12 THE STRUCTURE AND FUNCTION OF THE IMMUNE SYSTEM
INTRODUCTION
Of all the body systems, the immune system is one of the most complex. Unlike a discrete organ system, such as the pulmonary system, the immune system is not confined to one organ but is spread throughout the entire body. It is composed of several levels, and numerous cell types and molecules work together to provide immune responses. The immune system and its associated responses have many functions, but the most important one is to protect the body from foreign substances. In fact, the word ‘immunity’ is derived from the Latin word immūnitas, literally meaning ‘exemption’, referring to a body that is free from disease.
For a foreign substance such as a microorganism to enter the body it must pass through highly effective physical barriers, such as the skin, and chemical barriers, such as enzymes in gastric fluid that minimise the spread and impact of these agents. These first-line barriers are supplemented by rapidly activated biochemical and cellular responses — commonly referred to as inflammation — that limit damage and initiate repair. However, the most sophisticated component of the system is its ability to mount an adaptive response that specifically targets each foreign substance and uses various mechanisms to neutralise and destroy it. These responses are very powerful and increase the body’s long-term protective capacity against specific and highly dangerous infectious agents.
It is often difficult for students to grasp the structure of the immune system because of the vast assortment of cells and molecules involved in immune responses. Compounding this is the nomenclature — that is, the names designated to various immune components. This nomenclature has changed considerably as more and more components are discovered and the terms are often confusing. In addition, there are many misconceptions about the immune system. For instance, it is not uncommon to hear people comment that a person who has an infection has a ‘weak’ immune system, or that vaccinations are not safe or are linked to various disorders. In reality, the immune system is continually providing protective defences for the body. Moreover, during periods of heightened immune activity, such as when invading microorganisms are causing infection, the immune system is activated further to combat them and assist restoring the body back to homeostasis.
It is only over the last two decades that our understanding of the immune system has been greatly enhanced. However, many aspects are still not fully understood and, despite the sophistication of the immune system and technological improvements in therapies and treatment, the immune system is constantly under threat from foreign substances that may enter our bodies. Therefore, a fundamental and thorough understanding of each component of the immune system is required before looking at how they integrate to protect the body and provide immunity.
Chapters 12–15 explain the structure and function of the immune system, how it responds and how it can be affected by foreign substances. There are separate chapters on inflammation and fever, and infection, because these occur in large numbers of individuals in healthcare facilities, especially hospitalised patients. There is also a separate chapter on alterations to immune function across the life span — that is, factors that cause the immune system to overcompensate and those that attack the immune system directly.
We start by exploring the immune system, which provides insight into how the body protects itself and fights foreign invaders such as infectious microorganisms.
HUMAN DEFENCE MECHANISMS
We need first to define the term foreign substances, because it is used throughout Chapters 12–15 and there are several terms that can be considered under this broad heading. Foreign substances are anything that comes from outside the body and is not considered part of the body. For instance, the most commonly used example of foreign substances is that of infectious microorganisms such as bacteria, viruses, parasites and fungi. However, the term also refers to anything that enters the body and is recognised by the immune system as foreign, such as a transplanted organ. Therefore, the immune system is highly sophisticated at recognising what is foreign.
The human body is continually exposed to a large variety of conditions that can result in damage, such as sunlight, pollutants, agents that can cause physical trauma and infectious agents (bacteria, viruses, fungi and parasites). Damage may also arise from within, such as cancers. The damage may be at the level of a single cell, which can be easily repaired, or at the level of multiple cells, tissues or organs, which can result in severe alterations, leading to disease and potentially death.
The immune system can be divided broadly into two sections that operate on different levels but work cooperatively to protect the body from foreign substances:
 Innate immunity — also called natural, native or non-specific immunity — is a natural resistance that is present at birth and protects the individual by providing a natural barrier, activating cells and molecules that limit and destroy the capacity of foreign substances to enter and spread throughout the body.
Innate immunity — also called natural, native or non-specific immunity — is a natural resistance that is present at birth and protects the individual by providing a natural barrier, activating cells and molecules that limit and destroy the capacity of foreign substances to enter and spread throughout the body. Adaptive immunity — also called acquired and specific immunity — develops as the individual is exposed to foreign substances. It is a slower performing system than the innate system, yet is more powerful and precise, as it selectively targets foreign substances that it has identified.
Adaptive immunity — also called acquired and specific immunity — develops as the individual is exposed to foreign substances. It is a slower performing system than the innate system, yet is more powerful and precise, as it selectively targets foreign substances that it has identified.While adaptive immunity is commonly referred to as the immune response, the two forms of immunity should be considered as an overall system that provides protection, because without innate immunity, adaptive immunity would not be able to protect the body.
Innate immunity
Innate immunity includes all components of the immune system except those that can specifically recognise and remember foreign substances that have been encountered. These are shown in Figure 12-1. Innate immune responses are nonspecific; that is, they provide a generalised response to foreign substances. The system does not need to identify what a specific particle is in order to be activated. Following on from this, the innate immune system does not remember a particular foreign substance, because it never identifies it in the first place.
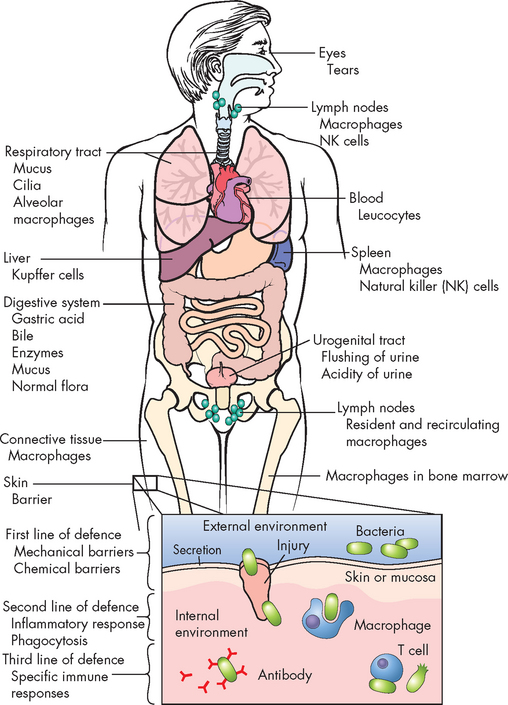
FIGURE 12-1 Innate immunity throughout the body.
This includes the first line of defence, physical barriers (skin, mucous membranes) and the second line of defence, inflammation and phagocytosis. If a foreign substance enters the system, second-line defence mechanisms are activated to limit the spread.
Source: Based on Damjanov I. Pathology for the health professions. 3rd edn. Philadelphia: Saunders; 2006.
Innate immunity consists of two levels of protection:
 The skin and mucous membranes form a physical barrier that is often referred to as the first line of defence — in most instances this physical barrier successfully prevents the foreign substance from actually penetrating further into the body. This first line of defence is remarkably effective and when it is damaged can leave the body vulnerable to invasion (for example, a skin wound becoming infected).
The skin and mucous membranes form a physical barrier that is often referred to as the first line of defence — in most instances this physical barrier successfully prevents the foreign substance from actually penetrating further into the body. This first line of defence is remarkably effective and when it is damaged can leave the body vulnerable to invasion (for example, a skin wound becoming infected). The second line of defence consists of a range of cells, chemicals and processes that are activated if a foreign substance has broken through the first line of defence. These include the phagocytes and natural killer cells (cellular components) and cytokines (signalling proteins), as well as inflammation and fever (see Chapter 13).
The second line of defence consists of a range of cells, chemicals and processes that are activated if a foreign substance has broken through the first line of defence. These include the phagocytes and natural killer cells (cellular components) and cytokines (signalling proteins), as well as inflammation and fever (see Chapter 13).The following sections look at the components of innate immunity.
Epithelial barriers
Epithelial cells are those that line cavities and the body surface, such as the skin. The physical barriers that cover the external parts of the human body offer considerable protection from damage and invasion by foreign substances. The external body covering of the skin consists of tightly associated epithelial cells and this is continuous with the mucous membranes that line the gastrointestinal, respiratory and genitourinary tracts. When foreign substances attempt to penetrate these physical barriers, they may be removed by mechanical means — that is, sloughed off with dead skin cells as they are routinely replaced, expelled by coughing or sneezing, vomited from the stomach or flushed from the urinary tract by urine. Epithelial cells of the upper respiratory tract also produce mucus and have hair-like cilia that trap and move foreign substances upwards to be expelled by coughing or sneezing (see Chapter 24). Additionally, the low temperature on the body’s surface (which is typically lower than the core temperature) generally inhibits microorganisms, most of which routinely require temperatures near 37°C for efficient growth.
Epithelial surfaces also secrete substances intended to trap or destroy pathogens. Mucus, sweat, saliva, tears and cerumen (earwax) are all examples of biochemical secretions that can trap potential invaders and contain substances that will kill microorganisms. In addition, sweat, tears and saliva contain an enzyme (lysozyme) that attacks the cell walls of gram-positive bacteria. Sebaceous glands in the skin also secrete fatty acids and lactic acid that kill bacteria and fungi. These glandular secretions create an acidic (pH 3 to 5) and inhospitable environment for most bacteria. Epithelial cells secrete small molecular weight proteins, generically termed antimicrobial peptides, which are toxic to bacteria, fungi and viruses.
