Intake
Digestion, Absorption, Transport, and Excretion of Nutrients
The Gastrointestinal Tract
One of the primary considerations for a complete nutrition assessment is to consider the three-step model of “ingestion, digestion, and utilization.” In this model, consideration is given to each step to identify all areas of inadequacy or excess. If there is any reason why a step is altered from physical, biochemical, or behavioral-environmental causes, the astute nutrition provider must select an appropriate nutrition diagnosis for which intervention is required. Intake and assimilation of nutrients should lead to a desirable level of nutritional health.
The gastrointestinal tract (GIT) is designed to (1) digest protein, carbohydrates, and lipids from ingested foods and beverages; (2) absorb fluids, micronutrients, and trace elements; and (3) provide a physical and immunologic barrier to microorganisms, foreign material, and potential antigens consumed with food or formed during the passage of food through the GIT. In addition, the GIT participates in many other regulatory, metabolic, and immunologic functions that affect the entire body.
The human GIT is well suited for digesting and absorbing nutrients from a tremendous variety of foods, including meats, dairy products, fruits, vegetables, grains, complex starches, sugars, fats, and oils. Depending on the nature of the diet consumed, 90% to 97% of food is digested and absorbed; most of the unabsorbed material is of plant origin. Compared with ruminants and animals with a very large cecum, humans are considerably less efficient at extracting energy from grasses, stems, seeds, and other coarse fibrous materials. Humans lack the enzymes to hydrolyze the chemical bonds that link the molecules of sugars that make up plant fibers. Fibrous foods and any undigested carbohydrates are fermented to varying degrees by bacteria in the human colon, but only 5% to 10% of the energy needed by humans can be derived from this process (Engylst and Englyst, 2005).
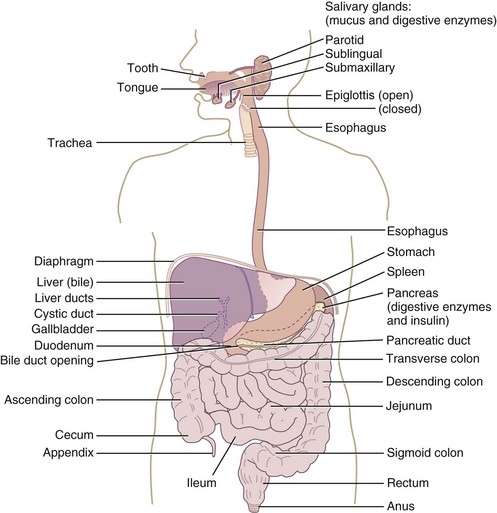
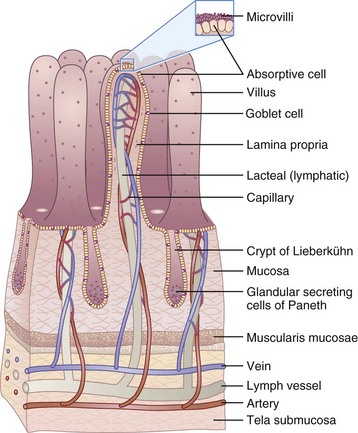
The GIT extends from the mouth to the anus and includes the oropharyngeal structures, esophagus, stomach, liver and gallbladder, pancreas, and small and large intestine. It is one of the largest organs in the body, has the greatest surface area, has the largest number of immune cells, and is one of the most metabolically active tissues in the body (Figure 1-1). The human intestine is about 7 m long and configured in a pattern of folds, pits, and fingerlike projections called villi. The villi are lined with epithelial cells and even smaller, cylindrical extensions called microvilli. The result is a tremendous increase in surface area compared with that expected from a smooth, hollow cylinder (Figure 1-2). The cells lining the intestinal tract have a life span of approximately 3 to 5 days, and then they are sloughed into the lumen and “recycled,” adding to the pool of available nutrients. The cells are fully functional only for the last 2 to 3 days as they migrate from the crypts to the distal third of the villi.
The health of the body depends on a healthy, functional GIT. Because of the unusually high metabolic activity and requirements of the GIT, the cells lining it are more susceptible than most tissues to micronutrient deficiencies; protein-energy malnutrition; and damage resulting from toxins, drugs, irradiation, or interruption of its blood supply. Approximately 45% of the energy requirement of the small intestine and 70% of the energy requirement of cells lining the colon are supplied by nutrients passing through its lumen. After only a few days of starvation, the GIT atrophies (i.e., the surface area decreases and secretions, synthetic functions, blood flow, and absorptive capacity are all reduced). Resumption of food intake, even with less than adequate calories, results in cellular proliferation and return of normal GI function after only a few days. Optimum function of the human GIT seems to depend on a constant supply of foods rather than on consumption of large amounts of foods interrupted by prolonged fasts.
Brief Overview of Digestive and Absorptive Processes
The sight, smell, taste, and even thought of food start the secretions and movements of the GIT. In the mouth, chewing reduces the size of food particles, which are mixed with salivary secretions that prepare them for swallowing. A small amount of starch is degraded by salivary amylase, but this overall carbohydrate digestion is minimal. The esophagus transports food and liquid from the oral cavity and pharynx to the stomach. In the stomach, food is mixed with acidic fluid and proteolytic and lipolytic enzymes. Small amounts of lipid digestion take place, and some proteins are changed in structure or partially digested to large peptides (Soybel, 2005). When food reaches the appropriate consistency and concentration, the stomach allows its contents to pass into the small intestine, where most digestion takes place. Alcohol, the exception, is absorbed through the stomach.
In the first 100 cm of small intestine, a flurry of activity occurs, resulting in the digestion and absorption of most ingested food. Here the presence of food stimulates the release of hormones that stimulate the production and release of powerful enzymes from the pancreas and small intestine and bile from the liver and gallbladder. Starches and proteins are reduced to smaller-molecular-weight carbohydrates and small to medium-size peptides. Dietary fats are reduced from visible globules of fat to microscopic droplets of triglycerides, then to free fatty acids and monoglycerides. Enzymes from the brush border of the small intestine further reduce the remaining carbohydrates to monosaccharides and peptides to single amino acids, dipeptides, and tripeptides (Keller and Layer, 2005). Together with salivary and gastric secretions, secretions from the pancreas, small intestine, and gallbladder contribute 7 to 9 L of fluid in a day, approximately three to four times more fluid than is normally consumed orally. All but 100 to 150 mL of the total fluid entering the lumen is reabsorbed.
The movement of ingested and secreted material in the GIT is regulated primarily by peptide hormones, nerves, and enteric muscles. Along the remaining length of the small intestine, almost all the macronutrients, minerals, vitamins, trace elements, and fluid are absorbed before reaching the colon. The colon and rectum absorb most of the remaining fluid delivered from the small intestine. The colon absorbs electrolytes and only a small amount of remaining nutrients.
Most nutrients absorbed from the GIT enter the portal vein for transport to the liver, where they may be stored, transformed into other substances, or released into circulation. End products of most dietary fats are transported into the bloodstream via the lymphatic circulation.
Remaining fiber, resistant starch, sugar, and amino acids are fermented in the brush border of the colon. Fermentation of the remaining carbohydrates results in the production of short-chain fatty acids (SCFAs) and gas. SCFAs help maintain normal mucosal function, salvage a small amount of energy from some of the residual carbohydrates and amino acids, and facilitate the absorption of salt and water (Englyst and Englyst, 2005). Some of the carbohydrate and fiber resistant to digestion in the upper GIT serve as “prebiotic” material by producing SCFAs, decreasing the colonic pH, and increasing the mass of “helpful” bacteria (Macfarlane et al., 2008). Prebiotic substances support the symbiotic relationship between the GIT and its microbiological environment.
The large intestine provides temporary storage for waste products. The distal colon, rectum, and anus control defecation.
