21 ALTERATIONS OF MUSCULOSKELETAL FUNCTION ACROSS THE LIFE SPAN
INTRODUCTION
The musculoskeletal system is subject to a large number of disorders that affect people of all ages. Congenital conditions affect the newborn; the major cause of dysfunction through to adulthood is trauma; and in the elderly the effects of reducing bone density or accumulated wear and tear on the skeleton cause fractures and failure of joints.
Musculoskeletal injuries have a major impact on patients, families and the community because of the increased support necessary to counteract the physical and psychological effects of reduced capability, pain and decreased quality of life. There are also financial and economic impacts, from direct costs of diagnosis and treatments to costs related to the loss of employment and decreased productivity.
MUSCULOSKELETAL INJURIES
Musculoskeletal trauma is referred to as the ‘neglected disease’. The 2004–2005 National Health Survey of Australia indicated that 648,000 people had sustained an injury in the previous 4 weeks,1 and between 1999 and 2003 1 of every 9 hospitalisations in New Zealand was attributed to trauma.2 Furthermore, trauma, often resulting in musculoskeletal injuries, is the leading cause of death in people aged 1 to 34 years for all races and socioeconomic levels.
Skeletal trauma
Fractures
A fracture is a break in a bone. A bone breaks when force is applied that exceeds its tensile or compressive strength. The incidence of fractures varies for individual bones according to age and gender, with the highest incidence of fractures occurring in young males (between the ages of 15 and 24) and older persons (65 years and older). Fractures of healthy bones, particularly the tibia, clavicle and lower humerus, tend to occur in young people and to be the result of trauma. Fractures of the hands and feet are usually caused by accidents in the workplace. The incidence of fractures of the upper femur, upper humerus, vertebrae and pelvis is highest in older adults and is often associated with osteoporosis. Hip fractures, the most serious outcome of osteoporosis, are occurring much more frequently because the populations of Australia and New Zealand are ageing.3
Classification of fractures
Fractures can be classified as complete or incomplete and open or closed (see Figure 21-1). In a complete fracture the bone is broken all the way through, whereas in an incomplete fracture the bone is damaged but still in one piece. Complete and incomplete fractures also can be called open (formerly referred to as compound) if the skin is broken and closed (formerly called simple) if it is not. A fracture in which a bone breaks into two or more fragments is termed a comminuted fracture. Fractures are also classified according to the direction of the fracture line: a linear fracture runs parallel to the long axis of the bone; an oblique fracture occurs at an oblique angle to the shaft of the bone; a spiral fracture encircles the bone; and a transverse fracture occurs straight across the bone.
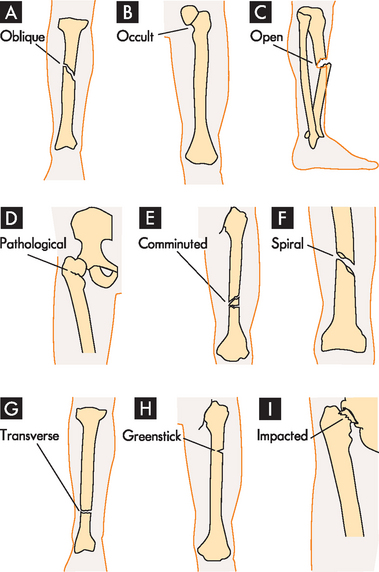
FIGURE 21-1 Examples of types of bone fractures.
A Oblique: fracture at oblique angle across both cortices. Cause: direct or indirect energy, with angulation and some compression. B Occult: fracture that is hidden or not readily discernible. Cause: minor force or energy. C Open: skin broken over fracture; possible soft-tissue trauma. Cause: moderate-to-severe energy that is continuous and exceeds tissue tolerances. D Pathological: transverse, oblique or spiral fracture of bone weakened by tumour pressure or presence. Cause: minor energy or force, which may be direct or indirect. E Comminuted: fracture with two or more pieces or segments. Cause: direct or indirect moderate-to-severe force. F Spiral: fracture that curves around cortices and may become displaced by twist. Cause: direct or indirect twisting energy or force with distal part held or unable to move. G Transverse: horizontal break through bone. Cause: direct or indirect energy towards bone. H Greenstick: break in only one cortex of bone. Cause: minor direct or indirect energy. I Impacted: fracture with one end wedged into opposite end of inside fractured fragment. Cause: compressive axial energy or force directly to distal fragment.
Source: Based on Mourad L. Musculoskeletal system. In Thompson JM et al. (eds). Mosby’s clinical nursing. 7th edn. St Louis: Mosby; 2002.
Incomplete fractures tend to occur in the more flexible, growing bones of children. The three main types of incomplete fractures are greenstick, torus and bowing fractures. A greenstick fracture perforates one cortex and splinters the spongy bone. The name is derived from the similar appearance of the damage sustained by a young tree branch (a green stick) when it is bent sharply. The outer surface is disrupted, but the inner surface remains intact. Greenstick fractures typically occur in the proximal metaphysis or diaphysis of the tibia, radius and ulna. In a torus fracture, the cortex buckles but does not break. Bowing fractures usually occur when longitudinal force is applied to bone. This type of fracture is common in children and usually involves the paired radius–ulna or fibula–tibia. A complete diaphyseal fracture occurs in one of the bones of the pair, which disperses the stress sufficiently to prevent a complete fracture of the second bone, which bows rather than breaks. A bowing fracture resists correction (reduction) because the force necessary to reduce it must be equal to the force that bowed it. Treatment of bowing fractures is also difficult because the bowed bone interferes with reduction of the fractured bone. Types of fractures are summarised in Table 21-1.
| TYPE OF FRACTURE | DEFINITION |
|---|---|
| Typical complete fractures | |
| Closed | Non-communicating wound between bone and skin |
| Open | Communicating wound between bone and skin |
| Comminuted | Multiple bone fragments |
| Linear | Fracture line parallel to long axis of bone |
| Oblique | Fracture line at an angle to long axis of bone |
| Spiral | Fracture line encircling bone (as a spiral staircase) |
| Transverse | Fracture line perpendicular to long axis of bone |
| Impacted | Fracture fragments pushed into each other |
| Pathological | Fracture at a point where bone has been weakened by disease (e.g. by tumours or osteoporosis) |
| Avulsion | A fragment of bone connected to a ligament or tendon breaks off from the main bone |
| Compression | Fracture wedged or squeezed together on one side of bone |
| Displaced | Fracture with one, both or all fragments out of normal alignment |
| Extracapsular | Fragment close to the joint but remains outside the joint capsule |
| Intracapsular | Fragment within the joint capsule |
| Typical incomplete fractures | |
| Greenstick | Break in one cortex of bone with splintering of inner bone surface; commonly occurs in children and the elderly |
| Torus | Buckling of cortex |
| Bowing | Bending of bone |
| Stress | Microfracture |
| Transchondral | Separation of cartilaginous joint surface (articular cartilage) from main shaft of bone |
Fractures may be further classified by cause as pathological, stress or transchondral fractures. A pathological fracture is a break at the site of a pre-existing abnormality, usually by force that would not fracture a normal bone. Any disease process that weakens a bone (especially the cortex) predisposes the bone to pathological fracture. Pathological fractures are commonly associated with tumours, osteoporosis, infections and metabolic bone disorders.
Stress fractures occur in normal or abnormal bone that experiences repeated stress, such as occurs during athletics. The stress is less than the stress that usually causes a fracture. Two types of stress fractures are recognised: fatigue fracture and insufficiency fracture. A fatigue fracture is caused by abnormal stress or torque applied to a bone with normal ability to deform and recover. Fatigue fractures usually occur in individuals who engage in a new or different activity that is both strenuous and repetitive (e.g. joggers, skaters, dancers, military recruits). Because gains in muscle strength occur more rapidly than gains in bone strength, the newly developed muscles place exaggerated stress on the bones that are not yet ready for the additional stress. The imbalance between muscle and bone development causes microfractures to develop in the cortex. If the activity is controlled and increased gradually, new bone formation catches up to the increased demands and microfractures do not occur. Runners employ the 10% rule to help avoid this problem, increasing their distance (or time) by 10% per week.
Insufficiency fractures are stress fractures that occur in bones lacking the normal ability to deform and recover; a fracture can occur as a result of normal weight-bearing or activity. Rheumatoid arthritis, osteoporosis, Paget’s disease, osteomalacia, rickets, hyperparathyroidism and radiation therapy all cause bone to lose its normal ability to deform and recover — that is, the stress of normal weight-bearing or activity fractures the bone. Many of these conditions are referred to later in this chapter.
A transchondral fracture consists of break-up and separation of a portion of the articular cartilage that covers the end of a bone at a joint as a result of trauma (joint structures are discussed in Chapter 20). Single or multiple sites may be fractured and the fragments may consist of cartilage alone or cartilage and bone. Typical sites of transchondral fracture are the distal femur, ankle, kneecap, elbow and wrist. Transchondral fractures are most prevalent in adolescents.
PATHOPHYSIOLOGY
When a bone is broken, the periosteum and blood vessels in the cortex, marrow and surrounding soft tissues are disrupted. Bleeding occurs from the damaged ends of the bone and from the neighbouring soft tissue. The volume of blood lost can be significant. For example, a simple break of the humerus accounts for 200–300 mL of blood loss, a simple break of the femur loses 500–1000 mL of blood and a unilateral break of the pelvis can bleed 1000–1500 mL. A clot (haematoma) forms within the medullary canal, between the fractured ends of the bone and beneath the periosteum (see Figure 21-2). Because blood flow to the injured area is disrupted (there is no oxygen supply) bone tissue immediately adjacent to the fracture dies. This dead tissue (along with any debris in the fracture area) stimulates an intense inflammatory response characterised by vasodilation, increased permeability allowing exudation of plasma, and infiltration by inflammatory leucocytes, growth factors and mast cells that simultaneously decalcify the fractured bone ends.
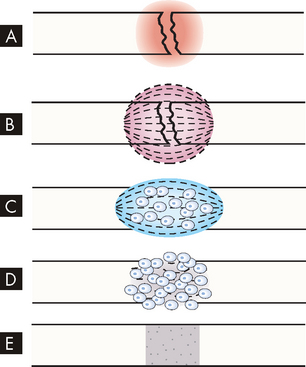
FIGURE 21-2 Bone healing (schematic representation).
A Bleeding at broken ends of the bone with subsequent haematoma formation. B Organisation of haematoma into fibrous network. C Invasion of osteoblasts, lengthening of collagen strands and deposition of calcium. D Callus formation; new bone is built up as osteoclasts destroy dead bone. E Remodelling is accomplished as excess callus is reabsorbed and trabecular bone is laid down.
Source: Monohan FD et al. Phipps’ medical-surgical nursing: health and illness perspectives. 8th edn. St Louis: Mosby; 2007.
Within 48 hours after the injury, new blood vessels grow (in a process called angiogenesis) from surrounding soft tissue and the marrow cavity into the fracture area and blood flow to the entire bone increases. Phagocytic cells begin cleaning up the debris (there are many dead red blood cells in the haematoma). Fibroblasts (collagen-forming cells) and osteoblasts (bone-forming cells) migrate into the damaged area (see Figure 21-2). The fibroblasts lay down collagen to form a fibrocartilaginous callus and the osteoblasts produce matrix, which they mineralise to form spongy bone. The osteoblasts migrate inwards to mineralise the whole callus, forming a bony callus. The bone is effectively ‘splinted’ at this stage, which takes from 3 to 10 weeks to achieve. As the repair process continues, remodelling occurs (for months), during which unnecessary callus is resorbed and trabeculae are formed along lines of stress (see Figure 21-3). The final structure is a response to the mechanical stress experienced by the bone. Bone is unique among all body tissues (apart from the liver) in that it forms new bone, not scar tissue, when it heals after a fracture.
CLINICAL MANIFESTATIONS
The signs and symptoms of a fracture include unnatural alignment (deformity), swelling, muscle spasm, tenderness, pain, impaired sensation and decreased mobility. The position of the bone segments is determined by the pull of attached muscles, gravity, and the direction and size of the force that caused the fracture.
Immediately after a bone is fractured, there is usually numbness in the fracture site because of trauma to the nerve or nerves at the site. The numbness may last up to 20 minutes, during which time the injured person may use the fractured bone or bones to crawl or move from the area. It is also possible to reduce (realign) the fracture during this time without any anaesthetic. However, once the numbness disappears, the subsequent pain is quite severe and incapacitating until relieved with medication and treatment of the fractured bones. The pain is related to muscle spasms at the fracture site, overriding of the fracture segments or damage to adjacent soft tissues.
Pathological fractures usually cause changes in the angle of a limb or its apparent point of articulation (the point about which it bends compared to the body), painless swelling or generalised bone pain. Stress fractures are generally painful during and after activity. The pain is usually relieved by rest. Stress fractures also cause local tenderness and soft-tissue swelling. Transchondral fractures may be entirely asymptomatic or may be painful during movement. Range of motion in the joint is limited and movement may produce audible clicking sounds (crepitus).
EVALUATION AND TREATMENT
Treatment of a displaced fracture involves realigning the bone fragments (reduction) close to their normal anatomic position and holding the fragments in place (immobilisation) so that bone union can occur. Several methods are available to reduce a fracture: closed manipulation, traction and open reduction. Adequate immobilisation is often all that is required for healing of fractures that are not misaligned.
Vitamin D and fracture risk
The beneficial effects of vitamin D on fracture risk are attributed to two explanations: (1) the decrease in bone loss in older persons; and (2) the increase in muscle strength and balance mediated through vitamin D receptors in muscle tissue.
In addition, vitamin D has been correlated with a significant reduction (22%) in the risk of falling in older people. Pooled analyses reveal that higher doses of 700–800 IU/day are better for reducing fractures than 400 IU/day. Previously, the recommendation for vitamin D in middle-aged and older adults was 400–600 IU/day. With new data and the uncertainty of intake recommendations, higher doses may be more effective (i.e. 700–800 IU/day). However, because calcium was administered in combination with vitamin D in all but one of the higher-dose vitamin D trials, the independent effects of vitamin D alone could not be determined. We still need further research into whether and in what dose calcium adds value to fracture prevention with vitamin D.
Source: Bischoff-Ferrari HA et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005; 293:2257–2264.
Most fractures can be reduced by closed manipulation and reduction. The bone is moved or manipulated into place without opening the skin. Closed reduction is used when the contour of the bone is in fair alignment and can be maintained well with immobilisation.
Traction may be used to accomplish or maintain reduction. When bone fragments are displaced (not in their anatomic position), weights are used to apply firm, steady traction (pull) and countertraction (pull in the other direction on the other side of the break) to the long axis of the bone. Traction stretches and fatigues muscles that have pulled the bone fragments out of place, allowing the distal fragment to align with the proximal fragment. Traction can be applied to the skin (skin traction), directly to the involved bone or distal to the involved bone (skeletal traction). Skin traction is used to a limit of 5 kg of pulling force to realign the fragments or when the traction will be used for brief times only, such as before surgery or, for children with femoral fractures, for 3–7 days before applying a cast. A traction boot is applied to the skin, closed with self-adhering straps and then weights are attached to the foot area of the traction boot. In skeletal traction, a pin or wire is drilled through the bone below the fracture site and a traction bow, rope and weights are attached to the pin or wire to apply tension and to provide the pulling force to overcome the muscle spasm and help realign the fracture fragments.
Open reduction is a surgical procedure that exposes the fracture site; the fragments are brought into alignment under direct visualisation. Some form of prosthesis, screw, plate, nail or wire is used most often to maintain the reduction (internal fixation). External fixation, a system of surgically placed pins and stabilising bars, is another method of maintaining fracture alignment. Bone grafts, using donor bone from the individual (autograft), cadaver (allograft) or bone substitutes (ceramic composites, bioactive cement), can fill voids in the bone.
Splints and plaster casts are used to immobilise and hold a reduction in place. Improper reduction or immobilisation of a fractured bone may result in non-union, delayed union or malunion:
Dislocation and subluxation
Dislocation and subluxation are usually caused by trauma. Dislocation is the temporary displacement of one or more bones in a joint in which the opposing bone surfaces lose contact entirely. If the contact between the opposing bone surfaces is only partially lost, the injury is called a subluxation.
Dislocation and subluxation are most common in persons younger than 20 years of age and are generally associated with fractures. However, they may be the result of congenital or acquired disorders that cause: (1) muscular imbalance, as occurs with congenital dislocation of the hip or neurological disorders; (2) failure of the articulating surfaces of the bones to match, as occurs with rheumatoid arthritis (see later in the chapter); or (3) joint instability.
The joints most often dislocated or subluxated are the joints of the shoulder, elbow, wrist, finger, hip and knee. The shoulder joint most often injured is the glenohumeral joint. Traumatic dislocation of the elbow joint is common in the immature skeleton. In adults, an elbow dislocation is usually associated with a fracture of the ulna or head of the radius. Traumatic dislocation of the wrist usually involves the distal ulna and carpal bones. Any one of the eight carpal bones can be dislocated after an injury. Dislocation in the hand usually involves the metacarpophalangeal and interphalangeal joints.
Considerable trauma is needed to dislocate the hip. Anterior hip dislocation is rare; it is caused by forced abduction, for example, when an individual lands on their feet after falling from an elevated height. Posterior dislocation of the hip can occur as a result of a car accident in which the flexed knee strikes the dashboard, causing the head of the femur to be pushed posteriorly from the hip joint.
The knee is an unstable weight-bearing joint that depends heavily on the soft-tissue structures around it for support. It is exposed to many different types of motion (flexion, extension, rotation) and is one of the most commonly injured joints. A knee dislocation can be anterior, posterior, lateral, medial or rotary. It is usually the result of an injury that occurs during sports activities. In addition, the meniscus within the knee joint may become damaged, usually by trauma.
PATHOPHYSIOLOGY
Dislocations and subluxations are often accompanied by fracture because stress is placed on areas of bone not usually subjected to stress. In addition, as the bone separates from the joint, it may bruise or tear adjacent nerves, blood vessels, ligaments, supporting structures and soft tissue. Dislocations of the shoulder may damage the shoulder capsule and the axillary nerve. Damage to axillary nerves can causes anaesthesia to a small area of the upper arm and paralysis of the deltoid muscle. Dislocations may also disrupt circulation, leading to ischaemia (low blood supply) and possibly permanent disability of the affected extremity’s tissues.
CLINICAL MANIFESTATIONS
Signs and symptoms of dislocations or subluxations include pain, swelling, limitation of motion and joint deformity. Pain may be caused by the presence of inflammatory chemicals (such as bradykinin; see Chapter 13) and exudate in the joint or associated tendon and ligament injury. Joint deformity is usually caused by muscle contractions that exert pull on the dislocated or subluxated joint. Limitation of motion results from swelling in the joint (with associated pain) or the displacement of bones.
EVALUATION AND TREATMENT
Evaluation of dislocations and subluxations is based on clinical manifestations and X-ray imaging. Treatment consists of reduction and immobilisation for 2–6 weeks and exercises to maintain normal range of motion in the joint. Depending on which joint is injured, healing is usually complete within months to sometimes years.
