30 ALTERATIONS OF RENAL FUNCTION AND FLUID BALANCE ACROSS THE LIFE SPAN
INTRODUCTION
Alterations to the urinary system involve diseases and disorders of the kidneys and urinary structures. Many people will experience a pathophysiological disorder of the urinary system at some point in their life, with infection of the lower urinary structures — a urinary tract infection — being one of the most common disorders. Blockages in the urinary tract, such as kidney stones, are also relatively common. Renal function can be impaired by disorders of the kidneys themselves or by many other systemic diseases. Because the kidneys filter the blood, they are directly linked to every other organ system. Therefore, insult to the kidneys causing injury, whether acute or chronic, can be life-threatening.
The incidence and type of renal and urinary tract disorders experienced by children varies with age and maturation; newborn disorders may involve congenital malformations. During childhood, the kidney and urinary structures continue to develop, so renal dysfunction may be associated with mechanisms and manifestations that differ from those found in adults.
URINARY TRACT OBSTRUCTION
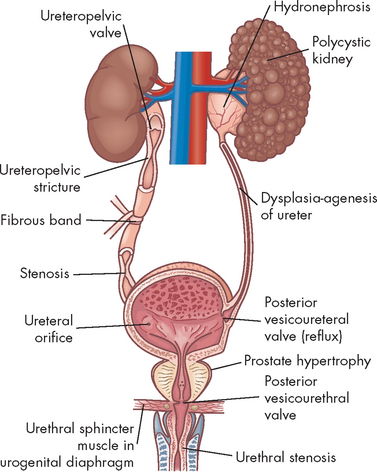
Urinary tract obstruction is an interference with the flow of urine at any site along the urinary tract (see Figure 30-1). An obstruction may be anatomical or functional. It can impede flow proximal (upstream) to the blockage, dilate the urinary system (as it becomes filled with urine that is not being drained), increase the risk for infection and compromise renal function. Anatomical changes in the urinary system caused by obstruction are referred to as obstructive uropathy. The severity of an obstructive uropathy is determined by:
Obstructions may be relieved or partially alleviated by correction of the obstruction, although permanent impairments occur if a complete or partial obstruction persists over a period of weeks to months or longer.
For the purposes of this discussion, we will consider obstructions in the upper and lower urinary tract: this demarcates an anatomical difference but also treatment differences. We start with the upper urinary structures.
Upper urinary tract obstruction
Common causes of upper urinary tract obstruction include:
Obstruction of the upper urinary tract causes dilation of the ureter, renal pelvis, and calyces proximal to the site of urinary blockage. Dilation of the ureter is referred to as hydroureter (an accumulation of urine in the ureter), and dilation of the renal pelvis and calyces (within the kidneys) proximal to a blockage leads to hydronephrosis (enlargement of the renal pelvis and calyces) or ureterohydronephrosis (dilation of both the ureter and pelvicaliceal system) (see Figures 30-2 and 30-3).
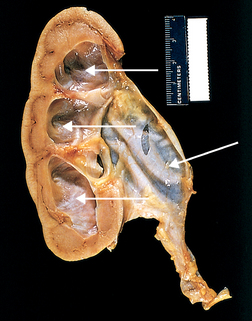
Hydronephrosis of the kidney, with marked dilation of the pelvis and calyces and thinning of the renal parenchyma (arrows).
Source: Kumar V. Robbins & Cotran pathological basis of disease. 8th edn. Philadelphia: Saunders; 2010.
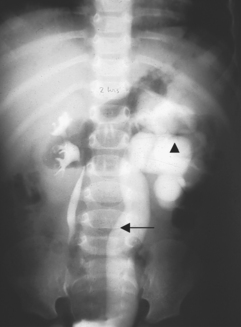
FIGURE 30-3 Ureterohydronephrosis.
Intravenous contrast dye highlights the dilated ureter (arrow) and renal pelvis (triangle).
Source: Zitelli BJ, Davis HW. Atlas of pediatric physical diagnosis, 5th edn. Philadelphia: Elsevier; 2007.
Dilation of the upper urinary tract is an early response to obstruction. It reflects smooth muscle hypertrophy and accumulation of urine above the level of blockage affecting the distal nephron within approximately 7 days. By 14 days, obstruction has adversely affected both distal and proximal aspects of the nephron. Within 28 days, the glomeruli of the kidney have been damaged and the renal cortex and medulla are reduced in size (thinned). Tubular damage (damage to the nephron tubules) initially decreases the kidney’s ability to concentrate urine, causing an increase in urine volume despite a decrease in the glomerular filtration rate (GFR). The affected kidney is unable to conserve sodium, bicarbonate and water or to excrete hydrogen or potassium, leading to metabolic acidosis and dehydration (see Chapter 29).
The magnitude of this damage, and the kidney’s ability to recover normal homeostatic function, is affected by the severity and duration of the obstruction. With complete obstruction, damage to the renal tubules occurs in a matter of hours and irreversible damage occurs within 4 weeks. Nevertheless, even in the face of a complete obstruction, the kidney may recover at least partial homeostatic function, provided the blockage is removed within about 2 months.1 The recovery requires a period of approximately 4 months. Partial obstruction, in the absence of renal infection, leads to subtler but ultimately permanent impairments including loss of the kidney’s ability to concentrate urine, reabsorb bicarbonate, excrete ammonia or regulate metabolic acid–base balance. This can lead to acute kidney injury and failure, which we discuss later in the chapter.
We now turn to some of the common causes of upper urinary tract obstruction.
Kidney stones
Calculi, or urinary stones, are masses of crystals, protein or other substances that are a common cause of urinary tract obstruction in adults. The incidence and prevalence of kidney stones in Australia is unknown as there is no nationwide collection of statistics.3 However, it has been estimated that about 4–8% of Australians will have kidney stones at some time in their life — the risk of developing a kidney stone is about 1 in 10 for males and 1 in 35 for females.4 The risk increases if there is a family history or if the patient is Indigenous, has type II diabetes mellitus or is older. Most persons develop their first stone before the age of 50 years. Individuals who regularly consume an adequate volume of water and those who are physically active are at reduced risk compared with individuals who are inactive or consume lower volumes of fluid. (Note that when we discuss fluid intake in relation to renal function, we mean water, tea and similar fluids; alcoholic and caffeinated drinks do not contribute to the healthy function of the kidneys.)
Urinary calculi can be described according to the primary minerals (salts) that make up the stones. The most common stone types include calcium oxalate or phosphate (70–80%), struvite (magnesium, ammonium and phosphate) (15%) and uric acid (7%).
PATHOPHYSIOLOGY
Human urine contains many ions that are capable of precipitating (becoming solid) from solution and forming a variety of salts. The salts form crystals that can grow into stones. Crystallisation is the process by which crystals grow from a small nidus (nucleus) to larger stones in the presence of supersaturated urine. Supersaturation is an important component of kidney stone formation and refers to a solution that contains more dissolved substances than can be dissolved in the water. This can be demonstrated by adding salt to a glass of water until it accumulates and will not dissolve into the solution any more. Intermittent periods of supersaturation after the ingestion of a meal or during times of dehydration are sufficient for stone growth in many individuals. In addition, the renal tubules and papillae have many surfaces that may attract a crystalline nidus and contribute to stone formation. This means that under the right conditions (such as insufficient fluid intake), a person may be prone to developing these stones.
The pH of urine also influences the risk of precipitation and calculus formation. An alkaline urinary pH (pH > 7) significantly increases the risk of calcium phosphate stone formation, whereas acidic urine (pH < 7) increases the risk of uric acid stone formation.
The size of a stone determines the likelihood that it will pass through the urinary tract and be excreted through micturition.5 A stone that is smaller than 5 mm in size has about a 50% chance of spontaneous passage, whereas a stone that is 1 cm has almost no chance of spontaneous passage. Nevertheless, the person with ureteral dilation from the previous passage of a stone may be able to excrete larger stones when compared with the person experiencing an initial obstructing calculus.
CLINICAL MANIFESTATIONS
Renal colic, described as moderate to severe pain often originating in the flank and radiating to the groin, usually indicates obstruction of the renal pelvis or proximal ureter.6 Colic that radiates to the lateral flank or lower abdomen typically indicates obstruction in the mid-ureter, and bothersome lower urinary tract symptoms (urgency, frequent voiding, urge incontinence) indicate obstruction of the lower ureter or ureterovesical junction. The pain can be severe and incapacitating and may be accompanied by nausea and vomiting. Gross or microscopic haematuria (blood in the urine) may be present.
EVALUATION AND TREATMENT
The evaluation and diagnosis of urinary calculi is based on presenting symptoms and history combined with a physical assessment, imaging studies and possibly a functional study of renal pelvic and ureteral pressures.7 The age of the person with a first stone episode, stone analysis and presence of complicating factors including hyperparathyroidism or recent gastrointestinal or genitourinary surgery are important contributors to kidney stone diagnosis. Urinalysis (including pH) is obtained and a 24-hour urine is completed to identify calcium salts. In addition, every effort is made to retrieve and analyse calculi that are passed spontaneously or retrieved through aggressive intervention, in order to send them for pathological examination, thereby confirming the type of stone. An ultrasound, intravenous pyelogram (dye image of the urinary structures) or CT scan is obtained to determine the location of the calculi, the severity of obstruction and associated obstructive uropathy (disease of the urinary system).8
The goals of treatment are to reduce the size of stones already formed and prevent new stone formation. The components of treatment include: (1) reducing the concentration of stone-forming substances by increasing urine flow rate with high fluid intake; (2) decreasing the amount of stone-forming substances in the urine by decreasing dietary intake or endogenous production or by altering urine pH;9 and (3) removing stones using endoscopy or ultrasonic or laser lithotripsy to fragment stones for excretion in the urine.10
Lower urinary tract obstruction
Obstructive disorders of the lower urinary tract are primarily related to storage of urine in the bladder or emptying of urine through the bladder outlet. The causes of the obstruction include both neurogenic (neurological in origin) and anatomical alterations or, in some instances, a combination of both. Incontinence is a common symptom; types of incontinence are reviewed in Table 30-1.
Table 30-1 TYPES OF INCONTINENCE
| TYPE | DESCRIPTION |
|---|---|
| Urge incontinence (most common in older adults) | Involuntary loss of urine associated with an abrupt and strong desire to void (urgency); often associated with involuntary contractions of the detrusor muscle |
| Stress incontinence (most common in women under 60 years who have borne children, and men who have had prostate surgery) | Involuntary loss of urine during coughing, sneezing, laughing or other physical activity associated with increased abdominal pressure |
| Overflow incontinence | Involuntary loss of urine with overdistension of the bladder; associated with neurological lesions below S1, polyneuropathies and urethral obstruction (i.e. an enlarged prostate in men) |
| Mixed incontinence (most common in older women) | Combination of both stress and urge incontinence |
| Functional incontinence | Involuntary loss of urine due to dementia or immobility |
Source: Agency for Health Care Policy and Research. Overview: urinary incontinence in adults, clinical practice guideline update. Rockville, MD; 1996. Available at www.ahrq.gov/clinic/uiovervw.htm.
Neurogenic bladder
Neurogenic bladder is a general term for bladder dysfunction caused by neurological disorders. The types of dysfunction are related to the sites in the nervous system that control sensory and motor bladder function. The location of the damage determines how the bladder will act. Lesions (abnormal tissue) that develop in motor neurons of the brain and spinal cord result in a loss of coordinated neuromuscular contraction and overactive or hyperreflexive bladder function. Lesions in the sacral area of the spinal cord or peripheral nerves result in underactive, hypotonic or atonic (flaccid) bladder function, often with loss of bladder sensation.
