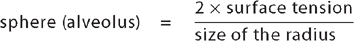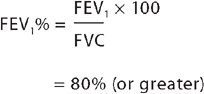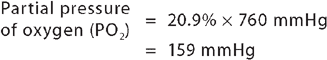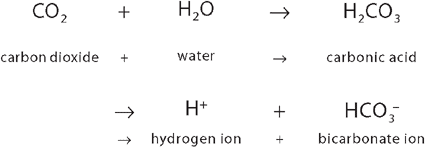24 THE STRUCTURE AND FUNCTION OF THE PULMONARY SYSTEM
INTRODUCTION
The importance of the pulmonary system in sustaining life has been known for millennia. The ancient Greeks had a rudimentary understanding of air and the relationship to breathing,1 and there are passages in the Bible that make reference to breathing and continuing life. However, it was not until the mid-eighteenth century that scientific experimentation was sophisticated enough to differentiate gases in the atmosphere. The pioneering work was done by two chemists: Joseph Priestley, who demonstrated that oxygen was essential for life and metabolic processes; and Antoine Lavoisier, who isolated oxygen from the atmosphere and found that carbon dioxide was the by-product of metabolism.2 Their work allowed others to further our understanding of the pulmonary system and associated diseases.
The pulmonary system consists of the lungs, airways, chest wall and pulmonary circulation. The primary function is the exchange of gases between the external environment (air) and the blood and onto the cells. The movement of air from the environment to the body cells involves several stages. There are three critical steps in this process and it is vital that you are able to differentiate each one because alterations to homeostasis can affect all three or each one independently. The three critical steps are:
The first two functions are carried out by the pulmonary system and the third by the cardiovascular system (which is discussed in Chapter 22). Normally, the pulmonary system functions efficiently under a variety of conditions and with little energy expenditure. However, alterations to the pulmonary system, cardiovascular system or circulating blood can impact greatly on the ability of the lungs to exchange oxygen and carbon dioxide with the atmosphere and the body cells, sometimes with deleterious systemic effects.
In this chapter we explore the anatomy and physiology of the pulmonary system, while Chapter 25 is devoted to alterations in the pulmonary system.
THE STRUCTURE OF THE PULMONARY SYSTEM
The pulmonary system is made up of two lungs (including the alveoli where gases are exchanged with the blood), the associated airways (nose, nasal cavity, mouth, pharynx, larynx, trachea, bronchi and bronchioles), blood vessels that serve them (pulmonary arteries, pulmonary capillaries, pulmonary veins and bronchial arteries) and the chest wall, also referred to as the thoracic cage. The lungs are divided into lobes: three in the right lung (upper, middle, lower) and two in the left lung (upper, lower). The apex of the heart, which extends into the left side of the thorax, means that the left lung is smaller than the right. Each lobe is further divided into segments and lobules. The space between the lungs, which contains the heart, great vessels and oesophagus, is called the mediastinum. A set of passageways deliver air to each section of the lung. The lung tissue that surrounds the airways supports them, preventing their distortion or collapse as gas moves in and out during ventilation.
Functional differences exist between these structures and they can be divided into two zones: the conducting zone, which includes all the passageways that lead from the mouth and nose into the chest cavity; and the respiratory zone, the sites of gas exchange — or what are commonly referred to as the lungs (see Figures 24-1 and 24-2). In clinical practice the terms upper respiratory tract and lower respiratory tract are frequently used to distinguish anatomical components when pulmonary disorders are present. For instance, lower respiratory tract infections are often more serious than upper respiratory tract infections. However, to adequately understand the physiological differences, the terms conducting zone and respiratory zone are more pertinent.
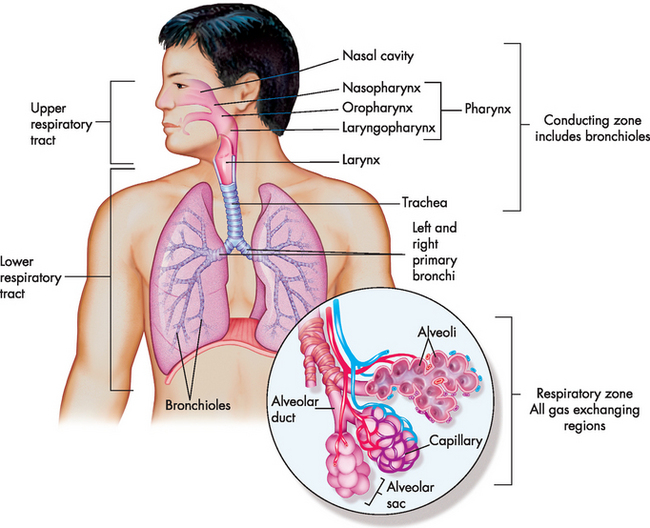
FIGURE 24-1 The structures of the pulmonary system.
The conducting airways from the mouth and nose to the lungs are visible. The enlargement in the circle depicts gas exchange units with corresponding blood vessels.
Source: Based on Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
FIGURE 24-2 Gross anatomy of the chest cavity.
The frontal section of chest has been preserved and the lungs’ position in the thorax is shown along with the other structures, diaphragm, heart and ribs.
Source: Based on Mason RJ. Murray & Nadel’s textbook of respiratory medicine. 4th edn. Philadelphia: Saunders; 2005.
The conducting zone
The conducting zone consists of the nose, mouth, nasal passage (cavity), pharynx, larynx, trachea, bronchi and bronchioles. The primary role of the conducting zone is to facilitate the movement of air into and out of the respiratory zone, the site where gas exchange occurs. These conducting airways are not simply hollow tubes that allow air to move into the lungs; in fact, they have other crucial roles to filter, warm and humidify the air. These are important properties, such that air reaching the respiratory zone is cleaned of foreign particles (for instance, dust and microorganisms), warmed to match body temperature and has moisture added, which all combine to facilitate gas exchange in the respiratory zone.
At rest, during normal breathing, air enters the nose through the nostrils and then into the nasal cavity (see Figure 24-3). Superiorly the bones of the skull and inferiorly the hard palate line this cavity. It is continuous with the upper aspect of the pharynx. The mouth provides an alternative route for air, but also can be utilised for ventilation during periods of increased ventilatory drive.
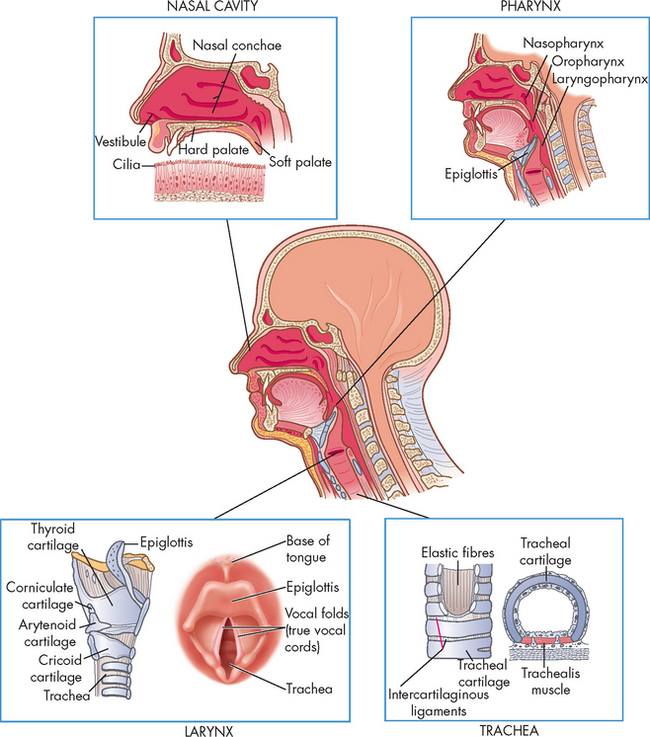
FIGURE 24-3 The structures of the upper airway.
A cross-section of the upper airways highlighting the anatomical features of the nasal cavity, pharynx, larynx and trachea.
Source: Based on Thompson JM et al. Mosby’s clinical nursing. 5th edn. St Louis: Mosby; 2002; and Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007.
The pharynx has three regions: (1) nasopharynx, (2) oropharynx and (3) laryngopharynx. The nasopharynx lies above where food is passed from the mouth, while the oropharynx and laryngopharynx both provide passageways for ingested food and air. These structures are lined with ciliated mucosa (a mucus-secreting membrane that contains hair-like projections [cilia] to move the mucus across the surface). This enables the inspired air to be warmed and humidified and importantly foreign particles are removed. The mouth and oropharynx are used for ventilation when the nose is obstructed or when increased flow is required during periods of increased ventilatory drive such as during exercise. It must be noted that filtering and humidifying are not as efficient during mouth breathing.
The larynx connects the laryngopharynx with the trachea and is the junction between the upper and lower airways. The upper respiratory tract contains all structures from the mouth to the start of the trachea, which is different from the conducting zone, which extends beyond this region. The main purposes of the larynx are for vocalisation (speech) and directing swallowed food and inspired air into the oesophagus and trachea, respectively. The larynx is formed from a scaffold of different cartilage pieces that are supported in place by surrounding ligaments and membranes. The larynx moves during voice production and swallowing, and the cartilage aids with this movement and importantly prevents collapse of the larynx during inspiration. The most prominent of these cartilage structures is the thyroid cartilage, which projects anteriorly and is commonly referred to as the Adam’s apple. It is more obvious and larger in males due to greater growth during puberty.
Immediately superior to the larynx, the epiglottis moves down during swallowing to cover the larynx and prevent food and liquid from entering the lungs (there is a complete discussion of swallowing in Chapter 26). The hollow space inside the larynx encompasses two pairs of folds: the false vocal cords (supraglottis, meaning above the glottis) and the true vocal cords (the slit-shaped space between the cords, forming the glottis; see Figure 24-3). The internal laryngeal muscles control vocal cord length and tension and the external laryngeal muscles move the larynx as a whole. Both sets of muscles are important to swallowing, ventilation and vocalisation.3 The internal muscles contract during swallowing to prevent aspiration into the trachea. These muscles also contribute to voice pitch.
The trachea, which is supported by a C-shaped cartilage, connects the larynx to the bronchi. The common term for the trachea is the ‘windpipe’ and you may have seen individuals who have had a tracheostomy (ostomy, from the Greek word ‘stoma’ meaning mouth; so forming a hole in the trachea). The procedure consists of making a surgical incision into the trachea and inserting an artificial airway (tracheostomy tube), which facilitates breathing. The trachea is composed of cartilage, connective tissue and smooth muscle, which provides an important combination of strength and flexibility. Rigidity is necessary, such that pressure changes in the lungs and airways do not cause the trachea to collapse during breathing. On the other hand, the posterior part of the trachea (which contains only smooth muscle and not cartilage) provides flexibility to allow the oesophagus to bulge anteriorly so that food can be swallowed. It is because the cartilage is incomplete around the diameter of the trachea that it is described as being C-shaped. Extending inferiorly, the trachea branches into two main airways, or bronchi (singular, bronchus), at the carina (see Figure 24-1). The carina contains nerve fibres that are very sensitive and, when stimulated by foreign particles such as pollution or microorganisms, cause powerful coughing and bronchospasm.4,5
The right and left main bronchi enter the lungs at the hila (which literally means roots of the lungs and there are two, which individually are called hilum). The hila are an important region because this is where the main pulmonary vessels, major bronchi and lymphatic vessels are situated. Compared with the left, the right main bronchus is more vertical, wider and shorter, which typically results in aspiration of foreign objects, such as food and liquids, entering the right lung. The bronchial walls have three layers: an epithelial lining, a smooth muscle layer and a connective tissue layer. The epithelial lining of the bronchi contains single-celled exocrine glands (the mucus-secreting goblet cells) and ciliated cells.