Support structures
Sprains and strains of tendons and ligaments
Tendon and ligament injuries can accompany fractures and dislocations. A tendon is a fibrous connective tissue that attaches skeletal muscle to bone. A ligament is a band of fibrous connective tissue that connects bones where they meet in a joint. Tendons and ligaments support the bones and joints and either allow or limit motion. Tendons and ligaments can be torn, ruptured or completely separated from bone at their points of attachment.
A tear in a tendon is commonly known as a strain. Major trauma can tear or rupture a tendon at any site in the body. Most commonly injured are the tendons of the hands and feet, knee (patellar), upper arm (biceps and triceps), thigh (quadriceps), ankle and heel (Achilles).
Ligament tears are commonly known as sprains. Ligament tears and ruptures can occur at any joint but are most common in the wrist, ankle, elbow and knee joints. A complete separation of a tendon or ligament from its bony attachment site is known as an avulsion and is commonly seen in young athletes, especially sprinters, hurdlers and runners.
Strains and sprains are classified as first degree (least severe), second degree and third degree (most severe).
PATHOPHYSIOLOGY
When a tendon or ligament is torn, an inflammatory exudate (a fluid that has been filtered from the blood, containing inflammatory chemicals) develops between the torn ends. Later, granulation tissue containing macrophages (to remove the damaged tissue), fibroblasts (to make collagen) and capillary buds (growing new blood vessels — angiogenesis) grows inwards from the surrounding soft tissue and cartilage to begin the repair process. Within 4–5 days after the injury, collagen formation begins. At first, collagen formation is random and disorganised. As the collagen fibres become associated with pre-existing tendon fibres, they become organised to run along the lines of stress. Eventually the new and surrounding tissues fuse into a single mass. As reorganisation takes place, the healing tendon or ligament separates from the surrounding soft tissue. Usually a healing tendon or ligament lacks sufficient strength to withstand strong pull for at least 4–5 weeks after the injury (a ruptured Achilles may take 6 months to heal). If strong muscle pull does occur during this time, the tendon or ligament ends may separate again, causing the tendon or ligament to heal in a lengthened shape with an excessive amount of scar tissue that renders the tendon or ligament functionless.
CLINICAL MANIFESTATIONS
Tendon and ligament injuries are painful and are usually accompanied by soft-tissue swelling, changes in tendon or ligament contour and dislocation or subluxation of bones. The pain is generally sharp and localised and tenderness persists over the distribution of the tendon or ligament. Depending on the tendon or ligament involved, such injuries may result in decreased mobility, instability and weakness of the affected joints, even with prompt treatment.
EVALUATION AND TREATMENT
Evaluation is based on clinical manifestations, stress radiography, arthroscopy (using an endoscope to view the interior of the tissue) or arthrography (an X-ray examination in which a contrast medium is used to better visualise the damage). When possible, treatment consists of suturing the tendon or ligament ends closely together. If this is not possible because of the extent of damage, tendon or ligament grafting may be necessary. Long-term rehabilitation exercises help ensure regaining of nearly normal functions, but recovery may be complicated by posttraumatic arthritis.
Tendonitis, epicondylitis and bursitis
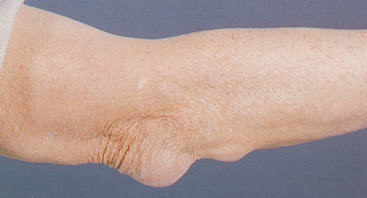
Trauma can cause painful inflammation of tendons (tendonitis) and bursae (bursitis). Other causes of damage to tendons include reduced tissue perfusion, mechanical irritation, crystal deposits, postural misalignment and hypermobility in a joint. The histopathology of common conditions such as lateral epicondylitis (‘tennis elbow’) and medial epicondylitis (‘golfer’s elbow’) is a degenerative process (see Figure 21-4)4 brought about by submaximal overload of the tendon. Achilles tendonitis is inflammation of the Achilles tendon, one that is often inflamed.
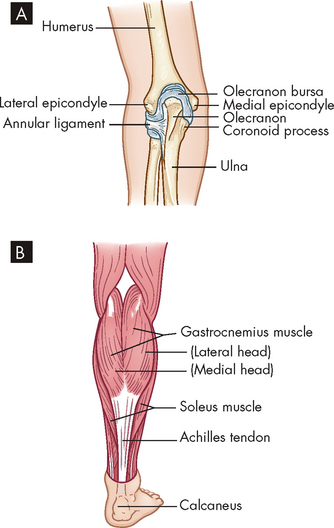
FIGURE 21-4 Tendonitis and epicondylitis.
A Medial or lateral epicondyles of humerus, site of epicondylitis. B Achilles tendon, site of commonly occurring tendonitis.
Epicondylitis is inflammation of a tendon where it attaches to a bone at its origin. Epicondylar areas of the humerus, radius or ulna and around the knee are most often inflamed. Lateral epicondylitis, commonly called ‘tennis elbow’ (although most affected people are not tennis players), is likely caused by irritation of the extensor carpi radialis brevis tendon and the resulting degradation. Medial epicondylitis, referred to as ‘golfer’s elbow’, is inflammation of the medial humeral epicondyle (see Figure 21-4). Epicondylitis is also related to work activities that involve cyclic flexion and extension of the elbow or cyclic pronation, supination, extension and flexion of the wrist that generate loads to the elbow and forearm region. A longitudinal study indicates that three sets of risk factors affect the incidence of epicondylitis. They include biochemical constraints and psychosocial and personal factors (including social support at work).5
Bursae are small sacs lined with synovial membrane and filled with synovial fluid that act to provide a slippery, cushioning surface to reduce friction for tissues of the body. They are typically located between tendons, muscles and bones near major joints in the body. Acute bursitis occurs primarily in the middle years and is caused by trauma. Chronic bursitis can result from repeated trauma. Septic bursitis is caused by wound infection or bacterial infection of the skin overlying the bursae. Bursitis commonly occurs in the shoulder, hip, knee and elbow (see Figure 21-5).
PATHOPHYSIOLOGY
In addition to tearing of the tendon, evidence also exists of tissue degeneration and disorganised collagen formation.6 Initial inflammatory changes cause swelling of the area, limiting movements and causing pain. Microtears cause bleeding, oedema and pain in the involved tendon or tendons. At times, after repeated inflammations, calcium may be deposited in the tendon. The calcium is usually spontaneously reabsorbed by the body.
Usually bursitis is an inflammation that is reactive to overuse or excessive pressure. The inflamed bursal sac becomes engorged and the inflammation can spread to adjacent tissues. The inflammation may decrease with rest, ice and aspiration of the fluid. (Inflammation is discussed in Chapter 13.)
CLINICAL MANIFESTATIONS
Clinical manifestations are usually localised to one side of the joint. Generally there is local tenderness and more pain with active motion than with passive motion. With tendonitis, the pain is localised over the involved tendon. Pain and sometimes weakness limit joint movement. The onset of pain may be gradual or sudden in bursitis and pain may limit active movement in the joint. Shoulder bursitis impairs arm abduction. Bursitis in the knee produces pain when climbing stairs, and crossing the legs is painful in bursitis of the hip. Lying on the side of the inflamed bursa is also very painful. Signs of infectious bursitis may include the presence of a puncture site, warmth and erythema (red appearance of the skin due to dilation of capillaries), prior corticosteroid injection, severe inflammation or an adjacent source of infection, such as an infected total joint replacement.
EVALUATION AND TREATMENT
The evaluation of tendonitis, epicondylitis and bursitis is based on clinical manifestations, physical examination, arthroscopy, arthrography and possibly MRI. Treatment includes immobilisation of the joint with a sling, splint or cast; systemic analgesics; ice or heat applications; or local injection of an anaesthetic and a corticosteroid to reduce inflammation. Physical therapy to prevent loss of function begins after acute inflammation subsides.
Muscle strains
Mild injury such as muscle strain is usually seen after traumatic or sports injuries. Muscle strain is a general term for local muscle damage. It is often the result of sudden, forced motion causing the muscle to become stretched beyond normal capacity. Strains often involve the tendon as well. Muscles are ruptured more often than tendons in young people; the opposite is true in the older population. Muscle strain may be chronic when the muscle is repeatedly stretched beyond its usual capacity. There is evidence of tissue disruption with subsequent signs of muscle regeneration and connective tissue repair when a biopsy is performed. Haemorrhage into the surrounding tissue and signs of inflammation also may be present. Regardless of the cause of trauma, muscle cells are usually able to regenerate. Regeneration may take up to 6 weeks and the affected muscle should be protected during that time. Degrees of acute muscle strain, together with their manifestations and treatment, are summarised in Table 21-2.
| TYPE | MANIFESTATIONS | TREATMENT |
|---|---|---|
| First degree (example: bench press in untrained athlete) | Muscle overstretched | Ice should be applied 5 or 6 times in the first 24–48 hours; gradual resumption of full weight-bearing after initial rest for up to 2 weeks; exercises individualised to specific injury |
| Second degree (example: any muscle strain with bruising and pain) | Muscle intact with some tearing of fibres, pain | Treatment similar to that for first-degree strains |
| Third degree (example: traumatic injury) | Caused by tearing of fascia | Surgery to approximate ruptured edges; immobilisation and non-weight-bearing for 6 weeks |
Myoglobinuria
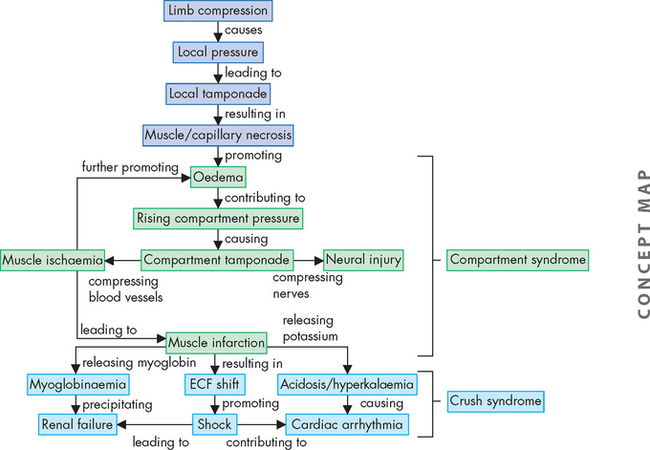
Myoglobinuria, also called rhabdomyolysis, can be a life-threatening complication of severe muscle trauma or secondary to a rare, genetically linked condition known as malignant hyperthermia. Myoglobinuria is so named because the principal manifestation of the condition is an excess of myoglobin (an oxygen-carrying intracellular muscle protein) in the urine. Muscle damage, with disruption of the sarcolemma (cell membrane of the muscle fibre), releases the myoglobin, which acts as a nephrotoxin (a substance that is toxic to nephrons) and may cause acute renal failure (see Chapter 30).
The most severe form is often called crush syndrome. Less severe local forms are called compartment syndromes. Crush syndrome first gained notoriety in the reports of injuries seen after the London air raids in World War II. More recently, it has been reported in individuals found unresponsive and immobile for long periods, usually after a drug overdose. Myoglobinuria also can be seen after viral infections, administration of cholesterol-lowering drugs known as statins, certain anaesthetic agents, cocaine, amphetamines, heroin, alcoholism with subsequent muscle tremors, tetanus, heat stroke, electrolyte disturbances and fractures. Excessive muscular activity also has been implicated in reports of myoglobinuria in athletes (such as long-distance runners and skiers) and military recruits. Status epilepticus, electroconvulsive therapy and high-voltage electrical shock are also associated with severe and sometimes fatal myoglobinuria.
PATHOPHYSIOLOGY
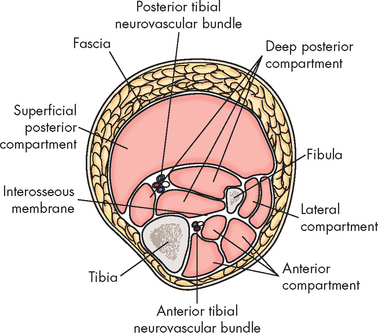
The primary requirement to develop myoglobinuria is damage to muscle fibres allowing the release of myoglobin. This damage may occur directly, as in the case of trauma, or as a result of any event that injures the sarcolemma. In the case of compartment syndromes the usual cause is ischaemia. The muscles of the limbs are organised within their non-elastic fascias. Many of the blood vessels (and nerves) are located deep to the fascia (see Figure 21-6). Thus any event that raises the pressure within the fascia can result in a compartment syndrome, as the increased pressure is occurring within the enclosed space of the muscle compartment. An increase in compartment volume may be caused by haematoma associated with a fracture or trauma and associated inflammation causing oedema. Even the weight of a limp extremity can generate enough pressure to reduce venous return. With continuing arterial supply the volume of the compartment increases. Whatever the cause, as the pressure within the compartment rises, the circulation becomes further compromised. Muscle necrosis occurs within 4–8 hours after releasing myoglobin. The myoglobin is toxic to the tubular cells of the kidneys. The pathogenesis of compartment syndrome and crush syndrome is outlined in Figure 21-7.
CLINICAL MANIFESTATIONS
When myoglobin is released from the muscle cells into the circulation, it can cause a visible, dark reddish brown pigmentation of the urine.7 Only 200 grams of muscle need be damaged to cause visible changes in the urine. The damaged cells also release intracellular enzymes, potassium and phosphate into the serum. One of the enzymes, creatine kinase (involved in creating phosphocreatine, an ATP store), may reach 2000 times normal levels (normal levels are 5–25 U/mL for women and 5–35 U/mL for men). The risk of renal failure correlates directly with the amount of serum creatine kinase, potassium and phosphorus levels in the blood.
EVALUATION AND TREATMENT
The manifestation of myoglobinuria for those with the genetic condition of malignant hyperthermia occurs during anaesthesia. Clinicians need to carefully assess the background of these individuals to diagnose potential malignant hyperthermia — a family history of anaesthetic problems and previous untoward anaesthetic experiences (muscle cramping, unexplained fevers, dark urine) are criteria that require further clarification before administration of a volatile anaesthetic, such as halothane, or the muscle relaxant succinylcholine.
Priorities in treatment of myoglobinuria include identifying and treating the underlying disorder and preventing life-threatening renal failure. Malignant hyperthermia and myoglobinuria can be treated by infusing dantrolene sodium. Diluting the pigment using intravenous fluids and administration of mannitol, sodium bicarbonate and frusemide to ‘flush’ the kidneys have been advocated to prevent renal failure. Secondary problems include electrolyte imbalance, volume depletion, acidosis, hyperuricaemia, hyperkalaemia and calcium imbalance. These need specific treatment. Short-term dialysis also may be necessary.
Compartment syndromes may require emergency treatment when blood flow to the affected extremity is compromised because of increased compartmental pressure, leading to ischaemia and oedema.8 When clinical evaluation is inconclusive, the rising compartment pressure can be directly measured by inserting a wick catheter, needle or slit catheter into the muscle. Pressures greater than 30 mmHg (normal = 0–8 mmHg) impair capillary flow.9 When conservative treatment fails to relieve the pressure, a fasciotomy may be necessary.9,10 In this procedure the fascia is incised parallel to the muscle. Because of the risk of infection and the added complication of wound closure, this procedure is one of last resort. Compartments often affected are the anterior tibial and deep posterior tibial compartments in the leg and the gluteal compartments in the buttocks.
DISORDERS OF BONE AND JOINTS
Metabolic bone diseases
Metabolic bone disease is characterised by abnormal bone structure that is caused by an altered metabolism, which may be the result of genetics, diet or hormones.
Osteoporosis
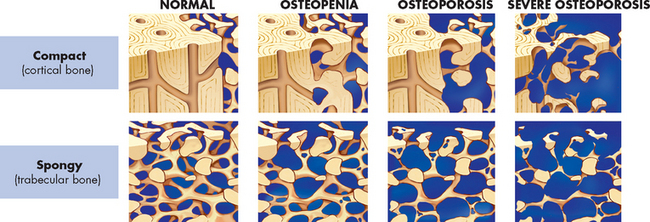
As the populations of Australia and New Zealand age, the incidence of osteoporosis will increase. Osteoporosis, or porous bone, is a disease in which bone tissue is normally mineralised but the mass (density) of bone is decreased and the structural integrity of trabecular (spongy) bone is impaired. Cortical (compact) bone becomes more porous and thinner, making bone weaker and prone to fractures (see Figures 21-8 and 21-9). The World Health Organization (WHO) has defined postmenopausal osteoporosis based on the bone density.11 Individual bone density is compared with the mean bone mineral density of a young-adult reference population; in other words, the degree of loss of bone mineral density (osteoporosis) is compared to the optimal level of that of a young adult. Figure 21.10 shows the progression from normal bone mineral density to severe osteoporosis. The disease can be: (1) generalised, involving major portions of the axial skeleton; or (2) regional, involving one segment of the appendicular skeleton.
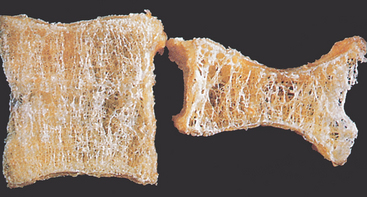
Osteoporotic vertebral body (right) shortened by compression fractures compared with a normal vertebral body. Note that the osteoporotic vertebra has a characteristic loss of horizontal trabeculae and thickened vertical trabeculae.
Source: Kumar V et al. Robbins & Cotran pathologic basis of disease. 8th edn. Philadelphia: Saunders; 2010.
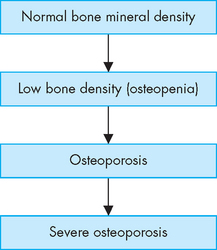
FIGURE 21-10 The progression from normal bone mineral density through various stages to severe osteoporosis.
With each stage, there is further loss of bone mineral density.
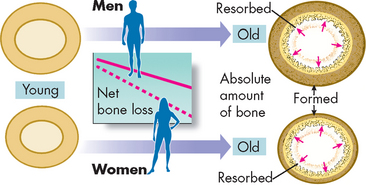
Throughout a lifetime bone responds to the stresses placed on it through the process of remodelling (see Chapter 20). This process involves removal of old bone (resorption) and creation of new bone (formation). Weight-bearing exercise increases bone mineral density. During childhood and the teenage years, new bone is added faster than old bone is removed. Consequently, bones become larger, heavier and denser. Peak bone mass or maximum bone density and strength is reached around age 30. After age 30, bone resorption slowly exceeds bone formation. In women, bone loss is most rapid in the first years after menopause, but persists throughout the postmenopausal years. Men lose bone density with ageing but because they begin with a higher bone density and their rate of loss is less than that of women, they reach osteoporotic levels at an older age than do women (see the box ‘Health alert: osteoporosis and men’).
