6 THE STRUCTURE AND FUNCTION OF THE NEUROLOGICAL SYSTEM
INTRODUCTION
The human nervous system is a remarkable structure responsible for the body’s ability to interact with the environment and for controlling activities of the internal organs. The nervous system regulates, coordinates and literally drives the other systems of the body. It oversees the functions of the body, while constantly receiving input to assess performance across every body structure and function. There is a constant flow of information towards the nervous system that is processed in the brain and then, if required, is acted on to bring about a change in function. The nervous system achieves this remarkable feat through a network of complex structures that transmit signals — both electrically and chemically — between the body’s many organs and tissues and the brain. In Chapter 2 we considered that homeostasis can often be maintained locally if only small changes occur; however, larger changes are detected by the nervous system, which regulates and maintains the body in homeostatic balance.
ORGANISATION OF THE NERVOUS SYSTEM
Although the nervous system functions as a unified whole, it can be divided according to structure and function. Structurally, the nervous system is divided into the central nervous system and the peripheral nervous system (see Figure 6-1). The central nervous system (commonly abbreviated to CNS) consists of the brain and spinal cord, enclosed within the protective cranium (cranial vault) and vertebrae, respectively. The peripheral nervous system (abbreviated to PNS) is composed of all the cranial and spinal nerves. The central nervous system receives afferent information from sensory neurons, and sends information in an efferent or outward direction using motor neurons (see Figure 6-2). So the peripheral nerve pathways are differentiated into afferent pathways (ascending pathways), which carry sensory impulses inwards or towards the central nervous system, and efferent pathways (descending pathways), which innervate skeletal muscle or effector organs, and transmit motor impulses outwards or away from the central nervous system.
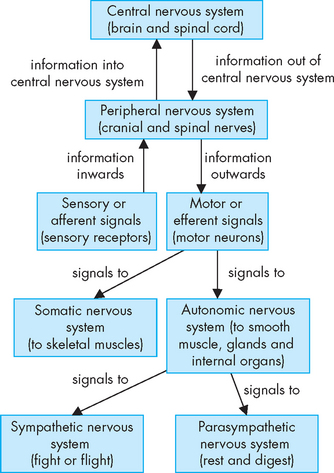
FIGURE 6-1 Functional divisions of the nervous system.
The central nervous system is composed of the brain and spinal cord. All other nerves belong to the peripheral nervous system. The divisions of the peripheral nervous system are divided into sensory and motor, with further divisions in the motor neurons.
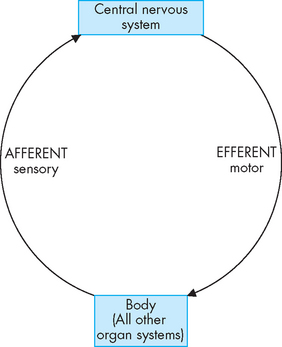
FIGURE 6-2 Peripheral nerve pathways.
The central nervous system receives afferent or inward information from sensory neurons in all body systems, and sends information in an efferent or outward direction back to the systems using motor neurons. These motor neurons regulate all of the body’s functions.
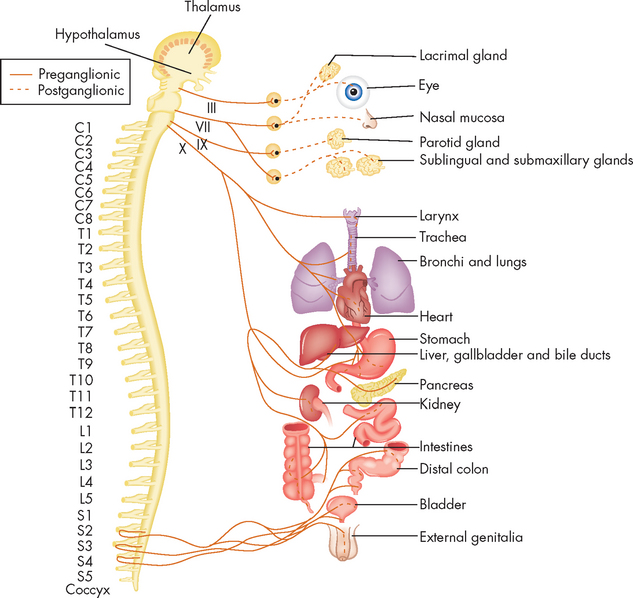
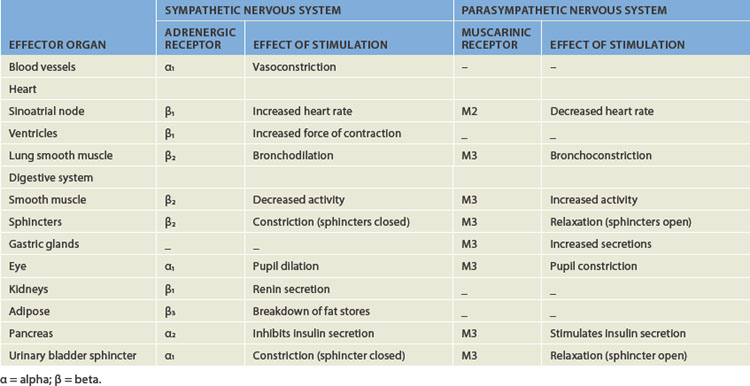
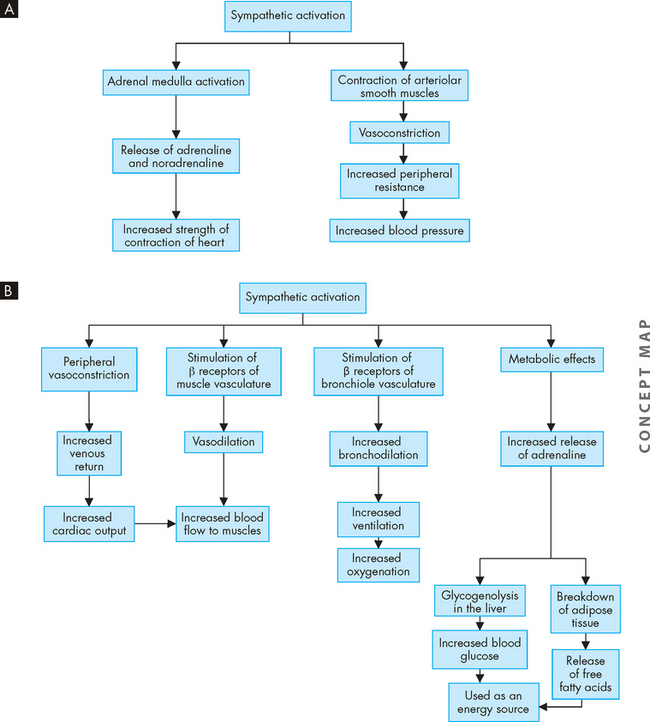
Functionally, the peripheral nervous system can be divided into the somatic nervous system and the autonomic nervous system. The somatic nervous system (somatic meaning body) consists of pathways that regulate voluntary motor control (namely, skeletal muscle). The autonomic nervous system (autonomic meaning automatic or involuntary) is involved with regulation of the body’s internal environment through involuntary control of organ systems. For instance, heart rate is controlled by the autonomic nervous system as we cannot consciously control our heart rate. In contrast, breathing is controlled by both the autonomic and somatic nervous systems. We can consciously change our breathing, such as taking deep breaths when trying to relax, but at most other times the regulation of breathing is entirely under the influence of the autonomic nervous system. The autonomic nervous system is further divided into sympathetic and parasympathetic divisions. Organs innervated by specific components of the nervous system are called effector organs.
CELLS OF THE NERVOUS SYSTEM
Two basic types of cells constitute nervous tissue: neurons and neuroglia (supporting cells). The neuron is the primary cell of the nervous system, whereas neuroglial cells such as astrocytes (in the central nervous system) and Schwann cells (in the peripheral nervous system) provide structural support and nutrition for the neurons.1
Neurons
Neurons, also referred to as nerve cells, are the functional units of the nervous system. They are complex structures that may be anatomically very different, yet have a common function — to transmit nerve impulses. They can send nerve impulses independently or work in units. Neurons detect environmental and internal body changes and initiate body responses to maintain homeostasis.
The structure of neurons
A neuron has three components: a cell body (soma) and the thin processes of the cell; the dendrites; and the axon (see Figure 6-3). The cell body contains the nucleus, cytoplasm and other normal organelles of a cell. There are also neurofibrils, which are collections of neurofilaments within the cell that extend away from the cell body to assist with carrying substances produced in the cell body along the axon. The dendrites are branching extensions that receive signals and carry them towards the cell body. Axons are long projections from the cell body that carry nerve impulses away from the cell. When the signal reaches the dendrite, it travels to the cell body and is then transmitted through the axon so that it can reach another neuron or another cell. In this way nerve impulses can be sent to various parts of the body.
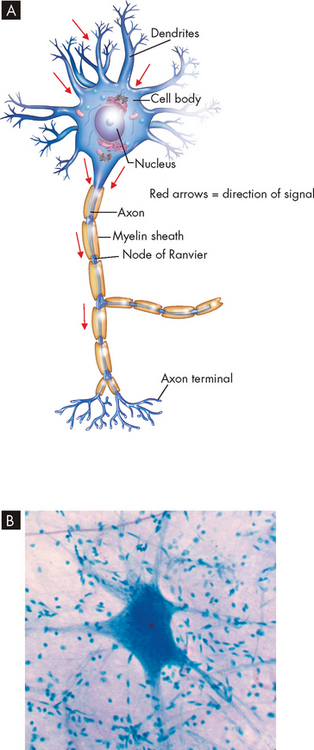
FIGURE 6-3 The general structure of a neuron.
A A neuron consists of dendrites (receive signals), a cell body (or soma) and an axon (send signals). B A photomicrograph of a neuron.
Source: B Thibodeau GA. The human body in health & disease. 5th edn. St Louis: Mosby; 2010.
Neurons can be classified according to the number of dendrites that branch from the cell body. Some neurons have only one dendrite, typical of sensory neurons in the cranial and spinal nerves. Others have two dendrites, but by far the most common are the multipolar neurons, which have multiple dendrites and one axon, typical of motor neurons (nerves that innervate a muscle).
A typical neuron has only one axon, which may be covered with a segmented layer of lipid material called myelin, an insulating substance. This entire membrane is referred to as the myelin sheath (see Figure 6-3). The myelin sheaths are interrupted at regular intervals by the nodes of Ranvier. Axons branch extensively at the nodes of Ranvier. Myelin is an extremely important component of the nervous system; without it, nerve impulses would not be as fast, and the ability of neurons to send signals to one discrete area might be impeded. We examine the function of myelin later in the chapter when discussing the nerve impulse.
The function of neurons
Functionally, there are three types of neurons: (1) sensory neurons, which send afferent information towards the central nervous system; (2) interneurons, which are between sensory and motor; and (3) motor neurons, which transmit efferent signals outwards from the central nervous system.
 Sensory neurons carry impulses from peripheral sensory receptors to the central nervous system. There are a range of different sensory neurons, each being capable of sensing a different type of stimulus (see Table 6-1). For example, thermoreceptors located throughout the skin detect temperature, while a chemoreceptor in the mouth (taste bud) can detect a particular chemical such as acid or sour, and other chemoreceptors located in the walls of blood vessels can sense the amount of oxygen in the blood. Some body regions have more sensory neurons than others; for example, the fingertips and lips have more sensory neurons than the legs and feet. Nerve impulses are continually being sent to the central nervous system with information about the external environment and, equally importantly, the condition of the internal environment. The central nervous system controls bodily functions in response to this constant supply of sensory information.
Sensory neurons carry impulses from peripheral sensory receptors to the central nervous system. There are a range of different sensory neurons, each being capable of sensing a different type of stimulus (see Table 6-1). For example, thermoreceptors located throughout the skin detect temperature, while a chemoreceptor in the mouth (taste bud) can detect a particular chemical such as acid or sour, and other chemoreceptors located in the walls of blood vessels can sense the amount of oxygen in the blood. Some body regions have more sensory neurons than others; for example, the fingertips and lips have more sensory neurons than the legs and feet. Nerve impulses are continually being sent to the central nervous system with information about the external environment and, equally importantly, the condition of the internal environment. The central nervous system controls bodily functions in response to this constant supply of sensory information. Interneurons transmit impulses from neuron to neuron — that is, they assist in the transmission between sensory and motor neurons. They are located solely within the central nervous system and provide the billions of connections between neurons in the brain.
Interneurons transmit impulses from neuron to neuron — that is, they assist in the transmission between sensory and motor neurons. They are located solely within the central nervous system and provide the billions of connections between neurons in the brain. Motor neurons transmit impulses away from the central nervous system to an effector (i.e. skeletal muscle or organ). In skeletal muscle, the innervation of the neuron to the muscle forms the neuromuscular junction. The function of the neuromuscular junction is discussed in Chapter 20.
Motor neurons transmit impulses away from the central nervous system to an effector (i.e. skeletal muscle or organ). In skeletal muscle, the innervation of the neuron to the muscle forms the neuromuscular junction. The function of the neuromuscular junction is discussed in Chapter 20.Table 6-1 TYPES OF SENSORY NEURONS
| TYPE OF SENSORY RECEPTOR | LOCATION | DETECTS |
|---|---|---|
| Thermoreceptor | Skin | Temperature (e.g. cool, hot) |
| Nociceptor | Skin, viscera, muscle | Pain (damage) |
| Mechanoreceptor | Skin, viscera, muscle, ear | Touch, pressure, stretch, vibration (hearing) |
| Chemoreceptor | Blood vessels, brain, mouth, nose | Chemicals (e.g. acid, sour), taste, smell |
| Proprioceptor | Muscle | Body positioning |
| Photoreceptors | Eye | Light (vision) |
Neuroglia
Neuroglia (from the Greek glia meaning glue — so the neuroglia are like a ‘nerve glue’) are the cells that support the neurons of the central nervous system. They comprise approximately half of the total brain and spinal cord volume, and are ten times more numerous than neurons. There are different types of neuroglia that are responsible for several different functions. They assist the neurons, which need to be maintained in optimal working condition, otherwise body functions may be affected. Within the central nervous system the neuroglia consist of four types:
 Astrocytes have a star-like appearance (hence the name astro) and fill the spaces between the neurons, as well as filling the spaces left by dead neurons. They also surround blood vessels to provide structural support and assist in supplying nutrients to the neurons.
Astrocytes have a star-like appearance (hence the name astro) and fill the spaces between the neurons, as well as filling the spaces left by dead neurons. They also surround blood vessels to provide structural support and assist in supplying nutrients to the neurons. Oligondendrocytes deposit myelin around the neuronal axons to electrically isolate the neurons within the central nervous system.
Oligondendrocytes deposit myelin around the neuronal axons to electrically isolate the neurons within the central nervous system. Microglia assist the immune system to protect the brain by using phagocytosis to engulf debris (see Chapter 12 for details of phagocytosis).
Microglia assist the immune system to protect the brain by using phagocytosis to engulf debris (see Chapter 12 for details of phagocytosis). Ependymal cells line the cerebrospinal fluid-filled cavities and are involved in the production of cerebrospinal fluid, which circulates around the brain and spinal cord (see the later discussion on protective structures of the central nervous system).
Ependymal cells line the cerebrospinal fluid-filled cavities and are involved in the production of cerebrospinal fluid, which circulates around the brain and spinal cord (see the later discussion on protective structures of the central nervous system).In the peripheral nervous system, the Schwann cells undertake myelination of axons. The appearance and characteristics of the neuroglia are shown in Figure 6-4 and Table 6-2.
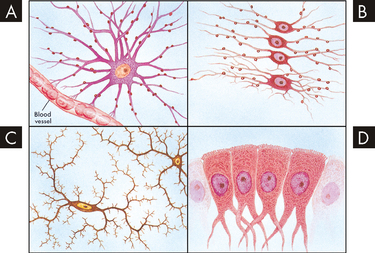
FIGURE 6-4 Types of neuroglial cells.
A Astrocyte. B Oligondendrocytes. C Microglia. D Ependymal cells.
Source: Based on Chipps E, Clanin N, Campbell V. Neurologic disorders. St Louis: Mosby; 1992.
Table 6-2 NEUROGLIAL CELLS OF THE NERVOUS SYSTEM
| CELL TYPE | PRIMARY FUNCTION |
|---|---|
| Central nervous system | |
| Astrocytes | |
| Ependymal cells | |
| Microglia | |
| Oligodendrocytes | |
| Peripheral nervous system | |
| Schwann cells | |
Source: Some data from Martinez Banaclocha MA. Magnetic storage of information in the human cerebral cortex: a hypothesis for memory. Int J Neurosci 2005; 115(3):329–337.
NERVE INJURY AND REGENERATION
Mature neurons do not divide to form new cells like most other body cells. As a result, injury to a neuron can cause permanent loss of function. This is very important for clinical practice as patients who have neuronal damage, such as occurs with a stroke, will lose function due to death or damage of the neurons that supply that part of the body.
If neurons in the central nervous system are damaged, there will be no regeneration. The regeneration of axonal constituents in the central nervous system is limited by an increased incidence of scar formation and the different nature of myelin formed by the oligodendrocytes. However, neurons in the peripheral nervous system do have the ability to regenerate, although this depends on a number of factors (see Figure 6-5). When the axon is severed, degeneration occurs in the distal part of the axon: (1) a characteristic swelling appears within the portion of the axon distal to the cut; (2) the neurofilaments hypertrophy (cell size becomes larger); (3) the myelin sheath shrinks and disintegrates; and (4) the axon degenerates and disappears. The myelin sheaths reform into Schwann cells that line up in a column between the cut and the effector organ.
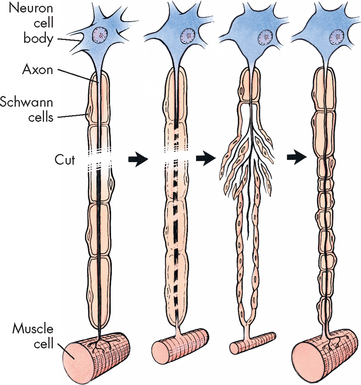
FIGURE 6-5 Repair of a peripheral nerve fibre.
When cut, a damaged motor axon can regrow to its distal connection only if the Schwann cells remain intact (to form a guiding tunnel) and if scar tissue does not block its way.
At the proximal end of the injured axon (towards the cell body), similar changes occur but only back to the next node of Ranvier. During the repair process, the cell increases its metabolic activity, protein synthesis and mitochondrial activity. Approximately 7–14 days after the injury, new terminal sprouts project from the proximal segment and may enter the remaining Schwann cell pathway. Figure 6-5 contains a more detailed representation of these events. This process is limited to myelinated fibres.
