16 THE STRUCTURE AND FUNCTION OF THE HAEMATOLOGICAL SYSTEM
INTRODUCTION
Blood is essential for life. It consists of different types of blood cells suspended in a fluid known as plasma. An adequate supply of oxygen and nutrients is required for the survival of all body cells, and blood provides these substances to all of the body’s tissues and organs. It also transports carbon dioxide and other cellular waste products so that they can be removed from the body — waste products must be removed to prevent toxic effects. Red blood cells are the most numerous blood cells, accounting for the red appearance of blood. Oxygen diffuses from the lungs to the red blood cells to be used at body tissues, while carbon dioxide diffuses from the tissues to the red blood cells to be eliminated at the lungs. The plasma portion of blood transports the nutrients and waste products. In addition, it also contains a number of proteins involved in blood clotting. A number of different types of white blood cells provide protection from foreign invaders such as bacteria and viruses. Platelets are the smallest cellular component of the blood and are involved in preventing blood loss if blood vessels are injured. Blood flows through kilometres of vessels wound throughout the body and blood cells must adapt to the different conditions found in arteries, veins and capillaries.
Like all cells in the body, blood cells have a limited life span. The spleen is an important organ in the haematological system and it is responsible for removing the majority of old or non-functional blood cells. Blood cells are manufactured in the bone marrow and under normal circumstances this process is tightly controlled so that cell production is equal to cell loss. An increase or decrease in the rate of blood cell production can occur in response to various physiological challenges or can be a primary cause of haematological disease.
We start by examining what blood is made of, particularly the different cells that perform an enormous number of functions essential for homeostasis.
COMPONENTS OF THE HAEMATOLOGICAL SYSTEM
The composition of blood
Blood consists of various cells that circulate in the cardiovascular system suspended in plasma, which is approximately 90% water and 10% dissolved substances (solutes). The blood volume amounts to about 5.5 L in a 70 kg adult — or approximately 8% of body weight. The continuous movement of blood around the body guarantees that critical components are available to carry out their chief functions:
Each of these functions is essential in contributing to homeostasis of the body.
Plasma and plasma proteins
In adults, plasma accounts for 50–55% of blood volume (see Figure 16-1). Plasma consists of water and a variety of organic (e.g. proteins) and inorganic (e.g. electrolytes) substances. The concentration of these substances varies depending on diet, metabolic demand, hormones and vitamins.
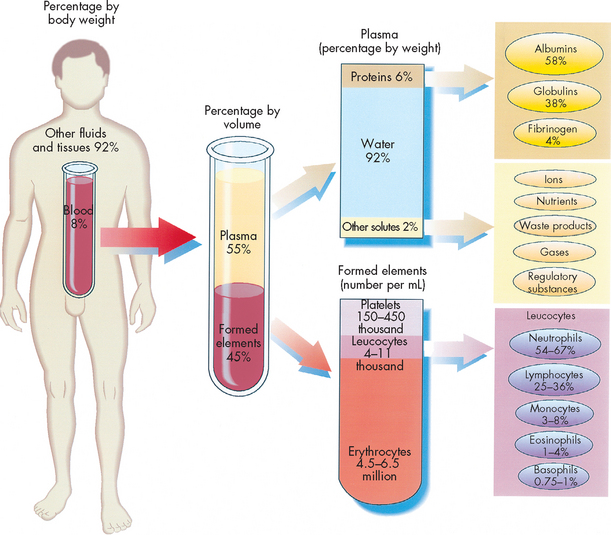
FIGURE 16-1 The composition of whole blood.
Approximate values for the components of blood in a normal adult.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
Plasma contains a large number of different plasma proteins that vary in structure and function, most of which are produced by the liver. The most abundant plasma protein is albumin (about 60% of total plasma protein). Albumin serves as a carrier molecule both for the normal components of blood and for drugs. A hormone or drug carried through the blood on albumin effectively becomes larger-sized and is therefore less able to be metabolised or filtered out of the bloodstream. In addition to albumin, other transport proteins specifically bind and carry a variety of substances including iron (transferrin), lipids and steroid hormones (lipoproteins) and vitamins (e.g. retinol-binding protein).
The most essential role of albumin is control of the passage of water and solutes through the capillaries. Albumin molecules are large and do not diffuse freely through the vascular endothelium; thus they maintain the critical colloidal osmotic pressure (or oncotic pressure) that regulates the passage of water and solutes into the surrounding tissues (see Chapter 22). In the case of decreased production (e.g. some liver diseases such as cirrhosis) or excessive loss of albumin (e.g. certain kidney diseases), the reduced oncotic pressure leads to excessive movement of fluid and solutes into the tissue and decreased blood volume.
Another important group of plasma proteins is the globulins. These are not all produced in the liver — immunoglobulins are produced by plasma cells in the lymph nodes and other lymphoid tissues. Plasma cells develop from immunologically stimulated B lymphocytes. These globulins are specific to the function of the immune system and were discussed in Chapter 12. Other proteins are involved in defence or protection against infection, including other antibodies and complement proteins.
One other essential constituent of plasma is the numerous clotting factors (which are proteins) that promote coagulation and stop bleeding from damaged blood vessels. Fibrinogen is the most plentiful of the clotting factors and is the precursor of the fibrin clot. The processes involved in blood clotting are discussed fully in the following sections.
Plasma also contains ions (electrolytes) that participate in the control of cell function, osmotic pressure and blood pH. These include sodium, potassium, calcium, chloride and phosphate. Electrolytes are discussed in Chapters 1 and 29.
Plasma versus serum
There is a significant difference between plasma and serum. Plasma is the fluid in which blood cells are suspended as blood flows around the body, whereas serum is the fluid that can be separated from blood cells after blood has been allowed to clot. Hence serum is similar to plasma except that the clotting factors (especially fibrinogen) have been consumed in the process of clot formation.
Knowing the distinction between plasma and serum is important with regard to collecting blood samples for pathology tests. Some blood tests require serum, whereas others need plasma. In addition, some tests require anticoagulated whole blood as the testing material.
Serum samples are required for certain laboratory tests that may be affected by the presence of fibrinogen. In order to obtain serum, a blood sample collected from a patient is placed in a plain tube. The blood in the tube will clot over time. After the blood has clotted, the tube is centrifuged (spun at very high speed) to allow the blood cells and fluid portions to separate. The cells will occupy the lower half of the tube, while the fluid will occupy the top half — this fluid is the serum.
Where plasma or whole blood is required, blood samples are collected in tubes that contain an anticoagulant to prevent the blood from clotting. When the tube is centrifuged, the fluid that separates from the blood cells is plasma. Some anticoagulants, such as EDTA (ethylene diamine tetra acetic acid) and sodium citrate, work by binding to calcium, thereby making the calcium unavailable for the blood-clotting process. Hence, when calcium is bound to the anticoagulant, clotting cannot occur. Heparin, another type of anticoagulant used in blood collection tubes, works by binding to and activating antithrombin III (a substance naturally present in the blood) to create a potent anticoagulant.
The details of the anticoagulant contained within a tube are written on an exterior label on the tube. The tubes also have colour-coded caps (tops) for ease of identification. Anticoagulants that are commonly used for different types of blood tests include:
It is important that blood collection tubes containing an anticoagulant are filled with the correct amount of blood (indicated by a fill line on the side of the tube). Overfilled or underfilled tubes will have an incorrect concentration of anticoagulant in the sample (that is, the proportion of anticoagulant to the proportion of blood) and this may affect the test results, especially for blood clotting studies.
Cellular components of blood
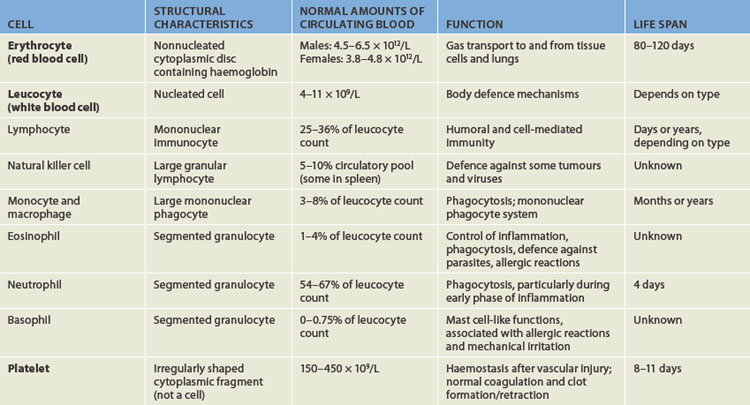
The cellular elements of blood are broadly classified as red blood cells (erythrocytes), white blood cells (leucocytes), which have several different types, and platelets (thrombocytes) (see Figure 16-2). The components of blood are listed in Table 16-1.
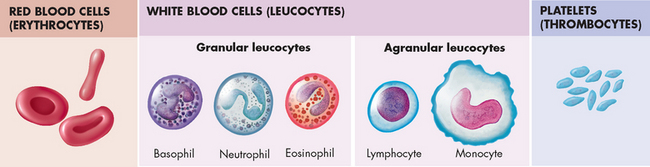
FIGURE 16-2 The formed elements of blood.
Red blood cells (erythrocytes), white blood cells (leucocytes) and platelets (thrombocytes) constitute the formed elements of blood.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
Erythrocytes
Erythrocytes (red blood cells) are the most abundant cells of the blood, occupying approximately 48% of the blood volume in men and about 42% in women. The volume of blood occupied by red cells is known as the haematocrit or packed cell volume. It can be expressed as a percentage or as a ratio between red blood cells and whole blood. The two ways of expressing the haematocrit are easily interchangeable — 48% is the same as 0.48. The 0.48 refers to 0.48 litres of red cells per litre of whole blood. The actual number of erythrocytes is approximately 4.5–6.5 × 1012 per litre of blood in males; the value is slightly lower for females (see Table 16-1). This equals 4,500,000,000,000 to 6,500,000,000,000 erythrocytes per litre of blood in males. For simplicity, this is usually expressed as 4.5–6.5 × 1012/L.