The major risks for those with osteoporosis are fractures. By the age of 90, about 17% of males have had a hip fracture, compared with 32% of females. Over half of all adults hospitalised for hip fracture do not return to their former level of functioning.12 In Australia the lifetime risk of a fracture due to osteoporosis after 50 years of age is 42% for women and 27% for men.13
Osteoporosis in men
With the emphasis on osteoporosis in women, the cellular and molecular aspects of male idiopathic osteoporosis (idiopathic meaning cause unknown) are poorly understood. The major difference in bone physiology between males and females is in the level of gonadal hormones. Although hypogonadism is related to bone loss in men, and androgen levels decline with age in men, it is not at all clear that reduced androgen levels are related to bone loss in older men. Testosterone is possibly anabolic at the bone level, and testosterone increases muscle mass, which indirectly results in higher bone density. In peripheral tissue, testosterone is converted to oestrogen, which prevents excessive bone resorption. Oestrogen is necessary to bone in men as well as in women. Men who have a deficiency of the enzyme that converts testosterone to oestrogen develop osteoporosis and are excessively tall because of the failure to fuse growth plates. Thus, oestrogen plays a vital role in the maintenance of bone in men as well as women. Some studies have shown that for a given bone mineral density, males and females have the same fracture risk, although other studies demonstrate a higher fracture risk in men with a higher bone mineral density than in women.
Source: Byers RJ et al. Review. J Endocrinol 2002; 168(3):353–362; Seeman E. Pathogenesis of bone fragility in women and men. Lancet 2002; 359(9320):1841–1850; Vescini F et al. Does bone mineral density predict fractures comparatively in women and men? J Endocrin Invest 2005; 28(10 suppl):48–51.
Vertebral fractures also occur in the later years of life; however, they are more difficult to ascertain because people are unaware of the fracture. The degree of compression necessary to define a vertebral fracture is not standardised.13 Thus, the true prevalence is unknown, but fractures do increase in frequency by the sixth and seventh decades. Vertebral fracture prevalence in men is close to that in women.14 Osteoporosis is the foremost underlying cause of fractures in the elderly. It affects more than half of women aged 60 and older and nearly one-third of men aged 60 and older in both New Zealand15 and Australia.16 Total costs related to osteoporosis are estimated at A$7 billion annually in Australia17 and at NZ$1.5 billion annually in New Zealand.18
Osteoporosis is most common in smaller statured people. Interestingly, larger people have a lower incidence of osteoporosis because their skeletons have become more massive through the process of remodelling and achieved a higher peak bone mass.19 The cause of generalised osteoporosis remains uncertain.
Bone strength is not defined by bone mass alone (as measured by bone mass density) but also by the microscopic structure of the bone. Thus, other variables include mineral crystal size and shape, brittleness, vitality of the bone cells, structure of the bone proteins, integrity of the trabecular network and the ability to repair tiny cracks.20,21 In spongy bone, the positioning of trabecular structures is important in providing strength. Because bone density relates to quantity of bone, quality of bone is not accurately identified by bone density testing. Therefore, bone density testing may or may not accurately identify those who will go on to suffer a fracture.
Osteoporosis is a complex, multifactorial chronic disease that often progresses silently for decades until fractures occur. It is the most common disease that affects bone. It is not necessarily a consequence of the ageing process because some elderly people retain strong, relatively dense bones.22 A progressive loss of bone mass may continue until the skeleton is no longer strong enough to support itself. Eventually, bones can fracture spontaneously. As bone becomes more fragile, falls or bumps that would not have caused fracture previously now do cause a fracture. Osteoporosis appears to be most severe in the spine, wrists and hip (see Figure 21-11).
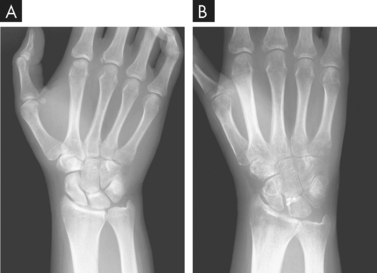
FIGURE 21-11 Osteoporosis as a result of disuse.
A X-ray taken just before wrist ligament reconstruction. B X-ray obtained 2 months later. Notice the extent of the osteopenia evident in the second image.
Source: Resnick D, Kransdorf M. Bone and joint imaging. 3rd edn Philadelphia: Saunders; 2004.
Postmenopausal osteoporosis is bone loss that occurs in middle-aged and older women. It can occur because of oestrogen deficiency as well as oestrogen-independent age-related mechanisms (e.g. secondary causes such as hyperparathyroidism and decreased mechanical stimulation) (see Figure 21-12). Oestrogen deficiency can also increase with stress, excessive exercise (particularly weight-bearing activities) and low body weight. Postmenopausal changes include a substantial increase in bone removal. There is an imbalance between the activities of the osteoclasts (bone destroyers) and osteoblasts (bone formers). Oestrogen helps osteoclast apoptosis (programmed cell death), so its decline is associated with survival of the bone-removing osteoclasts. Other causes may include a combination of inadequate dietary calcium intake and lack of vitamin D, possibly decreased magnesium, lack of exercise (particularly weight-bearing exercise), low body mass and family history.23 Excessive phosphate intake, chiefly through the intake of soft drinks and junk foods, interferes with the calcium/phosphate balance. Glucocorticoids (e.g. cortisone) also induce osteoporosis.
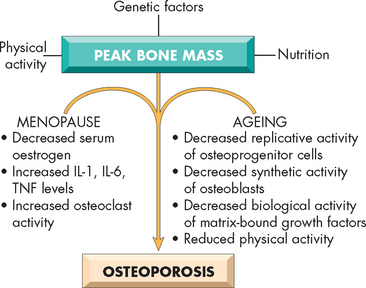
FIGURE 21-12 The pathophysiology of postmenopausal and senile osteoporosis.
Source: Kumar V et al. Robbins & Cotran pathologic basis of disease. 8th edn. Philadelphia: Saunders; 2010.
Oestrogen replacement can slow bone loss around the time of menopause; however, osteoporosis and fractures are still common in older women who have used oestrogen continuously since menopause.23,24 It has been found that serum androgens may influence bone density in women.14,25 Androgens (i.e. testosterone) have long been recognised as stimulants of bone formation. Increasing age in both men and women is associated with declining levels of oestrogen and androgen, leading to losses in bone mineral density. In addition, progesterone deficiency may be related to osteoporosis. Decreases in weight-bearing exercise are associated with osteoporosis as well. Other risk factors are identified in the box ‘Risk factors for osteoporosis’.
Intake of dietary minerals is important for skeletal health. Reduced intake or malabsorption of dietary minerals is a factor in the development of osteoporosis.26 Calcium absorption from the intestine decreases with age and studies of individuals with osteoporosis show that their calcium intake is lower than that of age-matched controls. Other mineral deficiencies may also be important, including magnesium. Deficiencies of vitamins, particularly vitamins C and D, and both deficiencies and excesses of protein also contribute to bone loss. Excessive intake of caffeine, phosphorus, alcohol and nicotine along with low body weight (less than 57 kg) have also been considered risk factors. In addition, significant differences in the trace elements (zinc, copper, manganese) have been noted in the bones and hair of unaffected individuals compared to those with osteoporosis.27
Skeletal homeostasis depends on a narrow range of plasma calcium and phosphate concentrations, which are maintained by the endocrine system. Therefore, endocrine dysfunction ultimately can cause metabolic bone disease. In addition to declining levels of sex steroids, the hormones most commonly associated with osteoporosis are parathyroid hormone, cortisol, thyroid hormone and growth hormone (see Figure 21-13). (Endocrine function is discussed in Chapters 10 and 11.)
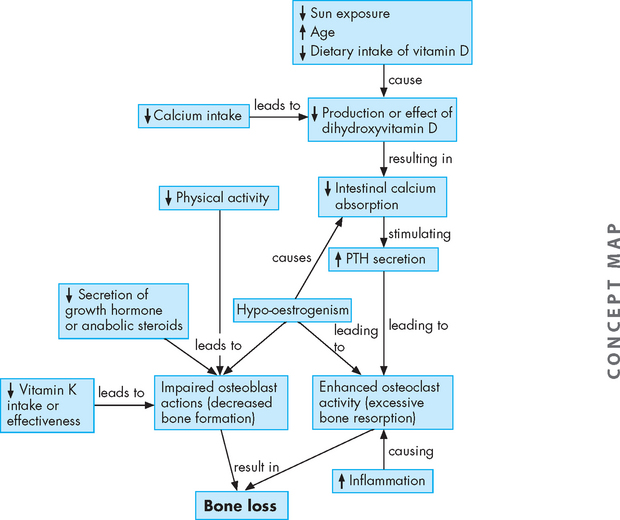
FIGURE 21-13 The pathophysiology of osteoporosis.
The changes that lead to bone loss. Dihydroxyvitamin D = vitamin D component that aids calcium absorption; PTH = parathyroid hormone.
Source: Based on Rakel D. Integrative medicine. 2nd edn. Philadelphia: Saunders; 2007.
Iatrogenic osteoporosis sometimes develops temporarily in individuals receiving large doses of heparin, perhaps because heparin promotes bone resorption by decreasing collagen synthesis or by increasing collagen breakdown. Osteoporosis caused by heparin therapy usually resolves when therapy ceases. Other medications increasing the risk of osteoporosis include glucocorticoids, lithium (used to treat bipolar disorder), methotrexate and cyclophosphamide (used to treat leukaemia and other cancers), anticonvulsants and cyclosporin (an immune suppressant used after organ transplantation).
Regional osteoporosis — osteoporosis confined to a region or segment of the appendicular skeleton — usually has a known cause. Classic regional osteoporosis is associated with disuse or immobilisation of a limb because of fractures, motor paralysis or bone or joint inflammation.28 A negative calcium balance develops early and continues throughout the period of immobilisation. After 8 weeks of immobilisation, significant osteoporosis is present, although it may develop earlier in those younger than 20 years or older than 50 years. A uniform distribution of osteoporosis has also been observed as a result of weightlessness in astronauts and individuals treated with air suspension therapy.
PATHOPHYSIOLOGY
It has been emphasised that remodelling is a normal feature of bone. Osteoclasts (bone-destroying cells) and osteoblasts (bone-building cells) are continually working to maintain bone with a structure that is responsive to and structurally able to withstand the stresses it experiences. To understand osteoporosis it is useful to have some knowledge of the interrelationship between osteoclasts and osteoblasts. Ultimately the number of osteoblasts is controlled by hormones, cytokines (intracellular communication molecules that control cell activity — cyto for cell and kines for action) and other chemical messengers. There is one cytokine that appears particularly important. It exerts its effect by binding to a receptor on osteoclast precursor cells (cells that combine to form osteoclasts) causing them to multiply and become activated. The bone matrix creates a decoy receptor for this same cytokine. If the cytokine binds to the decoy receptor there will be no effect on the osteoclasts. Thus the balance between the amounts of cytokine, the number of receptors on the osteoclast precursors and the number of decoy receptors determines the rate at which bone is resorbed. Any alteration to this balance can lead to osteoporosis.
Glucocorticoid-induced osteoporosis is characterised by increased bone resorption and decreased bone formation. Glucocorticoids (e.g. cortisone) increase formation of the cytokine and reduce production of its decoy receptor by osteoblasts.
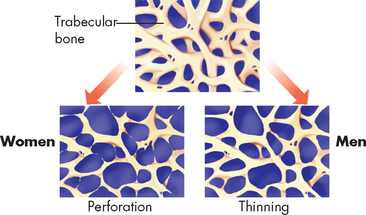
Age-related bone loss begins in the fourth decade. The cause remains unclear, but it is known that decreased serum growth hormone and insulin-like growth factor levels (both of which stimulate osteoclasts), along with increased binding of the cytokine and decreased production of the decoy receptor, affect osteoblast and osteoclast function. Loss of trabecular bone in men proceeds with thinning of trabecular bone rather than complete loss, as is noted in women (see Figure 21-14).29 Men have approximately 30% greater bone mass than women, which may be a factor in their later involvement with osteoporosis (see Figure 21-15). In addition, men have a more gradual decrease in testosterone and oestrogen (and possibly progesterone), thereby maintaining their bone mass longer than women.30 The reduction in weight-bearing activity with increasing age is another factor promoting bone loss.
CLINICAL MANIFESTATIONS
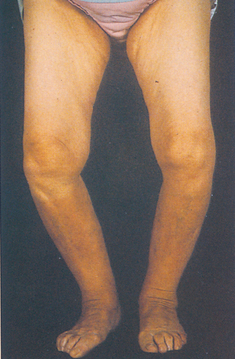
The specific clinical manifestations of osteoporosis depend on the bones involved. The most common manifestations, however, are pain and bone deformity. Unfortunately the condition develops insidiously and these manifestations occur only in an advanced disease state. By the time a person experiences symptoms there is little possibility of them being able to rebuild their skeleton. Fractures are likely to occur because the trabeculae of spongy bone become thin and sparse and compact bone becomes porous. As the bones lose volume, they become brittle and weak and may collapse or become misshapen. Vertebral collapse causes kyphosis (from the Greek kyphos meaning hump) and diminishes height (see Figure 21-16). Fractures of the long bones (particularly the femur and humerus), distal radius, ribs and vertebrae are most common. Fracture of the neck of the femur — so-called broken hip — tends to occur in older or elderly women with osteoporosis. Fatal complications of fractures include fat or pulmonary embolism, pneumonia, haemorrhage and shock. Approximately 20% of individuals may die as a result of surgical complications. Male osteoporosis is usually secondary osteoporosis. The following seem to help prevent primary osteoporosis: adequate dietary intake of calcium, vitamin D, magnesium and possibly boron; a regular regimen of weight-bearing exercise; and avoidance of tobacco, glucocorticoids and alcoholism.
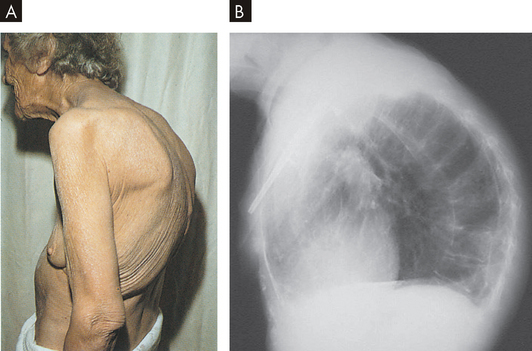
A This elderly woman’s condition was caused by a combination of spinal osteoporotic vertebral collapse and chronic degenerative changes in the vertebral column. B The X-ray demonstrates the marked curvature of the spine seen in kyphosis. The head and neck are bent forward and the total chest volume is markedly reduced.
Source: A Kamal A, Brocklehurst JC. Color Atlas of geriatric medicine. 2nd edn. St Louis: Mosby; 1992. B Klatt EC. Robbins & Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
EVALUATION AND TREATMENT
Generally, osteoporosis is detected on X-rays as increased radiolucency (transparency) of bone. By the time abnormalities are detected by radiological examination, up to 25–30% of bone tissue may have been lost.
Dual X-ray absorptiometry (DXA) (commonly known as the bone density test) is the gold standard for detecting and monitoring osteoporosis. Ultrasound is more cost-effective but it does not directly measure the fracture risk sites. Quantitative CT scans are also helpful. Other evaluation procedures include tests for levels of serum calcium, phosphorus and alkaline phosphatase and protein electrophoresis. Serum and urinary biochemical markers are useful in monitoring bone turnover.31
The goals of osteoporosis treatment are to slow down the rate of calcium and bone loss and to stop the deterioration before it progresses too far. Treatment includes increasing the dietary intake of calcium to 1500 mg/day along with vitamin D supplements to increase the intestinal absorption of calcium. High intake of phosphorus may neutralise calcium, interfering with its benefits. Magnesium supplementation may increase bone growth by stimulating cytokine activity in bone.32,33
Postmenopausal women may be given oestrogen and progestins to prevent bone loss. However, combined oestrogen–progestin therapy increases the risk for invasive breast cancer, heart disease, stroke and pulmonary embolism and is not warranted for routine osteoporosis prevention. Other steroid agents — for example, raloxifene, a selective oestrogen receptor modulator that provides the beneficial effects of oestrogen on bone without the negative effects on breast and endometrial tissue — may also be prescribed.
Regular, moderate weight-bearing exercise can slow down the bone loss and, in some cases, reverse demineralisation because the mechanical stress of exercise stimulates bone formation. It is important to reduce the risk of falls and enhance bone quality. An exercise program to enhance strength has the added benefits of reducing the risk of falls and promoting bone quality.
Newer treatments for osteoporosis — strontium and teriparatide
A Cochrane Database review showed a 37% reduction in vertebral fractures and a 14% reduction in non-vertebral fractures with 2 grams of strontium ranelate daily in a treatment population. An increase in bone mineral density was shown at all sites after 2–3 years in studied populations. Lower doses were also superior to the placebo and the highest doses revealed the greatest reduction in vertebral fractures. Strontium ranelate did not increase the risk of gastritis or death but slightly increased vascular and nervous system side effects.
Teriparatide is a recombinant form of parathyroid hormone used as a bone formation drug and increases both bone mass and strength. Teriparatide significantly reduces the risk of fracture. Much research is still needed on both therapies to understand benefits versus risks.
Source: O’Donnell S et al. Strontium ranelate for preventing and treating postmenopausal osteoporosis. Cochrane Database Syst Rev 2006; 18(4):CD005326; Delmas PD et al. Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone 2006; 39(2):237–243.
New medications formulated to prevent or treat osteoporosis are currently being prescribed and evaluated. There are new treatments that may rebuild the skeleton (see the box ‘Health alert: newer treatments for osteoporosis — strontium and teriparatide’). The anabolic or bone-building drug parathyroid hormone has been widely studied and the results are encouraging. Parathyroid hormone directly stimulates bone formation, particularly in trabecular bone.34
Osteomalacia
Osteomalacia is a metabolic disease characterised by reduced mineralisation of osteoid in mature compact and spongy bone. In osteomalacia, the remodelling cycle proceeds normally through osteoid formation, but mineral calcification and deposition do not occur. Bone volume remains unchanged, but the replaced bone consists of soft osteoid instead of rigid bone. Rickets is similar to osteomalacia in pathogenesis, but it occurs in the growing bones of children, whereas osteomalacia occurs in adult bone.
Many factors contribute to the development of osteomalacia, but the most important is a deficiency of vitamin D. The major risk factors in vitamin D deficiency are diets deficient in vitamin D, decreased endogenous production of vitamin D, intestinal malabsorption of vitamin D, renal tubular diseases and anticonvulsant therapy. Classic vitamin D deficiency is rare in Australasia because of the availability of vitamin D in dairy products and bread.
PATHOPHYSIOLOGY
Crystallisation of minerals in osteoid requires adequate concentrations of calcium and phosphate. When the concentrations are too low, crystallisation (and hence ossification) does not proceed normally.
Vitamin D deficiency disrupts mineralisation because vitamin D normally regulates and increases the absorption of calcium ions from the intestine. A lack of vitamin D causes the plasma calcium concentrations to fall. Low plasma calcium levels stimulate increased synthesis and secretion of parathyroid hormone. Although the increase in circulating parathyroid hormone raises the plasma calcium concentration, it also stimulates increased renal clearance of phosphate. When the concentration of phosphate in the bone decreases below a critical level, mineralisation cannot proceed normally.
