28 THE STRUCTURE AND FUNCTION OF THE URINARY SYSTEM
INTRODUCTION
The kidneys are the main organs of the urinary system and they are primarily responsible for the excretion of waste products from the body. These waste products include metabolites formed during chemical reactions, toxins in the blood and excess electrolytes that are eliminated from the body in the form of urine. The kidneys maintain a stable internal environment for optimal cell and tissue metabolism by filtering the blood and eliminating only the undesirable components, which means that most of the fluid is returned to the bloodstream. Therefore, not only do the kidneys produce urine, they also assist in facilitating a stable blood environment by contributing to homeostasis of the overall blood volume. Urine formation is a complex process that involves many steps, yet the fundamental principles of fluid and solute movement, such as filtration, diffusion and active transport should be familiar to you (see Chapter 3).
The other major component of the urinary system is the urinary bladder, which temporarily stores the urine that it receives from the kidneys. There are also tubes that transport urine between these structures, including two ureters that permit urine flow between the two kidneys and bladder and a urethra that travels from the bladder to outside the body.
However, the kidneys are integral to homeostatic functions other than urine production. They are centrally involved in balancing electrolyte and water transport, conserving nutrients and regulating acids and bases. They also have an endocrine function and secrete hormones that are involved in blood pressure regulation, erythrocyte production and calcium metabolism. In males only, the urinary system is anatomically linked with the reproductive system, but this is discussed in Chapter 31.
We commence our exploration of the urinary system by looking at the anatomy of the kidneys.
THE STRUCTURE OF THE KIDNEYS
External anatomy
The kidneys are two bean-shaped organs located on the posterior abdominal wall outside the peritoneal cavity. The kidney beans that some people eat are very similar in shape to the actual kidneys, hence the name. The kidneys lie on either side of the vertebral column (spine) with their upper and lower poles extending from near the twelfth thoracic vertebra to the third lumbar vertebra (see Figure 28-1). An adult kidney weighs approximately 130–150 g and is 11 cm long, 5–6 cm wide and 3–4 cm thick. The shape of the kidney arises because the lateral surface is convex and the medial surface is concave with a cleft in the middle called the hilum, where blood vessels, nerves and the joining tubes to the bladder, the ureters, enter the kidney. Each kidney is surrounded by a layer of fibrous connective tissue in a capsule (the renal capsule), which is embedded in a mass of fat. The capsule and this perirenal fat layer are covered with a double layer of renal fascia, fibrous tissue that attaches the kidney to the posterior abdominal wall. The cushion of fat, the ribs and the position of the kidney between the abdominal organs and muscles of the back protect the kidneys from trauma (see Figure 28-2). The right kidney is slightly lower than the left; it is displaced downwards by the superior position of the liver.
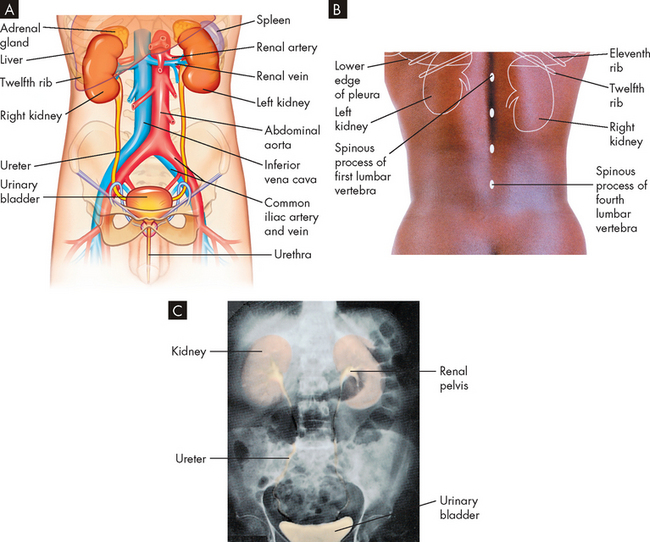
FIGURE 28-1 Organs of the urinary system.
A Anterior view of kidneys, ureters, bladder and urethra. Other anatomical structures are also shown in reference to the urinary system. B Posterior view of the position of the kidneys in relation to the ribs and vertebrae. C X-ray with contrast showing the position and size of the urinary organs.
Source: Based on Thibodeau GA. The human body in health & disease. 4th edn. St Louis: Mosby; 2005.
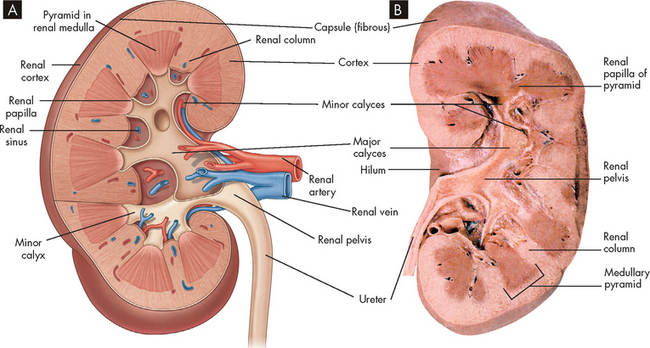
FIGURE 28-2 Cross-section of the kidney.
A Schematic of the internal structures of the kidney. B Photograph of a cross-section of a human kidney.
Source: A Based on Drake R, Vogl W, Mitchell A. Gray’s anatomy for students. 2nd edn. Philadelphia: Churchill Livingstone; 2010. B Based on Thibodeau GA. The human body in health & disease. 5th edn. St Louis: Mosby; 2010.
Internal anatomy
The kidney has three distinct sections, when viewing a frontal section: the cortex, medulla and pelvis. The outer layer of the kidney is called the cortex, while the medulla forms the inner part of the kidney and consists of regions call pyramids, which are cone-shaped structures. The renal columns separate the pyramids and the papillae are the inward projections of each pyramid. The pyramids extend into the renal pelvis, which has a striped appearance. The minor and major calyces are chambers that receive urine and form the entry into the renal pelvis (see Figure 28-2). The pelvis connects to the ureters for urine to flow into the bladder for storage.
The nephrons
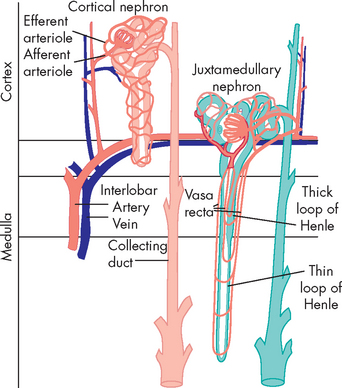
The nephron is the structural and functional unit of the kidney (see Figure 28-3). Each kidney contains approximately 1.2 million nephrons. The nephrons are the microscopic units that produce urine. We can actually survive with only one kidney, and a considerable number of nephrons need to be damaged before insufficient urine is produced, demonstrating that nephrons are remarkably efficient units at filtering waste.
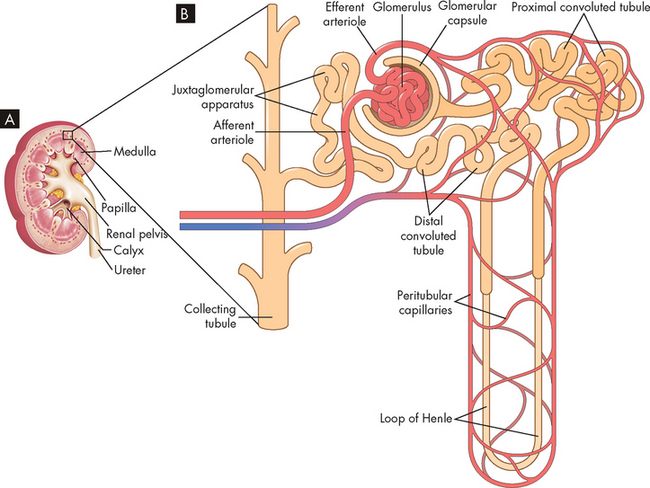
FIGURE 28-3 The location and components of the nephron.
A Magnified wedge cut from a renal pyramid showing the nephron’s structures. B Schematic of the nephron including the blood supply and the tubules down to the collecting tubule.
