Chapter 15 Preventing the spread of infection
Introduction
Infections acquired as a result of healthcare have a major impact on patients/clients and healthcare providers. For patients/clients an infection causes anxiety and discomfort, delays recovery and may result in long-term morbidity (ill health) or even death. Quality healthcare is a basic expectation; the public and government reasonably expect that people will not acquire disease because of their treatment or care. Control of infection is a responsibility shared by all healthcare personnel; however, nurses stand in the front line because of their close ‘hands on’ contact with patients/clients. Therefore, nurses in all areas of clinical practice including hospitals, residential homes, health centres and people’s homes provide care for those who are at risk of infection or those who already have an infection. The Nursing and Midwifery Council Code of professional conduct: standards for conduct, performance and ethics (NMC 2004) requires nurses to ensure that no action they undertake is detrimental to the safety and well-being of those in their care. Nurses must therefore understand why infections occur, how they are transmitted, and the precautions and methods necessary to prevent the development and spread of infection.
This chapter provides an overview of microorganisms and outlines the incidence of some infectious diseases. It considers the sequence of events that spread infectious diseases and the key features of disease development. The body’s defence mechanisms are briefly considered. The next section focuses on the practices required to prevent and control the spread of infection, exploring standard infection control precautions and then additional precautions, including isolation precautions and aseptic technique. Finally, it examines the nurse’s role in the collection of microbiology specimens for the laboratory.
Overview of microbiology
Microorganisms are tiny living organisms only visible under a microscope, apart from some fungi. Categories of microorganisms include algae, fungi, protozoa, bacteria, mycoplasma, rickettsia, chlamydia, viruses and prions. Microorganisms are found in:
For microorganisms to survive in any environment, they must have suitable physical and chemical conditions, nutrients and freedom from hostile competitors. The human body is populated by an extraordinary number of microorganisms, an estimated 1 3 1014 bacteria compared to 1 3 1013 body cells (Tortora et al 2003). These microorganisms (referred to as normal flora or commensals) can benefit the host by providing nutrients, aiding in food digestion and preventing the establishment of more dangerous microorganisms. Normal flora do not populate the entire human body but are located in certain regions, e.g. the skin, mucous membranes and the intestinal tract. Some areas of the body are completely devoid of microbial populations, e.g. the urinary tract, blood and the lungs.
Epidemiology of infectious diseases
Infectious diseases are caused by microorganisms and those that cause infectious disease are known as pathogens. An infectious disease that is transmissible from one person to another is called a communicable disease. Communicable diseases range from relatively mild illnesses such as the common cold to debilitating and lethal conditions such as human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), tuberculosis and malaria which together accounted for 5.7 million deaths in 2001 (World Health Organization [WHO] 2002). Communicable diseases are categorized according to their frequency and distribution:
Healthcare-associated infections
Infectious diseases (infections) can be divided into two categories:
A hospitalized patient may have either type. Community-acquired infections are those that are present or incubating at the time of hospital admission whereas a healthcare-associated infection manifests itself 72 hours or more after admission, and includes infections not apparent until after discharge (Scottish Executive 2002).
Incidence of healthcare-associated infections
In England, healthcare-associated infections account for 5000 deaths per year and are also a significant contributing factor in a further 15000 deaths annually (DH 2003). Approximately 9% of patients entering Scottish hospitals will develop one or more infections during their hospitalization. This is equivalent to over 33000 infections a year (NHS Quality Improvement Scotland 2003). The growing resistance of many microorganisms to antibiotic drugs has exacerbated this situation, making treatment of infections increasingly difficult.
Types and incidence of healthcare-associated infections
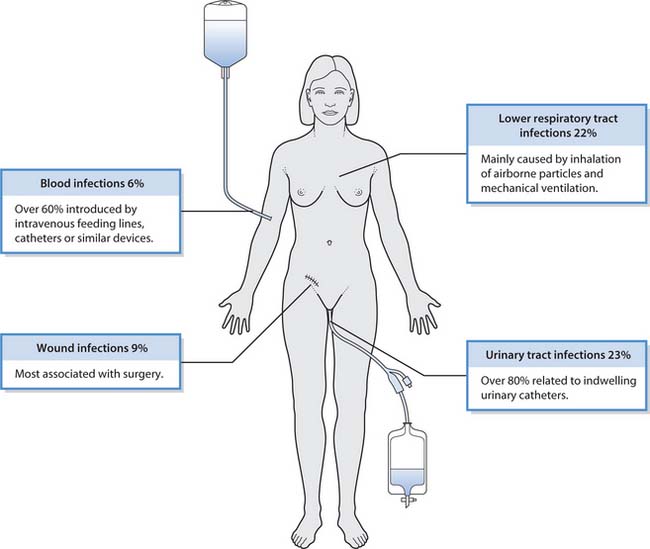
Infections resulting from surgical or medical treatment are called iatrogenic infections. In hospitalized patients, iatrogenic infections are frequently attributed to invasive procedures and indwelling medical devices which bypass the body’s first line of defence (Fig. 15.1).
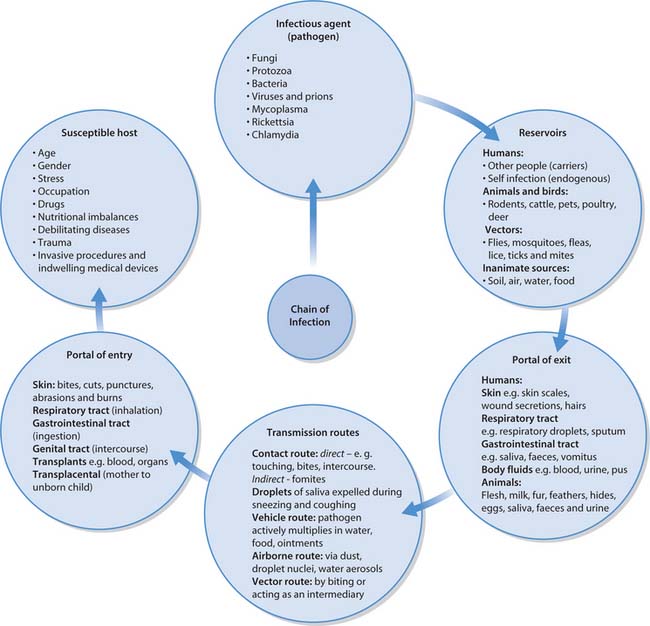
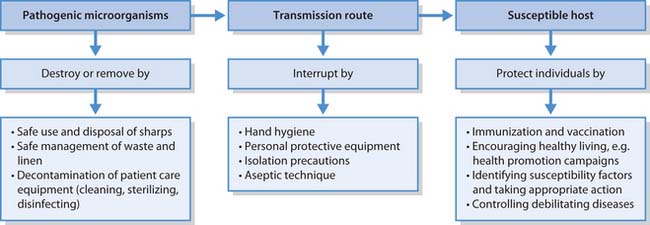
Chain of infection
The spread of infectious diseases follows a well-known sequence of events that can be compared to a chain with six links, frequently referred to as the ‘chain of infection’ (see Fig. 15.2). If all links remain intact and in the correct sequence, then the infection will be transmitted. An infection will not result if the sequence is interrupted. Understanding the characteristics of each link of the chain provides the fundamental knowledge necessary to prevent and control infection.
Infectious agent (pathogen)
The relationship between humans and microorganisms is usually one of balanced conflicts between the host’s ability to resist infection and the ability of the microorganism to cause disease. Some microbial species are very adept at avoiding or surviving the body’s defence mechanisms. For example, some possess surface structures that attach and anchor them to host cells. Others produce toxins (poisons) that target specific body cells and tissues. A few species manufacture enzymes that dissolve the host’s tissues, e.g. necrotizing fasciitis – ‘flesh-eating bacteria’.
Reservoirs
The place(s) where pathogens are provided with nutrients and suitable environmental conditions for their survival and multiplication is called the reservoir. Reservoirs may be human, animal or non-living (see Fig. 15.2). However, the principal reservoirs for most infectious diseases are human carriers. A carrier is a person who is, or has been, colonized with a particular pathogen and can transmit it to others who may then become ill. Various carrier states exist:
Humans can also become infected with micro-organisms that are part of their own normal flora. This is referred to as an endogenous infection and occurs when the normal flora that inhabit one site are transferred to another, e.g. when microorganisms from the colon gain access to the normally sterile urinary tract and cause a urinary tract infection.
Portals of exit and entry
The portal of exit is the route by which a pathogen leaves its reservoir. Some pathogens may leave the host using more than one exit route, e.g. the chickenpox virus exits via respiratory droplets and secretions from skin lesions. Portal of entry refers to the path by which an infectious agent invades a new host. The portal of entry is usually the same as the portal of exit (see Fig. 15.2).
Transmission of infection
To cause disease, a pathogen must be able to survive transfer from its reservoir to a susceptible host. Transmission of pathogens can occur by five main routes (see Fig. 15.2). In healthcare-associated infections the following routes are frequently implicated.
