Medical Nutrition Therapy for Lower Gastrointestinal Tract Disorders
Dietary interventions for many diseases of the intestinal tract are primarily designed to alleviate symptoms and to correct nutrient deficiencies. However, nutrition interventions play a preventative and therapeutic role in several conditions, such as diverticular disease and treatment of some types of constipation. Celiac disease (CD) is the only gastrointestinal (GI) condition for which dietary modification is the primary treatment. Careful assessment of the nature and severity of the primary GI problem is necessary to identify the nutrition diagnosis and appropriate interventions. Assessment may include evaluating the frequency and amount of nutrients consumed, medical and surgical history, medications used, subjective experiences with foods, and depth of understanding of the relationship between diet and the GI problem. The GI assessment should include information on the duration and severity of the disorder; its effect on digestion, secretion, and absorption of nutrients; and its effect on symptoms and complications. Meal consistency, frequency, and size, as well as other characteristics of the diet, may then be altered to fit the patient’s needs.
Common Intestinal Problems
It is important to understand some of the common GI processes and symptoms that occur in healthy people prior to discussing the nutrition issues relating to lower GI tract (GIT) disorders. The interaction of diet with intestinal gas, flatulence, constipation, and diarrhea provides insight when considering the more serious disorders.
Intestinal Gas and Flatulence
Air is commonly swallowed (aerophagia), and other gasses are produced within the GIT by digestive processes and bacteria. These gases are either expelled through belching (eructation) or passed rectally (flatus). Intestinal gases include nitrogen (N2), oxygen (O2), carbon dioxide (CO2), hydrogen (H2), and in some persons methane (CH4). Some of these gasses are absorbed into the circulation and then exhaled from the lungs.
Approximately 200 mL of gas is present in the healthy GIT. Humans excrete an average of 700 mL of gas each day, but are capable of moving considerably more through the GIT. The amount of intestinal gas varies greatly among individuals and from one day to the next. When patients complain about “excessive gas,” or flatulence, they may be referring to increased volume or frequency of belching or passage of rectal gas. They may also complain of abdominal distention or cramping associated with the accumulation of gases in the upper or lower GIT. However, the perception of gas and the degree of symptoms experienced by an individual do not necessarily correlate with the amount of gas that is actually in the GIT (Azpiroz, 2005; Morken et al., 2007). Inactivity, decreased GI motility, aerophagia, dietary components, and certain GI disorders can alter the amount of intestinal gas and individual symptoms. Aerophagia can be avoided to some degree by eating slowly, chewing with the mouth closed, limiting gum chewing, and refraining from drinking through straws. Movement of gas through the GIT may be enhanced with upright stance, mild exercise, or abdominal massage.
Gas production occurs in the stomach and small intestine from bacterial fermentation of carbohydrates, and can result in abdominal discomfort and distention. The colonies of bacteria in the small bowel are normally present in limited numbers, but various conditions can lead to overgrowth of bacteria, potentially causing diarrhea, bloating, distention, or other symptoms. Because the small intestine is less tolerant of gas than the colon, this distention may cause pain. The movement of gas into the small intestine and beyond is slowed by high-calorie, high-fat meals. Slowed excretion or retained gas may contribute to the perception of distention or bloating with large meals in normal circumstances and with the abdominal discomfort that is experienced in functional GI disorders, such as irritable bowel syndrome (IBS) (Azpiroz, 2005; Harder et al, 2006). Functional GI disorders present symptoms that are not explained by a known structural, infectious, or metabolic cause.
Increased amounts of H2 and CO2—and sometimes CH4—in rectal gas can lower the fecal pH, causing excessive colonic bacterial fermentation and malabsorption of fermentable substrate. The amounts and types of gases produced may depend on the mix of microorganisms in the individual’s colon. Consumption of large amounts of dietary fiber (especially soluble fiber), resistant starches, lactose in persons who are lactase deficient, or modest amounts of fructose or sugar alcohols (such as sorbitol) may result in increased gas production in the colon and increased flatulence (Beyer et al., 2005). In the United States consumption of fruit juices, fruit drinks, and high-fructose corn syrup (HFCS) in soft drinks and confections has increased significantly in recent years. Fructose is normally well absorbed when consumed in the form of sucrose or as small amounts of HFCS, but not so well when consumed as the only or predominant sugar in the diet (see Chapter 1). In children 10-20 g of fructose, or 25 g in adults, is sufficient to result in malabsorption.
Medical Nutrition Therapy
When assessing a patient it is important to differentiate between increased production of gas and gas that is not being passed. Likewise, it is important to consider why a patient may have new or increased symptoms. A thorough review of the patient’s medical history considers predisposing factors and treatment of underlying conditions before implementing nutrition therapy.
One of the direct nutrition considerations is development of lactose intolerance. Recent viral or GI infection may provoke temporary or even permanent impairment in the ability to digest lactose, and appropriate diet modifications can improve symptoms. A dramatic change in diet, such as adoption of a high-fiber diet, can also alter gas production. Foods that contain raffinose (a complex sugar resistant to digestion), such as beans, cabbage, Brussels sprouts, broccoli, asparagus and some whole grains, can increase gas production.
Altered bowel flora occur over time after an increase in dietary fiber. Although there are no randomized studies regarding the best way to implement high-fiber diets, a gradual introduction of fiber with adequate fluid consumption appears to reduce complaints of gas. Inactivity, dysmotility, constipation, or partial obstruction may be contributing to the inability to move normal amounts of gas as produced. Increased physical activity or exercise may help, if an underlying obstruction or dysmotility is not present.
Constipation
Constipation, commonly defined as difficult or infrequent passage of stool (Cook et al., 2009), is one of the most common intestinal maladies in Western societies, and may occur in 5% to 25% of the population or more (Müller-Lissner, 2009). Prevalence of constipation has been reported in as high as 50% to 80% of patients taking opioids daily for chronic pain, and may occur despite laxative use (Bell et al., 2009; Tuteja et al., 2010).
Although several definitions for constipation are based on frequency, difficulty, or consistency of stool, the sensation of “feeling constipated” may be enough to warrant intervention. Often, patients are troubled more by the physical discomfort of straining, hard stools, or incomplete evacuation than by the infrequency of bowel movements. In adults, normal stool weight is approximately 100 to 200 g daily, and normal frequency may range from one stool every 3 days to three times per day. Normal transit time through the GIT ranges from approximately 18 to 48 hours.
Children normally have more frequent stools, ranging from an average of two to three stools daily for the first few months of life to approximately one and a half bowel movements daily by age 3. As many as one third of children from ages 6 to 12 years complain of constipation in any given year (Biggs and Dery, 2006). Children may exhibit vomiting, abdominal pain, anorexia, or encopresis (involuntary passage of stool or fecal soiling).
Pathophysiology
Constipation may be caused by lifestyle factors (inadequate hydration, lack of exercise) or other medical conditions. Treatments differ based on the cause of constipation. Box 29-1 outlines numerous factors that may contribute to constipation.
The most common causes of constipation in otherwise healthy persons include repeatedly ignoring the urge to defecate, lack of fiber in the diet, insufficient fluid intake, inactivity, or use of certain medications. Individuals who believe that it is necessary to have scheduled and frequent bowel movements, yet ignore dietary and other recommendations for maintaining laxation, may be at risk for overuse of medications. When the desired stool frequency or timing of defecation does not occur, they may try to compensate with the use of medications and enemas. Chronic use of stimulant laxatives may damage the structure and innervation of the colon. Opioid medications bind to motility receptors in the gut, and chronic use may lead to constipation, delayed gastric emptying, nausea, and abdominal pain (Holzer, 2009).
Medical Treatment for Adults
It is important to first rule out serious neurologic, GI, or endocrine disorders, or constipation caused by medications. After this is done, the first approach to treat mild and functional constipation is to ensure adequate dietary fiber, exercise, and heeding the urge to defecate. Patients who depend on laxatives are usually encouraged to use milder products, reducing the dose until withdrawal is complete.
When constipation persists despite lifestyle interventions, medications that promote regular bowel movements may be prescribed (Emmanuel et al., 2009). Anionic surfactants such as docusate sodium or docusate potassium are used as stool softeners to make bowel movements easier to pass. Osmotic agents such as magnesium hydroxide, sorbitol, and lactulose draw fluid into the bowel. Polyethylene glycol is an isosmotic agent that treats constipation by keeping the water it is taken within the gut rather than it being absorbed. Bisacodyl and senna compounds have stimulant activity on bowel motility and also act to prevent water absorption. Lubiprostone is a prostaglandin E1 derivative that increases fluid secretion by the epithelial cells of the GIT (Ramkumar and Rao, 2005). Impactions of stool require evacuation and a more stringent preventive and maintenance program, including combinations of medications, fluids, activity, or enemas.
Medical Treatment for Infants and Children
Approximately 3% to 5% of all pediatric outpatient visits are related to chronic constipation. In the most severe cases of functional constipation with frequent stool retention, the rectum becomes insensitive to distention, and encopresis may result. After disease is ruled out, treatment includes laxatives, lubricants, adequate dietary fiber, and fluid. A careful history and physical examination followed by parent and child education, behavioral intervention, and appropriate use of laxatives often leads to dramatic improvement (Biggs and Dery, 2006) (see Chapter 18).
Medical Nutrition Therapy
Primary nutrition therapy for constipation in otherwise healthy people is consumption of adequate amounts of dietary fiber, both soluble and insoluble, as well as fluids. Fiber increases colonic fecal fluid, microbial mass (which accounts for 60% to 70% of stool weight), stool weight and frequency, and the rate of colonic transit. With adequate fluid, fiber may soften stools and make them easier to pass. Unfortunately, most adults and children in the United States chronically consume only about half the amount of fiber recommended by the Institute of Medicine (14 g/1000 kcal). Adult women should consume approximately 25 g of fiber daily, men approximately 38 g, and children from 19 to 25 g daily.
Dietary fiber refers to edible plant materials not digested by the enzymes in the GIT. It consists of cellulose, hemicelluloses, pectins, gums, lignin, starchy materials, and oligosaccharides that are partially resistant to digestive enzymes. Fiber can be provided in the form of whole grains, fruits, vegetables, legumes, seeds, and nuts. These foods are also high in prebiotics, substances that are not digested by humans and fuel colonic microflora. Appendix 41 lists the fiber content of foods.
Different from fiber, residue refers to the end result of digestive, secretory, absorptive, and fermentative processes. Increasing dietary fiber may result in increased fecal output, but increasing dietary lactose (a fiber-free food) in a person who is a lactose malabsorber also increases fecal weight (residue).
Every 10 g of carbohydrate reaching the colon may be fermented into as much as 1000 mL of gas. Thus transition to a diet pattern that meets guidelines for fiber often requires substantial change. A high-fiber therapeutic diet may need to exceed 25 to 38 g/day. The high-fiber diet in Box 29-2 provides more than the amount of fiber recommended. Amounts greater than 50 g/day are not necessary and may increase abdominal distention and excessive flatulence.
Bran and powdered fiber supplements may be helpful in persons who cannot or will not eat sufficient amounts of fibrous foods. Several of these concentrates are palatable and can be added to cereals, yogurts, fruit sauces, juices, or soups. Cooking does not destroy fiber, although the structure may change. Consumption of at least eight 8-oz glasses (∼2 L) of fluids daily is recommended to facilitate the effectiveness of a high-fiber intake. Gastric obstruction and fecal impaction may occur when boluses of fibrous gels or bran are not consumed with sufficient fluid to disperse the fiber.
Recommendations for increased dietary fiber for laxation should not be implemented in patients with neuromuscular disorders, dysmotility syndromes, chronic opioid use, pelvic floor disorders or other serious GI disease (Schiller, 2008). In some conditions, such as neuromuscular disorders, a specific laxative medication regimen is a necessary part of care.
Diarrhea
Diarrhea is characterized by the frequent evacuation of liquid stools, usually exceeding 300 mL, accompanied by an excessive loss of fluid and electrolytes, especially sodium and potassium. Diarrhea occurs when there is accelerated transit of intestinal contents through the small intestine, decreased enzymatic digestion of foodstuffs, decreased absorption of fluids and nutrients, increased secretion of fluids into the GIT, or exudative losses.
Types of Diarrhea and Their Pathophysiology
Diarrhea may be related to inflammatory disease; infections with fungal, bacterial, or viral agents; medications; over consumption of sugars or other osmotic substances; or insufficient or damaged mucosal absorptive surface.
Exudative diarrheas are always associated with mucosal damage, which leads to an outpouring of mucus, fluid, blood, and plasma proteins, with a net accumulation of electrolytes and water in the gut. Prostaglandin and cytokine release may be involved. The diarrheas associated with Crohn’s disease, ulcerative colitis (UC), and radiation enteritis are often exudative
Osmotic diarrheas occur when osmotically active solutes are present in the intestinal tract and are poorly absorbed. Examples include the diarrhea that accompanies dumping syndrome or that which follows lactose ingestion in the person with a lactase deficiency.
Secretory diarrheas are the result of active intestinal secretion of electrolytes and water by the intestinal epithelium, resulting from bacterial exotoxins, viruses, and increased intestinal hormone secretion. Unlike osmotic diarrhea, fasting does not relieve secretory diarrhea.
Malabsorptive diarrhea results when a disease process impairs digestion or absorption to the point that fat and other nutrients appear in the stool in increased amounts. Excess fat in the stool is called steatorrhea. Diarrhea occurs because of the osmotic action of these nutrients and the action of the bacteria on the nutrients that pass into the colon. Malabsorptive diarrhea occurs when there is not enough healthy absorptive area, inadequate production or interrupted flow of bile and pancreatic enzymes, or there is rapid transit, such as in inflammatory bowel disease (IBD) or after extensive bowel resection. Box 29-3 lists diseases and conditions associated with malabsorption and diarrhea.
Medication-induced diarrheas are frequent in hospitalized and long-term care patients. Medications such as lactulose (used in the management of hepatic encephalopathy) and sodium polystyrene sulfonate with sorbitol (used to treat hyperkalemia) create increased bowel movements as part of their mechanism of action. Some antibiotics have direct effects on GI function (see Chapter 9). For example, as a motilin agonist, erythromycin increases lower GI motility; clarithromycin and clindamycin also increase GI secretions.
In the normal GIT, bacterial “salvage” from sloughed intestinal cells and undigested foodstuffs converts osmotically active molecules (carbohydrate and amino acids) to gases and short-chain fatty acids (SCFAs). Absorption of the SCFAs facilitates absorption of electrolytes and water from the colon. Broad-spectrum antibiotics decrease the number of bacteria in the bowel and may result in increased osmotically active molecules, reduced absorption of electrolytes and water, and diarrhea.
Some antibiotics allow opportunistic proliferation of pathogenic organisms normally suppressed by competitive organisms in the GIT. The organisms or the toxins produced by some opportunistic organisms can cause colitis and increased secretion of fluid and electrolytes. The treatment of Escherichia coli and several other organisms has been implicated in antibiotic-associated diarrhea (AAD) (Schroeder, 2005). Overall, infection with Clostridium difficile is the most common cause of AAD, especially among patients who receive antibiotics within health care facilities. C. difficile is the leading cause of nosocomial (hospital-acquired) diarrhea in the United States (O’Keefe, 2010). This infection may cause colitis, secretory diarrhea, severe dilation of the colon (toxic megacolon), perforation of the bowel wall, peritonitis, or even death (Sánchez-Pérez et al., 2010).