Abnormalities occur in both spongy and compact bone. Trabeculae in spongy bone become thinner and fewer, whereas haversian systems in compact bone develop large channels and become irregular. Because osteoid continues to be produced but not mineralised, abnormal quantities of osteoid build up, coating the trabeculae and the linings of the haversian canals. Excessive osteoid can also accumulate in areas beneath the periosteum. The excess of osteoid leads to gross deformities of the long bones, spine, pelvis and skull.
CLINICAL MANIFESTATIONS
Osteomalacia causes varying degrees of diffuse skeletal pain and tenderness. Pain is noted particularly in the hips and the individual may be hesitant to walk. Muscular weakness is common and may contribute to a waddling gait. Bone fractures and vertebral collapse occur with minimal trauma. Low back pain may be an early complaint, but pain may also involve the ribs, feet, other areas of the vertebral column and other sites. Uraemia (the presence of nitrogen wastes in the blood; see Chapter 30) may be present in renal osteodystrophy (the skeletal changes of chronic kidney failure; see Chapter 30).
EVALUATION AND TREATMENT
Laboratory tests of blood and urine are often ordered first. Findings could include: normal or low serum calcium, a serum phosphate level that is usually over 1.8 mmol/L (normal range is 0.8–1.45 mmol/L), low levels of phosphate in the urine, low serum 25-hydroxy vitamin D (the activated form of vitamin D), elevated parathyroid hormone levels and elevated serum alkaline phosphatase (an enzyme that removes phosphate groups from many different molecules). Radiographical findings show pseudofractures (false fractures) and radiolucent bands perpendicular to the surface of involved bones. A bone biopsy is used to evaluate the presence of renal osteodystrophy to determine bone aluminium deposits.
Paget’s disease
Paget’s disease (osteitis deformans) is a state of increased metabolic activity in bone characterised by abnormal and excessive bone remodelling, both resorption and formation. Chronic accelerated remodelling eventually enlarges and softens the affected bones. Paget’s disease can occur in any bone but most often affects the vertebrae, skull, sacrum, sternum, pelvis and femur. The disease process may occur in one or more bones without causing significant clinical manifestations.
Paget’s disease occurs with increasing frequency in people as they age; it is rarely identified before 50 years of age and reaches a prevalence of almost 10% in the ninth decade of life. Men are more often affected than women at a ratio of 1.8 to 1. The disease is often symptomless and diagnosis is made by X-ray and radioisotope bone scan. Autopsy data from England and Germany indicate that approximately 3–4% of the population older than 40 years of age have Paget’s disease. It is most prevalent in Australia, Great Britain, New Zealand and the United States. The disease affects several members of the same family in 5–25% of cases.
The cause of Paget’s disease is unknown, but there appears to be a strong genetic component.35 A viral connection to Paget’s disease has also been proposed.36
PATHOPHYSIOLOGY
Paget’s disease is a focal process that begins with frantic excessive osteoclastic resorption of spongy bone, followed by furious deposition of bone by large numbers of osteoblasts. The deposited bone is disorganised rather than lamellar and is soft as a result. The trabeculae diminish and bone marrow is replaced by extremely vascular fibrous tissue.
Paget’s disease causes lesions that may be solitary or occur in multiple sites. Lesions tend to localise in the axial skeleton, including the skull, spine and pelvis. If the disease becomes more widespread, the proximal femur and tibia may become involved.
CLINICAL MANIFESTATIONS
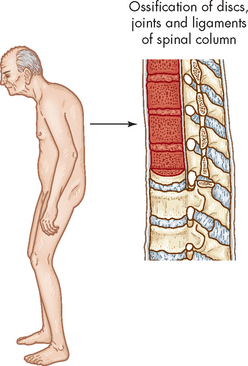
Paget’s disease varies in presentation from a single lesion to involvement of multiple bones. The manifestations depend on which bones are affected. In the skull, abnormal remodelling is first evident in the frontal or occipital regions; then it encroaches on the outer and inner surfaces of the entire skull. The skull thickens and assumes an asymmetric shape. Thickened segments of the skull may compress areas of the brain, producing altered mentality and dementia. Growth of new bone putting pressure on cranial nerves causes sensory abnormalities, impaired motor function, deafness (because of involvement of the middle ear ossicles or compression of the auditory nerve), atrophy of the optic nerve and obstruction of the lacrimal duct. Headache is commonly noted.
In the spinal column the vertebral bodies collapse leading to kyphosis. In long bones, resorption begins in the subchondral regions of the epiphysis and extends into the metaphysis and diaphysis. Softening of the femur and tibia causes them to bow. Stress fractures are common in the lower extremities and they often heal poorly with excessive and poorly distributed callus.
EVALUATION AND TREATMENT
Evaluation of Paget’s disease is made on the basis of characteristic bone deformities and radiographic findings of irregular bone trabeculae with a thickened and disorganised pattern. Early disease is detected by bone scanning that shows increased metabolic activity. Alkaline phosphatase and urinary hydroxyproline (a derivative of the amino acid proline, involved in collagen synthesis) are elevated.
Many individuals require no treatment if the disease is localised and does not cause symptoms. Treatment during active disease is for pain relief, prevention of deformity or fracture. Bisphosphonates are the treatment of choice: they bind to bone minerals, rapidly reducing resorption.
Osteochondroses
The osteochondroses are a series of childhood diseases involving areas of significant tensile or compressive stress (i.e. tibial tubercle, Achilles insertion, hip). They are characterised into two groups according to cause. The first group are caused by localised death of bone (osteonecrosis) in an apophyseal or epiphyseal centre (e.g. Legg-Calvé-Perthes disease), while the second group is the result of abnormalities of mineralisation of cartilage due to a genetically determined normal variation or trauma (e.g. Osgood-Schlatter disease).
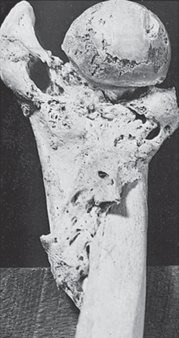
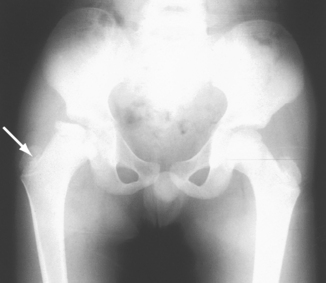
Legg-Calvé-Perthes disease
Legg-Calvé-Perthes disease is a common osteochondrosis usually occurring in children between the ages of 3 and 10 years, with a peak incidence at 6 years. The disorder affects both legs in 10–20% of children and boys are affected five times more often than girls, perhaps because boys have a more poorly developed blood supply to the femoral head (hip joint) than do girls of the same age. The role of genetics is unclear, but family history is positive in 20% of cases.
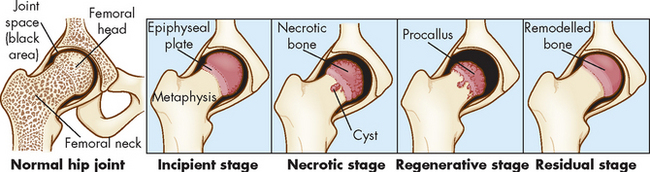
This disease which runs its natural course in 2–5 years, is presumably produced by recurrent interruption of the blood supply to the femoral head. The ossification centre first becomes necrotic (osteonecrosis) and then is gradually replaced by live bone.
Several causative theories have been proposed, including a generalised disorder of epiphyseal cartilage growth, thyroid deficiency, trauma, infection and blood-clotting disorders. However increases in thrombotic disorders in children with Legg-Calvé-Perthes were not found.37 Children are often delayed in skeletal age by 2 years, making some believe that Legg-Calvé-Perthes is actually a systemic skeletal dysplasia. Another study has shown the risk of Legg-Calvé-Perthes is five times greater in children exposed to passive smoke than those who are not.38
The primary feature of Legg-Calvé-Perthes is an avascular necrosis of the epiphyseal growth centre in the femoral head. The disease has four stages:
Injury or trauma precedes the onset in approximately 30–50% of children with Legg-Calvé-Perthes. For several months the child complains of a limp and pain that can be referred to the knee, inner thigh and groin. The pain is usually aggravated by activity and relieved by rest and anti-inflammatory drugs.
The typical physical findings include spasm on rotation of the hip, limitation of internal rotation and abduction (movement away from the centre of the body) and hip flexion–adduction deformity. If the child is walking, an abnormal gait termed an antalgic abductor lurch, or ‘Trendelenburg’ gait, is apparent. Associated muscle atrophy may occur.
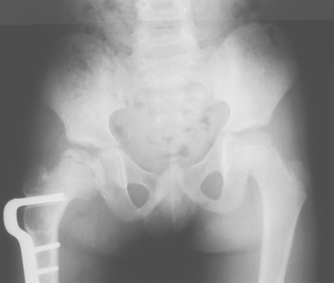
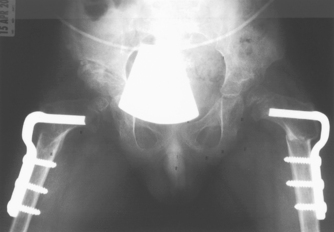
The goals of treatment are to reduce deformity, preserve the soundness of the femoral head and acetabulum, and maintain spasm-free and pain-free range of motion in the hip joint. Currently, most children can be managed with anti-inflammatory medications and activity modification during periods of synovitis (inflammation of the synovial membrane). Serial radiographs are obtained to monitor the progress of the disease and to ensure that the femoral head remains in contact with the acetabulum. Surgery may be necessary if the femoral head becomes subluxated or displaced from its normal position in the acetabulum (see Figures 21-18 and 21-19).39–41 4 6 Children older than 6 years of age (by bone age) have a worse prognosis due to poorer remodelling potential. Older children require surgery more often to avoid poor structural agreement of the femoral head in the acetabulum (congruence). Poor congruence predisposes to early osteoarthritis, with nearly 50% requiring hip replacement by age 40.
Osgood-Schlatter disease
Osgood-Schlatter disease causes microfractures of the tubercle of the tibia (the insertion point of the patellar tendon) and associated patella tendonitis. The disease occurs most often in preadolescents and adolescents who participate in sports and is more prevalent in boys than in girls. It is one of the most common ailments reported in adolescents involved in sports.42
The severity of the lesion varies from mild tendonitis to a complete separation of part of the tibial tubercle. The mildest form of Osgood-Schlatter disease causes ischaemic (avascular) necrosis in the region of the tibial tubercle, with excessive cartilage formation during the stages of repair. In more severe cases, the abnormality involves a true apophyseal separation of the tibial tubercle with avascular necrosis.
The child complains of pain and swelling in the region around the patellar tendon and tibial tubercle, which becomes prominent and is tender to direct pressure. The pain is most severe after physical activity that involves vigorous quadriceps contraction (jumping or running) or direct local trauma to the tibial tubercle area.
The goal of treatment is to decrease the stress at the tubercle. Often a period of 4–8 weeks of restriction from strenuous physical activity is sufficient. Bracing with a tubercle band can be very helpful. If the pain is not relieved, a cast or knee immobiliser is required, a situation that is particularly difficult if the condition is bilateral. Gradual return to activity is permitted after 8 weeks, but a further 8 weeks is necessary before strenuous physical activity to allow for revascularisation, healing and ossification of the tibial tubercle.39,43 With skeletal maturity and closure of the apophysis, Osgood-Schlatter disease resolves.
Scoliosis
Scoliosis is principally a lateral deviation of the spine. There are three main types of scoliosis: (1) idiopathic (unknown cause); (2) congenital (due to bone deformity); and (3) teratological (because of another systemic syndrome such as cerebral palsy). Eighty per cent of all scoliosis is idiopathic, which may have a genetic component. True structural scoliotic deformity involves not only a side-to-side curve but also rotation; curves without rotation may result from another cause such as unequal limb length or splinting from pain (see Figure 21-20). Although girls and boys are equally affected, once the curve becomes more than 20°, girls are five times more likely to be affected. Ninety-eight per cent of curves are apex right thoracic. If a left thoracic curve appears in the adolescent with idiopathic scoliosis, MRI is performed to rule out a neurological aetiology. MRI should also be used to assess kypho- (round back) scoliosis, loss of abdominal reflexes, children who also have exertional headaches or a congenital curve.44
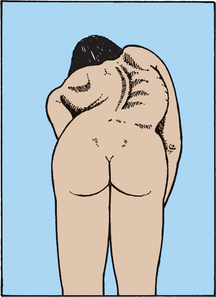
FIGURE 21-20 Rotation and curvature of scoliosis.
Scoliosis screening involves viewing the individual from behind, which discloses scapular asymmetry caused by not only curvature but also true rotation of the spine.
Idiopathic curves increase while a child is growing and progression can be very rapid during growth spurts. When idiopathic curves become 25° or greater and the child is skeletally immature, bracing is required. Curves of more than 50° will progress after skeletal maturity, so spinal fusion is required to stop progression. Early diagnosis is therefore necessary so that bracing can be attempted in the hopes of halting progression before the need for surgery. Children are required to wear the brace for 16 hours per day and gaining full compliance can be difficult. Nevertheless, bracing is the only non-operative measure known to slow scoliotic progression. Chiropractic manipulation, physical therapy, exercise and diet regimens have not been shown to alter natural history. Bracing is less successful in teratological or congenital curves; therefore, these conditions may require surgical intervention more often.
Disorders of joints
Joint disorders are usually accompanied by joint inflammation and hence may be referred to as inflammatory joint diseases. Interestingly, osteoarthritis has been previously referred to as a non-inflammatory joint disease; however, because there are some inflammatory processes involved, osteoarthritis may now be referred to as an inflammatory condition.45
Inflammatory joint disease
Inflammatory joint disease is commonly called arthritis. Typical of inflammatory joint disease is inflammatory damage or destruction in the synovial membrane or articular cartilage and systemic signs of inflammation: fever, leucocytosis (elevated numbers of leucocytes), malaise, anorexia and increased levels of fibrinogen in the blood.
Inflammatory joint disease can be infectious or non-infectious. In infectious inflammatory joint disease, invasion of the joint by bacteria, mycoplasmas (bacteria without a cell wall), viruses, fungi or protozoa (single-celled animals) causes inflammation. These agents gain access to the joint through a traumatic wound, surgical incision or contaminated needle, or they can be delivered by the bloodstream from sites of infection elsewhere in the body — typically bones, heart valves or blood vessels. There are two causes of non-infectious inflammatory joint disease: (1) inappropriate immune reactions and (2) deposition of crystals of monosodium urate in the synovial fluid. Rheumatoid arthritis and ankylosing spondylitis (from the Greek ankylos, bent, and spondylos, meaning vertebrae) are non-infectious inflammatory diseases caused by immune reactions and possibly hypersensitivity reactions;46,47 gouty arthritis is a non-infectious inflammatory disease caused by crystal deposition.
Rheumatoid arthritis
Rheumatoid arthritis is a systemic, inflammatory autoimmune disease associated with swelling and pain in multiple joints (autoimmune diseases are described in Chapter 15). Because this is an autoimmune condition it tends to have a bilateral presentation. The condition first affects the synovial membrane, which lines the joint cavity (see Figure 20-9). Eventually, inflammation may spread to the articular cartilage, fibrous joint capsule and surrounding ligaments and tendons, causing pain, joint deformity and loss of function (see Figure 21-21). The joints most commonly affected are in the fingers, feet, wrists, elbows, ankles and knees, but the shoulders, hips and cervical spine may also be involved, as well as the tissues of the lungs, heart, kidneys and skin.
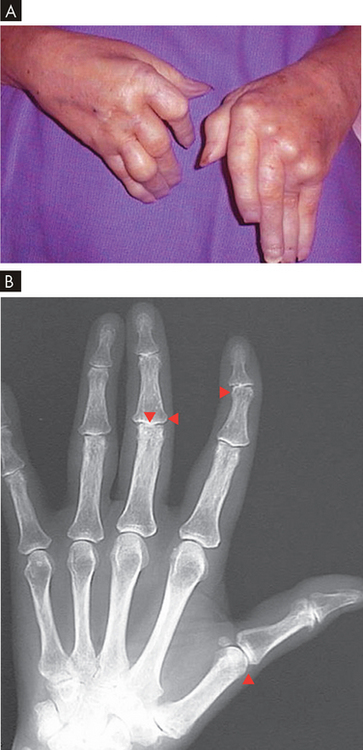
FIGURE 21-21 Rheumatoid arthritis of the hand.
A Note swelling from chronic synovitis of the metacarpophalangeal joints, marked ulnar drift, subcutaneous nodules and subluxation of the metacarpophalangeal joints with extension of the proximal interphalangeal joints and flexion of the distal joints. Note also the deformed position of the thumb. B An X-ray of a patient with rheumatoid arthritis. There is joint narrowing (triangles) and lateral deformation.
Source: A Klatt EC. Robbins & Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
Rheumatoid arthritis affects 1–2% of adults and, like most autoimmune diseases, develops most often in women, with a female/male ratio of 3:1. There is evidence of hormonal involvement because disease symptoms lessen during pregnancy and intensify in the postpartal period. The frequency of rheumatoid arthritis increases from the third decade onwards, affecting 5% or more of the population aged 70 years and older. Besides inflammation of the joints, rheumatoid arthritis can cause fever, malaise, rash, lymph node or spleen enlargement and Raynaud’s phenomenon (transient lack of circulation to the fingertips and toes).
Despite intensive research, the cause of rheumatoid arthritis remains obscure. It is likely to be a combination of genetic factors interacting with inflammatory mediators. Long-term smoking and a positive family history are associated with the development of rheumatoid arthritis.48,49 Rheumatoid arthritis also has seasonal variations and is worse in the winter months.
PATHOPHYSIOLOGY
Cartilage damage in rheumatoid arthritis is the result of at least three processes: (1) neutrophils and other cells in the synovial fluid become activated, breaking down the surface layer of articular cartilage; (2) cytokines (see Chapter 12), particularly TNF-α, stimulate the release of pro-inflammatory compounds (especially IL-1) and cause the chondrocytes to attack cartilage; and (3) the synovium digests nearby cartilage, releasing inflammatory molecules.
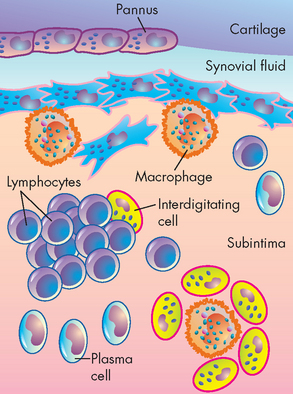
Several types of leucocytes are attracted out of the circulation and to the synovial membrane. The inflammatory phagocytes (neutrophils, macrophages) ingest the immune complexes and are stimulated to release powerful enzymes that degrade synovial tissue and articular cartilage (see Figure 21-22). The immune system’s B and T lymphocytes are also activated. The B lymphocytes are stimulated to produce more rheumatoid factors (auto-antibodies) and the T lymphocytes produce enzymes that amplify and continue the inflammatory response (see Figure 21-23).
