Medical Nutrition Therapy for Adverse Reactions to Food
Food Allergies and Intolerances
Sections of this chapter were written by Sherry Hubbard, RD, for the previous edition of this text.
There is growing evidence that adverse reactions to food (ARFs) are more prevalent than in the past, with a defined increase in severity and scope. Changes in the modern diet and environmental influences interacting with genetic predisposition have been implicated in the escalation of ARFs and the parallel rise in other chronic disorders such as asthma and autoimmune diseases. Estimates suggest that 20% of the population alters their diet because of perceived ARFs (Sicherer and Sampson, 2010). ARFs are implicated in many conditions as a result of the involvement of major organ systems, including the digestive tract, the respiratory system, and the skin. The management of ARFs is complex because of the diverse response by which the body reacts to food constituents and the multifaceted nature of the mechanisms involved. The clinical relevance of ARFs should be carefully assessed and evaluated in the nutrition care process, because this can greatly affect a person’s quality of life.
definitions
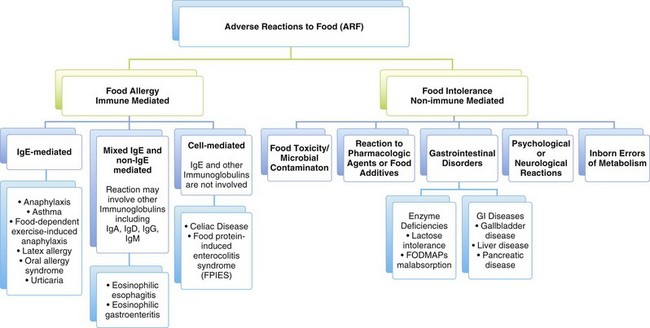
It is important to comprehend the language of ARFs because it can be a source of confusion and misunderstanding. The following definitions are used in this chapter. Adverse reactions to food (ARFs) encompass both food allergies and food intolerances, both of which can result in distressing symptoms and adversely affect health.
Food allergy or hypersensitivity is an adverse immune mediated reaction to a food, usually a food protein or hapten (a small molecule that can elicit an immune response only when attached to a large carrier protein). The symptoms are caused by the individual’s unique response to the food, not by the food itself. For example, a person who is allergic to a food such as peanuts can develop life-threatening anaphylaxis after consuming a very small amount of peanuts, whereas other individuals have no adverse response to eating peanuts. Furthermore, the symptoms of the allergy in one individual can differ greatly from those in another in response to the same food. It has been estimated that food allergy affects up to 4% of the population, with a greater prevalence in childhood that is estimated at almost 8% (Chafen et al., 2010; National Institute of Allergy and Infectious Diseases [NIAID], 2010). Symptoms of food allergy are noted in Box 27-1.
Food intolerance is an adverse reaction to a food that does not involve the immune system and occurs because of the way the body processes the food or components in the food. It may be caused by a toxic, pharmacologic, metabolic, digestive, psychologic, or idiopathic reaction to a food or chemical substances in that food. For example, an individual can be intolerant of milk not because of an allergy to milk protein, but because of an inability to digest lactose; see Chapter 29 for discussion of lactose intolerance).
Food sensitivity refers to an ARF or component of the food when it is not clear whether the reaction is due to food allergy or intolerance. The umbrella term food sensitivity has been used interchangeably with food allergy and food intolerance, but does not give any indication as to the cause of the person’s symptoms (Joneja, 2003). An emerging hypothesis called sensitivity-related illness poses that an individual who is exposed to some type of toxicant or insult may then become sensitive to food, inhalants, or chemicals (Genuis, 2010).
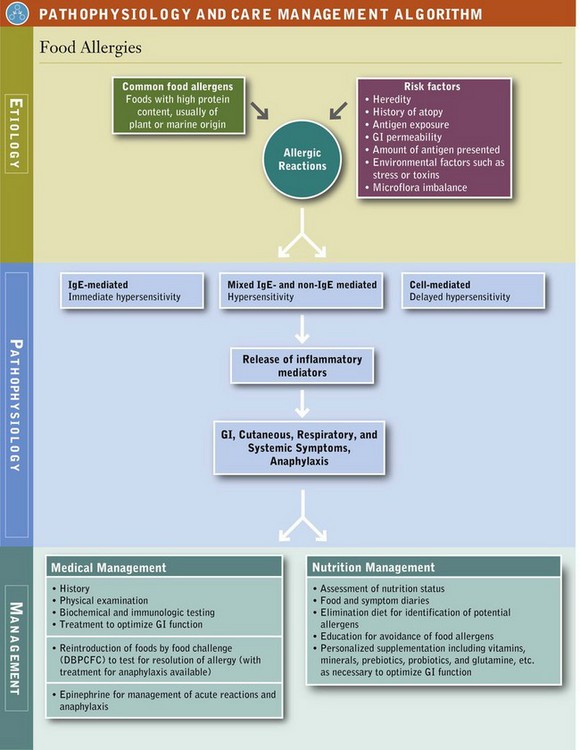
ARFs illustrate the critical importance of appreciating “biochemical uniqueness” as a core clinical concept in an integrative nutrition assessment. Numerous factors, including genetics, intestinal barrier integrity, resident intestinal microflora, stress, psychological factors, and environmental and physiological influences, affect an individual’s unique response to food or a component of food and its ultimate interpretation by the body as either “friend” or “foe” (Figure 27-1).
The immune system functions to clear the body of foreign substances or antigens such as viruses, bacteria, cancer cells, and other pathogens and disease-causing agents. Normally, when food antigens interact with cells of the immune system, they are dispelled from the body without an adverse reaction, unlike when a pathogenic virus or bacterium is expelled and there is a noticeable inflammatory reaction from microbial infection. Food comes from foreign material, either plant or animal, that our immune systems typically perceive as “foreign but safe” as a result of a process of oral mucosal tolerance that occurs when we digest and absorb food. Tolerance indicates that an individual is clinically and immunologically tolerant of the food (NIAID and NIH, 2010).
Etiology
Food allergy has a hereditary component that it is not yet clearly defined. Atopy is a condition of genetic predisposition to produce excessive immunoglobulin (Ig) E antibodies in response to an allergen. Atopic individuals, usually identified in infancy, and confirmed by positive skin-prick test, are characterized by severe IgE-mediated reactions to dander, pollens, food, or other environmental factors, which present as food allergy, atopic dermatitis (eczema), atopic conjunctivitis, atopic rhinitis, or asthma. A study of Finnish children showed that up to the age of 4 years, children with two parents reporting any type of allergic reactions were three times as likely to have a food allergy as were children with allergy-free parents. Children with one allergic parent were twice as likely to have a food allergy (Pyrhönen et al., 2010). However, genetic susceptibility alone does not completely explain the prevalence of food allergy; other environmental influences (external, maternal, and gastrointestinal [GI] environment) and interactions between the host and the environment need to be considered.
Antigen Exposure
Exposure to food antigens in the digestive tract, followed by immune regulation or suppression, is a prerequisite for the development of tolerance to the food, or oral tolerance (Burks et al., 2008). Food allergy is believed to occur when oral tolerance fails. Ongoing research is focused on how oral tolerance develops and is maintained (Brandtzaeg, 2010). The amount of antigen and environmental factors also influence the development of food allergy. The effects of food antigens and other antigens may be additive. Clinical symptoms of food allergy may increase when inhalant allergies are exacerbated by seasonal or environmental changes. Similarly, the effects of environmental factors, such as early exposure to microbes, toxins, tobacco smoke, stress, exercise, and cold may exacerbate the clinical symptoms of food allergy.
Maternal Diet and Early Infant Feeding
The initial exposure to an antigen may occur during pregnancy or lactation or in early infancy. The food does not have to be ingested by the infant directly. Postnatal sensitization may occur with exposure to food allergens by inhalation, skin contact, or ingestion. In fact, there is increasing evidence that many allergic reactions to food are initiated by exposure to food antigens through routes other than the digestive tract. (Lack, 2008). Food allergen sensitization can be a result of exposure to a food antigen through breast milk. More likely, it occurs from environmental exposure (skin or air) causing initial sensitization that is followed by continued exposure to antigens from the mother’s milk.
Gastrointestinal Microbiota
GI permeability and microbiota are critical influences in allergic disease. Both increased intestinal permeability; also referred to as “leaky gut” and the presence of excessive amounts of abnormal bacteria or “dysbiosis” influence gut immune function. This gut immune function is in the gut-associated lymphoid tissue (GALT), the largest mass of lymphoid tissue in the body. GI permeability is thought to be greatest in early infancy and to decline with intestinal maturation. A leaky gut with altered permeability and possibly dysbiosis allows antigen penetration and presentation to the GALT lymphocytes and sensitization (Groschwitz and Hogan, 2009). Other conditions such as GI disease, malnutrition, prematurity, and immunodeficiency may also be associated with increased gut permeability and risk of development of food allergy. See Fig. 39-3 in Chapter 39.
