonomic functions (such as sustained independent breathing) are maintained, but the individual has no awareness of their environment. It should be noted that cognitive cerebral functions cannot occur without a functioning reticular activating system. Therefore, if an individual sustains permanent damage to their reticular activating system, arousal is not possible.
An altered level of arousal (awareness) with acute onset may be caused by various factors (i.e. alteration to brain structure, metabolic disturbances or psychogenic alteration — one that has a psychological origin). Structural causes are divided according to whether the original location of the pathological condition is above or below the tentorial plate (a dural fold that separates the cerebrum from the cerebellum; see Figure 8-12). Pathological processes include infectious, vascular, neoplastic, traumatic, congenital (developmental), degenerative and metabolic causes. Metabolic causes are further divided into hypoxia, electrolyte disturbances, hypoglycaemia, drugs and toxins (both endogenous and exogenous). Alterations in arousal range from slight drowsiness to coma.
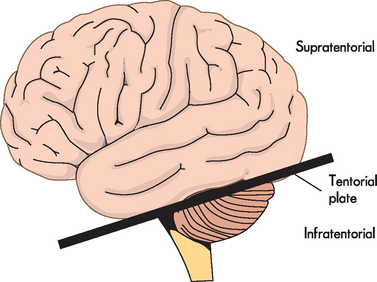
Note that the tentorial plate is a fold of dura that separates the cerebral hemispheres from the cerebellum, but is used to differentiate between neurological insults.
PATHOPHYSIOLOGY
Processes above the tentorial plate (supratentorial) produce changes in arousal by either diffuse or localised dysfunction. Disease processes may produce diffuse dysfunction (e.g. encephalitis — inflammation of the brain) and may affect the cerebral cortex or the underlying sub-cortical white matter. Localised dysfunction generally is caused by masses that directly impinge on deep structures of the diencephalon or that secondarily compress these structures in the process of herniation. Such localised destructive processes directly impair function of the thalamus or hypothalamus.
Disorders outside the brain but within the cranial vault can produce diffuse dysfunction. Examples include neoplasms, closed-head trauma with subsequent subdural bleeding and accumulation of pus in the subdural space. Disorders within the brain substance — bleeding, infarcts and emboli, and tumours — function primarily as masses.
With processes below the tentorial plate (infratentorial), arousal declines by direct destruction of the reticular activating system and its pathways or of the entire brainstem either by direct invasion or by indirect impairment of its blood supply. In addition, decreased awareness may result from compression of the reticular activating system by a disease process. This compression may result from direct pressure or compression as structures either expand or herniate. Causes include accumulations of blood or pus, neoplasms and demyelinating disorders.
CLINICAL MANIFESTATIONS AND EVALUATION
The cause of an altered level of arousal may be functional or organic. Functional conditions arise when no discrete anatomical location can be found for the alteration — for instance, headaches and insomnia. In contrast, organic conditions come about when there are structural alterations that can be located — for instance, brain tumours, bleeding or contusions. Both conditions may contribute to an altered level of arousal.
Further distinctions can be made between metabolic factors and structural factors (see Table 8-2). Metabolic factors include changes in biochemistry that affect brain function. An example is when acute liver failure occurs — the patient is unable to metabolise ammonia, which in elevated concentrations is neurotoxic and can lead to coma. Structural factors are similar to organic factors — they are areas where cellular damage has occurred and this alters central nervous system (CNS) homeostasis.
Table 8-2 CLINICAL MANIFESTATIONS OF METABOLIC AND STRUCTURAL CAUSES OF COMA
| MANIFESTATIONS | METABOLICALLY INDUCED COMA | STRUCTURALLY INDUCED COMA |
|---|---|---|
| Blink to threat (cranial nerves II, VII) | Equal | Asymmetric |
| Optic discs (cranial nerve II) | Flat, good pulsation | Papilloedema (optical disc swelling) |
| Extraocular movement (cranial nerves III, IV, VI) | Roving eye movements; normal doll’s eyes | Nerve palsy (paralysis of body part) |
| Pupils (cranial nerves II, III) | Equal and reactive, may be dilated (e.g. atropine), pinpoint (e.g. opiates) or midposition and fixed | Asymmetric or nonreactive; may be midposition (midbrain injury), pinpoint (pons injury), large (tectal injury) |
| Corneal reflex (cranial nerves V, VII) | Symmetric response | Asymmetric response |
| Grimace to pain (cranial nerve VII) | Symmetric response | Asymmetric response |
| Motor function movement | Symmetric | Asymmetric |
| Tone | Symmetric | Spastic, flaccid, especially if asymmetric |
| Posture | Symmetric | Decorticate, especially if symmetric; decerebrate, especially if asymmetric |
| Deep tendon reflexes | Symmetric | Asymmetric |
| Babinski’s sign | Absent or symmetric response | Present |
| Sensation | Symmetric | Asymmetric |
Patterns of clinical manifestations help in determining the extent of brain dysfunction and serve as indices for identifying increasing or decreasing central nervous system function. The types of manifestations suggest the cause of the altered arousal state (see Table 8-3). Five categories of neurological function are critical to the evaluation process:
Table 8-3 DIFFERENTIAL CHARACTERISTICS OF STATES CAUSING COMA
| MECHANISM | MANIFESTATIONS |
|---|---|
| Supratentorial mass lesions compressing or displacing the diencephalon or brainstem | |
| Infratentorial mass of destruction causing coma | |
| Metabolic coma |
Level of consciousness
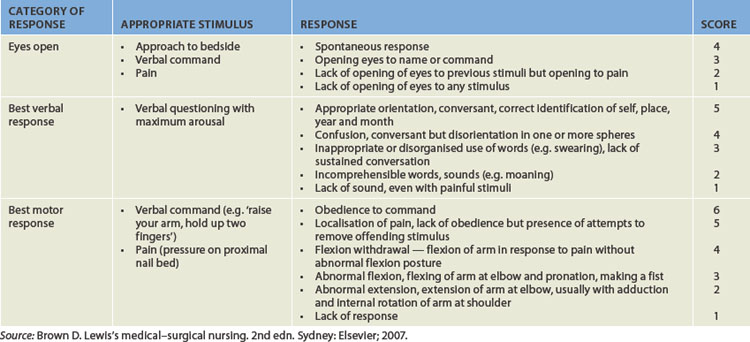
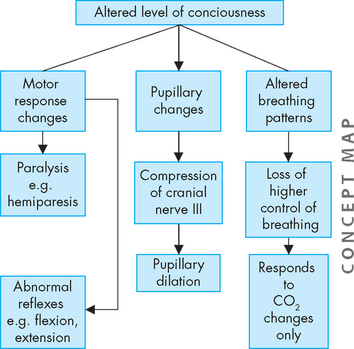
The level of consciousness is the most critical clinical index of nervous system function, with alterations indicating either improvement or deterioration of the individual’s condition. The most common way of assessing an individual’s level of consciousness is to use an objective assessment tool such as the Glasgow coma scale (GCS; see Table 8-4). The scale assesses an individual’s ability to open their eyes and their verbal and motor responses. Collectively, these provide information about the individual’s level of consciousness. A person who is alert and oriented to self, others, place and time is considered to be functioning at the highest level of consciousness, which implies full use of all the person’s cognitive capacities. From this normal alert state, levels of consciousness diminish in stages, each of which is clinically defined (see Table 8-5). In addition, decreases in the level of consciousness can alter many physiological processes. Some of the major processes are shown in Figure 8-13 and discussed below.
Table 8-5 LEVELS OF ALTERED CONSCIOUSNESS
| STATE | DEFINITION |
|---|---|
| Confusion | Loss of ability to think rapidly and clearly; impaired judgement and decision making |
| Disorientation | Beginning loss of consciousness; disorientation to time followed by disorientation to place and impaired memory; lost last is recognition of self |
| Lethargy | Limited spontaneous movement or speech; easy arousal with normal speech or touch; may or may not be oriented to time, place or person |
| Obtundation | Mild to moderate reduction in arousal (awakeness) with limited response to the environment; falls asleep unless stimulated verbally or tactilely; answers questions with minimum response |
| Stupor | A condition of deep sleep or unresponsiveness from which the person may be aroused or caused to open eyes only by vigorous and repeated stimulation; response is often withdrawal or grabbing at stimulus |
| Coma | No verbal response to the external environment or to any stimuli; noxious stimuli such as deep pain or suctioning do not yield motor movement |
| Light coma | Associated with purposeful movement on stimulation |
| Coma | Associated with non-purposeful movement only on stimulation |
| Deep coma | Associated with unresponsiveness or no response to any stimulus |
Breathing pattern
Characteristic breathing patterns help evaluate the neuroanatomical level of brain dysfunction and coma (see Figure 8-14). Rate, rhythm and pattern should be evaluated. Breathing patterns can be categorised as hemispheric or brainstem breathing patterns (see Table 8-6).