Contact transmission
This is direct, when pathogens are transferred by bodily contact between an infected and uninfected person, e.g. to nurses during bathing or when turning people. Contact transmission may also be indirect, when fomites (inanimate objects) act as an intermediary in the transfer of pathogens, e.g. contaminated instruments or needles. Contaminated hands of healthcare personnel may also transmit infection indirectly and proper handwashing and changing of gloves between patient contacts are essential to prevent transmission of infection by this route.
Droplet transmission
This occurs when an infected person (the reservoir) releases contaminated respiratory secretions into the air when coughing, sneezing and talking, or is subjected to procedures such as suctioning and bronchoscopy. Infected respiratory droplets are propelled short distances (usually a metre or less) and can enter the nasal passages, mouth and conjunctiva of another person, or they can settle on inanimate objects in close proximity to the infected person.
Airborne transmission
This route involves the dissemination of tiny dried particles (usually 5 mm or less) of evaporated respiratory secretions (called droplet nuclei). In contrast to droplet transmission in which the particles travel only short distances, in airborne transmission the particles may remain suspended in the air for long periods and can be widely dispersed on air currents. When inhaled by susceptible people, their small size allows them to penetrate the lungs from where they can initiate infection. In hospitals, special air handling and ventilation systems are required to contain infections transmitted by this route.
Susceptible host
The human body has numerous defence mechanisms for resisting the entry and multiplication of pathogens (see Fig. 15.4, p. 398). These mechanisms normally prevent infection unless the pathogen is particularly virulent (liable to cause disease). Lack of resistance to infectious diseases is known as susceptibility. A number of factors reduce an individual’s resistance to infectious diseases (Box 15.1). Hospitalized patients are especially susceptible to contracting infections because of their underlying disease, drugs that they may be receiving such as antimicrobial or immunosuppressive agents and procedures that breach the skin and mucous membranes, e.g. surgery, the insertion of indwelling urinary catheters or intravenous catheters (see Box 15.2).
Age
Congenital infections develop during gestation, while neonatal infections occur in the first 28 days of life. The source of the microorganisms may be the mother’s vaginal flora, other infected neonates in a baby unit or the hands of hospital staff. As children grow up, they encounter an increasing variety of social environments and may consequently develop common childhood infections. At the other end of the age spectrum, older adults are prone to infections, mainly due failing immune responses and chronic or debilitating diseases.
Gender
Anatomical differences between males and females explain why urinary tract infections are more common in females than males because the shorter female urethra provides microorganisms with easier access to the bladder (see Ch. 20).
Stress
Prolonged physical or emotional stress alters the body’s hormonal balance and reduces resistance to disease. Stress increases the output of cortisol from the adrenal cortex, which suppresses both inflammatory and immune processes. Stress-compromised people often suffer outbreaks of oral or genital herpes lesions or become susceptible to more severe diseases (see Ch. 11).
Occupation
Infectious disease is a persistent hazard of certain occupations, usually when there is increased exposure to pathogens rather than diminished host resistance. Healthcare professionals are frequently exposed to patients/clients shedding virulent human pathogens, veterinary surgeons, agricultural workers and those working in meat processing industries are likely to have a higher incidence of diseases spread by animals and sex industry workers are prone to infections transmitted by sexual intercourse.
Drugs
Both recreational and prescribed drugs can increase susceptibility to infection:
Nutritional imbalance
Infections may also be linked to vitamin and protein deficiencies, and this might partly explain why many infectious diseases are higher in parts of the world where undernutrition is widespread.
Debilitating diseases or trauma
Normal defences can be delayed or suppressed by debilitating diseases, e.g. leukaemia, diabetes, kidney and liver diseases, and AIDS. The risk of infection may be increased by some therapies, e.g. radiotherapy and chemotherapy, which can severely depress white blood cell counts. Trauma, e.g. burns, damages the body’s surface defences, predisposing to invasion by microorganisms.
Salmonella food poisoning
Salmonella food poisoning can cause outbreaks of infection in the community and in healthcare premises such as hospitals. The illness is usually self-limiting and short lived but in a proportion of people (usually at the extremes of life) the illness can be life threatening.
In 1984 an outbreak of salmonella food poisoning occurred at a psychiatric hospital in Wakefield, affecting 355 patients and 109 staff. Many of the patients were elderly and 19 died. The investigation that followed traced the source to cold roast beef. The meat had been removed from the kitchen refrigerator in the morning, sliced and then left out for 10 hours before being served.
Between December 2001 and January 2002 an outbreak of salmonella infection occurred in a ward at a busy general hospital in Glasgow, affecting eight patients and two members of staff. Three of the elderly patients consequently died. An investigation into the outbreak (Scottish Executive Health Department 2002) identified that the source of the infection was likely to have been a patient who had the infection on admission. The infection was likely to have then been transmitted to the other patients by one or more of the following routes:
Infectious disease process and associated terminology
There are several possible outcomes when an individual encounters pathogenic microorganisms:
Infectious diseases are often classified according to their severity, duration and the extent by which they spread throughout the body:
Box 15.3 Characteristics of local infections
| System affected | Signs and symptoms |
|---|---|
| Skin | Inflammation: redness, pain, swelling and heat |
| Respiratory tract | Increased respiratory tract secretions |
| Cough | |
| Sore throat | |
| Difficulty in breathing (dyspnoea) | |
| Urinary tract | Pain on passing urine (dysuria) |
| Frequency | |
| Urgency | |
| Urine appears cloudy, possibly ‘bloody’ and may have a ‘.shy’ smell | |
| Gastrointestinal tract | Abdominal pain |
| Nausea | |
| Vomiting | |
| Diarrhoea | |
| Poor appetite | |
| Central nervous system | Confusion |
| Drowsiness | |
| Stiff neck | |
| Headache | |
| Intolerance of light (photophobia) |
Box 15.4 The four phases of an acute infectious disease
Sometimes it is difficult to detect an infection, for example, in the very young, older adults, those with communication problems and people with mental health problems or a learning disability. The expected signs of infection may not always be obvious and therefore nurses need to be alert to subtle changes in behaviour that may indicate presence of an infection (Box 15.5).
Host defence mechanisms
If microorganisms never encountered resistance from the host, then people would be constantly ill and die from infectious diseases, and eventually the human race would become extinct. In most cases, however, host defence mechanisms are very effective at keeping microbial invaders out. They can be thought of as an army consisting of three lines of defence. If the enemy (the pathogen) breaks through the first line of defence, it will encounter and hopefully be stopped by the second line of defence. If the pathogen manages to escape the first two lines of defence, there is a third line ready to attack it.
The first two lines of defence are referred to as non-specific resistance and are ways in which the body attempts to protect itself against injury and all substances that are harmful to it including pathogenic microorganisms. These comprise external defences and the inflammatory process (see below). The third line of defence is specific to particular microorganisms (called specific resistance) and involves white blood cells (B- and T-lymphocytes) and the production of antibodies that protect the host from one particular foreign substance (see Fig. 15.3).
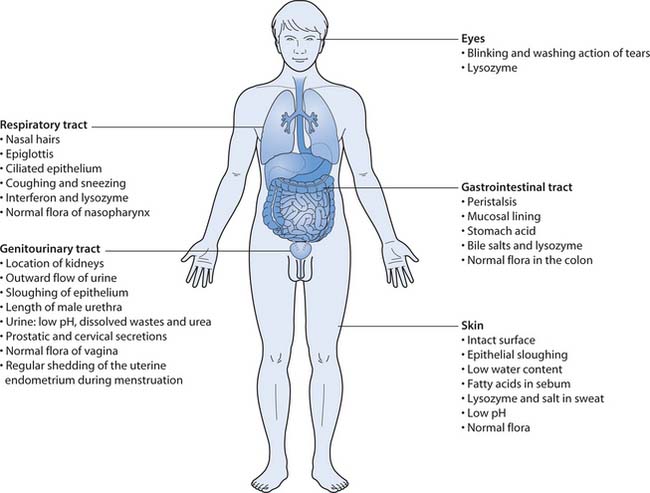
External defences
The integrity of body surfaces forms an effective barrier to the initial lodgement or penetration by microorganisms. This first line of defence to microbial invasion depends on mechanical, chemical and microbial barriers that combat any attack (see Fig. 15.4).
Inflammation
Inflammation is the second line of defence and is triggered when injury or infection damages tissues. The cardinal signs of inflammation are:
Loss of function can also occur as a consequence of swelling and pain. In apparent contradiction to the signs and symptoms observed, the inflammatory response is beneficial because it attempts to initially destroy the pathogen and then, if possible, remove it and its by-products from the body. Inflammation limits spread in the body by confining the agent to one specific area. Finally, the process repairs and replaces damaged tissues.