Erythrocytes are primarily responsible for tissue oxygenation. Haemoglobin (Hb) is a specialised protein contained within erythrocytes that carries gases. Mature erythrocytes lack a nucleus and other cytoplasmic organelles (such as mitochondria), so they cannot produce proteins or carry out the variety of cellular functions that a complete cell performs. Also, they cannot undergo mitotic division because they have no nucleus — hence they have a limited life span of approximately 120 days. Aged or damaged erythrocytes are removed from the circulation and replaced by new cells produced in the bone marrow.
The erythrocyte’s size and shape are ideally suited to its function as a gas carrier. It is a small disc (see Figure 16-3) approximately 6–8 μm in diameter (1 mm = 1000 μm) with two unique properties:
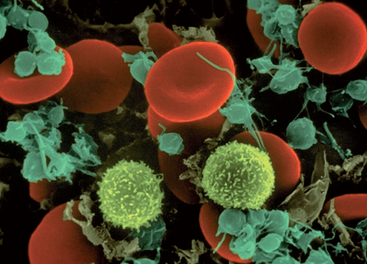
FIGURE 16-3 A scanning electron microscopy image of an erythrocyte.
Red cells are smooth and concave.
Source: Copyright by Dennis Kunkel Microscopy Inc.
The flattened biconcave shape provides a relatively large surface area that is optimal for gas diffusion into and out of the cell, as there is effectively more cell membrane for the volume of the cell than there would be if the same cell were spherical. During its life span, the erythrocyte repeatedly circulates throughout the entire circulatory system, including through leaky blood vessels in the splenic sinusoids and capillaries that are only 2 μm in diameter. Although the erythrocyte is 6–8 μm in diameter, its ability to undergo reversible deformity that enables it to assume a more compact torpedo-like shape, squeeze through the microcirculation and return to normal shape afterwards when reaching larger blood vessels.
Leucocytes
Leucocytes (white blood cells) defend the body against organisms that cause infection and remove debris, including dead or injured host cells of all kinds (see Figure 16-4). Although leucocytes are transported in the circulation, they act primarily in the tissues. The average adult has approximately 4–11 × 109 leucocytes per litre of blood.
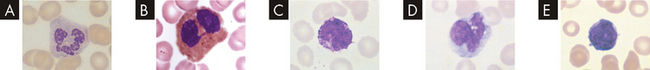
An example of leucocytes in a human blood smear. A Neutrophil. B Eosinophil. C Basophil with obscured nucleus. D Typical monocyte showing vacuolated cytoplasm and cerebriform nucleus. E Lymphocyte.
Source: Rodak BF et al. Hematology: clinical principles and applications. 3rd edn. St Louis: Saunders; 2007.
Leucocytes are classified according to structure as either granulocytes or agranulocytes (with or without granules in their cytoplasm) and according to function as either phagocytes or immunocytes. The granulocytes, which include neutrophils, basophils and eosinophils, are all phagocytes. (Phagocytic action was described in Chapter 12.) Of the agranulocytes, monocytes and macrophages are phagocytes, whereas lymphocytes are immunocytes (cells that create immunity; see Chapter 12).
Granulocytes
Granulocytes have many membrane-bound granules in their cytoplasm. These granules contain enzymes capable of killing microorganisms and degrading debris ingested during phagocytosis. The granules also contain powerful substances with inflammatory and immune functions. These substances, along with the digestive enzymes, are released from granulocytes in response to specific stimuli and have vascular and intercellular effects. Granulocytes are capable of amoeboid movement, which enables migration through vessel walls (called diapedesis) and then moving to sites where their action is needed (refer to Chapter 13 for this function).
Neutrophils are the most numerous and best understood of the granulocytes. Neutrophils constitute about 55% of the total leucocyte count in adults. They are important for protecting the body against bacterial infection. Neutrophils are the chief phagocytes of early inflammation. Soon after bacterial invasion or tissue injury, neutrophils migrate out of the capillaries and into the inflamed site, where they ingest and destroy microorganisms and debris and then die themselves after just 1 or 2 days. The dissolution of dead neutrophils releases digestive enzymes from their cytoplasmic granules. These enzymes dissolve cellular debris and prepare the site for healing.
Eosinophils, which have large, coarse granules, constitute only 1–4% of the normal leucocyte count in adults.1 Unlike neutrophils, eosinophils ingest antigen-antibody complexes and are induced by IgE-mediated hypersensitivity reactions to attack parasites (see Chapter 15). The eosinophil granules contain a variety of enzymes that help to control inflammatory processes. Eosinophils circulate in the blood for up to 8 hours and then become located next to mucosal surfaces, particularly of the respiratory and gastrointestinal systems.
Eosinophils have an important role in protecting the body against parasites such as worms. An individual with a parasitic infection may have a raised eosinophil count — that is, the number of eosinophils present in the blood is greater than normal; this is called eosinophilia. During type I hypersensitivity, allergic reactions and asthma are characterised by eosinophilia, which may be involved in control but may also contribute to the destructive inflammatory processes observed in the lungs of asthmatics.
Basophils, which make up less than 1% of the leucocytes, are structurally and functionally similar to the mast cells found throughout extravascular tissue (see Chapter 13).2 Like mast cells, basophils have cytoplasmic granules that contain vasoactive amines (e.g. histamine) and an anticoagulant (heparin). The release of histamine promotes vasodilation and attraction of other leucocytes, which results in an increased number of leucocytes in the local area. The heparin prevents the blood from clotting, thereby encouraging blood flow that further enhances delivery of blood cells to the site of infection.
Agranulocytes
Agranulocytes — monocytes, macrophages and lymphocytes — have a somewhat misleading name, as they do actually contain some cytoplasmic granules, but only a few in comparison to granulocytes. Monocytes and macrophages make up the mononuclear phagocyte system. Both monocytes and macrophages participate in the immune and inflammatory response, being powerful phagocytes. They also ingest dead or defective host cells, particularly blood cells. Lymphocytes also participate in the immune and inflammatory response, but are not phagocytic. Lymphocytes, monocytes and macrophages work together to protect the body from foreign invaders.
Monocytes are immature macrophages. Monocytes are formed and released by the bone marrow into the bloodstream. As they mature, monocytes migrate into a variety of tissues (e.g. liver, spleen, lymph nodes, peritoneum, gastrointestinal tract) and fully mature into tissue macrophages. Other monocytes may mature into macrophages and migrate out of the vessels in response to infection or inflammation.
Lymphocytes constitute approximately 36% of the total leucocyte count and are the primary cells of the immune response (see Chapter 12). Most lymphocytes circulate in the blood for a short time and then reside in lymphoid tissues as mature T cells, B cells or plasma cells. Natural killer cells are another type of lymphocyte that kill some types of tumour cells and some virus-infected cells (see Chapter 12). They develop in the bone marrow and circulate in the blood.
Platelets
Platelets (also called thrombocytes) are the smallest of the blood cells (see Figure 16-5). They are not true cells, but are disc-shaped cytoplasmic fragments of a large cell that resides in the bone marrow known as a megakaryocyte. Platelets are essential for blood coagulation and control of bleeding. They lack a nucleus and therefore have no DNA and are incapable of mitotic division. They do, however, contain cytoplasmic granules capable of releasing substances when stimulated by injury to a blood vessel to facilitate haemostasis.
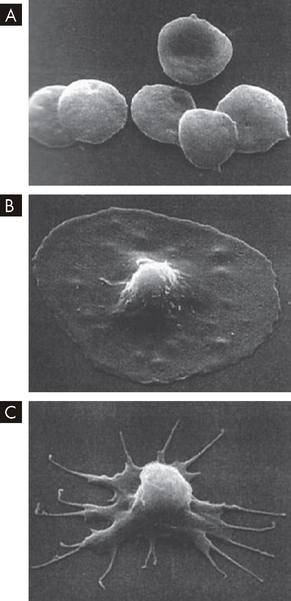
FIGURE 16-5 Scanning electron micrograph images of platelets.
A Free platelets. B Platelet adhesion. C Platelet activation.
Source: Courtesy of JG White, MD, Regents’ Professor, Department of Laboratory Medicine and Pathology, University of Minnesota School of Medicine. From DeLee JC, Drez D, Miller MD. DeLee and Drez’s orthopaedic sports medicine. 3rd edn. Philadelphia: Saunders; 2009.
There are approximately 150–450 × 109 platelets per litre of circulating blood. An additional one-third of the body’s available platelets are in a reserve pool in the spleen, which can be released into the circulation if necessary to enhance the process of haemostasis. A platelet circulates for approximately 10 days, then ages or is involved in the formation of haemostatic plugs, and then is removed by macrophages of the mononuclear phagocytic system (discussed in the next section), mostly in the spleen.
Lymphoid organs
The lymphoid system is closely integrated with the circulatory system. The role of lymphoid organs in the immune response is discussed in Chapter 12. Lymphoid organs are sites of residence, proliferation, differentiation, or a function of lymphocytes and mononuclear phagocytes (monocytes, namely the macrophages). The liver also has important haematological functions, including the production of clotting factors (described in Chapter 26).
The spleen
The spleen is one of the largest of the lymphoid organs. It is a site of fetal haematopoiesis (blood cell production), phagocytes within the spleen filter and cleanse the blood, splenic lymphocytes mount an immune response to blood-borne microorganisms and it serves as a blood reservoir.