Enzymes in Digestion
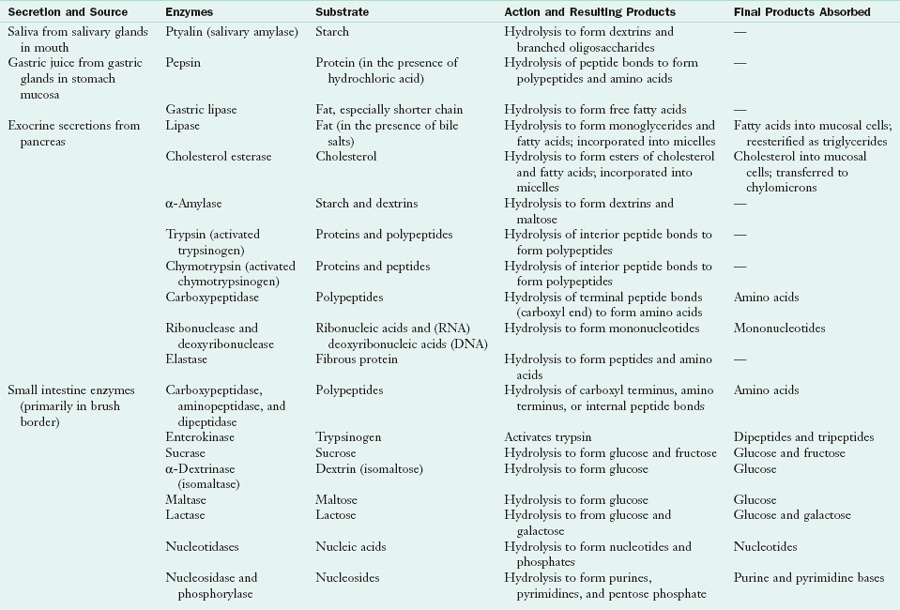
Digestion of food is accomplished by enzymatic hydrolysis. Cofactors such as hydrochloric acid, bile, and sodium bicarbonate facilitate the digestive and absorptive processes. Digestive enzymes are synthesized in specialized cells in the mouth, stomach, pancreas, and small intestine and are released into the lumen. Some enzymes are localized in the lipoprotein membranes of the mucosal cells and attach to their substrates as they enter the cell. Table 1-1 summarizes the GI enzymes and their functions in the small intestine.
Except for fiber and some carbohydrates, digestion and absorption are essentially completed in the small intestine. No digestive enzymes are secreted from the large intestine. Although water, monosaccharides, vitamins, minerals, and alcohol are usually absorbed in their basic form, often they must be unbound from other molecules or attached to carriers before absorption. Generally, the carbohydrates, lipids, and proteins must be converted to their simple constituents by digestive enzymes before they are absorbed (see Chapter 3).
Regulators of Gastrointestinal Activity: Nerves, Neurotransmitters, and Neuropeptide Hormones
GI movement, including contraction, mixing, and propulsion of luminal contents, is the result of the coordinated activity of enteric nerves, extrinsic nerves, endocrine cells, and smooth muscle. The neural mechanisms include (1) an intrinsic system consisting of two layers of nerves embedded in the gut wall and (2) an external system of nerve fibers running to and from the central and autonomic nervous systems. Mucosal receptors in the gut wall are appropriately sensitive to the composition of the chyme (a semiliquid substance of acid, fatty acids, and amino acids) and lumen distention (i.e., fullness) and send impulses through submucosal and mesenteric nerves.
Neurotransmitters and neuropeptides with small molecular weights signal nerves to contract or relax muscles, increase or decrease fluid secretions, or change blood flow. The GIT then largely regulates its own motility and secretory activity. However, signals from the central nervous system can override the enteric system and affect GI function. Hormones, neuropeptides, and neurotransmitters in the GIT not only affect GI function but also have an effect on other nerves and tissues in many parts of the body. Some examples of neurotransmitters released from enteric nerve endings are listed in Table 1-2. In people with GI disease (e.g., infections, inflammatory bowel disease, irritable bowel syndrome), the enteric nervous system may be overstimulated, resulting in abnormal secretion, altered blood flow, increased permeability, and altered immune function.
TABLE 1-2
Examples of Neurotransmitters and Their Actions
| Neurotransmitter | Site of Release | Primary Action |
| GABA | Central nervous system | Relaxes lower esophageal sphincter. |
| Norepinephrine | Central nervous system, spinal cord, sympathetic nerves | Decreases motility, increases contraction of sphincters, inhibits secretions. |
| Acetylcholine | Central nervous system, autonomic system, other tissues | Increases motility, relaxes sphincters, stimulates secretion. |
| Neurotensin | GI tract, central nervous system | Inhibits release of gastric emptying and acid secretion. |
| Serotonin (5-HT) | GI tract, spinal cord | Facilitates secretion and peristalsis. |
| Nitric oxide | Central nervous system, GI tract | Regulates blood flow, maintains muscle tone, maintains gastric motor activity. |
| Substance P | Gut, central nervous system, skin | Increases sensory awareness (mainly pain), and peristalsis. |
5-HT, 5-hydroxytryptamine; GABA, α-aminobutyric acid; GI, gastrointestinal.
Autonomic innervation is supplied by the sympathetic fibers that run along blood vessels and by the parasympathetic fibers in the vagal and pelvic nerves. In general, sympathetic neurons, which are activated by fear, anger, and stress, tend to slow transit of GI contents by inhibiting neurons affecting muscle contraction and inhibiting secretions. The parasympathetic nerves innervate specific areas of the alimentary tract. For example, the sight or smell of food stimulates vagal activity and subsequent secretion of acid from parietal cells scattered along the walls of the stomach. The GIT also sends signals that are perceived as colicky pain, sharp pain, nausea, urgency or gastric fullness, or gastric emptiness by way of the vagal and spinal nerves. Inflammation, dysmotility, and various types of intestinal damage may intensify these perceptions.
Primary Neuropeptide Hormones
Regulation of the GIT involves numerous peptide hormones that can act locally or distally. These regulators can act locally in an autocrine, paracrine, or endocrine role by traveling through the blood to their target organs. More than 100 peptide hormones and hormone-like growth factors have been identified. Their actions are often complex and extend well beyond the GIT. Some of the hormones (e.g., of the cholecystokinin [CCK] and somatostatin family) also serve as neurotransmitters between neurons.
The GIT secretes more than 30 families of neuropeptide hormones and is the largest endocrine organ in the body (Rehfeld, 2004). GI hormones are involved in initiating and terminating feeding, bringing on sensations of hunger and satiety, increasing or decreasing movements of the GIT, enhancing or retarding esophageal and gastric emptying, regulating blood flow and permeability, regulating immune functions, and stimulating the growth of cells (within and beyond the GIT). Ghrelin, a neuropeptide secreted from the stomach, and motilin, a related hormone secreted from the duodenum, send a “hungry” message to the brain. Once food has been ingested, hormones PYY 3-36, CCK, glucagon-like polypeptide-1 (GLP-1), oxyntomodulin, pancreatic polypeptide, and gastrin-releasing polypeptide (bombesin) send signals to decrease hunger and increase satiety (Stanley et al., 2005). Some of the GI hormones, including some of those that affect satiety, also tend to slow gastric emptying and decrease secretions (e.g., somatostatin). Other GI hormones (e.g., motilin) increase motility.
The signaling agents of the GIT are also involved in several metabolic functions. The neuropeptides glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 are called incretin hormones because they help lower blood sugar by facilitating insulin secretion, decreasing gastric emptying, and increasing satiety. Several of these neuropeptide hormones and analogs are used in management of obesity, inflammatory bowel disease, diarrhea, diabetes, GI malignancies, and other conditions. This area of research is critically important.
Some functions of the hormones that affect GI cell growth, deoxyribonucleic acid (DNA) synthesis, inflammation, proliferation, secretion, movement, or metabolism have not been fully identified (Kahn and Ghia, 2010). Knowledge of major hormone functions becomes especially important when the sites of their secretion or action are diseased or removed in surgical procedures or when hormones and their analogs are used to suppress or enhance some aspect of GI function. The key GIT hormones are summarized in Table 1-3.