Neurological disorders that develop above the pontine (in the pons) micturition centre result in detrusor hyperreflexia, also known as an uninhibited or reflex bladder. The bladder empties automatically when it becomes full and the external sphincter functions normally. Because the pontine micturition centre remains intact, there is coordination between detrusor muscle contraction and relaxation of the urethral sphincter. Stroke, traumatic brain injury, dementia and brain tumours are examples of disorders that cause this condition. Symptoms include urine leakage and incontinence.
Neurological lesions that occur below the pontine micturition centre but above the sacral micturition centre (between C2 and S1) cause a loss of pontine coordination of detrusor muscle contraction and external sphincter relaxation, so both the bladder and the sphincter are contracting at the same time, causing a functional obstruction of the bladder outlet.11 Spinal cord injury, multiple sclerosis, Guillain-Barré syndrome and disc problems are causes of neurogenic bladder. There is diminished bladder relaxation during storage, with small urine volumes and high pressure within the urinary bladder. This results in an overactive bladder syndrome (see below) with symptoms of frequency, urgency and urge incontinence and increased risk for urethral turbulence and urinary tract infection.
Lesions that involve the sacral (region of the spinal cord) micturition centre (below S1; may also be termed cauda equina syndrome) or peripheral nerve lesions result in an acontractile detrusor or atonic bladder with retention of urine and distention. This is an underactive bladder syndrome and may have symptoms of stress and overflow incontinence. Multiple sclerosis and peripheral polyneuropathies (damage to multiple peripheral nerves) are associated with this disorder.
Overactive bladder syndrome
Overactive bladder syndrome is a syndrome of detrusor overactivity characterised by involuntary detrusor contractions during the bladder filling phase that may be spontaneous or provoked.12 There is coordination between the contracting bladder and external sphincter, but the detrusor is too weak to empty the bladder, resulting in urinary retention with overflow or stress incontinence. Overactive bladder syndrome affects many older men and women. Diagnosis is usually made by evaluation of symptoms. Urodynamic evaluation confirms the diagnosis. Anti-muscarinic drugs (that act on cholinergic receptors to control bladder function; also called anti-cholinergics) are the most common treatment and, in intractable cases, surgery is recommended.13 When left untreated, overactive bladder syndrome impairs health and quality of life, causes depression and leads to social isolation; in the elderly it may increase risk for falls and urinary tract infection.14
Obstructions to urine flow
Anatomical causes of resistance to urine flow include urethral stricture, prostatic enlargement in men and pelvic organ prolapse in women. Symptoms of obstruction are more common in men and include:
A urethral stricture is a narrowing of its lumen. It occurs when infection, injury or surgical manipulation produces a scar that reduces the calibre of the urethra.15 The vast majority of urethral strictures occur in men; they are rare in women.16 The severity of obstruction is influenced by its location within the urethra, its length and the minimum calibre of urethral lumen within the stricture. Specifically, proximal urethral strictures cause more severe obstruction than do strictures of the distal urethra, longer strictures tend to be more obstructive and the magnitude of blockage is in reverse proportion to the urethral diameter.
Prostate enlargement is caused by acute inflammation, benign prostatic hyperplasia or prostate cancer (see Chapter 32). Each of these disorders can cause encroachment on the urethra with obstruction to urine flow.
Partial obstruction of the bladder outlet or urethra initially causes an increase in the force of detrusor contraction. If the blockage persists, afferent nerves within the bladder wall are adversely affected, leading to urinary urgency and, in some cases, overactive detrusor contractions (a myogenic cause of overactive bladder). When obstruction persists, there is an increased deposition of collagen within the smooth muscle bundles of the detrusor muscle, possibly in an attempt to increase the force of its contraction strength. Ultimately, the bladder wall loses its ability to stretch and accommodate urine and the detrusor loses its ability to contract efficiently.
EVALUATION AND TREATMENT
Although the history and physical examination are critical to the evaluation of lower urinary tract disorders, it must be remembered that no symptom or cluster of symptoms has been identified that accurately differentiates the various causes of these disorders. For example, symptoms such as urgency, urge incontinence, frequent urination and nocturia may develop because of overactive bladder or either increased or decreased bladder outlet resistance. Reduced resistance is associated with the symptom of stress incontinence (incontinence with coughing or sneezing) and symptoms of increased resistance are similar to bladder outlet obstruction, including poor force of urinary stream, hesitancy and feelings of incomplete bladder emptying.
Various diagnostic tests assist with evaluation. The amount of urine left in the bladder is measured by catheterisation within 5–15 minutes of urination or through a bladder ultrasound machine that measures bladder height and width to provide an approximation of urine within the vesicle. This measurement may be combined with uroflowmetry, a graphic representation of the force of the urinary stream expressed as millilitres voided per second. Each of these measurements assesses the lower urinary tract’s efficiency in evacuating urine through micturition, but neither differentiates poor detrusor contraction strength from obstruction as a cause of urinary retention.
Obstruction that is not adequately managed pharmacologically may require bladder neck incision. This consists of transurethral sphincterotomy (surgical incision of the striated sphincter in the urethra) in order to relieve obstruction.
Prostate enlargement is managed by treating the underlying cause of the prostate enlargement with medication or surgery. Acute prostatitis (inflammation of the prostate gland) is initially managed by broad-spectrum antibiotics until the results of a urine culture are obtained. Urinary retention may require transient placement of a suprapubic catheter (a catheter placed in the bladder through the skin above the pubic bone). The management of benign prostatic hyperplasia and treatment options for prostate cancer are presented in Chapter 32.
Urethral stricture is treated with urethral dilation accomplished by using a steel instrument shaped like a catheter (urethral sound) or a series of incrementally increasing catheter-like tubes. Long, dense strictures typically require surgical repair to prevent recurrence.
URINARY TRACT INFECTION
Causes of urinary tract infection
A urinary tract infection (commonly referred to by the initials UTI) is an inflammation of the urinary epithelium usually caused by bacteria from gut flora. A UTI can occur anywhere along the urinary tract including the urethra, prostate, bladder, ureter or kidney. Many people are at risk of developing a UTI, including premature newborns, pre-pubertal children, sexually active and pregnant women, women treated with antibiotics that disrupt vaginal flora, oestrogen-deficient postmenopausal women, individuals with indwelling catheters, and those with diabetes mellitus, neurogenic bladder or urinary tract obstruction. Cystitis (see below) is more common in women because of the shorter urethra and the closeness of the urethra to the anus (increasing the possibility of bacterial contamination from the intestines). Up to 50% of women may have a lower UTI at some time in their life.17
Several factors normally combine to protect against UTIs. Most bacteria are washed out of the urethra during micturition. The low pH and high osmolality of urea and secretions from the epithelial lining of the urethra provide a bactericidal effect. The ureterovesical junction closes during bladder contraction, preventing reflux of urine to the ureters and kidneys. Both the longer urethra and prostatic secretions decrease the risk of infection in men.
Types of urinary tract infection
Acute cystitis
Acute cystitis is an inflammation of the bladder and is the most common site of UTI. The morphological appearance of the bladder through cystoscopy describes different types of cystitis. With mild inflammation, the mucosa is hyperaemic (red; see Figure 30-4). More advanced cases may show diffuse haemorrhage (termed haemorrhagic cystitis), pus formation or suppurative exudates (termed suppurative cystitis) on the epithelial surface of the bladder. Prolonged infection may lead to sloughing of the bladder mucosa with ulcer formation (termed ulcerative cystitis). The most severe infections may cause necrosis of the bladder wall (termed gangrenous cystitis). Generally, infections are mild, without complications and occur in individuals with a normal urinary tract; such an infection is termed an uncomplicated UTI. A complicated UTI develops when there is an abnormality in the urinary system or there is a health problem that compromises host defences or response to treatment.
PATHOPHYSIOLOGY
The most common infecting microorganisms are Escherichia coli and less commonly Klebsiella, Proteus, Pseudomonas, Staphylococcus, fungi, viruses, parasites or tubercular bacilli (see Chapter 14). Bacterial contamination of the normally sterile urine usually occurs by retrograde movement of gram-negative bacilli into the urethra and bladder and then to the ureter and kidneys. Some women may be genetically susceptible to certain strains of E. coli attachment.18 Infection initiates an inflammatory response and the symptoms of cystitis. The inflammatory oedema in the bladder wall stimulates discharge of stretch receptors initiating symptoms of bladder fullness with small volumes of urine and producing the urgency and frequency of urination associated with cystitis.
CLINICAL MANIFESTATIONS
Many individuals with bacteriuria (presence of bacteria in the urine) are asymptomatic and the elderly have the highest risk. Clinical manifestations of cystitis, however, usually include frequency, urgency, dysuria (painful urination) and suprapubic and low back pain. Haematuria, cloudy urine and flank pain are more serious symptoms. Approximately 10% of individuals with bacteriuria have no symptoms and 30% of individuals with symptoms do not have bacteria in the urine. Elderly individuals with cystitis may be asymptomatic or demonstrate confusion or vague abdominal discomfort. The elderly with recurrent UTI and other concurrent illness have a higher risk of mortality.19
EVALUATION AND TREATMENT
Infections are diagnosed by urine culture of specific microorganisms with counts of 10,000/mL or more from freshly voided urine. Risk factors, such as urinary tract obstruction, should be identified and treated. Evidence of bacteria from urine culture and antibiotic sensitivity warrants treatment with a microorganism-specific antibiotic. A single large dose of antibiotic or a 3-day course may be effective when symptoms are of short duration and there are no complications; 3–7 days of treatment is most common; and elderly people with obstructive disorders may require 7–14 days of treatment. From 20% to 25% of women have relapsing infection within 7–10 days requiring prolonged antibiotic treatment.20 Follow-up urine cultures should be obtained 1 week after initiation of treatment and at monthly intervals for 3 months. Clinical symptoms are frequently relieved, but bacteriuria may still be present. Repeat cultures should be obtained every 3–4 months until 1 year after treatment for evaluation of recurrent infection.21 Sexually active women should be educated about the importance of passing urine after sexual activity, which assists in ensuring that foreign microorganisms, which may have been introduced to the urethra during intimacy, are removed with the urine. This is a simple and effective method of preventing recurrent infections in cases where there is no underlying structural abnormality.
Urinary tract infection and antibiotic resistance
Uncomplicated urinary tract infection occurs primarily in sexually active women, with fewer cases among older and pregnant women and older men. The leading cause of UTI is Escherichia coli and antibiotics are the mainstay of treatment. Of major concern is the worldwide emergence of bacterial strains resistant to specific antibiotics in both hospital- and community-acquired infections. The resistance is caused in part by high human use of antibiotics and antibiotics in animal feed. Rates of resistance are highest in regions with the highest rates of prescription; ampicillin and trimethoprim-sulfamethoxazole have a high rate of resistance. Risks for resistance include diabetes mellitus, recent hospitalisation and specific antibiotic resistance rates in a community of greater than 20%. However, reliable data regarding the true prevalence of resistance in a community are often lacking. Other factors to consider in choice of antibiotic treatment include infection severity, complicated or uncomplicated UTI, accuracy of diagnosis, broad-spectrum antibiotic sensitivity (i.e. trimethoprim-sulfamethoxazole, ampicillin and cephalothin) versus narrow-spectrum antibiotic sensitivity (i.e. nitrofurantoin and ciprofloxacin), cost-effectiveness and instructing consumers about the correct use of antibiotics.