Nerve regeneration depends on many factors, such as the location of the injury, the type of injury, the inflammatory responses and the process of scarring. The closer the injury to the cell body of the nerve, the greater the chance that the nerve cell will die and not regenerate. In the peripheral nervous system, a crushing injury allows recovery more fully than does a cut injury. Crushed nerves sometimes recover fully, whereas cut nerves form connective tissue scars that block or slow regenerating axonal branches.
THE NERVE IMPULSE
Neurons generate and conduct electrical and chemical impulses as their means of communication. This is achieved through the movement of electrolytes, predominately sodium and potassium, across the cell membrane. The change in the concentration of these electrolytes causes a change in electrical charge, and this permits the propagation of an electrical impulse. An unexcited neuron, one that has not been stimulated, maintains an electrically neutral state between inside and outside the cell, which is referred to as the resting membrane potential. When the neuron is stimulated, the membrane potential is altered and a nerve impulse, or more correctly an action potential, is generated; this then flows to all parts of the neuron. The action potential response occurs only when the stimulus is strong enough; if it is too weak, the membrane remains unexcited. This property is termed the all-or-none response, as all action potentials of the neuron are of the same size.
In this section, we also consider how a neuron is able to communicate with another cell using synaptic transmission, whereby the signal travels from the neuron across a small space known as a synapse, resulting in a response in the next cell.
As you read through the details of these processes, you may be wondering why this information is so important for someone working with patients in the healthcare system. Having a good understanding of how neurons communicate using the action potential and synaptic transmission will assist you in clinical practice, as it will allow you to:
 appreciate why drugs might have side effects that interact with nerve transmission — a mistake here may be fatal for the patient, so you should be able to think critically about the potential effects of drugs, based on your knowledge of normal neuron function.
appreciate why drugs might have side effects that interact with nerve transmission — a mistake here may be fatal for the patient, so you should be able to think critically about the potential effects of drugs, based on your knowledge of normal neuron function.Membrane potentials
When a neuron is at rest (unexcited or not signalling), there are differences between the intracellular and extracellular concentrations of ions (or electrolytes), such that there is a concentration gradient across the cell membrane. Important ions involved in neuronal function are sodium (Na+), potassium (K+) and calcium (Ca2+). The positive charge associated with each of these ions is relevant to how the neurons work, because the movement of the ion means that there is a change in the amount of positive charge in the area. Knowing how these ions contribute to the function of the neuron will give you valuable insight as to why these ions are monitored closely in patients. If the concentration of sodium, potassium or calcium is altered in the body, it can be detrimental for neurons and become life-threatening for the patient.
Because there are different concentration gradients across the cell membrane for ions, there exists a difference in electrical potential across the cell membrane. This is referred to as the membrane potential and it can be measured in millivolts (mV).
Resting membrane potential
In a neuron at rest, sodium ions are more concentrated in the extracellular fluid, and potassium ions are in greater concentration in the intracellular fluid (see Figure 6-6). When the neuron is resting, a few of the potassium channels in the cell membrane are actually open, which allows small amounts of potassium to diffuse down its concentration gradient and ‘leak out’ or exit the cell. As a result of the positively charged potassium exiting the cell, the inside of the cell becomes negative. Consequently, the recording of the electrical potential is actually a negative number, due to there being a loss of positive charge from inside the cell. There is a range of electrical potentials in neurons, but the average value at rest is approximately −70 millivolts (see Figure 6-7A, stage 1).
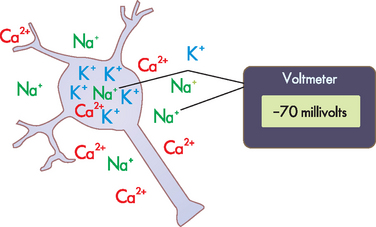
FIGURE 6-6 Normal concentration gradient of sodium, potassium and calcium ions between the intracellular fluid of a neuron at rest and the surrounding extracellular fluid.
These differences in ion concentrations between inside and outside of the cell produce changes in membrane voltage, which can be measured using a voltmeter.
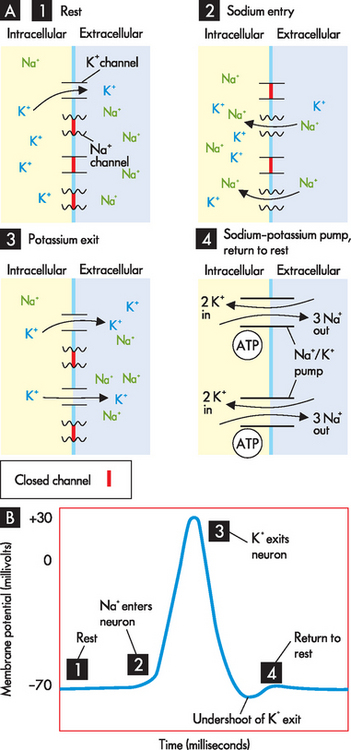
FIGURE 6-7 The action potential.
A Opening and closing of sodium and potassium ion channels occurs throughout the action potential. B Changes in membrane potential with the action potential. Stages: 1 Rest — resting membrane potential; a few potassium channels open. 2 Depolarisation — sodium channels open; sodium enters the cell. 3 Repolarisation —potassium channels open; potassium exits the cell (sodium channels are shut). 4 Return to resting membrane position.
Action potential
Neurons are excitable and their resting membrane potential changes in response to stimuli. For example, a thermoreceptor in your hand that detects cold will be stimulated when you grasp a cold drink from the fridge. This stimulus changes into an electrical signal that is interpreted by the nervous system through the generation of an action potential. The stimulus causes sodium channels in the neuron cell membrane to open. As there is a higher concentration of sodium in the extracellular fluid compared with the intracellular fluid, sodium diffuses down its concentration gradient and enters the cell. These positively charged sodium ions make the inside of the cell become more positive, so that the membrane potential moves from −70 millivolts towards zero. This is referred to as depolarisation. The membrane potential actually continues upwards to about +30 millivolts (see Figure 6-7A, stage 2).
When the membrane potential reaches +30 millivolts, this causes closing of the sodium channels, so that further sodium entry is prohibited. At the same time, the voltage change causes the potassium channels to open and thus potassium moves down its concentration gradient and exits the cell. The positively charged potassium ions exiting the cell cause the inside of the cell to become less positive, so that the membrane potential returns down to where it started (–70 millivolts). This is known as repolarisation (see Figure 6-7A, stage 3).
When the action potential is completed, the sodium–potassium pump located in the cell membrane returns the membrane to the resting potential by pumping potassium back into the cell and sodium out of the cell, in order to restore the normal concentration gradients (see Figure 6-7A, stage 4). Three molecules of sodium are pumped out for every two potassium ions returned to the cell in one cycle of the pump. The sodium–potassium pump requires energy in the form of ATP as molecules are being transported against their concentration gradients (see Chapter 3 for details). Note that there is a brief undershoot of the graph in Figure 6-7B, which occurs because the potassium channels close slowly, so that slightly more potassium is allowed to exit than is necessary. This is termed hyperpolarisation and it is corrected by the action of the sodium–potassium pump.
During the action potential, there are two different refractory periods during which the neuron cannot respond to a stimulus in the same way that it could when it was resting:
 The absolute refractory period occurs during sodium entry (depolarisation), and when a neuron is in this stage it is completely unable to respond to another stimulus. Remember that the neuron responded to the initial stimulus by opening the sodium channels, which led to changes in the membrane potential; the neuron is incapable of responding again during the absolute refractory period as the sodium channels are already open.
The absolute refractory period occurs during sodium entry (depolarisation), and when a neuron is in this stage it is completely unable to respond to another stimulus. Remember that the neuron responded to the initial stimulus by opening the sodium channels, which led to changes in the membrane potential; the neuron is incapable of responding again during the absolute refractory period as the sodium channels are already open. The relative refractory period occurs after the sodium channels are shut (commencement of repolarisation) and during the later parts of the action potential. During this time, it is possible, although more difficult, to restimulate the neuron.
The relative refractory period occurs after the sodium channels are shut (commencement of repolarisation) and during the later parts of the action potential. During this time, it is possible, although more difficult, to restimulate the neuron.The refractory periods are important because they allow the neuron to be ready for the next action potential. Remember that there are changes in electrical charge and the refractory period allows the neuron to be electrically neutral again; in this way, the nervous system has the ability to recognise differences in the strength of different stimuli.
Synapses
Neurons are not physically contiguous with one another. There is a very small region between adjacent neurons called a synapse (see Figure 6-8). These synapses are crucial to the nervous system communicating not only within itself, but also with effector organs. They enable information to be spread to many different areas of the brain, which aids in the rapid regulation of bodily processes. In addition, they also allow electrical signals to be converted to chemical signals. Impulses are transmitted across a synapse by chemical messengers. The neurons that conduct a nerve impulse are named according to whether they relay the impulse towards the synapse by the presynaptic neurons or away from the synapse by the postsynaptic neurons. The synapse is usually formed by the axon of the presynaptic neuron and the dendrite of the postsynaptic neuron so that the signal can travel in only one direction across the synapse. The synaptic cleft is the region between the neurons that contains extracellular fluid.
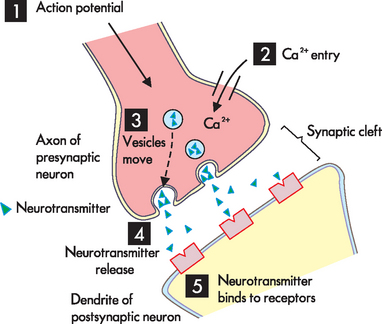
1 The action potential arrives at the axon terminal. 2 Rather than sodium entry, calcium channels open and calcium enters the axon. 3 The increase in calcium causes the synaptic vesicles to move to the presynpatic membrane. 4 The neurotransmitter is released using exocytosis. 5 The neurotransmitter diffuses across the synapse and binds with receptors on the postsynaptic neuron.
Signals are transmitted across the synapse by chemicals stored inside small vesicles in the axon terminal. These chemicals are known as neurotransmitters, and they allow the transmission of an action potential across the synapse to the postsynaptic neuron. When an action potential originates in a presynaptic neuron it travels down the axon to the synapse. As the impulse reaches the axon terminal, rather than sodium entering the cell, the voltage changes here cause calcium channels to open (see Figure 6-8). Calcium moves from the higher concentration outside the cell to inside the cell by entering the axon terminal. The entry of calcium into the axon terminal causes the vesicles to move to the cell membrane and release their neurotransmitter by exocytosis (refer to Chapter 3). Once released from the vesicles, the neurotransmitter from the presynaptic neuron diffuses across the synaptic cleft and binds to receptor sites on the cell membrane of the postsynaptic neuron.2 These steps of neuron communication are summarised in Table 6-3.
Table 6-3 SIGNALLING FUNCTIONS OF NEURONS
| STAGE | WHAT THE NEURON IS DOING | WHAT PROCESSES ARE INVOLVED |
|---|---|---|
| Rest | ||
| Resting membrane potential | At rest, not signalling | A few potassium ion channels are open |
| Signalling | ||
| Action potential | Receiving a signal at the dendrite and then sending it out through the axon | Opening and closing of the sodium and potassium ion channels |
| Synaptic transmission | Sending a signal from the axon across the synapse to another cell | Opening of the calcium ion channels; neurotransmitter released by exocytosis |
Neurotransmitters
When a neurotransmitter interacts with the receptor, this is referred to as binding. The binding of a neurotransmitter at the receptor site is specific — in other words, a neurotransmitter can bind only to the specific receptor for that neurotransmitter. When the neurotransmitter binds with the receptor, it causes changes in the postsynaptic neuron by opening particular ion channels (such as sodium, potassium or chloride). This allows ion movement into or out of the postsynaptic neuron (depending on the ion’s concentration gradient), which results in a change in that neuron’s membrane potential, so that it is no longer at rest. Two possible scenarios may result:
The result depends on the type of neurotransmitter released and the binding to receptors on the membrane of the postsynaptic neuron. Basically, in some cases, propagation of the action potential occurs that excites the neuron. These neurotransmitters are called excitatory neurotransmitters. Alternatively, binding of other neurotransmitters on receptors on the postsynaptic neuron delays or stops the propagation of the action potential, thereby inhibiting the nerve transmission. Neurotransmitters that inhibit the action potential are known as inhibitory neurotransmitters.
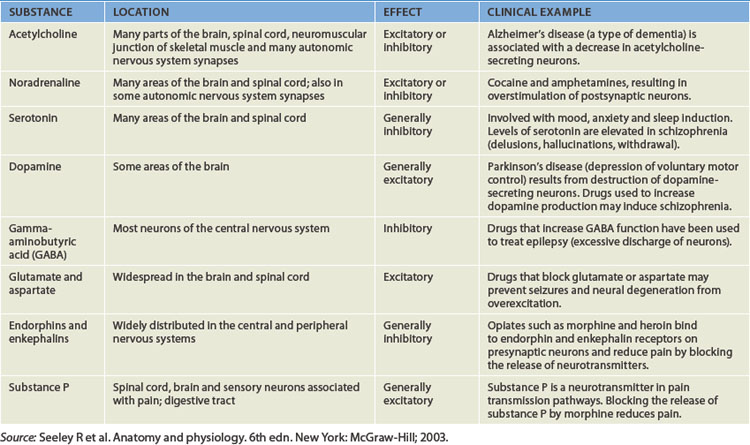
A range of substances are neurotransmitters (see Table 6-4), with acetylcholine and noradrenaline being particularly important for the central nervous system, as well as having some specific roles in the peripheral nervous system (see the section on the autonomic nervous system later in the chapter). Other neurotransmitters with important roles within the central nervous system include dopamine, serotonin, gamma (γ)-aminobutyric acid (GABA), glutamate, aspartate, enkephalins, endorphins and substance P. Many of these transmitters have more than one function and can be either excitatory or inhibitory, depending on the receptor site where the neurotransmitter binds.3 For example, noradrenaline in the brain probably helps regulate mood, functions in dreaming sleep and maintains arousal. It is worth remembering that a substance is classified as a neurotransmitter if it is stored in synaptic vesicles and released by a neuron; the same substance may also be considered a hormone when it is released into the bloodstream. Neurotransmitter substances are listed in Table 6-4.
Myelin
The speed of nerve transmission is greatly influenced by myelin. Where there is myelin, the velocity (speed) of nerve impulses increases. This occurs because myelin acts as an insulator that allows ions to flow between segments rather than along the entire length of the membrane. In this way, the action potential jumps between the gaps in myelin, called nodes of Ranvier, increasing the velocity. This mechanism is referred to as saltatory conduction. For instance, if you place your foot on a sharp object, the detection of the stimuli is very rapid and you will quickly move your foot away. In an adult, the nerve transmission has to travel approximately 1 metre from the foot to the central nervous system and back 1 metre again for the nerve impulses to activate the muscles to move your foot. The total distance travelled is about 2 metres and this occurs rapidly due to the presence of myelin in the nerves. In fact, the speed of nerve transmission in myelinated neurons can be up to 50 times faster than in nerves without myelin! In addition, conduction velocities depend not only on the myelin coating but also on the diameter of the axon. Larger diameter axons transmit impulses at a faster rate, thereby speeding up the nerve impulses even more.
THE CENTRAL NERVOUS SYSTEM
The brain
The human brain enables a person to reason, function intellectually, express personality and mood, and interact with the environment. The mass of the brain is approximately 1.5 kilograms. It requires a constant blood supply for the transport of oxygen and nutrients and receives approximately 15% to 20% of the total cardiac output. The major divisions of the brain are:
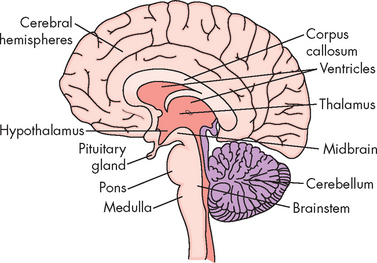
FIGURE 6-9 Schematic of the human brain.
Source: Stern TA et al. Massachusetts General Hospital comprehensive clinical psychiatry. St Louis: Mosby; 2008.
The constituents of these divisions are listed in Table 6-5.
Table 6-5 THE MAIN DIVISIONS OF THE CENTRAL NERVOUS SYSTEM
| MAIN REGION | CONTAINS |
|---|---|
| Cerebral hemispheres | |
| Diencephalon | |
| Brainstem | |
| Cerebellum | Cerebellum |
The different divisions of the brain are associated with different functions, but attributing specific functions to definite regions of the brain is not simple, as there is substantial overlap, with many functions being coordinated in several areas. However, for clinical considerations, functional specificity is very useful for localising pathological conditions in different regions of the brain.
The general, larger-scale appearance of nervous tissue depends on the presence of cellular structures. The grey matter is actually slightly darker tissue, due to the localisation of cell bodies from many neurons. The organelles within the cell body contribute to this grey appearance. On the other hand, white matter consists mainly of axons, and is lighter than the grey matter (see Figure 6-10). The white matter also contains myelinated axons, which have a fatty lipid sheath surrounding them that contributes to the white appearance.
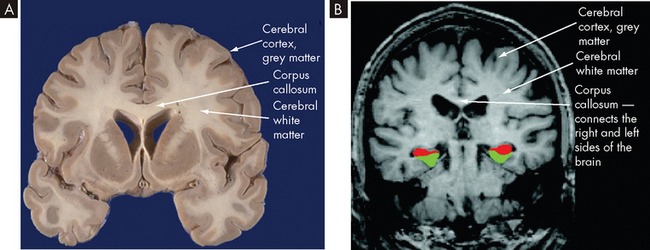
FIGURE 6-10 Coronal imaging through the cerebral cortex, showing grey and white matter.
A Brain cut demonstrating the cerebral cortex (grey matter) and corpus callosum (white matter). B Magnetic resonance image (MRI) scan of the brain. The cerebral cortex and corpus callosum are shown, as are the differences in the white and grey matter. (Red = hippocampus; green = parahippocampus.)
Source: A http://library.med.utah.edu/WebPath/HISTHTML/NEURANAT/CNS213A.html; B Dickerson BC et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 2004; 56(1): 27–35.
Collections of cell bodies are often organised in a local area, due to a similarity of function between those neurons. Where a group of cell bodies is found within the central nervous system, it is referred to as the nuclei — an example being the basal nuclei. A cluster of cell bodies within the peripheral nervous system is a ganglion, such as the sympathetic chain ganglion. A collection of axons in the peripheral nervous system is referred to as a plexus.
The cerebral hemispheres
The cerebral hemispheres — or cerebrum — comprise the largest portion of the brain. This is the structure that most people would recognise as the brain, and it is divided broadly into the cerebral cortex, cerebral white matter and the basal nuclei.