Pathophysiology
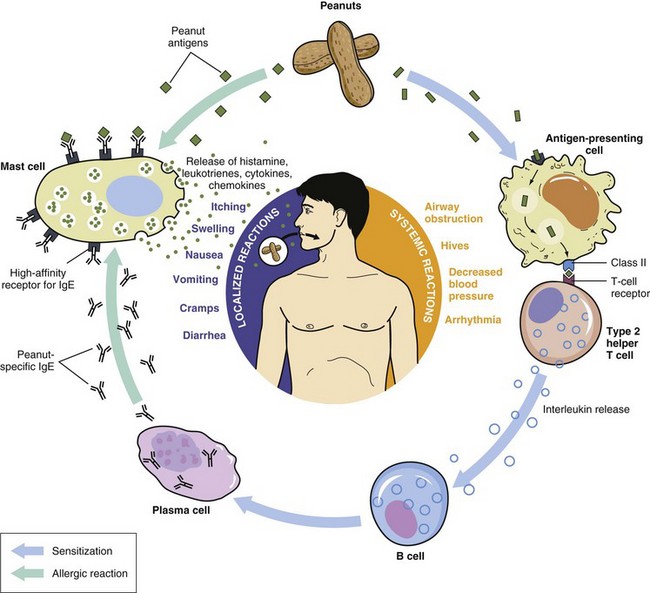
In allergy, the immune system unleashes defensive chemicals (inflammatory mediators) in response to something (in this case, food) that should not cause a response. The immune system mistakenly identifies food as a threat and mounts an attack against it. Sensitization occurs on the first exposure of the immune cells to the allergen and there are no symptoms of reaction. Thereafter, whenever that same foreign material enters the body, the immune system responds to this threat in the same manner. Because individuals can develop immunologic sensitization as evidenced by the production of allergen-specific IgE without having clinical symptoms upon exposure to those foods, a IgE-mediated food allergy requires both the presence of sensitization and the development of specific signs and symptoms on exposure to the food. Sensitization alone is not sufficient to define food allergy (NIAID and NIH, 2010; Boyce et al., 2011; Vickery et al., 2011) (Figure 27-2).
The combination of an allergen with allergen-specific IgE fixed to tissue mast cells or circulating basophils causes the release of chemical mediators, including histamine, enzymes, lipid-derived prostaglandins, interleukins, and others. When released, these inflammatory mediators can cause itching, pain, reddening, tissue swelling, contraction of smooth muscle, vasodilation, and fluid secretions. Manifestations, which are most often systemic, may involve multiple organs and systems (see Pathophysiology and Care Management Algorithm: Food Allergies).
Immune System Cells
Lymphocytes are the “command and control” cells of the immune system and include two important groups: B cells, arising from stem cells in the bone marrow, and T cells. T cells also originate from stem cells, but are later transported to the thymus gland where they mature. These two types of cells function as the basis for the humoral immune response and for cell-mediated immunity
Monocytes and macrophages are primarily phagocytes that engulf foreign material, break it apart, and display specific molecules of the material on their surfaces. making them antigen-presenting cells (APC). The antigenic component displayed on the surface is an epitope, and is recognized by T cells. T cells respond by generating a cytokine message that stimulates their differentiation.
T cells, often called T “helper” cells (Th cells) differentiate into Th-1 cells or Th-2 cells which have different roles in the immune response under different circumstances, and secrete different sets of cytokines. Th1 cells regulate the activities of the B cells to produce antibodies and direct damage to target cells, resulting in the destruction of antigens. This function is useful in defending against bacteria, viruses, and other pathogenic cells. Th2 cells mediate the allergic response by regulating the production by B cells of IgE sensitized to food allergens.
These allergen-specific antibodies attach themselves to mast cells (in the lungs, skin, tongue and linings of the nose and intestinal tract) or basophils (in circulation). On second exposure to the allergen, the sensitized IgE antibodies and the allergen form antibody-antigen complexes that activate granulocytes
Granulocytes contain intracellular granules, or small vessels that are storage depots for defense chemicals or inflammatory mediators that protect the body from invading pathogens. When these granulocytes are activated, they degranulate and release these inflammatory mediators such as histamine, prostaglandins, leukotrienes, and cytokines. Each of the mediators has a specific effect on local tissues and at distant sites, resulting in the symptoms of allergy. Degranulation of other granulocytes such as neutrophils and eosinophils attracted to the reaction site by mediators such as chemokines causes release of additional inflammatory chemicals, which further enhance the allergic response, resulting in increased symptom severity.
The humoral immune response is mediated by antibodies and has a major role in food allergy. Antigen-specific antibodies are produced by the B lymphocytes (B cells) in response to the antigen presented. The union of an antigen and antibody results in the degranulation of mast cells or basophils and the release of chemical inflammatory mediators, or direct cellular damage, which in turn causes symptoms. Each antibody contains a globulin protein; because of their association with the immune system, they are referred to as immunoglobulins (Ig). Five distinct classes of antibodies have been identified: IgA, IgD, IgE, IgG, and IgM. Each Ig has a specific function in immunity reactions (Box 27-2).
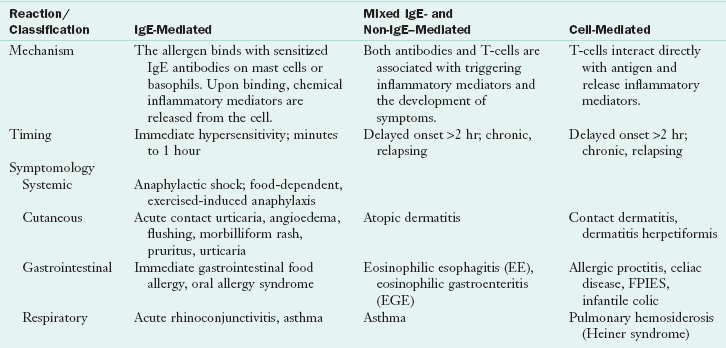
IgE-Mediated Reactions
IgE-mediated food allergic reactions usually are rapid in onset, occurring within minutes to a few hours of exposure. The methods of exposure include inhalation, skin contact and ingestion. A wide range of symptoms has been attributed to this type of food allergy; and frequently involve the GI, cutaneous, or respiratory systems, and can range from mild hives to life-threatening anaphylaxis Table 27-1).
A few foods account for the vast majority of IgE-mediated allergic reactions: milk, eggs, peanuts, tree nuts, soy, wheat, fish, and shellfish. However, any food is capable of eliciting an IgE-mediated reaction after a person has been sensitized to it. Food-induced anaphylaxis, oral allergy syndrome (OAS), immediate GI hypersensitivity, latex-food allergy syndrome, and exercise-induced anaphylaxis are IgE-mediated immune reactions.
Food-Induced Anaphylaxis
Food-induced anaphylaxis is an acute, often severe, and sometimes fatal immune response that usually occurs within a limited period following exposure to an antigen. Multiple organ systems are affected. Symptoms can include respiratory distress, abdominal pain, nausea, vomiting, cyanosis, arrhythmia, hypotension, angioedema, urticaria, diarrhea, shock, cardiac arrest, and death. The vast majority of fatal anaphylactic reactions to foods in adults in North America involve peanuts or tree nuts; in children anaphylaxis to other foods such as egg and milk are reported more frequently. People with known anaphylactic reactions to food allergens should carry and be prepared to use injected epinephrine via an injectable adrenaline at all times. Epinephrine is the drug of choice to reverse an allergic reaction, even with asthma (Franchini et al, 2010). Delayed use of epinephrine has been associated with an increased risk of biphasic reactions in which a recurrence of symptoms 4 to 12 hours after the initial anaphylactic reaction may be fatal.
Oral Allergy Syndrome
The oral allergy syndrome (OAS), or pollen-food syndrome (PFS), results from direct contact with food allergens and is confined almost exclusively to the oropharynx and rarely involves other target organs (Hoffmann and Burks, 2008). Sensitization occurs through the respiratory system or skin (Fernandez-Rivas et al., 2006). The reaction to foods occurs as a result of the presence of an antigen within the food with a structure similar to that of the pollen. The primary sensitization is to the pollen, not the food. Symptoms are rapid and appear within minutes upon ingestion of the offending food. They include itching and irritation of oral tissues along with swelling and sometimes blisters, and most often subside within 30 minutes. OAS is most commonly seen in individuals with coexisting seasonal allergic rhinitis to birch, ragweed, or grass pollens following ingestion of specific fruits, vegetables, and some nuts. (Geroldinger-Simic et al, 2011.) The cooked fruit or vegetable is often tolerated because the reactions are caused by predominately heat-labile proteins cross-reacting with pollen proteins. However, this is not always the case and a careful history and questioning about the food is important (Kondo and Urisu, 2009). Box 27-3 lists the foods and pollens most commonly linked with OAS.
Immediate Gastrointestinal Hypersensitivity
A range of GI symptoms can develop within minutes to 2 hours after ingestion of an offending food and may include nausea, vomiting, diarrhea, and abdominal pain. More than half of patients with food allergy have GI reactions that are mediated by IgE-dependent and independent mechanisms involving mast cells, eosinophils, and other immune cells (Bischoff and Crowe, 2005). The GI manifestations can involve eosinophilic esophagitis, or may occur in conjunction with allergic symptoms outside the digestive tract such as respiratory (wheezing) or skin symptoms (urticaria) (Sicherer and Sampson, 2010).