Source: Nicolle L et al. Uncomplicated urinary tract infection in women. Current practice and the effect of antibiotic resistance on empiric treatment. Can Fam Physician 2006; 52:612–618; Chulain MN et al. Antimicrobial resistance in E. coli associated with urinary tract infection in the west of Ireland. Ir J Med Sci 2005; 174(4):6–9; Mehnert-Kay SA. Diagnosis and management of uncomplicated urinary tract infections. Am Fam Physician 2005; 72(3):451–456.
Acute pyelonephritis
Pyelonephritis is an infection of the renal pelvis and interstitium. Common causes are summarised in Table 30-2. Urinary obstruction and reflux of urine from the bladder (vesicoureteral reflux) are the most common underlying risk factors. One or both kidneys may be involved. Most cases occur in women. The responsible microorganism is usually E. coli, Proteus or Pseudomonas. The latter two microorganisms are more commonly associated with infections after urethral instrumentation or urinary tract surgery. These microorganisms also split the urea molecule into ammonia, making alkaline urine that increases the risk of stone formation.
Table 30-2 COMMON CAUSES OF PYELONEPHRITIS
| PREDISPOSING FACTOR | PATHOLOGICAL MECHANISMS |
|---|---|
| Kidney stones | Obstruction and stasis of urine contributing to bacteriuria and hydronephrosis; irritation of the epithelial lining with entrapment of bacteria |
| Vesicoureteral reflux | Chronic reflux of urine up the ureter and into the kidney during micturition, contributing to bacterial infection |
| Pregnancy | Dilation and relaxation of the ureter with hydroureter and hydronephrosis; partly caused by obstruction from an enlarged uterus and partly from ureteral relaxation caused by higher progesterone levels |
| Neurogenic bladder | Neurological impairment interfering with normal bladder contraction with residual urine and ascending infection |
| Instrumentation | Introduction of organisms into the urethra and bladder by catheters and endoscopes introduced into the urinary tract for diagnostic purposes |
| Female sexual trauma | Movement of organisms from the urethra into the bladder with infection and retrograde spread to the kidney |
PATHOPHYSIOLOGY
The infection is probably spread by ascending microorganisms along the ureters, but spread may also occur by way of the bloodstream. The inflammatory process is usually focal and irregular, primarily affecting the pelvis, calyces and medulla. The infection causes medullary infiltration of white blood cells with renal inflammation, renal oedema and purulent urine (containing pus). In severe infections, localised abscesses may form in the medulla and extend to the cortex (see Figure 30-5). Primarily affected are the tubules; the glomeruli are usually spared. Necrosis of renal papillae can develop. After the acute phase, healing occurs with deposition of scar tissue and atrophy of affected tubules. The number of bacteria decreases until the urine again becomes sterile. Acute pyelonephritis rarely causes acute kidney injury.22
CLINICAL MANIFESTATIONS
The onset of symptoms is usually acute, with fever, chills and flank or groin pain. Symptoms characteristic of a UTI, including frequency, dysuria and flank tenderness, may precede systemic signs and symptoms.
EVALUATION AND TREATMENT
Differentiating symptoms of cystitis from those of pyelonephritis by clinical assessment alone is difficult. The specific diagnosis is established by urine culture, urinalysis and clinical signs and symptoms. White blood cell casts (collection of white blood cells in the urine) indicate pyelonephritis, but they are not always present in the urine. Complicated pyelonephritis requires blood cultures and urinary tract imaging.23
Uncomplicated acute pyelonephritis responds well to 2–3 weeks of microorganism-specific antibiotic therapy. Follow-up urine cultures are obtained at 1 and 4 weeks after treatment if symptoms recur. Antibiotic-resistant microorganisms or re-infection may occur in cases of urinary tract obstruction or reflux.
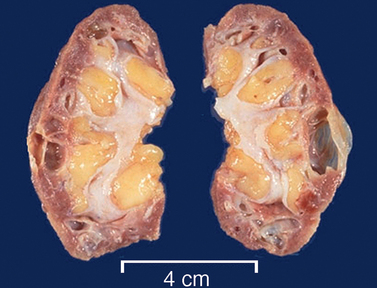
Chronic pyelonephritis
Chronic pyelonephritis is a persistent or recurrent infection of the kidney leading to scarring of the kidney. One or both kidneys may be involved (see Figure 30-6). The specific cause of chronic pyelonephritis is difficult to determine. Recurrent infections from acute pyelonephritis may be associated with chronic pyelonephritis. Generally, chronic pyelonephritis is more likely to occur in patients who have renal infections associated with some type of obstructive pathological condition, such as renal stones and vesicoureteral reflux.
PATHOPHYSIOLOGY
Chronic urinary tract obstruction starts a process of progressive inflammation, altered renal pelvis and calyces, destruction of the tubules, atrophy or dilation and diffuse scarring and finally impaired urine-concentrating ability, leading to chronic kidney disease. The lesions of chronic pyelonephritis cause inflammation and fibrosis in the interstitial spaces between the tubules. Causes other than chronic pyelonephritis include drug toxicity from analgesics such as aspirin and paracetamol, ischaemia, irradiation, and immune-complex diseases.
CLINICAL MANIFESTATIONS
The early symptoms of chronic pyelonephritis are often minimal and may include hypertension, frequency, dysuria and flank pain. Persistent pyelonephritis can progress to chronic kidney disease and end-stage kidney disease (see ‘Chronic kidney disease’ below).
EVALUATION AND TREATMENT
Urinalysis, intravenous pyelography and ultrasound are used diagnostically. Treatment is related to the underlying cause. Obstruction must be relieved. Antibiotics may be given, with prolonged antibiotic therapy for recurrent infection.
Childhood urinary tract infections
Childhood urinary tract infections are often seen in primary care settings and can cause significant longer-term morbidity f not treated. Children younger than 2 years often have few, nonspecific signs of infection, including fever, irritability, poor feeding, failure to thrive and diarrhoea. Obtaining a proper urine sample and culture is vital because true infections require radiographical studies. Antibiotic prophylaxis is promoted because of the link between vesicoureteral reflux, recurrent UTIs, and renal scarring and hypertension. Prophylactic antibiotics may be required until children are 3 or 4 years old, especially when there is risk of damage from reflux. Surgical management is sought only when medical management has failed and there are recurrent infections and pyelonephritis or poor renal growth.
Source: Chang SL, Shortliffe LD. Pediatric urinary tract infections. Pediatr Clin North Am 2006; 53(3):379–400; Zorc JJ, Kiddoo DA, Shaw KN. Diagnosis and management of pediatric urinary tract infections. Clin Microbial Rev 2005; 18(2):417–422; Zorc JJ et al. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics 2005; 116(3):644–648.
UTI in the paediatric population
Urinary tract infections are rare in newborns and when they do occur they are usually caused by bacteria from the bloodstream that have settled in the urinary tract. Urinary tract infections in children are most common in 7- to 11-year-old girls (about 8%) as a result of perineal bacteria, especially E. coli, ascending the urethra.24,25 Individual susceptibility, bacterial virulence and the host’s anatomy (presence of reflux, obstruction, stasis or stones) affect the severity of the disease. An abnormal urinary tract is particularly susceptible to infection.26 As well as considering these structural abnormalities, these young girls and their parents should be educated about appropriate toileting habits, to ensure that bacteria from the anus are not wiped towards the urethral opening (i.e. the need to wipe from front to back).
Cystitis, or infection of the bladder, results in mucosal inflammation and congestion. This causes detrusor muscle hyperactivity and a resulting decrease in bladder capacity. It may also cause distortion of the ureterovesical junction leading to transient reflux of infected urine up the ureters, causing acute or chronic pyelonephritis.27
Differentiating whether an infection is in the bladder or the kidneys is difficult based on symptoms alone. Infants may be asymptomatic or develop fever, lethargy, vomiting, diarrhoea or jaundice. Children may present with fever of undetermined origin, frequency, urgency, dysuria, enuresis (see later in the chapter) or incontinence in a previously dry child, abdominal pain and sometimes haematuria. Acute pyelonephritis usually causes chills, fever and flank or abdominal pain, along with enlarged kidney(s) caused by inflammatory oedema. Chronic pyelonephritis may be asymptomatic.
Diagnosis of UTI is by urine culture and urinalysis. A positive urinalysis result requires urine culture. Diagnostic imaging may be necessary to rule out obstructions, renal scarring or functional abnormalities.28
With treatment, UTI symptoms are usually relieved in 1–2 days and the urine becomes sterile. Antibiotics sensitive to the microorganism are required in some cases. If there is no improvement in 2 days, the child should be re-evaluated.
GLOMERULAR DISORDERS
The onset of glomerular disease may be sudden or insidious. Damage to the glomerulus is the result of inflammatory processes initiated by immune responses, metabolic disorders or circulatory disturbances. Most glomerular diseases are the result of immune dysregulation mediated by type II (tissue-specific) or type III (immune complex-mediated) hypersensitivity reactions (see Chapter 15). Damage to the glomerulus results in changes in the GFR and capillary wall structure, with proteinuria and haematuria (protein or blood in the urine, respectively).29 Severe glomerular disease is usually associated with diffuse lesions and may cause oliguria (low urine output), hypertension and chronic kidney disease. Focal lesions tend to produce less severe clinical symptoms.
Glomerular damage reduces glomerular membrane surface area, glomerular capillary blood flow and driving hydrostatic pressure (see Chapter 28). Injury to the glomeruli causes various signs and symptoms as a result of changes in capillary wall structure and the GFR. Proteinuria is caused by increased permeability of glomerular capillaries. Haematuria results from increased glomerular permeability or bleeding along the nephron, which results in red blood cells in the urine.
A reduced GFR during glomerular disease is evidenced by elevated plasma urea and creatinine concentration (see Chapter 28). Oedema, caused by excessive sodium and water retention, may require the use of diuretics or dialysis to reduce the excess volume of fluid due to inadequate urine output. The volume expansion that accompanies salt and water retention leads to hypertension. During the first few weeks, the major life-threatening problems are acute kidney injury with fluid, electrolyte and acid–base imbalances; and acute hypertension that may cause hypertensive encephalopathy; circulatory failure; and pulmonary oedema.
Glomerulonephritis
Glomerulonephritis is an inflammation of the glomerulus. The inflammation can be caused by numerous factors, including immunological abnormalities, ischaemia, free radicals, drugs, toxins, vascular disorders and systemic diseases such as diabetes mellitus and systemic lupus erythematosus. Immunological alterations are the most common cause of glomerular injury (see Figure 30-7). Glomerular disease is the most common cause of chronic and end-stage kidney disease.30
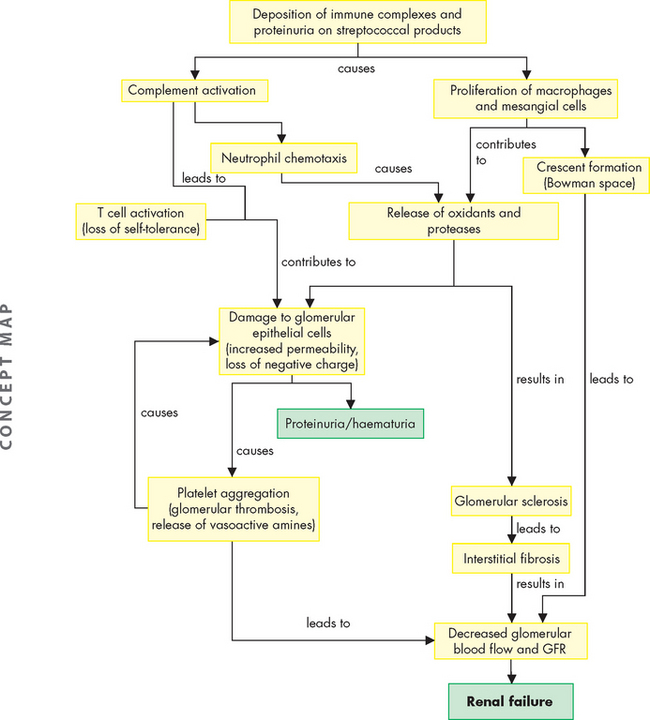
FIGURE 30-7 Mechanisms of glomerular injury.