The spleen is a concave organ that weighs approximately 150 g and is about the size of a fist. It is located in the left upper abdominal cavity, curved around a portion of the stomach. Strands of connective tissue (trabeculae) extend throughout the spleen from the splenic capsule, dividing it into compartments that contain masses of lymphoid tissue called splenic pulp. The spleen is interlaced with many blood vessels, some of which can distend to store blood.
Blood that circulates through the spleen first encounters the white splenic pulp, which consists of masses of lymphoid tissue containing lymphocytes and macrophages. The white pulp forms clumps around the splenic arterioles and is the chief site of immune and phagocytic function within the spleen. Here blood-borne antigens encounter lymphocytes, initiating an immune response (see Chapter 12).
Some of the blood continues through the microcirculation and enters highly distensible storage areas called venous sinuses. Most of the blood, however, oozes through the capillary walls into the principal site of splenic filtration, the red pulp. Here the resident macrophages of the mononuclear phagocytic system phagocytose damaged or old blood cells of all kinds (but mainly erythrocytes), microorganisms and particles of debris. Haemoglobin from phagocytosed erythrocytes is broken down, as discussed in the section on normal destruction of aged erythrocytes. Blood that filters through the red pulp of the spleen then moves through the venous sinuses and into the portal circulation.
The venous sinuses (and the red pulp) can store more than 300 mL of blood. Sudden reductions in blood pressure cause the sympathetic nervous system to stimulate constriction of the sinuses and expel as much as 200 mL of blood into the venous circulation, helping to restore blood volume or pressure in the circulation.
You can actually survive without a spleen and there are many individuals without a spleen who live healthy lives. If the spleen is removed surgically (known as a splenectomy), a number of haematological effects may be seen. For example, leucocytosis (high levels of circulating leucocytes) often occurs after splenectomy, so the spleen may exert some control over the rate of proliferation of leucocyte cells. After splenectomy, iron levels in the circulation are decreased, immune function is diminished and the blood contains more structurally defective blood cells than normal.
Lymph nodes
Structurally, lymph nodes are part of the lymphatic system (see Chapter 22). Thousands of lymph nodes are clustered around the lymphatic veins, which collect interstitial fluid from the tissues and transport it as fluid, known as lymph, back into the cardiovascular system near the heart. Although the lymph nodes are structurally associated with the cardiovascular system, functionally lymph nodes are part of the haematological and immune systems because large numbers of lymphocytes, monocytes and macrophages develop or function within the lymph nodes. As the lymph filters through the bean-shaped lymph nodes clustered in the inguinal, axillary and cervical regions of the body, it is cleansed of foreign particles and microorganisms by the monocytes and macrophages. The microorganisms in lymph stimulate the resident lymphocytes to develop into antibody-producing plasma cells. During an infection, the rate of proliferation of macrophages within the nodes is so great that the nodes enlarge and become tender.
Each lymph node is enclosed in a fibrous capsule (see Figure 16-6), with strands of connective tissue (trabeculae) extending inwards, dividing the node into several compartments. Reticular fibres divide the compartments into smaller sections and trap and store large numbers of lymphocytes, monocytes and macrophages. The node has an outer cortex area and an inner medullary area. Within the cortex are germinal centres or separate masses of lymphoid tissue. Lymph enters the node, slowly filters through its sinuses and leaves through the efferent lymphatic vessel.
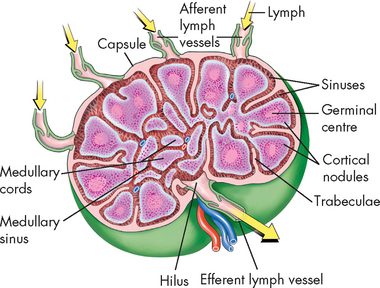
FIGURE 16-6 Cross-section of lymph node.
Several afferent valved lymphatics bring lymph to the node. A single efferent lymphatic leaves the node at the hilus. Note that the artery and vein also enter and leave at the hilus. Arrows show direction of lymph flow.
Source: Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The mononuclear phagocyte system
The mononuclear phagocyte system consists of the cells that originate in the bone marrow, differentiate to monocytes that are transported by the bloodstream and finally settle in the tissues as mature macrophages.3 The main examples of these cells are the monocytes and macrophages. Table 16-2 lists the various names given to macrophages localised in specific tissues.
Table 16-2 THE MONONUCLEAR PHAGOCYTE SYSTEM
| NAME OF CELL | LOCATION |
|---|---|
| Monocyte macrophages | Bone marrow and peripheral blood |
| Kupffer cells (macrophages) | Liver |
| Alveolar macrophages | Lungs |
| Macrophages | Bone marrow |
| Fixed and free macrophages | Spleen and lymph nodes |
| Pleural and peritoneal macrophages | Serous cavities |
| Microglial cells | Nervous system |
| Mesangial cells | Kidneys |
| Osteoclasts | Bone |
| Langerhans cells | Skin |
| Dendritic cells | Lymphoid tissue |
The cells of the mononuclear phagocyte system are mostly in the liver and spleen, and function by ingesting and destroying (phagocytic process) foreign substances. They are important in the defence against bacteria and other microorganisms in the bloodstream. In addition, it cleanses the blood of old, injured or dead erythrocytes, leucocytes, platelets, coagulation products, antigen-antibody complexes and macromolecules. These cells also process and present foreign antigens to the immune system (see Chapter 12).
The origin and life span of all the tissue macrophages named in Table 16-2 are not precisely known. Once monocytes leave the circulation, they do not return. In the tissues, monocytes differentiate into macrophages without dividing and can survive for many months or perhaps even years. Under normal circumstances, macrophages show little evidence of mitotic division, but production can be rapidly elevated in response to need, as in infection.
THE DEVELOPMENT OF BLOOD CELLS
We now turn to the development of blood cells, which when altered can cause systemic problems that affect many body functions.
Haematopoiesis
The typical adult requires about 100 billion new blood cells per day. Blood cell production, termed haematopoiesis, is constantly ongoing, occurring in the liver and spleen of the fetus and in the bone marrow after birth. This process involves the stimulation of populations of young undeveloped cells (i.e. undifferentiated) to undergo mitotic division (i.e. proliferation) and maturation into mature haematological cells.4 The process of cells changing their appearance (morphology) and function as they develop is known as differentiation. During the stages of production of blood cell types, proliferation occurs a few times and then ceases, while differentiation continues. Erythrocytes and neutrophils generally differentiate fully before entering the blood, but monocytes and lymphocytes do not. When considering the process of cell development, it is possible to visualise a line of growth from a stem cell through to immature forms and eventually to the mature end-stage cell. A collection of cells at different stages of development for a particular cell type (from stem cell to immature cell to mature cell) is known as a cell line, or lineage.
Haematopoiesis continues throughout life and importantly increases in response to disease and certain conditions. For instance, haemorrhage, haemolytic anaemia (in which erythrocytes are destroyed), chronic infection and other disorders that deplete blood cells all cause an increase in blood cell production. This response is a homeostatic mechanism that can be investigated by clinicians to determine the severity of a condition or as a general guide to locating alterations such as the source of infection. In general, long-term stimuli, such as chronic disease, cause a greater increase in haematopoiesis than acute conditions, such as haemorrhage.
Bone marrow
Bone marrow is confined to the cavities of bone. It consists of blood vessels, nerves, mononuclear phagocytes, blood cells in various stages of differentiation and fatty tissue. Adults have two kinds of bone marrow: (1) red or active (haematopoietic) marrow (also called myeloid tissue) and (2) yellow or inactive marrow. The large quantities of fat in inactive marrow make it yellow. Not all bones contain active marrow. In adults, active marrow is found primarily in the flat bones of the pelvis (34%), vertebrae (28%), cranium and mandible (13%), sternum and ribs (10%) and in the extreme proximal portions of the humerus and femur (4–8%). Inactive marrow predominates in cavities of other bones. (Bones are discussed further in Chapter 20.)
Haematopoietic marrow receives oxygen and nutrients needed for cellular differentiation from the primary arteries of the bones. Branches of these arteries terminate in a capillary network that coalesces into large venous sinuses, which eventually drain into a central vein. Haematopoietic marrow and fat fill the spaces surrounding the network of venous sinuses. Newly produced blood cells traverse narrow openings in the venous sinus walls and thus enter the circulation. Normally, cells do not enter the circulation until they have differentiated to a certain extent, but premature release occurs in certain diseases.
Cellular differentiation
The bone marrow contains a population of haematopoietic stem cells known as haemocytoblasts. These are stem cells that have partially differentiated (see Figure 16-7)5,6 such that they are committed to become a blood cell — they have the capacity to differentiate into any of the haematological cell populations, but can no longer differentiate into other cell types, like nerve or muscle cells.
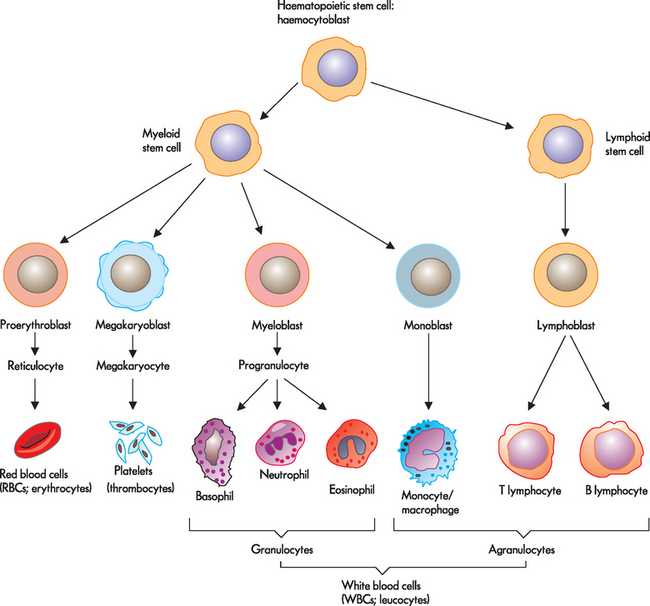
FIGURE 16-7 Differentiation of a stem cell into red blood cells, platelets and white blood cells.
