Medical Nutrition Therapy for Upper Gastrointestinal Tract Disorders
Digestive disorders are among the most common problems in health care. More than 50 million visits are made annually to ambulatory care facilities for symptoms related to the digestive system. More than 10 million endoscopies and surgical procedures involving the gastrointestinal tract (GIT) are performed each year (Cherry et al., 2008). Dietary habits and specific food types can play an important role in the onset, treatment, and prevention of many GI disorders. Nutrition therapy is vital in prevention and treatment of malnutrition, deficiencies, and conditions that can develop from GIT disease, such as secondary osteoporosis or anemia. Additionally, diet and lifestyle modifications can improve nutritional well being and quality of life by decreasing symptoms, health care visits, and associated costs. Table 28-1 describes disorders of the upper GIT, their typical symptoms, and nutritional consequences.
TABLE 28-1
Upper Gastrointestinal Disorders and Nutritional Consequences
| Gastrointestinal Condition | Common Symptoms | Possible Nutritional Consequences |
| Achalasia | Aperistalsis; delayed or incomplete relaxation of the lower esophageal sphincter in response to swallowing; dysphagia | Decreased nutritional intake leading to malnutrition, weight loss, nutrient deficiencies; considered a premalignant disorder |
| Cancer of the oral cavity, esophagus, or stomach | Asymptomatic, or difficulty chewing, swallowing, epigastric discomfort, delayed gastric emptying | Anorexia, decreased variety of foods, weight loss, change in food textures; may require surgery, radiation, chemotherapy, enteral feeding |
| Dumping syndrome after gastrectomy, pyloroplasty, fundoplication, Roux-en-Y gastric bypass surgery | Early satiety, bloating, nausea; weak, lightheaded, sweaty; later symptoms such as reactive hypoglycemia and possible cramping, diarrhea | Decreased intake, malabsorption of nutrients, weight loss, nutrient deficiencies |
| Duodenal ulcer | Pain several hours after meals; may be relieved by eating | Perceived food intolerances, increased or decreased food intake |
| Dyspepsia | Upper abdominal discomfort, bloating, especially after meals | Possible decreased intake of food variety or energy intake, gastric acid suppression may lead to nutrient malabsorption and deficiencies. |
| Esophageal stricture or tumor | Asymptomatic, or difficulty swallowing foods; solids may especially cause discomfort | Reduced energy and nutrient intake, weight loss. |
| Gastric ulcer | Vague epigastric discomfort associated with eating | Decreased intake in general, or of selected foods |
| GERD | Acid taste, increased belching, hoarseness, dry cough, burning sensation in upper middle of chest, sometimes spasm, difficulty swallowing, bloating | Reduced quality and quantity of dietary intake, gastric acid suppression may lead to nutrient malabsorption and deficiencies |
| Gastroparesis | Abdominal bloating, decreased appetite/anorexia, nausea and vomiting, fullness, early satiety, halitosis, and postprandial hypoglycemia | Reduced energy and nutrient intake, decreased nutrient use resulting from hyperglycemia, dehydration Severe cases may benefit from feeding-tube placement. |
Assessment Parameters
Nutritional screening and careful evaluation of patients with upper GI disorders guide the patient’s overall plan of care. Unintentional weight loss over time is the single most useful parameter, with severe malnutrition indicated by a loss of 2% or more of usual body weight in 1 week, 5% or more during 1 month, or 10% or more during 6 months. Other assessments of nutritional risk include percent of ideal body weight and body mass index. Patients who have severe weight loss benefit from beginning nutritional support early, sometimes prior to or during other medical treatments.
During the initial assessment, the clinician should also obtain an assessment of the patient’s weight history, changes in appetite, nausea, vomiting, diarrhea, problems with chewing or swallowing, typical daily dietary intake, use of supplemental nutrition (oral, enteral, or parenteral), food allergies or intolerances, use of supplements (vitamins, minerals, herbs, probiotics, or protein powders), use of stool-bulking agents or laxatives, and medications. Intolerance to various foods, inadequate intake, and malabsorption can lead to nutrient deficiencies and increased morbidity. Common laboratory values, such as B12, folate, ferritin, and 25-hydroxy vitamin D, may be useful in the initial assessment and monitoring. Other laboratory values may be useful, particularly when malabsorption or insufficient intake of certain nutrients is suspected. Patients with gastric surgeries or gastric acid suppression are at higher risk for nutrient deficiencies, such as iron or B12. In patients with gastric surgeries, deficiencies may manifest early or develop over time.
The Esophagus
The esophagus is a tubular organ, approximately 25 cm long, that is lined with both tubular and striated muscles. Swallowing triggers peristalsis—waves of coordinated muscles contractions. As a bolus of food is moved voluntarily from the mouth to the pharynx, the upper sphincter relaxes, the food moves into the esophagus, and peristaltic waves move the bolus down the esophagus; the lower esophageal sphincter (LES) relaxes to allow the food bolus to pass into the stomach (Figure 28-1). From start to finish, this process generally takes 5 seconds when in an upright position, and up to 30 seconds when in a supine position (Cordova-Fraga, 2008).
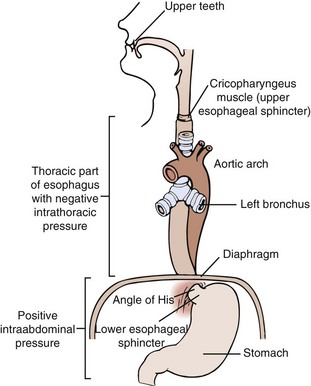
FIGURE 28-1 Normal esophagus. (Modified from Price SA, Wilson LM: Pathophysiology: clinical concepts of disease processes, ed 6, St Louis, 2003, Mosby.)
The normal esophagus has a multitiered defense system that prevents tissue damage from exposure to gastric contents, including LES contraction, normal gastric motility, esophageal mucus, tight cellular junctions, and cellular pH regulators. Dysphagia (difficulty in swallowing) may be by obstruction, inflammation, or abnormal upper esophageal sphincter function that causes derangement of the swallowing mechanism. Skeletomuscular disorders and motility disorders may result in dysphagia. For example, achalasia is characterized by a failure of esophageal neurons, resulting in loss of ability to relax the LES and normal peristalsis. Odynophagia (painful swallowing) may interfere with nutritional intake in some patients with oral or esophageal cancers.
Gastroesophageal Reflux and Esophagitis
Regurgitation occurs in approximately half of infants in the first few months of life; most cases resolve after the first year. Reflux of gastric contents into the esophagus is a normal physiologic event that occurs daily in healthy individuals (Orlando, 2008). In gastroesophageal reflux disease (GERD) episodes of reflux overwhelm esophageal protective mechanisms and result in symptoms such as heartburn, a burning sensation in the esophagus, or inflammation with erosion of the lining of the esophagus. Approximately 7% to 8% of the U.S. population experiences heartburn daily, and 20% to 40% of adults report symptoms of GERD at least once weekly. The prevalence of GERD in children may range from approximately 2% to 20% (Gold, 2006).
The types of GERD can be distinguished by esophagogastroduodenoscopy (EGD), which uses a fiberoptic endoscope to directly visualize the esophagus, stomach, and duodenum. EGD can be useful in determining the success of treatment in erosive GERD (Yuan and Hunt, 2009). Erosive GERD is generally associated with more severe and prolonged symptoms, compared with the nonerosive esophageal reflux disease (Orlando, 2008). Some people experience GERD symptoms primarily in the evening (nocturnal GERD), which may occur as a result of decreased salivary secretions and swallowing, decreased GI motility, prolonged exposure to acid, and being in the supine position (Gerson and Fass, 2009).