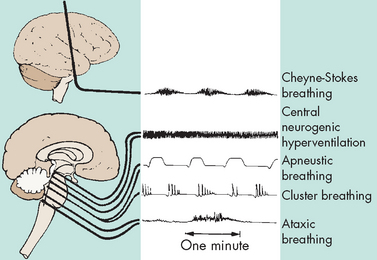
FIGURE 8-14 Abnormal breathing patterns with the corresponding level of anatomical insult.
Source: Urden LD, David JK, Lough ME. Thelan’s critical care nursing: diagnosis and management. 5th edn. St Louis: Mosby; 2006.
Table 8-6 ABNORMAL BREATHING PATTERNS
| BREATHING PATTERN | DESCRIPTION | LOCATION OF INJURY |
|---|---|---|
| Hemispheric breathing patterns | ||
| Normal | After a period of hyperventilation that lowers the arterial carbon dioxide pressure (PaCO2), the individual continues to breathe regularly but with a reduced depth. | Response of the nervous system to an external stressor — not associated with injury to the CNS |
| Post-hyperventilation apnoea | Associated with diffuse bilateral metabolic or structural disease of the cerebrum | |
| Cheyne-Stokes breathing | The breathing pattern has a smooth increase (crescendo) in the rate and depth of breathing (hyperpnoea), which peaks and is followed by a gradual smooth decrease (decrescendo) in the rate and depth of breathing to the point of apnoea, when the cycle repeats itself. The hyperpnoeic phase lasts longer than the apnoeic phase. | Bilateral dysfunction of the deep cerebral or diencephalic structures, seen with supratentorial injury and metabolically induced coma states |
| Brainstem breathing patterns | ||
| Central neurogenic hyperventilation | A sustained, deep, rapid but regular pattern (hyperpnoea) occurs, with a decreased PaCO2 and a corresponding increase in pH and PaO2. | May result from CNS damage or disease that involves the midbrain and upper pons; seen after increased intracranial pressure and blunt head trauma |
| Apneustic breathing | A prolonged inspiratory cramp (a pause at full inspiration) occurs; a common variant of this is a brief end-inspiratory pause of 2 or 3 seconds, often alternating with an end-expiratory pause. | Indicates damage to the respiratory control mechanism located at the pontine level; most commonly associated with pontine infarction but documented with hypoglycaemia, anoxia and meningitis |
| Cluster breathing | A cluster of breaths has a disordered sequence with irregular pauses between breaths. | Dysfunction in the lower pontine and high medullary areas |
| Ataxic breathing | Completely irregular breathing occurs, with random shallow and deep breaths and irregular pauses. Often the rate is slow. | Originates from a primary dysfunction of the medullary neurons controlling breathing |
| Gasping breathing pattern (agonal gasps) | A pattern of deep ‘all-or-none’ breaths is accompanied by a slow respiratory rate. | Indicative of a failing medullary respiratory centre |
With normal breathing, a neural centre in the forebrain (cerebrum) produces a rhythmic pattern. When consciousness decreases, lower brainstem centres regulate the breathing pattern, responding only to changes in PaCO2 levels. The result is the irregular breathing associated with post-hyperventilation apnoea.
Cheyne-Stokes breathing results from an increased ventilatory response to carbon dioxide stimulation, causing hypercapnia and diminished ventilatory stimulus. Changes in PaCO2 produce irregular breathing, contributing to ‘overbreathing’ with carbon dioxide stimulation. The PaCO2 level then decreases to below normal and breathing stops until the carbon dioxide reaccumulates to bring the PaCO2 level back to normal. With opiate or sedative drug overdose, the respiratory centre in the brainstem is depressed so the rate of breathing gradually decreases until respiratory failure occurs. Central neurogenic breathing is also a ventilatory response to carbon dioxide and is characterised by deep and rapid breathing. It is an abnormal breathing pattern and often an indicator of poor prognosis. Ataxic breathing occurs when the injury has extended to or is associated with the medulla. It is characterised by a lack of breathing coordination and breathing appears to be irregular, hence the name. It is associated with an increase in mortality. In summary, the more inferior (towards the feet) the level of insult in the brainstem, the poorer the prognosis.
Pupillary changes
Anatomically, brainstem areas that control arousal are adjacent to areas that control the pupils. In this way, clinicians examine pupillary changes to determine the extent of injury because the pupillary changes indicate the presence and level of brainstem dysfunction (see Figure 8-15). For example, severe ischaemia and hypoxia usually produce dilated, fixed pupils.
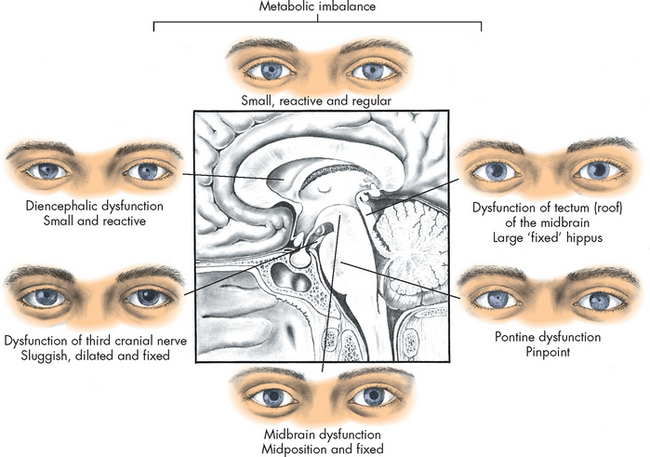
FIGURE 8-15 Pupillary changes due to injury at different levels of the brain.
The pupillary changes correspond to the level of consciousness.
The third cranial nerve (oculomotor) controls pupillary constriction, and compression of the nerve causes contralateral dilation of the pupil. This occurs because the oculomotor nerve is compressed when there is an increase in intracranial pressure, which may be a precursor to uncal herniation (refers to the movement of the uncus (medial part of the temporal lobe) down, which increases pressure on the midbrain of the brainstem). Therefore, pupillary changes are crucial to a neurological assessment of a patient who is experiencing a decreased level of consciousness.
Some drugs affect pupils and must be considered in evaluating individuals in comatose states. For instance, large concentrations of atropine (used as an anticholinergic agent) cause full dilation and fixing of the pupils. Opiates, such as morphine, cause pinpoint pupils. Severe barbiturate intoxication may produce fixed pupils.
Oculomotor responses
Resting, spontaneous and reflexive eye movements change at various levels of brain dysfunction. For instance, individuals with metabolically induced coma generally retain ocular reflexes even when other signs of brainstem damage are present. Therefore, evaluation of the movement of the eyes is also essential for thorough assessment of the level of consciousness.
Motor responses
Motor responses help evaluate the level of brain dysfunction and determine the most severely damaged side of the brain. The pattern of response noted may be: (1) purposeful; (2) inappropriate, generalised motor movement; or (3) not present at all. Motor signs indicating loss of cortical inhibition that are commonly associated with decreased consciousness include reflex grasping, reflex sucking and rigidity. Abnormal flexor (decorticate) and extensor (decerebrate) responses in the upper and lower extremities are defined in Table 8-7 and illustrated in Figure 8-16.