Source: Based on Herlihy B. The human body in health and illness. 3rd edn. St Louis: Saunders; 2007.
As with all stem cells, haematopoietic stem cells are self-renewing (they have the ability to proliferate or reproduce, without further differentiation or maturation) so that a relatively constant population of stem cells is available. This means that when production of blood cells is necessary, the haematopoietic stem cell will proliferate into two cells: one remains in the bone marrow, while the other undergoes differentiation into a mature blood cell. The haematopoietic stem cell may develop into a myeloid stem cell, whereby the cell undergoes development and maturation within the bone marrow, or a lymphoid stem cell, whereby the cell undergoes maturation outside the bone marrow. During further stages of differentiation, some haematopoietic stem cells will become haematopoietic progenitor (immature) cells. Progenitor cells retain the capacity to continue proliferating, but are committed to further differentiation into particular types of haematological cells: lymphoid (B cells, T cells, natural killer cells), granulocyte/monocyte (granulocytes, monocytes, macrophages) and megakaryocyte/erythroid (platelets, erythrocytes) progenitor cells. These progenitor cells are also referred to as colony-forming units.
Several cytokines participate in haematopoiesis, particularly colony-stimulating factors (or haematopoietic growth factors), which stimulate the proliferation of progenitor cells and their progeny and initiate the maturation events necessary to produce fully mature cells. Multiple cell types, including endothelial cells, fibroblasts and lymphocytes, produce these important colony-stimulating factors. In addition, the hormones erythropoietin and thrombopoietin stimulate the production of erythrocytes and platelets, respectively.
When neutrophils are released to the peripheral blood they either circulate or adhere to the walls of the blood vessels (often called the marginating storage pool), mainly in areas where blood flow is slow. These cells can be rapidly mobilised to move into tissues and mucous membranes when needed, such as during bacterial infection. Cells from the circulating pool join the marginating pool to replace the cells that have migrated out of the capillaries. In addition, immature neutrophils can be released from the bone marrow when the body is challenged by infection, and in this way the bone marrow can be considered as having a storage component for the neutrophil cell line. Thus a raised white cell count (leucocytosis) can be seen in response to bacterial infection. In this case, the increase in the white cell count is due to an increase in the numbers of neutrophils circulating in the blood (known as neutrophilia).
Approximately one-third of platelets are stored in the spleen (known as splenic sequestration) while the remainder circulate. Under certain conditions, the level of circulating haematological cells needs to be rapidly replenished. Haematopoiesis in the bone marrow can be accelerated by expansion of the bone marrow, faster differentiation of the daughter cells or faster proliferation of stem cells.
The development of erythrocytes
For almost 100 years it was believed that erythrocytes developed from lymphocytes that were transformed in the spleen. It was not until the 1950s that the bone marrow was identified as the site of erythropoiesis, or development of red blood cells (see Figure 16-8). Approximately 1% of the body’s circulating erythrocyte mass normally is generated every 24 hours.
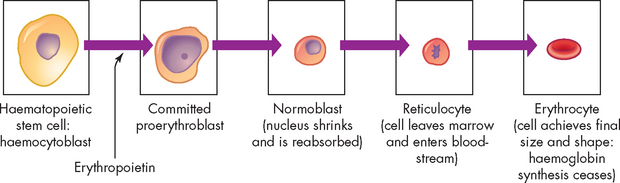
FIGURE 16-8 Erythrocyte differentiation.
Erythrocyte differentiation from large, nucleated stem cell to small, nonnucleated erythrocyte.
In the confines of the bone marrow some haematopoietic stem cells will differentiate into large, nucleated proerythroblasts, which are committed to producing erythrocytes. The proerythroblast differentiates through several intermediate forms of erythroblast and normoblast while progressively eliminating most intracellular structures, including the nucleus. Haemoglobin will be produced in large quantities and the cell becomes more compact as it matures, eventually taking on the shape and characteristics of an erythrocyte.
The last immature form is the reticulocyte, which contains a mesh-like (reticular) network of ribosomal RNA that is visible microscopically after staining with certain dyes. Reticulocytes remain in the marrow for approximately 1 day and are released into the venous sinuses. They continue to mature in the bloodstream and may travel to the spleen for several days of additional maturation. The normal reticulocyte count is 1% of the total red blood cell count. Therefore, the reticulocyte count is a useful clinical index of erythropoietic activity: a raised reticulocyte count would be consistent with increased production of red cells in the bone marrow, whereas a low reticulocyte count indicates a failure to produce sufficient new red cells.
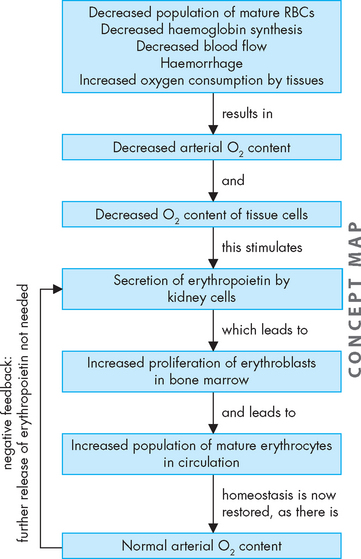
Most steps of this process are primarily under the control of the hormone erythropoietin.7 In healthy humans, the total volume of circulating erythrocytes remains surprisingly constant. In conditions of tissue hypoxia, erythropoietin is secreted by the kidneys (see Figure 16-9). It causes a compensatory increase in erythrocyte production if the oxygen content of blood decreases because of anaemia, high altitude or pulmonary disease. The normal rate of production (an amazing 2.5 million erythrocytes per second) can increase (to an even more startling 17 million per second) under anaemic or low-oxygen states. The body responds to reduced oxygenation of blood in two ways:
Haemoglobin production
Haemoglobin is the protein contained within red cells that is responsible for carrying oxygen from the lungs to the tissues. Under normal circumstances red cells are saturated with haemoglobin and a single erythrocyte may contain as many as 300 million haemoglobin molecules. Haemoglobin consists of four globin protein chains, each of which contains a haem group — hence the name. Each haem group contains an iron atom. It is the iron contained within the haem of haemoglobin that binds to and releases oxygen.
Haemoglobin is responsible for the red colour of erythrocytes. As erythrocytes are much more numerous than other blood cells, blood appears red in colour. Arterial blood contains oxyhaemoglobin and has a bright red colour, whereas venous blood has more deoxyhaemoglobin and has a darker red colour by comparison.
Haemoglobin A is the specific type of haemoglobin that occurs in adults. A form of haemoglobin with a slightly different structure, haemoglobin F, is found in the fetus. Haemoglobin F in the fetus has a stronger attraction for oxygen than haemoglobin A found in the mother, facilitating the movement of oxygen from the mother’s erythrocytes to those of the fetus. These (and other) types of haemoglobin exist to cope with the different oxygenation conditions found in the embryo, fetus and atmospheric environment.
Molecules other than oxygen can competitively bind to haemoglobin. Carbon monoxide (CO) directly competes with oxygen to bind to iron, with an ability that is about 200-fold greater than oxygen. Thus, even a small amount of CO can dramatically decrease the ability of haemoglobin to bind and transport oxygen. Haemoglobin also binds carbon dioxide (CO2), but at a binding site separate from where oxygen binds. Therefore, oxygen and carbon dioxide do not directly compete for binding sites on the haemoglobin.
In addition to transporting oxygen, haemoglobin has an important role to play in transporting carbon dioxide produced in the tissues back to the lungs for expiration, as well as in maintaining the acid–base balance in the blood (buffering). The role of haemoglobin in the transport of oxygen and carbon dioxide is discussed in detail in Chapter 24, while acid–base balance is explored in Chapter 29.
Erythrocytes may play a role in the maintenance of vascular relaxation. Nitric oxide (NO) produced by blood vessels is a major mediator of relaxation and dilation of the vessel walls. In the lungs, haemoglobin can bind oxygen to iron and nitric oxide to globin. As haemoglobin transfers its oxygen to tissues, it may also shed small amounts of nitric oxide, contributing to dilation of the blood vessels and assisting oxygen delivery. (See Chapter 13 for the role of nitric oxide in inflammation.)
Nutritional requirements for erythropoiesis
Normal development of erythrocytes and synthesis of haemoglobin depend on an optimal biochemical state and adequate supplies of protein, vitamins and minerals. Additional specific nutritional requirements for the production of erythrocytes are iron, vitamin B12 (cobalamin) and folate. It is important to note that these minerals and vitamins are required by other cells of the body as well as erythrocytes. If these components are lacking for a prolonged time, erythrocyte production slows and anaemia (an insufficient number of functional erythrocytes) may result (see Chapter 17).
Iron cycle
Approximately 67% of total body iron is bound to haem in erythrocytes (haemoglobin) and muscle cells (myoglobin) and approximately 30% is stored in mononuclear phagocytes (i.e. macrophages) and hepatic cells as either ferritin or haemosiderin. The remaining 3% (less than 1 mg) is lost daily in urine, sweat, bile and epithelial cells shed from the gut. Iron is transported in the blood bound to transferrin, a glycoprotein produced primarily by the liver. Iron that will be used for haemoglobin production is carried by transferrin to the bone marrow, where it binds to transferrin receptors on erythroblasts. The iron is transported inside the erythroblast to the mitochondria, the site of haemoglobin production, where an enzyme incorporates it into a molecule to form haem.
