Cervical Intervertebral Discs
The IVDs of the cervical spine constitute more than 25% of the superior-to-inferior length of this region, and they help to allow the large amount of motion that occurs here. Recall that there are no IVDs between the occiput and atlas and between the atlas and axis. The C2-3 interbody joint is the first such joint to possess an IVD. Therefore the C3 spinal nerve is the most superior nerve capable of being affected by IVD protrusion.
Mendel and colleagues (1992) studied the innervation of the cervical IVDs and found sensory nerve fibers throughout the anulus fibrosus. No nerves were found in the nucleus pulposus. The sensory fibers were most numerous in the middle third (from superior to inferior) of the anulus. The size and myelination of many of the nerve fibers and their end receptors were consistent with nerves that transmit pain. In addition, pacinian corpuscles and Golgi tendon organs were found in the posterolateral aspect of the disc. These authors’ findings help to confirm that the anulus fibrosus is a pain-sensitive structure. Furthermore, their findings of Golgi tendon organs and pacinian corpuscles indicate that the cervical discs are involved in proprioception, thereby enabling the central nervous system to monitor the mechanical status of the IVDs. These authors hypothesized that the arrangement of the sensory receptors may allow the IVD to sense peripheral compression or deformation and also alignment between adjacent vertebrae.
The posterior aspect of each cervical IVD has less height from superior-to-inferior than the anterior aspect of the IVD (Lu et al., 1999). In addition, the upright posture of humans takes a toll on the IVDs (Liang et al., 2011) and the IVDs of the cervical region become thinner with age. This is because the cervical IVDs dehydrate earlier in life than those of the thoracic and lumbar regions, and by the age of 45 the nucleus pulposus is difficult to distinguish from the anulus fibrosus. Such cervical IVD thinning also puts additional loads on the Z joints (Hussain et al., 2010a,b). Evaluation of IVD thinning associated with degeneration can be done reliably from standard x-rays if rigorous, standardized methods of evaluation are used (Côté et al., 1997). Furthermore, horizontal and vertical clefts can develop in these aging cervical IVDs. These clefts cause bulging and protrusion of the hardened cervical cartilaginous end plates. These end plate herniations are the predominant type of IVD herniations in the cervical region (Kokubun, Sakurai, & Tanaka, 1996). Like IVD herniations in the lumbar region, cervical disc herniations usually regress with time (Bush et al., 1997). However, the higher the concentration of cartilaginous end plates in the herniation, the slower is the process of IVD resorption. As in the lumbar region, repetitive flexion and extension, vibration (both generally as part of a person’s occupation), and loading of the cervical spine (as with traumatic injuries) increase the likelihood of cervical IVD herniation (Gregory & Callaghan, 2011).
As the cervical IVDs narrow, the uncinate processes enlarge. As a result, by age 40 the uncinate processes help to create a barrier that in turn helps to prevent lateral and posterolateral herniation of the IVD (Bland, 1989). However, the uncinate processes can develop osteophytes that can cause stenosis of the vertebral canal and IVF, leading to radiculopathy of osteophytic origin (Bland, 1989).
MRI has been shown to be effective in evaluating the status of the IVD (Forristall, Marsh, & Pay, 1988). Viikari-Juntara and colleagues (1989) also found that ultra–low-field MRI is useful in identifying posterior disc displacement below the level of C4. These MRI units are less expensive, and as resolution improves, they may be more frequently used in place of standard x-ray procedures. However, tears of the anulus fibrosus frequently are undetected on cervical MRI (Schellhas et al., 1996), and the importance of a thorough and accurate clinical history and physical examination is essential for the correct diagnosis of cervical pain of discogenic origin, with or without radiculopathy.
The basic anatomy of the cervical IVDs differs from that of the IVDs throughout the remainder of the spine. The anulus fibrosus is a single crescent-shaped piece of fibrocartilage that is thick anteriorly and extremely thin posteriorly (Fig. 5-26). Those interested in the detailed anatomy of the IVDs should refer to the sections of Chapters 2 and 14 devoted to the gross and microscopic anatomy of these clinically relevant structures.
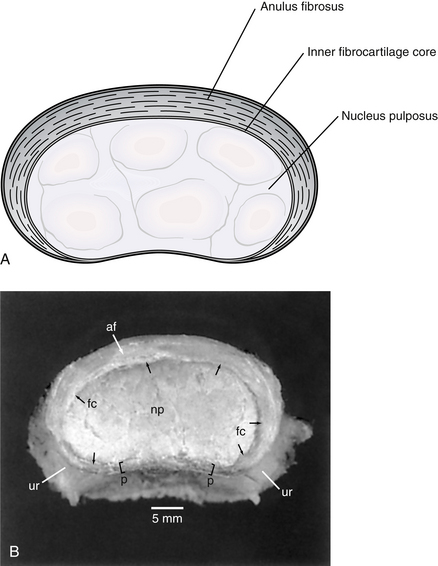
FIG. 5-26 A, Illustration and, B, dissection showing superior view of a cervical intervertebral disc demonstrating the crescent-shaped anulus fibrosus in the cervical region. Notice that the anulus is extremely thin posteriorly. af, anulus fibrosus; fc, inner fibrocartilage core; np, nucleus pulposus; p, posterior aspect of the anulus fibrosus (the posterior longitudinal ligament has been removed); ur, uncinate region. (B, From Mercer S & Bogduk N. [1999]. The ligaments and anulus fibrosis of the human adult cervical intervertebral discs. Spine, 24, 619-628.)
Ranges of Motion of the Cervical Spine
Although cervical ranges of motion can be measured reliably (Nilsson, Christensen, & Hartvigsen, 1996), one must keep in mind that measurements made on different days of both active (performed by an individual’s own muscular activity) and passive (performed by another person holding the subject’s head and moving the spine through the range of motion to be measured) ranges of motion of the same individual can vary considerably. Variations of 12 to 20 degrees can be found in flexion-extension, lateral flexion, and axial rotation (Christensen & Nilsson, 1998). One must also keep in mind that range of motion in the cervical region normally decreases after 25 years of age, with the greatest decreases in motion occurring between 30 to 39 and 40 to 49 years of age (Panjabi et al., 2005; Demaille-Wlodyka et al., 2007). In addition, forward flexion and left and right axial rotation decrease as neck circumference increases and lateral flexion decreases in relation to a ratio of neck height and circumference (i.e., lateral flexion decreases with a “short, fat neck”) (Reynolds et al., 2009). Small decreases in cervical ranges of motion may not affect an individual’s daily life because a healthy person uses only a relatively small amount of the total cervical ranges of motion during their activities of daily living (Bible et al., 2010).
Atlanto-Occipital Joint
The right and left atlanto-occipital joints together form an ellipsoidal joint that allows movement in flexion, extension, and, to a lesser extent, left and right lateral flexion (Table 5-5). A little rotation also occurs between occiput and atlas (Standring et al., 2008). Extension is limited by the opposition of the posterior aspect of the superior articular processes of the atlas with the bone of the occiput’s condylar fossa. Flexion is limited by soft tissue stops, such as the posterior atlanto-occipital membrane.
Table 5-5
Approximate Ranges of Motion at the Atlanto-Occipital Joints
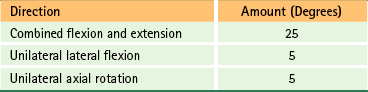
From White AW & Panjabi MM. (1990). Clinical biomechanics of the spine. Philadelphia: JB Lippincott; and Panjabi et al. (2001a). The cortical shell architecture of human cervical vertebral bodies. Spine, 26, 2478-2484.
Table 5-6 lists the muscles that produce the most flexion, extension, and lateral flexion between the occiput and atlas and between the atlas and axis.
Table 5-6
Muscles Producing Flexion, Extension, and Lateral Flexion at Occiput, C1-2
| Movement | Muscles |
| Flexion | Longus capitis Rectus capitis anterior |
| Extension | Rectus capitis posterior major and minor Obliquus capitis superior Semispinalis and spinalis capitis Longissimus capitis Splenius capitis Trapezius Sternocleidomastoid |
| Lateral flexion | Rectus capitis lateralis Semispinalis capitis Longissimus capitis Splenius capitis Sternocleidomastoid Trapezius |
Atlanto-Axial Joints
Motion occurs at all three (median and left and right lateral) atlanto-axial joints simultaneously. The most motion occurs in axial rotation (Table 5-7), which is limited by the alar ligaments (see earlier discussion). Because the superior articular process of C2 is convex superiorly and with the presence of hyaline cartilage the inferior articular facet of C1 is convex inferiorly, the anterior and posterior gliding that accompanies axial rotation also is accompanied by descent of the atlas. This moves the upper joint surface (i.e., inferior facet of C1) inferiorly, which conserves the amount of capsule necessary to accommodate the large amount of unilateral axial rotation that can occur at this joint. In addition, the descent of the atlas, as its inferior articular processes move along the superior articular processes of the axis, allows added rotation to occur between the two segments (Standring et al., 2008).
Table 5-7
Approximate Ranges of Motion at the Atlanto-Axial Joint
| Direction | Amount (Degrees) |
| Combined flexion and extension | 20 |
| Unilateral lateral flexion | 5 |
| Unilateral axial rotation | 28-40 |
From White AW & Panjabi MM (1990). Clinical biomechanics of the spine. Philadelphia: JB Lippincott; and Panjabi et al. (2001a). The cortical shell architecture of human cervical vertebral bodies. Spine, 26, 2478-2484.
Muscles that produce rotation at this joint include the following: obliquus capitis inferior, rectus capitis posterior major, splenius capitis, and the contralateral sternocleidomastoid.
Lower Cervicals
The ranges of motion for the cervical region from C2-3 through C7-T1 are given in Table 5-8.
Table 5-8
Total Range of Motion of Cervical Vertebrae (C2-T1)∗
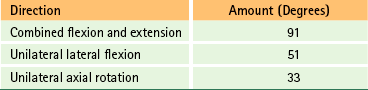
∗Ranges are for C2-3 through C7-T1 and do not include occiput, C1, and C1-2 (see Tables 5-5 and 5-7 for upper cervical ranges of motion).
Values calculated from White AW & Panjabi MM. (1990). Clinical biomechanics of the spine. Philadelphia: JB Lippincott.
Usually extension is somewhat greater than flexion. Extension is limited below by the inferior articular processes of C7 entering a groove below the superior articular processes of T1. Flexion is limited by the lip on the anterior and inferior aspects of the cervical vertebral bodies pressing against the beveled surface of the anterior and superior aspects of the vertebral bodies immediately below (Standring et al., 2008).
Rotation with Lateral Flexion
Lateral flexion of the cervical spine is accompanied by rotation of the C2-7 vertebral bodies (the occiput-atlas and atlas-axis articulations perform uniquely) into the concavity formed by the lateral flexion (vertebral body rotation to the same side as lateral flexion) (Panjabi et al., 2001b). For example, right lateral flexion of the cervical region is accompanied by right rotation of the vertebral bodies. This phenomenon is known as coupled motion and occurs because the superior articular processes of cervical vertebrae not only face superiorly but also are angled slightly medially. This arrangement forces some rotation with any attempt at lateral flexion. The posture during initiation of motion and age affect coupled motion patterns (Edmondston et al., 2005; Demaille-Wlodyka et al., 2007) and other, more subtle, coupled motions occur in the cervical region (Malmstrom et al., 2006). A comprehensive discussion of vertebral coupled motions is beyond the scope of this text. A more thorough discussion of coupled motion is found in Chapter 2.
Nerves, Vessels, Anterior Neck Muscles, and Viscera of the Cervical Region
The vertebral artery is so closely related to the cervical spine that it is discussed before the nerves of the neck. The remaining arteries of the neck are covered later in this chapter.
The vertebral artery is the first branch of the subclavian artery. It enters the foramen of the TP of C6 and ascends through the remaining foramina of the TPs of the cervical vertebrae (Fig. 5-27; see also Fig. 5-24). Continuing, it passes through the foramen of the TP of C1, winds medially around the superior articular process of the atlas, and passes beneath the posterior atlanto-occipital membrane (see Fig. 5-16). The vertebral artery then pierces the dura and arachnoid and courses superiorly through the foramen magnum to unite with the vertebral artery of the opposite side. The union of the two vertebral arteries forms the basilar artery.
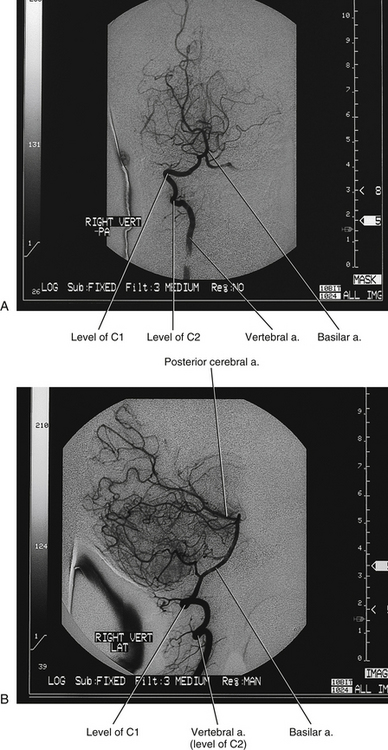
FIG. 5-27 A, Posterior-anterior and, B, lateral angiograms of the right vertebral artery. A radiopaque dye has been injected into the vertebral artery and x-ray films have been taken. The vertebral artery can be seen as it courses superiorly through the foramina of the TPs of C6 through C1. Notice the normal tortuosity seen as the vertebral artery passes laterally at C2 to reach the foramen of the TP of C1. The vertebral artery also is tortuous as it passes around the superior articular process of C1 and then passes superiorly to enter the foramen magnum. It then unites with the vertebral artery of the opposite side to form the basilar artery. Several branches of the basilar artery can be seen, and its termination as the posterior cerebral arteries also can be seen.
Each vertebral artery is approximately 4.5 mm in diameter. The left and right arteries often are asymmetric, with one artery much larger than the other (Figure 5-28). When this is the case the larger artery is called the “dominant artery” and the smaller artery is called the “minor artery” (George & Laurian, 1987). George and Laurian (1987) reported the incidence of asymmetric vertebral arteries to be 59.2%, with the left vertebral artery being dominant 35.8% of the time and the right vertebral artery dominant 23.4% of the time. However, a study of 1013 Korean patients undergoing CT angiography found a lower incidence of asymmetric vertebral arteries (total asymmetric vertebral arteries 32.1%; left-dominant 22.3%, right-dominant, 9.8%) (Hong et al., 2008). Females appear to have a higher incidence of asymmetry of the vertebral arteries than men (Peterson et al., 2010). The minor artery can be small and occasionally is absent (in which case a well-developed anastomosis is found).

FIG. 5-28 Cervical arteriogram showing asymmetric vertebral arteries (arrowheads). The larger vertebral artery on the reading left (patient’s right) is termed the “major vertebral artery” and the smaller artery on the reading right is the “minor vertebral artery.” The right common carotid artery is the largest ascending artery, seen on the reading left ascending from the bottom of the panel. This same artery (i.e., right common carotid artery) is seen branching superiorly from the brachiocephalic trunk. (From George B & Laurian C. [1987]. The vertebral artery: pathology and surgery. New York: Springer-Verlag.)
The First Part of the Vertebral Artery
The vertebral artery can be divided into four parts (Standring et al., 2008). The first part of the vertebral artery begins at the artery’s origin from the subclavian artery and continues until it passes through the foramen of the TP of C6. The first part courses between the longus colli and scalenus anterior muscles before reaching the TP of C6. In a study of 36 vertebral arteries, Taitz and Arensburg (1989) found that 18 (50%) were tortuous to some degree in the first segment. Currently there is debate as to whether or not tortuosity of a vertebral artery may cause a decrease in flow to the structures supplied by it. However, to date no clinical significance has been ascribed to mild-to-moderate tortuosity of the vertebral artery. True anomalies of the origin of the vertebral artery are relatively rare. However, the most common anomaly is an origin from the aortic arch (4%), with the anomalous vertebral artery usually arising between the left common carotid and left subclavian arteries.
The first part of the vertebral artery is accompanied by several venous branches that become the vertebral vein in the lower cervical region. It is also accompanied by a large branch and several small branches from the more posteriorly located inferior cervical ganglion or, when present, the cervicothoracic ganglion (stellate ganglion, present 80% of the time). These branches form a plexus of nerves around the vertebral artery. This plexus is discussed in more detail later in this chapter.