Upon entering the lungs, the main bronchi divide into lobar bronchi (secondary), three on the right and two on the left (see Figure 24-4). These provide connections for the lobes of the lungs. The bronchi continue branching to form smaller bronchi (segmental bronchi — tertiary) that have less cartilage and ciliated cells, but more smooth muscle in the walls. These branch even more to form bronchioles, which are literally ‘little bronchi’. The bronchioles are important because they not only conduct air to and from the lungs and the environment, but are also responsible for control of airflow by either dilating or constricting. This is explained in more detail later in the chapter. Successive bronchiole branching occurs until they form terminal bronchioles, which are the smallest.
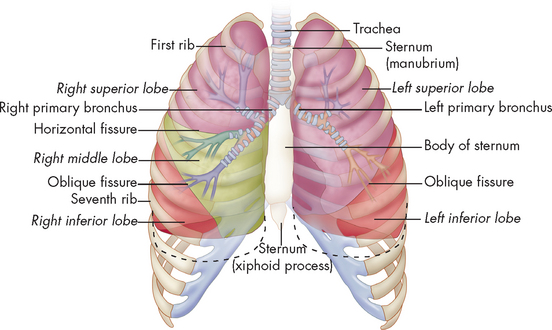
FIGURE 24-4 Conducting airways of the lungs.
The lobes of the lungs inside the chest wall. The trachea is an airway that branches to form a treelike formation of bronchi and bronchioles. Note that the right lung has three lobes and the left lung has two lobes and the dotted line refers to inspiration depth.
Source: Thibodeau GA. The human body in health & disease. 5th edn. St Louis: Mosby; 2010.
A useful analogy for the conducting passageways is that of a large tree. From the ground, the trunk is synonymous with the trachea and the branches arising from the trunk resemble bronchi and bronchioles spreading out through the lungs. The small branches and leaves are similar to the respiratory zone providing an increased surface area to facilitate the exchange of gas with the blood. In fact, there are 23 levels of branching and these can be seen in Figure 24-5. The successive branching of the airways occurs with changes in the bronchial wall (see Figure 24-6), as the layers of epithelium become thinner to the extent that only a thin membrane remains where gas exchange occurs.
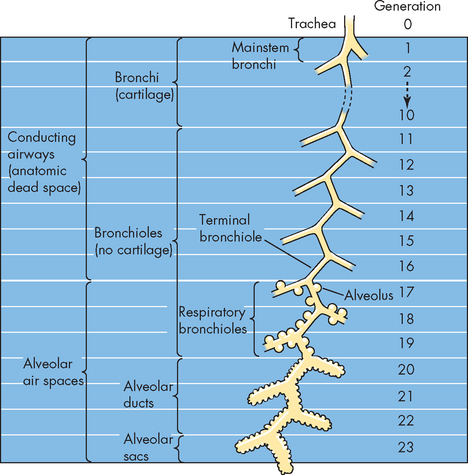
FIGURE 24-5 Airway generations from the trachea to the alveolus.
Source: Boron WF, Boulpaep EL. Medical physiology. 2nd edn. Philadelphia: Saunders; 2009.
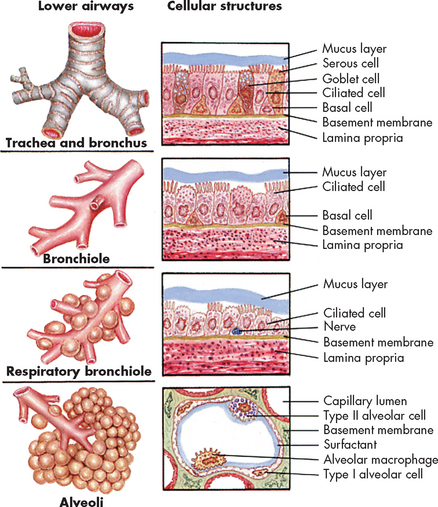
FIGURE 24-6 Changes in the bronchial wall with progressive branching.
Source: Wilson SF, Thompson JM. Respiratory disorders. St Louis: Mosby; 1990.
An important aspect is the way that the pulmonary system filters and cleans the air to ensure that the gas-exchange units are sterile. Within the air that we inhale are a range of potential foreign particles that may cause infections or irritation to the lungs. The pulmonary system is very efficient at removing these foreign particles from entering the alveoli. This starts at the nasal cavity, which contains turbinates and hairs that trap foreign particles. Along the upper respiratory tract, the mucosa also traps foreign particles and the mucus that lines the tract is propelled upwards out of the upper airways using cilia — this is referred to as the mucociliary escalator. This mucus is either swallowed or coughed out, thereby cleaning the pulmonary system. Furthermore, as the conducting zone changes to the respiratory zone, the defence mechanisms of the pulmonary system change from a series of mechanical barriers to cells of the inflammatory and immune systems, macrophages, which ingest foreign substances to prevent contamination of the lungs. For more details of the pulmonary defence mechanisms see Table 24-1.
Table 24-1 PULMONARY DEFENCE MECHANISMS
| STRUCTURE OR SUBSTANCE | MECHANISM OF DEFENCE |
|---|---|
| Nasal hairs and turbinates | Trap and remove foreign particles, some bacteria and noxious gases from inspired air |
| Irritant receptors in nostrils | Stimulation by chemical or mechanical irritants triggers sneeze reflex, which results in rapid removal of irritants from nasal passages |
| Upper respiratory tract mucosa | Maintains constant temperature and humidification of gas entering the lungs; traps and removes foreign particles, some bacteria and noxious gases from inspired air |
| Mucus blanket | Protects trachea and bronchi from injury; traps most foreign particles and bacteria that reach the lower airways |
| Cilia | Propel mucus blanket and entrapped particles towards the oropharynx, where they can be swallowed or expectorated |
| Irritant receptors in trachea and large airways | Stimulation by chemical or mechanical irritants triggers cough reflex, which results in removal of irritants from the lower airways |
| Alveolar macrophages | Ingest and remove bacteria and other foreign particles from the alveoli by phagocytosis |
The respiratory zone
The respiratory zone consists of structures that are involved in gas exchange. These include the respiratory bronchioles, alveolar ducts and alveoli (singular, alveolus) (see Figures 24-1 and 24-7). The respiratory bronchioles, branching from the terminal bronchioles, have fewer ciliated and mucus-producing cells than the conducting zone and more epithelial cells than the conducting zone, and mainly consist of one layer of epithelial cells, thereby allowing gas exchange. The alveolar ducts are distal to the respiratory bronchioles and although some gas exchange occurs here, the majority of gas exchange takes place in the alveoli.
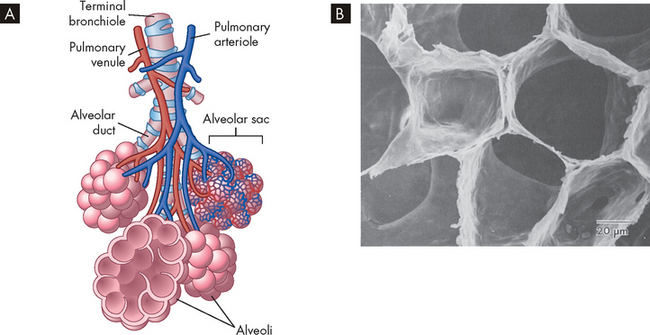
A Bronchioles subdivide to form tiny tubes called alveolar ducts, which end in clusters of alveoli called alveolar sacs. B Alveolar shape.
Source: A Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010. B Mason RJ. Murray & Nadel’s textbook of respiratory medicine. 4th edn. Philadelphia: Saunders; 2005.
The alveoli are the primary gas-exchange units of the lung, where oxygen enters the blood and carbon dioxide is removed (see Figure 24-7). Alveoli are approximately only 0.2 mm in diameter; however, there are about 300 million in the adult lungs and therefore the surface area is very large. In fact, if the lungs were spread out flat they would be approximately the size of a singles tennis court!6 This surface area is further enhanced by tiny passages called pores of Kohn, which permit some air to pass through the septa (plural for septum, a thin membrane between two cavities) from alveolus to alveolus, promoting collateral ventilation and even distribution of air among the alveoli. The alveoli are composed of two major types of epithelial cells:
 type I alveolar cells (or type I pneumocytes), which are flat-shaped squamous cells that provide structure of the alveolar wall and are specialised for gas exchange
type I alveolar cells (or type I pneumocytes), which are flat-shaped squamous cells that provide structure of the alveolar wall and are specialised for gas exchangeTherefore, while there are somewhat similar numbers of type I and type II cells, it is type I alveolar cells that constitute the majority of the surface area involved in gas exchange.
Surfactant is a lipoprotein (a combination of lipid and protein) that coats the inner surface of the alveolus and lowers alveolar surface tension at the end of expiration, which helps prevent lung collapse.3,8,9 Type I alveolar cells comprise about 40% of all alveolar cells; however, they provide 90% of the surface area of the alveoli. In contrast, type II cells account for 60% of alveolar cells and are widely spread through the alveolar surface, but cover less than 10% of the surface area (see Figure 24-8).10 Both these cell types are formed late in pregnancy and are the primary reason why premature infants are unable to survive before 24 weeks of gestational age (see the ‘Paediatrics’ box later in the chapter for more details).
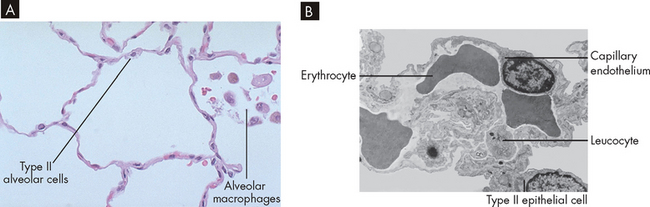
FIGURE 24-8 Alveolar wall components.
A Microscopic view of alveoli with type II cells and alveolar macrophages visible. B Microscopic view of alveoli with adjacent capillary.
Source: A Klatt EC. Robbins and Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010. B Leslie KO, Wick, MR (eds). Lung anatomy. In: Practical pulmonary pathology: a diagnostic approach. Churchill Livingstone; 2005. Reproduced from Nagaishi with permission.
Like the bronchi, the alveoli contain cellular components of inflammation and immunity, particularly the alveolar macrophages (see Figure 24-8). The alveolar macrophages move about the alveoli scavenging foreign particles that may enter the alveoli if not filtered and removed from the conducting zone. It should be noted that a huge amount of potential contaminants are in the inspired air and while the conducting zone is very effective in removing the majority of these from the inspired air, some may still reach the alveoli. Therefore, the immune system can rapidly counteract any foreign invasion and in this way the lungs are sterile. Alveolar macrophages remove foreign particles by phagocytosis (see Chapter 13), and the ingested foreign material is removed through the lymphatics (see Table 24-1 for a comparison with other pulmonary system defences).