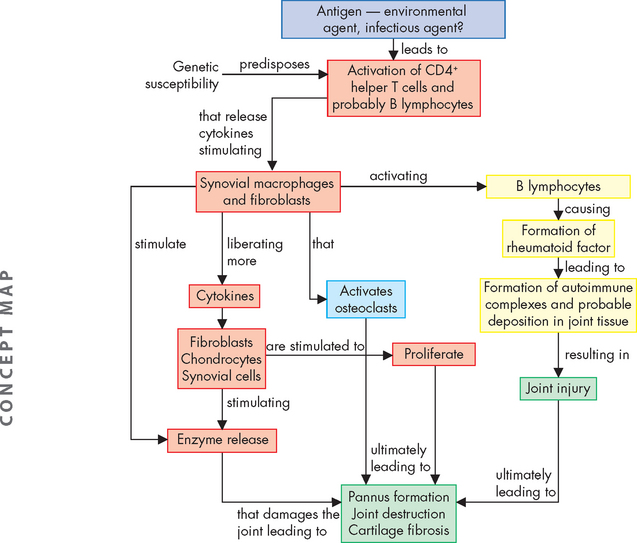
FIGURE 21-23 Emerging model of pathogenesis of rheumatoid arthritis.
Rheumatoid arthritis is an autoimmune disease of a genetically susceptible host triggered by an unknown antigenic agent. This chronic autoimmune reaction occurs with activation of CD4+ helper T cells, possibly other lymphocytes, and the local release of inflammatory cytokines and mediators that eventually destroys the joint. T cells stimulate cells in the joint to produce cytokines that are key mediators of synovial damage. Apparently, immune complex deposition also plays a role. TNF-α and IL-1, as well as some other cytokines, stimulate synovial cells to proliferate and produce other mediators of inflammation and enzymes that all contribute to destruction of cartilage. Pannus is a mass of synovium and synovial stroma with inflammatory cells, granulation tissue and fibroblasts that grows over the articular surface and causes its destruction.
Inflammatory and immune processes have several damaging effects on the synovial membrane. The synovial membrane thickens and puts pressure on nearby small venules, reducing the supply of blood. The reduced circulation, coupled with the increased metabolism from the proliferation and enlargement of the cells in the synovial membrane, leads to a local hypoxia and metabolic acidosis. Acidosis stimulates the release of enzymes from synovial cells into the surrounding tissue that break down tissue, initiating erosion of the articular cartilage and causing inflammation in the supporting ligaments and tendons.
Inflammation causes haemorrhage, coagulation and fibrin deposition on the synovial membrane, in the intracellular matrix and in the synovial fluid. Over denuded areas of the synovial membrane, fibrin develops into granulation tissue called pannus. (Granulation tissue is the initial tissue produced in the process of healing; see Chapter 13.) Pannus formation does not lead to synovial or articular regeneration but rather to formation of scar tissue, which immobilises the joint.
CLINICAL MANIFESTATIONS
The onset of rheumatoid arthritis is usually insidious, although as many as 15% of cases have an acute onset. Rheumatoid arthritis begins with general systemic manifestations of inflammation, including fever, fatigue, weakness, anorexia, weight loss and generalised aching and stiffness. Local manifestations also appear gradually over a period of weeks or months. Typically, the joints become painful, tender and stiff. Pain early in the disease is caused by pressure from swelling. Later in the disease, pain is caused by sclerosis of subchondral bone and new bone formation. Stiffness usually lasts for about an hour after rising in the morning and is thought to be related to synovitis. Initially the joints most commonly involved are the metacarpophalangeal joints (base of the finger), proximal interphalangeal joints (middle of the finger) and wrists, with later involvement of larger weight-bearing joints (refer to Figure 21-29).
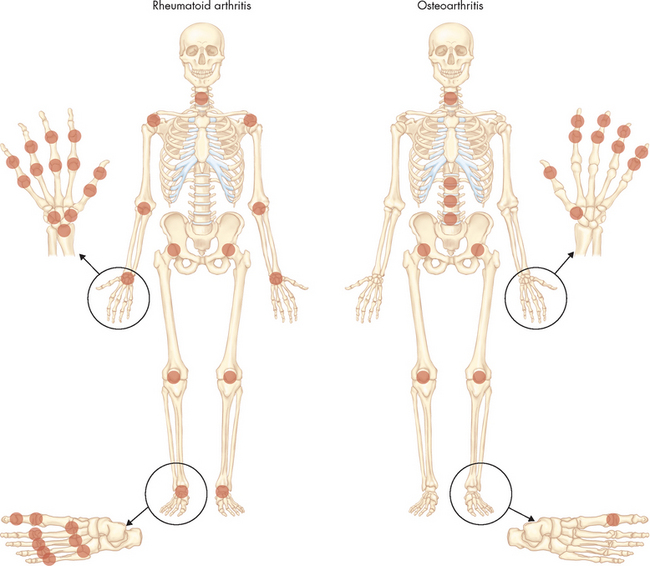
FIGURE 21-29 The distribution of involved joints in the two most common forms of arthritis — rheumatoid arthritis and osteoarthritis.
Dark circles are shown over the involved joint areas.
Source: Goldman L. Cecil medicine. 23rd edn. Philadelphia: Saunders; 2008.
Joint swelling, which is widespread and symmetric, is caused by increasing amounts of inflammatory exudate (leucocytes, plasma, plasma proteins) in the synovial membrane, hyperplasia (an increase in cell numbers) of inflamed tissues and formation of new bone. On palpation, the swollen joint feels warm and the synovial membrane feels boggy. The skin over the joint may have a ruddy, cyanotic hue and may look thin and shiny.
An inflamed joint may lose some of its mobility. Even mild synovitis can lead to loss of range of motion, which becomes evident after inflammation subsides. Extension becomes limited and is eventually lost if flexion contractures form. Loss of range of motion can progress to permanent deformities of the fingers, toes and limbs, including ulnar deviation of the hands, boutonnière and swan-neck deformities of the finger joints, plantar subluxation of the metatarsal heads of the foot and hallux valgus (angulation of the great toe towards the other toes). Flexion contractures of the knees and hips are also common.
Joint deformities cause the physical limitations experienced by those with rheumatoid arthritis (see Figure 21-21). Loss of joint motion is quickly followed by secondary atrophy of the surrounding muscles. With secondary muscle atrophy, the joint becomes unstable, which further aggravates joint pathology.
Two complications of chronic rheumatoid arthritis are caused by excessive amounts of inflammatory exudate in the synovial cavity. One complication is the formation of cysts in the articular cartilage or subchondral bone. Occasionally, these cysts communicate with the skin surface (usually the sole of the foot) and can drain through passages called fistulae (singular; fistula). The second complication is rupture of a cyst or of the synovial joint itself, usually caused by strenuous physical activity that places excessive pressure on the joint. Rupture releases inflammatory exudate into adjacent tissues, thereby spreading inflammation.
Extrasynovial rheumatoid nodules, or swellings, are observed in areas of pressure or trauma in 20% of individuals with rheumatoid arthritis. Each nodule is a collection of inflammatory cells surrounding a central core of fibrinoid and cellular debris. T lymphocytes are the main leucocytes in the nodule. B lymphocytes, plasma cells and phagocytes are found around the edges. Nodules are most often found in subcutaneous tissue over the extensor surfaces of the elbows and fingers. Less common sites are the scalp, back, feet, hands, buttocks and knees.
Rheumatoid nodules may also invade the skin, cardiac valves, pericardium, pleura, lung parenchyma and spleen. These nodules are identical to those encountered in some individuals with rheumatic fever and are characterised by central tissue necrosis surrounded by proliferating connective tissue. Also noted are large numbers of lymphocytes and occasional plasma cells. Acute glaucoma may result with nodules forming on the sclera. Pulmonary involvement may result in diffuse pleuritis (inflammation of the pleura) or multiple intraparenchymal nodules (within the tissue of the lungs). Diffuse pulmonary fibrosis may occur because of immunologically mediated immune complex deposition.
Rheumatoid nodules within the heart may cause valvular deformities, particularly of the aortic valve leaflets, and pericarditis (inflammation of the pericardium). Lymphadenopathy (swelling) of the nodes close to the affected joints may develop. Rheumatoid nodules within the spleen result in splenomegaly (enlarged spleen). Blood vessels may show an acute inflammatory response as is noted in other immunological/inflammatory states. Thromboses in involved vessels may give rise to myocardial infarctions (see Chapter 23), cerebrovascular occlusions (see Chapter 9), mesenteric infarction (often causing necrosis in the gut), kidney damage (typical of systemic autoimmune conditions) and vascular insufficiency in the hands and fingers (Raynaud’s phenomenon). The vascular changes are primarily noted in individuals receiving steroid therapy; thus, there is some concern that the therapy may play a role in initiating these lesions. Changes in skeletal muscle are often noted in the form of nonspecific atrophy secondary to joint dysfunction.
EVALUATION AND TREATMENT
Evaluation of rheumatoid arthritis is done by physical examination, X-ray of the joint and serological tests for rheumatoid factor and circulating immune complexes. The American College of Rheumatology lists the following diagnostic criteria for rheumatoid arthritis that have widespread use, including within Australia and New Zealand:
The presence of four or more of the numbered criteria is diagnostic of rheumatoid arthritis. Criteria 1–4 with joint signs or symptoms must be present for 6 weeks.
Treatment can be nonsurgical or surgical. Nonsurgical treatment includes resting the inflamed joint and whole-body rest for several hours daily; use of hot and cold packs; physical therapy; patient education; aggressive, early intervention using disease-modifying antirheumatic drugs and biological response modifiers; a diet high in kilojoules and vitamins; corticosteroids; and anti-inflammatory drugs taken orally or injected into the joint. Intra-articular injection of a radionuclide can be used to treat synovitis. Surgical synovectomy may be done early in the disease to decrease inflammatory effusion and remove pannus. Surgery is used to correct deformity or mechanical deficiency in intermediate or late stages of the disease and includes arthrodesis (surgical fusion of the two bones so the joint becomes immoveable), arthroplasty (surgical repair of the joint) or total joint replacement. There is evidence that total fasting substantially reduces joint pain, swelling, morning stiffness and other symptoms in individuals with rheumatoid arthritis.
Juvenile rheumatoid arthritis
Juvenile rheumatoid arthritis is the childhood form of rheumatoid arthritis and accounts for 5% of all cases of rheumatoid arthritis. Juvenile rheumatoid arthritis has three distinct modes of onset: oligoarthritis (fewer than three joints), polyarthritis (more than three joints) and Still’s disease (severe systemic onset). Juvenile rheumatoid arthritis differs from rheumatoid arthritis in several ways:
Many children with oligoarthritis who are ‘seronegative’ (whose blood tests negative for rheumatoid factor or antinuclear antibody) will resolve their symptoms over time. Systemic onset, or ‘seropositivity’, of the disease is more likely consistent with lifelong arthritis. Long-term studies have shown that one-third to half of patients with juvenile rheumatoid arthritis have active disease 10 years later. Treatment is therefore supportive, not curative. Non-steroidal anti-inflammatories are a mainstay, and methotrexate (typically an anti-cancer medication) is also being used with success. The aims are to minimise inflammation and deformity.
New rheumatoid arthritis treatments
Innovative new therapies for rheumatoid arthritis treatment continue to emerge. In addition to pharmaceutical agents, biological and genetic agents are gaining increasing attention. New treatments are targeted at specific cytokines, inhibition of chemokines, and complement activation, and investigational therapies include T cell or T cell receptor vaccination. High-dose intravenous immunoglobulins and plasmapheresis are also being used. Experimental intra-articular gene transfer may one day prove successful as a ‘gene scalpel’ for removing inflammatory synovial tissue.
Source: Breeveld FC, Kalden JR. Appropriate and effective management of rheumatoid arthritis. Ann Rheum Dis 2004; 63:627–633; Zhang H et al. Elimination of rheumatoid synovium in situ using a Fas ligand ‘gene scalpel’. Arthritis Res Ther 2005; 7(6):R1235–R1243.
Ankylosing spondylitis
Ankylosing spondylitis is a chronic, inflammatory joint disease characterised by stiffening and fusion (ankylosis) of the spine and sacroiliac joints. Like rheumatoid arthritis, ankylosing spondylitis is a systemic, autoimmune disease. However, the two conditions respond to different antigens. In the case of rheumatoid arthritis the synovial membrane is affected, whereas in ankylosing spondylitis it is the point at which the tendons and ligaments attach to bone that is affected (known as the enthesis). The end result is fibrosis, ossification and fusion of the joint, usually beginning with the sacroiliac joints and progressing up the vertebral column.
Ankylosing spondylitis usually develops in late adolescence and young adulthood, with peak incidence at about 20 years of age, and affects slightly more men than women. There is a wide range of presentations from asymptomatic sacroiliitis (inflammation of the sacroiliac joint) to progressive disease that affects many body systems.
Secondary ankylosing spondylitis affects older age groups and is often associated with other inflammatory diseases (e.g. psoriatic arthropathy, inflammatory bowel disease, Reiter’s syndrome — a reactive arthritis).
The cause of ankylosing spondylitis is unknown, but a genetic predisposition to the disease has been suggested.51
PATHOPHYSIOLOGY
Ankylosing spondylitis begins with inflammation of fibrocartilage in cartilaginous joints (see Chapter 14) between the sacrum and ilia (plural of ilium). Inflammatory cells infiltrate the joint and begin to damage fibrocartilage and bone in the joint structures. Repair of the damage is mediated by fibroblasts that synthesise and secrete collagen. This collagen forms scar tissue that is eventually calcified. With time, all the cartilaginous structures of the joint are replaced by ossified scar tissue, causing the joint to fuse or lose flexibility.
The eroded bone is repaired in the normal process of bone repair and remodelling (see Chapter 20). The new bone has to grow outwards to connect with the ligaments that have been eroded by the inflammatory response and, as a consequence, the shape of the vertebral bodies changes — they lose their concave anterior contour and appear square. The spine assumes the classic bamboo spine appearance of ankylosing spondylitis (see Figure 21-24).
CLINICAL MANIFESTATIONS
The most common signs and symptoms of early ankylosing spondylitis are low back pain and stiffness that may be persistent or intermittent. It is often worse after prolonged rest and is alleviated by physical activity. Early morning stiffness usually accompanies the low back pain and the individual typically has difficulty sitting up or twisting the spine. Forward flexion, rotation and lateral flexion of the spine are restricted and painful. Early pain and resultant loss of motion are caused by the underlying inflammation and reflex muscle spasm rather than by soft tissue or bony fusion.
As the disease progresses, the normal convex curve of the lower spine (lumbar lordosis) diminishes and concavity of the upper spine (kyphosis) increases. The individual becomes increasingly stooped. The thoracic spine becomes rounded, the head and neck are held forward on the shoulders and the hips are flexed (see Figure 21-16).
Inflammation in the tendon insertions of the muscles of the chest wall can cause pleuritic chest pain and restricted chest movement. The pain is usually worse on inspiration. Movement in the diaphragm is normal and full. Pressure on the anterior chest wall over the sternum, ribs and costal cartilages may show tenderness. Tenderness over the pelvic brim may cause discomfort at night and interfere with sleep because turning onto the iliac crests causes pain. Tenderness over the ischial tuberosities may make sitting on hard seats unbearable. Tenderness in the heels may contribute to a limp or cautious placement of the feet during walking.
Along with low back pain, many individuals have peripheral joint involvement, uveitis, fibrotic changes in the lungs and cardiomegaly, aortic incompetence, amyloidosis and Achilles tendonitis. Symptoms may include fatigue, weight loss, low-grade fever, hypochromic anaemia and an increased erythrocyte sedimentation rate.
EVALUATION AND TREATMENT
Diagnosis of ankylosing spondylitis is made from the history and physical examination, X-ray and serum analysis for the presence of the relative antigen (HLA-B27). The erythrocyte sedimentation rate and C-reactive protein are elevated throughout the disease. Alkaline phosphatase levels are often elevated. Early precise diagnosis allows implementation of a usually effective, conservative, lifelong treatment.
Treatment of individuals with ankylosing spondylitis is directed at controlling pain, maintaining mobility and controlling inflammation. Prevention of deformity and maintenance of mobility require a continuous program of physical therapy. Exercises are performed several times each day to maintain chest expansion, full extension of the spine and complete range of motion in the proximal joints.
Non-steroidal anti-inflammatory drugs often provide temporary symptom relief within 48 hours. Analgesic medications are prescribed to suppress some of the pain and stiffness and to facilitate exercise. The medications do not prevent disease progression, but they do provide relief from symptoms. Biological response modifying agents, such as infliximab, which inhibits TNF-α, may be useful in treating ankylosing spondylitis.52,53 Surgical procedures, such as osteotomy, total hip replacement and cervical spinal fusion, and radiation therapy are sometimes used to provide relief for individuals with end-stage disease or intolerable deformity. Individuals should stop smoking to lessen pulmonary problems.
Gout
Gout is a syndrome caused by defects in uric acid metabolism and characterised by inflammation and pain of the joints. Either excessive uric acid production or underexcretion of uric acid by the kidneys will cause hyperuricaemia. When the uric acid reaches a certain concentration in fluids, it crystallises, forming insoluble crystals that are deposited in connective tissues throughout the body. Crystallisation in synovial fluid causes acute, painful inflammation of the joint, a condition known as gouty arthritis. With time, crystal deposition in subcutaneous tissues causes the formation of small, white nodules, or tophi, that are visible through the skin. Crystal aggregates deposited in the kidneys can form urate renal stones and lead to renal failure.
Gout is predominantly a disease of men. The peak age of onset in males is between 40 and 60 years, whereas it is somewhat later in females. The risk of developing gouty arthritis is similar in males and females for a particular urate concentration. The plasma urate concentration is the single most important determinant of the risk of developing gout (see Table 21-3).
Table 21-3 MEAN URATE CONCENTRATIONS BY AGE AND GENDER
| CHARACTERISTIC | MEAN URATE LEVELS |
|---|---|
The solubility of urate is critical to the development of crystals. Urate is more soluble in plasma and urine than in synovial fluid. The solubility of urate also decreases with decreasing temperature. Initial deposits are often in the joint of the great toe where temperatures are lower than the body core. The pathways of production of uric acid are shown in Figure 21-25.
PATHOPHYSIOLOGY
The pathophysiology of gout is closely linked to purine metabolism (or cellular metabolism of purines — adenine and guanine from DNA and RNA) and kidney function. At the cellular level, purines are used for synthesis of several products, including ATP and nucleic acids. Uric acid is a breakdown product of purine nucleotides (urate synthesis and elimination are illustrated in Figure 21-25). Some individuals with gout have an accelerated rate of purine synthesis accompanied by an overproduction of uric acid. Even with restricted purine consumption, these individuals continue to overproduce uric acid. Other individuals break down purine nucleotides at an accelerated rate that also results in an overproduction of uric acid. In addition, production of uric acid can be caused by an increased turnover of nucleic acids, which is associated with an increased turnover of cells at other body sites. The increased turnover of nucleic acids leads to increased levels of uric acid with a compensatory increase in purine synthesis.
Most uric acid is eliminated from the body through the kidneys. Urate is filtered at the glomerulus and undergoes both reabsorption, as well as being excreted into urine. In primary gout, urate excretion by the kidneys is sluggish, which may result from a decrease in glomerular filtration of urate or increased urate reabsorption. In addition, monosodium urate crystals are deposited in renal interstitial tissues, causing impaired urine flow. (Kidney function is described in Chapter 28.)