TABLE 1-3
Functions of Major Gastrointestinal Hormones
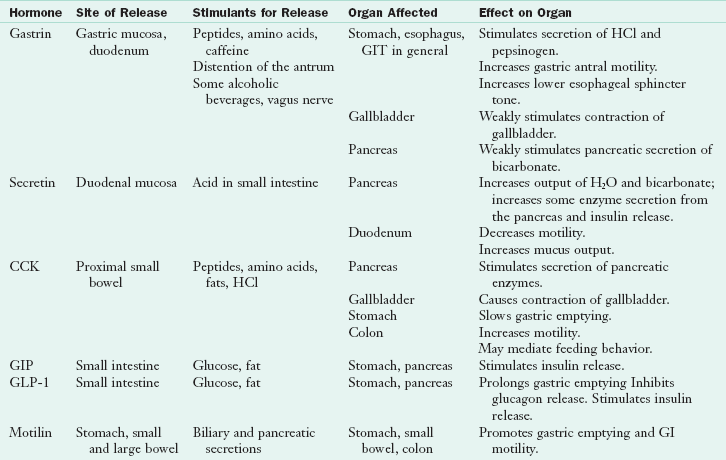
CCK, Cholecystokinin; GI, gastrointestinal; GIP, glucose-dependent insulinotropic polypeptide; GIT, gastrointestinal tract; GLP-1, glucagon-like polypeptide; H2O, water; HCl, hydrochloric acid.
Gastrin, a hormone that stimulates gastric secretions and motility, is secreted primarily from endocrine “G” cells in the antral mucosa of the stomach. Secretion is initiated by (1) distention of the antrum after a meal; (2) impulses from the vagus nerve such as those triggered by the smell or sight of food; and (3) the presence in the antrum of secretagogues such as partially digested proteins, fermented alcoholic beverages, caffeine, or food extracts (e.g., bouillon). When the lumen gets more acidic, feedback involving other hormones inhibits gastrin release (Schubert, 2009). Gastrin binds to receptors on parietal cells and histamine-releasing cells to stimulate gastric acid, to receptors on chief cells to release pepsinogen, and to receptors on smooth muscle to increase gastric motility.
Secretin, the first hormone to be named, is released from “S” cells in the wall of the proximal small intestine into the bloodstream. It is secreted in response to gastric acid and digestive end products in the duodenum, stimulates the pancreas to secrete water and bicarbonate into the duodenum, and inhibits gastric acid secretion and emptying (the opposite of gastrin). Neutralized acidity protects the duodenal mucosa from prolonged exposure to acid and provides the appropriate environment for intestinal and pancreatic enzyme activity. The human receptor is found in the stomach and ductal and acinar cells of the pancreas. In different species, other organs may express secretin, including the liver, colon, heart, kidney, and brain (Chey and Chang, 2003).
Small bowel mucosal “I” cells secrete cholecystokinin (CCK), an important multifunctional hormone released in response to the presence of protein and fat. Receptors for CCK are in pancreatic acinar cells, pancreatic islet cells, gastric somatostatin-releasing D cells, smooth muscle cells of the GIT, and the central nervous system. Major functions of CCK are to (1) stimulate the pancreas to secrete enzymes, some bicarbonate, and water; (2) stimulate gallbladder contraction; (3) increase colonic and rectal motility; (4) slow gastric emptying; and (5) increase satiety (Keller and Layer, 2005). CCK is also widely distributed in the brain and plays a role in neuronal functioning (Deng et al, 2010).
GLP-1 and GIP, released from the intestinal mucosa in the presence of meals rich in glucose and fat, stimulate insulin synthesis and release. GLP-1 also decreases glucagon secretion, delays gastric emptying, and may help promote satiety. GLP-1 and GIP are examples of incretin hormones, which help keep blood glucose from rising excessively after a meal (Nauck, 2009). This may explain why a glucose load received enterally results in less of an increase in blood glucose than when an equal amount of glucose is received intravenously.
Motilin is released by endocrine cells in the duodenal mucosa during fasting to stimulate gastric emptying and intestinal motility. Erythromycin, an antibiotic, has been shown to bind to motilin receptors; thus analogs of erythromycin and motilin have been used as therapeutic agents to treat delayed gastric emptying (De Smet et al., 2009).
Somatostatin, released by D cells in the antrum and pylorus, is a hormone with far-reaching actions. Its primary roles are inhibitory and antisecretory. It decreases motility of the stomach and intestine and inhibits or regulates the release of several GI hormones. Somatostatin and its analog, octreotide, are being used to treat certain malignant diseases (Van Op Den Bosch et al., 2009) as well as numerous GI disorders such as diarrhea, short bowel syndrome, pancreatitis, dumping syndrome, and gastric hypersecretion.
Digestion in the Mouth
In the mouth, the teeth grind and crush food into small particles. The food mass is simultaneously moistened and lubricated by saliva. Three pairs of salivary glands—the parotid, submaxillary, and sublingual glands—produce approximately 1.5 L of saliva daily. A serous secretion containing amylase (ptyalin) begins the digestion of starch. This digestion is minimal, and the amylase becomes inactive when it reaches the acidic contents of the stomach. Another type of saliva contains mucus, a protein that causes particles of food to stick together and lubricates the mass for swallowing. The oropharyngeal secretions also contain a lipase that is capable of digesting a minimal amount of fat.
The masticated food mass, or bolus, is passed back to the pharynx under voluntary control, but throughout the esophagus the process of swallowing (deglutition) is involuntary. Peristalsis then moves the food rapidly into the stomach (see Chapter 41 for detailed discussion on swallowing).
Digestion in the Stomach
Food particles are propelled forward and mixed with gastric secretions by wavelike contractions that progress forward from the upper portion of the stomach (fundus), to the midportion (corpus), and then to the antrum and pylorus. In the stomach gastric secretions are mixed with food and beverages. An average of 2000 to 2500 mL of gastric juice is secreted daily. The gastric secretions contain hydrochloric acid (secreted by the parietal cells in the walls of the fundus and corpus), a protease, gastric lipase, mucus, intrinsic factor (a glycoprotein that facilitates vitamin B12 absorption in the ileum), and the GI hormone gastrin. The protease is pepsin, which is also secreted from glands in the fundus and corpus. It is secreted in an inactive form, pepsinogen, which is converted by hydrochloric acid to its active form. Pepsin is active only in the acid environment of the stomach and serves primarily to change the shape and size of some of the proteins in a normal meal.
An acid-stable lipase is secreted into the stomach by chief cells. Although this lipase is considerably less active than pancreatic lipase, it contributes to the overall processing of dietary triglycerides. Gastric lipase is more specific for triglycerides composed of medium- and SCFAs, but the normal diet contains few of these fats. Lipases secreted in the upper portions of the GIT may have a relatively important role in the liquid diet of infants; but, when pancreatic insufficiency occurs, it becomes apparent that lingual and gastric lipases are not sufficient to prevent lipid malabsorption (Keller and Layer, 2005). In the process of gastric digestion, most of the food becomes semiliquid chyme, which is 50% water. Gastric secretions are also important in increasing the availability and downstream absorption of vitamin B12, calcium, iron, and zinc (Soybel, 2005).
When food is consumed, significant numbers of microorganisms are also consumed. The stomach pH is quite low, ranging from about 1 to 4. The combined actions of hydrochloric acid and proteolytic enzymes result in a significant reduction in the concentration of microorganisms ingested. Some microbes may escape and enter the intestine if consumed in sufficient concentrations or if achlorhydria, gastrectomy, GI dysfunction or disease, poor nutrition, or drugs that suppress acid secretions are present. This may increase the risk of bacterial overgrowth in the intestine.
The stomach continuously mixes and churns food and normally releases the mixture in small quantities into the small intestine. The amount emptied with each contraction of the antrum and pylorus varies with the volume and type of food consumed, but only a few milliliters are released at a time. The presence of food in the intestine and regulatory hormones provide feedback to slow gastric emptying.