The pulmonary and bronchial circulation
The pulmonary circulation facilitates gas exchange, delivers nutrients to lung tissues, acts as a reservoir for the left ventricle and serves as a filtering system that removes clots, air and other debris from the circulation.
Although the entire cardiac output from the right ventricle goes into the lungs, the pulmonary circulation has a lower pressure and resistance than the systemic circulation. Pulmonary arteries are exposed to about one-fifth the pressure of the systemic circulation (mean pulmonary artery pressure is approximately 15 mmHg compared to a mean arterial pressure in the aorta of 95 mmHg; see Figure 22-38). Usually about one-third of the pulmonary vessels are filled with blood (referred to as being ‘perfused’) at any given time. More vessels become perfused when right ventricular cardiac output increases. Therefore, increased delivery of blood to the lungs does not normally increase mean pulmonary artery pressure.
The arterioles divide at the terminal bronchioles to form a network of pulmonary capillaries around the alveoli. Capillary walls consist of an endothelial layer and a thin basement membrane, which often fuses with the basement membrane of the alveoli. Therefore, very little physical separation exists between blood in the capillary and gas in the alveolus.
The shared alveolar and capillary walls compose the alveolar–capillary membrane (air–blood interface) (see Figure 24-9). Gas exchange occurs across this membrane. With normal perfusion, approximately 100 mL of blood in the pulmonary capillary bed is spread very thinly over 70–100 m2 of alveolar surface area. Any alteration to the membrane, such as that which occurs with the disease emphysema (see Chapter 25), will impact on normal gas exchange.
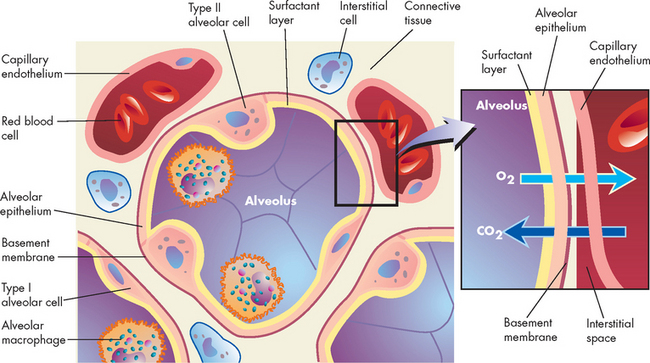
FIGURE 24-9 Cross-section of an alveoli (alveolar–capillary membrane).
Inset shows a magnified view of the respiratory membrane composed of the alveolar wall (fluid coating, epithelial cells, basement membrane), interstitial fluid and wall of a pulmonary capillary (basement membrane, endothelial cells). The gases carbon dioxide and oxygen diffuse across the respiratory membrane.
The bronchial circulation is part of the systemic circulation and it supplies nutrients and oxygen to the cells of the conducting zone, large pulmonary vessels and membranes (pleurae) that surround the lungs. Not all of the capillaries drain into the bronchial venous system. Some empty into the pulmonary vein and contribute to the normal venous mixture of oxygenated and deoxygenated blood.
The chest wall and pleura
The chest wall consists of the skin, ribs and intercostal muscles (between the ribs). The chest wall has two important functions: (1) to coordinate with the diaphragm (the primary muscle involved in breathing) in performing the muscular work of breathing; and (2) to provide protection for the heart and lungs from injury. The thoracic cavity is contained by the chest wall and encases the lungs (see Figure 24-10). When individuals have alterations to their pulmonary system, investigation of the condition is often conducted using chest X-rays. These can be taken in different positions (upright or supine) and there are many distinguishing features that can be observed. Figure 24-11 outlines some of the pulmonary and cardiovascular anatomical features of a normal chest X-ray.
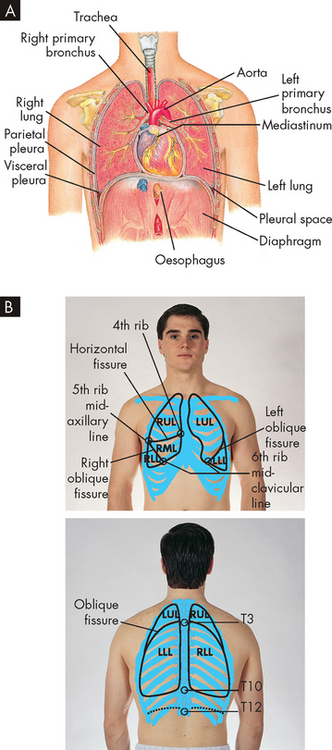
FIGURE 24-10 Thoracic (chest) cavity and related structures.
A The thoracic (chest) cavity is divided into three subdivisions (left and right pleural divisions and mediastinum) by a partition formed by a serous membrane called the pleura. B Anatomical position of the lungs in the chest.
Source: A Thibodeau GA, Patton KT. Anatomy & physiology. 6th edn. St Louis: Mosby; 2007. B Jarvis C. Physical examination and health assessment. 5th edn. St Louis: Saunders; 2008.
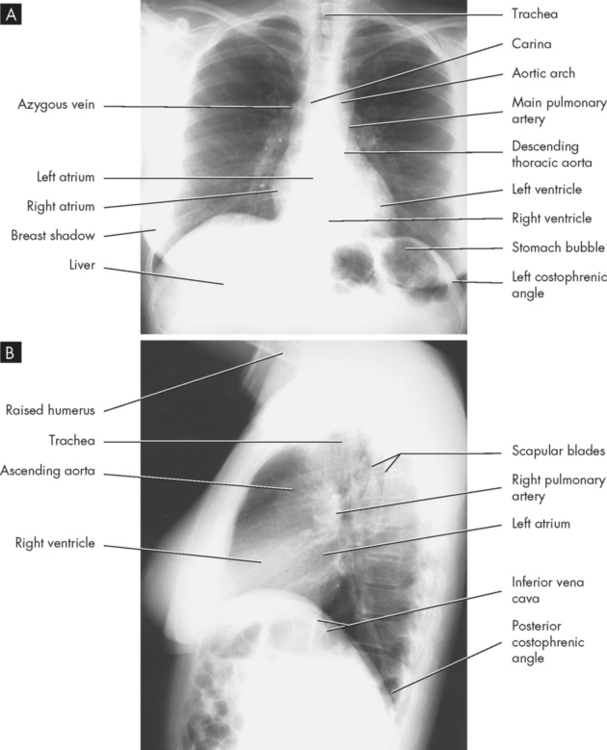
FIGURE 24-11 Normal anatomy on the female chest X-ray.
A Upright posterior-anterior position. B Lateral position.
Source: Based on Mettler FA. Essentials of radiology. 2nd edn. Philadelphia: Saunders; 2004.
A serous membrane called the pleura adheres firmly to the lungs and then folds over itself and attaches firmly to the chest wall. The membrane covering the lungs is the visceral pleura; that lining the thoracic cavity is the parietal pleura. The area between the two pleurae is called the pleural space (or pleural cavity). Normally, only a thin layer of fluid secreted by the pleura (pleural fluid) fills the pleural space, lubricating the pleural surfaces and allowing the two layers to slide over each other without separating. The pressure in the pleural space is usually negative or subatmospheric (below that of the atmosphere, or about −4 to −10 mmHg). This negative pressure is important because it assists with keeping the lungs from collapsing and moving away from the chest wall.
THE FUNCTION OF THE PULMONARY SYSTEM
The pulmonary system has three primary functions: (1) to ventilate the alveoli; (2) to allow gases to diffuse into and out of the blood; and (3) to perfuse the lungs so that the organs and tissues of the body receive blood that is rich in oxygen and low in carbon dioxide. These functions are influenced by chemical and neural input to the nervous system, which controls the movement of the chest wall muscles, thereby affecting gas transport which will impact on the blood flow in the lungs. Each of these components of the pulmonary system contributes to one or more of these functions, and we explore them in more detail below.
The mechanics of breathing
The physical aspects of inspiration and expiration are known collectively as the mechanics of breathing and involve: (1) major and accessory muscles of inspiration and expiration; (2) elastic properties of the lungs and chest wall; and (3) resistance to airflow through the conducting zone. Alterations in any of these properties increase the work of breathing or the metabolic energy needed to achieve adequate ventilation of alveoli and oxygenation of the blood.
Major and accessory muscles
The major muscles of inspiration are the diaphragm and the external intercostal muscles (muscles between the ribs) (see Figure 24-12). The diaphragm is a dome-shaped muscle that separates the abdominal and thoracic cavities. When it contracts and flattens downwards, it increases the volume of the thoracic cavity. This creates a slight negative pressure, which draws air into the lungs through the upper airways and trachea. Contraction of the external intercostal muscles elevates the anterior portion of the ribs and increases the volume of the thoracic cavity by increasing its front-to-back (anterior-posterior) diameter. Although the external intercostals may contract during quiet breathing, inspiration at rest is usually achieved by the diaphragm only.
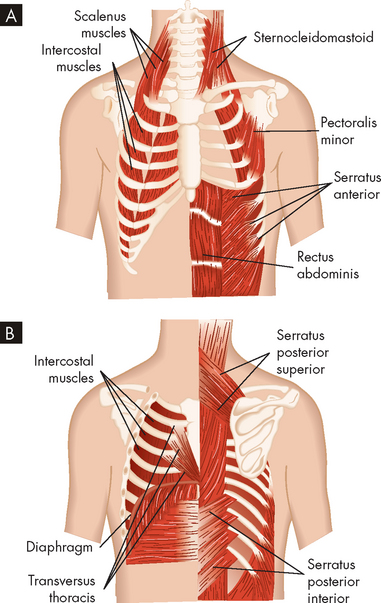
FIGURE 24-12 Muscles of ventilation.
A Anterior view. B Posterior view.
Source: Based on Thompson JM et al. Mosby’s clinical nursing. 5th edn. St Louis: Mosby; 2002.
The accessory muscles of inspiration are the sternocleidomastoid and scalene muscles. Like the external intercostals, these muscles enlarge the thorax by increasing its anterior-posterior diameter. The accessory muscles assist inspiration when minute volume (the volume of air inspired and expired per minute; see ‘Ventilation’ later in the chapter) is high; for example, during strenuous exercise or when the work of breathing is increased because of disease. However, the accessory muscles do not increase the volume of the thorax as efficiently as the diaphragm, which is the main muscle of ventilation. The movement of these accessory muscles may be observed in a patient who is experiencing breathing difficulties.
There are no major muscles of expiration because normal, quiet expiration is passive and requires no muscular effort. The relaxation of the diaphragm causes it to move back towards the thoracic cavity, which decreases the volume and air moves out. Also, the properties of the smooth muscle and elastic fibres within the respiratory zone structures enable them to recoil passively during expiration. The accessory muscles of expiration, the abdominal and internal intercostal muscles, assist expiration when minute volume is high, during coughing, when airway obstruction is present or during forced expiration (such as blowing into a balloon). When the abdominal muscles contract, intra-abdominal pressure increases, pushing up the diaphragm and decreasing the volume of the thorax. The internal intercostal muscles pull down the anterior ribs, decreasing the anterior-posterior diameter of the thorax.