Pathophysiology
The pathophysiology of GERD is complex. The most common underlying mechanisms are thought to be reduced LES pressure, inadequate esophageal tissue defense, direct mucosal irritants, decreased gastric motility, and increased intraabdominal pressure. LES pressure decreases during pregnancy (heartburn affects up to 80% of women in their third trimester), in women taking progesterone-containing oral contraceptives, and even in the late stage of a normal menstrual cycle (Dowswell and Neilson, 2008).
The pressure of the LES may be influenced by other conditions, including hiatal hernia, scleroderma (a disease that involves hardening and tightening of skin and connective tissues), and hypersecretory diseases such as Zollinger-Ellison syndrome. Transient LES relaxations, which are induced by distension of the proximal stomach (the same stimulus for belching) are common in GERD. Patients with chronic respiratory disorders, such as chronic obstructive pulmonary disease, are at risk for GERD because of frequent increases in intraabdominal pressure. Muscle relaxants and nonsteroidal antiinflammatory drugs (NSAIDs) are the primary offending medications implicated in GERD.
The presentation of GERD symptoms varies, but may include reflux of gastric secretions, heartburn, substernal pain, belching, and esophageal spasm. In children, vomiting, dysphagia, refusal to eat, or complaints of abdominal pain may be present (Hassall, 2005). Manifestations such as pharyngeal irritation, frequent throat clearing, hoarseness, and worsening of asthmatic symptoms may also occur. The frequency and severity of symptoms do not always predict the severity or complications of the disease, and may not correlate with endoscopic findings. Some patients have few overt symptoms and relatively significant disease; others may have considerable discomfort without erosive, long-standing consequences.
Prolonged acid exposure can result in esophagitis (inflammation of the esophagus), esophageal erosions, ulceration, scarring, stricture, and in some cases dysphagia (see Pathophysiology and Care Management Algorithm: Esophagitis). Acute esophagitis may be caused by reflux, ingestion of a corrosive agent, viral or bacterial infection, intubation, radiation, or eosinophilic infiltration. Eosinophilic esophagitis is characterized by an isolated, severe eosinophilic infiltration of the esophagus manifested by GERD-like symptoms that may be caused by an immune response. See Chapter 27. Irritants such as smoking and large doses or chronic use of aspirin or NSAIDs can increase the risk of esophagitis (Pera et al., 2005).
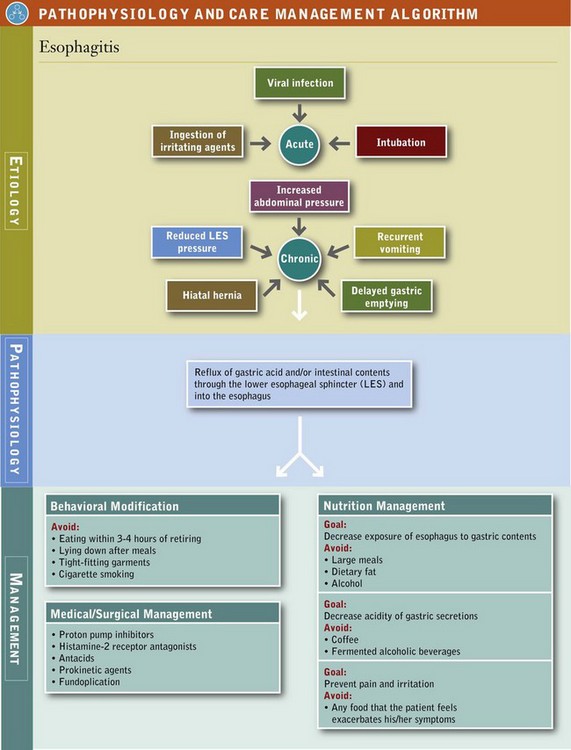
The severity of the esophagitis resulting from gastroesophageal reflux is influenced by the composition, frequency, and volume of the gastric reflux; length of exposure of the esophagus to the gastric reflux; the health of the mucosal barrier; and the rate of gastric emptying. Symptoms of esophagitis and GERD may impair the ability to consume an adequate diet, and interfere with sleep, work, social events, and the overall quality of life.
A common contributor to gastroesophageal reflux and esophagitis is hiatal hernia. The presence of hiatal hernia is not synonymous with reflux, but it increases the likelihood of symptoms and complications. The esophagus passes through the diaphragm by way of the esophageal hiatus or ring. The attachment of the esophagus to the hiatal ring may become compromised, allowing a portion of the upper stomach to move above the diaphragm. The most common type of hiatal hernia is the sliding hernia, and the less common form is the paraesophageal hernia (Figure 28-2).
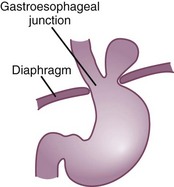
FIGURE 28-2 Hiatal hernia. (Modified from Price SA, Wilson LM: Pathophysiology: clinical concepts of disease processes, ed 6, St Louis, 2003, Mosby.)
When acid reflux occurs with a hiatal hernia, the gastric contents remain above the hiatus longer than normal. The prolonged acid exposure increases the risk of developing more serious esophagitis (Orlando, 2008). Because increases in intragastric pressure force acidic stomach contents up into the esophagus, persons with hiatal hernia may experience difficulty when lying down or bending over. Epigastric pain occurs in the upper middle region of the abdomen after large, energy-dense meals. Weight reduction and decreasing meal size decreases the negative consequences of hiatal hernia.
Barrett’s esophagus (BE) is a precancerous condition in which the normal squamous epithelium of the distal esophagus is replaced by an abnormal columnar epithelium known as specialized intestinal metaplasia. Certain risk factors may prompt physicians to consider testing for BE, including prolonged history of GERD symptoms (>5 years), white race, male sex, older age (>50 years), and family history of BE or adenocarcinoma of the esophagus. It is estimated that 5% to 15% of persons with GERD have BE (Lichtenstein et al., 2007; Pera et al., 2005). Both GERD and BE increase a patient’s risk for adenocarcinoma of the esophagus. The incidence of adenocarcinoma of the esophagus is rising at a rate exceeding all other cancers in the US–4% to 10% annually (Okoro and Wang, 2010).
Medical and Surgical Management
The primary medical treatment of esophageal reflux is suppression of acid secretion. Proton pump inhibitors (PPIs), which decrease acid production by the gastric parietal cell, are most effective (Rohof et al., 2009), but milder forms of reflux are sometimes managed by H2 receptor (a type of histamine receptor on the parietal cell) antagonists and antacids. The aim in acid-suppression therapy is to raise the gastric pH above 4 during periods when reflux is most likely to occur. (See “Gastritis and Peptic Ulcers” later in this chapter for side effects.) Prokinetic agents, which increase propulsive contractions of the stomach, may be used in persons who have delayed gastric emptying. Refer to Table 28-2 for medications commonly used in upper GI disorders.
TABLE 28-2
Some Common Medications Used in the Treatment of Upper Gastrointestinal Tract Disorders
| Type of Medication | Common Names | Medication Function |
| Proton pump inhibitor | Omeprazole Lansoprazole Esomeprazole Pantoprazole Dexlansoprazole Rabeprazole |
Inhibits acid secretion |
| H2 blocker | Cimetidine Ranitidine Famotidine Nizatidine |
Blocks the action of histamine on parietal cells, decreasing the production of acid |
| Prokinetic | Erythromycin Metoclopramide Domperidone |
Increases contractility of the stomach and shortens gastric emptying time |
| Antisecretory | Octreotide (somatostatin analogue) Somatostatin |
Inhibits release of insulin and other gut hormones. Slows rate of gastric emptying and small intestine transit time; increases intestinal water and sodium absorption |
| Antidumping | Acarbose | Delays carbohydrate digestion by inhibiting alpha-glycoside hydrolase, which interferes with conversion of starch to monosaccharides |
| Antigas agent | Simethicone | Lowers surface tension of gas bubbles |
| Antacids | Magnesium, calcium, or aluminum bound to carbonate or phosphate | Buffers gastric acid |
Raising the head of the bed by 6 to 8 inches can reduce likelihood of nocturnal reflux. Frequent bending over should be avoided. Obesity is a contributing factor to GERD and hiatal hernia because it increases intragastric pressure, and weight loss may reduce acid contact time in the esophagus, leading to decreased reflux symptoms. Box 28-1 lists modifications that are aimed at enhancing esophageal acid clearance, minimizing the occurrence of reflux, or both. Lifestyle interventions alone are unlikely to suffice, except in mild cases of GERD.