Table 8-7 ABNORMAL MOTOR RESPONSES WITH DECREASED RESPONSIVENESS
| MOTOR RESPONSE | DESCRIPTION | LOCATION OF INJURY |
|---|---|---|
| Abnormal motor responses, upper extremity flexion with or without extensor responses in the leg (decorticate rigidity) | Slowly developing flexion of the arm, wrist and fingers with adduction in the upper extremity, and extension, internal rotation and plantar flexion of the lower extremity | Suggest hemispheric damage above midbrain |
| Extensor responses in the upper and lower extremities (decerebrate posturing, decerebrate rigidity) | ||
| Extensor responses in the upper extremities accompanied by flexion in the lower extremities | Indicates pontine level dysfunction | |
| Flaccid state with little or no motor response to stimuli | Damage to lower pons |
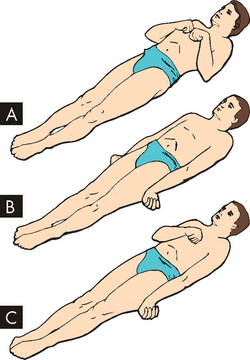
FIGURE 8-16 Decorticate and decerebrate responses to painful stimuli.
A Decorticate response. Flexion of arms, wrists and fingers with adduction in upper extremities; extension, internal rotation and plantar flexion in lower extremities. B Decerebrate response. All four extremities in rigid extension, with hyperpronation of forearms and plantar extension of feet. C Decorticate response on right side of body and decerebrate response on left side of body.
Source: Rudy EB (ed.). Advanced neurological and neurosurgical nursing. St Louis: Mosby; 1984.
Vomiting
Yawning, vomiting and hiccups are complex reflex-like motor responses that are integrated by neural mechanisms in the lower brainstem. These responses may be produced by compression or diseases involving tissues of the medulla oblongata (e.g. infection, neoplasm, infarct) but also occur relative to other more benign stimuli to the vagal nerve.
Most CNS disorders produce nausea and vomiting. Vomiting without nausea indicates direct involvement of the central neural mechanism. Vomiting often accompanies CNS injuries that: (1) involve the vestibular nuclei (involved in balance and spatial orientation) or its immediate projections, particularly when double vision (diplopia) is also present; (2) impinge directly on the floor of the fourth ventricle; or (3) produce brainstem compression secondary to increased intracranial pressure.
Post-coma unresponsiveness
Essentially, when brain damage is severe, individuals will experience a decreasing level of consciousness often leading to coma and death (see Table 8-5). However, individuals who survive and remain in a prolonged deep coma are in a post-coma unresponsiveness state.7 Previously, this was termed persistent vegetative state, but this description has been changed in Australia because it implies that the state is permanent and the word ‘vegetative’ was considered too negative.8 In contrast, post-coma unresponsiveness better describes the level of consciousness of the individual following coma. It has been defined as:
The post-coma unresponsive individual has emerged from coma — that is, sleep–wake cycles are observable over time — but only brainstem reflexes are present. There is a complete unawareness of self or the surrounding environment and cerebral function is lost. The individual’s vital autonomic functions, such as blood pressure and breathing, are maintained without support. There is bowel and bladder incontinence. The individual does not speak any comprehensible words or follow commands. Recovery is unlikely if the state persists for 12 months.
In some individuals, a gradual return to some level of responsiveness occurs. These individuals are considered to be in a minimally responsive state. In this state, individuals may have cognitively mediated behaviour that initiates a minimal level of purposeful response. For instance, these individuals may be able to respond to simple commands with, say, finger movement, as opposed to reflex responses.8,9 It should be noted that the chances of these individuals returning to full neurological function are remote.
Finally, some individuals exhibit a coma-like appearance but do not have any alteration of level of arousal or cognition. These individuals have locked-in syndrome. This is quite distinct from post-coma unresponsiveness and the minimally responsive state, because there is only specific damage to the brainstem without damage to the cerebrum.7,8 Therefore, the efferent pathways are disrupted and the individual has a limited capacity to communicate with the environment. The individual cannot communicate through speech or body movement but is fully conscious, with intact cognitive function. The person retains eye movement and blinking as a means of communication.
Brain death
Brain death occurs when the brain is damaged so completely that it can never recover and cannot maintain the body’s internal homeostasis. On postmortem examination, the brain is autolysing (self-digesting) or already autolysed, because the brain tissue has died.
Brain death has occurred when there is no evidence of function above the foramen magnum10 — that is, in the cerebral hemispheres or brainstem — for an extended period. The abnormality of brain function must result from structural or known metabolic disease and must not be caused by a depressant drug, alcohol poisoning or hypothermia. For brain death to occur there must be severe brain injury with sustained increased ICP above systemic blood pressure, such that intracranial blood flow ceases.11
In Australia and New Zealand brain death guidelines have been determined. They state that:
Determination of brain death requires that there is unresponsive coma, the absence of brain-stem reflexes and the absence of respiratory centre function, in the clinical setting in which these findings are irreversible. In particular, there must be definite clinical or neuro-imaging evidence of acute brain pathology (e.g. traumatic brain injury, intracranial haemorrhage, hypoxic encephalopathy) consistent with the irreversible loss of neurological function.11
The following pre-conditions must be present before clinical determination of brain death:
The clinical testing of brain death requires two medical practitioners with specific experience and qualifications to perform two separate clinical examinations of the brain death test. The clinical test examines three main categories: coma, absence of brainstem reflexes and absence of breathing. The test and responses for brain death determination are included in Table 8-8. It should be noted that if any test results are not conclusive, the patient cannot be confirmed as brain dead. Furthermore, if brain death cannot be determined conclusively from clinical testing, demonstration of an absence of intracranial blood flow can be used via imaging techniques (see Figure 8-17).11 The Australian and New Zealand Intensive Care Society (ANZICS) recommends that in children brain death studies have the same criteria as for adults, but with a longer observation period.
Table 8-8 BRAIN DEATH CLINICAL TESTS
| TYPE OF CLINICAL TEST | CLINICAL TEST | RESPONSES THAT INDICATE BRAIN DEATH |
|---|---|---|
| Coma | Apply noxious stimuli to elicit motor response (e.g. periorbital rub, sternal rub). | Absence of movement |
| Brainstem reflexes | ||
| Pupillary light reflex (cranial nerves II & III) | Shine a bright light into the eye and observe pupillary constrictor response. | No pupillary constriction response |
| Corneal reflex (cranial nerves V & VII) | Touch the corneas with soft cotton wool or gauze and examine the eye response. | No blinking or withdrawal response |
| Reflex response in trigeminal distribution (cranial nerves V & VII) | Apply pain over the trigeminal distribution, e.g. pressure over the supraorbital nerve. | No facial or limb movement |
| Vestibulo-ocular reflex (cranial nerves III, IV, VI & VIII) | No eye movement in response to the cold water; the eyes remain in the midline within the socket | |
| Gag reflex (cranial nerves IX & X) | Stimulate the posterior pharyngeal wall. | No gag response |
| Cough/tracheal reflex (cranial nerve X) | Stimulate the tracheobronchial wall. | No cough response observed |
| Apnoea testing | No breathing effort is seen during testing | |
Source: Australian and New Zealand Intensive Care Society. The ANZICS statement on death and organ donation. 3rd edn. Melbourne: ANZICS; 2008.
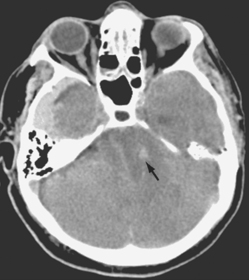
FIGURE 8-17 Brain death study.
A CT scan of a 73-year-old man showing compression of the brainstem (arrow). Note the lack of gyri and sulci as the brain has swollen following global neuronal death.
Source: Imaging consult. Elsevier. 2009.
Seizures
A seizure results from a sudden, explosive, disorderly discharge of cerebral neurons and is characterised by a rapid, transient alteration in brain function, usually involving motor, sensory, autonomic or psychic clinical manifestations and altered level of arousal. Neurons both produce and transmit electrical impulses and the rate of neuronal electrical signalling is different according to the activity of the individual. For instance, during sleep, electrical activity is reduced compared to when performing complex tasks such as talking to a passenger while driving a motor vehicle. Nonetheless, the neuronal electrical discharges are ordered and coordinated. During a seizure, the electrical impulses are uncoordinated and many neurons are firing simultaneously at a faster rate than normal, which produces a brief disruption in the brain’s electrical functions. Seizure disorders represent a syndrome, however, and not a specific disease entity. The alteration in level of arousal is temporary. It should be noted that the term convulsion is sometimes applied to seizures, but it should be applied to the jerky, contract-relax (more correctly termed, tonic-clonic) movement associated with some seizures.