Alveolar surface tension
Surface tension occurs at any gas–liquid interface and refers to the tendency for liquid molecules that are exposed to air to adhere to one another. This phenomenon can be seen in the way water drops ‘bead’ when splashed on a waterproof surface. Within a sphere, such as an alveolus, surface tension tends to make expansion difficult. Laplace’s law shows the relationship among pressure to inflate the alveolus, the alveolus surface tension and radius:
or, using terms, P = 2T/r, where P = pressure required to inflate a sphere, T = surface tension and r = radius. Simply put, as the radius of the sphere (alveolus) becomes smaller, more and more pressure is required to inflate it. If the alveoli were lined with only a water-like fluid, breathing would be extremely difficult.
Alveolar ventilation, or distention, is made possible by surfactant, which lowers surface tension by coating the air–liquid interface in the alveoli. Surfactant has a detergent-like effect that separates the liquid molecules, thereby decreasing alveolar surface tension. Surfactant lines the alveolar side of the alveolar–capillary membrane and reverses Laplace’s law, thereby allowing alveoli to inflate easily. If surfactant is not produced in adequate quantities, alveolar surface tension increases, which results in alveolar collapse, decreased lung expansion, increased work of breathing and severe gas-exchange abnormalities.
The decrease in surface tension caused by surfactant is also responsible for keeping the alveoli free of fluid. In the absence of surfactant, water tends to move into the alveoli, which can lead to the life-threatening condition of pulmonary oedema (see Chapter 25). Also, insufficient surfactant production can contribute to the breathing difficulties experienced by premature babies.
Elastic properties of the lung and chest wall
The lung and chest wall have elastic properties that permit expansion during inspiration and the return to resting volume during expiration. The effect is produced by elastin fibres in the alveolar walls and surrounding the small airways and pulmonary capillaries, as well as the surface tension at the alveolar air–liquid interface. The elasticity of the chest wall is the result of the configuration of the bones and muscles.
Elastic recoil is the tendency of the lungs to return to the resting state after inspiration, in a similar way that an elastic band springs back into place after being stretched. Normal elastic recoil permits passive expiration, eliminating the need for major muscles of expiration. Passive elastic recoil may be insufficient during laboured breathing — that is, when minute volume is high and when the accessory muscles of expiration may be needed. The accessory muscles are used also if disease compromises elastic recoil (for example, in emphysema) or blocks the conducting airways.
However, elastic recoil is not confined to the lungs: the chest wall also has elastic recoil. But this recoil is in the opposite direction — that is, it tends to pull the chest wall outwards, away from the lungs. In fact, the tendency of the outward elastic recoil of the chest wall is balanced by the tendency of the lungs to recoil or inwardly collapse around the hila. The negative pressure of the pleural space (between the parietal and visceral pleurae) is indicative of the different elastic recoils in the chest wall and lungs (that is, in opposite directions).
The balance between the outward recoil of the chest wall and inward recoil of the lungs occurs at the end of expiration, where the functional residual capacity (the amount of air remaining in the lungs after normal tidal expiration) is reached. During inspiration, the diaphragm and intercostal muscles contract, air flows into the lungs, due to the negative pressure (compared to outside the lungs), and the chest wall expands. Muscular effort is needed to overcome the resistance of the lungs to expansion. During expiration, the muscles relax and the elastic recoil of the lungs causes the thorax to decrease in volume until, once again, balance between the chest wall and lung recoil forces is reached (see Figure 24-13).
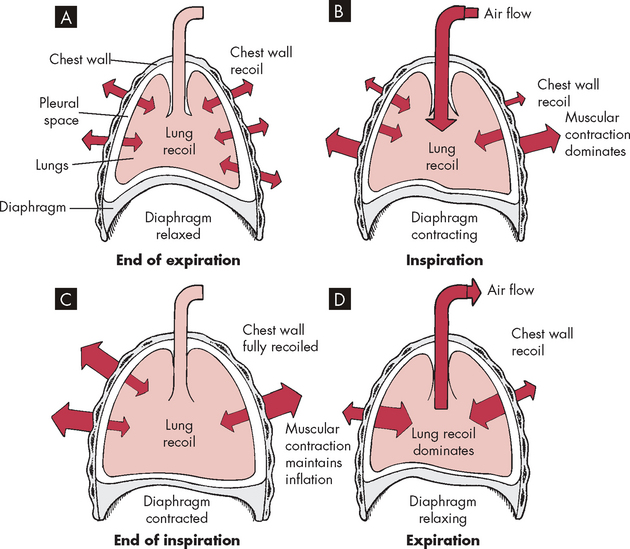
FIGURE 24-13 The interaction of forces during inspiration and expiration.
A Outward recoil of the chest wall equals inward recoil of the lungs at the end of expiration. B During inspiration, contraction of respiratory muscles, assisted by chest wall recoil, overcomes the tendency of the lungs to recoil. C At the end of inspiration, respiratory muscle contraction maintains lung expansion. D During expiration, respiratory muscles relax, allowing elastic recoil of the chest wall to deflate the lungs.
Compliance is the measure of lung and chest wall distensibility (stretchiness) and is defined as volume change per unit of pressure change. It represents the relative ease with which these structures can be stretched and is therefore the opposite of elasticity. Compliance is determined by alveolar surface tension and the elastic recoil of the lung and chest wall.
Increased compliance indicates that the lung or chest wall is abnormally easy to inflate and has lost some elastic recoil. A decrease indicates that the lung or chest wall is abnormally stiff or difficult to inflate. Compliance is increased in the normal ageing process and in diseases such as emphysema, and is decreased in the acute respiratory distress syndrome, pneumonia, pulmonary oedema and fibrosis (these disorders are described in Chapter 25).
Airway resistance
Airway resistance, which is similar to resistance to blood flow (described in Chapter 22), is determined by the length, radius and cross-sectional area of the airways and density, viscosity and velocity of the gas (Poiseuille’s law). Airway resistance is normally very low. Most of the resistance occurs in the upper airways: half to two-thirds of total airway resistance occurs in the nose. The next highest resistance is in the oropharynx and larynx. Airway resistance increases when the diameter of the airways decreases. However, there is very little resistance in the bronchioles and alveoli of the lungs when considered in total because of their large cross-sectional area — that is, there are millions of tubes compared to the upper airways that have larger diameters but are essentially single passageways.
Bronchoconstriction (narrowing of the airways), which increases airway resistance, may occur following release of neurotransmitters from some parasympathetic nerve terminals. However, direct sympathetic nerve innervation of the airways is sparse and bronchodilation (dilation of the airways), which decreases resistance to airflow, is caused by β2 (beta)-adrenergic receptor stimulation, primarily through circulating adrenaline (see Chapter 6).7 Airway resistance can also be increased by oedema of the bronchial mucosa, such as that which occurs during an asthmatic episode, and by airway obstructions such as mucus, tumours or foreign substances.
The work of breathing
The work of breathing is determined by the muscular effort required for ventilation — that is, the energy cost. Normally very low, the work of breathing may increase considerably in diseases that disrupt the equilibrium between forces exerted by the lung and chest wall. More muscular effort is required when lung compliance decreases (for example, pulmonary oedema), chest wall compliance decreases (such as in obesity or spinal deformity) or airways are obstructed by bronchospasm or mucous plugging (for example, asthma or bronchitis).11 An increase in the work of breathing can result in a marked increase in oxygen consumption and an inability to maintain adequate ventilation.
Ventilation
Ventilation is the mechanical movement of gas or air into and out of the lungs. It is often misnamed respiration, which is actually the exchange of oxygen and carbon dioxide during cellular metabolism. ‘Respiratory rate’ is actually the ventilatory rate, or the number of times gas is inspired and expired per minute. However, the term respiratory rate is often used in clinical practice, and you should understand that this term refers only to what we externally observe in the chest wall movement. At rest, the ventilatory rate is approximately 12 breaths per minute, but can range from 8 to 16 breaths per minute, depending on the resting state. For example, during sleep, ventilatory rate declines but is enough to sustain respiration.
The size or amount of gas inspired or exhaled each breath is the tidal volume. The name derives from the tide of the oceans; that is, the rhythmical movement back and forth analogous to air moving in and out of the lungs. The inspired and exhaled volumes are approximately the same and for our purposes we consider them equal. The tidal volume usually refers to the resting volume and in adults it is approximately 500 mL of air. However, the amount of effective ventilation is calculated by multiplying the ventilatory rate (breaths per minute) by the volume or amount of air per breath (litres per breath: tidal volume). This is called the minute volume, because it is the amount of air breathed each minute and is expressed in litres per minute (L/min).
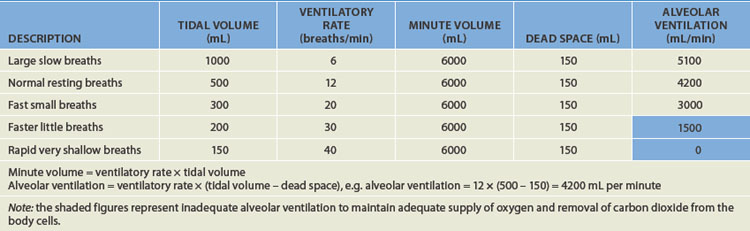
The minute volume provides an approximation of the overall ventilation, but it does not account for dead space — the volume of air that does not reach the alveoli and therefore is not involved in gas exchange. The dead space in an adult is approximately 150 mL of air and is the air that resides in the conducting airways and so does not reach the gas-exchange zone. Alveolar ventilation takes into account the ‘wasted’ dead space air and is the ventilation that occurs at the alveoli: it provides a more accurate picture of the actual gas exchange. For instance, it is possible that, despite adequate minute volume, the alveolar ventilation is not sufficient for satisfactory gas exchange and therefore oxygenation. Table 24-2 provides an overview of six different tidal volume and ventilatory rate combinations with the same minute volume but vastly different alveolar ventilation rates. This issue is addressed again in Chapter 25.
There are many different lung volumes and often information about these volumes aids in the diagnosis of disease and the evaluation of therapy. Pulmonary function tests are used to determine the lung volumes and, importantly, the flow rates that individuals can generate. Spirometry (literally meaning ‘to measure breathing’) is the mainstay of determining pulmonary function. Any individual who has experienced breathing difficulties, such as asthma, will have undertaken spirometry evaluation of lung function. The different lung volumes and capacities (combinations of lung volumes) are graphically represented in Figure 24-14; Table 24-3 explains how each is derived.
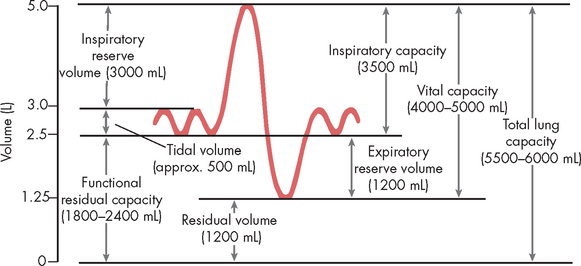
FIGURE 24-14 Respiratory volumes and capacities as determined using spirometry.
The dynamic lung volumes that can be measured by simple spirometry are the tidal volume, inspiratory reserve volume, expiratory reserve volume, inspiratory capacity and vital capacity. The static lung volumes are the residual volume, functional residual capacity and total lung capacity. Static lung volumes cannot be measured by simple spirometry and require separate methods of measurement (e.g. inert gas dilution, nitrogen washout or whole-body plethysmography).