Source: A Based on Thibodeau GA. The human body in health & disease. 5th edn. St Louis: Mosby; 2010. B Based on Stoelting RK, Miller RD. Basics of Anesthesia. 5th edn. Churchill Livingstone; 2006.
The nephron is a tubular structure with an enlarged unit called the glomerular capsule or Bowman’s capsule at one end. Inside this capsule is a clumped network of capillaries called the glomerulus where blood enters the nephron to commence the filtration process. Collectively, these structures are termed the renal corpuscle (glomerular capsule and glomerulus). The glomerular capsule has two layers: a parietal (outer) layer and a visceral (inside) layer. The parietal layer is the external component and is composed of simple squamous epithelium. The visceral layer attaches to the glomerular capillaries and has specialised epithelial cells called podocytes. These cells allow the movement of fluid from the capillaries and into the capsule (discussed below).
The glomerular capsule is continuous with a series of tubes called the proximal convoluted tubule, the loop of Henle and the distal convoluted tubule. The proximal and distal components refer to their location relative to the renal corpuscle, with the loop of Henle connecting these two tubules. The distal convoluted tubule drains into the collecting duct, which contributes to the formation of urine. The proximal and convoluted tubules are twisted and folded back, while the loop of Henle is considerably longer and has two limbs — a descending arm and an ascending arm. In addition, the renal tubules are lined with a single layer of epithelial cells. The structure and function of these epithelial cells are slightly different along the length of the tubules, which permits specific functions in the production of urine. This is discussed later in the chapter when urine production is explained. Urine production starts at the glomerular capsule and continues through the proximal convoluted tubule, the loop of Henle, the distal convoluted tubule and the collecting duct.
The kidney has two major types of nephrons: cortical and juxtamedullary (juxta meaning ‘near’; see Figure 28-4). Cortical nephrons (85% of all nephrons) are located in the cortex and extend only partially into the medulla. Juxtamedullary nephrons (15% of all nephrons) lie close to and extend deep into the medulla and are important in the kidney’s production of concentrated urine. The major structural difference between the two types of nephrons is the length of the loop of Henle. In cortical nephrons, the loop is short, whereas in juxtamedullary nephrons the loop may extend the whole length of the medulla.
The glomerular filtration membrane
The glomerular filtration membrane filters blood components from the capillary into the glomerular capsule. The membrane has three components: (1) the endothelium of the capillary, (2) the visceral layer of the glomerular capsule with podocytes and (3) a basement membrane between these two layers (see Figure 28-5). The capillary endothelium is composed of cells in continuous contact with the basement membrane of the glomerular capsule and contains pores. These pores are numerous and are called fenestrae (from the Latin word for window). These fenestrations allow plasma to flow through without the blood proteins, which are too large to fit through the fenestrations. The basement membrane, between the capillary and the podocytes, further prevents proteins from entering the capsule. The podocytes have filtration slits, which are gaps between foot processes that attach to the basement membrane forming an elaborate network of intercellular clefts.1,2 The fluid flows through these filtration slits into the glomerular capsule and forms the primary urine, called filtrate. These sophisticated structures separate the blood of the glomerular capillaries from the fluid (filtrate) in the glomerular capsule.
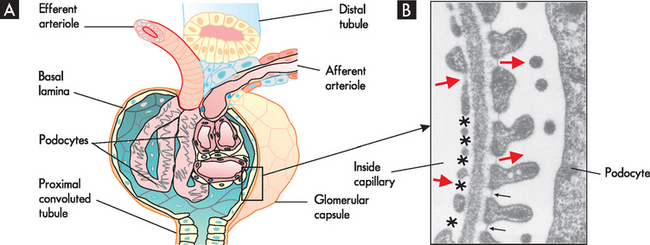
FIGURE 28-5 The filtration membrane of the glomerulus.
A The arrangement of the glomerulus and glomerular capsule showing the podocytes. B An electron micrograph showing a cross-section through the filtration barrier. The endothelium on the inside of the capillary has large multiple fenestrations (see asterisks) and the multiple foot processes of the podocyte, which are separated by filtration slits (arrows). Large red arrows indicate the direction of fluid flow from capillary into the glomerular capsule.
Source: Boron WF, Boulpaep EL. Medical physiology. 2nd edn. Philadelphia: Saunders; 2009.
Blood supply to the kidneys and nephrons
The kidneys are highly vascular organs, meaning that they have a high blood flow and there are many blood vessels within the kidneys (see Figure 28-6). The kidneys receive about 25% of total cardiac output — or approximately 1000 mL to 1200 mL of blood per minute. This is necessary to allow adequate filtration of the blood and this process needs to be continuous. When this blood flow is reduced, the kidneys often result in injury and if the blood flow is severely restricted, this can lead to acute kidney injury (Chapter 30). Blood flow to the kidneys and nephrons is shown in Figure 28-7.
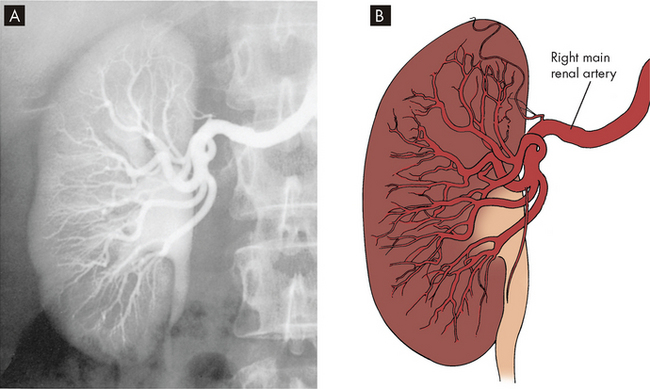
FIGURE 28-6 Blood vessels within the kidneys.
A Renal angiogram. B Diagram of renal blood vessels.
Source: Wein AJ et al. Campbell-Walsh urology. 9th edn. Philadelphia: Saunders; 2007.
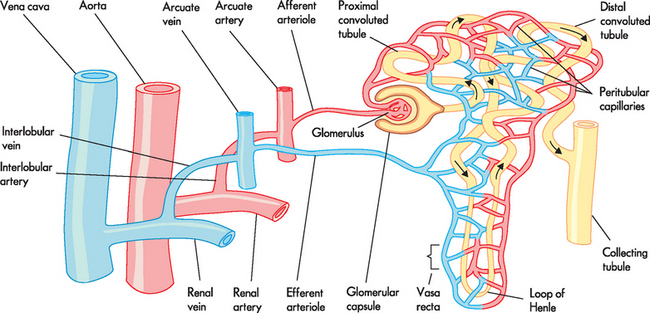
FIGURE 28-7 Blood supply to the kidney and nephron.
Source: Based on Copstead-Kirkhorn L-EC, Banasik JL. Pathophysiology. 4th edn. Philadelphia: Saunders; 2009.
The renal arteries arise from the abdominal aorta, divide into anterior and posterior branches at the renal hilum, then subdivide into lobar arteries supplying blood to the lower, middle and upper thirds of the kidney. The interlobar arteries are more subdivisions that travel down renal columns between the pyramids towards the cortex. The arcuate arteries are branches of the interlobar arteries at the cortical medullary junction, which arch over the base of the pyramids and run parallel to the surface of the kidney. From these arises the arteriole that enters the glomerular capsule, called the afferent arteriole (afferent meaning ‘carrying towards’), which connects to the glomerular capillaries, where filtration occurs. After the capillaries exit the capsule, they form the efferent arteriole (efferent meaning ‘carrying away’). The afferent and efferent arterioles are unique in the body because this is the only time that arterioles both feed and drain a capillary. The efferent arteriole forms a network of capillaries that intertwine with the tubules of the nephron. These are termed the peritubular capillaries; they are a low-pressure system that permits reabsorption of fluid and solute from the tubules. In this way, all the structures of the nephron have a close relationship with capillaries — the glomerular capsule has the glomerular capillaries, while the remaining structures are associated with the peritubular capillaries.
The venous system is essentially the reverse of the arterial supply: the peritubular capillaries drain into an arcuate vein joining to an interlobar vein and then into the renal vein, which eventually empties into the inferior vena cava.