Seizures are common — approximately 10% of people worldwide have one seizure during their lifetime.12 In addition, it is estimated that 50% of those who have one seizure will have more seizures. If seizures are recurrent, without any underlying correctable cause, the individual will be diagnosed with epilepsy. The World Health Organization estimates that 50 million people worldwide have epilepsy. The vast majority (up to 90%) are found in developing countries.12 In Australia, there are a reported 200,000 people with epilepsy.13 For approximately half of all people with epilepsy the onset occurs before 20 years of age, and there is another peak around 60 years of age.13 The prevalence of epilepsy is 5–10 persons per 1000.14
PATHOPHYSIOLOGY
Any disorder that alters the neuronal environment may cause seizure activity, so, theoretically, anyone may experience a seizure. The seizure threshold of some persons, however, apparently is genetically lower.
Diseases or other processes that involve the nervous system can produce a seizure disorder. The onset may indicate the presence of an ongoing primary neurological disease. Aetiological factors in seizures generally include (1) cerebral lesions, (2) biochemical disorders, (3) cerebral trauma and (4) epilepsy, which can result from the following conditions:
Causes of recurrent seizures are age-related (see Table 8-9).
Table 8-9 CAUSES OF SEIZURES IN DIFFERENT AGE GROUPS
| AGE AT ONSET | PROBABLE CAUSE |
|---|---|
| Neonates (< 1 month) | |
| Infants and children (> 1 month and < 12 years) | |
| Adolescents (12–18 years) | |
| Young adults (18–35 years) | |
| Older adults (> 35 years) |
Note: febrile refers to fever; idiopathic means the cause is unknown.
Source: Lownstein DH. Seizures and epilepsy. In: Kasper DL et al (eds). Harrison’s principles of internal medicine. 16th edn. Vol 2. New York: McGraw-Hill; 2005.
Seizures may be precipitated by a range of events. For instance, all of the following can result in seizure activity: hypoglycaemia; fatigue or lack of sleep; emotional or physical stress; febrile illness; large amounts of water ingestion; constipation; use of stimulant drugs; withdrawal from depressant drugs (including alcohol); hyperventilation; and some environmental stimuli, such as blinking lights, a poorly adjusted television screen, loud noises, certain music, certain odours or merely being startled.
Epilepsy is now thought to be the result of complex genetic mutations with environmental effects that cause abnormalities in brain synapses or connections, or an imbalance in chemicals that the brain uses to send signals or abnormal nerve connections made while attempting to repair itself after injury.15 A group of neurons may exhibit a paroxysmal (short, frequent attacks) depolarisation shift and function as an epileptogenic focus. These neurons are hypersensitive and are more easily activated by conditions that change their depolarisation threshold. Examples include hyperthermia, hypoxia, hypoglycaemia, hyponatraemia, repeated sensory stimulation and certain sleep phases.
Epileptogenic neurons fire more and more frequently. When the intensity reaches a threshold point, cortical excitation spreads. Excitation of the sub-cortical, thalamic and brainstem areas corresponds to the tonic phase (muscle contraction with increased muscle tone) and is associated with loss of consciousness. The clonic phase (alternating contraction and relaxation of muscles) begins when inhibitory neurons in the cortex, anterior thalamus and basal nuclei react to the cortical excitation. The seizure discharge is interrupted, producing intermittent muscle contractions that gradually decrease and finally cease. The epileptogenic neurons are exhausted.
During seizure activity, oxygen is consumed at a high rate — about 60% over normal. Although cerebral blood flow also increases, oxygen is rapidly depleted, along with glucose, and lactate accumulates in brain tissue. Continued, severe seizure activity holds the potential for progressive brain injury and irreversible damage.
Types of seizure disorders
The International League Against Epilepsy (www.ilae-epilepsy.org) has attempted to modify the International Classification of Epileptic Seizures without consensus. Seizures are classified in different ways: by clinical manifestations, site of origin, EEG findings or response to therapy. In addition, a number of terms are used to describe seizure activity and these are defined in Table 8-10. There are more than 40 different types of seizures; however, the most common classification is to divide them into generalised and partial seizures, which are then further subdivided again. A simplified version of the International Classification of Epileptic Seizures is presented in Figure 8-18 and discussed below.
Table 8-10 TERMINOLOGY APPLIED TO SEIZURES
| TERM | DEFINITION |
|---|---|
| Aura | A partial seizure experienced as a peculiar sensation preceding the onset of generalised seizure that may take the form of gustatory, visual or auditory experience or a feeling of dizziness, numbness or just ‘a funny feeling’ |
| Prodroma | Early clinical manifestations, such as malaise, headache or a sense of depression, which may occur hours to a few days before the onset of a seizure |
| Tonic phase | A state of muscle contraction in which there is excessive muscle tone |
| Clonic phase | A state of alternating contraction and relaxation of muscles |
| Post-ictal phase | The time period immediately following the cessation of seizure activity |
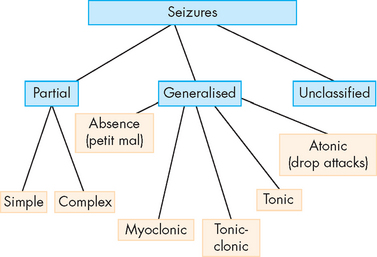
FIGURE 8-18 Classification of epileptic seizures.
See the text for an explanation of the different types of seizures.
Source: Modified from the International Classification of Epileptic Seizures.
The primary difference between generalised and partial seizures is where the epileptic activity occurs:
 Partial, or focal, seizures usually involve only a portion of the brain. These are the most common type of seizure; approximately 60% of people who have seizures have partial seizures — mostly teenagers and the older population. The seizures are often subtle and the person may seem not to be aware of their environment, without any other clinical manifestation.
Partial, or focal, seizures usually involve only a portion of the brain. These are the most common type of seizure; approximately 60% of people who have seizures have partial seizures — mostly teenagers and the older population. The seizures are often subtle and the person may seem not to be aware of their environment, without any other clinical manifestation. In contrast, generalised seizures, as the name implies, involve widespread epileptic activity in both hemispheres of the brain. They usually result in unconsciousness. It should be noted that partial seizures can progress to generalised seizures in some cases.
In contrast, generalised seizures, as the name implies, involve widespread epileptic activity in both hemispheres of the brain. They usually result in unconsciousness. It should be noted that partial seizures can progress to generalised seizures in some cases.Partial seizures can be subdivided as follows:
 Simple partial seizures. Simple partial seizures involve a short-lived seizure with no loss of consciousness or awareness. They usually involve only one area of the brain; the area of neuronal epileptic activity will dictate the type of symptoms the person will experience. For instance, if the seizure focus is in the sensory area of the brain, the person may experience numbness or pins and needles in a body area. In addition, some people may have an aura immediately before the seizure. This is a distinct warning sign that a seizure is imminent and it can be visual, motor or sensory — for instance, the person may have a strange taste in their mouth. Auras have not been fully explained, but it is likely that the anatomical location of seizure origin in the brain is related to the type of seizure.
Simple partial seizures. Simple partial seizures involve a short-lived seizure with no loss of consciousness or awareness. They usually involve only one area of the brain; the area of neuronal epileptic activity will dictate the type of symptoms the person will experience. For instance, if the seizure focus is in the sensory area of the brain, the person may experience numbness or pins and needles in a body area. In addition, some people may have an aura immediately before the seizure. This is a distinct warning sign that a seizure is imminent and it can be visual, motor or sensory — for instance, the person may have a strange taste in their mouth. Auras have not been fully explained, but it is likely that the anatomical location of seizure origin in the brain is related to the type of seizure. Complex partial seizures. In contrast to simple partial seizures, with complex partial seizures consciousness and arousal are lowered, which can make the person appear confused. The most common location of the epileptic focus is either the temporal or the frontal lobe of the brain. The other defining characteristic feature is that the person experiencing the seizure may respond to vocalisations but demonstrate repetitive, odd actions, such as fidgeting, saying unusual sounds or walking around. The time frame for the seizure is 3 minutes or less.
Complex partial seizures. In contrast to simple partial seizures, with complex partial seizures consciousness and arousal are lowered, which can make the person appear confused. The most common location of the epileptic focus is either the temporal or the frontal lobe of the brain. The other defining characteristic feature is that the person experiencing the seizure may respond to vocalisations but demonstrate repetitive, odd actions, such as fidgeting, saying unusual sounds or walking around. The time frame for the seizure is 3 minutes or less.Generalised seizures can be subdivided as follows:
 Absence seizures. Absence seizures, previously referred to as petit mal seizures, are brief episodes (less than 10 seconds) where the person may stare without facial expression and be unresponsive. These seizures commence in childhood and it may appear that the child is daydreaming.