A spectrum of non-pathogenic bacteria, collectively known as normal bacterial flora, resides on the body’s surfaces. The normal flora contributes to our innate protection against microorganisms that cause disease — these are pathogens. Colonisation of the lower gut by normal flora begins quickly after birth and the number and concentration of microorganisms increases progressively during the first year of life. Many of these microorganisms help digest fatty acids, large polysaccharides and other dietary substances; produce vitamin K; and assist in the absorption of various ions, such as calcium, iron and magnesium. They also produce chemicals that inhibit colonisation by pathogens. When individuals are exposed to antibiotic treatment this can alter the normal intestinal flora, decreasing its protective activity, and allow an overgrowth of pathogenic microorganisms, such as the yeast Candida albicans or the bacteria Clostridium difficile. This is often the cause of diarrhoea in individuals on antibiotics. The bacterium Lactobacillus is a major constituent of the normal vaginal flora in healthy women. This microorganism produces chemicals (hydrogen peroxide, lactic acid and other molecules) that help prevent infections of the vagina and urinary tract by other bacteria and yeast. Diminished colonisation with lactobacilli (e.g. as a result of prolonged antibiotic treatment) increases the risk for urinary tract infections or vaginal infections.
Cellular components
Phagocytes
The word phagocyte comes from the Greek word phagein meaning to eat and cyte referring to cells. Therefore, these cells consume other cells or foreign substances. Specifically, phagocytes engulf pathogens and debris, such as dead or damaged cells that are no longer required. Phagocytosis refers to the process of consuming cells and is described in detail in Chapter 13.
The phagocytes are types of white blood cells, or leucocytes. There are five types of white blood cells and all of these are required for normal immunity. The phagocytes consist of two types of white blood cells: neutrophils and monocytes. These cells originate from bone marrow and are released into the blood, then move into the tissues when required. Neutrophils are the first-line defenders of the immune system and move rapidly to the site of infection in large numbers and initiate phagocytosis. Monocytes circulate in the blood for about 3 days and are considered an immature form of white blood cell. However, when monocytes migrate into the tissues to provide immune-fighting capabilities, they become macrophages. These supplement and eventually replace the neutrophils and macrophages can survive for longer periods of time. Collectively, monocytes and macrophages are referred to as the mononuclear phagocyte system and are located in diverse sites throughout the body (see Figure 12-1). These cells are described in more detail in Chapter 13 as they are intricately tied to inflammation.
Natural killer cells
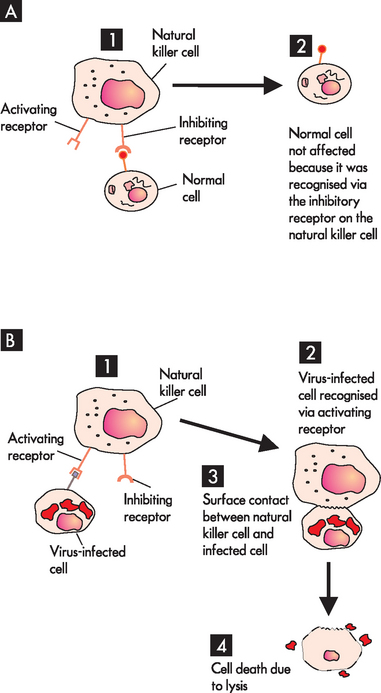
The main function of natural killer cells, often abbreviated to NK cells, is to recognise and eliminate cells infected with viruses and abnormal host cells, specifically cancer cells. They are derived from a type of white blood cell called a lymphocyte (see Chapter 16 for blood cell types). This is important because there are two other lymphocytes and these are the primary cells of adaptive immunity. As the name implies, natural killer cells have the ability to kill cells that are abnormal without the need for specific recognition via adaptive immunity. Natural killer cells have receptors that allow them to recognise differences between infected or tumour cells and normal cells (see Figure 12-2). If a natural killer cell binds to a target cell through activating receptors, it produces several molecules that can kill the target.
Chemical mediators
Cytokines
Apart from the cellular components of innate immunity, there are numerous chemical mediators that enable the immune and inflammatory cells to function more efficiently and assist in their coordination. Collectively, these chemical mediators are referred to as cytokines. The word cytokine is derived from the Greek word cyto meaning cell and kinos meaning movement; therefore, cytokines are molecules that affect other cells (termed cell signalling).
The classification of cytokines has been problematic. Difficulties arose because cytokines have similar biological and structural functions to hormones, but unlike hormones, cytokines can act on the cell that releases them (autocrine) and on nearby cells (paracrine) yet not on distant cells (endocrine). In addition, the term interleukin was used initially to describe cytokines as these chemicals target leucocytes. However, many different cells secrete chemicals and are targets of cytokines: the terms used to describe them arose primarily from the cell responsible — for example, monokines (monocytes), lymphokines (lymphocytes) and chemokines (chemical attractors). The naming system now in use is that cytokines are signalling molecules, mainly proteins that are involved in nearly all aspects of immunity and inflammation. They are integral to promoting growth, differentiation of cells and activating functions that aid in regulation of immunity. At least 70 have been identified and the number is climbing as more signalling molecules are discovered.1
The majority of important cytokines are classified as interleukins or interferons (see Table 12-1), although some critical cytokines are not classified as either. Many of these same cytokines are produced by cells of the adaptive immune system in response to specific antigens and are discussed later in this chapter.
Table 12-1 KEY CYTOKINES AND RECEPTORS THAT INFLUENCE THE IMMUNE RESPONSE
| CYTOKINE | PRIMARY SOURCE | PRIMARY FUNCTION |
|---|---|---|
| Interleukins (IL) | ||
| IL-1 | APCs | Stimulates T cells to proliferation and differentiation; induces acute phase proteins in inflammatory response; endogenous pyrogen |
| IL-2 | TH1 cells, NK cells | Stimulates proliferation and differentiation of T cells and NK cells |
| IL-4 | TH2 cells, mast cells | Induces B-cell proliferation and differentiation; up-regulates MHC class II expression; induces class-switch to IgE |
| IL-5 | TH2 cells, mast cells | Induces eosinophil proliferation and differentiation; induces B-cell proliferation and differentiation |
| IL-6 | TH2 cells, APCs | Induces B-cell proliferation and differentiation; induces acute phase proteins in inflammatory disease |
| IL-7 | Thymic epithelial cells, bone marrow stromal cells | Major cytokine for induction of B- and T-cell proliferation and differentiation in the central lymphoid organs |
| IL-8 | Macrophages | Chemotactic factor for neutrophils and T cells |
| IL-10 | TH cells, B cells | Inhibits cytokine production; activator of B cells |
| IL-12 | B cells, APCs | Induces NK-cell proliferation; increase production of IFN-γ |
| IL-13 | TH2 cells | IL-4-like properties; decreases inflammatory responses |
| Interferons (INF) | ||
| IFN-α, IFN-β | Macrophages, some virally infected cells | Antiviral; increases expression of MHC class I; activates NK cells |
| IFN-γ | TH1 cells, NK cells, Tc cells | Increases expression of MHC class II; activates macrophages and NK cells |
| Tumour necrosis factor (TNF) | ||
| TNF-α (cachectin) | Macrophages | IL-1-like properties; induces cellular proliferation |
| TNF-β (lymphotoxin) | Tc cells | Kills some cells; increases phagocytosis by macrophages and neutrophils |
| Transforming growth factor (TGF) | ||
| TGF-β | Lymphocytes, macrophages, fibroblasts | Chemotactic for macrophages; increases macrophage IL-1 production; stimulates wound healing |
APCs = antigen-presenting cells; TH cells = helper T cells; NK cells = natural killer cells; Tc cells = cytotoxic T cells; MHC = major histocompatibility complex.
Source: McCance KL. Pathophysiology: the biologic basis for disease in adults and children. 5th edn. St Louis: Mosby; 2005.
Interferons
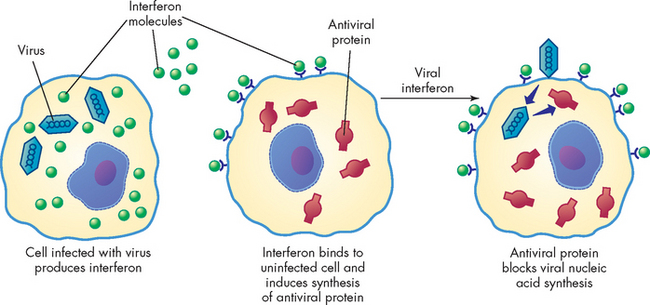
Interferons are a family of cytokines that protect against viral infections. Different types of cells produce different kinds of interferons: macrophages and other cells are the primary producers of both interferon alpha (IFN-α) and interferon beta (IFN-β), whereas lymphocytes release interferon gamma (IFN-γ). Interferon alpha and interferon beta induce the production of antiviral proteins, thereby conferring protection on uninfected cells (see Figure 12-3). Interferon gamma enhances the inflammatory response by increasing the activity of macrophages. This cytokine also facilitates the development of the acquired immune response against viral antigens on infected cells.