Specific resistance
The third line of defence is the body’s immune response, which is triggered when antigens enter the body. An antigen is any material that the body recognizes as foreign, including pathogenic microorganisms, pollen, dust, food components, drugs, insect venom and transplanted tissues. Two processes work together to combat and destroy a specific antigen: cell-mediated immunity and humoral immunity.
This type of immunity results in the production of antibodies and usually confers lifelong resistance to the antigen. For further discussion of these processes, readers should consult their anatomy and physiology textbook.
Development of immunity
The formation of antibodies is the basis of immunization against disease and can be either active or passive.
Active immunity
This provides long-term protection against specific microorganisms. It occurs following an infectious disease when antibodies are produced within the body (called natural active immunity) or when the person receives a vaccine which stimulates the immune system to produce specific antibodies against a particular agent (called artificial active immunity) (Box 15.6).
Passive immunity
This provides temporary protection against micro-organisms. Natural passive immunity is acquired by the developing fetus when it receives maternal antibodies in utero, or by baby when it receives maternal antibodies contained in colostrum and breast milk. Artificial passive immunity is acquired when a person receives antibodies contained in anti-sera or gamma globulin, e.g. hepatitis B immune globulin is given to protect those who have been exposed to the hepatitis B virus.
Infection control
Infection control refers to the numerous measures that are taken to prevent infections from occurring in healthcare facilities and aims to destroy or remove sources of pathogenic microorganisms by:
These measures break links in the chain of infection. Two tiers of infection control measures are in operation:
Standard precautions
This section explores standard precautions, which provide guidelines on:
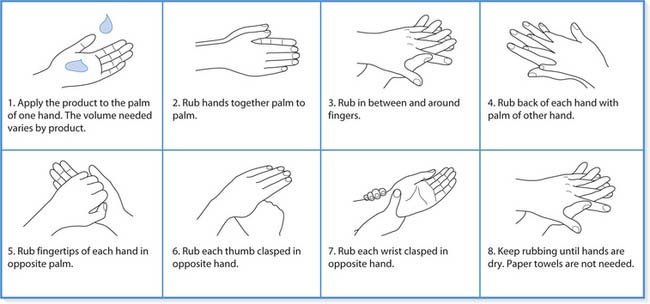
Hand hygiene
Many infections are spread by contact and the hands are the major vehicles in transfer of potential pathogens in healthcare settings from:
In the mid-19th century, studies by both Ignaz Semmelweis and Oliver Wendell Holmes identified handwashing as the most important feature in preventing transmission of infection in healthcare facilities. Infection control guidelines from national and international organizations have repeatedly acknowledged and supported the view that handwashing remains the most effective measure in reducing the incidence of healthcare-associated infections.
The purpose of handwashing is to remove potentially pathogenic microorganisms from the skin, of which there are two categories:
Indications for handwashing
Hands must be washed whenever there is a chance that they may have become contaminated and any time when there is a risk of transmitting infection to others. Figure 15.6 shows the areas of hands prone to harbouring microorganisms. It is important to be aware that handwashing is one of the most important infection prevention practices and, if not washed at appropriate times, the hands can put patients, residents and clients at risk (Box 15.7). Furthermore, contaminated hands can be a danger not only to the practitioner but also to their colleagues, friends and family members.
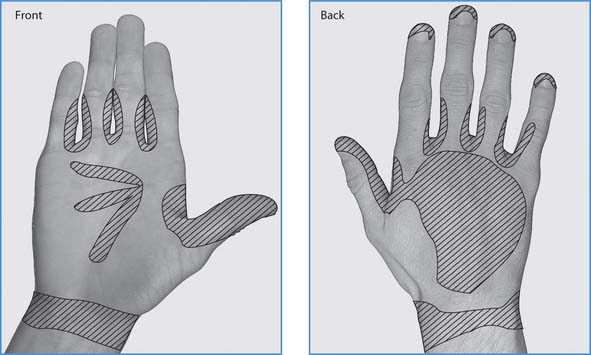
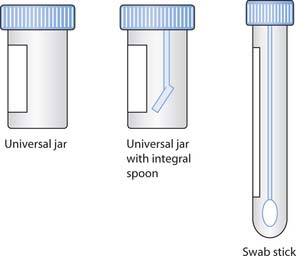
Fig. 15.6 Areas of hands prone to harbouring microorganisms
(reproduced with permission from Inglis 2003)
Types of handwashing and preparations used
There are three types of hand hygiene used in clinical settings, each of which uses different preparations and is appropriate in different situations.
Handwashing with plain soap and running water (social handwashing)
Plain soap has detergent properties and effectively removes dirt, most organic substances and transient flora from the skin. Handwashing with plain soap and water for 10–15 seconds and rinsing in running water is used routinely in clinical areas (Box 15.8). If the hands are heavily soiled with dirt, blood or other organic material, e.g. when gloves have been torn, handwashing for several minutes may be necessary. It important to recognize that some bacteria grow on soap bars, especially if they are allowed to remain wet (Hateley 2003). Soap bars must therefore be kept dry or liquid soap from a dispenser used instead.
Handwashing
Prerequisites to handwashing
Handwashing with soap and water
Hands are rubbed together vigorously for a minimum of 10–15 seconds, paying particular attention to the tips of the fingers, thumbs and areas between the fingers (see below).
Skin care
Regular hand decontamination can cause skin dryness. The regular use of moisturizing hand cream increases skin hydration and replaces depleted skin fats. If a particular soap, antiseptic preparation or alcohol product causes skin irritation, the occupational health team should be consulted (DH 2001, CDC 2002).
Handwashing with antiseptic preparations and running water (aseptic handwashing)
Antiseptic preparations, e.g. chlorhexidine, povidone–iodine and triclosan, have the same action as plain soap with the additional benefit of killing or inhibiting the growth of resident microorganisms. Some antiseptics continue to perform these actions for several hours after the hands are washed. Washing the hands with an antiseptic preparation is appropriate in high-risk situations, i.e. before invasive procedures or contact with clients who have compromised immunity and are therefore highly susceptible to infection (see Box 15.8).
Alcohol-based hand rubs
These contain 70% alcohol and emollients (moisturizing agents to counteract the drying effect of the alcohol) and some contain an antiseptic (Hateley 2003). They act rapidly and kill or inhibit the growth of both transient and resident microorganisms. Their use can offer a practical and acceptable alternative to handwashing in certain clinical situations, e.g. between surgical cases in high-volume settings (Fig. 15.7). However, they are ineffective if hands are visibly soiled with dirt, blood or other matter (DH 2001). It is important to remember that alcohol is flammable and toxic if swallowed.
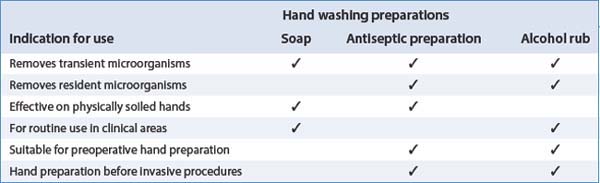
Types of handwashing preparation and their indications
Handwashing preparations and indications for their use are summarized in Table 15.1.
Table 15.1 Handwashing preparations and their indications (from Wilson 2001)
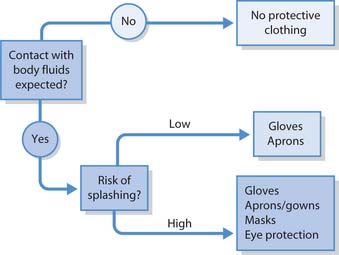
Personal protective equipment
Personal protective equipment (PPE) includes disposable gloves, gowns, aprons, eye protection and masks. They are worn to protect staff and patients from pathogenic microorganisms in both healthcare and community settings. The decision to use or wear PPE is based on a risk assessment (see Ch. 13) associated with a specific patient care activity or intervention (NICE 2003) (Fig. 15.8).
Disposable gloves
The purpose of wearing gloves is to:
Types of gloves and their uses
Gloves are available in a variety of materials including natural rubber latex (NRL), synthetic latex and vinyl. The appropriate type of glove is determined by the activity to be undertaken:
Polythene gloves should not be used because of their permeability and tendency to damage easily, exposing both the patient and healthcare practitioner to microbial contamination (Pratt et al 2000, Clark et al 2002).
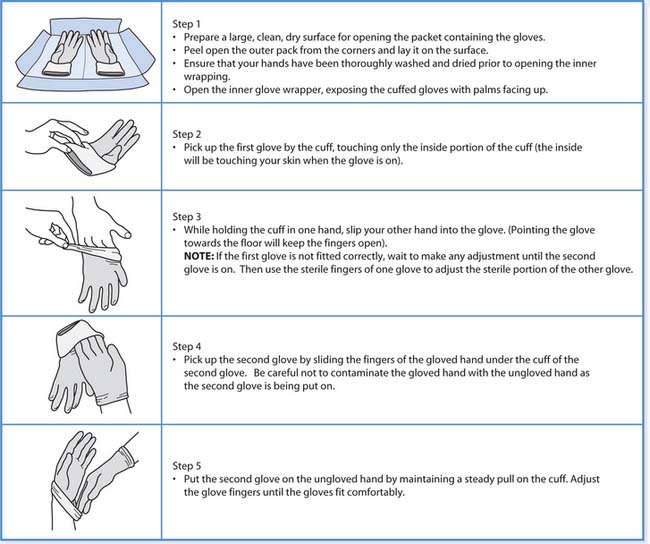
Gloves can be either sterile or non-sterile and choice is based on the type of activity to be undertaken. Sterile gloves must be worn for any invasive procedure and the technique for putting them on is outlined in Box 15.9. Non-sterile gloves are suitable for activities involving contact with body fluids, e.g. handling urine, wiping up spillage and some ‘clean techniques’ such as administering injections.