Source: McCance KL, Huether SE. Pathophysiology: the biologic basis for disease in adults and children. 6th edn. St Louis: Mosby; 2010.
Types of glomerulonephritis
The classification of glomerulonephritis can be described according to cause, pathological lesions, disease progression (acute, rapidly progressive, chronic) or clinical presentation. In nearly all types of glomerulonephritis, the epithelial or podocyte layer of the glomerular capillary membrane is disturbed with changes in membrane permeability. This often results in an inadequate GFR and so individuals with glomerulonephritis are at an increased risk of acute kidney injury.
Acute glomerulonephritis
Acute glomerulonephritis is often associated with a streptococcal infection (acute poststreptococcal glomerulonephritis). The disease begins abruptly and usually occurs 7–10 days after a streptococcal infection of the skin (impetigo: see Chapter 19) or the throat (pharyngitis) and commonly affects children (see below). Glomerular injury is immune-mediated with streptococcal antigen-antibody complexes depositing in the glomerular basement membrane. The antigen-antibody complex activates complement and the release of inflammatory mediators that damage endothelial and epithelial cells lying on the basement membrane.31
Symptoms usually occur 10–21 days after infection and include haematuria, red blood cell casts, proteinuria, a decreased GFR, oliguria, hypertension, oedema around the eyes or feet and ankles and, occasionally, ascites or pleural effusions. There are immune complex deposits in the glomerulus, which thickens the glomerular membrane and contributes to the decreased GFR. More severe renal disease is observed after a prolonged infection and before antibiotic therapy. Most individuals, especially children, recover without significant loss of renal function or recurrence of the disease.
Chronic glomerulonephritis
Chronic glomerulonephritis encompasses several glomerular diseases with a progressive course leading to chronic kidney disease. There may be no history of renal disease before the diagnosis. Hypercholesterolaemia and proteinuria have been associated with progressive glomerular and tubular injury. The proposed mechanism is related to glomerulosclerosis (scarring or hardening of the glomerulus) and interstitial injury.32 The primary cause may be difficult to establish because advanced pathological changes may obscure specific disease characteristics. Diabetes mellitus and systemic lupus erythematosus are secondary causes of chronic glomerular injury.33
PATHOPHYSIOLOGY
Two types of immune mechanisms commonly contribute to glomerular injury: (1) deposition of circulating soluble antigen-antibody complexes, often with complement components; and (2) formation of antibodies specific for the glomerular basement membrane. The severity of glomerular damage is related to the size, number, location (focal or diffuse), duration of exposure and type of antigen-antibody complexes.
Activation of biochemical mediators of inflammation (complement, leucocytes, fibrin) begins after the antibody or antigen-antibody complexes have localised in the glomerular capillary wall. Complement is deposited with the antibodies and its activation can cause cell lysis or serve as a chemotactic stimulus for attraction of leucocytes.34 The phagocytes further the inflammatory reaction by releasing lysosomal enzymes, reactive oxygen species and cytokines, which damage glomerular cell walls and contribute to proliferation of the extracellular matrix causing a decrease in glomerular blood flow and the GFR.35
The inflammatory processes also alter membrane permeability enhancing filtration of proteins into the filtrate. Membrane damage can lead to platelet aggregation and degranulation, whereby platelets release substances that increase glomerular permeability, permitting the passage of larger protein molecules or red blood cells into the urine and causing proteinuria or haematuria. The coagulation system also may be activated and lead to fibrin deposition in the glomerular space, contributing to crescent formation (the deposition of substances in the glomerular space forming the shape of a crescent moon; see Figure 30-8). Renal blood flow decreases and glomerular filtration is reduced.
CLINICAL MANIFESTATIONS
Injury to the glomeruli causes various signs and symptoms consequent to changes in glomerular capillary wall structure and the GFR. Two major changes distinctive of more severe glomerulonephritis are: (1) haematuria with red blood cell casts; and (2) proteinuria exceeding 3–5 g/day with albumin as the major protein.
Several disorders may produce haematuria, because bleeding can occur anywhere along the urinary tract. The characteristics of haematuria from red blood cells escaping through the glomerular membrane include smoky brown–tinged urine, red blood cell casts and an accompanying proteinuria. Bleeding from sites lower in the urinary tract may produce pink- or red-coloured urine.
After 10–20 years, chronic kidney disease usually begins to develop, followed by nephrotic syndrome (see below) and an accelerated progression to end-stage kidney disease. Symptom patterns vary depending on the underlying cause. Corticosteroids usually do not change the course of chronic glomerular disease and dialysis or kidney transplantation ultimately may be needed.
EVALUATION AND TREATMENT
The diagnosis of glomerular disease is confirmed by the progressive development of clinical manifestations and laboratory findings of abnormal urinalysis with proteinuria, red blood cells, white blood cells and casts. Microscopic evaluation from renal biopsy provides a specific determination of renal injury and the type of pathological condition.
Management of glomerulonephritis is related to treating the primary disease, preventing or minimising immune responses and correcting accompanying problems, such as oedema, hypertension and hyperlipidaemia. Specific treatment regimens are necessary for particular types of glomerulonephritis. Antibiotic therapy is essential for the management of underlying infections that may be contributing to ongoing antigen-antibody responses. Corticosteroids decrease antibody synthesis and suppress inflammatory responses. Cytotoxic agents (for instance, cyclophosphamide) may be used to suppress the immune response.
Nephrotic syndrome
Nephrotic syndrome is the excretion of 3.5 g or more of protein in the urine per day and is characteristic of glomerular injury.
Secondary forms of nephrotic syndrome occur in systemic diseases, including diabetes mellitus and systemic lupus erythematosus. When present as a secondary complication with renal diseases, nephrotic syndrome often signifies a more serious prognosis.
PATHOPHYSIOLOGY
Disturbances in the glomerular basement membrane lead to increased permeability to protein. Loss of plasma proteins, particularly albumin, occurs across the injured glomerular filtration membrane.36 Hypoalbuminaemia (low levels of albumin in the blood) results from urinary loss of albumin combined with a diminished synthesis of replacement albumin by the liver. Albumin is lost in the greatest quantity because of its high plasma concentration and low molecular weight (relatively small size). Decreased dietary intake of protein from anorexia or malnutrition or accompanying liver disease may also contribute to lower levels of plasma albumin. Loss of albumin stimulates lipoprotein synthesis by the liver and hyperlipidaemia. Loss of immunoglobulins may increase susceptibility to infections.
CLINICAL MANIFESTATIONS
Many clinical manifestations of nephrotic syndrome are related to loss of serum proteins. They include oedema, hyperlipidaemia and vitamin D deficiency.36,37 Vitamin D deficiency is related to loss of serum transport proteins and decreased vitamin D activation by the kidneys. Alterations in coagulation factors cause hypercoagulability and may lead to thromboembolic events.38
EVALUATION AND TREATMENT
Nephrotic syndrome is diagnosed when the protein level in a 24-hour urine collection is greater than 3.5 grams (normal range < 0.3 grams per day). Serum albumin decreases (to less than 30 g/L; normal range 36–47 g/L) and serum cholesterol, phospholipids and triglycerides increase. Fatty substances may be present in the urine. The specific pathological condition is identified by renal biopsy.
Nephrotic syndrome is commonly treated with a normal-protein (i.e. 1 g/kg body weight/day), low-fat diet, salt restriction, diuretics, immunosuppression and heparin. When diuretics are used, care must be taken to observe for hypovolaemia (low blood volume) and hypokalaemia (low blood potassium) or, alternatively, potassium toxicity may occur in the presence of acute kidney injury. Aldactone may be combined with loop diuretics to suppress aldosterone activity to conserve potassium.
Paediatric glomerular disorders
Glomerular disorders in children can present in different ways including glomerulonephritis, immunoglobin A (IgA) nephropathy and the more rare nephrotic syndrome and haemolytic uraemic syndrome. The disease can be acute or chronic but rarely leads to acute kidney injury (as it does in adults).
Glomerulonephritis
Chronic glomerulonephritis accounts for about 30–50% of chronic kidney disease in children and is the causative factor for most school-age and teenage children who require dialysis and kidney transplantation.
Poststreptococcal glomerulonephritis
Acute poststreptococcal glomerulonephritis is one of the most common immune complex-mediated renal diseases in children. The sudden onset of gross haematuria, oedema, hypertension and acute kidney injury occurs after a throat or skin infection with certain strains of group A β-haemolytic streptococci.
Pharyngeal infections are most common during cold weather. Skin infections from impetigo, infected insect bites or varicella sores usually occur during warm weather.
Glomerulonephritis develops with the deposition of antigen-antibody complexes in the glomerulus. The immune injury results in activation of complement and inflammation, which increases glomerular capillary permeability and loss of the negative charge. These changes lead to haematuria and proteinuria.
Symptoms of varying severity develop about 1–3 weeks after the streptococcal infection. As many as half the children affected are asymptomatic and show only microscopic haematuria with otherwise normal renal function. The most severely affected develop acute kidney injury with oliguria.
The onset of symptoms in the child is abrupt and consists of flank or mid-abdominal pain, irritability, general malaise and fever. Acute hypertension may cause headache, vomiting, somnolence and other central nervous system manifestations, including seizures. Cardiovascular symptoms are related to circulatory overload and are compounded by hypertension. These include dyspnoea, tachypnoea and an enlarged, tender liver.
The disease usually runs its course in 1 month, but urine abnormalities may be found for up to 1 year after the onset. Prolonged proteinuria and an abnormal GFR indicate an unfavourable prognosis. More than 95% recover completely. Treatment is symptom specific.
Immunoglobulin A nephropathy
Immunoglobulin A (IgA) nephropathy is the most common form of glomerulonephritis worldwide and occurs more often in males. It is characterised by deposition mainly of immunoglobulin IgA and some IgM and complement proteins in glomerular capillaries. No systemic immunological disease is evident.39 Deposits of IgA cause immune injury to the glomerulus that is usually reversible.
Children with the disease have recurrent gross haematuria, often after a respiratory infection. Most continue to have microscopic haematuria between the attacks of gross haematuria and have a mild proteinuria as well. Treatment is supportive because kidney damage is generally insignificant. However, approximately 20% of affected children develop the progressive form of the disease, with hypertension and decreasing renal function. These children eventually require dialysis and transplantation.