Absence seizures. Absence seizures, previously referred to as petit mal seizures, are brief episodes (less than 10 seconds) where the person may stare without facial expression and be unresponsive. These seizures commence in childhood and it may appear that the child is daydreaming. Myoclonic seizures. Myoclonic refers to the muscle jerks that are brief and usually involve one or a group of muscles. In this case the person does not lose consciousness but may fall or drop an item if they were holding it in their hands.
Myoclonic seizures. Myoclonic refers to the muscle jerks that are brief and usually involve one or a group of muscles. In this case the person does not lose consciousness but may fall or drop an item if they were holding it in their hands. Tonic-clonic seizures. Tonic-clonic seizures, previously referred to as grand mal seizures, are the type of seizure that the general population associates with epilepsy. The person experiencing the seizure loses consciousness, then their body stiffens from a generalised muscle contraction (tonic phase), which is rapidly followed by a strong rhythmical movement of the limbs that appears jerking-like (clonic phase). One distinguishing characteristic is the often loud, sudden cry that people vocalise before commencement of the tonic phase. This occurs when a volume of air is forced past the vocal cords immediately before the tonic contraction. The seizure lasts for about 2–3 minutes and is accompanied by a confused, tired phase after the convulsions have ceased. This is referred to as the post-ictal phase.
Tonic-clonic seizures. Tonic-clonic seizures, previously referred to as grand mal seizures, are the type of seizure that the general population associates with epilepsy. The person experiencing the seizure loses consciousness, then their body stiffens from a generalised muscle contraction (tonic phase), which is rapidly followed by a strong rhythmical movement of the limbs that appears jerking-like (clonic phase). One distinguishing characteristic is the often loud, sudden cry that people vocalise before commencement of the tonic phase. This occurs when a volume of air is forced past the vocal cords immediately before the tonic contraction. The seizure lasts for about 2–3 minutes and is accompanied by a confused, tired phase after the convulsions have ceased. This is referred to as the post-ictal phase. Tonic seizures. As the name implies, the person experiences an increase in muscle tone and the body will stiffen. These seizures can cause considerable injury if the person falls after the onset of the seizure.
Tonic seizures. As the name implies, the person experiences an increase in muscle tone and the body will stiffen. These seizures can cause considerable injury if the person falls after the onset of the seizure. Atonic seizures. The ‘a’ before tonic refers to without. Therefore, during these seizures the person loses muscle tone, often causing them to fall to the ground if they were standing. There is usually no loss of consciousness and recovery is rapid.
Atonic seizures. The ‘a’ before tonic refers to without. Therefore, during these seizures the person loses muscle tone, often causing them to fall to the ground if they were standing. There is usually no loss of consciousness and recovery is rapid.Epilepsy surgery stands the test of time
Theodore and Kelley, researchers at the National Institute of Neurological Disorders and Stroke (NINDS), obtained information from 48 people and families about individuals who had right or left temporal lobe surgery for seizures between 1965 and 1974. Twenty-one were free of seizures that caused loss of consciousness, and three had been free of these types of seizures for 19 years. The others died or were never completely free of seizures causing loss of consciousness. Theodore and Kelley believe this type of surgery is underutilised because individuals shy away from brain surgery as a treatment option. The decision to pursue surgical treatment must balance the potential benefit of seizure control and the effect on cognitive function.
Source: Hamberger MJ, Drake EB. Cognitive functioning following epilepsy surgery. Curr Neurol Neurosci Rep 2006; 6(4):319–326; Kelley K, Theodore WH. Prognosis 30 years after temporal lobectomy. Neurology 2005; 64:1974–76.
Any seizure that does not follow the pattern of either a partial or a generalised seizure type is referred to as an unclassified seizure, also known as an atypical seizure. Most unclassified seizures occur in early childhood and are not associated with a loss of consciousness.
EVALUATION AND TREATMENT
The health history, physical examination and laboratory tests of blood and urine can identify systemic diseases known to promote seizures. Radiographic studies (CT and MRI scans) and cerebrospinal fluid examination (lumbar puncture to sample the CSF) help identify neurological diseases associated with seizures. Therefore, most tests are to exclude cranial pathology that may explain the seizure activity. When these tests have ruled out any extraneous cause of the epilepsy, an EEG is used to assess the type of seizure and determine its focus.
Treatment involves correcting or controlling the cause and, if none is identified, administering anti-seizure medications to suppress seizure activity. Counselling also may be of value. Prevention of epilepsy is the direction that research has taken.16
Seizures in childhood may occur due to many reasons. Certain types of seizures may have a genetic component or familial predisposition, or they can result from maternal diseases or congenital structural anomalies of the CNS. From the newborn period through to childhood, asphyxia (choking — difficulty breathing), intracranial haemorrhage, CNS infections, injury, electrolyte imbalances and inborn errors of metabolism may cause seizures. Often the cause of seizures is unknown.
The incidence of epilepsy varies greatly with age and is estimated to be 0.5–1% of children, with onset occurring during infancy or childhood. It is estimated that 30% of epilepsy diagnoses occur within the first 4 years of life. One of the most common types of seizures in childhood is that of febrile seizures. They are estimated to occur in up to 8% of all children.17 They are classified as seizure accompanied by fever (> 38°C) in the absence of CNS infection or other conditions that may induce convulsions, such as electrolyte disturbances or head trauma.
It was often thought that the rate of core temperature increase was the reason for the seizure, but this has been shown to be erroneous.18 It is more likely that febrile seizures arise from a combination of genetic and environmental factors. Genetic mutations in sodium channel receptor genes have been demonstrated in children who experience febrile seizures.17
The seizures are often generalised tonic-clonic seizures lasting for approximately 2 minutes. They usually only occur once during the febrile state, but children with abnormal radiological or EEG findings may have an increased risk of epilepsy. Partial seizures are seen in about 15% of febrile seizures.18
In the majority of cases, no specific treatment is required. The core temperature should be reduced and the child should be observed for a period of time until normothermia returns. It has been recommended that rapid reduction in body temperature using paracetamol or ibuprofen does not reduce the incidence or recurrence of febrile seizures.17 Approximately 1–2% of children who experience febrile seizures will be diagnosed with epilepsy.19
Cognitive disorders
In healthcare settings, many patients experience changes in their cognitive function and often it is difficult to understand the pathophysiological alterations that have brought on these conditions. For instance, following surgery with a general anaesthetic (substances that induce unconsciousness), many patients, especially the elderly, may experience behavioural changes that are not usually associated with that particular individual. In fact, some patients may have psychotic episodes that appear to have a psychiatric origin. However, in the vast majority of cases, the problem is an alteration to the neurological system, precipitated by environmental factors such as pain or sleep deprivation leading to a confused state. It is important to consider these conditions and to differentiate between delirium, dementia and depression, as these may overlap.
In addition, there is an increasing incidence of autism in children (see later in the chapter), which is a developmental disorder that affects executive function. This means that higher-level control of actions, especially new and novel actions, may be impaired.
Acute confusional states
The term confusion is used by health professionals to describe a patient’s mental status. It means that the person does not have the ability to be clear in their thoughts and is related to cognitive dysfunction. Therefore, it can be applied to many clinical situations.
Acute confusional states result from cerebral dysfunction secondary to a range of different states (see Table 8-11) — for instance, drug intoxication, metabolic disorder, anaesthetics or nervous system disease. Withdrawal from alcohol, barbiturate or other sedative drug ingestion is a common cause. Acute confusional states may begin either suddenly or gradually, depending on the amount of exposure to the toxin. These states often occur with febrile illnesses, systemic diseases (e.g. heart failure), head injury, anaesthesia or certain focal cerebral lesions. Delirium is one of the most common acute confusional states and is often under-diagnosed.