The Second Part of the Vertebral Artery
The second part of the vertebral artery is the region that passes superiorly through the foramina of the TPs of C6 to C1 (Fig. 5-29; see also Figs. 5-24 and 5-27). It usually enters the foramen of the TP of C6 (89.8% of the time), but may enter at any level (e.g., C5, 6.3%; C7, 3.0%). The entrance is symmetric from left to right 85% of the time and is asymmetric 15% of the time (Francke et al., 1980). When asymmetry is present, the arteries have only very rarely been found to enter the foramina of the TPs more than two levels apart, and the right artery almost always has been found to enter at the lower level (Francke et al., 1980; Gluncic et al., 1999). When the vertebral artery originates from the arch of the aorta it usually enters the foramen of the TP at C5 or C4.
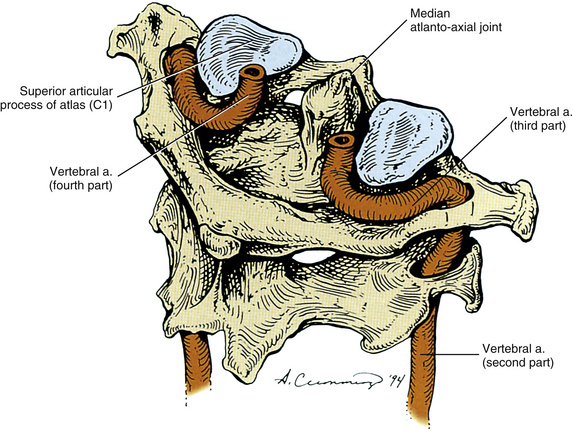
FIG. 5-29 The second, third, and fourth parts of the left and right vertebral arteries. Notice that each vertebral artery courses laterally between C2 and C1. It then courses posteriorly and medially at the level of C1, and finally passes superiorly and medially to reach the foramen magnum.
In its ascent through the foramina of the TPs of the C6 to C1 vertebrae, the second part of the vertebral artery passes approximately 1 mm posterior and lateral to the uncinate processes of C2 to C6 vertebrae. More specifically, the distance of the vertebral artery from the uncinate processes gradually decreases during the ascent of the vertebral artery from C6 to C3. This may predispose the vertebral artery to compression from bone spurs (osteophytes) of the uncinate processes in the midcervical region (Ebraheim et al., 1997c). In addition, the second part of the vertebral artery passes anterior to the C2 to C6 cervical spinal nerves and ventral rami, which course from medial to lateral in the grooves (gutters) for the spinal nerves of their respective cervical TPs (see Fig. 5-33).
Occasionally the second part of the vertebral artery may become tortuous between any two TPs. Such tortuousness increases with age. Whether this tortuosity is congenital, acquired (secondary to atherosclerosis), or a combination of both has yet to be determined. However, Oga and colleagues (1996) found that severe disc degeneration, as sometimes seen in cervical spondylotic myelopathy, causes the distance between adjacent vertebrae to decrease. This decreased intervertebral distance occasionally can result in an increase in tortuosity of the second part of the vertebral artery. On rare occasions a tortuous vertebral artery causes a widening of the foramen of the TP (Taitz & Arensburg, 1989; Schima et al., 1993). Severe trauma to the cervical region leading to partial or complete anterior dislocation of one vertebra on another can lead to complete occlusion of flow through the second part of the vertebral artery (Giacobetti et al., 1997).
The second part of the vertebral artery normally makes a rather dramatic lateral curve (usually 45 degrees but up to 90 degrees) after passing through the foramen of the TP of the axis (see Figs. 5-16 and 5-29). This allows the artery to reach the more laterally placed TP of the atlas. Taitz and Arensburg (1989) found that 4 of 36 vertebral arteries (11%) showed marked kinking or tortuosity at the foramen of the TP of the axis.
A sympathetic nerve plexus surrounds the second through fourth parts of the vertebral artery. External to the sympathetic plexus, a dense connective tissue sheath covers the second part of the vertebral artery. Irritation of the vertebral artery sympathetic plexus (as can occur from hypertrophy of the closely related uncinate processes) has been associated with posterior occipital headaches, dizziness, and various pupillary changes, but not with a change of blood flow through the vertebral artery or any of the intracranial vessels (George & Laurian, 1987). The second part of the vertebral artery is accompanied by an interconnected venous plexus, which is most highly developed at the superior and inferior-most ends of this part of the artery (Schmidt & Pierson, 1934; Meyer, Yoshida, & Sakamoto, 1967; Nagashima & Iwama, 1972).
The dynamics of blood flow through the left and right vertebral arteries between the axis and the atlas remain rather poorly understood. Until recently, the general consensus was that extension combined with rotation of the head to one side normally impaired blood flow through the second part of the vertebral artery of the opposite side, the constriction occurring between the axis and atlas (Taitz, Nathan, & Arensburg, 1978). However, Haynes and colleagues (2002) found that usually there is no compression or stenosis of the vertebral artery with atlanto-axial rotation. Yi-Kai and colleagues (1999) determined that extreme extension and extension with rotation resulted in decreased flow in both vertebral arteries. Licht and colleagues (1998) found a decrease in flow in the vertebral artery contralateral to rotation and for the first time documented an increase in flow on the ipsilateral side of rotation. Mitchell (2003) found a decrease in flow through both the left and right vertebral arteries (more in the contralateral vessel) with maximal rotation, especially in those arteries with underlying pathology (e.g., atherosclerosis). Therefore maximal rotation and extension seem to decrease flow through the vertebral arteries, but submaximal rotation seems to have less of an effect.
The Third Part of the Vertebral Artery
The third part of the vertebral artery normally has a tortuous course. It begins as the artery passes through the foramen of the TP of the atlas (see Fig. 5-29). Here it is located posterior and medial to the rectus capitis lateralis muscle. Immediately the vertebral artery curves farther posteriorly and medially around the superior articular process of C1. It reaches the posterior arch of the atlas, where it lies in the groove for the vertebral artery of the posterior arch. The dorsal ramus of the first cervical nerve (suboccipital nerve) passes between the vertebral artery and the posterior arch of the atlas in this region. The artery then passes inferior to the posterior atlanto-occipital membrane (see Fig. 5-16). This membrane may form an ossified bridge for the artery, which, when present, passes from the posterior arch of the atlas to the lateral mass. This bony bridge is known as a posterior ponticle and was discussed earlier in this chapter (see Fig. 5-10, F).
The Fourth Part of the Vertebral Artery
The fourth part of the vertebral artery begins as the artery passes beneath the bridge of the posterior atlanto-occipital membrane. It continues anteriorly, superiorly, and medially from the posterior atlanto-occipital membrane, and ascends along the anterolateral aspect of the spinal dura mater. At the level of the foramen magnum, each vertebral artery pierces the dura and arachnoid mater and continues ascending within the subarachnoid space. The left and right vertebral arteries continue to gradually course medially along the anterior aspect of the medulla and inferior pons, where they unite to form the single basilar artery. The basilar artery ascends along the anterior aspect of the pons and midbrain, until it helps to form the cerebral arterial circle (of Willis) by ending as the left and right posterior cerebral arteries.
Along its course, each vertebral artery forms muscular branches, osteoarticular branches, meningeal branches, and spinal rami; the latter enter the IVFs to help supply the vertebral body, posterior arch, and soft tissue structures within the vertebral canal (including the nerve roots and rootlets). The fourth part of the vertebral artery has several branches. Each vertebral artery develops a branch that unites with its pair from the opposite side to form a single anterior spinal artery. The anterior spinal artery supplies the anterior aspect of the spinal cord throughout its length. Each vertebral artery then produces a posterior spinal artery. The left and right posterior spinal arteries remain separate as they course along the posterior aspect of the spinal cord (see Chapter 3 for both anterior and posterior spinal arteries). Each vertebral artery then forms a posterior inferior cerebellar artery that supplies a portion of the medulla and the inferior aspect of the cerebellum.
As mentioned, the right and left vertebral arteries unite at the level of the inferior pons to form the basilar artery. The basilar artery provides the left and right posterior superior cerebellar arteries. These arteries course posteriorly around the medulla to supply the medulla, inferior pons, and inferior one half to two thirds of the cerebellum. The basilar artery also sends the left and right internal acoustic (auditory) arteries to the internal acoustic meatus of each side. After passing through the meatus, each of these arteries supplies the middle and inner ear structures of each side. As the basilar artery ascends along the anterior aspect of the pons, it sends many small pontine branches to the pons. The basilar artery then gives off the left and right superior cerebellar arteries that course posteriorly, supplying the superior pons and inferior aspect of the cerebral peduncles of the midbrain before continuing posteriorly to supply the superior aspect of the cerebellum. The basilar artery ends at the level of the superior pons by dividing into the left and right posterior cerebral arteries. The posterior cerebral arteries participate in the cerebral arterial circle (of Willis) and then continue posteriorly to supply the occipital lobes of the cerebral cortex and the inferior portion of the temporal lobes.
Microscopic Anatomy
Each vertebral artery is thin walled, but its structure is quite similar to all other arteries (Wilkinson, 1972). As with all typical arteries, the vertebral artery is composed of three layers. From internal to external these layers are as follows: tunica intima, tunica media, and tunica adventitia (Fig. 5-30). An internal elastic lamina separates the intima from the media, and an external elastic lamina separates the media from the adventitia. The tunica intima is the most delicate layer; the tunica media is more rugged and has elastic fibers within it. The tunica media and external elastic lamina are well developed in the vertebral artery below the foramen magnum. After piercing the dura and arachnoid mater at about the level of the foramen magnum, the adventitia decreases in thickness, the external elastic lamina disappears, and the tunica media loses most of its elastic fibrils.
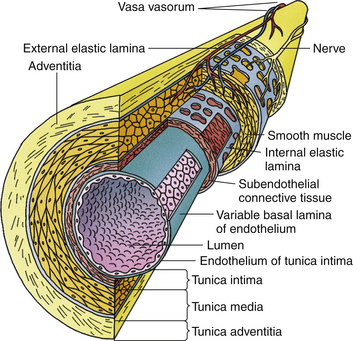
FIG. 5-30 Illustration depicting histologic composition of a large artery (e.g., vertebral artery). Notice the outer tunica adventitia, the large central tunica media, and the inner tunica intima.
As with all large arteries, much potential pathology can affect the walls of the vertebral artery; primary among these is atherosclerosis. The usual lesion of atherosclerosis is a smooth plaque that can be found anywhere within the first and second parts of the artery. These plaques may calcify. Hemorrhage in plaques has been observed in the vertebral artery, but ulceration of plaques only occurs in approximately 4% of the lesions, and is less common than such ulceration found in the carotid arteries. Possible complications to atherosclerosis include local thrombosis, ischemia to structures supplied downstream of the lesion, embolism, and stenosis leading to decreased flow.
As mentioned, the intima is the most delicate layer of any artery and severe trauma or plaque formation may cause this layer to tear and separate from the media. Such tearing of the intima causes rapidly flowing blood to be forced between the intima and media, causing dissection of the intima from the media. The vertebral artery is not exempted from such pathology. Causes of dissection include trauma, dissection associated with preexisting arterial disease (e.g., atherosclerosis, which is the most common cause of vertebral artery dissection), and spontaneous dissection. With respect to trauma, there are four regions of the vertebral artery where such lesions have been identified. These are as follows: at the entrance of the vertebral artery to the foramen of the TP of C6, as the artery passes through the foramina of the TPs of C6 to C2, at the C1 foramen of the TP, and as the vertebral artery perforates the dura mater (George & Laurian, 1987). An intimal tear also may lead to the formation of a thrombus at the site of the tear. The thrombus can extend proximally or distally from the tear, and embolism formation from the thrombus is possible. Alternatively, a local hematoma also may form at the tear site. Such hematoma formation can result in subsequent stenosis or occlusion of the artery. If dissection causes only narrowing, as opposed to complete occlusion, the condition usually either improves or completely resolves. If complete occlusion occurs, the condition will usually not resolve (George & Laurian, 1987). Like most intracranial arteries, the thinner walls of the portion of the vertebral artery distal to its entrance to the dura mater predispose this portion of the vertebral artery to aneurysm formation after dissection.
Difference between the Vertebral Artery and Other Arteries
Intracranial arteries have thinner walls than extracranial arteries of comparable size. More specifically, the media and adventitial layers of intracranial arteries are narrower and have fewer elastic fibers in them. Wilkinson (1972) found that the structure of the vertebral artery changed dramatically just proximal to its point of penetration through the dura mater. Up to this point the structure of the vertebral artery was found to be similar to most extracranial vessels, and distal to this point its structure resembled that of intracranial vessels. More precisely, the adventitia and media of the intracranial portion of the vertebral artery were found to be thinner and had fewer elastic fibrils, and the external elastic lamina “was either absent completely or represented by sparse single elastic fibrils only” (Wilkinson, 1972). The changes were not found to be complete until approximately 5 mm “past the point of dural perforation” (Wilkinson, 1972). The intima and internal elastic lamina were found to be similar in the extracranial and intracranial regions of the vertebral artery. These findings are similar to those reported for the intracranial and extracranial portions of the internal carotid artery (Ratinov, 1964).
Nerves of the Cervical Region
A thorough understanding of patients presenting with neck pain can be achieved only if clinicians know those structures capable of nociception (pain perception). Also, clinicians first must understand how pathologic conditions, aberrant movement, or pressure affecting these structures can result in nociception, and then how the patient perceives that nociception. Knowledge of the innervation of the cervical region provides an understanding of the structures that are pain sensitive and the way in which this nociceptive information is transmitted to the central nervous system. Consequently, this topic is significant to clinicians dealing with pain of cervical origin.
Rootlets, Roots, Dorsal Root Ganglia, Spinal Nerves, and Rami
The dorsal and ventral rootlets of the cervical region leave the spinal cord and unite into dorsal and ventral roots (see Chapter 3). The lengths of cervical nerve roots increase from C4 to C8 (Yabuki & Kikuchi, 1996), and the positions of the dorsal root ganglia vary from being proximally to distally located within the IVF. No relationship has been found among the varying positions of the dorsal root ganglia within the cervical IVFs and patients’ symptoms (Yabuki & Kikuchi, 1996), although with age-related spinal degeneration the dorsal root ganglia have been found to be “distorted” (indented as a result of compression by neighboring structures) and to exibit an increase in inflammatory mast cell density (Boyd-Clark, Briggs, & Galea, 2004). The dorsal and ventral roots unite within the region of the IVF to form the spinal nerve (Fig. 5-31). The spinal nerve is short and almost immediately divides into a dorsal ramus (posterior primary division) and a ventral ramus (anterior primary division).
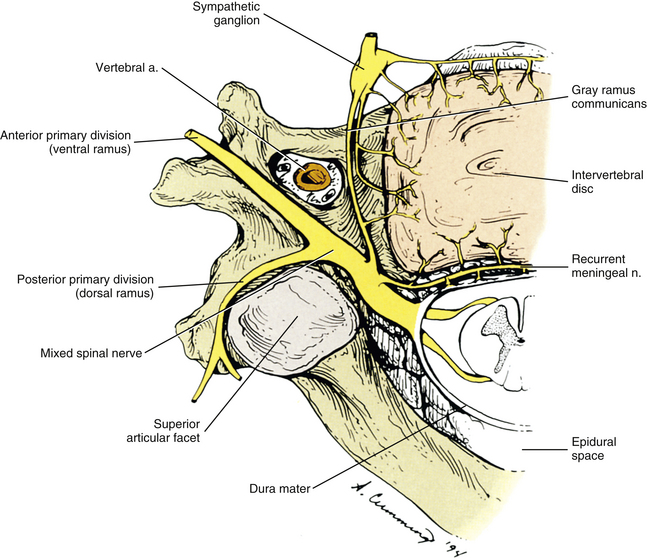
FIG. 5-31 Superior view of a typical cervical segment showing the neural elements. Notice the dorsal and ventral roots, spinal nerve, and posterior and anterior primary divisions (dorsal and ventral rami). The posterior primary division can be seen dividing into a medial and a lateral branch. The recurrent meningeal nerve is shown entering the intervertebral foramen. Fibers arising from the middle cervical ganglion and the gray communicating ramus also are shown. Notice that these fibers supply the anterior and lateral aspects of the intervertebral disc, vertebral body, and anterior longitudinal ligament. The sympathetic plexus that surrounds the vertebral artery is shown in Figure 5-32.