Most of a liquid meal empties within 1 to 2 hours, and most of a solid meal empties within 2 to 3 hours. When eaten alone, carbohydrates leave the stomach the most rapidly, followed by protein, fat, and fibrous food. In a meal with mixed types of foods, emptying of the stomach depends on the overall volume and characteristics of the foods. Liquids empty more rapidly than solids, large particles empty more slowly than small particles, and concentrated food tends to empty more slowly than low-calorie meals. These factors are important considerations for practitioners who counsel patients with nausea, vomiting, diabetic gastroparesis, or partial obstruction or for practitioners monitoring patients after GI surgery or malnourished patients.
The lower esophageal sphincter (LES) above the entrance to the stomach prevents reflux of gastric contents into the esophagus. The pyloric sphincter in the distal portion of the stomach helps regulate the exit of gastric contents, preventing backflow of chyme from the duodenum into the stomach. Emotional changes, food, GI regulators, and irritation from nearby ulcers may alter the performance of strictures. Certain foods and beverages may alter LES pressure, permitting reflux of stomach contents back into the esophagus (see Chapter 28).
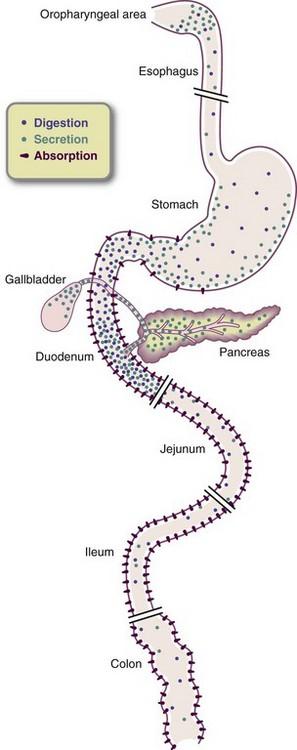
Digestion in the Small Intestine
The small intestine is the primary site for digestion of foods and nutrients. The small intestine is divided into the duodenum, the jejunum, and the ileum (Figure 1-2). The duodenum is approximately 0.5 m long, the jejunum is 2 to 3 m, and the ileum is 3 to 4 m. Most of the digestive process is completed in the duodenum and upper jejunum, and the absorption of most nutrients is largely complete by the time the material reaches the middle of the jejunum. The acidic chyme from the stomach enters the duodenum, where it is mixed with duodenal juices and the secretions from the pancreas and biliary tract. As a result of the secretion of bicarbonate-containing fluid from the pancreas and dilution from other secretions, acid chyme is neutralized. Enzymes of the small intestine and pancreas operate more effectively in a more neutral pH.
The entry of partially digested foods, primarily fats and protein, stimulates the release of several hormones that in turn stimulate the secretion of enzymes and fluids and affect GI motility and satiety. Bile, which is predominantly a mixture of water, bile salts, and small amounts of pigments and cholesterol, is secreted from the liver and gallbladder. Through their surfactant properties, the bile salts facilitate the digestion and absorption of lipids, cholesterol, and fat-soluble vitamins. Bile acids are also regulatory molecules; they activate the vitamin D receptor and cell-signaling pathways in the liver and GIT that alter gene expression of enzymes involved in the regulation of energy metabolism (Hylemon et al., 2009). It is now known that bile acids play an important role in hunger and satiety.
The pancreas secretes potent enzymes capable of digesting all of the major nutrients, and enzymes from the small intestine help complete the process. The primary lipid-digesting enzymes secreted by the pancreas are pancreatic lipase and colipase. Proteolytic enzymes include trypsin and chymotrypsin, carboxypeptidase, aminopeptidase, ribonuclease, and deoxyribonuclease. Trypsin and chymotrypsin are secreted in their inactive forms and are activated by enterokinase (also known as enteropeptidase), which is secreted when chyme contacts the intestinal mucosa. Pancreatic amylase serves to hydrolyze large starch molecules eventually into units of approximately two to six sugars. Enzymes lining the brush border of the villi further break down the carbohydrate molecules into monosaccharides before absorption. Varying amounts of resistant starches and most ingested dietary fiber escape digestion in the small intestine and may add to fibrous material available for fermentation by colonic microbes (Englyst and Englyst, 2005).
Intestinal contents move along the small intestine at a rate of 1 cm per minute, taking from 3 to 8 hours to travel through the entire intestine to the ileocecal valve; along the way, remaining substrates continue to be digested and absorbed. The ileocecal valve, like the pyloric valve, serves to limit the amount of intestinal material passed back and forth from the small intestine to the colon. A damaged or nonfunctional ileocecal valve results in the entry of significant amounts of fluid and substrate into the colon and increased chance for microbial overgrowth in the small intestine (see Chapter 28).
The Small Intestine: Primary Site of Nutrient Absorption
The primary organ of nutrient and water absorption is the small intestine, which has an expansive absorptive area. The surface area is attributable to its extensive length, as well as to the organization of the mucosal lining. The small intestine has characteristic folds in its surface called valvulae conniventes. These convolutions are covered with fingerlike projections called villi (Figure 1-3), which in turn are covered by microvilli, or the brush border. The combination of folds, villous projections, and microvillous border creates an enormous absorptive surface of approximately 200 to 300 m2. The villi rest on a supporting structure called the lamina propria. Within the lamina propria, which is composed of connective tissue, the blood and lymph vessels receive the products of digestion.
Each day, on average, the small intestine absorbs 150 to 300 g of monosaccharides, 60 to 100 g of fatty acids, 60 to 120 g of amino acids and peptides, and 50 to 100 g of ions. The capacity for absorption in the healthy individual far exceeds the normal macronutrient and caloric requirements. In the small intestine, all but 1 to 1.5 L of the 7 or 8 L of fluid secreted from the upper portions of the GIT, in addition to 1.5 to 3 L of dietary fluids, is absorbed by the time the contents reach the end of the small intestine. Approximately 95% of the bile salts secreted from the liver and gallbladder are reabsorbed as bile acids in the distal ileum. Without recycling bile acids from the GIT (enterohepatic circulation), synthesis of new bile acids in the liver would not keep pace with needs for adequate digestion. Bile salt insufficiency becomes clinically important in patients who have resections of the distal small bowel and diseases affecting the small intestine, such as Crohn disease, radiation enteritis, and cystic fibrosis. The distal ileum is also the site for vitamin B12 (with intrinsic factor) absorption.
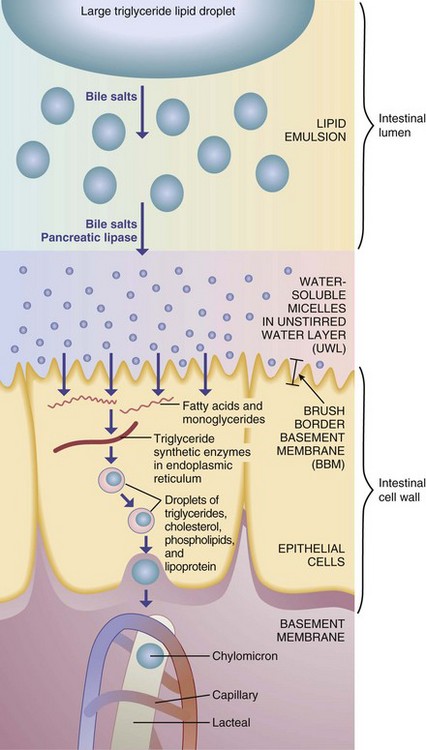
Emulsification of fats in the small intestine is followed by their digestion, primarily by pancreatic lipase, into free fatty acids and monoglycerides. Pancreatic lipase typically cleaves the first and third fatty acid, leaving one attached to the middle glycerol carbon. When the concentration of bile salts reaches a certain level, they form micelles (small aggregates of fatty acids, monoglycerides, cholesterol, bile salts, and other lipids), which are organized with the polar ends of the molecules oriented toward the watery lumen of the intestine. The products of lipid digestion are rapidly solubilized in the central portion of the micelles and carried to the intestinal brush border (Figure 1-4).