Table 8-11 COMMON CAUSES OF THE ACUTE CONFUSIONAL STATE
| Intoxications | Alcohol; prescription, over-the-counter and illegal drugs |
| Withdrawal states | Alcohol, sedative-hypnotic drugs |
| Nutritional deficiencies | Thiamine, vitamin B12, folate, niacin |
| Metabolic disorders | Electrolyte and acid–base disturbances; hepatic, renal, pancreatic disease |
| Infections | Pneumonia, urinary tract infection, sepsis, AIDS |
| Endocrine disorders | Hypo- and hyperthyroidism, hypo- and hyperglycaemia, hypo- and hyperadrenocorticism |
| Structural brain disease | Traumatic brain injury, seizure disorders, stroke, subarachnoid or intracerebral haemorrhage, epidural or subdural haematoma, encephalitis, brain abscess |
| Postoperative states | Anaesthesia, electrolyte disturbances, fever, hypoxia, analgesics |
Source: Jacobson JL, Jacobson AM. Psychiatric secrets. 2nd edn. Hanley & Belfus; 2001.
PATHOPHYSIOLOGY
Acute confusional states arise from disruption of a widely distributed neural network involving the reticular activating system of the upper brainstem and its projections into the thalamus, basal nuclei and specific association areas of the cortex and limbic areas. In most cases, both cerebral hemispheres are affected.
Trauma to the brain or conditions that increase ICP can lower consciousness and cause deficits in cognition. Most metabolic disturbances that produce an acute confusional state interfere with neuronal metabolism or synaptic transmission. This may be due to a lowering of oxygen and glucose delivery, thereby suppressing global neuronal function. Many drugs and toxins also interfere with neurotransmission function at the synapse.
CLINICAL MANIFESTATIONS
The predominant feature of an acute confusional state is impaired or lost concentration. The person is highly distractible and unable to concentrate on incoming sensory information or on any one particular mental or motor task.
The onset of an acute confusion state is usually abrupt. The first clinical manifestations are difficulty in concentration, restlessness, irritability, insomnia and poor appetite. Later there are misperceptions, illusions and hallucinations. Obsessions, compulsive behaviour and rituals may be evident.
In other acute confusional states, the individual exhibits decreases in mental function, specifically alertness, attention span, accurate perception, interpretation of the environment and reaction to the environment. Forgetfulness is prominent and the individual is frequently sleepy.
Delirium, a major type of acute confusion state, typically develops over 2–3 days and is seen initially with slurred speech, altered level of consciousness, hallucinations and restlessness. Some persons experience seizures. Unpleasant, even terrifying, dreams may occur.
Delirium has been estimated to affect up to 20% of the hospitalised elderly population.20,21 In a fully developed delirium state, the individual is completely inattentive and perceptions are grossly altered, with extensive misperception and misinterpretation. Hallucinations may be present. The person appears distressed and often perplexed; conversation is incoherent. Tremor and high levels of restless movement are common. Violent behaviour may be present, but not always. The individual usually cannot sleep, is flushed and has dilated pupils, a rapid pulse (tachycardia), core body temperature elevation and profuse sweating (diaphoresis). Delirium typically abates suddenly or gradually in 2–3 days, although occasionally delirium states persist for weeks.
EVALUATION AND TREATMENT
The initial goals in evaluating an acute confusional state are to:
A complete history and physical examination, as well as laboratory tests (electrocardiogram and blood, urine, CSF and radiological studies) are needed. These are performed to exclude causes, such as those listed in Table 8-11. Once the cause is established, treatment is directed at controlling the primary disorder, with supportive measures used as appropriate (see Figure 8-19).
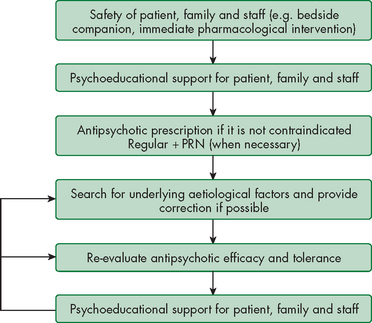
FIGURE 8-19 Delirium management flowchart.
The management of delirium is aimed at reducing the symptoms and treating the underlying causes.
Source: Walsh D et al. Palliative medicine. Philadelphia: Saunders; 2009.
The determination between delirium and dementia needs to be addressed. The primary differences are that delirium is a reversible condition, associated with acute confusion that normally has an underlying cause — when this is rectified, the delirium abates. In contrast, dementia is a progressive and irreversible condition that is associated with pathological changes in the central nervous system.
Dementia
Dementia is a progressive failure of many cerebral functions not caused by impaired level of consciousness.22 The result may be a decrease in orienting, memory, language and executive attentional networks. Because of declining intellectual ability, the individual exhibits alterations in behaviour. Dementia is a major neurodegenerative condition that slowly affects the ability of the person to function normally and is one of the most prevalent disabling conditions in older Australians.
Currently, almost 30 million people worldwide live with dementia — more than the combined populations of Australia and New Zealand. In 2008, it was estimated that approximately 240,000 people in Australia had dementia.23 However, because this is a condition associated with ageing, it is predicted that by 2050 almost one million Australians will have dementia.24 The most prevalent dementia is Alzheimer’s disease, which is discussed in detail in Chapter 9.
PATHOPHYSIOLOGY
There are more than 100 different conditions that can cause dementia.25 Mechanisms leading to dementia include degeneration, compression, atherosclerosis and trauma. Genetic predisposition is associated with the degenerative diseases, including Alzheimer’s disease and Huntington’s disease. Central nervous system infections, including the human immunodeficiency virus (HIV) and slow-growing viruses associated with Creutzfeldt-Jakob disease, are associated with dementia in addition to changes in motor function. Progressive dementias produce nerve cell degeneration, brain atrophy and hypoperfusion of particular areas of the brain, such as the parietal and temporal lobes (see Figure 8-20).
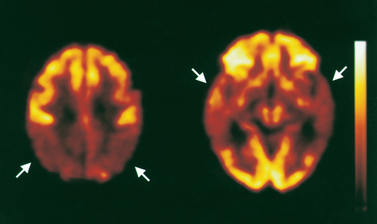
FIGURE 8-20 Metabolic deficits in Alzheimer’s disease.
The positron emission tomography (PET) scan shows parietotemporal hypoperfusion or hypoactivity indicated by the arrows. The darker regions are areas of low metabolic activity.
Source: Bradley WG. Neurology in clinical practice. 5th edn. Philadelphia: Butterworth-Heinemann; 2008. Courtesy of Dr C Meltzer.
CLINICAL MANIFESTATIONS
There are many clinical manifestations of dementia, but the onset is always gradual. One of the first signs is short-term memory loss, with mood swings and a decline in the individual’s ability to perform all their activities of daily living. As the condition progresses, the individual may experience the following three conditions:
 Agnosia: a defect of pattern recognition — a failure to recognise the form and nature of objects. Agnosia can be tactile, visual or auditory, but generally only one sense is affected.
Agnosia: a defect of pattern recognition — a failure to recognise the form and nature of objects. Agnosia can be tactile, visual or auditory, but generally only one sense is affected. Dysphasia: an impairment of comprehension or production of language. Comprehension or use of symbols, in either written or verbal language, is disturbed or lost. The condition in dementia patients leads to aphasia, which is total loss of the comprehension or production of language. Expressive dysphasia involves primarily expression deficits — that is, the individual may be able to speak, yet the sentences are not clear and may not make sense. Receptive dysphasia occurs when the individual can speak, yet does not understand what is being spoken to them.
Dysphasia: an impairment of comprehension or production of language. Comprehension or use of symbols, in either written or verbal language, is disturbed or lost. The condition in dementia patients leads to aphasia, which is total loss of the comprehension or production of language. Expressive dysphasia involves primarily expression deficits — that is, the individual may be able to speak, yet the sentences are not clear and may not make sense. Receptive dysphasia occurs when the individual can speak, yet does not understand what is being spoken to them.As the dementia worsens, the individual’s personality may change and mood swings become common. The person with dementia does not recognise their family members and their manner may be flat or aggressive. Towards the late stage of dementia, the individual loses memory capacity, cannot perform activities of daily living like feeding and requires full care. The condition progresses to coma and ultimately death, most commonly due to chest infections.
EVALUATION AND TREATMENT
Establishing the cause for dementia may be complicated, but individuals with clinical manifestations of dementia should be evaluated with laboratory and neuropsychological testing to identify underlying conditions that may be treatable. Unfortunately, no specific cure exists for most progressive dementias. Therapy is directed at maintaining and maximising the use of the remaining capacities, restoring functions if possible and accommodating for lost abilities. Helping the family to understand the process and to learn ways to assist the individual is essential.