C. difficile occurs in 50% of hospitalized patients with a stay longer than 4 weeks (DeLegge and Berry, 2009). In the mid 1990s, the incidence of C. difficile was reported to be between 30-40 cases per 100,000 patients, but by 2005 the incidence doubled to 84 cases per 100,000 patients (DeLegge and Berry, 2009). Additionally, resistant strains of C. difficile are less susceptible to treatment with antimicrobials, and cause a more severe form of the disease with increased health care costs, and higher mortality (O’Keefe, 2010).
C. difficile is a spore-forming organism, and the spores are resistant to common disinfectant agents. The spore-forming ability of C. difficile allows the organism to be spread inadvertently to other patients by health care providers (iatrogenic infection) if strict infection control procedures are not followed. The presence of this infection is detected by analysis of a stool sample for the presence of the toxin produced by the organisms. Clindamycin, penicillins, and cephalosporins are associated most often with the development of C. difficile infection. Its occurrence depends on the number of antibiotics used, the duration of exposure to antibiotics, and the patient’s overall health. Chronic suppression of stomach acid with proton-pump inhibitor medications during broad-spectrum antibiotic therapy may also increase susceptibility to C. difficile infection (Howell et al., 2010; Linsky et al., 2010).
With human immunodeficiency virus (HIV) and other immune deficiency states, several factors contribute to the diarrhea, including the toxic effects of medications, proliferation of opportunistic organisms, and the GI manifestations of the disease itself (Kulkarni et al., 2009) (see Chapter 38). Increased risk of opportunistic infection is also associated with use of antineoplastic agents (such as chemotherapy) or with malnutrition.
Medical Treatment
Because diarrhea is a symptom, not a disease, the first step in medical treatment is to identify and treat the underlying problem. The next goal is to manage fluid and electrolyte replacement. In cases of severe diarrhea, restoring fluid and electrolyte is first priority. Electrolyte losses, especially potassium and sodium, should be corrected early by using oral glucose electrolyte solutions with added potassium. Oral rehydration solutions (ORS) work because they contain concentrations of sodium and glucose that are optimal for interaction with the sodium-glucose transport (SGLT) proteins in the intestinal epithelial cells. With intractable diarrhea, especially in an infant or young child, parenteral feeding may be required. Parenteral nutrition (PN) may even be necessary if exploratory surgery is anticipated or if the patient is not expected to resume full oral intake within 5 to 7 days (see Chapter 14).
Supplementation with probiotics shows some promise to prevent recurrence of C. difficile but there is inadequate data to recommend probiotics as a primary treatment for C. difficile infections. (Gao et al., 2010; Lawrence et al., 2005 Pillai 2008); see New Directions: Probiotics for the Right Balance of Bugs.
Products that combine probiotic microorganisms and a prebiotic fiber source have been described as synbiotics for their synergistic effects. However, there are no controlled studies that have systematically investigated the effectiveness of probiotics alone compared with synbiotics. There is a need for controlled studies to understand which strains of probiotics should be provided, as well as type and amount of prebiotic fibers.
Although there is a long history of safe use of many strains of probiotics in foods in healthy humans, there is a limited body of evidence on the use of large doses of concentrated probiotic supplements, especially of specific strains that exhibit greater resistance to gastric acid or have increased ability to proliferate in the GIT. There is very limited safety data to support the use of concentrated probiotic supplements in patients with immunocompromised states, critical illness, or when probiotics are administered directly into the small intestine, as with jejunal feeding tubes. There have been a number of case reports of hospitalized patients receiving concentrated strains of probiotics that have become septic because of infection in the bloodstream with the very same strain of probiotic being administered (Whelan and Myers, 2010). In a review of cases of adverse events related to probiotic administration in hospitalized patients, 25% of those adverse events resulted in the death of the patient (Whelan and Myers, 2010). In a large double-blind, randomized study of a high-dose multispecies probiotic administered via jejunal feeding tube in patients with severe acute pancreatitis, there were significantly more deaths in the patients who received probiotics compared with those receiving the inactive placebo (Besselink et al., 2008).
Probiotic preparations hold promise as an adjunctive or primary treatment in several gastrointestinal conditions, but there is a need for additional studies before routine use of these preparations is adopted, especially for hospitalized or immunocompromised patients. The studies to date have been relatively small, have used varying doses and strains of probiotic microorganisms, and there remains much to be learned about true effectiveness, differences between probiotic strains, possible benefits of coadministration of prebiotics, best doses, safety, and cost-benefit of using probiotics.
Medical Nutrition Therapy
All nutrition interventions related to diarrhea must be viewed within the context of the underling pathologic condition responsible for the diarrhea. Replacement of necessary fluids and electrolytes is the first step, using ORSs, soups and broths, vegetable juices, and isotonic liquids. Restrictive diets, such as the BRAT diet made up of bananas, rice, applesauce, and toast, are nutrient poor and there is no evidence that they are necessary during acute diarrheal illness. However, some clinicians recommend a progression of starchy carbohydrates such as cereals, breads, and low-fat meats, followed by small amounts of vegetables and fruits, followed by fats. The goal with this progression is to limit large amounts of hyperosmotic carbohydrates that may be maldigested or malabsorbed, foods that stimulate secretion of fluids, and foods that speed the rate of GI transit.
Sugar alcohols, lactose, fructose, and large amounts of sucrose may worsen osmotic diarrheas. Because the activity of the disaccharidases and transport mechanisms decrease during inflammatory and infectious intestinal disease, sugars may need to be limited, especially in children (Robayo-Torres et al., 2006). It is important to remember that malabsorption is only one potential cause of diarrhea, and diarrhea may occur without significant malabsorption of macronutrients (carbohydrate, fat, and protein). Absorption of most nutrients occurs in the small intestine; diarrhea related to colonic inflammation or disease preserves the absorption of most ingested nutrients.
Minimal fiber and low-residue diets are rarely indicated (Table 29-1). Patients are encouraged to resume a regular diet as tolerated that contains moderate amounts of soluble fiber. The metabolism of fiber and resistant starches by colonic bacteria leads to production of SCFAs, which in physiologic quantities serve as a substrate for colonocytes, facilitate the absorption of fluid and salts, and may help to regulate GI motility (Binder, 2010).
TABLE 29-1
Food to Limit in a Low-Fiber (Minimal Residue) Diet
| Food | Comments |
| Lactose (in lactose malabsorbers) | 6-12 g is normally tolerated in healthy lactase-deficient individuals, but may not be in some individuals. |
| Fiber (quantities >20 g) | Modest amounts (10-15 g) may help maintain normal consistency of gastrointestinal (GI) contents and normal colonic mucosa in healthy states and GI disease. |
| Resistant starch (especially raffinose and stachyose found in legumes) | |
| Sorbitol, mannitol, and xylitol (excess, >10 g/day) | |
| Fructose (excess, 20-25 g/meal) | |
| Sucrose (excess, >25-50 g/meal) | Well tolerated in moderate amounts; large amounts may cause hyperosmolar diarrhea or decreased fecal pH with fermentation to short-chain fatty acids. |
| Caffeine | Increases GI secretions, colonic motility. |
| Alcoholic beverages (especially wine and beer) | Increase GI secretions. |
Fibrous material tends to slow gastric emptying, moderate overall GI transit, and pull water into the intestinal lumen. Providing fiber to patients with diarrhea does increase the volume of stool, and in some cases (such as small intestine bacterial overgrowth [SIBO]) can initially increase gas and bloating. Modest intake of prebiotic components and soluble fibers such as pectin or gum slows transit through the GIT.
Several probiotics have been tested for preventing AAD in children; risk reduction was higher from Saccharomyces boulardii than for Lactobacillus GG or Lactobacillus bifidus and Streptococcus thermophilus (Szajewska, 2006). Studies are needed to find the combination of probiotics, prebiotics, and antibiotics that works most effectively in each situation (Teitelbaum, 2005).
Severe and chronic diarrhea is accompanied by dehydration and electrolyte depletion. If also accompanied by prolonged infectious, immunodeficiency, or inflammatory disease, malabsorption of vitamins, minerals, and protein or fat may also occur, and nutrients may need to be replaced parenterally or enterally. In some forms of infectious diarrheas, loss of iron from GI bleeding may be severe enough to cause anemia. Nutrient deficiencies themselves cause mucosal changes such as decreased villi height and reduced enzyme secretion, further contributing to malabsorption. As the diarrhea begins to resolve, the addition of more normal amounts of fiber to the diet may help to restore normal mucosal function, increase electrolyte and water absorption, and increase the firmness of the stool.
Food in the lumen is needed to restore the compromised GIT after disease and periods of fasting. Early refeeding after rehydration reduces stool output and shortens the duration of illness. Micronutrient replacement or supplementation may also be useful for acute diarrhea, probably because it accelerates the normal regeneration of damaged mucosal epithelial cells.
Treating Diarrhea in Infants and Children
Acute diarrhea is most dangerous in infants and small children, who are easily dehydrated by large fluid losses. In these cases replacement of fluid and electrolytes must be aggressive and immediate. Standard ORS recommended by the World Health Organization and the American Academy of Pediatrics contain a 2% concentration of glucose (20 g/L), 45 to 90 mEq/L of sodium, 20 mEq/L of potassium, and a citrate base (Table 29-2).
TABLE 29-2
Oral Rehydration Solution: Composition and Recipes
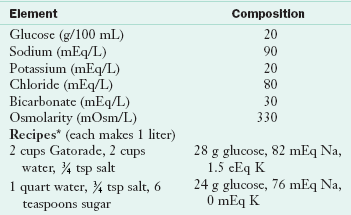
Recipes from Parrish CR: The Clinician’s guide to short bowel syndrome, Pract Gastroenterol 29:67, 2005.
*The solution should be made fresh every 24 hr.
Data from Krenitsky J, McCray S: University of Virginia Health System Nutrition Support Traineeship Syllabus, Charlottesville, Va, 2010, University of Virginia Health System; World Health Organization: Guidelines for cholera control, WHO/COD/Ser/80.4, Rev 1, Geneva, 1986.
Newer reduced-osmolarity solutions (200-250 mOsm/L) have advantages over the traditional WHO-recommended ORS in treating acute diarrhea in children (Atia and Buchman, 2009). The use of reduced-osmolarity ORS in children with acute diarrhea resulted in decreased need for intravenous therapy, significant reduction in stool output, and decreased vomiting when compared with standard WHO-recommended ORS (Atia and Buchman, 2009). Commercial solutions such as Pedialyte, Infalyte, Lytren, Equalyte, and Rehydralyte typically contain less glucose and slightly less salt and are available in pharmacies, often without prescription. Oral rehydration therapy is less invasive and less expensive than intravenous rehydration and, when used with children, allows parents to assist with their children’s recovery.
A substantial proportion of children 9 to 20 months of age can maintain adequate intake when offered either a liquid or a semisolid diet continuously during bouts of acute diarrhea. Even during acute diarrhea, the intestine can absorb up to 60% of the food eaten. Some practitioners have been slow to adopt the practice of early refeeding after severe diarrhea in infants despite evidence that “resting the gut” is actually more damaging. Thus prescription of the typical hospital “full liquid” or “clear liquid” diet, which is commonly high in fructose, lactose, and other sugars, is inappropriate for recovery from diarrhea.
Gastrointestinal Strictures and Obstruction
Intestinal tumors or scarring from GI surgeries, IBD, peptic ulcer, or radiation enteritis may partially or completely obstruct the GIT or cause dysfunctional segments. Obstructions may be partial or complete, and may occur in the stomach (gastric outlet obstruction), small intestine or large intestine. Symptoms include bloating, abdominal distention and pain, and sometimes nausea and vomiting.
Pathophysiology
People with gastroparesis, Crohn’s disease, scars, adhesions, dysmotility, or volvulus are all prone to obstruction. Partial or complete obstructions are not usually caused by foods in an otherwise healthy individual; however, when sections of the GIT are partially obstructed or not moving appropriately, foods may contribute to obstruction.
Although there are no controlled studies that have investigated different diets and the frequency of obstructive symptoms, it is believed that fibrous plant foods can contribute to obstructions because the fiber in the foods may not be completely chewed or reduced in size enough to pass through abnormal or narrowed segments of the GIT.
Medical Nutrition Therapy
Most clinicians would recommend patients prone to obstructions to chew food well and avoid excessive fiber intake. In addition, potato skins, citrus fruits, persimmons, and similar foods should be avoided by edentulous patients.
With a partial obstruction, the patient may be able to tolerate easily digestible foods and liquids, depending on the location of stricture or obstruction in the GIT. A more proximal (closer to the mouth) blockage may require a semisolid or liquid diet. However, the more distal (closer to the anus) the blockage, the less likely altering the consistency of the diet will help.
During complete obstruction, symptoms are more severe. Patients may be intolerant of oral intake and also of their own secretions. Intensive intervention, such as surgery, may be required for complete obstruction. In some cases, enteral feeding beyond the point of obstruction may be feasible, but if enteral feeding is not possible for a prolonged period, PN may be needed. Working with the patient and physician is necessary to determine the nature, site, and duration of the obstruction, so that nutrition therapy can be individualized.
Diseases of the Small Intestine
Celiac Disease (Gluten-Sensitive Enteropathy)
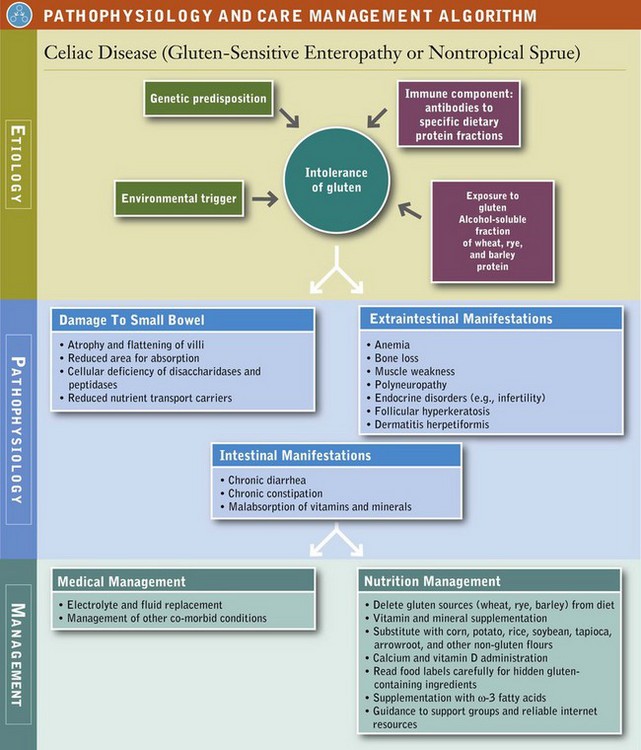
Celiac disease (CD), or gluten-sensitive enteropathy, is characterized by a combination of four factors: (1) genetic susceptibility, (2) exposure to gluten, (3) an environmental “trigger,” and (4) an autoimmune response. Gluten refers to specific peptide fractions of proteins (prolamines) found in wheat (glutenin and gliadin), rye (secalin), and barley (hordein). These peptides are generally more resistant to complete digestion by GI enzymes and may reach the small intestine intact. In a normal, healthy intestine, these peptides are harmless. However, in persons with CD these peptides travel from the intestinal lumen, across the intestinal epithelium, and into the lamina propria where they can trigger an inflammatory response that results in flattening of intestinal villi and elongation of the crypt cells (secretory cells), along with a more general systemic immune response (Kagnoff, 2007).