Source: Based on Miller RD. Miller’s anesthesia. 6th edn. Churchill Livingstone; 2005.
Table 24-3 RESPIRATORY VOLUMES AND CAPACITIES
| DESCRIPTION AND MEASUREMENT | |
|---|---|
| Respiratory volumes | |
| Tidal volume (VT) | The amount of air inspired or exhaled in a normal resting breath |
| Inspiratory reserve volume (IRV) | The maximal amount of air that can be inhaled after tidal volume |
| Expiratory reserve volume (ERV) | The maximal amount of air that can be exhaled after tidal volume |
| Residual volume (RV) | The amount of air that remains in the lungs after a maximal exhalation |
| Respiratory capacities | |
| Inspiratory capacity (IC) | The amount of air inspired by maximum inspiratory effort after tidal volume, expiration IC = VT + IRV |
| Vital capacity (VC) | The amount of air that can be forcibly expired after a maximal inspiration, VC = VT + IRV + ERV |
| Functional residual capacity (FRC) | The amount of air left in the lungs after normal tidal expiration, FRC = ERV + RV |
| Total lung capacity (TLC) | The volume of air occupying the lungs after maximum inhalation, TLC = VT + IRV + ERV + RV |
Two primary variables used to determine lung volume and flow changes are (1) the forced expiratory volume in the first second of exhalation, abbreviated to FEV1 and (2) the forced vital capacity (FVC), or the maximal amount of air that can be expelled from the lungs in one breath. These two variables, and the ratio of them, provide important information about the type of pulmonary alteration, the effects of medication (specifically bronchodilators — medication that causes an increased opening of the bronchioles) and the extent of disease (see Figure 24-15). The relationship between FEV1 and FVC can be calculated using this equation:
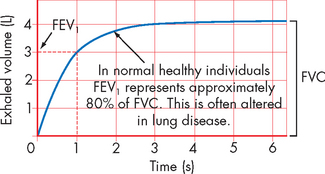
FIGURE 24-15 Representative spirometry of forced exhalation.
FVC is the forced vital capacity, the maximal amount of air that can be forcibly exhaled in one breath. FEV1 is the forced expiratory volume in the first second of forced vital capacity.
Source: Based on Brown R. Management of the patient with asthma. In: Krouse JH, Derebery MJ, Chadwick SJ (eds). Managing the allergic patient. Philadelphia: Saunders; 2007.
This indicates that for a healthy individual, 80% of their forced vital capacity can be exhaled within the first second. Pulmonary function test values change over the life span and are influenced by height, sex, age and race. Usually, the individual’s results are compared against predicted values. FEV1 and FVC are addressed again in Chapter 25, when characterising alterations in pulmonary function.
Control of ventilation
The air that we breathe contains many gases; however, only two gases are actively involved in respiration — oxygen and carbon dioxide. Of these, carbon dioxide has a greater influence over the chemical control of ventilation and is under tight control. This can be easily demonstrated by trying to hold your breath for an extended period of time: the impetus to breathe rises more strongly from the increase in carbon dioxide level in the blood rather than the decrease in oxygen levels. Below we explore the neural and chemical control of ventilation in more detail.
Breathing is usually involuntary, because homeostatic changes in the ventilatory rate and tidal volume are adjusted automatically by the nervous system to maintain normal gas exchange. Voluntary breathing is necessary for many activities, such as talking, singing, laughing and deliberately holding one’s breath. The control of ventilation is complex and requires input from many sources — neural (autonomic and voluntary or higher centre control), mechanical (stretch and irritant receptors in the lungs and muscles involved in ventilation) and chemical (predominately carbon dioxide and oxygen levels in the blood and cerebrospinal fluid). The coordination of ventilation occurs in the medulla and pons of the brainstem, and a constant supply of information is required to make the fine adjustments needed to maintain adequate ventilation. These factors are summarised in Figure 24-16.
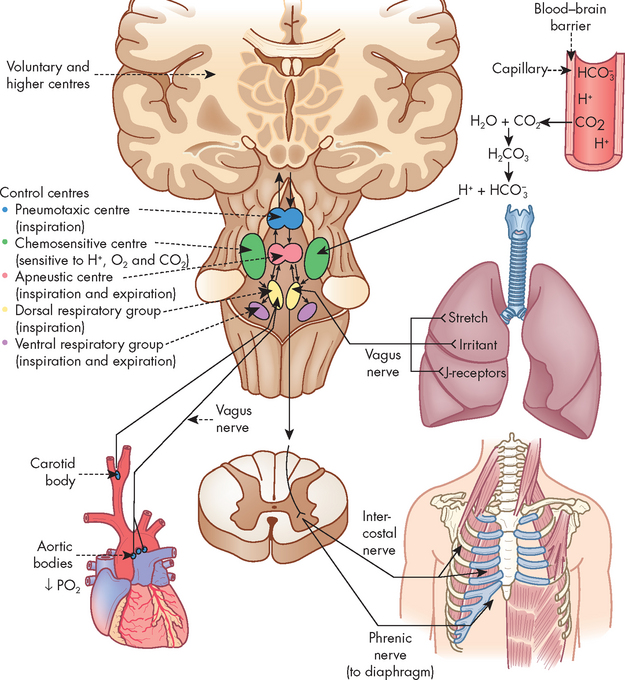
FIGURE 24-16 Respiratory centres in the brain and the multiple inputs for the control of ventilation.
Neural control
The respiratory centre in the brainstem controls ventilation by transmitting impulses to the respiratory muscles, causing them to contract and relax. The respiratory centre is composed of several groups of neurons: the dorsal respiratory group, the ventral respiratory group, the apneustic centre and the pneumotaxic centre.3,8,9 The exact nature of each group is not entirely understood; however, it is thought that the automatic ventilatory rhythm is set by the dorsal respiratory group (located in the medulla), which receives afferent input (sensory nerve) from peripheral chemoreceptors in the carotid and aortic bodies and from several different types of receptors in the lungs (see below). The ventral respiratory group (also located in the medulla) contains both inspiratory and expiratory neurons and is almost inactive during normal, quiet ventilation, becoming active when increased ventilatory effort is required. The pneumotaxic centre and apneustic centre, situated in the pons (above the medulla), do not generate primary rhythm but, rather, act as modifiers of the rhythm established by the medullary centres. It should be noted that the pattern of breathing can be influenced by many factors, such as emotions, pain and disease.
Mechanical control
Three types of lung receptors send impulses from the lungs to the dorsal respiratory group:
The lung is innervated by the autonomic nervous system; however, the primary neural control of the bronchioles comes from the parasympathetic fibres which travel in the vagus nerve to the lung (the structure and function of the autonomic nervous system is covered in detail in Chapter 6). The parasympathetic division controls airway diameter (interior diameter of the airway lumen) by causing bronchoconstriction of the smooth muscles. In contrast, the sympathetic nerve fibres are sparse, and bronchodilation is mediated by stimulation of β2-receptors responding to circulating adrenaline. Constriction occurs if the irritant receptors in the airway epithelium are stimulated by irritants in inspired air (such as dust and pollen), by endogenous substances (such as histamine, serotonin, prostaglandins) and by many drugs (such as β-blockers).
Chemical control
Chemoreceptors monitor the pH, carbon dioxide level in the arterial blood (PaCO2, with the lower case ‘a’ referring to arterial) and oxygen level in the arterial blood (PaO2). There are two groups of chemoreceptors: (1) central chemoreceptors located in the brainstem near the respiratory centres; and (2) peripheral chemoreceptors located in the aortic and carotid bodies (see Figure 24-16).
Central chemoreceptors monitor arterial blood indirectly by sensing changes in the pH (hydrogen ion content) of cerebrospinal fluid. They are sensitive to hydrogen ion concentration, or the amount of acid, in the cerebrospinal fluid. The pH of the cerebrospinal fluid reflects arterial pH because carbon dioxide in arterial blood can diffuse across the blood–brain barrier (the capillary wall separating blood from cells of the central nervous system) into the cerebrospinal fluid until the carbon dioxide level is equal on both sides. Carbon dioxide that has entered the cerebrospinal fluid combines with water to form carbonic acid (a weak acid), which subsequently dissociates into hydrogen ions that are capable of stimulating the central chemoreceptors. In this way, carbon dioxide regulates ventilation through its impact on the pH (hydrogen ion content) of the cerebrospinal fluid.3,8,9
If alveolar ventilation is inadequate or there is a substantial increase in the metabolic rate, the carbon dioxide level in the blood increases. Carbon dioxide diffuses across the blood–brain barrier until the level of carbon dioxide in the blood and cerebrospinal fluid reaches equilibrium. As the central chemoreceptors sense the resulting decrease in pH (which is actually an increase in acid), they stimulate the respiratory centre to increase the depth and rate of ventilation. Increased ventilation causes the carbon dioxide level of arterial blood to decrease below that of the cerebrospinal fluid, and carbon dioxide diffuses back out of the cerebrospinal fluid and pH returns to normal levels. The homeostatic control of this mechanism is outlined in Figure 24-17.
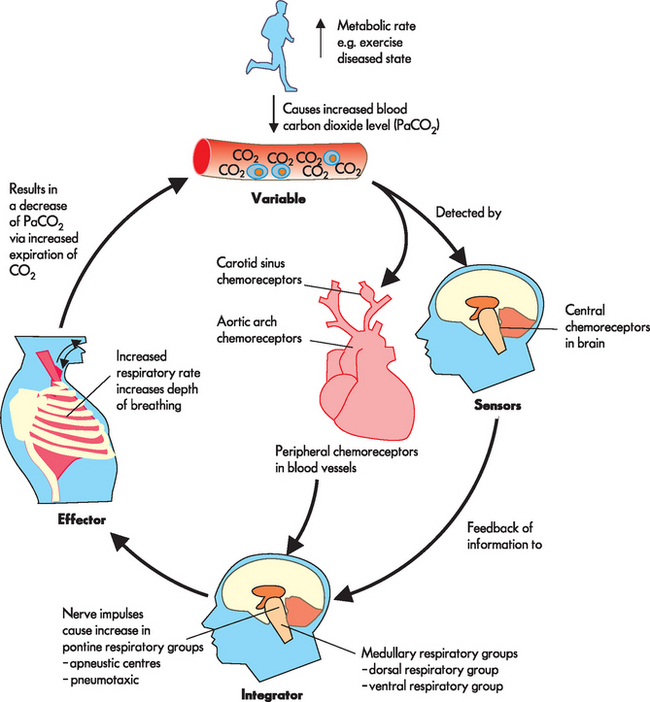
FIGURE 24-17 Homeostatic control responding to an increase in carbon dioxide levels in the blood.
Source: Based on Patton KT, Thibodeau GA. Anatomy & physiology. 7th edn. St Louis: Mosby; 2010.
Changes in the chemical control of breathing during sleep
There are multiple sites of central carbon dioxide chemosensitivity in the brainstem and there may be specialised chemosensory sites that function only during certain sleep states. Chemical control of ventilation, related to both hypercapnia and hypoxia, appears to be blunted during sleep. This blunting increases with obesity (especially at altitude) and is severely disordered in those with sleep apnoea. Congestive heart failure and hypertension are also associated with abnormal breathing responses during sleep.