Depression
Depression is a mood disorder that can involve affective, cognitive and physiological manifestations. In older individuals, depression is often under-diagnosed and can be misinterpreted as dementia, due to the lack of initiative, insomnia, increased confusion and general apathy. Furthermore, it has been estimated that depression or depressive symptoms are present in approximately 40% of patients with dementia. Depression is discussed in more detail in Chapter 39.
Exercising the body can benefit the mind
Exercise prompts nerve cells to multiply and strengthens their connections, and it protects them from harm. In animal studies, exercise (voluntary running) has been shown to cause neurons to regenerate (neurogenesis). This has been shown in areas of the brain that are involved in memory, such as the hippocampus. Also, in young mice, exercise-induced neurogenesis was found in areas of the brain that had been damaged with radiation, similar to that which occurs with radiotherapy for brain tumours. Therefore, the benefits of exercise may extend to the brain and nerves that are diseased or damaged in persons with Alzheimer’s disease, Parkinson’s disease and spinal cord injury. Release of neurotrophic factors (nerve growth factors) appears to account for the exercise effect, but further research is needed to fully understand the effects of exercise on the brain.
Source: Brownee C. Buff and brainy exercising the body can benefit the mind. Sci News 2006; 169(8):122–124; Dishman RK et al. Neurobiology of exercise. Obesity (Silver Spring) 2006; 14(3):345–356; van Praag H et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci 1999 96(23):13427–13431; Naylor AS et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci 2008; 105(38):14,632–14,637.
Autism spectrum disorders
The autism spectrum disorders are a group of conditions that have the common neurological deficits of communication, social interaction and stereotyped behaviour. The disorder of autism is the most common; however, the spectrum is included to demonstrate that no two children display a classic presentation. For instance, the spectrum includes children with Asperger’s syndrome and pervasive developmental disorder.
There is an increasing incidence of children with autism spectrum disorders in countries such as Australia and New Zealand.26–28 4 6 In Australia, the diagnosis rates for autism spectrum disorders have risen significantly, reflecting the changes in the rest of the world over the last 10–15 years.28 This has been attributed to a combination of changing diagnostic criteria and an increased awareness among health professionals. In Australia, it is estimated that some 11,700 children under the age of 16 years are on the autism spectrum, with up to 125,000 people having some form of autism spectrum disorder. This means that approximately 1 in 160 children has an autism spectrum disorder.28,29 More male children are diagnosed with autism than females, with an average male/female ratio of 3.8:1.27–29 4 6
PATHOPHYSIOLOGY
The exact cause of autism spectrum disorders is unknown. These complex neurodevelopmental disorders have a biological basis. There is a likely genetic component, although this has not been fully identified.30 The predominance in males compared to females is highly suggestive of a genetic link. The two main theories regarding autism spectrum disorder genes are those genes (1) involved in brain development and (2) responsible for neurotransmitter function.
Increasingly, there is evidence that particular regions of the brain are the cause of autism spectrum disorders. Neuroanatomical imaging indicates that there may be abnormalities in several areas of the brain, including the amygdala, hippocampus, septum, mamillary bodies and cerebellum.26 The brains of people with an autism spectrum disorder have been shown to be slightly larger and heavier than people without the disorder.31 In addition, some of the brain regions have neurons that seem to be developmentally immature. There appears to be widespread brain dysfunction at both the cortical and the sub-cortical levels.
It has been suggested that environmental factors play a role in the development of autism spectrum disorders. Problems during the perinatal period, such as maternal illness, are likely to be involved. Low birth weight, prematurity and events during delivery have not consistently been shown to lead to an increased incidence of autism spectrum disorders. It was suggested in the 1990s that childhood immunisations might affect the rates of autism spectrum disorders, but this has conclusively been shown to be false, despite parental concerns.26
CLINICAL MANIFESTATIONS
Children with an autistic spectrum disorder have a triad of impairments (see Figure 8-21). This includes, but is not limited to:
The social, behavioural and cognitive deficits are often present in early childhood (under the age of 3 years); however, they may be subtle and parents may not recognise these manifestations. The first contact with health professionals is often due to speech delays, which are more noticeable.
EVALUATION AND TREATMENT
Evaluation is difficult because of the need for a comprehensive, multidisciplinary assessment, which is often not available to parents. In many cases, the child may be demonstrating signs of autism, yet parents do not perceive differences, often meaning that diagnosis is delayed. Despite the likelihood that the disorders are biologically based, diagnosis is derived from developmental testing and behavioural observation. There are no specific treatments, but early intervention with behavioural modification programs have been shown to significantly improve cognitive, behavioural and intellectual development in children with autism spectrum disorders.
ALTERATIONS IN MOTOR FUNCTION
Movements are complex patterns of activity controlled by the central nervous system. General motor dysfunctions may produce changes in muscle tone, movement and complex motor performance.
Alterations in muscle tone
Normal muscle tone involves a slight resistance to passive movement. Throughout the range of motion, the resistance is smooth, constant and even. The abnormalities of muscle tone can be divided into either decreased tone (hypotonia) or increased tone (hypertonia) (see Table 8-12).
Table 8-12 ALTERATIONS IN MUSCLE TONE
| ALTERATIONS | CHARACTERISTICS | CAUSE |
|---|---|---|
| Hypotonia | ||
| Thought to be caused by decreased muscle spindle activity as a result of decreased excitability of neurons | ||
| Flaccidity | Associated with limp, atrophied muscles and paralysis | Occurs typically when nerve impulses necessary for muscle tone are lost |
| Hypertonia | ||
| Results when the lower motor unit reflex arc continues to function but is not mediated or regulated by higher centres | ||
| Spasticity | A gradual increase in tone causing increased resistance until tone suddenly reduces, which results in clasp-knife phenomenon | Exact mechanism unclear; appears to arise from an increased excitability of the alpha motor neurons |
| Rigidity | Muscle resistance to passive movement of a rigid limb that is uniform in both flexion and extension throughout the motion | Occurs as a result of constant, involuntary contraction of muscle |
Hypotonia
In hypotonia (decreased muscle tone), passive movement of a muscle occurs with little or no resistance. Hypotonia or flaccidity (a state in which the muscle may be moved rapidly without resistance) occurs when the nerve impulses needed for muscle tone are lost, such as in spinal cord injury or stroke.
Individuals with hypotonia tire easily or are weak. They may have difficulty rising from a sitting position, sitting down without using arm support, and walking up and down stairs, as well as an inability to stand on their toes. Because of their weakness, accidents during walking and self-care activities are common. The joints become hyperflexible, so persons with hypotonia may be able to assume positions that require extreme joint mobility.
The muscle mass atrophies because of decreased neural input entering the motor unit and muscles appear flabby and flat. Muscle cells are gradually replaced by connective tissue and fat.
Hypertonia
In hypertonia (increased muscle tone), passive movement of a muscle occurs with resistance.
Spasticity results from hyperexcitability of the stretch reflexes and is associated with damage to the motor, premotor and supplementary motor areas, as well as lateral corticospinal tract damage (review using Figure 6-19). Increased deep tendon reflexes (hyperreflexia) and the spread of reflexes accompany it.
Rigidity produced by tonic reflex activity mediated by motor neurons may be continuous or intermittent. The involved muscles are firm and tense; the increase in muscle movement is even and uniform throughout the range of passive movement.
The muscles may atrophy because of decreased use. However, hypertrophy occasionally occurs as a result of the overstimulation of muscle fibres. Overstimulation occurs when the motor unit reflex arc remains intact and functioning but is not inhibited by higher centres. This causes continual muscle contraction, resulting in enlargement of the muscle mass and firm muscles.
Alterations in movement
Movement requires a change in the contractile state of muscles. Abnormal movements occur when central nervous system dysfunctions alter muscle innervation. Researchers have found that dopamine, a neurotransmitter in the brain, apparently functions in several movement disorders. Some result from too little dopamine activity, whereas others result from too much. Still others are not primarily related to dopamine function.