The juxtaglomerular apparatus
Another important anatomical component of the nephron that needs to be discussed is the juxtaglomerular apparatus. A group of specialised cells known as juxtaglomerular cells (which release a hormone called renin; see later in the chapter) are located around the afferent arteriole where it enters the renal corpuscle (see Figure 28-8). These cells lie within the arteriole wall; they detect the blood pressure within the vessel and contain granules that secrete renin when stimulated. Located within the distal tubule are the macula densa (sodium-sensing cells), which detect the amount of sodium within the distal tubule (see Figure 28-8). Together, the juxtaglomerular cells and macula densa cells form the juxtaglomerular apparatus, which is important in sensing the properties of fluid flowing through that region. The juxtaglomerular apparatus is vitally important in the control of blood pressure and the rate at which glomerular filtration occurs.3,4
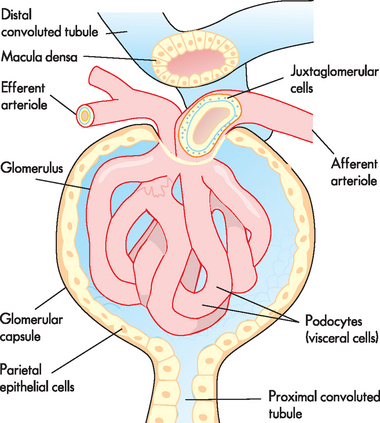
FIGURE 28-8 The juxtaglomerular apparatus.
The juxtaglomerular cells of the afferent arteriole are granular cells within the wall of the arteriole. The macula densa are cells located in the ascending limb of the loop of Henle, adjacent to the juxtaglomerular cells. Collectively, they form the juxtaglomerular apparatus.
KIDNEY FUNCTION
We now turn our attention to kidney physiology and the formation of urine.
Urine formation
The formation of urine involves many steps. The kidneys are relatively non-selective in what they filter from the blood. Except for large molecules like proteins, the majority of fluid and solutes are filtered into the nephrons, then must be returned to the bloodstream. The sophistication with the process is the balancing act by the nephrons to control both the volume of urine produced and the concentration of solutes in the urine. There are three main processes in the formation of urine:
These processes are summarised in Figure 28-9.
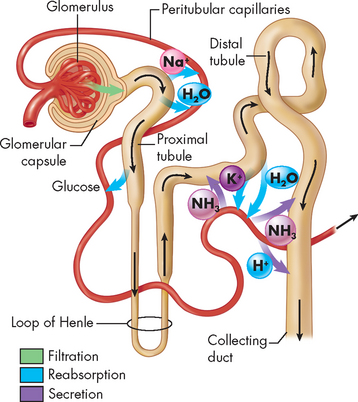
FIGURE 28-9 A summary of urine formation.
Includes the processes of filtration, reabsorption and secretion. See coloured arrows.
Source: Based on Thibodeau GA. The human body in health & disease. 5th edn. St Louis: Mosby; 2010.
We now explore how these processes work and coordinate to produce urine. We start with the glomerulus, where the first process occurs.
Glomerular filtration
Filtration is a passive process whereby fluid and solutes move across a membrane (see Chapter 3). Fluid and solutes are forced across the glomerular filtration membrane by hydrostatic pressure; the filtrate (fluid and solutes that are filtered) contains electrolytes (such as sodium, chloride and potassium) and organic molecules (such as creatinine, urea and glucose) in the same concentrations as in plasma. While large proteins cannot cross the glomerular filtration membrane, some small proteins (amino acids) may be filtered. Like other capillary membranes, the glomerulus is freely permeable to water and relatively impermeable to large substances such as plasma proteins. However, the glomerulus is more efficient at filtering fluid than other capillaries because of the fenestrations and the higher pressure in the glomerular capillaries. This allows more substances to exit the capillaries at the nephrons than at other capillaries in the body.
The hydrostatic pressure within the capillaries is the major force for moving water and solutes across the filtration membrane and into the glomerular capsule (see Figure 28-10). Two forces oppose the filtration effects of the glomerular capillary hydrostatic pressure: (1) the hydrostatic pressure in the glomerular space; and (2) the oncotic pressure of the glomerular capillary blood. Because the fluid in the glomerular capsule normally contains only small amounts of protein, it does not usually have a large oncotic influence on the plasma of the glomerular capillary.
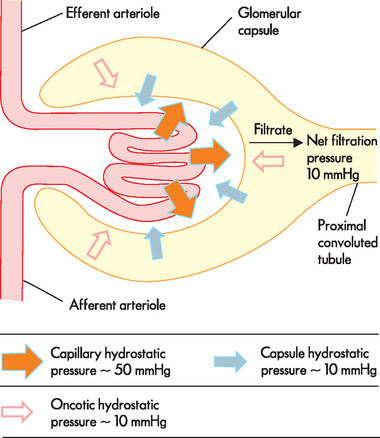
FIGURE 28-10 Glomerular filtration pressures.
The larger arrows represent the hydrostatic pressure within the capillary and the smaller arrows are forces opposing the hydrostatic pressure: hydrostatic pressure in the glomerular capsule and blood oncotic pressure. This results in a net fluid pressure that forces fluid out of the capillary. Pressures are approximate values only.
The combined effect of forces favouring and forces opposing filtration determines the filtration pressure. The net filtration pressure is the sum of forces favouring and opposing filtration. The estimated values contributing to the forces of filtration are presented in Figure 28-10. Overall, there is a net outward pressure that allows substances to move from the capillary into the glomerular capsule.
Tubular reabsorption
Thus far, blood has entered the glomerulus, via the afferent arteriole, and the majority of plasma has filtered across into the glomerular capsule. From here, the filtrate, which is actually primary urine, flows into the proximal convoluted tubule. Fortunately, nearly all the filtered plasma (99%) is reabsorbed into the blood — otherwise, we would very quickly deplete our blood volume! In fact, by the end of the proximal convoluted tubule, about 60–70% of the filtered water has been reabsorbed and large amounts of electrolytes (such as sodium, potassium, bicarbonate, calcium and phosphate) and other molecules (such as glucose and amino acids) have also been reabsorbed.
Tubular reabsorption along the tubules is due to processes such as simple diffusion, facilitated diffusion, active transport, co-transport and osmosis (see Chapter 3). The pressure in the peritubular capillaries is low so that filtrate will be reabsorbed from the tubules. Some molecules, particularly glucose, are reabsorbed using active transport which requires specific protein carriers to return filtered glucose to the blood. Because there are only a certain number of protein carriers available, reabsorption can be limited. Glucose should not normally appear in the urine because it is able to be fully reabsorbed. When it does appear in urine it is often due to the transporters not being able to move the glucose back into the blood or because there is too much glucose in the filtrate. The transporters cannot keep up and some glucose will not be reabsorbed. This phenomenon is referred to as the transport maximum.
We turn now to how reabsorption occurs in the different sections of the tubules, starting with the proximal convoluted tubule.
The proximal convoluted tubule
The proximal tubular lumen consists of one layer of cuboidal cells. This is the only surface inside the nephron where the cells are covered with microvilli (a brush border). This greatly expands the surface area of the tubule and enhances its reabsorptive function. The most important electrolyte that is reabsorbed is sodium. The reabsorption of sodium occurs by active transport using the sodium–potassium–ATP pump. While this pump requires energy in the form of ATP, it greatly enhances the movement of other electrolytes and water out of the proximal convoluted tubule and into the peritubular capillaries (see Figure 28-11). Water, most electrolytes (potassium, chloride, calcium and phosphate) and organic substances (glucose and amino acids) are co-transported with sodium, which means that when sodium is reabsorbed, other substances are also reabsorbed. This process does not require ATP and therefore is very efficient.
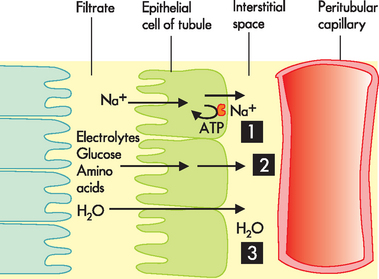
FIGURE 28-11 Reabsorption at the proximal convoluted tubule.