Unique rootlets, roots, and dorsal root ganglia: The posterior rootlets of C1 are unique. They are so thin that frequently they are mistaken for arachnoidal strands during dissection (Edmeads, 1978). Stimulation of the C1 rootlets has been found to cause orbital pain (superior rootlets of C1), frontal pain (middle rootlets), and vertex pain (lower rootlets). Conditions such as tumors of the posterior cranial fossa, herniations of the cerebellar tonsils through the foramen magnum, bony anomalies of the craniovertebral junction, and possibly prolonged muscle tightness can cause irritation of the sensory rootlets or root of C1. Irritation of these rootlets or root may, in turn, refer pain to the regions just mentioned (Edmeads, 1978; Darby & Cramer, 1994).
Great variation exists in the distribution of rootlets in the cervical region. More specifically, anastomoses frequently exist between rootlets of adjacent spinal cord segments. These anastomoses occur 61% of the time in the cervical spinal cord, compared with 7% in the thoracic region and 22% in the lumbar cord (Moriishi et al., 1989; Tanaka et al., 2000). This is clinically significant because sensory impulses conducting nociceptive (pain) sensations through the dorsal root ganglion at one vertebral level may enter the spinal cord at the next spinal cord segment above or below. The pain sensations in such cases may be perceived one segment “off,” adding to the body’s already difficult task of pain localization (Darby & Cramer, 1994). These anastomoses also complicate the presentation of radicular pain by disrupting the normal dermatomal pattern of innervation by dorsal roots and dorsal root ganglia (see Chapter 11).
Recall that the cell bodies of all afferent nerve fibers are located in the dorsal root ganglia (DRG), which are also known as the spinal ganglia. These ganglia, with the exception of those of the C1 and C2 cord segments, are located within the IVFs. The C1 DRG may be absent; however, when present, it usually is found lying on the posterior arch of the atlas (Standring et al., 2008).
The C2 DRG is located between the posterior arch of the atlas and the lamina of C2; more exactly, it is located posterior and medial to the lateral atlanto-axial joint. It contains the cell bodies of sensory fibers innervating the median atlanto-axial joint, the lateral atlanto-axial joint, and a large part of the neck and scalp, extending from the posterior occipital region to the vertex and occasionally even to the coronal suture of the skull (Bogduk, 1982). Another unique characteristic of the C2 DRG is that it is the only such ganglion normally located outside the dura. The ganglion normally fills 76% of the superior-to-inferior space between the posterior arch of the atlas and the lamina of C2. It is vulnerable to compression in this location. In addition to compression during prolonged extension of the upper cervical region, spondylosis of the lateral atlanto-axial joint may contact the C2 DRG and further increase its vulnerability to compression. The morphologic changes that occur in this ganglion after compression have been shown to be similar to those associated with compressive neuropathy of peripheral nerves (Lu & Ebraheim, 1998). The prominent and predictable location of the C2 DRG also has enabled investigators to study the effects of localized anesthesia on the C2 DRG (Bogduk, 1989a), allowing for a better understanding of the importance of the second cervical nerve in suboccipital headaches.
Dorsal rami: The dorsal rami (posterior primary divisions) generally are smaller than the ventral rami (anterior primary divisions). Recall that each dorsal ramus exits the spinal nerve just lateral to the IVF (see Fig. 5-31). After exiting the IVF, the dorsal ramus curves posteriorly, close to the anterolateral aspect of the articular pillar. In fact, the dorsal rami of C4 and C5 produce a groove on the lateral aspect of the articular pillars of the C4 and C5 vertebrae. On reaching the posterior and lateral aspect of the superior articular process, each dorsal ramus quickly divides into a medial and lateral branch (see Fig. 5-31).
Some of the most important structures innervated by the dorsal rami are the deep back muscles. The deeper and more segmentally oriented transversospinalis muscles receive innervation from the medial branch of the dorsal rami. The longer and more superficial erector spinae muscles are innervated by the lateral branch of the dorsal rami. Other structures innervated by the medial branch include the Z joints and the interspinous ligaments. The lateral branches of the dorsal rami of the upper cervical nerves (except C1) continue posteriorly, after innervating the erector spinae and splenius capitis and cervicis muscles, to supply sensory innervation to the skin of the neck. The dorsal rami of C6, C7, and C8 usually do not have cutaneous branches (Kasai et al., 1989).
The dorsal ramus of the C1 spinal nerve is unique. The C1 nerve exits the vertebral canal by passing above the posterior arch of the atlas. It quickly divides into a ventral and a dorsal ramus. The dorsal ramus (suboccipital nerve) runs between the posterior arch of the atlas and the vertebral artery. It does not divide into medial and lateral branches, but rather curves superiorly for a short distance (≈1 cm) and terminates by providing motor innervation to the suboccipital muscles. It also sends a communicating branch to the dorsal ramus of C2. Some authors have described an inconsistent cutaneous branch that runs to the posterolateral scalp (Standring et al., 2008), although other detailed studies have not reproduced this finding (Bogduk, 1982).
The C2 spinal nerve branches into a dorsal and a ventral ramus posterior to the lateral atlanto-axial joint. The dorsal ramus loops superiorly around the inferior border of the obliquus capitis inferior muscle and then divides into medial, lateral, superior communicating, inferior communicating, and a branch to the obliquus capitis inferior. The lateral branch of the dorsal ramus of C2 helps to supply motor innervation to the longissimus capitis, splenius capitis, and semispinalis capitis muscles (Bogduk, 1982). The medial branch of the dorsal ramus of C2 is large and is called the greater occipital nerve. This nerve receives a communicating branch from the third occipital nerve before piercing the large semispinalis capitis muscle. The greater occipital nerve always pierces the semispinalis capitis muscle and pierces the trapezius muscle 20% of the time. However, the distance from the midline that this nerve pierces the neck muscles to reach the subcutaneous tissue of the scalp is variable (5 to 28 mm from the midline) (Becser, Bovim, & Sjaastad, 1998). As it pierces the semispinalis muscle, the greater occipital nerve usually is accompanied by the occipital artery, which it contacts approximately 54% of the time with the occipital artery frequently (70.4% of instances of contact) loosely coiling several times around the greater occipital nerve (Janis, Hatef, & Reece, 2010). As the nerve reaches the scalp it is sometimes (when the nerve does not pierce the trapezius muscle) thought to be protected from compression during contraction of the trapezius muscle by passing through a protective aponeurotic sling associated with the insertions of the trapezius and SCM muscles onto the superior nuchal line (Bogduk, 1982). After passage through the sling, the greater occipital nerve courses superiorly and divides into several terminal branches. These branches provide a broad area of sensory innervation extending from the occipital region medially to the region superior to the mastoid process and posterior to the ear laterally. Superiorly they supply sensory innervation to the scalp from the region of the posterior occiput to as far as the skull’s coronal suture (Bogduk, 1982). Terminal branches of the greater occipital nerve also provide sensory branches to the occipital and transverse facial arteries.
Disorders of the upper cervical spine, including irritation of the greater occipital nerve or the C2 ganglion, can cause headaches (Edmeads, 1978; Bogduk et al., 1985; Bogduk, 1986b, 1989a). Causes of irritation to the nerve or ganglion include direct trauma to the posterior occiput; entrapment between traumatized or hypertonic cervical muscles, particularly the semispinalis capitis (Edmeads, 1978; Mosser et al., 2004); and vascular pulsation of the occipital artery (Janis, Hatef, & Reece, 2010). Hyperextension injuries to the neck, especially during rotation, also can compress the C2 ganglion between the posterior arch of the atlas and the lamina of the axis.
The C3 spinal nerve is the most superior nerve to pass through an IVF. Within the lateral aspect of the IVF the C3 nerve branches into a dorsal and a ventral ramus. The dorsal ramus of C3 passes posteriorly between the C2 and C3 TPs, where it divides into deep and superficial medial branches, a lateral branch, and a communicating branch with the C2 dorsal ramus (Bogduk, 1982). The superficial medial branch of the dorsal ramus is known as the third occipital nerve. This nerve courses around the lower part of the C2-3 Z joint from anterior to posterior. The deep surface of the third occipital nerve provides articular branches to the C2-3 Z joint (Bogduk & Marsland, 1986). Because of its close relationships with the bony elements of the C2-3 IVF, the third occipital nerve has been implicated by one investigator as the cause of the headaches that frequently accompany generalized osteoarthritis of the cervical spine (Trevor-Jones, 1964). After supplying the C2-3 Z joint, the third occipital nerve courses superiorly; pierces the semispinalis capitis, splenius capitis, and trapezius muscles; and then assists the greater occipital nerve (C2) in its sensory innervation of the suboccipital region (Bogduk, 1982). Dash and colleagues (2005) have proposed that compression of the third occipital nerve, as it passes through the muscles of the posterior neck, may be a source of headaches in some patients.
The deep medial branch of the C3 dorsal ramus helps to supply the uppermost multifidus muscles. The lateral branch of the C3 dorsal ramus helps to supply the more superior of the fourth through sixth layers of neck muscles (e.g., longissimus capitis, splenius capitis, semispinalis capitis). In addition, the C3 dorsal ramus also helps to supply the C2-3 (via the dorsal ramus itself, the third occipital nerve, or a communicating branch) and the C3-4 (via the deep medial branch) Z joints. The atlanto-occipital joints and the median and lateral atlanto-axial joints are innervated by the C1 and C2 ventral rami, respectively (Bogduk, 1982). Bogduk and Marsland (1986) reported on the relief of occipital and suboccipital headaches by local anesthetic block of the third occipital nerve in 10 consecutive patients with headaches of suspected cervical origin. They suggested that the cause of the headaches was traumatic arthropathy or degenerative joint disease of the C2-3 Z joints and stated that their findings “may reflect an actual high incidence in the community of a condition that has remained unrecognized by specialists dealing with headache, and perhaps misdiagnosed as tension headache.” They also mentioned that C1-2 joints may be another cause of cervical headache but thought that further investigation was necessary before differentiation between C1-2 and C2-3 headaches could be performed accurately.
Injury to structures of the upper cervical spine can result in pain referral to the occipital regions innervated by the dorsal rami of the upper three cervical nerves. Upper cervical injury can also refer to regions of the head innervated by the trigeminal nerve, because the central processes of the upper three cervical sensory nerves enter the upper cervical spinal cord and converge on neurons of the spinal tract and spinal nucleus of the trigeminal nerve. This region of the spinal cord has been called the trigemino-cervical nucleus (Bogduk et al., 1985). The specific location of pain referral depends on the central neurons stimulated by the incoming cervical fibers. Therefore after injury to the upper cervical region, pain can be interpreted as arising from as far away as the anterior aspect of the head (neurons of trigeminal nerve stimulated by afferents from the C2 ventral ramus) or the suboccipital region to the scalp above the vertex of the skull (region innervated by [C1] C2 and C3 dorsal rami).
The spinal nerves of C4 through C8 exit through their respective IVFs (e.g., C4 through the C3-4 IVF, C8 through the C7-T1 IVF). The dorsal rami are quickly given off and pass posteriorly, medial to the posterior intertransversarii muscles, which they supply. They then divide into medial and lateral branches. The medial branches of C4 and C5 (occasionally C6) divide into a superficial and a deep branch. The dorsal rami of (C6) C7 and C8 do not divide and only have deep medial branches. The superficial branches help to supply the semispinalis cervicis and capitis muscles and then send cutaneous fibers to provide sensory innervation to the skin of the posterior neck. The deep medial branches of the dorsal rami course to the multifidi muscles, where they provide a specific innervation. Each nerve supplies those muscle fibers that attach to the spinous process of a segmental level numbered one less than the nerve. Therefore the C5 deep medial branch supplies those multifidus fibers that insert onto the C4 spinous process (Bogduk, 1982). The deep medial branches of C4 to C8 also supply the Z joints. Each deep medial branch sends a rostral branch to the Z joint above and a caudal branch to the Z joint below. These branches run along the dorsal aspect of the joints within the pericapsular fibrous tissue (Bogduk, 1982). The lateral branches of the C4 to C8 dorsal rami help to supply the semispinalis capitis, longissimus cervicis, splenius cervicis, and iliocostalis cervicis muscles (C8).
Because many structures of the cervical region that can produce pain receive their sensory supply from dorsal rami, certain diagnostic procedures and therapies for neck and head pain have been directed specifically at these nerves (Bogduk, 1989a, b).
Ventral rami: Each ventral ramus of the cervical region leaves its spinal nerve of origin and then exits the spine by passing posterior to the vertebral artery and then between the anterior and posterior intertransversarii muscles. The cervical ventral rami innervate the anterior muscles of the cervical spine, including the longus capitis, longus colli, and rectus capitis anterior and lateralis muscles. The atlanto-occipital joints and the median and lateral atlanto-axial joints are innervated by the C1 and C2 ventral rami, respectively (Bogduk, 1982).
Bogduk (1981) stated that abnormal position (subluxation) of a lateral atlanto-axial joint, compressing the C2 ventral ramus, is the most likely cause of neck-tongue syndrome. This syndrome includes suboccipital pain with simultaneous numbness of the tongue on the same side. The author explained the tongue numbness by the fact that some proprioceptive fibers to the tongue accompany the hypoglossal nerve and then pass through the ventral ramus of C2. Such “numbness” is analogous to that reported in Bell palsy, in which the proprioceptive fibers of the seventh cranial nerve give the sensation of numbness over a region of the face that receives its sensory innervation from the trigeminal nerve.
The cervical ventral rami also help to supply the vertebral bodies, ALL, and anterior aspect of the IVD with sensory innervation. These latter structures also receive sensory innervation from fibers arising from the sympathetic chain (see Fig. 5-31) and from the autonomic fibers associated with the vertebral artery (Bogduk, Windsor, & Inglis, 1988; Groen, Baljet, & Drukker, 1990). A thorough understanding of the specific sensory innervation to the anterior structures of the spine is important because these structures can be damaged during an extension injury or during the acceleration portion of an acceleration-deceleration injury (Foreman & Croft, 1992; Croft, 2009). Therefore the autonomic fibers associated with the recurrent meningeal nerve, the sympathetic chain itself, and the vertebral artery are listed in the following discussion.
The ventral rami of cervical spinal nerves also form the cervical and brachial plexuses, which innervate the anterior neck and upper extremities. These neural elements are discussed at the end of this section.
Recurrent Meningeal Nerve
The recurrent meningeal nerves also are known as the sinuvertebral nerves. In the cervical region, each nerve originates from the ventral ramus and then receives a contribution from the gray communicating ramus and other sympathetic nerves that run with the vertebral artery (Groen, Baljet, & Drukker, 1990) (Fig. 5-32; see also Fig. 5-31). The recurrent meningeal nerve then courses medially, through the medial aspect of the IVF and anterior to the spinal dura. This nerve supplies the posterior aspect of the IVD, PLL, anterior spinal dura mater (Groen, Baljet, & Drukker, 1990), posterior vertebral bodies, and uncovertebral joints (Xiuqing, Bo, & Shizhen, 1988). Usually the recurrent meningeal nerve supplies these structures at the level at which it enters the vertebral canal and then continues superiorly to innervate the same structures at the vertebral level above, although the distribution varies (Groen, Baljet, & Drukker, 1990).