The surface of the cerebral hemispheres, the cerebral cortex, is covered with convolutions called gyri (singular = gyrus), which greatly increase the surface area and the number of neurons. It should be stressed that this is important for fitting billions of neurons into a tight space. Grooves between adjacent gyri are termed sulci, and deeper grooves are known as fissures. The two cerebral hemispheres, left and right, are separated by the longitudinal fissure. The surface of each hemisphere is divided into lobes named after the region of the skull under which it lies — frontal, parietal, occipital and temporal — with each having a left and right (see Figure 6-11).
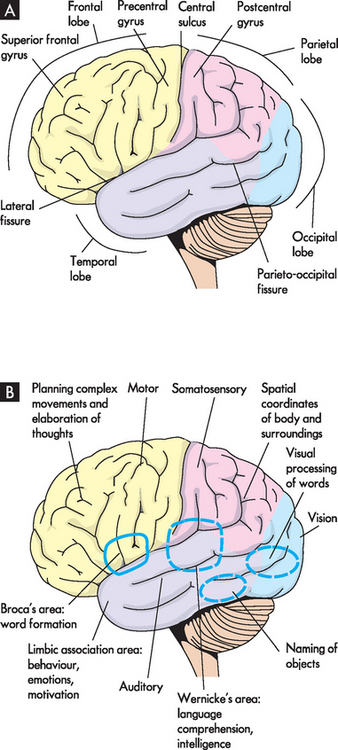
FIGURE 6-11 The cerebral hemispheres, lateral surface.
A Structural divisions. B Functional areas.
Source: A Based on Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010. B Based on Guyton AC, Hall JE. Textbook of medical physiology. 11th edn. Philadelphia: Saunders; 2006.
The principle of contralateral control occurs with the brain, meaning that the left side of the brain receives sensory input and controls the motor activities of the right side of the body, and vice versa. This is because the neurons that communicate with the cerebral cortex actually cross over to the other side of the body (the cross-over usually occurs in the spinal cord or brainstem). The crossover is referred to as where the neurons decussate.
Interestingly, it is common for one side of the brain to be more dominant than the other; a concept known as cerebral lateralisation. If the left side of the brain is dominant, then logical and analytical skills are enhanced, such as mathematics and language. The left side of the brain is dominant in controlling the writing skills of most of our population, as the majority of people are right-handed. If the right side is dominant, then creative and emotional activities are more pronounced, such as art, music and spatial relationships. Although one side of the brain tends to dominate, it also communicates extensively with the other side. If the dominant side is damaged, then the non-dominant side may be able to increase its control over that function.
The cerebral cortex
The cerebral cortex is the outer or superficial layer of the cerebral hemispheres and contains the cell bodies of neurons (the grey matter). This is the critical part of the brain for conscious control, as sensory information must reach the cerebral cortex for conscious awareness, and conscious control of muscles originates in the cerebral cortex. Many functions do not require conscious thought, and these are located in brain areas other than the cerebral cortex. This is a way of ‘filtering out’ information, so that the cerebral cortex is not bombarded with information that can be controlled by other brain centres. The cerebral cortex is also referred to as a ‘higher brain’ region, due to its role in conscious thought. Processes that do not require conscious thought and the same degree of integration are coordinated by ‘lower brain’ regions (such as the brainstem).
The posterior margin of the frontal lobe (located posterior to the forehead) is on the central sulcus and it borders inferiorly on the lateral sulcus (see Figure 6-11). The prefrontal cortex is responsible for goal-oriented behaviour (e.g. ability to concentrate), short-term or recall memory, the elaboration of thought and inhibition of the limbic areas of the central nervous system. The premotor cortex is involved in programming motor movements — the name premotor indicates that this is where movements are thought about, before they actually occur. This area also contains the cell bodies that form part of the basal nuclei (described in the next section). The frontal eye fields, which are involved in controlling eye movements, are located on the middle frontal gyrus.
The primary motor cortex is an important region of the frontal lobe. It is located along the precentral gyrus and is responsible for the conscious control of voluntary muscle activities. It receives information from the premotor cortex described above. The primary motor cortex then signals down to the skeletal muscles to cause movement. The axons travelling from the cell bodies in and on either side of this gyrus project fibres (axons) that form the corticospinal tracts (discussed in the next section) that descend into the spinal cord. The Broca’s speech area is on the inferior frontal gyrus. It is usually on the left hemisphere and is responsible for the motor aspects of speech (movements of muscles that allow words to be formed).
The parietal lobe (located posterior to the frontal lobe) lies within the borders of the central, parieto-occipital and lateral sulci (see Figure 6-11). This lobe contains the major area for somatosensory input, located primarily along the postcentral gyrus, which is adjacent to the primary motor area. The parietal lobe receives sensory information and then integrates and interprets it. For example, you may feel a smooth, round object with your fingers, and sensory integration allows you to recognise it as a ball.
The occipital lobe (at the most posterior part of the brain) lies caudal to the parieto-occipital sulcus and is superior to the cerebellum (see Figure 6-11). The primary visual cortex is located in this region and receives input from the retinas. Most of this lobe is involved in visual association, so that you can interpret and associate structures that you see.
The temporal lobe lies inferior to the lateral fissure (inside the skull near the ears; see Figure 6-11). The primary auditory cortex and its related association area lie deep within the lateral sulcus on the superior temporal gyrus. The Wernicke’s area, along with adjacent portions of the parietal lobe, constitutes a sensory speech area. This area is responsible for reception and interpretation of speech, which is closely linked with hearing. The temporal lobe is also involved in memory consolidation and smell.
Cerebral tracts
Inside the cerebral hemispheres are numerous tracts or axons (white matter; see Figure 6-12). This white matter lies beneath the cerebral cortex and is composed of myelinated nerve fibres. Lying directly beneath the longitudinal fissure is a mass of white matter pathways called the corpus callosum. This structure connects the two cerebral hemispheres and is essential in coordinating activities between the hemispheres (see also Figure 6-10).
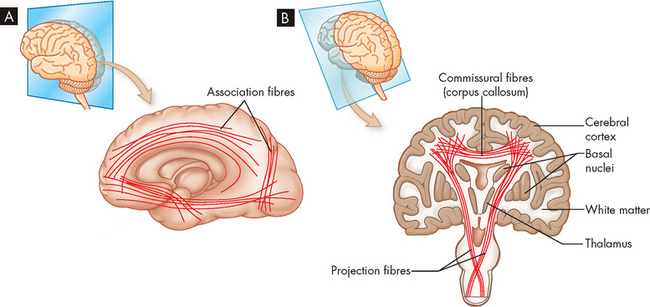
A Lateral perspective, showing various association fibres. B Frontal (coronal) perspective, showing commissural fibres that make up the corpus callosum and the projection fibres that communicate with lower regions of the nervous system.
Source: Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
The association fibres are a range of fibres that connect regions of the brain between gyri and between lobes. There are extensive amounts of association fibres that allow effective communication and coordination between brain regions. For example, the communication between the motor and sensory areas allows appropriate motor response to sensory inputs.
Basal nuclei
The major cerebral nuclei are called basal nuclei (nuclei referring to a collection of cell bodies in the central nervous system). The basal nuclei are found deep in the white matter and functionally include a number of nuclei that connect to different regions of the brain. The basal nuclei have direct and indirect interconnections with the thalamus, premotor cortex, reticular formation and spinal cord. The exact functions of the basal nuclei are not yet fully understood, but they are believed to influence muscular activity by exerting a fine-tuning effect on motor movements and inhibiting unnecessary movement. Note that the basal nuclei were previously known as the basal ganglia.
The limbic system
The limbic system is a group of structures, many of which are in the cerebral hemispheres and surrounding the corpus callosum, which influence emotions through connections in the prefrontal cortex. The system is composed of many regions including parts of the prefrontal cortex, hippocampus, amygdala and thalamus. Its principal effects are believed to be involved in basic behavioural responses, visceral reaction to emotions, feeding behaviours, biological rhythms and sense of smell (see Figure 6-13). This system is powerful and has a great influence on how we interpret our environment and experiences, such as whether something makes us happy or not.
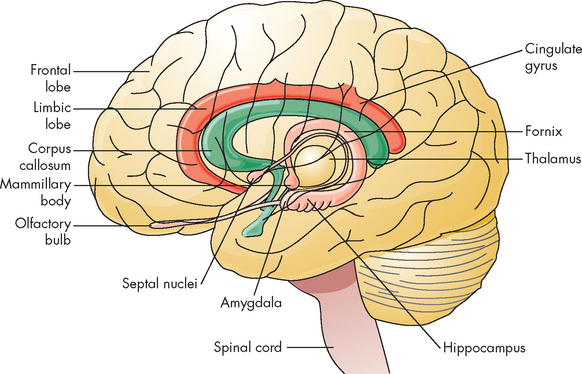
FIGURE 6-13 The limbic system.
The limbic system is composed of a group of structures deep in the brain that are important in memory and emotion. These structures include the limbic lobe, amygdala, fornix, hippocampus, olfactory cortex and portions of the thalamus.
Source: Based on Copstead L-EC, Banasik JL. Pathophysiology. 4th edn. Philadelphia: Saunders; 2010.
The diencephalon
The diencephalon, surrounded by the cerebrum, consists mainly of the thalamus and hypothalamus (see Figure 6-9). The thalamus borders and surrounds the third ventricle. It is a major integrating centre for afferent impulses to the cerebral cortex. Various sensations are perceived at this level, but processing by the cerebral cortex is required for interpretation. The thalamus serves also as a relay centre for information from the basal nuclei and cerebellum to the appropriate motor area.
Neuroimaging techniques
Functional and structural neuroimaging techniques have reached a level of sophistication where they can be used in diagnosing brain dysfunctions by looking at levels of brain activity in specific areas. The technologies include positron emission tomography (PET), functional magnetic resonance imaging (fMRI), single-photon emission computed tomography (SPECT) and magnetic resonance spectroscopy (MRS). In dyslexia, autism and attention-deficit-hyperactivity disorder, abnormal brain symmetry or abnormal interactions between or within lobes are evident. Researchers are starting to apply these techniques and others to psychiatry and cognitive neuroscience problems. They are evaluating problems such as how brain areas interact in individuals with psychoses or hallucinations, how they recover from neurotrauma and the advancement of neurodegenerative diseases.
Source: Gore JC et al. Integration of fMRI, NIROT and ERP studies of human brain function. J Magn Reson Imaging 2006; 24:507–513; Weiller C et al. Role of functional imaging in neurological disorders. J Magn Reson Imaging 2006; 23(6):840–850; Roffman JL et al. Neuroimaging-genetic paradigms: a new approach to investigate the pathophysiology and treatment of cognitive deficits in schizophrenia. Harv Rev Psychiatry 2006; 14(2):78–91.
The hypothalamus forms the base of the diencephalon. The hypothalamus functions to: (1) maintain a constant internal environment; and (2) implement behavioural patterns. Integrative centres control autonomic nervous system function, regulate body temperature and endocrine function, and regulate emotional expression. The hypothalamus exerts its influence through the endocrine system, as well as through neural pathways (see Box 6-1).
The brainstem
The brainstem is the vital centre of the brain. Damage to this area affects basic life functions such as breathing and blood pressure control. It is divided into the midbrain (the superior portion of the brainstem), the pons and the medulla oblongata (at the most inferior part). The medulla is continuous with the spinal cord (see Figure 6-9).
 The midbrain is composed of several structures, including the superior and inferior colliculi, the red nucleus and substantia nigra, and the cerebral peduncles. The superior colliculi are involved with voluntary and involuntary visual motor movements (e.g. the ability of the eyes to track moving objects in the visual field); while the inferior colliculi accomplish similar motor activities but involve movements affecting the auditory system (e.g. positioning the head to improve hearing). The red nucleus receives ascending sensory information from the cerebellum and projects a minor motor pathway to the cervical part of the spinal cord. The substantia nigra, which produces the neurotransmitter dopamine, may also be considered part of the basal nuclei. Other notable structures of this region are the nuclei of cranial nerves III (called oculomotor) and IV (called trochlear), and the cerebral aqueduct, which carries cerebrospinal fluid.
The midbrain is composed of several structures, including the superior and inferior colliculi, the red nucleus and substantia nigra, and the cerebral peduncles. The superior colliculi are involved with voluntary and involuntary visual motor movements (e.g. the ability of the eyes to track moving objects in the visual field); while the inferior colliculi accomplish similar motor activities but involve movements affecting the auditory system (e.g. positioning the head to improve hearing). The red nucleus receives ascending sensory information from the cerebellum and projects a minor motor pathway to the cervical part of the spinal cord. The substantia nigra, which produces the neurotransmitter dopamine, may also be considered part of the basal nuclei. Other notable structures of this region are the nuclei of cranial nerves III (called oculomotor) and IV (called trochlear), and the cerebral aqueduct, which carries cerebrospinal fluid. The pons is easily recognised by its bulging appearance below the midbrain and above the medulla. Primarily it transmits information from the cerebellum to the brainstem and between the two cerebellar hemispheres. The nuclei of the cranial nerves V (trigeminal) to VIII (vestibulocochlear) are located in this structure.
The pons is easily recognised by its bulging appearance below the midbrain and above the medulla. Primarily it transmits information from the cerebellum to the brainstem and between the two cerebellar hemispheres. The nuclei of the cranial nerves V (trigeminal) to VIII (vestibulocochlear) are located in this structure. The medulla oblongata forms the lowest portion of the brainstem. Reflex activities, such as heart rate, breathing, blood pressure, coughing, sneezing, swallowing and vomiting, are controlled in this area. The nuclei of cranial nerves IX (glossopharyngeal) to XII (hypoglossal) also are located in this region. The medulla is the most important region of the brain to keep you alive. Damage to other areas of the brain may cause alterations in function, yet not be fatal; however, injury to the medulla is likely to damage the neurons that control the vital functions of the cardiovascular and respiratory systems, which usually results in death.
The medulla oblongata forms the lowest portion of the brainstem. Reflex activities, such as heart rate, breathing, blood pressure, coughing, sneezing, swallowing and vomiting, are controlled in this area. The nuclei of cranial nerves IX (glossopharyngeal) to XII (hypoglossal) also are located in this region. The medulla is the most important region of the brain to keep you alive. Damage to other areas of the brain may cause alterations in function, yet not be fatal; however, injury to the medulla is likely to damage the neurons that control the vital functions of the cardiovascular and respiratory systems, which usually results in death.A collection of nerve cell bodies (nuclei) within the brainstem makes up the reticular formation (see Figure 6-14). The reticular formation is a large network of connected tissue that contains portions of vital reflexes, such as those controlling cardiovascular function and ventilation. The reticular formation is essential for maintaining wakefulness and therefore is referred to as the reticular activating system. We now discuss the sleep process and the role of the reticular activating system.
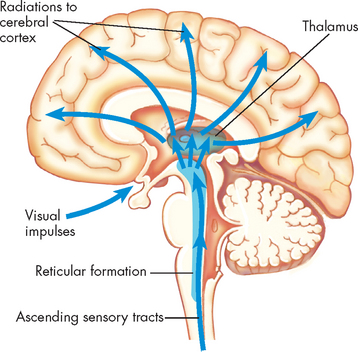
FIGURE 6-14 The reticular activating system.
System consists of nuclei in the brainstem reticular formation plus fibres that conduct to the nuclei from below and fibres that conduct from the nuclei to widespread areas of the cerebral cortex. Functioning of the reticular activating system is essential for consciousness.
The sleep process
Sleep is a temporary state of unconsciousness from which the individual can be aroused either spontaneously or by an external stimulus such as an alarm clock, a baby crying or light in the room. It is distinguished from the unconsciousness of coma, whereby arousal cannot be prompted (consciousness is discussed in Chapter 8). The brain remains constantly active during sleep. Interestingly, the brain consumes the same amount of glucose and oxygen whether awake or asleep, indicating that sleep does not reduce the nutrient or energy requirements of the brain.
Normal sleep has two phases that alternate through the night, as distinguished by electroencephalogram (EEG) (recording of the electrical activity of the cerebral cortex from the skull surface):
Rapid eye movement (REM) sleep occurs about every 90 minutes beginning 1–2 hours after NREM sleep begins. The EEG pattern is similar to the normal awake pattern and the brain is very active. The changes associated with REM sleep include increased parasympathetic activity and variable sympathetic activity associated with rapid eye movement; muscle relaxation; loss of temperature regulation; altered heart rate, blood pressure and breathing rate; penile erection in men and clitoral engorgement in women; release of steroids; and many memorable dreams. Respiratory control appears largely independent of metabolic requirements and oxygen variation. Loss of normal voluntary muscle control in the tongue and upper pharynx may produce some respiratory obstruction. Cerebral blood flow increases. The exact reason for all these changes is not fully understood and researchers are exploring the physiological and pathophysiological implications.
Non-rapid eye movement (NREM) sleep is divided into four stages (I–IV) from light to deep sleep.4 NREM sleep accounts for 75–80% of sleep time in adults. Sympathetic tone is decreased and parasympathetic activity is increased during NREM sleep, which creates a state of reduced activity. The basal metabolic rate falls by 10–15%; the core temperature decreases 0.5° to 1.0°C; the heart rate decreases by 10–30 beats per minute; and there are also decreases in the breathing rate, blood pressure and muscle tone. During the various stages, cerebral blood flow to the brain decreases and growth hormone is released, with secretion of corticosteroids (such as cortisol and aldosterone) and catecholamines (adrenaline and noradrenaline) depressed.
The importance of sleep
Sleep is restful and allows restoration. The essential and unavoidable nature of sleep suggests that there must be distinct advantages to the body. After insufficient sleep, there is an increased amount of sleep at the next opportunity, usually at a higher intensity (seen on the EEG).5 Sleep is an important part of the consolidation of memories,6 which makes it critical for students to undertake an appropriate pattern of sleep throughout their studies. In addition, sleep deprivation can result in impaired function of some regions of the brain that are involved in learning.7 During the deepest stage of NREM sleep, serotonin is released, which actually maintains the wellbeing of the brain.8 The role of sleep in setting daily rhythms of hormone secretion is discussed in Chapter 34.
Control of the sleep–wake cycle
The sleep control centre is coordinated in the hypothalamus (in a region known as the suprachiasmatic nucleus), which sets and regulates the circadian rhythms of body function around a 24-hour cycle. The hypothalamus receives information from the environment regarding the amount of light (via the eyes), and other sources assist, including alarm clocks and social cues (such as the pattern of sleep following dinner). In addition, the pineal gland (within the brain) also receives light information from the eyes and secretes melatonin, which interacts with the hypothalamus to induce drowsiness, thus marking the onset of sleep. The details of these processes and their interactions are still under investigation.