Paresis (weakness) is partial paralysis with incomplete loss of muscle power. Paralysis is loss of motor function so that a muscle group is unable to overcome gravity. The way to describe paresis and paralysis is by using a prefix that describes the limbs involved. Therefore, hemiparesis and hemiplegia refer to paresis and paralysis of the upper and lower extremities on one side. Paraplegia is paralysis of the lower extremities — individuals with spinal cord injuries in the lower spinal cord have paraplegia; and quadriplegia refers to paralysis of all four limbs — it occurs in individuals who have spinal cord injuries at the cervical level.
Alterations in cerebral homeostasis
 Cerebral blood flow is the total blood flow to the brain and is approximately 15–20% of total cardiac output.
Cerebral blood flow is the total blood flow to the brain and is approximately 15–20% of total cardiac output. Changes in oxygen and carbon dioxide blood concentrations are the most critical factors in altering blood vessel diameter.
Changes in oxygen and carbon dioxide blood concentrations are the most critical factors in altering blood vessel diameter. There are three main volumes that occupy the cranial vault: blood, cerebrospinal fluid and brain tissue.
There are three main volumes that occupy the cranial vault: blood, cerebrospinal fluid and brain tissue. The Monro-Kellie hypothesis refers to the relationship between the blood, cerebrospinal fluid and brain tissue. Changes in one volume need to be offset by changes in the other two volumes to maintain a stable pressure.
The Monro-Kellie hypothesis refers to the relationship between the blood, cerebrospinal fluid and brain tissue. Changes in one volume need to be offset by changes in the other two volumes to maintain a stable pressure. Increased intracranial pressure may result from oedema, excess cerebrospinal fluid, haemorrhage or tumour growth. When intracranial pressure approaches arterial pressure, hypoxia and hypercapnia produce brain damage.
Increased intracranial pressure may result from oedema, excess cerebrospinal fluid, haemorrhage or tumour growth. When intracranial pressure approaches arterial pressure, hypoxia and hypercapnia produce brain damage. Cerebral oedema is an increase in the fluid content of the brain resulting from infection, haemorrhage, tumour, ischaemia, infarct or hypoxia.
Cerebral oedema is an increase in the fluid content of the brain resulting from infection, haemorrhage, tumour, ischaemia, infarct or hypoxia. The shifting or herniation of brain tissue from one compartment to another disrupts the blood flow of both compartments and damages brain tissue.
The shifting or herniation of brain tissue from one compartment to another disrupts the blood flow of both compartments and damages brain tissue. There are four types of cerebral oedema: vasogenic oedema, cytotoxic oedema, ischaemic oedema and interstitial oedema.
There are four types of cerebral oedema: vasogenic oedema, cytotoxic oedema, ischaemic oedema and interstitial oedema.Alterations in cognitive function
 Full consciousness is an awareness of oneself and the environment, with an ability to respond to external stimuli with a wide variety of responses.
Full consciousness is an awareness of oneself and the environment, with an ability to respond to external stimuli with a wide variety of responses. A decreased level of arousal occurs by diffuse bilateral cortical dysfunction, bilateral sub-cortical reticular formation, brainstem dysfunction and localised hemispheric dysfunction.
A decreased level of arousal occurs by diffuse bilateral cortical dysfunction, bilateral sub-cortical reticular formation, brainstem dysfunction and localised hemispheric dysfunction. Infratentorial alterations produce changes in arousal by direct destruction of the reticular activating system and its pathways.
Infratentorial alterations produce changes in arousal by direct destruction of the reticular activating system and its pathways. Pupillary changes reflect changes in the level of brainstem function, drug action and response to hypoxia and ischaemia.
Pupillary changes reflect changes in the level of brainstem function, drug action and response to hypoxia and ischaemia. Loss of cortical inhibition associated with decreased consciousness produces abnormal flexor and extensor movements.
Loss of cortical inhibition associated with decreased consciousness produces abnormal flexor and extensor movements. Post-coma unresponsiveness refers to an altered level of consciousness with a complete lack of responses that suggest a cognitive component, preservation of the sleep–wake cycles and cardiorespiratory function and partial or complete preservation of hypothalamic and brainstem autonomic functions.
Post-coma unresponsiveness refers to an altered level of consciousness with a complete lack of responses that suggest a cognitive component, preservation of the sleep–wake cycles and cardiorespiratory function and partial or complete preservation of hypothalamic and brainstem autonomic functions. Brain death occurs when the brain is damaged so completely that it can never recover and cannot maintain the body’s internal homeostasis.
Brain death occurs when the brain is damaged so completely that it can never recover and cannot maintain the body’s internal homeostasis. Seizures represent a sudden, chaotic discharge of cerebral neurons, with transient alterations in brain function. Seizures may be generalised or focal and can result from cerebral lesions, biochemical disorders, trauma or epilepsy.
Seizures represent a sudden, chaotic discharge of cerebral neurons, with transient alterations in brain function. Seizures may be generalised or focal and can result from cerebral lesions, biochemical disorders, trauma or epilepsy. Epilepsy is now thought to be the result of complex genetic mutations with environmental effects that cause abnormalities in brain wiring or an imbalance in chemicals that the brain uses to send signals or abnormal nerve connections made while attempting to repair itself after injury.
Epilepsy is now thought to be the result of complex genetic mutations with environmental effects that cause abnormalities in brain wiring or an imbalance in chemicals that the brain uses to send signals or abnormal nerve connections made while attempting to repair itself after injury. There are more than 40 different types of seizures, with the most common classification split into partial and generalised seizures.
There are more than 40 different types of seizures, with the most common classification split into partial and generalised seizures. Febrile seizures occur in about 8% of all children and are thought to be a combination of genetic and environmental factors. Genetic mutations in sodium channel receptor genes have been demonstrated in children who experience febrile seizures.
Febrile seizures occur in about 8% of all children and are thought to be a combination of genetic and environmental factors. Genetic mutations in sodium channel receptor genes have been demonstrated in children who experience febrile seizures. Acute confusional states are characterised chiefly by a loss of detection and, in the case of delirium, an intense autonomic nervous system hyperactivity.
Acute confusional states are characterised chiefly by a loss of detection and, in the case of delirium, an intense autonomic nervous system hyperactivity. Acute confusional states result from cerebral dysfunction secondary to a range of different states — for example, drug intoxication, metabolic disorder, anaesthetics or nervous system disease.
Acute confusional states result from cerebral dysfunction secondary to a range of different states — for example, drug intoxication, metabolic disorder, anaesthetics or nervous system disease. They arise from disruption of a widely distributed neural network involving the reticular activating system of the upper brainstem and its projections into the thalamus, basal nuclei and specific association areas of the cortex and limbic areas. In most cases, both cerebral hemispheres are affected.
They arise from disruption of a widely distributed neural network involving the reticular activating system of the upper brainstem and its projections into the thalamus, basal nuclei and specific association areas of the cortex and limbic areas. In most cases, both cerebral hemispheres are affected. Dementia is a progressive failure of many cerebral functions not caused by impaired level of consciousness. It is a major neurodegenerative condition that slowly affects the ability of the person to function normally.
Dementia is a progressive failure of many cerebral functions not caused by impaired level of consciousness. It is a major neurodegenerative condition that slowly affects the ability of the person to function normally. Dementia may result in a decrease in orienting, memory, language and executive attentional networks.
Dementia may result in a decrease in orienting, memory, language and executive attentional networks. Expressive dysphasia involves primarily expression deficits — that is, the individual may be able to speak, yet the sentences are not clear and may not make sense. Receptive dysphasia occurs when the individual can speak, yet does not understand what is being spoken to them.
Expressive dysphasia involves primarily expression deficits — that is, the individual may be able to speak, yet the sentences are not clear and may not make sense. Receptive dysphasia occurs when the individual can speak, yet does not understand what is being spoken to them.Rade was a 24-year-old male involved in a serious motor vehicle accident. He was the driver and sole occupant of the car and was speeding when he crashed into a roadside pole. He was not wearing a seatbelt. At the scene, the ambulance crew found that he had an 8 cm laceration of the forehead and was unconscious, with a Glasgow coma score of 3 (no eye movement, no vocalisation and motor movement). His pupils were non-reactive to light but were fixed and dilated at 6 mm. The crew inserted an artificial airway, which enabled mechanical ventilation. Then they urgently transported Rade to the nearest acute care hospital, where, after a thorough clinical investigation, it was determined that he was brain dead.