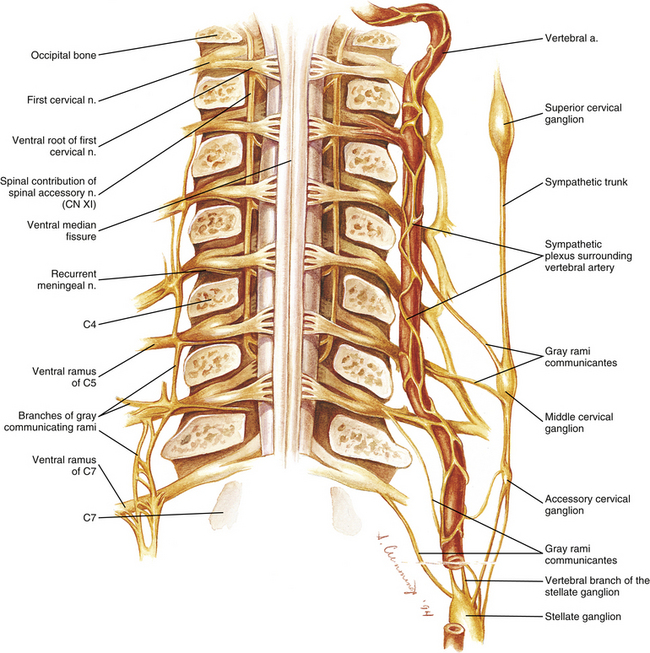
FIG. 5-32 Sympathetic plexus surrounding the vertebral artery. The pedicles have been cut coronally, and the vertebral bodies and transverse processes have been removed to reveal an anterior view of the neural elements. Right, One vertebral artery was spared. Notice several branches from the stellate ganglion coursing to this vertebral artery. The largest of these branches is the vertebral branch of the stellate ganglion (sometimes known as the “vertebral nerve”). Also, notice several gray communicating rami (GRs) contributing to the vertebral artery sympathetic plexus. Left, Components of this plexus after the vertebral artery has been removed. Notice that the GRs branch considerably and sends twigs to join branches of adjacent GRs. In addition, each GR sends twigs to ventral rami of the same level, the level above, and the level below. Other twigs of the plexus unite with branches of the ventral rami to form recurrent meningeal nerves. The recurrent meningeal nerves, in turn, course medially to enter the vertebral canal. Branches of the plexus also innervate the vertebral artery itself by passing into the arterial walls (see text for further details). The ventral rami of the spinal nerves can be seen uniting to form the cervical and brachial plexuses on the right side of the illustration. Notice that the vertebral artery is sending a small arterial branch to the C2 spinal nerve. This branch can be seen dividing into anterior and posterior radicular arteries. These branches, which are normally found at each vertebral level, have been removed from the remaining levels to display the neural elements more clearly.
More than one recurrent meningeal nerve usually is present at each vertebral level (Groen, Baljet, & Drukker, 1990). The recurrent meningeal nerves of the cervical region probably carry both vasomotor fibers, derived from the sympathetic contribution, and general somatic afferent fibers (including nociceptive fibers), arising from the ventral rami (Bogduk, Windsor, & Inglis, 1988).
The recurrent meningeal nerves of C1, C2, and C3 have relatively large meningeal branches that ascend to the posterior cranial fossa. As they course superiorly to reach the posterior cranial fossa, they supply the atlanto-axial joint complex (also supplied by the ventral ramus of C2), tectorial membrane, components of the cruciform ligament, and alar ligaments (Bogduk, Windsor, & Inglis, 1988). Once in the posterior cranial fossa, they help to supply the cranial dura mater, including that in the region of the clivus, which is supplied by the recurrent meningeal nerve of C3 (Bogduk, Windsor, & Inglis, 1988). These meningeal branches probably are related to the pain referral patterns associated with disorders of the upper cervical ligaments (and possibly the atlanto-axial joints) and occipital headache (Standring et al., 2008).
Cervical Sympathetics
This section focuses on those aspects of the sympathetic nervous system most closely related to the general anatomy of the cervical spine. The specific anatomy of the cervical sympathetics is discussed in Chapter 10. The cervical sympathetic chain lies anterior to the longus colli and longus capitis muscles and deep to the prevertebral fascia (Civelek et al., 2008). The sympathetic trunk takes a slight medial to lateral course (10.4 ± 3.8 degrees) as it ascends the cervical region (Ebraheim et al., 2000). It is composed of three ganglia: superior, middle, and inferior. The superior and inferior (cervicothoracic) ganglia are almost always present, but the middle is more variable. The superior ganglion is by far the largest (12.5 ± 1.5 mm long and 5.3 ± 0.6 mm wide), and it is positioned inferior to the occiput and anterior to the TPs of C2 and C3, although it has been consistently found at the level of C4 in unembalmed specimens (Civelek et al., 2008). The middle cervical ganglion is present approximately 48% to 74% of the time, and approximately 10% of middle cervical ganglia are composed of two small ganglia (Civelek et al., 2008; Saylam et al., 2009). When the middle cervical ganglion is present, it usually lies anterior to the TP of C6, but can be found from the TPs of C5-C7 (Civelek et al., 2008). Usually the inferior ganglion unites with the first thoracic ganglion to form the cervicothoracic (stellate) ganglion, located just inferior to the TP of C7 at the level of C7 (40%), the C7-T1 IVD (25%), or the T1 level (35%) (Saylam et al., 2009).
The relationships of the sympathetic plexus surrounding the vertebral artery are complex (see Fig. 5-32). Because of the intimate relationship of this plexus with the vertebral artery and the spinal structures innervated by this plexus, it is discussed here. Chapter 10 also discusses this plexus in the context of the entire autonomic nervous system.
The plexus surrounding the vertebral artery has been called the vertebral nerve (Edmeads, 1978). Other authors (Gayral & Neuwirth, 1954; Xiuqing et al., 1988) state that of the nerves surrounding the vertebral artery, the vertebral nerve is the largest of the several branches that arise from the cervicothoracic (stellate) ganglion to follow the vertebral artery through the foramen of the TP of C6. The term “vertebral branch of the stellate ganglion” (or “vertebral branch of the inferior cervical ganglion” when arising from that ganglion) is now preferred to “vertebral nerve” (Tubbs et al., 2007). The vertebral branch of the stellate ganglion is a gray communicating ramus connecting the stellate ganglion (90%) or the inferior cervical ganglion (10%, then called the vertebral branch of the inferior cervical ganglion) to the anterior primary division (ventral ramus) of C6 or C7. The nerve usually courses from the stellate ganglion to the posteromedial aspect of the vertebral artery, accompanying the artery through the foramen of the transverse process of C6. Approximately 50% of the time the nerve provides branches to the adjacent Z joint capsules and intervertebral discs, and much less frequently provides branches to the anterior aspect of the cervical dura mater (15%) or the deep component of the vertebral artery sympathetic nerve plexus (10%) (Tubbs et al., 2007). This section uses the term vertebral branch of the stellate ganglion only when discussing the previously mentioned large branch of that ganglion. The term vertebral plexus of nerves is used to refer to the neural network surrounding the vertebral artery.
The literature describing the vertebral plexus of nerves is rather confusing, indicating that nerve tracing techniques will need to be used in the future to clarifiy the origins and components of the plexus. When taken as a whole, the literature seems to indicate that the vertebral plexus is composed of superficial and deep components. The superficial component is primarily made up of (larger) gray communicating rami, such as the vertebral branch of the stellate ganglion, just described, that follow the artery superiorly to join the ventral rami of C3 to C6 (see Fig. 5-32). The deeper plexus is more dense and is composed of smaller branches of the cervical sympathetic chain (including contributions from the stellate ganglion).
In addition to the vertebral branch of the stellate ganglion, the superficial component of the plexus receives a branch (or branches) from the middle cervical ganglion. Sometimes branches from intermediate ganglia also join the plexus above the level of C6 (Xiuqing, Bo, & Shizhen, 1988). The branch from the middle cervical ganglion runs laterally to either the C5-6 or the C4-5 uncovertebral joint before reaching the vertebral artery. The superior part of the plexus surrounding the vertebral artery is joined by branches directly from the ventral rami of C1 and C2 (Bogduk, Lambert, & Duckworth, 1981) and C3 (Xiuqing, Bo, & Shizhen, 1988). Branches of the superficial component of the vertebral artery nerve plexus also supply sensory innervation to the lateral aspects of the cervical IVDs (Bogduk, Windsor, & Inglis, 1988).
The deeper and denser plexus of nerves surrounding the vertebral artery is derived from small branches of the stellate ganglion (and approximately 10% of the time additional small branches of the vertebral branch of the stellate ganglion), middle and intermediate cervical ganglia, and cervical ventral rami. These fibers form vascular branches that create a dense neural plexus around the vertebral artery. The vertebral arteries themselves have been found to be capable of producing pain. The afferents for their nociceptive sensation course with the autonomic fibers. Therefore irritation of these fibers by degenerative spur formation of the upper cervical uncovertebral or Z joints may be a cause of headaches (Edmeads, 1978).
Nerves of the Anterior Neck
This section and the sections that follow discuss the neural, muscular, vascular, and visceral structures of the anterior neck. Even though an extensive description of the anatomy of the anterior neck is beyond the scope of this text, the mentioned structures are so intimately related to the cervical spine that covering them in adequate detail is important. Also, flexion and extension injuries to the cervical region, commonly known as whiplash injuries, are prevalent (Croft, 2009). Such injuries vary considerably in severity and can result in damage to a variety of anatomic structures. Injury to the ALL, PLL, interspinous ligament (ligamentum nuchae), IVDs, vertebral end plates, odontoid process, spinous processes, Z joints, muscles, esophagus, sympathetic trunk, temporomandibular joint, cranium, and brain all have been reported, either through experimental studies or during clinical examination, after flexion and extension injury to the cervical region (Bogduk, 1986a). In addition, proper examination of the cervical region includes an examination of the anterior neck. Therefore the following sections describe the most clinically relevant relationships of the anterior neck, beginning with the nerves.
The nerves of the anterior neck include the ventral rami of the cervical nerves. These ventral rami constitute the cervical and brachial (including T1) plexuses (Fig. 5-33). Also, several cranial nerves (CNs) are found in the anterior neck. These include the glossopharyngeal (CN IX), vagus (CN X), accessory (CN XI), and hypoglossal (CN XII) nerves. The cervical and brachial plexuses are discussed in modest detail, and the most relevant points of CNs IX through XII are covered.
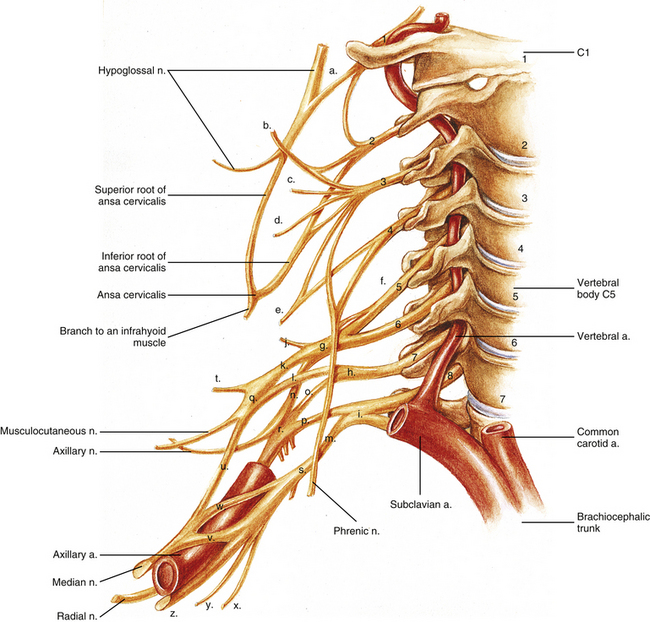
FIG. 5-33 Nerves of the cervical region, including the cervical plexus and the brachial plexus. Notice that the anterior primary divisions (ventral rami) exit posterior to the vertebral artery. The anterior primary divisions of C1 through C4 (with a contribution from C5 to the phrenic nerve) form the cervical plexus, and the anterior primary divisions of C5 through T1 form the roots of the brachial plexus. The following structures are identified: a, anterior primary division (ventral ramus) of C1, uniting with the hypoglossal nerve; b, lesser occipital nerve; c, great auricular nerve (receives contributions from both C2 and C3 ventral rami); d, transverse cervical nerve, also known as the transverse cutaneous nerve of the neck (also receives contributions from both C2 and C3 ventral rami); e, supraclavicular nerve (common trunk of origin for lateral, intermediate, and medial supraclavicular nerves); f, dorsal scapular nerve from C5 ventral ramus would be given off here; g, upper (superior) trunk of the brachial plexus; h, middle trunk; i, lower (inferior) trunk; j, suprascapular nerve; k, anterior division of upper trunk of the brachial plexus; l, anterior division of middle trunk; m, anterior division of lower trunk; n, posterior division of upper trunk of the brachial plexus; o, posterior division of middle trunk; p, posterior division of lower trunk; q, lateral cord of the brachial plexus; r, posterior cord; s, medial cord; t, lateral pectoral nerve; u, contribution of the lateral cord to the median nerve; v, contribution of the medial cord to the median nerve; w, variant additional contribution of medial cord to the median nerve; x, medial brachial cutaneous nerve (medial cutaneous nerve of the arm); y, medial antebrachial cutaneous nerve (medial cutaneous nerve of the forearm); z, ulnar nerve. The medial pectoral nerve is shown arising from the inferior aspect of (s), the medial cord. From proximal to distal, the upper subscapular, thoracodorsal, and lower subscapular nerves are shown arising from, r, the posterior cord. The long thoracic nerve, which arises from the ventral rami of C5, C6, and C7, is not shown in this illustration.
Ventral ramus of C1: This ramus passes laterally around the superior articular process of the atlas. It lies anterior to the vertebral artery in this region and runs medial to the artery as the nerve passes medial to the rectus capitis lateralis muscle (which it supplies) to exit above the TP of the atlas. The ventral ramus of C1 receives some fibers from the ventral ramus of C2, and together these fibers join the hypoglossal nerve. Some fibers of the ventral ramus of C1 follow the hypoglossal nerve proximally and help provide sensory innervation to the dura mater of the posterior cranial fossa (Agur & Dalley, 2013). However, most fibers of the ventral ramus of C1 continue distally along CN XII and then form several branches that leave CN XII. The first such branch participates in the ansa cervicalis and is known as the superior (upper) root of the ansa cervicalis (descendens hypoglossi). The next branch is the nerve to the thyrohyoid, which innervates the thyrohyoid muscle. The nerve to the geniohyoid is the last branch. It innervates the muscle of the same name.
Cervical plexus: The cervical plexus can be divided into sensory and motor portions. The sensory portion of the cervical plexus is more superficially placed than the motor portion. The named nerves of the sensory portion (see Fig. 5-33) are formed deep to the SCM by the union of individual C2 to C4 ventral rami. The named nerves course around the posterior surface of the SCM and emerge from behind its midpoint in proximity to one another. They then proceed in different directions to reach their respective destinations. The named nerves of the sensory (superficial) part of the cervical plexus and their ventral rami of origin are reviewed in Table 5-9 (see also Fig. 5-33).
Table 5-9
Sensory Portion of Cervical Plexus
| Nerve | Cord Segments | Destination |
| Lesser occipital nerve | C2, C3 | Mastoid region and superior aspect of ear |
| Great auricular nerve | C2, C3 | Ear and region overlying angle of mandible |
| Transverse cervical | C2, C3 | Anterior neck |
| Supraclavicular nerve | C3, C4 | Medial, intermediate, and lateral branches to skin over clavicle and deltoid muscle |
The motor portion of the cervical plexus lies deep to the sensory portion and is located within the anterior triangle of the neck. The motor portion comprises the ansa cervicalis (see Fig. 5-33). The two limbs (roots) of the ansa cervicalis are the following:
• C1 ventral ramus (see preceding discussion). Provides separate motor innervation to the thyrohyoid and geniohyoid muscles (the nerves to these muscles may also contain C2 and C3 fibers that ascend from the inferior root of the ansa [Banneheka, 2008], see immediately below) and also forms the superior root of the ansa cervicalis (descendens hypoglossi).
• C2 and C3 rami. Combine to form the inferior root of the ansa cervicalis (descendens cervicalis).
Together the superior and inferior roots combine to form the ansa cervicalis. Branches of this neural loop provide motor innervation to all of the infrahyoid (strap) muscles (i.e., both bellies of the omohyoid, sternohyoid, and sternothyroid), except the thyrohyoid muscle, which is supplied by the ventral ramus of C1.
The phrenic nerve also is considered to be a part of the cervical plexus. It arises from the ventral rami of C3, C4, and C5, with C4 providing the most significant contribution. The phrenic nerve provides motor and sensory innervation to the diaphragm. Occasionally an accessory phrenic nerve arises from the ventral rami of C5 and C6. When present, the accessory phrenic nerve branches from the nerve to the subclavius and courses to the diaphragm.
Brachial plexus: The brachial plexus (see Fig. 5-33) is formed by the ventral rami of C5 through T1. The ventral rami that participate in forming the brachial plexus are called the “roots” of the brachial plexus. The ventral rami (or roots of the plexus) form trunks, the trunks form anterior and posterior divisions, the divisions form cords, and the cords end as terminal branches.