CHRONIC KIDNEY DISEASE
Chronic kidney disease is a complex disease that can be caused by a variety of different pathophysiological conditions. It is a progressive loss of renal function over a period of months or years and develops as a complication of systemic diseases (e.g. hypertension or diabetes mellitus) or as a complication of many renal diseases (i.e. chronic glomerulonephritis, chronic pyelonephritis or chronic obstruction). Chronic kidney disease decreases the GFR and tubular functions with changes manifest throughout all organ systems.40
Chronic kidney disease is a significant problem in Australia: approximately 1 in 3 adults have an increased risk of developing the disease.41 The increased risk is linked in a large part to lifestyle diseases such as obesity and type II diabetes mellitus, and whether the person smokes, has hypertension and a family history, is older than 50 years of age and is Indigenous. Unfortunately, 1 in 7 Australians over the age of 25 years has at least one clinical sign of chronic kidney disease, such as proteinuria or increased creatinine.4,42 However, most people do not know that they have the disease because the kidneys can compensate adequately until a significant amount of nephron damage has occurred. Due to the strong connection between chronic kidney disease and cardiovascular disease, most individuals will die of cardiovascular causes rather than end-stage kidney disease. Nonetheless, chronic kidney disease causes a large number of deaths each year and is the seventh most common cause of death in Australia.4 As with most other diseases, Indigenous Australians experience greater rates of death and disability than non-Indigenous Australians.42
We begin our exploration of chronic kidney disease by examining the different stages of the disease.
Stages of chronic kidney disease
Stages of chronic kidney disease can generally be described according to the level of GFR. There are progressive reductions in the GFR as the disease progresses and, if not managed adequately, this may cause end-stage kidney disease in which less than 10% of renal function remains and dialysis or kidney transplant is required to sustain life. Table 30-3 summarises the progressive stages of chronic kidney disease as a measure of declining GFR. Azotaemia is increased serum urea levels and, often, increased creatinine levels as well. Uraemia is a syndrome of chronic kidney disease and includes elevated blood urea and creatinine levels accompanied by fatigue, anorexia, nausea, vomiting, pruritus and neurological changes. Uraemia represents the numerous consequences related to chronic kidney disease, including retention of toxic wastes, deficiency states and electrolyte disorders. Both azotaemia and uraemia indicate an accumulation of nitrogenous waste products in the blood.
Table 30-3 CLASSIFICATION OF CHRONIC KIDNEY DISEASE
| STAGE | DESCRIPTION | TREATMENT |
|---|---|---|
| 1 | Normal kidney function — normal or relatively high GFR (> 90 mL/min) | Further investigation for chronic kidney disease may be indicated in those at increased risk: Cardiovascular risk reduction: |
| 2 | Mild kidney damage — mild reduction in GFR (60–89 mL/min) | |
| 3 | Moderate kidney damage — moderate reduction in GFR (30–59 mL/min) | As above, plus: |
| 4 | Severe kidney damage — severe reduction in GFR (15–29 mL/min) | As above, plus referral to a renal specialist is usually indicated for physical and psychosocial preparation for renal replacement therapy (dialysis, transplantation) or conservative medical management. |
| 5 | End-stage kidney disease — established kidney failure (< 15 mL/min) | |
| Normal GFR is approximately 125 mL/min. | ||
Source: Kidney Health Australia. National chronic kidney disease strategy. Melbourne; 2006; Kidney Health Australia. Chronic kidney disease management in General Practice. Melbourne; 2007.
PATHOPHYSIOLOGY
The kidneys have a remarkable ability to adapt to loss of nephron mass.43 Symptomatic changes resulting from increased creatinine, urea, potassium and alterations in salt and water balance usually do not become apparent until renal function declines to less than 25% of normal, at which time adaptive renal reserves have been exhausted.
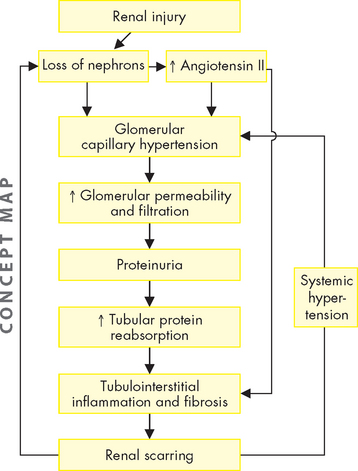
The intact nephron hypothesis proposes that loss of nephron mass with progressive kidney damage causes the surviving nephrons to sustain normal kidney function — this means that the remaining nephrons have an increased productivity to compensate for those that no longer contribute to renal function. These nephrons are capable of a compensatory hypertrophy and expansion or hyperfunction in their rates of filtration, reabsorption and secretion and can maintain a constant rate of excretion in the presence of an overall declining GFR. The intact nephron hypothesis explains adaptive changes in solute and water regulation that occur with advancing chronic kidney disease. Although the urine of an individual with chronic kidney disease may contain abnormal amounts of protein and red and white blood cells or casts, the major end products of excretion are similar to those of normally functioning kidneys until the advanced stages of end-stage kidney disease (see below), when there is a significant reduction of functioning nephrons.44,45 The continued loss of functioning nephrons and the adaptive hyperfiltration (more than usual amounts of substances being filtered at the glomerulus) probably results in further nephron injury and ultimately results in uraemia and end-stage kidney disease.45 Progression of chronic kidney disease is thought to be associated with common pathophysiological processes regardless of the initial disease (see Figure 30-9).46
The factors that contribute to the pathogenesis of chronic kidney disease are complex and involve the interaction of many cells, cytokines and structural alterations. Two factors that have consistently been recognised to advance renal disease are proteinuria and angiotensin II.47–50 48 49 50 Glomerular hyperfiltration and increased glomerular capillary permeability lead to proteinuria. Proteinuria contributes to tubule injury by accumulating in the interstitial spaces and activating complement proteins and other mediators and cells, such as macrophages, that promote inflammation and progressive fibrosis. Angiotensin II activity is elevated with progressive nephron injury. Angiotensin II promotes glomerular hypertension and hyperfiltration caused by efferent arteriolar vasoconstriction and also promotes systemic hypertension. The chronically high glomerular pressure increases glomerular capillary permeability, contributing to proteinuria. Angiotensin II also may promote the activity of inflammatory cells and growth factors that participate in the scarring of the tubules.
CLINICAL MANIFESTATIONS
The clinical manifestations of chronic kidney disease are often described using the term uraemia. Uraemia refers to the accumulation of nitrogenous wastes from protein metabolism as well as the systemic symptoms caused by a decline in renal function with the accumulation of toxins in the plasma. Sources of toxins include end products of protein metabolism, alterations in electrolytes, metabolic acidosis and intestinal absorption of toxins produced by gut bacteria. Uraemia represents a pro-inflammatory state with many systemic effects.51 Generally, the symptoms include hypertension, anorexia, nausea, vomiting, diarrhoea, weight loss, pruritus (itching), oedema, anaemia and neurological and skeletal changes.
Creatinine and urea clearance
Creatinine is constantly released from muscle and excreted primarily by glomerular filtration. In chronic kidney disease, as the GFR declines, the plasma creatinine level increases by a reciprocal amount to maintain a constant rate of excretion. As the GFR continues to decline, plasma creatinine concentration increases. The clearance of urea follows a similar pattern, but urea is both filtered and reabsorbed and varies with the state of hydration. However, as the GFR decreases, plasma urea concentration increases.
Fluid and electrolyte balance
Fluid and electrolyte balance is significantly disturbed with chronic kidney disease, especially as the disease progresses. When the GFR decreases to 25%, there is an obligatory loss of up to 40 mmol of sodium per day with osmotic loss of water. Dietary intake must be maintained to prevent sodium deficits and volume depletion. As the GFR continues to decline, there is also loss of tubular function to dilute and concentrate the urine and urine-specific gravity becomes fixed at about 1.010 (the specific gravity of distilled water is 1.000). Ultimately, the kidney loses its ability to regulate sodium and water balance. Both sodium and water are retained, contributing to oedema and hypertension.
With the onset of oliguria, as end-stage kidney disease occurs, total body potassium can increase to life-threatening levels and must be controlled by dialysis.
Musculoskeletal system
Bone and skeletal changes develop with alterations in calcium and phosphate metabolism. These changes begin when the GFR decreases to 25% or less. Hypocalcaemia is accelerated by impaired renal synthesis of vitamin D with decreased intestinal absorption of calcium. Renal phosphate excretion also decreases and the increased serum phosphate binds calcium, further contributing to hypocalcaemia.
Hyperlipidaemia is common among individuals with chronic kidney disease. There is a high ratio of low-density lipoprotein (LDL) to high-density lipoprotein (HDL) with accelerated atherosclerosis and vascular calcification. Uraemia causes a deficiency in lipoprotein lipase and decreased hepatic triglyceride lipase. Decreased lipolytic activity results in a reduction in HDL. Apolipoprotein B is also elevated, thereby accelerating atherogenesis.52
Cardiovascular system
Cardiovascular disease is a major cause of morbidity and mortality in chronic kidney disease. Pro-inflammatory mediators, oxidative stress and metabolic derangements are significant contributors.53 Hypertension is the result of excess sodium and fluid volume. Elevated renin also stimulates the secretion of aldosterone, increasing sodium reabsorption. Hyperlipidaemia promotes atheromatous plaque formation. Endothelial cell dysfunction and calcium deposits lead to a loss of vessel elasticity and vascular calcification. The resulting vascular disease increases the risk for coronary heart disease, left ventricular hypertrophy, heart failure, stroke and peripheral vascular disease in individuals with uraemia. Declining erythropoietin production causes anaemia, thereby increasing demands for cardiac output and adding to cardiac workload.
Immune system
Immune system dysregulation with immune suppression, deficient response to vaccination and increased risk for infection develops with chronic kidney disease. Chemotaxis, phagocytosis, antibody production and cell-mediated immune responses are suppressed. Malnutrition, metabolic acidosis and hyperglycaemia may amplify immunosuppression.
Neurological system
Neurological symptoms are common and progressive with chronic kidney disease. Symptoms may include headache, drowsiness, sleep disorders, impaired concentration, memory loss and impaired judgement. In advanced stages of end-stage kidney disease, symptoms may progress to seizures and coma.
Digestive system
Gastrointestinal complications are common in individuals with chronic kidney disease. Nonspecific symptoms include anorexia, nausea and vomiting. Uraemia can cause bad breath caused by the breakdown of urea by salivary enzymes. Malnutrition in these patients is common.
Insulin resistance is common in uraemia and as chronic kidney disease progresses the ability of the kidney to degrade insulin is reduced and the half-life of insulin is prolonged (which means that insulin’s effects last for longer than usual). Individuals with diabetes mellitus and chronic kidney disease need to carefully manage their insulin dosages. Low-protein diets and renal replacement therapy improve insulin sensitivity.54
Integumentary system
Skin changes are associated with other complications that develop with chronic kidney disease. Anaemia can cause pallor and bleeding into the skin and results in haematomas and ecchymosis (bruises). The skin can take on a sallow colour.
EVALUATION AND TREATMENT
Evaluation of chronic kidney disease is based on the history and presenting signs and symptoms. Elevated serum creatinine and serum urea concentrations are consistent with chronic kidney disease. Ultrasound, CT scan or plain X-ray films will show small kidney size. Renal biopsy confirms the diagnosis.