1. Sodium (Na+) is actively reabsorbed using an ATP pump. 2. Electrolytes and other solutes are reabsorbed using facilitated diffusion and are greatly enhanced by the sodium concentration changes due to active transport. 3. Water moves via osmosis in the same direction as the solute. Therefore, most of the electrolytes and water are reabsorbed by the time the filtrate leaves the proximal convoluted tubule.
The osmotic force generated by active sodium transport promotes the passive diffusion of water out of the tubular lumen and into the peritubular capillaries (remember the general rule that where sodium goes, water follows — on the condition that the membrane must actually be permeable to both sodium and water). Passive transport of water is further enhanced by the elevated oncotic pressure of the blood in the peritubular capillaries, which is created by the previous filtration of water at the glomerulus. As the filtrate moves through the proximal convoluted tubule, approximately 65% of water is reabsorbed. In a healthy person, glucose is able to filter at the glomerulus and is then completely reabsorbed in the proximal convoluted tubule, such that glucose does not appear in the urine.
The loop of Henle
The loop of Henle has descending and ascending arms that project down into the medulla. The descending arm has a thin segment composed of thin squamous cells with no active transport capabilities. However, this section is very permeable to water and water moves out via osmosis — approximately 15% of water is reabsorbed in the loop of Henle. In contrast, the ascending loop is not permeable to water, but allows sodium to move out using both active and passive transport mechanisms.
The distal convoluted tubule and collecting duct
By the time the filtrate has entered the distal convoluted tubule, the majority of water and solute reabsorption has occurred. The distal convoluted tubule and collecting duct are involved in adjusting the solute concentration and the amount of water, and this is mainly done under hormonal control. The distal convoluted tubule is poorly permeable to water but readily reabsorbs ions and contributes to the dilution of the tubular fluid. The end of the distal convoluted tubule and the collecting duct are permeable to water as controlled by antidiuretic hormone (ADH: diuresis = production of urine, so antidiuresis = less production of urine). This means that in the presence of ADH, water is reabsorbed from the urinary filtrate so that more water returns to the blood (see below). Alternatively, in someone who is well-hydrated, ADH is not released and in the kidneys the excess water remains in the filtrate through the collecting duct and can exit the body with the urine.
Sodium is readily reabsorbed by the later segment of the distal convoluted tubule and collecting duct under the regulation of the hormone aldosterone. Potassium is actively secreted in these segments and is also controlled by aldosterone and other factors related to the concentration of potassium in body fluids.
Tubular secretion
The final process in the production of urine is tubular secretion (see Figure 28-9). This occurs when toxins and by-products of metabolism are moved into the tubules from the peritubular capillaries for elimination. It is essentially reabsorption in reverse and is a valuable method for allowing additional substances to be moved from the blood into the filtrate. Remember that the process of filtration occurs only at the glomerular capsule, so in order for other substances to pass from the blood into urinary filtrate after that, secretion is necessary. Furthermore, filtration is a general, non-selective process whereby many substances enter the urinary filtrate, but secretion along the tubules is a selective process whereby substances that need to be removed from the blood can become part of the urine.
Substances are synthesised in the tubular cells and secreted into the filtrate and urine. A variety of substances may be secreted, including creatinine, urea, excess hydrogen ions (acid), potassium and ammonia. Furthermore, the kidneys are an important organ in removing drugs from the body; these drugs are secreted in the tubules to enter the urinary filtrate.
Urine concentration
Urine can be hypotonic, isotonic or hypertonic, but body fluids must stay constant with an osmolality of approximately 300 mOsm/kg. The kidneys have a sophisticated mechanism whereby they can regulate urine concentration and volume. This is achieved principally by the juxtamedullary nephrons, which have long loops of Henle that project down into the medulla. The structural features of the medullary loops of Henle allow the kidneys to concentrate urine and conserve water for the body. There exists an osmotic gradient from the cortex to the medulla, where the osmolality of the interstitial fluid becomes progressively more concentrated the deeper into the medulla. Therefore, the increased length of the longer loops of Henle means that there is more distance for water reabsorption to occur, which results in more concentrated urine. In addition, the transition of the filtrate into the final urine reflects the concentrating ability of the loops. It should be noted that the efficiency of water conservation is related to the length of the loops: the longer the loops, the greater the ability to concentrate the urine. Final adjustments in urine composition are made by the distal convoluted tubule and collecting duct according to body needs.
Antidiuretic hormone
The distal convoluted tubule in the cortex receives the hypo-osmotic urine from the ascending limb of the loop of Henle. The concentration of the final urine is controlled by ADH, which is secreted from the posterior lobe of the pituitary gland (neurohypophysis). The osmoreceptors in the hypothalamus sense changes in blood osmolality and, when osmolality increases, ADH is released. ADH is activated when renin causes the activation of angiotensin II by stimulating the hypothalamus (see Figure 28-12). Other external stimuli, such as surgery, pain, exercise and heat exposure, cause ADH release, as the body will want to preserve water content in these instances. ADH increases water permeability and reabsorption in the last segment of the distal convoluted tubule and along the entire length of the collecting duct, which pass through the medulla. The water diffuses out of the tubule and returns to the systemic circulation. The excreted urine can be very concentrated, such that the volume is normally reduced to about 1% of what was filtered at the glomerulus. Therefore, ADH causes an individual to produce concentrated urine.
In the absence of ADH, water diuresis — an increase in excretion of highly dilute urine — takes place. The distal convoluted tubules and collecting duct become impermeable to water. Water remains in the tubular lumen and is excreted as a dilute and large volume of urine. Because ADH has no effect on sodium reabsorption, sodium continues to be actively transported from the distal tubule.
Diuretics as a factor in urine flow
A diuretic is any agent that enhances the flow of urine. Clinically, diuretics interfere with renal sodium reabsorption and reduce extracellular fluid volume. Diuretics are commonly used to treat hypertension and oedema caused by heart failure, cirrhosis and renal failure. Diuretics are useful in these conditions, as they cause fluid to be lost from the body in the urine (as a result, less fluid remains in the bloodstream to lessen the hypertension), as well as to draw in some fluid from surrounding tissues in sites of oedema.
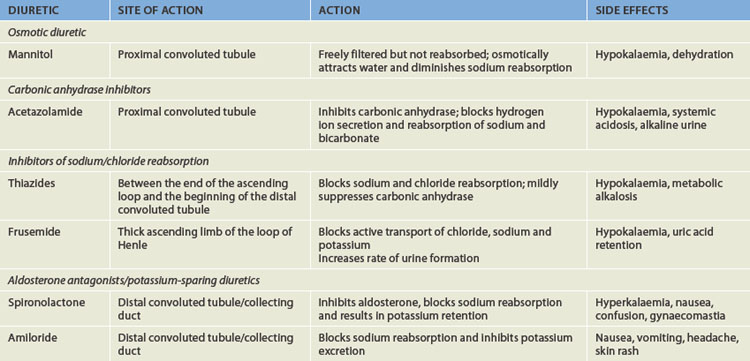
Diuretics are divided into four general categories: (1) osmotic diuretics that increase water loss by osmosis; (2) carbonic anhydrase inhibitors that inhibit carbonic anhydrase — an enzyme that causes urine to become more acidic by also causing reabsorption of sodium. Thus inhibiting this allows sodium and the water that follows to exit the body; (3) inhibitors of loop sodium or chloride transport (which decrease reabsorption and therefore increases urine volume); and (4) aldosterone antagonists and potassium-sparing diuretics — aldosterone increases sodium and water reabsorption; preventing aldosterone actions inhibits reabsorption. Aldosterone antagonists and potassium-sparing diuretics are usually placed together as many of them share the same properties. The physiological mechanisms related to each category are summarised in Table 28-1.