The brachial plexus is discussed in more detail in the following section. Where appropriate, the spinal cord segments that contribute to the formation of the individual named nerves are included in parentheses following the named nerves, for example, radial nerve (C5,6,7,8,T1).
The ventral rami of C5 and C6 form the upper (superior) trunk of the brachial plexus. The ventral ramus of C7 remains free of the complex relationships seen in the other rami and forms the middle trunk by itself. The C8 and T1 ventral rami converge to form the lower (inferior) trunk. A few important branches arise from the ventral rami before they form trunks. The first is the dorsal scapular nerve, which branches from the C5 ramus and provides motor innervation to the rhomboid major and minor muscles and occasionally to the levator scapulae muscle. Branches of the fifth, sixth, and seventh ventral rami form the long thoracic nerve (of Charles Bell), which innervates the serratus anterior muscle.
The suprascapular nerve branches from the upper trunk. (Therefore it is derived from C5 and C6.) It courses through the scapular notch (beneath the superior transverse scapular ligament) to innervate the supraspinatus and infraspinatus muscles. The suprascapular nerve also sends articular twigs to the shoulder joint and the acromioclavicular joint. The nerve to the subclavius muscle (C5,6), which also branches from the upper trunk (C5 and C6), supplies the small muscle of the same name. The nerve to the subclavius usually sends a communicating branch to the phrenic nerve (usually from the C5 contribution).
The trunks divide into anterior and posterior divisions. The anterior divisions of the upper and middle trunks unite to form the lateral cord. The anterior division of the lower trunk remains alone to form the medial cord, and all the posterior divisions unite to form the posterior cord.
The cords of the brachial plexus are named according to their anatomic relationship to the axillary artery (e.g., lateral cord is lateral to the artery). The cords themselves have branches. The lateral cord has a branch called the lateral pectoral nerve (C5,6,7), which innervates both the pectoralis major and minor muscles. The medial cord branches into the medial pectoral nerve (C8,T1), which innervates the pectoralis minor muscle, and a few branches may help to supply the pectoralis major (Standring et al., 2008). The posterior cord gives off the superior or upper (C5,6) and inferior or lower (C5,6) subscapular nerves and the thoracodorsal (middle subscapular) nerve (C6,7,8). The upper subscapular nerve supplies the subscapularis muscle. The thoracodorsal nerve supplies the latissimus dorsi muscle, and the inferior subscapular nerve supplies the teres major muscle and helps to supply the subscapularis muscle.
The cords end as terminal branches of the brachial plexus. The lateral cord divides into the musculocutaneous nerve (C5,6,7) and a large contributing branch to the median nerve (C[5],6,7). The musculocutaneous nerve provides motor innervation to the flexor muscles of the arm and sensory innervation to the lateral forearm. The median nerve is discussed in more detail in the following section.
The medial cord provides the medial brachial (C8,T1) and medial antebrachial (C8,T1) cutaneous nerves (sensory to arm and forearm, respectively) before dividing into the ulnar nerve (C[7],8,T1) and the medial cord contribution to the median (C8,T1) nerve. The ulnar nerve sends articular branches to the elbow and wrist, motor fibers to one and a half muscles of the forearm, and the majority of the intrinsic muscles of the palm. The ulnar nerve is also sensory to the medial distal forearm and medial hand (medial palm, fifth digit, ulnar side of the fourth digit).
Recall that both lateral and medial cords participate in the formation of the median nerve (C[5],6,7,8,T1). This nerve provides articular branches to the elbow and wrist joints, motor innervation to the majority of the muscles of the anterior forearm, and innervation to five muscles of the palm (three thenar muscles, first two lumbricals). In addition, the median nerve provides sensory innervation to the lateral aspect (radial side) of the palm, the anterior aspect of the first three and a half digits, and the distal aspect of the posterior surface of the first three and a half digits. However, the sensory innervation to the hand is subject to significant variation.
The posterior cord ends by dividing into the axillary (C5,6) and radial nerves (C5,6,7,8,T1). The axillary nerve courses through the quadrangular space (i.e., space between the teres minor, teres major, long head of the triceps muscles, and surgical neck of the humerus), supplying motor innervation to the teres minor and the deltoid muscles. In addition, the axillary nerve provides sensory innervation to the upper lateral aspect of the arm.
The radial nerve provides motor and sensory innervation to the posterior arm and forearm. It also gives articular branches to the elbow and wrist joints. In addition, the radial nerve provides sensory innervation to the lateral aspect of the dorsum of the hand and the dorsal aspect of the first three and a half digits (except for the distal portions of these digits that are innervated by the median nerve). Chapter 9 provides additional information on the large terminal branches of the brachial plexus.
Vagus nerve: The vagus nerve exits the jugular foramen of the posterior cranial fossa and courses inferiorly throughout the entire length of the neck. Accompanying the vagus nerve in its course through the neck are the internal jugular vein and the internal and common carotid arteries. These structures are wrapped in a fibrous tissue sheath known as the carotid sheath. The vagus nerve is located within the posterior aspect of the carotid sheath between the internal jugular vein, which is lateral to it, and the internal carotid artery, which is medial to it. Inferiorly the vagus nerve lies between the internal jugular vein and the common carotid artery.
The vagus nerve has several branches in the neck:
• Pharyngeal branch. This nerve participates in the pharyngeal plexus, which supplies motor and sensory innervation to the pharynx.
• Superior laryngeal nerve. This nerve divides into two branches. The first, the internal laryngeal nerve, pierces the thyrohyoid membrane to provide sensory innervation to laryngeal structures above the true vocal folds. The second branch, the external laryngeal nerve, runs inferiorly to innervate the cricothyroid muscle and also helps to supply the inferior constrictor muscle with motor innervation.
• Nerve to the carotid body. This nerve supplies sensory innervation to the chemoreceptor of the same name. It also may help to innervate the carotid sinus, the baroreceptor located at the bifurcation of the common carotid artery into the internal and external carotid arteries.
• Cardiac nerves. Several cardiac nerves enter the thorax and participate in the cardiac plexus of nerves. The cervical cardiac nerves of the vagus provide parasympathetic innervation to the heart.
• Recurrent laryngeal nerve. This nerve loops around the subclavian artery (from anterior to posterior) on the right to run in the groove between the trachea and the esophagus (tracheo-esophageal groove). It provides motor innervation to all the muscles of vocalization with the exception of the cricothyroid muscle, which is innervated by the external laryngeal nerve. The left recurrent laryngeal nerve wraps around the arch of the aorta (from anterior to posterior) just lateral to the ligamentum arteriosum and continues superiorly in the left tracheo-esophageal groove.
Cranial nerves IX, XI, and XII: As with the vagus nerve, the glossopharyngeal nerve (CN IX) exits the posterior cranial fossa by passing through the jugular foramen. It courses along the posterior pharynx just lateral to the stylopharyngeus muscle, which it supplies. CN IX enters the pharynx together with the stylopharyngeus muscle by passing between the superior and middle constrictor muscles and terminates on the posterior third of the tongue. This branch supplies both general sensation and taste sensation to this region of the tongue. The glossopharyngeal nerve also participates in the pharyngeal plexus of nerves. This plexus supplies both motor and sensory innervation to the pharynx. In addition, CN IX with the vagus nerve supplies sensory fibers to the carotid body and sinus. It also provides the parasympathetic fibers that eventually become the lesser petrosal nerve. This nerve synapses in the otic ganglion, and the postganglionic fibers supply secretomotor fibers to the parotid gland.
The accessory and hypoglossal nerves (CNs XI and XII) are located in the superior neck just behind the posterior belly of the digastric muscle. The accessory (spinal accessory) nerve enters the carotid triangle by coursing behind the posterior belly of the digastric and enters the posterior aspect of the SCM. It innervates this muscle before continuing posteriorly to supply the trapezius muscle.
The hypoglossal nerve (CN XII) enters the neck dorsal to the posterior belly of the digastric, courses anteriorly and slightly inferiorly, and then exits the neck by passing medial to the intermediate tendon of the digastric muscle. It continues deep to the mylohyoid muscle and supplies the intrinsic and extrinsic (except the palatoglossus muscle, supplied by CN X, pharyngeal branch) muscles of the tongue.
Muscles of the Anterior Neck
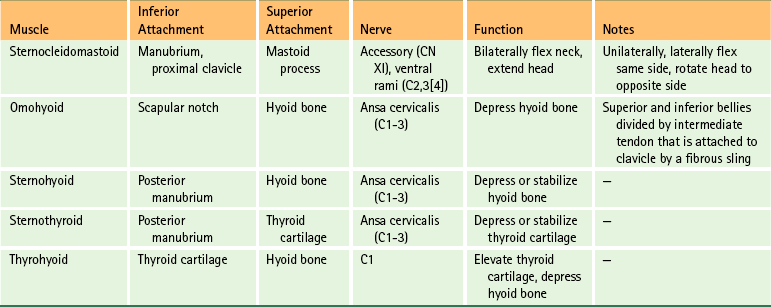
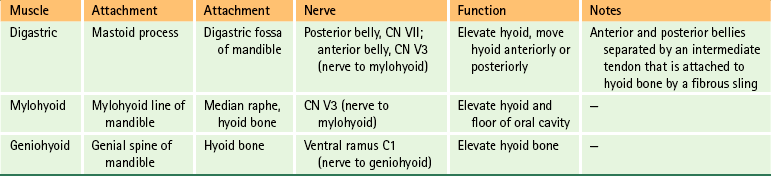
The muscles of the anterior neck (Fig. 5-34) can conveniently be divided into those below the hyoid bone (infrahyoid muscles) and those above the hyoid bone (suprahyoid muscles). The salient features of these two groups of muscles are listed in Tables 5-10 and 5-11 for easy reference.
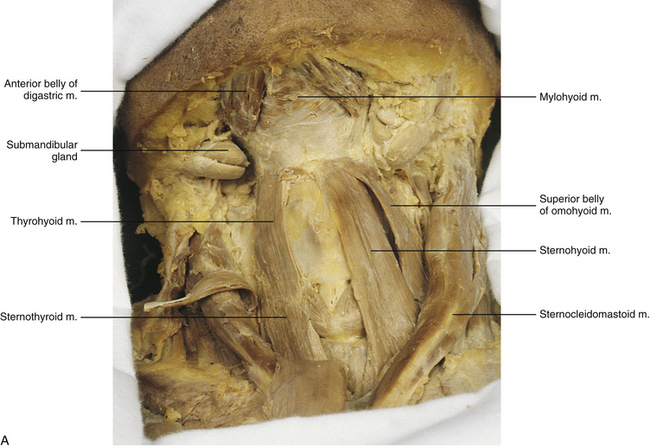
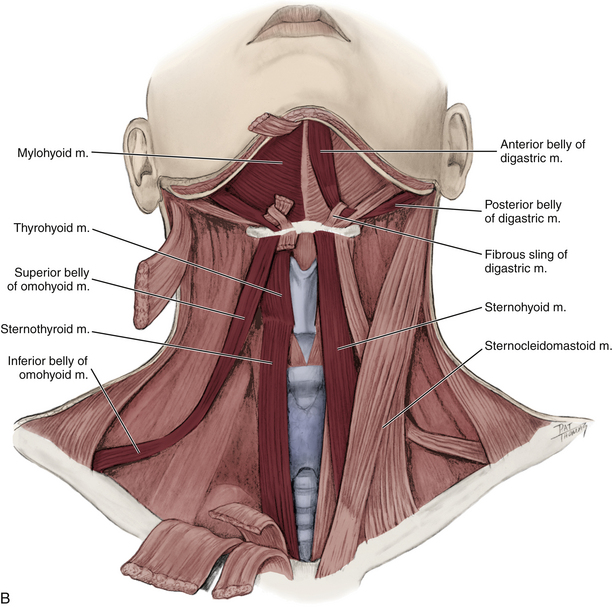
FIG. 5-34 A, Anterior dissection of the neck. The suprahyoid and infrahyoid muscles are shown. The sternohyoid muscle has been removed on the right side of the cadaver (left side of figure) to demonstrate the sternothyroid and thyrohyoid muscles more clearly. B, Illustration showing the suprahyoid and infrahyoid muscles. The fibrous sling attaching the intermediate tendon of the omohyoid to the clavicle is not shown.
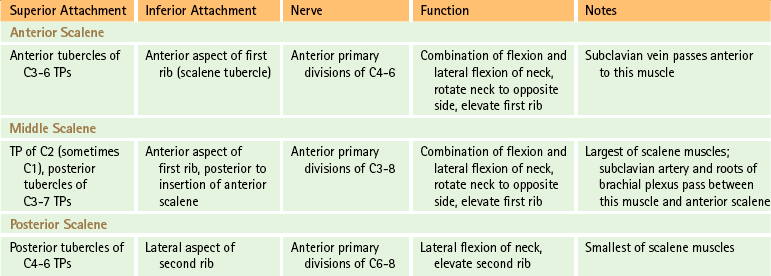
In addition to the infrahyoid and suprahyoid muscles, the SCM and scalene muscles also are associated with the anterior aspect of the cervical spine and neck (Figs. 5-35 and 5-36). The principal features of the scalene muscles are listed in Table 5-12 and illustrated in Figure 5-36. Because of its importance, the SCM is discussed next.
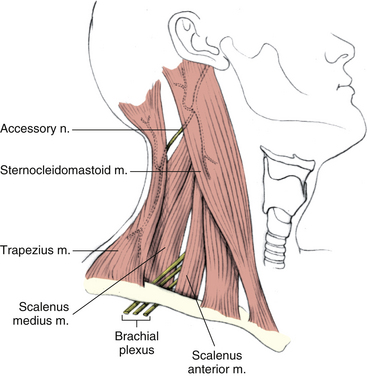
FIG. 5-35 Lateral view of the neck demonstrating the sternocleidomastoid muscle. (From Mathers LH. [1996]. Clinical anatomy principles. St Louis: Mosby.)
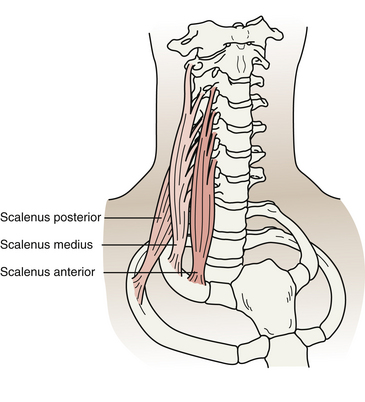
FIG. 5-36 Anterior, middle, and posterior scalene muscles. (From Salvo S. [2003]. Massage therapy principles and practice [2nd ed.]. Philadelphia: WB Saunders.)
Sternocleidomastoid Muscle
The SCM, or sternomastoid muscle, is a prominent and important muscle of the cervical region (see Fig. 5-35). Its origin, insertion, innervation, and function are listed in Table 5-10. As shown in the table, when it contracts unilaterally, the SCM laterally flexes the neck to the same side and rotates the face to the opposite side. Occasionally this muscle becomes abnormally tight, holding the neck in the laterally flexed and contralaterally rotated position. This is known as torticollis, or “wry neck.” Torticollis is caused by a variety of factors, including prolonged exposure (e.g., a night of sleep) in the presence of a cool breeze, congenital torticollis, neurologic torticollis, and torticollis of unknown causes (idiopathic). The pain arising from neurologic and idiopathic torticollis most likely results from more than nociception arising from the muscles or the Z joints, but probably also has a mechanism involving the central nervous system (Kutvonen, Dastidar, & Nurmikko, 1997).
In addition to its innervation from the accessory nerve (CN XI), the SCM receives some fibers of innervation from the ventral rami of C2-4. These are thought to be primarily proprioceptive, although some authors believe that a small proportion of motor fibers also may be present. The presence of motor fibers from cervical ventral rami explains reports of individuals retaining some SCM function after their CN XI had been severed.
Vascular Structures of the Anterior Neck
Lymphatics of the Head and Neck
Lymphatics of the face and head drain inferiorly into the pericervical lymphatic collar. This collar consists of a series of connected lymph nodes, which form a chain that encircles the junction of the head and the neck. The collar consists of the following groups of nodes (from posterior to anterior): occipital, postauricular (retroauricular), preauricular, submandibular, and submental. These lymph nodes are drained by lymphatic channels that eventually drain into the deep cervical lymph nodes, located along the internal jugular vein. The deep cervical lymph nodes empty into the thoracic duct on the left side and the right lymphatic duct on the right side.