Although we have examined several components of innate immunity, these are discussed in more detail in our discussion of the inflammatory response in Chapter 13. Inflammation forms the second line of defence when foreign substances have penetrated the epithelial barriers; it is also a major component of innate immunity and is therefore crucial to your understanding of the body’s defences. Furthermore, the components of innate immunity cross over to assist and work with components of adaptive immunity and are essential to coordinated defence responses.
Adaptive immunity
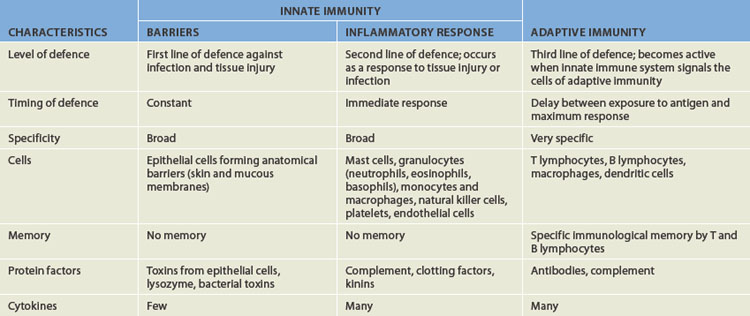
The third line of defence is adaptive immunity, often called the immune response. It is specific and has memory, so that it can confer permanent or long-term protection against particular foreign substances, such as microorganisms. Many components of innate resistance are necessary for the development of the adaptive immune response. Conversely, products of the adaptive immune response protect the individual by activating components of innate resistance. Thus, both systems are essential for complete protection against foreign substances. The two systems are compared in Table 12-2.
The main defining characteristic of adaptive immunity is the ability to specifically recognise various foreign substances and to remember them. This is very important because it accelerates the immune response the next time that a particular foreign substance is encountered. It should be noted that adaptive immunity, while very powerful and targeted, responds more slowly compared to innate immunity (namely, the inflammatory processes). Nonetheless, when activated, adaptive immunity is highly specific at protecting the body. The first aspect that we look at is how the immune system differentiates between normal and foreign cells.
Antigens
The immune system is continually challenged by a spectrum of substances. One of the primary features of adaptive immunity is the ability to recognise ‘self’ from ‘non-self’ — that is, to recognise normal body cells and tissues from what is foreign.
Each cell in the body can be identified as self. These cells are not normally attacked by the immune system because they are recognised as self and so immune responses are not triggered. The cells in the body contain a marker (self-marker) that consists of molecules (proteins) of the major histocompatibility complex (MHC). There are two classes: MHC class I and MHC class II. MHC class I proteins are found on all cells in the body with a nucleus and can inform the immune system when they are infected or are cancerous. This can be seen in how natural killer cells respond to infected cells (refer to Figure 12-2). MHC class II proteins are found on cells that aid the immune system to recognise what is foreign. The cells that contain class II proteins are called antigen-presenting cells and they are vital to initiating immune responses;2 they are discussed later in the chapter.
The immune system recognises non-self cells via antigens. Simply put, antigens are the substances that determine whether an immune response will be initiated — they are identifiers for the immune system. Non-self cells can be infectious microorganisms, such as bacteria, viruses, fungi and parasites, or non-infectious agents, such as pollen and foods. Furthermore, drugs and other body tissues and fluids contain antigens that can be identified as foreign. That is why organ transplants are recognised as foreign, and a transplant recipient must be medicated with immunosuppressive drugs that dampen the usual immune response. The antigen can be the whole non-self cell or a protein on the foreign body surface.
A useful analogy to understand antigens is that of personal characteristics that we use to identify individuals. For instance, say you are meeting someone you have not met before. They inform you that they have blonde hair. When you go to meet them, you search for someone with blonde hair, but see only black-haired individuals. After some time, you spot a blonde-haired individual and greet them. In this example, self are analogous with black hair, as you ignored them, and blonde hair is similar to an antigen. You identified the individual by their hair colour (antigen) and made contact with them. Similarly, the immune system identifies what is foreign and what is self, and accordingly identifies what action (or not) needs to be taken.
To be antigenic, part of a molecule’s chemical structure must be recognised and become bound to an antibody or to specific receptors on a lymphocyte in a lock-and-key manner. The precise area of the molecule that is recognised is called its antigenic determinant, or epitope. The size of an antigenic determinant is relatively small, perhaps just a few amino acids or simple sugars. A large molecule (e.g. protein, polysaccharide, nucleic acid) usually contains multiple and diverse antigenic determinants. Thus, the immune response against the molecule may consist of a mixture of specific antibodies against several of these determinants.
Certain criteria influence the degree to which an antigen is immunogenic (its ability to induce an immune response). These include: (1) foreignness to the host; (2) adequate size; (3) adequate chemical complexity; and (4) being present in sufficient quantity. These criteria are important for the development of vaccines, which must be highly immunogenic to produce protective immune responses against pathogenic microorganisms.
An antigen that fulfils all four criteria except foreignness is considered a self-antigen and does not normally elicit an immune response. Thus, most individuals are tolerant to their own antigens. Some pathogens are successful because they develop the capacity to mimic self-antigens and avoid inducing an immune response. In Chapter 15, we discuss specific diseases resulting from a breakdown of tolerance that leads to an individual’s immune system attacking its own antigens (these are autoimmune diseases).
Molecular size also contributes to an antigen’s immunogenicity. In general, large molecules such as proteins, polysaccharides and nucleic acids are most immunogenic. Smaller molecules such as amino acids, monosaccharides and fatty acids tend to be unable to induce an immune response. Many molecules in this size range can function as haptens: that is, antigens that are too small to activate the immune system by themselves but become immunogenic after combining with larger molecules that function as carriers for the hapten. For example, the antigens of poison ivy are haptens, but they initiate allergic responses in individuals after binding to larger proteins in the skin. Antigens that induce an allergic response are also called allergens.
Even if an antigen fulfils all these criteria, the quality and intensity of the immune response may still be affected by a variety of additional factors. For example, the route and vehicle of antigen entry or administration are critical to the immunogenicity of some antigens. This has important clinical implications. The most common routes for clinical administration of antigens in the form of vaccines are intravenous, intraperitoneal, subcutaneous, intranasal and oral. Each route preferentially stimulates a different set of lymphocyte-containing (lymphoid) tissues and therefore results in the induction of different types of immune responses. For some vaccines, the route may affect the protectiveness of the immune response, so that the individual is protected if immunised by one route, but may be less protected if administered through a different route (e.g. oral versus injected polio vaccines).
Immunogenicity of an antigen also may be altered by being delivered along with substances that stimulate the immune response; these substances are known as adjuvants. Finally, the genetic make-up of the individual can play a critical role in the immune system’s ability to respond to many antigens. Some individuals appear to be unable to respond to immunisation with a particular antigen, whereas they respond well to other antigens. For instance, a small percentage of the population may fail to produce a measurable immune response to a common vaccine, despite multiple injections. An individual’s immune response can be affected by the person’s age, nutritional status, genetic background and reproductive status, as well as by exposure to traumatic injury, concurrent disease or the use of immunosuppressive medications.
Cells of the immune system
The immune response involves several different cells that can be broadly divided into lymphocytes and antigen-presenting cells. The lymphocytes account for approximately 30% of all white blood cells. Lymphocytes are produced from stem cells, called haematopoietic stem cells, which are involved in the production of all blood cells. These are further differentiated into lymphoid stem cells that are responsible for the development of two types of lymphocytes: B lymphocytes (B cells), which produce antibodies that enter the blood and react with the antigen; and the T lymphocytes (T cells), which attack the antigen directly. Both cells are extremely specific, so that each individual B or T cell recognises only one specific antigen. In contrast, antigen-presenting cells are responsible for ingesting antigens and presenting them to the T cell lymphocytes to induce an immune response. The cells are summarised in Figure 12-4.
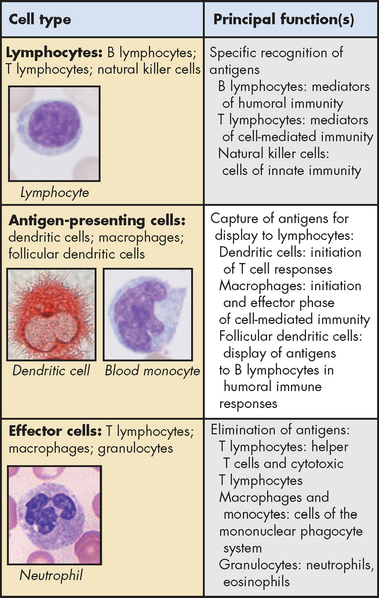
FIGURE 12-4 Principal cells of the immune system.
The major cell types involved in immune responses and their functions are shown. Micrographs in the left panels illustrate the morphology of some of the cells of each type.
Source: Townsend CM. Sabiston textbook of surgery. 18th edn. Philadelphia: Saunders; 2007. From Abbas A, Lichtman AH. Basic immunology: functions and disorders of the immune system. 2nd edn. Philadelphia: Elsevier; 2006.