Glomerular filtration regulation
As already mentioned, the kidneys usually receive 1000–1200 mL of blood per minute. With a normal haematocrit (concentration of red blood cells in the blood) of 45%, about 600–700 mL of blood flowing through the kidneys per minute is plasma. From the renal plasma flow, 20% (approximately 120–140 mL/min) is filtered at the glomerulus and passes into glomerular capsule. The amount of fluid filtered over time is known as the glomerular filtration rate (GFR) and it is measured in millilitres per minute (mL/min). The GFR is directly related to the perfusion pressure of the glomerular capillaries; therefore as the pressure increases, so does the GFR, and as the pressure decreases, the GFR is also reduced. The normal GFR in adults is approximately 120–125 mL/min. Note that these values are combined for both kidneys and, in a healthy person, each kidney receives approximately half of the blood flow and contributes half of the GFR.
The total volume of fluid filtered by the glomeruli averages 180 L per day or approximately 120 mL/min, which is a phenomenal amount considering the size of the kidneys. Because only about l.5 L of urine is excreted per day, 99% of the filtrate is reabsorbed into the peritubular capillaries and returned to the blood. The factors determining the GFR are directly related to the pressures that favour or oppose filtration. For example, if the afferent arteriole constricts, blood flow decreases with a corresponding drop in glomerular pressure. The GFR then decreases and body fluids are conserved, meaning that less fluid is excreted in the urine and more is retained in the blood. Conversely, constriction of the efferent arteriole increases the net filtration pressure and the GFR increases. When both afferent and efferent arterioles constrict, little change occurs in filtration pressure, but renal blood flow is reduced and so is the GFR.
Pathophysiological conditions, such as kidney stones, can obstruct the outflow of urine. This can cause a retrograde increase in pressure at the glomerular capsule and a decrease in the GFR. Excessive loss of protein-free fluid from vomiting, diarrhoea, use of diuretics or excessive sweating can increase glomerular capillary oncotic pressure and decrease the GFR. Renal disease can also cause changes in pressure relationships by altering capillary permeability and the surface area available for filtration (these issues are discussed in more detail in Chapter 30).
The GFR is directly related to renal blood flow. However, it is advantageous for the kidneys to have a constant blood pressure and flow. Therefore, there are mechanisms that control renal blood flow and hence GFR. These mechanisms can be separated into intrinsic and extrinsic controls.
Intrinsic control
In the kidneys, a local mechanism tends to keep the rate of renal blood flow and therefore the GFR fairly constant over a range of arterial pressures between 80 mmHg and 180 mmHg (see Figure 28-13). This occurs because smooth muscle in the arteriole wall will contract (causing vasoconstriction) when the systemic blood pressure increases and will relax (causing vasodilation) when the systemic blood pressure drops. Therefore, both the renal blood flow and the GFR are relatively constant. This is called renal autoregulation and it is crucial to the GFR and hence urine output. The purpose of autoregulation of blood flow is to prevent large changes in the GFR when there are increases or decreases in systemic blood pressure. This enables the kidneys to still be effective in filtering the blood to maintain homeostasis, despite the alterations in blood delivery to the kidneys. Solute and water excretion and thus blood volume are regulated when arterial pressure changes.5
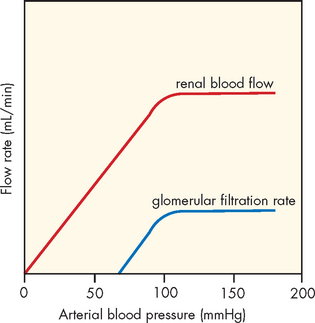
FIGURE 28-13 Autoregulation of renal blood flow.
Blood flow and the GFR are kept constant when arterial pressure is approximately between 80 mmHg and 180 mmHg. However, decreases in renal blood flow directly affect the GFR.
Source: Levy MN. Berne & Levy principles of physiology. 4th edn. Philadelphia: Mosby; 2006.
Another mechanism assists renal autoregulation. As the GFR in an individual nephron increases or decreases, the macula densa cells in the distal convoluted tubule (of the juxtaglomerular apparatus) sense the increasing or decreasing amounts of filtered sodium. When the GFR and sodium content increases, the macula densa cells stimulate afferent arteriolar vasoconstriction, which causes a decrease in the GFR. The opposite occurs with decreases in the GFR and sodium at the macula densa. By these mechanisms, renal blood flow is autoregulated, irrespective of systemic blood pressure, and the GFR keeps relatively stable. Together, these processes are vital for ensuring that the blood is undergoing constant filtering by the kidney.
Extrinsic control
The extrinsic control mechanisms that control the GFR work to maintain a stable and constant systemic blood pressure. Two mechanisms are responsible for extrinsic control: the sympathetic nervous system and hormonal control.
Sympathetic nervous system control
The blood vessels of the kidneys are innervated by the autonomic nervous system through sympathetic fibres that cause vasoconstriction and decrease renal blood flow. The afferent and efferent arterioles are richly innervated. There is no significant parasympathetic innervation.
When systemic arterial pressure decreases to below 80 mmHg, increased renal sympathetic nerve activity activates the carotid sinus and the baroreceptors of the aortic arch (see Chapter 22). This stimulates vasoconstriction of the afferent arteriole and decreases both renal blood flow and the GFR. The decreased renal blood flow also reduces excretion of sodium and water, promoting an increase in blood volume and thus an increase in systemic pressure.
This mechanism is particularly important in many hospitalised patients. Hypoxia (low tissue oxygen levels) influences renal blood flow by stimulating the chemoreceptors of the carotid and aortic bodies. This decreases renal blood flow due to sympathetic stimulation. Haemorrhage also induces intense sympathetic stimulation and vasoconstriction, and both the GFR and blood flow are reduced. Therefore, patients with hypoxia or haemorrhage can result in substantial changes in their blood flow to the kidneys, which can have deleterious effects on renal function. Remember that if the kidneys are unable to adequately filter the blood, then homeostasis of the blood cannot be maintained, and this can have a significant impact on health.
The renin-angiotensin-aldosterone system
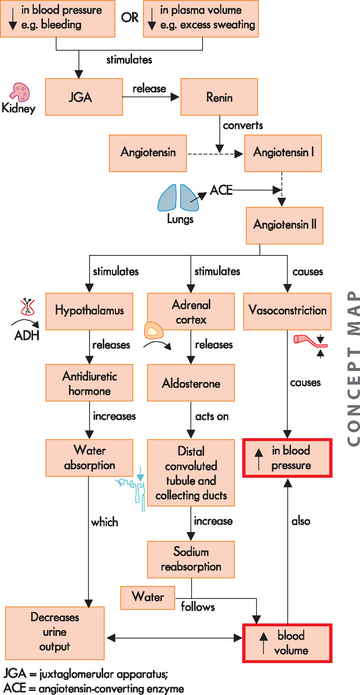
The major hormonal regulator of renal blood flow is the renin-angiotensin-aldosterone system, which can increase systemic arterial pressure and change renal blood flow (see Figure 28-14). Renin is an enzyme formed and stored in the cells of the arterioles of the juxtaglomerular apparatus (see Figure 28-8). Several complex physiological mechanisms stimulate the release of renin, including decreased blood pressure in the afferent arterioles (which reduces the stretch of the juxtaglomerular cells), decreased sodium chloride concentration in the distal convoluted tubule and sympathetic nerve stimulation of β (beta)-adrenergic receptors on the juxtaglomerular cells.6 Renin is released from the juxtaglomerular cells into the blood and acts on the plasma protein angiotensinogen, which is produced by the liver. This interaction converts angiotensinogen to angiotensin I. An enzyme called angiotensin-converting enzyme (ACE), which is found in the capillaries of the lungs, converts angiotensin I to angiotensin II. Angiotensin II has a number of effects, chiefly to increase systemic blood pressure and restore plasma volume. It is a powerful vasoconstrictor that causes the smooth muscle in the arterioles to constrict.
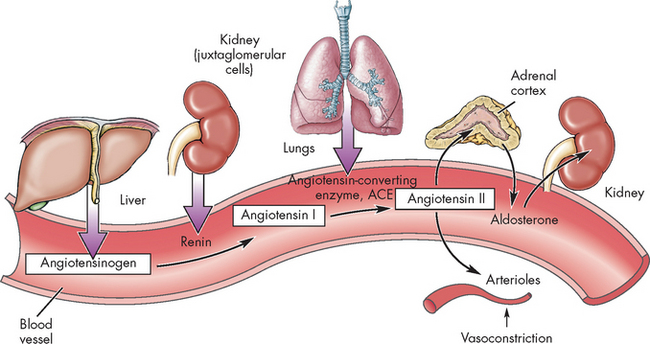
FIGURE 28-14 The renin-angiotensin-aldosterone system.