Lymphatic drainage of the nasopharynx, retropharyngeal region, and nasal cavities communicates with the lymphatic drainage of the upper cervical region. Retrograde flow of lymph from inflamed tissues of the pharynx or sinuses has been implicated in causing inflammation of the synovial linings of the atlano-occipital and atlanto-axial joints. The inflammation can result in joint effusion and joint instability that can subsequently lead to neurologic deficits, attributable to the close proximity of the spinal cord and exiting neural structures to the upper cervical joints. This scenario has been referred to as “Grisel’s Syndrome” (Menezes & Traynelis, 2008).
Major Arteries of the Anterior Neck
The major arteries of the anterior neck (Fig. 5-37) begin in the base (root) of the neck. The root of the neck is the region bounded by the first thoracic vertebra, first rib, and manubrium of the sternum. The principal arteries of this region are the right and left subclavian and the right and left common carotid. The right subclavian and right common carotid arteries are branches of the brachiocephalic trunk from the aortic arch. The left subclavian and left common carotid arteries branch directly from the aortic arch.
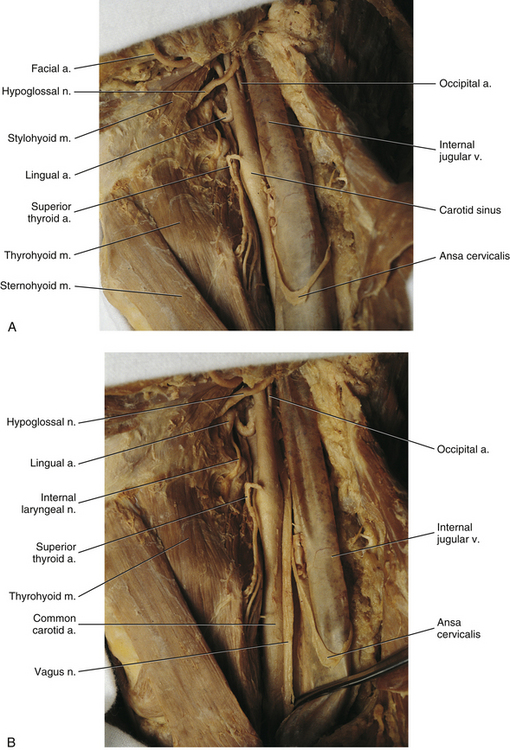
FIG. 5-37 A, Anterolateral dissection of the neck showing the internal jugular vein, common carotid artery, and external carotid artery and several of its branches. B, The internal jugular vein has been moved laterally to reveal the left vagus nerve, which lies posteriorly between the internal jugular vein and common carotid artery. Superior to the bifurcation of the common carotid artery, the vagus nerve lies between the internal jugular vein and internal carotid artery. A and B, The ansa cervicalis can be seen looping across the internal jugular vein.
Subclavian arteries: The branches of the first part of the subclavian artery (from its origin to the medial border of the anterior scalene muscle) are listed next:
1. Vertebral artery. This artery enters the foramen of the TP of C6 and ascends the cervical region through the foramina of the TPs of the remaining five cervical vertebrae. This artery was discussed in detail earlier in this chapter.
2. Internal thoracic artery. Also known as the internal mammary artery, this vessel passes inferiorly into the thorax along the posterior aspect of the anterior thoracic wall.
3. Thyrocervical trunk. This artery has several branches:
a. Inferior thyroid artery. This branch provides the ascending cervical artery before supplying the inferior aspect of the thyroid gland. The ascending cervical artery helps to supply the neck musculature and the posterior elements of the cervical vertebrae.
b. Superficial (transverse) cervical artery. This artery supplies the superficial and deep back muscles of the cervical and upper thoracic regions.
c. Suprascapular artery. This artery supplies several muscles of the scapula.
The following are branches of the second part of the subclavian artery (between the medial and lateral borders of the anterior scalene muscle).
4. Costocervical trunk. This short artery divides into two branches:
a. Deep cervical artery. This branch helps to supply the posterior neck musculature and the posterior arches of the cervical vertebrae.
b. Highest intercostal artery. This branch courses to the first two intercostal spaces.
5. Dorsal scapular artery. This is a branch from the third part of the subclavian (between the lateral aspect of the anterior scalene muscle and the first rib) and is only present when there is no deep branch of the superficial (transverse) cervical artery. It supplies the superficial and deep back muscles of the upper thoracic region.
Carotid arteries: The common carotid artery divides into the internal and external carotid arteries (see Fig. 5-37). This bifurcation usually occurs at the level of the C3-C4 intervertebral disc (see Table 1-1). Before its bifurcation, the common carotid artery expands to form the carotid sinus, which contains baroreceptors to monitor blood pressure. At the bifurcation of the common carotid artery into its internal and external branches, the carotid body is found. The carotid body is responsible for monitoring oxygen and carbon dioxide concentration in the blood. The carotid sinus and body are innervated by CNs IX and X.
Each internal carotid artery ascends the neck, passing approximately 3 mm anterior to the atlas (usually just medial to the region of the lateral mass in front of the C1 foramen of the transverse process) (Currier et al., 2008), to enter the cranial cavity via the carotid foramen (canal). The internal carotid artery then supplies the orbit, pituitary gland, and a large part of the frontal, parietal, and temporal lobes of each cerebral hemisphere.
The external carotid artery is responsible for the blood supply to the neck and face (both superficial and deep face) (see Fig. 5-37). The branches of the external carotid artery are listed next:
1. Superior thyroid artery. This artery courses to the thyroid gland. The superior laryngeal artery branches from the superior thyroid artery. This artery pierces the thyrohyoid membrane with the internal laryngeal nerve and helps supply the larynx.
2. Ascending pharyngeal artery. This is a long artery of small diameter that ascends between the internal and external carotid arteries and supplies the pharynx.
3. Lingual artery. This is a tortuous artery that runs to the tongue by passing deep to the mylohyoid and hyoglossus muscles.
4. Facial artery. This is another tortuous artery that courses to the anterior face; it runs deep to the submandibular gland. (Note: Sometimes the lingual and facial arteries arise from a common faciolingual [linguofacial] trunk.)
5. Occipital artery. This branch courses to the occiput. It is “held” against the external carotid artery by the hypoglossal nerve (CN XII).
6. Posterior auricular artery. This artery runs to and supplies the region posterior to the ear.
The external carotid artery ends by dividing into the:
7. Superficial temporal artery. This large artery courses superiorly to the temporal region and divides into frontal and parietal branches.
8. Maxillary artery. This artery supplies the structures within the infratemporal fossa (deep face), nasal cavity, palate, maxilla, and superior aspect of the pharynx.
Major Veins of the Anterior Neck
A large part of the scalp and the superior and lateral face are drained by the external jugular vein. This vein is formed by the union of the posterior auricular vein and the posterior division of the retromandibular vein. The external jugular vein empties into the subclavian vein.
The veins in the central region of the face and the deep structures of the head and neck drain into the internal jugular vein (see Fig. 5-37). This vein is formed at the jugular foramen by the union of the sigmoidal and inferior petrosal dura mater venous sinuses of the cranial cavity.
More specifically, the central region of the face is drained by the facial vein (anterior facial vein). This vein ends by passing in front of the submandibular gland to join the anterior branch of the retromandibular vein (posterior facial vein). The union of these two veins forms the common facial vein. The common facial vein, in turn, empties into the internal jugular vein. The internal jugular vein joins the subclavian vein to form the brachiocephalic vein. The right and left brachiocephalic veins then unite to form the superior vena cava, which empties into the right atrium.
The anterior jugular veins (right and left) drain the anterior neck. They may communicate in the midline low in the neck close to the region between the left and right clavicular heads (the jugular fossa). The anterior jugular vein drains into either the external jugular or the subclavian vein.
Viscera of the Anterior Neck
The pharynx and esophagus lie in the midline and allow for passage of food from the oral cavity through the thorax and eventually to the abdomen. The larynx and trachea lie anterior to the esophagus and function in vocalization and passage of air to and from the lungs.
The thyroid gland lies in contact with the anterolateral aspect of the inferior larynx and superior trachea. This gland has two lobes, a right and a left, which are united in the midline by the isthmus. The isthmus covers the anterior aspect of the second to fourth tracheal rings. The superior and inferior thyroid arteries provide the blood supply to the thyroid gland. Sympathetics (vasomotor) reach the gland via the middle cervical ganglion. Parasympathetics (uncertain function) are supplied by the laryngeal branches of the vagus nerve.
The four small parathyroid glands (two on each side) are located on the posterior aspect of the thyroid gland.
References
Agur, A.M., Dalley, A.F. Grant's atlas of anatomy, ed 13. Baltimore: Lippincott Williams & Wilkins; 2013.
Alix, M.E., Bates, D.K. A proposed etiology of cervicogenic headache: the neurophysiologic basis and anatomic relationship between dura mater and the rectus posterior capitis minor muscle. J Manipulative Physiol Ther. 1999;22:534–539.
Aprill, C., Bogduk, N. The prevalence of cervical zygapophyseal joint pain. Spine. 1992;17:744–747.
Aprill, C., Dwyer, A., Bogduk, N. Cervical zygapophyseal joint pain patterns II: a clinical evaluation. Spine. 1990;15:458–461.
Bagnall, K.M., Harris, P.F., Jones, P.R.M. A radiographic study of the human fetal spine. 2. The sequence of development of ossification centres in the vertebral column. J Anat. 1977;124:791–802.
Bailey, P., Casamajor, L. Osteo-arthritis of the spine as a cause of compression of the spinal cord and its roots. J Nerv Ment Dis. 1911:588–609.
Banneheka, S. Anatomy of the ansa cervicalis: nerve fiber analysis. Anat Sci Int. 2008;83:61–67.
Barnsley, L., et al. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20:20–26.
Becser, N., Bovim, G., Sjaastad, O. Extracranial nerves in the posterior part of the head: anatomic variations and their possible clinical significance. Spine. 1998;23:1435–1441.
Bednar, D.A., Parikh, J., Hummel, J. Management of type-2 odontoid process fractures in geriatric patients; a prospective study of sequential cohorts with attention to survivorship. J Spinal Disord. 1995;8:166–169.
Behrsin, J., Briggs, C. Ligaments of the lumbar spine: a review. Surg Radiol Anat. 1988;10:211–219.
Bible, J.E., et al. Normal functional range of motion of the cervical spine during 15 activities of daily living. J Spinal Disord Tech. 2010;23:15–21.
Billmann, F., Le Minor, J.M. Transverse foramen of the atlas (C1) anteriorly unclosed: a misknown human variant and its evolutionary significance. Spine (Phila Pa 1976). 2009;34:E422–E426.
Bland, J. Disorders of the cervical spine. Philadelphia: WB Saunders; 1987.
Bland, J. The cervical spine: from anatomy to clinical care. Med Times. 1989;117:15–33.
Bogduk, N. An anatomical basis for the neck-tongue syndrome. J Neurol Neurosurg Psychiatry. 1981;44:202–208.
Bogduk, N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7:319–330.
Bogduk, N. The anatomy and pathophysiology of whiplash. Clin Biomech. 1986;1:92–101.
Bogduk, N. Headaches and the cervical spine (editorial). Cephalalgia. 1986;4:7–8.
Bogduk, N. Local anesthetic blocks of the second cervical ganglion: a technique with application in occipital headache. Cephalalgia. 1989;1:41–50.
Bogduk, N. The rationale for patterns of neck and back pain. Patient Management. 1989;13:17–28.
Bogduk, N. Clinical anatomy of the lumbar spine, 3rd ed. London: Churchill Livingstone; 1997.
Bogduk, N. Diagnostic blocks. In: Clark C.R., ed. The cervical spine. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005:255–260.
Bogduk, N., et al. Cervical headache. Med J Aust. 1985;143:202–207.
Bogduk, N., Lambert, G., Duckworth, J. The anatomy and physiology of the vertebral nerve in relation to cervical migraine. Cephalalgia. 1981;1:11–24.
Bogduk, N., Marsland, A. On the concept of third occipital headache. J Neurol Neurosurg Psychiatry. 1986;49:775–780.
Bogduk, N., Marsland, A. The cervical zygapophysial joints as a source of neck pain. Spine. 1988;13:610–617.
Bogduk, N., Windsor, M., Inglis, A. The innervation of the cervical intervertebral discs. Spine. 1988;13:2–8.
Boulos, A.S., Lovely, T.J. Degenerative cervical spondylolisthesis: diagnosis and management in five cases. J Spinal Disord. 1996;9:241–245.
Boyd-Clark, L.C., Briggs, C.A., Galea, M.P. Segmental degeneration in the cervical spine and associated changes in dorsal root ganglia. Clin Anat. 2004;17:468–477.
Boyle, J.J.W., Singer, K.P., Milne, N. Morphological survey of the cervicothoracic junctional region. Spine. 1996;21:544–548.
Bozbuga, M., et al. Morphometric evaluation of subaxial cervical vertebrae for surgical application of transpedicular screw fixation. Spine (Phila Pa 1976). 2004;29:1876–1880.
Buna, M., et al. Ponticles of the atlas: a review and clinical perspective. J Manipulative Physiol Ther. 1984;7:261–266.
Bush, K., et al. The pathomorphologic changes that accompany the resolution of cervical radiculopathy: a prospective study with repeat magnetic resonance imaging. Spine. 1997;22:183–186.
Cattrysse, E., et al. In vitro three dimensional morphometry of the lateral atlantoaxial articular surfaces. Spine (Phila Pa 1976). 2008;33:1503–1508.
Cave, A.J.E. On the occipito-atlanto-axial articulations. J Anat. 1934;68:416–423.
Cave, A.J.E., Griffiths, J.D., Whiteley, M.M. Osteo-arthritis deformans of Luschka joints. Lancet. 1955;1:176–179.
Chaynes, P., et al. Microsurgical anatomy of the internal vertebral venous plexuses. Surg Radiol Anat. 1998;20:47–51.
Chiba, K., et al. Treatment protocol for fractures of the odontoid process. J Spinal Disord. 1996;9:267–276.
Chikuda, H., et al. Radiographic analysis of the cervical spine in patients with retro-odontoid pseudotumors. Spine (Phila Pa 1976). 2009;34:E110–E114.
Chikuda, H., et al. Acute cervical spinal cord injury complicated by preexisting ossification of the posterior longitudinal ligament: a multicenter study. Spine (Phila Pa 1976). 2011;36:1453–1458.
Chistyakov, A.V., et al. Motor and somatosensory conduction in cervical myelopathy and radiculopathy. Spine. 1995;20:2135–2140.
Christensen, H.W., Nilsson, N. Natural variation of cervical range of motion: a one-way repeated-measures design. J Manipulative Physiol Ther. 1998;21:383–387.
Civelek, E., et al. Surgical anatomy of the cervical sympathetic trunk during anterolateral approach to cervical spine. Eur Spine J. 2008;17:991–995.
Côté, P., et al. Apophysial joint degeneration, disc degeneration, and sagittal curve of the cervical spine. Spine. 1997;22:859–864.
Cramer, G., et al. Identification of the anterior longitudinal ligament on cadaveric spines and comparison with appearance on MRI. Proceedings of the 1996 International Conference on Spinal Manipulation (ICSM); 1996. [pp 151–152].
Cramer, G., et al. Morphometric evaluation of the anterior longitudinal ligament on cadaveric spines by means of magnetic resonance imaging. J Chiropract Educ. 1998;11:141.
Cramer, G., et al. The effects of side posture positioning and spinal adjusting on the lumbar Z joints: a randomized controlled trial of 64 subjects. Spine. 2002;27:2459–2466.
Crawford, C.M., Cassidy, J.D., Burns, S. Cervical spondylotic myelopathy: a report of two cases. Chiropract J Aust. 1995;25:101–110.
Croft, A.C. Cervical acceleration/deceleration trauma: a reappraisal of physical and biochemical events. J Neuromuscular Syst. 1993;1:45–51.
Croft, A.C. Whiplash and mild traumatic brain injuries. Coronado: Spine Research Institute of San Diego Press; 2009.
Currier, B.L., et al. Relationship of the internal carotid artery to the anterior aspect of the C1 vertebra: implications for C1-C2 transarticular and C1 lateral mass fixation. Spine (Phila Pa 1976). 2008;33:635–639.