Lymphocytes
The immune response occurs in two phases. Immature lymphocytes are produced in the primary (central) lymphoid organs (see Figure 12-5) and, when matured into B- and T-cell lymphocytes, have the ability to react against almost any antigen that will be encountered throughout life. It is estimated that B and T cells can collectively recognise millions of different antigenic determinants. When the individual is exposed to an antigen this induces a process that provides further development of only a small group of specialised B and T cells against that particular antigen.
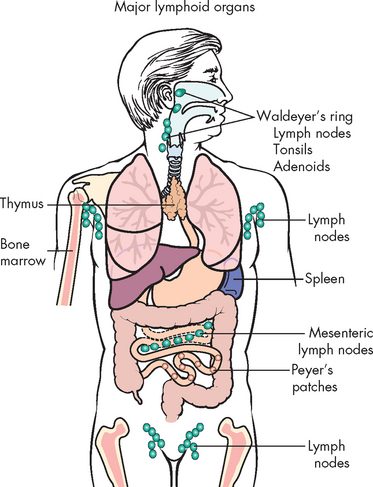
FIGURE 12-5 Lymphoid tissue throughout the body.
Immature lymphocytes migrate through central (primary) lymphoid tissues: the bone marrow (central lymphoid tissue for B lymphocytes) and the thymus (central lymphoid tissue for T lymphocytes). Mature lymphocytes later reside in the T and B lymphocyte-rich areas of the peripheral (secondary) lymphoid tissues.
Source: Based on Damjanov I. Pathology for the health professions. 3rd edn. Philadelphia: Saunders; 2006.
B Lymphocytes
The classification of lymphocytes as either B or T is an unusual one. The letter B derives from an organ in birds called the bursa of Fabricius. This was found to be responsible for the maturation of B lymphocytes. Humans have no discrete bursa, but the bone marrow makes up the human bursal equivalent and serves as the primary lymphoid organ (see Figure 12-5) for B cell development.
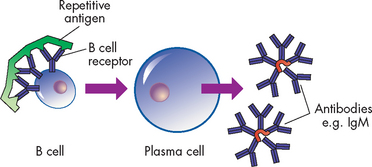
Lymphocytes destined to become B cells circulate through the bone marrow, where they are exposed to hormones that, without the presence of antigen, induce proliferation and differentiation into B cells. Each B cell, however, responds to only one specific antigen. They exit the bone marrow and take up residence in other lymphoid organs (secondary lymphoid organs) as immunocompetent (mature) B cells, which means that they have the ability to develop an immune response but have not been activated. Once exposed to an antigen, B lymphocytes can differentiate into plasma cells and memory cells (see Figure 12-6). Plasma cells are fully differentiated B cells and secrete antibodies, which bind to the antigens to produce an immune response. Memory cells are long-living cells that remain inactive until subsequent exposure to the same antigen.3 Upon subsequent exposure to that particular antigen, memory cells do not require much further differentiation and rapidly become new plasma cells (see Figure 12-7).
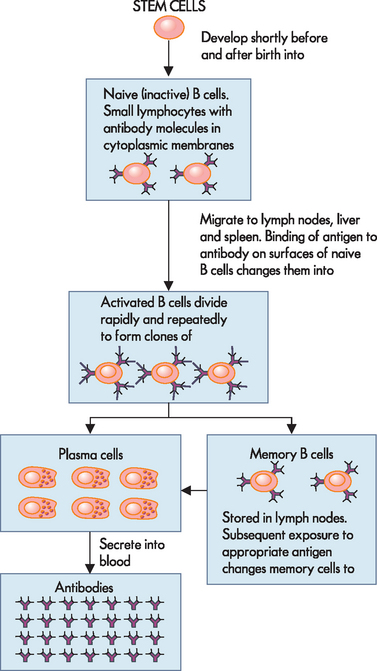
FIGURE 12-6 Differentiation of B cells.
Stem cells produce naive B cells that migrate to the secondary lymphoid tissues and bind to an antigen to become activated B cells. These mature into plasma cells, which secrete antibodies specific for the antigen, and memory cells, which accelerate the process when re-exposed to that specific antigen.
Source: Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
T Lymphocytes
The process of T cell proliferation and differentiation is similar to that for B cells.4 The primary lymphoid organ for T cell development is the thymus (see Figure 12-5). Lymphoid stem cells journey through the thymus, where, under the guidance of thymus hormones and without the presence of antigen, they are driven to undergo cell division and simultaneously produce receptors against the diversity of antigens the individual will encounter throughout life. They exit the thymus through the blood vessels and lymphatics as mature (immunocompetent) T cells with antigen-specific receptors on the cell surface and take up residence in secondary lymphoid organs (see Figure 12-5).
There are several different subsets of T cells including: memory cells with similar functions to the B memory cells; regulatory T cells (also called suppressor cells), which help regulate T cell immunity; cytotoxic T cells (also called killer T cells), which directly attack antigenic cells; and helper T cells, which assist the activation of both B and T cells. All these cells are discussed later in the chapter when exploring how the immune response is activated and coordinated.
Antigen-presenting cells
The other cells that are required by the immune system are cells that present antigens to lymphocytes such that an immune response can be initiated. Antigens that enter the bloodstream or lymphatics encounter a variety of antigen-presenting cells. These include macrophages, dendritic cells and B cells. These cells are responsible for engulfing the antigen, breaking it up and presenting antigenic fragments to the T cells.5 Dendritic cells come from bone marrow and are similar to macrophages. They have extensive projections, similar to dendrites of neurons, and are located throughout the body. In addition, B cells can also be antigen-presenting cells. Therefore, B cells produce plasma and memory cells; however, when activated by helper T cells, B cells can present antigens to the T cells to induce an immune response.
HUMORAL AND CELL-MEDIATED IMMUNITY
The adaptive immune response has two arms: antibodies and T cells, both of which protect against infection (see Figure 12-8).2 Antibodies circulate in the blood and bind to antigens on infectious agents. This interaction can result in either the direct inactivation of the microorganism or the activation of a variety of inflammatory mediators that will destroy the pathogen. Antibodies are primarily responsible for protection against many bacteria and viruses. This arm of the immune response is termed humoral immunity. Humoral pertains to body fluids and in this case refers to the production and secretion of antibodies that circulate in those body fluids.
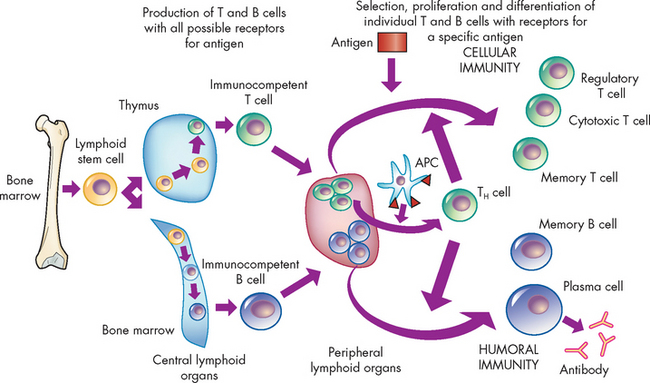
FIGURE 12-8 An overview of the adaptive immune response.
The immune response arises from cellular divisions and differentiation stages resulting in either immunocompetent T cells from the thymus or immunocompetent B cells from the bone marrow. These cells have never encountered foreign antigen. The immunocompetent cells enter the circulation and migrate to the secondary lymphoid organs (e.g. spleen and lymph nodes), where they take up residence in B and T cell-rich areas. When exposed to a foreign antigen the cells commence differentiating into the different cell lines. The antigen is usually processed by antigen-presenting cell (APC) for presentation to helper T cells (TH cells). The intercellular cooperation among antigen-presenting cells, helper T cells and immunocompetent T and B cells results in a second stage of cellular proliferation and differentiation, which results in an active cellular immunity or humoral immunity, or both. Cellular immunity is mediated by a population of ‘effector’ T cells that can kill targets (cytotoxic T cells) or regulate the immune response (regulatory T cells), as well as a population of memory T cells that can respond more quickly to a second challenge with the same antigen. Humoral immunity is mediated by a population of soluble proteins (antibodies) produced by plasma cells and by a population of memory B cells that can produce more antibody rapidly to a second challenge with the same antigen.
Lymphocyte T cells also undergo differentiation during an immune response and develop into several subpopulations of effector T cells that react directly with antigen on the surface of foreign substances or infectious agents (see Figure 12-4). These subpopulations of T cells aid the immune response and are termed cell-mediated immunity, because the immune response is carried out by cells.
The success of an acquired immune response depends on the functions of both the humoral and the cellular-mediated responses, as well as the appropriate interactions between them.
Humoral immune response
The primary functions of B cell differentiation are to (1) produce plasma cells that secrete antibodies in response to antigens and (2) to produce memory cells that accelerate the process when that particular antigen is encountered again. Therefore, to ensure adequate understanding of the humoral immune response, the types and effects of antibodies need to be understood.