Management of chronic kidney disease is multifactorial and involves several healthcare professionals. The mainstay of management (if the kidney disease does not necessitate transplant or dialysis) involves dietary control, including moderate protein restriction, sodium and fluid evaluation, potassium restriction, adequate kilojoule intake, management of dyslipidaemias and erythropoietin as needed. Angiotensin-converting enzyme (ACE) inhibitors or receptor blockers are often used to provide renal protection and to control systemic hypertension.55 End-stage kidney disease related to diabetic nephropathy can often be significantly reduced with control of hyperglycaemia by intense insulin therapy (mainly dialysis).56 However, when end-stage kidney disease is established, dialysis and kidney transplantation are required to sustain life.57 Figure 30-10 and Table 30-3 provide an overview of the management of chronic kidney disease.
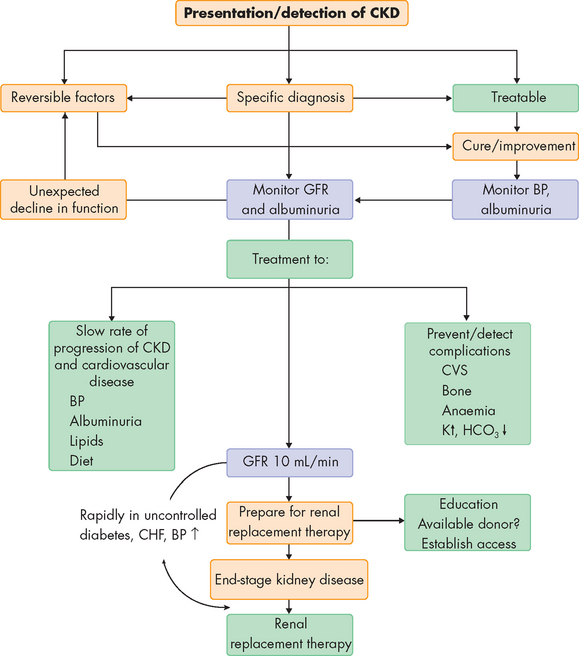
FIGURE 30-10 Management of chronic kidney disease.
BP = blood pressure; CHF = congestive heart failure; CVS = cardiovascular system; CKD = chronic kidney disease; GFR = glomerular filtration rate.
Source: Goldman L. Cecil medicine. 23rd edn. Philadelphia: Saunders; 2007.
In Australia in 2007, approximately 16,750 people received renal replacement therapy (mainly dialysis) for end-stage kidney disease, and about 7000 had a functioning kidney transplant, with more than 9000 receiving different forms of dialysis. Each day, 5 Australians commence dialysis or transplantation to stay alive.4,58,59 Approximately 11% of all dialysis is performed in the home.60
ACUTE KIDNEY INJURY
In contrast to chronic kidney disease, acute kidney injury refers to a sudden decline in kidney function occurring over hours to days that inhibits the ability to regulate fluid, electrolyte and acid-base balance.61 Previously, the term acute renal failure was used; however, there were more than 30 different definitions in the literature and this made the finding and diagnosis of acute renal failure difficult to determine, and inconsistent.62 Therefore, the Acute Dialysis Quality Initiative and the Acute Kidney Injury Network collaborated to provide a consensus on the definition, proposing that acute kidney injury more accurately reflects the spectrum of changes that can occur prior to and during failure.62,63 Furthermore, a classification system was devised, which outlined the progressive kidney alterations that lead to failure. This is termed the RIFLE staging system:
This system more accurately describes the range of stages that may occur (see Table 30-4).
Table 30-4 RIFLE CLASSIFICATION FOR ACUTE KIDNEY INJURY
| CATEGORY | SERUM CREATININE/GFR | URINE OUTPUT |
|---|---|---|
| Risk | Increased creatinine × 1.5 or decrease in GFR > 25% | < 0.5 mL/kg/hr ≥ 6 hours |
| Injury | Increased creatinine × 2 or decrease GFR > 50% | < 0.5 mL/kg/hr ≥ 12 hours |
| Failure | Increased creatinine × 3 or decrease in GFR > 75% | < 0.3 mL/kg/hr ≥ 24 hours or anuria ≥ 12 hours |
| Loss | Complete loss of kidney function > 4 weeks | |
| End-stage | End-stage kidney disease > 3 months |
Acute kidney injury occurs rapidly with a reduction in the GFR and elevation of blood urea and plasma creatinine. It is usually associated with oliguria (urine output of less than 0.5 mL/kg/hr), although in some cases urine output may be normal or increased. Fluid is still filtered at the glomerulus but there is an alteration in tubular secretion or reabsorption. Most types of acute kidney injury are reversible if diagnosed and treated early. Acute kidney injury can be classified as prerenal, intrarenal or postrenal (obstructive) (see Figure 30-11 and Table 30-5).64
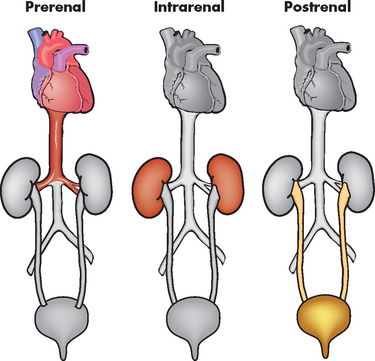
FIGURE 30-11 Prerenal, intrarenal and postrenal acute kidney injury.
Source: Brown D, Edwards H. Medical–surgical nursing. Sydney: Mosby; 2007.
Table 30-5 CLASSIFICATION OF ACUTE KIDNEY INJURY
| LOCATION | POSSIBLE CAUSES |
|---|---|
| Prerenal |
• Haemorrhage leading to significant blood loss (trauma, gastrointestinal bleeding, complications of childbirth)
|
| Intrarenal | |
| Postrenal |
PATHOPHYSIOLOGY
Prerenal acute kidney injury is the most common cause of acute kidney injury and is caused by impaired renal blood flow, so the underlying cause is actually prior to the kidney (in terms of direction of blood flow). The GFR declines because of the decrease in filtration pressure. Poor perfusion can result from renal vasoconstriction, hypotension, hypovolaemia, haemorrhage or inadequate cardiac output. Acute prerenal kidney injury may occur when chronic kidney disease exists if a sudden stress is imposed on already marginally functioning kidneys. Failure to restore blood volume or blood pressure and oxygen delivery causes cell injury.
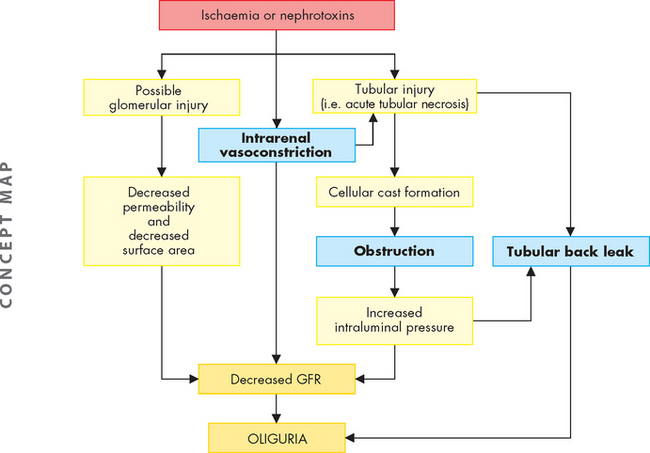
Intrarenal acute kidney injury usually results from acute tubular necrosis. Acute tubular necrosis caused by ischaemia occurs most often after surgery (40–50% of cases) but is also associated with sepsis, obstetric complications, severe trauma including severe burns, and nephrotoxins (radiocontrast media and some antibiotics). Hypotension associated with hypovolaemia produces ischaemia, generating toxic oxygen-free radicals that cause cell swelling, injury and necrosis.65 Dehydration, advanced age, concurrent chronic kidney disease and diabetes mellitus tend to enhance nephrotoxicity from either antibiotics or radiocontrast media. Aminoglycosides (neomycin, gentamicin, tobramycin) and other antibiotics tend to accumulate in the renal cortex and may not cause kidney injury until after treatment is complete. Necrosis caused by nephrotoxins is usually uniform and limited to the proximal tubules. Ischaemic necrosis tends to be patchy and may be distributed along any part of the nephron.
Three pathophysiological explanations have been proposed to account for the oliguria of acute tubular necrosis.64,66 All three mechanisms probably contribute to oliguria in varying combinations and degrees throughout the course of the disease (see Figure 30-12). These theories67 are as follows:
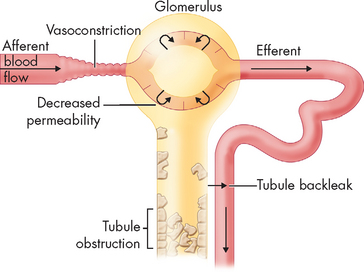
FIGURE 30-13 The mechanism of tubular back-leak leading to acute kidney injury.
Source: Goldman L. Cecil medicine. 23rd edn. Philadelphia: Saunders; 2007.
Postrenal acute kidney injury usually occurs with urinary tract obstruction that affects the kidneys bilaterally (e.g. bladder outlet obstruction, prostatic hypertrophy, bilateral ureteral obstruction). A pattern of several hours of anuria (no urine output) with flank pain followed by polyuria (increased urine output) is a characteristic finding. This type of acute kidney injury can occur after diagnostic catheterisation of the ureters, a procedure that may cause oedema of the tubular lumen.
CLINICAL MANIFESTATIONS
The clinical progression of acute kidney injury with recovery of renal function occurs in three phases: oliguria, diuresis and recovery. Oliguria begins within 1 day after a hypotensive event and lasts 1–3 weeks, but it may regress in several hours or extend for several weeks, depending on the duration of ischaemia or severity of injury or obstruction. Acute kidney injury can present with non-oliguria, particularly with intrarenal acute kidney injury associated with nephrotoxins. The urine output may vary in volume, but the urea and creatinine concentrations increase.
As renal function improves, increase in urine volume (diuresis) is progressive. During the early diuretic phase, the tubules are still damaged and recovering their function. Fluid and electrolyte balance must be carefully monitored and excessive urinary losses replaced.
Serial measurements of plasma creatinine provide an index of renal function during the recovery phase. Return to normal status may take from 3 to 12 months although some individuals do not have full recovery of a normal GFR or tubular function.
EVALUATION AND TREATMENT
The diagnosis of acute kidney injury is related to the cause of the disease. A history of surgery, trauma or cardiovascular disorders is common and exposure to nephrotoxins must be considered. Obstructive uropathies (i.e. an enlarged prostate) also need consideration. The diagnostic challenge is to differentiate prerenal acute renal failure from intrarenal acute kidney injury and some evidence is available from urinalysis, plasma creatinine and urea. Prevention of acute renal failure is a major treatment factor and involves maintenance of fluid volume before and after surgery or diagnostic procedures and use of vasoactive drugs (which affect the diameter of the blood vessels) or diuretics.68
The primary goal of therapy is to maintain the individual’s life until renal function has been recovered. Management principles directly related to physiological alterations generally include:
Renal replacement therapy in the form of haemodialysis may be indicated. The mortality rate is greater than 30%69 and is associated with the underlying cause of acute kidney injury.