The exact process by which crystals of monosodium urate are deposited in joints and induce gouty arthritis is unknown, but several mechanisms may be involved, including the following:
The monosodium urate crystals may form in the synovial fluid or in the synovial membrane, cartilage or other connective tissues in joints and elsewhere, such as in the heart, earlobes and kidneys. Evidence suggests that an acute attack of gout is the result of the formation of crystals rather than the releasing of the crystals from connective tissues into the synovial fluid.
Monosodium urate crystals can stimulate and perpetuate the inflammatory response (see Figure 21-26), during which neutrophils are attracted out of the circulation and phagocytose (ingest) the crystals. The neutrophils subsequently die and release both the crystals and the lysosomal enzymes that cause tissue damage and further stimulate inflammation.
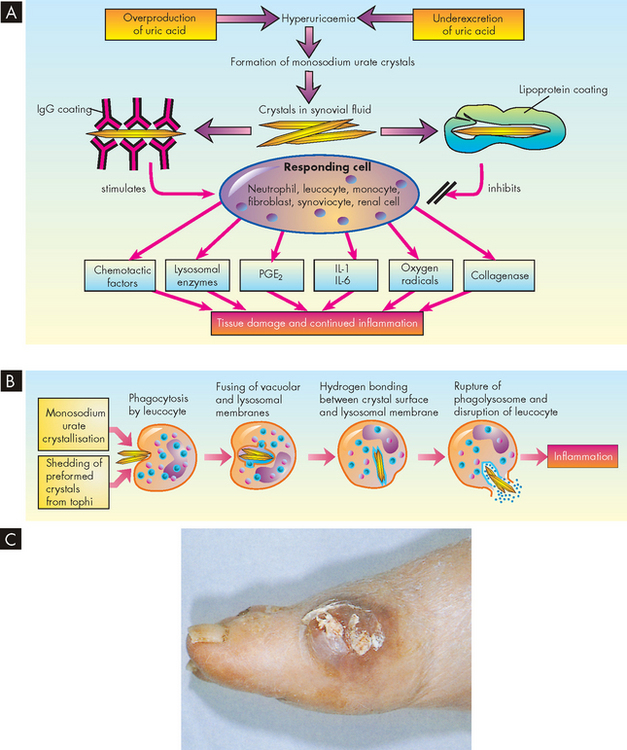
FIGURE 21-26 The pathogenesis of acute gouty arthritis.
A Depending on the urate crystal coating, a variety of cells may be stimulated to produce a wide range of inflammatory mediators. IgG = immunoglobulin G; PGE2 = prostaglandin E2; IL = interleukin. B The sequence of events in the production of inflammatory response to urate crystals. C Gouty tophus on the right foot.
Source: C Dieppe PA et al. Arthritis and rheumatism in practice. London: Gower; 1991.
CLINICAL MANIFESTATIONS
Gout is manifested by: (1) an increase in serum urate concentration (hyperuricaemia); (2) recurrent attacks of monarticular arthritis (inflammation of a single joint); (3) deposits of monosodium urate monohydrate (tophi) in and around the joints; (4) renal disease involving glomerular, tubular and interstitial tissues and blood vessels; and (5) the formation of renal stones. These manifestations appear in three clinical stages:
Trauma is the most common aggravating factor. The great toe is subject to chronic strain in walking and subsequently an acute gout attack may follow long walks. Trauma associated with occupations such as truck driving also may precipitate an attack.
Attacks of gouty arthritis occur abruptly, usually in a peripheral joint (see Figure 21-26C). The primary symptom is severe pain. Approximately 50% of initial attacks occur in the metatarsophalangeal joint of the great toe (a condition known as podogra). The other 50% involve the heel, ankle, instep of the foot, knee, wrist or elbow. The pain is usually noted at night. Within a few hours the affected joint becomes hot, red and extremely tender and may be slightly swollen. Lymphangitis (inflammation of lymph vessels) and systemic signs of inflammation (leucocytosis, fever, elevated sedimentation rate) are occasionally present. Untreated, mild attacks usually subside in several hours but may persist for 1 or 2 days. Severe attacks may persist for several days or weeks. When the individual recovers, the symptoms resolve completely. The helix of the ear is the most common site of tophi, which are the characteristic diagnostic lesions of chronic gout.
Tophi do not usually appear until at least 10 years after the first gout attack. They produce irregular swellings of the fingers, hands, knees and feet. Tophi commonly form lumps along the ulnar surface of the forearm, the tibial surface of the leg, the Achilles tendon and olecranon bursae. Tophi may produce marked limitation of joint movement and eventually cause grotesque deformities of the hands and feet. Although the tophi themselves are painless, they often cause progressive stiffness and persistent aching of the affected joint. Tophi in the upper extremities may cause nerve compressions, such as carpal tunnel syndrome, while tophi in the lower extremities may cause tarsal tunnel syndrome. They also may erode and drain through the skin.
Kidney stones (see Chapter 30) are 1000 times more prevalent in individuals with primary gout than in the general population. The stones may be any size, from the size of a grain of sand or a piece of gravel to much larger deposits. Renal stones can form in the collecting tubules, pelvis or ureters, causing obstruction, dilation and atrophy of the more proximal tubules and leading eventually to acute renal failure. Stones deposited directly in renal interstitial tissue initiate an inflammatory reaction that leads to chronic renal disease and progressive renal failure.
TREATMENT
The first objective of gout treatment is to terminate the acute gouty attack as promptly as possible. Once the inflammatory process has subsided, attention is directed to prevent recurring attacks, prevent or reverse complications associated with urate deposits in the joints and kidneys, and prevent formation of kidney stones. Acute gouty arthritis is treated with anti-inflammatory drugs. The drugs of choice are colchicine, non-steroidal anti-inflammatory agents (especially indomethacin) and allopurinol. Colchicine is useful in those unable to take non-steroidal anti-inflammatories. Once infection has been ruled out, hydrocortisone may be injected into the joint to relieve pain. Ice also may relieve some of the inflammation of the joint. Weight-bearing on the involved joint is to be avoided until the acute attack subsides. After the attack the individual is put on a low-purine diet, with high fluid intake to increase urinary output. Uricosuric drugs (e.g. probenecid or sulfinpyrazone) increase the excretion of urate by blocking its reabsorption by the kidney tubules. Antihyperuricaemic drugs (e.g. allopurinol) reduce serum urate concentrations by inhibiting the formation of urate.
Osteoarthritis
Osteoarthritis is effectively a wearing out of the joint (see Figure 21-27). It therefore predominantly affects the weight-bearing joints. Osteoarthritis tends to occur in men and women older than 40 years of age and becomes more common with increasing age. It is a leading cause of pain and disability in the elderly. Although incidence rates are quite similar in men and women, women are more severely affected. It usually occurs in those who put exceptional stress on joints, as do obese people, gymnasts, long-distance runners and marathoners, and basketball, soccer and football players. Many of these people develop osteoarthritis at earlier ages than usual. A previously torn anterior cruciate ligament or meniscectomy (surgical removal of the meniscus of the knee) increases the risk for accelerated osteoarthritis of the knee.54
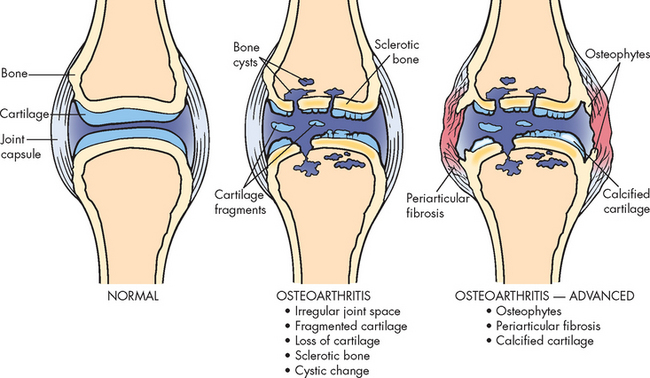
A schematic of the pathology of osteoarthritis. Fragmentation and loss of cartilage denude the bone, which undergoes sclerosis (stiffening). Osteophytes (bone spurs) form on the lateral sides and protrude into the soft tissue, causing irritation, inflammation and fibrosis (excessive fibrous connective tissue).
Source: Damjanov I. Pathology for health professionals. 3rd edn. St Louis: Saunders; 2006.
Osteoarthritis is characterised by localised loss and damage of articular cartilage, new bone formation of joint margins (osteophytosis), subchondral bone changes, variable degrees of mild synovitis and thickening of the joint capsule (see Figure 21-28). Pathology centres on load-bearing areas. Advancing disease reveals narrowing of the joint space because of cartilage loss, osteophytes (bone spurs) and sometimes changes in the subchondral bone. Osteoarthritis can arise in any synovial joint but is commonly found in the hips, hands and spine (see Figure 21-29). It involves a complex interaction of cytokines, growth factors, matrix molecules and enzymes.
PATHOPHYSIOLOGY
Articular cartilage is normally a dynamic tissue: the chondrocytes (cartilage-forming cells) continuously replace the tissue in the same way that bone is continually replaced. In osteoarthritis this process becomes disrupted — the primary defect in osteoarthritis is loss of articular cartilage.55 Early in the disease, articular cartilage loses its glistening appearance, becoming yellow-grey or brownish grey. As the disease progresses, surface areas of the articular cartilage flake off and deeper layers develop longitudinal fissures that extend to the subchondral bone. Synovial fluid fills the fissures and may enter the underlying bone, forming cysts. The cartilage becomes thin and may be absent over some areas, leaving the underlying bone (subchondral bone) unprotected. Consequently, the unprotected subchondral bone becomes sclerotic (dense and hard). Fragments of bone and cartilage (joint mice) become free-floating and enter the joint cavity. Formation of new bone and cysts usually occurs near the joint margins, forming osteophytes. As the joint loses its integrity there is trauma to the synovial membrane causing a nonspecific inflammation, or synovitis.
CLINICAL MANIFESTATIONS
Clinical manifestations of osteoarthritis typically appear during the fifth or sixth decade of life, although asymptomatic, articular surface changes will have been occurring for 10 or 20 years. Pain that is initially described as aching and difficult to localise and is aggravated by weight-bearing is a common presentation of the disease. It is usually aggravated by use of the joint and relieved by resting the joint. Later in the course of the disease night pain may be experienced that is not relieved by rest and may be accompanied by paraesthesias (numbness, tingling or prickling).
Sometimes pain is referred to another part of the body. For example, osteoarthritis of the lumbosacral spine may mimic sciatica, causing severe pain in the back of the thigh along the course of the sciatic nerve. Osteoarthritis in the lower cervical spine may cause brachial neuralgia (pain in the arm) aggravated by movement of the neck. Osteoarthritic conditions in the hip cause pain that may be referred to the lower thigh and knee area. Sleep deprivation adds to the stress of the chronic pain of osteoarthritis.
RISK FACTORS FOR OSTEOARTHRITIS
Body weight and osteoarthritis
Longitudinal studies have shown obesity to be a major risk factor in developing osteoarthritis of the knee. In addition to altered biomechanics, increased weight may make subchondral bone stiffer and thus less capable of handling joint impact loading.
Source: Powell A et al. Obesity: a preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Br J Sports Med 2005; 39:4–5.
Physical examination of the person with osteoarthritis usually shows general involvement of both peripheral and central joints. Peripheral joints most often involved are in the hands, wrists, knees and feet. Central joints most often afflicted are in the lower cervical spine, lumbosacral spine, shoulders and hips (see Figure 21-29).
Joint structures are capable of generating a limited number of signs and symptoms. The primary signs and symptoms of joint disease are pain, stiffness, enlargement or swelling, tenderness, limited range of motion, muscle wasting, partial dislocation and deformity (see the box ‘Risk factors: osteoarthritis’). Range of motion is limited to some degree, depending on the extent of cartilage degeneration and any swelling of the affected joint. Frequently, joint motion is accompanied by crepitus (a crackling sound or grating sensation in a joint).
As osteoarthritis of the lower extremity progresses, the person may begin to limp noticeably (see Figure 21-30). Having a limp is distressing because it affects the person’s independence and ability to do usual activities of daily living. The affected joint is also more symptomatic after use, such as at the end of a period of strenuous activity.
EVALUATION AND TREATMENT
Evaluation consists of clinical assessment and radiological studies, CT scan, arthroscopy and MRI. Treatment is either conservative or surgical. Conservative treatment includes rest of the involved joint until inflammation, if present, subsides; range of motion to prevent joint capsule contraction; use of a cane, crutches or walker to decrease weight-bearing; and analgesic and anti-inflammatory drug therapy to reduce swelling and pain. In addition, weight loss is recommended if obesity is present (obese people are five times more likely to have osteoarthritis of the knees and twice as likely to have osteoarthritis of the hips as people of normal weight; see the box ‘Health alert: body weight and osteoarthritis’). Intra-articular injection of hyaluronic acid has also been successful in decreasing knee pain with osteoarthritis.56 Speculation regarding the use of the cartilage-supporting agents glucosamine and chondroitin has prompted mixed results from studies, some claiming benefit and others finding no effect.57–59 4 6 Surgery is used to improve joint movement, correct deformity or malalignment or implant an artificial joint (see Figure 21-31).
FIGURE 21-31 Musculoskeletal anatomy of the hip.
A An artificial hip is shown at a 4.5 year follow-up X-ray alongside B, an anatomical drawing of an actual hip joint.
Source: A Resnick D, Kransdorf M. Bone and joint imaging. 3rd edn Philadelphia: Saunders; 2004. B Goldman L. Cecil medicine. 23rd edn. Philadelphia: Saunders; 2008. From Polley HF, Hunder GG (eds). Rheumatalogic interviewing and physical examination of the joints. 2nd edn. Philadelphia: Saunders; 1978.
The intervention rate (which is much lower than the incidence rate) for major joint surgery is 1.9 per 1000 in Australia and 1.2 per 1000 in New Zealand. These rates are increasing.60
Infectious bone disease
Infectious bone disease is expensive, difficult to treat and often culminates in extensive physical disability. Several factors contribute to the difficulty in treating bone infection:
Osteomyelitis
Osteomyelitis (osteo meaning bone and myelo meaning marrow) is an infection of the bone and marrow that can be caused by many infective agents (see Box 21-1). Antibiotic drugs and often surgical interventions are used to fight these infections. With modern treatments morbidity and mortality resulting from osteomyelitis have fallen drastically. With present management, serious long-term effects occur for less than 15% of cases.
Box 21-1 CAUSATIVE MICROORGANISMS OF OSTEOMYELITIS ACCORDING TO AGE
Osteomyelitis is usually caused by bacteria; however, fungi, parasites and viruses can also cause bone infection. The infection may originate from the bloodstream or from a surgical procedure (Figure 21-32).
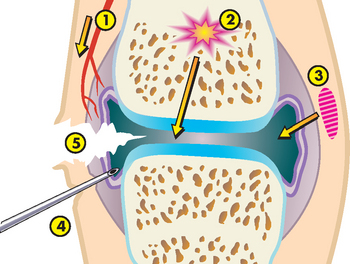
FIGURE 21-32 The routes of infection to the joint.
(1) Via the blood stream. (2) Dissemination from osteomyelitis. (3) Spread from an adjacent soft-tissue infection. (4) Diagnostic or therapeutic measures. (5) Penetrating damage by puncture or cutting.
Exogenous osteomyelitis is an infection that enters from outside the body — for example, through open fractures, penetrating wounds or surgical procedures. The infection spreads from soft tissues into adjacent bone.
Endogenous osteomyelitis (also referred to as haematogenous osteomyelitis) is caused by pathogens carried in the blood from sites of infection elsewhere in the body. The infection spreads from bone to adjacent soft tissues. Endogenous osteomyelitis is commonly found in infants, children and the elderly. In infants, incidence rates among males and females are approximately equal. In children and older adults, however, males are more commonly affected.
Osteomyelitis in children usually begins as an abscess in the metaphysis of a long bone where blood flow is sluggish and bacteria can collect. The periosteum may peel off the affected bone leading to necrosis of the bone. New bone can develop inside the now extended periosteum. These changes may be visualised by X-ray and signify the need for surgical debridement as well as antibiotic treatment.
In adults, endogenous osteomyelitis is more common in the spine, pelvis and small bones. Microorganisms reach the vertebrae through arteries, veins or lymphatic vessels. The spread of infection from pelvic organs to the vertebrae is well documented. Vaginal, uterine, ovarian, bladder and intestinal infections can lead to iliac or sacral osteomyelitis.
Cutaneous, sinus, ear and dental infections are the primary sources of bacteria in endogenous bone infections. Soft-tissue infections, disorders of the gastrointestinal tract, infections of the genitourinary system and respiratory infections are also sources of bacterial contamination. In addition, infections that occur after total joint replacements are sometimes the cause. The vulnerability of a specific bone depends on the anatomy of its vascular supply. Staphylococcus aureus is the usual cause of osteomyelitis.61,62
Exogenous osteomyelitis can be caused by human bites or fist blows to the mouth. Superficial animal or human bites inoculate local soft tissue with bacteria that later spread to underlying bone. Deep bites can introduce microorganisms directly onto bone. The most common infecting organism in human bites is Staphylococcus aureus. In animal bites, the most common infecting organism is Pasteurella multocida, which is part of the normal mouth flora of cats and dogs.
Direct contamination of bones with bacteria can also occur in open fractures or dislocations with an overlying skin wound. Intervertebral disc surgery and surgical procedures involving insertion of foreign objects such as metal plates or artificial joints are associated with exogenous osteomyelitis. Local injections and venous punctures are significant causes of exogenous osteomyelitis. Exogenous osteomyelitis of the arm and hand bones tends to occur in those who abuse drugs. In general, persons who are chronically ill, have diabetes or alcoholism or are receiving large doses of steroids or immunosuppressive drugs are particularly susceptible to exogenous osteomyelitis or recurring episodes of this disease.
PATHOPHYSIOLOGY
Regardless of the source of the pathogen, the pathological features of bone infection are similar to those in any other body tissue. The invading pathogen provokes an intense inflammatory response. As always, the inflammatory response dilates blood vessels flowing to the affected area and constricts those leading away from it, leading to vascular engorgement. An associated increase in permeability causes oedema. Leucocytes attend, releasing inflammatory chemicals and phagocytosing bacteria; abscesses form. Once inflammation is initiated, the small terminal vessels thrombose and exudate seals the bone’s canaliculi. Inflammatory exudate extends into the metaphysis and the marrow cavity and through small metaphyseal openings into the cortex.
In children, exudate that reaches the outer surface of the cortex forms abscesses that lift the periosteum off underlying bone. Lifting of the periosteum disrupts blood vessels that enter bone through the periosteum, depriving underlying bone of its blood supply; this leads to necrosis of the affected bone, producing sequestrum, an area of devitalised bone. Lifting of the periosteum also stimulates the osteoblasts into intense activity as they lay down new bone that can partially or completely surround the infected bone. This layer of new bone surrounding the infected bone is called an involucrum. Openings in the involucrum allow the exudate to escape into surrounding soft tissue and ultimately through the skin by way of sinus tracts (see Figure 21-33).