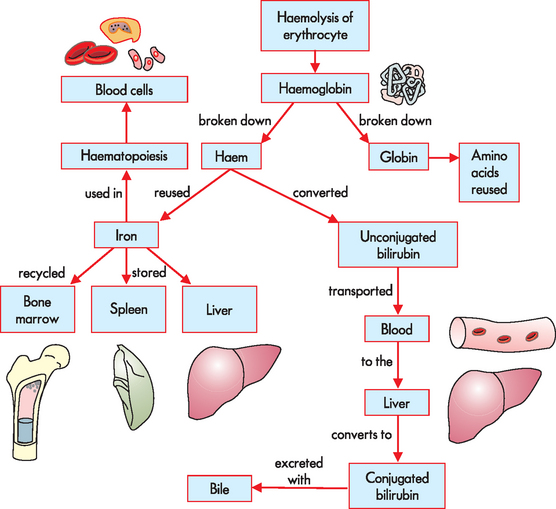
Aged or damaged erythrocytes are removed from the bloodstream by macrophages of the mononuclear phagocyte system (described above), largely in the spleen. Here, macrophages undergo phagocytosis of the erythrocytes, which results in release of the haemoglobin. The haemoglobin molecule is broken down and the iron is stored as ferritin or haemosiderin. The stored iron is released into the bloodstream, where it binds to transferrin so that it can be recycled (see Figure 16-10).
Iron balance is maintained through controlled absorption at the intestines. Regulation of iron transport across the plasma membrane of gastrointestinal epithelial cells is related to the cell’s iron content and the overall rate of erythropoiesis. If the body’s iron stores are low or the demand for erythropoiesis increases, iron is transported rapidly through the intestinal epithelial cell and into the plasma. If body stores are high and erythropoiesis is not increased, iron crosses the epithelial cell plasma membrane passively and is temporarily stored as ferritin. Excretion of iron occurs when the epithelial cells of the intestinal mucosa slough off.
Normal destruction of aged erythrocytes
Although mature erythrocytes lack nuclei, mitochondria and endoplasmic reticulum, they do have cytoplasmic enzymes capable of producing small quantities of ATP. ATP provides the energy required to maintain cell function and cell membrane flexibility, which is needed to allow the erythrocyte to fold on entering small blood vessels. As the erythrocyte ages, less ATP is available to maintain cell membrane function. The older (senescent) red cell becomes increasingly fragile and loses its reversible deformability, becoming susceptible to rupture while passing through the narrow regions of the microcirculation. Additionally, the cell membrane of aged erythrocytes undergoes rearrangement such that phospholipid normally only found within the cell membrane is exposed on the cell surface. This is recognised by receptors on macrophages (primarily in the spleen), which selectively remove the red cells. If the spleen has reduced function or is absent, macrophages in the liver (Kupffer cells) take over.
During digestion of haemoglobin in the macrophage, the breakdown of haem produces the waste product bilirubin, which is transported to the liver and metabolised further to be secreted with the bile into the intestines (see Figure 16-10). Bacteria in the intestinal lumen transform bilirubin into urobilinogen. Most urobilinogen is excreted in faeces, although some also reaches the blood and is excreted by the kidneys in the urine, which can be detected by urinalysis.
The development of leucocytes
All leucocytes arise from stem cells in the bone marrow (their pathways of differentiation are shown in Figure 16-7). The process of leucocyte production is known as leucopoiesis. Lymphoid progenitor cells develop into lymphocytes, which are released into the bloodstream to undergo further maturation in the primary and secondary lymphoid organs (see Chapter 12). Monocyte progenitors develop into monocytic cells, which continue maturing into macrophages after release into the bloodstream and entrance into various tissues. Progenitor cells for granulocytes fully mature in the marrow into neutrophils, eosinophils and basophils and are released into the blood.
The bone marrow selectively retains immature granulocytes as a reserve pool that can be rapidly mobilised in response to the body’s needs. Further maturation is under the control of several haematopoietic growth factors, including interleukins, granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF).
Leucocyte production increases in response to infection, to the presence of steroids and to the reduction or depletion of reserves in the marrow. It is also associated with strenuous exercise, convulsive seizures, heat, intense radiation, increased heart rate, pain, nausea and vomiting and stress.
The development of platelets
Platelets are derived from large cells found in the bone marrow known as megakaryocytes. As with other blood cells megakaryocytes originate from a stem cell and have a number of progenitor stages (see Figure 16-7).8,9 The process of platelet production is known as thrombopoiesis. During thrombopoiesis, the megakaryocyte progenitor is programmed to undergo a cell cycle during which DNA replication occurs, but anaphase and cytokinesis are blocked (see Chapter 5). Thus, the megakaryocyte nucleus enlarges (containing up to 100-fold more than the normal amount of DNA) without cellular division. Concurrently, the numbers of cytoplasmic organelles (e.g. internal membranes, granules) increase and the cell develops cellular surface elongations and branches that progressively fragment into platelets. Like erythrocytes, platelets released from the bone marrow lack nuclei.
Thrombopoietin, a hormone growth factor, is the main regulator of the circulating platelet mass. Thrombopoietin is primarily produced by the liver and induces platelet production in the bone marrow. Platelets express receptors for thrombopoietin and when circulating platelet levels are normal, thrombopoietin is adsorbed onto the platelet surface and prevented from accessing the bone marrow and stimulating further platelet production.
An optimal number of platelets and committed platelet precursors (megakaryoblasts) in the bone marrow is maintained primarily by thrombopoietin produced by the liver and kidneys, together with other factors such as GM-CSF. These factors affect the rate of differentiation into megakaryocytes and the rate of platelet release. About two-thirds of platelets enter the circulation and the remainder reside in the splenic pool. Platelets circulate in the blood stream for about 10 days before beginning to lose their ability to carry out biochemical processes. These senescent (ageing) platelets are removed from the circulation in the spleen by mononuclear cell phagocytosis.
THE MECHANISMS OF HAEMOSTASIS
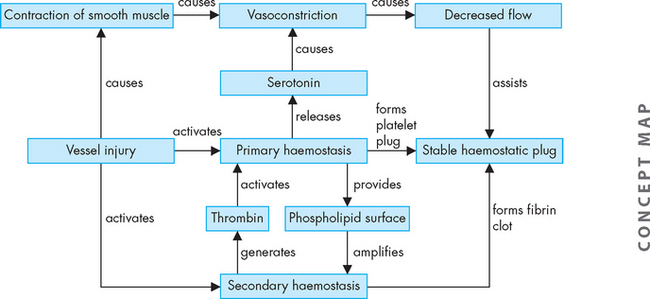
Haemostasis means arrest of bleeding. As a result of haemostasis, damaged blood vessels may maintain a relatively steady state of blood volume, pressure and flow. Three main processes occur in haemostasis to prevent further loss of blood:
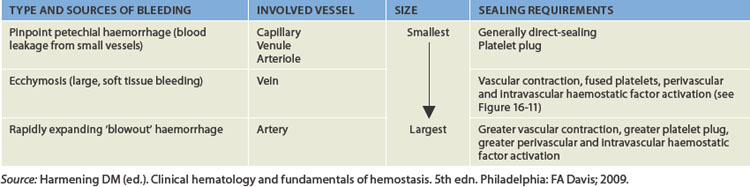
Although damage to small vessels can be repaired relatively quickly with limited blood loss, damage to large vessels depends on powerful vasoconstriction to decrease the blood flow and hence blood loss (see Table 16-3).
In the following sections, we discuss how the blood vessels, platelets and clotting factors achieve haemostasis.
The function of platelets and blood vessels
 contribute to regulation of blood flow at a damaged site through induction of vasoconstriction (vasospasm)
contribute to regulation of blood flow at a damaged site through induction of vasoconstriction (vasospasm) adhere to damaged blood vessels and initiate platelet-to-platelet interactions resulting in the formation of a platelet plug to stop further bleeding
adhere to damaged blood vessels and initiate platelet-to-platelet interactions resulting in the formation of a platelet plug to stop further bleedingPlatelets normally circulate freely as disc-shaped particles, suspended in plasma, in an unactivated state, which means that they are ready to be involved in haemostasis, but do not undergo any changes unless stimulated. Endothelial cells lining the vessels produce nitric oxide and prostacyclin I2 (PGI2) to inhibit platelet activation allowing free circulation. This is very important, because in this way, platelets are able to travel through the blood, while not contributing to haemostasis unless it is necessary; therefore, unwanted clotting is prevented.