Profilins and Allergy to Latex
Allergy to latex or natural rubber is common. Up to 50% of latex-sensitive individuals can respond with allergic symptoms when exposed to cross-reactive food allergens (Blanco, 2003). In the pollen-food-latex syndrome, cross reactivity occurs between the food antigen and various latex antigens found in many items such as latex gloves, clothing, children’s toys, and other articles in the immediate environment.
Profilins are proteins, present in all eukaryotic cells, that form allergens from pollen, latex, and plant foods (Santos and Van Ree, 2011). As a food allergen, profilin usually elicits mild oral allergy syndrome, is not modified by processing, but may be linked with allergy to melons, banana, tomato, and many of the OAS foods (see Box 27-3) (Santos and Van Ree, 2011; Condemi, 2002). Potential therapies such as curcumin may help control the allergic response (Kurup et al., 2007).
Food-Dependent, Exercise-Induced Anaphylaxis
Food-dependent, exercise-induced anaphylaxis (FDEIA) is a distinct form of physical allergy in which an offending food triggers an anaphylactic reaction only when the individual exercises within 2 to 4 hours after eating (DuToit, 2007). The food may not be problematic in the absence of exercise. It appears to be more common in adolescent girls and young women. Celery, seafood, a gliadin component in wheat, and other foods have been reported as offending agents (Morita et al., 2009). In FDEIA, the combination of a sensitizing food and exercise precipitates symptoms, possibly related to increased GI permeability, blood-flow redistribution, and increased osmolality (Robson-Ansley and Toit, 2010). The prevalence and causative agents and effective methods of diagnosis in FDEIA continue to be explored.
Non–IgE-Mediated or Mixed Antibody Reactions
The contribution of non–IgE-mediated immunologic reactions to food hypersensitivity continues to be investigated. It has been postulated that non-IgE antigen-antibody complexes may play a role in food-related inflammatory diseases. These include various forms of colitis, enteritis with bleeding, malabsorptive disorders, ulceration, and chronic pneumonitis (Heiner syndrome). Non–IgE-mediated antibody reactions may also be involved in celiac disease, protein-losing enteropathies, eosinophilic esophagitis (EE), eosinophilic gastroenteritis (EGE), and ulcerative colitis. Multiple components of the immune system are likely to be involved with different underlying mechanisms.
Eosinophilic Esophagitis and Eosinophilic Gastroenteritis
EE and EGE are characterized by eosinophilic infiltration of the esophagus, stomach, or intestines with peripheral eosinophilia. Both conditions can have serious consequences and distinction is important because it may influence therapy (Rothenberg, 2004). Many studies have indicated that food allergies are responsible, and almost half of the patients who present with EGE have atopic features (Eroglu et al., 2009; Roy-Ghanta et al., 2008). Identification of specific offending allergens is not always possible. A comprehensive elimination diet may improve symptoms of EE (Kagalwalla et al., 2006; Spergel et al., 2005). EGE can occur at any age and symptoms can easily be mistaken for functional GI disorders. Nutrition assessment is important with either condition because the implementation of an elimination diet aimed at identifying and excluding food antagonists can be most helpful.
Cell-Mediated Reactions
Cell-mediated immunity is non–IgE-mediated and acts in reponse to viruses, fungi, tumor cells, and other foreign cells through its production of the controller T lymphocytes (T helper or Th cells.) Th cells are involved in most aspects of the immune response, from directing other immune cells, to responding to the recognition of a foreign antigen. However they have no cytotoxic or phagocytotic activity themselves.
When an antigen stimulates a T-cell response, the T cells produce cytokines which cause them to differentiate into Th-1 cells or Th-2 cells. Specific cytokines secreted by allergen-driven Th2 cells may induce B cells to produce IgE antibodies. The IgE antibodies attach to specific receptors on the surface of mast cells or basophils. Coupling of the specific antigen with the IgE on the mast cell or basophil surface starts a series of reactions that result in the release of the inflammatory mediators stored within the mast cell and basophil granules.
Manipulating the Th1 and Th2 immune response for allergy prevention and possible protection against Th1 type autoimmune disease and Th2-mediated atopic disease is a current area of investigation. The model of Th1 and Th2 immunity will continue to evolve, moving beyond the simplistic interpretation of protective versus allergic response in view of more recent evidence on the complexities of T helper cells and cytokine production (Durrant and Metzger, 2010).
Food Protein–Induced Enterocolitis Syndromes (FPIES)
An example of a cell-mediated reaction is food protein–induced enterocolitis syndrome (FPIES) which is most commonly seen in formula-fed infants, and is typically provoked by cow’s milk or soy protein-based formula (Mehr et al., 2009). A response to sheep or goat’s milk is less common but can also occur (Järvinen and Chatchatee, 2009.)
Occasionally FPIES is seen in breast-fed infants, presumably caused by milk proteins from the mother’s diet crossing into her milk. The infant reacts with emesis, diarrhea, poor growth, and lethargy. In protein-induced proctocolitis, bloody and mucus-laden stools are also seen. Food-specific IgE antibodies have no value in this diagnosis; confirmation of FPIES is challenging because it mimics other GI inflammatory disorders. Infants should be switched to an extensively hydrolyzed casein formula. If they do not tolerate that, they may require an elemental formula. Breast-fed infants should remain on the breast, and the mother should eliminate cow’s milk from her diet. FPIES is usually transient and resolves after a few weeks to months.
Food Intolerances
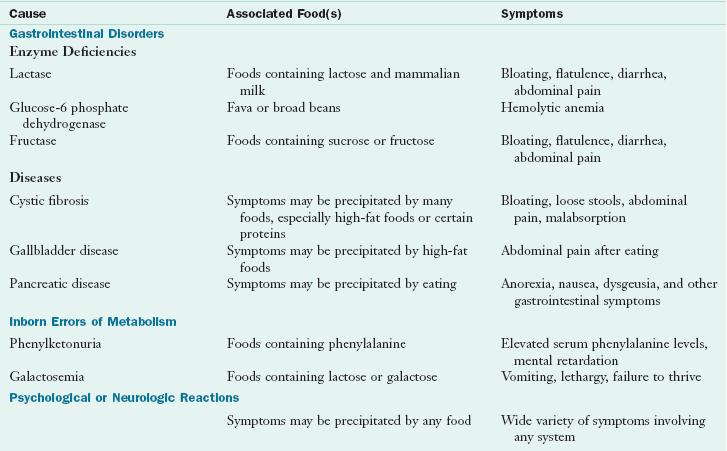
Food intolerances (nonallergic food sensitivities) are ARFs caused by nonimmunologic mechanisms including toxic, pharmacologic, metabolic, or idiosyncratic reactions. Food intolerances are much more common than food allergies. Clinically, it is important to distinguish food intolerance from immune-mediated food allergy. Symptoms caused by food intolerances are often similar to food allergy and include GI, cutaneous, and respiratory manifestations. See Table 27-2.
Lactose Intolerance
Intolerance to the disaccharide lactose is the most common ARF, and most cases result from a genetically influenced reduction of intestinal lactase. Half of the world’s population has hypolactasia (Jarvela et al., 2009). Abdominal bloating and cramping, flatulence, and diarrhea occur usually several hours following lactose ingestion. Because the symptoms are similar, lactose intolerance is often confused with allergy to cow’s milk; however, some individuals who are allergic to cow’s milk can also have respiratory or anaphylactic reactions. Deficiencies of lactase and other carbohydrate-digesting enzymes and their management are discussed further in Chapter 29.
Carbohydrate Intolerances
Carbohydrates, sugars, starches, and polysaccharides are complex in structure and must be broken down by enzymes for optimal digestion, absorption, and assimilation. Adverse reactions can occur if there is a deficiency of enzymes responsible for the breakdown of carbohydrates, especially disaccharides.
Maldigestion and malabsorption of the fructo-, oligo-, di-, and monosaccharides and polyols (FODMAPs) may also occur (Gibson and Shepherd, 2010). Included are the sugars and the polyols sorbitol, maltitol, and others. Intolerances lead to diarrhea, cramping, and flatulence. They appear to be more common in individuals who have an underlying functional GI disorder, such as irritable bowel syndrome. GI symptoms reported after the ingestion of fruit juice may be related to fructose intolerance, a problem from widespread use of high-fructose corn syrups in food manufacturing and processing (see Chapter 29 for discussion of the FODMAPs diet.) Tools are available for assessment of FODMAPs intake (Barrett and Gibson, 2010.)