Arousal from sleep involves increased activity in the brainstem (the reticular activating system). Prior to waking, sensory information that ascends from the body periphery provides the reticular activating system with inputs such as an increasing amount of light or noise in the morning (refer to Figure 6-14); it also receives the sudden loud noise of someone calling you or an alarm clock. The reticular activating system then communicates with other brain regions, the most important being the cerebral cortex, to restore consciousness (awakening). During sleep, the flow of communication between the cerebral cortex and the reticular activating system is inhibited. The hypothalamus also has an important role in initiating sleep and coordinates this essential function by receiving information on the amount of light from the eyes, as well as the amount of melatonin secreted from the pineal gland.
The cerebellum
The cerebellum (literally meaning ‘little brain’) is located inferior to the occipital lobe of the cerebral hemispheres (see Figure 6-9) and is composed of grey and white matter; its outside surface is convoluted like the surface of the cerebrum. It is divided into two lobes (left and right) connected by the vermis.
The cerebellum is responsible for reflexive, involuntary fine-tuning of motor control for precise activities such as throwing a ball directly to a target, and the delicate functions of the hands of a neurosurgeon or an expert musician. It is also involved in maintaining balance and posture through extensive neural connections with the medulla through the inferior cerebellar peduncle and with the midbrain through the superior cerebellar peduncle. These roles allow precise control of regular activities, such that conscious control is not needed. In this way, you can avoid having to consciously think about not falling over when you walk, as the cerebellum controls that function.
A major portion of the descending motor pathways (corticospinal tracts) cross to the other side, or decussate, at the medulla. These pathways, together with other areas of decussation in the central nervous system, are the basis for the phenomenon of contralateral control.
The spinal cord
The spinal cord is the portion of the central nervous system that lies within the vertebral canal and is surrounded and protected by the vertebral column (spine). The spinal cord is a long nerve cable that connects the brain and body, and is responsible for somatic and autonomic reflexes, motor pattern control centres, and sensory and motor modulation. It continues from the medulla oblongata and ends at the level of the first or second lumbar vertebra in adults (see Figure 6-15). The end of the spinal cord, the conus medullaris, is cone-shaped. Grossly, the spinal cord is divided into vertebral sections (7 cervical, 12 thoracic, 5 lumbar, 5 sacral and 1 coccygeal) that correspond to paired nerves — one on the left and one on the right of the body (see Figure 6-15). Note that although there are 7 cervical vertebrae, there are actually 8 cervical nerves.
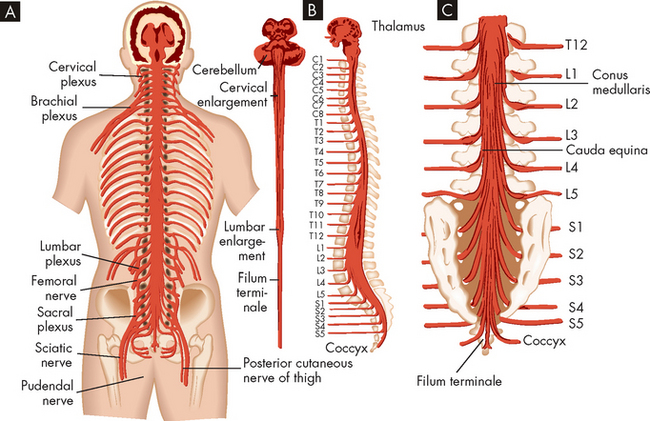
FIGURE 6-15 Spinal cord within the vertebral canal and exiting spinal nerves.
A Posterior view of the brainstem and spinal cord in situ with spinal nerves and plexus. B Lateral view of brainstem and spinal cord. C Enlargement of caudal area showing termination of the spinal cord (conus medullaris) and group of nerve fibres constituting the cauda equina.
Source: Redrawn from Ruby EB (ed.). Advanced neurological and neurosurgical nursing. St Louis: Mosby; 1984.
A cross-section of the spinal cord (see Figure 6-16) is characterised by a butterfly-shaped inner core of grey matter (containing nerve cell bodies). The central canal lies in the centre of this region and extends through the spinal cord from its origin in the fourth ventricle, and contains cerebrospinal fluid. The grey matter of the spinal cord contains interneurons and axons from sensory neurons (whose cell bodies lie in the dorsal root ganglion) and nerve cell bodies for efferent pathways that leave the spinal cord by way of spinal nerves.
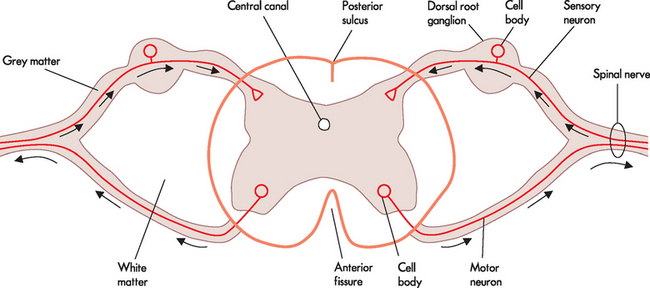
FIGURE 6-16 Cross-section through the spinal cord.
Note the outer white matter and inner grey matter. Sensory neurons are located in the dorsal or posterior side, while motor neurons are located in the ventral or anterior side. Arrows indicate the direction of action potentials. The sensory and motor neurons are located together in the spinal nerve.
Reflexes
Neural circuits in the spinal cord, when activated, display specific sets of motor responses. Reflex arcs form basic units that respond to stimuli and provide protective circuitry for motor output. They are referred to using many different terms. First, there are four aspects of all reflexes: they are involuntary, rapid and require a stimulus to trigger them, and the reflex response is similar every time it is activated. The structures needed for a reflex arc are a receptor, a sensory (afferent) neuron and an integration centre (within the central nervous system), followed by a motor (efferent) neuron and an effector muscle or gland. The receptor senses the stimuli and the sensory neuron transmits that signal as an action potential to the site of integration within the central nervous system. In response, the motor neuron relays the signal to the effector, which is often to a muscle. When the reflex arc occurs through the spinal cord, it is classified as a spinal reflex.
The two main reflex arcs are the stretch and withdrawal reflexes:
 The stretch reflex is the simplest of the spinal reflex arcs and contains only two neurons, meaning that there is only one synapse between the two neurons (monosynaptic). An example of this is a common reflex test performed in clinical practice: the patellar reflex or knee jerk. When the patellar ligament is tapped, the extensor muscle stretches, sending an afferent signal to the spinal cord. This triggers motor neurons that cause the quadriceps to contract, which completes the reflex arc (see Figure 6-17).
The stretch reflex is the simplest of the spinal reflex arcs and contains only two neurons, meaning that there is only one synapse between the two neurons (monosynaptic). An example of this is a common reflex test performed in clinical practice: the patellar reflex or knee jerk. When the patellar ligament is tapped, the extensor muscle stretches, sending an afferent signal to the spinal cord. This triggers motor neurons that cause the quadriceps to contract, which completes the reflex arc (see Figure 6-17). The withdrawal reflex involves multiple synapses (polysynaptic). These reflexes are associated with involuntary removal from a painful or injury-provoking stimuli — for instance, removing your hand when you touch a hot stove or pulling your leg up when you step on a sharp object (see Figure 6-18). The polysynaptic reflex arcs are usually associated with the sensation of pain, as nociceptive fibres (neurons that carry pain signals; see Chapter 7) transmit the signal to the cerebral cortex, where the individual becomes aware of the pain. It is important to realise that this occurs after the reflex, as the distance to the brain is longer than the reflex and the sensation of pain needs to be interpreted in the cerebral cortex. These processes take time, and the withdrawal reflex working through a reflex arc means that the body is already removed from pain prior to awareness. This means the reflexes are protective and prevent further injury to the body (see Chapter 7).
The withdrawal reflex involves multiple synapses (polysynaptic). These reflexes are associated with involuntary removal from a painful or injury-provoking stimuli — for instance, removing your hand when you touch a hot stove or pulling your leg up when you step on a sharp object (see Figure 6-18). The polysynaptic reflex arcs are usually associated with the sensation of pain, as nociceptive fibres (neurons that carry pain signals; see Chapter 7) transmit the signal to the cerebral cortex, where the individual becomes aware of the pain. It is important to realise that this occurs after the reflex, as the distance to the brain is longer than the reflex and the sensation of pain needs to be interpreted in the cerebral cortex. These processes take time, and the withdrawal reflex working through a reflex arc means that the body is already removed from pain prior to awareness. This means the reflexes are protective and prevent further injury to the body (see Chapter 7).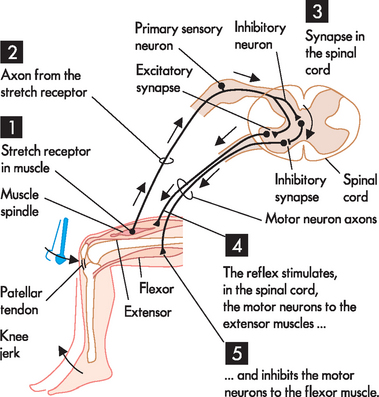
FIGURE 6-17 The stretch or knee-jerk reflex.
A stretch to the muscle stimulates the stretch receptor. 1 The stretch receptor within the quadriceps muscle detects the stretch caused by tapping the patellar tendon. 2 The stretch receptor sends an afferent signal to the spinal cord. 3 In the spinal cord, there is an excitatory synapse with the motor neuron that innervates the quadriceps muscle, as well as an inhibitory synapse with the motor neuron for the hamstrings. 4 As a result, the quadriceps muscle contracts (extensor muscle) 5 Also, the hamstring muscle relaxes, which prevents it from limiting the action of the quadriceps.
Source: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. 2nd edn. Philadelphia: Saunders; 2009.
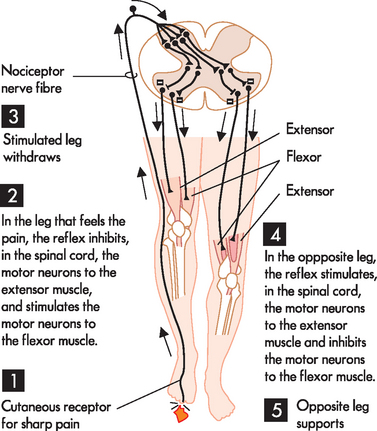
FIGURE 6-18 The withdrawal reflex.
A painful stimulus to the right foot elicits a withdrawal reflex. 1 The cutaneous nociceptor is triggered by the sharp object and sends an afferent signal to the spinal cord. 2 The interneurons in the spinal cord relay motor efferent signals to the flexor muscles and inhibit the extensor muscles in the right leg. 3 Collectively, this causes the right leg to withdraw from the painful source. 4 At the same time, interneuron connections in the spinal cord relay efferent signals to the opposite leg that cause the extensor muscles to contract and flexor muscles to relax. 5 This causes the opposite leg to stabilise your position so that you do not fall over.
Source: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. 2nd edn. Philadelphia: Saunders; 2009.
In addition to the spinal reflexes, which are associated with the somatic nervous system, there are many reflexes associated with the autonomic nervous system. These occur without conscious control and involve organ responses, such as increasing the heart rate when frightened. The autonomic reflexes are controlled by the sympathetic and parasympathetic nervous systems and are discussed with individual body systems in later chapters.
Sensory pathways
Afferent pathways transmit information from peripheral receptors, through the spinal cord and eventually terminate in the cerebral cortex or cerebellum, or both. The three clinically important spinal afferent pathways are the posterior (dorsal) column, the anterior spinothalamic tract and the lateral spinothalamic tract (see Figure 6-19). The posterior part of the spinal cord carries information about where the body is positioned in space, called proprioception. An example of this is knowing where your arms and legs are positioned relative to your body without having to see them. The anterior spinothalamic tract carries vague touch information, while the lateral spinothalamic tract is responsible for pain and temperature.
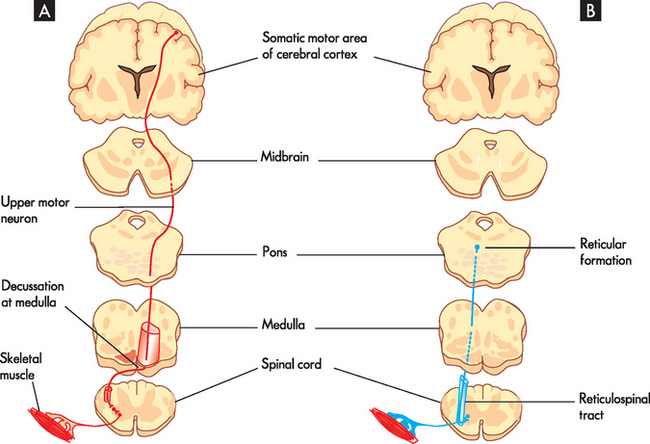
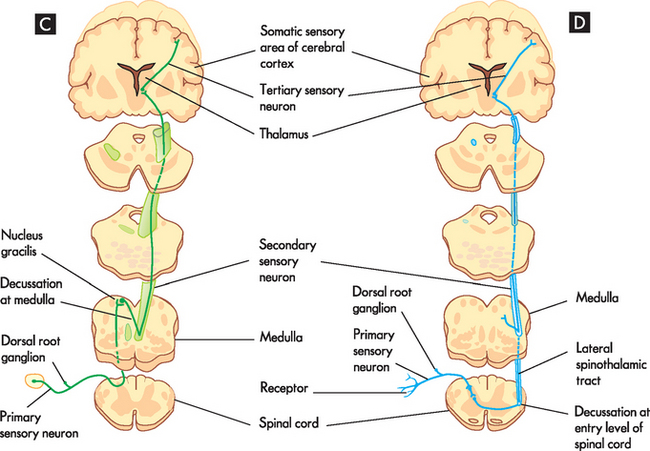
FIGURE 6-19 Somatic motor and sensory pathways (or tracts).
A Lateral corticospinal tract sending signals to skeletal muscles. B Reticulospinal pathway sending signals to muscles involved in posture. C Dorsal column, transmits touch and propioception. D Lateral spinothalamic tract, transmits pain and temperature signals.
Source: Based on Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Motor pathways
Efferent pathways primarily relay information from the cerebral hemispheres to the brainstem or spinal cord. The initiation of movement occurs in neurons within the central nervous system, which synapse with interneurons, which then synapse with motor neurons before projecting into the periphery. Motor neurons directly influence muscles. Their cell bodies lie in the grey matter of the brainstem and spinal cord, but their processes extend out of the central nervous system and into the peripheral nervous system. Muscle activity (i.e. stimulation and contraction) is regulated by nerve impulses. Motor neurons innervate one or more muscle cells, forming the neuromuscular junction (this is discussed in greater detail in Chapter 14).
The four clinically relevant motor pathways are the lateral corticospinal, corticobulbar, reticulospinal and vestibulospinal tracts.9 The corticospinal and corticobulbar pathways are essentially the same tract and their cell bodies originate in and around the precentral gyrus — this is the region where most motor information is initiated. These motor neurons are involved in precise motor movements. The reticulospinal tract (see Figure 6-19) controls motor movement by inhibiting and exciting spinal activity. The vestibulospinal tract arises from a vestibular nucleus in the pons (responsible for balance) and causes the muscles of the body to rapidly contract. For example, this becomes activated when an individual starts to fall backwards.
Protective structures of the central nervous system
The cranium and vertebral column
The cranium (skull) is composed of eight bones. The cranial vault encloses and protects the brain and its associated structures. The floor of the cranial vault is irregular and contains many foramina (openings) for cranial nerves, blood vessels and the spinal cord to exit. The vertebral column (see Figure 6-15) provides physical protection for the spinal cord by the bones of the spine.
The meninges
Surrounding the brain and extending down the spinal column beyond the end of the spinal cord are three protective membranes: the dura mater, the arachnoid mater and the pia mater. Collectively they are called the meninges (see Figure 6-20A).
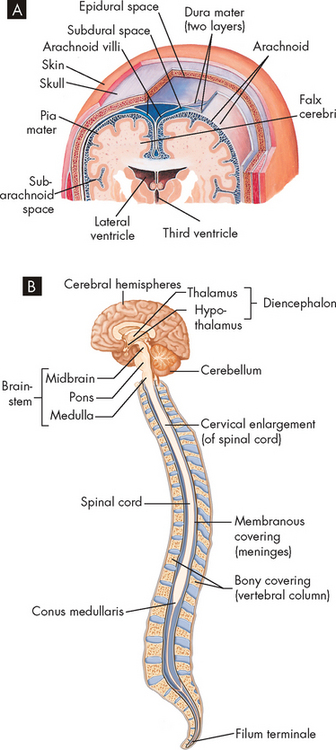
FIGURE 6-20 Meninges of the brain and spinal cord.
A Layers of meninges. Note that the meninges seen here are also continuous with the spinal cord. B The filum terminale is the extension of the pia mater, which assists in anchoring the spinal cord inside the vertebral column.
Source: Based on Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
The dura mater
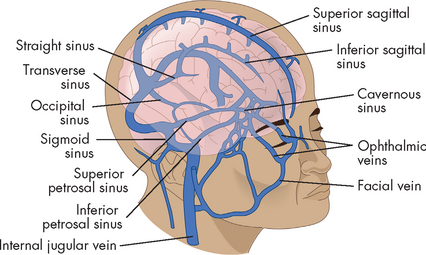
The dura mater (meaning literally ‘hard mother’) is the outermost layer, and is composed of two sublayers: the outermost layer, the periosteum (endosteal layer), forms the lining inside the skull; and the inner layer (meningeal layer) forms the outside cover of both the brain and the spinal cord. Usually, the periosteal and meningeal layers are close together, but in some places there are venous sinuses between them, which are regions that drain venous blood.
The meningeal layer is responsible for forming rigid membranes that support and separate various brain structures, as well as limiting the movement of the brain. One of these membranes, the falx cerebri, dips between the two cerebral hemispheres along the longitudinal fissure. The falx cerebri is anchored anteriorly to the base of the brain at the ethmoid bone. The falx cerebelli is a small membrane located between the left and right lobes of the cerebellum. The tentorium cerebelli, a common landmark, is a membrane that separates the cerebellum below from the cerebral structures above.
Epidural injections are made into the space just external to the dural covering of the spinal cord. The ligamentum flavum provides an internal lining of the vertebral column, and so the tip of the epidural needle is inserted through the ligamentum flavum to the epidural space, without penetrating the dural layer, and the drug is injected into this space.
The arachnoid mater
Internal to the dura mater lies the arachnoid mater, a spongy weblike structure that loosely follows the contours of the cerebral structures. The subdural space lies between the dura and arachnoid. Many small bridging veins that have little support traverse the subdural space. Their disruption results in a subdural haematoma (see Chapter 9). The subarachnoid space lies between the arachnoid and the pia mater and contains cerebrospinal fluid. This space is very vascular and if bleeding occurs here it can result in severe damage to the brain (see Chapter 9). In addition, the subarachnoid space is the site for intrathecal injections in the spinal cord.