Source: Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J 2006; 13(4):203–210; Patil SP et al. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 2007; 102(2):547–556; Arzt M, Bradley TD. Treatment of sleep apnea in heart failure. Am J Respir Crit Care Med 2006; 173(12):1300–1308; Shigemitsu M et al. Nocturnal oxygen therapy prevents progress of congestive heart failure with central sleep apnea. Int J Cardiol 2007; 115(3):354–360; Smith ML, Pacchia CF. Sleep apnoea and hypertension: role of chemoreflexes in humans. Exp Physiol 2007; 92(1):45–50.
Central chemoreceptors are sensitive to very small changes in the pH of cerebrospinal fluid (equivalent to a 1–2 mmHg change in carbon dioxide levels) and can maintain a normal carbon dioxide level under many different conditions, including strenuous exercise. If inadequate ventilation, or hypoventilation, is long term (such as in chronic obstructive pulmonary disease), these receptors may become insensitive to small changes in carbon dioxide levels in the blood and ‘reset’ to a higher level. The carbon dioxide in the blood then needs to rise to a level above that which it normally regulates before an increase in ventilatory drive will be instigated.
Peripheral chemoreceptors are somewhat sensitive to changes in carbon dioxide levels and pH but are sensitive primarily to oxygen levels in arterial blood. As oxygen levels in the blood and pH decrease, peripheral chemoreceptors, particularly the carotid bodies, send afferent signals to the respiratory centre to increase ventilation. However, the oxygen level of the blood must drop well below normal (from 100 mmHg to approximately 60 mmHg) before the peripheral chemoreceptors have much influence on ventilation. If this situation occurs with an increase in carbon dioxide levels as well, ventilation increases much more than it would in response to either abnormality alone. Also, the peripheral chemoreceptors become the major stimulus to ventilation when the central chemoreceptors are reset by chronic hypoventilation.3
Gas transport
Gas transport, the delivery of oxygen to the cells of the body and the removal of carbon dioxide, has four steps:
Steps in the transport of carbon dioxide occur in reverse order:
It is important to understand that disruption at any step can lead to inadequate gas exchange at the cellular level.
The diffusion of oxygen across the alveoli into the blood and carbon dioxide from the blood into the alveoli occurs due to the change in concentration gradient, with gases moving from an area of higher to lower concentration. This occurs even when the gases are dissolved in liquid (blood and cerebrospinal fluid) or in body tissues. To gain a more complete understanding of diffusion, we now explore the physical properties of gases.
Gas pressure
The atmosphere is composed of many gases. The most prevalent gases are nitrogen and oxygen, which together account for 99% of all gases in the atmosphere (see Table 24-4). Other gases in smaller concentrations include carbon dioxide. These gases are made up of millions of molecules moving randomly and colliding with each other. When a gas is within a closed space (such as the lungs), the molecules collide with the walls of the space in which they are contained. All of these collisions exert pressure. If the same number of gas molecules are contained in a small container and a large container, the pressure will be greater in the small container because more collisions occur in the smaller space (see Figure 24-18). If there are many gases in a confined space, the pressure exerted by each individual gas, the partial pressure, is directly proportional to the percentage of that gas in the mixture of gases. For instance, there is more nitrogen in the atmosphere than oxygen, so the partial pressure of nitrogen is greater. Heat increases the speed of the molecules, which also increases the number of collisions and therefore the pressure.
Table 24-4 THE COMPOSITION OF GAS PARTIAL PRESSURES IN THE ATMOSPHERE AND HOW THEY CHANGE IN THE LUNGS
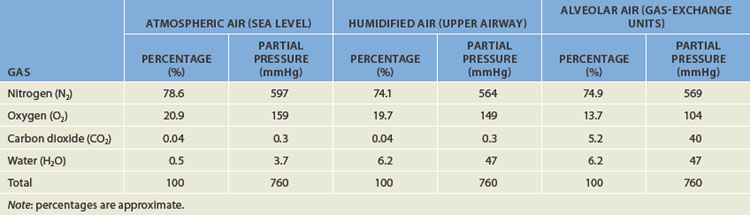
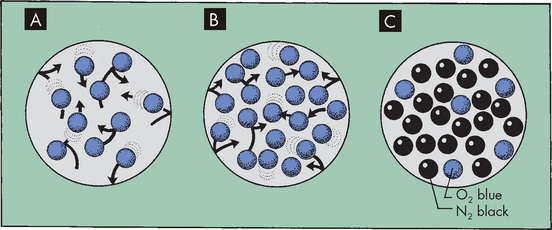
FIGURE 24-18 The relationship between the number of gas molecules and the pressure exerted by the gas in an enclosed space.
A Theoretically, 10 molecules of the same gas exert a total pressure of 10 within the space. B If the number of molecules is increased to 20, total pressure is 20. C If there are different gases in the space, each gas exerts a partial pressure that is directly proportional to the percentage of that gas in the total. For instance, the partial pressure of nitrogen will be greater than oxygen as there is more nitrogen than oxygen.
Barometric pressure (which is actually atmospheric pressure) is the pressure exerted by gas molecules in the air at specific altitudes. At sea level, barometric pressure is 760 mmHg and is the sum (total) of the pressure exerted by all the gases in the air at sea level. The partial pressure of a gas is calculated as:
Therefore, the partial pressure of the respiratory gases will change throughout the lungs and in the blood. As oxygen and carbon dioxide diffuses across membranes this will affect the partial pressure. The compositions of atmospheric air and alveolar air are shown in Table 24-4.
Another important factor for gas exchange is water vapour. The amount of water vapour contained in a gas mixture is determined by the temperature of the gas, but is unrelated to barometric pressure. Gas that enters the lungs becomes saturated with water vapour (humidified) as it passes through the upper airway. At body temperature (approximately 37°C), water vapour exerts a pressure of 47 mmHg. The partial pressure of water vapour must be subtracted from the barometric pressure before the partial pressure of other gases in the mixture can be determined. In saturated air at sea level, the partial pressure of oxygen is therefore (760 mmHg − 47 mmHg) × 0.209 = 149 mmHg. See Table 24-4 for how this changes the partial pressures of other gases.
Distribution of ventilation and perfusion
Effective gas exchange depends on an approximately even distribution of gas (ventilation) and blood (perfusion) in all portions of the lungs. The lungs are actually suspended from the hila in the thoracic cavity. When an individual is in an upright position (sitting or standing), gravity pulls the lungs down towards the diaphragm and compresses their lower portions, commonly referred to as the bases. The alveoli in the upper portions, specifically the apices, of the lungs contain a greater residual volume of gas and are larger and less numerous than those in the lower portions. Because surface tension increases as the alveoli become larger, the larger alveoli in the upper portions of the lung are more difficult to inflate (that is, they are less compliant) than the smaller alveoli in the lower portions of the lung. Therefore, during ventilation most of the tidal volume is distributed to the bases of the lungs, where compliance is greater. Remember that an increased compliance indicates that the lungs are easier to inflate.
The heart pumping blood to the lungs is also affected by gravity. As blood is pumped into the lung apices of an upright individual, some blood pressure is dissipated in overcoming gravity. As a result, blood pressure at the apices is lower than that at the bases. Because greater pressure results in greater perfusion, the bases of the lungs are better perfused than the apices. Thus, ventilation and perfusion are greatest in the same lung portions, the lower lobes. However, if a standing or sitting individual assumes a supine position, the apex and base of the lungs are at a similar level and ventilation is relatively more uniformly distributed. In addition, the posterior region of the lungs receives more blood than the anterior, due to the effects of gravity.
Distribution of perfusion in the pulmonary circulation is also affected by alveolar pressure (gas pressure in the alveoli). The pulmonary capillary bed differs from the systemic capillary bed in that it is surrounded by gas-containing alveoli. If the gas pressure in the alveoli exceeds the blood pressure in the capillary, the capillary collapses and flow ceases. This is most likely to occur in portions of the lung where blood pressure is lowest and alveolar gas pressure is greatest; that is, at the apex of the lung.
The lungs are divided into three zones on the basis of relationships among all the factors affecting pulmonary blood flow:
 In zone I, alveolar pressure exceeds pulmonary arterial and venous pressures. The capillary bed collapses and normal blood flow ceases. Normally zone I is a very small part of the lung at the apex.
In zone I, alveolar pressure exceeds pulmonary arterial and venous pressures. The capillary bed collapses and normal blood flow ceases. Normally zone I is a very small part of the lung at the apex. In zone II, alveolar pressure is greater than venous pressure but not arterial pressure. Blood flows through zone II, but it is impeded to a certain extent by alveolar pressure. Zone II is normally above the level of the left atrium.
In zone II, alveolar pressure is greater than venous pressure but not arterial pressure. Blood flows through zone II, but it is impeded to a certain extent by alveolar pressure. Zone II is normally above the level of the left atrium. In zone III, both arterial and venous pressures are greater than alveolar pressure and blood flow is not affected by alveolar pressure. Zone III is in the base of the lung.
In zone III, both arterial and venous pressures are greater than alveolar pressure and blood flow is not affected by alveolar pressure. Zone III is in the base of the lung.Blood flow through the pulmonary capillary bed increases in regular increments from the apex to the base. Alveolar pressure plus the forces of gravity, arterial blood pressure and venous blood pressure affect the distribution of perfusion, as shown in Figure 24-19.
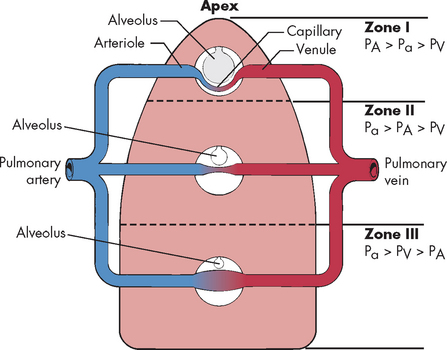
FIGURE 24-19 Gravity and alveolar pressure.
Effects of gravity and alveolar pressure on pulmonary blood flow in the three lung zones. In zone I, alveolar pressure (PA) is greater than arterial and venous pressure, and no blood flow occurs. In zone II, arterial pressure (Pa) exceeds alveolar pressure, but alveolar pressure exceeds venous pressure (PV). Blood flow occurs in this zone, but alveolar pressure compresses the venules (venous ends of the capillaries). In zone III, both arterial and venous pressures are greater than alveolar pressure and blood flow fluctuates depending on the difference between arterial and venous pressure.
Although both blood flow and ventilation are greater at the base of the lungs than at the apices, they are not perfectly matched in any zone. Perfusion exceeds ventilation in the bases and ventilation exceeds perfusion in the apices of the lung. The relationship between ventilation and perfusion is expressed as a ratio called the ventilation/perfusion ratio (V/Q). The normal ventilation/perfusion ratio is approximately 0.8, which means that perfusion usually exceeds ventilation under normal conditions. Alterations to the ventilation/perfusion ratio are discussed in Chapter 25.
Oxygen transport
Approximately 1000 mL (1 litre) of oxygen is transported to the cells of the body each minute. Oxygen is transported in the blood in two forms: a small amount dissolves in plasma (1.5%), as oxygen does not readily dissolve in water; and the remainder binds to haemoglobin (Hb) molecules (98.5%). Without haemoglobin, oxygen would not reach the cells in amounts sufficient to maintain normal metabolic function.