At the surface of the unstirred water layer (UWL), the slightly acidic and watery plate that forms a boundary between the intestinal lumen and the brush border membranes, the lipids detach from the micelles. Remnants of the micelles return to the lumen for further transport. The monoglycerides and fatty acids are thus left to make their way across the lipophobic UWL to the more lipid-friendly membrane cells of the brush border. Lipids are taken up and transported through the endoplasmic reticulum and Golgi apparatus where fatty acids are re-esterified to triglyceride. Triglycerides are packaged, along with other lipids, into chylomicrons, which are released into the lymphatic circulation. Cholesterol absorption is facilitated by a protein transport system specific to cholesterol and not to other sterols (Hui et al., 2008; Lammert and Wang, 2005).
Absorptive and Transport Mechanisms
Absorption is complex, combining the intricate process of active transport with the simple process of passive diffusion. In absorption, nutrients pass through the intestinal mucosal cells (enterocytes or colonocytes) and make their way into the venous system to the liver or into the lymphatic circulation. Diffusion involves random movement through openings in or between the membranes of the mucosal cell walls using channel proteins (passive diffusion) or carrier/transport proteins in facilitated diffusion (Figure 1-5).
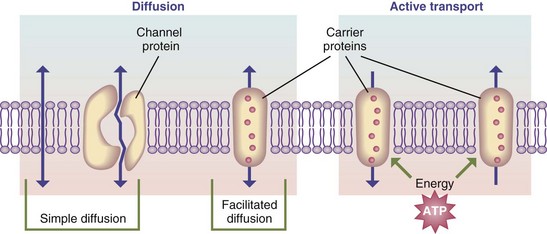
FIGURE 1-5 Transport pathways through the cell membrane, as well as basic transport mechanisms. ATP, Adenosine triphosphate.
Active transport involves the input of energy to move ions or other substances, in combination with a transport protein, across a membrane against an energy gradient. Some nutrients may share the same carrier and thus compete for absorption. Transport or carrier systems can also become saturated, slowing the absorption of the nutrient. A notable example of such a carrier is intrinsic factor, which is responsible for the absorption of vitamin B12 (see Chapters 3 and 33).
Some molecules are moved from the intestinal lumen into mucosal cells by means of pumps, which require a carrier and energy from adenosine triphosphate. The absorption of glucose, sodium, galactose, potassium, magnesium, phosphate, iodide, calcium, iron, and amino acids occurs in this manner.
Pinocytosis has been described as the “drinking in,” or engulfing, by the epithelial cell membrane of a small drop of intestinal contents. Pinocytosis allows large particles such as whole proteins to be absorbed in small quantities. The movement of foreign proteins across the GIT into the bloodstream, where they cause allergic reactions, may be the result of pinocytosis. The immunoglobulins from breast milk are probably absorbed through pinocytosis.
The Large Intestine
The large intestine is approximately 1.5 m long and consists of the cecum, colon, and rectum. Mucus secreted by the mucosa of the large intestine protects the intestinal wall from excoriation and bacterial activity and provides the medium for binding the feces together. Bicarbonate ions secreted in exchange for absorbed chloride ions help to neutralize the acidic end products produced from bacterial action. Approximately 2 L of fluids are taken in food and beverages during the day, and 7 to 9 L of fluid is secreted along the GIT. Under normal circumstances, most of that fluid is absorbed in the small intestine and approximately 1 to 1.5 L of fluid enters the large intestine. Only approximately 100 mL remain to be excreted in the feces.
The large intestine is also the site of bacterial fermentation of remaining carbohydrates and amino acids, synthesis of a small amount of vitamins, storage, and excretion of fecal residues. Colonic contents move forward slowly at a rate of 5 cm/h, and some remaining nutrients may be absorbed.
Defecation, or expulsion of feces through the rectum and anus, occurs with varying frequency, ranging from three times daily to once every 3 or more days. Average stool weight is in the range of 100 to 200 g, and mouth-to-anus transit time may vary from 18 to 72 hours. The feces generally consist of 75% water and 25% solids, but the proportions vary greatly. Approximately two thirds of the contents of the wet weight of the stool is bacteria, with the remainder coming from GI secretions, mucus, sloughed cells, and undigested foods. A diet that includes abundant fruits, vegetables, legumes, and whole grains typically results in a shorter overall GI transit time, more frequent defecation, and larger and softer stools.
Bacterial Action
The gut microflora make up a complex community that is estimated to involve thousands of species of microorganisms (Frank and Pace, 2008). At birth the GIT is essentially sterile, but accumulation of various microorganisms soon takes place. Lactobacillus organisms are the chief component of the GIT flora until an infant begins to eat solid foods. Escherichia coli then become predominant in the distal ileum, and the primary colonic flora are anaerobic, with species of the genus Bacteroides occurring most frequently. Lactobacilli are also present in the stools of most people who consume an ordinary mixed diet; but differences in the host’s genome, dietary intake, hygiene, and medical and surgical history affect the kind of flora in the GIT (Table 1-4).
TABLE 1-4
Most Common Microbes Colonizing the Gastrointestinal Tract
| Bacteria | Lactobacilli | Fungi |
| Acinetobacter | Peptostreptococcus | Candida |
| Bacteroides | Porphyromonas | |
| Bifidobacterium | Prevotella | Parasites |
| Clostridium | Propionibacterium | Blastocystis |
| Corynebacterium | Pseudomonas | Endolimax |
| Eubacterium | Staphylococcus | Entamoeba coli |
| Enterobacteriaceae | Streptococcus A, B, C, F, G | E. hartmanni |
| Enterococcus | Streptococcus bovis | E. polecki |
| Fusobacterium | Streptococcus | Iodamoeba |
| Helicobacter | Veillonella | Trichomonas hominis |
Modified from Walter J. Ecological role of Lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research, Appl Environ Microbiol 74:4985, 2008.
Normally relatively few bacteria remain in the stomach or small intestine after meals because bile, hydrochloric acid, and pepsin work as germicides. However, decreased gastric secretions can increase the risk of inflammation of the gastric mucosa (gastritis), increase the risk of bacterial overgrowth in the small intestine, or increase the numbers of microbes reaching the colon. An acid-tolerant bacterium is known to infect the stomach (Helicobacter pylori) and may cause gastritis and ulceration in the host (see Chapter 28).
Bacterial action is most intense in the large intestine. Following a meal, dietary fiber, resistant starches, remaining bits of amino acids, and mucus sloughed from the intestine are fermented in the colon. Colonic bacteria contribute to the formation of gases (e.g., hydrogen, carbon dioxide, nitrogen, and in some individuals methane) and SCFAs (e.g., acetic, propionic, butyric, and some lactic acids). Colonic bacteria continue the digestion of some materials that have resisted previous digestive activity. During the process, several nutrients are formed by bacterial synthesis. These nutrients are used to varying degrees by GI mucosal cells but usually contribute little to meeting the nutrient requirements of the human host. Examples of nutrients produced include vitamin K, vitamin B12, thiamin, and riboflavin.
Increased consumption of prebiotic material may lead to an increase in SCFAs and in the microbial mass—in particular, indigenous Bifidobacteria and Lactobacilli species are thought to be beneficial. Prebiotic carbohydrates typically refer to oligosaccharides from vegetables, grains, and legumes; chicory, Jerusalem artichokes, soybeans, and wheat bran are the best dietary sources. Prebiotics provide a healthy gut “ecosystem” with beneficial health effects (Roberfroid et al, 2010.)
Bacterial action also may result in the formation of potentially toxic substances such as ammonia, indoles, amines, and phenolic compounds such as indolacetate, tyramine, histamine, and cresol (MacFarlane, 2008). Some of the gases and organic acids contribute to the odor of feces.