The term gluten sensitivity is commonly used to describe persons with nonspecific symptoms, without the immune response characteristic of CD or the consequential intestinal damage. Gluten intolerance describes individuals who have symptoms, and who may or may not have CD. These two terms are used to describe symptoms such as nausea, abdominal cramps, or diarrhea after ingesting gluten. Patients who experience these symptoms should generally be advised against following a gluten-free (GF) diet without having a workup to exclude or confirm a diagnosis of CD because (1) there may be an underlying medical condition for which a GF diet is not the treatment; (2) after following a GF diet for months or years, it is difficult to diagnose CD; and (3) although generally a healthy way to eat, a GF diet can be expensive and restrictive.
Pathophysiology
The “triggers” of CD are not well understood, but stressors (illness, inflammation, etc.) are thought to play a role. When CD remains untreated, the immune and inflammatory response eventually results in atrophy and flattening of villi. Over time, the process can cause enough damage to the intestinal mucosa to compromise normal secretory, digestive, and absorptive functions, leading to impaired micronutrient and macronutrient absorption (Chand and Mihas, 2006). Cells of the villi become deficient in the disaccharidases and peptidases needed for digestion and also in the carriers needed to transport nutrients into the bloodstream (see Figure 29-1). The disease primarily affects the proximal and middle sections of the small bowel, although the more distal segments may also be involved (Bonamico et al., 2008).
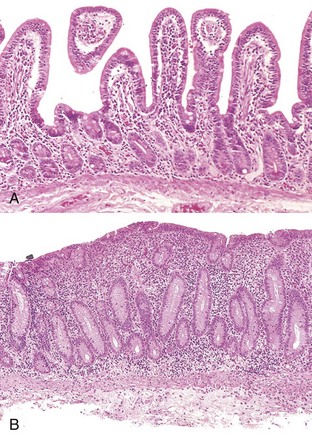
FIGURE 29-1 CD (gluten-sensitive enteropathy). A, Peroral jejunal biopsy specimen of diseased mucosa shows severe atrophy and blunting of villi, with a chronic inflammatory infiltrate of the lamina propria. B, Normal mucosal biopsy. (From Kumar V and others: Robbins and Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders.)
The prevalence of CD has been underestimated in the past and now is considered to affect at least 1 in 133 persons in the United States. The onset and first occurrence of symptoms may appear any time from infancy to adulthood, but the peak in diagnosis occurs between the fourth and sixth decade. The disease may become apparent when an infant begins eating gluten-containing cereals. In some, it may not appear until adulthood, when it may be triggered or unmasked during GI surgery, stress, pregnancy, or viral infection. Or it may be discovered as a result of evaluation for another suspected problem. Approximately 20% of cases are diagnosed after the age of 60 years.
The presentation in young children is likely to include the more “classic” GI symptoms of diarrhea, steatorrhea, malodorous stools, abdominal bloating, apathy, fatigue, and poor weight gain. Although GI-related symptoms are often thought to be most common, an increasing number of patients present without GI symptoms. Fifty percent of celiac patients have few or no obvious symptoms, and some are overweight at presentation (Venkatasubramani et al., 2010). CD is frequently misdiagnosed as irritable bowel syndrome (IBS), lactase deficiency, gallbladder disease, or other disorders not necessarily involving the GIT, because the presentation and onset of symptoms vary so greatly.
Patients may present with one or more of a host of conditions associated with CD: anemias, generalized fatigue, weight loss or failure to thrive, osteoporosis, vitamin or mineral deficiencies, and (although rare) GI malignancy. Dermatitis herpetiformis, yet another manifestation of CD, presents as an itchy skin rash; its presence is diagnostic of CD. Box 29-4 lists conditions associated with CD. Persons who are diagnosed late in life, who cannot or will not comply with the diet, or who were diagnosed as children but told they would grow out of it are at a higher risk for experiencing long-term complications from CD (Nachman et al., 2010).
Assessment
The diagnosis of CD is made by a combination of clinical, laboratory, and histologic evaluations. Persons suspected of having CD should be evaluated for the overall pattern of symptoms and family history. Biopsy of the small intestine is the gold standard for diagnosis (Chand and Mihas, 2006). An intestinal biopsy positive for CD generally shows villous atrophy, increased intraepithelial lymphocytes, and crypt cell hyperplasia. Biopsy is not used for initial screening because of its cost and invasiveness.
Several serologic tests are used for screening. These tests identify the presence of antibodies in the blood, such as anti-tissue transglutaminase (anti-TTG), and anti-endomysial antibodies, and deaminated gliadin peptide. The sensitivity and specificity of these tests are 90% to 99% (Rostom et al., 2005). There is a higher incidence of immunoglobulin (Ig) A deficiency in patients with CD; thus physicians often measure IgA levels if serologic findings are normal but the overall clinical picture suggests CD. Using capsule endoscopy to image the entire intestinal mucosa can show inflammation related to CD, but is not currently used in the initial diagnosis (El-Matary et al., 2009). Because dietary change alters diagnostic results, initial evaluation should be done before the person has eliminated gluten-containing foods from his or her diet. Serologic tests may also be used to monitor the response of a newly diagnosed patient treated with a GF diet.
Lifelong, strict adherence to a GF diet is the only known treatment for CD. See Box 29-5 for a list of safe, questionable and unsafe choices on the GF diet. The GF diet greatly diminishes the autoimmune process, and the intestinal mucosa usually reverts to normal or near normal. Within 2 to 8 weeks of starting the GF diet, most patients report that their clinical symptoms have abated. Histologic, immunologic, and functional improvements may take months to years, depending on the duration of the disease, age of the subject, and degree of dietary compliance. With strict dietary control, levels of the specific antibodies usually become undetectable within 3 to 6 months in most persons. In some individuals, recovery may be slow or incomplete.
A small percentage of patients are “nonresponders” to diet therapy. Inadvertent gluten intake is the most common offender, but another coexisting disorder may be present (such as pancreatic insufficiency, IBS, bacterial overgrowth, fructose intolerance, other GI maladies, or unknown causes). For nonresponders, intensive interviewing to identify a source of gluten contamination or treatment of another underlying disease may resolve the symptoms. Diagnosis of refractory celiac disease is made when patients do not respond or respond only temporarily to a GF diet, and all external causes have been ruled out, including inadvertent gluten ingestion. Patients with refractory disease may respond to steroids, azathioprine, cyclosporine, or other medications classically used to suppress inflammatory or immunologic reactions (see Pathophysiology and Care Management Algorithm: Celiac Disease).
Several novel treatments for CD are being studied for their potential as alternative therapies. Researchers seek to treat CD by reducing gluten exposure (by digestion from enzymes), decreasing uptake of gluten (by tightening junctions between intestinal epithelial cells), altering the immune response to gluten, or repairing intestinal injury.
Medical Nutrition Therapy
Elimination of gluten peptides from the diet is the only treatment of CD. The diet omits all dietary wheat, rye, and barley, which are the major sources of the prolamin fractions
In general, patients should be assessed for nutrient deficiencies before supplementation is initiated. In all newly diagnosed patients, the clinician should consider checking levels of ferritin, red blood cell folate, and 25-hydroxy vitamin D. If patients present with more severe symptoms, such as diarrhea, weight loss, malabsorption, or signs of nutrient deficiencies (night-blindness, neuropathy, prolonged prothrombin time, etc.), other vitamins such as fat-soluble vitamins (A, E, K) and minerals (zinc) should be checked.
The healing of the intestinal mucosa that occurs after initiation of a GF diet improves nutrient absorption, and many patients who eat well-balanced GF diets do not need nutritional supplementation. However, most specialty GF products are not fortified with iron and B vitamins like other grain products, so the diet may not be as complete without at least partial supplementation. Anemia should be treated with iron, folate, or vitamin B12, depending on the nature of the anemia. Patients with malabsorption may benefit from a bone density scan to assess for osteopenia or osteoporosis. Calcium and vitamin D supplementation are likely to be beneficial in these patients. Electrolyte and fluid replacement is essential for those dehydrated from severe diarrhea.
Those who continue to have malabsorption should take a general vitamin-mineral supplement to at least meet DRI recommendations. Lactose and fructose intolerance sometimes occur secondary to CD, and sugar alcohols are not well absorbed, even in a healthy gut. A low-lactose or low-fructose diet may be useful in controlling symptoms, at least initially. Once the GIT returns to more normal function, lactase activity may also return, and the person can incorporate lactose and dairy products back into the diet.
In general, many fruits, vegetables, grains, meats, and dairy products that are plain and unseasoned are safe to eat. Oats were once thought to be questionable for persons with CD; however, extensive studies have shown that they are safe in the GF diet as long as they are pure, uncontaminated oats (Garsed and Scott, 2007). A very small population of patients with CD may not tolerate even pure oats. In general, patients do not need to be advised against including GF oats in their diet unless they have demonstrated intolerance to GF oats.
Flours made from corn, potatoes, rice, soybean, tapioca, arrowroot, amaranth, quinoa, millet, teff, and buckwheat can be substituted in recipes. Patients can expect differences in textures and flavors of common foods using the substitute flours, but new recipes can be quite palatable once the adjustment is made. In GF baked goods, gums such as xanthan, guar, and cellulose can be used to provide the elasticity needed to trap leavening gases in baked goods.
A truly GF diet requires careful scrutiny of the labels of all bakery products and packaged foods. Gluten-containing grains are not only used as a primary ingredient but may also be added during processing or preparation of foods. For example, hydrolyzed vegetable protein can be made from wheat, soy, corn, or mixtures of these grains.
The diet for the person with CD requires a major lifestyle change because of the change from traditional grains in the diet. A tremendous number of foods made with wheat (in particular breads, cereals, pastas, and baked goods) are a common part of a Western diet. However, there is increasing awareness among food companies and restaurants of the expanding demand for GF foods, and these businesses are responding. The individual and family members should all be taught about label reading, safe food additives, food preparation, sources of cross contamination (such as toasters, condiment jars, bulk bins, and buffets), and hidden sources of gluten (such as medications and communion wafers) to be compliant. Box 29-6 provides sources of hidden gluten and cross-contamination. Eating in cafeterias, restaurants, vending outlets, street markets, at friends’ homes, and at social events can be challenging, especially initially.
To avoid misinterpretation of information, newly diagnosed patients should be started with an in-depth instruction from a registered dietitian on the GF diet, along with reliable resources for further guidance and support. Persons with CD generally need several education or counseling sessions with a registered dietitian knowledgeable in the disease management (American Gastroenterological Association, 2006; Case, 2005). Box 29-7 lists CD resources.
Marked gut improvement and a return to normal histologic findings occurs in the majority of patients after an average of 2 years (Hutchinson et al., 2010). Patients who are able to follow the GF diet closely have a better overall response.
Tropical Sprue
Tropical sprue is an acquired diarrheal syndrome with malabsorption that occurs in many tropical areas (Nath, 2005). In addition to diarrhea and malabsorption, anorexia, abdominal distention, and nutritional deficiency as evidenced by night blindness, glossitis, stomatitis, cheilosis, pallor, and edema can occur. Anemia may result from iron, folic acid, and vitamin B12 deficiencies.
Pathophysiology
Diarrhea appears to be an infectious type, although the precise cause and the sequence of pathogenic events remains unknown. The syndrome may include bacterial overgrowth, changes in GI motility, and cellular changes in the GIT. Identified intestinal organisms may differ from one region of the tropics to the next. As in CD, the intestinal villi may be abnormal, but the surface cell alterations are much less severe. The gastric mucosa is atrophied and inflamed, with diminished secretion of hydrochloric acid and intrinsic factor.
Medical Treatment
Treatment of tropical sprue typically includes use of broad-spectrum antibiotics, folic acid, fluid, and electrolytes.
Medical Nutrition Therapy
Nutrition management includes restoration and maintenance of fluids, electrolytes, macronutrients, and micronutrients, and introduction of a diet that is appropriate for the extent of malabsorption (see “Diarrhea” earlier in this chapter). Along with other nutrients, B12 and folate supplementation may be needed if deficiency is identified. Nutritional deficiency increases susceptibility to infectious agents, further aggravating the condition.
Intestinal Brush-border Enzyme Deficiencies
Intestinal enzyme deficiency states involve deficiencies of the brush-border disaccharidases that hydrolyze disaccharides at the mucosal cell membrane. Disaccharidase deficiencies may occur as (1) rare congenital defects such as the sucrase, isomaltase, or lactase deficiencies seen in the newborn; (2) generalized forms secondary to diseases that damage the intestinal epithelium (e.g., Crohn’s disease or CD); or, most commonly, (3) a genetically acquired form (e.g., lactase deficiency) that usually appears after childhood but can appear as early as 2 years of age. For this chapter, only lactose malabsorption is described in detail (see Chapter 44 for a discussion of inborn metabolic disorders).
Lactose Intolerance
Lactose intolerance is the syndrome of diarrhea, abdominal pain, flatulence, or bloating occurring after lactose consumption. Secondary lactose intolerance can develop as a consequence of infection of the small intestine, inflammatory disorders, HIV, or malnutrition. In children it is typically secondary to viral or bacterial infections. Lactose malabsorption is commonly associated with other GI disorders, such as IBS, which is not surprising because lactose intolerance is so common.
Of the adult worldwide population, 70%, especially African, Hispanic, Asian, South American, and Native American populations, demonstrate lactose malabsorption. However, the prevalence of lactose intolerance in the United States has not yet been accurately estimated because of limitations in the current studies (Suchy et al., 2010). Typically, lactase activity declines exponentially at weaning to about 10% of the neonatal value. Lactose malabsorption and intolerance has been reported to be low in children below age six, but increases throughout childhood, peaking at age 10-16.
There is little evidence that lactose intolerance increases with increasing adult age (Suchy et al, 2010). Even in adults who retain a high level of lactase levels (75% to 85% of white adults of Western European heritage), the quantity of lactase is approximately half that of other saccharidases such as sucrase, α-dextrinase, or glucoamylase. The decline of lactase is commonly known as hypolactasia; the adult form involves down-regulation after weaning (Järvelä 2005)and may have a relationship with an increased risk of colon cancer in some populations (Rasinperä et al. 2005). See Focus On: Lactose Intolerance—NOT an Uncommon Anomaly.
Pathophysiology
When large amounts of lactose are consumed, especially by persons who have little remaining lactase enzyme or with concurrent GI problems, loose stools or diarrhea can occur. As is the case with any malabsorbed sugar, lactose may act osmotically, and increase fecal water, as well as provide a substrate for rapid fermentation by intestinal bacteria, which may result in bloating, flatulence, and cramps. Malabsorption of lactose is due to a deficiency of lactase, the enzyme that digests the sugar in milk. Lactose that is not hydrolyzed into galactose and glucose in the upper small intestine passes into the colon, where bacteria ferment it to SCFAs, carbon dioxide, and hydrogen gas.
Medical Treatment
Lactose malabsorption is diagnosed by (1) an abnormal hydrogen breath test, or (2) an abnormal lactose tolerance test. During a hydrogen breath test, the patient is given a standard dose of lactose after fasting, and breath hydrogen is measured. If lactose is not digested in the small intestine, it passes into the colon where it is fermented by microbes to SCFAs, CO2, and hydrogen. Hydrogen is absorbed into the bloodstream and is exhaled through the lungs. The breath hydrogen test shows increased levels 60 to 90 minutes after lactose ingestion.
During a lactose tolerance test, a dose of lactose is given and if the individual has sufficient lactase enzyme, blood sugar will rise, reflecting the digestion of lactose to galactose and glucose. If the individual is lactose intolerant (lactase deficient) blood sugar will not rise because the lactose is not absorbed; it passes into the colon and GI symptoms may appear. The lactose tolerance test was originally based on an oral dose of lactose equivalent to the amount in 1 quart of milk (50 g). Recently, doses lower than 50 g of lactose have been used to approximate more closely the usual consumption of lactose from milk products.