Food Additives or Pharmacologic Reactions
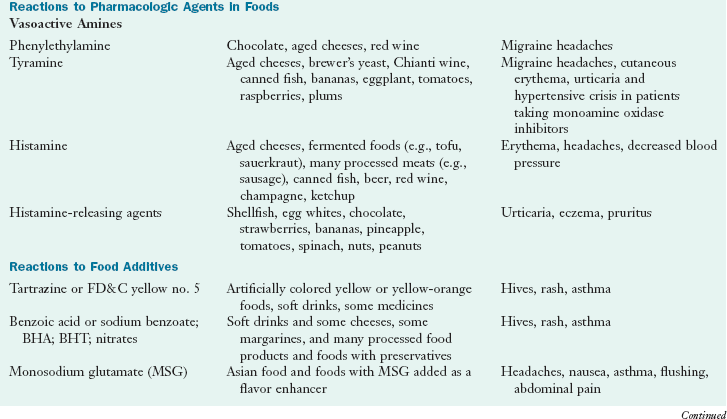
An adverse reaction may be to a food additive or pharmacologically active component in that food. Research should clarify nutritional concerns, including underlying mechanisms, genetic susceptibilities, risks from medications, food processing techniques, and food labeling. A wide range of allergic-like symptoms can result from ingestion of biogenic amines such as histamine and tyramine; salicylates; carmine (cochineal extracts); artificial food dyes and colorings such as FD & C #5; and preservatives such as benzoic acid, sodium benzoate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), nitrates, sulfites, and monosodium glutamate (MSG) (Joneja, 2003).
Ingestion of foods with a high histamine content, including fermented foods such as tofu and sauerkraut, aged cheeses, processed meats and fish, alcoholic beverages (champagne and red wine), and old food, may result in symptoms indistinguishable from food allergy, because histamine is an important mediator responsible for IgE-mediated hypersensitivity reactions. Foods such as strawberries, egg whites, shellfish, and some food additives (e.g., tartrazine) and preservatives (e.g., benzoates) stimulate histamine release from mast cells. Histamine intolerance or sensitivity may be suspected when an allergic cause has been ruled out (Maintz and Novak, 2007). A deficiency of the enzymes diamine oxidase or histamine-N-methyltransferase and a genetic defect in histamine metabolism have been implicated (Maintz and Novak, 2007).
Tyramine is formed from the amino acid tyrosine and can cause adverse reactions in individuals who are taking monoamine oxidase inhibitors (MAOIs), which inhibit the breakdown of tyramine. This is an example of a potentially serious ARF caused by a drug-food interaction. Fortunately, the MAOIs are used infrequently today. Tyramine is found in some fermented foods such as aged cheeses, wines, vinegars, and naturally in bananas, eggplant, raspberries, plums, and tomatoes. Ingestion may cause migraine headaches or chronic hives in tyramine-sensitive individuals, with the response being dose-dependent (Joneja, 2003). See Box 9-3 in Chapter 9 and Chapter 41.
Reactions to sulfites are most common in asthmatics and result in a range of symptoms in sulfite-sensitive individuals. These can include dermatitis, urticaria, hypotension, abdominal pain, diarrhea, and life-threatening asthmatic and anaphylactic reactions. Chronic respiratory and skin problems can also be due to sulfite sensitivity (Vally et al., 2009). The mechanisms remain unclear.
Adverse reactions to MSG were originally reported as the “Chinese restaurant syndrome” because of its use in Chinese cooking. Complaints of headache, nausea, flushing, abdominal pain, and asthma occurred after ingestion. Glutamates are found naturally in tomatoes, Parmesan cheese, mushrooms, and other foods. Results from double-blind, placebo-controlled food challenges (DBPCFC) found symptoms from MSG to be neither persistent, clear, consistent, nor serious (Geha et al., 2000; Williams and Woessner, 2009). Considering the debate about this common flavoring agent, dietetic practitioners should be aware of MSG sensitivity.
Food Toxins and Microbial Contaminants
Other causes of food intolerance can be mistaken for food allergy. Food toxicity or food poisoning results from microbial contamination of food and can cause nausea, vomiting, diarrhea, abdominal pain, headache, and fever. Most episodes are self-limiting and should be distinguished from food allergy through a thorough history. Pseudoallergic or anaphylactoid reactions to food can result from ingredients that mimic the effects of mast cell degranulation, but do not involve the production of antibodies (Reese et al., 2009). Some adverse reactions are triggered by physiologic reactions to foods that result from a heightened sensory response to foods.
Unclear Adverse Reactions
The role of food allergy or intolerance in behavioral disorders (anxiety, depression, and mood disorders), neurologic disorders (migraine headache), musculoskeletal disorders (fibromyalgia, chronic fatigue syndrome), irritable bowel syndrome, and many other clinical conditions is emerging. Even if a food-symptom relationship is not proven but food avoidance is perceived as helpful because of personal experience, appropriate therapy can optimize nutritional status (Hepworth, 2010). Psychological ARFs exist and are often prevalent in individuals with underlying psychiatric disorders (Kelsay, 2003).
Assessment
Diagnosis of ARFs requires identification of the suspected food or food ingredient, proof that the food causes an adverse response, and verification of immune- or nonimmune-mediated response. The first diagnostic tool is the detailed clinical history, followed by appropriate testing. Biochemical tests can rule out nonallergenic causes of symptoms. Tests that may be useful include a complete blood count and differential; stool tests for reducing substances, ova, parasites, or occult blood; breath hydrogen tests; intestinal permeability tests; genetic tests for celiac disease and gluten sensitivity profiles ; tests for small intestinal bacterial overgrowth (SIBO); and a sweat chloride test for cystic fibrosis (see Chapters 8, 28, 29 and 35). Tests to diagnose adverse reactions to food and identify the immune response should not be used alone, but rather in conjunction with history, physical, and nutrition assessment. See Table 27-3 for a complete description of tests.
TABLE 27-3
Tests Used in the Assessment of Adverse Reactions to Foods

ALCAT, Antigen leukocyte cellular antibody test; BAT, basophil activation test; CAP-FEIA, CAP-fluorescein-enzyme immunoassay; CRD, component-resolved diagnostics; DBPCFC, double-blind, placebo-controlled food challenge; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; MD, medical doctor; MRT, mediator release test; RAST, radioallergosorbent test; sIg, secretory immunoglobulin.
Immunologic Testing
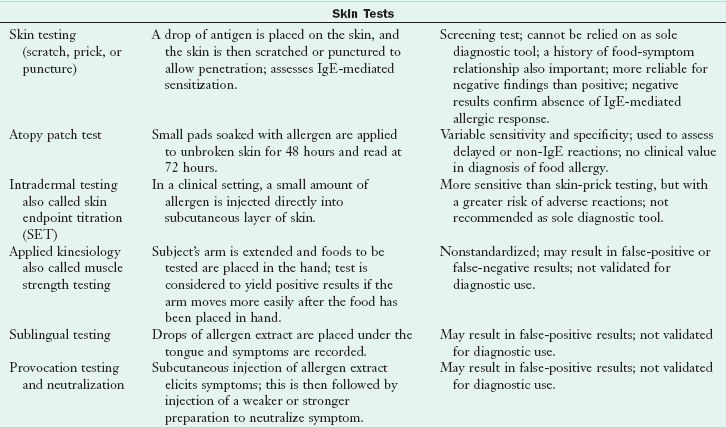
In skin-prick tests, the skin is pricked and a food allergen is placed under the skin in contact with allergen-specific IgE (bound to the surface of cutaneous mast cells). These tests are the most economic immunologic tests, providing results within 15 to 30 minutes. Comparison with the positive control (histamine) and the negative control (usually the solute used for the antigen or saline) provide parameters necessary for accurate readings (see Figure 27-3). All skin-prick tests are compared with the control wheal. Test wheals that are 3 mm greater than the negative control usually indicate a positive result. Negative skin-prick tests have good negative predictive accuracy and suggest the absence of an IgE-mediated reaction. Positive skin-prick test results, however, indicate only the possibility of food allergy. In the patient with a suspected food allergy, the skin-prick test is useful in supporting the diagnosis. For children younger than 2 years of age, the skin test is reserved to confirm immunologic mechanisms after symptoms have been confirmed by a positive test result from a food challenge or when the history of the reaction is impressive.
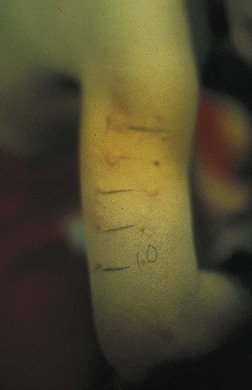
FIGURE 27-3 A skin-prick test showing the wheal and flare of the reaction to the allergen as compared with the reaction to the histamine control at the bottom.
In children with atopic dermatitis, skin-prick testing for food allergens is contraindicated because of the high reactivity of the skin, leading to false-positive reactions and the real danger of sensitization to the allergen through inflamed skin (Lack, 2008) (Figure 27-4).
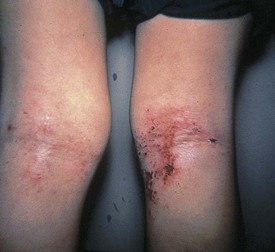
FIGURE 27-4 Atopic eczema: An immunoglobulin E–mediated skin reaction to a food allergen. Commonly seen on the back of knees and the inside of elbows.
All foods that test positive must correlate with a strong exposure history or be proven to cause allergic reactions through food challenges before they can be considered allergenic. The most common food allergens (milk, egg, peanut, soy, wheat, shellfish, fish, and tree nuts) account for most of the positive food skin-prick tests (Nowak-Wegrzyn and Sampson, 2006).