The interactions between the innate and acquired immune systems evolve based on one’s genetic heritage and exposure to a myriad of environmental substances over a lifetime. Malnutrition, exposure to toxic agents, and disease may affect the relationships among the physical and immunologic components of the GIT and the tremendous number of substances that reside or pass through its lumen (Quigley, 2010). Each individual’s GI immune system, therefore, has a formidable task. It must (1) mount and subsequently turn off an attack against transient invading pathogens that make their way into the GIT; (2) prevent antigenic components of peptides from producing allergic responses locally and systemically; and (3) tolerate the thousands of different species of “normal” bacteria that reside in the GIT, their secretions, and degradation into cell wall components, DNA fragments, and peptides.
Several diseases may be exacerbated by or even caused by disruption of the tenuous harmony between the GIT and the contents of its lumen. Interactions among the host immune system, host genome, diet, and GI microflora may be linked with several infectious and inflammatory bowel diseases, allergies, immune disorders, metabolic disorders, and neoplasms (O’Keefe, 2008; Tappenden and Deutsch, 2007). In addition to the therapeutic use of antibiotics and antiinflammatory or immunosuppressive agents, attention is being given to the therapeutic potential of probiotic, prebiotic, and synbiotic products.
Probiotics are foods or concentrates of live organisms that contribute to a healthy microbial environment and suppress potential harmful microbes. Knowledge of their role in preventing and treating a host of GI and systemic disorders has expanded tremendously (Snelling, 2005).
Prebiotics are oligosaccharide components of the diet (e.g., fructo-oligosaccharides, inulin) that are the preferred energy substrates of “friendly” microbes in the GIT. When prebiotics, other sources of soluble dietary fiber, and other carbohydrates resistant to digestion are fermented by bacteria in the distal ileum and colon, they produce SCFAs that serve as fuel for the cells lining the GIT. SCFAs also serve as regulatory agents for several GI and host functions (Roberfroid et al, 2010.)
Synbiotics are a combination of probiotics and prebiotics. Synbiotics are long-chain, inulin-type fructans as compared with short-chain derivatives. These fructans, extracted from chicory roots, are prebiotic food ingredients that are fermented to lactic acid and SCFAs in the gut lumen. Synbiotics may be useful for early prevention or treatment of allergic disease (van de Pol et al, 2011.)
Colonic Salvage of Malabsorbed Energy Sources and Short-Chain Fatty Acids
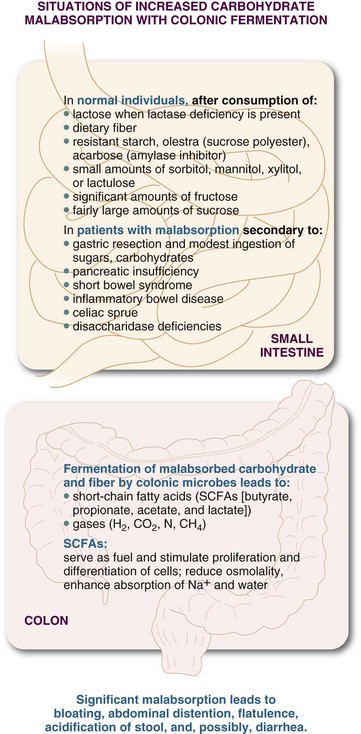
Normally, varying amounts of some small-molecular-weight carbohydrates and amino acids remain in the chyme after leaving the small intestine. Accumulation of these small molecules could become osmotically important were it not for the action of bacteria in the colon. The disposal of residual substrates through production of SCFAs is called colonic salvage (Figure 1-6). SCFAs produced in fermentation are rapidly absorbed and take water with them. They also serve as fuel for the colonocytes and gut microbes, stimulate colonocyte proliferation and differentiation, enhance the absorption of electrolytes and water, and reduce the osmotic load of malabsorbed sugars. SCFAs may also help slow the movement of GI contents and participate in several other regulatory functions.
The ability to salvage carbohydrates is limited in humans. Colonic fermentation normally disposes of 20 to 25 g of carbohydrate over 24 hours. Excess amounts of carbohydrate and fermentable fiber in the colon can cause increased gas production, abdominal distention, bloating, pain, increased flatulence, decreased colonic pH, or even diarrhea. Over time, adaptation seems to occur in individuals consuming diets high in fiber that are resistant to human digestive enzymes. Current recommendations are for the consumption of approximately 24 to 38 g of dietary fiber per day from fruits, vegetables, legumes, seeds, and whole grains for (1) maintaining the health of the cells lining the colon, (2) preventing excessive intracolonic pressure, (3) preventing constipation, and (4) maintaining a stable and healthful microbial population.
Digestion and Absorption of Specific Types of Nutrients
Most dietary carbohydrates are consumed in the form of starches, disaccharides, and monosaccharides. Starches, or polysaccharides, usually make up the greatest proportion of carbohydrates. Starches are large molecules composed of straight or branched chains of sugar molecules that are joined together, primarily in 1-4 or 1-6 linkages. Most of the dietary starches are amylopectins, the branching polysaccharides, and amylose, the straight chain–type polymers.
Dietary fiber is also largely made of chains and branches of sugar molecules, but in this case the hydrogens are positioned on the beta (opposite) side of the oxygen in the link instead of the alpha side. That humans have significant ability to digest starch, but not most fiber, exemplifies the “stereospecificity” of enzymes.
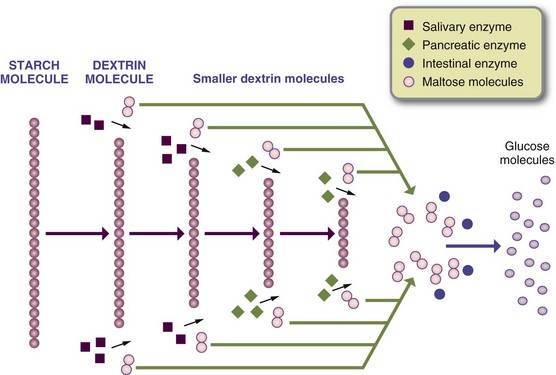
In the mouth, the enzyme salivary amylase (ptyalin) operates at a neutral or slightly alkaline pH and starts the digestive action by hydrolyzing a small amount of the starch molecules into smaller fragments (Figure 1-7). Amylase deactivates after contact with hydrochloric acid. If digestible carbohydrates remained in the stomach long enough, acid hydrolysis could eventually reduce most of them into monosaccharides. However, the stomach usually empties before significant digestion can take place. By far, most carbohydrate digestion occurs in the proximal small intestine.
Pancreatic amylase breaks the large starch molecules at 1-4 linkages to create maltose, maltotriose, and “alpha-limit” dextrins remaining from the amylopectin branches. Enzymes from the brush border of the enterocytes further break the disaccharides and oligosaccharides into monosaccharides. For example, maltase from the mucosal cells breaks down the disaccharide maltose into two molecules of glucose. These outer-cell membranes also contain the enzymes sucrase, lactase, and isomaltase (or a-dextrinase), which act on sucrose, lactose, and isomaltose, respectively (Figure 1-8).
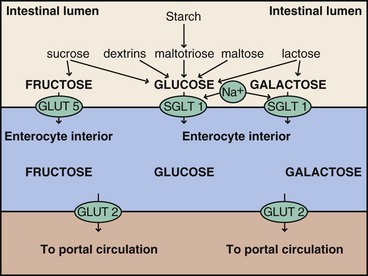
FIGURE 1-8 Starch, sucrose, maltotriose, and galactose are digested to their constituent sugars. Glucose and galactose are transported through the apical brush border membrane of the enterocyte by a sodium-dependent transporter, glucose (galactose) cotransporter; and fructose is transported by glucose transporter (GLUT) 5. Glucose, fructose, and galactose are transported across the serosal membrane by the sodium-independent transporter, GLUT2.
The resultant monosaccharides (i.e., glucose, galactose, and fructose) pass through the mucosal cells and into the bloodstream via the capillaries of the villi, where they are carried by the portal vein to the liver. At low concentrations, glucose and galactose are absorbed by active transport, primarily by a sodium-dependent transporter called the glucose (galactose) cotransporter. At higher luminal concentrations of glucose, glucose transporter (GLUT) 2 becomes a primary facilitative transporter into the intestinal cell. Fructose is more slowly absorbed and uses GLUT5 and the facilitative transporter from the lumen. GLUT2 is used to transport both glucose and fructose across the intestinal cell membranes into the blood (Kellett and Brot-Laroche, 2005).