Demonstrated lactose malabsorption does not always indicate a person will be symptomatic. Many factors play a role, including the amount of lactose ingested, the residual lactase activity, the ingestion of food in addition to lactose, the ability of the colonic bacteria to ferment lactose, and the sensitivity of the individual to the lactose fermentation products (Suchy et al., 2010). Consumption of small amounts should be of little consequence because the SCFAs are readily absorbed and the gases can be absorbed or passed. Larger amounts, usually greater than 12 g/day, consumed in a single food (the amount typically found in 240 mL of milk) may result in more substrate entering the colon than can be disposed of by normal processes (Suchy et al., 2010). Because serving sizes of milk drinks are increasing and more than one source of lactose might be consumed in the same meal, the amounts of lactose consumed may be more important than in years past.
Medical Nutrition Therapy
Management of lactose intolerance requires dietary change. The symptoms are alleviated by reduced consumption of lactose-containing foods. Persons who avoid dairy products may need calcium and vitamin D supplementation or must be careful to get nondairy sources of these nutrients. A completely lactose-free diet is not necessary in lactase-deficient persons. Most lactose maldigesters can consume some lactose (up to 12 g/day) without major symptoms, especially when taken with meals or in the form of cheeses or cultured dairy products (Shaukat et al., 2010); see Chapter 3 and Table 29-3.
TABLE 29-3
Lactose Content of Common Foods
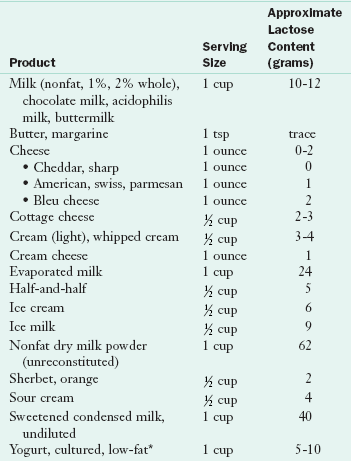
*Note: Although yogurt does contain lactose, cultured yogurt is generally well tolerated by those with lactose intolerance
Many adults with intolerance to moderate amounts of milk can ultimately adapt to and tolerate 12 g or more of lactose in milk (equivalent to 240 mL of full-lactose milk) when introduced gradually, in increments, over several weeks. Incremental or continuous exposure to increasing quantities of fermentable sugar can lead to improved tolerance, not as a consequence of increased lactase enzyme production but perhaps by altered colonic flora. This has been shown with lactulose, a nonabsorbed carbohydrate that is biochemically similar to lactose (Bezkorovainy 2001). Individual differences in tolerance may relate to the state of colonic adaptation. Regular consumption of milk by lactase-deficient persons may increase the threshold at which diarrhea occurs.
Lactase enzyme and milk products treated with lactase enzyme (e.g., Lactaid) are available for lactase maldigesters who have discomfort with milk ingestion. Commercial lactase preparations may differ in their effectiveness. Fermented milk products, such as aged cheeses and yogurts, are well tolerated because the lactose content is low. Tolerance of yogurt may be the result of a microbial galactosidase in the bacterial culture that facilitates lactose digestion in the intestine. The presence of galactosidase depends on the brand and processing method. Because this microbial enzyme is sensitive to freezing, frozen yogurt may not be as well tolerated. Although the addition of probiotics may change this, evidence is lacking (Levri et al., 2005).
Fructose Malabsorption
Consumption of fructose in the United States, especially from fruit juices, fruit drinks, and HFCS in soft drinks and confections, has increased significantly in recent years. The human small intestine has a limited ability to absorb fructose, compared with the ability to rapidly and completely absorb glucose. Breath hydrogen testing has revealed that up to 75% of healthy people will incompletely absorb a large quantity of fructose (50 g) taken alone (Barrett and Gibson, 2007). Absorption of fructose is improved when it is ingested with glucose (such as in sucrose) because glucose absorption stimulates pathways for fructose absorption.
Pathophysiology
Although fructose malabsorption is common in healthy people, its appearance appears to depend on the amount of fructose ingested. In one study, more than 50% of people had a positive breath hydrogen test after a 25-g load, whereas 73% had a positive breath hydrogen test after a 50-g load (Beyer et al., 2005). Although some degree of fructose malabsorption may be normal, those with coexisting GI disorders may be more likely to experience GI symptoms after fructose ingestion. Patients with IBS and visceral hypersensitivity may be more sensitive to gas, distension, or pain from fructose malabsorption, whereas those with SBBO may experience symptoms from normal amounts of fructose.
Medical Nutrition Therapy
People with fructose malabsorption and those patients with GI conditions that experience symptoms of fructose malabsorption may not have problems with foods containing balanced amounts of glucose and fructose, but may need to limit or avoid foods containing large amounts of free fructose (Beyer et al., 2005). Pear, apple, mango, and Asian pear are notable in that they have substantially more “free fructose” (more fructose than glucose) (Barrett and Gibson, 2007). Additionally, most dried fruits and fruit juices may pose a problem in larger amounts because of the amount of fructose provided per serving. Foods sweetened with HFCS (as opposed to sucrose) are also more likely to cause symptoms. Hepatic fructose metabolism is similar to ethanol, in that they both serve as substrates for de novo lipogenesis, thus promoting hepatic insulin resistance, dyslipidemia, and hepatic steatosis (Lustig, 2010). The degree of fructose intolerance and tolerance to the symptoms of fructose malabsorption are so variable that tolerable intake of these foods must generally be individualized with each patient.
Inflammatory Bowel Diseases
The two major forms of inflammatory bowel disease (IBD) are Crohn’s disease and ulcerative colitis (UC). Both Crohn’s disease and UC are relatively rare disorders, but they result in frequent use of health care resources. The prevalence is approximately 130 cases per 100,000 persons for Crohn’s disease and 100 per 100,000 for UC. The onset of IBD occurs most often in patients 15 to 30 years of age, but for some it occurs later in adulthood. Both sexes are equally affected. IBD occurs more commonly in developed areas of the world, in urban compared with rural environments, and in northern compared with southern climates.
Crohn’s disease and ulcerative colitis (UC) share some clinical characteristics, including diarrhea, fever, weight loss, anemia, food intolerances, malnutrition, growth failure, and extraintestinal manifestations (arthritic, dermatologic, and hepatic). In both forms of IBD, the risk of malignancy increases with the duration of the disease. The reasons for the increased risk are not firmly established but are likely related to the increased inflammatory and proliferative state and nutritional factors. Although malnutrition can occur in both forms of IBD, it is more of a lifelong concern in patients with Crohn’s disease. The features that distinguish the forms of the disease in terms of genetic characteristics, clinical presentation, and treatment are discussed in Table 29-4.
Crohn’s Disease and Ulcerative Colitis
Crohn’s disease may involve any part of the GIT, but approximately 50% to 60% of cases involve both the distal ileum and the colon. Only the small intestine or only the colon is involved in 15% to 25% of cases. Disease activity in UC is limited to the large intestine and rectum. In Crohn’s disease, segments of inflamed bowel may be separated by healthy segments, whereas in UC the disease process is continuous (Figure 29-2). Mucosal involvement in Crohn’s disease is transmural in that it affects all layers of the mucosa; in UC the disease normally is limited to the mucosa. Crohn’s disease is characterized by abscesses, fistulas, fibrosis, submucosal thickening, localized strictures, narrowed segments of bowel, and partial or complete obstruction of the intestinal lumen. Bleeding is more common in UC.
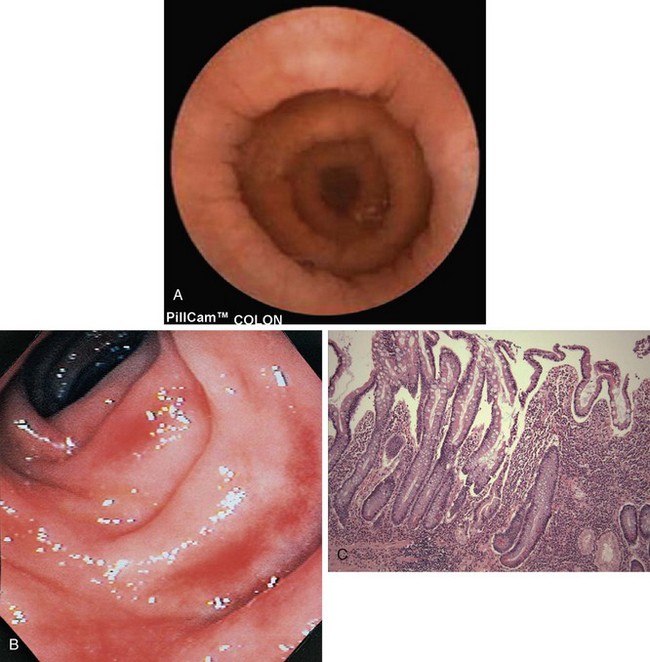
FIGURE 29-2 A, Normal colon, B, ulcerative colitis, C, Crohn’s disease. (A, From Fireman, Z., & Kopelman, Y. (2007). The colon—the latest terrain for capsule endoscopy. Digestive and Liver Disease, 39(10), 895-899. B, From Black JM, Hawks JH: Medical-surgical nursing: clinical management for positive outcomes, ed 8, St. Louis, 2009, Saunders. C, From McGowan, CE, Lagares-Garcia, JA, & and Bhattacharya, B. (2009). Retained capsule endoscope leading to the identification of small bowel adenocarcinoma in a patient with undiagnosed Crohn disease. Annals of Diagnostic Pathology, 13(6), 390-393.)
Pathophysiology
The cause of IBD is not completely understood, but it involves the interaction of the GI immunologic system and genetic and environmental factors. The genetic susceptibility is now recognized to be diverse, with a number of possible gene mutations that affect risk and characteristics of the disease. The diversity in the genetic alterations among individuals may help explain differences in the onset, aggressiveness, complications, location, and responsiveness to different therapies as seen in the clinical setting (Shih and Targan, 2008). The major environmental factors include resident and transient microorganisms in the GIT and dietary components.
The genes affected (e.g., C677T mutation related to methylene-tetrahydrofolate reductase) normally play a role in the reactivity of the host GI immune system to luminal antigens such as those provided by intestinal flora and the diet. In animal models inflammatory disease does not occur without the intestinal flora. Normally, when an antigenic challenge or trauma occurs, the immune response rises to the occasion; it is then turned off and continues to be held in check after the challenge resolves. In IBD, increased exposure, decreased defense mechanisms, or decreased tolerance to some component of the GI microflora occur. Inappropriate inflammatory response and an inability to suppress it play primary roles in the disease. For example, one of the genes affected in Crohn’s disease is the NOD2/CARD15 gene, which codes for a small peptide that interacts with a host of GI bacteria. Failure to produce that peptide may result in abnormal immune responses (Mueller and Macpherson, 2006).
The inflammatory response (e.g., increased cytokines and acute-phase proteins, increased GI permeability, increased proteases, and increased oxygen radicals and leukotrienes) results in GI tissue damage (Sanders, 2005). In IBD either the regulatory mechanisms are defective or the factors perpetuating the immune and acute-phase responses are enhanced, leading to tissue fibrosis and destruction. The clinical course of the disease may be mild and episodic or severe and unremitting (see Pathophysiology and Care Management Algorithm: Inflammatory Bowel Disease).
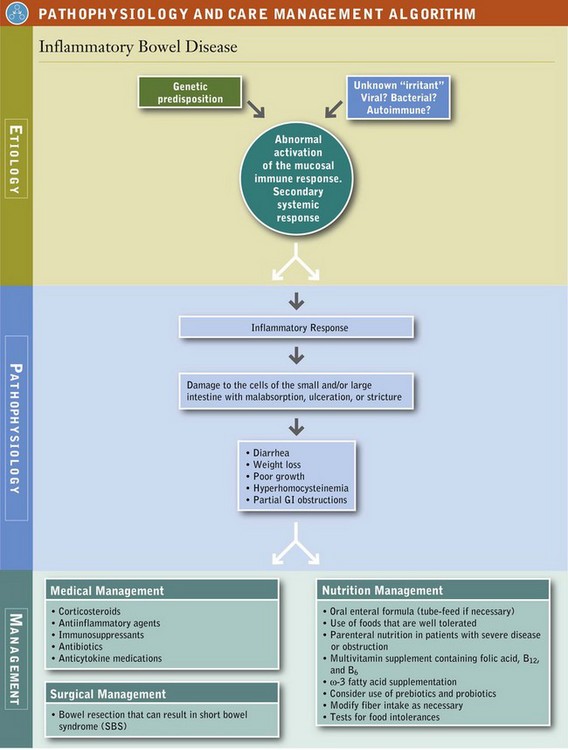
Diet is an environmental factor that may trigger relapses of IBD. Foods, microbes, individual nutrients, and incidental contaminants provide a huge number of potential antigens, especially considering the complexity and diversity of the modern diet. Malnutrition can affect the function and effectiveness of the mucosal, cellular, and immune barriers; diet can also affect the type and relative composition of the resident microflora. Several nutrients (e.g., dietary lipids) can affect the intensity of the inflammatory response.
Food allergies and other immunologic reactions to specific foods have been considered in the pathogenesis of IBD and its symptoms; however, the incidence of documented food allergies, compared with food intolerances, is relatively small. The permeability of the intestinal wall to molecules of food and cell fragments is likely increased in inflammatory states, allowing the potential for increased interaction of antigens with host immune systems (Müller et al., 2005).
Food intolerances occur more often in persons with IBD than in the population at large, but the patterns are not consistent among individuals or even between exposures from one time to the next. Reasons for specific and nonspecific food intolerances are abundant and are related to the severity, location, and complications associated with the disease process. Partial GI obstructions, malabsorption, diarrhea, altered GI transit, increased secretions, food aversions, and associations are but a few of the problems experienced by persons with IBD. However, neither food allergies nor intolerances fully explain the onset or manifestations in all patients (see Chapter 27).
Medical Management
The goals of treatment in IBD are to induce and maintain remission and to improve nutrition status. Treatment of the primary GI manifestations appears to correct most of the extraintestinal features of the disease as well. The most effective medical agents include corticosteroids, antiinflammatory agents (aminosalicylates), immunosuppressive agents (cyclosporine, azathioprine, mercaptopurine), antibiotics (ciprofloxacin and metronidazole), and monoclonal tumor necrosis factor antagonists (anti-TNF) (infliximab, adalimumab, certolizumab, and natalizumab), agents that inactivate one of the primary inflammatory cytokines. Anti-TNF is normally used in severe cases of Crohn’s disease and fistulas, but it has not been shown to be effective in UC.
Investigations of various treatment modalities for the acute and chronic stages of IBD are ongoing, and include new forms of existing drugs, as well as new agents targeted to regulate cytokines, eicosanoids, or other mediators of the inflammatory and acute-phase response (Caprilli et al., 2006; Travis et al., 2006). ω-3 fatty acid supplements (fish oil capsules) in Crohn’s disease significantly reduce disease activity (Turner et al., 2009). Use of fish oil supplements in UC appears to result in a significant medication-sparing effect, with reductions in disease activity and increased time in remission reported (Seidner et al., 2005). Use of foods and supplements containing prebiotics and probiotic cultures are being investigated because each has the potential to alter both the GI microflora and the immunologic response at the gut level (Dotan and Rachmilewitz, 2005).