Technique for putting on sterile gloves
Removal of gloves
Gloves are changed and discarded as follows:
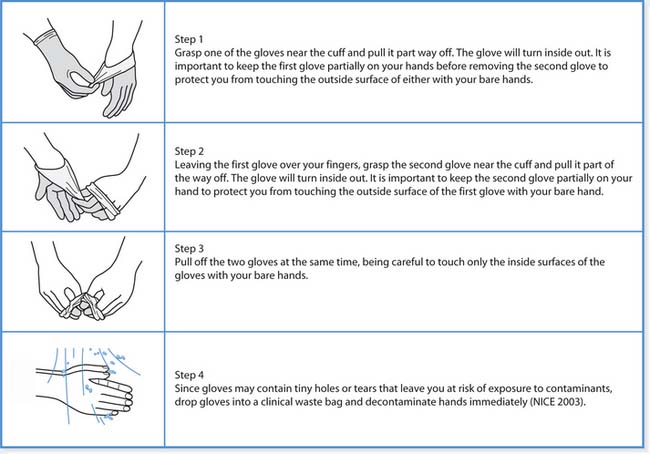
It is important that gloves are always treated as single-use items and discarded following removal. Wearing gloves does not replace the need for handwashing because gloves may have small defects, may be torn during use or the hands may have become contaminated during removal. Therefore, hand hygiene is essential before their use and after their removal. The technique for removing gloves is outlined in Box 15.10 (p. 405).
Glove removal
Health risks associated with glove use
Pratt et al (2000) and Clark et al (2002) advise that gloves should not be worn unnecessarily as their prolonged and indiscriminate use may lead to skin sensitivity and adverse reactions. Guidelines for reducing latex allergy are shown in Box 15.11.
Latex allergy is a reaction to certain proteins in natural rubber latex. The amount of latex exposure needed to produce sensitization or an allergic reaction is unknown although increasing exposure to latex proteins increases the risk of developing allergic symptoms.
Symptoms occurring in sensitized people are variable and usually begin within minutes of exposure but they sometimes develop hours later. Mild reactions involve skin redness, urticaria (rashes) and itching. More severe reactions may involve respiratory symptoms, e.g. nasal congestion, sneezing, itchy watery eyes, sore throat and asthma. Rarely, anaphylactic shock may occur; however, this life-threatening reaction is seldom the first sign of latex allergy.
The National Institute for Occupational Safety and Health (1997) recommend:
Many products, in both the home and healthcare facilities, contain natural rubber latex (Table 15.2). Patients/clients may also have allergies and sensitivity to latex and admission to a healthcare facility may put them at risk (Box 15.12). NICE (2003) advise that any sensitivity to natural latex rubber in patients, carers or healthcare personnel must be documented and alternatives gloves made available.
Table 15.2 Products containing latex
| Household objects | Medical devices |
|---|---|
Gowns and aprons
The purpose of wearing water-repellent protection is to:
When to wear gowns and aprons
Water-repellent gowns are used for procedures where there is a risk of extensive splashing of blood, body fluids, secretions or excretions onto the skin or clothing, e.g. in the operating theatre, dealing with trauma cases in the Emergency Department or during childbirth.
Disposable plastic aprons are worn to protect the front of the uniform from soiling, wetting or contamination that may occur during procedures involving close/direct contact with patients/clients such as:
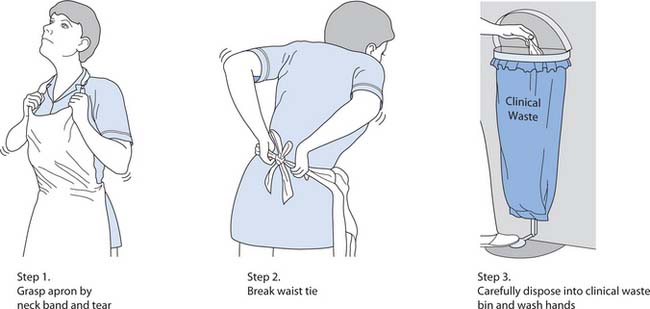
In many healthcare facilities, different coloured aprons are used for different activities. Plastic aprons must be worn as single-use items, i.e. for one procedure or episode of patient care (NICE 2003). Care should be taken when removing aprons (see Fig. 15.9, p. 407).
Masks and eye protection
The purpose of masks and eye protection is to protect the wearer where there is a danger of pathogens in blood or other body substances splashing, splattering and spraying onto the mouth, nose and eyes, e.g. dental and operating theatre procedures, airway suctioning, obstetrical procedures.
Eye protection equipment, such as face shields, goggles and spectacles, must be optically clear (scratch- and mark-free), anti-fog and distortion-free, close-fitting and shielded at the sides. They are identified either as reusable after cleaning or for single-use only.
The use and efficacy of masks have been debated for many years. It used to be common practice to wear masks for many procedures, e.g. wound dressings; however, it is now recognized that their use contributes little to patient/client or staff safety. Unless the mask fits closely around the mouth and nose, microorganisms can escape around its edges (Wilson 2001). The movements of the wearer, length of facial hair and level of the voice when speaking, all affect the mask’s close fit (Leonas & Jones 2003). Furthermore, if worn for long periods, its filtering efficiency is impaired because moisture collects in the fabric and interrupts the passage of air through the mask (Belkin 1997).
Masks are worn to protect healthcare practitioners when there is a danger of blood-borne pathogens or other body substances splashing or spraying into the practitioner’s mouth and nose. Their use is also indicated when caring for susceptible clients, e.g. people with compromised immunity (DH 2001) or in situations when micro-organisms may be transmitted from patients via the airborne route, e.g. meningococcal meningitis (Hateley 2003) (Box 15.13, p. 407). It is recommended that if healthcare workers are likely to be exposed to either tuberculosis or severe acute respiratory syndrome (SARS) a particulate filter personal respiratory protection device (close-fitting masks capable of filtering 0.3 mm particles) should be used. If they are not available then it is better to wear a facemask than no protection (Health Protection Agency 2004, Occupational Safety and Health Administration 2004).
[Adapted from Health Protection Agency (2004)]
Correct use of masks
Safe use and disposal of sharps
In healthcare settings, injuries from needles or other sharp instruments pose a serious danger in transmitting blood-borne viruses such as HIV and hepatitis B and C to healthcare personnel. These infections can be potentially life threatening but are preventable.
What are sharps?
The term ‘sharps’ refers to any sharp instrument or object used in the delivery of care including:
Most reported sharps injuries involve nursing staff; however, other healthcare personnel including doctors, laboratory, domestic and portering staff may also be involved (Exposure Prevention Information Network [EPINet] 2004) (see Box 15.14).
Box 15.14 Incidence and effects of needlestick injuries to healthcare workers
In the UK, an estimated 100000 needlestick accidents occur annually to healthcare workers (NHS Scotland 2005). The majority of reported needlestick injuries are as follows:
Although needlestick injuries may result in local trauma, the principal associated health risk is transmission of blood-borne viral diseases, in particular hepatitis B and C and HIV. The incidence of infection following a needlestick injury varies depending on the virus (DH 2001):
The emotional impact of a needlestick injury can be severe and long lasting, even when infection is not transmitted. Not knowing the infection status of the source patient can accentuate the healthcare worker’s stress (Bandolier 2003) and this impact can be particularly severe when the injury involves exposure to HIV. In one study of 20 healthcare workers with HIV exposure, 11 reported severe distress, 7 had persistent moderate distress and 2 resigned from their posts as a result of the exposure (Henry et al 1990).
How injuries occur
Many injuries occur when staff are using and disposing of sharps, for example when:
Preventing sharps injuries
The assessment and management of risks associated with the use of sharps is paramount in health and safety promotion as well as in infection control. Injuries from sharps can be avoided by handling and disposing of them safely (Box 15.15).
Safe handling and disposal of sharps
National and international guidelines are consistent in their recommendations for the safe use and disposal of sharp instruments and needles.
Sharps containers
Disposing of sharps into sharps containers
Managing injuries and exposure to body fluids
Exposure to blood from a sharps injury, bite or splashing into the eyes, mouth or broken skin must always be followed up because of the risk of infection from blood-borne viruses (Box 15.16).