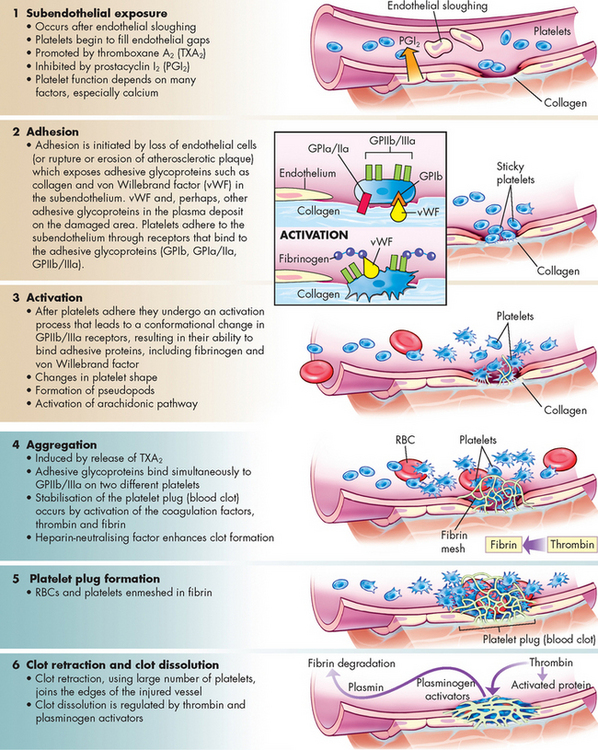
When a vessel is damaged, platelet activation may be initiated.10,11 Activated platelets radically change shape, develop spikes termed pseudopod-like cytoplasmic extensions and become sticky. Activation proceeds through a process of increasing platelet adhesion, aggregation and activation. Initially, platelets adhere weakly to the vessel wall, followed by increased strength of adherence to the vessels and aggregation between platelets. This creates a platelet plug at the site of injury. The process of platelet plug formation is known as primary haemostasis. The platelet plug is not secure and can be easily dislodged, so a more permanent meshwork of platelets and fibrin subsequently develops (see Figure 16-11). The plasma enzyme system that produces insoluble fibrin strands at the wound site is known as secondary haemostasis. The primary and secondary haemostasis systems work together to prevent blood loss at sites of vascular injury.
The process of platelet activation can begin in several ways. If the vessel lining remains intact in an area of inflammation, the endothelial cells may become activated and begin expressing new proteins on their surface, which enable platelets to adhere to the endothelium. As inflammation progresses, the platelets adhere to each other by binding to fibrinogen. Platelets are therefore able to aggregate together by forming platelet-fibrinogen-platelet bridges.
Rupture of the endothelial layer during blood vessel damage results in exposure of the underlying matrix that contains collagen and von Willebrand factor (vWF) (see Figure 16-11). Platelets adhere strongly to both collagen and vWF, so exposure of the subendothelium initiates platelet adhesion. Progressively the platelets undergo further aggregation through platelet-to-platelet adhesion involving further fibrinogen bridging between adjacent platelets.
As a result of interactions with the subendothelial matrix, as well as exposure to inflammatory mediators produced by the endothelium and other cells, the platelets become activated. Activation results in dynamic changes in platelet shape from smooth spheres to those with spiny projections and also causes the granules contained within platelets to release their contents (known as degranulation, or the platelet-release reaction). This results in various potent substances being shed from platelets into the surrounding environment. Many of these molecules have roles to play in haemostasis.
Platelets are capable of releasing numerous substances that contribute to haemostasis. These include ADP, serotonin and calcium:
 ADP reacts with specific receptors on platelets to induce further adherence (and subsequent release-reaction) of nearby platelets. The activated platelets aggregate together to cause a platelet plug to seal the injured endothelium.
ADP reacts with specific receptors on platelets to induce further adherence (and subsequent release-reaction) of nearby platelets. The activated platelets aggregate together to cause a platelet plug to seal the injured endothelium. Serotonin functions like histamine and has immediate effects on smooth muscle in the vascular endothelium, causing an immediate temporary constriction of the injured vessel (see Chapters 13 and 22). Vasoconstriction reduces blood flow and diminishes bleeding. However, this lasts for only a short time (up to 30 minutes), as vasodilation soon follows, permitting the inflammatory response to proceed.
Serotonin functions like histamine and has immediate effects on smooth muscle in the vascular endothelium, causing an immediate temporary constriction of the injured vessel (see Chapters 13 and 22). Vasoconstriction reduces blood flow and diminishes bleeding. However, this lasts for only a short time (up to 30 minutes), as vasodilation soon follows, permitting the inflammatory response to proceed. Calcium is necessary for many of the intracellular signalling mechanisms that control platelet activation and is essential for the enzymes of the secondary haemostasis system to function.
Calcium is necessary for many of the intracellular signalling mechanisms that control platelet activation and is essential for the enzymes of the secondary haemostasis system to function.Activated platelets also produce thromboxane A2 (TXA2), which causes vasoconstriction and promotes attraction of additional platelets to contribute to plug formation. In addition, platelets release some clotting factors (such as fibrinogen and factor V), which enhance the process of coagulation.
If blood vessel injury is minor, haemostasis is achieved temporarily by formation of the platelet plug, which usually forms within 3–5 minutes of injury. Platelet plugs seal the many minute ruptures that occur daily in the microcirculation, particularly in capillaries. If there are too few platelets or if platelets do not function properly, damaged capillaries may leak, resulting in numerous small haemorrhagic areas under the skin and tissues. When this occurs under the skin a purple discolouration results (purpura).
The function of clotting factors
A blood clot is a meshwork of protein strands that stabilises the platelet plug. Although other cells such as erythrocytes and leucocytes may become trapped in the clot, they do not contribute to its formation (see Figure 16-12). The strands are made of fibrin, which is produced by secondary haemostasis (coagulation).12 This system is traditionally described by the coagulation cascade model, comprising inactive enzymes or coagulation factors that are produced by the liver and are circulating in the blood; these coagulation factors activate each other in a sequence of complex reactions after an initiating event (e.g. blood vessel damage). The end result of this series of reactions is the conversion of fibrinogen to fibrin to form a stable blood clot. This is important, as the platelet plug formed by primary haemostasis is not stable and can be dislodged easily.
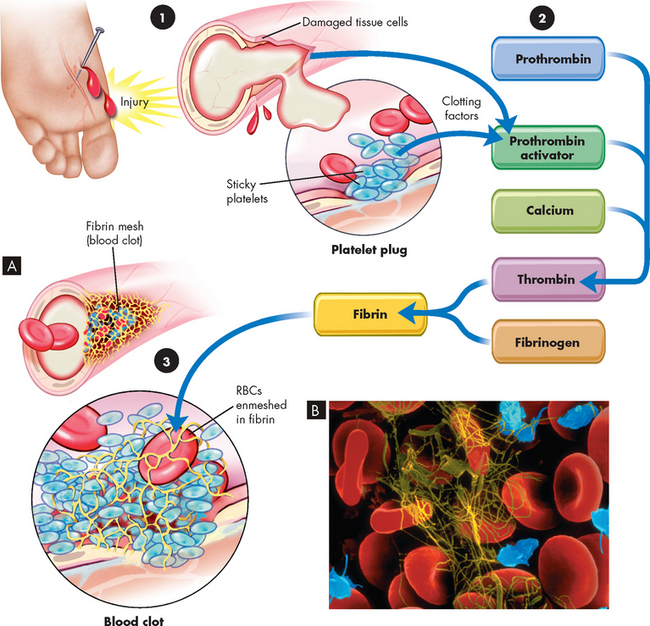
FIGURE 16-12 The blood clotting mechanism.
A The clotting mechanism involves release of platelet factors at the injury site, formation of thrombin and trapping of red blood cells (RBCs) in fibrin to form a clot. B An electron micrograph showing entrapped red blood cells in a fibrin clot.
Source: A Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007. B Copyright by Dennis Kunkel Microscopy, Inc.
While it is necessary that an efficient system exists to prevent blood loss, it is equally important that clot formation can be stopped when required. Although excessive bleeding (haemorrhage) can be life-threatening — as blood volume will be dramatically reduced and the individual will not be able to adequately oxygenate — in clinical practice unwanted clotting is far more common. Unwanted clot formation (thrombosis) can have serious and fatal consequences. For instance, clot formation in the coronary arteries can lead to acute myocardial infarction (discussed in Chapter 23), pulmonary system clots can cause infarction of lung tissue (see Chapter 25) and clots in cerebral arteries can lead to stroke (refer to Chapter 9). All these conditions can be fatal. Therefore, the coagulation system has many intricate control mechanisms to either promote or inhibit clot formation and under normal circumstances a balance exists between these processes.
The coagulation system is usually presented as two pathways of initiation (intrinsic and extrinsic pathways) that join in a common pathway (see Figure 16-13). The intrinsic pathway is activated when factor XII in plasma contacts subendothelial substances such as collagen, which are exposed by vascular injury. Factor XIIa becomes activated and can then activate factor XI and a sequence of reactions follows. The extrinsic pathway is activated when tissue factor, a substance exposed by vascular damage, reacts with clotting factor VII. Both the intrinsic and extrinsic pathways lead to the common pathway and activation of factor X, which converts prothrombin to thrombin. Thrombin then converts fibrinogen to fibrin. Thrombin also activates factor XIII, which cross-links the fibrin strands to form a stable clot formed of a meshwork of fibrin. This fibrin mesh traps the blood components and prevents them from exiting the vessel wound.
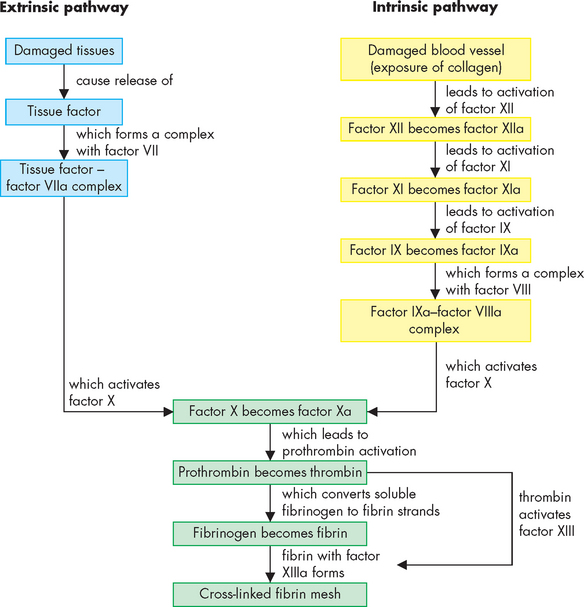
FIGURE 16-13 A simplified schematic of the steps involved in blood clotting.