Surgical Management
In Crohn’s disease, surgery may be necessary to repair strictures or remove portions of the bowel when medical management fails. Approximately 50% to 70% of persons with Crohn’s disease will undergo surgery related to the disease. Surgery does not cure Crohn’s disease, and recurrence often occurs within 1 to 3 years of surgery. The chance of needing subsequent surgery in the patient’s life is approximately 30% to 70%, depending on the type of surgery and the age at first operation. Major resections of the intestine may result in varying degrees of malabsorption of fluid and nutrients. In extreme cases patients may have extensive or multiple resections, resulting in short-bowel syndrome (SBS) and dependence on PN to maintain adequate nutrient intake and hydration.
With UC, approximately 20% of patients have a colectomy and removal of the colon, and this resolves the disease. Inflammation does not occur in the remaining GIT. Whether a colectomy is necessary depends on the severity of the disease and indicators of increased cancer risk. After a colectomy for UC, surgeons may create an ileostomy with an external collection pouch and an internal abdominal reservoir fashioned with a segment of ileum or an ileoanal pouch, which spares the rectum, to serve as a reservoir for stool. The internal Koch pouch may also be used (see Chapter 14).
Medical Nutrition Therapy
Persons with IBD are at increased risk of nutrition problems for a host of reasons related to the disease and its treatment. Thus the primary goal is to restore and maintain the nutrition status of the individual patient. Foods, dietary and micronutrient supplements, enteral and PN may all be used to accomplish that mission. Oral diet and the other means of nutrition support may change during remissions and exacerbations of the disease.
Persons with IBD often have fears and misconceptions regarding GI symptoms and the role of food. Patients are also often confused by dietary advice from associates, various media, and health care providers. Education is a key form of nutrition intervention. There is no single dietary regimen for reducing symptoms or decreasing the flares in IBD. Diet and specific nutrients play a supportive role in maintaining nutrition status, limiting symptom exacerbations, and supporting growth in pediatric patients.
The ability of parenteral or enteral nutrition to induce remission of IBD has been debated for several years. Evaluation is confounded by the natural course of IBD with exacerbations and remissions, and by the genetic diversity of the patients. Studies have generally concluded that (1) nutrition support may bring about some clinical remission when used as a sole source of treatment; (2) “complete bowel rest” using PN is not necessarily required; (3) enteral nutrition has the potential to feed the intestinal epithelium and alter GI flora and is the preferred route of nutrition support; (4) enteral nutrition may temper some elements of the inflammatory process, serve as a valuable source of nutrients needed for restoration of GI defects, and be steroid sparing; (5) children benefit from the use of enteral nutrition to maintain growth and reduce the dependence on steroids that may affect growth and bone disease (Dray and Marteau, 2005; Lochs, 2006; Sanderson and Croft, 2005). Patients and caretakers must be very committed when using enteral nutrition formulas or tube feeding because it takes 4 to 8 weeks before one sees the clinical effects.
Timely nutritional support is a vital component of therapy to restore and maintain nutritional health. Malnutrition itself compromises digestive and absorptive function by increasing the permeability of the GIT to potential inflammatory agents. PN is not as nutritionally complete, has increased risk of infectious complications, and is more expensive than enteral nutrition. However, PN may be required in patients with persistent bowel obstruction, fistulas, and major GI resections that result in SBS where enteral nutrition is not possible.
Energy needs of patients with IBD are not greatly increased (unless weight gain is desired). Generally when disease activity increases basal metabolic rate, physical activity is greatly curtailed and overall energy needs are not substantially changed.
Protein requirements may be increased, depending on the severity and stage of the disease and the restoration requirements. Inflammation and treatment with corticosteroids induce a negative nitrogen balance and cause a loss of lean muscle mass. Protein losses also occur in areas of inflamed and ulcerated intestinal mucosa via defects in epithelial tight junctions. See Fig. 39-3 in Chapter 39. To maintain positive nitrogen balance, 1.3-1.5 g/kg/day of protein is recommended.
Supplemental vitamins, especially folate, B6, and B12, may be needed as well as minerals and trace elements to replace stores or for maintenance because of maldigestion, malabsorption, drug-nutrient interactions, or inadequate intake (Zezos et al., 2005). Diarrhea can aggravate losses of zinc, potassium, and selenium. Patients who receive intermittent corticosteroids may need supplemental calcium and vitamin D. Patients with IBD are at increased risk of osteopenia and osteoporosis; 25-OH vitamin D levels and bone density should be routinely monitored.
In daily life people with IBD may have intermittent “flares” of the disease characterized by partial obstructions, nausea, abdominal pain, bloating, or diarrhea. Many patients report specific, individualized food intolerances. Patients are sometimes advised to eliminate the foods they suspect are responsible for the intolerance. Often, the patient becomes increasingly frustrated as the diet becomes progressively limited and symptoms still do not resolve. Malnutrition is a significant risk in patients with IBD, and an overly restricted diet only increases the likelihood of malnutrition and weight loss.
During acute and severe exacerbations of the disease, the diet is tailored to the individual patient. In patients with rapid intestinal transit, extensive bowel resections, or extensive small bowel disease, absorption may be compromised. Here, excessive intake of lactose, fructose, or sorbitol may contribute to abdominal cramping, gas, and diarrhea; and high fat intake may result in steatorrhea. However, the incidence of lactose intolerance is no greater in patients with IBD than in the general population. Patients with IBD who tolerate lactose should not restrict lactose-containing foods because they can be a valuable source of high quality protein, calcium, and vitamin D.
Patients with strictures or partial bowel obstruction benefit from a reduction in dietary fiber or limited food particle size. Small, frequent feedings may be tolerated better than large meals. Small amounts of isotonic, liquid oral supplements may be valuable in restoring intake without provoking symptoms. In cases in which fat malabsorption is likely, supplementation with foods made with medium-chain triglycerides (MCTs) may be useful in adding calories and serving as a vehicle for fat-soluble nutrients. However, these products are expensive and may be less effective than more basic treatments.
Factors associated with of the development of IBD in epidemiologic studies include increased sucrose intake, lack of fruits and vegetables, a low intake of dietary fiber, use of red meat and alcohol, and altered ω-6/ω-3 fatty acid ratios. Yet dietary interventions to modify these factors during IBD flares have not resulted in significant improvements (Rajendran and Kumar, 2010).
The same foods that are responsible for GI symptoms (gas, bloating, and diarrhea) in a normal, healthy population are likely to be the triggers for the same symptoms in patients with mild stages of IBD or those in remission.
Patients receive nutritional information from a variety of sources, including support groups, Internet news groups, the audio and printed media, well-meaning friends, and food supplement salespersons. The information is sometimes inaccurate or exaggerated, or it may pertain only to one individual’s situation. The health care provider can help patients sort out the role of foods in normal, everyday GI disturbances and in IBD and teach them how to evaluate valid nutrition information from unproven or exaggerated claims. Patients’ participation in the management of their disease may help to reduce not only the symptoms of the disease but the associated anxiety level as well.
Probiotic foods and supplements have been investigated as potential therapeutic agents for IBD because of their ability to modify the microbial flora and modulate gut inflammatory response. High-dose probiotic supplements (e.g., VSL#3) improved disease activity in patients with UC who had pouchitis, inflammation in the ileal pouch surgically formed after colectomy (Holubar et al., 2010). However, a different probiotic supplement at a lower dose did not significantly reduce symptoms (Holubar et al., 2010). Probiotic supplements also appear to be useful for induction and extension of remissions in pediatric and adult UC (Guandalini, 2010; Mallon et al., 2007).
Although probiotics appear useful in UC, probiotic studies to date have not demonstrated significant improvement in Crohn’s disease activity in adults or pediatric patients, nor do probiotic supplements appear to prolong remission in Crohn’s disease (Butterworth et al., 2008; Guandalini, 2010).
Regular intake of prebiotic foods such as oligosaccharides, fermentable fibers, and resistant starches can alter the mixture of microorganisms in the colonic flora, feeding lactobacillus and bifidobacteria to provide competition to and theoretically suppress pathogenic or opportunistic microflora. Additionally, fermentation of prebiotics leads to increased production of SCFAs, theoretically creating a more acidic and less favorable environment for opportunistic bacteria.
Use of probiotics and prebiotics may serve to prevent small intestine bacterial overgrowth in predisposed individuals and to treat diarrhea. Additional study is needed to identify the dose, the most effective prebiotic and probiotic foods, the form in which they can be used for therapeutic and maintenance purposes, and their relative value compared with other therapies (Penner et al., 2005).
Microscopic Colitis
Injury of the colon caused by UC, Crohn’s disease, infections, radiation injury, and ischemic insult to the colon all present with abnormalities such as edema, redness, bleeding, or ulcerations that are visible on colonoscopy examination. Microscopic colitis is characterized by inflammation that is not visible by inspection of the colon during colonoscopy, and is only apparent when the colon’s lining is biopsied and then examined under a microscope.
There are two types of microscopic colitis. In lymphocytic colitis, there is an accumulation of lymphocytes within the lining of the colon. In collagenous colitis, there is also a layer of collagen (like scar tissue) just below the lining. Some experts believe that lymphocytic colitis and collagenous colitis represent different stages of the same disease. Symptoms include chronic, watery diarrhea, mild abdominal cramps, and pain.
More than 30% of patients report weight loss (Simondi et al., 2010). Patients with microscopic colitis can have diarrhea for months or years before the diagnosis is made. The cause of microscopic colitis is unknown. Microscopic colitis appears more frequently in patients aged 60-70 years, and collagenous colitis occurs more frequently in females (Jobse et al., 2009; Tysk et al., 2008).
Patients with CD are 70 times more likely than the normal population to develop microscopic colitis (Green et al., 2009). Patients with CD and microscopic colitis have more severe villous atrophy and frequently require steroids or immunosuppressant therapies in addition to a GF diet to control diarrhea. Research is underway to determine possible effective treatments for microscopic colitis, including corticosteroids and immunosuppressive agents. Medical nutrition therapy is supportive in nature with efforts to maintain weight and nutrition status, avoid symptom exacerbation, and maintain hydration.
Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is characterized by chronically recurring abdominal discomfort or pain and altered bowel habits. Other common symptoms include bloating, feelings of incomplete evacuation, presence of mucus in the stool, straining or increased urgency (depending on the type of presentation), and increased GI distress associated with psychosocial distress.
IBS is one of the most common reasons for primary care visits and consultations with gastroenterologists in the United States. IBS occurs in approximately 15% of women and 10% of men; however, it is estimated that only 25% to 50% of those with symptoms actually seek treatment. Typically, symptoms first occur between adolescence and the fourth decade of life, but many persons do not bring the problem to the attention of a physician. Persons with IBS often have increased absenteeism from school and work, decreased productivity, increased health care costs, and decreased quality of life as a result of their symptoms.
Diagnosis is based on international consensus criteria (Rome criteria) and diagnostic algorithms that help separate other medical or surgical disorders that manifest with similar symptoms (Malagelada, 2006). According to the criteria, symptoms of abdominal discomfort must be present for at least 3 days per month for the past 3 months, include at least two of three features: (1) discomfort relieved by defecation, (2) onset associated with a change in frequency of stool, and (3) onset associated with a change in form of the stool. Diagnosis further categorizes the syndrome into one of three subtypes: diarrhea predominant, constipation predominant, or mixed. Small intestinal bacterial overgrowth (SIBO) has been described in a significant number of patients with IBS, particularly diarrhea-predominant IBS (Ghoshal et al., 2010a). Positive hydrogen or lactulose breath tests have been reported in 22% to 54% of patients with IBS (Ford and Spiegel, 2009; Lombardo et al., 2010). Prevalence of CD has been reported as fourfold greater in individuals diagnosed with IBS than in individuals without IBS, likely because of misdiagnosis of IBS, and screening for CD has been determined to be cost-effective in this population (Ford and Spiegel, 2009).
Pathophysiology
The normal enteric nervous system is sensitive to the presence, chemical composition, and volume of foods in the GIT, and also responds to a variety of inputs from the central nervous system (see Chapter 1). Increased awareness and sensitivity of the GIT to internal and external stimuli and altered motility appear to be primary features of IBS (Malagelada, 2006). Persons with IBS have heightened enteric sensitivity and motility in response to usual GI and environmental stimuli. They react more significantly than normal persons to intestinal distention, dietary changes, and psychosocial factors. IBS is considered a functional disorder, because it is a diagnosis by exclusion and is based on symptoms, not structural or biochemical abnormality. It is commonly described as a “brain-gut disorder” because of the association with serotonin.
The mediators of GI responses may be abnormal secretion of peptide hormones or signaling agents (e.g., neurotransmitters secreted in response to the hormones); but altered handling of intestinal gas, microbial flora, SIBO, and other contributors affect some forms of IBS. Post-infectious IBS typically appears abruptly after gastroenteritis and is essentially managed with the same approach as other forms of IBS (Ghoshal et al., 2010b). In addition to stress and dietary patterns, factors that may worsen symptoms include (1) excess use of laxatives and other over-the-counter medications; (2) antibiotics; (3) caffeine; (4) previous GI illness; and (5) lack of regularity in sleep, rest, and fluid intake. In patients with a strong family history of allergy, hypersensitivity to certain foods may aggravate IBS; a trial of food elimination and challenge may be justified (see Chapter 27).
Medical Management
The first step in management of IBS and other functional GI disorders includes first validating the reality of the patient’s complaints and establishing an effective clinician-patient relationship. Care should be tailored to help the patient deal with the symptoms and the factors that may trigger them. Education, medications, pain management, counseling, and diet each play a role in care. Depending on the predominant pattern and severity of the symptoms, medications may include those that affect GI motility, visceral hypersensitivity, or psychological symptoms. Relaxation and stress-reduction techniques may also be useful.
Osmotic laxatives are commonly used to treat constipation, although they have not been thoroughly studied. Agents that affect how the GIT responds to serotonin (5-hydroxytryptophane [5-HT], a major mediator in the sensory and motility functions of the enteric nervous system) are under investigation. Two major 5-HT receptors, 5-HT3 antagonists, and 5-HT4 agonists have been targeted for use in treating patients with different forms of IBS. 5-HT3 antagonists have shown some success in women with diarrhea-predominant IBS, whereas 5-HT4 agonists serve as prokinetic agents that stimulate peristalsis of the small and large intestine and are used in the management of constipation-predominant IBS. A number of other agents are being evaluated. Low-dose loperamide is commonly effective in patients who have diarrhea-predominant IBS.
Antispasmotic agents have been used to treat pain associated with IBS, but have not been thoroughly studied in randomized trials. Tricyclic antidepressants in low doses have been shown to reduce symptoms in some cases.
Medical Nutrition Therapy
The goals of nutrition therapy for IBS are to ensure adequate nutrient intake, tailor the diet for the specific GI pattern of IBS, and explain the potential roles of foods in the management of symptoms. There is little scientific evidence for restricting particular foods. Large meals and certain foods may be poorly tolerated, such as excess quantities of dietary fat, caffeine, lactose, fructose, sorbitol, and alcohol. This is especially true in persons with diarrhea-predominant IBS or mixed IBS.
Most fiber studies in the IBS population to date have numerous short-comings, such as a strong placebo effect (Heizer et al., 2009). Some patients with constipation-predominant IBS may benefit from fiber in the form of bulk laxatives (e.g., psyllium) (Bijkerk et al., 2009). Supplementation of insoluble fiber, such as wheat bran, may actually worsen symptoms. Consumption of adequate fluid is recommended, especially when powdered fiber supplements are used.