The release of renin affects many organs. These are summarised here; see the text for details.
Source: Herlihy B. The human body in health and illness. 3rd edn. St Louis: Saunders; 2007.
Although angiotensin II is effective in increasing systemic blood pressure, it is degraded rapidly and therefore its effect is only temporary, so it activates the secretion of other hormones, namely aldosterone and ADH (see Chapter 10). Aldosterone, released from the adrenal cortex, acts on the distal convoluted tubule to reabsorb water and sodium — when sodium is reabsorbed, water is reabsorbed too, as water usually follows sodium. This causes an increase in blood volume and a subsequent increase in blood pressure. ADH is released from the hypothalamus, stimulating the thirst centre and increasing water reabsorption at the distal convoluted tubule and collecting duct. This preservation of water also causes an increase in intravascular volume and assists in maintaining water balance throughout the body. Therefore, the release of renin causes many physiological effects that work to increase blood pressure to normal and restore water balance. The renin-angiotensin-aldosterone system is a vital link in the intimate relationship between the cardiovascular and urinary systems.
It is of note that a large percentage of individuals with hypertension control their blood pressure pharmacologically by taking medications called angiotensin-converting enzyme (ACE) inhibitors. These drugs work by blocking the conversion of angiotensin I to angiotensin II, thereby causing vasodilation and a reduction in blood pressure. ACE inhibitors have names that are easy to remember by the ending ‘-pril’ — for example, captopril, enalapril and ramipril. Individuals who are taking ACE inhibitors often complain of a cough. This is thought to be related to the suppression of the angiotensin-converting enzyme in the lungs.
URINE
Urine is normally clear yellow or amber in colour. It obtains its yellowish colour from a pigment of haemoglobin breakdown. Changes in colour often occur due to drugs, foods or vitamin supplements. Cloudiness may indicate the presence of bacteria, cells or high solute concentration. The pH ranges from 4.6 to 8.0, but it is normally acidic, providing protection against bacteria. Because the kidneys have such an important role in removing excess acid from the blood, it is expected that the urine will be acidic.
Specific gravity is a measure of substance concentration. Distilled water that has no solutes has a specific gravity of 1.000. In contrast, any substance that contains solute, such as urine, will have a greater weight than distilled water and the specific gravity will be greater than 1.000. The specific gravity of urine ranges from 1.001 to 1.035, depending on the amount of solutes. For instance, the first passing of urine in the morning after waking will have a higher specific gravity than that obtained after drinking a large volume of water. The odour of urine is slightly aromatic, but urine can become pungent if left exposed to air. This is caused by an increase in ammonia due to bacterial contamination.
Measures of renal function
To adequately assess renal function, determining the GFR would be appropriate. If the GFR is lowered, we know that this will affect the production of urine and therefore the body’s ability to excrete waste products is impaired. However, there is no specific test to measure the GFR; rather, there are tests that provide an estimate of this based on the excretion of a particular substance. In clinical practice, many patients have renal impairment. This may be due to a variety of reasons, but some of the main causes include an alteration to renal blood flow such as bleeding, direct damage to the kidneys (infection and diseases), obstruction of the urinary structures that transport urine (kidney stones) and the ageing process.
One of the first measures of renal function is the amount of urine produced. The volume of urine produced is crucial to adequate clearance and excretion of waste products. For instance, if there is damage to the kidneys, usually urine output (mL/hour) will be affected and the volume reduced. Therefore, a simple measure can inform the clinician that there may be an alteration to renal function. In addition, the chemical and physical composition of urine and blood provides vital clues to how well the kidneys are excreting waste. In the following sections we look at three common tests used to assess renal function: urinalysis (analysis of the urine) and two blood measures — urea and creatinine.
Urinalysis
Urinalysis provides clues as to the composition of the urine and therefore indicates how effectively the kidneys are processing waste. The test usually consists of a chemical reagent dipstick that is placed in fresh urine. The microscopic and chemical properties of the urine are determined by the change in colour of the reagent dipstick. The usual tests measure pH, red blood cells, white blood cells, protein, glucose, specific gravity and ketone bodies. Normal urine does not contain glucose or blood cells and only occasionally contains traces of protein, usually in association with rigorous exercise. However, detection of glucose, blood, protein or ketone bodies in urine should be investigated further (see Table 28-2).
Table 28-2 NORMAL RENAL FUNCTION TESTS
| TEST | NORMAL VALUE | INTERPRETATION |
|---|---|---|
| Urine | ||
| Colour | Amber-yellow | Drugs and foods may change colour |
| Turbidity | Clear | Purulent matter will make cloudy |
| pH | 4.6–8.0 | Bacteria create an alkaline urine |
| Specific gravity (density of distilled water = 1.000) | Represents concentrating ability or density of urine in relation to density of water (i.e. higher when contains glucose or protein; lower with dilute urine) | |
| Adults | 1.010–1.025 | |
| Infants | 1.010–1.018 | |
| Blood | Negative | Bleeding along urinary tract |
| Microscopic urine | ||
| Bacteria | None | Infection |
| Red blood cells | Negative | Bleeding along urinary tract |
| White blood cells | Negative | Urinary tract infection |
| Crystals | Negative | May have potential for stones |
| Fat | Negative | Can be associated with nephrosis |
| Casts | Occasional | A few are normal, may represent renal disease |
| Urinary chemistry | ||
| Bilirubin | Negative | Increases may cause dark orange colour |
| Ketones | Negative | Represents an increase in fat metabolism |
| Glucose | Negative | Usually signifies hyperglycaemia, may indicate diabetes mellitus |
| Protein | Negative-trace | Dysfunction of the glomerulus |
| Normal serum values | ||
| Urea | 3.0–8.0 mmol/L | Elevated with diseased kidneys |
| Creatinine | Elevated with decreased GFR | |
| Adult male | 0.06–0.12 mmol/L | |
| Adult female | 0.05–0.11 mmol/L | |
| Child <12 years | 0.04–0.08 mmol/L | |
| Potassium | 3.8–4.9 mmol/L | Elevated in renal failure |
Source: The Royal College of Pathologists of Australasia. RCPA Manual. 6th edn. Available at www.rcpamanual.edu.au, accessed June 2010.
Urea
Urea is found in both urine and the blood. It is formed due to protein metabolism in the liver. However, it is excreted by the kidneys, and therefore increases in urea in the blood indicate that renal function is not optimal. Urea is a major constituent of urine along with water. The glomerulus freely filters urea and tubular reabsorption depends on urine flow rate, with less reabsorption at higher flow rates.
The concentration of urea in the blood reflects glomerular filtration and urine-concentrating capacity. Urea is filtered at the glomerulus and therefore the blood concentration of urea increases when glomerular filtration drops. Because urea is reabsorbed by the blood through the permeable tubules, the blood concentration of urea rises in states of dehydration and acute and chronic renal failure when passage of fluid through the tubules slows. Urea also changes as a result of altered protein intake and protein catabolism. The normal range for urea in the blood of an adult is 3–8 mmol/L. In this case, a blood test is used to assess the function of the kidneys.
Creatinine
Creatinine is a natural substance and by-product of muscle metabolism and is released into the blood at a relatively constant rate (regardless of the level of physical activity). Creatinine is freely filtered at the glomerulus and a small amount is secreted by the tubules. Like urea, the level of creatinine closely matches the GFR. Creatinine clearance provides a good measure of the GFR because only one blood sample is required in addition to a 24-hour volume of urine. The normal level of creatinine in the plasma is 0.05–0.12 mmol/L; however, this is dependent on the muscle mass of the individual and males have slightly higher values than females. Patients with suspected kidney damage will have their creatinine and urea blood levels measured to ascertain the level of injury and also to indicate when to provide therapies, such as dialysis.