The intrinsic pathway is initiated by processes within (intrinsic to) the blood vessel lumen. The extrinsic pathway is initiated by processes outside the blood vessel lining, such as the collagen underneath the blood vessel endothelium. Damage to a blood vessel would usually activate both pathways. The common pathway starts with activation of factor X, and leads to the conversion of soluble fibrinogen into fibrin strands that become cross-linked into a stable fibrin mesh. Importantly, several steps in all three pathways are dependent on calcium (available in the blood). Furthermore, several steps are enhanced by substances from platelets, such that processes in the formation of the platelet plug and the fibrin mesh overlap to contribute to haemostasis. The small ‘a’ beside the factor number indicates the active form.
The coagulation system is complex, with a large number of alternative activators and inhibitors. There is also interaction between the intrinsic and extrinsic pathways so that an activated member of one pathway may activate a member of the other pathway (e.g. activated factor VII of the extrinsic pathway can directly activate factor IX of the intrinsic pathway). Tissue factor is a powerful stimulant of the coagulation system and produces a greater amount of fibrin clot at a faster rate than the intrinsic system.
Activated platelets are important participants in clotting. During activation, phospholipids in the platelet cell membrane undergo change resulting in the formation of several important complexes of clotting factors. In this way, platelets contribute to coagulation.
The interaction of the different contributing mechanisms in blood clot formation is shown in Figure 16-14.
Natural substances that limit coagulation and platelet plug formation
A variety of substances, some of which are products of the coagulation system itself, control coagulation.13 For example, thrombin can bind to the endothelial cell surface to activate protein C in plasma. This leads to inhibition of some coagulation factors, which serves as a means to control fibrin production. Thrombin therefore not only promotes clot formation by converting fibrinogen to fibrin, but also acts to control the clotting process.
Antithrombin III is a powerful naturally occurring anticoagulant. As suggested by its name, this molecule inhibits thrombin; however, it also inhibits most other coagulation enzymes as well. When antithrombin III combines with heparin, the inhibitory activity to thrombin is increased 2000–10,000 times. Heparin is found naturally in the body and is secreted from intact endothelial cells and mast cells; in addition, heparin is also administered therapeutically as a rapid-acting anticoagulant.
Tissue factor pathway inhibitor is another naturally occurring anticoagulant that helps to control coagulation. It is produced in the liver, like most proteins associated with coagulation, but is also found in platelets. Tissue factor pathway inhibitor inhibits the extrinsic coagulation pathway by acting against the tissue factor–activated factor VII complex. It can also inhibit activated factor X.
Prostacyclin is produced by intact endothelial cells. It promotes vasodilation and inhibits platelet activation processes when there is no endothelial cell damage; in this way, unnecessary platelet plug formation is prevented. In addition, when endothelial cells are intact the underlying collagen is not exposed, which means that the platelets are unable to adhere to the vessel wall.
In platelets, an enzyme called cyclo-oxygenase (COX-1) converts arachidonic acid to TXA2. Aspirin irreversibly inhibits COX-1 in platelets, decreasing production of TXA2 and decreasing platelet activation, making it an effective antiplatelet drug in patients at risk of arterial thrombosis. However, aspirin also affects other body cells, leading to side effects such as gastric irritation. Hence, when aspirin is used to prevent thrombosis, it is given in low doses.
Clot retraction and fibrinolysis
After a clot is formed, it undergoes clot retraction as it ‘solidifies’. Fibrin strands shorten, becoming denser and stronger, which pulls the edges of the injured vessel wall closer and seals the site of injury. Retraction is facilitated by the large numbers of platelets trapped within the fibrin meshwork. The platelets contract and ‘pull’ the fibrin threads closer together while releasing a factor that stabilises the fibrin. Contraction expels protein-free serum from the fibrin meshwork. This process usually begins within a few minutes after a clot has formed, and most of the serum is expressed within 20–60 minutes. The process of clot retraction is important in making the clot more secure.
Fibrinolysis (breakdown) of blood clots is necessary in order to remove a clot once the vessel is undergoing repair. It is carried out by the fibrinolytic system (see Figure 16-15).14 Another plasma protein, plasminogen, is converted to plasmin by plasminogen activator as well as activated factor XII. Plasmin is an enzyme that dissolves clots by degrading fibrin into fibrin-degradation products. The fibrinolytic system removes clotted blood from tissues and dissolves small clots (thrombi) in blood vessels. This is important for the restoration of normal blood flow. A balance between the amounts of thrombin and plasmin in the circulation maintains normal coagulation and lysis.
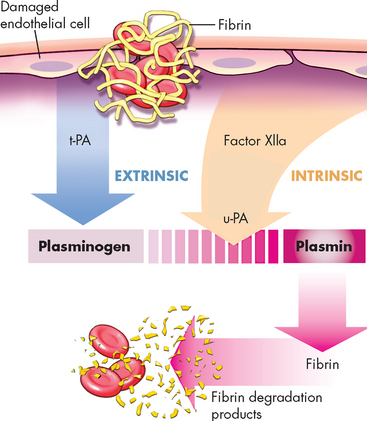
FIGURE 16-15 The fibrinolytic system.
The central reaction is the conversion of plasminogen to the enzyme plasmin. Conversion of plasminogen is achieved by the action of tissue plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA) as well as activated factor XII (factor XIIa). Plasmin splits fibrin in the clot into fibrin-degradation products.
BLOOD GROUPS
There are hundreds of different known blood group antigens found on red blood cells; however, only a few of these are generally considered to be clinically significant. The two most important blood group systems are the ABO system and the Rhesus (Rh) system.
It is important to review the terms antigen and antibody when discussing blood groups. An antigen is a molecule that is capable of being recognised as foreign by an immune system, whereas an antibody is a protein (immunoglobulin) produced by an immune system after recognising a foreign antigen. The antibody that is produced is manufactured to specifically bind to the antigen that has been recognised. Refer to Chapter 12 for further discussion of antigens and antibodies.
If blood from one individual is transfused to another, there is a risk that the donor red cells may have different antigens to the recipient. In the case of an ABO blood group difference (incompatibility), this can cause a haemolytic transfusion reaction, which can be fatal. This is why the ABO blood group system is the most clinically significant.
The ABO system
There are four ABO blood groups:
The ABO blood groups are determined by the presence or absence of certain sugar molecules attached to the surface of red blood cells. Individuals with the A antigen (N-acetygalactosamine) attached to their red blood cells are group A. Individuals with the B antigen (galactose) are group B. Some individuals have both A and B antigens on their red blood cells and hence are group AB. Finally, individuals with neither antigen are group O (refer to Table 5-1). The inheritance of the ABO blood groups is discussed in Chapter 5.
At birth, antibodies to ABO antigens are not found in the plasma. Ingestion of bacteria with food or from the surrounding environment exposes the immune system to antigens that are almost identical to the ABO antigens found on erythrocytes. If an individual is exposed to an antigen that they do not possess, an antibody to that antigen will be made. For example, a group A person does not have the group B antigen and therefore will manufacture an antibody to the B antigen (known as an anti-B antibody). A group B person will similarly acquire an anti-A antibody. A group O person, lacking both A and B antigens, will acquire both anti-A and anti-B antibodies, whereas a group AB person, having both A and B antigens, will not acquire any ABO antibodies. Antibodies that are acquired in this way are known as naturally occurring antibodies. Table 16-4 provides a summary of the ABO blood group system and shows the different ABO antibodies that are found in the plasma of individuals of different ABO blood groups.
The presence of naturally occurring ABO blood group system antibodies creates a potentially dangerous situation with respect to blood transfusions. For example, if a group A recipient is given group B blood, a haemolytic transfusion will occur, because the recipient already possesses anti-B antibodies in their plasma. These antibodies will bind to the B antigen on the transfused erythrocytes. Complement will be activated (see Chapter 18) and the red cells will be lysed (ruptured). This can cause a series of physiological reactions leading to shock, coagulopathy and renal failure. An acute transfusion reaction can be fatal.
For this reason, great care must be taken when transfusing blood to ensure that the correct blood group is given to the correct patient (that is, the transfused blood must be compatible with the patient’s blood). Group O blood does not have either A or B antigens and thus it is possible to transfuse this blood type to a recipient with any ABO blood type without the risk of an ABO-related acute transfusion reaction. Table 16-5 shows the donor blood groups that are compatible with recipients of different blood groups. Note that someone who is type O can receive blood only from type O, yet can donate to all types (universal donor). On the other hand, someone who is type AB can receive from all types (universal recipient), but donate only to another type AB.
Table 16-5 ABO BLOOD GROUP COMPATIBILITY FOR BLOOD TRANSFUSION
| PATIENT’S BLOOD GROUP | COMPATIBLE DONOR |
|---|---|
| O | O |
| A | A, O |
| B | B, O |
| AB | AB, B, A, O |
The Rhesus system
The naming of the Rhesus (Rh) blood group system originates from the work of pioneer blood transfusion scientists who found a blood group antigen on human erythrocytes that was immunologically similar to an antigen found on the red cells of Rhesus monkeys. The human antigen is known as the D antigen. If the D antigen is present, the red cells are typed as Rh-positive; if the D antigen is not present, the cells are typed as Rh-negative. This is much simpler than the ABO grouping, as the only options regarding the Rh group is to be positive (with the antigen) or negative (not possessing the antigen). Approximately 85% of Caucasians are Rh-positive and 15% are Rh-negative.