In adults, this complication is rare because the periosteum is firmly attached to the cortex and resists displacement. Instead, infection disrupts and weakens the cortex, which predisposes the bone to pathological fracture.
CLINICAL MANIFESTATIONS
Clinical manifestations of osteomyelitis vary with the age of the individual, the site of involvement, the initiating event, the infecting organism and whether the infection is acute, subacute or chronic:
 Acute osteomyelitis causes abrupt onset of inflammation. If an acute infection is not completely eliminated, the disease may become subacute or chronic.
Acute osteomyelitis causes abrupt onset of inflammation. If an acute infection is not completely eliminated, the disease may become subacute or chronic. In the chronic stage, infection develops slowly or is silent between exacerbations. The microorganisms persist in small abscesses or fragments of necrotic bone and produce occasional flare-ups of acute osteomyelitis.
In the chronic stage, infection develops slowly or is silent between exacerbations. The microorganisms persist in small abscesses or fragments of necrotic bone and produce occasional flare-ups of acute osteomyelitis.The progression from acute to subacute osteomyelitis may be the result of inadequate or inappropriate therapy or the development of drug-resistant microorganisms.
In children, radiographic bone changes take 2–3 weeks to develop. Initially, osteomyelitis presents as pain, swelling and warmth. Children will often present with fever, an elevated white blood cell count (50–70%), elevated C-reactive protein (98%) and elevated erythrocyte sedimentation rate (90%). Blood culture is positive in only 40% of cases. Without changes on plain radiograph, bone scans can help define the location of infection. In infants, where osteomyelitis can be multifocal in up to 40% of cases, bone scans identify other locations of infection that may need surgical intervention (see Figure 21-34).
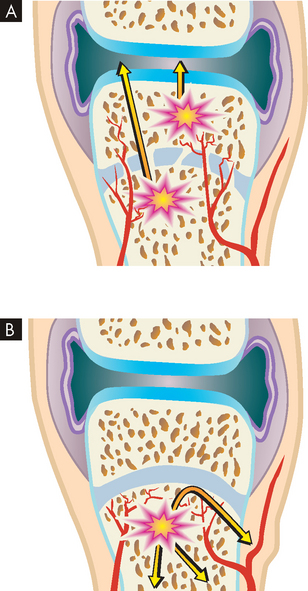
FIGURE 21-34 The pathogenesis of acute osteomyelitis differs with age.
A In infants younger than 1 year the epiphysis is nourished by arteries penetrating through the physis, allowing development of the condition within the epiphysis. B In children up to 15 years of age, the infection is restricted to below the physis because of interruption of the vessels.
Treatment of osteomyelitis consists of appropriate antibiotic management for 6 weeks. If blood cultures are negative, bone aspirate must determine the bacterial cause of the infection. If bony changes exist on plain radiographs, surgical debridement accompanies antibiotic treatment.
In the adult, osteomyelitis has an insidious onset. The symptoms are usually vague and include fever, malaise, anorexia, weight loss, and pain in and around the infected areas. Oedema may or may not be evident. Recent infection (urinary, respiratory, skin) or instrumentation (catheterisation, cystoscopy [endoscopy of the urinary bladder], myelography [X-ray visualisation of the spinal cord after injection of contrast medium], discography [X-ray of the spinal column disc/s after injection of contrast medium]) usually precedes the onset of symptoms.
Single or multiple abscesses (Brodie’s abscesses) characterise subacute or chronic osteomyelitis. Brodie’s abscesses are well-defined lesions 1–4 cm in diameter, usually in the ends of long bones and surrounded by dense ossified bone matrix. The abscesses are thought to develop when the infectious microorganism has become less virulent or the individual’s immune system is resisting the infection somewhat successfully.
In exogenous osteomyelitis, signs and symptoms of soft-tissue infection predominate. Inflammatory exudate in the soft tissues disrupts muscles and supporting structures and forms abscesses. Low-grade fever, lymphadenopathy (enlargement of the lymph nodes), local pain and swelling usually occur within days of contamination by a puncture wound.
EVALUATION AND TREATMENT
Laboratory data show an elevated white cell count. Radiographic studies include radionuclide bone scanning, CT scans and MRI. MRI with gadolinium contrast shows both bone and soft tissue, providing more accurate assessment of infection.63
Treatment of osteomyelitis includes antibiotics and debridement with bone biopsy. Each individual is treated according to their signs. Biodegradable antibiotic-impregnated bioabsorbable beads have benefited many individuals.64,65 Chronic conditions may require surgical removal of the inflammatory exudate followed by continuous wound irrigation with antibiotic solutions in addition to systemic treatment with antibiotics. Implants for total joint replacements may be removed to treat the infected joint more thoroughly.
Septic arthritis
Septic arthritis is an infection of the joint space. This condition is always a surgical emergency. The bacteria, and the activities of white cells (leucocytes) fighting the bacteria, can quickly destroy the articular cartilage of the joint and affect the blood supply to the epiphyseal bone nearby. Neither of these complications is easy to treat, and either one can lead to a lifetime of disability.
Septic arthritis can occur primarily or secondarily to osteomyelitis that breaks out of the metaphysis of the bone into the joint space. The metaphysis of the paediatric hip, shoulder, proximal radius and distal lateral tibia are all located within the joint capsule and therefore osteomyelitis in these regions must be carefully monitored for secondary septic arthritis. The most common sites for septic arthritis are the knees, hips, ankles and elbows.
Children with septic arthritis present with severe joint pain, ‘pseudoparalysis’ (apparent loss of muscle power without actual paralysis) or marked guarding to motion of the joint, inability to bear weight and malaise, often with anorexia. Children appear quite ill with this diagnosis. Non-pyogenic (not pus forming) arthritis, such as juvenile rheumatoid arthritis, can be difficult to distinguish clinically from septic arthritis because both can lead to malaise and an elevated erythrocyte sedimentation rate. An elevation in C-reactive protein, fever and complete inability to bear weight is more common with septic arthritis. Blood cultures are positive in 30–40% of cases. Culture taken from the affected joint positive for pus defines the diagnosis and determines the bacterial aetiology. As in osteomyelitis, Staphylococcus aureus is the most common bacterial cause.
After surgical debridement of the joint, antibiotics are required for 2–3 weeks. Long-term follow-up to assess damage to the joint cartilage or bone is required.
DISORDERS OF SKELETAL MUSCLE
Muscle and associated soft-tissue damage through trauma and overuse is an issue faced by many athletes and sportspeople. Muscle weakness and fatigue are common symptoms. In many cases, neural, traumatic and psychogenic causes are the reason for the failure to generate force (weakness) or maintain force (fatigue) seen in myopathies. Muscular symptoms also arise from a variety of causes unrelated to the muscle itself. Secondary muscular phenomena (contracture, stress-related muscle tension, immobility) are common disorders that influence muscular function. The ability of the nervous system to modify or control motor performance means that it has a large effect on muscular function. In this section we restrict our discussion to inherited and acquired disorders.
Contractures
Contractures can be physiological or pathological. A physiological muscle contracture occurs even though there is no action potential in the sarcolemma. Muscle shortening occurs because of failure of the calcium pump in the presence of plentiful ATP. A physiological contracture is seen in McArdle’s disease and malignant hyperthermia. The contracture is usually temporary if the underlying pathology is reversed.
A pathological muscle contracture is a permanent muscle shortening caused by muscle spasm or weakness. Heel cord (Achilles tendon) contractures are examples of pathological contractures. They are associated with plentiful ATP and occur in spite of a normal action potential. The most common form of contracture is seen in conditions such as muscular dystrophy and central nervous system injury. Contractures may also develop secondary to scar tissue contraction in the flexor tissues of a joint. This type of contracture is most common after a burn injury. An example could be contracture of burned tissues in the palm of the hand leading to a flexion contracture of the fingers.
Stress-induced muscle tension
Abnormally increased muscle tension has been associated with chronic anxiety as well as a variety of stress-related muscular symptoms, including neck stiffness, back pain and headache. Tension-type headaches have a very high prevalence. There is no convincing description of the pathophysiology of stress-induced muscle tension.
Various forms of treatment have been used to reduce the muscle tension associated with stress. Biofeedback, progressive relaxation training, yoga and meditation are examples of stress-reduction therapies. Biofeedback uses an integrated electromyogram to make recordings from the skin surface. It is particularly useful in individuals who have a connection between skeletal muscle tension and pain. Progressive relaxation training emphasises the individual’s ability to perceive the difference between tension and relaxation. This technique involves sequential tensing and a relaxing environment. The individual is taught to practise this routine daily, often with the use of audio instructions. By teaching the individual to recognise excessive contraction of skeletal muscle, the idea is to enhance the person’s ability to relax specific muscle groups to relieve tension and thus reduce central nervous system arousal, as well as autonomic nervous system arousal.
Disuse atrophy
The term disuse atrophy describes the reduction in normal size of muscle fibres after prolonged inactivity from bed rest, trauma (resulting in application of a cast) or local nerve damage. It is an example of the body responding to use (or lack of use). Decreased muscle activity reduces muscle mass through reduced protein synthesis and increased proteolysis (breakdown of protein), probably by reactive oxygen radical regulation.66 The effects of lack of use can become apparent rather quickly. With bed rest, the normal individual loses muscle strength from baseline levels at a rate of 3% per day. Bed rest is also associated with cardiovascular, skeletal and other organ system changes. Having a cast in place on a limb produces noticeable atrophy in a month. Also, as people age, their muscles atrophy and become weaker, a condition known as sarcopenia.
Measures to prevent atrophy include frequent forceful isometric muscle contractions (contractions without muscle shortening) and passive lengthening exercises. If reuse is not restored within 1 year, regeneration of muscle fibres becomes impaired.
Fibromyalgia
Fibromyalgia is a chronic musculoskeletal syndrome characterised by diffuse pain, fatigue and tender points (increased sensitivity to touch). The absence of systemic or localised inflammation and the presence of fatigue and disturbed sleep are common. However, fibromyalgia has often been misdiagnosed or completely dismissed by clinicians due to the similar clinical presentations to other conditions (see the list below). A common misdiagnosis has been chronic fatigue syndrome. Eighty to ninety per cent of individuals affected are women and the peak age is 30–50 years. While the incidence is unknown, the prevalence is reported to be 2% and increases with age.67 Although more common than rheumatoid arthritis, its cause is still unknown.
The aetiology of fibromyalgia has been debated for more than a century. It is unlikely that it is caused by a single factor. The most common precipitating factors include the following:
Certain rheumatic diseases, such as rheumatoid arthritis or systemic lupus erythematosus, may coexist if not initially present with fibromyalgia.68
PATHOPHYSIOLOGY
It is unproven but has long been suspected that muscle is the end organ responsible for the pain and fatigue. Some studies have documented metabolic alterations — lower ATP, lower adenosine diphosphate (ADP) and higher concentrations of adenosine monophosphate — and more alterations in the number of capillaries and fibre area in individuals with fibromyalgia than in study control subjects. Most studies have demonstrated that increased muscle tenderness in fibromyalgia is a result of generalised pain intolerance, possibly related to functional abnormalities within the central nervous system (see Figure 21-35).69
A chronic stress response may be involved in producing lower levels of serotonin (a neurotransmitter). There is increasing evidence that fibromyalgia involves the sympathetic nervous system. Individuals with fibromyalgia may have an adrenal hyporesponsiveness.
CLINICAL MANIFESTATIONS
The prominent symptom of fibromyalgia is diffuse, chronic pain. The locations of 9 pairs of (18) tender points for diagnostic classification of fibromyalgia are shown in Figure 21-36. Tenderness in 11 of these 18 points is necessary for diagnosis, along with a history of diffuse pain. The only reliable finding on examination is the presence of multiple tender points. The pain often begins in one location, especially the neck and shoulders, but then becomes more generalised. People describe the pain as burning or gnawing. Fatigue is profound. The effect on everyday life is considerable. Fatigue is most notable when arising from sleep and in mid-afternoon. Headaches and memory loss are common complaints. There is a strong association between fibromyalgia, Raynaud’s phenomenon and irritable bowel syndrome. Individuals with fibromyalgia are light sleepers and wake frequently.
FIGURE 21-36 The location of specific tender points for diagnostic classification of fibromyalgia.
Source: Freundlich B, Leventhal L. The fibromyalgia syndrome. In: Schumacher HR Jr, Klippel JH, Koopman WJ (eds). Primer on the rheumatic diseases. 11th edn. Atlanta: Arthritis Foundation; 1997.
Almost 25% of individuals seek psychological support for depression. Anxiety, particularly in regard to their diagnosis and future, is almost universal.
EVALUATION AND TREATMENT
As manifestations of chronic, generalised pain and fatigue are present in many musculoskeletal (e.g. rheumatic) disorders, these disorders should be discounted before diagnosis of fibromyalgia. Treatment should be highly individualised.70
No one regimen of medication has been proven to treat fibromyalgia successfully. Certain central nervous system active medications, most notably the tricyclic antidepressants, amitriptyline and cyclobenzaprine, have performed significantly better than placebos in controlled trials.71 These medications administered 1–2 hours before bedtime provide a better sleep. Amitriptyline significantly improves pain, morning stiffness and sleep but not tender points. Low-impact exercise in the amounts suggested for normal health and fitness may also help. One of the most important aspects of treatment is education and reassurance (see Box 21-2).
Box 21-2 EDUCATING AND PROVIDING REASSURANCE FOR INDIVIDUALS WITH FIBROMYALGIA
INTEGRATIVE ISSUES RELATED TO THE MUSCULOSKELETAL SYSTEM
Lower back pain
Lower back pain is a common health issue and a considerable problem for many individuals in Australia and New Zealand. Approximately 80% of individuals will experience lower back pain in their lifetime, but for 90% the pain will be short-lived. Back pain is the second most common symptom reported at general practitioners. It has been estimated that more than 5 million Australians72 and approximately 1 million New Zealanders73 have lower back pain. Furthermore, it has been estimated that the cost of lower back pain in Australia is more than $1 billion annually.74
Lower back pain affects the area between the lower rib cage and gluteal muscles and often radiates into the thighs. About 1% of individuals with acute lower back pain have sciatica — pain along the distribution of a lumbar nerve root. Sciatica is often accompanied by neurosensory and motor deficits, such as tingling, numbness and weakness. Men and women are equally affected, with women reporting lower back symptoms more often after 60 years of age. In addition, back pain is common in children, especially in the adolescent years. The increase in back pain in children has been associated with heavy, inappropriate schoolbags and inappropriate posture when sitting.
PATHOPHYSIOLOGY
Most cases of lower back pain are idiopathic and no precise diagnosis is possible. The local processes involved in lower back pain range from tension caused by tumours or disc prolapse, bursitis, synovitis, rising venous and tissue pressure (found in degenerative joint disease), abnormal bone pressures, problems with spinal mobility, inflammation caused by infection (as in osteomyelitis), bony fractures or ligamentous sprains to pain referred from viscera or the posterior peritoneum. General processes resulting in lower back pain include bone diseases such as osteoporosis or osteomalacia.
Risk factors include occupations that require repetitious lifting in the forward bent-and-twisted position; exposure to vibrations caused by vehicles or industrial machinery; obesity; and cigarette smoking. Osteoporosis increases the risk of spinal compression fractures and may be why elderly women report more symptoms than men. Genetic predispositions for lower back pain have also been reported.
The most commonly encountered causes of lower back pain include lumbar disc herniation, degenerative disc disease, spondylosis and spinal stenosis. Anatomically, lower back pain must come from innervated structures, but deep pain is widely referred and varies. The nucleus pulposus has no intrinsic innervation, but when extruded or herniated through a prolapsed disc, it irritates the dural membranes and causes pain referred to the segmental area. The interspinous bursae can be a source of pain between L3, L4, L5 and S1, but also may affect L1, L2 and L3 spinous processes. The anterior and posterior longitudinal ligaments of the spine and the interspinous and supraspinous ligaments are abundantly supplied with pain receptors, as is the ligamentum flavum. All of these ligaments are vulnerable to traumatic tears (sprains) and fracture. Muscle injury may contribute to lower back pain, with sprains and strains the most common diagnoses.
EVALUATION AND TREATMENT
Diagnosis of lower back pain is based on physical examination, electromyelography, CT scans with or without myelography, MRI and nerve conduction studies. Most individuals with acute lower back pain benefit from a nonspecific short-term treatment of bed rest, analgesic medications, exercises, physical therapy and education. Surgical treatments, specifically discectomy and spinal fusions, are used for individuals not responding to medical management. Individuals with chronic lower back pain are also prescribed anti-inflammatory and muscle-relaxant medications and are instructed to follow exercise programs. Aerobic exercises are a popular treatment and seem to be more effective than traction or lower back exercises. Spinal surgery has a limited role in curing chronic lower back pain.
Bone pain
Pain is associated with trauma to the skeletal system. Although no nociceptors (pain receptors) have been found within the osteon, the periosteum and endosteum are richly supplied. Pain may be experienced due to stimulation of these receptors, the release of inflammatory chemicals (e.g. bradykinin), the presence of any oedema or the spasm of muscles. Bone pain is also associated with many metabolic diseases.
The pain can be very severe, and in many conditions attempts to manage the pain can dominate the treatment of the condition. The pain of osteoarthritis becomes more evident as the condition progresses. Early in the course of the condition it is aggravated by use and relieved by rest, but later it may become persistent and no longer relieved by rest. With scoliosis the pain is described as very severe and often related to the degree of curvature. Patients with Paget’s experience a pain that is usually described as dull, but may be shooting and knifelike. Various other conditions, such as multiple myeloma, pancreatitis and sickle cell disease, can give rise to a bone pain that may be debilitating. With multiple myeloma the pain is usually precipitated by movement, while in pancreatitis bone pain may be severe enough to require the use of narcotics.
In children, bone and joint pain is associated with a wide variety of conditions such as rheumatic fever, systemic lupus erythematosus, juvenile rheumatoid arthritis, bursitis, osteomyelitis, osteosarcoma, acute leukaemia and some viral conditions like measles, influenza and chickenpox.
Myasthenia gravis
Myasthenia gravis is a very rare autoimmune condition that is outlined here because it illustrates the specificity of the immune response and normal cellular function in the region of the neuromuscular junction.
The disease process begins when the immune system recognises the acetylcholine receptor of the neuromuscular junction as an antigen. The B cells of the immune system are stimulated and produce antibodies that specifically bind to the receptor. This binding blocks the receptor and stimulates a local inflammatory response. The muscle fibre responds by withdrawing the affected receptors from the sarcolemma and producing new ones that are displayed in the neuromuscular junction. Cells bearing receptors for neurotransmitters and hormones normally have a continual process of recycling the receptors where old ones are internalised and new ones are produced. In the case of the muscle fibre, as soon as new receptors are displayed they are bound by antibodies. This action highlights both the specificity and the effectiveness of antibodies. The muscle fibre eventually becomes exhausted and reduces its rate of production of receptors.