The pia mater
Unlike the dura mater and arachnoid mater, the delicate pia mater adheres to the contours of the brain and spinal cord. It provides support for the rich network of blood vessels serving brain tissue. The choroid plexuses, structures that produce cerebrospinal fluid, arise from the pial membrane. The spinal cord is anchored to the vertebrae by extension of the meninges known as the filum terminale (see Figure 6-20B). The meninges continue beyond the end of the spinal cord to the lower portion of the sacrum. Cerebrospinal fluid contained within the subarachnoid space also circulates down to the second sacral vertebra.
Cerebrospinal fluid and the ventricular system
Cerebrospinal fluid (CSF) is a clear, colourless fluid similar to blood plasma and interstitial fluid, but it has a different composition to these other fluids. The intracranial and spinal cord structures float in cerebrospinal fluid and are thereby provided some protection from trauma. The buoyant properties of the cerebrospinal fluid also prevent the brain from tugging on meninges, nerve roots and blood vessels. The constituents of cerebrospinal fluid are listed in Table 6-6. Between 125 mL and 150 mL of cerebrospinal fluid is circulating within the ventricles (small cavities) of the brain and subarachnoid space at any given time.
Table 6-6 THE COMPOSITION OF CEREBROSPINAL FLUID
| CONSTITUENT | NORMAL VALUE |
|---|---|
| Sodium (Na+) | 148 mmol/L |
| Potassium (K+) | 2.9 mmol/L |
| Chloride (Cl−) | 125 mmol/L |
| Bicarbonate (HCO3−) | 22.9 mmol/L |
| Glucose (fasting) | 2.8–4.4 mmol/L (60% of plasma glucose) |
| pH | 7.3 |
| Protein | 0.15–0.45g/L |
| Albumin | 80% |
| Globulin | 6–10% |
| White blood cells | 0–6/mm3 |
| Red blood cells | 0 |
Four ventricles within the brain serve to provide buoyancy and allow circulation of the cerebrospinal fluid: two lateral ventricles (one within each of the right and left cerebral hemispheres); the third ventricle between the left and right sides of the thalamus; and the fourth ventricle between the cerebellum and the brainstem (see Figure 6-21). A choroid plexus in each ventricle produces the cerebrospinal fluid. These plexuses are characterised by a rich network of blood vessels (supplied by the pia mater) that lie close to the ependymal cells (a type of neuroglial cell) of the ventricles.
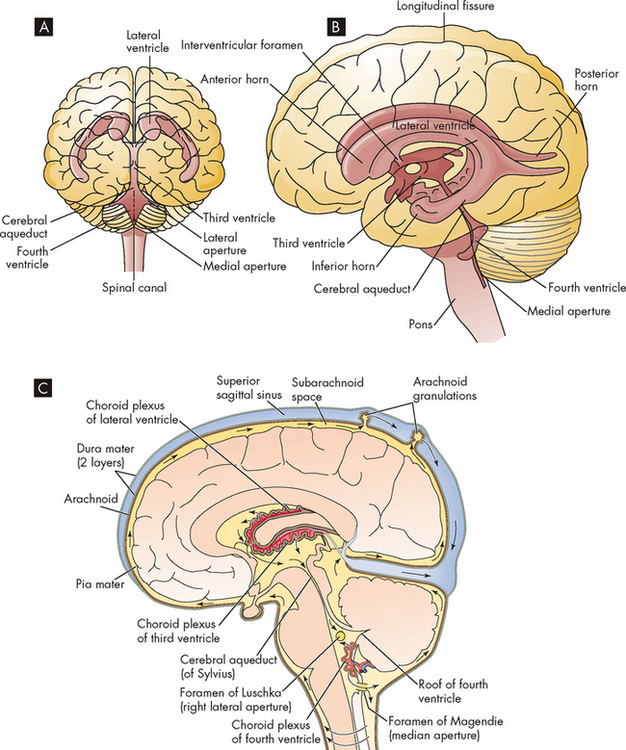
FIGURE 6-21 The ventricles within the brain and the circulation of cerebrospinal fluid.
A Frontal view of the ventricles. B Lateral view of the ventricles. C Each ventricle contains a choroid plexus, which secretes cerebrospinal fluid. The CSF escapes from the fourth ventricle and into the subarachnoid space.
Source: A & B Copstead L-EC, Banasik JL. Pathophysiology. 4th edn. Philadelphia: Saunders; 2010. C Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. 2nd edn. Philadelphia: Saunders; 2009.
The cerebrospinal fluid exerts pressure within the brain and spinal cord. When a person is lying down, cerebrospinal fluid pressure is about 5–14 mmHg, or 6.8–19 cmH2O, but this can double when the person sits up. Beginning in the lateral ventricles, the cerebrospinal fluid flows through the interventricular foramen into the third ventricle and passes through the cerebral aqueduct into the fourth ventricle. From the fourth ventricle the cerebrospinal fluid then continues to the subarachnoid spaces of the brain and spinal cord. The cerebrospinal fluid does not accumulate; instead, it is reabsorbed into the venous circulation through the arachnoid villi. Approximately 600 mL of cerebrospinal fluid is produced and reabsorbed daily. The arachnoid villi protrude from the arachnoid space, through the dura mater, and lie within the blood flow of the venous sinuses. The villi function as one-way valves directing cerebrospinal fluid outflow into the blood but preventing blood flow into the subarachnoid space. Thus, cerebrospinal fluid is formed from the blood, and after circulating throughout the central nervous system, it returns to the blood.
It should be noted that increases in cerebrospinal fluid pressure can cause severe damage to the brain: this pathophysiological condition, known as hydrocephalus, is examined in Chapter 8.
The blood–brain barrier
The blood–brain barrier describes cellular structures that inhibit some potentially harmful substances in the blood from entering the interstitial spaces of the brain or cerebrospinal fluid. In this way, the neurons that are particularly sensitive to change are protected from the changing environment of the blood, as only some substances from the blood can reach the neurons. The tight junctions between endothelial cells of capillaries in the brain are important in limiting the flow of substances out of the blood and into the cerebrospinal fluid. The astrocytes also assist with this barrier (see Figure 6-22). Metabolites, electrolytes and chemicals can cross into the brain to varying degrees. This has substantial implications for drug therapy because certain types of antibiotics and chemotherapeutic drugs show a greater propensity than others for crossing this barrier — which can limit the choices of drugs that can reach the brain. Importantly, not all potentially harmful substances are blocked by this barrier; for example, alcohol can easily pass through the blood–brain barrier, where it can affect the brain.
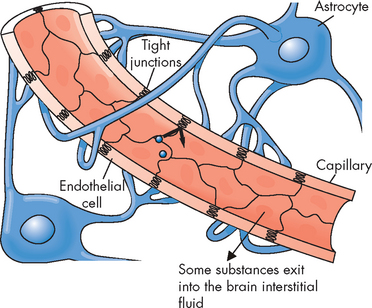
FIGURE 6-22 Tight junctions between brain capillary endothelial cells prevent substances from passing between the endothelial cell lining and thereby entering the neural tissue.
Astrocytes have foot processes on the capillary that help to maintain the integrity of the blood–brain barrier.
Source: Copstead L-EC, Banasik JL. Pathophysiology. 4th edn. Philadelphia: Saunders; 2010.
Blood supply of the central nervous system
Blood supply to the brain
The brain receives approximately 15–20% of cardiac output, or 800–1000 mL of blood flow per minute. This constant delivery is vital to brain function — without it, the brain rapidly starves of oxygen and nutrients and the person will be rendered unconscious within a matter of minutes. Carbon dioxide is the primary regulator for blood flow within the central nervous system. It is a potent vasodilator and its effects ensure an adequate blood supply. This is most graphically illustrated when an individual hyperventilates (breathes rapidly and deeply). If the individual continues to hyperventilate, the carbon dioxide levels will decrease as it is expelled from the body, cerebral blood flow will decrease and the individual may become light-headed and feel faint.
The brain derives its arterial supply from two systems: the internal carotid arteries and the vertebral arteries (see Figure 6-23). The internal carotid arteries supply a proportionately greater amount of blood to the brain. They take their origin from the common carotid arteries, enter the cranium through the base of the skull and pass through the cavernous sinus. After giving rise to some small branches, these arteries divide into the anterior and middle cerebral arteries. The vertebral arteries originate at the subclavian arteries and pass through the transverse foramina of the cervical vertebrae, entering the cranium through the foramen magnum. They join at the junction of the pons and medulla to form the basilar artery. The basilar artery divides at the level of the midbrain to form paired posterior cerebral arteries.
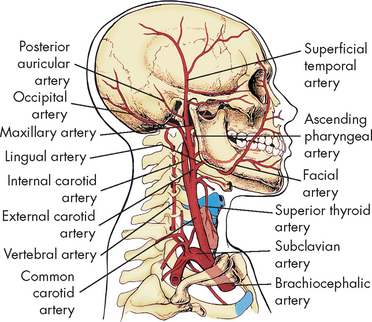
FIGURE 6-23 Major arteries of the head and neck.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The circle of Willis, named after the British physician Thomas Willis (see Figure 6-24), provides an alternative route for blood flow when one of the contributing arteries is obstructed (collateral blood flow). The circle of Willis is formed by the posterior cerebral arteries, posterior communicating arteries, internal carotid arteries, anterior cerebral arteries and anterior communicating artery. The anterior cerebral, middle cerebral and posterior cerebral arteries leave the circle of Willis and extend to various brain structures.
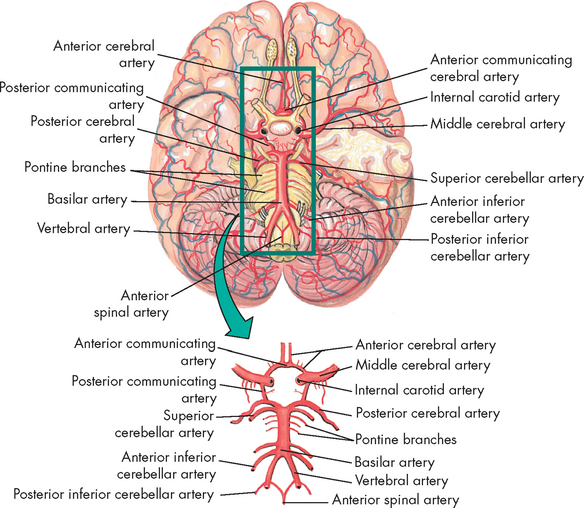
FIGURE 6-24 Arteries at the base of the brain.
The arteries that compose the circle of Willis are the two anterior cerebral arteries, joined to each other by the anterior communicating artery and two short segments of the internal carotids, off of which the posterior communicating arteries connect to the posterior cerebral arteries.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Cerebral venous drainage does not parallel its arterial supply, whereas the venous drainage of the brainstem and cerebellum does parallel the arterial supply of these structures. The cerebral veins are classified as superficial and deep veins. The veins drain into venous plexuses and dural sinuses (formed between the dural layers) and eventually join the internal jugular veins at the base of the skull (see Figure 6-25). Adequacy of venous outflow can significantly affect intracranial pressure. For example, individuals with head injuries who turn or let their heads fall to the side partially occlude venous return, and the intracranial pressure can increase because of decreased flow through the jugular veins.
Blood supply to the spinal cord
The spinal cord derives its blood supply from branches off the vertebral arteries and from branches from various regions of the aorta (see Figure 6-26). The anterior spinal artery and the paired posterior spinal arteries branch off from the vertebral artery at the base of the cranium and descend alongside the spinal cord. Arterial branches from vessels exterior to the spinal cord follow the spinal nerve through the intervertebral foramina, pass through the dura and divide into the anterior and posterior radicular arteries.
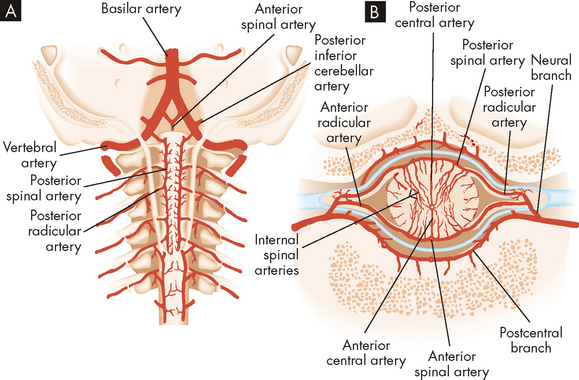
FIGURE 6-26 Arteries of the spinal cord.
A Arteries of the cervical cord exposed from the rear. B Arteries of the spinal cord diagrammatically shown in horizontal section.
Source: Redrawn from Ruby EB (ed.). Advanced neurological and neurosurgical nursing. St Louis: Mosby; 1984.
The radicular arteries eventually connect to the spinal arteries. Branches from the radicular and spinal arteries form plexuses whose branches penetrate the spinal cord, supplying the deeper tissues. Venous drainage parallels the arterial supply closely and drains into venous sinuses located between the dura and periosteum of the vertebrae.
THE PERIPHERAL NERVOUS SYSTEM
The peripheral nervous system consists of all the neurons outside of the brain and spinal cord. Importantly, the cranial and spinal nerves, including their branches and ganglia (cell bodies located in the peripheral nervous system as opposed to nuclei in the central nervous system), constitute the peripheral nervous system, and these nerves are important connections between the central nervous system and the body extremities. A peripheral nerve (cranial or spinal) is composed of individual axons wrapped in a myelin sheath.
There are 12 pairs of cranial nerves (paired = one left, one right; see Figure 6-27) that connect to nuclei in the brain and brainstem. A description of the function of each is listed in Table 6-7. Most of these cranial nerves are mixed nerves, which means that axons and dendrites of both sensory and motor neurons are located together, although some are purely sensory or purely motor. These nerves, while originating from the brain, are considered part of the peripheral nervous system. They are vitally important in clinical practice. Testing each cranial nerve is relatively simple and abnormalities in the responses of the different nerves provides clues to the clinician about the location of nerve damage in the brain. For instance, cranial nerve III is the oculomotor nerve and is responsible for pupillary size and shape. To test the nerve, the clinician simply shines a light into the individual’s eyes, one at a time: the pupil should constrict due to the increase in light. However, if pupil constriction occurs in one eye but not the other, this is often an indication of an increase in intracranial pressure in the opposite side of the brain and should be investigated more thoroughly immediately as it could be life-threatening. Therefore, clinical tests of cranial nerves provide the clinician with information about abnormal function and the anatomical location of injury to the brain.
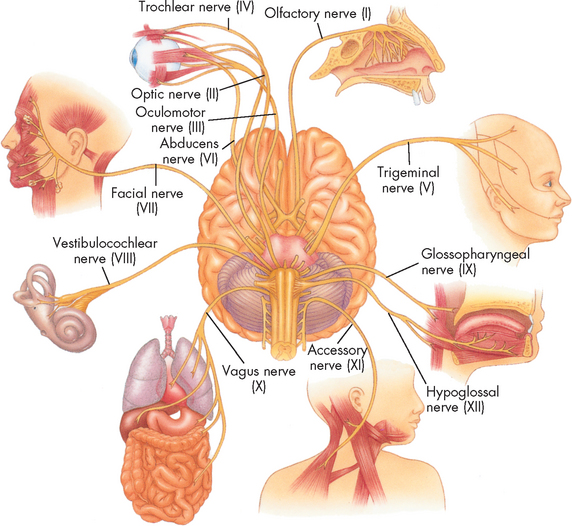
FIGURE 6-27 The 12 cranial nerves.
Ventral surface of the brain showing attachment of the cranial nerves.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Table 6-7 THE FUNCTIONS OF THE 12 CRANIAL NERVES
| NERVE | TYPE | FUNCTION |
|---|---|---|
| I Olfactory | Sensory | Sense of smell |
| II Optic | Sensory | Sense of sight |
| III Oculomotor | Mixed (mostly motor) | Movement of eyeball, raising of eyelid, change in pupil size |
| IV Trochlear | Mixed (mostly motor) | Movement of eyeball |
| V Trigeminal | Mixed | Chewing of food; sensations in face, scalp, cornea (eye) and teeth |
| VI Abducens | Mixed (mostly motor) | Movement of eyeball |
| VII Facial | Mixed | Facial expressions; secretion of saliva and tears; taste; blinking |
| VIII Vestibulocochlear | Sensory | Sense of hearing and balance |
| IX Glossopharyngeal | Mixed | Swallowing, secretion of saliva; taste; sensory for the reflex regulation of blood pressure; part of the gag reflex |
| X Vagus | Mixed | Visceral muscle movement and sensations, especially movement and secretion of the digestive system; sensory for reflex regulation of blood pressure |
| XI Accessory | Mixed (mostly motor) | Swallowing; head and shoulder movement; speaking |
| XII Hypoglossal | Mixed (mostly motor) | Speech and swallowing |
The 31 pairs of spinal nerves derive their names from the vertebral level where they exit. There are eight cervical spinal nerves. The first cervical nerve exits above the first cervical vertebra and the rest of the spinal nerves exit below their corresponding vertebrae. From the thoracic region (and inferiorly), nerves correspond to the vertebral level above their exit (see Figure 6-28).
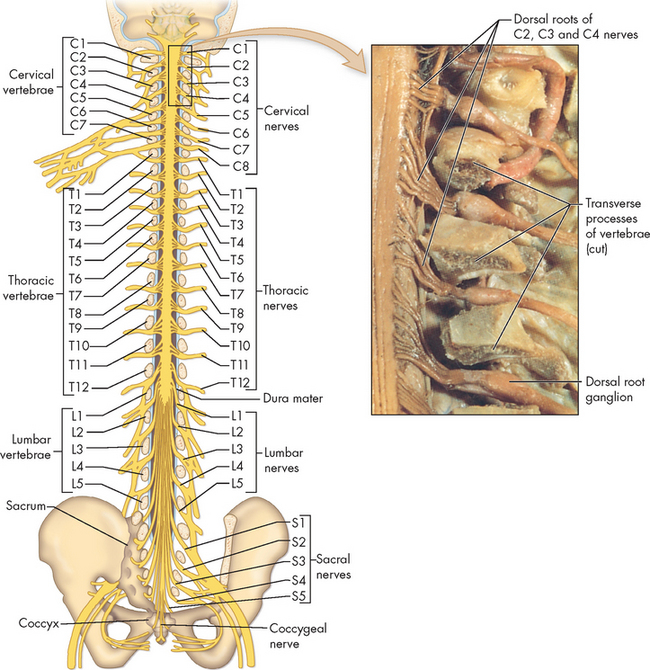
FIGURE 6-28 Peripheral nerves.
Inset is a dissection of the cervical segment of the spinal cord showing emerging cervical nerves. The spinal cord is viewed from behind (posterior aspect).
Source: Thibodeau GA, Patton KT. The human body in health & disease. 5th edn. St Louis: Mosby; 2010.