Beyond the ABO blood group system, the Rh blood group system is the next most clinically significant. It is a complex system with more than 45 antigens described. The D antigen is the most immunogenic of the Rh antigens, meaning that if an individual who is Rh-negative is exposed to Rh-positive blood (e.g. by transfusion or pregnancy), there is a high probability that an anti-D antibody will be made by the immune system. This could cause a haemolytic reaction (lysis or destruction of erythrocytes) if there is another exposure to Rh-positive blood at a later date.
Because the ABO blood group is the most important in clinical practice, followed by the Rh antigen, blood grouping tests generally only test for these antigens. Other blood group antigens are tested for only if required as part of further laboratory investigation.
The universal donor
Donors with the blood type O Rh-negative are highly sought by transfusion services. This is because this blood group can be transfused to any recipient and is therefore referred to as the universal donor. Group O blood can be given to any ABO blood type recipient and because the blood is also Rh-negative there is no chance of a reaction should the recipient have a circulating anti-D antibody. Furthermore, the transfusion of Rh-negative blood will not stimulate the immune system to produce an anti-D antibody if the recipient is Rh-negative. Group O Rh-negative blood is particularly useful in emergency situations where blood needs to be transfused as a life-saving measure and there is insufficient time to perform a blood group analysis. For example, helicopter medical retrieval services in Australia carry group O Rh-negative blood in specially designed coolers to accident sites, so that blood transfusion can be given as quickly as possible should this be necessary.15,16
PAEDIATRICS AND HAEMATOLOGICAL FUNCTION
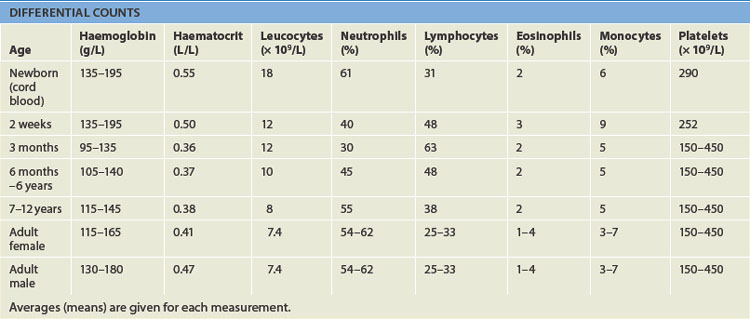
Blood cell counts tend to rise above adult levels at birth and then decline gradually throughout childhood. Table 16-6 lists normal ranges during infancy and childhood. The immediate rise in values is the result of accelerated haematopoiesis during fetal life, the increased numbers of cells that result from the trauma of birth and cutting of the umbilical cord.
Average blood volume in the full-term neonate is 85 mL/kg of body weight, higher than adults (about 70 mL/kg). The premature infant has a slightly larger blood volume of 90 mL/kg of body weight, with the mean increasing to 150 mL/kg during the first few days after birth. In both full-term and premature infants, blood volume decreases during the first few months. Thereafter, the average blood volume is 75–77 mL/kg, which is similar to that of older children and adults.
The hypoxic intrauterine environment prior to birth stimulates erythropoietin production in the fetus and accelerates fetal erythropoiesis, producing polycythaemia (excessive proliferation of erythrocyte precursors) in the newborn. After birth, the oxygen from the lungs saturates arterial blood and more oxygen is delivered to the tissues. In response to the change from a placental to a pulmonary oxygen supply during the first few days of life, levels of erythropoietin and the rate of blood cell formation decrease. The active rate of fetal erythropoiesis is reflected by the large numbers of immature erythrocytes (reticulocytes) in the peripheral blood of full-term neonates. After birth, the number of reticulocytes decreases about 50% every 12 hours, so it is rare to find an elevated reticulocyte count after the first week of life. During this period of rapid growth, the rate of erythrocyte destruction is greater than that in later childhood and adulthood. In full-term infants, the normal erythrocyte life span is 60–80 days and in premature infants it may be as short as 20–30 days. In children and adolescents erythrocyte life span is the same as that in adults — 120 days.
The fall in haemoglobin and haematocrit values after birth is more marked in premature infants than it is in full-term infants. In preschool and school-aged children, haemoglobin, haematocrit and red blood cell counts gradually rise. Metabolic processes within erythrocytes of neonates differ significantly from those found in erythrocytes of normal adults. The relatively young population of erythrocytes in newborns consumes greater quantities of glucose than do erythrocytes in adults.
The lymphocytes of children tend to have more cytoplasm and less compact nuclear chromatin than do the lymphocytes of adults. A possible explanation is that children tend to have more frequent viral infections, which are associated with atypical lymphocytes. Minor infections, in which the child fails to exhibit clinical manifestations of illness, and the administration of immunisations may also account for the lymphocyte changes. At birth the lymphocyte count is high and it continues to rise during the first year of life. Then it steadily declines until the lower value seen in adults is reached. It is unknown whether these developmental variations are physiological or a pathological response to frequent viral infection and immunisations in children.
The neutrophil count, like the lymphocyte count, is high at birth and rises during the first days of life. After 2 weeks, the neutrophil count falls to within or below the normal adult range. By approximately 4 years of age, the neutrophil count is the same as that of an adult.
The eosinophil count is high in the first year of life and higher in children than in teenagers or adults. Monocyte counts too are high in the first year of life but then decrease to adult levels. Platelet counts in full-term neonates are comparable with platelet counts in adults and remain so throughout infancy and childhood.
AGEING AND THE HAEMATOLOGICAL SYSTEM
Blood composition changes little with age. Erythrocyte life span in the elderly is normal, although erythrocytes are replenished more slowly after bleeding, probably because of iron depletion. Total serum iron, total iron-binding capacity and intestinal iron absorption are all decreased somewhat in the elderly. Iron deficiency is often responsible for the low haemoglobin levels noted in the elderly. The plasma membranes of erythrocytes become increasingly fragile, with portions being lost, presumably because of physical trauma inflicted during circulation.
Lymphocyte function decreases with age (see Chapter 12), causing changes in cellular immunity and some decline in T cell function. The humoral immune system is less able to respond to challenges to the immune system.
No changes in platelet numbers or structure have been observed in the elderly, yet evidence shows that platelet adhesiveness probably increases. Although fibrinogen levels and factors V, VII and IX tend to be increased in the elderly, evidence concerning hypercoagulability is inconclusive.
Components of the haematological system
 The major functions of blood are the delivery of oxygen and nutrients to the tissues, the removal of wastes from tissues, providing protection against microorganisms and injury, and the maintenance of acid–base balance.
The major functions of blood are the delivery of oxygen and nutrients to the tissues, the removal of wastes from tissues, providing protection against microorganisms and injury, and the maintenance of acid–base balance. Plasma proteins are involved in blood clotting, microbial defence, transportation of molecules and regulation of biological processes.
Plasma proteins are involved in blood clotting, microbial defence, transportation of molecules and regulation of biological processes. Plasma is the fluid found in non-clotted blood, whereas serum is the fluid obtained from clotted blood.
Plasma is the fluid found in non-clotted blood, whereas serum is the fluid obtained from clotted blood. Erythrocytes are the most abundant blood cell and their main role is to provide oxygen to the tissues.
Erythrocytes are the most abundant blood cell and their main role is to provide oxygen to the tissues. The main role of leucocytes is to defend the body against foreign organisms, but they also have roles in destroying tumour cells and dead or dying cells.
The main role of leucocytes is to defend the body against foreign organisms, but they also have roles in destroying tumour cells and dead or dying cells. Leucocytes can be classified according to structure as granulocytes (neutrophils, eosinophils and basophils) or agranulocytes (monocytes and lymphocytes) or according to function as phagocytes (neutrophils, eosinophils, basophils, monocytes) or immunocytes (lymphocytes).
Leucocytes can be classified according to structure as granulocytes (neutrophils, eosinophils and basophils) or agranulocytes (monocytes and lymphocytes) or according to function as phagocytes (neutrophils, eosinophils, basophils, monocytes) or immunocytes (lymphocytes). The spleen is one of the largest lymphoid organs and is important for the removal of old or damaged blood cells, microorganisms and debris.
The spleen is one of the largest lymphoid organs and is important for the removal of old or damaged blood cells, microorganisms and debris.The development of blood cells
 The different types of blood cells all originate from haematopoietic stem cells, which are stimulated by cytokines to develop along a particular pathway.
The different types of blood cells all originate from haematopoietic stem cells, which are stimulated by cytokines to develop along a particular pathway. Iron, vitamin B12 and folate are the three most important nutritional requirements for red blood cell development.
Iron, vitamin B12 and folate are the three most important nutritional requirements for red blood cell development.The mechanisms of haemostasis
 The components that are important in haemostasis are platelets, the clotting factors, the fibrinolytic system, natural anticoagulants and the vasculature.
The components that are important in haemostasis are platelets, the clotting factors, the fibrinolytic system, natural anticoagulants and the vasculature. Activated platelets release numerous substances that can activate further platelets or are involved in haemostasis.
Activated platelets release numerous substances that can activate further platelets or are involved in haemostasis. Secondary haemostasis consists of a system of plasma enzymes that result in the formation of a stable fibrin clot.
Secondary haemostasis consists of a system of plasma enzymes that result in the formation of a stable fibrin clot.Blood groups
 Individuals have naturally occurring antibodies to ABO blood group antigens in their plasma according to their ABO blood group.
Individuals have naturally occurring antibodies to ABO blood group antigens in their plasma according to their ABO blood group.Louise is a 24-year-old outdoor sports enthusiast. As a consequence of falling recently while mountain biking, Louise has presented with a laceration in her leg that has become badly infected. Louise has also developed a fever. As part of the clinical management for Louise, a number of laboratory tests have been requested, including a full blood count. The haematology laboratory has completed the full blood count and the results show that Louise’s white cell count is raised well above the reference range.