Serum Antibody Tests
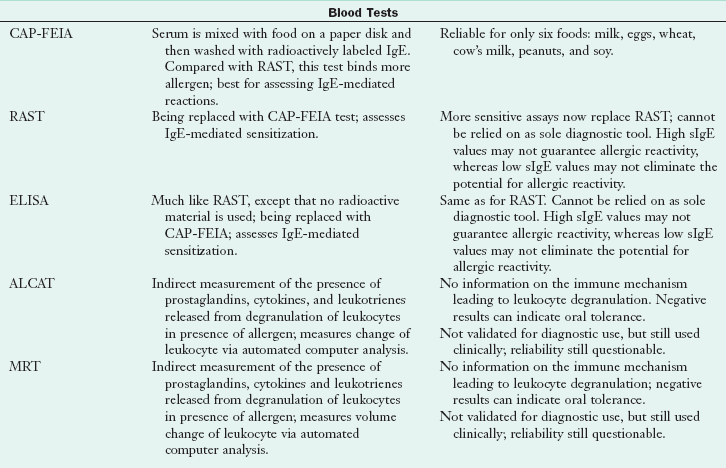
Food allergen–specific serum IgE testing is used to identify foods that may be causing the allergic response. The radioallergosorbent test (RAST) and the enzyme-linked immunosorbent assay (ELISA), both IgE tests, are being replaced by the CAP–fluorescein-enzyme immunoassay (FEIA) The CAP-FEIA is a blood test that provides a quantitative assessment of allergen-specific IgE antibodies; higher levels of antibodies are predictors of clinical symptoms. The CAP-FEIA test has been approved for only six foods: egg, milk, peanut, fish, wheat, and soy (soy is still not as predictive) (Sampson, 2004). It is fairly effective as shown by testing known food-allergic children whose food allergies had been previously proven with DBPCFCs. Test results should be followed with either food elimination and challenge or DBPCFCs to complete the diagnostic process (Sampson, 2004). It should be noted that CAP-FEIA or skin testing results for IgE sensitization may remain positive even after the child has “outgrown” the allergy, and the food can be eaten without symptoms.
Other Tests
Many attempts have been made to suggest that immunoglobulin G (IgG) is an indicator of allergy, especially measured by the IgG4 fraction of the immunoglobulin. However, a positive IgG4 response to foods only indicates repeated exposure to components that are recognized as foreign proteins by the immune system; thus the clinical usefulness of IgG4 testing is questionable (Stapel et al., 2008).
Some tests indirectly measure the amount of cytokines released by lymphocytes and granulocytes upon degranulation in response to food antigen exposure. Examples of these tests are the antigen leukocyte cellular antibody test (ALCAT) and the mediator release test (MRT). These tests do not measure IgE-mediated responses. They may be useful in identifying problematic foods in cell-mediated or delayed reactions, but should be followed up with appropriate food elimination and clinical observation of the patient. (NIAID and NIH, 2010).
Medical Nutrition Therapy
A nutrition-focused physical examination and a complete nutrition assessment (see Chapters 4, 6, 8 and Appendices 29 and 30) should be performed. Gathered information should include the time of food ingestion relative to the onset of symptoms, a description of the most recent symptoms, a list of suspected foods, and an estimate of the quantity of food required to cause a reaction. Prenatal history, early feeding practices, and exposure is also important in a thorough history.
Measurements for infants and children should be plotted on a growth chart and evaluated in relationship to earlier measurements. Because decreased weight-for-height measurements may be related to malabsorption or food allergy or intolerance, patterns of growth and their relationship to the onset of symptoms should be explored. Clinical signs of malnutrition should be assessed, including the evaluation of fat and muscle stores.
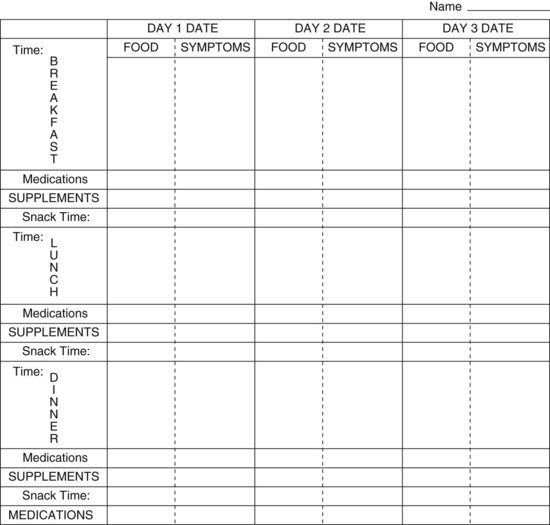
Food and Symptom Diary
A 7- to 14-day food and symptom diary is a very useful tool for uncovering ARFs (Figure 27-5). This diary can also be used to identify possible nutrient insufficiencies and deficiencies. The food and symptom diary should include the time the food is eaten, the quantity and type of food, all food ingredients if possible, the time symptoms appear relative to the time of food ingestion, and any supplements or medications taken before or after the onset of symptoms. Other influences such as stress, physical exercise, elimination and sleep patterns can provide valuable information in piecing together the factors that affect ARFs.
The location where the reaction occurred can even be informative, providing unexpected insights into possible food sources of allergen exposure. Or the information obtained can indicate something other than a food reaction. A reaction that appears to be caused by a food when the food allergen cannot be found may actually be caused by a pet or a chemical or other environmental factor. The more information obtained about the adverse reaction, the more useful the diary. The 1- to 2-week food and symptom diary also can serve as a baseline for future interventions.
Food-Elimination Diets
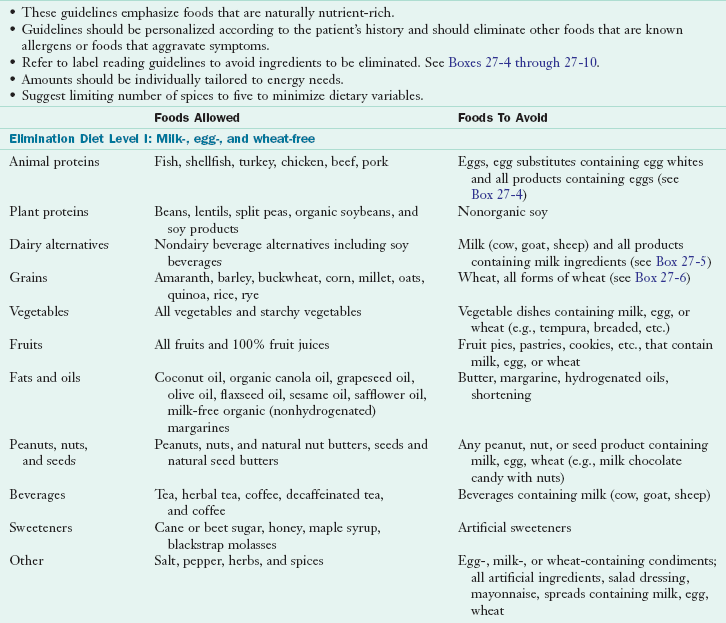
Food elimination is a useful tool in the diagnosis and management of ARFs when used in conjunction with a thorough history and nutrition assessment. With a standard elimination diet, suspect foods are eliminated from the diet for a specified period, usually 4 to 12 weeks, followed by a reintroduction and food-challenge phase. All forms (i.e., cooked, raw, and protein derivatives) of a suspected food are removed from the diet, and a food and symptom record is kept during the elimination phase. This record is used to ensure that all forms of suspected foods have been eliminated from the diet and to evaluate the nutritional adequacy of the diet. Elimination diets should be personalized, and may entail eliminating only one or two suspect foods at a time to see if there is improvement in symptoms. If multiple foods are suspected, a variation of the “strict” elimination diet shown in Table 27-4 could be used. Any food on the list that is suspect should be substituted with a food that is not likely to cause a reaction.
Elemental formulas, medical foods or hypoallergenic formulas can also be used for additional nutrition support with the elimination diet. An elemental formula provides high-quality calories in an easily digestible form and helps to optimize nutritional status. Because of low palatability and high cost, this should be reserved for the most restrictive cases.
After the designated elimination phase, foods are systematically reintroduced into the diet one at a time to determine any adverse reactions while the person is carefully monitored. If symptoms persist with careful avoidance of suspect foods, other causes for the symptoms should be considered. If a positive result has been obtained on a skin test or allergen-specific IgE blood test and symptoms improve unequivocally with the elimination of the food, that food should be eliminated from the diet until an oral food challenge is appropriate. The oral food challenge will prove or disprove a food symptom relationship. If symptoms improve with only the elimination of multiple foods, multiple food challenges are necessary.