Managing needlestick injuries and exposure to body fluids
Healthcare facilities have protocols to follow if a sharp injury or exposure to body fluids occurs.
First aid
Following any accidental exposure to blood or other body fluids by needlestick, another sharp object or a splash of fluid then:
There is no evidence to show that using antiseptics or encouraging the wound to bleed reduces the risk of a blood-borne infection (CDC 2003a). However, many organizations and healthcare facilities within the UK suggest that this should carried out as a first aid measure and this is supported by the WHO (2003).
Management
Whether post-exposure treatment is indicated following exposure to blood or other body fluids depends on a number of factors including:
Management of waste
Management of healthcare waste is a crucial aspect of infection control. It is a statutory requirement for healthcare facilities to comply with international, national and local legislation and regulations that relate to the segregation, handling, transportation and final disposal of waste.
Classification of waste
Waste generated from healthcare facilities is classified as clinical or non-clinical waste.
Clinical (hazardous) waste
This is generated from many sources including healthcare, veterinary and pharmaceutical establishments. Because of its toxic, infectious or dangerous content it may be hazardous to healthcare personnel, members of the public and the environment. Consequently, special precautions are required to treat and dispose of it safely (Health Services Advisory Committee [HSAC] 1999).
Domestic waste
A considerable proportion of the waste generated in clinical areas is not hazardous to those who come into contact with it and can be safely disposed of as household waste. It is important that this waste is not sent for incineration in order to minimize both disposal costs and damage to the environment (Table 15.3).
Table 15.3 Categories, containment, treatment and disposal of waste from clinical areas (from HSAC (1999))
| Clinical (hazardous) waste | Container colour and disposal |
|---|---|
| Items soiled by blood or other body fluids : Include wound dressings, swabs, disposable gloves and aprons, materials used to clean up spillages; contaminated waste from patients with transmissible infectious diseases, e.g. tuberculosis and salmonella; disposable nappies from babies, infants, toddlers and others with infectious diarrhoeal diseases | Yellow or orange bag |
| Incineration or heat disinfection followed by landfill | |
| Sharps : Include discarded needles and syringes, cartridges, contaminated broken glass and other disposable sharp instruments including scalpels, razors, lancets, sharp tips of intravenous administration sets, stitch cutters | Yellow sharps container |
| Incineration | |
| Pharmaceutical products and chemical wastes: Include expired and unwanted medicines, cartridges from drug infusion devices, cytotoxic drugs | Collected by ward/community pharmacist who makes arrangements for disposal |
| Body waste products: Include urine, faeces, body secretions and excretions, plus disposables used for their collection, e.g. bedpans, bedpan liners, vomit bowls | Discharged into sewerage system via sluice, lavatory or macerator |
| Sanpro (sanitary products) waste: Includes disposable nappies, incontinence pads, stoma bags, urine containers | Yellow bag with black stripes |
| May be incinerated or sent direct to landfill | |
| Domestic (non-hazardous) waste | Container and destination |
| General waste: Includes dead flowers, used hand towels, food (small quantities only), paper wrappings from packs, magazines and newspapers | Black bag at source although some facilities may use clear, green, buff or white bags |
| Sent direct to landfill[AU1] | |
| Cardboard boxes | Folded flat for collection, then direct to recycling unit or landfill |
| Confidential waste : Paperwork containing sensitive information relating to patients or staff | Green/brown bag at source |
| Shredded or incinerated | |
| If shredded then recycling unit or landfill | |
| Glass bottles and jars | When empty, place in a strong box (mark box ‘Glass with Care’); put into designated container |
| Recycling unit or landfill | |
| Aerosols, pressurized containers, batteries: May pose a safety and/or environmental risk, i.e. may contain CFCs, prescription medicines, flammable liquids or be explosive in nature | Placed in separately identified containers |
Safe handling of clinical waste in healthcare facilities
Disposing of waste safely and economically depends on correct segregation of different types of waste at the point of generation. Waste is bagged, packaged or containerized and must clearly indicate the contents (see Box 15.17).
[From HSAC (1999)]
Safe handling of clinical waste
Dealing with clinical waste bags
Safe handling of clinical waste in people’s homes
The amount of clinical waste generated by patients/clients in their own homes is much smaller and can usually be disposed of as normal household waste. Used needles, e.g. those used by people with insulin-dependent diabetes, must not be discarded as household waste. Instead, arrangements with local hospitals, clinics, pharmacies or local authorities need to be made regarding their disposal (HSAC 1999). Healthcare personnel, e.g. community nurses and dialysis technicians, who generate clinical waste while treating patients in their homes are obliged by the Health and Safety at Work Act (1992) to transport and dispose of the waste safely (see Box 15.18).
Management of linen
Used linen, e.g. clothing, towels, sheets, pillowslips, should be laundered:
In healthcare facilities, linen that is soiled with blood, excreta or other body fluids, or contaminated with microorganisms from infectious patients, needs to be handled carefully in order to prevent:
The laundering process should remove evidence of previous use and significantly reduce microbial counts so that the risk of infection to subsequent users is negligible.
Categories of hospital linen
The NHS Executive guidelines (1995) for hospital laundering recommend that laundry should be sorted into three categories (Table 15.4). These recommendations are uncertain for the following reasons:
Table 15.4 Laundering of hospital linen (from NHS Executive 1995)

Dealing with used linen
Healthcare facilities use different terminology when categorizing, segregating and treating linen.
Student activities
Think about your current placement and:
Safe handling of linen
To prevent the risk of cross-infection it is important that linen in healthcare facilities is handled in the same way for all patients/clients. PPE should be used when handling linen, as follows:
Safe handling of soiled linen
Linen is handled with minimal agitation and shaking to avoid the dispersal of microorganisms into the air and onto people or objects in the vicinity.
By careful handling of used linen the nurse can break the chain of infection, thereby protecting patients from healthcare-associated infections (see Box 15.20).
Breaking the chain of infection using standard precautions
Staff Nurse Jones was assigned to care for Mr Green, a patient who had an open draining wound on his left lower leg. When a sample of pus was sent to the laboratory for analysis, the microorganism meticillin (methicillin)-resistant Staphylococcus aureus (MRSA) was isolated. Prior to making Mr Green’s bed, Staff Nurse Jones washed his hands. Clean linen and a collection bag for linen were placed at the patient’s bed. To remove the soiled linen from the bed, Staff Nurse Jones took the following actions:
Staff Nurse Jones applied principles of infection control to contain the infectious microorganisms at many points in the chain of infection as shown below.
| Link in the chain | Nursing action to break the chain |
|---|---|
| Infectious agent : Presently unknown. Could be Salmonella, | Interrupted the microorganisms’ transmission route by implementing contact isolation precautions |
| Shigella, etc. Awaiting confirmation from the laboratory | |
| Cohorted the two children with a similar infection in the same room with its own en-suite facilities | |
| Assignment of one nurse to care for the two children to reduce the risk of transmitting the infection to others in the unit | |
| Reservoir: Gastrointestinal tract | The nurse was aware that the microorganisms could easily spread to other children by direct/indirect contact |
| Portal of exit: Diarrhoea | Faeces and vomit were discarded directly into the en-suite lavatory |
| Mode of transmission: Direct contact, especially via the hands of the children and healthcare personnel. Indirect contact with contaminated surfaces/equipment | The nurse wore gloves and disposable apron for all contact with body excretions and used proper handwashing techniques following removal of gloves and apron |
| Linen was handled carefully and placed directly into the laundry bag; waste was discarded into a yellow waste bag | |
| Visitors were instructed to wash their hands before leaving the room | |
| All patient-care equipment was decontaminated before it was removed from the room | |
| Portal of entry: Mouth | The nurse ensured that both children carefully washed and dried their hands following each episode of diarrhoea |
| The nurse encouraged the children to refrain from putting fingers and objects into their mouths. | |
| Susceptible host | The infection was not transmitted to other children in the unit due to adherence to infection control measures |
Decontamination of patient-care equipment
Patient-care equipment can act as an intermediary (fomite) in transferring infectious microorganisms from one person to another. It is therefore important that shared or reusable patient-care equipment is decontaminated, i.e. made safe by removing, inhibiting or destroying microorganisms, after use. The term ‘decontamination’ includes sterilization, disinfection and cleaning. These methods confer different levels of microbial safety on items processed.
Levels of decontamination
The appropriate level of decontamination depends on the risk that the equipment may present in transmitting infection. The following factors are critical to that interrelationship:
The risk of transmission can be categorized as high, medium, low or minimal (see Table 15.5).