Food intolerances and allergies should be evaluated objectively because patients may unnecessarily limit large groups of foods, resulting in frustration and an incomplete diet (Kalliomäki, 2005; Seibold, 2005). In clinical practice, it can be very difficult to determine if a patient’s symptoms are truly from an adverse reaction to food. Systematically eliminating and reintroducing foods can be useful in determining if a patient is truly reacting to a food. A double-blind, placebo-controlled food challenge may be helpful but it is time-consuming and labor intensive (Heizer et al., 2009). See Chapter 27.
Foods with fiber, resistant starches, and oligosaccharides may serve as prebiotic foods, which favor the maintenance of healthy microflora and resistance to pathogenic infections. Results of initial studies on the use of prebiotic and probiotic supplements have been mixed. More studies comparing types of organisms, doses, and subtypes of IBS are needed. Additionally, potential benefits of prebiotics may be outweighed by poor absorption.
Some probiotic supplements may offer benefits in IBS. However, the randomized controlled trials that have been conducted have been small and have produced variable results depending on the type and dose of the probiotic as well as the individual population studied (Aragon et al., 2010). One study evaluated different doses of Bifidobacterium infantis in women diagnosed with IBS (Whorwell et al., 2006). The group treated with the higher dose of probiotic reported a significant improvement in abdominal pain or discomfort, bloating and distension, sensation of incomplete evacuation, passage of gas, straining, and bowel habit satisfaction.
A diet low in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) has been theorized to be useful (Shepherd et al., 2008). The low FODMAP diet limits foods that contain fructose, lactose, fructo- and galactooligosaccharides (fructans and galactans), and sugar alcohols (sorbitol, mannitol, xylitol and maltitol). FODMAPs are poorly absorbed in the small intestine, highly osmotic, and rapidly fermented by bacteria. Limiting the amount of FODMAPs per meal has been shown to reduce GI symptoms in patients with IBS (Gibson and Shepherd 2010). However, a cutoff value for acceptable amounts of FODMAPs has not been well defined, and is likely patient specific. See Table 29-5 for foods containing FODMAPs as well as dietary instructions.
TABLE 29-5
Foods Containing FODMAPs, and Low FODMAP Diet Instructions

FODMAP, Fermentable oligo-, di-, and monosaccharides and polyols.
Adapted from Gibson PR, Shepherd SJ: Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach, J Gastroenterol Hepatol 25:252, 2010.
Peppermint oil also shows promise. One randomized controlled trial showed significant improvement in abdominal symptoms for individuals supplementing with peppermint oil (Ford et al., 2008).
The nutrition practitioner can work with the person with IBS to identify his or her concerns and perceptions, review the characteristics of the disease and the potential role of various foods, and teach the client how to reduce food-related symptoms. Sometimes clients become trapped in a vicious cycle in which anxiety about food, GI distress, and social embarrassment leaves them with an unnecessarily restrictive diet, declining nutrition status, and worsening symptoms. Reassurance and the gradual return to a good diet with limitations of only irritating foods can greatly improve quality of life.
Diverticular Disease
Diverticulosis is the condition of having saclike herniations (diverticula) in the colonic wall. The incidence of diverticulosis increases with age. Sigmoid involvement occurs in almost all cases; right-sided colonic involvement occurs in Asians, but it is rare in whites. Most persons are asymptomatic. However, 15% to 20 % of persons with diverticulosis experience colicky pain; approximately 5% experience inflammation and diverticulitis.
Pathophysiology
The cause of diverticulosis has not been clearly elucidated. A combination of colonic structure, motility, genetics, and a lifelong low-fiber intake results in increased intracolonic pressures (Parra-Blanco, 2006; Salzman and Lillie, 2005). The pressures result from attempts to propel small, dry, hard fecal material through the lumen of the bowel. Theoretically, circular muscles completely close around the fecal material when the stools are small and longitudinal muscles contract, attempting to push the contents distally. Increased pressure allows herniations of the mucosal wall to develop through weaker segments of the colon. See Fig. 29-3. This theory is supported by multiple human and animal studies. In general, diverticular disease is relatively rare in countries where a high-fiber diet is part of the lifelong pattern, and increasing where there is “Westernization” of the diet with high intake of refined foods (Salzman and Lillie, 2005). Lack of exercise may also contribute.
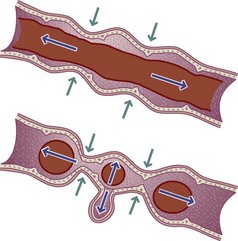
FIGURE 29-3 Mechanism by which low-fiber, low-bulk diets might generate diverticula. Where the colon contents are bulky (top), muscular contractions exert pressure longitudinally. If the fecal contents are small in diameter (bottom), contractions can produce occlusion and exert pressure against the colon wall, which may produce a diverticular hernia.
Medical and Surgical Treatment
Complications of diverticular disease range from painless, mild bleeding and altered bowel habits to diverticulitis. Diverticulitis includes a spectrum of inflammation, abscess formation, acute perforation, acute bleeding, obstruction, and sepsis. Treatment typically includes antibiotics and oral intake as tolerated. A modified diet or bowel rest may be indicated based on the patient’s degree of illness, desire to eat, and likelihood of imminent surgery (Salzman and Lillie, 2005). Colon cleansers that cause hard stools, constipation, and straining are not recommended. Approximately 10% to 25% of patients with diverticulosis develop diverticulitis, and approximately one fourth to one third of those admitted to hospitals require surgery.
Medical Nutrition Therapy
At one time it was thought that “roughage” (dietary fiber) aggravated diverticular disease; thus the classic diet therapy was low in fiber. It is now recognized that a high-fiber diet, in combination with adequate hydration, promotes soft, bulky stools that pass more swiftly and require less straining with defecation. High-fiber intakes have been found to relieve symptoms for most patients, and exercise appears to aid in preventing constipation.
Patients may require extensive encouragement to adopt the high-fiber approach. Fiber intake should be increased gradually because it may cause bloating or gas. These side effects usually disappear within 2 to 3 weeks. Recommended intakes of dietary fiber, preferably from foods, are 25 g/day for adult women and 38 g/day for men. If an individual cannot or will not consume the necessary amount of fiber, methylcellulose and psyllium fiber supplements have been used with good results. Adequate fluid intake (e.g., 2 to 3 L daily) should accompany the high-fiber intake.
During an acute flare of diverticulitis, a low-residue diet or PN may be required initially, followed by a gradual return to a high-fiber diet. Historically, health care providers have advised patients with diverticular disease to avoid seeds, nuts, or skins of plant matter to prevent complications or after bouts of diverticulitis. A recent 18-year study found no association between nut, corn, or popcorn consumption and diverticular bleeding (Strate et al., 2008). In fact, the researchers reported an inverse association between nut and popcorn consumption and the risk of diverticulitis. There is no data to support restriction.
Intestinal Polyps and Colon Cancer
In the United States and worldwide, colorectal cancer is the third most common cancer in adults and is also the second most common cause of cancer death. However, the number of new cases of colon cancer decreased 3% for men and 2.2% for women during the past decade. There are approximately 142,500 new cases of colorectal cancer per year, and the incidence is higher in men than women (National Cancer Institute and U.S. National Institutes of Health, 2010). The highest rates are seen in whites of northern European origin. Rates in Africa and Asia are lower, but they tend to rise with migration and westernization.
Pathophysiology
Factors that increase the risk of colorectal cancer include family history, long-term presence of IBD, familial polyposis, adenomatous polyps, and several dietary components. Polyps are considered precursors of colon cancers (see Chapter 37 for more details). Patterns of dietary practices rather than specific nutrients may be more predictive of the risk of developing colorectal cancer. Dietary risk factors include increased meat, fat, and alcohol intake; obesity; and inadequate intake of several micronutrients, fruits, vegetables, and whole grains. Food preparation methods may also influence the carcinogenic potential of meats and fatty foods (McGarr et al., 2005; Raju and Cruz-Correa, 2006).
Use of aspirin and nonsteroidal antiinflammatory agents and exercise appear to be protective (Raju and Cruz-Correa, 2006).
Micronutrients considered protective in epidemiologic and cohort studies include vitamin D, folate, calcium, and selenium. There have been several types of supportive studies regarding the protective role of fruits and vegetables as a group, individual plant foods, high-fiber grains, ω-3 fatty acids, several antioxidants, and phytochemicals; but the data are not always consistent. The use of prebiotics and probiotics alters colonic microflora, induces glutathione transferase, increases butyrate content of the stool, reduces toxic and genotoxic compounds, and in animal models reduces the development of some precancerous lesions (McGarr et al., 2005).
Medical Management
Patients diagnosed with colorectal polyps or cancer may require moderate to significant interventions, including medications, radiation therapy, chemotherapy, colonic surgery, or enteral or PN support.
Medical Nutrition Therapy
Recommendations from cancer organizations that publish public health messages or consensus statements include notations that target colon cancer. These recommendations typically include sufficient exercise; weight maintenance or reduction; modest and balanced intake of lipids; adequate intake of micronutrients from fruits, vegetables, legumes, whole grains, and dairy products; and limited use of alcohol. Supplements are normally encouraged if the diet is not adequate. The diet for cancer survivors typically follows these prevention guidelines (see Chapter 37).
Nutritional Consequences of Intestinal Surgery
Small Bowel Resections and Short-Bowel Syndrome
Short-bowel syndrome (SBS) can be defined as inadequate absorptive capacity resulting from reduced length or decreased functional bowel after resection. A loss of 70% to 75% of small bowel usually results in SBS, defined as 100-120 cm of small bowel without a colon, or 50 cm of small bowel with the colon remaining. A more practical definition of SBS is the inability to maintain nutrition and hydration needs with normal fluid and food intake, regardless of bowel length.
Patients with SBS often have complex fluid, electrolyte, and nutritional management issues (Parrish, 2005). Consequences of SBS include malabsorption of micronutrients and macronutrients, frequent diarrhea, steatorrhea, dehydration, electrolyte imbalances, weight loss, and growth failure in children. Other complications include gastric hypersecretion, oxalate renal stones, and cholesterol gallstones. Individuals who eventually need long-term PN have increased risk of catheter infection, sepsis, cholestasis, and liver disease, and reduced quality of life associated with chronic intravenous nutrition support (Diamanti et al., 2007).
Pathophysiology
The most common reasons for major resections of the intestine in adults include Crohn’s disease, radiation enteritis, mesenteric infarct, malignant disease, and volvulus (Parrish, 2005). In the pediatric population most cases of SBS result from congenital anomalies of the GIT, atresia, volvulus, or necrotizing enterocolitis.
Duodenal Resection: Resections of the duodenum (≈10 in) are rare, which is fortunate as it is the preferred site for absorption of key nutrients such as iron, zinc, copper, and folate. The duodenum is a key player in the digestion and absorption of nutrients, as it is the portal of entry for pancreatic enzymes and bile salts. See Chapter 1.
Jejunal Resections: The jejunum (6-10 ft) is responsible for a large portion of nutrient absorption. Normally most digestion and absorption of food and nutrients occurs in the first 100 cm of small intestine. Jejunal enterohormones play key roles in digestion and absorption. Cholecystokinin (CCK) stimulates pancreatic secretion and gallbladder contraction, and secretin stimulates secretion of bicarbonate from the pancreas. Gastric inhibitory peptide slows gastric secretion and gastric motility, whereas vasoactive inhibitory peptide inhibits gastric and bicarbonate secretion. What remains to be digested or fermented and absorbed are small amounts of sugars, resistant starch, fiber, lipids, dietary fiber, and fluids. After jejunal resections, the ileum typically adapts to perform the functions of the jejunum. The motility of the ileum is comparatively slow, and hormones, secreted in the ileum and colon help to slow gastric emptying and secretions. Because jejunal resections result in reduced surface area and faster intestinal transit, the functional reserve for absorption of micronutrients, excess amounts of sugars (especially lactose), and lipids is reduced.
Ileal Resections: Significant resections of the ileum, especially the distal ileum, produce major nutritional and medical complications. The distal ileum is the only site for absorption of bile salts and the vitamin B12-intrinsic factor complex. The ileum also absorbs a major portion of the 7-10 L of fluid ingested and secreted into the GIT daily (see Chapter 1). The ileocecal valve, at the junction of the ileum and cecum, maximizes nutrient absorption by controlling the rate of passage of ileal contents into the colon and preventing reflux of colonic bacteria, which may decrease risk for SIBO.
Although malabsorption of bile salts may appear to be benign, it creates a cascade of consequences. If the ileum cannot “recycle” bile salts secreted into the GIT, hepatic production cannot maintain a sufficient bile salt pool or the secretions to emulsify lipids. The gastric and pancreatic lipases are capable of digesting some triglycerides to fatty acids and monoglycerides, but, without adequate micelle formation facilitated by bile salts, lipids are poorly absorbed. This can lead to malabsorption of fats and fat-soluble vitamins A, D, E, and K. In addition, malabsorption of fatty acids results in their combination with calcium, zinc, and magnesium to form fatty acid–mineral soaps, thus leading to their malabsorption as well. To compound matters, colonic absorption of oxalate is increased, leading to hyperoxaluria and increased frequency of renal oxalate stones. Relative dehydration and concentrated urine, which are common with ileal resections, further increase the risk of stone formation (see Chapter 36).
The colon (≈5 ft long) is responsible for reabsorbing 1-1.5 L of electrolyte-rich (particularly sodium and chloride) fluid each day, but is capable of adapting to increase this capacity to 5-6 L daily. Preservation of colon is key to maintaining hydration status. If the patient has any colon left, malabsorption of bile salts can act as a mucosal irritant, increasing colonic motility with fluid and electrolyte losses. Consumption of high-fat diets with ileal resections and retained colon may also result in the formation of hydroxy fatty acids, which also increase fluid loss. Cholesterol gallstones occur because the ratio of bile acid, phospholipid, and cholesterol in biliary secretions is altered. Dependence on PN increases the risk of biliary “sludge,” secondary to decreased stimulus for evacuation of the biliary tract (see Chapter 30).
Medical and Surgical Management of Resections
The first step in management is assessment of the remaining bowel length from patient records or interview. Assessment should quantify dietary intake as well as stool and urine output over 24 hours. Medications and hydration status should be assessed. Medications may be prescribed to slow GI motility, decrease secretions, or treat bacterial overgrowth. The primary “gut slowing” medications include loperamide, and, if needed, narcotic medications. Somatostatin and somatostatin analogs; glucagon-like polypeptide 2; growth hormone; and other hormones with antisecretory, antimotility, or trophic actions have been studied for use to slow both motility and secretions. Surgical procedures such as creation of reservoirs (“pouches”) to serve as a form of colon, intestinal lengthening, and intestinal transplant have been performed to help patients with major GI resections (Shatnawei et al., 2010). Intestinal transplant is very complex and is reserved for gut failure, or when patients develop significant complications from PN.
Medical Nutrition Therapy
Most patients who have significant bowel resections require PN initially to restore and maintain nutrition status. The duration of PN and subsequent nutrition therapy will be based on the extent of the bowel resection, the health of the patient, and the condition of the remaining GIT. In general, older patients with major ileal resections, patients who have lost the ileocecal valve, and patients with residual disease in the remaining GIT do not fare as well. Enteral feeding provides a trophic stimulus to the GIT; PN is used to restore and maintain nutrient status. Some may require lifetime PN to maintain adequate fluid and nutrition status.
The more extreme and severe the problem, the slower the progression to a normal diet. Small, frequent, mini meals (6 to 10 per day) are likely to be better tolerated than larger feedings (Matarese et al., 2005; Parrish, 2005). Tube feeding may be useful to maximize intake when a patient would not typically eat, such as nighttime (see Chapter 14). Because of malnutrition and disuse of the GIT, the digestive and absorptive functions of the remaining GIT may be compromised, and malnutrition itself will delay postsurgical adaptation. The transition to more normal foods may take weeks to months, and some patients may never tolerate normal concentrations or volumes of foods.