Antibodies
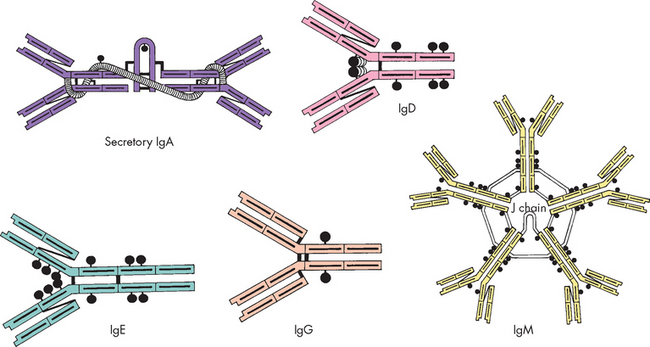
Antibodies, also called immunoglobulin (Ig), are protein compounds found in body fluids that are produced by mature B cells (plasma cells) in response to a challenge by an antigen. The term immunoglobulin is used for all molecules that are known to have specificity for antigen, whereas antibody is generally used to denote one particular set of immunoglobulins known to have specificity for a particular antigen. There are five classes of immunoglobulins (IgG, IgA, IgM, IgD and IgE), which are characterised by antigenic, structural and functional differences (see Figure 12-9).
 IgG is the most abundant class, constituting up to 80% of the immunoglobulin in the blood and accounting for most of the protective activity against infections. As a result of selective transport across the placenta, maternal IgG is the major class of antibody found in the blood of the fetus and newborn.
IgG is the most abundant class, constituting up to 80% of the immunoglobulin in the blood and accounting for most of the protective activity against infections. As a result of selective transport across the placenta, maternal IgG is the major class of antibody found in the blood of the fetus and newborn. IgA is found in the blood and body fluids such as tears, saliva and digestive fluids. It accounts for about 10–15% of all antibodies. IgA is important in preventing infectious microorganisms from attaching to epithelial barriers. A small number of individuals do not produce IgA, but the reasons for this are unknown.
IgA is found in the blood and body fluids such as tears, saliva and digestive fluids. It accounts for about 10–15% of all antibodies. IgA is important in preventing infectious microorganisms from attaching to epithelial barriers. A small number of individuals do not produce IgA, but the reasons for this are unknown. IgM is found in the blood, is the largest of the immunoglobulins and accounts for 5–10% of antibodies. It is the first antibody produced during the initial, or primary, response to antigen. IgM is produced early in neonatal life and its synthesis may be increased as a response to infection in utero.
IgM is found in the blood, is the largest of the immunoglobulins and accounts for 5–10% of antibodies. It is the first antibody produced during the initial, or primary, response to antigen. IgM is produced early in neonatal life and its synthesis may be increased as a response to infection in utero. IgD is found in low concentrations in the blood. Its primary function is as an antigen receptor on the surface of early B cells and for maturation of B cells. Its exact mechanisms are unclear.
IgD is found in low concentrations in the blood. Its primary function is as an antigen receptor on the surface of early B cells and for maturation of B cells. Its exact mechanisms are unclear. IgE is normally at very low concentrations in the circulation. It has very specialised functions as a mediator of many common allergic responses (see Chapter 15) and in the defence against parasitic infections. In individuals with allergies, often IgE antibody levels in the blood are high.
IgE is normally at very low concentrations in the circulation. It has very specialised functions as a mediator of many common allergic responses (see Chapter 15) and in the defence against parasitic infections. In individuals with allergies, often IgE antibody levels in the blood are high.Antigen binding
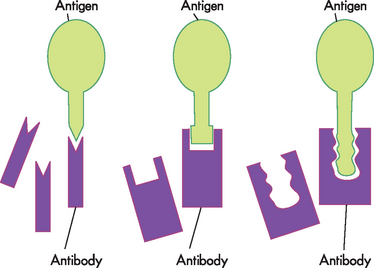
As can be seen, antibodies have different shapes and sizes (refer to Figure 12-9). They also have specific sections called antigen-binding fragments, which join with antigens when activated. The chemical nature of the particular amino acids in those sites and the shape of the site determine the specificity towards a particular antigen. The antigen that will bind most strongly must have complementary chemistry and topography with the binding site formed by the antibody. The antigen fits into this binding site with the specificity of a key into a lock and is held there by the chemical interaction (see Figure 12-10). It should be noted that most antigens have multiple epitopes — that is, antigenic sites. This means that antigens can bind with more than one antibody, which is advantageous to limiting the spread and proliferation of pathogenic microorganisms.
The function of antibodies
The chief function of antibodies is to protect the individual from infection. The mechanism can be either direct or indirect (see Figure 12-11). Directly, antibodies can affect infectious agents or their toxic products by neutralisation (inactivating or blocking the binding of antigen to receptors), agglutination (clumping insoluble particles that are in suspension) or precipitation (making a soluble antigen into an insoluble precipitate). Indirectly, antibodies activate components of innate resistance, including the complement system and phagocytes (this is discussed in more detail in Chapter 13).
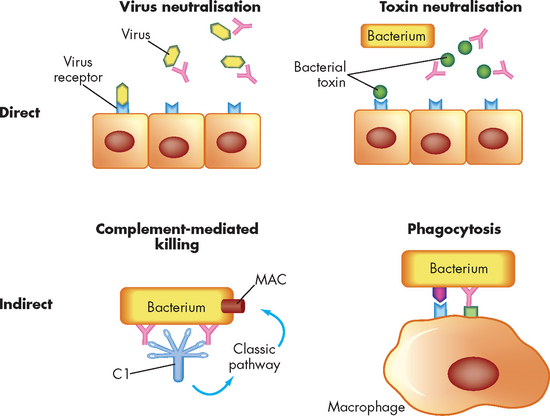
FIGURE 12-11 The direct and indirect functions of antibodies.
Activities of antibodies can be direct (through the action of antibody alone) or indirect (requiring activation of other components of inflammation). Direct means they include neutralisation of viruses or bacterial toxins before they bind to receptors on the surface of the host’s cells. Indirect means they include activation of the classical complement pathway through C1 resulting in formation of the membrane-attack complex (MAC) or by increased phagocytosis of bacteria opsonised with antibody and complement components bound to appropriate surface receptors on the phagocyte.
Direct effects
To cause infection, many pathogens must attach to specific receptors on the host’s cells. For instance, influenza viruses (see Chapter 14 for examples) must attach to specific receptors on respiratory epithelial cells; and the bacteria Neisseria gonorrhoeae (which causes gonorrhoea) must attach to specific sites on urogenital epithelial cells. Antibodies may protect the host by covering sites on the microorganism that are needed for attachment, thereby preventing infection.
Many viral infections can be prevented by vaccination with inactivated or attenuated (weakened) viruses to induce neutralising antibody production at the site of the virus’ entrance into the body. A good indication of the degree of protection against viral infection is the level of antibodies found in the blood, which is called antibody titre. This is used clinically to determine the effectiveness of the vaccine.
Some bacteria secrete toxins that harm humans. For instance, specific bacterial toxins cause the symptoms of tetanus or diphtheria. Most toxins bind to surface molecules on the host’s cells and damage those cells. Protective antibodies can bind to the toxins, prevent their interaction with the host cells and neutralise their biological effects. Detection of the presence of an antibody response against a specific toxin (antibodies referred to as antitoxins) can aid in the diagnosis of diseases. Antibodies that neutralise bacterial toxins can be induced to confer immunity against bacterial pathogens by means of immunisation. To prevent harming the recipient of immunisation, bacterial toxins are chemically inactivated so that they have lost most of their harmful properties but still retain their immunogenicity. Therefore, an immune response is produced, without the individual developing full symptoms of the toxin or the disease.
Indirect effects
Antibodies can also work by binding to antigens that cause activation of the inflammatory response (see Figure 12-12). These bindings cause an increase in opsonisation, which is a process that leads to enhanced phagocytosis and activation of the complement system, which may lead to destruction of the pathogen.
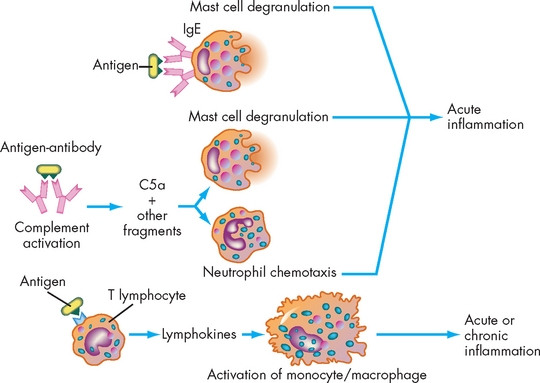
FIGURE 12-12 Antigen antibody activation of the inflammatory response.
Immunological factors may activate inflammation through three mechanisms: (1) IgE can bind to the surface of a mast cell and, after binding antigen, induce the cell’s degranulation; (2) antigen and antibody can activate the complement system, releasing anaphylatoxins and chemotactic factors, especially C5a, which result in mast cell degranulation and neutrophil chemotaxis; and (3) antigen may react with T lymphocytes, resulting in the production of lymphokines, which may contribute to the development of either acute or chronic inflammation.
IgE
IgE is a special class of antibody that protects the individual from infection with large parasites. However, when IgE is produced against relatively innocuous environmental antigens it is also the primary cause of common allergies (e.g. hay fever, dust allergies, bee stings). The role of IgE in allergies is discussed further in Chapter 15.