Of patients with severe GERD, 5% to 10% do not respond to medical therapy. They may be treated surgically with fundoplication, a procedure in which the fundus of the stomach is wrapped around the lower esophagus to limit reflux. Use of tobacco products is contraindicated with reflux. Smoking tobacco products decreases LES pressure and prolongs acid clearance by decreasing salivation. Smoking also compromises GIT integrity and increases the risk of esophageal and other cancers (see Clinical Insight: Smoking and Gastrointestinal Function).
Medical Nutrition Therapy
Certain diet and lifestyle changes may relieve symptoms in some patients with GERD. The main factors are caffeine, alcohol, tobacco, and stress. Other dietary factors include dietary fat, chocolate, coffee, onions, peppermint, spices, citrus foods, wine, and carbonated beverages.
The role of spices in the pathologic conditions related to upper GI disorders is not clear. In patients with GI lesions, foods highly seasoned with chili powder and pepper may cause discomfort. The type of chili and amount of capsaicin consumed make the difference (Milke et al., 2006). Foods such as carminatives (peppermint and spearmint) may lower LES pressure. While fermented alcoholic beverages (such as beer and wine) stimulate the secretion of gastric acid and should be limited, coffee may be used in small amounts.
Limiting or avoiding aggravating foods may improve symptoms in some individuals. There is no need to eliminate foods if they do not affect symptoms (El-Serag et al., 2005). Chewing gum has been shown to increase salivary secretions, which help to raise esophageal pH, but no studies prove efficacy when compared with other lifestyle changes.
For patients with severe esophagitis, a low-fat, liquid diet initially minimizes esophageal distention, passes more easily through any strictured areas, and empties readily from the stomach. Foods with an acidic pH including citrus juices, tomatoes, and soft drinks cause pain when the esophagus is already inflamed and should be avoided.
Identification and treatment of the main mechanism underlying the GERD is the first line of therapy. Large, high-fat meals delay gastric emptying and prolong acid secretion; avoiding those conditions prior to going to bed is often useful. Lifestyle modifications, including change in dietary practices, weight loss, smoking cessation, and elevation of the head of the bed, can reduce symptoms (see Box 28-1).
Oral Cancer and Surgeries
The patient diagnosed with cancer of the oral cavity, pharynx, or esophagus may present with existing nutritional problems and dysphagia or odynophagia secondary to the tumor mass, obstruction, oral infection, or ulceration. Nutritional deficits may be compounded by the treatment, which commonly involves surgical resection, radiation, or chemotherapy. Chemotherapy may produce nausea, vomiting, and anorexia (see Chapter 37). Chewing, swallowing, salivation, and taste acuity are often altered. Extensive dental decay, osteoradionecrosis, and infections may also occur.
Surgery of the Mouth or Esophagus
Surgery of the mouth or esophagus may be necessary to remove tumors. Thus it may be necessary to provide nutrition using liquid supplements. Patients who are unable to take adequate nutrition orally for an extended time, such as those with extensive disease or those requiring major surgery, are likely to benefit from a gastrostomy tube placement. The enteral route of nutrition is preferred; however, if the GIT is not functional, parenteral nutrition can be provided (see Chapter 14).
Tonsillectomy: Tonsils are lymphatic tissue. Mild inflammation of the tonsils is considered a natural part of the efforts of the immune system to fight infection. Rarely, the physician may remove the tonsils if they are too large and obstruct the ability to breathe, or for the purpose of reducing the number and frequency of ear infections, tonsillitis, and sinusitis. Cold, mild-flavored, soft, moist foods bring the most comfort to the patient and offer the most protection against unexpected bleeding from the surgical area. The patient can typically consume a normal diet within 3 to 5 days.
Medical Nutrition Therapy
When the patient is unable to meet energy and protein needs orally for prolonged periods, tube feeding should be considered. Gastrostomy feedings can be total or supplemental; many nutritionally complete formulas are available (see Appendix 32). Enteral tube feedings are most commonly provided as ready-to-feed formulas, which are convenient and nutritionally complete. To add variety to the diet, ordinary foods such as fruits can be puréed and mixed with water until liquefied. Table foods may be prepared in a blender, but maintaining nutritional adequacy, sanitation, and a viscosity that will not clog feeding tubes is too labor-intensive to be practical for most patients or their families.
Fluid intake, artificial saliva solutions, and saline rinses may be used to prevent dry mouth. Topical anesthetics can be used to relieve pain. Because narcotic pain medications delay gastric emptying and constipation, extra fluids and a bowel regimen (stool softeners, laxatives) may be necessary.
The Stomach
The mucosa of the stomach and duodenum is protected from the proteolytic actions of gastric acid and pepsin by a coating of mucus secreted by glands in the epithelial walls from the lower esophagus to the upper duodenum. The mucosa is also protected from bacterial invasion by the digestive actions of pepsin and hydrochloric acid (HCl) and the mucus secretions. HCl is secreted by the parietal cells in response to stimuli by gastrin, acetylcholine, and histamine. The mucus contains acid-neutralizing bicarbonate, and additional bicarbonate is provided by the pancreatic juice secreted into the intestinal lumen. Production of mucus is stimulated by the action of prostaglandins.
Dyspepsia
Dyspepsia (indigestion) refers to nonspecific, persistent upper-abdominal discomfort or pain. The discomfort may be related to organic causes such as esophageal reflux, gastritis, peptic ulcer, gallbladder disease, or other identifiable pathologic conditions. Because of the variety of presentations and symptoms, dyspepsia may overlap with other problems such as GERD or irritable bowel syndrome, anxiety, and depression. Diet, stress, and other lifestyle factors may contribute to the symptoms.
Functional dyspepsia (nonulcer dyspepsia) describes persistent or recurrent upper GI discomfort, without underlying pathologic conditions. Symptoms of functional dyspepsia are reported in approximately 15% to 20% of adults per year and may include vague abdominal discomfort, bloating, early satiety, nausea, and belching. Underlying mechanisms are not entirely clear; visceral hypersensitivity to acid or distention, impaired gastric accommodation, altered brain-gut axis, and abnormal gastric motility and emptying have all been considered (Fajardo et al., 2005).
Medical Nutrition Therapy
Dietary and lifestyle management is the same as for GERD. Excessive volumes of food or high intake of fat, sugar, caffeine, spices, or alcohol are commonly implicated but have not been confirmed in all cases. Delayed emptying and increased sensation of fullness are common features. Reduction of dietary fat intake, use of smaller meals, diets of low caloric density, and achieving a healthy weight may be helpful (Pilichiewicz et al., 2009). Because alcoholic beverages may alter GI functions in a number of ways, limiting intake is recommended. Mild exercise enhances movement of foodstuffs through the GIT and increases one’s sense of well being. Because periods of persistent stress may contribute to functional GI disorders, behavioral management and emotional support may also help. If symptoms persist, further evaluation should be pursued to identify the underlying cause.