Individuals with myasthenia gravis will suffer progressive muscle weakness usually initially causing drooping of the eyelids and later resulting in a generalised weakness. Medication seeks to counteract the cause of the condition. Acetylcholine esterase inhibitors (e.g. neostigmine) increase the effectiveness of the released acetylcholine and prednisone reduces the inflammatory response. Because this is an autoimmune condition, the person faces medication for the rest of their life.
Muscular dystrophy
The muscular dystrophies are a group of familial disorders that cause degeneration of skeletal muscle fibres. Ongoing genetic research has helped improve detection of carriers and define not only the inheritance pattern but also the DNA sequence of the various types. The most common singular type, Duchenne’s muscular dystrophy, is discussed here.
PATHOPHYSIOLOGY
Duchenne’s muscular dystrophy is a myopathy caused by mutations in a gene located on the short arm of the X chromosome. This mutation causes a protein thought to be responsible for maintaining the cytoskeleton of the muscle cell to be produced with an abnormal structure, to be reduced or to be absent (see Figure 21-37). The same protein also occurs in the brain and about one-third of people with Duchenne’s muscular dystrophy show mental retardation. As an X-linked inherited disorder, Duchenne’s muscular dystrophy affects only boys, with any male child of a known female carrier having a 50% risk of showing the condition. The overall incidence is 1:3500 male births.
FIGURE 21-37 Duchenne’s muscular dystrophy.
A Patient with late-stage Duchenne’s muscular dystrophy showing severe muscle loss. B Transverse section of gastrocnemius muscle from a normal boy. C Transverse section of gastrocnemius muscle from a boy with Duchenne’s muscular dystrophy. Normal muscle fibre is replaced with fat and connective tissue.
Source: Jorde LB et al. Medical genetics. 3rd edn updated. St Louis: Mosby; 2006.
CLINICAL MANIFESTATIONS
Duchenne’s muscular dystrophy causes muscle bulk to reduce through removal of muscle fibres. In the younger child the fibres regenerate, but become non-functional with time. Fibrous connective tissue and fat eventually replace muscle fibres. Duchenne’s muscular dystrophy is usually identified at about 3 years of age, with parents noting slow motor development or regression of motor tasks. Sitting, standing and walking become laboured and the child is clumsy, falls frequently and has difficulty climbing stairs.
Muscular weakness always begins in the pelvic girdle, causing a waddling gait. Hypertrophy (enlargement) of the calf muscles is apparent in 80% of cases. The method of rising from the floor by ‘climbing up the legs’ (Gowers’ sign) is characteristic and is caused by weakness of the lumbar and gluteal muscles. The foot assumes a talipes equinovarus position (rotated internally) and the child tends to walk on the toes because of weakness of the muscles in the front of the lower leg (tibialis and peroneus). The deep tendon reflexes are usually depressed or absent. Contractures and wasting of the muscles lead to muscular atrophy and deformity of the skeleton. Scoliosis can occur and is relentlessly progressive; curves of more than 20° are treated surgically to maintain pulmonary function and to slow the progression to a wheelchair.
Children usually lose their ability to walk by age 8–10 years. Progressive osteopenia (low bone density), due to inactivity, leads to pathological fractures. Studies cite that bisphosphonates, such as those used in osteogenesis imperfecta or osteoporosis, slow bone loss.71 Death, usually from progressive pulmonary or cardiac weakness, ensues by the 20s. Only 25% of individuals with Duchenne’s reach the age of 21 years.
EVALUATION AND TREATMENT
Diagnosis is confirmed by measurement of the serum enzyme, creatine kinase. Creatine kinase is increased to more than 20 times the normal level because it is liberated into the bloodstream with muscle death.
Although there is no effective cure for Duchenne’s muscular dystrophy, maintaining function for as long as possible is the primary goal. Activity helps maintain muscle function, but strenuous exercise may hasten the breakdown of muscle fibres. Both Muscular Dystrophy Australia and the Muscular Dystrophy Association of New Zealand note the possibility of treatment with steroids to maintain muscle strength and function. This treatment significantly lengthens the period of time a child can still walk but does not alter the life span. Range-of-motion exercises, bracing and surgical release of contracture deformities are used to maintain normal function. Genetic counselling is recommended. With X-linked inheritance, male siblings of an affected child have a 50% chance of being affected and female siblings have a 50% chance of being carriers.
Because of its tragic course, prenatal screening for Duchenne’s muscular dystrophy is encouraged. Possible female carriers are urged to have serum creatine kinase levels determined, which can be elevated in 60–80% of those affected. Female carriers have an increased risk of developing dilated cardiomyopathy (enlarged heart) later in life.
Congenital defects
Clubfoot
Clubfoot, or congenital equinovarus, describes a deformity in which the forefoot is adducted and supinated (turned inwards and ‘face up’; see Table 21-4) and the heel is in varus (turned inwards) and equines (points down) (see Figures 21-38 and 21-39). Clubfoot deformity can be positional (correctable passively), idiopathic or teratological (as a result of another syndrome, such as spina bifida). Idiopathic clubfoot usually occurs in 1:1000 live births, with males twice as likely as females to be affected. Incidence of clubfoot shows ethnic variation; for example, in the Polynesian Islands incidence is close to 75:1000 live births.75
Table 21-4 TERMS USED TO DESCRIBE FOOT ABNORMALITIES
| TERM | DEFINITION |
|---|---|
| Position | |
| Abduction | Lateral deviation away from the midline of the body |
| Adduction | Lateral deviation towards the midline of the body |
| Eversion | Twisting of the foot outwards along its long axis |
| Inversion | Twisting of the foot inwards on its long axis |
| Dorsiflexion | Bending of the foot upwards and backwards |
| Plantar flexion | Bending of the foot downwards and forwards |
| Abnormality | |
| Talipes | Congenital abnormality of the foot (clubfoot) |
| Pes | Acquired deformity of the foot |
| Varus | Inversion and adduction of the heel and forefoot |
| Valgus | Eversion and abduction of the heel and forefoot |
| Equinus | Plantar flexion of the foot in which the heel is lower than the toes |
| Calcaneus | Dorsiflexion of the foot in which the heel is lower than the toes |
| Planus | Flattening of the medial longitudinal arch of the foot (flatfoot) |
| Cavus | Elevation of the medial longitudinal arch of the foot (high arch) |
| Equinovarus | Coexistent equinus and varus deformities |
| Calcaneovarus | Coexistent calcaneus and varus deformities |
| Equinovalgus | Coexistent equinus and valgus deformities |
| Calcaneovalgus | Coexistent calcaneus and valgus deformities |
Note: The positions listed can all be achieved by voluntary movement of the normal foot; an abnormality exists if the foot is fixed in one or more of the positions while at rest.
EVALUATION AND TREATMENT
In idiopathic clubfoot, manipulation and casting above the knee, as described by Ponseti,76 begun soon after birth and correctly done, can correct the forefoot deformity in more than 95% of cases.77 Hindfoot equinus often requires lengthening of the Achilles tendon, which can be performed in a clinic under local anaesthetic. Achilles tenotomy (cutting the tendon) can be safely performed with local anaesthetic until 8 or 9 months of age. After this age, a formal lengthening and repair under general anaesthesia is required. Bracing is required until age 3. Idiopathic feet that are not correctable by these procedures require surgical posteromedial release, which includes lengthening of the Achilles, posterior tibialis and flexor tendons, and surgical release of the capsules of the ankle, subtalar and midfoot joints. Some sculpting of the bones around the ankle (most often the talus and calcaneus) is usually necessary to align the foot. Teratological feet are usually stiffer and up to 90% require posteromedial release. From 25% to 50% of children requiring posteromedial release may need a second operative procedure with growth; a large number of those with teratological feet also may need a second procedure.
Developmental dysplasia of the hip
Developmental dysplasia of the hip describes imperfect development of the hip joint and can affect the femoral head or the acetabulum, or both. Although most often present congenitally, dysplasia may develop later in the newborn or infant period. Like clubfoot, developmental dysplasia of the hip can be idiopathic or teratological. Teratological hips (because of another cause such as cerebral palsy or spina bifida) are more difficult to treat and often need operative intervention. In idiopathic developmental dysplasia of the hip, 70% of cases involve the left side only, 10–15% are bilateral and girls are four times as likely to be affected. A positive family history, breech presentation and oligohydramnios (low amniotic fluid) all predispose children to developmental dysplasia of the hip. Children in these groups are considered high risk and must be carefully evaluated with physical examination and, possibly, ultrasound.78
Variants of idiopathic developmental dysplasia of the hip are dislocated hip (no contact between the femoral head and acetabulum), subluxated hip (partial contact only) and acetabular dysplasia (the femoral head is located properly but the acetabulum is shallow). Idiopathic instability of the hip ranges from 3:1000 to 7:1000, but a true dislocation is only 1:1000.
EVALUATION AND TREATMENT
Clinical examination is the mainstay of diagnosis. The examination must be performed on a relaxed infant for accuracy. A positive Ortolani’s sign (hip dislocated, but reducible) or Barlow’s sign (hip reduced, but dislocatable) is an absolute indication for treatment. Other indicators for further evaluation are limitation of abduction79 or apparent shortening of the femur (Galeazzi’s sign). Asymmetric skin folds at the groin crease may also be observed.
In children younger than 4 months old, bracing with a Pavlik harness is successful in 90% of cases. A Barlow-positive hip (hip reduced, but dislocatable) is easier to treat with a Pavlik harness and success reaches 95–98%. An Ortolani-positive hip (hip dislocated, but reducible) must be followed closely with ultrasound and exam; the success rate with Pavlik is 70% in this situation. If a stable reduction is not attained within 2–3 weeks of treatment, the Pavlik harness should be abandoned. A partially reduced hip puts pressure on the rim of the acetabulum by the femoral head and can worsen dysplasia (abnormal cell proliferation and growth) and make treatment more difficult. In older children or cases where the Pavlik harness has failed, closed reduction of the hip and spica (body) casting under general anaesthesia is required. The spica cast is worn for 3 months. Children older than 12 months require surgery on the joint, femur or acetabulum, or all three (see Figure 21-40). The incidence of excellent outcome falls steadily with age, emphasising the need for early diagnosis and treatment.
Non-accidental trauma
It is estimated that more than 55,000 children are abused per year in Australia and more than 13,000 are abused per year in New Zealand. The rate of child abuse in both countries is showing an increasing trend. Maltreatment may be psychological, sexual or physical. Thirty per cent of children who have been physically abused are seen by an orthopaedist. Accurate and appropriate referrals to child protection agencies are not only legally mandated but also essential for the wellbeing of the child. An abused child who is returned to the same situation without intervention has a 10–15% chance of subsequent mortality.
Children who are not yet walking and present with a long bone fracture have more than a 75% chance of that fracture being caused by non-accidental trauma.80 ‘Corner’ metaphyseal fractures (where a small fragment shears off the side of the metaphysis) are nearly always indicative of abuse, but occur only 25% of the time. Fractures at multiple stages of healing also suggest abuse; however, osteogenesis imperfecta or other causes of systemic osteomalacia must be ruled out. The most common presentation is a transverse tibia fracture. After walking age, only 2% of long bone fractures are the result of non-accidental trauma.81
Musculoskeletal injuries
 The most serious musculoskeletal injury is a fracture. A bone can be completely or incompletely fractured. A closed fracture leaves the skin intact. An open fracture has an overlying skin wound. The direction of the fracture line can be linear, oblique, spiral or transverse. Greenstick, torus and bowing fractures are examples of incomplete fractures that occur in children. Stress fractures occur in normal or abnormal bone that is subjected to repeated stress. Fatigue fractures occur in normal bone subjected to abnormal stress. Normal weight-bearing can cause an insufficiency fracture in abnormal bone.
The most serious musculoskeletal injury is a fracture. A bone can be completely or incompletely fractured. A closed fracture leaves the skin intact. An open fracture has an overlying skin wound. The direction of the fracture line can be linear, oblique, spiral or transverse. Greenstick, torus and bowing fractures are examples of incomplete fractures that occur in children. Stress fractures occur in normal or abnormal bone that is subjected to repeated stress. Fatigue fractures occur in normal bone subjected to abnormal stress. Normal weight-bearing can cause an insufficiency fracture in abnormal bone. Dislocation is complete loss of contact between the surfaces of two bones. Subluxation is partial loss of contact between two bones. As a bone separates from a joint, it may damage adjacent nerves, blood vessels, ligaments, tendons and muscle.
Dislocation is complete loss of contact between the surfaces of two bones. Subluxation is partial loss of contact between two bones. As a bone separates from a joint, it may damage adjacent nerves, blood vessels, ligaments, tendons and muscle.Disorders of bones and joints
 In osteoporosis the density or mass of bone is reduced because the bone-remodelling cycle is disrupted.
In osteoporosis the density or mass of bone is reduced because the bone-remodelling cycle is disrupted. Avascular diseases of the bone are collectively referred to as osteochondroses and are caused by an insufficient blood supply to growing bones.
Avascular diseases of the bone are collectively referred to as osteochondroses and are caused by an insufficient blood supply to growing bones. Legg-Calvé-Perthes disease is one of the most common osteochondroses. This disorder is characterised by epiphyseal necrosis or degeneration of the head of the femur, followed by regeneration or recalcification.
Legg-Calvé-Perthes disease is one of the most common osteochondroses. This disorder is characterised by epiphyseal necrosis or degeneration of the head of the femur, followed by regeneration or recalcification. Osgood-Schlatter disease is characterised by tendonitis of the anterior patellar tendon and inflammation or partial separation of the tibial tubercle caused by chronic irritation, usually as a result of overuse of the quadriceps muscles. The condition is seen primarily in muscular, athletic adolescent males.
Osgood-Schlatter disease is characterised by tendonitis of the anterior patellar tendon and inflammation or partial separation of the tibial tubercle caused by chronic irritation, usually as a result of overuse of the quadriceps muscles. The condition is seen primarily in muscular, athletic adolescent males. Scoliosis is a lateral curvature of the spinal column that can be caused by congenital malformations of the spine, poliomyelitis, skeletal dysplasias, spastic paralysis and unequal leg length, but it is most often idiopathic.
Scoliosis is a lateral curvature of the spinal column that can be caused by congenital malformations of the spine, poliomyelitis, skeletal dysplasias, spastic paralysis and unequal leg length, but it is most often idiopathic. Because of improved imaging technology, inflammation has been identified as an important feature of osteoarthritis.
Because of improved imaging technology, inflammation has been identified as an important feature of osteoarthritis. Rheumatoid arthritis is an inflammatory joint disease characterised by inflammatory destruction of the synovial membrane, articular cartilage, joint capsule, and surrounding ligaments and tendons. Rheumatoid nodules may also invade the skin, lung and spleen, and involve small and large arteries. Rheumatoid arthritis is a systemic disease that affects the heart, lungs, kidneys and skin, as well as the joints.
Rheumatoid arthritis is an inflammatory joint disease characterised by inflammatory destruction of the synovial membrane, articular cartilage, joint capsule, and surrounding ligaments and tendons. Rheumatoid nodules may also invade the skin, lung and spleen, and involve small and large arteries. Rheumatoid arthritis is a systemic disease that affects the heart, lungs, kidneys and skin, as well as the joints. Juvenile rheumatoid arthritis is an inflammatory joint disorder characterised by pain and swelling. Large joints are most commonly affected.
Juvenile rheumatoid arthritis is an inflammatory joint disorder characterised by pain and swelling. Large joints are most commonly affected. Ankylosing spondylitis is a chronic, inflammatory joint disease characterised by stiffening and fusion of the spine and sacroiliac joints. It is a systemic, immune inflammatory disease.
Ankylosing spondylitis is a chronic, inflammatory joint disease characterised by stiffening and fusion of the spine and sacroiliac joints. It is a systemic, immune inflammatory disease. Gout is a syndrome caused by defects in uric acid metabolism, with high levels of uric acid in the blood and body fluids. Uric acid crystallises in the connective tissue of a joint where it initiates inflammatory destruction of the joint.
Gout is a syndrome caused by defects in uric acid metabolism, with high levels of uric acid in the blood and body fluids. Uric acid crystallises in the connective tissue of a joint where it initiates inflammatory destruction of the joint. Osteoarthritis is a common, age-related disorder of the synovial joints. The primary defect is loss of articular cartilage.
Osteoarthritis is a common, age-related disorder of the synovial joints. The primary defect is loss of articular cartilage.Disorders of skeletal muscle
 A pathological contracture is permanent muscle shortening caused by muscle spasticity, as seen in central nervous system injury or severe muscle weakness.
A pathological contracture is permanent muscle shortening caused by muscle spasticity, as seen in central nervous system injury or severe muscle weakness. Stress-induced muscle tension can be treated using progressive relaxation training and biofeedback to reduce muscle tension.
Stress-induced muscle tension can be treated using progressive relaxation training and biofeedback to reduce muscle tension.Integrative issues related to the musculoskeletal system
Paediatrics
 The muscular dystrophies are a group of genetically transmitted diseases characterised by progressive atrophy of skeletal muscles. There is an insidious loss of strength in all forms of the disorder with increasing disability and deformity. The most common type is Duchenne’s muscular dystrophy.
The muscular dystrophies are a group of genetically transmitted diseases characterised by progressive atrophy of skeletal muscles. There is an insidious loss of strength in all forms of the disorder with increasing disability and deformity. The most common type is Duchenne’s muscular dystrophy. Clubfoot is a common deformity in which the foot is twisted out of its normal shape or position. Clubfoot can be positional, idiopathic or teratological.
Clubfoot is a common deformity in which the foot is twisted out of its normal shape or position. Clubfoot can be positional, idiopathic or teratological. Developmental dysplasia of the hip is an abnormality in the development of the femoral head or acetabulum, or both. Like clubfoot, it can be idiopathic or teratological. It is a serious and disabling condition in children if not diagnosed and treated.
Developmental dysplasia of the hip is an abnormality in the development of the femoral head or acetabulum, or both. Like clubfoot, it can be idiopathic or teratological. It is a serious and disabling condition in children if not diagnosed and treated. The presence of soft-tissue injury, corner fractures and multiple fractures at different stages of healing is extremely helpful for making a diagnosis of non-accidental trauma.
The presence of soft-tissue injury, corner fractures and multiple fractures at different stages of healing is extremely helpful for making a diagnosis of non-accidental trauma.Cveta is 60 years old and experienced menopause 10 years ago. She remembers having her first period at the age of 16 years. Throughout her life she has maintained a slim appearance, which may be due in part to her smoking, which has been at the rate of a pack of cigarettes a day since her late teens. Cveta spent the first 30 years of her life in Yugoslavia before emigrating to Australia. Although she now has a good diet, this was not always the case. When she lived in Yugoslavia vegetables were plentiful, but dairy products were scarce. She has not had a very active life, but she enjoys her life in Brisbane as the climate allows her to be outside frequently. Lately, she has felt the odd twinge of back pain and her 35-year-old daughter has wondered whether her mother is a little shorter than previously. This week Cveta experienced a visit to the local Accident and Emergency Department after she slipped and fell on a wet floor, breaking her left radius just above the wrist. Cveta has been referred back to her general practitioner for a check-up as the magnitude of the trauma would not have been expected to break a bone.