B cell antigen receptor
Another form of antibody serves as an antigen receptor on the B cell, the B cell receptor. Its role is to recognise the antigen and communicate that information to the cell’s nucleus. Therefore, the B cell receptor complex consists of antibody bound to the cell surface and molecules within the cell involved in intracellular signalling. B cell receptors on the surface of B cells that have not yet reacted with antigen are membrane-associated IgM and IgD immunoglobulins that have identical specificities. After having reacted with antigen, the B cell receptor on the developing plasma cell may change to other classes of antibody.
Cell-mediated immune response
There are several types of mature T cells, each with a different function. Memory cells induce the secondary immune response; others, such as macrophages, secrete cytokines that activate or signal other cells; cytotoxic cells attack antigens directly and destroy cells with foreign antigens; and regulatory cells, primarily helper T cells, control both cell-mediated and humoral immune responses. T cells are particularly important in protection against viruses, tumours and pathogens that are resistant to killing by normal neutrophils and macrophages. They are also absolutely essential for the development of most humoral responses.
The process by which T cells recognise and destroy a target is highly complex and requires an understanding of three different concepts: the T cell receptor complex, antigen presentation molecules and CD molecules. Defects in any of these will lead to major defects in cell-mediated immunity and potentially lead to the individual’s death (see Chapter 15).
T cell recognition of a target cell
T cell receptor complex
T lymphocytes use an antigen receptor that is similar to the B cell receptor. The T cell receptor complex is composed of an antibody-like protein and a group of accessory proteins that are involved in signalling to the nucleus. Although the components of the T cell receptor resemble an antibody, they are encoded by different genes. All of the T cell receptors on a single T cell are identical in structure and specificity.
Antigen presentation molecules
Unlike B cells, T cells do not react with soluble antigens. Protein antigen must be presented in a specific manner on the surface of the target cell; it must be held by molecules of the major histocompatibility complex. The immune system uses MHC class I and II molecules to recognise normal cells from infected or tumour cells. MHC proteins are encoded on chromosome 6 and in humans are referred to as human leucocyte antigens (HLAs) as they were first discovered on leucocytes (white blood cells). They are unique to each individual and this explains why transplanted organs are rejected by the recipients, even when they are closely tissue-matched.
The T cells use the MHCs to distinguish antigens in the body. Specifically, helper T cells use the complexes to aid in the initiation of an immune response against foreign substances, via the antigen presentation molecules.
CD molecules
Cells express a large number of molecules on their surfaces, many of which are important in the immune response. Many of the molecules are part of a nomenclature that uses the prefix ‘CD’ (cluster of differentiation) followed by a number (e.g. CD1 or CD2). The list of CD molecules is constantly increasing (it is currently in excess of 250). Both T and B cells express CD molecules that assist in immune function and can be used to define the subsets of T cells (CD4 helper T cells; CD8 cytotoxic T cells). In this chapter we focus on a small number of highly important examples to illustrate the immensely complicated, but highly effective, interactions that take place to produce a protective immune response.
T lymphocyte function
Cytotoxic T lymphocytes
Cytotoxic T lymphocytes are responsible for the cell-mediated destruction of tumour cells or cells infected with viruses. The cytotoxic T cell must directly adhere to the target cell through an antigen presented by MHC class I molecules and appropriate CD molecules (see Figure 12-13). Cytotoxic T cell–mediated killing is therefore class I restricted. Because of the cellular distribution of MHC class I molecules, cytotoxic T cells can recognise antigens on the surface of almost any type of cell that has been infected by a virus or has become cancerous. Most cytotoxic T cell killing also requires CD8 on the cytotoxic T cell, which binds to MHC class I on the target. After attachment to a target cell, killing occurs by induction of apoptosis (programmed cell death, but in this case the cytotoxic T cell binds to the antigen membrane and releases cytotoxic chemical, which causes lysis).
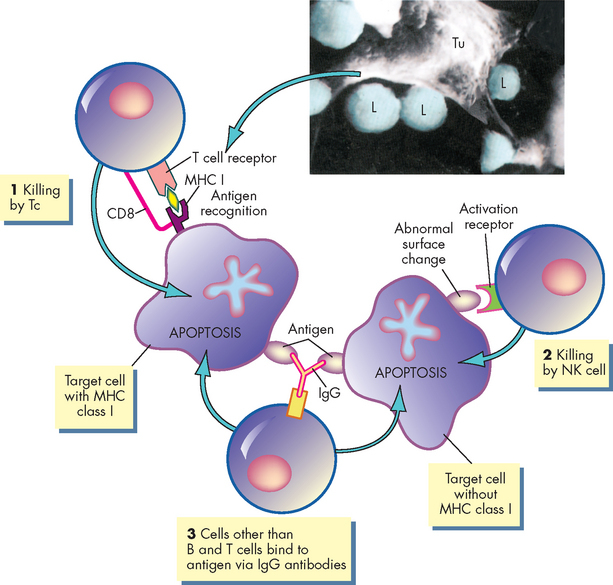
FIGURE 12-13 Cytotoxic T cell mechanisms.
Several cells have the capacity to kill abnormal (e.g. virally infected, cancerous) target cells. (1) Cytotoxic T (Tc) cells recognise endogenous antigens presented by MHC class I molecules. The Tc cell mobilises multiple killing mechanisms that induce apoptosis of the target cell. (2) Natural killer (NK) cells identify and kill target cells through receptors that recognise abnormal surface changes. Natural killer cells specifically kill targets that do not express surface MHC class I molecules. (3) Several cells, including macrophages, can kill by IgG antibodies binding to foreign antigen on the target cell. The macrophages then initiate killing. The insert is a scanning electron microscopic view of Tc cells (L) attacking a much larger tumour cell (Tu).
Source: Inset Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Other cells that kill abnormal cells
Apart from cytotoxic T cells, natural killer cells also target antigens in a similar manner to cytotoxic T cells (see Figure 12-13). T cells can also activate macrophages via the production of cytokines. The cytokines (particularly interferon-gamma) stimulate the macrophage to become a more efficient phagocyte and increase production of proteolytic enzymes and other antimicrobial substances.
Regulatory T lymphocytes
Regulatory T cells are a group of T cells that control the immune response.6 Some regulatory T cells suppress immune responses. This population is a mixture of cells that affect the immune response in multiple ways: some affect the recognition of antigen and others suppress the proliferative steps that follow antigen recognition. The most characterised is the helper T cell, which is necessary for the development of most humoral and cellular immune responses. The human immunodeficiency virus (HIV) specifically infects and destroys helper T cells, thus leading to the onset of life-threatening infections.
Helper T lymphocytes
Regardless of whether an antigen primarily induces a cellular or humoral immune response, antigen-presenting cells usually must present antigen to helper T cells (TH cells; see Figure 12-14).7 This extremely important role involves three distinct steps:
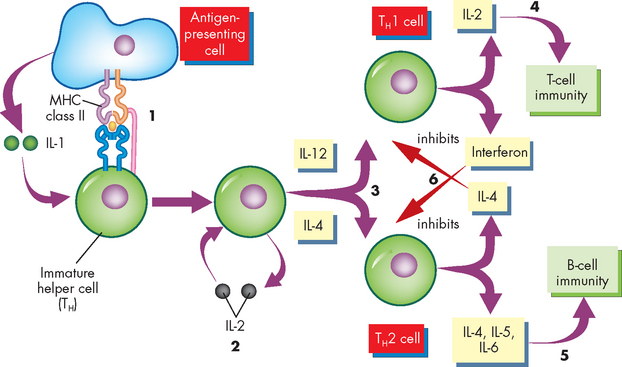
FIGURE 12-14 The development of helper T cells.
An antigen-presenting cell presents antigen to an immature helper T cell (precursor). (1) An antigen signal is produced by the interaction of the T cell receptor with antigen presented by MHC class II molecules. Cytokines, particularly IL-1, produced by the antigen-presenting cell provide a second signal. (2) In response to these signals, the immature helper T cell begins producing the cytokine IL-2, which binds with the same cell to accelerate differentiation and proliferation. (3) IL-12 assists differentiation into the TH1 cell, whereas IL-4 assists differentiation into the TH2 cell. (4) The TH1 cell produces cytokines that assist in the differentiation of cytotoxic T cells (e.g. IL-2), whereas (5) the TH2 cell produces cytokines that activate B cell differentiation (e.g. IL-4, IL-5, IL-6). (6) TH1 and TH2 cells affect each other through the production of inhibitory cytokines: interferon will inhibit the development of TH2 cells, and IL-4 will inhibit the development of TH1 cells. IL = interleukin.
When T cells develop in the thymus, two different populations are produced. T cells that are destined to become TH cells emerge from the thymus with a characteristic cell surface protein, called CD4. Cells destined to become cytotoxic T cells have a different cell surface protein, called CD8. The role of CD4 and CD8 is to help the interaction between T cells and antigen-presenting cells by reacting with antigen-presenting molecules. Thus, the T cell receptor and CD4 or CD8 both attach to the antigen-presenting molecules on the surface of another cell. CD4 can only interact with MHC class II molecules, whereas CD8 reacts only with MHC class I molecules.