Maximum adaptation of the GIT may take 1-2 years after surgery. Adaptation improves function, but it does not restore the intestine to normal length or capacity. Whole nutrients are the most important stimuli of the GIT. Other nutritional measures have also been studied as a means of hastening the adaptive process and decreasing malabsorption, but their evidence for use is limited. For example, glutamine is the preferred fuel for small intestinal enterocytes and thus may be valuable in enhancing adaptation. Nucleotides (in the form of purines, pyrimidine, ribonucleic acid) may also enhance mucosal adaptation, but unfortunately they are often lacking in parenteral and enteral nutritional products. SCFAs (e.g., butyrate, propionate, acetate) produced from microbial fermentation of carbohydrate and fibers are major fuels for the colonic epithelium.
Patients with jejunal resections and an intact ileum and colon will likely adapt quickly to normal diets. A normal balance of protein, fat, and carbohydrate sources is satisfactory. Six small feedings with avoidance of lactose, large amounts of concentrated sweets, and caffeine may help to reduce the risk of bloating, abdominal pain, and diarrhea. Because the typical American diet may be nutritionally lacking and use of some micronutrients may be marginal, patients should be advised that the quality of their diet is of utmost importance. A multivitamin and mineral supplement may be required to meet all their nutritional needs.
Patients with ileal resections require increased time and patience in the advancement from parenteral to enteral nutrition. Because of losses, fat-soluble vitamins, calcium, magnesium, and zinc may need to be supplemented. Dietary fat may need to be limited, especially in those with remaining colon. Small amounts at each feeding are more likely to be tolerated and absorbed.
MCT products add to the caloric intake and serve as a vehicle for lipid-soluble nutrients. Because boluses of MCT oil (e.g., taken as a medication in tablespoon amounts) may add to the patient’s diarrhea, it is best to divide the doses equally in feedings throughout the day. Fluid and electrolytes, especially sodium, should be provided in small amounts and frequently.
In patients with SBS an oral diet or enteral nutrition plus the use of gut-slowing medications should be maximized to prevent dependence on PN. Frequent meals, removal of osmotic medications and foods, use of oral hydration therapies, and other interventions should be pursued. In some cases, overfeeding in an attempt to compensate for malabsorption results in further malabsorption, not only of ingested foods and liquids but also of the significant amounts of GI fluids secreted in response to food ingestion. Patients with an extremely short bowel may depend on parenteral solutions for at least part of their nutrient and fluid supply. Small, frequent snacks provide some oral gratification for these patients, but typically they can supply only a portion of their fluid and nutrient needs (see Chapter 14 for discussion of home PN).
Small Intestine Bacterial Overgrowth
Small intestine bacterial overgrowth (SIBO) is a syndrome characterized by over-proliferation of bacteria within the small bowel. There are a number of physiologic processes that normally limit the amount of small intestine bacterial colonies. Gastric acid, bile, and pancreatic enzymes have bacteriostatic and bactericidal action within the small bowel. Normal propulsive action of bowel motility “sweeps” bacteria into the distal bowel. The ileocecal valve prevents migration of the large numbers of colonic bacteria into the small intestine. SIBO has also been referred to as “blind loop syndrome” because one cause of bacterial overgrowth can result from stasis of the intestinal tract as a result of obstructive disease, strictures, radiation enteritis, or surgical procedures that leaves a portion of bowel without normal flow (a blind loop or Roux limb).
Pathophysiology
Frequently, more than one of the normal homeostatic defenses must be impaired before small intestine bacteria overproliferate to the point that symptoms develop. Chronic use of medications that suppress gastric acid allow more bacteria to survive passage into the small bowel. Liver diseases or chronic pancreatitis can decrease the production or flow of bile and pancreatic enzymes into the bowel. Gastroparesis, narcotic medications, or bowel dysmotility disorders decrease peristalsis and can impair the ability to propel bacterial to the distal bowel. Surgical resection of the distal ileum and ileocecal valve can result in retrograde proliferation of colonic bacteria.
One of the most common symptoms of SIBO is chronic diarrhea from fat maldigestion. Bacteria within the small bowel deconjugate bile salts resulting in impaired formation of micelles and thus impaired fat digestion and steatorrhea. Carbohydrate malabsorption occurs because of injury to the brush border secondary to the toxic effects of bacterial products and consequent enzyme loss. The expanding numbers of bacteria use the available vitamin B12 and other nutrients for their own growth, and the host becomes deficient. Bacteria within the small bowel produce folic acid as a byproduct of their metabolism, and vitamin B12 deficiency with normal or elevated serum folic acid is common. Bloating and distention are also frequently reported in SIBO, resulting from the action of bacteria on carbohydrates with production of hydrogen and methane within the small bowel.
Medical Treatment
Treatment is directed toward control of the bacterial growth with antibiotics, probiotics, prebiotics, and in some cases surgical modification of the blind loop.
Medical Nutrition Therapy
Part of the problem with bacterial overgrowth in the small intestine is that carbohydrates reaching the site where microbes are harbored serve as fuel for their proliferation, with subsequent increased production of gases and organic acids. At least theoretically a diet that limits refined carbohydrates that are readily fermented such as refined starches and sugars (e.g., lactose, fructose, alcohol sugars) and substitutes whole grains, and vegetables, can limit the proliferation and increase motility. See Table 29-5: Low FODMAP Diet.
Limited studies are available as to the effectiveness of diets and probiotic and prebiotic materials in the prevention and treatment of altered GI motility, strictures, abnormal anatomy of the GIT, and the presence of opportunistic organisms in the colon (C. difficile and other organisms). Because vitamin B12 may be lost in fermentation and some dietary nutrients may be lacking, an assessment of the medical problem and the patient’s dietary intake is in order. If bile salts are being degraded, as in the case of blind loop syndrome, MCTs may be helpful if they provide a source of lipid and energy.
Fistulas
A fistula is an abnormal passage between two organs or between an organ and the skin. An enterocutaneous (EC) fistula is an abnormal passage beginning at the bowel and exiting at the skin. Fistulas may occur as a result of prenatal developmental error, trauma, surgery, or inflammatory or malignant disease processes. Most EC fistulas occur after surgery and usually manifest 7-10 days postoperatively. Fistulas of the intestinal tract can be serious threats to nutrition status because large amounts of fluid and electrolytes are lost and malabsorption and infection can occur.
Medical Treatment
Fluid and electrolyte balance must be restored, infection must be brought under control, and aggressive nutrition support may be necessary to permit spontaneous closure or to maintain optimal nutritional status prior to surgical closure.
Medical Nutrition Therapy
Nutrition management of patients with EC fistulas can be very challenging. Either PN, tube feeding, oral diet, or a combination, are used in patients with fistulas. The success rate of the chosen method depends on multiple variables, including the location of the fistula, the presence of abscesses or obstructions, the length of functional bowel, the ability to manage fistula output, and the patient’s overall condition (Willcutts, 2010).
Ileostomy or Colostomy
Patients with severe UC, Crohn’s disease, colon cancer, or intestinal trauma frequently require the surgical creation of an opening from the body surface to the intestinal tract to permit defecation from the intact portion of the intestine. When the entire colon, rectum, and anus must be removed, an ileostomy, or an opening of the ileum at the abdominal wall, is performed. If only the rectum and anus are removed, a colostomy can provide entrance to the colon. In some cases a temporary opening may be made to allow surgery and healing of more distal parts of the intestinal tract.
The opening, or stoma, eventually shrinks to the size of a nickel. The output from the stoma depends on its location. The consistency of the stool from an ileostomy is liquid (effluent), whereas that from a colostomy ranges from mushy to fairly well formed. Stool from a colostomy on the left side of the colon is firmer than that from a colostomy on the right side. Odor is a major concern of the patient with an ileostomy or colostomy; however, an ileostomy effluent usually has a weakly acidic odor that is not unpleasant.
Medical Treatment
Patients with a permanent colostomy or ileostomy require sympathetic understanding from the entire health care team. Acceptance of the condition and the problems involved in maintaining bowel regularity is usually difficult. Nursing personnel, especially enterostomal therapists (who specialize in the care of stomas), play a major role in supporting and teaching patients with ostomies. Having these patients meet other people who have undergone similar surgery may help with the adjustment. Eventually they may be encouraged by the realization that in the future they will not have the multiple hospitalizations or chronic disabilities that accompanied their intestinal disease.
Medical Nutrition Therapy
Malodorous stools may be caused by steatorrhea or partial digestion or bacterial fermentation of foodstuffs. SCFAs, sulfur-containing compounds, ammonia, methane, and other end products can produce odors. Because an individual patient may have different flora, types and amounts of gases and odors may differ among patients and with different dietary practices. Patients learn to observe their stools to determine which foods to eliminate; this differs from one patient to the next.
Foods that tend to cause odor from a colostomy are legumes, onions, garlic, cabbage, eggs, fish, some medications, as well as some vitamin and mineral supplements. Persistent odor may be attributable to poor stoma hygiene or to an ileostomy complication that allows bacterial overgrowth in the ileum. Deodorants are available, and modern pouch appliances are odor-proof. Gas production may cause the pouch to become tense and distended, and accidental dislodgment is likely. The nutritional recommendations for reducing flatulence, presented in this chapter, may be helpful for patients with colostomies.
The normal output from the ileum to the colon is in the range of 750 mL to 1.5 L in the intact GIT. After a colectomy and creation of an ileostomy, adaptation occurs within 1 to 2 weeks. Fecal output will lessen, and stools will become less liquid. Reduction in stool volume may not occur to the same extent in patients who have had an ileal resection in addition to a colectomy. Depending on the amount of ileum resected, the ileal output may be 1.5 to 5 times greater than that of the patient who has had only a colectomy. Patients with ileostomies have an above-average need for salt and water to compensate for excessive losses in stool. Inadequate water intake can result in small urine volumes and a predisposition for renal calculi. A normal diet provides adequate sodium, and patients should be instructed to drink at least 1 L more than their ostomy output daily.
The patient with a normal, functional ileostomy usually does not become nutritionally depleted. Surgical procedures such as ileostomy may require specific dietary changes but no greater energy intake; caloric expenditures in these patients are similar to those of normal subjects. Those who also undergo resection of the terminal ileum need vitamin B12 supplementation or intravenous injections. Patients with an ileostomy may have low vitamin C and folate intakes because of low fresh vegetable and fruit intakes, and they require supplementation.
Patients with ileostomies should be guided by physiologic reasons for intolerance of foods and not by anecdotal reports. Because gastric emptying may be more rapid and foodstuffs are not fermented to the same degree after colostomy, absorption of nutrients may be somewhat better from cooked, shredded, or pureed fruits and vegetables. Because it is possible for a food bolus to get caught at the point where the ileum narrows as it enters the abdominal wall, it is important to warn the patient to avoid very fibrous vegetables and to chew all food well. Other than this, patients with either an ileostomy or a colostomy should be encouraged to follow their normal diet, omitting only foods known to cause problems.
Ileal Pouch after Colectomy
As an alternative to creation of an ileostomy for persons who have had their colons removed, surgeons can create a reservoir using a portion of the distal ileum. Folds of the ileum are joined together to create a small pouch, which is then connected to the rectum and ileum. This is called an ileal pouch–anal anastomosis. The most common pouch is the J-pouch, but S and W pouches are sometimes created using additional folds of ileum. Like the colon, the pouch develops a microflora capable of at least partially fermenting fiber and carbohydrate. Because the reservoir is smaller than the colon, bowel movements are likely to occur more frequently than normal (i.e., between four and eight times daily). A Koch pouch is a type of applianceless ileostomy that uses an internal reservoir with a one-way valve, constructed from a loop of intestine, that is attached to the abdominal wall with a skin-level stoma. Patients must insert a tube or catheter into the stoma to open the valve and allow drainage of ileostomy contents. The technical difficulties of surgical construction and the potential for complications has led to decreased use of the Koch pouch in favor of the J-pouch with anal anastomosis.
Medical Treatment
Vitamin B12 injections are usually required because, as in SBBO, the microbes may compete for and bind intraluminal vitamin B12. Other problems commonly reported include obstruction; inflammation of the pouch; and increased stool output, frequency, and gas. The incidence of obstruction may be lessened with attention to particle size of fibrous foods, chewing thoroughly, and consuming small meals frequently throughout the day. Stool frequency and volume do not return to normal, however. The normal, intact colon absorbs 80% to 90% of the liter or so of fluid entering from the ileum, leaving only 100 to 200 mL. After surgery the remaining ileum does adapt to a small degree by increasing efficiency of fluid absorption, but even after adaptation, fluid output is always in the range of 300 to 600 mL.
Pouchitis is an inflammation of the mucosal tissue forming the pouch. The associated pathologic changes have been described as being somewhat similar to that of IBD (e.g., UC). The cause of pouchitis is not entirely clear, but it may be related to selected bacterial overgrowth, bile salt malabsorption, or insufficient SCFA production. Antibiotics are the primary form of therapy, but experiments with different types of dietary fiber, prebiotics and probiotics, and other nutrient components have been used successfully to reduce the incidence (Guarner, 2005; Meier and Steuerwald, 2005).
Medical Nutrition Therapy
There are few controlled studies regarding diet and ileal pouch. Food intolerances are common but relatively mild (Steenhagen et al., 2006). The same dietary measures that are used by others to reduce excessive stool output (reduced caffeine, lactose avoidance in lactase-deficient persons, limitation of fructose and sorbitol) will likely reduce stool volume and frequency in persons with pouches. Adequate fluid and electrolyte intake are especially important because of the increase in intestinal losses.
Rectal Surgery
Nutrition care after rectal surgery such as hemorrhoidectomy should be directed toward maintaining an intake that will allow wound repair and prevent infection of the wound by feces. The frequency of stools is minimized by the use a minimum-residue diet (see Table 29-1). Chemically defined diets are low in residue, and their use can reduce stool volume and frequency to as little as 50 g every 6 days, making the surgical construction of a temporary colostomy unnecessary. A normal diet is resumed after healing is complete, and the patient is instructed about the benefits of eating a high-fiber diet to avoid constipation in the future.
University of Virginia Division of Gastroenterology and Hepatology
www.uvahealth.com/celiacsupport
Crohn’s and Colitis Foundation of America
National Digestive Diseases Information Clearinghouse
http://digestive.niddk.nih.gov/ddiseases/pubs/ileostomy/index.htm
http://www.nlm.nih.gov/medlineplus/tutorials/colostomy/htm/index.htm
References
American Gastroenterological Association (AGA) Institute. Medical position statement on the diagnosis and management of celiac disease. Gastroenterol. 2006;131:1977.
Aragon, G, Graham, DB. Probiotic therapy for irritable bowel syndrome. Gastroenterol Hepatol. 2010;6:39.
Atia, AN, Buchman, AL. Oral rehydration solutions in non-cholera diarrhea: a review. Am J Gastroenterol. 2009;104:2596.
Azpiroz, F. Intestinal gas dynamics: mechanisms and clinical relevance. Gut. 2005;54:893.
Barrett, JS, Gibson, PR. Clinical ramifications of malabsorption of fructose and other short-chain carbohydrates. Practical Gastroenterol. 2007;31:51.
Bell, TJ, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of US and European Patient Survey (PROBE 1). Pain Med. 2009;10:35.
Besselink, MGH, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651.
Beyer, PL, et al. Fructose intake at current levels in the United States may cause gastrointestinal distress in normal adults. J Am Diet Assoc. 2005;105:1559.