URINARY STRUCTURES
The ureters
The urine formed by the nephrons flows from the distal convoluted tubule and collecting duct through the renal papillae (projections of the duct) and into the calyces and is collected in the renal pelvis (see Figure 28-2). From the renal pelvis, urine is funnelled into the ureters (see Figure 28-15). The word ureter comes from the Greek word ‘to make water’. The ureters are thin tubes that transport urine from the kidneys to the bladder. Each adult ureter is approximately 30 cm long and is composed of long, intertwining muscle bundles. The lower ends pass obliquely through the posterior aspect of the bladder wall. The close approximation of muscle cells permits the direct transmission of electrical stimulation and the resulting peristaltic activity (like the intestines in the digestive system) propels urine into the bladder. Peristaltic activity is affected by urine volume such that increasing flow rates increase peristalsis. Peristalsis is maintained even when the ureters are denervated (does not have a nerve supply), so ureters can be transplanted. The upper part of the ureter is innervated by the tenth thoracic nerve roots, with referred pain to the umbilicus. The innervation of lower segments arises from the sacral nerves, with referred pain to the vulva or penis.
The bladder
The urinary bladder is a bag made of smooth muscle fibres that is a temporary storage area for urine (see Figure 28-16). It would be inappropriate to continually pass urine, as it is constantly being formed; therefore, the bladder stores urine until ready for urination. The bladder is situated in the pelvic cavity, behind the symphysis pubis. The position of the bladder varies with age and gender. In females, it is in an anterior position compared to the vagina and uterus. In males, it is anterior to the rectum. The bladder is lined with transitional epithelium (cells that can change shape; see Chapter 3) and as it fills with urine, the walls distend and the layers of transitional epithelium slide past each other and become thinner. This epithelium maintains an important barrier function to prevent movement of water and solutes between the urine and the blood.7 The smooth muscle layer is called the detrusor muscle and it is important in the process of passing urine (see below — micturition), and the trigone is a smooth triangular area between the openings of the two ureters and the urethra. The bladder has a profuse blood supply, accounting for the bleeding that readily occurs with trauma, surgery or inflammation.
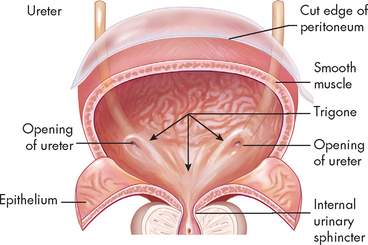
FIGURE 28-16 The urinary bladder.
Source: Based on Thibodeau GA. The human body in health & disease. 5th edn. St Louis: Mosby; 2010.
When the kidneys are damaged or diseased such that metabolic waste is not cleared from the blood and urine production declines, kidney failure may ensue. This usually occurs when renal function declines by about 85–90%. If this does occur, there are two treatment options: dialysis and kidney transplant. Dialysis refers to the artificial cleaning of the blood and it provides a substitution for the kidneys, without performing all renal processes — for instance, the production of renal hormones. Once dialysis commences, it continues for the individual’s life, or until a kidney transplant is performed. There are two types of dialysis:
There are disadvantages and advantages to both types of dialysis. For example, haemodialysis requires the individual to be attached to a dialysis machine for many hours while the blood is continuously washed. The individual must restrict their fluid intake between dialysis treatments. Peritoneal dialysis requires a large volume of fluid to reside in the peritoneal cavity, which can provide a heavy feeling to many individuals; however, dietary restrictions are not as strict compared with haemodialysis. The options for individuals are based on both medical and lifestyle considerations.
Source: www.kidney.org.au.
The bladder can greatly expand and hold large volumes of urine. In most cases, when the bladder contains approximately 500 mL of urine, the individual will have the urge to urinate. However, the bladder can hold considerably more urine and in some cases has a volume in excess of 1 L. You may well have experienced this — the bladder can be palpated and in some cases seen as the distension moves superiorly into the abdominal cavity.
The urethra
The urethra extends from the inferior side of the bladder to the outside of the body. A ring of smooth muscle forms the internal urethral sphincter at the junction of the urethra and the bladder. The external urethral sphincter is composed of skeletal muscle and is under voluntary control. The entire urethra is lined with mucus-secreting glands. The female urethra is short (3–4 cm) compared with the male urethra (18–20 cm); for this reason, females are more prone to urinary tract infections. In males, the urethra has three segments: (1) the prostatic urethra is closest to the bladder — it passes through the prostate gland and contains the openings of the ejaculatory ducts (note that the prostatic urethra ‘tunnels’ through the prostate gland without mixing with the contents of the prostate gland); (2) the membranous urethra passes through the floor of the pelvis; and (3) the cavernous segment forms the remainder of the tube — it is surrounded by erectile tissue and contains the openings of the bulbourethral mucous glands.
MICTURITION
The process of emptying urine from the bladder is called micturition (see Figure 28-17). This term is not used extensively in clinical practice — terms such as ‘voiding’ and ‘urinating’ are more commonly used. The process is involuntary in children and in many older individuals, and in pregnancy micturition may be affected.
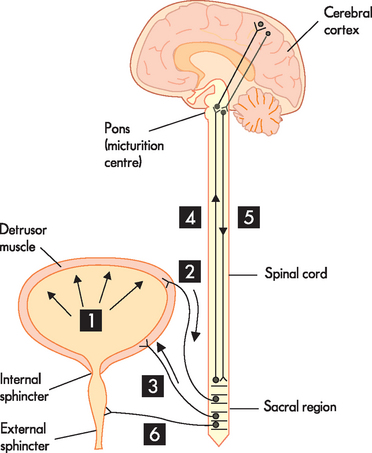
1. As the bladder fills with urine it causes stretch receptors on the bladder wall to initiate action potentials. 2. Nerve impulses are sent to the sacral region (S2–S4) of the spinal cord with increasing frequency as the bladder fills. 3. Parasympathetic fibres at the sacral level are activated, causing the detrusor muscle to contract. 4. As urine continues to fill the bladder, nerve impulses are sent with increased frequency to the pons (micturition centre) and the cerebral cortex, which ascertains the urge to urinate. 5. Signals sent back to the sacral region inhibit the external sphincter and the individual can consciously prevent urination. 6. When the individual wants to urinate, nerve impulses are transmitted to the pontine micturition centre, coordinating the detrusor muscle to contract and simultaneously reducing nerve impulses to the internal and external sphincters, which cause them to relax and the individual passes urine.
There are a number of stages to micturition and it commences when the bladder wall is stretched due to urine filling the bladder. This initiates the micturition reflex, which is controlled by the micturition centre in the pons. Nerve impulses, stimulated by mechanoreceptors responding to the stretching of tissue, are sent to the sacral level of the spinal cord. This releases action potentials of the parasympathetic fibres of the autonomic nervous system that contract the detrusor muscle of the bladder. At the same time, the internal urethral sphincter relaxes and the individual feels the urge to void. Impulses from higher brain centres inhibit the relaxing of the external urinary sphincter and while the individual has the urge to void, conscious control inhibits micturition. Small children are unable to control when they empty their bladders, as they have not yet learnt how to perform this conscious control. As urine accumulates in the bladder, the urge to void becomes greater; at an appropriate time, conscious control allows relaxation of the external urinary sphincter and urine flows out. During micturition, contraction of the bladder compresses the lower end of the ureter, preventing reflux, which is important in preventing infections.
PAEDIATRICS AND RENAL FUNCTION
Glomerular filtration in infants does not reach adult values until 1–2 years of age, and newborns have a decreased ability to efficiently remove excess water and solutes. Their shorter loops of Henle also decrease concentrating ability and produce more dilute urine than that produced by adults. These normal developmental processes result in a narrow safety margin for fluid and electrolyte balance when there is any disturbance such as diarrhoea, infection, improper feeding or fluid replacement. An increased risk of toxicity accompanies drug administration.8,9
AGEING AND THE URINARY SYSTEM
There is a slow decline in the GFR in most individuals, but generally it is not significant enough to lead to severe loss of renal function. The number of nephrons decreases and degenerative changes can occur, so nephrons are less able to concentrate urine, with decreases in the ability to tolerate dehydration or excessive water loads. Reabsorption of glucose may be delayed and drugs eliminated by the kidneys can accumulate in the plasma, causing toxic reactions; drug dosage should be carefully evaluated. Impairment in renal blood flow, hormonal regulatory systems and use of medications may alter sodium and water balance. Furthermore, the elderly often have impaired thirst, and water intake may alter water balance.10–12 4 6
The effects of ageing on the urinary system are summarised in Figure 28-18.