Gastritis and Peptic Ulcers
Gastritis and peptic ulcers result when infectious, chemical, or neural abnormalities disrupt mucosal integrity of the stomach. The most common cause is Helicobacter pylori infection, a gram-negative bacteria that is somewhat resistant to the acidic medium of the stomach. H. pylori infection induces inflammation from both innate and systemic immune response. Olfactomedin 4 is a glycoprotein that has been found to be up-regulated in H. pylori–infected patients, leading to expression of proinflammatory cytokines or chemokines through Nod1- and Nod2-mediated nuclear factor (NF)-κB activation; this inhibits host immune response and contributes to persistence of H. pylori colonization (Liu et al., 2010).
The prevalence of H. pylori infection generally correlates with geography and the socioeconomic status of the population. It ranges from approximately 10% in developed countries to 80% to 90% in developing countries. Although gastritis is a characteristic observation, only 10% to 15% of those infected by the organism develop symptomatic ulceration, and approximately 1% develop gastric cancer (Ernst et al., 2006; Fennerty, 2005).
H. pylori infection is responsible for most cases of chronic inflammation of the gastric mucosa and peptic ulcer, gastric cancer, and atrophic gastritis (chronic inflammation with deterioration of the mucous membrane and glands) resulting in achlorhydria and loss of intrinsic factor (Israel and Peek, 2006; Selgrad et al., 2008). The infection does not resolve spontaneously, and risks of complications increase with duration of the infection. Other factors affect the risk of pathologic consequences, including the patient’s age at onset of the initial infection, the specific strain and concentration of the organism, genetic factors related to the host, and the lifestyle and overall health of the patient. The infection is typically confined to the mucosa of the stomach. Treatment of H. pylori typically involves the use of two or three antibiotics and acid-suppressing medications. Doing so ameliorates the gastritis, reduces the conditions that favor carcinogenesis, and may improve digestive function (Bytzer and O’Morain, 2005; Guzzo et al., 2005) (see Focus On: The Changing Face of Helicobacter pylori and Gastric Cancer).
Other Forms of Gastritis
Chronic use of aspirin or other NSAIDs, steroids, alcohol, erosive substances, tobacco, or any combination of these factors may compromise mucosal integrity and increase the chance for acquiring acute or chronic gastritis. Eosinophilic gastritis may also contribute to some cases of gastritis (Whittingham and Mackay, 2005). See Chapter 27. Poor nutrition and general poor health may contribute to the onset and severity of the symptoms and can delay the healing process.
Acute gastritis refers to rapid onset of inflammation and symptoms. Chronic gastritis may occur over a period of months to decades, with waxing and waning of symptoms. Gastritis may manifest by a number of symptoms, including nausea, vomiting, malaise, anorexia, hemorrhage, and epigastric pain. Prolonged gastritis may result in atrophy and loss of stomach parietal cells, with a loss of secretion of HCl (achlorhydria) and intrinsic factor, resulting in pernicious anemia.
Recent studies emphasize the importance of considering side effects of chronic acid suppression either from disease or chronic use of acid-suppressing medication such as PPIs (Katz, 2010). These include reduction of gastric secretion of HCl, which has been shown to reduce absorption of nutrients such as B12, calcium, and nonheme iron, which rely on intragastric proteolysis to make them bioavailable (McColl, 2009). Acid suppression may increase incidence of some bone fractures (Gray et al., 2010), as well as increase risk for intestinal infection, as gastric acidity is a front-line barrier to microbial invasion (Ali et al., 2009; Linsky et al., 2010).
Peptic Ulcers
Normal gastric and duodenal mucosa is protected from the digestive actions of acid and pepsin by the secretion of mucus, the production of bicarbonate, the removal of excess acid by normal blood flow, and the rapid renewal and repair of epithelial cell injury. Peptic ulcer refers to an ulcer that occurs as a result of the breakdown of these normal defense and repair mechanisms. Typically more than one of the mechanisms must be malfunctioning for symptomatic peptic ulcers to develop. Peptic ulcers typically show evidence of chronic inflammation and repair processes surrounding the lesion.
The primary causes of peptic ulcers are H. pylori infection, gastritis, the use of aspirin, other NSAIDs and corticosteroids, and severe illness (see “Stress Ulcers” later in this chapter) (see Pathophysiology and Care Management Algorithm: Peptic Ulcer) (Israel and Peek, 2006). Life stress may lead to behaviors that increase peptic ulcer risk. Excessive use of concentrated forms of ethanol can damage gastric mucosa, worsen symptoms of peptic ulcers, and interfere with ulcer healing. However, modest doses of alcoholic beverages in otherwise healthy persons do not appear to cause peptic ulcers. Consumption of beer and wine increases gastric secretions, whereas low concentrations of alcohol may not. Use of tobacco products decreases bicarbonate secretion, decreases mucosal blood flow, exacerbates inflammation, and is associated with additional complications of H. pylori infection. Other risk factors include gastrinoma and Zollinger-Ellison syndrome (see Chapter 30).
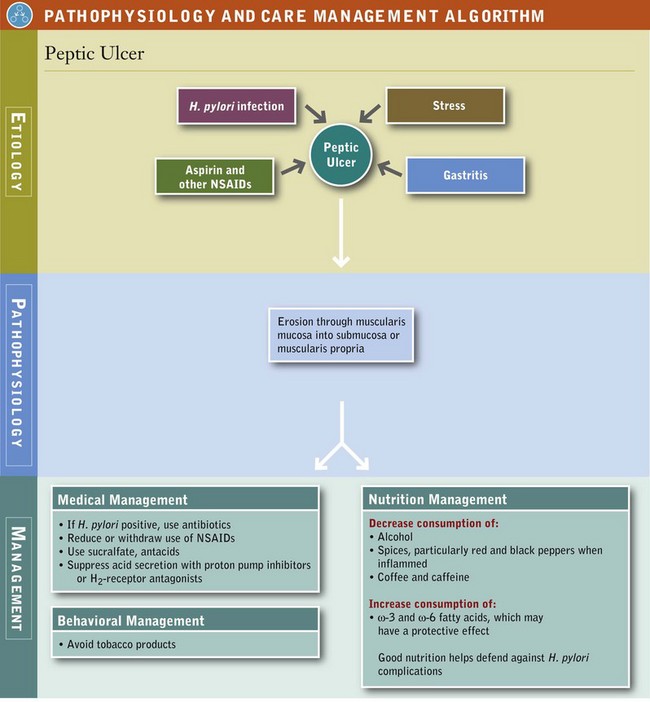
As a result of earlier screening for H. pylori and early recognition of the symptoms and risk factors associated with peptic ulcers, their incidence and prevalence and the number of surgical procedures related to them have decreased markedly in the last three decades.
Peptic ulcers normally involve two major regions: gastric and duodenal. Uncomplicated peptic ulcers in either region may present with signs similar to those associated with dyspepsia and gastritis. Abdominal pain or discomfort is characteristic of both gastric and duodenal ulcers, although anorexia, weight loss, nausea and vomiting, and heartburn may occur slightly more often in persons with gastric ulcers. In some patients peptic ulcers are asymptomatic.
Complications of hemorrhage and perforation contribute significantly to the morbidity and mortality of peptic ulcers. Ulcers can perforate into the peritoneal cavity or penetrate into an adjacent organ (usually the pancreas), or they may erode an artery and cause massive hemorrhage. Melena refers to black, tarry stools that are common in peptic ulcer disease, especially in older adults. Melena may suggest either acute or chronic upper GI bleeding.
Gastric versus Duodenal Ulcers
Although gastric ulcers can occur anywhere in the stomach, most occur along the lesser curvature of the stomach (Figure 28-3). Gastric ulcers typically are associated with widespread gastritis, inflammatory involvement of parietal (acid-producing) cells, and atrophy of acid- and pepsin-producing cells with advancing age. In some cases gastric ulceration develops despite relatively low acid output. Antral hypomotility, gastric stasis, and increased duodenal reflux are common in gastric ulcer and, when present, may increase the severity of the gastric injury. With a gastric ulcer, hemorrhage and overall mortality are higher than with a duodenal ulcer.