Table 15.5 Categories of decontamination related to risk (adapted from Ayliffe et al 1999, WHO 2003)
| Risk | Examples | Level of decontamination required |
|---|---|---|
| High : Entry or penetration into the vascular system, body cavities or tissues | Surgical and invasive procedures | Sterilization |
| Intravenous cannulation | ||
| Urinary catheterization | ||
| Injections | ||
| Medium : In contact with mucous membranes, body fluids or other potentially infectious material | Thermometers | Disinfection: chemical or thermal |
| Body fluid spills | ||
| Reusable bedpans and urine bottles | ||
| Crockery, feeding bottles | ||
| Vaginal specula | ||
| Low : Items in contact with intact skin | Sinks and washbowls | Cleaning – clean with detergent, rinse and dry |
| Lavatory seats | Disinfection – for people with known infections | |
| Blood pressure cuffs | ||
| Mattresses | ||
| Hoists | ||
| Minimal: Not in direct contact with patients | Furniture | Cleaning – including damp dusting, wet mopping, dust-attractant mopping, vacuum cleaning |
| Floors | ||
| Walls | ||
| Ceilings |
Cleaning
This is a physical process that involves decontaminating an item or surface with a detergent solution followed by thorough drying. Cleaning contributes to infection control because it physically removes organic materials, e.g. blood, other body fluids, soil or dust, in which micro-organisms can survive. Although cleaning does not necessarily destroy microorganisms, it significantly reduces their numbers and is suitable for low and minimal risk items.
Unless cleaning is carried out competently, infectious microorganisms may be redistributed to other sources and sites (see Box 15.21). Cleaning is also essential prior to disinfection and sterilization, otherwise microorganisms trapped in organic material may survive further processing (see WHO 2003).
Box 15.21  EVIDENCE-BASED PRACTICE
EVIDENCE-BASED PRACTICE
Good cleaning practices
Although environmental cleaning in most healthcare facilities is the duty of cleaning staff, nursing staff have ultimate responsibility to ensure that the standard of cleaning adheres to national guidelines. As nurses are often required to clean patient-care equipment (e.g. blood pressure equipment, washbowls) and blood and body fluid spillages, it is important that they understand fundamental cleaning principles. Correct handling of cleaning solutions, use of cleaning cloths and ensuring that items are dried after cleaning is paramount. PPE, i.e. gloves and aprons, are worn for cleaning and once the task is completed, the hands are washed before carrying out other duties.
Cleaning solutions
When detergent is dissolved in water, it breaks up and dissolves or suspends grease, oil and other foreign matter, thus facilitating its removal. Detergents are available in various forms, e.g. powders, liquids, sprays, gels and wipes.
Cleaning cloths
Drying after cleaning
This is essential because bacteria thrive in moisture. Greaves (1985) showed that washing bowls are still contaminated by bacteria when stored wet and may present an infection risk to the next user.
Cleaning the hospital environment
This is carried out routinely to ensure a clean, dust-free hospital environment. Visible dirt contains microorganisms and cleaning helps to eliminate them. The main methods of removing organic materials are:
The frequency with which wet cleaning should be carried out depends on the situation, for example:
Sterilization
Sterilization is the complete destruction of all living microorganisms including bacterial spores (spores are a resistant casing produced by several species of bacteria that enables them to survive in adverse conditions, e.g. heat, cold, drying, and most chemicals). When something is sterile, it is devoid of microbial life.
Creutzfeldt–Jakob disease (CJD) and variant CJD present a serious cross-infection risk as the micro-organisms resist normal decontamination methods. It is important to refer to local policy regarding decontamination processes.
Sterilization is necessary for all high-risk procedures using the methods shown in Box 15.22 (p. 414). Sterilized items need to be stored correctly and checked prior to opening (see Box 15.23).
Box 15.22 Sterilization methods
Disinfection
Disinfection is the destruction or removal of micro-organisms to a level that is unlikely to cause infection. It does not guarantee complete removal of all micro--organisms because bacterial spores can still survive, i.e. some forms of microbial life may still be present after disinfection.
Disinfection is necessary for all medium-risk procedures and for equipment used for those with transmissible infections (see Table 15.5). Disinfection can be achieved by thermal and chemical methods (see Box 15.24).
Thermal disinfection
Suitable for items that can withstand heat and moisture but do not need to be sterile. Examples of thermal disinfection equipment used in clinical settings include bedpan washers, dishwashers and laundry machines. Although not frequently used in hospitals, boiling is sometimes used to disinfect medium-risk equipment, e.g. feeding bottles, vaginal specula, ear syringes.
Chemical disinfection
The performance of chemical disinfectants depends on several factors including temperature, contact time, concentration, pH, presence of organic or inorganic matter and the numbers and resistance of the microorganisms. Thermal disinfection is more reliable and should be used in preference to chemicals wherever possible (Wilson 2001). Although chemical disinfectants are widely used and perceived to be safe, there are many drawbacks as identified in the list below:
Numerous chemical disinfectants are available to decontaminate equipment and the environment. How-ever, in healthcare facilities the number available is strictly limited (Table 15.6). Furthermore, the use of chemical disinfectants is regulated by the Control of Substances Hazardous to Health (COSHH) Regulations (1999) which are designed to protect against risks to health from hazardous substances in the workplace (see Ch. 13). Each healthcare facility has an infection control policy/manual that gives information about procedures for decontamination (Box 15.25).
Table 15.6 Chemical disinfectants used in healthcare facilities
| Group | Uses | Precautions |
|---|---|---|
| Alcohol (60–90%), e.g. isopropyl alcohol, methylated spirit | Clean equipment, e.g. trolleys, tabletops, glass mercury thermometers, external surfaces of stethoscopes | Flammable: use in well-ventilated areas, keep away from heat sources, electrical equipment, flames, hot surfaces |
| Toxic: avoid inhalation | ||
| Peracetic acid, e.g. Nu-cidex®, Steris-system® | Delicate instruments, e.g. flexible endoscopes, anaesthesia and respiratory equipment, items damaged by heat | Strong smell and should be used in well-ventilated areas |
| May damage rubber and brass after prolonged immersion | ||
| Hypochlorite (bleach), e.g. Domestos®, Chloros®, Milton®, Presept®, Haz-tabs® | Environment | Corrosive to metals |
| Non-metallic equipment | Must not be mixed with acids as they may release chlorine gas | |
| Disinfection of material contaminated with blood and body fluids | Can be irritating to the skin, eyes, and respiratory tract | |
| Phenolic (carbolic acid), e.g. Clearsol®, Stericol®, Hycolin® | Environment | Absorbed by rubber and plastics |
| Can cause severe burning of the skin or mucous membranes |
Additional precautions
These are used in addition to the standard precautions discussed above; aseptic technique and isolation precautions are explained in this section.
Aseptic technique
Patients in healthcare facilities often acquire infections as a result of invasive clinical procedures which breach the body’s normal defence mechanisms, making the tissues vulnerable to invasion by microorganisms. For example:
Aseptic technique is often referred to as ‘sterile technique’ or ‘no-touch technique’. It includes practices used to render and keep objects and areas sterile, i.e. free of all microorganisms including bacterial spores. Aseptic technique is routinely carried out in a wide range of hospital and community settings.
Indications for aseptic technique
Aseptic technique is carried out during any invasive clinical procedure that enters or penetrates a vulnerable body site such as the vascular system, a sterile body cavity or tissue. Examples of invasive clinical procedures include:
Components of aseptic technique
The components of aseptic technique include all the key elements of standard precautions and also focus on:
Box 15.26  EVIDENCE-BASED PRACTICE
EVIDENCE-BASED PRACTICE
Antiseptic agents
Antiseptic agents (antiseptics) are chemical solutions that reduce or destroy microorganisms on the skin or mucous membranes without causing damage or irritation. They are used to clean the skin before invasive procedures and also as hand cleansing agents for healthcare workers.
The principles of aseptic technique are shown in Box 15.27.
Principles of aseptic technique
Aseptic technique may vary slightly but the basic principles are similar.
Preparation
Opening sterile packs and supplies and organizing the work surface
Clean technique
Clean technique is a version of aseptic technique. The goal of clean technique is to exclude pathogens from a susceptible site, whereas that of aseptic technique is to exclude all microorganisms (Burton & Engelkirk 2004). When used in conjunction with a ‘no-touch’ technique (not touching the susceptible body area with non-sterile items) clean technique is used for:
Clean technique includes all the key elements of standard precautions including:
Isolation precautions
Microorganisms cause a wide variety of infections and, for most, standard precautions are adequate to prevent their transmission to healthcare personnel and other patients. However, for patients known to have, or are suspected to have, highly transmissible infections or are colonized by dangerous pathogens, additional precautions are needed. In the past, these were referred to as ‘barrier nursing’, but are now known as isolation precautions. Some healthcare facilities use the term ‘source isolation’ to indicate that the patient is the source of the infection and to distinguish them from ‘protective isolation’, the precautions which may be required for patients who are highly vulnerable to infection.
Principles of isolation nursing
The theory and practice of isolation nursing focus on interrupting the transmission route of microorganisms. Consideration is given to the following elements.
Patient accommodation
A key component of isolation is the appropriate placement of the patient. Some healthcare facilities have infectious disease or isolation units where patients are nursed in single rooms with en-suite facilities, controlled airflow systems and cared for by a team of specialist infection control practitioners. In the UK there are several high-security units for treating patients with highly communicable infections, e.g. Lassa fever and Ebola fever.