Spinal nerves contain both sensory and motor neurons and are called mixed nerves. They arise as rootlets lateral to the anterior and posterior horns of the spinal cord. These two spinal nerve roots converge in the region of the intervertebral foramen to form the spinal nerve trunk. Shortly after converging, the spinal nerve divides into anterior and posterior rami (branches). The anterior rami (except the thoracic) initially form plexuses (networks of nerve fibres), which then branch into the peripheral nerves. Instead of forming plexuses, the thoracic nerves pass through the intercostal spaces and innervate regions of the thorax.
The main spinal nerve plexuses innervate the skin and the underlying muscles of the limbs. The brachial plexus, for example, is formed by the last four cervical nerves (C5 to C8) and the first thoracic nerve (T1). The brachial plexus innervates the nerves of the arm, wrist and hand. The lumbar plexus (L2 to L4) and sacral plexus (L5 to S5) contain nerves that innervate the anterior and posterior portions of the lower body, respectively.
THE AUTONOMIC NERVOUS SYSTEM
Although all the components of the nervous system are vital to homeostasis, the autonomic nervous system is probably the most important division to control physiological function throughout the body such that our body systems are in homeostatic balance. The autonomic nervous system is one of the main efferent systems from the peripheral nervous system to the body cells and organs. The autonomic nervous system consists of two main divisions:
A third branch, the enteric nervous system, is specific to the digestive system and is discussed in Chapter 26.
Whereas the other main branch of the peripheral nervous system, the somatic nervous system, innervates skeletal muscles, the autonomic nervous system innervates smooth muscle of internal organs, blood vessels and other parts of the body that are not controlled by conscious thought, such as the pupils of the eye. Interestingly, some afferent information also ascends through this system from the body viscera. However, it is the motor control or efferent function that we will consider in detail. Many neurons of the autonomic nervous system travel in the spinal nerves and certain cranial nerves. The autonomic nervous system coordinates and maintains homeostasis among visceral (internal) organs, such as regulation of cardiac muscle, smooth muscle and the glands of the body. This system is considered an involuntary system because we generally cannot will these functions to happen.
Both divisions of the autonomic nervous system operate using a two-neuron system consisting of preganglionic neurons (myelinated) and postganglionic neurons (unmyelinated). Recall that a ganglion is a collection of cell bodies in the peripheral nervous system. There are two distinctions for each of the autonomic system branches: for the sympathetic nervous system, the ganglia are near the spinal cord; and for the parasympathetic nervous system, the ganglia are near the effector organs. This arrangement contrasts with the somatic nervous system, where a single motor neuron travels from the central nervous system to the innervated muscle. The central nervous system regulates and coordinates the activities of the autonomic nervous system — there are autonomic control areas in the hypothalamus, spinal cord and reticular formation. Central nervous system pathways interconnect all these areas to allow for effective communication and coordination of function.
Anatomy of the sympathetic nervous system
The sympathetic nervous system mobilises energy stores in times of need and is referred to as coordinating the ‘fight or flight’ response. This response should be well known to you already, as it is activated when the body is placed under some threat — for instance, a dog has barked and chased you, or you have been suddenly frightened and been ready to either flee from a situation (flight) or combat the person who has startled you (fight). The sympathetic division is innervated by cell bodies located from the first thoracic (T1) to the second lumbar (L2) regions of the spinal cord and therefore is called the thoracolumbar division (see Figure 6-29). The preganglionic axons of the sympathetic division form synapses shortly after leaving the cord in the sympathetic ganglia or chain. At the sympathetic ganglia, most preganglionic neurons then synapse with their respective postganglionic neuron, which innervates target organs or effectors throughout the body (see Figure 6-30).
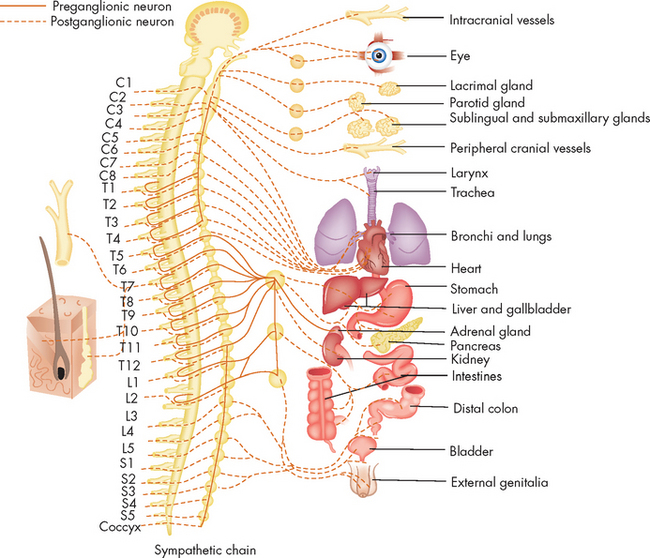
FIGURE 6-29 Sympathetic division of the autonomic nervous system.
Source: Redrawn from Ruby EB (ed.). Advanced neurological and neurosurgical nursing. St Louis: Mosby; 1984.
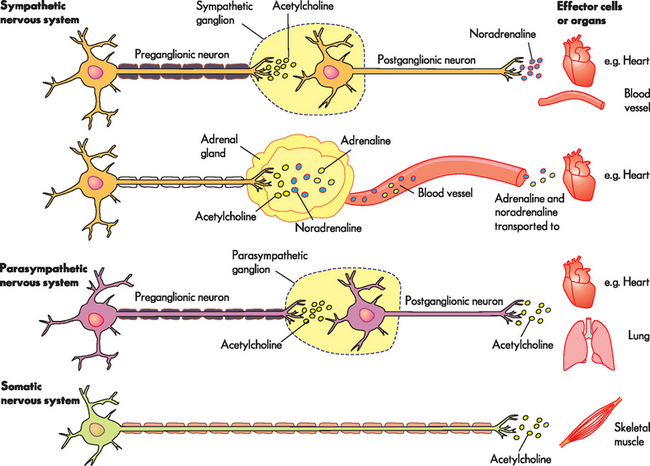
FIGURE 6-30 Comparison of neurotransmitters and receptors between the sympathetic and parasympathetic nervous systems.
Although not part of the autonomic nervous system, the somatic nervous system is included due to its similarity. In each case, the cell body of the first neuron is located in the central nervous system.
Some preganglionic sympathetic neurons innervate the cells of the adrenal medulla, which produce adrenaline and noradrenaline. Because preganglionic sympathetic fibres are myelinated, action potentials travel quickly to the adrenal medulla and innervation causes the release of adrenaline and noradrenaline (see Figure 6-30). It should be noted that when these substances are released by the adrenal medulla into the bloodstream, they are hormones; however, when they are released by neurons and travel across the synapse, they are neurotransmitters despite being exactly the same substance. The effect of neurotransmitters is short-lasting, whereas release of the same substances as hormones allows for longer-lasting effects at the target organs. This enhancement of the sympathetic nervous system by release of hormones is unique: there is no equivalent in the parasympathetic nervous system. It also contributes to the sympathetic nervous system’s role in a body-wide stress response, necessary for the protection of the individual. (The concept of stress and its effects on the body are discussed fully in Chapter 34.)
Anatomy of the parasympathetic nervous system
The parasympathetic nervous system conserves and restores energy and is referred to as the ‘rest and digest’ system. The nerve cell bodies of this division are located in the cranial nerve nuclei and in the sacral region of the spinal cord and therefore constitute the craniosacral division (see Figure 6-31). Unlike the sympathetic branch, the preganglionic fibres in the parasympathetic division are quite long as they travel close to the organs they innervate before forming synapses with the relatively short postganglionic neurons. Parasympathetic nerves arising from nuclei in the brainstem travel to the viscera of the head, thorax and abdomen within cranial nerves, such as the oculomotor (III), facial (VII), glossopharyngeal (IX) and vagus (X) nerves.
Neurotransmitters and receptors
There are distinct differences in the types and actions of the neurotransmitters of the autonomic nervous system. It is very important that you understand these differences, as a variety of drugs used in clinical practice act on these neurotransmitters and receptors. For instance, individuals with asthma often require a drug called salbutamol, which acts on specific receptors in the lungs that when stimulated cause enlargement of the airways (bronchodilation). These receptors are stimulated by the neurotransmitters adrenaline and noradrenaline, and hence salbutamol is called an adrenergic agonist because it mimics the effects of the neurotransmitter adrenaline. Therefore, it is crucial that you understand the neurotransmitters and receptors of the autonomic nervous system.
Neurotransmitters and receptors of the sympathetic nervous system
The sympathetic nervous system has three main neurotransmitters: acetylcholine, adrenaline and noradrenaline. Acetylcholine is released from the sympathetic preganglionic fibres. In contrast, most postganglionic sympathetic fibres release noradrenaline (with some adrenaline as well) and thus are described as being adrenergic. The adrenaline (and noradrenaline) released as hormones by the adrenal medulla have the same effects on target cells, as they bind to the same receptor types. There is one exception: sweat glands that are innervated by the sympathetic nervous system. The postganglionic fibres release acetylcholine to stimulate the release of sweat. The reason for this remains unknown.
Adrenaline and noradrenaline are known together as catecholamines. These catecholamines stimulate two major classes of adrenergic receptors: α-(alpha)adrenergic receptors (α1 and α2); and β-(beta)adrenergic receptors (β1, β2 and β3). The adrenergic receptors are structurally similar to each other, with slight differences that still allow the catecholamines to bind (see Figure 6-32). Cells of the effector organs have particular types of receptors located on their surface; a cell may actually have more than one type of adrenergic receptor (see Table 6-8). α1-adrenergic receptors are found on the smooth muscle of blood vessels, so that when adrenaline or noradrenaline binds to the receptors, it causes contraction of the smooth muscle, resulting in vasoconstriction (a narrowing of the blood vessels). Most of the α-adrenergic receptors on effector organs belong to the α1 class. α2-adrenergic receptors are located in the pancreas to inhibit secretion of insulin.
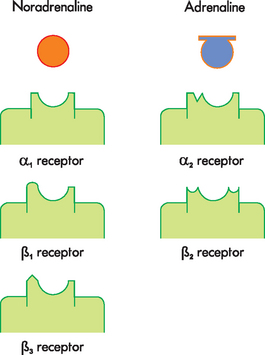
FIGURE 6-32 Schematic representation of adrenergic neurotransmitters and receptors.
Noradrenaline and adrenaline are structurally very similar and both are capable of binding to all adrenergic receptor subtypes (although only some particular receptor types are found on target cells). Pharmacological agents can be targeted to specific receptor subtypes, limiting their interactions and adverse effects at other receptor types on other target cells. (α = alpha; β = beta.)
β1-adrenergic receptors are located mainly on the heart, both in the conduction system and in the muscle of the atria and ventricles. The effects of catecholamines on the heart are increased heart rate and contractility. β1-receptors are also located on the kidney and cause it to release renin (which leads to an increase in blood pressure). β2-adrenergic receptors are located in smooth muscle of the respiratory system and cause bronchodilation; they are also located in other organs, including arterioles and cause vasoconstriction. β3 receptors are located on adipose cells.
Neurotransmitters and receptors of the parasympathetic nervous system
In the parasympathetic nervous system, both the preganglionic and the postganglionic fibres release acetylcholine. Because the neurotransmitter is acetylcholine, these neurons are known as cholinergic.
Acetylcholine works through cholinergic receptors, which are broadly categorised into nicotinic and muscarinic receptors (see Figure 6-33). The nicotinic receptors are located at the first synapse (at the ganglia) of both the sympathetic and the parasympathetic nervous systems. Although it is not a part of the autonomic nervous system, the synapse between neuron and voluntary skeletal muscle (of the somatic nervous system) also has nicotinic receptors (see Figure 6-30). When considering drugs that interact with nicotinic receptors, it is important to remember that these nicotinic receptors are found in both the autonomic and the somatic nervous systems and can affect both systems.
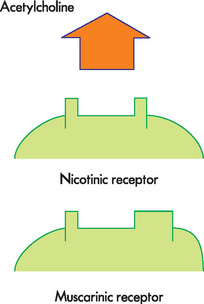
FIGURE 6-33 Schematic representation of cholinergic receptors.
Acetylcholine interacts with nicotinic and muscarinic receptors.
Acetylcholine is the neurotransmitter released at the effector organs of the parasympathetic nervous system, but these effector organs have the muscarinic receptors (see Table 6-8). Muscarinic receptors are located in the heart, and the effects of acetylcholine cause a slowing of the heart rate. Muscarinic receptors are also distributed throughout the body in smooth muscle and glands, and are found within the central nervous system as well as the parasympathetic nervous system.
Physiology of the autonomic nervous system
Many body organs are innervated by both the sympathetic and the parasympathetic nervous systems. The two divisions usually cause opposing responses; for example, sympathetic stimulation of the heart causes increased heart rate, while parasympathetic stimulation slows the heart rate; and whereas the sympathetic nervous system widens (dilates) airways and increases respiratory rate, the parasympathetic nervous system causes airways to narrow (constrict) and the respiratory rate to slow. The sympathetic nervous system does not always increase organ activity, however, as sympathetic stimulation of the stomach causes decreased digestion, whereas parasympathetic stimulation increases digestive activities.
Sympathetic nervous system: functions
In general, sympathetic stimulation promotes responses for the protection of the individual — hence the ‘fight or flight’ response. For example, sympathetic activity increases blood glucose levels and temperature and raises blood pressure.
In emergency situations, a generalised and widespread discharge of the sympathetic system occurs, known as sympathetic mass discharge. This is accomplished by an increased firing frequency of sympathetic fibres and by activation of sympathetic fibres normally at rest (fibres to the sweat glands, pilomotor muscles and the adrenal medulla, as well as vasodilator fibres to muscle).
Regulation of blood vessel tone is considered the single most important function of the sympathetic nervous system, as maintaining adequate blood pressure is essential to ensure appropriate blood flow to organs (Figure 6-34 illustrates some of the most important functions of the sympathetic nervous system). The sympathetic nervous system can actually cause both vasodilation (widening of blood vessels) and vasoconstriction (narrowing of blood vessels) at the same time in vessels of different parts of the body. This marvellous feat allows blood flow to be directed to tissues that need it most — for instance, vasoconstriction limits the amount of blood being supplied to the skin and digestive system, and simultaneous vasodilation in the heart, skeletal muscles and respiratory system maximises blood flow to those areas, thereby increasing their potential performance.
Parasympathetic nervous system: functions
Increased parasympathetic activity promotes rest and tranquillity and is characterised by a reduced heart rate and enhanced and increased digestion. Stimulation of the vagus nerve (cranial nerve X) in the gastrointestinal tract increases peristalsis and secretion, as well as relaxation of the sphincters. The vagus nerve has widespread functions in innervating the organs of the abdomen. Activation of parasympathetic fibres in the head (provided by cranial nerves III, VII and IX) causes constriction of the pupils, tear secretion and increased salivary secretion. Stimulation of the sacral division of the parasympathetic system contracts the urinary bladder and facilitates the process of genital erection.
The parasympathetic system lacks the generalised and widespread response of the sympathetic system. In Table 6-8 you will notice that some areas do not have parasympathetic receptors. For instance, most blood vessels involved in the control of blood pressure are innervated by sympathetic nerves. To decrease blood pressure, therefore, it is more important to block or paralyse the continuous (tonic) discharge of the sympathetic system than to promote parasympathetic activity.
SENSORY FUNCTION
Although the role of neurons and how they conduct action potentials has been discussed throughout the chapter, in this section we focus on the specialised role of a group of sensory neurons that are involved in our sense organs. Different neurons sense different stimuli; sensory neurons are particularly abundant in the skin to sense the external environment and are also located in deeper body tissues to sense the internal environment. In all cases, the sensory neuron converts the stimulus into action potentials, which must travel from the sensory neuron to the central nervous system. Sensory information is interpreted in the brain and reaches the cerebral cortex for stimuli that lead to conscious awareness. In this way, the body monitors itself by providing sensory information to the central nervous system. The most critical sensation is the ability to detect pain, and this is discussed fully in Chapter 7. Finally, you may be wondering where to find a similar focus on the motor function of neurons — this is discussed in the musculoskeletal system (Chapter 20).
Somatosensory function
Touch
Receptors sensitive to touch are present in the skin and are particularly abundant in the fingers and lips. The sensation of touch involves several neuron types, so that a variety of physical stimuli can be sensed and distinguished. In broad terms, these mechanoreceptors sense mechanical stimuli such as light touch, deep pressure, stretching and vibration. Because a sensitive area of the skin, such as the hands, has a large number of mechanoreceptors, it is easily possible to distinguish between these different types of touch stimulus. In addition, most of these sensations evoke affective or emotional responses that determine whether the sensation is unpleasant, pleasant or neutral.
Temperature
Neurons that sense temperature are known as thermoreceptors, with some being specialised to sense cold and others to sense warm. The more extreme the temperature, the more action potentials the neuron will send. Putting your hand into an esky filled with icy water will cause cold thermoreceptors in your skin to send many action potentials to the central nervous system, giving you thermal awareness of the cold water.
Chemical
The chemoreceptors are a diverse group of neurons that detect the proportion of particular chemicals. Obvious examples of chemoreceptors are the ‘taste buds’ on the tongue that detect sour, salty, sweet and bitter, and those located within the nasal cavity that allow you to distinguish scents as diverse as peppermint and putrid in the air.
We also have other chemoreceptors that have a vital role in maintaining normal body function. Chemoreceptors located in blood vessels sense particular substances in the blood such as the proportions of oxygen, carbon dioxide and acid; while chemoreceptors located in the brain detect substances within the cerebrospinal fluid. Detecting these substances is essential, so that any alterations can be corrected to restore homeostasis. We look at the specialised functions of these chemoreceptors in Chapters 22 and 24.
Vision
The eyes are complex sense organs responsible for vision. Within a protective casing, each eye has receptors, a lens system for focusing light on the receptors and a system of nerves for conducting impulses from the receptors to the brain.
The wall of the eye is formed of the sclera, choroid and retina (see Figure 6-35). The sclera is the thick, white, outermost layer. It becomes transparent at the cornea in the central anterior region that allows light to enter the eye. The choroid is the deeply pigmented middle layer that prevents light from scattering inside the eye. The iris, part of the choroid, has a round opening, the pupil, through which light passes. Smooth muscle fibres control the size of the pupil so that it adjusts to bright or dim light, and close or distant vision.
FIGURE 6-35 Internal anatomy of the eye.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The innermost layer of the eye, the retina, contains millions of rods and cones — special photoreceptors that sense light. These synapse with neurons that exit the back of the eye at the optic nerve and project to the brain. There are no photoreceptors where the optic nerve leaves the eyeball; this creates the optic disc, or blind spot. Vision is based on light that enters the eye and projects onto photoreceptors. Rods are used for peripheral and dim light vision, and are most abundant at the periphery. Cones, densest in the centre of the retina, detect colour and detail. Lateral to each optic disc is the fovea centralis, which contains only cones — in order for you to see an object in clear focus, that image must project directly onto the fovea centralis (see Figure 6-35).