STRUCTURAL ABNORMALITIES
Variations from the normal anatomical structure of the urinary tract occur in 10–15% of the total population. These abnormalities range from minor, non-pathological or easily correctable anomalies to those that are incompatible with life. For example, the kidneys may fail to ascend from the pelvis to the abdomen, causing ectopic kidneys — which usually function normally. The kidneys may also fuse as they ascend, causing a single, U-shaped horseshoe kidney. Approximately one-third of individuals with horseshoe kidneys are asymptomatic and the most common problems are infection, stone formation and, rarely, renal malignancies.70,71 Collectively, structural anomalies of the renal system account for approximately 45% of cases of kidney injury leading to failure in children and many are linked to gene defects.72,73
Hypospadias
Hypospadias is a congenital condition in which the urethral meatus is located on the ventral side or undersurface of the penis. The meatus can be located anywhere on the glans, on the penile shaft, at the base of the penis, the penoscrotal junction or the perineum (see Figure 30-14). This is the most common anomaly of the penis; it occurs in about 1 in 230 infant boys in Australia74 and the incidence appears to be increasing.75 The cause of this condition is multifactorial and includes maternal intake of progestin androgen synthesis, advanced maternal age and environmental factors.75
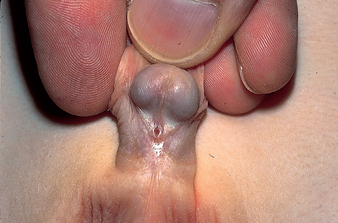
Source: Courtesy H. Gil Rushton, MD, Children’s National Medical Center, Washington, DC. From Hockenberry MJ, Wilson D. Wong’s nursing care of infants and children. 8th edn. St Louis: Mosby; 2007.
The goals for corrective surgery on the child with hypospadias are: (1) a straight penis when erect to facilitate intercourse as an adult; (2) a uniform urethra of adequate calibre to prevent spraying during urination; (3) a cosmetic appearance satisfactory to the individual; and (4) repair completed in as few procedures as possible. Surgery is most effective, psychologically as well as physically, when performed between 4 and 8 months of age.76
Polycystic kidneys
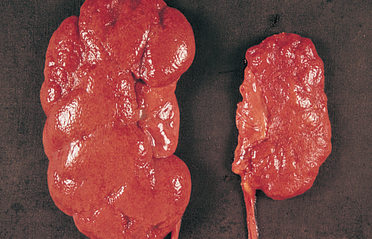
Polycystic kidney disease is an autosomal dominant (PDK1 or PDK2 gene) and autosomal recessive inherited disorder. It occurs in about 1 in 1000 live births.77 The affected kidney has large fluid-filled cysts that include the tubules and collecting ducts (see Figure 30-15). Other organs also may have cysts, including the liver, pancreas and ovaries. Hypertension, heart valve defects and cerebral and aortic aneurysms may develop. Symptoms may not develop until adulthood. Cyst formation is related to tubular cell proliferation, basement membrane remodelling and fluid accumulation with obstruction.
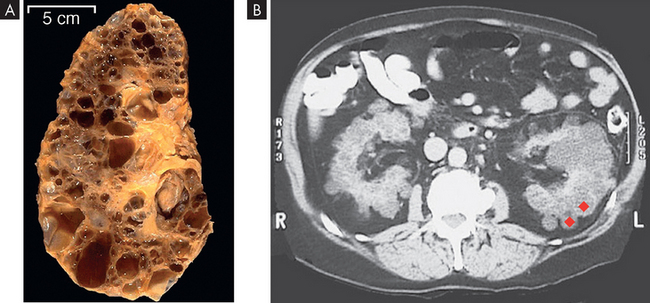
FIGURE 30-15 Polycystic kidneys.
A This kidney was substantially enlarged and weighed 3 kg with large cysts. B The cysts (red diamond shapes on CT scan) are not present at birth but develop slowly over time. Chronic kidney disease occurs later in life, approximately at 50 years of age.
Source: Klatt EC. Robbins & Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
Renal agenesis
Renal agenesis (the absence of one or both kidneys) may be unilateral or bilateral and randomly occurring or clearly hereditary. The kidney is usually polycystic and dysplastic (abnormal cells). The condition may occur as an isolated entity or as a problem associated with other urological disorders.78
Unilateral renal agenesis occurs in approximately 1 in 1000 live births. Males are more often affected and it is usually the left kidney that is absent. The single remaining kidney is often completely normal so that the child can expect a normal, healthy life. By the time the child is several years old, the volume of this kidney may approach twice the normal size. In some instances, however, the single kidney is abnormally formed and associated with abnormalities of its collecting system.
Bladder disorders
Vesicoureteral reflux
Vesicoureteral reflux is the retrograde flow of urine from the bladder into the ureters. Reflux allows infected urine from the bladder to be repeatedly swept up into the kidneys. The reflux perpetuates infection by preventing complete emptying of the bladder and allows the maximal intravesical pressure to be transmitted to the renal calyces and pyramids. The combination of reflux and infection is an important cause of pyelonephritis, especially in children younger than 5 years.
Vesicoureteral reflux occurs more often in girls by a ratio of 10:1. Its incidence is approximately 1 in 1000 children. Siblings of those affected have a 50% chance of having reflux, but children with parents who had childhood reflux have almost a 70% chance of reflux.79 Although reflux is considered abnormal at any age, the shortness of the submucosal segment of the ureter during infancy and childhood renders the anti-reflux mechanism relatively inefficient and delicate. Thus reflux is seen commonly in association with infections during early childhood but rarely in older children and adults.
PATHOPHYSIOLOGY
Primary reflux results from a congenitally abnormal or ectopic insertion of the ureter into the bladder. It develops in association with infection, malformations of the ureterovesical junction (see Figure 30-16), increased intravesical pressures or surgery on the ureterovesical junction.
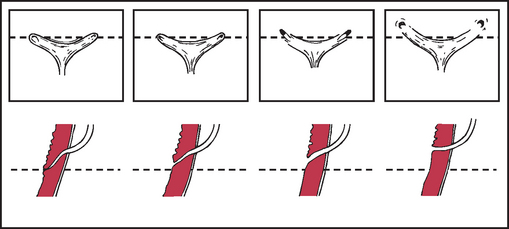
FIGURE 30-16 Normal and abnormal configuration of the ureterovesical junction.
Shown from left to right, progressive lateral displacement of the ureteral orifices and shortening of the intramural tunnels. (Top) Endoscopic appearance. (Bottom) Sagittal view through the intramural ureter.
Source: Behrman R et al (eds). Nelson textbook of pediatrics. 16th edn. Philadelphia: Saunders; 2000.
CLINICAL MANIFESTATIONS
Children with reflux have recurrent urinary tract infections or unexplained fever, poor growth and development, irritability and feeding problems. The family history reveals reflux or urinary tract infections, pain with voiding and signs of urinary obstruction or nephropathy.
EVALUATION AND TREATMENT
In addition to the history of recurrent urinary tract infection and other symptoms, a voiding cystourethrogram is the primary diagnostic procedure. Most children with vesicoureteral reflux respond to non-operative management aimed at prevention and treatment of infection. Spontaneous remission of mild reflux may occur in 30–60% of children younger than 5 years. Recurrent infection requires surgical intervention or endoscopic injection. In cases of severe reflux, early surgical intervention may be indicated to prevent renal scarring.80
Enuresis
Enuresis refers to the involuntary passage of urine by a child who is beyond the age when voluntary bladder control should have been acquired. Bladder control is usually accomplished by most children before the age of 4 or 5 years. However, toilet training is largely determined by cultural beliefs and the practices of parents. In 80% of children, enuresis occurs at night only, in which case it is called nocturnal enuresis. Wetness during the day is called diurnal enuresis.
In primary enuresis, the child has never been continent. In secondary enuresis or acquired enuresis, the child has experienced a period of dryness of at least 3–6 months after toilet training and then becomes incontinent. Secondary enuresis may be diurnal or nocturnal, or a combination of both.
The incidence of enuresis is difficult to determine because it is not a problem that parents readily share with others and because definitions vary according to cultural norms and family practices. Some families start toilet training before 1 year of age and expect continence by the age of 1 to 1½ years, whereas other families do not expect dryness earlier than 5 years. The incidence of enuresis in children older than 5 years ranges from 10% to 20%. Boys represent more cases of enuresis than girls by a ratio of 3:2. Teenage and adult enuresis is rare and usually is a continuation of childhood bed-wetting.
PATHOGENESIS
A combination of factors is likely to be responsible for enuresis.81 Organic causes account for 2–10% of cases and include urinary tract infection; neurological disturbances; congenital defects of the meatus, urethra and bladder neck; and allergies. Disorders that increase the normal output of urine, such as diabetes mellitus and diabetes insipidus, or disorders that impair the concentrating ability of the kidneys, such as chronic kidney disease, must be considered in the evaluation of enuresis.
Genetic factors as a cause of enuresis are likely and the condition shows a familial tendency. Bed-wetting occurs with high frequency among parents, siblings and other near relatives of symptomatic children.
Other problems may be associated with enuresis, such as perinatal anoxia, central nervous system trauma, seizures, developmental delay, urinary tract infection and bladder trauma or surgery. Difficult sleep arousal, prolonged rapid eye movement (REM) sleep intervals and the presence of obstructive sleep apnoea syndrome (see Chapter 25) are also associated with nocturnal enuresis.82 Stressful psychological situations, such as a new sibling, may cause enuresis to develop.
TREATMENT
Therapeutic management of enuresis includes fluid management, diet therapy, drugs (desmopressin, an antidiuretic), treatment of obstructive sleep apnoea and behavioural modification therapy.83,84 The main goals of therapy should be to have the child awaken and get up to use the toilet during the night to preserve self-esteem and to relieve psychological stress.83,84
TUMOURS
Renal tumours
Kidney cancers were diagnosed in almost 2300 Australians in 2005, accounting for 2.3% of all cancers.85 The rate in males was almost double that in females. Approximately 850 people die each year of kidney cancer in Australia, with projections that the rate will increase slightly in the future. Almost one-fifth of kidney cancers can be attributed to smoking and excess alcohol consumption.85
There are a number of different types of kidney tumours. Renal adenomas (benign tumours) are uncommon but are increasing in number. The tumours are encapsulated and are usually located near the cortex of the kidney. Because they can become malignant, they need to be surgically removed. Renal cell carcinoma is the most common renal neoplasm (85% of all renal neoplasms). Five-year survival is less than 50% and less than 2% with metastasis.86
PATHOGENESIS
Renal cell carcinomas are adenocarcinomas (see Chapter 36) that usually arise from tubular epithelium (lining cells) commonly in the renal cortex (see Figure 30-17). They are classified according to cell type and extent of metastasis. Confinement within the renal capsule, together with treatment, is associated with a better survival rate. The tumours usually occur unilaterally. About 25% of individuals with renal cell carcinoma present with metastasis — this means that the cancer has already spread to other organs, prior to it being diagnosed.87
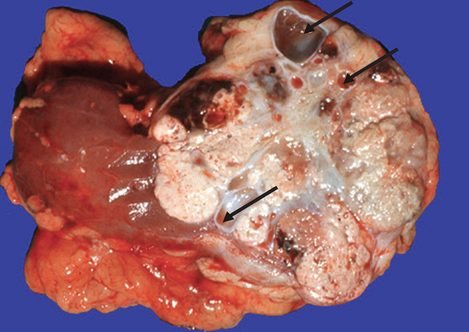
FIGURE 30-17 Renal cell carcinoma.
The carcinoma is located on the pole of the kidney and is large but defined. It contains cysts (arrows) and has grown over a number of years.
Source: Klatt EC. Robbins & Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
A moderate association has been identified between tobacco use, obesity, hypertension and the incidence of renal cell carcinoma.88 The aetiology is unknown.