Cusick, J. Monitoring of cervical spondylotic myelopathy. Spine. 1988;13:877–880.
Czervionke, L., et al. Cervical neural foramina: correlative anatomic and MR imaging study. Radiology. 1988;169:753–759.
Danziger, J., Bloch, S. The widened cervical intervertebral foramen. Radiology. 1975;116:671–674.
Darby, S., Cramer, G. Chapter 3: Pain generators and pain pathways of the head and neck. In: Curl D., ed. Chiropractic approach to head pain. Baltimore: Williams & Wilkins; 1994:55–73.
Dash, K.S., Janis, J.E., Guyuron, B. The lesser and third occipital nerves and migraine headaches. Plast Reconstr Surg. 2005;115:1752–1758. [discussion 1759–1760].
Dean, N.A., Mitchell, B.S. Anatomic relation between the nuchal ligament (ligamentum nuchae) and the spinal dura mater in the craniocervical region. Clin Anat. 2002;15:182–185.
Demaille-Wlodyka, S., et al. Cervical range of motion and cephalic kinesthesis: ultrasonographic analysis by age and sex. Spine (Phila Pa 1976). 2007;32:E254–E261.
Dong, Y., et al. Quantitative anatomy of the lateral mass of the atlas. Spine. 2003;28:860–863.
Doual, J.M., Ferri, J., Laude, M. The influence of senescence on craniofacial and cervical morphology in humans. Surg Radiol Anat. 1997;19:175–183.
Dupuis, P.R., et al. Radiologic diagnosis of degenerative lumbar spinal instability. Spine. 1985;10:262–276.
Dvorak, J., Panjabi, M. Functional anatomy of the alar ligament. Spine. 1987;12:183–189.
Dwyer, A., Aprill, C., Bogduk, N. Cervical zygapophyseal joint pain patterns. I. A study in normal volunteers. Spine. 1990;15:453–457.
Ebraheim, N.A., Biyani, A., Salpietro, B. Zone III fractures of the sacrum: a case report. Spine. 1996;21:2390–2396.
Ebraheim, N.A., et al. Cartilage and synovium of the human atlanto-odontoid joint. Acta Anat. 1997;159:48–56.
Ebraheim, N.A., et al. Quantitative anatomy of the cervical facet and the posterior projection of its inferior facet. J Spinal Disord. 1997;10:308–316.
Ebraheim, N.A., et al. Anatomic considerations for uncovertebral involvement in cervical spondylosis. Clin Orthop Rel Res. 1997;334:200–206.
Ebraheim, N.A., et al. Anatomic basis of the anterior surgery on the cervical spine: relationships between uncus-artery-root complex and vertebral artery injury. Surg Radiol Anat. 1998;20:389–392.
Ebraheim, N., et al. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine. 2000;25:1603–1606.
Edmeads, J. Headaches and head pains associated with diseases of the cervical spine. Med Clin North Am. 1978;62:533–544.
Edmondston, S.J., et al. Influence of cranio-cervical posture on three-dimensional motion of the cervical spine. J Manipulative Physiol Ther. 2005;10:44–51.
Ehara, S. Relationship of elongated anterior tubercle to incomplete segmentation in the cervical spine. Skeletal Radiol. 1996;25:243–245.
Farmer, J.C., Wisneski, R.J. Cervical spine nerve root compression. Spine. 1994;19:1850–1855.
Fesmire, F., Luten, R. The pediatric cervical spine: development anatomy and clinical aspects. J Emerg Med. 1989;7:133–142.
Fletcher, G., et al. Age-related changes in the cervical facet joints: studies with cryomicrotomy, MR and CT. AJNR. 1990;11:27–30.
Foreman, S.M., Croft, A.C. Whiplash injuries: the cervical acceleration/deceleration syndrome. Baltimore: Williams & Wilkins; 1992.
Forristall, R., Marsh, H., Pay, N. Magnetic resonance imaging and contrast CT of the lumbar spine: comparison of diagnostic methods of correlation with surgical findings. Spine. 1988;13:1049–1054.
Francke, J.P., et al. The vertebral arteries: atlanto-axial and intracranial segments. Anat Clin (Surg Radiol Anat). 1980;2:229–242.
Frank, E. HLA-DR expression on arachnoid cells. Spine. 1995;20:2093–2096.
Friedrich, K.M., et al. High-field magnetic resonance imaging of meniscoids in the zygapophyseal joints of the human cervical spine. Spine (Phila Pa 1976). 2007;32:244–248.
Friedrich, K.M., et al. Reference data for in vivo magnetic resonance imaging properties of meniscoids in the cervical zygapophyseal joints. Spine (Phila Pa 1976). 2008;33:E778–E783.
Fujiwara, K., et al. Morphometry of the cervical spinal cord and its relation to pathology in cases with compression myelopathy. Spine. 1988;13:1212–1216.
Gates, D. Correlative spinal anatomy. Lakemont, Ga: CHB Printing and Binding; 1980.
Gayral, L., Neuwirth, E. Oto-neuro-ophthalmologic manifestations of cervical origin, posterior cervical sympathetic syndrome of Barré-Lieou. NY State J Med. 1954;54:1920–1926.
George, B., Laurian, C. The vertebral artery: pathology and surgery. New York: Springer-Verlag, p 258; 1987.
Giacobetti, F.B., et al. Vertebral artery occlusion associated with cervical spine trauma. Spine. 1997;22:188–192.
Gluncic, V., et al. Anomalous origin of both vertebral arteries. Clin Anat. 1999;12:281–284.
Gottleib, M.S. Absence of symmetry in superior articular facets on the first cervical vertebra in humans: implications for diagnosis and treatment. J Manipulative Physiol Ther. 1994;17:314–320.
Gregory, D.E., Callaghan, J.P. Does vibration influence the initiation of intervertebral disc herniation? An examination of herniation occurrence using a porcine cervical disc model. Spine (Phila Pa 1976). 2011;36:E225–E231.
Groen, G., Baljet, B., Drukker, J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282–296.
Hack, G.D., et al. Anatomic relation between the rectus capitis posterior minor muscle and the dura mater. Spine. 1995;20:2484–2486.
Hack, G.D., et al. In response. Spine. 1995;20:925–926.
Hackney, D.B. Normal anatomy. Top Magn Reson Imaging. 1992;4:1–6.
Hadley, L.A. The covertebral articulations and cervical foramen encroachment. J Bone Joint Surg. 1957;39-A:910–920.
Haffajee, M.R., Thompson, C., Govender, S. The supraodontoid space or “apical cave” at the craniocervical junction: a microdissection study. Clin Anat. 2008;21:405–415.
Hallgren, R.C., Cattrysse, E., Zrull, J.M. In vitro characterization of the anterior to posterior curvature of the superior articular facets of the atlas as a function of age. Spine J. 2011;11:241–244.
Halliday, D.R., et al. Torn cervical ligaments: necropsy examination of the normal cervical region of the spinal column. J Trauma. 1964;4:219–232.
Hankinson, T.C., Anderson, R.C. Craniovertebral junction abnormalities in Down syndrome. Neurosurgery. 2010;66:32–38.
Hasue, M., et al. Anatomic study of the interrelation between lumbosacral nerve roots and their surrounding tissues. Spine. 1983;8:50–58.
Haynes, M.J., et al. Vertebral arteries and cervical rotation: modeling and magnetic resonance angiography studies. J Manipulative Physiol Ther. 2002;25:370–382.
Herrera, M., Puchades-Ortis, A. Correspondence: morphology of the articular process of the sixth cervical vertebra in humans. J Anat. 1998;192:309–311.
Ho, P.S., et al. Ligamentum flavum: appearance on sagittal and coronal MR images. Radiology. 1988;168:469–472.
Holmes, A., et al. Changes in cervical canal spinal volume during in vitro flexion-extension. Spine. 1996;21:1313–1319.
Hong, J.T., et al. Analysis of anatomical variations of bone and vascular structures around the posterior atlantal arch using three-dimensional computed tomography angiography. J Neurosurg Spine. 2008;8:230–236.
Hukuda, S., et al. Large vertebral body, in addition to narrow spinal canal, are risk factors for cervical myelopathy. J Spinal Disord. 1996;9:177–186.
Humphreys, B.K., et al. Investigation of connective tissue attachments to the cervical spinal dura mater. Clin Anat. 2003;16:152–159.
Humphreys, S.C., et al. The natural history of the cervical foramen in symptomatic and asymptomatic individuals aged 20-60 years as measured by magnetic resonance imaging. Spine. 1998;23:2180–2184.
Hussain, M., et al. Reduction in segmental flexibility because of disc degeneration is accompanied by higher changes in facet loads than changes in disc pressure: a poroelastic C5-C6 finite element investigation. Spine J. 2010;10:1069–1077.
Hussain, M., et al. Patterns of height changes in anterior and posterior cervical disc regions affects the contact loading at posterior facets during moderate and severe disc degeneration: a poroelastic C5-C6 finite element model study. Spine (Phila Pa 1976). 2010;35:E873–E881.
Iizuka, H., et al. The characteristics of bony ankylosis of the facet joint of the upper cervical spine in rheumatoid arthritis patients. Eur Spine J. 2009;18:1130–1134.
Imai, S., et al. An ultrastructural study of calcitonin gene-related peptide-immunoreactive nerve fibers innervating the rat posterior longitudinal ligament. Spine. 1997;22:1941–1947.
Ito, T., et al. Cervical spondylotic myelopathy. Spine. 1996;21:827–833.
Janis, J.E., et al. Neurovascular compression of the greater occipital nerve: implications for migraine headaches. Plast Reconstr Surg. 2010;126:1996–2001.
Ji-Hong, F., et al. Variable morphology of the axis vertebrae in 100 specimens: implications for clinical palpation and diagnostic imaging. J Manipulative Physiol Ther. 2010;33:125–131.
Johnson, G.M., Zhang, M., Jones, G. The fine connective tissue architecture of the human ligamentum nuchae. Spine. 2000;25:5–9.
Jonsson E. (2000). Back pain, neck pain. SBU Project Group 2000, Summary & Conclusions, Report 145.
Jovanovic, M. A comparative study of the foramen transversarium of the sixth and seventh cervical vertebrae. Surg Radiol Anat. 1990;12:167–172.
Kadri, P.A., Al-Mefty, O. Anatomy of the nuchal ligament and its surgical applications. Neurosurgery. 2007;61:301–304. [discussion 304].
Kapandji, I.A. The physiology of the joints. Annotated diagrams of the mechanics of the human joints, 6th ed. Edinburgh: Churchill Livingstone; 2008.
Karaikovic, E.E., et al. Morphologic characteristics of human cervical pedicles. Spine. 1997;22:493–500.
Karau, P.B., et al. Anatomy and prevalence of atlas vertebrae bridges in a Kenyan population: an osteological study. Clin Anat. 2010;23:649–653.
Kasai, T., et al. Cutaneous branches from the dorsal rami of the cervical nerves, with emphasis on their positional relations to the semispinalis cervicis. Okajimas Folia Anat Jpn. 1989;66:153–160.
Kasai, T., et al. Growth of the cervical spine with special reference to its lordosis and mobility. Spine. 1996;21:2067–2073.
Kaufman, R., Glenn, W. Rheumatoid cervical myelopathy evaluation by computerized tomography with multiplanar reconstruction. J Rheumatol. 1983;10:42–54.
Kawaguchi, Y., et al. Pathomechanism of myelopathy and surgical results of laminoplasty in elderly patients with cervical spondylosis. Spine. 2003;28:2209–2214.
Kazan, S., et al. Anatomical evaluation of the groove for the vertebral artery in the axis vertebrae for atlanto-axial transarticular screw fixation technique. Clin Anat. 2000;13:237–243.
Kettler, A., Werner, K., Wilke, H.J. Morphological changes of cervical facet joints in elderly individuals. Eur Spine J. 2007;16:987–992.
Kinalski, R., Kostro, B. Planimetric measurements of intervertebral foramina in cervical spondylosis. Polish Med J. 1971;10:737–742.
Kissel, P., Youmans, J. Post-traumatic anterior cervical osteophyte and dysphagia: surgical report and literature review. J Spinal Disord. 1992;5:104–107.
Koebke, J., Brade, H. Morphological and functional studies on the lateral joints of the first and second cervical vertebrae in man. Anat Embryol. 1982;161:265–275.
Kokubun, S., Sakurai, M., Tanaka, Y. Cartilaginous end plate in cervical disc herniation. Spine. 1996;21:190–195.
Kos, J., Hert, J., Sevcik, P. Meniscoids of the intervertebral joints [in Czech]. Acta Chir Orthop Traumatol Cech. 2002;69:149–157.
Kurokawa, R., et al. Altered blood flow distribution in the rat spinal cord under chronic compression. Spine (Phila Pa 1976). 2011;36:1006–1009.
Kutvonen, O., Dastidar, P., Nurmikko, T. Pain in spasmodic torticollis. Pain. 1997;69:279–286.
Kwon, B.K., et al. Morphologic evaluation of cervical spine anatomy with computed tomography: anterior cervical plate fixation considerations. J Spinal Disord Tech. 2004;17:102–107.
Lang, J.K., Buchfelder, M. Radiofrequency neurotomy for headache stemming from the zygapophysial joints C2/3 and C3/4. Cen Eur Neurosurg. 2010;71:75–79.
Lefebvre, S., et al. Unstable os odontoideum in young children. J CCA. 1993;37:141–144.
Lehto, E.M., et al. The clinical picture of atlantoaxial subluxation changes when atlantoaxial impaction develops. Joint Bone Spine. 2010;77:159–164.
Le Minor, J., Kahn, E., Di Paola, R. Osteometry by computer aided image analysis: application to the human atlas. Gegenbaurs Morphol Jahrb (Leipzig). 1989;135:865–874.
Liang, Q.Q., et al. Prolonged upright posture induces degenerative changes in intervertebral discs of rat cervical spine. Spine (Phila Pa 1976). 2011;36:E14–E19.
Licht, P., et al. Triplex ultrasound of vertebral artery flow during cervical rotation. J Manipulative Physiol Ther. 1998;21:27–31.
Liguoro, D., Vandermeersch, B., Guerin, J. Dimensions of cervical vertebral bodies according to age and sex. Surg Radiol Anat. 1994;16:149–155.
Liu, J., et al. Quantitative changes in the cervical neural foramen resulting from axial traction: in vivo imaging study. Spine J. 2008;8:619–623.
Lohiya, G.S. Os odontoideum: chronic neck pain after car accident; failure of two posterior atlanto-axial arthrodesis—medicolegal issues in the occupational setting. J Manipulative Physiol Ther. 1993;16:475–480.
Loudon, J.K., Ruhl, M., Field, E. Ability to reproduce head position after whiplash injury. Spine. 1997;22:865–868.
Louis, R. Spinal stability as defined by the three column spine concept. Anat Clin. 1985;7:33–42.
Lord, S.M., et al. Chronic cervical zygapophysial joint pain after whiplash. Spine. 1996;21:1737–1745.
Lu, J., et al. Anatomic basis for anterior spinal surgery: surgical anatomy of the cervical vertebral body and disc space. Surg Radiol Anat. 1999;21:235–239.
Lu, J., Ebraheim, N. Anatomic considerations of C2 nerve root ganglion. Spine. 1998;23:649–652.
Malmstrom, E.M., et al. Primary and coupled cervical movements: the effect of age, gender, and body mass index. A 3-dimensional movement analysis of a population without symptoms of neck disorders. Spine (Phila Pa 1976). 2006;31:E44–E50.
Marcelis, S., et al. Cervical spine: comparison of 45° and 55° anteroposterior oblique radiographic projections. Radiology. 1993;188:253–256.
Matsuda, Y., et al. Atlanto-occipital hypermobility in subjects with Down's syndrome. Spine. 1995;20:2283–2286.
Matsunaga, S., et al. Effects of strain distribution in the intervertebral discs on the progression of ossification of the posterior longitudinal ligaments. Spine. 1996;21:184–189.
McCormack, B.M., Weinstein, P.R. Cervical spondylosis. WJM. 1996;165:43–51.
McLain, R.F. Mechanoreceptor endings in human cervical facet joints. Spine. 1994;19:495–501.
Mendel, T., et al. Neural elements in human cervical intervertebral discs. Spine. 1992;17:132–135.