As shown in Figure 6-36, nerve impulses pass through the optic nerves to the optic chiasm. The nerves from the inner (nasal) halves of the retinas cross to the opposite side and join fibres from the outer halves of the retinas to form the optic tracts. The fibres of the optic tracts travel to the primary visual cortex in the occipital lobe of the brain.10 Damage to different areas of the optic nerve cause blindness in different visual fields.
FIGURE 6-36 Visual pathways and defects.
Source: Modified from Thompson JM et al. Mosby’s clinical nursing. 5th edn. St Louis: Mosby; 2002.
Light entering the eye is focused on the retina by the lens — a flexible, biconvex, crystal-like structure. The lens divides the anterior chamber into the aqueous and vitreous chambers. Aqueous humour fills the aqueous chamber, helps maintain internal eye pressure and provides nutrients to the lens and cornea. Aqueous humour is secreted by the ciliary processes and reabsorbed into the canal of Schlemm. The vitreous chamber is filled with a gel-like substance called vitreous humour. Vitreous humour helps to prevent the eyeball from collapsing inward.
Six extrinsic eye muscles allow gross eye movements and permit eyes to follow a moving object (see Figure 6-37). The external structures protecting the eye include the eyelids (palpebrae), conjunctiva and lacrimal apparatus. The eyelids are used to control the amount of light reaching the eyes, and the conjunctiva lines the eyelids. Tears released from the lacrimal apparatus bathe the surface of the eye and prevent friction, maintain hydration and wash out foreign substances.
Hearing
The external auditory canal is surrounded by the bones of the cranium. The opening (meatus) of the canal is just above the mastoid process. The air-filled sinuses (called mastoid air cells) of the mastoid process promote conductivity of sound between the external and middle ear.
The ear is divided into three areas: (1) the external ear, (2) the middle ear and (3) the inner ear. While all three structures are involved with hearing, the inner ear has an additional function in equilibrium.
The external ear is composed of the pinna (auricle), which is the visible portion of the ear, and the external auditory canal, a tube that leads to the middle ear (see Figure 6-38). Sound waves entering the external auditory canal hit the tympanic membrane (eardrum) and cause it to vibrate. The tympanic membrane separates the external ear from the middle ear.
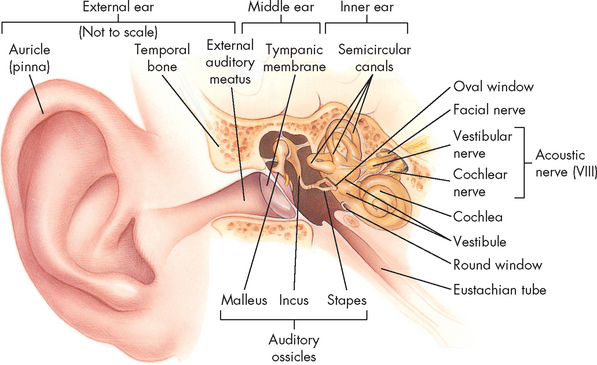
External, middle and inner ears. (Anatomical structures are not drawn to scale.)
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The middle ear is composed of the tympanic cavity, a small chamber in the temporal bone. Three ossicles (small bones known as the malleus, incus and stapes) transmit the vibrations of the tympanic membrane to the inner ear. When the tympanic membrane moves, the malleus moves with it and transfers the vibration to the incus, which passes it on to the stapes. The stapes presses against the oval window, a small membrane of the inner ear. The movement of the oval window sets the fluids of the inner ear in motion (see Figure 6-39).
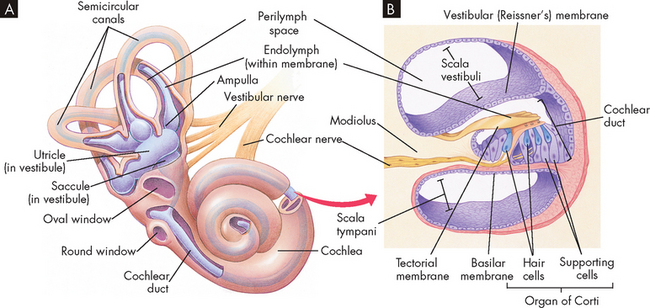
A The bony labyrinth (orange) is the hard outer wall of the entire inner ear and includes the semicircular canals, vestibule and cochlea. Within the bony labyrinth is the membranous labyrinth (purple), which is surrounded by perilymph and filled with endolymph. Each ampulla in the vestibule contains a crista ampullaris that detects changes in head position and sends sensory impulses through the vestibular nerve to the brain. B The inset shows a section of the membranous cochlea. Hair cells in the organ of Corti detect sound and send the information through the cochlear nerve. The vestibular and cochlear nerves join to form the eighth cranial nerve.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The eustachian tube connects the middle ear with the throat. Normally flat and closed, the eustachian tube opens briefly when a person swallows or yawns, and it equalises the pressure in the middle ear with atmospheric pressure. Equalised pressure permits the tympanic membrane to vibrate freely.
The inner ear is a system of osseous labyrinths (bony, mazelike chambers) filled with perilymph. The bony labyrinth is divided into the cochlea, the vestibule and the semicircular canals (see Figure 6-38). Suspended in the perilymph is the endolymph-filled membranous labyrinth.
Within the cochlea is the organ of Corti, which contains hair cells (hearing receptors). Sound waves that reach the cochlea through vibrations of the tympanic membrane, ossicles and oval window set the cochlear fluids into motion. Receptor cells on the basilar membrane are stimulated when their hairs are bent or pulled by the movement. Once stimulated, hair cells transmit impulses along the cochlear nerve (a division of the vestibulocochlear nerve) to the auditory cortex of the temporal lobe in the brain for interpretation of sound.
The semicircular canals and vestibule of the inner ear contain equilibrium receptors. In the semicircular canals the dynamic equilibrium receptors respond to changes in the direction of movement. Within each semicircular canal is the crista ampullaris, a receptor region composed of a tuft of hair cells covered by a gelatinous cupula. When the head is rotated, the endolymph in the canal lags behind and moves in the direction opposite to the head’s movement. The hair cells are stimulated and impulses are transmitted through the vestibular nerve (a division of the vestibulocochlear nerve) to the cerebellum.
The vestibule in the inner ear contains maculae — receptors essential to the body’s sense of static equilibrium. As the head moves, otoliths (small pieces of calcium salts) move in a gel-like material in response to changes in the pull of gravity. The otoliths pull on the gel, which in turn pulls on the hair cells in the maculae. Nerve impulses in the hair cells are triggered and transmitted to the brain. Thus, the ear not only permits the hearing of a large range of sounds but also assists with maintaining balance through the sensitive equilibrium receptors.
Olfaction and taste
Olfaction (smell) is a function of cranial nerve I. Taste (gustation) is a function of multiple nerves in the tongue, soft palate, uvula, pharynx and upper oesophagus innervated by cranial nerves VII and IX. Dysfunctions of smell and taste may occur separately or jointly. The strong relationship between smell and taste creates the sensation of flavour. If either sensation is impaired, the perception of flavour is altered. Olfactory structures are illustrated in Figure 6-40.
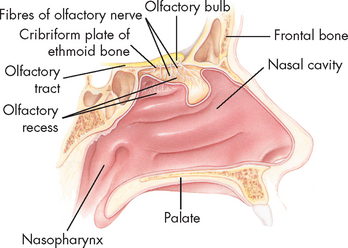
FIGURE 6-40 Olfactory structures.
Midsagittal section of the nasal area shows the location of the major olfactory structures.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Olfactory cells, located in the olfactory epithelium, are the receptor cells for smell. Seven different primary classes of olfactory stimulants have been identified: (1) camphoraceous, (2) musky, (3) floral, (4) peppermint, (5) ethereal (such as essential oils), (6) pungent and (7) putrid. The primary sensations of taste are (1) sour, (2) salty, (3) sweet and (4) bitter. Taste buds sensitive to each of the primary sensations are located in specific areas of the tongue.11
Alterations of sensory function
Visual dysfunction
Alterations in ocular movements
Abnormal ocular movements result from dysfunction of cranial nerves III (oculomotor), IV (trochlear) or VI (abducens), and include nystagmus and strabismus:
Alterations in visual acuity
Visual acuity is the ability to see objects in sharp detail. With advancing age, the lens of the eye becomes less flexible and adjusts slowly, and the sclera changes shape, so that visual acuity declines with age.
 Retinal detachment occurs as fluid accumulates and separates the retina from underlying tissues, and may result from diabetes.
Retinal detachment occurs as fluid accumulates and separates the retina from underlying tissues, and may result from diabetes. Glaucoma is characterised by high intraocular pressures (greater than 12–20 mmHg), usually due to excessive fluid. Fluid may be unable to be circulated or drained adequately. This may occur acutely, with a sudden rise in intraocular pressure causing pain and visual disturbances. Increased pressure on the optic nerve blocks its blood supply, leading to nerve destruction. More than 300,000 Australians have glaucoma, and those with diabetes are at increased risk of developing the condition.12
Glaucoma is characterised by high intraocular pressures (greater than 12–20 mmHg), usually due to excessive fluid. Fluid may be unable to be circulated or drained adequately. This may occur acutely, with a sudden rise in intraocular pressure causing pain and visual disturbances. Increased pressure on the optic nerve blocks its blood supply, leading to nerve destruction. More than 300,000 Australians have glaucoma, and those with diabetes are at increased risk of developing the condition.12 Age-related macular degeneration (AMD) is a severe and irreversible loss of vision and a major cause of blindness in older individuals. Hypertension, cigarette smoking, diabetes mellitus and age over 60 are risk factors. AMD includes abnormal blood vessel growth, leakage of blood or serum, retinal detachment and loss of photoreceptors, resulting in loss of central vision. This may be treated by laser photocoagulation.
Age-related macular degeneration (AMD) is a severe and irreversible loss of vision and a major cause of blindness in older individuals. Hypertension, cigarette smoking, diabetes mellitus and age over 60 are risk factors. AMD includes abnormal blood vessel growth, leakage of blood or serum, retinal detachment and loss of photoreceptors, resulting in loss of central vision. This may be treated by laser photocoagulation.Alterations in refraction
Alterations in refraction are the most common visual problem, and may be due to the focusing power of the lens or structural abnormalities. The major symptoms are blurred vision and headache.
 Myopia, or nearsightedness, occurs when light rays are focused in front of the retina when the person is looking at a distant object (the person can see near objects clearly but distant objects are blurry; see Figure 6-41).
Myopia, or nearsightedness, occurs when light rays are focused in front of the retina when the person is looking at a distant object (the person can see near objects clearly but distant objects are blurry; see Figure 6-41). Hyperopia, or farsightedness, occurs when light rays are focused behind the retina when a person is looking at a near object (the person can see distant objects clearly but close objects are blurry; see Figure 6-41).
Hyperopia, or farsightedness, occurs when light rays are focused behind the retina when a person is looking at a near object (the person can see distant objects clearly but close objects are blurry; see Figure 6-41).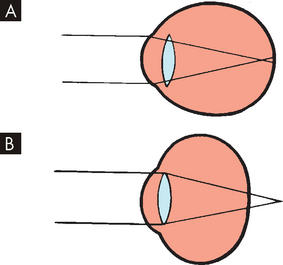
FIGURE 6-41 Alterations in refraction.
A Myopic eye. Parallel rays of light are brought to a focus in front of the retina. B Hyperopic eye. Parallel rays of light come to a focus behind the retina in the unaccommodative eye.
Source: Stein HA, Slatt BJ, Stewin RM. The ophthalmic assistant: fundamentals and clinical practice. 5th edn. St Louis: Mosby; 1998.
Alterations in colour vision
Normal sensitivity to colour diminishes with age because of the progressive yellowing of the lens that occurs with ageing. All colours become less intense, although colour discrimination for blue and green is greatly affected. Colour vision deteriorates more rapidly for individuals with diabetes mellitus than for the general population. Abnormal colour vision may also be caused by colour blindness, an inherited trait. Colour blindness affects 8% of the male population and 0.5% of the female population. Although many forms of colour blindness exist, most commonly the affected individual cannot distinguish red from green.13
Neurological disorders causing visual dysfunction
Vision may be disrupted at many points along the visual pathway, causing various defects in the visual field. Visual changes may cause defects or blindness in the entire visual field or in half of a visual field (hemianopia). Figure 6-36 illustrates the many areas along the visual pathway that may be damaged and the associated visual changes. Injury to the optic nerve causes same-side blindness. Injury to the optic chiasm (the X-shaped crossing of the optic nerves) can cause various defects, depending on the location of the injury.
External eye structure disorders
Infection and inflammatory responses are the most common conditions affecting the supporting structures of the eyes. Conjunctivitis is an inflammation of the conjunctiva caused by bacteria, viruses, allergies or chemical irritants.14
 Acute bacterial conjunctivitis (pinkeye) is highly contagious and often caused by Staphylococcus, Haemophilus, Streptococcus pneumoniae and Moraxella catarrhalis, although other bacteria may be involved.
Acute bacterial conjunctivitis (pinkeye) is highly contagious and often caused by Staphylococcus, Haemophilus, Streptococcus pneumoniae and Moraxella catarrhalis, although other bacteria may be involved. Viral conjunctivitis is caused by an adenovirus. Common symptoms are watering, redness and photophobia.
Viral conjunctivitis is caused by an adenovirus. Common symptoms are watering, redness and photophobia.Preventing spread of the microorganism with hand-washing and use of separate towels is important. Bacterial conjunctivitis is treated with antibiotics.
Auditory dysfunction
Impaired hearing is the most common sensory defect. The major categories of auditory dysfunction are conductive hearing loss, sensorineural hearing loss, mixed hearing loss and functional hearing loss.15 Hearing loss may range from mild to profound. Auditory changes caused by ageing are common and incremental.
Conductive hearing loss
A conductive hearing loss occurs when a change in the outer or middle ear impairs conduction of the sound from the outer ear to the inner ear. Conditions that commonly cause a conductive hearing loss include impacted cerumen (wax) or foreign bodies lodged in the ear canal, eustachian tube dysfunction, otitis media and acute viral otitis media. Symptoms of conductive hearing loss include diminished hearing and the individual speaking softly due to hearing his or her voice, conducted by bone, as loud.
Sensorineural hearing loss
A sensorineural hearing loss is caused by impairment of the organ of Corti or its central connections. Causes include noise exposure, ageing, Ménière’s disease, ototoxicity and diabetes.16 Congenital and neonatal sensorineural hearing loss may be caused by maternal rubella, ototoxic drugs, prematurity, traumatic delivery and congenital hereditary malfunction. Diagnosis is often made when delayed speech development is noted.
Presbycusis is the most common form of sensorineural hearing loss and is especially common in elderly people. Its cause may be atrophy of the basal end of the organ of Corti, loss of auditory receptors, vascular changes or stiffening of the basilar membranes. Drug ototoxicities (drugs that cause destruction of auditory function) have been observed after exposure to alcohol, the antibiotic gentamycin, and other substances. The initial effect is tinnitus (ringing in the ear), followed by a progressive high-tone sensorineural hearing loss that is permanent.
Ménière’s disease
Ménière’s disease is a disorder of the middle ear with an unknown aetiology. There is excessive endolymph and associated pressure that disrupts both vestibular and hearing functions. Recurring symptoms include profound vertigo, nausea and vomiting associated with deafness, and tinnitus (ringing in the ears). Dietary salt has been shown to cause attack of vestibular and hearing alteration, and it appears that stress may also precipitate attacks. Therefore, reduction of dietary salt and diuretics can be used to reduce body salt content. Stress management is also advised. Surgery is used only in severe cases, where symptoms are debilitating. Unfortunately, there is no definitive treatment and the aim of treatment should be to alleviate symptoms, termed symptomatic treatment.17
Ear infections
 Otitis externa is the most common infection of the outer ear and may be acute or chronic.18 The most common cause of acute infections are bacterial microorganisms including Pseudomonas, Escherichia coli and Staphylococcus aureus. Infection usually follows prolonged exposure to moisture (swimmer’s ear). The earliest symptoms are inflammation with pruritus, swelling and clear drainage, progressing to purulent drainage with obstruction of the canal. Acidifying solutions and topical antibiotics are used for treatment.
Otitis externa is the most common infection of the outer ear and may be acute or chronic.18 The most common cause of acute infections are bacterial microorganisms including Pseudomonas, Escherichia coli and Staphylococcus aureus. Infection usually follows prolonged exposure to moisture (swimmer’s ear). The earliest symptoms are inflammation with pruritus, swelling and clear drainage, progressing to purulent drainage with obstruction of the canal. Acidifying solutions and topical antibiotics are used for treatment. Otitis media is the most common infection of infants and children.19 Most children have one episode by 3 years of age. The most common pathogens include Streptococcus pneumoniae and Haemophilus influenzae. Predisposing factors include sinusitis, adenoidal hypertrophy and immune deficiency. Breast-feeding is a protective factor. Recurrent acute otitis media may be genetically determined.20 Acute otitis media is associated with ear pain, fever, irritability, inflamed tympanic membrane and fluid in the middle ear. The tympanic membrane progresses from erythema to opaqueness with bulging as fluid accumulates. Otitis media with effusion is the presence of fluid in the middle ear. Treatment includes symptom management, particularly of pain, with watchful waiting, antimicrobial therapy for severe illness and placement of tympanotomy tubes when there is persistent bilateral effusion and significant hearing loss.21 Complications include mastoiditis, brain abscess, meningitis and chronic otitis media with hearing loss. Persistent middle ear effusions may affect speech, language and cognitive abilities.
Otitis media is the most common infection of infants and children.19 Most children have one episode by 3 years of age. The most common pathogens include Streptococcus pneumoniae and Haemophilus influenzae. Predisposing factors include sinusitis, adenoidal hypertrophy and immune deficiency. Breast-feeding is a protective factor. Recurrent acute otitis media may be genetically determined.20 Acute otitis media is associated with ear pain, fever, irritability, inflamed tympanic membrane and fluid in the middle ear. The tympanic membrane progresses from erythema to opaqueness with bulging as fluid accumulates. Otitis media with effusion is the presence of fluid in the middle ear. Treatment includes symptom management, particularly of pain, with watchful waiting, antimicrobial therapy for severe illness and placement of tympanotomy tubes when there is persistent bilateral effusion and significant hearing loss.21 Complications include mastoiditis, brain abscess, meningitis and chronic otitis media with hearing loss. Persistent middle ear effusions may affect speech, language and cognitive abilities.Olfactory and taste dysfunction
Olfactory dysfunctions can be mild or detrimental. In the person with anosmia — a complete loss of smell — less variety may be incorporated into food choices, which may impact negatively on nutritional status. Malnutrition is a concern in some groups within our community (refer to Chapter 27), and anosmia may contribute to the condition. Of more immediate concern is the inability to smell harmful substances such as fire, chemicals and spoiled foods, which may have a substantial impact on the individual. A less severe condition is hyposmia, an impaired sense of smell.