The sodium-dependent transport of monosaccharides is the reason why sodium-glucose drinks are used to rehydrate infants with diarrhea or athletes who have lost too much fluid. Glucose is transported from the liver to the tissues, although some glucose is stored in the liver and muscles as glycogen. Most of the fructose, as in the case of galactose, is transported to the liver, where it is converted to glucose. Consumption of large amounts of lactose (especially in individuals with a lactase deficiency), fructose, stachyose, raffinose, or alcohol sugars (e.g., sorbitol, mannitol, or xylitol) can result in considerable amounts of these sugars passing unabsorbed into the colon (Beyer et al., 2005) and may cause increased gas and loose stools. Fructose is found naturally in many fruits (e.g., in sucrose and high-fructose corn syrup) but is likely to produce symptoms only if consumed as the single monosaccharide or if the food has an abundance of fructose compared with glucose (as in the case of apple juice).
Some forms of carbohydrates (i.e., cellulose, hemicellulose, pectin, gum, and other forms of fiber) cannot be digested by humans because neither their salivary nor pancreatic amylase has the ability to split the linkages connecting the constituent sugars. These carbohydrates pass relatively unchanged into the colon, where they are partially fermented by bacteria in the colon. However, unlike humans, cows and other ruminants can subsist on high-fiber food because of the bacterial digestion of these carbohydrates that takes place in the rumen. Other resistant starches and sugars are also less well digested or absorbed by humans; thus their consumption may result in significant amounts of starch and sugar in the colon. The resistant starches and some types of dietary fiber are fermented into SCFAs and gases. Starches resistant to digestion tend to include plant foods with a high protein and fiber content such as those from legumes and whole grains. One form of dietary fiber, lignin, is made of cyclopentane units and is neither readily soluble nor fermentable.
Proteins
Protein intake in the Western world ranges from approximately 50 to 100 g daily, and a good deal of the protein consumed is from animal sources. Additional protein is added all along the GIT from GI secretions and cells sloughed from GI tissues. The GIT is one of the most active synthetic tissues in the body, and the life span of enterocytes migrating from the crypts of the villi until they are shed is only 3 to 4 days. The number of cells shed daily is in the range of 10 to 20 billion cells. The latter accounts for an additional 50 to 60 g of protein that is digested and “recycled” and contributes to the daily supply. In general, animal proteins are more efficiently digested than plant proteins, but human GI physiology allows for very effective digestion and absorption of large amounts of ingested protein sources.
Protein digestion begins in the stomach, where some of the proteins are split into proteoses, peptones, and large polypeptides. Inactive pepsinogen is converted into the enzyme pepsin when it contacts hydrochloric acid and other pepsin molecules. Unlike any of the other proteolytic enzymes, pepsin digests collagen, the major protein of connective tissue. Most protein digestion takes place in the upper portion of the small intestine, but it continues throughout the GIT (Soybel, 2005). Any residual protein fractions are fermented by colonic microbes.
Contact between chyme and the intestinal mucosa stimulates release of enterokinase, an enzyme that transforms inactive pancreatic trypsinogen into active trypsin, the major pancreatic protein-digesting enzyme. Trypsin in turn activates the other pancreatic proteolytic enzymes. Pancreatic trypsin, chymotrypsin, and carboxypeptidase break down intact protein and continue the breakdown started in the stomach until small polypeptides and amino acids are formed.
Proteolytic peptidases located on the brush border also act on polypeptides, breaking them down into amino acids, dipeptides, and tripeptides. The final phase of protein digestion takes place in the brush border, where some of the dipeptides and tripeptides are hydrolyzed into their constituent amino acids by peptide hydrolases.
End products of protein digestion are absorbed as both amino acids and small peptides. Several transport molecules are required for the different amino acids, probably because of the wide differences in the size, polarity, and configuration of the different amino acids. Some of the transporters are sodium- or chloride-dependent, and some are not. Considerable amounts of dipeptides and tripeptides are also absorbed into intestinal cells using a peptide transporter, a form of active transport (Daniel, 2004). Absorbed peptides and amino acids are transported to the liver via the portal vein for metabolism by the liver and are released into the general circulation.
The presence of antibodies to many food proteins in the circulation of healthy individuals indicates that immunologically significant amounts of large intact peptides escape hydrolysis and can enter the portal circulation. The exact mechanisms that cause a food to become an allergen are not entirely clear, but these foods tend to be high in protein, to be relatively resistant to complete digestion, and to produce an immunoglobulin response (see Chapter 27). With new technology, it is possible to map and characterize allergenic peptides; this will eventually lead to safe immunotherapy treatments (Lin et al., 2009).
Almost all protein is absorbed by the time it reaches the end of the jejunum, and only 1% of ingested protein is found in the feces. Small amounts of amino acids may remain in the epithelial cells and are used for synthesis of new proteins, including intestinal enzymes and new cells.
Lipids
Approximately 97% of dietary lipids are in the form of triglycerides, and the rest are in the form of phospholipids and cholesterol. Only small amounts of fat are digested in the mouth with lingual lipase and in the stomach from the action of gastric lipase (tributyrinase). Gastric lipase hydrolyzes some triglycerides, especially short-chain triglycerides (such as those found in butter), into fatty acids and glycerol. However, most fat digestion takes place in the small intestine as a result of the emulsifying action of bile salts and hydrolysis by pancreatic lipase. As in the case of carbohydrates and protein, the capacity for digestion and absorption of dietary fat is in excess of ordinary needs.
Entrance of fat and protein into the small intestine stimulates the release of CCK and enterogastrone, which inhibit gastric secretions and motility, thus slowing the delivery of lipids. As a result, a portion of a large, fatty meal may remain in the stomach for 4 hours or longer. In addition to its many other functions, CCK stimulates biliary and pancreatic secretions. The combination of the peristaltic action of the small intestine and the surfactant and emulsification action of bile reduces the fat globules into tiny droplets, thus making them more accessible to digestion by the most potent lipid-digesting enzyme, pancreatic lipase (Keller and Layer, 2005).
Bile is a liver secretion composed of bile acids (primarily conjugates of cholic and chenodeoxycholic acids with glycine or taurine), bile pigments (which color the feces), inorganic salts, some protein, cholesterol, lecithin, and many compounds such as detoxified drugs that are metabolized and secreted by the liver. From its storage organ, the gallbladder, approximately 1 L of bile is secreted daily in response to the stimulus of food in the duodenum and stomach.
The free fatty acids and monoglycerides produced by digestion form complexes with bile salts called micelles. The micelles facilitate passage of the lipids through the watery environment of the intestinal lumen to the brush border (see Figure 1-4). The micelles release the lipid components and are returned to the gut lumen. Most of the bile salts are actively reabsorbed in the terminal ileum and returned to the liver to reenter the gut in bile secretions. This efficient recycling process is known as the enterohepatic circulation. The pool of bile acids may circulate from 3 to 15 times per day, depending on the amount of food ingested.
In the mucosal cells the fatty acids and monoglycerides are reassembled into new triglycerides. A few are further digested into free fatty acids and glycerol and then reassembled to form triglycerides. These triglycerides, along with cholesterol, fat-soluble vitamins, and phospholipids, are surrounded by a lipoprotein coat, forming chylomicrons (see Figure 1-4). The lipoprotein globules pass into the lymphatic system instead of entering portal blood and are transported to the thoracic duct and emptied into the systemic circulation at the junction of the left internal jugular and left subclavian veins. The chylomicrons are then carried through the bloodstream to several tissues, including liver, adipose tissue, and muscle. In the liver, triglycerides from the chylomicrons are repackaged into very low-density lipoproteins and transported primarily to the adipose tissue for metabolism and storage.
The fat-soluble vitamins A, D, E, and K are also absorbed in a micellar fashion, although water-soluble forms of vitamins A, E, and K supplements and carotene can be absorbed in the absence of bile acids.