Oral Food Challenge
An oral food challenge is conducted in a supervised medical setting once symptoms have resolved and when the person is not taking any antihistamines. Foods are challenged one at a time on different days while the person is carefully observed in a medical setting for the recurrence of symptoms, thus eliminating confusion. The three types of food challenges are an open food challenge, which allows the food to be given openly; a single-blind, placebo-controlled food challenge, in which the food is hidden from the patient with at least one placebo; and a double-blind, placebo-controlled food challenge (DBPCFC), in which the food is hidden from the patient and the clinician and is presented with at least one to three placebos. Increasing amounts of the offending food should be given every 15 to 60 minutes until there is a convincing, but not life-threatening, response. The goal is to ingest 6 to 10 g of dry food or 80 mL of liquid food mixed in a masking food the patient tolerates (Nowak-Wegrzyn and Sampson, 2006). A person with a positive challenge response must be given appropriate medications to stop symptoms and be observed for an additional 1 to 2 hours. Those who are observed to have a negative challenge response should also be observed for an additional 1 to 2 hours because a reaction may occasionally occur later than expected. The amount of food tolerated under observation can then be offered at home.
The DBPCFC provides objective results by eliminating outside influences; it is the standard when attempting to establish a food and symptom relationship and to confirm a food allergy. Each DBPCFC must be personalized. Single foods (e.g., applesauce, grape juice) or tolerated food combinations can “hide” a suspect food. The product must mask any hint of the flavor, color, or texture of the suspect food or allergen. The patient should not be able to detect the differences between the “reactive” food and the placebo food. Because severe reactions can occur during a challenge, a physician must be in attendance with emergency supplies measured and ready to be administered.
After a negative DBPCFC, an open challenge should be given. In this challenge the patient is given a serving of the suspect food. Interestingly, reactions have occurred during the open challenge that did not occur in the blind challenge. Occasionally symptoms may accompany the last presentation if the threshold is greater than indicated by the history. Most allergic reactions occur within 2 hours of the challenge. Non–IgE-mediated reactions may occur more than 24 hours after challenge. Monitoring of the patient should continue during this time.
If there is a clear history of a life-threatening anaphylactic reaction after eating a specific food, that food should not be challenged unless there is sufficient evidence that the person is no longer reacting to the allergen and skin test or allergen-specific IgE blood test results are negative, and then only in a controlled medical setting where epinephrine is available. Because of the risk of severe reactions and the lack of standardization of the testing procedure, many clinicians are questioning the use of the DBPCFC to document a food allergy reaction (Mullin et al., 2010).
Avoidance of Unsafe Food
Although many food intolerances may allow some ingestion of the offending food, food allergies usually do not. Total avoidance of the unsafe food (food allergen) is the only proven treatment for food allergy. Food immunotherapy vaccine is a possible future treatment meant to complement food allergen avoidance, but these vaccines are still experimental (Sicherer and Sampson, 2010).
Recent research has produced encouraging evidence that specific oral tolerance induction (SOTI) can be achieved by introducing the culprit food via the digestive tract in minute then increasing quantities for an extended period (Clark et al., 2009; Zapatero et al., 2008). Allergic individuals and their families need guidelines and suggestions for avoiding allergenic foods and ingredients, substituting permissible foods for restricted foods in meal planning and preparation, and selecting nutritionally adequate replacement foods (Joneja, 2007).
Additional food characteristics may be relevant. For example, recent studies suggest that 70% to 80% of young children allergic to milk or eggs can tolerate baked (heat-denatured) forms of the protein, but not the unbaked form. It is suggested that these children make IgE antibodies primarily to conformational epitopes (antigenic determinants on the surface of the food proteins that are recognized by the immune system) and represent those children who will naturally outgrow their food allergies (Sicherer and Sampson, 2010).
To help identify and avoid offending foods, allergy-specific lists that describe foods to avoid, state key words for ingredient identification, and present acceptable substitutes are useful and necessary in counseling (Boxes 27-4 through 27-8). Caretakers and school personnel working with the food-allergic child should be cautioned to read labels carefully before purchasing or serving food. The Food Allergy and Anaphylaxis Network, a nonprofit organization created to support the food-allergic child, has worked with board-certified allergists and dietitians to develop an excellent education program for day-care or school programs. Food substitutions can be challenging when working within school food program guidelines and special help may be necessary.
Food ingredients to be avoided may be hidden in the diet in unfamiliar forms. When a food-sensitive person ingests a hidden allergen, the most common reason is that the “safe” food was contaminated. This may happen as a result of using common serving utensils such as at an ice cream parlor, salad bar, or deli (where the meat slicer may be used to slice both meat and cheese). Manufacturing plants or restaurants may use the same equipment to produce two different products (e.g., peanut butter and almond butter); despite cleaning, traces of an allergen may remain on the equipment between uses. Alternatively, a restaurant may use the same oil to fry both potatoes and fish (Box 27-9).
In addition, the food may have been genetically modified, changing its allergenicity. Here again, label reading is essential. See Focus On: Genetically Modified (GM) Foods.
Another situation that may lead to the unknowing ingestion of an allergenic food occurs when one product is used to make a second product, and only the ingredients of the second product are listed on the food label. An example is the listing of mayonnaise as an ingredient in a salad dressing without specifically listing egg as an ingredient of the mayonnaise. Labels must be read often to ensure that ingredients have not changed in the processing of the food (Box 27-10).
When foods are removed from the diet, alternative nutrient sources must be provided. Table 27-5 defines the levels of nutritional risk based on the types of food removed from the diet. For example, when eggs are omitted, other foods must provide choline, vitamin D, protein, and energy.
TABLE 27-5
Nutritional Risk in food Allergy Management
| Level of Risk | Food Characteristics/Examples |
| Low risk | Any food that can easily be eliminated with minimum or no nutritional risk to the patient; protein, calorie, and nutrient consumption is adequate. Example: Avoidance of a specific fruit or vegetable |
| Moderate risk | Any food that may be encountered frequently throughout the food supply yet the elimination of which does not significantly limit food choices or vital nutrient sources; questionable adequacy of protein, calorie, and nutrient consumption. Example: Avoidance of fish, crustaceans, or tree nuts |
| Complex risk | Any food that permeates the food supply, providing a significant source of specific nutrients that are not readily available through other foods that are a part of the normal diet, the elimination of which results in a significant lifestyle and dietary change because of the difficulty of avoiding that food and products containing that food; adequate protein, calorie, and nutrient consumption unlikely. Example: Avoidance of wheat, soy, egg, milk, peanuts, or multiple foods |
Healing the Gut and Restoring Immune Balance
Because 70% of immune cells are located in the gut-associated lymphatic tissue (GALT), efforts to restore gut health should improve immune function and modulate allergic responses. Besides eliminating problematic foods, other measures include optimizing stomach acidity and enzyme function; identifying and treating intestinal pathogens such as bacteria, yeasts, and parasites; restoring intestinal barrier function; and repleting nutritional stores (see Chapters 28 and 29). Sometimes after the gut is healed it is possible to institute a rotation diet where foods identified as causing allergic reactions can be consumed on a planned “rotating” basis without symptoms developing. There is some preliminary research to suggest that rotation diets in combination with probiotics may be useful in the management of food intolerances in patients with diarrhea-dominant irritable bowel syndrome (Drisko, et al, 2006). See Clinical Insight: Rotation Diets—Where’s the Science? Are they Clinically Useful?
Nutritional Adequacy
The nutritional adequacy of the diet should be monitored on a regular basis by conducting an ongoing evaluation of the patient’s growth, nutrition status, and food records. The omission of foods from the diet on the basis of proper or improper diagnosis can and has threatened the nutritional status of the allergic individual (Noimark and Cox, 2008). Malnutrition and poor growth may occur in children who consume inadequate elimination diets. Vitamin and mineral supplementation may be needed to prevent this, especially when multiple foods are omitted. Nutrition assessment needs to be done regularly. Because food is an important part of a person’s culture, the social aspects of eating can make adherence to an elimination diet difficult. Continued support from health care providers is needed to minimize the effect of dietary changes on family and social life. The strategies listed in Box 27-11 can help families and individuals cope with food allergies.
It was previously thought that most children would “outgrow” their food allergies by 3 years of age; however, it is becoming apparent that this is not the case. Only 11% of egg-allergic and 19% of milk-allergic children resolved their allergies by 4 years of age. However, almost 80% resolved these allergies by age 16 (Savage et al., 2007; Skripak et al., 2007).
This is not true for peanut allergy, which is considered a persistent allergy lasting a lifetime for most children (Sicherer and Sampson, 2010). Sensitization procedures show some promise (Stahl and Rans, 2011.) While about 20% of children with peanut allergy will outgrow their early allergy to peanut, it appears that, once outgrown, frequent peanut ingestion is recommended to maintain the tolerance (NIAID, 2010.)
Preventing Food Allergy
Intensive research is being focused on the pathogenesis and prevention of allergic disease, including the role of genetics and environmental factors such as early dietary exposures and feeding practices. Allergy prevention guidelines have gradually shifted away from allergen avoidance to examination of the role of specific dietary factors in the development and prevention of allergic disease (Jennings and Prescott, 2010).