In other healthcare facilities, patients are isolated in single rooms within a ward. These settings are less secure than isolation units due to the close proximity of other patients and the frequent contact that healthcare practitioners have with both infected and non-infected patients. Furthermore, the availability of single rooms with en-suite facilities may be limited and, for most patients, rooms and bathrooms must often be shared. When a single room is unavailable, patients with the same microorganism may share a room, a practice referred to as ‘cohorting’.
Patient movement
A practice that impinges on isolation measures is moving isolation patients/clients between wards or other healthcare facilities. Glynn et al (1997) identified that five to seven moves were not uncommon in some hospitals. This practice interferes with measures to prevent, control and contain infection and should be avoided. If a patient has to be moved, then it is important that the receiving unit/ward is notified of their impending arrival and the infection control measures required.
Psychological effects of isolation
Society protects itself from those who would do it harm by isolating people: those who have committed serious crimes are isolated in prison; infectious patients are isolated in hospital. In prisons, the degree of isolation varies from high-security confinement to an open approach; however, in hospitals, whatever the degree of isolation it can be a disturbing experience for the patient. In response to their isolation, some patients may become demanding, fussy or irritable. Many patients express feelings of loneliness, abandonment, inferiority and boredom when isolated (Wilson 2001). Isolated patients have significantly higher levels of anxiety and depression, and lower self-esteem and sense of control. In order to promote patients’ wellbeing they need to be informed about their condition, its symptoms and treatment, the control measures and their rationale, together with advice about their responsibilities (Lewis et al 1999) (Box 15.28).
The impact of isolation
Susan is 20 years old and has a mild learning disability. She is admitted to hospital for investigations. Three days later she develops a fever and a vesicular skin rash. Suspecting that she may have contracted chickenpox and to prevent the transmission of the virus to others, Susan is isolated in one of the ward’s single rooms. Isolation in a separate room can be a frightening and anxious time for patients, their relatives and visitors.
It is important to consider the ethical issues relating to confidentiality when caring for people with infectious diseases. Maintaining patient confidentiality is another important concern in isolation nursing (Box 15.29).
Maintaining confidentiality
Isolation procedures are necessary to reduce the risk of infection to healthcare personnel and other patients. However, by their very nature, they may indicate the type of infection, resulting in a breach of patients’ confidentiality.
Student activities
Read the NMC Code of Professional Conduct (2004, clause 5) and Chapter 7. Then think about the questions below.
Categories of isolation
The three categories of isolation (transmission-based precautions) are:
These precautions may be combined for diseases that have multiple routes of transmission, e.g. chickenpox which can be transmitted both by the airborne route and by direct contact with vesicle fluid or respiratory secretions.
Whether used alone or in combination, transmission-based precautions are always used in addition to standard precautions, i.e. by wearing clean non-sterile gloves when touching blood, body fluids, secretions, excretions and contaminated items, and handwashing after removal of gloves. If splashing of blood or body fluids is anticipated then eye protection, masks and plastic aprons are worn. Extra care is taken when handling equipment, sharp items, linen and waste.
Airborne precautions
Airborne precautions are necessary for infections transmitted by the inhalation of droplet nuclei, e.g. tuberculosis, measles and chickenpox.
Isolation (transmission-based) precautions are as follows:
Droplet precautions
Infections are transmitted by contact with respiratory secretions and large droplets expelled during coughing and sneezing, e.g. mumps, diphtheria and whooping cough. These infections are also spread by direct contact with contaminated items in the patient’s immediate environment.
Isolation (transmission-based) precautions are as follows:
Contact precautions
Infections are transmitted by direct contact with patients or by indirect contact with surfaces or equipment, e.g. MRSA; some enteric, skin and respiratory infections.
Isolation (transmission-based) precautions are as follows:
Breaking the chain of infection using transmission-based precautions
Two young children in a paediatric unit start vomiting and have profuse diarrhoea. Suspecting that the condition may be infectious, both children are moved into a double room and contact isolation precautions initiated. The room has its own toilet facilities and supplies of hand soap, disposable gloves, paper towels, plastic aprons, yellow waste bags, a laundry bag and patient-care equipment. One nurse is assigned to care for both children and wears gloves and a disposable plastic apron when in contact with faeces and vomit, e.g. when changing soiled bed linen, assisting the children with personal hygiene measures. Following each care activity, the nurse removes her gloves and discards them directly into the waste receptacle and thoroughly washes her hands. The nurse applied principles of infection control to contain the infectious organism at many points in the chain of infection as shown in the box below.
| Link in the chain | Nursing action to break the chain |
|---|---|
| Infectious agent : Presently unknown. Could be Salmonella, Shigella, etc. Awaiting confirmation from the laboratory | Interrupted the microorganisms’ transmission route by implementing contact isolation precautions |
| Cohorted the two children with a similar infection in the same room with its own en-suite facilities | |
| Assignment of one nurse to care for the two children to reduce the risk of transmitting the infection to others in the unit | |
| Reservoir: Gastrointestinal tract | The nurse was aware that the microorganisms could easily spread to other children by direct/indirect contact |
| Portal of exit: Diarrhoea | Faeces and vomit were discarded directly into the en-suite lavatory |
| Mode of transmission: Direct contact, especially via the hands of the children and healthcare personnel. Indirect contact with contaminated surfaces/equipment | The nurse wore gloves and disposable apron for all contact with body excretions and used proper handwashing techniques following removal of gloves and apron |
| Linen was handled carefully and placed directly into the laundry bag; waste was discarded into a yellow waste bag | |
| Visitors were instructed to wash their hands before leaving the room | |
| All patient-care equipment was decontaminated before it was removed from the room | |
| Portal of entry: Mouth | The nurse ensured that both children carefully washed and dried their hands following each episode of diarrhoea |
| The nurse encouraged the children to refrain from putting fingers and objects into their mouths. | |
| Susceptible host | The infection was not transmitted to other children in the unit due to adherence to infection control measures |
Protective isolation
This type of isolation is also known as ‘reverse barrier nursing’ or ‘neutropenic isolation’. Certain patients are at increased risk of microbial infections from both endogenous and exogenous sources. This is due to compromised defences such as in severe burns, leukaemia, organ transplants, immunosuppressed states and radiation treatment. Premature infants are also highly susceptible to infection.
Isolation (transmission-based) precautions are as follows:
Specimen collection
Many different specimens are collected from patients and used to diagnose or follow the progress of infectious diseases. The most common clinical specimens that nurses take for sending to the microbiology laboratory are listed in Box 15.31.
It is important that the specimen is of the highest quality and collected safely:
Collection of clinical specimens
When collecting a clinical specimen for microbiology it must be collected in a manner that will prevent its contamination with either the patient’s/client’s or healthcare professional’s microorganisms. It is important therefore, that standard precautions are implemented:
Sterile containers are always used for the collection of specimens (Fig. 15.10). Care needs to be taken to avoid contaminating the inside of the container or its lid when collecting the specimen, and to ensure that the outside of the container is not contaminated by the contents. The container’s lid should be closed tightly to prevent leakage during transportation to the laboratory.
A sufficient quantity of material must be obtained to provide enough material for all the diagnostic tests required. The specimen container is labelled and accompanied by a request form. As a minimum, labels should contain the patient’s name, hospital identification number, ward number/name or the requesting doctor’s name, the specimen type and the date and time of collection. The specimen container is placed in a double self-sealing bag with one compartment containing the request form and the other the specimen.
When specimens are regarded as infection hazards, e.g. from people with hepatitis B or C, or HIV, they may present an infection risk to portering and laboratory staff; in such cases the specimen container and request form are labelled with biohazard labels.
Transport of specimens
Specimens should be delivered to the laboratory promptly so that the results accurately represent the number and types of organisms present at the time of collection. If delivery to the laboratory is delayed, the pathogens may die or any indigenous flora (non-pathogens) may overgrow, inhibit or kill the pathogens. If the specimen cannot be transported to the laboratory immediately then it may be refrigerated at 4°C (Hateley 2003). However, the refrigerator must be used for specimens only – it must not contain foods or medicines. Blood cultures are never refrigerated but stored at body temperature, in an incubator if necessary.
| Activity | Must wash hands | Not necessary |
|---|---|---|
| Centers for Disease Control and Prevention | www.cdc.gov |
| Available July 2006 | |
| EngenderHealth | www.engenderhealth.org/ip/index.html |
| Available July 2006 | |
| Eurosurveillance | www.eurosurveillance.org/search/search-02.asp |
| Available July 2006 | |
| Evidence Based Practice in Infection Control (EPIC) | www.epic.tvu.ac.uk |
| Available July 2006 | |
| Health Protection Agency | www.hpa.org.uk/infections |
| Available July 2006 | |
| Health Protection Scotland | www.hps.scot.nhs.uk |
| Available July 2006 | |
| Hospital eTool | www.osha.gov/SLTC/etools/hospital/index.html |
| Available July 2006 | |
| Infection Control Nurses Association | www.icna.co.uk/ |
| Available July 2006 | |
| Medline Plus | www.nlm.nih.gov/medlineplus/infectioncontrol.html |
| Available July 2006 | |
| National Institute for Health and Clinical Excellence (NICE) | www.nice.org.uk/page.aspx?o=CG002 |
| Available July 2006 | |
| Infection Control | |
| NHS Plus | www.nhsplus.nhs.uk/nhsstaff/infection.asp |
| Available July 2006 | |
| Practical guidelines for infection control in health care facilities (WHO) | www.wpro.who.int/sars/docs/practicalguidelines/practical_guidelines.pdf |
| Available July 2006 |
Ayliffe GJA, Babb JR, Taylor LJ. Hospital-acquired infection, 3rd edn. Oxford: Butterworth-Heinemann, 1999.