Menezes, A.H., Traynelis, V.C. Anatomy and biomechanics of normal craniovertebral junction (a) and biomechanics of stabilization (b). Childs Nerv Syst. 2008;24:1091–1100.
Mercer, S., Bogduk, N. Intra-articular inclusions of the cervical synovial joints. Br J Rheumatol. 1993;32:705–710.
Mercer, S.R., Bogduk, N. Clinical anatomy of ligamentum nuchae. Clin Anat. 2003;16:484–493.
Meseke, C.A., Duray, S.M., Brillon, S.R. Principal components analysis of the atlas vertebra. J Manipulative Physiol Ther. 2008;31:212–216.
Meyer, J.S., Yoshida, K., Sakamoto, K. Autonomic control of cerebral blood flow measured by electromagnetic flowmeters. Neurology. 1967;17:638–648.
Meyer, S.A., et al. Cervical spinal stenosis and stingers in collegiate football players. Am J Sports Med. 1994;22:158–166.
Miles, K.A., Finlay, D. Is prevertebral soft tissue swelling a useful sign in injury of the cervical spine? Injury. 1988;19:177–179.
Milz, S., et al. Fibrocartilage in the transverse ligament of the human atlas. Spine. 2001;26:1765–1771.
Mitchell, B.S., Humphreys, B.K., O'Sullivan, E. Attachments of the ligamentum nuchae to cervical posterior spinal dura and the lateral part of the occipital bone. J Manipulative Physiol Ther. 1998;21:145–148.
Mitchell, J.A. Changes in vertebral artery blood flow following normal rotation of the cervical spine. J Manipulative Physiol Ther. 2003;26:347–351.
Moriishi, J., et al. The intersegmental anastomoses between spinal nerve roots. Anat Rec. 1989;224:110–116.
Mosser, S.W., et al. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg. 2004;113:693–697. [discussion 698–700].
Motegi, H., et al. Proliferating cell nuclear antigen in hypertrophied spinal ligaments. Spine. 1998;23:305–310.
Muhle, C., et al. In vivo changes in the neuroforaminal size at flexion-extension and axial rotation of the cervical spine in healthy persons examined using kinematic magnetic resonance imaging. Spine. 2001;26:e287–e293.
Murphy, F., Simmons, J.C., Brunson, B. Ruptured cervical discs, 1939-1972. Clin Neurosurg. 1973;20:9–17.
Musha, Y., Mizutani, K. Cervical myelopathy accompanied with hypoplasia of the posterior arch of the atlas: case report. J Spinal Disord Tech. 2009;22:228–232.
Nagashima, C., Iwama, K. Electrical stimulation of the stellate ganglion and the vertebral nerve. J Neurosurg. 1972;36(6):756–762.
Nagayoshi, R., et al. Evaluation of occipitocervical subluxation in rheumatoid arthritis patients, using coronal-view reconstructive computed tomography. Spine (Phila Pa 1976). 2009;34:E879–E881.
Nilsson, N., Christensen, H.W., Hartvigsen, J. The interexaminer reliability of measuring passive cervical range of motion, revisited. J Manipulative Physiol Ther. 1996;19:302–305.
Oga, M., et al. Tortuosity of the vertebral artery in patients with cervical spondylotic myelopathy: risk factor for the vertebral artery injury during anterior cervical decompression. Spine. 1996;21:1085–1089.
Oliver, J., Middleditch, A. Functional anatomy of the spine. Oxford, UK: Butterworth Heinemann; 1991.
Omura, K., et al. Cervical myelopathy caused by calcium pyrophosphate dehydrate crystal deposition in facet joints. Spine. 1996;21:2372–2375.
Onan, O.A., Heggeness, M.H., Hipp, J.A. A motion analysis of the cervical facet joint. Spine. 1998;23:430–439.
Orofino, C., Sherman, M.S., Schecter, D. Luschka's joint: a degenerative phenomenon. J Bone Joint Surg. 1960;42-A:853–858.
Ouedraogo, D.D., et al. Predominant cervical involvement in patients with psoriatic arthritis: report of two cases. Joint Bone Spine. 2007;74:175–178.
Paik, N.C. Bilateral cervical spondylolysis of C7. Spine J. 2010;10:e10–e13.
Pal, G.P., et al. Trajectory architecture of the trabecular bone between the body and the neural arch in human vertebrae. Anat Rec. 1988;222:418–425.
Panjabi, M., et al. Flexion, extension, and lateral bending of the upper cervical spine in response to alar ligament transections. J Spinal Disord. 1991;4:157–167.
Panjabi, M.M., et al. The cortical shell architecture of human cervical vertebral bodies. Spine. 2001;26:2478–2484.
Panjabi, M.M., et al. Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine. 2001;26:2692–2700.
Panjabi, M., Oxland, T., Parks, E. Quantitative anatomy of cervical spine ligaments. Part I. Upper cervical spine. J Spinal Disord. 1991;4:270–276.
Panjabi, M., Oxland, T., Parks, E. Quantitative anatomy of cervical spine ligaments. Part II. Middle and lower cervical spine. J Spinal Disord. 1991;4:277–285.
Panjabi, M.M., et al. Cervical spine kinematics and clinical instability. In: Clark C.R., ed. The cervical spine. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005:55–78.
Paolini, S., Lanzino, G. Anatomical relationships between the V2 segment of the vertebral artery and the cervical nerve roots. J Neurosurg Spine. 2006;5:440–442.
Pech, P., et al. The cervical neural foramina: correlation on microtomy and CT anatomy. Radiology. 1985;155:143–146.
Pei-Feng, J., et al. Morphological asymmetry of the atlas and its clinical implications. J Manipulative Physiol Ther. 2011;34:463–467.
Peterson, C., et al. Prevalence of hyperplastic articular pillars in the cervical spine in relationship with cervical lordosis. J Manipulative Physiol Ther. 1999;22:390–394.
Peterson, C., et al. Vertebral artery hypoplasia: prevalence and reliability of identifying and grading its severity on magnetic resonance imaging scans. J Manipulative Physiol Ther. 2010;33:207–211.
Pettersson, K., et al. Disc pathology after whiplash injury. Spine. 1997;22:283–288.
Pfirrmann, C.W., et al. MR morphology of alar ligaments and occipitoatlantoaxial joints: study in 50 asymptomatic subjects. Radiology. 2001;218:133–137.
Przybylski, G.J., et al. Human anterior and posterior cervical longitudinal ligaments possess similar tensile properties. J Orthop Res. 1996;14:1005–1008.
Przybylski, G.J., et al. Quantitative anthropometry of the subatlantal cervical longitudinal ligaments. Spine. 1998;23:893–898.
Ratinov, G. Extradural intracranial portion of the carotid artery. Arch Neurol. 1964;10:66–73.
Renaudin, J., Snyder, M. Chip fracture through the superior articular facet with compressive cervical radiculopathy. J Trauma. 1978;18:66–67.
Reynolds, J., et al. Cervical range of movement in relation to neck dimension. Eur Spine J. 2009;18:863–868.
Ross, J.K., Bereznick, D.E., McGill, S.M. Atlas-axis facet asymmetry: implications in manual palpation. Spine. 1999;24:1203.
Safran, M.R. Nerve injury about the shoulder in athletes, part 2: long thoracic nerve, spinal accessory nerve, burners/stingers, thoracic outlet syndrome. Am J Sports Med. 2004;32:1063–1076.
Sakaura, H., et al. Preservation of the nuchal ligament plays an important role in preventing unfavorable radiologic changes after laminoplasty. J Spinal Disord Tech. 2008;21:338–343.
Samartzis, D., et al. A revisitation of distractive-extension injuries of the subaxial cervical spine: a cadaveric and radiographic soft tissue analysis. Spine (Phila Pa 1976). 2010;35:395–402.
Saylam, C.Y., et al. Neuroanatomy of cervical sympathetic trunk: a cadaveric study. Clin Anat. 2009;22:324–330.
Schellhas, K.P., et al. Cervical discogenic pain: prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine. 1996;21:300–311.
Schiff, D.C., Parke, W.W. The arterial supply of the odontoid process. J Bone Joint Surg Am. 1973;55:1450–1456.
Schima, W., et al. Case report; cervical intervertebral foramen widening caused by vertebral artery tortuosity. Diagnosis with MR and colour-coded Doppler sonography. Br J Radiol. 1993;66:165–167.
Schimmel, D., Newton, T., Mani, J. Widening of the cervical intervertebral foramen. Neuroradiology. 1976;12:3–10.
Schmidt, H.P., Pierson, J.C. The intrinsic regulation of the blood vessels of the medulla oblongata. Am J Physiol. 1934;10:241–263.
Schulte, T.L., et al. Intra-articular meniscoid folds in thoracic zygapophysial joints. Spine (Phila Pa 1976). 35, 2010.
Senol, U., et al. Anteroposterior diameter of the vertebral canal in cervical region: comparison of anatomical, computed tomographic, and plain film measurements. Clin Anat. 2001;14:15–18.
Shingyouchi, Y., Nagahama, A., Niida, M. Ligamentous ossification of the cervical spine in late middle-aged Japanese men. Spine. 1996;21:2474–2478.
Shinomiya, K., et al. Isolated muscle atrophy of distal upper extremity in spinal compression disorders. J Spinal Disord. 1995;7:311–316.
Shinomiya, K., et al. An analysis of the posterior epidural ligament role on the cervical spinal cord. Spine. 1996;21:2081–2088.
Sosner, J., Fast, A., Kahan, B.S. Odontoid fracture and C1-C2 subluxation in psoriatic cervical spondyloarthropathy. Spine. 1996;21:519–521.
Standring, S., et al. Gray's anatomy: the anatomical basis of clinical practice, 40th ed. Edinburgh: Churchill Livingstone; 2008.
Sunderland, S. Meningeal-neutral relations in the intervertebral foramen. J Neurosurg. 1974;40:756–763.
Taitz, C. Anatomical observations of the developmental and spondylotic cervical spinal canal in South African blacks and whites. Clin Anat. 1996;9:395–400.
Taitz, C. Bony observations of some morphological variations and anomalies of the craniovertebral region. Clin Anat. 2000;13:354–360.
Taitz, C., Arensburg, B. Erosion of the foramen transversarium of the axis. Acta Anat. 1989;134:12–17.
Taitz, C., Nathan, H. Some observations on the posterior and lateral bridge of the atlas. Acta Anat. 1986;127:212–217.
Taitz, C., Nathan, H., Arensburg, B. Anatomical observations of the foramina transversaria. J Neurol Neurosurg Psychiatry. 1978;41:170–176.
Takeshita, K., et al. The nuchal ligament restrains cervical spine flexion. Spine (Phila Pa 1976). 2004;29:E388–E393.
Tanaka, N., et al. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine. 2000;25:286–291.
Tang, X.Y., et al. Anatomic study of the synovial folds of the occipito-atlanto-axial joints. Clin Anat. 2007;20:376–381.
Taylor, J.R. Ligaments and annulus fibrosus of cervical discs. Spine. 1999;24:627–628.
Tondury, G. Zur anatomie der Halswirbelsaule. Gibt es Uncovertebralgelenke? Z Anat EntwGesch. 1943;112:448–459.
Tonetti, J., et al. Elastic reinforcement and thickness of the joint capsules of the lower cervical spine. Surg Radiol Anat. 1999;21:35–39.
Torg, J.S., et al. Neurapraxia of the cervical spinal cord with transient quadriplegia. J Bone Joint Surg. 1986;68-A:1354–1370.
Trevor-Jones, R. Osteo-arthritis of the paravertebral joints of the second and third cervical vertebrae as a cause of occipital headaches. S Afr Med J. 1964:392–394. [May].
Tubbs, R.S., et al. The vertebral nerve revisited. Clin Anat. 2007;20:644–647.
Tubbs, R.S., et al. The transverse occipital ligament: anatomy and potential functional significance. Neurosurgery. 2010;66:1–3. [discussion 3].
Uhrenholt, L., et al. Degenerative and traumatic changes in the lower cervical spine facet joints. Scand J Rheumatol. 2008;37:375–384.
Van Dyke, D., Gahagan, C. Down's syndrome cervical spine abnormalities and problems. Clin Pediatr. 1988;27:415–418.
Verska, J.M., Anderson, P.A. Os odontoideum. Spine. 1997;22:706–709.
Viikari-Juntara, E., et al. Evaluation of cervical disc degeneration with ultralow field MRI and discogrophy: an experimental study on cadavers. Spine. 1989;14:616–619.
Von Lanz, T. Uber die ruckenmarkshaute. I. Die konstruktive form der harten haut des menschlichen ruckenmarkes und ihrer bander. Arch Entwickl Mech Org. 1929;118:252–307.
von Ludinghausen, M., et al. Accessory joints between basiocciput and atlas/axis in the median plane. Clin Anat. 2005;18:558–571.
Wang, A., et al. Cervical chordoma presenting with intervertebral foramen enlargement mimicking neurofibroma CT findings. J Comput Assist Tomogr. 1984;8:529–532.
Wang, P., et al. Ossification of the posterior longitudinal ligament of the spine. Spine. 1999;24:142–144.
Webb, A.L., et al. Synovial folds of the lateral atlantoaxial joints: in vivo quantitative assessment using magnetic resonance imaging in healthy volunteers. Spine (Phila Pa 1976). 2009;34:E697–E702.
Webb, A.L., et al. Synovial folds—a pain in the neck? Man Ther. 2011;16:118–124.
Webb, A.L., Rassoulian, H., Mitchell, B.S. Morphometry of the synovial folds of the lateral atlanto-axial joints: the anatomical basis for understanding their potential role in neck pain. Surg Radiol Anat. 2012;34:115–124.
Weiglein, A.H., Schmidberger, H.R. The radio-anatomic importance of the colliculus atlantis. Surg Radiol Anat. 1998;20:209–214.
Wiegand, R., et al. Cervical spine geometry correlated to cervical degenerative disease in a symptomatic group. J Manipulative Physiol Ther. 2003;26:341–346.
White, A.W., Panjabi, M.M. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1990.
Wight, S., Osborne, N., Breen, A.C. Incidents of ponticulus posterior of the atlas in migraine and cervicogenic headache. J Manipulative Physiol Ther. 1999;22:15–20.
Wilkinson, I.M.S. The vertebral artery. Arch Neurol. 1972;27:392–396.
Xiuqing, C., Bo, S., Shizhen, Z. Nerves accompanying the vertebral artery and their clinical relevance. Spine. 1988;13:1360–1364.
Xu, R., et al. Morphology of the second cervical vertebra and the posterior projection of the C2 pedicle axis. Spine. 1995;20:259–263.
Yabuki, S., Kikuchi, S. Positions of dorsal root ganglia in the cervical spine. Spine. 1996;21:1513–1517.
Yamada, H., et al. Direct innervation of sensory fibers from the dorsal root ganglion of the cervical dura mater of rats. Spine. 1998;23:1524–1530.
Yamada, K., et al. High serum levels of menatetrenone in male patients with ossification of the posterior longitudinal ligament. Spine. 2003;28:1789–1793.
Yenerich, D.O., Haughton, V.M. Oblique plane MR imaging of the cervical spine. J Comput Assist Tomogr. 1986;5:823–826.
Yi-Kai, L., et al. Changes and implications of blood flow velocity of the vertebral artery during rotation and extension of the head. J Manipulative Physiol Ther. 1999;22:91–95.
Yilmazlar, S., et al. Clinical importance of ligamentous and osseous structures in the cervical uncovertebral foraminal region. Clin Anat. 2003;16:404–410.
Yilmazlar, S., et al. Details of fibroligamentous structures in the cervical unco-vertebral region: an obscure corner. Surg Radiol Anat. 2003;25:50–53.
Yoo, J.U., et al. Effect of cervical spine motion on the neuroforaminal dimensions of the human cervical spine. Spine. 1992;17:1131–1136.
Yu, S., Sether, L., Haughton, V.M. Facet joint menisci of the cervical spine: correlative MR imaging and cryomicrotomy study. Radiology. 1987;164:79–82.
Zumpano, M.P., Jagos, C.S., Hartwell-Ford, S. A cadaveric survey exploring the variation, prevalence, sex bias, and tissue type of the soft tissue bridge between rectus capitis posterior minor and the posterior atlanto-occipital membrane. J Neuromusculoskeletal Syst. 2002;10:133–140.