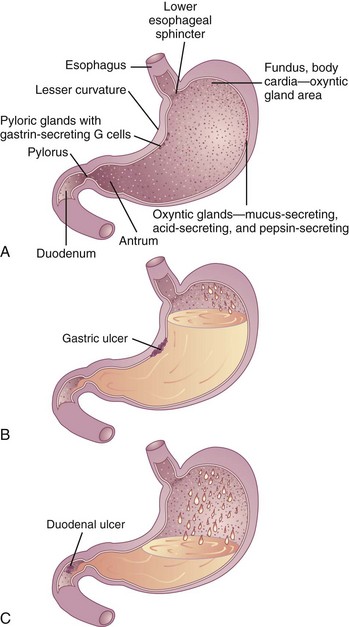
FIGURE 28-3 Diagram showing A, the normal stomach and duodenum; B, a gastric ulcer; and C, a duodenal ulcer.
A duodenal ulcer is characterized by increased acid secretion, nocturnal acid secretion, and decreased bicarbonate secretion. Most duodenal ulcers occur within the first few centimeters of the duodenal bulb, in an area immediately below the pylorus. Gastric outlet obstruction occurs more commonly with duodenal ulcers than with gastric ulcers, and gastric metaplasia (i.e., replacement of duodenal villous cells with gastric-type mucosal cells) may occur with duodenal ulcer related to H. pylori.
Medical and Surgical Management of Ulcers
Peptic Ulcers: The primary cause of gastritis and peptic ulcers is H. pylori infection; therefore the primary focus of treatment in most cases is the eradication of this organism with the appropriate antibiotic and acid suppressive regimen. As a result of the ability to recognize and eradicate H. pylori, surgical intervention for peptic ulcer management is less frequent, although emergent and elective surgeries are still needed for complications. Interventions may include endoscopic, open, and laparoscopic procedures to treat individual lesions, to partial gastrectomy and selective vagotomies. One measure includes regular use of protective foods that contain phenolic antioxidants such as cranberries or ginger extracts (Zingiber officinale), which may have the capacity to help eradicate H. pylori (Siddaraju and Dharmesh, 2007; Vattem et al., 2005).
Stress Ulcers: Stress ulcers may occur as a complication of severe burns, trauma, surgery, shock, renal failure, or radiation therapy. A primary concern with stress ulceration is the potential for significant hemorrhage. Gastric ischemia with GI hypoperfusion, oxidative injury, reflux of bile salts and pancreatic enzymes, microbial colonization, and mucosal barrier changes have also been implicated. The true mechanisms are not completely understood, but the use of antioxidant compounds shows promise (Zhu and Kaunitz, 2008).
Stress ulcers that bleed can be a significant cause of morbidity in critically ill patients, but knowledge of effective prevention and treatment is still incomplete. Sucralfate, acid suppressives, and antibiotics as necessary are used for prophylaxis and therapy (Kallet and Quinn, 2005; Stollman and Metz, 2005). Efforts to prevent gastric ulcers in stressed patients have focused on preventing or limiting conditions leading to hypotension and ischemia and coagulopathies. Avoiding NSAIDs and large doses of corticosteroids is also beneficial. Providing oral or enteral feeding (when possible) increases GI vascular perfusion and stimulates secretion and motility.
Medical Nutrition Therapy
In persons with atrophic gastritis, vitamin B12 status should be evaluated because a lack of intrinsic factor and acid results in malabsorption of this vitamin (see Chapters 3 and 33). Low acid states result in reduced absorption of iron, calcium, and other nutrients because of the role of gastric acid in increasing their bioavailability. In the case of iron-deficiency anemia, other causes may be the presence of H. pylori and gastritis. Eradication of H. pylori has resulted in improved absorption of iron and increased ferritin levels (Hershko and Ronson, 2009).
For several decades dietary factors have gained or lost favor as a significant component in the cause and treatment of dyspepsia, gastritis, and peptic ulcer disease. There is little evidence that specific dietary factors cause or exacerbate gastritis or peptic ulcer disease. Protein foods temporarily buffer gastric secretions, but they also stimulate secretion of gastrin, acid, and pepsin. Milk or cream, which in the early days of peptic ulcer management was considered important in coating the stomach, is no longer considered medicinal.
The pH of a food has little therapeutic importance, except for patients with existing lesions of the mouth or the esophagus. Most foods are considerably less acidic than the normal gastric pH of 1 to 3. The pH of orange juice and grapefruit is 3.2 to 3.6, and the pH of commonly used soft drinks ranges from approximately 2.8 to 3.5. On the basis of their intrinsic acidity and amount consumed, fruit juices and soft drinks are not likely to cause peptic ulcers or appreciably interfere with healing. Some patients express discomfort with ingestion of acidic foods, but the response is not consistent among patients, and in some, symptoms may be related to heartburn.
Consumption of large amounts of alcohol may cause at least superficial mucosal damage and may worsen existing disease or interfere with treatment of the peptic ulcer. Modest consumption of alcohol does not appear to be pathogenic for peptic ulcers unless coexisting risk factors are also present. On the other hand, beers and wines significantly increase gastric secretions and should be avoided in symptomatic disease.
Both coffee and caffeine stimulate acid secretion and may also decrease LES pressure; however, neither has been strongly implicated as a cause of peptic ulcers outside of the increased acid secretion and discomfort associated with their consumption.
When very large doses of certain spices are fed orally or placed intragastrically without other foods, they increase acid secretion and cause small, transient superficial erosions, inflammation of the mucosal lining, and altered GI permeability or motility. Most often incriminated are chili, cayenne, and black peppers (Milke et al., 2006). Small amounts of chili pepper or its pungent ingredient, capsicum, may serve to increase mucosal protection by increasing production of mucus; but large amounts may cause superficial mucosal damage, especially when consumed with alcohol. Interestingly, another spice, curcumin, through its antiinflammatory activity that inhibits NF-κB pathway activation may be a chemopreventive candidate against H. pylori–related cancer (Zaidi et al., 2009).
The synergy of food combinations may inhibit the growth of H. pylori. Food provides an interesting alternative to therapies that include antibiotics, PPIs, and bismuth salts (Kennan et al., 2010). Studies suggest that green tea, broccoli sprouts, black currant oil, and kimchi (fermented cabbage) help with H. pylori eradication. Probiotics containing lactobacillus and bifidobacterium have also been studied for prevention, management, and eradication of H. pylori (Lionetti et al., 2010; Sachdeva and Nagpal, 2009). More controlled studies with different foods and combinations of probiotics would be beneficial.
Omega-3 and omega-6 fatty acids are involved in inflammatory, immune, and cytoprotective physiologic conditions of the GI mucosa, but they have not yet been found to be effective for treatment. Long-term clinical trials have not been performed. Overall, a high-quality diet without nutrient deficiencies may offer some protection and may promote healing. Persons being treated for gastritis and peptic ulcer disease should be advised to avoid foods that exacerbate their symptoms, and to consume a nutritionally complete diet with adequate dietary fiber from fruits and vegetables.
Carcinoma of the Stomach
Because symptoms are slow to manifest themselves and the growth of the tumor is rapid, carcinoma of the stomach is frequently overlooked until it is too late for a cure. Loss of appetite, strength, and weight frequently precede other symptoms. In some cases achylia gastrica (absence of HCl and pepsin) or achlorhydria (absence of HCl in gastric secretions) may exist for years before the onset of gastric carcinoma.