Bandolier. 2003 Evidence-based healthcare: needlestick injuries. Online: www.jr2.ox.ac.uk/bandolier/Extraforbando/needle.pdf.
Belkin NL. The evolution of the surgical mask: filtering efficiency versus effectiveness. Journal of Infection Control and Hospital Epidemiology. 1997;18(1):49-56.
Burton RW, Engelkirk PG. Microbiology for the health sciences, 7th edn. Williams & Wilkins, Philadelphia: Lippincott, 2004.
Centers for Disease Control and Prevention. 2002 Guideline for hand hygiene in healthcare settings: MMWR Recommendations and Reports. Online: www.cdc.gov/handhygiene.
Centers for Disease Control and Prevention. 2003a Exposure to blood: what healthcare personnel need to know. Online: http://www.cdc.gov/ncidod/dhqp/pdf/bbp/Exp_to_Blood.pdf. Available July 2006.
Centers for Disease Control and Prevention. 2003b Guidelines for environmental infection control in health-care facilities. Online: http://www.cdc.gov/ncidod/dhqp/gl_environinfection.html. Available July 2006.
Clark L, Smith W, Young L. Protective clothing: principles and guidance. London: Infection Control Nurses Association, 2002.
Department of Health. Standard principles for preventing hospital-acquired infections. Journal of Hospital Infection. 2001;47(Suppl):S21-S37.
Department of Health. 2003 Winning ways: working together to reduce healthcare associated infection in England. DH, London. Online: www.dh.gov.uk/assetRoot/04/06/46/89/04064689.pdf.
Engender Health. http://www.engenderhealth.org/ip/index.html.
Exposure Prevention Information Network (EPINet). 2004. Online: http://www.needlestickforum.net/3epinet/latestresults.htm. Available July 2006.
Glynn A, Ward V, Wilson J. Hospital-acquired infection. Surveillance policies and practice. London: Public Health Laboratory Service, 1997.
Gould D, Brooker C. Applied microbiology for nurses. Basingstoke: Palgrave Macmillan, 2000.
Government of Ontario, Canada.
Greaves A. We’ll just freshen you up my dear. Nursing Times. 1985;6(Suppl):3-8.
Hateley P. Infection control. In: Brooker C, Nicol M, editors. Nursing adults: the practice of caring. Edinburgh: Mosby; 2003:253-270.
Health Canada. 1998 Infection control guidelines (supplement). Canadian Medical Association, Ottawa. Online: www.phac-aspc.gc.ca/publicat/ccdr-rmtc/99vol25/25s4/index.html.
Health Protection Agency. 2004 Information on face masks and respirators. Online: www.hpa.org.uk/infections/topics_az/SARS/maskFAQs.htm.
Health Services Advisory Committee. Safe disposal of clinical waste, 2nd edn. Norwich: TSO, 1999.
Henry K, Campbell S, Jackson B, et al. Long-term follow-up of health care workers with work-site exposure to human immunodeficiency virus [letter to the editor]. Journal of the American Medical Association. 1990;263(13):1765-1766.
Inglis TJJ. Microbiology and infection, 2nd edn. Edinburgh: Churchill Livingstone, 2003.
Leonas KK, Jones CR. The relationship of fabric properties and bacterial filtering efficiency for selected surgical masks. Journal of Textile and Apparel, Technology and Management. 3(2), 2003.
Lewis AM, Gammon J, Hosein I. The pros and cons of isolation and containment. Journal of Hospital Infection. 1999;43:19-23.
McDonald LL, Pugliese G. Laundry service. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. Baltimore: Williams and Wilkins, 1996.
National Institute for Occupational Safety and Health. 1997 Preventing allergic reactions to natural rubber latex in the workplace. NIOSH Publication No. 97–135 (June 1997). Online: http://www.cdc.gov/niosh/topics/latex. Available July 2006
National Institute for Health and Clinical Excellence. 2003 Infection control: prevention of healthcare-associated infections in primary and community care. NICE, London. Online: www.nice.org.uk/pdf/Infection_control_fullguideline.pdf.
NHS Executive. Hospital laundry arrangements for used and infected linen. HSG(95)18. London: HMSO, 1995.
NHS Quality Improvement Scotland. Improving clinical care in Scotland: healthcare associated infection (HAI) infection control. Edinburgh: NHS QIS, 2003.
NHS Scotland. 2005 Needlestick injuries: sharpen your awareness. Scottish Executive, Edinburgh. Online. Available: http://www.show.scot.nhs.uk/sehd/publications/nisa/nisa-00.htmAvailable July 2006.
Nursing and Midwifery Council. 2004 Code of professional conduct: standards for conduct, performance and ethics. NMC, London. Online: www.nmc-uk.org/aFramedisplay.aspx?documentID=201.
Occupational Safety and Health Administration. 2004 Tuberculosis. HealthCare Wide Hazards Module. Online: www.osha.gov/SLTC/etools/hospital/hazards/tb/tb.html.
Pratt RJ, Pellowe C, Loveday HP, et al. 2000 Epic phase 1: the development of national evidence-based guidelines for preventing hospital-acquired infections in England. Standard Principles: Technical Report. London: Thames Valley University. Online: www.epic.tvu.ac.uk.
Scottish Executive. 2002 Preventing infections acquired while receiving healthcare, 2002–2005. Online: www.scotland.gov.uk/library5/health/preventinfect.pdf.
Scottish Executive Health Department. 2002 The Watt Group Report. A review of the outbreak of salmonella at the Victoria Infirmary. Online: www.scotland.gov.uk/library5/health/twgr.pdf.
The Scottish Office. 1999 Hospital acquired infection: a framework for a national surveillance for the NHS in Scotland. Online: www.scotland.gov.uk/library2/doc15/hai-00.asp.
Tortora GJ, Funke BR, Case CL. Microbiology: an introduction, 8th edn. San Francisco: Benjamin Cummings, 2003.
Wilson J. Infection control in clinical practice, 2nd edn. Edinburgh: Baillière Tindall, 2001.
World Health Organization. 2002 Scaling up the response to infectious diseases: report on infectious diseases 2002. WHO, Geneva. Online: www.who.int/infectious-disease-report/2002/introduction.html.
World Health Organization. 2003 Practical guidelines for infection control in health care facilities. WHO, Geneva. Online: www.wpro.who.int/sars/docs/practicalguidelines/practical_guidelines.pdf.
Burton RW, Engelkirk PG. Microbiology for the health sciences, 7th edn. Philadelphia: Lippincott Williams and Wilkins, 2004.
Gould D, Brooker C. Applied microbiology for nurses. Basingstoke: Palgrave Macmillan, 2000.
Inglis TJJ. Microbiology and infection, 2nd edn. Edinburgh: Churchill Livingstone, 2003.
Kowalak JP, Hughes SA, Mills JE. Best practices: a guide to excellent nursing care. Philadelphia: Lippincott Williams and Wilkins, 2002.
Tortora GJ, Funke BR, Case CL. Microbiology: an introduction, 8th edn. San Francisco: Benjamin Cummings, 2003.
Wilson J. Infection control in clinical practice, 2nd edn. Edinburgh: Baillière Tindall, 2001.
 CRITICAL THINKING
CRITICAL THINKING REFLECTIVE PRACTICE
REFLECTIVE PRACTICE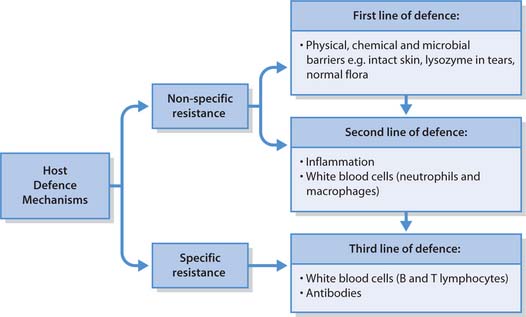
 NURSING SKILLS
NURSING SKILLS
 HEALTH PROMOTION
HEALTH PROMOTION FIRST AID
FIRST AID ETHICAL ISSUES
ETHICAL ISSUES