Diffusion across the alveolar–capillary membrane
The alveolar–capillary membrane is ideal for oxygen diffusion because it has a large total surface area (70–100 m2) and is very thin (0.5 micrometre [μm] — this is about 50 times thinner than an average human hair!). In addition, the partial pressure of oxygen molecules in alveolar gas (PAO2, the capital ‘A’ referring to alveolar) is much greater than in capillary blood, a condition that promotes rapid diffusion down the concentration gradient from the alveolus into the capillary. The partial pressure of oxygen in mixed venous (deoxygenated) or pulmonary artery blood (PVO2, the ‘V’ standing for venous) is approximately 40 mmHg as it enters the capillary — this is the venous blood which has returned to the heart from the systemic circulation. The alveolar oxygen partial pressure is approximately 100 mmHg at sea level. Therefore, a pressure gradient of 60 mmHg facilitates the diffusion of oxygen from the alveolus into the capillary. This can be seen in Figure 24-20 with the changes in the partial pressure of oxygen in inspired air, during transit throughout the body and in expired air.
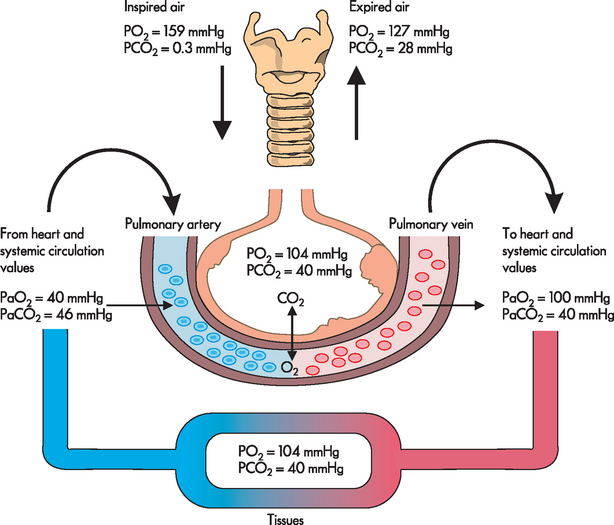
FIGURE 24-20 The changes in the partial pressures of respiratory gases.
The partial pressures for oxygen and carbon dioxide are approximations only. There will be subtle differences in each breath and dependent on the activity of the individual. The numbers shown are average values near sea level. The values of PaO2 and PaCO2 fluctuate from breath to breath.
Source: Based on Thompson JM et al. Mosby’ clinical nursing. 5th edn. St Louis: Mosby; 2002.
Blood remains in the pulmonary capillary at the alveoli for about 0.75 seconds, but only 0.25 seconds is required for the oxygen concentration to equilibrate (equalise) across the alveolar–capillary membrane. Therefore, oxygen has ample time to diffuse into the blood, even during increased cardiac output, which speeds blood flow and shortens the time the blood remains in the capillary.
Determinants of arterial oxygenation
As oxygen diffuses across the alveolar–capillary membrane, it dissolves in the plasma, where it exerts pressure (the partial pressure of oxygen in arterial blood, or PaO2, the small ‘a’ standing for arterial, as opposed to a capital ‘A’). As the PaO2 increases, oxygen moves from the plasma into the red blood cells (erythrocytes) and binds with haemoglobin molecules. Oxygen continues to bind with haemoglobin until the haemoglobin binding sites are filled or fully saturated (meaning that no more oxygen can bind to the haemoglobin). Each molecule of haemoglobin can bind four molecules of oxygen, with millions of haemoglobin molecules found in each red blood cell. Oxygen then continues to diffuse across the alveolar–capillary membrane until the PaO2 (oxygen dissolved in plasma) and PAO2 (alveolar oxygen) equilibrate, eliminating the pressure gradient across the alveolar–capillary membrane. At this point, diffusion ceases (see Figure 24-20). In this way, oxygen is transported to the body cells and the reverse process occurs to unload oxygen from haemoglobin so that it can diffuse from the blood into the interstitium and ultimately the cells (see Figure 24-21).
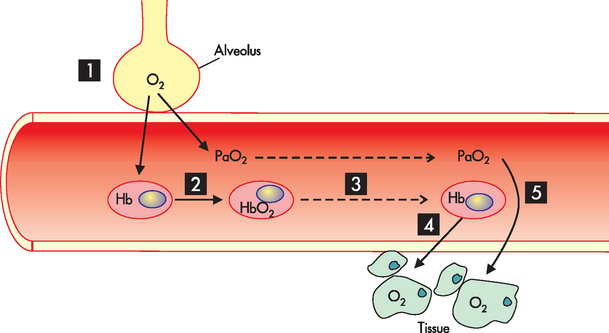
FIGURE 24-21 Oxygen transport.
Steps in the process are as follows. (1) Oxygen in the alveolus diffuses down the concentration gradient into the capillary. (2) Oxygen binds to haemoglobin to form oxyhaemoglobin (HbO2) and is dissolved in the blood (PaO2). (3) Oxygen is transported to all body tissues. (4) Oxygen dissociates from haemoglobin. (5) Oxygen diffuses down the concentration gradient into the body tissues.
In clinical practice, the amount of oxygen in the arterial blood (PaO2) can be measured in the blood by obtaining an arterial blood gas (ABG) measurement. This provides vital information about the individual’s oxygenation status. In addition, the percentage of the available haemoglobin that is bound to oxygen, or the oxygen saturation (SaO2), can be measured. However, more routine is the use of pulse oximetry, which provides an indirect non-invasive measure of oxygen saturation. The pulse oximeter uses a pulse to detect the presence of haemoglobin in blood that is saturated or desaturated with oxygen. However, it should be noted that oxygen saturation measured with a pulse oximeter (SpO2) is an approximation of the directly measured oxygen saturation (SaO2) and there may be differences between the values.
Because haemoglobin transports all but a small fraction of the oxygen carried in arterial blood, changes in haemoglobin concentration affect the oxygen content of the blood. Decreases in haemoglobin concentration below the lower limit of the reference range (males: 130 g/L; females: 115 g/L) reduce the oxygen content and increases in haemoglobin concentration may increase the oxygen content, minimising the impact of impaired gas exchange. In fact, increased haemoglobin concentration is a major compensatory mechanism in pulmonary diseases that impair gas exchange. For this reason, measurement of haemoglobin concentration is important in assessing individuals with pulmonary disease. If cardiovascular function is normal, the body’s initial response to low oxygen content is to elevate the heart rate and stroke volume, which will increase cardiac output. In individuals who also have cardiovascular disease, this compensatory mechanism will not be optimal, making increased haemoglobin concentration an even more important compensatory mechanism. (Haemoglobin structure and function are described in Chapter 16.)
The oxygen–haemoglobin dissociation curve
The importance of haemoglobin in gas exchange is paramount. Haemoglobin has the ability to both bind and unbind oxygen, making the process of oxygen transportation very efficient. Oxygen binds to haemoglobin and becomes oxyhaemoglobin, abbreviated as HbO2 (see Figure 24-21). Oxygen binding occurs more readily following subsequent bindings — that is, the affinity of haemoglobin for oxygen increases. Once haemoglobin cannot accept more oxygen it is termed fully saturated. At the body tissues, the haemoglobin then releases its oxygen (and is thus called deoxyhaemoglobin). Oxygen is then available to diffuse into the cells. In this way, the oxygen binds to haemoglobin in the lungs and is released at the body tissues where it is needed.
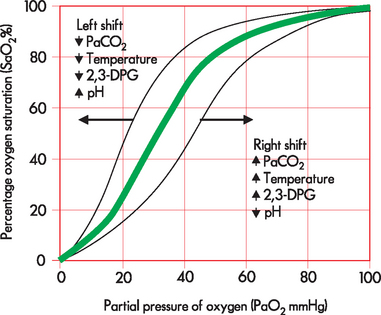
The oxygen–haemoglobin dissociation curve (see Figure 24-22) describes the relationship between the partial pressure of oxygen (PaO2) and the oxygen bound to haemoglobin (the degree of haemoglobin saturation). The distinctive S-shaped curve has a flattened portion at the top, which represents the binding of oxygen with haemoglobin in the lungs (the association portion). Above 70 mmHg only small amounts of oxygen binding occur with increasing PaO2. This is advantageous, because at low PaO2 levels, binding and releasing of oxygen from haemoglobin will still occur normally. However, at rest, when the PaO2 is 100 mmHg the haemoglobin is 98% saturated and further increases in PaO2 do not result in increased SaO2. The steep portion of the curve represents the unloading of oxygen from haemoglobin at the tissue level. This is also advantageous to efficient respiration because small decrements in PaO2 result in substantial unloading of oxyhaemoglobin and diffusion of oxygen into the tissues.
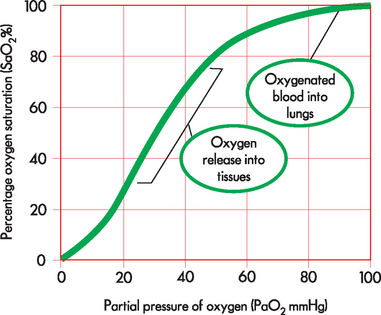
FIGURE 24-22 The oxygen–haemoglobin dissociation curve.
The horizontal or flat segment of the curve at the top of the graph is the arterial or association portion, or that part of the curve where oxygen is bound to haemoglobin, and occurs in the lungs (‘oxygenated blood in lungs’ circle). This portion of the curve is flat because partial pressure changes of oxygen between 60 and 100 mmHg do not significantly alter the percentage saturation of haemoglobin with oxygen. If the relationship between SaO2 and PaO2 were linear (in a downward-sloping straight line) instead of being flat between 60 and 100 mmHg, there would be inadequate saturation of haemoglobin with oxygen. The steep part of the oxygen–haemoglobin dissociation curve represents the rapid dissociation of oxygen from haemoglobin that occurs in the tissues. During this phase there is rapid diffusion of oxygen from the blood into tissue cells (‘oxygen release into tissues’ circle)
Several factors can alter the binding and release of oxygen from haemoglobin, causing the curve to shift to the right or left (see Figure 24-23). A shift to the right results in a decreased affinity in haemoglobin for oxygen, or an increase in the ease with which oxyhaemoglobin dissociates and oxygen moves into the cells. A shift to the left results in an increased affinity in haemoglobin for oxygen, which promotes oxygen binding to haemoglobin in the lungs and inhibits release at the tissues. The factors that affect the affinity include the amount of oxygen dissolved in the blood (PaO2), the amount of carbon dioxide dissolved in the blood (PaCO2), temperature, pH and a chemical in erythrocytes called 2,3-diphosphoglycerate, abbreviated to 2,3-DPG (see Figure 24-23).
Carbon dioxide transport
Carbon dioxide is carried in the blood in three ways:
This relationship can be seen in the following equation:
The diffusion gradient for carbon dioxide in the lung is only approximately 6 mmHg (venous carbon dioxide is 46 mm Hg and alveolar carbon dioxide is 40 mmHg) (see Figure 24-20). Yet carbon dioxide is so soluble in the alveolar–capillary membrane that the carbon dioxide in the blood quickly diffuses into the alveoli, where it is removed from the lung during expiration. Diffusion of carbon dioxide in the lung is so efficient that diffusion defects that cause hypoxaemia (low oxygen concentrations in the blood) do not as readily cause hypercapnia (excessive carbon dioxide levels in the blood).