The sense of taste can be impaired by injury. Altered taste may be attributed to impaired smell associated with injury near the hippocampus. Hypogeusia is a decrease in taste sensation, whereas ageusia is an absence of the sense of taste. These disorders result from cranial nerve injuries and can be specific to the area of the tongue innervated. These conditions can also contribute to the likelihood of developing malnutrition.
GROWTH AND DEVELOPMENT OF THE NERVOUS SYSTEM
Environmental influences have a significant role in nervous system development. Nutrition, hormones, oxygen levels and external stimulation all affect normal growth. The proper proportions of essential nutrients are necessary for proliferation of the nervous system tissue. Maternal lifestyle, nutrition and state of health also have a crucial impact on nervous system development at certain critical periods of maturation.
The growth and development of the brain occurs rapidly during weeks 15–20 of gestation and again at week 30 through the first year of life, reflecting the development and multiplication of neurons. The head is the fastest growing body part during infancy. One half of postnatal brain growth is achieved by the first year and is 90% complete by age 6 years. The cortex thickens with maturation and the sulci deepen as cortical functions develop. Cerebral blood flow and oxygen consumption is about twice that of the adult brain during these years.
The bones of the infant’s skull are separated at the suture lines forming two fontanels or ‘soft spots’: one diamond-shaped anterior fontanel and one triangular-shaped posterior fontanel. The fontanels allow for expansion of the rapidly growing brain. The posterior fontanel may be open until 2–3 months of age; the anterior fontanel normally does not fully close until 18 months of age (see Figure 6-42). Abnormal intracranial conditions, such as those characterised by increased intracranial pressure, may also result in distention or bulging of the fontanels and an increased head circumference in excess of that expected with normal growth. Healthcare providers carefully monitor the fontanels for 2 years and head growth during the first 5 years by measuring head circumference and comparing the results with a standardised growth chart.
FIGURE 6-42 Cranial sutures and fontanels in infancy.
Fibrous union of suture lines and interlocking of serrated edges (occurs by 6 months; solid union requires approximately 12 years).
Human neurological functioning is primarily at a sub-cortical level at birth (impulses are handled by the brainstem and spinal cord). Many reflex patterns mediated by brainstem and spinal cord mechanisms are present at birth and then disappear at predictable times during infancy. Absence of expected reflex responses at the appropriate age indicates general depression of central or peripheral motor functions. Asymmetric responses may indicate lesions in the motor cortex or may occur with fractures of bones after traumatic delivery or postnatal injury. As the infant matures, the neonatal reflexes disappear in a predictable order as voluntary motor functions supersede them. Abnormal persistence of these reflexes is seen in infants with developmental delays or with central motor lesions.
AGEING AND THE NERVOUS SYSTEM
A number of changes occur in the neurological system with ageing. These changes include both structural and functional alterations.22–26 23 24 25 26
Several anatomical changes occur with the normal ageing process. The total amount of brain size and weight decreases, particularly in the frontal region, and there is an accompanying narrowing of the gyri and widening of the sulci, and an increase in the size of the ventricles. The meninges undergo fibrosis and thickening. The permeability of the blood–brain barrier also increases — this may allow the sensitive neurons to be exposed to a greater number of substances, which may be detrimental.
At a cellular level, there is a decrease in the number of neurons — interestingly, this is not consistently related to changes in mental function. Less myelin is observed. The decreased number of dendritic processes and synaptic connections may contribute to less memory, and an imbalance in the amount and distribution of neurotransmitters can lead to functional changes.
In parallel with the structural changes that occur as we age, there are some corresponding changes in neurological function, although these changes vary significantly with individuals. Memory impairments occur, as do sleep disturbances. There is a decrease in neuromuscular control, with a change in gait and posture. Cognitive alterations are also associated with chronic disease.
Finally, progressive deterioration of sensory function accompanies the ageing process. In the eye, there is a decrease in pupil accommodation and colour vision. The cornea becomes thicker and less curved, and the lens thickens and becomes more opaque. There is also a loss of the number of rods in the retina. In the ear, the cochlear hair cells degenerate, and there is a loss of neurons in the spiral ganglia and organ of Corti. A decrease in the sense of vibration results in less hearing ability. A loss of proprioception caused by vestibular dysfunction and neuropathy may increase the risk of falls and injury. After the age of 80, there is a decrease in sensitivity to odours, which may be hazardous if toxic substances cannot be detected. The senses of olfaction and smell and amount of salivation are lessened, which can decrease the enjoyment of eating and contribute to some elderly people preferring simple diets rather than a wide variety of foods.
Organisation of the nervous system
 The structural divisions of the nervous system are the central nervous system and peripheral nervous system. The central nervous system is contained within the brain and spinal cord.
The structural divisions of the nervous system are the central nervous system and peripheral nervous system. The central nervous system is contained within the brain and spinal cord.Cells of the nervous system
 The neuron and neuroglial cells constitute nervous tissue. The neuron is specialised to transmit and receive electrical and chemical impulses, whereas the neuroglial cell provides supportive functions.
The neuron and neuroglial cells constitute nervous tissue. The neuron is specialised to transmit and receive electrical and chemical impulses, whereas the neuroglial cell provides supportive functions. The neuron is composed of a cell body, dendrites and an axon. A myelin sheath around the axon of neurons forms an insulation that allows quicker nerve impulse conduction.
The neuron is composed of a cell body, dendrites and an axon. A myelin sheath around the axon of neurons forms an insulation that allows quicker nerve impulse conduction. A number of different neuroglia are found in the central nervous system, including: astrocytes, which provide physical support; ependymal cells, which produce cerebrospinal fluid; and microglia, which are phagocytes. Oligodendrocytes form the myelin sheath in the central nervous system, while Schwann cells perform this function in the peripheral nervous system.
A number of different neuroglia are found in the central nervous system, including: astrocytes, which provide physical support; ependymal cells, which produce cerebrospinal fluid; and microglia, which are phagocytes. Oligodendrocytes form the myelin sheath in the central nervous system, while Schwann cells perform this function in the peripheral nervous system.The nerve impulse
 Neurons at rest have a stable resting membrane potential. This electrical potential changes during neuronal communication.
Neurons at rest have a stable resting membrane potential. This electrical potential changes during neuronal communication. The action potential occurs when the neuron is stimulated. It consists of sodium influx (depolarisation), followed by potassium efflux (repolarisation), a brief undershoot of potassium and finally restoration of the resting membrane potential.
The action potential occurs when the neuron is stimulated. It consists of sodium influx (depolarisation), followed by potassium efflux (repolarisation), a brief undershoot of potassium and finally restoration of the resting membrane potential. Neurotransmitters are responsible for chemical conduction across the synapse. The neurotransmitter is released from the presynaptic neuron, diffuses across the synapse and binds to receptors on the postsynaptic neuron. This results in a change in the resting membrane potential in the postsynaptic neuron.
Neurotransmitters are responsible for chemical conduction across the synapse. The neurotransmitter is released from the presynaptic neuron, diffuses across the synapse and binds to receptors on the postsynaptic neuron. This results in a change in the resting membrane potential in the postsynaptic neuron.The central nervous system
 The brain is contained within the cranial vault and is divided into four regions: (1) the cerebral hemispheres, (2) the diencephalon, (3) the brainstem and (4) the cerebellum.
The brain is contained within the cranial vault and is divided into four regions: (1) the cerebral hemispheres, (2) the diencephalon, (3) the brainstem and (4) the cerebellum. The cerebral hemispheres have an outer cortex, which allows conscious perception of internal and external stimuli, thought and memory processes, and voluntary control of skeletal muscles. Deep to the cerebral cortex are the cerebral tracts of white matter, which communicate between different regions of the cerebral cortex, and basal nuclei, which function in movement control.
The cerebral hemispheres have an outer cortex, which allows conscious perception of internal and external stimuli, thought and memory processes, and voluntary control of skeletal muscles. Deep to the cerebral cortex are the cerebral tracts of white matter, which communicate between different regions of the cerebral cortex, and basal nuclei, which function in movement control. The centre for voluntary control of skeletal muscle movements is located along the precentral gyrus in the frontal lobe, whereas the centre for perception is along the postcentral gyrus in the parietal lobe. Broca’s area (inferior frontal gyrus) and Wernicke’s area (superior temporal gyrus) are major speech centres.
The centre for voluntary control of skeletal muscle movements is located along the precentral gyrus in the frontal lobe, whereas the centre for perception is along the postcentral gyrus in the parietal lobe. Broca’s area (inferior frontal gyrus) and Wernicke’s area (superior temporal gyrus) are major speech centres. The limbic system consists of neurons from a variety of brain regions and is the important region for control of emotions.
The limbic system consists of neurons from a variety of brain regions and is the important region for control of emotions. The diencephalon, including the thalamus and hypothalamus, processes incoming sensory data. It is an important relay area for sending sensory information on to the cerebral hemispheres.
The diencephalon, including the thalamus and hypothalamus, processes incoming sensory data. It is an important relay area for sending sensory information on to the cerebral hemispheres. The brainstem is divided into the midbrain, pons and medulla oblongata. The midbrain is primarily a relay centre for motor and sensory tracts, as well as a centre for auditory and visual reflexes. The pons is involved in communicating with the cerebellum. The medulla is the most important brain region, in that it contains the control centres for heart rate and breathing. Damage to the medulla is likely to be fatal.
The brainstem is divided into the midbrain, pons and medulla oblongata. The midbrain is primarily a relay centre for motor and sensory tracts, as well as a centre for auditory and visual reflexes. The pons is involved in communicating with the cerebellum. The medulla is the most important brain region, in that it contains the control centres for heart rate and breathing. Damage to the medulla is likely to be fatal. Sleep may be divided into REM and non-REM stages, each of which has its own series of stages. While asleep, an individual progresses through REM and non-REM (slow wave) sleep in a predictable cycle.
Sleep may be divided into REM and non-REM stages, each of which has its own series of stages. While asleep, an individual progresses through REM and non-REM (slow wave) sleep in a predictable cycle. REM sleep is associated with enhanced parasympathetic activity and fluctuating sympathetic nervous system activity. Non-REM sleep is characterised by increased parasympathetic activity and decreased sympathetic activity. Non-REM sleep accounts for 75–80% of sleep.
REM sleep is associated with enhanced parasympathetic activity and fluctuating sympathetic nervous system activity. Non-REM sleep is characterised by increased parasympathetic activity and decreased sympathetic activity. Non-REM sleep accounts for 75–80% of sleep. Restorative, reparative and growth processes occur during sleep. Sleep deprivation can cause profound changes in personality and functioning.
Restorative, reparative and growth processes occur during sleep. Sleep deprivation can cause profound changes in personality and functioning. Sleep is coordinated by the pineal gland, melatonin and the hypothalamus. Arousal from sleep occurs due to the actions of the reticular activating system in the brainstem.
Sleep is coordinated by the pineal gland, melatonin and the hypothalamus. Arousal from sleep occurs due to the actions of the reticular activating system in the brainstem. The cerebellum is involved in the coordination and refinement of skeletal muscle movement that can be performed without conscious control.
The cerebellum is involved in the coordination and refinement of skeletal muscle movement that can be performed without conscious control. The spinal cord consists of an inner grey matter (in a ‘butterfly shape’) and outer white matter. It contains most of the nerve fibres that connect the brain with the periphery.
The spinal cord consists of an inner grey matter (in a ‘butterfly shape’) and outer white matter. It contains most of the nerve fibres that connect the brain with the periphery. Sensory pathways ascend through the spinal cord, while motor pathways descend down the spinal cord. Each pathway will decussate (cross to the other side) between the body periphery and the brain.
Sensory pathways ascend through the spinal cord, while motor pathways descend down the spinal cord. Each pathway will decussate (cross to the other side) between the body periphery and the brain. The central nervous system is protected by the scalp, bony cranium, meninges, vertebral column and cerebrospinal fluid (CSF). This fluid is formed from blood components in the choroid plexuses of the ventricles and is reabsorbed in the arachnoid villi (located in the dural venous sinuses) after circulating through the brain and subarachnoid space.
The central nervous system is protected by the scalp, bony cranium, meninges, vertebral column and cerebrospinal fluid (CSF). This fluid is formed from blood components in the choroid plexuses of the ventricles and is reabsorbed in the arachnoid villi (located in the dural venous sinuses) after circulating through the brain and subarachnoid space. The meninges are thin layers of membrane that cover and provide support. The outermost layer is the dura mater, the middle layer is the arachnoid mater and the pia mater is the layer against the brain tissue.
The meninges are thin layers of membrane that cover and provide support. The outermost layer is the dura mater, the middle layer is the arachnoid mater and the pia mater is the layer against the brain tissue. The blood–brain barrier is provided by tight junctions between the cells of brain capillaries and the astrocytes, which are surrounding supporting cells (neuroglia).
The blood–brain barrier is provided by tight junctions between the cells of brain capillaries and the astrocytes, which are surrounding supporting cells (neuroglia). The paired carotid and vertebral arteries supply blood to the brain and connect to form the circle of Willis. The major branches projecting from the circle of Willis are the anterior, middle and posterior cerebral arteries. Drainage of blood from the brain is accomplished through the venous sinuses and jugular veins.
The paired carotid and vertebral arteries supply blood to the brain and connect to form the circle of Willis. The major branches projecting from the circle of Willis are the anterior, middle and posterior cerebral arteries. Drainage of blood from the brain is accomplished through the venous sinuses and jugular veins.The peripheral nervous system
 The peripheral nervous system relays information from the central nervous system to muscle and effector organs through cranial and spinal nerve tracts arranged in fascicles (multiple fascicles bound together form the peripheral nerve).
The peripheral nervous system relays information from the central nervous system to muscle and effector organs through cranial and spinal nerve tracts arranged in fascicles (multiple fascicles bound together form the peripheral nerve).The autonomic nervous system
 The autonomic nervous system is responsible for maintaining a steady state in the internal environment. Two opposing divisions make up the autonomic nervous system: (1) the sympathetic nervous system responds to stress by mobilising energy stores and prepares the body to defend itself; and (2) the parasympathetic nervous system conserves energy and the body’s resources.
The autonomic nervous system is responsible for maintaining a steady state in the internal environment. Two opposing divisions make up the autonomic nervous system: (1) the sympathetic nervous system responds to stress by mobilising energy stores and prepares the body to defend itself; and (2) the parasympathetic nervous system conserves energy and the body’s resources. The sympathetic nervous system uses adrenaline as a neurotransmitter at the effector organs. This system also innervates the adrenal medulla to cause a release of adrenaline and noradrenaline into the blood to travel throughout the body, thereby complementing the effects of direct neural innervation at target tissues.
The sympathetic nervous system uses adrenaline as a neurotransmitter at the effector organs. This system also innervates the adrenal medulla to cause a release of adrenaline and noradrenaline into the blood to travel throughout the body, thereby complementing the effects of direct neural innervation at target tissues.Sensory function
 Mechanoreceptors detect different types of touch including pressure, stretch and vibration, while thermoreceptors detect warm and cold.
Mechanoreceptors detect different types of touch including pressure, stretch and vibration, while thermoreceptors detect warm and cold. Chemoreceptors in the mouth and nose detect chemicals that contribute to the senses of taste and smell. Vital chemoreceptors within the blood and brain are essential for monitoring oxygen, carbon dioxide and pH.
Chemoreceptors in the mouth and nose detect chemicals that contribute to the senses of taste and smell. Vital chemoreceptors within the blood and brain are essential for monitoring oxygen, carbon dioxide and pH. Proprioception is the position and location of the body and its parts. Proprioceptors are located in the inner ear, joints and ligaments.
Proprioception is the position and location of the body and its parts. Proprioceptors are located in the inner ear, joints and ligaments. The wall of the eye has three layers: the sclera, choroid and retina. The retina contains photoreceptors known as rods and cones that receive light through the lens and then convey signals to the optic nerve and subsequently to the visual cortex of the brain.
The wall of the eye has three layers: the sclera, choroid and retina. The retina contains photoreceptors known as rods and cones that receive light through the lens and then convey signals to the optic nerve and subsequently to the visual cortex of the brain. Alterations in visual acuity can be caused by cataracts, retinal detachment, glaucoma and macular degeneration.
Alterations in visual acuity can be caused by cataracts, retinal detachment, glaucoma and macular degeneration. Conjunctivitis is a common infection of the eye, and can be acute or chronic, bacterial, viral or allergic. Redness, oedema, pain and lacrimation are common symptoms.
Conjunctivitis is a common infection of the eye, and can be acute or chronic, bacterial, viral or allergic. Redness, oedema, pain and lacrimation are common symptoms. The ear is composed of external, middle and inner structures. The external structures are the pinna, auditory canal and tympanic membrane. The tympanic cavity (containing three bones: the malleus, incus and stapes), oval window, eustachian tube and fluid compose the middle ear and transmit sound vibrations to the inner ear.
The ear is composed of external, middle and inner structures. The external structures are the pinna, auditory canal and tympanic membrane. The tympanic cavity (containing three bones: the malleus, incus and stapes), oval window, eustachian tube and fluid compose the middle ear and transmit sound vibrations to the inner ear. The inner ear includes the bony and membranous labyrinths that transmit sound waves through the cochlea to the division of cranial nerve VIII. The semicircular canals and vestibule help maintain balance through the equilibrium receptors.
The inner ear includes the bony and membranous labyrinths that transmit sound waves through the cochlea to the division of cranial nerve VIII. The semicircular canals and vestibule help maintain balance through the equilibrium receptors. Sensorineural hearing loss develops with impairment of the organ of Corti or its central connections.
Sensorineural hearing loss develops with impairment of the organ of Corti or its central connections.Growth and development of the nervous system
 Growth and development of the brain occur most rapidly during fetal development and during the first year of life.
Growth and development of the brain occur most rapidly during fetal development and during the first year of life.Matthew and Vanessa are students. Their lecturer has just announced that the exam scheduled for the following week is quite difficult and so they should all ensure that they study well. Matthew and Vanessa decide to study together and discuss what they have already learned about the nervous system to assist each other’s understanding. First, they form some general definitions and descriptions of the main branches of the nervous system. Next, they spend some time chatting about the differences between an action potential, which travels along the axon, and transmission across the synapse. Matthew has a fairly good understanding of some roles of the main divisions of the brain, while Vanessa can explain the structure of the regions of the spinal cord quite well.
Answer the following questions as though you were participating in the study session with Matthew and Vanessa.