To mature into a functional helper cell, the TH cell must receive several signals, including the T cell receptor binding to antigen and CD4 binding to MHC class II. If all the appropriate signalling pathways are activated, the cell will differentiate into a functional TH cell.
Additional signals are provided by cytokines. At this early stage of TH cell differentiation, interleukin-1 (IL-1) secreted by the antigen-presenting cell provides this signal. The TH cell then produces interleukin-2 (IL-2), which is secreted and acts in an autocrine (self-stimulating) fashion to induce further maturation and proliferation of the TH cell. Without IL-2 production, the TH cell cannot efficiently mature into a functional helper cell.
At this point, TH cells undergo differentiation into either TH1 or TH2 cells (see Figure 12-14). These subsets have different functions: TH1 cells appear to provide more help in developing cell-mediated immunity, whereas TH2 cells provide more help for developing humoral immunity. The two TH subsets differ considerably in the spectrum of cytokines produced by each. Additionally, each TH cell may suppress the other so that the immune response may favour either antibody formation with suppression of a cell-mediated response, or the opposite. For example, antigens derived from viral or bacterial pathogens and those derived from cancer cells seem to induce a greater number of TH1 cells relative to TH2 cells, whereas antigens derived from multi-cellular parasites and allergens are hypothesised to result in the production of more TH2 cells. Many antigens, however, produce excellent humoral and cell-mediated responses simultaneously.
INDUCTION OF THE IMMUNE RESPONSE
The final aspect of the immune system is to examine how an immune response is commenced. The antigen is central to this process. Immune responses are triggered by antigens and this activates the B and T cells to differentiate into their respective cell subsets. This generally occurs in lymphoid organs called secondary (peripheral) lymphoid organs in which antigen selectively reacts with B or T cells. The secondary lymphoid organs include the spleen, lymph nodes, adenoids, tonsils, Peyer’s patches (intestines) and appendix (see Figure 12-5). Under the control of a variety of cytokines (see Table 12-1) and complex cellular interactions, the selected B or T cells further proliferate and differentiate into plasma cells that produce antibody, T cells that can attack cellular targets or B or T memory cells that will respond more quickly to a second exposure to the same antigen (see Figure 12-15).
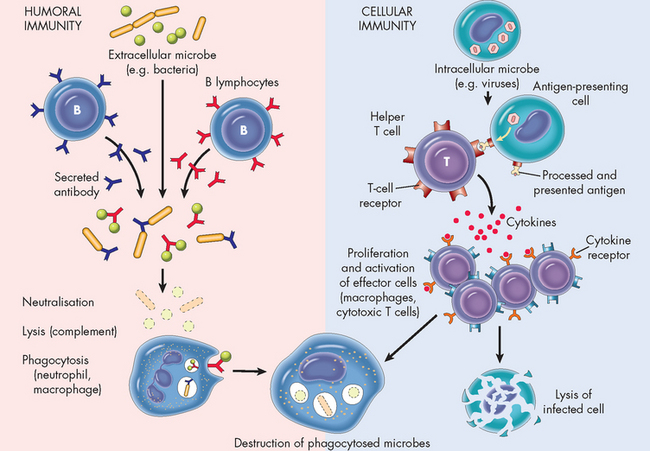
FIGURE 12-15 Humoral and cell-mediated immunity from presentation of the antigen to destruction of the pathogen.
Source: Based on Kumar V et al. Robbins & Cotran pathologic basis of disease. 7th edn. Philadelphia: Saunders; 2005.
The immune response to antigen has classically been divided into two phases — the primary and secondary responses — which are most easily demonstrated by measuring concentrations of circulating antibody over time (see Figure 12-16). After a single initial exposure to most antigens, there is a latent period, or lag phase, during which B and T cell differentiation and proliferation occurs. After approximately 5 to 7 days, IgM antibody is detected in the circulation. The lag phase is the time necessary for the production of the B and T cell lines. This is the primary immune response, characterised typically by initial IgM followed by IgG against the same antigen. The quantity of IgG may be about equal to or less than the amount of IgM. If no further exposure to the antigen occurs, the circulating antibody is catabolised (broken down) and measurable quantities fall. The individual’s immune system, however, has been primed.
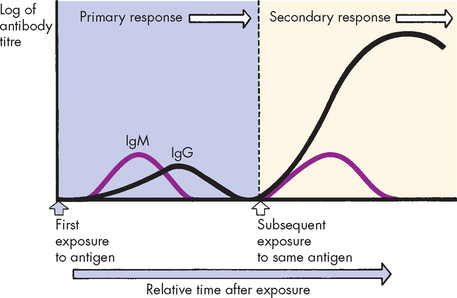
FIGURE 12-16 Primary and secondary immune responses.
The initial administration of antigen induces a primary response during which IgM is initially produced, followed by IgG. Another administration of the antigen induces the secondary response in which IgM is transiently produced and larger amounts of IgG are produced over a longer period of time.
A second challenge by the same antigen results in the secondary immune response, which is characterised by the more rapid production of a larger amount of antibody than the primary response. The rapidity of the secondary immune response is the result of B and T memory cells that do not require further differentiation (see Figure 12-8). IgM may be transiently produced in the secondary response, but IgG production is increased considerably, making it the predominant antibody class. If the antigenic challenge is in the form of a vaccine or occurs through natural infection, the level of protective IgG may remain elevated for decades.
Paediatrics and the immune function
When born, human infants are immunologically immature with deficiencies in antibody production, phagocytic activity and complement activity. However, in the last trimester the fetus can produce a primary immune response (IgM; T cell independent) to antigenic challenge in utero and to infections (e.g. cytomegalovirus and rubella virus), but cannot produce sufficient IgG response, and only limited amounts of IgA can be detected. In most cases, maternal antibodies provide protection within the fetal circulation (see Figure 12-17).
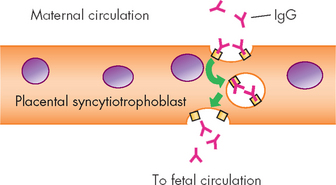
FIGURE 12-17 Transport of IgG across the syncytiotrophoblast.
The human placenta is covered with a specialised multinucleate cell, the syncytiotrophoblast. Transport of maternal IgG across the syncytiotrophoblast and into the fetal circulation is an active process. Maternal IgG binds to receptors on the surface of the syncytiotrophoblast and is internalised by the process of endocytosis (engulfing the cells). Receptors on the syncytiotrophoblast are specific for IgG and do not bind to other classes of immunoglobulins. Interaction of IgG protects the antibody from lysosomal digestion during transport of the vacuole across the cell. On the fetal side of the syncytiotrophoblast, IgG is released by exocytosis (release of contents outside of the cell).
At birth, total IgG levels are near adult levels (see Figure 12-18). After birth, antibody titres (levels) drop as maternal antibody is broken down, reaching a minimum at 5 to 6 months; transient hypogammaglobulinaemia (low antibody levels) can then occur. Recurrent respiratory tract infections are common during this transient period of immune insufficiency, as the immune system is not sufficiently activated to combat foreign substances, such as bacteria and viruses.
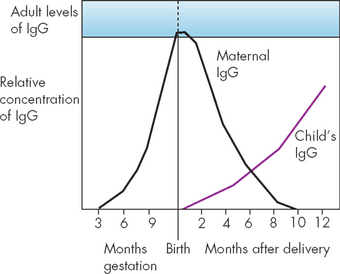
FIGURE 12-18 Antibody levels in umbilical cord blood and in neonatal circulation.
Early in gestation, maternal IgG begins active transport across the placenta and enters the fetal circulation. At birth, the fetal circulation may contain nearly adult levels of IgG, which is almost exclusively from the maternal source. The fetal immune system has the capacity to produce IgM and small amounts of IgA before birth. After delivery, maternal IgG is rapidly destroyed and neonatal IgG production increases.
Antibodies in breast milk may protect the nursing newborn against these infectious disease agents. Colostrum found in breast milk produces colostral antibodies, which provide the newborn with passive immunity against gastrointestinal infections. They do not provide systemic immunity because they do not cross the newborn’s gut into the bloodstream after the first 24 hours of life. Maternal antibodies that pass across the placenta into the fetus before birth provide passive systemic immunity.
AGEING AND THE IMMUNE SYSTEM
It is well known that immune function decreases with age, with diminished T cell function and antibody responses to antigenic challenge. In contrast, circulating autoantibodies and immune complexes increase. The thymus reaches maximum size at sexual maturity and then shrinks to be small by middle age — by 45–50 years of age, the thymus is only 15% of maximum size. Thymic hormone production drops, as does the organ’s ability to mediate T cell differentiation. Therefore, T cell function deteriorates, although the number of T cells does not drop.
Those individuals older than 60 years of age have decreased delayed hypersensitivity responses, decreased T cell–mediated responses to infections and decreased T cell activity.8
Human defence mechanisms
 Foreign substances are anything that the immune system recognises as not normal cells or tissue and so activates immune responses.
Foreign substances are anything that the immune system recognises as not normal cells or tissue and so activates immune responses. Inflammation is a rapid, non-specific defence that is effective at limiting the spread of foreign substances and activating parts of adaptive immunity.