Pregnancy and Infancy
The traditional approach for dietary allergy prevention has been avoidance of food allergens in the maternal diet and early postnatal period. However, there is a lack of evidence that maternal dietary restrictions during pregnancy help prevent atopic disease in infants. Food restriction to avoid antigen exposure while breast-feeding does not appear to prevent atopic disease, with the possible exception of atopic eczema (Greer et al., 2008). However, recent research indicates that exposure to food antigens in the “safe” environment of pregnancy and in breast milk is more likely to lead to tolerance rather than sensitization to those foods in the infant. Current trials on infant feeding are attempting to elucidate the concept of oral tolerance and define the effect that delayed introduction of solids and allergenic foods has on development of allergic disease.
Breastfeeding
Breast milk contains a host of immunologically active compounds such as transforming growth factor–beta, lactoferrin, lysozymes, long-chain fatty acids, antioxidants, and secretory IgA (sIgA), all of which have an effect on immune development, including oral tolerance, and help to reinforce the gut-epithelial barrier (Brandtzaeg, 2009; Jennings and Prescott, 2010). Breastfeeding without any maternal dietary restrictions is strongly encouraged, although the exact role of breastfeeding in allergy prevention is unclear. There is evidence that exclusive breastfeeding for at least 3 months protects against wheezing in early life (Greer et al., 2008). For infants at high risk of developing atopic disease (infants with a first-degree relative with allergy) exclusive breastfeeding for at least 4 months is recommended (Host et al., 2008). Continuation of breastfeeding through the time when solid foods are introduced is believed to help prevent the development of food allergy (Greer et al., 2008).
Sensitivity to breast milk is rare but has been reported. Allergens in the mother’s diet such as cow’s milk, eggs, and peanuts can pass into the breast milk and cause sensitization and then an allergic reaction in the exclusively breast-fed infant. Food challenges to each food will determine food symptom relationships. The mother eats a suspect food before nursing, and the infant is observed for symptoms for up to 24 hours after nursing. If a food is judged to yield a positive test result through challenge, that food is eliminated from the mother’s diet and she is encouraged to continue breastfeeding. The nutritional adequacy of the mother’s diet should be monitored when food groups are omitted from her diet.
Choice of Infant Formula
In infants at high risk of developing atopic disease who are not exclusively breastfed for 4 to 6 months, a partially hydrolyzed or extensively hydrolyzed formula to replace a cow’s milk formula is recommended. Extensively hydrolyzed formulas may be more protective than partially hydrolyzed formulas in prevention of atopic disease (Greer et al., 2008). Soy-based infant formulas offer no advantage for the purpose of allergy prevention and some infants may react adversely to these formulas. Amino acid–based formulas may be used in allergy, but have not been adequately studied for atopy prevention. See Boxes 27-5 and 27-8.
Solid Food Introduction
It is recommended that solid foods or complementary foods other than breastmilk or formula should not be introduced until 4 to 6 months of age. There is no convincing evidence that delaying introduction beyond this time prevents the development of atopic disease, and this also pertains to the introduction of foods that are considered to be highly allergenic such as peanuts, eggs, and fish (Greer et al., 2008, Jennings and Prescott, 2010). Although early exposure to some food antigens, such as wheat and gluten, is being promoted as a method of encouraging oral tolerance to them, this technique has not been proven to be effective (Poole et al., 2006).
Early Diet and Immunomodulatory Factors
Dietary factors in early life may influence asthma and allergic disease development. The immunoregulatory network in newborns is orchestrated not only by microbial products but also by dietary constituents such as vitamin A, vitamin D, ω-3 fatty acids, folate, and other micronutrients (Brandtzaeg, 2010).
Antioxidants
Diets high in antioxidants such as β-carotene, vitamin C, vitamin E, zinc, and selenium may prevent the development of food allergies. There have been positive associations found between maternal antioxidant status in pregnancy and cord blood immune responses (West et al, 2010). Higher maternal intake of green and yellow vegetables, citrus fruit, and β-carotene during pregnancy was significantly associated with a reduced risk of eczema, but not wheezing, in the infants. Maternal vitamin E consumption was inversely related to the risk of infantile wheeze, but not eczema (Miyake et al., 2010). Thus optimizing food sources of antioxidants from fruit and vegetable intake during pregnancy may be an effective effort for allergy risk reduction.
Folate
Folate deficiency has been associated with several disorders characterized by enhanced activation of the cellular, Th1-type immune response (Husemoen et al., 2006). An intriguing development has been the recognition of epigenetic effects of dietary folate in the development of asthma (Jennings and Prescott, 2010). Impaired folate metabolism may be related to the development of atopy, although the significance is not clear, because one study demonstrated that prenatal folate supplementation was associated with increased childhood wheezing (Miller, 2008), whereas another found the opposite (Matsui and Matsui, 2009).
Pre- and Probiotics
Prebiotics include the nondigestible, fermentable oligosaccharides that stimulate the growth and activity of bacteria in the colon, whereas probiotics are live microorganisms that impart health benefits to the host. Their role in allergy prevention has not been thoroughly studied and should elucidate the effect of the individual strain, timing, dose, and environmental factors that affect colonization and host genetic factors. By supplementing during pregnancy 1 month before delivery, or providing the infant with 6 months’ treatment of probiotic therapy either from the nursing mother or with direct supplementation, the incidence of infant food allergy–related atopic eczema may be reduced (Rautava et al., 2005). However, administration of probiotics to infant feeds for prevention of allergic disease requires further investigation and studies have not yielded consistent results (Osborn and Sinn, 2007).
Polyunsaturated Fatty Acids (PUFA)
The role of polyunsaturated fatty acids (ω-3 and ω-6 PUFAs) in allergy development has been the subject of investigation because PUFAs have effects on immune function and inflammation. Some studies have suggested that maternal consumption of fish oil in pregnancy protects against the development of asthma, eczema, and allergic sensitization. However, a recent systematic review indicated that supplementation with ω-3 and ω-6 oils is unlikely to play an important role for the primary prevention of sensitization or allergic disease (Anandan et al., 2009). Further studies are needed to elucidate the role of fatty acids in allergy prevention and their role in the inflammatory cascade. In the meantime, inclusion of both plant (flaxseeds, hempseeds, chia seeds, purslane, organic soybeans, walnuts) and animal food sources (safe wild fish) of ω-3 PUFAs in the maternal diet can be encouraged.
Vitamin D
It has been proposed that the increase in the development of food allergy in children may be due to an increased prevalence of vitamin D deficiency. Deficiency of this vitamin in a developmentally critical period increases susceptibility to colonization of the gut with abnormal intestinal microbial flora and GI infections, contributing to an abnormally porous gut and inappropriate exposure of the immune system to dietary allergens. Vitamin D helps promote immunoregulation through T-cell differentiation and has been found to be associated with a reduced risk of infant wheezing (Jennings and Prescott, 2010). Preliminary studies suggest that early correction of vitamin D deficiency might promote mucosal immunity, healthy microbial ecology, and allergen tolerance, and may prevent food allergy development (Vassallo and Camargo, 2010).
Food Allergy and Anaphylaxis Network
The American Latex Allergy Association
http://www.latexallergyresources.org/
American Academy of Allergy, Asthma, and Immunology
References
Anandan, C, et al. ω 3 and 6 oils for primary prevention of allergic disease: systematic review and meta-analysis. Allergy. 2009;64:840.
Barrett, JS, Gibson, PR. Development and validation of a comprehensive semi-quantitative food frequency questionnaire that includes FODMAP intake and glycemic index. J Am Diet Assoc. 2010;110:1469.
Bischoff, S, Crowe, SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128:1089.
Blanco, C. Latex-fruit syndrome. Curr Allergy Asthma Rep. 2003;3:47.
Boyce, JA, et al. Guidelines for the diagnosis and management of food allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Am Diet Assoc. 2011;111:17.
Brandtzaeg, P. Food allergy: separating the science from the mythology. Nat Rev Gastroenterol Hepatol. 2010;7:380.
Brandtzaeg, P. “ABC” of mucosal immunology. Nestle Nutr Workshop Ser Pediatr Prog. 2009;64:23.
Burks, AW, et al. Oral tolerance, food allergy, and immunotherapy: implications for future treatment. J Allergy Clin Immunol. 2008;121:1344.
Chafen, JJS, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848.
Clark, AT, et al. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009;64:1218.
Condemi, J. Allergic reactions to natural rubber latex at home, to rubber products, and to cross-reacting foods. J Allergy Clin Immunol. 2002;110:S107.
Dona, A, Arvanitoyannis, IS. Health risks of genetically modified foods. Crit Rev Food Sci Nutr. 2009;49:164.
Drisko, J, et al. Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J Am Coll Nutr. 2006;25:514.
Durrant, DM, Metzger, DW. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol Invest. 2010;39:526.
DuToit, G. Food-dependent exercise-induced anaphylaxis in childhood. Pediatr Allergy Immunol. 2007;18:455.
Eroglu, Y, et al. Pediatric eosinophilic esophagitis: single-center experience in northwestern USA. Pediatr Int. 2009;51:531.
Fernandez-Rivas, M, et al. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006;118:481.
Finamore, A, et al. Intestinal and peripheral immune response to MON810 maize ingestion in weaning and old mice. J Agriculture Food Chem. 2008;56:11533.
Franchini, S, et al. Emergency treatment of asthma. N Engl J Med.. 2010;363:2567.