Consumption of fruits, vegetables, and selenium appears to have a modest role in the prevention of GI cancers, whereas alcohol consumption and overweight increase the risk (van den Brandt and Goldbohm, 2006). Other factors that may increase the risk of gastric cancer include chronic infection with H. pylori, smoking, intake of highly salted or pickled foods, or inadequate amounts of micronutrients (Lynch et al., 2005).
Malignant neoplasms of the stomach can lead to malnutrition as a result of excessive blood and protein losses or, more commonly, because of obstruction and mechanical interference with food intake. Most cancers of the stomach are treated by surgical resection; thus part of the nutritional considerations includes partial or total resection of the stomach, a gastrectomy.
Medical Nutrition Therapy
The dietary regimen for carcinoma of the stomach is determined by the location of the cancer, the nature of the functional disturbance, and the stage of the disease. Gastrectomy is one of the possible therapies, and some patients may experience difficulties with nutrition after surgery. The patient with advanced, inoperable cancer should receive a diet that is adjusted to his or her tolerances, preferences, and comfort. Anorexia is almost always present from the early stages. In the later stages of the disease, the patient may tolerate only a liquid diet. If a patient is unable to tolerate oral feeding, consideration should be given to using an alternate route, such as a gastric or intestinal feeding tube, or in the case of the inability to feed enterally, parenteral nutrition. The nutritional support for the patient should be in line with the patient’s goals of care.
Gastric Surgeries
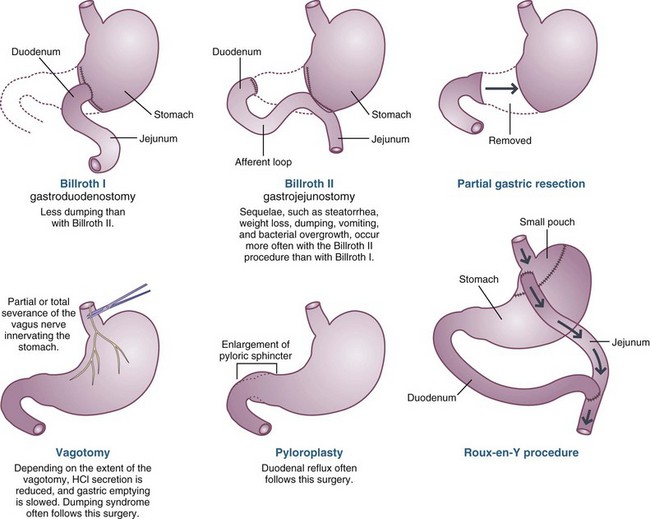
Gastric surgeries are performed less frequently today, because of increased recognition and improved treatment of H. pylori and acid secretion. However, partial or total gastrectomy may still be necessary for patients with ulcer disease that does not respond to therapy, or with malignancy (Figure 28-4). Gastric surgeries performed for weight loss, or bariatric surgeries, are becoming increasingly common. These surgeries, such as Roux-en-Y, gastric bypass, gastric banding, vertical banding gastroplasty, and jejunoileal bypass, are designed to induce malnutrition through volume restriction, malabsorption, or both (see Chapter 22).
Types of Surgeries
A partial gastrectomy involves removal of the gastrin-secreting antrum, as much as 75% of the distal stomach. During surgery, the remnant stomach may be reattached to the duodenum, a Billroth I, or to the side of the jejunum, a Billroth II. In a Billroth II, the duodenal stump is preserved, allowing for the continued flow of bile and pancreatic enzymes into the intestines.
Vagotomy, with or without gastric resection, was developed after it was demonstrated that the vagus nerve was not only responsible for motility of the stomach but also stimulated the parietal cells in the proximal stomach to secrete acid. Truncal vagotomy, complete severing of the vagus nerve on the distal esophagus, decreases acid secretion by parietal cells in the stomach and decreases their response to gastrin, but it also creates poor gastric emptying. When truncal vagotomy is performed, a drainage procedure such as pyloroplasty is performed to allow better gastric emptying of solids. A parietal cell vagotomy (partial or selective) divides and severs only the vagus nerve branches that affect the proximal stomach where gastric acid secretion occurs, whereas the antrum and pylorus remain innervated, and gastric emptying can proceed more normally.
Total gastrectomy is performed for malignancies that affect the middle or upper stomach. The entire stomach is removed and usually reconstructed with the Roux-en-Y method. The total gastrectomy, by definition, involves a functional vagotomy, eliminating acid production.
Postoperative Medical Nutrition Therapy
After most types of gastric surgery, oral intake of foods and fluids is initiated as soon as it is determined that the patient’s GIT is functioning. Small, frequent feedings of ice or water are initiated, followed by liquids and easily digested solid foods, after which the patient can progress to a regular diet. If the surgery requires an extended period for healing, or the patient is unable to tolerate an oral diet, the patient may be fed through a feeding tube, such as a jejunostomy (see Chapter 14).
Understanding the surgery performed and patient’s resulting anatomy is paramount to providing proper nutritional care. Nutritional complications after gastric surgeries are varied. Complications such as obstruction, dumping, abdominal discomfort, diarrhea, and weight loss may occur, depending on the nature and extent of the disease and surgical interventions (see Figure 28-4). Patients may have difficulty regaining normal preoperative weight because of inadequate food intake related to (1) early satiety, (2) symptoms of dumping syndrome (see later in this chapter), or (3) malabsorption of nutrients.
Patients with certain gastric surgeries, such as Roux-en-Y, are set up for impaired digestion and absorption caused by a mismatch in timing of entry of food into the small intestine and the release of bile and pancreatic enzymes. Patients who were lactose tolerant before gastric surgery may experience relative lactase deficiency, either because food enters the small intestine further downstream or because the rate of transit through the proximal small intestine is increased. Because of the complications of reflux or dumping syndrome associated with traditional gastrectomies, other procedures are used, including truncal, selective, or parietal cell vagotomy, pyloromyotomy, antrectomy, Roux-en-Y esophagojejunostomy, loop esophagojejunostomy, and pouches or reservoirs made from jejunal or ileocecal segments (Tomita, 2005.)
Over the long term, anemia, osteoporosis, and select vitamin and mineral deficiencies may occur as a result of malabsorption or limited dietary intake. Iron deficiency may be attributable to loss of acid secretion. Gastric acid normally facilitates the reduction of iron compounds, allowing their absorption. Rapid transit and diminished contact of dietary iron with sites of iron absorption can also lead to iron-deficiency anemia.
Vitamin B12 deficiency may cause a megaloblastic anemia. If the amount of gastric mucosa is reduced, intrinsic factor may not be produced in quantities adequate to allow for complete vitamin B12 absorption, and pernicious anemia may result. Bacterial overgrowth in the proximal small bowel or in the afferent loop contributes to vitamin B12 depletion because bacteria compete with the host for use of the vitamin. Therefore after gastrectomy patients should receive prophylactic vitamin B12 supplementation (injections) or take synthetic oral supplementation.
Dumping Syndrome
The dumping syndrome is a complex GI and vasomotor response to the presence of larger-than-normal quantities of hypertonic foods and liquids in the proximal small intestine. Dumping syndrome usually occurs as a result of surgical procedures that allow excessive amounts of liquid or solid foods to enter the small intestine in a concentrated form. Milder forms of dumping may occur to varying degrees in persons without surgical procedures, and most of the symptoms can be reproduced in normal individuals by infusing a loading dose of glucose into the jejunum (Ukleja, 2005). Dumping may occur as a result of total or partial gastrectomy, manipulation of the pylorus, after fundoplication, vagotomy, and after some gastric bypass procedures for obesity (Ukleja, 2005). As a result of better medical management of peptic ulcers, use of selective vagotomies, and newer surgical procedures to avoid complications, classic dumping is less frequently encountered in clinical practice.