Under normal conditions approximately 95% to 97% of ingested fat is absorbed into lymph vessels. Because of their shorter length and thus increased solubility, fatty acids of 8 to 12 carbons (i.e., medium-chain fatty acids) can be absorbed directly into colonic mucosal cells without the presence of bile and micelle formation. After entering mucosal cells, they are able to go directly without esterification into the portal vein, which carries them to the liver.
Increased motility, intestinal mucosal changes, pancreatic insufficiency, or the absence of bile can decrease absorption of fat. When undigested fat appears in the feces, the condition is known as steatorrhea (see Chapter 29. Medium-chain triglycerides (MCTs) have fatty acids 8 to 12 carbons long; MCTs are clinically valuable for individuals who lack necessary bile salts for long-chain fatty acid metabolism and transport. Supplements for clinical use are normally provided in the form of oil or a dietary beverage with other macronutrients and micronutrients.
For the nutrition care process, several nutrition diagnoses can be identified. The following list provides examples:
Vitamins and Minerals
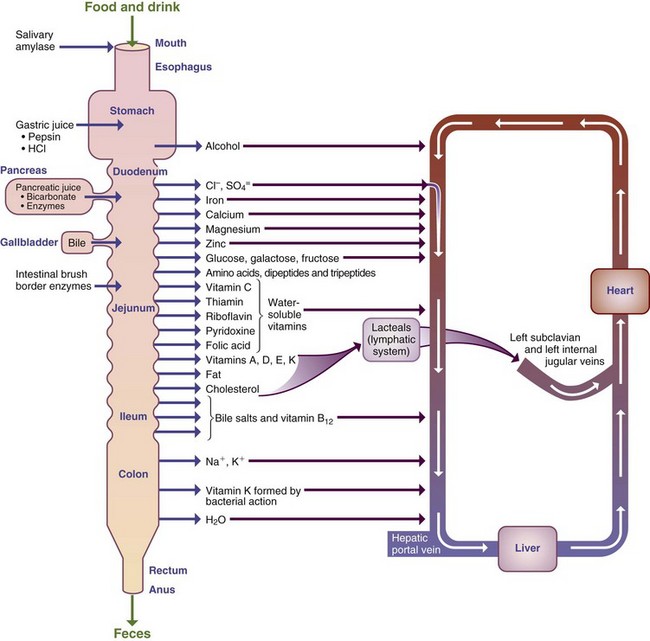
Vitamins and minerals from foods are made available as macronutrients and are digested and absorbed across the mucosal layer, primarily in the small intestine (Figure 1-9). Besides adequate passive and transporter mechanisms, various factors affect the bioavailability of vitamins and minerals, including the presence or absence of other specific nutrients, acid or alkali, phytates, and oxalates. Each day approximately 8 to 9 L of fluid is secreted from the GIT and serves as a solvent, a vehicle for chemical reactions, and a medium for transfer of several nutrients.
At least some vitamins and water pass unchanged from the small intestine into the blood by passive diffusion, but several different mechanisms might be used to transport individual vitamins across the GI mucosa. Drugs are absorbed by a number of mechanisms, but often by passive diffusion. Thus drugs may share or compete with mechanisms for the absorption nutrients into intestinal cells (see Chapter 9).
Mineral absorption is more complex, especially the absorption of the cation minerals. These cations, such as selenium, are made available for absorption by the process of chelation, in which a mineral is bound to a ligand—usually an acid, an organic acid, or an amino acid, so that it is in a form absorbable by intestinal cells.
Iron and zinc absorption share several characteristics in that the efficiency of absorption partly depends on the needs of the host. They also use at least one transport protein, and each has mechanisms to increase absorption when stores are inadequate. Because phytates and oxalates from plants impair the absorption of both iron and zinc, absorption is better when animal sources are consumed. The absorption of zinc is impaired with disproportionately increased amounts of magnesium, calcium, and iron. Calcium absorption into the enterocyte occurs through channels in the brush border membrane, where it is bound to a specific protein carrier for transportation across the basolateral membrane. The process is regulated by the presence of vitamin D. Phosphorus is absorbed by a sodium phosphorus cotransporter, which is also regulated by vitamin D or low phosphate intake.
The GIT is the site of important interactions among minerals. Supplementation with large amounts of iron or zinc may decrease the absorption of copper. In turn, the presence of copper may lower iron and molybdenum absorption. Cobalt absorption is increased in patients with iron deficiency, but cobalt and iron compete and inhibit one another’s absorption. These interactions are probably the result of an overlap of mineral absorption mechanisms.
Minerals are transported in blood bound to protein carriers. The protein binding is either specific (e.g., transferrin, which binds with iron, or ceruloplasmin which binds with copper) or general (e.g., albumin, which binds with a variety of minerals). A fraction of each mineral is also carried in the serum as amino acid or peptide complexes. Specific protein carriers are usually not completely saturated; the reserve capacity may serve as a buffer against excessive exposure. Toxicity from minerals usually results only after this buffering capacity is exceeded.
References
Beyer, P, et al. Fructose intake at current level in the United States may cause gastrointestinal distress in normal adults. J Am Diet Assoc. 2005;105:1559.
Chey, WY, Chang, TM. Secretin, 100 years later. J Gastroenterol. 2003;38:1025.
Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Ann Rev Physiol. 2004;66:361.
Deng, PY, et al. Cholecystokinin facilitates glutamate release by increasing the number of readily releasable vesicles and releasing probability. J Neurosci. 2010;30:5136.
De Smet, B, Mitselos, A, Depoortere, I. Motilin and ghrelin as prokinetic drug targets. Pharmacol Ther. 2009;123:207.
Englyst, KN, Englyst, HN. Carbohydrate bioavailability. Br J Nutr. 2005;94:1.
Frank, DN, Pace, NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;1:4.
Hui, DY, et al. Development and physiological regulation of intestinal lipid absorption. III. Intestinal transporters and cholesterol absorption. Am J Physiol Gastrointest Liver Physiol. 2008;294:G839.
Hylemon, PB, et al. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509.
Kahn, WI, Ghia, JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19.
Keller, J, Layer, P. Human pancreatic endocrine response to nutrients in health and disease. Gut. 2005;54:1.
Kellett, G, Brot-Laroche, E. Apical GLUT 2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056.
Lammert, F, Wang, DO. New insights into the genetic regulation of intestinal cholesterol absorption. Gastroenterology. 2005;128:718.
Lin, J, et al. Microarrayed allergen molecules for diagnostics of allergy. Methods Mol Biol. 2009;524:259.
Macfarlane, GT, et al. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. Ann Microbiol. 2008;104:305.
Nauck, MA. Unraveling the science of incretin biology. Am J Med. 2009;122S:S3.
O’Keefe, SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51.
Quigley, EM. Prebiotics and probiotics: modifying and mining the microbiota. Pharmacol Res. 2010;61:213.
Rehfeld, JF. A centenary of gastrointestinal endocrinology. Horm Metab Res. 2004;36:735.
Roberfroid, M, et al. Prebiotic effects: metabolic and health benefits. Brit J Nutr. 2010;104:1S.
Schubert, ML. Hormonal regulation of gastric acid secretion. Curr Gastroenterol Rep. 2009;10:523.
Snelling, AM. Effects of probiotics on the gastrointestinal tract. Curr Opin Infect Dis. 2005;18:420.
Soybel, DI. Anatomy and physiology of the stomach. Surg Clin North Am. 2005;85:875.
Stanley, S, et al. Hormonal regulation of food intake. Physiol Rev. 2005;85:1131.
Tappenden, KA, Deutsch, AS. The physiological relevance of the intestinal microbiota—contributions to human health. J Am Coll Nutr. 2007;26:679S.
van de Pol, MA, et al. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy. 2011;66:39.
Van Op Den Bosch, J, et al. The role of somatostatin, structurally related peptides and somatostatin receptors in the gastrointestinal tract. Regul Pept. 2009;156:1.