Geha, R, et al. Multicenter, double-blind, placebo-controlled, multiple-challenge evaluation of reported reactions to monosodium glutamate. J Allergy Clin Immunol. 2000;106:973.
Genuis, SJ. Sensitivity related illness: the escalating pandemic of allergy, intolerance and chemical sensitivity. Sci Total Environ. 408, 2010. [60-47].
Gibson, PR, Shepherd, SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25:252.
Geroldinger-Simic, M, et al. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG(4) antibodies. J Allergy Clin Immunol. 2011;127:616.
Greer, FR, et al. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183.
Groschwitz, KR, Hogan, SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3.
Hepworth, K. Eating disorders today—not just a girl thing. J Christ Nurs. 2010;27:236.
Hofmann, A, Burks, AW. Pollen food syndrome: update on the allergens. Curr Allergy Asthma Rep. 2008;8:413.
Host, A, et al. Dietary prevention of allergic diseases in infants and small children. Amendment to previous published articles in Pediatric Allergy and Immunology 2004, by an expert group set up by the Section on Pediatrics. Europ Acad Allergology Clin Immunology, Pediatr Allergy Immunol. 2008;19:1.
Husemoen, LL, et al. The association between atopy and factors influencing folate metabolism: is low folate status causally related to the development of atopy? Int J Epidemiol. 2006;35:954.
Järvinen, KM, Chatchatee, P. Mammalian milk allergy: clinical suspicion, cross-reactivities and diagnosis. Curr Opin Allergy Clin Immunol. 2009;9:251.
Jarvela, I, et al. Molecular genetics of human lactase deficiencies. Ann Med. 2009;41:568.
Jennings, S, Prescott, SL. Early dietary exposures and feeding practices: role in pathogenesis and prevention of allergic disease? Postgrad Med J. 2010;86:94.
Joneja, JMV. Dealing with food allergies in babies and children. Boulder, CO: Bull Publishing Company; 2007.
Joneja, JMV. Dealing with food allergies: a practical guide to detecting culprit foods and eating a healthy, enjoyable diet. Boulder, CO: Bull Publishing Company; 2003.
Kagalwalla, AF, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097.
Kelsay, K. Psychological aspects of food allergy. Curr Allergy Asthma Rep. 2003;3:41.
Kondo, Y, Urisu, A. Oral allergy syndrome. Allergol Int. 2009;58:485.
Kurup, VP, et al. Immune response modulation by curcumin in a latex allergy model. Clin Mol Allergy. 2007;5:1.
Lack, G. Epidemiological risks for food allergy. J Allergy Clin Immunol. 2008;121:1331.
Maintz, L, Novak, N. Getting more and more complex: the pathophysiology of atopic eczema. Eur J Dermatol. 2007;17:267.
Matsui, EC, Matsui, W. Higher serum folate levels are associated with a lower risk of atopy and wheeze. J Allergy Clin Immunol. 2009;123:1253.
Mehr, S, et al. Food protein-induced enterocolitis syndrome: 16-year experience. Pediatrics. 2009;123:e459.
Miller, RL. Prenatal maternal diet affects asthma risk in offspring. J Clin Invest. 2008;118:3265.
Miyake, Y, et al. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65:758.
Morita, E, et al. Food-dependent exercise-induced anaphylaxis-importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Allergol Int. 2009;58:493.
Mullin, GE, et al. Testing for food reactions: the good, the bad and the ugly. Nutr Clin Prac. 2010;25:192.
NIAID-Sponsored Expert Panel, Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored Expert Panel. J Allerg Clin Immunol. 2010;126(6 Suppl):S1. www.niaid.nih.gov/topics/foodallergy/clinical/pages/default.aspx [Accessed April, 2011].
Noimark, L, Cox, HE. Nutritional problems related to food allergy in childhood. Pediatr Allergy Immunol. 2008;19:188.
Nowak-Wegrzyn, A, Sampson, H. Adverse reactions to foods. Med Clin North Am. 2006;90:1.
Osborn DA, Sinn JK: Probiotics in infants for prevention of allergic disease and food hypersensitivity, Cochrane Database Syst Rev 17(4):CD006475, 2007.
Pessler, F, Nejat, M. Anaphylactic reaction to goat’s milk in a cow’s milk-allergic infant. Pediatr Allergy Immunol. 2004;15:183.
Poole, JA, et al. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics. 2006;117:2175.
Pusztai, A, Bardocz, S. GMO in animal nutrition: potential benefits and risks. In: Mosenthin R, Zentek J, Zebrowska T, eds. Biology of nutrition in growing animals. St Louis: Elsevier, 2005.
Pyrhönen, K, et al. Heredity of food allergies in an unselected child population: an epidemiological survey from Finland. Pediatr Allergy Immunol. 2011;22(1pt2):e124.
Rautava, S, et al. New therapeutic strategy for combating the increasing burden of allergic disease: probiotics—a Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota (NAMI) Research Group report. J Allergy Clin Immunol. 2005;116:1.
Reese, I, et al. Diagnostic approach for suspected pseudoallergic reaction to food ingredients. J Dtsch Dermatol Ges. 2009;7:70.
Robson-Ansley, P, Toit, GD. Pathophysiology, diagnosis and management of exercise-induced anaphylaxis. Curr Opin Allergy Clin Immunol. 2010;10:312.
Rothenberg, ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004;113:11.
Roy-Ghanta, S, et al. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6:531.
Sampson, H. Update on food allergy. J Allergy Clin Immunol. 2004;113:5.
Santos, A, Van Ree, R. Profilins: mimickers of allergy or relevant allergens? Int Arch Allergy Immunol.. 2011;155:191.
Savage, JH, et al. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120:1413.
Sicherer, SH, Sampson, HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116.
Skripak, JM, et al. The natural history of Ig-E mediated cow’s milk allergy. J Allergy Clin Immunol. 2007;120:1172.
Spergel, JM, et al. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336.
Stahl, MC, Rans, TS. Potential therapies for peanut allergy. Ann Allergy Asthma Immunol. 2011;106:179.
Stapel, SO, et al. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy. 2008;63:793.
Teuber, SS, Porch-Curren, C. Unproved diagnostic and therapeutic approaches to food allergy and intolerance. Curr Opin Allergy Clin Immunol. 2003;3:217.
Vally, H, et al. Clinical effects of sulphite additives. Clin Exp Allergy. 2009;39:1643.
Vassallo, MF, Camargo, CA. Potential mechanisms for the hypothesized link between sunshine, vitamin D and food allergy in children. J Allergy Clin Immunol. 2010;126:217.
Vickery, BP, et al. Pathophysiology of food allergy. Pediatr Clin N Am. 2011;58:363.
West, CE, et al. Role of diet in the development of immune tolerance in the context of allergic disease. Curr Opinion Pediatr. 2010;22:635.
Williams, AN, Woessner, KM. Monosodium glutamate ‘allergy’: menace or myth? Clin Exp Allergy. 2009;39:640.
Zapatero, L, et al. Oral desensitization in children with cow’s milk allergy. J Invest Allergol Clin Immunol. 2008;18:389.
Zolla, L, et al. Proteomics as a complementary tool for identifying unintended side effects occurring in transgenic maize seeds as a result of genetic modifications. J Proteome Res. 2008;7:1850.

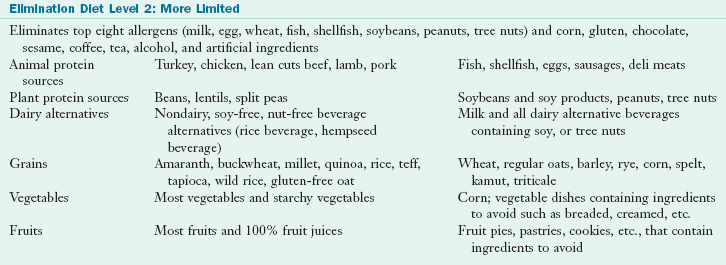

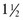 tsp Ener G Egg Replacer (ENERG-G Foods, Inc.) + 1 Tbsp of water
tsp Ener G Egg Replacer (ENERG-G Foods, Inc.) + 1 Tbsp of water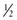 tsp baking powder + 1 Tbsp liquid + 1 Tbsp vinegar
tsp baking powder + 1 Tbsp liquid + 1 Tbsp vinegar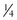 c warm water
c warm water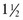 Tbsp water +
Tbsp water + 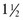 Tbsp oil + 1 tsp baking powder
Tbsp oil + 1 tsp baking powder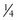 c soft tofu, beaten
c soft tofu, beaten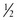 tsp oil +
tsp oil + 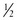 tsp baking powder + 2 Tbsp milk, water, or fruit juice.
tsp baking powder + 2 Tbsp milk, water, or fruit juice.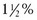 , 1%,
, 1%, 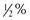 , skim, evaporated, condensed)
, skim, evaporated, condensed)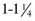 c rye flour
c rye flour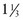 c rolled oats or oat flour
c rolled oats or oat flour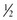 c potato flour + 1/2 c rye flour
c potato flour + 1/2 c rye flour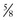 c potato starch
c potato starch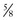 c rice flour + 1/3 c rye flour
c rice flour + 1/3 c rye flour