Inflammation is a rapid, non-specific defence that is effective at limiting the spread of foreign substances and activating parts of adaptive immunity. Epithelial barriers are physical structures, such as the skin surface and mucosal membranes, which provide protection from the environment.
Epithelial barriers are physical structures, such as the skin surface and mucosal membranes, which provide protection from the environment. Phagocytes are cells that engulf and digest foreign substances as well as diseased and dead cells. They include neutrophils, monocytes and macrophages.
Phagocytes are cells that engulf and digest foreign substances as well as diseased and dead cells. They include neutrophils, monocytes and macrophages. For both the innate and the adaptive immune systems to function, chemicals are released to regulate the immune response. Cytokines is the term referring to these mediators and includes a vast array of different substances that are integral to normal immune function.
For both the innate and the adaptive immune systems to function, chemicals are released to regulate the immune response. Cytokines is the term referring to these mediators and includes a vast array of different substances that are integral to normal immune function. Adaptive immunity is a state of protection, primarily against infectious agents, which differs from inflammation by being slower to develop, being more specific and having memory that makes it much longer lived.
Adaptive immunity is a state of protection, primarily against infectious agents, which differs from inflammation by being slower to develop, being more specific and having memory that makes it much longer lived. The adaptive immune response is most often initiated by cells of the innate system. These cells process and present portions of invading pathogens (i.e. antigens) to lymphocytes in peripheral lymphoid tissue.
The adaptive immune response is most often initiated by cells of the innate system. These cells process and present portions of invading pathogens (i.e. antigens) to lymphocytes in peripheral lymphoid tissue. The production of B and T lymphocytes with receptors against millions of antigens that may possibly be encountered in an individual’s lifetime occurs in the fetus in the primary lymphoid organs — the thymus for T cells and portions of the bone marrow for B cells.
The production of B and T lymphocytes with receptors against millions of antigens that may possibly be encountered in an individual’s lifetime occurs in the fetus in the primary lymphoid organs — the thymus for T cells and portions of the bone marrow for B cells. Immunocompetent T and B cells migrate from the primary lymphoid organs into the circulation and secondary lymphoid organs to await antigen.
Immunocompetent T and B cells migrate from the primary lymphoid organs into the circulation and secondary lymphoid organs to await antigen. Antigens are molecules that react with components of the immune response, such as antibodies and receptors on B and T cells. Most antigens can induce an immune response.
Antigens are molecules that react with components of the immune response, such as antibodies and receptors on B and T cells. Most antigens can induce an immune response. The antigenic-determinant, or epitope, is the precise chemical structure with which an antibody or B/T cell receptor reacts.
The antigenic-determinant, or epitope, is the precise chemical structure with which an antibody or B/T cell receptor reacts. Self-antigens are antigens on an individual’s own cells. The individual’s immune system does not normally recognise self-antigens as immunogenic.
Self-antigens are antigens on an individual’s own cells. The individual’s immune system does not normally recognise self-antigens as immunogenic. Very small antigens may not normally be immunogenic (haptens) unless they are bound to a larger molecular weight molecule (carrier).
Very small antigens may not normally be immunogenic (haptens) unless they are bound to a larger molecular weight molecule (carrier). Antigen is processed in the antigen-presenting cell and presented on the cell surface by molecules of the major histocompatibility complex (MHC). The particular MHC molecule (class I or class II) that presents antigen determines which cell will respond to that antigen.
Antigen is processed in the antigen-presenting cell and presented on the cell surface by molecules of the major histocompatibility complex (MHC). The particular MHC molecule (class I or class II) that presents antigen determines which cell will respond to that antigen. The adaptive immune response is mediated by two different types of lymphocytes: B lymphocytes and T lymphocytes. Each has distinct functions: B cells are responsible for humoral immunity, which is mediated by circulating antibodies; and T cells are responsible for cell-mediated immunity, in which they kill targets directly or stimulate the activity of other leucocytes.
The adaptive immune response is mediated by two different types of lymphocytes: B lymphocytes and T lymphocytes. Each has distinct functions: B cells are responsible for humoral immunity, which is mediated by circulating antibodies; and T cells are responsible for cell-mediated immunity, in which they kill targets directly or stimulate the activity of other leucocytes. Immature B cells differentiate when exposed to an antigen to become either plasma cells (which release antibodies) or memory cells (which accelerate the process on further antigenic exposure).
Immature B cells differentiate when exposed to an antigen to become either plasma cells (which release antibodies) or memory cells (which accelerate the process on further antigenic exposure). Immature T cells differentiate when exposed to an antigen to become cytotoxic T cells (which kill infected cells), memory cells (which accelerate the process on further antigenic exposure), helper T cells (which assist the immune response) or regulatory T cells (which often suppress the immune response).
Immature T cells differentiate when exposed to an antigen to become cytotoxic T cells (which kill infected cells), memory cells (which accelerate the process on further antigenic exposure), helper T cells (which assist the immune response) or regulatory T cells (which often suppress the immune response).Humoral and cell-mediated immunity
 Antibodies are plasma glycoproteins that can be classified by chemical structure and biological activity as IgG, IgM, IgA, IgE or IgD.
Antibodies are plasma glycoproteins that can be classified by chemical structure and biological activity as IgG, IgM, IgA, IgE or IgD. Direct effects result from the binding of antibody directly to a harmful antigen or infectious agent. These include inhibition of processes that are necessary for infection, such as the reaction of an infectious agent with a particular cell in the body, or neutralisation of harmful bacterial toxins.
Direct effects result from the binding of antibody directly to a harmful antigen or infectious agent. These include inhibition of processes that are necessary for infection, such as the reaction of an infectious agent with a particular cell in the body, or neutralisation of harmful bacterial toxins. Indirect effects result from activation of inflammation by antibodies. These include opsonisation to increase phagocytosis, killing the infectious agent through activation of complement and widespread activation of inflammation through the production of biologically active complement components.
Indirect effects result from activation of inflammation by antibodies. These include opsonisation to increase phagocytosis, killing the infectious agent through activation of complement and widespread activation of inflammation through the production of biologically active complement components. Antibodies of the systemic immune system function internally, in the bloodstream and tissues. Antibodies, primarily secretory IgA, are abundant in the secretions of mucous membranes.
Antibodies of the systemic immune system function internally, in the bloodstream and tissues. Antibodies, primarily secretory IgA, are abundant in the secretions of mucous membranes. There are several types of mature T cells: cytotoxic T cells, regulatory T cells including T helper and T suppression, and memory cells.
There are several types of mature T cells: cytotoxic T cells, regulatory T cells including T helper and T suppression, and memory cells. T cells have antigen-specific receptors (T cell receptors) that must ‘see’ antigen presented on cell surfaces by special antigen-presenting molecules of the MHC.
T cells have antigen-specific receptors (T cell receptors) that must ‘see’ antigen presented on cell surfaces by special antigen-presenting molecules of the MHC. Cytotoxic T cells bind to and kill cellular targets such as cells infected with viruses or cancer cells.
Cytotoxic T cells bind to and kill cellular targets such as cells infected with viruses or cancer cells. Natural killer cells have some characteristics of cytotoxic T cells and are important for killing target cells in which viral infection or malignancy has resulted in the loss of cellular MHC molecules.
Natural killer cells have some characteristics of cytotoxic T cells and are important for killing target cells in which viral infection or malignancy has resulted in the loss of cellular MHC molecules. The T cell ‘sees’ the presented antigen through the T cell receptor and accessory molecules: CD4 or CD8. CD4 is found on helper T cells and reacts specifically with MHC class II. CD8 is found on cytotoxic T cells and reacts specifically with MHC class I.
The T cell ‘sees’ the presented antigen through the T cell receptor and accessory molecules: CD4 or CD8. CD4 is found on helper T cells and reacts specifically with MHC class II. CD8 is found on cytotoxic T cells and reacts specifically with MHC class I. A subgroup of helper T cells (TH2 cells) helps B cells respond to antigen and develop into antibody-secreting plasma cells.
A subgroup of helper T cells (TH2 cells) helps B cells respond to antigen and develop into antibody-secreting plasma cells.Induction of the immune response
 The response to antigen can be divided into two phases: the primary and secondary responses. The primary immune response of humoral immunity is usually dominated by IgM, with lesser amounts of IgG. The secondary immune response has a more rapid production of a larger amount of antibody, predominantly IgG.
The response to antigen can be divided into two phases: the primary and secondary responses. The primary immune response of humoral immunity is usually dominated by IgM, with lesser amounts of IgG. The secondary immune response has a more rapid production of a larger amount of antibody, predominantly IgG.Paediatrics and the immune function
 Mechanisms of self-defence are naturally somewhat deficient in the fetus, the neonate and the elderly individual.
Mechanisms of self-defence are naturally somewhat deficient in the fetus, the neonate and the elderly individual.Jamila is a 19-year-old student. She is talking to her friend, Anji, about her expectation that she will develop the flu (influenza) again this winter because she ‘always gets the flu’ and believes she has a weak immune system. However, Anji has attended immunology lectures and says that she thinks Jamila is incorrect in what she is stating — in fact, the immune system is continually responding to an enormous array of different pathogenic microorganisms and is very effective in responding to these through both innate and adaptive immunity.