Symptoms can be divided into early, mid, and late stages of dumping of foods and beverages into the small intestine. Early dumping is characterized by both GI and vasomotor symptoms, whereas late dumping is predominantly characterized by vascular symptoms. Characteristics and severity of symptoms vary between patients. In early dumping, patients may experience abdominal fullness and nausea within 10-30 minutes of eating a meal. These symptoms are attributed to accelerated gastric emptying of hyperosmolar solution into the small bowel, and resultant fluid shifts from the circulation into the bowel. It is thought that patients with these early dumping symptoms are experiencing a decrease in peripheral vascular resistance and perhaps visceral pooling of blood.
In the intermediate stage, from 20 minutes to more than 1 hour after eating, patients may experience abdominal bloating, increased flatulence, crampy abdominal pain, and explosive diarrhea. These symptoms are likely related to the malabsorption of carbohydrates and other foodstuffs and the subsequent fermentation of the substrates entering the colon (see Chapter 29).
Late dumping, occurring from 1 to 3 hours after a meal, is characterized by vascular symptoms, related to reactive hypoglycemia. Rapid delivery, as well as hydrolysis and absorption of carbohydrates, produces an exaggerated rise in insulin level with a subsequent decline in blood glucose level (see Chapter 31). Patients may experience flushing, rapid heartbeat, faintness, and sweating, and feel the need to sit or lie down. They may feel anxious, weak, shaky, or hungry, and have difficulty concentrating. The rapid changes in blood glucose and the secretion of gut peptides, glucose insulinotropic polypeptide, and glucagon-like polypeptide-1 appear to be at least partly responsible for the late symptoms (Ukleja, 2005).
Medical Management
Medical intervention typically involves dietary changes as the initial treatment, and they are usually effective. In 3% to 5% of patients, severe dumping persists despite dietary change. In these patients, medications may be used to slow gastric emptying and delay transit of food through the GIT. Some, such as acarbose, inhibit alpha glycoside hydrolase and interfere with carbohydrate absorption, and octreotide, a somatostatin analogue, inhibits insulin release. See Table 28-2 for common medications. Rarely, surgical intervention is used to treat dumping syndrome.
Medical Nutrition Therapy
Patients with dumping syndrome may experience weight loss and malnutrition caused by inadequate intake, malabsoption or a combination of both. The prime objective of nutrition therapy is to restore nutrition status and quality of life. Proteins and fats are better tolerated than carbohydrates because they are hydrolyzed more slowly into osmotically active substances. Simple carbohydrates such as lactose, sucrose, and dextrose are hydrolyzed rapidly; thus quantities should be limited, but complex carbohydrates (starches) can be included in the diet. Liquids enter the jejunum rapidly; thus some patients may have problems tolerating liquids with meals. Patients with severe dumping may benefit from limiting the amount of liquids taken with meals, and taking liquids between meals, without solid food. Lying down immediately after meals may also decrease the severity of symptoms.
The use of fiber supplements, particularly pectin or gums (e.g., guar) can be beneficial in managing dumping syndrome because of their ability to form gels with carbohydrates and delay GI transit. Patients may need to be taught true portion sizes of foods, especially carbohydrate foods such as juices, soft drinks, desserts, and milk. The exchange lists given in Appendix 34 can be used to calculate carbohydrate intake and teach the patient about carbohydrate control.
Postgastrectomy patients often do not tolerate lactose, but small amounts (e.g., 6 g or less per meal) may be tolerated at one time. Patients typically do better with cheeses or unsweetened yogurt than with fluid milk. Nondairy milks are also useful. Vitamin D and calcium supplements may be needed when intake is inadequate. Commercial lactase products are available for those with significant lactose malabsorption (see Chapter 29).
When steatorrhea (greater than 7% of dietary fat excreted in stool) exists, reduced fat formulas or pancreatic enzymes may be beneficial. Box 28-2 provides general nutrition guidelines for patients with dumping syndrome after gastric surgery; however, each diet must be adjusted based on a careful dietary and social history from the patient.
Gastroparesis
Gastroparesis, or delayed gastric emptying, is a complex and potentially debilitating condition. The nature of gastroparesis is complex in part because gastric motility is orchestrated by a variety of chemical and neurologic factors. Viral infection, diabetes, and surgeries are the most common causes for gastroparesis; however, more than 30% of cases are idiopathic. Numerous classes of clinical conditions are associated with gastroparesis, including mechanical obstructions, metabolic or endocrine disorders, acid-peptic diseases, gastritis, postgastric surgery, disorders of gastric smooth muscle, psychogenic disorders, and neuropathic disorders. Clinical symptoms may include abdominal bloating, decreased appetite and anorexia, nausea and vomiting, fullness, early satiety, halitosis, and postprandial hypoglycemia.
Diagnosis and Medical Management
The gold-standard measure of gastric emptying rate is scintigraphy, a nuclear test of gastric emptying. This consists of the patient ingesting a radionucleotide-labeled meal (such as an egg labeled with 99mtechnetium), and scintigraphic images are taken over time (generally 4 hours) to assess the rate of gastric emptying.
Numerous symptoms of gastroparesis can affect oral intake, and the management of these symptoms generally improves nutritional status. Treatment of nausea and vomiting is perhaps the most vital, and prokinetics and antiemetics are the primary medical therapies (see Table 28-2). Metoclopramide and erythromycin are medications that may be used to promote gastric motility. Small bowel bacterial overgrowth, ileal brake (the slowing effect on intestinal transit and appetite of undigested nutrients, often fat, reaching the ileum), or formation of a bezoar (concentration of undigested material in the stomach) are other factors that may affect nutritional status.
Bezoar formation may be related to undigested food such as cellulose, hemicellulose, lignin and fruit tannins (phytobezoars), or medications (pharmacobezoars) such as cholestyramine, sucralfate, enteric coated aspirin, aluminum-containing antacids, and bulk-forming laxatives. Treatment of bezoars includes enzyme therapy (such as papain or cellulose), lavage, and sometimes endoscopic therapy to mechanically break up the bezoar. Most patients respond to some combination of medication and dietary intervention; however, unresponsive and more severe cases many benefit from placement of an enteral tube, such as a percutaneous endoscopic gastrostomy (PEG) with jejunal extension or a PEG and percutaneous endoscopic jejunostomy (Parrish and Yoshida, 2005). These tube combinations allow nutrition to bypass the stomach while providing an alternative route for venting of gastric secretions, which may relieve nausea and vomiting.
Medical Nutrition Therapy
The primary dietary factors that affect gastric emptying (in order of clinical importance) are volume, liquids versus solids, hyperglycemia, fiber, fat, and osmolality (Maljaars et al., 2007). Larger volumes of food that create stomach distension (approximately 600 mL) have been shown to delay gastric emptying and increase satiety (Oesch et al., 2006). Generally, patients benefit from smaller, more frequent meals. Patients with gastroparesis often have preserved emptying of liquids, as they empty, in part, by gravity and do not require antral contraction. Shifting the diet to more pureed and liquefied foods is often useful. A number of medications (such as narcotics and anticholinergics) slow gastric emptying. Moderate to severe hyperglycemia (serum blood glucose >200 mg/dL) may acutely slow gastric motility, with long-term detrimental effects on gastric nerves and motility. Laboratory data considered in initial assessment include glycosylated hemoglobin A1c (if diabetes is present), ferritin, vitamin B12, and 25-OH vitamin D.