The diffusion of carbon dioxide out of the blood is enhanced by oxygen binding with haemoglobin in the lung. As haemoglobin binds with oxygen, the amount of carbon dioxide carried by the blood decreases. Thus, in the tissue capillaries, oxygen release from haemoglobin facilitates the binding of carbon dioxide, and conversely the binding of oxygen to haemoglobin in the lungs facilitates the release of carbon dioxide from the blood. This effect of oxygen on carbon dioxide transport is known as the Haldane effect.9
ANATOMICAL DEVELOPMENT OF THE PULMONARY SYSTEM
Over the life span there are numerous changes to the pulmonary system. These changes are both anatomical and functional. The fetal lungs are immature and do not have definite alveoli like those of the adult. In fact, the alveolar stage, which signifies the commencement of alveoli development, begins at about 36 weeks of gestation. Moreover, these alveoli are not the same as the adult form.
The ability of premature infants to survive before 24 weeks of gestation is severely limited because of the lack of lung development. The epithelial lining has sufficiently thinned at around 24 weeks, which permits gas exchange, and type II cells that produce surfactant, facilitating ventilation, also appear at this time. The final stage of lung development continues until approximately 2 years of age; however, the number of alveoli and, therefore the surface area of the lungs, increases throughout development until adulthood.13
The newborn’s lungs are considerably different from the adult’s. At birth, an infant has approximately 25 million alveoli compared to the 300 million in adulthood. The reduced alveoli number means that surface area is also considerably reduced (3–4 m2 compared to 75–100 m2 in the adult). In addition, there are fewer pores of Kohn (passageways between successive alveoli) and a thicker alveolar–capillary membrane compared to adulthood. Therefore, airway resistance is higher than in adulthood (about 16-fold difference) and there is less available lung volume (150–200 mL compared to 5000 mL in the adult). The infant compensates for this smaller volume by increasing the ventilatory rate.13,14 Overall, the infant’s lungs are functional yet still immature. They have to undergo considerable development to increase the surface area and available alveoli.
AGEING AND THE PULMONARY SYSTEM
With increasing age, the pulmonary system changes in many ways from younger adulthood. These include changes in elasticity in the chest wall and changes in the respiratory zone and gas-exchange capacity. Chest wall compliance decreases due to stiffer ribs and loss of elastin, which causes an increase in the work of breathing and decreases the strength of the muscles involved in ventilation. Therefore, this causes an increase in compliance and residual volume.
While newborns have relatively immature lungs at birth and development continues in the first years of life, elderly individuals gradually lose lung function through a variety of mechanisms. Often there is a decrease in the number of alveoli, although this does not always occur. However, there is a decrease in the number of cilia, which reduces mucus clearance and can expose the elderly individual to pathogenic infections. The pulmonary capillary network decreases and there is a reduction in pulmonary arterial blood flow, which lowers the diffusion capacity and decreases gas exchange. This often results in ventilation–perfusion mismatches, reducing the partial pressure of oxygen (PaO2). Therefore, the ventilatory capacity of the elderly is markedly reduced compared to their younger counterparts.
These changes in the pulmonary system with ageing are summarised in Figure 24-24.
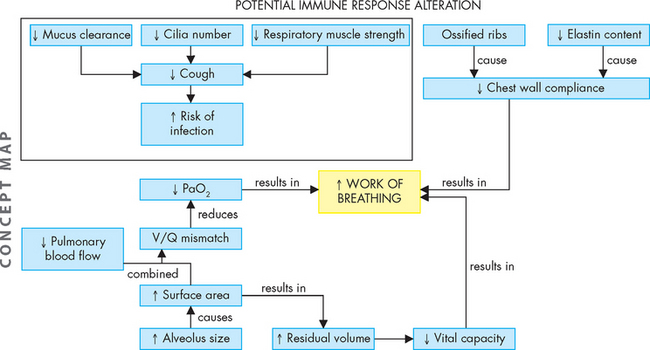
FIGURE 24-24 A concept map of the changes in the pulmonary system that can occur with ageing.
Note that these changes do not always lead to dysfunction, but rather highlight that the capacity of the pulmonary system is altered as individuals become older.
The structure of the pulmonary system
 The pulmonary system consists of the lungs, airways, chest wall and pulmonary and bronchial circulation.
The pulmonary system consists of the lungs, airways, chest wall and pulmonary and bronchial circulation. Air is inspired and expired through the conducting zone, which include the nasopharynx, oropharynx, trachea, bronchi and bronchioles to the sixteenth division.
Air is inspired and expired through the conducting zone, which include the nasopharynx, oropharynx, trachea, bronchi and bronchioles to the sixteenth division. The respiratory zone consists of structures that are involved in gas exchange. These include the respiratory bronchioles, alveolar ducts and alveoli.
The respiratory zone consists of structures that are involved in gas exchange. These include the respiratory bronchioles, alveolar ducts and alveoli. The chief gas-exchange units of the lungs are the alveoli. The membrane that surrounds each alveolus and contains the pulmonary capillaries is called the alveolar–capillary membrane.
The chief gas-exchange units of the lungs are the alveoli. The membrane that surrounds each alveolus and contains the pulmonary capillaries is called the alveolar–capillary membrane. The gas-exchange airways are served by the pulmonary circulation, a separate division of the circulatory system. The bronchi and other lung structures are served by a branch of the systemic circulation called the bronchial circulation.
The gas-exchange airways are served by the pulmonary circulation, a separate division of the circulatory system. The bronchi and other lung structures are served by a branch of the systemic circulation called the bronchial circulation.The function of the pulmonary system
 The pulmonary system enables oxygen to diffuse into the blood and carbon dioxide to diffuse out of the blood.
The pulmonary system enables oxygen to diffuse into the blood and carbon dioxide to diffuse out of the blood. The majority of ventilation is involuntary. It is controlled by the sympathetic and parasympathetic divisions of the autonomic nervous system, which adjust airway calibre (by causing bronchial smooth muscle to contract or relax) and control the rate and depth of ventilation.
The majority of ventilation is involuntary. It is controlled by the sympathetic and parasympathetic divisions of the autonomic nervous system, which adjust airway calibre (by causing bronchial smooth muscle to contract or relax) and control the rate and depth of ventilation. Lung receptors monitor the mechanical aspects of ventilation. Irritant receptors sense the need to expel unwanted substances, stretch receptors sense lung volume (lung expansion) and J-receptors sense pulmonary capillary pressure.
Lung receptors monitor the mechanical aspects of ventilation. Irritant receptors sense the need to expel unwanted substances, stretch receptors sense lung volume (lung expansion) and J-receptors sense pulmonary capillary pressure. Chemoreceptors in the circulatory system and brainstem sense the effectiveness of ventilation by monitoring the pH status of cerebrospinal fluid and the oxygen content of arterial blood.
Chemoreceptors in the circulatory system and brainstem sense the effectiveness of ventilation by monitoring the pH status of cerebrospinal fluid and the oxygen content of arterial blood. Successful ventilation involves the mechanics of breathing: the muscles of inspiration and expiration, alveolar surface tension, elastic properties of the lungs and chest wall, and resistance to airflow.
Successful ventilation involves the mechanics of breathing: the muscles of inspiration and expiration, alveolar surface tension, elastic properties of the lungs and chest wall, and resistance to airflow. The major muscle of inspiration is the diaphragm. When the diaphragm contracts, it moves downwards, creating a vacuum in the thoracic cavity that causes air to flow into the lungs.
The major muscle of inspiration is the diaphragm. When the diaphragm contracts, it moves downwards, creating a vacuum in the thoracic cavity that causes air to flow into the lungs. Type II alveolar cells produce surfactant, a lipoprotein that lines the alveoli. Surfactant reduces alveolar surface tension and permits the alveoli to expand as air flows in.
Type II alveolar cells produce surfactant, a lipoprotein that lines the alveoli. Surfactant reduces alveolar surface tension and permits the alveoli to expand as air flows in. Compliance is the ease with which the lungs and chest wall expand during inspiration. Lung compliance is ensured by an adequate production of surfactant, whereas chest wall expansion depends on elasticity.
Compliance is the ease with which the lungs and chest wall expand during inspiration. Lung compliance is ensured by an adequate production of surfactant, whereas chest wall expansion depends on elasticity. Elastic recoil is the tendency of the lungs and chest wall to return to their resting state after inspiration.
Elastic recoil is the tendency of the lungs and chest wall to return to their resting state after inspiration. The elastic recoil forces of the lungs and chest wall are in opposition, creating negative pressure in the pleural space.
The elastic recoil forces of the lungs and chest wall are in opposition, creating negative pressure in the pleural space. Gas transport depends on ventilation of the alveoli, diffusion across the alveolar–capillary membrane, perfusion of the pulmonary and systemic capillaries, and diffusion between systemic capillaries and tissue cells.
Gas transport depends on ventilation of the alveoli, diffusion across the alveolar–capillary membrane, perfusion of the pulmonary and systemic capillaries, and diffusion between systemic capillaries and tissue cells. Efficient gas exchange depends on an even distribution of ventilation and perfusion within the lungs. In the upright individual, both ventilation and perfusion are greatest in the bases of the lungs because the alveoli in the bases are more compliant (the resting volume is low) and perfusion is greater in the bases due to gravity.
Efficient gas exchange depends on an even distribution of ventilation and perfusion within the lungs. In the upright individual, both ventilation and perfusion are greatest in the bases of the lungs because the alveoli in the bases are more compliant (the resting volume is low) and perfusion is greater in the bases due to gravity. Almost all the oxygen that diffuses into pulmonary capillary blood is transported by haemoglobin, located within red blood cells. The remainder of the oxygen is transported dissolved in plasma.
Almost all the oxygen that diffuses into pulmonary capillary blood is transported by haemoglobin, located within red blood cells. The remainder of the oxygen is transported dissolved in plasma. Oxygen enters the body by diffusing down the concentration gradient, from high concentrationsy in the alveoli to lower concentrations in the pulmonary capillaries. Diffusion ceases when alveolar and capillary oxygen pressures equilibrate.
Oxygen enters the body by diffusing down the concentration gradient, from high concentrationsy in the alveoli to lower concentrations in the pulmonary capillaries. Diffusion ceases when alveolar and capillary oxygen pressures equilibrate.Jack is 33 years old and has recently undertaken his yearly physical check-up for the fire brigade. He is fit, exercising 5 times a week for 60 minutes on each occasion. The following measures were recorded at his check-up: weight 76 kg, height 182 cm, resting heart rate 56 beats/minute, blood pressure 116/78 mmHg. Jack reported that he has no physical ailments. As part of the check-up, a pulmonary examination was undertaken. This comprised observation of chest movement and ventilation, palpation of the chest wall with hands over the ribcage at the front and back, and auscultation (listening with a stethoscope) to breath sounds over the lung fields. The following observations were noted:
Jack passed his physical check-up and can continue with his fire-fighting role for another year.