CLINICAL MANIFESTATIONS
The classical clinical manifestations of renal tumours are haematuria, flank pain, palpable flank mass and weight loss. However, it should be noted that these symptoms occur in fewer than 10% of cases. Furthermore, they represent an advanced stage of disease, whereas earlier stages are often silent. The most common sites of metastasis are the lungs, lymph nodes, liver, bone, thyroid and central nervous system.89
EVALUATION AND TREATMENT
Diagnosis is based on the clinical symptoms, plain X-ray films of the abdomen, intravenous pyelography (whereby contrast dye is intravenously injected to show the urinary structures), renal angiography and CT scan. Treatment for localised disease is surgical removal of the affected kidney (called a radical nephrectomy) with combined use of chemotherapeutic agents. Radiation therapy may also be used.
Childhood renal cancer
Nephroblastoma (also referred to as Wilms’ tumour) is an embryonal tumour of the kidney (see Figure 30-18). The tumour is the fifth most common childhood cancer and the most common solid tumour occurring in children. It accounts for about 7% of all childhood cancers. The peak incidence occurs between 2 and 3 years of age.
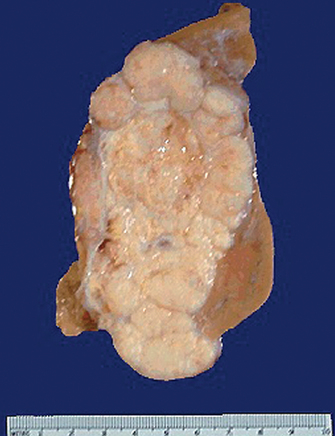
The tumour is clearly visible and is a large circumscribed mass.
Source: Klatt EC. Robbins & Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
PATHOGENESIS
Nephroblastoma has both sporadic (random-like) and inherited origins. The sporadic form occurs in children with no known genetic predisposition. Inherited cases, which are relatively rare, are transmitted in an autosomal dominant fashion (see Chapter 37).
Of children who have nephroblastoma, 18% also have a number of congenital anomalies, including aniridia (lack of an iris in the eye), hemihypertrophy (an asymmetry of the body) and genitourinary malformations (i.e. horseshoe kidneys, hypospadias, ureteral duplication, polycystic kidneys).90
CLINICAL MANIFESTATIONS
Most nephroblastomas usually present as enlarging asymptomatic abdominal masses before the age of 5 years. Many tumours are actually discovered by the child’s parent, who feels or notices an abdominal swelling, usually while dressing or bathing the child. The child appears healthy and thriving. Other presenting complaints include vague abdominal pain (37%), haematuria — blood in the urine — (18%) and fever (22%).91
Nephroblastoma may occur in any part of the kidney and varies greatly in size at the time of diagnosis. The tumour generally appears as a solitary mass surrounded by a smooth, fibrous external capsule and may contain cystic or haemorrhagic areas.
EVALUATION AND TREATMENT
On physical examination, the tumour feels firm, non-tender and smooth and is generally confined to one side of the abdomen. Diagnosis is based on surgical biopsy. Abdominal CT or MRI scans and laboratory studies are used to evaluate the presence or absence of metastasis. The most common sites of metastasis are regional lymph nodes and the lungs. Metastases also occur in the liver, brain and bone.
Primary treatment is usually surgical exploration and resection or chemotherapy and then surgical resection. Survival approaches 90% for localised disease and 70% for metastatic disease.92
Bladder tumours
Urinary bladder cancer accounts for 2.3% of all cancers in Australia and is the tenth most common malignancy. Approximately 2300 people develop bladder cancer each year and 909 die from the cancer.85 There is a projected increase in bladder cancer in the future, with more males developing the disease than females. Bladder cancer is most common in males over the age of 60 years, and transitional cell carcinoma is the most common bladder malignancy.
PATHOGENESIS
The risk of primary bladder cancer is greater among people who smoke.93 Bladder cancer can result from genetic alterations in normal bladder epithelium.94 Metastasis is usually to lymph nodes, liver, bones or lungs. Staging for bladder carcinoma follows the TNM system described in Chapter 36. Secondary bladder cancer develops by invasion of cancer from bordering organs, such as cervical carcinoma in women or prostatic carcinoma in men.
CLINICAL MANIFESTATIONS
Gross painless haematuria is the classical clinical manifestation of bladder cancer. Episodes of haematuria tend to recur and they are often accompanied by bothersome lower urinary tract symptoms including daytime voiding frequency, nocturia, urgency and urge urinary incontinence. Flank pain may occur if tumour growth obstructs the ureters connecting to the bladder. Bothersome lower urinary tract symptoms are particularly intense in individuals with carcinoma in situ (remaining confined to the area).
EVALUATION AND TREATMENT
Urinalysis for evidence of haematuria in the absence of infection provides a useful screening tool for high-risk patients. Several bladder tumour antigen-testing systems have been developed for screening, but they have proved more useful in monitoring patients with known cancer as compared to being used for primary screening. Urine cytology (pathological analysis of sloughed cells within the urine) is completed in individuals with evidence of haematuria from unknown causes; cystoscopy (direct inspection of the bladder) with tissue biopsy confirms the diagnosis. Transurethral resection or laser ablation, combined with intrabladder chemotherapy, is effective for superficial tumours, but radical cystectomy (surgical removal of the entire bladder) with urinary diversion and adjuvant chemotherapy is required for locally invasive tumours.
Urinary tract obstruction
 Obstruction can occur anywhere in the urinary tract and it may be anatomic or functional, including renal stones, an enlarged prostate gland or urethral strictures. The most serious complications are hydronephrosis, hydroureter, ureterohydronephrosis and infection caused by the accumulation of urine behind the obstruction.
Obstruction can occur anywhere in the urinary tract and it may be anatomic or functional, including renal stones, an enlarged prostate gland or urethral strictures. The most serious complications are hydronephrosis, hydroureter, ureterohydronephrosis and infection caused by the accumulation of urine behind the obstruction. Persistent obstruction of the bladder outlet leads to residual urine volumes, low bladder wall compliance and risk for vesicoureteral reflux and infection.
Persistent obstruction of the bladder outlet leads to residual urine volumes, low bladder wall compliance and risk for vesicoureteral reflux and infection. Kidney stones are caused by supersaturation of the urine with precipitation of stone-forming substances, changes in urine pH or urinary tract infection.
Kidney stones are caused by supersaturation of the urine with precipitation of stone-forming substances, changes in urine pH or urinary tract infection.Urinary tract infection
Glomerular disorders
 Glomerular disorders are a group of related diseases of the glomerulus that can be caused by immune responses, toxins or drugs, vascular disorders and other systemic diseases.
Glomerular disorders are a group of related diseases of the glomerulus that can be caused by immune responses, toxins or drugs, vascular disorders and other systemic diseases. Acute glomerulonephritis commonly results from inflammatory damage to the glomerulus as a consequence of immune reactions after a streptococcal infection.
Acute glomerulonephritis commonly results from inflammatory damage to the glomerulus as a consequence of immune reactions after a streptococcal infection. Chronic glomerulonephritis is related to a variety of diseases that cause deterioration of the glomerulus and a progressive loss of renal function.
Chronic glomerulonephritis is related to a variety of diseases that cause deterioration of the glomerulus and a progressive loss of renal function. Immune mechanisms in glomerulonephritis are the deposition of antigen-antibody complexes, often with complement components, and the formation of antibodies specific for the glomerular basement membrane.
Immune mechanisms in glomerulonephritis are the deposition of antigen-antibody complexes, often with complement components, and the formation of antibodies specific for the glomerular basement membrane. Nephrotic syndrome is the excretion of at least 3.5 g protein (primarily albumin) in the urine per day because of glomerular injury with increased capillary permeability and loss of membrane negative charge. Its principal signs are hypoproteinuria, hyperlipidaemia and oedema. The liver cannot produce enough protein to adequately compensate for urinary loss.
Nephrotic syndrome is the excretion of at least 3.5 g protein (primarily albumin) in the urine per day because of glomerular injury with increased capillary permeability and loss of membrane negative charge. Its principal signs are hypoproteinuria, hyperlipidaemia and oedema. The liver cannot produce enough protein to adequately compensate for urinary loss.Chronic kidney disease
 Chronic kidney disease represents a progressive loss of renal function. Plasma creatinine levels gradually become elevated as the GFR declines; sodium is lost in the urine; potassium is retained; acidosis develops; calcium metabolism and phosphate metabolism are altered and erythropoietin production is diminished. All organ systems are affected by chronic kidney disease.
Chronic kidney disease represents a progressive loss of renal function. Plasma creatinine levels gradually become elevated as the GFR declines; sodium is lost in the urine; potassium is retained; acidosis develops; calcium metabolism and phosphate metabolism are altered and erythropoietin production is diminished. All organ systems are affected by chronic kidney disease.Acute kidney injury
 Acute kidney injury refers to the range of changes that can be associated with a rapid decline in renal function. Acute kidney injury is classified as prerenal, intrarenal or postrenal and is usually accompanied by oliguria with elevated blood urea and plasma creatinine levels.
Acute kidney injury refers to the range of changes that can be associated with a rapid decline in renal function. Acute kidney injury is classified as prerenal, intrarenal or postrenal and is usually accompanied by oliguria with elevated blood urea and plasma creatinine levels. Prerenal acute kidney injury is caused by decreased renal perfusion with a decreased GFR, ischaemia and tubular necrosis.
Prerenal acute kidney injury is caused by decreased renal perfusion with a decreased GFR, ischaemia and tubular necrosis.Structural abnormalities
 Congenital renal disorders affect 10–15% of the population. These disorders range in severity from minor, easily correctable anomalies to those incompatible with life.
Congenital renal disorders affect 10–15% of the population. These disorders range in severity from minor, easily correctable anomalies to those incompatible with life. Hypospadias is a congenital condition in which the urethral meatus can be located anywhere on the ventral surface of the glans, the penile shaft, the midline of the scrotum or the perineum.
Hypospadias is a congenital condition in which the urethral meatus can be located anywhere on the ventral surface of the glans, the penile shaft, the midline of the scrotum or the perineum. Ureteropelvic junction obstruction causes urethral obstruction by a malformation of junctional smooth muscle.
Ureteropelvic junction obstruction causes urethral obstruction by a malformation of junctional smooth muscle. Polycystic kidneys is an inherited disorder that results in large, fluid-filled cysts within the kidneys.
Polycystic kidneys is an inherited disorder that results in large, fluid-filled cysts within the kidneys. Renal agenesis is the failure of a kidney to grow or develop. The condition may be unilateral or bilateral and may occur as an isolated entity or in association with other disorders.
Renal agenesis is the failure of a kidney to grow or develop. The condition may be unilateral or bilateral and may occur as an isolated entity or in association with other disorders.Tumours
 Renal cell carcinoma is the most common renal neoplasm. The larger neoplasms tend to metastasise to the lungs, liver and bone.
Renal cell carcinoma is the most common renal neoplasm. The larger neoplasms tend to metastasise to the lungs, liver and bone.Ali is 67 years old and has chronic kidney disease. He worked as a manual labourer, often smoking during outdoor work, as he mainly worked alone. In addition, Ali drinks heavily and consumes at least 6 cans of beer each night. He was diagnosed with hypertension 15 years ago and his medication dosage has increased over that time. More recently, he has been short of breath, has periods of nausea and vomiting, and has been generally tired. At a check-up with his renal specialist, it is found that Ali has large concentrations of protein in his urine, his creatinine level is 497 mmol/L and his estimated GFR is only 11 mL/min. The specialist recommends that Ali commences dialysis.