Fiber, particularly pectin, can slow gastric emptying and increase risk of bezoar formation in patients who are susceptible. It is prudent to advise patients to avoid high-fiber foods and fiber supplements. The size of the fibrous particles, not the amount of fiber, is more important in bezoar risk (e.g., potato skins versus bran). This and the resistance to chewing are factors in bezoar formation. Examination of the patient’s dentition is very important because patients who have missing teeth, a poor bite, or are edentulous are at greater risk. People even with good dentition have swallowed and passed food particles up to 5-6 cm in diameter (potato skins, seeds, tomato skins, peanuts).
Fat is a powerful inhibitor of stomach emptying primarily mediated by cholecystokinin (Goetze et al., 2007); however, many patients tolerate fat well in liquid form. Fat should not be restricted in patients who are struggling to meet their daily caloric needs. Studies have demonstrated a slowing effect of highly osmotic foods on gastric emptying, but, compared with other interventions, dietary manipulation of osmolarity is not clinically effective (Parrish, 2007).
American Gastrointestinal Association
American College of Gastroenterology
International Foundation for Functional Gastrointestinal Disorders
http://www.aboutgimotility.org/
National Digestive Diseases Information Clearinghouse
http://digestive.niddk.nih.gov/
References
Ali, T, et al. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122:896.
Bytzer, P, O’Morain, C. Treatment of Helicobacter pylori. Helicobacter. 2005;10:40S.
Cherry, DK, et al. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Report. 2008;3:1.
Cordova-Fraga, T. Effects of anatomical position on esophageal transit time: a biomagnetic diagnostic technique. World J Gastro. 2008;14:5707.
Dowswell T, Neilson JP: Interventions for heartburn in pregnancy, Cochrane Database Syst Rev 4:CD007065, 2008.
El-Serag, HB, et al. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut. 2005;54:11.
Ernst, PB, et al. The translation of Helicobacter pylori basic research to patient care. Gastroenterol. 2006;130:188.
Fajardo, NR, et al. Frontiers in functional dyspepsia. Curr Gastroenterol Report. 2005;7:289.
Fennerty, MB. Helicobacter pylori: why it still matters in 2005. Cleveland Clinic J Med. 2005;72:S1.
Gerson, LB, Fass, R. A systematic review of the definitions, prevalence, and response to treatment of nocturnal gastroesophageal reflux disease. Clin Gastro Hepatol. 2009;7:372.
Goetze, O, et al. The effect of macronutrients on gastric volume responses and gastric emptying in humans: a magnetic resonance imaging study. Am J Physiol. 2007;292:G11.
Gold, BD. Is gastroesophageal reflux disease really a life-long disease: do babies who regurgitate grow up to be adults with GERD complications? Am J Gastroenterol. 2006;101:641.
Gray, SL, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Int Med. 2010;170:765.
Guzzo, JL, et al. Severe and refractory peptic ulcer disease: the diagnostic dilemma: case report and comprehensive review. Dig Dis Sci. 2005;50:1999.
Hassall, E. Decisions in diagnosing and managing chronic gastroesophageal reflux disease in children. J Pediatr. 2005;146:S3.
Hershko, C, Ronson, A. Iron deficiency, Helicobacter infection and gastritis. Acta Haematol. 2009;122:97.
Israel, DA, Peek, RM. The role of persistence in Helicobacter pylori pathogenesis. Curr Opin Gastroenterol. 2006;22:3.
Kallet, RH, Quinn, TE. The GIT and ventilator-associated pneumonia. Resp Care. 2005;50:910.
Kamangar, F, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445.
Katz, MH. Failing the acid test: benefits of proton pump inhibitors may not justify the risks for many users. Arch Int Med. 2010;170:747.
Kennan, JI, et al. Individual and combined effects of foods on Helicobacter pylori growth. Phytother Res. 2010;24:1229.
Leong, WL, et al. Association of intestinal granulomas with smoking, phenotype, and serology in Chinese patients with Crohn’s’s disease. Am J Gastroenterol. 2006;101:1024.
Lichtenstein, DR, et al. Role of endoscopy in the management of GERD. Gastro Endo. 2007;66:219.
Linsky, A, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Int Med. 2010;170:772.
Lionetti, E, et al. Role of probiotics in pediatric patients with Helicobacter pylori infection: a comprehensive review of the literature. Helicobacter. 2010;15:79.
Liu, W, et al. Olfactomedin 4 down-regulates innate immunity against Helicobacter pylori infection. Proc Natl Acad Sci U S A. 2010;107:11056.
Lynch, HT, et al. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90:114.
Mahid Suhal, S, et al. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462.
Maljaars, J, et al. The GIT: neuroendocrine regulation of satiety and food intake. Alimentary Pharmacol Ther. 2007;26:241S.
McColl, KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol. 2009;104:S5.
Milke, P, et al. Gastroesophageal reflux in healthy subjects induced by two different species of chilli (Capsicum annum). Dig Dis. 2006;24:184.
Oesch, S, et al. Effect of gastric distension prior to eating on food intake and feelings of satiety in humans. Physiol Behav. 2006;87:903.
Okoro, NI, Wang, KK. Changing faces of Barrett’s esophagus: implications for adenocarcinoma. Gastroenterol. 2010;138:1620.
Orlando, RC. Pathophysiology of gastroesophageal reflux disease. J Clin Gastroenterol. 2008;42:584.
Parrish, CR, Yoshida, CM. Nutrition intervention for the patient with gastroparesis: an update. Pract Gastroenterol. 2005;29:29.
Parrish, CR. Nutrition concerns for the patient with gastroparesis. Current Gastro Rep. 2007;9:295.
Pera, M, et al. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:151.
Pilichiewicz, AN, et al. Relationship between symptoms and dietary patterns in patients with functional dyspepsia. Clin Gastro Hepatol. 2009;7:317.
Rohof, WO, et al. Pathophysiology and management of gastroesophageal reflux disease. Minerva Gastroenterol Dietologica. 2009;55:289.
Sachdeva, A, Nagpal, J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol & Hep. 2009;1:45.
Selgrad, M, et al. Dyspepsia and Helicobacter pylori. Dig Dis. 2008;26:210.
Siddaraju, MN, Dharmesh, SM. Inhibition of gastric H+, K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale. Mol Nutr Food Res. 2007;51:324.
Stollman, N, Metz, DC. Pathophysiology and prophylaxis of stress ulcer in intensive care unit patients. J Crit Care. 2005;20:35.
Tomita, R. A novel surgical procedure of vagal nerve, lower esophageal sphincter, and pyloric sphincter-preserving nearly total gastrectomy reconstructed by single jejunal interposition, and postoperative quality of life. Hepato-Gastroenterol. 2005;52:1895.
Ukleja, A. Dumping syndrome: pathophysiology and treatment. Nutr Clin Pract. 2005;20:517.
Van den Brandt, PA, Goldbohm, RA. Nutrition in the prevention of gastrointestinal cancer. Best Pract Res Clin Gastroenterol. 2006;20:589.
Vattem, DA, et al. Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry. Asia Pacific J Clin Nutr. 2005;14:120.
Whiteman, DC, et al. Association of Helicobacter pylori infection with reduced risk for esophageal cancer is independent of environmental and genetic modifiers. Gastroenterol. 2010;139:73.
Whittingham, S, Mackay, IR. Autoimmune gastritis: historical antecedents, outstanding discoveries, and unresolved problems. Int Rev Immunol. 2005;24:1.
Wong, T, et al. Barrett’s surveillance identifies patients with early esophageal adenocarcinoma. Am J Med. 2010;123:462.
Yuan, Y, Hunt, RH. Evolving issues in the management of reflux disease? Curr Opin Gastroenterol. 2009;25:342.
Zaidi, SF, et al. Modulation of activation-induced cytidine deaminase by curcurmin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter. 2009;14:588.
Zhu, A, Kaunitz, J. Gastroduodenal mucosal defense. Curr Gastroenterol Rep. 2008;10:548.