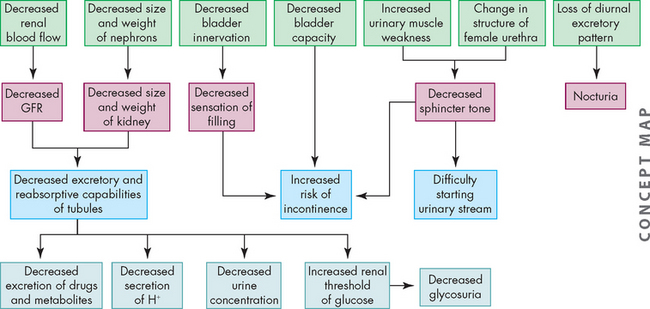
FIGURE 28-18 The effects of ageing on urinary and renal function.
Source: Based on Copstead-Kirkhorn L-EC, Banasik JL. Pathophysiology, 4th edn. Philadelphia: Saunders; 2009.
The structure of the kidneys
 The kidneys are paired structures lying bilaterally between the twelfth thoracic and third lumbar vertebrae.
The kidneys are paired structures lying bilaterally between the twelfth thoracic and third lumbar vertebrae. The right kidney is slightly lower than the left; it is displaced downwards by the superior position of the liver.
The right kidney is slightly lower than the left; it is displaced downwards by the superior position of the liver. The nephron is the urine-forming unit of the kidney and is composed of the glomerulus, proximal convoluted tubule, loop of Henle, distal convoluted tubule and collecting duct. Each kidney contains approximately 1.2 million nephrons.
The nephron is the urine-forming unit of the kidney and is composed of the glomerulus, proximal convoluted tubule, loop of Henle, distal convoluted tubule and collecting duct. Each kidney contains approximately 1.2 million nephrons. The glomerulus contains loops of capillaries. The capillary walls serve as a filtration membrane for the formation of primary urine.
The glomerulus contains loops of capillaries. The capillary walls serve as a filtration membrane for the formation of primary urine. The proximal convoluted tubule is lined with microvilli to increase surface area and enhance reabsorption.
The proximal convoluted tubule is lined with microvilli to increase surface area and enhance reabsorption. The hairpin loop of Henle transports solutes and water, contributing to the production of concentrated urine as more reabsorption of water occurs here.
The hairpin loop of Henle transports solutes and water, contributing to the production of concentrated urine as more reabsorption of water occurs here.Kidney function
 The major function of the nephrons is urine formation, which involves the processes of glomerular filtration, tubular reabsorption and tubular secretion.
The major function of the nephrons is urine formation, which involves the processes of glomerular filtration, tubular reabsorption and tubular secretion. Glomerular filtration is favoured by capillary hydrostatic pressure and opposed by oncotic pressure in the capillary and hydrostatic pressure in glomerular capsule. The net filtration pressure is the sum of forces favouring and opposing filtration and there is a net outward pressure that allows substances to move from the capillary into the glomerular capsule.
Glomerular filtration is favoured by capillary hydrostatic pressure and opposed by oncotic pressure in the capillary and hydrostatic pressure in glomerular capsule. The net filtration pressure is the sum of forces favouring and opposing filtration and there is a net outward pressure that allows substances to move from the capillary into the glomerular capsule. The proximal convoluted tubule reabsorbs about 60–70% of the filtered sodium and water and 90% of other electrolytes.
The proximal convoluted tubule reabsorbs about 60–70% of the filtered sodium and water and 90% of other electrolytes. The distal convoluted tubule and collecting duct adjust the solute concentration and the amount of water that needs to be reabsorbed. In addition, sodium is reabsorbed by the distal convoluted tubule and collecting duct under the regulation of the hormone aldosterone. Potassium is actively secreted in these segments and is also controlled by aldosterone.
The distal convoluted tubule and collecting duct adjust the solute concentration and the amount of water that needs to be reabsorbed. In addition, sodium is reabsorbed by the distal convoluted tubule and collecting duct under the regulation of the hormone aldosterone. Potassium is actively secreted in these segments and is also controlled by aldosterone. Tubular secretion is the final process in the formation of urine. Toxins and by-products of metabolism are moved from the peritubular capillaries into the tubules for elimination. It is essentially reabsorption in reverse.
Tubular secretion is the final process in the formation of urine. Toxins and by-products of metabolism are moved from the peritubular capillaries into the tubules for elimination. It is essentially reabsorption in reverse. The concentration of the final urine is a function of the level of antidiuretic hormone, which stimulates the distal convoluted tubule and collecting duct to reabsorb water.
The concentration of the final urine is a function of the level of antidiuretic hormone, which stimulates the distal convoluted tubule and collecting duct to reabsorb water. The glomerular filtration rate is the filtration of plasma per unit of time (mL/min) and is directly related to the perfusion pressure of renal blood flow.
The glomerular filtration rate is the filtration of plasma per unit of time (mL/min) and is directly related to the perfusion pressure of renal blood flow. Blood flow through the glomerular capillaries is maintained at a constant rate in spite of a wide range of arterial pressures.
Blood flow through the glomerular capillaries is maintained at a constant rate in spite of a wide range of arterial pressures. Autoregulation of renal blood flow and neural regulation of vasoconstriction maintain a constant GFR.
Autoregulation of renal blood flow and neural regulation of vasoconstriction maintain a constant GFR. Renin is an enzyme secreted from the juxtaglomerular apparatus and causes the generation of angiotensin, a potent vasoconstrictor. This occurs when renin acts on angiotensinogen, which permits the conversion of angiotensinogen to angiotensin I. Angiotensin-converting enzyme converts angiotensin I to angiotensin II, increasing systemic blood pressure and restoring plasma volume. The renin-angiotensin-aldosterone system is thus a regulator of renal blood flow.
Renin is an enzyme secreted from the juxtaglomerular apparatus and causes the generation of angiotensin, a potent vasoconstrictor. This occurs when renin acts on angiotensinogen, which permits the conversion of angiotensinogen to angiotensin I. Angiotensin-converting enzyme converts angiotensin I to angiotensin II, increasing systemic blood pressure and restoring plasma volume. The renin-angiotensin-aldosterone system is thus a regulator of renal blood flow.Urine
 Urine is normally clear yellow or amber in colour and its pH ranges from 4.6 to 8.0. Urine is slightly aromatic in odour but can become pungent if exposed to air due to an increase in ammonia from bacterial contamination.
Urine is normally clear yellow or amber in colour and its pH ranges from 4.6 to 8.0. Urine is slightly aromatic in odour but can become pungent if exposed to air due to an increase in ammonia from bacterial contamination. Urinalysis is performed to measure both microscopic and chemical properties of the urine. It is used to provide clues about the cause of the renal dysfunction.
Urinalysis is performed to measure both microscopic and chemical properties of the urine. It is used to provide clues about the cause of the renal dysfunction.Urinary structures
 The ureters are thin tubes that extend from the renal pelvis to the posterior wall of the bladder. Urine flows through the ureters by means of peristaltic contraction of the muscles.
The ureters are thin tubes that extend from the renal pelvis to the posterior wall of the bladder. Urine flows through the ureters by means of peristaltic contraction of the muscles.Micturition
Paediatrics and renal function
Angela is studying nursing at university and is trying to work out why urine volume varies according to the volume of fluids ingested and the level of physical activity of the individual. She has just completed a practical in the laboratory where three individuals had to either drink 1 L of water, drink 1 L of water and exercise for 30 minutes, or just exercise for 30 minutes without ingesting any water. All the individuals had to measure the volume of their urine every 30 minutes for the next 2 hours. Angela noticed that the individual who drank water only produced the largest volume of urine, followed by the individual who exercised and drank fluid, with the exercise-only individual producing the lowest volume of urine.