Bezkorovainy, A. Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr. 2001;73:399S.
Biggs, WS, Dery, WH. Evaluation and treatment of constipation in infants and children. Am Fam Physician. 2006;73:469.
Bijkerk, CJ, et al. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ (Clinical Research Ed). 2009;339:b3154.
Binder, HJ. Role of colonic short-chain fatty acid transport in diarrhea. Ann Rev Physiol. 2010;72:297.
Bonamico, M, et al. Duodenal bulb biopsies in celiac disease: a multicenter study. J Pediatr Gastroenterol Nutr. 2008;47:618.
Butterworth AD, et al: Probiotics for induction of remission in Crohn’s disease, Cochrane Database Syst Rev (Online) CD006634, 2008.
Caprilli, R, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations. Gut. 2006;55(Suppl 1):i36.
Case, S. The gluten-free diet: how to provide effective education and resources. Gastroenterol; 2005;128.
Chand, N, Mihas, AA. Celiac disease: current concepts in diagnosis and treatment. J Clin Gastroenterol. 2006;40:3.
Cook, IJ, et al. Chronic constipation: overview and challenges. Neurogastroenterol Motil. 2009;21(Suppl 2):1.
DeLegge, MH, Berry, A. Enteral feeding: should it be continued in the patient with clostridium difficile enterocolitis? Practical Gastroenterol. 40, 2009.
Diamanti, A, et al. Prevalence of life-threatening complications in pediatric patients affected by intestinal failure. Transplant Proc. 2007;39:1632.
Dotan, I, Rachmilewitz, D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr Opin Gastroenterol. 2005;21:426.
Dray, X, Marteau, P. The use of enteral nutrition in the management of Crohn’s disease in adults. JPEN. 2005;29:S166.
El-Matary, W, et al. Diagnostic characteristics of given video capsule endoscopy in diagnosis of celiac disease: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2009;19:815.
Emmanuel, AV, et al. Pharmacological management of constipation. Neurogastroenterol Motil. 2009;21(Suppl 2):41.
Ford, AC, et al. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651.
Ford, AC, Spiegel, BMR. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279.
Ford, AC, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313.
Gao, XW, et al, Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010. Accessed 2010 from. http://www.ncbi.nlm.nih.gov/pubmed/20145608
Garsed, K, Scott, BB. Can oats be taken in a gluten-free diet? A systematic review. Scand J Gastroenterol. 2007;42:171.
Ghoshal, UC, et al. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16:40.
Ghoshal, UC, et al. Bugs and irritable bowel syndrome: the good, the bad and the ugly. J Gastroenterol Hepatol. 2010;25:244.
Gibson, PR, Shepherd, SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252.
Green, PH. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol. 2009;7:1210.
Guandalini, S. Update on the role of probiotics in the therapy of pediatric inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6:47.
Guarner, F. Inulin and oligofructose: impact on intestinal diseases and disorders. Br J Nutr. 2005;93(Suppl 1):S61.
Guyonnet, D, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475.
Harder, H, et al. Effect of high- and low-caloric mixed liquid meals on intestinal gas dynamics. Dig Dis Sci. 2006;51:140.
Heizer, WD, et al. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109:1204.
Hickson, M, et al. Use of probiotic Lactobacillus preparation to prevent diarrhea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
Holubar SD, et al: Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis, Cochrane Database Syst Rev 6:CD001176, 2010.
Holzer, P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009;155:11.
Howell, MD, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Int Med. 2010;170:784.
Hutchinson, JM, et al. Long-term histological follow-up of people with coeliac disease in a UK teaching. QJM. 2010;103:511.
Järvelä, IE. Molecular genetics of adult-type hypolactasia. Ann Med. 2005;37:179.
Jobse, P, et al. Collagenous colitis: description of a single centre series of 83 patients. Eur J Int Med. 2009;20:499.
Kagnoff, MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41.
Kalliomäki, MA. Food allergy and irritable bowel syndrome. Curr Opin Gastroenterol. 2005;21:708.
Kulkarni, SV, et al. Opportunistic parasitic infections in HIV/AIDS patients presenting with diarrhea by the level of immunosuppression. Indian J Med Res. 2009;130:63.
Lawrence, SJ, et al. Probiotics for recurrent Clostridium difficile disease. J Med Microbiol. 2005;54:905.
Levri, KM, et al. Do probiotics reduce adult lactose intolerance? A systematic review. J Fam Pract. 2005;54:613.
Linsky, A, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Int Med. 2010;170:772.
Lochs, H. To feed or not to feed? Are nutritional supplements worthwhile in active Crohn’s disease? Gut. 2006;55:306.
Lombardo, L, et al. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010;8:504.
Lustig, RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110:1307.
Malagelada, JR. A symptom-based approach to making a positive diagnosis of irritable bowel syndrome with constipation. Int J Clin Pract. 2006;60:57.
Mallon P, et al: Probiotics for induction of remission in ulcerative colitis, Cochrane Database Syst Rev CD005573, 2007.
Matarese, LE, et al. Short bowel syndrome: clinical guidelines for nutrition management. Nutr Clin Pract. 2005;20:493.
McGarr, SE, et al. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. J Clin Gastroenterol. 2005;39:98.
Meier, R, Steuerwald, M. Place of probiotics. Curr Opin Crit Care. 2005;11:318.
Morken, MH, et al. Intestinal gas in plain abdominal radiographs does not correlate with symptoms after lactulose challenge. Eur J Gastroenterol Hepatol. 2007;19:589.
Mueller, C, Macpherson, AJ. Layers of mutualism with commensal bacteria protect us from intestinal inflammation. Gut. 2006;55:276–284.
Müller, S, et al. Anti-saccharomyces cerevisiae antibody titers are stable over time in Crohn’s patients and are not inducible in murine models of colitis. World J Gastroenterol. 2005;11:6988.
Müller-Lissner, S. The pathophysiology, diagnosis, and treatment of constipation. Deutsches Ärzteblatt International. 2009;106:424.
Nachman, F, Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig Liver Dis 2010. Accessed 2010 from. http://www.ncbi.nlm.nih.gov/pubmed/20399159
Nath, SK. Tropical sprue. Curr Gastroenterol Reports. 2005;7:343.
National Cancer Institute and U.S. National Institutes of Health. Colon and rectal cancer. Accessed 1 July 2010 from http://www.cancer.gov/cancertopics/types/colon-and-rectal, 2010.
O’Keefe, SJD. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16:139.
Parra-Blanco, A. Colonic diverticular disease: pathophysiology and clinical picture. Digestion. 2006;73(Suppl 1):47.
Parrish, CR. The clinician’s guide to short bowel syndrome. Pract Gastroenterol. 2005;29:67.
Penner, R, Fedorak, RN. Probiotics and nutraceuticals: non-medicinal treatments of gastrointestinal diseases. Curr Opin Pharmacol. 2005;5:596.
Pillai A, Nelson R: Probiotics for treatment of Clostridium difficile-associated colitis in adults, Cochrane Database Syst Rev CD004611, 2008.
Rajendran, N, Kumar, D. Role of diet in the management of inflammatory bowel disease. World J Gastroenterol. 2010;16:1442.
Raju, R, Cruz-Correa, M. Chemoprevention of colorectal cancer. Dis Colon Rectum. 2006;49:113.
Ramkumar, D, Rao, SSC. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100:936.
Rasinperä, H, et al. The C/C-13910 genotype of adult-type hypolactasia is associated with an increased risk of colorectal cancer in the Finnish population. Gut. 2005;54:643.
Robayo-Torres, CC, et al. Disaccharide digestion: clinical and molecular aspects. Clin Gastroenterol Hepatol. 2006;4:276.
Rostom, A, et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterol. 2005;128:S38.
Salzman, H, Lillie, D. Diverticular disease: diagnosis and treatment. Am Fam Phys. 2005;72:1229.
Sánchez-Pérez, M, et al. Toxic megacolon secondary to Clostridium difficile colitis. Case report. Revista De Gastroenterologia De Mexico. 2010;75:103.
Sanders, DSA. Mucosal integrity and barrier function in the pathogenesis of early lesions in Crohn’s disease. J Clin Pathol. 2005;58:568.
Sanderson, IR, Croft, NM. The anti-inflammatory effects of enteral nutrition. J Parenter Enteral Nutr. 2005;29:S134.
Schiller, LR. Nutrients and constipation: cause or cure? Pract Gastroenterol. 2008;32:43.
Schroeder, MS. Clostridium difficile-associated diarrhea. Am Fam Physician. 2005;71:921.
Seibold, F. Food-induced immune responses as origin of bowel disease? Digestion. 2005;71:251.
Seidner, DL, et al. An oral supplement enriched with fish oil, soluble fiber, and antioxidants for corticosteroid sparing in ulcerative colitis: a randomized, controlled trial. Clin Gastroenterol Hepatol. 2005;3:358.
Shatnawei, A. Intestinal failure management at the Cleveland Clinic. Arch Surg. 2010;145:521.
Shaukat, A, Systematic review: effective management strategies for lactose intolerance. Ann Int Med 2010. Accessed 2010 from. http://www.ncbi.nlm.nih.gov/pubmed/20404262
Shepherd, SJ, et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765.
Shih, DQ, Targan, SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2008;14:390.
Simondi, D, et al. A retrospective study on a cohort of patients with lymphocytic colitis. Revista Española De Enfermedades Digestivas. 2010;102:381.
Steenhagen, E, et al. Sources and severity of self-reported food intolerance after ileal pouch-anal anastomosis. J Am Diet Assoc. 2006;106:1459.
Strate, LL, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300:907.
Suchy, FJ, et al. National Institutes of Health Consensus Development Conference: lactose intolerance and health. Ann Int Med. 2010;152:792.
Szajewska, H, et al. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367.
Teitelbaum, JE. Probiotics and the treatment of infectious diarrhea. Pediatr Infect Dis J. 2005;24:267.
Travis, SPL, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut. 2006;55(Suppl 1):i16.
Turner D et al: ω 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease, Cochrane Database Syst Rev CD006320, 2009.
Tuteja, AK, Biskupiak, J. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil. 2010;22:424.
Tysk, C, et al. Diagnosis and management of microscopic colitis. World J Gastroenterol. 2008;14:7280.
Venkatasubramani, N, et al, Obesity in pediatric celiac disease. J Pediatr Gastroenterol Nutr 2010. Accessed 2010 from. http://www.ncbi.nlm.nih.gov/pubmed/20479683
Whelan, K, Myers, CE. Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr. 2010;91:687.
Whorwell, PJ, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581.
Willcutts, K. The art of fistuloclysis: nutritional management of enterocutaneous fistulas. Pract Gastroenterol. 2010.
Zezos, P, et al. Hyperhomocysteinemia in ulcerative colitis is related to folate levels. World J Gastroenterol. 2005;11:6038.
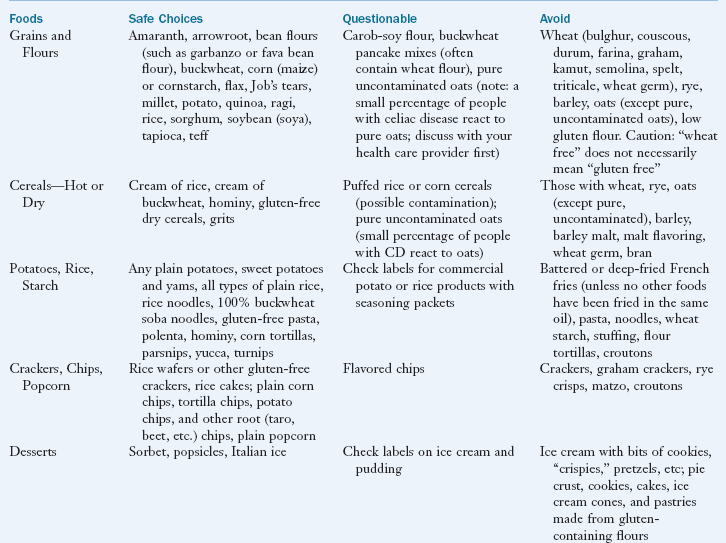
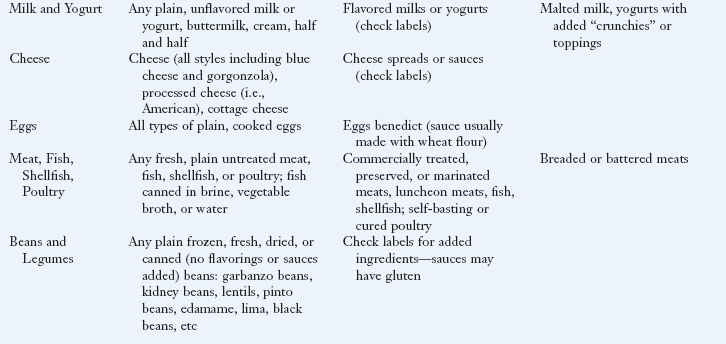
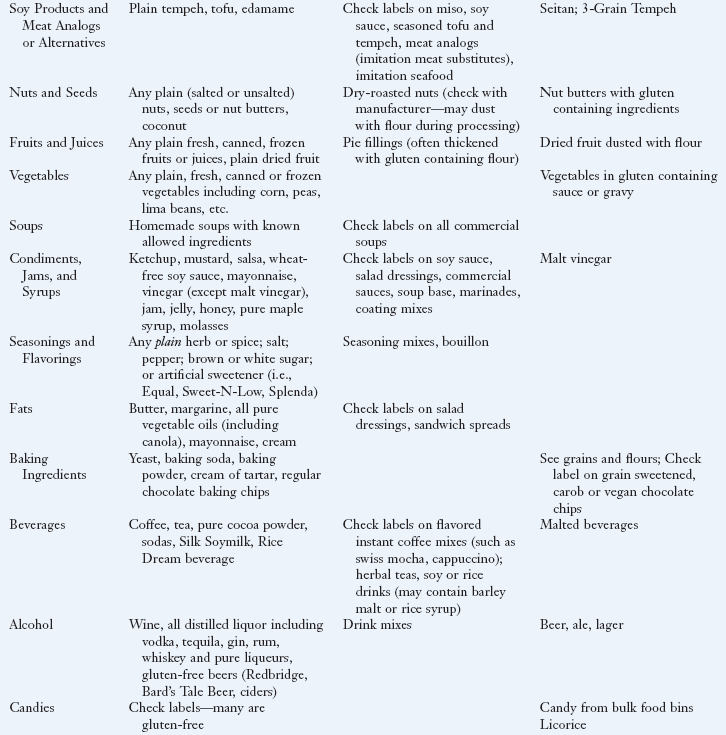
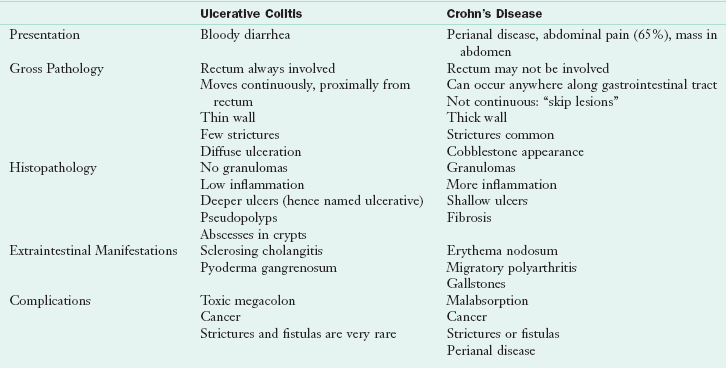
 cup vanilla ice cream
cup vanilla ice cream